Abstract
Purpose
The molecular targets for castration-resistant prostate cancer (CRPC) are unknown because the disease inevitably recurs, and therapeutic approaches for CRPC patients remain less well understood. We sought to investigate regulatory mechanisms that result in increased therapeutic resistance, which is associated with neuroendocrine (NE) differentiation of prostate cancer and linked to dysregulation of the androgen-responsive pathway.
Experimental Design
The underlying intracellular mechanism that sustains the oncogenic network involved in NE differentiation and therapeutic resistance of prostate cancer was evaluated to investigate and identify effectors. Multiple sets of samples with prostate adenocarcinomas and CRPC were assessed via immunohistochemistry and other assays.
Results
We demonstrated that leukemia inhibitory factor (LIF) was induced by androgen deprivation therapy (ADT) and was upregulated by ZBTB46 in prostate cancer to promote CRPC and NE differentiation. LIF was found to be induced in prostate cancer patients after ADT and was associated with enriched nuclear ZBTB46 staining in high-grade prostate tumors. In prostate cancer cells, high ZBTB46 output was responsible for the activation of LIF-signal transduction and activator of transcription 3 (STAT3) signaling and NE-like features. The abundance of LIF was mediated by ADT-induced ZBTB46 through a physical interaction with the regulatory sequence of LIF. Analysis of serum from patients showed that cases of higher tumor grade and metastatic prostate cancer exhibited higher LIF titers.
Conclusions
Our findings suggest that LIF is a potent serum biomarker for diagnosing advanced prostate cancer and that targeting the ZBTB46-LIF axis may therefore inhibit CRPC development and NE differentiation after ADT.
Keywords: androgen deprivation therapy (ADT) resistance, leukemia inhibitory factor (LIF), castration-resistant prostate cancer (CRPC), neuroendocrine (NE) differentiation, ZBTB46
Introduction
To date, prostate cancer therapies have attempted to suppress androgen receptor (AR) activity, but despite initial responses, recurrent castration-resistant prostate cancer (CRPC) eventually ensues (1). A subset of patients with advanced CRPC may progress to an AR-suppressed phenotype and histologically exhibit strong neuroendocrine (NE) characteristics called castration-resistant neuroendocrine prostate cancer (CRPC-NE) (2). CRPC-NE has been demonstrated to be resistant to androgen deprivation therapy (ADT) and displays a high metastatic propensity with an average survival of less than a year (3). Currently, there are no targeted therapies available that can lead to more effective treatments for CRPC-NE patients (4). Chemotherapeutic strategies and a new generation of drugs that target AR signaling often involve management to increase the efficacy of CRPC-NE treatment; however, side effects and the development of treatment resistance remain problematic (5). Therefore, identifying consensus targets not involving AR signaling could lead to more curative therapies for CRPC-NE.
Several molecular mechanisms have been implicated in ligand-induced NE differentiation events, including activation of phosphoinositide 3-kinase (PI3K) (6), mitogen-activated protein kinase (MAPK) (7), and signal transducer and activator of transcription (STAT) pathways (8). Dysregulation of STAT signaling at the level of ligands, receptors, or effectors has been shown to promote the initiation and progression of many malignancies (9,10). Leukemia inhibitory factor (LIF) activates both the Janus kinase (JAK)/STAT and RAS/MAPK pathways by mediating a specific LIF receptor (LIFR) (11). LIF is overexpressed in breast cancer and activates STAT3 signaling, leading to the induction of proliferation, epithelial-mesenchymal transition (EMT), and resistance to drug responses (12,13). In the prostate microenvironment, activation of LIF-STAT signaling occurs specifically through the phosphorylation of STAT3 and is involved in the appearance of metastasis and aggressive processes (8,14). Our understanding of the mechanisms by which LIF is stimulated in prostate cancer is incomplete; in particular, its involvement in CRPC development and NE differentiation and its contribution to ADT-induced drug resistance are unclear.
In prostate cancer cells, prolonged exposure to interleukin (IL)-6 has been shown to reduce AR expression (15) and induce resistance to AR-targeted treatment (16). LIF is a member of the IL-6 cytokine family and regulates embryonic stem cell self-renewal (17). LIF is a particularly important factor during critical stages of central nervous system development (18) and can induce neuronal plasticity in pancreatic cancer (19). LIF stimulates the differentiation of neuroglial cells and induces their migration by activating JAK/STAT3/AKT signaling (19). LIF acts as a key differentiation regulator of stem cells and as a neuropoietic cytokine (20,21). LIF also promotes the differentiation of an NE phenotypic switch in murine corticotropic cells (22). LIF signaling is often aberrantly activated in later stages of advanced metastatic cancers (23); however, it is not known whether LIF contributes to the development of CRPC or NE differentiation in prostate cancer patients following ADT. Herein, we addressed this question by focusing on regulatory mechanisms between LIF and the progression of CRPC and NE differentiation during the dysregulation of AR signaling pathways.
Our earlier study demonstrated that ADT stimulated the abundance of a prostatic tumor promoter (ZBTB46) and promoted EMT through the transcriptional regulation of SNAI1 (24). Herein, we demonstrated that the abundance of ZBTB46 was involved in LIF induction by directly and physically interacting with LIF. It is not clear whether LIF is particularly associated with CRPC development and NE differentiation following additional AR-targeted therapy. We identified consensus molecular pathways in prostate cancer cells modulated by the LIF-induced CRPC and NE-like phenotype via activated ZBTB46. We demonstrated that silencing of LIF by LIF-knockdown or treatment with an LIF inhibitor could suppress tumor growth and NE marker expression. Our results thus have potential implications for the development of effective treatment strategies for advanced prostate cancer.
Materials and Methods
Cell culture
The prostate adenocarcinoma cell line LNCaP, castration-resistant adenocarcinoma cell line C4–2, and AR-suppressed prostate cancer cell line PC3 (25) were obtained from the ATCC (MD, USA) and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Invitrogen). The RasB1 cell line (an aggressive cell line expressing a constitutively active Ras in DU145 cells and isolated from a bone metastasis) was provided by Dr. Kathleen Kelly (NCI/NIH, MD, USA) and maintained as described previously (24,26–28). The small-cell neuroendocrine carcinoma (SCNC) cell line NCI-H660 was purchased from the ATCC and cultured in RPMI 1640 medium supplemented with 0.005 mg/ml insulin (Sigma-Aldrich), 0.01 mg/ml transferrin (Sigma-Aldrich), 30 nM sodium selenite (Sigma-Aldrich), 10 nM hydrocortisone (Sigma-Aldrich), 10 nM ß-estradiol (Sigma-Aldrich), 4 mM L-glutamine (Invitrogen), and 5% FBS. Dihydrotestosterone (DHT) (Sigma-Aldrich) and LIF protein (R&D Systems) were used to treat cells at 10 nM and 200 ng/ml, respectively, for 24 h in a 10% charcoal-stripped serum (CSS)-containing medium. The AR antagonist enzalutamide (MDV3100) (Selleck) and the first-in-class steroidal LIF inhibitor EC330 (MedChemExpress) were used to treat cells at concentrations of 10 μM and 35 nM, respectively, for 24 h in 10% FBS-containing medium.
Immunohistochemical (IHC) staining
We collected 18 cases of clinical tissue samples from prostate cancer patients who were treated before and after ADT from Taipei Medical University-Wan Fang Hospital (Taipei, Taiwan). Tissue microarray (TMA) sections, including 16 normal prostatic epithelial samples, 100 primary prostate adenocarcinomas, and eight SCNCs were collected from Duke University School of Medicine (Durham, NC, USA). Tissue samples were obtained and used according to protocols approved by the Taipei Medical University-Joint Institutional Review Board (approval no. N201711067) and the Duke University School of Medicine-Institutional Review Board (protocol ID: Pro00070193) in accordance with Declaration of Helsinki and U.S. Common Rule. IHC staining of ZBTB46, LIF, Chromogranin A (CgA/CHGA), neuron-specific enolase (NSE/ENO2), Ki67, phosphorylated (p)-STAT3, and cleaved caspase-3 was performed using the antibodies listed in Supplemental Table S4. The pathological diagnoses and Gleason score of these cases were microscopically reconfirmed by two pathologists (Wei-Yu Chen and Qingfu Zhang). The intensity was denoted as 0 (negative), 1+ (weakly positive), 2+ (moderately positive), and 3+ (strongly positive). H-score values (range 0~300) were calculated according to the following formula: [(% cells with an intensity of 1+) + 2 × (% cells with an intensity of 2+) + 3 × (% cells with an intensity of 3+)].
Invasion assay
For the invasion assay, LNCaP and C4–2 cells stably expressing the LIF expression vector or PC3 and RasB1 cells with LIF knockdown were resuspended at a concentration of 2 × 105 cells/ml in FBS-containing medium. Matrigel™ (BD Biosciences)-coated transwell dishes were prepared by adding 200 μl of Matrigel™ diluted 10-fold in FBS-containing medium. Cells that had invaded the Matrigel™-coated transwells after 10 h were fixed and stained with a 0.5% crystal violet fixative solution for 15 min. Invaded cells on the underside of the membrane were quantified in five medium-power fields for each replicate in triplicate.
Proliferation assay
LNCaP and C4–2 cells stably expressing the LIF expression vector or PC3 and RasB1 cells with LIF knockdown were seeded at a density of 2 × 103 cells/well in 96-well plates. LNCaP, C4–2, PC3, and NCI-H660 cells were treated with 0, 10, 20, 30, 35, 40, 50 and 100 nM EC330 for 24 h and analyzed using a Cell Proliferation Assay Kit (Promega cat. # G4000) according to the manufacturer’s protocol. For the experiment, multiple wells were assessed at each time point and then averaged. The cells were stained with a 0.5% crystal violet fixative solution for 15 min, rinsed in distilled water, and dissolved in 50% ethanol containing 0.1 M sodium citrate. The absorbance was quantified at a wavelength of OD 550 nm on a plate reader.
Colony-formation assay
A colony-formation assay was performed using single-cell suspensions of C4–2 and LNCaP cells stably expressing the LIF expression vector or PC3 and RasB1 cells with LIF knockdown. Cells were seeded at a density of 500 cells/well in 6-well plates in triplicate and incubated for 14 days at 37 °C in a humidified incubator. Following incubation in a 0.5% crystal violet fixative solution for 15 min, colonies of more than 50 μm in diameter were counted and quantified for each replicate in triplicate.
Tumorigenicity assays in mice
Animal work was performed in accordance with a protocol approved by the Taipei Medical University Animal Care and Use Committee (approval no. LAC-2017–0269, Taiwan). For the tumorigenicity assays, 5-week-old male nude mice (NLAC, Taiwan) were subcutaneously injected with 2.5 × 106 LIF shRNA vector-transfected PC3 cells in 50% Matrigel™ (BD Biosciences). For EC330 treatment, mice were injected with PC3 or NCI-H660 cells for 1 month or 18 days, respectively, and then treated with 2.5 mg/kg EC330 or DMSO as the control by intraperitoneal injection once every two days. The tumor size was measured weekly or twice a week, respectively, with calipers, and the tumor volume was calculated using the following formula: tumor volume = (4/3) x (L/2) (W/2)2, where L is the length and W is the width. The results are presented as the mean ± standard error (SE) for each experimental group.
Immunofluorescence (IF) staining
A CRPC TMA section was obtained from Duke University School of Medicine using LIF (Abcam cat. # ab135629,) and CgA (Abcam cat. # ab15160) antibodies at 1:100 and 1:150 dilutions, respectively. Tissue sections were washed with PBS containing 0.1% Tween-20, incubated with Alexa-488- and/or Alexa-568-conjugated immunoglobulin G (IgG) in 2% BSA for 30 min at room temperature, and finally washed and mounted using Fluoro-gel II anti-fade reagent with DAPI (Electron Microscopy Sciences cat. # 17984–24). Fluorescent signal images were captured using an inverted and/or upright fluorescence Axioplan microscope (Zeiss).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using an EZ magna ChIP A kit (Millipore cat. # 17–10086) with a modified protocol (29). For each sample, 107 cells in 10-cm dishes were treated with DHT (10 nM) or MDV3100 (10 μM) in 10% CSS- or FBS-containing medium as indicated for 10 h. Nuclear extract preparation, immunoprecipitation, and DNA-purification steps were performed according to the protocol provided by Millipore. Quantitative (q)PCR was performed in triplicate with 1 μl of eluted chromatin. Enrichment is presented as a percentage of the total input normalized to IgG. ChIP antibodies and PCR primers are listed in Supplemental Table S5.
Promoter reporter assay
For promoter reporter assays, cells in 12-well plates (5 × 104 cells/well) were transiently cotransfected with 1 μg of the promoter reporter and 1 μg of the cDNA expression vector or 100 nM siRNA followed by DHT (10 nM) or MDV3100 (10 μM) treatment for 24 h in 10% CSS- or FBS-containing medium, respectively. Reporter activity was analyzed as the relative median fluorescence intensity (MFI) values for the green fluorescence protein (GFP) and was measured by FACS using FACS Diva software and normalized to the value of the vehicle.
Enzyme-linked immunosorbent assay (ELISA) assay
Sera from healthy donors (20 samples), patients with benign prostatic hyperplasia (BPH) (20 samples), patients with prostate cancer (20 samples), and prostate cancer patients with bone metastasis (20 samples) were collected from the Chinese PLA General Hospital (Beijing, China). All human sera were collected in June and July of 2018. The eight BPH patients and 18 prostate cancer patients who had been tested for LIF serum levels were selected for LIF, CgA, and NSE examination by IHC staining. Written informed consent was obtained from all patients, and the study was approved by the Ethics Committee and Institutional Review Board of the Chinese PLA General Hospital in accordance with Declaration of Helsinki. LIF titration in serum was performed with the Legend Max™ Human LIF ELISA Kit (BioLegend cat. # 443507) according to the supplier’s instructions.
Statistical Analysis
All data are presented as the mean ± standard error of the mean (SEM). Statistical calculations were performed with GraphPad Prism analytical tools. Differences between individual groups were determined by Student’s t-test or one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test for comparisons among three or more groups. A log-rank test was used for the survival curve analysis. The method for determining cutoffs was predecided for half of the patients, and p values <0.05 were considered statistically significant.
Results
The LIF-STAT3 pathway induces NE differentiation
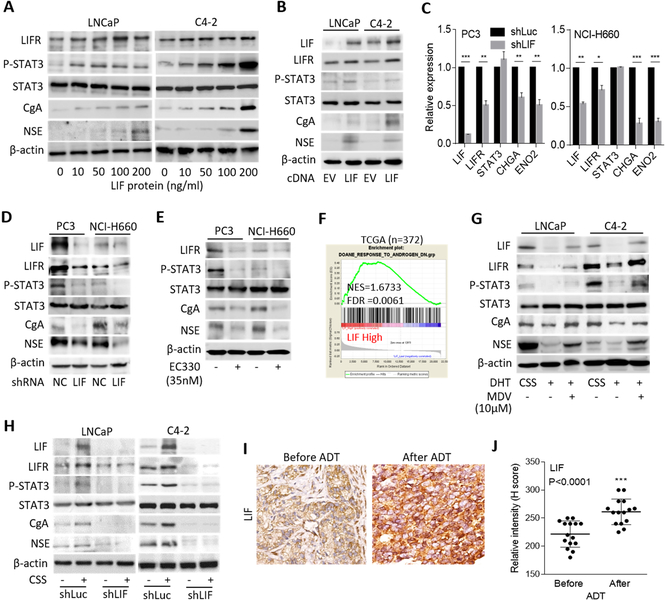
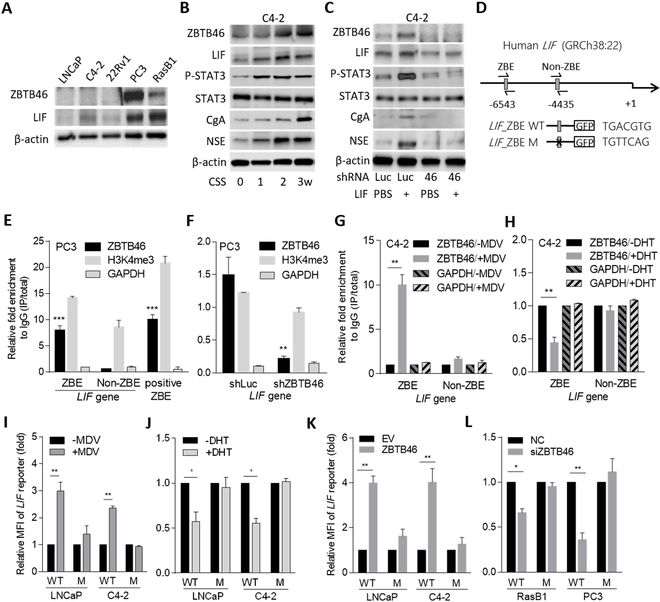
To better understand the transcriptome profile consistent with CRPC-NE, we checked the levels of messenger (m)RNAs of AR, LIF, and NE markers (chromogranin A (CgA/CHGA) and neuron-specific enolase (NSE/ENO2)) expression in a panel of the cell lines. We found that the AR-suppressed PC3 and RasB1 cells and SCNC cell line NCI-H660 expressed lower AR and higher LIF and NE markers than did the prostate adenocarcinoma cell lines LNCaP and C4–2 (Supplementary Fig. S1A–D). We sought to determine whether activation of the LIF-STAT3 pathway could modulate the NE differentiation of prostate cancer. We investigated the effect of LIF on the NE differentiation of adenocarcinoma cells and observed an induction of p-STAT3, LIFR, and NE markers in LNCaP and C4–2 cells treated with increased LIF protein (Fig. 1A and Supplementary Fig. S1E). To determine if LIF overexpression could induce NE differentiation, we generated LNCaP and C4–2 cells that stably express LIF complementary (c)DNA and found that forced expression of LIF significantly induced the mRNA and protein levels of LIFR and NE markers (Supplementary Fig. S1F and Fig. 1B). In contrast, decreases in mRNA levels of the LIFR and NE markers were observed in PC3 and NCI-H660 cells with LIF knockdown (Fig. 1C). Western blotting confirmed that the reduction of p-STAT3, LIFR, and NE markers was positively associated with LIF knockdown in PC3 and NCI-H660 cells (Fig. 1D). Moreover, the mRNA and protein levels of the LIFR and NE markers were decreased in PC3 and NCI-H660 cells when the cells were treated with EC330, an LIF inhibitor (Supplementary Fig. S1G and Fig. 1E). These results support that an abundance of LIF and activation of the LIF-STAT3 pathway induces NE differentiation of prostate cancer cells.
Figure 1.
ADT induces LIF abundance and NE differentiation of prostate cancer cells. (A) Western blotting for LIFR, p-STAT3, STAT3, CgA, and NSE in LNCaP and C4–2 cells treated with LIF protein at various concentrations for 24 h. (B) Protein levels of LIF, LIFR, p-STAT3, STAT3, CgA, and NSE in LNCaP and C4–2 cells following stable expression of an LIF cDNA vector. (C) mRNA levels of LIF, LIFR, STAT3, CHGA, and ENO2 in PC3 and NCI-H660 cells following stable expression of an LIF shRNA vector. (D) Western blotting for LIF, LIFR, p-STAT3, STAT3, CgA, and NSE in PC3 and NCI-H660 cells stably expressing the LIF shRNA vector. (E) Western blotting for LIFR, p-STAT3, STAT3, CgA, and NSE in PC3 and NCI-H660 cells treated with EC330 (35 nM) for 24 h. (F) GSEA of the TCGA prostate cancer dataset showing that higher LIF expression was associated with an androgen-inactivated gene signature. NES, normalized enrichment score; FDR, false discovery rate. (G) Effects of DHT and MDV3100 (MDV), relative to that of the vehicle (methanol or DMSO), on the expression of LIF, LIFR, p-STAT3, STAT3, CgA, and NSE proteins in LNCaP and C4–2 cells cultured with 10% CSS-containing medium. (H) LIF, LIFR, p-STAT3, STAT3, CgA, and NSE protein levels in control (Luc) and LIF-knockdown LNCaP or C4–2 cells and those treated with androgen withdrawal (CSS) for 1 week. (I and J) IHC staining (I) and analysis (J) of cytoplasmic LIF in prostate cancer tissue sections from patients before and after ADT treatment. The 18 samples were obtained from Taipei Medical University-Wan Fang Hospital. Scale bars, 100 μm. Statistical analysis by the two-tailed Student’s t-test. Data from the quantification of mRNA are presented as the mean ± SEM, n=3. * p<0.05, ** p<0.01, *** p<0.001.
Inhibition of AR signaling induces LIF and is associated with NE differentiation
To assess whether the abundance of LIF was mediated by ADT, we analyzed LIF expression with signatures that reflect AR signaling components in the Taylor and TCGA prostate cancer datasets by gene set enrichment analyses (GSEAs). We found that tissues expressing low levels of LIF were significantly associated with gene signatures of upregulated androgen-responsiveness (30,31) (Supplementary Fig. S2A), whereas high levels of LIF were associated with a downregulated androgen-responsive signature (32) (Fig. 1F). We further analyzed LIF expression by z-score analysis, which was grouped based on relative AR activities defined by the levels of gene signatures regulated by androgens (30,31). We found that higher LIF expression was associated with the downregulation of androgen-responsive genes (Supplementary Fig. S2B), whereas tissues that expressed low levels of LIF had gene signatures associated with androgen upregulation (Supplementary Fig. 2C). We validated the expression of LIF in AR-positive LNCaP and C4–2 cells relative to the AR signaling response. We found that cells treated with the AR ligand DHT had lower levels of LIF, LIFR, p-STAT3, and NE markers than did cells with androgen withdrawal (after culturing in CSS-containing medium) (Fig. 1G). In contrast, higher expression levels of LIF, LIFR, p-STAT3, and NE markers were found in DHT-treated cells exposed to the AR antagonist MDV3100 (Fig. 1G). We further performed LIF knockdown in LNCaP and C4–2 cells and treated the cells with androgen withdrawal. The results showed that LIF-knockdown reduced LIF, LIFR, p-STAT3, and NE markers regardless of the treatment with CSS-containing medium (Fig. 1H, Supplementary Fig. S2D, E), confirming that the abundance of LIF was involved in NE differentiation after ADT. We next analyzed samples consisting of tissue specimens from 18 prostate cancer patients before and after undergoing ADT, collected from Taipei Medical University-Wan Fang Hospital (Taipei, Taiwan). We found that the cytoplasmic LIF was increased in prostate tumors from patients who had received ADT compared with the corresponding levels in the same patients before ADT treatment (Fig. 1I, J). These results suggest that ADT or inhibition of AR signaling induces LIF expression, which is associated with NE differentiation of prostate cancer.
LIF promotes a malignant phenotype in prostate cancer
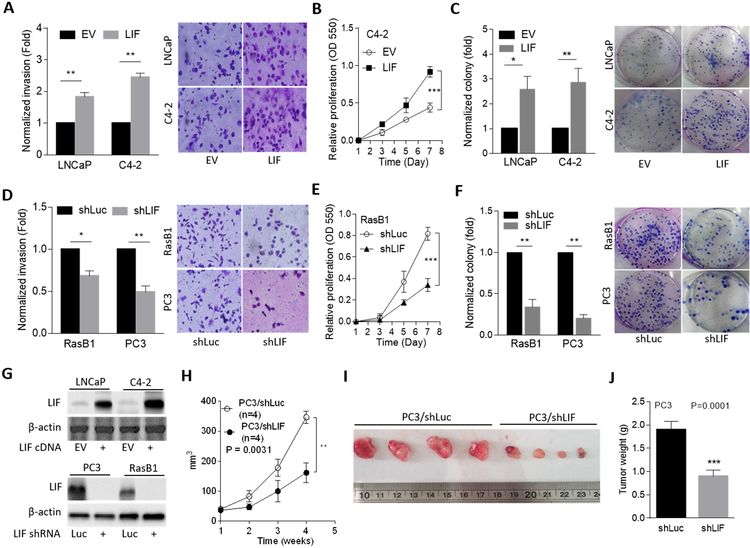
To investigate the functional relevance of LIF-mediated induction of the malignant phenotype in nonmetastatic cell lines, LNCaP and C4–2 cells were overexpressed with LIF. We found that cells with ectopic LIF expression had induced cell invasiveness through Matrigel (Fig. 2A) and exhibited higher cell proliferation and colony formation than did cells carrying the empty vector (Fig. 2B, C and Supplementary Fig. S3A). In contrast, when RasB1 and PC3 metastatic cell lines were exposed to LIF knockdown, cell invasiveness decreased (Fig. 2D). We also found that LIF knockdown significantly reduced the proliferation rate of RasB1 and PC3 cells (Fig. 2E and Supplementary Fig. S3B) and reduced colony formation in RasB1 and PC3 cells (Fig. 2F). LIF expression was confirmed by Western blotting in cells harboring LIF alterations (Fig. 2G). These results were supported by further in vivo experiments. Mice that were administered subcutaneous injections of PC3 cells with LIF knockdown had significantly reduced tumor formation and tumor weights compared to those in mice injected with PC3 cells carrying the empty vector (Fig. 2H–J). These data suggest that LIF acts as a tumor promoter that induces tumor formation in prostate cancer cells, thus promoting a variety of invasive properties and the growth rate of prostate cancer cells.
Figure 2.
LIF induces malignant progression of prostate cancer cells. (A) Invasion assay in stable cell lines containing either a control (empty vector; EV) or LIF-expressing vector. Selected images are shown on the right. (B) Proliferation assay in stably LIF-expressing C4–2 cells. (C) Colony formation by LNCaP and C4–2 cells stably transfected with the EV or LIF-expressing vector. (D) Invasion assay in RasB1 and PC3 cells by stable transfection of either a control (shLuc) or an LIF-targeted shRNA (shLIF) vector. (E and F) Proliferation (E) and colony formation (F) of RasB1 or PC3 cells stably transfected with shLuc or shLIF. (G) Confirmation of the overexpression (top) or knockdown (bottom) efficiency by an LIF-expressing vector or LIF shRNA in stable cell lines. (H-J) Growth (H), images (I), and weights (J) of tumor xenografts in male nude mice 4 weeks after subcutaneous inoculation with PC3 cells stably expressing shLuc or shLIF. n=4 mice per group. Data from the invasion, proliferation, and colony formation assays are presented as the mean ± SEM from three independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
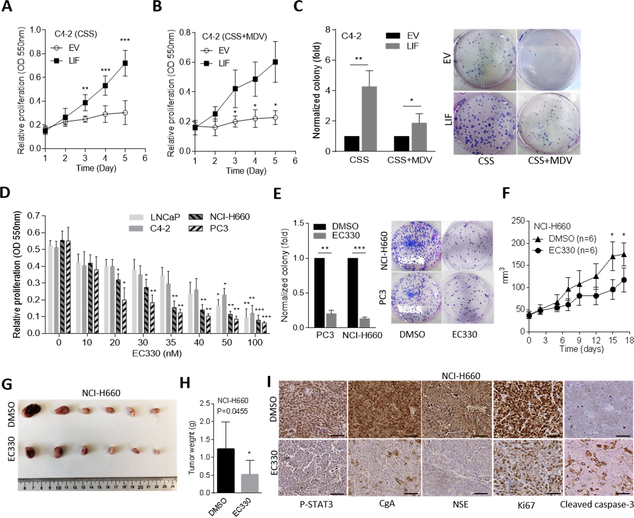
LIF induces prostate cancer resistance to AR-targeted therapeutics
To examine the biological effect of LIF after ADT, we cultured AR-positive cells with CSS-containing medium to mimic an androgen-deprivation condition. Notably, overexpression of LIF in C4–2 and LNCaP cells significantly promoted cell proliferation and colony formation regardless of androgen withdrawal or being combined with MDV3100 treatment (Fig. 3A–C and Supplementary Fig. S3C–E), suggesting that an abundance of LIF could induce tumor growth irrespective of AR signaling inhibition in AR-positive cells. Moreover, we treated cells with an LIF inhibitor and found that AR-negative PC3 and NCI-H660 cells had greater drug sensitivity than AR-positive LNCaP and C4–2 cells (Fig. 3D). We also found a significant reduction of colony formation in PC3 and NCI-H660 cells or in C4–2 and LNCaP cells overexpressing LIF following treatment with an LIF inhibitor (Fig. 3E and Supplementary Fig. S3F, G). We further studied the role of the LIF inhibitor in suppressing the tumor growth of AR-negative PC3 and NCI-H660 cells in vivo. We found that in mice administered subcutaneous injections of PC3 and NCI-H660 cells, treatment with the LIF inhibitor produced significant decreases in the tumor growth rate and tumor weights (Fig. 3F–H and Supplementary Fig. S4A–C). IHC analyses showed that LIF inhibitor-treated tumors had reduced p-STAT3, NE (CgA and NSE) and proliferation (Ki67) markers and had gained an apoptotic marker (cleaved caspase-3) (Fig. 3I and Supplementary Fig. S4D–F). These data suggest that targeting LIF might suppress CRPC-NE progression and efficiently inhibit tumor growth in prostate cancer cells.
Figure 3.
LIF promotes tumor growth and enzalutamide resistance, and LIF inhibitor reduces NE marker expression. (A and B) Proliferation assay in C4–2 cells treated with androgen withdrawal (A, charcoal-stripped serum (CSS)) or the combination of androgen withdrawal and enzalutamide (10 μM) (B, CSS+MDV3100), n=8. (C) Quantification and images of the colony formation of C4–2 cells with androgen withdrawal (CSS) or combined androgen withdrawal with MDV3100 (10 μM) treatment (CSS+MDV) for 6 days following empty vector (EV) or LIF-expressing vector overexpression. (D) Proliferation assays in LNCaP, C4–2, PC3, and NCI-H660 cells treated with an LIF inhibitor (EC330) at the indicated concentrations for 24 h, n=8. (E) Colony formation of PC3 and NCI-H660 cells with EC330 (35 nM) treatment for 6 days. (F-H) Tumor growth analysis of NCI-H660 cells subcutaneously inoculated into male nude mice followed by treatment with EC330 (2.5 mg/kg). Tumor sizes were monitored twice a week (F), and images (G) and tumor weights (H) were also measured at the end of the experiment. n=6 mice per group. (I) IHC staining of subcutaneous tumors with antibodies specific for p-STAT3, CgA, NSE, Ki67, and cleaved caspase-3 in tumor-bearing mice from F. Scale bars represent 100 μm. Data from the proliferation and colony formation assays are presented as the mean ± SEM from three independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
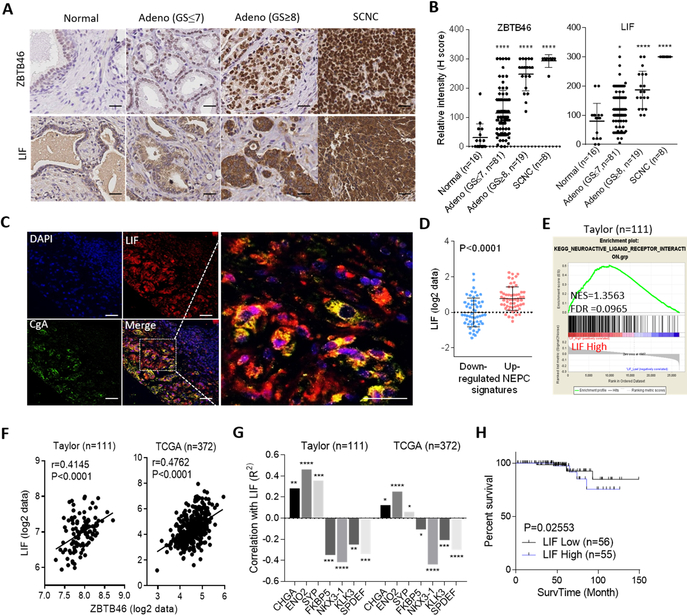
LIF associates with ZBTB46 expression and contributes to CRPC-NE progression
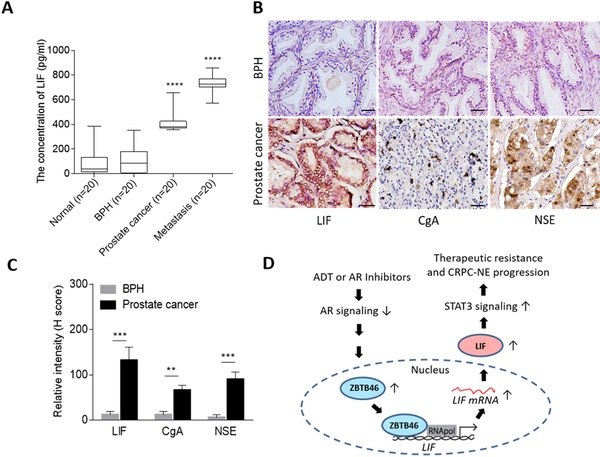
We have previously shown that hormonal therapy for prostate cancer enhances ZBTB46 overexpression (24). ZBTB46 is a lineage-specific transcription factor that is crucial for EMT and metastasis of prostate cancer (24). We hypothesized that the underlying mechanism by which LIF induces NE differentiation after ADT is associated with the abundance of ZBTB46. We analyzed 16 normal prostatic epithelial samples, 100 primary prostate adenocarcinomas, and eight SCNCs from a prostate cancer TMA collected from the Department of Pathology at Duke University School of Medicine (NC, USA). IHC analyses showed that cytoplasmic LIF was associated with increased nuclear ZBTB46 and had greater abundances in high-grade tumors and SCNC samples (Fig. 4A, B). Quantitative coIF staining of the CRPC TMA confirmed that in high-grade prostate tumors, LIF expression was restricted to NE-like (CgA-positive) tumor cells (Fig. 4C). Moreover, the abundance of LIF was positively associated with gene signatures of CRPC-NE-differentiation (3) and neuronal development, as confirmed by z-score analyses and GSEAs (Fig. 4D, E and Supplementary Fig. S5A, B). Furthermore, the mean expression correction was validated in the Taylor and TCGA prostate cancer datasets, showing that LIF was positively correlated with ZBTB46 and the expression of NE markers but was inversely correlated with androgen-responsive genes (FKBP5, NKX3.1, KLK3, and SPDEF) (Fig. 4F, G). In studying the clinical relevance of LIF, we found that prostate tumors with either higher ZBTB46 or higher LIF expression were associated with metastasis (Supplementary Fig. S5C, D) and a high pathological grade in the Gleason score (PathGGS) (Supplementary Fig. S5E, F). Moreover, Kaplan-Meier analyses showed that patients with tumors exhibiting higher LIF gene expression had a shorter survival than those with low LIF expression in the Taylor prostate cancer dataset (Fig. 4H). These results indeed supported an association of the induction of LIF and ZBTB46 expression with aggressive prostate cancer.
Figure 4.
LIF is positively associated with the induction of ZBTB46 and NE markers in clinical samples. (A) IHC staining of ZBTB46 and LIF of a prostate cancer TMA from Duke University School of Medicine. Scale bars, 100 μm. (B) Relative intensities of the ZBTB46- and LIF-positive staining statuses in prostate cancer TMA sections containing normal tissues (n=16), adenocarcinomas with a Gleason score ≤ 7 (n=81), adenocarcinomas with a Gleason score ≥ 8 (n=19), and SCNCs (n=8) from Duke University School of Medicine by H-score analysis. (C) IF staining of a CRPC TMA from Duke University School of Medicine showing coexpression of LIF and CgA in the same tumor cells. Scale bars, 50 μm. (D and E) Z-score analyses (D) and GSEA (E) of the Taylor prostate dataset showing that higher LIF expression in prostate tissues was positively associated with a CRPC-NE response signaling gene set (D) and neuronal development signature (E). (F and G) Pearson correlation analysis of LIF with ZBTB46, NE markers, and androgen-response gene mRNA levels in clinical tissue samples from the Taylor and TCGA prostate cancer datasets. Significance was determined using a two-tailed test. * vs. LIF. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. (H) Survival analysis of patients in the Taylor dataset (n=111) based on LIF expression. A log-rank test was used for the survival curve analysis.
LIF-induced NE differentiation is ZBTB46-dependent
We hypothesized that LIF stimulates malignant progression through the upregulation of ZBTB46 after ADT. To address this hypothesis, we measured ZBTB46 and LIF expression in a variety of nonmetastatic and metastatic prostate cancer cell lines. We found that AR-negative PC3 and RasB1 cells, which readily metastasize to bone (28,33), had higher ZBTB46 and LIF expression levels than cell lines that do not metastasize, such as AR-positive LNCaP, C4–2, and 22Rv1 cells (Fig. 5A). Regarding our recent study demonstrating the induction of ZBTB46 following ADT (24), we next examined whether ADT-induced ZBTB46 was associated with LIF abundance and NE differentiation following ADT. We found that long-term treatment with CSS-containing medium induced LIF and was associated with ZBTB46, p-STAT3, and NE marker stimulation in C4–2 cells (Fig. 5B). We further confirmed that such signaling activation was mediated by ZBTB46, as we found that ZBTB46 knockdown was able to inhibit the expression of LIF and NE markers, even in cells treated with LIF protein (Supplementary Fig. S6A). We then analyzed the expression of LIF and NE markers after stably introducing a ZBTB46 cDNA vector into C4–2 cells. Increased ZBTB46 expression significantly induced the expression of LIF and NE marker mRNAs, and better induction was observed in cells treated with LIF protein (Supplementary Fig. S6B). Immunoblots of extracts from ZBTB46-knockdown C4–2 cells confirmed the reduction of LIF, p-STAT3 and NE markers, regardless of whether the cells were treated with LIF protein (Fig. 5C). Interestingly, we also observed a positive feedback of the LIF-STAT3 pathway in inducing ZBTB46 abundance because increased LIF was found to induce ZBTB46 expression in control cells (Fig. 5C and Supplementary Fig. S6A, B). These results indicate that stimulating the LIF-STAT3 pathway promotes NE differentiation, which is associated with ZBTB46 expression in prostate cancer cells.
Figure 5.
The induction of LIF is regulated by ZBTB46 in prostate cancer cells following ADT. (A) Western blotting of ZBTB46 and LIF from a panel of prostate cancer cells. (B) Western blotting of ZBTB46, LIF, p-STAT3, STAT3, CgA, and NSE in C4–2 cells after treatment with androgen withdrawal (CSS) at each time point. (C) Abundances of ZBTB46, LIF, p-STAT3, STAT3, CgA, and NSE protein levels in control (Luc) and ZBTB46-knockdown C4–2 cells and those treated with LIF protein (200 ng/ml) or vehicle (PBS) for 24 h. (D) Schematic of the predicted ZBTB46 response element (ZBE) or a non-ZBTB46 response element (non-ZBE) and an introduced binding site mutant in regulatory sequence reporter constructs of human LIF. (E) ChIP assays in PC3 cells. Antibodies against H3K4me3 and GAPDH served as the positive and negative controls, respectively. Precipitated DNA was quantified by qRT-PCR of ZBE, non-ZBE, and positive ZBE sites. Enrichment is presented as a percentage of the total input followed by normalization to immunoglobulin G (IgG). * vs. non-ZBE. (F) ChIP assays in PC3 cells following control (Luc) or ZBTB46 knockdown. * vs. shLuc. (G and H) ChIP assays in C4–2 cells following MDV3100 (10 μM, left) or DHT (10 nM, right) treatment for 10 h. (I and J) Relative MFIs of the wild-type (WT) and mutant (M) LIF reporter in LNCaP and C4–2 cells after treatment with MDV3100 (I) and DHT (J). (K and L) Relative WT and M LIF reporter activities in response to ZBTB46 overexpression in LNCaP and C4–2 cells (K) or ZBTB46 knockdown in RasB1 and PC3 cells (L). Quantification of the mRNA and ChIP data and MFIs are presented as the mean ± SEM from three independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
ZBTB46 binds directly to the LIF regulatory sequence and enhances its transcription
We next examined whether the LIF abundance is upregulated by ZBTB46. Using ZBTB46-specific siRNA in RasB1 and PC3 cells, we observed a reduction in LIF mRNA (Supplementary Fig. S6C), whereas overexpression of ZBTB46 cDNA in LNCaP and C4–2 cells resulted in an abundance of LIF mRNA (Supplementary Fig. S6D). We hypothesized that ZBTB46 could transcriptionally activate LIF in prostate cancer cells by directly binding to the ZBTB46 response element (TGACGTC) (34) on the LIF promoter. When analyzing the putative ZBTB46 response element in the LIF regulatory sequence, we found a candidate response element for ZBTB46 at nucleotide −6543 relative to the LIF transcriptional start site (Fig. 5D). To test whether ZBTB46 directly bound to LIF, we performed ChIP assays in PC3 cells and found increased ZBTB46-binding activity at the putative ZBTB46-binding site (ZBE) on the LIF regulatory sequence and at a positive ZBE compared with a non-ZBTB46-binding site (non-ZBE) (Fig. 5E). Importantly, decreased ZBTB46-binding activity was found at the putative ZBE in the presence of ZBTB46-knockdown (Fig. 5F). Moreover, the ZBTB46-binding signal was enriched in C4–2 cells after MDV3100 treatment (Fig. 5G) but was reduced after DHT treatment of the cells (Fig. 5H), which is consistent with our hypothesis that ADT-regulated ZBTB46 mediates LIF transcription. We next performed reporter assays with an LIF regulatory sequence construct containing the ZBE (Fig. 5D). We generated a mutation at the putative ZBE and performed reporter assays in RasB1 and PC3 cells and found that compared with the ZBE wild-type, the ZBE mutant reduced the reporter activity (Supplementary Fig. S6E). We also found significantly increased reporter activity in MDV3100-treated LNCaP and C4–2 cells (Fig. 5I), and decreased reporter activity was observed upon DHT treatment (Fig. 5J). Moreover, the ZBE mutant disrupted the ability of MDV3100 and DHT, respectively, to induce and reduce reporter activity in the promoter assays (Fig. 5I, J). Furthermore, the ZBE mutant abolished the ability of ectopic ZBTB46 to induce reporter activity in LNCaP and C4–2 cells (Fig. 5K) and disrupted the ability of ZBTB46 knockdown to reduce reporter activity in RasB1 and PC3 cells (Fig. 5L). These data are consistent with a mechanism whereby ZBTB46-enhanced LIF transcription is site-specific and AR signaling-dependent.
LIF titers in serum as a diagnostic and prognostic biomarker for prostate cancer
To definitively assess whether LIF is a diagnostic and prognostic biomarker for prostate cancer, we validated LIF titers in the sera from a cohort including healthy donors (n=20), patients with BPH (n=20), patients with prostate tumors (n=20), and prostate cancer patients with metastatic tumors (n=20) from the Chinese PLA General Hospital (Beijing, China). We found that healthy donors and BPH patients displayed a low level of LIF in serum (<200 pg/mL), whereas the patients with tumors displayed a higher level of LIF in serum (>400 pg/mL) (Fig. 6A). Interestingly, patients with bone metastasis showed robust LIF quantities in serum (>700 pg/mL) (Fig. 6A). These data revealed that LIF titration of serum can serve as a biomarker to predict patients with high-grade or metastatic prostate cancer. To better understand the connection between NE markers in prostate tumors and LIF levels in serum, we collected prostate tissue samples for LIF and NE marker examination from the same patients who had been tested for LIF serum levels. The results demonstrated that compared with BPH patients, prostate cancer patients with higher LIF serum levels were positively associated with induced LIF and NE marker expression in their prostate tissues (Fig. 6B, C). In summary, LIF titers in serum were associated with prostate cancer progression and could help in classifying prostate cancer patients with metastasis or CRPC-NE development. Our data support the connection between ADT-induced ZBTB46 and STAT3 pathway stimulation in therapeutic resistance and NE differentiation of prostate cancer through activated LIF. Mechanistically, the abundance of LIF was upregulated by ADT resistance-induced ZBTB46 by direct and physical interaction with LIF (Fig. 6D).
Figure 6.
LIF is a potent diagnostic and predictive biomarker for prostate cancer. (A) Measurement of LIF levels in human serum from healthy donors (n=20), patients with BPH (n=20), prostate cancer (n=20), and patients with bone metastatic tumors (n=20) collected from the Chinese PLA General Hospital. * vs. Normal. (B and C) IHC staining (B) and relative intensities (C) of samples from eight BPH patients and 18 prostate cancer patients collected from (A) for LIF, CgA, and NSE examination. Scale bars, 100 μm. ** p<0.01, *** p<0.001. (D) Proposed model of LIF-mediated therapeutic resistance and NE differentiation of prostate cancer. An anti-androgen or AR antagonist inactivates AR signaling and induces ZBTB46 expression. Induced ZBTB46 enhances malignant progression and is involved in the development of CRPC-NE by an activated LIF-STAT3 pathway.
Discussion
Consistent with our earlier studies of patients with ADT, prostate adenocarcinoma cells are likely to change into a NE-like phenotype, which is AR signaling suppressed and terminally resistant to AR-targeted therapies (2,35,36). Herein, we identified that the ADT resistance-induced ZBTB46-LIF pathway is involved in therapeutic resistance and NE differentiation of prostate cancer. We provide evidence showing that ZBTB46 is involved through the inactivation of AR signaling and activation of the LIF-STAT3 pathway in prostate cancer cells. This finding, together with the observation that induction of LIF is related to increased malignancy in vitro and in vivo and NE marker expression, suggests that LIF might act as an important regulator in CRPC-NE development. These results are consistent with previous findings in which LIF was originally demonstrated to play an important role in the differentiation and proliferation of murine macrophage cells and to belong to a neuropoietic cytokine family that contributes to neural development (37).
We have previously shown that ZBTB46 overexpression can promote AR-independent proliferation under conditions of androgen withdrawal or in the presence of enzalutamide (24), which provides insights into the oncogenic role of ZBTB46 in prostate cancer after ADT. We investigated the mechanisms underlying how hormonal therapy enhances the overexpression of ZBTB46 in malignant progression. Our study confirmed a novel positive association between ZBTB46 activity and LIF levels in prostate cancer tissues and cells. Under androgen regulation, low levels of ZBTB46 are an essential transcriptional factor for maintaining LIF-STAT3 signaling, while the loss of androgen signaling or inhibition of AR signaling causes LIF-enhanced therapeutic resistance and CRPC characteristics through the upregulation of ZBTB46. We also found that LIF activation drives malignant progression and NE-like reprogramming in prostate cancer by activating STAT3 signaling. Consistently, our results support that LIF functions as an oncogene and participates in the progression of many malignancies (38,39).
Prostate cancer frequently becomes refractory to hormone therapy because the AR is often mutated or absent from recurrent tumors, thus rendering androgen-independent prostate cancer (1,40). STAT signaling normally activates an androgen response in prostate cancer epithelial cells, and androgen-independent prostate cancer cells exhibit aberrations in STAT3 signaling that promote cell proliferation (41,42). Our study elucidated a link between NE differentiation and ADT-induced ZBTB46 that is associated with activation of the LIF-STAT3 pathway. Although we demonstrated that LIF is a direct target of ZBTB46, our results show that an increase in LIF protein can also induce ZBTB46, suggesting a positive feedback loop between ZBTB46 and LIF-STAT3 signaling. In addition to the involvement of the IL-6 cytokine axis in prostate cancer resistance to AR antagonists (43,44), we showed that targeting AR activity induces both ZBTB46 and LIF; it is possible that factors downstream of STAT3 signaling are involved in the androgen-resistance phenotype, partly through induction of ZBTB46. Because of the oncogenic function of ZBTB46 observed in prostate cancer cells (24), the induction of LIF expression by suppression of AR signaling would provide a selective advantage to cells that stimulate STAT3 signaling during CRPC-NE progression.
Our results not only have potential therapeutic implications by targeting intracellular ZBTB46-LIF signaling in prostate cancer but also clinical implications by suggesting that LIF titration can serve as a diagnostic and predictive marker in serum. We demonstrated that the increased LIF titers in serum were associated with prostate cancer progression and could aid in classifying prostate cancer patients in terms of metastasis or NE differentiation. Although most CRPC-NE patients do not respond to androgen-targeted therapies and chemotherapeutic agents (5,45), our study provides the potential to develop new diagnostic and therapeutic options for CRPC patients by providing a rationale for the use of an LIF inhibitor to combat therapeutic resistance and NE differentiation of prostate cancer. Although LIF is broadly expressed in the gall bladder, appendix, urinary bladder and some other organs(46–48), we showed that LIF is variably expressed in high-grade and metastatic prostate cancer compared with the expression in the normal prostate or BPH. Since abnormal LIF was also shown to be expressed during the progression of other tumors or under inflammatory conditions(49,50), we should pay attention to potentially false positive results when patients present prostate cancer and other diseases such as appendicitis or cholecystitis at the same time. In summary, our study supports a molecular and therapeutic basis for the ability of LIF to regulate drug resistance and NE-like differentiation in prostate cancer. We determined a molecular mechanism of prostate cancer lineage plasticity that elucidates an abnormal LIF-STAT3 pathway to facilitate NE differentiation through the activation of ADT-induced ZBTB46 signaling.
Supplementary Material
Translational relevance.
We show the underlying mechanisms that contribute to androgen deprivation therapy (ADT) resistance and reveal the pivotal mechanisms responsible for the development of castration-resistant prostate cancer (CRPC). We investigated the oncogenic role of leukemia inhibitory factor (LIF), which promotes therapeutic resistance and neuroendocrine (NE) differentiation in ADT-treated prostate cancer. This research is important because it potentially integrates the impact of common hormonal therapy resistance observed in CRPC patients. This property is supported by results from clinical samples exhibiting a direct link between increased LIF expression in prostate tissue samples with ADT resistance and metastasis. Importantly, we elucidate the effect of a first-in-class steroidal LIF inhibitor in reducing the tumor growth rate and NE marker expression by inhibiting the LIF-STAT3 pathway. Our findings propose a novel link between NE differentiation and ADT-induced LIF expression and provide new biomarkers and therapeutic targets for advanced prostate cancer.
Acknowledgments
Financial support
This work was jointly supported by grants from the National Institutes of Health (1R01 CA172603–01A, 1R01 CA205001–01, 1R01 CA181242–01A1, 1U54 CA217297–01, 1R01 CA212403–01A1, 1R01 CA200853–01A1, and 1R01 CA195505), Department of Defense Prostate Cancer Research Program (W81XWH-11–1-0227, W81XWH-12–1-0206, PC150382, and PC150382), Prostate Cancer Foundation, UCLA SPORE in Prostate Cancer (2P50CA092131–11A1), and the Stand-up-to-Cancer Dream Team award to J.H.; the Ministry of Science and Technology of Taiwan (MOST105–2628-B-038–006-MY3, MOST106–2918-I-038 −001, and MOST107–2628-B-038–001), the National Health Research Institute of Taiwan (NHRI-EX108–10702BI), and the “TMU Research Center of Cancer Translational Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan to Y.N.L.
Footnotes
Conflicts of interest: The authors disclose no potential conflicts of interest.
References
- 1.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013;32:5501–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res 2009;1:148–62 [PMC free article] [PubMed] [Google Scholar]
- 3.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A 2008;105:12182–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadal R, Schweizer M, Kryvenko ON, Epstein JI, Eisenberger MA. Small cell carcinoma of the prostate. Nat Rev Urol 2014;11:213–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez-Canas I, Juarranz MG, Collado B, Rodriguez-Henche N, Chiloeches A, Prieto JC, et al. Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate 2005;63:44–55 [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Adam RM, Freeman MR. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res 2002;62:1549–54 [PubMed] [Google Scholar]
- 8.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate 2000;42:88–98 [DOI] [PubMed] [Google Scholar]
- 9.Kamohara H, Ogawa M, Ishiko T, Sakamoto K, Baba H. Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: Involvement of regulation of LIF and its receptor expression. Int J Oncol 2007;30:977–83 [PubMed] [Google Scholar]
- 10.Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, et al. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol 2005;37:2284–96 [DOI] [PubMed] [Google Scholar]
- 11.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998;12:2048–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estrov Z, Samal B, Lapushin R, Kellokumpu-Lehtinen P, Sahin AA, Kurzrock R, et al. Leukemia inhibitory factor binds to human breast cancer cells and stimulates their proliferation. J Interferon Cytokine Res 1995;15:905–13 [DOI] [PubMed] [Google Scholar]
- 13.Zeng H, Qu J, Jin N, Xu J, Lin C, Chen Y, et al. Feedback Activation of Leukemia Inhibitory Factor Receptor Limits Response to Histone Deacetylase Inhibitors in Breast Cancer. Cancer Cell 2016;30:459–73 [DOI] [PubMed] [Google Scholar]
- 14.Izumi K, Fang LY, Mizokami A, Namiki M, Li L, Lin WJ, et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med 2013;5:1383–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ. p300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer Res 2005;65:5965–73 [DOI] [PubMed] [Google Scholar]
- 16.Feng S, Tang Q, Sun M, Chun JY, Evans CP, Gao AC. Interleukin-6 increases prostate cancer cells resistance to bicalutamide via TIF2. Mol Cancer Ther 2009;8:665–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daheron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004;22:770–8 [DOI] [PubMed] [Google Scholar]
- 18.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron 2009;64:61–78 [DOI] [PubMed] [Google Scholar]
- 19.Bressy C, Lac S, Nigri J, Leca J, Roques J, Lavaut MN, et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res 2018;78:909–21 [DOI] [PubMed] [Google Scholar]
- 20.He Z, Li JJ, Zhen CH, Feng LY, Ding XY. Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation. Acta Pharmacol Sin 2006;27:80–90 [DOI] [PubMed] [Google Scholar]
- 21.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 2015;26:533–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefana B, Ray DW, Melmed S. Leukemia inhibitory factor induces differentiation of pituitary corticotroph function: an immuno-neuroendocrine phenotypic switch. Proc Natl Acad Sci U S A 1996;93:12502–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A 2003;100:8621–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WY, Tsai YC, Siu MK, Yeh HL, Chen CL, Yin JJ, et al. Inhibition of the androgen receptor induces a novel tumor promoter, ZBTB46, for prostate cancer metastasis. Oncogene 2017;36:6213–24 [DOI] [PubMed] [Google Scholar]
- 25.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011;71:1668–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YN, Yin J, Barrett B, Sheppard-Tillman H, Li D, Casey OM, et al. Loss of Androgen-Regulated MicroRNA 1 Activates SRC and Promotes Prostate Cancer Bone Metastasis. Mol Cell Biol 2015;35:1940–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F, Chen WY, et al. Transforming growth factor-beta promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene 2015;34:4767–76 [DOI] [PubMed] [Google Scholar]
- 28.Chen WY, Tsai YC, Yeh HL, Suau F, Jiang KC, Shao AN, et al. Loss of SPDEF and gain of TGFBI activity after androgen deprivation therapy promote EMT and bone metastasis of prostate cancer. Sci Signal 2017;10 [DOI] [PubMed] [Google Scholar]
- 29.Liu YN, Abou-Kheir W, Yin JJ, Fang L, Hynes P, Casey O, et al. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol 2012;32:941–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A 2002;99:11890–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Jones SJ, Marra MA, Sadar MD. Identification of genes targeted by the androgen and PKA signaling pathways in prostate cancer cells. Oncogene 2006;25:7311–23 [DOI] [PubMed] [Google Scholar]
- 32.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006;25:3994–4008 [DOI] [PubMed] [Google Scholar]
- 33.Chang YS, Chen WY, Yin JJ, Sheppard-Tillman H, Huang J, Liu YN. EGF Receptor Promotes Prostate Cancer Bone Metastasis by Downregulating miR-1 and Activating TWIST1. Cancer Res 2015;75:3077–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meredith MM, Liu K, Kamphorst AO, Idoyaga J, Yamane A, Guermonprez P, et al. Zinc finger transcription factor zDC is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J Exp Med 2012;209:1583–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Chen CJ, Wang JK, Hsia E, Li W, Squires J, et al. Neuroendocrine differentiation of prostate cancer. Asian J Androl 2013;15:328–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu CD, Choo R, Huang J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol 2015;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 2006;26:12089–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellokumpu-Lehtinen P, Talpaz M, Harris D, Van Q, Kurzrock R, Estrov Z. Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer 1996;66:515–9 [DOI] [PubMed] [Google Scholar]
- 39.Dakhova O, Rowley D, Ittmann M. Genes upregulated in prostate cancer reactive stroma promote prostate cancer progression in vivo. Clin Cancer Res 2014;20:100–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 2001;93:1687–97 [DOI] [PubMed] [Google Scholar]
- 41.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem 2002;277:7076–85 [DOI] [PubMed] [Google Scholar]
- 42.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog 2011;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel R, Fleming J, Mui E, Loveridge C, Repiscak P, Blomme A, et al. Sprouty2 loss-induced IL6 drives castration-resistant prostate cancer through scavenger receptor B1. EMBO Mol Med 2018;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Culig Z Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol 2014;2:231–8 [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 2007;14:531–47 [DOI] [PubMed] [Google Scholar]
- 46.Graf U, Casanova EA, Cinelli P. The Role of the Leukemia Inhibitory Factor (LIF) - Pathway in Derivation and Maintenance of Murine Pluripotent Stem Cells. Genes (Basel) 2011;2:280–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hisaka T, Desmouliere A, Taupin JL, Daburon S, Neaud V, Senant N, et al. Expression of leukemia inhibitory factor (LIF) and its receptor gp190 in human liver and in cultured human liver myofibroblasts. Cloning of new isoforms of LIF mRNA. Comp Hepatol 2004;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omori N, Evarts RP, Omori M, Hu Z, Marsden ER, Thorgeirsson SS. Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab Invest 1996;75:15–24 [PubMed] [Google Scholar]
- 49.Morel DS, Taupin JL, Potier M, Deminiere C, Potaux L, Gualde N, et al. Renal synthesis of leukaemia inhibitory factor (LIF), under normal and inflammatory conditions. Cytokine 2000;12:265–71 [DOI] [PubMed] [Google Scholar]
- 50.Liu SC, Chang YS. Role of leukemia inhibitory factor in nasopharyngeal carcinogenesis. Mol Cell Oncol 2014;1:e29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.