Abstract
The economic consequences of bovine diarrhea are serious. Few long‐term epidemiological data are available concerning the causative pathogens of bovine diarrhea in Japan. From 2002 to 2011, surveillance of enteric pathogens was performed in cows of various breed and age from 302 farms in which diarrhea had occurred in Yamagata Prefecture, Japan. Differences between dairy and beef cows in the number of cases of diarrhea and rates of infection by Salmonella spp. and Eimeria spp. were found. Clinical symptoms (duration of epidemic, hematochezia and complications) caused by bovine rotavirus infection were milder than those caused by bovine coronavirus infection.
Keywords: cows, diarrhea, enteric pathogens, epidemiology
List of Abbreviations
- BCV
bovine coronavirus
- BTV
bovine torovirus
- BVDV
bovine viral diarrhea virus
- E. coli
Escherichia coli
- RVA
rotavirus A
- RVB
rotavirus B
- RVC
rotavirus C
Young calves have immature immune systems and are easily infected with pathogens. In particular, Japanese Black calves have genetic factors that make them more susceptible to stillbirth and perinatal weak calf syndrome 1. Hence, they are highly susceptible to infection and may die from diarrhea or pneumonia within a few weeks of birth 2. Diarrhea causes economic losses in adult commercial dairy cows because they yield less milk and shipment of milk must be stopped during treatment and in commercial beef cows because they do not gain sufficient body weight. Therefore, the economic losses associated with diarrheas in cattle are significant including, in addition to the above, morbidity, mortality and costs of treatment 3.
The causative agents of diarrhea in cows can be divided into two categories: infectious and non‐infectious agents 4. Infectious agents include enteric pathogens such as viruses, bacteria, parasites, protozoa and mycotoxins. Non‐infectious agents are diverse and include poor hygiene, various stresses, overfeeding, indigestion, poor diet, intestinal deficiency, inflammation and malabsorption. Diarrhea caused by enteric pathogens is often exacerbated by other factors such as environment, other etiologic agents, management and host factors 5. Although various tools are used to prevent infection with enteric pathogens—such as vaccination, disinfection and antibiotic use—the most effective means of preventing pathogen exposure is biosecurity 6.
Bovine rotavirus A, BCV, Escherichia coli, Cryptosporidium spp., and Eimeria spp. are common diarrhea‐causing pathogens in young calves 7, 8. In contrast, bovine RVA, RVB and RVC, BCV, BVDV and Salmonella spp. have been identified in epidemic diarrheas in adult cows 9, 10, 11, 12, 13, 14, 15, 16, 17, 18. The characteristics of diarrhea caused by these enteric pathogens are summarized in Table S1.
To our knowledge, few long‐term systematic epidemiological surveillance studies have investigated the causative pathogens of diarrhea in dairy and beef cows. In the present study, we performed detailed surveillance of enteric pathogens in diarrheal fecal samples obtained from 302 farms in Yamagata Prefecture, located in the southwest of the Tohoku region on Honshu Island, Japan from 2002 to 2011. During this period, there were three separate episodes of diarrhea in adult dairy cows on the same farm from 2002 to 2003. We investigated the clinical symptoms of cows in these three episodes to determine the characteristics of BCV‐ and RV‐associated diarrhea.
Fecal samples were collected from 302 cases of endemic diarrhea in 159 dairy and 143 beef farms over a period of 10 years. The samples originated from 123 adult dairy cows, 40 adult commercial beef cows, 36 dairy calves and 103 beef calves. The species of dairy and beef cattle were Holstein and Japanese Black, respectively. The stool samples were diluted 1:10 in PBS and clarified by low‐speed centrifugation at 3000 g for 10 min. They were then used in the following series of diagnostics tests for causative pathogens: antigen detection kits (Dipstic‐Rota and Dipstic‐Adeno; Eiken, Tokyo, Japan) for bovine RVA and adenovirus, standard techniques for Salmonella spp. and E. coli and a saturated saline floatation method for Eimeria spp. Viral RNA was extracted from 10% fecal suspensions using a High Pure Virus RNA Kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. Viral RNAs were tested for the presence of BCV, bovine RVB, RVC, BTV and BVDV by RT‐PCR using a One‐Step RT‐PCR Kit (Qiagen, Gaithersburg, CA, USA) with specific pairs of primers (Table S2). The frequency of diarrhea‐causing pathogens of cattle detected by these diagnostics tests during the study period was analyzed according to breed and age of the cows. In addition, the frequency of detection of BCV and RVs in adult dairy and commercial beef cows was also analyzed every year.
During the study, BCV infections were detected in 71/123 adult dairy cows (57.7%) and 25/40 commercial beef cows (62.5%) (Fig. 1). In adult dairy cows, bovine RVs were identified in 24 cases (19.5%), including five of RVA (4.1%), two of co‐infection with RVA and RVB (1.6%), ten of RVB (8.1%) and seven of RVC (5.7%). By contrast, Eimeria spp. were detected in five samples (12.5%) from commercial beef cows. Furthermore, Salmonella spp. were detected in five samples (4.1%) from adult dairy cows only. No causative pathogens were detected in 21 (17.1%) and 6 (15.0%) cases from adult dairy and commercial beef cows, respectively. During the 10 years of the study, two peaks of BCV infections in adult dairy cows with diarrhea were consistently observed: from October to December and from March to May (Figs.2, 3). In adult dairy cows, bovine RV infections, including RVA, RVB, and RVC infections, were also identified from November to April every year, except for 2006. In commercial beef cows, BCV infections peaked in winter (from November to February) every year, except for 2011.
Figure 1.
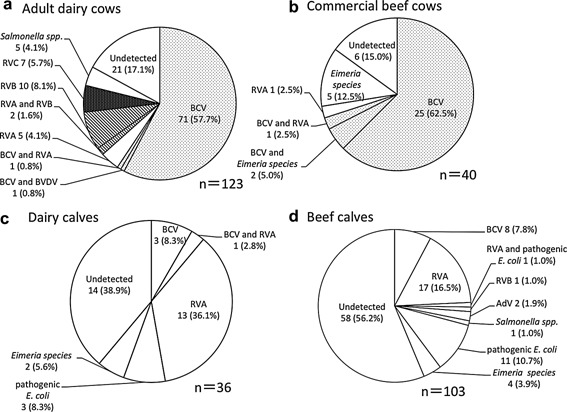
Frequency of detection of diarrhea‐causing pathogens in dairy and beef cows in Yamagata Prefecture, Japan, from 2002 to 2011.
Figure 2.
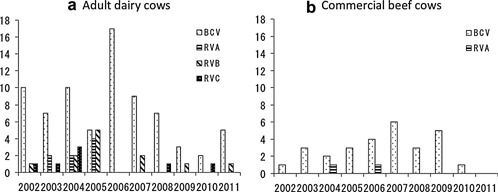
Annual data on enteric pathogens detected in (a) adult dairy and (b) commercial beef cows in Yamagata Prefecture, Japan, from 2002 to 2011.
Figure 3.
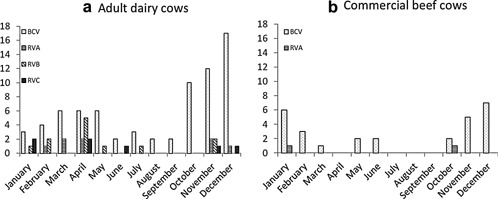
Seasonal changes in enteric pathogens detected in (a) adult dairy and (b) commercial beef cows in Yamagata Prefecture, Japan, from 2002 to 2011.
Including complex infections with other pathogens, bovine RVA was detected most frequently at rates of 14/36 (38.9%) and 18/103 (17.5%) samples from dairy and beef calves, respectively (Fig. 1). Next frequency were pathogenic E. coli and BCV, each of which were detected in samples from three dairy calves (8.3%) and from eleven (10.7%) and eight (7.8%) beef calves, respectively. No enteric pathogens were detected in 14 (38.9%) and 58 (56.2%) cases of diarrhea in dairy and beef calves, respectively. Diarrhea in calves was usually caused by a combination of infectious agents, including viruses, bacteria and protozoa, and non‐infectious agents, such as insufficient intake of colostrum, poor sanitation, stress and cold weather 19. Therefore, it was more difficult to identify a single causative agent of diarrhea in calves than it was in adult cows. Because our study did not include a diagnostic test for Cryptosporidium spp., one of the major diarrhea‐associated pathogens in calves in Japan 20, we cannot exclude the possibility that infection with Cryptosporidium spp. was responsible for the diarrhea in some of the calves for which no pathogen was detected.
On 17 January 2002, some lactating cows on one of the study farms developed acute diarrhea; within 2 days more of the lactating cows on the same farm also became ill. The diarrheal feces were liquid and brownish, but not bloody. The epidemic of diarrhea on this farm continued for 8 days. The affected cows had reduced milk yield without respiratory symptoms. Samples from all five affected lactating cows were positive for bovine RVB. Later, on 21 November 2002, a number of lactating cows on the same farm again developed diarrhea; within the following 5 days all lactating cows on this farm developed diarrhea. The diarrheal feces from this outbreak were liquid and bloody and this epidemic continued for 14 days. All affected cows had respiratory symptoms and, on average, a 14% reduction in milk yield. Samples from the five affected lactating cows were positive for BCV. A third epidemic began on 15 December 2003; within 4 days all lactating cows had liquid and brownish, but not bloody, feces and an average 9% reduction in milk yield, but without respiratory symptoms on this occasion. This epidemic lasted for 9 days. Samples from all four affected cows were positive for bovine RVC. Comparison of the clinical symptoms associated with these repeated epidemics of diarrhea on the same farm revealed characteristic symptoms of infectious diarrhea caused by bovine RVs and BCV, as shown in Table 1.
Table 1.
Frequency and clinical symptoms of BCV, bovine RVB, and RVC infections
| Clinical symptoms | BCV | RVB | RVC |
|---|---|---|---|
| Diarrheal feces | Liquid and bloody | Liquid and brownish | Liquid and brownish |
| Reduction in milk yield | 14% | + | 9% |
| Respiratory symptoms | Nasal discharge leakage | − | − |
| Number of affected animals | All dairy cattle and calf | Half dairy cattle | All dairy cattle |
| Peak day of epidemic | Day 6 | Day 2 | Day 4 |
| Duration of epidemic | 14 days | 8 days | 9 days |
+, yes; −, no.
In this study, regardless of the variety of cattle, BCV was the most frequently detected pathogen, particularly in winter, in adult cows with diarrhea. The finding that BCV was the major cause of epizootic adult cow diarrhea, often referred to as winter dysentery, supports findings reported previously 21. Bovine RVA is considered a major causative pathogen of diarrhea in neonatal calves; it can also infect adult cows, but this is unusual 11, 16. Some adult cows in this study were infected with bovine RVA. Our previous studies have shown that bovine RVB and RVC, rather than BCV, are associated with epidemic diarrhea in adult cows in Japan 14, 15. The data presented here confirm that bovine RVB and RVC, followed by BCV, are frequently detected in adult cows with diarrhea, especially dairy cows. In addition, both bovine RVs and BCV were detected in adult dairy cows with diarrhea, mostly in winters. These data suggest that, especially during winter, adult dairy cows with diarrhea should be tested for both BCV and bovine RVs. Furthermore, by comparing with three separate outbreaks of diarrhea on the same dairy farm, we identified clear differences in clinical symptoms—such as duration of diarrhea epidemic, hematochezia and complications—between bovine RVs and BCV infections. Briefly, the diarrhea caused by bovine RV infection tends to be milder than that caused by BCV infection. These differences in clinical symptoms may help to distinguish between bovine RV and BCV infections in cows.
With the exception of bovine RV infections, there were three clear differences in characteristics of cases of diarrhea between dairy and beef cows: the overall prevalence, and incidence of infections with Salmonella spp. and Eimeria spp.. Possible explanations for the greater incidence of cases in adult dairy than in commercial beef cows include differences in contacts between farms, such as equipment sharing and movement of people and vehicles between farms, all of which may play a major role in transmission of infectious agents 22. In contrast, there were more cases in beef calves than in dairy calves. There are several possible explanations for this difference. First, Japanese Black calves have a neonatal mortality rate of approximately 4.5% and in approximately half the neonates that die shortly after birth the cause is not apparent 1, 2. This so‐called stillbirth/perinatal weak calf syndrome 23 is characterized by low body weight, anemia, depression, weakness, variable body temperature, reddened and crusty muzzles and astasia; the syndrome is in part associated with genetic factors 1, 2. These calves are highly susceptible to infection and die with symptoms of diarrhea or pneumonia within a few weeks of birth 2. Second, clinical reports of Salmonella spp. infection occurred only in adult dairy cows and not in commercial beef cows. Adult dairy cows that are shedding this pathogen in their feces are typically asymptomatic 18. However, dairy cows are fed high‐protein concentrated food, including non‐degradable protein that bypasses the rumen, to increase milk production. This could cause an imbalance in the physiological functions of the rumen, which might increase susceptibility to Salmonella spp. infection 24. In addition, stress factors such as calving and milking may induce more severe diarrhea in dairy cows than in beef cows. Third, Eimeria spp. infections were identified only in commercial beef cows. Japanese beef cows are usually fed a diet that is low in vitamin A 25, 26, 27. In particular, no vitamin A is added to their feed during the middle finishing period (16–18 months old) 28. Many dietary factors, including vitamin A, are essential for gut and immune system development. In addition, low vitamin A intake is known to depress the responses of T‐lymphocytes to mitogens in vitro 29, 30. Moreover, deficiency of vitamin A is known to increase susceptibility of poultry to infection with enteric parasites such as coccidia 31, 32. Thus, because of their low vitamin A intake, commercial beef cows may be more susceptible to coccidiosis than adult dairy cows.
In conclusion, our 10 year surveillance study revealed differences in the type and prevalence of enteric pathogens depending on the age and type of cattle. This practical information may contribute to more accurate diagnosis and effective prevention of diarrhea in cows.
DISCLOSURE
All authors declare that they have no conflicts of interests.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web‐site.
Table S1: Characteristics of diarrhea caused by several representative enteric pathogens in cattle
Table S2: List of primers used in the detection of diarrhea‐causing viruses
ACKNOWLEDGMENT
This study was supported in part by a grant from the National Institute of Animal Health, NARO.
REFERENCES
- 1. Ogata Y., Nakao T., Takahashi K., Abe H., Misawa T., Urushiyama Y., Sakai J. ( 1999) Intrauterine growth retardation as a cause of perinatal mortality in Japanese Black beef calves. Zentralbl Veterinarmed A 46: 327–34. [DOI] [PubMed] [Google Scholar]
- 2. Takasu M., Shirota K., Ohba Y., Nishii N., Murase T., Miyazawa K., Kitagawa H. ( 2008) Thymic hypoplasia in Japanese black calves with stillbirth/perinatal weak calf syndrome. J Vet Med Sci 70: 1173–7. [DOI] [PubMed] [Google Scholar]
- 3. Megan E.S., Mangkey A.B., Sandy R., Rocky J.B., Wendy B., Hemant N., Binu V., Loyd S., Barbara S., Alfonso C. ( 2012) Development and performance evaluation of calf diarrhea pathogen nucleic acid purification and detection workflow. J Vet Diagn Invest 24: 945–53. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi K. ( 1996) Diseases of the intestine. Textbook of Veterinary Internal Medicine. In: Murakami D, Motoyoshi S, Hasegawa A, Kawamura S, Naito Y, Maede Y eds. Tokyo: Buneido Press, pp. 189–202. [Google Scholar]
- 5. Radostits O.M., Gay C.C., Blood D.C., Hinchcliffe K.W. ( 2000) Diseases of the alimentary tract. In: Veterinary Medicine: a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 9th edn. London: Harcourt–Brace, pp. 234–46. [Google Scholar]
- 6. Sakai T. ( 2000) Hygiene management. Livestock Hygiology. In: Sugano S., Kamata S., Sakai T., Oshida T. eds. Tokyo: Buneido Press, pp. 27–9. [Google Scholar]
- 7. De Rycke J., Bernard S., Laporte J., Naciri M., Popoff M.R., Rodolakis A. ( 1986) Prevalence of various enteropathogens in the feces of diarrheic and healthy calves. Ann Rech Vet 17: 159–68. [PubMed] [Google Scholar]
- 8. De la Fuente R., Luzon M., Ruiz‐Santa‐Quiteria J.A., Garcia A., Cid D., Orden J.A., Garcia S., Sanz R., Gomez‐Bautista M. ( 1999) Cryptosporidium and concurrent infections with other major enteropathogens in 1 to 30‐day‐old diarrheic dairy calves in central Spain. Vet Parasitol 80: 179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saif L.J., Brock K.V., Redman D.R., Kohler E.M. ( 1991) Winter dysentery in dairy herds: electron microscopic and serological evidence for an association with coronavirus infection. Vet Rec 128: 447–9. [DOI] [PubMed] [Google Scholar]
- 10. Chang K.O., Parwani A.V., Smith D., Saif L.J. ( 1997) Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. J Clin Microbiol 35: 2107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato M., Nakagomi T., Tajima K., Ezura K., Akashi H., Nakagomi O. ( 1997) Isolation of serotype G8, P6[1] bovine rotavirus from adult cattle with diarrhea. J Clin Microbiol 97: 1266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carman S., van Dreumel T., Ridpath J., Hazlett M., Alves D., Dubovi E., Tremblay R., Bolin S., Godkin A., Anderson N. ( 1998) Severe acute bovine viral diarrhea in Ontario, 1993‐1995. J Vet Diagn Invest 10: 27–35. [DOI] [PubMed] [Google Scholar]
- 13. Fukutomi T., Tsunemitsu H., Akashi H. ( 1999) Detection of bovine coronaviruses from adult cows with epizootic diarrhea and their antigenic and biological diversities. Arch Virol 144: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsunemitsu H., Morita D., Takaku H., Nishimori T., Imai K., Saif L.J. ( 1999) First detection of bovine group B rotavirus in Japan and sequence of its VP7 gene. Arch Virol 144: 805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mawatari T., Taneichi A., Kawagoe T., Hosokawa M., Togashi K., Tsunemitsu H. ( 2004) Detection of a bovine group C rotavirus from adult cows with diarrhea and reduced milk production. J Vet Med Sci 66: 887–90. [DOI] [PubMed] [Google Scholar]
- 16. Fukai K., Takahashi T., Tajima K., Koike S., Iwane K., Inoue K. ( 2007) Molecular characterization of a novel bovine group Arotavirus. Vet Microbiol 20: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Natsuaki S., Goto K., Nakamura K., Yamada M., Ueo H., Komori T., Shirakawa H., Utinuno Y. ( 2007) Fatal winter dysentery with severe anaemia in an adult cow. J Vet Med Sci 69: 957–60. [DOI] [PubMed] [Google Scholar]
- 18. Edrington T.S., Ross T.T., Callaway T.R., Martinez C.H., Hume M.E., Genovese K.J., Poole T.L., Anderson R.C., Nisbet D.J. ( 2008) Investigation into the seasonal salmonellosis in lactating dairy cattle. Epidemiol Infect 136: 381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith G.W. ( 2009) Treatment of calf diarrhea: oral fluid therapy. Vet Clin North Am Food Anim Pract 25: 55–72. [DOI] [PubMed] [Google Scholar]
- 20. Murakoshi F., Tozawa Y., Inomata A., Horimoto T., Wada Y., Kato K. ( 2013) Molecular characterization of Cryptosporidium isolates from calves in Ishikari District, Hokkaido, Japan. J Vet Med Sci 75: 837–40. [DOI] [PubMed] [Google Scholar]
- 21. Kanno T., Hatama S., Ishihara R., Uchida I. ( 2007) Molecular analysis of the S glycoprotein gene of bovine coronaviruses isolated in Japan from 1999 to 2006. J Gen Virol 88: 1218–24. [DOI] [PubMed] [Google Scholar]
- 22. Stanković B., Hristov S., Bojkovski J., Zlatanović Z., Maksimović N., Todorović‐Joksimović M., Davidović V. ( 2011) The possibility of dairy farms isolation assessment—biosecurity aspect. Biotechnol Anim Husb 27: 1425–31. [Google Scholar]
- 23. Radostits O.M., Gay C.C., Blood D.C., Hinchcliffe K.W. ( 2000) Specific diseases of uncertain etiology. In: Veterinary Medicine: a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. London: Harcourt–Brace, pp. 1763–6. [Google Scholar]
- 24. Uchida I. ( 2013) Bovine salmonellosis. In: Akashi H., Eguchi M., Kamio T., Kamomae H., Sakai Y., Haga T., Manabe N. eds. Buiatrics, 3rd edn. Tokyo: Kindai Press, pp. 262–4. [Google Scholar]
- 25. Oka A., Maruo Y., Miki T., Yamasaki T., Saito T. ( 1998) Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Sci 48: 159–67. [DOI] [PubMed] [Google Scholar]
- 26. Oka A., Dohgo T., Juen M., Saito T. ( 1998) Effects of vitamin A on beef quality, weight gain, and serum concentrations of thyroid hormones, insulin‐like growth factor‐I, and insulin in Japanese black steers. Anim Sci Technol 9: 90–9. [Google Scholar]
- 27. Nade T., Hirabara S., Okumura T., Fujita K. ( 2003) Effects of vitamin A on carcass composition concerning younger steer fattening of Wagyu cattle. Asian‐Aust J Anim Sci 16: 353–8. [Google Scholar]
- 28. Irie M., Inno Y., Ishizuka Y., Nishioka T., Morita T. ( 2006) Vitamins A and E in carcass fat from Japanese black and F1 cross cattle. Asian‐Aust J Anim Sci 19: 1266–70. [Google Scholar]
- 29. Friedman A., Sklan D. ( 1989) Impaired T lymphocyte immune response in vitamin A depleted rats and chicks. Br J Nutr 62: 439–49. [DOI] [PubMed] [Google Scholar]
- 30. Sklan D., Melamed D., Friedman A. ( 1994) The effect of varying levels of dietary vitamin A on immune response in the chick. Poult Sci 73: 843–7. [DOI] [PubMed] [Google Scholar]
- 31. Chew B.P. ( 1995) Antioxidant vitamins affect food animal immunity and health. J Nutr 125: 18045–85. [DOI] [PubMed] [Google Scholar]
- 32. Dalloul R.A., Lillehoj H.S., Shellem T.A., Doerr J.A. ( 2003) Intestinal immunomodulation by vitamin A deficiency and lactobacillus‐based probiotic in Eimeria acervulina infected chickens broiler. Avian Dis 47: 1313–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher’s web‐site.
Table S1: Characteristics of diarrhea caused by several representative enteric pathogens in cattle
Table S2: List of primers used in the detection of diarrhea‐causing viruses


