Abstract
Brain responses to low plasma glucose may be key to understanding the behaviors that prevent severe hypoglycemia in type 1 diabetes. This study investigated the impact of long duration, hypoglycemia aware type 1 diabetes on cerebral blood flow responses to hypoglycemia. Three-dimensional pseudo-continuous arterial spin labeling magnetic resonance imaging was performed in 15 individuals with type 1 diabetes and 15 non-diabetic controls during a two-step hyperinsulinemic glucose clamp. Symptom, hormone, global cerebral blood flow and regional cerebral blood flow responses to hypoglycemia were measured. Epinephrine release during hypoglycemia was attenuated in type 1 diabetes, but symptom score rose comparably in both groups. A rise in global cerebral blood flow did not differ between groups. Regional cerebral blood flow increased in the thalamus and fell in the hippocampus and temporal cortex in both groups. Type 1 diabetes demonstrated lesser anterior cingulate cortex activation; however, this difference did not survive correction for multiple comparisons. Thalamic cerebral blood flow change correlated with autonomic symptoms, and anterior cingulate cortex cerebral blood flow change correlated with epinephrine response across groups. The thalamus may thus be involved in symptom responses to hypoglycemia, independent of epinephrine action, while anterior cingulate cortex activation may be linked to counterregulation. Activation of these regions may have a role in hypoglycemia awareness and avoidance of problematic hypoglycemia.
Keywords: Arterial spin labeling, cerebral blood flow, hypoglycemia, thalamus, type 1 diabetes
Introduction
Hypoglycemia is a virtually inevitable side-effect of insulin therapy. In health, plasma glucose is maintained within narrow limits, ensuring that the majority of individuals without diabetes remain hypoglycemia naïve. If plasma glucose concentrations start to fall, insulin secretion is suppressed and glucagon, epinephrine, growth hormone and cortisol secretion is increased, arresting further glucose decline.1–3 The brain plays a central role in maintaining this homeostasis. Animal data have shown that the ventral medial hypothalamus controls counterregulatory hormone release and suppression, through local glucose sensing.4–6 Functional neuroimaging has allowed further exploration of the brain regions involved in hypoglycemia and counterregulation in man. Positron emission tomography (PET) and magnetic resonance imaging (MRI) provide in vivo techniques to examine changes in regional cerebral blood flow (CBF), tissue perfusion and glucose metabolism as surrogate markers of brain activity. These methods have shown that in healthy man, increased perfusion of the hypothalamus occurs as plasma glucose falls, within the euglycemic range and significantly before the peripheral counterregulatory response.7 The hypothalamus is part of a core network recruited to detect and reverse glucopenia.7,8 In non-diabetic (ND) adults, thalamic and cortical structures, such as the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), and dorsolateral prefrontal cortex (DLPFC), involved in arousal, sensory relay, executive function, and feeding behavior, have all been shown to be activated during hypoglycemia.8–13 Deactivation has been reported in the temporal lobes and hippocampus, regions involved in memory and speech.9,14 However, the effects of hypoglycemia in individuals with long duration type 1 diabetes (T1D) and intact hypoglycemia awareness remain unclear. Brain responses to hypoglycemia may underpin the behavioral and decision-making processes involved in hypoglycemia detection and avoidance. Despite a deficient glucagon and catecholamine counterregulatory response,15 the majority of people with T1D continue to be symptomatic of their hypoglycemia and avoid frequent severe hypoglycemic episodes.16 As part of ongoing research into mechanisms of hypoglycemia awareness, we designed this study to explore the differences in brain responses to hypoglycemia between hypoglycemia naïve, ND individuals, and hypoglycemia experienced, type 1 individuals, with intact awareness of hypoglycemia. To do this reliably, we used three-dimensional pseudo-continuous arterial spin labeling (3D pCASL), a method that employs a long (1.5–2 s) train of radiofrequency pulses in tandem with background suppressed, rapid imaging sequences to enhance the signal-to-noise ratio of CBF measurements. This technique is designed to provide improved sensitivity to perfusion and enhanced spatial resolution in comparison to conventional continuous and pulsed ASL methods, with no radiation exposure or exogenous contrast.17,18
Material and methods
Study participants
Thirty, right-handed, non-obese participants, aged 18–60 years, were recruited from diabetes clinics at King's College Hospital and via e-mail advertisement to students and staff at King's College London. Fifteen participants had T1D with intact awareness of hypoglycemia (Gold Score ≤ 2),19 and 15 were ND controls. No participant in either group had renal impairment (estimated glomerular filtration rate >60 ml/min/1.73 m2), history or current evidence of vascular disease, neurological conditions expected to produce MRI changes, previous significant head injury, a major psychological diagnosis, or any contraindications to MRI. The protocol was approved by the Dulwich Research Ethics Committee (National Research Ethics Service, London, UK), in accordance with the Declaration of Helsinki. Each participant gave written informed consent.
Study protocol
Consenting eligible volunteers avoided alcohol, caffeine and strenuous activity for 48 h prior to study. Participants with T1D were admitted to the National Institute for Health Research and Wellcome Trust King's Clinical Research Facility the evening before their scan, after their evening meal. Multiple daily injection participants omitted their evening dose of basal insulin; those using continuous subcutaneous insulin infusion remained on their insulin pump until replaced with intravenous (IV) insulin. A variable rate insulin infusion was used to maintain blood glucose between 90 and 144 mg/dL (5–8 mmol/L) overnight with venous sampling every 30–60 min, to avoid nocturnal hypoglycemia and ensure a fasting glucose for the day of study between 90 and 144 mg/dL (5–8 mmol/L). After 10 pm, participants were only permitted water or preemptive hypoglycemia treatment if blood glucose fell to 81 mg/dL (4.5 mmol/L) but otherwise did not consume any food or drink until after the scan. The study was rescheduled if blood glucose fell below 54 mg/dL (3.0 mmol/L).
In the morning, a hyperinsulinemic clamp was commenced in all participants to stabilize glucose concentrations for at least 60 min prior to allocated scanning time. Target glucose was 90 mg/dL (5 mmol/L). A new, primed IV insulin infusion (Actrapid; Novo-Nordisk, UK) replaced the overnight insulin at a maintenance rate of 1.5 mU kg−1 minute−1 with a variable rate 20% glucose solution (Baxter, UK). An IV cannula was inserted into a left dorsal hand vein. A heated thermal pack with CE marking (Conformité Européene; indicative of compliance with European safety requirements), was applied to warm the hand, enabling arterialized venous blood sampling.20,21 Plasma glucose was measured every 5 min, using a glucose oxidase analyzer (YSI 2300 STAT PLUS, Yellow Springs Instruments, Yellow Springs, OH, USA). Participants were placed on the scanner table in the supine position and provided with earplugs and earphones to reduce noise exposure. The head was stabilized and participants were asked to remain as still as possible to avoid artefact. Within the scanner plasma glucose was held at 90 mg/dL (5 mmol/L) for approximately 30 min, during which two 3D pCASL scans, ASL 1 and ASL 2 were acquired consecutively, each approximately 5 min duration. On completion of the euglycemic phase, plasma glucose was lowered over a period of 15–20 min. Once 47 mg/dL (2.6 mmol/L) was achieved and maintained for approximately 20 min, ASL 3 and ASL 4 were acquired. After each 3D pCASL scan, blood samples were collected for counterregulatory hormone measurements and participants reported autonomic and neuroglycopenic symptoms on a seven-point visual analogue scale, using a button box. Participants rated the extent to which they experienced hypoglycemic symptoms, where 1 = not at all and 7 = severely. Seven symptoms were classified as autonomic (anxiety, pounding heart, shaking, tingling, sweating, hunger, and nausea) and four as neuroglycopenic (drowsiness, irritability, visual disturbance, and confusion).10,22,23
Participants were blinded to their plasma glucose throughout. After completion of the scanning protocol, IV insulin was stopped and IV glucose and oral carbohydrate used to restore normoglycemia promptly. Following a meal, glucose was stabilized and participants were provided with support to avoid hypoglycemia for the next 48 h prior to discharge.
Biochemical analysis
Plasma glucose was measured throughout the study with a glucose oxidase analyzer (YSI 2300 STAT PLUS, Yellow Springs Instruments). Epinephrine and norepinephrine were analyzed by high-performance liquid chromatography with electrochemical detection.24 Automated immunoassay was used to analyze cortisol (Siemens Centaur XPT), growth hormone, and free insulin (Siemens Immulite 2000 XPi).
Statistical analysis of non-imaging data
Statistical analyses were performed using SPSS version 22 (IBM). Continuous demographic data were compared using unpaired two-sample Student t-tests. The Chi-squared test was used to compare categorical gender data. Within-group symptom scores, mean glucose concentrations, and hormonal responses were analyzed using paired two-sample Student t-tests. Unpaired Student t-tests were used for between-group comparisons. Data are presented as mean ± SD unless otherwise stated. A p value < 0.05 was considered statistically significant.
MRI parameters
MRI images were acquired using a 3 Tesla GE Healthcare MR750 scanner (GE Medical Systems, Milwaukee, WI, USA). Radiofrequency was transmitted with the scanner body coil, while signal was received with a 12-channel receive-only head coil. After an initial localizer scan, high-resolution anatomical images were acquired with an adapted 3D T1-weighted magnetization-prepared rapid acquisition with gradient echo sequence, with the following parameters: 1.2 mm isotropic resolution, repetition time 7.312 ms, echo time 3.01 ms, and inversion time 450 ms. CBF maps were acquired using a 3D pseudo-continuous Arterial Spin Labeling pulse sequence (3D pCASL), to determine changes in regional resting perfusion. The sequence used four non-selective radiofrequency pulses for background suppression; a labeling time of 1.5 s and a post labeling delay of 1.5 s. Four control-label pairs were collected. Following the post-labeling delay, images were acquired using a 3D Fast-Spin Echo Stack-of-spiral sequence, with an effective resolution of 2 × 2 × 3 mm. A proton density image was also acquired in the same series, to enable the computation of quantitative CBF maps.25
Statistical analysis of neuroimaging data
CBF maps were analyzed using Statistical Parametric Mapping (SPM Version 12, University College London, London, UK). As part of this process, the maps were transformed to the standard space of the Montreal Neurological Institute using a custom built software package called Automatic Software for ASL Processing (ASAP, Department of Neuroimaging, KCL).26 For each participant, two CBF maps obtained at euglycemia (ASL 1 and ASL 2) and hypoglycemia (ASL 3 and ASL 4) were averaged and smoothed (smoothing kernel 8 mm full width at half maximum). Global CBF change between euglycemia and hypoglycemia was measured from the mean of all gray-matter voxels in the brain volume in both conditions. Differences within and between groups (T1D and ND) were compared using a paired and unpaired Student t-test, respectively. To identify regional CBF change between euglycemia and hypoglycemia, a voxel-wise paired t-test (within the SPM framework) was performed within each group. Only those clusters that remained statistically significant after family-wise error (FWE) correction for multiple comparisons (p < 0.05) are reported. Clusters of significant change were determined using the “cluster-extent” criterion (pFWE < 0.05) at an uncorrected voxel-wise cluster-forming threshold of p < 0.005. CBF maps from both groups and both states (euglycemia and hypoglycemia) were then analyzed using a 2 × 2 flexible factorial analysis of variance (ANOVA) model within SPM-12, to assess the interaction of group and glycemic state across the whole brain. Again, significance was defined as any result on the map which survived FWE correction on the basis of cluster extent (pFWE < 0.05) using an uncorrected voxel-wise cluster-forming threshold of p < 0.005. To identify areas commonly recruited by both groups in response to hypoglycemia, we performed a conjunction analysis, wherein the null hypotheses of no activation in both ND and T1D were jointly rejected. Post hoc interaction and conjunction analyses of four pre-specified regions of interest (ROIs)—the thalamus, ACC, OFC, and hippocampus—were performed using small volume correction to spatially constrain multiple comparisons correction. Additional Bonferroni correction for the number of ROIs was applied, giving a critical alpha of p < 0.0125. These regions were selected based on a review of literature establishing key cerebral regions involved in the response to hypoglycemia.8–14,27,28 Regional masks were created using the Wake Forest University School of Medicine, (WFU) PickAtlas.29 A gray-matter mask was used in each analysis and global CBF was added to each model as a covariate to control for the effect of global perfusion. Correlation analyses were then used to identify regional CBF associations with hormone and symptom responses to hypoglycemia. CBF change in the thalamus, ACC, OFC, and hippocampus were correlated with mean increase in epinephrine and symptom score (total, autonomic, and neuroglycopenic) across both groups, using SPSS version 22 (IBM). Global CBF was again used as a covariate.
Results
Participant characteristics
Fifteen participants with T1D and 15 ND control participants, matched for age, gender, and body mass index, were studied (Table 1). T1D had long duration diabetes (24.0 ± 12.8 years), HbA1c of 7.6 ± 1.0%, intact hypoglycemia awareness (Gold score 1.5 ± 0.5). Severe hypoglycemia (SH) rates were low in T1D, 0.2 ± 0.6 episodes per year.
Table 1.
Participant characteristics.
| Controls n = 15 | T1D n = 15 | p | |
|---|---|---|---|
| Age, years | 40.1 ± 11.7 | 39.1 ± 13.5 | 0.852 |
| Gender Female Male | 8 7 | 9 6 | 0.713 |
| BMI, kg/m2 | 25.0 ± 2.8 | 24.7 ± 4.0 | 0.838 |
| HbA1c % mmol/mol | 5.2 ± 0.3 33.8 ± 2.9 | 7.6 ± 1.0 59.7 ± 11.2 | <0.001 |
| T1D duration, years | – | 24.0 ± 12.8 | – |
| Gold score | – | 1.5 ± 0.5 | – |
Continuous data are presented as mean ± SD and categorical data as n. Gold score is a measure of hypoglycemia awareness whereby a score of 1 or 2 denotes hypoglycemia awareness.19 SD: standard deviation; BMI: body mass index; HbA1c: hemoglobin A1C.
Glucose and insulin concentrations
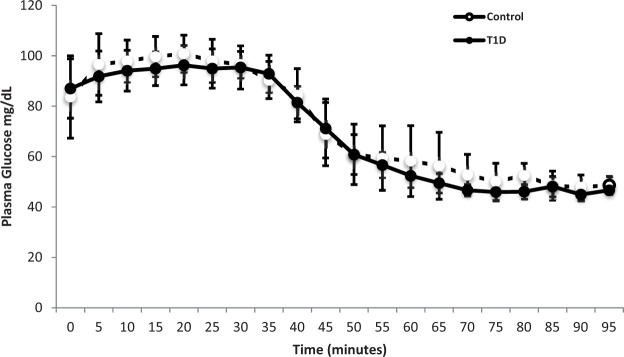
Plasma glucose targets were achieved with no significant difference between the groups, p = 0.69 (Figure 1). Mean glucose concentrations during the euglycemic phase pCASL scan acquisition were not different between groups 97 ± 8 mg/dL (5.4 ± 0.4 mmol/L) and 96 ± 6 mg/dL (5.4 ± 0.4 mmol/L), for ND and T1D, respectively (p = 0.69). Corresponding concentrations during the hypoglycemic phase pCASL scan acquisition were 48 ± 4 mg/dL (2.7 ± 0.2 mmol/L) and 47 ± 2 mg/dL (2.6 ± 0.1 mmol/L), also not different between groups (p = 0.21). Insulin concentrations were comparable between groups throughout the study (p = 0.42).
Figure 1.
Glucose concentrations (mg/dL) presented as mean ± SD, during hyperinsulinemic euglycemic hypoglycemic clamp, non-diabetic controls shown in open circles and T1D shown in closed circles. Between-group differences analyzed by unpaired Student t-test, p = 0.69. T1D: type 1 diabetes.
Symptomatic and hormonal responses to hypoglycemia
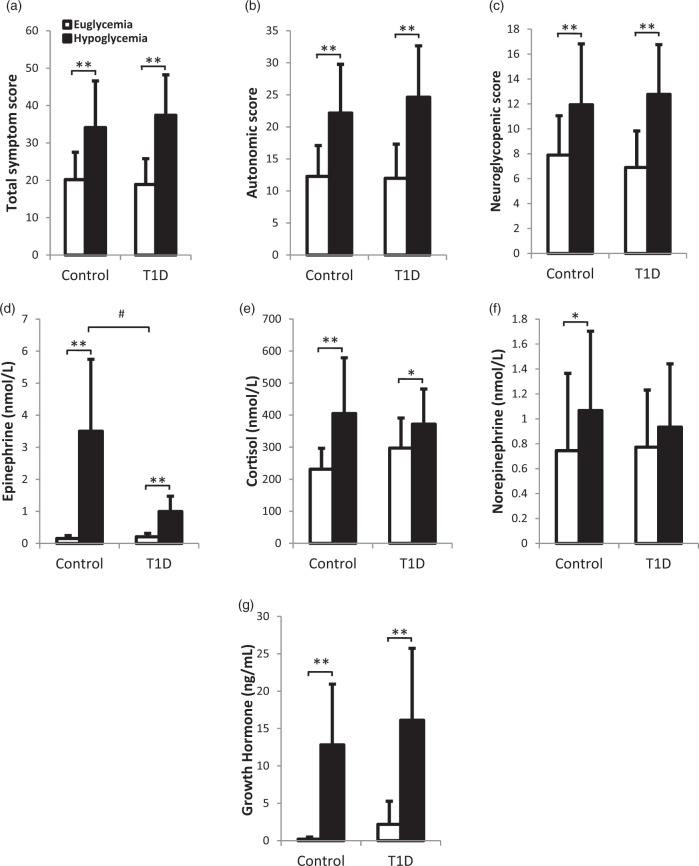
Symptom scores increased significantly in response to hypoglycemia in both groups (Figure 2(a) to (c), euglycemia vs. hypoglycemia mean ± SD: ND, total score 20.2 ± 7.3 vs. 34.1 ± 12.5 (p < 0.001), autonomic score 12.3 ± 4.8 vs. 22.2 ± 7.6 (p < 0.001), neuroglycopenic score 7.9 ± 3.2 vs. 11.9 ± 4.9 (p < 0.002); T1D, total score 18.9 ± 6.9 vs. 37.4 ± 10.8 (p < 0.001), autonomic score 12.0 ± 5.3 vs. 24.6 ± 8.0 (p < 0.001), neuroglycopenic score 6.9 ± 2.9 vs. 12.8 ± 4.0 (p < 0.001)). There were no significant differences in these responses between the two groups (Δ symptom score, hypoglycemia–euglycemia: ND vs. T1D total p = 0.25, autonomic p = 0.35, neuroglycopenic p = 0.19). Epinephrine concentrations increased in response to hypoglycemia in both groups (p < 0.005), but to a significantly lesser extent in T1D (Figure 2(d), ND vs. T1D mean epinephrine concentration increase during hypoglycemia; 3.0 ± 2.2 nmol/L vs. 1.2 ± 0.9 nmol/L, p = 0.009). Cortisol concentrations increased in both groups (p < 0.05) (Figure 2(e)), with a greater increase in ND that did not reach significance (p = 0.05). Norepinephrine concentrations increased in ND (p = 0.02), with no significant increase seen in T1D (p = 0.20) and no significant differences between the two groups' responses (p = 0.35) (Figure 2(f)). Growth hormone increased significantly in both groups (p < 0.005) with no significant difference between the ND and T1D response to hypoglycemia (p = 0.69) (Figure 2(g)).
Figure 2.
Hypoglycemia-induced changes in (a) total symptom score, (b) autonomic symptom score, (c) neuroglycopenic symptom score, (d) epinephrine, (e) cortisol, and (f) norepinephrine, (g) growth hormone, during euglycemic phase (mean values for ASL scan 1 and ASL scan 2, open bars) and hypoglycemic phase (mean values for ASL 3 and 4, black bars). Mean values ± SD are depicted, *p < 0.05 and **p < 0.005 for euglycemia versus hypoglycemia, #p < 0.005 non-diabetic control versus T1D response to hypoglycemia (Δ hypoglycemia–euglycemia). T1D: type 1 diabetes.
Global CBF response to hypoglycemia
In the euglycemic state (baseline), no significant differences in global CBF between T1D and ND were observed (T1D: 41.89 ± 9.3 ml/100 g/min, ND: 45.77 ± 11.3 ml/100 g/min, p = 0.31). Global CBF increased in response to hypoglycemia in T1D, while a non-significant rise was seen in ND (T1D: 6.8% ± 9.2, p = 0.01, ND: 5.5% ± 11.2, p = 0.07). There was no significant difference between the two groups' global blood flow response to hypoglycemia (p = 0.8).
Regional CBF
Whole-brain voxel-wise within-group analyses
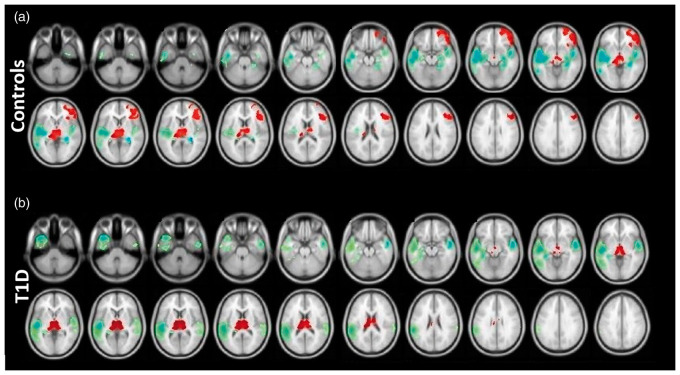
ND exhibited a significant increase in CBF in the thalamus, right OFC, and right DLPFC, with a decrease in the temporal cortex and hippocampus bilaterally, in response to hypoglycemia (Figure 3(a)). T1D demonstrated a significant increase in CBF in the thalamus, with a decrease in the temporal cortex and left hippocampus (Figure 3(b)).
Figure 3.
Effect of hypoglycemia on cerebral blood flow, within-group analysis of (a) non-diabetic controls and (b) T1D. Statistical parametric maps projected onto brain images showing significant rise (red) and significant fall (blue–green) in CBF. A voxel-wise two-sided paired t-test was performed on each group to identify the effect of hypoglycemia; clusters of significant change were determined using the “cluster-extent” criterion (pFWE < 0.05) at an uncorrected voxel-wise cluster-forming threshold of p < 0.005. T1D: type 1 diabetes.
Effect of diabetes: Between-group analyses
Whole-brain analysis using a repeated measures ANOVA to assess differences in response to hypoglycemia between ND and T1D, showed no significant interaction.
Region of interest (ROI) analysis in pre-selected regions (thalamus, ACC, OFC, and hippocampus) demonstrated a significant glucose-state by group interaction, reflecting a greater increase of regional CBF within the ACC of ND compared to T1D (ACC ND vs. T1D interaction p = 0.037, cluster size 518 voxels, peak voxel coordinates 2, 38, 0 (Table 2)); however, this did not survive Bonferroni correction for the number of ROIs (critical alpha of p < 0.0125). No significant differences were seen in the remaining predetermined ROIs, the thalamus, OFC, or hippocampus.
Table 2.
Characteristics of clusters identified by SPM-12 using small volume correction to spatially constrain multiple comparisons correction in pre-defined regions.
| Analysis | Cluster size | x | y | z | p | Bonferroni critical α | |
|---|---|---|---|---|---|---|---|
| Anterior cingulate | Interaction ND > T1D | 208 | 2 | 38 | 0 | 0.037 | 0.0125 |
| Thalamus | Conjunction | 1325 | 6 | −12 | 4 | 0.002* | 0.0125 |
| Orbitofrontal cortex | Conjunction | 522 | 46 | 40 | −16 | 0.009* | 0.0125 |
Interaction (ND response greater than T1D response) and conjunction analyses (common ND and T1D response). Peak voxel coordinates (x, y, z) given in Montreal Neurological Institute space. T1D: type 1 diabetes; ND: non-diabetic; ROI: region of interest.
Significant p value after Bonferroni correction for the number of ROI tests (critical alpha p < 0.0125).
Conjunction analyses: Between-group commonality
Conjunction analyses across the whole brain demonstrated that the thalamus was commonly recruited by ND and T1D during hypoglycemia (FWE cluster-corrected p = 0.028, cluster size 1875 voxels, peak voxel coordinates 6, −12, 4).
Small-volume correction in four pre-defined regions (ACC, thalamus, OFC, and hippocampus) confirmed a common response (i.e., a significant conjunction) in ND and T1D within the thalamus (p = 0.002, cluster size 1875 voxels, peak voxel coordinates 6, −12, 4; Table 2) and additionally the OFC (p = 0.009, cluster size 1481 voxels, peak voxel coordinates 46, 40, −16; Table 2). Both regions survived Bonferroni correction for the number of ROI tests (critical alpha pFWE < 0.0125). Common responses were not seen in the ACC or hippocampus.
Correlation analyses
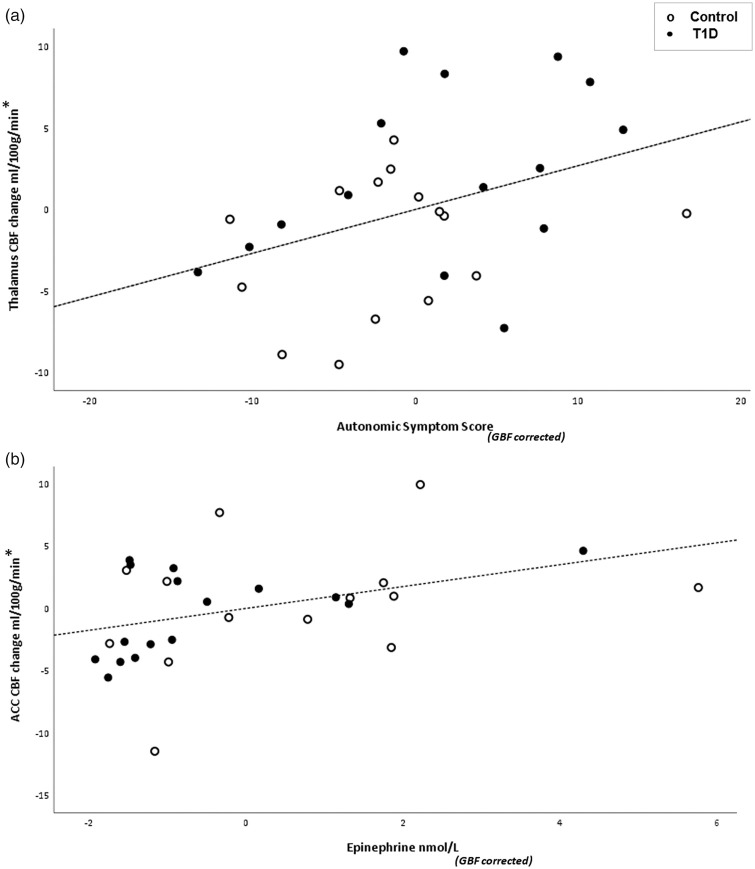
Using partial correlation across groups, with change in global CBF as a covariate, a significant correlation was observed between thalamic CBF and autonomic symptom score (Figure 4(a); R = 0.381, p = 0.041, degrees of freedom (df) = 27) and ACC CBF and epinephrine (Figure 4(b); R = 0.391, p = 0.036, df = 27). No other significant correlations were seen.
Figure 4.
(a) Correlation between mean thalamic CBF change and mean autonomic symptom score difference between euglycemia and hypoglycemia across groups, GBF as covariate (*GBF corrected) R = 0.381, p = 0.041, degrees of freedom (df) = 27. (b) Correlation between mean ACC CBF change and mean epinephrine difference between euglycemia and hypoglycemia across groups, GBF as covariate (*GBF corrected) R = 0.391, p = 0.036, df = 27. Non-diabetic controls shown in open circles and T1D shown in closed circles. T1D: type 1 diabetes; CBF: cerebral blood flow; GBF: global blood flow.
Discussion
We have shown that individuals with long duration T1D and intact hypoglycemia awareness have robust symptom, global and thalamic responses to hypoglycemia despite diminished epinephrine responses. Most individuals with T1D are able to subjectively detect hypoglycemia (75%–80%).16,30 We carefully selected type 1 participants with preserved symptomatic awareness of hypoglycemia to reflect the majority of the population. The groups were otherwise well-matched, and glucose targets were achieved demonstrating an equivalent and effective hypoglycemia stimulus. Symptom scores increased in response to hypoglycemia similarly in T1D and ND, and notably autonomic symptoms were not reduced despite a significantly weaker epinephrine rise in T1D. An impaired epinephrine response to hypoglycemia has been described in individuals with a long history of T1D.15,31 Despite an attenuated epinephrine response, our participants were still able to generate a normal symptomatic response to hypoglycemia. Although this dissociation has been reported previously, such as in adrenalectomized patients,32 and by Dagogo-Jack et al.33 who achieved symptom but not epinephrine response restoration in adults with impaired awareness of hypoglycemia (IAH) after hypoglycemia avoidance, our present data demonstrate the neuro-anatomical associations of these responses by showing a correlation between thalamic activation and autonomic symptoms and between ACC activation and epinephrine response. Differential changes in these regions may have a role in the development of IAH.11,27,34
Global CBF to the brain increased similarly in both groups in response to hypoglycemia. Early xenon-clearance studies reported an increase in global CBF in ND participants35,36 and T1D35 during hypoglycemia. Notably, CBF was measured at mean plasma glucose concentrations of 20–40 mg/dL (1.1–2.2 mmol/L), much lower than described here. At mild hypoglycemia of 65 mg/dL (3.6 mmol/L), Lubow et al.37 and Segel et al.38 found no difference in global CBF between euglycemia and hypoglycemia in ND participants. At moderate hypoglycemia, 50 mg/dL (2.8 mmol/L), Wiegers et al.27 reported a trend toward an increase of 5% (p = 0.08) in seven type 1 participants with good awareness of hypoglycemia and no change in seven control participants (p = 0.70). Our findings of a significant increase in global CBF in response to hypoglycemia in T1D (p = 0.01, n = 15), and a non-significant increase in our ND controls (p = 0.07, n = 15), may reflect a larger sample size, compared to the Wiegers study. In contrast, Teves et al. reported a fall in CBF in the “cerebrum, brainstem, and cerebellum” using water PET in nine ND controls at 54 mg/dL (3.0 mmol/L). There is no obvious reason why the difference in technique should contribute to this discrepancy. The increase in global CBF seen at moderate hypoglycemia in our study is similar to that observed with hypoxia.39 As both states represent impaired supplies for metabolism in the brain, we speculate that the rise in global CBF may well be a compensatory response to increase delivery of oxygen and glucose. Our data do not support the view that differences in global CBF responses are directly linked to epinephrine release, as both groups had a similar rise in global CBF, while epinephrine responses were different.
The key brain regions activated in response to hypoglycemia were the thalamus, OFC, and DLPFC, with deactivation in the temporal cortex and hippocampus. These findings are compatible with regional responses described in ND adults by Teves et al.9 and Arbelaez et al.12 Fittingly, a fall in glucose triggers the redistribution of blood flow to areas that govern arousal, response to stressful stimuli, feeding behavior, and memory. Our finding of a significant and similar increase in thalamic blood flow during hypoglycemia in both the ND group and type 1 participants is also in keeping with previous studies.9–12,27 Traditionally, the principal role of the thalamus has been the relay of motor and sensory stimuli to frontal and other cortical structures via neuronal networks.40 Later work suggested thalamic amplification of cortical connectivity41 and involvement in arousal, attention, and consciousness,42,43 key factors in hypoglycemia awareness. Our data suggest that the thalamus has a key role in hypoglycemia responses. A correlation between thalamic activation and epinephrine response to hypoglycemia has been reported in ND individuals.10,11 We did not see a significant correlation between thalamic CBF and epinephrine in the ND and T1D groups we tested; instead we found thalamic activation correlated with autonomic symptom score. This together with the suggestion that the thalamus adapts incoming stimuli before transmission to the cortex depending on behavioral condition, put forward by Sherman et al.44 leads us to speculate that the thalamus may be involved in the generation of an appropriate symptom response despite limited epinephrine.
Mangia et al.11 compared ND controls with T1D participants with IAH; however, their study did not include type 1 participants with intact awareness of hypoglycemia. Their IAH group had lesser thalamic activation than their ND comparators, which is compatible with our hypothesis that preserved thalamic activity may be related to the perception of symptoms during a diminished epinephrine response. Arbelaez et al.34 found enhanced thalamic perfusion during hypoglycemia in a ND model of IAH and suggested an inhibitory role of the thalamus. Importantly, they studied individuals exposed to a relatively brief interval of hypoglycemia, rather than the recurrent hypoglycemia experienced over a prolonged time by type 1 individuals with IAH, which may explain the difference in thalamic activity. Nonetheless their data provide further evidence that the thalamus is involved in the symptomatic response to hypoglycemia. Our data are similar to those of Wiegers et al.,27 who compared a single ASL scan at euglycemia and hypoglycemia, demonstrating thalamic activation in their ND and type 1 participants with intact hypoglycemia awareness. In both their study and ours, no evidence of significant hypothalamic activation was found. The hypothalamus has an important role in glucose sensing4; however, it is a small organ and its neuroimaging data are susceptible to partial volume effects. This may explain why certain studies describing thalamic activation have not observed concurrent hypothalamic activation.9,11,34 However, it is also possible that the hypothalamus, with its known complement of glucose responsive neurons, is more activated at the onset of hypoglycemia or during a changing glucose concentration, than in late established hypoglycemia. Page et al.7 described an increase in hypothalamic blood flow but in response to a changing and much lesser degree of hypoglycemia than in our study and did not report an associated thalamic response. Likewise, Musen et al.8 identified an increase in Blood Oxygen Level Dependent (BOLD) signal within the hypothalamus using a different technique to that described herein, also during a lesser degree of hypoglycemia. The ROIs we selected represented functional associations we are particularly interested in, namely sensory input and adaptation (thalamus), autonomic function (ACC), behavior (OFC), and memory (hippocampus) and have been repeatedly shown to respond to hypoglycemia in studies with similar methodology to our own.9,11,12
Within our control group, we observed hypoglycemia-induced activation of the OFC and DLPFC, regions involved in executive function, decision-making, feeding behavior, and reward pathways. The OFC forms part of the orbital and medial prefrontal network (OMPFC), believed to coordinate complex cognitive, emotional, and volitional behaviours45 with extensive visceromotor connections with the thalamus and limbic structures.46 The OMPFC and connecting structures have also been implicated in integrating sensory input and distributing cortical output to autonomic structures.9 The DLPFC is thought to be part of a similar network highly involved in the guidance of behavior. Relative diversion of CBF to frontal regions may contribute to an appropriate behavioral response to hypoglycemia, such as food-seeking. Hypoglycemia-induced frontal activation did not reach significance when we performed the within-group analysis in T1D; however, any frontal changes in CBF may have been lost due to the strict statistical threshold applied. Indeed, our conjunction ROI analysis quantitatively comparing the ND and T1D response to hypoglycemia showed that there was a common response to hypoglycemia within the OFC, suggesting that frontal structures, involved in feeding behavior and reward, are recruited in the response to hypoglycemia in individuals with diabetes. Hypoglycemia-induced deactivation of the temporal cortex and hippocampus was noted in both groups, regions involved in processing of sensory input and memory. This may explain the memory impairment seen with acute hypoglycemia.47
The between-group analysis comparing the two groups' responses to hypoglycemia across the whole brain showed no significant differences, consistent with similar symptom and global responses. This voxel-wise whole-brain analysis was less sensitive at detecting pre-specified regional changes in blood flow due to correction for multiple comparisons and multiple thresholding. To address this, we performed an ROI analysis to compare each group's response to hypoglycemia within a priori defined brain structures and found a difference within the ACC. Although this difference did not survive correction for multiple comparisons in our data, one other study reported reduced ACC activation during hypoglycemia in T1D (n = 7) when compared to ND controls (n = 6) using BOLD functional MRI during hypoglycemia; however, hypoglycemia awareness status was not reported.8 The ACC is thought to have a role in autonomic regulation and interoception (internal awareness) and has been shown to be activated during hypoglycemia in ND and type 1 individuals.7–10,28 Our data showing a positive correlation between ACC activation and epinephrine response implies that the ACC may be particularly linked to epinephrine secretion.
In conclusion, we have shown that individuals with long duration T1D and preserved hypoglycemia awareness have an increase in global and thalamic blood flow during hypoglycemia. Their responses to this physiological stressor are comparable to their ND counterparts despite prior hypoglycemia experience and reduced epinephrine secretion. These individuals, with 24 years of diabetes and reasonable glycemic control, have experienced multiple episodes of mild hypoglycemia over the years. This may explain the diminished epinephrine response but importantly the symptom response, vital to reducing the risk of SH, remained intact. Even autonomic symptoms, traditionally thought to be mediated via epinephrine, increased significantly in response to hypoglycemia and were, in fact, associated with thalamic activity. Overall, our results suggest that symptomatic hypoglycemia awareness and protective counterregulatory responses are mediated via different, but linked pathways. Autonomic symptoms may be linked to the thalamus while the ACC may be involved in the counterregulatory epinephrine response. We hypothesize that the hypoglycemia-driven increase in thalamic blood flow seen in T1D is related to the perception and preservation of hypoglycemia-induced symptoms despite limited epinephrine and ACC response. These data provide a potential mechanism by which individuals with long duration diabetes preserve the subjective symptomatic awareness that protects them from SH. Further study of these brain regions with a specific focus on awareness status would provide a better understanding of the brain responses in individuals with problematic hypoglycemia.
Acknowledgements
The authors thank the participants; the clinical research staff at the NIHR and Wellcome Trust King’s Clinical Research Facility, Louisa Green and John Lord Villajin; the laboratory staff at Viapath, King’s College Hospital, Tracy Dew, Gemma Cross, Andrew Given, and Joseph Molloy; and the radiographers and administrative staff at the Clinical Neuroimaging Sciences Department Centre, King’s College London.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Diabetes UK (grant number 13/0004653).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MN recruited the participants. MN, BMW, MLB, AP, and PC performed the study. MN, FOZ, and OO analyzed the neuroimaging data. MN, and MB collated and analyzed study data. SMC and IAM analyzed the catecholamine data. MN, SAA, FOZ, and PC wrote the manuscript. MN, SAA, FOZ, OO, PC, IAM, and SMC reviewed and edited the manuscript. MN, SAA, FOZ, OO, and PC contributed to conception and design of the research and to interpreting the data. PC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Fanelli C, Pampanelli S, Epifano L, et al. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia 1994; 37: 797–807. [DOI] [PubMed] [Google Scholar]
- 2.Amiel SA, Simonson DC, Tamborlane WV, et al. Rate of glucose fall does not affect counterregulatory hormone responses to hypoglycemia in normal and diabetic humans. Diabetes 1987; 36: 518–522. [DOI] [PubMed] [Google Scholar]
- 3.Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991; 260: E67–E74. [DOI] [PubMed] [Google Scholar]
- 4.Borg MA, Sherwin RS, Borg WP, et al. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997; 99: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrimmon RJ, Shaw M, Fan X, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008; 57: 444–450. [DOI] [PubMed] [Google Scholar]
- 6.Evans ML, McCrimmon RJ, Flanagan DE, et al. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 2004; 53: 2542–2551. [DOI] [PubMed] [Google Scholar]
- 7.Page KA, Arora J, Qiu M, et al. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 2009; 58: 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musen G, Simonson DC, Bolo NR, et al. Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab 2008; 93: 1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teves D, Videen TO, Cryer PE, et al. Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A 2004; 101: 6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teh MM, Dunn JT, Choudhary P, et al. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage 2010; 53: 584–592. [DOI] [PubMed] [Google Scholar]
- 11.Mangia S, Tesfaye N, De Martino F, et al. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 2012; 32: 2084–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbelaez AM, Su Y, Thomas JB, et al. Comparison of regional cerebral blood flow responses to hypoglycemia using pulsed arterial spin labeling and positron emission tomography. PLoS One 2013; 8: e60085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbelaez AM, Rutlin JR, Hershey T, et al. Thalamic activation during slightly subphysiological glycemia in humans. Diabetes Care 2012; 35: 2570–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal JM, Amiel SA, Yaguez L, et al. The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes 2001; 50: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 15.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983; 32: 134–141. [DOI] [PubMed] [Google Scholar]
- 16.Hendrieckx C, Hagger V, Jenkins A, et al. Severe hypoglycemia, impaired awareness of hypoglycemia, and self-monitoring in adults with type 1 diabetes: Results from Diabetes MILES-Australia. J Diabetes Complications 2017; 31: 577–582. [DOI] [PubMed] [Google Scholar]
- 17.Silva AC, Kim SG. Pseudo-continuous arterial spin labeling technique for measuring CBF dynamics with high temporal resolution. Magn Reson Med 1999; 42: 425–429. [DOI] [PubMed] [Google Scholar]
- 18.Wu WC, Jiang SF, Yang SC, et al. Pseudocontinuous arterial spin labeling perfusion magnetic resonance imaging – a normative study of reproducibility in the human brain. Neuroimage 2011; 56: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 19.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg GE, Keller U. Sampling of arterialized heated-hand venous blood as a noninvasive technique for the study of ketone body kinetics in man. Metabolism 1982; 31: 1–5. [PubMed] [Google Scholar]
- 21.Brooks DC, Black PR, Arcangeli MA, et al. The heated dorsal hand vein: an alternative arterial sampling site. JPEN J Parenter Enteral Nutr 1989; 13: 102–105. [DOI] [PubMed] [Google Scholar]
- 22.Hepburn DA, Deary IJ, Frier BM, et al. Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM. Factor-analysis approach. Diabetes Care 1991; 14: 949–957. [DOI] [PubMed] [Google Scholar]
- 23.Deary IJ, Hepburn DA, MacLeod KM, et al. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia 1993; 36: 771–777. [DOI] [PubMed] [Google Scholar]
- 24.Forster CD, Macdonald IA. The assay of the catecholamine content of small volumes of human plasma. Biomed Chromatogr 1999; 13: 209–215. [DOI] [PubMed] [Google Scholar]
- 25.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mato Abad V, Zelaya F, García-Polo P, et al. Toolbox for coregistration and spatial normalisation of CBF maps acquired with ASL for group comparison studies. Magn Reson Mater Phys Biol Med 2013; 26(Suppl 1): 512. [Google Scholar]
- 27.Wiegers EC, Becker KM, Rooijackers HM, et al. Cerebral blood flow response to hypoglycemia is altered in patients with type 1 diabetes and impaired awareness of hypoglycemia. J Cereb Blood Flow Metab 2017; 37: 1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn JT, Choudhary P, Teh MM, et al. The impact of hypoglycaemia awareness status on regional brain responses to acute hypoglycaemia in men with type 1 diabetes. Diabetologia 2018; 61: 1676–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 30.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 31.Fanelli CG, Paramore DS, Hershey T, et al. Impact of nocturnal hypoglycemia on hypoglycemic cognitive dysfunction in type 1 diabetes. Diabetes 1998; 47: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 32.Altorfer RM, Ziegler WH, Froesch ER. Insulin hypoglycaemia in normal and adrenalectomized subjects: comparison of metabolic parameters and endocrine counter regulation. Acta Endocrinol (Copenh) 1981; 98: 413–419. [DOI] [PubMed] [Google Scholar]
- 33.Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994; 43: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 34.Arbelaez AM, Powers WJ, Videen TO, et al. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 2008; 57: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neil HA, Gale EA, Hamilton SJ, et al. Cerebral blood flow increases during insulin-induced hypoglycaemia in type 1 (insulin-dependent) diabetic patients and control subjects. Diabetologia 1987; 30: 305–309. [DOI] [PubMed] [Google Scholar]
- 36.Tallroth G, Ryding E, Agardh CD. Regional cerebral blood flow in normal man during insulin-induced hypoglycemia and in the recovery period following glucose infusion. Metabolism 1992; 41: 717–721. [DOI] [PubMed] [Google Scholar]
- 37.Lubow JM, Pinon IG, Avogaro A, et al. Brain oxygen utilization is unchanged by hypoglycemia in normal humans: lactate, alanine, and leucine uptake are not sufficient to offset energy deficit. Am J Physiol Endocrinol Metab 2006; 290: E149–E153. [DOI] [PubMed] [Google Scholar]
- 38.Segel SA, Fanelli CG, Dence CS, et al. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes 2001; 50: 1911–1917. [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Liu P, Pascual JM, et al. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab 2012; 32: 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 1988; 68: 649–742. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt LI, Wimmer RD, Nakajima M, et al. Thalamic amplification of cortical connectivity sustains attentional control. Nature 2017; 545: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinomura S, Larsson J, Gulyas B, et al. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996; 271: 512–515. [DOI] [PubMed] [Google Scholar]
- 43.Sturm W, de Simone A, Krause BJ, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia 1999; 37: 797–805. [DOI] [PubMed] [Google Scholar]
- 44.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 2002; 357: 1695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999; 877: 383–396. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 1995; 363: 642–664. [DOI] [PubMed] [Google Scholar]
- 47.Allen KV, Pickering MJ, Zammitt NN, et al. Effects of acute hypoglycemia on working memory and language processing in adults with and without type 1 diabetes. Diabetes Care 2015; 38: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]