Abstract
HBoV is an emergent virus, which is frequently detected as a co‐infective agent. However, it can cause disease on its own. It is associated with respiratory and diarrhoeal illness in children and adults, whether immunocompetent or immunocompromised. We report HBoV infection in a child post‐liver transplantation, who presented with persistent fever and mild tachypnea, 3 weeks after a successful transplant. She recovered spontaneously with no graft dysfunction.
Keywords: bocavirus, liver transplant, paediatric
Abbreviations
- CMV
cytomegalovirus
- CRP
C‐reactive protein
- CT
computed tomography
- EBV
Epstein‐Barr virus
- HBoV
human bocavirus
- IF
immunofluorescent
- PCR
polymerase chain reaction
- RVP,
respiratory viral panel
1. Introduction
HBoV is a recently discovered virus of the Parvoviridae family that has been recognized as a co‐infective virus along with other established viruses causing respiratory tract infection.1 It has been associated with respiratory tract and/or diarrhoeal illnesses in children and adults, although having a predilection for children, in both immunocompetent and immunocompromised patients.1
After solid organ transplant, children are immunocompromised. They are predominantly on calcineurin inhibitor‐based immunosuppressive regimens, with or without corticosteroids, to avoid graft rejection. This puts them at increased risk of infections. Although with improved molecular diagnostic techniques, newer viruses have been detected with increasing incidence, more so in the transplant population.2 We report our experience with HBoV following paediatric liver transplantation.
2. Case Report
A 15‐month‐old girl underwent liver transplant for decompensated chronic liver disease, secondary to underlying biliary atresia. Her pretransplant clinical course had been complicated by sepsis with renal impairment. Her initial sepsis was attributed to intra‐abdominal collections. She had episodes of prolonged fever even after her collections were drained, and a respiratory multiplex (PCR‐based test) to look for viruses, from nasopharyngeal aspirates, was negative (3 weeks prior to transplant). She had eventual full recovery.
In terms of immunosuppression, intravenous basiliximab was used as a renal‐sparing agent in the immediate post‐transplant period with tacrolimus being introduced later. Intravenous mycophenolate mofetil was used as adjuvant immunosuppression, with corticosteroids. Her course was complicated by biopsy‐proven acute cellular rejection on the 7th postoperative day, requiring pulsed intravenous methylprednisolone (10 mg/kg/day). Steroids were subsequently weaned off. She made steady progress post‐transplant, with normal graft function.
She developed persistent low‐grade pyrexia up to 38.5°C (101.3 Fahrenheit), 3 weeks after her transplant. Clinically, she had mild tachypnea even when not febrile, with normal breath sounds on examination. She had mild ascites, which was improving from her preliver transplant state, thus unlikely contributing to her tachypnea. Her abdomen was otherwise soft and non‐tender. There was no diarrhoea or other clinical focus of infection. Her inflammatory markers were unremarkable—full blood count showed a white cell count of 9.72×109/L (normal range 5.00‐15.00), with mild lymphopenia at 2.00×109/L (normal range 6.00‐9.00), without neutropenia. CRP was minimally raised, at 14 mg/L.
Investigations were done to look for a source of the fever. This included a blood and urine culture which returned negative. An ultrasound of the abdomen did not show significant intra‐abdominal collections or abscesses. Chest X‐ray showed patchy bibasal atelectasis (Figure 1), with mild peribronchial haziness, without any consolidation. A nasal swab sample sent for immunofluorescence for common respiratory viruses was negative as well.
Figure 1.
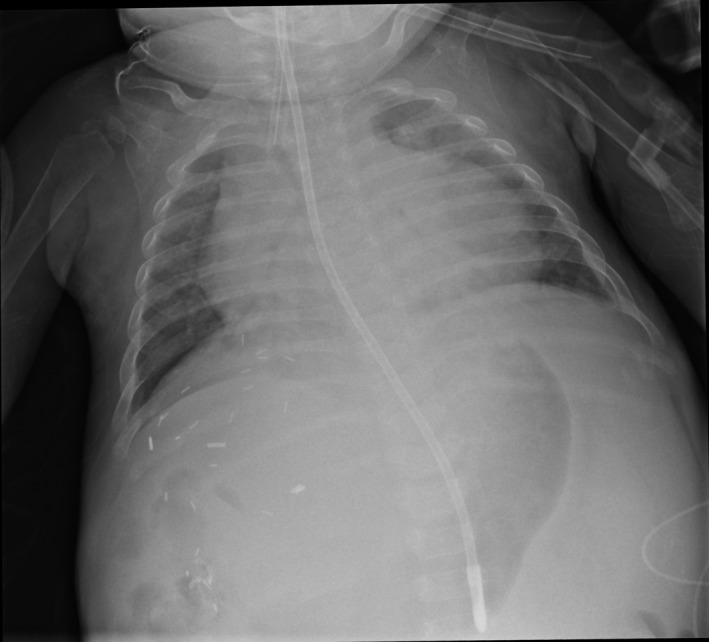
Mild bibasal atelectasis on plain radiograph of the chest
Prior to transplant, her CMV serostatus was positive, and EBV was negative. Her donor was CMV seropositive, and thus she was on intravenous ganciclovir initially and subsequently on oral valganciclovir from the immediate post‐transplant period. At the time of persistent fevers, EBV and CMV PCR results were negative.
She remained clinically well, but was started empirically on broad‐spectrum antibiotics, while cultures were pending. As the fever persisted, a CT scan of the abdomen was obtained to look for collections that could have been missed earlier. This showed a possible collection; however, when drained, it did not show any evidence of bacterial or fungal infection; and her fever persisted despite this drainage. Her CT scan also showed bilateral atelectasis that was seen in her chest X‐ray. A respiratory multiplex PCR to look for other viruses was also sent off from nasopharyngeal fluid.
The PCR (the xTAG RVP FAST v2 nucleic acid multiple test) for HBoV was positive, with no other viruses detected (negative for influenza, parainfluenza, and coronavirus groups). Her fever settled after a week, and she was well at discharge. We did not have to modify her immunosuppression, and her graft function remains good on follow‐up with no other clinical concerns.
3. Discussion
First described in 2005, HBoV is a recently discovered virus1 and clinical information is still being gathered regarding the role of HBoV. Studies in otherwise well and immunocompromised children are just emerging in the last few years, and in the latter mostly in oncology/stem cell transplant patients.
Clinical presentation is still not clearly defined, but has been associated with respiratory tract infections (both upper and lower tracts) as well as gastroenteritis in children.3 It is also commonly seen as a co‐infection3 and found shed in the stool and respiratory secretions of asymptomatic children.4
However, in recent years, it has been emerged that HBoV alone is pathogenic and can cause disease in immunocompetent children, most commonly presenting with respiratory symptoms, such as wheeze and bronchiolitis.4
In our patient, there were many potential sources of fever, one being the abdominal collection which was drained. We acknowledge that she was on broad‐spectrum antibiotics and hence cultures might be negative. However, her fever persisted for a week after, despite drainage and antibiotics, leading us to conclude HBoV as the most likely pathogen causing her fever. While we recognize HBoV still has unclear clinical significance, in our patient, we think HBoV is most likely pathogenic given the consistent respiratory symptoms, single pathogen isolated, with no other source to explain symptoms.
Interesting to note is that she did have respiratory samples sent for IF as well as for multiplex testing 3 weeks prior to transplant, which were negative. Thus, it is likely that HBoV was acquired and not a reflection of asymptomatic carriage. Hence, the new detection of HBoV coinciding with her respiratory symptoms and fever further supports our hypothesis that HBoV is pathogenic.
We do acknowledge the limitation that we did not perform subsequent advanced testing such as serological analysis, quantitative single real‐time PCR, immunofluorescence assays or sequencing to confirm HBoV‐positive results. Unfortunately, these advanced tests are not routinely offered in our laboratory and available only at highly specialized diagnostic laboratories overseas. However, that would incur a high cost, and our patient was an overseas patient from a neighbouring country with limited financial resources.
The value and the role of quantitative assay in assessing the severity of the HBoV disease are also debated.5, 6 More importantly, the patient's fever settled after a week, and she was discharged well. Thus, we did not pursue further advanced analysis of subsequent respiratory samples by PCR or other methods to confirm the HBoV or gain information on duration of viral shedding in the respiratory tract or of blood samples to monitor for HBoV dissemination. We also did not send blood samples to assess for viraemia.
The message that we want to give across through the case is, even in the absence of availability or requirement of specific antiviral therapy in the given setting, being vigilant about newer emerging viruses that can potentially cause severe disease is needed to avoid unnecessary and often expensive investigations. The HBoV is associated with higher hospitalization days,6 can cause neurological infections,7 and may contribute to chronic disease in adulthood as it can persist and reactive during later period of life.8
There are a few reports of HBoV infections in immunocompromised hosts wherein they have caused respiratory or diarrhoeal illness, and rarely even life‐threatening conditions in the stem cell transplant population. Severe diarrhoeal illness was reported in a 9‐year‐old boy with underlying erythropoietic protoporphyria, who had undergone orthotopic liver transplant and previous stem cell transplant, 9 months and 4 months, respectively, prior to presentation.9 HBoV has also been shown to cause disseminated illness in a child, post‐stem cell transplant.10 HBoV infection has also been postulated to be related to hepatitis in a child with T‐cell immunodeficiency.11
There are no reports of HBoV‐associated respiratory infections in children after liver transplantation. Our patient had an atypical presentation with pyrexia and only mild tachypnea—she had no cough nor symptoms or signs of a lower respiratory tract infection. The commonly available IF‐based testing for respiratory viruses was negative as well. The diagnosis was achieved only with the extended PCR‐based test. Her chest X‐ray did not show any significant changes, although there were subtle findings of bibasal atelectasis (Figure 1). These are frequently seen in children with significant ascites preliver transplant, especially in the immediate post‐transplant period, as they are weaned off mechanical ventilation. Moreover, our patient had significant ascites pretransplant which was actually improving when her persistent fevers began, thus unlikely to explain her tachypnea.
Our experience highlights the importance of awareness to have a lower threshold for screening for atypical newer pathogens, which could potentially cause infectious complications post‐liver transplantation. Our patient will add to the international experience of HBoV in transplant recipients.
4. Conclusion
As far as we are aware, there are few reports of bocavirus infections in solid organ transplant recipients in children. In post‐transplant patients, where immunosuppression is crucial to avoid rejection, there is a need to be vigilant about infections, which can result in poor outcomes. There is a need to be aware of newer emerging viruses which can potentially cause severe disease. Although no specific antiviral therapy is available or required in this setting, identification of the cause of illness post‐liver transplant can potentially avoid unnecessary and often expensive investigations.
Authors' ContributionS
MY Tan and SV Karthik: Initiated and drafted the report; LN Tan, M Aw, and SH Quak: Involved in patient care and critical input on draft case report. All authors contributed to the writing, reviewing, editing, and approval of the case report submitted.
Acknowledgments
We thank Dr Dimple Rajgor for her assistance in editing, formatting, and submission of the manuscript for publication.
Tan MY, Tan LN, Aw MM, Quak, SH , Karthik SV. Bocavirus infection following paediatric liver transplantation. Pediatr Transplant, 2017;21:e12840. doi: 10.1111/petr.12840
References
- 1. Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund‐Venermo M. Human bocavirus‐the first 5 years. Rev Med Virol. 2012;22:46–64. [DOI] [PubMed] [Google Scholar]
- 2. Waggoner JJ, Soda EA, Deresinski S. Rare and emerging viral infections in transplant recipients. Clin Infect Dis. 2013;57:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schildgen O, Muller A, Allander T, et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21:291–304, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ursiç T, Petrovec M. Chapter 15 ‐ Human Bocavirus In: Ergönül O, Can Fs, Madoff L, Akova M, eds. Emerging Infectious Diseases. Amsterdam: Academic Press; 2014:191–201. [Google Scholar]
- 5. Ghietto LM, Majul D, Ferreyra Soaje P, et al. Comorbidity and high viral load linked to clinical presentation of respiratory human bocavirus infection. Arch Virol. 2015;160:117–127. [DOI] [PubMed] [Google Scholar]
- 6. Principi N, Piralla A, Zampiero A, et al. Bocavirus infection in otherwise healthy children with respiratory disease. PLoS One. 2015;10:e0135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noorbakhsh S, Monavari HR, Tabatabaei A. Neurological manifestaions in acute onset of viral gastroentritis. J AIDS Clin Res. 2013;4:189. [Google Scholar]
- 8. Ringshausen FC, Tan A, Allander T, et al. Frequency and clinical relevance of human bocavirus infection in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Vries JJ, Bredius RG, Van Rheenen PF, et al. Human bocavirus in an immunocompromised child presenting with severe diarrhea. J Clin Microbiol. 2009;47:1241–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schenk T, Strahm B, Kontny U, Hufnagel M, Neumann‐Haefelin D, Falcone V. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis. 2007;13:1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kainulainen L, Waris M, Soderlund‐Venermo M, Allander T, Hedman K, Ruuskanen O. Hepatitis and human bocavirus primary infection in a child with T‐cell deficiency. J Clin Microbiol. 2008;46:4104–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]


