Abstract
Respiratory infections in infancy may protect against developing Th2‐mediated allergic disease (hygiene hypothesis). To estimate the relative contribution of particular viruses to the development of the immune system and allergic disease, we investigated longitudinally the prevalence of respiratory viral infections in infants. One hundred and twenty‐six healthy infants were included in this prospective birth cohort study in their first year of life. Physical examination was performed and nasal brush samples were taken during routine visits every 6 months and during an upper respiratory tract infection (URTI) (sick visits). The prevalence of respiratory viral infections in infants with URTI, infants with rhinitis without general malaise and infants without nasal symptoms was studied. Rhinovirus was the most prevalent pathogen during URTI and rhinitis in 0‐ to 2‐year‐old infants (∼40%). During URTI, also respiratory syncytial virus (∼20%) and coronavirus (∼10%) infections were found, which were rarely detected in infants with rhinitis. Surprisingly, in 20% of infants who did not present with nasal symptoms, rhinovirus infections were also detected. During routine visits at 12 months, a higher prevalence of rhinovirus infections was found in infants who attended day‐care compared with those who did not. We did not observe a relation between breast‐feeding or smoking by one or both parents and the prevalence of rhinovirus infections. The parental history of atopy was not related to the prevalence of rhinovirus infection, indicating that the genetic risk of allergic disease does not seem to increase the chance of rhinovirus infections. In conclusion, rhinovirus infection is the most prevalent respiratory viral infection in infants. It may therefore affect the maturation of the immune system and the development of allergic disease considerably.
Keywords: allergy, infant, day‐care, prevalence, respiratory infection, rhinovirus, RSV
The human immune system can respond in various ways to environmental factors. Two of the main responses are the production of T helper 1 (Th1) cytokines during viral and bacterial infections and Th2 cytokine production upon parasite infection 1, 2, 3). Particularly in the Western world, there has been an increase in the number of subjects who respond to common allergens such as grass pollen and house dust mite, a response which is characterized by Th2 cytokine production (4). A change in exposure to pathogens may underlie this increased prevalence of allergic disease.
The infant immune system differs from that of adults as it is skewed towards a Th2 response after birth, developing into a normal adult Th1 cytokine profile in subsequent years (5). As postulated in the hygiene hypothesis (6), frequent infections during childhood may affect this maturation process. Th1 cytokine production during infection may skew the infant immune response from Th2 to Th1. As a consequence, infants suffering from many infections may have a diminished risk of developing Th2‐mediated allergic disease.
Several types of infection may account for the reduced risk of allergic disease. Lactobacilli colonizing the gut immediately after birth and bacterial infection or exposure to bacterial products during childhood may reduce the risk of allergic disease (7, 8). Because viruses induce the majority of respiratory tract infections, these pathogens have been most extensively studied in relation to allergic disease (9). Epidemiological studies have demonstrated a reduced risk of allergic disease in children with older siblings and children attending day‐care, who are known to be all heavily exposed to viral infections (10, 11). Recently, the first direct evidence for this protective effect was provided by a study of Illi and colleagues, who found a twofold reduction of asthma in children of school age with repeated viral respiratory tract infections in the first 3 years of life (12).
Some data show that viral respiratory tract infections can trigger the development of allergic disease. The high risk of wheezing symptoms after bronchiolitis in infants induced by respiratory syncytial virus (RSV) has suggested the possibility of the subsequent development of asthma. Indeed, Sigurs and colleagues were able to show that children with bronchiolitis had an increased risk of allergic disease at age 7 (13). But others were not able confirm these observations. Although Stein and colleagues found an increased risk of recurrent wheezing in 7‐year‐old children after bronchiolitis, no increased risk of allergic disease was found at age 14 (14).
The VIGALL (Dutch abbreviation for ‘Virus mediated allergy’) birth cohort study aims at investigating the effect of upper respiratory tract infections (URTI) on the development of allergic disease and the maturation of the infant immune system. Both the prevalence of a particular virus and the host cellular antiviral immune response probably determine the general contribution of the virus infection on the maturation of the immune system. Initially, then, we need to investigate the prevalence of viruses during URTI in infants. In adults and children of school age, rhinovirus is the most prevalent inducer of respiratory pathology, which is found in up to 80% of patients with a common cold (15, 16) and during the majority of asthma exacerbations (17). In infants, viruses have mainly been studied during bronchiolitis, but they have hardly been studied at all during URTI. RSV, and occasionally parainfluenzavirus (PIV) and influenzavirus infections, have been mainly found in bronchiolitis 18, 19, 20). Only recently, rhinovirus infections have been reported in common colds in infants (21). In this paper, we analyzed the prevalence of respiratory viruses in 0‐ to 2 year‐old infants in relation to severity of symptoms, day‐care attendance and the age of the child.
Methods
Participants
The aim of the VIGALL study was to investigate the relation between viral URTI and the development of allergy during childhood. One hundred and twenty‐six healthy infants, living in the Rotterdam area (the Netherlands), were included in this prospective birth cohort study and were followed until 2 years of age. Recruitment of children took place at the Department of Obstetrics (Sophia's Children Hospital), at health centres, and among participants of the PIAMA birth cohort study (22). Eighty‐six infants (68%) were selected who had a family history of atopy, defined as allergic disease in one or both parents. Parents with self‐reported asthma, hay‐fever, house dust mite allergy, or pet allergy were considered to be allergic. This was established with a validated screening questionnaire (23). Forty infants were selected with a negative family history of atopy; infants of whom both parents reported not to have allergic disease. The Erasmus University Medical Ethics Committee in Rotterdam approved the study design and parents of all infants gave informed consent.
Data collection
Information was collected on birth characteristics (duration of pregnancy, gender, birth weight, numbers of siblings, season of birth), indoor environmental and lifestyle factors (smoke exposure, breast‐feeding, day‐care attendance) using questionnaires completed by the parents when the child reached 3, 12 and 24 months. General symptoms of illness such as rhinorrhoea, cough, fever, and symptoms of allergic disease such as skin rash and wheezing were scored by the parents on weekly symptom cards.
Parents were asked to contact the study doctor when their child had signs of an URTI, defined as rhinorrhoea and fever, general malaise, sleeping difficulties or loss of appetite. During the subsequent sick visits, infants were considered to have a common cold and were assigned to the ‘URTI group’. Physical examination was performed and nasal brush samples were taken. A medical history was taken on general illness symptoms (fever, general malaise, loss of appetite), upper respiratory tract disease (rhinorrhoea, sore throat), lower respiratory tract disease (wheeze, cough, dyspnoea), skin rash and numbers of upper respiratory tract infections in the preceding follow‐up period. During sick visits, infants were grouped around 6 (2–9), 12 (10–15), 18 (16–21) and 24 (22–29) months of age.
Children visited the Sophia's Children Hospital for routine visits at the age of 6, 12, 18 and 24 months. A physical examination was performed, and a medical history and nasal brush sample were taken. During routine visits, infants were assigned to two groups. Infants with rhinorrhoea, but without signs of fever, general malaise, sleeping difficulties and loss of appetite were assigned to the ‘rhinitis group’. Children without rhinorrhoea were considered not to have upper respiratory tract disease and were assigned into the ‘nasal‐symptom‐free group’.
Parentally reported episodes of rhinorrhoea
The numbers of rhinorrhoea episodes that infants at 12 and 24 months of age had suffered from in the preceding year of life were calculated from the weekly symptom cards. Missing data were obtained from medical histories taken at 6, 12, 18 and 24 months of age. Infants were classified into three groups: (1) infants with 0–3 episodes a year, (2) infants with 3–8 episodes a year and (3) infants with continuous rhinorrhoea, defined as rhinorrhoea for more than 16 weeks a year.
Day‐care facilities
Infants were considered to attend day‐care when they were cared for regularly by relatives or foster parents and have contact with small numbers of children (<5 children) other than their siblings, and when infants attended large day‐care facilities (usually >10 children).
Viral diagnostics in nasal brushes
Cells were harvested from the nasal cavity with a cytobrush (Medscand Medical, Malmö, Sweden) and processed as previously described (24). Cells were collected in 7 ml of RPMI‐1640 medium (Life Technologies, Breda, the Netherlands). After centrifugation, 4 ml of supernatant was used for the isolation of influenzavirus, parainfluenzavirus, RSV, adenovirus, cytomegalovirus (CMV), enterovirus and echovirus and 3 ml was frozen and stored at −80°C. Cells were stained with fluorescent labeled antiviral antibodies to detect RSV, influenzavirus, parainfluenzavirus and adenovirus. Rhinovirus and coronavirus were detected by the isolation of viral RNA from 0.5 ml of frozen nasal brush supernatant using the MagnaPure LC Instrument (Roche Applied Science, Penzberg, Germany) and amplification by RT‐PCR followed by hybridization with either rhinovirus‐ or coronavirus‐specific radiolabeled probes (25).
Statistical analysis
For the purposes of a simultaneous evaluation of the prevalence of individual viruses, logistic regression was used (GEE estimation using the GENMOD module from SAS; SAS Institute Inc., Cary, NC, USA), allowing for differences between, as well as within, individuals. The age of the child, symptom severity, season of sampling (winter: October to March, summer: April to September), a family history of atopy and day‐care attendance were the factors examined. Fisher's exact test was used to evaluate the relation between rhinovirus prevalence and several genetic and environmental factors at 12 and 24 months separately. The McNemar test was used to compare prevalences of different viruses within symptom and age groups. Kaplan–Meier survival analysis was used to examine differences between children who dropped out at 12, 18 or 24 months compared with those who completed the study. Differences were considered statistically significant if p was ≤0.05.
Results
Patient characteristics
One hundred and twenty‐six infants were followed for the first 2 years of life and examined during routine and sick visits. Table 1 shows the number of visits as well as the clinical symptoms of the child during the visit. None of the infants were admitted to hospital due to severe lower respiratory tract infection. Forty‐two children (33%) dropped out for various reasons. Fifteen children dropped out at 12 months of age, 22 children at 18 months and 5 children at 24 months of age. No differences were found between the children who dropped out and those who did not in terms of family history of atopy, day‐care attendance, numbers of episodes of rhinorrhoea, and development of atopic dermatitis in the preceding follow‐up period (data not shown).
Table 1.
Clinical symptoms in infants (%) during routine and sick visits
| Sick visits | Routine visits | ||
|---|---|---|---|
| URTI | Rhinitis | Nasal‐symptom‐free | |
| n | 80 | 133 | 221 |
| Rhinorrhoea | 80 (100%) | 133 (100%) | 0 (0%) |
| Loss of appetite | 47 (59%) | 0 (0%) | 4 (2%) |
| General malaise | 66 (83%) | 0 (0%) | 4 (2%) |
| Fever | 40 (50%) | 0 (0%) | 0 (0%) |
| Wheeze | 14 (18%) | 13 (10%) | 5 (2%) |
| Cough | 68 (85%) | 76 (57%) | 41 (19%) |
URTI: Upper respiratory tract infection.
Prevalence of respiratory viruses in infants
The prevalence of viral respiratory pathogens was examined in infants with URTI, with rhinitis or without nasal symptoms separately around 6, 12, 18 and 24 months of age (Table 2). During URTI, a viral infection was diagnosed in 58% (6 months) to 80% (24 months) of the infants. At 6 months of age, rhinoviruses (27%), RSV (18%) and coronaviruses (21%) were mainly found during URTI. In children of 12 months and older, the most prevalent pathogen during URTI was rhinovirus (43% at 12 months to 60% at 24 months). In infants with rhinitis, a positive virus diagnosis was found in 34% (18 months) to 58% (12 months) and in infants without nasal symptoms in 16% (24 months) to 33% (6 months). These were mainly rhinoviruses (85% and 75% of virus infections, respectively). At 6 months of age in the nasal‐symptom‐free group, in addition to rhinovirus, significantly more CMV was detected compared with PIV, influenzavirus, adenovirus, and enterovirus. Multiple‐virus infections were diagnosed in 15% of infants with URTI, 8% of infants with rhinitis and 3% of infants without nasal symptoms. Rhinovirus and coronavirus infections were mostly found in combination with any other virus.
Table 2.
Prevalences of respiratory viruses in infants with URTI, with rhinitis or infants without nasal symptoms at 6, 12, 18 and 24 months of age
| n | Any virus detected (%) | Rhino‐virus (%) | RSV (%) | Corona‐virus (%) | PIV (%)* | Influenza‐virus (%)† | CMV (%) | Other viruses‡ (%) | |
|---|---|---|---|---|---|---|---|---|---|
| URTI | |||||||||
| 6 months | 33 | 19 (58) | 9 (27) | 6 (18) | 7 (21) | 2 (6) | 2 (6) | 1 (3)¶ | 2 (6)** |
| 12 months | 14 | 11 (79) | 6 (43) | 3 (21) | 0 (0)* | 2 (14) | 1 (7) | 0 (0)** | 0 (0)** |
| 18 months | 28 | 20 (71) | 15 (54) | 3 (11)¶ | 1 (4)¶ | 0 (0)¶ | 1 (4)¶ | 0 (0)¶ | 2 (7)¶ |
| 24 months | 5 | 4 (80) | 3 (60) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rhinitis | |||||||||
| 6 months | 46 | 25 (54) | 19 (41) | 1 (2)¶ | 3 (7)¶ | 3 (7)¶ | 0 (0)¶ | 3 (7)¶ | 1 (2)¶ |
| 12 months | 33 | 19 (58) | 17 (52) | 1 (3)¶ | 1 (3)¶ | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 1 (3)¶ |
| 18 months | 29 | 10 (34) | 9 (31) | 1 (3)** | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 2 (7)** | 1 (3)¶ |
| 24 months | 25 | 13 (52) | 12 (48) | 1 (4)¶ | 1 (4)¶ | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ |
| Nasal‐symptom‐free | |||||||||
| 6 months | 70 | 23 (33) | 12 (17) | 2 (3)** | 3 (4)** | 1 (1)¶ | 1 (1)¶ | 9 (13)§ | 2 (3)¶ |
| 12 months | 64 | 20 (31) | 18 (28) | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 3 (5)¶ | 1 (2)¶ |
| 18 months | 38 | 12 (32) | 10 (26) | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 0 (0)¶ | 2 (5)** | 0 (0)¶ |
| 24 months | 49 | 8 (16) | 7 (14) | 0 (0)** | 1 (2) | 0 (0)** | 0 (0)** | 0 (0)** | 0 (0)** |
Prevalences were calculated as a percentage of total numbers of infants (n) per age and symptom group.
RSV: respiratory syncytial virus; PIV: parainfluenzavirus; CMV: cytomegalovirus.
One PIV1, 2 PIV2 and 5 PIV3 infections.
Four influenza virus A and 1 influenza B infection.
Adenovirus and enterovirus.
p < 0.01 CMV vs. PIV and vs. influenza virus, p < 0.05 vs. ‘other viruses’.
p < 0.01 vs. rhinovirus.
p < 0.05 vs. rhinovirus.
Viral prevalence in relation to symptomatology and age of the child
Multiple logistic regression was conducted to investigate whether the age of the child and the severity of symptoms affected viral prevalences in infants. Age‐ and severity‐related differences in viral prevalences did not depend on day‐care attendance, a family history of atopy and season of sampling.
Rhinovirus Rhinovirus was the most prevalent inducer of respiratory infection in all symptom groups (Fig. 1a, Table 2). The prevalence of rhinovirus in the URTI group increased with age from 27% at 6 months to 60% at 24 months and remained unchanged in the rhinitis group (31% at 18 months – 52% at 12 months) and in infants without nasal symptoms (14% at 24 months – 28% at 12 months). No statistically significant differences were found between age categories in any symptom groups. We did observe significantly more rhinovirus in the URTI group and rhinitis group than in infants without nasal symptoms (p < 0.001 and p = 0.001, respectively), whereas the prevalence of rhinovirus did not differ between the URTI and rhinitis groups.
Figure 1.
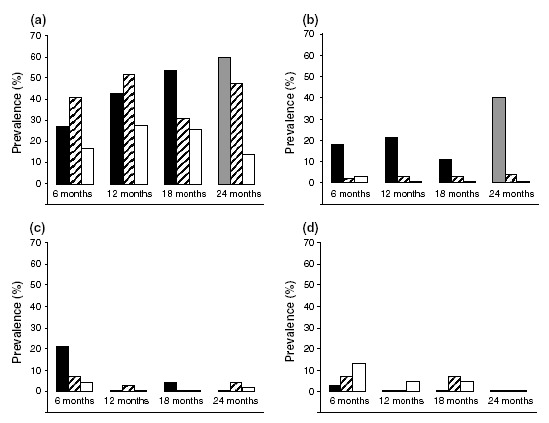
Prevalences of rhinovirus (a), RSV (b), coronavirus (c) and CMV (d) in infants with URTI (black bars), rhinitis (cross‐hatched bars) or without nasal symptoms (white bars) related to the age of the child. Data represented by the gray bar are based on only a few infants (n = 5).
RSV A significantly higher prevalence of RSV was found in infants with URTI than in infants with rhinitis or in infants without nasal symptoms (p = 0.001 and p < 0.001, respectively; Fig. 1b, Table 2). No differences in prevalence of RSV were found between age groups.
Coronavirus Coronavirus was predominantly found during URTI episodes in 6‐month‐old infants (21%), decreasing to 0% at 24 months of age (p = 0.02; Fig. 1c, Table 2). Significantly more coronavirus infections were found in the URTI group than in the rhinitis and nasal‐symptom‐free groups (p = 0.05 and p = 0.004, respectively).
CMV Some CMV infections were detected in children without nasal symptoms. Prevalences decreased gradually with age from 13% at 6 months to 0% at 24 months (p = 0.001; Fig. 1d, Table 2). CMV infections were significantly more prevalent in the nasal‐symptom‐free group compared with the URTI group (p = 0.01) but did not differ from infants in the rhinitisgroup.
Genetic and environmental factors in rhinovirus prevalence
During routine visits (rhinitis and symptom‐free groups), the majority of the viruses found were rhinoviruses. We therefore examined the relationship between several genetic and environmental factors and the prevalence of rhinovirus during routine visits at 12 and 24 months of age separately.
At the age of 12 months, a higher prevalence of rhinovirus was found in children in day‐care (51%) compared with other children (19%; p = 0.01; Table 3). No relation was found between the presence or absence of siblings and rhinovirus prevalence. We were able to confirm the reliability of parental symptom scores since the prevalence of rhinovirus increased from 21% in children with 0–3 episodes of rhinorrhoea to 60% in children with continuous rhinorrhoea during the preceding year of life (ptrend = 0.01). Simultaneous evaluation using logistic regression of the significant factors (day‐care attendance and rhinorrhoea episodes) showed that both these factors are significant predictors of increased rhinovirus prevalence. A trend for similar relations was observed between day‐care attendance and episodes of rhinorrhoea and rhinovirus prevalence at the age of 24 months, but this did not attain statistical significance (data not shown). Breast‐feeding, smoking parents, season of birth, gender and a family history of atopy did not affect the prevalence of rhinovirus during routine visits at 12 and 24 months. Comparable results were obtained when genetic and environmental factors were related to a positive diagnosis for any virus in infants at 12 and 24 months of age (data not shown).
Table 3.
Prevalence of rhinovirus at 12 months of age in relation to genetic and environmental factors
| Rhinovirus positive/n (%)* | p‐value | ||
|---|---|---|---|
| Day‐care attendance | Yes | 31/61 (51%) | |
| No | 4/21 (19%) | 0.01 | |
| Siblings | Yes | 19/42 (45%) | |
| No | 17/47 (36%) | 0.40 | |
| 0–3 episodes of rhinorrhoea | 7/34 (21%) | ||
| 3–8 episodes of rhinorrhoea | 17/40 (43%) | ||
| Continuous rhinorrhoea | 12/20 (60%) | 0.01† | |
| Breast‐feeding | Yes | 12/22 (55%) | |
| No | 21/61 (34%) | 0.13 | |
| Smoking parents | Yes | 7/22 (32%) | |
| No | 29/58 (50%) | 0.21 | |
| Girls | 19/46 (41%) | ||
| Boys | 18/51 (35%) | 0.68 | |
| Born in winter‡ | 12/27 (44%) | ||
| Born in summer | 25/70 (36%) | 0.49 | |
| Family history of atopy | Yes | 26/70 (37%) | |
| No | 11/26 (42%) | 0.81 |
n not always 97 due to occasional missing data.
Test for trend.
Winter: October–March, Summer: April–September.
Discussion
In the VIGALL birth cohort study, we found that rhinovirus was the most prevalent viral pathogen in infants from 0 to 2 years. Rhinovirus was found in ∼40% of children with URTI and also in ∼40% of children with rhinitis without general malaise. Rhinovirus was even detected in ∼20% of infants without nasal symptoms.
RSV and parainfluenzavirus have usually been considered the most important viral pathogens in infants because these viruses have been found to induce lower respiratory tract infection (bronchiolitis) 18, 19, 20). However, the importance of rhinovirus in infants has been underestimated. It is only in recent years that PCR techniques have become available. These techniques can detect rhinoviruses more accurately than the viral culture methods used previously (26). In adults, rhinovirus has already been shown to be the most prevalent viral pathogen during URTI (15, 16). We have now shown that rhinovirus is the most frequently detected viral pathogen during URTI and rhinitis in infants as well. This is in accordance with recent data from the Finnish Otitis Media Cohort Study that observed similar high rates of rhinovirus infection in infants during episodes of URTI and acute otitis media (21, 27). It can therefore be concluded that rhinovirus frequently infects both infants and adults.
Increased prevalences of rhinovirus were found in children attending day‐care. Epidemiological studies indicated that frequent respiratory tract infection in infants, associated with day‐care attendance or the numbers of siblings, may reduce the risk of developing allergic disease 10, 11, 12). On the basis of extensive virus diagnostics, this study found prevalences of viral infections in infants in day‐care, in particular rhinovirus, which were ∼2.7 times higher than those for other children. We therefore concluded that the indirect indications of protection by recurrent respiratory infections in epidemiological studies, such as by day‐care attendance, are probably rhinovirus infections to a large extend. A well‐known protective factor for lower respiratory infections during childhood is breast‐feeding whereas a parental history of allergic disease and exposure to tobacco smoke have been described as risk factors (22, 28, 29). Remarkably, in contrast to lower respiratory tract infections, the same studies showed that breast‐feeding, a parental history of allergic disease and exposure to tobacco smoke did not relate to upper respiratory tract infections. In line with these reports, we also did not observe a relation between a family history of atopy, breast‐feeding or smoking by one or both parents and the prevalence of rhinovirus infections during routine visits at 12 months.
At the age of 6 months, RSV and coronaviruses were also found in addition to rhinovirus in infants with URTI. Only a few coronavirus infections were detected in children of 12 months and older. Below the age of 24 months, the prevalence of RSV remained ∼20%. This is likely to decrease during later childhood because prevalences up to 13% have been reported in adults (30). At the age of 18 months, most children (75%) will have been infected with RSV at least once (31). The prevalence of rhinovirus increases slowly to more than 50%. In adults, a prevalence of 80% has been reported for rhinovirus during the common cold (16). This indicates that rhinovirus prevalence found in infants in the present study may increase further. Why does the prevalence of rhinovirus remain unaltered or even increase gradually, whereas the prevalence of RSV decreases with age? For RSV, which has few different serotypes, humoral and cellular responses may possibly protect the child from frequent re‐infections. By contrast, over 100 different serotypes of rhinovirus are present (32). Re‐infection with rhinovirus is therefore likely to occur with a new serotype for which the child does not yet have induced humoral and cellular responses.
Frequent viral infections are believed to be important modulators of the natural maturation of the infant immune system from Th2‐ to Th1‐skewed and may affect the development of allergic disease (5, 12). This maturation is likely to result from repeated stimulation by pathogens, rather than from a single viral infection. The general effect of a particular virus is dependent on both the prevalence of the virus and on the type of host immune response upon infection. RSV is capable of inducing a general inflammatory response in infants which largely induces an immune response towards Th1 (33). Since now rhinovirus has been found to be more prevalent than RSV in infants, the question arises to what extend rhinovirus infections are able to influence the infant immune maturation and modulation. We are currently investigating what type of immune response a rhinovirus infection can induce in infants and whether this type of immune response differs between infants with URTI and rhinitis (van Benten et al., unpublished data).
Interestingly, rhinovirus was also detected in infants without nasal symptoms (∼20%). It is unlikely that the high prevalence of rhinovirus results from RT‐PCR detection that is too sensitive, because this assay is not designed to detect a single viral particle. Rhinovirus detection could indicate imminent or past infection because ∼70% of the infants had symptoms of URTI or rhinitis during the week before or after sampling.
In the other children without nasal symptoms, rhinovirus could have colonized the nasal mucosa without inducing symptoms. This is in accordance with a recent Finish study that detected picornavirus in 20% of nasopharyngeal samples from children without any past or recent respiratory infection (34). As the nasal mucosa is colonized by bacteria, it is likely that it can also harbor respiratory viruses (35). These rhinovirus infections may perhaps only induce clinical symptoms when the host immune system is temporarily compromised. It is unclear whether these subclinical rhinovirus infections may also affect immune maturation in infants.
In conclusion, rhinovirus infection has been found to be the most prevalent respiratory viral infection, in ∼40% of infants with URTI and rhinitis, and it may well stimulate the maturation of immune system and prevent the development of allergic disease. More information is subsequently needed on the immune modulating capacity of rhinovirus infections in this context.
Acknowledgments
We would like to thank all parents and children for their participation in the VIGALL study. This study was supported by the Dutch Asthma Foundation (Nr 93.96.1) and the Netherlands Organization for Health Science (Nr 940‐35‐025). We received an additional research grant from the Foundation ‘Vereniging Trustfonds Erasmus Universiteit Rotterdam’ in the Netherlands (Nr 97030).
References
- 1. Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev 1999: 12: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finkelman FD, Urban JF Jr The other side of the coin: the protective role of the TH2 cytokines. J Allergy Clin Immunol 2001: 107: 772–80. [DOI] [PubMed] [Google Scholar]
- 3. Hunter CA, Reiner SL. Cytokines and T cells in host defense. Curr Opin Immunol 2000: 12: 413–8. [DOI] [PubMed] [Google Scholar]
- 4. Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence? Lancet 2001: 357: 1821–5. [DOI] [PubMed] [Google Scholar]
- 5. Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen‐specific T‐cell memory in atopic and normal children. Lancet 1999: 353: 196–200. [DOI] [PubMed] [Google Scholar]
- 6. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989: 299: 1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2‐year‐old children. Clin Exp Allergy 1999: 29: 342–6. [DOI] [PubMed] [Google Scholar]
- 8. Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 2000: 320: 412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denny FW Jr The clinical impact of human respiratory virus infections. Am J Respir Crit Care Med 1995: 152 (Suppl. 4 Part 2): S4–12. [DOI] [PubMed] [Google Scholar]
- 10. Kramer U, Heinrich J, Wjst M, Wichmann HE. Age of entry to day nursery and allergy in later childhood. Lancet 1999: 353: 450–4. [DOI] [PubMed] [Google Scholar]
- 11. Ball TM, Castro‐Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day‐care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000: 343: 538–43. [DOI] [PubMed] [Google Scholar]
- 12. Illi S, Von Mutius E, Lau S, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 2001: 322: 390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000: 161: 1501–7. [DOI] [PubMed] [Google Scholar]
- 14. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999: 354: 541–5. [DOI] [PubMed] [Google Scholar]
- 15. Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998: 36: 539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arruda E, Pitkaranta A, Witek TJ jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol 1997: 35: 2864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995: 310: 1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000: 342: 232–9. [DOI] [PubMed] [Google Scholar]
- 19. Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children 5 years old. J Infect Dis 1997: 175: 807–13. [DOI] [PubMed] [Google Scholar]
- 20. Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 1999: 159: 785–90. [DOI] [PubMed] [Google Scholar]
- 21. Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty‐four months of age. Pediatr Infect Dis J 2001: 20: 574–81. [DOI] [PubMed] [Google Scholar]
- 22. Koopman LP, Smit HA, Heijnen ML, et al. Respiratory infections in infants: interaction of parental allergy, child care, and siblings‐the piama study. Pediatrics 2001: 108: 943–8. [DOI] [PubMed] [Google Scholar]
- 23. Lakwijk N, Van Strien RT, Doekes G, Brunekreef B, Gerritsen J. Validation of a screening questionnaire for atopy with serum IgE tests in a population of pregnant Dutch women. Clin Exp Allergy 1998: 28: 454–8. [DOI] [PubMed] [Google Scholar]
- 24. Godthelp T, Holm AF, Fokkens WJ, et al. Dynamics of nasal eosinophils in response to a nonnatural allergen challenge in patients with allergic rhinitis and control subjects: a biopsy and brush study. J Allergy Clin Immunol 1996: 97: 800–11. [DOI] [PubMed] [Google Scholar]
- 25. Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of patients with acute community‐acquired sinusitis by reverse transcription‐PCR. J Clin Microbiol 1997: 35: 1791–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hyypia T, Puhakka T, Ruuskanen O, Makela M, Arola A, Arstila P. Molecular diagnosis of human rhinovirus infections: comparison with virus isolation. J Clin Microbiol 1998: 36: 2081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol 2002: 66: 263–8. [DOI] [PubMed] [Google Scholar]
- 28. Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States. Third National Health Nutrition Examination Survey, 1988 to 1994. Pediatrics 1998: 101: E8. [DOI] [PubMed] [Google Scholar]
- 29. Cushing AH, Samet JM, Lambert WE, et al. Breastfeeding reduces risk of respiratory illness in infants. Am J Epidemiol 1998: 147: 863–70. [DOI] [PubMed] [Google Scholar]
- 30. Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza‐like illness: an observational study. Lancet 2001: 358: 1410–6. [DOI] [PubMed] [Google Scholar]
- 31. Harsten G, Prellner K, Lofgren B, Heldrup J, Kalm O, Kornfalt R. Serum antibodies against respiratory tract viruses: a prospective three‐ year follow‐up from birth. J Laryngol Otol 1989: 103: 904–8. [DOI] [PubMed] [Google Scholar]
- 32. Van Kempen M, Bachert C, Van Cauwenberge P. An update on the pathophysiology of rhinovirus upper respiratory tract infections. Rhinology 1999: 37: 97–103. [PubMed] [Google Scholar]
- 33. Brandenburg AH, Kleinjan A, Van Het Land B, et al. Type 1‐like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol 2000: 62: 267–77. [PubMed] [Google Scholar]
- 34. Nokso‐Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkaranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol 2002: 66: 417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gluck U, Gebbers JO. The nose as a bacterial reservoir: important differences between the vestibule and cavity. Laryngoscope 2000: 110 (Suppl. 3 Part 1): S426–8. [DOI] [PubMed] [Google Scholar]


