Abstract
Respiratory viral infection is known to be a significant cause of asthma exacerbation. Eosinophils have been considered to play an important role in the pathogenesis of virus‐induced asthma exacerbations. To determine how often asthma exacerbation is caused by virus infections and to examine the relationship between eosinophilia and asthma episode, we investigated 64 children who experienced asthma attacks between October 1999 and March 2000. We used rapid enzyme immunoassays to detect antigens of respiratory syncytial virus (RSV), influenza A virus, and adenovirus in nasopharyngeal secretions (NPS) of these children, and enumerated eosinophils in the blood and NPS. We detected RSV in 27% and influenza A virus in 17% of the patients. No adenovirus infection or RSV/influenza A co‐infection was detected. RSV‐infected children were younger (3.85 ± 0.83 years old) than influenza A virus‐infected patients (5.23 ± 1.34 years old). Eighty‐two per cent of patients in the RSV group and 36% of patients in the influenza A virus group had moderate‐to‐severe asthma episodes (p < 0.05). In RSV‐infected children, the eosinophil counts in NPS were higher in the ‘severe’ group, and younger patients had a greater number of eosinophils in their NPS than older patients (p < 0.05). These trends were not found in influenza A virus patients. In conclusion, our results indicate that, compared with influenza A virus‐induced asthma attacks, RSV infection had a higher probability of being associated with asthma exacerbation in infants and younger children and induced attacks of greater severity. The increase in the number of eosinophils in the NPS of RSV‐infected children may be responsible, in part, for these differences.
Keywords: asthma exacerbation, RSV, influenza A virus, eosinophils, NPS
Viral respiratory tract infections frequently cause wheezing in children with asthma and, in some cases, may even precipitate the development of asthma (1). Recently, because of improved detection techniques, viral infections have been increasingly recognized as a cause of asthma exacerbation in children. A recent study of schoolchildren showed that 80–85% of episodes of acute asthma are associated with a respiratory viral infection (2). Many studies show that respiratory virus infections precipitate acute exacerbation of asthma and are the commonest reason for admission to hospital (3, 4).
The pathogenesis of asthma is complex, but a prominent feature is the migration of eosinophils into the respiratory mucosa (5) and the local release of various chemical mediators.
We aimed to establish the frequency with which asthma exacerbations are caused by respiratory syncytial virus (RSV), influenza A virus or adenovirus infections. If the eosinophil count of the nasopharyngeal secretions (NPS) changed during acute respiratory viral infection, we studied its relationship with severity of illness, patient age, and the eosinophil count in peripheral blood.
Subjects and methods
Subjects
From October 1999 to March 2000, 64 asthmatic children with acute exacerbation were enrolled. The diagnosis of asthma and classification of asthma attack severity were based on standard criteria (6). According to the patients' signs and symptoms, they were divided into three groups: mild, moderate, and severe.
The age of the patients ranged from 4 months to 15 years (mean age 4.14 ± 3.55 years), and 35 were girls. They attended the pediatric department of Showa University Hospital with signs and symptoms of wheezing and lower respiratory tract illness, with or without fever. Most needed hospitalization. When the patient entered the hospital, a detailed history was taken and a physical examination was carried out. Approximately 5 min after nebulized bronchodilation had been performed, the NPS of the patient was obtained by placing a sterile polyethylene catheter into the nasopharynx, followed by aspiration into a mucus trap. The tubing was then flushed with 2 ml of saline solution. Viral antigen detection was performed on the NPS within 30 min of its collection. Blood was collected for eosinophil counts.
Viral studies
The NPS of the patients were tested for viral antigens of RSV, influenza A virus, and adenovirus. The diagnosis of RSV infection was made using Abbott Testpack RSV (Abbott Laboratories, IL, USA); diagnosis of influenza A virus was performed using Directigen Flu A (Becton‐Dickinson Microbiology Systems, MD, USA); and adenovirus was detected using adenovirus antigen rapid diagnostic kit (SA Scientific, Inc., USA). All are rapid enzyme immunoassays designed for the direct detection of viral antigen in respiratory secretions.
NPS smears for eosinophils
After obtaining NPS, a sterile cotton swab was used to transfer mucoid secretions onto a glass slide, which was then immediately immersed in methanol. After 30 min, secretions were treated with the ‘1‐min method’ for eosinophils (Torii Pharm. Co. Ltd, Tokyo, Japan). Three or more fields from each slide were examined and the total number of eosinophils per 100 cells counted was recorded.
Statistical analysis
Statistical analysis was carried out using statview. The means of different groups were compared with the unpaired t‐test. The relationship between NPS and virus infection, age, and eosinophil count in blood, were analyzed using Pearson's correlation coefficient. Analysis of frequency data was performed by using the chi‐square test. A p‐value of ≤0.05 was considered significant.
Results
Virus antigens were detected in 44% of specimens from the wheezing children. Of these, 17 patients were positive for RSV (27%) and 11 patients were positive for influenza A virus (17%). No adenovirus infection was detected and no patient was positive for two viruses. Among the virus‐infected asthma patients, those with RSV were younger than those with influenza A (Fig. 1). Fifty‐nine per cent of the RSV‐infected children and 45% of the influenza A virus‐infected children were < 2 years old. The average age was 3.85 ± 0.83 vs. 5.23 ± 1.34 years old for RSV‐ and influenza A virus‐infected patients, respectively. These differences did not reach statistical significance (t = 0.909, p> 0.05).
Figure 1.
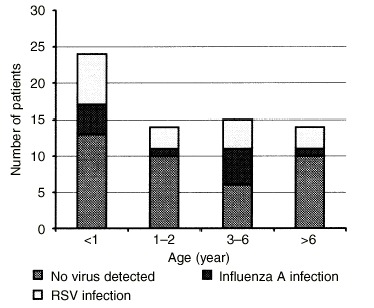
Percentage of virus infections in different age‐groups of children. Among the patients <2 years old, 26% had respiratory syncytial virus (RSV) infection compared with 13% who had influenza A virus infection. Within the patients ≥3 years old, 24% of the asthma attacks were induced by RSV infection; 20% were induced by influenza A virus infection.
Compared to children with asthma exacerbation caused by influenza A, those with RSV infections had asthma of greater severity. Among the RSV‐infected patients, 82% were judged to have moderate or severe asthma compared to 36% of those infected with influenza A (chi‐square = 4.312, p< 0.05). Symptoms of wheeze, wet deep cough, shortness of breath, and stridor lasted longer in RSV‐infected children than in those infected with influenza A. Nine of the 17 RSV‐infected children (53%) needed therapy with BDP or sodium cromoglycate, compared to 36% in the influenza A group.
The percentage of eosinophils in NPS smears was higher in the group of RSV‐infected patients with severe asthma (Fig. 2). The greater the severity of RSV infection, the higher the NPS eosinophil counts (although this trend was not significant). No such trend was observed in other patients with exacerbation of asthma (including the influenza A‐infected group).
Figure 2.
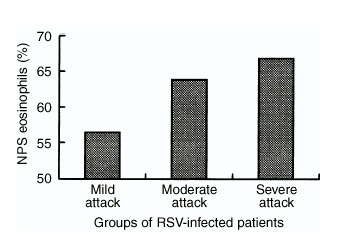
The percentage of eosinophil counts in the nasopharyngeal secretions (NPS) of the patients infected with respiratory syncytial virus (RSV). The eosinophil count of the NPS from the patients in the severe attack group is the highest, but no such trend was observed in the influenza A virus‐infected patients or in other patients with asthma exacerbation. There was a positive correlation between severity of RSV infection and number of eosinophils in the patients' sputum.
Within the RSV‐infected group, the patients with higher blood eosinophil counts were older than the patients with lower blood eosinophil counts (correlation coefficient=0.52, p< 0.05) (Fig. 3a). However, there was an inverse relationship between age and NPS eosinophil counts (Fig. 3b): the younger RSV‐infected patients had a greater number of eosinophils in their NPS than the older children. This relationship was statistically significant (correlation coefficient=−0.58, p< 0.05). This relationship between eosinophil number and NPS was not found among the influenza A‐infected or other asthmatic patients.
Figure 3.
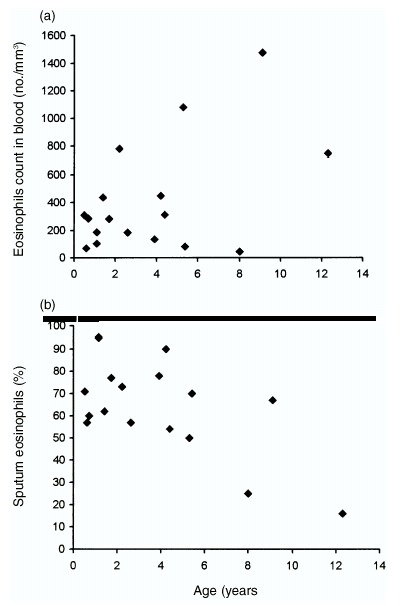
(a) The relationship between age and blood eosinophil counts in respiratory syncytial virus (RSV)‐infected patients. The younger the RSV‐infected patients, the lower the percentage of eosinophils in their peripheral blood (p<0.05). (b) There was an inverse relationship between age and number of eosinophils in nasopharyngeal secretions (NPS) in RSV‐positive patients: the younger the RSV‐infected patients, the greater the number of eosinophils detected in sputum (p<0.05).
Discussion
A seasonal variation of virus‐induced asthma exacerbation, especially in childhood asthma, has been observed. Regular outbreaks occur between October and April, when our study was carried out. In the present study, three types of virus, known to infect and replicate in the lower respiratory tract, were analysed in children with asthma exacerbation.
Adenovirus infection is not an important trigger of asthma attack in children of any age. Consequently, we did not identify asthmatic children with adenovirus infection in our study. RSV is one of the most important respiratory pathogens and is also the most common cause of asthma attack in infants and young children. In children < 2 years of age, RSV infection was frequently found in association with acute wheezing and respiratory distress, often requiring hospitalization. In older children, RSV infection was also commonly associated with wheezing. However, rhinovirus, coronavirus, and influenza A virus are also significant factors in the exacerbation of wheezing.
In our study, RSV antigen was detected in 17 patients (27%), which is similar (31%) to the proportion infected with RSV and identified in a previous study carried out in Japan (7). Our study also revealed that among the virus‐infected children, 61% of the asthma exacerbation was caused by RSV and 49% by influenza A virus infection. It was observed that RSV‐infected patients were younger, mostly < 2 years old (58%), and exhibited more severe clinical symptoms. The findings of the present study therefore parallel those seen in previous studies of RSV inducing an asthma episode (8).
In our research we confirmed the significant inverse relationship between age and number of NPS eosinophils in RSV‐infected asthmatic children, i.e. there were a greater number of eosinophils in the NPS of younger RSV‐infected patients than in older patients, although the older patients had a greater number of eosinophils in their peripheral blood. The observation that infants and younger children with RSV infection have symptoms of greater severity could be partly attributed to the increased number of eosinophils in the respiratory tract. Experiments with animals and clinical studies have implied that eosinophilia is associated with viral infections in asthma (9, 10). A massive infiltration of eosinophils has been observed in asthmatic patients during viral infection (11). Thus, eosinophil infiltration is considered to be a crucial element of the pathology leading to clinical exacerbation of asthma. Furthermore, recent research has proposed that analysis of the respiratory mucosa secretion is a more accurate diagnostic test than measurement of blood eosinophils for detecting airway eosinophilic inflammation (12). Therefore, our result from testing NPS eosinophils was more reliable for reflecting the airway inflammation of asthmatic children.
On the other hand, influenza A virus is also a virus that can induce asthmatic attack, although the asthmatic attack caused by influenza A virus is less common and less severe than that caused by RSV infection. In an ovalbumin sensitization mouse model, eosinophilia was not discovered in the lung tissue and not observed in the airway after infection with influenza A virus (13). In our study we did not detect eosinophilia in the NPS or in blood among influenza A‐infected patients, in contrast to that caused by RSV infection.
The results of this study indicated that RSV infection was more likely to be associated with asthma exacerbation in infants and younger children than influenza A virus, and would potentially lead to a more severe illness. The increased number of airway eosinophils may partly contribute to this.
References
- 1. Pattenmore PK, Johnston SL, Bardin PG. Viruses as precipitations and symptoms. I Epidemiology. Clin Exp Allergy 1992: 22: 325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston SL, Pattenmore PK, Sanderson G, et al. Com‐munity study of the role of viral infections in exacerbations of asthma in 9–11‐year‐old children. BMJ 1995: 310: 1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995: 95: 500–5. [PubMed] [Google Scholar]
- 4. Noble V, Murray M, Webb MSC, Alexander J, Swarbrick J, Milner AD. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child 1997: 76: 315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol 1988: 81: 776–81. [DOI] [PubMed] [Google Scholar]
- 6. Guidelines for the Diagnosis and Management of Asthma. Expert Panel Report. NIH publication no. 97–405. Bethesda, MD: National Institutes of Health, 1997. [Google Scholar]
- 7. Saijo M, Ishii T, Kobubo M, Takahashi Y. Respiratory syncytial infection in lower respiratory tract and asthma attack in hospitalized children in North Hokkaido, Japan. Acta Paediatr Jpn 1993: 35: 233–7. [DOI] [PubMed] [Google Scholar]
- 8. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999: 354: 541–5. [DOI] [PubMed] [Google Scholar]
- 9. Schwarza J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection re‐sults in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 1997: 100: 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duff AL, Pomeranz ES, Gelber LE, et al. Risk factors for acute wheezing in infants and children: viruses, pass‐ive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993: 92: 535–40. [PubMed] [Google Scholar]
- 11. Pizzichini E, Pizzichini MMM, Efthimiadis A, Dolovich J, Harfreave FE. Measuring airway influenza in asthma; eosinophilic cationic protein in induced sputum compared with peripheral blood. J Allergy Clin Immunol 1997: 99: 539–44. [DOI] [PubMed] [Google Scholar]
- 12. Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airway influenza during rhinovirus colds in normal and asthmatic subjects. Am J Respir Crit Care Med 1995: 151: 879–86. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki S, Suzuki Y, Yamamoto N, et al. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen‐exposed mice. J Allergy Clin Immunol 1998: 102: 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


