Abstract
Background and aims: the increasing prevalence of strains resistant to antimicrobial agents is a critical issue for the management of Helicobacter pylori infection. This study aimed to evaluate, in Italian naïve patients, H. pylori antibiotic resistance trends and their potential predictive factors during the last decade. Methods: consecutive Italian naïve H. pylori positive patients, referred from General Practitioners to our Unit from January 2009 to January 2019 to perform an upper gastrointestinal endoscopy (UGIE), were considered. Each patient underwent 13C-urea breath test (13C-UBT) and UGIE with multiple biopsies to perform rapid urease test (RUT), culture/susceptibility test (vs. clarithromycin, metronidazole, levofloxacin), and histopathological examination. H. pylori status was assessed through CRM (composite reference method: at least two tests positive or only culture positive). Results: between 2009 and 2014, 1763 patients were diagnosed as H. pylori positive, 907 were naïve with antibiogram available. Between 2015 and 2019, 1415 patients were diagnosed as H. pylori positive, antibiotic susceptibility test was available in 739 naïve patients. H. pylori primary antibiotic resistance rates in the first and second five-year period were, respectively, clarithromycin 30.2% (95% CI 27.2–33.3), 37.8% (95% CI 34.2–41.4); metronidazole 33.3% (95% CI 30.2–36.5), 33.6% (95% CI 30.2–37.1); levofloxacin 25.6% (95% CI 22.8–28.5), 33.8% (95% CI 37.4–47.4), double resistance clarithromycin-metronidazole 18.9% (95% CI 16.4–21.6), 20.7% (95% CI 17.8–23.8). The increase of the resistance rates to clarithromycin and levofloxacin in naïve patients was statistically significant (p < 0.05). Although eradication rates for sequential therapy in the 10 years considered were 93.4% (95% CI 92–94.6) and 87.5% (95% CI 85.7–89) at per-protocol (PP) and intention-to-treat (ITT) analysis, respectively, they showed a significant decrease in the second five-year period. Conclusions: this data highlights an increase in primary H. pylori antibiotic resistance and strongly suggests the importance of drug susceptibility testing also in naïve patients.
Keywords: H. pylori, antibiotic resistance, first line therapy
1. Introduction
Helicobacter pylori infection is correlated to upper gastrointestinal diseases such as peptic ulcers, gastric mucosa associated lymphoid tissue lymphoma (MALT), and gastric cancer [1]. Antibiotic resistance is an increasing problem for eradication therapies, the trending abuse of antibiotics is probably the cause of this issue. The selective pressure of the antibiotic intake causes modification in the genetic pattern of H. pylori that stays stable generation after generation [2]. Clarithromycin is a key antibiotic in H. pylori eradication regimens, it is a macrolide and inhibits protein synthesis by binding to the 23S rRNA component of the 50S subunit of the ribosome. Clarithromycin resistance is due to several point mutations in 23S rRNA gene; A2143G, A2142G, and A2142C represent > 90% of the observed mutations with confirmed clinical relevance [3,4]. Metronidazole is also involved in the eradication of the bacterium. It is a 5-nitroimidazole activated by H. pylori nitroreductase enzyme. In particular, the inactivation of rdxA (encodes an oxygen-insensitive NADPH nitroreductase) and frxA (encodes a NADPH flavin oxidoreductase) genes is highly associated with metronidazole resistance in H. pylori [5]. Levofloxacin, a fluoroquinolone used in rescue therapeutic regimens, interacts with type II topoisomerases preventing the unwinding of DNA and DNA replication. Mutation in GyrA or GyrB genes are linked to levofloxacin resistance in H. pylori [6]. In 2017 the World Health Organization published a list of antibiotic resistant “priority pathogens”, a catalogue of bacteria that pose the greatest threat to human health, and clarithromycin resistant H. pylori was categorized as a high-priority bacterium [7]. Resistance to fluoroquinolones can also impair the efficacy of eradication regimens [8,9,10], whereas resistance to nitroimidazole can be partially overcome in vivo when used in quadruple therapies [11]. Antibiotic agents used for H. pylori eradication are also widely and improperly used to treat other infections [7,8,12]. For this reason, antibiotic resistance develops continuously, so it is very important to carry out periodic assessments of H. pylori primary antibiotic resistance rates and to monitor the efficacy of first line treatments [13,14,15,16], thus helping clinicians in selecting the most appropriate therapy in their setting [17]. Current Italian guidelines suggest sequential or Pylera® therapy as first line treatments [8,9,18]. Sequential therapy consists of 5 days of a dual therapy with PPI (proton pump inhibitor) and amoxicillin both twice a day followed by 5 days of a triple therapy with PPI, clarithromycin, and metronidazole all twice a day. Pylera® therapy consists of three Pylera® tablets four times a day with PPI twice a day for 10 days. The aim of our study was therefore to evaluate, in Italian naïve H. pylori positive patients: (1) resistance rates trends for clarithromycin, metronidazole and levofloxacin over two five-year periods, from 2009 to 2014 vs. 2015 to 2019; (2) which factors are potentially correlated with primary H. pylori drug resistance; (3) the effectiveness of sequential therapy.
2. Results
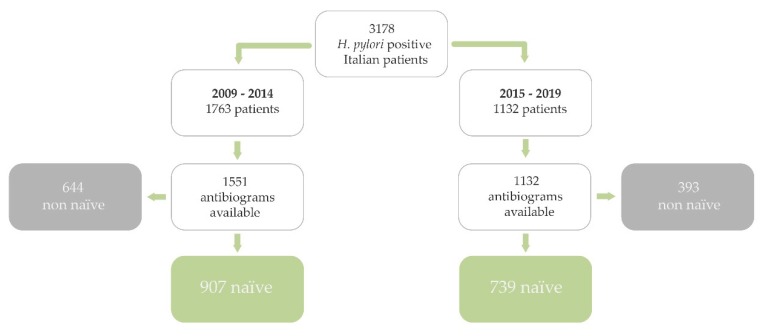
A total of 3178 Italian patients were infected, 1646 were naïve with antibiogram available (M/F: 646/1000; median age: 51 years, range 18–85 years). A total of 1763 Italian H. pylori positive patients underwent endoscopy between 2009 and 2014, and antibiotic susceptibility test was available in 1551, 907 were naïve; whilst 1415 Italian H. pylori positive patients underwent endoscopy between 2015 and 2019, and antibiogram was available in 1132, 739 were naïve (Figure 1).
Figure 1.
Population flow chart. Only Helicobacter pylori positive naive patients born in Italy were considered.
Population features are reported in Table 1a,b and Table 2.
Table 1.
(a) Population features. (b) Population features.
| (a) | ||||||
|---|---|---|---|---|---|---|
| Population Features | 2009–2014 | % | 95% CI | 2015–2019 | % | 95% CI |
| Patients | 907 | 739 | ||||
| Male | 358 | 39.5 | 36.3–42.7 | 288 | 39.0 | 35.4–42.6 |
| Female | 549 | 60.0 | 57.3–63.7 | 451 | 61.0 | 57.4–64.6 |
| Age mean | 51.5 | 53 | ||||
| BMI mean | 24.3 | 25 | ||||
| Smokers | 205 | 22.6 | 19.9–25.5 | 158 | 21.4 | 18.5–24.5 |
| Alcohol | 135 | 14.9 | 12.6–17.4 | 99 | 13.4 | 11.0–16.1 |
| Cardioaspirin | 68 | 7.5 | 5.9–9.4 | 56 | 7.6 | 5.8–9.7 |
| Familiarity for gastric cancer | 118 | 13.0 | 10.9–15.4 | 89 | 12.0 | 9.8–14.6 |
| Compulsory education | 362 | 39.0 | 36.7–43.2 | 276 | 37.3 | 33.8–40.9 |
| High school | 362 | 39.9 | 36.7–43.2 | 311 | 42.1 | 38.5–45.7 |
| Graduation | 183 | 20.2 | 17.6–22.9 | 152 | 20.6 | 17.7–23.7 |
| Chief town | 397 | 43.8 | 40.5–47.1 | 284 | 38.4 | 34.9–42.0 |
| Emilia Romagna | 737 | 81.3 | 78.6–83.7 | 593 | 80.2 | 77.2–83.1 |
95% CI: 95% Confidence Interval. BMI: Body Mass Index.
| (b) | |||
|---|---|---|---|
| Population Features | TOT | % | 95% CI |
| Patients | 1646 | ||
| Male | 646 | 39.2 | 36.9–41.7 |
| Female | 1000 | 60.8 | 58.3–63.1 |
| Age mean | 52.3 | ||
| BMI mean | 24.6 | ||
| Smokers | 363 | 22.1 | 20.1–24.1 |
| Alcohol | 234 | 14.2 | 12.6–16.0 |
| Cardioaspirin | 124 | 7.5 | 6.3–8.9 |
| Familiarity for gastric cancer | 207 | 12.6 | 11.0–14.3 |
| Compulsory education | 638 | 38.8 | 36.4–41.2 |
| High school | 673 | 40.9 | 38.5–43.3 |
| Graduation | 335 | 20.4 | 18.4–22.4 |
| Chief town | 681 | 41.4 | 39.0–43.8 |
| Emilia Romagna | 1330 | 80.8 | 78.8–82.7 |
TOT: totals from 2009 to 2019. 95% CI: 95% Confidence Interval. BMI: Body Mass Index.
Table 2.
Endoscopic reports.
| UGIE Reports (2009–2019) | No. | % | 95% CI |
|---|---|---|---|
| NUD | 1413 | 85.8 | 84.0–87.4 |
| PUD | 204 | 12.3 | 10.8–14.0 |
| MALT lymphoma | 20 | 1.2 | 0.7–1.8 |
| Gastric cancer | 9 | 0.5 | 0.2–1.0 |
PUD: peptic ulcer (ulceration 5 mm in diameter) or mucosal erosions (superficial lesion 4 mm) in the stomach or duodenum. NUD: no macroscopic lesions detected. MALT: mucosal-associated lymphoid tissue.
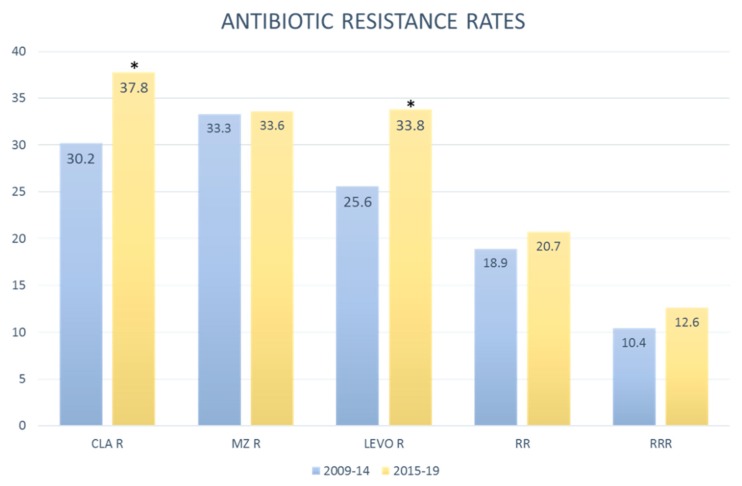
Data collected from 2009 to 2014 were compared to data collected from 2015 to 2019 to analyze primary drug resistance trends (Figure 2). Antibiotic resistance rates in patients diagnosed in the first and second five-year period were, respectively, clarithromycin 30.2% (95% CI 27.2–33.3) and 37.8% (95% CI 34.2–41.4); metronidazole 33.3% (95% CI 30.2–36.5) and 33.6% (95% CI 30.2–37.1); levofloxacin 25.6% (95% CI 22.8–28.5) and 33.8% (95% IC 30.4–47.4). Double resistance clarithromycin-metronidazole 18.9% (95% CI 16.4–21.6) and 20.7% (95% CI 17.8–23.8) triple resistance clarithromycin-metronidazole-levofloxacin 10.4% (95% CI 8.5–12.5) and 12.6% (95% CI 10.3–15.2).
Figure 2.
H. pylori antibiotic resistance rates in 2009–2014 and 2015–2019. (* Statistically significant). CLA: clarithromycin. Mz: metronidazole. Levo: levofloxacin. R: resistant. RR: resistant to clarithromycin and metronidazole. RRR: resistant to clarithromycin, metronidazole and levofloxacin.
Total resistance data are shown in Table 3 and Table 4 for 2009–2014 and 2015–2019, respectively.
Table 3.
Primary resistance patterns in H. pylori strains collected in 2009–2014.
| 907 Naïve | No. | % | 95% CI |
|---|---|---|---|
| ClaR, MzR, LevoR | 94 | 10.4 | 8.5–12.5 |
| ClaR, MzR, LevoS | 77 | 8.5 | 6.8–10.5 |
| ClaR, MzS, LevoR | 31 | 3.4 | 2.3–4.8 |
| ClaR, MzS, LevoS | 72 | 7.9 | 6.3–9.9 |
| ClaS, MzR, LevoR | 46 | 5.1 | 3.7–6.7 |
| ClaS, MzR, LevoS | 85 | 9.4 | 7.6–11.5 |
| ClaS, MzS, LevoR | 61 | 6.7 | 52.0–8.6 |
| ClaS, MzS, LevoS | 441 | 48.6 | 45.3–51.9 |
| Cla R tot | 274 | 30.2 | 27.2–33.3 |
| Cla S tot | 633 | 69.8 | 66.7–72.8 |
| Mz R tot | 302 | 33.3 | 30.2–36.5 |
| Mz S tot | 605 | 66.7 | 63.5–69.8 |
| Levo R tot | 232 | 25.6 | 22.8–28.5 |
| Levo S tot | 675 | 74.4 | 71.5–77.2 |
| ClaR, MzR tot | 171 | 18.9 | 16.4–21.6 |
R: resistant. S: susceptible. Cla: clarithromycin. Mz: metronidazole. Levo: levofloxacin.
Table 4.
Primary resistance patterns in H. pylori strains collected in 2015–2019.
| 739 Naive | No. | % | 95% CI |
|---|---|---|---|
| ClaR, MzR, LevoR | 93 | 12.6 | 10.3–15.2 |
| ClaR, MzR, LevoS | 60 | 8.1 | 6.3–10.3 |
| ClaR, MzS, LevoR | 46 | 6.2 | 4.6–8.2 |
| ClaR, MzS, LevoS | 80 | 10.8 | 8.7–13.3 |
| ClaS, MzR, LevoR | 46 | 6.2 | 4.6–8.2 |
| ClaS, MzR, LevoS | 49 | 6.6 | 4.9–8.7 |
| ClaS, MzS, LevoR | 65 | 8.8 | 6.9–11.1 |
| ClaS, MzS, LevoS | 300 | 40.6 | 37.0–44.2 |
| Cla R tot | 279 | 37.8 | 34.2–41.4 |
| Cla S tot | 460 | 62.2 | 58.6–65.8 |
| Mz R tot | 248 | 33.6 | 30.2–37.1 |
| Mz S tot | 491 | 66.4 | 62.9–69.8 |
| Levo R tot | 250 | 33.8 | 30.4–37.4 |
| Levo S tot | 489 | 66.2 | 62.6–69.6 |
| ClaR, MzR tot | 153 | 20.7 | 17.8–23.8 |
R: resistant. S: susceptible. Cla: clarithromycin. Mz: metronidazole. Levo: levofloxacin.
Although past antibiotic abuse is a key factor for the increase in H. pylori antibiotic resistance [15], we investigated other factors. Patients diagnosed in the second five-year period (2014–2019) had a higher probability to be clarithromycin and levofloxacin resistant. Female gender was correlated to metronidazole and double (clarithromycin + metronidazole) resistance, with a subsequent high probability of treatment failure. Age was correlated with clarithromycin resistance (younger than 50 years old), levofloxacin and triple resistance (older than 50 years old). Active smokers had higher probability of being resistant to metronidazole.
3. Discussion
The treatment of H. pylori infection has become complicated by the increasing trend in antimicrobial resistance worldwide [19,20] primarily because antibiotic agents used for H. pylori eradication are also widely used to treat other infections [7,8,12]. Antibiotic resistance develops continuously, so it is very important to carry out periodic assessments of H. pylori primary antibiotic resistance rates and to monitor the efficacy of first line treatments [13,14,15,16]. Aims of this study were to evaluate the prevalence of primary resistance to clarithromycin, metronidazole, and levofloxacin and to assess the effectiveness of sequential therapy over a 10 years period. The resistance rates to clarithromycin and levofloxacin had both a statistically significant increase (p < 0.05). Despite this, the general eradication rate of the sequential therapy was still optimal, being constantly higher than 90% (PP 93.4%, 95% CI 92–94.6; ITT 87.5%, 95% CI 85.7–89). Only in patients harboring resistant strains to both clarithromycin and metronidazole, the eradication rate was suboptimal (PP 83.6%, 95% CI 78.7–87.5; ITT 77%, 95% CI 71.8–81.5). Nevertheless, it is important to stress that eradication rates had a significant decrease in the second five-year period, going from PP 95.3% (95% CI 93.6–96.5) and ITT 91% (95% CI 88.9–92.7) to PP 90.4% (95% CI 87.6–92.8) and ITT 82.1% (95% CI 78.8–85.1) (p < 0.05). Data of patients of the second five period who took Pylera® therapy have already been published in a previous ad hoc study [21]. We investigated the role of factors potentially related with bacterial resistance (Table 5a,b).
Table 5.
(a) Factors potentially correlated with resistance rates. (b) Factors potentially correlated with resistance rates.
| (a) | |||
|---|---|---|---|
| Variables | Patterns of Resistance | OR | p Value |
| 2015–2019 | Cla R | 1.4 | 0.001 * |
| Mz R | 1.01 | 0.910 | |
| Levo R | 1.48 | 0.000 * | |
| ClaR, MzR | 1.23 | 0.348 | |
| ClaR, MzR, LevoR | 1.24 | 0.158 | |
| Sex female | Cla R | 1.22 | 0.069 |
| Mz R | 1.78 | 0.000 * | |
| Levo R | 0.99 | 0.927 | |
| ClaR, MzR | 1.47 | 0.003 * | |
| ClaR, MzR, LevoR | 1.18 | 0.309 | |
| Age > 50 years | Cla R | 0.79 | 0.027 ** |
| Mz R | 1 | 0.7054 | |
| Levo R | 1.38 | 0.003 * | |
| ClaR, MzR | 1 | 0.8291 | |
| ClaR, MzR, LevoR | 1.1 | 0.003 * | |
| BMI > 25 | Cla R | 0.9 | 0.367 |
| Mz R | 0.9 | 0.280 | |
| Levo R | 1.06 | 0.608 | |
| ClaR, MzR | 0.9 | 0.113 | |
| ClaR, MzR, LevoR | 0.88 | 0.401 | |
| Smokers | Cla R | 1.1 | 0.447 |
| Mz R | 1.87 | 0.000 * | |
| Levo R | 1.01 | 0.927 | |
| ClaR, MzR | 0.97 | 0.828 | |
| ClaR, MzR, LevoR | 0.96 | 0.816 | |
OR: Odd Ratio. BMI: Body Mass Index. * Statistically significant. ** protective.
| (b) | |||
|---|---|---|---|
| Variables | Patterns of Resistance | OR | p Value |
| Daily alcohol consumption | Cla R | 1.01 | 0.954 |
| Mz R | 0.84 | 0.282 | |
| Levo R | 1.06 | 0.701 | |
| ClaR, MzR | 0.94 | 0.715 | |
| ClaR, MzR, LevoR | 1.02 | 0.725 | |
| Familiarity for gastric cancer | Cla R | 0.94 | 0.689 |
| Mz R | 0.95 | 0.733 | |
| Levo R | 0.93 | 0.699 | |
| ClaR, MzR | 0.94 | 0.744 | |
| ClaR, MzR, LevoR | 0.87 | 0.556 | |
|
Level of education (till middle school) |
Cla R | 0.86 | 0.153 |
| Mz R | 0.99 | 0.899 | |
| Levo R | 1.04 | 0.724 | |
| ClaR, MzR | 0.96 | 0.742 | |
| ClaR, MzR, LevoR | 1.04 | 0.809 | |
| Residence (main city) | Cla R | 0.92 | 0.409 |
| Mz R | 1 | 0.637 | |
| Levo R | 1.08 | 0.469 | |
| ClaR, MzR | 0.89 | 0.375 | |
| ClaR, MzR, LevoR | 1.04 | 0.797 | |
| PUD | Cla R | 0.8 | 0.070 |
| Mz R | 0.77 | 0.120 | |
| Levo R | 0.85 | 0.350 | |
| ClaR, MzR | 0.74 | 0.157 | |
| ClaR, MzR, LevoR | 0.78 | 0.380 | |
OR: Odd Ratio. PUD: peptic ulcer disease.
The correlation between the year of H. pylori infection diagnosis (2015–2019) with clarithromycin and levofloxacin resistance confirms the significant increase in these antibiotics resistance rates previously observed [17]. Isolates collected from women patients are more prone to metronidazole and double clarithromycin/metronidazole resistance; in fact, macrolides and nitroimidazoles are widely used to treat urinary tract infections in women, inducing a selective pressure on H. pylori strains [2,22]. Age was correlated with clarithromycin resistance (younger than 50 years old), levofloxacin, and triple resistance (older than 50 years old) [22], this could be due to patients being prescribed more antibiotics (especially fluoroquinolones) [23,24] at an older age. Smokers had higher probability to be infected by a strain resistant to metronidazole; the correlation between smoke and therapy failure has been described in literature [25,26], so this observation is very interesting to be further investigated.
4. Materials and Methods
4.1. Patients
This was a retrospective, single-center study (Sant’Orsola Hospital, Bologna, Italy) evaluating consecutive Italian patients referred by their physicians to our unit for upper endoscopy. Naïve (never treated for H. pylori) H. pylori positive patients diagnosed from January 2009 to January 2019 were considered. The exclusion criteria were (1) age 18 years; (2) previous gastric surgery; (3) use of PPI or antibiotics in the 2 weeks before the endoscopy; (4) known allergy to macrolides, nitroimidazoles, or penicillins. Each patient provided us personal information such as age, weight, height, smoking habits, daily intake of alcohol, familiarity for gastric cancer, educational qualifications, and region of birth and residence. All participants provided written informed consent. The study was approved by the local Ethical Committee and performed according to guidelines for Good Clinical Practice [27] and the Declaration of Helsinki [28].
4.2. Endoscopy and H. pylori Assessment
Prior to the endoscopy all patients were subjected to 13C-urea breath test to detect H. pylori infection. The 13C-UBT was performed after an overnight fast. A baseline breath sample was obtained, and 75 mg of 13C urea with citric acid (1.5 g) was administered as an aqueous solution. Another breath sample was collected 30 min after the test solution was administered. Breath samples were analyzed with non-dispersive infrared spectroscopy (HeliFAN Plus, Fischer Analytic Instruments, Germany). The results of the test were considered as positive if the difference between the baseline sample and the 30-min sample exceeded 4 parts per 1000 of 13CO2, according to the manufacturer’s instructions. During endoscopy biopsy specimens (two from the antrum, two from the corpus, one from incisura angularis) were taken for histology (haematoxylin-eosin for pathological assessment and Giemsa for H. pylori staining). One bioptic sample from the antrum was used for rapid urease test (RUT) as routinely performed in our center, and one additional antral biopsy was used for bacterial culture and drug-susceptibility test. H. pylori status was assessed through CRM (composite reference method: at least two tests positive or only culture positive). Treatment success was evaluated by using a standard 13C-urea breath test performed 6–8 weeks after treatment ended. In the event of an early interruption of eradication therapy, 13C-UBT was performed after at least 7 days of treatment. Patients undergoing therapy for fewer than 7 days were considered as drop-outs, and those who did not undergo 13C-UBT testing after treatment were considered as lost to follow-up evaluation. Based on endoscopic reports, for the purposes of the study, patients with either a peptic ulcer (ulceration 5 mm in diameter) or mucosal erosions (superficial lesion 4 mm) in the stomach or duodenum were grouped together as “peptic ulcer disease” (PUD). Non-ulcer dyspepsia was diagnosed when no macroscopic lesions were detected at endoscopy and patients were included in “non-ulcer disease” (NUD) group.
4.3. Antibiotics Susceptibility Test for H. pylori
Biopsy specimens collected for bacterial culture were streaked immediately onto commercial selective medium Pylori Agar (BioMérieux Italia S.p.A., Italy). The plates were incubated under microaerobic conditions at 37 °C for 3–5 days. Once incubated, the colonies resembling H. pylori were identified by oxidase, catalase, and urease tests. Suspensions from the primary plates were prepared in sterile saline solution to McFarland opacity standard 4, approximately 109 colony forming units (CFU)/mL to perform an E-Test (BioMérieux Italia S.p.A., Italy). A total of three agar plates for every H. pylori strain were streaked in three directions with a swab dipped into each bacterial suspension to produce a lawn of growth. Three E-Test strips (clarithromycin 0.016–256 ug/mL, metronidazole 0.016–256 ug/mL, and levofloxacin 0.008–32 ug/mL) were placed each onto a separate plate, which was incubated immediately in a microaerobic atmosphere at 37 °C for 72 h. A fourth plate was used as positive control. Clarithromycin, metronidazole, and levofloxacin resistance break points for the minimal inhibitory concentration (MIC) are greater than 0.5 mg/L, greater than 8 mg/L, and greater than 1 mg/L, respectively, according to the updated recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [29]. From 2015 Eucast established that clarithromycin MICs between >0.25 and ≤0.5 were to be considered as “indeterminate”, suggesting not to administer the drug in this case, so these strains were considered as “resistant” in this study [30]. Drug susceptibility test was not performed for amoxicillin and tetracycline because in Europe the resistance rate is lower than 1% [16].
4.4. Chemotherapy for H. pylori
According to Italian Guidelines and European Guidelines [8,18] the first line therapies were sequential or Pylera® therapy. Sequential therapy consist of 5 days of a dual therapy with 40 mg PPI twice a day (before breakfast and dinner) and 1000 mg of amoxicillin twice a day (after breakfast and dinner); followed by 5 days of a triple therapy with 40 mg PPI twice a day (before breakfast and dinner) and clarithromycin 500 mg and metronidazole 500 mg both twice a day (after breakfast and dinner). From 2016 Pylera® was available, a three in one capsule containing 140 mg bismuth subcitrate potassium, 125 mg metronidazole, and 125 mg tetracycline. Pylera® therapy is therefore a 10 days quadruple therapy with 20 mg PPI twice a day (before breakfast and dinner) plus three Pylera® capsules four times a day (after breakfast, lunch, dinner and before bedtime).
4.5. Potential Predictive Factors of Antibiotic Resistance
Many factors were investigated as potentially correlated to resistance rates. Age (>50 years or ≤50 years), sex, BMI (>25 or ≤25), smoking habits (at least one cigarette a day), alcohol consumption (at least one glass a day), familiarity for gastric cancer (first degree relatives), educational level (until middle school or above middle school), City of residence, endoscopic findings (PUD or NUD), and year of H. pylori infection diagnosis (2009–2014 vs. 2015–2019).
4.6. Statistical Analysis
Means and their 95% confidence intervals were calculated as suggested by Newcombe et al. [29]. Eradication rates were calculated both by intention-to-treat (ITT) analysis, including all the enrolled patients, and by per-protocol (PP) analysis, including patients who took more than 90% of their medications and completed follow-up evaluation. Comparisons among patient subgroups were performed using the Chi-square test (Yates correction when appropriate), odd ratio calculator. A p level less than 0.05 was considered significant. Statistical analysis was performed with MedCalc19.1.
5. Conclusions
The constant increase of resistance rates is a serious problem to be solved. Since virtually all H. pylori eradication regimens are based on antimicrobials used also for other infectious diseases, setting up regular monitoring of primary resistance for H. pylori (as well as for other microorganisms) should be considered. This would improve the use of appropriate antimicrobial agents and also it would provide an indirect indicator of their use (or abuse) in the population.
Acknowledgments
The authors received no financial support for the research or publication of this article.
Author Contributions
Guarantor of the article: D.V. and A.Z. conceived the study. D.V. and G.F. included patients in the study. M.P. and L.S. performed the microbiological analyses. I.M.S. performed data management and statistical analysis. I.M.S., G.F., M.P. and L.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
All Authors have no conflict of interest to disclose.
References
- 1.McColl K.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Ailloud F., Didelot X., Woltemate S., Pfaffinger G., Overmann J., Bader R.C., Schulz C., Malfertheiner P., Suerbaum S. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat. Commun. 2019;10:2273. doi: 10.1038/s41467-019-10050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Francesco V., Zullo A., Hassan C., Giorgio F., Rosania R., Ierardi E. Mechanisms of Helicobacter pylori antibiotic resistance: An updated appraisal. World J. Gastrointest. Pathophysiol. 2011;2:35–41. doi: 10.4291/wjgp.v2.i3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Francesco V., Zullo A., Giorgio F., Saracino I., Zaccaro C., Hassan C., Ierardi E., Di Leo A., Fiorini G., Castelli V., et al. Change of point mutations in Helicobacter pylori rRNA associated with clarithromycin resistance in Italy. J. Med. Microbiol. 2014;63:453–457. doi: 10.1099/jmm.0.067942-0. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.M., Kim N., Kwon Y.H., Nam R.H., Kim J.M., Park J.Y., Lee Y.S., Lee D.H. rdxA, frxA, and efflux pump in metronidazole-resistant Helicobacter pylori: Their relation to clinical outcomes. J. Gastroenterol. Hepatol. 2018;33:681–688. doi: 10.1111/jgh.13906. [DOI] [PubMed] [Google Scholar]
- 6.Rimbara E., Noguchi N., Kawai T., Sasatsu M. Fluoroquinolone resistance in Helicobacter pylori: Role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter. 2012;17:36–42. doi: 10.1111/j.1523-5378.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization: List of Bacteria for Which New Antibiotics Are Urgently Needed. [(accessed on 18 November 2019)];2017 Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/
- 8.Fallone C.A., Chiba N., van Zanten S.V., Fischbach L., Gisbert J.P., Hunt R.H., Jones N.L., Render C., Leontiadis G.I., Moayyedi P., et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P., Megraud F., O’morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 10.Thung I., Aramin H., Vavinskaya V., Gupta S., Park J.Y., Crowe S.E., Valasek M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malfertheiner P., Bazzoli F., Delchier J.C., Celiñski K., Giguère M., Rivière M., Mégraud F., Pylera Study Group Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 12.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 13.Megraud F., Coenen S., Versporten A., Kist M., Lopez-Brea M., Hirschl A.M., Andersen L.P., Goossens H., Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 14.Dang B.N., Graham D.Y. Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 2017;14:383–384. doi: 10.1038/nrgastro.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell B.G., Schellevis F., Stobberingh E., Goossens H., Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of antibiotic resistance in helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatta L., Scarpignato C., Fiorini G., Belsey J., Saracino I.M., Ricci C., Vaira D. Impact of primary antibiotic resistance on the effectiveness of sequential therapy for Helicobacter pylori infection: Lessons from a 5-year study on a large number of strains. Aliment. Pharmacol. Ther. 2018;47:1261–1269. doi: 10.1111/apt.14597. [DOI] [PubMed] [Google Scholar]
- 18.Zagari R.M., Romano M., Ojetti V., Stockbrugger R., Gullini S., Annibale B., Farinati F., Ierardi E., Maconi G., Rugge M., et al. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig. Liver Dis. 2015;47:903–912. doi: 10.1016/j.dld.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Vakil N., Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133:985–1001. doi: 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Zullo A., Perna F., Hassan C., Ricci C., Saracino I., Morini S., Vaira D. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment. Pharmacol. Ther. 2007;25:1429–1434. doi: 10.1111/j.1365-2036.2007.03331.x. [DOI] [PubMed] [Google Scholar]
- 21.Fiorini G., Zullo A., Saracino I.M., Gatta L., Pavoni M., Vaira D. Pylera and sequential therapy for first-line Helicobacter pylori eradication: A culture-based study in real clinical practice. Eur. J. Gastroenterol. Hepatol. 2018;30:621–625. doi: 10.1097/MEG.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 22.Löfmark S., Edlund C., Nord C.E. Carl Erik Nord. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 2010;50:S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 23.Mcquiston Haslund J., Rosborg Dinesen M., Sternhagen Nielsen A.B., Llor C., Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand. J. Prim. Health Care. 2013;31:235–240. doi: 10.3109/02813432.2013.844410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji Z., Han F., Meng F., Tu M., Yang N., Zhang J. The association of age and antibiotic resistance of Helicobacter pylori: A Study in Jiaxing City, Zhejiang Province, China. Medicine. 2016;95:e2831. doi: 10.1097/MD.0000000000002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camargo M.C., Piazuelo M.B., Mera R.M., Fontham E.T., Delgado A.G., Yepez M.C., Ceron C., Bravo L.E., Bravo J.C., Correa P. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol. Latinoam. 2007;37:238–245. [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T., Matsuo K., Ito H., Sawaki A., Hirose K., Wakai K., Sato S., Nakamura T., Yamao K., Ueda R., et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am. J. Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.ICH Steering Committee Principles for clinical evaluation of new antihypertensive drugs: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Int. Dig. Health Legis. 1997;48:231–234. [PubMed] [Google Scholar]
- 28.World Medical Association WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. [(accessed on 19 November 2019)]; Available online: http://www.wma.net/en/30publications/10policies/b3.
- 29.Newcombe R.G., Altman D.G. Proportion and Their Differences. In: Altman D.G., Machin D., Trevor N.B., Gardner M.J., editors. Statistics with Confidences. 2nd ed. BMJ Books; London, UK: 2000. [Google Scholar]
- 30.Clinical Breakpoints-Breakpoints and Guidance. [(accessed on 18 November 2019)]; Available online: http://www.eucast.org/clinical_breakpoints.