Abstract
Osimertinib is a tyrosine kinase inhibitor (TKI) of the mutated epidermal growth factor receptor (EGFRm) with observed efficacy in patients with brain metastases. Brain exposure and drug distribution in tumor regions are important criteria for evaluation and confirmation of CNS efficacy. The aim of this PET study was therefore to determine brain distribution and exposure of 11C-labelled osimertinib administered intravenously in subjects with an intact blood–brain barrier. Eight male healthy subjects (age 52 ± 8 years) underwent one PET measurement with 11C-osimertinib. The pharmacokinetic parameters Cmax (brain) (standardized uptake value), Tmax (brain) and AUC0–90 min brain/blood ratio were calculated. The outcome measure for 11C-osimertinib brain exposure was the total distribution volume (VT). 11C-osimertinib distributed rapidly to the brain, with higher uptake in grey than in white matter. Mean Cmax, Tmax and AUC0–90 min brain/blood ratio were 1.5 (range 1–1.8), 13 min (range 5–30 min), and 3.8 (range 3.3–4.1). Whole brain and white matter VT were 14 mL×cm−3 (range 11–18) and 7 mL×cm−3 (range 5–12). This study in healthy volunteers shows that 11C-osimertinib penetrates the intact blood–brain barrier. The approach used further illustrates the role of molecular imaging in facilitating the development of novel drugs for the treatment of malignancies affecting the brain.
Keywords: Brain metastasis, microdose, PET, epidermal growth factor receptor, blood–brain barrier
Introduction
Lung cancer has long been the most common cause of death from cancer in the world. Of all lung cancers, non-small cell lung cancer (NSCLC) is the most common and represents 80% to 85% of all cases. At time of diagnosis, approximately 70% of patients with NSCLC already have locally advanced or metastatic disease not amenable to surgical resection.1
During the past decade, the understanding of the critical role of the epidermal growth factor receptor (EGFR) pathway and subsequent development of EGFR-targeted tyrosine kinase inhibitors (EGFR-TKI) have led to significant advancements in the treatment of NSCLC. EGFR-TKIs are now the established first line therapy in patients with NSCLC having sensitizing mutations in EGFR (EGFRm), the most common of which are L858R and deletions in exon 19 [Ex19del].2,3 Selective inhibition of EGFR-TKI has demonstrated objective response in approximately 70% of patients with advanced NSCLC harboring the sensitivity mutations.
The two major problems in NSCLC are that albeit an initial response to first or second generation EGFR-TKIs, patients subsequently develop resistance to therapy, with a median time to progression of ∼10 months.4 In about 30–40% of cases, patients also develop brain metastases during treatment with EGFR-TKIs.5–9 In approximately 50% of those initially responsive to first or second generation EGFR-TKI patients, disease progression is associated with the emergence of a secondary EGFR mutation, T790M in exon 20 of the EGFR that confers resistance to therapy.10
Osimertinib is a potent, oral and irreversible third-generation EGFR-TKI that has shown to provide clinical benefit to patients with advanced NSCLC harboring the single sensitivity mutations as well as the resistance mutation following prior therapy with first or second generation EGFR-TKIs.11,12 Osimertinib has shown efficacy superior to that of first generation EGFR-TKIs (erlotinib and gefitinib) in the first-line treatment of EGFR mutation-positive advanced NSCLC (median PFS of 18.9 months compared to 10 months with erlotinib/gefitinib) and also a reduced risk of metastases.4,13,14 Importantly, a number of clinical reports has described that osimertinib can provide rapid and sustained effect on brain metastases in individual cases13,15–17 and reduced risk of CNS progression compared with standard EGFR-TKIs.18 Thus, further understanding of the pharmacological properties of osimertinib that enable clinical efficacy for the treatment of CNS metastases is warranted.
In experimental animals, osimertinib has demonstrated higher brain exposure than gefitinib or erlotinib at clinically relevant doses and has, moreover, been found to induce sustained tumor regression in an EGFR mutation-positive PC9 (exon 19 deletion) mouse brain metastases model.19 PET studies performed in non-human primates using 11C-osimertinib (N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-11C-methoxy-5-(4-(1-methyl-1H-indol-3-yl)pyrimidin-2-ylamino)phenyl)acrylamide) have also shown brain exposure at the level of established CNS-drugs.19,20 This paradigm, applied to human subjects, might provide important data to further evaluate the effect of osimertinib in patients with brain metastases including those with leptomeningeal metastases.
The aim of the present study was to examine the blood–brain barrier (BBB) penetration, distribution and brain exposure of 11C-osimertinib in healthy human subjects who have an intact BBB. For this purpose, 11C-osimertinib was administered intravenously in microdoses (1.1–1.4 μg) to eight male subjects. The exposure and regional distribution of 11C-osimertinib in brain was measured with a high-resolution PET system and quantified using standard pharmacokinetic parameters as well as a kinetic analysis with compartmental modeling.
Methods
Subjects
Eight healthy male subjects (mean age 52 years, range 44–62 years) participated in the study. The subjects were recruited through advertisement in a local newspaper. They were healthy according to medical history, physical examination, ECG, vital signs, routine blood laboratory health screen and urine test for drug abuse. All subjects had normal collateral circulation between radial and ulnar arteries in at least one hand assessed with the Allen's test. No anatomical brain abnormalities were detected by magnetic resonance imaging (MRI). The study was approved by the Ethical Committee of the Stockholm region, by the Radiation Safety Committee of the Karolinska University Hospital, and by the Medical Products Agency in Sweden (Trial registration: Clinical trial EudraCT no. 2016-004160-19). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all subjects who participated in the study.
Magnetic resonance imaging
MRI scans were performed with a GE DISCOVERY MR750 system (General Electric, USA). The MR protocol included T2-weighted and FLAIR sequences used for clinical evaluation and exclusion of pathology. The third T1-weighted sequence was used for delineation of anatomically defined brain regions of interests (ROIs). The T1-weighted sequence was a 3D FSPGR BRAVO with the following parameters: TR: 8.1 ms, TE: 3.2 ms, FOV: 256 mm, Matrix: 256 × 256, Slice thickness: 1.0 mm, Pixel size: 1 mm × 1 mm, Flip angle: 12, Prep Time: 450 ms, NEX: 1.
PET experimental procedures
Individualized plaster helmets were made for each subject and used with a head fixation system. The subject was placed recumbent with his head in the PET system. A cannula was inserted into the left or right cubital vein and another cannula into the radial artery on the opposite side. A sterile physiological phosphate buffer (pH 7.4) solution containing 11C-osimertinib was injected as a bolus within 10 s into the antecubital vein. The cannula was then immediately flushed with 10 ml saline.
PET measurements were performed with a high-resolution research tomograph (HRRT) system (Siemens Medical Imaging). List mode data were acquired for 93 min and reconstructed into frames of increasing duration (9 × 10 s, 2 × 15 s, 3 × 20 s, 4 × 30 s, 4 × 1 min, 4 × 3 min, 12 × 6 min) using the ordinary Poisson-3D-ordered subset expectation maximization algorithm, with 10 iterations and 16 subsets including modelling of the point spread function. The corresponding in-plane resolution with ordinary Poisson-3D-ordered subset expectation maximization point spread function was 1.5 mm in the centre of the field of view and 2.4 mm at 10-cm off-centre directions.21 Attenuation correction was acquired with a 6-min transmission measurement using a single 137Cs source.
Arterial blood sampling was performed automatically using a blood sampling system (ABSS, Allogg, Sweden) for the first 10 min and manually thereafter. Region-of-interest analysis was done using the automated anatomical labeling template.
Radiolabelling of osimertinib
No-carrier-added 11C–CH4 was produced using 16.5 MeV protons in the 14N(p,α)11C nuclear reaction on a mixture of nitrogen and hydrogen gas (10% hydrogen). 11C–CH4 was converted to 11C–CH3I by radical iodination in a gas-phase recirculation system and swept in a stream of helium through a heated glass column containing silver triflate impregnated on graphpac to produce 11C–CH3OTf. The radiolabeling agent was bubbled through a solution of precursor (AZ13774738, 0.6–1.0 mg) and aqueous sodium hydroxide (0.5 M, 5 µL) in acetone (400 µL) at room temperature. After 3 min, the reaction mixture was diluted with mobile phase and purified by semi-preparative HPLC using a reversed-phase C-18 column (ACE-C18, 5 µm, 7.8 × 300 mm, Waters) with MeCN/ammonium formate 45:55 (v/v) and sodium ascorbate (0.5 g/L) as eluent at 6 mL/min. The column outlet was connected with an UV absorbance detector (λ = 254 nm) in series with a detector for radioactivity. The purified product was diluted in sterile water (50 mL) containing sodium ascorbate (1 mg/mL) and was subsequently loaded on to an Oasis® HLB 1cc cartridge (Waters) previously conditioned with ethanol (10 mL) and sterile water containing sodium ascorbate (10 mL, 1 mg/mL). After trapping the product, the cartridge was rinsed with sterile water containing sodium ascorbate (8 mL, 1 mg/mL) and 11C-osimertinib was then eluted with 30% ethanol in propylene glycol (3 mL) into 12 ml phosphate-buffered saline (12 mL; pH 7.4) and sodium ascorbate (100 mg). The solution was filtered through a Millex-GV filter unit (0.22 μm) (Millipore, Billerica, MA, USA).
The radiochemical purity was >99%. The mean and standard deviation of the administered mass of 11C-osimertinib was 1.26 ± 0.12 μg (range 1.1 to 1.4 μg). The mean administered radioactivity was 347 ± 51 MBq (range 285 to 435 MBq) and the mean molar activity at time of injection was 141 ± 23 GBq/μmol (range 127 to 182 GBq/μmol).
Radiometabolite analysis
The unchanged fraction 11C-osimertinib and its radioactive metabolites in human plasma were measured using a protein precipitation sample preparation technique in combination with a reversed-phase radio-HPLC method. The blood samples (2 mL) were taken 2, 6, 10, 20 and 30 min after injection of 11C-osimertinib and plasma (0.7 mL) was separated from blood by centrifuging at 6000g for 2 min. The obtained plasma was mixed with 0.98 mL of acetonitrile and centrifuged at 2000g for 4 min and the supernatant was diluted with 2 mL water and injected into an on-line radio-HPLC system to determine the fraction. The amount of radioactivity in blood, in plasma and left in precipitate was measured separately with a NaI(Tl) well counter (PerkinElmer 2480 Wizard2™, Waltham, MA, USA).
An Agilent 1200 series HPLC instrument with a manual injection valve (7725i, Rheodyne), 5.0 ml loop and a radiation detector (Oyokoken, S-2493Z) housed in a shield of 50 mm thick lead was used for metabolite measurements (the accumulation time of radiation detector was 10 s). Data collection and control of the LC system were performed using chromatographic software (ChemStation Rev. B.04.03; Agilent). Chromatographic separation was performed with an ACE 5 C18-HL column, (250 mm × 10 mm I.D) by gradient elution. Acetonitrile (A) and 10 mM ammonium format (B) were used as the mobile phase at 5.0 mL/min, according to the following program: 0−3.0 min, (A/B) 60:40 → 80:20 v/v; 3.0−5.0 min, (A/B) 80:20 v/v; 5.1−6.0 min, (A/B) 90:10 v/v. The area of each obtained peak in the radiochromatogram was expressed as a percentage of the sum of the areas of all detected radioactive peaks (with decay correction).
Calculations
Several descriptive pharmacokinetic parameters were calculated. These include Cmax (brain), Tmax (brain) and AUC0–90 min, brain/blood ratio. In addition, the standard uptake value (SUV = [kBq • cm−3/(MBq injected/body weight]) and the percentage of the injected dose (ID%) in brain at Tmax were calculated.
Moreover, a quantitative analysis of 11C-osimertinib brain exposure was performed using two-tissue (2T) and one-tissue (1T) compartment models (CM) for reversible radioligand binding, as well as using the 2TCM for irreversible radioligand binding, i.e. the rate constant k4 was set to zero (Supplementary Figure S1 and Table S1).22 For that purpose, the arterial plasma curve was corrected for radioactive metabolites and used as input function. Correction for radioactive metabolites was performed using a population-based approach. The individual data of the fraction of parent radioligand in the plasma were fitted in a hierarchical Bayesian framework with individual and population level parameters, with weakly informative priors, to ensure consistency across individual fitted and extrapolated curves.23 The software used for fitting was CmdStan.24,25 In this framework, the parent fraction data were described by an empirical model curve consisting of a linear mixture of the Hill and Richards equations.26 Blood volume correction was performed assuming a blood volume fraction of 5%. The primary outcome measure of the compartment analysis was the total distribution volume (VT), which is the ratio at steady state between the concentration of the radioligand in brain and the concentration of the radioligand in plasma. The compartment analyses were performed using PMOD v 3.2. The Akaike information criterion27 and F statistics were used to identify the statistically preferred model configuration.
Results
All eight subjects participated in the study according to the protocol. There were no adverse or clinically detectable pharmacologic effects in any of the eight subjects. One subject experienced an episode of vaso-vagal reaction after placement and removal of the arterial cannula. No significant changes in vital signs were otherwise observed. For technical reasons, part of the list mode PET data was missing in one subject. This subject was excluded from the kinetic analysis.
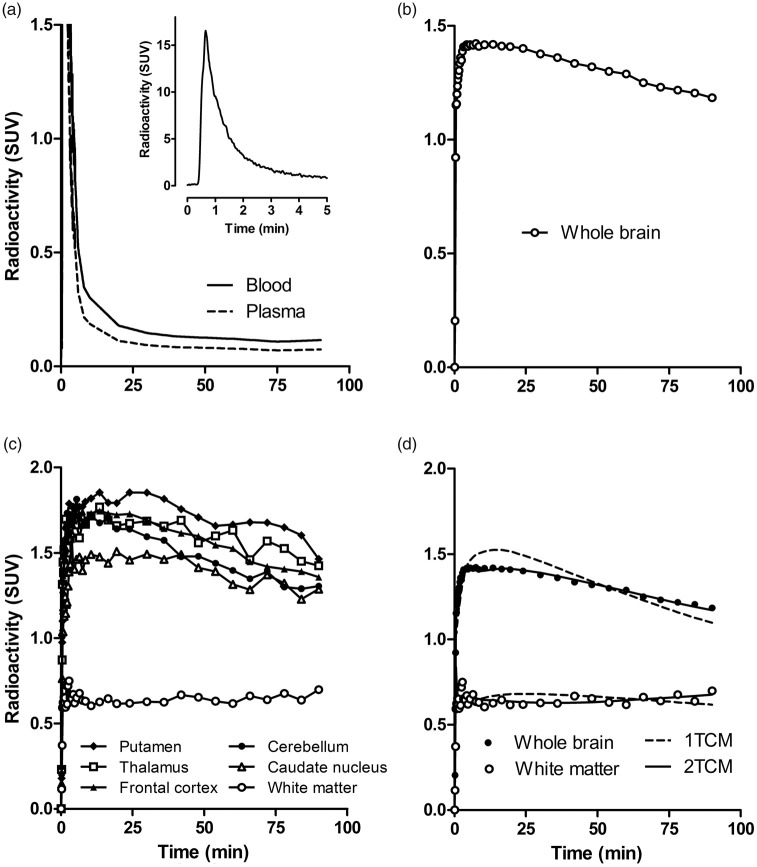
After i.v. administration of 11C-osimertinib, radioactivity appeared rapidly in brain (Figure 1). Injected radioactivity in blood reached its peak within 1 min after radioligand injection, followed by a rapid decrease during the initial 5 min of measurement. The rate of elimination of radioactivity from plasma was slow at later time (25–90 min) after injection (Figure 2(a)).
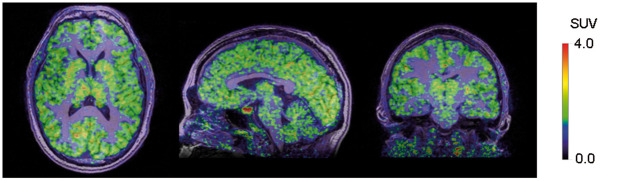
Figure 1.
Representative PET images for 11C-osimertinib overlaid on an MRI in a human subject.
Figure 2.
Time activity curves for 11C-osimertinib in a human subject (subject 4). (a) Total radioactivity concentration in blood and radioactivity concentration for parent radioligand in plasma. Inset shows blood radioactivity concentration during the initial five minutes after injection. Whole brain (b) and regional radioactivity (c). Experimental values for the whole brain and white matter with corresponding fitted curves as obtained by the 1TCM and 2TCM (d).
The mean Tmax was 13 min (range 5–30 min), a time at which 2.2 ± 0.2% of injected radioactivity was in brain. The Cmax was 1.5 ± 0.2 SUV (range 1–1.8) (Figure 2(b)), and the AUC0–90 min brain/blood ratio was 3.8 ± 0.3 (range 3.3–4.1).
Brain radioactivity was generally higher in grey matter than in white matter (Figures 1 and 2(c)). Among grey matter regions, 11C-osimertinib binding was highest in putamen followed by thalamus, frontal cortex, temporal cortex, caudate, and cerebellum (Figure 2(c)).
The HPLC analysis of unchanged 11C-osimertinib and radioactive metabolites was only performed up to 30 min due to the low radioactivity concentration in the plasma samples. The fraction of unchanged 11C-osimertinib in plasma was 73 ± 8% at 30 min.
The metabolite-corrected arterial input function generated with the population-based approach (Supplementary Figure S2) was used for the subsequent compartment analyses. Based on AIC and F statistics, the 2TCM was statistically preferred over the 1TCM for whole brain and gray matter regions, whereas the 1TCM was sufficient to describe curves for white matter (Figure 2(d); Supplementary Table S2). The regional influx rate constant, K1, the rate constants k2, k3, and k4, and VT values are presented in Table 1. Mean VT of the whole brain was 14 mL × cm−3 (range 11–18). Among gray matter regions, VT was highest for the putamen (17 mL × cm−3, range 13–24) and lowest for cerebellum (13 mL × cm−3, range 10–16). Mean VT of white matter was 7 mL × cm×3 (range 5–12).
Table 1.
Rate constants (K1, k2, k3, k4) and total volume of distribution (VT) for 11C-osimertinib obtained using a two-tissue compartment model.
| Brain region | K1 (mL·cm−3·min−1) | k2 (min−1) | k3 (min−1) | k4 (min−1) | VT (mL·cm−3) |
|---|---|---|---|---|---|
| Caudate nucleus | 0.16 (0.11–0.18) | 0.03 (0.01–0.05) | 0.13 (0.02–0.21) | 0.34 (0.05–1.99) | 15 (11–21) |
| Cerebellum | 0.22 (0.14–0.24) | 0.13 (0.09–0.18) | 0.28 (0.15–0.40) | 0.04 (0.03–0.04) | 13 (10–16) |
| Frontal cortex | 0.21 (0.14–0.23) | 0.09 (0.04–0.28) | 0.27 (0.12–0.58) | 0.08 (0.03–0.24) | 15 (12–22) |
| Insular cortex | 0.21 (0.13–0.30) | 0.33 (0.06–1.62) | 0.49 (0.12–1.62) | 0.03 (0.02–0.05) | 16 (12–23) |
| Limbic cortices | 0.18 (0.12–0.21) | 0.11 (0.05–0.20) | 0.29 (0.16–0.55) | 0.04 (0.03–0.06) | 14 (10–19) |
| Occipital cortex | 0.30 (0.21–0.37) | 0.73 (0.10–1.78) | 0.96 (0.23–1.84) | 0.03 (0.02–0.05) | 16 (13–20) |
| Parietal cortex | 0.23 (0.18–0.27) | 0.18 (0.04–0.56) | 0.41 (0.11–0.87) | 0.05 (0.03–0.08) | 16 (13–22) |
| Putamen | 0.21 (0.14–0.24) | 0.06 (0.02–0.16) | 0.21 (0.06–0.59) | 0.07 (0.04–0.21) | 17 (13–24) |
| Temporal cortex | 0.20 (0.13–0.24) | 0.14 (0.06–0.41) | 0.37 (0.15–0.97) | 0.04 (0.03–0.08) | 16 (12–22) |
| Thalamus | 0.23 (0.14–0.34) | 0.38 (0.03–1.91) | 0.51 (0.14–2.07) | 0.04 (0.02–0.06) | 16 (13–23) |
| Whole brain | 0.17 (0.12-0.19) | 0.08 (0.04-0.14) | 0.19 (0.11-0.29) | 0.04 (0.03-0.07) | 14 (11-18) |
Note: Data are presented as mean and range for seven subjects.
For all subjects and regions examined, estimates of k4 were consistently above zero (Table 1). The standard 2TCM was statistically preferred over a 2TCM with irreversible binding to the second compartment, i.e. k4 set to zero (Supplementary Table S3).
Discussion
This PET-study was designed to measure the brain exposure of 11C-labelled osimertinib administered intravenously at microdoses in healthy subjects who have an intact BBB. The primary objective was to obtain quantitative pharmacokinetic parameters that provide a measure of BBB penetration and brain exposure. In addition, kinetic analysis with compartmental modeling was performed using a metabolite corrected arterial input function. Here, the quantitative outcome measure was the total distribution volume (VT) which indicates the ratio between the concentration of the radioligand in brain and plasma at steady state.
Pharmacokinetic analysis
The pharmacokinetic parameters obtained indicate that 11C-osimertinib crosses the blood–brain barrier rapidly, with a mean Tmax of 13 min, at which time approximately 2% of the injected radioactivity had entered the brain and was approximately four times higher than the blood exposure. The Tmax, Cmax and AUC0–90 min brain/blood ratio values were in the same range as those previously reported for reference CNS drugs using the same methodology in non-human primates.20 These pharmacokinetic observations and the similarities to established CNS-drugs support the view that osimertinib has excellent brain exposure and hence potential for the treatment of brain metastases in EGFRm NSCLC patients. The BBB penetration can of course be also dependent on the lipophilicity and protein binding of a drug. Osimertinib is a lipophilic compound (calculated LogP = 4.8) with a plasma protein binding of 94.7% measured in vitro.28 Previous studies have shown that the recovery of osimertinib in human plasma and human serum albumin is particularly low even using the equilibrium dialysis method.29 For this reason, the plasma protein binding was not measured in this study.
To examine the metabolism of 11C-osimertinib in vivo, HPLC analysis was performed on arterial plasma samples. The fraction of unchanged 11C-osimertinib in plasma was measured only for the first 30 min after injection, due to low plasma radioactivity, most likely related to the large volume of distribution of osimertinib.30 The unchanged radioligand represented approximately 70% of the total radioactivity in plasma at 30 min, suggesting that 11C-osimertinib is rather stable in plasma. In support of these observations, previous studies with 14C-labelled osimertinib performed in healthy volunteers at steady state after oral administration have indicated that no more than 10% of the total radioactivity is related to metabolites of osimertinib and that the Tmax of the two major metabolites was 33 and 42 h, respectively, after IV administration of osimertinib.30 Therefore, after IV administration of 11C-osimertinib, because of the slow kinetics of the two major metabolites, there is a low likelihood of observing relevant fractions of radiometabolites in plasma and brain within the short time frame of a PET measurement.
Brain distribution and quantification of 11C-osimertinib binding
The distribution of 11C-osimertinib in the brain of healthy volunteers showed that the radioactivity is distributed across all regions of the brain (Figures 1 and 2(c)). The kinetic analysis was performed using compartmental models for reversible and irreversible radioligand binding.
In all grey matter regions, the 2TCM with four rate constants (reversible model) was the statistically preferred model, indicating that the kinetics of 11C-osimertinib in the healthy brain can be described by two compartments with passive diffusion of the radioligand between compartments and without irreversible binding or metabolic trapping. Osimertinib has been shown in experimental animals to bind to the EGFR sensitizing mutation and the resistant T790M mutation in an irreversible manner.29 The reversible nature of the binding kinetics of 11C-osimertinib observed in the present study within the time frame of the PET measurements is reasonably explained by the lack of the EGFRm receptor in the brain of healthy volunteers.
The K1 values obtained by 2TCM were similar to those reported for established neuroreceptor radioligands such as 11C-raclopride31 and in line with the early Tmax of osimertinib. The lowest K1 value was obtained for the caudate (0.11 mL · cm−3 · min−1) and the highest for the occipital cortex (0.37 mL · cm−3 · min−1). In the remaining regions, K1 ranged between 0.12 and 0.30 mL · cm−3 · min−1. Assuming a cerebral blood flow of 50 mL ·100 g · min−1 and a density of brain tissue of 1 g · cm−3, the average first pass extraction of 11C-osimertinib for the whole brain would be approximately 30%. This estimate of first pass extraction is again similar to the one reported for 11C-raclopride31 and consistent with the presence of an intact BBB.
A population-based approach was used to obtain a metabolite-corrected arterial input function for the kinetic analysis. The whole brain VT was 14 mL · cm−3, indicating a high partition coefficient between brain and plasma, for instance in comparison with well-established radioligands such as 11C-raclopride (VT ∼0.3–2 mL · cm−3 depending on the brain region)32 or 11C-flumazenil (VT ∼3–6 mL · cm−3 depending on the brain region).33 Regional VT values ranged between 13 and 17 mL · cm−3, indicating that 11C-osimertinib is rather homogeneously distributed in gray matter.
The reversible type of kinetic behavior observed for 11C-osimertinib is a common observation for radioligands that bind specifically to brain receptors, transporters or enzymes. In such setting, the two compartments are thought to represent non-displaceable and specific binding. On the other hand, in white matter, the preferred quantification model was the 1TCM. This observation does not support the existence of a specific binding compartment in white matter. Taken together, it cannot be excluded that a fraction of 11C-osimertinib binding in gray matter may represent specific binding to non-mutated or wild-type EGFR.
Specific binding of osimertinib
Biochemical characterization of the drug has shown that osimertinib displays nearly 200-fold greater affinity for the EGFR with T790M/L858R mutation (apparent IC50 of 1 nM) than for the EGFR wild-type (apparent IC50 184 nM).12 EGFR is widely expressed in the normal brain in neurons (cortical pyramidal cells and Purkinje cells of cerebellum) and ependymal cells.34,35 The broad distribution of 11C-osimertinib in the human brain is therefore consistent with the wide expression of the EGFR. However, considering the low apparent affinity of osimertinib for the wild-type EGFR, it cannot be excluded that the uptake observed in the healthy brain could be non-specific.
Future directions
This study has examined the pharmacokinetic properties of 11C-osimertinib in healthy subjects. Considering the affinity of osimertinib towards the T790M/L858R EGFRm+, an ongoing study of the kinetic behavior of 11C-osimertinib in patients with metastatic NSCLC and brain metastases (NCT03463525) will provide additional information on the pharmacodynamic potential of osimertinib for the treatment of brain metastases.
Conclusions
This study showed that 11C-osimertinib enters the brain rapidly with an exposure of 2% of injected dose at Tmax (range from 1.7 to 2.4%). This brain exposure is similar to that for well-established CNS drugs. The pharmacokinetic observations of the present study, the similarity with established CNS-drugs, along with recent evidence of CNS efficacy, support the view that osimertinib has potential for the treatment of EGFRm-positive NSCLC patients with brain metastases. The approach presented in this study further illustrates the role of molecular imaging in facilitating the development of novel drugs for the treatment of malignancies.
Supplemental Material
Supplemental Material for A PET study in healthy subjects of brain exposure of 11C-labelled osimertinib – A drug intended for treatment of brain metastases in non-small cell lung cancer by Andrea Varrone, Katarina Varnäs, Aurelija Jucaite, Zsolt Cselényi, Peter Johnström, Magnus Schou, Ana Vazquez-Romero, Mohammad M Moein, Christer Halldin, Andrew P Brown, Karthick Vishwanathan and Lars Farde in Journal of Cerebral Blood Flow & Metabolism
Acknowledgments
The authors thank the staff of the PET-Centre at Karolinska Institutet for their technical support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by AstraZeneca, project number: ESR-17-12920.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andrea Varrone has received research grants from the Swedish Science Council, the Michael J Fox Foundation, Vinnova, Åhlens Foundation, a private donation and the Swedish Brain Foundation. Aurelija Jucaite, Zsolt Cselenyi, Peter Johnström, Magnus Schou, Andrew P Brown, Karthick Vishwanathan and Lars Farde are employed and own stocks of AstraZeneca. Katarina Varnäs has received a consulting fee from AstraZeneca. Christer Halldin, Ana Vazquez-Romero, and Mohammad Mahdi Moein declare no conflict of interest.
Authors' contributions
The specific contribution of each author is described as follows: Andrea Varrone: Contributing to conception and design, acquisition of data, interpretation of data; drafting and revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Katarina Varnäs: Contributing to analysis and interpretation of data; drafting and revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Aurelija Jucaite: Contributing to conception and design, interpretation of data; drafting and revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Zsolt Cselényi: Contributing to conception and design, analysis and interpretation of data; revising the manuscript; approving the final content of the manuscript. Peter Johnström: Contributing to conception and design, acquisition of data, interpretation of data; drafting and revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Magnus Schou: Contributing to conception and design, acquisition of data, interpretation of data; revising the manuscript; approving the final content of the manuscript. Ana Vazquez-Romero: Contributing to acquisition of data; drafting the manuscript; approving the final content of the manuscript. Mohammad Mahdi Moein: Contributing to acquisition, analysis and interpretation of data; drafting the manuscript; approving the final content of the manuscript. Christer Halldin: Contributing to interpretation of data; revising the manuscript and approving the final content of the manuscript. Andrew P Brown: Contributing to conception and design, interpretation of data; revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Karthick Vishwanathan: Contributing to conception and design, interpretation of data; revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript. Lars Farde: Contributing to conception and design, interpretation of data; revising the manuscript, as well as enhancing its intellectual content; approving the final content of the manuscript.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Bonomi PD. Implications of key trials in advanced nonsmall cell lung cancer. Cancer 2010; 116: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 2.Heydt C, Michels S, Thress KS, et al. Novel approaches against epidermal growth factor receptor tyrosine kinase inhibitor resistance. Oncotarget 2018; 9: 15418–15434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahluwalia MS, Becker K, Levy BP. Epidermal growth factor receptor tyrosine kinase inhibitors for central nervous system metastases from non-small cell lung cancer. Oncologist 2018; 23: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vansteenkiste J, Reungwetwattana T, Nakagawa K, et al. CNS response to osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFR-TKI sensitising mutation (EGFRm)-positive advanced non-small cell lung cancer (NSCLC): data from the FLAURA study. Ann Oncol 2017; 28: x189. [Google Scholar]
- 5.Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007; 242: 882–888. [DOI] [PubMed] [Google Scholar]
- 6.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010; 16: 5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrieta O, Villarreal-Garza C, Zamora J, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011; 6: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol 2014; 4: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015; 88: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005; 2: e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 2014; 57: 8249–8267. [DOI] [PubMed] [Google Scholar]
- 12.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014; 4: 1046–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koba T, Kijima T, Takimoto T, et al. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation: two case reports. Medicine 2017; 96: e6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 15.Reichegger H, Jochum W, Forbs D, et al. Rapid intracranial response to osimertinib in a patient with epidermal growth factor receptor T790M-positive adenocarcinoma of the lung. Oncol Res Treat 2016; 39: 461–463. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers A, Jensen L, Akerley W. Durable response to osimertinib in EGFR mutated T790M wildtype non-small cell lung cancer with leptomeningeal metastases: a case report. Lung Cancer 2017; 114: 68–69. [DOI] [PubMed] [Google Scholar]
- 17.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018; 36: 2702–2709. [DOI] [PubMed] [Google Scholar]
- 18.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 2018; 36: 3290–3297. [DOI] [PubMed] [Google Scholar]
- 19.Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130–5140. [DOI] [PubMed] [Google Scholar]
- 20.Schou M, Varnäs K, Lundquist S, et al. Large variation in brain exposure of reference CNS drugs: a PET study in nonhuman primates. Int J Neuropsychopharmacol 2015; 18: pyv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varrone A, Sjöholm N, Eriksson L, et al. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging 2009; 36: 1639–1650. [DOI] [PubMed] [Google Scholar]
- 22.Varnäs K, Varrone A, Farde L. Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn 2013; 40: 267–279. [DOI] [PubMed] [Google Scholar]
- 23.Gelman A, Carlin JB, Stern HS, et al. Hierarchical models. Bayesian data analysis. Boca Raton, FL, USA: CRC Press Taylor & Francis Group, 2013, pp.101–138.
- 24.Carpenter B, Gelman A, Hoffman MD, et al. Stan: a probabilistic programming language. J Stat Software 2017; 76: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stan Development Team. CmdStan: the command-line interface to Stan, 2015. Available at: https://mc-stan.org/users/interfaces/cmdstan.
- 26.Bindslev N. Hill in hell. Drug-acceptor interactions modeling theoretical tools to test and evaluate experimental equilibrium effects. Routledge: Taylor and Francis Group, 2008, pp.257–282.
- 27.Akaike H. A new look at the statistical model identification. IEEE Transac Automatic Control 1974; 19: 716–723. [Google Scholar]
- 28.European Medicine Agency. Tagrisso – product information, www.ema.europa.eu/en/medicines/human/EPAR/tagrisso (2016, accessed 17 February 2016).
- 29.Dickinson PA, Cantarini MV, Collier J, et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos 2016; 44: 1201–1212. [DOI] [PubMed] [Google Scholar]
- 30.Vishwanathan K, So K, Thomas K, et al. Absolute bioavailability of osimertinib in healthy adults. Clin Pharmacol Drug Dev 2019; 8: 198–207. [DOI] [PubMed] [Google Scholar]
- 31.Farde L, Eriksson L, Blomquist G, et al. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET – a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 1989; 9: 696–708. [DOI] [PubMed] [Google Scholar]
- 32.Schain M, Fazio P, Mrzljak L, et al. Revisiting the Logan plot to account for non-negligible blood volume in brain tissue. EJNMMI Res 2017; 7: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odano I, Halldin C, Karlsson P, et al. [18F]flumazenil binding to central benzodiazepine receptor studies by PET – quantitative analysis and comparisons with [11C]flumazenil. Neuroimage 2009; 45: 891–902. [DOI] [PubMed] [Google Scholar]
- 34.Birecree E, King LE, Jr., Nanney LB. Epidermal growth factor and its receptor in the developing human nervous system. Brain Res Dev Brain Res 1991; 60: 145–154. [DOI] [PubMed] [Google Scholar]
- 35.Werner MH, Nanney LB, Stoscheck CM, et al. Localization of immunoreactive epidermal growth factor receptors in human nervous system. J Histochem Cytochem 1988; 36: 81–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A PET study in healthy subjects of brain exposure of 11C-labelled osimertinib – A drug intended for treatment of brain metastases in non-small cell lung cancer by Andrea Varrone, Katarina Varnäs, Aurelija Jucaite, Zsolt Cselényi, Peter Johnström, Magnus Schou, Ana Vazquez-Romero, Mohammad M Moein, Christer Halldin, Andrew P Brown, Karthick Vishwanathan and Lars Farde in Journal of Cerebral Blood Flow & Metabolism