Abstract
A total of 54 broiler flocks during the first two weeks of life was used to investigate the incidence of avian pathogenic E. coli in Egypt; 28 isolates (51.85%) were revealed by colony morphology and biochemical identification which then investigated for their serogroups and only 18/28 isolates were serotyped. The most prevalent serotypes were O115, O142, O158, O55, O125, O114, O27, O20, and O15. By application of polymerase chain reaction (PCR), 83.3% (15/18) of the serotyped isolates were confirmed to be E. coli, and 93.3% (14/15), 46.6% (7/15), and 20% (3/15) of isolates harbored the iss, iutA, and fimH genes, respectively. Virulence testing of the selected 13 APEC isolates on the specific-pathogen-free (SPF) chicks revealed them to be highly virulent (15.4%), moderately virulent (23.1%), and avirulent (61.5%); however, all isolates (100%) were extremely virulent towards SPF embryonated chicken eggs. Antibiotic resistance (100% of isolates (n = 13)) was observed for ampicillin, amoxycillin–clavulanic acid, and tetracyclines, colistin (92.31%; 12/13), doxycycline and spiramycin (84.62%; 11/13), florfenicol (69.23%; 9/13), cefotaxime (61.54%; 8/13), and ciprofloxacin (53.85%; 7/13). The highest percentage of sensitivity (53.85% of isolates; 7/13) was recorded for ofloxacin and enrofloxacin followed by gentamycin (46.15%; 6/13). The results suggest that the diagnosis of APEC with PCR is rapid and more accurate than traditional methods for E. coli identification; moreover, the presence or absence of iss, iutA, and/or fimH genes is not an indicator of in vivo pathogenicity of APEC. Thus, further studies, including a wider range of virulence genes and gene sequencing, are required. In addition, serotyping has no effect on the virulence of APEC.
Keywords: E. coli, APEC, serotyping, PCR, virulence gene, antibiotics, broilers, resistance
1. Introduction
Escherichia coli (E. coli) is a Gram-negative bacterium of the family Enterobacteriaceae [1]. It is a commensal microorganism found in the intestine of humans and animals; however, it may induce illness so they are classified to commensal and pathogenic E. coli with further classification of pathogenic group to two pathotypes, diarrheagenic E. coli (DEC) and extraintestinal pathogenic E. coli (ExPEC), that cause various diseases in both humans and animals [2]. Avian colibacillosis, caused by ExPEC, is one of the major bacterial diseases in the poultry industry that has gained immense attention worldwide [3]. It is responsible for various disorders, including colisepticemia, air sacculitis, peritonitis, perihepatitis, pericarditis, omphalitis, coligranuloma, enteritis, synovitis, swollen head syndrome, and osteomyelitis [4], which eventually lead to total or partial condemnation of carcasses and expensive antibiotic treatment [3]. Animals of various ages are susceptible to avian colibacillosis; adults are more prone to infection. Moreover, yolk sac infection and high mortality rate in the baby chicks or embryos were recorded following the penetration of eggshells with E. coli, as well as spreading of the organism during egg hatching, laying, or oviduct formation [3,5,6]. Furthermore, it is important to consider the potential for its zoonotic transmission through poultry reservoirs [7]. Hence, the European Union has enforced food safety legislation, which usually constitutes a blueprint for the bill in third countries [8,9]. For determining the virulence of E. coli strains, its inoculation into embryos or 1-day-old chicks is followed as a golden standard test [10], whereas serotyping remains the most frequently used diagnostic method in laboratories, although it only allows the identification of a limited number of avian pathogenic E. coli (APEC) strains [11]. Moreover, the prevalence of a certain serotype is linked with the geographical localization of a flock [3]; hence, various molecular typing methods have been employed to study APEC, but none has revealed a specific genotype [12]. Several virulence genes have been associated with APEC including adhesin factors such as (fimH) that relate to the adhesion to the avian upper respiratory tract [13], as well as iss gene, which is known for its increasing ability to survive in the serum and it has been found more frequently among the pathogenic strains than the nonpathogenic ones, [14]. The iutA gene, one of the five genes of the aerobactin operon, encodes an outer membrane protein involved in the high binding affinity for iron [15,16]. Previously, the frequency of these genes was higher in APEC isolates obtained from other countries. Number and combination patterns of the virulence-associated genes in E. coli strains correlate with the virulence factors [17]; nevertheless, numerous studies have demonstrated that these virulence factors are rarely present in the same isolate and they can occur either individually or polygenically with varying frequencies in the clinical isolates [18,19]. This study aims to investigate the prevalence of APEC in baby chicks using traditional laboratory methods, followed by confirmatory molecular techniques, and assess their virulence in specific pathogen-free (SPF) 1-day-old chicks and embryonated chicken eggs (ECEs) and the correlation of in vivo virulence with studied virulence genes (iss, iutA, and fimH) or serotypes. Finally, the isolates are subjected to antibiotic sensitivity testing.
2. Results
2.1. Bacterial Isolation, Identification, and Serogrouping
The cultural morphology on MacConkey and Eosin Methylene Blue (EMB) agars and biochemical identification revealed that 51.85% (28/54) of the examined broiler chick flocks were positive to E. coli. Serological identification revealed that 18/28 isolates of E. coli were serotyped (64.3%), whereas 10/28 isolates were untypable (35.7%). The most prevalent serotype was O115 4/18 (22.2%); followed by O142 3/18 (16.66%); O158, O55, O125, and O114 2/18 (11.11% for each); and O27, O20, and O15 1/18 (5.55% for each).
2.2. Polymerase Chain Reaction (PCR) Using E. coli Species-Specific Gene and Virulence Genes (iss, iutA, and fimH)
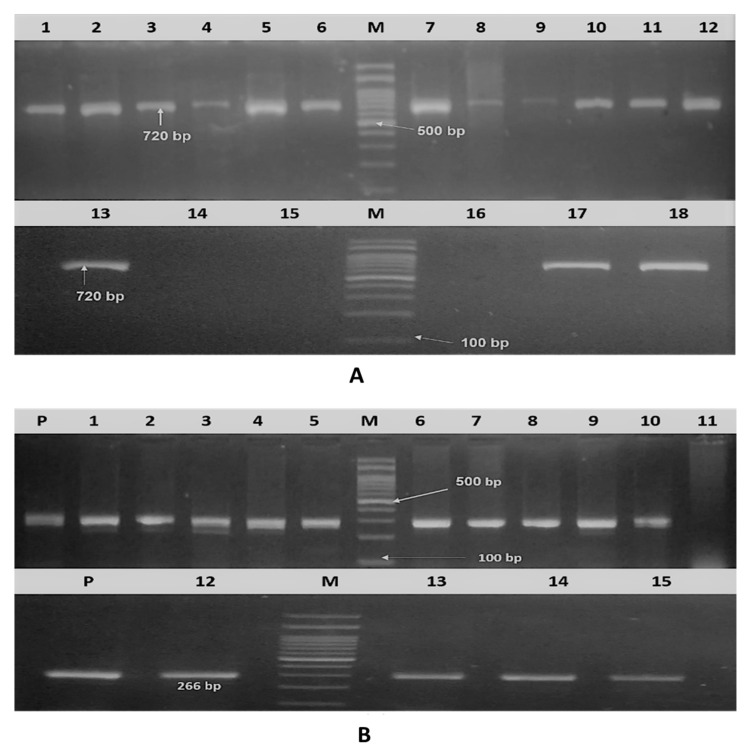
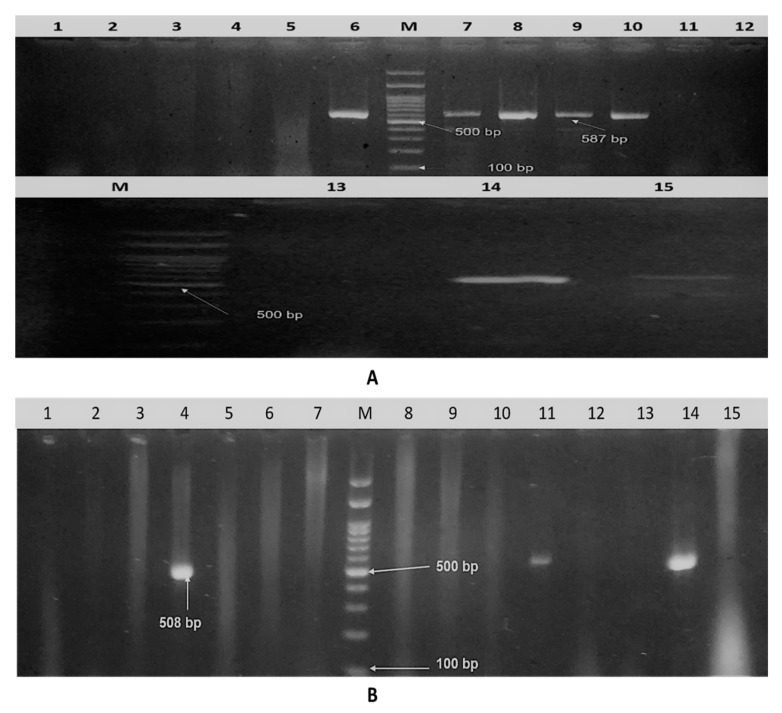
Of the 18 (83.3%) isolates, 15 were confirmed to be E. coli (positive for phoA gene; Figure 1A). Considering the virulence genes, the iss gene was detected in 14/15 isolates (93.3%), 7/15 (46.6%) were positive for the iutA gene, and 3/15 (20%) were positive for the fimH gene, as presented in (Figure 1B and Figure 2A,B). Various gene combinations were observed as following: iss gene was alone present in 6/15 of isolates (40%), iutA in 1/15 isolates (6.7%), iss plus fimH in 2/15 (13.3%), iss plus iutA in 5/15 (33.3%), and the three virulence genes were detected in 1/15 isolates (6.7%).
Figure 1.
Agarose gel electrophoresis. (A) Amplified PhoA gene of isolated APEC (18 serotyped isolates). Lane M: DNA molecular weight ladder (100 bp ladder), lane 1–18: isolates and positive sample at 720 bp. (B) Amplified iss gene of 15 molecularly confirmed APEC. Lane M: DNA molecular weight ladder (100 bp ladder), lanes p: positive control (266 bp), lane 1–15: samples.
Figure 2.
Agarose gel electrophoresis. (A) Amplified iutA gene of isolated APEC. Lane M: DNA molecular weight ladder (100 bp ladder), lanes 1–15: samples and positive samples at 587 bp. (B) Amplified fimH gene of isolated APEC. Lane M: DNA molecular weight ladder (100 bp ladder), lane 1–15: samples, and positive sample at 508 bp.
2.3. Results of Virulence Assessment in Specific Pathogen-Free (SPF) 1-day-old Chicks and Embryonated Chicken Eggs (SPF ECEs)
In vivo virulence assessment of thirteen E. coli isolates that represent different combinations of serotypes and virulence genes was performed (Table 1). Five isolates recorded the mortality rate as 20%, 40%, 40%, 60%, and 100%, respectively, with lesions including pericarditis, perihepatitis, air sacculitis, liver necrosis, and pneumonia, whereas the other 8 isolates recorded no mortalities but postmortem lesions included mild air sacculitis at the 7th day post-inoculation (PI) of SPF 1-day old chicks. In SPF ECEs, all 13 isolates resulted in (40–100%) embryo mortality, with the highest mortality rate at day 2 PI; cranial and skin hemorrhages and liver necrosis were recorded.
Table 1.
Different patterns of serotypes and virulence genes, and in vivo virulence assessment of APEC isolates.
| Serial No. | Isolate Code No. | Serotype | Virulence Gene Content | In Vivo Virulence Assays | |
|---|---|---|---|---|---|
| SPF 1-day-old Chicks a | SPF ECEs b | ||||
| 1 | 9 | O115 | iss | L | H |
| 2 | 16 | O115 | iss + iutA | L | H |
| 3 | 41 | O158 | iss + fimH | M | H |
| 4 | 50 | O158 | iss + fimH + iutA | L | H |
| 5 | 28 | O114 | iss + iutA | M | H |
| 6 | 54 | O114 | iutA | L | H |
| 7 | 5 | O125 | iss | H | H |
| 8 | 2 | O27 | iss | M | H |
| 9 | 7 | O55 | iss + fimH | L | H |
| 10 | 39 | O55 | iss + iutA | H | H |
| 11 | 15 | O20 | iss + iutA | L | H |
| 12 | 32 | O142 | iss + iutA | L | H |
| 13 | 49 | O15 | iss | L | H |
| 14 | Control c | - | - | - | - |
| 15 | Control negative d | - | - | - | - |
a SPF 1-day-old chicks (n = 5) were inoculated with each sample subcutaneously and were observed for 7 days post-infection to record the mortality rate. H: highly virulent (highly pathogenic isolates produced mortality or severe lesions including pericarditis, perihepatitis, air sacculitis, and liver necrosis in more than 50% of the challenged chicks), M: moderately virulent (were nonlethal and produced lesions in fewer than 50% of the inoculates), L: low virulent (produced no mortality and only occasional lesions in the air sacs) [20]. b 10-day-old SPF ECEs (n = 10) were inoculated with each sample via the allantoic sac route and were observed for 6 days post-inoculation to record the mortality rate. H: highly virulent strain resulted in mortality rate of >29% of ECEs; M: moderately virulent strains reported 10–29% mortality, and L: low virulent strains induced <10% mortality [13,21]. c Control for each assay (1-day-old chicks or ECEs) were sham inoculated with saline by the same route and dose; neither mortality nor post-mortem lesions were observed. d Control negative for each assay (1-day-old chicks or ECEs were not inoculated).
2.4. Results of Antibiotic Sensitivity Testing
Susceptibility of 13 E. coli isolates to 13 antibiotics was recorded and revealed that 7/13 (53.84%) of isolates were sensitive to ofloxacin and enrofloxacin, followed by gentamicin (6/13; 46.15%), ciprofloxacin and florfenicol (3/13; 23.08%), doxycycline, neomycin, and cefotaxime (1/13; 7.69%). All isolates recorded 100% resistance to ampicillin, amoxycillin–clavulinic acid, and tetracycline, followed by colistin (92.31%), spiramycin and doxycycline (84.62%), and florfenicol (69.23%); moreover, the intermediate susceptibility was showed by 53.85% of isolates for neomycin, 30.77% for cefotaxime, 23.08% for ciprofloxacin, and 15.38% for ofloxacin. Two isolates (code no. 50 and 7) showed resistance to all tested antibiotics, as listed in Table 2.
Table 2.
Results of antibiotic sensitivity testing for APEC isolates.
| Antibiotic a | APEC Isolates (Code No.) | % of Isolates b | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 16 | 41 | 50 | 28 | 54 | 5 | 2 | 7 | 39 | 15 | 32 | 49 | R | I | S | |
| Ampicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | 100 | 0 | 0 |
| Amoxycillin–clavulanic acid | R | R | R | R | R | R | R | R | R | R | R | R | R | 100 | 0 | 0 |
| Cefotaxime | R | I | I | R | R | R | R | R | I | R | S | R | I | 61.54 | 30.77 | 7.69 |
| Colistin | R | R | R | R | R | R | R | R | R | R | S | R | R | 92.31 | * | 7.69 |
| Tetracyclines | R | R | R | R | R | R | R | R | R | R | R | R | R | 100 | 0 | 0 |
| Doxycycline | S | R | R | R | R | R | R | I | R | R | R | R | R | 84.62 | 7.69 | 7.69 |
| Gentamicin | R | S | R | R | S | S | S | R | R | S | S | R | R | 53.85 | 0 | 46.15 |
| Neomycin | R | I | R | I | S | R | I | I | R | I | I | R | I | 38.46 | 53.85 | 7.69 |
| Spiramycin | R | R | I | R | R | R | I | R | R | R | R | R | R | 84.62 | 15.38 | 0 |
| Florfenicol | R | I | R | R | R | R | S | S | R | S | R | R | R | 69.23 | 7.69 | 23.7 |
| Ofloxacin | I | S | S | R | I | S | R | R | R | S | S | S | S | 30.77 | 15.38 | 53.85 |
| Enrofloxacin | R | S | S | R | R | S | R | R | R | S | S | S | S | 46.15 | 0 | 53.85 |
| Ciprofloxacin | R | I | S | R | R | I | R | R | R | S | S | I | R | 53.84 | 23.08 | 23.08 |
a Oxoid Laboratories, Basingstoke, Hampshire, England, and Lot No. 2230562. b R: resistant, I: intermediate sensitive, and S: sensitive to antibiotics, according to [22]. * Zone diameters for colistin were interpreted according to what was suggested by [23] as {R ≤ 11 mm, S ≥14 mm, as well as 12–13 mm, were considered susceptible}.
3. Discussion
In this study, bacteriological investigation for APEC was performed on 54 broiler flocks in the northern delta of Egypt during the first two weeks of age to determine the most prevalent serotype and virulence genes associated to these serotypes; furthermore, in vivo assessment of the virulence of certain selected isolates and their in vitro antibiotic sensitivity testing was carried out.
Although all examined diseased field broiler flocks (n = 54) received antibiotics during the last three days just before sampling, 51.85% of these flocks were positive to APEC by culturing and biochemical identification; it may indicate antibiotic resistance in the field. On the other hand, negative flocks (48.45%) may exhibit APEC but it is possible to suppress using antibiotics; unfortunately, this is what happens in the field before coming into clinical laboratories. The most common isolated serotype was O115 (14.2%); followed by O142 (10.7%); O158, O55, O125, and O114 (7.1% for each); and O27, O20, and O15 (3.5% for each); whereas, O1, O2, and O78 serotypes represent 0%. Nevertheless, similar findings regarding serotype prevalence were recorded by Abdeltawab et al. [24], Abd El-Haleem et al. [25], and Roshdy et al. [26]; however, the geographical localization of a flock may affect the prevalence of certain serotype. The observable decreased rate of O78 incidence may be attributed to the use of vaccines containing this serotype [11].
The observed percentage of the untypable isolates (35.7%) by O sero-grouping may be the result from autoagglutination or an incomplete antisera panel [27]; this was in accordance with the study by Eid et al. [28] and Abd El-Haleem et al. [25]. Eighteen APEC isolates were identified by serotyping; nevertheless, the use of the PCR technique revealed that only 15/18 (83.3%) isolates were positive for phoA gene (species-specific gene), and this method was repeated thrice for best results. These results indicate that molecular identification is more accurate and faster than the traditional method of bacteriological identification (colony morphology, biochemical, and serotyping); Eid et al. [28] reported similar findings.
Holland et al. [29] and Chui et al. [30] mentioned that the detection of specific microorganisms depends on virulence genes, and neglecting the species-specific gene is not recommended due to the presence of microorganisms that have similar virulence traits rather than the target microorganism itself. So in this study, the detection of the species-specific gene is considered the main step to complete the investigation of virulence genes. The iss, fimH, and iutA genes were selected due to their common prevalence in APEC isolated from broiler samples via PCR [19,31,32,33]. Our findings revealed that the most prevalent virulence gene was iss (93.3%), followed by iutA (46.6%), and fimH gene (20%); this highest incidence of iss and iutA genes among the pathogenic cases was in accordance with that in the study by Rodriguez-Siek et al. [34] (81.5% and 80.2%, respectively) and Rocha et al. [35] (73.8% and 45.9%, respectively). In contrast, Delicato et al. [19] detected the iutA gene with a higher percentage (63%) than the iss gene (38.5%).
The adhesion gene (fimH) showed low prevalence (20%), which was in accordance with the result (33.3%) reported by Mbanga and Nyararai [33]; though it was 98.1% [35] and 96.5% [19], these varying proportions were observed as this gene is not the sole adhesion factor. Several other genes such as P fimbrial adhesins, F11, Curli fimbriae, and other adhesins [36] possess adhesion properties; these are commonly found in APEC isolated from septicemic cases [37], and play a pivotal role in stabilizing APEC infection.
Several studies compared different models of pathogenicity testing, for example, the study of Gibbs et al. [38], which stated that intravenous, subcutaneous, and intratracheal challenges of chickens were strongly correlated with the embryo lethality assay. Moreover, the latter can discriminate between highly virulent, moderately virulent, and avirulent isolates of avian E. coli by determining the morality percentage [13].
Virulence assessment of APEC isolates in 1-day-old chicks or embryos is considered as the gold standard test to detect virulent strains according to the ability of APEC isolates to kill chicks or embryos in each model [39]. The virulence of 13 APEC isolates that were selected to be representative of different serotypes and virulence gene content in this study was compared using these two models.
In subcutaneously inoculated SPF 1-day-old chicks, two isolates were classified as highly virulent, three isolates were moderately virulent, and the remaining eight isolates were avirulent and showed mild air sacculitis without mortalities. In contrast, all the 13 tested isolates were highly virulent following the allantoic sac inoculation of SPF ECEs. This variation in mortality and the virulence between the embryo and chick models may indicate that the survival frequency of chicken embryo against colibacillosis lesions is less than that of chicks [40], which may be a result of the highly developed immune system of chicks than that of the embryo.
All 13 isolates, with variable serotypes, were highly virulent according to the SPF ECEs model; furthermore, in 1-day-old chicks, different isolates of the same serotype revealed distinct virulence indices; for example, two isolates of each of O114 and O158 were low and moderately virulent, thus indicating that serotyping has no effect on the virulence of APEC. Previous reports [11,41] proved that serotyping did not distinguish between the pathogenic and nonpathogenic E. coli.
In addition, it was observed that some highly virulent isolates in the SPF 1-day-old chick model had only one virulence gene, whereas others with all three investigated genes did not result in mortality (low virulent). Hence, no correlation existed between the number of virulence genes and virulence that agreed with the findings of previous studies [11,19], which reported that the presence of a single or even a combination of virulence genes is not enough to investigate most of APEC. Moreover, there are other genes that may be integrated in virulence other than iss, iutA, and fimH, and a sequence variation of the same gene may also play a role. Moreover, Wang et al. [17] suggested the presence of a real interaction among the APEC virulence factors; however, this role has not yet been established. Therefore, further studies are warranted to investigate these points.
In poultry production, the primary step for controlling losses resulting from APEC infection is to use antimicrobial therapy; however, the antimicrobial resistance increased with time among several bacterial species and resulted in major health threats [42]. Studying the susceptibility of 13 APEC isolates for 13 antibiotics revealed that the organism was susceptible to ofloxacin and enrofloxacin (53.85%), followed by gentamicin (46.15%), ciprofloxacin and florfenicol (23.08%), and eventually, neomycin, cefotaxime, and doxycycline (7.69%); however, 92.31% of the isolates exhibited resistance for colistin and 84.62% for spiramycin and doxycycline. Interestingly, resistance to all tested antibiotics was recorded for two isolates and three isolates had sensitivity to only 1/13 of the antibiotics. Moreover, all isolates (100%) exhibited resistance to ampicillin, amoxycillin–clavulanic acid, and tetracyclines. This issue is of great concern and a high-risk worldwide as even mild bacterial infections cannot be treated with antibiotics. Sepehri and Abbass-Zadeh [43] reported that the highest resistance was recorded for oxytetracycline, flumequine, neomycin, difloxacin, and enrofloxacin. Moreover, Ozawa et al. [44] reported that more than 70% isolates were resistant to ampicillin and tetracycline; this is of importance as penicillins and tetracyclines, in addition to sulfonamides and streptomycin, are the oldest drugs used against infectious bacterial diseases. Hence, resistance to these drugs was expected [45,46], and uncontrolled use of antimicrobials in poultry production for disease treatment or prevention may result in drug resistance, which was reported in our study. The use of antimicrobials as medication should only be for infectious cases to prevent their misuse, and applying biosecurity measures to overcome infection and decreasing the need for these antibiotics are crucial measures for public health.
4. Materials and Methods
In this study, all experimental procedures were performed according to the Local Experimental Animal Care Committee and were approved by the ethics board of the Institutional Committee of the Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Egypt. All efforts were made to minimize the suffering of animals.
4.1. Sample Collection
Liver, heart blood, and spleen pooled samples were collected from 54 broiler chicken flocks during the first 2 weeks of age from the governorates (Alexandria and El-Behera) of North Delta, Egypt, under a complete aseptic condition in the laboratory of the Poultry and Fish Diseases Department, Faculty of Veterinary Medicine, Alexandria University. For bacteriological examination, these samples were collected from live, diseased cases (5–7 chicks/flock) by postmortem examination; they exhibited omphalitis, perihepatitis, pericarditis, and air sacculitis as listed in Table 3.
Table 3.
The history of investigated broiler chick flocks.
| Code No. | Location | Total Bird No. | Age (days) | Breed | PM Lesions | Antibiotics during Last 3 days |
|---|---|---|---|---|---|---|
| 1 | Beheira | 2000 | 14 | Balady | Air sacculitis, unabsorbed yolk sac, and precipitation of urates on ureters | Florfenicol |
| 2 | Beheira | 4000 | 12 | Arbo Acres | Air sacculitis and fibrinous pericarditis and perihepatitis | Tylosin and colistin |
| 3 | Beheira | 6000 | 11 | Ross | Air sacculitis, pneumonia, fibrinous pericarditis, and perihepatitis and unabsorbed yolk sac | Tylosin and florfenicol |
| 4 | Beheira | 6000 | 9 | Balady | Unabsorbed yolk sac | Ciprofloxacin and florfenicol |
| 5 | Beheira | 5000 | 9 | Arbo Acres | Air sacculitis and whitish diarrhea | Sulphadiazine sodium plus trimethoprim and ciprofloxacin |
| 6 | Beheira | 26,000 | 6 | Cobb | Air sacculitis and unabosorbed yolk sac | Tiamulin and colistin |
| 7 | Beheira | 6000 | 12 | Ross | Air sacculitis and fibrinous pericarditis and perihepatitis, unabsorbed yolk sac | Ciprofloxacin and colistin |
| 8 | Beheira | 6000 | 14 | Cobb | Caseated blug at tracheal bifurcation and pneumonia | Florfenicol |
| 9 | Beheira | 2500 | 12 | Cobb | Ricketts and unabsorbed yolk sac | Florfenicol |
| 10 | Beheira | 100 | 4 | Cobb | Air sacculitis, fibrinous pericarditis and perihepatitis, unabsorbed yolk sac | Colistin |
| 11 | Beheira | 9000 | 14 | Cobb | Air sacculitis, septicemia, and unabsorbed | Tilmicosin and colistin |
| 12 | Beheira | 1000 | 10 | Ross | Unabsorbed yolk sac, air sacculitis | Tilmicosin and colistin |
| 13 | Beheira | 1000 | 7 | Cobb | Air sacculitis and pneumonia | Cefotaxim injection |
| 14 | Beheira | 6000 | 6 | Ross | Omphalitis, air sacculitis | Florfenicol |
| 15 | Beheira | 100 | 7 | Cobb | Pneumonia, air sacculitis, and omphalitis | Colistin |
| 16 | Beheira | 10,000 | 12 | Cobb | Enteritis and air sacculitis | Tylosin and colistin |
| 17 | Beheira | 450 | 11 | Cobb | Enteritis and air sacculitis | Colistin and doxy |
| 18 | Beheira | 1000 | 15 | Hybrid | Precipitation of urates on ureters | Sulphadiazine sodium plus trimethoprim |
| 19 | Beheira | 5000 | 9 | Ross | Air sacculitis, pneumonia, fibrinous pericarditis and perihepatitis and precipitation of urates on ureters | Sulphadiazine sodium plus trimethoprim |
| 20 | Beheira | 4000 | 7 | Ross | Air sacculitis and enteritis | Sulphadiazine sodium plus trimethoprim |
| 21 | Beheira | 10 | Cobb | Enteritis | Sulphadiazine sodium plus trimethoprim | |
| 22 | Beheira | 2700 | 15 | Ross | Pneumonia and airsacculitis | Florfenicol |
| 23 | Beheira | 3400 | 14 | Ross | Air sacculitis, fibrinous pericarditis and perihepatitis | Florfenicol |
| 24 | Beheira | 2000 | 15 | Cobb | Enteritis | Colistin and doxycycline |
| 25 | Beheira | 1500 | 14 | Ross | Enteritis | Colistin and tylosin |
| 26 | Beheira | 1000 | 13 | Cobb | Enteritis | Colistin and doxycycline |
| 27 | Beheira | 4500 | 15 | Cobb | Mycotoxicosis, air sacculitis, fibrinous pericarditis, and perihepatitis | Sulphadiazine sodium plus trimethoprim and cefotaxime |
| 28 | Alexanderia | 9000 | 15 | Cobb | Lesions of Gumboro disease and enteritis | Florfenicol |
| 29 | Beheira | 10,000 | 5 | Ross | Enteritis | Florfenicol and tylosin |
| 30 | Alexanderia | 8000 | 15 | Cobb | Necrotic enteritis | Florfenicol |
| 31 | Beheira | 7000 | 9 | Ross | Enteritis | Florfenicol |
| 32 | Beheira | 10,000 | 10 | Cobb | Enteritis | Tylosin and colistin |
| 33 | Beheira | 10,000 | 15 | Ross | Lesions of NewCastle and omphalitis | Doxycycline and colistin |
| 34 | Beheira | 1000 | 15 | Cobb | Enteritis | Doxycycline and colistin |
| 35 | Beheira | 1000 | 14 | Cobb | Air sacculitis | Florfenicol and doxycycline |
| 36 | Beheira | 2700 | 15 | Ross | NewCastle and Gumboro diseases IBD | Florfenicol |
| 37 | Beheira | 1000 | 6 | Cobb | Omphalitis, airsacculitis | Colistin and tylosin |
| 38 | Beheira | 6000 | 15 | Cobb | Air sacculitis, fibrinous pericarditis and perihepatitis | Florfenicol |
| 39 | Beheira | 5000 | 6 | Arbo Acres | Air sacculitis | Florfenicol and doxycycline |
| 40 | Beheira | 5000 | 6 | Arbo Acres | Air sacculitis | Ciprofloxacin and florfenicol |
| 41 | Beheira | 4000 | 3 | Ross | Omphalitis | Doxycycline and colistin |
| 42 | Beheira | 2000 | 4 | Ross | Omphalitis | Florfenicol |
| 43 | Beheira | 600 | 13 | Arbo Acres | Pneumonia, air sacculitis, fibrinous pericarditis and perihepatitis | Florfenicol |
| 44 | Beheira | 4000 | 4 | Ross | Enteritis | Tylosin and colistin |
| 45 | Beheira | 8500 | 14 | Cobb | Airsacculitis | Doxycycline and colistin |
| 46 | Beheira | 10 | Arbo Acres | Air sacculitis, fibrinous pericarditis, perihepatitisand omphalitis | Ciprofloxacin and florfenicol | |
| 47 | Beheira | 9000 | 2 | Arbo Acres | Omphalitis | Florfenicol and doxycycline |
| 48 | Beheira | 5000 | 3 | Cobb | Pneumonia, air sacculitis, fibrinous pericarditis, and perihepatitis | Tylosin and colistin |
| 49 | Beheira | 3000 | 6 | Cobb | Air sacculitis, fibrinous pericarditis, perihepatitisand unabsorbed yolk sac | Tylosin and colistin |
| 50 | Beheira | 5000 | 10 | Cobb | Air sacculitis and omphalitis | Doxycycline and colistin |
| 51 | Beheira | 1000 | 15 | Arbo Acres | Necrotic enteritis and air sacculitis | Ciprofloxacin and florfenicol |
| 52 | Beheira | 10,000 | 10 | Cobb | Air sacculitis | Doxycycline and colistin |
| 53 | Beheira | 1000 | 15 | Cobb | Air sacculitis | Florfenicol |
| 54 | Beheira | 9000 | 15 | Arbo Acres | Enteritis and slight airsacculitis | Doxycycline and colistin |
4.2. Bacteriological Isolation, Identification, and Serotyping
A loopful sample from each specimen was inoculated into the nutrient broth and was incubated at 37 °C for 24 h; thereafter, a loopful of inoculated broth was streaked on MacConkey agar plates and incubated for 24 h at 37 °C. Suspected lactose fermented colonies were isolated and streaked on eosin methylene blue media (EMB). Next, suspected colonies from EMB were isolated and subcultured into nutrient agar slants and were incubated at 37 °C for 24 h and then stored at 4 °C until use for further biochemical testing such as indole, methyl red, citrate utilization, urease, triple sugar iron (TSI), catalase, and Voges Proskauer tests.
In total, 28 isolates that were identified biochemically as E. coli were subjected to serological identification using slide agglutination test according to Lee et al. [47] using specific eight polyvalent, then 43 monovalent group O somatic antisera (Denka Seiken Co., Ltd. Tokyo, Japan), as shown in Table 4. The serotyping was performed in the serology unit, Animal Health Research Institute, Dokki, Egypt.
Table 4.
Group O somatic antisera for serological identification of E. coli isolates.
| Polyvalent Sera | Monovalent Sera | ||||||
|---|---|---|---|---|---|---|---|
| Polyvalent 1 | O1 | O26 | O86 | O111 | O119 | O127 | O128 |
| Polyvalent 2 | O44 | O55 | O125 | O126 | O146 | O166 | |
| Polyvalent 3 | O18 | O114 | O142 | O151 | O157 | O158 | |
| Polyvalent 4 | O6 | O27 | O78 | O148 | O159 | O168 | |
| Polyvalent 5 | O20 | O25 | O63 | O153 | O167 | ||
| Polyvalent 6 | O8 | O15 | O115 | O169 | |||
| Polyvalent 7 | O28 | O112 | O124 | O136 | O144 | ||
| Polyvalent 8 | O29 | O143 | O152 | O164 | |||
4.3. Polymerase Chain Reaction (PCR) Using E. coli General Gene and Virulence Genes (iss, iutA, and fimH)
Eighteen serotyped APEC isolates were tested for the presence of phoA gene, encoding E. coli species, by PCR technique. Moreover, virulence genes including iss, iutA, and fimH were detected on PCR-confirmed APEC isolates.
DNA extracts were prepared by the boiling method according to Sambrook et al. [48]. PCR reaction comprising 1 µL of extracted DNA was used with PCR Master Mix (10 µL; 2X TOP simple™ dyemix–ntaq cat.#p510t), forward primer (1 µL), reverse primer (1 µL ), and distilled water up to a volume of 20 µL. Primer sequence and cycling conditions are listed in Table 5 and Table 6.
Table 5.
Oligonucleotide primer sequences.
| Gene | Primer Sequence (5′-3′) | Amplified Product (bp) | Reference | |
|---|---|---|---|---|
| Species-specific | phoA | CGATTCTGGAAATGGCAAAAG CGTGATCAGCGGTGACTATGAC |
720 bp | [49] |
| Virulence | iss | ATG TTA TTT TCT GCC GCT CTG CTA TTG TGA GCA ATA TAC CC |
266 bp | [50] |
| iutA | ATGAGCATATCTCCGGACG CAGGTCGAAGAACATCTGG |
587 bp | [51] | |
| fimH | TGCAGAACGGATAAGCCGTGG GCAGTCACCTGCCCTCCGGTA |
508 bp | [52] | |
Table 6.
Cycling conditions for PCR
| Gene | Initial Denaturation | Denaturation | Annealing | Extension | Number of Cycles | Final Extension | |
|---|---|---|---|---|---|---|---|
| Species-specific | phoA | 94 °C 5 min |
94 °C 30 s |
58 °C 45 s |
72 °C 45 s |
35 | 72 °C 10 min |
| Virulence | Iss | 94 °C 5 min |
94 °C 30 s |
58 °C 45 s |
72 °C 45 s |
35 | 72 °C 10 min |
| fimH | 95 °C 2 min |
94 °C 30 s |
58 °C 30 s |
72 °C 1 min |
33 | 72 °C 7 min |
|
| iutA | 94 °C 3 min |
94 °C 1 min |
55 °C 1 min |
72 °C 30 s |
30 | 72 °C 7 min |
|
4.4. Virulence Testing APEC Isolates
The virulence of 13 APEC isolates was assessed; these isolates were selected to be representatives according to the serotype and virulence genes content (isolate no. 9, 16, 41, 50, 28, 54, 5, 2, 7, 39, 15, 32, and 49), as listed in Table 1 by the following models:
One-day-old specific pathogen-free (SPF) chick model: Seventy-five 1-day-old SPF chicks, supplied by a SPF farm at Al-fayoum province, Egypt, were divided into 15 groups (n = 5) for {13 isolates, 2 control groups (one sham-challenged subcutaneously with saline and the other non-challenged}. All chicks were reared in separate cages with food and water supplied ad-libitum. According to Wang et al. [17], each chick in every group was inoculated subcutaneously with 0.2 mL of APEC isolate suspension (1 × 108 CFU/mL), calculated according to the McFerland standard [53]. Deaths and clinical signs of illness were recorded four times daily for 7 days post-inoculation (PI). The surviving chicks were killed at 7th day PI and the lesions were recorded. APEC isolates were classified, on the basis of their virulence degree for one day-old chicks, as follows: (a) highly pathogenic isolates—produced mortality or severe lesions including pericarditis, perihepatitis, air sacculitis, and liver necrosis in more than 50% of the challenged chicks, (b) intermediate pathogens—were nonlethal and produced lesions in fewer than 50% of the inoculates, and (c) low pathogens—produced no mortality and only occasional lesions in the air sacs [20].
Specific pathogen-free (SPF) embryonated chicken eggs (ECEs) model: Fifteen groups of SPF ECEs (n = 10 eggs) for the same 13 isolates and 2 additional control groups (one group inoculated with saline and the other non-inoculated) were used in an embryo lethality test. According to Wooly et al. and Nolan et al. [13,21], 0.2 mL containing 500 CFU of each isolate (calculated according to McFerland standard [53]) was inoculated into 10 SPF ECEs through the allantoic sac (10-day old embryos); thereafter, by daily candling for 7 days PI, the mortality was recorded to classify APEC strains as follows: highly virulent strain resulted in mortality rate of >29% of ECEs, moderately virulent strains reported 10%–29% mortality, and <10% mortality was observed in a virulent strain.
4.5. In Vitro Antibiotic Sensitivity Testing of E. coli Isolates
Fifteen molecularly confirmed APEC isolates were tested by disc diffusion on Mueller–Hinton agar (MHA) for sensitivity to 13 different antibiotics (Oxoid Laboratories, Basingstoke, Hampshire, England, and Lot No. 2230562), according to Finegold and Martin [54]. The tested antibiotics (discs) included ampicillin (AMP/10 µg), amoxycillin–clavulanic acid (AMC/30 µg), cefotaxime (CTX/30 µg), colistin (CT/10 µg), tetracyclines (TE/30 µg), doxycycline (DO/30 µg), gentamicin (CN/10 µg), neomycin (N/30 µg), spiramycin (SP/100 µg), florfenicol (FFC/30 µg), ofloxacin (OFX/5 µg), enrofloxacin (ENR/5 µg), and ciprofloxacin (CIP/5 µg). According to zone diameter interpretive standards for Enterobacteriaceae [22], inhibition zone diameters were measured and were evaluated. Zone diameters of colistin were interpreted according to what was suggested by [23]: {resistant ≤ 11 mm, sensitive ≥ 14 mm, as well as 12−13 mm, were considered susceptible}.
5. Conclusions
All tested APEC isolates (n = 13) in the northern delta of Egypt exhibited resistance to ampicillin, amoxycillin–clavulanic acid, and tetracyclines; moreover, two isolates (O55 and O158) showed resistance to all tested antibiotics. Of the isolates, 53.3% showed sensitivity to ofloxacin and enrofloxacin. Nine E. coli serotypes (O115, O142, O158, O55, O125, O114, O27, O20, and O15) were the most circulated ones. The molecular diagnosis of APEC is faster and more accurate than the traditional microbiological methods. In this study, it was difficult to correlate between studied virulence genes (iss, fimH, and iutA) and/or serotyping with in vivo virulence assessment models. Hence, further studies are warranted to investigate a wider range of virulence genes for their possible use in judging the virulence of APEC.
Author Contributions
Conceptualization and Design, A.M.A., N.A.E.-S., D.S.K., and M.E.S.; Methodology, A.M.A., N.A.E.-S., D.S.K., and M.E.S.; Data Analysis, M.E.A.E.-H.; Writing and Reviewing, M.E.A.E.-H., A.A.S., A.H.M., H.E., A.K., and R.H.S. wrote and revised the paper. All authors have read and approved the final manuscript.
Funding
The authors extended their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project (RG-1440-120).
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Tenaillon O., Skurnik D., Picard B., Denamur E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 2.Filho H.K., Brito K., Cavalli L., Brito B. Avian pathogenic Escherichia coli (APEC)—An update on the control. In: Méndez-Vilas A., editor. The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs. Formatex Research Center; Badajoz, Spain: 2015. pp. 598–618. [Google Scholar]
- 3.Kabir S.M.L. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan L.K., Barnes H.J., Vaillancourt J.P., Abdul¬Aziz T., Logue C.M. Colibacillosis. Mosby-Wolf Publication Ltd.; London, UK: 2013. [Google Scholar]
- 5.Rahman M., Samad M., Rahman M., Kabir S. Bacterio-pathological studies on salmonellosis, colibacillosis and pasteurellosis in natural and experimental infections in chickens. Bangladesh J. Vet. Med. 2004;2:1–8. doi: 10.3329/bjvm.v2i1.1926. [DOI] [Google Scholar]
- 6.Jordan F.T., Williams N.J., Wattret A., Jones T. Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. Vet. Rec. 2005;157:573–577. doi: 10.1136/vr.157.19.573. [DOI] [PubMed] [Google Scholar]
- 7.Markland S.M., LeStrange K.J., Sharma M., Knie K.E. Old Friends in New Places: Exploring the Role of xtraintestinal E. coli in Intestinal Disease and Foodborne Illness. Zoonoses Public Health. 2015 doi: 10.1111/zph.12194. [DOI] [PubMed] [Google Scholar]
- 8.Bondoc I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part One: The Role of European Institutions in Laying Down and Passing Laws Specific to the Veterinary Sanitary and Food Safety Area. Universul Juridic, Supliment, 12–15. [(accessed on 24 July 2019)];2016 Available online: http://revista.universuljuridic.ro/supliment/european-regulation-veterinary-sanitary-food-safety-area-component-european-policies-safety-food-products-protection-consumer-interests-2007-retrospective/
- 9.Bondoc I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Two: Regulations. Universul Juridic, Supliment, 16–19. [(accessed on 24 July 2019)];2016 Available online: http://revista.universuljuridic.ro/supliment/european-regulation-veterinary-sanitary-food-safety-area-component-european-policies-safety-food-products-protection-consumer-interests-2007-retrospective-2/
- 10.Kunert F.H., Carvalho D., Grassotti T., Soares B., Rossato J., Cunha A., Brito K., Cavalli L., Brito B. Avian pathogenic Escherichia coli-methods for improved diagnosis. Worlds Poult. Sci. J. 2015;71:249–258. doi: 10.1017/S0043933915000264. [DOI] [Google Scholar]
- 11.Schouler C., Schaeffer B., Brée A., Mora A., Dahbi G., Biet F., Oswald E., Mainil J., Blanco J., Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbial. 2012;50:1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dziva F., Stevens M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37:355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- 13.Wooley R.E., Gibbs P.S., Brown T.P., Maurer J.J. Chicken embryo lethality assay for determining the virulence of avian Escherichia coli isolates. Avian Dis. 2000;44:318–324. doi: 10.2307/1592546. [DOI] [PubMed] [Google Scholar]
- 14.Pfaff-Mcdonough S.J., Horne S.M., Giddings C.W., Ebert J.O., Doetkott C., Smith M.H., Nolan L.K. Complement resistance-related traits among Escherichia coli isolates from apparently healthy birds and birds with colibacillosis. Avian Dis. 2000;44:23–33. doi: 10.2307/1592504. [DOI] [PubMed] [Google Scholar]
- 15.Johnson T.J., Siek K.E., Johnson S.J., Nolan L.K. DNA Sequence of a colv Plasmid and Prevalence of Selected Plasmid-Encoded Virulence Genes among Avian Strains. J. Bacteriol. 2006;188:745. doi: 10.1128/JB.188.2.745-758.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellata M., Touchman J.W., Curtiss R. Full sequence and comparative analysis of the plasmid papec-1 of avian pathogenic E. coli chi7122 (O78:K80:H9) PLoS ONE. 2009;4:4232. doi: 10.1371/journal.pone.0004232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Tang P., Tan D., Wang L., Zhang S., Qiu Y., Dong R., Liu W., Huang J., Chen T., et al. The pathogenicity of chicken pathogenic Escherichia coli is associated with the numbers and combination patterns of virulence-associated genes. Open J. Vet. Med. 2015;5:243–254. doi: 10.4236/ojvm.2015.512033. [DOI] [Google Scholar]
- 18.Vandekerchove D., Vandemaele F., Adriaensen C., Zaleska M., Hernalsteens J.P., De Baets L., Butaye P., Van Immerseel F., Wattiau P., Laevens H., et al. Virulence-associated traits in avian Escherichia coli: Comparison between isolates from colibacillosis-affected and clinically healthy layer flocks. Vet. Microbiol. 2005;108:75–87. doi: 10.1016/j.vetmic.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Delicato E.R., De Brito B.G., Gaziri L.C., Vidotto M.C. Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet. Microbiol. 2003;94:97–103. doi: 10.1016/S0378-1135(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger J., Fries P., Cloud S., Wilson R. In vitro and in vivo characterization of avian Escherichia coli. II. Factors associated with pathogenicity. Avian Dis. 1985;29:1094–1107. doi: 10.2307/1590464. [DOI] [PubMed] [Google Scholar]
- 21.Nolan L.K., Wooley R.E., Brown J., Spears K.R., Dickerson H.W., Dekich M. Comparison of a complement resistance test, a chicken embryo lethality test, and the chicken lethality test for determining virulence of avian Escherichia coli. Avian Dis. 1992;36:395–397. doi: 10.2307/1591518. [DOI] [PubMed] [Google Scholar]
- 22.ClSI (Clinical Laboratory Standards Institute) Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement M100-S23. ClSI (Clinical Laboratory Standards Institute); Wayne, PA, USA: 2013. [Google Scholar]
- 23.Galani I., Kontopidou F., Souli M., Rekatsina P.D., Koratzanis E., Deliolanis J., Giamarellou H. Colistin susceptibility testing by E test and disk diffusion methods. Int. J. Antimicrob. Agent. 2008;31:434–439. doi: 10.1016/j.ijantimicag.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Abdeltawab A., Maarouf A.A., Abd El Al S., El-Hofy F.A., El Mougy E. Detection of some virulence genes of avian pathogenic E. coli by polymerase chain reaction. Benha Vet. Med. J. 2014;26:159–176. [Google Scholar]
- 25.Abd El-Haleem Y.F. Master’s Thesis. Faculty of Veterinary Medicine, Zagazig University; Zagazig City, Egypt: 2000. Some Epidemiological Studies on Escherichia coli in Poultry Farms. [Google Scholar]
- 26.Roshdy H., El-Aziz S.A., Refai M. Incidence of E. coli in chickens and ducks in different governorates in Egypt. In Proceedings of the 1st Conference of Animal Health Research Institute Association; 2012. [(accessed on 19 December 2019)]. Available online: https://scholar.cu.edu.eg/?q=hanem/files/publication49_1_9.pdf. [Google Scholar]
- 27.Ozaki H., Murase T. Multiple routes of entry for Escherichia coli causing colibacillosis in commercial layer chickens. J. Vet. Med. Sci. 2009;71:1685–1689. doi: 10.1292/jvms.001685. [DOI] [PubMed] [Google Scholar]
- 28.Eid H.I., Algammal A.M., Nasef S.A., Elfeil W.K., Mansour G.H. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens. Asian J. Anim. Vet. Adv. 2016;11:350–356. [Google Scholar]
- 29.Holland J.L., Louie L., Simor A.E., Louie M. PCR detection of Escherichia coli O157:H7 directly from stools: Evaluation of commercial extraction methods for purifying fecal DNA. J. Clin. Microbial. 2000;38:4108–4113. doi: 10.1128/JCM.38.11.4108-4113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chui L., Couturier M.R., Chiu T., Wang G., Olson A.B., Mcdonald R.R., Antonishyn N.A., Horsman G., Gilmour M.W. Comparison of Shiga toxin-producing Escherichia coli detection methods using clinical stool samples. J. Mol. Diagnost. 2010;12:469–475. doi: 10.2353/jmoldx.2010.090221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paixao A.C., Ferreira A.C., Fontes M., Themudo P., Albuquerque T., Soares M.C., Fevereiro M., Martins L., Corrêa de Sá M.I. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates. Poult. Sci. 2016;95:1646–1652. doi: 10.3382/ps/pew087. [DOI] [PubMed] [Google Scholar]
- 32.Subedi M., Luitel H., Devkota B., Bhattarai R.K., Phuyal S., Panthi P., Shrestha A., Chaudhary D.K. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet. Res. 2018;14:113. doi: 10.1186/s12917-018-1442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbanga J., Nyararai Y.O. Virulence gene profiles of avian pathogenic Escherichia coli isolated from chickens with colibacillosis in Bulawayo, Zimbabwe. Onderstepoort J. Vet. Res. 2015;82:e1–e8. doi: 10.4102/ojvr.v82i1.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Siek K.E., Giddings C.W., Doetkott C., Johnson T.J., Fakhr M.K., Nolan L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151:2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- 35.Rocha A.C., Rocha S.L., Lima-Rosa C.A., Souza G.F., Moraes H.L., Salle F.O., Moraes L.B., Salle C.T. Genes associated with pathogenicity of avian Escherichia coli (APEC) isolated from respiratory cases of poultry. Pesq. Vet. Bras. 2008;28:183–186. doi: 10.1590/S0100-736X2008000300009. [DOI] [Google Scholar]
- 36.Nakazato G., Campos T.A.D., Stehling E.G., Brocchi M., Silveira W.D.D. Virulence factors of avian pathogenic Escherichia coli (APEC) Pesq. Vet. Bras. 2015;29:479–486. doi: 10.1590/S0100-736X2009000700001. [DOI] [Google Scholar]
- 37.Van Den Bosch J.F., Hendriks J.H., Gladigau I., Willems H.M., Storm P.K., De Graaf F.K. Identification of F11 fimbriae on chicken Escherichia coli strains. Infect. Immun. 1993;61:800. doi: 10.1128/IAI.61.3.800-806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbs P.S., Petermann S.R., Wooley R.E. Comparison of several challenge models for studies in avian colibacillosis. Avian Dis. 2004;48:751–758. doi: 10.1637/7176-030404R. [DOI] [PubMed] [Google Scholar]
- 39.Dho M., Lafont J.P. Escherichia coli colonization of the trachea in poultry: Comparison of virulent and avirulent strains in gnotoxenic chickens. Avian Dis. 1982;26:787–797. doi: 10.2307/1589865. [DOI] [PubMed] [Google Scholar]
- 40.Gibbs P.S., Wooley R.E. Comparison of the intravenous chicken challenge method with the embryo lethality assay for studies in avian colibacillosis. Avian Dis. 2003;47:672–680. doi: 10.1637/7011. [DOI] [PubMed] [Google Scholar]
- 41.Ewers C., Antão E.-M., Diehl I., Philipp H.C., Wieler L.H. Intestine and Environment of the Chicken as Reservoirs for Extraintestinal Pathogenic Escherichia coli Strains with Zoonotic Potential. Appl. Environ. Microbiol. 2009;75:184–192. doi: 10.1128/AEM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miles T.D., Mclaughlin W., Brown P.D. Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Vet. Res. 2006;2:7. doi: 10.1186/1746-6148-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sepehri G., Abbass-Zadeh H. Prevalence of bacterial resistance to commonly used antimicrobials among Escherichia coli isolated from chickens in Kerman Province of Iran. J. Med. Sci. 2006;6:99–102. [Google Scholar]
- 44.Ozawa M., Harada K., Kojima A., ASAI T., Sameshima T. Antimicrobial susceptibilities, serogroups, and molecular characterization of avian pathogenic Escherichia coli isolates in Japan. Avian Dis. 2008;52:392–397. doi: 10.1637/8193-120907-Reg. [DOI] [PubMed] [Google Scholar]
- 45.Singer R.S., Hofacre C.L. Potential impacts of antibiotic use in poultry production. Avian Dis. 2006;50:16–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- 46.Aidara-Kane A., Angulo F.J., Conly J.M., Minato Y., Silbergeld E.K., Mcewen S.A., Collignon P.J. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Cont. 2018;7:7. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee G.Y., Jang H.I., Hwang I.G., Rhee M.S. Prevalence and classification of pathogenic Escherichia coli isolated from fresh beef, poultry, and pork in Korea. Int. J. Food Microbiol. 2009;134:196–200. doi: 10.1016/j.ijfoodmicro.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 1989. [Google Scholar]
- 49.Hu Q., Tu J., Han X., Zhu Y., Ding C., Yu S. Development of multiplex PCR assay for rapid detection of Riemerella anatipestifer, Escherichia coli, and Salmonella enterica simultaneously from ducks. J. Microbiol. Method. 2011;87:64–69. doi: 10.1016/j.mimet.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Yaguchi K., Ogitani T., Osawa R., Kawano M., Kokumai N., Kaneshige T., Noro T., Masubuchi K., Shimizu Y. Virulence factors of avian pathogenic Escherichia coli strains isolated from chickens with colisepticemia in Japan. Avian Dis. 2007;51:656–662. doi: 10.1637/0005-2086(2007)51[656:VFOAPE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 51.Moulin-Schouleur M., Schouler C., Tailliez P., Kao M.R., Bree A., Germon P., Oswald E., Mainil J., Blanco M., Blanco J. Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. J. Clin. Microbiol. 2006;44:3484–3492. doi: 10.1128/JCM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiba M.R., Yano T., Leite Dda S. Genotypic characterization of virulence factors in Escherichia coli strains from patients with cystitis. Rev. Inst. Med. Trop. Sao Paulo. 2008;50:255–260. doi: 10.1590/S0036-46652008000500001. [DOI] [PubMed] [Google Scholar]
- 53.Mcfarland J. The nephelometer: An instrument for media used for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 1907;14:1176–1178. doi: 10.1001/jama.1907.25320140022001f. [DOI] [Google Scholar]
- 54.Finegold S.M., Martin S. Diagnostic Microbiology 6th ed the C.V. Mosby Company, St. Louis Tranto, London. Wiener Tierarstilichmschr. 1982;6:233. [Google Scholar]