Abstract
Nanophotocatalyst MnTiO3 powders were synthesized by sol-gel technique and characterized by means of X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), as well as their thermal behavior has been studied by differential thermal and thermogravimetric analyses (DTA/TGA). Powders morphology was analyzed by means of Transmission electron microscopy (TEM). Effect of various process parameters like amount of photocatalyst, dye concentration, solution pH and irradiation time on the extent of removal of dye were studied in detail. The photo-degradation was relatively higher using lower amount (0.005 g) of semiconductor, reached rate of 75% and 70% after 240 min for mixed MnTiO3/TiO2 and pure MnTiO3 nanocatalysts. The kinetic model of photocatalytic degradation of MB follows pseudo-first-order kinetic with a high correlation coefficient values (R2 > 0.95). These results underline the use of effective, low-cost and easily available MnTiO3 photocatalyst for the decomposition of pollutants to water under natural sunlight.
Keywords: Inorganic chemistry, Materials chemistry, MnTiO3, Solar light, Methylene blue dye, Photocatalysis, Nanocrystalline
Inorganic chemistry; Materials chemistry, MnTiO3; Solar light; Methylene blue dye; Photocatalysis; Nanocrystalline.
1. Introduction
The residual dyes from textile, pharmaceutical or dye intermediates industries are considered non-biodegradable organic pollutants introduced into the natural water resources or wastewater treatment systems [1, 2, 3]. In particular, the discharge of effluents containing dyes into the water environment is undesirable, due to their color which generates toxic products through hydrolysis; oxidation or other chemical reactions in the wastewater phase [3, 4, 5]. At present, the photocatalysis process allows performing the efficient degradation of variety of organic pollutants in aqueous wastes [6, 7, 8, 9, 10]. This process is based on the use of semiconductor materials, which upon activation with suitable light sources give rise to the formation of various reactive species. Among various semiconductor photo-catalysts, the perovskite type ABO3 oxides (A and B are two cations of different sizes) have received considerable attention for their good abilities towards water splitting and organic pollutant degradation under visible light or UV irradiation [11, 12, 13]. Particularly, manganese titanate MnTiO3 is the most stable perovskite type oxide and a favorable material with strong adsorption in the visible region which appear suitable for photocatalysis applications [14, 15, 16, 17]. However, He et al. have reported the photodegradation of aqueous methyl orange on MnTiO3 powder at different initial pH and it was found that this catalyst showed promise for use in many applications in heterogeneous catalysis and decontamination of the environment [16].
The present work reports the photocatalytic degradation of methylene blue (MB) in aqueous solution using MnTiO3 nanoparticles as photocatalyst under sunlight radiation. A comparative photocatalytic activity of MnTiO3 powders, synthesized by the sol-gel process following two methods of preparation, has been investigated. The effects of different operational parameters on the degradation of dye have also been studied along with their reaction kinetics.
2. Experimental methods
2.1. Preparation and characterization of nanocatalyst
MnTiO3 nanopowders were synthesized according to the previously reported sol-gel methods [16, 18].
-
•
Sol-gel method 1 (SG-1): 0.01 mol MnCl2.4H2O dissolved into distilled water was reacted with 0.02 mol NaOH to produce Mn(OH)2. Then 0.01 mol of TiO2 (P-25 powder: mixture of 70% Rutile and 30% Antase) was added to the above solution. The mixed solution was further refluxed for 12 h at 75 °C. The supernatant was then decanted, and the solid residue was washed three times with distilled water, dried in air at 100 °C for 12 h and calcined in air at 900 °C for 6 h.
-
•
Sol-gel method 2 (SG-2): An appropriate amount of Manganese chloride tetrahydrate MnCl4. 4H2O (0.6 g) was dissolved in distilled water with addition 0.12 mL of nitric acid HNO3, 1.6 mL acetic acid CH3COOH and then 1 ml of titanium isopropoxide, Ti[O(C3H7)]4 was added to the above mixture and a white precipitate was formed immediately. The mixture was then stirred for 12 h maintaining the temperature at 50 °C. The resulting semi-transparent colloidal suspension solution was evaporated at 80 °C. The obtained powder was dried and treated at 900 °C for 6 h.
The X-ray diffraction (XRD) measurement was carried out at room temperature using a Philips X-Pert Pro diffractometer. The TEM micrographs were obtained on a JEOL 2011 transmission electron microscope with an accelerating voltage of 200 kV. The infrared spectra were recorded with a NICOLET 5700-FTIR spectrometer equipped with an attenuated total reflection (ATR) accessory. Thermoanalytical measurements (TG/DTA) were performed using a SETARAM TG-DTA92 microbalance and the thermograms were recorded between 30 and 1200 °C with a heating rate of 10 °C/min.
2.2. Photocatalytic activity
The photocatalytic activities of pure MnTiO3 and mixed MnTiO3/TiO2 were tested in five neck pyrex flask type reactor under sunlight irradiation. 0.005 g of the photocatalysts were introduced into the 50 ml aqueous solution of methylene blue (1 × 105- M, pH = 6.4). The decomposed dye solutions were centrifuged at 4000 rpm for 10 min and for the measurement of UV-visible absorbance (OPTIMA UV-vis spectrometer SP-3000 Plus). The photo-degradation rate of methylene blue (MB) was calculated by the following Eq. (1) [19]:
| (1) |
where A0 is the absorbance of initial MB; At is the absorbance of the solution after illumination at time t. According to first order kinetics reaction, rate constant k (min−1) was determined by using the following relation (2) [20]:
| (2) |
where C0 and Ct are concentration at the beginning and at a certain time, t is the irradiation time. Adsorption kinetics investigations were carried out by agitating 50 mL of MB (1 × 10−5 M) dye solution of known initial concentration with 0.005 g of adsorbent prepared by SG-1 and SG-2 routes (pH = 6.4).
3. Results and discussion
3.1. Catalyst characterization
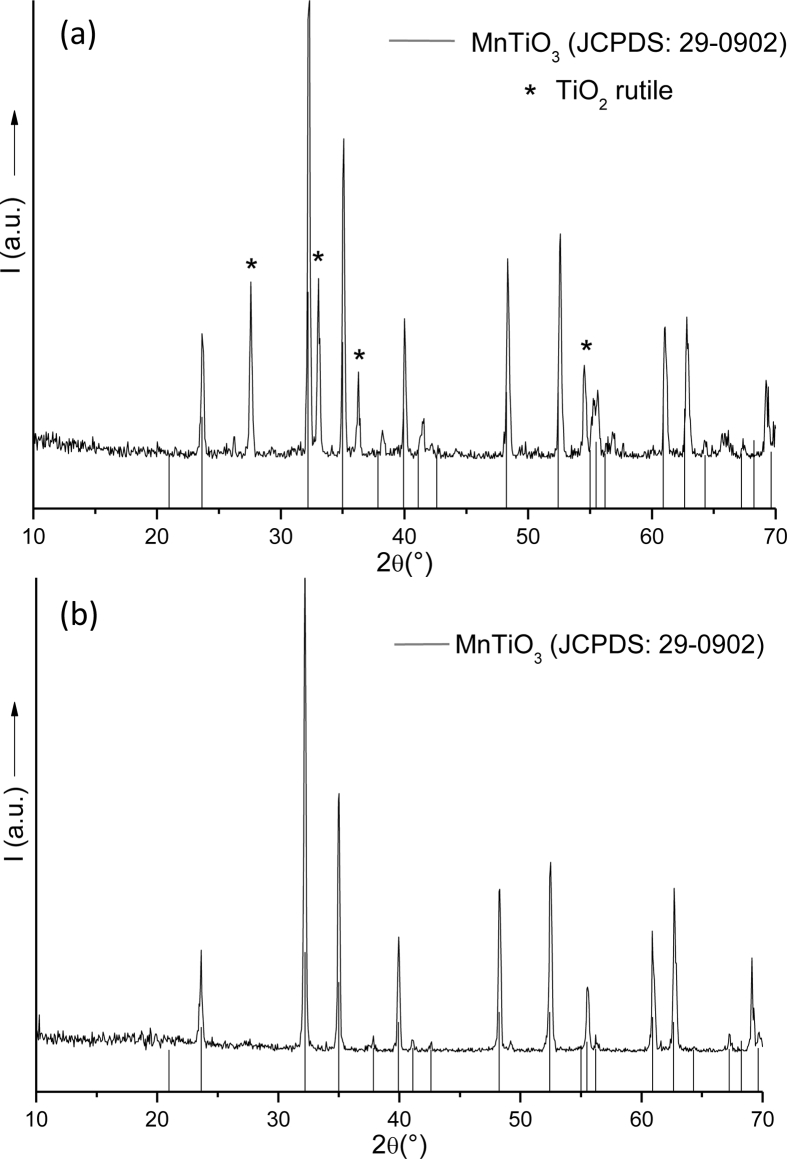
Figure 1(a) and (b) present the XRD patterns of MnTiO3 powders synthesized by the both routes SG-1 and SG-2 respectively. The X-ray diffraction pattern in Figure 1(a) suggests the crystallisation of rhombohedral MnTiO3 phase (JCPDS: 29-0902) [21] along with amount of TiO2 rutile phase (2θ = 27.59, 33.12, 36.22 and 54.61) (∗ marked peaks, JCPDS: 78-1508) [22]. The semi quant % of MnTiO3 and TiO2 are for powder prepared by sol-gel, method 1 (SG-1) are 77.6% and 22.4% respectively. Figure 1(b) shows the XRD pattern of the product synthesized by using Titanium isopropoxide as precursor (SG-2). All the reflection peaks can be indexed to rhombohedral pyrophanite phase MnTiO3 (JCPDS 29-0902). No peaks corresponding to rutile phase are detected in the XRD pattern, which shows the high purity of the product.
Figure 1.
X-ray diffraction pattern for MnTiO3 prepared using (a) Titanium dioxide powder P-25 (SG-1) or (b) Titanium isopropoxide as precursor (SG-2).
The crystallite sizes of the particles were estimated from XRD peak half widths using the Scherrer Eq. (3) [23]:
| (3) |
where D is the average crystallite diameter, λ is the X-ray wavelength, b is the full width at half maximum (FWHM) of the peak corrected for instrumental broadening and θ is the Braggs' angle. The calculated values average particle diameters for MnTiO3 powders synthesized by sol-gel method SG-1 and SG-2 were approximately 29.2 and 35.5 nm respectively.
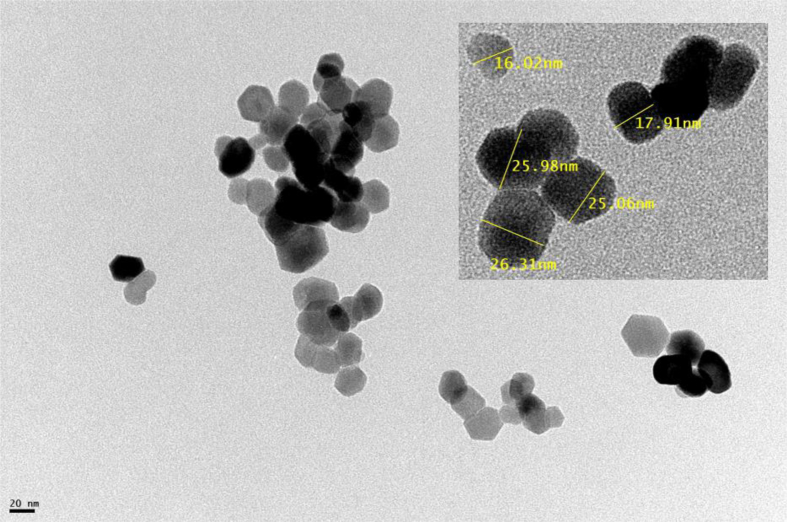
The TEM micrographs of MnTiO3 sample are illustrated in Figure 2. It is found that the nanoparticles possess almost spherical shape. These spherical nano assemblies are discrete and well defined with limited aggregation and a narrow size distribution ranging between 15–35 nm. The result agrees well with that of the XRD analysis.
Figure 2.
TEM picture of MnTiO3 nanopowder prepared by sol-gel method 2 and calcined at 900 °C.
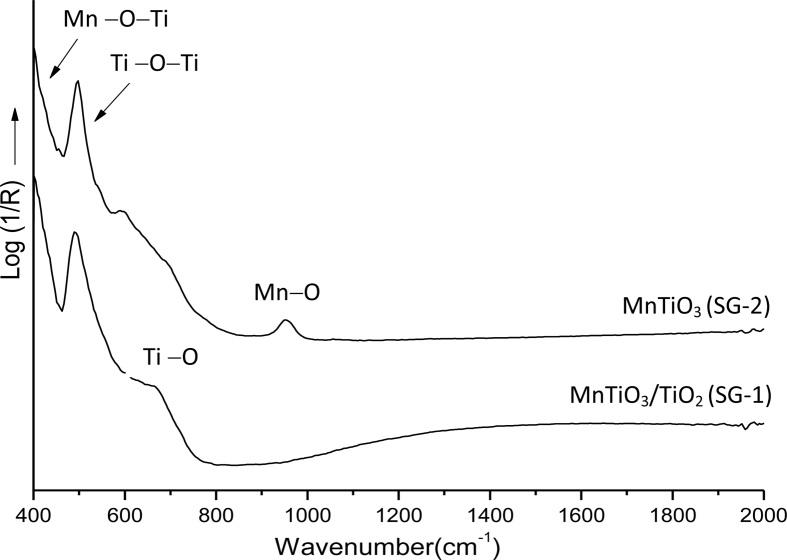
Room temperature IR/ATR spectra recorded for MnTiO3 samples prepared by both synthesis routes (SG-1 and SG-2) and measured in the range of 400–2000 cm−1 spectral region are shown in Figure 3. Their spectra are characterized with main absorption bands of MnTiO3. The bands centered at 407, 494, and 656 cm−1 were attributed to the Ti–O bond vibrations. The bands at 407 and 494 cm−1 can be assigned to Mn–O–Ti and O–Ti–O vibrational modes, respectively [24]. The band centered around 657 cm−1 observed in SG-1 sample can be also attributed to corresponding Ti–O modes of crystalline rutile TiO2. The band appearing at 970 cm−1 is due to Mn–O bonds for SG-2 sample [25].
Figure 3.
IR/ATR spectra of samples prepared by SG-1 and SG-2 methods, after heating at 900 °C.
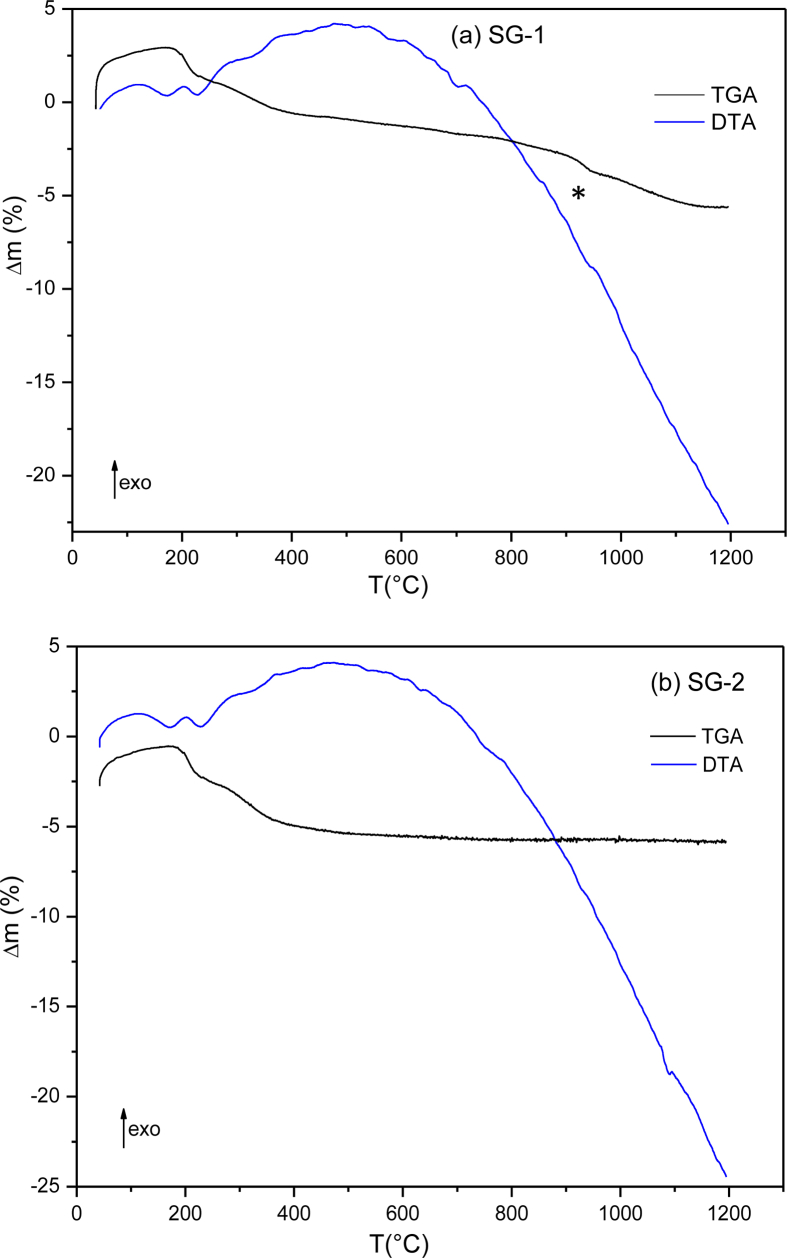
To investigate the domains of stability and decomposition of MnTiO3 treated at 900 °C, Thermogravimetric and differential thermal analysis (TGA and DTA) of samples prepared by SG-1 and SG-2 methods were performed. The thermograms in Figure 4 show no thermal effects accompanied by mass losses. The endothermic peak between 80 °C and 250 °C observed for both synthesis sol-gel routes can be attributed to dehydration of water molecules. In the temperature range of 250–450 °C, no weight loss was exhibited, that proves the absence of hydroxyl groups and/or organic residues such as alkoxy groups in the powder of MnTiO3. It showed that no significant changes were observed both in DTA and TGA curves even up to 1200 °C. As we expected, the thermal stability of MnTiO3 was improved effectively. For the compound prepared by sol-gel method 1, were titanium dioxide powder used as starting precursor, a slight endothermic effect is identified accompanied with low mass loss observed around 1000 °C (Figure 4(a)). From the foregoing XRD characterization, the presence of titanium dioxide TiO2 was observed in the calcined powder SG-1 at 900 °C. According to these observations, we can hypothesize that the endothermic peak around 1000 °C corresponds to the decomposition or loss of TiO2 rutile phase.
Figure 4.
TG-DTA curves of the as-synthesized nanopowders obtained by (a) SG-1 and (b) SG-2 methods.
3.2. Photocatalytic degradation of MB
To understand photocatalytic degradation of methylene blue dye using semi-conductor MnTiO3 nanoparticles as catalyst, we studied the influence of several factors on the degradation process including contact time, initial concentration of dye, photocatalyst loading, solution pH and nature of light source.
-
•
Effect of contact time
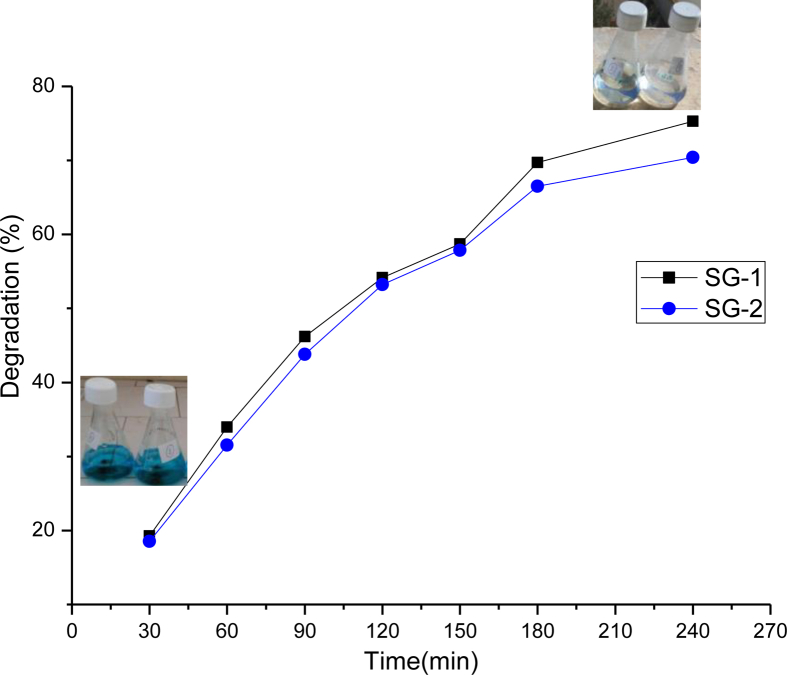
Adsorption of methylene blue on MnTiO3 nanocatalyst surface, obviously, is a function of the contact time, which is corresponding to the time of adsorption, or the state of saturation of the absorbant surface by the absorbate (dye). Figure 5 presents the effect of contact time on the removal of methylene blue using 0.005 g of catalysts in aqueous solution. The result show that the catalysis of photodecomposition of methylene blue (MB) occurs. It was observed as shown in Figure 5, the efficiency of MB decolorization using the individual MnTiO3 catalyst (SG-2) is substantially identical as compared with the mixture MnTiO3/TiO2 catalyst (SG-1). However, for the mixed photocatalyst MnTiO3/TiO2, the degradation was quite high (75%) as compared with the pure MnTiO3 nanoparticule (70%) after 240 min irradiation under sunlight.
-
•
Effect of the dye concentration
Figure 5.
Effect of contact time on MB dye removal onto MnTiO3-TiO2 (SG-1) and (b) MnTiO3 (SG-2) catalysts.
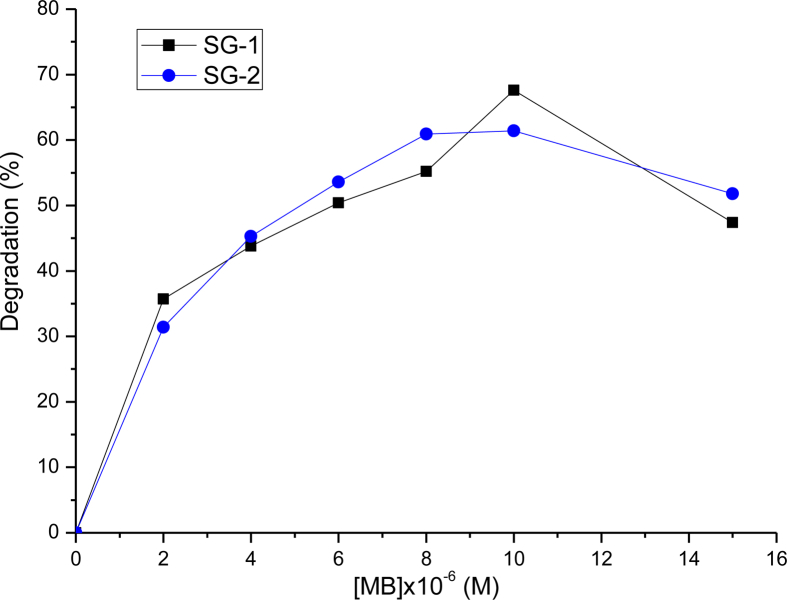
The influence of the initial dye concentration on the decolorization of MB was studied under sunlight irradiation by varying the initial dye concentrations from 2 × 10−6 M to 1.5 × 10−5 M in the presence of 0.005 g of photocatalyst. From Figure 6, it can be seen that the removal efficiency of dye increases with increasing initial concentration of the dye until 1 × 10−5 M after 240 min irradiation time. The adsorption capacity is higher at lower concentration because of the higher availability of more active sites for MB molecules to be adsorbed on the surface MnTiO3 photo-catalyst. When dye concentration increases, number of dye molecules adsorbed on catalyst surface increases. Increasing the dye concentration also caused the dye molecules to adsorb light and the photons never reached MnTiO3 surface; finally, it causes reduced efficiency of dye removal [26, 27]. Thus, the photodegradation of MB was found better at low concentration, i.e [MB]o ≤ 1 × 10−5 M.
-
•
Effect of catalyst dose
Figure 6.
Effect of initial MB dye concentration on the adsorption of MB onto nanosizes catalysts, prepared by SG-1 and SG-2 methods.
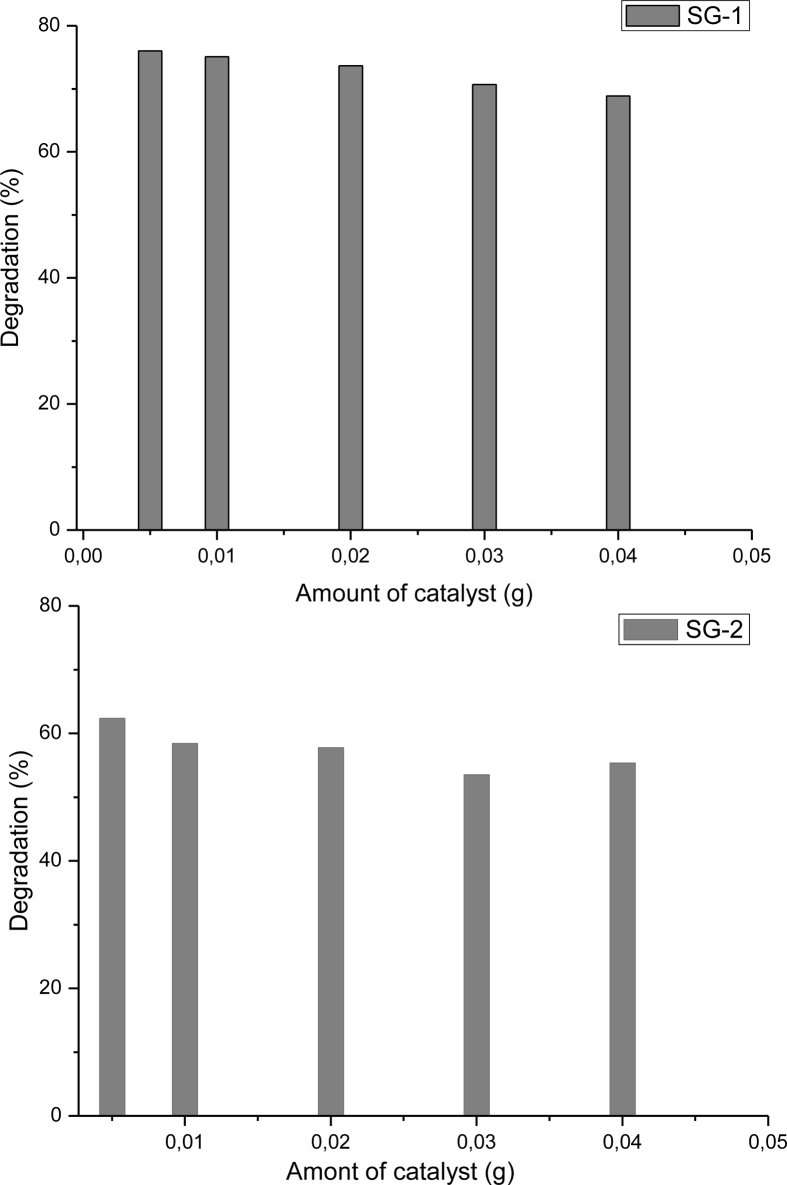
The degradation percentage of MB dye under visible light irradiation by different amounts of photo catalyst were used (0.005 g–0.04 g), was examined. As it can be seen in Figure 7, the rate of photo-degradation was higher using lower amount (0.005 g) of semiconductor, 76% of MB degradation for MnTiO3/TiO2 catalyst obtained by SG-1 (Figure 7(a)) and 62.5% of MB degradation for MnTiO3 catalyst prepared by SG-2 (Figure 7(b)). Whereas, the presence of TiO2 improved the reaction photodegradation of MB dye. It has been reported that TiO2 represent a good photocatalytic activity for the decomposition of organic pollutants [28, 29]. Beyond 0.005 g of MnTiO3 nanoparticle, the percentage degradation of MB dye increases only slightly up to 0.04 g. These observations can be rationalized both in terms of availability of active sites on MnTiO3 surface and the light penetration of photo-activating light into the system [30]. At higher catalyst loading may be due to the deactivation of activated molecules by collision with ground state catalysts, thus reducing the rate of reaction [31].
-
•
Effect of pH
Figure 7.
Effect of MnTiO3 catalyst dose prepared by (a) SG-1 and (b) SG-2 method on the methylene blue removal.
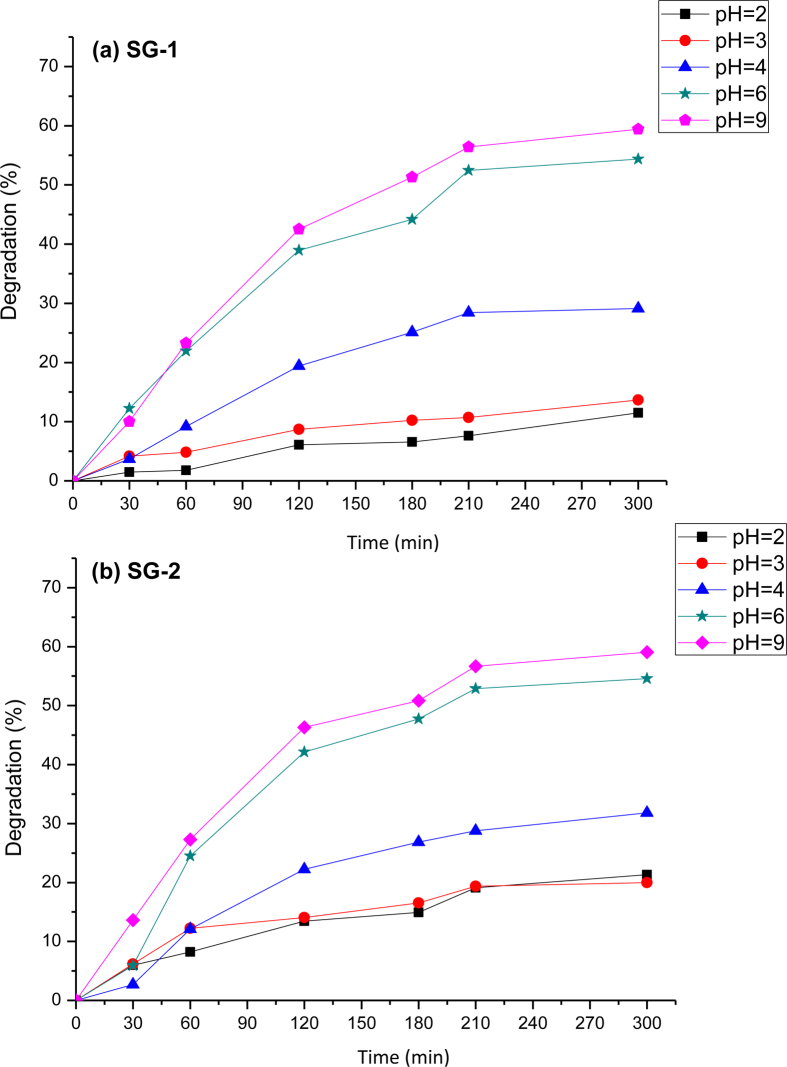
Another important parameter in the photocatalytic reactions is the pH of the solution. It has a significant effect on the surface charge of the photocatalyst [32]. The pH of methylene blue solution was adjusted by adding HCl or NaOH (1 M). The effect of the solution pH on the rate of photocatalytic degradation was investigated within the range of 2–9 at constant dye concentration (1 × 10−5 M) and catalyst amount 0.005g/50mL MnTiO3/TiO2 (Figure 8(a)) or MnTiO3 (Figure 8(b)). The removal of dye by adsorption onto nanosized MnTiO3 was found to be slow at the initial period of contact time, and then to become rapid with the increase of contact time. An increase in the degradation of MB dye was observed with increasing the pH from 2 to 9. The percentage of removal increases from 15% in pH = 2–60% in pH = 9 of the solution, for the two nanosized photocatalytic materials prepared by SG-1 and SG-2 routes.
-
•
Effect of light intensity
Figure 8.
Influence of pH on the photocatalytic degradation onto catalysts prepared by (a) SG-1 and (b) SG-2 methods.
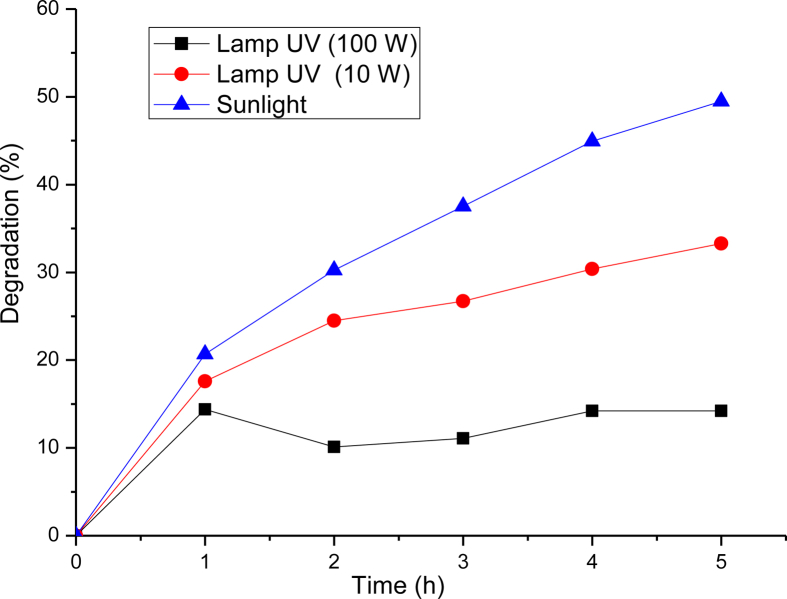
Intensity of light and the effect of the radiation wavelength both have affect on the rate of photo-degradation of pollutants [33, 34, 35]. The light intensity was varied by changing the source of irradiation. For that, two kinds of lamps 10 W UV-C (λ = 254 nm) and 100 W UV-A (λ = 356 nm) were used. Figure 9 illustrates the effect of the variation of light intensity on the degradation of MB dye. From the observed data, it is evident that the degradation of methylene blue using solar light irradiation was more efficient than that with artificial UV light irradiation. The percent (%) of degradation of methylene blue was significantly improved with UV-C radiation compared to UV-A radiation. An increase in light intensity did not result in any significant corresponding increase in the degradation efficiency. This probably means that the optimum number of photons required for effective photocatalytic degradation was attained under UV 10 W. Therefore, an increase in photon number under UV 100 W did not produce any major change in the degradation efficiency [36, 37].
-
•
Adsorption kinetics
Figure 9.
Effect of solar/UV light source on the degradation of MB dye.
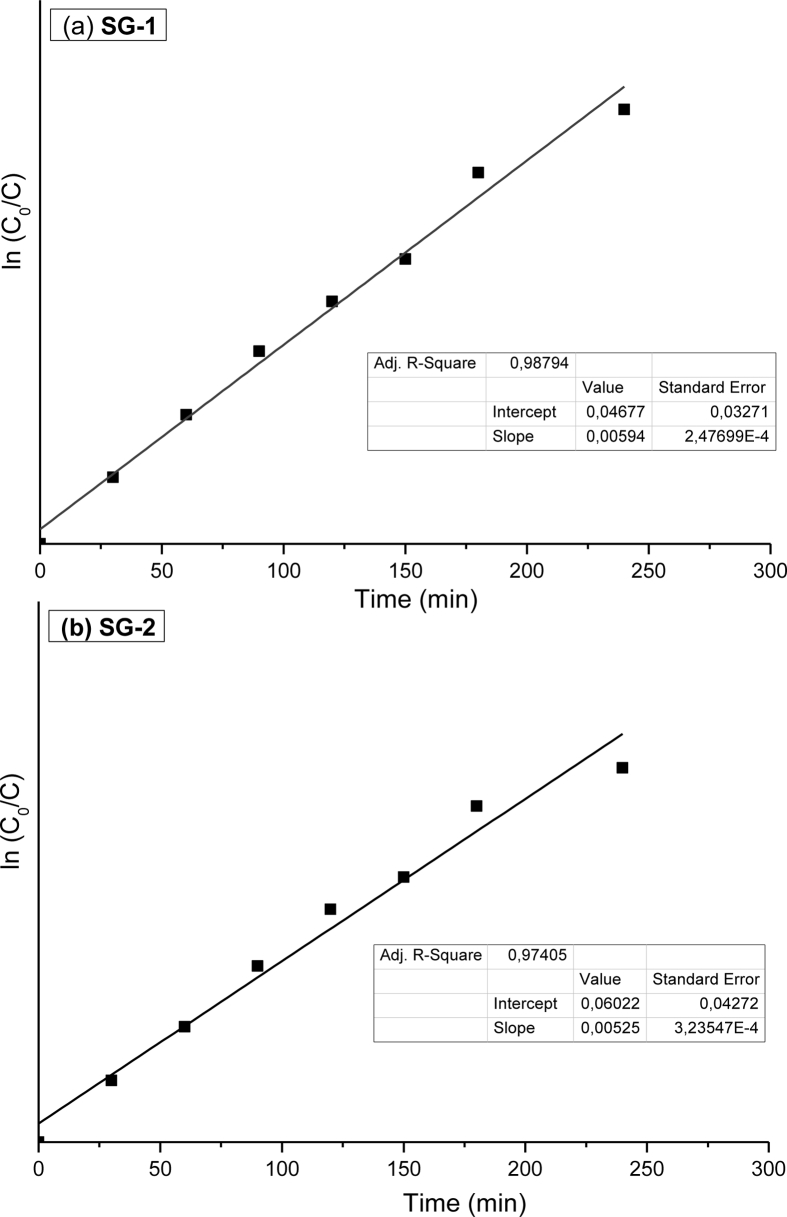
The kinetics of an adsorption process provides information about the mechanism of adsorption. The pseudo-first order kinetic equation given by the Langmuir-Hinshelwood model at low initial concentrations [16] was tested. Figures. 10(a) and (b) show the reaction kinetics of MB degradation in the presence of pure MnTiO3 (obtained by SG-2 route) and MnTiO3-TiO2 (prepared by SG-1 synthesis). By plotting ln(C0/C) vs t, a straight line was obtained. From the graphs, it is seen that the photocatalytic methylene blue dye degradation follow the first order-kinetics as the correlation constant for the fitted line to be R2 > 0.95. A good correlation to the first order reaction kinetics (R2 = 0.988 for MnTiO3-TiO2 and R2 = 0.974 for MnTiO3) was found. The calculated pseudo-first-order rate constants of MnTiO3-TiO2 and pure MnTiO3 are 5.96 10−3 min−1 and 5.25 10−3 min−1 respectively. The results further demonstrated that MnTiO3 photocatalyst exhibits good photo-reactivity which corroborates the corresponding degradation efficiency.
Figure 10.
Pseudo-first-order kinetics plots of MB adsorption on with (a) MnTiO3-TiO2 (SG-1) and (b) MnTiO3 (SG-2).
4. Conclusions
In summary, pure MnTiO3 or mixed MnTiO3/TiO2 nanoparticules have been successfully prepared by sol-gel method. The structure and morphology of these nanoparticles were studied using X-ray diffraction and high resolution transmission electron microscopy analysis. The particles are in the average crystallite size range of 20–30 nm. The experiments of degrading MB confirmed that the obtained catalysts can perform significant photocatalytic activity even if it is irradiated by solar light. Therefore, the MnTiO3 nanoparticule is a good candidate in the treatment of industrial wastewater to eliminate organic pollutants, which cause severe threat to environment.
Declarations
Author contribution statement
Suhila Alkaykh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Aïcha Mbarek: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Elbashir E. Ali-Shattle: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Crosby D.G. Oxford University Press; New York: 1998. Environmental Toxicology and Chemistry. [Google Scholar]
- 2.Forgacs E., Crestile T., Oros G. Removal of synthetic dyes from waste water. A review. Environ. Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Banat I.M., Nigam P., Singh D., Marchant R. Microbial decolorization of textile-dye-containing effluents: a review. Bioresour. Technol. 1996;58:217–227. [Google Scholar]
- 4.Kant R. Textile dyeing industry an environmental hazard. Univ. Inst. Fashion Tech. 2012;4:22–26. [Google Scholar]
- 5.Zollinger H. Color Chemis- Try. VCH Publishers; New York, USA: 1987. Synthesis, properties of organic dyes and pigments; pp. 92–102. [Google Scholar]
- 6.Hoffmann M.R., Martin S.T., choi W., Bahnemann D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995;95:69–96. [Google Scholar]
- 7.Bhatkhande D.S., Garkar V.G., Beenackers A.A.C.M. Surfactant-assisted sol-gel synthesis of TiO2 with uniform particle size distribution. J. Chem. Technol. Biotechnol. 2001;77:102–116. [Google Scholar]
- 8.Hauas A.A., Lachheb H.A., Mohamed K.A., Elaloui E.A., Guillard C., Herrmann J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. 2001;B31:145–157. [Google Scholar]
- 9.Mei Ling C., Mohamed A.R., Bhatia S. Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere. 2004;57:547–554. doi: 10.1016/j.chemosphere.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Jain R., Shirkarwar S. Photocatalytic removal of hazardous dye cyanosine from industrial waste using titanium dioxide. J. Hazard Mater. 2008;152:216–220. doi: 10.1016/j.jhazmat.2007.06.119. [DOI] [PubMed] [Google Scholar]
- 11.Labhasetwar N., Saravanan G., Megarajan S.K., Manwar N., Khobragade R., Doggali P., Grasset F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015;16:36002–36015. doi: 10.1088/1468-6996/16/3/036002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J., Guo L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. 2012;22:592–615. [Google Scholar]
- 13.Kanhere P., Chen Z. A review on visible light active perovskite-based photocatalysts. Molecules. 2014;19:19995–20022. doi: 10.3390/molecules191219995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong W., Wang D., Jiang L., Zhu H., Huang H., Li J., Zhao H., Li C., Chen B., Deng G. Synthesis of F doping MnTiO3 nanodiscs and their photocatalytic property under visible light. Mater. Lett. 2013;98:265–268. [Google Scholar]
- 15.Bae K.N., Noh S.I., Ahn H.J., Seong T.Y. Effect of MnTiO3 surface treatment on the performance of dye-sensitized solar cells. Mater. Lett. 2013;96:67–70. [Google Scholar]
- 16.He H.Y., Dong W.X., Zhang G.H. Photodegradation of aqueous methyl orange on MnTiO3 powder at different initial pH. Res. Chem. Intermed. 2010;36:995–1001. [Google Scholar]
- 17.Enhessari M., Parviz A., Karamali E., Ozaee K. Synthesis, characterisation and optical properties of MnTiO3 nanopowders. J. Exp. Nanosci. 2012;7:327–335. [Google Scholar]
- 18.de Haart L.G.J., de Vries A.J., Blasse G. Photoelectrochemical properties of MgTiO3 and other titanates with the ilmenite structure. Mater. Res. Bull. 1984;19:817–824. [Google Scholar]
- 19.Zhang X., Zhou G., Zhang H., Wu C., Song H. Characterization and activity of visible light–driven TiO2 photocatalysts co-doped with nitrogen and lanthanum. Transition Meter. Chem. 2011;36:217–222. [Google Scholar]
- 20.Vasanth Kumar K., Porkodi K., Selvaganapathi A. Constrain in solving Langmuir-Hinshelwood kinetic expression for the photocatalytic degradation of Auramine O aqueous solutions by ZnO catalyst. Dyes Pigments. 2007;75:246–249. [Google Scholar]
- 21.Liferovich R.P., Mitchell R.H. Rhombohedral ilmenite group nickel titanates with Zn, Mg, and Mn: synthesis and crystal structures. Phys. Chem. Miner. 2005;32:442–449. [Google Scholar]
- 22.Selman A.M. Studies on the influence of growth time on the rutile TiO2 nanostructures prepared on Si substrates with fabricated high-sensitivity and fast-response p-n heterojunction photodiode. Am. J. Nano Res. Appl. 2016;4(3):23–32. [Google Scholar]
- 23.Scherrer P. Nanoscience and the scherrer equation versus the scherrer-gottingen equation. Nach. Ges. Wiss. Göttingen. 1918;26/9:98–100. [Google Scholar]
- 24.Harizanov O., Ivanova T., Harizanova A. Study of sol–gel TiO2 and TiO2–MnO obtained from a peptized solution. Mater. Lett. 2001;49(3/4):165–171. [Google Scholar]
- 25.Sharma Y.K., Kharkwal M., Uma S., Nagarajan R. Synthesis and characterization of titanates of the formula MTiO3 (M = Mn, Fe, Co, Ni and Cd) by co-precipitation of mixed metal oxalates. Polyhedron. 2009;28(3):579–585. [Google Scholar]
- 26.Mengyue Z., Shifu C., Yaowu T.C. Photocatalytic degradation of organophosphous pesticides using thin films of TiO2. J. Chem. Technol. Biotechnol. 1995;64:339–344. [Google Scholar]
- 27.Ming L. Treatment of dye aqueous solution by UV/TiO2 process with applying bias potential. Water Sci. 2000;36:106–189. [Google Scholar]
- 28.Liu C., Li R., Zhang W. Adsorption property and photocatalytic activity of sol-gel prepared porous TiO2 using PEG1000 as template. Appl. Mech. Mater. 2011;48–49:153–156. [Google Scholar]
- 29.Yao J., Bai Y., Chen N., Takahashi M., Yoko T. Sol-gel preparation, characterization, and photocatalytic activity of macroporous TiO2 thin films. J. Am. Ceram. Soc. 2011;94(4):1191–1197. [Google Scholar]
- 30.Matthews R.W. Process Engineering for pollution control and waste minimization. Water Res. 1990;24:653–660. doi: 10.1007/s11356-017-8936-2. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann J.M. Heterogeneous photocatalysis: an Emerging discipline involving multiphase systems. Catal. Today. 1995;24(1-2):157–164. [Google Scholar]
- 32.Haque M.M., Bahnemann D., Muneer M. Photocatalytic degradation of organic pollutants: mechanisms and kinetics photocatalytic degrad. Org. Pollut. Mech. Kinet. 2012;3:294–326. [Google Scholar]
- 33.Ahmed S., Rasul M.G., Martens W.N., Brown R.J., Hashib M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination. 2010;261(1-2):3–18. [Google Scholar]
- 34.Puma G.L., Yue P.L. Effect of the radiation wavelength on the rate of photocatalytic oxidation of organic pollutants. Ind. Eng. Chem. Res. 2002;41:5594–5600. [Google Scholar]
- 35.Kaneco S., Rahman M.A., Suzuki T., Katsumata H., Ohta K. Optimization of solar photocatalytic degradation conditions of bisphenol A in water using titanium dioxide. J. Photochem. Photobiol. A. 2004;163:419–424. [Google Scholar]
- 36.Wilson Anku W., Osei-Bonsu Oppong S., Kumar Shukla S., Penny Govender P. Comparative photocatalytic degradation of monoazo and diazo dyes under simulated visible light using Fe3+/C/S doped-TiO2 nanoparticles. Acta Chim. Slov. 2016;63:380–391. doi: 10.17344/acsi.2016.2385. [DOI] [PubMed] [Google Scholar]
- 37.Reza K.M., Kurny A., Gulshan F. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl. Water Sci. 2017;7:1569–1578. [Google Scholar]