Abstract
Background
Gestational diabetes mellitus (GDM) is the most common metabolic disorder that can occur during pregnancy and is associated with a long-term risk of both maternal and neonatal comorbidities. This study aimed to investigate the association between echocardiographic epicardial adipose tissue (EAT) and the risk for GDM during the early second trimester of pregnancy.
Method
We recruited all singleton pregnancies between January 2014 and December 2018 at 16 weeks + 0 days to 19 weeks + 6 days. We then used generalized linear models to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for EAT as a potential predictor for GDM. Receiver-operating-characteristic (ROC) analysis was then conducted to investigate the discriminative capacity of any individual maternal factor for the prediction of GDM.
Results
In total, our study involved 314 pregnant women with GDM and 1832 pregnant women without GDM. Multivariate regression analysis revealed that EAT thickness (OR = 2.87; 95% CI: 2.49–3.31) was significantly associated with the presence of GDM (P < 0.001). Furthermore, EAT thickness was also significantly associated with a range of adverse outcomes in the GDM group, including large size for gestational age, neonatal hypoglycemia, admission to the neonatal intensive care unit, preterm delivery, and hyperbilirubinemia (P < 0.001). ROC analysis revealed that the area under the curve was 0.790 (95% CI: 0.768–0.812). When the cutoff value for EAT thickness was set to 5.49 mm, the sensitivity was 95.2% and the specificity was 50.5%.
Conclusions
Echocardiographic EAT thickness is positively and significantly associated with both the risk of GDM and adverse outcomes related to GDM. Echocardiographic EAT has the potential to predict GDM prior to actual clinical diagnosis.
Keywords: Gestational diabetes mellitus, Epicardial adipose tissue, Prediction, Echocardiography
Background
Gestational diabetes mellitus (GDM), the most common metabolic disorder of pregnancy, is a condition in which carbohydrate intolerance develops during pregnancy. The offspring of women with GDM are at an increased risk of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia. The prevalence of GDM varies from 1 to 20%, and is rising worldwide, parallel to the increased prevalence of obesity and type 2 diabetes mellitus (T2DM) [1]. As reported previously, the prevalence of GDM in a population of pregnant women usually reflects the prevalence of T2DM in that particular population [2].
It is important to predict GDM early in pregnancy to enable early interventions that could prevent GDM and reduce adverse outcomes. Currently, there are no established guidelines for the prediction of GDM and no effective modalities for the prevention of its future development prior to actual diagnosis. According to existing literature, excessive body weight/obesity is the main risk factor for GDM [3]. Clinicians usually use body mass index (BMI) to assess maternal obesity during pregnancy. However, previous studies found that BMI may not be a good predictor for GDM around the first trimester [4, 5], due to its inability to reflect the accumulation and mass of the visceral adipose tissue (VAT) [6]. Recently, a promising echocardiographic parameter, epicardial adipose tissue (EAT), has emerged that could complement the use of BMI to assess risk for GDM. EAT is closely related to metabolic syndrome and diabetes [7, 8], and is an independent predictor of visceral adiposity, as measured by echocardiography [9]. This study aimed to investigate the association between the EAT thickness and GDM, and assess the efficacy of EAT thickness as a potential predictor for GDM at 16 weeks + 0 days to 19 weeks + 6 days before the diagnosis of GDM.
Methods
Study design, setting, and population
This prospective cohort study was conducted at the First Affiliated Hospital of China Medical University. All participants were admitted to our obstetric clinic between January 2014 and December 2018. All participants provided written informed consent and the study protocol was approved by the Medical Ethics Review Board of China Medical University (Shenyang, Liaoning, China).
Criteria
Participants were eligible if they: (1) had a singleton pregnancy; (2) had their first pregnancy visit before 19 weeks + 6 days (gestational age was determined by ultrasound within 3 months of pregnancy confirmation); (3) signed the informed consent form and provided a complete medical history. Participants were not eligible if they had a history of diabetes (including GDM in previous pregnancies), hypertension, or cardiovascular diseases.
Data collection between 16 weeks + 0 days and 19 weeks + 6 days
A range of anthropometric parameters were measured for each participant, including weight, height, heart rate, systolic blood pressure, and diastolic blood pressure; we also calculated the BMI [10]. At each visit, we also recorded the family history of diabetes as a parent or sibling may have been diagnosed as having diabetes in the interval since the previous visit [11].
A peripheral blood sample was collected from each participant before 19 weeks + 6 days; samples were collected in a vacutainer collection tube. We used the blood samples to determine the lipid profile of each participant, including triglyceride, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C); these parameters were determined with an auto-analyzer (AU1000; Olympus, Tokyo, Japan).
Maternal echocardiography was also performed between 16 weeks + 0 days and 19 weeks + 6 days. All images were obtained using a Philips iE33 system (Philips Medical Systems, Bothell, WA, USA) with a 1.5/5 MHz phased array probe. Maternal EAT thickness was measured by echocardiography in the parasternal long-axis view at the level of the fold of Rindfleisch, between the free wall of the right ventricle and the anterior surface of the ascending aorta [12].
Data collection between 24 weeks + 0 days and 28 weeks + 0 days
A two-hour 75-g oral glucose tolerance test (OGTT) was used to test for GDM; these tests were carried out after an overnight fast. The following morning, we tested the fasting plasma glucose concentration and then asked the participant to drink 250–300 mL of water containing 75 g of sugar. We then determined plasma glucose levels 1 hour and 2 hours later [13].
GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Groups [13]. A diagnosis of GDM was made when one or more of the test parameters equaled or exceeded the following cut points: fasting 92 mg/dL (5.1 mmol/L), 1-h 180 mg/dL (10.0 mmol/L), or 2-h 153 mg/dL (8.5 mmol/L).
Follow-up of neonatal outcomes in GDM patients
Adverse neonatal outcomes were recorded after delivery, including large size for gestational age, neonatal hypoglycemia, admission to the neonatal intensive care unit (NICU), preterm delivery, and hyperbilirubinemia. Large size for gestational age was defined as the birth weight at or above the gestational age-specific 90th percentile [3]. Neonatal hypoglycemia was defined as a blood glucose concentration < 47 mg/dL (< 2.6 mmol/L) [14]. Preterm was defined as delivery before 37 weeks of pregnancy [15]. Hyperbilirubinemia was defined as a bilirubin level that, at any time after birth, exceeded the hour-specific phototherapy treatment threshold recommended in the American Academy of Pediatrics’ clinical practice guideline on the management of neonatal hyperbilirubinemia [16].
Statistical analysis
Statistical analysis was performed using STATA version 14.0 software. Continuous parameters were expressed as the mean ± standard deviation. Non-normally distributed parameters were expressed as the median. Differences of normally distributed continuous parameters between groups were analyzed using the independent-samples t-test. Differences of non-normally distributed parameters between groups were analyzed using the Mann-Whitney U test. Differences of categorial parameters between groups were analyzed using Pearson’s chi-squared test. Odds ratio (OR) and 95% confidence intervals (CIs) of individual maternal factors, as potential predictors for GDM, were calculated using generalized linear models. Receiver-operating-characteristic (ROC) analysis was conducted to assess the discriminative capacity of any individual maternal factor for the prediction of GDM. A two-tailed P < 0.05 was used to define statistical significance.
Results
Baseline maternal characteristics
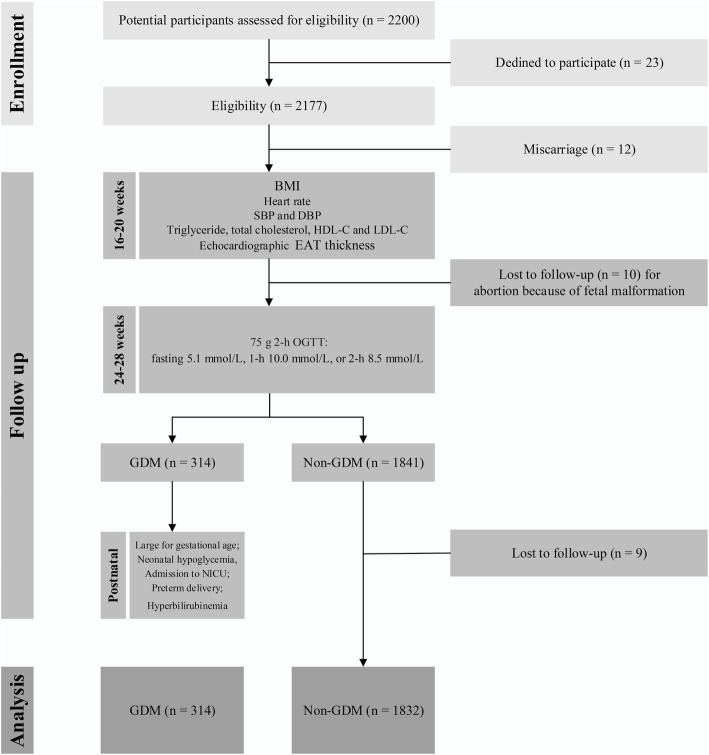
A total of 2146 mothers met our eligibility criteria and were included in the main analysis; 314 of these mothers had GDM and 1832 did not (Fig. 1). Table 1 shows the baseline clinical characteristics of the participants as compared between the GDM and control groups. There were significant differences between the two groups with respect to maternal age, BMI, lipid profiles (triglyceride, total cholesterol, and HDL-C), and EAT thickness (P = 0.018, P = 0.026, P = 0.018, P = 0.015, P = 0.007, P < 0.001, respectively). Overall, 47, 102, 113, and 52 participants received maternal echocardiography at 16–17, 17–18, 18–19, and 19–20 weeks of gestation in the GDM group. In the control group, 310, 628, 611, and 283 participants received maternal echocardiography at 16–17, 17–18, 18–19, and 19–20 weeks of gestation.
Fig. 1.
Study flow diagram. BMI, body mass index; DBP, diastolic blood pressure; EAT, epicardial adipose tissue; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NICU, neonatal intensive care unit; OGTT, oral glucose tolerance test; SBP, systolic blood pressure
Table 1.
Baseline characteristics of participants in GDM and control groups
| GDM (n = 314) | Control (n = 1832) | P | |
|---|---|---|---|
| Maternal age (years) | 32.10 ± 3.02 | 31.66 ± 3.01 | 0.018 |
| Height (m) | 1.63 ± 0.04 | 1.63 ± 0.05 | 0.106 |
| Weight (kg) | 60.42 ± 7.25 | 59.76 ± 7.11 | 0.128 |
| BMI (kg/m2) | 22.86 ± 3.07 | 22.46 ± 2.87 | 0.026 |
| Gravidaa | 1 | 1 | 0.504 |
| Parity | 0 | 0 | 0.734 |
| Family history of diabetes | 21 (7%) | 94 (5%) | 0.258 |
| Heart rate (beats/minute) | 74.26 ± 8.04 | 73.36 ± 9.11 | 0.100 |
| SBP (mmHg) | 115.71 ± 5.71 | 115.20 ± 6.33 | 0.184 |
| DBP (mmHg) | 73.05 ± 6.00 | 72.87 ± 5.45 | 0.585 |
| Triglyceride | |||
| mg/dL | 233.68 ± 61.59 | 224.81 ± 61.61 | 0.018 |
| mmol/L | 2.65 ± 0.70 | 2.55 ± 0.70 | 0.018 |
| Total cholesterol | |||
| mg/dL | 240.92 ± 29.22 | 236.45 ± 30.19 | 0.015 |
| mmol/L | 6.26 ± 0.76 | 6.15 ± 0.78 | 0.015 |
| HDL-C | |||
| mg/dL | 68.10 ± 9.01 | 69.31 ± 6.99 | 0.007 |
| mmol/L | 1.76 ± 0.23 | 1.79 ± 0.18 | 0.007 |
| LDL-C | |||
| mg/dL | 129.37 ± 22.12 | 130.94 ± 21.82 | 0.239 |
| mmol/L | 3.36 ± 0.58 | 3.40 ± 0.57 | 0.239 |
| EAT thickness (mm) | 6.57 ± 0.79 | 5.49 ± 1.05 | < 0.001 |
BMI Body mass index, DBP Diastolic blood pressure, EAT Epicardial adipose tissue, GDM Gestational diabetes mellitus, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, SBP Systolic blood pressure. All the parameters (except for gravida, parity, and family history) were expressed as the mean ± standard deviation. Gravida and parity were expressed as the median
aGravida describes the total number of confirmed pregnancies that a woman has had, regardless of the outcome
Regression analysis for the presence of GDM
The results of all regression analyses are summarized in Table 2. Univariate regression analysis revealed that maternal age (OR = 1.05, 95% CI: 1.01–1.09), BMI (OR = 1.05, 95% CI: 1.01–1.09), triglyceride (OR = 1.23, 95% CI: 1.04–1.46), total cholesterol (OR = 1.21, 95% CI: 1.04–1.41), HDL-C (OR = 0.42, 95% CI: 0.22–0.79), and EAT thickness (OR = 2.92, 95% CI: 2.54–3.36) were significantly associated with the presence of GDM. Multivariate regression analysis further revealed that EAT thickness (OR = 2.87, 95% CI: 2.49–3.31) were significantly associated with the presence of GDM (P < 0.001).
Table 2.
Results of univariate and multivariate regression analysis for the presence of GDM
| Univariate regression | Multivariate regression | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Maternal age (years) | 1.05 (1.01–1.09) | 0.018 | 1.03 (0.98–1.07) | 0.223 |
| BMI (kg/m2) | 1.05 (1.01–1.09) | 0.026 | 1.03 (0.98–1.08) | 0.230 |
| Triglyceride (mg/dL) | 1.23 (1.04–1.46) | 0.018 | 1.16 (0.96–1.40) | 0.130 |
| Total cholesterol (mg/dL) | 1.21 (1.04–1.41) | 0.015 | 1.06 (0.90–1.26) | 0.483 |
| HDL-C (mg/dL) | 0.42 (0.22–0.79) | 0.007 | 0.42 (0.21–0.83) | 0.011 |
| EAT thickness (mm) | 2.92 (2.54–3.36) | < 0.001 | 2.87 (2.49–3.31) | < 0.001 |
BMI Body mass index, CI Confidence interval, EAT Epicardial adipose tissue, GDM Gestational diabetes mellitus, HDL-C High-density lipoprotein cholesterol, OR Odds ratio
ROC analysis
ROC analysis was performed to verify whether EAT thickness could predict GDM. The ROC curve is shown in Fig. 2. The area under the curve was 0.790 (95% CI: 0.768–0.812). When the cutoff value was set to 5.49 mm, the sensitivity was 95.2% and the specificity was 50.5%.
Fig. 2.
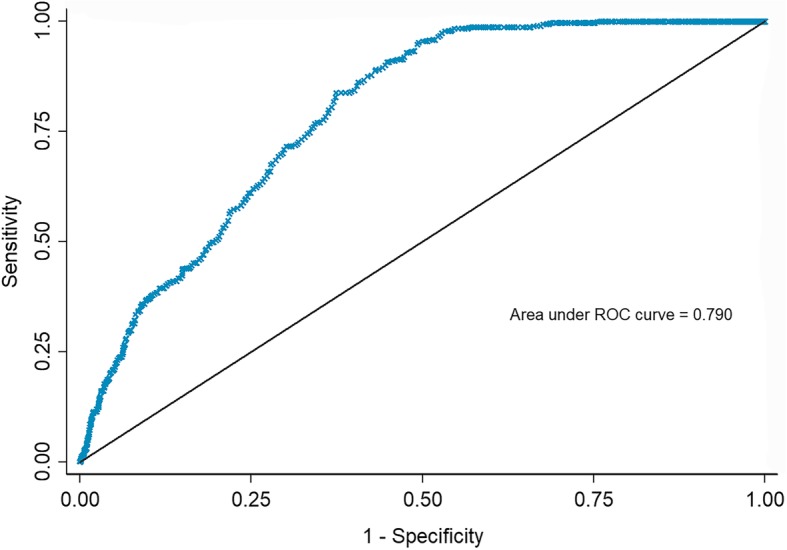
Receiver operating characteristic (ROC) curves for EAT thickness for the prediction of GDM. EAT, epicardial adipose tissue; GDM, gestational diabetes mellitus
The association of EAT thickness with adverse outcomes in the GDM group
Table 3 shows that the EAT was significantly higher in patients with adverse outcomes when compared with the patients without adverse outcomes in the GDM group (P < 0.001). Regression analysis further revealed that EAT thickness was a significant risk factor for large size for gestational age (OR = 3.47, 95% CI: 2.29–5.26), neonatal hypoglycemia (OR = 3.10, 95% CI: 1.78–5.37), admission to NICU (OR = 4.38, 95% CI: 2.81–6.84), preterm delivery (OR = 4.67, 95% CI: 2.84–7.68), and hyperbilirubinemia (OR = 4.04, 95% CI: 2.24–7.28) (all P < 0.001). Overall, EAT thickness was a significant risk factor for adverse outcomes in GDM patients (OR = 8.28, 95% CI: 5.10–13.43, P < 0.001).
Table 3.
Association of EAT thickness with adverse outcomes in the GDM group (n = 314)
| Cases with adverse outcomes | With adverse outcomes | Without adverse outcomes | P* | OR (95%CI) | P# | |
|---|---|---|---|---|---|---|
| Total | 91 (29%) | 7.29 ± 0.66 | 6.27 ± 0.64 | < 0.001 | 8.28 (5.10–13.43) | < 0.001 |
| Large size for gestational age | 54 (17%) | 7.19 ± 0.80 | 6.43 ± 0.73 | < 0.001 | 3.47 (2.29–5.26) | < 0.001 |
| Neonatal hypoglycemia | 23 (7%) | 7.25 ± 0.73 | 6.51 ± 0.77 | < 0.001 | 3.10 (1.78–5.37) | < 0.001 |
| Admission to NICU | 54 (17%) | 7.28 ± 0.65 | 6.41 ± 0.74 | < 0.001 | 4.38 (2.81–6.84) | < 0.001 |
| Preterm delivery | 41 (13%) | 7.36 ± 0.53 | 6.44 ± 0.76 | < 0.001 | 4.67 (2.84–7.68) | < 0.001 |
| Hyperbilirubinemia | 23 (7%) | 7.39 ± 0.64 | 6.50 ± 0.77 | < 0.001 | 4.04 (2.24–7.28) | < 0.001 |
CI Confidence interval, EAT Epicardial adipose tissue, GDM Gestational diabetes mellitus, NICU Neonatal intensive care unit, OR odds ratio
P*: P-value of independent-samples t-test
P#: P-value of univariate regression between epicardial adipose tissue thickness and adverse outcomes using generalized linear models
Discussion
Possessing the ability to predict GDM as early as possible is a very important goal and has been pursued by researchers over many years as this could allow for early lifestyle changes and/or nutritional interventions to prevent GDM. Unfortunately, pregnancy is a complex and dynamic process, involving profound changes in energy and nutrient metabolism to sustain fetal development and growth, and to meet the requirements of labor and lactation. To date, although many risk factors for GDM have been identified (e.g., maternal age, obesity, and lipid profiles), no validated tool exists to predict the risk of GDM.
Clinicians usually use BMI, VAT, and subcutaneous adipose tissue (SAT), to assess obesity when trying to predict GDM. However, SAT is not a good predictor for GDM during the first trimester [17, 18]; neither is BMI [4, 5, 19]. Over recent years, several studies have revealed that VAT could be a good predictor for GDM in the first and second trimesters [18, 19]. It remains unclear whether EAT, as a special type of VAT, exerts a similar performance when screening for GDM.
EAT has a range of functions, including lipogenic capacity [20]. A number of researchers have focused on the adverse effects of EAT and confirmed this parameter as a marker of diabetic risk [8]. A previous study also revealed that EAT thickness was significantly higher in women with a history of GDM than controls [21]. Subsequently, two cross-sectional studies found a difference in EAT thickness when comparing between GDM and control groups during the second trimester [22, 23]. In these two previous studies, EAT thickness was measured at 24–28 gestational weeks (GW) at the time of GDM diagnosis. However, this is not the appropriate time for clinicians to make early interventions to prevent GDM. Therefore, we designed this study to identify whether EAT can act as a potential predictor during the early second trimester.
EAT thickness can be measured by echocardiography during pregnancy. This measurement has been proven to be both accurate and reproducible [12]. Abnormal levels of blood sugar in GDM patients usually appear after 20 GW [24]. Hence, we measured EAT thickness at 16 weeks + 0 days to 19 weeks + 6 days, a time point at which hyperglycemia is generally not present. This measurement was therefore taken 1 to 2 months prior to diagnosis, and would therefore be highly beneficial in that it could identify patients who have a risk of GDM and provide clinicians with sufficient time to develop appropriate treatment strategies.
Our analysis revealed that EAT thickness was significantly increased in the GDM group compared to the control group when measured between 16 weeks + 0 days and 19 weeks + 6 days. Furthermore, higher EAT thickness was significantly associated with adverse outcomes in GDM patients. We propose that there are two mechanisms that might be responsible for the increased EAT thickness in GDM patients. Firstly, the higher levels of retinol-binding protein 4 and lower levels of adiponectin secreted by adipose tissue, including EAT, prior to 16 GW [25, 26], could cause insulin resistance (IR), the main mechanisms underlying GDM [27]. Secondly, during the first-second trimester, EAT releases higher levels of pro-inflammatory adipokines (RBP4, hs-CRP, fatty acid-binding protein-4, leptin, and visfatin), and lower levels of anti-inflammatory adipokines (omentin-1 and adiponectin); these may participate in the chronic low-grade state of inflammation that has previously been confirmed to be associated with GDM [26, 28].
Pregnancy can be viewed as a cardiovascular stress test in that the development of certain complications has the potential to reveal a woman’s susceptibility for future vascular or metabolic disease [29]. A previous study showed that GDM patients who had an increased EAT thickness during pregnancy were associated with subclinical atherosclerosis 6 years postpartum [21, 30]. The association between EAT and postpartum cardiovascular diseases therefore needs to be addressed further in future research. We aim to set up a new study, with a longer follow-up duration, to determine whether patients at a high-risk of GDM, as identified by EAT, are associated with T2DM and cardiovascular diseases. This type of study will allow us to implement preventive measures for this particular population.
Limitations
There are some limitations to our study that need to be considered. For example, all of our participants were Asian women. It is possible that race may be an important consideration when analyzing the relationship between EAT and the risk of disease [31]. Furthermore, we did not analyze data relating to inflammatory biomarkers; such data may be beneficial with regards to the efficacy of the EAT thickness model during the early second trimester.
Conclusion
Collectively, our findings indicate that echocardiographic EAT thickness is positively and significantly associated with GDM risk and adverse outcomes related to GDM. Echocardiographic EAT has the potential to be a predictor for GDM prior to actual clinical diagnosis.
Acknowledgments
None.
Abbreviations
- BMI
Body mass index
- CIs
Confidence intervals
- GDM
Gestational diabetes mellitus
- GW
Gestational week
- HDL-C
High-density lipoprotein cholesterol
- IR
Insulin resistance
- LDL-C
Low-density lipoprotein cholesterol
- OR
Odds ratio
- T2DM
Type 2 diabetes mellitus
- VAT
Visceral adipose tissue.
Authors’ contributions
JL drafted the manuscript. GS participated in manuscript writing, data collection, and data analysis. TM participated in project development and manuscript writing. GZ participated in data collection and data analysis. All authors read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81871173). The funder had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Review Board of China Medical University (Shenyang, Liaoning, China). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jing Liu, Email: liujing9936@163.com.
Guang Song, Email: songg84@163.com.
Tao Meng, Email: mengtao201011@163.com.
Ge Zhao, Email: zhao_ge_cmu@163.com.
References
- 1.Bevier WC, Jovanovic-Peterson L, Peterson CM. Pancreatic disorders of pregnancy. Diagnosis, management, and outcome of gestational diabetes. Endocrinol Metab Clin N Am. 1995;24(1):103–138. doi: 10.1016/S0889-8529(18)30055-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 4.Kumru P, Arisoy R, Erdogdu E, Demirci O, Kavrut M, Ardic C, Aslaner N, Ozkoral A, Ertekin A. Prediction of gestational diabetes mellitus at first trimester in low-risk pregnancies. Taiwan J Obstet Gynecol. 2016;55(6):815–820. doi: 10.1016/j.tjog.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Correa PJ, Venegas P, Palmeiro Y, Albers D, Rice G, Roa J, Cortez J, Monckeberg M, Schepeler M, Osorio E, et al. First trimester prediction of gestational diabetes mellitus using plasma biomarkers: a case-control study. J Perinat Med. 2019;47(2):161–168. doi: 10.1515/jpm-2018-0120. [DOI] [PubMed] [Google Scholar]
- 6.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2(4):367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 7.Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111(1):73–78. doi: 10.1016/j.amjcard.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu B, Li Y, Jing X, Deng S, Yan Y, She Q. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18(1):3. doi: 10.1186/s12933-019-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16(4):887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Song G, Meng T, Zhao G, Guo S. Weight retention at six weeks postpartum and the risk of gestational diabetes mellitus in a second pregnancy. BMC Pregnancy Childbirth. 2019;19(1):272. doi: 10.1186/s12884-019-2423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry OA, Beischer NA. Long-term implications of gestational diabetes for the mother. Baillieres Clin Obstet Gynaecol. 1991;5(2):461–483. doi: 10.1016/S0950-3552(05)80107-5. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Weinert LS. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33(7):e97. doi: 10.2337/dc10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AF. Hypoglycaemia of the newborn: a review. Bull World Health Organ. 1997;75(3):261–290. [PMC free article] [PubMed] [Google Scholar]
- 15.Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin N Am. 2019;48(3):479–493. doi: 10.1016/j.ecl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Subcommittee on H Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 17.De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW, Ray JG. First-trimester maternal abdominal adiposity predicts Dysglycemia and gestational diabetes mellitus in Midpregnancy. Diabetes Care. 2016;39(1):61–64. doi: 10.2337/dc15-2027. [DOI] [PubMed] [Google Scholar]
- 18.Saif Elnasr I, Ammar H. Ultrasound markers for prediction of gestational diabetes mellitus in early pregnancy in Egyptian women: observational study. J Matern Fetal Neonatal Med. 2020;1–7. 10.1080/14767058.2019.1678132. [Epub ahead of print]. [DOI] [PubMed]
- 19.D’Ambrosi F, Rossi G, Soldavini CM, Di Maso M, Carbone IF, Cetera GE, Colosi E, Ferrazzi E. Ultrasound assessment of maternal adipose tissue during 1st trimester screening for aneuploidies and risk of developing gestational diabetes. Acta Obstet Gynecol Scand. 2020. 10.1111/aogs.13800. [Epub ahead of print]. [DOI] [PubMed]
- 20.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14(12):1013–1022. [PubMed] [Google Scholar]
- 21.Caliskan M, Caklili OT, Caliskan Z, Duran C, Ciftci FC, Avci E, Gullu H, Kulaksizoglu M, Koca H, Muderrisoglu H. Does gestational diabetes history increase epicardial fat and carotid intima media thickness? Echocardiography. 2014;31(10):1182–1187. doi: 10.1111/echo.12597. [DOI] [PubMed] [Google Scholar]
- 22.Nar G, Inci S, Aksan G, Unal OK, Nar R, Soylu K. The relationship between epicardial fat thickness and gestational diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):120. doi: 10.1186/1758-5996-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yavuz A, Akkurt MO, Yalcin S, Karakoc G, Varol E, Sezik M. Second trimester fetal and maternal epicardial fat thickness in gestational diabetic pregnancies. Horm Metab Res. 2016;48(9):595–600. doi: 10.1055/s-0042-111435. [DOI] [PubMed] [Google Scholar]
- 24.Boriboonhirunsarn D, Sunsaneevithayakul P, Nuchangrid M. Incidence of gestational diabetes mellitus diagnosed before 20 weeks of gestation. J Med Assoc Thai. 2004;87(9):1017–1021. [PubMed] [Google Scholar]
- 25.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, Miled A, Grissa A, Jerbi M, Tabka Z, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91(10):4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo-Almoros A, Hang T, Peiro C, Soriano-Guillen L, Egido J, Tunon J, Lorenzo O. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc Diabetol. 2019;18(1):140. doi: 10.1186/s12933-019-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667–1672. doi: 10.1016/0002-9378(91)90012-G. [DOI] [PubMed] [Google Scholar]
- 28.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol. 2003;15(6):465–471. doi: 10.1097/00001703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Li JW, He SY, Liu P, Luo L, Zhao L, Xiao YB. Association of gestational diabetes mellitus (GDM) with subclinical atherosclerosis: a systemic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:132. doi: 10.1186/1471-2261-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salami SS, Tucciarone M, Bess R, Kolluru A, Szpunar S, Rosman H, Cohen G. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn Dis. 2013;23(3):281–285. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.