Abstract
Background
In myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), altered membrane excitability often occurs in exercising muscles demonstrating muscle dysfunction regardless of any psychiatric disorder. Increased oxidative stress is also present in many ME/CFS patients and could affect the membrane excitability of resting muscles.
Methods
Seventy-two patients were examined at rest, during an incremental cycling exercise and during a 10-min post-exercise recovery period. All patients had at least four criteria leading to a diagnosis of ME/CFS. To explore muscle membrane excitability, M-waves were recorded during exercise (rectus femoris (RF) muscle) and at rest (flexor digitorum longus (FDL) muscle). Two plasma markers of oxidative stress (thiobarbituric acid reactive substance (TBARS) and oxidation–reduction potential (ORP)) were measured. Plasma potassium (K+) concentration was also measured at rest and at the end of exercise to explore K+ outflow.
Results
Thirty-nine patients had marked M-wave alterations in both the RF and FDL muscles during and after exercise while the resting values of plasma TBARS and ORP were increased and exercise-induced K+ outflow was decreased. In contrast, 33 other patients with a diagnosis of ME/CFS had no M-wave alterations and had lower baseline levels of TBARS and ORP. M-wave changes were inversely proportional to TBARS and ORP levels.
Conclusions
Resting muscles of ME/CFS patients have altered muscle membrane excitability. However, our data reveal heterogeneity in some major biomarkers in ME/CFS patients. Measurement of ORP may help to improve the diagnosis of ME/CFS.
Trial registration Ethics Committee “Ouest II” of Angers (May 17, 2019) RCB ID: number 2019-A00611-56
Keywords: Muscle excitability, Oxidative stress, Potassium outflow, Myalgic encephalomyelitis/chronic fatigue syndrome
Background
Adult patients with chronic fatigue may be suffering from chronic fatigue syndrome, also known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). ME/CFS often occurs in the absence of any disease that could be responsible for body fatigue and is characterized by intense fatigue which is worsened by physical/mental activity and is often associated with post-exertional malaise (PEM) [1, 2]. Current definitions of ME/CFS do not use biological markers.
Several body systems including the muscular and nervous systems are affected in ME/CFS [3–5]. Potential causes of muscle dysfunction in ME/CFS patients may include oxidative stress with reduced heat-shock protein production. Experimental evidence indicates that exercising skeletal muscles are often affected in ME/CFS because marked alterations in myopotential occur in response to direct muscle stimulation (M-wave) [5–11]. M-wave alterations show impaired muscle membrane excitability and begin early in the exercising muscles before culminating during the 10-min recovery period [6–8]. In contrast, M-wave alterations are absent in healthy subjects in whom the myopotential amplitude often increases with the incremental pedalling force [12, 13]. No data are available on altered excitability in the resting muscles of ME/CFS patients.
Fulle et al. [7] observed a dysregulation of the Na+/K+ ATPase pump in ME/CFS patients and suggested that this dysregulation could result from increased fluidity of the sarcoplasmic reticulum membrane in relation to the excessive production of reactive oxygen species (ROS). Indeed, an increase in both resting levels and exercise-induced production of ROS is often found in ME/CFS patients [8–11, 14–16]. In these studies, thiobarbituric acid reactive substance (TBARS), an element related to overall redox equilibrium and a marker of lipid peroxidation, was used on several occasions to measure oxidative stress. Conversely, oxidation-reduction potential (ORP) per se was not addressed in this context. ORP reflects the capacity of an aqueous medium to either release or accept electrons produced by chemical reactions. ORP therefore measures the overall balance between oxidants and reductants in a system. When the oxidant activity exceeds reductant activity, a high ORP value is measured [17–19].
Because oxidative stress conditions are detected in the blood of ME/CFS patients, we hypothesized that M-wave alterations may not only affect the exercising muscles but also affect resting ones. We therefore investigated the presence of altered muscle excitability in exercising and resting muscles of patients with a clinical diagnosis of ME/CFS. We simultaneously recorded M-waves in the rectus femoris (RF) leg muscle and flexor digitorum longus (FDL) forearm muscle, during and after an incremental cycling exercise. The redox status of the patients was also assessed by measuring resting plasma levels of TBARS and ORP.
Methods
Study population
Subjects aged ≥ 18-years referred to our hospital for the diagnosis of chronic fatigue were recruited between April 2018 and June 2019. Inclusion criteria included: at least 6 months of chronic fatigue and at least four symptoms of inability as required for the diagnosis of ME/CFS [1, 2]. Exclusion criteria were: a history of any other pathology confounding the ME/CFS diagnosis, including multiple sclerosis, auto-immune disease (lupus and sicca syndrome), diabetes, congestive heart disease, non-treated hypothyroidism, inflammatory muscle disease, or a significant psychiatric illness (e.g. major depression or psychosis) or pregnancy. Patients with drug abuse during the previous 12 months were also excluded.
The study protocol was approved by the French institutional review boards for human studies (ANSM and CPP Ouest II) and written consent was obtained from all patients to measure their maximal capacity at work.
Electromyography (EMG) recording and analysis
Bipolar Ag–AgCl surface electrodes (Medtronic, 13 L 20 Skovlunde, Denmark) were used to measure EMG voltage from the RF and FDL muscles on the dominant side of the body, as in previous studies [8–13]. The EMG signal was amplified (Nihon Kohden, Tokyo, Japan; common mode rejection ratio, 90 dB; input impedance, 100 mOhm; gain, 1000–5000) with a frequency band ranging from 10‒10,000 Hz. Compound muscle mass action potentials (M-waves) were evoked by direct muscle stimulation, using a constant-current neurostimulator (Grass, Quincy, MA, USA) delivering supramaximal shocks with 0.1 ms rectangular pulses through an isolation unit. Stimulating skin electrodes were fixed perpendicularly to the muscle axis from either side of the recording electrodes. An oscilloscope (model DSO 400; Gould, Ballainvilliers, France) was used to measure the average M-waves from eight successive potentials and to calculate the peak M-wave amplitude and its duration, and the conduction time, that is the time between the stimulus artefact and the peak.
Maximal handgrip strength
Maximal handgrip strength was also measured to determine the degree of force failure. Maximal handgrip strength was measured with the wrist in the neutral position with a pronated forearm to hold the handgrip device (model 5401; Takei Scientific Instruments Co Ltd, Niigata-City, Japan), as recommended by de Ponte et al. [20]. Study participants were instructed to perform three maximal handgrips sustained for 3 s. The highest handgrip strength of three contractions, expressed in Newtons (N), was considered the maximum. Each forearm was tested. The reference values were those reported by Steiber [21].
Blood measurements
Five ml of heparinized blood was taken from each subject to measure the biochemical variables. Potassium concentration was measured at rest and at the end of exercise to evaluate K+ outflow in relation to exercise. Two blood markers of oxidative stress (TBARS and ORP) were used. Plasma TBARS concentration was measured according to the method of Uchiyama and Mihara [22]. Plasma ORP was measured using a combined platinum electrode with an Ag/AgCL reference and a potentiostat (ArrowDox; Lazar Research Laboratories, Los Angeles CA, USA). Electrode calibration was performed using 10 different molarities of HCl. Two to three measurements of each plasma sample, separated by a 1-h interval, were performed. Between each measurement, the ORP of pure water was recorded and used to determine the shift with time of the rest value provided by the electrode, which was eventually used to correct the ORP sample values. The results are the average of 2–3 replications with a standard deviation of < 5%.
Exercise protocol
All patients underwent an exercise session on a cycle ergometer up to their maximal work rate supported for a 1-min period, often limited by muscle fatigue. As in our previous studies [8–11, 13], the protocol consisted of: (i) a 2-min rest period, during which all variables were measured and venous blood samples collected; (ii) a 1-min 20-W work load period used to reach the 1 Hz cycling frequency; (iii) a work period; and (iv) a 10-min recovery period. During the work period, the load was increased by 20 W every 60 s until fatigue obliged the subject to stop the exercise. Peak oxygen uptake (VO2max) was measured at the maximal work rate (Wmax) as was the maximal increase in heart rate. M-waves were recorded at 20, 60, 100 W, and at the maximal work rate reached. The ergometer was then unloaded and the subject continued to cycle for a 2-min recovery period to facilitate venous blood return from the legs. During recovery, M-waves were recorded at 2, 5 and 10 min. During the exercise session and the post-exercise recovery period the right forearm remained totally relaxed, the hand being placed on the ergocycle handlebar without grasping.
Statistical analyses
Power analysis for determination of sample size was founded on an assumption of 95% confidence intervals [95% CI] and 80% power. The Holm-Sidak test was used for both pairwise comparisons and comparisons versus a control situation (rest) to determine the significance of changes in M-wave amplitude and duration. The Student’s t-test was used to assess intergroup differences in M-wave changes between resting values of TBARS and ORP, and maximal changes in M-wave amplitude and duration. Linear regressions between maximal M-wave variations and resting levels of TBARS and ORP were investigated.
Results
Seventy-two patients were included in the study. After completion of the whole exercise trial, two groups of patients were distinguished: (i) 39 ME/CFS patients without comorbidities with M-wave alterations (group 1); and (ii) 33 ME/CFS patients who did not present M-wave changes (group 2). In group 2, 15 patients suffered from other diseases (obstructive sleep apnoea (n = 4), ankylosing spondylitis (n = 2), sequelae of poliomyelitis (n = 1), Lyme disease (n = 4), Ehlers Danlos syndrome (n = 2) or a psychiatric disorder (burn out) (n = 2)).
There was no difference in age, sex ratio, exercise power characteristics and maximal handgrip strength between the two groups (Table 1), the later observation suggesting that the patients reached the same level of muscle fatigue. There was also no difference in the onset of fatigue. However, the maximal increase in plasma K+ concentration, measured at the end of exercise (Delta K+ max), was lower and resting TBARS and ORP levels were higher in group 1 vs. group 2.
Table 1.
Characteristics of the study population
| Group 1 (N = 39) | Group 2 (N = 33) | |
|---|---|---|
| Age (years) | 43 ± 3 | 47 ± 2 |
| Weight (kg) | 64 ± 2 | 68 ± 2 |
| Sex ratio (F/M) | 28/11 | 17/16 |
| Onset of fatigue (months) | 78 ± 19 | 69 ± 14 |
| TBARS rest (nmol/ml) | 1.36 ± 0.03 | 1.01 ± 0.05*** |
| ORP (mV) | 165 ± 5 | 127 ± 4*** |
| VO2max (mlO2/min/kg) | 19 ± 1 | 19 ± 1 |
| VO2max (% predicted) | 67 ± 3 | 63 ± 3 |
| Maximal work rate (W) | 124 ± 5 | 118 ± 7 |
| Maximal work rate (% predicted) | 90 ± 3 | 83 ± 3 |
| HR max (bpm) | 151 ± 3 | 148 ± 3 |
| Delta K+ max (mmol/l) | 0.42 ± 0.07 | 0.65 ± 0.07** |
| Handgrip strength (N | 278 ± 22 | 315 ± 33 |
| Handgrip strength (% predicted) | 62 ± 7 | 71 ± 7 |
Group 1 had marked changes in M-wave amplitude and duration whereas no M-wave changes were measured in group 2. All values shown are mean ± standard deviation
TBARS thiobarbituric acid reactive substance, ORP oxidation reduction potential, VO2 oxygen uptake, HR heart rate
Maximal increase in plasma potassium (K+) concentration was measured at the end of exercise. Asterisks denote significant intergroup difference (**p < 0.01; ***p < 0.001)
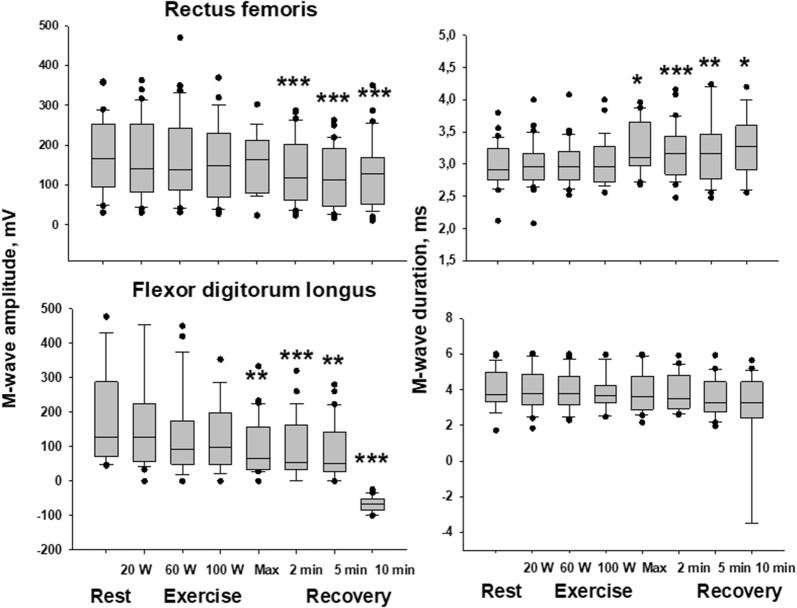
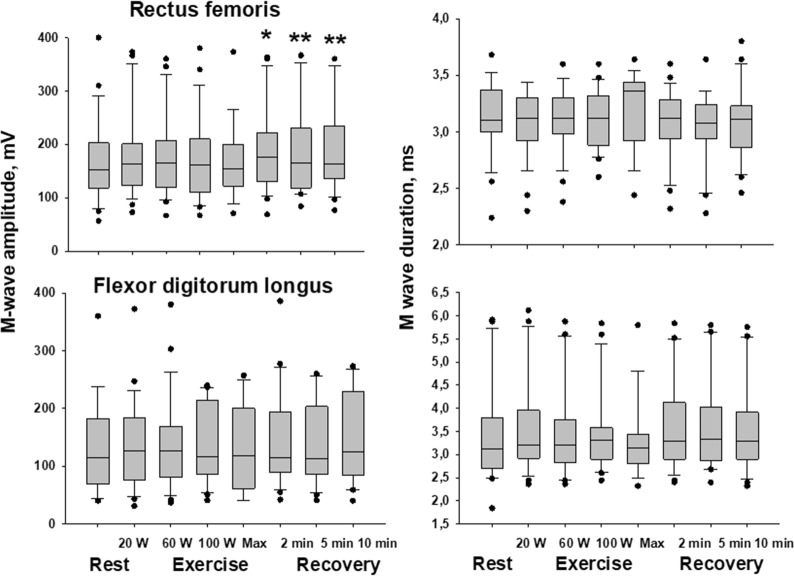
Maximal M-wave variations measured in the RF and FDL muscles are shown in Table 2. In group 1, M-wave alterations were present in both the exercising (RF) and resting (FDL) muscles. More specifically, the M-wave amplitude and length and the conduction time were affected in RF whereas only the M-wave amplitude varied in FDL. The kinetics of the M-wave changes in group 1 are shown in Fig. 1: compared to resting values, significant changes in M-wave amplitude (F value > 12) occurred close to the end of exercise and often progressed until the end of the recovery period. A significant increase in M-wave duration (F = 4.42) only occurred in RF. In group 2, the M-wave amplitude tended to increase in RF (F = 10.24) with no change in duration or conduction time. No M-wave change occurred in FDL (Table 2 and Fig. 2).
Table 2.
M-wave characteristics
| Group 1 (N = 39) | Group 2 (N = 33) | |
|---|---|---|
| Rectus femoris | ||
| Delta M wave amplitude (%) | − 41 ± 4† | + 14 ± 3*** |
| Delta M wave duration (%) | + 18 ± 3# | + 2 ± 3*** |
| Delta M wave latency (ms) | + 2.65 ± 0.17 | + 0.07 ± 0.04*** |
| Flexor digitorum longus | ||
| Delta M wave amplitude (%) | − 65 ± 4† | + 22 ± 5***,# |
| Delta M wave duration (%) | + ± 4 | + 8 ± 2 |
| Delta M wave latency (ms) | + 0.26 ± 0.07 | + 0.04 ± 0.05** |
Maximal variations in M-wave amplitude and duration expressed as a percentage of resting values. Latencies measured at rest and at the end of the post-exercise recovery period. Symbols indicate that the changes in each group differ significantly from control values (i.e. rest data) (#p < 0.01; †p < 0.001). Asterisks denote significant intergroup differences (***p < 0.001). Post-exercise variations in M-wave latencies are not significant
Fig. 1.
Time course of changes in M-wave amplitude and duration during exercise (rectus femoris) and at rest (flexor digitorum longus) in group 1 patients. Median values are given at rest, at the four steps of exercise, and at the 2nd, 5th, and 10th min of post-exercise recovery. Asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) indicate significant decreases in M-wave amplitude and increased duration
Fig. 2.
Time course of changes in M-wave amplitude and duration during exercise (rectus femoris) and at rest (flexor digitorum longus) in group 2 patients. Median values are given at rest, at the four steps of exercise, and at the 2nd, 5th, and 10th min of post-exercise recovery. Asterisks (*p < 0.05; **p < 0.01) indicate significant increases in M-wave amplitude
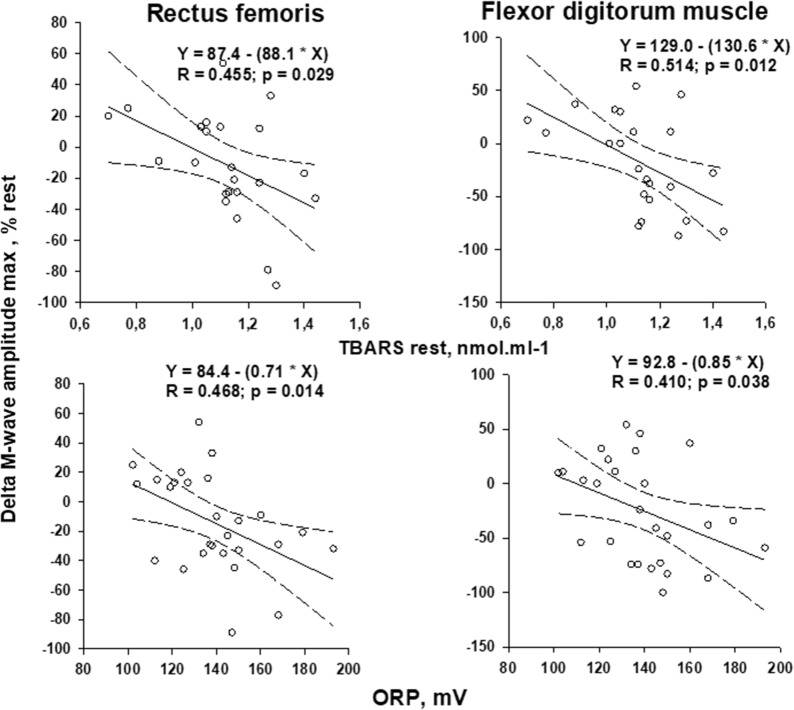
Regarding plasma markers, a significant positive correlation was found between TBARS and ORP values (Fig. 3). When plotting all values measured in both groups of patients (i.e. maximal variations in M-wave amplitude vs. resting levels of TBARS or ORP), negative linear regressions were found (Fig. 4). No significant correlations were found between M-wave changes in the two muscle groups and K+ outflow magnitude.
Fig. 3.
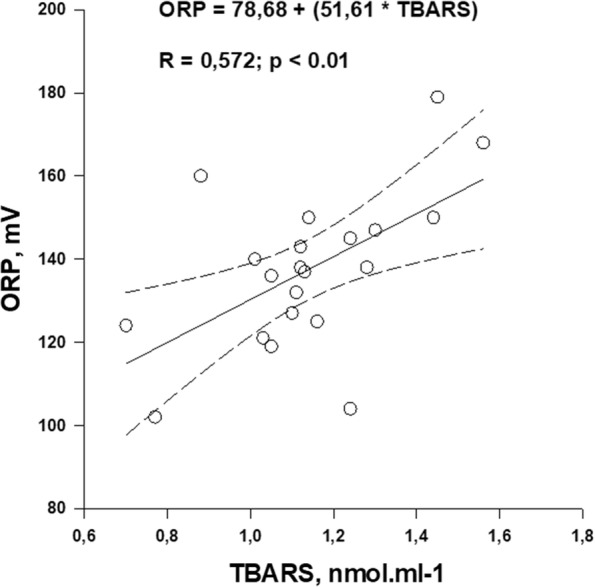
Regression with 95% CIs obtained between resting TBARS and ORP values. The regression equation and significance against zero of the R coefficient value is indicated
Fig. 4.
Maximal variations in M-wave amplitude expressed as a percentage of resting values plotted against resting levels of TBARS and ORP. Linear regressions with 95% CIs are shown as well as the regression equations and the significance against zero of the R coefficient value
Discussion
This study distinguished two groups of patients with ME/CFS. In group 1, M-wave alterations were found during and after exercise in both the exercising leg muscle and the resting forearm muscle. In the exercising leg muscle, a decreased in amplitude and longer duration of M-waves were measured whereas in the resting forearm muscle only an alteration in M-wave amplitude was observed. By referring to the redox data presented here, our study shows that high ORP and TBARS values were associated with disorders in both exercising and resting muscles. Finally, exercise-induced K+ outflow was reduced and M-wave alterations were proportional to the magnitude of oxidative stress. In group 2, with no M-wave alterations, low baseline levels of TBARS and ORP were measured. It should be noted however that half the subjects in this group suffered from comorbidities which could contribute to body fatigue.
There are several important strengths of this study. First, we used a population with homogeneous criteria for ME/CFS. Second, all patients completed a blinded exercise protocol.
The main limitation to this study is its monocentric nature. Thus, we could not exclude potential referral bias.
The present observations confirm the existence of redox disorders in many ME/CFS patients [3–11, 14–16]. These data also show that abnormal redox status is generally present in ME/CFS patients with abnormal muscle membrane excitability, in contrast to patients with no muscle abnormalities. We therefore conclude that high ORP values can be used to distinguish these two groups of patients. A recent study has already reported that plasma ORP changes give a rapid, accurate and user-friendly measurement of increased oxidative stress [23]. We confirm this idea and conclude that ORP measurement is cheaper, quicker to perform and provides information on the overall imbalance between oxidants and anti-oxidants whereas TBARS measurements need complex biochemical processing and only explore a single element of the overall redox equilibrium.
The present study also shows that the decreased M-wave amplitude in both exercising and resting muscles was proportional to the magnitude of oxidant/anti-oxidant imbalance in plasma, which supports the primary hypothesis of Fulle et al. [14]. This also supports the hypothesis that M-wave alterations in non-exercising muscles result from a systemic disorder of muscle membrane excitability due to dysregulation of the oxidant/anti-oxidant balance in blood. Increased ROS production following exercise inhibits Na+–K+ pump activity [7], reducing the K+ outflow and muscle membrane excitability [24]. Previous studies have shown that increased plasma oxidant level is associated with reduced K+ outflow from exercising muscles in healthy subjects [25] and ME/CFS patients [10]. In the present study, it should be noted that K+ outflow measured at the end of exercise was higher in group 2 vs. group 1. This indicates that the dysregulation of oxidant/anti-oxidant status in plasma alters the ionic flux through the muscle membrane, which may partly promote altered muscle membrane excitability.
The existence of a disorder of muscle excitability after exercise could partly explain the occurrence of PEM reported by many ME/CFS patients. However in our series of patients PEM was present in both groups. Pietrangelo et al. [26] hypothesized that patients with ME/CFS are subjected to problems of muscle aging (sarcopenia) and that intergroup differences in biological data (M-wave, redox status, K+ outflow) may have resulted from differences in gene expression. Gene expression subtypes have been reported in ME/CFS patients [27] and the authors isolated 40 abnormal gene pathways. However, the intergroup differences between oxidative abnormalities could not result from age and/or sex differences as reported in healthy subjects by Fano et al. [28] because in our study these parameters were similar in the two groups. Furthermore, the comorbidities present in group 2 patients cannot explain the absence of marked oxidative damage. Indeed, high levels of oxidative stress are often measured in patients with ankylosing spondylitis [29] or Lyme disease [30], these comorbidities being present in six of our patients. Additionally, fatigue that leads to physical deterioration is another important symptom that is observed in 90% of patients with post-polio syndrome, probably resulting from spine frontal horn motor neuron damage during acute poliovirus infection [31]. However this comorbidity was only reported in one of our patients. We therefore have no satisfactory explanation for the intergroup differences observed here.
Conclusion
Future studies in different patient populations may help to further refine the biological criteria of ME/CFS and in particular address the reason(s) for the presence or absence of peripheral muscle fatigue in patients. Our data also highlight the heterogeneity of some biomarker measurements in patients with a diagnosis of ME/CFS. Finally, our data support the use of ORP to address the redox stress in a ME/CFS context with diagnostic relevance.
Acknowledgements
None.
Abbreviations
- CFS
Chronic fatigue syndrome
- EMG
Electromyography
- FDL
Flexor digitorum longus
- K+
Potassium
- ME
Myalgic encephalomyelitis
- M-wave
Evoked muscle action potential
- ORP
Oxidation–reduction potential
- PEM
Post-exertional malaise
- RF
Rectus femoris
- ROS
Reactive oxygen species
- TBARS
Thiobarbituric acid reactive substance
- VO2max
Maximal oxygen uptake
- Wmax
Maximal work rate
Authors’ contributions
YJ, FR, EF and RG designed the research study, analyzed the data and wrote the paper. YJ, EF, FR, CCharpin, SR, CS, NA, NK and CCriado performed the acquisition of data. YJ, FR, and EF analyzed and interpreted the data. All authors read and approved the final manuscript.
Funding
This study did not receive any funding body for its design, analysis and interpretation of data, or for writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee “Ouest II” of Angers (May 17, 2019) RCB ID: number 2019-A00611-56 and the study was carried out according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). Procedures were carried out with the adequate understanding and written consent of the subjects.
Consent for publication
Our manuscript does not contain any identifiable individual’s data in any form (including individual details, images or videos).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carruthers BM. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. J Clin Pathol. 2007;60:117–119. doi: 10.1136/jcp.2006.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine of the National Academies . Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations. Washington: National Academies Press; 2015. [PubMed] [Google Scholar]
- 3.Gerwyn M, Maes M. Mechanisms explaining muscle fatigue and muscle pain in patients with myalgic encephalomyelitis chronic fatigue syndrome (ME/CFS): a review of recent findings. Curr Rheumatol Rep. 2017;19:1. doi: 10.1007/s11926-017-0628-x. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford G, Panning P, Newton JL. Understanding muscle dysfunction in chronic fatigue syndrome. J Aging Res. 2016 doi: 10.1155/2016/2497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jammes Y, Retornaz F. Understanding neuromuscular disorders in chronic fatigue syndrome. F1000 Research. 2019 doi: 10.12688/f1000research.18660.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samii A, Wassermann EM, Ikoma K, Mercuri B, George MS, O’Fallon A, et al. Decreased post-exercise facilitation of motor evoked potentials in patients with chronic fatigue syndrome or depression. Neurology. 1996;47:1410–1414. doi: 10.1212/WNL.47.6.1410. [DOI] [PubMed] [Google Scholar]
- 7.Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fano G. Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromusc Disorders. 2003;13:479–484. doi: 10.1016/S0960-8966(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 8.Jammes Y, Steinberg JG, Mambrini O, Brégeon F, Delliaux S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 9.Jammes Y, Steinberg JG, Delliaux S. Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J Intern Med. 2012;272:74–84. doi: 10.1111/j.1365-2796.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 10.Jammes Y, Steinberg JG, Guieu R, Delliaux S. Chronic fatigue syndrome with history of severe infection combined altered blood oxidant status, and reduced potassium efflux and muscle excitability at exercise. Open J Intern Med. 2013;3:98–105. doi: 10.4236/ojim.2013.33023. [DOI] [Google Scholar]
- 11.Fenouillet E, Vigouroux A, Steinberg JG, Chagvardieff A, Retornaz F, Guieu R, et al. Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. J Transl Med. 2016;31(14):251. doi: 10.1186/s12967-016-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnaud S, Zattara Hartmann MC, Tomei C, Jammes Y. Correlation between muscle metabolism and changes in M-wave and surface electromyogram: dynamic constant load leg exercise in untrained subjects. Muscle Nerve. 1997;20:1197–1199. doi: 10.1002/(SICI)1097-4598(199709)20:9<1197::AID-MUS20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Jammes Y, Zattara Hartmann MC, Caquelard F, Arnaud S, Tomei C. Electromyographic changes in vastus lateralis during dynamic exercise. Muscle Nerve. 1997;20:247–249. doi: 10.1002/(SICI)1097-4598(199702)20:2<247::AID-MUS21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Fulle S, Mecocci P, Fanó G, Vecchiet I, Vecchini A, Racciotti D, et al. Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic Biol Med. 2000;29:1252–1259. doi: 10.1016/S0891-5849(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 15.Richards RS, Roberts TK, McGregor NR, Dunstan RH, Butt HL. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000;5:35–41. doi: 10.1179/rer.2000.5.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335:151–154. doi: 10.1016/S0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bobe G, Cobb TJ, Leonard SW, Aponso S, Bahro CB, Koley D, et al. Increased static and decreased capacity oxidation-reduction potentials in plasma are predictive of metabolic syndrome. Redox Biol. 2017;12:121–128. doi: 10.1016/j.redox.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels RC, Jun H, Tiba MH, McCracken B, Herrera-Fierro P, Collinson M, et al. Whole blood redox potential correlates with progressive accumulation of oxygen debt and acts as a marker of resuscitation in a swine hemorrhagic shock model. Shock. 2018;49:345–351. doi: 10.1097/SHK.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Ponte ÁM, Guirro EC, Pletsch AH, Dibai-Filho AV, Brandino HE, Guirro RR. The forearm positioning changes electromyographic activity of upper limb muscles and handgrip strength in the task of pushing a load cart. J Body Mov Ther. 2015;19:597–603. doi: 10.1016/j.jbmt.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Steiber N. Strong or weak handgrip? Normative reference values for the German population across the life course stratified by sex, age, and body height. PLoS ONE. 2016;11:e0163917. doi: 10.1371/journal.pone.0163917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchiyama M, Mihara M. Determination of malonedialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 23.Polson D, Villalba N, Freeman K. Optimization of a diagnostic platform for oxidation-reduction potential (ORP) measurement in human plasma. Redox Rep. 2018;23:125–129. doi: 10.1080/13510002.2018.1456000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjøgaard G. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol Scand Suppl. 1990;593:1–63. [PubMed] [Google Scholar]
- 25.Hermann A, Sitdikova GF, Weiger TM. Oxidative stress and maxi calcium-activated potassium (BK) channels. Biomolecules. 2015;5:1870–1911. doi: 10.3390/biom5031870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietrangelo T, Fulle S, Coscia F, Gigliotti PV, Fanò Illic G. Old muscle in young body: an aphorism describing the chronic fatigue syndrome. Eur J Transl Myol. 2018;28:7688. doi: 10.4081/ejtm.2018.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr JR, Petty R, Burke B, Gough J, Fear D, Sinclair LI, et al. Gene expression subtypes in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Infect Dis. 2008;197:1171–1184. doi: 10.1086/533453. [DOI] [PubMed] [Google Scholar]
- 28.Fanò G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22:345–351. doi: 10.1023/A:1013122805060. [DOI] [PubMed] [Google Scholar]
- 29.Pishgahi A, Abolhasan R, Danaii S, Amanifar B, Soltani-Zangbar MS, Zamani M, et al. Immunological and oxidative stress biomarkers in ankylosing spondylitis patients with or without metabolic syndrome. Cytokine. 2020;128:155002. doi: 10.1016/j.cyto.2020.155002. [DOI] [PubMed] [Google Scholar]
- 30.Peacock BN, Gherezghiher TB, Hilario JD, Kellermann GH. New insights into Lyme disease. Redox Biol. 2015;5:66–70. doi: 10.1016/j.redox.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pastuszak Ż, Stępień A, Tomczykiewicz K, Piusińska-Macoch R, Galbarczyk D, Rolewska A. Post-polio syndrome. Cases report and review of literature. Neurol Neurochir Pol. 2017;51:140–145. doi: 10.1016/j.pjnns.2017.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.