Abstract
Background
Previously, family-based designs and high-risk pedigrees have illustrated value for the discovery of high- and intermediate-risk germline breast cancer susceptibility genes. However, genetic heterogeneity is a major obstacle hindering progress. New strategies and analytic approaches will be necessary to make further advances. One opportunity with the potential to address heterogeneity via improved characterization of disease is the growing availability of multi-source databases. Specific to advances involving family-based designs are resources that include family structure, such as the Utah Population Database (UPDB). To illustrate the broad utility and potential power of multi-source databases, we describe two different novel family-based approaches to reduce heterogeneity in the UPDB.
Methods
Our first approach focuses on using pedigree-informed breast tumor phenotypes in gene mapping. Our second approach focuses on the identification of families with similar pleiotropies. We use a novel network-inspired clustering technique to explore multi-cancer signatures for high-risk breast cancer families.
Results
Our first approach identifies a genomewide significant breast cancer locus at 2q13 (p=1.6×10−8, LOD equivalent 6.64). In the region, IL1A and IL1B are of particular interest, key cytokine genes involved in inflammation. Our second approach identifies five multi-cancer risk patterns. These clusters include expected co-aggregations (such as, breast with both prostate, ovarian, and melanoma), and also identify novel patterns, including uterine, thyroid, and bladder cancers.
Conclusions
Our results suggest pedigree-informed tumor phenotypes can map genes for breast cancer, and that various different cancer pleiotropies exist for high-risk breast cancer pedigrees.
Impact
Both methods illustrate the potential for decreasing etiological heterogeneity that large, population-based multi-source databases can provide.
Keywords: familial breast cancer, tumor heterogeneity, family history of cancer, pan-cancer, multi-cancer risk
Introduction
The use of the family study design, and high-risk pedigrees in particular, was instrumental in the discovery of germline breast cancer susceptibility genes and our understanding of their pleiotropies.1,2 However, breast cancers, like other complex diseases, have many sources of heterogeneity that can hinder gene discovery. Efforts to identify additional etiological risk factors are hampered by these complexities and new methods to identify and reduce sources of heterogeneities are needed to identify novel disease loci. Deconstructing within-site heterogeneity and identification of across-site pleiotropies will require large multi-source data resources and computational techniques to mine them. Many large multi-source data resources are currently under development throughout the US and the world3–9, providing potential opportunities for a new wave of discoveries. In Utah, an established statewide multi-source database (the Utah Population Database, UPDB) with linked biobank resources exists. Here, we will describe two different novel family-based approaches using the UPDB, designed to address heterogeneity and identify pleiotropies, to illustrate the broad utility of multi-source databases.
Fundamentally necessary to family studies are data for relationship structure and disease, as well as knowledge of population expectations of disease. The former is critical for defining phenotypes that cluster in families and therefore has potential power for genetic discovery. The UPDB is currently the only statewide resource in the United States that links statewide genealogies (5 million [M] records that span 3–16 generations) with a statewide Surveillance Epidemiology and End-Results (SEER) Program cancer registry (Utah Cancer Registry [UCR], since 1966). Hence, it allows for both family construction and designation of significant clustering of disease. Other data sources are also linked to the UPDB (https://uofuhealth.utah.edu/huntsman/utah-population-database/data/), including: electronic medical records (1996-present); historical census data (1880; 1900–1940); vital statistics (1905-present); residential histories (back to 1900); linkages to environmental measures (geographic-based); biobanks. This multi-source database is unique and can be harnessed for many designs to study cancer risk and survivorship across the life span and across generations10–14.
Breast cancer is a prime example of a common, complex disease. Substantial etiological heterogeneity exists both within and across breast cancers in high-risk pedigrees. Reducing heterogeneity is an important design issue in family-based genetic research. For example, even within high-risk pedigrees, the discovery of BRCA1 and BRCA2 (BRCA1/2) required restriction to early-onset disease to clarify segregation.15,16 It is well-established that gene expression varies across tumors, and hence tumor expression phenotypes may hold promise for deconstructing heterogeneity. In breast cancer, tumor gene expression has been shown to differentiate tumors into intrinsic subtypes (Luminal A, Luminal B, HER2-enriched, and Basal-like),17,18 of which Basal-like has increased BRCA1 susceptibility.19 The first approach we will describe integrates tumor expression phenotypes with gene mapping in high-risk pedigrees. This approach is made possible by record-linkages between genealogy, cancer diagnoses, hospital medical records and biobanks, all available via the UPDB. We previously defined quantitative tumor expression phenotypes associated with high-risk pedigrees not attributed to BRCA1/2, and illustrated power for mapping breast cancer loci in one large pedigree.20 Here we apply the same approach to a second large, high-risk breast cancer pedigree.
Cancer pleiotropies are a well-accepted phenomenon, and crucial to genetic counselling for accurate risk predictions. In breast cancer, pleiotropies are known to vary by the risk gene involved (Figure 1). Hence, characterizing families by their patterns of familial cancer risk could provide new opportunities to identify families with similar genetic risk factors. Gene mapping focusing on multi-cancer patterns could also elucidate molecular factors that underlie pleiotropies. For example, Basal-like breast tumors show more gene expression similarities to high grade serous ovarian cancer than other breast tumor types21,22. The multiple linked data sources in the UPDB provide a platform to describe multi-cancer patterns of familial risk. Furthermore, links to biorepositories could support investigations into the molecular factors underlying pleiotropies, and links to environmental data investigations to shared exposures. In the second approach, we illustrate how data driven methods make it possible to uncover familial multi-cancer signatures. We recently introduced this novel multi-cancer clustering technique and defined four familial multi-cancer signatures in high-risk bladder cancer families.23 Here we focus on multi-cancer signatures for high-risk breast cancer families.
Figure 1.
Familial multi-cancer susceptibilities for a) BRCA1; b) BRCA2. Cancers: BR breast; PR prostate; CRC colorectal; OV ovarian; PA pancreas; MEL melanoma; FA Fanconi anemia. Source: https://ghr.nlm.nih.gov.
Material and Methods
The Utah Population Database
The vast majority of individuals residing in Utah are represented in the UPDB.24–27 Core to the UPDB is an immense genealogy that is record-linked to many other statewide datasets (including the UCR), with annual updates. The full genealogical data set contains nearly 5 M people with 28 M records and the linking of multiple distinct records for a specific person allows the UPDB to depict the life history of an individual based on medical and administrative data. There are currently 336,000 cancer records from the UCR with diagnoses beginning in 1966 that are linked to the UPDB. The UPDB is linked to the pathology records of two healthcare systems (University of Utah and Intermountain Healthcare) that together serve over 85% of the state, and facilitates access to over 4 M formalin-fixed paraffin-embedded (FFPE) tissue blocks linked to clinical data. It is also linked to external data repositories using a statewide federated ID, including ~85% of outpatient claims in the state of Utah (1996 – present).
The data contained in the UPDB may be used for biomedical and health-related research. It is a rich and unique resource for cancer research that can support genetic, epidemiologic, public health, and healthcare delivery studies. Overseeing ethical approvals for use of the UPDB data for research is the Resource for Genetic and Epidemiological Research (RGE) body, which was established by Executive Order of the Governor of Utah in 1982. RGE administers access to UPDB through a formal review process to ensure the protection of privacy and confidentiality of the persons and data held in UPDB, and protects the interests of the data contributors.28 A summary list of data contributors can be found in Supplementary Table 2.
Approach 1: Reducing heterogeneity: Breast cancer gene-mapping using a tumor expression phenotype
Breast cancer pedigrees were identified in the UPDB using record linkage between the 16-generation genealogy and statewide cancer records from the UCR. High-risk status was defined as a statistical excess of breast cancer compared UPDB internal rates (p<0.05). Pedigrees known to be attributable to BRCA1/2 from previous Utah studies were removed (i.e. screen positive or linked to chromosomes 17q21 or 13q13). Record linkage between the UPDB and pathology records in the University of Utah and Intermountain Healthcare systems allowed identification of pathology records and archived tissue blocks. We pursued matched tumor and GU FFPE tissues for 25 high-risk pedigrees. GU refers to tissue that is histologically determined to contain 0% tumor. In the absence of peripheral blood, DNA extracted from GU tissue can be used for germline (inherited) DNA (see Supplementary material for more detail). Eleven of the 25 pedigrees contained at least 15 cases for whom tumor blocks were available. These 11 pedigrees were selected for tumor and germline experiments. Tumor RNA was used for gene expression and GU DNA for germline genotyping. Tumor gene expression was measured using the PAM50 RT-qPCR research assay.29 We used the OmniExpress high-density SNP array for germline genotyping. Quality control included: duplicate check, sex check, SNP call-rate (95%), sample call rate (90%), and failure of Hardy-Weinberg equilibrium (p≤1×10−5). All women were of European ancestry. Ethical approvals for the study were governed by RGE and IRBs at the University of Utah (IRB_00096990) and Intermountain Healthcare (IRB_1015580).
We previously used a set of population-based breast tumors30 and identified five principal components from the 50 PAM50 classifier genes, referred to as dimensions PC1—PC531. PC3 and PC5 were shown to be significantly different between the population and the pedigree tumors and hence potentially powerful for gene mapping in pedigrees. Here we concentrate on high-risk pedigree 1822 (Figure 2) and dimension PC3 as the phenotype of interest. Tumors in pedigree 1822 were identified as the most significantly different of all 11 pedigrees to population tumors for PC3 (p=4.0×10−5).20 Germline DNA were available for 46 breast cancer cases and tumor RNA for 31. As previously described32, we considered breast cancer cases with tumors in the top decile of PC3 in the population as “extreme”, resulting in 10 PC3-extreme breast cancer cases for gene mapping in pedigree 1822.
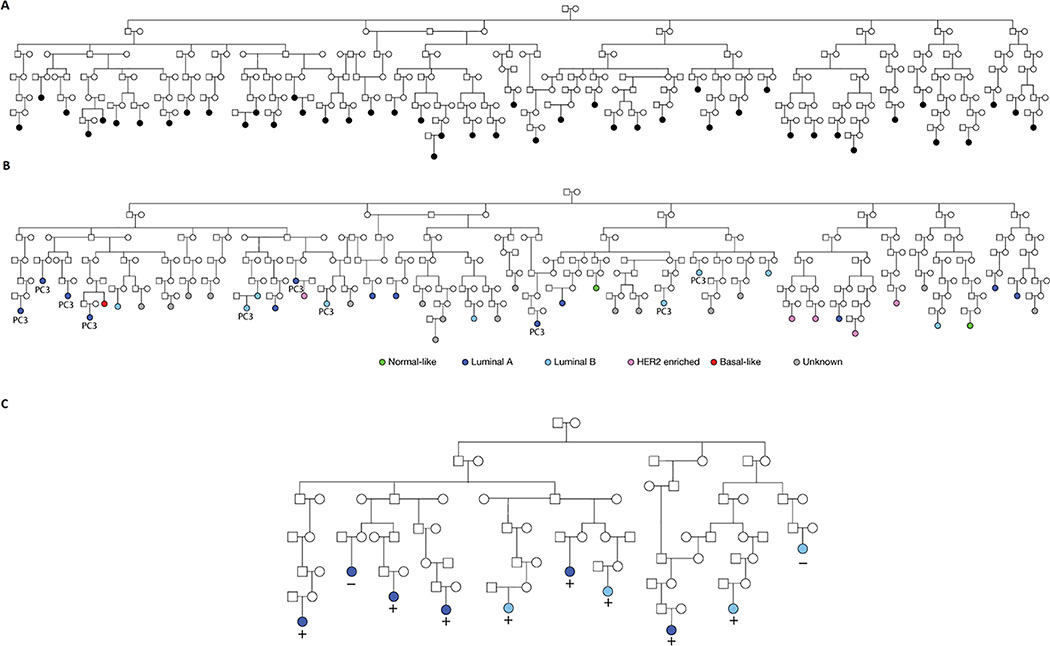
Figure 2 -.
Pedigree 1822 - Panel A: 46 breast cancer cases with germline DNA available (colored black). Panel B: 31 breast cancer cases with tumor expression data (colored green, light/dark blue, purple or red). Grey color indicates no tumor data. “PC3” indicates cases whose tumors were established to be extreme for PC3. Panel C: The reduced pedigree structure for 1822 based on the 10 PC3-extreme breast cancer cases. These 10 were the focus of the SGS gene mapping
We used Shared Genomic Segment (SGS) analysis33, a single pedigree method which identifies chromosomal identity-by-state (IBS) sharing at consecutive SNPs. Segregation from a common ancestor is implied if the observed IBS sharing is significantly longer than expected by chance.34,35 To address any residual heterogeneity, sharing evidence is assessed over all possible subsets. Statistical significance was determined empirically using a gene-drop approach. Briefly, a gene-drop assigns haplotypes randomly to pedigree founders under the null hypothesis (i.e. according to a population distribution –we used 1000Genomes Project36 data for our linkage disequilibrium [LD] model37). Mendelian segregation and recombination are simulated through the pedigree structure38 to generate genotypes for all pedigree members. We used the established Rutgers genetic map39 for simulating recombination events. For each simulated configuration of genotypes in the pedigree, shared segments are assessed and result in one genomewide expectation of sharing under the null hypothesis. The gene-drop procedure is repeated to generate a null distribution of sharing from which an empirical estimate of significance for the observed sharing is made. For accurate interpretation, a genomewide significance threshold is established which corrects for the subsets within the pedigree and the whole genome framework. After 1 M simulations, a gamma distribution is fit to the observed p-values across the genome. The genomewide significance threshold is derived from this distribution using the theory of large deviations.40
Approach 2. Identifying pleiotropic patterns- Multi-cancer signatures for familial breast cancer
High-risk breast cancer families were the focus of the clustering to identify multi-cancer pleiotropies. Linked genealogical, demographic, and cancer data from the UPDB were used. First, all individuals with breast cancer (‘probands’) and their first- (FDR), second- (SDR) and third-degree relatives (TDR) were identified using the UPDB. Only family members known to reside in Utah for at least one year from 1966 – 2017 were included. We identified 27,635 probands with at least one TDR and 1,696,913 family members. Second, this set was reduced to only families with at least 10 relatives to allow for family risk assessment. Familial risk for a cancer type was measured using Standardized Incidence Risk (SIR) ratios accounting for the sex, age, birth-cohort, and person-years of the pedigree members (for a detailed description of SIR calculations, see Supplemental Methods). Person-years were calculated using the minimum of the first year residing in Utah or 1966 to the year of first cancer diagnosis, last year of residence in Utah (due to death or migration), or 2017. Last, a total of 5,045 families (including 326,024 family members) were determined as high-risk for breast cancer, defined as a statistical excess of cases compared to the age and sex adjusted internal rates of the UPDB (p<0.05). These were the basis of our study. This study was approved by IRBs at the University of Utah (IRB_00088870, IRB_00079328).
Each of the 5,045 high-risk breast cancer families were further characterized by risk for 25 additional cancer types (26 total, including breast cancer). Other cancers were selected based on SEER Site codes and frequency (see Supplementary Table 1 for detailed information).41
Two risk metrics were used to capture a family’s multi-cancer signature. First, wSIR, the SIR weighted by the p-value. This incorporated both the magnitude and significance of the familial risk, and was calculated using the following equation. This metric allowed us to include, but down-weight, SIR values that were not significantly different than the overall population.
Where p is the p-value, i is the family, and j is the cancer type.
For robustness, and to avoid bias due to large SIRs (especially for rare cancers), we imposed a maximum value such that any wSIR values larger than the 90th percentile were set to the 90th percentile value across all families for the cancer type.
where 90 indicates the 90th percentile for cancer j.
Second, we included a dichotomous indicator of risk (ISIR). Families were considered to have “high risk” status for a cancer type (ISIR =1) if the SIR was statistically significant (p<0.05) and “population risk” (ISIR =0) otherwise. As all families were selected to be high-risk for breast cancer by design, we substituted the ISIR for breast with an indicator variable for male breast cancer. Our final matrix included 52 risk metrics per family (26 wSIR and 26 ISIR).
Clustering was performed on the 5,045 × 52 data matrix (families x risk metrics). A Gower general coefficient (ade4 R package) was used as the distance metric for clustering as it allows for the simultaneous use of our two risk metric types (wSIR continuous, ISIR categorical [detailed information can be found in the supplement]). We used partitioning around medoids (PAM or K-medoids clustering package in R42) to measure similarities between the multi-cancer risk signatures of families. K was selected by running a series of iterative models from k=2 to k=20 and using Silhouette (Supplementary Figure 1) and elbow plots to identify the point of diminishing improvement in average Silhouette width.
Bootstrapping was used to evaluate the reproducibility of the clustering (clustboot function in R) with 200 random draws. Results from each draw were transformed into a consensus matrix using the ward linkage algorithm and the (consensusmatrix function in R) and then plotted in a heatmap used for visualization. The results for k=5 were stable (Supplementary Figure 2).
Each cluster in the matrix represents a familial multi-cancer configuration (FMC) signature for high-risk breast cancer families. To describe and compare these clusters (FMCs), we used Cox proportional hazard models to estimate cluster specific differences in cancer incidence and their 95% CIs using the R package survival. All models controlled for birth year and sex.
Results
Approach 1. Reducing heterogeneity: Breast cancer gene-mapping using a tumor expression phenotype
Figure 2 illustrates pedigree 1822, showing the 46 breast cancer cases with germline DNA available (panel 1) and the subset of 31 with tumor expression data (panel 2). Their intrinsic subtype (the usual purpose of the PAM50) are also indicated for comparison. The ten PC3-extreme breast cancer cases used in the SGS analyses are shown in panel 3. The SGS genomewide significance threshold for 1822 was determined to be α=2.0×10−8, and one 0.6 Mb region at chromosome 2q13 surpassed this (p=1.6×10−8, from 113.2—113.8 Mb). This segment was shared by 8 of the 10 extreme PC3 breast cancer cases and was inherited through 38 meioses (Figure 2, panel 3). Ten genes are contained in the 2q13 locus: TTL; POLR1B; CHCHD5; SLC20A1; NT5DC4; CKAP2L; IL1A; IL1B; IL37; and IL36G.
We explored fine-mapping of the 2q13 locus within the pedigree by assessing the possibility that the shared haplotype inherited to others. We defined the eight SGS sharers as ‘core sharers’ and ranked all other breast cancer cases with genotype data based on their IBS sharing with them at this locus. We sequentially added these breast cancer cases to core sharers based on their ranking, and re-assessed SGS sharing across the full set after each addition. Figure 3 shows how the possible sharing narrows as cases are added. As a post-hoc analysis, this cannot be formally tested for significance, but it indicates there may be that an additional 15 cases who inherit the same 120,567 bp region. This reduced region contains only NT5DC4, CKAP2L, IL1A and IL1B.
Figure 3 –
LHS y-axis indicates the number of individuals sharing. From the established 8 ‘core sharers’, individuals are added, thus range is from 9 individuals to 46 (total cases with DNA). The RHS y-axis indicates the number of meioses separating the set of sharers on each row. The x-axis indicates the SNP markers across 2q13. Each horizontal rectangle is a shared segment, the color indicating the p-value for the segment (green highly significant, to red not-significant). The number in the white box on the green rectangles indicates the number of simulations more extreme than the observed segment. At 23 sharers (15 additional to the core 8), significance disappears and returns to that expected.
Approach 2. Identifying pleiotropic patterns- Multi-cancer signatures for familial breast cancer
The 5,045 high-risk breast cancer families in the UPDB ranged in size from 10 to 284 relatives (FDR, SDR, and TDR). Figure 4 shows the hazard rate ratios (HRR) for all 5,045 familial breast cancer families relative to the Utah population and for each familial multi-cancer configuration (FMC1–5). The clustering algorithm identified five family types based on their multi-cancer risks: FMC1 (2,159 families, 42.8%), FMC2 (657, 13.0%), FMC3 (625, 12.4%), FMC4 (1,004, 19.9%), and FMC5 (600, 11.9%). While, by definition, all clusters contained a statistical excess of breast cancer, the magnitude of breast cancer risk varied across clusters (see Table 1): FMC1 HRR=3.05 (95% CI, 2.98 – 3.18), FMC2 HRR =4.32 (4.14 – 4.50), FMC3 HRR=3.79 (3.64 – 3.94), FMC4 HRR =6.16 (5.96 – 6.37), FMC5 HRR=3.24 (3.12 – 3.37).
Figure 4 –
Familial Multiphenograms illustrating the patterns of familial cancer risk across the five high-risk familial multi-cancer configurations (FMC). The ‘Overall’ panel shows the fold difference in risk for all familial breast cancer families relative to the control population. The X-axis is truncated at 2.5 and values larger than 2.5 are noted within the horizontal bars on the chart. Panels FMC1 – FMC5 show the unique familial cancer patterns by FMC. These patterns differ significantly from each other and the overall pattern of cancer clustering in familial breast cancer families.
Table 1.
The overall hazard rate ratios (HRR) and 95% confidence intervals for 5045 familial breast cancer families relative to population controls by cancer type are displayed in column 2. Columns 3 – 7 show the HRs and 95% CIs by familial multi-cancer configuration (FMC), illustrating the heterogeneity in cancer risk by FMC.
| Overall | FMC1 | FMC2 | FMC3 | FMC4 | FMC5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CIs | HR | 95% CIs | HR | 95% CIs | HR | 95% CIs | HR | 95% CIs | HR | 95% CIs | |
| Breast | 3.64 | (3.57, 3.70) | 3.05 | (2.98, 3.12) | 4.32 | (4.14, 4.50) | 3.79 | (3.64, 3.94) | 6.16 | (5.96, 6.37) | 3.24 | (3.12, 3.37) |
| Ovary | 1.17 | (1.09, 1.26) | 0.19 | (0.15, 0.24) | 0.61 | (0.46, 0.82) | 0.72 | (0.57, 0.92) | 0.17 | (0.11, 0.28) | 6.10 | (5.64, 6.61) |
| Larynx | 0.99 | (0.93, 1.05) | 0.41 | (0.37, 0.46) | 0.69 | (0.56, 0.85) | 4.93 | (4.58, 5.31) | 0.19 | (0.14, 0.27) | 0.75 | (0.64, 0.88) |
| Melanoma of the Skin | 1.09 | (1.05, 1.13) | 0.76 | (0.72, 0.80) | 4.17 | (3.95, 4.40) | 0.83 | (0.75, 0.92) | 0.59 | (0.53, 0.67) | 0.94 | (0.86, 1.02) |
| Prostate | 1.09 | (1.06, 1.11) | 1.07 | (1.03, 1.10) | 1.08 | (1.01, 1.16) | 1.05 | (0.99, 1.12) | 1.20 | (1.13, 1.27) | 1.11 | (1.05, 1.17) |
| Acute Myeloid Leukemia | 1.00 | (0.91, 1.10) | 0.87 | (0.77, 0.99) | 1.30 | (1.02, 1.65) | 0.97 | (0.75, 1.24) | 1.34 | (1.08, 1.65) | 1.00 | (0.80, 1.24) |
| Acute Lymphocytic Leukemia | 1.06 | (0.97, 1.15) | 1.14 | (1.02, 1.27) | 1.57 | (1.28, 1.93) | 0.85 | (0.66, 1.09) | 0.92 | (0.73, 1.17) | 0.74 | (0.58, 0.94) |
| Hodgkin - Nodal | 1.04 | (0.91, 1.19) | 0.95 | (0.79, 1.14) | 1.20 | (0.83, 1.72) | 1.53 | (1.15, 2.04) | 1.14 | (0.83, 1.57) | 0.75 | (0.51, 1.08) |
| NHL - Nodal | 1.06 | (1.00, 1.11) | 1.05 | (0.98, 1.12) | 1.20 | (1.05, 1.39) | 0.91 | (0.79, 1.05) | 1.20 | (1.06, 1.36) | 0.98 | (0.87, 1.11) |
| Colon | 1.00 | (0.97, 1.03) | 0.96 | (0.91, 1.00) | 1.06 | (0.96, 1.16) | 1.02 | (0.93, 1.11) | 1.10 | (1.01, 1.20) | 1.03 | (0.95, 1.11) |
| Thyroid | 1.01 | (0.95, 1.07) | 1.03 | (0.95, 1.12) | 1.23 | (1.04, 1.45) | 0.78 | (0.65, 0.94) | 0.93 | (0.79, 1.10) | 1.04 | (0.89, 1.20) |
| Cervical | 0.80 | (0.74, 0.86) | 0.76 | (0.69, 0.84) | 0.98 | (0.80, 1.19) | 1.02 | (0.86, 1.21) | 0.84 | (0.70, 1.01) | 0.60 | (0.49, 0.74) |
| Uterine | 1.11 | (1.05, 1.17) | 1.05 | (0.97, 1.12) | 1.08 | (0.93, 1.27) | 1.17 | (1.02, 1.34) | 1.39 | (1.22, 1.57) | 1.06 | (0.93, 1.20) |
| Lung and Bronchus | 0.84 | (0.80, 0.88) | 0.77 | (0.72, 0.82) | 0.92 | (0.81, 1.05) | 1.06 | (0.95, 1.18) | 0.94 | (0.84, 1.06) | 0.77 | (0.68, 0.86) |
| Stomach | 0.92 | (0.84, 1.01) | 0.87 | (0.76, 0.98) | 1.25 | (0.99, 1.58) | 0.92 | (0.73, 1.17) | 0.98 | (0.78, 1.24) | 0.87 | (0.70, 1.08) |
| Soft Tissue including Heart | 1.02 | (0.90, 1.15) | 1.03 | (0.87, 1.21) | 1.31 | (0.94, 1.81) | 1.03 | (0.75, 1.43) | 1.14 | (0.84, 1.55) | 0.69 | (0.48, 0.98) |
| Kidney and Renal Pelvis | 0.89 | (0.83, 0.96) | 0.83 | (0.75, 0.91) | 0.99 | (0.80, 1.22) | 1.00 | (0.83, 1.20) | 0.87 | (0.72, 1.06) | 0.97 | (0.82, 1.15) |
| Testis | 1.05 | (0.92, 1.19) | 1.00 | (0.84, 1.20) | 1.55 | (1.13, 2.12) | 1.04 | (0.74, 1.47) | 1.09 | (0.79, 1.50) | 0.80 | (0.56, 1.15) |
| Pancreas | 1.04 | (0.97, 1.11) | 0.98 | (0.89, 1.07) | 1.12 | (0.93, 1.36) | 1.06 | (0.89, 1.26) | 1.24 | (1.05, 1.46) | 1.03 | (0.88, 1.21) |
| Esophagus | 0.88 | (0.77, 1.01) | 0.74 | (0.61, 0.90) | 0.79 | (0.51, 1.21) | 1.16 | (0.85, 1.60) | 1.31 | (0.98, 1.76) | 0.84 | (0.60, 1.16) |
| Liver | 0.83 | (0.71, 0.96) | 0.68 | (0.55, 0.85) | 0.58 | (0.34, 1.01) | 0.89 | (0.60, 1.32) | 1.14 | (0.81, 1.61) | 1.17 | (0.85, 1.59) |
| Brain | 0.98 | (0.90, 1.06) | 0.90 | (0.80, 1.02) | 1.13 | (0.89, 1.43) | 1.01 | (0.81, 1.25) | 1.03 | (0.83, 1.27) | 1.05 | (0.86, 1.27) |
| Cranial Nerves Other Nervous System | 0.94 | (0.86, 1.03) | 0.89 | (0.79, 1.01) | 0.92 | (0.70, 1.21) | 1.05 | (0.84, 1.32) | 0.99 | (0.79, 1.25) | 1.00 | (0.81, 1.24) |
| Myeloma | 1.03 | (0.95, 1.13) | 0.98 | (0.87, 1.10) | 1.26 | (0.99, 1.60) | 1.08 | (0.86, 1.36) | 1.07 | (0.85, 1.34) | 1.02 | (0.83, 1.26) |
| Small Intestine | 1.01 | (0.87, 1.17) | 0.97 | (0.79, 1.19) | 1.22 | (0.82, 1.83) | 0.97 | (0.65, 1.45) | 0.95 | (0.63, 1.42) | 1.07 | (0.75, 1.51) |
| Urinary Bladder | 0.99 | (0.94, 1.04) | 0.96 | (0.90, 1.03) | 1.04 | (0.90, 1.22) | 1.02 | (0.89, 1.17) | 1.05 | (0.92, 1.20) | 0.94 | (0.83, 1.07) |
Separating high-risk breast cancer families into clusters with similar patterns of multi-cancer risk uncovered many differences in effect sizes of cancer risks (including opposing directions), and identified previously undiscovered pleiotropic associations (Table 1, Figure 4 and Supplementary Figure 3). We found that the risk of ovarian cancer, an established co-aggregation with breast cancer for known risk genes, varied widely by cluster. Ovarian cancer risk for each of the five FMCs was significantly different than the risk estimated from all families together (overall HRR=1.17, Table 1). FMC5 captured extreme increased risk (HRR=6.10), while the remaining four FMCs showed negative associations (significant decreased risk, Table 1, Figure 4). Melanoma, another established cancer associated with breast cancer, was found to vary widely across clusters (Table 1, Figure 4). Novel co-aggregations were also evident. There is established association for larynx cancer, nor a signal for risk to larynx cancer when all high-risk breast cancer families were considered together. However, significant risks (increased and decreased) were seen for larynx cancer in all 5 FMCs (e.g. FMC3 HRR=4.93, FMC4 HRR=0.19, Table 1).
Prostate cancer risk was consisℓtent and modest (1.05 to 1.20) across all clusters, significantly elevated in four of the FMCs, and borderline in the fifth. Some cancers were consistently absent: bladder, brain, cranial nerves and other nervous system (CNS), myeloma, and small intestine. The remaining cancers provided patterns that differentiated FMCs. Families in FMC1 were at moderately increased risk for prostate cancer and acute lymphocytic leukemia (ALL) and had decreased risk for 11 cancers (Figure 4; Table 1), with notable decreases in ovarian (HRR=0.19; 95%CI 0.15 – 0.24) and cancer of the larynx (HRR=0.41; 95% CI 0.37 – 0.46). The FMC2 cluster alone showed strong co-aggregation of melanoma (HRR= 4.17; 95% CI 3.95 – 4.40) and moderate increases in risk for cancers that are usually seen in adolescents, such as testicular, thyroid, non-Hodgkin lymphoma, acute lymphocytic leukemia, acute myeloid leukemia (Figures 4; Table 1). This cluster had increased risk for eight cancer sites, the highest of the FMCs, and decreased risk for two sites, the lowest of the FMCs. FMC3 was the only cluster to exhibit substantial and significant risk for cancer of the larynx (HRR=4.93; 95% CI 4.58 – 5.31) and Hodgkin’s lymphoma (HRR=1.53; 95% CI 1.15 – 2.04). Families in FMC4 had an increased risk of uterine cancer (HRR = 1.39; 95% CI 1.22 – 1.57), the lowest risk of cancer of the larynx (HRR=0.19; 95% CI 0.14 – 0.27) and ovary (HRR=0.17; 95% CI 0.11 – 0.28). Lastly, the FMC5 cluster was the only to capture strong co-aggregation with ovarian cancer (HRR= 6.10; 95% CI 5.64 – 6.61).
Discussion
Large multi-source database resources are being developed in several healthcare systems across the United States and country-wide initiatives are becoming more common across the world43–45. Each of these immense resources has its particular strength and together these resources hold the potential for paradigm shifting opportunities in Population Science research. However, these will only be realized with consummate advances in computational approaches to interrogate the data. In Utah, a strength of the UPDB is an immense genealogy linked to a statewide health data. Here we have described two different novel approaches that focus on high-risk pedigrees to understand and address etiologic heterogeneity and define pleiotropic patterns. Both rely on the UPDB to provide the necessary linked databases of genealogy, cancer data, demographic and medical/clinical information. These data are available on nearly the entire population of Utah starting with the original European settlers of Utah in the 1800s (the earliest records) and extending to current residents of the state (where all sources of records are represented). The UPDB is a dynamic resource that continues to expand as the population grows and as linked data sources develop. For example, a recent SEER-funded pilot project by the UCR illustrated a 73.6% success rate for identifying FFPE tumor blocks for breast cancers diagnosed from 2000 to 2015 across the state. Such streamlining of tumor acquisition by the UCR would further benefit UPDB studies.
The techniques and findings here rely on a large multi-source population database and cannot easily be replicated. However, the Statistics Sweden Multigeneration Register, which has been used by extensively to identify familial associations between concordant and discordant cancers,46,47 is one potential data source that could be used to test the reproducibility of our findings. Notably, previous genetic discoveries using UPDB have proven generalizable, such as for breast cancer (BRCA1/BRCA2), neurofibromatosis type I (NF1), familial adenomatous polyposis coli (APC), and melanoma (CDKN2A). Once other large databases become ready, the methods described here may enable and accelerate the path to discovery elsewhere. Conversely, our methods also have the potential to be broadened, for example, to explore genetic pleiotropy through multiple primaries.22,48
In Approach 1 we highlighted a strategy for reducing heterogeneity, and utilized a novel tumor expression phenotype, PC3, previously shown to be increased in high-risk pedigrees in the UPDB20. We performed gene mapping in a large high-risk pedigree that contained an unusual number of breast cancer cases whose tumors were extreme for PC3. Using SGS, a method specifically designed for identifying segregating haplotypes in very large families33,35, we identified a 0.6 Mb genomewide significant segment in pedigree 1822 at 2q13 (p=1.6×10−8, LOD equivalent 6.64). A post-hoc search for additional carriers (not restricted to those with tumor data) indicates the region may only be 120 kb. Only four genes are contained in the smaller region, and of particular interest are IL1A and IL1B. Interleukins are key regulators of inflammation and immune response with roles in cell growth, angiogenesis and regulation of inflammatory process, and therefore strong candidate genes for breast cancer risk and mortality. In case-control studies, IL1B SNPs have been associated with breast cancer risk.49,50 IL1B has also been studied as a candidate for metastatic progression, particularly with respect to invasiveness and the epithelial-mesenchymal transition,51–57 as well as resistance to therapy.58 IL1A has been shown to play a role in chronic inflammation driving tumorigenesis and chemotherapy resistance.59 With these compelling candidates, the natural next step will be to sequence the shared haplotype for functional variants.
In Approach 2 we highlighted the ability to identify pleiotropies and described five familial multi-cancer configurations (FMC) for high-risk breast cancer families. This novel network-inspired approach simultaneously considered risk of multiple cancer types to classify families into clusters with similar patterns of familial cancer risk. Several cancer types that have previously been shown to co-aggregate with breast were identified in the signatures of our agnostic clustering approach (prostate, ovary, uterine and melanoma).60–62 However, we show that these risks may vary widely across clusters (ovarian and melanoma, in particular). New co-aggregations were also identified. Notably, risk for larynx cancer (FMC3 HRR = 4.93) and lymphomas (FMC3 Hodgkins HRR=1.53 and FMC2 ALL HRR=1.57). These findings improve resolution and our understanding of cancer family risks and have potential implications for screening and prevention. Also, while it is common for familial studies to focus only on increased risk, we also considered cancers with decreased risk. Isolating patterns of extreme decrease in risk, such as the multiple cancers at decreased risk in FMC1, could aid in the discovery of etiological factors that have opposing pleiotropic effects (i.e., a genetic mutation that increases risk for one cancer but is protective for others) or are single cause-single phenotype relationships. Another interesting pattern that may provide avenues to better understand etiology was identified in FMC2 which showed increased risk for several cancers often seen in adolescent and young adults. Other studies have shown similar clustering patterns: Hodgkin’s lymphoma and other lymphoid neoplasms;,10,63–65 testicular and non-Hodgkin’s lymphoma;66 and testicular, breast, and melanoma67. Our multi-cancer signatures of risk have the potential to improve characterization of different subtypes of breast cancer and provide new avenues to explore common etiological pathways including gene-environment factors. Subtypes provide the potential to reduce heterogeneity and increase power. The method could also be extended to non-cancer phenotypes that may have an underlying genetic link to cancer, such as Parkinsons disease.61 Cancer is a complex phenotype and by embracing large multi-source databases and computational tools, such as machine learning, it will be possible to seek out important combinations, beyond individual factors, to further our knowledge of the disease.
The goal of both approaches was to increase homogeneity to improve genetic studies, the first by defining cases within a pedigree that are similar and second by selecting groups of pedigrees that are similar (and indicative of genetics, rather than environment). It is important to note that findings from both approaches are sensitive to parameters of the methods. In Approach 1, the phenotype used to select cases is critical to power (extreme-PC3, previously shown to cluster in pedigrees). Without restriction, there is no signal at 2q13, or elsewhere in the genome. We note that sharing in the 8 cases in 1822 (p=1.6×10−8) compares in significance to the best single BRCA1 pedigree published (equivalent p=6.2×10−8)68 or best BRCA2 pedigree (p=1.8×10−5)2. In Approach 2, as with all clustering techniques, the clusters are sensitive to the distance metrics and weighting scheme used. This is important to consider when interpreting findings. To improve authenticity and generalizability and reduce spurious patterns, these parameters can be grounded with domain-specific knowledge or logical theories.
Large, population-based, multi-faceted databases, such as the UPDB, represent a new era for Population Sciences. Together with novel approaches, such as we have described here, these will play a critical role in advancing knowledge of cancer risk, elucidating the interplay between factors at the molecular level to individual interactions with the environment, and determine how these factors vary between people. Datasets which link family structure will also allow for important questions about the transgenerational nature of disease. We have illustrated that tumor phenotypes identified using high-risk status can map genes for breast cancer, and that various different cancer pleiotropies exist in high-risk breast cancer pedigrees. These types of discoveries will offer new avenues for defining germline susceptibilities, cancer prevention and multi-cancer risk management.
Supplementary Material
Acknowledgments
Financial Support
Research reported in this publication was supported by the National Institutes of Health K12 Award, 1K12HD085852-01 received by HA Hanson; National Institutes of Health K07 Award 1K07CA230150-01, and HCI Cancer Center Support Grant (grant number P30CA042014) received by HA Hanson. The Utah Cancer Registry is funded by the National Cancer Institute’s SEER Program, Contract No. HHSN261201800016I, the US Center for Disease Control and Prevention’s National Program of Cancer Registries, Cooperative Agreement No. NU58DP0063200, with additional support from the University of Utah and Huntsman Cancer Foundation.
Footnotes
Conflicts of Interests:
The authors declare no potential conflicts of interest.
References
- 1.Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science (New York, NY). 1994;265(5181):2088–2090. [DOI] [PubMed] [Google Scholar]
- 3.Wood GC, Chu X, Manney C, et al. An electronic health record-enabled obesity database. BMC Medical Informatics and Decision Making. 2012;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE--An integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–395. [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins IM, Siadaty MS, Lyman J, et al. Data mining and clinical data repositories: Insights from a 667,000 patient data set. Computers in Biology and Medicine. 2006;36(12):1351–1377. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie MD, Denny JC, Crawford DC, et al. Robust Replication of Genotype-Phenotype Associations across Multiple Diseases in an Electronic Medical Record. The American Journal of Human Genetics. 2010;86(4):560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brüggenjürgen B, Burkowitz J, Willich SN. Utilisation of medical resources of patients with pain undergoing an outpatient opioid therapy. Gesundheitswesen. 2007;69(6):353–358. [DOI] [PubMed] [Google Scholar]
- 8.Webster PC. Sweden’s health data goldmine. CMAJ. 2014;186(9):E310–E310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins R What makes UK Biobank special? The Lancet. 2012;379(9822):1173–1174. [DOI] [PubMed] [Google Scholar]
- 10.Kerber RA, O’Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103(9):1906–1915. [DOI] [PubMed] [Google Scholar]
- 11.Martin C, Leiser CL, O’Neil B, et al. Familial Cancer Clustering in Urothelial Cancer: A Population-Based Case–Control Study. JNCI: Journal of the National Cancer Institute. 2017;110(5):527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson HA, Horn KP, Rasmussen KM, Hoffman JM, Smith KR. Is Cancer Protective for Subsequent Alzheimer’s Disease Risk? Evidence From the Utah Population Database. The Journals of Gerontology: Series B. 2016;72(6):1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soisson S, Ganz PA, Gaffney D, et al. Long-term, adverse genitourinary outcomes among endometrial cancer survivors in a large, population-based cohort study. Gynecologic Oncology. 2018;148(3):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou JY, Hanson HA, Ramsay JM, et al. Fine Particulate Matter and Respiratory Healthcare Encounters among Survivors of Childhood Cancers. International Journal of Environmental Research and Public Health. 2019;16(6):1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. [DOI] [PubMed] [Google Scholar]
- 16.Tavtigian SV, Simard J, Rommens J, et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996;12(3):333–337. [DOI] [PubMed] [Google Scholar]
- 17.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. [DOI] [PubMed] [Google Scholar]
- 18.Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18(16):4465–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorski JJ, James CR, Quinn JE, et al. BRCA1 transcriptionally regulates genes associated with the basal-like phenotype in breast cancer. Breast Cancer Res Treat. 2010;122(3):721–731. [DOI] [PubMed] [Google Scholar]
- 20.Madsen MJ, Knight S, Sweeney C, et al. Reparameterization of PAM50 expression identifies novel breast tumor dimensions and leads to discovery of a genomewide significant breast cancer locus at 12q15. Cancer Epidemiol Biomarkers Prev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Rice MS, Zabor EC, Tworoger SS. Examining the common aetiology of serous ovarian cancers and basal-like breast cancers using double primaries. British Journal of Cancer. 2017;116(8):1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson HA, Leiser CL, Martin C, et al. Redefining the Bladder Cnacer Phenotype using Patterns of Fmailia Risk. medRxiv. 2019. [Google Scholar]
- 24.Bean LL, May DL, Skolnick M. The Mormon historical demography project. Hist Methods. 1978;11(1):45–53. [DOI] [PubMed] [Google Scholar]
- 25.Bishop DT, Skolnick MH. Genetic epidemiology of cancer in Utah genealogies: a prelude to the molecular genetics of common cancers. J Cell Physiol Suppl. 1984;3:63–77. [DOI] [PubMed] [Google Scholar]
- 26.Skolnick MBL, Dintelman S, Mineau GP. A computerized family history database system. Sociol Social Res. 1979;63:506–523. [Google Scholar]
- 27.O’Brien E, Rogers AR, Beesley J, Jorde LB. Genetic structure of the Utah Mormons: comparison of results based on RFLPs, blood groups, migration matrices, isonymy, and pedigrees. Hum Biol. 1994;66(5):743–759. [PubMed] [Google Scholar]
- 28.Wylie JE, Mineau GP. Biomedical databases: protecting privacy and promoting research. Trends Biotechnol. 2003;21(3):113–116. [DOI] [PubMed] [Google Scholar]
- 29.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweeney C, Bernard PS, Factor RE, et al. Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(5):714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camp NJ, Madsen MJ, Herranz J, et al. Re-interpretation of PAM50 gene expression as quantitative tumor dimensions shows utility for clinical trials: application to prognosis and response to paclitaxel in breast cancer. Breast cancer research and treatment. 2019;175(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen MJ, Knight S, Sweeney C, et al. Reparameterization of PAM50 Expression Identifies Novel Breast Tumor Dimensions and Leads to Discovery of a Genome-Wide Significant Breast Cancer Locus at 12q15. Cancer Epidemiology Biomarkers & Prevention. 2018;27(6):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waller RG, Darlington TM, Wei X, et al. Novel Pedigree Analysis Implicates DNA Repair And Chromatin Remodeling In Multiple Myeloma Risk. bioRxiv. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas A, Camp NJ, Farnham JM, Allen-Brady K, Cannon-Albright LA. Shared genomic segment analysis. Mapping disease predisposition genes in extended pedigrees using SNP genotype assays. Ann Hum Genet. 2008;72(Pt 2):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight S, Abo RP, Abel HJ, et al. Shared genomic segment analysis: the power to find rare disease variants. Ann Hum Genet. 2012;76(6):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genomes Project C, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abel HJ, Thomas A. Accuracy and computational efficiency of a graphical modeling approach to linkage disequilibrium estimation. Stat Appl Genet Mol Biol. 2011;10:Article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas A Assessment of SNP streak statistics using gene drop simulation with linkage disequilibrium. Genetic epidemiology. 2010;34(2):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matise TC, Chen F, Chen W, et al. A second-generation combined linkage physical map of the human genome. Genome Res. 2007;17(12):1783–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11(3):241–247. [DOI] [PubMed] [Google Scholar]
- 41.Norris D, Stone J. WHO classification of tumours of haematopoietic and lymphoid tissues. Geneva: WHO; 2008. [Google Scholar]
- 42.Kaufman L, Rousseeuw PJ. Partitioning Around Medoids (Program PAM). In: Finding Groups in Data.1990:68–125. [Google Scholar]
- 43.Polubriaginof FCG, Vanguri R, Quinnies K, et al. Disease Heritability Inferred from Familial Relationships Reported in Medical Records. Cell. 2018;173(7):1692–1704.e1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machluf Y, Tal O, Navon A, Chaiter Y. From Population Databases to Research and Informed Health Decisions and Policy. Front Public Health. 2017;5:230–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer A-M, Olshan AF, Green L, et al. Big data for population-based cancer research: the integrated cancer information and surveillance system. N C Med J. 2014;75(4):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weires M, Bermejo JL, Sundquist J, Hemminki K. Clustering of concordant and discordant cancer types in Swedish couples is rare. European Journal of Cancer. 2011;47(1):98–106. [DOI] [PubMed] [Google Scholar]
- 47.Frank C, Sundquist J, Yu H, Hemminki A, Hemminki K. Concordant and discordant familial cancer: Familial risks, proportions and population impact. International journal of cancer. 2017;140(7):1510–1516. [DOI] [PubMed] [Google Scholar]
- 48.Mauguen A, Zabor EC, Thomas NE, Berwick M, Seshan VE, Begg CB. Defining Cancer Subtypes With Distinctive Etiologic Profiles: An Application to the Epidemiology of Melanoma. J Am Stat Assoc. 2017;112(517):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng S, Lu B, Ruan W, Zhu Y, Sheng H, Lai M. Genetic polymorphisms and breast cancer risk: evidence from meta-analyses, pooled analyses, and genome-wide association studies. Breast cancer research and treatment. 2011;127(2):309–324. [DOI] [PubMed] [Google Scholar]
- 50.He B, Zhang Y, Pan Y, et al. Interleukin 1 beta (IL1B) promoter polymorphism and cancer risk: evidence from 47 published studies. Mutagenesis. 2011;26(5):637–642. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Yepez EA, Ayala-Sumuano JT, Lezama R, Meza I. A novel beta-catenin signaling pathway activated by IL-1beta leads to the onset of epithelial-mesenchymal transition in breast cancer cells. Cancer letters. 2014;354(1):164–171. [DOI] [PubMed] [Google Scholar]
- 52.Escobar P, Bouclier C, Serret J, et al. IL-1beta produced by aggressive breast cancer cells is one of the factors that dictate their interactions with mesenchymal stem cells through chemokine production. Oncotarget. 2015;6(30):29034–29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh K, Lee O-Y, Park Y, Seo MW, Lee D-S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer. 2016;16(1):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voigt C, May P, Gottschlich A, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(49):12994–12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castano Z, San Juan BP, Spiegel A, et al. IL-1beta inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nature cell biology. 2018;20(9):1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tulotta C, Lefley DV, Freeman K, et al. Endogenous Production of IL1B by Breast Cancer Cells Drives Metastasis and Colonization of the Bone Microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(9):2769–2782. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Reza I, Diaz L, Barrera D, et al. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Proinflammatory Cytokines IL-1beta and TNF-alpha. Journal of immunology research. 2019;2019:6384278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendoza-Rodriguez MG, Ayala-Sumuano JT, Garcia-Morales L, Zamudio-Meza H, Perez-Yepez EA, Meza I. IL-1beta Inflammatory Cytokine-Induced TP63 Isoform NP63alpha Signaling Cascade Contributes to Cisplatin Resistance in Human Breast Cancer Cells. International journal of molecular sciences. 2019;20(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Lee JS, Jie C, et al. HER2 Overexpression Triggers an IL1alpha Proinflammatory Circuit to Drive Tumorigenesis and Promote Chemotherapy Resistance. Cancer research. 2018;78(8):2040–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goggins W, Gao W, Tsao H. Association between female breast cancer and cutaneous melanoma. International journal of cancer. 2004;111(5):792–794. [DOI] [PubMed] [Google Scholar]
- 61.Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology (Cambridge, Mass). 2006;17(5):582–587. [DOI] [PubMed] [Google Scholar]
- 62.Kar SP, Beesley J, Amin Al Olama A, et al. Genome-Wide Meta-Analyses of Breast, Ovarian, and Prostate Cancer Association Studies Identify Multiple New Susceptibility Loci Shared by at Least Two Cancer Types. Cancer Discov. 2016;6(9):1052–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linabery AM, Erhardt EB, Richardson MR, et al. Family history of cancer and risk of pediatric and adolescent Hodgkin lymphoma: A Children’s Oncology Group study. International journal of cancer. 2015;137(9):2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. American journal of epidemiology. 2012;176(12):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang D, Alston RD, Eden TO, Birch JM. Cancer risks among relatives of children with Hodgkin and non-Hodgkin lymphoma. International journal of cancer. 2008;123(6):1407–1410. [DOI] [PubMed] [Google Scholar]
- 66.Nordsborg RB, Meliker JR, Wohlfahrt J, Melbye M, Raaschou-Nielsen O. Cancer in first-degree relatives and risk of testicular cancer in Denmark. International journal of cancer. 2011;129(10):2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Yu H, Hemminki O, Försti A, Sundquist K, Hemminki K. Familial Associations in Testicular Cancer with Other Cancers. Scientific Reports. 2018;8(1):10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldgar DE, Cannon-Albright LA, Oliphant A, et al. Chromosome 17q linkage studies of 18 Utah breast cancer kindreds. American journal of human genetics. 1993;52(4):743–748. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.