Abstract
The lack of inclusion of comorbidities in animal models of stroke may underlie the limited development of therapy in stroke. Previous studies in mice deficient of CD36, an immune receptor, indicated its contribution to stroke-induced inflammation and injury in hyperlipidemic conditions. The current study, therefore, tested whether pharmacological inhibition of CD36 provides neuroprotection in hyperlipidemic stroke. The hyperlipidemic mice subjected to stroke showed an exacerbation of infarct size and profound brain swelling. However, post-stroke treatment with CD36 inhibitors did not reduce, and in some cases worsened, acute stroke outcome, suggesting potential benefits of elevated CD36 in the post-stroke brain in a hyperlipidemic condition. On the other hand, chronic treatment of a CD36 inhibitor prior to stroke significantly reduced stroke-induced brain swelling. There was a trend toward infarct reduction, although it did not reach statistical significance. The observed benefit of preventative CD36 inhibition is in line with previously reported smaller infarct volume and swelling in CD36 KO mice. Thus, the current findings suggest that insights gained from the genetic models should be carefully considered before the implementation of pharmacological interventions, as a potential therapeutic strategy may depend on preventative treatment or a post-stroke acute treatment paradigm.
Keywords: Brain swelling, CD36 inhibition, hyperlipidemia, ischemic stroke, salvianolic acid B
Introduction
Stroke-induced brain injury leads to severe physical disability associated with increased mortality. Despite tremendous effort, clinical trials based on acute neuroprotection strategies have been unsuccessful (http://www.strokecenter.org/trials/).1 To overcome this translational roadblock, inclusion of comorbidities in the experimental animal models of stroke has emerged to closely mimic the conditions in patients.2–8 However, it would be necessary to use the animal models with comorbidities to validate the molecular targets in order to develop an effective stroke therapy.
CD36 is a class B scavenger receptor initially discovered in platelets and expressed in various cell types including microglia, astrocytes, and microvascular endothelium in brain and monocytes/macrophages, cardiac and skeletal muscle, adipocytes, and epithelia of retina, breast, and kidney in periphery. By binding with several ligands such as oxidized or modified low density lipoprotein (LDL), long-chain fatty acid, thrombospondin-1 and -2, fibrillar β-amyloid, and apoptotic cells, it plays multiple roles in oxidative stress, inflammation, innate immunity, and apoptosis.9–11 Past studies have demonstrated an association between CD36 function and stroke pathology. Accordingly, we previously reported a contributing role of CD36 in stroke-induced inflammation and brain injury by demonstrating attenuated acute stroke outcome in CD36 KO mice.12 Furthermore, the absence of CD36 in a hyperlipidemia model profoundly reduced brain swelling through CD36-mediated foam cell formation, a key step for atherosclerotic lesion development.4,13 Given the fact that CD36 functions in uptake of oxidized lipid ligands and fatty acid translocase,14–16 our findings indicate that CD36 could serve as a potential target to improve stroke outcome in hyperlipidemic conditions.
Several pharmacological inhibitors of CD36 have been identified. We have previously shown that treatment of mice with the SS31 peptide, a new class of antioxidants, attenuated ischemia-induced glutathione (GSH) depletion in the cortex and reduced infarct size in normal metabolic mice. The protective effect of SS31 was abrogated in CD36 KO mice, indicating SS31 acts through CD36-mediated pathways.17 Hexarelin, an hexapeptide belonging to the family of growth hormone-releasing peptides (GHRPs), also has been reported to reduce CD36-mediated uptake of oxLDLs through binding site competition18 and resulted in a marked decrease in atherosclerotic lesions.19 Another established CD36 antagonist is salvianolic acid B (SAB). SAB is a polyphenolic antioxidant isolated from a Chinese herb, Danshen and was shown to enhance neurogenesis in ischemic stroke.20 SAB also reduces oxLDL uptake and prevents foam cell formation in vitro21 and reduces CD36 expression in macrophages or adipose tissue in hyperlipidemic or obese mice, establishing SAB as an effective in vivo CD36 antagonist.21,22
The findings from hyperlipidemic CD36 KO mice suggest that CD36 is a potential therapeutic target. To test this hypothesis, this study evaluated the effect of CD36 inhibitors against stroke in this comorbid condition. Here we report that, contrary to our hypothesis, post-stroke treatments of CD36 inhibitors did not provide any benefit and even resulted in worse outcomes in hyperlipidemic stroke. By contrast, a chronic CD36 inhibition prior to stroke significantly reduced stroke-induced brain swelling in the hyperlipidemic mice. Careful consideration prior to implementation of these genetically identified targets is suggested for strategizing pharmacological intervention.
Methods
Animals
Procedures for the use of animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medicine in accordance with the IACUC, National Institutes of Health, and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. C57BL/6 and ApoE knockout (ApoE KO, C57 background) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred at the institute’s animal facility that monitored and maintained temperature, humidity, and a 12-h light/dark cycle. A maximum of five mice were housed in a single cage with an individual ventilating system and irradiated bedding (The Anderson, Maumee, OH). Sterilized food and water were freely accessible in each cage.
In vivo study design
Mice were randomized to a specific diet and surgery. Animals’ identity and treatment were blinded to the persons who performed surgery, cut brains, and assessed outcomes. Sample size for stroke outcome measurements was calculated a priori (n = 8/group) by predicting detectable differences to reach power of 0.80 at a significance level of <0.05, assuming a 45% difference in mean and a 30% SD at the 95% confidence level.
Animal models of hyperlipidemia
Six-week-old male C57 and ApoE KO mice were fed a normal diet (ND) or Western diet (WD, 42% fat with 0.15% (wt/wt) cholesterol, 88137, Harlan Teklad, Madison, WI) for eight weeks.4,23 Plasma cholesterol levels were measured using a commercially available colorimetric assay kit (BioVision., Milpitas, CA) as previously reported.4,23
Transient middle cerebral artery occlusion
Mice were subjected to 30 min proximal middle cerebral artery occlusion (MCAO) using an intraluminal thread method according to previously reported methods.3,23 Briefly, mice were anesthetized with a mixture of isoflurane/oxygen/nitrogen and a 6–0 Teflon-coated black monofilament surgical suture (coating length, 5–6 mm; diameter, 0.23 or 0.25 mm, 602356PK10, 602556PK10, Doccol, Redland, CA) was inserted into the exposed external carotid artery, advanced into the internal carotid artery, and wedged into the Circle of Willis to obstruct the origin of MCA. The filament was advanced until we observed a sufficient drop of cerebral blood flow (CBF). Reperfusion was confirmed at the time of filament withdrawal. The CBF was measured by Laser-Doppler flowmetry (Periflux System 5010; Perimed, Järfälla, Sweden) prior to stroke, during occlusion period, and 10 min after reperfusion. Animals exhibiting greater than 80% reduction of pre-ischemic baseline CBF (20% of pre-stroke baseline) during MCAO and greater than 80% of pre-stroke baseline at 10 min of reperfusion were included in the study. Accordingly, 11.0% of animals were excluded in this study due to the insufficient CBF reduction and/or reperfusion. At three days after MCAO, animals were euthanized for histology and molecular analyses.
Post-stroke treatment of SS31, CD36 antibody, hexarelin, and SAB
Each CD36 targeting agent was dissolved in phosphate-buffered saline (PBS) and treated to separate cohorts of hyperlipidemic mice. SS31 (5 mg/kg) was treated by intraperitoneal (i.p.) injection at 0 h, 6 h, 24 h, and 48 h after MCAO. CD36 antibody (1 mg/kg, BD Biosciences, San Jose, CA) was administered intravenously using a retro-orbital injection at 0 h and 24 h-post ischemia. Hexarelin (GenScript, Piscataway, NJ) was initially administered at a dose of 100 µg/kg intravenously immediately after reperfusion with subsequent intraperitoneal injection at 6 h, 24 h, and 48 h-post stroke. SAB (Chengdu Biopurify Phytochemicals Ltd, Chengdu, China) was neutralized (adjusted to pH 7.0 with hydrochloric acid) and administered by i.p. at low (100 mg/kg) or high (500 mg/kg) dose at 0 h, 6 h, 24 h, and 48 h-post ischemic mice. Mouse IgA (ThermoFisher Scientific, Waltham, MA) was treated for isotype control of CD36 antibody and vehicle (PBS) was injected at the time of administration of SS31, hexarelin and SAB as controls.
Pre-stroke treatment of SAB
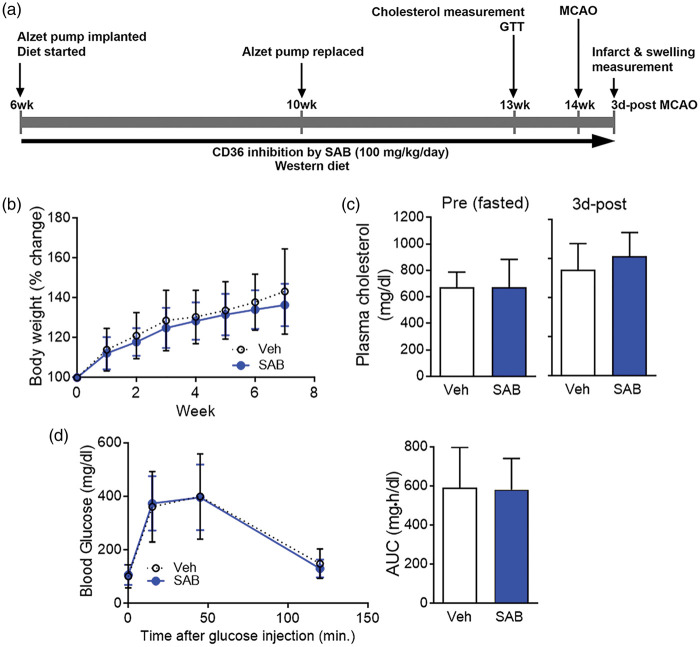
The ability of SAB to inhibit oxLDL uptake and its stability at 37℃ up to 1 month were tested for its stability during chronic administration using Alzet pumps.22 Six-week-old ApoE KO mice were implanted with Alzet mini pumps (Alzet Osmotic Pumps, Cupertino, CA) containing either vehicle or SAB (infusion rate, 100 mg/kg/day) under the dorsal skin. Animals were fed a WD for eight weeks. During the diet intervention, the Alzet pump was replaced at four weeks of diet. At seven weeks of diet, the glucose tolerance tests were performed and plasma was collected for cholesterol measurement. The mice were subjected to 30 m-MCAO at the end of eight weeks of diet (see the timeline in Figure 4(a)).
Figure 4.
SAB pre-treatment did not alter body weight gain, plasma cholesterol, and blood glucose levels in hyperlipidemic mice. Low dose (100 mg/kg/day) of SAB was infused by implanted Alzet pump in the ApoE KO (AKO) mice during diet intervention and several physiological parameters were determined in the mice before or after stroke. (a) Timeline for the SAB pre-treatment. (b) Body weight gain during diet intervention with SAB treatment. (c) Plasma cholesterol levels from the mice at the end of seven weeks of diet before (pre) and after (3 d-post) stroke. (d) GTT and the area under the curve (AUC) at seven weeks of diet intervention. Data are expressed as mean ± SD, n = 16–17/group for BW and GTT, n = 7–10/group for plasma cholesterol. Veh, WD/AKO mice implanted with vehicle Alzet pump; SAB, WD/AKO mice implanted with SAB Alzet pump.
Glucose tolerance test
Glucose tolerance tests were performed at seven weeks of WD intervention according to method described.8,22 Baseline blood glucose concentrations were measured in overnight fasted animals using a glucometer (Ascensia Contour, Bayer, Whippany, NJ, USA). D-glucose (2 mg/g body weight) was injected intraperitoneally and blood glucose levels were measured at 15, 30, 45, and 120 min.
Tissue section strategy
According to our previous study,3 sections were collected from the brain regions spanning about 7 mm rostrocaudal (+3.1 mm to −4.1 mm from bregma). The brain block containing an entire infarct region was serially cryosectioned at 20 µm thickness at 600 µm intervals, thus a total of 13 sections were used for infarct volume and swelling measurement. Tissues between the intervals were sectioned and collected for each hemisphere to perform molecular analyses. This unbiased sectioning strategy facilitated measurement of infarct/swelling and gene/protein in the same animals, thereby allowing correlation analyses between histological and molecular parameters.
Infarct volume and edema measurement
Infarct volume, corrected for brain swelling, was determined in the serial sections using a phase contrast method and analyzed by Axiovision software (Zeiss, Germany) as we previously reported.3,24 Infarct volume was determined by integrating the volume of each section obtained by infarct area (mm2) × distance (0.6 mm), which was then adjusted by the ratio of contralateral and ipsilateral size to normalize swelling components. To determine % infarct volume, the adjusted infarct volume was divided by the contralateral size. Percent hemispheric swelling (% HS) was calculated from the difference in volume between two hemispheres and then divided by contralateral hemispheric volume.25
IgG immunohistochemistry
Vascular permeability was determined by IgG immunohistochemistry.26 Frozen brain sections (+1.3 mm, +0.1 mm, and −1.1 mm from bregma) were fixed with 4% paraformaldehyde for 15 min and collected on gelatin-coated slides. After incubation in methanol containing 0.3% H2O2 for 30 min to block endogenous hydroperoxidase, the sections were incubated in 1% bovine serum albumin and 10% normal goat serum for 1 h. The sections were labeled with anti-mouse IgG antibody (1:1000, Vector Lab., Burlingame, CA) for overnight at 4℃. After washing with PBS, the sections were incubated with avidin/biotinylated peroxidase (ABC reagent, Vector Lab., Burlingame, CA) for 1 h at room temperature and colorized by incubating in diaminobenzidine (Sigma, MO). The IgG density was quantified using Image J software.
CD36 protein measurement
Total protein concentrations were measured by the Bradford method. Protein was loaded on a NuPAGE 4-12% Bis-Tris Gel (Life Technology) and transferred onto polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The membrane was incubated in blocking buffer (Li-cor, Lincoln, NE) for 1 h followed by overnight incubation at 4℃ with CD36 (For peritoneal macrophages, AF2519, 1:1000, R & D Systems, Minneapolis, MN; For brain, 2479S, 1:1000, Cell signaling, MA) or β-Actin (sc-1615, 1:10,000, Santa Cruz Biotechnology, Santa Cruz, CA) antibody in blocking buffer. The membrane was washed with tris-buffered saline containing 0.05% Tween-20 followed by incubating with appropriate secondary antibodies conjugated with Alexa Fluor 680 (A21088, Life Technologies) or IRDye® 680RD (926-68071, Li-cor) in blocking buffer for 1 h. Then each protein’s specific band was visualized using the Odyssey Imaging System (Li-cor). Western blots were performed in multiple gels. To normalize inter-blot variability, identical samples were loaded in each blot as an internal control and the density of the internal standard sample was used to standardize other samples in multiple blots.
Data analyses
Infarct volume and percent hemispheric swelling were reported as mean ± 95% confidence intervals. Body weights, blood glucose levels, CBF changes, and all other molecular analyses were presented as mean ± standard deviation (SD). Comparison between two groups was statistically evaluated using Student’s t-test. Multiple comparisons were made using analysis of variance (ANOVA). In analyses that involve more than one factor (i.e. effect of stroke (contralateral vs. ipsilateral) and effect of drug (Veh vs. SAB)), two-way ANOVA was used followed by a post hoc Bonferroni’s correction. Correlation analyses between infarct volume and % swelling were made using linear regression, and slope differences among groups were further analyzed. Differences were considered statistically significant at p < 0.05.
Results
Severe hyperlipidemia increases stroke-induced injury with profound brain swelling
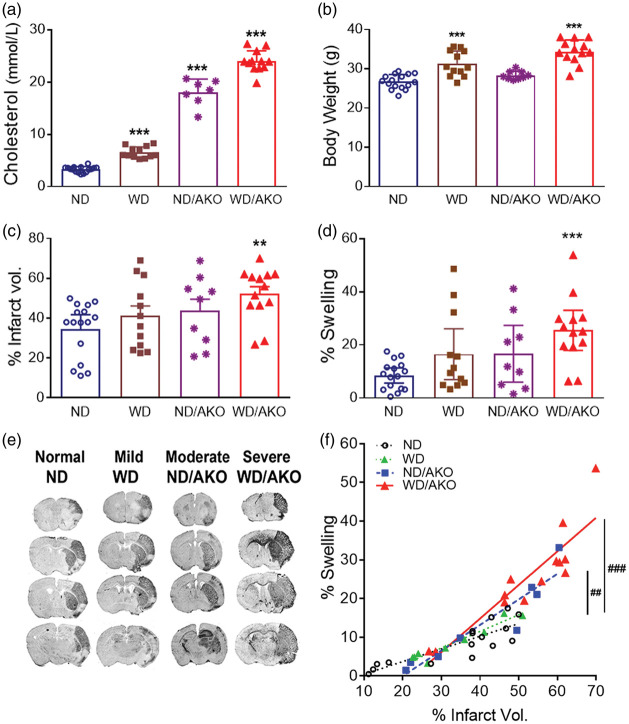
To determine the effect of hyperlipidemia on brain injury in stroke, hyperlipidemic mice were generated in C57 or ApoE KO mice were fed either a ND or high-fat/cholesterol diet (WD) for eight weeks. Elevated plasma cholesterol levels confirmed that genetic and diet interventions generated normal (ND), mild (WD), moderate (ND/AKO), and severe (WD/AKO) hyperlipidemia (Figure 1(a)). There were significant body weight gains in WD fed C57 and ApoE KO mice (Figure 1(b)). Stroke severity was similar among the groups with comparable CBF reduction during MCAO and reperfusion (CBF reduction (%), ND, 85.0 ± 4.9, WD, 83.7 ± 2.8; ND/AKO, 88.4 ± 5.1; WD/AKO, 86.8 ± 5.4; CBF reperfusion (%), ND, 139.3 ± 31.8, WD, 124.3 ± 23.5; ND/AKO, 121.6 ± 15.6; WD/AKO, 116.5 ± 25.8). Compared to normal mice (ND), severe hyperlipidemic mice (WD/AKO) demonstrated significant increases in % infarct volume (p < 0.01, Figure 1(c)) with profound brain swelling (p < 0.001, Figure 1(d)). Regardless of the severity of hyperlipidemia, there were significant correlation between % infarct volume and swelling in all groups (ND, r2 = 0.5947, p < 0.001; WD, r2 = 0.8674, p < 0.001; ND/AKO, r2 = 0.7231, p < 0.01; WD/AKO, r2 = 0.6785, p < 0.001, Figure 1(f)). The two most hyperlipidemic groups showed significant slope differences compared to the normal group (ND/AKO vs. ND, p < 0.01; WD/AKO vs. ND, p < 0.001, Figure 1(f)). The severe hyperlipidemic mice (WD/AKO) were used for further studies to investigate the effect of CD36 inhibition.
Figure 1.
Hyperlipidemia exacerbates stroke-induced injury with profound brain edema. Different degrees of hyperlipidemia were generated in C57BL/6 or ApoE KO (AKO) mice by feeding a normal (ND) or Western diet (WD). (a,b), Fasting blood cholesterol levels at seven weeks of diet (a) and body weight at the end of diet intervention (b). Data are expressed as mean ± SD, n = 7–16/group, ***p < 0.001 vs. ND, one-way ANOVA, (c, d), % infarct volume (c) and swelling (d) were determined at three days post-stroke brain. Data are expressed at the 95% confidence interval, n = 7–16/group, **,***p < 0.01, 0.001 vs. ND, one-way ANOVA (e), Images of serially sectioned brains (f), Correlation analyses between % infarct volume and swelling. n = 8–16/group, ##,###p < 0.01, 0.001 in correlation slope vs. ND. ND, normal diet fed C57BL/6; WD, Western diet fed C57BL/6; ND/AKO, normal diet fed AKO, WD/AKO, Western diet fed AKO, represent mild, moderate, and severe hyperlipidemic conditions.
Post-stroke CD36 inhibition is not effective to reduce ischemic injury in hyperlipidemic condition
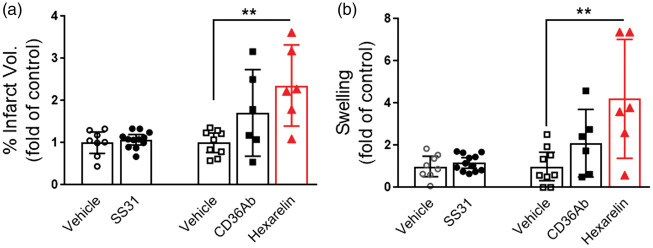
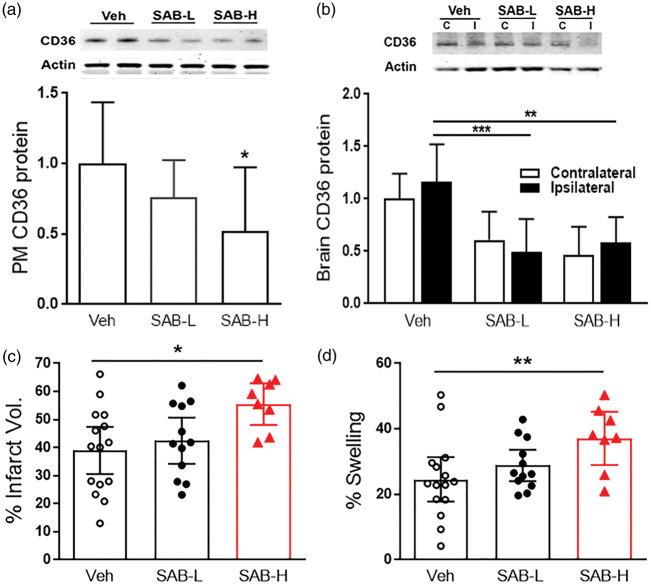
Previous studies in CD36 KO mice demonstrated that the absence of CD36 in hyperlipidemic conditions reduced stroke-induced brain injury and swelling.4,23 To corroborate the finding from the genetic CD36 KO mice, we further tested the effect of pharmacological inhibition of CD36 in reducing infarct size and swelling in hyperlipidemic stroke. We treated stroked mice with three CD36 inhibitors and assessed acute outcome at three days post-stroke. Compared to vehicle-treated mice, SS31 did not attenuate infarct volume and brain swelling in the severe hyperlipidemic mice (ApoE KO mice fed WD). Administration of the CD36 antibody and hexarelin resulted in either no effect or increased infarct size and swelling as compared to vehicle-treated hyperlipidemic mice (Figure 2), suggesting potential benefits of the elevated CD36 in the post-stroke brain in hyperlipidemic mice.4 Previously, we reported that SAB effectively reduces CD36-mediated lipid uptake in macrophages and adipocytes in non-stroked mice with severe hyperlipidemia.21,22 To determine the effect of SAB in hyperlipidemic stroke, the severe hyperlipidemic mice were subjected to focal ischemia, and treated with low- (100 mg/kg) or high-dose (500 mg/kg) of SAB at 0 h, 6 h, 24 h, and 48 h-post stroke. Peritoneal macrophages isolated from these SAB-treated severe hyperlipidemic mice showed a dose-dependent reduction of CD36 protein levels (Figure 3(a)). The low- and high-doses of SAB significantly decreased brain CD36 expression in ipsilateral side (Figure 3(b)). SAB also reduced the CD36 expression in the contralateral hemisphere with a statistical significance in high dose (p < 0.05, Figure 3(b)). The low-dose of SAB did not alter the injury size and swelling, but high-dose of SAB significantly increased the ischemic injury with an enhanced brain swelling (Figure 3(c) and (d)). The unexpected, but consistent results with hexarelin treatment suggest that elevated CD36 in hyperlipidemia may have a potential benefit against stroke and that post-stroke inhibition of CD36 should not be used for the reduction of stroke injury in hyperlipidemic condition.
Figure 2.
Post-stroke treatment of SS31, CD36Ab, or Hexarelin worsened acute stroke injury in hyperlipidemic condition. Each CD36 targeting agents were administered in WD/AKO mice up to 48 h-post stroke and ischemic outcomes were measured in the stroked brain. (a,b), % infarct volume (a) and % swelling (b) were measured in the ischemic brain of WD/AKO mice at three days post-stroke. Data are expressed at the 95% confidence interval, n = 6–12/group, **p < 0.01 vs. Vehicle, one-way ANOVA.
Figure 3.
Post-stroke SAB treatment worsened acute stroke injury in hyperlipidemic condition. Low (100 mg/kg, SAB-100) – or high (500 mg/kg, SAB-500) – dose of SAB was administered in WD/AKO mice at 0, 6, 24, and 48 h-post stroke and CD36 expression levels in peritoneal macrophages and ischemic injury in stroked brain were determined. (a,b), CD36 protein expression levels in peritoneal macrophages (a) and brain (b) by Western blot. Data are expressed as mean ± SD, n = 5–11/group, (c,d), % Infarct volume (c) and % swelling (d) were measured in the ischemic brain at three days post-stroke. Data are expressed at the 95% confidence interval, n = 8–15/group, *,**p < 0.05, 0.01 vs. vehicle (Veh) one-way ANOVA.
Preventative inhibition of CD36 is effective to reduce stroke-induced brain swelling in hyperlipidemic stroke
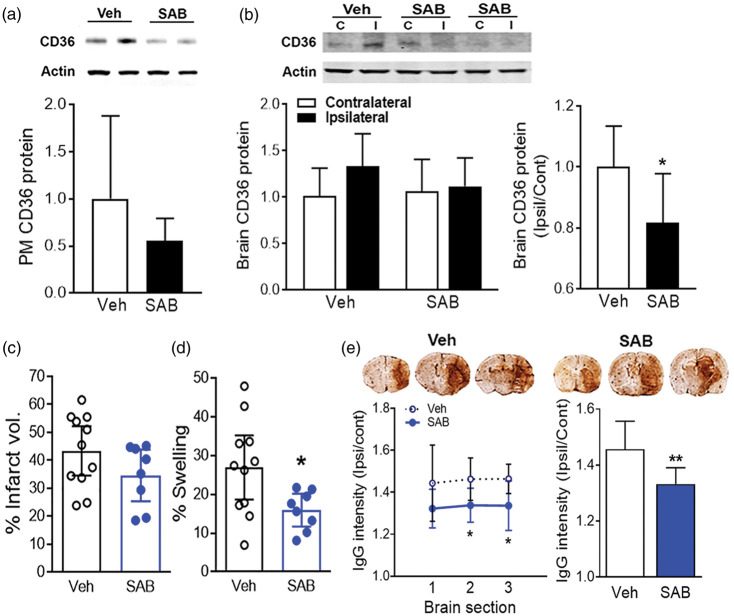
Previously reported reduction of stroke-induced brain injury and swelling in hyperlipidemic stroke were performed in animals with CD36 deficiency.4 Since genetic deletion of CD36 reflects the chronic absence of CD36 before and after stroke, we treated hyperlipidemic mice with SAB chronically (Figure 4(a)). Chronic infusion of SAB (100 mg/kg daily) during the induction of hyperlipidemia did not alter body weight gain compared to vehicle-infused mice (Figure 4(b)). The SAB pre-treatment also did not change plasma cholesterol levels and glucose clearance rates in the hyperlipidemic mice (Figure 4(c) and (d)), indicating that treatment of SAB in young six-week-old mice did not induce overall physiological parameters. The chronic SAB treatment prior to and after stroke reduced CD36 protein levels in peritoneal macrophages (Figure 5(a)). In the brain, the stroke-induced CD36 protein levels were decreased in SAB treatment group (Figure 5(b)). The chronic treatment of SAB caused a moderate reduction in infarct size, but did not reach a statistical significance (Figure 5(c)). On the other hand, the treatment significantly reduced the brain swelling (Figure 5(d)) and reduced IgG leakage in the ischemic brain (Figure 5(e)).
Figure 5.
SAB pre-treatment reduced stroke-induced swelling in hyperlipidemic condition. Low dose (100 mg/kg/day) of SAB was infused by implanted Alzet pump in the ApoE KO mice during diet intervention, and CD36 expression levels in peritoneal macrophages and ischemic injury in stroked brain were determined. (a,b), CD36 protein expression levels in peritoneal macrophages (a) and brain (b) by Western blot. Data are expressed as mean ± SD, n = 7–10/group, Student t-test. (c,d), % Infarct volume (c) and % swelling (d) were measured in the ischemic brain at three days post-stroke. Data are expressed at the 95% confidence interval, n = 8–11/group, *p < 0.05 vs. veh, student t-test. (e) IgG immunostaining using brain sections. The IgG intensity of each section, +1.9 mm (1), +0.7 mm (2), and −0.5 mm (3) (left line graph) and average intensity of the three sections (right bar graph). Data are expressed as mean ± SD, n = 8/group, *,**p < 0.05, 0.01 vs. veh, student t-test. Veh, ApoE KO mice implanted with Alzet pump infusing vehicle; SAB, ApoE KO mice implanted with Alzet pump infusing SAB.
Discussion
In the absence of effective translation in stroke studies, multi-modal approaches have been suggested to target the heterogeneous nature of stroke pathology. We previously validated CD36, a multifunctional receptor that functions in oxidative stress, inflammation, innate immunity, and apoptosis, as a potential multi-modal target in stroke.12 Due to its affinity for lipid-based ligands, including long-chain fatty acids and oxidized or modified lipids (OxLDL and mLDL), we further established the role of CD36 in stroke outcome in hyperlipidemic stroke.4,23 Thus, the present study tested our working hypothesis that CD36 is a viable pharmacological target to reduce brain injury and swelling in hyperlipidemic stroke.
Consistent with previous reports,4,23 the combined genetic ApoE KO mice and WD intervention caused an elevation of plasma cholesterol levels. The elevated plasma cholesterol levels were positively correlated with stroke severity, especially brain swelling, in the two most severely hyperlipidemic mice (Figure 1(d) and (f)). By using several previously identified pharmacological agents that antagonize or block uptake of lipid-based ligands,2,17,18 we tested the efficacy of CD36 inhibitors with expected benefits in hyperlipidemic stroke. One intriguing observation from this study is that the mice treated with CD36 antagonists after stroke consistently showed either no protection or a worse outcome. Despite previously reported effects of cell permeable peptide SS31 on the reduction of infarct size in normal C57BL/6 mice,17 the treatment was not effective in hyperlipidemic mice, demonstrating differential outcomes depending on normal metabolic status vs. comorbid conditions. Furthermore, administration of hexarelin and high dose of SAB even exacerbated stroke outcome. This unexpected result indicates a potential benefit of stroke-induced CD36 expression in hyperlipidemic conditions. Although the underlying mechanism for the potential benefit is not clear at present, it may relate to CD36’s controversial role. In an earlier study, CD36 was recognized as an inflammatory receptor during an acute phase of stroke.4,12 However, CD36 interaction with phosphatidylserine moieties of dying cells27 elicits engulfment of apoptotic cell bodies and resolves inflammation.28,29 The apparent context-dependent role of CD36 may also derive from CD36 expression in different types of cells (microvascular endothelial cells, monocytes, and microglia). While the current study did not address phagocytosis in hyperlipidemic stroke, we and others previously reported the beneficial effect of CD36 mediated-phagocytosis in ischemic stroke and also in intracerebral hemorrhage.30–33 Therefore, the presence of different drug efficacy in normal vs. comorbid stroke suggests the importance of target validation across different metabolically compromised conditions.
Besides metabolic status, the absence of neuroprotection by acute post-stroke treatment of CD36 inhibitors in hyperlipidemic conditions was unexpected, since our genetic studies using CD36 KO mice were also conducted in a hyperlipidemic condition.4 The discrepancy prompts an investigation on the SAB effect of prolonged inhibition of CD36 before stroke. SAB is a well characterized in vivo CD36 antagonist21 and was established for its stability during chronic Alzet pump infusion and in vivo efficacy in reducing oxidized lipid uptake.22 In this preventative SAB treatment paradigm, we observed significant reduction of the hyperlipidemia-exacerbated brain swelling and IgG leakage. While the presence of non-specific staining from severely injured tissues that may affect the staining intensity is not entirely excluded, IgG staining is generally accepted method to indicate the extravascular leakage. Stroke-induced brain edema is another pathological event that can influence stroke outcome and has been considered a life-threatening complication during infarct development.34,35 Consistently, we observed disproportional enlargement of swelling over infarct volume in comorbid conditions.4,8 Thus, selective attenuation in brain swelling in the hyperlipidemic stroke by preventative SAB treatment suggests genetic inhibition closely reflects preventative, but not acute post-stroke, treatment, and consideration of the preventative approach to effectively target CD36.
While the study demonstrated the benefit of CD36 inhibition in stroke severity by a preventative, but not post-stroke, treatment, the protective mechanism that underlies the preventative CD36 inhibition is unclear. However, the efficacy of SAB in improving metabolic functions has been reported in mice fed a high-fat diet by the reduction of visceral fat accumulation and improves insulin resistance.22 Thus, the chronic inhibition of CD36 is likely normalizing metabolic dysfunctions in a severe hyperlipidemic condition, resulting in reduced stroke-induced brain swelling. CD36 is multi-functional receptor and the functions are derived from CD36 interaction with available ligands at different stages of stroke and different cell types in the brain. The current study did not distinguish the different cell types that would be affected by SAB inhibition. Thus, further studies are granted to determine the impact of CD36 inhibition using conditional CD36 knock-out mice in cell-specific manner. Additional caveat is that there are no statistically significant differences in acute behavior outcome in vehicle vs. SAB-treated animals (data not shown), possibly due to the complications arise from surgical wound for MCAO preparation, severe body weight loss during the critical post-stroke period, and low sensitivity of neurological scoring. Our previously established stroke recovery model with rigorous longitudinal behavior testing during acute, subacute and recovery phases up to six-month post-stroke also demonstrated that infarct size is not well correlated with acute behavior impairment.24,36 It suggests the importance to address the effect of preventative CD36 inhibition in comorbid conditions on motor and cognitive functional recovery in comprehensive stroke stages.
In summary, hyperlipidemia exacerbated stroke-induced infarct volume with disproportionally enlarged swelling. Contrary to our prediction, post-stroke treatment of CD36 inhibitors in hyperlipidemic mice did not provide protection, and in some cases worsened stroke outcome. In contrast, chronic pretreatment significantly reduced hyperlipidemia-exacerbated brain swelling following stroke. The absence of benefits by post-stroke CD36 inhibition suggests a beneficial role of CD36 in hyperlipidemic conditions. Furthermore, reduced swelling by preventative, but not post-stroke, treatment suggests that genetically identified CD36 targets closely mimic the condition of preventative chronic inhibition. Therefore, the preclinical findings provide critical insights that targeting CD36 depends on peripheral metabolic status (normal vs. hyperlipidemia) and stroke stages (post-stroke treatment vs. preventative chronic inhibition). Furthermore, they demonstrate that acute post-stroke CD36 inhibition does not necessarily reflect biological consequences of genetic inhibition. Careful consideration of possible target inhibition based on genetic studies is suggested for effective pharmacological intervention strategies to treat stroke.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Health awards [grant number, NINDS R01NS077897 (SC) and R01NS095359-10 (SC)] and the Burke Foundation (SC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Eunhee Kim generated hyperlipidemic mice, characterized and performed MCAO, molecular analyses and wrote the manuscript; Jiwon Yang contributed to acute and chronic treatment CD36 inhibitors; Keun Woo Park contributed to molecular and biochemical assessment; and Sunghee Cho designed the study and wrote the manuscript.
References
- 1.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 2.Cho S, Yang J. What do experimental models teach us about comorbidities in stroke? Stroke 2018; 49: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim E, Tolhurst AT, Cho S. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J Neuroinflammation 2014; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim E, Tolhurst AT, Qin LY, et al. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci 2008; 28: 4661–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ergul A, Abdelsaid M, Fouda AY, et al. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab 2014; 34: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ergul A, Hafez S, Fouda A, et al. Impact of comorbidities on acute injury and recovery in preclinical stroke research: focus on hypertension and diabetes. Transl Stroke Res 2016; 7: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElAli A, Doeppner TR, Zechariah A, et al. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 2011; 42: 3238–3244. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Yang J, Park KW, et al. Inhibition of VEGF Signaling Reduces Diabetes-Exacerbated Brain Swelling, but Not Infarct Size, in Large Cerebral Infarction in Mice. Transl Stroke Res 2018; 9: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 2001; 108: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho S, Kim E. CD36: a multi-modal target for acute stroke therapy. J Neurochem 2009; 109(Suppl 1): 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol 2007; 39: 2012–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci 2005; 25: 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 2000; 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abumrad NA, el-Maghrabi MR, Amri EZ, et al. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 1993; 268: 17665–17668. [PubMed] [Google Scholar]
- 15.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274: 19055–19062. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein RL, Li W, Park YM, et al. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc 2010; 121: 206–220. [PMC free article] [PubMed] [Google Scholar]
- 17.Cho S, Szeto HH, Kim E, et al. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 2007; 282: 4634–4642. [DOI] [PubMed] [Google Scholar]
- 18.Demers A, McNicoll N, Febbraio M, et al. Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J 2004; 382: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marleau S, Harb D, Bujold K, et al. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J 2005; 19: 1869–1871. [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Tang MK, Zhang Y, et al. Effect of salvianolic acid B on neural cells damage and neurogenesis after brain ischemia-reperfusion in rats. Yao Xue Xue Bao 2007; 42: 716–721. [PubMed] [Google Scholar]
- 21.Bao Y, Wang L, Xu Y, et al. Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 2012; 223: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Park KW, Cho S. Inhibition of the CD36 receptor reduces visceral fat accumulation and improves insulin resistance in obese mice carrying the BDNF-Val66Met variant. J Biol Chem 2018; 293: 13338–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim E, Febbraio M, Bao Y, et al. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol 2012; 71: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim E, Woo MS, Qin L, et al. Daidzein augments cholesterol homeostasis via ApoE to promote functional recovery in chronic stroke. J Neurosci 2015; 35: 15113–15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin TN, He YY, Wu G, et al. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 1993; 24: 117–121. [DOI] [PubMed] [Google Scholar]
- 26.ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 2014; 15: 6453–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem 2002; 277: 38503–38516. [DOI] [PubMed] [Google Scholar]
- 28.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ 1998; 5: 563–568. [DOI] [PubMed] [Google Scholar]
- 29.Savill J, Dransfield I, Gregory C, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965–975. [DOI] [PubMed] [Google Scholar]
- 30.Ballesteros I, Cuartero MI, Pradillo JM, et al. Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARgamma and 5-LO-dependent pathways. J Leukoc Biol 2014; 95: 587–598. [DOI] [PubMed] [Google Scholar]
- 31.Woo MS, Wang X, Faustino JV, et al. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann Neurol 2012; 72: 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo MS, Yang J, Beltran C, et al. Cell Surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem 2016; 291: 23654–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007; 61: 352–362. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis 1999; 42: 209–216. [DOI] [PubMed] [Google Scholar]
- 35.Schwab S, Aschoff A, Spranger M, et al. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurology 1996; 47: 393–398. [DOI] [PubMed] [Google Scholar]
- 36.Qin L, Jing D, Parauda S, et al. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J Neurosci 2014; 34: 2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]