ABSTRACT
Fragment 450–650 of the spike (S) protein (S450–650) of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) contains epitopes capable of being recognized by convalescent sera of SARS patients. Vaccination of mice with recombinant S450–650 (rS450–650) can induce Abs against SARS‐CoV, although the titer is relatively low. In the present study, a fusion protein linking a fragment (residues 39–272) of murine calreticulin (CRT) to S450–650 in a prokaryotic expression system was created. Compared with target antigen alone, the recombinant fusion product (rS450–650‐CRT) has much improved hydrophilicity and immunogenicity. The S450–650‐specific IgG Abs of BALB/c mice subcutaneously immunized with rS450–650‐CRT were in substantially higher titer (approximately fivefold more). Furthermore, the fusion protein, but not rS450–650 alone, was able to elicit S450–650‐specific IgG responses in T cell deficient nude mice. Given that rCRT/39–272 can drive the maturation of bone‐marrow‐derived dendritic cells, directly activate macrophages and B cells, and also elicit helper T cell responses in vivo, we propose that fragment 39–272 of CRT is an effective molecular adjuvant capable of enhancing target Ag‐specific humoral responses in both a T cell‐dependent and independent manner. Fusion protein rS450–650‐CRT is a potential candidate vaccine against SARS‐CoV infection.
Keywords: Spike protein, severe acute respiratory syndrome‐associated coronavirus, calreticulin, antibody
List of Abbreviations:
- APC
antigen‐presenting cell
- CD
cluster of differentiation
- CRT
calreticulin
- DC
dendritic cell
- E
envelope
- E. coli
Escherichia coli
- EGFP
enhanced green fluorescence protein
- ER
endoplasmic reticulum
- FITC
fluorescein isothiocyanate
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- IPTG
isopropyl b‐D‐thiogalactoside
- IL
interleukin
- LPS
lipopolysaccharide
- M
membrane
- N
nucleocapsid
- OPD
ortho‐phenylenediamine
- PBST
PBS containing 0.05% Tween‐20
- rCRT/39–272
recombinant murine CRT amino acid 39–272
- rS450–650
recombinant S protein amino acid 450–650
- rS450–650‐CRT
recombinant fusion protein between S450–650 and CRT/39–272
- rEGFP
recombinant enhanced green fluorescence protein, S, spike
- SARS‐CoV
severe acute respiratory syndrome‐associated coronavirus
- s.c.
subcutaneous
Severe acute respiratory syndrome is an infectious disease caused by SARS‐CoV (1). The genome of SARS‐CoV encodes several structural proteins, including the S glycoprotein, N protein, M glycoprotein and small E protein, which play synergistic roles in viral infectivity and pathogenicity (1, 2). S protein, with 1255 amino acid residues, is the largest structural protein in the virus. It is a type I transmembrane glycoprotein consisting of two domains, S1 and S2 (2). The former contains a receptor‐binding domain responsible for viral binding to the receptor on target cells (3, 4, 5, 6, 7). Former studies have shown that the S protein is a major antigen recognized by protective neutralizing antibodies in patients (3, 4, 5, 6). We have previously expressed fragment 450–650 of the S protein (rS450–650) in E. coli and demonstrated that SARS patients mount early and strong humoral responses against this polypeptide (3, 8, 9). However, the solubility and immunogenicity of rS450–650 is relatively poor, which compromises its use as a vaccine candidate (10).
Calreticulin, expressed mainly in the ER of cells, contains 416 amino acids and folds into three domains, a lectin‐like N domain (residues 1–197), a proline rich P domain (residues 198–308) and a calcium‐binding C domain (residues 309–416) (reviewed in reference 11). It is one of the key molecular chaperones in the ER as well as a homeostatic controller of amounts of cytosolic and ER calcium. Additionally, CRT is recognized to be one of the heat shock proteins that have potent immunobiological activity (11). We have recently shown that a recombinant fragment of murine CRT (rCRT/39–272) covering its partial N and P domains is a potent activator of B cells and macrophages via the Toll like receptor‐4 and CD14 pathway (12). When fused to EGFP, CRT/39–272 greatly improves humoral responses against EGFP in both BALB/c and T cell deficient nude mice (12). By using DNA vaccines encoding fusion proteins between CRT and target antigens such as tumor antigen E7, N protein of SARS‐CoV and Bacillus anthracis protective antigen domain IV, previous investigators have also observed that CRT can function as a molecular adjuvant (13, 14, 15, 16). In the present study, we prepared a soluble recombinant fusion protein (rS450–650‐CRT) between S450–650 and CRT/39–272 and observed that it has much better immunogenicity than rS450–650 alone.
MATERIALS AND METHODS
Animal immunization and serum collection
Female BALB/c and BALB/c‐nu mice of 6–8 weeks of age were obtained from the Academy of Military Medical Sciences (Beijing, China) and housed in a specific pathogen‐free barrier facility. The mice were immunized s.c. once with 30 μg recombinant protein rCRT/39–272, rS450–650, rS450–650‐CRT or rCRT/39–272 (15 μg) + rS450–650 (15 μg) in PBS at the base of the tail. Mouse blood was collected by tail bleeding at different time points post immunization and the sera kept at −20 °C until use.
Molecular biology reagents
High fidelity Taq DNA polymerase was purchased from TaKaRa Biotech (Shiga, Japan). Restriction enzymes and T4 ligase were from Invitrogen, (Carlsbad, CA, USA). A kit for DNA extraction and purification was from Qiagen (Hilden, Germany). The E. coli strain of BL21 (DE3) was from Stratagene (La Jolla, CA, USA). The Ni‐nitrilotriacetic acid (Ni‐NTA) resin was from Novagen (Darmstadt, Germany). The cell transfection reagent was from Vigorous Biotech (Beijing, China).
Expression and purification of recombinant proteins in Escherichia coli
Preparation of expression plasmids encoding for S450–650 and CRT/39–272 was performed as previously described (3, 8, 10, 12). After digestion with HindIII and XhoI (Promega, Madison, WI, USA), the CRT DNA fragment was cloned into the HindIII and XhoI sites of pET28a‐S450–650 to generate pET28a‐S450–650/CRT. Plasmid constructs were confirmed by DNA sequencing.
Expression and purification of recombinant proteins was essentially the same as previously described (10, 12). Briefly, E. coli BL21 (DE3) cells harboring plasmid pET28a‐S450–650, pET28a‐CRT, or pET28a‐S450–650/CRT were cultured in 1L 2YT medium containing kanamycin (30 μg/mL) at 37 °C. When the cell density had reached 0.8–1.0 (optical density 600), IPTG (Sigma‐Aldrich, St Louis, MO, USA) was added to a final concentration of 0.1 mM, and the bacteria cultured for a further 3.5 hr at 37 °C. The culture was then harvested by centrifugation and the cell pellet suspended in 40 mL binding buffer (500 mM NaCl, 20 mM Tris‐HCl, 5 mM imidazole, pH 7.9). After sonication (4 s pulse, 4 s pause, 200 W 50 times), the lysed cells were centrifuged at 5000 g for 15 min at 4 °C. The supernatant was incubated with 2 mL Ni sepharose (GE Healthcare, Uppsala, Sweden) at 4 °C for 1 hr. The sepharose was poured into a column and washed with 100 mL wash buffer (500 mM NaCl, 20 mM Tris‐HCl, 20 mM imidazole, pH 7.9) and then the recombinant protein eluted with elute buffer (500 mM NaCl, 20 mM Tris‐HCl, 500 mM imidazole, pH 7.9). The final products were dialyzed with PBS (pH 7.2) and stored at −20°C before use.
S450–650‐based enzyme‐linked immunosorbent assays
S450–650‐based ELISAs were performed according to the protocol previously described (8, 9). Briefly, ELISA plates were coated at 4 °C overnight with 2 μg/mL rS450–650 in carbonate buffer (pH 9.6). The wells were then incubated with 2% BSA in PBS for 2 hr at 37 °C, and then washed five times with PBST. Serum samples from immunized mice were diluted in dilution buffer (0.1% BSA in PBS). 100 μL of each dilution was added to each well and the plates incubated for 90 min at 37 °C. After washes with PBST, the plates were incubated with 100 μL HRP‐labeled goat anti‐mouse IgG, IgG1 or IgG2a antibody (Southern Biotech, Birmingham, AL, USA) 1/4000 diluted in dilution buffer for 1 hr at 37 °C. OPD substrate (100 μL /well) was added after five washes with PBS‐T and incubated at room temperature. 50 μL of 2M H2SO4 solution was added to each well to stop the reaction, and the optical density was immediately read at 492 nm.
Bone marrow‐derived dendritic cells and flow cytometric analysis
Bone marrow was flushed out of the femora and tibiae of BALB/c mice and incubated at a starting concentration of 5 × 106 cells/mL in R10 medium in 6‐well flat bottomed plates (Falcon, Oxnard, CA, USA) at 37 °C, 5% CO2 for 3 hr. Non‐adherent cells were removed before recombinant mouse GM‐CSF (rmGM‐CSF, PeproTech EC, London, UK) was added to the culture (20 ng/mL). On day 3, half of the medium was replaced with fresh medium containing rmGM‐CSF. On day 5, adherent cells were harvested as bone‐marrow‐derived immature DCs and examined microscopically and also by flow cytometry for expression of CD11c.
For indirect immunofluorescence staining, cells were collected, washed with 1% BSA (Sigma, St Louis, MO, USA) in PBS and the pellets (1 × 106 cells /tube) incubated for 30 min at 4 °C with 1 μL phycoerythrin‐conjugated rat anti‐mouse CD11c and also FITC‐conjugated Abs against mouse CD40 FITC‐conjugated isotype control Abs (Biolegend, Franklin Lakes, NJ, USA). After washes, the cells were subjected to analysis using a fluorescence‐activated cell sorter (FACScan, Becton‐Dickinson, Rutherford, NJ, USA).
Statistical analysis
All experiments were repeated at least three times and the results expressed as mean ± SD of the mean. Statistical analysis was performed using the independent‐samples t‐test or two‐side paired t‐test between groups using the SPSS 14.0 program (SPSS, Chicago, IL, USA). Differences were considered statistically significant at P≤ 0.05.
RESULTS
Prokaryotic expression and purification of rS450–650‐calreticulin
Preparation of expression vectors pET28a‐S450–650 and pET28a‐CRT/39–272 encoding for S450–650 and murine CRT/39–272, respectively, has been described previously (3, 10, 12). In the present study, a new DNA construct, namely pET28a‐S450–650‐CRT, encoding a fusion protein (rS450–650‐CRT) between S450–650 and murine CRT/39–272 with a histidine tag was created. All three recombinant proteins were successfully expressed in E. coli. As illustrated in Figures 1a–1c, following IPTG induction rCRT/39–272, a highly soluble polypeptide, was present in the lysate of E. coli cells harboring pET28a‐CRT/39–272, whilst rS450–650 was less soluble and mainly expressed in the inclusion bodies of pET28a‐S450–650‐harboring bacteria. The fusion protein rS450–650‐CRT was found in both cell lysate and inclusion bodies of transduced E. coli cells. All three recombinant products were purified using Ni‐columns, and homogeneity of the resultant products was more than 90% as assessed by SDS‐PAGE 12% gel electrophoresis (Fig. 1d).
Figure 1.
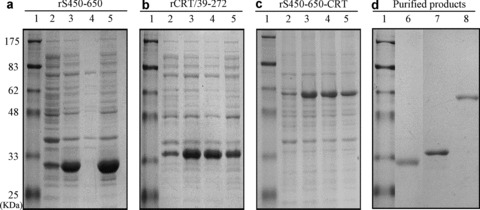
Expression and purification of recombinant proteins. Samples of different fractions of E. coli harboring (a) pET28a‐S450–650, (b) pET28a‐CRT/39–272 or (c) pET28a‐S450–650‐CRT after induction with IPTG were run in a Coomassie‐blue‐stained SDS–PAGE 12% gel, with protein molecular weight markers in lane 1. Total lysates of cells before and after IPTG induction were run in lanes 2 and 3 respectively; the supernatant and pellet of the lysate of cells after IPTG induction were in lanes 4 and 5. (d)After purification steps, the resultant products of rS450–650 (lane 6), rCRT/39–272 (lane 7) and rS450–650‐CRT (lane 8) were analyzed by SDS‐PAGE electrophoresis and visualized by Coomassie blue staining.
Immunogenicity of rS450–650‐calreticulin
Initial immunogenicity evaluation of the recombinant fusion protein was carried out by comparing its ability to elicit S450–650‐specific Abs in vivo with rS450–650 alone and a mixture of equal proportions of rS450‐650 and rCRT/39–272. Figure 2 shows that the fusion protein was by far the most effective immunogen for inducing S450–650‐specific IgG responses in BALB/c mice. More detailed analysis on the IgG Abs thus produced was then carried out. Serum samples from BALB/c mice (five per group) 28 days post s.c. immunization with rS450–650, rCRT/39–272 or rS450–650‐CRT (30 μg/mouse) were assayed by ELISAs. The rS450–650‐specific serum Ab titers of the rS450–650‐CRT group were approximately fivefold higher than those of the rS450–650 group (Fig. 3a). Target Ag‐specific IgGs of the rS450–650‐CRT group were of both IgG1 and IgG2a isotypes, whilst specific IgG2a was hardly detectable in the sera of the rS450–650‐immunized mice (Fig. 3b, c).
Figure 2.
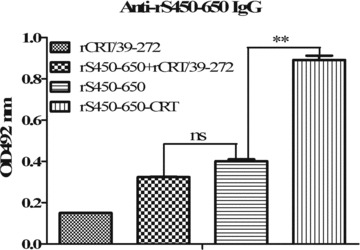
Immunogenicity of fusion protein rS450–650‐CRT vs rS450–650 and a mixture of rS450–650 + rCRT/39–272. Serum samples from BALB/c mice (5/group) immunized s.c. 14 days earlier with rCRT/39–272, rS450–650, rS450–650‐CRT or an equal proportion mixture rS450–650 plus rCRT/39–272 were serially diluted and analyzed, in triplicate wells, using rS450–650‐based ELISAs. The detection Abs were goat‐anti‐mouse IgG with OPD as substrate, and the results expressed as mean absorbance at 492 nm±SD.**P < 0.01; ***P < 0.001; ns, not significant.
Figure 3.
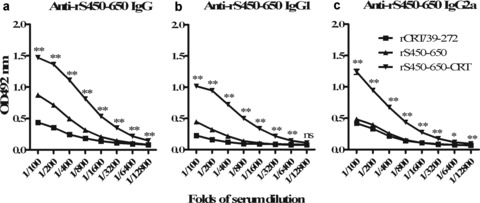
Titration of mouse antisera against rS450–650. Serum samples from mice immunized s.c. 28 days earlier with rCRT/39–272, rS450–650, rS450–650‐CRT were serially diluted and analyzed, in triplicate wells, using rS450–650‐based ELISAs. The detection Abs were (a) goat‐anti‐mouse IgG, (b) IgG1 or (c) IgG2a with OPD as substrate. The results are expressed as mean absorbance at 492 nm ± SD. *P≤ 0.05; **P < 0.01 (comparing with the rCRT/39–272 group); ns, not significant.
Induction of S450–650‐specific IgG antibodies by rS450–650‐ calreticulin in nude mice
It has previously been demonstrated by this group that rCRT/39–272 is able to activate B cells and trigger Ig production and IgG class switching in the absence of T cell help both in vitro and in vivo (12). It was of interest to know whether rS450–650‐CRT can also induce S450–650‐specific IgG in T‐cell‐deficient mice. BALB/c‐nu mice were vaccinated s.c. with either rCRT/39–272, rS450–650 or rS450–650‐CRT and then monitored for serum IgG Abs against S450–650. The fusion protein, but not rS450–650 or rCRT/39–27, successfully induced S450–650‐specific IgG production in nude mice (Fig. 4).
Figure 4.
Induction of S450–650‐specific IgG production in BALB/c‐nu mice by vaccination with rS450–650‐CRT. Groups of BALB/c‐nu mice were s.c. immunized at the base of the tail with rCRT/39–272, rS450–650 or rS450–650/CRT (30 μg/mouse, in 50 μL PBS). Serum samples, collected 21 days later, were assayed for anti‐S450–650 IgG using ELISA. The detection Abs were HRP‐labeled goat‐anti‐mouse IgG with OPD as substrate and the results are expressed as mean absorbance at 492 nm ± SD. **P < 0.01; ***P < 0.001; ns, not significant.
Ability of rCRT/39–272 and rS450–650‐calreticulin to induce dendritic cell maturation
The potent adjuvanticity of rCRT/39–272 can be partially explained by its direct activating effect on B lymphocytes (12). However, there are other possible ways for it to enhance target Ag‐specific humoral lresponses in vivo. After all, adjuvants are typically characterized by their ability to activate professional APCs, such as DCs and macrophages, rather than B or T cells. Bone marrow‐derived mouse DCs were stimulated with rCRT/39–272, or rS450–650‐CRT, or LPS, or rEGFP for 24 hrs and then analyzed by flow cytometry for CD40 expression, which is regarded as a marker for DC maturation (17). As illustrated in Figure 5, the percentage of CD40+ cells of the groups treated with rCRT/39–272 (24.5%) or rS450–650‐CRT (18.6%) was considerably higher than that of the rEGFP control group (6.8%), thus confirming rCRT/39–272 and rS450–650‐CRT as potent activators of murine DCs. This is further supported by the fact that rS450–650‐CRT as well as rCRT/39–272, but not rEGFP, were able to induce production of IL‐12 and IL‐1β by DCs in vitro (data not shown). The ability of rCRT/39–272 and rS450–650‐CRT to activate DC is not due to endotoxin contamination because the recombinant proteins used in this study were passed through polymyxin B agarose to remove endogenous LPS that could have come from the E. coli system.
Figure 5.
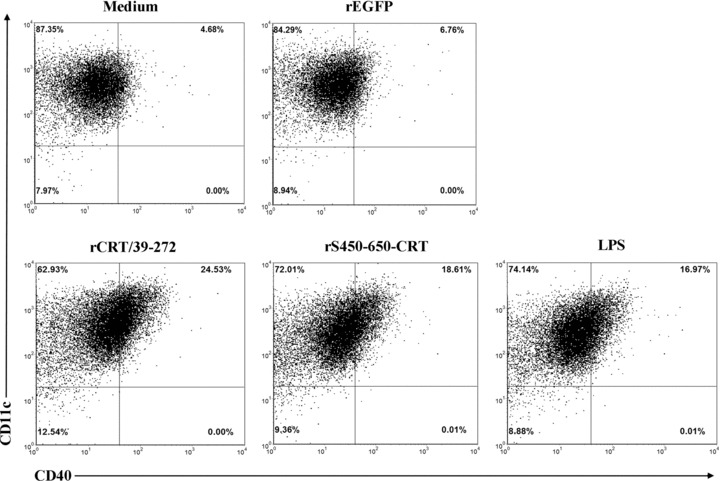
rCRT/39–272 and rS450–650‐CRT induce maturation of dendritic cells in vitro. Bone‐marrow‐ derived mouse immature DCs were stimulated with, or without, LPS (10 μg/mL), rCRT/39–272 (30 μg/mL), rS450–650‐CRT (30 μg/mL) or rEGFP (30 μg/mL) for 24 hrs in R10 medium at 37 °C with 5% CO2. The cells were then double stained with FITC‐conjugated anti‐CD40 mAb and PE‐labeled mAb against CD11c.
DISCUSSION
Newly emerging pathogens such as SRAS‐CoV and avian influenza viruses are of major concern for public health today. The development of more effective vaccines (and adjuvants) against such infectious agents is urgently needed. Our results reported herein show that fusion protein rS450–650‐CRT exhibits much more potent immunogenicity than rS450–650 alone in terms of eliciting rS450–650‐specific IgG responses in vivo. It should be noted, however, that whether such Abs exhibit any neutralizing effect against SARS‐CoV infection remains to be tested by using either live virus or pseudo‐virus systems.
Physical linkage between rS450–650 and CRT/39–272 is necessary for the improved immunogenicity, because a mixture of rS450–650 and rCRT/39–272 was no more immunogenic than rS450–650 alone (Fig. 2). Another advantage of rS450–650‐CRT over rS450–650 as an immunogen is its better hydrophilicity. When preparing rS450–650, renaturation steps were necessary after Ni‐column purification and the resultant product had to be maintained at a relatively low concentration in order to avoid protein aggregation and precipitation. By contrast, no renaturation steps are necessary for preparation of rS450–650‐CRT and the final product is less likely to form aggregates in PBS.
Although our previous work showed that rCRT/39–272 is able to activate B cells and macrophages in vitro (12), data on DC activation by rCRT/39–272 and rCRT‐S450–650 allows a more panoramic view of the molecular mechanisms underlying the extraordinary adjuvanticity of this prokaryotically expressed soluble calreticulin fragment. It is also of importance to mention that, in addition to its stimulatory effects on B cells and DCs, rCRT/39–272 can also induce CD4 helper T cell responses in mice. In a previous study using draining lymph node cells from BALB/c mice after s.c. immunization with rCRT/39–272, we were able to establish highly sensitive CRT‐specific CD4+ helper T cell lines (manuscript in preparation). Based upon the above observation, we propose that recombinant CRT may function as a molecular adjuvant through several different pathways that may result in synergistic effect in vivo. Firstly, APCs are known to express different receptors (e.g. CD14 and CD91) for CRT (18, 19, 20, 21); this would facilitate more efficient capture and uptake of CRT‐linked antigens. Secondly, soluble CRT directly activates DCs (Fig. 5) and macrophages (12), thereby leading to more efficient antigen processing and presentation. Thirdly, CRT in fusion proteins functions as a carrier protein and activates CD4+ helper T cells that are capable of providing cognate help for antigen‐specific B cells. Finally, the CRT portion of the fusion protein directly activates B cells and triggers their IgG class switching even in the absence of T cell help (Ref. 12 and Fig. 4). The genomes of many viruses (e.g. SARS‐CoV and influenza viruses) undergo substantial mutation, which can diminish T cell epitopes in the viral proteins, resulting in escape of the virus from immune detection by T lymphocytes (22, 23, 24). In this scenario, the ability of vaccines to induce IgG responses in hosts deficient in cognate helper T cells can be valuable.
Because calreticulin is a widely expressed self‐antigen, its use as a molecular adjuvant is inevitably embedded with the possibility of triggering (or exacerbating) immunopathological reactions in vivo. Previous investigators have observed increased concentrations of CRT‐specific serum IgG Abs in patients with systemic lupus erythematosus and rheumatoid arthritis (25, 26). However, it is unclear whether such Abs participate in the pathological damage to the host or function as part of the immunoregulatory network. When rCRT/39–272 was employed to immunize different strains of mice, rats and rabbits, with or without Freund's adjuvant, high titer IgG Abs were obtained in these animals with no accompanying signs of autoimmune disorders (data not shown). In one experiment, BALB/c mice remained healthy for 6 months after four doses of s.c. immunization with rCRT/39–272 (data not shown), arguing against the possibility that recombinant CRT causes autoimmune damage in vivo.
Previous investigators have exploited the adjuvanticity of CRT by using it as a molecular adjuvant in DNA vaccines. Cheng and colleagues found that intradermal immunization with a DNA vaccine encoding a fusion protein between CRT, or CRT fragments, and the E7 tumor antigen was more efficient at eliciting E7‐specific CD8+ T cells and protecting against E7‐expressing tumors in C57BL/6 mice (13, 14). Park and coworkers prepared a DNA vaccine encoding a fusion protein between CRT and Bacillus anthracis protective antigen domain IV and showed that it much enhanced antibody responses to the target Ag (15). Kim and colleagues also demonstrated that a DNA vaccine encoding CRT linked to the N protein of the SARS‐CoV is capable of generating strong N‐specific humoral and cellular immunity (16). It should be noted, however, that proteins expressed by DNA vaccines may be retained in the endoplasmic reticulum or Golgi after synthesis, thus limiting their ability to induce an antibody response. Moreover, intracellularly expressed CRT may not be as efficient as soluble extracellular CRT in exerting APC conditioning and in activating B cells in vivo.
Nucleocapsid protein, another major structural protein of SARS‐CoV, is capable of eliciting strong humoral and cellular immune response in patients and in experimental animals (2, 8, 27). Unlike the S protein, which contains neutralizing epitopes, the N protein cannot induce neutralizing Abs in vivo because it is located inside the viral particles. On the other hand, the S, M and E proteins of SARS‐CoV play synergistic roles in viral infection (2) and Abs against these viral proteins are thought to have a synergistic effect in combating the infectivity of SARS‐CoV. Thus, a recombinant fusion polypeptide containing CRT/39–272 and the major B cell epitopes in the S, M and E proteins of SARS‐CoV may comprise a more favorable vaccine design.
In conclusion, rS450–650‐CRT has several advantages over rS450–650, including its immunogenicity, stability in solution and simplicity of production. Given that rCRT/39–272 is able to activate human peripheral blood mononuclear cells in vitro (12), this CRT fragment could be exploited as a molecular adjuvant in the preparation of SARS‐CoV vaccines for humans.
DISCLOSURE
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by grants from the Program for Changjiang Scholars and Innovative Research Team in University (IRT1075), the National Foundation of Natural Science of China (30890142/31070781) and National Key Basic Research Programs (2010CB529102).
Present address for XQ: Department of Physiology, Gannan Medical University, China.
REFERENCES
- 1. Rota PA., Oberste MS., Monroe SS., Nix WA., Campagnoli R., Icenogle JP., Penaranda S., Bankamp B., Maher K., Chen MH., Tong S., Tamin A., Lowe L., Frace M., DeRisi JL., Chen Q., Wang D., Erdman DD., Peret TC., Burns C., Ksiazek TG., Rollin PE., Sanchez A., Lick S., Holloway B., Limor J., McCaustland K., Olsen‐Rasmussen M., Fouchier R., Gunther S., Osterhaus AD., Drosten C., Pallansch MA., Anderson LJ., Bellini WJ. (2003) Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–9. [DOI] [PubMed] [Google Scholar]
- 2. Marra MA., Jones SJ., Astell CR., Holt RA., BrooksWilson A., Butterfield YS., Khattra J., Asano JK., Barber SA., Chan SY., Cloutier A., Coughlin SM., Freeman D., Girn N., Griffith OL., Leach SR., Mayo M., McDonald H., Montgomery SB., Pandoh PK., Petrescu AS., Robertson AG., Schein JE., Siddiqui A., Smailus DE., Stott JM., Yang GS., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth TF., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples GA., Tyler S., Vogrig R., Ward D., Watson B., Brunham RC., Krajden M., Petric M., Skowronski DM., Upton C., Roper RL. (2003) The genome sequence of the SARS‐associated coronavirus. Science 300: 1399–404. [DOI] [PubMed] [Google Scholar]
- 3. Zhao JC., Wang W., Jia RJ., Yuan ZH., Zhao ZD., Xu XJ., Lv P., Zhang Y., Gao XM. (2007) A study on antigenicity and receptor‐binding ability of fragment 450–650 of the spike protein of SARS‐corona virus. Virology 359: 362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong SK., Li W., Moore MJ., Choe H., Farzan M. (2004) A 193‐amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin‐converting enzyme 2. J Biol Chem 279: 3197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babcock GJ., Esshaki DJ., Thomas WD Jr., Ambrosino DM. (2004) Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol 78: 4552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He Y., Lu H., Siddiqui P., Zhou Y., Jiang S. (2005) Receptor‐binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation‐ dependent epitopes that induce highly potent neutralizing antibodies. J Immunol 174: 4908–15. [DOI] [PubMed] [Google Scholar]
- 7. Zhou T., Wag H., Luo D., Rowe T., Wang Z., Hogan RJ., Qiu SH., Bunzel RJ., Huang G.Q., Mishra V., Voss TG., Kimberly R., Luo M. (2004) An exposed domain in the severe acute respiratory syndrome coronavirus spike protein induces neutralizing antibodies. J Virol 78: 7217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao JC., Wang W., Wang WL., Zhao ZD., Zhang Y., Lv P., Ren FR., Gao XM. (2007) Comparison of IgG responses against the spike and nucleocapsid proteins of SARS‐coronavirus in patients with severe acute respiratory syndrome. Clin Vaccine Virology. 14: 839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao JC., Wang W., Wang GF., Yuan ZH., Jia RJ., Xu X., Ren FR., Zhao ZD., Gao XM. (2005) Development and evaluation of n enzyme‐linked immunosorbent assay for detection of antibodies against the spike protein of SARS‐coronavirus. J Clin Virol 33: 12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao JC., Zhao ZD., Wang W., Gao XM. (2005) Prokaryotic expression, refolding, and purification of fragment 450–650 of the spike protein of SARS‐coronavirus. Protein Expr Purif 39: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michalak M., Groenendyk J., Szabo E., Gold LI., Opas M. (2009) Calreticulin, a multi‐process calcium‐buffering chaperone of the endoplasmic reticulum. Biochem J 417: 651–66. [DOI] [PubMed] [Google Scholar]
- 12. Hong C., Qiu X., Zhong ZY., Huang QR., Li Y., Zhang Y., Lv P., Gao XM. (2010) Functional analysis of a recombinant calreticulin fragment 39–272: Implications for immunobiological activities of calreticulin in health and disease. J Immunol 185: 4561–9. [DOI] [PubMed] [Google Scholar]
- 13. Cheng WF., Hung CF., Chai CY., Hsu KF., He L., Ling M., Wu TC. (2001) Tumor‐specific immunity and anti‐angiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest 108: 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng WF., Hung CF., Chen CA., Lee CN., Su YN., Chai CY., Boya DA., Hsieh CY., Wu TC. (2005) Characterization of DNA vaccines encoding the domains of calreticulin for their ability to elicit tumor‐specific immunity and anti‐angiogenesis. Vaccine 23: 3864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park YS., Lee JH., Hung CF., Wu TC., Kim TW. (2008) Enhancement of antibody responses to Bacillus anthracis protective antigen domain IV by use of calreticulin as a chimeric molecular adjuvant. Infect Immun 76: 1952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim TW., Lee JH., Hung CF., Peng S., Roden R., Wang MC., Viscidi R., Tsai YC., He LM., Chen PJ., Boyd DA., Wu TC. (2004) Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol 78: 4638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koch BF., Stanzl U., Jennewein P., Janke K., Heufler C., Kimpgen E., Romani N., Schuler G. (1996) High level IL‐12 production by murine dendritic cells: upregulation via MHCclass II and CD40 molecules and downregulation by IL‐4 and IL‐10. J Exp Med 184(2): 741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binder RJ., Strivastava P. (2007) HSP‐APC interactions: initiation of immune responses In: Asea A.A., De Maio A. (eds.)Heat shock proteins: potent mediators of inflammation and immunity. New York : Springer , pp. 131–45. [Google Scholar]
- 19. Duus K., Pagh RT., Holmskov U., Hojrup P., Skov S., Houen G. (2007) Interaction of calreticulin with CD40 ligan, TRIAL and Fas ligand. Scand J Immunol 66: 501–7. [DOI] [PubMed] [Google Scholar]
- 20. Basu S., Binder RJ., Ramalingam T., Srivastava PK. (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 4: 303–13. [DOI] [PubMed] [Google Scholar]
- 21. Berwin B., Hart JP., Rice S., Gass C., Pizzo SV., Post SR., Nicchitta CV. (2003) Scavenger receptor‐A mediates gp96/GRP94 and calreticulin internalization by antigen‐presenting cells. EMBO J 22: 6127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berkhoff EG., Boon AC., Nieuwkoop NJ., Fouchier RA., Sintnicolaas K., Osterhaus AD., Rimmelzwaan GF. (2004) A mutation in the HLA‐B*2705‐restricted NP383–391 epitope affects the human influenza A virus‐specific cytotoxic T‐lymphocyte response in vitro . J Virol 78: 5216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimmelzwaan G.F., Boon AC., Voeten JT., Berkhoff EG, Fouchier RA., Osterhaus AD. (2004) Sequence variation in the influenza A virus nucleoprotein associated with escape from cytotoxic T lymphocytes. Virus Res 103: 97–100. [DOI] [PubMed] [Google Scholar]
- 24. Wahl A., McCoy W., Schafer F., Bardet W., Buchli R., Fremont DH., Hildebrand WH. (2009) T‐cell tolerance for variability in an HLA class I‐presented influenza A virus epitope. J Virol 83: 9206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Berg RH., Siegert CE., Faber‐Krol MC., Huizinga TW., van Es LA., Daha MR. (1998) Anti‐C1q receptor/calreticulin autoantibodies in patients with systemic lupus erythematosus (SLE). Clin Exp Immunol 111: 359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boehm J., Orth T, Van Nguyen P., Soling HD. (1994) Systemic lupus erythematosus is associated with increased auto‐antibody titers against calreticulin and grp94, but calreticulin is not the Ro/SS‐A antigen. Eur J Clin Invest 24: 248–57. [DOI] [PubMed] [Google Scholar]
- 27. Zhao JC., Huang QR., Wang W., Zhang Y., Lv P., Gao XM. (2007) Identification and characterization of helper T cell epitopes in the nucleocapsid protein of SARS‐CoV. J Virology 81: 6079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


