Abstract
Forsythoside A is a polyphenolic constituent of the fruits of Forsythia suspensa Vahl. which is widely used as an antiinflammatory agent in traditional Chinese medicine. In the present study, the effects of forsythoside A on cell infection by avian infectious bronchitis virus were assessed. A real‐time fluorescence quantitative PCR assay was used to determine mRNA content of IBV N gene. The pretreatment of cells with forsythoside A, adding forsythoside A post infection of cells, and treatment of virus with forsythoside A were analysed. The inhibitory effect of forsythoside A was confirmed by infecting primary chicken embryo kidney cells. Infected cells were inhibited by forsythoside A treatment. The data indicated that forsythoside A has the potential to prevent IBV infection in vitro. Copyright © 2010 John Wiley & Sons, Ltd.
Keywords: forsythoside A, IBV, inhibition, CEK cell, in vitro
INTRODUCTION
Infectious bronchitis virus (IBV), the causative agent of infectious bronchitis (IB), has been effectively controlled by the extensive use of vaccines but still remains a major economic problem since first reported in 1931. IBV has already caused major health concerns in the poultry industry around the world (Cavanagh, 2003). Like many other coronaviruses, IBV is an enveloped virus with a large, positive stranded RNA genome about 27–32 kilobases long (Lai and Cavanagh, 1997). The IBV virion is built from four structural proteins, including the nucleocapsid (N) protein with which the genomic RNA is packed, the spike (S) protein that forms the prominent coronavirus spikes, the M protein which is the most abundant component of coronavirus, and the envelope (E) protein which is a minor but yet critical component in virion assembly (de Haan et al., 1998). In addition, N protein, combined with the viral RNA with high affinity (Spencer and Hiscox, 2006), is abundantly expressed in infected cells.
The sporadic occurrence of infectious bronchitis (IB) has been reported frequently in China, although the application of vaccine in most of the chicken farms has decreased the large‐scale outbreaks of IB in China (Ren et al., 2009). Therefore, the use of vaccines alone is not sufficient to control IB (Gelb et al., 2005; Jackwood et al., 2005; Liu et al., 2006; Bochkov et al., 2006). In China, the government prohibits the use of antiviral drugs in food animals, so more attention is being paid to traditional antiviral herbs, and many reports are confirming the potential use of Chinese traditional antiviral herbs in inhibiting the virus (Yamasaki et al., 1993; Chen et al., 2003; Mori et al., 1999; Li et al., 2009).
The fruits of Forsythia suspensa Vahl. (Oleaceae) are widely used in traditional Chinese medicine to treat inflammation, pyrexia or emesis. Forsythoside A is the active ingredient of Forsythia suspensa. Endo et al. (1981) first extracted forsythoside, and Nishibe et al. (1982) successfully obtained forsythoside from weeping forsythia, and found it strongly inhibited pathogenic bacteria such as Staphylococcus aureus. Forsythoside also inhibited syncytial virus and coxsackie virus in vitro (Hu et al., 2001).
Currently, the detection and serotype analysis of IBV is performed by RT‐PCR and restriction fragment length polymorphism analysis (Kwon et al., 1993) or by sequencing RT‐PCR products of the S1 gene (Kingham et al., 2000). In this study, real‐time fluorescence quantitative PCR was used to analyse the IBV N gene. Real‐time fluorescence quantitative PCR is sensitive and accurate in the determination of copy numbers of DNA or cDNA (Zhang and Shen, 2003) and was therefore used to investigate the inhibiting effects of forsythoside A.
MATERIALS AND METHODS
Virus and cells.
IBV strain M41 (China Institute of Veterinary Drug Control) was adapted and propagated in chicken embryo kidney (CEK) cells. Primary cultures of CEK cells from 19‐day‐old chicken embryo were prepared with standard techniques. The CEK cell monolayers were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, USA) supplemented with penicillin–streptomycin and 10% fetal bovine serum (FBS) at 37 °C in CO2 incubator.
Cytotoxic assay of forsythoside A.
The CEK cells suspension was added to 96‐well culture plates (Costar/Corning USA) at a density of 0.1 mL per well and cultured to allow the formation of cell monolayers at 37°C in CO2 incubator. After 48 h, it was replaced with DMEM containing 3% fetal bovine serum (FBS) and culture continued for 24 h. After washing with PBS three times, forsythoside A (Biomedical R&D Center in Liaoning Province) was dissolved in DMEM and the diluted forsythoside A was put in wells (0.2 mL per well) at eight repeats of each concentration. A normal control was set. Morphological changes of cells were observed by light microscope.
Treatment of CEK cells prior to virus infection.
The CEK cells in 96‐well plates or 24‐well plates (Costar/Corning USA) were treated for 1 h with serially diluted forsythoside A (0.16 mm, 0.32 mm, 0.64 mm). Eight replicates were used for each concentration. The cells were infected with IBV at a multiplicity of infection of 0.001 for 1 h, and after washing with PBS three times, DMEM was applied. Mock and infected cells were prepared for subsequent experiments. Cytopathic effects (CPE) were observed in the infected cells.
Treatment of infected cells.
To analyse the effect of forsythoside A on infected cells, the CEK cell monolayers were infected with IBV at a multiplicity of infection of 0.001 at 37°C for 1 h. The cell monolayers were then washed with PBS three times and the infected cells treated with serially diluted forsythoside A.
Treatment with forsythoside A.
To analyse the effect of forsythoside A, IBV cultures were incubated with serially diluted forsythoside A at 37°C for 1 h. The CEK cell monolayers were infected with IBV at a multiplicity of infection of 0.001 at 37°C for 1 h. After washing with PBS three times, DMEM with 3% FBS was added cultured at 37°C.
Preparation of total cellular RNA.
Total cellular RNA in the three groups was extracted using a rapid RNA extracting kit (centrifugation column type, Promega), according to the manufacturer's instructions.
Determination of IBV N gene expression by real‐time reverse transcription PCR.
The RT system included 1 mg extracted RNA (4.8 mL), 0.5 mL Oligo dT primer (1 mg/mL), 1 mL dNTP mixture (10 mm each), 0.2 mL RNase inhibitor (40 U/mL), 2 mL MgCl2 (25 mm/mL), 0.5 mL reverse transcriptase M‐MLV (200 U/mL) and 1 mL 10× RT buffer. The mixture was kept at 42°C for 30 min, 99°C for 5 min and 4°C for 10 min prior to PCR.
According to the published sequence of IBV strain M41 (GenBank EU116941) and avian β‐actin (GenBank L08165), the primers p1: 5′‐AAACCAGTCCCAGATGCT‐3′; p2: 5′‐GAGGAATGAAATCCCAACG‐3′; p3: 5′‐CCACAGCTGCCTCTAGCTCT‐3′; and p4: 5′‐ACATCTGCTGGAAGGTGGAC‐3′ were designed. While P1 and P2 were used to amplify the IBV N open reading frame, P3 and P4 amplified the avian β‐actin. The PCR system included 10 µL SYBR Green PCR mix; 0.2 µL 10 pmol/µL upstream and downstream primers respectively; 0.2 µL referencing dye; 2 µL cDNA templates, and 7.4 µL water. The PCR was performed at 95°C for 10 min, and 30 cycles of 94°C for 1 min, 60°C for 1 min. The PCR products were separated by 1% agarose gel electrophoresis and detection and amplification of RNA levels measured using Stepone Applied Biosystems equipment. The results were statistically analysed according to the method of Livak and Schmittgen (2001).
RESULTS AND DISCUSSION
Currently, IBV causes highly contagious diseases in chickens and is a constant threat to the poultry industry. Vaccination is a key strategy for the prevention and control of IB in poultry, but is not always successful. Antiviral therapy is a possible strategy for treating IBV infection, although there are few effective antiviral drugs available. The purpose of the study was to identify whether forsythoside A can inhibit IBV infection in vitro, and if so, to investigate its mode of action.
Cell‐toxicity of forsythoside A
The effects of forsythoside A on CEK cell viability and proliferation were determined using CEK cell monolayers. The maximum non‐toxic concentrations of forsythoside A diluted in DMEM was found to be greater than 1.28 mm. Previously, it has been shown that forsythoside A has an antiviral effect on the syncytial virus when used at a concentration of 0.624 mm (Hu et al., 2001). In this study, forsythoside A showed a direct anti‐IBV effect at a concentration of 0.64 mm, as shown by real‐time PCR assay and observation of the cytopathic effects. Although forsythoside A did not inhibit IBV infection after virus absorption, and pretreatment of the cells with forsythoside A resulted in only partial inhibition of IBV infectivity, the sensitivity of cellular factors such as the surface receptors of IBV to the drug remains under investigation. Two approaches were used to analyse virus infection, and both real‐time PCR and CPE gave rise to similar results, which confirms the feasibility of these results.
Forsythoside A treatment of CEK cells prior to infection inhibits IBV production
When forsythoside A was applied to the infected cells, as shown in Fig. 1a, it did not decrease IBV infectivity at any of the concentrations tested, as determined by CPE. The IBV N gene was detected and it was found that viral loads decreased with increasing concentrations of forsythoside A. The inhibiting effect of forsythoside A on virions was analysed up to concentrations of 0.64 mm. No reduction in IBV‐N was observed at 0.16 mm and 0.32 mm samples, but partial inhibition of the infectivity was seen at 0.64 mm, as determined by real‐time fluorescence quantitative PCR (Fig. 2). It is speculated that the result may be caused by a decrease in the number of cells.
Figure 1.
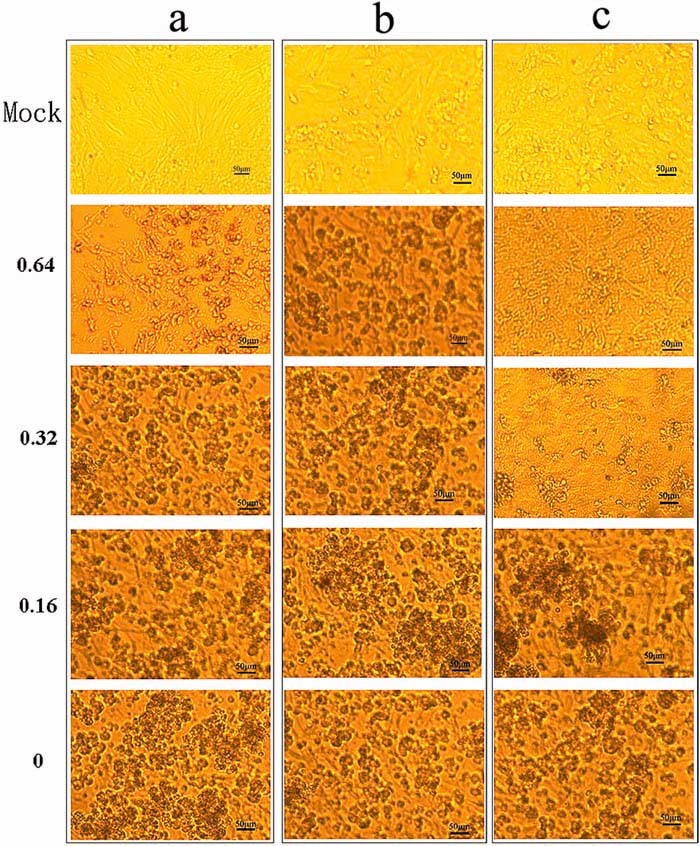
The cytopathic effects in CEK cells infected with progeny IBV from cells treated with the concentrations of forsythoside A (0.64 mm, 0.32 mm, 0.16 mm and 0) indicated to the left. (a) Pretreatment of CEK cells with forsythoside A. (b) The effect of forsythoside A on infected CEK cells; (c) Treatment of virus with forsythoside A. This figure is available in colour online at http://wileyonlinelibrary.com/journal/ptr
Figure 2.
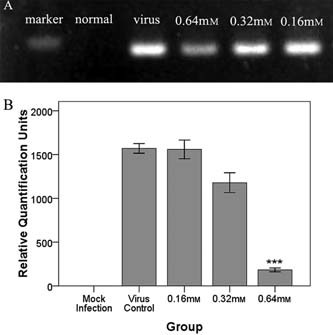
The effect of forsythoside A pretreatment on CEK cells. (A) PCR product shown by agarose gel electrophoresis. Real‐time PCR product of IBV N gene in cells pretreated with forsythoside A is shown. (B) Contents of IBV N gene measured by real‐time PCR in cells pretreated with forsythoside A. It shows the mRNA contents of IBV N gene in the mock infection sample, IBV control and forsythoside A groups (0.16 mm, 0.32 mm, 0.64 mm, CT values were normalized by β‐actin). Error line represents the standard deviation (SD) (n = 3). Asterisk indicates the comparative results of mRNA levels (signified by RQ unit) between 0.64 mm sample and IBV control sample (***p < 0.001, SPSS.16.0 used).
Forsythoside A has a direct virucidal effect on IBV
Partial inhibition of the infectivity was seen at 0.32 mm and complete inhibition at 0.64 mm of forsythoside A, respectively, as shown by observation of CPE (Fig. 1c). The antiviral effect of forsythoside A was confirmed by real‐time PCR analysis. The trend in reduced viral yield was seen by a reduction in virus titre and IBV N gene expression, compared with untreated IBV infected cells. At a concentration of 0.64 mm, the production of virus progeny was abrogated (Fig. 3).
Figure 3.
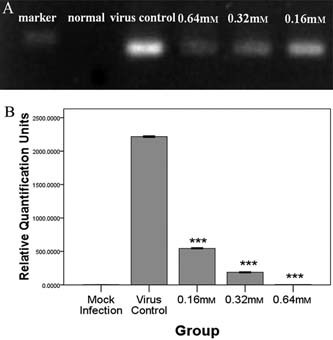
Histogram showing the content of IBV treated with forsythoside A directly. (A) Direct effect of forsythoside A on virions detected by real‐time PCR, the PCR product of IBV N gene is shown by agarose gel electrophoresis. (B) mRNA levels of IBV N gene measured by real‐time PCR on direct effect of forsythoside A on virions. It shows the mRNA contents of IBV N gene in the mock infection group, IBV control and forsythoside A groups (0.16 mm, 0.32 mm, 0.64 mm). Error line represents the standard deviation (SD) (n = 3). Asterisk indicates the comparative results of mRNA levels (signified by RQ unit) between each concentration of forsythoside A group and IBV control (***p < 0.001).
Forsythoside A treatment does not reduce progeny virus production in IBV‐infected
Forsythoside A, when added to the infected cells, did not decrease IBV infectivity after 36 h at any concentration analysed, as determined by observations of CPE (Fig. 1b). The lack of inhibitory effect was confirmed by real‐time PCR (data not shown).
These results indicate that forsythoside A inhibits IBV infection in vitro, in a dose‐dependent manner. It displayed a direct virucidal effect, but had no effect on IBV‐infected cells. However, when a high dose of forsythoside A (0.64 mm) was used to treat CEK cells prior to IBV infection, it was able partially to inhibit the infectivity of IBV. These data suggest the potential use of forsythoside A as an antiviral agent against IBV, although the mechanisms remain unclear. It has effects on cell signalling that could lead to changes in virus replication, so further studies could focus on changes induced by forsythoside A on the signal pathways relevant to IBV infection.
Conflict of Interest
The authors have declared that there is no conflict of interest.
Acknowledgements
The research was supported by Natural Science Foundation of Beijing (No. 6092004), Academic Human Resource Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality and National Natural Science Foundation (No. 30771625 and 30972215).
Huawei Li and Jufu Wu contributed equally to this paper.
Huawei Li and Jufu Wu contributed equally to this paper.
Contributor Information
Zhongwen Zhang, Email: zwzhang9288@sina.com.
Guojuan Wu, Email: wgj9288@sina.com.
REFERENCES
- Bochkov YA, Batchenko GV, Shcherbakova LO, Borisov AVL, Drygin VV. 2006. Molecular epizootiology of avian infectious bronchitis in Russia. Avian Pathol 35: 379–393. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. 2003. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol 32: 567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang L, Zhang N et al. 2003. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother 47: 2810–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan CAM, Kuo L, Masters PS, Vennema H, Rottier PJM. 1998. Coronavirus particle assembly: primary structure requirements of the membrane protein. J Virol 72: 6838–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Takahashi K, Abe T, Hikino H. 1981. Structure of Forsythoside A, an Antibacterial Principle of Forsythia suspensa Leaves. Heterocycles 16: 1311–1314. [Google Scholar]
- Gelb J Jr, Weisman Y, Ladman BS, Meir R. 2005. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000). Avian Pathol 34: 194–203. [DOI] [PubMed] [Google Scholar]
- Hu KJ, Xu KJ, Wang Y H et al. 2001. Empirical study of forsythoside anti‐virus in vitro . Chin J Tradit Med Sci Technol 8: 889 (in Chinese). [Google Scholar]
- Jackwood MW, Hilt DA, Lee CW et al. 2005. Data from 11 years of molecular typing infectious bronchitis virus field isolates. Avian Dis 49: 614–618. [DOI] [PubMed] [Google Scholar]
- Kingham BF, Keeler CL Jr, Nix WA, Ladman BS, Gelb J Jr. 2000. Identification of avian infectious bronchitis virus by direct automated cycle sequencing of the S‐1 gene. Avian Dis 44: 325–335. [PubMed] [Google Scholar]
- Kwon HM, Jackwood MW, Gelb J Jr. 1993. Differentiation of infectious bronchitis virus serotypes using polymerase chain reaction and restriction‐fragment‐length polymorphism analysis. Avian Dis 37: 194–202. [PubMed] [Google Scholar]
- Lai MM, Cavanagh D. 1997. The molecular biology of coronaviruses. Adv Virus Res 48: 1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yin JH, Sui XW, Li GX, Ren XF. 2009. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro . Avian Pathol 38: 215–221. [DOI] [PubMed] [Google Scholar]
- Liu SW, Chen JF, Han ZX et al. 2006. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol 35: 394–399. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real2time quantitative PCR and the 2‐ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mori K, Kido T, Daikuhara H et al. 1999. Effect of Hochu‐ekki‐to (TJ‐41), a Japanese herbal medicine, on the survival of mice infected with influenza virus. Antivir Res 44: 103–111. [DOI] [PubMed] [Google Scholar]
- Nishibe S, Okabe K, Tsukamoto H, Sakushima A, Hisada S. 1982. The structure forsythoside isolated from Forsythia suspensa . Chem Pharm Bull 30: 1048–1050. [DOI] [PubMed] [Google Scholar]
- Ren X, Yin J, Ma D, Li G. 2009. Characterization and membrane gene‐based phylogenetic analysis of avian infectious bronchitis virus Chinese strain HH06. Virus Genes 38: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KA, Hiscox JA. 2006. Characterisation of the RNA binding properties of the coronavirus infectious bronchitis virus nucleocapsid protein amino‐terminal region. FEBS Lett 580: 5993–5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Otake T, Mori H et al. 1993. Screening test of crude drug extract on anti‐HIV activity. Yakugaku Zasshi 113: 818–824. (In Japanese) [DOI] [PubMed] [Google Scholar]
- Zhang B, Shen LS. 2003. The application and development of real‐time fluorescent quantitative PCR. Foreign Medical Sciences (section of Clinical Biochemistry and Laboratory Medicine) 6: 327–329. [Google Scholar]


