Abstract
Asthma is a common disease in childhood, and might predispose for chronic obstructive respiratory morbidity in adolescence and adulthood. Various early‐life risk factors might influence the risk of wheezing, asthma, and lower lung function in childhood. Cohort studies demonstrated that lower respiratory tract infections in the first years of life are associated with an increased risk of wheezing and asthma, while the association with lung function is less clear. Additionally, the gut and airway microbiome might influence the risk of wheezing and asthma. The interaction between respiratory tract infections and the microbiome complicates studies of their associations with wheezing, asthma, and lung function. Furthermore, the causality behind these observations is still unclear, and several other factors such as genetic susceptibility and the immune system might be of importance. This review is focused on the association of early‐life respiratory tract infections and the microbiome with wheezing, asthma, and lung function, it is possible influencing factors and perspectives for future studies.
Keywords: asthma, children, epidemiology, microbiome, respiratory tract infections, wheeze
1. INTRODUCTION
Asthma is a common disease in childhood and has a worldwide prevalence of 5‐10%.1 In preschool children, the prevalence of asthma related symptoms, such as wheezing and shortness of breath, is even higher.1 Results of birth cohort studies of the last decades suggest that part of the origins of childhood asthma occur in early life.2, 3, 4, 5, 6, 7, 8, 9, 10 Lower lung function or asthma in childhood might predispose to chronic obstructive respiratory diseases, including COPD, in adolescence and adult life.11, 12 Therefore, identifying risk factors in early life is important to understand the development of lower lung function and asthma and allow prevention of disease. Recent studies suggest that early‐life respiratory tract infections could lead to airway obstruction and hyperreactivity,13 and subsequently increased risk of persistent lower lung function and asthma. Also, exposure to microorganisms seems associated with either an increased risk or lower risk of atopic diseases, depending on the type of bacteria, the composition of the microbiome, or both.
In this review, we describe the current knowledge from cohort studies on the role of early‐life respiratory tract infections and the microbiome in the development of asthma.
2. RESPIRATORY TRACT INFECTIONS
Respiratory tract infections occur most frequently in early childhood, which is also the age with the most rapid development of the immune and respiratory system.14, 15, 16 The prevalence varies per type of respiratory tract infection. In developed countries, the incidence of pneumonia in children until the age of 5 years is 0.05 episodes per child year.17 Symptoms of an upper respiratory tract infection, such as sore throat and rhinorrhea, in children until the age of 5 years are more common, with a reported prevalence of 5.06 episodes per child year in the United States.18
The diagnosis of early‐life respiratory tract infections in studies that examine the relation of respiratory tract infections with wheezing, asthma, or lung function varies largely, and is mainly divided into respiratory tract infections diagnosed in the laboratory or physician‐diagnosed. For laboratory proven respiratory tract infections, nasal samples are taken either during symptoms of an acute respiratory tract infection,19, 20, 21, 22, 23, 24, 25, 26, 27 or at scheduled time points.19, 24, 28 These are mainly analyzed for either human rhinovirus (HRV) or respiratory syncytial virus (RSV), the most common viral pathogens, and more rarely for other viruses, such as adenovirus, coronavirus, or influenza virus. The difference in timing of sampling could potentially lead to biased results, since the prevalence of a virus during an acute respiratory infection is most likely not comparable to the prevalence of a virus during an asymptomatic period. Studies that used physician‐diagnosed respiratory tract infections in early‐life as outcomes used either questionnaires or standardized registries. Some prospective cohort studies used questionnaires in which parents were asked whether their child has had a specific infection in a specific period of time.29, 30, 31 Alternatively, studies use ICD‐codes or hospital (admission) registries to identify children with respiratory tract infections.32, 33, 34, 35, 36, 37 Such differences in definitions could lead to differences in effect sizes, which is also illustrated by a cohort study that compared RSV infections in children that visited the outpatient clinic, the emergency department or who were hospitalized34 in relation to asthma diagnosis. Children with RSV infections in these three different clinical groups had a 1.86‐, 2.41‐, and 2.82‐fold increased risk for asthma, respectively, compared with children without a hospital visit for RSV infections. This is in line with another retrospective cohort study that showed that children with RSV infections who were at the outpatient clinic or prolonged hospitalized had a 1.38 versus 2.5921 increased odds for recurrent wheeze at the age of 5 years, respectively, compared with those without an RSV infection. The use of questionnaires or registries has the advantage of easily accessible data at relatively low cost, but could possibly lead to misclassification. Viral sampling is more reliable in terms of accurate diagnosis, but whether sampling during symptoms of an acute respiratory tract infection is comparable to sampling at scheduled times is unclear. Additionally, costs will be higher and logistics are more complex. Thus, in large observational population‐based cohort studies, questionnaires or registries are cost‐effective methods to assess respiratory tract infections, while smaller studies using viral sampling provide information on specific agents.
In preschool children, asthma is difficult to diagnose and mainly based on the occurrence of wheezing. Previous population‐based or high risk cohorts showed that HRV, RSV, or bronchiolitis in the first 1‐3 years of life are associated with an up to 13‐fold increased risk of preschool wheezing.20, 21, 22, 23, 28, 30, 38 One cohort study showed that hospitalization for RSV was associated with an increased risk of recurrent wheezing at the age of 18 years.39 Cohort studies or case‐control studies that focused on childhood asthma at age 4‐13 years showed that HRV, RSV, or bronchiolitis in the first year, the first 2 or the first 3 years of life were associated with an increased risk of asthma with odds ratios ranging from 1.39 to 13.55.19, 29, 32, 33, 34, 35, 36, 37, 40 Some studies examined a group of, and not individual, respiratory tract infections only. A case‐control study showed that hospitalization for any respiratory tract infection in the first year of life was associated with a 1.5‐fold increased risk of asthma at the age of 5 years,35 while a high‐risk cohort study demonstrated that wheezy and febrile lower respiratory tract infections in the first year of life were associated with an increased risk of asthma at the age of 5 of 10 years. However, these latter associations seemed only present when the child had allergic sensitization before the age of 2 years.41, 42 It has also been suggested that the number of respiratory tract infections, rather than a specific infection, is associated with an increased risk of asthma.43 Thus, it might be that individual and type of respiratory infections are less important for the development of preschool wheezing or childhood asthma than groups and number of respiratory tract infections.
Only a few studies have examined the effect of early‐life respiratory tract infections on lung function. Population‐based cohorts, but also a high‐risk cohort and a case‐control study showed that respiratory tract infections in the first or the first three years of life were associated with a lower lung function, specifically Forced Expiratory Volume in 1 s (FEV1), Forced Expiratory Flow after exhaling 50% of the Forced Vital Capacity (FEF50), FEV1/FVC, Midexpiratory Flow (FEF2575), or Peek Expiratory Flow (PEF) mostly at age 6‐8 years,24, 25, 26 although one prospective cohort study assessed lung function multiple times between the ages of 11‐26 years.44 The decrease in lung function varied between −62.8 and −80 mL for absolute values, and −2.5% to −20% for percentage predicted values. However, two population‐based cohorts found no associations of lower respiratory tract infections before the age of 2 years or pneumonia or whooping cough in the first 7 years of life with lung function at the age of 10 years,31 or decline in FEV1 between the age of 35 and 45,45 respectively. It could be argued that respiratory tract infections in later childhood might not have the same adverse effect as in earlier childhood, explaining why no association was found in the latter study. Further studies on the role of early respiratory tract infections with later life wheezing, asthma, and lung function are warranted.
The vast majority of the studies focused on respiratory tract infections in the first 1‐3 years of life. It is speculated that respiratory tract infections in early life most probably have the greatest adverse effect, since both the immune system and the respiratory system are still under development in this stage of life.15, 16 Early‐life respiratory tract infections could lead to a disturbance in the development of both systems leading to persistent adaptations, and risk of respiratory morbidity in later life.
It remains unclear whether lower respiratory tract infections influence the risk of lower lung function, asthma and wheezing, or vice versa. Some evidence on causality is provided by two randomized controlled trials (RCT) comparing palivizumab with placebo in preterm infants. Both demonstrated that compared with the placebo, palivizumab decreased the risk of later wheezing, either in the first year of life46 or at the age of 2‐5 years.47 However, some studies that measured early‐life lung function suggest the opposite direction of causality. One prospective cohort study showed that increased bronchial responsiveness in infancy was associated with increased risk of severe bronchiolitis48 and another showed that children with a lower respiratory system compliance, and higher resistance at the age of 2 months, were at greater risk for hospitalization for an RSV infection and wheeze after the infection.49 However, the latter study group demonstrated that the association of HRV in the first year of life with wheezing at the age of 4 years remained significant after adjusting for lung function measurements at the age of 2 months.28 Additionally, another cohort study demonstrated that lower respiratory tract infections in the first year of life were associated with an increased respiratory rate at 1 year of age, and that recurrent lower respiratory tract infections were associated with a lower tidal volume and increased lung clearance index, irrespective of lung function measured at the age of 6 weeks.50 To examine the direction of causality, further longitudinal studies with detailed information on respiratory tract infections and lung function measures early and later in life are urgently needed to provide more insight in the causal direction of these associations.
3. THE HUMAN MICROBIOME
The microbiome could be defined as the community of micro‐organisms living in or on the human body.51 Two locations of the microbiome are of great interest in relation to wheezing, asthma, and lung function, namely the airway and gut microbiome. Analyses of bacterial communities are mostly performed by 16S rRNA gene sequencing, identifying different bacteria. Challenges in measuring the airway microbiome lie in the relative low density of bacterial communities when compared to the gut microbiome. Additionally, the lower airway microbiome is difficult to sample and carries the risk of contamination of the upper respiratory tract microbiome.52 When studying the microbiome, it is possible to focus on microbiome diversity or composition, or on specific bacteria. For microbiome diversity, the richness and evenness of species is estimated, for example by using the Shannon index or the Simpson's diversity index.53 Here, richness reflects the number of different species, while evenness reflects how even these different species are distributed. Another approach is to model bacterial community compositions, to form distinguishable groups.54 If specific bacteria are used as the exposure, it is possible to focus on bacterial groups or specific bacteria. Some of these bacteria might have a beneficial effect on wheezing, asthma, and lung function, such as Bifidobacterium spp, while others might have a negative effect, such as Clostridium difficile. The use of different methods for the determination of the microbiome makes it difficult to compare studies. Also, the question remains whether the focus should be on specific bacteria, or rather on the total composition of the microbiome. Characterizing specific bacteria is used more commonly, and is less complicated in terms of both analysis and interpretation but is likely to be an over simplification of the health effects of the entire microbial composition.
3.1. Airway microbiome
The composition of the airway microbiome changes in the first years of life. Nasopharyngeal samples in healthy subjects around the age of 2 months are mostly dominated by Staphylococcus and Corynebacterium, but this frequency decreases with increasing age. In contrast, Alloicoccus and Moraxella colonization is low at the age of 2 months, but is increased at the age of 12 months.55 Interestingly, when an acute respiratory illness had occurred in between the two sample periods, a transition to Moraxella or a stable colonization with Moraxella was most commonly present. Other than respiratory illness, exposure to pets, daycare attendance, siblings, and antibiotic use in the 4 weeks prior to sampling were associated with nasopharyngeal colonization, showing mostly higher rates of Haemophilus and Moraxella colonization. Additionally, a prospective cohort study collected samples of the hypopharyngeal microbiome at the age of 1 week, 1 month, and 3 months, and showed that the microbiome at the age of 1 week represented over 60% of the microbiome at 3 months.56 Thus, although various factors can influence the airway microbiome, it is also likely that the microbiome formation in later life is determined by early colonization.
Various airway microbiota have been linked to later life asthma and wheezing. A prospective high‐risk cohort study showed that Streptococcus colonization at the age of 2 months was associated with an increased risk of chronic wheeze at the age of 5 years,55 while another prospective cohort study showed that in neonates, S. pneumoniae, M. catharallis, and H. influenza were associated with an increased risk of wheezing at the age of 5 years.57 Additionally, some cross‐sectional studies have compared the lung microbiome obtained by bronchoalveolar lavage or broncho‐epithelial brush of asthmatics and healthy controls. Those studies showed that the bronchi of both children and adults with asthma contained more Haemophilus spp,58 and that that the bacterial concentration and diversity were higher in adults with suboptimally controlled asthma,59 compared with children or adults without asthma. To date, it is not clear whether differences in the microbiome of the airways and lungs precede asthma, or whether the disease itself is the cause of these changes.
3.2. Gut microbiome
The composition of the gut microbiome changes during the first years of life, mostly as an effect of the changing diet in the same period.60 The introduction of supplementary feeding, the introduction of solids and the start of weaning61 are important time periods in which the microbial composition changes. These time periods should be taken into account when deciding at which age to sample feces for measuring the gut microbiome, or when interpreting results of different studies.
Previously published human studies have linked the gut microbiome with the development of atopic diseases such as asthma. A prospective cohort study showed that C. difficile colonization at the age of 1 month was associated with an increased risk of recurrent wheeze until the age of 2 years, but also with eczema and atopic sensitization at the same age.62 However, only the presence of C. difficile, not the concentration in the feces was associated with these outcomes. Other bacteria, such as bifobacteria, B. fragilis species, E. coli, and lactobacilli were not associated with recurrent wheeze. A birth cohort study characterized the bacterial composition at the age of 1 month and 6 months within three distinct groups.54 The group with a lower abundance of bacteria such as Bifidobacteria and Lactobacillus, but a higher abundance of fungi such as Candida had the highest risk of asthma, predominantly multisensitized atopy at the age of 2 years, and asthma at the age of 4 years. No difference was observed between the three groups in the risk of atopy at the age of 2 years, defined as an IgE level above 0.35 IU mL−1. A comparison of stool samples at the age of 4 years between non‐wheeze, non‐sensitized controls, and wheezy‐sensitized cases showed no difference in the microbiome composition.63 Both a prospective cohort and a substudy of an RCT showed that the gut microbiome until the age of 1 month is also associated with asthma development at the age of 6 or 7 years.64, 65 The microbiome at the age of 12 months however was not associated with asthma. In summary, previous studies support the hypothesis that mostly the microbiome in early life is important for the development of wheezing and asthma at a later age.64
4. RELATION BETWEEN RESPIRATORY TRACT INFECTIONS AND THE AIRWAY MICROBIOME
It is likely that respiratory tract infections and the airway microbiome influence each other. Respiratory tract infections could have an effect on the microbiome both during, and after the infection.66 Additionally, it is possible that the microbiome colonization of the airway could increase the risk of a subsequent respiratory tract infection.67 Hypopharyngeal colonization with S. pneumoniae, H. influenza, or M. catharralis in the first 3 years of life is associated with an increased risk of pneumonia and bronchitis at the age of 4 years, providing evidence for the latter. It has also been shown that early Streptococcus colonization is associated with an increased risk of a lower respiratory tract infection at an earlier age, while Moraxella colonization is associated with an upper respiratory tract infection at an earlier age.55 These findings suggest that respiratory tract infections might not only influence the airway microbiome, but also vice versa. This further complicates the relationship of either with wheezing, asthma, and lung function, and the understanding of this association.
5. INFLUENCING FACTORS
Several factors could be of influence in the associations of respiratory tract infections or the microbiome with wheezing, asthma, and lung function. The difference between the influencing factors is of importance in the matter of possible prevention or treatment strategies.
5.1. Environmental factors
Mode of delivery has been suggested as a possible intermediate factor in the relationship between the microbiome and risk of asthma.68, 69, 70, 71, 72, 73 Children born through a caesarian section are likely to have a lower gut microbiome diversity, especially in the first 3 months of life. Mode of delivery might also influence the airway microbiome, although the association of mode of delivery with the airway microbiome has been studied less. Antibiotic use could be related to both the associations of respiratory tract infections with wheezing, asthma, and lung function, and to the associations of the microbiome with these outcomes. Respiratory tract infections could lead to increased antibiotic use, which thereby has as an intermediate effect on wheezing, asthma, and lung function.74, 75 The use of antibiotics could also have an effect on the composition of the gut microbiome, and therefore act as a confounder in the associations of the microbiome with asthma or wheezing.76, 77, 78 Confounding by indication or reverse causation can complicate studies on the effect of the microbiome. For example, it has been suggested that the use of antibiotics is a results of confounding by indication, meaning that only respiratory tract infections themselves have an effect on wheezing, asthma, and lung function, and that a observed adverse effect of antibiotic use on these outcomes is solely because of the direct relation between the use of antibiotics and respiratory tract infections.75
5.2. Intrinsic factors
An explaining factor for the associations of respiratory tract infections with wheezing, asthma and lung function is genetic susceptibility. The 17q21 locus, the strongest known susceptibility locus for asthma, was demonstrated to be associated with HRV wheezing in early life as well, but not with RSV wheezing illness.79 Similarly, CDHR3 gene variation increases risk of childhood asthma with severe exacerbations,80 with an increased susceptibility to rhinovirus C infections as a possible underlying mechanism.81 Genetic differences in immune response to infections are also of interest. Some single nucleotide polymorphisms (SNPs) have been identified to be associated with respiratory tract infections and asthma or airway hyperreactivity, including picornavirus with atopic asthma and airway hyperreactivity, and RSV with atopic asthma and airway hyperreactivity.82 A cohort study of children with RSV demonstrated that genes coding for the Interleukin (IL) pathway, specifically IL4 which might promote allergic inflammation and asthma, are associated with both RSV infection and wheezing, suggesting a potential role for genetic differences in immune responses to infections.83 The immune system could also be an explaining factor in the associations of the microbiome with asthma and wheezing. The gut‐associated lymphoid tissue is an important factor in the immune system, and plays a role in the development of the gastro‐intestinal immune system.84 Additionally, the birth cohort study that demonstrated that one of three distinct groups of bacterial composition of the gut microbiome was associated with a higher risk of asthma and atopy, also showed that this same group was associated with CD4+ cell dysfunction. Specifically, CD4+IL4+ cells are upregulated, as is the concentration of IL‐4 released, which could contribute to airway inflammation.54 This could possibly mean that the risk of asthma and wheezing due to differences in the gut microbiome might be mediated by differences in the immune system. The immune system could be a true underlying causal factor in these associations, meaning that the immune system could influence both the risk of respiratory tract infections or alter the microbiome, and influence the risk of wheezing, asthma, and lung function separately. Alternatively, the immune system might be a mediating factor in the association of respiratory tract infections or the microbiome with wheezing, asthma, and lung function. Further studies on the role of the immune system are needed to disentangle these associations.
The associations between respiratory tract infections and wheezing, asthma, and lung function might be modified by some factors such as the atopic status characterized by sensitization or parental asthma or atopy. In a prospective cohort study, associations of wheezy or febrile lower respiratory tract infections was only found if children were sensitized by the age of 2 years, defined as a positive skin prick test for either food or inhalant allergens.41, 42 Some differences in the effect estimates for bronchiolitis with asthma were also found when children with and without atopic parents were compared, although for both groups the effect estimates were significant (odds ratios 3.11 vs 1.66).40
The role of genetics, the immune system and atopy in the associations of the microbiome or respiratory tract infections with wheezing, asthma, and lung function should be explored further.
6. FUTURE RESEARCH
The role of respiratory tract infections and the microbiome has been of increasing interest in the past decade, but population‐based cohort studies on its associations with wheezing, asthma, and lung function are scarce. Population‐based prospective cohort studies with frequent measures of respiratory tract infections and the microbiome in early life are needed to better understand the causal pathway between respiratory tract infections and the microbiome, and the development of chronic obstructive respiratory diseases in later life. Although the associations of respiratory tract infections with wheezing, asthma, and lung function has been studied more commonly, the question still remains whether observed associations are causal, or whether children with a lower lung function at birth or at risk of asthma are more likely to develop respiratory tract infections before asthma occurs. Additional to frequent early‐life measures of respiratory tract infections and the microbiome, population‐based prospective cohort studies with lung function measurements in infancy could provide more insight. Large studies or meta‐analyses might be of interest to take heterogeneity or small effect estimates into account. Studies should also focus on the relationship between the microbiome and respiratory tract infections, and their combined associations with chronic obstructive respiratory diseases. Additional to bacteria and viruses influencing each other, it is also possible that there is an interaction between bacteria and fungi in the airway. Studies on this subject, as well as the role of fungi alone, are scarce and should be addressed in future studies. Also, contamination of lower respiratory tract sampling with bacteria from the upper respiratory tract remains a problem. However, it has also been suggested that in healthy persons, the bacterial communities in the upper and lower respiratory tract resemble each other. RCT's within this research area are scarce, but could provide more insight in the causal pathway. Identifying true causal factors, as opposed to confounding and intermediating factors, is the first step toward development of preventive strategies or specific treatment options.
7. CONCLUSION
Results of cohort studies of the last decades show that mostly lower respiratory tract infections in the first years of life are associated with an increased risk of wheezing and asthma and lower lung function in later life. The presence of specific bacteria in the airway and gut in the first years of life, or the composition of the gut microbiome seem associated with an increased risk of wheezing and asthma only. The airway microbiome and respiratory tract infections could influence each other, which complicates their relation as well as the understanding of their associations with later life chronic obstructive respiratory diseases (Figure 1). More detailed population‐based, prospective cohort studies taking influencing factors into account, as well as RCT's, are needed to further study the causal effect of respiratory tract infections and the microbiome in early life on later life wheezing, asthma, and lung function. Specifically, longitudinal analysis of both the gut and airway microbiome to identify critical periods, early lung function measurements to establish causality in the association of respiratory tract infections with wheezing, asthma, and lung function, and the relation between the microbiome, respiratory tract infections, and the immune system are needed. This could ultimately allow targeted treatment and prevention strategies aiming at reducing respiratory morbidity at the long term.
Figure 1.
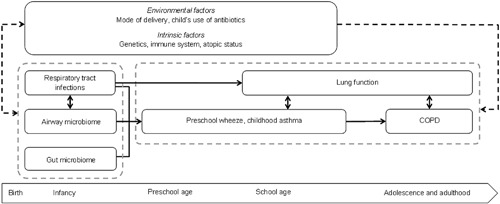
Pathways leading from respiratory tract infections and the microbiome in early life, to chronic obstructive respiratory diseases across the life course, and influencing factors. Chronic obstructive pulmonary disease (COPD)
ORCID
Liesbeth Duijts http://orcid.org/0000-0001-6731-9452
ACKNOWLEDGMENTS
Dr Vincent W.V. Jaddoe received grants from the Netherlands Organization for Health Research and Development (VIDI o16.136.3610) and the European Research Council (ERC‐2014‐CoG‐648916). Dr Liesbeth Duijts received funding from the co‐funded program ERA‐Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project, Horizon 2020 (grant agreement no 696295; 2017), ZonMW The Netherlands (no 529051014; 2017)). Dr Klaus Bønnelykke received funding from The Lundbeck Foundation (Grant no R163‐2013‐16235) and NIH (NHLBI R01 HL129735). The project received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 733206 (LIFECYCLE). The researchers are independent from the funders. The study sponsors had no role in the writing of this report.
van Meel ER, Jaddoe VWV, Bønnelykke K, de Jongste JC, Duijts L. The role of respiratory tract infections and the microbiome in the development of asthma: A narrative review. Pediatric Pulmonology. 2017;52: 1363–1370. 10.1002/ppul.23795
REFERENCES
- 1. Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet. 2006; 368:733–743. [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Bmj. 1991; 303:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherrill DL, Guerra S, Wright AL, Morgan WJ, Martinez FD. Relation of early childhood growth and wheezing phenotypes to adult lung function. Pediatr Pulmonol. 2011; 46:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hancox RJ, Poulton R, Greene JM, McLachlan CR, Pearce MS, Sears MR. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009; 64:228–232. [DOI] [PubMed] [Google Scholar]
- 5. Canoy D, Pekkanen J, Elliott P, et al. Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax. 2007; 62:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brostrom EB, Akre O, Katz‐Salamon M, Jaraj D, Kaijser M. Obstructive pulmonary disease in old age among individuals born preterm. Eur J Epidemiol. 2013; 28:79–85. [DOI] [PubMed] [Google Scholar]
- 7. Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2013; 7:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duijts L, Reiss IK, Brusselle G, de Jongste JC. Early origins of chronic obstructive lung diseases across the life course. Eur J Epidemiol. 2014; 29:871–885. [DOI] [PubMed] [Google Scholar]
- 9. Martinez FD. Early‐life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016; 375:871–878. [DOI] [PubMed] [Google Scholar]
- 10. Dratva J, Zemp E, Dharmage SC, et al. Early life origins of lung ageing: early life exposures and lung function decline in adulthood in two European cohorts aged 28‐73 years. PLoS ONE. 2016; 11:0145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Postma DS, Rabe KF. The asthma‐COPD overlap syndrome. N Engl J Med. 2015; 373:1241–1249. [DOI] [PubMed] [Google Scholar]
- 12. McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. New Engl J Med. 2016; 374:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005; 18:541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holt PG. Programming for responsiveness to environmental antigens that trigger allergic respiratory disease in adulthood is initiated during the perinatal period. Environ Health Perspect. 1998; 106:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Vries E, de Groot R, de Bruin‐Versteeg S, Comans‐Bitter WM, van Dongen JJ. Analysing the developing lymphocyte system of neonates and infants. Eur J Pediatr. 1999; 158:611–617. [DOI] [PubMed] [Google Scholar]
- 16. Dunnill MS. Postnatal growth of the lung. Thorax. 1962; 17:329–333. [Google Scholar]
- 17. Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008; 86:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chonmaitree T, Revai K, Grady JJ, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008; 46:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubner FJ, Jackson DJ, Evans MD, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017; 139:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Winter JJ, Bont L, Wilbrink B, van der Ent CK, Smit HA, Houben ML. Rhinovirus wheezing illness in infancy is associated with medically attended third year wheezing in low risk infants: results of a healthy birth cohort study. Immun Inflamm Dis. 2015; 3:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory‐confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013; 13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med. 2008; 178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Callaghan‐Gordo C, Bassat Q, Diez‐Padrisa N, et al. Lower respiratory tract infections associated with rhinovirus during infancy and increased risk of wheezing during childhood. A cohort study. PLoS ONE. 2013; 8:69370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guilbert TW, Singh AM, Danov Z, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011; 128:532–538. e531‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zomer‐Kooijker K, van der Ent CK, Ermers MJ, et al. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS ONE. 2014; 9:e87162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cassimos DC, Tsalkidis A, Tripsianis GA, et al. Asthma, lung function and sensitization in school children with a history of bronchiolitis. Pediatr Int. 2008; 50:51–56. [DOI] [PubMed] [Google Scholar]
- 27. Bonnelykke K, Vissing NH, Sevelsted A, Johnston SL, Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015; 136:81 –86.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Gugten AC, van der Zalm MM, Uiterwaal CS, Wilbrink B, Rossen JW, van der Ent CK. Human rhinovirus and wheezing: short and long‐term associations in children. Pediatr Infect Dis J. 2013; 32:827–833. [DOI] [PubMed] [Google Scholar]
- 29. Ramsey CD, Gold DR, Litonjua AA, Sredl DL, Ryan L, Celedon JC. Respiratory illnesses in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007; 119:150–156. [DOI] [PubMed] [Google Scholar]
- 30. Garcia‐Marcos L, Mallol J, Sole D, et al. Pneumonia and wheezing in the first year: an international perspective. Pediatr Pulmonol. 2015; 50:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haland G, Lodrup Carlsen KC, Mowinckel P, et al. Lung function at 10 yr is not impaired by early childhood lower respiratory tract infections. Pediatr Allergy Immunol. 2009; 20:254–260. [DOI] [PubMed] [Google Scholar]
- 32. James KM, Gebretsadik T, Escobar GJ, et al. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013; 132:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin HW, Lin SC. Environmental factors association between asthma and acute bronchiolitis in young children‐a perspective cohort study. Eur J Pediatr. 2012; 171:1645–1650. [DOI] [PubMed] [Google Scholar]
- 34. Carroll KN, Wu P, Gebretsadik T, et al. The severity‐dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009; 123:1055–1061. 1061 e 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montgomery S, Bahmanyar S, Brus O, Hussein O, Kosma P, Palme‐Kilander C. Respiratory infections in preterm infants and subsequent asthma: a cohort study. BMJ Open. 2013; 3:004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balekian DS, Linnemann RW, Hasegawa K, Thadhani R, Camargo CA, Jr. Cohort study of severe bronchiolitis during infancy and risk of asthma by age 5 years. J Allergy Clin Immunol Pract. 2017; 5:92–96. [DOI] [PubMed] [Google Scholar]
- 37. Stensballe LG, Simonsen JB, Thomsen SF, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009; 123:131–137. e131. [DOI] [PubMed] [Google Scholar]
- 38. Romero JR, Stewart DL, Buysman EK, Fernandes AW, Jafri HS, Mahadevia PJ. Serious early childhood wheezing after respiratory syncytial virus lower respiratory tract illness in preterm infants. Clin Ther. 2010; 32:2422–2432. [DOI] [PubMed] [Google Scholar]
- 39. Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010; 65:1045–1052. [DOI] [PubMed] [Google Scholar]
- 40. Brandao HV, Vieira GO, Vieira TO, et al. Acute viral bronchiolitis and risk of asthma in schoolchildren: analysis of a Brazilian newborn cohort. J Pediatr (Rio J). 2016; 93:223 –229. [DOI] [PubMed] [Google Scholar]
- 41. Kusel MM, de Klerk NH, Kebadze T, et al. Early‐life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007; 119:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012; 39:876–882. [DOI] [PubMed] [Google Scholar]
- 43. Bonnelykke K, Vissing NH, Sevelsted A, Johnston SL, Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015; 136:81–86. e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015; 135:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marossy AE, Strachan DP, Rudnicka AR, Anderson HR. Childhood chest illness and the rate of decline of adult lung function between ages 35 and 45 years. Am J Respir Crit Care Med. 2007; 175:355–359. [DOI] [PubMed] [Google Scholar]
- 46. Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013; 368:1791–1799. [DOI] [PubMed] [Google Scholar]
- 47. Simoes EA, Carbonell‐Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. Palivizumab Long‐Term Respiratory Outcomes Study G. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010; 126:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chawes BL, Poorisrisak P, Johnston SL, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol. 2012; 130:354–361. e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zomer‐Kooijker K, Uiterwaal CS, van der Gugten AC, Wilbrink B, Bont LJ, van der Ent CK. Decreased lung function precedes severe respiratory syncytial virus infection and post‐respiratory syncytial virus wheeze in term infants. Eur Respir J. 2014; 44:666–674. [DOI] [PubMed] [Google Scholar]
- 50. Gray DM, Turkovic L, Willemse L, et al. Lung function in african infants in the drakenstein child health study. Impact of lower respiratory tract illness. Am J Respir Crit Care Med. 2017; 195:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ipci K, Altintoprak N, Muluk NB, Senturk M, Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Eur Arch Otorhinolaryngol. 2017; 274:617–626. [DOI] [PubMed] [Google Scholar]
- 52. Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017; 15:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li K, Bihan M, Yooseph S, Methe BA. Analyses of the microbial diversity across the human microbiome. PLoS ONE. 2012; 7:32118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016; 22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015; 17:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mortensen MS, Brejnrod AD, Roggenbuck M, et al. The developing hypopharyngeal microbiota in early life. Microbiome. 2016; 4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007; 357:1487–1495. [DOI] [PubMed] [Google Scholar]
- 58. Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010; 5:8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011; 127:372–381. e371‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noverr MC, Huffnagle GB. The “microflora hypothesis” of allergic diseases. Clin Exp Allergy. 2005; 35:1511–1520. [DOI] [PubMed] [Google Scholar]
- 61. Cooperstock M. Intestinal flora of infants. In: Hentges D, editor. Human Intestinal microflora in health and disease. New York: Academic Press; 1983. pp 77–99. [Google Scholar]
- 62. Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007; 56:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murray CS, Tannock GW, Simon MA, et al. Fecal microbiota in sensitized wheezy and non‐sensitized non‐wheezy children: a nested case‐control study. Clin Exp Allergy. 2005; 35:741–745. [DOI] [PubMed] [Google Scholar]
- 64. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014; 44:842–850. [DOI] [PubMed] [Google Scholar]
- 65. van Nimwegen FA, Penders J, Stobberingh EE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011; 128:948–955. e941‐943. [DOI] [PubMed] [Google Scholar]
- 66. Korten I, Mika M, Klenja S, et al. Interactions of respiratory viruses and the nasal microbiota during the first year of life in healthy infants. mSphere. 2016; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vissing NH, Chawes BL, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med. 2013; 188:1246–1252. [DOI] [PubMed] [Google Scholar]
- 68. Sordillo JE, Zhou Y, McGeachie MJ, et al. Factors influencing the infant gut microbiome at age 3–6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. 2017; 139:482–491. e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brumbaugh DE, Arruda J, Robbins K, et al. Mode of delivery determines neonatal pharyngeal bacterial composition and early intestinal colonization. J Pediatr Gastroenterol Nutr. 2016; 63:320–328. [DOI] [PubMed] [Google Scholar]
- 71. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants' life: a systematic review. BMC Gastroenterol. 2016; 16:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Madan JC, Hoen AG, Lundgren SN, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6‐week‐old infants. JAMA Pediatr. 2016; 170:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martin R, Makino H, Cetinyurek Yavuz A, et al. Early‐life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE. 2016; 11:e0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murk W, Risnes KR, Bracken MB. Prenatal or early‐life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011; 127:1125–1138. [DOI] [PubMed] [Google Scholar]
- 75. Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta‐analysis. Eur Respir J. 2011; 38:295–302. [DOI] [PubMed] [Google Scholar]
- 76. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016; 22:713–722. [DOI] [PubMed] [Google Scholar]
- 77. Russell SL, Gold MJ, Reynolds LA, et al. Perinatal antibiotic‐induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015; 135:100–109. [DOI] [PubMed] [Google Scholar]
- 78. Fouhy F, Guinane CM, Hussey S, et al. High‐throughput sequencing reveals the incomplete, short‐term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012; 56:5811–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Caliskan M, Bochkov YA, Kreiner‐Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood‐onset asthma. N Engl J Med. 2013; 368:1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bonnelykke K, Sleiman P, Nielsen K, et al. A genome‐wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014; 46:51–55. [DOI] [PubMed] [Google Scholar]
- 81. Bochkov YA, Watters K, Ashraf S, et al. Cadherin‐related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci USA. 2015; 112:5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Daley D, Park JE, He JQ, et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma‐related phenotypes. J Allergy Clin Immunol. 2012; 130:1284–1293. [DOI] [PubMed] [Google Scholar]
- 83. Tapia LI, Ampuero S, Palomino MA, et al. Respiratory syncytial virus infection and recurrent wheezing in Chilean infants: a genetic background? Infect Genet Evol. 2013; 16:54–61. [DOI] [PubMed] [Google Scholar]
- 84. Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007; 62:1223–1236. [DOI] [PubMed] [Google Scholar]


