Abstract
Rabs have been reported to be involved in the carcinogenesis process and in the progression of cancer. However, it is unclear whether or not Rab9 is associated with the development of cancer. In the present study, we aimed to investigate the role of Rab9 in the biological functions of gastric cancer cells. The gastric cancer cell lines AGS and MKN45 were transfected with siRNA-Rab9 to block the expression of Rab9. The cell viability, proliferation, migration, invasion, and apoptosis were examined using Cell Count Kit-8, colony formation, wound healing, Transwell, and flow cytometry assays, respectively. Our data showed that silencing of Rab9 significantly inhibited the viability, proliferation, migration, and invasion abilities of AGS and MKN45 cells. Moreover, transfection with siRab9 promoted the rate of apoptosis in AGS and MKN45 cells through regulating the Bcl-2–Bax axis and the Caspase cascade. We also found that silencing of Rab9 inhibited activation of the Akt signaling pathway by downregulating the phosphorylation level of Akt. In conclusion, our data suggest that Rab9 plays an oncogenic role in the progression of gastric cancer, providing a potential target for the treatment of gastric cancer.
Keywords: gastric cancer, Rab9, proliferation, invasion, Akt signaling pathway
Introduction
Gastric cancer is the most common type of gastrointestinal system tumor, with high rates of morbidity and mortality.1,2 It is estimated that there are approximately 951 600 new cases each year, resulting in 723 100 deaths.1,3 Despite improvement in treatment methods, the 5-year survival rate of patients with gastric cancer is still less than 30%. In advanced patients, it is even lower at 5% to 20%.4,5 At present, surgical resection is the most effective strategy for the treatment of gastric cancer, but more than 60% of patients experience tumor metastasis or recurrence after surgery. This is the primary cause of death and poor prognosis of patients with gastric cancer.6,7 Recently, increasing studies have focused on finding biomarkers and targets for gastric cancer in order to develop new treatment strategies.
The Rab family, a class of small GTPases belonging to the Ras superfamily, serves as a key regulator in intracellular trafficking.8,9 Recently, emerging evidence reveal that some members of the Rab family play key roles in the carcinogenesis process and the progression of cancers.10-13 For instance, Rab27A has been reported to be deregulated in cancer, and its overexpression could promote the growth and metastasis of breast cancer.14,15 Li et al find that Rab40b is correlated with metastasis and prognosis of patients with gastric cancer as well as proving that it plays an oncogenic role in the progression of gastric cancer.16 Rab22A as a target of miR-204-5p is involved in the growth of gastric cancer cells.17 As a member of the Rab family, Rab9 (also known as Rab9A) has been revealed to participate in the endosome-to-trans-Golgi Network transport and is involved in the endolysosomal system.9 Recently, Liu et al report that Rab9 is upregulated in breast cancer and promotes tumor proliferation and invasion.18 However, whether Rab9 is associated with the development of gastric cancer is unclear.
Herein, we aimed to illustrate the relationship between Rab9 and gastric cancer and explore the underlying mechanisms. We found that silencing of Rab9 inhibited the proliferation, migration, and invasion of gastric cancer cells, as well as promotion of apoptosis by regulating the Bcl-2–Bax axis and Caspase cascade. Our data highlighted an oncogenic role of Rab9 in the progression of gastric cancer, providing a potential target for treatment.
Materials and Methods
Cell Culture and Transfection
The human gastric cancer cell lines AGS and MKN45 were bought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin (Sigma Aldrich, Darmstadt, Germany), and 0.1 mg/mL streptomycin (Sigma Aldrich). When the cell confluence in the 6-well plate reached 80%, the culture medium was replaced with an antibiotic-free medium. Then, cells were transfected with small-interfering RNA (siRNA) using liposome according to the instructions. The siRNA sequences for Rab9 were synthesized from Oligobio (Beijing, China). The sequences were as follows: siRNA1: 5′-CACAGTCAATCTTCACCGA-3′; siRNA2: 5′-CCGAGGATAGGTCAGATCA-3′; siRNA3: 5′-GACAACGGCGACTATCCTT-3′. The scramble sequence was used as negative control (NC). Cells without any treatment were used as the control group.
Real-Time Fluorescence Quantitative Polymerase Chain Reaction Assay
After being transfected for 24 hours, an ultrapure RNA extraction kit (CWBIO, Beijing, China) was used to extract total RNA from cells. Then, RNA was reverse transcribed for cDNA, which was used as the template in real-time fluorescence quantitative polymerase chain reaction. The expression level of Rab9 was detected using a SYBR Premix Ex Taq II kit (Takara, Shiga, Japan). The primers used in this study were synthesized from GENEWIZ (Suzhou, China). The sequences were as follows: Rab9: F: 5′-CGACCCTCTCTCTGTCCTCA-3′, R: 5′-GCAGCAGTCAGAACCTCTGT-3′. β-actin was used as internal normalization standards: F: 5′-CCCGAGCCGTGTTTCCT-3′, R: 5′-GTCCCAGTTGGTGACGATGC-3′. The obtained data were analyzed using the 2−ΔΔCt method.
Western Blot
After transfection for 48 hours, cells were lysed in RIPA Lysis Buffer (CWBIO) to extract protein that was quantified using the BCA kit (CWBIO). Subsequently, 20 µg protein of each sample was isolated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and then electrotransferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, Massachusetts). The membrane was blocked with 5% defatted milk for 1 hour and incubated with primary antibodies (dilution, 1:1000; Proteintech Group, Illinois) overnight at 4°C. After that, the membrane was incubated with horseradish peroxidase–conjugated secondary antibodies (dilution, 1:3000; Proteintech Group) for 1 hour at room temperature. Signals were observed using an enhanced chemiluminescence kit (CWBIO).
Cell Count Kit-8 Assay
Cells transfected with siRNA for 24 hours were grown in a 96-well plate at a density of 1 × 103 cells per well and cultured at 37°C. Cell viability was detected every 24 hours. Before detection, 10 µL of Cell Count Kit-8 (CCK8) reagent (Beijing Solarbio Science & Technology, Beijing, China) was added to each well and incubated at 37°C for an additional 1.5 hours. The optical density value was measured at 450 nm using an enzyme standard instrument.
Colony Formation Assay
After being transfected with siRNA for 24 hours, cells were seeded in 35-mm dishes at a density of 1 × 103 cells per dish and cultured at 37°C for 2 to 3 weeks. When visible colonies had formed, the medium was removed, and the colonies were washed using phosphate-buffered saline (PBS). 4% paraformaldehyde was used to fix the colonies followed by staining with 0.1% crystal violet for 30 minutes. The number of colonies was quantified and then compared between groups.
Wound-Healing Assay
Wound-healing assay was performed to assess the migration ability of AGS and MKN45 cells after transfection with siRNA for 24 hours. Cells were seeded in a 6-well plate at a density of 5 × 105 cells/well. After culturing overnight, the cell surfaces were scratched with a pipette tip. The cells were washed 3 times with PBS, cultured in serum-free medium at 37°C for 24 hours, and photographed. The wound closure was measured using ImageJ software (version 1.5, National Institutes of Health, Bethesda, Maryland, USA).
Transwell Assay
Transwell chambers (Millipore) with or without Matrigel (BD Bioscience, San Jose, California) were conducted to examine cell invasion or migration ability of AGS and MKN45 cells. In brief, the upper chambers were precoated with Matrigel for the invasion assay. Cells suspended in the serum-free medium (1 × 105 cells) were placed in the upper chamber, and 1 mL of medium containing 10% FBS was added to the lower chamber. After incubating at 37°C overnight, the remaining cells in the upper chamber were wiped off with a cotton swab. The invaded cells were fixed with 4% paraformaldehyde for 30 minutes followed by staining with 0.1% crystal violet for 20 minutes. The number of invaded cells was counted under the microscope and photographed (magnification, ×100). The migration assay procedure was similar to that of the invasion assay. However, the Transwell chambers did not require coating with Matrigel, and the number of cells was 5000.
Flow Cytometry Assay
Flow cytometry assay was used for the assessment of apoptosis in AGS and MKN45 cells transfected with siRNA. After being transfected for 24 hours, cells were harvested and cultured in serum-free medium for an additional 24 hours for starvation. After starvation, cells were resuspended with 1× binding buffer, blended with Annexin V/fluoresceine isothiocyanate (FITC) (5 μL), and incubated for 5 minutes in the dark. Subsequently, cells were stained with 10 µL of propidium iodide and measured using a flow cytometer (BD FACSC anto II; BD Biosciences). The rate of apoptosis was analyzed by Flowjo software (version 7.6.5, Tree Star Inc., San Carlos, CA).
Gelatin Zymogram Assay
Cells were seeded in a 6-cm plate at a density of 2.5 × 106 cells per dish for transfection with siRNA. After being transfected for 24 hours, cells were cultured in serum-free medium for another 24 hours and then removed to collect the culture supernatant. Then, the culture supernatant was electrophoresed by 10% SDS-PAGE containing 0.5 mg/mL gelatin and incubated in a buffer at 37°C for 40 hours. The fully incubated gel was rinsed in double distilled water and then stained in 0.25% Coomassie Brilliant Blue R250 for 4 hours at room temperature. Subsequently, the gel was decolorized at room temperature, scanned using the Image Scanner (Amersham Pharmacia GE, Little Chalfont, Buckinghamshire, UK), and analyzed using ImageQuant TL V2003 software.
Statistical Analysis
The data in this study were obtained from triple independent assays and expressed as the mean ± standard deviation. Statistical analysis was conducted using GraphPad Prism 7.0 (GraphPad Soft-ware, Inc, La Jolla, California). Differences between groups were analyzed using the Student t test or 1-way analysis of variance followed by the least-significant difference (LSD) post hoc test. A P value< .05 was considered to be statistically significant.
Results
Silencing of Rab9 Inhibits the Proliferation and Colony Formation Abilities of Gastric Cancer Cells
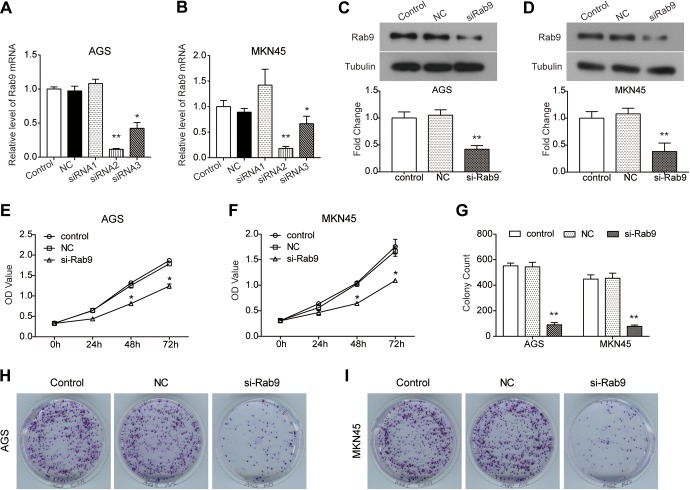
In order to investigate the role of Rab9 in the progression of gastric cancer, loss-of-function assays were performed. As shown in Figure 1A and B, 3 siRNA sequences were transfected into AGS and MKN45 cells, and the expression of Rab9 was significantly blocked by siRNA2# (P < .01) and siRNA3# (P < .05). Since siRNA2# possessed greater knockdown efficiency, it was used in subsequent experiments. The protein expression of Rab9 was also inhibited in AGS and MKN45 cells by transfection with siRNA compared to NC (P < .01; Figure 1C and D). We found that in comparison with NC, the viability of AGS cells was decreased by the depletion of Rab9 (P < .05; Figure 1E). A similar depression in cell viability was also observed in MKN45 cells transfected with si-Rab9 (P < .05; Figure 1F). Moreover, results from the colony formation assay further confirmed the inhibitory effect of silencing Rab9 on cell growth. As shown in Figure 1G to I, silencing Rab9 inhibited the colony-formation ability of AGS and MKN45 cells (P < .01).
Figure 1.
Silencing of Rab9 decreases the viability and proliferation of gastric cancer cells. A and B, After being transfected with small-interfering RNA (siRNA)-Rab9,1-3 the expression level of Rab9 messenger RNA (mRNA) was detected using real-time polymerase chain reaction (RT-PCR) assay in AGS (A) and MKN45 (B) cells. C and D, The expression of Rab9 protein was detected using Western blot assay in AGS (C) and MKN45 (D) cells. E and F, Cell Count Kit-8 (CCK8) assay was conducted to detect cell viability of AGS (E) and MKN45 (F) cells treated with siRNA. G-I, Cell proliferation ability was also examined using colony formation assay. Data are presented as the mean ± standard deviation (SD). Results were obtained in 3 replicates. *P < .05, **P < .01.
Silencing of Rab9 Inhibits the Migration and Invasion Abilities of Gastric Cancer Cells
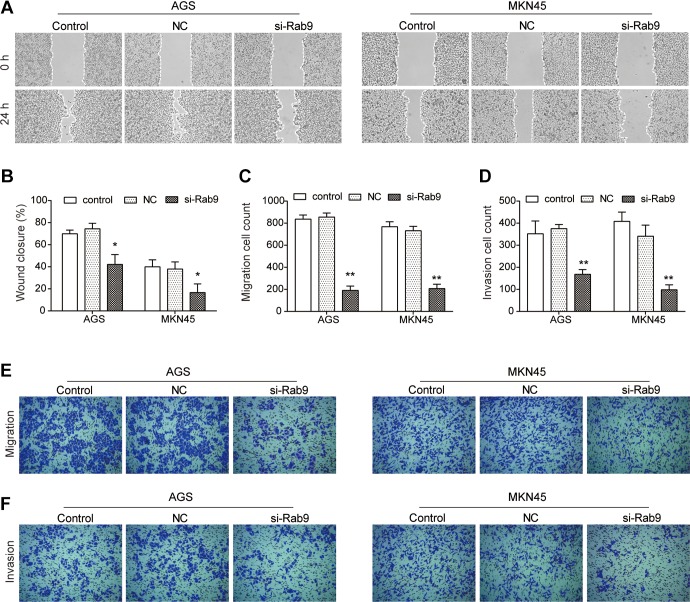
To further assess the effect of si-Rab9 on the metastasis of gastric cancer, wound-healing and Transwell assays were performed. As indicated in Figure 2A and B, the migration ability of both AGS and MKN45 cells was decreased by si-Rab9 in comparison with NC (P < .05). Results from the Transwell migration assay further indicated that AGS cells transfected with si-Rab9 showed more than 70% decreased migration ability, compared to NC cells (P < .01; Figure 2C and E). Similarly, the migration ability of MKN45 cells transfected with si-Rab9 also decreased significantly (P < .01; Figure 2C and E). Additionally, the invasion ability of AGS and MKN45 cells was significantly inhibited by silencing Rab9 (P < .01; Figure 2D and F).
Figure 2.
Knockdown of Rab9 inhibits the migration and invasion abilities of gastric cancer cells. A and B, Wound-healing assay was performed to examine the migration ability of AGS and MKN45 cells after Rab9 was silenced. C and E, Transwell assay also performed to assess the migration ability of AGS and MKN45 cells after Rab9 was silenced. D and F, After Rab9 being silenced, the invasion ability of AGS and MKN45 cells was detected using Transwell invasion assay. Data are presented as the mean ± standard deviation (SD). Results were obtained in 3 replicates. *P < .05, **P < .01.
Silencing of Rab9 Promotes Apoptosis in Gastric Cancer Cells by Regulating the Bcl-2–Bax Axis and Caspase Cascade
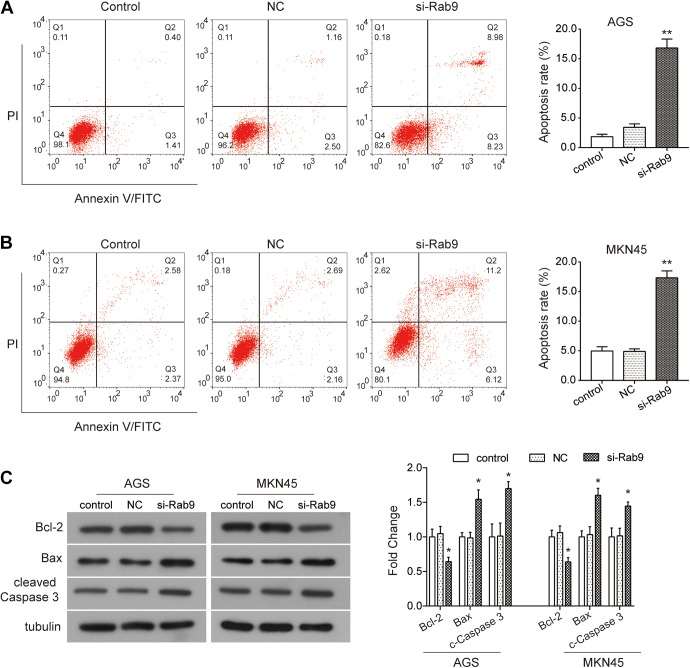
To investigate whether Rab9 affects the survival of gastric cancer cells, flow cytometry was used to detect the apoptotic rate of gastric cancer cells transfected with si-Rab9. As indicated in Figure 3A, silencing Rab9 clearly promoted the rate of apoptosis in AGS cells (P < .01). Similarly, the rate of apoptosis in MKN45 cells was also enhanced by the knockdown of Rab9 as compared to NC (P < .01; Figure 3B). Subsequently, we detected the changes in expression of apoptosis-related proteins by Western blot assay. Our data showed that the expression of antiapoptotic protein Bcl-2 was significantly downregulated by silencing Rab9 in both AGS and MKN45 cells, while the expression of proapoptotic protein Bax and cleaved Caspase 3 was upregulated (P < .05; Figure 3C). Collectively, these results indicate that the depletion in Rab9 promotes apoptosis in gastric cancer cells through regulation of the Bcl-2–Bax axis and Caspase cascade.
Figure 3.
Depletion of Rab9 promotes apoptosis in gastric cancer cells. A and B, After being transfected with siRab9, the rate of apoptosis was examined using flow cytometry assay. C, The expression of apoptosis-related proteins Bcl-2, Bax, and cleaved Caspase3 was examined using Western blot. Data are presented as the mean ± standard deviation (SD). Results were obtained in 3 replicates. *P < .05, **P < .01.
Knockdown of Rab9 Suppresses Matrix Metalloproteinase 2 and the Akt Signaling Pathway
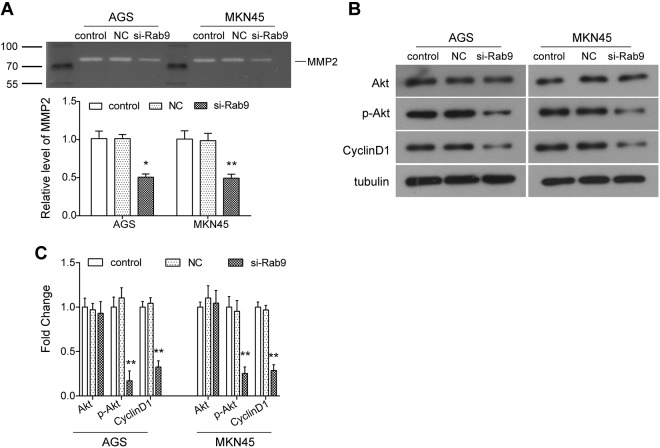
We have previously demonstrated that Rab9 regulates the migration and invasion of gastric cancer cells. Therefore, considering the pivotal roles of matrix metalloproteinases (MMPs) in cell adhesion and tumor metastasis, the expression changes of MMP2 were examined in this study. As indicated from gelatin zymogram assay, we observed that the level of MMP2 was significantly diminished in cells transfected with si-Rab9 as compared to NC (P < .05; Figure 4A). Moreover, the expression of key components in the Akt signaling pathway was also examined using Western blot assay. Our data showed that compared to the NC group, there was no significant change in the expression of total Akt after Rab9 was silenced in AGS and MKN45 cells, while the phosphorylation level of Akt (p-Akt) was significantly decreased (P < .05; Figure 4B and C). Additionally, the expression of its downstream protein, Cyclin D1, was also downregulated accordingly in gastric cancer cells transfected with si-Rab9 (P < .05; Figure 4B and C). These data suggest that the Akt signaling pathway may be involved in the functional mechanism of Rab9 in gastric cancer.
Figure 4.
Silencing of Rab9 inhibits the expression of matrix metalloproteinase 2 (MMP2) and activation of Akt signaling pathway. A, The level of MMP2 was examined using gelatin zymogram assay in gastric cancer cells after Rab9 was silenced. B and C, The expression of key components of Akt signaling pathway was detected using Western blot. Data are presented as the mean ± standard deviation (SD). Results were obtained in 3 replicates. *P < .05, **P < .01.
Discussion
It is well known that the tumorigenesis is caused by the interaction of multiple genes and that tumor metastasis is one of the primary causes of poor prognosis of patients.19 RabGTPases have been demonstrated to be frequently dysregulated in cancers.20,21 This is associated with tumor metastasis and is also the leading cause of cancer-related deaths.11 Jeong et al report that Rab25 could enhance the invasion ability of breast and ovarian cancer cells by regulating the integrin/EGFR/VEGF-A/Snail axis.22 Overexpression of Rab27A is revealed to inhibit proliferation, migration, and invasion of gastric cancer HGC-27 cells.3 In the present study, we investigated the effects of Rab9 on the biological characteristics of gastric cancer AGS and MKN45 cells.
Rab9 has been revealed to be upregulated in breast cancer tissues and plays an oncogenic role in breast cancer cells.18 In the current study, after silencing Rab9 by siRNA interference, CCK8 and colony formation assays showed a significant decrease in the viability and proliferation of gastric cancer cells, highlighting a potential progrowth role of Rab9 in gastric cancer in vitro. Moreover, our data showed that the migration and invasion abilities of AGS and MKN45 cells were also inhibited by the depletion of Rab9 in vitro. Consistent with previous results,18 Rab9 may serve as an oncogene in gastric cancer. However, further study should be performed to verify the oncogenic role of Rab9 in the progression of gastric cancer in vivo and investigate the significance of Rab9 in the prognosis of gastric cancer.
Apoptosis is a key regulatory method to control cell growth. It is well known that evading apoptosis is a hallmark of tumor cells, which is also the key to malignant transformation of cells and the carcinogenesis process. Therefore, in order to further study the regulation of Rab9 in the growth of gastric cancer, apoptosis in AGS and MKN45 cells was examined after Rab9 was knocked down. We observed that the rate of apoptosis, confirmed through flow cytometry, was significantly promoted by si-Rab9 in both AGS and MKN45 cells, which was further supported by the results from Western blot. Gastric cancer cells transfected with si-Rab9 displayed a significant depression in the expression of Bcl-2 as well as an increase in the expression of Bax and cleaved Caspase3. As a pivotal apoptosis-related protein, Bcl-2-Bax axis is a regulator that controls cell fate. An increase in the level of Bax and a decrease in Bcl-2 can trigger cell death.23 Caspase 3 is the most critical apoptosis-executing protease.24 As a result of these studies, we concluded that the depletion of Rab9 promoted apoptosis of gastric cancer through regulation of the Bcl-2/Bax axis and Caspase cascade, resulting in suppression of tumor growth.
It is well known that activated MMP2, a key member of the MMP family, is revealed to degrade the basement membrane, which is an essential step for the tumor metastasis process.25,26 Considering the pivotal role of MMP2 in tumor metastasis, we examined the level of MMP2 using gelatin zymogram assay. We found that the level of MMP2 was significantly decreased in Rab9 silenced cells compared to the NC group, indicating that Rab9 may modulate tumor invasion by regulating the level of MMP2. Additionally, the Akt signaling pathway plays a central role in cancer growth, metastasis, and survival. As a result, Akt has been confirmed as an effective therapeutic target for cancer treatment.27-29 Previous studies reveal that the Akt signaling pathway is involved in the function of Rabs in tumor progression.30,31 For instance, Zhang et al report that upregulation of Rab25 promotes the metastasis of bladder cancer by activating the Akt pathway.30 In this study, our data showed that silencing Rab9 suppressed the activation of the Akt signaling pathway by decreasing the phosphorylation of Akt. Collectively, the Akt signaling pathway is also involved in the oncogenic role of Rab9 in the progression of gastric cancer.
In summary, the present study highlights a potential oncogenic role of Rab9 in the progression of gastric cancer in vitro, which the regulation of the Akt signaling pathway possibly contributes to. Targeting Rab9 may be a potential method for the therapy of gastric cancer. We will further investigate the functional role of Rab9 in gastric cancer in vivo in our following study.
Abbreviations
- CCK8
Cell Count Kit-8
- FBS
fetal bovine serum
- NC
negative control
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- siRNA
small-interfering RNA
- MMP
matrix metalloproteinase
- PBS
phosphate-buffered saline.
Footnotes
Authors’ Note: Y.Z., F.S., and J.D. contributed to conception and design. All authors contributed to administrative support; provision of study materials or patients; data analysis and interpretation; and final approval of manuscript. F.S. and M.W. contributed to collection and assembly of data. Y.Z. and J.D. contributed to manuscript writing. No patient consent is needed for this study. Our study did not require an ethical board approval because it did not contain human or animal trials. All data in this study are available upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Key Research and Development Plan of Shandong Province (No. 2017GSF19104) and Shandong Traditional Chinese Medicine Science and Technology Development Plan (No. 2017-061).
ORCID iD: Jian Ding  https://orcid.org/0000-0002-5530-8075
https://orcid.org/0000-0002-5530-8075
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Smith JP, Nadella S, Osborne N. Gastrin and gastric cancer. Front Endocrinol. 2017;8(2):801–811. [Google Scholar]
- 3. Li Y, Chen S, Shan Z, et al. MiR-182-5p improves the viability, mitosis, migration and invasion ability of human gastric cancer cells by down-regulating RAB27A. Biosci Rep. 2017;37(3): BSR20170136. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Röcken C, Warneke V. Molecular pathology of gastric cancer. Der Pathologe. 2012;207(1):608–612. [DOI] [PubMed] [Google Scholar]
- 5. Rebecca S, Jiemin M, Zhaohui Z, Ahmedin J. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 6. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roviello F, Caruso S, Neri A, Marrelli D. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol. 2013;39(12):1309–1316. [DOI] [PubMed] [Google Scholar]
- 8. Yan Z, Harald S. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128(17):3171–3176. [DOI] [PubMed] [Google Scholar]
- 9. Kucera A, Bakke O, Progida C: The multiple roles of Rab9 in the endolysosomal system. Commun Integr Biol. 2016;9(4):e1204498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chano T, Avnet S. RAB39A: a Rab small GTPase with a prominent role in cancer stemness. J Biochem. 2018;164(1):9–14. [DOI] [PubMed] [Google Scholar]
- 11. Villagomez FR, Medina-Contreras O, Cerna-Cortes JF, Patino-Lopez G. The role of the oncogenic Rab35 in cancer invasion, metastasis, and immune evasion, especially in leukemia. Small GTPases. 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Rui F, Jia F, He S, Tang L. Functional implications of Rab27 GTPases in Cancer. Cell Commun Signal. 2018;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang XZ, Cui SZ, Zeng LS, et al. Overexpression of Rab1B and MMP9 predicts poor survival and good response to chemotherapy in patients with colorectal cancer. Aging (Albany NY). 2017;9(3):914–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang JS, Wang FB, Zhang QG, Shen ZZ, Shao ZM. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6(3):372–382. [DOI] [PubMed] [Google Scholar]
- 15. Angélique B, Sophie K, Fabien R, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(1):4920–4930. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Jia Q, Wang Y, Li F, Jia Z, Wan Y. Rab40b upregulation correlates with the prognosis of gastric cancer by promoting migration, invasion, and metastasis. Med Oncol. 2015;32(4):126. [DOI] [PubMed] [Google Scholar]
- 17. Zhang B, Yin Y, Hu Y, et al. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med Oncol. 2015;32(1):331. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Wang X, Zhang Z, Xiao B, An B, Zhang J. The overexpression of Rab9 promotes tumor progression regulated by XBP1 in breast cancer. Onco Targets Ther. 2019;12:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Wang H, Ni Q, et al. Effects of silencing Rab27a gene on biological characteristics and chemosensitivity of non-small cell lung cancer. Oncotarget. 2017;8(55):94481–94492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65(7):2516–2519. [DOI] [PubMed] [Google Scholar]
- 21. Wan JC, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795(2):110–116. [DOI] [PubMed] [Google Scholar]
- 22. Jeong BY, Cho KH, Jeong KJ, et al. Rab25 augments cancer cell invasiveness through a β1 integrin/EGFR/VEGF-A/Snail signaling axis and expression of fascin. Exp Mol Med. 2018;50(1):e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown R. The bcl-2 family of proteins. Brit Med Bullet. 1997;53(1):466–477. [DOI] [PubMed] [Google Scholar]
- 24. Heng Z, Yenari MA, Danye C, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2010;85(4):1026–1036. [DOI] [PubMed] [Google Scholar]
- 25. Mook ORF, Frederiks WM, Noorden CJFV. The role of gelatinases in colorectal cancer progression and metastasis. Biochimica Et Biophysica Acta. 2009;1705(2):69–89. [DOI] [PubMed] [Google Scholar]
- 26. Safranek J, Pesta M, Holubec L, et al. Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung tissue of patients with non-small cell lung cancer (NSCLC) and benign pulmonary disease. Antican Res. 2009;29(2):2513–2517. [PubMed] [Google Scholar]
- 27. Vara JÁF, Casado E, Castro JD, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer ☆. Can Treat Rev. 2004;30:193–204. [DOI] [PubMed] [Google Scholar]
- 28. Mundi PS, Sachdev J, Mccourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. British J Clin Pharm. 2016;82(2):943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nitulescu GM, Margina D, Juzenas P, et al. Akt inhibitors in cancer treatment: the long journey from drug discovery to clinical use (Review). Int J Oncol. 2016;48(1):869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiaxing Z, Jinhuan W, Jian L, et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3β/Snail signaling. Carcinogenesis. 2013;34(10):2401–2408. [DOI] [PubMed] [Google Scholar]
- 31. Guo B, Wang W, Zhao Z, et al. Rab14 Act as oncogene and induce proliferation of gastric cancer cells via AKT signaling pathway. Plos One. 2017;12(1):e0170620. [DOI] [PMC free article] [PubMed] [Google Scholar]