Abstract
A strain of porcine epidemic diarrhoea virus (PEDV), namely HLJBY, was isolated in Heilongjiang province, China. To provide insight into the understanding of the phylogenetic and the current epidemiological status of PEDV, PEDV HLJBY was compared with CV777 and other PEDV strains deposited in the GenBank. The homology between the entire genomic nucleotide sequences of PEDV HLJBY and CV777 was 97.7%. The homology of M gene was the highest (99.0%). However, the homology of ORF3 gene was 97.7%, and protein of ORF3 was 90.1%. In addition, HLJBY showed the highest nucleotide identity (99.9%) with PEDV‐SX/China/2017 strain and lowest similarity (91.2%) to PEDV/Belgorod/dom/2008 strain. We analysed the changes in S gene and its protein of PEDV HLJBY with 65 historic PEDV strains. The highest nucleotide identity was 99.9% compared with PEDV‐SX/China/2017 strain, and the lowest nucleotide identity was 60.0% compared with PEDV/Belgorod/dom/2008 strain. The length of deduced amino acid sequences of S proteins varied from 1,372 to 1,390 amino acids (aa). Compared with most aa sequences of S proteins, HLJBY exhibited 5 aa deletions (position 55, 59–61, 144). Analysis and comparison of open reading frame 3 (ORF3) proteins between HLJBY strain and other PEDV strains were also focused in this study. We revealed that the length of deduced amino acid sequences of ORF3 proteins was 80–224 aa among tested strains and the identity of HLJBY ORF3 amino acids with other PEDV strains was 71.4%–98.9%. ORF3 protein of both HLJBY strain and PEDV‐SX/China/2017 strain consists of 91 aa, with 133 aa deletions at their C' end in relation to the other tested PEDV strains. The phylogenetic tree based on different proteins or genes resulted in different phylogenetic groups. For pathogenicity evaluation of PEDV HLJBY strain, colostrum deprivation piglets were challenged with PEDV HLJBY, and PEDV reference strain CV777 as a control, the results showed that animals challenged with either of these PEDV strains developed diarrhoea, and histopathological examination of small intestines of challenged animals showed acute viral enteritis with villous atrophy in either PEDV HLJBY‐P10 or PEDV CV777‐P8 inoculated piglets.
Keywords: genomic sequence, HLJBY strain, open reading frame 3, phylogenetic analysis, porcine epidemic diarrhoea virus, S protein
1. INTRODUCTION
Porcine epidemic diarrhoea virus (PEDV) is an enveloped virus including a positive‐sense single‐strand RNA genome, which can cause a devastating enteric disease characterized with dehydration and watery diarrhoea (Pensaert & de Bouck, 1978). PEDV is the causative agent of porcine epidemic diarrhoea (PED), which has high mortality in suckling piglets (Debouck & Pensaert, 1980; Pijpers, Nieuwstadt, Terpstra, & Verheijden, 1993). This disease was initially documented in the United Kingdom in 1971. Since 1990s, PED was not serious. However, outbreak of PED suddenly occurred in the United States, Canada and Mexico causing huge economic losses (Mole, 2013; Stevenson et al., 2013; Vlasova et al., 2014). In addition, PED caused tremendous economic losses to the swine industry in Europe and Asia, including China, Korea and Japan (Kocherhans, Bridgen, Ackermann, & Tobler, 2001; Sun et al., 2012).
PEDV is a nonsegmented and infectious RNA virus. The genome of PEDV is 27‐33 kb in length containing a 5ꞌcap and a 3ꞌpolyadenylated tail (Pensaert & de Bouck, 1978). In addition, the genome includes seven open reading frames (ORFs) encoding three nonstructural proteins (replicase 1a,1b and ORF3) and four structural proteins (the spike (S), envelope (E), membrane (M) and nucleoprotein (N)). These proteins arrange in the order of 5ꞌ‐replicase(1a/1b)‐S‐ORF3‐E‐M‐N‐3ꞌ (Kocherhans et al., 2001).
To reveal the characteristic and the diversity between PEDV strains currently circulating in China and other PEDV strains outside, the complete genomic sequence of PEDV HLJBY strain was determined and analysed, and the pathogenicity of PEDV HLJBY strain in newborn piglets was also evaluated.
2. MATERIALS AND METHODS
2.1. Virus, cell culture and virus passages
PEDV HLJBY strain was isolated from the intestinal contents of a piglet with diarrhoea from Heilongjiang province, China at 2011. Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM, HyClone) supplemented with 8% foetal bovine serum (FBS, GIBCO) and were maintained in maintenance medium (DMEM supplemented with 2% FBS) at 37 °C in a 5% CO2 incubator. Strain HLJBY was passaged ten times in Vero cells. PEDV N‐specific antibody used in immunofluorescence assay (IFA) was gifted by Professor Xiang Mao. PEDV reference strain CV777 was purchased from China Institute of Veterinary Drug Control.
2.2. RNA extraction and RT‐PCR
When PEDV‐infected Vero cells showed 70%–80% cytopathic effect (CPE), cell culture flasks were frozen and thawed three times, and cell debris was pelleted by centrifugation for 30 min at 12,000 rpm. Culture supernatants were collected and used for preparation of viral RNA. Total RNA was extracted using Trizol reagent (Vazyme Biotech) according to the manufacturer's instructions. Total RNA was used for synthesis of cDNA with HiScript reverse transcriptase (HiScript II 1st Strand cDNA synthesis kit; Vazyme Biotech) according to the manufacturer's instructions.
2.3. Sequencing of the viral genome
Specific primers for PEDV were designed based on PEDV‐CV777 genome (Table 1). PCR was performed to amplify for the six overlapping DNA fragments using LAmp DNA polymerase (Vazyme Biotech). The expected bands in agarose electrophoresis of PCR products were excised, and a GenClean Column gel extraction kit (Generay Biotech) was used to purify the synthesized DNA, and the DNA products were cloned into pJET1.2 vector (Thermo). The positive clone was sequenced by Biotechnology Co, Ltd. The validated genome sequence of PEDV HLJBY strain was submitted to GenBank and acquired accession number: KP403802.1.
Table 1.
Oligonucleotide primers used for amplification of the complete genome of PEDV HLJBY by reverse transcription–polymerase chain reaction
| Sequence (5ꞌ−3ꞌ) | Nucleotide position | Product size(bp) | |
|---|---|---|---|
| 1F | ACTTAAAGAGATTTTCTATCTACGGATA | 1 | 6,146 |
| 1R | AAACACTGTAATTAAATTACGTGACAT | 6,146 | |
| 2F | ATACTGTTTTTGATCATGGCACTGGTA | 5,934 | 4,130 |
| 2R | AGAATTTGCTTTCCACCAAAATTCTTA | 10,066 | |
| 3F | TAATGGTATGACAACAGTGGGCAATAC | 9,964 | 6,505 |
| 3R | AATTAGTGCACATAGAGACCTCATCCA | 16,469 | |
| 4F | GCTCAGTACCTTTTCTCCACTGTCAAT | 16,408 | 4,123 |
| 4R | GCACTAGCAACTTACCATTCTTCAACA | 20,531 | |
| 5F | GCATAAGGCTACAGTTGTTGTTAATTT | 20,469 | 4,630 |
| 5R | ATAATAAGCTGCTTTACCATTGAGAAA | 25,099 | |
| 6F | TTGCACTGTTTAGAGCGTCTTCTTTGA | 24,927 | 3,000 |
| 6R | GTGTATCCATATCAACACCGTCAGGTC | 27,927 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.4. Sequence analysis
Sequence data was employed to assemble and analyse by DNAStar software package (DNAStar Inc.). The Clustal W method was used to analyse multiple sequence alignments. The MegAlign program was applied to construct phylogenetic trees by the neighbour‐joining method. The nucleotide and the amino acid sequences of PEDV HLJBY strain were compared with the corresponding sequences of PEDV strains deposited in the GenBank database. The PEDV strains used in this study were shown in Table 2. The sequences of 66 complete genomes, along with their fully sequenced S genes and ORF3 genes of PEDV strains were used for sequence alignments and phylogenetic analyses. Nucleotide sequences of full‐genomes, ORF3 genes and S genes of PEDV strains were aligned using the ClustalX 2.0 program (Thompson, Gibson, Plewniak, Jeanmougin, & Higgins, 1997).
Table 2.
Nucleotide and amino acid sequence identity (%) of different regions of the PEDV HLJBY genome compared with those of the other strains
| Strain | GenBank Accession No. | Location | Date | HLJBY identity | |||
|---|---|---|---|---|---|---|---|
| genome* | S aa | ORF3 aa | |||||
| 1 | HLJBY | KP403802.1 | China | 2011 | 100.0 | 100.0 | 100.0 |
| 2 | CV777 | AF353511.1 | Swizerland | 2001 | 97.7 | 95.9 | 90.1 |
| 3 | 85–7‐C40 | KY486714.1 | China | 2017 | 97.2 | 94.7 | 71.4 |
| 4 | 85–7‐mutant1 | KX839247.1 | China | 2016 | 97.2 | 95.0 | 71.4 |
| 5 | AH2012/12 | KU646831.1 | China | 2016 | 96.8 | 92.7 | 91.2 |
| 6 | AJ1102 | JX188454.1 | China | 2012 | 97.0 | 93.1 | 91.2 |
| 7 | B5‐HB2017 | MF807952.1 | China | 2017 | 96.6 | 93.9 | 92.3 |
| 8 | BJ−2011–1 | JN825712.1 | China | 2011 | 96.9 | 92.8 | 92.3 |
| 9 | CH/FJND−3/2011 | JQ282909.1 | China | 2011 | 97.0 | 93.3 | 91.2 |
| 10 | CH/FJZZ−9/2012 | KC140102.1 | China | 2012 | 96.9 | 93.3 | 91.2 |
| 11 | CH/GDZHDM/1401 | KR153326.1 | China | 2015 | 96.8 | 93.0 | 92.3 |
| 12 | CH/GDZHDM/1401 | KX016034.1 | China | 2016 | 96.8 | 93.0 | 92.3 |
| 13 | CH/HNLH/2015 | KT199103.1 | China | 2015 | 96.7 | 93.3 | 92.3 |
| 14 | CH/HNZZ47/2016 | KX981440.1 | China | 2016 | 96.8 | 92.9 | 92.3 |
| 15 | CH/JLDH/2016 | MF346935.1 | China | 2017 | 96.8 | 93.2 | 91.2 |
| 16 | CH/JXJA/2017 | MF375374.1 | China | 2017 | 96.7 | 92.6 | 92.3 |
| 17 | CH/S | JN547228.1 | China | 2011 | 97.5 | 96.1 | 92.3 |
| 18 | CH/YNKM−8/2013 | KF761675.1 | China | 2013 | 97.0 | 93.0 | 91.2 |
| 19 | CH/ZJCX−1/2012 | KF840537.1 | China | 2013 | 96.9 | 93.1 | 92.3 |
| 20 | CHN/SH−2016−4/2016 | MG837012.1 | China | 2018 | 95.7 | 93.2 | 92.3 |
| 21 | CO/P14/IC | KU558702.1 | China | 2016 | 96.8 | 92.7 | 97.5 |
| 22 | COL/Cundinamarca/2014 from Colombia | KU569509.1 | America | 2016 | 96.8 | 92.8 | 92.3 |
| 23 | FR/001/2014 | KR011756.1 | France | 2015 | 97.1 | 95.9 | 92.3 |
| 24 | GDS23 | MH107322.1 | China | 2018 | 96.8 | 93.2 | 91.2 |
| 25 | JS‐HZ2012 | KC210147.1 | China | 2012 | 97.0 | 92.7 | 92.3 |
| 26 | LNCT2 | KT323980.1 | China | 2015 | 96.8 | 95.9 | 92.3 |
| 27 | MEX/104/2013 | KJ645708.1 | America | 2014 | 96.7 | 92.9 | 92.3 |
| 28 | NPL‐PEDv/2013 | KJ778615.1 | America | 2014 | 96.9 | 93.0 | 92.3 |
| 29 | OH8593−14 | KP641662.1 | America | 2015 | 96.7 | 92.7 | 92.3 |
| 30 | OH9097−14 | KP641663.1 | America | 2015 | 96.7 | 93.3 | 92.3 |
| 31 | OH15962 | KJ584361.1 | America | 2014 | 96.9 | 93.2 | 92.3 |
| 32 | PC21A | KR078299.1 | America | 2015 | 96.8 | 93.1 | 92.3 |
| 33 | PC22A‐P100‐C6 | KU893871.1 | America | 2016 | 96.7 | 93.1 | 92.3 |
| 34 | PC273/O | MG837058.1 | America | 2018 | 96.7 | 92.7 | 92.3 |
| 35 | PEDV 1842/2016 ITA | KY111278.1 | Italy | 2016 | 96.9 | 93.1 | 92.3 |
| 36 | PEDV/Belgorod/dom/2008 | MF577027.1 | Russia | 2017 | 91.2 | 95.7 | 92.3 |
| 37 | PEDV/MEX/PUE/01/2015 | MH004421.1 | Mexico | 2018 | 96.1 | 60.0 | 92.3 |
| 38 | PEDV/MEX/QRO/02/2017 | MH013466.1 | Mexico | 2018 | 96.0 | 92.5 | 92.3 |
| 39 | PEDV/USA/Minnesota125/2015 | KU982980.1 | America | 2016 | 96.6 | 92.8 | 91.2 |
| 40 | PEDV/USA/NorthDakota93/2015 | KU982970.1 | America | 2016 | 96.7 | 92.4 | 92.3 |
| 41 | PEDV/USA/Oklahoma133/2015 | KU982968.1 | America | 2016 | 96.6 | 93.0 | 92.3 |
| 42 | PEDV‐LNsy | KY007140.1 | China | 2016 | 96.7 | 92.6 | 92.3 |
| 43 | PEDV‐SX | KY420075.1 | China | 2017 | 99.9 | 93.0 | 98.9 |
| 44 | PEDV‐WS | KM609213.1 | China | 2014 | 96.7 | 99.9 | 92.3 |
| 45 | TC PC168‐P2 | KM392226.1 | America | 2014 | 96.8 | 93.0 | 92.3 |
| 46 | USA/2014/IL/20697 P7 | KT591944.1 | America | 2015 | 97.1 | 93.0 | 92.3 |
| 47 | USA/Colorado/2013 | KF272920.1 | America | 2013 | 96.9 | 95.8 | 92.3 |
| 48 | USA/IL20697/2014 Passage 5 | KT860508.1 | America | 2015 | 97.1 | 93.1 | 92.3 |
| 49 | USA/Illinois98/2013 | KJ645690.1 | America | 2014 | 96.7 | 95.8 | 92.3 |
| 50 | USA/Illinois259/2014 from USA | KR265785.1 | America | 2015 | 96.7 | 93.1 | 92.3 |
| 51 | USA/Indiana34/2013&America | KJ645641.1 | America | 2014 | 96.7 | 93.0 | 92.3 |
| 52 | USA/Iowa/18984/2013 | KF804028.1 | America | 2013 | 96.9 | 93.0 | 92.3 |
| 53 | USA/Kansas46/2013 | KJ645650.1 | America | 2014 | 96.7 | 93.0 | 92.3 |
| 54 | USA/Kansas431/2014 from USA | KR265819.1 | America | 2015 | 96.7 | 92.9 | 92.3 |
| 55 | USA/Minnesota76/2013 | KJ645671.1 | America | 2014 | 96.7 | 93.0 | 92.3 |
| 56 | USA/Minnesota90/2013 | KJ645682.1 | America | 2014 | 96.7 | 93.1 | 91.2 |
| 57 | USA/Missouri373/2014 from USA | KR265844.1 | America | 2015 | 96.7 | 93.0 | 92.3 |
| 58 | USA/MO/2014/03293 | KM975741.1 | America | 2014 | 97.2 | 92.3 | 92.3 |
| 59 | USA/NC/2013/35140 | KM975735.1 | America | 2014 | 96.8 | 92.7 | 92.3 |
| 60 | USA/Nebraska287/2014 from USA | KR265765.1 | America | 2015 | 96.7 | 93.0 | 92.3 |
| 61 | USA/Nebraska288/2014 from USA | KR265803.1 | America | 2015 | 96.7 | 92.9 | 92.3 |
| 62 | USA/NorthCarolina66/2013 | KJ645662.1 | America | 2014 | 96.7 | 92.8 | 92.3 |
| 63 | USA/Ohio123/2014 | KJ645699.1 | America | 2014 | 96.7 | 93.0 | 92.3 |
| 64 | USA/OK10240−8/2017 | MG334555.1 | America | 2017 | 95.1 | 93.0 | 92.3 |
| 65 | USA/Tennesse56/2013 | KJ645654.1 | America | 2014 | 96.7 | 94.7 | 92.3 |
| 66 | YN90 | KT021231.1 | China | 2015 | 96.9 | 93.0 | 91.2 |
Abbreviations: aa, Amino acid.
* Bold value indicates highest identity.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.5. Immunofluorescence assay (IFA)
Vero cells grown on coverslips were infected with 0.1 multiplicity of infection (MOI) PEDV for 6, 12, 24 and 36 hr, respectively. Vero cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X‐100 at room temperature (RT) for 10 min, then blocked with 3% BSA for 1h and incubated with PEDV N‐specific antibody for 2 hr at 37°C. The cells were washed with PBS for three times and then incubated with a goat anti‐rabbit secondary antibody conjugated to Alexa Fluor 488 (Invitrogen) and DAPI (Beyotime). The fluorescence was visualized under a Leica inverted fluorescence microscope (Leica, DMi8), after washing three times with PBS.
2.6. Experimental challenge in piglets
Fifteen newborn piglets without colostrum were purchased from one pig farm and were free of PEDV, transmissible gastroenteritis virus, porcine deltacoronavirus, and porcine rotavirus. The piglets were divided randomly into three groups: the sham‐inoculated control group (n = 5), CV777‐P8‐inoculated group (n = 5), and HLJBY‐P10‐inoculated group (n = 5). The piglets were fed commercial milk replacer (8 times daily). The piglets in the challenge groups received an oral 1 ml dose of 107.0 TCID50/ml of PEDV CV777 or HLJBY. The sham‐inoculated pigs were given DMEM medium (1 ml) orally. All animals were monitored for mortality and signs of vomiting and/or diarrhoea (observed and recorded for every 3 hr during whole experiment). All piglets were euthanized at 7 d post‐challenge and checked for macroscopic and microscopic lesions. The amount of PEDV N antigen in the small intestines of all experimental animals was determined by immunofluorescence. The animal experiments were approved by the Animal Care and Use Committee of Yangzhou University (approval ID: SYXK (Su) 2007–0005).
3. RESULTS
3.1. Complete genomic characterization of PEDV HLJBY strain
To reveal the characteristics of PEDV HLJBY strain and determine more precisely the relationship among the PEDV strains currently circulating in China and those from other nations, the full‐length genome sequence of strain HLJBY was deduced by combining the sequences of several overlapping cDNA fragments. The genome sequence of strain HLJBY was 27,953 nucleotides (nt) in length, excluding the 3ꞌ poly(A) tail. The genomic organization was typical of previously sequenced PEDV strains and was summarized as 5ꞌUTR‐ORF1a/1b‐S‐ORF3‐E‐M‐N‐3ꞌUTR (Figure 1).
Figure 1.
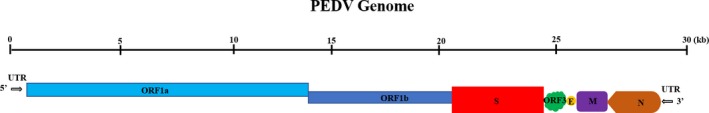
Schematic representation of the PEDV genome [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Of note, compared with classical PEDV CV777, the genome of HLJBY contains four deleted nucleotides or regions including 72 nt, 89–93 nt, 3403–3426 nt, 21092–21094 nt, respectively. The four deleted nucleotides or regions were located at 5ꞌUTR, ORF1a/1b, S, ORF3, respectively. To investigate the molecular characteristics of PEDV HLJBY strain, UTR (5ꞌ and 3ꞌ) and the nucleotide and predicted amino acid sequences of the nonstructural and structural proteins (replicase ORF1a/1b, S, ORF3, E, M, N) of PEDV HLJBY strain were compared with CV777. As shown in Figure 2a and Table 3, the nucleotide of 5ꞌUTR of PEDV HLJBY had 5 nt deletions and 1 nt insertion and the identity is 96.0% compared with CV777. 24 nt deletions were found in ORF1a/1b of PEDV HLJBY (Figure 2b), and the nucleotide sequence identity was 98.0%, and the amino acid sequence identity was 98.0% (Table 3). 3 nt deletions showed in S of PEDV HLJBY (Figure 2c), and the nucleotide sequence identity was 97.0%, and the amino acid sequence identity was 96.0% (Table 3). 399 nt deletions exhibited in ORF3 of PEDV HLJBY (Figure 2d), and nucleotide sequence identity was 91.7%, and the amino acid sequence identity was 90.1% (Table 3). In protein E, the nucleotide sequence identity was 97.0%, and the amino acid sequence identity was 97.0% (Table 3), resulting in amino acid changes in E (11Val to Ala and 76 Val to Ile) (Figure 2e). In protein M, the nucleotide sequence identity was 99.0% and the amino acid sequence identity was 98.0% (Table 3), resulting in 4 amino acid changes in M (Figure 2f). In protein N, the nucleotide sequence identity was 98.0% and the amino acid sequence identity was 98.0% (Table 3), resulting in 9 amino acid changes in N (Figure 2g). The nucleotide sequence identity of 3ꞌUTR was 97.0% (Table 3).
Figure 2.
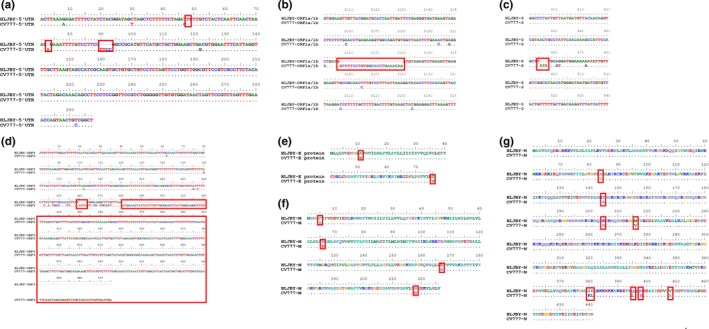
Genome‐scale comparison between the HLJBY strain and the CV777 strain. (a) The nucleotide of 5ꞌUTR of PEDV HLJBY had 5 nt deletions and 1 nt insertion. (b) 24 nt deletions were found in ORF1a/1b of PEDV HLJBY. (c) 3 nt deletions were found in S protein of PEDV HLJBY. (d) 399 nt deletions were showed in ORF3 of PEDV HLJBY. (e) The amino acid sequence of E protein had 2 aa mutations in PEDV HLJBY. (f) Protein M exhibited 4 aa changes in PEDV HLJBY. (g) Protein N experienced 9 aa changes in PEDV HLJBY [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Table 3.
Differences in genome organization between the HLJBY strain and the CV777 strain
| Gene | HLJBY strain | CV777 strain | identity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | Stop | Size (nt) | Size (aa) | Start | Stop | Size (nt) | Size (aa) | nt | aa | |
| 5'UTR | 1 | 292 | 292 | – | 1 | 296 | 296 | – | 96.0% | – |
| ORF1a/1b | 293 | 20,613 | 20,321 | 6,773 | 297 | 20,641 | 20,345 | 6,781 | 98.0% | 98.0% |
| S | 20,610 | 24,758 | 4,149 | 1,382 | 20,638 | 24,789 | 4,152 | 1,383 | 97.0% | 96.0% |
| ORF3 | 24,758 | 25,033 | 276 | 91 | 24,789 | 25,463 | 675 | 224 | 91.7% | 90.1% |
| E | 25,364 | 25,594 | 231 | 76 | 25,444 | 25,674 | 231 | 76 | 97.0% | 97.0% |
| M | 25,602 | 26,282 | 681 | 226 | 25,682 | 26,362 | 681 | 226 | 99.0% | 98.0% |
| N | 26,294 | 27,619 | 1,326 | 441 | 26,374 | 27,699 | 1,326 | 441 | 98.0% | 98.0% |
| 3ꞌUTR | 27,620 | 27,953 | 334 | – | 27,700 | 28,033 | 334 | – | 97.0% | – |
Abbreviations: aa, Amino acid; nt, Nucleotide.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Genome‐wide sequence comparison
The complete genome sequences of PEDV strains from different locations and years were compared, and the results revealed that HLJBY had a nucleotide identity of 99.9%–91.2% with other entire PEDV genomes available in GenBank (Table 2). HLJBY showed the highest nucleotide identity (99.9%) with PEDV‐SX/China/2017 strain, and lowest similarity (91.2%) to PEDV/Belgorod/dom/2008 strain. HLJBY shared 97.7% nucleotide similarity with CV777 and 97.5% nucleotide identity with CH/S/2011 strain, which was isolated from the same province with PEDV HLJBY, and 97.2% nucleotide similarity to CH/85‐7‐C40, CH/85‐7‐mutant1, and USA/MO/2014/03293, respectively.
3.3. The S protein
The nucleotide of HLJBY S gene was 4,149 nt, translating 1,382 amino acids. The deduced amino acid sequences of HLJBY S genes were compared with 65 historic PEDV strains. HLJBY S amino acid identity was 60.0%‐99.9% compared with other strains. The highest nucleotide identity was 99.9% compared with PEDV‐SX/China/2017 strain, and the lowest nucleotide identity was 60.0% compared with PEDV/Belgorod/dom/2008 strain. The length of deduced amino acid sequences of S protein was 1372–1390 aa. HLJBY harboured the same length with PEDV‐SX/China/2017 strain. We revealed that HLJBY S protein had insertions and deletions. Compared with S protein aa sequences of most PEDV strains, HLJBY showed 5 aa deletions (position 55, 59–61, 144) (Figures 3a and b). Only CH/85‐7‐C40 and CH/85‐7‐mutant1 had 4 aa insertions in position 115–118 and had ‘FEKVHVQ’ deletions at Cꞌ end (Figures 3b and c). PEDV‐LNsy/2017 had 4 aa insertions at position 626–629.
Figure 3.
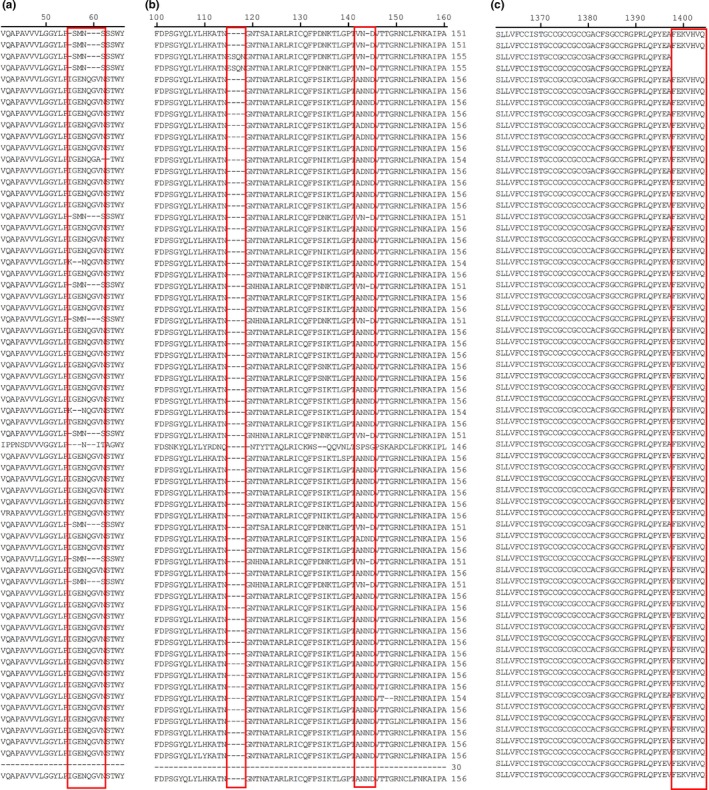
The S protein of PEDV HLJBY compared with 65 historic strains available in GenBank. (a) HLJBY showed 4 aa deletions in position 55 and 59–61. (b) HLJBY showed 1 aa deletion in position 144. CH/85‐7‐C40 and CH/85‐7‐mutant1 had 4 aa insertions in position 115–118. (c) CH/85‐7‐C40 and CH/85‐7‐mutant1 had ‘FEKVHVQ’ deletions at Cꞌ end [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
3.4. The ORF3 protein
The ORF3 protein (an accessory protein) was located between S and E proteins. The amino acid of HLJBY ORF3 identity was compared with 65 historic PEDV strains. The results revealed that the length of deduced amino acid sequences of ORF3 protein was 80–224 aa and HLJBY ORF3 amino acid identity was 71.4%‐98.9% compared with other PEDV strains. Both HLJBY and PEDV‐SX/China/2017 ORF3 proteins were 91 aa, which had 133 aa deletions at Cꞌ end compared with most of PEDV strains (Figure 4b). In addition, USA/2014/IL/20697 ORF3 protein was 143 aa (81 aa deletions at Cꞌ end), YN90/China/2015 ORF3 protein was 144 aa (80 aa deletions at Cꞌ end), and CO/P14/IC/2016 ORF3 protein was 80 aa, with 144 aa deletions at Cꞌ end (Figure 4b). CH/85‐7‐C40 and CH/85‐7‐mutant1 ORF3 protein had 70 aa deletions at Nꞌ end (Figure 4a).
Figure 4.
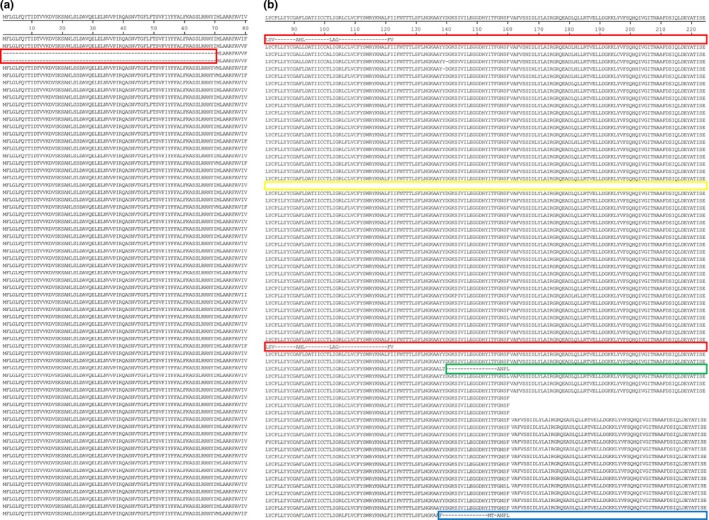
(a) CH/85‐7‐C40 and CH/85‐7‐mutant1 ORF3 proteins had 70 aa deletions at N' end. (b) HLJBY and PEDV‐SX/China/2017 ORF3 proteins were 91 aa, with 133 aa deletions at C' end (red). USA/2014/IL/20697 ORF3 protein was 143 aa (81 aa deletions at C' end [green]). YN90/China/2015 ORF3 protein was 144 aa (80 aa deletions at C' end [blue]). CO/P14/IC/2016 ORF3 protein was 80 aa, with 144 aa deletions at C' end (orange) [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
3.5. Phylogenetic analysis
To investigate the evolution of PEDV, phylogenetic analysis based on the entire genomic nucleotide sequences of PEDV HLJBY strain and 65 historic strains available in GenBank was performed. All PEDV strains were divided into three groups. The group Ⅰ held PEDV/Belgorod/dom/2008 only. PEDV HLJBY belonged to the group Ⅱ, which could be further divided into subgroup Ⅱa and Ⅱb. The group Ⅲ was further divided into subgroup Ⅲa and Ⅲb. The U.S. PEDV strains belonged to the subgroup Ⅲ. PEDV HLJBY did not clustered within the same group with the U.S. PEDV strains (Figure 5).
Figure 5.
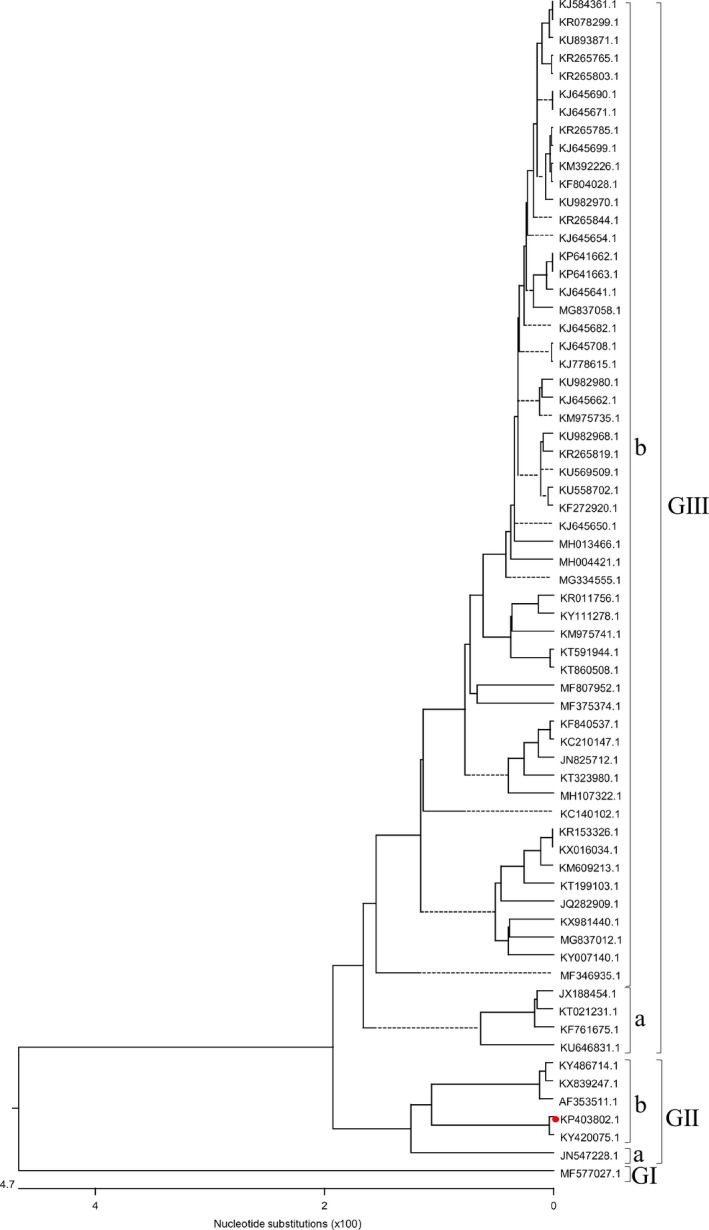
Phylogenetic analysis of the entire genomic nucleotide sequences of PEDV strains (red solid circle indicated PEDV HLJBY) [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
S gene of PEDV was a virulence gene of PEDV. The S protein of PEDV was a glycosylated fibrillary protein located on the surface of virus. The PEDV S gene was an important viral gene for studying genetic relatedness among isolates and epidemiology of PEDV (Chen et al., 2014; Gerber et al., 2014; Lee, 2015; Lee, Park, Kim, & Lee, 2010; Lee & Lee, 2014; Oh, Lee, Choi, & Lee, 2014). S protein plays a vital role in regulating the interaction between host cell receptor protein and virus and mediates virus into host cell, which elicits neutralizing antibody in host cells. The S protein is used as a target for the development of vaccine and antiviral drug. Therefore, phylogenetic analysis based on the S protein of PEDV HLJBY strain and 65 historic strains available in GenBank was performed. The phylogenetic tree for the S protein showed the similar grouping structure as the tree generated from the PEDV whole genomes. All S proteins of PEDV strains were divided into three groups. The S protein of HLJBY belonged to the group Ⅱ (Figure 6).
Figure 6.
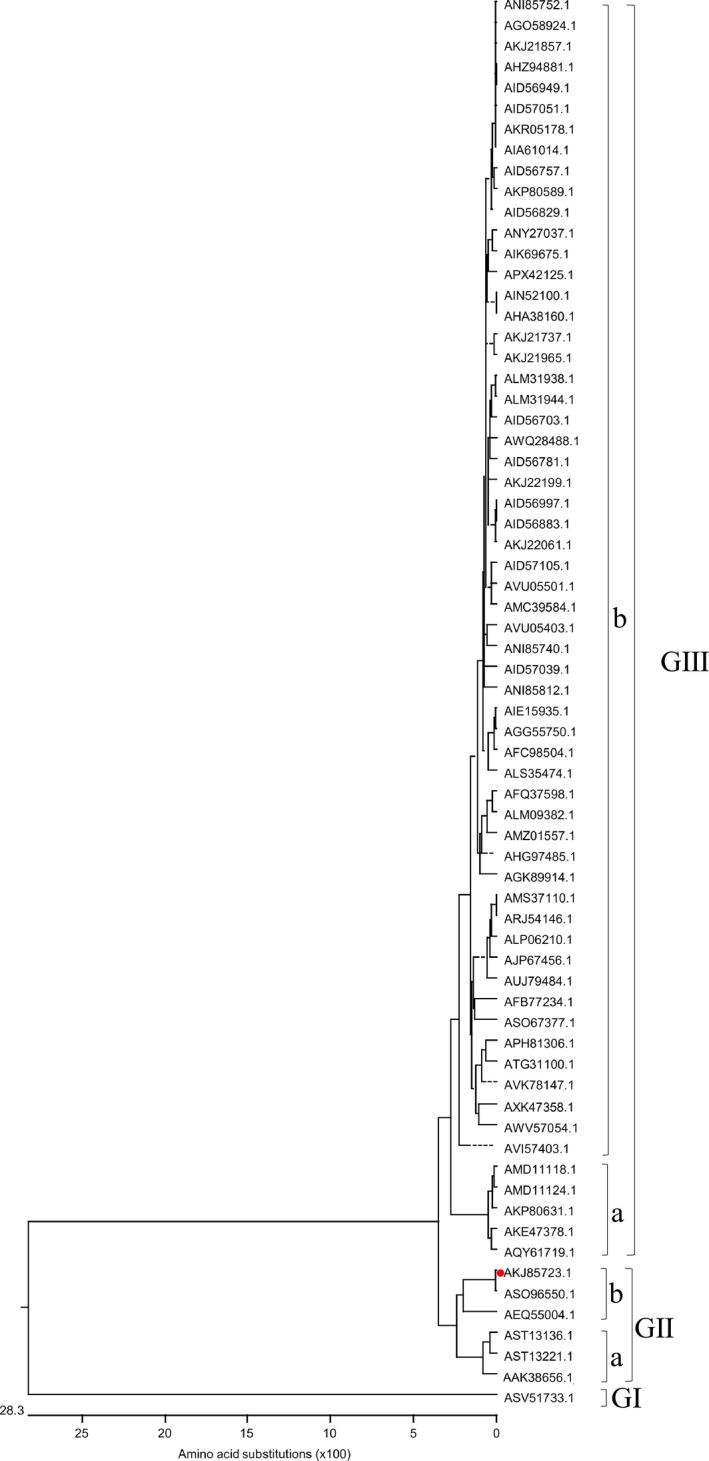
Phylogenetic analysis based on the S protein of PEDV HLJBY strain and 65 historic strains available in GenBank (red solid circle indicated PEDV HLJBY) [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
The ORF3 is the only auxiliary factor of PEDV. ORF3 may contribute to the virulence of PEDV. Hence, we analysed the phylogenetic tree based on the ORF3 proteins of PEDV HLJBY strain and 65 historic strains available in GenBank. The phylogenetic tree of the ORF3 proteins was different from the phylogenetic trees based on the S protein and genomic nucleotide sequences. All ORF3 proteins of PEDV strains were divided into two groups. the group Ⅰ harboured PEDV/Belgorod/dom/2008 and HLJBY strains. The group Ⅱ included the other PEDV strains (Figure 7).
Figure 7.
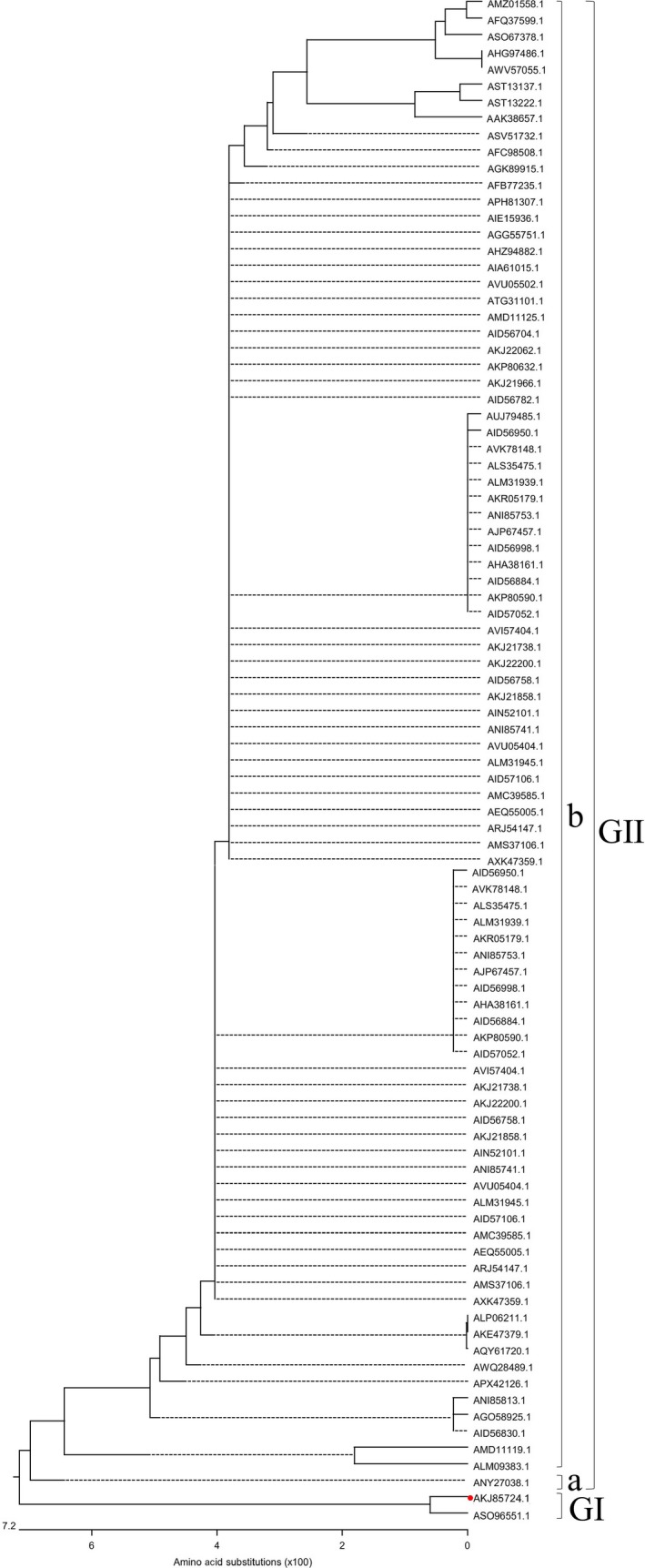
Phylogenetic analysis based on the ORF3 protein of PEDV HLJBY strain and 65 historic strains available in GenBank (red solid circle indicated PEDV HLJBY) [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
3.6. Biological characteristics of PEDV HLJBY strain in vitro
PEDV HLJBY strain can induce an apparent and classical cytopathic effect (CPE) in Vero cells, including cell fusion, cell detachment and multinucleated giant cell formation after 24 h post‐infection (hpi). The immunofluorescence of N protein to detect PEDV antigens was readily visible 6 hr after HLJBY inoculation. The apparent staining was distributed in the cytoplasm and a quick increase from 12 to 48 hpi (Figure 8a).
Figure 8.
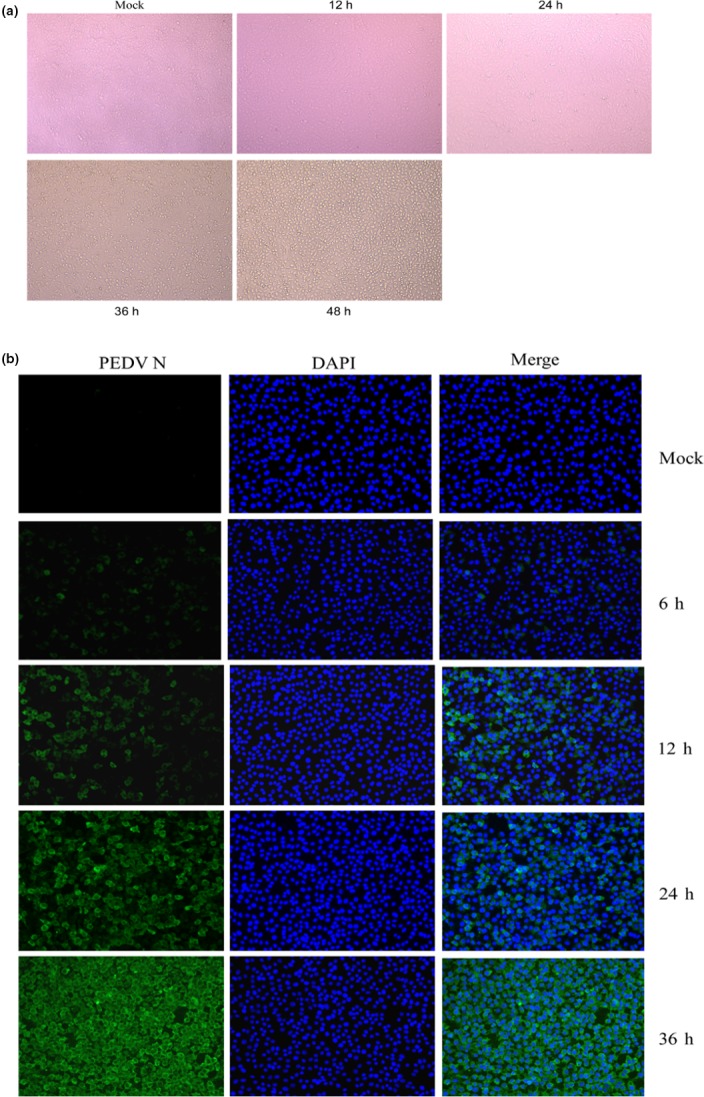
Infectivity of PEDV HLJBY strain in Vero cells. (a) CPE of HLJBY in Vero cells at different intervals. (b) Immunofluorescence of PEDV N (green) detected in infected Vero cells at different intervals [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
3.7. Pathogenicity of PEDV HLJBY strain in piglets
To determine the pathogenicity of PEDV HLJBY strain in newborn piglets, the piglets received an oral 1ml of 107.0 TCID50/ml of PEDV CV777 or HLJBY, while mock animals were inoculated with 1ml DMEM medium. Clinical signs were observed and recorded 8 times daily. During the acclimation period, all piglets had no clinical symptoms, and their faeces were normal. Following viral challenge, the mock piglets experienced no clinical signs. Meanwhile, all HLJBY‐P10‐challenged piglets and CV777‐P8‐ challenged piglets exhibited lethargy, and diarrhoeic faeces by 48 hpi and diarrhoea lasted till the end of the experiment, however, no deaths were observed during whole experiment. All experimental piglets were euthanized and necropsied at 7 d post‐inoculation. Necropsy examination revealed that HLJBY‐P10‐challenged piglets and CV777‐P8‐ challenged piglets displayed archetypal PED‐like lesions. The small intestines gave rise to the accumulation of a yellowish watery content and presented with thin‐walled (data not shown). Histopathological examination of small intestine showed acute viral enteritis with villous atrophy in either PEDV HLJBY‐P10 or PEDV CV777‐P8 inoculated pigs (Figures 9b and c). The small intestines of mock piglets were normal through histopathological examination (Figure 9a). PEDV antigens were mainly observed in the cytoplasm of epithelial cells in atrophied villi of the small intestines of piglets from both HLJBY and CV777 challenge groups (Figures 9e and f). PEDV antigens were not observed in the small intestines of mock piglets (Figure 9d).
Figure 9.
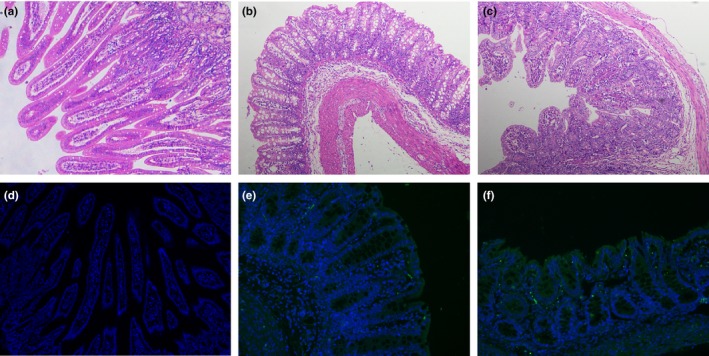
Microscopic small intestine lesions in piglets inoculated with PEDV HLJBY strain. (a) Small intestines from a mock piglet. The normal villous was observed in the mock‐inoculated piglet. (b) Small intestines from a piglet inoculated with PEDV CV777 strain, showed villous atrophy. (c) Small intestines from a piglet inoculated with PEDV HLJBY strain showed diffuse atrophic enteritis. (d–f) Detection of PEDV N antigen in the small intestines was performed using immunofluorescence (100× magnification). PEDV N was green (FITC) and DAPI was blue. (d) No PEDV N antigen was detected in the small intestines of the mock‐inoculated piglet. (e–f) Immunostaining of PEDV N antigen was detected in the epithelial cells of the small intestines in PEDV CV777 (e) and HLJBY (f) inoculated piglets [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
4. DISCUSSION
In recent years, PEDV has re‐emerged as one of the deadliest and most contagious pathogens in swine, causing large economic losses to the global swine industry. The biosecurity, vaccination and feedback are the fundamental methods for managing the disease, but these methods do not effectively eradicate and block PEDV (Chae et al., 2000; Lee et al., 2010; Li et al., 2012; Lowe et al., 2014; Olanratmanee, Kunavongkrit, & Tummaruk, 2010). In this study, we isolated one strain of PEDV from intestinal contents from PED‐affected pigs in China. The complete genome of PEDV named HLJBY was sequenced and then submitted to Genbank. To provide insight into the understanding of the genetic, phylogenetic and the current epidemiological status of PEDV, PEDV HLJBY was compared with CV777 and other PEDV strains. CV777‐based vaccine did not block the new PEDV variants circulated in China since 2011 effectively (Li et al., 2012). Therefore, the relationship of PEDV HLJBY with CV777 was analysed firstly. The results showed that the homology between the entire genomic nucleotide sequences of PEDV HLJBY and CV777 was 97.7%. Next, the homology of each component of PEDV HLJBY and CV777 was explored. The range of homology of each component of PEDV HLJBY and CV777 was 99.0%‐91.7%. The homology of M gene was the highest (99.0%), which demonstrated M gene was the most conservative. However, the homology of ORF3 gene between PEDV HLJBY and CV777 was 97.7%, and protein of ORF3 was 90.1%, which revealed the identify of ORF3 gene between PEDV HLJBY and CV777 was the lowest. Therefore, ORF3 gene might determine the different properties of PEDV HLJBY and CV777. The ORF3 is the only auxiliary factor of PEDV. It was predicated that ORF3 had four transmembrane regions and formed homology (Wang et al., 2012). ORF3 may contribute to the virulence of PEDV. The ORF3 was a key gene for PEDV culture in vitro. ORF3 gene was usually used to differentiate between field and vaccine‐derived isolates and altered the virulence of PEDV (Park et al., 2008). In addition, the PEDV S protein is an important viral gene for studying genetic relatedness among isolates and epidemiology of PEDV (Chen et al., 2014; Gerber et al., 2014; Lee, 2015; Lee et al., 2010; Lee & Lee, 2014; Oh et al., 2014). Nucleotide sequencing of S gene analysis revealed that 3 nt deletions were found in S gene of PEDV HLJBY compared with CV777 (Figure 2c) and the nucleotide sequence identity is 97.0%. The amino acid sequence identity of S protein was 96.0% between PEDV HLJBY and CV777. 3 nt deletions of S gene were located in the amino terminus of S gene, which was suitable to analyse the significant differences in epidemiology of PEDV(Li et al., 2012; Temeeyasen et al., 2014; Vlasova et al., 2014). S gene functions as a receptor‐binding domain(Belouzard, Millet, Licitra, & Whittaker, 2012). The 3 nt deletions might be leading to the change in structure and function of S protein. S gene might play a vital role in the genetic evolutionary process of PEDV. Therefore, it will be the focus of future research.
To further understand the status of PEDV HLJBY in the PEDV evolutionary and epidemiology, we analysed the relationship of PEDV HLJBY with 65 historic strains available in GenBank. Some researchers had analysed the sequences of PEDV strains, which gave a reference for us to understand the changes in evolutionary and epidemiology of PEDV. Our study will provide more information about diversity, evolution, and in particular, the epidemic characteristics of PEDV including HLJBY, which will give insight into the virulence of PEDV. First, the complete genome sequences of PEDV from different locations and years were compared, and the results revealed that HLJBY had the highest nucleotide identity (99.9%) with PEDV‐SX/China/2017 strain and lowest similarity (91.2%) to PEDV/Belgorod/dom/2008 strain. HLJBY shared 97.5% nucleotide identity with CH/S/2011 strain, which was isolated from the same province with HLJBY. Second,we emphasized the identity of S gene and protein of PEDV HLJBY in relation to other strains. The nucleotide of HLJBY S gene was 4,149 nt, translating 1,382 amino acids. The deduced amino acid sequence of HLJBY S gene was compared with 65 historic PEDV strains. The highest nucleotide identity was identified with PEDV‐SX/China/2017 strain, and the lowest nucleotide identity was 60.0% compared with PEDV/Belgorod/dom/2008 strain. The length of deduced amino acid sequences of S protein was 1372–1390 aa. HLJBY harboured the same length as PEDV‐SX/China/2017 strain. Compared with S aa sequences of most PEDV strains, HLJBY showed 5 aa deletions (position 55, 59–61, 144) (Figures 3a and b), which might be leading to the virulence change in PEDV HLJBY. Third, we focused on the analysis of ORF3. We revealed that the length of deduced amino acid sequences of ORF3 protein was 80–224 aa and HLJBY ORF3 amino acid identity was 71.4%‐98.9% compared with that of other PEDV strains. HLJBY and PEDV‐SX/China/2017 ORF3 protein were 91 aa, which had 133 aa deletions at Cꞌ end in relation to most of other PEDV strains.
In the phylogenetic tree of the whole genome, S aa and ORF3 aa, the phylogenetic tree based on both the whole genome and S aa was similar, but the phylogenetic tree of ORF3 aa was different from the phylogenetic trees for the S protein and genomic nucleotide sequences. All PEDV strains were divided into three groups based on the phylogenetic tree of the whole genome and S protein. In the phylogenetic tree of the whole genome and S protein, PEDV/Belgorod/dom/2008 strain located at group Ⅰ, and PEDV HLJBY belonged to the group Ⅱ. In the phylogenetic tree of ORF3 protein, the group Ⅰ only harboured PEDV/Belgorod/dom/2008 and HLJBY. The phylogenetic tree based on different proteins implied the different evolution events. This will provide further information for virus evolution. The phylogenetic trees suggested that PEDV HLJBY might originate from a genetic recombination event between pandemic strains and classical strains. We need to explore the mechanism and/or cause of the different phylogenetic trees based on different proteins or genes. This will lay a foundation for the recombination and evolution of PEDV.
Biological characteristics of PEDV HLJBY including the cytopathic effect, and pathogenicity, indicated that PEDV HLJBY induced an apparent and classical cytopathic effect (CPE) in Vero cells, and caused acute viral enteritis with villous atrophy in small intestine of challenged piglets. But all HLJBY‐P10 or CV777‐P8 inoculated piglets had no deaths during the challenge experiment. These results suggested that PEDV HLJBY might have similar pathogenicity in experimentally infected piglets compared with that of PEDV reference strain CV777.
In summary, we found that the evolutionary trees are different based on different proteins or genes of PEDV strains. This phenomenon needs further study. Pathogenicity evaluation of PEDV HLJBY indicated that PEDV HLJBY potentially possessed equal pathogenicity compared with that of PEDV reference strain CV777. This study might provide a reference for the evolutionary and variation of PEDV.
CONFLICT OF INTEREST
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICAL STATEMENT
All experimental protocols were approved by the Animal Care and Use Committee of Yangzhou University (approval ID: SYXK (Su) 2007–0005). All animal care and use protocols in this study were performed in accordance with the approved current guidelines.
ACKNOWLEDGEMENTS
We thank James Allen, PhD, for editing the English text of a draft of this manuscript. This work was supported by the Natural Science Foundation of Jiangsu Province (BK20180921), the China Postdoctoral Science Foundation (2018M632399), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Earmarked fund for Jiangsu Agricultural Industry Technology System (JATS[2018]221).
Huan C, Pan H, Fu S, et al. Characterization and evolution of the coronavirus porcine epidemic diarrhoea virus HLJBY isolated in China. Transbound Emerg Dis. 2020;67:65–79. 10.1111/tbed.13321
Contributor Information
Song Gao, Email: gsong@yzu.edu.cn.
Changhai Chen, Email: gsong@yzu.edu.cn, Email: cchai63@126.com.
REFERENCES
- Belouzard, S. , Millet, J. K. , Licitra, B. N. , & Whittaker, G. R. (2012). Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses, 4, 1011–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae, C. , Kim, O. , Choi, C. , Min, K. , Cho, W. S. , Kim, J. , & Tai, J. H. (2000). Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. The Veterinary Record, 147, 606–608. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Li, G. , Stasko, J. , Thomas, J. T. , Stensland, W. R. , Pillatzki, A. E. , … Zhang, J. (2014). Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. Journal of Clinical Microbiology, 52, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck, P. , & Pensaert, M. (1980). Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. American Journal of Veterinary Research, 41, 219–223. [PubMed] [Google Scholar]
- Gerber, P. F. , Gong, Q. , Huang, Y. W. , Wang, C. , Holtkamp, D. , & Opriessnig, T. (2014). Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Veterinary Journal, 202, 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans, R. , Bridgen, A. , Ackermann, M. , & Tobler, K. (2001). Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes, 23, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. (2015). Porcine epidemic diarrhea virus: An emerging and re‐emerging epizootic swine virus. Virology Journal, 12, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. K. , Park, C. K. , Kim, S. H. , & Lee, C. (2010). Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Research, 149, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , & Lee, C. (2014). Outbreak‐related porcine epidemic diarrhea virus strains similar to US strains, South Korea, 2013. Emerging Infectious Diseases, 20, 1223–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Li, H. , Liu, Y. , Pan, Y. , Deng, F. , Song, Y. , … He, Q. (2012). New variants of porcine epidemic diarrhea virus, China, 2011. Emerging Infectious Diseases, 18, 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, J. , Gauger, P. , Harmon, K. , Zhang, J. , Connor, J. , Yeske, P. , … Main, R. (2014). Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerging Infectious Diseases, 20, 872–874. 10.3201/eid2005.131628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole, B. (2013). Deadly pig virus slips through US borders. Nature, 499, 388 10.1038/499388a [DOI] [PubMed] [Google Scholar]
- Oh, J. , Lee, K. W. , Choi, H. W. , & Lee, C. (2014). Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Archives of Virology, 159, 2977–2987. 10.1007/s00705-014-2163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanratmanee, E. O. , Kunavongkrit, A. , & Tummaruk, P. (2010). Impact of porcine epidemic diarrhea virus infection at different periods of pregnancy on subsequent reproductive performance in gilts and sows. Animal Reproduction Science, 122, 42–51. 10.1016/j.anireprosci.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Park, S. J. , Moon, H. J. , Luo, Y. , Kim, H. K. , Kim, E. M. , Yang, J. S. , … Park, B. K. (2008). Cloning and further sequence analysis of the ORF3 gene of wild‐ and attenuated‐type porcine epidemic diarrhea viruses. Virus Genes, 36, 95–104. 10.1007/s11262-007-0164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert, M. B. , & de Bouck, P. (1978). A new coronavirus‐like particle associated with diarrhea in swine. Archives of Virology, 58, 243–247. 10.1007/BF01317606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers, A. , van Nieuwstadt, A. P. , Terpstra, C. , & Verheijden, J. H. (1993). Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. The Veterinary Record, 132, 129–131. 10.1136/vr.132.6.129 [DOI] [PubMed] [Google Scholar]
- Stevenson, G. W. , Hoang, H. , Schwartz, K. J. , Burrough, E. R. , Sun, D. , Madson, D. , … Yoon, K. J. (2013). Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc, 25, 649–654. [DOI] [PubMed] [Google Scholar]
- Sun, R. Q. , Cai, R. J. , Chen, Y. Q. , Liang, P. S. , Chen, D. K. , & Song, C. X. (2012). Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerging Infectious Diseases, 18, 161–163. 10.3201/eid1801.111259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeeyasen, G. , Srijangwad, A. , Tripipat, T. , Tipsombatboon, P. , Piriyapongsa, J. , Phoolcharoen, W. , … Nilubol, D. (2014). Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 21, 205–213. 10.1016/j.meegid.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Thompson, J. D. , Gibson, T. J. , Plewniak, F. , Jeanmougin, F. , & Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova, A. N. , Marthaler, D. , Wang, Q. , Culhane, M. R. , Rossow, K. D. , Rovira, A. , … Saif, L. J. (2014). Distinct characteristics and complex evolution of PEDV strains, North America, may 2013‐February 2014. Emerging Infectious Diseases, 20, 1620–1628. 10.3201/eid2010.140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Lu, W. , Chen, J. , Xie, S. , Shi, H. , Hsu, H. , … Sun, B. (2012). PEDV ORF3 encodes an ion channel protein and regulates virus production. FEBS Letters, 586, 384–391. 10.1016/j.febslet.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


