Abstract
In randomized clinical trials (RCTs), it is assumed that non-specific effects beyond action of pharmacological agents are roughly equivalent in drug and placebo treatment groups. Hence, since the inception of RCTs, drug efficacy is determined by comparing outcomes in active to those in placebo control arms. However, quantitation of efficacy is based on an unproven assumption, that drug and placebo responses are always additive. Response to treatment in RCTs can be differentially influenced by the perturbing influence of patient expectations, side-effects, and pharmacogenomic interactions in both drug and placebo arms. Ability to control for these effects requires understanding of when and where they arise, how to mitigate, analyze, and even leverage their impact. Here, we examine three factors that influence additivity: expectation, side-effects, and pharmacogenomics. Furthermore, to provide novel insights into non-additivity and solutions for managing it, we introduce systems pharmacogenomics, a network approach to integrating and analyzing the effects of the numerous interacting perturbations to which a patient is exposed in RCTs.
Introduction
In randomized placebo controlled clinical trials (RCTs), the placebo treatment arm is designed to capture and control for the non-specific variables that can influence clinical outcomes. These include regression to the mean, spontaneous remission, changes due to the natural course of the disease, and placebo effects. Placebo effects refer to clinical improvement derived from the patient’s interactions with the clinician, the information they receive with regard to their condition and treatment, and the therapeutic ritual performed in the clinical setting (1). Hence factors that influence clinical outcomes in RCTs derive from the “players” engaged in the therapeutic encounter and their emergent properties. From the patient’s characteristics, the physician’s appearance and manner, and the information communicated, to the mis-en-scene, and the pharmacological agent’s properties, this complexity of factors can make predicting that there will be a statistically significant drug-placebo difference difficult. It is this complexity that placebo controls, along with randomization and double-blinding, were designed to reduce, allowing us to use simple arithmetic to subtract the non-specific effects captured in the placebo control arm to reveal the efficacy of the drug. Hence, determination of drug efficacy relies on the assumption that the placebo and drug responses are additive (i.e., drug efficacy = drug response – placebo response).
For drugs like antimicrobials or chemotherapeutics that target well-defined exogenous pathogens or cellular processes not under the influence of placebo effects, the additivity assumption appears to hold, allowing us to establish successfully the efficacy of numerous medications (2-4). In contrast, treatments for conditions such as pain, anxiety, and depression, and a wide-variety of functional and central sensitization disorders defined less by pathophysiology and more by symptoms (e.g., neuropathic pain, chronic fatigue, irritable bowel, and restless leg syndromes), where the difference between drug and placebo response is often quite small due to large placebo responses (5, 6), it has been and is increasingly more difficult for drugs to demonstrate efficacy beyond placebo (7-10). Consequently, it is important to understand whether additivity holds in RCTs of these conditions and, if not, can systems pharmacogenomics – new quantitative approaches that account for and integrate large datasets of patients genomic characteristics, the myriad perturbations to which patients are exposed and their clinical outcomes – help us to address this challenge in future RCTs.
The role of expectation in shaping clinical response
“Rather than producing direct and unambiguous pharmacological effects on a subject’s pain or anxiety, drugs act primarily as amplifiers or inhibitors or placebo effects.”
Dinnerstein et al. 1966 (11)
Expectation has long been identified as a factor that influences response to placebo treatment. Expectation or “set” as it was termed in earlier studies, refers to the subject’s belief about the potential effects of the treatment. Expectations are shaped by past experience, conditioning, verbal (suggestions, instructions, and informed consent disclosures) and non-verbal (labelling, rituals, and symbols) cues. Expectations can be positive or negative, and can thus influence additivity between placebo and drug treatment (Figure). The modifying effects of expectation are not limited to placebo treatment, and numerous studies have demonstrated that exposures that modify expectation can influence subjective and physiological outcomes in the drug treatment arms, as well.
Figure:
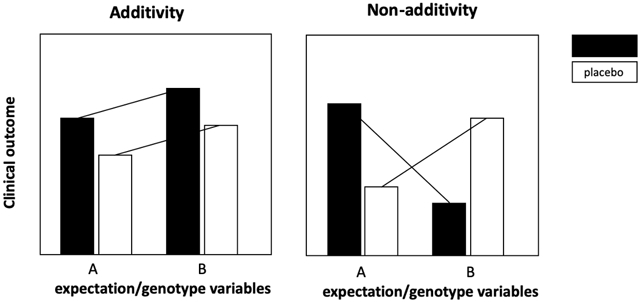
Expectation and genetic variation can result in differential effects in both the drug and placebo treatment arms that undermine the assumption of additivity.
When randomized clinical trials were established as the gold standard for determining drug efficacy shortly after World War II, clinicians and researchers began to realize that manipulating a subject’s expectation could influence the outcomes of RCTs. No sooner had Beecher in his landmark paper on “The Powerful Placebo” proposed a model of additivity, pointing out that the total ‘drug’ effect was equal to its ‘active’ effect plus its placebo effect, than the legitimacy of the model began to be called into question. Kast and Loesch demonstrated that the efficacy of pharmacologically active treatments was significantly enhanced when administered by a physician who displayed a positive endorsement of the efficacy of the drug (12). In 1964, Penick and Fisher, showed that administering epinephrine could have both stimulating and sedative effects depending on what information was conveyed to participants (13). Interestingly, these effects were not confined to subjective outcomes, but plasma fatty acid and glucose levels were also influenced by the set. Similarly, Lyerly et al. demonstrated that the effects of amphetamine and chloral hydrate compared to placebo varied depending on whether the participants were told they were receiving a sedative, receiving a stimulant, or given no instructions (14). Likewise, Dinnerstein et al. demonstrated surprising and differential effects of aspirin as a function of the instruction that the treatment was a tranquilizer or an energizer (15). Uhlenhuth et al. conducted a 2x2 factorial design study in which they examined meprobamate versus placebo and the effects of a positive, enthusiastic, and therapeutic attitude of the physician in the one direction versus an experimental, questioning attitude in the other direction (16). In this study patients diagnosed with neurosis were treated for anxiety. While they found that there was an interaction between the physician’s attitude and treatment, the “richness and complexity” of the clinical setting was underscored with the variation in the results at the three clinical sites, i.e., additivity varied across sites. Building on these studies, Moddell and Garett randomized participants to no-treatment (the appropriate control to be able to draw inferences about the effect of placebo treatment), placebo pentobarbital, or active pentobarbital. The unexpected outcome was an increase in the rate of high-amplitude finger tremor with placebo, while pentobarbital non-significantly reduced high-amplitude finger tremor. High-amplitude finger tremor also increased with no-treatment significantly. In this small study, Modell and Garett found that the directions of the placebo and drug effects were unexpected as the placebo increased negative symptoms, again reinforcing non-additivity (17). In these studies the physiologic effects were only evident among participants who received drug but not placebo, suggesting that modification of biological effects required a pharmacological substrate that could elicit a physical stimulus allowing the given instructions to shape the expectation and influence interpretation and perception of the drug effect (11).
In one of the largest placebo studies ever performed, the Trial of Asthma Patient Education (TAPE), researchers manipulated placebo effects in asthma using a 2x2 factorial plus a no-treatment-arm to examine montelukast versus placebo in one direction and enhanced versus neutral expectation in the other (18). The expectation enhancement arm included a scripted message plus a video emphasizing the positive attributes of the treatment and special blue and white capsule. In the low-expectation arm patients were given a neutral presentation on asthma and white capsules. The enhanced expectation intervention resulted in improved asthma control equivalent to the level observed in the montelukast arm and significantly better than the no-treatment arm. However, the expectation manipulation had no effect on objective lung function measures. Overall, lung function improved in the montelukast arm but not the placebo arm. These findings demonstrated that the drug and placebo responses were not simply additive in the placebo and drug treatment arms for an important outcome in asthma trials, viz., asthma control. Interestingly, headaches were more frequent among participants randomized to the enhanced expectation intervention in both the montelukast and placebo treatment arms, supporting the suggestibility of side-effects and again demonstrating lack of additivity in drug and placebo arms.
The observation, that expectations can modulate outcomes in RCTs, can and has been leveraged to improve clinical outcomes. In an RCT of oral immunotherapy for peanut allergy, Howe et al. randomized children to a positive “symptoms as positive signals” versus a “symptoms as side effects” mindset and found that participants in the positive mindset group were less anxious and more adherent to the oral immunotherapy treatment (19).
Studies that utilized neuroimaging coupled with expectation modification have advanced our understanding of non-additivity. Bingel et al. demonstrated that whereas a positive expectation of the analgesic effects of remifentanil resulted in enhanced analgesia and neural activity in regions of the brain involved with perception of pain intensity, the analgesic effect of remifentanil was completely abrogated by negative expectation while increasing hippocampal activity (20). Lidstone et al. further demonstrated that in patients with Parkinson disease, activation of dopaminergic signaling in the ventral striatum was strongest when the probability of receiving drug versus placebo treatment was high (75%), and this effect was correlated with response to active treatment and higher expectation for benefit from the treatment (21). Similarly in a meta-analysis of trials in major depressive disorder (MDD), a greater probability of receiving placebo (a surrogate for expectation) was associated with lower effectiveness of active treatment (22). Further, Pecina et al. demonstrated that patients with MDD who responded to active placebo (told “active antidepressant”) also responded to active antidepressant treatment and this treatment response was associated with activation of mu-opioid transmission in regions of the brain associated with MDD, stress, and emotional regulation (23). These studies not only indicate that additivity of placebo and drug responses vary with patient’s beliefs and expectation by trial, but also suggest that the effectiveness of removing placebo responders from RCTs using methodologies like the placebo-run may vary and the conditions under which these approaches will be effective need to be further understood.
Non-verbal or visual cues in the clinical setting, such as medication and drug labels, can also differentially affect outcomes in the drug and treatment arms. In a study of the effects of labelling in migraine, Kam-Hansen et al. labelled verum and placebo maxalt, as “placebo”, “maxalt” or “maxalt or placebo” (24). A no-treatment control was also included in the study. Whereas maxalt labelled “maxalt” was the most effective treatment, maxalt labelled “placebo” was no better than placebo labelled “maxalt.” This open versus hidden drug and placebo administration paradigm also provides an interesting corollary to the effect of non-verbal cues on expectations and symptoms (25). In the studies of Levine, Gordon, and Fields on pain and placebo with molar tooth extractions, they found that when administration of 6-8 mg of morphine was hidden from the view of the patient, the drug effect was equivalent to effect of placebo (saline) administered in plain view (26). Later studies revealed similar effects with open versus hidden administration of buprenorphine, tramadol, metamizole, and ketorolac (27). Importantly, these effects are not limited to pain; in post-operative patients, where diazepam was effective at reducing anxiety, hidden administration of diazepam was completely ineffective (25).
Taken together, these studies highlight how verbal and non-verbal cues can shape patient’s comprehension and emotional response, and guide their interpretation of physiological stimuli to be either positive and in favor of symptom reduction or negative resulting in nocebo (the opposite of placebo) effects. Although the finding that expectation matters has been replicated and elaborated in numerous subsequent studies, it has had little impact on the design and interpretation of clinical trials (28). Given the tight interconnectedness between active treatment and the ubiquity of information today, it is extremely difficult to control the nature of verbal and non-verbal information that patients enrolled in RCTs are exposed to. Further, challenges with patient recruitment, and the need for larger numbers of patients has resulted in the globalization of RCTs, introducing even more heterogeneity in expectations and complexity in their influence on clinical outcomes. Hence the potential for expectations to differentially impact treatment arms in RCTs remains a real and important concern.
The influence of side-effects and adverse events on outcomes in the placebo and drug treatment groups
Side-effects or adverse events (AE) among participants randomized to placebo treatment include, headache, bruising, nausea, and pain, and are common in RCTs. A recent systematic review of 1271 trials and 250,726 patients treated with placebo found that about half of the participants reported an AE and 5% dropped out because of an AE misattributed to the drug (29). A striking example of this latter point is in statin intolerance, where muscle-related AEs lead to discontinued treatment in 9-29% of observational studies and registries (30). There was, however, a similar incidence of muscle-related symptoms among placebo- and statin-treated patients in a meta-analysis of 26 RCTs (31). In a cross-over study in which patients who were intolerant to statins were randomized to either placebo or atorvastatin and then re-allocated to open-label atorvastatin, the rate of AE reporting increased significantly when participants were not blinded to statin treatment, compared to the double-blinded period where the rate of AE reported was equivalent between placebo and atorvastatin (32).
Physiological changes, whether they are direct drug effects or side-effects (e.g. yellowing of skin with beta-carotene treatment, bleeding with aspirin, or the taste of fish oil with omega-3 capsules), can cue patients that they are in the active treatment arm, frequently breaking-the-blind, and thus potentially enhancing the drug response, AE or side-effects. The influence of side-effects on the drug treatment outcomes was explored in a 2x2 factorial study in healthy normal participants who were given a painful stimulus with a drink plus or minus caffeine in one direction and information that the drink was a painkiller or placebo in the other direction (33). Without caffeine, there was no difference in the effect of the expectation manipulation; however, when caffeine was perceived as an analgesic, the participants experienced it as analgesic, compared to when they were told it was a placebo. Again, suggesting that side-effects are more likely to change the outcome when the participant experiences a physiological effect, no matter how non-specific that effect is. Another example of how side-effects can change drug efficacy was demonstrated in a study in which atropine, a drug that elicits dry mouth, was introduced to purposefully create a side-effect in both active drug (diclofenac) and matching placebo arms (34). In this study, Berna et al. found that without atropine, diclofenac was no better than placebo in eliciting analgesia, whereas atropine with diclofenac resulted in a 3-fold increase in analgesia. Notably, in this population of healthy volunteers, atropine did not enhance the analgesic effects of placebo, demonstrating once again, that drugs may induce somatosensory sensation which may be perceived as a signal that the drug is acting upon the dysfunctional neurologic site or sites per the instructions or suggestions of the clinician. As pointed out by Kirsch in a review of drug placebo additivity in depression, these subjective effects, induced by salient physiological responses to active drug, can enhance the placebo response in the drug but not placebo treatment arm, compromising additivity and possibly leading to overestimation of drug effects (35).
Enrichment window (36) and adaptive trial designs (37) have been introduced to manage several of the factors such as expectations, AE and side-effects, that lead to high placebo response and non-additivity. The Sequential Parallel Comparison Design (SPCD) (38), which after a pre-specified period of time in the trial, re-randomizes subjects assigned to placebo who do not respond to treatment (“non-responders”) to placebo or treatment, is one example of an adaptive trial design. Although these types of trials have the potential to minimize the traditional factors that influence non-additivity, they do not currently account for or manage pharmacogenomic effects.
Direct pharmacological effects on drug-placebo additivity
That drugs can modify placebo effects and response to placebo treatment in RCTs is not a novel concept. Starting in the 1970’s Levine et al. demonstrated that naloxone, a drug that inhibits opioid signaling, could also block placebo effects in pain (26). Building on these studies, Benedetti and Amanzio later reported that proglumide, a drug that inhibits cholecystokinin and hence increases opioid signaling, potentiated the placebo response in pain studies (39). Neuroimaging studies expanded our understanding of the pathways involved in placebo response identifying key brain regions activated in response to placebo treatment in pain (e.g., dorsolateral prefrontal cortex, nucleus accumbens, and periaqueductal grey) (40) and depression (rostral anterior cingulate, nucleus accumbens, and amygdala) (23, 41). Given this underlying neurochemistry, it would not be surprising that in addition to naloxone and proglumide, drugs that perturb opioid or dopaminergic signaling, e.g., by modifying the availability, metabolism, or signaling at the receptors of these neurotransmitters, might also perturb the placebo response resulting in either an enhancement or diminution of response to the therapy and, hence, unequal clinical effects in the active and inactive treatment arms (42, 43).
Genetics and non-additivity in RCTs
Building on placebo neuroimaging studies, candidate gene studies (44) and genome-wide association studies (45) suggest that variations in genes related to placebo neurotransmitter pathways, termed the placebome, could modify individual response to placebo treatment (42, 46). Interestingly, in a candidate gene analysis of subjective placebo response in asthma, Haug-Baltzell et al. reported a significant association between the placebome gene, TPH2, and subjective outcomes in the placebo treatment arm of four asthma studies (47). However, they negated these findings in asthma by combining them with trials in which a majority of patients with rheumatoid arthritis across five studies were randomized to placebo plus methotrexate in which not surprisingly there was no association with TPH2. Curiously, Haug-Baltzell then concluded that “previously reported placebo-response-associated variants do not predict patient outcomes in inflammatory disease phase III trial placebo arms.
When the placebome module was mapped to the interactome, the comprehensive, unbiased network of protein-protein interactions, it was found to overlap with or be in close proximity to disease modules or subnetworks of conditions with high placebo response (e.g., anxiety disorders, depression, and migraine), as well as with multiple drug targets (e.g., anti-anxiety medications, antidepressants, and analgesics) (48). In RCTs where participants are blinded to their treatment assignment, genetic variation in proteins with overlapping effects in disease, drug, and placebo pathways could have differential effects in drug and placebo treatment arms of an RCT (Figure). In other words, gene variation in placebome-related proteins has the potential to undermine the additivity assumption. A striking example of this theoretical outcome is in the recently reported GWAS of an RCT of duloxetine versus placebo for major depressive disorder (45). If placebo and drug treatments are, indeed, additive, one would at least expect to see a subset of the genome-wide significant and suggestive findings in the placebo treatment arm to be present in the drug treatment arm; however, this was not the case in this study. Interestingly genome-wide significant loci, which included STAC1 (a gene implicated in cognitive processes and functions related to expectation and associative learning), were observed in the placebo arm but not drug treatment arm. Several possibilities could account for the lack of overlap between genome-wide significant or suggestive loci in the placebo and duloxetine arms. First, there may be limited power due to the size of this study, with these overlapping effects only observed in larger studies. Second, genetic associations in the placebo arm of these studies may be spurious and not at all linked to placebo response; without a no-treatment control, this possibility is impossible to discern. Alternatively, duloxetine may perturb a genuinely STAC1-related placebo response, which would suggest a gene-disease/drug/placebo or gene-treatment interaction, again undermining the assumption of additivity. More GWAS will be required to determine which of these processes are important in order to understand genetic association differences in drug and placebo arms.
In candidate gene studies, gene-treatment interaction effects that contradict the assumption of additivity have been demonstrated in several studies of the placebome gene, catechol-O-methyltransferase or COMT. In an RCT of tolcapone (a COMT inhibitor), attention measures varied by COMT rs4680 genotype and randomized treatment allocation. Homozygotes of the low activity rs4680 variant (met/met) had higher outcomes in the placebo arm than homozygotes of the high-activity variant (val/val), (49). Conversely, in the tolcapone treatment arm, met/met individuals did worse than those with the val/val genotype. Although without a no-treatment control, it is not possible to conclude anything about the placebo effect, and it is well established that COMT has effects on executive function (50), we continue to support the notion that the additivity assumption would lead us to conclude that there was no difference between drug and placebo, as the drug and placebo responses are not simply additive (Figure).
In the NorCAPITAL trial of clonidine for the treatment of chronic fatigue syndrome, the original conclusion was the drug was not better than the placebo in improving the number of steps taken per day by the patients (51). When this study was reanalyzed based on COMT rs4680 genotype, a more nuanced picture emerged (52). Whereas val/val patients did better on placebo, those randomized to clonidine did worse, while there was no difference between drug and placebo for patients with the other COMT genotypes. This finding suggests that mutually exclusive processes were potentially operational in each of the arms of the trial, perhaps linked to the condition of chronic fatigue syndrome and the availability of catecholamines in the disease and placebogenic states, a hypothesis that was masked by the additivity assumption.
In the Women’s Health Study (WHS), an RCT of aspirin and vitamin E versus placebo for the prevention of cardiovascular disease and cancer, the original finding was that there was no difference between either of these drugs and placebo for both major outcomes (53). Reanalysis by COMT rs4680 revealed that there were significant differences by genotype in the placebo arm for cardiovascular disease outcomes, and that the direction of these effects changed in both the aspirin and vitamin E treatment arms (54). Specifically, met/met women benefited from randomization to aspirin or vitamin E with lower rates of cardiovascular disease compared to placebo. Conversely, val/val women had higher rates of cardiovascular disease with randomization to aspirin or vitamin E compared to placebo. Similar results were obtained for the effects of COMT rs4680 on vitamin E for the cancer prevention outcomes and these were replicated in another large clinical trial (55). Although we would not expect cardiovascular disease or cancer to be disease domains susceptible to placebo response, we note that these were prevention studies, where placebo treatment could reduce the effects of stress on chronic disease in some participants. Still, although we cannot attribute outcomes in the placebo arm to a placebo effect, the assumption that the responses in the placebo arm and drug treatment arm were additive led to the conclusion that the trials were null, missing potentially important pharmacogenomic interactions. Although COMT rs4680 is a common genetic variant with minor allele frequency among of 0.42 (56), it is not known how broadly and to what extent these and other pharmacogenomic effects influence outcomes in RCTs.
Differential effects between drug and placebo arms are not limited to genes implicated in placebo response: genes associated with disease, drug metabolism, or the mechanism of action of the drug may have differential effects in the placebo treatment arm likely unrelated to placebo effects. GUCY1A3, which encodes a subunit of guanylyl cyclase a receptor for nitric oxide; and LPA, which encodes a component of the plasma lipoprotein(a), both have differential effects in the placebo and aspirin treatment arms of the WHS resulting in significant gene-drug interactions that identified subpopulations for potential benefit in cardiovascular disease prevention (57, 58). In the Selenium and Vitamin E Prevention Trial, (SELECT), which was stopped early due to lack of efficacy, several genes (CAT, PRDX6, SOD2, SOD3, TXNRD2) associated with metabolism and transport of the anti-oxidants studied in this trial had differential effects in the placebo and drug treatment arms on rates of cancer. Although little is currently known about the scope, magnitude and relationship to gene dosage of these pharmacogenomic effects, these results support the use of pharmacogenomics in examining non-additivity and identifying subpopulations for differential benefit or harm, potentially improving the utility of RCTs.
Conclusions
As reviewed above, numerous factors influence outcomes in RCTs, from expectations and side-effects, to the pharmacological action of the drug and gene-treatment interactions. These factors are likely further compounded in global trials where geography, cultural diversity and more subtle differences in the patient populations, add to the complexity of the mix. Although it was hoped that double-blinding, randomization and placebo controls could mitigate the impact of these variables, the outcome of the clinical trials described in this paper, the increasing placebo response rate, and potential to miss subpopulations for benefit or harm, suggest that novel innovative approaches are warranted to optimize the design and analysis of some RCTs. Therefore, identifying the complex biomolecular systems within a patient that react and interact in response to multiple inputs in RCTs is an important next step in improving clinical trial design. Developing these new quantitative approaches to achieve integrated analyses that construct networks reflecting and accounting for the many perturbations to which patients are exposed and their net effects will require a systems based pharmacogenomic approach.
The goal of this review is to raise several challenges and concerns with non-additivity in RCTs, and to point out that our attempts to optimize and personalize treatment seldom take into account effects in the placebo treatment arm. The evidence provided in this review in support of understanding and harnessing placebo response has several important limitations. Non-additivity could be due to mechanisms beyond those we have discussed here including, overlapping regulatory pathways that could be disease-dependent, or factors related to the clinician’s bias. Many of the studies discussed in this review are small and most did not include a no-treatment arm, a key control for placebo studies. While the pharmacogenomic results discussed above are all from post-hoc analyses of trials and are therefore only hypothesis generating, the magnitude of these effects, therapeutic areas, type of drug and dosage that they influence, and their potential to confound the results and interpretation of RCTs, remain to be determined. In developing a group of genes important in understanding drug-placebo interactions, we recognize that the placebome as currently composed, is small and potentially biased. We anticipate that as more GWAS are conducted on placebo treatment arms, the placebome will grow to become a useful tool in guiding clinical trial design and improved selection of subpopulations that may benefit from active and as well as inactive treatments.
Although there are currently standard methods for assessing effect modification and confounding in clinical trials (59), in order to benefit from identification of drug-placebo pharmacogenomic interactions, we need to account for and leverage the genetic architecture of both drug and placebo responses. By moving away from additivity to a broader understanding of the potential for genetic effects in drug and placebo arms to mask the true benefits and harms of drugs in clinical trials, we propose that a systems pharmacogenomics approach – using network analysis to account for and integrate the many interacting effects – will allow us to manage non-additivity in the design and interpretation of past and prospective RCTs and thus improve precision in drug development and the treatment of disease (54).
Acknowledgements
We thank Ted J. Kaptchuk and Nancy R. Cook for helpful discussions.
Funding
This work was supported in part by National Institutes of Health grants HL61795, GM107618, and HG007690; and by American Heart Association grant D007382 to JL. KTH is funded by K01HL130625.
Footnotes
COI
All other authors declared no competing interests for this work.
References
- (1).Kaptchuk TJ & Miller FG Placebo effects in medicine. N Engl J Med 373, 8–9 (2015).10.1056/NEJMp1504023 [DOI] [PubMed] [Google Scholar]
- (2).Schlumberger M et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372, 621–30 (2015).10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- (3).Menezes P et al. Does haart efficacy translate to effectiveness? Evidence for a trial effect. PLoS One 6, e21824 (2011).10.1371/journal.pone.0021824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Miller FG, Colloca L & Kaptchuk TJ The placebo effect: Illness and interpersonal healing. Perspectives in biology and medicine 52, 518–39 (2009).10.1353/pbm.0.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Woolf CJ Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152, S2–15 (2011).10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wessely S, Nimnuan C & Sharpe M Functional somatic syndromes: One or many? Lancet 354, 936–9 (1999). 10.1016/S0140-6736(98)08320-2 [DOI] [PubMed] [Google Scholar]
- (7).Walsh BT, Seidman SN, Sysko R & Gould M Placebo response in studies of major depression: Variable, substantial, and growing. JAMA 287, 1840–7 (2002).10.1001/jama.287.14.1840 [DOI] [PubMed] [Google Scholar]
- (8).Tuttle AH et al. Increasing placebo responses over time in u.S. Clinical trials of neuropathic pain. Pain 156, 2616–26 (2015).10.1097/j.pain.0000000000000333 [DOI] [PubMed] [Google Scholar]
- (9).Khan A, Fahl Mar K & Brown WA Does the increasing placebo response impact outcomes of adult and pediatric adhd clinical trials? Data from the us food and drug administration 2000-2009. J Psychiatr Res 94, 202–7 (2017).10.1016/j.jpsychires.2017.07.018 [DOI] [PubMed] [Google Scholar]
- (10).Iovieno N & Papakostas GI Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: A meta-analysis. The Journal of clinical psychiatry 73, 1300–6 (2012).10.4088/JCP.11r07485 [DOI] [PubMed] [Google Scholar]
- (11).Dinnerstein AJ, Lowenthal M & Blitz B The interaction of drugs with placebos in the control of pain and anxiety. Perspect Biol Med 10, 103–17 (1966) [DOI] [PubMed] [Google Scholar]
- (12).Kast EC & Loesch J Influence of the doctor-patient relationship on drug action. The Illinois medical journal 119, 390–3 (1961) [PubMed] [Google Scholar]
- (13).Penick SB & Fisher S Drug-set interaction: Psychological and physiological effects of epinephrine under differential expectations. Psychosom Med 27, 177–82 (1965) [DOI] [PubMed] [Google Scholar]
- (14).Lyerly SB, Ross S, Krugman AD & Clyde DJ Drugs and placebos: The effects of instructions upon performance and mood under amphetamine sulphate and chloral hydrate. J Abnorm Psychol 68, 321–7 (1964) [DOI] [PubMed] [Google Scholar]
- (15).Dinnerstein AJ & Halm J Modification of placebo effects by means of drugs: Effects of aspirin and placebos on self-rated moods. J Abnorm Psychol 75, 308–14 (1970) [DOI] [PubMed] [Google Scholar]
- (16).Uhlenhuth EH, Canter A, Neustadt JO & Payson HE The symptomatic relief of anxiety with meprobamate, phenobarbital and placebo. Am J Psychiatry 115, 905–10 (1959).10.1176/ajp.115.10.905 [DOI] [PubMed] [Google Scholar]
- (17).Modell W & Garrett M Interactions between pharmacodynamic and placebo effects in drug evaluations in man. Nature 185, 538–9 (1960).10.1038/185538a0 [DOI] [PubMed] [Google Scholar]
- (18).Wise RA et al. Randomized trial of the effect of drug presentation on asthma outcomes: The american lung association asthma clinical research centers. J Allergy Clin Immunol 124, 436–44, 44e1-8 (2009).10.1016/j.jaci.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Howe LC et al. Changing patient mindsets about non-life-threatening symptoms during oral immunotherapy: A randomized clinical trial. The journal of allergy and clinical immunology In practice 7, 1550–9 (2019).10.1016/j.jaip.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bingel U et al. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 3, 70ra14 (2011).10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- (21).Lidstone SC et al. Effects of expectation on placebo-induced dopamine release in parkinson disease. Arch Gen Psychiatry 67, 857–65 (2010).10.1001/archgenpsychiatry.2010.88 [DOI] [PubMed] [Google Scholar]
- (22).Papakostas GI & Fava M Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in mdd. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 19, 34–40 (2009). 10.1016/j.euroneuro.2008.08.009 [DOI] [PubMed] [Google Scholar]
- (23).Pecina M et al. Association between placebo-activated neural systems and antidepressant responses: Neurochemistry of placebo effects in major depression. JAMA Psychiatry 72, 1087–94 (2015).10.1001/jamapsychiatry.2015.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kam-Hansen S et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science translational medicine 6, 218ra5 (2014).10.1126/scitranslmed.3006175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Benedetti F, Carlino E & Pollo A Hidden administration of drugs. Clin Pharmacol Ther 90, 651–61 (2011).10.1038/clpt.2011.206 [DOI] [PubMed] [Google Scholar]
- (26).Levine JD, Gordon NC, Smith R & Fields HL Analgesic responses to morphine and placebo in individuals with postoperative pain. Pain 10, 379–89 (1981) [DOI] [PubMed] [Google Scholar]
- (27).Amanzio M, Pollo A, Maggi G & Benedetti F Response variability to analgesics: A role for non-specific activation of endogenous opioids. Pain 90, 205–15 (2001) [DOI] [PubMed] [Google Scholar]
- (28).Frisaldi E, Shaibani A & Benedetti F Why we should assess patients' expectations in clinical trials. Pain Ther 6, 107–10 (2017).10.1007/s40122-017-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Howick J, Webster R, Kirby N & Hood K Rapid overview of systematic reviews of nocebo effects reported by patients taking placebos in clinical trials. Trials 19, 674 (2018).10.1186/s13063-018-3042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Stroes ES et al. Statin-associated muscle symptoms: Impact on statin therapy-european atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur Heart J 36, 1012–22 (2015).10.1093/eurheartj/ehv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cholesterol Treatment Trialists, C. et al. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–81 (2010).10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Gupta A et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the anglo-scandinavian cardiac outcomes trial-lipid-lowering arm (ascot-lla): A randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 389, 2473–81 (2017).10.1016/S0140-6736(17)31075-9 [DOI] [PubMed] [Google Scholar]
- (33).Bjorkedal E & Flaten MA Interaction between expectancies and drug effects: An experimental investigation of placebo analgesia with caffeine as an active placebo. Psychopharmacology (Berl) 215, 537–48 (2011).10.1007/s00213-011-2233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Berna C et al. Side effects can enhance treatment response through expectancy effects: An experimental analgesic randomized controlled trial. Pain 158, 1014–20 (2017).10.1097/j.pain.0000000000000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kirsch I Are drug and placebo effects in depression additive? Biol Psychiatry 47, 733–5 (2000) [DOI] [PubMed] [Google Scholar]
- (36).Merlo-Pich E, Alexander RC, Fava M & Gomeni R A new population-enrichment strategy to improve efficiency of placebo-controlled clinical trials of antidepressant drugs. Clin Pharmacol Ther 88, 634–42 (2010).10.1038/clpt.2010.159 [DOI] [PubMed] [Google Scholar]
- (37).Wason JMS, Brocklehurst P & Yap C When to keep it simple - adaptive designs are not always useful. BMC medicine 17, 152 (2019).10.1186/s12916-019-1391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Fava M, Evins AE, Dorer DJ & Schoenfeld DA The problem of the placebo response in clinical trials for psychiatric disorders: Culprits, possible remedies, and a novel study design approach. Psychotherapy and psychosomatics 72, 115–27 (2003).10.1159/000069738 [DOI] [PubMed] [Google Scholar]
- (39).Benedetti F, Amanzio M & Maggi G Potentiation of placebo analgesia by proglumide. Lancet 346, 1231 (1995).10.1016/s0140-6736(95)92938-x [DOI] [PubMed] [Google Scholar]
- (40).Ashar YK, Chang LJ & Wager TD Brain mechanisms of the placebo effect: An affective appraisal account. Annu Rev Clin Psychol 13, 73–98 (2017).10.1146/annurev-clinpsy-021815-093015 [DOI] [PubMed] [Google Scholar]
- (41).Sikora M, Heffernan J, Avery ET, Mickey BJ, Zubieta JK & Pecina M Salience network functional connectivity predicts placebo effects in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 1, 68–76 (2016).10.1016/j.bpsc.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hall KT, Loscalzo J & Kaptchuk T Pharmacogenomics and the placebo response. ACS chemical neuroscience 9, 633–5 (2018).10.1021/acschemneuro.8b00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Enck P, Bingel U, Schedlowski M & Rief W The placebo response in medicine: Minimize, maximize or personalize? Nat Rev Drug Discov 12, 191–204 (2013). 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- (44).Hall KT et al. Catechol-o-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PloS one 7, e48135 (2012).10.1371/journal.pone.0048135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Maciukiewicz M et al. Genome-wide association studies of placebo and duloxetine response in major depressive disorder. The pharmacogenomics journal 18, 406–12 (2018).10.1038/tpj.2017.29 [DOI] [PubMed] [Google Scholar]
- (46).Hall KT, Loscalzo J & Kaptchuk TJ Genetics and the placebo effect: The placebome. Trends Mol Med 21, 285–94 (2015).10.1016/j.molmed.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Haug-Baltzell A et al. Previously reported placebo-response-associated variants do not predict patient outcomes in inflammatory disease phase iii trial placebo arms. Genes and immunity 20, 172–9 (2019).10.1038/s41435-018-0018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wang RS, Hall KT, Giulianini F, Passow D, Kaptchuk TJ & Loscalzo J Network analysis of the genomic basis of the placebo effect. JCI insight 2, (2017). 10.1172/jci.insight.93911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Farrell SM, Tunbridge EM, Braeutigam S & Harrison PJ Comt val(158)met genotype determines the direction of cognitive effects produced by catechol-o-methyltransferase inhibition. Biol Psychiatry 71, 538–44 (2012).10.1016/j.biopsych.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hall KT, Loscalzo J & Kaptchuk T Systems pharmacogenomics: Gene, disease, drug and placebo interactions -- a case study in comt. Pharmacogenomics in press, (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sulheim D et al. Disease mechanisms and clonidine treatment in adolescent chronic fatigue syndrome: A combined cross-sectional and randomized clinical trial. JAMA Pediatr 168, 351–60 (2014).10.1001/jamapediatrics.2013.4647 [DOI] [PubMed] [Google Scholar]
- (52).Hall KT et al. Genetic variation in catechol-o-methyltransferase modifies effects of clonidine treatment in chronic fatigue syndrome. Pharmacogenomics J 16, 454–60 (2016).10.1038/tpj.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ridker PM et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 352, 1293–304 (2005).10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- (54).Hall KT et al. Polymorphisms in catechol-o-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol 34, 2160–7 (2014).10.1161/ATVBAHA.114.303845 ATVBAHA.114.303845 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hall KT et al. Comt and alpha-tocopherol effects in cancer prevention: Gene-supplement interactions in two randomized clinical trials. J Natl Cancer Inst, (2019).10.1093/jnci/djy204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).dbSNP. Database of single nucleotide polymorphisms (dbsnp). Released July 9, 2019 edn. (National Library of Medicine, Bethesda (MD):National Center for Biotechnology Information, 2019). [Google Scholar]
- (57).Hall KT et al. Genetic variation at the coronary artery disease risk locus gucy1a3 modifies cardiovascular disease prevention effects of aspirin. Eur Heart J, (2019).10.1093/eurheartj/ehz384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Chasman DI et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis 203, 371–6 (2009).10.1016/j.atherosclerosis.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Burt T & Dhillon S Pharmacogenomics in early-phase clinical development. Pharmacogenomics 14, 1085–97 (2013).10.2217/pgs.13.81 [DOI] [PMC free article] [PubMed] [Google Scholar]


