Abstract
Ebola hemorrhagic fever is a deadly disease caused by infection with one of the Ebola virus species. Although a significant progress has recently been made in understanding of Ebola virus biology and pathogenesis, development of effective anti‐Ebola treatments has not been very productive, compared to other areas of antiviral research (e.g., HIV and HCV infections). No approved vaccine or medicine is available for Ebola but several are currently under development. This review summarises attempts in identification, evaluation, and development of small‐molecule candidates for treatment of Ebola viral disease, including the most promising experimental drugs brincidofovir (CMX001), BCX4430, and favipiravir (T‐705).
Keywords: antiviral, filovirus, Ebola virus, Marburg virus, hemorrhagic fever
1. INTRODUCTION
Ebolavirus, Marburgvirus (MARV), and Cuevavirus are the only genera of the Filoviridae family of enveloped viruses with nonsegmented negative‐sense RNA genoms.1 They are causative agents of severe viral hemorrhagic fevers (VHFs) and are classified as biosafety level‐4 (BSL‐4) pathogens and Category A agents (in terms of bioterrorism).2 The taxonomy of the Filoviridae family has kept changing over time and several virus names and abbreviations have been created. Currently, five ebola species (earlier they were considered strains or subtypes of one species) are recognized, namely Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV), Taï Forest ebolavirus (TAFV), Reston ebolavirus (RESTV), and Bundibugyo ebolavirus (BDBV).1, 3 Especially, EBOV and SUDV are responsible for serious outbreaks of Ebola hemorrhagic fever (EHF), or Ebola virus disease (EVD), among humans and nonhuman primates in the regions of sub‐Saharan Africa. EBOV is the most virulent ebola virus with fatality rate ranging from 50 to 90%.
EVD was first identified in 1976 in Sudan (now South Sudan)4 and Zaire (now the Democratic Republic of the Congo)5 and 24 outbreaks were reported by the World Health Organization (WHO) since then through 2013.6 The transmissions from animals to humans are believed to involve direct contact with an infected wild animal or fruit bat, which are considered to be the most likely natural reservoir for Ebola virus.
The recent outbreak in West Africa,7 which started in March 2014, represents the biggest Ebola outbreak so far and it is considered to be the first Ebola epidemic the world has ever seen.6 It has brought a substantial attention of both scientific community and the public. Over 20,000 confirmed, probable, and suspected cases of EVD have been reported by WHO from six African countries (with main incidence in Guinea, Liberia, and Sierra Leone) and several isolated cases also from other countries (Spain and United States).8 The reported case fatality rate across the most‐affected countries is estimated to be at least 70%.9
VHFs caused by filoviruses are usually characterized by nonspecific flu‐like symptoms including high fever, severe headache, myalgia, and prostration, followed by gastrointestinal symptoms such as diarrhea, nausea, and vomiting, and further signs as bleeding, petechiae, rash, dry cough, chest pain, behavioral disorders, and seizures, resulting in multiorgan failure and, ultimately, death.10, 11, 12 Death, due to multiorgan failure and a syndrome resembling septic shock, typically occurs within 6–16 days after development of the clinical signs.13, 14
Commonly applied standard supportive care is based on replacement of the body fluids patients lose during the infection, and on treatment of other opportunistic infections. Currently, there are no approved drugs to treat EHF. Although several small‐molecule candidates were developed and approved for the treatment of various RNA virus infections,15 most of them did not show to be really potent in case of filovirus diseases. Only a handful of potential antiviral agents are in the pipeline for filovirus infections,16, 17, 18, 19, 20, 21, 22, 23 but these experimental drugs actually represent promising options for the prevention and treatment of EVD.
While development of EBOV vaccines is highly desirable, especially for protection of high‐risk groups (e.g., medical personal or family members of patients), vaccines cannot completely prevent single cases or even new EBOV outbreaks in remote areas of West Africa. The use of vaccines is, furthermore, accompanied with more or less serious adverse effects and it is not even clear how efficient it would be in areas endemic with various other serious human diseases (e.g., malaria), where, moreover, the local community may strongly disagree with vaccination. Development and use of humanized monoclonal antibodies (e.g., ZMapp)24 also has significant limitations, including stability, difficulty with transport, and problematic scale‐up production. For these reasons, usage of small‐molecule antivirals is invaluable approach in treatment and prevention of viral infections in general and development of potent small‐molecule anti‐EBOV agents is clearly of high priority.
The EBOV genome contains seven genes (NP, VP35 (where VP is viral protein), VP40, GP, VP30, VP24, and L) that encode the corresponding VPs. The filovirus replication complex consists of the genomic RNA molecule and four proteins: NP (nucleoprotein), VP30 (transcription activator), VP35 (polymerase cofactor), and L (RNA‐dependent RNA polymerase).16 The matrix proteins VP24 and VP40 connect glycoprotein (GP) (actually, its GP2 segment) to the central ribonucleoprotein.16 Number of steps in the filovirus replication cycle can, theoretically, be targeted with small‐molecule inhibitors, namely attachment of the virion to a cell‐surface receptor, fusion of the viral envelope with cellular membranes, replication/transcription process, assembly/maturation of new viral particles, and budding.16
This work summarizes discovery and identification of number of small molecules with important anti‐Ebola virus properties. Promising treatments based on antisense technology and RNA interference (RNAi) are also briefly mentioned while monoclonal antibodies (e.g., ZMapp)24 and Ebola virus vaccine25, 26, 27 development is not addressed in this review and can be found elsewhere.
2. NUCLEOSIDE AND NUCLEOTIDE ANALOGUES
This group of compounds is represented by structurally modified nucleosides and nucleotides with various modes of antiviral action. Acyclic nucleoside phosphonates, and compounds that can be metabolized to nucleotide analogues in cells are also included here.
Among nucleoside analogues, ribavirin (Virazole, Fig. 1), a broad‐spectrum antiviral drug, has received a lot of attention.28 Ribavirin has been reported to be active against some hemorrhagic fever viruses (e.g., Rift Valley fever virus and Crimean‐Congo hemorrhagic fever virus), but it had no in vitro or in vivo effects on Ebola and Marburg viruses.29 Later, number of structural adenosine analogues, for example, 3‐deazaaristeromycin (C‐c3Ado, Fig. 1) and 3‐deazaneplanocin A (c3‐Npc A, Fig. 1), were discovered to inhibit replication of EBOV in vitro by blocking S‐adenosyl‐l‐homocystein (SAH) hydrolase.30, 31, 32
Figure 1.
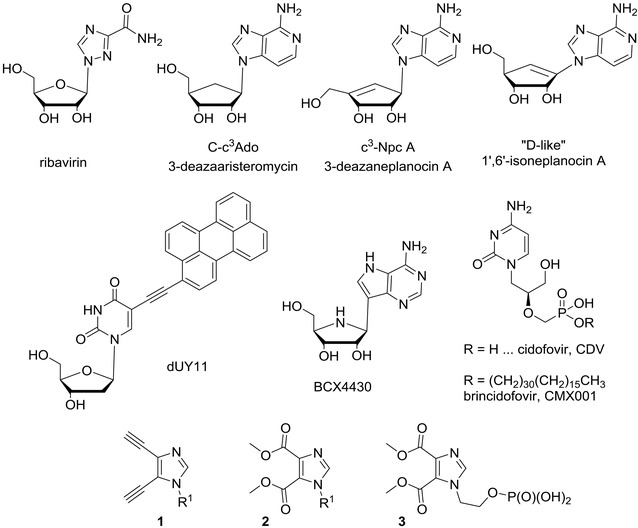
Chemical structures of nucleoside analogues with various modes of action: ribavirin (not active against EBOV); 3‐deazaaristeromycin, 3‐deazaneplanocin A and “d‐like” 1′,6′‐isoneplanocin A (SAH hydrolase inhibitors); dUY11 (fusion inhibitor); BCX4430, CDV, and CMX001 (viral transcription inhibitors); compounds 1–3 (unknown mechanism of action).
SAH hydrolase is a key enzyme in methylation reactions depending on S‐adenosylmethionine (SAM) as the methyl donor and it has a key role in the methylation of 5′‐end guanine of viral messenger RNA (regulation of capping process). Since the discovery of SAH hydrolase as a valuable pharmacological target for antiviral chemotherapy,33 a large variety of adenosine (Ado) analogues as potential SAH hydrolase inhibitors have been reported.34, 35, 36 Such inhibitors block the cleavage of S‐adenosyl‐l‐homocystein (SAH) into homocysteine (Hcy) and adenosine, which itself can be further metabolized into AMP, adenine, and inosine. As a consequence of the SAH hydrolase inhibition, SAH accumulates in the cell and leads to an inhibition of the SAM‐dependent methylation processes, including those that are required for the maturation (i.e., 5′‐capping) of viral mRNAs. As a consequence, maturation of viral mRNAs is suppressed, and so is the production of progeny virus particles.
3‐Deazaaristeromycin (C‐c3Ado, Fig. 1) was the first compound that demonstrated to cure mice from otherwise lethal EBOV infection.31 Bray et al.32 showed that 3‐deazaneplanocin A (Fig. 1), as a single inoculation of 1 mg/kg, given on the first or second day after virus infection, also afforded a significant protection of mice against a lethal infection with EBOV without causing acute toxicity. In later study,37 it was discovered that the protective effect of 3‐deazaneplanocin A might result from massively increased production of interferon‐α in Ebola‐infected, but not uninfected mice. SAH hydrolase inhibitors have received only limited clinical evaluation and should be further tested for their potential antifilovirus properties as they exert a broad‐spectrum antiviral activity and represent an attractive antiviral strategy.38
Ye and Schneller39 have reported 1′,6′‐isoneplanocin A enantiomers (e.g., “d‐like” 1′,6′‐isoneplanocin A, Fig. 1) as compounds potent against a variety of important viruses, including EBOV with submicromolar EC50 values (e.g., EC50 = 0.38 for the “d‐like” enantiomer, Fig. 1). The author also speculated that SAH hydrolase inhibition is not the only site of action of the “l‐like” enantiomer and more studies are needed to fully understand the antiviral potential of l‐like carbocyclic nucleosides.39
Rigid amphipathic fusion inhibitors (RAFIs),40 for example, compound dUY11 (Fig. 1), are uridine nucleoside analogues bearing a bulky hydrophobic group in the C‐5 position. RAFIs represent another group of synthetic compounds that inhibit infectivity of several unrelated enveloped viruses, including HCV and HSV‐1 and HSV‐2 at submicromolar range and with no cytotoxic or cytostatic effects (selectivity index > 3000).40 It was shown that RAFIs inhibit virion fusion as a result of their shape and amphipathicity. RAFIs should be further evaluated against other emerging viruses, such as EBOV and MARV.
Another promising EBOV therapy represents compound BCX4430 (Fig. 1),41 which has been reported in 2014 as a novel broad‐spectrum antiviral agent. It is an adenine analogue of the so‐called Immucillin H,42 a powerful transition‐state analogue43 inhibitor of purine nucleoside phosphorylase, which has a potential for treatment of human T‐cell leukemia and lymphoma. BCX4430 exhibits broad‐spectrum activity against numerous viruses, including filo‐, bunya‐, arena‐, paramyxo‐, corona‐, and flaviviruses. BCX4430 was shown to inhibit infection of distinct filoviruses in human cells and postexposure intramuscular administration of BCX4430 protected rodents both against EBOV and MARV viral disease.41 It, moreover, completely protected cynomolgus macaques from MARV infection when administered as late as 48 hr following infection. BCX4430 appeared to inhibit viral RNA polymerase function, acting probably as a non‐obligate RNA chain terminator.41 BCX4430 also effectively treated yellow fever virus (YFV) infection in a hamster model, even when treatment was initiated at the peak of viral replication.44 The first‐in‐man Phase I study to evaluate the safety, tolerability, and pharmacokinetic properties of BCX4430 administered via intramuscular injection in healthy volunteers was announced by BioCryst Pharmaceuticals (Durham, NC, USA) in the middle of December 2014.45
Brincidofovir (CMX001, BCV, HDP‐CDV, Fig. 1) is an oral nucleotide analogue with broad‐spectrum in vitro and in vivo antiviral activity against dsDNA viruses that effect humans,46 including adenoviruses,47, 48 poxviruses,49 and herpesviruses.50 CMX001 is a hexadecyloxypropyl prodrug of cidofovir (Fig. 1),51 acyclic nucleoside phosphonate52 approved by FAD for treatment of cytomegalovirus (CMV) infections. CMX001, being developed by Chimerix (Durham, NC, USA), has several key advantages compared to the parent compound: improved oral bioavailability, rapid transport across cell membranes leading to higher intracellular concentrations of the active species, greater potency, and elimination of nephrotoxicity.53, 54 Brincidofovir has actually received Fast Track designation from the FDA for treatment of CMV, adenovirus, and smallpox infections.
Quite surprisingly, investigational antiviral brincidofovir, which was considered by antiviral experts to be specific for treatment of DNA viral diseases, has been reported55 to show in vitro activity against EBOV and, thus, to have potential use in patients with EVD. While additional assessments of CMX001 in animal model studies are being conducted through the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), Chimerix announced in October 2014 that Emergency Investigational New Drug Applications (EIND) for brincidofovir were authorized by the US Food and Drug Administration (FDA) for EVD patients.55 CMX001 probably interferes with certain enzyme(s) of nucleos(t)ide metabolism, but its exact mechanism of EBOV inhibition remains to be clarified.
A class of imidazole nucleoside and nucleotide analogues, bearing either nitrile or ester groups at imidazole 4‐ and 5‐positions (compounds of general structure 1 and 2, Fig. 1), has been reported to inhibit replication of Lassa virus, severe acute respiratory syndrome (SARS) coronavirus, and EBOV in vitro, employing real‐time PCR.56 Acyclic nucleotide analogue 3 (Fig. 1), for example, showed IC50 of 12 μg/mL and CC50 of 75 μg/mL, suggesting that the reported activity may be linked to cytotoxicity. The IC50 values of all the compounds against EBOV ranged from 10 to 52 μg/mL and the mechanism of action of these analogues remained speculative.56
Favipiravir (T‐705, Fig. 2),57, 58 or 6‐fluoro‐3‐hydroxypyrazine‐2‐carboxamide, is a broad‐spectrum antiviral agent active against many RNA viruses, as alpha‐, arena‐, bunya‐, flavi‐, noro‐, orthomyxo‐, and picornaviruses. It was discovered and developed by Toyama Chemical Co. (Toyama, Japan) as anti‐influenza virus agent,59, 60 and is approved in Japan as an influenza treatment under the brand name Avigan. Favipiravir is currently undergoing Phase III clinical trials in the United States. The in vivo efficacy of T‐705 was recently confirmed in a mouse models for EBOV, when postexposure initiation of T‐705 administration completely prevented the lethal consequences.61, 62 In vitro, T‐705 is efficiently converted by cellular enzymes to its ribofuranosyl 5‐triphosphate (T‐705 RTP, Fig. 2), the active species that was suggested to selectively inhibit influenza virus RNA‐dependent RNA polymerase.60 T‐705 RTP is recognized by influenza A virus polymerase as an efficient substrate for incorporation to the RNA both as a guanosine and an adenosine analogue and its two consecutive incorporations were shown to prevent further primer extension.63 Baranovich et al.64 have reported lethal mutagenesis to be the key antiviral mechanism of T‐705, that also explains its broad‐spectrum antiviral activity. Favipiravir has been given to several Ebola patients and with its unique mechanism of action currently represents a very promising candidate for EVD treatment.
Figure 2.
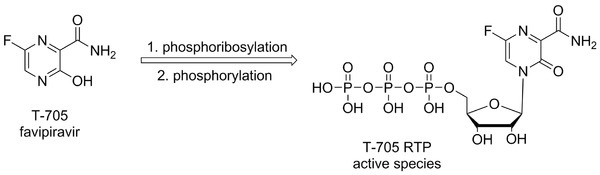
Chemical structure of favipiravir (T‐705) and mode of its enzymatic activation.
3. POTENTIAL VIRAL ENTRY INHIBITORS
This section includes structurally diverse compounds that are reported to inhibit the cell entry of filoviruses. Several of the agents discussed are repurposed FDA‐approved drugs.
Number of compounds of distinct structural features has been reported as potential entry inhibitors. EBOV entry requires functioning cholesterol transporter protein Niemann–Pick C1 (NPC1).65 It was shown that cells defective NPC1 function, which binds to the viral GP, are resistant to infection by EBOV and MARV. Small‐molecule inhibitors, derived from benzylpiperazine adamantane diamides (e.g., compounds 3.0 and 3.47, Fig. 3),66 have been described that interfere with GP binding to NPC1. Since this process is essential for EBOV infection, it seems to represent a good target for potential antiviral therapy.
Figure 3.
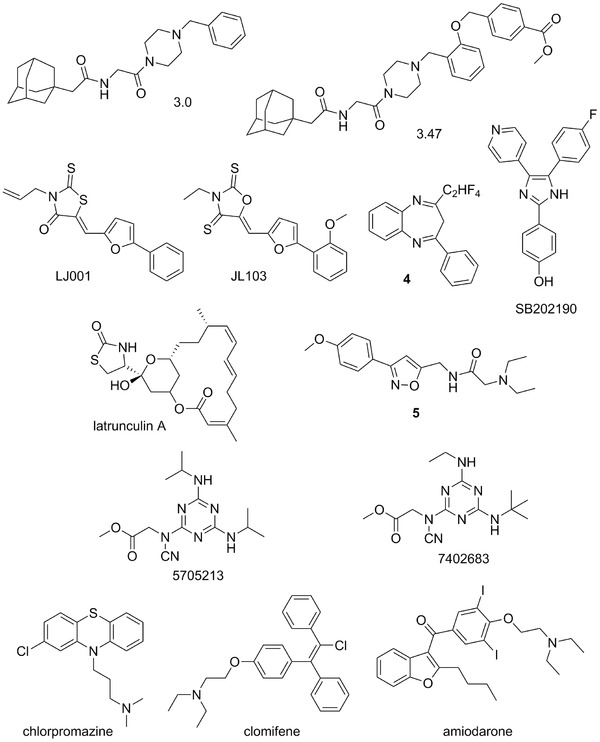
Chemical structures of potential viral entry inhibitors of EBOV: 3.0, 3.47, LJ001, JL103, compound 4, SB202190, latrunculin A, compound 5, 5705213, 7402683; FDA‐approved drugs chlorpromazine, clomifene, and amiodarone, representing examples of potential drug‐repurposing.
Wolf et al.67 have reported the discovery of promising broad‐spectrum antiviral agent, LJ001 (Fig. 3), active against an impressive number of enveloped viruses. It was effective against influenza A, filo‐, pox‐ arena‐, bunya‐, paramyxo‐, flaviviruses, and HIV, but had no effect on the infection of nonenveloped viruses. LJ001 intercalates into viral membranes preventing virus–cell fusion, but the host cells can overcome the toxic effects of LJ001 due to their repair by cellular lipid biosynthesis. The rhodanine derivative LJ001 was suggested to inhibit viral entry at a step after virus binding and before virus–cell fusion, but the molecular target and molecular mechanism remained elusive. Later, Vigant et al.68 identified the unsaturated fatty acid chains of viral membrane phospholipids as the major target of LJ001 antiviral activity. In the membrane bilayer, LJ001 generates singlet oxygen (1O2) and subsequent lipid oxidation results in changes to the biophysical properties of the viral membrane that disrupts the virus ability to undergo virus–cell fusion. Furthermore, elucidation of the mode of action and subsequent structure–activity relationship (SAR) optimization of LJ001 led to a new class of oxazolidine‐2,4‐dithiones, for example, compound JL103 (Fig. 3),68 as membrane‐targeted photosensitizers with increased potencies, 1O2 quantum yields, and red‐shifted absorption spectra.
A series of benzodiazepine compounds, represented by derivative 4 (Fig. 3), has been reported as potential entry inhibitors for filoviruses.69 Compound 4 was validated as an inhibitor of EBOV and MARV in cell‐based assays, with 50% inhibitory concentrations (IC50s) of 10 and 12 μM, respectively. It was hypothesized that it binds to the hydrophobic pocket of the EBOV GP1–GP2 interface and as a consequence inhibits EBOV infection of cells.
Pyridinyl imidazole inhibitors of p38 MAP kinase, for example, compound SB202190 (Fig. 3), were found to impair viral entry and reduce cytokine induction by EBOV.70 SB202190 reduced viral replication in macrophage‐like human THP‐1 cells with an IC50 = 4.73 μM and primary human monocyte derived dendritic cells (MDDCs) with an IC50 = 2.67 μM. Kinase, as well as phosphatase inhibitors may represent new leads and a unique strategy for antifilovirus therapeutic development and such compounds with reported anti‐EBOV activity have recently been reviewed in depth.21
Yermolina et al.71 have reported a novel group of selective inhibitors of filoviral entry that selectively inhibit the EBOV and MARV GP mediated infection of human cells. Extensive SAR study led to an identification of lead compound 5 (Fig. 3) as a selective inhibitor of filoviral entry with an IC50 of 30 μM.71 Also several natural products that are able to impair microfilament function, including latrunculin A (Fig. 3) and cytochalasins, were shown to be potent inhibitors of EBOV virus GP mediated entry and fusion.72
A novel high‐throughput screening (HTS) assay of some 5000 small molecules led to an identification of novel broad‐spectrum compounds able to block cathepsin L (CatL) cleavage of viral GPs derived from SARS‐CoV and EBOV, Hendra, and Nipah viruses that are required for their entry into the host cell.73 Parent compound 5705213 (Fig. 3) and its derivative 7402683 (Fig. 3) showed IC50s of 15 and 10 μM against EBOV‐GP, respectively, and are not cytotoxic.
Fullerene sugar balls represent a new class of biologically active compounds.74 Water‐soluble glycofullerenes were found to efficiently inhibit a DC‐SIGN‐dependent (DC‐SIGN is a C‐type lectin) cell infection by virus‐like particles.75 Several mannosylated fullerene sugar balls showed remarkable IC50s of 2 μM against EBOV and thus can be considered as a very promising tool to interfere with the EBOV entry.
Drug repurposing (or drug repositioning) is potential application of known compounds to new indications. Therefore, a systematic screening of FDA‐approved drugs could rapidly become available for a new indication in an emergency, including EBOV infections.76, 77, 78 Chlorpromazine (trademarketed as Thorazine, Largactil, and Megaphen, Fig. 3),79 a known psychotropic drug approved by the FDA, was reported as potential inhibitor of EBOV entry, possibly through inhibition of clathrin‐mediated endocytosis.80
FDA‐approved selective estrogen receptor modulators (SERMs), including clomifene (trademarked as Androxal, Clomid, and Omifin, Fig. 3) and toremifene (brand name Fareston) were identified as potent inhibitors of EBOV infection from an in vitro screening of readily available approved drugs.77, 81, 82 The authors suggested that mode of the action of SERMs did not involve classical pathways associated with the estrogen receptor, but instead, interfere with a late step in viral entry.81
Antiarrhytmic agents amiodarone (a multi‐ion channel inhibitor, Fig. 3) and dronedarone are other examples of FDA‐approved drugs that could be repurposed.83 Amiodarone84 was found to inhibit filovirus entry at concentrations (1.5–2.5 μg/mL) that are routinely reached in human serum during antiarrhythmic therapy.83 The above examples show that drug repurposing may be a viable approach for identification of potent anti‐EBOV therapeutics.
4. MISCELLANEOUS SMALL MOLECULES
Functional Genetics, Inc. (Gaithersburg, MD, USA) has reported a series of polyaromatic compounds active against distinct viruses. A small‐molecule inhibitor of filovirus infection, designated as FGI‐103 (Fig. 4),85 was identified via HTS of compound library from National Cancer Institute (NCI). FGI‐103 exhibited antiviral activity against wild‐type EBOV and SUDV, as well as multiple strains of MARV.85 Although the mechanism of its action is unknown, it was shown in the murine model of EBOV infection that FGI‐103 reduces viremia and viral burden in organ tissues and that it could be applicable for both prophylactic and therapeutic treatments.
Figure 4.
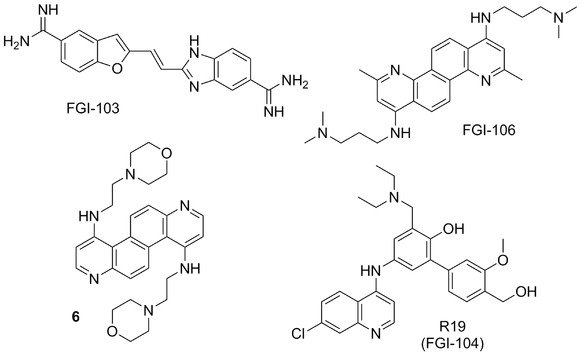
Chemical structures of antiviral inhibitors developed by Functional Genetics, Inc. (Gaithersburg, USA) and related compounds.
FGI‐106 (Fig. 4)86 is a diazachrysene (DAAC) based analogue that was discovered in a cell‐based HTS as a potent and broad‐spectrum inhibitor of lethal VHF pathogens, including EBOV, Rift Valley, and Dengue Fever viruses. FGI‐106 protected mice from otherwise lethal EBOV infection both in prophylactic and therapeutic settings. FGI‐106 also revealed potential inhibitory activity against other viral pathogens including HIV and HCV,86 but the precise mode of action remains unclear.22 The broad‐spectrum nature of the antiviral activity of DAAC‐based analogues may suggest targeting of a conserved host pathway.86
Conventional antivirals are designed to target virally encoded proteins/enzymes and mechanisms. The disadvantage of the conventional antivirals is often their toxicity to the host and development of the resistant viral strains rendering them relatively quickly ineffective. To prevent the resistance problem, combination therapies using a cocktail of drugs with various modes of actions were successfully introduced and approved. Host‐directed therapeutics represents another important approach to combat established, as well as emerging viral diseases.87 Such approach is based on targeting host to deny the viral pathogen the ability to cause disease.
Structural modification studies of the promising DAAC‐based inhibitors were performed,88, 89 and compound 6 (Fig. 4) was identified as highly efficacious EBOV and MARV inhibitor with IC50 values of 0.70 and 2.76 μM, respectively, with little or no associated cellular toxicity.89
Recently, a broad‐spectrum small‐molecule inhibitor of EBOV, FGI‐104 (structure originally not given),90 has been reported that might target host protein TSG101 that plays an essential role in the viral life cycle. The interaction of filovirus matrix protein VP40, the key VP that drives the budding process,91 with TSG101 facilitates the viral budding.92 In addition, FGI‐104 demonstrated inhibition of multiple emerging viruses (e.g., EBOV, Cowpox) and blood‐borne pathogens (e.g., HBV, HCV, HIV). In the patent,93 chaotically, FGI‐104 is a name used for the whole family of compounds and R19 (Fig. 4) is mentioned as the preferred compound listed there.
Retinoid thiosemicarbazone derivative, retinazone (RTZ, Fig. 5),94 was described as a broad‐spectrum antiviral agent active against HIV, HCV, VZV, and CMV. RTZ was found to be a potent suppressor of HCV RNA replicon replication.94 Later, RTZ has also been reported to be potent and efficacious inhibitor of EBOV with an IC50 value of 1.1 μM,95 but since the SI50 was only 3.4, the activity may be linked to cytotoxicity.
Figure 5.
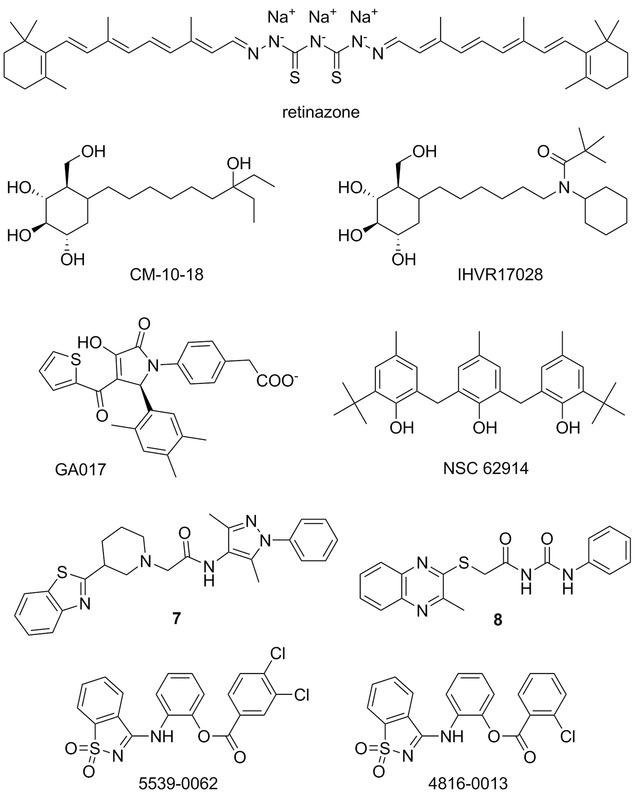
Miscellaneous small‐molecule agent with potential anti‐EBOV properties: retinazone; endoplasmic reticulum (ER) glucosidase inhibitors CM‐10‐18 and IHVR17028; GA017; antioxidant compound NSC 62914; compounds 7, 8, 5539‐0062, and 4816‐0013 as potential budding inhibitors.
Iminosugar 1‐deoxynojirimycin (DNJ) and its derivatives (as glucose mimics) can serve as glucosidase inhibitors and were shown to exhibit antiviral effects against a number of enveloped viruses. DNJ derivative CM‐10‐18 (Fig. 5) was shown to exhibit in vitro and in vivo inhibitory activity against endoplasmic reticulum (ER) α‐glucosidases I and II,96, 97 and demonstrated in vivo efficacy against lethal Dengue virus infection in mice.98 Further extensive SAR studies of CM‐10‐18 derivatives lead to an identification of novel iminosugars, for example, compound IHVR17028 (Fig. 5), that significantly reduced the mortality of MARV and EBOV infections in mice.99 A significant survival rate was, for example, observed for 25 mg/kg of IHVR17028 in a murine protection‐of‐death model of EBOV infection, when the treatment was initiated 4 hr post virus challenge.
The multifunctional VP35 is another attractive therapeutic target as it plays a critical role in Ebola viral replication, and knowledge of high‐resolution structures of the VP35 C‐terminal domain (termed VP35 IID)100 provides an opportunity for further structure‐based antiviral research.101 Using in silico and NMR‐based screening methods, Brown et al.102 identified several compounds, for example, representative compound GA017 (Fig. 5), capable of binding of VP35 IID with high affinity and specificity. Some of the compounds were also shown to inhibit a replication‐competent EBOV in a cell‐based assay.102
Recent HTS of a subset of FDA‐approved drugs has reported that also antimalarials amodiaquine and chloroquine were active in vitro and in vivo against EBOV in single digit micromolar range,76, 77 but the mechanism of action was unclear. Later it was shown103 that these compounds docked favorably in VP35 suggesting they may be targeting this VP. Furthermore, chloroquine was shown to block EBOV virus like particle entry at an IC50 ∼15 μM with selectivity index SI > 32.77
Small‐molecule screening for EBOV inhibitors leads to identification of NSC 62914 (Fig. 5).104 The compound acts as a scavenger of reactive oxygen species (ROS) and it upregulates oxidative stress induced genes. ROS contribute to the pathogenesis of a wide array of diseases including viral infections. NSC 62914 was shown to inhibit EBOV, MARV, Rift Valley fever virus, Lassa virus, and Venezuelan equine encephalitis virus in cell‐based assays, and in vivo it protected mice following challenge with EBOV or MARV.
Budding of a broad range of RNA viruses is facilitated by subversion of host proteins (e.g., Nedd4) by viral PPxY late budding domains expressed within the matrix proteins of these viruses. In silico design and subsequent SAR study resulted in an identification of lead compounds 7 and 8 (Fig. 5) with ability to inhibit these critical viral–host (PPxY‐Nedd4) interactions.105 In addition, compounds 7 and 8 exhibited antibudding activity against EBOV and other RNA viruses and can thus serve as the lead structures for the development of novel broad‐spectrum antivirals.
PTAP type L domain is another domain utilized by number of RNA viruses (e.g., Junin virus, EBOV, HIV‐1) during the budding process and, thus, recently identified PTAP inhibitors, such as compound 5593–0062 and its structural analogue 4816–0013 (Fig. 5), have the potential to act as potent broad‐spectrum, host‐oriented antiviral drugs.
5. SEQUENCE‐SPECIFIC ANTIVIRAL AGENTS
RNA viruses present a good target for the rapidly advancing field of sequence‐specific therapeutics.107, 108, 109, 110 Antisense strategy usually utilize single‐stranded DNA oligonucleotides to inhibit protein production by binding to specific sites on mRNA essential for translation, or by mediating the catalytic degradation of target mRNA,111 while double‐stranded RNA oligonucleotides, known as short‐interfering RNAs (siRNAs), also mediate the catalytic degradation of complementary mRNAs. Thus, both antisense and RNAi strategies can find therapeutic applications for treatment of highly pathogenic RNA viral infections.
Phosphorodiamidate morpholino oligomers (PMOs) were designed to inhibit translation of EBOV VP35, VP24, and L transcripts.112, 113 All anti‐EBOV PMOs reported showed reduced viral titer in cell cultures and provided complete protection to rodents when administered in both pre‐ and postexposure therapeutic regimens. PMOs also protected 75% of rhesus macaques in a prophylactic regimen.113
Sarepta Therapeutics (Cambridge, MA, USA, formerly AVI BioPharma) has developed PMO containing up to five positively charged linkages (PMOplus, Fig. 6)114, 115 that have significantly improved the stability, efficacy, specificity, delivery, and safety of antisense complexes. Chemical evolution of the antisense molecules led to the discovery of two new therapeutic agents, AVI‐7537 targeting the VP24 transcript of EBOV and AVI‐7288 targeting the NP transcript of MARV.116 The VP24 protein is an inhibitor of type I interferon responses. It also forms homodimers and binds to VP35 or NP and, thus, may play an important role in the switch from viral replication to transcription, a function that is critical to the viral life cycle. Inhibition of VP24 may lead to an efficient host response to viral infection.
Figure 6.
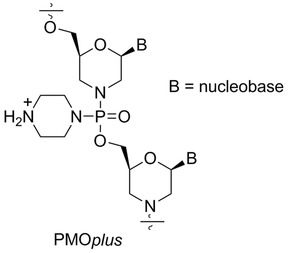
General structure of PMOplus.
Recently, Heald et al.117 evaluated the safety and pharmacokinetic properties of two combination drugs AVI‐6002 (a combination of AVI‐7537 and AVI‐7539) and AVI‐6003 (a combination of AVI‐7287 and AVI‐7288) that are under evaluation for postexposure prophylaxis of EBOV and MARV, respectively. Additional studies in nonhuman primates and humans are in progress to estimate the protective human doses.117
RNAi may also prove to be an effective and druggable therapy against filovirus infections.118, 119, 120 siRNAs targeting EBOV RNA polymerase, formulated in stable nucleic acid lipid particles (SNALPs),121 completely protected guinea pigs when administered shortly after an EBOV challenge.118 The siRNA proof‐of‐concept experiment in non‐human primates against a lethal Ebola virus infection showed 66% and full postexposure protection of rhesus monkeys and macaques, respectively.122 Although the observation of adverse events (as fever) in some subjects in a Phase I study caused TKM‐Ebola, siRNA developed by Tekmira Pharmaceuticals (Burnaby, Canada), to be placed on partial clinical hold, the FDA has still authorized its use in treating patients with confirmed or suspected EBOV infection under expanded access protocols.
6. CONCLUSIONS
The development of successful antiviral therapies to treat filovirus diseases is under way. The research programs should be facilitated by use of specific technologies and strategies,123, 124 as well as high‐throughput systems125, 126 and suitable animal models,127, 128, 129, 130 that have been recently reported. Especially, the use of laboratory animals is fundamental for the development of potent antifiloviral agents. Since guinea pigs are, for their size, less useful, newborn mice and immunodeficient adult mice represent a suitable model for preliminary testing of potential vaccines and antiviral agents.16 But namely use of non‐human primates (NHPs), in which filoviruses cause severe VHF, is crucial for the successful development of efficient anti‐EBOV treatments.16
All of the filoviral proteins (GP, L, NP, VP24, VP30, VP35, and VP40)16, 131 can potentially be chosen as a suitable target for development of druggable anti‐EBOV agents. For example, VP24 and VP35, and RNA‐dependent RNA polymerase L have been shown so far to be exploitable targets for potential antiviral therapy. It has also been demonstrated that host‐directed therapeutics, those targeting host proteins (e.g., TSG101, SAH hydrolase), represent another viable approach to combat various viral diseases. The number of small‐molecule inhibitors was shown to interfere with the filoviral entry/fusion step, but mode of action of many other inhibitors of viral replications is not yet known or fully understood.
Two potential small‐molecule antivirals were intended to be tested in human trials during the 2014 epidemic in Africa: brincidofovir (as orally bioavailable prodrug of cidofovir), an experimental drug originally developed by Chimerix to treat DNA viruses,55 and favipiravir from Toyama Chemicals, approved in Japan to treat influenza.132 These agents were considered to receive the Fast Track designation from FDA to speed up the development of potent anti‐EBOV drugs as much as possible.
Although this most frightening disease is endemic mainly to developing and third‐world African countries, the recent Ebola outbreak has triggered new drug‐ and vaccine‐development programs by a number of pharmaceutical companies, as well as by many academic research teams. It has become evident that remaining challenge for development of any VHF treatment currently is to move the most promising vaccine and drug candidates forward into human trials, so we are ready when the next Ebola outbreak strikes.
7. ABBREVIATIONS
- BDBV
Bundibugyo ebolavirus
- CMV
cytomegalovirus
- EBOV
Zaire ebolavirus or ebola
- EHF
Ebola hemorrhagic fever
- EVD
Ebola virus disease
- HTS
high‐throughput screening
- MARV
Marburgvirus
- PMO
phosphorodiamidate morpholino oligomer
- RAFI
rigid amphipathic fusion inhibitor
- RESTV
Reston ebolavirus
- SAH
S‐adenosyl‐l‐homocystein
- SAR
structure–activity relationship
- siRNA
short‐interfering RNA
- SNALP
stable nucleic acid lipid particle
- SUDV
Sudan ebolavirus
- TAFV
Taï Forest ebolavirus
- VHF
viral hemorrhagic fever
- VP
viral protein
ACKNOWLEDGMENTS
This work was supported by the subvention for development of research organization (Institute of Organic Chemistry and Biochemistry) RVO 61388963 and by Gilead Sciences, Inc. (Foster City, CA, USA).
Biography
Zlatko Janeba has been a group leader at the Institute of Organic Chemistry and Biochemistry (IOCB), Academy of Sciences of the Czech Republic in Prague since 2010. He obtained his Ph.D. in Chemistry from the same institute and the Institute of Chemical Technology Prague in 2001. He did his postdoctoral training at the Brigham Young University, Provo, Utah (Prof. M. J. Robins) and at the Northern Arizona University, Flagstaff, Arizona (Prof. P. F. Torrance). Before rejoining IOCB in 2008, he served for 3 years as principal research scientist at Moravek Biochemicals, Inc. in Brea, California. His main research interests have been the design, development, and biological evaluation of various classes of compounds with potent biological properties, with main focus on modified nucleosides and nucleotides. His research group utilizes modern synthetic and analytical methods to gain access to various types of nucleoside and nucleotide analogues with potential antiviral, antiparasitic, and immunomodulatory properties. He is a member of the International Society for Antiviral Research, International Society of Heterocyclic Chemistry, and International Society of Nucleosides, Nucleotides & Nucleic Acids.
REFERENCES
- 1. Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brister JR, Bukreyev AA, Chandran K, Davey RA, Dolnik O, Dye JM, Enterlein S, Hensley LE, Honko AN, Jahrling PB, Johnson KM, Kobinger G, Leroy EM, Lever MS, Mühlberger E, Netesov SV, Olinger GG, Palacios G, Patterson JL, Paweska JT, Pitt L, Radoshitzky SR, Saphire EO, Smither SJ, Swanepoel R, Towner JS, van der Groen G, Volchkov VE, Wahl‐Jensen V, Warren TK, Weidmann M, Nichol ST. Virus nomenclature below the species level: A standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch Virol 2013;158:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bray M. Defense against filoviruses used as biological weapons. Antiviral Res 2003;57:53–60. [DOI] [PubMed] [Google Scholar]
- 3. Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB. Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Arch Virol 2010;155:2083–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anonymous. Ebola haemorrhagic fever in Sudan, 1976 . Report of a WHO/International Study Team. Bull World Health Organ 1978;56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 5. Anonymous. Ebola haemorrhagic fever in Zaire, 1976 . Report of an International Commission. Bull World Health Organ 1978;56:271–293. [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Ebola virus disease Fact sheet No. 103. September 2014. http://www.who.int/mediacentre/factsheets/fs103/en/
- 7. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol 2014;95:1619–1624. [DOI] [PubMed] [Google Scholar]
- 8. http://www.who.int/csr/disease/ebola/situation-reports/en/
- 9. Kucharski AJ, Edmunds WJ. Case fatality rate for Ebola virus disease in west Africa. Lancet 2014;384:1260. [DOI] [PubMed] [Google Scholar]
- 10. Geisbert TW, Jahrling PB. Exotic emerging viral diseases: Progress and challenges. Nat Med 2004;10:S110–S121. [DOI] [PubMed] [Google Scholar]
- 11. Nkoghe D, Leroy EM, Toung‐Mve M, Gonzalez JP. Cutaneous manifestations of filovirus infections. Int J Dermatol 2012;51:1037–1043. [DOI] [PubMed] [Google Scholar]
- 12. West TE, von Saint André‐vonArnim A. Clinical presentation and management of severe Ebola virus disease. Ann Am Thorac Soc 2014;11:1341–1350. [DOI] [PubMed] [Google Scholar]
- 13. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011;377:849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoenen T, Groseth A, Callison J, Falzarano D, Feldmann H. Ebola virus: Unraveling pathogenesis to combat a deadly disease. Trends Mol Med 2006;12:206–215. [DOI] [PubMed] [Google Scholar]
- 15. Leyssen P, De Clercq E, Neyts J. Molecular strategies to inhibit the replication of RNA viruses. Antiviral Res 2008;78:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bray M, Paragas J. Experimental therapy of filovirus infections. Antiviral Res 2002;54:1–17. [DOI] [PubMed] [Google Scholar]
- 17. De Clercq E. A cutting‐edge view on the current state of antiviral drug development. Med Res Rev 2013;33:1249–1277. [DOI] [PubMed] [Google Scholar]
- 18. Wong G, Qiu X, Olinger GG, Kobinger GP. Post‐exposure therapy of filovirus infections. Trends Microbiol 2014;22:456–463. [DOI] [PubMed] [Google Scholar]
- 19. Friedrich BM, Trefry JC, Biggins JE, Hensley LE, Honko AN, Smith DR, Olinger GG. Potential vaccines and post‐exposure treatments for filovirus infections. Viruses 2012;4:1619–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Ying T, Yu F, Lu L, Jiang S. Development of therapeutics for treatment of Ebola virus infection. Microbes Infect 2015;17:109–117. [DOI] [PubMed] [Google Scholar]
- 21. Picazo E, Giordanetto F. Small molecule inhibitors of ebola virus infections. Drug Discov Today 2015;20;277–286. [DOI] [PubMed] [Google Scholar]
- 22. De Clercq E. Ebola virus (EBOV) infection: Therapeutic strategies. Biochem Pharmacol 2015;93:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother 2015;49:196–206. [DOI] [PubMed] [Google Scholar]
- 24. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther‐Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A, Morton J, Swope K, Bohorov O, Bohorova N, Goodman C, Kim D, Pauly MH, Velasco J, Pettitt J, Olinger GG, Whaley K, Xu B, Strong JE, Zeitlin L, Kobinger GP. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014;514:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bradfute SB, Dye JM, Bavari S. Filovirus vaccines. Hum Vaccin 2011;7:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoenen T, Groseth A, Feldmann H. Current Ebola vaccines. Expert Opin Biol Ther 2012;12:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marzi A, Feldmann H. Ebola virus vaccines: An overview of current approaches. Expert Rev Vaccines 2014;13:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graci JD, Cameron CE. Mechanism of action of ribavirin against distinct viruses. Rev Med Virol 2006;16:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad‐spectrum antiviral drug. Rev Infect Dis 1989;11:S750–S761. [DOI] [PubMed] [Google Scholar]
- 30. Huggins JW, Zhang Z, Monath TI. Inhibition of Ebola virus replication in vitro and in a SCID mouse model by S‐adenosylhomocysteine hydrolase inhibitors. Antiviral Res 1995;1:S122. [Google Scholar]
- 31. Huggins J, Zhang Z‐X, Bray M. Antiviral drug therapy of filovirus infections: S‐adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J Infect Dis 1999;179:S240–S247. [DOI] [PubMed] [Google Scholar]
- 32. Bray M, Driscoll J, Huggins JW. Treatment of lethal Ebola virus infection in mice with a single dose of an S‐adenosyl‐L‐homocysteine hydrolase inhibitor. Antiviral Res 2000;45:135–147. [DOI] [PubMed] [Google Scholar]
- 33. Montgomery JA, Clayton SJ, Thomas HJ, Shannon WM, Arnett G, Bodner AJ, Kion I‐K, Cantoni GL, Chiang PK. Carbocyclic analogue of 3‐deazaadenosine: A novel antiviral agent using S‐adenosylhomocysteine hydrolase as a pharmacological target. J Med Chem 1982;25:626–629. [DOI] [PubMed] [Google Scholar]
- 34. De Clercq E. S‐adenosylhomocysteine hydrolase inhibitors as broad‐spectrum antiviral agents. Biochem Pharmacol 1987;36:2567–2575. [DOI] [PubMed] [Google Scholar]
- 35. De Clercq E. Carbocyclic adenosine analogues as S‐adenosylhomocysteine hydrolase inhibitors and antiviral agents: Recent advances. Nucleosides Nucleotides 1998;17:625–634. [DOI] [PubMed] [Google Scholar]
- 36. De Clercq E. John Montgomery's Legacy: Carbocyclic adenosine analogues as SAH hydrolase inhibitors with broad‐spectrum antiviral activity. Nucleosides Nucleotides Nucleic Acids 2005;24:1395–1415. [DOI] [PubMed] [Google Scholar]
- 37. Bray M, Raymond JL, Geisbert T, Baker RO. 3‐Deazaneplanocin A induces massively increased interferon‐α production in Ebola virus‐infected mice. Antiviral Res 2002;55:151–159. [DOI] [PubMed] [Google Scholar]
- 38. De Clercq E. Antivirals and antiviral strategies. Nat Rev Microbiol 2004;2:704–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye W, Schneller SW. The enantiomers of the 1’,6’‐isomer of neplanocin A: Synthesis and antiviral properties. Bioorg Med Chem 2014;22:5315–5319. [DOI] [PubMed] [Google Scholar]
- 40. St. Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, Korshun VA, Tyrrell DLJ, Schang LM. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc Natl Acad Sci USA 2010;107:17339–17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. Protection against filovirus diseases by a novel broad‐spectrum nucleoside analogue BCX4430. Nature 2014;508:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kicska GA, Long L, Hörig H, Fairchild C, Tyler PC, Furneaux RH, Schramm VL, Kaufman HL. Immucillin H, a powerful transition‐state analog inhibitor of purine nucleoside phosphorylase, selectively inhibits human T lymphocytes. Proc Natl Acad Sci USA 2001;98:4593–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schramm V. Transition states, analogues, and drug development. ACS Chem Biol 2013;8:71−81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Julander JG, Bantia S, Taubenheim BR, Minning DM, Kotian P, Morrey JD, Smee DF, Sheridan WP, Babub YS. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a hamster model. Antimicrob Agents Chemother 2014;58:6607−6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. http://investor.shareholder.com/biocryst/releasedetail.cfm?ReleaseID=887581
- 46. Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti‐infect Ther 2014;12:1171–1178. [DOI] [PubMed] [Google Scholar]
- 47. Tollefson AE, Spencer JF, Ying B, Buller RML, Wold WSM, Toth K. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antivir Res 2014;112:38–46. [DOI] [PubMed] [Google Scholar]
- 48. Sandkovsky U, Vargas L, Florescu DF. Adenovirus: Current epidemiology and emerging approaches to prevention and treatment. Curr Infect Dis Rep 2014;16:416–423. [DOI] [PubMed] [Google Scholar]
- 49. Olson VA, Smith SK, Foster S, Li Y, Randall Lanier E, Gates I, Trost LC, Damona IK. In vitro efficacy of Brincidofovir against Variola virus. Antimicrob Agents Chemother 2014;58:5570–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Griffiths P, Lumley S. Cytomegalovirus. Curr Opin Infect Dis 2014;27:554–559. [DOI] [PubMed] [Google Scholar]
- 51. De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res 1987;8:261–272. [DOI] [PubMed] [Google Scholar]
- 52. De Clercq E, Holý A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat Rev Drug Discov 2005;4:928–940. [DOI] [PubMed] [Google Scholar]
- 53. Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RML, Painter GR, Wold WSM. Hexadecyloxypropyl‐cidofovir, CMX001, prevents adenovirus‐induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA 2008;105:7293–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, Brundage TM, Robertson AT, Godkin S, Momméja‐Marin H, Boeckh M. CMX001 to prevent cytomegalovirus disease in hematopoietic‐cell transplantation. New Engl J Med 2013;369:1227–1236. [DOI] [PubMed] [Google Scholar]
- 55. Chimerix . Chimerix announces emergency investigational new drug applications for brincidofovir authorized by FDA for patients with Ebola virus disease. Available at http://ir.chimerix.com/releasedetail.cfm?releaseid=874647 (accessed October 7, 2014).
- 56. Günther S, Asper M, Röser C, Luna LKS, Drosten C, Becker‐Ziaja B, Borowski P, Chen, H‐M , Hosmane RS. Application of real‐time PCR for testing antiviral compounds against Lassa virus, SARS coronavirus and Ebola virus in vitro. Antiviral Res 2004;63:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD. T‐705 (favipiravir) and related compounds: Novel broad‐spectrum inhibitors of RNA viral infections. Antiviral Res 2009:82:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T‐705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013;100:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti‐influenza virus compound T‐705. Antimicrob Agents Chemother 2002;46:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Furuta Y, Takahashi K, Kuno‐Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T‐705 against influenza virus. Antimicrob Agents Chemother 2005;49:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Post‐exposure efficacy of oral T‐705 (favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res 2014;104:153–155. [DOI] [PubMed] [Google Scholar]
- 62. Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz‐Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T‐705 (favipiravir) in a small animal model. Antiviral Res 2014;105:17–21. [DOI] [PubMed] [Google Scholar]
- 63. Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous basepairing and high substrate efficiency of T‐705 (favipiravir) ribofuranosyl 5′‐triphosphate towards influenza A virus polymerase. PLoS One 2013;8:e68347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baranovich T, Wong S‐S, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA. T‐705 (Favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013;87:3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 2011;477:340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. Small molecule inhibitors reveal Niemann–Pick C1 is essential for Ebola virus infection. Nature 2011;477:344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wolf MC, Freiberg AN, Zhang T, Akyol‐Ataman Z, Grock A, Hong PW, Li J, Watsona NF, Fang AQ, Aguilar HC, Porotto M, Honko AN, Damoiseaux R, Miller JP, Woodson SE, Chantasirivisal E, Fontanes V, Negrete OA, Krogstad P, Dasgupta A, Moscona A, Hensley LE, Whelan SP, Faull KF, Holbrook MR, Jung ME, Lee B. A broad‐spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci USA 2010;107:3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vigant F, Lee J, Hollmann A, Tanner LB, Akyol‐Ataman Z, Yun T, Shui G, Aguilar HC, Zhang D, Meriwether D, Roman‐Sosa G, Robinson LR, Juelich TL, Buczkowski H, Chou S, Castanho MARB, Wolf MC, Smith JK, Banyard A, Kielian M, Reddy S, Wenk MR, Selke M, Santos NC, Freiberg AN, Jung ME, Lee B. A mechanistic paradigm for broad‐spectrum antivirals that target virus‐cell fusion. PLoS Pathog 2013;9:e1003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Basu A, Li B, Mills DM, Panchal RG, Cardinale SC, Butler MM, Peet NP, Majgier‐Baranowska H, Williams JD, Patel I, Moir DT, Bavari S, Ray R, Farzan MR, Rong L, Bowlin TL. Identification of a small‐molecule entry inhibitor for filoviruses. J Virol 2011;85:3106–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johnson JC, Martinez O, Honko AN, Hensley LE, Olinger GG, Basler CF. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Antiviral Res 2014;107:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yermolina MV, Wang J, Caffrey M, Rong LL, Wardrop DJ. Discovery, synthesis, and biological evaluation of a novel group of selective inhibitors of filoviral entry. J Med Chem 2011;54:765–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yonezawa A, Cavrois M, Greene WC. Studies of ebola virus glycoprotein‐mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: Involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol 2005;79:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Elshabrawy HA, Fan J, Haddad CS, Ratia K, Broder CC, Caffrey M, Prabhakar BS. Identification of a broad‐spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high‐throughput screening assay. J Virol 2014;88:4353–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nierengarten I, Nierengarten J‐F. Fullerene sugar balls: A new class of biologically active fullerene derivatives. Chem Asian J 2014;9:1436–1444. [DOI] [PubMed] [Google Scholar]
- 75. Luczkowiak J, Muñoz A, Sánchez‐Navarro M, Ribeiro‐Viana R, Ginieis A, Illescas BM, Martín N, Delgado R, Rojo J. Glycofullerenes inhibit viral infection. Biomacromolecules 2013;14:431–437. [DOI] [PubMed] [Google Scholar]
- 76. Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, Green CE, Iyer LV, Dilks DD, Davey RA, Kolokoltsov AA, Jr Carrion R, , Patterson JL, Bavari S, Panchal RG, Warren TK, Wells JB, Moos WH, Burke RL, Tanga MJ. A systematic screen of FDA‐approved drugs for inhibitors of biological threat agents. PLoS One 2013;8:e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kouznetsova J, Sun W, Martínez‐Romero C, Tawa G, Shinn P, Chen CZ, Schimmer A, Sanderson P, McKew JC, Zheng W, García‐Sastre A. Identification of 53 compounds that block Ebola virus‐like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect 2014;3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Veljkovic V, Loiseau PM, Figadere B, Glisic S, Veljkovic N, Perovic VR, Cavanaugh DP, Branch DR. Virtual screen for repurposing approved and experimental drugs for candidate inhibitors of EBOLA virus infection. F1000Research 2015;4;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adams CE, Rathbone J, Thornley B, Clarke M, Borrill J, Wahlbeck K, Awad AG. Chlorpromazine for schizophrenia: A Cochrane systematic review of 50 years of randomised controlled trials. BMC Med 2005;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bhattacharyya S, Warfield KL, Ruthel G, Bavari S, Aman MJ, Hope TJ. Ebola virus uses clathrin mediated endocytosis as an entry pathway. Virol 2010; 401:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, Evans DeWald L, Schornberg KL, Scully C, Lehár J, Hensley LE, White JM, Olinger GG. FDA‐approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 2013;5:190ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shoemaker CJ, Schornberg KL, Delos SE, Scully C, Pajouhesh H, Olinger GG, Johansen LM, White JM. Multiple cationic amphiphiles induce a Niemann‐Pick C phenotype and inhibit ebola virus entry and infection. PLoS One 2013;8:e56265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, Dahlmann F, Pöhlmann S, Vondran FWR, David S, Manns MP, Ciesek S, von Hahn T. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother 2014;69:2123–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh B. Practical guidelines for clinicians who treat patients with amiodarone. Practice Guidelines Subcommittee, North American Society of Pacing and Electrophysiology. Arch Intern Med 2000;160:1741–1748. [DOI] [PubMed] [Google Scholar]
- 85. Warren TK, Warfield KL, Wells J, Enterlein S, Smith M., Ruthel G, Yunus AS, Kinch MS, Goldblatt M, Aman MJ, Bavari S. Antiviral activity of a small‐molecule inhibitor of filovirus infection. Antimicrob Agents Chemother 2010;54:2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aman MJ, Kinch MS, Warfield K, Warren T, Yunus A, Enterlein S, Stavale E, Wang P, Chang S, Tang O, Porter K, Goldblatt M, Bavari S. Development of a broad‐spectrum antiviral with activity against Ebola virus. Antivir Res 2009;83:245–251. [DOI] [PubMed] [Google Scholar]
- 87. Lee SMY, Yen HL. Targeting the host or the virus: Current and novel concepts for antiviral approaches against influenza virus infection. Antivir Res 2012;96:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Opsenica I, Burnett JC, Gussio R, Opsenica D, Todorovic N, Lanteri CA, Sciotti RJ, Gettayacamin M, Basilico N, Taramelli D, Nuss JE, Wanner L, Panchal RG, Šolaja BA, Bavari S. A chemotype that inhibits three unrelated pathogenic targets: The botulinum neurotoxin serotype a light chain, p. Falciparum malaria, and the ebola filovirus. J Med Chem 2011;54:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Selaković Ž, Opsenica D, Eaton B, Retterer C, Bavari S, Burnett JC, Šolaja BA, Panchal RG. A limited structural modification results in a significantly more efficacious diazachrysene‐based filovirus inhibitor. Viruses 2012;4:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kinch MS, Yunus AS, Lear C, Mao H, Chen H, Fesseha Z, Luo G, Nelson EA, Li L, Huang Z, Murray M, Ellis WY, Hensley L, Christopher‐Hennings J, Olinger GG, Goldblatt M. FGI‐104: A broad‐spectrum small molecule inhibitor of viral infection. Am J Transl Res 2009;1:87–98. [PMC free article] [PubMed] [Google Scholar]
- 91. Harty RN. No exit: Targeting the budding process to inhibit filovirus replication. Antiviral Res 2009;81:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu Y, Lee MS, Olson MA, Harty RN. Bimolecular complementation to visualize filovirus VP40‐host complexes in live mammalian cells: Toward the identification of budding inhibitors. Adv Virol 2011;341816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kinch M, Goldblatt M. Methods of inhibiting viral infection. 2009, WO 2009/091435 A2.
- 94. Kesel AJ. Broad‐spectrum antiviral activity including human immunodeficiency and hepatitis C viruses mediated by a novel retinoid thiosemicarbazone derivative. Eur J Med Chem 2011;46:1656–1664. [DOI] [PubMed] [Google Scholar]
- 95. Kesel AJ, Huang Z, Murray MG, Prichard MN, Caboni L, Nevin DK, Fayne D, Lloyd DG, Detorio MA, Schinazi RF. Retinazone inhibits certain bloodborne human viruses including Ebola virus Zaire. Antivir Chem Chemother 2014;23:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang J, Wang L, Ma D, Qu X, Guo H, Xu X, Mason PM, Bourne N, Moriarty R, Gu B, Guo JT, Block TM. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob Agents Chemother 2009;53:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chang J, Schul W, Butters TD, Yip A, Liu B, Goh A, Lakshminarayana SB, Alonzi D, Reinkensmeier G, Pan X, Qu X, Weidner JM, Wang L, Yu W, Borune N, Kinch MA, Rayahin JE, Moriarty R, Xu X, Shi PY, Guo JT, Block TM. Combination of alpha‐glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral Res 2011;89:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang J, Schul W, Yip A, Xu X, Guo JT, Block TM. Competitive inhibitor of cellular alpha‐glucosidases protects mice from lethal dengue virus infection. Antiviral Res 2011;92:369–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chang J, Warren TK, Zhao X, Gill T, Guo F, Wanga L, Comunale MA, Duc Y, Alonzi DS, Yu W, Ye H, Liu F, Guo J‐T, Mehta A, Cuconati A, Butters TD, Bavari S, Xu X, Block TM. Small molecule inhibitors of ER alpha‐glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res 2013;98:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, Amarasinghe GK. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci USA 2009;106:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Leung DW, Prins KC, Basler CF, Amarasinghe GK. Ebolavirus VP35 is a multifunctional virulence factor. Virulence 2010;1:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brown CS, Lee MS, Leung DW, Wang T, Xu W, Luthra P, Anantpadma M, Shabman RS, Melito LM, MacMillan KS, Borek DM, Otwinowski Z, Ramanan P, Stubbs AJ, Peterson DS, Binning JM, Tonelli M, Olson MA, Davey RA, Ready JM, Basler CF, Amarasinghe GK. In silico derived small molecules bind the filovirus VP35 protein and inhibit its polymerase cofactor activity. J Mol Biol 2014;426:2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ekins S, Freundlich JS, Coffee M. A common feature pharmacophore for FDA‐approved drugs inhibiting the Ebola virus. F1000Research 2014;3;277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Panchal RG, Reid SP, Tran JP, Bergeron AA, Wells J, Kota KP, Aman J, Bavari S. Identification of an antioxidant small‐molecule with broad‐spectrum antiviral activity. Antiviral Res 2012;93:23–29. [DOI] [PubMed] [Google Scholar]
- 105. Han Z, Lu J, Liu Y, Davis B, Lee MS, Olson MA, Ruthel G, Freedman BD, Schnell MJ, Wrobel JE, Reitz AB, Harty RN. Small‐molecule probes targeting the viral PPxY‐host Nedd4 interface blco egress of a broad range of RNA viruses. J Virol 2014;88:7294–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu J, Han Z, Liu Y, Liu W, Lee MS, Olson MA, Ruthel G, Freedmen BD, Harty RN. A host‐oriented inhibitor of Junin Argentine hemorrhagic fever virus egress. J Virol 2014;88:4736–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dias N, Stein CA. Antisense oligonucleotides: Basic concepts and mechanisms. Mol Cancer Ther 2002;1:347–355. [PubMed] [Google Scholar]
- 108. Spurgers KB, Sharkey CM, Warfield KL, Bavari S. Oligonucleotide antiviral therapeutics: Antisense and RNA interference for highly pathogenic RNA viruses. Antiviral Res 2008;78:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jain ML, Bruice PY, Szabó IE, Bruice TC. Incorporation of positively charged linkages into DNA and RNA backbones: A novel strategy for antigene and antisense agents. Chem Rev 2012;112:1284–1309. [DOI] [PubMed] [Google Scholar]
- 110. Sahin U, Karikó K, Türeci Ö. mRNA‐based therapeitics—Developing a new class of drugs. Nat Rev Drug Discov 2014;13:759–780. [DOI] [PubMed] [Google Scholar]
- 111. Chan JH, Lim S, Wong WS. Antisense oligonucleotides: From design to therapeutic application. Clin Exp Pharmacol Physiol 2006;33:533–540. [DOI] [PubMed] [Google Scholar]
- 112. Warfield KL, Swenson DL, Olinger GG, Nichols DK, Pratt WD, Blouch R, Stein DA, Aman MJ, Iversen PL, Bavari S. Gene‐specific countermeasures against Ebola virus based on antisense phosphorodiamidate morpholino oligomers. PLoS Pathog 2006;2:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Enterlein S, Warfield KL, Swenson DL, Stein DA, Smith JL, Gamble CS, Kroeker AD, Iversen PL, Bavari S, Muhlberger E. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob Agents Chemother 2006;50:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med 2010;16:991–994. [DOI] [PubMed] [Google Scholar]
- 115. Warren TK, Shurtleff AC, Bavari S. Advanced morpholino oligomers: A novel approach to antiviral therapy. Antiviral Res 2012;94:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, Panchal RG, Bavari S. Discovery and early development of AVI‐7537 and AVI‐7288 for the treatment of Ebola virus and Marburg virus infections. Viruses 2012;4:2806–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Heald AE, Iversen PL, Saoud JB, Sazani P, Charleston JS, Axtelle T, Wong M, Smith WB, Vutikullird A, Kaye E. Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against Ebola virus and Marburg virus: Results of two single‐ascending‐dose studies. Antimicrob Agents Chemother 2014;58:6639–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fowler T, Bamberg S, Möller P, Klenk H‐D, Meyer TF, Becker S, Rudel T. Inhibition of Marburg virus protein expression and viral release by RNA interference. J Gen Virol 2005;86:1181–1188. [DOI] [PubMed] [Google Scholar]
- 119. Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario‐Dicaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee ACH, Judge A, Jeffs LB, MacLachlan I. Postexposure protection of guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J Infect Dis 2006;193:1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thi EP, Mire CE, Ursic‐Bedoya R, Geisbert JB, Lee ACH, Agans KN, Robbins M, Deer DJ, Fenton KA, MacLachlan I, Giesbert TW. Marburg virus infection in nonhuman primates: Therapeutic treatment by lipid‐encapsulated siRNA. Sci Transl Med 2014;6:250ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti‐HBV activity of chemically modified siRNAs. Nat Biotechnol 2005;23:1002–1007. [DOI] [PubMed] [Google Scholar]
- 122. Geisbert TW, Lee ACH, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, MacLachlan I. Postexposure protection of non‐human primates against a lethal Ebola virus challenge with RNA interference: A proof‐of‐concept study. Lancet 2010;375:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Paragas J, Geisbert TW. Development of treatment strategies to combat Ebola and Marburg viruses. Expert Rev Anti Infect Ther 2006;4:67–76. [DOI] [PubMed] [Google Scholar]
- 124. Bausch DG, Sprecher AG, Jeffs B, Boumandouki P. Treatment of Marburg and Ebola hemorrhagic fevers: A strategy for testing new drugs and vaccines under outbreak conditions. Antiviral Res 2008;78:150–161. [DOI] [PubMed] [Google Scholar]
- 125. Hoenen T, Groseth A, Callison J, Takada A, Feldmann H. A novel Ebola virus expressing luciferase allows for rapid and quantitative testing of antivirals. Antiviral Res 2013;99:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Uebelhoer LS, Albariño CG, McMullan LK, Chakrabarti AK, Vincent JP, Nichol ST, Towner JS. High‐throughput, luciferase‐based reverse genetics systems for identifying inhibitors of Marburg and Ebola viruses. Antiviral Res 2014;106:86–94. [DOI] [PubMed] [Google Scholar]
- 127. Gowen BB, Holbrook MR. Animal models of highly pathogenic RNA viral infections: Hemorrhagic fever viruses. Antiviral Res 2008;78:79–90. [DOI] [PubMed] [Google Scholar]
- 128. Bradfute SB, Warfield KL, Bray M. Mouse models for Filovirus infections. Viruses 2012;4:1477–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections. Front Microbiol 2013;4:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Safronetz D, Geisbert TW, Feldmann H. Animal models for highly pathogenic emerging viruses. Curr Opin Virol 2013;3:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Feldmann H, Sanchez A, Geisbert TW. Filoviridae: Marburg and ebola viruses In: Knipe DM, Howley PM, Eds. Fields Virology, 6th ed Philadelphia: Lippincott, Williams and Wilkins; 2013. p 923–956. [Google Scholar]
- 132.Médecins Sans Frontiéres. http://www.msf.org/article/clinical-trial-potential-ebola-treatment-started-msf-clinic-guinea.


