Abstract
Background
The present study investigated the effects of chronic sodium bicarbonate (NaHCO3) ingestion on a single bout of high-intensity exercise and on acid-base balance during 7-day high-altitude exposure.
Methods
Ten recreationally active subjects participated in a pre-test at sea level and a 7-day hiking tour in the Swiss Alps up to 4554 m above sea level. Subjects received either a daily dose of 0.3 g/kg NaHCO3 solution (n = 5) or water as a placebo (n = 5) for 7 days. Anaerobic high-intensity exercise performance was assessed using the portable tethered sprint running (PTSR) test under normoxic and hypoxic conditions (3585 m). PTSR tests assessed overall peak force, mean force, and fatigue index. Blood lactate levels and blood gas parameters were assessed pre- and post-PTSR. Urinary pH and blood gas parameters were further analyzed daily at rest in early morning samples under normoxic and hypoxic conditions.
Results
There were no significant differences between the bicarbonate and control group in any of the PTSR-related parameters. However, urinary pH (p = 0.003, ηp2 = 0.458), early morning blood bicarbonate concentration (p < 0.001, ηp2 = 0.457) and base excess (p = 0.002, ηp2 = 0.436) were significantly higher in the bicarbonate group compared with the control group under hypoxic conditions.
Conclusions
These results indicate that oral NaHCO3 ingestion does not ameliorate the hypoxia-induced impairment in anaerobic, high-intensity exercise performance, represented by PTSR-related test parameters, under hypobaric, hypoxic conditions, but the maximal performance measurements may have been negatively affected by other factors, such as poor implementation of PTSR test instructions, pre-acclimatization, the time course of hypoxia-induced renal [HCO3−] compensation, changes in the concentrations of intra- and extracellular ions others than [H+] and [HCO3−], or gastrointestinal disturbances caused by NaHCO3 ingestion. However, chronic NaHCO3 ingestion improves blood bicarbonate concentration and base excess at altitude, which partially represent the blood buffering capacity.
Keywords: Chronic sodium bicarbonate supplementation, Baking soda, Moderate altitude, High-intensity exercise, Portable tethered sprint running test, Hypobaric hypoxia
Background
Consistent physical performance is indispensable for athletes during altitude training and for mountaineers climbing at moderate and high altitudes. Several studies have shown that altitude training can improve athletic performance, and it has thus become an accepted training method in various types of sports [1–3]. Furthermore, increasing numbers of people are travelling to moderate or high altitudes for trekking, climbing, mountaineering, or skiing activities. However, acute exposure to moderate and high altitudes above 1500 m acutely impairs physical performance [4]. Reduced exercise tolerance at altitude is caused by severe disruption to homeostasis resulting from a decline in arterial oxygen saturation (saO2) due to reduced oxygen pressure in the ambient and inspired air (PIO2) [5]. The reduced PIO2 leads to an increased respiratory rate, resulting among others in an acute respiratory alkalosis. Subsequent renal compensation of the acute respiratory alkalosis induces a reduction in blood bicarbonate ([HCO3−]) concentrations, and the resulting decline in the blood buffering capacity during altitude adaption has been suggested to have a significant effect on exercise performance at altitude, particularly above the lactate threshold [6–9]. These findings are supported by the fact that exercise-induced acidosis is more severe at altitude compared with sea level [6, 10].
Dietary strategies have previously been used to attenuate the impaired exercise performance under hypoxic conditions [11–13]. Among these, supplementation with sodium bicarbonate (NaHCO3) as an alkalotic buffer is an interesting approach to alleviate the elevated acidic stress during exercise above the lactate threshold under hypoxic conditions [11]. In normoxic conditions, NaHCO3 is proposed to enhance anaerobic exercise performance by increasing the availability of blood [HCO3−], thereby strengthening the physiochemical buffering capacity to reduce the rate of hydrogen cation ([H+]) production during exercise [11, 14, 15]. However, while the results of some studies have suggested that significant increases in [H+] may impair subsequent exercise performance by reducing the capacity for muscle force production [16, 17], the results of other studies suggest that exercise performance may be unaffected by disturbances in acid-base balance [18]; thus, there remains no consensus in the literature regarding the roles of acid-base status in anaerobic exercise performance and skeletal muscle fatigue. In addition, it has been suggested that the relative increase in glycolytic flux under hypoxic conditions increases [H+] production, which may be the underlying mechanism for the beneficial effect of NaHCO3 ingestion under hypoxic conditions [16, 19]. Furthermore, the removal of [H+] may be inhibited and the blood [HCO3−]-buffering capacity may be reduced [16, 19], due to the proposed lower [HCO3−] concentrations present under hypoxic conditions [8].
Few studies have examined the effect of NaHCO3 ingestion on anaerobic exercise performance at altitude, and the results of recent studies have been inconsistent. Flinn et al. [20] and Saunders et al. [21] found no effect of NaHCO3 supplementation on the power output of intermittent high-intensity exercise at simulated altitudes of 3000 m and 2500 m, respectively, while several studies have supported the assumption of a beneficial effect of NaHCO3 ingestion on anaerobic performance at altitude. Feriche Fernandez-Castanys et al. [22], Hauswirth et al. [23], and McLellan et al. [24] all described increased or constant exercise performance under acute altitude conditions in hypoxic chambers compared with sea-level performance in subjects receiving alkalizing agent supplements prior to exercise. In addition, Deb et al. [11, 19] and Gough et al. [16] reported positive effects of NaHCO3 under acute moderate hypoxic conditions at simulated altitude during intermittent and repeated high-intensity exercise. They conclude that NaHCO3 ingestion may offer an ergogenic strategy to mitigate hypoxia-induced declines in exercise performance. Most previous studies tested the effect of acute ingestion of a single dose of NaHCO3 shortly before exercise testing on anaerobic exercise performance, but the effects of chronic daily ingestion of NaHCO3 on anaerobic performance have also been evaluated. However, although chronic ingestion of NaHCO3 has been suggested to improve high-intensity work more effectively than acute ingestion [25–28] at sea level, especially during several-day training schedules, to the best of our knowledge, the influence of chronic NaHCO3 ingestion on exercise performance under hypoxic conditions has not been investigated to date. The aforementioned findings on the effects of NaHCO3 ingestion prior to high-intensity exercise under simulated altitude conditions raise questions regarding the potential practical implications of these results for mountaineering disciplines with a high anaerobic demand performed under hypobaric hypoxic conditions over a period of several days. Although mountaineering is mainly associated with aerobic performance [29], several disciplines are performed at moderate to high altitudes with high anaerobic demands, such as cross-country skiing [30, 31], alpine ski racing [32, 33], cross-country mountain biking and transalpine challenges [34, 35], and single- und multi-pitch climbing [36, 37]. These activities are affected by reduced exercise performance at altitude due to hypoxia, and may thus benefit from NaHCO3 ingestion to improve anaerobic exercise performance. However, there have been few studies regarding the beneficial effects of NaHCO3 ingestion during several-day altitude sojourns with high applicability to sport activities performed at moderate to high altitudes.
The present study aimed to analyze the effects of chronic ingestion of NaHCO3 on a single bout of high-intensity exercise and on the acid-base status during high-altitude exposure for 7 days. We hypothesized that chronic NaHCO3 ingestion would attenuate the hypoxia-induced impairment in anaerobic, high-intensity exercise performance under hypobaric, hypoxic conditions. We further hypothesized that daily chronic NaHCO3 ingestion would increase urinary pH levels and blood gas parameters during an altitude sojourn.
Methods
Participants
Fourteen healthy, non-specifically trained adult volunteers participated in the present study. Of these, four dropped out during the study due to medical problems (two because of moderate acute mountain sickness and two because of orthopedic problems). The results for the remaining 10 participants were analyzed (bicarbonate group: 4 men and 1 woman; control group: 4 men and 1 woman). Anthropometric data for participants in the bicarbonate and control groups are shown in Table 1. All participants underwent a medical screening before entering the study. Participants had to be in good health with no pre-existing altitude illnesses, cardiac or pulmonary conditions, and no musculoskeletal injuries that could interfere with mountaineering or running activities. All participants lived close to sea level. Because of the assumption that NaHCO3 [38] has a greater effect in recreationally trained compared with specifically trained persons, we set moderate physical fitness as an inclusion criterion. Exclusion criteria included acute muscular injuries or restrictions, chronic medication intake, alcohol consumption, acute infections, and preceding altitude sojourns above 2000 m in the 4 weeks prior to the investigation. The study was approved by the ethical committee of the Ruhr-Universität Bochum in accordance with the Declaration of Helsinki. Subjects gave their written informed consent after they had been informed of all experimental procedures and risks. Subjects were randomly assigned to either the bicarbonate supplementation (BIC) or the control group (CON).
Table 1.
Anthropometric data for subjects in the bicarbonate supplementation and control groups
| BIC (male: n = 4; female: n = 1) |
CON (male: n = 4; female: n = 1) |
|
|---|---|---|
| Age (years) | 25.0 ± 3.2 | 23.2 ± 2.3 |
| Body mass (kg) | 69.0 ± 12.2 | 73.3 ± 9.5 |
| Height (cm) | 173.7 ± 10.0 | 179.1 ± 7.9 |
| BMI (kg/m2) | 22.8 ± 1.7 | 22.8 ± 1.0 |
Data presented as mean ± standard deviation. BIC Bicarbonate supplementation, CON Control, BMI Body mass index. For further details see Materials and Methods section
Experimental design
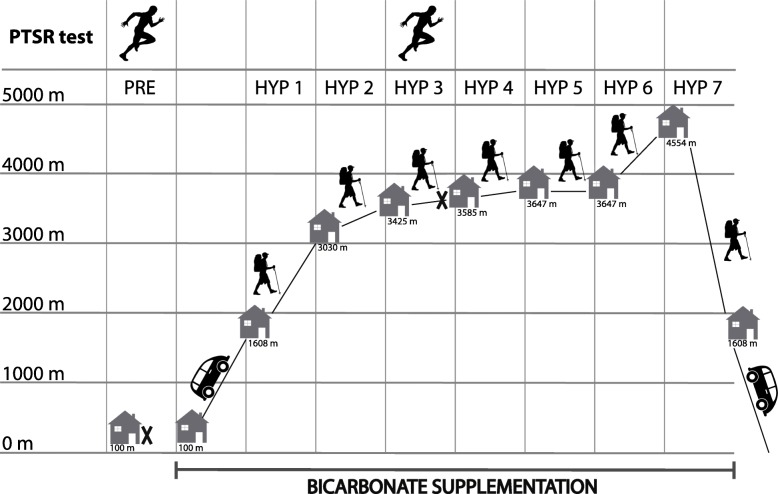
All test persons participated in a 7-day mountaineering tour (HYP1 – HYP7) at moderate to high altitude in the European Alps (Wallis, Switzerland). Glacier trekking was accomplished up to 4554 m above sea level and the sleeping heights were between 3030 m and 4554 m above sea level. Each participant passed a baseline test at sea level under normoxic conditions (NOR) before altitude exposure (elevation = 100 m). Anaerobic performance tests were performed at sea level (NOR) and after 3 days of altitude exposure (HYP3) at 3585 m above sea level (Fig. 1).
Fig. 1.
Experimental sequence. Sleeping heights and days of PTSR tests during a 7-day mountaineering tour. NOR = nomoxia, HYP = hypoxia, PTSR = portable tethered sprint running test
Supplementation
Based on commonly-used NaHCO3 doses [15, 39–42], the bicarbonate group received a NaHCO3 dose of 0.3 g/kg body mass daily, dissolved in 1 l water. On exercise testing days, NaHCO3 was administered 1 h before exercise testing. The control group drank 1 l water in the same time, as a placebo treatment. The groups received the respective treatments for 8 days, starting on the day when travelling to altitude exposure. After our subjects reported GI discomfort and GI symptoms seem to increase with increasing NaHCO3 dose, we decided to reduce the dose to 0.15 g/kg body mass on day five of supplementation.
Anaerobic performance test
Anaerobic performance was measured using a portable test device during the tethered sprint running (PTSR) test [43]. The PTSR test was chosen because it is simple, requires little space, and does not involve heavy and unwieldy equipment. These aspects were important because of the need to carry the test equipment and carry out the anaerobic testing under hypoxic conditions in the restricted spatial conditions of a mountain hut. Furthermore, NaHCO3 ingestion is recommended to enhance anaerobic exercise performance for short durations of about 1 min [41] and work performed at high intensity during continuous exercise has been shown to be enhanced by prior NaHCO3 ingestion [44]. We therefore assessed anaerobic performance using a single PTSR test of 60-s duration. For the test, participants ran with a belt round the waist to collect force measurements. The belt was attached to an inextensible static rope combined in series with a load cell and fixed to a pillar at a 90° angle to the subject’s waist height. Following a structured 10-min warm-up, the participant performed an all-out sprint for 60 s. Participants were instructed to sprint at maximal effort and pull the rope with full force. Study investigators provided strong verbal encouragement for the entire test duration and fluids were provided ad libitum, both before and after the 60-s sprint period. Force data were recorded in Newton (N) and the variables overall peak force (PF), lowest force (Fmin), and mean force (MF) were determined over the 60-s test duration. The PF was taken to be the highest 5-s force and Fmin the lowest 5-s force during the 60-s sprint period. MF was determined as the mean value over the entire 60-s test, and PF and MF were used in subsequent analyses. The fatigue level during the PTSR test was assessed using the fatigue index (FI), which was calculated as FI (%) = [(PF – Fmin)/PF] × 100, as recommended for Wingate tests [43]. Blood lactate levels were measured in 20-μl capillary blood samples collected before and 2, 4, 6, 8 and 10 min after PTSR testing. The samples were cooled and stored for up to 7 days until analysis (BIOSEN S-Line, EKF Diagnostics, UK). The maximum post-exercise lactate concentration (Lamax) of one PTSR test was used for statistical analyses.
Blood gas analysis
Capillary blood samples (100 μl) were taken from a hyperemized earlobe daily before breakfast (HYP1 – HYP7) and pre- and post-PTSR tests. Blood samples were immediately analyzed for blood gas parameters using a portable epoc® blood gas analyzer (Alere GmbH, Cologne, Germany). The oxygen and carbon dioxide partial pressure (PO2/PCO2), blood pH (pHb), oxygen saturation (saO2), blood bicarbonate concentration ([HCO3−]) and base excess (BE) were determined. We further calculated the difference between pre- and post-PTSR values (DIFF PTSR) for all blood gas parameters.
Urine pH
Urinary pH (pHu) was determined each day in spontaneous early morning urine samples (at least 5 ml of urine) using Neutralit® pH-indicator strips pH 5.0–10.0 (Merck, Darmstadt, Germany). pHu was measured throughout the experimental period (NOR; HYP1-HYP7) and served as a surrogate marker to assure that the supplementation had been conducted successfully [45].
Lake Louise AMS score
Acute mountain sickness (AMS) was assessed each morning using the Lake Louise AMS score (LLS) to ensure that it was assessed only after the minimum recommended altitude exposure of at least 6 h [46]. The LLS is a self-report questionnaire assessing the symptoms of headache, nausea/vomiting, fatigue, and dizziness/light-headedness. Each symptom item is scored from 0 (not present) to 3 (severe), and the sum score for the four symptom items was calculated (0–12 points) and used for statistical analyses. An individual sum score ≥ 3 and a headache score of ≥1 was mandatory for a positive AMS score. Mild AMS was defined as a LLS of 3–5 points, moderate AMS as 6–9 points, and severe AMS as 10–12 points, with headache and a recent altitude gain as conditions [46].
Body weight
Body weight was measured daily before breakfast using digital scales (Seca Clara 830, Seca Germany, Hamburg, Germany).
Statistical analysis
Data are presented as mean ± standard deviation. All departures from a normal distribution were identified using the Shapiro-Wilk test. We calculated ∆ values (HYP3 minus NOR) for all PTSR-related parameters to identify the influence of hypoxia on the respective parameters. Differences in ∆ values between BIC and CON conditions were assessed by two-sample t-tests. Cohen’s d (d) was used to calculate effect sizes, with 0.2 considered to indicate a small effect, 0.5 a medium effect, and 0.8 a large effect [47]. Non-normally distributed variables (∆ PCO2 PRE PTSR) were analyzed using Mann-Whitney-U-tests and effect sizes were calculated using correlation coefficients (r). The effect of conditions (BIC and CON) on the parameters PO2, PCO2, saO2, pHb, [HCO3−], BE, pHu, LLS, and body weight over time (NOR vs. HYP1 – HYP7) and pre and post-PTSR (NOR PRE PTSR vs. NOR POST PTSR; HYP PRE PTSR vs. HYP POST PTSR) were tested by two-way [condition x time] repeated-measures ANOVA. Violations of the assumption of sphericity were corrected for by Greenhouse-Geisser adjustments. Two-tailed t-tests were utilized as post hoc tests to indicate significant differences. A Bonferroni procedure was used (p*) to retain α = 0.05, and the significance level was set at p ≤ 0.05 in all comparisons. Effect sizes were calculated using partial η squared (ηp2) and interpreted as small (0.01), medium (0.06), and large (0.14), respectively [48]. The α level was set at p ≤ 0.05, and all analyses were conducted using SPSS 25 (IBM Corp., Armonk, NY, USA). The free software G*Power [49] was used to calculate required sample sizes and effect sizes.
Results
Anaerobic performance test
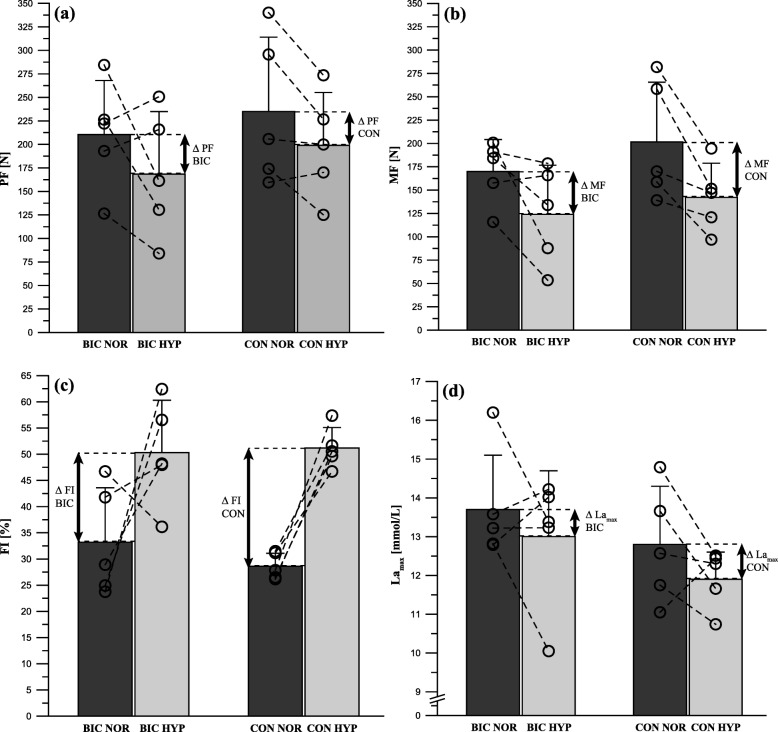
There were no significant differences between the bicarbonate and control groups in ∆PF (BIC: − 42.0 ± 68.3, CON: − 36.0 ± 36.3 N; p = 0.866, d = 0.11), ∆MF (BIC: − 46.0 ± 47.0, CON: − 59.5 ± 38.9 N; p = 0.634, d = 0.31), and ∆FI (BIC: 17.0 ± 19.8, CON: 22.5 ± 5.2%; p = 0.575, d = 0.38) (Fig. 2 a−c). There was also no difference between the groups in ∆Lamax (BIC: − 0.7 ± 1.9, CON: − 0.8 ± 1.5 mmol/L; p = 0.935, d = 0.05) (Fig. 2 d).
Fig. 2.
Performance measurements with (BIC) and without (CON) bicarbonate supplementation under normoxic (NOR) and hypoxic (HYP) conditions for (a) peak force (PF), (b) mean force (MF), and (c) fatigue index (FI), as well as the associated physiological response (d) maximum blood lactate (Lamax). Statistical analyses are for ∆ values only (HYP minus NOR), indicating intra-individual hypoxic-induced changes in performance parameters. Data points represent individual values (○). Bar charts are mean ± standard deviation. See Materials and methods for further details
Blood gas analysis
In term of blood gas parameters, there were significant differences between the two groups for ∆[HCO3−] PRE PTSR (p = 0.004, d = 2.53), ∆BE PRE PTSR (p = 0.001, d = 3.15), ∆pHb POST PTSR (p = 0.003, d = 2.65), ∆[HCO3−] POST PTSR (p = 0.001, d = 3.14), and ∆BE POST PTSR (p = 0.001, d = 3.16). There was also a significant difference for ∆pHb DIFF PTSR (p = 0.032, d = 0.30) (Table 2).
Table 2.
PTSR-related blood gas parameters in the bicarbonate supplementation and control groups under normoxia and hypoxia, and delta values
|
PO2 [mmHg] |
PCO2 [mmHg] |
saO2 [%] |
pHb | [HCO3−] [mmol/L] | BE [mmol/L] |
|||
|---|---|---|---|---|---|---|---|---|
|
N O R |
BIC | PRE PTSR | 99.6 ± 23.2 | 32.2 ± 10.7 | 97.6 ± 1.2 | 7.44 ± 0.04 | 21.7 ± 6.1 | −2.4 ± 5.6 |
| POST PTSR | 94.4 ± 11.4 | 38.3 ± 4.8 | 95.8 ± 1.5 | 7.26 ± 0.02 | 17.4 ± 1.8 | −9.7 ± 1.9 | ||
| DIFF PTSR | −5.2 ± 30.1 | 6.1 ± 11.0 | − 1.8 ± 2.2 | − 0.18 ± 0.04 | − 4.3 ± 6.0 | −7.3 ± 5.6 | ||
| CON | PRE PTSR | 82.6 ± 7.9 | 38.7 ± 3.1 | 96.1 ± 1.4 | 7.42 ± 0.02 | 24.9 ± 1.3 | 0.3 ± 1.1 | |
| POST PTSR | 90.2 ± 7.9 | 38.7 ± 3.8 | 95.5 ± 1.1 | 7.26 ± 0.02 | 17.6 ± 2.0 | − 9.5 ± 2.1 | ||
| DIFF PSTR | 7.6 ± 9.7 | − 0.0 ± 2.9 | − 0.7 ± 1.6 | − 0.15 ± 0.04 | −7.3 ± 1.3 | − 9.8 ± 1.7 | ||
|
H Y P |
BIC | PRE PTSR | 49.4 ± 2.5 | 31.6 ± 1.9 | 88.9 ± 1.7 | 7.52 ± 0.03* | 26.0 ± 2.1* | 3.2 ± 2.4* |
| POST PTSR | 57.3 ± 5.4 | 30.6 ± 1.8 | 89.4 ± 2.8 | 7.40 ± 0.03* | 18.8 ± 1.2* | − 6.0 ± 1.6* | ||
| DIFF PTSR | 7.8 ± 4.9 | − 1.0 ± 0.5 | 0.5 ± 2.6 | − 0.13 ± 0.01* | −7.2 ± 0.9 | − 9.2 ± 0.9 | ||
| CON | PRE PTSR | 54.2 ± 5.1 | 28.4 ± 2.2 | 89.6 ± 3.0 | 7.46 ± 0.01 | 20.1 ± 1.3 | − 3.8 ± 1.2 | |
| POST PTSR | 58.3 ± 3.3 | 28.4 ± 4.3 | 87.1 ± 1.6 | 7.29 ± 0.03 | 12.6 ± 1.5 | − 14.1 ± 1.9 | ||
| DIFF PTSR | 4.0 ± 7.7 | 0.0 ± 3.2 | −2.6 ± 3.8 | − 0.17 ± 0.03 | −7.4 ± 1.6 | −10.3 ± 1.9 | ||
| ∆ | BIC | PRE PTSR | −50.2 ± 23.0 | −0.6 ± 10.8 | −8.7 ± 2.3 | 0.08 ± 0.06 | 4.4 ± 5.1* | 5.6 ± 4.3* |
| POST PTSR | −37.2 ± 13.0 | − 7.7 ± 3.9 | −6.5 ± 2.9 | 0.14 ± 0.04 | 1.5 ± 1.6* | 3.7 ± 2.2* | ||
| DIFF PTSR | 13.0 ± 27.0 | −7.0 ± 10.9 | 2.3 ± 1.6 | 0.06 ± 0.38* | − 2.9 ± 5.7 | −1.9 ± 5.4 | ||
| CON | PRE PTSR | −28.3 ± 10.8 | − 10.4 ± 1.8 | −6.5 ± 3.6 | 0.04 ± 0.02 | −4.8 ± 0.6 | −4.1 ± 0.7 | |
| POST PTSR | −31.9 ± 9.8 | − 10.3 ± 4.1 | −8.4 ± 2.2 | 0.02 ± 0.05 | − 4.9 ± 2.4 | −4.6 ± 3.0 | ||
| DIFF PTSR | −3.6 ± 16.9 | 0.1 ± 4.0 | −1.9 ± 4.8 | −0.02 ± 0.05 | −0.1 ± 2.3 | −0.5 ± 3.1 | ||
Data presented as mean ± standard deviation. PO2 Oxygen partial pressure, PCO2 Carbon dioxide partial pressure, saO2 Oxygen saturation, pHb Blood pH value, [HCO3−] Blood bicarbonate concentration, BE Base excess, BIC Bicarbonate supplementation group (n = 5), CON Control (n = 5), NOR Normoxia, HYP Hypoxia, PTSR Portable tethered sprint running test, PRE PTSR Pre-PTSR values, POST PTSR Post-PTSR values, DIFF PTSR Difference between pre- and post-PTSR values (DIFF = POST – PRE). ∆ = HYP minus NOR. For further details see Materials and Methods section. *p < 0.05 vs. CON. For p-values see results section
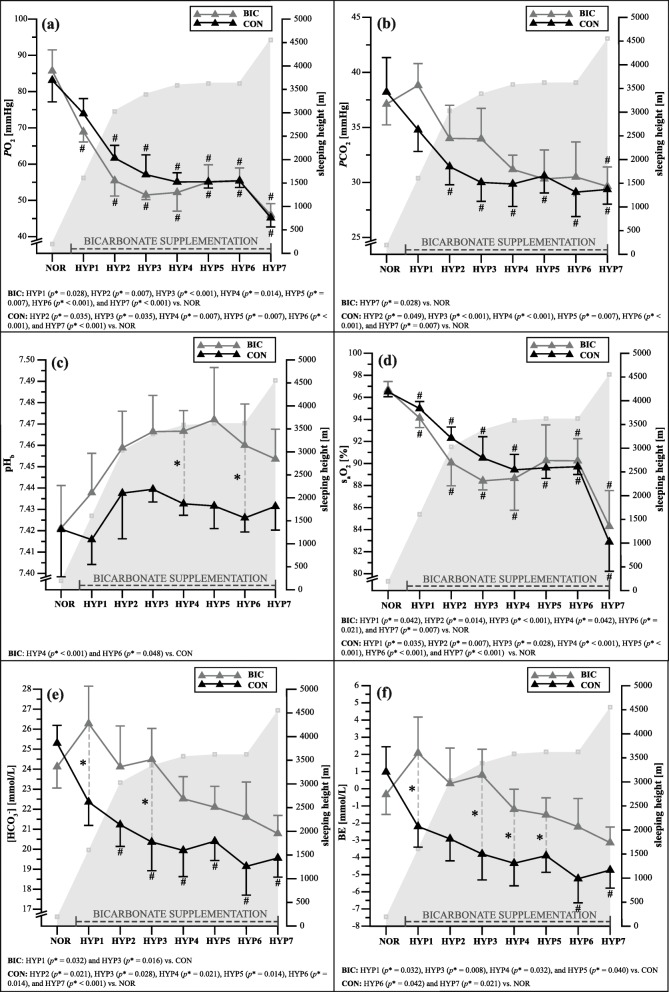
The early morning blood gas analysis measurements for BIC and CON are shown in Fig. 3. A significant [condition x time] interaction was detected on PCO2 (p = 0.003, ηp2 = 0.306), [HCO3−] (p < 0.001, ηp2 = 0.457), and BE (p = 0.002, ηp2 = 0.436), but not for PO2(p = 0.176, ηp2 = 0.184), saO2 (p = 0.227, ηp2 = 0.159), and pHb (p = 0.263, ηp2 = 0.141). Additionally, the [condition] effect was significant for PO2(p = 0.047, ηp2 = 0.407), pHb (p < 0.001, ηp2 = 0.912), [HCO3−] (p = 0.005, ηp2 = 0.646), and BE (p = 0.001, ηp2 = 0.743). A significant [time] effect was evident on change in PO2 (p < 0.001, ηp2 = 0.925), PCO2 (p < 0.001, ηp2 = 0.831), pHb (p < 0.001, ηp2 = 0.399), saO2 (p < 0.001, ηp2 = 0.867), [HCO3−] (p < 0.001, ηp2 = 0.774), and BE (p < 0.001, ηp2 = 0.695). Significant differences in pairwise comparisons and the associated p-values are shown in Fig. 3.
Fig. 3.
Early morning blood gas analysis measurements with (BIC) and without (CON) bicarbonate supplementation under normoxic (NOR) and hypoxic (HYP1-HYP7) conditions for (a) PO2, (b) PCO2, (c) pHb, (d) saO2, (e) [HCO3−], and (f) BE. Data is presented as mean ± standard deviation. Filled gray graphs represent sleeping heights above sea level before the daily measurement. * p ≤ 0.05 vs. CON; # p ≤ 0.05 vs. PRE. See Materials and Methods for further details
Urine pH
We found a main effect for pHu (p = 0.003, ηp2 = 0.458) as well as a significant [condition] (p < 0.001, ηp2 = 0.945) and [time] effect (p = 0.001, ηp2 = 0.544). Post hoc analyses showed significant differences in pHu between the bicarbonate and control groups at HYP3 (p* < 0.001), HYP4 (p* < 0.001), HYP5 (p* < 0.001), HYP6 (p* < 0.001), and HYP7 (p* < 0.001). Furthermore, there were significant differences in pHu between NOR and HYP1 (p* = 0.049), HYP2 (p* = 0.049), HYP3 (p* = 0.028), and HYP4 (p* = 0.028) in the bicarbonate group, but not in the control group (Fig. 4).
Fig. 4.
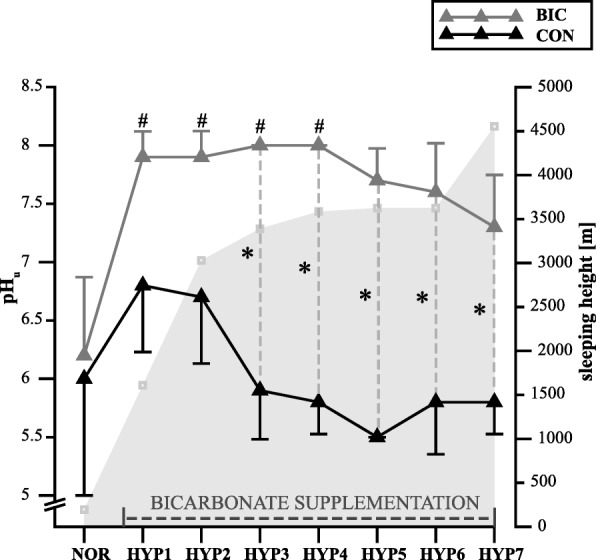
Early morning urinary pH values (means ± standard deviation) in subjects with (BIC) and without (CON) bicarbonate supplementation before (NOR) and during hypoxic exposure (HYP1-HYP7). * p ≤ 0.05 vs. CON; # p ≤ 0.05 vs. PRE. See Materials and Methods for further details
LLS
There was no main effect of interaction or effect of [condition] for LLS (BIC: HYP1: 2.0 ± 2.4, HYP2: 3.2 ± 2.4, HYP3: 2.4 ± 2.1, HYP4: 2.6 ± 3.0, HYP5: 2.0 ± 1.4, HYP6: 1.4 ± 1.5, HYP7: 1.8 ± 2.2; CON: HYP1: 1.0 ± 0.0, HYP2: 1.0 ± 0.7, HYP3: 0.8 ± 0.5, HYP4: 0.4 ± 06, HYP5: 0.4 ± 0.6, HYP6: 04 ± 0.6, HYP7: 0.6 ± 0.6; p = 0.266, ηp2 = 0.142), but there was a significant [time] effect for the LLS (p = 0.016, ηp2 = 0.268). Pairwise comparisons revealed a significant difference in LLS between HYP2 (2.1 ± 2.0) and HYP6 (0.9 ± 1.2, p = 0.049) for the total group.
Body weight
There was no significant interaction [condition x time], or effect of [condition] or [time] for body weight during the experimental period in the bicarbonate group (NOR: 69.0 ± 12.2; HYP1: 70.1 ± 12.2; HYP2: 69.5 ± 11.8; HYP3: 70.1 ± 11.9; HYP4: 70.0 ± 12.3; HYP5: 70.1 ± 12.3; HYP6: 69.2 ± 12.0; HYP7: 69.4 ± 11.9 kg) and in the control group (NOR: 73.3 ± 9.5; HYP1: 73.4 ± 9.0; HYP2: 72.8 ± 9.9; HYP3: 72.5 ± 9.7; HYP4: 73.0 ± 8.8; HYP5: 72.8 ± 9.0; HYP6: 72.7 ± 8.5; HYP7: 72.5 ± 9.1 kg; p = 0.344, ηp2 = 0.127).
Discussion
In the present study, we evaluated the effects of chronic NaHCO3 ingestion during 7 days of exposure to moderate and high altitudes on anaerobic performance parameters and laboratory blood and urine parameters. The primary finding of the study was that chronic NaHCO3 ingestion had a considerable impact on acid-base balance, which resulted in a higher alkalotic state; however, chronic NaHCO3 ingestion did not significantly influence PTSR-related performance outputs and associated physiological responses. A higher alkalotic acid-base balance prior to exercise under hypoxic conditions has been reported to be related to higher performance output and higher maximum blood lactate values after high-intensity exercise [11, 16, 19]. The suggested mechanism underlying the increased [H+] buffering from intramuscular to extramuscular compartments may lead to improved protection of intramuscular pH and increased anaerobic energy provision and glycogen utilization [16, 50]. However, although some outliers showed the expected increases in anaerobic performance outputs and the associated lactate response, the current study revealed no significant overall effect of chronic NaHCO3 ingestion on anaerobic performance outputs (indicated by ∆MF, ∆PF, and ∆FI) or on the related parameter ∆Lamax. This apparent discrepancy may be attributable to several factors.
Several recent studies reported increased or constant anaerobic exercise performance during acute altitude exposure in hypoxic chambers following supplementation with alkalizing agents prior to exercise [11, 16, 19, 22–24]. All these studies analyzed the effects of bicarbonate supplementation on different aspects of exercise performance under acute hypoxic conditions (between 15 min and 4 h of altitude exposure). In contrast, the present study assessed long-term altitude exposure over 7 days. We suggest that experimental acute hypoxia may not reflect the physiological acid-base responses to hypoxic conditions sufficiently, because the proposed lower [HCO3−] concentrations under hypoxic conditions [8] and the subsequently reduced [HCO3−] buffering capacity and anaerobic exercise performance [16, 17] should also take into account the time course of renal compensation of hypoxia-induced respiratory alkalosis, which is generally considered to be a slow-adapting mechanism affecting [HCO3−] concentrations after several hours or days. More specifically, renal [HCO3−] compensation has been shown to occur after 6 h and to be complete after 24 h of exposure to low to moderate altitudes, but to still be incomplete after 24 h of exposure to high altitudes [51]. In the present study, the enhanced renal [HCO3−] compensation was most obvious in the first 3 days of hypoxic exposure based on early morning urinary pH values. This finding complements the results of Ge et al. [51], who reported the renal [HCO3−] compensation based on early morning urinary pH values at simulated moderate altitudes of 1780, 2085, 2455 and 2800 m. They demonstrated that renal compensation was completed by 24 h at 1780, 2085 and 2455 m, but not at 2800 m. The time course of urinary pH values in our control group suggested that the renal [HCO3−] compensation at higher altitudes (between 2500 and 3500 m) seemed to be completed by 48 h. However, these results should be interpreted with caution because there was no significant increase of early morning urinary pH in the control group. This may be because of the high variability in pre-test urine and blood pH values. Urine and blood pH are influenced by several external factors such as nutrition, intake of dietary supplements, and high-intensity exercise [52]. Although subjects in the present study were asked to cease any special diets, supplements, and high-intensity exercise at least 2 days before the pre-testing, their nutrition was not controlled. Remer [53] showed a high impact of alkalizing-food intake on urine and blood pH values within 3 days, suggesting that nutrition should be standardized and controlled at least 3 days before anaerobic exercise testing in future investigations to reduce variability in urinary pH. In summary, we suggest that NaHCO3 ingestion during an altitude sojourn might be most effective when renal compensation of respiratory alkalosis is in progress or completed. However, to the best of our knowledge, the exact time course of renal compensation of hypoxia-induced respiratory alkalosis is unknown, and should be investigated in a controlled setting with at least 6 h of hypoxic exposure.
Notably, hypoxia-induced respiratory alkalosis is usually described as a desirable and important process that contributes to altitude adaption [54]. Chronic NaHCO3 ingestion thus contrasts with recommendations regarding the use of acetazolamide for the prevention of AMS when ascending to moderate and high altitudes [55]. Acetazolamide is a potent carbonic anhydrase inhibitor that increases minute ventilation and oxygenation and causes diuresis and renal [HCO3−] loss by enhancing central chemoreceptor output [56]. Via this mechanism, acetazolamide has been shown to provide prophylaxis for the symptoms of AMS in individuals ascending to high altitudes [55, 57]. Because NaHCO3 ingestion aims to compensate for the [HCO3−] loss rather than supporting hypoxia-induced diuresis and the associated renal [HCO3−] loss, we measured the AMS score every morning using the LLS in the present study, to control a potential higher risk of AMS due to NaHCO3 ingestion. However, chronic NaHCO3 ingestion had no significant effect on LLS values during the 7-day altitude sojourn, suggesting that it did not increase the risk of developing AMS symptoms. In addition, information on the effect of acetazolamide on exercise performance is still insufficient. Although acetazolamide was shown to impair submaximal and maximal exercise performances at sea level, its influences on submaximal and maximal exercise at altitude remain controversial [58]. We therefore conclude that NaHCO3 supplementation as an alkalotic buffer remains an interesting approach to alleviating acidic stress during exercise above the lactate threshold under hypoxic conditions, with no increase in the risk of AMS development.
The current dosage strategy may have been another possible reason for the lack of anaerobic performance enhancement by chronic NaHCO3 ingestion, which was contrary to our original hypothesis. We administered NaHCO3 according to a chronic schedule due to a lack of studies using chronic dosing schedules [16]; moreover, a chronic alkalotic state may meet the requirements of mountain sport disciplines better than single-dose NaHCO3, resulting in short-term performance enhancement. However, the effects of chronic NaHCO3 ingestion on anaerobic exercise performance improvements may have been hindered by the relatively long mountaineering exercise with altitude exposure, as well as the associated altitude adaption processes. Furthermore, most studies investigating the influence of NaHCO3 supplementation on anaerobic exercise performance under acute normobaric hypoxic conditions determined the individual time to peak [HCO3−] after NaHCO3 ingestion, and administered NaHCO3 in test trials at the participant’s pre-determined time to peak [HCO3−] to achieve the optimal performance changes [11, 16, 19]. The time course to peak blood [HCO3−] using liquid supplementation has previously been shown to range from 40 to 90 min [19]. Unfortunately, we were unable to assess the pre-determined time to peak [HCO3−] in the present study and we therefore decided to administer the daily NaHCO3 dose in 1 l of water 60 min before exercise testing. Although we assumed that this time frame met the requirements for maximizing the ergogenic effect of NaHCO3, it is possible that individualized supplementation strategies may be superior for the optimization of the performance-enhancing properties of NaHCO3.
It is also possible that the results might have differed if anaerobic performance tests had been performed at higher altitudes or for longer altitude exposures and associated daily NaHCO3 ingestion periods. We therefore intended to assess anaerobic performance after 7 days of NaHCO3 ingestion (HYP6) at 4554 m, but were unable to report the associated performance data due to computer crashes caused by high-altitude barometric pressure changes. Follow-ups to the current pilot study should thus focus on anaerobic performance tests at higher altitudes and after longer NaHCO3 ingestion periods, bearing in mind the potential computer-related problems caused by high altitude barometric pressure changes. Additionally, it has been proposed that performance of single sprints of short duration (up to 45 s) can be maintained in acute hypoxic conditions because of a shift toward anaerobic metabolism, whereas power output for tests with continuous or repeated high-intensity exercise longer than 45 s (such as the 3-min all-out critical power test and repeated sprints [11, 59, 60]), is often reduced in acute hypoxia [61, 62]. Therefore, we assumed that a 60-s continuous test protocol would be sufficient to assess changes in anaerobic exercise performance under hypoxic conditions. However, we presume that follow-up studies may involve the use of different test protocols, including assessments of all-out running for longer durations up to 3 min or repeated-sprint performance, to further investigate the results of impaired anaerobic exercise performance in hypoxia.
A further potential limitation of the present study and possible explanation for the lack of any significant effect of chronic NaHCO3 ingestion on ∆MF, ∆PF, ∆FI, and ∆Lamax may be the low test-power of the comparisons between the bicarbonate and control groups. An a priori power calculation indicated that a sample size of three participants per group would allow the detection of differences between the groups, based on an earlier study that reported significantly improved force in a 60-s anaerobic performance cycling test under normoxic conditions after chronic NaHCO3 ingestion, with a statistical power of 57% [63]. However, we acknowledge that the use of data from the abovementioned investigation resulted in a surprisingly low calculated sample size for detection of possible changes. Indeed, smaller effect sizes were found within the current investigation and a sample size of 10 participants resulted in a test power of 10% (6–14%) for the effect of NaHCO3 ingestion on PTSR-related parameters. In contrast, power calculations for the metabolic parameters measured in this study were associated with higher power values (11–99.8%). This indicates sufficient test power to analyze the effect of NaHCO3 supplementation, but an underpowered trial in terms of determining the effect of anaerobic exercise at altitude, making the detection of significant difference in ∆MF, ∆PF, ∆FI, and ∆Lamax between the bicarbonate and control groups highly unlikely. These small effects meant that we would probably not be able to detect differences in these parameters with the current sample size of 10 participants, and could therefore not exclude a type 2 error within our interpretation.
Furthermore, the high individual variations represented by individual trajectories in Fig. 2 in response to anaerobic exercise at altitude may have contributed to the lack of power for the effect of anaerobic exercise at altitude. Individual variations in this context may represent a previously suggested responder vs. non-responder phenomenon to intervention with NaHCO3 supplementation [64, 65] or exercise performance changes under hypoxic conditions [66, 67]. Another explanation for variations in the response to anaerobic exercise at altitude may be an unfamiliar exercise pattern, such that the participants were unable to properly implement the test instructions. It has already been shown that tethered sprinting reduces maximal velocity, flight time, and stride length, and increases contact time, compared with free sprinting [68, 69]. Therefore, it is possible that the PTSR test pattern may have prevented our participants from sprinting to their full potential, meaning that the maximal performance measurement may not reflect the participants’ true maxima. In addition, the inclusion of a single female participant in each group may have contributed to variability in the effect of anaerobic exercise at altitude; a general sex-related difference may have introduced additional heterogeneity. We therefore also performed statistical analyses on PTSR-related performance parameters in male participants alone, but found no significant differences in the outcomes from the results of analyzing the complete groups. Therefore, we decided to report the data from both groups, including the female participants, to achieve higher study power.
Nevertheless, we assumed that the possible undetected differences in PTSR-related performance parameters were likely to be too small to contribute to an anaerobic performance enhancement, and influences of other factors may have negatively affected the ergogenic effects of NaHCO3 ingestion. This assumption was supported by the higher [HCO3−] and BE values pre- and post-PTSR in the bicarbonate group compared with the control group, but the lack of any significant difference in exercise-induced difference between pre- and post-PTSR values. Recent studies reported an increased glycolytic energy contribution to exercise and improved anaerobic exercise performance following NaHCO3 ingestion under normoxic [70] and acute hypoxic conditions [16]. It has also been suggested that changes in blood pH and [HCO3−] are greater during exercise with NaHCO3 ingestion and the associated elevation of pre-exercise [HCO3−] and BE values, which is supposed to explain the increased anaerobic exercise performance in acute hypoxic conditions following NaHCO3 ingestion [19]. However, our data do not support these assumptions because despite higher [HCO3−] and BE values pre- and post-PTSR following NaHCO3 ingestion, only the exercise-induced difference between pre- and post-PTSR values for ∆pHb differed between conditions, indicating similar glycolytic energy contributions and exercise performance outputs irrespective of NaHCO3 ingestion, but a possible difference in respiratory contributions resulting in less-pronounced acidosis following NaHCO3 ingestion.
Participant acclimatization may also have had a negative influence of the ergogenic effect of NaHCO3 ingestion by negating the additional acidic load apparent in unacclimatized individuals. Although the results for saO2, [HCO3−], and BE do not suggest that our participants were already fully acclimatized at HYP3 when they performed the PTSR test at altitude, further studies are needed to prove the assumptions raised in the present pilot study. Furthermore, the unexpected lack of an ergogenic effect of NaHCO3 could be explained by the theory of strong ion difference (SID) [71]. The present findings refer to the Henderson-Hasselbach approach, which assumes that blood pH is determined by changes in [H+] and [HCO3−]. In contrast, the SID approach refers to the intra- and extracellular ions (e.g. chloride, potassium, sodium) and describes the difference between the concentrations of strong cations and strong anions. The SID is also suggested to have an independent effect on blood pH, and thus impair muscle performance by altering intra- or extra-cellular pH [71]. The SID approach may therefore explain the exercise-induced difference between pre- and post-PTSR values for ∆pHb between the bicarbonate and control groups with simultaneously similar developments of changes in PTSR-related parameters, [HCO3−], and BE. However, this conclusion should be interpreted with caution because we did not calculate the SID values in the present study, and future studies are needed to examine the influence of changes in the SID on anaerobic exercise performance at altitude.
PTSR performance parameters in this study might also have been influenced by gastro-intestinal (GI) disturbances. Negative GI symptoms caused by bicarbonate ingestion have been reported in the literature [21, 72, 73] and GI discomfort is suggested to have ergolytic effects on anaerobic performance [15, 19]. Unfortunately, we did not carry out any structured monitoring of GI discomfort in the current participants, which represents a limitation of this study. However, we asked the subjects—in daily individual unstructured interviews—about any GI disturbances after consuming the NaHCO3 solution; most participants in the bicarbonate group reported GI complaints after NaHCO3 ingestion. We mainly attributed these GI disturbances to the dose of NaHCO3 (0.3 g/kg). Although this dose has been recommended for NaHCO3 supplementation under normoxic and hypoxic conditions [15, 16, 39–42], smaller doses have been suggested for participants who display severe GI symptoms after NaHCO3 ingestion [16]. Given that GI discomfort seems to increase with increasing NaHCO3 dose [72], we decided to reduce the dose to 0.15 g/kg body mass on day five of supplementation, after which the subjects’ reported GI symptoms decreased. However, in retrospect, we would potentially recommend a reduction to the common dose of 0.20 g/kg body mass, rather than the pronounced reduction (by 50%) to 0.15 g/kg body mass. Finally, within the present study, the PTSR test under hypoxic conditions was performed at a dose of 0.3 g/kg, and the performance outputs may have been inhibited due to GI discomfort. In addition, it must be noted that GI problems are common at high altitude and are often reported, regardless of NaHCO3 ingestion [74, 75]. The reported GI discomfort in the present study may thus have been due to both NaHCO3 ingestion and altitude exposure. Further investigations under hypobaric hypoxic conditions should thus be performed using NaHCO3 at a lower dose or in a different dosage form; these should include controls for and monitoring of GI upset using structured daily self-reports [16]. Different dosage forms and strategies that have been reported to reduce GI side effects include the use of tablets or capsules (instead of liquid supplementation), serial loading [76], and co-ingestion of NaHCO3 with water and a high-carbohydrate meal [14]. Future studies should include the use of a placebo supplement for the control group; this aspect was not implemented in the present study and therefore constitutes a limitation of the study.
Finally, another limitation of the study is that it was not double-blinded. Although the study was originally designed with this in mind, the serious gastrointestinal problems reported by the participants forced us to inform the study investigators and participants regarding group affiliations, prior to reducing the NaHCO3 dose. Therefore, the present study provides the first results in this field, but further research is needed to confirm the findings of this investigation with regard to the effects of chronic NaHCO3 ingestion on anaerobic exercise performance under hypobaric, hypoxic conditions.
Practical applications
Although mountaineering is mainly associated with aerobic performance [29], the results of the current study will be applicable to other mountain sports disciplines performed at moderate to high altitudes. Previous studies demonstrated the need for a high level of anaerobic power during steep climbs and sprints in cross-country ski races [30], and cross-country sprint disciplines with maximal-effort durations of 2.5–3 min are also expected to have a significant anaerobic contribution [31]. Moreover, alpine ski races last for 45 s to 2 min, and anaerobic fitness has been identified as being of primary importance in alpine skiing [32, 33]. To the best of our knowledge, no studies have examined ski mountaineering, though the findings for alpine skiing may be transferable to ski touring and ski mountaineering. Additionally, anaerobic power has been suggested to be an important determinant of performance in cross-country and downhill mountain biking and transalpine challenges [34, 35, 77]. Unfortunately, no studies have examined the physiological requirements of disciplines such as multi-pitch rock, mixed, or ice climbing, and recent studies have focused on the physiology of difficult rock or indoor climbing. Performance in single-pitch climbing disciplines is mainly determined by anaerobic power and muscular strength [36, 37]; it might be necessary to consider whether multi-pitch climbing requires greater anaerobic power due to longer exercise duration. However, further studies are required to support these assumptions. The above-mentioned sport disciplines are thus affected by hypoxia-induced reduced exercise performance at altitude and may therefore benefit from a dietary strategy involving NaHCO3 ingestion to improve anaerobic exercise performance.
Conclusion
Due to methodological uncertainties, the present randomized controlled trial should be considered as a pilot presenting first results examining the effects of chronic NaHCO3 supplementation on anaerobic exercise performance during an altitude sojourn. The principal finding was that oral chronic NaHCO3 ingestion did not affect hypoxia-induced performance changes (∆MF, ∆PF, ∆FI) or ∆Lamax changes, but significantly increased the early-morning pHb, [HCO3−], and BE values, which partially represent the blood buffering capacity. A higher alkalotic state of the acid-base balance prior to exercise under hypoxic conditions is often associated with higher performance outputs and higher maximum blood lactate values after high-intensity exercise. Explanations for the apparent lack of any ergogenic effect of NaHCO3 ingestion include pre-acclimatization, the time course of hypoxia-induced renal [HCO3−] compensation, changes in intra- and extracellular ions others than [H+] and [HCO3−], or GI disturbances caused by NaHCO3 ingestion. Further, the present study provides important practical advices for future field investigations in this research area, such as a reduction of commonly used sodium bicarbonate doses to prevent for exercise decrements due to gastro-intestinal symptoms and the computer crashes caused by high-altitude barometric pressure changes.
Acknowledgments
We are grateful to all participants for participating in this study. We thank our laboratory staff, Michaela Rau, for contributions and support. We also thank Susan Furness, PhD, for editing a draft of this manuscript.
Abbreviations
- AMS
Acute mountain sickness
- BE
Base excess
- BIC
Bicarbonate supplementation group
- CON
Control group
- DIFF PTSR
Difference between pre- and post-PTSR values
- FI
Fatigue index
- GI
Gastro-intestinal
- [H+]
Hydrogen cation
- [HCO3−]
Blood bicarbonate
- HYP
Hypoxic conditions
- Lamax
Maximum post-exercise lactate concentration
- LLS
Lake Louise score
- MF
Mean force
- NaHCO3
Sodium bicarbonate
- NOR
Normoxic conditions
- PIO2
Oxygen pressure in the inspired air
- PF
Peak force
- pHb
Blood pH
- pHu
Urinary pH
- PCO2
Carbon dioxide partial pressures
- PO2
Oxygen partial pressures
- PTSR
Portable tethered sprint running test
- SID
Strong ion difference
Authors’ contributions
ML and PP conceptualized and designed the research project; ML performed experiments, acquired the data and conducted the statistical analysis; ML interpreted the results of the experiments with assistance from MdM and PP; ML prepared the figures and tables and wrote the manuscript with revisions from MdM and PP; all authors reviewed and agreed upon the final version of manuscript.
Funding
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-University Bochum.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the figshare data repository [doi: 10.6084/m9.figshare.8937995].
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the ethical committee of the Ruhr-University Bochum. All participants gave written informed consent prior to being enrolled in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mirjam Limmer, Email: mirjam.limmer@rub.de.
Markus de Marées, Email: markus.demarees@rub.de.
Petra Platen, Email: petra.platen@rub.de.
References
- 1.Bailey DM, Davies B. Physiological implications of altitude training for endurance performance at sea level: a review. Br J Sports Med. 1997;31(3):183–190. doi: 10.1136/bjsm.31.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billaut F, Gore CJ, Aughey RJ. Enhancing team-sport athlete performance. Sports Med. 2012;42(9):751–767. doi: 10.1007/BF03262293. [DOI] [PubMed] [Google Scholar]
- 3.WILBER RL. Application of altitude/hypoxic training by elite athletes. Med Sci Sports Exerc. 2007;39(9):1610–1624. doi: 10.1249/mss.0b013e3180de49e6. [DOI] [PubMed] [Google Scholar]
- 4.Baertsch P. Innere Medizin: Hoehenanpassung. Dtsch Z Sportmed. 2000;4:139–140. [Google Scholar]
- 5.Mazzeo RS. Physiological responses to exercise at altitude: an update. Sports Med. 2008;38(1):1–8. doi: 10.2165/00007256-200838010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Udo M, Chida M, Makiguchi K, Ichioka M, Muraoka I. Arterial blood gases, acid-base balance, and lactate and gas exchange variables during hypoxic exercise. Int J Sports Med. 1989;10(4):279–285. doi: 10.1055/s-2007-1024916. [DOI] [PubMed] [Google Scholar]
- 7.Coudert J. Anaerobic performance at altitude. Int J Sports Med. 1992;13(Suppl):1–5. doi: 10.1055/s-2007-1024604. [DOI] [PubMed] [Google Scholar]
- 8.Cerretelli P, Samaja M. Acid–base balance at exercise in normoxia and in chronic hypoxia. Revisiting the “lactate paradox”. Eur J Appl Physiol. 2003;90(5):431–448. doi: 10.1007/s00421-003-0928-x. [DOI] [PubMed] [Google Scholar]
- 9.Rusko HK, Tikkanen HO, Peltonen JE. Altitude and endurance training. J Sports Sci. 2004;22(10):928. doi: 10.1080/02640410400005933. [DOI] [PubMed] [Google Scholar]
- 10.Moncloa F, Carcelen A, Beteta L. Physical exercise, acid-base balance, and adrenal function in newcomers to high altitude. J Appl Physiol. 1970;28(2):151–155. doi: 10.1152/jappl.1970.28.2.151. [DOI] [PubMed] [Google Scholar]
- 11.Deb SK, Gough LA, Sparks SA, McNaughton LR. Determinants of curvature constant (W’) of the power duration relationship under normoxia and hypoxia: the effect of pre-exercise alkalosis. Eur J Appl Physiol. 2017;117(5):901–912. doi: 10.1007/s00421-017-3574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon OM, McGawley K, Nybäck L, Duckworth L, Barlow MJ, Woods D, et al. “Beet-ing” the mountain: a review of the physiological and performance effects of dietary nitrate supplementation at simulated and terrestrial altitude. Sports Med. 2017;47(11):2155–2169. doi: 10.1007/s40279-017-0744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front Physiol. 2017;8:401. doi: 10.3389/fphys.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Effect of sodium bicarbonate on HCO3-, pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2011;21(3):189–194. doi: 10.1123/ijsnem.21.3.189. [DOI] [PubMed] [Google Scholar]
- 15.McNaughton LR, Gough L, Deb S, Bentley D, Sparks SA. Recent developments in the use of sodium bicarbonate as an ergogenic aid. Curr Sports Med Rep. 2016;15(4):233–244. doi: 10.1249/JSR.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 16.Gough LA, Brown D, Deb SK, Sparks SA, McNaughton LR. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur J Appl Physiol. 2018;118(12):2489–2498. doi: 10.1007/s00421-018-3975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitts RH. The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc. 2016;48(11):2335–2338. doi: 10.1249/MSS.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 18.Westerblad H. Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc. 2016;48(11):2339–2342. doi: 10.1249/MSS.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 19.Deb SK, Gough LA, Sparks SA, McNaughton LR. Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur J Appl Physiol. 2018;118(3):607–615. doi: 10.1007/s00421-018-3801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flinn S, Herbert K, Graham K, Siegler JC. Differential effect of metabolic alkalosis and hypoxia on high-intensity cycling performance. J Strength Cond Res. 2014;28(10):2852–2858. doi: 10.1519/JSC.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 21.Saunders B, Sale C, Harris RC, Sunderland C. Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform. 2014;9(4):627–632. doi: 10.1123/ijspp.2013-0295. [DOI] [PubMed] [Google Scholar]
- 22.Feriche Fernandez-Castanys B, Delgado-Fernandez M, Alvarez GJ. The effect of sodium citrate intake on anaerobic performance in normoxia and after sudden ascent to a moderate altitude. J Sports Med Phys Fitness. 2002;42(2):179–185. [PubMed] [Google Scholar]
- 23.Hausswirth C, Bigard AX, Lepers R, Berthelot M, Guezennec CY. Sodium citrate ingestion and muscle performance in acute hypobaric hypoxia. Eur J Appl Physiol Occup Physiol. 1995;71(4):362–368. doi: 10.1007/BF00240418. [DOI] [PubMed] [Google Scholar]
- 24.McLellan T, Jacobs I, Lewis W. Acute altitude exposure and altered acid-base states. II. Effects on exercise performance and muscle and blood lactate. Eur J Appl Physiol Occup Physiol. 1988;57(4):445–451. doi: 10.1007/BF00417991. [DOI] [PubMed] [Google Scholar]
- 25.Edge J, Bishop D, Goodman C. Effects of chronic NaHCO3 ingestion during interval training on changes to muscle buffer capacity, metabolism, and short-term endurance performance. J Appl Physiol. 2006;101(3):918–925. doi: 10.1152/japplphysiol.01534.2005. [DOI] [PubMed] [Google Scholar]
- 26.Mc Naughton L, Thompson D. Acute versus chronic sodium bicarbonate ingestion and anaerobic work and power output. J Sports Med Phys Fitness. 2001;41(4):456–462. [PubMed] [Google Scholar]
- 27.Durkalec-Michalski K, Zawieja EE, Podgórski T, Łoniewski I, Zawieja BE, Warzybok M, et al. The effect of chronic progressive-dose sodium bicarbonate ingestion on CrossFit-like performance: a double-blind, randomized cross-over trial. PLoS One. 2018;13(5):e0197480. doi: 10.1371/journal.pone.0197480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes-Silva JP, Reale R, Franchini E. Acute and chronic effect of sodium bicarbonate ingestion on Wingate test performance: a systematic review and meta-analysis. J Sports Sci. 2019;37(7):762–771. doi: 10.1080/02640414.2018.1524739. [DOI] [PubMed] [Google Scholar]
- 29.Oelz O, Howald H, Di Prampero PE, Hoppeler H, Claassen H, Jenni R, et al. Physiological profile of world-class high-altitude climbers. J Appl Physiol. 1986;60(5):1734–1742. doi: 10.1152/jappl.1986.60.5.1734. [DOI] [PubMed] [Google Scholar]
- 30.Mahood NV, Kenefick RW, Kertzer R, Quinn TJ. Physiological determinants of cross-country ski racing performance. Med Sci Sports Exerc. 2001;33(8):1379–1384. doi: 10.1097/00005768-200108000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Losnegard T, Myklebust H, Hallen J. Anaerobic capacity as a determinant of performance in sprint skiing. Med Sci Sports Exerc. 2012;44(4):673–681. doi: 10.1249/MSS.0b013e3182388684. [DOI] [PubMed] [Google Scholar]
- 32.Hydren JR, Kraemer WJ, Volek JS, Dunn-Lewis C, Comstock BA, Szivak TK, et al. Performance changes during a weeklong high-altitude alpine ski-racing training camp in lowlander young athletes. J Strength Cond Res. 2013;27(4):924–937. doi: 10.1519/JSC.0b013e31827a9c62. [DOI] [PubMed] [Google Scholar]
- 33.Patterson C, Raschner C, Platzer H-P. The 2.5-minute loaded repeated jump test: evaluating anaerobic capacity in alpine ski racers with loaded countermovement jumps. J Strength Cond Res. 2014;28(9):2611–2620. doi: 10.1519/JSC.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 34.Wirnitzer KC, Kornexl E. Exercise intensity during an 8-day mountain bike marathon race. Eur J Appl Physiol. 2008;104(6):999–1005. doi: 10.1007/s00421-008-0855-y. [DOI] [PubMed] [Google Scholar]
- 35.Inoue A, Sa Filho AS, Mello FCM, Santos TM. Relationship between anaerobic cycling tests and mountain bike cross-country performance. J Strength Cond Res. 2012;26(6):1589–1593. doi: 10.1519/JSC.0b013e318234eb89. [DOI] [PubMed] [Google Scholar]
- 36.Bertuzzi RC. de Moraes, Franchini E, Kokubun E, kiss, Maria Augusta Peduti dal Molin. Energy system contributions in indoor rock climbing. Eur J Appl Physiol. 2007;101(3):293–300. doi: 10.1007/s00421-007-0501-0. [DOI] [PubMed] [Google Scholar]
- 37.Watts PB. Physiology of difficult rock climbing. Eur J Appl Physiol. 2004;91(4):361–372. doi: 10.1007/s00421-003-1036-7. [DOI] [PubMed] [Google Scholar]
- 38.Peart DJ, Siegler JC, Vince RV. Practical recommendations for coaches and athletes: a meta-analysis of sodium bicarbonate use for athletic performance. J Strength Cond Res. 2012;26(7):1975–1983. doi: 10.1519/JSC.0b013e3182576f3d. [DOI] [PubMed] [Google Scholar]
- 39.Deldicque L, Francaux M. Functional food for exercise performance: fact or foe? Curr Opin Clin Nutr Metab Care. 2008;11(6):774–781. doi: 10.1097/MCO.0b013e3283139489. [DOI] [PubMed] [Google Scholar]
- 40.Bishop D. Dietary supplements and team-sport performance. Sports Med. 2010;40(12):995–1017. doi: 10.2165/11536870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med. 2011;41(10):801–814. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Pendergast DR, Meksawan K, Limprasertkul A, Fisher NM. Influence of exercise on nutritional requirements. Eur J Appl Physiol. 2011;111(3):379–390. doi: 10.1007/s00421-010-1710-5. [DOI] [PubMed] [Google Scholar]
- 43.Limmer M, Berkholz A, M de M, Platen P. Reliability and Validity of a New Portable Tethered Sprint Running Test as a Measure of Maximal Anaerobic Performance. J Strength Cond Res. 2019; Volume Publish Ahead of Print (April 01):1–8. 10.1519/JSC.0000000000003119. [DOI] [PubMed]
- 44.Egger F, Meyer T, Such U, Hecksteden A. Effects of sodium bicarbonate on high-intensity endurance performance in cyclists: a double-blind, Randomized Cross-Over Trial. PLoS One. 2014;9(12):e114729. doi: 10.1371/journal.pone.0114729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch AA, Mulligan A, Bingham SA, Khaw K-T. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European prospective investigation into Cancer and nutrition (EPIC)-Norfolk population study. Br J Nutr. 2008;99(6):1335–1343. doi: 10.1017/S0007114507862350. [DOI] [PubMed] [Google Scholar]
- 46.Roach RC, Hackett PH, Oelz O, Bärtsch P, Luks AM, MacInnis MJ, et al. The 2018 Lake Louise Acute Mountain sickness score. High Alt Med Biol. 2018;19(1):4–6. doi: 10.1089/ham.2017.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9. 10.1037/0033-2909.112.1.155. [DOI] [PubMed]
- 48.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Erlbaum; 1988. [Google Scholar]
- 49.Faul F, Erdfelder E, Lang A-G, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 50.Percival ME, Martin BJ, Gillen JB, Skelly LE, MacInnis MJ, Green AE, et al. Sodium bicarbonate ingestion augments the increase in PGC-1α mRNA expression during recovery from intense interval exercise in human skeletal muscle. J Appl Physiol. 2015;119(11):1303–1312. doi: 10.1152/japplphysiol.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge R-L, Babb TG, Sivieri M, Resaland GK, Karlsen T, Stray-Gundersen J, et al. Urine acid-base compensation at simulated moderate altitude. High Alt Med Biol. 2006;7(1):64–71. doi: 10.1089/ham.2006.7.64. [DOI] [PubMed] [Google Scholar]
- 52.Poupin N, Calvez J, Lassale C, Chesneau C, Tome D. Impact of the diet on net endogenous acid production and acid-base balance. Clin Nutr. 2012;31(3):313–321. doi: 10.1016/j.clnu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Remer T. Influence of nutrition on acid-base balance—metabolic aspects. Eur J Nutr. 2001;40(5):214–220. doi: 10.1007/s394-001-8348-1. [DOI] [PubMed] [Google Scholar]
- 54.Swenson ER. Hypoxia and its Acid-Base consequences: from mountains to malignancy. Adv Exp Med Biol. 2016;903:301–323. doi: 10.1007/978-1-4899-7678-9_21. [DOI] [PubMed] [Google Scholar]
- 55.Ritchie ND, Baggott AV, Andrew Todd WT. Acetazolamide for the prevention of acute mountain sickness—a systematic review and meta-analysis. J Travel Med. 2012;19(5):298–307. doi: 10.1111/j.1708-8305.2012.00629.x. [DOI] [PubMed] [Google Scholar]
- 56.Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102(4):1313–1322. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- 57.Burtscher M, Gatterer H, Faulhaber M, Burtscher J. Acetazolamide pre-treatment before ascending to high altitudes: when to start? Int J Clin Exp Med. 2014;7(11):4378–4383. [PMC free article] [PubMed] [Google Scholar]
- 58.Posch AM, Dandorf S, Hile DC. The effects of acetazolamide on exercise performance at sea level and in hypoxic environments: a review. Wilderness Environ Med. 2018;29(4):541–545. doi: 10.1016/j.wem.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Gatterer H, Menz V, Untersteiner C, Klarod K, Burtscher M. Physiological factors associated with declining repeated sprint performance in hypoxia. J Strength Cond Res. 2019;33(1):211–6. [DOI] [PubMed]
- 60.Simpson LP, Jones AM, Skiba PF, Vanhatalo A, Wilkerson D. Influence of hypoxia on the power-duration relationship during high-intensity exercise. Int J Sports Med. 2015;36(2):113–119. doi: 10.1055/s-0034-1389943. [DOI] [PubMed] [Google Scholar]
- 61.Girard O, Brocherie F, Millet GP. Effects of altitude/hypoxia on single- and multiple-Sprint performance: a comprehensive review. Sports Med. 2017;47(10):1931–1949. doi: 10.1007/s40279-017-0733-z. [DOI] [PubMed] [Google Scholar]
- 62.McLellan TM, Kavanagh MF, Jacobs I. The effect of hypoxia on performance during 30 s or 45 s of supramaximal exercise. Eur J Appl Physiol Occup Physiol. 1990;60(2):155–161. doi: 10.1007/BF00846037. [DOI] [PubMed] [Google Scholar]
- 63.McNaughton L, Backx K, Palmer G, Strange N. Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol. 1999;80(4):333–336. doi: 10.1007/s004210050600. [DOI] [PubMed] [Google Scholar]
- 64.Froio de Araujo Dias G, da Eira Silva V, V de SP, Sale C, Giannini Artioli G, Gualano B, et al. Consistencies in Responses to Sodium Bicarbonate Supplementation: A Randomised, Repeated Measures, Counterbalanced and Double-Blind Study. PLoS One. 2015;10(11):e0143086. doi: 10.1371/journal.pone.0143086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hadzic M, Eckstein ML, Schugardt M. The impact of sodium bicarbonate on performance in response to exercise duration in athletes: a systematic review. J Sports Sci Med. 2019;18(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol. 1998;85(4):1448–1456. doi: 10.1152/jappl.1998.85.4.1448. [DOI] [PubMed] [Google Scholar]
- 67.Hamlin MJ, Manimmanakorn A, Creasy RH, Manimmanakorn N. Live high-train low altitude training: responders and non- responders. J Athl Enhanc. 2015;04(02):1–8.
- 68.Lockie RG, Murphy AJ, Spinks CD. Effects of resisted sled towing on sprint kinematics in field-sport athletes. J Strength Cond Res. 2003;17(4):760–767. doi: 10.1519/1533-4287(2003)017<0760:eorsto>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Maulder PS, Bradshaw EJ, Keogh JWL. Kinematic alterations due to different loading schemes in early acceleration sprint performance from starting blocks. J Strength Cond Res. 2008;22(6):1992–2002. doi: 10.1519/JSC.0b013e31818746fe. [DOI] [PubMed] [Google Scholar]
- 70.Lopes-Silva JP, Da Silva Santos JF, Artioli GG, Loturco I, Abbiss C, Franchini E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur J Sport Sci. 2018;18(3):431–440. doi: 10.1080/17461391.2018.1424942. [DOI] [PubMed] [Google Scholar]
- 71.Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol. 1978;33(1):9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 72.McNaughton LR. Bicarbonate ingestion: effects of dosage on 60 s cycle ergometry. J Sports Sci. 1992;10(5):415–423. doi: 10.1080/02640419208729940. [DOI] [PubMed] [Google Scholar]
- 73.Kahle LE, Kelly PV, Eliot KA, Weiss EP. Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res. 2013;33(6):479–486. doi: 10.1016/j.nutres.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slaney G, Cook A, Weinstein P. High altitude syndromes at intermediate altitudes: a pilot study in the Australian Alps. Med Hypotheses. 2013;81(4):547–550. doi: 10.1016/j.mehy.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Anand AC, Sashindran VK, Mohan L. Gastrointestinal problems at high altitude. Trop Gastroenterol. 2006;27(4):147–153. [PubMed] [Google Scholar]
- 76.Delextrat A, Mackessy S, Arceo-Rendon L, Scanlan A, Ramsbottom R, Calleja-Gonzalez J. Effects of three-day serial sodium bicarbonate loading on performance and physiological parameters during a simulated basketball test in Female University players. Int J Sport Nutr Exerc Metab. 2018;28(5):547–552. doi: 10.1123/ijsnem.2017-0353. [DOI] [PubMed] [Google Scholar]
- 77.Stapelfeldt B, Schwirtz A, Schumacher YO, Hillebrecht M. Workload demands in mountain bike racing. Int J Sports Med. 2004;25(4):294–300. doi: 10.1055/s-2004-819937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is available in the figshare data repository [doi: 10.6084/m9.figshare.8937995].