Summary
Transmission electron microscopy (TEM) is the only imaging technique allowing the direct visualization of viruses, due to its nanometer‐scale resolution. Between the 1960s and 1990s, TEM contributed to the discovery of many types of viruses and served as a diagnostic tool for identifying viruses directly in biological samples, either in suspension or in sections of tissues or mammalian cells grown in vitro in contact with clinical samples. The diagnosis of viral infections improved considerably during the 1990s, with the advent of highly sensitive techniques, such as enzyme‐linked immunosorbent assay (ELISA) and PCR, rendering TEM obsolete for this purpose. However, the last 20 years have demonstrated the utility of this technique in particular situations, due to its “catch‐all” nature, making diagnosis possible through visualization of the virus, without the need of prior assumptions about the infectious agent sought. Thus, in several major outbreaks in which molecular techniques failed to identify the infectious agent, TEM provided the answer. TEM is also still occasionally used in routine diagnosis to characterize infections not diagnosed by molecular assays. It is also used to check the microbiological safety of biological products. Many biopharmaceuticals are produced in animal cells that might contain little‐known, difficult‐to‐detect viruses. In this context, the “catch‐all” properties of TEM make it possible to document the presence of viruses or virus‐like particles in these products.
Keywords: electron microscopy, viral diagnosis, viral safety, virus
Abbreviations
- ELISA
enzyme‐linked immunosorbent assay
- EM
electron microscopy
- EMEA
European Medicines Agency
- FDA
Food and Drug Administration
- FPERT
fluorescent product‐enhanced reverse transcription
- LCMV
lymphocytic choriomeningitis virus
- PCR
polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SFTS
severe fever with thrombocytopenia syndrome
- TEM
transmission electron microscopy
1. HISTORY
The first transmission electron microscope was developed in the early 1930s by Ernst Ruska with his PhD supervisor, Max Knoll.1, 2 This microscope had a much higher resolution than any of the light microscopes available at the time and promised to revolutionize many aspects of science, including cell biology and virology. Ernst Ruska was a physicist (1986 Nobel prize winner in physics), but his younger brother, Helmut Ruska, who had trained in medicine, rapidly recognized the potential of this microscope for investigating the nature of viruses.3 In the early 1940s, viruses were classified according to their hosts and the clinical symptoms they caused. Despite the lack of established methods of biological sample preparation for transmission electron microscopy (TEM) at this time, Helmut Ruska was able to characterize the morphology of several viruses and he developed a rough viral classification based on the size and shape of the viral particles.4 TEM was rapidly adopted for its first major use in clinical virology: the differential diagnosis of smallpox, caused by the variola virus from the poxvirus family, and chickenpox, caused by the varicella‐zoster virus of the herpes family, based on investigations of fluid samples from the vesicles on the patients' skin.5 The chickenpox virus appeared to be spherical and 140 to 150 nm in diameter, with a central body, a structure clearly different from that of the much larger, brick‐shaped smallpox virus.
1.1. Role of the TEM in the discovery of viruses and routine diagnosis until the 1990s
The introduction of negative staining, based on aqueous suspensions of biological particles deposited on carbon‐coated grids and stained with heavy metals salts (such as uranyl acetate or phosphotungstic acid), improved the observation of viral particles, paving the way for the widespread use of TEM in basic virology and for the rapid diagnosis of viral infection6 (Figure 1A‐D). Negative staining clearly distinguishes the viral particle from the background and provides precise morphological information (concerning symmetry and the presence or absence of an envelope, for example), facilitating the specific identification of viruses, or at least their classification into morphologically similar groups. The use of TEM for viral studies peaked in the 1970s and 1980s, when this technique contributed to the discovery of many clinically important viruses, such as adeno‐, entero‐, paramyxo‐, and reoviruses, all of which were isolated and observed after propagation in cell cultures in vitro. Differences in virus size and fine structure were used as criteria for a more precise classification.7 However, TEM initially failed to detect agents for other diseases, such as hepatitis and gastroenteritis, because the causal viruses could not be propagated in cell cultures in vitro. Nevertheless, the application of TEM to “dirty” clinical samples, such as plasma, urine and feces in the 1970s constituted a major breakthrough for studies of these viruses.8 The etiologic agents of hepatitis B9 and A10 were detected in plasma and stool samples, respectively. The BK virus, a polyomavirus, was identified for the first time in the urine of patients undergoing renal transplantation.11 Rotaviruses were also identified by TEM as the main cause of epidemic gastroenteritis in humans and animals.12, 13 However, many other viruses were also found to cause gastroenteritis. The first of these viruses was the Norwalk virus, identified during an outbreak of gastroenteritis in Norwalk, Ohio, USA.14, 15 Viruses with a similar morphology were subsequently discovered elsewhere and called “Norwalk‐like” viruses, to reflect the similarity of their appearance on TEM,16 before being officially renamed “noroviruses.”17 Other viruses from the adenovirus,18 astrovirus,19, 20 and calicivirus21 families were also identified in the stool samples of children with gastroenteritis. TEM was, thus, widely used on negatively stained samples for routine diagnosis, as a rapid, “catch‐all” method for distinguishing between the diverse viruses potentially implicated in human gastroenteritis, providing a diagnosis within 15 minutes of the arrival of the sample in the laboratory.
Figure 1.

Diagnosis of viral infections by transmission electron microscopy (TEM). Panels A to C show negative‐staining TEM images of viruses present in human biological samples examined for routine diagnosis. Panels A and B illustrate rapid differential morphological diagnosis comparing a herpesvirus (A) and a parapoxvirus (B) in fluid recovered from skin vesicles. Panels C and D illustrate rapid differential morphological diagnosis for a rotavirus (C) and an adenovirus (D) in feces. Panel E and F show ultrathin sections of human tissue or cells (with high magnification in insets): E, parapoxvirus (Orf virus) infection on a human skin biopsy specimen; F, polyomavirus (BK virus) infection in cells obtained from a urine sample from a transplant recipient. The scale bars correspond to 100 nm (in A,C,D), 200 nm (in B,E) or 500 nm (F)
Although more time‐consuming, due to the need to embed a sample in resin and cut ultrathin sections with an ultramicrotome, the TEM has also proved useful in medical virology, in searches for viruses in tissues. Panels E and F in Figure 1 illustrate a parapoxvirus (Orf virus) visualized in a skin biopsy specimen from a patient with a severe finger ulcer22 and a polyomavirus (the BK virus) in cells collected from the urine of a renal transplant patient with nephropathy,23 respectively.
1.2. Declining the use of TEM for routine viral diagnosis since the 1990s
Major changes in the diagnosis of viral infections occurred in the 1990s, with the advent of more sensitive molecular techniques, such as enzyme‐linked immunosorbent assays (ELISAs) and polymerase chain reaction (PCR) in particular. Molecular techniques, with their advantages of greater sensitivity and the capacity to process large numbers of samples easily, replaced TEM in many areas of virological diagnosis. This was the case, in particular, for the diagnosis of viral gastroenteritis, for which molecular techniques capable of identifying most of the virus families involved in human gastroenteritis have been established.24, 25, 26, 27 A similar shift in practice occurred in veterinary medicine, with ELISAs and PCR progressively replacing TEM for the routine diagnosis of viral infections.28, 29, 30, 31 In human medicine, the use of TEM to differentiate between smallpox virus and the other viruses present in the fluids of cutaneous vesicles is no longer required, since the successful eradication of the variola virus in 1980 thanks to a worldwide vaccination program.32 It has been argued that TEM remains potentially useful for this application in a context of bioterrorism.33, 34 However, the risk of smallpox reappearing is extremely small, and even in the unlikely event of this happening, molecular techniques would undoubtedly outperform TEM for this diagnosis. Consequently, the number of laboratories making use of TEM for diagnostic purposes has decreased considerably.
2. CURRENT ROLE OF TEM IN THE DIAGNOSIS OF VIRAL INFECTION
TEM remains very useful for resolving certain diagnostic problems in medical virology, as clearly illustrated on several remarkable occasions over the last 20 years. In most of these cases, TEM was not used to characterize the virus directly in the patient sample, but after isolation of the virus from the clinical sample by propagation in a cell culture in vitro. During an outbreak of fatal respiratory disease in horses and influenza‐like illness in humans in Australia in 1995, TEM proved essential for the identification of a previously unknown virus, the Hendra virus, recognized as a member of the Paramyxoviridae family on the basis of its ultrastructure.35 A related virus, the Nipah virus, was also first identified by TEM on cerebrospinal fluid, during an outbreak of encephalitis in Malaysia and Singapore in 1999 in men who had been exposed to pigs.36, 37 The etiology of the severe acute respiratory syndrome (SARS) pandemic in Hong Kong and Southern China in 2003 was also initially determined by TEM. The causal virus was isolated in several laboratories around the world, by inoculating cell cultures with respiratory specimens, leading to the identification of a coronavirus on ultrathin TEM sections of these cells.38, 39 An outbreak of an unidentified rash in humans, associated with an illness in prairie dogs, occurred in the United States in 2003. TEM revealed the presence of a poxvirus in a cell culture isolate, and this virus was later identified as a monkeypox virus.40 The etiologic agent responsible for an acute severe fever with thrombocytopenia syndrome (SFTS) in six provinces of China in 2011 was diagnosed through the use of TEM to investigate cells cultured in the presence of blood samples from patients with the disease. TEM revealed the presence of virions with the characteristic morphological features of a bunyavirus.41 RNA sequence analysis revealed that this virus was, indeed, a new member of the genus Phlebovirus in the Bunyaviridae family.
TEM has also been successfully used to elucidate unexplained symptoms in small transmission clusters, and even in isolated cases. It was used, for example, in cases of graft dysfunction, fever, and altered mental status in transplant recipients in the United States in 2006. A virus from the Arenaviridae family was observed in ultrathin sections of Vero cells grown in the presence of a cerebrospinal fluid sample from one of these patients.42 PCR was then used to characterize the virus in more detail. It was identified as lymphocytic choriomeningitis virus (LCMV), an arenavirus transmitted by rodents. TEM was also used to identify West Nile virus in a skin specimen from a patient with an enigmatic hemorrhagic fever in the United States43 in 2006. The virus was identified by visualization in ultrathin sections of Vero cells grown in the presence of a homogenate of the skin biopsy specimen. Several cases of bunyavirus infection have also been diagnosed by TEM in patients bitten by mosquitoes or ticks in the United States and presenting with encephalitis44 or fever and fatigue syndrome.45 In Austria, in 2014, TEM identified picornaviruses in the urine samples of a group of neonates, all born at the same hospital and suffering from a sepsis‐like illness, after negative results were obtained from the initial microbiological tests.46 TEM also made possible the direct detection, in the brain tissues, of a polyomavirus (the JC virus) responsible for cases of fatal progressive multifocal leukoencephalopathy, in the absence of viral DNA in the cerebrospinal fluid47 (Figure 2). TEM is still occasionally useful in cases of human gastroenteritis, for the identification of new subtypes of causal viruses such as adenovirus,48 picornavirus,49 or calicivirus50 not recognized by molecular techniques.
Figure 2.
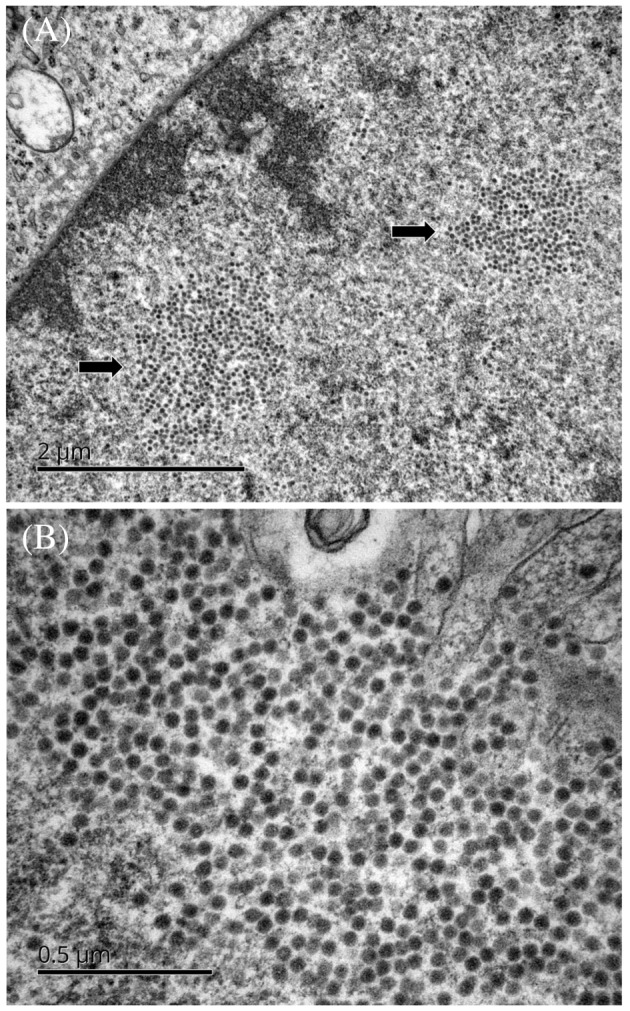
Detection by transmission electron microscopy (TEM) of a polyomavirus (JC virus) in ultrathin sections of a brain biopsy specimen from a patient with progressive multifocal leukoencephalopathy. A, Arrows indicate two areas of the cell nucleus containing numerous viral particles. B, Nuclear JC virus particles at high magnification. Scale bars represent 2 μm (A) or 0.5 μm (B)
Thus, in recent years, the role of TEM in the diagnosis of viral infections has shifted from routine use to an initial screening test for the identification of unknown infectious agents in particular outbreaks or viral transmission clusters. In such investigations, the underlying “catch‐all” principle of this technique is a major advantage for the recognition of an unknown agent, as viruses from various families have different morphological appearances, which are used as the basis of initial virus identification by TEM. This method allows an “open view,” sometimes revealing unexpected infectious agents, while molecular methods require previous knowledge of the virus to be tested. TEM has also the advantage of being able to potentially identify double or multiple infections caused by more than one virus, which could be missed by molecular or antigen tests. Moreover, the nature of the samples to be analyzed can be diverse, from body fluids or biopsies analyzed directly or after cell culture.
In some cases, TEM has also been used to confirm a diagnosis previously established with molecular techniques.51, 52, 53, 54 Figure 3 illustrates the detection of a reovirus strain in MRC5 cells cultured in the presence of urine and throat swab specimens from two children with unexplained neurologic symptoms of encephalitis. In this case, TEM confirmed the results of molecular techniques and contributed to the identification of a previously unknown reovirus strain as an etiologic agent of encephalitis.55
Figure 3.

Detection by transmission electron microscopy (TEM) of a reovirus in ultrathin sections of a cell line cultured in the presence of throat swab specimen from a child with an acute necrotizing encephalopathy. The white square in A corresponds to the area shown at high magnification in B. Scale bars represent 2 μm (A) or 200 nm (B)
In veterinary medicine, TEM has frequently proved useful for identification of the virus responsible for particular outbreaks of disease.56, 57, 58, 59, 60, 61, 62, 63, 64, 65 Immuno‐TEM with serum from convalescent domestic or wild animals has proved useful for the detection of unknown etiological agents, in situation in which alternative diagnostic methods are unsuccessful due to the lack of immunological reagents and primers.66
3. TEM IN VIRAL SAFETY
TEM is also considered an important method for the assessment of viral safety in biopharmaceutical products. TEM is recommended in the guidelines of the US Food and Drug Administration (FDA) and European Medicines Agency (EMEA), which also specify the materials to be tested, including cell lines, culture supernatants, and fermenter bulk harvests.67, 68 Although time‐consuming and of limited sensitivity, TEM is recommended, in particular, for its “catch‐all” properties, as a complementary approach to in vitro assays and molecular techniques. Current regulations for the use of animal‐derived components in biopharmaceutical products stipulate a number of source‐testing and manufacturing measures to be implemented, to minimize the potential risk of viral contamination. The in vitro assays for virus detection use a selection of cell lines with history of successful use for the detection of a wide range of potential virus contaminants.69 They can be used in the testing of culture medium in cell banks, and for the testing of raw materials, such as the bovine serum or other animal‐derived growth factors used in cell culture. However, infections can occur in cultured cells without a cytopathic effect, and such infections may be missed. Various other molecular assays can be used such as fluorescent product‐enhanced reverse transcription (FPERT),70 which is specific for retroviruses, or PCR‐based tests if specific risks have been identified during risk evaluation. By combining all these methods, it is possible to cover a broad spectrum of potential contaminants, although it is never possible to provide a 100% guarantee that no unwanted agents are present. In this context, TEM constitutes an additional check in the viral safety testing of biological products as it can document the possible presence of viruses or virus‐like particles in master cell banks or fermenter bulk harvests.
Rodent cell lines are widely used for the manufacture of recombinant proteins for pharmaceutical use in humans such as monoclonal antibodies, vaccines, and viral vectors for gene therapy. These cell lines have long been known to contain retroviral elements, because the rodent genome contains many copies of endogenous retrovirus‐like sequences.71 Most of the viral particles produced by these cells such as intracytoplasmic or intracisternal A‐type particles are defective and noninfectious (Figure 4). However, other particles such as C‐type particles bud at the cell surface and may infect nonrodent cells72 (Figure 5). Some murine retroviruses have been shown to be tumorigenic in primates,73 and cases of leukemia have been reported in children with severe combined immunodeficiency treated by gene therapy involving the use of murine retroviral vectors.74 All these elements demonstrate the relevance of tracking the presence of retroviruses in biological products derived from rodent cells. Reverse transcriptase assays performed on bulk harvests are often hampered by high background levels due to cell‐derived DNA polymerases.75 TEM may, therefore, help to document the presence of retrovirus‐like particles in these bulk harvests (Figure 6). TEM can also be used to gauge the concentration of viral particles to validate the clearance of retroviruses or any other virus suspected to be present in the master cell bank. Fortunately, the endogenous retroviruses present in the Chinese hamster ovary (CHO) cell line, the main rodent cell line used to produce biological products in the biotech industry, have been shown to be noninfectious.76 Testing requirements are now lower for this well‐characterized cell line than for other cell lines with which experience is more limited. Nevertheless, novel cell substrates, including insect cell lines in particular, are now being introduced into the biotech industry.77 Their use will carry new concerns about unknown viruses for which there is a potential risk of contamination, and TEM will undoubtedly be useful for documenting the presence of viruses or virus‐like particles in these cells and the products derived from them.
Figure 4.

Detection of intracellular retroviruses in rodent cells used for the production of biological products. The transmission electron microscopy (TEM) examination of ultrathin cell sections may reveal the presence of intracytoplasmic (A,B) or intracisternal (C,D) A‐type retroviral particles. Scale bars represent 200 nm in all panels
Figure 5.

Detection of retroviruses budding at the cell surface of rodent cells used for the production of biological products. These four ultrathin sections of cells examined by transmission electron microscopy (TEM) show C‐type retroviral particles budding at the plasma membrane and released into the extracellular medium. The viruses indicated by the arrows are shown at high magnification in the inset. Scale bars represent 200 nm (A,D) or 500 nm (B,C)
Figure 6.

Detection of retrovirus‐like particles by transmission electron microscopy (TEM) with negative staining in bulk harvests of rodent cells used for the production of biological products. Scale bars represent 200 nm in both panels
4. CONCLUDING REMARKS
In conclusion, although TEM is sometimes seen as a somewhat “old‐fashioned” technique, it still has an important role to play in virus detection. It is particularly useful for identifying unknown agents involved in particular outbreaks or transmission clusters. In routine diagnosis, it may be useful to confirm or even, in some cases, to guide the diagnosis of a viral infection. TEM can also be used to check the viral safety of biopharmaceutical products. This technique has several disadvantages, such as the cost of electron microscopes and their maintenance, the need for well‐trained microscopists, and time‐consuming analysis, particularly if the samples must be embedded in resin for the cutting of ultrathin sections. However, all the techniques available have benefits and disadvantages, and their complementary natures mean that there are advantages to be gained by using them in combination. In this respect, the principal advantage of TEM is its ability to provide an image of the virus, providing additional confidence in the result.
CONFLICT OF INTEREST
The authors have no competing interest.
ACKNOWLEDGEMENTS
We wish to thank Fabienne Arcanger, Sonia Georgeault, Christine Hayot, and Juliette Rousseau for their invaluable help with TEM techniques. The TEM photographs illustrating our manuscript are original images that were produced in our laboratory. The Electron Microscopy Laboratory of the University of Tours and the Tours University Hospital is part of the French IBiSA network.
Roingeard P, Raynal P‐I, Eymieux S, Blanchard E. Virus detection by transmission electron microscopy: Still useful for diagnosis and a plus for biosafety. Rev Med Virol. 2019;29:e2019 10.1002/rmv.2019
REFERENCES
- 1. Knoll M, Ruska E. Das elektronenmikroscop. Zeitschrift Fur Physik. 1932;78(5‐6):318‐339. [Google Scholar]
- 2. Ruska E. Nobel lecture: the development of the electron microscope and of electron microscopy. Biosci Rep. 1987;7(8):607‐629. [DOI] [PubMed] [Google Scholar]
- 3. Ruska H, von Borries B, Ruska E. Die bedeutung der ubermikroskopie fur die virusforschung. Arch Gesamte Virusforsch. 1939;1(1):155‐159. [Google Scholar]
- 4. Ruska H. Versuch zu einer ordnung der virusarten. Arch Gesamte Virusforsch. 1943;2(5):480‐498. [Google Scholar]
- 5. Nagler FP, Rake G. The use of the electron microscope in diagnosis of variola, vaccinia, and varicella. J Bacteriol. 1948;55(1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenner S, Horne RW. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959;34:103‐110. [DOI] [PubMed] [Google Scholar]
- 7. Tyrrell DA, Almeida JD. Direct electron‐microscopy of organ culture for the detection and characterization of viruses. Arch Gesamte Virusforsch. 1967;22(3):417‐425. [DOI] [PubMed] [Google Scholar]
- 8. Madeley CR. Viruses in the stools. J Clin Pathol. 1979;32(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dane DS, Cameron CH, Briggs M. Virus‐like particles in serum of patients with Australia antigen‐associated hepatitis. Lancet. 1970;1(7649):695‐698. [DOI] [PubMed] [Google Scholar]
- 10. Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: detection by immune electron microscopy of a virus‐like antigen associated with acute illness. Science. 1973;182(4116):1026‐1028. [DOI] [PubMed] [Google Scholar]
- 11. Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253‐1257. [DOI] [PubMed] [Google Scholar]
- 12. Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non‐bacterial gastroenteritis. Lancet. 1973;2(7841):1281‐1283. [DOI] [PubMed] [Google Scholar]
- 13. Flewett TH, Bryden AS, Davies H. Virus particles in gastroenteritis. Lancet. 1973;2(7844):1497. [DOI] [PubMed] [Google Scholar]
- 14. Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27‐nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10(5):1075‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapikian AZ. The discovery of the 27‐nm Norwalk virus: an historic perspective. J Infect Dis. 2000;181(suppl 2):S295‐S302. 10.1086/315584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caul EO, Appleton H. The electron microscopical and physical characteristics of small round human fecal viruses: an interim scheme for classification. J Med Virol. 1982;9(4):257‐265. [DOI] [PubMed] [Google Scholar]
- 17. Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147(8):1655‐1663. 10.1007/s007050200039 [DOI] [PubMed] [Google Scholar]
- 18. Morris CA, Flewett TH, Bryden AS, Davies H. Epidemic viral enteritis in a long‐stay children's ward. Lancet. 1975;1(7897):4‐5. [DOI] [PubMed] [Google Scholar]
- 19. Appleton H, Higgins PG. Viruses and gastroenteritis in infants. Lancet. 1975;1(7919):1297. [DOI] [PubMed] [Google Scholar]
- 20. Madeley CR, Cosgrove BP. 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;2(7932):451‐452. [DOI] [PubMed] [Google Scholar]
- 21. Madeley CR, Cosgrove BP. Caliciviruses in man. Lancet. 1976;1(7952):199‐200. [DOI] [PubMed] [Google Scholar]
- 22. Roingeard P, Machet L. Orf skin ulcer. New Engl J Med. 1997;337(16):1131 10.1056/NEJM199710163371605 [DOI] [PubMed] [Google Scholar]
- 23. Roingeard P, Al‐Najjar A, Buchler M, Goudeau A, Lebranchu Y. BK virus infection in a renal transplant recipient. Virologie. 1999;3(5):418‐419. [Google Scholar]
- 24. Medici MC, Martinelli M, Ruggeri FM, et al. Broadly reactive nested reverse transcription‐PCR using an internal RNA standard control for detection of noroviruses in stool samples. J Clin Microbiol. 2005;43(8):3772‐3778. 10.1128/JCM.43.8.3772-3778.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logan C, O'Leary JJ, O'Sullivan N. Real‐time reverse transcription‐PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J Clin Microbiol. 2006;44(9):3189‐3195. 10.1128/JCM.00915-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oka T, Katayama K, Hansman GS, et al. Detection of human sapovirus by real‐time reverse transcription‐polymerase chain reaction. J Med Virol. 2006;78(10):1347‐1353. 10.1002/jmv.20699 [DOI] [PubMed] [Google Scholar]
- 27. Logan C, O'leary JJ, O'sullivan N. Real‐time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J Virol Methods. 2007;146(1‐2):36‐44. 10.1016/j.jviromet.2007.05.031 [DOI] [PubMed] [Google Scholar]
- 28. Tang Y, Wang Q, Saif M. Development of a ssRNA internal control template reagent for a multiplex RT‐PCR to detect turkey astroviruses. J Virol Methods. 2005;126(1‐2):81‐86. 10.1016/j.jviromet.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 29. Rodak L, Valicek L, Smid B, Nevorankova Z. An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. Vet Microbiol. 2005;105(1):9‐17. 10.1016/j.vetmic.2004.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Poel WH, van der Heide R, Verschoor F, Gelderblom H, Vinje J, Koopmans MP. Epidemiology of Norwalk‐like virus infections in cattle in The Netherlands. Vet Microbiol. 2003;92(4):297‐309. 10.1016/S0378-1135(02)00421-2 [DOI] [PubMed] [Google Scholar]
- 31. Guo M, Evermann JF, Saif LJ. Detection and molecular characterization of cultivable caliciviruses from clinically normal mink and enteric caliciviruses associated with diarrhea in mink. Arch Virol. 2001;146(3):479‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Henderson DA. Countering the posteradication threat of smallpox and polio. Clin Infect Dis. 2002;34(1):79‐83. 10.1086/323897 [DOI] [PubMed] [Google Scholar]
- 33. Miller SE. Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: dos and don'ts. Ultrastruct Pathol. 2003;27(3):133‐140. [DOI] [PubMed] [Google Scholar]
- 34. Curry A, Appleton H, Dowsett B. Application of transmission electron microscopy to the clinical study of viral and bacterial infections: present and future. Micron. 2006;37(2):91‐106. 10.1016/j.micron.2005.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murray K, Selleck P, Hooper P, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268(5207):94‐97. 10.1126/science.7701348 [DOI] [PubMed] [Google Scholar]
- 36. Chua KB, Goh KJ, Wong KT, et al. Fatal encephalitis due to Nipah virus among pig‐farmers in Malaysia. Lancet. 1999;354(9186):1257‐1259. 10.1016/S0140-6736(99)04299-3 [DOI] [PubMed] [Google Scholar]
- 37. Chua KB, Wong EM, Cropp BC, Hyatt AD. Role of electron microscopy in Nipah virus outbreak investigation and control. Med J Malaysia. 2007;62(2):139‐142. [PubMed] [Google Scholar]
- 38. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953‐1966. 10.1056/NEJMoa030781 [DOI] [PubMed] [Google Scholar]
- 39. Goldsmith CS, Tatti KM, Ksiazek TG, et al. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10(2):320‐326. 10.3201/eid1002.030913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350(4):342‐350. 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 41. Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523‐1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354(21):2235‐1549. 10.1056/NEJMoa053240 [DOI] [PubMed] [Google Scholar]
- 43. Paddock CD, Nicholson WL, Bhatnagar J, et al. Fatal hemorrhagic fever caused by West Nile virus in the United States. Clin Infect Dis. 2006;42(11):1527‐1535. 10.1086/503841 [DOI] [PubMed] [Google Scholar]
- 44. Sexton DJ, Rollin PE, Breitschwerdt EB, et al. Life‐threatening Cache Valley virus infection. N Engl J Med. 1997;336(8):547‐549. 10.1056/NEJM199702203360804 [DOI] [PubMed] [Google Scholar]
- 45. McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367(9):834‐841. 10.1056/NEJMoa1203378 [DOI] [PubMed] [Google Scholar]
- 46. Strenger V, Diedrich S, Boettcher S, et al. Nosocomial outbreak of parechovirus 3 infection among newborns, Austria, 2014. Emerg Infect Dis. 2016;22(9):1631‐1634. 10.3201/eid2209.151497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Broutin L, Rousselot‐Denis C, Dartigeas C, et al. Diagnosis of progressive multifocal leukoencephalopathy in the absence of DNA from the JC virus in the cerebrospinal fluid. Virologie. 2006;20(5):287‐292. 10.1684/vir.2016.0669 [DOI] [PubMed] [Google Scholar]
- 48. Jones MS, Harrach B, Ganac RD, et al. New adenovirus species found in a patient presenting with gastroenteritis. J Virol. 2007;81(11):5978‐5984. 10.1128/JVI.02650-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45(7):2144‐2150. 10.1128/JCM.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hallström B, Lagerqvist N, Lind‐Karlberg M, et al. Complete genome sequence of a Sapporo virus GV.2 variant from a 2016 outbreak of gastroenteritis in Sweden. Genome Announc. 2017;5(5). pii: e01446‐16. 10.1128/genomeA.01446-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chung WH, Shih SR, Chang CF, et al. Clinicopathologic analysis of coxsackievirus a6 new variant induced widespread mucocutaneous bullous reactions mimicking severe cutaneous adverse reactions. J Infect Dis. 2013;208(12):1968‐1978. 10.1093/infdis/jit383 [DOI] [PubMed] [Google Scholar]
- 52. Lakis NS, Li Y, Abraham JL, et al. Novel poxvirus infection in an immune suppressed patient. Clin Infect Dis. 2015;61(10):1543‐1548. 10.1093/cid/civ643 [DOI] [PubMed] [Google Scholar]
- 53. Osadebe LU, Manthiram K, McCollum AM, et al. Novel poxvirus infection in 2 patients from the United States. Clin Infect Dis. 2015;60(2):195‐202. 10.1093/cid/ciu790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Springer YP, Hsu CH, Werle ZR, et al. Novel orthopoxvirus infection in an Alaska resident. Clin Infect Dis. 2017;64(12):1737‐1741. 10.1093/cid/cix219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ouattara LA, Barin F, Barthez MA, et al. Novel human reovirus isolated from children with acute necrotizing encephalopathy. Emerg Infect Dis. 2011;17(8):1436‐1444. 10.3201/eid1708.101528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prukner‐Radovcic E, Luschow D, Ciglar‐Grozdanic I, et al. Isolation and molecular biological investigations of avian poxviruses from chickens, a turkey, and a pigeon in Croatia. Avian Dis. 2006;50(3):440‐444. 10.1637/7506-012006R.1 [DOI] [PubMed] [Google Scholar]
- 57. Coyne KP, Jones BR, Kipar A, et al. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet Rec. 2006;158(16):544‐550. 10.1136/vr.158.16.544. [DOI] [PubMed] [Google Scholar]
- 58. Literak I, Smid B, Dubska L, Bryndza L, Valicek L. An outbreak of the polyomavirus infection in budgerigars and cockatiels in Slovakia, including a genome analysis of an avian polyomavirus isolate. Avian Dis. 2006;50(1):120‐123. 10.1637/7395-061605R.1 [DOI] [PubMed] [Google Scholar]
- 59. Matz‐Rensing K, Ellerbrok H, Ehlers B, et al. Fatal poxvirus outbreak in a colony of New World monkeys. Vet Pathol. 2006;43(2):212‐218. 10.1354/vp.43-2-212 [DOI] [PubMed] [Google Scholar]
- 60. Chan KW, Lin JW, Lee SH, et al. Identification and phylogenetic analysis of orf virus from goats in Taiwan. Virus Genes. 2007;35(3):705‐712. 10.1007/s11262-007-0144-6. [DOI] [PubMed] [Google Scholar]
- 61. Maeda Y, Shibahara T, Wada Y, et al. An outbreak of teat papillomatosis in cattle caused by bovine papilloma virus (BPV) type 6 and unclassified BPVs. Vet Microbiol. 2007;121(3‐4):242‐248. 10.1016/j.vetmic.2006.12.015 [DOI] [PubMed] [Google Scholar]
- 62. Gruber A, Grabensteiner E, Kolodziejek J, Nowotny N, Loupal G. Poxvirus infection in a great tit (Parus major). Avian Dis. 2007;51(2):623‐625. 10.1637/0005-2086(2007)51[623:PIIAGT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 63. Hess C, Maegdefrau‐Pollan B, Bilic I, et al. Outbreak of cutaneous form of poxvirus on a commercial turkey farm caused by the species fowlpox. Avian Dis. 2011;55(4):714‐718. 10.1637/9771-050511-Case.1 [DOI] [PubMed] [Google Scholar]
- 64. Cargnelutti JF, Flores MM, Teixeira FR, Weiblen R, Flores EF. An outbreak of pseudocowpox in fattening calves in southern Brazil. J Vet Diagn Invest. 2012;24(2):437‐441. 10.1177/1040638711435408 [DOI] [PubMed] [Google Scholar]
- 65. Cardeti G, Gruber CEM, Eleni C, et al. Fatal outbreak in Tonkean macaques caused by possibly novel orthopoxvirus, Italy, January 2015. Emerg Infect Dis. 2017;23(12):1941‐1949. 10.3201/eid2312.162098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lavazza A, Tittarelli C, Cerioli M. The use of convalescent sera in immune‐electron microscopy to detect non‐suspected/new viral agents. Viruses. 2015;7(5):2683‐2703. 10.3390/v7052683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Food and Drug Administration . Guidance for industry: A viral safety evaluation of biotechnology products derived from cell lines of human or animal origin; 1998. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073454.pdf
- 68. European Medicines Agency . Guideline on virus safety evaluation of biotechnological investigational medicinal products; 2008. https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003795.pdf.
- 69. Minor P. Adventitious agent issues. Dev Biol (Basel). 2001;106:409‐414. discussion 414‐6, 465‐75 [PubMed] [Google Scholar]
- 70. Arnold BA, Hepler RW, Keller PM. One‐step fluorescent probe product‐enhanced reverse transcriptase assay. Biotechniques. 1998;25(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 71. Weiss RA. Retroviruses produced by hybridomas. N Engl J Med. 1982;307(25):1587 10.1056/NEJM198212163072522 [DOI] [PubMed] [Google Scholar]
- 72. Lueders KK. Genomic organization and expression of endogenous retrovirus‐like elements in cultured rodent cells. Biologicals. 1991;1:1‐7. [DOI] [PubMed] [Google Scholar]
- 73. Donahue RE, Kessler SW, Bodine D, et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176(4):1125‐1135. 10.1084/jem.176.4.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hacein‐Bey‐Abina S, von Kalle C, Schmidt M, et al. LMO2‐associated clonal T cell proliferation in two patients after gene therapy for SCID‐X1. Science. 2003;302(5644):415‐419. 10.1126/science.1088547 [DOI] [PubMed] [Google Scholar]
- 75. Brorson K, Xu Y, Swann PG, et al. Evaluation of a quantitative product‐enhanced reverse transcriptase assay to monitor retrovirus in mAb cell‐culture. Biologicals. 2002;30(1):15‐26. 10.1006/biol.2001.0290 [DOI] [PubMed] [Google Scholar]
- 76. Shepherd AJ, Wilson NJ, Smith KT. Characterisation of endogenous retrovirus in rodent cell lines used for production of biologicals. Biologicals. 2003;31(4):251‐260. 10.1016/S1045-1056(03)00065-4 [DOI] [PubMed] [Google Scholar]
- 77. Aranha H. Current issues in assuring virological safety of biopharmaceuticals. Bioprocess International. 2012;10(3):12‐17. [Google Scholar]


