Abstract
Ultrasound shear wave elastography (SWE) can provide accurate in vivo measurements of the effect of advanced age on muscle elasticity. Our objective was to determine whether passive muscle elasticity was influenced by posture, chronological age, sex, body mass index, and clinical measures of upper extremity function for healthy adults. The dominant arm of 33 male and 33 female participants (ranging from 20-89 years old) was examined using a Supersonic Imagine Aixplorer ultrasound SWE system. The mean and standard deviation of shear wave velocity (SWV) was measured from elastography maps for five upper extremity muscles examined at rest: anterior deltoid (AD), biceps brachii (BB), clavicular (CL) and sternocostal (SC) region of the pectoralis major and middle trapezius (MT). Linear mixed models for each muscle were used to assess how SWV was influenced by humeral elevation, chronological age, sex, BMI and three functional measures. All significances are reported at α=0.05. Humeral elevation influenced shear wave velocity at a statistically significant level for AD, BB, SC and MT (all p<0.047). Chronological age was a significant predictor of mean SWV for the sternocostal region of the pectoralis major and the middle trapezius (both p<0.03). These same muscles were also less homogenous (based on their standard deviations) with increased age, particularly for female participants. Performance-based functional assessments of the upper extremity were predictors of mean SWV for the clavicular region of the pectoralis major (all p<0.04). These results suggest ultrasound SWE has potential utility for assessing age-related changes to muscle elasticity, but these associations were muscle-dependent.
Keywords: Muscle stiffness, age, sex, shear wave elastography, ultrasound, skeletal muscle
INTRODUCTION
Musculoskeletal disorders have a significant impact on society, with 28% of the world population reporting at least one rheumatic or musculoskeletal disease as of 2013 (Palazzo et al., 2014). Specific to the upper extremity, the majority of the adult world population experiences upper limb pain regularly, with ~ 20% of individuals having upper limb pain that lasts longer than a month at some point in their lifetime (Hill et al., 2010; Walker-Bone et al., 2004). The prevalence of these disorders is associated with decreased quality of life of older adults (Netuveli et al., 2006). Deficits in physical functioning (including reduced strength and mobility, increased pain, and increased stiffness) are also associated with advanced age (Buckwalter et al., 1993; Hill et al., 2010; Palazzo et al., 2014). A common functional limitation with certain musculoskeletal disorders and aging is increased joint and muscle stiffness. Increased stiffness inhibits individuals from participating in work and leisure activities necessary to maintain general health, financial security, and social relationships (Buckwalter et al., 1993). These deficits can progress to a partial or complete loss of independence during activities of daily living (Buckwalter et al., 1993; Hill et al., 2010; Larsson et al., 1997; McPhail et al., 2014; Palazzo et al., 2014).
The stiffness properties of muscle provide immediate protection for a muscle before reflex or voluntary control of the muscle occurs (Cui et al., 2008). This protection is important for older adults using their upper extremity to arrest a fall to the ground (DeGoede et al., 2003). Muscle stiffness is a structural property influenced both by tissue elasticity and muscle architecture (Cui et al., 2008). Muscles experience a shift in their fiber composition with increasing age (Claflin et al., 2011), resulting in progressive declines in the number and size of fast twitch muscle fibers (Lexell et al., 1988). Elderly adults also exhibit increased collagen and fat content of muscle (Gao et al., 2008; Gosselin et al., 1998; Visser et al., 2005). These intramuscular changes lead to sarcopenia (Lexell et al., 1988), increased muscle elasticity (Wood et al., 2014) and ultimately increased muscle stiffness (Gao et al., 2008; Gosselin et al., 1998).
Recent developments in ultrasound shear wave elastography (SWE) provides a reliable and valid assessment tool for quantifying in vivo muscle elasticity (Bercoff et al., 2004; Koo et al., 2014; Nordez and Hug, 2010; Shinohara et al., 2010). Increases in muscle elasticity across the lifespan have been identified with SWE for upper extremity muscles like the biceps brachii (Alfuraih et al., 2019; Eby et al., 2015) and supraspinatus (Baumer et al., 2018). However, age-related changes in muscle elasticity are muscle dependent given the gastrocnemius and soleus muscles respectively exhibit decreased or no change in elasticity with increased age (Akagi et al., 2015). These findings support the need to investigate the association between age and muscle elasticity across a broad range of muscles with SWE. Furthermore, these inconsistencies in age-related changes to muscle elasticity may reflect that chronological age is a poor indicator of an individual’s functional capabilities (Crane et al., 2013; Zemkova et al., 2016). In addition, prior studies investigating aging and muscle elasticity with SWE focus on obtaining average measures of muscle elasticity and do not consider age-related changes to the homogeneity of muscle elasticity within a given muscle (Domire et al., 2009).
Therefore, the objective of the current study was to assess whether changes in passive muscle elasticity for five upper extremity muscles (biceps brachii (BB), the sternocostal (SC) and clavicular (CL) regions of the pectoralis major, the anterior deltoid (AD), and the middle trapezius (MT)) are more reflective of chronological age or the functional status of the upper extremity. These muscles are all involved with stabilizing and moving the shoulder through its vast range of motion, but have varying fiber type compositions and force-length relationships as the arm is elevated (Srinivasan et al., 2007). We hypothesized that 1) humeral elevation would influence passive elasticity for each muscle differently, 2) functional status of the upper extremity is a better predictor of muscle elasticity than chronological age, and 3) muscle elasticity will become less homogenous with advanced age. Combined, this information will serve to establish a baseline for differentiating healthy aging from pathologic changes to muscle in older adults.
METHODS
Subjects
The University of Michigan Institutional Review Board approved all study procedures (IRB#: HUM00135096), and written informed consent was obtained from each participant prior to the collection of any data. Sixty-six healthy participants (33 males, 33 females; mean (SD) body mass index (BMI): 26.8 (4.4) kg/m2) ranging from age 20 to 89 years old were enrolled in the study (Table 1). Participants had no reported history of any orthopedic injuries or neuromuscular pathologies to the upper extremity. All participants were asked to self-report their shoulder pain and disability using the Shoulder Pain and Disability Index (SPADI).
Table 1:
Univariate linear regression models describing the relationship between chronological age and relevant covariates.
| Regression Coefficient |
Adjusted R2 | ||
|---|---|---|---|
| Intercept | Age (years) | ||
| Body Mass Index (kg/m2) | 23.7 (1.5)*** | 0.059 (0.026)* | 0.06 |
| Hand dynamometer (kg) | 47.5 (3.8)*** | −0.20 (0.07)** | 0.11 |
| Purdue pegboard time (s) | 16.6 (2.7)*** | 0.15 (0.05)** | 0.11 |
| Time to button blouse (s) | 14.2 (4.7)** | 0.37 (0.08)*** | 0.22 |
Significant parameters are bolded with an asterisk signifying significance level
p < 0.05,
p < 0.01,
p < 0.001
Experimental Protocol
Each participant was seated in the Biodex chair (Biodex Medical Systems, Shirley, New York). The dominant arm was placed in a plastic removable fiberglass cast extending from the hand to just below the shoulder. The cast was attached to a servomotor to support the arm against gravity. Within the cast, the elbow was fixed at 90 degrees and the wrist was neutral. The glenohumeral joint was aligned with the center of rotation of the servomotor by manually adjusting the height and base of the chair. The motor was controlled in real time using the Matlab Simulink Real Time Toolbox (2017a Mathworks Inc, Natick, MA), and allowed the position of the arm in relation to the trunk to be standardized for each subject.
Participants were examined in five arm postures all along the frontal plane: 105, 90, 75, 60 and 45 degrees of humeral elevation relative to the trunk (Figure 1). Five muscles around the upper extremity were examined as participants were relaxed, including the anterior deltoid (AD), biceps brachii (BB), clavicular (CL) and sternocostal (SC) region of the pectoralis major and middle trapezius (MT). The data were acquired in a randomized order, first by randomizing the examined muscle, and then by randomizing the humeral elevation angle.
Figure 1:
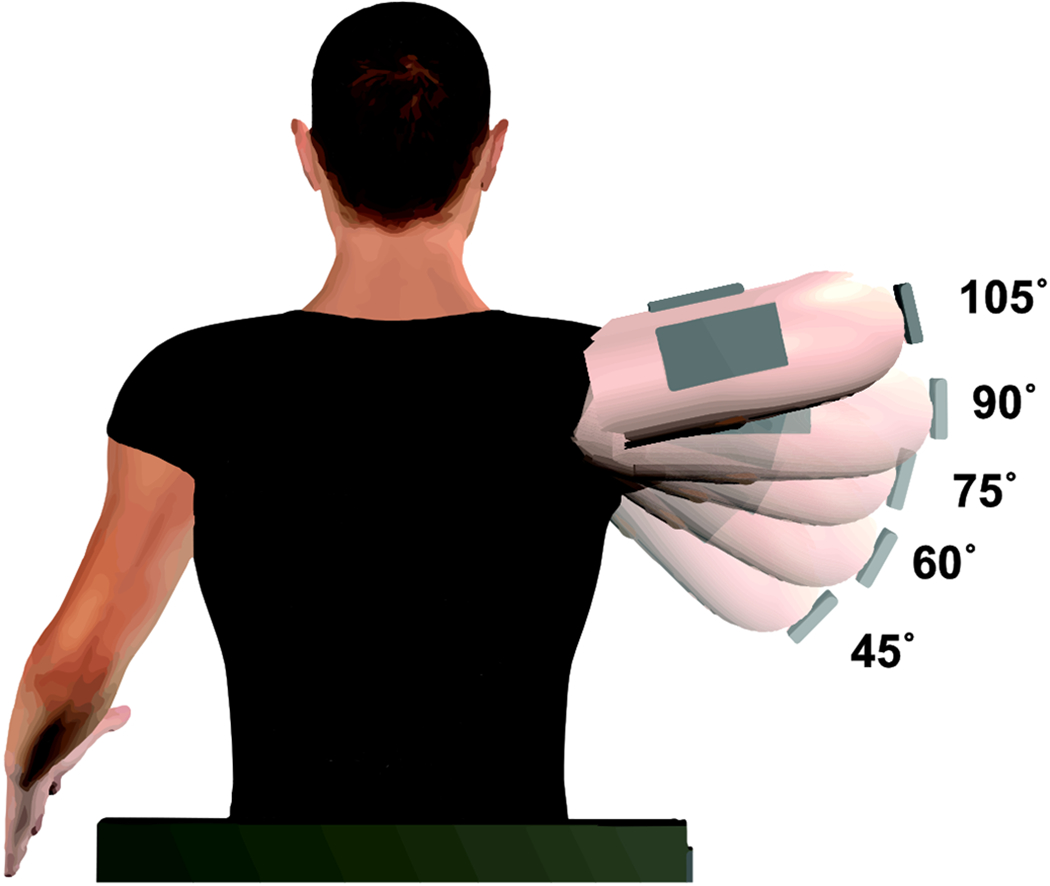
Schematic of experimental setup with the examined humeral elevation angles overlaid over each other.
A Supersonic Imagine Aixplorer ultrasound SWE system (Aix-en-Provence, France) connected to an SL15-4 linear transducer was used to acquire objective elastography measures from each muscle. At each position, the probe was placed over the belly of the muscle of interest. Three images were acquired from the muscle at each humeral elevation angle before continuing to the next angle. The transducer was removed from the skin and repositioned between each image acquisition. The position and orientation of the transducer was considered satisfactory when individual muscle fascicles could be clearly identified on the corresponding B-mode ultrasound image. These B-mode images were superimposed with an elastography color map positioned in the belly of the muscle (Figure 2). The color map provides pixel by pixel calculations of shear wave velocity (SWV). The size of the color map was consistent between subjects (2.5 cm x 1 cm), but the depth of the color map was changed depending on the anatomy of the examined muscle and participant. In total, 75 images were acquired from each participant. One experimenter collected all ultrasound images.
Figure 2:
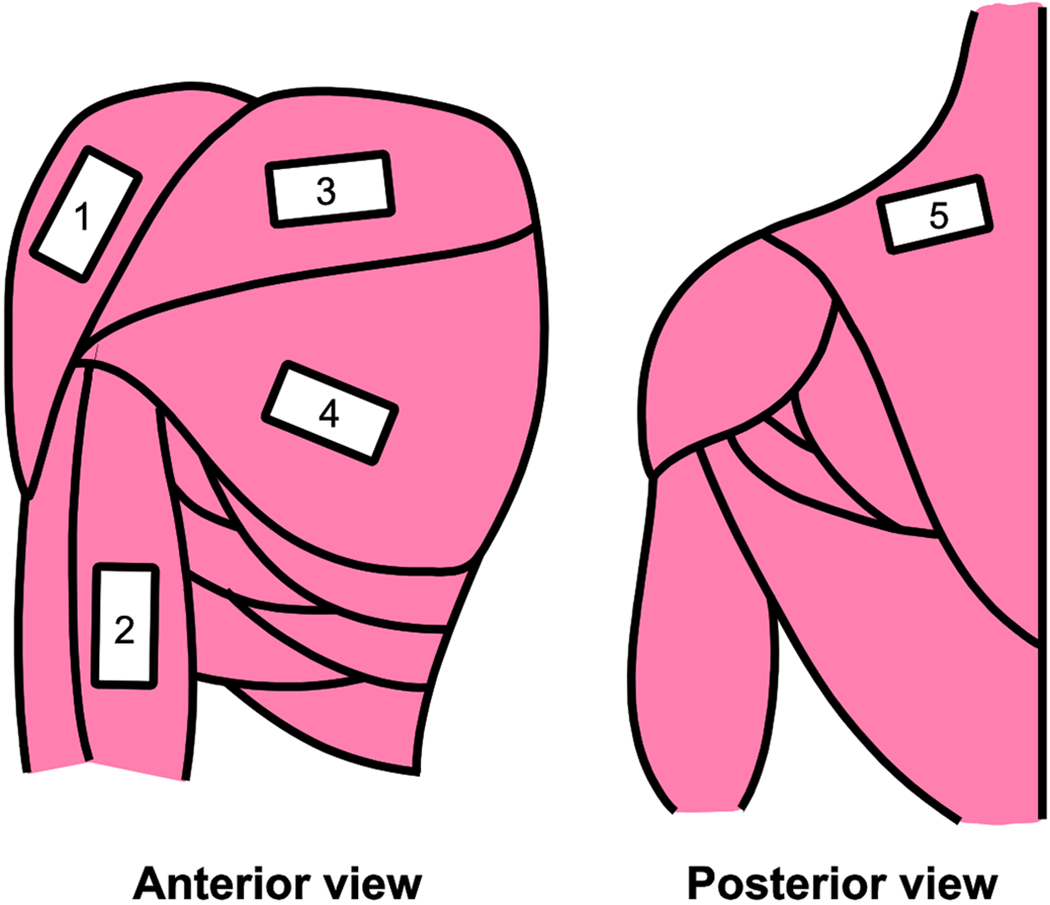
Ultrasound transducer locations for the anterior deltoid (AD), biceps brachii (BB), middle trapezius (MT), and the clavicular (CL) and sternocostal (SC) regions of the pectoralis major.
A series of functional measures previously correlated with upper extremity function in older adults (Onder et al., 2005; Onder et al., 2002) were acquired from each participant, including measures of manual coordination and dexterity, activities of daily living and hand grip strength (Martins et al., 2015; Poole and Mason, 2007). Manual dexterity and coordination were assessed by measuring the time to place 10 pegs using a Purdue Pegboard test (Lafayette Instruments, Lafayette, IN). This test was repeated three times on the dominant arm and averaged together. The ability to perform activities of daily living with the upper extremity was assessed by measuring the time to button a shirt. The shirt was placed on the back of a chair and time to put the shirt on and button six buttons were measured three times and averaged together. Finally, hand grip strength was measured using a hand dynamometer (Lafayette Instruments, Lafayette, IN). Each participant held their dominant arm parallel to the ground and straight in front of their chest while seated to complete the test and the maximum force from three trials was recorded.
Data and Statistical Analysis
All shear wave elastography images were exported from the ultrasound machine onto a DICOM server and the images were then objectively analyzed using a custom-written MATLAB algorithm previously described in detail (Lee et al., 2015; Leonardis et al., 2017). In general, the semi-automated algorithm is used to identify a region of interest on the ultrasound machine corresponding to the muscle belly and then extracts the SWV and the quality map for each pixel within the selected region for each image. This method avoids the inclusion of adipose tissue and aponeurosis in the color map that would bias the SWV measure. The algorithm calculated the mean and standard deviation of SWV within the region of interest for each image only using the pixels that achieved a quality map value above 0.7.
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). The effect of chronological age on BMI, dynamometer grip strength, Purdue Pegboard time, or time to button a blouse was evaluated using separate linear regression models for each covariate. Separate linear mixed effects models for each muscle were used to test our primary hypotheses that 1) humeral elevation would influence passive elasticity for each muscle differently, and 2) functional status of the upper extremity is a better predictor of SWV than chronological age. Average SWV was treated as an outcome measure, and the humeral elevation angle was treated as a within-subjects factor (45° – reference level, 60°, 75°, 90°, 105°). Each subject was treated as a random factor. Chronological age was treated as a continuous variable. The model was adjusted by sex (female – reference level), BMI and the three functional assessments to determine if these biologically relevant covariates were a better predictor of SWV than chronological age. We included age*sex and age*angle interaction terms within our statistical framework to determine if potential age-related changes in muscle elasticity differed by sex or humeral elevation angle. Bonferroni-corrected pairwise comparisons were performed to determine how mean SWV changes with respect to the humeral elevation angle. We also examined a secondary hypothesis that muscle elasticity will become less homogenous with advanced age using the same linear mixed model framework, but with the standard deviation of the SWV map as the outcome measure. For all analyses, a P-value < 0.05 was considered to be statistically significant.
RESULTS
All participants enrolled in this study self-reported no shoulder pain and disability using the SPADI patient-reported outcome measure, with an average score across all participants of 1.4 (out of 130). Across all participants, increased chronological age was significantly associated with increased BMI (p = 0.026), reduced hand grip strength (p = 0.004), and increased time to complete the Purdue Pegboard time (p = 0.003) or button a shirt/blouse (p < 0.001) (Table 1).
The effect of humeral elevation for each of the examined muscles’ SWV can be visualized using the shear wave elastography color maps, with typical data for younger and older males and females displayed in Figure 3. Brighter colors are indicative of higher SWVs, and cooler colors indicate lower SWVs. Postural differences can be observed by comparing each column, which represents one of five humeral elevation angles where ultrasound images were acquired. These images exhibit the general trends in our data, including increases in SWV of the biceps and sternocostal region of the pectoralis major with humeral elevation and that the older participants often had stiffer muscles than the younger participants. The clavicular region of the pectoralis major exhibits the highest SWV values of the examined muscles.
Figure 3:
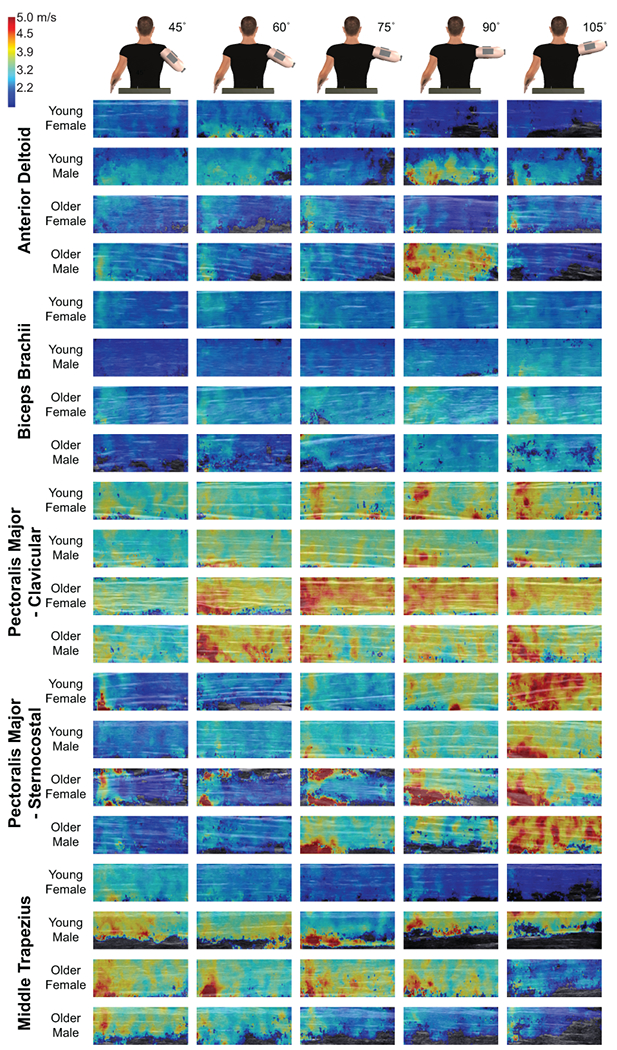
Typical shear wave elastography maps for the five examined muscles. For each muscle, each row shows the images from a representative young female, young male, older female, and older male participants. Each column indicates the resultant color map at a given humeral elevation angle. A legend for the color maps is shown in the upper right corner.
The influence of age, sex, humeral elevation angle, BMI, and upper extremity function on muscle elasticity was examined using linear mixed models for each muscle (Table 2). Across all participants, humeral elevation consistently influenced muscle elasticity (Figure 4). Increases in the humeral elevation angle resulted in a reduced SWV for the anterior deltoid and middle trapezius muscles. Humeral elevation angles of 75, 90 and 105 degrees were significantly associated with decreased SWV compared to 45 degrees for the anterior deltoid (all p< 0.047). The significant decrease in SWV for the MT was observed when the arm was elevated to 105 degrees when compared to humeral elevation angles of 45, 60, and 75 degrees (all p < 0.039). On the contrary, elevating the arm to 105 degrees significantly increased SWV for both the biceps brachii (both p < 0.001 when compared to 45 and 60 degrees) and sternocostal region of the pectoralis major (all p <0.001 when compared to 45, 60, 75, and 90 degrees). Humeral elevation was not a significant effector for the clavicular region of the pectoralis major (all p > 0.12).
Table 2:
Parameter estimates of mean shear wave velocity shown by each variable within the linear mixed effects model for each muscle.
| Measures | Mean Shear Wave Velocity (m/s) (* p < 0.05, ** p < 0.01, *** p < 0.001) |
||||
|---|---|---|---|---|---|
| AD | BB | CL | SC | MT | |
| Intercept | 2.0* | 2.8*** | 2.5*** | 1.6*** | 1.5*** |
| Age | 0.0072 | 0.0023 | 0.0039 | 0.0087* | 0.019** |
| Sex (Reference: Female) | |||||
| Male | −0.2 | −0.27 | −0.31 | −0.26 | 0.28 |
| Age* Sex (Reference: Female) | |||||
| Age*Male | −0.00079 | 0.0014 | 0.0022 | −0.00025 | −0.016* |
| Humeral Elevation Angle (Reference: 45 degrees) | |||||
| 60 degrees | −0.041 | −0.00006 | −0.019 | 0.15 | 0.053 |
| 75 degrees | −0.096 | 0.093 | 0.19 | 0.29 | 0.33 |
| 90 degrees | −0.0009 | 0.22* | 0.25 | 0.54** | 0.14 |
| 105 degrees | −0.16 | 0.19* | 0.22 | 0.79*** | 0.62* |
| Age*Humeral Elevation Angle (Reference: 45 degrees) | |||||
| Age*60 degrees | −0.0018 | 0.0012 | 0.0014 | −0.00089 | −0.0011 |
| Age*75 degrees | −0.0022 | 0.0016 | −0.0022 | 0.0014 | −0.0067 |
| Age*90 degrees | −0.0051 | 0.0007 | −0.004 | 0.0024 | −0.005 |
| Age*105 degrees | −0.0011 | 0.0012 | −0.0059 | 0.0051 | −0.017*** |
| BMI | 0.0072 | −0.02* | 0.012 | 0.011 | 0.008 |
| Dynamometer | 0.0098 | 0.0046 | 0.017* | 0.013* | 0.024* |
| Purdue Pegboard Time | −0.0013 | −0.0047 | −0.022* | 0.0017 | 0.017 |
| Time to Button Blouse | −0.0019 | 0.0024 | 0.017** | −0.0041 | −0.0056 |
Significant parameters are bolded with an asterisk signifying significance level
p < 0.05,
p < 0.01,
p < 0.001
Figure 4:
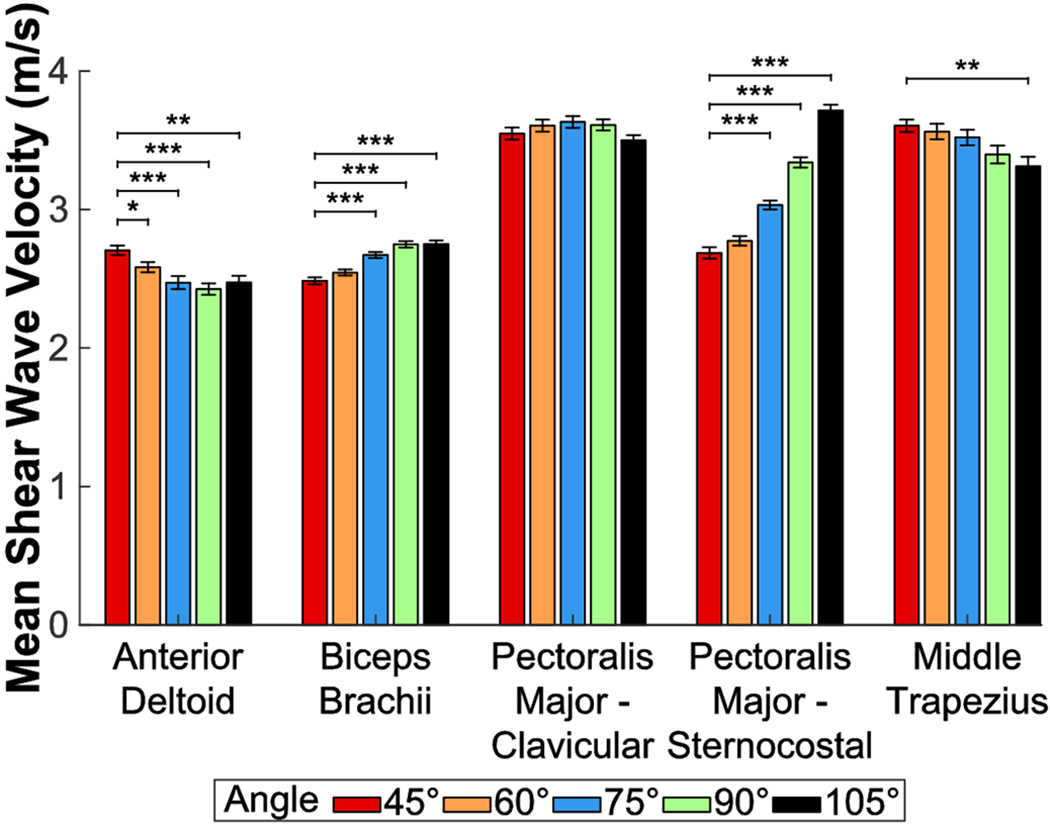
The effect of humeral elevation on the shear wave velocity (SWV) recorded at rest from all five examined muscles. Significant humeral elevation angles from each muscle’s linear mixed model (relative to 45 degrees) are signified with brackets and asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001).
Chronological age was compared to both mean SWV and the standard deviation of SWV for the five muscles in Figure 5. Chronological age was a significant predictor of SWV for both the sternocostal region of the pectoralis major (p = 0.011) and the middle trapezius (p = 0.006). The middle trapezius exhibited a significant age * sex interaction on SWV (p = 0.034), with increasing chronological age leading to greater SWV’s for females than males, and a significant age * humeral elevation angle interaction on SWV at 105 degrees (p < 0.001). Chronological age was not a significant effector for the anterior deltoid, biceps brachii or clavicular region (all p > 0.16). The standard deviation of the SWV indicates the homogeneity of muscle tissue within the SWV map. The middle trapezius muscle displayed increased standard deviations across the SWV maps with increasing chronological age (p = 0.006) (Table 3). The middle trapezius also exhibited a significant age * sex interaction on the standard deviation of SWV (p = 0.015), with females exhibited greater variability than males with increased age. Finally, the sternocostal region of the pectoralis major displayed a significant age*humeral elevation angle interaction at 105 degrees on the standard deviation of SWV (p = 0.038).
Figure 5:
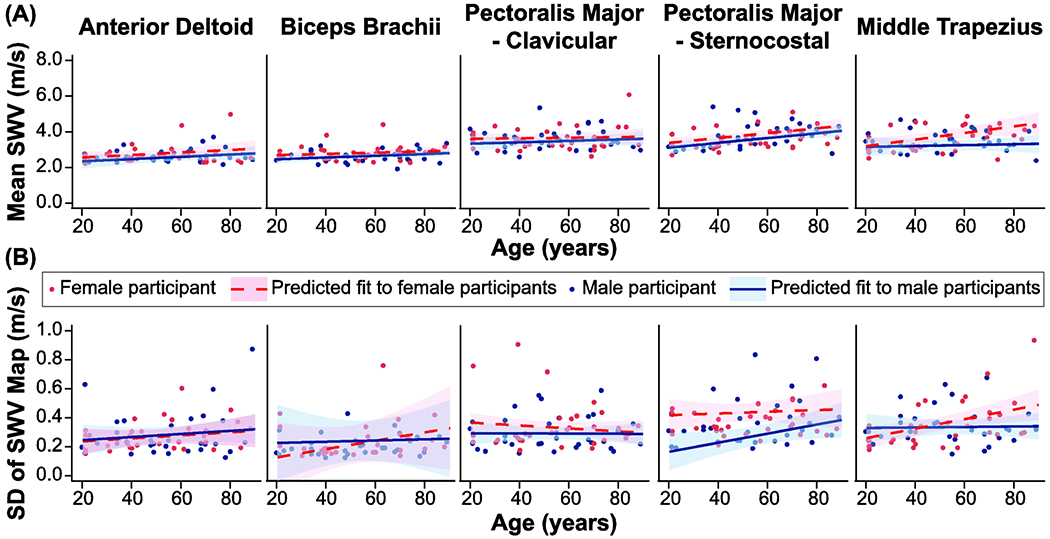
Influence of chronological age on (a) mean shear wave velocity (SWV) and (b) the standard deviation of the SWV map for five examined muscles (anterior deltoid, biceps brachii, clavicular and sternocostal regions of the pectoralis major, and middle trapezius). Blue filled circles represent data from male participants and red filled circles represent data from female participants. For each muscle, data is only displayed for the humeral elevation angle that produced the largest SWV for the group. The anterior deltoid and middle trapezius are displayed at 45 degrees, the clavicular region of the pectoralis major is displayed at 75 degrees, and the biceps brachii and sternocostal regions of the pectoralis major are displayed at 105 degrees. The resultant fit of the linear mixed model for each sex (blue solid line – male; red dashed line – female) with shaded regions indication 95% confidence intervals. For each muscle, the model was fit using the parameters provided in Table 3, and calculated using the median body mass index (26.5 kg/m2) and functional scores (hand dynamometer: 36.2 kg; Purdue Pegboard time: 22.1 sec; time to button shirt/blouse: 29.3 sec) for the entire group.
Table 3:
Parameter estimates for the standard deviation of the shear wave velocity maps shown by each variable within the linear mixed effects model for each muscle.
| Measures | SD of Shear Wave Velocity Map (m/s) (* p < 0.05, ** p < 0.01, *** p < 0.001) |
||||
|---|---|---|---|---|---|
| AD | BB | CL | SC | MT | |
| Intercept | 0.24* | 0.68* | 0.068 | 0.36* | 0.049 |
| Age | 0.001 | −0.0029 | 0.00022 | −0.0021 | 0.0033** |
| Sex (Reference: Female) | |||||
| Male | 0.0081 | 0.15 | −0.092 | −0.3** | 0.13 |
| Age* Sex (Reference: Female) | |||||
| Age*Male | 0.000026 | −0.0025 | 0.00095 | 0.0026 | −0.0031* |
| Humeral Elevation Angle (Reference: 45 degrees) | |||||
| 60 degrees | −0.077 | −0.45* | 0.0036 | −0.032 | 0.027 |
| 75 degrees | −0.042 | −0.44 | 0.081 | −0.1 | 0.075 |
| 90 degrees | −0.0037 | −0.43 | 0.051 | −0.18* | 0.036 |
| 105 degrees | 0.008 | −0.44 | 0.075 | −0.22** | 0.1 |
| Age*Humeral Elevation Angle (Reference: 45 degrees) | |||||
| Age*60 degrees | 0.0013 | 0.0058 | −0.00026 | −0.00057 | −0.00036 |
| Age*75 degrees | 0.00079 | 0.0055 | −0.0012 | 0.0011 | −0.0008 |
| Age*90 degrees | 0.00012 | 0.0055 | −0.00083 | 0.002 | −0.00004 |
| Age*105 degrees | −0.00008 | 0.0058 | −0.0014 | 0.0027* | −0.0014 |
| BMI | −0.00047 | −0.0063 | 0.0083*** | 0.0004 | 0.0072* |
| Dynamometer | −0.00058 | 0.00085 | 0.00042 | 0.0056 | −0.00090 |
| Purdue Pegboard Time | −0.0016 | −0.0009 | −0.00033 | 0.000048 | −0.00016 |
| Time to Button Blouse | 0.0016 | −0.00068 | 0.00045 | 0.0018 | −0.00034 |
Significant parameters are bolded with an asterisk signifying significance level
p < 0.05,
p < 0.01,
p < 0.001
Since chronological age is not indicative of an individual’s functional status, we also compared ultrasound SWE measures to each participant’s performance on three functional clinical assessments of the upper extremity. All three functional scores were significant effectors for the pectoralis major clavicular region (all p < 0.033). SWV was increased in participants with stronger hand grip strength, more difficulty completing the time to button a shirt/blouse test, and reduced time to complete the abbreviated Purdue Pegboard test. Increased hand grip strength using a hand dynamometer was a significant predictor of SWV for the sternocostal region of the pectoralis major and the middle trapezius (both p < 0.04).
Other biologically relevant factors that could influence SWV are sex and BMI. Sex was not a significant effector for mean SWV any muscle (all p > 0.17), but females exhibited a greater standard deviation of the SWV map for the sternocostal region of the pectoralis major (p = 0.003). BMI was only a significant effector for mean SWV of the biceps brachii (p = 0.013) where decreased BMI was associated with increased SWV. BMI was not significant for mean SWV for the other four muscles (all p > 0.35). Increased BMI was related to increased SWV standard deviations for both the clavicular region of the pectoralis major and the middle trapezius (both p < 0.028).
DISCUSSION
Ultrasound SWE is of growing interest in the evaluation of healthy and pathologic muscles. However, there is limited evidence regarding how relevant biological factors like age and sex, along with overall functional capabilities influence SWE assessments. This study provided new insights into these knowledge gaps by examining how in vivo elasticity measurements from five upper extremity muscle changes across the lifespan using ultrasound SWE. These muscle elasticity measures were examined against changes in humeral elevation, age, sex, BMI and functional measures. We hypothesized that 1) humeral elevation would influence passive elasticity for each muscle differently, 2) functional status of the upper extremity is a better predictor of SWV than chronological age, and 3) muscle elasticity will become less homogenous with advanced age. Our main findings were that humeral elevation influenced passive muscle elasticity for all muscles except the clavicular region of the pectoralis major and age was a significant predictor of muscle elasticity for only the middle trapezius and sternocostal region of the pectoralis major. We also observed muscle tissue homogeneity for the middle trapezius and sternocostal region of the pectoralis major was reduced with increased chronological age, particularly for female participants.
Humeral elevation had a significant effect on SWV for all examined muscles except for the clavicular region of the pectoralis major. Increased humeral elevation resulted in greater SWV for the biceps. SWV increases as muscles are passively stretched (Chernak et al., 2013; Koo et al., 2014), suggesting our observed changes in these muscles likely result from increasing passive muscle force as the arm was elevated. Increased SWV at higher elevation angles may allow these muscles to maintain passive shoulder stability at these positions. The muscle moment arms for the biceps and sternocostal region of the pectoralis major decrease with humeral elevation (Ackland et al., 2008; Landin et al., 2008), so increased passive force would be necessary to produce equal or greater torques at higher elevation angles. On the contrary, SWV was significantly reduced as humeral elevation increased for the anterior deltoid and middle trapezius muscles. This suggests the two muscles contribute more to shoulder stability at lower elevation angles and their muscle fibers are likely shortened as the shoulder becomes more elevated (Klein Breteler et al., 1999). The only muscle that was not influenced by humeral elevation was the clavicular region of the pectoralis major. This is consistent with previous findings that the clavicular region largely contributes to shoulder flexion and not elevation (Leonardis et al., 2017).
This study examined changes in muscle elasticity across the lifespan for five upper extremity muscles. Chronological age was only a significant predictor of muscle SWV for the sternocostal region of the pectoralis major and the middle trapezius. Both muscles exhibited increased mean SWVs with age, indicative of increasing muscle stiffness. Similarly, we observed increases in the variability of these SWV measures with increased age for both muscles, particularly for female participants. Combined, these changes may contribute to increased shoulder joint stiffness, pain, and loss of function often associated with advanced aging (Buckwalter et al., 1993; Eby et al., 2015). Healthy aging is also associated with altered muscle fiber composition, including a reduction in the number and size of type II muscle fibers (Claflin et al., 2011). Previous studies indicate that the middle trapezius is primarily a type I dominant muscle while the sternocostal region of the pectoralis major is primarily type II dominant muscle (Lindman et al., 1991; Srinivasan et al., 2007). Therefore, our results suggest that changes in muscle elasticity with advanced aging is not fiber type specific. We observed increased muscle elasticity variability for these muscles, which is reflective of muscle tissue becoming more heterogeneous with increased age (Domire et al., 2009). This may reflect changes to the extracellular matrix of muscle, including increased collagen cross-linking, and fat infiltration with age (Haus et al., 2007; Marcus et al., 2010). Future work is warranted to directly compare changes to intermuscular connective tissue with SWE-based measures of muscle elasticity.
Chronological age is a poor indicator of an individual’s functional capabilities, as active and sedentary older adults can exhibit significant biomechanical differences in strength, speed and power (Crane et al., 2013; Zemkova et al., 2016). Therefore, we also compared how changes in muscle elasticity relate to validated upper extremity functional assessments in older adults (Onder et al., 2005; Onder et al., 2002). This included the time to button a shirt or blouse and the hand grip strength. Overall, we only found the SWV of the clavicular region of the pectoralis major was significantly related to all three functional assessments. Hand grip strength was also significantly related to SWV of the middle trapezius. The timed blouse test required flexion of the shoulder to be able to successfully put on the shirt. Since the clavicular region of the pectoralis major contributes largely to shoulder flexion (Leonardis et al., 2017), the observed increase in time to put on a shirt or blouse may indicate a stiffening of this muscle. Reduced hand grip strength is predictive of impaired upper body strength (Bohannon, 1998), and the positive relationship between SWV of the middle trapezius and hand grip strength may be indicative of reduced muscle stiffness in these muscles as older adults become weaker with advanced age. Overall, these findings highlight the need to evaluate the functional capabilities of older adults when evaluating muscle elasticity with SWV.
Limitations of this work include that our study design was limited to movement of the glenohumeral joint within the frontal plane even though the shoulder joint functions can both translate and rotate in six degrees of freedom. Given age-related changes to muscle elasticity were found to be muscle dependent, our findings are limited to the five muscles examined here and do not apply to other muscles crossing the shoulder girdle. It was not feasible to directly assess muscle activity with electromyography and acquire ultrasound images; muscle activity was monitored using the B-mode images in real-time by the ultrasonographer to ensure the muscle was not contracting before collecting the elastography data. Since the muscles were only examined in a relaxed state, future work is needed to predict how age and function relate to muscle elasticity during volitional contraction. Finally, our study only examined the dominant arm of subjects, with the vast majority of participants being right-handed. Future work is needed to investigate potential hand dominance differences in material properties of fiber regions and how various pathologies of the shoulder joint influence material properties.
In conclusion, ultrasound SWE has potential utility to assess age-related changes to muscle but these associations were muscle-dependent. Chronological age was only significantly related to muscle SWV for the sternocostal region of the pectoralis major and the middle trapezius. Identifying associations between upper extremity muscle elasticity and biological factors like age, gender, BMI, and functional status can aid the development of normative insights into how the material properties of muscle change with advanced age. Our findings provide normative values for evaluating a variety of upper extremity disorders with ultrasound SWE. In particular, our results provide new insights into whether ultrasound SWE measures acquired from a given upper extremity muscle are more related to healthy aging or pathology. Ultrasound SWE can also help assess the efficacy of rehabilitation strategies to improve function and the overall quality of life of older adults (Eby et al., 2015; Koo et al., 2014).
ACKNOWLEDMENTS
This research was financially supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R03HD097704. The authors received assistance recruiting participants through the Human Subjects and Assessment Core of the University of Michigan Claude D. Pepper Older Americans Independent Center, which is supported by the National Institute of Aging under award number P30AG024824.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors do not have any financial or personal relationships to disclose that could have inappropriately biased this work.
REFERENCES
- Ackland DC, Pak P, Richardson M, Pandy MG, 2008. Moment arms of the muscles crossing the anatomical shoulder. Journal of anatomy 213, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi R, Yamashita Y, Ueyasu Y, 2015. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound in medicine & biology 41,2906–2912. [DOI] [PubMed] [Google Scholar]
- Alfuraih AM, Tan AL, O’Connor P, Emery P, Wakefield RJ, 2019. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res 31, 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer TG, Dischler J, Davis L, Labyed Y, Siegal DS, van Holsbeeck M, Moutzouros V, Bey MJ, 2018. Effects of age and pathology on shear wave speed of the human rotator cuff. J Orthop Res 36, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercoff J, Tanter M, Fink M, 2004. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 51, 396–409. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, 1998. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther 11, 258–260. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, Eyre DR, 1993. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am 75, 1533–1548. [DOI] [PubMed] [Google Scholar]
- Chernak LA, DeWall RJ, Lee KS, Thelen DG, 2013. Length and activation dependent variations in muscle shear wave speed. Physiological measurement 34, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin DR, Larkin LM, Cederna PS, Horowitz JF, Alexander NB, Cole NM, Galecki AT, Chen S, Nyquist LV, Carlson BM, Faulkner JA, Ashton-Miller JA, 2011. Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. Journal of applied physiology (Bethesda, Md. : 1985) 111, 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Macneil LG, Tarnopolsky MA, 2013. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J. Gerontol. A. Biol. Sci. Med. Sci 68, 631–638. [DOI] [PubMed] [Google Scholar]
- Cui L, Perreault EJ, Maas H, Sandercock TG, 2008. Modeling short-range stiffness of feline lower hindlimb muscles. J Biomech 41, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGoede KM, Ashton-Miller JA, Schultz AB, 2003. Fall-related upper body injuries in the older adult: a review of the biomechanical issues. J Biomech 36, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Domire ZJ, McCullough MB, Chen Q, An KN, 2009. Feasibility of using magnetic resonance elastography to study the effect of aging on shear modulus of skeletal muscle. J Appl Biomech 25, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby SF, Cloud BA, Brandenburg JE, Giambini H, Song P, Chen S, LeBrasseur NK, An KN, 2015. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 30, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS, 2008. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP, 1998. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. Journal of applied physiology (Bethesda, Md. : 1985) 85, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Haus JM, Carrithers JA, Trappe SW, Trappe TA, 2007. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. Journal of applied physiology (Bethesda, Md. : 1985) 103, 2068–2076. [DOI] [PubMed] [Google Scholar]
- Hill CL, Gill TK, Shanahan EM, Taylor AW, 2010. Prevalence and correlates of shoulder pain and stiffness in a population-based study: the North West Adelaide Health Study. International journal of rheumatic diseases 13, 215–222. [DOI] [PubMed] [Google Scholar]
- Klein Breteler MD, Spoor CW, Van der Helm FC, 1999. Measuring muscle and joint geometry parameters of a shoulder for modeling purposes. J Biomech 32, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Koo TK, Guo JY, Cohen JH, Parker KJ, 2014. Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin Biomech (Bristol, Avon) 29, 33–39. [DOI] [PubMed] [Google Scholar]
- Landin D, Myers J, Thompson M, Castle R, Porter J, 2008. The role of the biceps brachii in shoulder elevation. J Electromyogr Kinesiol 18, 270–275. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR, 1997. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272, C638–649. [DOI] [PubMed] [Google Scholar]
- Lee SS, Spear S, Rymer WZ, 2015. Quantifying changes in material properties of stroke-impaired muscle. Clin Biomech (Bristol, Avon) 30, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardis JM, Desmet DM, Lipps DB, 2017. Quantifying differences in the material properties of the fiber regions of the pectoralis major using ultrasound shear wave elastography. J Biomech 63, 41–46. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M, 1988. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. Journal of the neurological sciences 84, 275–294. [DOI] [PubMed] [Google Scholar]
- Lindman R, Eriksson A, Thornell LE, 1991. Fiber type composition of the human female trapezius muscle: enzyme-histochemical characteristics. Am J Anat 190, 385–392. [DOI] [PubMed] [Google Scholar]
- Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC, 2010. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14, 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins JC, Aguiar LT, Lara EM, Teixeira-Salmela LF, Faria CD, 2015. Assessment of grip strength with the modified sphygmomanometer test: association between upper limb global strength and motor function. Braz J Phys Ther 19, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail SM, Schippers M, Marshall AL, 2014. Age, physical inactivity, obesity, health conditions, and health-related quality of life among patients receiving conservative management for musculoskeletal disorders. Clinical interventions in aging 9, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netuveli G, Wiggins RD, Hildon Z, Montgomery SM, Blane D, 2006. Quality of life at older ages: evidence from the English longitudinal study of aging (wave 1). J Epidemiol Community Health 60, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordez A, Hug F, 2010. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J Appl Physiol (1985) 108, 1389–1394. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M, 2005. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J. Gerontol. A. Biol. Sci. Med. Sci 60, 74–79. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor M, 2002. Change in physical performance over time in older women: the Women’s Health and Aging Study. J. Gerontol. A. Biol. Sci. Med. Sci 57, M289–293. [DOI] [PubMed] [Google Scholar]
- Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S, 2014. The burden of musculoskeletal conditions. PLoS One 9, e90633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K, Mason H, 2007. Relationship between self-reported upper limb disability and quantitative tests in hand-arm vibration syndrome. Disabil Rehabil 29, 359–366. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Sabra K, Gennisson JL, Fink M, Tanter M, 2010. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve 42, 438–441. [DOI] [PubMed] [Google Scholar]
- Srinivasan RC, Lungren MP, Langenderfer JE, Hughes RE, 2007. Fiber type composition and maximum shortening velocity of muscles crossing the human shoulder. Clinical anatomy (New York, N.Y.) 20, 144–149. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB, 2005. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. A. Biol. Sci. Med. Sci 60, 324–333. [DOI] [PubMed] [Google Scholar]
- Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C, 2004. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum 51,642–651. [DOI] [PubMed] [Google Scholar]
- Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV, 2014. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. Journal of applied physiology (Bethesda, Md. : 1985) 117, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova E, Jelen M, Schickhofer P, Hamar D, 2016. Jumping From a Chair is a More Sensitive Measure of Power Performance In Older Adults Than Chair Rising. Experimental aging research 42, 418–430. [DOI] [PubMed] [Google Scholar]


