Summary
Acute respiratory tract infections are a major cause of morbidity and mortality and represent a significant burden on the health care system. Laboratory testing is required to definitively distinguish infecting influenza virus from other pathogens, resulting in prolonged emergency department (ED) visits and unnecessary antibiotic use. Recently available rapid point‐of‐care tests (POCT) may allow for appropriate use of antiviral and antibiotic treatments and decrease patient lengths of stay.
We undertook a systematic review to assess the effect of POCT for influenza on three outcomes: (1) antiviral prescription, (2) antibiotic prescription, and (3) patient length of stay in the ED.
The databases Medline and Embase were searched using MeSH terms and keywords for influenza, POCT, antivirals, antibiotics, and length of stay.
Amongst 245 studies screened, 30 were included. The majority of papers reporting on antiviral prescription found that a positive POCT result significantly increased use of antivirals for influenza compared with negative POCT results and standard supportive care. A positive POCT result also led to decreased antibiotic use. The results of studies assessing the effect of POCT on ED length of stay were not definitive.
The studies assessed in this systematic review support the use of POCT for diagnosis of influenza in patients suffering an acute respiratory infection. Diagnosis using POCT may lead to more appropriate prescription of treatments for infectious agents. Further studies are needed to assess the effect of POCT on the length of stay in ED.
Keywords: influenza, point of care, systematic review
Abbreviations used
- ED
emergency department
- ILI
influenza‐like illness
- LOS
length of stay
- POCT
point‐of‐care test
- RCT
randomised controlled trial
- RIDT
rapid influenza diagnostic test
1. INTRODUCTION
Respiratory infections are the third most common cause of death globally.1 Many of these are precipitated by viral infections either directly through damage to the host (influenzavirus, severe acute respiratory syndrome, and Middle East respiratory syndrome coronaviruses2, 3, 4) or indirectly through adverse effects on patients with other conditions such as asthma, chronic obstructive pulmonary disease, or cystic fibrosis.5 The World Health Organisation have reported that annual influenza epidemics result in an estimated 3 to 5 million cases of severe illness, and approximately 250 000 to 500 000 deaths annually.6
The current reference standard laboratory test for influenza diagnosis is quantitative reverse transcription PCR. Viral culture7, 8 is now uncommon, as it is time consuming and expensive and requires specifically trained operators.9, 10, 11 New rapid diagnostic tests forinfluenza are available that show sensitivity and specificity comparable to real‐time PCR assays12, 13 (Figure 1).
Figure 1.
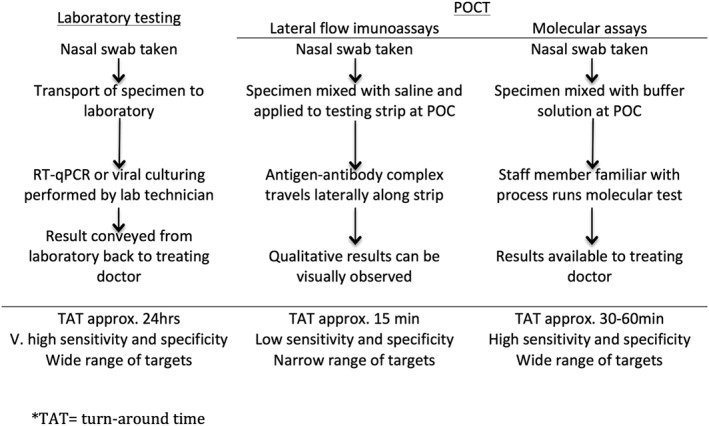
Generalised steps in different methods of diagnostic testing for influenza. POC, point of care; POCT, point of care tests; RT‐qPCR, quantitative reverse transcription PCR
We aimed to systematically review the literature to determine the effects of point‐of‐care testing for influenza on outcomes for patients, including (1) use of antivirals for influenza virus, (2) administration of antibiotics, and (3) length of stay (LOS) in the emergency department (ED) for patients presenting with symptoms of acute respiratory infection.
2. METHODS
2.1. Search strategy
The electronic databases Medline and Embase were independently searched from inception to December 31, 2017. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines.14 The references of the included studies and recent scientific review articles on influenza point‐of‐care test (POCT) diagnostics were manually searched for any additional relevant studies, and these were included. The database search strategy was created with a focus on terms for influenza AND point of care testing, and these were combined with terms for any of the targeted outcomes—antibiotic use OR antiviral use OR length of stay.
The specific search terms for influenza used for Embase included “exp influenza” [MeSh] OR “exp influenza virus” [MeSh]. By exploding these terms, we included all types of influenza as well as the different influenza subtypes. The search terms for point‐of‐care testing included “exp point of care testing” [MeSh] OR “exp rapid test” [MeSh] OR “influenza a rapid test” [MeSh] OR “ridt.” These terms contained subheadings that included brand names for the most commonly used commercial POCT for influenza.
For the antibiotic and antiviral component of the systematic review, the search terms used were “exp antivirus agent” [MeSh] OR “exp antiviral therapy” [MeSh] OR “exp antibiotic agent” [MeSh] OR “exp antibiotic therapy” [MeSh]. The search terms used for length of stay were “exp length of stay” [MeSh] or “los.”
The search terms for Medline for influenza were “exp Influenza, Human” [MeSh] OR “exp Orthomyoxviridae” [MeSh]. The search terms for point‐of‐care testing included “exp Point of Care Testing” [MeSh] OR “exp Point of Care Systems” [MeSh] OR “rapid test” OR “ridt” OR “radt” OR “influenza A rapid test.” The search terms for antivirals were “exp Antiviral Agents” [MeSh] OR “Oseltamivir” [MeSh] OR “antiviral ther*.” For antibiotics, we used the search terms “exp Anti‐Bacterial Agents” [MeSh] OR “antibiotic*” OR “antibacterial*.” The search terms used for length of stay were “exp Length of Stay” [MeSh] OR “LOS.”
Two reviewers (G.W. and S.S.B.) independently screened the title and abstracts of articles that were eligible using the above criteria. These were supplemented with additional papers that were searched manually using the references of the suitable articles. In cases where there was uncertainty about the eligibility and relevance of a particular article, a resolution was achieved through consensus between reviewers. We excluded scientific review articles, commentaries, Cochrane reviews, case studies, case series studies, analytical model studies, conference abstracts, and studies involving surveys. Studies published only in English were considered.
2.2. Study selection criteria
Studies were eligible for inclusion if they assessed the effect of POCT on the clinical management of the patient presenting to a physician in a hospital, acute medical centre, or house visit with influenza‐like illness (ILI). For this review, a POCT was defined as a commercially available diagnostic assay that detects influenza in respiratory samples. For a test to be considered as a POCT, it needed to be conducted immediately after specimen acquisition. Studies that did not specify when the test was performed were excluded, as they were presumed to not have been done at point of care. Tests that were not commercially available and that were used in routine influenza diagnosis such as enzyme‐linked immunosorbent assay serology were not included in this definition. Studies that used as reference standards viral cell culture, nucleic acid test, immunofluorescence, or diagnosis based on patient symptoms and signs were eligible.
For this systematic review, papers were excluded if the results of the POCT were withheld from the health care professional and not used for diagnosis. Attempts were made to contact authors if information or data were lacking in order to construct the tables.
2.3. Quality appraisal
The searches were performed by two investigators separately (G.W. and S.S.B.), with quality of the data assessed by two investigators according to Oxford Centre for Evidence‐based Medicine (OCEBM) guidelines.15 Papers included in this systematic review were eligible to be graded from levels 2 to 4 of the OCEBM Levels of Evidence. We considered a level 2–graded study as “excellent” quality, level 3 as “good,” and level 4 as “fair.”
3. RESULTS
3.1. Results of search
The database search yielded 245 publications after removal of duplicates (Figure 2). Titles and abstracts were screened, and 90 papers were assessed for further eligibility. Additional 10 studies were found through citation of relevant publications in the assessed papers. After full text assessment of study methods and outcomes, a total of 30 met the selection criteria and were included in this systematic review.
Figure 2.
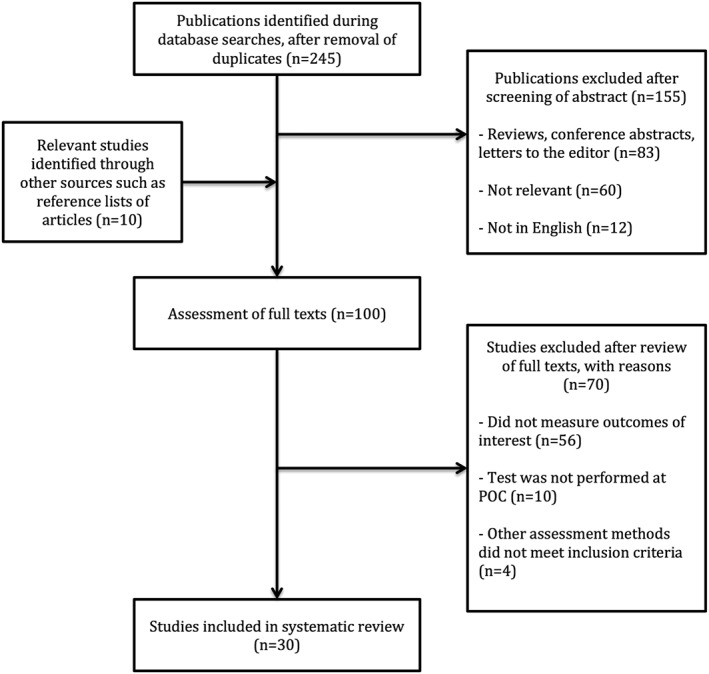
Literature search and selection process
Using the OCEBM Levels of Evidence Table,15 nine studies were graded level 2 or excellent quality, 12 were graded level 3 or good quality, and nine were graded level 4 or fair quality (Table S1, found in the Supporting Information). Results between studies of differing levels of evidence were examined qualitatively, and similar trends were observed between studies.
3.2. Description of studies
The included studies consisted of retrospective; prospective; and randomised, controlled trials. The POCT used is composed of both antigen‐based and molecular‐based techniques. Study methods included randomisation of patients to POCT or standard care, comparisons of preimplementation and postimplementation of POCTs, and analysis of positive and negative POCT results. The patient populations in this review included children (<18 years) and adults (≥18 years) from a wide range of study locations including Asia, Australia, Europe, and North America.
3.3. Antiviral prescription
There were 14 publications that measured the effect of POCT on antiviral prescription (Table 1). Of these, 12/14 (86%) reported increased antiviral use associated with a positive diagnosis by POCT.
Table 1.
Included studies assessing antiviral prescriptiona
| Authors, Year, Location | Population, Study Period, Patient Groups | POCT | Results |
|---|---|---|---|
| Brendish et al, 2017,16 UK16, b | A RCT enrolling 720 adults (>18 years) with acute respiratory illness at a large UK hospital during Jan‐July 2015, and Oct 2015‐April 2016. Patients randomly assigned either POCT (n = 362) or routine care (n = 358). | FilmArray Respiratory Panel | Significant, POCT+ vs standard+: 52/57 (91%) vs 24/37 (65%), P = 0.0026 |
| Nitsch‐Osuch et al, 2017,17 Polandb | A study comparing treatment of hospitalised children in a paediatric ward during two consecutive influenza seasons: Jan‐Mar 2015, when no RIDT were in use (n = 52), and Jan‐march 2016, when RIDT were implemented into routine practice (n = 63). | bioNexia Influenza A+B | Significant, POCT+ vs standard+: 7/11 (64%) vs 0/15 (0%), P < 0.05 |
| Trabattoni et al, 2017,24 Franceb , c | A single centre prospective observational study comparing two diagnostic strategies over 2 months. During Feb 2016 standard laboratory testing was used for diagnosis of influenza (n = 169), and in Mar 2016, a POCT was performed (n = 132). | Alere i Influenza A&B | Not significant, POCT vs standard: 7/132 (5.3%) vs 4/169 (2.4%), P = 0.22 |
| Li‐Kim‐Moy et al, 2016,18 Australiab , c | A retrospective review of 364 lab‐confirmed influenza cases presenting at a paediatric ED during Jan‐Dec 2009. Children (<18 y) were diagnosed with influenza by either POCT (n = 236) or standard testing (n = 65). | QuikVue Influenza A+B | Significant, POCT+ vs standard+: 109/236 (46.2%) vs 14/65 (21.5%), P = 0.001 |
| Chu et al, 2015,30 USAb | A retrospective study of 350 adult (>18 y) patients at a teaching hospital and medical centre in USA over two influenza seasons, Feb–Mar 2012 and January 2013. The first season was prior to implementation of POCT (n = 175) and in the second season POCT was used systematically (n = 175). | Simplexa Flu A/B & RSV Kit | Significant, pre‐POCT vs post‐POCT: 79/175 (45%) vs 97/175 (55%), P = 0.05 |
| González‐Del Vecchio et al, 2015,39 Spainb | Cohort of 217 children and adults influenza positive from a general teaching hospital in Spain over one influenza season, Jan–Mar 2014. Patients were divided into: A‐POCT negative, reference positive (n = 132) or B‐POCT positive (n = 85) | Xpect Flu A&B | Significant, A vs B: 89/132 (67.4%) vs 70/85 (82.3%), P = 0.02 |
| Blaschke et al, 2014,19 USAb , d | A retrospective study using data from the National Hospital Ambulatory Medical Care Survey over three influenza periods: Jan‐Apr 2007, Oct‐Dec 2008, and Jan‐Apr 2009. 1166 of an estimated 4.9 M eligible ED visits were sampled and examined in three groups: POCT+, POCT−, and Influenza (+) by standard test. | Not specified | Significant, POCT+ vs standard+: 56% vs 19%, P = 0.002Significant, POCT+ vs POCT−: 56% vs 2%, P < 0.0001 |
| Lim et al, 2014,23 Koreab | A retrospective review of medical records of 770 children (<15 y) hospitalised with laboratory confirmed influenza between Feb 2004 and June 2007. Different treatment groups were analysed and included patients receiving; oseltamivir only (n = 27), antibiotics‐only (n = 620), antibiotics/oseltamivir combination (n = 67), or standard supportive care (n = 56). | Directigen EZ Flu A+B Test Kit | Significant, oseltamivir only vs antibiotics only, antibiotics/oseltamivir combination, standard supportive care: likelihood of diagnosis by POCT− 22/27 (81.5%) vs 21/620 (3.4%) P < 0.001, 37/67 (55.2%) P = 0.017, 9/56 (16.1%) P < 0.001 |
| Suryaprasad et al, 2014,21 USA | A retrospective analysis of patients with ILI who presented to four US healthcare facilities during the May‐Dec 2009 period. A POCT was performed on 290 subjects within 48 h of symptom onset. | BinaxNOW® influenza A & B | Significant, POCT+ vs POCT−: 48/84 (57%) vs 37/206 (18%) (rate ratio 3.3, 95% CI 2.4, 4.6) |
| Theocharis et al, 2010,22 Greeceb | An observational study of patients with ILI who received house call visits from a network of doctors in Greece during Jan‐May 2009. 3412 visits were due to ILI, 184 of which had data available from a POCT. | Coris BioConcept Influ A&B Uni‐Strip | Significant, POCT+ vs POCT−: 74/97 (76.2%) vs 1/87 (1.1%), P < 0.01 |
| Jennings et al, 2009,20 Germanyb | A study analysing data from a standardised questionnaire that was used by paediatricians in Germany assessing children 1‐12 y with ILI. During the study period Jan‐April 2007, 16 907 patients were evaluated, 15 871 of which received a POCT. | Clearview Exact Influenza A+B | Significant, POCT+ vs standard+: 4618/7658 (60.1%) vs 178/725 (24.6%) |
| Falsey et al, 2007,37 USAb | Retrospective analysis of 166 adult (>18 y) hospitalised patients at a hospital in USA over four influenza seasons, Nov‐Apr 1999‐2003. Comparison of POCT positive (n = 86) and POCT negative/no POCT (n = 80) | Directigen Test Kit | Significant, POCT+ vs POCT−/no test: 63/86 (73%) vs 6/80 (8%), P < 0.001 |
| Poehling et al, 2006,25 USAb | A RCT enrolling children <5 y presenting with ILI in a Tennessee county over two consecutive influenza seasons (2003‐2004). All subjects (n = 468) had a nasal and throat swab obtained for PCR, and surveillance days were randomised to perform POCT (n = 205). | Quikvue influenza test | Not significant, POCT vs standard: 1/205 (0.5%) vs 0/263 (0%) |
| Bonner et al, 2003,31 USAb | An RCT of patients aged 2 mo to 21 y at a children's teaching hospital ED in USA over one influenza season, Jan‐Mar 2002. All patients (n = 391) received a POCT. Of 202 influenza positive patients, the physician was either made aware of the result (n = 96) or unaware (n = 106). | Flu OIA® | Significant, aware vs unaware: 18/96 (18.8%) vs 7/106 (6.6%), P < 0.02 |
Abbreviations: ED, emergency department; ILI, influenza‐like illness; POCT, point of care tests; RCT, randomised controlled trial; RIDT, rapid influenza diagnostic test.
Listed by year of publication, alphabetical.
Also assessed antibiotic prescription (Table 2).
Also assessed ED length of stay (Table 3).
Reported as weighted percentages, exact numbers not available.
A recent randomised controlled trial by Brendish et al16 found significantly higher prescription rates (91% vs 65%, P = 0.0026) amongst study groups diagnosed by POCT. Another recent study by Nitsch‐Osuch et al17 of preimplementation and postimplementation of POCTs showed increased antiviral use in post‐POCT patients (64% vs 0%, P < 0.05). Likewise, Li‐Kim‐Moy et al18 (46.2% vs 21.5%, P = 0.001); Blaschke et al19 (56% vs 19%, P = 0.002); and Jennings et al20 (60.1% vs 24.6%, 4618/7658 vs 178/725) all reported that influenza positive patients had significantly increased antiviral use amongst those diagnosed by a POCT compared with standard testing. Blaschke et al19 also demonstrated antiviral prescription was significantly higher in patients who received a positive POCT result in comparison with a negative POCT result (56% vs 2%, P < 0.0001), as did Suryaprasad et al21 (57% vs 18%, 48/84 vs 37/206) and Theocharis et al22 (76.2% vs 1.1%, P < 0.01). One study23 of treatment outcomes showed that patients who were prescribed oseltamivir only were significantly more likely to have been diagnosed by POCT compared with patients receiving antibiotics only, antibiotic/antiviral combination, or supportive care. Two studies found no significant effect of POCT on antiviral use.24, 25
3.4. Antibiotic prescription
Twenty‐six studies assessed the effect of POCT on antibiotic prescription rates (Table 2). Eleven (42.3%) of these papers17, 18, 19, 20, 26, 27, 28, 29, 30, 31, 32 compared POCT with standard testing and reported significant decreases in antibiotic use in patients receiving an influenza positive POCT diagnosis. In addition to comparing POCT and standard testing, three of the studies19, 27, 32 also assessed differences between positive and negative POCT results, demonstrating that patients positive for influenza by POCT were significantly less likely to receive antibiotics. Eight other studies (30.8%)22, 33, 34, 35, 36, 37, 38, 39 reported similar findings. There were six (23.1%) studies that found no significant changes in antibiotic prescription with POCT use.16, 24, 25, 40, 41, 42
Table 2.
Included studies assessing antibiotic prescriptiona
| Authors, Year, Location | Population, Study Period, Patient Groups | POCT | Results |
|---|---|---|---|
| Andrews et al, 2017,40 UK | Quasi‐randomised study assessing 606 adults (>16 y) at a single NHS centre over one influenza season, Jan‐July 2015. Standard testing used on odd days (n = 211), POCT used on even days (n = 334). | FilmArray Respiratory Panel | Not significant, POCT+ vs standard+ on decision to stop, remain off or reduce AB: 35.8% vs 32%, P < 0.05 |
| Brendish et al, 2017,16 UKb | An RCT enrolling 720 adults (>18 y) with acute respiratory illness at a large UK hospital during Jan‐July 2015, and Oct 2015 to April 2016. Patients randomly assigned either POCT (n = 362) or routine care (n = 358). | FilmArray Respiratory Panel | Not significant, POCT+ vs standard+: 301/360 (84%) vs 294/354 (83%), P = 0.96 |
| Nitsch‐Osuch et al, 2017,17 Polandb | A study comparing treatment of hospitalised children in a paediatric ward during two consecutive influenza seasons: Jan‐Mar 2015, when no RIDT were in use (n = 52), and Jan‐Mar 2016, when RIDT were implemented into routine practice (n = 63). | bioNexia Influenza A+B | Significant, POCT+ vs standard+: 7/11 (64%) vs 14/15 (93%), P < 0.05 |
| Trabattoni et al, 2017,24 Franceb , c | A single centre prospective observational study comparing two diagnostic strategies over 2 months. During Feb 2016 standard laboratory testing was used for diagnosis of influenza (n = 169), and in Mar 2016, a POCT was performed (n = 132). | Alere i Influenza A&B | Not significant, POCT vs standard: 39/132 (29.5%) vs 60/169 (35.5%), P = 0.32 |
| Jun et al, 2016,33 Koreac | A retrospective study conducted in the ED of a Korean university hospital over two influenza periods: Dec 2008 to Jan 2009, and Feb‐Mar 2013. Consisted of 342 paediatric patients (146 in period 1 and 196 in period 2), and 132 adult patients (61, period 1; 71, period 2). | Not specified | Significant, POCT+ vs POCT−: 3/17 (17.6%) vs 31/54 (57.4%), P < 0.05 |
| Li‐Kim‐Moy et al, 2016,18 Australiab , c | A retrospective review of 364 lab‐confirmed influenza cases presenting at a paediatric ED during Jan‐Dec 2009. Children (<18 y) were diagnosed with influenza by either POCT (n = 236) or standard testing (n = 65). | QuikVue Inluenza A+B | Significant, POCT+ vs standard+: 78/236 (33.1%) vs 35/65 (53.8%), P = 0.003 |
| Berthod et al, 2015,42 Switzerland | An RCT of adult patients presenting with ILI to two Swiss hospitals within 14 d of returning from a trip abroad. The study ran over a 4‐y period (Dec 2008 to Nov 2012), and patients randomly received either a POCT (n = 60) or standard care (n = 33) following a nasopharyngeal swap that was taken for laboratory confirmation. | Directigen Flu A+B rapid test | Not significant, POCT vs standard: 14/60 (23%) vs 13/33 (39%), P = 0.15 |
| Chu et al, 2015,30 USAb | A retrospective study of 350 adult (>18 y) patients at a teaching hospital and medical centre in the US over two influenza seasons, Feb–Mar 2012 and January 2013. The first season was prior to implementation of POCT (n = 175), and in the second season, POCT was used systematically (n = 175). | Simplexa Flu A/B & RSV Kit | Significant, pre‐POCT vs post‐POCT: 133/175 (76%) vs 110/175 (63%), P = 0.008 |
| González‐Del Vecchio 2015,39 Spainb | Cohort of 217 child and adult influenza positive from a general teaching hospital in Spain over one influenza season, Jan‐Mar 2014. Patients were divided into A‐POCT negative, reference positive (n = 132) or B‐POCT positive (n = 85) | Xpect Flu A&B | Significant, A vs B: 118/132 (89.3%) vs 57/85 (67.0%), P < 0.01 |
| Lacroix et al, 2015,34 France | A prospective study of febrile children (<5 y) presenting to a paediatric ED in France during a single influenza season. 170 subjects were included in the Jan‐Mar 2013 period, all of whom received diagnosis by POCT. | Quikvue influenza A+B | Significant, POCT+ vs POCT−: 4/80 (5%) vs 33/90 (36.6%), P < 0.05 |
| Tillekeratne et al, 2015,29 Sri Lanka | A prospective two phase study in the outpatient department of a Sri Lankan hospital. During the first phase (Mar‐Dec 2013), patients were attended by physicians who were not aware of their POCT results (n = 316). During the second phase (Jan‐Oct 2014), patients were attended by physicians who were made aware of POCT results (n = 241). | Veritor Flu A+B | Significant, IF positive patients phase 1 vs phase 2: 123/147 (83.7%) vs 43/69 (62.3%), P = 0.001 |
| Blaschke et al, 2014,19 USAb , d | A retrospective study using data from the National Hospital Ambulatory Medical Care Survey over three influenza periods: Jan‐Apr 2007, Oct‐Dec 2008, and Jan Apr 2009. 1166 of an estimated 4.9 M eligible ED visits were sampled and examined in three groups: POCT+, POCT−, and Influenza (+) by standard test. | Not specified | Significant, POCT+ vs standard+: 11% vs 23%, P = 0.05 significant, POCT+ vs POCT−: 11% vs 47%, P < 0.0001 |
| Jeong et al, 2014,27 Koreac | A retrospective review of data from 437 patients who were suffering from ILI and were discharged from the ED of a Korean hospital over two influenza seasons. In 2010‐2011 patients received standard care (n = 221), and in 2011‐2012, patients were diagnosed by POCT (n = 216). | Alere SD Bioline Influenza | Significant, POCT vs standard: 54/216 (25.0%) vs 97/221 (43.9%), P < 0.01significant, POCT+ vs POCT−: 7/73 (9.6%) vs 42/123 34.1%, P < 0.01 |
| Lim et al, 2014,23 Koreab | A retrospective review of medical records of 770 children (<15 y) hospitalised with laboratory confirmed influenza between Feb 2004 and June 2007. Different treatment groups were analysed and included patients receiving; oseltamivir only (n = 27), antibiotics only (n = 620), antibiotics/oseltamivir combination (n = 67), or standard supportive care (n = 56). | Directigen EZ Flu A + B | Significant, oseltamivir only vs antibiotics only, antibiotics/oseltamivir combination, standard supportive care: likelihood of diagnosis by POCT− 22/27 (81.5%) vs 21/620 (3.4%) P < 0.001, 37/67 (55.2%) P = 0.017, 9/56 (16.1%) P < 0.001 |
| Nitsch‐Osuch et al, 2013,26 Poland | A prospective cohort study conducted in three Warsaw primary care clinics over two influenza seasons of 2009/2010 and 2010/2011.256 children with ILI aged 0‐5 y were given either a POCT (n = 115), or standard care (n = 141). | Directigen EZ Flu A+B | Significant, POCT vs standard: 7% vs 16%, P = 0.032 |
| Theocharis et al, 2010,22 Greeceb | An observational study of patients with ILI who received house call visits from a network of doctors in Greece during Jan‐May 2009. 3412 visits were due to ILI, 184 of which had data available from a POCT. | Influ A&B Uni‐Strip Dry Swabs (C‐1512) | Significant, POCT+ vs POCT−: 4/97 (4.1%) vs 32/87 (36.8%), P < 0.01 |
| Jennings et al, 2009,20 Germanyb | A study analysing data from a standardised questionnaire that was used by paediatricians in Germany assessing children 1‐12 y with ILI. During the study period Jan‐April 2007, 16 907 patients were evaluated, 15 871 of which received a POCT. | Clearview Exact Influenza A+B | Significant, POCT+ vs standard+: 271/7658 (3.5%) vs 125/725 (17.2%) |
| Ozkaya et al, 2009,28 Turkey | A cross‐sectional, single blinded trial of children aged 3‐14 who presented to the ED of a Turkish paediatric hospital between November 2006 and Mar 2007. Patients were allocated into two groups that received either a POCT or a standard physical examination. | Influenza A/B Rapid Test | Significant, POCT vs standard: 32% vs 100%, P < 0.0001 |
| D'Heilly et al, June 2008,38 USA | Retrospective study of 311 adults patients at a medical centre in USA over two influenza seasons; Nov 2003 to May 2004 and Oct 2004 to May 2005. Compared positive (n = 78) and negative (n = 233) POCT results. | FLU OIA A/B | Significant, POCT+ vs POCT−: AB odds ratio = 0.20 (95% CI, 0.10‐0.42) |
| Falsey et al, 2007,37 USAb | Retrospective analysis of 166 adult (>18 y) hospitalised patients at a hospital in USA over four influenza seasons, Nov‐Apr 1999‐2003. Comparison of POCT positive (n = 86) and POCT negative/no POCT (n = 80) | Directigen | Significant, POCT+ vs POCT−/no test: 74/86 (86%) vs 79/80 (98.8%), P = 0.002 |
| Bhavnani et al, 2006,36 Thailand | A retrospective analysis of medical records from 300 patients of all ages selected from 5 outpatient departments in Thailand. Records were selected at a 1:2 ratio of ILI cases that were diagnosed as influenza positive (n = 106) or negative (n = 194) by POCT. | Quikvue influenza test | Significant, POCT+ vs POCT−: 77/106 (73%) vs 168/194 (87%), P = 0.003 |
| Benito‐Fernandez et al, 2006,35 Spainc | A prospective study of febrile infants (<36 months) presenting to a single paediatric ED in Spain. 206 patients received diagnosis by POCT, with no conformational laboratory testing performed. | Directigen Flu A+B Test Kit | Significant, POCT+ vs POCT−: 0/84 (0%) vs 47/122 (38.5%), P < 0.01 |
| Poehling et al, 2006,25 USAb | An RCT enrolling children <5 y presenting with ILI in a Tennessee county over two consecutive influenza seasons (2003‐2004). All subjects (n = 468) had a nasal and throat swab obtained for PCR, and surveillance days were randomised to perform POCT (n = 205). | Quikvue influenza® test | Not significant, POCT vs standard: 61/205 (29.8%) vs 75/263 (28.5%) |
| Iyer et al,41 2006, USAc | A prospective, quasi‐randomised controlled trial of febrile children aged 2‐24 months during two influenza periods. Diagnosis was determined by either POCT (n = 345) or standard test (n = 355), which was determined by alternating testing days. | Quikvue influenza test | Not significant, POCT vs standard: 54/345 (15.7%) vs 59/355 (16.6%) |
| Bonner et al, 2003,31 USAb | An RCT of patients aged 2 mo to 21 y at a children's teaching hospital ED in USA over one influenza season, Jan‐Mar 2002. All patients (n = 391) received a POCT. Of 202 influenza positive patients the physician was either made aware of the result (n = 96), or was unaware (n = 106). | Flu OIA | Significant, aware vs unaware: 7/96 (7.3%) vs 26/106 (24.5%), P < 0.001 |
| Esposito et al, 2003,32 Italy | An RCT of 957 paediatric (<15 y) patients at the ED of a teaching hospital in Italy over one influenza season, Jan‐Feb 2002. Patients were randomly allocated POCT/no POCT, and outcomes for POCT positive (n = 43), POCT negative (n = 435), and standard care (n = 479) were compared. | Quickvue Influenza Test | Significant, POCT+ vs POCT−, standard care: AB (%) 32.6 vs 64.8 (P < 0.0001), vs 61.8 (P = 0.0003) |
Abbreviations: ED, emergency department; POCT, point of care tests; RCT, randomised controlled trial.
Listed by year of publication, alphabetical.
Also assessed antiviral prescription (Table 1).
Also assessed ED length of stay (Table 3).
Reported as weighted percentages, exact numbers unavailable.
3.5. Length of stay (LOS)
Nine studies assessed the effect of POCT on ED length of stay (Table 3). Five (55.6%) of these reported significant reductions in time spent in ED in association with POCT use.
Table 3.
Included studies assessing ED length of staya
| Authors, Year, Location | Population, Study Period, Patient Groups | POCT | Results |
|---|---|---|---|
| Trabattoni et al, 2017,24 Franceb , c | A single centre prospective observational study comparing two diagnostic strategies over 2 months. During Feb 2016 standard laboratory testing was used for diagnosis of influenza (n = 169), and in Mar 2016, a POCT was performed (n = 132). | Alere i Influenza A&B | Significant, POCT vs standard (mean): 4.2 vs 6.1 h, P = 0.03 |
| Jun et al, 2016,33 Koreac | A retrospective study conducted in the ED of a Korean university hospital over two influenza periods: Dec 2008 to Jan 2009, and Feb‐Mar 2013. Consisted of 342 paediatric patients (146 in period 1 and 196 in period 2), and 132 adult patients (61, period 1; 71, period 2). | Not specified | Significant, POCT vs standard (mean): 4.8 vs 3.0 h (P = 0.001, period 1), 2.2 vs 1.7 h (P = 0.048, period 2) |
| Li‐Kim‐Moy et al, 2016,18 Australiab , c | A retrospective review of 364 lab‐confirmed influenza cases presenting at a paediatric ED during Jan‐Dec 2009. Children (<18 y) were diagnosed with influenza by either POCT (n = 236) or standard testing (n = 65). | QuikVue Inluenza A+B | Not significant, POCT+ vs standard+ (median): 2.7 vs 2.4 h, P = 0.53 |
| Soto et al, 2016,44 Spain | A retrospective study of 1057 adult patients attending the ED of a Barcelona hospital over two influenza seasons. Patients enrolled between Jan and Mar 2013 had samples analysed by standard PCR (n = 366), and between Jan and Mar 2014, patients were diagnosed by a POCT (n = 691). | Xpert Flu Assay | Significant, POCT vs standard (mean): 20.7 vs 28.1 h, P = 0.003 |
| Rogers et al, 2015,45 USA | Retrospective analysis of 1136 patients (3 mo to 21 y) at a tertiary care centre in the US over two influenza seasons, comparing pre‐ and post‐POCT implementation. In Nov 2011 to Jan 2012, patients received standard care (n = 365), and during Nov 2012 to Jan 2013, patients underwent POCT (n = 771). | FilmArray Rapid Respiratory Panel | Significant, pre‐ vs post‐POCT (mean): 4.3 vs 4.7 h, P < 0.002 |
| Jeong et al, 2014,27 Koreac | A retrospective review of data from 437 patients who were suffering from ILI and were discharged from the ED of a Korean hospital over two influenza seasons. In 2010‐2011, patients received standard care (n = 221), and in 2011‐2012, patients were diagnosed by POCT (n = 216). | SD Bioline Influenza Antigen Test | Significant, POCT vs standard (mean): 4.3 vs 3.6 h, P < 0.01 |
| Abanses et al, 2006,43 USA | Prospective analysis of 1007 febrile infants (3‐36 mo old) at a children's hospital ED in the US over one influenza season (Dec 2002 to Mar 2003). Compared patients receiving a POCT (n = 288) to standard care (n = 719). | Directigen Flu A + B | Significant, POCT vs standard care (mean): 2.6 vs 3.3 h (95% CI, 0.32‐1.00) |
| Benito‐Fernandez et al, 2006,35 Spainc | A prospective study of febrile infants (<36 mo) presenting to a single paediatric ED in Spain. 206 patients received diagnosis by POCT, with no conformational laboratory testing performed. | Directigen Flu A+B Test Kit | Significant, POCT+ vs POCT− (mean): 3.6 vs 7.8 h, P < 0.01 |
| Iyer et al, 2006,41 USAc | A prospective, quasi‐randomised controlled trial of febrile children aged 2‐24 months during two influenza periods. Diagnosis was determined by either POCT (n = 345) or standard test (n = 355), which was determined by alternating testing days. | Quikvue influenza test | Not significant, POCT vs standard (mean, 95% CI): 3.4 h (3.2‐3.5) vs 3.4 h (3.2‐3.6) |
Trabattoni et al24 (4.2 vs 6.1 hours, P = 0.03) and Abanses et al43 (2.6 vs 3.3 hours, 95% CI, 0.32‐1.00) demonstrated decreased mean ED LOS in prospective studies comparing POCT vs standard testing over a single influenza season. Similarly, in two retrospective studies comparing post‐ and pre‐POCT implementation, Soto et al44 reported a reduced mean ED LOS (20.7 vs 28.1 hours, P = 0.003) as did Rogers et al45 (4.3 vs 4.7 hours, P < 0.002). Fernandez et al35 also demonstrated a significant decrease in ED LOS, in this case between POCT(+) and POCT(−) patients (3.6 vs 7.84 hours, P < 0.01).
Two studies found no significant differences in the ED LOS of POCT and standard testing groups,18, 41 whilst two studies reported that POCT increased ED LOS. Jeong et al27 showed in a retrospective study the median length of ED stay was significantly longer after systematic implementation of POCT (4.3 vs 3.6 hours, P < 0.01), whilst Jun et al33 also reported paediatric mean LOS to be significantly longer when POCT were used (4.8 vs 3.04 hours, P = 0.001).
4. DISCUSSION
This is the first systematic review to examine the effects of using current, commercially available influenza POCT for diagnosis on the prescription of antibiotics and antivirals, and patient LOS in the ED.
Diagnosis of influenza positive patients by POCT resulted in significantly higher rates of antiviral prescription. A number of the studies comparing POC with standard testing16, 18, 40 attributed the increased antiviral use to faster turnaround time to diagnosis by POCT. Given that antivirals are of most clinical benefit if taken within 48 hours of symptom onset,10, 46, 47 this is a key point. In the studies comparing positive and negative POCT results, the increased antiviral use in influenza positive patients is likely due to the removal of diagnostic uncertainty, which also commonly causes patients to be overprescribed antibiotics.48 A positive result may eliminate unnecessary antibiotic prescription, whilst a negative POCT result may allow a bacterial infection to be treated promptly. This was evident in the 11 studies included in this review, which demonstrated that a positive POCT diagnosis led to decreased antibiotic use compared with a negative POCT result.19, 22, 27, 32, 33, 34, 35, 36, 37, 38, 39 Shortening or eliminating avoidable antibiotic use can be of benefit by reducing risk from antibiotic resistance on both a patient and large‐scale level.
Length of stay is an important variable to consider when evaluating the effectiveness and quality of patient care. Our systematic review highlights the potential of POCTs for influenza in reducing ED LOS; however, results were mixed, and two studies actually reported that POCT led to an increased LOS in ED. Jeong et al27 attributed longer ED stay to the significantly older age of their POCT cohort that may have had more comorbidities, whilst reasons for the other study are unclear.33 Emergency department LOS can be influenced by many factors including availability of beds, overcrowding, admission and discharge procedures, and the efficiency of ED staff. Thus, it may be that the benefits of POCT in terms of ED LOS are somewhat diminished and that this is reflected in the mixed findings of publications in this systematic review. Nonetheless, studies finding reduced ED stay associated with POCT are encouraging, as this can lead to improved clinical outcomes through prevention of nosocomial spread, and easing burden on the healthcare system. More significant reductions in ED LOS can likely be established by further monitoring as health care centres become more familiar with POC technologies.
Early antigen‐based POC tests are poor in terms of sensitivity, such as the Quikvue influenza test, which has a sensitivity of 74.0%, and a negative predictive value of 75.0%.34 Physicians are in many cases aware of such limitations and used confirmatory tests to avoid false‐negative results.18 Nonetheless, the findings of studies in this systematic review suggest that antigen‐based POCTs can still significantly affect clinical decision‐making and patient outcomes. Newer molecular‐based POCTs boast high sensitivity and specificity, meaning that results can be trusted by physicians.
Some of the studies using newer molecular POCT saw a limited effect on antibiotic prescription rates, with one group suggesting16 that in many cases, antibiotics were administered very early in patient assessment, even before POCT results were available. An outcome measuring the proportion of patients treated with brief courses of antibiotics may be more clinically relevant in these cases. At times, molecular POCTs were performed by research investigators rather than clinical staff,16, 40 suggesting that the study protocol was not initiated immediately upon ED admission. Adoption of new diagnostic technologies will require changes to management protocol and ED collaboration in order to maximise the benefits of improved clinical decision‐making and patient workflow.
This paper expands upon a 2014 systematic review,49 which assessed the effect of rapid viral diagnosis for children with acute febrile respiratory illness on a range of clinical outcomes including antibiotic use and length of hospital stay. The previous publication was a quantitative analysis of four randomised controlled trials, whereas this systematic review was a qualitative analysis of 30 studies, and thus, we were able to make vastly different conclusions. Our findings were similar to those of another publication50; however, these reviews differed in a number of key ways. The study of Ko and Drews50 is an expert opinion of studies published between January 2000 and June 2016. Their literature search was performed on PubMed only and did not specify criteria for selection of studies. The search for our systematic review was performed on two databases up to December 2017, yielding different results including six recent studies using molecular‐based POCTs. Our search was also supplemented by screening citations of included publications and relevant review papers for additional studies meeting our selection criteria.
Biases due to corporate interests are an important consideration when reviewing literature on the potential benefits of POCT. Because of this, the conflict of interest statements of all publications included in this systematic review were thoroughly checked. Two studies40, 41 declared potential conflicts of interest, as they were financially supported by commercial manufacturers of POCT, and three others19, 20, 45 disclosed professional relationships between contributing authors and such companies.
A limitation of this systematic review is that its focus is restricted to (1) influenza diagnosis only and (2) antiviral, antibiotic, and LOS outcomes. Whilst these outcomes are highly applicable for influenza management, new molecular POCTs such as the FilmArray Respiratory Panel can test for a range of viruses and bacteria simultaneously. Hence, these technologies have the potential to affect variables not covered in this review, and this is an area that may be targeted by future research.
In the past decade, numerous studies reporting the diverse use of nanoparticles in POCT have received great interest because of their unique chemical and physical properties.51 Several research groups have proposed simple colorimetric approaches based on controlled assembly of gold nanoparticles on the surface of viruses, including influenza.52, 53 The limit of detection of these proposed methods is up to 385 times lower than that of conventional enzyme‐linked immunosorbent assay.54 Such reports represent a step forward in the development of tools for the facile detection and surveillance of influenza viruses. Future integration of nanoparticles with specific biological markers will facilitate low‐cost, next generation diagnostic methods for on‐site detection of respiratory viruses.
The findings of this systematic review support the use of influenza POCT for patients with acute respiratory infection. The majority of studies reported that POCT is associated with more appropriate use of antivirals and antibiotics, which can lead to improved health outcomes for patients. Point‐of‐care tests may also reduce LOS in ED; however, further clinical trials are needed to properly assess the effect of rapid diagnostic technologies on this variable.
CONFLICT OF INTEREST
The authors have no competing interest.
Supporting information
Supplementary Table 1. Study quality assessment results
Egilmezer E, Walker GJ, Bakthavathsalam P, et al. Systematic review of the impact of point‐of‐care testing for influenza on the outcomes of patients with acute respiratory tract infection. Rev Med Virol. 2018;28:e1995 10.1002/rmv.1995
REFERENCES
- 1. World Health Organisation . Top 10 causes of death fact sheet No. 310.2018; http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed Feb 10, 2018.
- 2. Singh S. Middle East respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med. 2016;37(4):572‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taubenberger J, Morens D. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3(1):499‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tovey ER, Stelzer‐Braid S, Toelle BG, et al. Rhinoviruses significantly affect day‐to‐day respiratory symptoms of children with asthma. J Allergy Clin Immunol. 2015;135(3):663‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organisation . Influenza (Seasonal) Fact Sheet No. 211.2018; http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed Jan 15, 2018.
- 7.St. Geroge K. Diagnosis of influenza virus In: Kawaoka Y, Neumann G, eds. Influenza Virus: Methods and Protocols. Humana Press; 2012;53‐69. [Google Scholar]
- 8. Organization WH . WHO recommendations on the use of rapid testing for influenza diagnosis. 2005; http://www.who.int/influenza/resources/documents/rapid_testing/en/. Accessed February 10, 2018.
- 9. Gavin PJ, Thomson RB. Review of rapid diagnostic tests for influenza. Clin Appl Immunol Rev. 2004;4(3):151‐172. [Google Scholar]
- 10. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2011;60(1):1‐24. [PubMed] [Google Scholar]
- 11. Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta‐analysis. Ann Intern Med. 2012;156(7):500‐511. [DOI] [PubMed] [Google Scholar]
- 12. Popowitch EBMM. Performance characteristics of Xpert Flu/RSV XC assay. J Clin Microbiol. 2015;53(8):2720‐2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahrenbrock MG, Matushek S, Boonlayangoor S, Tesic V, Beavis KG, Charnot‐Katsikas A. Comparison of Cepheid Xpert flu/RSV XC and BioFire FilmArray for detection of influenza a, influenza B, and respiratory syncytial virus. J Clin Microbiol. 2016;54(7):1902‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher DLA, Tetzlaff J, Altman DG, PRISMA Group . Reprint—preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Phys Ther. 2009;89(9):873‐880. [PubMed] [Google Scholar]
- 15. Group OLoEW . The Oxford levels of evidence. http://www.cebm.net/index.aspx?o=5653. Accessed February 10, 2017.
- 16. Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point‐of‐care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open‐label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nitsch‐Osuch A, Kuchar E, Golebiak I, et al. Rapid influenza diagnostic tests improve suitability of antiviral treatment in hospitalized children. Adv Exp Med Biol 2017;968 (no pagination). [DOI] [PubMed] [Google Scholar]
- 18. Li‐Kim‐Moy J, Dastouri F, Rashid H, et al. Utility of early influenza diagnosis through point‐of‐care testing in children presenting to an emergency department. J Paediatr Child Health. 2016;52(4):422‐429. [DOI] [PubMed] [Google Scholar]
- 19. Blaschke AJ, Shapiro DJ, Pavia AT, et al. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J Pediatric Infect Dis Soc. 2014;3(2):112‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennings LC, Skopnik H, Burckhardt I, Hribar I, del Piero L, Deichmann KA. Effect of rapid influenza testing on the clinical management of paediatric influenza. Influenza Other Respi Viruses. 2009;3(3):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suryaprasad A, Redd JT, Ricks PM, et al. Effect of rapid influenza diagnostic testing on antiviral treatment decisions for patients with influenza‐like illness: southwestern U.S., May‐December 2009. Public Health Rep. 2014;129(4):322‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Theocharis G, Vouloumanou EK, Rafailidis PI, Spiropoulos T, Barbas SG, Falagas ME. Evaluation of a direct test for seasonal influenza in outpatients. Eur J Intern Med. 2010;21(5):434‐438. [DOI] [PubMed] [Google Scholar]
- 23. Lim JK, Kim TH, Kilgore PE, et al. The association between influenza treatment and hospitalization‐associated outcomes among Korean children with laboratory‐confirmed influenza. J Korean Med Sci. 2014;29(4):485‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trabattoni E, Le V, Pilmis B, et al. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an emergency department. Am J Emerg Med. 2017. [DOI] [PubMed] [Google Scholar]
- 25. Poehling KA, Zhu Y, Tang YW, Edwards K. Accuracy and impact of a point‐of‐care rapid influenza test in young children with respiratory illnesses. Arch Pediatr Adolesc Med. 2006;160(7):713‐718. [DOI] [PubMed] [Google Scholar]
- 26. Nitsch‐Osuch A, Stefanska I, Kuchar E, et al. Influence of rapid influenza test on clinical management of children younger than five with febrile respiratory tract infections. Adv Exp Med Biol. 2013;755:237‐241. [DOI] [PubMed] [Google Scholar]
- 27. Jeong HW, Heo JY, Park JS, Kim WJ. Effect of the influenza virus rapid antigen test on a physician's decision to prescribe antibiotics and on patient length of stay in the emergency department. PLoS One. 2014;9(11):e110978 10.1371/journal.pone.0110978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozkaya E, Cambaz N, Coskun Y, et al. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. 2009;98(10):1589‐1592. [DOI] [PubMed] [Google Scholar]
- 29. Tillekeratne LG, Bodinayake CK, Nagahawatte A, et al. Use of rapid influenza testing to reduce antibiotic prescriptions among outpatients with influenza‐like illness in southern Sri Lanka. Am J Trop Med Hyg. 2015;93(5):1031‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu HY, Englund JA, Huang D, et al. Impact of rapid influenza PCR testing on hospitalization and antiviral use: a retrospective cohort study. J Med Virol. 2015;87(12):2021‐2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonner A, Monroe K, Talley L, Klasner A, Kimberlin D. Impact of the rapid diagnosis of influenza on physician decision‐making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363‐367. [DOI] [PubMed] [Google Scholar]
- 32. Esposito S, Marchisio P, Morelli P, Crovari P, Principi N. Effect of a rapid influenza diagnosis. Arch Dis Child. 2003;88(6):525‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jun SH, Kim JY, Yoon YH, Lim CS, Ch HJ, Choi SH. The effect of the rapid antigen test for influenza on clinical practice in the emergency department: a comparison of periods before and after the 2009 H1N1 influenza pandemic. Signa Vitae. 2016;11(1):74‐89. [Google Scholar]
- 34. Lacroix S, Vrignaud B, Avril E, et al. Impact of rapid influenza diagnostic test on physician estimation of viral infection probability in paediatric emergency department during epidemic period. J Clin Virol. 2015;72:141‐145. [DOI] [PubMed] [Google Scholar]
- 35. Benito‐Fernandez J, Vazquez‐Ronco MA, Morteruel‐Aizkuren E, et al. Impact of rapid viral testing for influenza A and B viruses on management of febrile infants without signs of focal infection. Pediatr Infect Dis J. 2006;25(12):1153‐1157. [DOI] [PubMed] [Google Scholar]
- 36. Bhavnani D, Phatinawin L, Chantra S, Olsen SJ, Simmerman JM. The influence of rapid influenza diagnostic testing on antibiotic prescribing patterns in rural Thailand. Int J Infect Dis. 2006;11(4):355‐359. [DOI] [PubMed] [Google Scholar]
- 37. Falsey A, Murata Y, Walsh E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167(4):354‐360. [DOI] [PubMed] [Google Scholar]
- 38. D'Heilly SJ, Janoff EN, Nichol P, Nichol KL. Rapid diagnosis of influenza infection in older adults: influence on clinical care in a routine clinical setting. J Clin Virol. 2008;42(2):124‐128. [DOI] [PubMed] [Google Scholar]
- 39. González‐Del Vecchio M, Catalán P, de Egea V, et al. An algorithm to diagnose influenza infection: evaluating the clinical importance and impact on hospital costs of screening with rapid antigen detection tests. Eur J Clin Microbiol Infect Dis. 2015;34(6):1081‐1085. [DOI] [PubMed] [Google Scholar]
- 40. Andrews D, Chetty Y, Cooper BS, et al. Multiplex PCR point of care testing versus routine, laboratory‐based testing in the treatment of adults with respiratory tract infections: a quasi‐randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis. 2017;17(1):671 10.1186/s12879-017-2784-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iyer SB, Gerber MA, Pomerantz WJ, Mortensen JE, Ruddy RM. Effect of point‐of‐care influenza testing on management of febrile children. Acad Emerg Med. 2006;13(12):1259‐1268. [DOI] [PubMed] [Google Scholar]
- 42. Berthod D, Genton B, Hatz C, Blum J, de Vallière S. Ability of physicians to diagnose influenza and usefulness of a rapid influenza antigen test in febrile returning travelers: a randomized controlled trial. Travel Med Infect Dis. 2015;13(5):394‐399. [DOI] [PubMed] [Google Scholar]
- 43. Abanses JC, Dowd MD, Simon SD, Sharma V. Impact of rapid influenza testing at triage on management of febrile infants and young children. Pediatr Emerg Care. 2006;22(3):145‐149. [DOI] [PubMed] [Google Scholar]
- 44. Soto M, Sampietro‐Colom L, Vilella A, et al. Economic impact of a new rapid PCR assay for detecting influenza virus in an emergency department and hospitalized patients. PLoS One. 2016;11(1):e0146620 10.1371/journal.pone.0146620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogers BB, Shankar P, Jerris RC, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636‐641. [DOI] [PubMed] [Google Scholar]
- 46. Campion KSC, Keene O, Cooper C, et al. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352(9144):1877‐1881. [PubMed] [Google Scholar]
- 47. Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283(8):1016‐1024. [DOI] [PubMed] [Google Scholar]
- 48. McCullough AR, Pollack AJ, Plejdrup Hansen M, et al. Antibiotics for acute respiratory infections in general practice: comparison of prescribing rates with guideline recommendations. Med J Aust. 2017;207(2):65‐69. [DOI] [PubMed] [Google Scholar]
- 49. Doan QH, Kissoon N, Dobson S, et al. A randomized, controlled trial of the impact of early and rapid diagnosis of viral infections in children brought to an emergency department with febrile respiratory tract illnesses. J Pediatr. 2009;154(1):91‐95. [DOI] [PubMed] [Google Scholar]
- 50. Ko F, Drews SJ. The impact of commercial rapid respiratory virus diagnostic tests on patient outcomes and health system utilization. Expert Rev Mol Diagn. 2017;17(10):917‐931. [DOI] [PubMed] [Google Scholar]
- 51. Moulick A, Richtera L, Milosavljevic V, et al. Advanced nanotechnologies in avian influenza: current status and future trends—a review. Anal Chim Acta. 2017;983:42‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Zhang L, Wei W, et al. Colorimetric detection of influenza A virus using antibody‐functionalized gold nanoparticles. Analyst. 2015;140(12):3989‐3995. [DOI] [PubMed] [Google Scholar]
- 53. Zheng L, Wei J, Lv X, et al. Detection and differentiation of influenza viruses with glycan‐functionalized gold nanoparticles. Biosens Bioelectron. 2017;91:46‐52. [DOI] [PubMed] [Google Scholar]
- 54. Ahmed SR, Kim J, Suzuki T, Lee J, Park EY. Enhanced catalytic activity of gold nanoparticle‐carbon nanotube hybrids for influenza virus detection. Biosens Bioelectron. 2016;85:503‐508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Study quality assessment results


