Abstract
This review is the third update of the original review, published in 1999, on the application of matrix‐assisted laser desorption/ionization (MALDI) mass spectrometry to the analysis of carbohydrates and glycoconjugates and brings the topic to the end of 2004. Both fundamental studies and applications are covered. The main topics include methodological developments, matrices, fragmentation of carbohydrates and applications to large polymeric carbohydrates from plants, glycans from glycoproteins and those from various glycolipids. Other topics include the use of MALDI MS to study enzymes related to carbohydrate biosynthesis and degradation, its use in industrial processes, particularly biopharmaceuticals and its use to monitor products of chemical synthesis where glycodendrimers and carbohydrate–protein complexes are highlighted. © 2009 Wiley Periodicals, Inc., Mass Spec Rev 28:273–361, 2009
Keywords: MALDI, carbohydrates, glycoproteins, glycolipids, fragmentation, biopharmaceuticals, glycosyltransferases, glycosidases, time‐of‐flight
INTRODUCTION
This review is a continuation of the three earlier ones in this series on the application of matrix‐assisted laser desorption/ionization (MALDI) mass spectrometry to the analysis of carbohydrates and glycoconjugates (Harvey, 1999, 2006, 2008) and brings the coverage of the literature to the end of 2004. The review is intended to illustrate both developments in technology and in the way in which analysis of carbohydrates contributes to scientific knowledge in general.
Matrix‐assisted laser desorption/ionization (MALDI) continues to be a major technique for the analysis of carbohydrates although electrospray, particularly with quadrupole‐time‐of‐flight (Q‐TOF) instruments, is becoming increasingly popular. Advantages of the MALDI‐Q‐TOF combination for proteomics and glycomics have been stressed in a brief focus paper (Huang, 2003). Figure 1 shows the year‐by‐year increase in papers reporting use of MALDI MS for carbohydrate analysis for the period 1990–2004. As the review is designed to complement the earlier work, structural formulae, etc. that were presented earlier are not repeated. However, a citation to the structure in the earlier works is indicated by its number with a prefix (i.e., 1/x refers to structure x in the first review and 2/x to the second). Other reviews and review‐type articles directly concerned with, or including MALDI analysis of glycoconjugates that have been published during the review period include general reviews by Harvey (2003), Stuhler and Meyer (2004), and Zaia (2004), a review of capillary electrophoresis‐mass spectrometry for glycoscreening (Zamfir & Peter‐Katalinic, 2004) and a review on new methods for mass spectrometry of bioorganic macromolecules (Cristoni & Bernsrdi, 2003). More specific reviews include those on the glycosylation of Caenorhabditis elegans (Haslam & Dell, 2003), characterization of substituent distribution in starch and cellulose derivatives (Richardson & Gorton, 2003), derivatization of carbohydrates for chromatographic, electrophoretic, and mass spectral structural analysis (Lamari, Kuhn, & Karamanos, 2003), structure of bacterial lipopolysaccharides (Caroff & Karibian, 2003), analysis of post‐translational modifications (Cantin & Yates, 2004; Jensen, 2004; Seo & Lee, 2004), the use of MALDI MS to detect enantioselectivity in gas‐phase ion‐molecule reactions with carbohydrates such as cyclodextrins (Speranza, 2004), synthesis of heparan and heparin sulfate fragments (Poletti & Lay, 2003), analysis of protein glycation products (Horvat & Jakas, 2004; Kislinger, Humeny, & Pischetsrieder, 2004), carbohydrate biosensors (Jelinek & Kolusheva, 2004), synthesis and discovery of oligosaccharides and glycoconjugates for the treatment of disease (Macmillan & Daines, 2003), dendrimers in drug research (Boas & Heegaard, 2004), combinatorial carbohydrate synthesis (Baytas & Linhardt, 2004), chemical tagging strategies for proteome analysis (Leitner & Lindner, 2004), capillary electrophoresis of biopharmaceutical products (Kakehi, Kinoshita, & Nakano, 2002) and the use of mass spectrometry to study congenital disorders of glycosylation type IIx (Mills et al., 2003b).
Figure 1.

Yearly totals of the number of publications containing work relating to the application of MALDI mass spectrometry to carbohydrates and glycoconjugates.
INSTRUMENTATION
MALDI Interfaces to Trapped Ion Instruments
A comparison between spectra obtained with a conventional quadrupole ion trap (QIT) (electrospray ionization) and a MALDI‐QIT‐reflectron‐TOF instrument has shown that the latter instrument produces significant amounts of metastable fragmentation due to the longer (msec) time span of the QIT trapping process, an observation also made by Harvey et al. (2004) with released N‐glycans. Glycopeptides were completely desialylated in the process. Fragmentation (MS2) of glycopeptides produced fragments exclusively from the carbohydrate with the ESI‐QIT instrument with peptide fragmentation occurring in MS3, whereas the MALDI‐QIT‐reflectron‐TOF instrument yielded both types of fragmentation at the MS2 stage (Demelbauer et al., 2004).
High‐Pressure and Atmospheric Pressure MALDI (AP‐MALDI)
Coupling of both vacuum and atmospheric pressure MALDI (AP‐MALDI) ion sources with ion‐trap instruments has been reviewed (Moyer et al., 2003) and the latter technique investigated as a method for obtaining fragmentation spectra from carbohydrates. High‐pressure ion sources have been developed to cool ions in an attempt to reduce or eliminate fragmentation occurring either in‐source or post‐source. Such fragmentation is the primary factor limiting the use of vacuum MALDI for analysis of acidic glycans and can be considerably reduced with a high‐pressure ion source but at the cost of diminished sensitivity. Arabinosazone (1/40) has been used as the matrix for maltohexaose (α‐d‐Glcp‐(α‐d‐Glcp‐)4‐(1 → 4)‐α‐d‐Glcp, 1) in positive ion mode ([M + Na]+ ion) and for 6′‐sialyllactose (2) in negative mode ([M−H]−). The spectrum of the latter compound contained two abundant fragments, an O,2A3 and a C‐ion (Domon and Costello (1988) nomenclature, see first review, Scheme 3) whereas the major glycosidic fragments in the positive ion spectrum were produced by B or Y cleavages.


Because of the near physiological conditions attainable at atmospheric pressure with glycerol as the matrix, it has been possible to observe non‐covalent complexes between carbohydrates and synthetic peptides that were designed to mimic the binding sites of three members of the siglec family. 3′‐ (3) and 6′‐sialyllactose (2), were used as the carbohydrate ligands with 3‐sialyllactose maintaining its binding specificity for sialoadhesin (Von Seggern & Cotter, 2004). A new AP‐MALDI technique named atmospheric pressure infrared ionization from solution (AP‐IRIS) that is claimed to be 16 times a sensitive as AP UV‐MALDI has been implemented on a QIT mass spectrometer (Tan et al., 2004). The method operated in the absence of an extraction field and used a high power IR laser with aqueous glycerol as the matrix. Spectra consisting of [M + Na]+ ions were obtained from several high‐mannose and complex N‐linked glycans with much better signal/noise ratios than with corresponding amounts ionized by UV‐MALDI from 2,4,6‐trihydroxyacetophenone (THAP, 1/44).

Other Developments in Instrumentation and Techniques
Matrix‐assisted laser desorption/ionization (MALDI) plates sprayed with the hydrophobic 3M product, ScotchGard™, a water repellent material for coating fabrics, has the effect of reducing the MALDI spot size and improving sensitivity (Owen et al., 2003). For cleaning, the coating could easily be removed with methyl‐t‐butyl ether and the film reapplied to the clean plate. Experiments with the carbohydrate‐containing antibiotic, erythromycin A (4), showed increases in sensitivity of two‐ to threefold when ionized from the coated targets compared with signals from uncoated plates.

A microdeposition device has been constructed which mixes effluent from capillary electrochromatography or microcolumn liquid chromatography with the MALDI matrix and deposits the mixture onto the MALDI plate. Dextrin and N‐linked glycans from ribonuclease B were successfully analyzed (Tegeler et al., 2004). An array of 96 perforated nanovials manufactured by silicon microfabrication and filled with 40 nL of reversed‐phase beads has been developed as a MALDI target for peptide analysis. It was successfully used to examine peptides and glycopeptides from prostate‐specific antigen (Ekström et al., 2004).
Liquid chromatography‐mass spectrometry (LC‐MS) has the disadvantage that the entire sample is consumed after exiting the HPLC column. Lochnit and Geyer (2004) have used nano‐LC‐MALDI‐TOF MS to examine tryptic peptides and glycopeptides, a technique that allows more detailed MS investigation of the effluent to be made. The technique also produced enhanced detection of glycopeptides in the presence of peptides and improved characterization of the attached glycans by optimizing the experimental conditions.
MATRICES
Theory of Matrix Action
Investigations into the mechanism of proton transfer from dihydroxybenzoic acid (DHB) isomers continues. Yassin and Marynick (2003) have calculated the gas‐phase acidities of the radical cations of all six dihydroxybenzoic acid (DHB) isomers using density functional theory and found excellent agreement with experimental measurements. The 2,5‐isomer (1/26), the most effective matrix, was the least acidic (in this review the abbreviation DHB is used for the 2,5‐isomer, unless stated otherwise). The results indicate that, as proposed earlier (Gimon et al., 1992), deprotonation occurs at the phenolic rather than the acidic site as the result of resonance stabilization of the resulting ion. This conclusion is consistent with the theory proposed by Harvey (1993) that one of the reasons that the 2,5‐isomer is so effective a matrix is that it is the only one that can form a p‐benzoquinone‐type (5) structure after photochemical decarboxylation.

Sugars, however generally ionize as [M + Na]+ ions for which the factors governing formation are much less clear. A recent study by Antonopoulos et al. (2003) on the mechanism of attachment of sodium to glucose and its methylated derivatives found no simple correlation between ion yield and factors such as volatility and hydrophobicity. A study by Luxembourg et al. (2003) using the high resolution capability of matrix‐assisted secondary ion mass spectrometry has demonstrated crystal inhomogeneity among DHB crystals on MALDI targets. Some crystals were free of sodium whereas others contained sodium and sample. Yet other areas of the target contained mostly sodium and little matrix. The results were thought to explain the phenomenon of “sweet spots” frequently observed with MALDI targets.
Proton‐affinity values for 15 common matrices have been measured by a kinetic method. α‐Cyano‐4‐hydroxycinnamic acid (CHCA, 1/23) and DHB showed the lowest affinity whereas 2‐(4′‐hydroxyphenyl)azobenzoic acid (HABA, 1/32) and nor‐harmane (1/35) had the highest affinity. In general, the rank order was similar to that found by other investigators. With β‐cyclodextrin (6, cyclic‐(1 → 4)‐α‐d‐glucose)7 as the reference compound in negative ion mode, matrices with low proton affinity, such as DHB (204.4 ± 0.17), produced no [M−H]− ions whereas those with high affinity, such as nor‐harmane (233.0 ± 0.44), did (Mirza, Raju, & Vairamani, 2004b). Gas‐phase potassium binding energies of several MALDI matrices have also been published (Zhang et al., 2003b).

Fournier et al. (2003) have measured the ablation volume of DHB crystals under different laser powers and found that, although the ablation volume increased only slowly with increasing laser power, the signal rose dramatically. They interpreted their results as involving a very rapid ablation to produce a plume that was further ionized by the incoming laser beam. Frankevich et al. (2003) have discussed the role of photoelectrons on MALDI sensitivity. Suppression of photoelectrons with a film of insulating material on the target; the authors used Scotch Magic Tape; was found to produce an increase of up to two orders of magnitude in sensitivity and an improvement in resolution.
Simple Matrices
Bashir, Mutter, and Derrick (2003b) have investigated long‐chain esters of DHB as matrices and found some improvement in performance, particularly after the addition of iodine to those compounds that contained a double bond in the chain. The mechanism producing this enhancement in performance by the addition of iodine was unclear but the reason for the improved performance with some of the long‐chain compounds was thought to be the formation of micelles that enhanced the matrix–analyte interactions.
5‐Amino‐2‐mercapto‐1,3,4‐thiadiazole (AMT, 7) has been described as a new matrix for carbohydrates (Mirza et al., 2004a). Its performance was similar to that of DHB although slightly higher‐mass compounds could be ionized from dextran‐5000. Kéki et al. (2004) have ionized simple organic molecules such as a peracetylated isoflavone glycoside (8) from common matrices such as DHB doped with silver trifluoroacetate to give [M + Ag3]+ species. These ions were shown to be more stable than the corresponding [M + Ag]+ ions. The latter ions and their Cu+ counterparts, fragmented in a similar manner to those from Na+ and Li+ (Kéki et al., 2004). Carbon nanotubes, prepared from coal by an arc discharge have been reported as a novel matrix for low molecular weight peptides and β‐cyclodextrin (Xu et al., 2003). Spectra showed a very low background as the result of the absence of matrix peaks and ions produced by fragmentation. To obtain the spectrum, a well‐dispersed ethanolic solution of nanotubes was deposited onto the MALDI target and allowed to dry. The sample solution was then deposited onto the nanotubes and dried with hot air. Two papers reporting the use of fine cobalt powder for the analysis of lepidimoic acid (9) from okra pectic polysaccharide (Hirose et al., 2003) and of lepidimoide (10) from okra mucilage have appeared (Hirose, Endo, & Hasegawa, 2004).




Binary Matrices
A mixture of DHB and CHCA has been shown to give enhanced performance for the ionization of glycopeptides than use of the individual matrices. The mixed matrix showed better tolerance towards the presence of salts and impurities and improved peptide sequence coverage (Laugesen & Roepstorff, 2003). A thin layer of very homogeneous crystals has been produced by mixing THAP with nitrocellulose and used to analyze pectin digests (Mohamed, Christensen, & Mikkelsen, 2003).
Liquid Matrices
Atmospheric pressure MALDI (AP‐MALDI) has the advantage over vacuum MALDI of collisional stabilization of fragile molecules such as carbohydrates containing sialic acid residues. The technique is also compatible with the use of liquid matrices whose volatility presents problems under vacuum conditions. Von Seggern, Moyer, and Cotter (2003a) have compared water, glycerol, and nitrobenzyl alcohol (1/21) for ionization of sialylated glycans with an IR laser (Er:YAG, 2,490 nm) and have found that glycerol provides the longest lasting signal with the best signal/noise ratio. Water was too volatile to survive for long under the heat produced by the laser. Intact sialylated glycans were desorbed as [M−H]− and [M + Na]+ ions under negative and positive conditions respectively. In a further study (Von Seggern, Zarek, & Cotter, 2003b), the glycerol matrix was doped with alkali (Li, Na, K) alkaline earth (Ca) or transition metal cations (Ca, Co) for production of adducts containing one or two singly charged cation or one doubly charged cation. Adduction proved to be efficient with lithium or potassium being able to replace the more common sodium adduction when salts (Cl− or I−) containing these metals were used as the doping agents. Cobalt adduction was achieved by use of cobalt powder added to the glycerol. Fragmentation (see below) was obtained with an ion‐trap instrument.
The use of glycerol as the matrix with an IR laser under atmospheric pressure conditions has also enabled non‐covalent sugar–sugar complexes to be observed (Von Seggern & Cotter, 2003). The stability of the complexes varied with different sugars potentially due to the strengths of the hydrogen bonding networks. Fragmentation of the complexes was also a function of structure; some complexes, particularly those containing sialic acid, fragmented by loss of the acid before breakdown of the complex whereas complexes between neutral molecules tended preferentially to dissociate into monomer units.
Ionic Liquid Matrices
Ionic liquid matrices appear to show great promise for carbohydrate analysis. The matrix DHB‐butylamine (DHBB) was reported to give improved shot–shot reproducibility compared with solid matrices such as DHB, and reduced the relative standard deviation by 50%. It also produced much less fragmentation from compounds such as sialylated carbohydrates (Mank, Stahl, & Boehm, 2004). Greatly improved quantitative performance has also been reported by Zabet‐Moghaddam, Heinzle, and Tholey (2004).
DERIVATIVES
Derivatization techniques for analysis of carbohydrates have been reviewed (Gao et al., 2003; Suzuki et al., 2003b).
Reducing‐Terminal Derivatives
Reducing‐Terminal Derivatives Prepared by Reductive Amination
Advantages of analytical derivatization have been emphasized by Rosenfeld (2003) with, among others, examples from the reductive amination of carbohydrates for improving the interpretation of fragmentation spectra and for modifying behavior in chromatographic systems. MALDI is frequently used to monitor reducing‐terminal labeling, used to attach fluorophores or chromophores to carbohydrates for chromatographic detection as illustrated in a recent study of glycans from the Tamm‐Horsfall glycoprotein (Rohfritsch et al., 2004) and the use of 2‐AA‐derivatives to monitor glycosylation of therapeutic glycoproteins (Dhume et al., 2002). Benzylamine (2/9) derivatization was used by Morelle and Michalski (2004) mainly for ESI‐MS/MS analysis of small and N‐linked glycans. However, exoglycosidase sequencing was performed on a MALDI target and it was observed that the derivative did not inhibit the enzymic reaction. An, Franz, and Lebrilla (2003a) have derivatized several sugars, including N‐linked glycans released from ribonuclease B, with benzylamine and quaternized the product with methyl iodide as originally performed by Broberg, Broberg, and Duus (2000) but with a procedure not involving the use of resins. The product allowed separation of isomers by capillary electrophoresis (CE) and also gave strong MALDI spectra on account of the inbuilt charge.
It is possible to remove labels attached by reductive amination; thus, 2‐aminopyridine (2‐AP, 1/52)‐derivatized N‐glycans have been underivatized by a process that involved catalytic hydrogenation and hydrazinolysis using the method reported by Hase (1992) to give the reducing‐terminal amine which was converted to an aldehyde using the Sommlet reaction (hexamethylenetetramine and acetic acid) (Takahashi, Nakakita, & Hase, 2003a).
Reducing‐Terminal Derivatives Prepared by Other Methods
Phenylhydrazine (11) has been used to form hydrazone derivatives of N‐linked glycans for both MALDI and electrospray ionization. Because the derivatives were not reduced, as with reductive amination, no post‐derivatization clean‐up was necessary. Derivatives could be prepared on‐target by addition of a solution (0.5 µL), prepared by dissolving phenylhydrazine (1 µL) in 10 µL of water/methanol (4:1 v/v), directly to the glycan–matrix mixture on the MALDI target and allowing the mixture to dry under ambient conditions for 45 min. No osazone formation was reported and the derivatives produced [M + Na]+ ions. Post‐source decay (PSD) spectra were reported to be simpler than those of the underivatized glycans and to be dominated by B/Y internal fragments (Lattova & Perreault, 2003a,b).

Several hydrazones containing either a constitutive cationic charge, such as those formed from Girard's T reagent (1/55, quaternary ammonium) or from hydrazines carrying a guanidine group (e.g., 12), have been shown to produce increases in sensitivity and to suppress signals from peptides, thus allowing N‐linked glycans to be detected in the presence or tryptic peptides without extensive clean‐up (Shinohara et al., 2004). Differences in sensitivity among the derivatives were observed. Among those containing a charge, and which formed M+ ions, the pyridinium derivative (13) was the most efficient, producing a detection limit of 1 fmol. The guanidine derivatives formed [M + H]+ ions and some produced equal detection limits. It would appear that the most efficient derivatives possessed both good ion‐forming properties and high lipophilicity. Improved detection limits have also been achieved by use of hydrazide derivatives of the cyanine dyes Cy‐3 (14) or Cy‐5 (15) that contain a constituent positive charge (Kameyama et al., 2004). N‐glycans from chicken ovalbumin could be detected with near independence from the type of matrix. PSD spectra contained mainly Y‐type ions consistent with localization of the positive charge on the derivative.




Derivatives of Other Sites
Sialic acids can be stabilized for MALDI analysis by formation of methyl esters to remove the very labile acidic proton on their carboxyl groups. Migration of this hydrogen atom is largely responsible for lability of this carbohydrate. A recent example of methyl ester formation is in a study of the N‐linked glycosylation of the glycoprotein, N‐cadherin, from human melanoma cell lines (Ciolczyk‐Wierzbicka et al., 2004). The acids were first converted into their sodium salts with a Dowex AG50 resin (Na+ form) and these salts were then reacted with methyl iodide. Other examples are included below.
CLEAN‐UP OF SAMPLES PRIOR TO MALDI ANALYSIS
Removal of contaminants such as salts, buffers, etc. is essential to obtain high quality spectra. A variety of methods are used, the majority being dialysis, use of various membranes such as Nafion 117 (Börnsen, Mohr, & Widmer, 1995) or various resins. Methods for preparing samples for mass spectrometric analysis have been reviewed (Perreault, 2004). Some examples of those during the review period are listed below.
Membranes
Drop dialysis has been used in clean‐up of lipooligosaccharide (LOS) from Moraxelle catarrhalis by Luke et al. (2003) and a Nafion membrane for on‐probe clean‐up of O‐acetylated glucomannans from birch and aspen (Teleman et al., 2003).
Use of Resins
Dowex AG50 resins in various forms have been used for analysis of N‐glycans from the moss Physcomitrella patens (Viëtor et al., 2003) (AG 50W‐X2 form), deacylated lipopolysaccharide (LPS) from Vibrio parahaemolyticus strains (Hashii et al., 2003a,b) (50 W × 8, H+ form to remove cations) and extracellular polysaccharide (EPS) from Burkholderia cepacia (Cescutti et al., 2003) (50 W × 8–200). AG50 combined with AG3 (anions) in a gel loader tip have been used for N‐linked glycans (Hoja‐Lukowicz et al., 2000; Ciolczyk‐Wierzbicka et al., 2004). Porous graphitized carbon has been employed to desalt quaternized benzylamine derivatives (An, Franz, & Lebrilla, 2003a) and Hypercarb columns have been used by Mills et al. (2003b) for N‐linked glycans released from human plasma glycoproteins proteins and by Forno et al. (2004) for N‐ and O‐linked glycans from recombinant human granulocyte‐macrophage colony‐stimulating factor secreted by a Chinese hamster ovary (CHO) cell line. Several examples of the use of cellulose cartridges have been published; thus, Higai et al. used them for purification of N‐glycans from α1‐acid glycoprotein (AGP) (Higai et al., 2003a) and sialyl Lewis X antigen‐expressing glycoproteins secreted by human hepatoma cell line (Higai et al., 2003b) and Wada, Tajiri, and Yoshida (2004) have employed both cellulose or Sepharose for the separation of tryptic glycopeptides from tryptic peptides.
C18‐solid‐phase extraction followed by Carbograph column was used by Ohl et al. (2003) to remove detergents from N‐glycans. Salts, etc. ware eluted from the columns with water, neutral glycans with 25% MeCN and acidic glycans with 25% MeCN/0.05% trifluoroacetic acid (TFA). Amberlite IR‐120 (H+) was used by Frirdich et al. (2003) to purify core oligosaccharide from E. coli. Release of N‐glycans with protein‐N‐glycosidase F (PNGase F) is more efficient if the glycoprotein is pre‐digested with trypsin or other protease. However, the resulting peptides can interfere with subsequent analysis and are difficult to remove with octadecyl (C18) silica cartridges, cation exchange columns or graphitized carbon cartridges. To overcome this problem, Nakano, Kakehi, and Lee (2003b) have modified the amino groups of the peptides with 2,4,6‐trinitrobenzene‐1‐sulfonate (16) to render them more hydrophobic so that they can be more strongly retained on C18 or graphitized carbon.

Bioaffinity Clean‐Up
Affinity capture of glycoproteins to a MALDI target has been reported by Koopmann and Blackburn (2003). The target was coated with poly‐l‐lysine poly(ethylene glycol)‐biotin polymer followed by tetrameric neutravidin. This surface then acted as a capturing surface for biotinylated proteins which could be adsorbed and desalted by washing. MALDI spectra were obtained following addition of CHCA to the surface. Protein–protein interactions could also be studied with this treated target and, furthermore, it could be used to isolate glycoproteins by lectin binding. Thus, biotinylated wheat germ agglutinin bound to the surface was used to further capture fetuin. The resulting MALDI spectrum contained ions from the biotinylated lectin, neutravidin and fetuin.
On‐Target Fractionation
Glycopeptides, such as those obtained from protease digests, frequently give weak signals in the presence of hydrophobic peptides. A simple method for separating hydrophobic from hydrophilic peptides on a MALDI target has recently been devised (Kjellström & Jensen, 2003). An aqueous solution of the peptide mixture was placed on the target followed by an immiscible solvent, such as ethyl acetate with or without added matrix. The organic solvent extracted the hydrophobic peptides and was allowed to evaporate, after which, the residual aqueous phase was transferred to another part of the target and allowed to dry after addition of DHB. MALDI spectra could then be acquired from both the hydrophobic and hydrophilic fractions.
QUANTIFICATION
A paper on quantitation of glucose (1/4) in a study on enzyme kinetics has highlighted problems in the use of MALDI‐MS for quantitative work and how the technique can produce good quantitative results in spite of the popular belief that the technique is not quantitative. The inhomogeneity of the target and variations in the shot‐to‐shot laser intensity can be compensated for by averaging many shots from different target positions. It is also important to eliminate any spectrum in which the A/D converter is saturated. By use of a [U‐13C]‐labeled internal standard and measurement of the [M + Na]+ ions, a linear calibration curve was obtained with a correlation coefficient of r = 0.991. The mean standard deviation was 6% but the authors (Bungert, Heinzle, & Tholey, 2004b) state that this could be improved if more spectra were averaged although this would have increased the analysis time.
A MALDI method for the quantification of glucose from hydrolyzed starch has been reported. Sorbitol (1/42) was used as the internal standard and DHB provided the matrix even though it produced peaks in the same region, but not at the same mass as the analytes. Calibration curves were linear over the range 0.1–10 pmol added to the target and the method compared favorably with the normal colorimetric method for measuring glucose. Even though the MALDI results showed a greater degree of variability, this was more than compensated for by the speed of analysis (Grant et al., 2003).
A quantitative MALDI‐TOF MS method as also been developed for screening of ten pyranose oxidase variants. The isotopic labeled internal standard, [U‐13C]‐glucose and the ionic liquid matrix, DHB/pyridine, were used with aliquots of enzyme reaction mixtures without pre‐purification steps (Bungert et al., 2004a). The ionic, liquid matrix enabled spectra to be recorded from most areas of the target (only 7 out of 200 spots failed to produce a signal). The mean standard deviation for glucosone was 11.8% and the results were in good agreement with HPLC measurements.
FRAGMENTATION
Glycosidic and cross‐ring cleavages produce most of the fragment ions from carbohydrates. A third type of fragmentation mechanism has now been reported involving hexacyclic hydrogen rearrangements. The products were proposed to consist of unsaturated sugar rings lacking two oxygen atoms as shown in Scheme 1 (Spina et al., 2004).
Scheme 1.

Post‐Source Decay (PSD)
Post‐source decay (PSD) fragmentation of per‐acylated iso‐flavone glycosides (e.g., 8) cationized with various metals and hydrogen have shown that the number of fragment ions decreased in the order Li+=Na+ > Ag+ > Cu+ > H+ > K+ > Rb+=Cs+, roughly in line with earlier studies of underivatized carbohydrates (Kéki et al., 2003). Fragments were largely glucosidic and losses of acetic acid and ketene but, unusually, there were a few fragments in some of the metal‐cationized spectra that had lost the metal. Loss of acetic acid and ketene was highest for the lighter metals. The authors attributed this observation to elimination of metal acetates.
Neutral and some acidic carbohydrates have been shown to form stable adducts with chlorine that survive the MALDI process to give [M + Cl]− ions from matrices such as harmine (1/36) that have gas‐phase acidities lower than or close to that of HCl (Cai, Jiang, & Cole, 2003). Such adducts open the way for the production of negative ion fragmentation spectra from carbohydrates that, in many cases, produce more informative spectra than their positive ion counterparts. In the above publication, the adduct of the disaccharide d‐turanose (α‐d‐Glcp‐(1 → 3)‐d‐Fru, 17) was reported to fragment initially by loss of HCl to give a PSD spectrum containing both glycosidic and cross‐ring fragments arising from what was essentially a [M−H]− ion.

MS/MS analysis of N‐acetyllactosamine‐6,6′‐disulfate (18) and its 2′‐epimer produce different fragmentation patterns under several mass spectrometric methods (fast‐atom bombardment (FAB), ESI and MALDI‐PSD). The PSD spectra were characterized by an abundant ion at m/z 431 that was produced from the 2α‐epimer by elimination of both the N‐acetylamino‐group and the 6‐sulfate (Ohashi et al., 2004). Muzikar et al. (2004) have found that many carbohydrates containing an amino group at the reducing terminus show enhanced PSD fragmentation with production of more abundant cross‐ring fragments. N‐linked glycans are normally released enzymatically in this form but cross‐ring fragmentation of these branched compounds was not enhanced as much as that from linear compounds and was inferior to the high‐energy fragmentation obtained with a TOF/TOF mass spectrometer.

Collision‐Induced Decomposition (CID)
A novel method of ion isolation for CID has utilized IR‐induced fragmentation and consequent removal of unwanted ions by use of a Nd:YAG laser that shone directly into the analytical cell of a modified Fourier transform ion cyclotron resonance (FT‐ICR) mass spectrometer (Xie, Schubothe, & Lebrilla, 2003). The method differed from existing techniques in that it did not involve diverting the unwanted ions with magnetic or electric fields. The paper reported the isolation of γ‐cyclodextrin (1/65) from a mixture containing maltotetraose ((β‐(1 → 4)‐linked d‐glucose)4, 19) and maltohexaose both of which also produced fragments in the analytical cell prior to irradiation with the IR laser. Infrared multiphoton dissociation (IRMPD) of alkali metal‐adducted carbohydrates has also been studied in this instrument and compared with CID. Although higher energy fragmentation could be obtained with CID, IRMPD provided continuous energy transfer to the fragment ions with the result that more extensive fragmentation was achieved (Xie & Lebrilla, 2003).

Fragmentation of maltohexaose and high‐mannose glycans (positive ion) with a TOF‐TOF mass spectrometer with air as the collision gas has been shown to give high‐energy‐type fragmentation with the production of abundant cross‐ring cleavage ions and, in particular, X‐type cleavages not seen in the low energy spectra. Isomers of Man7GlcNAc2 (20) could be distinguished with respect to the antenna (3 or 6) to which the seventh mannose was attached by the O,4A3 and 3,5A3 ions (Mechref, Novotny, & Krishnan, 2003). Similar results have been reported by Morelle et al. (2004) with a series of smaller sugars. A study of the effect of DHB or CHCA on fragmentation patterns of native and permethylated oligosaccharides in a TOF/TOF mass spectrometer has shown that, whereas CHCA promoted mainly glycosidic cleavages, DHB initiated glycosidic, cross‐ring and internal cleavages with X‐type cross‐ring cleavages among the most abundant ions. Permethylation produced increased sensitivity and spectra that were easier to interpret. Studies with avidin from a DHB matrix allowed most of its N‐glycans to be identified (Stephens et al., 2004a). A comparison of matrices used for ionization of 2‐AP‐labeled complex N‐linked glycans with a TOF‐TOF mass spectrometer has shown that, whereas CHCA produced only sodiated fragments in MS2 spectra from [M + Na]+ parent ions, DHB produced both sodiated and protonated ions. It was suggested that the two matrices produced parent ions in different excited states (Kurogochi & Nishimura, 2004). However, CHCA was more effective than DHB in producing abundant PSD ions. Although providing additional information through the production of more cross‐ring cleavages, acidic glycans showed extensive fragmentation before reaching the collision cell. Sialylated glycans, however, can be stabilized by permethylation or methyl ester formation, as discussed above.

Negative ion fragmentation appears to be gaining more in popularity because of its ability to produce abundant cross‐ring cleavages. The [M−H]− ion generated by AP‐MALDI from 6′‐sialyl‐lactose (6′‐SL, 1) using a glycerol matrix, for example, yields the O,2A3 ion as the most abundant fragment (Von Seggern, Moyer, & Cotter, 2003a). C‐ions were prominent in the spectrum of this and larger sugars such as LSTa (Neu5Ac‐α‐(2 → 3)‐Gal‐β‐(1 → 3)‐GlcNAc‐β‐(1 → 3)‐Gal‐β‐(1 → 4)‐Glc, 21) and LSTb (Gal‐β‐(1 → 3)‐(Neu5Ac‐α‐(2 → 3))GlcNAc‐β‐(1 → 3)‐Gal‐β‐(1 → 4)‐Glc, 22) and the spectra easily allowed the location of sialic acids to be determined. Cross‐ring and C‐type fragments were also prominent in the spectra of lithiated adducts and, in particular, the spectra of lithium salts of sialic acids (Von Seggern, Zarek, & Cotter, 2003b). Fragmentation of the [M + Li]+ and [M + Co]+ ions showed no significant difference between 3′‐ (3) and 6′‐SL (2) but the spectra of the [M + 2Li]+ ions were different. In general, replacing the acidic proton of the sialic acid group by salt formation prevented ready loss of sialic acid under positive ion conditions and accentuated differences between the spectra of isomeric sugars containing sialic acid at different positions.

Multiple Successive Fragmentation (MSn)
Studies with a MALDI‐ion‐trap‐TOF instrument have shown that many of the fragments in the positive ion spectra of N‐glycans originate from multiple sites. In the spectra of high‐mannose glycans an ion formed by loss of the chitobiose core and 3‐antenna is diagnostic for the composition of the 6‐antenna. However, in the spectra of biantennary glycans (23), the abundant ion at the equivalent mass (m/z 712, Hex3‐HexNAc) was shown to be formed predominantly from the B5 ion (loss of the reducing‐terminal GlcNAc) and Gal‐GlcNAc from each antenna (Harvey et al., 2004).

Computer Analysis of Fragmentation Spectra
The programming language Java has been used to write a program for identifying the total monosaccharides present in a tryptic glycopeptide containing up to six O‐linked glycans from the hinge region of human serum immunoglobulin 1 (IgG1). The program also calculated the amount of each glycopeptide as a percentage of total peak area (Pouria et al., 2004).
A modification and extension to the StrOligo algorithm (Ethier et al., 2002) for assigning structures of N‐linked glycans from their CID spectra has been published (Ethier et al., 2003). Spectra were recorded with a MALDI‐Q‐TOF instrument, isotope‐stripped and examined for peaks corresponding to monosaccharide losses. The program then built a relationship tree that was analyzed with respect to fragment ion types and adduct. [M + Na]+, the ions usually encountered in MALDI spectra of carbohydrates are now catered for as well as the [M + H]+ ions featured in the original version of the program. Negative ion fragmentation was also accommodated. Greater attention to known biochemistry was incorporated in the new algorithm and the program was able to annotate spectra with the most probable structure.
A web‐based tool entitled GLYCO‐FRAGMENT, to be found at http://www.dkfz.de/spec/projekte/fragments/ accepts carbohydrate structures in the extended IUPAC nomenclature (http://www.chem.qmw.ac.uk/lupac/2carb/38.html), as developed for the carbohydrate database, CarbBank, and calculates the masses of all possible fragments. The presence of reducing‐terminal derivatives, various adducts such as sodium or lithium, persubstituted (e.g., permethyl) derivatives and substituents such as sulfate, acetate and phosphate are also accepted. B‐ and Y‐type fragments are listed by default and masses of C‐, Z‐ and cross‐ring fragments can be obtained on demand. Internal fragments are not catered for. Output is in the form of a list or, in “view as structure” mode, ions associated with cleavage of each bond are shown when the user moves a cursor over a specific glycosidic linkage (Lohmann & von der Lieth, 2003). GlycoSearchMS (http://www.dkfz.de/spec/glycosciences.de/sweetdb/ms/) compares each peak in a mass spectrum with calculated fragments from all structures in the SweetDB database and the best matches are displayed (Lohmann & von der Lieth, 2004). Constituent monosaccharides can be obtained from the program GlycoMod (http://www.expasy.ch/) as demonstrated by Ma et al. (2003a) to assign compositions to N‐glycans released from the glycoprotein rat selenoprotein P. Reviews of available databases relating to glycomics have been published (von der Lieth, 2004a,b).
STUDIES ON SPECIFIC CARBOHYDRATE TYPES
Polysaccharides
Work in this area is summarized in Table 1. A comparison of the behavior of dextrans in positive and negative ion MALDI has shown the expected formation of [M + Na]+ ions in positive mode whereas the negative ion spectra contained ions at [M‐1‐120]− as the result of fragmentation. Under ESI conditions on a Shimadzu‐Kratos SEQ instrument, positive ionization again produced [M + Na]+ ions but with a bias towards the lower masses. The negative ion spectra were dominated by extensive A‐type fragment ions. MALDI was reported to be more sensitive than ESI but ESI was more useful for structural studies (Cmelík, Stikarovská, & Chmelík, 2004). Comparisons of fragmentation modes for the [M + Na]+ ions from these compounds have shown that, whereas PSD, ISD, and CID all produced glycosidic cleavages, only ISD and CID produced significant cross‐ring cleavage. Localization of the charge to the reducing terminus by formation of a 1‐phenyl‐3‐methyl‐5‐pyrazolone derivative (see 1/61) restricted cleavage to the non‐reducing terminus (Bashir et al., 2004).
Table 1.
Use of MALDI MS for examination of carbohydrate polymers from plants, fungi, and lichens
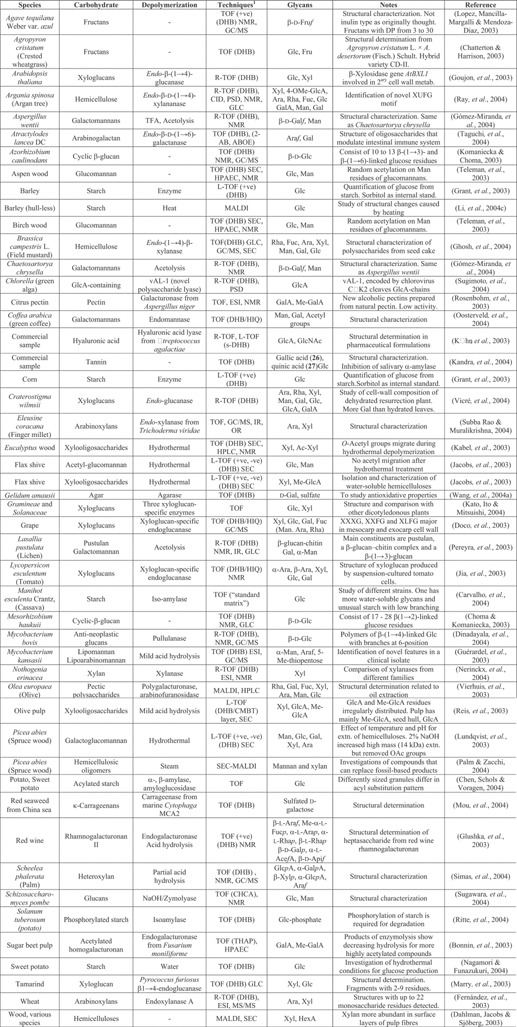
(1) Instrument (matrix) other technique, sample (derivative).
Polysaccharides from Plants
The β‐d‐fructan (inulin; for fructose, see 1/16) from dahlia tubers and glucose syrup from potatoes, together with similar polysaccharides from red onion and Jerusalem artichokes, were used as reference compounds by Stikarovská and Chmelík (2004) to evaluate the best matrices for these compounds. Linear‐MALDI‐TOF analysis gave stronger signals than reflectron‐TOF; DHB was found to be the best matrix for starch and THAP for inulin. Less effective matrices were CHCA, HABA, 3‐aminoquinoline (3‐AQ, 1/24) and sinapinic acid (1/48). Depolymerization of most of these very large molecules is necessary before MALDI analysis. Enzymatic treatment is usually used (details in Table 1) but heat treatment is also common. Acetylation of glucuronoxylans and glucomannans has received considerable attention (Table 1) with some evidence of acetyl group migration during depolymerization (Kabel et al., 2003). Acetylated glucuronoxylans and glucomannans have been recovered from flax shive following hydrothermal treatment but in this case, acetyl migration was not reported (Jacobs et al., 2003). Acetylation has also been found on the mannose residues of glucomannans from birch and aspen wood and appear to be randomly distributed (Teleman et al., 2003). MALDI‐TOF spectra from these two papers consisted of an extensive series of peaks with a mass separation of 42 units (masses up to 5,500) but considerable simplification was found after deacylation.
Fernández et al. (2003) have examined arabinoxylans from wheat by MALDI and ESI mass spectrometry and detected structures with up to 22 monosaccharide residues. As arabinose (1/2) and xylose (1/3) are isobaric, it was not possible to study the distribution of arabinose residues along the xylose backbone. However, this problem was overcome by permethylation; arabinose residues attracted three methyl groups, unsubstituted internal xylose residues, two and substituted residues one group.
Synthetic (Modified) Polysaccharides
Matrix‐assisted laser desorption/ionization (MALDI) conditions for determination of the substitution patterns in methyl‐ (Momcilovic et al., 2003b) and carboxymethyl‐cellulose (Momcilovic et al., 2003a) have been evaluated. Depolymerization was achieved both by use of enzymes or acid and the products fractionated by size‐exclusion chromatography (SEC). DHB, CHCA, HABA and indoleacrylic acid (IAA, 3/5) were tested as matrices for methylcellulose but little difference was found between them. The choice of solvent, however, did have a significant effect, probably due to differential solubility of the variously methylated compounds. Measurements on two acid‐depolymerized samples of methylcellulose with different degrees of substitution gave values that agreed well with the data supplied by the manufacturers. However, data from the carboxymethyl‐celluloses was less satisfactory even though samples with different degrees of substitution could easily be distinguished from each other.
A method has been published for detecting hydroxyethyl starch in urine. The compound is used as a plasma volume expander by athletes and has been banned by the International Olympic Committee. Partial acid hydrolysis of urine was carried out with TFA and the product was examined directly by MALDI‐TOF using super‐DHB (s‐DHB). The method was claimed to provide unambiguous identification in only 90 min (Gallego & Segura, 2004).
Cyclodextrins and Related Compounds
Of the matrices DHB, nor‐harmane (1/35), CHCA, HABA, IAA, and sinapinic acid, DHB and nor‐harmane were found by Xiong et al. (2003b) to give the strongest and best resolved spectra from macrocyclic polysaccharides containing from 19 to 25 sugar residues. Sinapinic acid gave a very weak signal and was only recorded in linear mode. The liquid matrix, CHCA/3‐AQ/glycerol also gave a strong and long‐lasting signal but with inferior resolution. Addition of alkali metal salts produced the expected adducts.
A glass slide containing a UV absorbing TiO2 sol–gel film from which imprinted α‐cyclodextrin (CD (cyclic‐(1 → 4)‐α‐d‐glucose)6, 24) molecules had been removed has been used to selectively adsorb α‐CD from a mixture of α‐, β‐, and γ‐CD. The α‐CD was detected directly by MALDI‐MS without the addition of extra matrix (Chen & Chen, 2004).

Experiments with α‐, β‐, and γ‐cyclodextrins have shown that the larger molecules have greater affinity for larger alkali metals suggesting that the products are inclusion complexes that can be ionized intact. Sinapinic acid was used as the matrix and under these conditions, dextran, a linear polymer, produced only [M + H]+ ions (Bashir et al., 2003a). Inclusion complexes with cyclodextrins have recently been used to investigate the composition of humic acids from Antarctica. The structure of humic acids is still largely unknown even though they are widely distributed in nature. Gajdošová et al. (2003) recorded the MALDI spectra of humic acids with and without γ‐cyclodextrin and found that several constituents appeared to form inclusion complexes. Thus, for example, a shift in the cyclodextrin peak by 66.0 mass units after addition of humic acid from a standard soil sample was interpreted as consistent with inclusion of a cyclopentadiene radical (C5H). Results from the soils from Antarctica were similar. MALDI spectra were recorded without a matrix to simplify the spectra. An investigation of the mechanism of the well‐known migration of alkyl‐silyl groups from the two‐ to the three position in cyclodextrins has shown that the receiving oxygen is that from the same ring as the migrating silyl group and not to the adjacent ring (Teranishi & Ueno, 2003).
Other Polysaccharides
Matrix‐assisted laser desorption/ionization (MALDI) sample preparation protocols for examination of Curdlan ((( → 3)‐β‐d‐Glc‐(1 → ))n, 25), a polyglucose obtained from the bacterium Alcaligenes faecalis var. myxogenes 10C3, have been reported. The crude sample was separated into a low molecular weight, water‐soluble portion and a high‐molecular weight, water‐insoluble portion. The low molecular weight portion was examined from DHB containing ammonium fluoride and gave two low‐mass (<4 kDa) polysaccharide distributions differing by 16 Da. The high molecular weight, water‐insoluble portion was found to produce good signals from a mixture of DHB and 3‐AQ in dimethylsulfoxide (DMSO) that was dried on a hot‐plate at 70°C. Ions with masses of up to 15,000 were observed (Chan & Tang, 2003). Spectra of extracellular polysaccharides from the colony‐forming prymnesiophyte algae Phaeocystis globosa and P. antarctica showed peaks of less than 1,000 Da, some of which had a repeat pattern of 192 Da but the monosaccharide units were not identified (Solomon et al., 2003).

Supplementation of a pre‐term formula with a mixture of galacto‐ and fructo‐oligosaccharides with a molecular weight range similar to that of carbohydrates found in human milk as determined by MALDI‐TOF analysis has been found to have a stimulating effect on the growth of bifidobacteria in the intestine and results in more frequent produced and softer stools. Thus, prebiotic mixtures such as the studied oligosaccharide mixture might help in improving intestinal tolerance to enteral feeding in pre‐term infants (Boehm et al., 2003).
Glycoproteins
Intact Glycoproteins
It is unusual for all but the simplest, low mass glycoproteins to produce MALDI‐TOF spectra with resolved glycoforms but broad peaks and significant mass differences between observed and calculated protein masses for larger glycoproteins suggest glycosylation. Thus, epoetins (erythropoietins), with several glycosylation sites occupied by sialylated complex glycans, produced only broad peaks with masses in the range of 30–35 kDa and half‐height peak widths of up to 5 kDa when examined by MALDI‐TOF (Stanley & Poljak, 2003). Deglycosylation, however, produced sharp peaks from the protein with only minimal tailing. Human follistatin expressed in CHO cells has two N‐linked glycosylation sites at Asn‐95 and 259. Its MALDI spectrum contained three peaks; the first at m/z 31,525 corresponded to the unglycosylated protein and the other two at m/z 33,804 and 35,600 were produced by unresolved glycopeptides. LC/MS analysis identified nine complex glycans (Hyuga et al., 2004). Several partially resolved peaks from 52 to 55.5 kDa suggest glycosylation of human β‐secretase catalytic domain, confirmed as N‐linked by digestion with PNGase F (Wang et al., 2004b). Reduction in mass of 1.4 kDa of a humanized anti‐HBs Fab fragment by treatment with Endo‐H gave a mass that was still 3.7 kDa above that of the protein. The authors speculated that the extra mass might indicate O‐linked glycosylation but no proof was provided (Ning et al., 2003a). Similarly, a reduction in the mass of approximately 4.5 kDa of a lysosomal serine carboxypeptidase from Trypanosoma cruzi following Endo‐H treatment suggested the presence of two to three N‐linked glycans (Parussini et al., 2003). Other carbohydrates detected by mass‐difference measurements of proteins before and after deglycosylation include GlcNAc in Cu/Zn‐superoxide dismutase from fungus Humicola lutea 103 (Dolashka‐Angelova et al., 2004b) and the high‐mannose glycan Man10GlcNAc2 in ovotransferrin expressed in Pichia pastoris (Mizutani et al., 2004).
Matrix‐assisted laser desorption/ionization (MALDI) analysis is also useful for detecting the absence (Perteguer et al., 2004) or modification of glycosylation. Thus, the mass of human transferrin produced in insect (Drosophila melanogaster S2) cells is consistent with lack of sialic acids on its N‐glycans (Lim et al., 2004).
N‐Linked Glycans
Analysis of derived glycopeptides and site occupancy
Although N‐linked glycans have long been known to attach to a consensus sequence consisting of an Asn‐Xxx‐Ser(Thr) motif, where Xxx can be any amino acid except proline, evidence is now emerging that Asn‐Xxx‐Cys can also act as a motif. Satomi, Shimonishi, and Takao (2004) have identified a third site based on this motif, occupied by approximately 2% of the glycosylation, in human transferrin.
Proteolysis, usually with trypsin, to localize each consensus sequence site to an individual glycopeptide is the classical method for determination of the glycosylation at each site. Detection of glycopeptides by HPLC following proteolysis is often difficult because of effects such as signal suppression. Monitoring of oxonium ions (163 for hexose, 204 for GlcNAc, etc.) is a standard method for tracking the glycopeptides and has been extended to monitoring with an ion trap mass spectrometer by generating them in the ion‐source region (Sullivan, Addona, & Carr, 2004). Peptide masses were confirmed by MALDI‐TOF MS after desalting with C18 ZipTips. A method for predicting peptide retention times in reversed‐phase HPLC has been published (Krokhin et al., 2004b) following examination of 346 tryptic peptides and deglycosylated glycopeptides identified by MALDI‐TOF MS from 17 proteins. Glycopeptides eluted slightly earlier than their deglycosylated versions. Liu, Feasley, and Regnier (2004) have evaluated the technique of diagonal chromatography which involves comparisons of HPLC chromatograms run under identical conditions before and after enzymatic removal of the glycans and have concluded that, although the technique worked well for detection of protein phosphorylation, retention times of the glycopeptides and derived peptides, identified by MALDI‐TOF MS, were too similar for diagonal chromatography to be a reliable technique for detecting glycopeptides. MALDI‐TOF MS has the advantage over ESI‐MS for the detection of glycopeptides in that the spectra usually only contain singly charged ions unlike ESI with its tendency to produce multiply charged ions. Glycopeptides are often revealed in MALDI profiles by their relatively high mass and by peak spacing corresponding to monosaccharide residues (e.g., 162 for hexose) (Krokhin et al., 2004a).
Rather than attempt detection of glycopeptides in the presence of peptides, some investigators employ fractionation techniques to isolate the glycopeptides before mass spectrometric analysis. For example, Wada et al. (2004) have developed a method whereby glycoproteins are reduced and alkylated, cleaved with trypsin and lysylendopeptidase and partitioned with cellulose or Sepharose to bind the glycopeptides. The isolated glycopeptides could then be examined by LC or linear MALDI‐TOF. MS/MS in an ion‐trap‐TOF instrument provided peptide fragments for protein identification and carbohydrate ladders that gave information on the glycan composition. The method was applied to transferrin, IgG and β2‐glycoprotein 1. A method has been reported for identification and quantification of N‐linked glycoproteins following their specific immobilization on a solid support (Zhang et al., 2003a). Cis‐diol groups in the carbohydrate were oxidized with periodate to aldehydes allowing the carbohydrate to be captured by hydrazide chemistry. The protein was then cleaved with trypsin or another suitable enzyme and the resulting peptides were released with PNGase F for analysis by MALDI or ESI mass spectrometry. Deuterium labeling of the peptide with succinic anhydride allowed quantitative studies to be made.
The frequent absence of glycopeptides in LC/MS analyses has been used to advantage in indicating which peptide might be glycosylated. Thus, peptides containing the consensus N‐glycosylation sites at Asn‐142, 173, and 178 were not observed by Hambrock et al. (2004) from the tryptic digest of the recombinant protein TSC‐36/Flik expressed in human cells suggesting that these sites were glycosylated.
An et al. (2003b) have used pronase to digest two well‐characterized glycoproteins and found that steric factors prevented complete digestion in the region of the glycosylation site leaving the carbohydrate attached to a short peptide. N‐glycan masses were obtained by MALDI‐FT MS from a separate experiment involving glycan removal by PNGase F and subtracted from those of the glycopeptides to obtain the peptide mass and, hence, its structure. The method was claimed to be more rapid that the traditional method of proteolysis and the results easier to interpret because no large peptides appeared in the final sample. The method was applied to the study of site heterogeneity of Xenopus laevis egg cortical granule lectin.
Asn to Asp conversion after PNGase digestion effectively labels the percentage of a given site that is occupied and is detected by a mass difference of one unit. The technique has been used, for example, to show glycosylation in 10 of the 11 consensus sequence sites of the SU component of avian sarcoma/leukosis virus envelope glycoprotein (Kvaratskhelia et al., 2004) and to identify occupied glycosylation sites in membrane‐bound glycoproteins. Normally this latter task is difficult because 2D sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) gels are ineffective in separating these compounds. To overcome the problem, the proteins were solubilized in guanidine–HCl, digested with trypsin and the glycopeptides were recovered by lectin binding. Following removal of the N‐glycans with PNGase F, the peptides with the Asn to Asp conversion were detected by MALDI‐Q‐TOF MS (Fan et al., 2004). Investigations of the spike glycoprotein from the SARS virus have revealed four occupied and one unoccupied N‐linked sites by molecular weight measurements made before and after glycan removal by PNGase F and by observing the Asn to Asp conversion (Ying et al., 2004). In a similar experiment, digestion of the glycoprotein N‐CAM with AspN and trypsin resulted in the identification of glycopeptides, each containing a single glycosylation site. They were separated by HPLC and analyzed by MALDI‐TOF MS. Five of the six sites were identified but glycopeptides from site Asn‐4 were missing. This site was eventually characterized by a direct MALDI‐FTICR analysis of the glycopeptides released in‐gel by trypsin (Albach et al., 2004). Incorporation of 18O into the aspartic acid from HO can be used to label the newly produced aspartic acid and was used by Barinka et al. (2004) to identify and confirm glycosylation on each of the 10 consensus sequence sites of glutamate carboxypeptidase II.
N‐Linked glycan composition from glycopeptide analysis
Measurement of glycan masses directly or by subtraction of the mass of the peptide from those of glycopeptides leads directly to the constituent isobaric monosaccharide composition as illustrated by a recent study of N‐glycan masses from the androgenic hormone of Porcellio scaber (Crustacea) (Grève et al., 2004). Sometimes, with large glycopeptides, peaks are poorly resolved but can often be improved by chemical treatments. Thus, for example, the core fragment of human luteinizing hormone gave a broad, poorly resolved peak (m/z 8,700–10,700) when examined from sinapinic acid with a linear TOF instrument (Jacoby, Kicman, & Iles, 2003). The compound consisted of two peptide chains, only one of which was glycosylated, linked with four disulfide bonds. These bonds were reduced on the MALDI target with dithiothreitol (DTT) to give a more fully resolved envelope of peaks from which the N‐linked glycan composition was deduced by subtraction of the peptide masses.
Glycan release
Most structural determinations of these compounds are performed on released glycans. Experimental details for chemical and enzymatic release of N‐glycans have been described (Merry & Astrautsova, 2003). Release with PNGase F (or PNGase A if the glycans contain fucose in α‐(1 → 3)‐linkage to the core GlcNAc) or hydrazine are the preferred enzymatic and chemical release methods respectively. No new chemical release techniques or modifications were found during the review period reflecting the increasing popularity of enzymatic release, particularly with PNGase F. Additionally, PNGase F release is not accompanied with the potential hazards of hydrazine. However, there is some controversy as to whether this enzyme releases all glycans, particularly from larger glycoproteins. Thus, it has been shown not to release Glc1Man9GlcNAc2 (28) from arylophorin under non‐denaturing conditions (Kim et al., 2003) and, in addition, Reinhold et al. (Zhu et al., 2004a) assert that PNGase F fails to release a subset of glycans from C. elegans and thus use hydrazine. Artifacts detected by MALDI‐TOF analysis after in‐gel release with PNGase F have included glycans with urea attached to the reducing terminus (Omtvedt et al., 2004) as the result of its inclusion in an earlier procedure for glycoprotein purification. Deglycosylation with trifluoromethanesulfonic acid, a method that leaves the protein intact for further study has been reviewed (Edge, 2003).



Applications of MALDI MS to the detailed structural determination of N‐linked glycans
The ability to carry out the classical method of exoglycosidase sequencing by performing the digestions on the MALDI target, with DHB as the matrix, has been demonstrated by Morelle and Michalski (2004) using the well‐studied glycoproteins α1‐antitrypsin and ribonuclease B. Gutternigg et al. (2004), however, preferred ATT as the matrix because of its neutrality.
A large number of studies have been conducted on the structural determination of N‐glycans from isolated glycoproteins and from intact organisms or tissues; these are summarized in Tables 2 and 3 respectively. Among the more unusual glycosylations to have been reported are the presence of Glc1Man9GlcNAc2 (28) in the insect Antheraea pernyi (Chinese oak silkworm) (Kim et al., 2003), the presence of 3‐O‐Me‐galactose and 3‐O‐Me‐GlcNAc (bisecting GlcNAc) in biantennary glycans from the marine snail Rapana venosa (Dolashka‐Angelova et al., 2004a) and 3‐O‐Me‐galactose and ‐mannose from high‐mannose glycans of the slug Arion lusitanicus (Gutternigg et al., 2004). 3‐ and 6‐difucosylation have been found on the core GlcNAc residue of Man3GlcNAc2Fuc2 (29) from Todarodes pacificus (Japanese flying squid) together with attachment of a β4‐linked galactose to the 6‐linked fucose (30) (Takahashi et al., 2003b). Paucimannosidic glycans with similar Gal‐β‐(1 → 4)‐Fuc and Gal‐β‐(1 → 4)‐Gal‐β‐(1 → 4)‐Fuc groups at the reducing‐terminal 6‐position have been found in keyhole limpet (Megathura crenulata) hemocyanin (Wuhrer et al., 2004c). An unusual difucosylated core structure Man3GlcNAc2dHex2) in which one of the fucose residues is attached to a mannose residue has been reported as a constituent of phaiodactylipin (phospholipase A2) from scorpion Anuroctonus phaiodactylus) venom (Valdez‐Cruz, Batista, & Possani, 2004). High‐mannose glycans including the first report of a glycan with a Gal‐β‐(1 → 6)‐Man moiety have been reported from keyhole limpet hemocyanin together with glycans with a Fuc‐α‐(1 → 3)‐GalNAc epitope which causes cross‐reactivity with Schistosoma mansoni glycans which share same epitope (Geyer et al., 2004). Some N‐glycans from C. elegans and related organisms are substituted with phosphorylcholine (PC, 3/13) which is thought to modulate the host immune response. Studies on their biosynthesis have shown that C. elegans microsomes transfer PC from l‐α‐dipalmitoylphosphatidylcholine (31) to both hybrid and complex N‐glycans containing GlcNAc (Cipollo et al., 2004a). HL60 cells treated with the glucosidase inhibitor n‐butyl‐deoxynojirimycin (NB‐DNJ, 32) produced a series of compounds with the structure of high‐mannose glycans but with only one GlcNAc residue as the result of cytosolic cleavage from misfolded glycoproteins that had been exported from the endoplasmic reticulum (Mellor et al., 2004b). A novel trisulfated hybrid glycan containing a GlcA residue capping the Gal‐GlcNAc antenna has been identified from bovine myelin glycoprotein P0 (Yan, Kitamura, & Nomura, 2003).
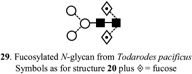



Table 2.
Use of MALDI MS for examination of N‐glycans from specific glycoproteins (see also Table 10, biopharmaceuticals)

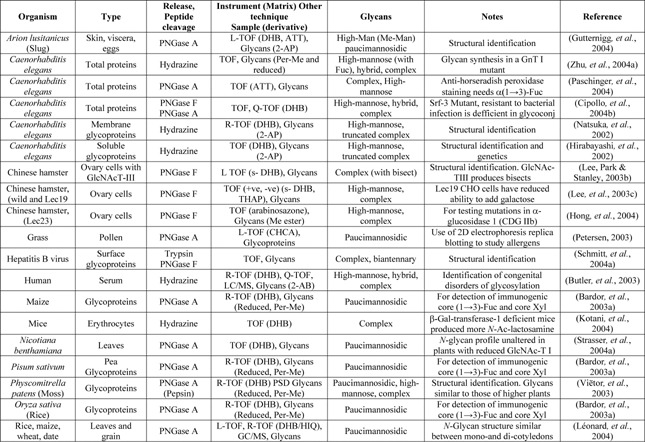
Table 3.
Use of MALDI MS for examination of N‐glycans from intact organisms, tissues or protein mixtures
Applications of MALDI MS to the study of N‐glycan function
The presence of N‐glycans on ribonuclease has been shown to reduce oligomerization (Gotte, Libonati, & Laurents, 2003). Engineered follitropins containing variable numbers of N‐ and O‐linked glycosylation sites, expressed in CHO cells, have been used to show that increasing numbers of attached carbohydrates lengthened the elimination half‐life by up to twofold. Numbers of attached carbohydrates were estimated by MALDI‐TOF measurements on the intact glycoproteins (Weenen et al., 2004). Gårdsvoll et al. (2004) have used site‐directed mutagenesis to remove the five N‐linked sites of soluble human urokinase receptor expressed in Drosophila S2 cells either individually or in various combinations and have shown than only when all glycans are removed was there an impaired level of secretion. Monitoring of the levels of glycosylation of the 35 kDa protein was made with MALDI‐TOF MS. Although there was incomplete resolution of the glycoforms, site occupation was clear by the appearance of peaks or shoulders coinciding with the calculated mass, assuming that the average mass of a glycan was 1,039 Da.
O‐linked Glycans
O‐Linked glycans continue to receive less attention than their N‐linked counterparts, one factor being their tendency to occur in groups that make site analysis difficult. As with N‐linked glycans, much structural analysis is conducted on released glycans. A method for the identification of sites modified by O‐GlcNAc that relies on mild β‐elimination followed by Michael addition with dithiothreitol and known as BEMAD has been described (Wells et al., 2002). The method was validated by mapping three previously identified O‐GlcNAc sites, as well as three novel sites, on Synapsin I purified from rat brain and on the Lamin B receptor and the nucleoporin Nup155.
Studies on intact glycoproteins and glycopeptides
Collision‐induced decomposition (CID) spectra of glycopeptides obtained by ESI from doubly charged ions frequently display prominent loss of the sugar moieties as the result of the labile protons. Czeszak et al. (2004) have used MALDI‐PSD on glycopeptides derivatized with a phosphonium group at the amino‐terminus and have shown that the resulting singly charged phosphonium ions and a‐type fragments retain the sugar. In contrast, when doubly charged ions were generated by proton addition, loss of the glycan was again seen. The method was claimed to be an effective alternative to electron‐capture dissociation (ECD) and was used to locate up to three GalNAc residues on the full tandem repeat peptide derived from the MUC5AC mucin. Kurogochi, Matsushita, and Nishimura (2004) have reported that LIFT‐TOF with DHB as the matrix is an ideal system for fragmenting glycopeptides (b and y series ions) and have used it to determine the O‐linked glycosylation sites in mucin‐type glycopeptides.
Up to six O‐linked glycosylation sites have been found in the hinge region of IgA1 from human serum (Tarelli et al., 2004). Tryptic digestion yielded a 33‐mer glycopeptide that was examined by MALDI‐TOF MS from THAP/ammonium citrate. This matrix gave more highly resolved spectra than s‐DHB or CHCA; the CHCA spectrum, in particular, was very poorly resolved (Pouria et al., 2004). Structural analysis was by exoglycosidase digestion (sialidase and galactosidase) and site analysis was performed on the degraded glycans by further cleavage with the endoproteases Glu‐C from Staphyloccus aureus and proteinase K from Tritirachium album (Tarelli et al., 2004). MALDI‐TOF analysis of the stalk‐region glycopeptide (APTPVPPPTGTPRPL) from murine CD8 has shown that all three threonine residues are glycosylated with HexNAc. Four peaks were obtained and attributed to molecules containing 0–3 additional hexose residues (Merry et al., 2003).
The 47 kDa portion of the 45/47 kDa glycoprotein from Mycobacterium tuberculosis expressed in Streptomyces lividans has been found to contain glycans on both the N‐ and C‐terminal tryptic peptide; MALDI‐TOF analysis showed from 0 to 9 hexose residues at the N‐terminus but the glycosylation site was not determined (Lara et al., 2004). The 13‐amino acid‐containing peptide isolated from Conus textile contains a Gal‐GalNAc residue attached to threonine but with an unknown linkage between the sugar rings. MALDI‐TOF analysis of the products of a β‐galactosidase digest on a synthetic analogue containing the common β‐d‐Gal‐(1 → 3)‐α‐d‐GalNAc‐carbohydrate showed that the enzyme was unable to remove the galactose residue suggesting the presence of α1 → 3‐linked galactose and this was confirmed by subsequent NMR analysis (Kang et al., 2004). Tryptic glycopeptides from surface glycoproteins of the hepatitis B virus were found by MALDI‐TOF MS and on‐target exoglycosidase digestion to be O‐glycosylated with Gal‐GalNAc or Neu5Ac‐Gal‐GalNAc. The N‐glycans were mainly biantennary complex (Schmitt et al., 2004a).
Applications of MALDI to the structural determination of O‐linked glycans
Studies on the structural determination of O‐glycans from isolated glycoproteins and from intact organisms or tissues are summarized in Tables 4 and 5 respectively. Studies on some specific structural types are summarized below.
Table 4.
Use of MALDI MS for examination of O‐glycans from specific glycopeptides

Table 5.
Use of MALDI MS for examination of O‐glycans from intact organisms or tissues

Mucins
The ability of MALDI MS to resolve complex mixtures of glycans has been utilized in studies of mucins from the jelly coats of amphibian eggs. Thus, three layers of the jelly coat from X. laevis have been shown to contain different but overlapping glycan structures; 40 neutral and 30 sulfated compounds were identified (Zhang et al., 2004b) by HPLC (PGC column), MALDI‐FT‐ICR, CID and the “catalog‐library” approach of CID peak matching reported earlier (Tseng, Hedrick, & Lebrilla, 1999). Thirty‐five neutral, sulfated and sialylated glycans have been identified from X. tropicalis by similar techniques combined with exoglycosidase digestion (Zhang et al., 2004a). Sialylated glycans were stabilized by formation of methyl esters. Sulfated core‐2 and core‐4 glycans have been identified by MALDI‐TOF MS and NMR from respiratory mucins from a patient suffering from chronic bronchitis but, unlike the structures of glycans identified earlier from a cystic fibrosis patient (Lo‐Guidice et al., 1994), these glycans contained no sialic acid (Degroote et al., 2003). MALDI‐TOF data were recorded in negative ion mode and no loss of sulfate was reported. Human intestinal mucins show an acidic gradient along the intestinal tract which may explain the region‐specific colonization of the gut by various bacteria (Robbe et al., 2003b). O‐Glycans of stomach mucosa have an antibacterial action against Helicobacter pylori by inhibiting biosynthesis of cholesteryl‐α‐d‐glucoside (identified by MALDI‐TOF MS), a major cell wall component (Kawakubo et al., 2004).
Glycosaminoglycans (GAGs)
Methods for the analysis of hyaluronan and its fragments have been reviewed (Capila & Sasisekharan, 2004).
α. Unsulfated GAGs. Hyaluronan oligomers ((( → 3)‐β‐d‐GlcNAcp‐(1 → 4)‐β‐d‐GlcAp‐(1 → ))n, 33), with masses up to 8 kDa, obtained from hyaluronic acid by the action of bovine testicular hyaluronidase have been examined by electrospray and MALDI‐TOF MS. Electrospray showed the presence of oligomers with both odd and even numbers of sugar units whereas MALDI and high‐performance anion exchange chromatography (HPAEC) showed only even numbered oligomers. The discrepancy was traced to the electrospray ion source which was producing cone‐voltage fragmentation. It was recommended that for ESI studies of compounds of this type, the cone voltage be kept low and precisely controlled (Prebyl et al., 2003). An LC/MS method for quantification of hyaluronic acid fragments in pharmaceutical preparations produced by the action of hyaluronate lyase from Streptococcus agalactiae has been reported, with negative ion MALDI‐TOF from DHB being used to record the glycan profiles of three fractions. The largest fraction showed peaks to 15 kDa (Kühn et al., 2003). A model disaccharide, ΔUA–GlcNAc (10 mmol), derived from heparan sulfate by heparitinase enzyme digestion and bearing an unsaturated 4,5‐uronic acid (ΔUA) at the non‐reducing end has been adducted to mercuric acetate and the product characterized by MALDI‐TOF MS, confirming the formation of a mercury adduct (m/z calc. 639.9, obs. 640 with the expected spread of 6 mass units for Hg198 to Hg204, consistent with the production of a cyclic 4,5‐mercurinium intermediate (Skidmore et al., 2004).

β. Sulfated GAGs. A common method for analysis of these glycans is the use of basic peptides for ion pairing as outlined in the earlier reviews in this series. Thus, for example, cleavage of heparin by controlled γ‐irradiation has produced fragments enriched in highly sulfated sequences which were examined by MALDI‐TOF MS using ion‐pairing with (Arg‐Gly)19‐Arg to stabilize the sulfates (Bisio et al., 2004). Studies by MALDI‐TOF MS and HPLC of the serine protease inhibitor and its chondroitinase and hyaluronidase digestion products have shown that, in acute inflammation, its chondroitin‐4 sulfate chain is both longer and undersulfated compared with control glycoproteins (Capon et al., 2003). Combined MALDI‐TOF and enzyme digestion have also been used by Kett and Coombe (2004) for GAG analysis.
Studies Involving Both N‐ and O‐Linked Glycans
A method for simultaneous analysis of both N‐ and O‐linked glycans has been published by Robbe et al. (2003a). Glycans were released by non‐reductive β‐elimination using the ammonia‐based method described earlier by Huang, Mechref, and Novotny (2001) and labeled by reductive amination with either 2‐aminoacridone (AMAC, 1/58) or 8‐aminonaphthalene‐1,3,6‐trisulphonic acid (ANTS, 34). They were then separated by gel electrophoresis, the bands containing the glycans were excised and the derivatized glycans were extracted for analysis by MALDI and electrospray MS. Comparisons between the electrophoresis profiles from the AMAC (uncharged) and ANTS (charged) derivatives provided an indication of the charge state of the glycan. The method was designed mainly for profiling mucin glycans and was applied to porcine gastric mucin and bovine submaxillary mucin.

A popular method for the independent study of both N‐ and O‐glycosylation is to remove the N‐linked glycans with, for example, PNGase F and then examine the O‐glycosylation, either by further release using β‐elimination or by measurements on the glycopeptides obtained from the O‐linked region (Forno et al., 2004; Trimble et al., 2004). The well‐established differential hydrazinolysis method was used by Gervais et al. (2003) to study N‐ and O‐linked glycosylation of human recombinant chorionic gonadotropin allowing O‐linked glycans to be identified for first time.
Function of O‐Linked Glycans
With the recent sequencing of several genomes, attention has become focused on how the complexity of higher organisms can be encoded by such a small number of genes. Post‐translational modifications and, in particular, glycosylation have emerged as important determinants of the control of biological systems. Lamarre‐Vincent and Hsieh‐Wilson (2003) have studied glycosylation of cyclic AMP‐responsive element‐binding protein, a transcription factor essential for long‐term memory and have shown the first link between O‐GlcNAc and information storage in the brain. O‐GlcNAc was detected by specific enzymatic radiolabeling with galactose from [3H]UDP‐galactose to the 4‐position of O‐GlcNAc. MALDI‐TOF analysis of the tryptic peptides was used to show that the enzyme was modified with two O‐GlcNAc residues.
Most organisms synthesize α‐linked‐polyglucans, such as glycogen, as an energy source when other reduced carbon compounds are insufficiently available but, to date, the method for the initiation of glycogen synthesis in prokaryotes has not been determined. In an attempt to rectify this gap in our knowledge, Albrecht et al. (2004) have produced the eukaryotic enzyme yeast glycogenin (Glg2p), a known initiator in fungi and animals, in E. coli and found it to be autocatalytically glucosylated at tyrosine residues 232 and 230 with from 4 to 25 glucose residues, as determined by MALDI‐TOF and MS/MS analysis. Further incubation with UDPglucose resulted in transfer of more than 36 additional glucose residues over 20 min as detected by MALDI‐TOF. In another study, the N‐terminal segment of potato X virus has been found to be glycosylated at the N‐terminus with galactose or fucose and that the presence of the sugar mediates the formation of a bound water shell on the virion surface (Baratova et al., 2004).
Glycoproteins and Disease
Glycan profiling by MALDI‐TOF MS for the detection of disease is gaining ground. For example, a new method has been developed for the extraction of the acute‐phase glycoprotein, α1‐acid glycoprotein from human serum and its N‐glycans have been compared in patients with inflammation or cancer and healthy controls. The disease states produced an increase in both the degree of branching and in the amount of fucosylation (Kremmer et al., 2004; Szöllosi et al., 2004). The amounts of fucosylated bi‐, tri‐, and tetra‐antennary glycans were also found to increase at the expense of unfucosylated triantennary glycans in this glycoprotein in patients with acute inflammation (Higai et al., 2003a). Sialylated glycans were stabilized for MALDI‐TOF analysis by formation of methyl esters. The same stabilization method was used by Flahaut et al. (2003) in a study on the effect of ethanol on transferrin glycosylation in chronic alcoholics has revealed patient‐dependent differences in both the level of sialylation and in the number of glycans attached to the molecule, possibly accounting for the controversial use of transferrin glycosylation as a marker for chronic ethanol consumption.
Detection of cancer biomarkers
N‐glycans from normal human pancreatic cells consist mainly of complex biantennary structures with small amounts of tri‐ and tetra‐antennary compounds and larger compounds with poly‐N‐acetyllactosamine extensions and extensive fucosylation (up to five fucose residues and eight Gal‐GlcNAc units as detected by MALDI‐TOF MS). Glycans from ribonuclease extracted from pancreatic adenocarcinoma tumor cells, on the other hand, contain fucosylated hybrid structures and biantennary glycans whose antennae terminated in GalNAc, thus providing a potential diagnostic test for the adenocarcinoma (Peracaula et al., 2003a). MALDI‐TOF analysis gave much better resolution of the large glycans than could be obtained by HPLC. Altered glycosylation has also been found associated with prostate cancer; glycans from prostate‐specific antigen (PSA) contained increased amounts of fucose and GalNAc in the antennae, again indicating a possible diagnostic test (Peracaula et al., 2003b). Another study found increased amounts of α‐(2 → 3)‐linked sialic acid in human poly‐sialic acid (PSA) suggesting that differential binding to Maackia amurensis agglutinin lectin could be used to differentiate prostate cancer from benign prostate hypertrophy (Ohyama et al., 2004). Masses of some sialylated glycans were reported from linear MALDI‐TOF spectra but most measurements were made on neutral glycans following linkage‐specific removal of sialic acid. Increased fucosylation has also been found in N‐linked glycans from human hepatoma (Higai et al., 2003b) and melanoma (Ciolczyk‐Wierzbicka et al., 2004) cell lines and a reduction in the concentration of fucosylated glycoproteins has been found in dogs undergoing treatment for lymphosarcoma (Xiong, Andrews, & Regnier, 2003a). An extensive study of the N‐ and O‐glycosylation of the human ovarian tumor marker CA125 has been reported. The bi‐, tri‐, and tetra‐antennary glycans were identified by MALDI‐TOF, MS/MS and FAB mass spectrometry as permethylated derivatives coupled with exoglycosidase digestion (Wong et al., 2003). Highly processed glycans have also been found on the breast tumor marker protein, gross cystic disease fluid protein‐15, than on the same protein from healthy controls (Caputo et al., 2003). Glycans were again examined as permethylated derivatives but, this time, by surface‐enhanced laser desorption/ionization (SELDI) mass spectrometry. MUC1 glycoprotein is overexpressed in breast cancer and O‐Glycans have been shown to control proteolysis by preventing proteolysis of the Thr3‐Ser4 bond if either amino acid is glycosylated (Hanisch et al., 2003). Tetrasialylated‐tetra‐antennary glycans carrying a β1 → 6 antenna appear to be associated with the development of cancer metastases as demonstrated by studies on integrins from metastatic and non‐metastatic human melanoma cell lines (Pochec et al., 2003). MALDI‐TOF profiles were obtained following in‐gel glycan release using the method described by Küster et al. (1997) as modified by Hoja‐Lukowicz et al. (2000). Bi‐ and tri‐antennary glycans have been identified attached to human chorionic gonadotropin produced in a choriocarcinoma cell line (JEG‐3); the biantennary glycans, which were useful markers, were unusual in having a branched 3‐antenna (35) rather than carrying both unbranched 3‐ and 6‐antenna (Takamatsu et al., 2004). The structure was confirmed by MALDI‐TOF analysis of exoglycosidase digestion products with an α‐mannosidase digestion following β‐galactosidase digestion to confirm the presence of the exposed mannose residue on the 6‐antenna. The study illustrates the danger of assigning structures directly by matching masses with those from a database or by using only the usual exoglycosidase sequence of β‐galactosidase followed by N‐acetylhexosaminidase which would incorrectly have suggested a normal biantennary structure such as 23.

Congenital diseases of glycosylation
Matrix‐assisted laser desorption/ionization‐time‐of‐flight (MALDI‐TOF) analysis of both the N‐glycans released from tryptic peptides and of the peptides themselves has shown that the asparagine residues of α1‐antitrypsin in patients suffering from congenital disorder of glycosylation (CDG) type I (deficiency in the initial protein glycosylation by Glc3Man9GlcNAc2, 36) are preferentially glycosylated in the order Asn‐46 > 247 > 83 (Mills et al., 2003a). Asparagine occupation was measured by observing the extent to which it was converted to aspartic acid during glycan removal by the enzyme PNGase F. In a newly characterized disorder, CDG‐1i, MALDI‐TOF and HPLC were used to show that some of the N‐glycans on fibroblasts consisted only of Man1GlcNAc2 and Man2GlcNAc2 (Thiel et al., 2003). Analysis by MALDI‐TOF and HPLC of serum glycoproteins from several patients with CDG type II syndromes (defective glycan processing) has been used to identify several enzyme defects. One patient was characterized with a mannosidase‐III deficiency that prevented biosynthesis of complex glycans as the result of failure to remove residual mannose residues from the 6‐antenna. The predominant hybrid glycan 37 was characterized by CID using a MALDI‐Q‐TOF instrument (Butler et al., 2003). α‐Mannosidase deficiency causes accumulation of mannose‐containing oligosaccharides (released from N‐glycans) in various tissues but the disease has been successfully treated by enzyme replacement therapy. Residual glycans were monitored in this study by MALDI‐TOF MS (Roces et al., 2004). A GlcNAc‐transferase‐II deficiency in another patient in the study by Butler et al. prevented elaboration of the 6‐antenna and resulted in the same biantennary glycans with a branched 3‐antenna (35) as observed in the choriocarcinoma cell line (JEG‐3) discussed above. In this case, however, the glycan was identified by negative ion MS/MS. Mass spectrometric methods for elucidating the pathogenesis of CDG type IIx have been reviewed with examples of transferrin and α1‐antitrypsin glycosylation (Mills et al., 2003b).


Other diseases
The hinge region of IgA1 has been shown by MALDI‐TOF MS to be under‐O‐glycosylated in patients with IgA neuropathy (Horie et al., 2003a). As upper respiratory tract infection often precedes hematurea and proteinurea in these patients, Horie et al. (2003b) have investigated the effect of tonsillectomy and steroid treatment and found that they produce increases in the extent of IgA O‐glycosylation. Again, analysis was by MALDI‐TOF with s‐DHB as the matrix. A method has been reported for detection of the biomarker, serum amyloid component, in human plasma or urine. Extraction was by monoclonal antibody binding and analysis by MALDI‐TOF MS from sinapinic acid. Several new, desialylated compounds were detected from three individuals (Kiernan et al., 2004). Krokhin et al. (2003) have identified, in a preliminary study, several high‐mannose and complex N‐glycans from the spike protein of the SARS virus taken directly from patients. The compounds were identified mainly with the aid of MS/MS on a Q‐TOF instrument.
Glycated Proteins (Non‐Enzymatic Attachment of Sugars)
Work with these compounds usually involves analysis of intact proteins that are modified with chemically attached glycan residues and are ideal subjects for the high mass resolving ability of MALDI‐TOF instruments. Analysis of β‐lactoglobulin glycation products formed from lactose and galactose has revealed lysine as the main binding site with additional binding at the amino‐terminal leucine residue and at Arg‐124. MALDI‐TOF analysis was found to be more efficient than LC/ESI‐MS for locating the binding sites (Fenaille et al., 2004). MALDI‐TOF has, for the first time, enabled kinetic data to be obtained on defined glycation products from specific sites of a glycated protein. Thus, lysozyme was incubated with d‐glucose for 1, 4, 8, or 16 weeks in phosphate‐buffered saline, digested with endoproteinase Glu‐C and the C‐ and N‐terminal peptides were used for the relative quantification of the initial Amadori product, N ε‐(carboxymethyl)lysine (38) and an imidazolone (39) formed from arginine (Kislinger et al., 2003). The preferred glycation sites in human serum albumin (HSA) have been determined as lysines 233, 276, 378, 525, and 545 following incubation of HSA with glucose, enzymatic digestion of the protein with either trypsin or endoproteinase Lys‐C, and examination of the resulting glycated peptides by LC/MS/MS and MALDI‐TOF from CHCA (Lapolla et al., 2004a). Glycation of casein by glucose, fructose or ribose has been characterized and shown not to affect its protective effects on human intestinal Caco‐2 cells (Jing & Kitts, 2004). A study of 20 Type 2 diabetic patients and 10 controls have shown that complications of diabetes are associated with a higher concentration of glyco‐oxidized variants of globin than observed in controls or patients without complications (Lapolla et al., 2004b).


Several other studies on advanced glycation end products (AGEs) have been reported. Yamada et al. (2004) have synthesized a collagen model peptide and subjected it to glycation with glucose, ribose and glyoxal (40). A dimeric peptide linked glyoxal‐lysine dimer (41) was isolated and identified by MALDI‐TOF as the first example of an intact glycation‐derived dimer. The same technique has been used to show that methylglyoxyl, produced during the Maillard reaction binds to Arg‐184 and Arg‐185 located within the binding site of bovine glutathione peroxidase and to deactivate the enzyme, resulting in an increase in the intracellular peroxidases responsible for oxidative cellular damage (Park et al., 2003b). AGEs from ribose were formed more rapidly when incubated with bovine serum albumin (BSA) than those from glucose or fructose as measured by binding to AGE receptors (Valencia et al., 2004). The use of in‐house‐developed PERL script software has been used to identify and localize AGEs on β2‐microglobulin (Cocklin et al., 2003; Zhang et al., 2003d). The program identifies masses, measured by MALDI‐TOF MS that correspond to those of peptides with an AGE modification. In other studies, the AGEs N ε‐(carboxymethyl)‐lysine, imidazolone A (42) and B (43), pyrraline (44) and 1‐alkyl‐2‐formyl‐3,4‐glycosyl pyrrole (45) have been found on the type 2 ryanodine receptor calcium‐release channel, accounting for decreased cardiac contractility in diabetes (Bidasee et al., 2003). The soluble AGE receptor (sRAGE), a member of the immunoglobulin superfamily, has been characterized from mouse and its N‐glycosylation identified by MALDI‐TOF MS (Hanford et al., 2004). In another study, measurements of protein molecular weights have added support to the conclusion that Amadori decomposition pathways are constrained in the presence of metal ion chelators and radical traps (Culbertson et al., 2003).




Although protein glycation is usually associated with pathology, as in the secondary effects of diabetes, the reaction is also involved in cooking where it changes the color (browning) and flavor of the food. Fay and Brevard (2004) have recently reviewed the use of mass spectrometry in the study of the involved Maillard reaction.
Peptidoglycans
One protein encoded by the 13 Drosophila peptidoglycan recognition proteins has been shown to possess enzymatic activity. MALDI‐TOF analysis of the degradation products demonstrated that the enzyme hydrolyses the lactylamide bond between the glycan chain and the cross‐linking peptides. The enzyme was, thus, classified as an N‐acetylmuramoyl‐l‐alanine amidase (Mellroth, Karlsson, & Steiner, 2003). HPLC and MALDI‐PSD were used by Antignac et al. (2003) to characterize the peptidoglycan from Neisseria meningitidis. The peptidoglycan was hydrolyzed with muramidase from Streptomyces globisporus. Fourteen of the 28 muropeptides separated by HPLC were found to be O‐acetylated to different degrees. Proteoglycan‐like glyconectins from three sponges, Microciona prolifera, Halichondria panicea, and Cliona celata have been examined by NMR and mass spectrometry. The structures were complicated and contained distinct acid‐resistant and acid‐sensitive domains. Some sugars were sulfated and others pyruvylated (Guerardel et al., 2004). Muropeptides derived from Escherichia coli peptidoglycan have been labeled with the fluorescent dyes ANTS, 7‐aminonaphthalene‐1,3‐disulfonic acid (ANDA, 46) or 4‐aminonaphthalene‐1‐sulfonic acid (ANSA, 47) and examined by fluorophore‐assisted carbohydrate electrophoresis (FACE). Results compared favorably, both qualitatively and quantitatively with earlier HPLC‐based methods. MALDI‐TOF MS was used to confirm the identity of the FACE separated glycans (Li, Höltje, & Young, 2004d).

Glycoconjugates from Bacteria
Some small anaerobic bacteria such as treponemes contain glycoconjugates that are different from the normal glycolipids. One of these glycoconjugates has been isolated from the oral spirochete, Treponema medium by phenol/water extraction and the carbohydrate removed enzymatically for structural identification by NMR and MALDI‐TOF MS. Fragmentation was performed with a TOF/TOF instrument and revealed the glycan sequence of a tetrasaccharide repeat unit containing the two amino acids ornithine (48) and Asp. The repeat unit ( → 4)‐β‐d‐GlcpNAc3NAcA‐(1 → 4)‐β‐d‐ManpNAc3NAOrn‐(1 → 3)‐β‐d‐GlcpNAc‐(1 → 3)‐α‐d‐Fucp4NAcp‐(1 → ) contained mannose that was amide‐linked to l‐ornithine and fucose linked to d‐aspartic acid (Hashimoto et al., 2003a).

Glycolipids
Lipo‐Poly‐ (LPS) and ‐Oligosaccharides (LOS)
Many examples of the application of MALDI‐based methods to analysis of these compounds have been published; these are summarized in Table 6 and only a few representative studies and some with more unusual features will be discussed here. Extractions are normally carried out with hot aqueous phenol. These compounds often contain many more monosaccharide constituents than are found in the glycoproteins, requiring additional techniques such as combined gas chromatography/mass spectrometry (GC/MS) and NMR for their analysis. Their generally acidic nature, conferred by Kdo glycans and phosphate groups, makes them ideal candidates for examination in negative ion mode.
Table 6.
Use of MALDI MS for examination of bacterial glycolipids

Intact LOS
Although small lipooligosaccharides can be examined directly, most have to be modified by hydrolysis of the large O‐glycan chain or by removal of much of the lipid component of the lipid A (1/18) moiety. Thus, for example, the backbone structures of the LOS from V. parahaemolyticus strains have been obtained following O‐ and N‐deacylation with hydrazine and dephosphorylation with TFA (Hashii et al., 2003a,b). Laser‐induced decomposition can be a problem or, in some cases, can yield useful structural information. Thus, the negative ion MALDI‐TOF spectrum from DHB of the short‐chain LOS from a strain of V. parahaemolyticus (3 kDa) following deacylation showed successive losses of phosphoethanolamine (49), hexuronic acid and phosphate (Hashii et al., 2003b). LOS from M. catarrhalis has been deacylated with hydrazine and examined in negative linear mode from DHB/hydroxy‐iso‐quinoline (HIQ). The spectrum showed several peaks produced by in‐source cleavage at the labile Kdo‐lipid A glycosidic bond (Luke et al., 2003). The lipid A molecule itself, examined in reflectron mode, was found to contain seven acyl groups and varying amounts of ethanolamine esterified to the two phosphate groups. Both MALDI‐TOF and nanospray MS/MS analysis of the de‐O‐acylated (hydrazine) LOS from Pseudoalteromonas haloplanktis TAC 125 grown at 15°C has shown the presence of three phosphate groups, two on the lipid A and one on the core oligosaccharide, whereas at 25°C, an additional phosphate was added to the core (Ummarino et al., 2003). The LOS from Pseudoalteromonas issachenkonii KMM 3549T has been found to contain what is thought to be the first example of a 4,7‐disubstituted heptose as determined mainly by NMR; MALDI‐TOF MS was used to delineate the lipid A acylation pattern (Silipo et al., 2004b). Two sets of ions were obtained as the result of cleavage of the labile Kdo‐lipid A bond.

Haemophilus ducreyi has been shown to be capable of incorporating sialic acids with N‐acyl groups larger than acetyl. However, the extent of sialylation dropped with increasing chain length, becoming non‐existent for sialic acid with chains above C8 (Goon et al., 2003). The LOS was deacylated with hydrazine and examined by MALDI‐TOF from DHB/HIQ in negative ion mode; positive ion MS/MS spectra were obtained with electrospray ionization.
Lipid A
Mild acid hydrolysis of the labile bond between Kdo and the lipid A moiety is the normal method for releasing lipid A from these glycolipids. A method combining thin‐layer chromatography (TLC) with both MALDI‐TOF and ESI‐MSn (up to MS4) has been applied to the analysis of lipid A from E. coli. The predominant fragmentation was loss of acyl groups but, whereas this appeared to occur by charge‐remote processes in the CID spectra, charge‐driven processes appeared to predominate in the MALDI spectra (Lee et al., 2004a).
Several novel lipid As have been discovered during the review period. The first example of a neutral lipid A has been isolated from the predatory bacterium Bdellovibrio bacteriovorus (Schwudke et al., 2003). The molecule consisted of a β‐(1 → 6)‐linked 2,3‐diamino‐2,3‐dideoxy‐d‐glucopyranose disaccharide carrying six hydroxylated fatty acids. α‐d‐mannopyranose residues occupied the two positions normally occupied by phosphates. A similar 2,3‐diamino‐2,3‐dideoxy‐d‐glucopyranose disaccharide carrying six hydroxylated fatty acids was found in the lipid A from Leptospira interrogans which causes a hemorrhagic fever known as Weil's disease. The molecule carried only one phosphate group which was mono‐methylated. The two hydroxy‐acyl groups attached to the diamino‐glucose residue I were esterified with unsaturated ω‐6‐12:1 and 14:1 fatty acids (Que‐Gewirth et al., 2004). 2,3‐Diamino‐2,3‐dideoxy‐d‐glucopyranose has also been found in lipid A from Bartonella henselae, a Gram‐negative bacterium that causes cat‐scratch disease (Zähringer et al., 2004) and in that from Mesorhizobium huakuii. In the latter compound, the phosphate group normally attached to the di‐NH2‐Glc moiety II was replaced by d‐glucuronic acid (Choma & Sowinski, 2004). A 4‐amino‐4‐deoxy‐l‐arabinopyranose‐1‐phosphate has been found at the reducing end of the lipid A from Burkholderia caryophylli (Molinaro et al., 2003). The negative ion MALDI‐TOF (DHB) spectrum showed considerable heterogeneity due to differing numbers of acyl groups. The proximal (reducing end) GlcN residue of lipid A from Rhizobium leguminosarum lacks a phosphate group and can be oxidized to 2‐amino‐2‐deoxy gluconate by an enzyme recently identified in the outer membrane of the bacterium (Que‐Gewirth et al., 2003). The first example of lipid A from an Agrobacterium species (A. tumefaciens strain C58, a plant pathogen) has revealed the presence of a normal bis‐phosphorylated glucosamine disaccharide backbone with several acyl variants. The main species was a penta‐acyl derivative bearing two 3‐OH‐14:0 fatty acids in ester linkage and two 3‐OH‐16:0 acids in amide linkage. The 3‐OH‐16:0 acid on GlcN II was esterified with 27‐OH‐28:0 that, in turn was esterified with a 3‐OH‐butyroyl residue (Silipo et al., 2004a). Other lipid A molecules to have been analyzed in the review period are listed in Table 6.
Core oligosaccharide
Mild acid hydrolysis is also the method normally used to obtain core oligosaccharides from LPS. Ethanolamine diphosphate has been identified for the first time and its position of substitution established in the LPS core of a rough‐type mutant of Pseudomonas aeruginosa (PAO1 ΔalgC). In addition, the paper reported a reinvestigation of the structure of the complete LPS core of wild‐type P. aeruginosa PAO1 and a reassignment of the phosphorylation sites (Kooistra et al., 2003). The LPS was complex because of the presence of esterified fatty acids that were removed by mild hydrazinolysis prior to analysis by ESI and MALDI. Phosphoethanolamine has also been found in strains of N. meningitidis (Tzeng et al., 2004) and 4,6‐O‐(1‐carboxy)‐ethylidine residues derived from pyruvic acid have been identified in the core of LOS from Pseudomonas stutzeri OX1 (Leone et al., 2004a).
O‐chain
The O‐chain from Bordetella avium LPS, obtained by mild acid hydrolysis to remove the lipid A followed by hydrofluorolysis to remove the core oligosaccharide, was found to consist only of a chain of the substituted 1 → 4 linked monosaccharide 2‐acetamido‐3‐(3‐hydroxybutanamido)‐2,3‐dideoxy‐β‐d‐glucopyranosyluronic acid (50). The negative ion MALDI‐TOF spectrum from DHB showed a series of peaks from 1 to 3.8 kDa (Larocque et al., 2003). Other examples of O‐chain analysis are cited in Table 6.

Extracellular polysaccharides (EPS)
Several examples of recent analyses of EPS are cited in Table 6 and only two representative examples will be expanded here. The structure of the repeating unit of the EPS from Butyrivibrio fibrisolvens strain H10b is a hexasaccharide carrying O‐acetyl and 1‐carboxyethyl groups and has been determined with a variety of techniques including NMR and circular dichroism (CD) following extraction with phenol. MALDI‐TOF MS of fractions from a partial acid hydrolysate was used to determine the carbohydrate sequence (Andersson, Cotta, & Kenne, 2003). B. cepacia, a bacterium causing lung infections was obtained from a cystic fibrosis patient and cultured on agar plates. Phenol was added to the harvested cells, stirred for 5 hr at 4°C and the EPS was precipitated with isopropanol. MALDI‐TOF and NMR were used to determine the structure of the repeating unit as → 5)‐β‐Kdop‐(2 → 3)‐β‐d‐Galp2Ac‐(1 → 4)‐α‐d‐Galp‐(1 → 3)‐β‐d‐Galp‐(1 → ). When grown on a mannitol‐rich medium, conditions that induce the formation of EPS, the bacteria produced a second compound with the structure ( → 6)‐β‐d‐Fruf‐(2 → ) (Cescutti et al., 2003).
Glycosphingolipids (GSL)
Studies on the glycan moiety following removal of the ceramide
Glycosphingolipids can be analyzed directly by MALDI‐TOF but the spectra are usually complicated because of heterogeneity in the ceramide portion of the molecule. Thus, many investigators use an enzyme such as ceramide glycanase to remove the ceramide. A recent example is a study of the effect of N‐alkyl analogues of deoxynojirimycin (DNJ), inhibitors of ceramide glucosyltransferase, on GSL structure. GSLs were cleaved with ceramide glycanase and labeled with 2‐AB (1/56) for analysis by HPLC and MALDI‐TOF (Mellor et al., 2004a). Carbohydrates substituted with phosphocholine (PC) and phosphoethanolamine (PE) have been released with endoglycoceramidase from glycosphingolipids of the pig parasitic nematode Ascaris suum and identified by MALDI, ESI, GC/MS (methylation analysis) and NMR (Friedl et al., 2003).
A rapid method for splitting glycosphingolipids into their three components (sugar, sphingosine and fatty acid) by use of a domestic microwave oven has been reported by Itonori et al. (2004b). The glycosphingolipids were heated with 0.1 M NaOH/MeOH for 2 min followed by 1 M HCl/MeOH for 45 sec. The alkaline methanolysis step produced the intermediate lysoglycosphingolipids virtually free of by‐products such as the O‐methyl ethers that are usually seen but the N‐acetylamino‐sugars needed re‐acetylation by reaction with acetic anhydride/pyridine at room temperature.
Studies by TLC and MALDI
Glycosphingolipids are frequently separated by TLC. Ivleva et al. (2004) have published a method for attaching TLC plates to a MALDI target and have obtained spectra of even fragile compounds such as gangliosides by using an FTICR instrument fitted with a vibrationally cooled MALDI ion source. Twenty matrices were investigated and the soft matrices, DHB, ATT and anthranilic acid (1/57) were found to produce the least sialic acid loss from gangliosides. TLC resolution was best preserved by using matrices in rapidly evaporating organic solvents.
Applications to the structural elucidation of glycosphingolipids
A novel GlcNAc‐α‐1 → HPO3 → 6‐Gal‐(1 → 1)‐ceramide (51) has been identified from the liver fluke, Fasciola hepatica, by MALDI‐TOF and electrospray‐MS/MS. HF treatment released the GlcNAc residue, confirming the GlcNAc‐phosphate link (Wuhrer et al., 2003b). Mammalian type globo‐ and iso‐globotriaosylceramides have also been found in this species obtained from infected sheep. MALDI‐TOF spectra were obtained from both the intact glycosphingolipids and the 2‐AP derivatives of their released glycans using ATT as the matrix, and the presence of antigenic terminal α‐galactose was demonstrated by on‐target incubation with α‐galactosidase (from chicken) (Wuhrer et al., 2004a). Ceramides carrying β‐Gal‐(1 → 6)‐β‐Gal‐(1 → 1)‐Cer and larger oligosaccharides with from one to three additional β1 → 6‐linked mannose residues represent a departure from the common glycosphingolipids that contain inositol phosphate found in many fungi and may account for the resistance of this species to the fungicide, Aureobadidin A (Aoki et al., 2004b). Measurements by SELDI MS of tetanus neurotoxin (53.7 kDa) incubated with the ganglioside GT1b, and observation of the intact protein/ganglioside complex, has shown that two carbohydrate binding sites are required for toxicity (Rummel et al., 2003).
Miscellaneous studies
Two photoaffinity probes (ganglioside GM2 labeled on the acyl chain with a p‐(3‐trifluoromethyl)diazirinyl‐phenyl group) (52), were used to probe the active site of GM2‐activator protein. After binding, and trypsinization, the tryptic peptides were examined by MALDI‐TOF and ESI‐MS and found to be bound to the most flexible region of the protein (Wendeler et al., 2004a). γ‐Radiolysis of glucosylceramide and galactosyl diglyceride resulted in the release of the sugars as shown by MALDI‐TOF MS (Shadyro et al., 2004). Other studies and details of the above work are summarized in Table 7.

Table 7.
Use of MALDI MS for examination of glycosphingolipids

Glycoinositolphosphosphingolipids (GIPSL)
Novel zwitterionic glycosphingolipids having the sugar chain capped with phospholcholine have been reported from the mycelia of filamentous fungi of Acremonium species (Aoki et al., 2004a). Structures were of the type PC → 6‐(Man‐(α1 → 6)‐Man‐(α1 → 6)‐GlcN‐(α1 → 2)‐Ins1‐P‐1‐Cer. A related compound without the attached glycan chain has been identified from the red alga Gracilaria verrucosa (Khotimchenko & Vas'kovsky, 2004).
Glycoglycerolipids
The glycoglycerolipid α‐d‐Glcp‐(1 → 3)‐α‐d‐Glcp‐(1 → 1)‐lipid where the lipid is glycerol containing a C15 acyl group at C‐2 and a C15 ether at C3 has been separated from the propionibacterium Propionibacterium propionicum by TLC and its structure determined by MALDI‐TOF, NMR, ESI/MS, and GC/MS. Related compounds acylated at C6 of the outer glucose residue and compounds lacking either this glucose or one fatty acid residue were also found (Pasciak et al., 2003). Glycoglycerolipids from the thermoplilic bacterium Meiothermus taiwanensis have been shown to have the structure α‐Galp‐(1 → 6)‐β‐Galp‐(1 → 6)‐β‐GalNAcyl‐(1 → 2)‐α‐Glc‐(1 → 1)Gro where Acyl = 17:0 or hydroxy‐17:0 (Yang et al., 2004). A similar compound from the thermophilic bacterium Thermus oshimai NTU‐063 has the structure β‐Glcp‐(1 → 6)‐β‐Glcp‐(1 → 6)‐β‐GlcpNAcyl‐(1 → 2)‐α‐Glcp‐(1 → 1)‐glycerol diester where the N‐acyl group is a 15:0 or 17:0 fatty acid and the glycerol‐attached fatty acids have chain lengths from 15:0 to 18:0. The MALDI‐TOF spectrum contained a range of peaks from m/z 1,300 to 1,550 (Lu et al., 2004a). Di‐mannosyl‐acyl monoglyceride in which the mannose residue adjacent to the glycerol contained an anteiso‐branched 15‐carbon acid at the 6‐position has been characterized from Rothia mucilaginosa, a pathogen responsible for several opportunistic infections. Unusually the acyl group attached to the glycerol was at C2 rather than the more usual C3 (Pasciak et al., 2004).
Lipomannans, Lipoarabinomannans, and Arabinomannans
The structure of a novel mannose‐capped lipoarabinomannan from Amycolatopsis sulphurea has been investigated with a range of techniques of which MALDI‐TOF (DHB, negative ion) showed an average molecular mass of around 10 kDa but with a broad, unresolved envelope of peaks with a half‐height width of approximately 5 kDa (Gibson et al., 2003). The lipoarabinomannan from the mycobacterium‐like organism Tsukamurella paurometabola has produced a MALDI‐TOF spectrum from s‐DHB with a broad peak with a mass range from 9 to 16 kDa. The structure was mainly determined by NMR. It lacked some of the features such as branching arabinan chains that are found in arabinomannans from mycobacteria but, nevertheless the molecule was claimed to be the most elaborated non‐mycobacterial arabinomannan identified to date (Gibson et al., 2004). Lipomannan and lipoarabinomannan from Mycobacterium kansasii has been shown to possess a manno‐oligosaccharide cap but to lack the phosphoinositol cap found in lipoarabinomannans from Mycobacterium smegmatis (Guérardel et al., 2003). Mechanistic studies on the biosynthesis of lipoarabinomannan from M. smegmatis have been conducted with the help of MALDI‐TOF analysis (Korduláková et al., 2003; Zhang et al., 2003c).

Other Glycolipids
Mass spectrometric methods for analysis of flavonoids have been reviewed (Prasain, Wang, & Barnes, 2004). Three glycosyltransferases (Pérez et al., 2004b) and two methyltransferases (Pérez et al., 2004a) involved in the biosynthesis of phenolic glycolipids from Mycobacterium tuberculosis have been characterized. MALDI‐TOF spectra of the glycolipids showed a range of produces with masses of around 1.5 kDa resulting from acyl heterogeneity. A new mannitol teichoic acid has been reported from the cell wall of the bacterium Brevibacterium sp. VKM Ac‐2118 isolated from a frozen late Pliocene layer (1.8–3 Myr) of Kolyma lowland, Russia. The structure consisted of 1,6‐poly(mannitol phosphate) with the majority of mannose residues carrying additional phosphate groups. Positive ion MALDI spectra from DHB gave peaks to 6 kDa (Potekhina et al., 2003). The structures of hexamannosides from mycobacterial species have been elucidated and found to contain up to four long‐chain acyl groups (Gilleron, Quesniaux, & Puzo, 2003). A typical structure is 53. Studies with M. smegmatis have shown that mannose metabolism, as revealed by studies on phosphoinositol mannosdes, is required for growth (Patterson et al., 2003). Mycoglycolipids have been isolated from the edible fungus Hypsizygus marmoreu and identified by GC/MS and MALDI‐TOF MS (Itonori, Aoki, & Sugita, 2004a). The biosynthesis of mycobacterial phosphatidyl mannosides has been revised with the help of MALDI‐TOF analysis (Morita et al., 2004). PIM4 (four mannose residues and two palmitate chains) present in mycobacterial phosphatidyl manno‐oligosaccharide, identified by PSD from CHCA, has been shown to be a natural antigen for CD1d‐restricted T cells (Fischer et al., 2004).
Glycosides
Although MALDI‐TOF MS is used quite extensively in this field, FAB still appears to be the most commonly encountered ionization technique. Glycosides identified by MALDI during the review period are listed in Table 8. Glucosylated heteropolyflavans (e.g., 54) from Sorghum bicolor have been examined by Krueger, Vestling, and Reed (2003); polymers with from three to seven flavan units and up to seven glucose residues were found. To overcome problems with estimation of the number of hydroxyl groups present due to the coincidence in mass with the [M + K]+ ion, samples were analyzed as cesium adducts by addition of cesium trifluoroacetate to the sample/matrix solution. Silver trifluoroacetate was also investigated but problems were encountered with cluster formation and the two mass unit difference between the silver isotopes. Over 28 triterpene saponins, 8 of them new, have been identified in a fraction, named QH‐B from the South American tree Quillaja saponaria. The 28 saponins formed 14 equilibrium pairs by the migration of an O‐acyl group between two adjacent positions on a fucosyl group (Nyberg, Baumann, & Kenne, 2003). The triterpene glycoside from the sea cucumber Stichopus mollis (Stichopodidae) was found by Moraes et al. (2004) to differ in structure from the corresponding glycosides from all other known stichopodids in possessing a sulfate group in the carbohydrate chain, prompting the authors to propose a reclassification to a new genus, Australostichopus Levin. Both MALDI and ESI in negative ion mode have been demonstrated to be capable of detecting carminic acid (55), the red dye isolated from cochineal, in paintings applied to canvas. Binding agents such as linseed oil did not interfere with the detection (Maier, Parera, & Seldes, 2004). MALDI‐TOF has also been used to study the biotransformation of ginsenoside Rb1 by 49 microbial strains (Dong et al., 2003).



Table 8.
Use of MALDI MS for examination of glycosides

Osmoregulated Periplasmic Glucans
These compounds are mixtures of cyclic, branched, and linear polymers of glucose containing various species‐different substituents. They accumulate in the periplasmic space of gram‐negative bacteria in response to low osmolarity. Lequette et al. (2004) have identified the gene that encodes for the protein that controls the backbone structure of these carbohydrates. Associated analysis of the carbohydrates with masses up to 3.5 kDa was performed by MALDI‐TOF from DHB or 3‐AQ.
GLYCOSYLATION AND OTHER REACTION MECHANISMS
Matrix‐assisted laser desorption/ionization‐time‐of‐flight mass spectrometry (MALDI‐TOF MS) has been used by many laboratories to monitor the products of enzyme reactions aimed at deducing mechanisms of action or characterization of newly discovered enzymes. Much of this work is summarized in Table 9. Desorption/ionization on silicon mass spectrometry (DIOS‐MS) or laser desorption without a matrix has been shown to be a viable alternative to MALDI for analysis of small molecules and carbohydrates and has been used to monitor the activity of α2 → 6‐sialyltransferase on the disaccharide Gal‐β‐(1 → 4)‐GlcNAc (Shen et al., 2004). Jobron et al. (2003) have immobilized N‐acetyllactosamine on a cellulose membrane and used it as an acceptor for assaying four glycosyltransferases. The trisaccharide produced by α1 → 3‐galactosyltransferase was identified by MALDI and the method was proposed as the basis of a high‐throughput technique for enzyme screening. A method for detecting the presence of O‐glycanase, an enzyme that cleaves the Galβ‐(1 → 3)‐GalNAcα‐(1 → ) link to serine or threonine, uses MALDI to detect the [M + Na]+ ion at m/z 406 corresponding to the released sugar (Akai et al., 2003).
Table 9.
Use of MALDI to study the products of enzyme action on carbohydrates

INDUSTRIAL APPLICATIONS
Food Industry
A MALDI‐TOF method for the simultaneous detection and measurement of sugars, ascorbic acid (57), citric acid (58), and sodium benzoate has been developed by Ayorinde, Bezabeh, and Delves (2003). The matrix was meso‐tetrakis‐(pentafluorophenyl)porphyrin (59), a compound with a molecular weight of 974 which is well above that of the target molecules. Sodium or potassium hydroxides were used as dopants. The method was particularly good at detecting the low molecular weight organic acids. MALDI‐TOF MS, from DHB also provides a method for detecting the presence of starch hydrolysates as adulterants in fruit juices (Cabálková et al., 2004). The action of the enzyme preparation, Olivex, used in the preparation of olive (Olea europaea) oil has been investigated. The preparation is mainly a pectinase that hydrolyses (1 → 4)‐linked galactan chains from the cell wall. However, it is unclear how this reaction improves the quality of the product (Vierhuis et al., 2003). A comparative study of five Rhizobium strains from different cropping areas of China has concluded that differences in the efficiency of nitrogen fixation is not related to differences in Nod factor structure (identified by MALDI‐TOF and ESI‐Q‐TOF MS) (Thomas‐Oates et al., 2003).


Biopharmaceutical Industry
Production of humanized antibodies and other biopharmaceuticals in plants and insect cell lines, as a safe and economically feasible alternative to production in animals, requires re‐engineering of the N‐glycan synthesis machinery to eliminate potentially antigenic α1 → 3‐fucose‐ and β1 → 2‐xylose‐containing glycans. MALDI‐TOF analysis is ideally placed for analysis of the resulting glycans and many examples are listed in Table 10. An analysis of human transferrin expressed in Lymantria dispar (gypsy moth), however, has shown the natural absence of core α1 → 3‐fucosylation, a normally common motif in insects (Choi et al., 2003b). To study the specificity of IgG and IgE antibodies against the α1 → 3‐fucose and β1 → 2‐xylose epitope, Bencúrová et al. (2004) have modified the relatively simple biantennary glycans on human transferrin to incorporate these epitopes. Products were monitored by MALDI‐TOF MS and antibody binding was reported to be greatest for the fucosylated glycans. Maeda et al. (2004) have replaced all of the asparagine residues of the N‐glycosylation sites in procarboxypeptidase Y by alanine and expressed the protein in P. pastoris. The resulting protein lacked N‐glycosylation but contained residual glucose, thought to be O‐linked, as detected by an increase in the MALDI mass from that expected from the amino acid sequence. Analysis of erythropoietins (EPO) with varying glycosylation has shown the fully sialylated tetra‐antennary glycan, identified by HPLC and MALDI‐TOF MS as its 4‐AA derivative, to be the major determinant of biological activity (Yuen et al., 2003). Sanz‐Nebot et al. (2003) have recorded MALDI spectra from intact human recombinant EPO and noted a drop in measured mass with increasing laser power which they attributed to loss of sialic acid within the ion source. MALDI‐TOF has been used to identify O‐glycans from a von Willebrand factor C domain produced in P. pastoris (O'Leary et al., 2004). Methods for their removal were investigated.
Table 10.
Use of MALDI analysis to monitor N‐glycosylation in biopharmaceuticals
A method for monitoring glycosylation in pharmaceuticals based on capillary electrophoreses of 3‐AA derivatives with structural confirmation by MALDI‐TOF MS has been published by Kamoda et al. (2004). MALDI and ion exchange chromatography have proved useful for checking the batch‐to‐batch variation in glycosylation of three recombinant gonadotropins. As an example, excellent consistency (3.5) was found for the degree of sialylation of 120 batches of recombinant thyroid stimulating hormone over a 3‐year period (Gervais et al., 2003).
Several reviews on the production of biopharmaceuticals and containing reference to MALDI‐TOF analysis have been published. These include production of human therapeutic proteins in yeasts (Bretthauer, 2003), yeasts and filamentous fungi (Gerngross, 2004) and lepidopteran cell lines (Tomiya et al., 2004). Methods for characterizing biological products after manufacturing changes have also been reviewed (Chirino & Mire‐Sluis, 2004).
Paper Industry
Slime deposits in paper mills, termed biofouling, is a problem in the paper industry and has prompted studies on the composition of their glycolipids. The EPS from Methylobacterium strains, responsible for “pink slime” have been shown by Verhoef et al. (2003) to consist of the repeating unit: ( → 3)‐[4,6‐O‐(1‐carboxyethylidene)]‐α‐d‐Galp‐(1 → 3)[4,6‐O‐(1‐carboxyethylidene)]‐α‐d‐Galp‐(1 → 3)‐α‐d‐Galp‐(1 → ).
CARBOHYDRATE SYNTHESIS
Sadly, many chemical papers appear to ignore details of the equipment and conditions used to obtain mass spectra of carbohydrates (and other compounds) even though conditions such as the choice of MALDI matrix are often essential in getting the compounds to “fly.” Although NMR experiments and equipment are usually described in great detail, information on mass spectrometry is frequently reduced to uninformative acronyms such as HRMALDIMS with no further details. Without knowledge of the equipment used to record the spectra it is difficult to judge if HR is indeed meaningful. The relegation of essential methods to “supplementary information” is also to be regretted. The absence of essential information such as the matrix from many publications is reflected in Table 11 (with apologies to authors from whose papers this information has been missed). “MALDI” is used for papers omitting to cite the type of instrument used to record the spectra. Most of the publications in this area relate to routine monitoring of reaction products and are summarized in Table 11. In addition to chemical reactions, enzymes are frequently used in this area because of their stereospecificity. Both transferases and glycosidases such as PNGase F used in their reversed mode to catalyze transglycosylations (Park, Lee, & Park, 2003a) can be used; however, evidence has been found to suggest that enzymes used for reverse hydrolysis reactions can have their activity reduced by Maillard glycation (Maitin & Rastall, 2004). As with the previous review, the two areas that are particularly relevant for monitoring are those of glycodendrimers and glycoprotein conjugates.
Table 11.
Use of MALDI MS for monitoring products of synthetic reactions

Synthesis of Multivalent Carbohydrates, Dendrimers and Glycoclusters
Many polyfunctional compounds have been used to build the basic scaffold of these molecules. Dendrimers built on such scaffolds include those on tris‐(2‐aminoethyl)‐amine (60) (Li et al., 2004e), trimesic acid (61) (Nishida et al., 2003), carbosilanes (62) (Boysen & Lindhorst, 2003), silsesquioxanes (63) (Gao et al., 2004), cyclotriveratrylene (64) (van Ameijde & Liskamp, 2003), tetra‐aryl porphorins (65) (Ballardini et al., 2003; Ahmed et al., 2004; Laville et al., 2004) and fullerene derivatives (Isobe et al., 2003). Further examples are in Table 11. Poly‐hydroxy compounds such as carbohydrates themselves have been used as the core for dendrimers. Thus, Wu and Kong (2004a) used ethylene glycol and glycerol to produce di‐ and trivalent (66) first order dendrimers with trisaccharides attached to each arm and Köhn et al. (2004) used glucose to produce pentavalent dendrimers containing either mono‐ or trisaccharides. Three first‐order dendrimers based on a cyclodextrin core containing fourteen Val, Phe or Val‐Phe residues have been synthesized. The amino acids were linked to the terminal residues of 3,3′‐iminobispropanolamine which was coupled through the central nitrogen to the 6‐iodo derivative of β‐cyclodextrin (Muhanna et al., 2003). First‐order dendrimers have also been synthesized by coupling 7, 14, or 21 Man—O—Ph—C≡C—CH2— groups to β‐cyclodextrin (Ortega‐Caballero, Giménez‐Martínez, & Vargas‐Berenguel, 2003) (67) giving glycodendrimers with carbohydrate moieties both at the core and periphery. Wu and Kong (2004a) have also synthesized related compounds by cyclization of nona‐ and dodecasaccharides to give dendrimers containing hexa‐ and octa‐saccharide rings. Schizophyllan ([ → Glc‐β‐(1 → 3)‐(Glc‐β‐(1 → 6)Glc‐β‐(1 → 3)‐Glc‐β‐(1 → 3)‐]n) has also been used as a backbone; β‐glucoside appendages were oxidized to give aldehyde groups which were coupled to aminoethyl‐β‐lactoside or α‐mannoside by reductive elimination (Hasegawa et al., 2004). There is also a report of a glycodendrimer, based on a polyphenylene scaffold with a ring of monosaccharides between the center and the periphery (Sakamoto & Müllen, 2004) (68).


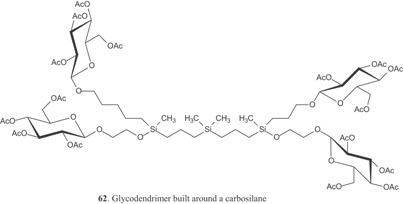





Some of the largest multivalent glycodendrimers are based around a scaffold consisting of amino acids or polyamidoamine (PAMAM). In one of the latter cases, MALDI‐TOF analysis of the products gave broad peaks with masses less than those predicted and attributed to incomplete synthesis of the PAMAM scaffold. Masses ranged from 10,000 to 132,000 Da (178 mannose residues) (Woller et al., 2003). Choudhury, Kitaoka, and Hayashi (2003) have used MALDI‐TOF for monitoring PAMAM glycodendrimers containing cellobiose. The dendrimer with 32 sugar units produced a spectrum (m/z 19,132) but the next generation glycodendrimer (64 sugar units, predicted mass for C1518H2656O892N250 = 38,661) failed to give a signal. Synthesis of large, fifth generation glycodendrimers with potentially 64 outer sugar residues (69) and sulfated analogues has been monitored by MALDI‐TOF MS from THAP (positive ion) and IAA (negative ion) to reveal that approximately 50 of the potential 64 sugars were actually incorporated (Kensinger et al., 2004). Sharp MALDI‐TOF peaks from spherical oligosaccharide‐β‐alanine‐poly(lysine) dendrimers containing 32 cellobiose (13,418 Da), maltose (13,472 Da), and lactose (13,507 Da) groups respectively, synthesized by Baigude et al. (2003), showed complete substitution of the amino groups by carbohydrate. Differences were found between the measured and calculated masses of larger maltose‐containing glycodendrimers (13 kDa) based of a poly(ornithine) scaffold with the higher experimental masses being attributed to instrumental factors. The MALDI‐TOF spectrum from CHCA of a third generation maltose‐proline‐poly(lysine) dendrimer (4.4 kDa) synthesized by Baigude et al. (2004b) gave three peaks attributed to the [M + H]+, [M + Na]+, and [M + K]+ ions. Attempted addition of a succinyl group to each maltose residue produced a MALDI spectrum with multiple peaks attributed to different numbers of attached succinyl groups and giving an average number of 13 attached groups. Finally a 24‐mer peptide was attached to produce a potential AIDS vaccine; MALDI‐TOF analysis indicated the addition of one and two such units. Self‐assembling dendrimers carrying four or eight GalNAc residues have been constructed on a bipyridyl core; addition of Cu(II)SO4 linked two bipyridyl units to give the dendrimer. It was characterized by MALDI‐TOF MS from DHB and THAP as the intact Cu(II)‐linked molecule (70) (Roy & Kim, 2003).
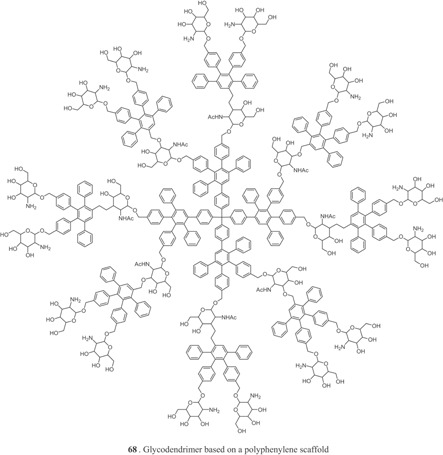
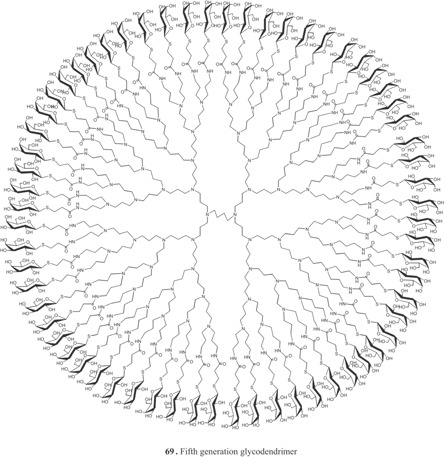
Synthesis of Carbohydrate–Protein Conjugates
Matrix‐assisted laser desorption/ionization‐time‐of‐flight (MALDI‐TOF) analysis is used in this area mainly to estimate the number of carbohydrate molecules bound to the protein. Conjugation of the glycans is frequently by squaric acid chemistry or by reductive amination. However, Lewis b hexasaccharide has been linked to human serum albumin (HSA) with a disuccinimidyl suberate spacer. The authors found that this spacer had a similar linking efficiency to squaric acid but had the advantage of a fast incorporation of the receptor saccharide to the carrier protein (Lahmann et al., 2004). Saksena, Ma, and Kovác (2003) have used SELDI to monitor the conjugation to BSA of di‐ through penta‐saccharides that mimic the upstream terminus of the O‐specific polysaccharide of Vibrio cholerae O:1, serotype Ogawa using squaric diester chemistry. SELDI monitoring of the reaction allowed the extent of conjugation to be monitored and stopped when the desired molar hapten‐BSA ratio had been reached. The accuracy of the mass measurement could be increased by using the carrier protein as the internal standard. Tri‐ and hexa‐galacturonates have been synthesized and coupled to BSA by reductive amination; MALDI analysis gave a molecular weight of 70.996 corresponding to 3.8 mol of ligand/mol of BSA (Clausen & Madsen, 2004). To develop antibodies to Saikosaponin A, a triterpenoid saponin from the root of Bupleuri radix, a plant used in Eastern medicine, Zhu et al. (2004b) have conjugated the saponins to BSA and determined the hapten number as 11 by MALDI from sinapinic acid. Concanavalin A has been labeled with a fluorescent probe at the active site by first binding a photolabile linker attached to mannose to the active site. The probe was then fixed to the protein by photoaffinity labeling, the mannose was removed by reduction of the disulfide link and the resulting thiol group used to attach various fluorescent reagents (Nagase et al., 2003). MALDI from sinapinic acid was used to monitor various stages of the reaction and to demonstrate that the final addition of the fluorescent group occurred to the extent of 70–90%. Nyame et al. (2003) have synthesized di‐ and trisaccharides bearing the LDN and LDNF epitopes and conjugated these, together with one carrying the Lewisx epitope, to BSA. MALDI‐TOF analysis showed that three to four molecules were coupled per mole of BSA. These and additional examples are listed in Table 11.

Carbohydrates as Synthetic Reagents
A cyclosophoraose (cyclic‐(1 → 2)‐β‐d‐glucan) named Cys, produced by soil micro‐organisms of the genus Rhizobium, has been found to act as a catalyst for the methanolysis of several compounds such as phosphatidylcholine (Lee & Jung, 2004). MALDI analysis of several of the reaction intermediates showed acylated cyclosophoraoses in the mass range 3,000–4,500. Carboxymethylated Cys from R. leguminosarum biovar trifolii could be used to complex and, consequently increase the aqueous solubility of hydrobenzoin and N‐acetyltryptophan (Lee et al., 2004c).
MISCELLANEOUS
A few additional papers documenting the use of MALDI analysis for carbohydrate analysis that do not fit the above categories, have been published. Thus, Microsin E492 (MccE492), an 84‐amino acid antimicrobial peptide from Klebsiella pneumoniae has been purified in a post‐translationally modified form from E. coli VCS257 harboring the pJAM229 plasmid or K. pneumoniae RCY492. The 831 Da modification was characterized by MS and NMR and found to consist of a trimer of N‐(2,3‐dihydroxybenzoyl)‐l‐serine linked via a C‐glycosidic bond to β‐d‐glucose that was O‐linked to the MccE492 (Thomas et al., 2004b). It has been proposed that tunicamycin, an inhibitor of d‐HexNAc‐1‐P‐translocases, exerts its action by coordinating the divalent metal cofactor at the active site. MALDI spectra of tunicamycin in the presence of several divalent metals contained ions with compositions of [Tun + Metal2]+ showing that one of the metal atoms was present as a metal chelate (Xu et al., 2004). The C‐terminus loop 13 of the Na+ glucose co‐transporter (SGLT1) has been shown to contain a binding site for inhibitory alkyl glucosides following photoaffinity labeling and MALDI‐TOF analysis (Raja, Kipp, & Kinne, 2004). Three hydroxyproline‐rich peptides of 15, 18, and 20 amino acids, containing several pentose residues have been identified as defense signaling peptides released at wound sites in tomato plants (Pearce & Ryan, 2003).
CONCLUSIONS
From the above, it can be seen that MALDI and particularly MALDI‐TOF is still a major analytical method for carbohydrate analysis. Even though electrospray ionization, particularly when coupled with CID, is being increasingly used, MALDI‐TOF usually provides a superior profile of constituent sugars because of the tendency of the technique to produce only singly charged ions. However, MALDI‐TOF MS of native acidic glycans is less satisfactory because of problems with prompt fragmentation. Electrospray causes less fragmentation of these compounds but tends to produce ions in different charge states from glycans with several acidic groups, thus giving a profile that is not representative of the glycan content. However, this problem, and the instability of acidic carbohydrates under MALDI conditions, can be overcome by derivatization of the carboxylic acid group of sialic acids but sulfates must be protected by other techniques such as ion pairing. Thus, both MALDI and electrospray have advantages and disadvantages for carbohydrate work and the best technique to use will be dictated by the problem to be solved. The work during the review period has shown that although MALDI is now relatively “mature,” it is expected that future editions of this review series will be able to report many improved methods for performing these analyses.
ABBREVIATIONS
- 2‐AA
2‐aminobenzoic acid
- 2‐AB
2‐aminobenzamide
- ABEE
aminobenzoic acid ethyl ester
- ABOE
aminobenzoic acid octyl ester
- A/D
analogue to digital
- AGE
advanced glycation end products
- AGP
α1‐acid glycoprotein
- AIDS
acquired immunodeficiency syndrome
- AMAC
aminoacridone
- AMP
adenosine monophosphate
- AMT
5‐amino‐2‐mercapto‐1,3,4‐thiadiazole
- ANDA
7‐aminonaphthalene‐1,3‐disulfonic acid
- ANSA
4‐aminonaphthalene‐1‐sulfonic acid
- ANTS
8‐aminonaphthalene‐1,3,6‐trisulfonic acid
- AP
aminopyridine
- AP‐MALDI
atmospheric pressure MALDI
- Api
apiose
- AQ
aminoquinoline
- Ara
arabinose
- Ara4N
4‐amino‐4‐deoxy‐l‐arabinopyranose
- Arg
arginine
- Asp
aspartic acid
- Asn
asparagine
- BSA
bovine serum albumin
- CAM
cell adhesion molecule
- CD
cyclodextrin or circular dichroism
- CE
capillary electrophoresis
- Cer
ceramide
- CDG
congenital disorder of glycosylation
- CHCA
α‐cyano‐4‐hydroxycinnamic acid
- CHO
Chinese hamster ovary
- CID
collision‐induced dissociation (decomposition)
- CMBT
5‐chloro‐2‐mercaptobenzothiazole
- CPS
capsular polysaccharide
- Cym
cymarose
- Cys
cysteine
- Da
Dalton
- DHB
dihydroxybenzoic acid (normally the 2,5‐isomer)
- DHBB
dihydroxybenzoic acid/butylamine
- DIOS
desorption/ionization on silicon
- DMSO
dimethylsulfoxide
- DNJ
deoxynojirimycin
- Dol
dolichol
- DP
degree of polymerization
- DTT
dithiothreitol
- ECD
electron capture dissociation
- Endo‐H
endoglycosidase‐H
- EPO
erythropoeitin
- EPS
extracellular polysaccharide
- ESI
electrospray ionization
- FAB
fast atom bombardment
- FACE
fluorophore‐assisted carbohydrate electrophoresis
- Fru
fructose
- FT
Fourier transform
- Fuc
fucose
- FucNAc
N‐acetylfucoseamine
- GAGS
glycosaminoglycans
- Gal
galactose
- GalA
galacturonic acid
- GalNAc
N‐acetylgalactosamine
- Gal‐T
galactosyltransferase
- GC/MS
gas chromatography/mass spectrometry
- GDP
guanosine‐5′‐diphosphate
- GIPSL
glycoinositolphosphosphingolipid
- Glc
glucose
- GLC
gas–liquid chromatography
- GlcA
glucuronic acid
- GlcN
glucosamine
- GlcNAc
N‐acetylglucosamine
- Glu
glutamic acid
- Gly
glycine
- GnT
GlcNAc transferase
- Gro
glycerol
- GSL
glycosphingolipid
- HABA
2‐(4′hydroxyphenyl)azobenzoic acid
- Hep
heptose
- HepP
heptose phosphate
- Hex
hexose
- HexA
hexuronic acid
- HexNAc
N‐acetylhexosamine
- HIQ
hydroxy‐iso‐quinoline
- HPAEC
high‐performance anion exchange chromatography
- HPLC
high‐performance liquid chromatography
- HR
high resolution
- HSA
human serum albumin
- IAA
indoleacrylic acid
- ICR
ion cyclotron resonance
- IgA (E, G, or M)
immunoglobulin A (E, G, or M)
- Ins
inositol
- IR
infrared
- IRIS
infrared ionization from solution
- IRMPD
infrared multiphoton dissociation
- IUPAC
International Union of Pure and Applied Chemistry
- Kdo
3‐deoxy‐d‐manno‐oct‐2‐ulosonic acid
- LC
liquid chromatography
- LOS
lipooligosaccharide
- LPS
lipopolysaccharide
- L‐TOF
linear‐TOF
- MALDI
matrix‐assisted laser desorption/ionization mass spectrometry
- Man
mannose
- ManNAc
N‐acetylmannosamine
- MS
mass spectrometry
- m/z
mass to charge ratio
- MUC
mucin
- NB‐DNJ
n‐butyl deoxynojirimycin
- N‐CAM
neural cell adhesion molecule
- Neu5Ac
N‐acetylneuraminic (sialic) acid
- Neu5Gc
N‐glycoylneuraminic acid
- NMR
nuclear magnetic resonance
- Orn
ornithine
- PAD
pulsed amperometric detection
- PAGE
polyacrylamide gel electrophoresis
- PAMAM
polyamidoamine
- PC
phosphatidyl choline or phosphoryl choline
- PE
phosphoethanolamine
- PEG
polyethylene glycol
- PEA
phosphoethanolamine
- Phe
phenylalanine
- PNGase
protein‐N‐glycosidase
- PSA
prostate‐specific antigen or polysialic acid
- PSD
post‐source decay
- Q
quadrupole
- QIT
quadrupole ion trap
- Qui
quinovose
- Rha
rhamnose
- R‐TOF
reflectron‐TOF
- SARS
severe acute respiratory syndrome
- SDS
sodium dodecyl sulfate
- SEC
size‐exclusion chromatography
- SELDI
surface‐enhanced laser desorption/ionization
- Ser
serine
- s‐DHB
super‐DHB
- SL
sialyl‐lactose
- sRAGE
soluble receptor for advanced glycation end products
- TFA
trifluoroacetic acid
- THAP
trihydroxyacetophenone
- Thr
threonine
- TLC
thin‐layer chromatography
- TOF, Tof
time‐of‐flight
- TT
tetanus toxoid
- UDP
uridine diphosph(ate)(o)
- UV
ultraviolet
- Val
valine
- X
xylose
- Xxx
any amino acid except proline
- Xyl
xylose
- YAG
yttrium aluminum garnet
Biographical Information
Dr. David J. Harvey obtained his Ph.D. from the University of Glasgow in 1968 and his D.Sc. in 1988. He spent 3 years at the Institute of Lipid Research, Baylor College of Medicine, Houston, Texas, USA from 1969, where he worked on the structural determination of drug metabolites, steroids, and sugar phosphates by GC/MS. In 1972, he returned to the UK as a Research Officer and later Wellcome Trust Senior Lecturer at the Department of Pharmacology, University of Oxford, where he applied GC/MS to the metabolism and pharmacokinetics of cannabinoids and to the structural determination of lipids. In 1990, he moved to the Department of Biochemistry, Oxford as Senior Research Fellow and later Reader in Glycobiology where he was involved in the development of new mass spectrometric techniques for the analysis of complex carbohydrates and glycolipids. He is currently a consultant to the Department of Biochemistry, Oxford, the Oxford‐Dublin Glycobiology Institute and is an Honorary Professorial Fellow at the University of Warwick, UK. He has served on the committee of the British Mass Spectrometry Society and has published more than 350 papers and reviews, mainly on mass spectrometry.
REFERENCES
- Aboitiz N, Cañada FJ, Huáková L, Kuzma M, Ken V, Jiménez‐Barbero J. 2004. Enzymatic synthesis of complex glycosaminotrioses and study of their molecular recognition by hevein domains. Org Biomol Chem 2: 1987–1994. [DOI] [PubMed] [Google Scholar]
- Adams EW, Ratner DM, Bokesch HR, McMahon JB, O'Keefe BR, Seeberger PH. 2004. Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology: Glycan‐dependent gp120/protein interactions. Chem Biol 11: 875–881. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Davoust E, Savoie H, Boa AN, Boyle RW. 2004. Thioglycosylated cationic porphyrins—Convenient synthesis and photodynamic activity in vitro . Tetrahedron Lett 45: 6045–6047. [Google Scholar]
- Akai R, Kinoshita M, Kakehi K, Lee YC. 2003. A method for detecting O‐glycanase in biological samples using a combination of MALDI‐TOF mass spectrometry and time‐resolved fluorimetry. Analyst 128: 440–446. [DOI] [PubMed] [Google Scholar]
- Akaike E, Tsutsumida M, Osumi K, Fujita M, Yamanoi T, Yamamoto K, Fujita K. 2004. High efficiency of transferring a native sugar chain from a glycopeptide by a microbial endoglycosidase in organic solvents. Carbohydr Res 339: 719–722. [DOI] [PubMed] [Google Scholar]
- Akbay P, Calis I, Heilmann J, Sticher O. 2003. Ionone, iridoid and phenylethanoid glycosides from Ajuga salicifolia . Z Naturforsch 58c: 177–180. [DOI] [PubMed] [Google Scholar]
- Albach C, Damoc E, Denzinger T, Schachner M, Przybylski M, Schmitz B. 2004. Identification of N‐glycosylation sites of the murine neural cell adhesion molecule NCAM by MALDI‐TOF and MALDI‐FTICR mass spectrometry. Anal Bioanal Chem 378: 1129–1135. [DOI] [PubMed] [Google Scholar]
- Albrecht T, Haebel S, Koch A, Krause U, Eckermann N, Steup M. 2004. Yeast glycogenin (Glg2p) produced in Escherichia coli is simultaneously glucosylated at two vicinal tyrosine residues but results in a reduced bacterial glycogen accumulation. Eur J Biochem 271: 3978–3989. [DOI] [PubMed] [Google Scholar]
- Alexander DC, Jones JRW, Tan T, Chen JM, Liu J. 2004. PimF, a mannosyltransferase of mycobacteria, is involved in the biosynthesis of phosphatidylinositol mannosides and lipoarabinomannan. J Biol Chem 279: 18824–18833. [DOI] [PubMed] [Google Scholar]
- Alpe M, Oscarson S. 2003. Synthesis of tetra‐ and pentasaccharides corresponding to the capsular polysaccharide of Streptococcus pneumoniae type 9A&L, 9N and 9A. Carbodydr Res 338: 2605–2609. [DOI] [PubMed] [Google Scholar]
- Alpe M, Oscarson S, Svahnberg P. 2003. Synthesis of Cryptococcus neoformans capsular polysaccharide structures. IV. Construction of thioglycoside donor blocks and their subsequent assembly. J Carbohydr Chem 22: 565–577. [Google Scholar]
- Amer H, Hofinger A, Kosma P. 2003. Synthesis of neoglycoproteins containing O‐methylated trisaccharides related to excretory/secretory antigens of Toxocara larvae. Carbohydr Res 338: 35–45. [DOI] [PubMed] [Google Scholar]
- An HJ, Franz AH, Lebrilla CB. 2003a. Improved capillary electrophoretic separation and mass spectrometric detection of oligosaccharides. J Chromatogr A 1004: 121–129. [DOI] [PubMed] [Google Scholar]
- An HJ, Peavy TR, Hedrick JL, Lebrilla CB. 2003b. Determination of N‐glycosylation sites and site heterogeneity in glycoproteins. Anal Chem 75: 5628–5637. [DOI] [PubMed] [Google Scholar]
- Andersen SM, Ling C‐C, Zhang P, Townson K, Willison HJ, Bundle DR. 2004. Synthesis of ganglioside epitopes for oligosaccharide specific immunoadsorption therapy of Guillian‐Barré syndrome. Org Biomol Chem 2: 1199–1212. [DOI] [PubMed] [Google Scholar]
- Andersson L, Cotta MA, Kenne L. 2003. Structural studies of the extracellular polysaccharide produced by Butyrivibrio fibrisolvens strain H10b. Carbohydr Res 338: 1571–1579. [DOI] [PubMed] [Google Scholar]
- André S, Unverzagt C, Kojima S, Frank M, Seifert J, Fink C, Kayser K, von der Lieth C‐J, Gabius H‐J. 2004. Determination of modulation of ligand properties of synthetic complex‐type biantennary N‐glycans by introduction of bisecting GlcNAc in silico, in vitro and in vivo . Eur J Biochem 271: 118–134. [DOI] [PubMed] [Google Scholar]
- Ångström J, Larsson T, Hansson GC, Karlsson K‐A, Henry S. 2004. Default biosynthesis pathway for blood group‐related glycolipids in human small intestine as defined by structural identification of linear and branched glycosylceramides in a group O Le(a‐b‐) nonsecretor. Glycobiology 14: 1–12. [DOI] [PubMed] [Google Scholar]
- Antignac A, Rousselle J‐C, Namane A, Labigne A, Taha M‐K, Boneca IG. 2003. Detailed structural analysis of the peptidoglycan of the human pathogen Neisseria meningitidis . J Biol Chem 278: 31521–31528. [DOI] [PubMed] [Google Scholar]
- Antonopoulos A, Bonnet P, Botek E, Debrun Jl, Hakim B, Herbreteau B, Morin‐Allory L. 2003. Study of the attachment of Na+ on glucose and on some of its methylated derivatives. Rapid Commun Mass Spectrom 17: 122–125. [DOI] [PubMed] [Google Scholar]
- Antonov AS, Afiyatullov SS, Kalinovsky AI, Ponomarenko LP, Dmitrenok PS, Aminin DL, Agafonova IG, Stonik VA. 2003. Mycalosides B‐I, eight new spermostatic steroid oligoglycosides from the sponge Mycale laxissima . J Nat Prod 66: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Aoki K, Sugiyama S, Dulaney JT, Itonori S, Sugita M. 2002. Classification into a novel mollu‐series of neutral glycosphingolipids from the lamp shell, Lingula unguis . J Oleo Sci 51: 463–472. [Google Scholar]
- Aoki K, Uchiyama R, Itonori S, Sugita M, Che F‐S, Isogai A, Hada N, Hada J, Takeda T, Kumagai H, Yamamoto K. 2004a. Structural elucidation of novel phosphocholine‐containing glycosylinositol‐phosphoceramides in filamentous fungi and their induction of cell death of cultured rice cells. Biochem J 378: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Uchiyama R, Yamauchi S, Katayama T, Itonori S, Sugita M, Hada N, Yamada‐Hada J, Takeda T, Kumagai H, Yamamoto K. 2004b. Newly discovered neutral glycosphingolipids in aureobasidin A‐resistant zygomycetes: Identification of a novel family of GALA‐series glycolipids with core Gal‐1‐6‐Gal‐1‐6‐Gal sequences. J Biol Chem 279: 32028–32034. [DOI] [PubMed] [Google Scholar]
- Argyropoulos NG, Sarli VC. 2004. Synthesis of a branched chain aza‐C‐disaccharide via the cycloaddition of a chiral nitrone to an alkene, both sugar derivatives. Tetrahedron Lett 45: 4237–4240. [Google Scholar]
- Arnold JC, Radcliffe CM, Wormald MR, Royle L, Harvey DJ, Crispin MDM, Dwek RA, Sim RB, Rudd PM. 2004. The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan binding lectin. J Immunol 173: 6831–6840. [DOI] [PubMed] [Google Scholar]
- Arosio D, Vrasidas I, Valentini P, Liskamp RMJ, Pieters RJ, Bernardi A. 2004. Synthesis and cholera toxin binding properties of multivalent GM1 mimics. Org Biomol Chem 2: 2113–2124. [DOI] [PubMed] [Google Scholar]
- Ayorinde FO, Bezabeh DZ, Delves IG. 2003. Preliminary investigation of the simultaneous detection of sugars, ascorbic acid, citric acid, and sodium benzoate in non‐alcoholic beverages by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom 17: 1735–1742. [DOI] [PubMed] [Google Scholar]
- Backinowsky LV, Abronina PI, Shashkov AS, Grachev AA, Kochetkov NK, Nepogodiev SA, Stoddart F. 2002. An efficient approach towards the convergent synthesis of fully‐carbohydrate mannodendrimers. Chem Eur J 8: 4412–4423. [DOI] [PubMed] [Google Scholar]
- Baigude H, Katsuraya K, Okuyama K, Tokunaga S, Uryu T. 2003. Synthesis of sphere‐type monodispersed oligosaccharide‐polypeptide dendrimers. Macromolecules 36: 7100–7106. [Google Scholar]
- Baigude H, Katsuraya K, Okuyama K, Hatanaka K, Ikeda E, Shibata N, Uryu T. 2004a. Synthesis of spherical and hemispherical sugar‐containing poly(ornithine) dendrimers. J Polym Sci A 42: 1400–1414. [Google Scholar]
- Baigude H, Katsuraya K, Okuyama K, Uryu T. 2004b. Synthesis of structurally‐controlled AIDS vaccine model with glyco‐peptide dendrimer scaffolds. Macromol Chem Phys 205: 684–691. [Google Scholar]
- Ballardini R, Colonna B, Gandolfi MT, Kalovidouris SA, Orzel L, Raymo FM, Stoddart JF. 2003. Porphyrin‐containing glycodendrimers. Eur J Org Chem 288–294. [Google Scholar]
- Bann JG, Bächinger HP, Peyton DH. 2003. Role of carbohydrate in stabilizing the triple‐helix in a model for a deep‐sea hydrothermal vent worm collagen. Biochemistry 42: 4042–4048. [DOI] [PubMed] [Google Scholar]
- Banoub J, El Aneed A, Cohen A, Martin P. 2004. Characterization of the O‐4‐phosphorylated and O‐5 substituted Kdo reducing end group and sequencing of the core oligosaccharide of Aeromonas salmonicida ssp salmonicida lipooligosaccharide using tandem mass spectrometry. Eur J Mass Spectrom 10: 715–730. [DOI] [PubMed] [Google Scholar]
- Baratova LA, Fedorova NV, Dobrov EN, Lukashina EV, Kharlanov AN, Nasonov VV, Serebryakova MV, Kozlovsky SV, Zayakina OV, Rodionova NP. 2004. N‐Terminal segment of potato virus X coat protein subunits is glycosylated and mediates formation of a bound water shell on the virion surface. Eur J Biochem 271: 3136–3145. [DOI] [PubMed] [Google Scholar]
- Bardor M, Faveeuw C, Fitchette A‐C, Gilbert D, Galas L, Trottein F, Faye L, Lerouge P. 2003a. Immunoreactivity in mammals of two typical plant glyco‐epitopes, core (1,3)‐fucose and core xylose. Glycobiology 13: 427–434. [DOI] [PubMed] [Google Scholar]
- Bardor M, Loutelier‐Bourhis C, Paccalet T, Cosette P, Fitchette A‐C, Vézina L‐P, Trépanier S, Dargis M, Lemieux R, Lange C, Faye L, Lerouge P. 2003b. Monoclonal C5‐1 antibody produced in transgenic alfalfa plants exhibits a N‐glycosylation that is homogenous and suitable for glyco‐engineering into human‐compatible structures. Plant Biotechnol J 1: 451–462. [DOI] [PubMed] [Google Scholar]
- Barinka C, Sácha P, Sklená J, Man P, Bezouska K, Slusher BS, Konvalinka J. 2004. Identification of the N‐glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci 13: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroca N, Schmidt RR. 2004. 2‐Nitro thioglycoside donors: Versatile precursors of β‐D‐glycosides of aminosugars. Org Lett 6: 1551–1554. [DOI] [PubMed] [Google Scholar]
- Bashir S, Derrick PJ, Critchley P, Gates PJ, Staunton J. 2003a. Martix‐assisted laser desorption time‐of‐flight mass spectrometry of dextran and dextrin derivatives. Eur J Mass Spectrom 9: 61–70. [DOI] [PubMed] [Google Scholar]
- Bashir S, Mutter R, Derrick PJ. 2003b. Matrix‐assisted laser desorption/ionization mass spectrometry with re‐engineered 2,5‐dihydroxybenzoic acid derivative. Analyst 128: 1452–1457. [DOI] [PubMed] [Google Scholar]
- Bashir S, Giannakopulos AE, Derrick PJ, Critchley P, Bottrill A, Padley HD. 2004. Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. A comparison of fragmentation patterns of linear dextran obtained by in‐source decay, post‐source decay and collision‐induced dissociation and the stability of linear and cyclic glucans studied by in‐source decay. Eur J Mass Spectrom 10: 109–120. [DOI] [PubMed] [Google Scholar]
- Batrakov SG, Konova IV, Sheichenko VI, Esipov SE, Galanina LA, Istratova LN, Sergeeva YE. 2004. Lipids of the zygomycete Absidia corymbifera F‐965. Phytochemistry 65: 1239–1246. [DOI] [PubMed] [Google Scholar]
- Baytas SN, Linhardt RJ. 2004. Combinatorial carbohydrate synthesis. Mini‐Rev Org Chem 1: 27–39. [Google Scholar]
- Bencúrová M, Rendi D, Fabini G, Kopecky E‐M, Altmann F, Wilson IBH. 2003. Expression of eukaryotic glycosyltransferases in the yeast Pichia pastoris . Biochimie 85: 413–422. [DOI] [PubMed] [Google Scholar]
- Bencúrová M, Hemmer W, Focke‐Tejkl M, Wilson IBH, Altmann F. 2004. Specificity of IgG and IgE antibodies against plant and insect glycoprotein glycans determined with artificial glycoforms of human transferrin. Glycobiology 14: 457–466. [DOI] [PubMed] [Google Scholar]
- Ben‐Menachem G, Kubler‐Kielb J, Coxon B, Yergey A, Schneerson R. 2003. A newly discovered cholesteryl galactoside from Borrelia burgdorferi . Proc Natl Acad Sci USA 100: 7913–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Yu Y, Cocklin RR, Zhang Y, Wang M, Dincer UD, Besch HRJ. 2003. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium‐release channels. Diabetes 52: 1825–1836. [DOI] [PubMed] [Google Scholar]
- Bisio A, Guglieri S, Frigerio M, Torri G, Vismara E, Cornelli U, Bensi D, Gonella S, De Ambrosi L. 2004. Controlled γ‐ray irradiation of heparin generates oligosaccharides enriched in highly sulfated sequences. Carbohydr Polym 55: 101–112. [Google Scholar]
- Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. 2003. Sialoside specificity of the siglec family assessed using novel multivalent probes. Identification of potent inhibitors of myelin‐associated glycoprotein. J Biol Chem 278: 31007–31019. [DOI] [PubMed] [Google Scholar]
- Blundell CD, DeAngelis PL, Day AJ, Almond A. 2004. Use of 15N‐NMR to resolve molecular details in isotopically‐enriched carbohydrates: Sequence‐specific observations in hyaluronan oligomers up to decasaccharides. Glycobiology 14: 999–1009. [DOI] [PubMed] [Google Scholar]
- Boas U, Heegaard PMH. 2004. Dendrimers in drug research. Chem Soc Rev 33: 43–63. [DOI] [PubMed] [Google Scholar]
- Bobrowicz P, Davidson RC, Li H, Potgieter TI, Nett JH, Hamilton SR, Stadheim TA, Miele RG, Bobrowicz B, Mitchell T, Rausch S, Renfer E, Wildt S. 2004. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: Production of complex humanized glycoproteins with terminal galactose. Glycobiology 14: 757–766. [DOI] [PubMed] [Google Scholar]
- Bode L, Beermann C, Mank M, Kohn G, Boehm G. 2004. Human and bovine milk gangliosides differ in their fatty acid composition. J Nutr 134: 3016–3020. [DOI] [PubMed] [Google Scholar]
- Boehm G, Fanaro S, Jelinek J, Stahl B, Marini A. 2003. Prebiotic concept for infant nutrition. Acta Paediatr 92: 64–67. [DOI] [PubMed] [Google Scholar]
- Boer H, Munck N, Natunen J, Wohlfahrt G, Söderlund H, Renkonen O, Koivula A. 2004. Differential recognition of animal type β4‐galactosylated and 3‐fucosylated chito‐oligosaccharides by two family 18 chitinases from Trichoderma harzianum . Glycobiology 14: 1303–1313. [DOI] [PubMed] [Google Scholar]
- Bonnin E, Le Goff A, van Alebeek G‐JWM, Voragen AGJ, Thibault J‐F. 2003. Mode of action of Fusarium moniliforme endopolygalacturonase towards acetylated pectin. Carbohydr Polym 52: 381–388. [Google Scholar]
- Boraston AB, Sandercock LE, Warren RA, Kilburn DG. 2003. O‐glycosylation of a recombinant carbohydrate‐binding module mutant secreted by Pichia pastoris . J Mol Microbiol Biotechnol 5: 29–36. [DOI] [PubMed] [Google Scholar]
- Borbás A, Csávás M, Szilágyi L, Májer G, Liptá A. 2004. Replacement of carbohydrate sulfates by sugar C‐sulfonic acid derivatives. J Carbohydr Chem 23: 133–146. [Google Scholar]
- Börnsen KO, Mohr MD, Widmer HM. 1995. Ion exchange and purification of carbohydrates on a Nafion(R) membrane as a new sample pretreatment for matrix‐assisted laser desorption‐ionization mass spectrometry. Rapid Commun Mass Spectrom 9: 1031–1034. [Google Scholar]
- Bousfield GR, Butnev VY, Butnev VY, Nguyen VT, Gray CM, Dias JA, MacColl R, Eisel L, Harvey DJ. 2004. Differential effects of subunit asparagine56 oligosaccharide structure on equine lutropin and follitropin hybrid conformation and receptor‐binding activity. Biochemistry 43: 10817–10833. [DOI] [PubMed] [Google Scholar]
- Boysen MMK, Elsner K, Sperling O, Lindhorst TK. 2003. Glycerol and glycerol glycol glycodendrimers. Eur J Org Chem 4376–4386. [Google Scholar]
- Boysen MMK, Lindhorst TK. 2003. ‘Sugaring’ carbosilane dendrimers via hydrosilylation. Tetrahedron 59: 3895–3898. [Google Scholar]
- Bretthauer RK. 2003. Genetic engineering of Pichia pastoris to humanize N‐glycosylation of proteins. Trends Biotechnol 21: 459–462. [DOI] [PubMed] [Google Scholar]
- Broberg S, Broberg A, Duus JØ. 2000. Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry of oligosaccharides derivatized by reductive amination and N,N‐dimethylation. Rapid Commun Mass Spectrom 14: 1801–1805. [DOI] [PubMed] [Google Scholar]
- Brocke C, Kunz H. 2003. Synthetic glycopeptides of the tandem repeat sequence of the epithelial mucin MUC4 with tumour‐associated carbohydrate antigens. Synlett 2052–2056. [Google Scholar]
- Brocke C, Kunz H. 2004. Synthetic tumor‐associated glycopeptide antigens from the tandem repeat sequence of the epithelial mucin MUC4. Synthesis 525–542. [Google Scholar]
- Broersen K, Voragen AGJ, Hamer RJ, de Jongh HHJ. 2004. Glycoforms of β‐lactoglobulin with improved thermostability and preserved structural packing. Biotechnol Bioeng 86: 78–87. [DOI] [PubMed] [Google Scholar]
- Bryan MC, Wong C‐H. 2004. Aminoglycoside array for the high‐throughput analysis of small molecule–RNA interactions. Tetrahedron Lett 45: 3639–3642. [Google Scholar]
- Bungert D, Bastian S, Heckmann‐Pohl DM, Giffhorn F, Heinzle E, Tholey A. 2004a. Screening of sugar converting enzymes using quantitative MALDI‐ToF mass spectrometry. Biotechnol Lett 26: 1025–1030. [DOI] [PubMed] [Google Scholar]
- Bungert D, Heinzle E, Tholey A. 2004b. Quantitative matrix‐assisted laser desorption/ionization mass spectrometry for the determination of enzyme activities. Anal Biochem 326: 167–175. [DOI] [PubMed] [Google Scholar]
- Buskas T, Li Y, Boons G‐J. 2004. The immunogenicity of the tumor‐associated antigen Lewisy may be suppressed by a bifunctional cross‐linker required for coupling to a carrier protein. Chem Eur J 10: 3517–3524. [DOI] [PubMed] [Google Scholar]
- Butler M, Quelhas D, Critchley AJ, Carchon H, Hebestreit HF, Hibbert RG, Vilarinho L, Teles E, Matthijs G, Schollen E, Argibay P, Harvey DJ, Dwek RA, Jaeken J, Rudd PM. 2003. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology 13: 601–622. [DOI] [PubMed] [Google Scholar]
- Cabálková J, Zídková J, Pibyla L, Chmelík J. 2004. Determination of carbohydrates in juices by capillary electrophoresis, high‐performance liquid chromatography, and matrix‐assisted laser desorption/ionization‐time of flight‐mass spectrometry. Electrophoresis 25: 487–493. [DOI] [PubMed] [Google Scholar]
- Cai Y, Jiang Y, Cole RB. 2003. Anionic adducts of oligosaccharides by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Anal Chem 75: 1638–1644. [DOI] [PubMed] [Google Scholar]
- Cantin GT, Yates JRI. 2004. Strategies for shotgun identification of post‐translational modifications by mass spectrometry. J Chromatogr A 1053: 7–14. [DOI] [PubMed] [Google Scholar]
- Capila I, Sasisekharan R. 2004. Methods for the analysis of hyaluronal and its fragments. In: Garg HG, Hales CA, editors. Chemistry and biology of hyaluronan. Amsterdam, Boston: Elsevier. pp 21–40. [Google Scholar]
- Capon C, Mizon C, Lemoine J, Rodié‐Talbère P, Mizon J. 2003. In acute inflammation, the chondroitin‐4 sulphate carried by bikunin is not only longer; it is also undersulphated. Biochemie 85: 101–107. [DOI] [PubMed] [Google Scholar]
- Caputo E, Camarca A, Moharram R, Tornatore P, Thatcher B, Guardiola J, Martin BM. 2003. Structural study of GCDFP/gp17 in disease versus physiological conditions using a proteomic approach. Biochemistry 42: 6169–6178. [DOI] [PubMed] [Google Scholar]
- Caroff M, Karibian D. 2003. Structure of bacterial lipopolysaccharides. Carbohydr Res 338: 2431–2447. [DOI] [PubMed] [Google Scholar]
- Carvalho LJCB, de Souza CRB, de Mattos Cascardo JC, Junior CB, Campos L. 2004. Identification and characterization of a novel cassava (Manihot esculenta Crantz) clone with high free sugar content and novel starch. Plant Mol Biol 56: 643–659. [DOI] [PubMed] [Google Scholar]
- Casas‐Solvas JM, Vargas‐Berenguel A, Capitan‐Vallvey LF, Santoyo‐Gonzalez F. 2004. Convenient methods for the synthesis of ferrocene‐carbohydrate conjugates. Org Lett 6: 3687–3690. [DOI] [PubMed] [Google Scholar]
- Cescutti P, Impallomeni G, Garozzo D, Sturiale L, Herasimenka Y, Lagatolla C, Rizzo R. 2003. Exopolysaccharides produced by a clinical strain of Burkholderia cepacia isolated from a cystic fibrosis patient. Carbohydr Res 338: 2687–2695. [DOI] [PubMed] [Google Scholar]
- Chan T‐WD, Tang KY. 2003. Analysis of a bioactive β‐(1‐3) polysaccharide (Curdlan) using matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom 17: 887–896. [DOI] [PubMed] [Google Scholar]
- Charbonnier F, Penadés S. 2004. A straightforward synthesis of 1‐adamantylmethyl glycosides, and their binding to cyclodextrins. Eur J Org Chem 3650–3656. [Google Scholar]
- Chatterton NJ, Harrison PA. 2003. Fructans in crested wheatgrass leaves. J Plant Physiol 160: 843–849. [DOI] [PubMed] [Google Scholar]
- Chen C‐T, Chen Y‐C. 2004. Molecularly imprinted TiO2‐matrix‐assisted laser desorption/ionization mass spectrometry for selectively detecting α‐cyclodextrin. Anal Chem 76: 1453–1457. [DOI] [PubMed] [Google Scholar]
- Chen L, Kong F. 2002. An efficient and practical synthesis of α‐(1‐3)‐linked mannohexaose and mannooctaose. J Carbohydr Chem 21: 341–353. [DOI] [PubMed] [Google Scholar]
- Chen Z, Schols HA, Voragen AGJ. 2004. Differently sized granules from acetylated potato and sweet potato starches differ in the acetyl substitution pattern of their amylose populations. Carbohydr Polym 56: 219–226. [Google Scholar]
- Cheng L, Tachibana K, Iwasaki H, Kameyama A, Zhang Y, Kubota T, Hiruma T, Tachibana K, Kudo T, Guo J‐M, Narimatsu H. 2004. Characterization of a novel human UDP‐GalNAc transferase, pp‐GalNAc‐T15. FEBS Lett 566: 17–24. [DOI] [PubMed] [Google Scholar]
- Chirino AJ, Mire‐Sluis A. 2004. Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol 22: 1383–1391. [DOI] [PubMed] [Google Scholar]
- Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU. 2003a. Use of combinatorial genetic libraries to humanize N‐linked glycosylation in the yeast Pichia pastoris . Proc Natl Acad Sci USA 100: 5022–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O, Tomiya N, Kim JH, Slavicek JM, Betenbaugh MJ, Lee YC. 2003b. N‐glycan structures of human transferrin produced by Lymantria dispar (gypsy moth) cells using the LdMNPV expression system. Glycobiology 13: 539–548. [DOI] [PubMed] [Google Scholar]
- Choma A, Komaniecka I. 2003. Characterisation of Mesorhizobium huakuii cyclic beta‐glucan. Acta Biochim Polon 50: 1273–1281. [PubMed] [Google Scholar]
- Choma A, Sowinski P. 2004. Characterization of Mesorhizobium huakuii lipid A containing both D‐galacturonic acid and phosphate residues. Eur J Biochem 271: 1310–1322. [DOI] [PubMed] [Google Scholar]
- Choudhury AK, Kitaoka M, Hayashi K. 2003. Synthesis of a cellobiosylated dimer and trimer and of cellobiose‐coated polyamidoamine (PAMAM) dendrimers to study accessibility of an enzyme, cellodextrin phosphorylase. Eur J Org Chem 2462–2470. [Google Scholar]
- Cieniewski‐Bernard C, Bastide B, Lefebvre T, Lemoine J, Mounier Y, Michalski J‐C. 2004. Identification of O‐linked N‐acetylglucosamine proteins in rat skeletal muscle using two‐dimensional gel electrophoresis and mass spectrometry. Mol Cell Proteomics 3: 577–585. [DOI] [PubMed] [Google Scholar]
- Ciolczyk‐Wierzbicka D, Amoresano A, Casbarra A, Hoja‐Lukowicz D, Litynska A, Laidler P. 2004. The structure of the oligosaccharides of N‐cadherin from human melanoma cell lines. Glycoconj J 20: 483–492. [DOI] [PubMed] [Google Scholar]
- Cipollo JF, Awad A, Costello CE, Robbins PW, Hirschberg CB. 2004a. Biosynthesis in vitro of Caenorhabditis elegans phosphorylcholine oligosaccharides. Proc Natl Acad Sci USA 101: 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollo JF, Awad AM, Costello CE, Hirschberg CB. 2004b. srf‐3, a mutant of Caenorhabditis elegans, resistant to bacterial infection and to biofilm binding, is deficient in glycoconjugates. J Biol Chem 279: 52893–52903. [DOI] [PubMed] [Google Scholar]
- Clausen M, Madsen R. 2004. Synthesis of oligogalacturonates conjugated to BSA. Carbohydr Res 339: 2159–2169. [DOI] [PubMed] [Google Scholar]
- Cmelík R, Stikarovská M, Chmelík J. 2004. Different behavior of dextrans in positive‐ion and negative‐ion mass spectrometry. J Mass Spectrom 39: 1467–1473. [DOI] [PubMed] [Google Scholar]
- Cocklin RR, Zhang Y, O'Neill KD, Chen NX, Moe SM, Bidasee KR, Wang M. 2003. Identity and localization of advanced glycation end products on human β2‐microglobulin using matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Anal Biochem 314: 322–325. [DOI] [PubMed] [Google Scholar]
- Cohen O, Kronman C, Velan B, Schafferman A. 2004. Amino acid domains control the circulatory residence time of primate acetylcholinesterases in rhesus macaques (Macaca mulatta). Biochem J 378: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin A, Florea D, Maes E, Cogálniceanu D, Strecker G. 2003. Comparative study of carbohydrate chains released from the oviducal mucins of the two closely related amphibian species Bombina bombina and Bombina variegata . Biochemie 85: 53–64. [DOI] [PubMed] [Google Scholar]
- Corsaro MM, Dal Piaz F, Lanzetta R, Naldi T, Parrilli M. 2004. Structure of lipid A from Pseudomonas corrugata by electrospray ionization quadrupole time‐of‐flight tandem mass spectrometry. Rapid Commun Mass Spectrom 18: 853–858. [DOI] [PubMed] [Google Scholar]
- Couto AC, Caffaro C, Uhrig ML, Kimura E, Peres VJ, Merino EF, Katzin AM, Nishioka M, Nonami H, Erra‐Balsells R. 2004. Glycosphingolipids in Plasmodium falciparum. Presence of an active glucosylceramide synthase. Eur J Biochem 271: 2204–2214. [DOI] [PubMed] [Google Scholar]
- Cristoni S, Bernsrdi LR. 2003. Development on new methodologies for the mass spectrometry study of bioorganic macromolecules. Mass Spectrom Rev 22: 369–406. [DOI] [PubMed] [Google Scholar]
- Cryan S‐A, Holohan A, Donohue R, Darcy R, O'Driscoll CM. 2004. Cell transfection with polycationic cyclodextrin vectors. Eur J Pharm Sci 21: 625–633. [DOI] [PubMed] [Google Scholar]
- Csávás M, Borbás A, Jánossy L, Lipták A. 2003. Synthesis of an arabinogalactan‐type octa‐ and two isomeric nonasaccharides. Suitable tuning of protecting groups. Tetrahedron Lett 44: 631–635. [Google Scholar]
- Culbertson SM, Vassilenko EI, Morrison LD, Ingold KU. 2003. Paradoxical impact of antioxidants on post‐Amadori glycoxidation. Counterintuitive increase in the yields of pentosidine and Nε‐carboxymethyllysine using a novel multifunctional pyridoxamine derivative. J Biol Chem 278: 38384–38394. [DOI] [PubMed] [Google Scholar]
- Culha M, Schell FM, Fox S, Green T, Betts TB, Sepaniak MJ. 2004. Evaluation of newly synthesized and commercially available charged cyclomaltooligosaccharides (cyclodextrins) for capillary electrokinetic chromatography. Carbohydr Res 339: 241–249. [DOI] [PubMed] [Google Scholar]
- Cutalo JM, Deterding LJ, Tomer KB. 2004. Characterization of glycopeptides from HIV‐ISF2 gp120 by liquid chromatography mass spectrometry. J Am Soc Mass Spectrom 15: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeszak X, Morelle W, Ricart G, Tétaert D, Lemoine J. 2004. Localization of the O‐glycosylated sites in peptides by fixed‐charge derivatization with a phosphonium group. Anal Chem 76: 4320–4324. [DOI] [PubMed] [Google Scholar]
- D'Agosto F, Charreyre MT, Pichot C, Dessalces G, Delolme F. 2004. Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry and nuclear magnetic resonance analyses of end‐functionalized saccharidic polymers: An example of a useful analytical technique combination. Rapid Commun Mass Spectrom 18: 664–672. [DOI] [PubMed] [Google Scholar]
- Dahlman O, Jacobs A, Sjöberg J. 2003. Molecular properties of hemicelluloses located in the surface and inner layers of hardwood and softwood pulps. Cellulose 10: 325–334. [Google Scholar]
- Damager I, Numao S, Chen H, Brayer GD, Withers SG. 2004. Synthesis and characterisation of novel chromogenic substrates for human pancreatic α‐amylase. Carbohydr Res 339: 1727–1737. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Pham T‐TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll‐like receptors 2 and 4. Infect Immun 72: 5041–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Kumar RS, Ayyadurai N, Kumar S, Sakthivel N. 2004. Polysaccharides of Pseudomonas pathovar strains that infect pea, tomato, and soya bean. Curr Microbiol 49: 35–41. [DOI] [PubMed] [Google Scholar]
- Davidson RC, Nett JH, Renfer E, Li H, Stadheim TA, Miller BJ, Miele RG, Hamilton SR, Choi B‐K, Mitchell TI, Wildt S. 2004. Functional analysis of the ALG3 gene encoding the Dol‐P‐Man: Man5GlcNAc2‐PP‐Dol mannosyltransferase enzyme of P. pastoris . Glycobiology 14: 399–407. [DOI] [PubMed] [Google Scholar]
- De Castro C, Bedini E, Garozzo D, Sturiale L, Parrilli M. 2004a. Structural determination of the O‐chain moieties of the lipopolysaccharide fraction from Agrobacterium radiobacter DSM. Eur J Org Chem 3842–3849. [Google Scholar]
- De Castro C, Molinaro A, Nunziata R, Lanzetta R, Parrilli M, Holst O. 2004b. A novel core region, lacking heptose and phosphate, of the lipopolysaccharide from the gram‐negative bacterium Pseudomonas cichorii (Pseudomonadaceae RNA group 1). Eur J Org Chem 2427–2435. [Google Scholar]
- de Souza AC, Kuil J, Maljaars CEP, Halkes KM, Vliegenthart JFG, Kamerling JP. 2004. Synthesis and conjugation of oligosaccharide analogues of fragments of the immunoreactive glycan part of the circulating anodic antigen of the parasite Schistosoma mansoni . Org Biomol Chem 2: 2972–2987. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL, Oatman LC, Gay DF. 2003. Rapid chemoenzymatic synthesis of monodisperse hyaluronan oligosaccharides with immobilized enzyme reactors. J Biol Chem 278: 35199–35203. [DOI] [PubMed] [Google Scholar]
- Degroote S, Maes E, Humbert P, Delmotte P, Lamblin G, Roussel P. 2003. Sulfated oligosaccharides isolated from the respiratory mucins of a secretor patient suffering from chronic bronchitis. Biochimie 85: 369–379. [DOI] [PubMed] [Google Scholar]
- Dell A, Chalabi S, Easton RL, Haslam SM, Sutton‐Smith M, Patankar MS, Lattanzio F, Panico M, Morris HR, Clark GF. 2003. Murine and human zona pellucida 3 derived from mouse eggs express identical O‐glycans. Proc Natl Acad Sci USA 100: 15631–15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demelbauer UM, Zehl M, Plematl A, Allmaier G, Rizzi A. 2004. Determination of glycopeptide structures by multistage mass spectrometry with low‐energy collision‐induced dissociation: Comparison of electrospray ionization quadrupole ion trap and matrix‐assisted laser desorption/ionization quadrupole ion trap reflectron time‐of‐flight approaches. Rapid Commun Mass Spectrom 18: 1575–1582. [DOI] [PubMed] [Google Scholar]
- Deng L, Wu H, Yu B, Jiang M, Wu J. 2004. Synthesis of OSW‐1 analogs with modified side chains and their antitumor activities. Bioorg Med Chem Lett 14: 2781–2785. [DOI] [PubMed] [Google Scholar]
- Dhume ST, Ebert MB, Saddic GN, Anumula KR. 2002. Monitoring glycosylation of therapeutic glycoproteins for consistency using highly fluorescent anthranilic acid. Methods Mol Biol 194: 127–142. [DOI] [PubMed] [Google Scholar]
- Dinadayala P, Lemassu A, Granovski P, Cérantola S, Winter N, Daffé M. 2004. Revisiting the structure of the anti‐neoplastic glucans of Mycobacterium bovis Bacille Calmette‐Guérin: Structural analysis of the extracellular and boiling water extract‐derived glucans of the vaccine substrains. J Biol Chem 279: 12369–12378. [DOI] [PubMed] [Google Scholar]
- Doco T, Williams P, Pauly M, O'Neill MA, Pellerin P. 2003. Polysaccharides from grape berry cell walls. Part II. Structural characterization of the xyloglucan polysaccharides. Carbohydr Polym 53: 253–261. [Google Scholar]
- Dolashka‐Angelova P, Beck A, Dolashki A, Beltramini M, Stevanovic S, Salvato B, Voelter W. 2003. Characterization of the carbohydrate moieties of the functional unit RvH1‐a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem J 374: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolashka‐Angelova P, Beck A, Dolashki A, Stevanovic S, Beltramini M, Salvato B, Hristova R, Velkova L, Voelter W. 2004a. Carbohydrate moieties of molluscan Rapana venosa hemocyanin. Micron 35: 101–104. [DOI] [PubMed] [Google Scholar]
- Dolashka‐Angelova P, Stevanovic S, Dolashki A, Angelova M, Serkedjieva J, Krumova E, Pashova S, Zacharieva S, Voelter W. 2004b. Structural and functional analysis of glycosylated Cu/Zn‐superoxide dismutase from the fungal strain Humicola lutea 103. Biochem Biophys Res Commun 317: 1006–1016. [DOI] [PubMed] [Google Scholar]
- Domon B, Costello CE. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB‐MS/MS spectra of glycoconjugates. Glycoconj J 5: 397–409. [Google Scholar]
- Dondoni A, Marra A. 2003. A new synthetic approach to mycobacterial cell wall α‐(1 → 5)‐D‐arabinofuranosyl C‐oligosaccharides. Tetrahedron Lett 44: 4067–4071. [Google Scholar]
- Dondoni A, Perrone D. 2003. A convenient synthesis of iminosugar‐C‐glycosides via organometallic addition to N‐benzyl‐N‐glycosylhydroxylamines. Tetrahedron 59: 4261–4273. [Google Scholar]
- Dondoni A, Giovannini PP, Massi A. 2004. Assembling heterocycle‐tethered C‐glycosyl and α‐amino acid residues via 1,3‐dipolar cycloaddition reactions. Org Lett 6: 2929–2932. [DOI] [PubMed] [Google Scholar]
- Dong A, Ye M, Guo H, Zheng J, Guo D. 2003. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata . Biotechnol Lett 25: 339–344. [DOI] [PubMed] [Google Scholar]
- Du Y, Gu G, Wei G, Hua Y, Linhardt RJ. 2003. Synthesis of saponins using partially protected glycosyl donors. Org Lett 5: 3627–3630. [DOI] [PubMed] [Google Scholar]
- Edge ASB. 2003. Deglycosylation of glycoproteins with trifluoromethanesulphonic acid: Elucidation of molecular structure and function. Biochem J 376: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov TA, Galkina TG, Balashova TA, Arsen'ev AS, Nikonorova AK, Babakov AV, Grishin EV. 2004. Phenolic glycoside isolated from seeds of the greater plantain (Plantago major L.). Dokl Biochem Biophys 396: 132–135. [DOI] [PubMed] [Google Scholar]
- Ekström S, Wallman L, Malm J, Becker C, Lilja H, Laurell T, Marko‐Varga G. 2004. Integrated selective enrichment target—A microtechnology platform for matrix‐assisted laser desorption/ionization‐mass spectrometry applied on protein biomarkers in prostate diseases. Electrophoresis 25: 3769–3777. [DOI] [PubMed] [Google Scholar]
- El‐Fasakhany FM, Ichihara‐Tanaka K, Uchimura K, Muramatsu T. 2003. N‐Acetylglucosamine‐6‐O‐sulfotransferase‐1: Production in the Baculovirus system and its applications to the synthesis of a sulfated oligosaccharide and to the modification of oligosaccharides in fibrinogen. J Biochem (Tokyo) 133: 287–293. [DOI] [PubMed] [Google Scholar]
- Erdemgil FZ, Hüsnü K, Baser C, Akbay P, Sticher O, Calisc I. 2003. Thalictricoside, a new phenolic compound from Thalictrum orientale . Z Naturforsch 58c: 632–636. [DOI] [PubMed] [Google Scholar]
- Ersöz T, Alipieva KI, Yalcin FN, Akbay P, Handjieva N, Dönmez AA, Simeon Popov S, Calis I. 2003. Physocalycoside, a new phenylethanoid glycoside from Phlomis physocalyx Hub.‐Mor. Z Naturforsch 58c: 471–476. [DOI] [PubMed] [Google Scholar]
- Ethier M, Saba JA, Ens W, Standing KG, Perreault H. 2002. Automated structural assignment of derivatized complex N‐linked oligosaccharides from tandem mass spectra. Rapid Commun Mass Spectrom 16: 1743–1754. [DOI] [PubMed] [Google Scholar]
- Ethier M, Saba JA, Spearman M, Krokhin O, Butler M, Ens W, Standing KG, Perreault H. 2003. Application of the StrOligo algorithm for the automated structure assignment of complex N‐linked glycans from glycoproteins using tandem mass spectrometry. Rapid Commun Mass Spectrom 17: 2713–2720. [DOI] [PubMed] [Google Scholar]
- Faijes M, Imai T, Bulone V, Planas A. 2004. In vitro synthesis of a crystalline (1 → 3,1 → 4)‐β‐D‐glucan by a mutated (1 → 3,1 → 4)‐β‐D‐glucanase from Bacillus. Biochem J 380: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, She Y‐M, Bagshaw RD, Callahan JW, Schachter H, Mahuran DJ. 2004. A method for proteomic identification of membrane‐bound proteins containing Asn‐linked oligosaccharides. Anal Biochem 332: 178–186. [DOI] [PubMed] [Google Scholar]
- Farkas E, Schmidt U, Thiem J, Kowalczyk J, Kunz M, Vogel M. 2003. Regioselective synthesis of galactosylated tri‐ and tetrasaccharides by use of β‐galactosidase from Bacillus circulans . Synthesis 699–706. [Google Scholar]
- Fay LB, Brevard H. 2004. Contribution of mass spectrometry to the study of the Maillard reaction in food. Mass Spectrom Rev 24: 487–507. [DOI] [PubMed] [Google Scholar]
- Fazio F, Wong C‐H. 2003. RuCl3‐promoted amide formation from azides and thioacids. Tetrahedron Lett 44: 9083–9085. [Google Scholar]
- Fazio F, Bryan MC, Lee H‐K, Chang A, Wong C‐H. 2004. Assembly of sugars on polystyrene plates: A new facile microarray fabrication technique. Tetrahedron Lett 45: 2689–2692. [Google Scholar]
- Fenaille F, Morgan F, Parisod V, Tabet J‐C, Guy PA. 2004. Solid‐state glycation of β‐lactoglobulin by lactose and galactose: Localization of the modified amino acids using mass spectrometric techniques. J Mass Spectrom 39: 16–28. [DOI] [PubMed] [Google Scholar]
- Fernández LEM, Obel N, Scheller HV, Roepstorff P. 2003. Characterization of plant oligosaccharides by matrix‐assisted laser desorption/ionization and electrospray mass spectrometry. J Mass Spectrom 38: 427–437. [DOI] [PubMed] [Google Scholar]
- Fernández M, Fragoso A, Cao R, Baños M, Ansorge‐Schumacher M, Hartmeier W, Villalonga R. 2004. Functional properties and application in peptide synthesis of trypsin modified with cyclodextrin‐containing dicarboxylic acids. J Mol Catal B Enzymatic 31: 47–52. [Google Scholar]
- Fini C, Talamo F, Cherri S, Coli M, Floridi A, Ferrara L, Scaloni A. 2003. Biochemical and mass spectrometric characterization of soluble ecto‐5′‐nucleotidase from bull seminal plasma. Biochem J 372: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SHE, Schaible UE. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d‐restricted T cells. Proc Natl Acad Sci USA 101: 10685–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flahaut C, Michalski JC, Danel T, Humbert MH, Klein A. 2003. The effects of ethanol on the glycosylation of human transferrin. Glycobiology 13: 191–198. [DOI] [PubMed] [Google Scholar]
- Forno G, Fogolin MB, Oggero M, Kratje R, Etcheverrigaray M, Conradt HS, Nimtz M. 2004. N‐ and O‐linked carbohydrates and glycosylation site occupancy in recombinant human granulocyte‐macrophage colony‐stimulating factor secreted by a Chinese hamster ovary cell line. Eur J Biochem 271: 907–919. [DOI] [PubMed] [Google Scholar]
- Fournier I, Marinach C, Tabet JC, Bolbach G. 2003. Irradiation effects in MALDI, ablation, ion production, and surface modifications. Part II: 2,5‐dihydroxybenzoic acid monocrystals. J Am Soc Mass Spectrom 14: 893–899. [DOI] [PubMed] [Google Scholar]
- Frankevich VE, Zhang J, Friess SD, Dashtiev M, Zenobi R. 2003. Role of electrons in laser desorption/ionization mass spectrometry. Anal Chem 75: 6063–6067. [DOI] [PubMed] [Google Scholar]
- Friedl CH, Lochnit G, Zähringer U, Bahr U, Geyer R. 2003. Structural elucidation of zwitterionic carbohydrates derived from glycosphingolipids of the porcine parasitic nematode Ascaris suum . Biochem J 369: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frirdich E, Lindner B, Holst O, Whitfield C. 2003. Overexpression of the waaZ Gene leads to modification of the structure of the inner core region of Escherichia coli lipopolysaccharide, truncation of the outer core, and reduction of the amount of O polysaccharide on the cell surface. J Bacteriol 185: 1659–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwaki T, Yamaguchi S, Tasaka M, Takayanagi M, Isobe M, Taketomi T. 2004. Evaluation of sphingolipids in vitreous bodies from a patient with Gaucher disease, using delayed extraction matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry. J Chromatogr B 806: 47–51. [DOI] [PubMed] [Google Scholar]
- Fujiyama K, Sakai Y, Misaki R, Yanagihara I, Honda T, Anzai H, Seki T. 2004. N‐linked glycan structures of human lactoferrin produced by transgenic rice. Biosci Biotechnol Biochem 68: 2565–2570. [DOI] [PubMed] [Google Scholar]
- Fukae K, Yamamoto N, Hatakeyama Y, Kajihara Y. 2004. Chemoenzymatic synthesis of diverse asparagine‐linked α‐(2 → 3)‐sialyloligosaccharides. Glycoconj J 21: 243–250. [DOI] [PubMed] [Google Scholar]
- Furuike T, Yamada K, Ohta T, Monde K, Nishimura S‐I. 2003. An efficient synthesis of a biantennary sialooligosaccharide analogue using a 1,6‐anhydro‐β‐lactose derivative as a key synthetic block. Tetrahedron 59: 5105–5113. [Google Scholar]
- Furukawa S, Mihara H, Ueno A. 2003. Sensing behavior of fluorescent cyclodextrin/peptide hybrids bearing a macrocyclic metal complex. Macromol Mol Commun 24: 202–206. [Google Scholar]
- Gajdošová D, Novotná K, Prošek P, Havel J. 2003. Separation and characterization of humic acids from Antarctia by capillary electrophoresis and matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry. Inclusion complexes of humic acids with cyclodextrins. J Chromatogr A 1014: 117–127. [DOI] [PubMed] [Google Scholar]
- Galan MC, Venot AP, Glushka J, Imberty A, Boons G‐J. 2003. Chemo‐enzymatic synthesis of conformationally constrained oligosaccharides. Org Biomol Chem 1: 3891–3899. [DOI] [PubMed] [Google Scholar]
- Gallego RG, Segura J. 2004. Rapid screening of plasma volume expanders in urine using matrix‐assisted laser desorption/ionisation time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom 18: 1324–1330. [DOI] [PubMed] [Google Scholar]
- Gallego RG, Dudziak G, Kragl U, Wandrey C, Kamerling JP, Vliegenthart JFG. 2003. Enzymatic synthesis of the core‐2 sialyl Lewis X O‐glycan on the tumor‐associated MUC1a' peptide. Biochimie 85: 275–286. [DOI] [PubMed] [Google Scholar]
- Ganesa C, Granda BW, Mattaliano RJ. 2003. Sialylation levels influence oligosaccharide quantitation. Analyzing response variability using high‐pH anion‐exchange chromatography and pulsed amperometric detection. BioPharm Int 16: 44–52. [Google Scholar]
- Gao XB, Yang JH, Huang F, Wu X, Li L, Sun CX. 2003. Progresses of derivatization techniques for analyses of carbohydrates. Anal Lett 36: 1281–1310. [Google Scholar]
- Gao Y, Eguchi A, Kakehi K, Lee YC. 2004. Efficient preparation of glycoclusters from silsesquioxanes. Org Lett 6: 3457–3460. [DOI] [PubMed] [Google Scholar]
- Gårdsvoll H, Werner F, Søndergaard L, Danø K, Ploug M. 2004. Characterization of low‐glycosylated forms of soluble human urokinase receptor expressed in Drosophila Schneider 2 cells after deletion of glycosylation‐sites. Protein Exp Purif 34: 284–295. [DOI] [PubMed] [Google Scholar]
- Gege C, Schneider MF, Schumacher G, Limozin L, Rothe U, Bendas G, Tanaka M, Schmidt RR. 2004. Functional microdomains of glycolipids with partially fluorinated membrane anchors: Impact on cell adhesion. ChemPhysChem 5: 216–224. [DOI] [PubMed] [Google Scholar]
- Gemmell N, Meo P, Osborn HMI. 2003. Stereoselective entry to β‐linked C‐disaccharides using a carbon‐Ferrier reaction. Org Lett 5: 1649–1652. [DOI] [PubMed] [Google Scholar]
- Germer A, Mügge C, Peter MG, Rottmann A, Kleinpeter E. 2003. Solution‐ and bound‐state conformational study of N,N,N‐triacetyl chitotriose and other analogous potential inhibitors of hevamine: Application of trNOESY and STD NMR spectroscopy. Chem Eur J 9: 1964–1973. [DOI] [PubMed] [Google Scholar]
- Gerngross TU. 2004. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat Biotechnol 22: 1409–1414. [DOI] [PubMed] [Google Scholar]
- Gervais A, Hammel Y‐A, Pelloux S, Lepage P, Baer G, Carte N, Sorokine O, Strub J‐M, Koerner R, Leize E, Van Dorsselaer A. 2003. Glycosylation of human recombinant gonadotrophins: Characterization and batch‐to‐batch consistency. Glycobiology 13: 179–189. [DOI] [PubMed] [Google Scholar]
- Geyer H, Wuhrer M, Kurokawa T, Geyer R. 2004. Characterization of keyhole limpet hemocyanin (KLH) glycans sharing a carbohydrate epitope with Schistosoma mansoni glycoconjugates. Micron 35: 105–106. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Ghosal P, Thakur S, Lerouge P, Loutelier‐Bourhis C, Driouich A, Ray B. 2004. Cell wall polysaccharides of Brassica campestris seed cake: Isolation and structural features. Carbohydr Polym 57: 7–13. [Google Scholar]
- Gibson KJ, Gilleron M, Constant P, Puzo G, Nigou J, Besra GS. 2003. Identification of a novel mannose‐capped lipoarabinomannan from Amycolatopsis sulphurea . Biochem J 372: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KJC, Gilleron M, Constant P, Brando T, Puzo G, Besra GS, Nigou J. 2004. Tsukamurella paurometabola lipoglycan, a new lipoarabinomannan variant with pro‐inflammatory activity. J Biol Chem 279: 22973–22982. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Quesniaux VFJ, Puzo G. 2003. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guérin and Mycobacterium tuberculosis H37Rv and its implication in toll‐like receptor response. J Biol Chem 278: 29880–29889. [DOI] [PubMed] [Google Scholar]
- Gimon ME, Preston LM, Solouki T, White MA, Russell DH. 1992. Are proton transfer reactions of excited states involved in UV laser desorption ionization? Org Mass Spectrom 27: 827–830. [Google Scholar]
- Glushka JN, Terrell M, York WS, O'Neill MA, Gucwa A, Darvill AG, Albersheim P, Prestegard JH. 2003. Primary structure of the 2‐O‐methyl‐α‐L‐fucose‐containing side chain of the pectic polysaccharide, rhamnogalacturonan II. Carbohydr Res 338: 341–352. [DOI] [PubMed] [Google Scholar]
- Gómez‐Miranda B, Prieto A, Leal JA, Ahrazem O, Jiménez‐Barbero J, Bernabé M. 2004. Differences among the cell wall galactomannans from Aspergillus wentii and Chaetosartorya chrysella and that of Aspergillus fumigatus . Glycoconj J 20: 239–246. [DOI] [PubMed] [Google Scholar]
- Goon S, Schilling B, Tullius MV, Gibson BW, Bertozzi CR. 2003. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc Natl Acad Sci USA 100: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Miura T, Hosaka D, Matsumoto H, Mizuno M, Ishida H, Inazu T. 2004. Rapid oligosaccharide synthesis on a fluorous support. Tetrahedron 60: 8845–8854. [Google Scholar]
- Gotoh M, Sato T, Kiyohara K, Kameyama A, Kikuchi N, Kwon Y‐D, Ishizuka Y, Iwai T, Nakanishi H, Narimatsu H. 2004. Molecular cloning and characterization of β1 → 4‐N‐acetylgalactosaminyltransferases IV synthesizing N,N′‐diacetyl‐lactosediamine. FEBS Lett 562: 134–140. [DOI] [PubMed] [Google Scholar]
- Gotte G, Libonati M, Laurents DV. 2003. Glycosylation and specific deamidation of ribonuclease B affect the formation of three‐dimensional domain‐swapped oligomers. J Biol Chem 278: 46241–46251. [DOI] [PubMed] [Google Scholar]
- Goujon T, Minic Z, El Amrani A, Lerouxel O, Aletti E, Lapierre C, Joseleau J‐P, Jouanin L. 2003. AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta‐xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J 33: 677–690. [DOI] [PubMed] [Google Scholar]
- Gould WR, Silveira JR, Tracy PB. 2004. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet‐derived cofactor. J Biol Chem 279: 2383–2393. [DOI] [PubMed] [Google Scholar]
- Grandjean C, Santraine V, Fardel N, Polidori A, Pucci B, Gras‐Masse H, Bonnet D. 2004. Efficient preparation of carbohydrate‐ and related polyol‐amphiphiles by hydrazone ligation. Tetrahedron Lett 45: 3451–3454. [Google Scholar]
- Grant GA, Frison SL, Yeung J, Vasanthan T, Sporns P. 2003. Comparison of MALDI‐TOF mass spectrometric to enzyme colorimetric quantification of glucose from enzyme‐hydrolyzed starch. J Agric Food Chem 51: 6137–6144. [DOI] [PubMed] [Google Scholar]
- Greiner LL, Watanabe H, Phillips NJ, Shao J, Morgan A, Zaleski A, Gibson BW, Apicella MA. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N‐acetylneuraminic acid that may mimic sialylated O‐linked glycans. Infect Immun 72: 4249–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grève P, Braquart‐Varnier C, Strub J‐M, Félix C, Van Dorsselaer A, Martin G. 2004. The glycosylated androgenic hormone of the terrestrial isopod Porcellio scaber (Crustacea). Gen Comp Endocrinol 136: 389–397. [DOI] [PubMed] [Google Scholar]
- Grishutin SG, Gusakov AV, Markov AV, Ustinov BB, Semenova MV, Sinitsyn AP. 2004. Specific xyloglucanases as a new class of polysaccharide‐degrading enzymes. Biochim Biophys Acta 1674: 268–281. [DOI] [PubMed] [Google Scholar]
- Groves P, Rasmussen MO, Molero MD, Samain E, Cañada FJ, Driguez H, Jiménez‐Barbero J. 2004. Diffusion ordered spectroscopy as a complement to size exclusion chromatography in oligosaccharide analysis. Glycobiology 14: 451–456. [DOI] [PubMed] [Google Scholar]
- Gu G, Du Y, Hu H, Jin C. 2003. Synthesis of 2‐chloro‐4‐nitrophenyl α‐L‐fucopyranoside: A substrate for α‐L‐fucosidase (AFU). Carbohydr Res 338: 1603–1607. [DOI] [PubMed] [Google Scholar]
- Gudlavalleti SK, Forsberg LS. 2003. Structural characterization of the lipid A component of Sinorhizobium sp. NGR234 rough and smooth form lipopolysaccharide. Demonstration that the distal amide‐linked acyloxyacyl residue containing the long chain fatty acids is conserved in Rhizobium and Sinorhizobium sp. J Biol Chem 278: 3957–3968. [DOI] [PubMed] [Google Scholar]
- Guerardel Y, Czeszak X, Sumanovski LT, Karamanos Y, Popescu O, Strecker G, Misevic GN. 2004. Molecular fingerprinting of carbohydrate structure phenotypes of three Porifera proteoglycan‐like glyconectins. J Biol Chem 279: 15591–15603. [DOI] [PubMed] [Google Scholar]
- Guérardel Y, Maes E, Briken V, Chirat F, Leroy Y, Locht C, Strecker G, Kremer L. 2003. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: Novel structural features and apoptosis‐inducing properties. J Biol Chem 278: 36637–36651. [DOI] [PubMed] [Google Scholar]
- Guillaumie F, Sterling JD, Jensen KJ, Thomas ORT, Mohnen D. 2003. Solid‐supported enzymatic synthesis of pectic oligogalacturonides and their analysis by MALDI‐TOF mass spectrometry. Carbohydr Res 338: 1951–1960. [DOI] [PubMed] [Google Scholar]
- Gutternigg M, Ahrer K, Grabher‐Meier H, Bürgmayr S, Staudacher E. 2004. Neutral N‐glycans of the gastropod Arion lusitanicus . Eur J Biochem 271: 1348–1356. [DOI] [PubMed] [Google Scholar]
- Halkes KM, St., Hilaire PM, Crocker PR, Meldal M. 2003. Glycopeptides as oligosaccharide mimics: High affinity sialopeptide ligands for sialoadhesin from combinatorial libraries. J Comb Chem 5: 18–27. [DOI] [PubMed] [Google Scholar]
- Hambrock HO, Kaufmann B, Müller S, Hanisch F‐G, Nose K, Paulsson M, Maurer P, Hartmann U. 2004. Structural characterization of TSC‐36/Flik. Analysis of two charge isoforms. J Biol Chem 279: 11727–11735. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Bobrowicz PB, Bobrowicz B, Davidson RC, Li H, Mitchell T, Nett JH, Rausch S, Stadheim TA, Wischnewski H, Wildt S, Gerngross TU. 2003. Production of complex human glycoproteins in yeast. Science 301: 1244–1246. [DOI] [PubMed] [Google Scholar]
- Hanashima S, Manabe S, Ito Y. 2003. Polymer—resin hybrid capture—release strategy for rapid oligosaccharide construction. Synlett 979–982. [Google Scholar]
- Haneda K, Tagashira M, Yoshino E, Takeuchi M, Inazu T, Toma K, Iijima H, Isogai Y, Hori M, Takamatsu S, Fujibayashi Y, Kobayashi K, Takeuchi M, Yamamoto K. 2004. Chemo‐enzymatic synthesis and structure‐activity study of artificially N‐glycosylated eel calcitonin derivatives with a complex type oligosaccharide. Glycoconj J 21: 377–386. [DOI] [PubMed] [Google Scholar]
- Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. 2004. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 279: 50019–50024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch F‐G, Schwientek T, Von Bergwelt‐Baildon MS, Schultze JL, Finn O. 2003. O‐Linked glycans control glycoprotein processing by antigen‐presenting cells: A biochemical approach to the molecular aspects of MUC1 processing by dendritic cells. Eur J Immunol 33: 3242–3254. [DOI] [PubMed] [Google Scholar]
- Harabagiu V, Simionescu B, Pinteala M, Merrienne C, Mahuteau J, Guégan P, Cheradame H. 2004. Synthesis and characterization of persilylated cyclodextrins. Carbohydr Polym 56: 301–311. [Google Scholar]
- Haritha B, Reddy VK, Oshikawa T, Yamashita M. 2004. A novel synthesis of deoxy phospha sugar‐sugar disaccharides. Tetrahedron Lett 45: 1923–1927. [Google Scholar]
- Harvey DJ. 1993. Quantitative aspects of the matrix‐assisted laser desorption mass spectrometry of complex oligosaccharides. Rapid Commun Mass Spectrom 7: 614–619. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 1999. Matrix‐assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spectrom Rev 18: 349–451. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 2003. Matrix‐assisted laser desorption/ionization mass spectrometry of carbohydrates and glycoconjugates. Int J Mass Spectrom 226: 1–35. [Google Scholar]
- Harvey DJ. 2006. Analysis of carbohydrates and glycoconjugates by matrix‐assisted laser desorption/ionization mass spectrometry: An update covering the period 1999–2000. Mass Spectrom Rev 25: 595–662. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 2008. Analysis of carbohydrates and glycoconjugates by matrix‐assisted laser desorption/ionization mass spectrometry: An update covering the period 2001–2002. Mass Spectrom Rev 27: 125–201. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Martin RL, Jackson KA, Sutton CW. 2004. Fragmentation of N‐linked glycans with a MALDI‐ion trap time‐of‐flight mass spectrometer. Rapid Commun Mass Spectrom 18: 2997–3007. [DOI] [PubMed] [Google Scholar]
- Hase S. 1992. Conversion of pyridylamino sugar chains to 1‐amino‐1‐deoxy derivatives, intermediates for tagging with fluorescein and biotin. J Biochem (Tokyo) 112: 266–268. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Fujisawa T, Numata M, Matsumoto T, Umeda M, Karinaga R, Mizu M, Koumoto K, Kimura T, Okumura S, Sakurai K, Shinkai S. 2004. Schizophyllans carrying oligosaccharide appendages as potential candidates for cell‐targeted antisense carrier. Org Biomol Chem 2: 3091–3098. [DOI] [PubMed] [Google Scholar]
- Hashii N, Isshiki Y, Iguchi T, Kondo S. 2003a. Structural analysis of the carbohydrate backbone of Vibrio parahaemolyticus O2 lipopolysaccharides. Carbohydr Res 338: 1063–1071. [DOI] [PubMed] [Google Scholar]
- Hashii N, Isshiki Y, Iguchi T, Kondo S. 2003b. Structural characterization of the carbohydrate backbone of the lipopolysaccharide of Vibrio parahaemolyticus O‐untypeable strain KX‐V212 isolated from a patient. Carbohydr Res 338: 2711–2719. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Asai Y, Jinno T, Adachi S, Kusumoto S, Ogawa T. 2003a. Structural elucidation of polysaccharide part of glycoconjugate from Treponema medium ATCC 700293. Eur J Biochem 270: 2671–2679. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Asai Y, Tamai R, Jinno T, Umatani K, Ogawa T. 2003b. Chemical structure and immunobiological activity of lipid A from Prevotella intermedia ATCC 25611 lipopolysaccharide. FEBS Lett 543: 98–102. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Dell A. 2003. Hallmarks of Caenorhabditis elegans N‐glycosylation: Complexity and controversy. Biochimie 85: 25–32. [DOI] [PubMed] [Google Scholar]
- He H, Gu G, Du Y. 2003. Synthesis of laminarin oligosaccharide derivatives having D‐arabinofuranosyl side‐chains. J Carbohydr Chem 22: 275–283. [Google Scholar]
- Heine H, Müller‐Loennies S, Brade L, Lindner B, Brade H. 2003. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur J Biochem 270: 440–450. [DOI] [PubMed] [Google Scholar]
- Henkensmeier D, Abele BC, Candussio A, Thiem J. 2004. Synthesis and characterisation of terminal carbohydrate modified poly(dimethylsiloxane)s. Macromol Chem Phys 205: 1851–1857. [Google Scholar]
- Hernández P, Tetaert D, Vergoten G, Debray H, del Carmen Jimenez M, Fernández G, Agundis C, Degand P, Zenteno E. 2004. Specificity of Amaranthus leucocarpus syn. hypocondriacus lectin for O‐glycopeptides. Biochim Biophys Acta 1674: 282–290. [DOI] [PubMed] [Google Scholar]
- Higai K, Azuma Y, Aoki Y, Matsumoto K. 2003a. Altered glycosylation of α1‐acid glycoprotein in patients with inflammation and diabetes mellitus. Clin Chim Acta 329: 117–125. [DOI] [PubMed] [Google Scholar]
- Higai K, Shibukawa K, Muto S, Matsumoto K. 2003b. Targeted proteo‐glycomics analysis of sialyl Lewis X antigen expressing glycoproteins secreted by human hepatoma cell line. Anal Sci 19: 85–92. [DOI] [PubMed] [Google Scholar]
- Hiki Y, Horie A, Yasuda Y, Iwase H, Sugiyama S. 2004. IgA nephropathy and tonsils—An approach from the structure of IgA1 produced by tonsillar lymphocytes. Acta Otolaryngol Suppl 555: 28–31. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J, Hayama K, Kaji H, Isobe T, Kasai K‐i. 2002. Affinity capturing and gene assignment of soluble glycoproteins produced by the nematode Caenorhabditis elegans . J Biochem (Tokyo) 132: 103–114. [DOI] [PubMed] [Google Scholar]
- Hirose K, Kosuge Y, Otomatsu T, Endo K, Hasegawa K. 2003. Preparation of a growth‐promoting substance, lepidimoic acid, from okra pectic polysaccharide. Tetrahedron Lett 44: 2171–2173. [Google Scholar]
- Hirose K, Endo K, Hasegawa K. 2004. A convenient synthesis of lepidimoide from okra mucilage and its growth‐promoting activity in hypocotyls. Carbohydr Res 339: 9–19. [DOI] [PubMed] [Google Scholar]
- Hiruma T, Togayachi A, Okamura K, Sato T, Kikuchi N, Kwon Y‐D, Nakamura A, Fujimura K, Gotoh M, Tachibana K, Ishizuka Y, Noce T, Nakanishi H, Narimatsu H. 2004. A novel human β1 → 3‐N‐acetylgalactosaminyltransferase that synthesizes a unique carbohydrate structure, GalNAcβ1 → 3GlcNAc. J Biol Chem 279: 14087–14095. [DOI] [PubMed] [Google Scholar]
- Hoja‐Lukowicz D, Ciolczyk D, Bergquist J, Litynska A, Laidler P. 2000. High‐mannose‐type oligosaccharides from human placental arylsulfatase A are core fucosylated as confirmed by MALDI MS. Glycobiology 10: 551–557. [DOI] [PubMed] [Google Scholar]
- Hojo H, Haginoya E, Matsumoto Y, Nakahara Y, Nabeshima K, Toole BP, Watanabe Y. 2003. The first synthesis of peptide thioester carrying N‐linked core pentasaccharide through modified Fmoc thioester preparation: Synthesis of an N‐glycosylated Ig domain of emmprin. Tetrahedron Lett 44: 2961–2964. [Google Scholar]
- Holland JW, Deeth HC, Alewood PF. 2004. Proteomic analysis of kappa‐casein micro‐heterogeneity. Proteomics 4: 743–752. [DOI] [PubMed] [Google Scholar]
- Holmén JM, Olson FJ, Karlsson H, Hansson GC. 2002. Two glycosylation alterations of mouse intestinal mucins due to infection by the parasite Nippostrongylus brasiliensis . Glycoconj J 19: 67–75. [DOI] [PubMed] [Google Scholar]
- Hong Y, Sundaram S, Shin D‐J, Stanley P. 2004. The Lec23 Chinese hamster ovary mutant is a sensitive host for detecting mutations in α‐glucosidase I that give rise to congenital disorder of glycosylation IIb (CDG IIb). J Biol Chem 279: 49894–49901. [DOI] [PubMed] [Google Scholar]
- Horie A, Hiki Y, Odani H, Yasuda Y, Takahashi M, Kato M, Iwase H, Kobayashi Y, Nakashima I, Maeda K. 2003a. IgA1 molecules produced by tonsillar lymphocytes are under‐O‐glycosylated in IgA nephropathy. Am J Kidney Dis 42: 486–496. [DOI] [PubMed] [Google Scholar]
- Horie A, Nakamura I, Yasuda Y, Odani H, Iwase H, Kobayashi Y, Hiki Y. 2003b. The structural alteration of O‐glycosylated hinge peptides in serum IgA1 before and after tonsillectomy in IgA nephropathy. Nephrology 8: A102–A103. [Google Scholar]
- Horvat S, Jakas A. 2004. Peptide and amino acid glycation: New insights into the Maillard reaction. J Peptide Sci 10: 119–137. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Nakagawa H, Nishiguchi S, Iwata K, Niikura K, Monde K, Nishimura S‐I. 2004. An engineered hyaluronan synthase. Characterization of recombinant human hyaluronan synthase 2 expressed in Escherichia coli . J Biol Chem 279: 2341–2349. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Mihara H, Ueno A. 2003. Fluorescence resonance energy transfer in a novel cyclodextrin‐peptide conjugate for detecting steroid molecules. Bioorg Med Chem Lett 13: 4305–4308. [DOI] [PubMed] [Google Scholar]
- Hossany RB, Johnson MA, Eniade AA, Pinto BM. 2004. Synthesis and immunochemical characterization of protein conjugates of carbohydrate and carbohydrate‐mimetic peptides as experimental vaccines. Bioorg Med Chem 12: 3743–3754. [DOI] [PubMed] [Google Scholar]
- Hua Y, Du Y, Yu G, Chu S. 2004a. Synthesis and biological activities of octyl 2,3‐di‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 3)‐2‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 4)‐2,3‐di‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 3)‐2‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 4)‐2,3‐di‐O‐sulfo‐β‐L‐fucopyranoside. Carbohydr Res 339: 2083–2090. [DOI] [PubMed] [Google Scholar]
- Hua Y, Gu G, Du Y. 2004b. Synthesis and biological activities of octyl 2,3,4‐tri‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 3)‐2,4‐di‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 3)‐2,4‐di‐O‐sulfo‐α‐L‐fucopyranosyl‐(1 → 3)‐2,4‐di‐O‐sulfo‐β‐L‐fucopyranoside. Carbohydr Res 339: 867–872. [DOI] [PubMed] [Google Scholar]
- Huang HH. 2003. Application of MALDI‐QTOF in proteomics and glycomics. Aust J Chem 56: 65. [Google Scholar]
- Huang Y, Mechref Y, Novotny MV. 2001. Microscale nonreductive release of O‐linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal Chem 73: 6063–6069. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang H, Shimizu N, Takeda T. 2003. Ardisimamillosides G and H, two new triterpenoid saponins from Ardisia mamillata . Chem Pharm Bull 51: 875–877. [DOI] [PubMed] [Google Scholar]
- Hussain LA, Dickens SH, Bowen RL. 2004. Effects of polymerization initiator complexation in methacrylated β‐cyclodextrin formulations. Dent Mater 20: 513–521. [DOI] [PubMed] [Google Scholar]
- Hyuga M, Itoh S, Kawasaki N, Ohta M, Ishii A, Hyuga S, Hayakawa T. 2004. Analysis of site‐specific glycosylation in recombinant human follistatin expressed in Chinese hamster ovary cells. Biologicals 32: 70–77. [DOI] [PubMed] [Google Scholar]
- Idakieva K, Stoeva S, Voelter W, Gielens C. 2004. Glycosylation of Rapana thomasiana hemocyanin. Comparison with other prosobranch (gastropod) hemocyanins. Comp Biochem Physiol B 138: 221–228. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Matsuhisa A, Ueno A. 2003. Efficient transport of saccharides through a liquid membrane mediated by a cyclodextrin dimer. Chem Eur J 9: 4907–4910. [DOI] [PubMed] [Google Scholar]
- Ikuta A, Mizuta N, Kitahata S, Murata T, Usui T, Koizumi K, Tanimoto T. 2004. Preparation and characterization of novel branched β‐cyclodextrins having β‐D‐galactose residues on the non‐reducing terminal of the side chains and their specific interactions with peanut (Arachis hypogaea) agglutinin. Chem Pharm Bull 52: 51–56. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Ide Y, Fujii S, Gu J, Honke K, Taniguchi N. 2003. Molecular cloning and characterization of human GnT‐IX, a Novel β1,6‐N‐acetylglucosaminyltransferase that is specifically expressed in the brain. J Biol Chem 278: 43102–43109. [DOI] [PubMed] [Google Scholar]
- Inamori K, Endo T, Gu J, Matsuo I, Ito Y, Fujii S, Iwasaki H, Narimatsu H, Miyoshi E, Honke K, Taniguchi N. 2004. N‐Acetylglucosaminyltransferase IX acts on the GlcNAcβ1,2‐Manα1‐Ser/Thr moiety, forming a 2,6‐branched structure in brain O‐mannosyl glycan. J Biol Chem 279: 2337–2340. [DOI] [PubMed] [Google Scholar]
- Isobe H, Mashima H, Yorimitsu H, Nakamura E. 2003. Synthesis of fullerene glycoconjugates through sulfide connection in aqueous media. Org Lett 5: 4461–4463. [DOI] [PubMed] [Google Scholar]
- Itonori S, Aoki K, Sugita M. 2004a. Glycosphingolipids in edible fungi and their biological activities. Foods Food Ingredients J Jpn 209: 211–218. [Google Scholar]
- Itonori S, Takahashi M, Kitamura T, Aoki K, Dulaney JT, Sugita M. 2004b. Microwave‐mediated analysis for sugar, fatty acid, and sphingoid compositions of glycosphingolipids. J Lipid Res 45: 574–581. [DOI] [PubMed] [Google Scholar]
- Ivannikova T, Bintein F, Malleron A, Juliant S, Cerutti M, Harduin‐Lepers A, Delannoy P, Augé C, Lubineau A. 2003. Recombinant (2 → 3)‐α‐sialyltransferase immobilized on nickel‐Agarose for preparative synthesis of sialyl Lewisx and Lewisa precursor oligosaccharides. Carbohydr Res 338: 1153–1161. [DOI] [PubMed] [Google Scholar]
- Ivleva VB, Elkin YN, Budnik BA, Moyer SC, O'Connor PB, Costello CE. 2004. Coupling thin‐layer chromatography with vibrational cooling matrix‐assisted laser desorption/ionization Fourier transform mass spectrometry for the analysis of ganglioside mixtures. Anal Chem 76: 6484–6491. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Zhang Y, Tachibana K, Gotoh M, Kikuchi N, Kwon Y‐D, Togayachi A, Kudo T, Kubota T, Narimatsu H. 2003. Initiation of O‐glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP‐N‐acetyl‐α‐D‐galactosamine:polypeptide N‐acetylgalactosaminyltransferase 2. J Biol Chem 278: 5613–5621. [DOI] [PubMed] [Google Scholar]
- Iyer S, Rele S, Grasa G, Nolan S, Chaikof EL. 2003. Synthesis of a hyaluronan neoglycopolymer by ring‐opening metathesis polymerization. Chem Commun 1518–1519. [DOI] [PubMed] [Google Scholar]
- Izquierdo L, Coderch N, Piqué N, Bedini E, Corsaro MM, Merino S, Fresno S, Tomás JM, Regué M. 2003. The Klebsiella pneumoniae wabG gene: Role in biosynthesis of the core lipopolysaccharide and virulence. J Bacteriol 185: 7213–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Okumura S, Yuasa H, Hashimoto H. 2003. Mannose‐BSA conjugates: Comparison between commercially available linkers in reactivity and bioactivity. J Carbohydr Chem 22: 317–329. [Google Scholar]
- Jacobs A, Palm M, Zacchi G, Dahlman O. 2003. Isolation and characterization of water‐soluble hemicelluloses from flax shive. Carbohydr Res 338: 1869–1876. [DOI] [PubMed] [Google Scholar]
- Jacoby ES, Kicman AT, Iles RK. 2003. Identification of post‐translational modifications resulting from LHβ polymorphisms by matrix‐assisted laser desorption time‐of‐flight mass spectrometric analysis of pituitary LHβ core fragment. J Mol Endocrinol 30: 239–252. [DOI] [PubMed] [Google Scholar]
- Janus L, Carbonnier B, Morcellet M, Ricart G, Crini G, Deratani A. 2003. Mass spectrometric characterization of a new 2‐hydroxypropyl‐β‐cyclodextrin derivative bearing methacrylic moieties and its copolymerization with 1‐vinyl‐2‐pyrrolidone. Macromol Biosci 3: 198–209. [Google Scholar]
- Jayaprakash KN, Fraser‐Reid B. 2004. One‐pot chemo‐, regio‐, and stereoselective double‐differential glycosidation mediated by lanthanide triflates. Org Lett 6: 4211–4214. [DOI] [PubMed] [Google Scholar]
- Jayaprakash KN, Lu J, Fraser‐Reid B. 2004. Synthesis of a key Mycobacterium tuberculosis biosynthetic phosphoinositide intermediate. Bioorg Med Chem Lett 14: 3815–3819. [DOI] [PubMed] [Google Scholar]
- Jelinek R, Kolusheva S. 2004. Carbohydrate biosensors. Chem Rev 104: 5987–6016. [DOI] [PubMed] [Google Scholar]
- Jensen ON. 2004. Modification‐specific proteomics: Characterization of post‐translational modifications by mass spectrometry. Curr Opin Chem Biol 8: 33–41. [DOI] [PubMed] [Google Scholar]
- Jeong EY, Lee JY, Park TH. 2004. Specificity of enzymatic in vitro glycosylation by PNGase F: A comparison of enzymatic and non‐enzymatic glycosylation. Enzyme Microb Technol 35: 587–591. [Google Scholar]
- Jia Z, Qin Q, Darvill AG, York WS. 2003. Structure of the xyloglucan produced by suspension‐cultured tomato cells. Carbohydr Res 338: 1197–1208. [DOI] [PubMed] [Google Scholar]
- Jing H, Kitts DD. 2004. Chemical characterization of different sugar‐casein Maillard reaction products and protective effects on chemical‐induced cytotoxicity of Caco‐2 cells. Food Chem Toxicol 42: 1833–1844. [DOI] [PubMed] [Google Scholar]
- Jobron L, Sujino K, Hummel G, Palcic MM. 2003. Glycosyltransferase assays utilizing N‐acetyllactosamine acceptor immobilized on a cellulose membrane. Anal Biochem 323: 1–6. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Park TH, Shuler ML. 2003a. Effect of silkworm hemolymph on N‐linked glycosylation in two Trichoplusia ni insect cell lines. Biotechnol Bioeng 83: 695–705. [DOI] [PubMed] [Google Scholar]
- Joosten JAF, Evers B, van Summeren RP, Kamerling JP, Vliegenthart JFG. 2003b. Synthesis of β‐D‐Galp‐(1 → 4)‐β‐D‐GlcpNAc‐(1 → 2)‐α‐D‐Manp‐(1 → O)(CH2)7CH3 mimics to explore the substrate specificity of sialyltransferases and trans‐sialidases. Eur J Org Chem 3569–3586. [Google Scholar]
- Kabel MA, de Waard P, Schols HA, Voragen AGJ. 2003. Location of O‐acetyl substituents in xylo‐oligosaccharides obtained from hydrothermally treated Eucalyptus wood. Carbohydr Res 338: 69–77. [DOI] [PubMed] [Google Scholar]
- Kahler CM, Datta A, Tzeng Y‐I, Carlson RW, Stephens DS. 2004. Inner core assembly and structure of the lipooligosaccharide of Neisseria meningitidis: Capacity of strain NMB to express all known immunotype epitopes. Glycobiology 15: 409–419. [DOI] [PubMed] [Google Scholar]
- Kajihara Y, Suzuki Y, Yamamoto N, Sasaki K, Sakakibara T, Juneja LR. 2004. Prompt chemoenzymatic synthesis of diverse complex‐type oligosaccharides and its application to the solid‐phase synthesis of a glycopeptide with Asn‐linked sialyl‐undeca‐ and asialo‐nonasaccharides. Chem Eur J 10: 971–985. [DOI] [PubMed] [Google Scholar]
- Kakehi K, Kinoshita M, Nakano M. 2002. Analysis of glycoproteins and the oligosaccharides thereof by high‐performance capillary electrophoresis—Significance in regulatory studies on biopharmaceutical products. Biomed Chromatogr 16: 103–115. [DOI] [PubMed] [Google Scholar]
- Kalashnikov PA, Kotel'nikov GV, Moiseeva SP, Topchieva IN. 2004. Inclusion complexes formed by sodium dodecyl sulfate and cyclodextrin‐based nanotubes. Colloid J 66: 542–546. [Google Scholar]
- Kalinovskii AI, Levina EV, Stonik VA, Dmitrenok PS, Andriyashchenko PV. 2004. Steroid polyols from the far eastern starfish Henricia sanguinolenta and H. leviuscula leviuscula . Russ J Bioorg Chem 30: 191–195. [DOI] [PubMed] [Google Scholar]
- Kamar M, Alvarez‐Manilla G, Abney T, Azadi P, Kolli VSK, Orlando R, Pierce M. 2004. Analysis of the site‐specific N‐glycosylation of β1,6 N‐acetylglucosaminyltransferase V. Glycobiology 14: 583–592. [DOI] [PubMed] [Google Scholar]
- Kameyama A, Kaneda Y, Yamanaka H, Yoshimine H, Narimatsu H, Shinohara Y. 2004. Detection of oligosaccharides labeled with cyanine dyes using matrix‐assisted laser desorption/ionization mass spectrometry. Anal Chem 76: 4537–4542. [DOI] [PubMed] [Google Scholar]
- Kamoda S, Nomura C, Kinoshita M, Nishiura S, Ishikawa R, Kakehi K, Kawasaki N, Hayakawa T. 2004. Profiling analysis of oligosaccharides in antibody pharmaceuticals by capillary electrophoresis. J Chromatogr A 1050: 211–216. [PubMed] [Google Scholar]
- Kandil FE, Grace MH, Seigler DS, Cheeseman JM. 2004. Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees Struct Funct 18: 518–528. [Google Scholar]
- Kandra L. 2003. α‐Amylases of medical and industrial importance. J Mol Struct 666–667: 487–498. [Google Scholar]
- Kandra L, Gyémánt G, Zajácz Á, Batta G. 2004. Inhibitory effects of tannin on human salivary α‐amylase. Biochem Biophys Res Commun 319: 1265–1271. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Alvarez‐Manilla G, Kamar M, Lee I, Lee J‐K, Troupe K, Zhang W, Osawa M, Pierce M. 2003. A novel β(1 → 6)‐N‐acetylglucosaminyltransferase V (GnT‐VB). FEBS Lett 554: 515–519. [DOI] [PubMed] [Google Scholar]
- Kang J, Low W, Norberg T, Meisenhelder J, Hansson K, Stenflo J, Zhou G‐P, Imperial J, Olivera BM, Rigby AC, Craig AG. 2004. Total chemical synthesis and NMR characterization of the glycopeptide tx5a, a heavily post‐translationally modified conotoxin, reveals that the glycan structure is α‐D‐Gal‐(1 → 3)‐α‐D‐GalNAc. Eur J Biochem 271: 4939–4949. [DOI] [PubMed] [Google Scholar]
- Kanipes MI, Kalb SR, Cotter RJ, Hozbor DF, Lagares A, Raetz CRH. 2003a. Relaxed sugar donor selectivity of a Sinorhizobium meliloti ortholog of the Rhizobium leguminosarum mannosyl transferase LpcC: Role of the lipopolysaccharide core in symbiosis of Rhizobiaceae with plants. J Biol Chem 278: 16365–16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanipes MI, Ribeiro AA, Lin S, Cotter RJ, Raetz CRH. 2003b. A mannosyl transferase required for lipopolysaccharide inner core assembly in Rhizobium leguminosarum. Purification, substrate specificity and expression in Salmonella waaC mutants. J Biol Chem 278: 16356–16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbarz MJ, Kalb SR, Cotter RJ, Raetz CRH. 2003. Expression cloning and biochemical characterization of a Rhizobium leguminosarum lipid A 1‐phosphatase. J Biol Chem 278: 39269–39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Sletten K, Westermark P. 2003. Biclonal systemic AL‐amyloidosis with one glycosylated and one nonglycosylated AL‐protein. Scand J Immunol 57: 319–323. [DOI] [PubMed] [Google Scholar]
- Karioti A, Skalts H, Heilmann J, Sticher O. 2003. Acylated flavonoid and phenylethanoid glycosides from Marrubium velutinum . Phytochemistry 64: 655–660. [DOI] [PubMed] [Google Scholar]
- Kasuya MCZ, Cusi R, Ishihara O, Miyagawa A, Hashimoto K, Sato T, Hatanaka K. 2004. Fluorous‐tagged compound: A viable scaffold to prime oligosaccharide synthesis by cellular enzymes. Biochem Biophys Res Commun 316: 599–604. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi Y, Tsumuraya Y, Hashimoto Y, Nakano H, Kovác P. 2003. In vitro biosynthesis of galactans by membrane‐bound galactosyltransferase from radish (Raphanus sativus L.) seedlings. Planta 217: 271–282. [DOI] [PubMed] [Google Scholar]
- Kato Y, Ito S, Mitsuishi Y. 2004. Study on the structures of xyloglucans using specific enzymes. Trends Glycosci Glycotechnol 16: 393–406. [Google Scholar]
- Kaufmann B, Müller S, Hanisch F‐G, Hartmann U, Paulsson M, Maurer P, Zaucke F. 2004. Structural variability of BM‐40/SPARC/osteonectin glycosylation: Implications for collagen affinity. Glycobiology 14: 609–619. [DOI] [PubMed] [Google Scholar]
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. 2004. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305: 1003–1006. [DOI] [PubMed] [Google Scholar]
- Kazuma K, Noda N, Suzuki M. 2003a. Malonylated flavonol glycosides from the petals of Clitoria ternatea . Phytochemistry 62: 229–237. [DOI] [PubMed] [Google Scholar]
- Kazuma K, Noda N, Suzuki M. 2003b. Flavonoid composition related to petal color in different lines of Clitoria ternatea . Phytochemistry 64: 1133–1139. [DOI] [PubMed] [Google Scholar]
- Kéki S, Deák G, Lévai A, Zsuga M. 2003. Post‐source decay matrix‐assisted laser desorption/ionization mass spectrometric study of peracetylated isoflavone glycosides cationized by protonation and with various metal ions. J Mass Spectrom 38: 1207–1209. [DOI] [PubMed] [Google Scholar]
- Kéki S, Nagy L, Deák G, Zsuga M, Somogyi L, Lévai A. 2004. Cationization of simple organic molecules by singly‐charged Ag cluster ions in matrix‐assisted laser desorption/ionization mass spectrometry: Metal cluster‐molecule interactions. J Am Soc Mass Spectrom 15: 879–883. [DOI] [PubMed] [Google Scholar]
- Kensinger RD, Yowler BC, Benesi AJ, Schengrund C‐L. 2004. Synthesis of novel, multivalent glycodendrimers as ligands for HIV‐1 gp120. Bioconj Chem 15: 349–358. [DOI] [PubMed] [Google Scholar]
- Kett WC, Coombe DR. 2004. A structural analysis of heparin‐like glycosaminoglycans using MALDI‐TOF mass spectrometry. Spectroscopy 18: 185–201. [Google Scholar]
- Khalaila I, Peter‐Katalinic J, Tsang C, Radcliffe CM, Aflalo ED, Harvey DJ, Dwek RA, Rudd PM, Sagi A. 2004. Structural characterization of the N‐glycan moiety and site of glycosylation in vitellogenin from the decapod crustacean Cherax quadricarinatus . Glycobiology 14: 767–774. [DOI] [PubMed] [Google Scholar]
- Khodair AI, Winterfeld GA, Schmidt RR. 2003. Conjugate addition of phenols to 2‐nitrogalactal—Synthesis of O‐(2‐acetamido‐2‐deoxygalactosyl)tyrosine. Eur J Org Chem 1847–1852. [Google Scholar]
- Khodair AI, Pachamuthu K, Schmidt RR. 2004. A convenient route to O‐glycosyl lactates via conjugate addition to 2‐nitroglycals: Ring closure to novel pyrano[2,3‐b][1,4]‐oxazines. Synthesis 53–58. [Google Scholar]
- Khotimchenko SV, Vas'kovsky VE. 2004. An inositol‐containing sphingolipid from the red alga Gracilaria verrucosa . Russ J Bioorg Chem 30: 168–171. [DOI] [PubMed] [Google Scholar]
- Kida T, Kikuzawa A, Nakatsuji Y, Akashi M. 2003. A facile synthesis of novel cyclodextrin derivatives incorporating one β‐(1,4)‐glucosidic bond and their unique inclusion ability. Chem Commun 3020–3021. [DOI] [PubMed] [Google Scholar]
- Kida T, Ohe T, Higashimoto H, Harada H, Nakatsuji Y, Ikeda I, Akashi M. 2004. Amphiphilic cyclodextrins as novel monosaccharide transport carriers through a bulk liquid membrane. Chem Lett 33: 258–259. [Google Scholar]
- Kiernan UA, Nedelkov D, Tubbs KA, Niederkofler EE, Nelson RW. 2004. Proteomic characterization of novel serum amyloid P component variants from human plasma and urine. Proteomics 4: 1825–1829. [DOI] [PubMed] [Google Scholar]
- Kim S, Hwang SK, Dwek RA, Rudd PM, Ahn YH, Kim E‐H, Cheong C, Kim SI, Park NS, Lee SM. 2003. Structural determination of the N‐glycans of a lepidopteran arylphorin reveals the presence of a monoglucosylated oligosaccharide in the storage protein. Glycobiology 13: 147–157. [DOI] [PubMed] [Google Scholar]
- Kim B‐M, Kim H, Raines RT, Lee Y. 2004. Glycosylation of onconase increases its conformational stability and toxicity for cancer cells. Biochem Biophys Res Commun 315: 976–983. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Humeny A, Peich CC, Zhang X, Niwa T, Pischetsrieder M, Becker C‐M. 2003. Relative quantification of N‐(carboxymethyl)lysine, imidazolone A, and the Amadori product in glycated lysozyme by MALDI‐TOF mass spectrometry. J Agric Food Chem 51: 51–57. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Humeny A, Pischetsrieder M. 2004. Analysis of protein glycation products by matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry. Curr Med Chem 11: 2185–2193. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Hojo H, Nakahara Y, Ishimizu T, Hase S. 2004. Synthesis and conformational characterization of the epidermal growth factor‐like domain of blood coagulation factor IX carrying xylosyl‐glucose. Glycoconj J 21: 197–203. [DOI] [PubMed] [Google Scholar]
- Kittur FS, Kumar ABV, Gowda LR, Tharanathan RN. 2003. Chitosanolysis by a pectinase isozyme of Aspergillus niger‐A non‐specific activity. Carbohydr Polym 53: 191–196. [Google Scholar]
- Kjellström S, Jensen ON. 2003. In situ liquid‐liquid extraction as a sample preparation method for matrix‐assisted laser desorption/ionization MS analysis of polypeptide mixtures. Anal Chem 75: 2362–2369. [DOI] [PubMed] [Google Scholar]
- Kleinert M, Röckendorf N, Lindhorst TK. 2004. Glyco‐SAMs as glycocalyx mimetics: Synthesis of L‐fucose‐ and D‐mannose‐terminated building blocks. Eur J Org Chem 3931–3940. [Google Scholar]
- Ko K, Tekoah Y, Rudd PM, Harvey DJ, Dwek RA, Spitsin S, Hanlon CA, Rupprecht C, Dietzschold B, Golovkin M, Koprowski H. 2003. Function and glycosylation of plant‐derived antiviral monoclonal antibody. Proc Natl Acad Sci USA 100: 3013–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhn M, Benito JM, Mellet CO, Lindhorst TK, García Fernández JM. 2004. Functional evaluation of carbohydrate‐centred glycoclusters by enzyme‐linked lectin assay: Ligands for concanavalin A. ChemBioChem 5: 771–777. [DOI] [PubMed] [Google Scholar]
- Koles K, van Berkel PHC, Mannesse MLM, Zoetemelk R, Vliegenthart JFG, Kamerling JP. 2004a. Influence of lactation parameters on the N‐glycosylation of recombinant human C1 inhibitor isolated from the milk of transgenic rabbits. Glycobiology 14: 979–986. [DOI] [PubMed] [Google Scholar]
- Koles K, van Berkel PHC, Pieper FR, Nuijens JH, Mannesse MLM, Vliegenthart JFG, Kamerling JP. 2004b. N‐ and O‐glycans of recombinant human C1 inhibitor expressed in the milk of transgenic rabbits. Glycobiology 14: 51–64. [DOI] [PubMed] [Google Scholar]
- Komaniecka I, Choma A. 2003. Isolation and characterization of periplasmic cyclic β‐glucans of Azorhizobium caulinodans . FEMS Microbiol Lett 227: 263–269. [DOI] [PubMed] [Google Scholar]
- Konishi T, Mitome T, Hatsushika H, Haque MA, Kotake T, Tsumuraya Y. 2004. Biosynthesis of pectic galactan by membrane‐bound galactosyltransferase from soybean (Glycine max Merr.) seedlings. Planta 218: 833–842. [DOI] [PubMed] [Google Scholar]
- Kooistra O, Bedoux G, Brecker L, Lindner B, Sánchez Carballo P, Haras D, Zähringer U. 2003. Structure of a highly phosphorylated lipopolysaccharide core in the ΔalgC mutants derived from Pseudomonas aeruginosa wild‐type strains PAO1 (serogroup O5) and PAC1R (serogroup O3). Carbohydr Res 338: 2667–2677. [DOI] [PubMed] [Google Scholar]
- Koopmann J‐O, Blackburn J. 2003. High affinity capture surface for matrix‐assisted laser desorption/ionisation compatible protein microarrays. Rapid Commun Mass Spectrom 17: 455–462. [DOI] [PubMed] [Google Scholar]
- Koprivova A, Stemmer C, Altmann F, Hoffmann A, Kopriva S, Gorr G, Reski R, Decker EL. 2004. Targeted knockouts of Physcomitrella lacking plant specific immunogenic N‐glycans. Plant Biotechnol J 2: 517–523. [DOI] [PubMed] [Google Scholar]
- Korduláková J, Gilleron M, Puzo G, Brennan PJ, Gicquel B, Mikusová K, Jackson M. 2003. Identification of the required acyltransferase step in the biosynthesis of the phosphatidylinositol mannosides of Mycobacterium species. J Biol Chem 278: 36285–36295. [DOI] [PubMed] [Google Scholar]
- Korekane H, Taguchi T, Sakamoto Y, Honke K, Dohmae N, Salminen H, Toivonen S, Helin J, Takio K, Renkonen O, Taniguchi N. 2003. Purification and cDNA cloning of UDP‐GlcNAc:GlcNAcβ1 → 3Galβ1 → 4Glc(NAc)‐R [GlcNAc → Gal]β1,6N‐acetylglucosaminyltransferase from rat small intestine: A major carrier of dIGnT activity in rat small intestine. Glycobiology 13: 387–400. [DOI] [PubMed] [Google Scholar]
- Kotani N, Asano M, Inoue N, Iwakura Y, Takasaki S. 2004. Polylactosamine synthesis and branch formation of N‐glycans in β1,4‐galactosyltransferase‐1‐deficient mice. Arch Biochem Biophys 426: 258–265. [DOI] [PubMed] [Google Scholar]
- Kotra LP, Zhang L, Fridman R, Orlando R, Mobashery S. 2002. N‐Glycosylation pattern of the zymogenic form of human matrix metalloproteinase‐9. Bioorg Chem 30: 356–370. [DOI] [PubMed] [Google Scholar]
- Kremmer T, Szöllösi É, Boldizsár M, Vincze B, Ludányi K, Imre T, Schlosser G, Vékey K. 2004. Liquid chromatographic and mass spectrometric analysis of human serum acid alpha‐1‐glycoprotein. Biomed Chromatogr 18: 323–329. [DOI] [PubMed] [Google Scholar]
- Kremnický L, Mastihuba V, Côté GL. 2004. Trichoderma reesei acetyl esterase catalyzes transesterification in water. J Mol Catal B Enzymatic 30: 229–239. [Google Scholar]
- Krokhin O, Li Y, Andonov A, Feldmann H, Flick R, Jones S, Stroeher U, Bastien N, Dasuri KVN, Cheng K, Simonsen JN, Perreault H, Wilkins J, Ens W, Plummer F, Standing KG. 2003. Mass spectrometric characterization of proteins from the SARS virus. A preliminary report. Mol Cell Proteomics 2: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokhin O, Ens W, Standing KG, Wilkins J, Perreault H. 2004a. Site‐specific N‐glycosylation analysis: Matrix‐assisted laser desorption/ionization quadrupole‐quadrupole time‐of‐flight tandem mass spectral signatures for recognition and identification of glycopeptides. Rapid Commun Mass Spectrom 18: 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokhin OV, Craig R, Spicer V, Ens W, Standing KG, Beavis RC, Wilkins JA. 2004b. An improved model for prediction of retention times of tryptic peptides in ion pair reversed‐phase HPLC. Its application to protein peptide mapping by off‐line HPLC‐MALDI MS. Mol Cell Proteomics 3: 908–919. [DOI] [PubMed] [Google Scholar]
- Krueger CG, Vestling MM, Reed JD. 2003. Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry of heteropolyflavan‐3‐ols and glucosylated heteropolyflavans in Sorghum [Sorghum bicolor (L.) Moench]. J Agric Food Chem 51: 538–543. [DOI] [PubMed] [Google Scholar]
- Kühn AV, Raith K, Sauerland V, Neubert RHH. 2003. Quantification of hyaluronic acid fragments in pharmaceutical formulations using LC‐ESI‐MS. J Pharmaceut Biomed Anal 30: 1531–1537. [DOI] [PubMed] [Google Scholar]
- Kulesza A, Frank CG, Aebi M, Vasella A. 2004. Synthesis of stable dolichylphosphomannose analogues. Helv Chim Acta 87: 3106–3118. [Google Scholar]
- Kuroda Y, Nakata M, Makino A, Matsumoto A, Ohashi K, Itahashi K, Takeuchi F, Goto M, Kojima N, Mizuochi T. 2002. Structural studies on IgG oligosaccharides of patients with primary Sjögren's syndrome. Glycoconj J 19: 23–31. [DOI] [PubMed] [Google Scholar]
- Kurogochi M, Nishimura S‐I. 2004. Structural characterization of N‐glycopeptides by matrix‐dependent selective fragmentation of MALDI‐TOF/TOF tandem mass spectrometry. Anal Chem 76: 6097–6101. [DOI] [PubMed] [Google Scholar]
- Kurogochi M, Matsushita T, Nishimura S‐I. 2004. Post‐translational modifications on proteins: Facile and efficient procedure for the identification of O‐glycosylation sites by MALDI‐LIFT‐TOF/TOF mass spectrometry. Angew Chem Intl Ed Engl 43: 4071–4075. [DOI] [PubMed] [Google Scholar]
- Küster B, Wheeler SF, Hunter AP, Dwek RA, Harvey DJ. 1997. Sequencing of N‐linked oligosaccharides directly from protein gels: In‐gel deglycosylation followed by matrix‐assisted laser desorption/ionization mass spectrometry and normal‐phase high performance liquid chromatography. Anal Biochem 250: 82–101. [DOI] [PubMed] [Google Scholar]
- Kvaratskhelia M, Clark PK, Hess S, Melder DC, Federspiel MJ, Hughes SH. 2004. Identification of glycosylation sites in the SU component of the avian sarcoma/leukosis virus envelope glycoprotein (subgroup A) by mass spectrometry. Virology 326: 171–181. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Lee YJ, Lee K, Kim KS. 2004. Synthesis of the trisaccharide repeating unit of the atypical O‐antigen polysaccharide from Danish Helicobacter pylori strains employing the 2′‐carboxybenzyl glycoside. Org Lett 6: 3901–3904. [DOI] [PubMed] [Google Scholar]
- La Ferla B, Bugada P, Cipolla L, Peri F, Nicotra F. 2004. Synthesis of imino sugar scaffolds for the generation of glycosidase inhibitor libraries. Eur J Org Chem 2451–2470. [Google Scholar]
- Lahmann M, Bülow L, Teodorovic P, Gybäck H, Oscarson S. 2004. Synthesis of the Lewis b hexasaccharide and HSA‐conjugates thereof. Glycoconj J 21: 251–256. [DOI] [PubMed] [Google Scholar]
- Lai X‐H, Ng S‐C. 2004. Preparation and chiral recognition of a novel chiral stationary phase for high‐performance liquid chromatography, based on mono(6A‐N‐allylamino‐6A‐deoxy)‐perfunctionalized β‐cyclodextrin and covalently bonded silica gel. J Chromatogr A 1031: 135–142. [DOI] [PubMed] [Google Scholar]
- Lamari FN, Kuhn R, Karamanos NK. 2003. Derivatization of carbohydrates for chromatographic, electrophoretic and mass spectrometric structure analysis. J Chromatogr B 793: 15–36. [DOI] [PubMed] [Google Scholar]
- Lamarre‐Vincent N, Hsieh‐Wilson LC. 2003. Dynamic glycosylation of the transcription factor CREB: A potential role in gene regulation. J Am Chem Soc 125: 6612–6613. [DOI] [PubMed] [Google Scholar]
- Lane SB, Tchedre KT, Nair MP, Thigpen AE, Lacko AG. 2004. Characterization of lecithin:cholesterol acyltransferase expressed in a human lung cell line. Protein Exp Purif 36: 157–164. [DOI] [PubMed] [Google Scholar]
- Lapolla A, Fedele D, Reitano R, Seraglia R, Traldi P, Marotta E, Tonani R. 2004a. Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom 15: 496–509. [DOI] [PubMed] [Google Scholar]
- Lapolla A, Tubaro M, Reitano R, Arico NC, Ragazzi E, Seraglia R, Vogliardi S, Traldi P, Fedele D. 2004b. The complexity of non‐enzymatic glycation product sets of human globins. Diabetologia 47: 1712–1715. [DOI] [PubMed] [Google Scholar]
- Lara M, Servín‐González L, Singh M, Moreno C, Cohen I, Nimtz M, Espitia C. 2004. Expression, secretion, and glycosylation of the 45‐ and 47‐kDa glycoprotein of Mycobacterium tuberculosis in Streptomyces lividans . Appl Environ Microbiol 70: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque S, Brisson J‐R, Thérisod H, Perry MB, Caroff M. 2003. Structural characterization of the O‐chain polysaccharide isolated from Bordetella avium ATCC 5086: Variation on a theme. FEBS Lett 535: 11–16. [DOI] [PubMed] [Google Scholar]
- Lattova E, Perreault H. 2003a. Labelling saccharides with phenylhydrazine for electrospray and matrix‐assisted laser desorption‐ionization mass spectrometry. J Chromatogr B 793: 167–179. [DOI] [PubMed] [Google Scholar]
- Lattova E, Perreault H. 2003b. Profiling of N‐linked oligosaccharides using phenylhydrazine derivatization and mass spectrometry. J Chromatogr A 1016: 71–87. [DOI] [PubMed] [Google Scholar]
- Lauc G, Dumic J, Flögel M, Lee YC. 2003. A new synthetic route for the preparation of glycoprobes. Anal Biochem 320: 306–309. [DOI] [PubMed] [Google Scholar]
- Lauer I, Foetisch K, Kolarich D, Ballmer‐Weber BK, Conti A, Altmann F, Vieths S, Scheurer S. 2004. Hazelnut (Corylus avellana) vicilin Cor a 11: Molecular characterization of a glycoprotein and its allergenic activity. Biochem J 383: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer‐Fields J‐L, Malkar NB, Richet G, Drauz K, Fields GB. 2003. Melanoma cell CD44 interaction with the 1(IV) 1263–1277 region from basement membrane collagen is modulated by ligand glycosylation. J Biol Chem 278: 14321–14330. [DOI] [PubMed] [Google Scholar]
- Laugesen S, Roepstorff P. 2003. Combination of two matrices results in improved performance of MALDI MS for peptide mass mapping and protein analysis. J Am Soc Mass Spectrom 14: 992–1002. [DOI] [PubMed] [Google Scholar]
- Laville I, Pigaglio S, Blais J‐C, Loock B, Maillard P, Grierson DS, Blais J. 2004. A study of the stability of tri(glucosyloxyphenyl)chlorin, a sensitizer for photodynamic therapy, in human colon tumoural cells: A liquid chromatography and MALDI‐TOF mass spectrometry analysis. Bioorg Med Chem 12: 3673–3682. [DOI] [PubMed] [Google Scholar]
- Leal S, Acosta‐Serrano A, Morris J, Cross GA. 2004. Transposon mutagenesis of Trypanosoma brucei identifies glycosylation mutants resistant to concanavalin A. J Biol Chem 279: 28979–28988. [DOI] [PubMed] [Google Scholar]
- Lee S, Jung S. 2003. Enantioseparation using cyclosophoraoses as a novel chiral additive in capillary electrophoresis. Carbohydr Res 338: 1143–1146. [DOI] [PubMed] [Google Scholar]
- Lee S, Jung S. 2004. Cyclosophoraose as a catalytic carbohydrate for methanolysis. Carbohydr Res 339: 461–468. [DOI] [PubMed] [Google Scholar]
- Lee H‐W, Park Y‐S, Choi J‐W, Yi S‐y, Shin W‐S. 2003a. Antidiabetic effects of chitosan oligosaccharides in neonatal streptozotocin‐induced noninsulin‐dependent diabetes mellitus in rats. Biol Pharm Bull 26: 1100–1103. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S‐H, Stanley P. 2003b. Antibodies that recognize bisected complex N‐glycans on cell surface glycoproteins can be made in mice lacking N‐acetylglucosaninyltransferase III. Glycoconj J 19: 211–219. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S‐H, Sundaram S, Raju TS, Shaper NL, Stanley P. 2003c. A mutation causing a reduced level of expression of six 4‐galactosyltransferase genes is the basis of the Lec19 CHO glycosylation mutant. Biochemistry 42: 12349–12357. [DOI] [PubMed] [Google Scholar]
- Lee C‐S, Kim Y‐G, Joo H‐S, Kim B‐G. 2004a. Structural analysis of lipid A from Escherichia coli O157:H7:K‐ using thin‐layer chromatography and ion‐trap mass spectrometry. J Mass Spectrom 39: 514–525. [DOI] [PubMed] [Google Scholar]
- Lee H, Hsu F‐F, Turk J, Groisman EA. 2004b. The PmrA‐regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica . J Bacteriol 186: 4124–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park H, Seo D, Choi Y, Jung S. 2004c. Synthesis and characterization of carboxymethylated cyclosophoraose, and its inclusion complexation behavior. Carbohydr Res 339: 519–527. [DOI] [PubMed] [Google Scholar]
- Leitner A, Lindner W. 2004. Current chemical tagging strategies for proteome analysis by mass spectrometry. J Chromatogr B 813: 1–26. [DOI] [PubMed] [Google Scholar]
- Léonard R, Kolarich D, Paschinger K, Altmann F, Wilson IBH. 2004. A genetic and structural analysis of the N‐glycosylation capabilities of rice and other monocotyledons. Plant Mol Biol 55: 631–644. [DOI] [PubMed] [Google Scholar]
- Leone S, Izzo V, Silipo A, Sturiale L, Garozzo D, Lanzetta R, Parrilli M, Molinaro A, Di‐Donato A. 2004a. A novel type of highly negatively charged lipooligosaccharide from Pseudomonas stutzeri OX1 possessing two 4,6‐O‐(1‐carboxy)‐ethylidene residues in the outer core region. Eur J Biochem 271: 2691–2704. [DOI] [PubMed] [Google Scholar]
- Leone S, Izzo V, Sturiale L, Garozzo D, Lanzetta R, Parrilli M, Molinaro A, Di Donato A. 2004b. Structure of minor oligosaccharides from the lipopolysaccharide fraction from Pseudomonas stutzeri OX1. Carbohydr Res 339: 2657–2665. [DOI] [PubMed] [Google Scholar]
- Lequette Y, Ödberg‐Ferragut C, Bohin J‐P, Lacroix J‐M. 2004. Identification of mdoD, an mdoG paralog which encodes a twin‐arginine‐dependent periplasmic protein that controls osmoregulated periplasmic glucan backbone structures. J Bacteriol 186: 3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina EV, Kalinovskii AI, Stonik VA, Dmitrenok PS. 2003. Steroidal polyols from Far‐Eastern starfishes Henricia sanguinolenta and H. leviuscula leviuscula . Russ Chem Bull 52: 1623–1628. [Google Scholar]
- Levina EV, Kalinovskii AI, Andriyashchenko PV, Dmitrenok PS, Evtushenko EV, Stonik VA. 2004. A new steroidal glycoside phrygioside A and its aglycone from the starfish Hippasteria phrygiana . Russ Chem Bull 53: 2634–2638. [Google Scholar]
- Lewis AL, Nizet V, Varki A. 2004. Discovery and characterization of sialic acid O‐acetylation in group B Streptococcus . Proc Natl Acad Sci USA 101: 11123–11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Kong F. 2004. Syntheses of arabinogalactans consisting of β‐(1 → 6)‐linked D‐galactopyranosyl backbone and α‐(1 → 3)‐linked L‐arabinofuranosyl side chains. Carbohydr Res 339: 1847–1856. [DOI] [PubMed] [Google Scholar]
- Li D, Park J‐H, Park J‐T, Park CS, Park K‐H. 2004a. Biotechnological production of highly soluble daidzein glycosides using Thermotoga maritima maltosyltransferase. J Agric Food Chem 52: 2561–2567. [DOI] [PubMed] [Google Scholar]
- Li J, Wang J, Czyryca PG, Chang H, Orsak TW, Evanson R, Chang C‐WT. 2004b. Application of glycodiversification: Expedient synthesis and antibacterial evaluation of a library of kanamycin B analogues. Org Lett 6: 1381–1384. [DOI] [PubMed] [Google Scholar]
- Li JH, Vasanthan T, Hoover R, Rossnagel BG. 2004c. Starch from hull‐less barley: IV. Morphological and structural changes in waxy, normal and high‐amylose starch granules during heating. Food Res Int 37: 417–428. [Google Scholar]
- Li S‐Y, Höltje J‐V, Young KD. 2004d. Comparison of high‐performance liquid chromatography and fluorophore‐assisted carbohydrate electrophoresis methods for analyzing peptidoglycan composition of Escherichia coli . Anal Biochem 326: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang X, Chu S, Yu K, Guan H. 2004e. Synthesis of cluster mannosides via a Ugi four‐component reaction and their inhibition against the binding of yeast mannan to concanavalin A. Carbohydr Res 339: 873–879. [DOI] [PubMed] [Google Scholar]
- Lim HJ, Kim YK, Hwang DS, Cha HJ. 2004. Expression of functional human transferrin in stably transfected drosophila S2 cells. Biotechnol Prog 20: 1192–1197. [DOI] [PubMed] [Google Scholar]
- Lin H, Walsh CT. 2004. A chemoenzymatic approach to glycopeptide antibiotics. J Am Chem Soc 126: 13998–14003. [DOI] [PubMed] [Google Scholar]
- Lin H, Thayer DD, Wong C‐H, Walsh CT. 2004. Macrolactamization of glycosylated peptide thioesters by the thioesterase domain of tyrocidine synthetase. Chem Biol 11: 1635–1642. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang L, Brock A, Wong C‐H, Schultz PG. 2003a. A method for the generation of glycoprotein mimetics. J Am Chem Soc 125: 1702–1703. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li L, Zhang H‐Y, Liang P, Wang H. 2003b. Inclusion complexation behavior of dyestuff guest molecules by a bridged bis(cyclomaltoheptaose)[bis(β‐cyclodextrin)] with a pyromellitic acid diamide tether. Carbohydr Res 338: 1751–1757. [DOI] [PubMed] [Google Scholar]
- Liu P, Feasley CL, Regnier FE. 2004. Optimization of diagonal chromatography for recognizing post‐translational modifications. J Chromatogr A 1047: 221–228. [DOI] [PubMed] [Google Scholar]
- Lochnit G, Geyer R. 2004. An optimized protocol for nano‐LC‐MALDI‐TOF‐MS coupling for the analysis of proteolytic digests of glycoproteins. Biomed Chromatogr 18: 841–848. [DOI] [PubMed] [Google Scholar]
- Logsdon NJ, Jones BC, Allman JC, Izotova L, Schwartz B, Pestka S, Walter MR. 2004. The IL‐10R2 binding hot spot on IL‐22 is located on the N‐terminal helix and is dependent on N‐linked glycosylation. J Mol Biol 342: 503–514. [DOI] [PubMed] [Google Scholar]
- Lo‐Guidice JM, Weiruszewski JM, Lemoine A, Verbert A, Rousset P, Lamblin G. 1994. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J Biol Chem 269: 18794–18813. [PubMed] [Google Scholar]
- Lohmann KK, von der Lieth C‐W. 2003. GLYCO‐FRAGMENT: A web tool to support the interpretation of mass spectra of complex carbohydrates. Proteomics 3: 2028–2035. [DOI] [PubMed] [Google Scholar]
- Lohmann KK, von der Lieth C‐W. 2004. GlycoFragment and GlycoSearchMS: Web tools to support the interpretation of mass spectra of complex carbohydrates. Nucleic Acids Res 32: W261–W266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MG, Mancilla‐Margalli NA, Mendoza‐Diaz G. 2003. Molecular structures of fructans from Agave tequilana Weber var. azul . J Agric Food Chem 51: 7835–7840. [DOI] [PubMed] [Google Scholar]
- Lu J, Fraser‐Reid B. 2004. Preparation, properties, and applications of n‐pentenyl arabinofuranosyl donors. Org Lett 6: 3051–3054. [DOI] [PubMed] [Google Scholar]
- Lu T‐L, Chen C‐S, Yang F‐L, Fung J‐M, Chen M‐Y, Tsay S‐S, Li J, Zou W, Wu H‐H. 2004a. Structure of a major glycolipid from Thermus oshimai NTU‐063. Carbohydr Res 339: 2593–2598. [DOI] [PubMed] [Google Scholar]
- Lu W, Leimkuhler C, Oberthür M, Kahne D, Walsh CT. 2004b. AknK is an L‐2‐deoxyfucosyltransferase in the biosynthesis of the anthracycline aclacinomycin A. Biochemistry 43: 4548–4558. [DOI] [PubMed] [Google Scholar]
- Lucas R, Angulo J, Nieto PM, Martín‐Lomas M. 2003. Synthesis and structural study of two new heparin‐like hexasaccharides. Org Biomol Chem 1: 2253–2266. [DOI] [PubMed] [Google Scholar]
- Lukacik P, Roversi P, White J, Esser D, Smith GP, Billington J, Williams PA, Rudd PM, Wormald MR, Harvey DJ, Crispin MDM, Radcliffe CM, Dwek RA, Evans DJ, Morgan BP, Smith RAG, Lea SM. 2004. Complement regulation at the molecular level: The structure of decay‐accelerating factor. Proc Natl Acad Sci USA 101: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz J, Jachymek W, Niedziela T, Dzieciatkowska M, Lakomska J, Miedzybrodzki R, Fortuna W, Szymaniec S, Misiuk‐Hojlo M, Lugowski C. 2003. Serological characterization of anti‐endotoxin serum directed against the conjugate of oligosaccharide core of Escherichia coli type R4 with tetanus toxoid. FEMS Immunol Med Microbiol 37: 59–67. [DOI] [PubMed] [Google Scholar]
- Luke NR, Allen S, Gibson BW, Campagnari AA. 2003. Identification of a 3‐deoxy‐D‐manno‐octulosonic acid biosynthetic operon in Moraxella catarrhalis and analysis of a KdsA‐deficient isogenic mutant. Infect Immun 71: 6426–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist J, Jacobs A, Palm M, Zacchi G, Dahlman O, Stålbrand H. 2003. Characterization of galactoglucomannan extracted from spruce (Picea abies) by heat‐fractionation at different conditions. Carbohydr Polym 51: 203–211. [Google Scholar]
- Luxembourg SL, McDonnell LA, Duursma MC, Guo X, Heeren RMA. 2003. Effect of local matrix crystal variations in matrix‐assisted ionization techniques for mass spectrometry. Anal Chem 75: 2333–2341. [DOI] [PubMed] [Google Scholar]
- Ma S, Hill KE, Burk RF, Caprioli RM. 2003a. Mass spectrometric identification of N‐ and O‐glycosylation sites of full‐length rat selenoprotein P and determination of selenide‐sulfide and disulfide linkages in the shortest isoform. Biochemistry 42: 9703–9711. [DOI] [PubMed] [Google Scholar]
- Ma X, Saksena R, Chernyak A, Kovác P. 2003b. Neoglycoconjugates from synthetic tetra‐ and hexasaccharides that mimic the terminus of the O‐PS of Vibrio cholerae O:1, serotype Inaba. Org Biomol Chem 1: 775–784. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang J, Kong F. 2003c. Concise synthesis of β‐GlcNAcp‐(1 → 6)‐α‐Manp‐(1 → 6)‐Manp and its dimer, β‐GlcNAcp‐(1 → 2)‐α‐Manp‐(1 → 6)‐Manp . Tetrahedron Asym 14: 2595–2603. [Google Scholar]
- Ma Z, Zhang J, Kong F. 2004. Facile synthesis of arabinomannose penta‐ and decasaccharide fragments of the lipoarabinomannan of the equine pathogen, Rhodococcus equi . Carbohydr Res 339: 1761–1771. [DOI] [PubMed] [Google Scholar]
- Macmillan D, Daines AM. 2003. Recent developments in the synthesis and discovery of oligosaccharides and glycoconjugates for the treatment of disease. Curr Med Chem 10: 2733–2773. [DOI] [PubMed] [Google Scholar]
- Madaj J, Jankowska M, Wisniewski A. 2004. Synthesis of cis‐(1 → 3)‐glycosides of allyl 2‐acetamido‐4,6‐O‐benzylidene‐2‐deoxy‐α‐D‐glucopyranoside. Carbohydr Res 339: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Maeda H, Chatani E, Koyama T, Sugiura M, Izumi H, Hayashi R. 2004. Indiscriminate glycosylation of procarboxypeptidase Y expressed in Pichia pastoris . Carbohydr Res 339: 1041–1045. [DOI] [PubMed] [Google Scholar]
- Maier MS, Parera SD, Seldes AM. 2004. Matrix‐assisted laser desorption and electrospray ionization mass spectrometry of carminic acid isolated from cochineal. Int J Mass Spectrom 232: 225–229. [Google Scholar]
- Maitin V, Rastall RA. 2004. Enzyme glycation influences product yields during oligosaccharide synthesis by reverse hydrolysis. J Mol Catal B Enzymatic 30: 195–202. [Google Scholar]
- Majumdar D, Zhu T, Boons G‐J. 2004. Synthesis of oligosaccharides on soluble high‐molecular‐weight branched polymers in combination with purification by nanofiltration. Org Lett 5: 3591–3594. [DOI] [PubMed] [Google Scholar]
- Mäki M, Järvinen N, Räbinä J, Maaheimo H, Mattila P, Renkonen R. 2003. Cloning and functional expression of a novel GDP‐6‐deoxy‐D‐talose synthetase from Actinobacillus actinomycetemcomitans . Glycobiology 13: 295–303. [DOI] [PubMed] [Google Scholar]
- Mamone G, Caira S, Garro G, Nicolai A, Ferranti P, Picariello G, Malorni A, Chianese L, Addeo F. 2003. Casein phosphoproteome: Identification of phosphoproteins by combined mass spectrometry and two‐dimensional gel electrophoresis. Electrophoresis 24: 2824–2837. [DOI] [PubMed] [Google Scholar]
- Mank M, Stahl B, Boehm G. 2004. 2,5‐Dihydroxybenzoic acid butylamine and other ionic liquid matrixes for enhanced MALDI‐MS analysis of biomolecules. Anal Chem 76: 2938–2950. [DOI] [PubMed] [Google Scholar]
- Manzoni L. 2003. Rapid synthesis of oligosaccharides using an anomeric fluorous silyl protecting group. Chem Commun 2930–2931. [DOI] [PubMed] [Google Scholar]
- Manzoni L, Castelli R. 2004. Synthesis of the Lewis a trisaccharide based on an anomeric silyl fluorous tag. Org Lett 6: 4195–4198. [DOI] [PubMed] [Google Scholar]
- Marceau M, Sebbane F, Ewann F, Collyn F, Lindner B, Campos MA, Bengoechea J‐A, Simonet M. 2004. The pmrF polymyxin‐resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP‐PhoQ two‐component system but not by PmrA‐PmrB, and is not required for virulence. Microbiology 150: 3947–3957. [DOI] [PubMed] [Google Scholar]
- Mårdberg K, Nyström K, Tarp MA, Trybala E, Clausen H, Bergström T, Olofsson S. 2004. Basic amino acids as modulators of an O‐linked glycosylation signal of the herpes simplex virus type 1 glycoprotein gC: Functional roles in viral infectivity. Glycobiology 14: 571–581. [DOI] [PubMed] [Google Scholar]
- Mari S, Posteri H, Marcou G, Potenza D, Micheli F, Cañada FJ, Jimenez‐Barbero J, Bernardi A. 2004. Synthesis, conformational studies and mannosidase stability of a mimic of 1,2‐mannobioside. Eur J Org Chem 5119–5125. [Google Scholar]
- Marin J, Violette A, Briand J‐P, Guichard G. 2004. Galactosylated 5‐hydroxylysine mimetics for glycopeptide synthesis. Eur J Org Chem 3027–3039. [Google Scholar]
- Marry M, Cavalier DM, Schnurr JK, Netland J, Yang Z, Pezeshk V, York WS, Pauly M, White AR. 2003. Structural characterization of chemically and enzymatically derived standard oligosaccharides isolated from partially purified tamarind xyloglucan. Carbohydr Polym 51: 347–356. [Google Scholar]
- Martín MT, Cruces MA, Alcalde M, Plou FJ, Bernabé M, Ballesteros A. 2004. Synthesis of maltooligosyl fructofuranosides catalyzed by immobilized cyclodextrin glucosyltransferase using starch as donor. Tetrahedron 60: 529–534. [Google Scholar]
- Matsunaga H, Sadakane Y, Haginaka J. 2004. Identification of disulfide bonds and site‐specific glycosylation in chicken α1‐acid glycoprotein by matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry. Anal Biochem 331: 358–363. [DOI] [PubMed] [Google Scholar]
- Mechref Y, Novotny MV, Krishnan C. 2003. Structural characterization of oligosaccharides using MALDI‐TOF/TOF tandem mass spectrometry. Anal Chem 75: 4895–4903. [DOI] [PubMed] [Google Scholar]
- Medzihradszky KF, Spencer DIR, Sharma SK, Bhatia J, Pedley RB, Read DA, Begent RHJ, Chester KA. 2004. Glycoforms obtained by expression in Pichia pastoris improve cancer targeting potential of a recombinant antibody‐enzyme fusion protein. Glycobiology 14: 27–37. [DOI] [PubMed] [Google Scholar]
- Mehndiratta P, Walton WJ, Hare JT, Pulido S, Parthasarathy G, Emmett MR, Marshall AG, Logan TM. 2004. Expression, purification, and characterization of avian Thy‐1 from Lec1 mammalian and Tn5 insect cells. Protein Exp Purif 33: 274–287. [DOI] [PubMed] [Google Scholar]
- Mellor HR, Neville DCA, Harvey DJ, Platt FM, Dwek RA, Butters TD. 2004a. Cellular effects of deoxynojirimycin analogues: Uptake, retention and inhibition of glycosphingolipid biosynthesis. Biochem J 381: 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor HR, Neville DCA, Harvey DJ, Platt FM, Dwek RA, Butters TD. 2004b. Cellular effects of deoxynojirimycin analogues: Inhibition of N‐linked oligosaccharide processing and generation of free glucosylated oligosaccharides. Biochem J 381: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellroth P, Karlsson J, Steiner H. 2003. A scavenger function for a Drosophila peptidoglycan recognition protein. J Biol Chem 278: 7059–7064. [DOI] [PubMed] [Google Scholar]
- Menéndez N, Nur‐e‐Alam M, Braña A, Rohr J, Salas JA, Méndez C. 2004. Tailoring modification of deoxysugars during biosynthesis of the antitumour drug chromomycin A. Mol Microbiol 53: 903–915. [DOI] [PubMed] [Google Scholar]
- Meng XB, Li H, Li Q, Cheng TM, Li ZJ. 2004. Assembly of a library of trisaccharides as mimics of sialyl Lewis X via random combination strategy. Chin Chem Lett 15: 379–382. [Google Scholar]
- Merry T, Astrautsova S. 2003. Chemical and enzymatic release of glycans from glycoproteins. In: Thibault P, Honda S, editors. Capillary electrophoresis of carbohydrates. Totowa, USA: Humana Press. pp 27–40. [DOI] [PubMed] [Google Scholar]
- Merry AH, Gilbert RJC, Shore DA, Royle L, Miroshnychenko O, Vuong M, Wormald MR, Harvey DJ, Dwek RA, Classon BJ, Rudd PM, Davis SJ. 2003. O‐Glycan sialylation and the structure of the stalk‐like region of the T cell co‐receptor CD8. J Biol Chem 278: 27119–27128. [DOI] [PubMed] [Google Scholar]
- Mikami T, Mizumoto S, Kago N, Kitagawa H, Sugahara K. 2003. Specificities of three distinct human chondroitin/dermatan N‐acetylgalactosamine 4‐O‐sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: Implication of differential roles in dermitan sulfate biosynthesis. J Biol Chem 278: 36115–36127. [DOI] [PubMed] [Google Scholar]
- Mills K, Mills PB, Clayton PT, Mian N, Johnson AW, Winchester BG. 2003a. The underglycosylation of plasma 1‐antitrypsin in congenital disorders of glycosylation type I is not random. Glycobiology 13: 73–85. [DOI] [PubMed] [Google Scholar]
- Mills PB, Mills K, Mian N, Winchester BG, Clayton PT. 2003b. Mass spectrometric analysis of glycans in elucidating the pathogenesis of CDG type IIx. J Inherit Metab Dis 26: 119–134. [DOI] [PubMed] [Google Scholar]
- Mirza SP, Raju NP, Madhavendra SS, Vairamani M. 2004a. 5‐Amino‐2‐mercapto‐1,3,4‐thiadiazole: A new matrix for the efficient matrix‐assisted laser desorption/ionization of neutral carbohydrates. Rapid Commun Mass Spectrom 18: 1666–1674. [Google Scholar]
- Mirza SP, Raju NP, Vairamani M. 2004b. Estimation of the proton affinity values of fifteen matrix‐assisted laser desorption/ionization matrices under electrospray ionization conditions using the kinetic method. J Am Soc Mass Spectrom 15: 431–435. [DOI] [PubMed] [Google Scholar]
- Misaki R, Nagaya H, Fujiyama K, Yanagihara I, Honda T, Seki T. 2003. N‐linked glycan structures of mouse interferon‐β produced by Bombyx mori larvae. Biochem Biophys Res Commun 311: 979–986. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Okamoto I, Fujita K, Yamamoto K, Hirose M. 2004. Structural and functional characterization of ovotransferrin produced by Pichia pastoris . Biosci Biotechnol Biochem 68: 376–383. [DOI] [PubMed] [Google Scholar]
- Mohamed SA, Christensen TMIE, Mikkelsen JD. 2003. New polygalacturonases from Trichoderma reesei: Characterization and their specificities to partially methylated and acetylated pectins. Carbohydr Res 338: 515–524. [DOI] [PubMed] [Google Scholar]
- Mokrzycki‐Issartel N, Bouchon B, Farrer S, Berland P, Laparra H, Madelmont J‐C, Theisen M. 2003. A transient tobacco expression system coupled to MALDI‐TOF‐MS allows validation of the impact of differential targeting on structure and activity of a recombinant therapeutic glycoprotein produced in plants. FEBS Lett 552: 170–176. [DOI] [PubMed] [Google Scholar]
- Molinaro A, Lindner B, De Castro C, Nolting B, Silipo A, Lanzetta R, Parrilli M, Holst O. 2003. The structure of lipid A of the lipopolysaccharide from Burkholderia caryophylli with a 4‐amino‐4‐deoxy‐L‐arabinopyranose‐1‐phosphate residue exclusively in glycosidic linkage. Chem Eur J 9: 1542–1548. [DOI] [PubMed] [Google Scholar]
- Momcilovic D, Wittgren B, Wahlund K‐G, Brinkmalm G, Karlsson J. 2003a. Sample preparation effects in matrix‐assisted laser desorption/ionisation time‐of‐flight mass spectrometry of partially depolymerised carboxymethyl cellulose. Rapid Commun Mass Spectrom 17: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Momcilovic D, Wittgren B, Wahlund K‐G, Karlsson J, Brinkmalm G. 2003b. Sample preparation effects in matrix‐assisted laser desorption/ionisation time‐of‐flight mass spectrometry of partially depolymerised methyl cellulose. Rapid Commun Mass Spectrom 17: 1116–1124. [DOI] [PubMed] [Google Scholar]
- Monteiro MA, Fortuna‐Nevin M, Farley J, Pavliak V. 2003. Phase‐variation of the truncated lipo‐oligosaccharide of Neisseria meningitidis NMB phosphoglucomutase isogenic mutant NMB‐R6. Carbohydr Res 338: 2905–2912. [DOI] [PubMed] [Google Scholar]
- Moraes G, Norhcote PC, Kalinin VI, Avilov SA, Silchenko AS, Dmitrenok PS, Stonik VA, Levin VS. 2004. Structure of the major triterpene glycoside from the sea cucumber Stichopus mollis and evidence to reclassify this species into the new genus Australostichopus . Biochem Syst Ecol 32: 637–650. [Google Scholar]
- Morelle W, Michalski J‐C. 2004. Sequencing of oligosaccharides derivatized with benzylamine using electrospray ionization‐quadrupole time of flight‐tandem mass spectrometry. Electrophoresis 25: 2144–2155. [DOI] [PubMed] [Google Scholar]
- Morelle W, Slomianny MC, Diemer H, Schaeffer C, Dorsselaer AV, Michalski JC. 2004. Fragmentation characteristics of permethylated oligosaccharides using a matrix‐assisted laser desorption/ionization two‐stage time‐of‐flight (TOF/TOF) tandem mass spectrometer. Rapid Commun Mass Spectrom 18: 2637–2649. [DOI] [PubMed] [Google Scholar]
- Mori T, Sekine Y, Yamamoto K, Okahata Y. 2004. Enzymatic preparation of biotinylated naturally‐occurring sialylglycan and its molecular recognition on a quartz‐crystal microbalance. Chem Commun 2692–2693. [DOI] [PubMed] [Google Scholar]
- Morita YS, Patterson JH, Billman‐Lacobe H, McConville MJ. 2004. Biosynthesis of mycobacterial phosphatidylinositol mannosides. Biochem J 378: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz SM, Ernst RK, Miller SI. 2004. PmrAB, a two‐component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol 186: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Jiang X, Liu Z, Guan H. 2004. Structural analysis of kappa‐carrageenan oligosaccharides released by carrageenase from marine cytophaga MCA‐2. J Food Biochem 28: 245–260. [Google Scholar]
- Moyer SC, Marzilli LA, Woods AS, Laiko VV, Doroshenko VM, Cotter RJ. 2003. Atmospheric pressure matrix‐assisted laser desorption/ionization (AP MALDI) on a quadrupole ion trap mass spectrometer. Int J Mass Spectrom 226: 133–150. [Google Scholar]
- Mueller M, Lindner B, Kusumoto S, Fukase K, Schromm AB, Seydel U. 2004. Aggregates are the biologically active units of endotoxin. J Biol Chem 279: 26307–26313. [DOI] [PubMed] [Google Scholar]
- Muhanna AMA, Ortiz‐Salmerón E, García‐Fuentes L, Giménez‐Martínez JJ, Vargas‐Brenguel A. 2003. Synthesis of peptide dendrimers based on a β‐cyclodextrin core with guest binding ability. Tetrahedron Lett 44: 6125–6128. [Google Scholar]
- Mulard LA, Guerreiro C. 2004. Total synthesis of a tetra‐ and two pentasaccharide fragments of the O‐specific polysaccharide of Shigella flexneri serotype 2a. Tetrahedron 60: 2475–2488. [Google Scholar]
- Mulder A, Jukovi A, van Leeuwen FWB, Kooijman H, Spek AL, Huskens J, Reinhoudt DN. 2004. Photocontrolled release and uptake of a porphyrin guest by dithienylethene‐tethered ‐cyclodextrin host dimers. Chem Eur J 10: 1114–1123. [DOI] [PubMed] [Google Scholar]
- Müthing J, Kemminer SE, Conradt HS, Sagi D, Nimtz M, Kärst U, Peter‐Katalinic J. 2003. Effects of buffering conditions and culture pH on production rates and glycosylation of clinical phase I anti‐melanoma mouse IgG3 monoclonal antibody R24. Biotechnol Bioeng 83: 321–334. [DOI] [PubMed] [Google Scholar]
- Muzikar J, Mechref Y, Huang Y, Novotny MV. 2004. Enhanced post‐source decay and cross‐ring fragmentation of oligosaccharides facilitated by conversion to amino derivatives. Rapid Commun Mass Spectrom 18: 1513–1518. [DOI] [PubMed] [Google Scholar]
- Na DH, Cho CK, Youn YS, Choi Y, Lee KR, Yoo SD, Lee KC. 2004. Capillary electrophoresis to characterize ricin and its subunits with matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Toxicon 43: 329–335. [DOI] [PubMed] [Google Scholar]
- Nagamori M, Funazukuri T. 2004. Glucose production by hydrolysis of starch under hydrothermal conditions. J Chem Technol Biotechnol 79: 229–233. [Google Scholar]
- Nagase T, Nakata E, Shinkai S, Hamachi I. 2003. Construction of artificial signal transducers on a lectin surface by post‐photoaffinity‐labeling modification for fluorescent saccharide biosensors. Chem Eur J 9: 3660–3669. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Nonaki T, Hojo H, Nakahara Y. 2003. Synthesis of an unnatural N‐glycan‐linked dolichyl pyrophosphate precursor. Biosci Biotechnol Biochem 67: 1761–1766. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Sakabe Y, Ikeda H, Ueno A. 2004. Cyclodextrin trimers as receptors for arranging ester and catalyst at optimized location to achieve enhancement of hydrolytic activity. Bioorg Med Chem Lett 14: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Nakano H, Kiso T, Okamoto K, Tomita T, Manan MBA, Kitahata S. 2003a. Synthesis of glycosyl glycerol by cyclodextrin glucanotransferases. J Biosci Bioeng 95: 583–588. [PubMed] [Google Scholar]
- Nakano M, Kakehi K, Lee YC. 2003b. Sample clean‐up method for analysis of complex N‐glycans released from glycopeptides. J Chromatogr A 1005: 13–21. [DOI] [PubMed] [Google Scholar]
- Nakano M, Kakehi K, Tsai M‐H, Lee YC. 2004. Detailed structural features of glycan chains derived from α1‐acid glycoproteins of several different animals: The presence of hypersialylated, O‐acetylated sialic acids but not disialyl residues. Glycobiology 14: 431–441. [DOI] [PubMed] [Google Scholar]
- Natsuka S, Adachi J, Kawaguchi M, Nakakita S‐i, Hase S, Ichikawa A, Ikura K. 2002. Structural analysis of N‐linked glycans in Caenorhabditis elegans . J Biochem (Tokyo) 131: 807–813. [DOI] [PubMed] [Google Scholar]
- Nelson A, Stoddart JF. 2003. Dynamic multivalent lactosides displayed on cyclodextrin beads dangling from polymer strings. Org Lett 5: 3783–3786. [DOI] [PubMed] [Google Scholar]
- Nelson A, Stoddart JF. 2004. Synthesis of lactoside glycodendrons using photoaddition and reductive amination methodologies. Carbohydr Res 339: 2069–2075. [DOI] [PubMed] [Google Scholar]
- Neretina OV, Fedorov SV, Gromova AS, Lutskii VI, El'kin YN. 2002. Flavonoids of Hedysarum setigerum . Chem Nat Compounds 38: 194–195. [Google Scholar]
- Nerinckx W, Broberg A, Duus J, Ntarima P, Parolis LAS, Parolis H, Claeyssens M. 2004. Hydrolysis of Nothogenia erinacea xylan by xylanases from families 10 and 11. Carbohydr Res 339: 1047–1060. [DOI] [PubMed] [Google Scholar]
- Ning D, Junjian X, Xunzhang W, Wenyin C, Qing Z, Kuanyuan S, Guirong R, Xiangrong R, Qingxin L, Zhouyao Y. 2003a. Expression, purification and characterization of humanized anti‐HBs Fab fragment. J Biochem (Tokyo) 134: 813–817. [DOI] [PubMed] [Google Scholar]
- Ning J, Yi Y, Yao Z. 2003b. An efficient method for the synthesis of 2,6‐branched galacto‐oligosaccharides and its applications to the synthesis of three tetrasaccharides and a hexasaccharide related to the arabinogalactans (AGs). Synlett 2208–2212. [Google Scholar]
- Nishida Y, Tsurumi T, Sasaki K, Watanabe K, Dohi H, Kobayashi K. 2003. Design and synthesis of C3‐symmetric LewisX antigen. Org Lett 5: 3775–3778. [DOI] [PubMed] [Google Scholar]
- Nomura K, Sakai I, Imanishi Y, Fujiki M, Miyamoto Y. 2004. Living ring‐opening metathesis polymerization of norbornenes containing acetyl‐protected carbohydrates using well‐defined molybdenum and ruthenium initiators. Macromol Mol Commun 25: 571–576. [Google Scholar]
- Nørbæk R, Aaboer DBF, Bleeg IS, Christensen BT, Kondo T, Brandt K. 2003. Flavone C‐glycoside, phenolic acid, and nitrogen contents in leaves of barley subject to organic fertilization treatments. J Agric Food Chem 51: 809–813. [DOI] [PubMed] [Google Scholar]
- Numao S, Damager I, Li C, Wrodnigg TM, Begum A, Overall CM, Brayer GD, Withers SG. 2004. In situ extension as an approach for identifying novel α‐amylase inhibitors. J Biol Chem 279: 48282–48291. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Lewis FA, Doughty BL, Correa‐Oliveira R, Cummings RD. 2003. Immunity to schistosomiasis: Glycans are potential antigenic targets for immune intervention. Exp Parasitol 104: 1–13. [DOI] [PubMed] [Google Scholar]
- Nyberg NT, Baumann H, Kenne L. 2003. Solid‐phase extraction NMR studies of chromatographic fractions of saponins from Quillaja saponaria . Anal Chem 75: 268–274. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Ohmae M, Kobayashi S. 2004a. Enzymatic glycosidation of sugar oxazolines having a carboxylate group catalyzed by chitinase. Carbohydr Res 339: 2769–2788. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Ohmae M, Kobayashi S. 2004b. Enzymatic synthesis of alternatingly 6‐O‐carboxymethylated chitotetraose by selective glycosidation with chitinase catalysis. Chem Lett 33: 694–695. [Google Scholar]
- Ohashi Y, Itoh Y, Kubota M, Hamada K, Ohashi M, Hirano T, Niwa H. 2004. Analysis of sugar epimers using mass spectrometry: N‐acetyllactosamine‐6‐6′‐disulfate and the 2′‐epimer. Eur J Mass Spectrom 10: 269–278. [DOI] [PubMed] [Google Scholar]
- Ohl C, Albach C, Altevogt P, Schmitz B. 2003. N‐Glycosylation patterns of HSA/CD24 from different cell lines and brain homogenates: A comparison. Biochimie 85: 565–573. [DOI] [PubMed] [Google Scholar]
- Ohta T, Miura N, Fujitani N, Nakajima F, Niikura K, Sadamoto R, Guo C‐T, Suzuki T, Suzuki Y, Monde K, Nishimura S‐I. 2003. Glycotentacles: Synthesis of cyclic glycopeptides, toward a tailored blocker of influenza virus hemagglutinin. Angew Chem Intl Ed Engl 42: 5186–5189. [DOI] [PubMed] [Google Scholar]
- Ohyama C, Hosono M, Nitta K, Oh‐eda M, Yoshikawa K, Habuchi T, Arai Y, Fukuda M. 2004. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 14: 671–679. [DOI] [PubMed] [Google Scholar]
- Oikawa M, Tanaka T, Kusumoto S, Sasaki M. 2004. Simple formylacetal (CH2) as a novel linker for saccharide synthesis on soluble‐polymer support. Tetrahedron Lett 45: 787–790. [Google Scholar]
- Ojeda R, Terentí O, de Paz J‐L, Martín‐Lomas M. 2004. Synthesis of heparin‐like oligosaccharides on polymer supports. Glycoconj J 21: 179–195. [DOI] [PubMed] [Google Scholar]
- Okada H, Fukushi E, Onodera S, Nishimoto T, Kawabata J, Kikuchi M, Shiomi N. 2003. Synthesis and structural analysis of five novel oligosaccharides prepared by glucosyltransfer from β‐D‐glucose 1‐phosphate to isokestose and nystose using Thermoanaerobacter brockii kojibiose phosphorylase. Carbohydr Res 338: 879–885. [DOI] [PubMed] [Google Scholar]
- O'Leary JM, Radcliffe CM, Willis AC, Dwek RA, Rudd PM, Downing AK. 2004. Identification and removal of O‐linked and non‐covalently linked sugars from recombinant protein produced using Pichia pastoris . Protein Exp Purif 38: 217–227. [DOI] [PubMed] [Google Scholar]
- Olivier A, Luna‐Carlos J, Cazaux F, Coqueret X. 2003. Structural evidence of grafting in electron beam irradiated starch‐allylurea blends. Macromol Symp 192: 227–238. [Google Scholar]
- Omtvedt LA, Royle L, Husby G, Sletten K, Radcliffe C, Dwek RA, Rudd PM, Harvey DJ. 2004. Artefacts formed by addition of urea to N‐linked glycans released with peptide‐N‐glycosidase F for analysis by mass spectrometry. Rapid Commun Mass Spectrom 18: 2357–2359. [DOI] [PubMed] [Google Scholar]
- Oosterveld A, Coenen GJ, Vermeulen NCB, Voragen AGJ, Schols HA. 2004. Structural features of acetylated galactomannans from green Coffea arabica beans. Carbohydr Polym 58: 427–434. [Google Scholar]
- Ootsubo M, Chiba T, Kobayashi Y, Hayashi H, Hayashi A, Onozaki K. 2004. Synthesis and biological activities in vitro and in vivo of glycosylated human interleukin‐1α, neoglyco IL‐1a, coupled with N‐acetyloneuraminyl‐galactose. Glycoconj J 20: 119–131. [DOI] [PubMed] [Google Scholar]
- Ortega‐Caballero F, Giménez‐Martínez JJ, Vargas‐Berenguel A. 2003. Diverse motifs of mannoside clustering on a β‐cyclodextrin core. Org Lett 5: 2389–2392. [DOI] [PubMed] [Google Scholar]
- Oshovsky GV, Verboom W, Fokkens RH, Reinhoudt DN. 2004. Anion complexation by glycocluster thioureamethyl cavitands: Novel ESI‐MS‐based methods for the determination of Ka values. Chem Eur J 10: 2739–2748. [DOI] [PubMed] [Google Scholar]
- Osipov AV, Astapova MV, Tsetlin VI, Utkin YN. 2004. The first representative of glycosylated three‐fingered toxins. Cytotoxin from the Naja kaouthia cobra venom. Eur J Biochem 271: 2018–2027. [DOI] [PubMed] [Google Scholar]
- Ostash B, Rix U, Remsing Rix LL, Liu T, Lombo F, Luzhetskyy A, Gromyko O, Wang C, Braña AF, Méndez C, Salas JA, Fedorenko V, Rohr J. 2004. Generation of new landomycins by combinatorial biosynthetic manipulation of the LndGT4 gene of the landomycin E cluster in S. globisporus . Chem Biol 11: 547–555. [DOI] [PubMed] [Google Scholar]
- Osumi K, Makino Y, Akaike E, Yamanoi T, Mizuno M, Noguchi M, Inazu T, Yamamoto K, Fujita K. 2004. Mucor hiemalis endo‐β‐N‐acetylglucosaminidase can transglycosylate a bisecting hybrid‐type oligosaccharide from an ovalbumin glycopeptide. Carbohydr Res 339: 2633–2635. [DOI] [PubMed] [Google Scholar]
- Ouchi T, Yamabe E, Hara K, Hirai M, Ohya Y. 2004. Design of attachment type of drug delivery system by complex formation of avidin with biotinyl drug model and biotinyl saccharide. J Control Release 94: 281–291. [DOI] [PubMed] [Google Scholar]
- Owen SJ, Meier FS, Brombacher S, Volmer DA. 2003. Increasing sensitivity and decreasing spot size using an inexpensive, removable hydrophobic coating for matrix‐assisted laser desorption/ionisation plates. Rapid Commun Mass Spectrom 17: 2439–2449. [DOI] [PubMed] [Google Scholar]
- Palm M, Zacchi G. 2004. Separation of hemicellulosic oligomers from steam‐treated spruce wood using gel filtration. Sep Purif Technol 36: 191–201. [Google Scholar]
- Palmacci ER, Plante OJ, Hewitt MC, Seeberger PH. 2003. Automated synthesis of oligosaccharides. Helv Chim Acta 86: 3975–3990. [Google Scholar]
- Park SJ, Lee JY, Park TH. 2003a. In vitro glycosylation of peptide (RKDVY) and RNase A by PNGase F. J Microbiol Biotechnol 13: 191–195. [Google Scholar]
- Park YS, Koh YH, Takahashi M, Miyamoto Y, Suzuki K, Dohmae N, Takio K, Honke K, Taniguchi N. 2003b. Identification of the binding site of methylglyoxal on glutathione peroxidase: Methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites Arg 184 and 185. Free Radic Res 37: 205–211. [DOI] [PubMed] [Google Scholar]
- Parussini F, MGarcía M, Mucci J, Agüero F, Sánchez D, Hellman U, Åslund L, Cazzulo JJ. 2003. Characterization of a lysosomal serine carboxypeptidase from Trypanosoma cruzi . Mol Biochem Parasitol 131: 11–23. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Rendic D, Lochnit G, Jantsch V, Wilson IBH. 2004. Molecular basis of anti‐horseradish peroxidase staining in Caenorhabditis elegans . J Biol Chem 279: 49588–49598. [DOI] [PubMed] [Google Scholar]
- Pasciak M, Holst O, Lindner B, Mordarska H, Gamian A. 2003. Novel bacterial polar lipids containing ether‐linked alkyl chains, the structures and biological properties of the four major glycolipids from Propionibacterium propionicum PCM 2431 (ATCC 14157T). J Biol Chem 278: 3948–3956. [DOI] [PubMed] [Google Scholar]
- Pasciak M, Holst O, Lindner B, Mierzchala M, Grzegorzewicz A, Mordarska H, Gamian A. 2004. Structural and serological characterization of the major glycolipid from Rothia mucilaginosa . Biochim Biophys Acta 1675: 54–61. [DOI] [PubMed] [Google Scholar]
- Pastene ER, Wilkomirsky T, Bocaz G, Havel J, Peric I, Vega M, Gonzalez M, Alderete YJ. 2001. USO de espectroscopia de rmn y maldi‐tof ms en la elucidacion estructural de flavonoides antioxidantes provenientes de la planta medicinal chilena Cheilanthes glauca (Cav.) Mett. Bol Soc Chil Quim 46: 449–457. [Google Scholar]
- Patterson JH, Waller RF, Jeevarajah D, Billman‐Jacobe H, McConville MJ. 2003. Mannose metabolism is required for mycobacterial growth. Biochem J 372: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Müller M, Schmidt RR. 2003. Reiterative intramolecular glycosylation supported by a rigid spacer. Eur J Org Chem 128–137. [Google Scholar]
- Pearce G, Ryan CA. 2003. Systemic signaling in tomato plants for defense against herbivores: Isolation and characterization of three novel defense‐signaling glycopeptide hormones coded in a single precursor gene. J Biol Chem 278: 30044–30050. [DOI] [PubMed] [Google Scholar]
- Pedersen NR, Wimmer R, Matthiesen R, Pedersen LH, Gessesse A. 2003. Synthesis of sucrose laurate using a new alkaline protease. Tetrahedron Asym 14: 667–673. [Google Scholar]
- Pellowska‐Januszek L, Dmochowska B, Skorupa E, Chojnacki J, Wojnowski W, Winiewski A. 2004. New class of quaternary ammonium salts, derivatives of methyl D‐glucopyranosides. Carbohydr Res 339: 1537–1544. [DOI] [PubMed] [Google Scholar]
- Peng D, Choudhury BP, Petralia RS, Carlson RW, Gu X‐X. 2004. Roles of 3‐deoxy‐D‐manno‐2‐octulosonic acid transferase from Moraxella catarrhalis in lipooligosaccharide biosynthesis and virulence. Infect Immun 73: 4222–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracaula R, Royle L, Tabarés G, Mallorquí‐Fernández G, Barrabés S, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. 2003a. Glycosylation of human pancreatic ribonuclease: Differences between normal and tumor states. Glycobiology 13: 227–244. [DOI] [PubMed] [Google Scholar]
- Peracaula R, Tabarés G, Royle L, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. 2003b. Altered glycosylation pattern allows the distinction between prostate‐specific antigen (PSA) from normal and tumor origins. Glycobiology 13: 457–470. [DOI] [PubMed] [Google Scholar]
- Pereyra MT, Prieto A, Bernabé A, Leal JA. 2003. Studies of new polysaccharides from Lasallia pustulata (L.) Hoffm. Lichenologist 35: 177–185. [Google Scholar]
- Pérez E, Constant P, Laval F, Lemassu A, Lanéelle M‐A, Daffé M, Guilhot C. 2004a. Molecular dissection of the role of two methyltransferases in the biosynthesis of phenolglycolipids and phthiocerol dimycoserosate in the Mycobacterium tuberculosis complex. J Biol Chem 279: 42584–42592. [DOI] [PubMed] [Google Scholar]
- Pérez E, Constant P, Lemassu A, Laval F, Daffé M, Guilhot C. 2004b. Characterization of three glycosyltransferases involved in the biosynthesis of the phenolic glycolipid antigens from the Mycobacterium tuberculosis complex. J Biol Chem 279: 42574–42583. [DOI] [PubMed] [Google Scholar]
- Peri F, Jiménez‐Barbero J, García‐Aparicio V, Tvaroka I, Nicotra F. 2004. Synthesis and conformational analysis of novel N(OCH3)‐linked disaccharide analogues. Chem Eur J 10: 1433–1444. [DOI] [PubMed] [Google Scholar]
- Perreault H. 2004. Oligosaccharides. In: Rosenfeld JM, editor. Sample preparation for hyphenated analytical techniques. Oxford, UK: Blackwell. pp 83–113. [Google Scholar]
- Perteguer MJ, Rodríguez E, Romarís F, Escalante M, Bonay P, Ubeira FM, Gárate MT. 2004. Minor interspecies variations in the sequence of the gp53 TSL‐1 antigen of Trichinella define species‐specific immunodominant epitopes. Mol Immunol 41: 421–433. [DOI] [PubMed] [Google Scholar]
- Petersen A. 2003. Two‐dimensional electrophoresis replica blotting: A valuable technique for the immunological and biochemical characterization of single components of complex extracts. Proteomics 3: 1206–1214. [DOI] [PubMed] [Google Scholar]
- Peyer C, Bonay P, Staudacher E. 2004. Purification and characterization of a β‐xylosidase from potatoes (Solanum tuberosum). Biochim Biophys Acta 1672: 27–35. [DOI] [PubMed] [Google Scholar]
- Pitchayawasin S, Isobe M. 2004. Mass spectrometric assignment of Smith degradation glycopeptides derived from ribonuclease B. Biosci Biotechnol Biochem 68: 1424–1433. [DOI] [PubMed] [Google Scholar]
- Plaza A, Piacente S, Perrone A, Hamed A, Pizza C, Bifulco G. 2004. Stemmosides C and D, two novel unusual pregnane glycosides from Solenostemma argel: Structural elucidation and configurational study by a combined NMR‐quantum mechanical strategy. Tetrahedron 60: 12205–12213. [Google Scholar]
- Plihal O, Sklenár J, Kmonícková J, Man P, Pompach P, Havlícek V, Kren V, Bezouska K. 2004. N‐glycosylated catalytic unit meets O‐glycosylated propeptide: Complex protein architecture in a fungal hexosaminidase. Biochem Soc Trans 32: 764–765. [DOI] [PubMed] [Google Scholar]
- Pochec E, Litynska A, Amoresano A, Casbarra A. 2003. Glycosylation profile of integrin α3β1 changes with melanoma progression. Biochim Biophys Acta 1643: 113–123. [DOI] [PubMed] [Google Scholar]
- Poletti L, Lay L. 2003. Chemical contributions to understanding heparin activity: Synthesis of related sulfated oligosaccharides. Eur J Org Chem 2999–3024. [Google Scholar]
- Potekhina NV, Shashkov AS, Evtushenko LI, Gavrish EY, Senchenkova SN, Stomakhin AA, Usov AI, Naumova IB, Stackebrandt E. 2003. A novel mannitol teichoic acid with side phosphate groups of Brevibacterium sp. VKM Ac‐2118. Eur J Biochem 270: 4420–4425. [DOI] [PubMed] [Google Scholar]
- Potluri VK, Hamilton AD, Karanikas CF, Bane SE, Xu J, Beckman EJ, Enick RM. 2003. The high CO2‐solubility of per‐acetylated α‐, β‐ and γ‐cyclodextrin. Fluid Phase Equilb 211: 211–217. [Google Scholar]
- Pouria S, Corran PH, Smith AC, Smith HW, Hendry BM, Challacombe SJ, Tarelli E. 2004. Glycoform composition profiling of O‐glycopeptides derived from human serum IgA1 by matrix‐assisted laser desorption ionization‐time of flight‐mass spectrometry. Anal Biochem 330: 257–263. [DOI] [PubMed] [Google Scholar]
- Prabhu A, Venot A, Boons G‐J. 2003. New set of orthogonal protecting groups for the modular synthesis of heparan sulfate fragments. Org Lett 5: 4975–4978. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Wang C‐C, Barnes S. 2004. Mass spectrometric methods for the determination of flavonods in biological samples. Free Radic Biol Med 37: 1324–1350. [DOI] [PubMed] [Google Scholar]
- Prebyl BS, Kaczmarek C, Tuinman AA, Baker DC. 2003. Characterizing the electrospray‐ionization mass spectral fragmentation pattern of enzymatically derived hyaluronic acid oligomers. Carbohydr Res 338: 1381–1387. [DOI] [PubMed] [Google Scholar]
- Preston A, Maxim E, Toland E, Pishko EJ, Harvill ET, Caroff M, Maskell DJ. 2003. Bordetella bronchiseptica PagP is a Bvg‐regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol Microbiol 48: 725–736. [DOI] [PubMed] [Google Scholar]
- Prosperi D, Ronchi S, Lay L, Rencurosi A, Russo G. 2004. Efficient synthesis of unsymmetrical ureido‐linked disaccharides. Eur J Org Chem 395–405. [Google Scholar]
- Pupo E, Phillips NJ, Gibson BW, Apicella MA, Hardy E. 2004. Matrix‐assisted laser desorption/ionization‐time of flight‐mass spectrometry of lipopolysaccharide species separated by slab‐polyacrylamide gel electrophoresis: High‐resolution separation and molecular weight determination of lipooligosaccharides from Vibrio fischeri strain HMK. Electrophoresis 25: 2156–2164. [DOI] [PubMed] [Google Scholar]
- Qin Q, Bergmann CW, Rose JKC, Saladie M, Kolli VSK, Albersheim P, Darvill AG, York WS. 2003. Characterization of a tomato protein that inhibits a xyloglucan‐specific endoglucanase. Plant J 34: 327–338. [DOI] [PubMed] [Google Scholar]
- Que‐Gewirth NLS, Lin S, Cotter RJ, Raetz CRH. 2003. An outer membrane enzyme that generates the 2‐amino‐2‐deoxy‐gluconate moiety of Rhizobium leguminosarum lipid A. J Biol Chem 278: 12109–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que‐Gewirth NLS, Ribeiro AA, Kalb SR, Cotter RJ, Bulach DM, Adler B, Saint Girons I, Werts C, Raetz CRH. 2004. A methylated phosphate group and four amide‐linked acyl chains in Leptospira interrogans lipid A: The membrane anchor of an unusual lipopolysaccharide that activates TLR2. J Biol Chem 279: 25420–25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja MM, Kipp H, Kinne RK. 2004. C‐terminus loop 13 of Na+ glucose cotransporter SGLT1 contains a binding site for alkyl glucosides. Biochemistry 43: 10944–10951. [DOI] [PubMed] [Google Scholar]
- Raman R, Myette JR, Shriver Z, Pojasek K, Venkataraman G, Sasisekharan R. 2003. The heparin/heparan sulfate 2‐O‐sulfatase from Flavobacterium heparinum. A structural and biochemical study of the enzyme active site and saccharide substrate specificity. J Biol Chem 278: 12167–12174. [DOI] [PubMed] [Google Scholar]
- Rasmussen MO, Hogg B, Bono J‐J, Samain E, Driguez H. 2004. New access to lipo‐chitooligosaccharide nodulation factors. Org Biomol Chem 2: 1908–1910. [DOI] [PubMed] [Google Scholar]
- Rauvolfová J, Kuzma M, Weignerová L, Fialová P, Pikrylová V, Pisvejcová A, Macková M, Kren V. 2004a. β‐N‐Acetylhexosaminidase‐catalysed synthesis of non‐reducing oligosaccharides. J Mol Catal B Enzymatic 29: 233–239. [Google Scholar]
- Rauvolfová J, Weignerová L, Kuzma M, Prikrylová V, Macková M, Pišvejcová A, Kren V. 2004b. Enzymatic synthesis of N‐acetylglucosaminobioses by reverse hydrolysis: Characterisation and application of the library of fungal β‐N‐acetylhexosaminidases. J Mol Catal B Enzymatic 29: 259–264. [Google Scholar]
- Ray B, Loutelier‐Bourhis C, Lange C, Condamine E, Driouich A, Lerouge P. 2004. Structural investigation of hemicellulosic polysaccharides from Argania spinosa: Characterisation of a novel xyloglucan motif. Carbohydr Res 339: 201–208. [DOI] [PubMed] [Google Scholar]
- Regué M, Hita B, Piqué N, Izquierdo L, Merino S, Fresno S, Benedí VJ, Tomás JM. 2004. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect Immun 72: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reipen T, Kunz H. 2003. Synthesis of peptide and glycopeptide partial structures of the homophilic recognition domain of epithelial cadherin. Synthesis 2487–2502. [Google Scholar]
- Reis A, Domingues MRM, Ferrer‐Correia AJ, Coimbra MA. 2003. Structural characterisation by MALDI‐MS of olive xylo‐oligosaccharides obtained by partial acid hydrolysis. Carbohydr Polym 53: 101–107. [Google Scholar]
- Remenyik J, Ragunath C, Ramasubbu N, Gyémánt G, Lipták A, Kandra L. 2003. Introducing transglycosylation activity into human salivary α‐amylase (HSA). Org Lett 5: 4895–4898. [DOI] [PubMed] [Google Scholar]
- Renaudie L, Daniellou R, Auge C, Le Narvor C. 2004. Enzymatic supported synthesis of lacto‐N‐neotetraose using dendrimeric polyethylene glycol. Carbohydr Res 339: 693–698. [DOI] [PubMed] [Google Scholar]
- Rencurosi A, Poletti L, Russo G, Lay L. 2003. Lipase‐catalysed regioselective acylations in combination with regioselective glycosylations as a strategy for the synthesis of oligosaccharides: Synthesis of a series of fucosyllactose building blocks. Eur J Org Chem 1672–1680. [Google Scholar]
- Richardson S, Gorton L. 2003. Characterisation of the substituent distribution in starch and cellulose derivatives. Anal Chim Acta 497: 27–65. [Google Scholar]
- Ritte G, Scharf A, Eckermann N, Haebel S, Steup M. 2004. Phosphorylation of transitory starch is increased during degradation. Plant Physiol 135: 2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter TK, Mong K‐KT, Liu H, Nakatani T, Wong C‐H. 2003. A programmable one‐pot oligosaccharide synthesis for diversifying the sugar domains of natural products: A case study of vancomycin. Angew Chem Intl Ed Engl 42: 4657–4660. [DOI] [PubMed] [Google Scholar]
- Robbe C, Capon C, Flahaut C, Michalski J‐C. 2003a. Microscale analysis of mucin‐type O‐glycans by a coordinated fluorophore‐assisted carbohydrate electrophoresis and mass spectrometry approach. Electrophoresis 24: 611–621. [DOI] [PubMed] [Google Scholar]
- Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta J‐P, Michalski J‐C. 2003b. Evidence of regio‐specific glycosylation in human intestinal mucins: Presence of an acidic gradient along the intestinal tract. J Biol Chem 278: 46337–46348. [DOI] [PubMed] [Google Scholar]
- Roces DP, Lüllmann‐Rauch R, Peng J, Balducci C, Andersson C, Tollersrud O, Fogh J, Orlacchio A, Beccari T, Saftig P, von Figura K. 2004. Efficacy of enzyme replacement therapy in α‐mannosidosis mice: A preclinical animal study. Hum Mol Genet 13: 1979–1988. [DOI] [PubMed] [Google Scholar]
- Rohfritsch PF, Rinnbauer M, Vliegenthart JFG, Kamerling JP. 2004. Donor specificity in the glycosylation of Tamm‐Horsfall glycoprotein: Conservation of the Sda determinant in pairs of twins. Glycobiology 14: 365–372. [DOI] [PubMed] [Google Scholar]
- Rosenbohm C, Lundt I, Christensen TMIE, Young NWG. 2003. Chemically methylated and reduced pectins: Preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohydr Res 338: 637–649. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JM. 2003. Derivatization in the current practice of analytical chemistry. Trends Anal Chem 22: 785–798. [Google Scholar]
- Rousseau C, Christensen B, Petersen TE, Bols M. 2004. Cyclodextrins containing an acetone bridge. Synthesis and study as epoxidation catalysts. Org Biomol Chem 43(6): 3476–3482. [DOI] [PubMed] [Google Scholar]
- Roy R, Kim JM. 2003. Cu(II)‐Self‐assembling bipyridyl‐glycoclusters and dendrimers bearing the Tn‐antogen cancer marker: Synthesis and lectin binding properties. Tetrahedron 59: 3881–3893. [Google Scholar]
- Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk‐Janssen D, Redwan E‐RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. 2003. Secretory IgA N‐ and O‐glycans provide a link between the innate and adaptive immune systems. J Biol Chem 278: 20140–20153. [DOI] [PubMed] [Google Scholar]
- Rummel A, Bade S, Alves J, Bigalke H, Binz T. 2003. Two carbohydrate binding sites in the Hcc‐domain of tetanus neurotoxin are required for toxicity. J Mol Biol 326: 835–847. [DOI] [PubMed] [Google Scholar]
- Sainlos M, Belmont P, Vigneron J‐P, Lehn P, Lehn J‐M. 2003. Aminoglycoside‐derived cationic lipids for gene transfection: Synthesis of kanamycin A derivatives. Eur J Org Chem 2764–2774. [Google Scholar]
- Sakamoto J, Müllen K. 2004. Sugars within a hydrophobic scaffold: Glycodendrimers from polyphenylenes. Org Lett 6: 4277–4280. [DOI] [PubMed] [Google Scholar]
- Saksena R, Ma X, Kovác P. 2003. One‐pot preparation of a series of glycoconjugates with predetermined antigen‐carrier ratio from oligosaccharides that mimic the O‐PS of Vibrio cholerae O:1, serotype Ogawa. Carbohydr Res 338: 2591–2603. [DOI] [PubMed] [Google Scholar]
- Sallas F, Niikura K, Nishimura S‐I. 2004. A practical synthesis of amphiphilic cyclodextrins fully substituted with sugar residues on the primary face. Chem Commun 596–597. [DOI] [PubMed] [Google Scholar]
- Samuelson LE, Sebby KB, Walter ED, Singel DJ, Cloninger MJ. 2004. EPR and affinity studies of mannose‐TEMPO functionalized PAMAM dendrimers. Org Biomol Chem 2: 3075–3079. [DOI] [PubMed] [Google Scholar]
- Samyn‐Petit B, Dubos J‐PW, Chirat F, Coddeville B, Demaizieres G, Farrer S, Slomianny M‐C, Theisen M, Delannoy P. 2003. Comparative analysis of the site‐specific N‐glycosylation of human lactoferrin produced in maize and tobacco plants. Eur J Biochem 270: 3235–3242. [DOI] [PubMed] [Google Scholar]
- Sanz‐Nebot V, Benavente F, Vallverdú A, Guzman NA, Barbosa J. 2003. Separation of recombinant human erythropoietin glycoforms by capillary electrophoresis using volatile electrolytes. Assessment of mass spectrometry for the characterization of erythropoietin glycoforms. Anal Chem 75: 5220–5229. [DOI] [PubMed] [Google Scholar]
- Sato K, Hada N, Takeda T. 2003a. Synthesis of new peptidic glycoclusters derived from β‐alanine. Tetrahedron Lett 44: 9331–9335. [Google Scholar]
- Sato T, Gotoh M, Kiyohara K, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, Yada T, Inaba N, Togayachi A, Kudo T, Asada M, Watanabe H, Imamura T, Kimata K, Narimatsu H. 2003b. Differential roles of two N‐acetylgalactosaminyltransferases, CSGalNAcT‐1, and a novel enzyme, CSGalNAcT‐2. Initiation and elongation in synthesis of chondroitin sulfate. J Biol Chem 278: 3063–3071. [DOI] [PubMed] [Google Scholar]
- Sato T, Gotoh M, Kiyohara K, Kameyama A, Kubota T, Kikuchi N, Ishizuka Y, Iwasaki H, Togayachi A, Kudo T, Ohkura T, Nakanishi H, Narimatsu H. 2003c. Molecular cloning and characterization of a novel human β1,4‐N‐acetylgalactosaminyltransferase, β4GalNAc‐T3, responsible for the synthesis of N,N′‐diacetyllactosediamine, GalNAcβ1 → 4GlcNAc. J Biol Chem 278: 47534–47544. [DOI] [PubMed] [Google Scholar]
- Sato M, Furuike T, Sadamoto R, Fujitani N, Nakahara T, Niikura K, Monde K, Kondo H, Nishimura S‐I. 2004a. Glycoinsulins: Dendritic sialyloligosaccharide‐displaying insulins showing a prolonged blood‐sugar‐lowering activity. J Am Chem Soc 126: 14013–14022. [DOI] [PubMed] [Google Scholar]
- Sato M, Sadamoto R, Niikura K, Monde K, Kondo H, Nishimura S‐I. 2004b. Site‐specific introduction of sialic acid into insulin. Angew Chem Intl Ed Engl 43: 1516–1520. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujita S, Kasuya MCZ, Hatanaka K, Yamagata T. 2004c. Display of azido glycoside on a sensor chip. Chem Lett 33: 580–581. [Google Scholar]
- Satomi Y, Shimonishi Y, Takao T. 2004. N‐glycosylation at Asn in the Asn‐Xaa‐Cys motif of human transferrin. FEBS Lett 576: 51–56. [DOI] [PubMed] [Google Scholar]
- Scheppokat AM, Bretting H, Thiem J. 2003a. Fast and efficient synthesis of a novel homologous series of L‐fucosylated trisaccharides using the Helix pomatia α‐(1 → 2)‐L‐galactosyltransferase. Carbohydr Res 338: 2083–2090. [DOI] [PubMed] [Google Scholar]
- Scheppokat AM, Morita M, Thiem J, Bretting H. 2003b. Enzymatic α(1 → 2)‐L‐fucosylation: Investigation of the specificity of the α(1 → 2)‐L‐galactosyltransferase from Helix pomatia . Tetrahedron Asym 14: 2381–2386. [Google Scholar]
- Scheppokat M, Sinnwell V, Thiem J, Bretting H. 2004. Facile synthesis of β(1 → 6)‐D‐galactosylated oligosaccharides employing the β(1 → 6)‐D‐GalT‐II from the albumen gland of the snails Helix pomatia . Synlett 1107–1111. [Google Scholar]
- Schmitt S, Glebe D, Tolle TK, Lochnit G, Linder D, Geyer R, Gerlich WH. 2004a. Structure of pre‐S2 N‐ and O‐linked glycans in surface proteins from different genotypes of hepatitis B virus. J Gen Virol 85: 2045–2053. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Ertan M, Holzgrabe U. 2004b. Chiral capillary electrophoresis: Facts and fiction on the reproducibility of resolution with randomly substituted cyclodextrins. Electrophoresis 25: 2801–2807. [DOI] [PubMed] [Google Scholar]
- Schröder NWJ, Schombel U, Heine H, Göbel UB, Zähringer U, Schumann RR. 2003. Acylated cholesteryl galactoside as a novel immunogenic motif in Borrelia burgdorferi Sensu Stricto . J Biol Chem 278: 33645–33653. [DOI] [PubMed] [Google Scholar]
- Schröder S, Schmidt U, Thiem J, Kowalczyk J, Kunz M, Vogel M. 2004. Synthesis of oligosaccharides as potential novel food components and upscaled enzymatic reaction employing the β‐galactosidase from bovine testes. Tetrahedron 60: 2601–2608. [Google Scholar]
- Schwudke D, Linscheid M, Strauch E, Appel B, Zähringer U, Moll H, Müller M, Brecker L, Gronow S, Lindner B. 2003. The obligate predatory Bdellovibrio bacteriovorus possesses a neutral lipid A containing α‐D‐mannoses that replace phosphate residues: Similarities and differences between the lipid As and the lipopolysaccharides of the wild type strain B. bacteriovorus HD100 and its host‐independent derivative HI100. J Biol Chem 278: 27502–27512. [DOI] [PubMed] [Google Scholar]
- Seo J, Lee K‐J. 2004. Post‐translational modifications and their biological functions: Proteomic analysis and systematic approaches. J Biochem Mol Biol 37: 35–44. [DOI] [PubMed] [Google Scholar]
- Shadyro O, Yurkova I, Kisel M, Brede O, Arnhold J. 2004. Formation of phosphatidic acid, ceramide, and diglyceride on radiolysis of lipids: Identification by MALDI‐TOF mass spectrometry. Free Radic Biol Med 36: 1612–1624. [DOI] [PubMed] [Google Scholar]
- Shafferman A, Chitlaru T, Ordentlich A, Velan B, Kronman C. 2004. A complex array of post‐translation modifications determines the circulatory longevity of acetylcholinesterase in a hierarchical manner. In: Silman A, Soreq H, Anglister L, Michaelson D, Fisher A, editors. Cholinergic mechanisms: Function and dysfunction. London New York: Taylor and Francis. pp 242–253. [Google Scholar]
- Shao N, Xue J, Guo Z. 2004. Chemical synthesis of a skeleton structure sperm CD52‐A GPI‐anchored glycopeptide. Angew Chem Intl Ed Engl 43: 1569–1573. [DOI] [PubMed] [Google Scholar]
- Shen Z, Go EP, Gamez A, Apon JV, Fokin V, Greig M, Ventura M, Crowell JE, Blixt O, Paulson JC, Stevens RC, Finn MG, Siuzdak G. 2004. A mass spectrometry plate reader: Monitoring enzyme activity and inhibition with a desorption/ionization on silicon (DIOS) platform. ChemBioChem 5: 921–927. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji‐Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. 2003. The absence of fucose but not the presence of galactose or bisecting N‐acetylglucosamine of human IgG1 complex‐type oligosaccharides shows the critical role of enhancing antibody‐dependent cellular cytotoxicity. J Biol Chem 278: 3466–3473. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Furukawa J, Niikura K, Miura N, Nishimura S‐I. 2004. Direct N‐glycan profiling in the presence of tryptic peptides on MALDI‐TOF by controlled ion enhancement and suppression upon glycan‐selective derivatization. Anal Chem 76: 6989–6997. [DOI] [PubMed] [Google Scholar]
- Shirasuka Y, Nakajima K, Asakura T, Yamashita H, Yamamoto A, Hata S, Nagata S, Abo M, Sorimachi H, Abe K. 2004. Neoculin as a new taste‐modifying protein occurring in the fruit of Curculigo latifolia . Biosci Biotechnol Biochem 68: 1403–1407. [DOI] [PubMed] [Google Scholar]
- Silchenko AS, Avilov SA, Kalinin VI, Kalinovsky AI, Stonik VA, Smirnov AV. 2004. Pseudostichoposide B—New triterpene glycoside with unprecedent type of sulfatation from the deep‐water North Pacific sea cucumber Pseudostichopus trachus . Nat Prod Res 18: 565–570. [DOI] [PubMed] [Google Scholar]
- Silipo A, De Castro C, Lanzetta R, Molinaro A, Parrilli M. 2004a. Full structural characterization of the lipid A components from the Agrobacterium tumefaciens strain C58 lipopolysaccharide fraction. Glycobiology 14: 805–815. [DOI] [PubMed] [Google Scholar]
- Silipo A, Leone S, Lanzetta R, Parrilli M, Sturiale L, Garozzo D, Nazarenko E, Gorshkova RP, Ivanova EP, Gorshkova NM, Molinaro A. 2004b. The complete structure of the lipooligosaccharide from the halophilic bacterium Pseudoalteromonas issachenkonii KMM 3549T . Carbohydr Res 339: 1985–1993. [DOI] [PubMed] [Google Scholar]
- Silipo A, Leone S, Molinaro A, Lanzetta R, Parrilli M. 2004c. The structure of the phosphorylated carbohydrate backbone of the lipopolysaccharide of the phytopathogen bacterium Pseudomonas tolaasii . Carbohydr Res 339: 2241–2248. [DOI] [PubMed] [Google Scholar]
- Silipo A, Molinaro A, Lanzetta R, Parrilli M, Lindner B, Holst O. 2004d. The structures of the lipid A moieties from the lipopolysaccharides of two phytopathogenic bacteria, Xanthomonas campestris pv. pruni and Xanthomonas fragariae . Eur J Org Chem 1336–1343. [Google Scholar]
- Silipo A, Sturiale L, Garozzo D, Castro C, Lanzetta R, Parrilli M, Grant WD, Molinaro A. 2004e. Structure elucidation of the highly heterogeneous lipid A from the lipopolysaccharide of the gram‐negative extremophile bacterium Halomonas magadiensis strain 21 M1. Eur J Org Chem 2263–2271. [Google Scholar]
- Simas FF, Gorin PAJ, Guerrini M, Naggi A, Sassaki GL, Delgobo CL, Iacomini M. 2004. Structure of a heteroxylan of gum exudate of the palm Scheelea phalerata (uricuri). Phytochemistry 65: 2347–2355. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. 2004. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 201: 197–207. [DOI] [PubMed] [Google Scholar]
- Skidmore MA, Patey SJ, Thanh NTK, Fernig DG, Turnbull JE, Yates EA. 2004. Attachment of glycosaminoglycan oligosaccharides to thiol‐derivatised gold surfaces. Chem Commun 2700–2701. [DOI] [PubMed] [Google Scholar]
- Skorupa E, Dmochowska B, Pellowska‐Januszek L, Wojnowski W, Chojnacki J, Wisniewski A. 2004. Synthesis and structure of selected quaternary N‐(1,4‐anhydro‐5‐deoxy‐2,3‐O‐isopropylidene‐D,L‐ribitol‐5‐yl)ammonium salts. Carbohydr Res 339: 2355–2362. [DOI] [PubMed] [Google Scholar]
- Solomon CM, Lessard EJ, Keil RG, Foy MS. 2003. Characterization of extracellular polymers of Phaeocystis globosa and P. antarctica . Marine Ecol Prog Ser 250: 81–89. [Google Scholar]
- Speranza M. 2004. Enantioselectivity in gas‐phase ion‐molecule reactions. Int J Mass Spectrom 232: 277–317. [Google Scholar]
- Spina E, Sturiale L, Romeo D, Impallomeni G, Garozzo D, Waidelich D, Glueckmann M. 2004. New fragmentation mechanisms in matrix‐assisted laser desorption/ionization time‐of‐flight/time‐of‐flight tandem mass spectrometry of carbohydrates. Rapid Commun Mass Spectrom 18: 392–398. [DOI] [PubMed] [Google Scholar]
- Sriraman R, Bardor M, Sack M, Vaquero C, Faye L, Fischer R, Finnern R, Lerouge P. 2004. Recombinant anti‐hCG antibodies retained in the endoplasmic reticulum of transformed plants lack core‐xylose and core‐α(1,3)‐fucose residues. Plant Biotechnol J 2: 279–287. [DOI] [PubMed] [Google Scholar]
- Stadelmaier A, Morath S, Hartung T, Schmidt RR. 2003. Synthesis of the first fully active lipoteichoic acid. Angew Chem Intl Ed Engl 42: 916–920. [DOI] [PubMed] [Google Scholar]
- Stanley SMR, Poljak A. 2003. Matrix‐assisted laser‐desorption time‐of flight ionization and high‐performance liquid chromatography‐electrospray ionization mass spectral analysis of two glycosylated recombinant epoetins. J Chromatogr B 785: 205–218. [DOI] [PubMed] [Google Scholar]
- Stephens E, Maslen SL, Green LG, Williams DH. 2004a. Fragmentation characteristics of neutral N‐linked glycans using a MALDI‐TOF/TOF tandem mass spectrometer. Anal Chem 76: 2343–2354. [DOI] [PubMed] [Google Scholar]
- Stephens E, Sugars J, Maslen SL, Williams DH, Packman LC, Ellar DJ. 2004b. The N‐linked oligosaccharides of aminopeptidase N from Manduca sexta. Site localization and identification of novel N‐glycan structures. Eur J Biochem 271: 4241–4258. [DOI] [PubMed] [Google Scholar]
- Stikarovská M, Chmelík J. 2004. Determination of neutral oligosaccharides in vegetables by matrix‐assisted laser desorption/ionization mass spectrometry. Anal Chim Acta 520: 47–55. [Google Scholar]
- Stowell SR, Dias‐Baruffi M, Penttilä L, Renkonen O, Nyame AK, Cummings RD. 2004. Human galectin‐1 recognition of poly‐N‐acetyllactosamine and chimeric polysaccharides. Glycobiology 14: 157–167. [DOI] [PubMed] [Google Scholar]
- Strasser R, Altmann F, Glössl J, Steinkellner H. 2004a. Unaltered complex N‐glycan profiles in Nicotiana benthamiana despite drastic reduction of β1,2‐N‐acetylglucosaminyltransferase I activity. Glycoconj J 21: 275–282. [DOI] [PubMed] [Google Scholar]
- Strasser R, Altmann F, Mach L, Glössl J, Steinkellner H. 2004b. Generation of Arabidopsis thaliana plants with complex N‐glycans lacking β1,2‐linked xylose and core α1,3‐linked fucose. FEBS Lett 561: 132–136. [DOI] [PubMed] [Google Scholar]
- Stuhler K, Meyer HE. 2004. MALDI: More than peptide mass fingerprints. Curr Opin Mol Ther 6: 239–248. [PubMed] [Google Scholar]
- Subba Rao MVSST, Muralikrishna G. 2004. Structural analysis of arabinoxylans isolated from native and malted finger millet (Eleusine coracana, ragi). Carbohydr Res 339: 2457–2463. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Takahashi S, Osumi M, Ohno N. 2004. Refinement of the structures of cell‐wall glucans of Schizosaccharomyces pombe by chemical modification and NMR spectroscopy. Carbohydr Res 339: 2255–2265. [DOI] [PubMed] [Google Scholar]
- Sugimoto I, Onimatsu H, Fujie M, Usami S, Yamada T. 2004. vAL‐1, a novel polysaccharide lyase encoded by chlorovirus CVK2. FEBS Lett 559: 51–56. [DOI] [PubMed] [Google Scholar]
- Suhr R, Lahmann M, Oscarson S, Thiem J. 2003a. Synthesis of dihydrodiosgenin glycosides as mimetics of bidesmosidic steroidal saponins. Eur J Org Chem 4003–4011. [Google Scholar]
- Suhr R, Pfefferkorn P, Weingarten S, Thiem J. 2003b. Synthesis of rhamnosylated diosgenyl glucosides as mimetics of cytostatic steroidal saponins from Ornithogalum saundersiae and Galtonia candicans . Org Biomol Chem 1: 4373–4379. [DOI] [PubMed] [Google Scholar]
- Sullivan B, Addona TA, Carr SA. 2004. Selective detection of glycopeptides on ion trap mass spectrometers. Anal Chem 76: 3112–3118. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Lee YC. 2004. Site‐specific N‐glycosylation of chicken serum IgG. Glycobiology 14: 275–292. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Shimazaki T. 2003. Preparation of a unique glucan with large intervals in molecular weight distribution. Controlled ring‐opening polymerization of O‐permethylcyclodextrin. Org Biomol Chem 1: 604–608. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Khoo K‐H, Chen C‐M, Chen H‐C, Lee YC. 2003a. N‐Glycan structures of pigeon IgG. A. major serum glycoprotein containing Galα1 → 4 termini. J Biol Chem 278: 46293–46306. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kelly JF, Locke SJ, Thibault P, Honda S. 2003b. Derivatization of carbohydrates. In: Thibault P, Honda S, editors. Capillary electrophoresis of carbohydrates. Totowa, USA: Humana Press. pp 41–70. [Google Scholar]
- Sweet CR, Williams AH, Karbarz MJ, Werts C, Kalb SR, Cotter RJ, Raetz CRH. 2004. Enzymatic synthesis of lipid A molecules with four amide‐linked acyl chains. LpxA acetyltransferases selective for an analog of UDP‐N‐acetylglucosamine in which an amine replaces the 3″‐hydroxyl group. J Biol Chem 279: 25411–25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllosi É, Kremmer T, Ludányi K, Imre T, Schlosser G, Boldizsár M, Vincze B, Vékey K. 2004. A novel method for the separation and purification of human serum acid alpha‐1‐glycoprotein. liquid chromatographic and mass spectrometric investigation of tryptic fragments. Chromatographia 60: S213–S219. [DOI] [PubMed] [Google Scholar]
- Taguchi I, Kiyohara H, Matsumoto T, Yamada H. 2004. Structure of oligosaccharide side chains of an intestinal immune system modulating arabinogalactan isolated from rhizomes of Atractylodes lancea DC. Carbohydr Res 339: 763–770. [DOI] [PubMed] [Google Scholar]
- Tait AS, Brown CJ, Galbraith DJ, Hines MJ, Hoare M, Birch JR, James DC. 2004. Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/DNA complexes in combination with microtubule disrupting anti‐mitotic agents. Biotechnol Bioeng 88: 707–721. [DOI] [PubMed] [Google Scholar]
- Takaha T, Yanase M, Takata H, Okada S, Smith SM. 2003. Potato D‐enzyme catalyzes the cyclization of amylose to produce cycloamylose, a novel cyclic glucan. J Biol Chem 271: 2902–2908. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Nakakita S, Hase S. 2003a. Conversion of pyridylamino sugar chains to corresponding reducing sugar chains. J Biochem (Tokyo) 134: 51–55. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Masuda K, Hiraki K, Yoshihara K, Huang H‐H, Khoo K‐H, Kato K. 2003b. N‐Glycan structures of squid rhodopsin. Existence of the α1‐3 and α1‐6 difucosylated innermost GlcNAc residue in a molluscan glycoprotein. Eur J Biochem 270: 2627–2632. [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Katsumata T, Inoue N, Watanabe T, Fujibayashi Y, Takeuchi M. 2004. Abnormal biantennary sugar chains are expressed in human chorionic gonadotropin produced in the choriocarcinoma cell line, JEG‐3. Glycoconj J 20: 473–481. [DOI] [PubMed] [Google Scholar]
- Takano Y, Kojima N, Nakahara Y, Hojo H, Nakahara Y. 2003. Solid‐phase synthesis of core 2 O‐linked glycopeptide and its enzymatic sialylation. Tetrahedron 59: 8415–8427. [Google Scholar]
- Takano Y, Hojo H, Kojima N, Nakahara Y. 2004. Synthesis of a mimic for the heterogeneous surface of core 2 sialoglycan‐linked glycoprotein. Org Lett 6: 3135–3138. [DOI] [PubMed] [Google Scholar]
- Tamura J, Tokuyoshi M. 2004. Synthesis of chondroitin sulfate E hexasaccharide in the repeating region by an effective elongation strategy toward longer chondroitin oligosaccharide. Biosci Biotechnol Biochem 68: 2436–2443. [DOI] [PubMed] [Google Scholar]
- Tan PV, Taranenko NI, Laiko VV, Yakshin MA, Prasad CR, Doroshenko VM. 2004. Mass spectrometry of N‐linked oligosaccharides using atmospheric pressure infrared laser ionization from solution. J Mass Spectrom 39: 913–921. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakahara Y, Hojo H, Nakahara Y. 2003. Studies directed toward the synthesis of protein‐bound GPI anchor. Tetrahedron 59: 4059–4067. [Google Scholar]
- Tarelli E, Smith AC, Hendry BM, Challacombe SJ, Pouria S. 2004. Human serum IgA1 is substituted with up to six O‐glycans as shown by matrix assisted laser desorption ionisation time‐of‐flight mass spectrometry. Carbohydr Res 339: 2329–2335. [DOI] [PubMed] [Google Scholar]
- Tegeler TJ, Mechref Y, Boraas K, Reilly JP, Novotny MV. 2004. Microdeposition device interfacing capillary electrochromatography and microcolumn liquid chromatography with matrix‐assisted laser desorption/ionization mass spectrometry. Anal Chem 76: 6698–6706. [DOI] [PubMed] [Google Scholar]
- Teleman A, Nordström M, Tenkanen M, Jacobs A, Dahlman O. 2003. Isolation and characterization of O‐acetylated glucomannans from aspen and birch wood. Carbohydr Res 338: 525–534. [DOI] [PubMed] [Google Scholar]
- Teng R, Ang C, McManus D, Armstrong D, Mau S, Bacic A. 2003. Regioselective acylation of ginsenosides by Novozyme 435. Tetrahedron Lett 44: 5661–5664. [Google Scholar]
- Teng R, Ang C, McManus D, Armstrong D, Mau S, Bacic A. 2004. Regioselective acylation of ginsenosides by Novozyme 435 to generate molecular diversity. Helv Chim Acta 87: 1860–1872. [Google Scholar]
- Teramoto N, Arai Y, Shibasaki Y, Shibata M. 2004. A facile synthesis of a novel polyacetal containing trehalose residue in the main chain. Carbohydr Polym 56: 1–6. [Google Scholar]
- Teranishi K. 2003. Practical and convenient modifications of the A,C‐secondary hydroxyl face of cyclodextrins. Tetrahedron 59: 2519–2538. [Google Scholar]
- Teranishi K, Nishiguchi T. 2004. Cyclodextrin‐bound 6‐(4‐methoxyphenyl)imidazo[1,2‐α]pyrazin‐3(7H)‐ones with fluorescein as green chemiluminescent probes for superoxide anions. Anal Biochem 325: 185–195. [DOI] [PubMed] [Google Scholar]
- Teranishi K, Ueno F. 2003. Mechanism of 2‐O–3‐O silyl migration in cyclomaltohexaose (α‐cyclodextrin). Tetrahedron Lett 44: 4843–4848. [Google Scholar]
- Tevyashova A, Sztaricskai F, Batta G, Herczegh P, Jeney A. 2004. Formation of squaric acid amides of anthracycline antibiotics. Synthesis and cytotoxic properties. Bioorg Med Chem Lett 14: 4783–4789. [DOI] [PubMed] [Google Scholar]
- Thiel C, Schwarz M, Peng J, Grzmil M, Hasilik M, Braulke T, Kohlschütter A, von Figura K, Lehle L, Körner C. 2003. A new type of congenital disorders of glycosylation (CDG‐Ii) provides new insights into the early steps of dolichol‐linked oligosaccharide biosynthesis. J Biol Chem 278: 22498–22505. [DOI] [PubMed] [Google Scholar]
- Thomas LJ, Panneerselvam K, Beattie DT, Picard MD, Xu B, Rittershaus CW, Marsh HC Jr, Hammond RA Qian J, Stevenson T, Zopf D, Bayer RJ. 2004a. Production of a complement inhibitor possessing sialyl Lewis X moieties by in vitro glycosylation technology. Glycobiology 14: 883–893. [DOI] [PubMed] [Google Scholar]
- Thomas X, Destoumieux‐Garzón D, Peduzzi J, Afonso C, Blond A, Birlirakis N, Goulard C, Dubost L, Thai R, Tabet J‐C, Rebuffat S. 2004b. Siderophore peptide, a new type of post‐translationally modified antibacterial peptide with potent activity. J Biol Chem 279: 28233–28242. [DOI] [PubMed] [Google Scholar]
- Thomas‐Oates J, Bereszczak J, Edwards E, Gill A, Noreen S, Zhou JC, Chen MZ, Miao LH, Xie FL, Yang Jk, Zhou Q, Yang SS, Li XH, Wang L, Spaink HP, Schlaman HRM, Harteveld M, Díaz Cl, van Brussel AAN, Camacho M, Rodríguez‐Navarro DN, Santamaría C, Temprano F, Acebes JM, Bellogín RA, Buendía‐Clavería AM, Cubo MT, Espuny MR, Gil AM, Gutiérrez R, Hidalgo A, López‐Baena FJ, Madinabeitia N, Medina C, Ollero FJ, Vinardell JM, Ruiz‐Sainz JE. 2003. A catalogue of molecular, physiological and symbiotic properties of soybean‐nodulating rhizobial strains from different soybean cropping areas of China. Syst Appl Microbiol 26: 453–465. [DOI] [PubMed] [Google Scholar]
- Toman R, Garidel P, Andra J, Slaba K, Hussein A, Koch M‐H, Brandenburg K. 2004. Physicochemical characterization of the endotoxins from Coxiella burnetii strain Priscilla in relation to their bioactivities. BMC Biochem 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiya N, Howe D, Aumiller JJ, Pathak M, Park J, Palter KB, Jarvis DL, Betenbaugh MJ, Lee YC. 2003. Complex‐type biantennary N‐glycans of recombinant human transferrin from Trichoplusia ni insect cells expressing mammalian β‐1,4‐galactosyltransferase and β‐1,2‐N‐acetylglucosaminyltransferase II. Glycobiology 13: 23–34. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Narang S, Lee YC, Betenbaugh MJ. 2004. Comparing N‐glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj J 21: 343–360. [DOI] [PubMed] [Google Scholar]
- Tran AX, Karbarz MJ, Wang X, Raetz CR, McGrath SC, Cotter RJ, Trent MS. 2004. Periplasmic cleavage and modification of the 1‐phosphate group of Helicobacter pylori lipid A. J Biol Chem 279: 55780–55791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble RB, Lubowski C, Hauer CRI, Stack R, McNaughton L, Gemmill TR, Kumar SA. 2004. Characterization of N‐ and O‐linked glycosylation of recombinant human bile salt‐stimulated lipase secreted by Pichia pastoris . Glycobiology 14: 265–274. [DOI] [PubMed] [Google Scholar]
- Tseng K, Hedrick JL, Lebrilla CB. 1999. Catalog‐library approach for the rapid and sensitive structural elucidation of oligosaccharides. Anal Chem 71: 3747–3754. [DOI] [PubMed] [Google Scholar]
- Tyagi NK, Kinne RKH. 2003. Synthesis of photoaffinity probes [2′‐iodo‐4′‐(3″‐trifluoromethyldiazirinyl)phenoxy]‐D‐glucopyranoside and [(4′‐benzoyl)phenoxy]‐D‐glucopyranoside for the identification of sugar‐binding and phlorizin‐binding sites in the sodium/‐glucose cotransporter protein. Anal Biochem 323: 74–83. [DOI] [PubMed] [Google Scholar]
- Tzeng YL, Datta A, Ambrose K, Lo M, Davies JK, Carlson RW, Stephens DS, Kahler CM. 2004. The MisR/MisS two‐component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis . J Biol Chem 279: 35053–35062. [DOI] [PubMed] [Google Scholar]
- Ummarino S, Corsaro MM, Lanzetta R, Parrilli M, Peter‐Katalinic J. 2003. Determination of phosphorylation sites in lipooligosaccharides from Pseudoalteromonas haloplanktis TAC 125 grown at 15°C and 25°C by nano‐electrospray ionization quadrupole time‐of‐flight tandem mass spectrometry. Rapid Commun Mass Spectrom 17: 2226–2232. [DOI] [PubMed] [Google Scholar]
- Valdez‐Cruz NA, Batista CVF, Possani LD. 2004. Phaiodactylipin, a glycosylated heterodimeric phospholipase A2 from the venom of the scorpion Anuroctonus phaiodactylus . Eur J Biochem 271: 1453–1464. [DOI] [PubMed] [Google Scholar]
- Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, Hughes TE. 2004. Advanced glycation end product ligands for the receptor for advanced glycation end products: Biochemical characterization and formation kinetics. Anal Biochem 324: 68–78. [DOI] [PubMed] [Google Scholar]
- van Alebeek GJ, van Scherpenzeel K, Beldman G, Schols HA, Voragen AGJ. 2003. Partially esterified oligogalacturonides are the preferred substrates for pectin methylesterase of Aspergillus niger . Biochem J 372: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ameijde J, Liskamp RMJ. 2003. Synthesis of novel trivalent amino acid glycoconjugates based on the cyclotriveratrylene (‘CTV’) scaffold. Org Biomol Chem 1: 2661–2669. [DOI] [PubMed] [Google Scholar]
- van Oort E, Lerouge P, de Heer PG, Seveno M, Coquet L, Modderman PW, Faye L, Aalberse RC, van Ree R. 2004. Substitution of Pichia pastoris‐derived recombinant proteins with mannose containing O‐ and N‐linked glycans decreases specificity of diagnostic tests. Int Arch Allergy Immunol 135: 187–195. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, Bank CMC, Nyame AK, Cummings RD, Deelder AM, van Die I. 2003. Schistosoma mansoni‐infected mice produce antibodies that cross‐react with plant, insect, and mammalian glycoproteins and recognize the truncated biantennary N‐glycan Man3GlcNAc2‐R. Glycobiology 13: 217–225. [DOI] [PubMed] [Google Scholar]
- Verhoef R, de Waard P, Schols HA, Siika‐aho M, Voragen AGJ. 2003. Methylobacterium sp. isolated from a Finnish paper machine produces highly pyruvated galactan exopolysaccharide. Carbohydr Res 338: 1851–1859. [DOI] [PubMed] [Google Scholar]
- Vicré M, Lerouxel O, Farrant J, Lerouge P, Driouich A. 2004. Composition and desiccation‐induced alterations of the cell wall in the resurrection plant Craterostigma wilmsii . Physiol Plant 120: 229–239. [DOI] [PubMed] [Google Scholar]
- Vierhuis E, Korver M, Schols HA, Voragen AGJ. 2003. Structural characteristics of pectic polysaccharides from olive fruit (Olea europaea cv moraiolo) in relation to processing for oil extraction. Carbohydr Polym 51: 135–148. [Google Scholar]
- Viëtor R, Loutelier‐Bourhis C, Fitchette A‐C, Margerie P, Gonneau M, Faye L, Lerouge P. 2003. Protein N‐glycosylation is similar in the moss Physcomitrella patens and in higher plants. Planta 218: 269–275. [DOI] [PubMed] [Google Scholar]
- Vo LH, Yen T‐Y, Macher BA, Hedrick JL. 2003. Identification of the ZPC oligosaccharide ligand involved in sperm binding and the glycan structures of Xenopus laevis vitelline envelope glycoproteins. Biol Reprod 69: 1822–1830. [DOI] [PubMed] [Google Scholar]
- Volc J, Sedmera P, Kujawa M, Halada P, Kubátová E, Haltrich D. 2004. Conversion of lactose to β‐D‐galactopyranosyl‐(1 → 4)‐D‐arabino‐hexos‐2‐ulose‐(2 → dehydrolactose) and lactobiono‐1,5‐lactone by fungal pyranose dehydrogenase. J Mol Catal B Enzymatic 30: 177–184. [Google Scholar]
- von der Lieth C‐W. 2004a. An endorsement to create open access databases for analytical data of complex carbohydrates. J Carbohydr Chem 23: 277–297. [Google Scholar]
- von der Lieth C‐W. 2004b. Bioinformatics for glycomics: Status, methods, requirements and perspectives. Brief Bioinformat 5: 164–178. [DOI] [PubMed] [Google Scholar]
- Von Seggern CE, Cotter RJ. 2003. Fragmentation studies of noncovalent sugar‐sugar complexes by infrared atmospheric pressure MALDI. J Am Soc Mass Spectrom 14: 1158–1165. [DOI] [PubMed] [Google Scholar]
- Von Seggern CE, Cotter RJ. 2004. Study of peptide‐sugar non‐covalent complexes by infrared atmospheric pressure matrix‐assisted laser desorption/ionization. J Mass Spectrom 39: 736–742. [DOI] [PubMed] [Google Scholar]
- Von Seggern CE, Moyer SC, Cotter RJ. 2003a. Liquid infrared atmospheric pressure matrix‐assisted laser desorption/ionization ion trap mass spectrometry of sialylated carbohydrates. Anal Chem 75: 3212–3218. [DOI] [PubMed] [Google Scholar]
- Von Seggern CE, Zarek PE, Cotter RJ. 2003b. Fragmentation of sialylated carbohydrates using infrared atmospheric pressure MALDI ion trap mass spectrometry from cation‐doped liquid matrixes. Anal Chem 75: 6523–6530. [DOI] [PubMed] [Google Scholar]
- Wada Y, Tajiri M, Yoshida S. 2004. Hydrophilic affinity isolation and MALDI multiple‐stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal Chem 76: 6560–6565. [DOI] [PubMed] [Google Scholar]
- Wagner M, Dziadek S, Kunz H. 2003. The (2‐phenyl‐2‐trimethylsilyl)ethyl‐(PTMSEL)‐linker in the synthesis of glycopeptide partial structures of complex cell surface glycoproteins. Chem Eur J 9: 6018–6030. [DOI] [PubMed] [Google Scholar]
- Wang F, Metcalf T, van der Wel H, West CM. 2003a. Initiation of mucin‐type O‐glycosylation in Dictyostelium is homologous to the corresponding step in animals and is important for spore coat function. J Biol Chem 278: 51395–51407. [DOI] [PubMed] [Google Scholar]
- Wang F, Nakouzi A, Angeletti RH, Casadevall A. 2003b. Site‐specific characterization of the N‐linked oligosaccharides of a murine immunoglobulin M by high‐performance liquid chromatography/electrospray mass spectrometry. Anal Biochem 314: 266–280. [DOI] [PubMed] [Google Scholar]
- Wang H, Tachibana K, Zhang Y, Iwasaki H, Kaneyama A, Cheng L, Guo J, Hiruma T, Togayachi A, Kudo T, Kikuchi N, Narimatsu H. 2003c. Cloning and characterization of a novel UDP‐GalNAc:polypeptide N‐acetylgalactosaminyltransferase, pp‐GalNAc‐T14. Biochem Biophys Res Commun 300: 738–744. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang X, Mou H, Guan H. 2004a. Anti‐oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J Appl Phycol 16: 333–340. [Google Scholar]
- Wang W, Reichert P, Beyer BM, Liu JJ, Lee J, Zhang L, Liu Y‐H, Taremi SS, Le HV, Strickland C. 2004b. Crystallization of glycosylated human BACE protease domain expressed in Trichoplusia ni . Biochim Biophys Acta 1698: 255–259. [DOI] [PubMed] [Google Scholar]
- Wang X, Karbarz MJ, McGrath SC, Cotter RJ, Raetz CR. 2004c. MsbA transporter‐dependent lipid A 1‐dephosphorylation on the periplasmic surface of the inner membrane: Topography of Francisella novicida LpxE expressed in Escherichia coli . J Biol Chem 279: 49470–49478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang X, Zhang L‐H, Ye X‐S. 2004d. A four‐component one‐pot synthesis of α‐gal pentasaccharide. Org Lett 6: 4415–4417. [DOI] [PubMed] [Google Scholar]
- Watt GM, Boons G‐J. 2004. A convergent strategy for the preparation of N‐glycan core di‐, tri‐, and pentasaccharide thioaldoses for the site‐specific glycosylation of peptides and proteins bearing free cysteines. Carbohydr Res 339: 181–193. [DOI] [PubMed] [Google Scholar]
- Watt GM, Lund J, Levens M, Kolli K, Jefferis R, Boons G‐J. 2003. Site‐specific glycosylation of an aglycosylated human IgG1‐Fc antobody protein generates neoglycoproteins with enhanced function. Chem Biol 10: 807–814. [DOI] [PubMed] [Google Scholar]
- Weenen C, Pena JE, Pollak SV, Klein J, Lobel L, Trousdale RK, Palmer S, Lustbader EG, Ogden RT, Lustbader JW. 2004. Long‐acting follicle‐stimulating hormone analogs containing N‐linked glycosylation exhibited increased bioactivity compared with O‐linked analogs in female rats. J Clin Endocrinol Metab 89: 5204–5212. [DOI] [PubMed] [Google Scholar]
- Wei G, Gu G, Du Y. 2003. Silver triflate. A mild alternative catalyst for glycosylation conditions using trichloroacetimidates as glycosyl donors. J Carbohydr Chem 22: 385–393. [Google Scholar]
- Weignerová L, Vavrusková P, Pisvejcová A, Thiem J, Kren V. 2003. Fungal β‐N‐acetylhexosaminidases with high β‐N‐acetylgalactosaminidase activity and their use for synthesis of β‐GalNAc‐containing oligosaccharides. Carbohydr Res 338: 1003–1008. [DOI] [PubMed] [Google Scholar]
- Weingarten S, Thiem J. 2003. Facile formation of novel carbohydrate‐amino acid conjugates by reductive amination. Synlett 1052–1054. [Google Scholar]
- Welinder KG, Larsen YB. 2004. Covalent structure of soybean seed coat peroxidase. Biochim Biophys Acta 1698: 121–126. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Cole RN, Cronshaw JM, Matunis MJ, Hart GW. 2002. Mapping sites of O‐GlcNAc modification using affinity tags for serine and threonine post‐translational modifications. Mol Cell Proteomics 1: 791–804. [DOI] [PubMed] [Google Scholar]
- Wendeler M, Hoernschemeyer J, Hoffmann D, Kolter T, Schwarzmann G, Sandhoff K. 2004a. Photoaffinity labelling of the human GM2‐activator protein. Mechanistic insight into ganglioside GM2 degradation. Eur J Biochem 271: 614–627. [DOI] [PubMed] [Google Scholar]
- Wendeler M, Hoernschemeyer J, John M, Werth N, Schoeniger M, Lemm T, Hartmann T, Kessler H, Sandhoff K. 2004b. Expression of the GM2‐activator protein in the methylotrophic yeast Pichia pastoris, purification, isotopic labeling, and biophysical characterization. Protein Exp Purif 34: 147–157. [DOI] [PubMed] [Google Scholar]
- Westerlind U, Westman J, Törnquist E, Smith CIE, Oscarson S, Lahmann M, Norberg T. 2004. Ligands of the asialoglycoprotein receptor for targeted gene delivery, part 1: Synthesis of and binding studies with biotinylated cluster glycosides containing N‐acetylgalactosamine. Glycoconj J 21: 227–241. [DOI] [PubMed] [Google Scholar]
- Westphal S, Kolarich D, Foetisch K, Lauer I, Altmann F, Conti A, Crespo JF, Rodríguez J, Enrique E, Vieths S, Scheurer S. 2003. Molecular characterization and allergenic activity of Lyc e 2 (β‐fructofuranosidase), a glycosylated allergen of tomato. Eur J Biochem 270: 1327–1337. [DOI] [PubMed] [Google Scholar]
- Weyermann J, Lochmann D, Georgens C, Rais I, Kreuter J, Karas M, Wolkenhauer M, Zimmer A. 2004. Physicochemical characterisation of cationic polybutylcyanoacrylat‐nanoparticles by fluorescence correlation spectroscopy. Eur J Pharm Biopharm 58: 25–35. [DOI] [PubMed] [Google Scholar]
- Wicklein D, Lindner B, Moll H, Kolarich D, Altmann F, Becker W‐M, Petersen A. 2004. Carbohydrate moieties can induce mediator release: A detailed characterization of two major timothy grass pollen allergens. Biol Chem 385: 397–407. [DOI] [PubMed] [Google Scholar]
- Winterfeld GA, Khodair AI, Schmidt RR. 2003. O‐Glycosyl amino acids by 2‐nitrogalactal concatenation—Synthesis of a mucin‐type O‐glycan. Eur J Org Chem 1009–1021. [Google Scholar]
- Woldemichael GM, Montenegro G, Timmermann BN. 2003. Triterpenoidal lupin saponins from the Chilean legume Lupinus oreophilus Phil. Phytochemistry 63: 853–857. [DOI] [PubMed] [Google Scholar]
- Woller EK, Walter ED, Morgan JR, Singel DJ, Cloninger MJ. 2003. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl‐functionalized dendrimers. J Am Chem Soc 125: 8820–8826. [DOI] [PubMed] [Google Scholar]
- Wong NK, Easton RL, Panico M, Sutton‐Smith M, Morrison JC, Lattanzio FA, Morris HR, Clark GF, Dell A, Patankar MS. 2003. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J Biol Chem 278: 28619–28634. [DOI] [PubMed] [Google Scholar]
- Wu Z, Kong F. 2004a. Synthesis of divalent β‐(1 → 6)‐branched (1 → 3)‐glucohexaose and trivalent β‐(1 → 6)‐branched (1 → 3)‐glucotriose. Carbohydr Res 339: 2761–2768. [DOI] [PubMed] [Google Scholar]
- Wu Z, Kong F. 2004b. Synthesis of 6‐branched cyclo‐(1 → 3)‐glucohexaose and glucooctaose. Synlett 2594–2596. [Google Scholar]
- Wu X, Schmidt RR. 2004. Solid‐phase synthesis of complex oligosaccharides using azidoglucose as a glycosyl acceptor. Eur J Org Chem 2826–2832. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Geyer H, von der Ohe M, Gerardy‐Schahn R, Schachner M, Geyer R. 2003a. Localization of defined carbohydrate epitopes in bovine polysialylated NCAM. Biochimie 85: 207–218. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Grimm C, Zähringer U, Dennis RD, Berkefeld CM, Idris MA, Geyer R. 2003b. A novel GlcNAcα1‐HPO3‐6Gal(1 → 1)ceramide antigen and alkylated inositol‐phosphoglycerolipids expressed by the liver fluke Fasciola hepatica . Glycobiology 13: 129–137. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Grimm C, Dennis RD, Idris MA, Geyer R. 2004a. The parasitic trematode Fasciola hepatica exhibits mammalian‐type glycolipids as well as Gal(β1 → 6)Gal‐terminating glycolipids that account for cestode serological cross‐reactivity. Glycobiology 14: 115–126. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Hokke CH, Deelder AM. 2004b. Glycopeptide analysis by matrix‐assisted laser desorption/ionization tandem time‐of‐flight mass spectrometry reveals novel features of horseradish peroxidase glycosylation. Rapid Commun Mass Spectrom 18: 1741–1748. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Robijn MLM, Koeleman CAM, Balog CIA, Geyer R, Deelder AM, Hokke CH. 2004c. A novel Gal(β1 → 4)Gal(β1 → 4)Fuc(α1 → 6)‐core modification attached to the proximal N‐acetylglucosamine of keyhole limpet haemocyanin (KLH) N‐glycans. Biochem J 378: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian M, Fatima Z, Zhang W, Fang J, Li H, Pei D, Loo J, Stevenson T, Wang PG. 2004. Identification of α‐galactosyl epitope mimetics through rapid generation and screening of C‐linked glycopeptide library. J Comb Chem 6: 126–134. [DOI] [PubMed] [Google Scholar]
- Xie Y, Lebrilla CB. 2003. Infrared multiphoton dissociation of alkali metal‐coordinated oligosaccharides. Anal Chem 75: 1590–1598. [DOI] [PubMed] [Google Scholar]
- Xie Y, Schubothe KM, Lebrilla CB. 2003. Infrared laser isolation of ions in Fourier transform mass spectrometry. Anal Chem 75: 160–164. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ning J. 2003. First synthesis of D‐mannose penta‐ and decasaccharides, the repeating unit and its dimer of the cell‐wall mannan of Candida kefyr IFO 0586. Tetrahedron Asym 14: 1275–1283. [Google Scholar]
- Xiong L, Andrews D, Regnier F. 2003a. Comparative proteomics of glycoproteins based on lectin selection and isotope coding. J Proteome Res 2: 618–625. [DOI] [PubMed] [Google Scholar]
- Xiong S, Zhao Z, Xin B, Wang G. 2003b. Characterization of macrocyclic polysaccharides using matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Rapid Commun Mass Spectrom 17: 1079–1083. [DOI] [PubMed] [Google Scholar]
- Xu S, Li Y, Zou H, Qiu J, Guo Z, Guo B. 2003. Carbon nanotubes as assisted matrix for laser desorption/ionization time‐of‐flight mass spectrometry. Anal Chem 75: 6191–6195. [DOI] [PubMed] [Google Scholar]
- Xu L, Appell M, Kennedy S, Momany FA, Price NPJ. 2004. Conformational analysis of chirally deuterated tunicamycin as an active site probe of UDP‐N‐acetylhexosamine:polyprenol‐P N‐acetylhexosamine‐1‐P translocases. Biochemistry 43: 13248–13255. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sasaki T, Niwa S, Oishi T, Murata M, Kawakami T, Aimoto S. 2004. Intact glycation end products containing carboxymethyl‐lysine and glyoxal lysine dimer obtained from synthetic collagen model peptide. Bioorg Med Chem Lett 14: 5677–5680. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ishida H, Kanamori A, Kannagi R, Kiso M. 2004. Synthesis and antigenic property of a novel sialyl 6‐O‐sulfo Lewis X neo‐glycolipid containing lactamized neuraminic acid. J Carbohydr Chem 23: 201–215. [Google Scholar]
- Yamamoto N, Sakakibara T, Kajihara Y. 2004. Convenient synthesis of a glycopeptide analogue having a complex type disialyl‐undecasaccharide. Tetrahedron Lett 45: 3287–3290. [Google Scholar]
- Yamamura T, Hada N, Kaburaki A, Yamano K, Takeda T. 2004. Synthetic studies on glycosphingolipids from Protostomia phyla: Total syntheses of glycosphingolipids from the parasite, Echinococcus multilocularis . Carbohydr Res 339: 2749–2759. [DOI] [PubMed] [Google Scholar]
- Yamanoi T, Tsutsumida M, Oda Y, Akaike E, Osumi K, Yamamoto K, Fujita K. 2004. Transglycosylation reaction of Mucor hiemalis endo‐β‐N‐acetylglucosaminidase using sugar derivatives modified at C‐1 or C‐2 as oligosaccharide acceptors. Carbohydr Res 339: 1403–1406. [DOI] [PubMed] [Google Scholar]
- Yan J, Kitamura K, Nomura M. 2003. A novel oligosaccharide on bovine peripheral myelin glycoprotein P0. J Saitama Med School 30: 121–129. [Google Scholar]
- Yang F, Du Y. 2003. A practical synthesis of a (1 → 6)‐linked β‐D‐glucosamine nonasaccharide. Carbohydr Res 338: 495–502. [DOI] [PubMed] [Google Scholar]
- Yang F, Hua Y, Du Y. 2003. Synthesis of biantennary β‐D‐(1 → 6) glucosamine oligosaccharides. Carbohydr Res 338: 1313–1318. [DOI] [PubMed] [Google Scholar]
- Yang F‐L, Lu C‐P, Chen C‐S, Chen M‐Y, Hsiao H‐L, Su Y, Tsay S‐S, Zou W, Wu S‐H. 2004. Structural determination of the polar glycoglycerolipids from thermophilic bacteria Meiothermus taiwansis . Eur J Biochem 271: 4545–4551. [DOI] [PubMed] [Google Scholar]
- Yassin FH, Marynick DS. 2003. Computational estimates of the gas‐phase acidities of dihydroxybenzoic acid radical cations and their corresponding neutral species. J Mol Struct 629: 223–235. [Google Scholar]
- Ying W, Hao Y, Zhang Y, Peng W, Qin E, Cai Y, Wei K, Wang J, Chang G, Sun W, Dai S, Li X, Zhu Y, Li J, Wu S, Guo L, Dai J, Wang J, Wan P, Chen T, Du C, Li D, Wan J, Kuai X, Li W, Shi R, Wei H, Cao C, Yu M, Liu H, Dong F, Wang D, Zhang X, Qian X, Zhu Q, He F. 2004. Proteomic analysis on structural proteins of severe acute respiratory syndrome coronavirus. Proteomics 4: 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoko‐o T, Wiggins CAR, Stolz J, Peak‐Chew SY, Munro S. 2003. An N‐acetylglucosaminyltransferase of the Golgi apparatus of the yeast Saccharomyces cerevisiae that can modify N‐linked glycans. Glycobiology 13: 581–589. [DOI] [PubMed] [Google Scholar]
- Yoon J‐W, Jeon E‐J, Jung IH, Min M‐J, Lee H‐Y, Kim M‐J, Baek J‐S, Lee H‐S, Park C‐S, Oh S, Park K‐H, Moon T‐W. 2003. Maltosyl‐erythritol, a major transglycosylation product of erythritol by Bacillus stearothermophilus maltogenic amylase. Biosci Biotechnol Biochem 67: 525–531. [DOI] [PubMed] [Google Scholar]
- Yoon S‐H, Fulton DB, Robyt JF. 2004a. Enzymatic synthesis of two salicin analogues by reaction of salicyl alcohol with Bacillus macerans cyclomaltodextrin glucanyltransferase and Leuconostoc mesenteroides B‐742CB dextransucrase. Carbohydr Res 339: 1517–1529. [DOI] [PubMed] [Google Scholar]
- Yoon S‐H, Shin Y, Chun KH, Shin JE. 2004b. Synthesis of GlcNAcp‐β‐(1 → 3)‐Galp‐α‐(1 → 2)‐6‐deoxy‐altroHepp‐α‐(1‐O‐propyl), an O‐antigenic repeating unit from C. jejuni O:23 and O:36. Bull Korean Chem Soc 25: 289–292. [Google Scholar]
- Yu H, Furukawa J‐I, Ikeda T, Wong C‐H. 2004. Novel efficient routes to heparin monosaccharides and disaccharides achieved via regio‐ and stereoselective glycosidation. Org Lett 6: 723–726. [DOI] [PubMed] [Google Scholar]
- Yuen C‐T, Storring PL, Tiplady RJ, Izquierdo M, Wait R, Gee CK, Gerson P, Lloyd P, Cremata JA. 2003. Relationships between the N‐glycan structures and biological activities of recombinant human erythropoietins produced using different culture conditions and purification procedures. Br J Haematol 121: 511–526. [DOI] [PubMed] [Google Scholar]
- Zabet‐Moghaddam M, Heinzle E, Tholey A. 2004. Qualitative and quantitative analysis of low molecular weight compounds by ultraviolet matrix‐assisted laser desorption/ionization mass spectrometry using ionic liquid matrices. Rapid Commun Mass Spectrom 18: 141–148. [DOI] [PubMed] [Google Scholar]
- Zähringer U, Lindner B, Knirel YA, van den Akker WMR, Hiestand R, Heine H, Dehio C. 2004. Structure and biological activity of the short‐chain lipopolysaccharide from Bartonella henselae ATC 49882T. J Biol Chem 279: 21046–21054. [DOI] [PubMed] [Google Scholar]
- Zaia J. 2004. Mass spectrometry of oligosaccharides. Mass Spectrom Rev 23: 161–227. [DOI] [PubMed] [Google Scholar]
- Zamfir A, Peter‐Katalinic J. 2004. Capillary electrophoresis‐mass spectrometry for glycoscreening in biomedical research. Electrophoresis 25: 1949–1963. [DOI] [PubMed] [Google Scholar]
- Zamyatina A, Sekljic H, Brade H, Kosma P. 2004. Synthesis and purity assessment of tetra‐ and pentaacyl lipid A of Chlamydia containing (R)‐3‐hydroxyicosanoic acid. Tetrahedron 60: 12117–12141. [Google Scholar]
- Zeng Y, Kong F. 2004. Synthesis of a hexasaccharide fragment of group E streptococci polysaccharide and the tetrasaccharide repeating unit of E. coli O7: K98:H6. Carbohydr Res 339: 1503–1510. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong F. 2003a. An efficient synthesis of a hexasaccharide—The repeating unit of the exopolysaccharide from Cryptococcus neoformans serovar A. Tetrahedron Lett 44: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong F. 2003b. Facile syntheses of the hexasaccharide repeating unit of the exopolysaccharide from Cryptococcus neoformans serovar A. Bioorg Med Chem 11: 4027–4037. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong F. 2003c. Synthesis of a hexasaccharide, the repeating unit of O‐deacetylated GXM of C. neoformans serotype A. Carbohydr Res 338: 1719–1725. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong F. 2003d. A general method for the synthesis of oligosaccharides consisting of α‐(1 → 2)‐ and α‐(1 → 3)‐linked rhamnan backbones and GlcNAc side chains. Tetrahedron 59: 1429–1441. [Google Scholar]
- Zhang H, Li X, Martin DB, Aebersold R. 2003a. Identification and quantification of N‐linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol 21: 660–666. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dyachokva E, Ha T‐K, Knochenmuss R, Zenobi R. 2003b. Gas‐phase potassium binding energies of MALDI matrices: An experimental and theoretical study. J Phys Chem A 107: 6891–6900. [Google Scholar]
- Zhang N, Torrelles JB, McNeil MR, Escuyer VE, Khoo K‐H, Brennan PJ, Chatterjee D. 2003c. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N‐terminal recognition region and a C‐terminal synthetic region. Mol Microbiol 50: 69–76. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cocklin RR, Bidasee KR, Wang M. 2003d. Rapid determination of advanced glycation end products of proteins using MALDI‐TOF‐MS and PERL script peptide searching algorithm. J Biomol Tech 14: 224–230. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Iwasaki H, Wang H, Kudo T, Kalka TB, Hennet T, Kubota T, Cheng L, Inaba N, Gotoh M, Togayachi A, Guo J, Hisatomi H, Nakajima K, Nishihara S, Nakamura M, Marth JD, Narimatsu H. 2003e. Cloning and characterization of a new human UDP‐N‐acetyl‐α‐D‐galactosamine: Polypeptide N‐acetylgalactosaminyltransferase, designated pp‐GalNAc‐T13, that is specifically expressed in neurons and synthesizes GalNA‐serine/threonine antigen. J Biol Chem 278: 573–584. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lindsay LL, Hedrick JL, Lebrilla CB. 2004a. Strategy for profiling and structure elucidation of mucin‐type oligosaccharides by mass spectrometry. Anal Chem 76: 5990–6001. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xie Y, Hedrick JL, Lebrilla CB. 2004b. Profiling the morphological distribution of O‐linked oligosaccharides. Anal Biochem 334: 20–35. [DOI] [PubMed] [Google Scholar]
- Zhou H, Groves JT. 2003. Hemodextrin: A self‐assembled cyclodextrin‐porphyrin construct that binds dioxygen. Biophys Chem 105: 639–648. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Park S‐H, Boucher S, Higgins E, Lee K, Edmunds T. 2004. N‐linked oligosaccharide analysis of glycoprotein bands from isoelectric focusing gels. Anal Biochem 335: 10–16. [DOI] [PubMed] [Google Scholar]
- Zhu X, Schmidt RR. 2003. Synthesis of α‐S‐linked glycopeptides in water containing solution. Tetrahedron Lett 44: 6063–6067. [Google Scholar]
- Zhu S, Hanneman A, Reinhold VN, Spence AM, Schachter H. 2004a. Caenorhabditis elegans triple null mutant lacking UDP‐N‐acetyl‐D‐glucosamine:α‐3‐D‐mannoside β1,2‐N‐acetylglucosaminyltransferase. Biochem J 382: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S‐H, Shimokawa S, Tanaka H, Shoyama Y. 2004b. Development of an assay system for saikosaponin a using anti‐saikosaponin a monoclonal antibodies. Biol Pharm Bull 27: 66–71. [DOI] [PubMed] [Google Scholar]
- Zhu X, Haag T, Schmidt RR. 2004c. Synthesis of an S‐linked glycopeptide analog derived from human Tamm‐Horsfall glycoprotein. Org Biomol Chem 2: 31–33. [DOI] [PubMed] [Google Scholar]
- Zhu X, Pachamuthu K, Schmidt RD. 2004d. Synthesis of novel S‐neoglycopeptides from glycosylthiomethyl derivatives. Org Lett 6: 1083–1085. [DOI] [PubMed] [Google Scholar]


