Abstract
Canine parvovirus type 2 (CPV‐2) is an important pathogen causing haemorrhagic enteritis in domestic dogs and wildlife worldwide. In early 2000, canine parvovirus type 2c (CPV‐2c) was first reported and subsequently became a predominant subtype circulating in Europe and the Americas. CPV‐2c has also been reported in Asia, including cases in China, India, Taiwan and Vietnam. However, CPV‐2c has never been reported in Thailand. In this study, we conducted viral enteric disease surveillance in dogs and cats in Thailand during 2016–2018. During 20 months of surveillance, 507 rectal swab samples were collected from dogs (n = 444) and cats (n = 63) with and without clinical signs. The samples were examined for parvovirus by using VP2 gene‐specific PCR for parvovirus. Our results showed that the positivity of canine parvovirus (CPV) was 29.95% and that of feline parvovirus (FPV) was 58.73%. In this study, we characterized 34 parvoviruses by VP2 gene sequencing. Moreover, two Thai‐CPV‐2 (Dog/CU‐24 and Cat/CU‐21) were characterized by whole genome sequencing. The phylogenetic results showed that Thai‐CPV‐2 had the highest nucleotide identities and clustered with Asian‐CPV‐2c but were in separate subclusters from the North American and European CPV‐2c. Similarly, whole genome analyses showed that Thai‐CPVs are closely related to Asian‐CPV‐2c, with unique amino acids at positions 297A, 324I, 370R and 426E. In summary, our results demonstrated the emergence of Asian‐CPV‐2c in dogs and cats in Thailand. Thus, the surveillance of CPV‐2 in domestic dogs and cats should be further conducted on a larger scale to determine the dynamics of predominant variants and their distributions in the country and in the Southeast Asia region.
Keywords: canine parvovirus, characterization, detection, emergence, Thailand
1. INTRODUCTION
Canine parvovirus type 2 (CPV‐2) is an important pathogen for domestic dogs and wildlife worldwide. CPV‐2, a non‐envelop, single‐stranded DNA virus, belongs to the family Parvoviridae. CPV‐2 causes acute haemorrhagic enteritis and myocarditis in dogs with high morbidity and frequent mortality (ranging 10%–90%). In 1977, it was first reported that CPV‐2 arose from feline panleukopenia virus (FPV) with at least six coding nucleotide differences in the VP2 gene. CPV‐2 can be further grouped into three antigenic variants, including CPV‐2a, CPV‐2b and CPV‐2c, based on unique amino acid residues at the positions 297 and 426 of VP2 (Buonavoglia et al., 2001). CPV‐2a and CPV‐2b were reported in 1979 and 1984, with unique amino acid residues as 426N and 426D, respectively. Both CPV‐2a and CPV‐2b variants are distributed worldwide and infect both dogs and cats but exhibit low pathogenicity in cats (Clegg et al., 2012). In 1990, CPV‐2a and CPV‐2b were replaced by two new variants of CPV‐2a (CPV‐2a‐297A) and CPV‐2b (CPV‐2b‐297A), with one unique amino acid substitution, S297A (Decaro et al., 2009). In 2000, CPV‐2c was first reported in Italy with one substitution at the VP2 gene (D426E) (Buonavoglia et al., 2001). Recently, CPV‐2c has been circulating predominantly in Europe and the Americas (Decaro & Buonavoglia, 2012). CPV‐2c has also been reported in Asia, including cases in China, India, Taiwan and Vietnam (Chiang, Wu, Chiou, Chang, & Lin, 2016; Nakamura et al., 2004; Nandi, Chidri, Kumar, & Chauhan, 2010; Zhao et al., 2016). It has also been reported that CPV‐2c can cause severe diseases in cats (Miranda, Parrish, & Thompson, 2014; Nakamura et al., 2001). In Thailand, CPV‐2a and CPV‐2b have been reported as major variants circulating in dogs (Phromnoi, Sirinarumitr, & Sirinarumitr, 2010), while CPV‐2c has never been reported in the country. In this study, CPV‐2c was detected in domestic dogs and cats during a viral enteric disease surveillance. This study is the first to report and characterize an emergence of Asian‐CPV‐2c in domestic dogs and cats in Thailand.
2. MATERIALS AND METHODS
From September 2016 to April 2018, the centre of excellence for emerging and re‐emerging infectious diseases in animals (CUEIDAs), Chulalongkorn University, conducted a viral enteric disease surveillance of domestic dogs and cats in Thailand. The surveillance was carried out in four provinces of Thailand under the animal use and care protocol # 1731074. Rectal swab samples were mainly collected from dogs and cats with acute haemorrhagic or watery diarrhoea, vomiting, fever and dehydration. During 20 months of surveillance, 507 rectal swab samples were collected from dogs (n = 444) and cats (n = 63) of young age (<1 year), adult (1–5 years) and older (>5 years) with vaccination history records. Of 444 canine samples, 366 samples from sick dogs and 78 from healthy dogs were collected. Of 63 feline samples, 60 samples from sick cats and three from healthy animals were collected. All samples were subjected to parvovirus identification by PCR specific to the VP2 gene, as previously described (Buonavoglia et al., 2001).
For parvovirus identification, viral DNA was extracted from rectal swab samples by using the QIAsymphony DSP viral/Pathogen mini kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. The viral DNA was stored at −20°C until used. PCR assay for parvovirus identification was conducted as previously described (Buonavoglia et al., 2001). The oligonucleotide primers specific to the VP2 gene were Hfor: 5′‐CAGGTGATGAATTGCTACA‐3′ and Hrev: 5′‐CATTTGGATAAACTGGTG GT‐3′, located at positions 3556–3575 and 4166–4185 of CPV‐2, respectively. In brief, PCR was performed in a final volume of 20 μl comprising 1 μl of DNA, 0.8 μM of each forward and reverse primer, 1× TopTaq Master Mix (Qiagen, Hilden, Germany), 1× CoralLoad, and distilled water. The PCR condition was set as initial denaturation step at 94°C for 3 min 40 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 45 s and extension at 72°C for 1 min and final extension at 72°C for 7 min. The expected size of parvovirus positive amplified product was 611 bp. Identification of CPV2 antigenic variants was performed by using PCR‐RFLP to differentiate CPV‐2c and CPV‐2a/CPV‐2b variants. The PCR product size was 583 bp of the coding capsid protein VP2. Then, the PCR product was digested with enzyme Mbo II (New England Biolabs, USA) that selectively recognizes the restriction site “GAAGA” (nucleotide 4062–4066 of the VP2 encoding gene). The CPV‐2c was digested into two fragments of 500 bp and 83 bp (Buonavoglia et al., 2001). The negative samples from CPV‐2c PCR‐RFLP assay were detected for CPV‐2a and CPV‐2b variants with specific primers (CPV‐2abF/CPV‐2abR and CPV‐2bF/CPV‐2bR) generating the product size of 681 bp and 427 bp, respectively (Pereira, Leal, & Durigon, 2007; Pereira, Monezi, Mehnert, D'Angelo, & Durigon, 2000) (Table S1). Concurrently, the CPV‐2a/CPV‐2b samples were confirmed by sequencing of the flanking region at amino acid position 426 to identify CPV‐2a or CPV‐2b variants.
For parvovirus characterization, we selected two parvoviruses (Dog/CU‐24 and Cat/CU‐21) for whole genome sequencing and the other 32 parvoviruses (CPV‐2 = 21, FPV = 11) for VP2 gene sequencing. The criteria for selecting these 34 viruses for genetic characterization were based on epidemiological and demographic data, such as age of dog, date of isolation, breed and vaccination history. The selection criteria for the two viruses for whole genome sequencing were based on the representatives of CPV‐2c from dogs (CU‐24) and cats (CU‐21). Parvovirus genome sequencing was conducted by using oligonucleotide primer sets previously described or new primer sets designed using the Primer 3 plus program (Table S1) (Buonavoglia et al., 2001; Koressaar & Remm, 2007; Untergasser et al., 2012). In brief, PCR was performed in a final volume of 30 μl comprising 2 μl of DNA, 0.4 μM of each forward and reverse primer, 1× TopTaq Master Mix, 1× CoralLoad, and distilled water. The PCR condition was set as initial denaturation at 94°C for 3 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 45 s, extension at 72°C for 2 min and final extension at 72°C for 7 min. PCR products were then purified and sequenced (1st Base Laboratories Sdn Bhd, Malaysia). Nucleotide sequences were assembled by using SeqMan software v.5.03 (DNASTAR Inc., Madison, WI).
For genetic analysis, pairwise comparison was conducted by using MegAlign software v.5.03 (DNASTAR Inc.). In brief, the nucleotide sequences and deduced amino acids of Thai‐CPV‐2 and FPV were aligned with those of vaccine and reference strains of CPV2‐a, CPV‐2b, CPV‐2c, CPV‐2a‐297A, CPV‐2b‐297A from the USA (CPV‐13/1981, CPV‐411b/1998, OH20219/2015), Japan (Y1), China (SC‐02/2011), India (KolkataD5/2014), Indonesia (HCM14/2013), Italy (288‐01/2001, 1‐99/1999), Vietnam (HCM7/2013) and Thailand (KU14/2008). Genetic analysis for CPV‐2 antigenic typing (VP2 at positions 297 and 426) and important amino acid determinants (VP2 at positions 300, 305, 321, 323, 324, 370, 371, 375) was conducted by the alignment of VP2 by using MEGA v6.06 and MegAlign software v.5.03 (DNASTAR Inc.). For the phylogenetic analysis, the partial VP2 gene sequences of Thai‐CPV‐2 and FPV were analysed with those of reference viruses. Vaccine and reference viruses, including CPV‐2‐vaccine strains (n = 3), CPV‐2 (n = 2), CPV‐2a (n = 3), CPV‐2b (n = 3), CPV‐2c (n = 14), CPV‐2a‐297A (n = 11), CPV‐2b‐297A (n = 7), FPV vaccine (n = 3), FPV‐G1 (n = 9), FPV‐G2 (n = 1) and FPV‐G3 (n = 3), were included in the phylogenetic analysis. The maximum clade credibility (MCC) tree of partial VP2 gene was constructed by BEAST 1.8 with the Bayesian Markov‐Chain Monte Carlo (BMCMC) algorithm. A strict clock model with coalescent constant population and HKY with gamma 4 substitution were used as model parameters (Drummond, Suchard, Xie, & Rambaut, 2012). The Bayesian MCMC chain lengths were 10,000,000 generations, with sampling every 10,000 generations. The tree iteration was discharged with 10% of the chains as burn‐in pattern by using a tree annotator, and the resulting MCC tree was drawn with FigTree software (v1.4.2) (Molecular evolution, phylogenetics and epidemiology, Edinburgh, Scotland, UK) (Figure 1). To determine the selective pressure on the partial VP2 (nucleotide positions 817–1314, amino acid positions 274–428), the ratio of non‐synonymous (dN) to synonymous (dS) substitutions was estimated using Mixed Effects Model of Evolution (MEME) within the HyPhy software package (Murrell et al., 2012). The significance levels were set at p = 0.1. The values dN/dS > 1, dN/dS = 1 and dN/dS < 1 were used to define positive selection, neutral mutations, and negative selection, respectively. A phylogenetic tree was also constructed by using maximum‐likelihood with bootstrap analysis of 1,000 replications using the MEGA v.6.06 program (Tamura, Dudley, Nei, & Kumar, 2007) (Figure S1).
Figure 1.
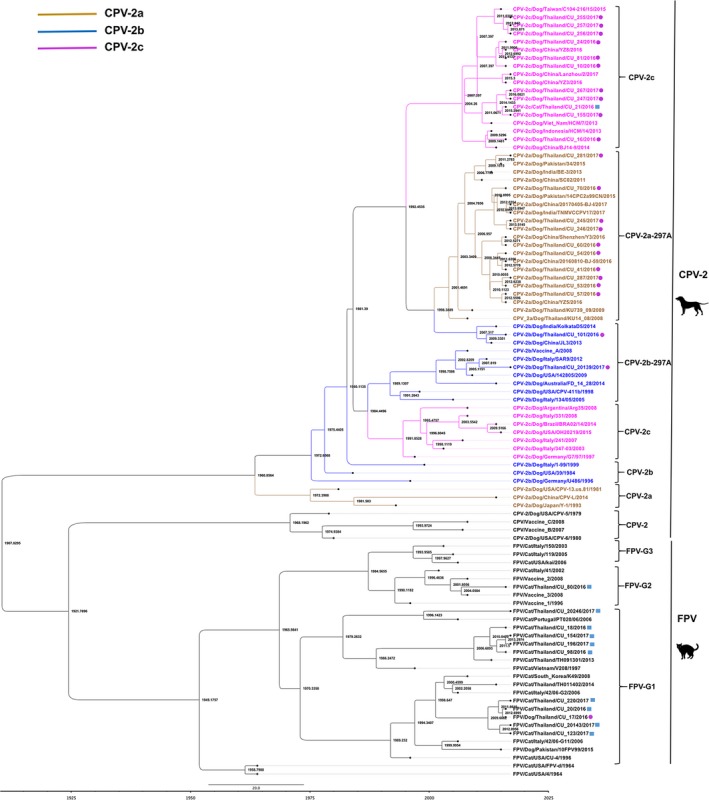
Phylogenetic tree of VP2 gene of canine parvovirus type 2 and feline parvovirus. Circles and squares represent Thai‐CPV‐2 and FPV, respectively. The phylogenetic tree was constructed by using the Beast program with Bayesian Markov‐Chain Monte Carlo (BMCMC), with 10,000,000 generations and an average standard deviation of split frequencies <0.10. Values on branches represent times of most recent common ancestor (TMRCA) among CPV‐2 antigenic types [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
3. RESULTS
From September 2016 to April 2018, a viral enteric disease surveillance of domestic dogs and cats was conducted in four provinces of Thailand. Of 444 canine samples and 63 feline samples subjected to parvovirus identification, the positivity of CPV‐2 in dogs was 29.95% (133/444) and that of FPV in cats was 58.73% (37/63), which were high in non‐vaccinated animals (44.59%). Moreover, animals of young age (<1 year) were more frequently infected with CPV‐2 (45.96%) (Tables 1 and 2). In this study, all samples were also examined for other important enteric viruses, including canine rotavirus (CRV) and canine coronavirus (CoV). We found coinfection of CPV‐2 and CRV (n = 1) as well as CPV‐2 and CoV (n = 22) in dogs. Additionally, coinfection of FPV and CoV was observed in two cats (data not shown).
Table 1.
Association between age and clinical presentations of CPV‐2 and FPV detection in this study
| Age | Dogs | Cats | ||
|---|---|---|---|---|
| CPV‐2 positive (%) | FPV positive (%) | |||
| Asymptomatic | Clinical sign | Asymptomatic | Clinical sign | |
| Young (<1 year) | 0/12 (0%) | 91/198 (45.96%) | 2/3 (66.67%) | 28/47 (59.57%) |
| Adult (1–5 years) | 3/63 (4.76%) | 23/104 (22.12%) | 0/0 (0%) | 6/11 (54.55%) |
| Older (>5 years) | 0/3 (0%) | 16/64 (25.00%) | 0/0 (0%) | 1/2 (50.00%) |
| 3/78 (3.84%) | 130/366 (35.52%) | 2/3 (66.67%) | 35/60 (58.33%) | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Association between vaccine history and clinical presentations of CPV‐2 and FPV detection in this study
| Vaccine history | Dogs | Cats | ||
|---|---|---|---|---|
| CPV‐2 positive (%) | FPV positive (%) | |||
| Asymptomatic | Clinical sign | Asymptomatic | Clinical sign | |
| Non‐vaccination | 0/67 (0%) | 103/231 (44.59%) | 2/3 (66.67%) | 34/53 (64.15%) |
| Completed | 3/11 (27.27%) | 27/135 (20.00%) | 0/0 (0%) | 1/7 (14.29%) |
| 3/78 (3.85%) | 130/366 (35.52%) | 2/3 (66.67%) | 35/60 (58.33%) | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In this study, we identified antigenic types of CPV‐2 as CPV‐2c (n = 62; 46.61%), CPV‐2a (n = 68; 51.13%) and CPV‐2b (n = 3; 2.26%) (Table S2). It is noted that both CPV‐2c and CPV‐2a were predominant variants and CPV‐2c has never been reported in Thailand. In this study, we selected 34 parvoviruses for genetic characterization. For CPV‐2, the viruses were subjected to VP2 gene (n = 21) and whole genome sequencing (n = 2; Dog/CU‐24 and Cat/CU‐21). For FPV, the viruses were subjected to VP2 gene sequencing (n = 11). The nucleotide sequences of the parvoviruses were submitted to the GenBank database under accession no. MH711880–MH711913 (Table 3). Pairwise comparisons of nucleotide and deduced amino acid sequences of Thai viruses were performed against those of vaccine and reference strains. Our results showed that the whole genomes of two Thai‐CPV‐2 (Dog/CU‐24 and Cat/CU‐21) had 99.90% nucleotide identity to each other and the highest nucleotide identities to Vietnam CPV‐2c (99.60% at WG, 99.90% at VP2) (Table S3). Within Thai‐CPV‐2, the VP2 gene is diverse with nucleotide identities of 99.80%–100% (CPV‐2c), 99.00%–99.20% (CPV‐2b‐297A) and 98.80%–99.00% (CPV‐2a‐297A) (Table S4). In this study, the overall dN/dS ratio for the partial VP2 of CPV‐2 and FPV was lower than 1 (0.296, 0.032), implying that the gene was under negative selection or purifying selection as the main evolutionary force.
Table 3.
Detailed descriptions of CPV‐2 and FPV characterized in this study
| Virus | Breed | Age of animal | Vaccine history | Clinical sign | Collection date | Location | Type of CPV/FPV | GenBank # |
|---|---|---|---|---|---|---|---|---|
| CPV | ||||||||
| Dog/Thailand/CU‐41/2016 | Mixed | 2 years | C | Asymptomatic | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711880 |
| Dog/Thailand/CU‐53/2016 | Pomeranian | 2 months | I | Diarrhoea | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711881 |
| Dog/Thailand/CU‐54/2016 | Yorkshire terrier | 1 years | C | Diarrhoea | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711882 |
| Dog/Thailand/CU‐57/2016 | Pomeranian | 2 months | I | Diarrhoea | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711883 |
| Dog/Thailand/CU‐60/2016 | Pomeranian | 2 months | I | Diarrhoea | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711884 |
| Dog/Thailand/CU‐70/2016 | Siberian husky | 4 months | I | Diarrhoea | Oct‐16 | Bangkok | CPV‐2a‐297A | MH711885 |
| Dog/Thailand/CU‐245/2017 | Mixed | 2 months | I | Diarrhoea | Apr‐17 | Bangkok | CPV‐2a‐297A | MH711886 |
| Dog/Thailand/CU‐246/2017 | Beagle | 2 months | I | Diarrhoea | Apr‐17 | Bangkok | CPV‐2a‐297A | MH711887 |
| Dog/Thailand/CU‐281/2017 | Mixed | 3 months | I | Diarrhoea | Sep‐17 | Bangkok | CPV‐2a‐297A | MH711888 |
| Dog/Thailand/CU‐287/2017 | Mixed | 1 year | I | Diarrhoea | Sep‐17 | Bangkok | CPV‐2a‐297A | MH711889 |
| Dog/Thailand/CU‐101/2016 | Pekingese | 2 months | I | Diarrhoea | Dec‐16 | Bangkok | CPV‐2b‐297A | MH711890 |
| Dog/Thailand/CU‐20139/2017 | Beagle | 2 months | I | Diarrhoea | Nov‐17 | Bangkok | CPV‐2b‐297A | MH711891 |
| Dog/Thailand/CU‐10/2016 | Beagle | 2 years | C | Asymptomatic | Sep‐16 | Bangkok | CPV‐2c | MH711892 |
| Dog/Thailand/CU‐16/2016 | Shih Tzu | 2 months | I | Diarrhoea | Sep‐16 | Bangkok | CPV‐2c | MH711893 |
| Dog/Thailand/CU‐24/2016 | Mixed | 2 years | C | Asymptomatic | Oct‐16 | Bangkok | CPV‐2c | MH711894 a |
| Dog/Thailand/CU‐81/2016 | Chihuahua | 2 months | I | Diarrhoea | Nov‐16 | Bangkok | CPV‐2c | MH711895 |
| Dog/Thailand/CU‐155/2017 | Pomeranian | 6 months | I | Diarrhoea | Jan‐17 | Bangkok | CPV‐2c | MH711896 |
| Dog/Thailand/CU‐247/2017 | Jack Russell | 2 months | I | Diarrhoea | Apr‐17 | Bangkok | CPV‐2c | MH711897 |
| Dog/Thailand/CU‐255/2017 | German Shepherd | 2 months | I | Diarrhoea | Jun‐17 | N.Ratchasima | CPV‐2c | MH711898 |
| Dog/Thailand/CU‐256/2017 | German Shepherd | 2 months | I | Diarrhoea | Jun‐17 | N.Ratchasima | CPV‐2c | MH711899 |
| Dog/Thailand/CU‐257/2017 | German Shepherd | 2 months | I | Diarrhoea | Jun‐17 | N.Ratchasima | CPV‐2c | MH711900 |
| Dog/Thailand/CU‐267/2017 | Mixed | 4 months | I | Diarrhoea | Jul‐17 | Tak | CPV‐2c | MH711901 |
| Cat/Thailand/CU‐21/2016 | Mixed | 5 months | I | Diarrhoea | Oct‐16 | Bangkok | CPV‐2c | MH711902 a |
| FPV | ||||||||
| Cat/Thailand/CU‐80/2016 | Mixed | 6 months | I | Diarrhoea | Nov‐16 | Bangkok | FPV‐G2 | MH711903 |
| Cat/Thailand/CU‐18/2016 | Mixed | 5 months | I | Diarrhoea | Sep‐16 | Bangkok | FPV‐G1 | MH711904 |
| Cat/Thailand/CU‐20/2016 | Mixed | 5 months | I | Diarrhoea | Sep‐16 | Bangkok | FPV‐G1 | MH711905 |
| Cat/Thailand/CU‐98/2016 | Mixed | 2 months | I | Diarrhoea | Dec‐16 | Bangkok | FPV‐G1 | MH711906 |
| Cat/Thailand/CU‐123/2017 | Mixed | 9 months | I | Asymptomatic | Jan‐17 | Chiang mai | FPV‐G1 | MH711907 |
| Cat/Thailand/CU‐154/2017 | Mixed | 3 months | I | Diarrhoea | Jan‐17 | Bangkok | FPV‐G1 | MH711908 |
| Cat/Thailand/CU‐196/2017 | Mixed | 1 year | I | Diarrhoea | Feb‐17 | Bangkok | FPV‐G1 | MH711909 |
| Cat/Thailand/CU‐220/2017 | Mixed | 3 months | I | Diarrhoea | Feb‐17 | Bangkok | FPV‐G1 | MH711910 |
| Cat/Thailand/CU‐20143/2017 | Mixed | 2 months | I | Diarrhoea | Nov‐17 | Bangkok | FPV‐G1 | MH711911 |
| Cat/Thailand/CU‐20246/2018 | Mixed | 5 months | I | Diarrhoea | Jan‐18 | Bangkok | FPV‐G1 | MH711912 |
| Dog/Thailand/CU‐17/2016 | Labrador retriever | 13 years | C | Diarrhoea | Sep‐16 | Bangkok | FPV‐G1 | MH711913 |
Whole genome sequence.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Phylogenetic analysis of the VP2 gene from Thai‐CPV‐2 showed that the viruses were clustered with CPV‐2c, CPV‐2a‐297A and CPV‐2b‐297A. The phylogenetic analysis indicated that Thai‐CPV‐2c was closely related to VietNam‐HCM7, Chinese‐YZ‐8, BJ14‐9, Taiwan‐C104 and Indonesia‐HCM but was in separate subclusters from the North American and European CPV‐2c (Figure 1 and Figure S1). Based on the MCC tree, the Asian‐CPV‐2c was estimated to separate from CPV‐2C of America and Europe since 1981. While, Thai‐CPV‐2c was started to evolved from other Asian‐CPV‐2c viruses (China, Taiwan, Vietnam and Indonesia) since 2004. The estimated nucleotide substitution rate of the partial VP2 was 1.1905 × 10−4 substitutions per site per year. 95% highest posterior densities (HPD) was 6.9511 × 10−5–1.6877 × 10−4). It is noted that the new variant CPV‐2b‐297A (n = 2) was clustered in a separate group in which one isolate (Dog/CU‐20139) was closely related to the vaccine strain (CPV‐2b/Vaccine), suggesting a virus of vaccine origin. The phylogenetic analysis of the VP2 gene of FPV was also performed, showing that Thai‐FPV was predominantly clustered with FPV‐G1 (n = 10), including one canine isolate (Dog/CU‐17). In contrast, one Thai‐FPV (Dog/CU‐80) was grouped in a distinct cluster (G2) with FPV vaccine strains (Figure S1). It is interesting to note that one dog isolate was clustered with FPV‐G1, suggesting FPV infection in a dog.
Genetic analyses of the genomes of Thai‐CPV‐2 and FPV were also conducted (Table 4). CPV‐2a, CPV‐2b and CPV‐2c variants were determined by genetic differences at VP2 position 426 as Asn (N), Asp (D) and Glu (E), respectively (Martella, Decaro, & Buonavoglia, 2006). In this study, the new variants CPV‐2a‐297A and CPV‐2b‐297A, had unique amino acids at positions 297A, 426N and 426D, which were also observed in reference viruses. Similarly, Thai‐CPV‐2c contained unique amino acids at positions 297A and 426E, which were observed in reference CPV‐2c. It is important to note that unique amino acid substitutions at positions Y324I and Q370R were only observed in the Asian strain CPV‐2c (VietNam‐HCM7, Chinese‐YZ‐8, BJ14‐9, Taiwan‐C104 and Indonesia‐HCM), including Thai‐CPV‐2c, but were not observed in American and European CPV2‐c (Table 4 and Figure 2).
Table 4.
Genetic analysis of deduced amino acids of Thai‐CPV‐2 and FPV in comparison to those of vaccine and reference strains
| Strain | Accession number | Year | Country | Amino acid position of VP2 gene | Type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typing | Important amino acids | |||||||||||||
| 297 | 426 | 300a | 305a | 321 | 323b | 324 | 370c | 371 | 375 | |||||
| Reference CPV | ||||||||||||||
| CPV‐2/Dog/USA/CPV‐5/1979 | EU659116 | 1979 | USA | S | N | A | D | N | N | Y | Q | A | N | CPV‐2 |
| CPV‐2/Dog/USA/CPV‐6/1980 | EU659117 | 1980 | USA | S | N | A | D | N | N | Y | Q | A | N | CPV‐2 |
| CPV‐2/Vaccine B (Nobivac;Intervet) | FJ197846 | 2007 | South Korea | S | N | A | D | N | N | Y | Q | A | N | CPV‐2/Vaccine |
| CPV‐2/Vaccine C (Vaccine06;Merial) | FJ222822 | N/A | N/A | A | D | G | Y | K | N | Y | Q | A | D | CPV‐2/Vaccine |
| CPV‐2a/Dog/USA/CPV‐13/1981 | EU659118 | 1981 | USA | S | N | G | Y | N | N | Y | Q | A | D | CPV‐2a |
| CPV‐2a/Dog/Japan/Y1/xxxx | D26079 | N/A | Japan | S | N | G | Y | N | N | Y | Q | A | D | CPV‐2a |
| CPV‐2a/Dog/Thailand/KU14/2008 | GQ379043 | 2008 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/China/SC02/2011 | JX660690 | 2011 | China | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2b/Dog/Italy/1‐99/1999 | MF177226 | 1999 | Italy | S | D | G | Y | N | N | Y | Q | A | D | CPV‐2b |
| CPV‐2b/Dog/USA/CPV‐411b/1998 | EU659121 | 1998 | USA | A | D | G | Y | N | N | Y | Q | A | D | CPV‐2b‐297A |
| CPV‐2b/Dog/India/KolkataD5/2014 | KP071953 | 2014 | India | A | D | G | Y | N | N | I | Q | A | D | CPV‐2b‐297A |
| CPV‐2b/Vaccine A (Duramune;Fort Dodge) | FJ222822 | N/A | N/A | A | D | G | Y | K | N | Y | Q | A | D | CPV‐2b/Vaccine |
| CPV‐2c/Dog/Italy/288‐01/2001 | MF177239 | 2001 | Italy | A | E | G | Y | N | N | Y | Q | A | D | CPV‐2c |
| CPV‐2c/Dog/USA/OH20219/2015 | MF457594 | 2015 | USA | A | E | G | Y | N | N | Y | Q | A | D | CPV‐2c |
| CPV‐2c/Dog/Vietnam/HCM/7/2013 | LC214969 | 2013 | Vietnam | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Indonesia/HCM/14/2013 | LC216909 | 2013 | Indonesia | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Taiwan/C104‐216/2015 | KX421787 | 2015 | Taiwan | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| This study: CPV | ||||||||||||||
| CPV‐2a/Dog/Thailand/CU 41/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 53/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 54/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 57/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 60/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 70/2016 | This study | 2016 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 245/2017 | This study | 2017 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 246/2017 | This study | 2017 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 281/2017 | This study | 2017 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2a/Dog/Thailand/CU 287/2017 | This study | 2017 | Thailand | A | N | G | Y | N | N | I | Q | A | D | CPV‐2a‐297A |
| CPV‐2b/Dog/Thailand/CU 101/2016 | This study | 2016 | Thailand | A | D | G | Y | N | N | I | Q | A | D | CPV‐2b‐297A |
| CPV‐2b/Dog/Thailand/CU 20139/2017 | This study | 2017 | Thailand | A | D | G | Y | K | N | Y | Q | A | D | CPV‐2b‐297A |
| CPV‐2c/Dog/Thailand/CU 10/2016 | This study | 2016 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 16/2016 | This study | 2016 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 24/2016 | This study | 2016 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 81/2016 | This study | 2016 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 155/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 247/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 255/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 256/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 257/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Dog/Thailand/CU 267/2017 | This study | 2017 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2c |
| CPV‐2c/Cat/Thailand/CU 21/2016 | This study | 2016 | Thailand | A | E | G | Y | N | N | I | R | A | D | CPV‐2ca |
| Reference FPV | ||||||||||||||
| FPV/Cat/USA‐4/1964 | EU659112 | 1964 | USA | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Cat/USA/kai/2006 | EU659115 | 2006 | USA | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Cat/Italy/42/06‐G2/2006 | EU498698 | 2006 | Italy | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Cat/Thailand/TH011402/2014 | KT357494 | 2014 | Thailand | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Dog/Pakistan/10FPV99/2015 | MF182903 | 2015 | Pakistan | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Vaccine 1 (PLI‐IV) | D88287 | N/A | N/A | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Vaccine 2 (Purevax;Merial) | EU498680 | N/A | N/A | S | N | A | D | N | D | Y | Q | A | D | |
| FPV/Vaccine 3 (Felocell;Pfizer) | EU498681 | N/A | N/A | S | N | A | D | N | D | Y | Q | A | D | |
| This study: FPV | ||||||||||||||
| FPV/Cat/Thailand/CU 80/2016 | This study | 2016 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G2 |
| FPV/Cat/Thailand/CU 18/2016 | This study | 2016 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 20/2016 | This study | 2016 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 98/2016 | This study | 2016 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 123/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 154/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 196/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 220/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 20143/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Cat/Thailand/CU 20246/2017 | This study | 2017 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1 |
| FPV/Dog/Thailand/CU 17/2016 | This study | 2016 | Thailand | S | N | A | D | N | D | Y | Q | A | D | FPV‐G1b |
N/A:notavailable.
A300G, D305Y amino acid residue related to the adaptation of the CPV variants to the feline host (Ikeda et al., 2000; Truyen, Evermann, Vieler, & Parrish, 1996).
D323N amino acid residue related to specific canine host (binding with canine receptor (TfR) (Chang, Sgro, & Parrish, 1992; Govindasamy, Hueffer, Parrish, & Agbandje‐McKenna, 2003).
Q370R amino acid residue related to host range, novel Asian variant (Govindasamy et al., 2003).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
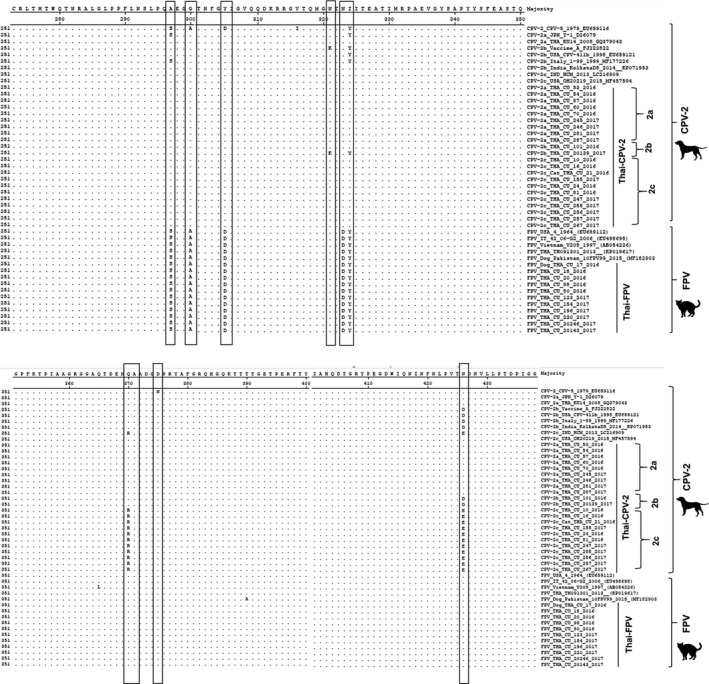
Amino acid alignment of VP2 protein of CPV‐2. Dots represent matched amino acid residues. Open boxes indicate amino acid substitutions
4. DISCUSSION
To our knowledge, this study is the first to report CPV‐2c in dogs and cats in Thailand. The infected animals showed clinical signs of acute haemorrhagic or watery diarrhoea. In this study, the positivity of CPV‐2 in dogs was 29.95% and that of FPV in cats was 58.73%, which were high in non‐vaccinated animals. This study also showed that CPV‐2 was predominantly detected in dogs of young age (<1 year). These results were similar to the previous report of CPV‐2 in puppies in Thailand (Sakulwira, Vanapongtipagorn, Theamboonlers, Oraveerakul, & Poovorawan, 2003). It is important to note that CPV‐2c could also be isolated from cats. Similar observations were also reported in other studies (Miranda et al., 2014; Nakamura et al., 2001). One FPV infection in a dog was observed, as also seen in a previous study of FPV infection in sick dogs in Pakistan in 2018 (Ahmed et al., 2018).
Nucleotide and amino acid comparison showed that the whole genomes of two Thai‐CPV‐2 strains had 99.90% nucleotide identity to each other and had highest nucleotide identities to Asian‐CPV‐2c from Vietnam. Similar studies reported Asian‐CPV‐2c in China and Taiwan (Chiang et al., 2016; Guo et al., 2013). Phylogenetic analysis showed that Thai‐CPV‐2c is closely related to Asian‐CPV‐2c, including VietNam‐HCM7, Chinese‐YZ‐8, BJ14‐9, Taiwan‐C104 and Indonesia‐HCM. These viruses were in separate subclusters from North American and European CPV‐2c. Our analysis suggested that the estimated time of the most recent common ancestor of Thai‐CPV‐2c subclusters was 2004 (Figure 1). The substitution rate of parvovirus in this study was in agreement with other studies (1.2 × 10−4–2.2 × 10−4 substitutions per site per year) (Hoelzer, Shackelton, Parrish, & Holmes, 2008; Pereira et al., 2007; Shackelton, Parrish, Truyen, & Holmes, 2005). Moreover, our data indicated that parvovirus (which is DNA virus) has high genomic substitution rate similar to other RNA viruses at approximately 10−4 substitutions per site per year (Duffy, Shackelton, & Holmes, 2008). Whole genome analysis indicated that Thai‐CPVs are closely related to Asian‐CPV‐2c with unique amino acids at position 297A, 370R and 426E of VP2, suggesting predominant Asian‐CPV‐2c in the country. It is also noted that unique amino acid substitutions at positions Y324I and Q370R were only observed in Asian strains of CPV‐2c. These unique amino acids (370R) might relate to receptor‐binding properties, suggesting species preference. Recent observations have also been reported in China and Taiwan (Chiang et al., 2016; Guo et al., 2013).
The identification of several types of CPV2 (CPV‐2c, new variant CPV‐2a‐297A, and new variant CPV‐2b‐297A) demonstrates diversity of CPV2 in Thailand. CPV‐2c is an emerging variant in the country and the Southeast Asia region. These findings will stimulate concern regarding whether currently used canine parvovirus vaccines will provide full protection against the new variant, Asian‐CPV‐2c. In summary, our results demonstrated the emergence of the new variant Asian‐CPV‐2c in dogs and cats in Thailand. Since cats can be infected with CPV‐2c, dogs can also be infected with FPV. Thus, veterinary practitioners should focus more attention on both CPV and FPV infections, especially interspecies transmission. In Thailand, the surveillance of CPV and FPV should be further conducted on a larger scale to determine the dynamics of predominant variants and their distributions. This information will aid early diagnosis and the development of future strategies for domestic animal vaccination.
Supporting information
ACKNOWLEDGEMENTS
We would like to thank the Royal Golden Jubilee (RGJ) Ph.D. program and the Thailand Research Fund for granting a scholarship to the first author (RGJ‐PHD/0056/2557). This project was financially supported by the research fund under the 90th Anniversary Chulalongkorn University (Ratchadaphiseksomphot Endowment Fund) (GCUGR1125614077D). Chulalongkorn University provided financial support to the Center of Excellence for Emerging and Re‐emerging Infectious Diseases in Animals. The Thailand Research Fund (TRF) supported the corresponding author as a TRF Senior Scholar (RTA6080012). We also thank the Overseas Academic Presentation Scholarship for Graduate Students, Chulalongkorn University.
Charoenkul K, Tangwangvivat R, Janetanakit T, et al. Emergence of canine parvovirus type 2c in domestic dogs and cats from Thailand. Transbound Emerg Dis. 2019;66:1518–1528. 10.1111/tbed.13177
REFERENCES
- Ahmed, N. , Riaz, A. , Zubair, Z. , Saqib, M. , Ijaz, S. , Nawaz‐Ul‐Rehman, M. S. , … Mubin, M. (2018). Molecular analysis of partial VP‐2 gene amplified from rectal swab samples of diarrheic dogs in Pakistan confirms the circulation of canine parvovirus genetic variant CPV‐2a and detects sequences of feline panleukopenia virus (FPV). Virology Journal, 15, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia, C. , Martella, V. , Pratelli, A. , Tempesta, M. , Cavalli, A. , Buonavoglia, D. , … Carmichael, L. (2001). Evidence for evolution of canine parvovirus type 2 in Italy. Journal of General Virology, 82, 3021–3025. [DOI] [PubMed] [Google Scholar]
- Chang, S. F. , Sgro, J. Y. , & Parrish, C. R. (1992). Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. Journal of Virology, 66(12), 6858–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, S. Y. , Wu, H. Y. , Chiou, M. T. , Chang, M. C. , & Lin, C. N. (2016). Identification of a novel canine parvovirus type 2c in Taiwan. Virology Journal, 13, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg, S. R. , Coyne, K. P. , Dawson, S. , Spibey, N. , Gaskell, R. M. , & Radford, A. D. (2012). Canine parvovirus in asymptomatic feline carriers. Veterinary Microbiology, 157, 78–85. [DOI] [PubMed] [Google Scholar]
- Decaro, N. , & Buonavoglia, C. (2012). Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Veterinary Microbiology, 155, 1518–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Desario, C. , Parisi, A. , Martella, V. , Lorusso, A. , Miccolupo, A. , … Buonavoglia, C. (2009). Genetic analysis of canine parvovirus type 2c. Virology, 385, 5–10. [DOI] [PubMed] [Google Scholar]
- Drummond, A. J. , Suchard, M. A. , Xie, D. , & Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution, 29, 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, S. , Shackelton, L. A. , & Holmes, E. C. (2008). Rates of evolutionary change in viruses: Patterns and determinants. Nature Reviews Genetics, 9, 267. [DOI] [PubMed] [Google Scholar]
- Govindasamy, L. , Hueffer, K. , Parrish, C. R. , & Agbandje-McKenna, M. (2003). Structures of host range-controlling regions of the capsids of canine and feline parvoviruses and mutants. Journal of Virology, 77(22), 12211–12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L. , Yang, S. L. , Chen, S. J. , Zhang, Z. , Wang, C. , Hou, R. , … Yan, Q. G. (2013). Identification of canine parvovirus with the Q370R point mutation in the VP2 gene from a giant panda (Ailuropoda melanoleuca). Virology Journal, 10, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer, K. , Shackelton, L. A. , Parrish, C. R. , & Holmes, E. C. (2008). Phylogenetic analysis reveals the emergence, evolution and dispersal of carnivore parvoviruses. The Journal of General Virology, 89, 2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y. , Mochizuki, M. , Naito, R. , Nakamura, K. , Miyazawa, T. , Mikami, T. , & Takahashi, E. (2000). Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology, 278(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Koressaar, T. , & Remm, M. (2007). Enhancements and modifications of primer design program Primer3. Bioinformatics, 23, 1289–1291. [DOI] [PubMed] [Google Scholar]
- Martella, V. , Decaro, N. , & Buonavoglia, C. (2006). Evolution of CPV‐2 and implication for antigenic/genetic characterization. Virus Genes, 33, 11–13. [DOI] [PubMed] [Google Scholar]
- Miranda, C. , Parrish, C. R. , & Thompson, G. (2014). Canine parvovirus 2c infection in a cat with severe clinical disease. Journal of Veterinary Diagnostic Investigation, 26, 462–464. [DOI] [PubMed] [Google Scholar]
- Murrell, B. , Wertheim, J. O. , Moola, S. , Weighill, T. , Scheffler, K. , & Kosakovsky Pond, S. L. (2012). Detecting individual sites subject to episodic diversifying selection. PLoS Genetics, 8, e1002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K. , Sakamoto, M. , Ikeda, Y. , Sato, E. , Kawakami, K. , Miyazawa, T. , … Mochizuki, M. (2001). Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clinical and Diagnostic Laboratory Immunology, 8, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, M. , Tohya, Y. , Miyazawa, T. , Mochizuki, M. , Phung, H. T. , Nguyen, N. H. , … Akashi, H. (2004). A novel antigenic variant of canine parvovirus from a Vietnamese dog. Archives of Virology, 149, 2261–2269. [DOI] [PubMed] [Google Scholar]
- Nandi, S. , Chidri, S. , Kumar, M. , & Chauhan, R. S. (2010). Occurrence of canine parvovirus type 2c in the dogs with haemorrhagic enteritis in India. Research in Veterinary Science, 88, 169–171. [DOI] [PubMed] [Google Scholar]
- Pereira, C. A. , Leal, E. S. , & Durigon, E. L. (2007). Selective regimen shift and demographic growth increase associated with the emergence of high‐fitness variants of canine parvovirus. Infection, Genetics and Evolution, 7, 399–409. [DOI] [PubMed] [Google Scholar]
- Pereira, C. A. , Monezi, T. A. , Mehnert, D. U. , D'Angelo, M. , & Durigon, E. L. (2000). Molecular characterization of canine parvovirus in Brazil by polymerase chain reaction assay. Veterinary Microbiology, 75, 127–133. [DOI] [PubMed] [Google Scholar]
- Phromnoi, S. , Sirinarumitr, K. , & Sirinarumitr, T. (2010). Sequence analysis of VP2 gene of canine parvovirus isolates in Thailand. Virus Genes, 41, 23–29. [DOI] [PubMed] [Google Scholar]
- Sakulwira, K. , Vanapongtipagorn, P. , Theamboonlers, A. , Oraveerakul, K. , & Poovorawan, Y. (2003). Prevalence of canine coronavirus and parvovirus infections in dogs with gastroenteritis in Thailand. Veterinární Medicína‐Czech, 48, 163–167. [Google Scholar]
- Shackelton, L. A. , Parrish, C. R. , Truyen, U. , & Holmes, E. C. (2005). High rate of viral evolution associated with the emergence of carnivore parvovirus. Proceedings of the National Academy of Sciences, 102, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Dudley, J. , Nei, M. , & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Truyen, U. , Evermann, J. F. , Vieler, E. , & Parrish, C. R. (1996). Evolution of canine parvovirus involved loss and gain of feline host range. Virology, 215(2), 186–189. [DOI] [PubMed] [Google Scholar]
- Untergasser, A. , Cutcutache, I. , Koressaar, T. , Ye, J. , Faircloth, B. C. , Remm, M. , & Rozen, S. G. (2012). Primer3–new capabilities and interfaces. Nucleic Acids Research, 40, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Liu, H. , Ding, K. , Peng, C. , Xue, Q. , Yu, Z. , & Xue, Y. (2016). Occurrence of canine parvovirus in dogs from Henan province of China in 2009–2014. BMC Veterinary Research, 12, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


