Abstract
Background
The clinical relevance of parallel detection of multiple viruses by real‐time polymerase chain reaction (RT‐PCR) remains unclear. This study evaluated the association between the detection of multiple viruses by RT‐PCR and disease severity in children with bronchiolitis.
Methods
Children less than 2 years of age with clinical symptoms of bronchiolitis were prospectively included during three winter seasons. Patients were categorized in three groups based on disease severity; mild (no supportive treatment), moderate (supplemental oxygen and/or nasogastric feeding), and severe (mechanical ventilation). Multiplex RT‐PCR of 15 respiratory viruses was performed on nasopharyngeal aspirates.
Results
In total, 142 samples were obtained. Respiratory Syncytial virus (RSV) was the most commonly detected virus (73%) followed by rhinovirus (RV) (30%). In 58 samples (41%) more than one virus was detected, of which 41% was a dual infection with RSV and RV. In RSV infected children younger than 3 months, disease severity was not associated with the number of detected viruses. Remarkably, in children older than 3 months we found an association between more severe disease and RSV mono‐infections.
Conclusion
Disease severity in children with bronchiolitis is not associated with infection by multiple viruses. We conclude that other factors, such as age, contribute to disease severity to a larger extent. Pediatr Pulmonol. 2012; 47:393–400. © 2011 Wiley Periodicals, Inc.
Keywords: bronchiolitis, rhinovirus, respiratory syncytial virus, disease severity
INTRODUCTION
In young children, bronchiolitis is a common presentation of viral lower respiratory tract infections. Human Respiratory Syncytial Virus (RSV) is the most frequently identified virus with detection rates up to 40–85% in infants hospitalized for respiratory infections during winter epidemics.1, 2 About 1–2% of children infected by RSV need to be hospitalized, of which 6–11% require intensive care admission.3, 4 Other viruses which are frequently detected in young children with acute respiratory tract infections include: Rhinovirus (RV), human metapneumovirus (hMPV), parainfluenza virus (PIV), influenza virus (IV), adenovirus (AdV), enterovirus (EV) and human bocavirus (hBoV).5, 6, 7
Children with bronchiolitis show a great variability in disease severity. Although prematurity, congenital heart diseases, chronic lung disease, and immune deficiencies are known risk factors for severe RSV infection, half of the children admitted at an intensive care unit were born at term and healthy.8 It is still not completely understood why some children develop a more severe course of disease than others. Both host and viral factors contribute to viral pathogenesis and disease severity is the result of a dynamic interplay between these factors.
The use of real‐time polymerase chain reaction (RT‐PCR) has greatly improved the ability to diagnose viral respiratory infections. PCR based methods are more sensitive than conventional detection methods such as viral culture and antigen detection. Furthermore, RT‐PCR has enabled the identification of viruses which are normally difficult to detect by conventional methods and has allowed the simultaneous detection of multiple pathogens in one sample.9
As a result of the latter, the detection of viral co‐infection in children with lower respiratory tract infections has increased from 5 to 10% using conventional methods to 10–30%.5, 10, 11 However, the clinical implications of these co‐infections remain unclear. Some reports have suggested that infection with multiple viruses results in a more severe course of disease, while others have described that disease severity did not differ between infections caused by one or multiple viruses.10, 12, 13 In addition, the presence of viruses in asymptomatic children suggests that a positive viral PCR does not necessarily indicate a causative relationship.14
In this study, we aim to examine whether infection with multiple viruses results in increased disease severity in young children with bronchiolitis. Therefore, we prospectively studied the association between the detection of multiple viral pathogens by RT‐PCR and disease severity in young children with bronchiolitis included during three consecutive winter seasons.
METHODS
Patients
Children younger than 2 years of age with clinical symptoms of bronchiolitis presenting to the emergency department or the departments of pediatrics of the Radboud University Nijmegen Medical Center or Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, were prospectively included between November and April (winter season) in the years 2006–2009. Bronchiolitis was defined as an acute infection of the lower airways, characterized by increased respiratory effort (tachypnea and/or use of accessory respiratory muscles) and expiratory wheezing and/or crackles and/or apnea. Medical history and demographic data were collected from questionnaires and medical records. The study was approved by the Committee on Research involving Human Subjects of the University Nijmegen Medical Centre and written informed consent was obtained from all parents. Within 24 hr after admission a nasopharyngeal aspirate was collected and stored at −80°C for virological characterization. Patients were classified into three different groups based on the severity of disease. The mild group was children without hypoxia or severe feeding problems. The moderate group included children requiring hospitalization for supplemental oxygen and/or nasogastric feeding. Supplemental oxygen was given at oxygen saturations below 93% measured by pulsoximetry. Finally, children requiring mechanical ventilation were included in the severe group.
Collection of Nasopharyngeal Aspirates
A nasopharyngeal aspirate was collected by introducing a catheter, connected to a collection tube and aspiration system, through one of the nostrils into the nasopharyngeal cavity. Then, 1.5 ml of saline was instilled into the catheter and, while slowly retracting the catheter, the nasopharyngeal fluid was aspirated in a collection tube. Subsequently the catheter was flushed with 1 ml of saline and added to the collection fluid. The samples were cooled and transported to the laboratory. The nasopharyngeal aspirate was centrifuged at 500g for 10 min at 4°C and the supernatant was frozen at −80°C.
Virus Detection in Nasopharyngeal Secretions
Samples were analyzed by multiplex PCR as previously described.9 Briefly, upon thawing, nucleic acids were extracted from each sample, using the MagNA Pure LC and the MagNA‐Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Almere, The Netherlands) according to the instructions of the manufacturer. A multiplex RT‐PCR assay containing 15 different viral pathogens was used. This assay was designed for the detection of specific viral genomes belonging to IV type A and B, coronavirus (CoV) 229E and OC43, hBoV, EV, AdV, parechovirus (PeV), PIV types 1–4, hMPV, RV, and RSV. An internal control consisting of Phocine Herpesvirus (PhPV, IC DNA control) and Equine Arthritis Virus (EAV, IC RNA control) was included in the assay. RNA was reverse transcribed to cDNA using the TaqMan Reverse Transcription Reagents kit (Applied Biosystems, Nieuwerkerk Ad Ijssel, NL) in a 50 µl reaction mix containing 20 µl of nucleic acid isolate and random hexamers as primers, according to the manufacturer's instructions. PCRs were performed on the LightCycler 480 instrument using LightCycler 480 Probes Master Mix (Roche Diagnostics). Validated primer/probe‐mixes were purchased from Diagenode (Liège, Belgium) and used according to the manufacturer's instructions. Cycling conditions were 95°C for 5 min, followed by 50 cycles of 95°C for 15 sec and 55°C for 15 sec and 72°C for 20 sec. The amount of virus was recorded semi‐quantitatively based on the cycle threshold value (Ct value).
Statistical Analysis
Values are expressed as percentages for discrete/categorical variables and as median and interquartile range (IQR) for continuous variables. As the data were not normally distributed, Kruskal–Wallis tests were performed to compare age, birth weight, and symptoms duration. Mann–Whitney U‐tests were used for individual comparisons between mild, moderate, and severe patients. Chi‐squared tests were performed to compare categorical data. A two sided value of P < 0.05 was considered statistically significant.
RESULTS
One hundred and forty two children were included. Table 1 shows the characteristics of the patients. The mean age was 4.5 months. The age distribution was: 76 children younger than 3 months (54%), 25 between 3 and 6 months (18%), 27 between 6 and 12 months (19%), and 14 between 1 and 2 years (10%). Children in the severe group were younger than those in the mild and moderate group (2.2 vs. 4.7 and 6.4 months, respectively; P < 0.001). Twenty‐seven children (19%) had underlying diseases, of which 18 (13%) were born prematurely (defined by a gestational age of 35 weeks or less) and 9 had a congenital heart defect (6%). Prematurity (27% vs. 6%; P = 0.02) and maternal smoking during pregnancy (33% vs. 12%; P = 0.01) was more often observed in the severe group compared to the moderate group. The lower day care attendance in the severe group can be explained by the lower age in this group. No other significant differences in clinical parameters were found between the patient groups.
Table 1.
Patient Characteristics
| Total group (N = 142) | Mild (N = 41) | Moderate (N = 64) | Severe (N = 37) | P‐value | |
|---|---|---|---|---|---|
| Age (mo), Mean ± SE | 4.5 ± 0.39 | 6.4 ± 0.81 | 4.7 ± 0.61 | 2.2 ± 0.36 | P < 0.0013 |
| Male | 87 (61) | 23 (56) | 38 (59) | 26 (70) | NS |
| Birth characteristics | |||||
| Birth weight (g), mean ± SE | 3261 ± 66 | 3202 ± 123 | 3405 ± 91 | 3094 ± 135 | NS |
| Prematurity1 | 18 (13) | 4 (10) | 4 (6) | 10 (27) | P = 0.0084 |
| Maternal smoking | 22/130 (17) | 5/39 (13) | 7/61 (12) | 10/30 (33) | P = 0.0245 |
| Breastfeeding | 81/138 (59) | 28/41 (68) | 34/61 (56) | 19/36 (53) | NS |
| Underlying diseases2 | |||||
| CHD | 9 (6) | 3 (7) | 2 (3) | 4 (11) | NS |
| Environmental factors | |||||
| Atopic disease | 18/140 (13) | 6 (15) | 9 (14) | 3/35 (9) | NS |
| Atopic family history | 76/136 (56) | 19/40 (48) | 38/62 (61) | 19/34 (56) | NS |
| Passive smoking | 19/134 (14) | 6/39 (15) | 7/63 (11) | 6/32 (19) | NS |
| Siblings | 110/141 (78) | 30 (73) | 47/63 (75) | 33 (89) | NS |
| Daycare attendance | 25/138 (18) | 10/38 (26) | 14/64 (22) | 1/36 (3) | P = 0.0186 |
| Presentation | |||||
| Days of illness before presentation, median (IQR) | 4.0 (3.0–6.0) | 4.0 (3.0–7.5) | 4.0 (3.0–6.0) | 5 (3.0–6.0) | NS |
Data are presented as number (%), unless otherwise specified.
Mo, months; g, grams; IQR, interquartile range; CHD, congenital heart defect.
Prematurity was defined as a gestation age of 35 weeks or less.
No patients with chronic lung diseases or immune deficiencies were included. Kruskal–Wallis or Chi‐squared tests were performed, and when significant, they were followed by Mann–Whitney U‐tests or Chi‐squared tests for individual comparisons. Individual statistical comparisons were as follows:
Mild versus moderate, P = 0.03; mild versus severe, P = 0.000; moderate versus severe, P = 0.01.
Moderate versus severe, P = 0.02.
Moderate versus severe, P = 0.01.
Mild versus severe, P = 0.001; moderate versus severe, P = 0.002.
Overall, 211 viruses were detected in 142 nasopharyngeal aspirates. Data are summarized in Table 2. In 4 of the 142 samples (3%) no virus was detected. The most frequently detected virus was RSV in 104 samples (73%), followed by RV, which was present in 43 samples (30%). Other respiratory viruses were found in less than 10% of the total group. RSV was detected in a similar frequency in the three severity groups (71–75%), while RV was found in 41, 32, and 16% of children with mild, moderate, and severe disease, respectively.
Table 2.
Distribution of Viruses Recovered From 142 Nasopharyngeal Aspirates From Children less than 2 Years of Age With Bronchiolitis
| Virus | Total (N = 142) | Mild (N = 41) | Moderate (N = 63) | Severe (N = 37) |
|---|---|---|---|---|
| RSV | 104 (73) | 29 (71) | 47 (75) | 28 (76) |
| RV | 43 (30) | 17 (41) | 20 (32) | 6 (16) |
| AdV | 13 (9) | 2 (5) | 7 (11) | 4 (11) |
| EV | 10 (7) | 5 (12) | 5 (8) | 0 |
| hMPV | 9 (6) | 4 (10) | 4 (6) | 1 (3) |
| IV‐A | 7 (5) | 1 (2) | 5 (8) | 1 (3) |
| hBoV | 6 (4) | 4 (10) | 2 (3) | 0 (0) |
| CoV | 8 (6) | 5 (12) | 2(3) | 1 (3) |
| PIV‐3 | 6 (4) | 2 (5) | 4 (6) | 0 |
| PeV | 5 (4) | 1 (2) | 4 (6) | 0 |
| No virus | 4 (3) | 0 | 0 | 4 (11) |
| 1 virus | 80 (56) | 18 (44) | 35 (56) | 26 (70) |
| > 1 virus | 58 (41) | 23 (56) | 28 (44) | 7 (19) |
| 2 viruses | 46 (32) | 18 (44) | 22 (35) | 6 (16) |
| 3 viruses | 10 (7) | 3 (7) | 6 (10) | 1 (3) |
| ≥ 4 viruses | 2 (1) | 2 (5) | 0 | 0 |
Data are presented as number (% of samples). Total exceeds 100% because of the detection of more than one virus per sample.
RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; EV, enterovirus; hMPV, human metapneumovirus; IV, Influenzavirus; hBoV, human bocavirus; CoV, coronavirus; PIV, para‐influenzavirus; PeV, parechovirus.
IV‐B, PIV type 1,2, and 4 were not detected and not mentioned in this table.
More than one virus was found in 58 of 142 subjects (41%). Figure 1 shows the distribution of viruses detected as a single infection or in combination with other viruses. RSV was detected as a single virus infection in 61 of RSV positive subjects (59%), followed by hMPV which was detected as a single virus in respectively 56% of hMPV positive samples. RV was detected as a single virus in 9 of 43 subjects with RV positive samples (21%). The other viruses were less frequently detected as single virus infections, of which hBoV, PeV, and AdV were only detected in combination with other viruses.
Figure 1.
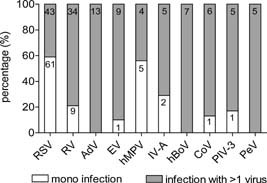
Frequencies of viruses detected as a single virus or in combination with other viruses. Numbers in bars represent the absolute numbers of infection per virus. RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; EV, enterovirus; hMPV, human metapneumovirus; IV, influenza virus; hBoV, human bocavirus; CoV, coronavirus; PIV, para‐influenza virus; PeV, parechovirus.
Infection with two or more viruses was more frequently found in children with mild and moderate disease than in those with severe disease (56 and 44%, respectively vs. 19%; P = 0.003). Infection with both RSV and RV was the most common combination of viruses, detected in 24 of the 58 infections caused by multiple viruses (41%), followed by the combination of Adv and RSV in 9 patients (16%).
Table 3 depicts the clinical characteristics and disease severity in children with either RSV mono‐ and multiple infections or children infected by one or more other viruses than RSV. Children with RSV mono‐infections were younger (2.0 months vs. 5.6 and 5.4 months; P = 0.001) and suffered from more severe disease than children with RSV multiple infections and children infected with other viruses than RSV. Children with RSV mono infections required more often mechanical ventilation compared to those with RSV and one or more other viruses (36% vs. 14%; P = 0.002). There was a trend toward lower Ct values, implicating higher viral load, in more severe disease in children with RSV multiple infections, but not in children with RSV mono‐infections.
Table 3.
Comparison of Patient Characteristics and Disease Severity in Patients With RSV Mono‐Infections, Multiple Infections Including RSV and Infections Caused by Viral Pathogens Excluding RSV
| RSV mono (N = 61) | RSV multiple (N = 43) | RSV negative (N = 38) | P‐values | |
|---|---|---|---|---|
| Age (mo), median (IQR) | 2.0 ± 0.39 | 5.6 ± 0.84 | 5.4 ± 0.80 | P < 0.0013 |
| Age < 3 mo | 46 (75%) | 15 (35%) | 15 (39%) | P < 0.0013 |
| Male | 41 (67%) | 25 (58%) | 21 (55%) | |
| Birth characteristics | ||||
| Birth weight (grams), mean ± SD | 3251 ± 778 | 3281 ± 700 | 3255 ± 817 | |
| Prematurity1 | 9 (15%) | 6 (14%) | 3 (8%) | |
| Maternal smoking | 12/59 (20%) | 6/40 (15%) | 4/31 (13%) | |
| Breastfeeding | 33/60 (53%) | 29/41 (71%) | 20/37 (54%) | |
| Underlying diseases2 | ||||
| CHD | 1 (2%) | 2 (5%) | 5 (13%) | P = 0.034 |
| Environmental factors | ||||
| Atopic disease | 6 (10%) | 6/42 (14%) | 6/37 (16%) | |
| Atopic family history | 34/59 (58%) | 19/42 (45%) | 23/35 (66%) | |
| Passive smoking | 9/59 (15%) | 4/42 (10%) | 6/33 (18%) | |
| Siblings | 50 (82%) | 33 (77%) | 27/37 (73%) | |
| Daycare attendance | 1 (2%) | 13/41 (32%) | 11/36 (31%) | P < 0.0013 |
| Viral load | ||||
| RSV Ct value, mean ± SD | 27.2 ± 5.1 | 28.3 ± 4.4 | ||
| Mild | 26.5 ± 7.2 | 29.2 ± 4.9 | ||
| Moderate | 27.2 ± 4.8 | 28.1 ± 3.7 | ||
| Severe | 27.5 ± 4.3 | 26.0 ± 3.4 | ||
| Disease severity | P = 0.0025 | |||
| Mild | 11 (18%) | 18 (42%) | 12 (32%) | |
| Moderate | 28 (46%) | 19 (44%) | 17 (45%) | |
| Severe | 22 (36%) | 6 (14%) | 9 (24%) | |
Data are presented as number (%), unless otherwise specified.
IQR, interquartile range; SD, standard deviation; CHD, congenital heart defect; mo, months.
Prematurity was defined as a gestation age of 35 weeks or less.
No patients with chronic lung diseases or immune deficiencies were included. Kruskall–Wallis non‐parametric or X2 tests were performed, and were, if significant, followed by Mann–Whitney U‐tests or Chi‐squared tests for individual comparisons.
RSV negative versus RSV mono and RSV negative versus RSV multiple.
RSV negative versus RSVmono.
RSV multiple versus RSV mono.
As the differences in age between the groups may have influenced our results, we also evaluated the association between disease severity and the detection of multiple viruses in children diagnosed with RSV bronchiolitis younger and older than 3 months (Fig. 2). Prematurity was more often observed in RSV infected children older than 3 months than in those below 3 months. Other risk factors were not different between these two age groups. Children younger than 3 months were less often infected by multiple viruses compared to children older than 3 months (25% vs. 65%). In children younger than 3 months disease severity was not associated with the number of detected viruses, while in children older than 3 months significantly less multiple infections were detected in the severe group compared to the mild group (33% vs. 84%; P < 0.01).
Figure 2.
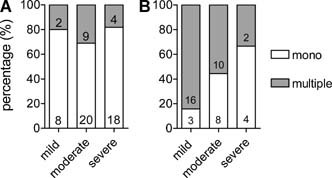
Percentages of infections caused by RSV (mono) or RSV and one or more other viruses (multiple) in children (A) younger and (B) older than 3 months of age for the different severity groups. Numbers in bars are absolute numbers.
DISCUSSION
In the present study, we evaluated the viral etiology in young children with bronchiolitis during three consecutive winter seasons and examined the association between the detection of two or more viruses by RT‐PCR and disease severity. Our main finding was that the detection of more than one virus is not associated with increased disease severity in children with bronchiolitis. These findings debate the cumulative effect of the detection of a certain virus on disease severity during co‐infection. In addition, viral load in children with single RSV infections is not associated with disease severity.
The amount of multiple infections (41%) in this study is consistent with current literature.5, 10, 11 However, in 97% of our samples at least one virus was detected which is high compared to other studies.15, 16 This may be explained by the inclusion period between November and April in which virus activity as reflected in the number of respiratory tract infections is highest in The Netherlands in combination with strict inclusion criteria of bronchiolitis. Furthermore, RT‐PCR is a sensitive method to detect viruses. As the detection of a virus by RT‐PCR does not necessarily mean that it causes symptoms, the presence of a virus, RV particularly, has to be interpreted carefully. Viral RNA is reported to be detectable in nasopharyngeal samples 4–5 weeks after infection.17, 18 RV has been detected in 12–35% of asymptomatic children,19, 20, 21 while asymptomatic carriage of other viruses, especially RSV, is uncommon with detection rates up to 5% only.7, 14, 18, 21, 22
In this study, infection with both RSV and RV was the most common combination of viral co‐infections. To date there has been little agreement on the effect of viral co‐infection on disease severity in children with bronchiolitis in which both RSV and RV are detected. Papadopoulos et al.13 demonstrated that the presence of RV in children with RSV bronchiolitis increased the risk for severe disease approximately five times. Although this study was supported by another study that also described dual viral infection as a relevant risk factor for intensive care admission,23 others did not find an additional effect of RSV/RV dual infections on disease severity compared to RSV single infections.15, 24
RSV has been described as the most common pathogen causing bronchiolitis and is associated with increased disease severity in young children and in those with underlying diseases.24, 25
In our study, children with RSV mono‐infections suffered from most severe disease. The young age of the children in the most severe group may have caused a bias in our results, since young age is a well‐known risk factor for severe RSV infection. This is supported by our findings that demonstrate that children with severe symptoms upon viral infection are younger than children with milder manifestations of viral infection. Correction for age is difficult, as age, disease severity, and multiple infections are all related to each other. To correct for age we performed analyses in children younger and older than 3 months.
In children younger than 3 months no association was found between disease severity and the number of detected viruses. The higher prevalence of prematurity in children older than 3 months compared to those below 3 months suggest that the youngest children are most susceptible for severe disease upon viral infection and that in older children other risk factors, such as prematurity, become more important contributors to disease severity.
Interestingly, in children older than 3 months, an association between severe disease and the presence of one virus was observed (Fig. 2). In line with this finding, Marguet et al.12 observed a shorter duration of hospitalization in children with RSV/RV dual infection compared to those with single RSV infection suggesting that infection with RV has no additional, and potentially a protective effect on disease severity. In addition, children with multiple viral infections including RSV did not suffer from more severe disease compared to those without RSV. This may be explained by either differences in age or type of immune response. The induced immune response upon viral infection may protect the host from infection with a second virus as has been proposed by Greer et al.26 who described a potential protective effect of RV through the stimulation of the interferon (IFN) stimulated genes inducing an antiviral state that prevents the patient from a more severe course of disease after second infection with a new virus.
We also observed an association between age and multiple infections. Less multiple infections were observed in younger children. This has been described before11 and possible explanations why detection of more than one virus is less frequently observed in the most youngest infants are; (1) less exposure to viruses due to less day‐care attendance, (2) the development of more and earlier severe symptoms upon single viral infection, and (3) partial protection against respiratory viruses because of protective maternal antibodies which disappear with age.
In addition to host factors, such as young age and underlying diseases, type of virus and viral load have been described to play a role in disease severity.27, 28 In our study, a trend toward higher RSV loads in children with more severe disease was observed in children with RSV multiple infection but not in those with RSV mono‐infections. Most studies have described a positive relation between higher RSV load and disease severity28, 29, 30, 31 In addition, an association of higher viral load with young age has been previously reported.32 Our sample size was rather small compared to these studies and age specific analyses for viral loads were therefore not performed. Finally, a number of limitations need to be considered. First, we only included children during the winter season, from November until April. This may have created a bias toward a higher incidence of RSV. While RV infections are present throughout the year with peaks in autumn and spring, RSV does rarely appear out of winter season in the Netherlands. Data from a registry of the Dutch Working Group on Clinical Virology showed that in 2006 and 2007 51% of the annual RV infections were detected between November until April compared to 94% of the annual RSV infections (published with permission of the Dutch Working Group on Clinical Virology). Second, we only included children presenting in a hospital, which may have caused a bias. Third, we analyzed infections with other viruses than RSV as a group (RSV negative infections) instead of analyzing them separately, because the low number of samples infected with other viruses than RSV. To further reveal the association between young age, multiple infections and disease severity in children with bronchiolitis, more large‐scale clinical studies in various health care settings are required.
To conclude, in this study we showed that disease severity in children with bronchiolitis is not associated with infection by multiple viruses. Remarkably, in children older than 3 months we found an association between more severe disease and RSV mono‐infections. Our results suggest that other factors than infection with multiple viruses contributes to disease severity, of which age is the most important risk factor.
Acknowledgements
The authors would like to thank all parents and children for their participation in the VIRGO study. Marieke Extercate is acknowledged for her contribution to the data collection of this study. The study was financially supported by the VIRGO consortium, an Innovative Cluster approved by the Netherlands Genomics Initiative and partially funded by the Dutch Government (BSIK 03012), The Netherlands.
REFERENCES
- 1. Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J 2004; 23: S11– S18. [DOI] [PubMed] [Google Scholar]
- 2. Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis‐associated hospitalizations among US children, 1980–1996. JAMA 1999; 282: 1440– 1446. [DOI] [PubMed] [Google Scholar]
- 3. Berger TM, Aebi C, Duppenthaler A, Stocker M. Prospective population‐based study of RSV‐related intermediate care and intensive care unit admissions in Switzerland over a 4‐year period (2001–2005). Infection 2009; 37: 109– 116. [DOI] [PubMed] [Google Scholar]
- 4. Purcell K, Fergie J. Driscoll Children's Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J 2004; 23: 418– 423. [DOI] [PubMed] [Google Scholar]
- 5. Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer‐Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real‐time polymerase chain reaction. Pediatr Infect Dis J 2008; 27: 589– 594. [DOI] [PubMed] [Google Scholar]
- 6. Canducci F, Debiaggi M, Sampaolo M, Marinozzi MC, Berre S, Terulla C, Gargantini G, Cambieri P, Romero E, Clementi M. Two‐year prospective study of single infections and co‐infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol 2008; 80: 716– 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J 2006; 25: 680– 686. [DOI] [PubMed] [Google Scholar]
- 8. Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006; 368: 312– 322. [DOI] [PubMed] [Google Scholar]
- 9. Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real‐time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2 3, and 4. J Clin Microbiol 2004; 42: 1564– 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvo C, Garcia‐Garcia ML, Blanco C, Vazquez MC, Frias ME, Perez‐Brena P, Casas I. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol 2008; 42: 268– 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr 2009; 98: 123– 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marguet C, Lubrano M, Gueudin M, Le RP, Deschildre A, Forget C, Couderc L, Siret D, Donnou MD, Bubenheim M, et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One 2009; 4: e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papadopoulos NG, Moustaki M, Tsolia M, Bossios A, Astra E, Prezerakou A, Gourgiotis D, Kafetzis D. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med 2002; 165: 1285– 1289. [DOI] [PubMed] [Google Scholar]
- 14. Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J 2008; 27: 1103– 1107. [DOI] [PubMed] [Google Scholar]
- 15. Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow‐Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon‐gamma response. Pediatr Infect Dis J 2005; 24: 605– 610. [DOI] [PubMed] [Google Scholar]
- 16. Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, Berardi R, Moretti C. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child 2010; 95: 35– 41. [DOI] [PubMed] [Google Scholar]
- 17. Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol 2004; 72: 695– 699. [DOI] [PubMed] [Google Scholar]
- 18. Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT‐PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol 2006; 78: 644– 650. [DOI] [PubMed] [Google Scholar]
- 19. Johnston SL, Sanderson G, Pattemore PK, Smith S, Bardin PG, Bruce CB, Lambden PR, Tyrrell DA, Holgate ST. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol 1993; 31: 111– 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nokso‐Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkaranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol 2002; 66: 417– 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts‐Mills TAE, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 1999; 159: 785– 790. [DOI] [PubMed] [Google Scholar]
- 22. van Gageldonk‐Lafeber AB, Heijnen ML, Bartelds AI, Peters MF, van der Plas SM, Wilbrink B. A case‐control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 2005; 41: 490– 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richard N, Komurian‐Pradel F, Javouhey E, Perret M, Rajoharison A, Bagnaud A, Billaud G, Vernet G, Lina B, Floret D, et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J 2008; 27: 213– 217. [DOI] [PubMed] [Google Scholar]
- 24. Mansbach JM, McAdam AJ, Clark S, Hain PD, Flood RG, Acholonu U, Camargo CA Jr. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med 2008; 15: 111– 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calvo C, Pozo F, Garcia‐Garcia M, Sanchez M, Lopez‐Valero M, Perez‐Brena P, Casas I. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three‐year prospective study. Acta Paediatr 2010; 99: 883– 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Do rhinoviruses reduce the probability of viral co‐detection during acute respiratory tract infections? J Clin Virol 2009; 45: 10– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosis S, Esposito S, Osterhaus AD, Tremolati E, Begliatti E, Tagliabue C, Corti F, Principi N, Niesters HGM. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol 2008; 42: 286– 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fodha I, Vabret A, Ghedira L, Seboui H, Chouchane S, Dewar J, Gueddiche N, Trabelsi A, Boujaafar N, Freymuth F. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol 2007; 79: 1951– 1958. [DOI] [PubMed] [Google Scholar]
- 29. Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis 2008; 62: 382– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 2005; 191: 1861– 1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, Bont L. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol 2010; 82: 1266– 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type‐specific real‐time RT‐PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004; 31: 123– 129. [DOI] [PMC free article] [PubMed] [Google Scholar]


