Abstract
The cochlear implant outcome is possibly improved by brain-derived neurotrophic factor treatment protecting spiral ganglion neurons. Implantation of genetically modified mesenchymal stem cells may enable the required long-term brain-derived neurotrophic factor administration. Encapsulation of mesenchymal stem cells in ultra-high viscous alginate may protect the mesenchymal stem cells from the recipient’s immune system and prevent their uncontrolled migration. Alginate stability and survival of mesenchymal stem cells in alginate were evaluated. Brain-derived neurotrophic factor production was measured and its protective effect was analyzed in dissociated rat spiral ganglion neuron co-culture. Since the cochlear implant is an active electrode, alginate–mesenchymal stem cell samples were electrically stimulated and alginate stability and mesenchymal stem cell survival were investigated. Stability of ultra-high viscous-alginate and alginate–mesenchymal stem cells was proven. Brain-derived neurotrophic factor production was detectable and spiral ganglion neuron survival, bipolar morphology, and neurite outgrowth were increased. Moderate electrical stimulation did not affect the mesenchymal stem cell survival and their viability was good within the investigated time frame. Local drug delivery by ultra-high viscous-alginate-encapsulated brain-derived neurotrophic factor–overexpressing mesenchymal stem cells is a promising strategy to improve the cochlear implant outcome.
Keywords: Hydrogel, cell-based drug delivery, neuroprotection, neurite outgrowth, spiral ganglion neurons
Introduction
Hearing impairment caused by sensorineural hearing loss affects millions of people worldwide and is expected to further increase in an aging society. Particularly, a limited ability of communication negatively influences the social, emotional, and also economic field of hearing impaired people.1,2 The implantation of a cochlear implant (CI) is the state-of-the-art therapy for patients suffering from severe to profound sensorineural hearing loss. The electrode array of the CI is inserted into the scala tympani of the inner ear to replace the functionless sensory hair cells and stimulates the peripheral part of the auditory nerve built by the spiral ganglion neurons (SGN). Due to hearing loss, a serious degeneration of the SGN has been observed in several animal studies3–5 as well as in humans.6–8 Since it is considered that one important factor for the efficiency of CI treatment is a high number of stimulated SGN,6 neuron degeneration should be prevented or at least reduced.
Another objective of current CI research for stimulation improvement is bridging the anatomical gap between electrode array and neurons by regeneration and guidance of the SGN peripheral processes.9–14
For both goals, protection of SGN from degeneration and regeneration of their dendrites, local neurotrophic factor (NTF) therapy is a promising strategy. For example, for brain-derived neurotrophic factor (BDNF), several studies detected a potential to protect SGN from degeneration and also a neurite regeneration in vitro and in vivo.12,13,15–19 Thereby a chronical delivery of the NTF is assumed to be crucial for the maintenance of the clinical benefits.20,21 Drug delivery systems tested in animal studies, such as intratympanic injection, round window application, (mini-) osmotic pump systems, or degradable NTF-eluting coatings, have disadvantages like need for repeated treatments due to short-term availability of the NTF or an increased risk for infections. A (stem) cell or gene therapy of the inner ear allows for chronical treatment. Yet, this strategy bears the risk for uncontrolled migration of cells or spread of genes (reviewed in literature2,22–25). Or extremely high levels of transgene protein expression can cause severe neurological symptoms and hearing loss.26 A continuous and controlled production of the NTF close to the target neurons combined with a reduced risk for cell or gene migration rendering repeated NTF applications or surgical interventions unnecessary would present potential benefits of an encapsulated cell-based drug delivery system. Cells can be genetically modified to overexpress a NTF (like BDNF) followed by an encapsulation in appropriate materials to avoid migration and shield the cells against the host immune system.
Since many years, alginate hydrogels are known as a promising biomaterial in the field of tissue engineering as either immobilization matrix for living cells,27 bulk material for complex three-dimensional scaffolds,28 and microcarriers.29 Isolated from bacteria or the cell wall of brown algae, the polysaccharide comprises different heterogeneous and homogeneous monomer blocks of α-l-guluronic acid and β-d-mannuronic acid. It is able to form a stable hydrogel under very mild conditions (room temperature, physiological pH, and no toxic compounds) after contact with polycations, such as calcium or barium. There is no alginate-degrading enzyme in humans, making alginate one of the most used materials for small cell-based implants in the scientific literature.30,31 Furthermore, a bidirectional transfer of small molecules is possible, leading to a supply with nutrients, growth factors, and oxygen. At the same time, waste products, CO2, and produced active molecules, such as insulin32 or BDNF,33 can be released. For biotechnological processes, alginate lyases allow the defined degradation of the liquid and gelled alginates.34,35 However, to fulfill the needs for medical applications, alginates with high molecular weight, low endotoxin levels, and sterility are mandatory. Several protocols are available and applied for raw material treatment and alginate purification to produce ultra-high viscous (UHV) alginate which fulfills above-mentioned requirements.36–38 UHV-alginates and the related high molecular weight of the polymer chains are preferable for in vivo applications, especially due to the reported decreased immunogenic potential and increased mechanical stability.39–41
For the treatment of hearing loss, alginate-encapsulated NTF-producing cells could be applied to the cochlea for example as solution before CI implantation, as polymerized beads beside, or as coating on the inserted electrode.42–44 Stem cells have advantageous characteristics for the application of drug delivering cells in the inner ear. Human bone marrow–derived mesenchymal stem cells (MSCs) have a neuroprotective effect on SGN per se,45,46 which is further increased by genetic modification for BDNF overexpression.47 MSCs also offer the opportunity for an individualized therapy with autologous cells. An implantation of allogenic or xenogeneic cells has a higher risk for rejection reactions and a possibly lower compliance by patients. MSCs are also a promising candidate for a successful encapsulation in alginate for drug delivery since their survival in this hydrogel has already been proven.30,48,49 A feasible inner ear drug delivery system additionally has to be resistant against electrical current since the CI therapy is based on an electrical stimulation (ES) of the neurons.
This study first investigated the stability of alginate for 4 weeks in inner ear relevant media. In addition, the neuroprotective and neurite regenerating effect of UHV-alginate-embedded BDNF-overexpressing MSCs (alginate–MSC) on SGN was tested in vitro using two drug delivery strategies: culture of SGN with adjacent adherent alginate layers as well as co-culture with floating alginate beads. Also the impact of ES on alginate stability and MSC survival was examined. Finally the survival of the alginate-encapsulated MSCs was observed over a period of 3 weeks.
Material and methods
BDNF-overexpressing human bone marrow-derived MSC
Ethical approval from the ethical committee of Hannover Medical School, Hannover, Germany, was obtained for the studies involving human bone marrow (565-2009, 565-2016). Collection of samples was performed in accordance with the Declaration of Helsinki after written informed consent of all donors. Personal information of donors was deleted.
MSC isolation and proliferation was performed as previously described.47 When a cell confluency of about 80% was achieved, they were passaged or prepared for encapsulation.
The MSCs were genetically modified using two lentiviral constructs based on the plasmid pRRL.PPT.SFFV which contains a red fluorescent marker gene (tdTomato red, kindly provided by Prof. Axel Schambach). Either the human BDNF gene was overexpressed (BDNF/pRRL) or, as a negative control (NC), a luciferase gene (K/pRRL) was used. Due to the required large amount of MSCs for the different experiments, two MSCs populations of one donor independently underwent the same protocol for genetic modification (1 and 2 infection). Subsequently, these MSCs were used for the performed experiments from passage 6 to 9.
MSC encapsulation in alginate
For long-term viability tests: MSCs without (K/pRRL) and with (BDNF/pRRL) the ability for BDNF production were harvested and gently mixed with UHV-alginate (final concentration 0.65% w/v concentration in saline, 1:1 mixture of alginate from Lessonia nigrescens and Lessonia trabeculata (now commercially available from Alginatec GmbH, Riedenheim, Germany) to a final concentration of 5 × 105 cells/mL alginate.
Alginate microcapsules were produced using the method described previously.50 In brief, the alginate–MSC suspension was transported under sterile conditions through a coaxial air flow nozzle (500 µm in diameter) and dropped into a crosslinking solution 20 mM BaCl2 (Sigma-Aldrich GmbH, Taufkirchen, Germany), 115 mM NaCl (Carl Roth GmbH & Co. KG, Karlsruhe, Germany), and 5 mM l-histidine (Genaxxon bioscience GmbH, Ulm, Germany) adjusted to a pH of 7 and filtered for sterility.33,51 Alginate–MSC beads were incubated for 15 min in the solution. Subsequently, beads were washed thoroughly with 0.9% saline (B.Braun Melsungen AG, Germany) to remove unbound barium ions.
For SGN co-culture: 10 µL of alginate–MSC solution (2500 MSCs) were dropped into crosslinking solution. Polymerization was performed for 20 min in the incubator at 37°C. Residues of crosslinking solution were removed by washing in saline.
Long-term stability of alginate
The stability of alginate gels in different media was examined in terms of swelling using alginate beads and mechanical stability using alginate layers. To analyze the gel stability under in vitro culture conditions, spiral ganglion cell (SGC)-medium (see below) was tested. Artificial perilymph (145 mM NaCl, 2.7 mM KCl, 2 mM MgSO4, 1.2 mM CaCl2, 5 mM HEPES, and 1 mg/mL serum albumin) was used to mimic the conditions in the inner ear. And 0.9% saline (B. Braun, Melsungen AG, Germany) was chosen as NC. Double distilled water was tested as positive control (PC) (D2H2O). Alginate beads were produced as described previously and placed in 1 mL of the named test solutions. The diameters of the alginate beads were measured as a parameter for bead stability/swelling using a fluorescence microscope (Nikon Eclipse TS300, Nikon Instruments, Amsterdam, Netherlands) and compared with the diameters after 1, 7, 14, 21, and 28 days of incubation (N = 20 for each time point). The fabrication of alginate gel layers, adapted from Gepp et al.,29 was carried out in a 24-well plate (Greiner Bio-One GmbH, Frickenhausen, Germany) treated with 200 µL of poly-l-lysine (pLL; Sigma-Aldrich) as a 1:5 dilution (v/v%) in phosphate buffered saline (PBS; Gibco, Karlsruhe, Germany) for 30 min at 37°C. And 300 µL of the alginate solution was added to the air-dried, pLL treated wells and gelled with 500 µL of crosslinking solution for 20 min at room temperature. Gelled alginate layers were washed three times with saline and subsequently compressed until 35% of strain with a deformation rate of 0.5 mm/s using TA.XTplus (Stable Micro Systems, Godalming, United Kingdom) to calculate the respective elastic modulus as the slope of the stress–strain curve (10%–30% of strain). Afterward, the alginate gel layers were incubated in 1 mL of test solutions for up to 28 days. Their mechanical stabilities were analyzed after 1, 7, 14, 21, and 28 days of incubation and compared to the initial stabilities at Day 0 (N = 8).
Spiral ganglion cell preparation
SGCs, including the SGN, were harvested from early postnatal (2–5 days) Sprague Dawley rats. The tissue preparation was performed in accordance with the German “Law on protecting animals” and with the Directive 86/609/EEC of the European Communities Council for protection of animals used for experimental purposes. Preparation and dissection of the spiral ganglia were previously described in detail.17 In brief, animals were decapitated, spiral ganglia prepared, collected, and the cells subsequently dissociated. Cell number was determined by Trypan blue exclusion assay in a Neubauer chamber. A cell number of 20 × 104 SGC/mL was adjusted in SGC-medium. This SGC-medium based on Panserin 401 (Pan-Biotech) supplemented with insulin (8.7 µg/mL; Biochrom Ltd), penicillin (30 U/mL; Biochrom Ltd), glucose (0.15%; B. Braun Melsungen AG), PBS (Ca2+/Mg2+-free PBS; 0.172 mg/mL; Gibco by Life Technologies), HEPES-buffer solution (23.43 µM; Invit-rogen), and N2-supplement (0.1 µL/mL; Invitrogen).
MSC encapsulation in alginate for SGC experiments
For encapsulation into alginate (see above), the MSCs were detached with trypsin and resuspended in MSC-medium for cell counting with a Fuchs-Rosenthal counting chamber. To concentrate the detached cells, the cell suspension was centrifuged, the supernatant was discarded, and a cell number of 1.5 × 106 MSCs/mL was adjusted and mixed in a ratio of 1:6 with the UHV-alginate. Thereby, the cell suspension was carefully dispersed in alginate to avoid air bubbles in the mixture and to ensure a homogeneous distribution of MSCs in the alginate. Occasional bubbles were removed by a short centrifugation. Finally, 250,000 MSCs/mL were encapsulated in the UHV-alginate.
Adjacent culture of SGN and alginate-encapsulated MSC: SGN morphology and neurite guidance
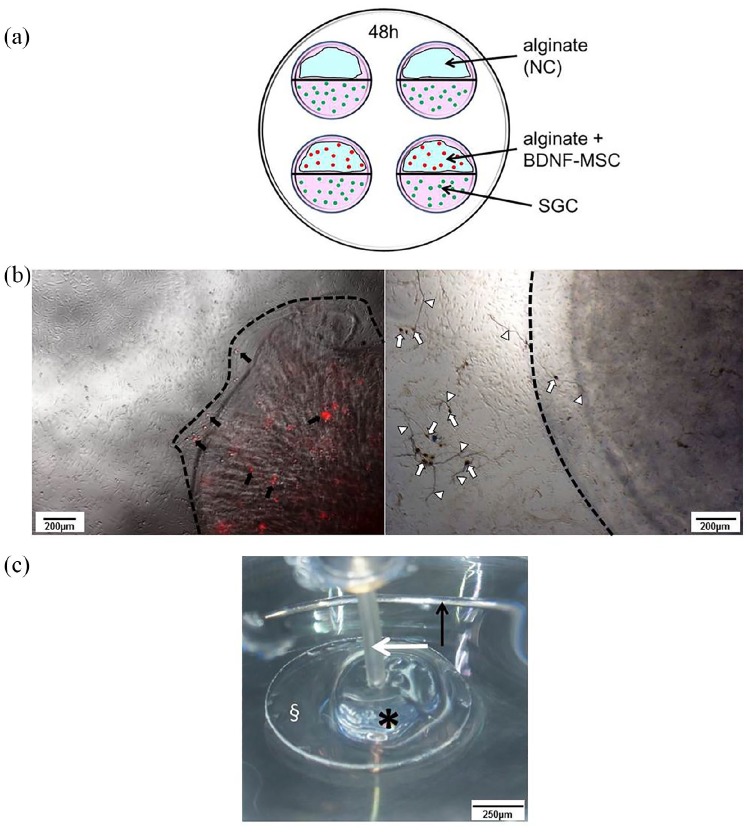
For detection of neuroprotection and possible neurite attraction by NTF-producing MSCs, a cell culture model allowing a cultivation of SGC beside UHV-alginate-encapsulated BDNF-producing MSCs was designed. A localized NTF-releasing source allows the analysis of the orientation of regenerated neurites to this source (Figure 1(a)). Petri dishes with four internal rings (10 Cellstar, Greiner Bio-One, Kremsmünster, Austria) were used. Internal wells were divided into two halves by a liquid blocker (ImmEdge Hydrophobic Barrier PAP Pen, Vector Laboratories, Burlingame, USA). One half was coated with pLL (1:10 in PBS) for 30 min at 37°C, with a subsequent drying step at room temperature, to provide a good adhesion of alginate. The other half was coated with poly-d/l-ornithine (0.1 mg/mL, Sigma-Aldrich) and laminin (0.01 mg/mL Natural Mouse Laminin; Invitrogen) to support attachment and growth of SGC. Two of the four internal wells per Petri dish were coated with a layer formed of 20 µL alginate, including 5000 MSCs on the pLL-side, while the other two were covered with pure alginate serving as NC. Alginate was crosslinked with BaCl2 for 20 min at 37°C in the incubator followed by washing with saline to remove remaining BaCl2. Subsequently, the half with polymerized alginate with and without MSCs was covered with MSC-medium and 50 µL SGC-suspension (1 × 104 SGC) was added to the other half. The Petri dish was transferred to the incubator to allow adhesion of the SGC. After 4 h, SGC adhered to the ornithine–laminin coating and medium was added to connect both compartments via a contact above the liquid blocker in the middle of the internal wells. Through this connection, molecules like the MSC-produced BDNF may diffuse over the liquid blocker to the SGN to potentially attract and guide regenerated neurites. Medium consisted of serum containing (10%) MSC-medium and serum deprived SGC-medium in equal parts.
Figure 1.
Performed experimental setups. (a) The setup for the adjacent culture of SGC (green dots) and encapsulated BDNF-producing MSCs (red dots) or pure alginate (negative control, NC) on Petri dishes with four internal rings. Internal wells were divided into two halves containing either alginate (blue) or SGC by a liquid blocker (black line). Medium (pink) connects both cell compartments at the central border. (b) Alginate–MSC beads. The left picture shows tdTomato-red fluorescent MSCs (some selected MSCs are indicated by black arrows) after encapsulation and bead (edge indicated by dashed line) formation. In co-culture, the SGCs are adherent to the well bottom while the MSC-bead floats. The right picture displays an example of a phase contrast image of the co-cultivation with fixed and neurofilament-stained SGN (dark brown, some somata and neurites are exemplarily marked with white arrows and arrowheads) and a floating bead (edge indicated by dashed line, added for illustration purpose). Scale bar: 200 µm. (c) The setup for electrical stimulation. Cover glass (§) with polymerized alginate–MSC mixture (asterisk) in a Petri dish. The active electrode (white arrow) was placed in the alginate–MSC mix and an annular electrode served as ground (black arrow). The Petri dish was filled up with MSC culture medium (not shown in the figure). Scale bar: 250 µm.
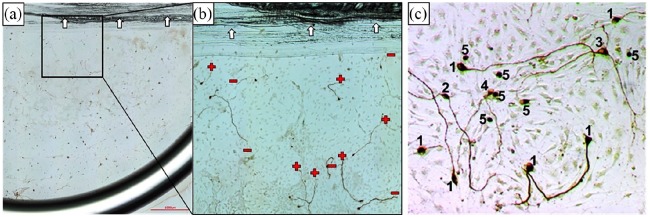
Side-by-side cultivation was performed for 48 h with subsequent fixation (1:1 methanol–acetone) followed by neuron-specific staining against neurofilament in accordance with the previously described protocol.17,18 Two repetitions were performed, each including two Petri dishes with tested conditions in double (N = 2, n = 4). Neuronal survival and morphology as well as number and length of neurites and their orientation were analyzed. For neuronal survival, each neuron regenerating a neurite of a minimum length of thrice of the soma diameter was counted as surviving. The different morphologies were classified as described before17,52 (Figure 2(c)). In short, neurons regenerating one neurite were classified as monopolar, while bipolar neurons have two neurites and multipolar neurons showed more than two regenerated neurites. Pseudomonopolar neurons have one neurite branching at a distance of one soma diameter from the cell body. Last distinguished category was neurons without neurite regeneration. For neurite analysis, all regenerated neurites per well were counted and the length of the longest neurite per neuron was measured. For neurite guidance, the orientation of each measured neurite at its endpoint was judged as oriented toward the alginate or alginate-embedded MSC side (+) of the well or directed to the opposite site (−) (Figure 2(a) and (b)). Neurite direction was then given as percentage of all analyzed neurites.
Figure 2.
(a) An image of the SGC side of the adjacent culture with alginate-encapsulated MSCs. Scale bar: 1000 µm. (b) A close-up for analysis with exemplarily marked orientation of the endpoint of the regenerated neurites (+: alginate-embedded MSC-oriented; −: away from alginate-embedded MSCs). Arrows indicate the liquid marker as border in the middle of the internal wells. (c) Examples of analyzed neuronal morphologies. Numbers indicate the distinguished morphologies: 1—monopolar, 2—bipolar, 3—multipolar, 4—pseudomonopolar, and 5—no neurites. Sharpness, brightness, and contrast were digitally improved in the sample image for visualization purposes.
Co-culture of SGN with MSC beads: neuronal survival
Besides the adjacent culture for the analysis of neuroprotection and neurite regeneration, a direct co-culture of UHV-alginate-encapsulated BDNF-overexpressing MSCs formed in beads was tested for neuroprotection of SGN in culture (Figure 1(b)).
A 96-well plate (TPP Techno Plastic Products AG, Trasadingen, Switzerland) was pre-coated with ornithine–laminin (see above), and 50 µL suspension of dissociated SGC were pipetted to the wells (1 × 104 SGC/well). One bead was applied per well for co-cultivation after SGC-solution and medium were added. To support the survival of both, SGC and MSCs, serum containing MSC-medium and serum-free SGC-medium were mixed 1:1. Controls were likewise performed in mixed media, thus all wells contained 5% serum. PC was treated with 50 ng/mL BDNF (human recombinant BDNF, from E. coli; Invitrogen), while NC received no additional substances. A seeding control for the detection of initial number of SGN was fixed 4 h after SGC seeding. After 48 h of cultivation, bead stability was controlled microscopically and SGC were fixed with 1:1 methanol–acetone followed by neuron-specific staining against neurofilament as described above. All conditions were performed in triplicate in each of the eight experiments (N = 8, n = 3). For quantification, if BDNF diffused out of the alginate into the medium, exemplarily six randomly chosen experiments were analyzed. The supernatants of the three MSC-bead wells of the chosen experiments were collected and pooled before fixation of the SGC. BDNF detection was performed by the enzyme-linked immunosorbent assay (ELISA) (human BDNF PicoKine ELISA Kit; Boster Biological Technology) as previously described in detail.45 For analysis of neuroprotection, the survival rate was calculated for the tested conditions. Surviving neurons (see adjacent culture) were counted per well and given as percentage of the average number of neurons in the seeding control.
Electrical stimulation of encapsulated MSC
Electrical stimulation was performed in a Petri dish with a central stimulating electrode, a peripheral ground electrode, and a custom made setup as previously described.53
The crosslinking of the alginate–MSC mixture was performed on a round glass slide (Ø 10 mm, Assistant®, Karl Hecht, Germany) placed in a 48-well plate. The glass slide was coated with pLL as described above and 100 µL of the alginate–MSC solution (250,000 MSCs/mL) were carefully dropped on the glass slide, covering the whole surface of the slide. Alginate was crosslinked with BaCl2 for 20 min at 37°C with a subsequent saline washing step, before the glass slide with the crosslinked alginate–MSCs was placed in the stimulation Petri dish and covered with MSC medium. The stimulation electrode was inserted centrally in the polymerized alginate, while the peripheral ground electrode surrounded the glass slide (Figure 1(c)). Embedded MSCs were stimulated at 1 kHz with 2000 (N = 14), 1000 (N = 2), 880 (N = 2), 660 (N = 5), or 330 (N = 10) µA amplitude using biphasic 800 µs pulses with 400 µs per phase and 120 µs interpulse gap for 24 h in an incubator. In each experiment, two cell chambers were stimulated simultaneously and two remained unstimulated, serving as NC. Before and after electrical stimulation, MSCs and alginate were microscopically (Olympus CKX53 Fluorescence with Olympus CellSens standard software) documented and controlled for alginate stability and for a potential reduction of red fluorescence from the tdTomato marker protein, indicative of damage of the stimulated alginate-embedded cells. Finally, alginate was lysed (Alginate Lyase, Sigma-Aldrich), and the number of viable MSCs was determined by Trypan blue exclusion assay in a Fuchs-Rosenthal counting chamber.
Viability testing of alginate-encapsulated MSC
Since the aim of the study is a chronic application of drug delivering cells, survival of the alginate-encapsulated MSCs was observed for 3 weeks in culture. Alginate beads with MSCs were cultivated in a culture medium composed of DMEM-HG basal medium (Biochrom Ltd), 20 mM HEPES (Biochrom Ltd), penicillin/streptomycin (1%, Biochrom Ltd), dexamethasone (0.1 µM; Sigma-Aldrich), ITS + supplement (Corning Inc.), ascorbate-2-phosphate (170 µM; Sigma-Aldrich), sodium pyruvate (1 mM, Biochrom Ltd), and proline (0.35 mM; Carl Roth Gmbh & Co. Kg). Viability of MSCs was measured before encapsulation, after Day 10 and Day 21 using the Trypan blue (Sigma-Aldrich) exclusion method. Before counting, beads were incubated at 37°C for 30 min with alginate lyase (1 mg/mL in PBS with Ca2+/Mg2+; Sigma-Aldrich) to release the MSCs (two independent experiments with two replicates; N = 2, n = 2).
Statistics
Data are given as mean ± standard error of mean (SEM). Due to small group size, unpaired t-test was performed for SGN data in the adjacent culture, except for multipolar neurons (no neuron counted in the pure alginate group) and the measured neurite length not following the normal distribution (D’Agostino & Pearson omnibus normality test), which were therefore analyzed with the Wilcoxon signed-rank test. The neuronal survival rate of MSC bead co-culture was analyzed with repeated-measures one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. To analyze a possible dependency between detected BDNF amount and neuronal survival rate, Pearson correlation was performed. Results for the MSC survival under electrical stimulation were first evaluated for normal distribution using the D’Agostino & Pearson test. Subsequently paired t-tests were performed for each condition and its relevant control.
All analyses were performed with GraphPad Prism® 5 software and a p-value < 0.05 was considered as significant difference between tested conditions.
Results
Alginate long-term stability
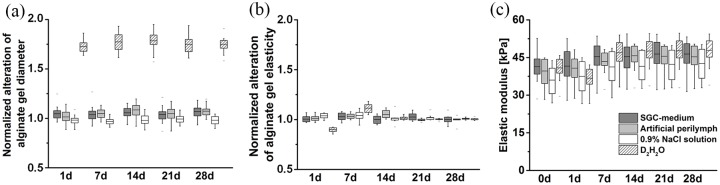
The bead diameter as parameter for bead stability indicated no signs of fragmentation or swelling in relevant media (SGC-medium and artificial perilymph). Only alginate beads incubated in D2H2O (PC) showed extreme swelling behavior at the beginning of the experiment (Day 1), proving the osmotic functionality of the produced alginate (Figure 3(a)). The elasticities of the alginate gel layers remained constant in SGC-medium and artificial perilymph. Small changes only occurred in hypoosmotic D2H2O within 7 days, which stabilized after 14 days (Figure 3(b) and (c)). Overall, there were no observations indicating bursting, fragmentation, extreme swelling, or shrinking of the alginate gels.
Figure 3.
Stability of alginate gels in different media. Analysis of alginate bead diameter over time revealed constant beads diameters especially in the relevant fluids SGC-medium and artificial perilymph, which is presented in (a). In double distilled water (D2H2O), the diameter increased, indicating the swelling of the alginate beads as PC. The alteration of elasticity of alginate layers is given as relative values in (b) and absolute values in (c). Elasticity of alginate layers remained constant in media relevant for cochlea applications (SGC-medium and artificial perilymph). (a) N = 20, (b) and (c) N = 8 for each experimental condition. Measurements were normalized in (a) and (b) to the initial (before contact with the studied media) bead diameter and layer elasticity, respectively.
All alginate–MSC beads co-cultured with SGC were investigated for stability by microscopic inspection at the end of the experiment. None of them showed signs of dissolution. In addition, some MSC beads were controlled for fluorescence marker production (Figure 1(b)), which was still visible after the co-cultivation in all examined beads.
Neuronal survival and morphology in adjacent culture
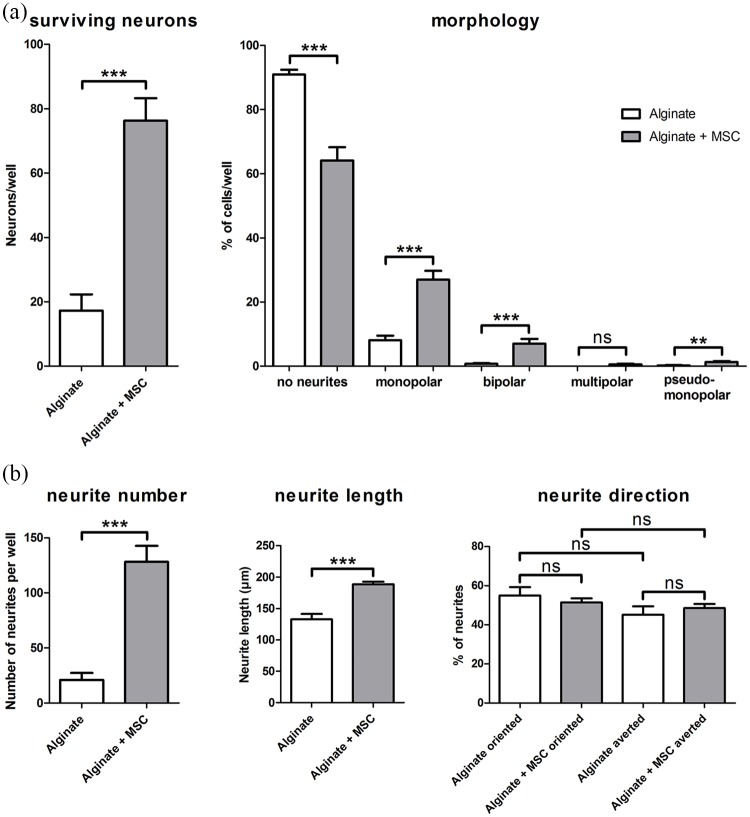
The number of surviving neurons was counted to analyze the neuroprotection of closely located, encapsulated, and BDNF-producing MSCs compared to pure alginate. In SGN-cultures of cell-free alginate, 17.25 ± 5.10 neurons per well were counted. In contrast, an inclusion of BDNF-producing MSCs into the alginate resulted in an increased number of 76.25 ± 7.01 neurons per well. This increase was significant when compared to the pure alginate (Figure 4(a)).
Figure 4.
The results for analysis of neuronal survival and morphologies are summarized in (a). Left graph: In comparison to cell-free alginate, survival of SGN was significantly increased by adjacent cultivation with alginate embedded, BDNF-producing MSCs (alginate + MSC). Right graph: the number of neurons without a regeneration of neurites (no neurites) was significantly reduced by the encapsulated MSCs, while the number of monopolar and bipolar neurons significantly increased. Neurons of multipolar or pseudomonopolar morphology were rarely detected. (b) The results for the analyzed neurite regeneration. An adjacent cultivation of SGN with alginate-encapsulated, BDNF-producing MSCs had a significant positive effect on the number of regenerated neurites and the measured length. There was no clear orientation of the neurite endings toward or away from the embedded MSCs. Data are presented as mean and standard error of mean (SEM). Asterisks indicate detected significances for the compared groups (mean ± SEM, **p < 0.01, ***p < 0.001; ns = not significant); N = 2, n = 4.
In addition, the proportional distribution of neuronal morphologies (for a representative image, see Figure 2(c)) was analyzed in these adjacent cultures (Figure 4(a)). The proportion of neurons without regeneration of neurites was significantly reduced by the embedded MSCs (90.93 ± 1.48% for alginate vs 64.14 ± 4.07% for alginate–MSC). In contrast, both the number of monopolar neurons (8.11 ± 1.40% vs 26.97 ± 2.74%) as well as the number of bipolar neurons (0.75 ± 0.24% vs 7.07 ± 1.44%) were significantly increased by the BDNF-producing MSCs. Neurons with multipolar or pseudomonopolar morphology were rarely detected in both cultures and only for pseudomonopolar neurons (0.21 ± 0.14% vs 1.28 ± 0.31%), not for multipolar neurons (0% vs 0.54 ± 0.25%), a significant increase in number was induced by the embedded MSCs.
Neurite regeneration in adjacent culture
The side-by-side cultivation of SGC with alginate–MSCs also enabled an analysis of a potential neurite attraction toward a fixed NTF source in the form of BDNF produced by the MSCs. The number of regenerated neurites per well as well as their length were significantly increased by the BDNF-overexpressing MSCs in alginate (Figure 4(b)) (number: 20.88 ± 6.53 neurites per well with alginate vs 128.10 ± 14.68 neurites per well with alginate–MSCs, length: 132.9 ± 8.47 µm alginate vs 188.7 ± 4.09 µm alginate–MSCs). To analyze a potential guidance toward a possible BDNF gradient, the orientation of the neurites at their endpoint was determined and given as percentage proportion of all analyzed neurites. There was no significant difference detected between neurite endings with an orientation to alginate or to alginate with BDNF-producing MSCs (54.91 ± 4.33% alginate vs 51.45 ± 2.06% alginate–MSCs). And there was no evidence for avoidance of one of them (45.09 ± 4.33% alginate vs 48.55 ± 2.06% alginate–MSCs) (Figure 4(b)).
Neuroprotection mediated by MSC beads
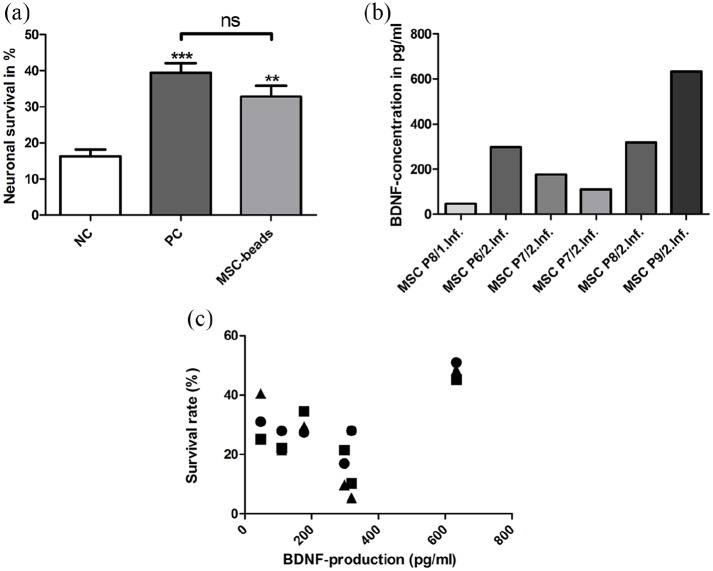
A neuroprotective effect of alginate-encapsulated BDNF-overexpressing MSCs in form of beads as a potential cell-based drug delivery system was investigated in SGC co-culture. In NC, a survival rate of 16.26 ± 3.05% was detected. The PC (50 ng/mL BDNF) resulted in a survival rate of 39.42 ± 4.21% while a co-cultivation with the beads increased the neuronal survival to 32.85 ± 5.09%. Both, the PC and MSC beads significantly protected the SGN from degeneration when compared to the NC (Figure 5(a)). In contrast, there was no significant difference detectable between MSC beads and PC.
Figure 5.
(a) SGNs are significantly protected from degeneration by co-culture with BDNF-producing MSC beads when compared to the NC without growth factor addition. This effect was comparable to the protection induced by 50 ng/mL BDNF in the PC. Asterisks above error bars indicate significant differences of PC and MSC beads compared to NC (mean ± SEM, **p < 0.01, ***p < 0.001; ns = not significant); N = 8, n = 3. (b) The concentration of BDNF in the supernatant was determined using ELISA. MSCs of different passages (P6–9) and two independent genetic modification schemes (1 and 2 infection) were encapsulated in alginate and applied as beads for co-cultivation for 48 h. The detected BDNF concentration in the supernatant varied widely from about 50–600 pg/mL. (c) Correlating the detected amount of BDNF in the culture supernatant with the neuronal survival rate of the corresponding three wells with beads, there was no consistency found. Pearson correlation: p = 0.45, r2 = 0.2.
BDNF detection
In all analyzed supernatants of the MSC-bead SGC-co-cultures, the BDNF concentrations were in a picogram per milliliter range. The concentrations varied widely from about 50 to 600 pg/mL (Figure 5(b)). Consequently, the cell-released BDNF concentration is notably lower than the recombinant protein concentration in the PC.
Correlation of BDNF and SGN survival
Since the detected BDNF amount in the analyzed supernatant of the co-cultures varied considerably, it was investigated if this variation was also reflected in the results of the neuronal survival. Therefore, a correlation analysis was performed (Figure 5(c)). There was no statistically significant dependency between BDNF amount and neuronal survival. Low as well as high amounts of BDNF were detected in supernatant of MSC-bead co-cultures with similar neuronal survival rates.
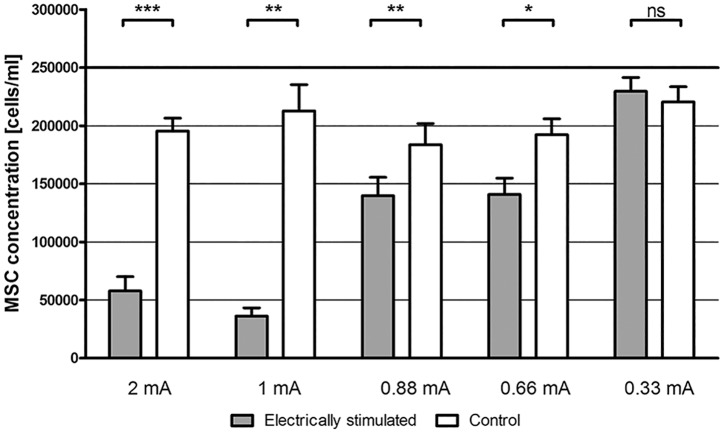
Electrical stimulation
Using 2 mA electric current, a highly significantly decreased cell number was observed for stimulated MSCs compared to non-stimulated controls (p < 0.0001) (Figure 6). In some cases of maximal stimulation (2 mA), the alginate directly located at the active electrode was charred. Stimulating with 1, 0.88, and 0.66 mA decreased the cell number as well but less prominently. Electrical stimulation with 0.33 mA did not change the cell number significantly compared to unstimulated controls (Figure 6).
Figure 6.
Electrical stimulation of alginate-encapsulated MSCs using high current levels decreased the MSC number significantly. The electrotoxicity increased with higher current levels. Electrical stimulation with 0.33 mA did not affect the MSC survival.
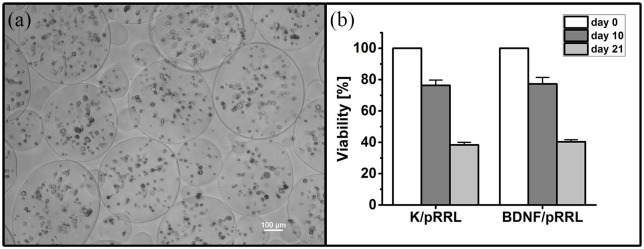
Viability of MSCs in alginate beads
The viability of MSCs of both genetic modifications (K/pRRL and BDNF/pRRL) was comparable after Day 0 (before encapsulation), Day 10, and Day 21. After Day 10, ~76% of the cells were viable, but this number dropped to ~40% after Day 21 (see Figure 7(a) and (b)). Microscopic images revealed that most MSCs were immobilized as single cells with only few aggregates.
Figure 7.
Viability of MSCs in alginate beads over 21 days. (a) A phase contrast image of encapsulated MSCs (BDNF/pRRL) in alginate beads at Day 21. The viability of MSC populations with control vector (K/pRRL) and genetic modification for BDNF production (BDNF/pRRL) is comparable at Day 0 (before encapsulation), Day 10, and Day 21, which is depicted in (b). On Day 10, the viability of both MSC populations was decreased to ~76% compared to the time of encapsulation. After Day 21, the viability dropped to ~40%. Data are presented as mean and mean ± standard error of mean (SEM, N = 2, n = 2). Scale bar in (a): 100 µm.
Discussion
A recently published study42 showed the neuroprotective effect of UHV-alginate-encapsulated BDNF-producing MSCs in a deafened guinea pig model. The here presented results validate the stability of the alginate and survival of the embedded MSCs for 3 weeks, the effects of electrical stimulation, and the positive effect on the neuronal morphology and neurite regeneration of SGN in vitro.
Alginate stability
An encapsulation of the genetically modified MSCs in UHV-alginate was possible and the red fluorescence of the MSCs enabled for vitality control of the MSCs through the transparent alginate.
The stability of the alginate gels in relevant media was verified by the observation of bead diameter and alginate layer elasticity. Shrinking or swelling of alginate beads would indicate osmotic activity what, in consequence, would stress embedded cells. Measurements up to 28 days after starting culture did not reveal changes in bead diameter leading to the conclusion that no changes in cell diameter would occur in the long-term of chronic implantation of beads. The elasticity of the alginate layers was also constant from experimental Day 1–28 in the tested media, indicating excellent mechanical properties for long-term applications. These observations coincide with a study from Zimmermann et al. reporting the long-term stability of alginate hydrogels in case of in vivo application.41 Only for the PC with D2H2O, small changes occurred at the beginning of the stability testing. The hypoosmotic environment of D2H2O resulted in swelling of the alginate gel. During the swelling process, the gel-network adsorbs water, expands and may break at certain strained binding sites. As a consequence, the alginate gel became softer and mechanically weaker (less elastic). However, after 14 days of incubation in the hypoosmotic condition (D2H2O), the elastic characteristics of the alginate gel stabilized. We hypothesize that the gel-network may have adapted to the swelled state after 2 weeks, so that cleaved bonds may have reassembled.
Amount and biological effect of released BDNF
BDNF was produced by the encapsulated cells and released from the UHV-alginate matrix into culture medium. The produced amount was high enough to be detectable even in the supernatant of the SGC–MSC–alginate co-culture, where it was not only continuously produced by the MSCs but also metabolized by the SGC. The detected amount was in a pg/mL range and varied widely (50–600 pg/mL). All experiments were performed following the same protocol. A defined number of cells was used for bead formation (MSCs) and neuronal survival testing (SGC). The variability of the BDNF amount could be explained by differences in the BDNF metabolization by the co-cultured SGC. For example, the amount of SGN differs in each preparation for an experiment, which is why a seeding control is performed and the neuronal survival is analyzed as survival rate (see methods). It is also conceivable that the number of transfected, BDNF-producing MSCs differed between the beads since the infection rate was about 80% (see fluorescence labeled and unlabeled MSCs in the bead in Figure 1(b)). Or the stress level during encapsulation of MSCs differed between the experiments, resulting in different BDNF amounts. Nevertheless, a significant protection of the SGN was detected and there was no correlation between higher and lower BDNF amounts in the supernatant and the neuronal survival. Low as well as high amounts of endogenously produced BDNF had a similar potential for neuroprotection. Compared to the previously proven concentration of 50 ng/mL BDNF of exogenous, recombinant BDNF for the best neuronal survival,18 the here detected amounts of endogenous in co-culture produced-BDNF were very low (pg/mL). But even in these low doses, the endogenous MSC-secreted BDNF had an equivalent neuroprotective effect, when compared with the 50 ng/mL exogenous BDNF of the PC. This is an effect confirming a previous study of our group with genetically modified fibroblasts.33 There, an amount of 0.05 ng/mL (⩠ 50 pg/mL) BDNF was detected in the supernatant of beads after 1 week (without co-cultivation) and had a neuroprotective effect when applied to SGN. In that study, a higher survival rate compared to the PC was achieved by the supernatant of UHV-alginate-encapsulated BDNF-overexpressing mouse fibroblasts than we could detect in co-culture for the UHV-alginate-embedded human MSCs. However, in the current study, a co-cultivation was performed to test the cell-based drug delivery in a model closer to in vivo/clinical conditions. This included a competition for nutrients between the cells and the application of human origin MSCs and BDNF-gen-expression.
In this study, additionally the neuronal morphology was analyzed for the adjacent culture and was affected by the cultivation of SGN with alginate–MSCs. The physiological morphology of SGN in the Rosenthal’s canal is mainly bipolar and rarely pseudomono- or multipolar.54,55 During the preparation and dissociation procedure, the existing neurites of the SGN are lost. In culture are some neurons able to regenerate neurites. This regrowth can be increased, for example, by NTF addition.17,52 The presence of the MSC-produced BDNF supported the neurite regeneration of the neurons leading to a reduction of the number of SGN with no neurites and an increased proportion of neurons showing a physiological morphology: mainly bipolar and rarely pseudomono- or multipolar. Altogether, the neuronal population was shifted to a significantly higher percentage of monopolar and bipolar neurons and a reduced proportion of neurons without regenerated neurites in the presence of MSCs. This finding is in accordance with our previous study analyzing the effect of a defined growth factor combination.17 In that study, 50 ng/mL of exogenous, recombinant BDNF had an equivalent effect on the SGN morphology as the here tested alginate–MSCs. A monopolar morphology of SGN due to loss of peripheral dendrites is found after deafness.8,56 These partly degenerated neurons stay connected with the brain stem via the axon and can be stimulated by CI. The amount of neurons with monopolar morphology was significantly increased in the co-culture with the MSCs. This may indicate the support of a specific regeneration of only one axon or dendrite, which cannot be distinguished in culture. At least two different types of neurons, the Type I and Type II SGN, are included in the dissociated cells of the SGC-culture. This may explain why some neurons only regenerate one neurite and have a monopolar morphology and others are able to regenerate more neurites showing a more physiological morphology. A support of a bipolar morphology is of great interest, since this is the most common physiological morphology of the CI-stimulated SGN.54 It may indicate a potential for BDNF-induced regeneration of dendrites, while protecting the axon against degeneration, what possibly improves the nerve electrode interface of the CI by neurite regeneration.11,14 The detected doses of MSC-secreted BDNF were much lower than the previously tested exogenous 50 ng/mL but showed a similar effect on the neuronal morphology. This may indicate that a continuous production and availability of low doses of BDNF produced by the MSCs can support the SGN similar to NTF-producing cells in the organ of Corti.
Besides neuronal survival and morphology, also the neurite length and number were positively influenced by the alginate–MSCs. Some in vivo studies already have proven the principle of NTF-induced dendrite regeneration into the scala tympani. But this regeneration was largely uncontrolled and rarely guided to the electrode.9–13 In the presence of the alginate-embedded MSCs, the number of regenerated neurites was about six times higher and the length of the regenerated neurites was significantly increased. This effect was shown in several studies for exogenous, recombinant BDNF.15,17,57 The adjacent culture of SGN and BDNF-producing MSCs allows the analysis of a guided growth of neurites in direction to this localized cell-based BDNF source. The orientation of the final position of the neurites at the end of the experiments was not affected by the MSCs. This finding could be explained by a too low diffusion gradient of BDNF between the connected cell compartments. Previous studies58,59 showed a neurite attraction following a NTF gradient in microfluidic systems. But Xie et al. could also detect no targeting of BDNF-secreting cells by regenerated neurites of SGN when cultured together.15 In comparison, Lee and Warchol60 showed an attraction of chick acoustic ganglia cell neurites toward localized matrigel-embedded HEK293-netrin-1-secreting cells in co-culture. Comparing this result, it has to be noted that they analyzed the overall neurite orientation, not the orientation at the neurite endings, and the different species used. The result might also implicate that BDNF is not the favorable NTF for neurite attraction. It may be necessary to focus on other factors or a combination of factors, such as BDNF and ciliary neurotrophic factor (CNTF)17 for neurite attraction.
Compared to other studies with cell-based drug delivery,15,33,43,44,61,62 the tested MSCs have the great advantage of being an autologous source. MSCs can be obtained, modified, and encapsulated from patients before they undergo a cochlear implantation. In the performed experiments, we tested different passages of the genetically modified MSCs and up to at least the ninth passage, the MSCs produced BDNF and had a neuroprotective effect on SGN. For clinical applications, MSCs could be produced in sufficient quantities for the treatment of each individual patient. Both independently performed genetic modification schemes had a neuroprotective effect in the experiments proving that the established protocol is functional and reproducible.
Electrical stimulation of alginate-embedded MSCs
Optimizing the CI outcome by use of alginate-encapsulated BDNF-overexpressing MSCs would always include a local application of this therapeutical approach. The alginate–MSCs would either be coated onto the electrode surface or applied as beads next to it or would be injected directly into the scala tympani. In all cases, the MSCs would directly be faced to the electrical stimulation of the implant. In the here used stimulation setup, the electrode was inserted into the alginate and therefore the maximum contact of electrode and cells was achieved. Parameters for safe stimulation of neurons using platinum electrodes in vivo have been determined by Shannon.63 The pulses used in the current study should theoretically be safe according to calculations by Hudak et al.64 The central electrode with an area of 1.56 ± 0.43 mm2 and a maximum pulse width of 400 μs could, according to the model, be operated at safe amplitude of 2.3 mA. Clinically used stimulation parameters range for current from about 10.2 to 2000 µA and for pulse width from 8 to 400 µs.53 Therefore, the maximum current of 2 mA with 400 µs phase width of biphasic pulses, as used in this study, induced much higher charge densities than actually used for CI stimulation.53 Even though the calculations state that 2 mA with 400 µs phase width of biphasic pulses should be safe, this stimulation resulted in a massive reduction of MSCs and in some cases in destruction of alginate but applying 0.33 mA did not affect MSC survival. This current level with a phase duration of 400 µs resulted in a charge density (σ) of 0.002 µC/cm2/phase at the alginate lateral surface and is much closer to clinically applied charge densities. The safety of this charge density is in line with the findings of Peter et al.,53 who investigated electrotoxicity on SGN using the same stimulation setup. When applying 0.004 µC/cm2/phase (i.e. 0.66 mA), the MSC survival was significantly decreased in the here presented study, whereas Peter reported a safe stimulation under 2.2 µC/cm2/phase. The discrepancy may be based on the much larger sample stimulated in our study. We stimulated an alginate drop of 1 mm diameter and the current density given is for the lateral surface of this alginate sample. In contrast, Peter et al. investigated the effect of the current density at 1–5 mm distance from the electrode. For sure, the charge density used in our experiments will have been much larger closer to the electrode and may have had a tremendous impact on the cell survival close to the electrode, whereas the cells with larger distance to the electrode were not affected by the current. Next to this, the alginate has—even though it is a hydrogel—a different density and viscosity than the cell culture medium in Peter’s experiments. One can speculate that the higher density causes reduced exchange of fluids and therefore reduced cooling effect around the electrode which may have led to increased temperature with negative effects on MSC survival. Since no reduced MSC number was detected in the unstimulated controls, we exclude that the sample diameter was too large and hindered nutrition diffusion. A positioning of MSC beads around the stimulating electrode instead of the electrode placed directly into the gel (⩠ coated electrode) might also be advantageous for MSC survival during stimulation. Since the electrical stimulation of CI patients varies widely from one patient to another and from one manufacturer to others, it is hard to say if the electrical stimulation via CI will affect the alginate-embedded MSCs and their BDNF release. The here performed experiments suggest that moderate electrical stimulation may not affect the MSCs. Further studies with more realistic stimulation paradigms applied for longer lasting intervals should be performed to ensure that the ES will not negatively affect the implanted MSCs.
MSC survival in UHV-alginate
The viability of encapsulated cells was determined after 10 and 21 days. A MSC Viability of ~76% was observed after 10 days cultivation, whereas the viabilities dropped to ~40% after 21 days. Microscopic images revealed a single cell distribution in the alginate capsules in combination with the minimalized cellular environment in alginate (no biochemical signals, no attachment). In general, encapsulation of stem cells is challenging due to the minimized cell matrix and cell interactions in hydrogels environment, which requires additional modification with, for example, signal peptides.65 Furthermore, there seems to be a strong dependency of the stem cell type and the resulting viability: high viabilities of neural stem cells were observed in alginates, whereas in the same hydrogel system, the viability of dental pulp stem cells decreased over time.66 Encapsulated bone marrow-derived MSCs with high viability (90% over 21 days) were reported in a study by Westhrin et al.67 Contrary, McKinney et al.68 reported decreasing viabilities of lentivirally modified bone marrow-derived MSCs in alginate (~50% at Day 21).
MSCs were in a suspension-like environment without integrin-triggered signal cascades, mainly as single cells. Apoptosis induced by inadequate or inappropriate cell–matrix interactions called anoikis69 might be the reason of the cell reduction. Furthermore, anoikis is one important challenge in MSC-based therapies as reviewed by Baldari.70 Increasing the cell density, adding proteins mediating integrin signal cascades in alginate (e.g. RGD-peptides71 or alginate/collagen mixtures), or using small multicellular aggregates would be the next steps for optimization of MSCs survival in alginate for long-term applications.
Conclusion
BDNF-overexpressing MSCs can be encapsulated in UHV-alginate and survive in alginate beads for the 21-day time period investigated. The overexpression of BDNF is not affected and the protein diffuses out of the alginate into the medium. The produced amount of BDNF is sufficient to protect SGN from degeneration and to induce neurite outgrowth. The UHV–alginate–MSC combination is not affected by CI-mimicking ES provided that given safety limits are respected. Therefore, we conclude that the encapsulation of BDNF-producing MSCs in UHV-alginate is a promising strategy to improve the CI outcome. The application as coating, injection, or beads into the implanted inner ear seems to be a feasible way for local drug delivery.
Acknowledgments
The authors thank Prof. Dr Axel Schambach for kindly providing the plasmid pRRL.PPT.SFFV (pRRL) and PD Dr Thilo Flörkemeier for the provision of the bone marrow samples, both from the Hannover Medical School. In addition, the authors thank Tanja Schubert for her support in neuron counting and neurite measurement, and Marvin Peter and Alexandra Rangnau for their assistance in electrical stimulation of alginate-encapsulated mesenchymal stem cells (MSCs).
Footnotes
Author contributions: V.S. and A.Ho. contributed to the study design; J.S., V.S., A.Ho., and M.M.G. contributed to the methodology; J.S., A.Ha., M.M.G., A.S., and A.Ho. contributed to the experiments; J.S., V.S., and M.M.G. contributed to the formal analysis; V.S., T.L., A.Ho., and M.M.G. contributed to the resources; J.S. drafted the article; J.S., V.S., A.Ho., A.Ha., T.L., M.M.G., and A.S. reviewed and edited the article; J.S., V.S., A.Ho., A.Ha., T.L., M.M.G., and A.S. contributed to the visualization; V.S. and A.Ho. contributed to the acquisition of funding.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval and patient consent: For the human bone marrow studies, ethical approval (565-2009, 565-2016) was obtained from the ethical committee of the Hannover Medical School, Hannover, Germany. The collection of bone marrow samples was performed after written informed consent of all donors and in accordance with the Declaration of Helsinki.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) by the projects HO 2058/13-1 to A.Ho. and SCHE 1663/2-1 to V.S. as well as through 1228/3-1. We acknowledge support by the German Research Foundation (DFG) and the Open Access Publication Fund of Hannover Medical School (MHH) for publication.
ORCID iD: Jana Schwieger  https://orcid.org/0000-0001-5010-6135
https://orcid.org/0000-0001-5010-6135
References
- 1. World Health Organization. Deafness and hearing loss, http://www.who.int/en/news-room/fact-sheets/detail/deafness-and-hearing-loss (2018, accessed 2 November 2018).
- 2. Gillespie LN, Richardson RT, Nayagam BA, et al. Treating hearing disorders with cell and gene therapy. J Neural Eng 2014; 11(6): 065001. [DOI] [PubMed] [Google Scholar]
- 3. Fransson A, de Medina P, Paillasse MR, et al. Dendrogenin A and B two new steroidal alkaloids increasing neural responsiveness in the deafened guinea pig. Front Aging Neurosci 2015; 7: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheper V, Paasche G, Miller JM, et al. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J Neurosci Res 2009; 87(6): 1389–1399. [DOI] [PubMed] [Google Scholar]
- 5. Konerding WS, Janssen H, Hubka P, et al. Encapsulated cell device approach for combined electrical stimulation and neurotrophic treatment of the deaf cochlea. Hear Res 2017; 350: 110–121. [DOI] [PubMed] [Google Scholar]
- 6. Seyyedi M, Viana LM, Nadol JB., Jr. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol 2014; 35(8): 1446–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol 1989; 98(6): 411–416. [DOI] [PubMed] [Google Scholar]
- 8. Liu W, Edin F, Atturo F, et al. The pre- and post-somatic segments of the human type I spiral ganglion neurons—structural and functional considerations related to cochlear implantation. Neuroscience 2015; 284: 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shibata SB, Cortez SR, Beyer LA, et al. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol 2010; 223(2): 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landry TG, Fallon JB, Wise AK, et al. Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity of cochlear implants. J Comp Neurol 2013; 521(12): 2818–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Senn P, Roccio M, Hahnewald S, et al. NANOCI—nanotechnology based cochlear implant with gapless interface to auditory neurons. Otol Neurotol 2017; 38(8): e224–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinyon JL, Tadros SF, Froud KE, et al. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci Transl Med 2014; 6(233): 233ra54. [DOI] [PubMed] [Google Scholar]
- 13. Leake PA, Hradek GT, Hetherington AM, et al. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 2011; 519(8): 1526–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bas E, Anwar MR, Goncalves S, et al. Laminin-coated electrodes improve cochlear implant function and post-insertion neuronal survival. Neuroscience 2019; 410: 97–107. [DOI] [PubMed] [Google Scholar]
- 15. Xie J, Pak K, Evans A, et al. Neurotrophins differentially stimulate the growth of cochlear neurites on collagen surfaces and in gels. Neural Regen Res 2013; 8(17): 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartnick CJ, Staecker H, Malgrange B, et al. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J Neurobiol 1996; 30(2): 246–254. [DOI] [PubMed] [Google Scholar]
- 17. Schwieger J, Warnecke A, Lenarz T, et al. Neuronal survival, morphology and outgrowth of spiral ganglion neurons using a defined growth factor combination. PLoS ONE 2015; 10(8): e0133680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wefstaedt P, Scheper V, Lenarz T, et al. Brain-derived neurotrophic factor/glial cell line-derived neurotrophic factor survival effects on auditory neurons are not limited by dexamethasone. Neuroreport 2005; 16: 2011–2014. [DOI] [PubMed] [Google Scholar]
- 19. Leake PA, Rebscher SJ, Dore’ C, et al. AAV-mediated neurotrophin gene therapy promotes improved survival of cochlear spiral ganglion neurons in neonatally deafened cats: comparison of AAV2-hBDNF and AAV5-hGDNF. J Assoc Res Otolaryngol 2019; 20(4): 341–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gillespie LN, Clark GM, Bartlett PF, et al. BDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res 2003; 71(6): 785–790. [DOI] [PubMed] [Google Scholar]
- 21. Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res 2008; 242(1–2): 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci 2005; 22(9): 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pettingill LN, Richardson RT, Wise AK, et al. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng 2007; 54(6 Pt. 1): 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H, Hao J, Li KS. Current strategies for drug delivery to the inner ear. Acta Pharm Sin B 2013; 3: 86–96. [Google Scholar]
- 25. Lee J-H, Lee MY, Lim Y, et al. Auditory disorders and future therapies with delivery systems. J Tissue Eng 2018; 9: 2041731418808455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akil O, Blits B, Lustig LR, et al. Virally mediated overexpression of glial-derived neurotrophic factor elicits age- and dose-dependent neuronal toxicity and hearing loss. Hum Gene Ther 2019; 30(1): 88–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smidsrod O, Skjakbrk G. Alginate as immobilization matrix for cells. Trends Biotechnol 1990; 8: 71–78. [DOI] [PubMed] [Google Scholar]
- 28. Habib A, Sathish V, Mallik S, et al. 3D printability of alginate-carboxymethyl cellulose hydrogel. Materials 2018; 11(3): 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gepp MM, Fischer B, Schulz A, et al. Bioactive surfaces from seaweed-derived alginates for the cultivation of human stem cells. J Appl Phycol 2017; 29: 2451–2461. [Google Scholar]
- 30. Heiligenstein S, Cucchiarini M, Laschke MW, et al. In vitro and in vivo characterization of nonbiomedical- and biomedical-grade alginates for articular chondrocyte transplantation. Tissue Eng Part C Methods 2011; 17(8): 829–842. [DOI] [PubMed] [Google Scholar]
- 31. Vanacker J, Luyckx V, Dolmans M-M, et al. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012; 33(26): 6079–6085. [DOI] [PubMed] [Google Scholar]
- 32. Ehrhart F, Mettler E, Böse T, et al. Biocompatible coating of encapsulated cells using ionotropic gelation. PLoS ONE 2013; 8(9): e73498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hütten M, Ehrhart F, Zimmermann H, et al. UHV-alginate as matrix for neurotrophic factor producing cells—a novel biomaterial for cochlear implant optimization to preserve inner ear neurons from degeneration. Otol Neurotol 2013; 34(6): 1127–1133. [DOI] [PubMed] [Google Scholar]
- 34. Breguet V, von Stockar U, Marison IW. Characterization of alginate lyase activity on liquid, gelled, and complexed states of alginate. Biotechnol Prog 2007; 23(5): 1223–1230. [DOI] [PubMed] [Google Scholar]
- 35. Formo K, Aarstad OA, Skjåk-Bræk G, et al. Lyase-catalyzed degradation of alginate in the gelled state: effect of gelling ions and lyase specificity. Carbohydr Polym 2014; 110: 100–106. [DOI] [PubMed] [Google Scholar]
- 36. Sondermeijer HP, Witkowski P, Woodland D, et al. Optimization of alginate purification using polyvinylidene difluoride membrane filtration: effects on immunogenicity and biocompatibility of three-dimensional alginate scaffolds. J Biomater Appl 2016; 31(4): 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmermann H, Zimmermann D, Reuss R, et al. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J Mater Sci Mater Med 2005; 16(6): 491–501. [DOI] [PubMed] [Google Scholar]
- 38. Tam SK, Dusseault J, Polizu S, et al. Impact of residual contamination on the biofunctional properties of purified alginates used for cell encapsulation. Biomaterials 2006; 27(8): 1296–1305. [DOI] [PubMed] [Google Scholar]
- 39. Tam SK, Dusseault J, Bilodeau S, et al. Factors influencing alginate gel biocompatibility. J Biomed Mater Res A 2011; 98(1): 40–52. [DOI] [PubMed] [Google Scholar]
- 40. Schneider S, Feilen PJ, Kraus O, et al. Biocompatibility of alginates for grafting: impact of alginate molecular weight. Artif Cells Blood Substit Immobil Biotechnol 2003; 31(4): 383–394. [DOI] [PubMed] [Google Scholar]
- 41. Zimmermann U, Thürmer F, Jork A, et al. A novel class of amitogenic alginate microcapsules for long-term immunoisolated transplantation. Ann N Y Acad Sci 2001; 944: 199–215. [DOI] [PubMed] [Google Scholar]
- 42. Scheper V, Hoffmann A, Gepp MM, et al. Stem cell based drug delivery for protection of auditory neurons in a guinea pig model of cochlear implantation. Front Cell Neurosci 2019; 13: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gillespie LN, Zanin MP, Shepherd RK. Cell-based neurotrophin treatment supports long-term auditory neuron survival in the deaf guinea pig. J Control Release 2015; 198: 26–34. [DOI] [PubMed] [Google Scholar]
- 44. Pettingill LN, Wise AK, Geaney MS, et al. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PLoS ONE 2011; 6(4): e18733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schulze J, Kaiser O, Paasche G, et al. Effect of hyperbaric oxygen on BDNF-release and neuroprotection: investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS ONE 2017; 12(5): e0178182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009; 3(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 47. Scheper V, Schwieger J, Hamm A, et al. BDNF-overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro. J Neurosci Res. Epub ahead of print 30 June 2019. DOI: 10.1002/jnr.24488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goren A, Dahan N, Goren E, et al. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J 2010; 24(1): 22–31. [DOI] [PubMed] [Google Scholar]
- 49. Herlofsen SR, Küchler AM, Melvik JE, et al. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng Part A 2011; 17(7–8): 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zimmermann H, Hillgärtner M, Manz B, et al. Fabrication of homogeneously cross-linked, functional alginate microcapsules validated by NMR-, CLSM- and AFM-imaging. Biomaterials 2003; 24(12): 2083–2096. [DOI] [PubMed] [Google Scholar]
- 51. Gepp M, Ehrhart F, Shirley S, et al. Dispensing of very low volumes of ultra high viscosity alginate gels: a new tool for encapsulation of adherent cells and rapid prototyping of scaffolds and implants. Biotechniques 2009; 46(1): 31–32, 34, 36–38 passim. [DOI] [PubMed] [Google Scholar]
- 52. Whitlon DS, Grover M, Tristano J, et al. Culture conditions determine the prevalence of bipolar and monopolar neurons in cultures of dissociated spiral ganglion. Neuroscience 2007; 146(2): 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peter MN, Warnecke A, Reich U, et al. Influence of in vitro electrical stimulation on survival of spiral ganglion neurons. Neurotox Res. Epub ahead of print 7 March 2019. DOI: 10.1007/s12640-019-00017-x. [DOI] [PubMed] [Google Scholar]
- 54. Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res 2011; 278(1–2): 2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ross MD, Burkel W. Multipolar neurons in the spiral ganglion of the rat. Acta Otolaryngol 1973; 76(6): 381–394. [DOI] [PubMed] [Google Scholar]
- 56. Rask-Andersen H, Liu W. Auditory nerve preservation and regeneration in man: relevance for cochlear implantation. Neural Regen Res 2015; 10(5): 710–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jin Y, Kondo K, Ushio M, et al. Developmental changes in the responsiveness of rat spiral ganglion neurons to neurotrophic factors in dissociated culture: differential responses for survival, neuritogenesis and neuronal morphology. Cell Tissue Res 2013; 351(1): 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wittig JH, Jr, Ryan AF, Asbeck PM. A reusable microfluidic plate with alternate-choice architecture for assessing growth preference in tissue culture. J Neurosci Methods 2005; 144(1): 79–89. [DOI] [PubMed] [Google Scholar]
- 59. Kunze A, Meissner R, Renaud P. Neurite guidance through 3D hydrogel layers in a microfluidic environment. In: 14th international conference on miniaturized systems for chemistry and life sciences (MicroTAS 2010), pp. 187–189, https://infoscience.epfl.ch/record/154050?ln=en [Google Scholar]
- 60. Lee KH, Warchol ME. Promotion of neurite outgrowth and axon guidance in spiral ganglion cells by netrin-1. Arch Otolaryngol Head Neck Surg 2008; 134(2): 146–151. [DOI] [PubMed] [Google Scholar]
- 61. Dazert S, Kim D, Luo L, et al. Focal delivery of fibro-blast growth factor-1 by transfected cells induces spiral ganglion neurite targeting in vitro. J Cell Physiol 1998; 177(1): 123–129. [DOI] [PubMed] [Google Scholar]
- 62. Wise AK, Fallon JB, Neil AJ, et al. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics 2011; 8(4): 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng 1992; 39(4): 424–426. [DOI] [PubMed] [Google Scholar]
- 64. Hudak EM, Kumsa DW, Martin HB, et al. Electron transfer processes occurring on platinum neural stimulating electrodes: calculated charge-storage capacities are inaccessible during applied stimulation. J Neural Eng 2017; 14(4): 046012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andersen T, Auk-Emblem P, Dornish M. 3D cell culture in alginate hydrogels. Microarrays 2015; 4: 133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hidalgo San Jose L, Stephens P, Song B, et al. Microfluidic encapsulation supports stem cell viability, proliferation, and neuronal differentiation. Tissue Eng Part C Methods 2018; 24(3): 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Westhrin M, Xie M, Olderøy MØ, et al. Osteogenic differentiation of human mesenchymal stem cells in mineralized alginate matrices. PLoS ONE 2015; 10(3): e0120374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McKinney J, Doan T, Wang L, et al. Therapeutic efficacy of intra-articular delivery of encapsulated human mesenchymal stem cells on early stage osteoarthritis. Eur Cell Mater 2019; 37: 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001; 13: 555–562. [DOI] [PubMed] [Google Scholar]
- 70. Baldari S, Di Rocco G, Piccoli M, et al. Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies. Int J Mol Sci 2017; 18(10): 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bidarra SJ, Barrias CC, Barbosa MA, et al. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules 2010; 11(8): 1956–1964. [DOI] [PubMed] [Google Scholar]