Abstract
The major constituent of the outer membrane of Gram-negative bacteria is lipopolysaccharide (LPS), which is comprised of lipid A, core oligosaccharide, and O antigen, which is a long polysaccharide chain extending into the extracellular environment. Due to the localization of LPS, it is a key molecule on the bacterial cell wall that is recognized by the host to deploy an immune defence in order to neutralize invading pathogens. However, LPS also promotes bacterial survival in a host environment by protecting the bacteria from these threats. This review explores the relationship between the different LPS glycoforms of the opportunistic pathogen Pseudomonas aeruginosa and the ability of this organism to cause persistent infections, especially in the genetic disease cystic fibrosis. We also discuss the role of LPS in facilitating biofilm formation, antibiotic resistance, and how LPS may be targeted by new antimicrobial therapies.
Keywords: lipopolysaccharide, O antigen, host–pathogen interactions, cystic fibrosis, biofilms, antimicrobial resistance, pyocin
1. Introduction
Pseudomonas aeruginosa is a Gram-negative bacterium that is a global threat to public health and is classified as one of the ESKAPE pathogens, a group of microorganisms with a high propensity for causing problematic, drug-resistant, nosocomial infections [1]. In the hospital setting, contamination of sinks, plumbing, and water are a significant reservoir for P. aeruginosa, and are often the source of an infection [2]. This bacterial species is versatile and can cause disease by colonizing a variety of human host sites, such as burn wounds, the urinary tract, and the respiratory system [3], but can also cause disease in plants [4,5]. P. aeruginosa is notorious as a significant cause of morbidity and mortality in those with cystic fibrosis (CF), an autosomal recessive genetic disorder causing ion imbalance in the lungs, which leads to a thick and sticky mucous that hinders mucociliary clearance of potential pathogens [6]. In the end-stage of the disease, P. aeruginosa is typically the dominant organism infecting the lung [7]. The success of P. aeruginosa as a pathogen is due to its intrinsic and acquired antibiotic resistance mechanisms, ability to establish robust biofilms, and repertoire of virulence factors, including a number of secreted enzymes and molecules causing extensive host tissue damage [3]. P. aeruginosa also expresses the major virulence factor lipopolysaccharide (LPS), which is an integral component of the archetypal cell envelope of most Gram-negative bacteria (GNB). GNB possess two membranes separated by the periplasmic milieu containing a thin layer of peptidoglycan [8]. Although there are a few exceptions, the outer membrane (OM) is an asymmetric bilayer of phospholipids on the periplasmic face and LPS on the extracellular face [9] (Figure 1). Inherent to its localization, LPS plays significant roles in interactions with the bacterium’s environment. Both the hydrophobic and polar nature of LPS contribute to a drastic decrease in membrane permeability in GNB; the lipid membrane impedes the passage of polar solutes, whereas the polar LPS groups repel lipophilic compounds [10]. LPS is conceptualized as consisting of three distinct domains: lipid A, core oligosaccharide, and O antigen (also called the O polysaccharide, O-antigen, or O-polysaccharide). These regions have both distinct and overlapping functions in bacterial physiology. This review focuses on the role of lipid A, core, and O antigen in the sensing of LPS by host defense systems, targeting by antimicrobials, and the pathogenesis of P. aeruginosa.
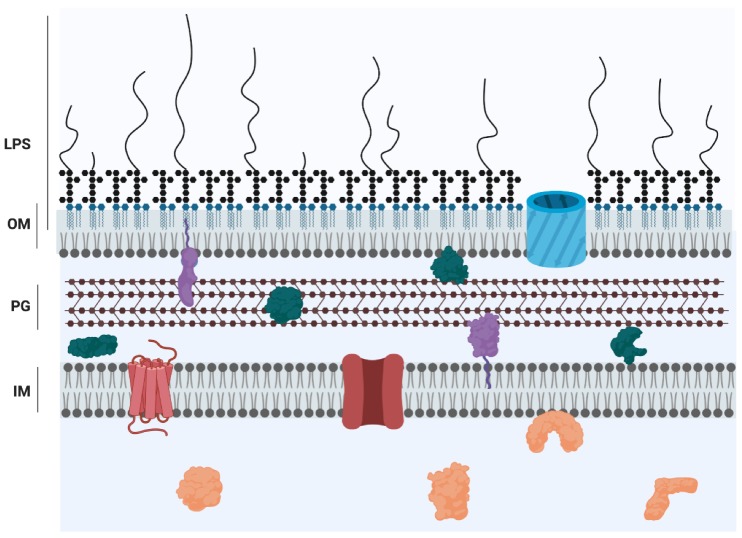
Figure 1.
Cartoon representation of the Gram-negative cell envelope. The inner membrane (IM) is a symmetric bilayer comprised of phospholipids while the outer membrane (OM) is an asymmetric bilayer containing phospholipids in the inner leaflet and LPS in the outer leaflet. The domains of LPS are represented as follows: lipid A, blue; core, black hexagons; O antigen, curved lines. The membranes are separated by the periplasmic space, which contains a thin layer of peptidoglycan (PG) [8]. Proteins are localized to all compartments and represented by the following colours: orange, cytoplasmic proteins; red, inner membrane proteins; purple, lipoproteins; green, periplasmic proteins; blue, outer membrane protein.
2. Structure of Lipid A, Core, and O Antigen
Lipid A is the hydrophobic portion of LPS that anchors the molecule in the OM. It is an acylated glucosamine disaccharide that is phosphorylated on the 1 and 4′ positions. The tight packing of lipid A in the OM constitutes a gel-like permeability barrier to small hydrophobic solutes [10]. Although the structure and synthesis of lipid A is generally conserved, the number of acyl chains, degree of phosphorylation, and presence of other modifications can vary, all of which have important implications for interactions with the bacterium’s environment or host (see below) [11]. The predominant lipid A of P. aeruginosa PAO1, a thoroughly studied laboratory-adapted strain, is shown in Figure 2.
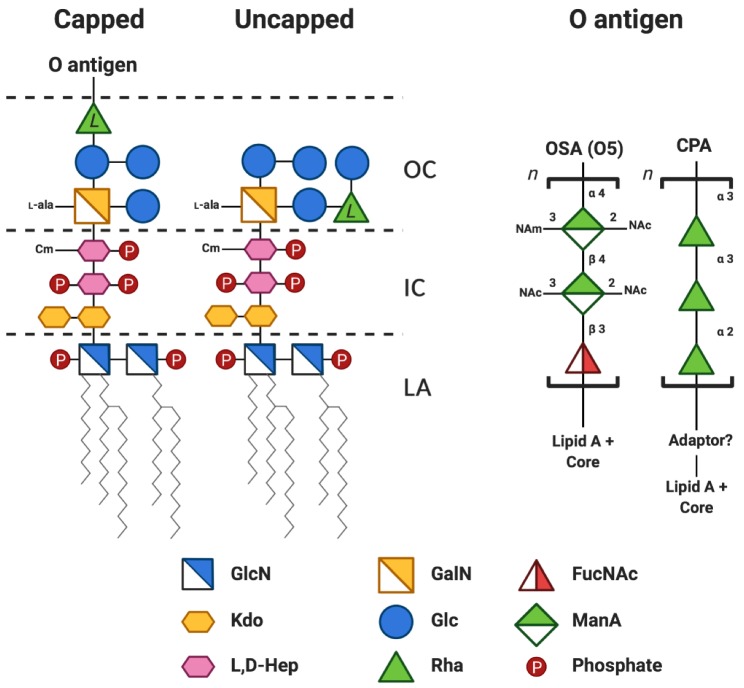
Figure 2.
Simplified chemical structure of P. aeruginosa PAO1 (serotype O5) lipopolysaccharide. The structure is adapted from several studies [12,13,14,15,16,17] and coloured according to the Symbol Nomenclature for Glycans (SNFG) [18,19]. A more detailed review of the chemical structure of P. aeruginosa LPS can be found in [17]. The lipid A-core region can be capped (or not) with a variable number of O antigen repeats. The predominant penta-acylated lipid A structure is shown. For clarity, the following modifications to the core sugars are not shown: the phosphorylation sites on the two heptose residues are depicted as monophosphorylated but may contain mono- di- or triphosphates; non-stoichiometric O-acetylation of the outer core sugars; the phosphate at position 2 in Hep II is non-stoichiometrically modified with phosphoethanolamine. The l-configuration of the rhamnose in the core is denoted by l to distinguish it from d-rhamnose found in the CPA repeat unit. A short sugar adapter may be present between CPA and the lipid A-core. OC, outer core; IC, inner core; LA, lipid A; GlcN, glucosamine; GalN, galactosamine; FucNAc, N-acetyl-d-fucosamine Kdo, 3-deoxy-d-manno-oct-2-ulosonic acid; Glc, glucose; ManA, manuronic acid; l,d-Hep, l-glycero-d-manno-heptose; Rha, rhamnose; Cm, 7-O-carbamoylation; l-Ala, 2-l-alanylation; n, variable number of repeats; NAm, N-amidino; NAc, N-acetyl.
The structure of the core oligosaccharide is overall more varied amongst GNB than lipid A, yet there are several conserved features. The core oligosaccharide is divided into two regions, the inner core and the outer core. The inner core typically contains Kdo (3-deoxy-d-manno-oct-2-ulosonic acid) covalently linked to several heptose residues (either l,d-Hep, or d,d-Hep), although some core structures contain Ko (d-glycero-d-talo-oct-2-ulopyranosonic acid) instead of Kdo, or no heptose [20]. The heptoses are the targets of modifications, such as the addition of phosphate and phosphoethanolamine. The outer core region varies among bacterial species, but typically contain hexoses and hexosamines. In P. aeruginosa, the core oligosaccharide is heavily phosphorylated and composed of Kdo, heptose, galactosamine, glucose, and rhamnose (Figure 2). The negative charges on the core provide membrane stability through bridging interactions with divalent cations [10] and the proper folding of some outer membrane proteins are dependent on specific protein-core interactions [21]. Indeed, in the literature, Escherichia coli mutants with truncations of the core oligosaccharide exhibit a number of OM defects and are particularly susceptible to hydrophobic antibiotics and anionic detergents [10,22]. However, the core is not necessarily essential for viability, since mutants of E. coli lacking any core sugars and containing only lipid IVA (a tetra-acylated, di-phosphorylated, di-glucosamine) have been isolated, albeit, some in the presence of compensatory mutations [23,24,25,26].
The O antigen is the long polysaccharide component of LPS, the length of which can vary from one to hundreds of sugars. O antigen is synthesized separately from the lipid A-core and later attached to it. Consequently, not every lipid A-core molecule is appended with O antigen before export. The result is a heterogeneous OM surface containing LPS with and without O antigen (Figure 1). LPS containing lipid A-core only or lipid A-core and O antigen are both exported to the cell surface. Those LPS molecules containing O antigen are termed “capped”, while the ones lacking O antigen are termed “uncapped”. In the literature, bacteria containing O antigen-capped LPS are often described as “smooth” whereas those devoid of O antigen are termed “rough”. These terms refer to the smooth and rough colony morphologies of the bacteria when grown on solid media, rather than the properties of the bacterial membrane [27]. The O antigen polymer is comprised of repeating sugar units that are highly variable in structure between and within species [11]. In E. coli alone there are more than 180 known unique O antigen structures [28]. This variability is the basis of the intraspecies classification system, known as O-serotyping, which categorizes strains based on the specific O antigen presented on the cell surface. Serotyping of P. aeruginosa was originally developed using immunochemical assays but has now been supplemented with genetic methods such as PCR and sequencing. [27,29]. We guide the reader to Lam et al. [27] for a perspective on the history, benefits, and challenges of different serotyping methods in P. aeruginosa. The heterogeneity of O antigen structures is the result of differences in the identities of the sugars in the repeat unit, the linkages between them, the presence or absence of side branches, and non-stoichiometric modifications. P. aeruginosa can simultaneously produce two O antigens: the common polysaccharide antigen (CPA) and the O-specific antigen (OSA) [27,30]. CPA has a common, conserved structure, consisting of repeating units of →3)d-Rha(α1→3)d-Rha(α1→2)d-Rha(α1→ whereas the OSA structure is variable, and therefore the determinant used in serotyping to segregate this bacterial species into many groups [16,31].
3. Interactions of LPS with the Host Immune System
3.1. Animal and Plant Receptors Recognize LPS and Mount an Immune Response
The surface exposure of LPS and the conservation of certain structural features across species make it a primary elicitor of host defenses. In mammals, LPS is a microbe-associated molecular pattern (MAMP) that can be a potent activator of the host innate immune response by inducing the activation of signal transduction cascades, which invariably lead to the production of proinflammatory cytokines [32]. Over-activation of these pathways can cause the life-threatening syndrome septic shock. Extracellularly, LPS monomers are extracted from GNB or their outer membrane vesicles (OMVs) by LPS-binding protein (LBP) and transferred to soluble or membrane-bound CD14. LPS is then transferred to MD-2/TLR4 monomers, inducing dimerization and the activation of Mal/MyD88-dependent signaling. Alternatively, endocytosis of activated complexes induces TRIF-dependent signaling and a different immunological response [32]. Within the cytosol, LPS is bound directly by caspases (caspase 4 and caspase 5 in humans, caspase 11 in mice), which in turn activate GSDMD (gasdermin family protein). GSMD forms pores on the plasma membrane, which induces cell pyroptosis and facilitates the release of interleukins [33].
The main interaction of LPS with MD-2/TLR4 is through the acyl chains of lipid A, which pack within a pocket of MD-2. The C2 acyl chain protrudes from this pocket and forms a dimerization interface for a stretch of hydrophobic amino acids within TLR4. The phosphates of the GlcN residues also contribute to ionic interactions that are necessary for optimal agonism [34]. Although the interactions of inner core Kdo residues are not essential for dimerization of the MD-22/TLR42/LPS2 complex, lipid A molecules containing Kdo tend to be more potent agonists than their cognate lipid A molecules lacking Kdo [35]. In general, hypo-acylated and hypo-phosphorylated forms of lipid A are less potent agonists, or even antagonists, of TLR4 signalling [36,37,38]. However, this does not always hold true, as a clinical isolate of Burkholderia cenocepacia is known to be able to induce MD2-TLR4 activation (albeit to a lesser extent than E. coli hexa-acylated LPS) despite only expressing tetra and penta-acylated lipid A. In this case, the longer acyl chains and aminoarabinosylation of lipid A seem to compensate for this hypo-acylation [39]. The lipid A isolated from P. aeruginosa CF isolates are typically hexa- and hepta-acylated, which apparently are more potent in eliciting inflammatory responses compared to the pentacylated lipid A typically found in laboratory-adapted strains or those derived from non-CF-related infections. The acylation pattern is associated with disease severity since hepta-acylated lipid A is associated with late-stage CF infections [14,40,41,42].
In addition to TLR4, a number of studies have implicated another membrane receptor, the cystic fibrosis transmembrane conductance regulator (CFTR), in recognition of P. aeruginosa LPS. The CFTR is an important pathogen recognition molecule because it extracts LPS from the bacterial membrane and activates an inflammatory response via nuclear translocation of NF-κB [43]. The outer core oligosaccharide was identified as the ligand for CFTR and interacts with the first extracellular loop of this protein [44,45,46]. Individuals with CF are homozygous for CFTR alleles that negatively affect the transport, processing, or function of this ion channel. The most common of these alleles is the ΔF508 mutation [47]. Experiments using epithelial cell lines carrying either the wildtype or ΔF508 variants of CFTR indicated that the internalization of P. aeruginosa was reduced when mutant but not wildtype CFTR was expressed, suggesting that CFTR mutations may promote P. aeruginosa infection [44]. In contrast, CFTR-dependent internalization of P. aeruginosa in corneal epithelial cells is necessary for this bacterium to cause keratitis [48,49,50]. Similarly, in Salmonella enterica serovar Typhi, entry into intestinal epithelial cells is also mediated by CFTR, and it has been documented that CF patients possessing mutant forms of this protein might be protected from contracting typhoid fever [51].
Plants are also able to sense LPS and activate signaling pathways that ultimately lead to an innate immune response that includes reactive oxygen species (ROS) bursts, callose deposition, nitric oxide production, and transcription of defense-related genes [52,53,54]. The mechanisms underlying the recognition of LPS have only recently started to be understood. In Arabidopsis thaliana, a bulb-type S-domain 1 receptor-like kinase, termed LORE (lipooligosaccharide-specific reduced elicitation), was found to mediate the sensing of LPS and trigger a pathogen response. This LORE-dependent response was induced by LPS from P. aeruginosa, P. syringae, and Xanthomonas campestris, but not from E. coli, S. enterica, or Burkholderia spp. LORE was initially thought to minimally interact with lipid A, and this interaction was enhanced by the presence of the core oligosaccharide, but not the O antigen [53]. However, a follow up investigation determined that LORE senses medium chain-3-hydroxy-fatty acids (mc-3-OH-FA). Although these fatty acids are a component of Pseudomonas lipid A and other pseudomonal compounds, only free mc-3-OH-FA is sensed by LORE. The apparent sensing of LPS by LORE seems to be due to contamination of LPS purifications with minute amounts of mc-3-OH-FA. Indeed, purified LORE interacts with mc-3-OH-FA [55].
Thus far, LORE homologs are confined to the Brassicaceae family, hence other undefined receptors are likely to be responsible for sensing LPS, or similar metabolites, in other plant families. In rice (Oryza sativa), OsCERK1, the receptor for chitin oligomers and peptidoglycan, also appears to mediate sensing of LPS from several bacterial species, including P. aeruginosa [56]. However, whether LPS is the specific ligand of this receptor has not been shown and requires further investigation. Two A. thaliana proteins related to LBP, AtLBR-1 and AtLBR-2, were recently discovered and shown to bind LPS. Mutants of AtLBR-1 and AtLBR-2 were deficient in some of the typical LPS responses and AtLBR-2 also appears to respond to P. aeruginosa LPS by inducing a number of genes related to defence against pathogens [57,58]. Despite these advances in understanding LPS sensing in plants, much work needs to be done to elucidate the species-specific events that lead to induction of plant immune responses.
3.2. LPS Stimulates and Inhibits Host-Mediated Bacterial Defences
LPS is an inducer of the complement system, a cascade of proteins that recognizes microbes and induces localized inflammatory responses, phagocytosis, and deposition of the pore-forming membrane attack complex (MAC). The complement cascade can be activated by the classical mannose-binding lectin, as well as alternative pathways [59]. Lipid A, core, and O antigen activate one or more of these pathways but bacteria expressing long O antigen chains are usually more resistant to serum than their O antigen-deficient isogenic mutants [60,61,62,63,64,65,66]. However, specific chain lengths of O antigen have been shown to be important in conferring resistance [67,68,69,70,71,72,73]. Nonetheless, some bacteria are resistant to serum-killing effects in the absence of O antigen, e.g., Brucella melitensis [74]. In P. aeruginosa, the long but not the very long chains of OSA are necessary for serum resistance, and the total loss of regulation of O antigen chain length in mutant strains defective in the expression of the wzz gene results in attenuation in a mouse model of pneumonia [72,73]. Interestingly, a serum-resistant P. aeruginosa mutant derived from a serum-sensitive CF isolate displayed an increase in the production of long OSA chains, further supporting the role of chain length in this organism [75]. The exact role of O antigen in conferring serum resistance may vary between organisms, but the activation of MAC (complement proteins C5 to C9) away from the bacterial membrane [76,77,78,79], inefficient convertase formation due to blocking of C3b-factor B binding sites [80], and poor interaction of certain polysaccharides with C3b have all been observed as contributing factors to serum resistance [76,80]. Additionally, the antibody response to bacterial polysaccharides can also be detrimental to the effectiveness of complement-mediated killing. As reported by Wells et al., the serum of some patients with chronic bronchiecstasis were shown to have inhibited killing of P. aeruginosa due to increased anti-O antigen IgG2 antibody titers. The authors hypothesized that the increase in O antigen-specific IgG2 blocked complement deposition or other antibodies from reaching the cell surface [81]. Importantly, this inhibitory effect was correlated with decreased lung function and mirror early studies that found a similar relationship between elevated anti-O antigen IgG2 with poor prognosis in CF patients [82,83].
The presence of O antigen also protects bacteria from phagocytosis and has been demonstrated for many organisms [84,85,86,87,88,89,90,91]. Once engulfed, O antigen may facilitate bacterial survival or delay the onset of recognition of immune receptors. This is particularly important for pathogens that replicate intracellularly, such as Brucella, which has been shown to delay lysosome fusion with phagosomes and delay apoptosis in an O antigen-dependent manner [92,93]. The opsonization of bacterial surfaces by lectins facilitates microbial killing and clearance by phagocytosis. In the lung, P. aeruginosa encounters opsonizing lectins that target LPS, such as surfactant protein A (SP-A) and surfactant protein D (SP-D). P. aeruginosa strains that are able to glycosylate pilin with O antigen subunits are more frequently identified in CF isolates compared to those in non-CF isolates [94], and this modification was shown to increase bacterial fitness by providing resistance to opsonization by SP-A and SP-D. When combined with the observations that the lungs of CF patients are typically deficient in SP-A, SP-D, and other LPS-targeting lectins, this points to one possible reason why P. aeruginosa is a particularly good pulmonary pathogen [95].
LPS can also stimulate neutrophils to release neutrophil extracellular traps (NETs) that sequester invading pathogens. The current literature suggests that the CF lung is enriched with NETs, and one hypothesis is that this may drive selection for mucoid P. aeruginosa (overproduction of the biofilm polysaccharide alginate), a hallmark of chronic infection isolates [96]. Under conditions that mimic those found in the tissues, the release of NETs (so-called “NETosis”) is induced by P. aeruginosa LPS, presumably in an O antigen-specific manner [97].
4. LPS Influences Bacterial Physiology
4.1. OMV Biogenesis and Packaging
Outer membrane vesicles (OMVs) are produced by deliberate blebbing of the Gram-negative OM and are enriched with various biomolecules. OMVs have been reported to play important roles in cell–cell communication, antibiotic resistance, biofilm structure, and long-range delivery of public goods, toxins, and virulence factors [98]. Not surprisingly, since these vesicles are derived from LPS-rich membranes, OMV production is intricately linked to LPS structure. A major contributor to OMV biogenesis in P. aeruginosa is the production of the Pseudomonas Quinolone Signal (PQS). PQS is one of the molecules of the complex Pseudomonas quorum sensing circuit, which regulates P. aeruginosa group behaviours, virulence factor production, and biofilm formation. The highly hydrophobic PQS is exported to the OM, promoting its own excretion in OMVs by interacting with Lipid A acyl chains and phosphates, which induces membrane curvature [99,100,101,102]. Remodeling of lipid A in response to environmental cues also influences OMV biogenesis. Recent experiments in S. Typhimurium revealed that lipid A species modified with l-4-aminoarabinose and phosphoethanolamine were less likely to be incorporated into OMVs, whereas lipid A that was hepta- or penta-acylated were enriched in OMVs. This differential incorporation correlates with the geometry of the lipid species (more cylindrical versus conical, respectively) and consequently their propensity to induce membrane curvature and vesiculation [103,104].
O antigen also plays a role in OMV biogenesis but is poorly understood. First, it was noticed by Kadurugamuwa and Beveridge [105] that naturally occurring P. aeruginosa OMVs contained the anionic OSA, but not the neutral CPA, leading them to propose that charge repulsion of the O antigen chains induces membrane curvature and membrane budding. A similar observation was made in Porphyromonas gingivalis, wherein the anionic A-LPS was enriched in OMVs compared to the neutral O-LPS. The proteins carried by OMVs of P. aeruginosa and P. gingivalis are altered in the absence of the anionic O antigens, suggesting they are involved in the selective protein sorting process [106,107]. P. gingivalis proteins are linked to A-LPS through the type IX secretion system and may be a means of directing this sorting [108], but would be by other means in P. aeruginosa, which lacks this system. The presence or absence of O antigen also has implications for the kinetics of entry into host cells. OMVs derived from O antigen+ E. coli enter the cells faster and through lipid raft endocytosis whereas those from O antigen− E. coli are endocytosed slower through clathrin-coated pits [109].
4.2. The Role of LPS in Planktonic and Biofilm Modes of Growth
O antigen is necessary for effective swimming and swarming motility in many bacteria [110,111,112,113,114,115,116,117,118,119,120], which has been demonstrated in genetic studies that investigated the effect on motility when genes involved in various steps of the O antigen synthesis and assembly pathway are deleted. Our group reported the phenotypes of several such mutants in P. aeruginosa. Firstly, deletion of the protein responsible for attaching O antigen to the core only expresses lipid A-core on the surface and results in the loss of swimming and swarming motility due to a substantial decrease in flagella assembly [121]. Secondly, deletions in P. aeruginosa genes that result in a truncated core region (and the blocking of attachment of O antigen) are defective in swarming and swimming on semi-solid media, but this is not due to defects in flagella synthesis or function [122]. The defect is apparently due to increased cell hydrophobicity, leading to stronger cell–cell association, which was supported by further studies of cell physical properties using atomic force microscopy [122,123,124]. Similarly, O antigen may mediate surface translocation by acting as a surfactant or by increasing the “wettability” of the cell surface [118].
Bacteria often grow as biofilms, which are complex communities encased in an intricate polymeric structure composed of polysaccharides, DNA, proteins, and lipids that protect the cells from external stress. In contrast to the motile planktonic mode of growth, biofilms are usually attached to biotic or abiotic surfaces and are a major cause of persistent infection. P. aeruginosa is a model organism for studying biofilms. In fact, it was the first organism implicated in a medically associated biofilm when cell aggregates were observed in the sputum of CF patients [125]. The O antigen is linked to biofilm formation, although, whether biofilm production is positively or negatively affected by the presence of O antigen varies between bacterial species and may be influenced by the surface tested [126].
The P. aeruginosa LPS structure is highly dynamic during biofilm growth, and the production of different chemotypes may be beneficial for certain stages of biofilm development. Overall, the literature suggests that CPA is more important than OSA in establishing robust biofilms. In vitro experiments showed that as P. aeruginosa adapts to the biofilm mode of growth, the production of OSA but not CPA is decreased [127]. However, strong selective pressure for an OSA-deficient phenotype will eventually lead to mutations in OSA biosynthesis [128]. In the CF lung, OSA expression is usually lost by acquiring mutations in the biosynthetic gene clusters, while CPA expression is more stable [129]. One study by our group showed that mutations that result in the loss of O antigen, but leave an intact core, produce biofilms with a similar structure and biomass. However, differences were noted in a mutant strain (Δrmd) where only the synthesis of CPA, but not OSA, was disrupted. Between 16 h and 48 h of biofilm growth, the density of cells and exopolysaccharides was gradually reduced, suggesting a defect in biofilm maturation [107]. These results were substantiated in another study that observed the restoration of biofilm maturation when CPA synthesis was rescued in a CPA-deficient isolate, PA14 [130]. The importance of CPA in biofilms is also demonstrated by its link to the secondary messenger cyclic-di-GMP, the so-called “master regulator”, that induces the physiological changes necessary to switch from motile planktonic growth to sessile biofilm growth. The CPA O antigen chain length was decreased by a cyclic-di-GMP-responsive methyltransferase, WarA, suggesting that CPA modification may be involved in the switch to a biofilm growth [131]. Interestingly the rhamnose-rich CPA is similar to the O antigens of many phytopathogens [132,133,134], raising the possibility that CPA evolved to allow P. aeruginosa to develop biofilms on plants and soil. The role of OSA in biofilm biogenesis is less clear, but one proteomics study presented intriguing data to indicate that the proteins that regulate OSA length are overproduced upon attachment to a glass wool surface, suggesting a role that OSA plays in the early stages of biofilm development [135]. Once biofilms are established, the production of long OSA chains may no longer be necessary. In line with this, very long OSA chain lengths are downregulated in mucoid P. aeruginosa (a hallmark of chronic infection) [136]. It is noteworthy that the link between OSA and biofilm development has mostly been studied in PAO1 (serotype O5). Since each serotype has a unique polysaccharide structure that will have different physiochemical properties, how OSA influences biofilm development in other serotypes should be explored.
5. Antimicrobials Target LPS
5.1. Phages and Pyocins
The extension of LPS into the extracellular milieu makes it a prime receptor for many bacteriophages. LPS is therefore integrally linked to the phage life cycle and bacteria experience strong selective pressure by phage predation to remodel their LPS. Some phages may recognize the O antigen or the core OS, either exclusively or in addition to outer membrane proteins. Accordingly, phages can be highly specific for a given O antigen serotype, or can have a broader host range if they recognize more conserved constituents of LPS [137]. A number of LPS-specific phages that target P. aeruginosa have been described in the literature as well as phage-resistant mutants arising from mutations in LPS biosynthesis genes [138,139,140,141,142,143,144,145,146,147]. In other cases, phage resistance may arise when temperate bacteriophages encode proteins that modify the O antigen, conferring resistance to superinfection [148]. The P. aeruginosa temperate bacteriophage D3 encodes a peptide that inhibits the host O antigen polymerase, allowing a separate phage polymerase to dominate and produce O antigen with a β linkage instead of an α linkage between O units, resulting in seroconversion [141,149,150]. Clearly, phage predation can influence the LPS phenotype of bacteria and drive O antigen diversity. Understanding phage–LPS interactions is important because LPS-specific phages often encode enzymes that degrade or modify the O antigen in order to gain access to the cell membrane. These enzymes may be useful in developing novel narrow-spectrum antimicrobial therapies [151]. For instance, Olszak et al. showed that a polysaccharide lyase from phage LKA1 degrades P. aeruginosa serotype O5 OSA, and this sensitized bacteria to serum complement, reduced virulence in a wax moth larvae infection model, and disrupted biofilms [147].
Bacteriocins are proteinaceous antibiotics produced by bacteria for intra- or inter-species killing. P. aeruginosa produces a number of bacteriocins, termed S- R-, and F-pyocins. The S-pyocins are analogous to the colicins produced by E. coli: They are proteins that hijack outer membrane proteins to gain access to the cell and exert their killing effects via a toxin domain. Producers of S-pyocins are protected from self-killing by immunity proteins that block the cytotoxic activity. R- and F-pyocins (also called tailocins) evolved from contractile and flexible phage tails, respectively, and kill by puncturing the bacterial membrane and inducing depolarization [152]. The lectin-like bacteriocins (L-pyocins; Llb) are comprised of one or two monocot mannose-binding lectin domains (MMBL) and may kill at the OM surface by blocking the function of BamA, a protein of the β-barrel assembly machinery [153,154]. All three classes of pyocins have been shown to interact with different LPS constituents. Some S- and L-pyocins bind CPA to target their other membrane receptors, and the loss of CPA decreases killing efficiency by these bacteriocins [155,156]. In contrast, the R-pyocins exclusively recognize LPS and do not have secondary OMP receptors. Using defined LPS mutants, different subtypes of R-pyocins were determined to recognize different core constituents [157,158]. For instance, the terminal GlcIV of the uncapped glycoform is part of the receptor for the R3-pyocin since strains lacking this residue are resistant to R3-mediated killing [158]. The recent structure of N-terminally truncated R2 pyocin suggests that a “foot domain” binds the core and that patches of mutations within specific loops of this domain drive specificity. Additionally, a distal “head domain” may bind O antigen [159]. Characterization of these LPS recognition domains may allow researchers to alter these killing particles to target a strain of choice and develop new antimicrobials. Importantly, the presence of LPS capped with OSA can protect bacteria from R pyocins, presumably by blocking access to the core [157]. In fact, the loss of O antigen due to mutations acquired during biofilm growth can result in sensitivity to self-produced R pyocins and a so-called “culture-impaired” phenotype, i.e., a drastically reduced ability to grow in liquid media [128]. O antigen likely also protects P. aeruginosa from the killing effects of S pyocins since studies in enterics have shown that the O antigen chain length and density are important factors in protecting them against colicins [160,161,162]. Interestingly, since a high proportion of CF isolates are susceptible to at least one subtype of R pyocin, the use of pyocin cocktails could potentially be viewed as highly targeted therapeutics to treat chronic infections in CF patients [163]. Indeed, the efficacy of a number of pyocins has been demonstrated in in vivo animal models [164,165,166,167].
Pyocins apparently play complex roles in establishing P. aeruginosa communities. The presence of R pyocins was shown to result in increased attachment and biofilm formation in susceptible strains at certain concentrations, but the mechanisms have yet to be determined [168]. Could pyocin-mediated killing drive changes to LPS that affect biofilm development (see above), or is this the result of a general stress response? In a separate study, R pyocins produced by one CF strain were necessary to outcompete another in a biofilm competition assay. When both strains lacked the ability to produce R pyocins, or when isolates producing the same R-pyocin subtype were grown together, the bacteria coexisted as a patchwork (adjacent communities) of individual strains [169]. Hence, these studies show that LPS-mediated pyocin susceptibility drives changes in the biofilm architecture and community, which may have downstream effects on disease outcomes.
5.2. LPS-Mediated Antibiotic Resistance
A myriad of oral, intravenous, and inhaled antibiotics are used to treat CF patients infected with P. aeruginosa and these include both monotherapies and combined antibiotic treatments [170]. Among these antibiotics are colistin (polymyxin E) and tobramycin (an aminoglycoside), whose efficacy is directly related to the LPS structure. In light of the increasing number of infections caused by multi-drug resistant Gram-negative bacteria, polymyxins have re-emerged in the clinic as last resort antibiotics. Polymyxins are cationic antimicrobial peptides (CAMPs) that target GNB through electrostatic interactions with lipid A and core phosphates, which is necessary for the self-mediated uptake of these antibiotics through the OM [171]. Accordingly, GNB defend against polymyxins by modifying the charge of their LPS through addition of positively charged moieties to lipid A phosphates. The best-described modifications in the literature are addition of l-4-aminoarabinose (l-Ara4N) and phosphoethanolamine (PEtN). These modification systems are controlled by complex networks of two-component regulators that sense magnesium, iron, zinc, cationic antimicrobial peptides, and pH [172]. The proteins for the synthesis and transfer of l-Ara4N are encoded chromosomally by the arn operon while PEtN addition is encoded chromosomally by eptA or the plasmid-borne mcr, which has garnered global concern due to its ability to mobilize colistin resistance [173]. P. aeruginosa can modify lipid A with both l-Ara4N and PEtN, but only l-Ara4N confers polymyxin resistance [174,175]. Although other mutations may play a role in polymyxin resistance, in vitro evolution studies point to the primary role of aminoarabinsoylation in establishing a trajectory towards high-level resistance. Firstly, high-level colistin resistance does not evolve in the absence of a functional arn operon [176]. Secondly, mutations in the two component systems controlling expression of the arn operon typically evolve first, and are necessary for synergistic interactions with mutations in other LPS biosynthesis genes, namely those involved in lipid A and core biosynthesis [177,178]. The acylation pattern of lipid A can also confer polymyxin resistance. PagL expression, which removes the 3-O-linked acyl chains from lipid A in the OM, is induced by polymyxin B and increases resistance to this CAMP only in an already resistant strain that constitutively aminoarabinosylates its lipid A. PagL-mediated resistance is due to decreased penetration of polymyxin B penetration through the OM, owing to the fewer available hydrophobic interactions with an underacylated lipid A [179]. Colistin insensitivity may also arise without modification of LPS. Yokota et al. showed that an inoculation effect can increase the MIC of colistin, which was attributed to the release of LPS either from dead cells or from OMVs [180]. These results agree with those of Manning and Kuehn, who showed that OMVs could protect bacteria by sequestering AMPs [181].
Since aminoglycosides also interact with the OM and enter the bacterial cell through self-promoted uptake, aminoarabinosylation of lipid A similarly confers resistance to these antibiotics. Importantly, the chelation of divalent cations and acidification of biofilms by extracellular DNA induces the arn operon and increases the aminoglycoside resistance of P. aeruginosa [182,183]. Additionally, loss of O antigen side chains is correlated with resistance to aminoglycosides, possibly by reducing binding to the cell surface [184] and membrane permeabilization [185].
5.3. New Classes of Antibiotics Target the LPS Biosynthesis Machinery of P. aeruginosa
New strategies are desperately needed to treat P. aeruginosa and other Gram-negative pathogens. Since LPS is essential to almost all GNB, the biosynthesis pathways are attractive targets for antimicrobial development, especially since these pathways often use substrates not found in humans. Inhibitors of the first committed step of lipid A biosynthesis, LpxC, have been designed with both broad and narrow spectrum antimicrobial activity (reviewed in [186]). Among these antimicrobials, a Pseudomonas-specific inhibitor developed by Achaogen was the only one to advance to Phase 1 clinical trials [187]. However, this compound was abandoned due to dose-limiting cardiovascular toxicity. Further development of this compound yielded several new leads, but the cardiovascular toxicity and narrow therapeutic window re-emerged in pre-clinical animal models [188]. Additionally, the potential for gaining resistance to these compounds was deemed too great to proceed [187]. Unfortunately, Achaogen filed for bankruptcy in April 2019, so the future development of these compounds remains uncertain [189].
A macrocyclic peptidomimetic antibiotic developed by Polyphor, termed Murepavadin (POL7080), has specific activity against Pseudomonas spp. This antibiotic targets LptD, an OMP of the LPS transport machine that, along with LptE, transports LPS from the periplasmic side of the OM to the outer leaflet [190]. The specificity of the antibiotic is due to targeting of a region of the periplasmic domain of LptD that is unique to pseudomonads [191]. Intravenous murepavadin has undergone Phase II clinical studies in patients with ventilator-associated bacterial pneumonia and non-cystic fibrosis associated bronchiecstasis but Phase III trials were halted after higher than expected renal toxicity was observed. In a 4 September 2019 news release, Polyphor has indicated that an inhalable murepavadin for treatment of P. aeruginosa infection in cystic fibrosis patients is expected to begin clinical trials in 2020 [192]. Concerningly, resistance to this compound may already exist and/or drive resistance to other antimicrobials. Romano et al. reported that resistance (4–32-fold change in MIC) to POL7080 can develop through pmrB mutations that upregulate the arn operon, resulting in decreased binding to the cell surface and the development of cross-resistance to colistin [193].
6. Concluding Remarks
In this review, we have described how LPS contributes to the pathogenesis of P. aeruginosa by (i) interacting with host receptors, (ii) inhibiting host defence systems, (iii) influencing the biogenesis of biofilms and OMVs, and (iv) mediating resistance to antimicrobials (Figure 3). Although the role of the lipid A, core, and O antigen moieties in these processes have been extensively studied, a comprehensive understanding of the interplay between LPS and pathogenesis will require further research. For instance, interactions of LPS with host proteins has been the focus of many studies, but recent research suggests that glycan–glycan interactions between bacteria and their hosts may be more relevant than previously realized [194,195,196]. The advent of new glycan arrays could facilitate the screening and discovery of novel LPS interactions and yield new insights into pathogenic processes [194,197,198].
Figure 3.
Summary of relevant P. aeruginosa O antigen, core, and lipid A interactions with antimicrobials and host defences. Arrows indicate binding of, or activation by, a specific LPS region while the flat-headed arrows indicate inhibition. The O antigen is coloured red, the core is coloured yellow, and lipid A is coloured blue.
The LPS glycoforms expressed on the cell surface can influence the biofilm mode of growth, yet the underlying mechanisms are poorly understood. Although differences in cell adhesion/cohesion may explain the propensity of a strain to form biofilms [122,123,124], the possibility that LPS may interact with matrix material also needs to be investigated. For instance, could CPA promote biofilm maturation by coordinating protein or polysaccharide components within the matrix? The role of LPS in biofilm development has mostly been studied on short time scales, under laboratory conditions. Since changes to LPS occur over the course of a chronic infection, the consequences of these changes on biofilm physiology should also be studied and with more clinically relevant systems. Additionally, other OSA polysaccharides may have more pronounced roles in biofilm development and should be investigated. O antigen also influences the selective packaging of cargo into OMVs, which are another component of biofilms. How O antigen is involved in this process is not understood and represents a significant gap in our knowledge. Unraveling this mechanism and characterizing the proteins that are deliberately packaged into OMVs may give clues to the function of OMVs within planktonic and biofilm communities.
A comprehensive understanding of LPS biosynthetic enzymes, gene regulatory pathways, and structure will be essential to developing new antimicrobials against P. aeruginosa and other Gram-negative pathogens. Although some promising compounds targeting LPS biosynthesis and transport have been developed, overcoming the development of resistance is a major hurdle that must be considered. The generation of therapeutics that inhibit the Lipid A modification pathways will be indispensable to mitigating CAMP resistance, while the continued characterization of “natural” antimicrobials that use LPS as a receptor, such as phages and bacteriocins, may be an effective way to develop targeted therapeutics. Finally, although LPS-based P. aeruginosa vaccines had only limited success in the past [199], this may still prove to be a promising prophylactic treatment since the use of OMVs and recombinant bacterial glycosylation pathways to produce glycoconjugates has made these vaccines safer, more effective, and cheaper to produce [200].
Author Contributions
Conceptualization, S.M.H., J.S.L., and C.M.K.; writing—original draft preparation, S.M.H.; writing—review and editing, S.M.H., J.S.L., and C.M.K.; funding acquisition, C.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC was funded by operating grants from the Canadian Institutes of Health Research (CIHR) awarded to C.M.K (PJT 156111).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Rice L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Bedard E., Prevost M., Deziel E. Pseudomonas aeruginosa in premise plumbing of large buildings. Microbiologyopen. 2016;5:937–956. doi: 10.1002/mbo3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gellatly S.L., Hancock R.E.W. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 4.Rahme L.G., Stevens E.J., Wolfort S.F., Shao J., Tompkins R.G., Ausubel F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 5.Schroth M.N., Cho J.J., Green S.K., Kominos S.D., Publishing M.S. Epidemiology of Pseudomonas aeruginosa in agricultural areas. J. Med. Microbiol. 2018;67:1191–1201. doi: 10.1099/jmm.0.000758. [DOI] [PubMed] [Google Scholar]
- 6.Cohen T.S., Prince A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Toole G.A. Cystic fibrosis airway microbiome: Overturning the old, opening the way for the new. J. Bacteriol. 2018;200:e00561-17. doi: 10.1128/JB.00561-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson J.C., Zimmerman S.M., Crofts A.A., Boll J.M., Kuhns L.G., Herrera C.M., Trent M.S. The power of asymmetry: Architecture and assembly of the Gram-negative outer membrane lipid bilayer. Annu. Rev. Microbiol. 2016;70:255–278. doi: 10.1146/annurev-micro-102215-095308. [DOI] [PubMed] [Google Scholar]
- 10.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitfield C., Trent M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 12.Bhat R., Marx A., Galanos C., Conrad R.S. Structural studies of lipid A from Pseudomonas aeruginosa PAO1: Occurrence of 4-amino-4-deoxyarabinose. J. Bacteriol. 1990;172:6631–6636. doi: 10.1128/jb.172.12.6631-6636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadovskaya I., Brisson J.R., Lam J.S., Richards J.C., Altman E. Structural elucidation of the lipopolysaccharide core regions of the wild-type strain PAO1 and O-chain-deficient mutant strains AK1401 and AK1012 from Pseudomonas aeruginosa serotype O5. Eur. J. Biochem. 1998;255:673–684. doi: 10.1046/j.1432-1327.1998.2550673.x. [DOI] [PubMed] [Google Scholar]
- 14.Ernst R.K., Yi E.C., Guo L., Lim K.B., Burns J.L., Hackett M., Miller S.I. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 15.Kooistra O., Bedoux G., Brecker L., Lindner B., Sanchez Carballo P., Haras D., Zahringer U. Structure of a highly phosphorylated lipopolysaccharide core in the ∆algC mutants derived from Pseudomonas aeruginosa wild-type strains PAO1 (serogroup O5) and PAC1R (serogroup O3) Carbohydr. Res. 2003;338:2667–2677. doi: 10.1016/j.carres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Bystrova O.V., Knirel Y.A., Lindner B., Kocharova N.A., Kondakova A.N., Zähringer U., Pier G.B. Structures of the core oligosaccharide and O-units in the R- and SR-type lipopolysaccharides of reference strains of Pseudomonas aeruginosa O-serogroups. FEMS Immunol. Med. Microbiol. 2006;46:85–99. doi: 10.1111/j.1574-695X.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 17.Knirel Y.A., Bystrova O.V., Kocharova N.A., Zähringer U., Pier G.B. Review: Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J. Endotoxin Res. 2006;12:324–336. doi: 10.1177/09680519060120060201. [DOI] [PubMed] [Google Scholar]
- 18.Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D., Stanley P., Hart G., Darvill A., Kinoshita T., et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 2015;25:1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neelamegham S., Aoki-Kinoshita K., Bolton E., Frank M., Lisacek F., Lutteke T., O’Boyle N., Packer N.H., Stanley P., Toukach P., et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology. 2019;29:620–624. doi: 10.1093/glycob/cwz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holst O. Structure of the Lipopolysaccharide Core Region. In: Knirel Y.A., Valvano M.A., editors. Bacterial Lipopolysaccharides: Structure, Chemical Synthesis, Biogenesis and Interaction with Host Cells. Springer Vienna; Vienna, Austria: 2011. pp. 21–39. [Google Scholar]
- 21.Arunmanee W., Pathania M., Solovyova A.S., Le Brun A.P., Ridley H., Baslé A., van den Berg B., Lakey J.H. Gram-negative trimeric porins have specific LPS binding sites that are essential for porin biogenesis. Proc. Natl. Acad. Sci. USA. 2016;113:E5034–E5043. doi: 10.1073/pnas.1602382113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagnout C., Sohm B., Razafitianamaharavo A., Caillet C., Offroy M., Leduc M., Gendre H., Jomini S., Beaussart A., Bauda P., et al. Pleiotropic effects of rfa-gene mutations on Escherichia coli envelope properties. Sci. Rep. 2019;9:9696. doi: 10.1038/s41598-019-46100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith T.C., Aggarwal P., Mamat U., Lindner B., Woodard R.W. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem. Biol. 2006;1:33–42. doi: 10.1021/cb0500015. [DOI] [PubMed] [Google Scholar]
- 24.Mamat U., Meredith T.C., Aggarwal P., Kuhl A., Kirchhoff P., Lindner B., Hanuszkiewicz A., Sun J., Holst O., Woodard R.W. Single amino acid substitutions in either YhjD or MsbA confer viability to 3-deoxy-d-manno-oct-2-ulosonic acid-depleted Escherichia coli. Mol. Microbiol. 2008;67:633–648. doi: 10.1111/j.1365-2958.2007.06074.x. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds C.M., Raetz C.R.H. Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry. 2009;48:9627–9640. doi: 10.1021/bi901391g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein G., Lindner B., Brabetz W., Brade H., Raina S. Escherichia coli K-12 Suppressor-free mutants lacking early glycosyltransferases and late acyltransferases: Minimal lipopolysaccharide structure and induction of envelope stress response. J. Biol. Chem. 2009;284:15369–15389. doi: 10.1074/jbc.M900490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam J.S., Taylor V.L., Islam S.T., Hao Y., Kocíncová D. Genetic and functional diversity of Pseudomonas aeruginosa lipopolysaccharide. Front. Microbiol. 2011;1:118. doi: 10.3389/fmicb.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DebRoy C., Fratamico P.M., Yan X., Baranzoni G., Liu Y., Needleman D.S., Tebbs R., O’Connell C.D., Allred A., Swimley M., et al. Comparison of O-antigen gene clusters of all O-serogroups of Escherichia coli and proposal for adopting a new nomenclature for O-typing. PLoS ONE. 2016;11:e0147434. doi: 10.1371/journal.pone.0147434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thrane S.W., Taylor V.L., Lund O., Lam J.S., Jelsbak L. Application of whole-genome sequencing data for O-specific antigen analysis and in silico serotyping of Pseudomonas aeruginosa isolates. J. Clin. Microbiol. 2016;54:1782–1788. doi: 10.1128/JCM.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera M., Bryan L.E., Hancock R.E., McGroarty E.J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: Analysis of lipopolysaccharide chain length. J. Bacteriol. 1988;170:512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokota S., Kaya S., Sawada S., Kawamura T., Araki Y., Ito E. Characterization of a polysaccharide component of lipopolysaccharide from Pseudomonas aeruginosa IID 1008 (ATCC 27584) as D-rhamnan. Eur. J. Biochem. 1987;167:203–209. doi: 10.1111/j.1432-1033.1987.tb13324.x. [DOI] [PubMed] [Google Scholar]
- 32.Weiss J., Barker J. Diverse pro-inflammatory endotoxin recognition systems of mammalian innate immunity. F1000Research. 2018;7 doi: 10.12688/f1000research.13977.1. F1000 Faculty Rev-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathinam V.A.K., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nat. Immunol. 2019;20:527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park B.S., Song D.H., Kim H.M., Choi B.-S., Lee H., Lee J.-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 35.Cochet F., Peri F. The Role of Carbohydrates in the lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017;18:2318. doi: 10.3390/ijms18112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teghanemt A., Zhang D., Levis E.N., Weiss J.P., Gioannini T.L. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 37.Korneev K.V., Arbatsky N.P., Molinaro A., Palmigiano A., Shaikhutdinova R.Z., Shneider M.M., Pier G.B., Kondakova A.N., Sviriaeva E.N., Sturiale L., et al. Structural relationship of the lipid A acyl groups to activation of murine Toll-like receptor 4 by lipopolysaccharides from pathogenic strains of Burkholderia mallei, Acinetobacter baumannii, and Pseudomonas aeruginosa. Front. Immunol. 2015;6:595. doi: 10.3389/fimmu.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjar A.M., Ernst R.K., Tsai J.H., Wilson C.B., Miller S.I. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 39.Di Lorenzo F., Kubik L., Oblak A., Lore N.I., Cigana C., Lanzetta R., Parrilli M., Hamad M.A., De Soyza A., Silipo A., et al. Activation of human Toll-like receptor 4 (TLR4).myeloid differentiation factor 2 (MD-2) by hypoacylated lipopolysaccharide from a clinical isolate of Burkholderia cenocepacia. J. Biol. Chem. 2015;290:21305–21319. doi: 10.1074/jbc.M115.649087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernst R.K., Adams K.N., Moskowitz S.M., Kraig G.M., Kawasaki K., Stead C.M., Trent M.S., Miller S.I. The Pseudomonas aeruginosa lipid A deacylase: Selection for expression and loss within the cystic fibrosis airway. J. Bacteriol. 2006;188:191–201. doi: 10.1128/JB.188.1.191-201.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst R.K., Hajjar A.M., Tsai J.H., Moskowitz S.M., Wilson C.B., Miller S.I. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J. Endotoxin Res. 2003;9:395–400. doi: 10.1177/09680519030090060201. [DOI] [PubMed] [Google Scholar]
- 42.SenGupta S., Hittle L.E., Ernst R.K., Uriarte S.M., Mitchell T.C. A Pseudomonas aeruginosa hepta-acylated lipid A variant associated with cystic fibrosis selectively activates human neutrophils. J. Leukoc. Biol. 2016;100:1047–1059. doi: 10.1189/jlb.4VMA0316-101R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder T.H., Lee M.M., Yacono P.W., Cannon C.L., Gerceker A.A., Golan D.E., Pier G.B. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc. Natl. Acad. Sci. USA. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pier G.B., Grout M., Zaidi T.S., Olsen J.C., Johnson L.G., Yankaskas J.R., Goldberg J.B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pier G.B., Grout M., Zaidi T.S., Goldberg J.B. How mutant CFTR may contribute to Pseudomonas aeruginosa infection in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996;154:S175–S182. doi: 10.1164/ajrccm/154.4_Pt_2.S175. [DOI] [PubMed] [Google Scholar]
- 46.Pier G.B., Grout M., Zaidi T.S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser-Pitt D., O’Neil D. Cystic fibrosis-a multiorgan protein misfolding disease. Future Sci. OA. 2015;1 doi: 10.4155/fso.15.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaidi T.S., Lyczak J., Preston M., Pier G.B. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect. Immun. 1999;67:1481–1492. doi: 10.1128/iai.67.3.1481-1492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaidi T., Mowrey-McKee M., Pier G.B. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Investig. Ophthalmol. Vis. Sci. 2004;45:4066–4074. doi: 10.1167/iovs.04-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaidi T., Bajmoczi M., Zaidi T., Golan D.E., Pier G.B. Disruption of CFTR-dependent lipid rafts reduces bacterial levels and corneal disease in a murine model of Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2008;49:1000–1009. doi: 10.1167/iovs.07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyczak J.B., Zaidi T.S., Grout M., Bittner M., Contreras I., Pier G.B. Epithelial cell contact-induced alterations in Salmonella enterica serovar Typhi lipopolysaccharide are critical for bacterial internalization. Cell. Microbiol. 2001;3:763–772. doi: 10.1046/j.1462-5822.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 52.Zeidler D., Zahringer U., Gerber I., Dubery I., Hartung T., Bors W., Hutzler P., Durner J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ranf S., Gisch N., Schaffer M., Illig T., Westphal L., Knirel Y.A., Sanchez-Carballo P.M., Zahringer U., Huckelhoven R., Lee J., et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015;16:426–433. doi: 10.1038/ni.3124. [DOI] [PubMed] [Google Scholar]
- 54.Shang-Guan K., Wang M., Htwe N.M.P.S., Li P., Li Y., Qi F., Zhang D., Cao M., Kim C., Weng H., et al. Lipopolysaccharides trigger two successive bursts of reactive oxygen species at distinct cellular locations. Plant Physiol. 2018;176:2543–2556. doi: 10.1104/pp.17.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kutschera A., Dawid C., Gisch N., Schmid C., Raasch L., Gerster T., Schaffer M., Smakowska-Luzan E., Belkhadir Y., Vlot A.C., et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science. 2019;364:178–181. doi: 10.1126/science.aau1279. [DOI] [PubMed] [Google Scholar]
- 56.Desaki Y., Kouzai Y., Ninomiya Y., Iwase R., Shimizu Y., Seko K., Molinaro A., Minami E., Shibuya N., Kaku H., et al. OsCERK1 plays a crucial role in the lipopolysaccharide-induced immune response of rice. New Phytol. 2018;217:1042–1049. doi: 10.1111/nph.14941. [DOI] [PubMed] [Google Scholar]
- 57.Iizasa S., Iizasa E., Matsuzaki S., Tanaka H., Kodama Y., Watanabe K., Nagano Y. Arabidopsis LBP/BPI related-1 and -2 bind to LPS directly and regulate PR1 expression. Sci. Rep. 2016;6:27527. doi: 10.1038/srep27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iizasa S., Iizasa E., Watanabe K., Nagano Y. Transcriptome analysis reveals key roles of AtLBR-2 in LPS-induced defense responses in plants. BMC Genom. 2017;18:995. doi: 10.1186/s12864-017-4372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajic G., Degn S.E., Thiel S., Andersen G.R. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34:2735–2757. doi: 10.15252/embj.201591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J. Immunol. 1974;112:935–940. [PubMed] [Google Scholar]
- 61.Grossman N., Leive L. Complement activation via the alternative pathway by purified Salmonella lipopolysaccharide is affected by its structure but not its O-antigen length. J. Immunol. 1984;132:376–385. [PubMed] [Google Scholar]
- 62.Burns A.M., Hull S.I. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect. Immun. 1998;66:4244–4253. doi: 10.1128/iai.66.9.4244-4253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins M.S., Ladehoff D.K., Mehton N.S., Noonan J.S. Opsonic and protective activity of five human IgM monoclonal antibodies reactive with lipopolysaccharide antigen of Pseudomonas aeruginosa. FEMS Microbiol. Immunol. 1990;2:263–268. doi: 10.1016/0928-8244(90)90021-J. [DOI] [PubMed] [Google Scholar]
- 64.Schreiber J.R., Cooper L.J., Diehn S., Dahlhauser P.A., Tosi M.F., Glass D.D., Patawaran M., Greenspan N.S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J. Infect. Dis. 1993;167:221–226. doi: 10.1093/infdis/167.1.221. [DOI] [PubMed] [Google Scholar]
- 65.Man-Kupisinska A., Swierzko A.S., Maciejewska A., Hoc M., Rozalski A., Siwinska M., Lugowski C., Cedzynski M., Lukasiewicz J. Interaction of mannose-binding lectin with lipopolysaccharide outer core region and its biological consequences. Front. Immunol. 2018;9:1498. doi: 10.3389/fimmu.2018.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasperkiewicz K., Swierzko A.S., Bartlomiejczyk M.A., Cedzynski M., Noszczynska M., Duda K.A., Michalski M., Skurnik M. Interaction of human mannose-binding lectin (MBL) with Yersinia enterocolitica lipopolysaccharide. Int. J. Med. Microbiol. 2015;305:544–552. doi: 10.1016/j.ijmm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Hong M., Payne S.M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol. Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 68.Bravo D., Silva C., Carter J.A., Hoare A., Alvarez S.A., Blondel C.J., Zaldívar M., Valvano M.A., Contreras I. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J. Med. Microbiol. 2008;57:938–946. doi: 10.1099/jmm.0.47848-0. [DOI] [PubMed] [Google Scholar]
- 69.Murray G.L., Attridge S.R., Morona R. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J. Bacteriol. 2006;188:2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray G.L., Attridge S.R., Morona R. Inducible serum resistance in Salmonella typhimurium is dependent on wzz(fepE)-regulated very long O antigen chains. Microbes Infect. 2005;7:1296–1304. doi: 10.1016/j.micinf.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Murray G.L., Attridge S.R., Morona R. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 2003;47:1395–1406. doi: 10.1046/j.1365-2958.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- 72.Kintz E., Scarff J.M., DiGiandomenico A., Goldberg J.B. Lipopolysaccharide O-antigen chain length regulation in Pseudomonas aeruginosa serogroup O11 strain PA103. J. Bacteriol. 2008;190:2709–2716. doi: 10.1128/JB.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivanov I.E., Kintz E.N., Porter L.A., Goldberg J.B., Burnham N.A., Camesano T. A Relating the physical properties of Pseudomonas aeruginosa lipopolysaccharides to virulence by atomic force microscopy. J. Bacteriol. 2011;193:1259–1266. doi: 10.1128/JB.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez-Prada C.M., Nikolich M., Vemulapalli R., Sriranganathan N., Boyle S.M., Schurig G.G., Hadfield T.L., Hoover D.L. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 2001;69:4407–4416. doi: 10.1128/IAI.69.7.4407-4416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohno A., Isii Y., Tateda K., Matumoto T., Miyazaki S., Yokota S., Yamaguchi K. Role of LPS length in clearance rate of bacteria from the bloodstream in mice. Microbiology. 1995;141:2749–2756. doi: 10.1099/13500872-141-10-2749. [DOI] [PubMed] [Google Scholar]
- 76.Schiller N.L., Joiner K.A. Interaction of complement with serum-sensitive and serum-resistant strains of Pseudomonas aeruginosa. Infect. Immun. 1986;54:689–694. doi: 10.1128/iai.54.3.689-694.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grossman N., Schmetz M.A., Foulds J., Klima E.N., Jimenez-Lucho V.E., Leive L.L., Joiner K.A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J. Bacteriol. 1987;169:856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joiner K.A., Grossman N., Schmetz M., Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J. Immunol. 1986;136:710–715. [PubMed] [Google Scholar]
- 79.Joiner K.A., Schmetz M.A., Goldman R.C., Leive L., Frank M.M. Mechanism of bacterial resistance to complement-mediated killing: Inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect. Immun. 1984;45:113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jimenez-Lucho V.E., Joiner K.A., Foulds J., Frank M.M., Leive L. C3b generation is affected by the structure of the O-antigen polysaccharide in lipopolysaccharide from salmonellae. J. Immunol. 1987;139:1253–1259. [PubMed] [Google Scholar]
- 81.Wells T.J., Whitters D., Sevastsyanovich Y.R., Heath J.N., Pravin J., Goodall M., Browning D.F., O’Shea M.K., Cranston A., De Soyza A., et al. Increased severity of respiratory infections associated with elevated anti-LPS IgG2 which inhibits serum bactericidal killing. J. Exp. Med. 2014;211:1893–1904. doi: 10.1084/jem.20132444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pressler T., Pedersen S.S., Espersen F., Høiby N., Koch C. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin. Exp. Immunol. 1990;81:428–434. doi: 10.1111/j.1365-2249.1990.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kronborg G., Pressler T., Fomsgaard A., Koch C., Høiby N. Specific IgG2 antibodies to Pseudomonas aeruginosa lipid A and lipopolysaccharide are early markers of chronic infection in patients with cystic fibrosis. Infection. 1993;21:297–302. doi: 10.1007/BF01712448. [DOI] [PubMed] [Google Scholar]
- 84.Engels W., Endert J., Kamps M.A., van Boven C.P. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 1985;49:182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.March C., Cano V., Moranta D., Llobet E., Perez-Gutierrez C., Tomas J.M., Suarez T., Garmendia J., Bengoechea J.A. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS ONE. 2013;8:e56847. doi: 10.1371/journal.pone.0056847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan Y.-J., Lin T.-L., Hsu C.-R., Wang J.-T. Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect. Immun. 2011;79:997–1006. doi: 10.1128/IAI.00906-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saldías M.S., Ortega X., Valvano M.A. Burkholderia cenocepacia O antigen lipopolysaccharide prevents phagocytosis by macrophages and adhesion to epithelial cells. J. Med. Microbiol. 2009;58:1542–1548. doi: 10.1099/jmm.0.013235-0. [DOI] [PubMed] [Google Scholar]
- 88.Eder K., Vizler C., Kusz E., Karcagi I., Glavinas H., Balogh G.E., Vigh L., Duda E., Gyorfy Z. The role of lipopolysaccharide moieties in macrophage response to Escherichia coli. Biochem. Biophys. Res. Commun. 2009;389:46–51. doi: 10.1016/j.bbrc.2009.08.082. [DOI] [PubMed] [Google Scholar]
- 89.Burns S.M., Hull S.I. Loss of resistance to ingestion and phagocytic killing by O(-) and K(-) mutants of a uropathogenic Escherichia coli O75:K5 strain. Infect. Immun. 1999;67:3757–3762. doi: 10.1128/iai.67.8.3757-3762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams P., Lambert P.A., Haigh C.G., Brown M.R. The influence of the O and K antigens of Klebsiella aerogenes on surface hydrophobicity and susceptibility to phagocytosis and antimicrobial agents. J. Med. Microbiol. 1986;21:125–132. doi: 10.1099/00222615-21-2-125. [DOI] [PubMed] [Google Scholar]
- 91.Duerr C.U., Zenk S.F., Chassin C., Pott J., Gutle D., Hensel M., Hornef M.W. O-antigen delays lipopolysaccharide recognition and impairs antibacterial host defense in murine intestinal epithelial cells. PLoS Pathog. 2009;5:e1000567. doi: 10.1371/journal.ppat.1000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandez-Prada C.M., Zelazowska E.B., Nikolich M., Hadfield T.L., Roop R.M., 2nd, Robertson G.L., Hoover D.L. Interactions between Brucella melitensis and human phagocytes: Bacterial surface O-Polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect. Immun. 2003;71:2110–2119. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porte F., Naroeni A., Ouahrani-Bettache S., Liautard J.-P. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 2003;71:1481–1490. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kus J.V., Tullis E., Cvitkovitch D.G., Burrows L.L. Significant differences in type IV pilin allele distribution among Pseudomonas aeruginosa isolates from cystic fibrosis (CF) versus non-CF patients. Microbiology. 2004;150:1315–1326. doi: 10.1099/mic.0.26822-0. [DOI] [PubMed] [Google Scholar]
- 95.Korfhagen T.R. Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2001;25:668–672. doi: 10.1165/ajrcmb.25.6.f221. [DOI] [PubMed] [Google Scholar]
- 96.Rahman S., Gadjeva M. Does NETosis contribute to the bacterial pathoadaptation in cystic fibrosis? Front. Immunol. 2014;5:378. doi: 10.3389/fimmu.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pieterse E., Rother N., Yanginlar C., Hilbrands L.B., van der Vlag J. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front. Immunol. 2016;7:484. doi: 10.3389/fimmu.2016.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mashburn-Warren L., Howe J., Garidel P., Richter W., Steiniger F., Roessle M., Brandenburg K., Whiteley M. Interaction of quorum signals with outer membrane lipids: Insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 2008;69:491–502. doi: 10.1111/j.1365-2958.2008.06302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schertzer J.W., Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio. 2012;3:e00297-11. doi: 10.1128/mBio.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Florez C., Raab J.E., Cooke A.C., Schertzer J.W. Membrane distribution of the pseudomonas quinolone signal modulates outer membrane vesicle production in Pseudomonas aeruginosa. MBio. 2017;8:e01034-17. doi: 10.1128/mBio.01034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li A., Schertzer J.W., Yong X. Molecular conformation affects the interaction of the Pseudomonas quinolone signal with the bacterial outer membrane. J. Biol. Chem. 2019;294:1089–1094. doi: 10.1074/jbc.AC118.006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elhenawy W., Bording-Jorgensen M., Valguarnera E., Haurat M.F., Wine E., Feldman M.F. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. MBio. 2016;7:e00940-16. doi: 10.1128/mBio.00940-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonnington K.E., Kuehn M.J. Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in Salmonella during environmental transitions. MBio. 2016;7:e01532-16. doi: 10.1128/mBio.01532-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kadurugamuwa J.L., Beveridge T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haurat M.F., Aduse-Opoku J., Rangarajan M., Dorobantu L., Gray M.R., Curtis M.A., Feldman M.F. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 2011;286:1269–1276. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy K., Park A.J., Hao Y., Brewer D., Lam J.S., Khursigara C.M. Influence of O polysaccharides on biofilm development and outer membrane vesicle biogenesis in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2014;196:1306–1317. doi: 10.1128/JB.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lasica A.M., Ksiazek M., Madej M., Potempa J. The type IX secretion system (T9SS): Highlights and recent insights into its structure and function. Front. Cell. Infect. Microbiol. 2017;7:215. doi: 10.3389/fcimb.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Donoghue E.J., Sirisaengtaksin N., Browning D.F., Bielska E., Hadis M., Fernandez-Trillo F., Alderwick L., Jabbari S., Krachler A.M. Lipopolysaccharide structure impacts the entry kinetics of bacterial outer membrane vesicles into host cells. PLoS Pathog. 2017;13:e1006760. doi: 10.1371/journal.ppat.1006760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noh J.-G., Jeon H.-E., So J.-S., Chang W.-S. Effects of the Bradyrhizobium japonicum waaL (rfaL) gene on hydrophobicity, motility, stress tolerance, and symbiotic relationship with soybeans. Int. J. Mol. Sci. 2015;16:16778–16791. doi: 10.3390/ijms160816778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiku K., Tsunemi K., Yamamoto M., Ohnishi-Kameyama M., Yoshida M., Ishii T., Taguchi F., Iwaki M., Ichinose Y., Ono H. Defects in D-rhamnosyl residue biosynthetic genes affect lipopolysaccharide structure, motility, and cell-surface hydrophobicity in Pseudomonas syringae pathovar glycinea race 4. Biosci. Biotechnol. Biochem. 2013;77:505–510. doi: 10.1271/bbb.120736. [DOI] [PubMed] [Google Scholar]
- 112.Zhou X., Liu B., Shi C., Shi X. Mutation of a Salmonella serogroup-C1-specific gene abrogates O7-antigen biosynthesis and triggers NaCl-dependent motility deficiency. PLoS ONE. 2014;9:e106708. doi: 10.1371/journal.pone.0106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan Q., Hu X., Wang N. The novel virulence-related gene nlxA in the lipopolysaccharide cluster of Xanthomonas citri ssp. citri is involved in the production of lipopolysaccharide and extracellular polysaccharide, motility, biofilm formation and stress resistance. Mol. Plant Pathol. 2012;13:923–934. doi: 10.1111/j.1364-3703.2012.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Post D.M.B., Yu L., Krasity B.C., Choudhury B., Mandel M.J., Brennan C.A., Ruby E.G., McFall-Ngai M.J., Gibson B.W., Apicella M.A. O-antigen and core carbohydrate of Vibrio fischeri lipopolysaccharide: Composition and analysis of their role in Euprymna scolopes light organ colonization. J. Biol. Chem. 2012;287:8515–8530. doi: 10.1074/jbc.M111.324012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morgenstein R.M., Clemmer K.M., Rather P.N. Loss of the WaaL O-antigen ligase prevents surface activation of the flagellar gene cascade in Proteus mirabilis. J. Bacteriol. 2010;192:3213–3221. doi: 10.1128/JB.00196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clemmer K.M., Bonomo R.A., Rather P.N. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bowden M.G., Kaplan H.B. The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol. Microbiol. 1998;30:275–284. doi: 10.1046/j.1365-2958.1998.01060.x. [DOI] [PubMed] [Google Scholar]
- 118.Toguchi A., Siano M., Burkart M., Harshey R.M. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: Critical role for lipopolysaccharide. J. Bacteriol. 2000;182:6308–6321. doi: 10.1128/JB.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han Y., Han X., Wang S., Meng Q., Zhang Y., Ding C., Yu S. The waaL gene is involved in lipopolysaccharide synthesis and plays a role on the bacterial pathogenesis of avian pathogenic Escherichia coli. Vet. Microbiol. 2014;172:486–491. doi: 10.1016/j.vetmic.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 120.Canals R., Jimenez N., Vilches S., Regue M., Merino S., Tomas J.M. The UDP N-acetylgalactosamine 4-epimerase gene is essential for mesophilic Aeromonas hydrophila serotype O34 virulence. Infect. Immun. 2006;74:537–548. doi: 10.1128/IAI.74.1.537-548.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abeyrathne P.D., Daniels C., Poon K.K.H., Matewish M.J., Lam J.S. Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J. Bacteriol. 2005;187:3002–3012. doi: 10.1128/JB.187.9.3002-3012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lindhout T., Lau P.C.Y., Brewer D., Lam J.S. Truncation in the core oligosaccharide of lipopolysaccharide affects flagella-mediated motility in Pseudomonas aeruginosa PAO1 via modulation of cell surface attachment. Microbiology. 2009;155:3449–3460. doi: 10.1099/mic.0.030510-0. [DOI] [PubMed] [Google Scholar]
- 123.Lau P.C.Y., Dutcher J.R., Beveridge T.J., Lam J.S. Absolute quantitation of bacterial biofilm adhesion and viscoelasticity by microbead force spectroscopy. Biophys. J. 2009;96:2935–2948. doi: 10.1016/j.bpj.2008.12.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lau P.C.Y., Lindhout T., Beveridge T.J., Dutcher J.R., Lam J.S. Differential lipopolysaccharide core capping leads to quantitative and correlated modifications of mechanical and structural properties in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2009;191:6618–6631. doi: 10.1128/JB.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Høiby N. A short history of microbial biofilms and biofilm infections. APMIS. 2017;125:272–275. doi: 10.1111/apm.12686. [DOI] [PubMed] [Google Scholar]
- 126.Ruhal R., Antti H., Rzhepishevska O., Boulanger N., Barbero D.R., Wai S.N., Uhlin B.E., Ramstedt M. A multivariate approach to correlate bacterial surface properties to biofilm formation by lipopolysaccharide mutants of Pseudomonas aeruginosa. Colloids Surf. B. Biointerfaces. 2015;127:182–191. doi: 10.1016/j.colsurfb.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 127.Beveridge T.J., Makin S.A., Kadurugamuwa J.L., Li Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 128.Penterman J., Nguyen D., Anderson E., Staudinger B.J., Greenberg E.P., Lam J.S., Singh P.K. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 2014;6:293–300. doi: 10.1016/j.celrep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lam M.Y.C., McGroarty E.J., Kropinski A.M., MacDonald L.A., Pedersen S.S., Hoiby N., Lam J.S. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J. Clin. Microbiol. 1989;27:962–967. doi: 10.1128/jcm.27.5.962-967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hao Y., Murphy K., Lo R.Y., Khursigara C.M., Lam J.S. Single-nucleotide polymorphisms found in the miga and wbpx glycosyltransferase genes account for the intrinsic lipopolysaccharide defects exhibited by Pseudomonas aeruginosa PA14. J. Bacteriol. 2015;197:2780–2791. doi: 10.1128/JB.00337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McCarthy R.R., Mazon-Moya M.J., Moscoso J.A., Hao Y., Lam J.S., Bordi C., Mostowy S., Filloux A. Cyclic-di-GMP regulates lipopolysaccharide modification and contributes to Pseudomonas aeruginosa immune evasion. Nat. Microbiol. 2017;2:17027. doi: 10.1038/nmicrobiol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bedini E., De Castro C., Erbs G., Mangoni L., Dow J.M., Newman M.-A., Parrilli M., Unverzagt C. Structure-dependent modulation of a pathogen response in plants by synthetic O-antigen polysaccharides. J. Am. Chem. Soc. 2005;127:2414–2416. doi: 10.1021/ja0452166. [DOI] [PubMed] [Google Scholar]
- 133.Clifford J.C., Rapicavoli J.N., Roper M.C. A rhamnose-rich O-antigen mediates adhesion, virulence, and host colonization for the xylem-limited phytopathogen Xylella fastidiosa. Mol. Plant Microbe Interact. 2013;26:676–685. doi: 10.1094/MPMI-12-12-0283-R. [DOI] [PubMed] [Google Scholar]
- 134.Rapicavoli J.N., Blanco-Ulate B., Muszyński A., Figueroa-Balderas R., Morales-Cruz A., Azadi P., Dobruchowska J.M., Castro C., Cantu D., Roper M.C. Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nat. Commun. 2018;9:390. doi: 10.1038/s41467-018-02861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crouzet M., Claverol S., Lomenech A.-M., Le Senechal C., Costaglioli P., Barthe C., Garbay B., Bonneu M., Vilain S. Pseudomonas aeruginosa cells attached to a surface display a typical proteome early as 20 minutes of incubation. PLoS ONE. 2017;12:e0180341. doi: 10.1371/journal.pone.0180341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cross A.R., Goldberg J.B. Remodeling of O Antigen in mucoid Pseudomonas aeruginosa via transcriptional repression of wzz2. MBio. 2019;10:e02914-18. doi: 10.1128/mBio.02914-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nobrega F.L., Vlot M., de Jonge P.A., Dreesens L.L., Beaumont H.J.E., Lavigne R., Dutilh B.E., Brouns S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018;16:760–773. doi: 10.1038/s41579-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 138.Kropinski A.M., Chan L., Jarrell K., Milazzo F.H. The nature of Pseudomonas aeruginosa strain PAO bacteriophage receptors. Can. J. Microbiol. 1977;23:653–658. doi: 10.1139/m77-098. [DOI] [PubMed] [Google Scholar]
- 139.Jarrell K., Kropinski A.M. Identification of the cell wall receptor for bacteriophage E79 in Pseudomonas aeruginosa strain PAO. J. Virol. 1977;23:461–466. doi: 10.1128/jvi.23.3.461-466.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jarrell K.F., Kropinski A.M. Pseudomonas aeruginosa bacteriophage phi PLS27-lipopolysaccharide interactions. J. Virol. 1981;40:411–420. doi: 10.1128/jvi.40.2.411-420.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuzio J., Kropinski A.M. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J. Bacteriol. 1983;155:203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Temple G.S., Ayling P.D., Wilkinson S.G. Isolation and characterization of a lipopolysaccharide-specific bacteriophage of Pseudomonas aeruginosa. Microbios. 1986;45:81–91. [PubMed] [Google Scholar]
- 143.Yokota S., Hayashi T., Matsumoto H. Identification of the lipopolysaccharide core region as the receptor site for a cytotoxin-converting phage, phi CTX, of Pseudomonas aeruginosa. J. Bacteriol. 1994;176:5262–5269. doi: 10.1128/jb.176.17.5262-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pan X., Cui X., Zhang F., He Y., Li L., Yang H. Genetic evidence for O-specific antigen as receptor of Pseudomonas aeruginosa phage K8 and its genomic analysis. Front. Microbiol. 2016;7:252. doi: 10.3389/fmicb.2016.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lim W.S., Phang K.K.S., Tan A.H.-M., Li S.F.-Y., Ow D.S.-W. Small colony variants and single nucleotide variations in Pf1 region of PB1 phage-resistant Pseudomonas aeruginosa. Front. Microbiol. 2016;7:282. doi: 10.3389/fmicb.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li L., Pan X., Cui X., Sun Q., Yang X., Yang H. Characterization of Pseudomonas aeruginosa phage K5 genome and identification of its receptor related genes. J. Basic Microbiol. 2016;56:1344–1353. doi: 10.1002/jobm.201600116. [DOI] [PubMed] [Google Scholar]
- 147.Olszak T., Shneider M.M., Latka A., Maciejewska B., Browning C., Sycheva L.V., Cornelissen A., Danis-Wlodarczyk K., Senchenkova S.N., Shashkov A.S., et al. The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci. Rep. 2017;7:16302. doi: 10.1038/s41598-017-16411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Taylor V.L., Fitzpatrick A.D., Islam Z., Maxwell K.L. The diverse impacts of phage morons on bacterial fitness and virulence. Adv. Virus Res. 2019;103:1–31. doi: 10.1016/bs.aivir.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 149.Newton G.J., Daniels C., Burrows L.L., Kropinski A.M., Clarke A.J., Lam J.S. Three-component-mediated serotype conversion in Pseudomonas aeruginosa by bacteriophage D3. Mol. Microbiol. 2001;39:1237–1247. doi: 10.1111/j.1365-2958.2001.02311.x. [DOI] [PubMed] [Google Scholar]
- 150.Taylor V.L., Udaskin M.L., Islam S.T., Lam J.S. The D3 bacteriophage α-polymerase inhibitor (Iap) peptide disrupts O-antigen biosynthesis through mimicry of the chain length regulator Wzz in Pseudomonas aeruginosa. J. Bacteriol. 2013;195:4735–4741. doi: 10.1128/JB.00903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roach D.R., Donovan D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage. 2015;5:e1062590. doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Michel-Briand Y., Baysse C. The pyocins of Pseudomonas aeruginosa. Biochimie. 2002;84:499–510. doi: 10.1016/S0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- 153.Ghequire M.G.K., De Mot R. LlpB represents a second subclass of lectin-like bacteriocins. Microb. Biotechnol. 2019;12:567–573. doi: 10.1111/1751-7915.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghequire M.G.K., Swings T., Michiels J., Buchanan S.K., De Mot R. Hitting with a BAM: Selective killing by lectin-like bacteriocins. MBio. 2018;9:e02138-17. doi: 10.1128/mBio.02138-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCaughey L.C., Grinter R., Josts I., Roszak A.W., Waloen K.I., Cogdell R.J., Milner J., Evans T., Kelly S., Tucker N.P., et al. Lectin-like bacteriocins from Pseudomonas spp. utilise D-rhamnose containing lipopolysaccharide as a cellular receptor. PLoS Pathog. 2014;10:e1003898. doi: 10.1371/journal.ppat.1003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McCaughey L.C., Josts I., Grinter R., White P., Byron O., Tucker N.P., Matthews J.M., Kleanthous C., Whitchurch C.B., Walker D. Discovery, characterization and in vivo activity of pyocin SD2, a protein antibiotic from Pseudomonas aeruginosa. Biochem. J. 2016;473:2345–2358. doi: 10.1042/BCJ20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Köhler T., Donner V., van Delden C. Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J. Bacteriol. 2010;192:1921–1928. doi: 10.1128/JB.01459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kocincova D., Lam J.S. A deletion in the wapB promoter in many serotypes of Pseudomonas aeruginosa accounts for the lack of a terminal glucose residue in the core oligosaccharide and resistance to killing by R3-pyocin. Mol. Microbiol. 2013;89:464–478. doi: 10.1111/mmi.12289. [DOI] [PubMed] [Google Scholar]
- 159.Salazar A.J., Sherekar M., Tsai J., Sacchettini J.C. R pyocin tail fiber structure reveals a receptor-binding domain with a lectin fold. PLoS ONE. 2019;14:e0211432. doi: 10.1371/journal.pone.0211432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Van der Ley P., de Graaff P., Tommassen J. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J. Bacteriol. 1986;168:449–451. doi: 10.1128/jb.168.1.449-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoa Tran E.N., Papadopoulos M., Morona R. Relationship between O-antigen chain length and resistance to colicin E2 in Shigella flexneri. Microbiology. 2014;160:589–601. doi: 10.1099/mic.0.074955-0. [DOI] [PubMed] [Google Scholar]
- 162.Sharp C., Boinett C., Cain A., Housden N.G., Kumar S., Turner K., Parkhill J., Kleanthous C. O-antigen-dependent colicin insensitivity of uropathogenic Escherichia coli. J. Bacteriol. 2019;201:e00545-18. doi: 10.1128/JB.00545-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Redero M., Lopez-Causape C., Aznar J., Oliver A., Blazquez J., Prieto A.I. Susceptibility to R-pyocins of Pseudomonas aeruginosa clinical isolates from cystic fibrosis patients. J. Antimicrob. Chemother. 2018;73:2770–2776. doi: 10.1093/jac/dky261. [DOI] [PubMed] [Google Scholar]
- 164.Scholl D., Martin D.W.J. Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model. Antimicrob. Agents Chemother. 2008;52:1647–1652. doi: 10.1128/AAC.01479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith K., Martin L., Rinaldi A., Rajendran R., Ramage G., Walker D. Activity of pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2012;56:1599–1601. doi: 10.1128/AAC.05714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McCaughey L.C., Ritchie N.D., Douce G.R., Evans T.J., Walker D. Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci. Rep. 2016;6:30201. doi: 10.1038/srep30201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Paskevicius S., Starkevic U., Misiunas A., Vitkauskiene A., Gleba Y., Razanskiene A. Plant-expressed pyocins for control of Pseudomonas aeruginosa. PLoS ONE. 2017;12:e0185782. doi: 10.1371/journal.pone.0185782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oliveira N.M., Martinez-Garcia E., Xavier J., Durham W.M., Kolter R., Kim W., Foster K.R. Biofilm formation as a response to ecological competition. PLoS Biol. 2015;13:e1002191. doi: 10.1371/journal.pbio.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oluyombo O., Penfold C.N., Diggle S.P. Competition in biofilms between cystic fibrosis isolates of Pseudomonas aeruginosa is shaped by R-pyocins. MBio. 2019;10:e01828-18. doi: 10.1128/mBio.01828-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tümmler B. Emerging therapies against infections with Pseudomonas aeruginosa. F1000Research. 2019;8 doi: 10.12688/f1000research.19509.1. F1000 Faculty Rev-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hancock R.E. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 172.Simpson B.W., Trent M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019;17:403–416. doi: 10.1038/s41579-019-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang H., Srinivas S., Xu Y., Wei W., Feng Y. Genetic and biochemical mechanisms for bacterial lipid A modifiers associated with polymyxin resistance. Trends Biochem. Sci. 2019;44:973–988. doi: 10.1016/j.tibs.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 174.Nowicki E.M., O’Brien J.P., Brodbelt J.S., Trent M.S. Extracellular zinc induces phosphoethanolamine addition to Pseudomonas aeruginosa lipid A via the ColRS two-component system. Mol. Microbiol. 2015;97:166–178. doi: 10.1111/mmi.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y.-Y., Chandler C.E., Leung L.M., McElheny C.L., Mettus R.T., Shanks R.M.Q., Liu J.-H., Goodlett D.R., Ernst R.K., Doi Y. Structural modification of lipopolysaccharide conferred by mcr-1 in Gram-Negative ESKAPE pathogens. Antimicrob. Agents Chemother. 2017;61:e00580-17. doi: 10.1128/AAC.00580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lo Sciuto A., Imperi F. Aminoarabinosylation of lipid A is critical for the development of colistin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018;62:e01820-17. doi: 10.1128/AAC.01820-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dosselmann B., Willmann M., Steglich M., Bunk B., Nubel U., Peter S., Neher R.A. Rapid and consistent evolution of colistin resistance in extensively drug-resistant Pseudomonas aeruginosa during morbidostat culture. Antimicrob. Agents Chemother. 2017;61:e00043-17. doi: 10.1128/AAC.00043-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jochumsen N., Marvig R.L., Damkiaer S., Jensen R.L., Paulander W., Molin S., Jelsbak L., Folkesson A. The evolution of antimicrobial peptide resistance in Pseudomonas aeruginosa is shaped by strong epistatic interactions. Nat. Commun. 2016;7:13002. doi: 10.1038/ncomms13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Han M.-L., Velkov T., Zhu Y., Roberts K.D., Le Brun A.P., Chow S.H., Gutu A.D., Moskowitz S.M., Shen H.-H., Li J. Polymyxin-induced lipid A deacylation in Pseudomonas aeruginosa perturbs polymyxin penetration and confers high-level resistance. ACS Chem. Biol. 2018;13:121–130. doi: 10.1021/acschembio.7b00836. [DOI] [PubMed] [Google Scholar]
- 180.Yokota S.-I., Hakamada H., Yamamoto S., Sato T., Shiraishi T., Shinagawa M., Takahashi S. Release of large amounts of lipopolysaccharides from Pseudomonas aeruginosa cells reduces their susceptibility to colistin. Int. J. Antimicrob. Agents. 2018;51:888–896. doi: 10.1016/j.ijantimicag.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 181.Manning A.J., Kuehn M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mulcahy H., Charron-Mazenod L., Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wilton M., Charron-Mazenod L., Moore R., Lewenza S. Extracellular DNA acidifies biofilms and induces aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:544–553. doi: 10.1128/AAC.01650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kadurugamuwa J.L., Lam J.S., Beveridge T.J. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob. Agents Chemother. 1993;37:715–721. doi: 10.1128/AAC.37.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schurek K.N., Marr A.K., Taylor P.K., Wiegand I., Semenec L., Khaira B.K., Hancock R.E.W. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008;52:4213–4219. doi: 10.1128/AAC.00507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Erwin A.L. Antibacterial drug discovery targeting the lipopolysaccharide biosynthetic enzyme LpxC. Cold Spring Harb. Perspect. Med. 2016;6:a025304. doi: 10.1101/cshperspect.a025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Krause K.M., Haglund C.M., Hebner C., Serio A.W., Lee G., Nieto V., Cohen F., Kane T.R., Machajewski T.D., Hildebrandt D., et al. Potent LpxC inhibitors with in vitro activity against multi-drug resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019;63:e00977-19. doi: 10.1128/AAC.00977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cohen F., Aggen J.B., Andrews L.D., Assar Z., Boggs J., Choi T., Dozzo P., Easterday A.N., Haglund C.M., Hildebrandt D.J., et al. Optimization of LpxC inhibitors for antibacterial activity and cardiovascular safety. ChemMedChem. 2019;14:1560–1572. doi: 10.1002/cmdc.201900287. [DOI] [PubMed] [Google Scholar]
- 189.Achaogen Inc. Achaogen Plans for Near-Term Sale Using Structured Process through Chapter 11 of the U.S. Bankruptcy Code. [(accessed on 26 November 2019)]; Available online: http://investors.achaogen.com/news-releases/news-release-details/achaogen-plans-near-term-sale-using-structured-process-through.
- 190.Srinivas N., Jetter P., Ueberbacher B.J., Werneburg M., Zerbe K., Steinmann J., Van der Meijden B., Bernardini F., Lederer A., Dias R.L.A., et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 191.Andolina G., Bencze L.-C., Zerbe K., Muller M., Steinmann J., Kocherla H., Mondal M., Sobek J., Moehle K., Malojcic G., et al. A peptidomimetic antibiotic interacts with the periplasmic domain of Lptd from Pseudomonas aeruginosa. ACS Chem. Biol. 2018;13:666–675. doi: 10.1021/acschembio.7b00822. [DOI] [PubMed] [Google Scholar]
- 192.Polyphor Polyphor Announces Financial Results for the First Half 2019 and Realigns Strategy. [(accessed on 26 November 2019)]; Available online: https://www.polyphor.com/news/corporate-news-details/?newsid=1820271.
- 193.Romano K.P., Warrier T., Poulsen B.E., Nguyen P.H., Loftis A.R., Saebi A., Pentelute B.L., Hung D.T. Mutations in pmrB Confer Cross-Resistance between the LptD inhibitor POL7080 and colistin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019;63:e00511-19. doi: 10.1128/AAC.00511-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Day C.J., Tran E.N., Semchenko E.A., Tram G., Hartley-Tassell L.E., Ng P.S.K., King R.M., Ulanovsky R., McAtamney S., Apicella M.A., et al. Glycan:glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells. Proc. Natl. Acad. Sci. USA. 2015;112:E7266–E7275. doi: 10.1073/pnas.1421082112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Belotserkovsky I., Brunner K., Pinaud L., Rouvinski A., Dellarole M., Baron B., Dubey G., Samassa F., Parsot C., Sansonetti P., et al. Glycan-glycan interaction determines Shigella tropism toward human T lymphocytes. MBio. 2018;9:e02309-17. doi: 10.1128/mBio.02309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tran E.N.H., Day C.J., Poole J., Jennings M.P., Morona R. Specific blood group antibodies inhibit Shigella flexneri interaction with human cells in the absence of spinoculation. Biochem. Biophys. Res. Commun. 2019 doi: 10.1016/j.bbrc.2019.10.091. [DOI] [PubMed] [Google Scholar]
- 197.Briard J.G., Jiang H., Moremen K.W., Macauley M.S., Wu P. Cell-based glycan arrays for probing glycan-glycan binding protein interactions. Nat. Commun. 2018;9:880. doi: 10.1038/s41467-018-03245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Geissner A., Reinhardt A., Rademacher C., Johannssen T., Monteiro J., Lepenies B., Thepaut M., Fieschi F., Mrazkova J., Wimmerova M., et al. Microbe-focused glycan array screening platform. Proc. Natl. Acad. Sci. USA. 2019;116:1958–1967. doi: 10.1073/pnas.1800853116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hoggarth A., Weaver A., Pu Q., Huang T., Schettler J., Chen F., Yuan X., Wu M. Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa. Drug Des. Dev. Ther. 2019;13:909–924. doi: 10.2147/DDDT.S189847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kay E., Cuccui J., Wren B.W. Recent advances in the production of recombinant glycoconjugate vaccines. NPJ Vaccines. 2019;4:16. doi: 10.1038/s41541-019-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]