Summary
Viral diseases like influenza, AIDS, hepatitis, and Ebola cause severe epidemics worldwide. Along with their resistant strains, new pathogenic viruses continue to be discovered so creating an ongoing need for new antiviral treatments. RNA interference is a cellular gene‐silencing phenomenon in which sequence‐specific degradation of target mRNA is achieved by means of complementary short interfering RNA (siRNA) molecules. Short interfering RNA technology affords a potential tractable strategy to combat viral pathogenesis because siRNAs are specific, easy to design, and can be directed against multiple strains of a virus by targeting their conserved gene regions. In this review, we briefly summarize the current status of siRNA therapy for representative examples from different virus families. In addition, other aspects like their design, delivery, medical significance, bioinformatics resources, and limitations are also discussed.
Keywords: antiviral, clinical trials, delivery, design, limitations, resources, RNAi, siRNA, virus
Abbreviations used
- BKV
polyomavirus BK
- DENV
Dengue virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HIVsirDB
HIV siRNA database
- HPV
human papillomavirus
- INFV
influenza virus
- MARV
Marburg virus
- RISC
RNA‐induced silencing complex
- RNAi
RNA interference
- RSV
respiratory syncytial virus
- SARS‐CoV
SARS coronavirus
- siRNA
short interfering RNA
- T‐Ag
T antigen
- UTR
untranslated region
- VACV
vaccinia virus
- VIRsiRNAdb
viral siRNA database
- VIRsiRNApred
viral siRNA predictor
- WNV
West Nile virus
1. INTRODUCTION
Because of their high mutation rates, viruses have the potential to elude host defense systems as well as antiviral drugs and vaccines. Thus, development of new and alternate antiviral therapies has become important.1, 2 During the past decade, scientists have widely used the cellular RNA interference (RNAi) pathway approach to target a number of viral genes to restrain their expression.3, 4 In this pathway, long double‐stranded RNA (dsRNA) precursors are split into short interfering RNAs (siRNAs) following which the RNA‐induced silencing complex includes one of the siRNA strands and slices the complementary target mRNA using ATP.5, 6 Short interfering RNA technology has been exploited to target disease‐causing genes as well as for functional studies.7, 8 Also, this strategy can target diverse types of viruses as even a tiny viral genome can provide several targetable regions. For example, siRNAs directed against different genes of deadly viruses like human immunodeficiency virus (HIV),9, 10 influenza virus (INFV),11, 12 hepatitis B virus (HBV),13 SARS coronavirus (SARS‐CoV),14, 15 human papillomavirus (HPV),16 and West Nile virus (WNV)17 in infected cells displayed encouraging results in inhibiting viral replication. Researchers have also used multiple siRNAs simultaneously to augment viral inhibition in a coordinated approach.18, 19 Short interfering RNAs for various human viruses like respiratory syncytial virus (RSV), hepatitis C virus (HCV), HBV, and HIV are also appearing in clinical trials, which further elucidate their importance in inhibiting viral infections.20 Thus, siRNAs have emerged as practically modular and adaptable therapeutics for treating viral infections. In this review, we will discuss the use of siRNAs against different viral families, their therapeutic applications, and design and delivery considerations. Further limitations of siRNAs as antivirals and the remedial measures are also discussed.
2. VIRUSES
Viruses are tiny obligate intracellular parasites, having either an RNA or a DNA genome enclosed by a virus‐coded protein coat. Viruses depend on host cells for proliferation. They are classified on account of shape, genome structure, or mode of replication, and many classes of these pathogens cause a large number of diseases in different organisms.21 Different species of viruses have varying types of infection processes; however, there are many general steps22, 23 that are briefly summarized in Figure 1. Short interfering RNAs directed against specific viral/host genes can block the virus life cycle at any of the steps.
Figure 1.
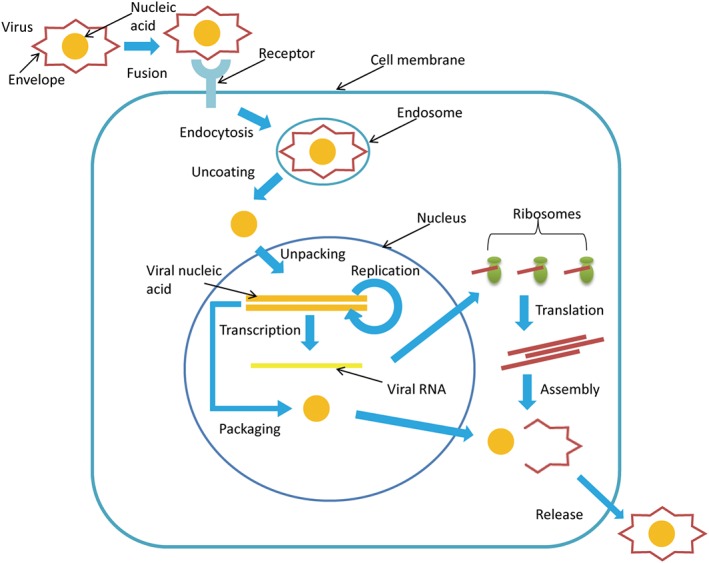
The typical different stages of virus life cycle. (1) Attachment: In this step, the viral envelope glycoproteins attach to certain host cell membrane receptors. (2) Endocytosis: Here, the viral contents are taken up by the host cell. (3) Uncoating: Degradation of the viral capsid by host cell enzymes. (4) Growth: It involves translation and replication of the viral genes. (5) Assembly: The viral proteins assemble to enclose the viral genome. (6) Release: The mature virus particles escape from the host cell by budding/lysis. In RNA viruses, however, the viral genome is usually not integrated into the host genome, and hence, their RNA molecules are directly used as mRNAs for translation
3. RNA INTERFERENCE
RNAi is a cellular mechanism wherein small molecules of RNA hinder the expression of a particular gene(s) via counteracting the corresponding mRNA molecules that possess nucleotide sequences complimentary to the small RNA.24 Historically, RNAi was identified by different terms such as quelling, cosuppression, and posttranscriptional gene silencing. In 1998, Mello and Fire illustrated a strong gene silencing caused by injecting dsRNA into Caenorhabditis elegans. 25 Further findings in the field of RNAi have been pictorially summarized in Figure 2.
Figure 2.
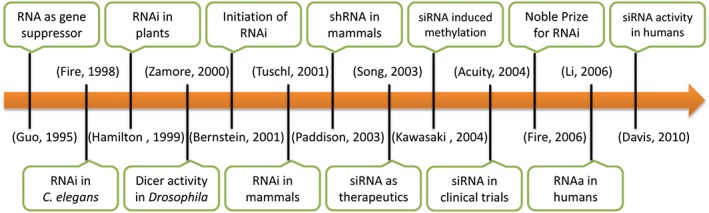
Timeline depicting the sequential progression in the field of RNA interference (RNAi). shRNA, short hairpin RNA; siRNA, short interfering RNA
The RNAi pathway processes dsRNA into 21 to 30 nucleotide‐long RNA molecules that act as a module of a silencing machinery to distinctively suppress expression/function of an intended gene/genomic region (Figure 3). In particular, the silencing pathway involves chopping of dsRNA into siRNA that are typically 21 to 25 base pairs long dsRNA having dinucleotide overhangs on the 3′ termini. One of the siRNA strands (guide strand) is then incorporated into an RNA‐induced silencing complex that degrades the target mRNA.5, 26 The siRNAs resulting from the original longer dsRNA are different from microRNAs as the latter in general have partial base pairing with a target mRNA and restrain the expression of several different genes having related sequences. Short interfering RNAs also differ from short hairpin RNAs as the latter have a hairpin turn and their expression in cells is usually achieved by means of bacterial/viral vectors.27
Figure 3.
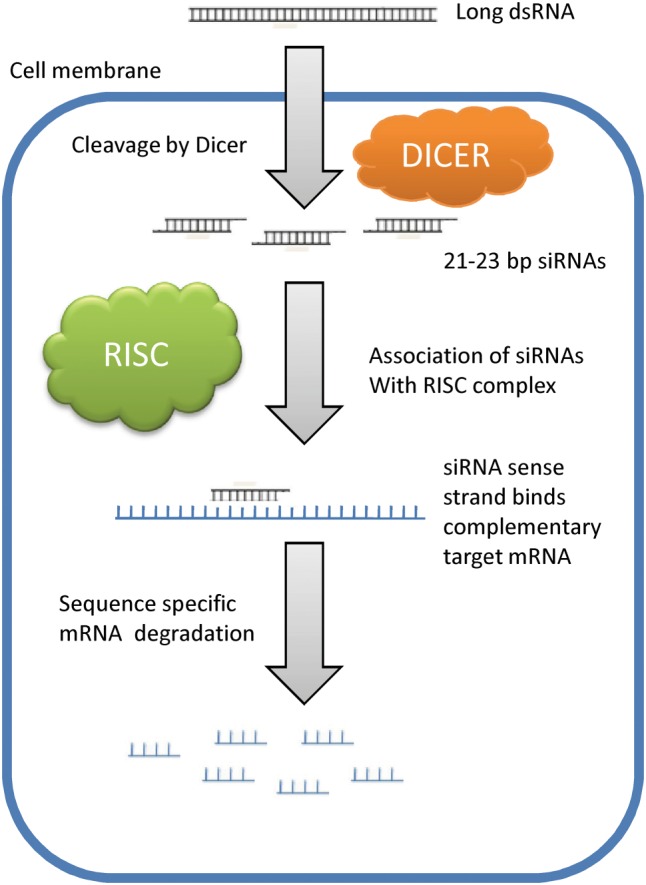
In the RNA interference mechanism, double‐stranded RNA (dsRNA) is chopped into short (21‐25 nucleotides) interfering RNA (siRNA) molecules possessing dinucleotide overhangs on the 3′ termini. One siRNA strand is integrated into the RNA‐induced silencing complex (RISC) that finally degrades the complementary target mRNA
Short interfering RNAs characteristically base‐pair completely and cause mRNA cleavage in a precise target region.28 MicroRNAs are generated from introns or their own genes. Gene silencing can take place through mRNA cleavage or thwarting mRNA translation. The role of microRNAs is to regulate gene expression. In the plant kingdom, RNAi is broadcasted by the transportation of siRNAs amid cells via plasmodesmata.29 RNAi was described as the “breakthrough of the year” in30 2002. RNAi is also believed to be a component of a primitive immune system based on nucleic acid recognition.31 RNAi shields human cells from pathogenic viruses by silencing viral genes.32, 33 Moreover, small RNAs can cause genomic imprinting or facilitate in delineating tissue‐specific transcription prototypes by adapting conformation of certain genome regions.34
4. ANTIVIRAL POTENTIAL OF siRNAs
Short interfering RNAs can be used against all types of viral genomes, be it double‐ or single‐stranded DNA/RNA. Also, several siRNAs can be used concurrently to maintain an extended antiviral effect. The following examples (Table 1) further illustrate the development of siRNA therapeutics as a novel antiviral strategy.
Table 1.
Development of antiviral short interfering RNAs by various researchers against a range of pathogenic viruses
| S. No. | Virus Family | Genome Type | Reference |
|---|---|---|---|
| 1 | Papillomaviridae | DNA viruses | 16, 35, 36 |
| 2 | Polyomaviridae | 37, 38 | |
| 3 | Poxviridae | 39, 40, 41 | |
| 4 | Hepadnaviridae | 42, 43, 44 | |
| 5 | Herpesviridae | 45, 46, 47 | |
| 6 | Reoviridae | Double‐stranded RNA viruses | 48, 49 |
| 7 | Arenaviridae | Negative‐strand RNA viruses | 50, 51, 52 |
| 8 | Paramyxoviridae | 53, 54, 55 | |
| 9 | Rhabdoviridae | 56, 57, 58 | |
| 10 | Bunyavirales | 59, 60, 61 | |
| 11 | Filoviridae | 62, 63, 64 | |
| 12 | Orthomyxoviridae | 65, 66, 67 | |
| 13 | Picornaviridae | Positive‐strand RNA viruses | 68, 69, 70 |
| 14 | Togaviridae | 71, 72 | |
| 15 | Coronaviridae | 15, 73, 74 | |
| 16 | Flaviviridae | 75, 76, 77 | |
| 17 | Hepeviridae | 78, 79, 80 | |
| 18 | Retroviridae | 81, 82, 83, 84 |
4.1. DNA viruses
4.1.1. Papillomaviridae
This taxonomic group consists of nonenveloped DNA viruses. Infection by papillomaviruses can cause benign or cancerous tumors.85 Viral oncogenes of HPV types 16 and 18 can cause cervical cancer. To counteract this, siRNAs have been engaged against the antiapoptotic HPV E6 oncogene, resulting in selective and substantial cell death of HeLa cancer cells.86 Short interfering RNAs have also been used in several combinations against HPV‐16 and HPV‐18 targeting their E6 or E7 gene resulting in considerable reduction in viral replication in HeLa cell line. It is noteworthy to mention that the siRNAs did not show any harmful effect on control cells, which indicates meager off‐target effects.87 This illustrates that siRNA treatment has the potential to suppress the progression of cervical cancer.
4.1.2. Polyomaviridae
Polyomaviruses have a double‐stranded circular DNA genome.88 They are clinically relevant because they cause Merkel cell carcinoma, a very aggressive type of squamous cancer. Merkel cell polyomavirus T antigen (T‐Ag) protein is involved in replication and plays a major role in viral infection. Merkel cell polyomavirus activity can be restrained through rationally designed siRNA molecules for the treatment of Merkel cell carcinoma at the genome level.37 Similarly, the polyomavirus BK (BKV) has been found to induce malignant transformation. Repression of the T‐Ag oncogene has been shown to hinder the transformation of the cells. Short interfering RNAs designed to target the BKV T‐Ag were reported to restrain its expression in pRPc cell lines. Blocking of T‐Ag results in diminished growth rate of BKV‐transformed cells and thus suppresses tumorigenicity.38
4.1.3. Poxviridae
Poxviruses have a single, linear, double‐stranded DNA and infect both vertebrates and invertebrates. Vaccinia virus (VACV) is the quintessential member of the Poxviridae.89 The VACV produces a dsRNA binding protein, E3L, which hampers host defense mechanisms like interferon. E3L‐specific siRNAs inhibited virus replication in HeLa cells by 98% as compared with control infection.39 In addition, both early and late gene expressions of VACV could be blocked by siRNA treatment.90
4.1.4. Hepadnaviridae
Hepadnaviridae include enveloped viruses with a partially double‐stranded genome. The viruses of this family can cause liver infections in animals including humans.91 Hepatitis B virus is the most well‐known member of this group, as it is one of the leading causes of liver cirrhosis and hepatocellular carcinoma. Short interfering RNA developed against the surface antigen region significantly reduced the level of viral transcripts as well as the secretion of viral antigens in mice.92
4.1.5. Herpesviridae
This family includes important pathogenic viruses like Epstein‐Barr virus and herpes simplex virus. Epstein‐Barr virus is responsible for the maintenance of the tumor phenotype in many cancer types. It was found that EBNA1 is universally expressed in all Epstein‐Barr virus–associated tumors. Short interfering RNAs generated against the EBNA1 mRNA are able to inhibit its translation and thus block tumor survival in HeLa cells.93 Similarly, the herpes simplex virus glycoprotein E is responsible for cell‐to‐cell spread and immune evasion. Targeting glycoprotein E with siRNAs suppressed its expression and function in HaCaT cells.94
4.2. dsRNA viruses
4.2.1. Reoviridae
Reoviruses have a genome of about 10 segments of dsRNA. Plasmid‐based vectors expressing siRNAs targeting the μNS, μ2, and σNS genes of the T3D strain of reovirus considerably blocked multiple steps in the viral replication machinery in 293T cells.48 These studies further illustrate the usefulness of siRNAs both as therapeutic agents and as important means for the investigation of relationship between structure and function of viral proteins.
4.3. Negative‐strand RNA viruses
4.3.1. Arenaviridae
Arenaviruses contain a segmented RNA genome with 2 single‐stranded ambisense RNAs.95 Arenaviruses are often responsible for fatal hemorrhagic fever in people, and there is a lack of effective medications to tackle their infection. The lymphocytic choriomeningitis virus is a key model used in the investigation of arenavirus linked pathogenesis. Short interfering RNAs directed against L polymerase and Z viral mRNAs stall viral growth in HEK 293T host cells.50
4.3.2. Paramyxoviridae
Paramyxoviridae are negative‐sense single‐stranded RNA viruses.96 The RSV is an important member of this group. It causes lung infection in young and the aged people worldwide. Short interfering RNAs targeting the RSV P gene in a BALB/c mice model showed less lung pathogenesis or pulmonary inflammation and generated a strong antiviral reaction when later given RSV.97
4.3.3. Rhabdoviridae
Rabies virus (RV) is a member of family Rhabdoviridae and causes fatal zoonotic disease in both humans and animals. It is responsible for 50 000 to 55 000 human deaths annually in Asia and Africa.56 Brandao et al used siRNAs against rabies virus nucleoprotein mRNA, which decreased virus titers in BHK‐21 cells.57 Other workers developed siRNAs targeting rabies virus glycoprotein and nucleoprotein showing remarkable knockdown effects and significant inhibition of RV multiplication and release.56
4.3.4. Bunyavirales
This order of viruses, previously known as the Bunyaviridae family, includes enveloped, segmented negative‐stranded RNA viruses.98 Because of lack of effective vaccines and therapies for bunyaviruses in humans, siRNAs afford an attractive alternative. For example, the model Hazara virus of genus Nairovirus was inhibited by siRNAs targeting the NP gene in A549 cells.59 Similarly, siRNAs developed against the agnoprotein of John Cunningham virus, the etiological agent of the demyelinating progressive multifocal leukoencephalopathy disease, proved to be an effective inhibitor of John Cunningham virus infection in nude mice.99
4.3.5. Filoviridae
The Filoviridae family comprises filamentous single‐stranded negative‐sense RNA viruses such as Ebola and Marburg virus (MARV), which can cause severe disease in humans.100 Short interfering RNA targeting the Zaire ebolavirus nucleoprotein decreased viral titers after infection in 293T cells.62 Short interfering RNAs were used to degrade MARV nucleocapsid transcripts (NP, VP35, and VP30) in HeLa CCL‐2 cells. Down‐regulation of VP30 also caused a strong decline in the expression of other MARV proteins and virus release, suggesting the role of VP30 in viral transcription and replication.63
4.3.6. Orthomyxoviridae
Influenza virus causes one of the most common infections in humans. Although some antiviral drugs are available, their use is limited by possible emergence of resistant virus. Ge et al found that siRNAs pertaining to conserved regions of the INFV genome can inhibit its replication in C57BL/6 mice.101, 102
4.4. Positive‐strand RNA viruses
4.4.1. Picornaviridae
Picornaviruses are nonenveloped RNA viruses and have an icosahedral capsid. Coxsackievirus and poliovirus are two of the well‐known viruses in this group.103 Treatment with siRNAs significantly decreased cell death in parallel with a reduction in coxsackievirus B3 replication in HeLa cells. Also, the efficient coxsackievirus B3–specific siRNA displayed antiviral activity in other related enteroviruses such as CVB1, CVB5, CVB6, coxsackievirus A9, and Echo6 in HeLa cells.104
4.4.2. Togaviridae
This group of viruses contains linear, single‐stranded, positive‐sense RNA.105 One of its members, the Chikungunya virus, is the causative agent of chikungunya fever, which has emerged as an important arboviral infection of public health concern. Short interfering RNAs targeting the conserved segments of nsP3 and E1 mRNAs of the pathogen were able to drastically reduce the virus titer in Vero cells.71
4.4.3. Coronaviridae
Severe acute respiratory syndrome, also known as SARS‐CoV, is a member of the Coronaviridae family whose members have single‐stranded positive‐sense RNA genomes. Short interfering RNAs targeting the 3′ untranslated regions (UTRs) of the pathogen inhibit the replication of SARS‐CoV in Vero‐E6 cells.106 Li et al demonstrated that siRNAs used against SARS‐CoV provided relief from viral fever, decreased viral levels, and lower acute diffuse alveoli damage in macaques.107
4.4.4. Flaviviridae
This family encompasses spherical enveloped viruses with linear, single‐stranded positive RNA genome. It includes many important human viruses like HCV, Dengue virus (DENV), and WNV.108 Hepatitis C virus infection can cause permanent damage to the liver, hepatocellular carcinoma, and death. Seo et al targeted multiple segments of the 5′ UTR of the virus genome using siRNA and achieved up to 85% reduction in activity in Huh‐7 cells.109 In a similar approach, siRNAs were designed against WNV 3′ UTR and then expressed from a plasmid‐based system in Vero cells resulting in suppression of WNV replication in a sequence‐specific manner and also indicating the function of 3′ UTR in WNV pathogenesis.110 Dengue virus causes severe disease that threatens public health globally in tropical and subtropical places. Exogenously introduced siRNA directed against the conserved 5′ cyclization sequence segment of the DENV genome effectively decreased the viral titers of multiple DENV strains in mice illustrating the potential of siRNAs to tackle genetically varied dengue strains.111 Zika virus had recently posed as a global health threat prompting intense research to control the pathogen.112 Scientists have found that the conserved 3′ UTR of the virus genome plays a crucial function in its replication. Therefore, rationally designed siRNAs against the 3′ UTRs have been predicted to inhibit the pathogen.113, 114 Studies have also shown that siRNA‐directed silencing of host endoplasmic reticulum membrane complex protein components halted the replication of multiple Zika virus strains in HeLa cells.115
4.4.5. Hepeviridae
Hepeviridae mainly includes hepatitis E virus, a key source of water‐borne hepatitis in adults that has particularly high mortality in pregnant women. Short interfering RNAs developed against the helicase and replicase genes of hepatitis E virus were found to be effective in inhibiting virus replication in A549 as well as HepG2 cells.78, 79
4.4.6. Retroviridae
Retroviridae is a family of enveloped single‐stranded RNA viruses that replicate in a host cell through the process of reverse transcription.116 It has been noted that siRNAs can inhibit HIV‐1 growth by aiming the genes for the host CD4 cell receptor, or the viral Gag and Nef proteins in Magi‐CCR5, HeLa‐CD4, and H9 T cells. Studies report that siRNA molecules efficiently hinder preintegration as well as postintegration infection phases in the HIV life cycle.117
5. CLINICAL TRIALS
Because of the large therapeutic potential offered by siRNAs against the pathogenic viruses, many of them are being considered for medical use in the near future. A few siRNA‐based antivirals have already entered clinical trials, eg, ALN‐RSV01 for RSV targeting its nucleocapsid gene (phase II),118 NucB1000 directed against 4 different targets (Pre‐C, Pre‐S1, Pre‐S2, and X) of HBV (phase I),119 SPC3649 directed against miR‐122 for HCV (phase II),120 pHIV7‐shI‐TAR‐CCR5RZ targeting multiple genes (Tat, Tar, and CCR5) for HIV (phase I),121 and TKM‐Ebola directed against multiple transcripts (L, VP24, and VP35) of EBOV (phase I).122
RNAi therapy has also been proved to be useful against animal viruses such as Marek's disease virus in chicken,123 foot and mouth disease virus in pigs,124 and O'nyong‐nyong virus in mosquitoes.125 The significance of RNAi in the treatment of viral infections was further reviewed.20, 126 Figure 4 briefly summarizes the role of gene silencing in combating pathogenic viruses.
Figure 4.
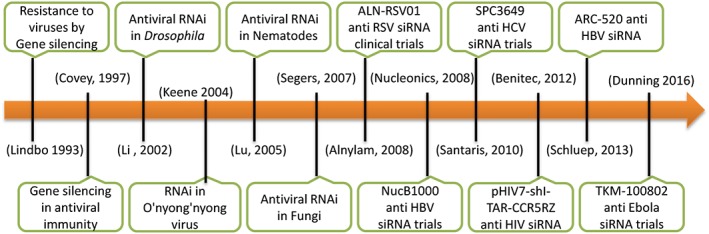
Timeline summarizing different stages in the course of development of RNA interference (RNAi)–based therapeutics against viruses. HBV, hepatitis B virus; HCV, hepatitis C virus; siRNA, short interfering RNA
6. DESIGN
Designing siRNAs with high antiviral activity is a challenging task. There are many features that influence siRNA efficacy including GC (guanine‐cytosine) content, nucleotides at siRNA termini, thermodynamic properties, siRNA structure, and accessibility of the target site.127, 128 Also, the siRNAs should be developed against conserved target sites to prevent viral escape due to mutations.129 It has been suggested that a single target site might not be enough for durable viral silencing so using multiple siRNAs against several target sites can also prevent viral escape.19 In addition, chemically modified nucleotides can also be used to enhance the stability or reduce off‐target effects of siRNAs,130, 131, 132 eg, modified siRNAs used against HBV infection were found to have significantly increased half‐life and activity in human serum as compared with the unmodified siRNAs.133
7. DELIVERY
Unaided siRNAs are not capable to penetrate the cellular membrane due to their negative charge. Besides, siRNAs have been shown to elicit immune response and are vulnerable to nuclease enzymes. Thus, suitable delivery agents are required to efficiently transport the siRNAs to the target cells. Delivery of siRNAs to the desired cells can lessen the amount of siRNA necessary for silencing and also circumvent off‐target effects.20
Short interfering RNAs are delivered in a number of ways, eg, by encapsulation in synthetic vehicles such as cationic liposomes/nanoparticles or siRNAs conjugated to cell penetrating peptides or specific antibodies against the infected cells.134 Liposomes are frequently used as delivery mediums for a wide range of drugs including siRNAs. This method often involves cationic lipids to overcome the negative charge associated with siRNAs.135 Lately, stable nucleic acid lipid particles have been used to efficiently stabilize and transport siRNAs.136 Morrissey et al used stable nucleic acid lipid particles to deliver siRNAs against HBV, which efficiently inhibited the virus in mice.137 Next, the polymer‐based siRNA delivery vehicles (polyplexes) afford high structural and physicochemical flexibility while shielding the siRNAs against nucleases.138 Likewise, inorganic nanoparticles generated from calcium phosphate, gold, carbon, and iron oxides are sometimes preferred to transport siRNAs due to their small size and increased permeability in comparison with liposomes and polyplexes. Occasionally, surface ligands are incorporated with the nanoparticles to enhance selective targeting of the diseased cells.139 Plasmid and viral vectors are also used as expression cassettes for sustained silencing effect. However, use of plasmids limits the delivery efficiency as compared with the viral vectors.140, 141 Alternatively, direct injection into the infected tissue may also help in targeting the specific cells.134
8. BIOINFORMATICS RESOURCES
Although a large number of databases, design, and prediction algorithms are available for mammalian siRNAs, very few bioinformatics resources have been developed so far regarding viral siRNAs despite their huge potential and data availability. The viral siRNA database, available at http://crdd.osdd.net/servers/virsirnadb, covers the details of siRNAs targeting 42 important human viruses. The database provides not only detailed information about siRNA sequence, target virus and gene, cell line, assay, and inhibition but also useful siRNA analysis tools including siTarAlign that aligns the siRNA sequence with genome sequences of representative viruses.142 On similar lines, the HIV siRNA database, available at http://crdd.osdd.net/raghava/hivsir, furnishes details of experimentally tested siRNAs/short hairpin RNAs aiming diverse HIV genome segments. Further, the “HivsirMut” subdatabase, accessible at http://crdd.osdd.net/raghava/hivsir/hiv-esc-seq.php, provides information of escape mutations together with nucleotide mismatch amid the target genes and the siRNA molecules and their effect on siRNA efficacy.143
As all siRNAs developed to inhibit a certain gene are not equally successful, a variety of wide‐ranging siRNA design rules and prediction methods have been published. The most basic procedures for siRNA design were grounded on frequency of certain nucleotides at multiple locations in siRNA sequence as anticipated by Elbashir et al,144 Reynolds et al,145 Ui‐Tei et al,146 Amarzguioui et al,147 and Jagla et al.148 siVirus web server, accessible at http://sivirus.rnai.jp, makes use of these guidelines to select effective siRNAs against viruses. siVirus provides siRNA sequences directed against conserved regions of viruses like HIV, HCV, INFV, and SARS‐CoV with minimum off‐target effects.149 Although many machine learning techniques like boosted genetic programming,150 artificial neural network,151 and support vector machine152 have been used to design mammalian siRNAs, however, their performances were not satisfactory for viral siRNAs. This may be due to the fact that these methods were not trained on viral siRNAs. Viral siRNA predictor, available at http://crdd.osdd.net/servers/virsirnapred, the original algorithm for calculating inhibition potential of viral siRNAs, is a support vector machine–based method for predicting the activity of viral siRNA. This algorithm was developed using published viral siRNAs using many features such as nucleotide frequency, thermodynamic factors, and nucleotide location to predict the competence of siRNAs targeting pathogenic viruses.153 These online resources (Table 2) will facilitate the researchers in selecting or designing efficient siRNAs for antiviral therapeutic development.
Table 2.
Bioinformatics resources dedicated to the analysis, prediction, and archival of antiviral short interfering RNAs (siRNAs)
| S. No. | Resource | Description | URL | Reference |
|---|---|---|---|---|
| 1 | siVirus | Antiviral siRNA design | http://sivirus.rnai.jp/ | 149 |
| 2 | HIVsirDB | HIV siRNA database | http://crdd.osdd.net/raghava/hivsir | 143 |
| 3 | VIRsiRNAdb | Viral siRNA database | http://crdd.osdd.net/servers/virsirnadb/ | 142 |
| 4 | VIRsiRNApred | Viral siRNA prediction | http://crdd.osdd.net/servers/virsirnapred/ | 153 |
9. LIMITATIONS AND FUTURE IMPLICATIONS
Short interfering RNA–mediated gene silencing has surfaced as a potent strategy to study cellular networks as well as to precisely knockdown the disease causing factors. However, siRNAs are confronted by a few shortcomings like virus escape, inefficient cellular uptake, poor stability, off‐target effects, and immunostimulation.154, 155, 156 Virus escape can be overcome by targeting conserved viral genes149 or those factors involved in negative‐feedback regulation.3 Cellular uptake can be improved by using synthetic nanoparticles composed of polymers, lipids, and conjugates and also by incorporating cell‐specific targeting ligands in the carriers.140 As far as siRNA stability is concerned, chemical modifications like the 2′‐fluoro and thioate linkages may be used to prolong the half‐life of siRNAs.157 Also, detailed identification of the cellular pathways of immunorecognition of RNA can allow the development of methods to avoid immunostimulatory oligonucleotide motifs during siRNA design.158 Also, multiple siRNA expression vectors may be used to maximize the long‐term inhibition.159 It would also be helpful to make use of bioinformatics approaches to identify potential target sites as well as to design siRNAs with optimum features for preliminary experiments.160
CONFLICT OF INTEREST
The authors have no competing interest.
ACKNOWLEDGEMENTS
Authors are thankful to Biomedical Informatics Center, Sher‐i‐Kashmir Institute of Medical Sciences (SKIMS), and the Indian Council of Medical Research (ICMR) project entitled “Second Phase of Biomedical Informatics Centre” for supporting the work.
Qureshi A, Tantray VG, Kirmani AR, Ahangar AG. A review on current status of antiviral siRNA . Rev Med Virol. 2018;28:e1976 10.1002/rmv.1976
REFERENCES
- 1. Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet. 2008;9:267‐276. 10.1038/nrg2323 [DOI] [PubMed] [Google Scholar]
- 2. Sanjuan R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. J Virol. 2010;84:9733‐9748. 10.1128/JVI.00694-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonard JN, Schaffer DV. Antiviral RNAi therapy: emerging approaches for hitting a moving target. Gene Ther. 2006;13:532‐540. 10.1038/sj.gt.3302645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haasnoot J, Berkhout B. Nucleic acids‐based therapeutics in the battle against pathogenic viruses. Handb Exp Pharmacol. 2009;243‐263. 10.1007/978-3-540-79086-0_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filipowicz W. RNAi: the nuts and bolts of the RISC machine. Cell. 2005;122:17‐20. 10.1016/j.cell.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 6. Woessmann W, Damm‐Welk C, Fuchs U, Borkhardt A. RNA interference: new mechanisms for targeted treatment? Rev Clin Exp Hematol. 2003;7:270‐291. [PubMed] [Google Scholar]
- 7. Monga I, Qureshi A, Thakur N, Gupta AK, Kumar M. ASPsiRNA: a resource of ASP‐siRNAs having therapeutic potential for human genetic disorders and algorithm for prediction of their inhibitory efficacy. G3 (Bethesda). 2017;7:2931‐2943. 10.1534/g3.117.044024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thakur A, Qureshi A, Kumar M. vhfRNAi: a web‐platform for analysis of host genes involved in viral infections discovered by genome wide RNAi screens. Mol Biosyst. 2017;13:1377‐1387. 10.1039/c6mb00841k [DOI] [PubMed] [Google Scholar]
- 9. Singh SK. RNA interference and its therapeutic potential against HIV infection. Expert Opin Biol Ther. 2008;8:449‐461. 10.1517/14712598.8.4.449 [DOI] [PubMed] [Google Scholar]
- 10. Rossi JJ. RNAi as a treatment for HIV‐1 infection. Biotechniques. 2006. Suppl: 25‐29. [DOI] [PubMed] [Google Scholar]
- 11. Ge Q, McManus MT, Nguyen T, et al. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc Natl Acad Sci U S A. 2003;100:2718‐2723. 10.1073/pnas.0437841100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakazawa M, Kadowaki SE, Watanabe I, Kadowaki Y, Takei M, Fukuda H. PA subunit of RNA polymerase as a promising target for anti‐influenza virus agents. Antiviral Res. 2008;78:194‐201. 10.1016/j.antiviral.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 13. Konishi M, Wu CH, Wu GY. Inhibition of HBV replication by siRNA in a stable HBV‐producing cell line. Hepatology. 2003;38:842‐850. 10.1053/jhep.2003.50416 [DOI] [PubMed] [Google Scholar]
- 14. Shi Y, Yang DH, Xiong J, Jia J, Huang B, Jin YX. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. 2005;15:193‐200. 10.1038/sj.cr.7290286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng B, Lui YW, Meng S, Cao C, Hu Y. Identification of effective siRNA blocking the expression of SARS viral envelope E and RDRP genes. Mol Biotechnol. 2006;33:141‐148. 10.1385/MB:33:2:141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bousarghin L, Touze A, Gaud G, et al. Inhibition of cervical cancer cell growth by human papillomavirus virus‐like particles packaged with human papillomavirus oncoprotein short hairpin RNAs. Mol Cancer Ther. 2009;8:357‐365. 10.1158/1535-7163.MCT-08-0626 [DOI] [PubMed] [Google Scholar]
- 17. Kumar P, Lee SK, Shankar P, Manjunath N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006;3:e96. 10.1371/journal.pmed.0030096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Mahato RI. siRNA pool targeting different sites of human hepatitis B surface antigen efficiently inhibits HBV infection. J Drug Target. 2008;16:140‐148. 10.1080/10611860701878750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ter Brake O, t Hooft K, Liu YP, Centlivre M, von Eije KJ, Berkhout B. Lentiviral vector design for multiple shRNA expression and durable HIV‐1 inhibition. Mol Ther. 2008;16:557‐564. 10.1038/sj.mt.6300382 [DOI] [PubMed] [Google Scholar]
- 20. Shah PS, Schaffer DV. Antiviral RNAi: translating science towards therapeutic success. Pharm Res. 2011;28:2966‐2982. 10.1007/s11095-011-0549-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhong P, Agosto LM, Munro JB, Mothes W. Cell‐to‐cell transmission of viruses. Curr Opin Virol. 2013;3:44‐50. 10.1016/j.coviro.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker TS, Olson NH, Fuller SD. Adding the third dimension to virus life cycles: three‐dimensional reconstruction of icosahedral viruses from cryo‐electron micrographs. Microbiol Mol Biol Rev. 1999;63:862‐922. table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim CW, Chang KM. Hepatitis C virus: virology and life cycle. Clin Mol Hepatol. 2013;19:17‐25. 10.3350/cmh.2013.19.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217‐239. 10.1146/annurev-biophys-083012-130404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature. 1998;391:806‐811. 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- 26. Zamore PD, Haley B. Ribo‐gnome: the big world of small RNAs. Science. 2005;309:1519‐1524. 10.1126/science.1111444 [DOI] [PubMed] [Google Scholar]
- 27. Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550‐553. 10.1126/science.1068999 [DOI] [PubMed] [Google Scholar]
- 28. Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118‐126. 10.1016/j.tcb.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 29. Kawasaki H, Taira K. MicroRNA‐196 inhibits HOXB8 expression in myeloid differentiation of HL60 cells. Nucleic Acids Symp Ser (Oxf). 2004;211‐212. 10.1093/nass/48.1.211 [DOI] [PubMed] [Google Scholar]
- 30. Couzin J. Breakthrough of the year. Small RNAs make big splash. Science. 2002;298:2296‐2297. 10.1126/science.298.5602.2296 [DOI] [PubMed] [Google Scholar]
- 31. Gitlin L, Andino R. Nucleic acid‐based immune system: the antiviral potential of mammalian RNA silencing. J Virol. 2003;77:7159‐7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gitlin L, Karelsky S, Andino R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature. 2002;418:430‐434. 10.1038/nature00873 [DOI] [PubMed] [Google Scholar]
- 33. Lecellier CH, Dunoyer P, Arar K, et al. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557‐560. 10.1126/science.1108784 [DOI] [PubMed] [Google Scholar]
- 34. Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364‐370. 10.1038/nature02875 [DOI] [PubMed] [Google Scholar]
- 35. Jonson AL, Rogers LM, Ramakrishnan S, Downs LS Jr. Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention in a mouse model of cervical cancer. Gynecol Oncol. 2008;111:356‐364. 10.1016/j.ygyno.2008.06.033 [DOI] [PubMed] [Google Scholar]
- 36. Kuner R, Vogt M, Sultmann H, et al. Identification of cellular targets for the human papillomavirus E6 and E7 oncogenes by RNA interference and transcriptome analyses. J Mol Med (Berl). 2007;85:1253‐1262. 10.1007/s00109-007-0230-1 [DOI] [PubMed] [Google Scholar]
- 37. Hoque KM, Azim MF, Mia MR, et al. Design of potential siRNA molecules for T antigen gene silencing of Merkel cell polyomavirus. Bioinformation. 2012;8:924‐930. 10.6026/97320630008924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabbioni S, Callegari E, Spizzo R, et al. Anticancer activity of an adenoviral vector expressing short hairpin RNA against BK virus T‐ag. Cancer Gene Ther. 2007;14:297‐305. 10.1038/sj.cgt.7701014 [DOI] [PubMed] [Google Scholar]
- 39. Dave RS, McGettigan JP, Qureshi T, Schnell MJ, Nunnari G, Pomerantz RJ. siRNA targeting vaccinia virus double‐stranded RNA binding protein [E3L] exerts potent antiviral effects. Virology. 2006;348:489‐497. 10.1016/jvirol200601.013 [DOI] [PubMed] [Google Scholar]
- 40. Vigne S, Duraffour S, Andrei G, Snoeck R, Garin D, Crance JM. Inhibition of vaccinia virus replication by two small interfering RNAs targeting B1R and G7L genes and their synergistic combination with cidofovir. Antimicrob Agents Chemother. 2009;53:2579‐2588. 10.1128/AAC.01626-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vigne S, Germi R, Duraffour S, et al. Specific inhibition of orthopoxvirus replication by a small interfering RNA targeting the D5R gene. Antivir Ther. 2008;13:357‐368. [PubMed] [Google Scholar]
- 42. He Y, Jiang Y, Wang G, et al. Inhibition of HBV‐DNA replication and expression by siRNA based on magnetic nanoparticles transferring in HepG2 2.2.15 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:543‐548. 10.3969/j.issn.1672-7347.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 43. Jenke AC, Wilhelm AD, Orth V, Lipps HJ, Protzer U, Wirth S. Long‐term suppression of hepatitis B virus replication by short hairpin RNA expression using the scaffold/matrix attachment region‐based replicating vector system pEPI‐1. Antimicrob Agents Chemother. 2008;52:2355‐2359. 10.1128/AAC.00067-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang J, Feng SS, Wang S, Chen ZY. Evaluation of cationic nanoparticles of biodegradable copolymers as siRNA delivery system for hepatitis B treatment. Int J Pharm. 2010;400:194‐200. S0378‐5173(10)00656‐3. 10.1016/j.ijpharm.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 45. Oh ST, Kim M, Lee SK. Maintenance of the viral episome is essential for the cell survival of an Epstein‐Barr virus positive gastric carcinoma cell line. Arch Pharm Res. 2009;32:729‐736. 10.1007/s12272-009-1512-7 [DOI] [PubMed] [Google Scholar]
- 46. Zhen Z, Bradel‐Tretheway B, Sumagin S, Bidlack JM, Dewhurst S. The human herpesvirus 6 G protein‐coupled receptor homolog U51 positively regulates virus replication and enhances cell‐cell fusion in vitro. J Virol. 2005;79:11914‐11924. 10.1128/JVI791811914-11924.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoon JS, Kim SH, Shin MC, et al. Inhibition of herpesvirus‐6B RNA replication by short interference RNAs. J Biochem Mol Biol. 2004;37:383‐385. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi T, Chappell JD, Danthi P, Dermody TS. Gene‐specific inhibition of reovirus replication by RNA interference. J Virol. 2006;80:9053‐9063. 10.1128/JVI.00276-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez T, Rojas M, Ayala‐Breton C, Lopez S, Arias CF. Reduced expression of the rotavirus NSP5 gene has a pleiotropic effect on virus replication. J Gen Virol. 2005;86:1609‐1617. 10.1099/Vir0.80827-80820 [DOI] [PubMed] [Google Scholar]
- 50. Sanchez AB, Perez M, Cornu T, de la Torre JC. RNA interference‐mediated virus clearance from cells both acutely and chronically infected with the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2005;79:11071‐11081. 10.1128/JVI791711071-11081.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muller S, Gunther S. Broad‐spectrum antiviral activity of small interfering RNA targeting the conserved RNA termini of Lassa virus. Antimicrob Agents Chemother. 2007;51:2215‐2218. 10.1128/AAC.01368-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Artuso MC, Ellenberg PC, Scolaro LA, Damonte EB, Garcia CC. Inhibition of Junin virus replication by small interfering RNAs. Antiviral Res. 2009;84:31‐37. 10.1016/j.antiviral.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang W, Yang H, Kong X, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56‐62. 10.1038/nm1174 [DOI] [PubMed] [Google Scholar]
- 54. Otaki M, Sada K, Kadoya H, Nagano‐Fujii M, Hotta H. Inhibition of measles virus and subacute sclerosing panencephalitis virus by RNA interference. Antiviral Res. 2006;70:105‐111. 10.1016/j.antiviral.2006.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deffrasnes C, Cavanagh MH, Goyette N, et al. Inhibition of human metapneumovirus replication by small interfering RNA. Antivir Ther. 2008;13:821‐832. [PubMed] [Google Scholar]
- 56. Meshram CD, Singh NK, Sonwane AA, et al. Evaluation of single and dual siRNAs targeting rabies virus glycoprotein and nucleoprotein genes for inhibition of virus multiplication in vitro. Arch Virol. 2013;158:2323‐2332. 10.1007/s00705-013-1738-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brandao PE, Castilho JG, Fahl W, et al. Short‐interfering RNAs as antivirals against rabies. Braz J Infect Dis. 2007;11:224‐225. [DOI] [PubMed] [Google Scholar]
- 58. Sonwane AA, Dahiya SS, Saini M, Chaturvedi VK, Singh RP, Gupta PK. Inhibition of rabies virus multiplication by siRNA delivered through adenoviral vector in vitro in BHK‐21 cells and in vivo in mice. Res Vet Sci. 2012;93:498‐503. 10.1016/j.rvsc.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 59. Flusin O, Vigne S, Peyrefitte CN, Bouloy M, Crance JM, Iseni F. Inhibition of Hazara nairovirus replication by small interfering RNAs and their combination with ribavirin. Virol J. 2011;8:249 10.1186/1743-422X-8-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soldan SS, Plassmeyer ML, Matukonis MK, Gonzalez‐Scarano F. La Crosse virus nonstructural protein NSs counteracts the effects of short interfering RNA. J Virol. 2005;79:234‐244. 10.1128/JVI791234-244.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Radhakrishnan S, Gordon J, Del Valle L, Cui J, Khalili K. Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection. J Virol. 2004;78:7264‐7269. 10.1128/JVI.78.13.7264-7269.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Groseth A, Hoenen T, Alimonti JB, et al. In vitro evaluation of antisense RNA efficacy against filovirus infection, by use of reverse genetics. J Infect Dis. 2007;196 Suppl 2: S382‐389. 10.1086/520604 [DOI] [PubMed] [Google Scholar]
- 63. Fowler T, Bamberg S, Moller P, et al. Inhibition of Marburg virus protein expression and viral release by RNA interference. J Gen Virol. 2005;86:1181‐1188. 10.1099/Vir0.80622-80620 [DOI] [PubMed] [Google Scholar]
- 64. Thi EP, Mire CE, Lee AC, et al. Lipid nanoparticle siRNA treatment of Ebola‐virus‐Makona‐infected nonhuman primates. Nature. 2015;521:362‐365. 10.1038/nature14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou H, Jin M, Yu Z, et al. Effective small interfering RNAs targeting matrix and nucleocapsid protein gene inhibit influenza A virus replication in cells and mice. Antiviral Res. 2007;76:186‐193. 10.1016/jantiviral200707.002 [DOI] [PubMed] [Google Scholar]
- 66. Suzuki H, Saitoh H, Suzuki T, Takaku H. Baculovirus‐mediated bispecific short‐hairpin small‐interfering RNAs have remarkable ability to cope with both influenza viruses A and B. Oligonucleotides. 2009;19:307‐316. 10.1089/oli.2009.0189 [DOI] [PubMed] [Google Scholar]
- 67. Cheng C, Yao L, Chen A, et al. Inhibitory effect of small interfering RNA specific for a novel candidate target in PB1 gene of influenza A virus. J Drug Target. 2009;17:133‐139. 10.1080/10611860802473048 [DOI] [PubMed] [Google Scholar]
- 68. Tan EL, Tan TM, Tak Kwong Chow V, Poh CL. Inhibition of enterovirus 71 in virus‐infected mice by RNA interference. Mol Ther. 2007;15:1931‐1938. 10.1038/sj.mt.6300287 [DOI] [PubMed] [Google Scholar]
- 69. Lu WW, Hsu YY, Yang JY, Kung SH. Selective inhibition of enterovirus 71 replication by short hairpin RNAs. Biochem Biophys Res Commun. 2004;325:494‐499. 10.1016/j.bbrc.2004.10.062 [DOI] [PubMed] [Google Scholar]
- 70. Saulnier A, Pelletier I, Labadie K, Colbere‐Garapin F. Complete cure of persistent virus infections by antiviral siRNAs. Mol Ther. 2006;13:142‐150. 10.1016/j.ymthe.2005.07.697 [DOI] [PubMed] [Google Scholar]
- 71. Dash PK, Tiwari M, Santhosh SR, Parida M, Lakshmana Rao PV. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem Biophys Res Commun. 2008;376:718‐722. 10.1016/j.bbrc.2008.09.040 [DOI] [PubMed] [Google Scholar]
- 72. Siu RW, Fragkoudis R, Simmonds P, et al. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: characterization, origin, and frequency‐dependent functions of virus‐derived small interfering RNAs. J Virol. 2011;85:2907‐2917. 10.1128/JVI.02052-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y, Li T, Fu L, et al. Silencing SARS‐CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141‐146. 10.1016/S0014-5793(04)00087-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74. Wang Z, Ren L, Zhao X, et al. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J Virol. 2004;78:7523‐7527. 10.1128/JVI.78.14.7523-7527.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Subramanya S, Kim SS, Abraham S, et al. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J Virol. 2010;84:2490‐2501. 10.1128/JVI.02105-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ye C, Abraham S, Wu H, Shankar P, Manjunath N. Silencing early viral replication in macrophages and dendritic cells effectively suppresses flavivirus encephalitis. PLoS One. 2011;6:e17889 10.1371/journal.pone.0017889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bai F, Wang T, Pal U, Bao F, Gould LH, Fikrig E. Use of RNA interference to prevent lethal murine West Nile virus infection. J Infect Dis. 2005;191:1148‐1154. 10.1086/428507 [DOI] [PubMed] [Google Scholar]
- 78. Kumar A, Panda SK, Durgapal H, Acharya SK, Rehman S, Kar UK. Inhibition of hepatitis E virus replication using short hairpin RNA (shRNA). Antiviral Res. 2010;85:541‐550. 10.1016/j.antiviral.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 79. Huang F, Hua X, Yang S, Yuan C, Zhang W. Effective inhibition of hepatitis E virus replication in A549 cells and piglets by RNA interference (RNAi) targeting RNA‐dependent RNA polymerase. Antiviral Res. 2009;83:274‐281. 10.1016/j.antiviral.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang F, Zhou J, Yang Z, et al. RNA interference inhibits hepatitis E virus mRNA accumulation and protein synthesis in vitro. Vet Microbiol. 2010;142:261‐267. 10.1016/jvetmic200910.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Naito Y, Nohtomi K, Onogi T, et al. Optimal design and validation of antiviral siRNA for targeting HIV‐1. Retrovirology. 2007;4:80 10.1186/1742-4690-4-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bobbin ML, Burnett JC, Rossi JJ. RNA interference approaches for treatment of HIV‐1 infection. Genome Med. 2015;7:50 10.1186/s13073-015-0174-y174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu C, Liang Z, Kong X. Efficacy analysis of combinatorial siRNAs against HIV derived from one double hairpin RNA precursor. Front Microbiol. 2017;8:1651 10.3389/fmicb.2017.01651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chang LJ, Liu X, He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 2005;12:1133‐1144. 10.1038/sj.gt.3302509 [DOI] [PubMed] [Google Scholar]
- 85. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17‐27. 10.1016/j.virol.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 86. Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe‐Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus‐positive cancer cells. Oncogene. 2003;22:5938‐5945. 10.1038/sj.onc.1206894 [DOI] [PubMed] [Google Scholar]
- 87. Chang JT, Kuo TF, Chen YJ, et al. Highly potent and specific siRNAs against E6 or E7 genes of HPV16‐ or HPV18‐infected cervical cancers. Cancer Gene Ther. 2010;17:827‐836. 10.1038/cgt2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Johne R, Buck CB, Allander T, et al. Taxonomical developments in the family Polyomaviridae. Arch Virol. 2011;156:1627‐1634. 10.1007/s00705-011-1008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bahar MW, Graham SC, Stuart DI, Grimes JM. Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13. Structure. 2011;19:1011‐1020. 10.1016/j.str.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lantermann M, Schwantes A, Sliva K, Sutter G, Schnierle BS. Vaccinia virus double‐stranded RNA‐binding protein E3 does not interfere with siRNA‐mediated gene silencing in mammalian cells. Virus Res. 2007;126:1‐8. 10.1016/j.virusres.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 91. Robertson BH, Margolis HS. Primate hepatitis B viruses—genetic diversity, geography and evolution. Rev Med Virol. 2002;12:133‐141. 10.1002/rmv.348 [DOI] [PubMed] [Google Scholar]
- 92. Giladi H, Ketzinel‐Gilad M, Rivkin L, Felig Y, Nussbaum O, Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol Ther. 2003;8:769‐776. [DOI] [PubMed] [Google Scholar]
- 93. Yin Q, Flemington EK. siRNAs against the Epstein Barr virus latency replication factor, EBNA1, inhibit its function and growth of EBV‐dependent tumor cells. Virology. 2006;346:385‐393. 10.1016/j.virol.2005.11.021 [DOI] [PubMed] [Google Scholar]
- 94. Bhuyan PK, Kariko K, Capodici J, et al. Short interfering RNA‐mediated inhibition of herpes simplex virus type 1 gene expression and function during infection of human keratinocytes. J Virol. 2004;78:10276‐10281. 10.1128/JVI.78.19.10276-10281.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Anon MC, Grau O, Segovia ZM, Franzefernandez MT. RNA composition of Junin virus. J Virol. 1976;18:833‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Franke J, Essbauer S, Ahne W, Blahak S. Identification and molecular characterization of 18 paramyxoviruses isolated from snakes. Virus Res. 2001;80:67‐74. [DOI] [PubMed] [Google Scholar]
- 97. Zhang W, Tripp RA. RNA interference inhibits respiratory syncytial virus replication and disease pathogenesis without inhibiting priming of the memory immune response. J Virol. 2008;82:12221‐12231. 10.1128/JVI.01557-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Elliott RM. Emerging viruses: the Bunyaviridae. Mol Med. 1997;3:572‐577. [PMC free article] [PubMed] [Google Scholar]
- 99. Matoba T, Orba Y, Suzuki T, et al. An siRNA against JC virus (JCV) agnoprotein inhibits JCV infection in JCV‐producing cells inoculated in nude mice. Neuropathology. 2008;28:286‐294. 10.1111/j.1440-1789.2007.00878.x [DOI] [PubMed] [Google Scholar]
- 100. Kiley MP, Bowen ET, Eddy GA, et al. Filoviridae: a taxonomic home for Marburg and Ebola viruses? Intervirology. 1982;18:24‐32. [DOI] [PubMed] [Google Scholar]
- 101. Ge Q, Eisen HN, Chen J. Use of siRNAs to prevent and treat influenza virus infection. Virus Res. 2004;102:37‐42. 10.1016/j.virusres.2004.01.013 [DOI] [PubMed] [Google Scholar]
- 102. Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus‐infected mice by RNA interference. Proc Natl Acad Sci U S A. 2004;101:8676‐8681. 10.1073/pnas.0402486101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Santti J, Vainionpaa R, Hyypia T. Molecular detection and typing of human picornaviruses. Virus Res. 1999;62:177‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahn J, Jun ES, Lee HS, et al. A small interfering RNA targeting coxsackievirus B3 protects permissive HeLa cells from viral challenge. J Virol. 2005;79:8620‐8624. 10.1128/JVI79138620-8624.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sidwell RW, Smee DF. Viruses of the Bunya‐ and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101‐111. [DOI] [PubMed] [Google Scholar]
- 106. Wu CJ, Huang HW, Liu CY, Hong CF, Chan YL. Inhibition of SARS‐CoV replication by siRNA. Antiviral Res. 2005;65:45‐48. 10.1016/j.antiviral.2004.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li BJ, Tang Q, Cheng D, et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11:944‐951. 10.1038/nm1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Paula T, Pablo R, Eugenia V, et al. New drug targets for hepatitis C and other Flaviviridae viruses. Infect Disord Drug Targets. 2009;9:133‐147. [DOI] [PubMed] [Google Scholar]
- 109. Seo MY, Abrignani S, Houghton M, Han JH. Small interfering RNA‐mediated inhibition of hepatitis C virus replication in the human hepatoma cell line Huh‐7. J Virol. 2003;77:810‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Anthony KG, Bai F, Krishnan MN, Fikrig E, Koski RA. Effective siRNA targeting of the 3′ untranslated region of the West Nile virus genome. Antiviral Res. 2009;82:166‐168. 10.1016/j.antiviral.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 111. Stein DA, Perry ST, Buck MD, et al. Inhibition of dengue virus infections in cell cultures and in AG129 mice by a small interfering RNA targeting a highly conserved sequence. J Virol. 2011;85:10154‐10166. 10.1128/JVI.05298-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017;390:2099‐2109. 10.1016/S0140-6736(17)31450-2 [DOI] [PubMed] [Google Scholar]
- 113. Hashem M, Shuvo MA. A computational approach to design potential antiviral RNA for 3'UTR post transcriptional gene silencing of different strains of Zika virus. J Young Pharm. 2017;9. [Google Scholar]
- 114. Gupta AK, Kaur K, Rajput A, et al. ZikaVR: an integrated Zika virus resource for genomics, proteomics, phylogenetic and therapeutic analysis. Sci Rep. 2016;6:32713 10.1038/srep32713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Savidis G, McDougall WM, Meraner P, et al. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep. 2016;16:232‐246. 10.1016/jcelrep201606.028 [DOI] [PubMed] [Google Scholar]
- 116. Kim FJ, Battini JL, Manel N, Sitbon M. Emergence of vertebrate retroviruses and envelope capture. Virology. 2004;318:183‐191. 10.1016/j.virol.2003.09.026 [DOI] [PubMed] [Google Scholar]
- 117. Novina CD, Murray MF, Dykxhoorn DM, et al. siRNA‐directed inhibition of HIV‐1 infection. Nat Med. 2002;8:681‐686. 10.1038/nm725 [DOI] [PubMed] [Google Scholar]
- 118. DeVincenzo J, Lambkin‐Williams R, Wilkinson T, et al. A randomized, double‐blind, placebo‐controlled study of an RNAi‐based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107:8800‐8805. 10.1073/pnas.0912186107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gish RG, Satishchandran C, Young M, Pachuk C. RNA interference and its potential applications to chronic HBV treatment: results of a phase I safety and tolerability study. Antivir Ther. 2011;16:547‐554. 10.3851/IMP1798 [DOI] [PubMed] [Google Scholar]
- 120. Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR‐122. Nucleic Acids Res. 2014;42:609‐621. 10.1093/nar/gkt852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Davidson BL, McCray PB Jr. Current prospects for RNA interference‐based therapies. Nat Rev Genet. 2011;12:329‐340. 10.1038/nrg2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Choi JH, Croyle MA. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs. 2013;27:565‐583. 10.1007/s40259-013-0046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lambeth LS, Zhao Y, Smith LP, Kgosana L, Nair V. Targeting Marek's disease virus by RNA interference delivered from a herpesvirus vaccine. Vaccine. 2009;27:298‐306. 10.1016/j.vaccine.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 124. Chen W, Liu M, Jiao Y, et al. Adenovirus‐mediated RNA interference against foot‐and‐mouth disease virus infection both in vitro and in vivo. J Virol. 2006;80:3559‐3566. 10.1128/JVI8073559-3566.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Keene KM, Foy BD, Sanchez‐Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong‐nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:17240‐17245. 10.1073/pnas.0406983101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Umbach JL, Cullen BR. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 2009;23:1151‐1164. 10.1101/gad.1793309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kurreck J. siRNA efficiency: structure or sequence‐that is the question. J Biomed Biotechnol. 2006; 2006;83757 10.1155/JBB/2006/83757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang X, Varma RK, Beauchamp L, Magdaleno S, Sendera TJ. Selection of hyperfunctional siRNAs with improved potency and specificity. Nucleic Acids Res. 2009;37:e152 10.1093/nar/gkp864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee HS, Ahn J, Jee Y, et al. Universal and mutation‐resistant anti‐enteroviral activity: potency of small interfering RNA complementary to the conserved cis‐acting replication element within the enterovirus coding region. J Gen Virol. 2007;88:2003‐2012. 10.1099/Vir0.82633-82630 [DOI] [PubMed] [Google Scholar]
- 130. Jackson AL, Burchard J, Leake D, et al. Position‐specific chemical modification of siRNAs reduces “off‐target” transcript silencing. RNA. 2006;12:1197‐1205. 10.1261/rna.30706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kenski DM, Butora G, Willingham AT, et al. siRNA‐optimized modifications for enhanced in vivo activity. Mol Ther Nucleic Acids. 2012;1:e5 10.1038/mtna.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dar SA, Thakur A, Qureshi A, Kumar M. siRNAmod: a database of experimentally validated chemically modified siRNAs. Sci Rep. 2016;6:20031 10.1038/srep20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Morrissey DV, Blanchard K, Shaw L, et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349‐1356. 10.1002/hep.20702 [DOI] [PubMed] [Google Scholar]
- 134. Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492‐503. 10.1208/s12248-010-9210-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tatiparti K, Sau S, Kashaw SK, Iyer AK. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials (Basel). 2017;7 10.3390/nano7040077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen Y, Huang L. Tumor‐targeted delivery of siRNA by non‐viral vector: safe and effective cancer therapy. Expert Opin Drug Deliv. 2008;5:1301‐1311. 10.1517/17425240802568505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti‐HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002‐1007. 10.1038/nbt1122 [DOI] [PubMed] [Google Scholar]
- 138. Khurana B, Goyal AK, Budhiraja A, Aora D, Vyas SP. Lipoplexes versus nanoparticles: pDNA/siRNA delivery. Drug Deliv. 2013;20:57‐64. 10.3109/10717544.2012.752419 [DOI] [PubMed] [Google Scholar]
- 139. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967‐977. 10.1038/nmat3765 [DOI] [PubMed] [Google Scholar]
- 140. Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129‐138. 10.1038/nrd2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lu PY, Woodle MC. Delivering small interfering RNA for novel therapeutics. Methods Mol Biol. 2008;437:93‐107. 10.1007/978-1-59745-210-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Thakur N, Qureshi A, Kumar M. VIRsiRNAdb: a curated database of experimentally validated viral siRNA/shRNA. Nucleic Acids Res. 2012;40:D230‐D236. 10.1093/nar/gkr1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tyagi A, Ahmed F, Thakur N, Sharma A, Raghava GP, Kumar M. HIVsirDB: a database of HIV inhibiting siRNAs. PLoS One. 2011;6:e25917 10.1371/journal.pone.0025917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21‐nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494‐498. 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- 145. Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326‐330. 10.1038/nbt936 [DOI] [PubMed] [Google Scholar]
- 146. Ui‐Tei K, Naito Y, Takahashi F, et al. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32:936‐948. 10.1093/nar/gkh247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Amarzguioui M, Prydz H. An algorithm for selection of functional siRNA sequences. Biochem Biophys Res Commun. 2004;316:1050‐1058. 10.1016/j.bbrc.2004.02.157 [DOI] [PubMed] [Google Scholar]
- 148. Jagla B, Aulner N, Kelly PD, et al. Sequence characteristics of functional siRNAs. RNA. 2005;11:864‐872. 10.1261/rna.7275905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Naito Y, Ui‐Tei K, Nishikawa T, Takebe Y, Saigo K. siVirus: web‐based antiviral siRNA design software for highly divergent viral sequences. Nucleic Acids Res. 2006;34:W448‐450 10.1093/nar/gkl214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Saetrom P. Predicting the efficacy of short oligonucleotides in antisense and RNAi experiments with boosted genetic programming. Bioinformatics. 2004;20:3055‐3063. 10.1093/bioinformatics/bth364 [DOI] [PubMed] [Google Scholar]
- 151. Huesken D, Lange J, Mickanin C, et al. Design of a genome‐wide siRNA library using an artificial neural network. Nat Biotechnol. 2005;23:995‐1001. 10.1038/nbt1118 [DOI] [PubMed] [Google Scholar]
- 152. Peek AS. Improving model predictions for RNA interference activities that use support vector machine regression by combining and filtering features. BMC Bioinformatics. 2007;8:182 10.1186/1471-2105-8-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Qureshi A, Thakur N, Kumar M. VIRsiRNApred: a web server for predicting inhibition efficacy of siRNAs targeting human viruses. J Transl Med. 2013;11:305 10.1186/1479-5876-11-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen SH, Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. Eur J Clin Invest. 2011;41:221‐232. 10.1111/j.1365-2362.2010.02400.x [DOI] [PubMed] [Google Scholar]
- 155. Thakur A, Fitzpatrick S, Zaman A, et al. Strategies for ocular siRNA delivery: potential and limitations of non‐viral nanocarriers. J Biol Eng. 2012;6:7 10.1186/1754-1611-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Aigner A. Applications of RNA interference: current state and prospects for siRNA‐based strategies in vivo. Appl Microbiol Biotechnol. 2007;76:9‐21. 10.1007/s00253-007-0984-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Schlee M, Hornung V, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463‐470. 10.1016/j.ymthe.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 159. Wang S, Shi Z, Liu W, Jules J, Feng X. Development and validation of vectors containing multiple siRNA expression cassettes for maximizing the efficiency of gene silencing. BMC Biotechnol. 2006;6:50 10.1186/1472-6750-6-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sachidanandam R. RNAi as a bioinformatics consumer. Brief Bioinform. 2005;6:146‐162. [DOI] [PubMed] [Google Scholar]


