Abstract
Aim
To further investigate the mechanism behind the antitumor properties of berberine regarding lipid metabolism.
Methods
Cell viability, proliferation, and apoptosis assays were performed to determine the antigrowth effects of berberine in vitro. Ectopic xenograft models in Balb/c nude mice were established to determine the antitumor effects of berberine in vivo.
Results
Berberine inhibited cell viability and proliferation of MGC803 human gastric cancer cell lines in a time- and dose-dependent manner. Berberine induced apoptosis of MGC803 and increased the apoptotic rate with higher doses. Berberine induced the accumulation of fatty acid of MGC803 and suppressed the protein expression of FABPs and PPARα. The FABP inhibitor BMS309403 recapitulated the effects of berberine on MGC803 cells. In the xenograft model, berberine significantly decreased the tumor volume and tumor weight and induced apoptosis in tumor tissues. Berberine significantly elevated the fatty acid content and inhibited the expression of FABPs and PPARα in the MGC803 xenograft models.
Conclusion
Berberine exerted anticancer effects on human gastric cancer both in vitro and in vivo by inducing apoptosis, which was due to the reduced protein expression of FABPs and the accumulation of fatty acid.
1. Introduction
An estimated 4.3 million new cancer cases and 2.9 million new cancer deaths occurred in China in 2018. China has lower cancer incidence but a 30% and 40% higher cancer mortality than the UK and USA, among which 36.4% of the cancer-related deaths were from the digestive tract cancers (stomach, liver, and esophagus cancer) and have relatively poorer prognoses [1]. Stomach cancer happened worldwide and is responsible for over 1,000,000 new cases in 2018 and an estimated 783,000 deaths (equating to 1 in every 12 deaths globally), making it the fifth most frequently diagnosed cancer and the third leading cause of cancer death [2]. The latest China cancer report in 2018 showed that gastric cancer ranks second in the incidence and mortality of cancer in China. One reason for the higher mortality in China may be the low-stage cancers at diagnosis and nonuniformed clinical cancer treatment strategies performed by different regions [1]. Therefore, it is important to determine a new specific target for gastric cancer and develop a newly adjuvant drug.
Lipids are widely distributed in cellular organelles and are critical components of all membranes. In addition to their role as structural components, lipids in membranes also serve important functions. Lipids not only function as second messengers to transduce signals within cells but also serve as important energy sources when nutrients are limited [3]. Accumulating experiments have demonstrated that lipid metabolism is substantially reprogrammed in cancers [4–6]. The obvious feature of lipid metabolism in cancers is an increased rate of lipogenesis and mitochondrial fatty acid β-oxidation. Gastric cancer demonstrates a similar tendency and displays typical changes regarding the varieties of metabolites involved in lipid metabolism [7], suggesting that the lipid metabolic pathway might be a potential pathway for gastric cancer therapy.
FABPs, members of the superfamily of lipid-binding proteins, are key lipid metabolic enzymes responsible for the regulation of fatty acid uptake and intracellular transport [8]. It was reported that FABPs were closely associated with cancer development and were upregulated in many cancers such as renal cell carcinoma [9], hepatocellular carcinoma [10], and breast cancer [11], which indicates the potential role of FABPs as the tumor markers and therapeutic targets in some cases.
Traditional Chinese medicine (TCM) has been practiced for thousands of years and now is widely accepted as an alternative treatment for cancers [12]. Berberine, extracted from Chinese herbs such as Coptis, is traditionally used to treat gastrointestinal infections such as bacterial diarrhea in China for a long time. Recent investigations have discovered its properties against many diseases including obesity, hypolipidemia [13], and gastric cancers [14]. Our previous study proved that berberine could attenuate the proliferation and induce cell cycle arrest of gastric cancer cell lines by targeting the AMPK/HNF4α/WNT5a pathway [15]. It was also confirmed that berberine inhibited the growth and induced apoptosis of gastric cancer cell lines, which effects were linked to inhibition of EGFR signals [14]. However, whether berberine exerted growth inhibition effects against tumors through regulating the lipid metabolism remained to be elucidated.
Therefore, we conducted this study to further investigate the mechanism behind the antitumor properties of berberine regarding lipid metabolism.
2. Materials and Methods
2.1. Materials and Reagents
Berberine chloride with a purity of more than 98%, isolated from Chinese herbal Huanglian, was purchased from Sigma-Aldrich, United States. Antibodies against FABP4, FABP5, and Bax were obtained from Cell Signaling Technologies (Boston, MA, United States). Rabbit antibodies against Bcl-2 and cleaved-caspase3 were purchased from ABclonal (China). Antibodies against GAPDH and β-actin were gained from Hubei Biossci Biological Co., Ltd. Rabbit antibodies against PPARα and Bax were bought from proteintech Biotech Co., Ltd. Triglyceride detection kit and total cholesterol detection kit were obtained from Nanjing Jiancheng Bioengineering Institute. Annexin V-FITC/PI apoptosis detection kit was purchased from Biolegend Biotech Co., Ltd. The TUNEL detection kit was gained from Yeasen Biotech Co., Ltd.
2.2. Cell Culture
The gastric cancer cell line MGC803 was purchased from Wuhan Servicebio Technology Co., Ltd. MGC803 cells were routinely cultured in the RPMI-1640 medium (HyClone, China) supplemented with 10% fetal bovine serum (FBS) (SiJiQing, China) and 1% penicillin-streptomycin solution (Beijing Solarbio Science & Technology Co., Ltd.) at 37°C in a humidified atmosphere with 5% CO2.
2.3. MTT Assays
The cytotoxicity of berberine against MGC803 cells was determined with 3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) assay. Cells with a number of 4000 cells per well were seeded in 96-well culture plates. After well adhered, the cells were starved with the RPMI-1640 medium supplemented with no FBS and 1% penicillin-streptomycin solution for 12 h and subsequently treated with different concentrations of berberine for 24 h, 48 h, or 72 h. Then, after removing the supernatant medium of each well, a volume of 100 μl MTT solution with a concentration of 0.5 mg/ml was added to it, followed by incubation for 4 h at 37°C. The MTT solution was then removed, and another 150 μl DMSO solution was added into each well. After shaking at a lower speed for 30 minutes to fully dissolve the crystals, the absorbance of each well at 570 nm was measured using an enzyme-linked immunoassay (Synergr2 BioTek, United States).
2.4. Apoptosis Assays
For apoptosis detection, MGC803 cells were dual stained using the Annexin V-FITC/PI apoptosis detection kit. Briefly, after treatment with berberine or inhibitor, cells were washed twice with PBS and trypsinized with trypsin solution without EDTA. Collected cells were then washed twice with cold PBS, resuspended in cold 1x binding buffer, and dual stained with Annexin V-FITC and PI in the dark at room temperature for 5 minutes. Flow cytometry was immediately performed on cells using the BD Accuri C6 flow cytometer (BD Biosciences) and analyzed using FlowJo v.10 software. Besides, TUNEL assays were also performed to detect the apoptosis induced by berberine in the tumor tissues in xenografts. All the TUNEL assays were conducted following the instructions of the TUNEL apoptosis detection kit(Alexa Fluor 488) provided by Yeasen Biotech Co., Ltd.
2.5. Measurement of Total Cholesterol and Triglyceride Content
The total cholesterol content and the triglyceride content of MGC803 cells and its supernatants and tumor tissues from MGC803 xenograft models were tested using total cholesterol content detection kit and the triglyceride content detection kit purchased from Nanjing Jiancheng Bioengineering Institute. Following the instructions, the supernatants, the serum sample, cell homogenate (107 cells/200 μl), and tumor tissue homogenate (10 mg/90 μl) were added to 96-well plates with a volume of 2.5 μl per well. And then the operation fluid provided by the detection kit with a volume of 250 μl was added into per well above. The mixture was incubated at 37°C for 10 min. The absorbance of the wells was measured at 510 nm using the enzyme-linked immunoassay (Synergr2 BioTek, United States).
2.6. In Vivo Xenograft Models
Four-week-old female Balb/c nude mice weighed about 13–16 g were purchased from Hunan Slake Jingda Experimental Co., Ltd. (Hunan, China) and were maintained under specific pathogen-free atmosphere using a laminar airflow rack and had free access to sterilized food and autoclaved water. After a week of adjustable feeding, those mice were injected subcutaneously with MGC803 cells about 107 cells per mouse into the right flank. Seventy-two hours later, a mass of more than 5 mm in maximal diameter in all mice was identifiable. Subsequently, the MGC803 xenograft mouse models were randomly assigned into two groups (control group and berberine group) followed by treatment with intragastric administration of normal saline (N group) or berberine (100 mg/kg, berberine group), respectively, for 18 days, and then all animals were sacrificed and the tumor tissues were abstracted and weighed. Furthermore, the weight and the tumor length and width were measured every three days. And the tumor volume were calculated with the following formula: tumor volume (mm³) = [tumor length (mm) ∗ tumor width (mm)2]/2. All animal experiments were performed according to the institutional guidelines and regulations. All animal studies were approved by the Huazhong University of Science and Technology Institutional Animal Care and Use Committee (S787).
2.7. Western Blot
The total proteins were extracted from the cultured MGC803 cells and the xenograft tumor tissues, respectively. The proteins were then separated by electrophoresis on a polyacrylamide gel and transferred to the nitrocellulose filter membrane (the NC membrane) (0.45 μm or 0.22 μm, Millipore, United States). After blocking with 5% no-fat milk (Wuhan Servicebio Technology Co., Ltd) for about 1 h, the membranes were incubated overnight at 4°C with primary antibodies, anti-FABP4 (1 : 1000), anti-FABP5 (1 : 1000), anticleaved caspased3 (1 : 1000), anti-Bcl-2 (1 : 1000), anti-Bax (1 : 1500), anti-PPARα (1 : 1000), anti-GAPDH (1 : 10000), and anti-β-actin (1 : 5000). After having been washed three times with TBST solution, the membranes were incubated with the second antibody (anti-rabbit IgG (H + L) (DyLight™ 800 4x PEG Conjugate) #5151 for 1 : 30000 or anti-mouse IgG (H + L) (DyLight™ 800 4x PEG Conjugate) #5257 for 1 : 15000) at room temperature in the dark for 1 h and subsequently were scanned and visualized with a near-infrared double color laser imaging system (Odyssey, Lincoln, NE, United States).
2.8. Statistical Analysis
All experiments were conducted at least three times. All data were expressed as means ± SD and were plotted by GraphPad Prism 6 software. Statistical analysis was performed using GraphPad Prism 6 software. One-way analysis of variance (ANOVA) or the independent sample t-test was conducted to determine the significance between groups. A value with P < 0.05 was considered statistical significance.
3. Results
3.1. Berberine Suppressed the Proliferation of MGC803 Cells
To evaluate the bioactivity of berberine, we performed MTT assays to test the growth inhibition effect of berberine on MGC803 cells. As we can see from Figure 1, berberine significantly retarded the proliferation of MGC803 cells in a time-dependent manner (24 h, 48 h, and 72 h). Besides, Figure 1 also demonstrates that the higher the doses of berberine were, the stronger the inhibition effects were. Berberine inhibited the proliferation of MGC803 cells in a dose-dependent manner (0–100 μM). Therefore, berberine displayed antigrowth properties against gastric cancer MGC803 cells. Considering the cytotoxicity of berberine and given that a certain number of MGC803 cells should be maintained for further experiments, half-maximal inhibitory concentration (IC50, 40 μM) and 1/2 IC50 (20 μM) and 1/4 IC50 (10 μM) of BBR on MGC803 cells at 24 h was chosen for the following experiments.
Figure 1.
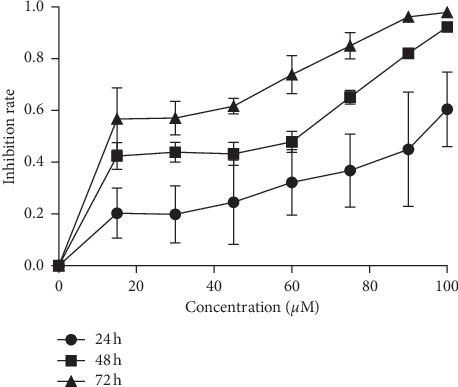
Berberine suppressed the proliferation of MGC803 cells. After treatment with berberine for 24 h, 48 h, and 72 h, the cell growth was determined by MTT assays. Berberine inhibited the growth of MGC803 cells in a time-dependent manner (24 h, 48 h, and 72 h) and in a dose-dependent manner (0–100 μM).
3.2. Berberine Induced Apoptosis of MGC803 Cells
Apoptosis, which maintains homeostasis through programmed cell death controlled by genes, has long been considered as the biggest challenge in the onset of cancer since it was first proposed by Kerr et al. in 1972 [16]. To explore the mechanisms behind the growth inhibition effects of berberine on MGC803 cells, we conducted the apoptosis assays on MGC803 cells which were treated with or without berberine. We took advantage of Annexin V-FITC/PI apoptosis detection kit to test the apoptotic rate of MGC803 cells induced by berberine. As it is seen in Figures 2(a) and 2(b), berberine induced apoptosis of MGC803 cells and increased the number of apoptotic cells. Besides, treatment with berberine with a dose of 40 μmol/L exerted greater apoptotic effects to MGC803 cells when compared to the other groups, so the higher the concentration of berberine was, the greater the apoptotic effects were. Moreover, we also detected the changes in the protein expression level of apoptosis-related proteins. As it is demonstrated in Figures 2(c) and 2(d), berberine decreased the protein level of antiapoptosis protein Bcl-2 while increased the expression level of proapoptotic protein Bax and activated the caspase3 protein. Thus, berberine inhibited cell survival by promoting the mitochondrial apoptosis pathways.
Figure 2.
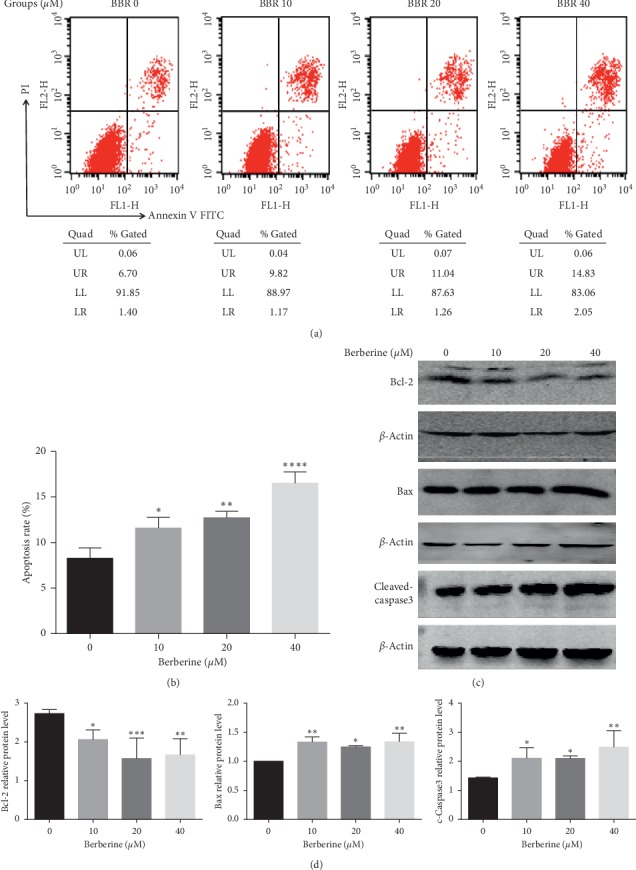
Berberine induced apoptosis of MGC803 cells. (a) Berberine treatment at 10, 20, and 40 μm for 24 h promoted the apoptosis of MGC803 cells, as detected by flow cytometry and statistically analyzed in (b). (c) Berberine treatment at 10, 20, and 40 μm for 24 h downregulated the protein expression of antiapoptosis protein Bcl-2 and promoted the protein expression of proapoptosis protein Bax and activated the expression of caspase3 protein. (d) The protein expression of apoptosis-related proteins of MGC803 cells after berberine treatment was quantified. ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05 vs controls.
3.3. Berberine Altered the Lipid Contents and Key Lipid Metabolic Enzymes in MGC803 Cells
It was pointed out that except for acting as infrastructural elements, lipids, and their metabolism exert key points of control over the Bcl-2 family-regulated mitochondrial apoptotic process [17], which suggested a link between lipids and apoptosis. Recent experiments also declared that berberine regulated lipid metabolism in many diseases such as type 2 diabetes and hepatoma carcinoma cells [18, 19]. Therefore, to investigate the tentative mechanism of how berberine regulated gastric cancer cells, the lipid contents and key lipid metabolic enzymes of MGC803 cells with or without berberine treatment were tested. It was concluded from Figures 3(a) and 3(b) that berberine elevated the contents of triglyceride in the supernatant as well as in MGC803 cells. And these increasing effects were enhanced with higher doses of berberine. Besides, we also detected the level of total cholesterol of MGC803 cells and their supernatant. However, the results in Figures 3(a) and 3(b) show that berberine with a concentration of 10 μmol/L or 20 μmol/L increased the level of total cholesterol of MGC803 cells while berberine with a dose of 40 μmol/L exerted no effects to MGC803 cells. And the level of total cholesterol in the supernatant of MGC803 cells was displayed with no significance after berberine treatment. These might also demonstrate the antitumor effects of berberine, given that route-specific outside-in delivery of cholesterol promotes GC progression [20]. Given that fatty acids of most cells were stored in triglyceride (TG) in cytosolic lipid droplets [21], these results might also suggest that berberine decreased the exogenous fatty acid uptake and lowered the usage of endogenous fatty acid-triglyceride.
Figure 3.
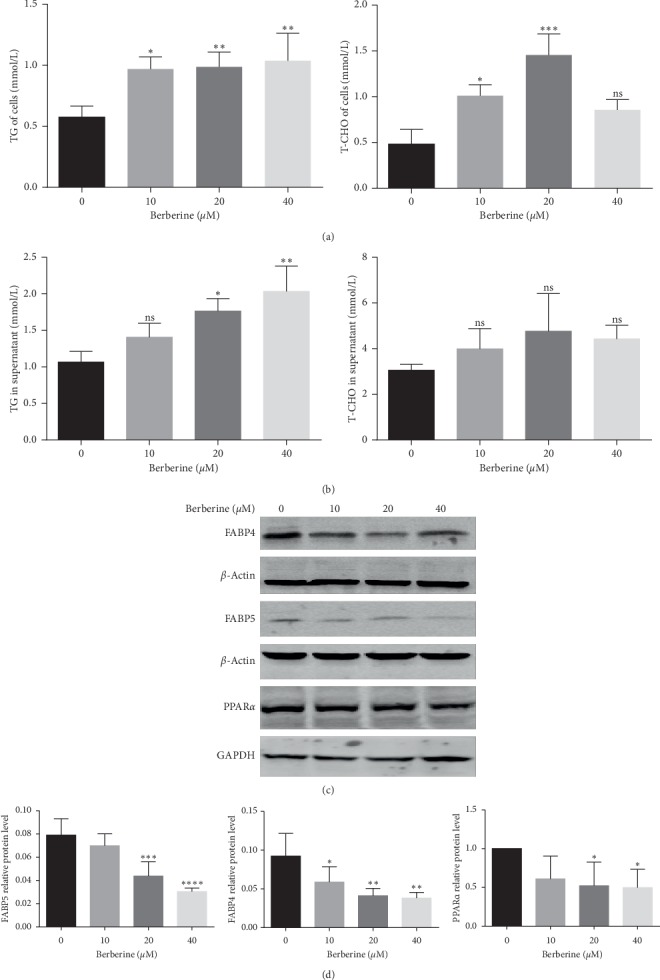
Berberine altered the lipid contents and key lipid metabolic enzymes of MGC803 cells. (a) Berberine treatment at 10, 20, and 40 μM for 24 h increased the level of TG of MGC803 cells. Berberine treatment at 10 and 20 μM for 24 h increased the level of T-CHO while 40 μM had no effects on the level of T-CHO of MGC803 cells. (b) Berberine treatment at 10, 20 and 40 μM for 24 h increased the level of TG while had no effects on the level of T-CHO in the supernatant of MGC803 cells. (c) Berberine treatment at 20 and 40 μM for 24 h suppressed the protein expression of key lipid metabolic enzymes FABP4, FABP5, and PPARα in MGC803 cells. (d) The quantification of protein expression of key lipid metabolic enzymes FABP4, FABP5, and PPARα in MGC803 cells. ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, and ns means no statistical significance.
Evidence showed that fatty acid uptake across the plasma membrane occurred partially via a protein-mediated process involving several fatty acid-binding proteins (FABPs) known as fatty acid transporters [22, 23]. FABPs are members of the superfamily of lipid-binding proteins, which are responsible for the regulation of fatty acid uptake and intracellular transport [8]. So, we tested the protein level of fatty acid-binding protein component FABP4 and FABP5. It is found in Figures 3(c) and 3(d) that berberine decreased the protein level of both FABP4 and FABP5 of MGC803 cells.
Furthermore, we also determined the expression level of the peroxisome proliferator-activated receptor (PPAR) component PPARα, which channels excess FAs into the FA oxidation pathway, thus playing a vital role in lipid metabolism [24, 25]. Increased PPARα expression could stimulate lipolysis, which in turn reduces lipid deposition [26]. Here as it is shown in Figures 3(c) and 3(d), the protein level of PPARα was suppressed by berberine in MGC803 cells, which might account for the elevated level of fatty acids of MGC803 cells caused by berberine treatment. On the whole, berberine regulated the fatty acid metabolism of MGC803 gastric cancer cells.
3.4. The FABP Inhibitor, BMS309403, Could Partially Recapitulate the Effects of Berberine on MGC803 Cells
Emerging evidence suggested that FABPs were associated with the development and progression of cancers [9–11]. A FABP inhibitor, BMS309403, was used to investigate if it could generate a similar effect to that seen in the berberine-treated cells. MGC803 cells were pretreated with a dose of 20 μmol/L BMS309403 for 10 minutes. And then its supernatant was replaced with the complete medium (the BMS group) or 20 μmol/L berberine (the BB group) for 24 h.
MTT assays were conducted to measure the antiproliferation effects of BMS309403 on MGC803 cells. The results showed that BMS309403 significantly inhibited the growth of MGC803 cells (P < 0.05) (Figure 4(a)). Moreover, treatment with BMS309403 plus berberine exerted greater growth inhibition effects than treating with BMS309403 alone (Figure 4(a)), which might lead to the probability that berberine exerted antiproliferation effects partially via inhibiting the FABP pathway.
Figure 4.
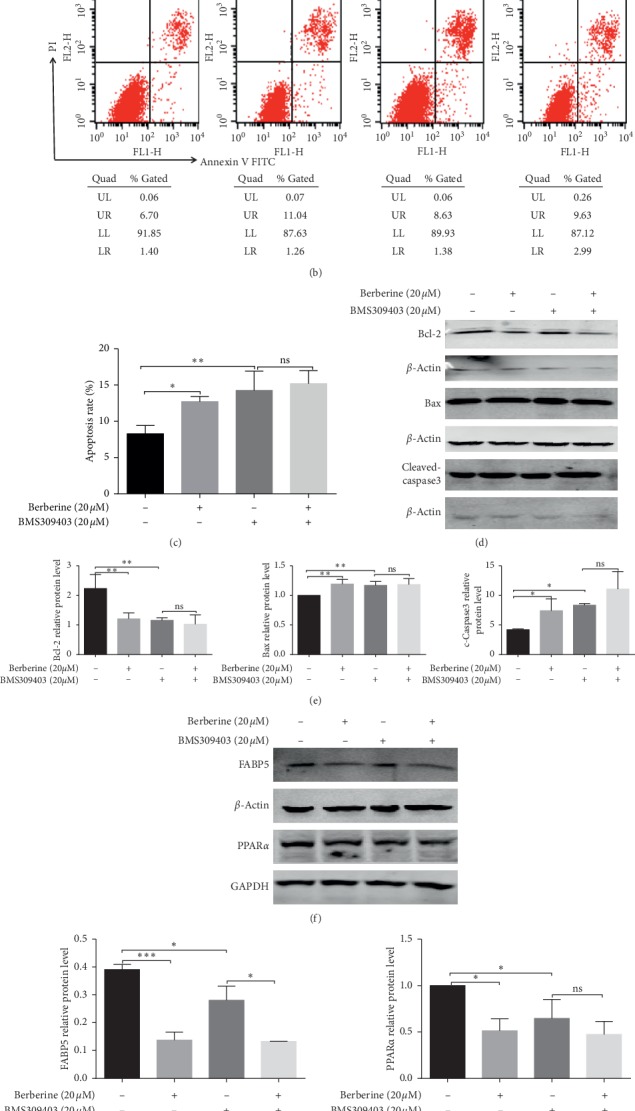
The FABP inhibitor, BMS309403, could partially recapitulate the effects of berberine on MGC803 cells. (a) BMS309403 suppressed the proliferation of MGC803 cells. (b) BMS309403 induced the apoptosis of MGC803 cells, and they were statistically analyzed in (c). (d) BMS309403 treatment downregulated the protein expression of antiapoptosis protein Bcl-2 and promoted the protein expression of proapoptosis protein Bax and activated the expression of caspase3 protein, and these apoptosis-related proteins were quantified in (e). (f) BMS309403 inhibited the protein expression of key lipid metabolic enzymes FABP5 and PPARα. (g) The quantification of the protein expression of key lipid metabolic enzymes FABP5 and PPARα in MGC803 cells after BMS309403 treatment. (h) BMS309403 elevated the cellular contents and supernatant contents of the triglyceride of MGC803 cells. ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, and ns means no statistical significance.
Besides, to further investigate whether the FABP pathway involved in fatty acid metabolism was responsible for the apoptosis-induced effect of berberine on MGC803 cells, we evaluated the apoptosis rate of MGC803 cells after BMS309403 treatment with or without berberine. It was displayed that BMS309403 significantly elevated the apoptotic rate of MGC803 cells (Figures 4(b) and 4(c)). Besides, berberine also enhanced the protein expression of proapoptosis protein Bax, activated the cleaved-caspase3, and meantime lowered the protein level of antiapoptosis protein Bcl-2 (Figures 4(d) and 4(e)). These indicated that the reduction of the FABPs could result in the trigger of the mitochondrial apoptosis pathway of MGC803 cells. Besides, we also detected the apoptosis-induced effects of berberine on MGC803 cells which were pretreated with BMS309403. As it is seen from Figures 4(e) and 4(f), the level of apoptosis-related proteins including the Bcl-2, Bax, and cleaved-caspase 3 between the BMS group and the BB group was found with no significance, indicating that the decrease of the protein level of FABPs was responsible for the berberine-induced apoptosis effects on MGC803 cells.
Furthermore, the triglyceride contents in supernatant and cancer cells were also increased upon BMS309403 treatment in Figure 4(h), suggesting that the reduction of FABPs might result in the accumulation of fatty acids on MGC803 cells and its supernatants. However, the triglyceride content in supernatant and cancer cells between the BMS group and the BB group was displayed with no significance, indicating that the decrease of the level of FABPs was responsible for the berberine-induced accumulation of fatty acids in MGC803 cells and its supernatants.
We also detected the protein level of PPARα of MGC803 cells which were pretreated with BMS309403 followed by replacement treatment with a complete medium or berberine. Results showed that BMS309403 significantly lowered the expression of PPARα, and there displayed no significance between the BMS group and the BB group in terms of the protein level of PPARα (Figures 4(f) and 4(g)), indicating that the decreased expression of FABPs could result in the reduction of PPARα protein and that berberine decreased the expression of PPARα, at least, partially via targeting FABPs. Therefore, consistent with those observed after berberine treatment, BMS309403 also caused the accumulation of fatty acids and the retardation of tumor growth and induced apoptosis of MGC803 cells.
3.5. Berberine Inhibited Tumor Growth in the MGC803 Xenograft Models
MGC803 xenograft model was established to explore the potential therapeutic effects of berberine in vivo. As it is shown in Figures 5(a) and 5(b), berberine decreased the tumor size and tumor weight (0.16 ± 0.02 g) (P < 0.05) in the MGC803 xenograft models when compared to the control group (0.32 ± 0.04 g). During this experiment, berberine decreased the growth rate of tumors in MGC803 xenografts (Figure 5(c)). Meantime, hematoxylin and eosin (H&E) staining of tumor sections in MGC803 xenografts was performed, and the results displayed that tumors treated with berberine were demonstrated with enlarged intercellular spaces and decreased cell density (Figure 5(e)). Moreover, during this experiment, there existed no significance in terms of weight of mice between the berberine group and the N group (Figure 5(d)), indicating that berberine exerted no side effects on the weight of mice in this experiment. Our research coordinated with other research studies that berberine induced 46.58% inhibition of tumor compared to the control group, showing that berberine significantly reduced the GC tumor incidence and berberine-treated mice displayed significant tumor growth retardation compared with the control group [27].
Figure 5.
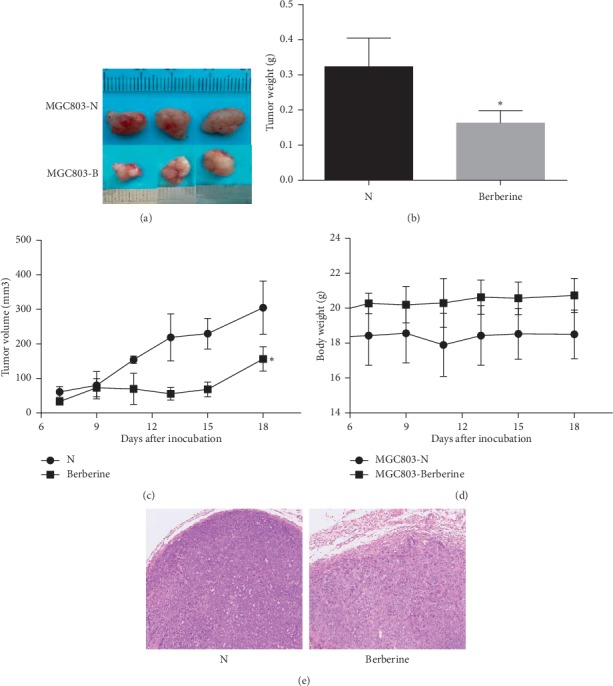
Berberine suppressed tumor growth in the MGC803 xenograft models. (a) Berberine (100 mg/kg/d, intragastric administration) retarded MGC803 xenograft tumor growth. (b) Tumors were removed from the sacrificed mice and weighted. Berberine decreased tumor weight. (c) The tumor volume in the treatment group and the control group. Berberine suppressed tumor growth. (d) Berberine had no effects on the body weights of treated mice. (e) Hematoxylin and eosin (H&E) staining of tumor sections in MGC803 xenografts. ∗P < 0.05.
3.6. Berberine Induced Tumor Apoptosis in the MGC803 Xenograft Models
TUNEL assays were also conducted to detect the apoptosis effects induced by berberine in tumor tissues of MGC803 xenografts. As it is displayed in Figure 6(a), treatment with 100 mg/kg/d berberine could lead to a greater apoptosis rate of tumor tissues when compared to the control groups. Besides, we also test the protein level of apoptosis-related proteins of tumor tissues, making use of western blot assays. It was showed that berberine elevated the protein expression level of proapoptotic protein Bax while lowered the level of antiapoptosis protein Bcl-2 when comparing to the control group (Figures 6(b) and 6(c)). Meantime, the protein level of the activated caspase3 protein was also detected and was demonstrated with an increase in tumor tissues of mice which were treated with berberine (Figures 6(b) and 6(c)). So, it could be concluded that berberine induced apoptosis of tumors in the MGC803 xenograft models.
Figure 6.
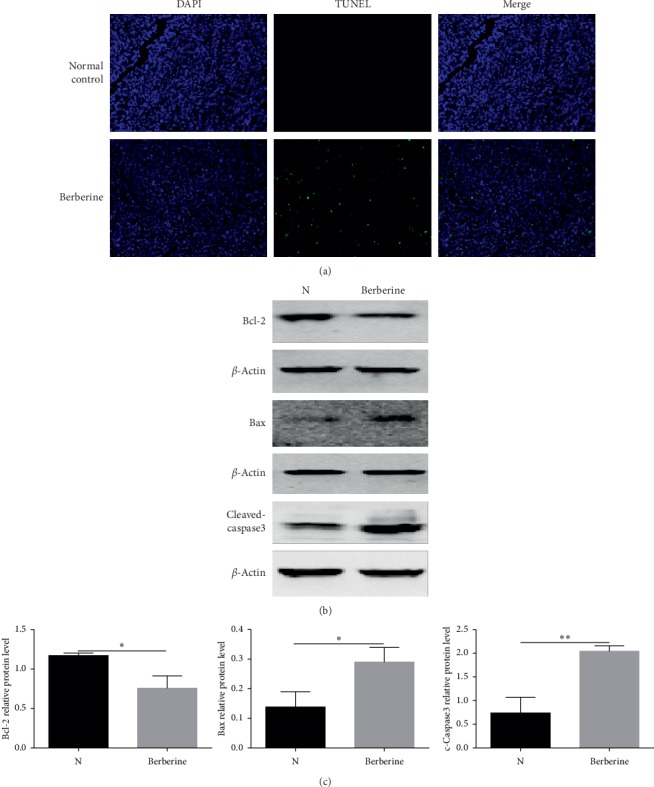
Berberine induced apoptosis of tumors in the MGC803 xenograft models. (a) Berberine treatment induced apoptosis of MGC803 xenograft tumors as detected by TUNEL assays. (b) Berberine downregulated the protein expression of antiapoptosis protein Bcl-2, promoted the protein expression of proapoptosis protein Bax, and activated the expression of caspase3 protein of MGC803 xenograft tumors. (c) The protein expression of apoptosis-related proteins of tumors in MGC803 xenograft models was quantified. ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
3.7. Berberine Altered the Lipid Contents and Key Lipid Metabolic Enzymes in the MGC803 Xenograft Models
We also tested the lipid contents of tumors and the lipid contents in serum in MGC803 xenografts. As it is presented in Figure 7(a), the level of the triglyceride of tumor tissues was much higher in the berberine group than that in the control group. Treatment with berberine was also found to elevate the level of triglyceride in the serum of MGC803 xenografts in Figure 7(b). However, the level of total cholesterol of tumors and the total cholesterol in serum was displayed with no significance between the berberine group and the control group (Figures 7(a) and 7(b)). Furthermore, the key lipid metabolic enzymes involved in the regulation of fatty acid metabolism were also detected (Figures 7(c) and 7(d)). It was found that the protein expression level of PPARα of tumor sections was retarded by berberine in the MGC803 xenograft models. Besides, it was also demonstrated that the expression level of both FABP4 and FABP5 was downregulated by treatment with berberine in tumor tissues of MGC803 xenografts. Therefore, it was concluded that berberine regulated fatty acid metabolism in the MGC803 xenograft models.
Figure 7.
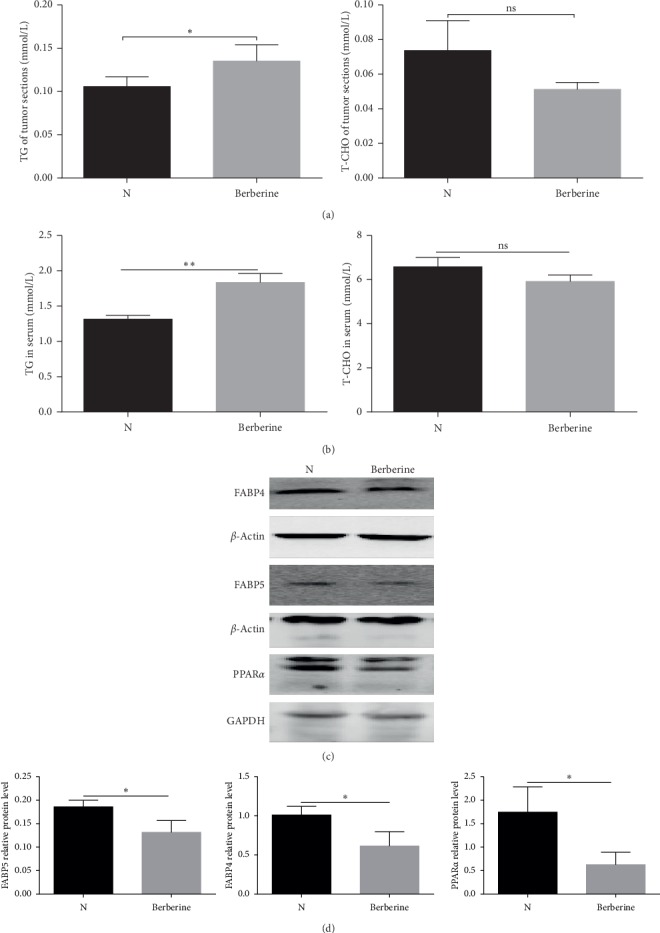
Berberine altered the lipid contents and key lipid metabolic enzymes in the MGC803 xenograft models. (a) Berberine treatment increased the level of TG of MGC803 xenograft tumors. Berberine had no effects on the level of T-CHO of MGC803 xenograft tumors. (b) Berberine treatment elevated the level of TG in the serum of MGC803 xenografts. Berberine had no effects on the level of T-CHO in the serum of MGC803 xenografts. (c) Berberine suppressed the protein expression of key lipid metabolic enzymes FABP4, FABP5, and PPARα of MGC803 xenograft tumors. (d) The quantification of key lipid metabolic enzymes FABP4, FABP5, and PPARα of tumor tissues from MGC803 xenografts. ∗∗P < 0.01, ∗P < 0.05, and ns means no statistical significance.
4. Discussion
According to the traditional Chinese medicine theories, Coptidis Rhizoma is routinely used to remove damp-heat and fire which are regarded as external pathogenic factors that cause diseases. Although the pathological concept of cancer first appeared in modern medical theories, it is still easy to discover some descriptions of cancer-like symptoms in ancient medical records in China. In the ancient Chinese medical monographs “ZhongZangJing,” cancer was described as “Yong, Yang, Chuang, and Zhong,” which are caused by intracorporal retention of various pathogens including heat and dampness [28]. Therefore, the use of clearing heat and dampness of the traditional Chinese medicine Coptidis Rhizoma and its abstract berberine indicates its potential therapeutic use for treating cancer.
Accumulating experiments have contributed to the antitumor properties of Coptidis Rhizoma extract and its active component berberine. Berberine could downregulate IL-8 expression through inhibition of the EGFR/MEK/ERK pathway to suppress cell invasiveness and growth in triple-negative breast cancer cells [29]. Berberine also could inhibit angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway [30]. Besides, berberine was also illustrated to promoted apoptosis of colorectal cancer via regulation of the long noncoding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU-Binding Factor 1 (AUF1)/B-Cell CLL/Lymphoma 2 (Bcl-2) Axis [31]. Here, in this study, we observed that berberine retarded the growth of human gastric cancer cells and induced apoptosis through the mitochondrial apoptosis pathway. These berberine-induced apoptotic effects likely arose, in part, from the effects of berberine on cellular lipid homeostasis [32].
Lipids which mainly include fatty acids and cholesterol are vital sources of energy metabolism in the human body [33]. Studies suggested that to meet the needs of biosynthesis during high levels of proliferation, the reprogramming of fatty acid (FA) metabolism becomes essential in cancer cells [34]. The abnormal fatty acid metabolism affects numerous vital cellular processes including cell growth, proliferation, and survival [17, 35], thus playing a crucial role in cancer development and progression [36, 37]. And it has been proposed that increased storage of fatty acids in neutral lipids (such as TGs) could lead to a reduction in available fatty acids, which was used for membrane building blocks or signaling lipids and inhibited cellular proliferation [23, 36], just the same with that observed in MGC803 cells after berberine treatment. We found that the berberine treatment of MGC803 cells led to the accumulation of fatty acid-triglyceride. Mounting evidence highlights the membrane as an equally important factor in the successful induction of the death response, though the mitochondrial apoptotic process heavily relies on its protein components [17, 23]. Unger et al. found that excess free fatty acids in the cytoplasm are harmful to cells: they can disrupt mitochondrial membrane integrity [38]. In our experiments, we found that treatment with berberine induced an accumulation of fatty acids and activated Bcl-2 family-regulated mitochondrial apoptotic process in MGC803 cells. These findings suggested that the accumulation of fatty acids was responsible for the activation of the mitochondrial apoptotic process.
Cancer cells are often in a metabolically challenging atmosphere with scarce availability of oxygen and nutrients, and such metabolic stress can lead to changes in the balance between the endogenous synthesis and exogenous uptake of fatty acids, which are needed for membrane biogenesis, energy production, and protein modification of cells [24]. It was pointed out that hypoxic cancer cells might turn to fatty acid uptake pathways to compensate for the repressed glucose-based de novo fatty acid synthesis [39]. The long-chain fatty acid uptake across the plasma membrane occurs mainly via several fatty acid-binding proteins (FABPs), which positively correlates with rates of fatty acid transport [23]. Nieman et al. showed that adipocytes surrounding tumor cells provide energy through supplying fatty acids to cancer cells in the FABP-dependent mechanism [40]. FABPs are cytoplasmic proteins, which could bind and transfer long-chain fatty acids to different intracellular destinations [41]. FABPs are recognized as a protective sink for long-chain fatty acids defending cells from injuries by excessive lipid accumulation [23]. Here, we found that the protein level of FABPs was downregulated and the accumulation of triglyceride was also induced by berberine in MGC803 cells and in MGC803 xenograft models. These results indicated that the reduction of FABPs resulted from berberine treatment could lead to the accumulation of fatty acids.
Recently, more and more researches have focused on the role of FABPs in the development and progression of a variety of cancers. The epidermal-type fatty acid-binding protein-5 (FABP-5) gene acts as a key molecule in the development and progression of a variety of tumors like breast cancer [42] and hepatocellular carcinoma [43]. Studies have shown that the upregulation of FABP5 stimulates fatty acid transport, which finally induces cell growth and survival [44]. It was also presented that the silence of the FABP5 gene might attenuate the invasiveness of gastric cancer cells, detain cell proliferation, and arrest the cell cycle in the G0/G1 phase, leading to a significant increase in apoptosis [45]. BMS309403 is a well-designed inhibitor of FABPs (FABP3-5, and −7), which interacts with the fatty acid-binding pocket to inhibit the binding of fatty acids [23, 46]. In our studies, the BMS309403 phenocopy was identical to the effects of berberine on lipid metabolism and cell apoptosis in MGC803 cells. We discovered that the FABP inhibitor BMS309403 could result in the accumulation of triglyceride, induce apoptosis, and suppress the growth of MGC803 cells. Our results correspond with the results that exogenous FABP reduces active caspase 3 expressions in HepG2 cells, which effect is reversed in the presence of BSM309403 [47].
In summary, we found that treatment with berberine inhibited gastric cancer growth in vitro and in vivo by suppressing the expression of FABPs, which led to the accumulation of fatty acids and apoptosis of gastric cancer cells. Our experiments provided new insight into the potential pharmacological application of berberine as an antitumor agent and also indicated new clues for biomarkers or therapeutic targets of lipid metabolism in tumors. Our studies also enriched the metabolic regulatory mechanism of berberine against gastric cancer.
5. Conclusion
Berberine, a natural isoquinoline alkaloid isolated from herbal plants such as Coptis, exerted anticancer effects both in vitro and in vivo by inducing apoptosis, due to the accumulation of fatty acids and the reduced expression of FABPs, suggesting that berberine was a promising novel anticancer agent.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (nos. 81673757 and 81573787).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Lingli Li and Ping Yi designed the study. Lingli Li, Ze Peng, Lijun Xu, and Xin Zou conducted the experiments. Lingli Li wrote the manuscript. Yan Yu plotted the manuscript. Ping Yi, Qian Hu, and Dongmei Huang revised the manuscript. All authors approved the final version to be published.
Supplementary Materials
Figure 2(c): all immunoblots of apoptosis-related proteins Bcl-2, Bax, and cleaved-caspase3 of MGC803 cells after berberine treatment. Figure 3(c): all immunoblots of key lipid metabolic enzymes FABP4, FABP5, and PPARα in MGC803 cells after berberine treatment. Figure 4(d): All immunoblots of Bcl-2, Bax, and cleaved-caspase3 of MGC803 cells after BMS309403 treatment. Figure 4(f): all immunoblots of key lipid metabolic enzymes FABP5 and PPARα of MGC803 cells after BMS309403 treatment. Figure 6(b): all immunoblots of apoptosis-related proteins Bcl-2, Bax, and cleaved-caspase3 of MGC803 xenograft tumors. Figure 7(c): all immunoblots of key lipid metabolic enzymes FABP4, FABP5, and PPARα of MGC803 xenograft tumors.
References
- 1.Feng R.-M., Zong Y.-N., Cao S.-M., Xu R.-H. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Communications. 2019;39(1):p. 22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Cheng C., Geng F., Cheng X., Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Communications. 2018;38(1):p. 27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusu P., Shao C., Neuerburg A., et al. GPD1 specifically marks dormant glioma stem cells with a distinct metabolic profile. Cell Stem Cell. 2019;25(2):241–257. doi: 10.1016/j.stem.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Hao Y., Li D., Xu Y., et al. Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinformatics. 2019;20(S7) doi: 10.1186/s12859-019-2734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X., Zhao L., Yang H., et al. Pyruvate kinase M2 interacts with nuclear sterol regulatory element-binding protein 1a and thereby activates lipogenesis and cell proliferation in hepatocellular carcinoma. Journal of Biological Chemistry. 2018;293(17):6623–6634. doi: 10.1074/jbc.ra117.000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao S., Zhou L. Gastric cancer: metabolic and metabolomics perspectives (Review) International Journal of Oncology. 2017;51(1):5–17. doi: 10.3892/ijo.2017.4000. [DOI] [PubMed] [Google Scholar]
- 8.Boiteux G., Lascombe I., Roche E., et al. A-FABP, a candidate progression marker of human transitional cell carcinoma of the bladder, is differentially regulated by PPAR in urothelial cancer cells. International Journal of Cancer. 2009;124(8):1820–1828. doi: 10.1002/ijc.24112. [DOI] [PubMed] [Google Scholar]
- 9.Nagao K., Shinohara N., Smit F., et al. Fatty acid-binding protein 7 may be a marker and therapeutic targets in clear cell renal cell carcinoma. BMC Cancer. 2018;18(1) doi: 10.1186/s12885-018-5060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao J., Yan F., Zhang Y., et al. Expression of adipocyte/macrophage fatty acid-binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Research. 2018;78(9):2343–2355. doi: 10.1158/0008-5472.can-17-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson K. J., Austin R. G., Nazari S. S., Gersin K. S., Iannitti D. A., McKillop I. H. Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver International. 2018;38(6):1074–1083. doi: 10.1111/liv.13639. [DOI] [PubMed] [Google Scholar]
- 12.Xiang Y., Guo Z., Zhu P., Chen J., Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Medicine. 2019;8(5):1958–1975. doi: 10.1002/cam4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y., Xia M., Yan H., et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. British Journal of Pharmacology. 2018;175(2):374–387. doi: 10.1111/bph.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Yang S., Cai X., et al. Berberine inhibits EGFR signaling and enhances the antitumor effects of EGFR inhibitors in gastric cancer. Oncotarget. 2016;7(46):76076–76086. doi: 10.18632/oncotarget.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q., Li L., Zou X., Xu L., Yi P. Berberine attenuated proliferation, invasion, and migration by targeting the AMPK/HNF4α/WNT5A pathway in gastric carcinoma. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr J. F. R., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with Wideranging implications in tissue kinetics. British Journal of Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T., Saghatelian A. Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids. 2013;1831(10):1542–1554. doi: 10.1016/j.bbalip.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Wei S., Zhang M., Yu Y., et al. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitate-incubated HepG2 cells through suppression of the HNF-4α miR122 pathway. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152097.e152097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y., Yuan X., Zhang F., et al. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-11860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang W., Huang S., Lee Y., et al. Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival. Oncotarget. 2017;8(1):p. 692. doi: 10.18632/oncotarget.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farese R. V., Walther T. C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139(5):855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonen A., Luiken J. J. F. P., Glatz J. F. C. Regulation of fatty acid transport and membrane transporters in health and disease. Molecular and Cellular Biochemistry. 2002;239(1-2):181–192. doi: 10.1023/a:1020511125085. [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Wang K., Kuang S., et al. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death & Disease. 2018;9(6) doi: 10.1038/s41419-018-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munir R., Lisec J., Swinnen J. V., Zaidi N. Lipid metabolism in cancer cells under metabolic stress. British Journal of Cancer. 2019;120(12):1090–1098. doi: 10.1038/s41416-019-0451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C.-H., Olson P., Evans R. M. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 26.Wei C.-C., Wu K., Gao Y., Zhang L.-H., Li D.-D., Luo Z. Magnesium reduces hepatic lipid accumulation in yellow catfish (Pelteobagrus fulvidraco) and modulates lipogenesis and lipolysis via PPARA, JAK-STAT, and AMPK pathways in hepatocytes. The Journal of Nutrition. 2017;147(6):1070–1078. doi: 10.3945/jn.116.245852. [DOI] [PubMed] [Google Scholar]
- 27.Yi T., Zhuang L., Song G., Zhang B., Li G., Hu T. Akt signaling is associated with the berberine-induced apoptosis of human gastric cancer cells. Nutrition and Cancer. 2015;67(3):523–531. doi: 10.1080/01635581.2015.1004733. [DOI] [PubMed] [Google Scholar]
- 28.Wang N., Tan H.-Y., Li L., Yuen M.-F., Feng Y. Berberine and Coptidis Rhizoma as potential anticancer agents: recent updates and future perspectives. Journal of Ethnopharmacology. 2015;176:35–48. doi: 10.1016/j.jep.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Kim S., You D., Jeong Y., et al. Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomedicine. 2018;50:43–49. doi: 10.1016/j.phymed.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Jin F., Xie T., Huang X., Zhao X. Berberine inhibits angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway. Pharmaceutical Biology. 2018;56(1):665–671. doi: 10.1080/13880209.2018.1548627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai W., Mu L., Cui Y., et al. Berberine promotes apoptosis of colorectal cancer via regulation of the long non-coding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU-Binding factor 1 (AUF1)/B-cell CLL/lymphoma 2 (Bcl-2) axis. Medical Science Monitor. 2019;25:730–738. doi: 10.12659/msm.912082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishi K., Suzuki K., Sawamoto J., et al. Inhibition of fatty acid synthesis induces apoptosis of human pancreatic cancer cells. Anticancer Research. 2016;36(9):4655–4660. doi: 10.21873/anticanres.11016. [DOI] [PubMed] [Google Scholar]
- 33.Ni K., Wang D., Xu H., et al. miR-21 promotes non-small cell lung cancer cells growth by regulating fatty acid metabolism. Cancer Cell International. 2019;19(1) doi: 10.1186/s12935-019-0941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X.-H., Ren X.-H., Liang X.-H., Tang Y.-L. Roles of fatty acid metabolism in tumourigenesis: beyond providing nutrition (review) Molecular Medicine Reports. 2018;18(6):5307–5316. doi: 10.3892/mmr.2018.9577. [DOI] [PubMed] [Google Scholar]
- 35.Ni T., He Z., Dai Y., Yao J., Guo Q., Wei L. Oroxylin A suppresses the development and growth of colorectal cancer through reprogramming of HIF1α-modulated fatty acid metabolism. Cell Death & Disease. 2017;8(6) doi: 10.1038/cddis.2017.261.e2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Currie E., Schulze A., Zechner R., Walther T. C., Farese R. V. Cellular fatty acid metabolism and cancer. Cell Metabolism. 2013;18(2):153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaiser R. A., Pessia A., Ateeb Z., et al. Integrated targeted metabolomic and lipidomic analysis: a novel approach to classifying early cystic precursors to invasive pancreatic cancer. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-46634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unger R. H., Clark G. O., Scherer P. E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2010;1801(3):209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Bensaad K., Favaro E., Lewis C. A., et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Reports. 2014;9(1):349–365. doi: 10.1016/j.celrep.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 40.Nieman K. M., Kenny H. A., Penicka C. V., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golaszewska K., Harasim Symbor E., Polak-Iwaniuk A., Chabowski A. Serum fatty acid-binding proteins as a potential biomarker in atrial fibrillation. Journal of Physiology and Pharmacology. 2019;70(1) doi: 10.26402/jpp.2019.1.11. [DOI] [PubMed] [Google Scholar]
- 42.Yang D., Li Y., Xing L., et al. Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression. Cell Communication and Signaling. 2018;16(1) doi: 10.1186/s12964-018-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan L., Xiao H., Liao R., et al. Fatty acid binding protein 5 promotes tumor angiogenesis and activates the IL6/STAT3/VEGFA pathway in hepatocellular carcinoma. Biomedicine & Pharmacotherapy. 2018;106:68–76. doi: 10.1016/j.biopha.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Guaita-Esteruelas S., Gumà J., Masana L., Borràs J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Molecular and Cellular Endocrinology. 2018;462:107–118. doi: 10.1016/j.mce.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhao G., Wu M., Wang X., Du Z., Zhang G. Effect of FABP5 gene silencing on the proliferation, apoptosis and invasion of human gastric SGC-7901 cancer cells. Oncology Letters. 2017;14(4):4772–4778. doi: 10.3892/ol.2017.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczocha M., Glaser S. T., Deutsch D. G., Lennarz W. J. Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of the National Academy of Sciences. 2009;106(15):6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laouirem S., Sannier A., Norkowski E., et al. Endothelial fatty liver binding protein 4: a new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene. 2019;38(16):3033–3046. doi: 10.1038/s41388-018-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2(c): all immunoblots of apoptosis-related proteins Bcl-2, Bax, and cleaved-caspase3 of MGC803 cells after berberine treatment. Figure 3(c): all immunoblots of key lipid metabolic enzymes FABP4, FABP5, and PPARα in MGC803 cells after berberine treatment. Figure 4(d): All immunoblots of Bcl-2, Bax, and cleaved-caspase3 of MGC803 cells after BMS309403 treatment. Figure 4(f): all immunoblots of key lipid metabolic enzymes FABP5 and PPARα of MGC803 cells after BMS309403 treatment. Figure 6(b): all immunoblots of apoptosis-related proteins Bcl-2, Bax, and cleaved-caspase3 of MGC803 xenograft tumors. Figure 7(c): all immunoblots of key lipid metabolic enzymes FABP4, FABP5, and PPARα of MGC803 xenograft tumors.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


