Abstract
Shrimp aquaculture has grown rapidly over several decades to become a major global industry that serves the increasing consumer demand for seafood and has contributed significantly to socio‐economic development in many poor coastal communities. However, the ecological disturbances and changes in patterns of trade associated with the development of shrimp farming have presented many of the pre‐conditions for the emergence and spread of disease. Shrimp are displaced from their natural environments, provided artificial or alternative feeds, stocked in high density, exposed to stress through changes in water quality and are transported nationally and internationally, either live or as frozen product. These practices have provided opportunities for increased pathogenicity of existing infections, exposure to new pathogens, and the rapid transmission and transboundary spread of disease. Not surprisingly, a succession of new viral diseases has devastated the production and livelihoods of farmers and their sustaining communities. This review examines the major viral pathogens of farmed shrimp, the likely reasons for their emergence and spread, and the consequences for the structure and operation of the shrimp farming industry. In addition, this review discusses the health management strategies that have been introduced to combat the major pathogens and the reasons that disease continues to have an impact, particularly on poor, small‐holder farmers in Asia.
Keywords: disease emergence, health management, shrimp, small‐scale farmers, virus
Introduction
Over the past three decades, shrimp farming in Asia has expanded rapidly from a very low base of traditional, low‐density polyculture to become a vibrant export industry currently valued at more than $US8 billion (FAO 2006). Industry growth, fuelled by commercial investment, government support and expanding global commodity markets, has been a major contributor to socio‐economic development in Asia, particularly in poor coastal communities. However, the rapid expansion of the industry has also been a source of significant environmental and sociological disturbances associated with changes in land use, the ecology of aquatic species and patterns of global trade. Not surprisingly, a major consequence of these disturbances has been the emergence and spread of disease. Commencing in the late 1980s, a succession of previously unknown diseases emerged in farmed shrimp, in both Asia and the Americas, and spread rapidly across international boundaries to impact very significantly on production (Fig. 1). Since 1994, it has been estimated that annual losses globally as a result of disease, primarily caused by viral pathogens, have been as high as $US3 billion (Lundin 1995; Lightner 2003). Although there has been a marked recovery in recent years, disease remains a major concern for the sustainability and profitability of the industry. This review will discuss the nature of these viral pathogens, the likely reasons for their emergence and spread, and the consequences of pandemic disease, both good and bad, for the structure and operation of the shrimp farming industry. The review will also discuss health management strategies that have been introduced to combat the major pathogens and the reasons that disease continues to have an impact, particularly on poor, small‐holder farmers in Asia.
Figure 1.
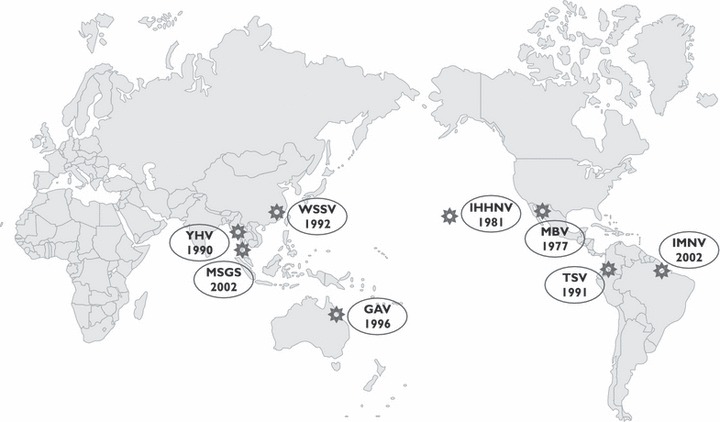
History of the emergence of the major pathogens of farmed shrimp. GAV, gill‐associated virus; IHHNV, infectious hypodermal and haematopoietic necrosis virus; IMNV, infectious myonecrosis virus; MBV, monodon baculovirus; MSGS, monodon slow growth syndrome; TSV, Taura syndrome virus; WSSV, white spot syndrome virus; YHV, yellow head virus.
Major pathogens of farmed shrimp
More than 20 viruses have been reported to infect marine shrimp. Many have not been associated with clinical signs of disease and some have been observed only by electron microscopy and are poorly characterised (Table 1). Seven viral pathogens of marine shrimp are currently listed by the World Organization for Animal Health (OIE) as causing notifiable aquatic animal diseases and two are under study for listing (OIE 2008). By international agreement, diseases listed by the OIE should be reported by member countries and are subject to specified health measures that are intended to limit disease spread and assure sanitary safety of international trade in aquatic animals and their products. Six of the seven OIE‐listed marine shrimp viruses have been reported to occur in Asia.
Table 1.
Major viruses infecting marine shrimp
| Virus | Abbreviation | Family‡ | Genus‡ | Genome | Known distribution |
|---|---|---|---|---|---|
| White spot syndrome virus† | WSSV | Nimaviridae | Whispovirus | dsDNA | Asia, Americas |
| Yellow head virus† | YHV | Roniviridae | Okavirus | (+) ssRNA | Asia, Central America |
| Gill‐associated virus | GAV | Roniviridae | Okavirus | (+) ssRNA | Australia, Asia, Pacific |
| Taura syndrome virus† | TSV | Dicistroviridae | Unassigned | (+) ssRNA | Americas, Asia |
| Infectious myonecrosis virus† | IMNV | Totiviridae | Unassigned | (+) ssRNA | South America, Asia |
| Baculovirus penaei† | BP | Baculoviridae | Unassigned | dsDNA | Asia |
| Monodon baculovirus† | MBV | Baculoviridae | Nucleopolyhedrosisvirus | dsDNA | Asia, Australia, Africa, Americas |
| Infectious hypoderma and haematopoietic necrosis virus† | IHHNV | Parvoviridae | Brevidensovirus | ssDNA | Asia, Australia, Africa, Americas, Pacific |
| Hepatopancreatic parvovirus | HPV | Parvoviridae | Brevidensovirus | ssDNA | Asia, Australia, Africa, Americas |
| Mourilyan virus | MoV | Bunavirus‐like | Unassigned | (−) ssRNA | Australia, Asia |
| Laem Singh virus | LSNV | Leuteovirus‐like | Unassigned | (+) ssRNA | Asia |
| Spawner‐isolated mortality virus | SMV | Parvoviridae | Unassigned | ssDNA | Australia, Asia |
| Baculoviral midgut gland necrosis virus | BMNV | Baculoviridae | Unassigned | dsDNA | Asia, Australia |
| Lymphoid organ vacuolization virus | LOVV | Togavirus‐like? | Unassigned | (+) ssRNA? | Americas |
†OIE (2008) listed.
‡International Committee on Taxonomy of Viruses.
White spot syndrome virus
White spot syndrome virus (WSSV) is by far the most devastating pathogen of farmed shrimp. It infects all cultured penaeids and has been responsible for much of the economic impact of disease on production globally. White spot disease (WSD) was first reported in June 1992 in cultured kuruma shrimp (Penaeus japonicus Bate, 1888) in the Fujian Province of China and in nearby Taiwan (Zhan et al. 1998; Jiang 2001). The disease spread rapidly north and south along the coast of China, affecting each of the major production species (P. japonicus, Penaeus monodon Fabricius, 1798 and Penaeus chinensis Osbeck, 1765). From March 1993, outbreaks were reported in several prefectures in Japan, commencing in farms that had imported P. japonicus juveniles from China (Nakano et al. 1994). The disease also first appeared in Korea in 1993 (Park et al. 1998) and by 1994 had spread to most shrimp‐farming countries throughout South and South‐East Asia. The first recorded outbreak of WSSV in the Americas was at a farm in Texas in November 1995. A nearby processing plant that imported frozen shrimp from Asia was considered to be the likely source (Lightner et al. 1997). In 1997 and 1998, WSSV was detected in wild shrimp stocks in South Carolina and in bait prawns in Texas. The major epizootic of WSD in the Americas commenced in Nicaragua, Honduras and Guatemala in mid‐January 1999. Subsequently, the disease was reported in Panama in March, Ecuador in May and had reached Peru by October 1999 (Alday de Graindorge 2000). It is now enzootic in all shrimp‐farming regions throughout much of Central and South America. The original source of emergence of WSSV in East Asia is not known, but it does not appear to be a natural shrimp pathogen and may have been introduced to shrimp broodstock via an unusual source of live or frozen feed.
White spot disease commonly results in 80–100% mortality within 5–10 days of the first appearance of clinical signs (Chou et al. 1995). Infected shrimp display reddish‐pinkish discolouration, appear lethargic, cease feeding and congregate at the pond edges. White spots are commonly observed under the cuticle of diseased shrimp, but are not pathognomonic, and similar signs can occur as a result of bacterial infection (Takahashi et al. 1994; Goarant et al. 2000; Wang et al. 2000b). White spot syndrome virus infects cells of ectodermal and mesodermal origin and the most characteristic histological lesion is the appearance of eosinophilic Cowdry A‐type inclusions in hypertrophied nuclei with marginated chromatin that become lightly basophilic late in infection (Wongteerasupaya et al. 1995b; Chang et al. 1996). The virus replicates and is assembled in the nucleus of cells and is not occluded as a result of infection (Wang & Chang 2000).
White spot syndrome virus is a large, ovaloid, DNA virus (120–150 nm × 270–290 nm) with a lipid envelope that features an unusual tail‐like appendage (1995a, 1995b). It is distantly related to other large DNA viruses and has been classified taxonomically as the only member of the genus Whispovirus in the newly formed family Nimaviridae (Vlak et al. 2005). Virions comprise at least 45 structural proteins that are arranged in three morphologically distinct layers (Tsai et al. 2004; Li et al. 2007). The nucleocapsid, a helical, bacilliform structure (∼70 nm × ∼300 nm) containing the circular, double‐stranded DNA (dsDNA) genome and nine proteins, lies at the core of the virions. The nucleocapsid proteins include a basic DNA‐binding protein (VP15) and a giant protein (VP664), which forms the stacked ring subunits and appears to protrude through the overlaying tegument (Leu et al. 2005; Witteveldt et al. 2005). The tegument layer is loosely associated with both the nucleocapsids and the envelope and is composed of at least four structural proteins including the major capsid protein VP26 and the minor proteins VP36A, VP39A and VP95 (Tsai et al. 2006; Xie et al. 2006). The envelope, which forms the 6–7‐nm‐thick outer layer, comprises a tri‐laminar lipid membrane and at least 28 viral proteins, many of which contain transmembrane domains. The major envelope proteins VP19 and VP28, which protrude from the outer surface of the membrane, have been shown to induce a protective response in shrimp (2004a, 2004b).
The ∼305 kbp WSSV genome encodes ∼180 long, open reading frames (ORF) and nine homologous regions containing direct repeats, inverted repeats and palindromes (van Hulten et al. 2001; Yang et al. 2001). Significant variations in genome size have been reported between different isolates, primarily as a result of large sequence deletions (up to 13 kb) in the region between the DNA polymerase and the protein kinase genes (Marks et al. 2004). Variations in the genome sequence have been used in molecular epidemiological studies to trace the movement of WSSV and to identify sources of infection (Wongteerasupaya et al. 2003; Dieu et al. 2004; Hoa et al. 2005; Musthaq et al. 2006). There is also evidence that different isolates vary significantly in virulence (Marks et al. 2005) and that passaging of WSSV in different hosts can alter both pathogenicity and the sequences of tandem repeat regions (Waikhom et al. 2006). Nevertheless, the overall nucleotide sequence identity between isolates is >99% (Marks et al. 2004) and variations do not appear to affect the performance of the polymerase chain reaction (PCR) tests commonly used for WSSV detection (Kiatpathomchai et al. 2005).
White spot syndrome virus has a very broad host range in decapod crustaceans. In marine shrimp, the virus occurs commonly as a low‐level persistent infection in the absence of clinical signs, but rapid increases in viral load, precipitated by physiological stress, salinity change or lower temperatures, can lead to disease and mass mortalities in ponds (Peng et al. 1998; Vidal et al. 2001; Du et al. 2006; Granja et al. 2006; Liu et al. 2006). There is evidence that WSSV replicates most efficiently at 23–28°C (Guan et al. 2003; Du et al. 2006; Granja et al. 2006; Reyes et al. 2007) and that high water temperatures prevent the onset of disease (Rahman et al. 2006). The virus also survives for longer periods in seawater at lower temperatures (Momoyama et al. 1998) and seawater temperatures <30°C are conducive to higher prevalence of WSSV infection in wild shrimp populations (Rodriguez et al. 2003; Withyachumnarnkul et al. 2003). All farmed marine shrimp species are susceptible to WSD. Other decapod crustaceans, although universally susceptible to infection, may not develop clinical signs (Jiravanichpaisal et al. 2001). Indeed, some crab and crayfish species have been reported to carry very high viral loads in the absence of disease and may be important reservoirs, sustaining the virus in the natural environment and representing potential sources of infection in shrimp ponds. Other invertebrates, such as polychaetes, bivalves, rotifers, artemia, copepods and some insect larvae, as well as microalgae, can accumulate high levels of viable WSSV in the absence of demonstrated virus replication and may act as mechanical vectors of infection (2004, 2007; Liu et al. 2007).
White spot syndrome virus infection can be transmitted either horizontally or vertically. Horizontal infection of shrimp and other crustaceans has been demonstrated experimentally by exposure to infected water or by ingestion of infected tissue (Chou et al. 1998; Kanchanaphum et al. 1998; Supamattaya et al. 1998; Corbel et al. 2001; Soto et al. 2001). Ingestion appears to be the more effective means of transmission in shrimp (Soto & Lotz 2001). Vertical transmission from infected broodstock to early life stages appears to be a common source of infection. Although there is evidence of WSSV in the muscles and connective tissues of gonads, infection of sperm has not been reported. Oogonia and oocytes may be positive by histology or in situ hybridisation, but mature eggs are not (Lo et al. 1997; Mohan et al. 1997). This suggests that the virus in developing oocytes does not survive maturation and that transovarial transmission is unlikely. Rather, WSSV appears to be transmitted transovum by surface contamination of the egg (Lo et al. 1997; Lo & Kou 1998). Both horizontal and vertical transmission of WSSV infection can occur in the absence of disease.
Yellow head virus
Yellow head disease was first reported in farmed black tiger shrimp (P. monodon) in central Thailand in 1990 and it remains the most virulent of the shrimp viruses, commonly causing total crop loss within 3–5 days of the first appearance of visible signs of the disease in a pond (Limsuwan 1991; Boonyaratpalin et al. 1993). From central Thailand, the disease spread to southern areas on the eastern and western coasts of the Gulf of Thailand, reaching the far south by February 1992 (Chantanachookin et al. 1993). Yellow head disease or yellow head virus (YHV) has since been reported from many shrimp‐farming countries in Asia, including India, Indonesia, Malaysia, the Philippines, Sri Lanka, Vietnam and Taiwan (Mohan et al. 1998; Wang & Chang 2000; WB, NACA, WWF, FAO 2001; Natividad et al. 2002). There is also recent evidence of the presence of YHV in Penaeus vannamei Boone, 1931 and Penaeus stylirostris Stimpson, 1874 shrimp from the north‐west coast of Mexico (de la Rosa‐Velez et al. 2006). Several yellow‐head‐like viruses have also been reported. Gill‐associated virus (GAV) is a closely related virus that has been associated with a disease called mid‐crop mortality syndrome (MCMS) in P. monodon shrimp in Australia (Spann et al. 1997; Munday & Owens 1998; Cowley et al. 1999; Callinan & Jiang 2003; Callinan et al. 2003). Yellow‐head‐related viruses have also been reported in P. monodon from Thailand and in P. japonicus from Taiwan (Wang et al. 1996; Soowannayan et al. 2003). A survey of shrimp samples from Mozambique, India, Thailand, Malaysia, Indonesia, the Philippines, Vietnam, Taiwan and Australia has revealed that YHV is one of at least six genetic lineages (genotypes) of related viruses that occur commonly in farmed P. monodon throughout the Indo‐Pacific region (Wijegoonawardane et al. 2008a). However, only YHV (genotype one) has been detected in shrimp with typical signs of yellow head disease. Of the other genotypes, only GAV (genotype two) has been associated with any form of disease. The original source of YHV infection in Thai shrimp in 1990 has not been determined, but it has been suggested that it may have previously been responsible for similar serious epizootics in Indonesia, Malaysia, China and the Philippines and possibly for the crash of the P. monodon industry in Taiwan in 1986–1987 (Lightner 1996b). The close genetic relationship of YHV to other genotypes in the YHV complex suggests that it is most likely a natural infection of wild penaeid or palemonid shrimp (Wijegoonawardane et al. 2008a).
Yellow head disease affects shrimp in early to late juvenile stages and is characterised by rapid feed consumption followed by a cessation of feeding and the congregation of disoriented and moribund shrimp at the pond edge, with rapidly accelerating mortalities. The disease derives its name from the bleached or pale‐yellow appearance of the cephalothorax, resulting from discolouration of the underlying hepatopancreas, but this condition is not always apparent (Chantanachookin et al. 1993; Flegel et al. 1995). Disease associated with GAV infection is far less severe, with mortalities developing more slowly and rarely reaching 100% and, although moribund shrimp commonly develop a reddish appearance, the pale discolouration typical of yellow head disease does not occur. Yellow head virus is also far more virulent that GAV (>106‐fold by LD50) in experimental infections in P. monodon (Nusra Sittidilokratna and Peter Walker, unpublished data, 2007). Yellow head virus infects tissues of ectodermal and mesodermal origin and causes severe necrosis, particularly in the lymphoid organ and gills, with prominent nuclear pyknosis and heterokaryosis and densely basophilic spherical cytoplasmic inclusions evident in histological sections (Chantanachookin et al. 1993; Lu et al. 1995). Similar histological lesions are seen in moribund shrimp infected with GAV. Yellow head virus and GAV replicate in the cytoplasm in which long filamentous pre‐nucleocapsids are abundant, and virions bud into cytoplasmic vesicles in densely packed paracrystalline arrays for egress at the cytoplasmic membrane (Boonyaratpalin et al. 1993; Chantanachookin et al. 1993; Spann et al. 1997).
The YHV is a rod‐shaped, enveloped positive‐sense single‐stranded RNA (ssRNA) virus (40–60 nm ×∼150–200 nm) with a helical nucleocapsid and prominent knob‐like surface projections (Chantanachookin et al. 1993; 1995a, 1995b; Nadala et al. 1997; Tang & Lightner 1999). The virus is distantly related to other large ssRNA viruses infecting vertebrates and fish, including the coronaviruses, toroviruses, arteriviruses and bafniviruses, and, together with GAV, YHV is currently classified as the species Gill‐associated virus in the genus Okavirus, family Roniviridae, order Nidovirales (Walker et al. 2005; Schultze et al. 2006). A roni‐like virus has also been described recently and associated with mortalities in the freshwater crab (Eriochier sinensis Milne‐Edwards, 1854) in China, but its relationship to YHV has not yet been determined (Zhang & Bonami 2007). The YHV virions contain three major structural proteins. The nucleocapsid contains the ssRNA genome and the nucleoprotein (p20), which encapsidates and protects the RNA. The envelope comprises a tri‐laminar lipid membrane and two glycoproteins, gp116 and gp64, which penetrate the membrane to form the spike‐like projections on the virion surface. Glycoprotein gp116 has been identified as the target for virus‐neutralising antibodies in vitro and in vivo (Assavalapsakul et al. 2005; Sittidilokratna et al. 2009). There is evidence that suggests that gp116 may be preferentially suppressed in persistently infected shrimp that survive YHV infection (Chaivisuthangkura et al. 2008). Glycoprotein gp116 must perform a critical function in viral infection, but the domain structure has not been defined and the role of gp64 is presently unexplored. There is also evidence that a small triple‐membrane‐spanning protein (gp22) is a very minor component of YHV virions (Nusra Sittidilokratna and Peter Walker, unpublished data, 2007).
The 26 662 nt YHV genome is polyadenylated and contains four long ORF (Sittidilokratna et al. 2008). ORF1a and ORF1b overlap and encode non‐structural enzymes involved in replication and transcription. ORF1b is expressed only as an extension of ORF1a as a result of a ribosomal frame‐shift at a predicted pseudoknot structure in the overlap region (Sittidilokratna et al. 2002). ORF1a encodes papain‐like and cysteine‐like proteases that are required for post‐translational auto‐processing of the large pp1a and pp1ab polyproteins (Sittidilokratna et al. 2008). ORF1b encodes several replication enzymes, including helicase, polymerase, exonuclease, endonuclease and methyl transferase domains (Cowley et al. 2000a; Sittidilokratna et al. 2002). ORF2 encodes the p20 nucleoprotein (Sittidilokratna et al. 2006). ORF3 encodes a long polyprotein (pp3) that is processed post‐translationally to generate the envelope glycoproteins gp116 and gp64 and the small triple‐membrane‐spanning protein gp22 of unknown function (Jitrapakdee et al. 2003; Nusra Sittidilokratna and Peter Walker, unpublished data, 2007). Downstream of ORF3, the long 3′ untranslated region contains a fifth small ORF (ORF4) that does not appear to be functional (Sittidilokratna et al. 2008).
The 26 235 nt GAV genome is very similar in organisation to that of YHV, but there is some evidence that ORF4 (which is somewhat longer than the ORF4 in YHV) may be functional (Cowley & Walker 2002, 2008). The overall nucleotide sequence identity between the YHV and GAV genomes is ∼79%, varying from ∼74% in ORF3 to ∼82% in ORF1b (Sittidilokratna et al. 2008). There is a similar level of sequence identity between YHV and each of the other four genotypes, but genotypes three and six are more closely related to GAV than genotypes four and five (Wijegoonawardane et al. 2008a). Analysis of a short region in ORF1b has indicated that nucleotide sequence variation between isolates within a genotype is generally within 2–3%, except for genotype five, which is more diverse (Wijegoonawardane et al. 2008a). The molecular basis of the differences in pathogenicity and virulence among the genotypes is not known. The level of diversity between the genotypes is sufficient to require careful design of PCR tests to ensure the required level of test specificity. Polymerase chain reaction (PCR) tests have been described for the genotype‐specific detection of YHV, detection and differentiation of YHV and GAV, and group‐specific detection of each of the known genotypes in the YHV complex (Wongteerasupaya et al. 1997; Cowley et al. 2004; Wijegoonawardane et al. 2008b). However, recent observations indicate that genetic recombination occurs with high frequency between some YHV genotypes in natural populations of shrimp, suggesting that genotype assignments and the association of genotype with virulence is not straightforward (Wijegoonawardane et al. 2009).
Although yellow head disease outbreaks have been reported only in P. monodon, natural and/or experimental YHV infection has been reported in several other commonly farmed species of penaeid shrimp, including P. vannamei, P. stylirostris, Penaeus merguiensis De Man, 1888, Penaeus setiferus Linnaeus, 1767, Penaeus aztecus Ives, 1891 and Penaeus duorarum Burkenroad, 1939 (Chantanachookin et al. 1993; Lu et al. 1994; Lightner et al. 1998; de la Rosa‐Velez et al. 2006). A yellow‐head‐like virus has also been reported in P. japonicus in Taiwan (Wang et al. 1996). There is evidence of natural and/or experimental YHV infection in wild penaeid and palemonid shrimp and krill (Acetes sp.) found within ponds or nearby canals (Flegel et al. 1997b; Longyant et al. 2006). However, the severity of disease varies, with some susceptible shrimp displaying mild signs and surviving up to 30 days after experimental infection (Longyant et al. 2005). Freshwater palemonid shrimp (Machrobrachium spp.) appear to be resistant to infection, although a mild infection has been established experimentally in one species (Machrobrachium sintangese De Man, 1879) (Longyant et al. 2005). A survey of 16 species of crabs collected from the vicinity of shrimp farms in Thailand detected no evidence of either natural infection or experimental susceptibility (Longyant et al. 2006). Other genotypes in the YHV complex have been detected almost exclusively in P. monodon. The prevalence of GAV in wild and farmed populations of P. monodon in eastern Australia approaches 100% (Cowley et al. 2000b; Walker et al. 2001). Penaeus merguiensis, P. japonicus and P. esculentus Haswell, 1879 are also susceptible to experimental GAV infection, but pathogenicity varies (Spann et al. 2000). Although P. esculentus co‐cultivated with P. monodon have been reported to be infected, there is no evidence that GAV infection occurs commonly in any species other than P. monodon. All other genotypes have been detected exclusively in P. monodon, but a survey of other potential hosts has not been conducted (Wijegoonawardane et al. 2008a). The evidence to date suggests that penaeid shrimp are the natural hosts of YHV and other viruses in the YHV complex, and that low‐level, persistent infections occur commonly in healthy P. monodon populations throughout the Indo‐Pacific region.
Yellow head virus and GAV can be transmitted horizontally by injection, immersion or ingestion of infected shrimp tissue and by co‐habitation with infected shrimp (Lu et al. 1994; Flegel et al. 1995; Lightner et al. 1998; Walker et al. 2001; Longyant et al. 2006). In shrimp that survive experimental challenge, the infection can persist for up to 30 days following recovery, and perhaps for life. Recent evidence suggests that preferential suppression of expression of envelope glycoprotein gp116 is associated with viral persistence (Chaivisuthangkura et al. 2008). There is no direct evidence that YHV is transmitted vertically. However, vertical transmission of GAV has been demonstrated experimentally (Cowley et al. 2002). High levels of GAV infection have been detected in spermatophores, seminal fluid, vas deferens and mature ovarian tissue (Walker et al. 2001; Callinan et al. 2003). Infection can originate from either the male or female parent, and the virus is most likely to be transmitted transovum on the egg surface (Cowley et al. 2002). The high prevalence of infection in healthy P. monodon postlarvae throughout the Indo‐Pacific region suggests that viruses in the YHV complex are maintained in a cycle of low‐level, life‐long chronic infections by vertical transmission and persistence (Walker et al. 2001; Spann et al. 2003). Virus amplification and associated disease outbreaks appear to occur as a result of physiological stress induced by poor water quality or other environmental factors (Flegel et al. 1997a; de la Vega et al. 2004). The much higher virulence of YHV compared with GAV and other genotypes (>106‐fold by LD50) ensures that the threshold of infection required for disease is far more easily obtained (Nusra Sittidilokratna and Peter Walker, unpublished data, 2008).
Taura syndrome virus
Taura syndrome first emerged in farmed white Pacific shrimp (P. vannamei) near the mouth of the Taura River in Ecuador in June 1992 (Jiminez 1992). It was originally attributed to the use of chemical fungicides in nearby banana plantations, but was subsequently shown to be of viral aetiology (Hasson et al. 1995). By 1996, Taura syndrome virus (TSV) had spread to Peru and north‐eastern Brazil, throughout the Pacific and Caribbean coasts of Central America, and to Florida and Texas in the USA (Lightner 1996a). It had also spread to P. vannamei breeding programs in Hawaii by 1994, but was subsequently eradicated (Brock et al. 1995; Brock 1997). The rapid spread of the virus has been attributed to international trade in postlarvae and broodstock. In 1998, TSV spread to Taiwan in P. vannamei broodstock imported from Central and South America (Tu et al. 1999; Yu & Song 2000). By 2004, following the dramatic amplification of P. vannamei production, TSV had become endemic in most major shrimp‐farming countries in East and South‐East Asia, including China, Korea, Thailand, Myanmar, Indonesia and Vietnam (Sunarto et al. 2004; Van 2004; Nielsen et al. 2005; Do et al. 2006). The impact of Taura syndrome on the shrimp‐farming industry in the Americas was estimated to be $US1–2 billion up to 2001 (Lightner 2003). The impact in Asia is yet to be systematically evaluated. The original source of TSV in Ecuador is unknown, but its apparent absence prior to 1992 and the radiant nature of its spread indicate that it is unlikely to be a natural infection of shrimp. Taura syndrome virus may well have crossed species from another invertebrate host in the farming environment.
Taura syndrome usually occurs in juvenile P. vannamei (0.1–5 g) within 14–40 days of stocking in nursery or grow‐out ponds, but it can also occur in postlarvae and adult shrimp (Lightner et al. 1995; Lightner 1996b). Acute, transition and chronic phases of TSV infection have been described (Hasson et al. 1999a). Diseased shrimp in the acute phase typically display reddish colouration, particularly in the tail fan and pleopods, as a result of expansion of the red chromatophores. Individuals have soft shells and an empty gut and usually die during moulting. Surviving shrimp entering the transition (recovery) phase display irregularly shaped melanised lesions on the cephalothorax, tail and appendages as a result of haemocyte accumulation, but may otherwise appear and behave normally. During the acute phase, cumulative mortalities may be as high as 95% (Brock 1997). Following moulting and recovery, shrimp enter the chronic phase in which they remain persistently infected in the absence of obvious clinical signs. Although there is evidence that TSV may be cleared from shrimp during the chronic phase, it may continue for at least 8–12 months, and possibly for life (Hasson et al. 1999b). Taura syndrome virus infects tissues of ectodermal and mesodermal origin, particularly the cuticular epithelium, subcuticular connective tissue and striated muscle, haematopoietic tissue, the lymphoid organ and the antennal gland (Lightner et al. 1995; 1999a, 1999b). The virus replicates in the cytoplasm, with evidence of TSV RNA in association with proliferating membranes and cytoplasmic vesicles (Srisuvan et al. 2006b).
Taura syndrome virus is a small, non‐enveloped, positive‐sense ssRNA virus with icosahedral architecture (31–32 nm diameter). It is similar to small insect viruses in the genus Cripavirus of the family Dicistroviridae (e.g. cricket paralysis virus, drosophila C virus) and is currently classified as an unassigned species (Taura syndrome virus) in this family (Christian et al. 2005). The TSV virions comprise the RNA genome, three major capsid proteins, VP1 (55 kDa), VP2 (40 kDa) and VP3 (24 kDa), and a minor protein VP0 (58 kDa) (Bonami et al. 1997; Mari et al. 2002). The functions of the individual capsid proteins are not yet known, but there is evidence that VP1 (also called CP2) binds to the laminin receptor/p40 protein, which is a common receptor for several arthropod‐borne RNA viruses (Senapin & Phongdara 2006). The 10 205 nt TSV RNA genome is polyadenylated and contains two long ORF flanked by long 5′ and 3′ untranslated regions and a long (207 nt) intergenic region (Mari et al. 2002). ORF1 encodes non‐structural proteins, including protease, helicase and RNA‐dependent RNA polymerase elements, and a domain that is homologous to IAP (inhibitor of apoptosis) sequences of animals and some other viruses. ORF2 encodes a long polyprotein from which the capsid proteins are generated by proteolytic cleavage. Translation of ORF2 appears to be from a single genome‐length messenger RNA (mRNA) and is mediated by an internal ribosomal entry site (IRES) at a predicted stem‐loop structure in the long intergenic region (Robles‐Sikisaka et al. 2001; Mari et al. 2002). Genetic and antigenic variations have been reported among TSV isolates in the major capsid protein precursor encoded in ORF2. At least two distinct lineages of TSV have been identified in shrimp from the Americas and variations at a locus in VP1 have been attributed to the failure of a standard diagnostic monoclonal antibody to detect some isolates (2002, 2005; Robles‐Sikisaka et al. 2002). Isolates from Asia also display variation in ORF2 sequences, but cluster separately from isolates from the Americas, possibly as two separate clades representing the early isolates from Taiwan and later isolates from Thailand, China, Myanmar and Korea (Chang et al. 2004; Nielsen et al. 2005; Do et al. 2006). This suggests that introductions of TSV to Asia from the Americas have occurred relatively infrequently and that the virus has been dispersed within Asia from locally established breeding facilities. A recent isolate from Belize may represent a third genetic lineage (Srisuvan et al. 2005; Lightner et al. 2007). There is also evidence of variations in virulence between TSV strains (2002, 2005) and of adaptation to growth in other shrimp species (Erickson et al. 2002; Chang et al. 2004).
Although P. vannamei is the major host of TSV, experimental infections have demonstrated susceptibility to infection in several other penaeid shrimp species, including P. stylirostris, P. setiferus, P. aztecus, P. duorarum, P. chinensis and P. monodon (Overstreet et al. 1997; Srisuvan et al. 2005). Natural infections have also been detected in P. stylirostris, P. monodon, P. japonicus, Metapenaeus ensis De Haan, 1844 and the freshwater shrimp, Macrobrachium rozenbergii De Man, 1879 (Erickson et al. 2002; Chang et al. 2004; Nielsen et al. 2005). However, susceptibility to disease varies. Clinical signs and mortalities have been reported following natural or experimental infection of P. stylirostris, P. schmitti, P. setiferus, P. monodon and M. ensis. Susceptibility to disease may also vary in selected lines of shrimp and for different strains of TSV (Brock 1997; Overstreet et al. 1997; Erickson et al. 2002; Chang et al. 2004; Nielsen et al. 2005; 2005, 2006a). Selected lines of TSV‐resistant P. vannamei have been developed (Argue et al. 2002; White et al. 2002; Xu et al. 2003; Srisuvan et al. 2006a).
Taura syndrome virus can be transmitted horizontally by injection, ingestion of infected tissue or exposure to infected shrimp or shrimp carcasses (Brock et al. 1995; Hasson et al. 1995; Lotz 1997; Lotz et al. 2003; Srisuvan et al. 2006a). Exposure to chronically infected shrimp can result in TSV transmission in the absence of disease (Lotz et al. 2003). Although considered likely to occur, vertical transmission of TSV has not been demonstrated experimentally (Lightner & Redman 1998a; Dhar et al. 2004). Mechanical transmission is also considered a likely mechanism for the local geographical spread of TSV infection between ponds or farms. Taura syndrome virus has been detected in the faeces of sea gulls collected from the edges of shrimp ponds during disease outbreaks (Garza et al. 1997). There are also reports that salinity‐tolerant water boatmen (Trichocorixa reticulata Guerin‐Meneville, 1857) carry TSV within the gut and may act as mechanical vectors (Lightner 1996a; Brock 1997; Dhar et al. 2004).
Infectious hypodermal and haematopoietic necrosis virus
Infectious hypodermal and haematopoietic necrosis (IHHN) first emerged in mid‐1981 as a viral disease causing mass mortalities (>90%) in juvenile and sub‐adult P. stylirostris farmed in super‐intensive raceways in Hawaii (Lightner et al. 1983a). Infectious hypodermal and haematopoietic necrosis virus (IHHNV) was subsequently detected in apparently healthy P. vannamei farmed in the same facility and shown not to cause mortalities or the clinical signs typical of IHHN (Bell & Lightner 1984). However, subsequent investigations demonstrated that IHHNV could cause a condition described as ‘runt deformity syndrome’ (RDS) in P. vannamei in which affected shrimp display cuticular deformities and irregular and slow growth (Kalagayan et al. 1991). Economic losses as a result of RDS in P. vannamei have been estimated to be 10–50% per crop (Lightner & Redman 1998b). Runt deformity syndrome has also been reported in cultured P. monodon infected with IHHNV, but the aetiology was not established (Primavera & Quinitio 2000) and subsequent studies have indicated that IHHNV infection causes no disease in P. monodon and has little or no impact on either growth or fecundity (Chayaburakul et al. 2005; Withyachumnarnkul et al. 2006).
Following the initial detection in Hawaii, IHHNV was found to be widely distributed in P. stylirostris and P. vannamei throughout shrimp‐farming regions of the Americas (Morales‐Covarrubias et al. 1999; Lightner 2003; Motte et al. 2003). It has also been reported to be highly prevalent in wild fisheries on the Pacific coast of the Americas and may have contributed to the collapse of the wild P. stylirostris fishery in the northern Gulf of California in 1990 (Lightner 1996a, 2003; Pantoja et al. 1999). In addition, IHHNV has been detected in P. stylirostris imported to French Polynesia and Guam for aquaculture (Costa et al. 1998; Tang & Lightner 2002) and in P. vannamei imported to China (Yang et al. 2007). In P. monodon, IHHNV is endemic in many Asian countries, including the Philippines, Singapore, Malaysia, Indonesia, Thailand and Taiwan (Lightner 1996a; Tang et al. 2003a; Flegel et al. 2004). Infectious hypodermal and haematopoietic necrosis virus or IHHNV‐related DNA sequences have been reported in P. monodon from Australia, Mauritius, Madagascar and Tanzania (Owens et al. 1992; Krabsetsve et al. 2004; Tang & Lightner 2006). There is genetic evidence that P. monodon broodstock imported to Hawaii from the Philippines were the source of the IHHNV epizootic in the Americas (Tang & Lightner 2002).
Infectious hypodermal and haematopoietic necrosis virus is a small (22 nm), non‐enveloped DNA virus with icosahedral symmetry (Bonami et al. 1990). It is closely related to small DNA viruses infecting Aedes spp. mosquitoes and is currently classified as the tentative species Penaeus stylirostris densovirus in the genus Brevidensovirus of the family Parvoviridae (Tattersall et al. 2005). Virions comprise four capsid proteins of 74, 47, 39 and 37.5 kDa and a ∼4.1 kb linear ssDNA genome that is primarily encapsidated in negative polarity (Bonami et al. 1990; Mari et al. 1993; Shike et al. 2000). The genome organisation is similar to other brevidensoviruses, comprising three long ORF of which the left ORF encodes replication initiator motifs and helicase and NTP‐binding domains of the major non‐structural protein (NS‐1). By analogy with other parvoviruses, the middle ORF may encode the N‐terminal region of a second non‐structural protein (NS‐2) that is expressed through an alternative splicing mechanism. The right ORF appears to encode the capsid proteins (Shike et al. 2000; Dhar et al. 2007). Significant genetic variation between IHHNV sequences has been observed with differences of up to ∼15% over a large (2.9 kb) region of the genome. Penaeus stylirostris and P. vannamei isolates originating from the Americas and Hawaii between 1982 and 1997 have been shown to cluster tightly (>99.6% similarity) and share a high level of identity (99.8%) with a P. monodon isolate collected from the Philippines in 1996 (Tang & Lightner 2002). A second genotype identified in P. monodon from Thailand and Taiwan shares 96.2% identity with the Philippine genotype (Tang et al. 2003a). Two other genotypes have been identified in healthy P. monodon; one was detected in shrimp from Tanzania and the other in shrimp from Madagascar, Mauritius and Australia (Tang et al. 2003a; Krabsetsve et al. 2004). These genotypes share ∼90% identity and differ from the Asian genotypes by 8–14%. The African and Australian genotypes have been shown to be integrated into the host genomic DNA. They have not been associated with histological lesions typical of IHHNV infection and experimental transmission studies suggest that they are not infectious for P. monodon or P. vannamei (Tang & Lightner 2006). However, other parvoviruses are known to cause latent infections, sometimes involving genome integration, and may be re‐activated by environmental factors or infection with unrelated DNA viruses (Tattersall et al. 2005; Geoffroy et al. 2006). Further work is required to determine the relationship between the integrated and free forms of IHHNV and the potential for reactivation of the African and Australian genotypes (Flegel 2006).
Infectious hypodermal and haematopoietic necrosis virus can be transmitted horizontally by injection, ingestion or exposure to infected water (Lightner et al. 1983a; Lotz 1997). Shrimp may be asymptomatic carriers of infection and may remain infected for life (Morales‐Covarrubias et al. 1999; Lightner 2003). Infectious hypodermal and haematopoietic necrosis virus can also be transmitted vertically from infected females, in which the virus has been detected in ovarian tissue and within developing oocytes, and high levels of infection can cause embryo development to abort (Lotz 1997; Motte et al. 2003). Selected lines of IHHNV‐resistant P. stylirostris have been developed (Weppe et al. 1992; Tang et al. 2000). There is also evidence of interference between IHHNV and WSSV in co‐infections, resulting in delayed or reduced mortalities (Tang et al. 2003b; Bonnichon et al. 2006).
Infectious myonecrosis virus
Infectious myonecrosis (IMN) is the most recently emergent of the major viral diseases of shrimp. It was first recognised in September 2002 in farmed P. vannamei at Pernambuco in the state of Piaui in north‐eastern Brazil (Lightner et al. 2004). By 2004, infectious myonecrosis virus (IMNV) had spread to other regions of north‐eastern Brazil and was subsequently detected in P. vannamei collected from an outbreak in the Situbondo District of East Java in Indonesia in May 2006 (Senapin et al. 2007). It is likely that the virus was introduced to Indonesia in broodstock imported from Brazil. By April 2007, IMNV had reached P. vannamei farming regions in south and north‐east Sumatra (Ketut Sugama, pers. comm., 2007).
Infectious myonecrosis virus is a small (40 nm) non‐enveloped, non‐segmented double‐stranded RNA (dsRNA) virus with icolahedral symmetry (Poulos et al. 2006). Phylogenetic analysis of RNA‐dependent RNA polymerase (RdRp) sequences and aspects of the genome organisation and likely transcription strategy indicate that it is most closely related to members of the genus Giadiavirus of the family Totiviridae (Poulos et al. 2006; Nibert 2007). Virions appear to contain several structural proteins, including a 106 kDa major capsid protein. The 7560 bp dsRNA genome contains two long ORF. ORF1 contains two 2A‐like proteolytic cleavage sites and encodes the coat protein and a dsRNA‐binding motif. ORF2 overlaps ORF1 and encodes the RdRp. A −1 ribosomal frame‐shift at a slippery GU‐rich heptamer appears to be facilitated by a predicted pseudoknot in the ORF1/ORF2 overlap to generate a read‐through polyprotein (Nibert 2007).
Experimental infection studies have demonstrated that P. vannamei, P. stylirostris and P. monodon are all susceptible to IMNV infection, but only P. vannamei was shown to be susceptible to disease with the isolate used (Tang et al. 2005). However, as P. vannamei and P. monodon are now commonly cultured in the same regions of Indonesia (Agus Sunarto, pers. comm., 2008) there remains a risk of adaptation to natural infection in P. monodon and associated disease. Infectious myonecrosis virus primarily infects skeletal muscle, but there is also evidence of infection in the lymphoid organs, hindgut and gills, and the virus has been detected in phagocytic cells within the hepatopancreas and heart (Tang et al. 2005). There is currently little other published information on the biology of IMNV infection.
Penaeus monodon‐type baculovirus
Monodon baculovirus (MBV), also called spherical baculovirus, was first observed in 1977 in adult P. monodon that were laboratory reared in Mexico from postlarvae imported from Taiwan (Lightner & Redman 1981; Lightner et al. 1983b). The virus was soon found to occur commonly in P. monodon populations from Taiwan, the Philippines and French Polynesia (Lightner et al. 1983b) and has subsequently been shown to be enzootic in P. monodon throughout its geographical range from east Africa and Madagascar in the west, through the Middle East and the Indian subcontinent, South‐East and East Asia, to Australia and islands of the South Pacific. It has also been reported in many countries to which P. monodon shrimp have been introduced, including West Africa, the Mediterranean, Tahiti, Hawaii, North and South America and the Caribbean (Lightner 1996b; OIE 2006). No systematic study of MBV strains has been conducted, but there is anecdotal evidence for the existence of different types and it has recently been reported that PCR tests designed to detect isolates from East Asia and South‐East Asia do not detect African strains (OIE 2006). Monodon baculovirus or MBV‐like viruses have been described in several other penaeid shrimp species, including Penaeus merguiensis, Penaeus plebejus Hess, 1865 and Metapenaeus bennettae Racek & Dall, 1965 from Australia (Lester et al. 1987; Doubrovsky et al. 1988; Spann & Lester 1996), Penaeus indicus Milne‐Edwards, 1837 from Vietnam (Hao et al. 1999), Penaeus penicillatus Alock, 1905 from Taiwan (Chen et al. 1989a) and P. indicus, Metapenaeus monoceros Fabricius, 1798 and Metapenaeus elegans De Man, 1907 from India (Vijayan et al. 1995; Manivannan et al. 2004). However, attempts to infect P. californiensis, P. stylirostris and P. japonicus by experimental exposure per os have not been successful (Lightner & Redman 1981; Fukuda et al. 1988) and observations of infection in species other than P. monodon have relied primarily on histology, electron microscopy and/or positive PCR reactions. A more detailed molecular analysis, together with bioassays, should be conducted to establish with more confidence the susceptible host range of MBV.
Monodon baculovirus can cause high mortalities in larvae (protozoea and mysis) and early postlarval stages (Natividad & Lightner 1992a). There are varied reports of the effect of MBV on the survival of juvenile and adult stages. Although MBV was implicated in the collapse of the P. monodon culture industry in Taiwan in 1987/1988 (Chen et al. 1989b; Lin 1989) and as the cause of mortalities in pond‐reared juveniles in Indonesia and Malaysia in the mid‐1980s (Nash et al. 1988), concomitant bacterial, parasitic or viral infections are now thought to be the likely cause of disease (Lightner et al. 1987; Chen et al. 1989a). Persistent infections in juvenile and adult stages occur commonly and appear to be tolerated without apparent signs of disease (Chen et al. 1989a; Fegan et al. 1991; Natividad & Lightner 1992b). Environmental or other stress has been identified as a significant factor in disease associated with MBV (Lightner et al. 1983b; Chen et al. 1989a; Fegan et al. 1991; OIE 2006). Co‐infection with other viruses, such as HPV, IHHNV and WSSV, has also been reported to occur commonly (Manivannan et al. 2002; Chayaburakul et al. 2004; Oanh et al. 2005; Natividad et al. 2006) and multiple infections may result in retarded growth (Chayaburakul et al. 2004; Flegel et al. 2004).
Monodon baculovirus is a large, bacilliform dsDNA virus that is classified as the tentative species Penaeus monodon nuclear polyhedrosis virus (PemoNPV) in the genus Nucleopolyhedrovirus of the family Baculoviridae (Theilmann et al. 2005). Enveloped virions (∼325 nm × 75 nm) mature in the nucleus and contain nucleocapsids (∼250–300 × 45–70 nm) of similar shape, but with one conical and one blunt end (Lightner et al. 1983b; Mari et al. 1993; Vickers et al. 2000). Naked nucleocapsids of similar dimensions are also detected in the cytoplasm and attached to the nuclear envelope, most likely in traverse from the plasma membrane following adsorption and endocytosis of the infecting virions (Johnson & Lightner 1988; Vickers et al. 2000). In the late stages of infection, virions in the nucleus are observed within spherical inclusion bodies comprising a paracrystalline network of small polyhedrin subunits, each ∼20 nm in diameter (Lightner et al. 1983b; Mari et al. 1993). The spherical shape of these inclusion bodies is the origin of the alternative common name of MBV (i.e. spherical baculovirus), although by scanning electorn microscopy they appear polyhedral (Flegel 2006). The 58 kDa polyhedron protein displays a high level of amino acid sequence identity with the polyhedron protein of Autographa californica MNPV (AcMNPV), the type species of the genus Nucleopolyhedrovirus (Chang et al. 1993; Lu et al. 1993; Mari et al. 1993). There are no published data available on the polypeptide composition of MBV virions or on the molecular characterisation of the viral DNA.
Monodon baculovirus can be transmitted by co‐habitation, feeding or exposure to homogenates of infected tissue (Lightner & Redman 1981; Natividad & Lightner 1992a; Paynter et al. 1992). All life stages, except eggs and nauplii, are susceptible to MBV infection. Infection occurs in the epithelial cells of the hepatopancreatic tubules and anterior midgut from which virus particles and inclusion bodies are released to enter the intestinal tract via the hepatopancreatic lumen (Lightner et al. 1983b; Johnson & Lightner 1988; Chen et al. 1989a). As for other baculoviruses, faecal–oral transmission is the most likely route of infection, with polyhedron providing environmental protection of virus released in inclusion bodies. Monodon baculovirus can be transmitted from broodstock to progeny, but there is no evidence of transovarial transmission and the evidence indicates that infection occurs by faecal contamination of eggs during spawning (Chen et al. 1992; OIE 2006). Good husbandry practices, including washing of fertilised eggs or nauplii with filtered seawater, formalin and iodophores, can be effective in breaking the transmission cycle (Chen et al. 1992).
Factors contributing to disease emergence in shrimp aquaculture
The ecology of viral disease emergence
The emergence and spread of new or previously quiescent viral diseases is an increasingly common phenomenon that has drawn serious attention in recent years. Much of this interest has been stimulated by the emergence of a range of human diseases of animal origin, such as HIV‐AIDS, Ebola and SARS, and recognition of the alarming prospect that human‐to‐human transmission of avian influenza could precipitate a devastating global pandemic (Webby & Webster 2003). However, there are many other examples of emerging viral diseases in humans, animals and plants, and there is now a growing understanding of the factors that cause disease emergence based on an appreciation of viruses as an integral part of the ecosystem (Morse 1993, 1995; Brown 1997; Mayer 2000).
Viruses are obligate parasites that normally exist in a biological cycle that involves a stable ecological association with one or more hosts (Wilcox & Gubler 2005). A virus must achieve efficient replication and progressive transmission of infection in an environment that is constantly changing because of individual differences in host genetics and the immune response of the host. These environmental variations are met by the virus by ongoing competitive selection of the most efficient mutants with suitable variant phenotypes, or through an inherent capacity for evasive behaviour (Holland 2006). In this way, viruses achieve a dynamic equilibrium that sustains a natural ecological balance with their host(s). This equilibrium does not a priori require the induction of pathology or disease. Many viruses infecting humans, animals or plants do not normally cause disease. Indeed, the absence of pathology or mortality may provide the virus with a better opportunity for replication and efficient ongoing transmission. Covert infections without disease commonly occur for viruses that have established a stable ecological niche (Hyatt et al. 2004; Walker 2004).
New diseases usually emerge as a consequence of a major shift in the environment of the virus that upsets this natural balance and leads to significant changes in the biology of infection (Morse 1993; Daszak et al. 2001; Hyatt et al. 2004). Such disturbances usually result, directly or indirectly, from anthropogenic influences on the ecosystem. Human social and industrial activities that have been implicated in disease emergence include increased urbanisation, the globalisation of trade, cultural and behavioural changes, the population of new environments, climate change, and the establishment and growth of new industries or industrial practices (Brown 1997; Mayer 2000). Changes of this nature can allow a virus, through necessity or opportunity, to occupy a new niche in which the ecological balance is temporarily lost (Hyatt et al. 2004).
There are two fundamentally different mechanisms by which new infectious diseases may emerge as a result of ecological imbalance. The first involves viruses (or other micro‐organisms) that have a natural association with a particular host, but do not normally cause disease. Through a shift in the natural ecological balance, a normally benign agent may become pathogenic in its natural host. Factors with the potential to cause such an imbalance include immunosuppression or reduced resistance to disease (induced by environmental stressors, chemicals, radiation or other infections), enhanced replication (induced by a shift in ambient temperature) or modified cell tropism (induced by a different route of exposure). The second mechanism involves a more dramatic change in viral ecology associated with cross‐species transmission. This complex process, by which a virus establishes in a new host, comprises four stages: (i) contact between a pathogen and a new, potentially susceptible host; (ii) transmission to and replication of the pathogen in the new host; (iii) sustained transmission of the pathogen between individuals of the new host; and (iv) genetic adaptation of the pathogen to achieve a new ecological balance in the new host (Childs et al. 2007). In the case of zoonotic diseases (human diseases derived from animals), the process by which cross‐species transmission occurs is termed ‘spill‐over’ (Childs et al. 2007). This requires that a virus is capable of completing the replication cycle in the new host and will be critically dependent on the availability of a suitable molecular landscape, including receptors and replication co‐factors provided by the new host cell. As the molecular landscape is most similar for closely related species, spill‐over is most likely to occur between phylogenetically related organisms. As the process of adaptation to a new host is assisted by genetic variability in the virus through mutation and/or recombination, it is also more likely that RNA viruses and some small DNA viruses with a high intrinsic replication error frequency will be involved in cross‐species transmission rather than large DNA viruses (Holland 1993). These likely trends are confirmed by the recent history of disease emergence involving cross‐species transmissions (Morse 1993).
Disease emergence in shrimp aquaculture
Shrimp aquaculture is an important seafood industry that has created new employment and contributed significantly to socio‐economic development in many countries in Asia and the Americas. However, large‐scale shrimp aquaculture also represents a change in human activity with the potential for very significant impacts on the environment and the ecology of pathogens. The displacement of shrimp from their natural offshore or estuarine environments to terrestrial, earthen ponds provides opportunities for exposure to pathogens that they may not naturally encounter. The use of artificial or alternative live feeds presents risks of inadvertent cross‐species transmission of contaminating pathogens. The culture conditions can often be stressful, particularly when water quality is poor or variable, compromising the natural defences of the shrimp and their capacity to contain infection. High stocking densities facilitate rapid transmission of infection and disease (Soto & Lotz 2001). Aquaculture has also led to a large and rapidly growing trade in live aquatic animals and fresh or frozen shrimp product, providing ready pathways for the rapid transboundary spread of disease (Lightner 1996b; Renault 1996; Yoshimitsu 1996). The emergence and spread of a growing array of shrimp diseases is a natural and predictable consequence of these practices.
Shrimp pathogens can be considered to fall into two categories: (i) those for which shrimp are the natural host in which they have existed in for, perhaps, millions of years; and (ii) those that have been introduced to shrimp as a result of a recent cross‐species transmission. Of the major viral pathogens of marine shrimp, WSSV, TSV and IMNV have almost certainly emerged through cross‐species transmission. Each of these viral pathogens appears to have spread from a single focal origin in Asia or South America and had not been detected in farmed or wild shrimp prior to disease emergence. Each also appears to represent a single evolving genetic lineage that reflects its focal origin. However, neither the original sources of infection nor the mechanism of first exposure have been determined conclusively for these important pathogens.
White spot syndrome virus has a very broad susceptible host range in decapod crustaceans and is now detected commonly in wild shrimp and crabs in many locations where shrimp are farmed. However, reports of WSSV in crustaceans outside of shrimp‐farming regions are rare and the virus has not been detected in surveys of crustaceans in Australia where there is no WSSV or WSD in farmed shrimp (East et al. 2004). The apparent absence of widespread WSSV infection in wild decapod crustaceans prior to the emergence of WSD in 1992, despite their susceptibility, suggests that the original source was a non‐decapod invertebrate from which the virus has ‘crossed’ and adapted to shrimp and other crustaceans. Alternatively, WSSV may have been introduced to shrimp from a decapod host that is relatively isolated from other susceptible species and in which disease does not occur. There is evidence of the rapid evolution of WSSV since its emergence in shrimp and some strains contain large deletions of the DNA sequence, indicating that these regions of the genome are non‐essential for efficient replication and transmission in shrimp (Dieu et al. 2004; Marks et al. 2005). The high rate of evolution and the severity of disease are characteristics often displayed by infectious agents that have undergone a temporary loss of ecological balance as a result of cross‐species transmission (Steinhauer & Holland 1987).
The mechanism of yellow head disease emergence in farmed shrimp is less clear. Like WSSV, YHV was not known prior to its appearance in Thailand in 1990 (Pasharawipas et al. 1997). However, YHV has a far more limited susceptible host range than WSSV, primarily infecting penaeid and palemonid shrimp, and it is now known that YHV is only one of six closely related genotypes in the yellow head complex that occur commonly in healthy P. monodon throughout its natural distribution in Africa, Asia, Australia and the Pacific (Wijegoonawardane et al. 2008a). Each of the other genotypes is either non‐pathogenic or far less virulent that YHV and there is evidence that some genotypes cluster geographically, suggesting the evolution of separate lineages in geographical isolation prior to aquaculture‐associated shrimp translocations. This suggests that P. monodon is very likely to be the natural host of other genotypes in this complex and may also be the natural host of YHV. If so, the explosive emergence of yellow head disease in Thailand in 1990 is perplexing. One possibility is that a mutation or other genetic process created a highly virulent strain in a formerly non‐pathogenic virus. However, this would imply that non‐virulent strains of the virulent YHV genotype should also continue to exist in P. monodon in Thailand and there has been no evidence of this to date. Another possibility is that yellow head disease has emerged in P. monodon as a result of cross‐species transmission from another similar shrimp species, perhaps a metapenaeid, in which it does not cause disease. If so, the natural host may be an important reservoir of YHV infection and a continuing source of spill‐over into farmed shrimp. Further investigation of the genetic basis of virulence and the susceptibility of other shrimp species may unravel the obscure origins of yellow head disease.
Infectious hypodermal and haematopoietic necrosis virus is almost certainly a natural inhabitant of P. monodon in which it does not appear to cause disease. It occurs at high prevalence throughout the natural geographical range of P. monodon and at least one genotype appears to have integrated into the host genome (Tang & Lightner 2006). There is also evidence of geographical clustering of IHHNV genotypes in Africa and Australia (Tang et al. 2003a; Krabsetsve et al. 2004). However, the history of disease emergence for IHHNV is also associated with cross‐species transmission, in this case as a result of exposure to Western Hemisphere penaeid shrimp species (P. stylirostris and P. vannamei) in which it does cause disease. Other viruses, such as MBV, Mourilyan virus (MoV) and hepatopancreatic parvovirus (HPV), also appear to be natural infections of penaeid shrimp (Flegel 2006), although there is some evidence that both HPV and IHHNV may have distant evolutionary origins in insects (Roekring et al. 2002). Shrimp viruses usually cause low‐level unapparent infections in their natural host and are commonly transmitted vertically. Disease emergence is usually the result of stress, high stocking density or other aspects of the culture system that disturb the natural cycle of infection.
Future disease emergence risks
Aquaculture practices provide abundant opportunities for the emergence and spread of disease and it is highly likely that new diseases will continue to emerge with significant impacts on production. The risk of future disease emergence is directly related to the opportunities created for shrimp to encounter new pathogens as the industry continues to expand and evolve. Live or frozen feeds, particularly those of crustacean or other invertebrate origin, present a particularly high risk of successful pathogen transfer because of the potentially high dose, the route of exposure and the higher likelihood of trans‐species adaptation. Polyculture of shrimp with other aquatic species, particularly invertebrates, also presents a high risk of pathogen transfer. The risk of exposure to new pathogens from natural sources in the environment will increase as shrimp aquaculture expands into new locations. It is perhaps not surprising that three of the major pathogens (WSSV, YHV and TSV) first emerged in countries that were leaders in shrimp culture development. Although the source of IMNV remains obscure, it is also not surprising that this virus first appeared at a time that the shrimp culture industry in Brazil was in a phase of rapid expansion. Africa now looms as a new and exciting opportunity for aquaculture development, but it is also the site of rich biodiversity and has been the source of emergence and re‐emergence of many important animal and human pathogens. The expansion of shrimp aquaculture into Africa may well provide opportunities for cross‐species transmission that will precipitate the emergence and spread of a new array of virulent shrimp pathogens.
As poikilotherms, marine invertebrates are highly sensitive to changes in ambient water temperatures and global warming and associated extreme weather events are likely to impact on many aspects of their ecology and the emergence and spread of disease (Marcogliese 2008). Climate change may also influence the locations and distribution of suitable farming sites, rendering some sites vulnerable to rising sea levels and severe weather, and placing other areas within the suitable climatic zone for efficient production. It could be argued that, for viruses such as WSSV, higher ambient water temperatures will reduce disease risks (Rahman et al. 2006). However, the disruptive environmental effect of rising global temperatures will undoubtedly present new opportunities for the emergence of new pathogens and lead to a generally greater risk of disease.
Impacts of disease in shrimp aquaculture
Socio‐economic impacts
The emergence and spread of disease in shrimp aquaculture has caused economic impacts on a scale rivalling that of many of the major emerging diseases of livestock and humans. It was estimated in 1996 that annual disease‐related losses in shrimp farming globally were ∼US$3000 million or 40% of the total production capacity of the industry (Israngkura & Sae‐Hae 2002). The most devastating impacts have followed the first emergence of each of the major pathogens, which was marked either by a pause in industry expansion or a dramatic drop in the level of production (Fig. 2). In Taiwan in 1987/1988, an epizootic attributed to MBV, but likely to have been a combination of disease and environmental degradation, saw a 70% fall in production and near‐total collapse of the industry (Lightner 1996a). It has been estimated that the combined economic losses as a result of IHHNV and TSV on wild and cultured shrimp fisheries in the Americas, from first emergence to 2001, totalled US$1.5–3.0 billion (Hasson et al. 1999a; Lightner 2003) In China in 1993, the emergence of WSSV resulted in an estimated production loss of US$420 million in a single season (Wei 2002). In Thailand, annual production losses peaked at US$650 million in 1994, falling marginally to US$600 million in 1997 (Timothy Flegel, pers. comm., 1998). Production losses of a similar magnitude have occurred in many shrimp‐farming countries in Asia (Walker 2004; Bondad‐Reantaso et al. 2005) and it has been estimated that the impact of WSSV throughout Asia in the first 10 years after its emergence in 1992 was of the order of US$4–6 billion (Lightner 2003). In the Americas, the emergence of WSSV in 1999–2000 also saw a rapid decline in production with immediate losses estimated at more than US$1 billion. The industry in many countries has never fully recovered (Lightner 2003).
Figure 2.
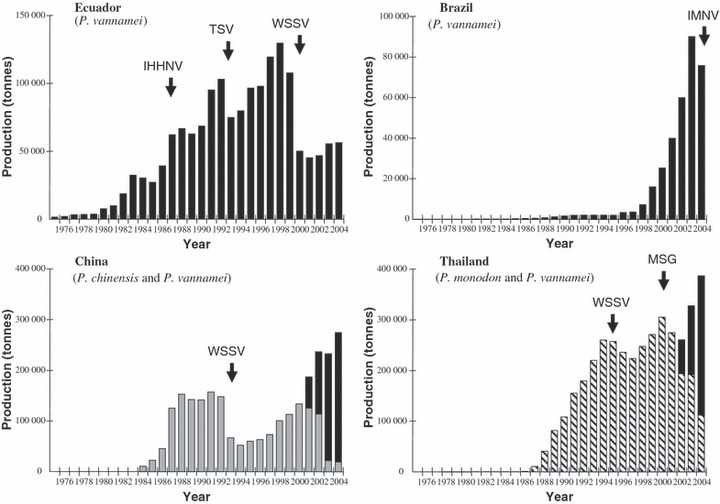
Effect of disease emergence on farmed shrimp production in countries in South America and Asia. IHHNV, infectious hypodermal and haematopoietic necrosis virus; IMNV, infectious myonecrosis virus; MSG, monodon slow growth; TSV, Taura syndrome virus; WSSV, white spot syndrome virus. ▪, P. vannamei;  , P. chinensis;
, P. chinensis;  , P. monodon.
, P. monodon.
Although losses resulting from disease have subsided since the period of peak impact during the 1990s, WSSV and other newly emerging pathogens continue to impact severely, particularly on the small‐holder farmers that comprise much of the industry in major producing countries such as Vietnam, Indonesia and India. Lost production as a result of disease impacts on the income and food security of small‐holder farmers, as well as job security for workers on larger farms and in hatcheries, feed mills and processing plants (Bondad‐Reantaso et al. 2005). As shrimp aquaculture supplies seafood markets in the USA, Europe and Japan, disease also impacts directly on export revenue with flow‐on impacts on socio‐economic development in the sustaining local communities in tropical coastal regions of many developing countries in Asia and the Americas.
Environmental impacts
Assessing the environmental impacts of aquatic animal diseases is a complex and difficult task and rigorous scientific assessments have rarely been conducted. Arthur and Subasinghe (2002) noted that potential impacts of aquatic animal diseases on wild populations and biodiversity could include changes in predator and prey populations, changes in host abundance, reduction in intra‐specific genetic variation, local exterpations and extinctions. However, despite the widespread occurrence of disease in shrimp ponds and evidence that major pathogens occur at relatively high prevalence in wild stocks, there is little available evidence that viral diseases have led to a decline in wild shrimp populations in Asia. This may be because of a combination of low population densities and the likely absence of extreme environment stressors that promote disease emergence in the farming system. It may also reflect a lack of objective data. Nevertheless, there is circumstantial evidence that IHHNV has impacted on populations of wild shrimp in the Americas, with P. stylirostris fisheries landings in the Gulf of Mexico falling to the lowest on record following the introduction of the virus in 1987 (Morales‐Covarrubias et al. 1999), and the risks of serious environmental consequences of known or newly emerging viral diseases should not be underestimated.
The indirect environmental impacts of shrimp disease are more clearly evident. Successive crop failures have led to the abandonment of affected ponds and the relocation of farming operations to new areas, compounding the problem of coastal zone and mangrove habitat degradation (Dierberg & Kiattisimkul 1996; Paez‐Osuna 2001). Abandoned ponds are rarely re‐habilitated for farming and either remain idle or are converted to non‐agricultural land uses, such as housing or manufacturing (Szuster 2006). Crop failures in disease‐prone coastal areas have also led to the development of low‐salinity husbandry practices, resulting in the conversion of irrigated paddy fields and the expansion of shrimp farming into inland areas. There have been concerns that this practice could lead to soil salinization, water pollution and increased competition between agriculture and aquaculture for water resources (Miller et al. 1999; Pongnak 1999; Ali 2006). In Thailand, such environmental concerns have led to a national ban of low‐salinity shrimp farming in inland areas (Szuster 2006).
Difficulties in maintaining reliable production of the major endemic farmed species in Asia have driven a major shift to exotic species. The white Pacific shrimp (P. vanammei), which is native to the west coast of the Americas, has been bred in captivity for many generations from stocks that are free of most known pathogens. Since 1998–1999, white Pacific shrimp have been used for large‐scale commercial production in Asia, rapidly becoming the major production species (Fig. 2). In 2004, it was estimated that 28 000 specific‐pathogen‐free (SPF) P. vannamei broodstock were exported from the hatcheries in Hawaii to Asia each month, translating to a possible 6 billion nauplii and 3 billion postlarve (Briggs et al. 2004). In 2006, P. vannamei production in Asia reached 1.81 million tonnes or 64.8% of total farmed shrimp production (FAO 2008). This may well represent the largest trans‐continental relocation of a single species in the history of the planet. Although P. vannamei are now commonly recovered off the coast of several Asian countries, and concerns have been expressed about their impact on native habitats (Arthington & Bluhdorn 1996; Szuster 2006), there has been little evidence to date of displacement of endemic species and the environmental consequences of this mass translocation remain uncertain.
Another potential indirect environmental impact is the use of antibiotics, disinfectants and other chemicals to prevent disease or to treat shrimp during disease outbreaks (Boyd & Massaut 1999; Paez‐Osuna 2001; Holmstrom et al. 2003; Cabello 2006; Biao & Kaijin 2007). A study conducted in 2000 indicated that 74% of shrimp farmers along the Thai coast used antibiotics in shrimp pond management. At least 13 different antibiotics were in use, mostly as prophylactics (Holmstrom et al. 2003). Subsequent attention to chemical residue testing and bans by importing countries has significantly reduced chemical use in shrimp‐farming operations. Although disinfectants, such as iodophores and hypochlorite, can be used to inactivate viruses contaminating surfaces (Danner & Merrill 2006), there are no commercially available prophylactics or treatments that have been shown to be effective against the major viral pathogens in infected shrimp.
Systemic improvements in response to disease
The impacts of disease on shrimp production and the environment, and the recognition that disease risks are inextricably linked to environmental conditions, have led to systemic improvements in the structure and regulated operation of the industry by international agencies, governments and farmers. Since the mid‐1990s, when disease impacts were most severe, there have been significant technological improvements, including sensitive pathogen screening tests and breeding programs for SPF and specific‐pathogen‐resistant (SPR) stock, and improvements to operational practices, such as structured biosecurity in hatchery and farming systems, and the adoption of Hazard Analysis and Critical Control Points (HACCP) principles and traceability and certification procedures throughout the production cycle (Jahncke et al. 2001; Lightner 2005). There has also been improved communication and cooperation between industry, environmental groups and governments, leading to international agreements on trading practices, increased industry regulation and the development of codes of conduct for sustainable development (Bondad‐Reantaso et al. 2005; FAO, NACA, UNEP, WB, WWF 2006). However, disease continues to impact significantly on production, particularly on small‐holder farmers, and health, environmental and product safety issues remain the highest priorities for the industry globally.
Current disease management strategies and their effectiveness
Principles of disease management in shrimp aquaculture
As invertebrates, shrimp lack the key components of the vertebrate adaptive immune response (e.g. immunoglobulins, major‐histocompatibility‐complex (MHC) antigens, T‐cell receptors) that provide a versatile mechanism for natural protection and allow for conventional vaccination against viruses (Arala‐Chaves & Sequeira 2000; Johnson et al. 2008). Strategies for health management in shrimp aquaculture are therefore based primarily on the principles of pathogen exclusion and the avoidance of environmental conditions that induce stress, stimulate viral replication or facilitate disease transmission. Effective pathogen exclusion practises require attention to all points in the shrimp production cycle, from spawning to harvest, at which viruses may be encountered or recycled into the environment (Fegan & Clifford 2001). For P. vannamei and P. stylirostris, the availability of SPF and SPR stock, bred in captivity under biosecure conditions, has provided a source of nauplii and postlarvae from which the major pathogens have been excluded (Lightner 2005). However, for most farmed shrimp species, including P. monodon, SPF stock are not widely available and broodstock continue to be sourced from the wild. As many of the viral pathogens of concern are common in wild stocks, stringent viral screening of broodstock and/or seed is an essential pathogen exclusion practice. On‐farm biosecurity includes pond preparation by drying and sunlight exposure to eliminate residual pathogens and aquatic crustaceans in soil, filtration and disinfection of pond water prior to stocking and prior to exchange during grow‐out, careful monitoring and control of water quality, fencing to exclude potential carrier crustaceans, netting to exclude birds that may feed on and distribute moribund shrimp, physical separation of pond implements, and treatment of effluents to prevent discharge of contaminated pond water into the environment (Fegan & Clifford 2001; Vanpatten et al. 2004; Subasinghe 2005). Monitoring of shrimp during grow‐out for early signs of disease, such as lethargy, gill fouling, unusual swimming behaviour, feeding patterns or colouration, and regular histological examination for evidence of infection is also commonly used to varying degrees by farmers.
Disease management for medium–large semi‐intensive farms
The implementation of effective disease management by medium–large semi‐intensive farmers has impacted very significantly on productivity and delivered a range of other benefits to farmers and the community. These include less reliance on wild fisheries as a source of broodstock, better management of land and water usage and treatment of effluents, decreased use of chemicals and antibiotics, and improved quality of record keeping from stocking to market. Biosecurity is now an integral aspect of farm and hatchery management for many medium–large farms and has largely been responsible for the recovery of the industry that has seen the volume of farmed shrimp production increase more than threefold from 2000 to 2006 (FAO 2006). Although this has been driven by the availability of SPF P. vannamei in China, Thailand, Indonesia and Vietnam, successful farming operations using SPF stock have been underpinned by, and continue to rely on, a more stringent approach to biosecurity.
Disease management for poorer small‐holder farmers
For low‐income, small‐holder farmers (<1 ha holding), who constitute a very significant sector of the industry in some Asian countries, limited education and lack of resources to implement comprehensive disease management practices are major barriers to achieving reliable production (Padiyar 2009). White spot syndrome virus continues to impact severely on small‐holder shrimp farmers and the importation of non‐SPF P. vannamei from the western hemisphere has seen the arrival of new viral pathogens, such as TSV and IMNV. These are now causing significant production losses in several countries and have the potential for further geographical spread and to infect other shrimp species (Chang et al. 2004; Srisuvan et al. 2005; Tang et al. 2005; Phalitakul et al. 2006; Senapin et al. 2007). Furthermore, although SPF P. vannamei offer some advantage as a source of high‐quality seed, they remain susceptible to infection by WSSV, YHV, TSV and other pathogens that are endemic throughout extensive farming systems in Asia.
In view of the importance of aquaculture in maintaining food security and sustaining livelihoods in poor rural and coastal communities, there has been growing international attention to the development and implementation of practical, affordable and effective measures to reduce disease and environmental impacts for small‐holder farmers. ‘Better Management Practices’ (BMP) have been developed by international teams of specialists in conjunction with industry and government. These BMP are based on published scientific information on the biology of infection, epidemiological studies to identify disease risk factors, empirical observations and successful industry practices, and provincial, national and international regulatory requirements (Subasinghe 2005; Mohan et al. 2008). In alignment with the International Principles for Responsible Shrimp Farming (FAO, NACA, UNEP, WB, WWF 2006), BMP are designed to support small producers to: (i) increase efficiency and productivity by reducing the risk of shrimp health problems; (ii) reduce or mitigate the impacts of farming on the environment; (iii) improve food safety and the quality of shrimp farm product; and (iv) improve the social benefits from shrimp farming and its social acceptability and sustainability (Mohan et al. 2008). Better Management Practices have been developed to provide guidance at all stages of the production cycle from hatchery operation to pond bottom preparation and water management prior to stocking, seed selection and stocking, and post‐stocking management of ponds (Subasinghe & Bondad‐Reantaso 2006; ADB, ACIAR, AwF, BRR, DKP, FAO, GTZ, IFC, MMAF, NACA, WWF 2007). Better Management Practices are often voluntary practices, but can also be used as a basis for local regulations or certification programmes. A critical aspect of the introduction of BMP has been the role of farmer groups and their effective linkage to the public sector (Mohan et al. 2008). The provision of science‐based information to farmer groups through effective networking and communication is an important key to success (Corsin et al. 2007). The successful application of BMP in the shrimp‐farming sector in Asia has led to the development of BMP for small‐scale farming of other aquatic species.
The availability of high‐quality seed is a critical factor in reducing the risk of crop failure during grow‐out. The increasing use of SPF P. vannamei broodstock as a source of seed has significantly improved seed quality for medium–large semi‐intensive farms, but small‐scale farmers continue to rely principally on seed obtained from wild spawners or other sources of non‐SPF boodstock. Polymerase chain reaction screening of broodstock, nauplii and/or postlarvae prior to stocking to eliminate the major viral pathogens, such as WSSV, is therefore a critical requirement to reduce the risk of disease during grow‐out (Hsu et al. 1999; Mushiake et al. 1999; Withyachumnarnkul 1999). This has been facilitated by the commercial availability of an increasing number of PCR test kits and the proliferation of government and private PCR service laboratories in shrimp‐farming regions. However, recent national proficiency testing surveys in India, Indonesia and Vietnam indicate that less than 50% of the participating laboratories were able to correctly identify standard positive and negative samples (Walker and Gudkovs, unpublished data, 2007). This deficiency is being addressed through measures such as training and the implementation of national registration and accreditation programs for PCR service laboratories. A more widespread adoption of simple, inexpensive and rapid pond‐side test formats may also prove to be a very useful adjunct to PCR screening by eliminating heavily infected postlarvae and providing small‐scale farmers with more control over seed quality (Sithigorngul et al. 2006; Patil et al. 2007).
Transboundary pathogen movements
One of the most difficult biosecurity issues facing the shrimp‐farming industry is transboundary movement of pathogens through trade in live shrimp (Subasinghe & Bondad‐Reantaso 2008). The rapid spread of WSSV and TSV within countries and regions, and between hemispheres, has been attributed primarily to the movement of live broodstock and seed. Shrimp seed (nauplii and postlarvae) are commonly transported freely within countries and there is a prolific international and transcontinental trade in both wild‐caught and domesticated shrimp broodstock. Each of the major viral infections of shrimp can occur as low‐level persistent infections in apparently healthy stock. Although PCR screening can be an effective method of detection, the vast numbers of animals being moved daily, the inadequacy of international and internal quarantine inspection capabilities, illegal trading in unscreened stock and the substitution of unscreened stock for certified SPF stock all contribute to a very high ongoing risk of transboundary disease spread. The potential for rapid translocation was first realized during the explosive WSSV emergence in Asia between 1992 and 1994, and has been demonstrated most recently by the emergence and spread of IMNV since 2002. The transfer of TSV and IMNV to Asia from the Americas has been a direct consequence of the importation of large numbers of P. vannamei broodstock, not all of which have been ‘SPF’, and the subsequent use of pond‐raised progeny in local breeding programs. This has occurred despite the knowledge and costly experience of previous pandemic disease spread.
The transboundary spread of viruses in frozen uncooked shrimp has also been an issue of concern (Durand et al. 2000). A total of 4.5 million tonnes of commodity shrimp were traded globally in 2006, ∼75% of which was transported from Asia to Europe and the USA (FAO 2006). Several studies have demonstrated the presence of live WSSV and YHV in imported frozen commodity shrimp and bait shrimp (Nunan et al. 1998; Durand et al. 2000; McColl et al. 2004; Hasson et al. 2006) and identified risks to both farming and the environment. The Sanitary and Phyto‐sanitary (SPS) Agreement of the World Trade Organization provides the basis on which member countries may promote free trade without increasing the risk of the introduction and spread of aquatic animal pathogens. To limit the potential for future disease spread, it is essential that national aquatic animal health policies and strategies provide the basis for the implementation of measures specified in the SPS and associated standards (OIE 2008).
Future prospects
New technologies
There are indications that at least partial solutions to problems of health management in shrimp aquaculture may lie in new technologies. Although conventional vaccination, mediated by antibodies, cytokines and lymphocytes, is not possible because of the lack of a complex adaptive immune system, there is growing evidence that shrimp use a range of mechanisms to respond to viruses and these may be exploited to prevent disease or limit the spread of infection.
The first suggestion of a form of induced anti‐viral immunity in shrimp was obtained when shrimp surviving natural or experimental WSSV infection were shown to remain resistant to experimental WSSV challenge for up to 4 months (Venegas et al. 2000; Wu et al. 2002). It was subsequently demonstrated that the injection of formalin‐inactivated WSSV or recombinant WSSV envelope proteins (VP26, VP28, VP19, VP292 or VP466) can also induce protection against experimental challenge, with VP28 apparently providing the most potent protection (Namikoshi et al. 2004; Witteveldt et al. 2004a; Vaseeharen et al. 2006; Ha et al. 2008). Protection can also be induced by oral delivery of formalin‐inactivated virus (Bright Singh et al. 2005) or recombinant WSSV envelope proteins expressed from bacterial plasmids or baculovirus vectors (Witteveldt et al. 2004b; Ha et al. 2008). The injection of DNA plasmids expressing envelope proteins VP28 and VP281 also induces protection, but DNA ‘vaccines’ expressing the nucleocapsid proteins VP15 or VP35 have failed to protect (Rout et al. 2007; Kumar et al. 2008). Relative protection levels using WSSV envelope proteins delivered directly or expressed from DNA are usually in the order of 50–90%, but immunity is relatively short lived, with a reported duration of approximately 4–7 weeks depending on the formulation. The mechanism by which WSSV proteins induce protection in shrimp is not well understood. Recent evidence suggests that anti‐viral phagocytosis in shrimp may be controlled by a Rab GTPase that is upregulated in WSSV‐resistant shrimp and forms a complex with β‐actin, tropomycin and the WSSV envelope protein VP466 (Wu & Zhang 2007; Wu et al. 2008). It has also been suggested that autophagy, a homeostatic mechanism by which cells digest and re‐cycle macromolecules and organelles by lysosomal digestion, may be involved (Johnson et al. 2008).
RNA interference (RNAi) is another anti‐viral strategy that has been shown to have the potential for application in shrimp. RNAi is a mechanism of post‐transcriptional gene silencing by which dsRNA, delivered into cells, is digested into small (∼21–23 nt) fragments that form a complex with cellular proteins that recognises, targets and guides the digestion of mRNA containing homologous sequences (Fire 1999; Tijsterman & Plasterk 2004; Su et al. 2008). It has been shown that RNAi is active in shrimp and that injection of dsRNA or short interfering (si)RNA homologous to viral mRNA can effectively block WSSV, TSV and YHV infection (Robalino et al. 2005; Yodmuang et al. 2006; Xu et al. 2007). Inhibition of viral infection can be complete when shrimp are challenged within 1 day of the dsRNA treatment by injection, but the long‐term durability of the protective effect has not yet been adequately assessed. A non‐specific anti‐viral immunity has also been induced in shrimp by long dsRNA or siRNA, but targeting to specific viral sequences, particularly essential replication genes, is far more potent (Robalino et al. 2004; Westenberg et al. 2005).
For each of these potential anti‐viral strategies, the core remaining problem is the development of practical and cost‐effective methods for application on a commercial scale. For protein‐based ‘vaccines’, the potency of oral delivery suggests that application in shrimp feed may be practicable, but there is a lack of published experimental evidence to demonstrate the efficacy of this approach. The most obvious potential application of RNAi is in the therapeutic treatment of infected broodstock in hatcheries to generate virus‐free progeny, either for farm production or domesticated breeding programs. The prolongation of anti‐viral effects by microparticle delivery of stable DNA constructs expressing envelope proteins and/or hairpin RNA also requires further exploration.
Development and implementation of national aquatic animal health strategies
The ongoing difficulties in the management of shrimp disease are reflected in other sectors of the aquaculture industry, particularly in Asia, which accounts for >90% of global aquaculture production (FAO 2006). It is now widely recognised that there is a need to address deficiencies in aquatic animal health management and to support sustainable aquaculture development through the implementation of national strategies that parallel those adopted in many countries for terrestrial animal diseases. These include such measures as the identification of national authorities with the responsibility and competence to supervise the implementation of aquatic animal health measures, national lists of reportable diseases, national aquatic animal health committees with the responsibility for coordinating and advising governments on disease issues, national surveillance and disease reporting programs, national contingency programs for dealing with disease emergencies, competent national and regional diagnostic facilities and planned programs for building capability and capacity (Bondad‐Reantaso et al. 2005). National strategies should be simple, practical and achievable within available resource allocations, and should not only consider farm‐level disease management, but also the control of transboundary pathogen movements, management of chemical use, food safety and compliance with regional and international agreements and standards.
Infrastructure development
The intersection of shrimp health and environmental sustainability, and the need to support socio‐economic development in poor coastal communities, should also lead governments to consider the need for infrastructure development in small‐holder shrimp‐farming regions. Commonly, shrimp ponds have proliferated in river estuaries in an unplanned and unstructured fashion without consideration of the need for appropriate water or land management. Water intake to ponds and effluent discharge are very often drawn from, and returned to, the same shared canal, and there is little cooperation in the event of disease outbreaks to avoid cross‐contamination of ponds. White spot syndrome virus is endemic in wild crustaceans in many of these farming systems and, notwithstanding strict adherence to BMP, it is doubtful if reliable production will ever be achieved without substantial investment in infrastructure. As poor small‐holder farmers lack the necessary resources, this will require significant investment by governments or corporate benefactors in the interest of poverty alleviation and environmental sustainability.
Attitudinal change
Effective disease management is a shared responsibility in which all stakeholders, from farmers to policy makers, must understand their roles and exercise responsible attitudes and behaviours. Cooperation between industry and government and adherence to policies and practices that serve long‐term sustainability and profitability rather than short‐term gains will be essential if past disease emergence scenarios and immense socio‐economic and environmental impacts are to be avoided in the future. The need for responsible behaviour applies particularly to the core problem of transboundary pathogen dispersal through irresponsible trade in live shrimp. This is a significant challenge as year‐to‐year profitability and survival are strong drivers of industry attitudes and lack of education and traditional beliefs are widespread in poor coastal communities. Achieving attitudinal change and compliance across this diverse trader sector, often operating beyond national jurisdictions, presents major challenges to policy makers and regulators. Nevertheless, attitudinal change can be achieved through effective communication and an increasing number of peak industry bodies and other farmer groups are engaging with government to assist policy formulation. Creating awareness of the importance of aquatic animal health management and its relevance to sustainable aquaculture development, aquatic animal trade and human food safety will greatly assist in achieving attitudinal changes in key stakeholders.
Conclusion
The pattern of emergence and the global spread of an increasing array of new viral diseases of shrimp is the logical consequence of the massive ecological shift and changes in trade patterns that have accompanied the establishment and growth of a new farming industry. Key aspects of the biology and epidemiology of infection are now known for most of the major pathogens and technological advances have enabled the production of SPF broodstock and provided sensitive detection tools that allow screening of seed to eliminate pathogens. Although new pathogens will almost certainly emerge as the industry continues to expand into new locations and responds to the challenges presented by global warming, we should now be far better prepared to limit impacts and contain the spread of disease. The primary constraints on the effective management of existing or future diseases lie in the will and capacity of governments and industry to respond with appropriate policies and practices and the necessary levels of investment in training and infrastructure development. International cooperation and productive alliances of governments, industry and the community will be required to underpin the goal of profitable and environmentally sustainable aquaculture development.
Acknowledgement
The authors would like to thank Dr Nusra Sittidilokratna and Dr Jeff Cowley, CSIRO Livestock Industries, Australia, for pre‐review editing of the manuscript.
References
- ADB, ACIAR, AwF, BRR, DKP, FAO, GTZ, IFC, MMAF, NACA, WWF (2007) A Practical Manual on Better Management Practices for Tambak Farming in Aceh. Asian Development Bank ETESP, Australian Centre for International Agriculture Research, Food and Agriculture Organization of the United Nations, International Finance Corporation of the World Bank Group, Banda Aceh. [Google Scholar]
- Alday de Graindorge V (2000) Disease management protocols and semi‐intensive shrimp farming in Ecuador. Global Aquaculture Advocate 3 (2): 17–19. [Google Scholar]
- Ali AMS (2006) Rice to shrimp: Land use/land cover changes and soil degradation in southwestern Bangladesh. Land Use Policy 23: 421–435. [Google Scholar]
- Arala‐Chaves M, Sequeira T (2000) Is there any kind of adaptive immunity in invertebrates? Aquaculture 191: 247–258. [Google Scholar]
- Argue BJ, Arce SM, Lotz JM, Moss SM (2002) Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura syndrome virus. Aquaculture 204: 447–460. [Google Scholar]
- Arthington AH, Bluhdorn DR (1996) The results of species interactions resulting from aquaculture operations In: Baird DJ, Beveridge M, Kelly L, Muir JF. (eds) Aquaculture and Water Resources Management, pp. 114–139. Blackwell Scientific Publications, Oxford. [Google Scholar]
- Arthur JR, Subasinghe RP (2002) Potential adverse socio‐economic and biological impacts of aquatic animal pathogens due to hatchery‐based enhancement of inland open‐water systems, and possibilities for their minimisation In: Arthur JR, Phillips MJ, Subasinghe RP, Reantaso MB, MacRae IH. (eds) Primary Aquatic Animal Health Care in Rural, Small‐scale, Aquaculture Development, pp. 113–126. FAO Fisheries Technical Paper No. 406, Rome. [Google Scholar]
- Assavalapsakul W, Tirasophon W, Panyim S (2005) Antiserum to the gp116 glycoprotein of yellow head virus neutralizes infectivity in primary lymphoid organ cells of Penaeus monodon . Diseases of Aquatic Organisms 63: 85–88. [DOI] [PubMed] [Google Scholar]
- Bell TA, Lightner DV (1984) IHHN virus: infectivity and pathogenicity studies in Penaeus stylirostris and Penaeus vannamei . Aquaculture 38: 185–194. [Google Scholar]
- Biao X, Kaijin Y (2007) Shrimp farming in China: operating characteristics, environmental impact and perspectives. Ocean and Coastal Management 50: 538–550. [Google Scholar]
- Bonami JR, Trumper B, Mari J, Brehelin M, Lightner DV (1990) Purification and characterization of the infectious hypodermal and haematopoietic necrosis virus of penaeid shrimps. Journal of General Virology 71: 2657–2664. [DOI] [PubMed] [Google Scholar]
- Bonami JR, Hasson KW, Mari J, Poulos BT, Lightner DV (1997) Taura syndrome of marine penaeid shrimp: characterization of the viral agent. Journal of General Virology 78: 313–319. [DOI] [PubMed] [Google Scholar]
- Bondad‐Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R et al. (2005) Disease and health management in Asian aquaculture. Veterinary Parasitology 132: 249–272. [DOI] [PubMed] [Google Scholar]
- Bonnichon V, Lightner DV, Bonami JR (2006) Viral interference between infectious hypodermal and hematopoietic necrosis virus and white spot syndrome virus in Litopenaeus vannamei . Diseases of Aquatic Organisms 72: 179–184. [DOI] [PubMed] [Google Scholar]
- Boonyaratpalin S, Supamattaya K, Kasornchandra J, Direkbusaracom S, Aekpanithanpong U, Chantanachookin C (1993) Non‐occluded baculo‐like virus, the causative agent of yellow head disease in the black tiger shrimp (Penaeus monodon). Fish Pathology 28: 103–109. [Google Scholar]
- Boyd CE, Massaut L (1999) Risks associated with the use of chemicals in pond aquaculture. Aquacultural Engineering 20: 113–132. [Google Scholar]
- Briggs M, Funge‐Smith S, Subasinghe R, Phillips M (2004) Introductions and Movement of Penaeus vannamei and Penaeus stylirostris in Asia and the Pacific. FAO Regional Office for Asia and the Pacific, Bangkok. [Google Scholar]
- Bright Singh IS, Manjusha M, Somnath Pai S, Phillip R (2005) Fenneropenaeus indicus is protected from white spot disease by oral administration of inactivated white spot syndrome virus. Diseases of Aquatic Organisms 66: 265–270. [DOI] [PubMed] [Google Scholar]
- Brock JA (1997) Special topic review: Taura syndrome, a disease important to shrimp farms in the Americas. World Journal of Microbiology and Biotechnology 13: 415–418. [Google Scholar]
- Brock JA, Gose R, Lightner DV, Hasson KA (1995) An overview on Taura syndrome, an important disease of farmed Penaeus vannamei In: Browdy CL, Hopkins JS. (eds) Swimming through Troubled Water: Proceedings of the Special Session on Shrimp Farming, Aquaculture ‘95, pp. 84–94. The World Aquaculture Society, Baton Rouge. [Google Scholar]
- Brown C (1997) Emerging diseases: what veterinarians need to know. Journal of Veterinary Diagnostic Investigation 9: 113–117. [DOI] [PubMed] [Google Scholar]
- Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental Microbiology 8: 1137–1144. [DOI] [PubMed] [Google Scholar]
- Callinan RB, Jiang L (2003) Fatal virus‐associated peripheral neuropathy and retinopathy in farmed Penaeus monodon in eastern Australia. II. Outbreak descriptions. Diseases of Aquatic Organisms 53: 195–202. [DOI] [PubMed] [Google Scholar]
- Callinan RB, Jiang L, Smith PT, Soowannayan C (2003) Fatal virus‐associated peripheral neuropathy and retinopathy in farmed Penaeus monodon in eastern Australia. I. Pathology. Diseases of Aquatic Organisms 53: 181–193. [DOI] [PubMed] [Google Scholar]
- Chaivisuthangkura P, Tejangkura T, Rukpratanporn S, Longyant S, Sithigorngul W, Sithigorngul P (2008) Preferential suppression of yellow head virus (YHV) envelope protein gp116 in shrimp that survive challenge with YHV. Diseases of Aquatic Organisms 79: 1–8. [DOI] [PubMed] [Google Scholar]
- Chang PS, Lo CF, Kou GH, Chen SN (1993) Purification and amplification of DNA from Penaeus monodon‐type baculovirus (MBV). Journal of Invertebrate Pathology 62: 116–120. [DOI] [PubMed] [Google Scholar]
- Chang PS, Lo CF, Wang YC, Kou GH (1996) Identification of white spot syndrome associated baculovirus (WSBV) target organs in the shrimp Penaeus monodon by in situ hybridization. Diseases of Aquatic Organisms 27: 131–139. [Google Scholar]
- Chang YS, Peng SE, Yu HT, Liu FC, Wang CH, Lo CF et al. (2004) Genetic and phenotypic variations of isolates of shrimp Taura syndrome virus found in Penaeus monodon and Metapenaeus ensis in Taiwan. Journal of General Virology 85: 2963–2968. [DOI] [PubMed] [Google Scholar]
- Chantanachookin C, Boonyaratpalin S, Kasornchandra J, Direkbusarakom S, Ekpanithanpong U, Supamataya K et al. (1993) Histology and ultrastructure reveal a new granulosis‐like virus in Penaeus monodon affected by yellow‐head disease. Diseases of Aquatic Organisms 17: 145–157. [Google Scholar]
- Chayaburakul K, Nash G, Pratanpipat P, Sriurairatana S, Withyachumnarnkul B (2004) Multiple pathogens found in growth‐retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Diseases of Aquatic Organisms 60: 89–96. [DOI] [PubMed] [Google Scholar]
- Chayaburakul K, Lightner DV, Sriurairattana S, Nelson KT, Withyachumnarnkul B (2005) Different responses to infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Penaeus monodon and P. vannamei . Diseases of Aquatic Organisms 67: 191–200. [DOI] [PubMed] [Google Scholar]
- Chen SN, Chang PS, Kou GH, Lightner DV (1989a) Studies on virogenesis and cytopathology of Penaeus monodon baculovirus (MBV) in the giant tiger prawn (Penaeus monodon) and the red tail prawn (Penaeus penicilatus). Fish Pathology 24: 89–100. [Google Scholar]
- Chen SN, Chang PS, Kou GH (1989b) Observation on pathogenicity and epizootiology of Penaeus monodon baculovirus (MBV) in cultured shrimp in Taiwan. Fish Pathology 24: 189–195. [Google Scholar]
- Chen SN, Chang PS, Kou GH (1992) Infectious route and eradication of Penaeus monodon baculovirus (MBV) in larval giant tiger prawns, Penaeus monodon In: Fulks W, Main KL. (eds) Diseases of Cultured Penaeid Shrimp in Asia and the United States, pp. 177–184. Oceanic Institute, Honolulu. [Google Scholar]
- Childs JE, Richt JA, Mackenzie JS (2007) Conceptualizing and partitioning the emergence process of zoonotic viruses from wildlife to humans. Current Topics in Microbiology and Immunology 315: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HY, Huang CY, Wang CH, Chaing HC, Lo CF (1995) Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Diseases of Aquatic Organisms 23: 165–173. [Google Scholar]
- Chou HY, Huang CY, Lo CF, Kou GH (1998) Studies on transmission of white spot syndrome associated baculovirus (WSBV) in Penaeus monodon and P. japonicus via waterborne contact and oral ingestion. Aquaculture 164: 263–276. [Google Scholar]
- Christian P, Carstens E, Domier L, Johnson J, Johnson K, Nakashima N et al. (2005) Family Dicistroviridae In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (eds) Virus Taxonomy, Eighth Report of the International Committee on Taxonomy of Viruses, pp. 783–788. Elsevier, Academic Press, Amsterdam. [Google Scholar]
- Corbel V, Zuprizal , Shi Z, Huang C, Sumartono , Arcier JM et al. (2001) Investigation on the potential hosts of the white spot syndrome virus (WSSV) in European waters, by experimental infection. Journal of Fish Diseases 24: 377–382. [Google Scholar]
- Corsin F, Giorgetti G, Mohan CV (2007) Contribution of science to farm‐level aquatic animal health management. Developments in Biologicals 129: 35–40. [PubMed] [Google Scholar]
- Costa R, Mermoud I, Mari J, Bonami JR, Hasson K, Lightner DV (1998) Investigations of Penaeus stylirostris disease (Syndrome 93) in New Caledonia, exploring a viral hypothesis. Aquaculture 164: 311–322. [Google Scholar]
- Cowley JA, Walker PJ (2002) The complete sequence of gill‐associated virus of Penaeus monodon prawns indicates a gene organisation unique among nidoviruses. Archives of Virology 147: 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley JA, Walker PJ (2008) Molecular biology and pathogenesis of roniviruses In: Perlman S, Gallagher T, Snijder EJ. (eds) Nidoviruses, pp. 361–377. ASM Press, Washington. [Google Scholar]
- Cowley JA, Dimmock CM, Wongteerasupaya C, Boonsaeng V, Panyim S, Walker PJ (1999) Yellow head virus from Thailand and gill‐associated virus from Australia are closely related but distinct viruses. Diseases of Aquatic Organisms 36: 153–175. [DOI] [PubMed] [Google Scholar]
- Cowley JA, Dimmock CM, Spann KM, Walker PJ (2000a) Gill‐associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri‐ and coronaviruses. Journal of General Virology 81: 1473–1484. [DOI] [PubMed] [Google Scholar]
- Cowley JA, Dimmock CM, Spann KM, Walker PJ (2000b) Detection of Australian gill‐associated virus (GAV) and lymphoid organ virus (LOV) of Penaeus monodon by RT‐nested PCR. Diseases of Aquatic Organisms 39: 159–167. [DOI] [PubMed] [Google Scholar]
- Cowley JA, Hall MR, Cadogan LC, Spann KM, Walker PJ (2002) Vertical transmission of gill‐associated virus (GAV) in the black tiger prawn Penaeus monodon . Diseases of Aquatic Organisms 50: 95–104. [DOI] [PubMed] [Google Scholar]
- Cowley JA, Cadogan LC, Wongteerasupaya C, Hodgson RAJ, Boonsaeng V, Walker PJ (2004) Multiplex RT‐nested PCR differentiation of gill‐associated virus (Australia) from yellow head virus (Thailand) of Penaeus monodon . Journal of Virological Methods 117: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner GR, Merrill P (2006) Disinfectants, disinfection, and biosecurity in aquaculture In: Scarfe AD, Lee CS, O’Bryen PJ. (eds) Aquaculture Biosecurity: Prevention, Control, and Eradication of Aquatic Animal Disease, pp. 91–128. Blackwell Publishing, Ames. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD (2001) Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica 78: 103–116. [DOI] [PubMed] [Google Scholar]
- Dhar AK, Cowley JA, Hasson KW, Walker PJ (2004) Genomic organization, biology, and diagnosis of Taura syndrome virus and yellowhead virus of penaeid shrimp. Advances in Virus Research 63: 347–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar AK, Lakshman DK, Natarajan S, Allnutt FCT, Van Beek NAM (2007) Functional characterization of putative promoter elements from infectious hypodermal and hematopoietic necrosis virus (IHHNV) in shrimp and in insect and fish cell lines. Virus Research 127: 1–8. [DOI] [PubMed] [Google Scholar]
- Dierberg FE, Kiattisimkul W (1996) Issues, impacts and implications of shrimp aquaculture in Thailand. Environmental Management 20: 649–666. [DOI] [PubMed] [Google Scholar]
- Dieu BT, Marks H, Siebenga JJ, Goldbach RW, Zuidema D, Duong TP et al. (2004) Molecular epidemiology of white spot syndrome virus within Vietnam. Journal of General Virology 85: 3607–3618. [DOI] [PubMed] [Google Scholar]
- Do JW, Cha SJ, Lee NS, Kim YC, Kim JW, Kim JD et al. (2006) Taura syndrome virus from Penaeus vannamei shrimp cultured in Korea. Diseases of Aquatic Organisms 12: 171–174. [DOI] [PubMed] [Google Scholar]
- Doubrovsky A, Paynter JL, Sambhi SK, Atherton JG (1988) Observations on the ultrastructure of baculovirus in Australian Penaeus monodon and Penaeus mergiuensis . Australian Journal of Marine and Freshwater Research 39: 743–749. [Google Scholar]
- Du HH, Li WF, Xu ZR, Kil ZS (2006) Effect of hyperthermia on the replication of white spot syndrome virus (WSSV) in Procambarus clarkia . Diseases of Aquatic Organisms 71: 175–178. [DOI] [PubMed] [Google Scholar]
- Durand SV, Tang KFJ, Lightner D (2000) Frozen commodity shrimp: potential avenue for introduction of white spot syndrome virus and yellow head virus. Journal of Aquatic Animal Health 12: 128–135. [Google Scholar]
- East IJ, Black PF, McColl KA, Hodgson RA, Bernoth EM (2004) Survey for the presence of white spot syndrome virus in Australian crustaceans. Australian Veterinary Journal 82: 236–240. [DOI] [PubMed] [Google Scholar]
- Erickson HS, Zarain‐Herzberg M, Lightner DA (2002) Detection of Taura syndrome virus (TSV) strain differences using selected diagnostic methods: diagnostic implications in penaeid shrimp. Diseases of Aquatic Organisms 52: 1–10. [DOI] [PubMed] [Google Scholar]
- Erickson HS, Poulos BT, Tang KF, Bradley‐Dunlop D, Lightner DA (2005) Taura syndrome virus from Belize represents a unique variant. Diseases of Aquatic Organisms 64: 91–98. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) (2006) The State of World Fisheries and Aquaculture. Food and Agricultural Organization of the United Nations, Rome. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) (2008) Fishstat Plus Version 2.32, 2000. Food and Agricultural Organization of the United Nations, Rome. [Google Scholar]
- FAO, NACA, UNEP, WB, WWF (2006) International Principles for Responsible Shrimp Farming. Network of Aquaculture Centres in Asia‐Pacific, Bangkok. [Google Scholar]
- Fegan DF, Clifford HC (2001) Health management for viral diseases in shrimp farms In: Browdy CL, Jory DE. (eds) The New Wave: Proceedings of the Special Session on Sustainable Shrimp Farming, Aquaculture 2001, pp. 168–198. The World Aquaculture Society, Baton Rouge. [Google Scholar]
- Fegan DF, Flegel TW, Sriurairatana S, Waiyakruttha M (1991) The occurrence, development and histopathology of monodon baculovirus in Penaeus monodon in southern Thailand. Aquaculture 96: 205–217. [Google Scholar]
- Fire A (1999) RNA‐triggered gene silencing. Trends in Genetics 15: 358–363. [DOI] [PubMed] [Google Scholar]
- Flegel TW (2006) Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture 258: 1–33. [Google Scholar]
- Flegel TW, Sriurairatana S, Wongteerasupaya C, Boonsaeng V, Panyim S, Withyachumnarnkul B (1995) Progress in characterization and control of yellow‐head virus of Penaeus monodon In: Browdy CL, Hopkins JS. (eds) Swimming Through Troubled Water, Proceedings of the Special Session on Shrimp Farming, Aquaculture ’95, pp. 76–83. World Aquaculture Society, Baton Rouge. [Google Scholar]
- Flegel TW, Boonyaratpalin S, Withyachumnarnkul B (1997a) Progress in research on yellow‐head virus and white‐spot virus in Thailand In: Flegel TW, MacRae JH. (eds) Diseases in Asian Aquaculture III, Fish Health Section, pp. 285–295. Asian Fisheries Society, Manila. [Google Scholar]
- Flegel TW, Sriurairatana S, Wongteerasupaya C, Boonsaeng V, Panyim S, Withyachumnarnkul B (1997b) Progress in characterization and control of yellow‐head virus of Penaeus monodon In: Flegel TW, Menasveta P, Paisarnrat S. (eds) Shrimp Biotechnology in Thailand, pp. 71–78. National Center for Genetic Engineering and Biotechnology, Bangkok. [Google Scholar]
- Flegel TW, Nielsen L, Thamavit V, Kongtim S, Pasharawipas T (2004) Presence of multiple viruses in non‐diseased cultivated shrimp at harvest. Aquaculture 240: 55–68. [Google Scholar]
- Fukuda H, Momoyama K, Sato T (1988) First detection of monodon baculovirus in Japan. Nippon Suisan Gakkaishi 54: 45–48. [Google Scholar]
- Garza JR, Hasson KW, Poulos BT, Redman RM, White BL, Lightner DV (1997) Demonstration of infectious Taura syndrome virus in the feces of sea gulls collected during an epizootic in Texas. Journal of Aquatic Animal Health 9: 156–159. [Google Scholar]
- Geoffroy MC, Chadeuf G, Orr A, Salvetti A, Everett RD (2006) Impact of the interaction between herpes simplex virus type 1 regulatory protein ICP0 and ubiquitin‐specific protease USP7 on activation of adeno‐associated virus type 2 rep gene expression. Journal of Virology 80: 3650–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goarant C, Brizard R, Marteau AL (2000) A white spot disease‐like syndrome in the Pacific blue shrimp (Litopenaeus stylirostris) as a form of bacterial shell disease. Aquaculture 183: 25–30. [Google Scholar]
- Granja CB, Vidal OM, Parra G, Salazar M (2006) Hyperthermia reduces viral load of white spot syndrome virus in Penaeus vannamei . Diseases of Aquatic Organisms 68: 175–180. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yu Z, Li C (2003) The effects of temperature on white spot syndrome infections in Marsupenaeus japonicus . Journal of Invertebrate Pathology 83: 257–260. [DOI] [PubMed] [Google Scholar]
- Ha YM, Gong SL, Nguyen TH, Ra CH, Kim KH, Nam YK et al. (2008) Vaccination of shrimp (Penaeus chinensis) against white spot syndrome virus (WSSV). Journal of Microbiology and Biotechnology 18: 964–967. [PubMed] [Google Scholar]
- Hao NV, Thuy DT, Loan LTT, Phi TT, Phuoc LH, Duong HHT et al. (1999) Presence of the two viral pathogens WSSV and MBV in three wild shrimp species (Penaeus indicus, Metapenaeus ensis and Metapenaeus lysianassa) cultured in the mangrove forest of Ca Mau Province. Asian Fisheries Science 12: 309–325. [Google Scholar]
- Hasson KW, Lightner DV, Poulos BT, Redman RM, White BM, Brock JA et al. (1995) Taura syndrome in Penaeus vannamei: demonstration of viral etiology. Diseases of Aquatic Organisms 28: 115–126. [Google Scholar]
- Hasson KW, Lightner DV, Mohney LL, Redman RM, Poulos BT, White BM (1999a) Taura syndrome virus (TSV) lesion development and the disease cycle in Pacific white shrimp Penaeus vannamei . Diseases of Aquatic Organisms 36: 81–93. [Google Scholar]
- Hasson KW, Lightner DV, Mohney LL, Redman RM, Poulos BT, White BM (1999b) Role of lymphoid organ spheroids in chronic Taura syndrome virus (TSV) infections in Penaeus vannamei . Diseases of Aquatic Organisms 38: 93–105. [Google Scholar]
- Hasson KW, Fan Y, Reisinger T, Venuti J, Varner PW (2006) White‐spot syndrome virus (WSSV) introduction into the Gulf of Mexico and Texas freshwater systems through imported, frozen bait‐shrimp. Diseases of Aquatic Organisms 71: 91–100. [DOI] [PubMed] [Google Scholar]
- Hoa TTT, Hodgson RAJ, Oanh DTH, Phuong NT, Preston NJ, Walker PJ (2005) Genotypic variations in tandem repeat DNA segments between ribonucleotide reductase subunit genes of white spot syndrome virus (WSSV) isolates from Vietnam In: Walker PJ, Lester RG, Bondad‐Reantaso MG. (eds) Diseases in Asian Aquaculture V, pp. 339–352. Fish Health Section, Asian Fisheries Society, Manila. [Google Scholar]
- Holland JJ (1993) Replication error, quasispecies populations, and extreme evolution rates of RNA viruses In: Morse SS. (ed.) Emerging Viruses, pp. 203–218. Oxford University Press, New York. [Google Scholar]
- Holland JJ (2006) Transitions in understanding RNA viruses: a historical perspective. Current Topics in Microbiology and Immunology 299: 371–401. [DOI] [PubMed] [Google Scholar]
- Holmstrom K, Graslund S, Wahlstrom A, Poungshompoo S, Bengtsson BE, Kautsky N (2003) Antibiotic use in shrimp farming and implications for environmental impacts and human health. International Journal of Food Science and Technology 38: 255–266. [Google Scholar]
- Hsu HC, Lo CF, Lin SC, Liu KF, Peng SE, Chang YS et al. (1999) Studies on effective PCR screening strategies for white spot syndrome virus (WSSV) detection in Penaeus monodon brooders. Diseases of Aquatic Organisms 39: 13–19. [DOI] [PubMed] [Google Scholar]
- Van Hulten MC, Witteveldt L, Peters S, Kloosterboer N, Tarchini R, Fiers M et al. (2001) The white spot syndrome virus DNA genome sequence. Virology 286: 7–22. [DOI] [PubMed] [Google Scholar]
- Hyatt AD, Daszak P, Cunningham AA, Field H, Gould AR (2004) Henipaviruses: gaps in the knowledge of emergence. EcoHealth 1: 25–38. [Google Scholar]
- Israngkura A, Sae‐Hae S (2002) A review of economic impacts of aquatic animal disease In: Arthur JR, Phillips MJ, Subasinghe RP, Reantaso MB, McCrae IH. (eds) Primary Aquatic Animal Health Care in Rural, Small‐scale Aquaculture Development. Technical Proceedings of the Asia Regional Scoping Workshop. FAO Fisheries Technical Paper 406. Dhaka, Bangladesh, pp. 55–61. Food and Agriculture Organization of the United Nations, Rome. [Google Scholar]
- Jahncke ML, Browdy CL, Schwarz MH, Segars A, Silva JL, Smith DC et al. (2001) Preliminary application of hazard analysis critical control point (HACCP) principles as a risk management tool to control exotic viruses at shrimp production and processing facilities In: Browdy CL, Jory DE. (eds) The New Wave: Proceedings of the Special Session on Sustainable Shrimp Farming, Aquaculture 2001, pp. 279–284. The World Aquaculture Society, Baton Rouge. [Google Scholar]
- Jiang Y (2001) P.R. China In: Subasinghe R, Arthur R, Phillips MJ, Reantaso M. (eds) Thematic Review on Management Strategies for Major Diseases in Shrimp Aquaculture. Proceedings of a workshop held in Cebu, Philippines. The World Bank, Network of Aquaculture Centers Asia‐Pacific, World Wildlife Fund and Food and Agricultural Organization of the United Nations Consortium Program on Shrimp Farming and the Environment, pp. 74–78. FAO, Rome. [Google Scholar]
- Jiminez R (1992) Sindrome de Taura (resumen) Acuacultura del Ecuador In: Jumenez R. (ed.) Revista Especializada de la Camara National de Acuacultura, pp. 1–16. Guayaquil; (in Spanish). [Google Scholar]
- Jiravanichpaisal P, Bangyeekhun E, Soderhall K, Soderhall I (2001) Experimental infection of white spot syndrome virus in freshwater crayfish Pacifastacus leniusculus . Diseases of Aquatic Organisms 47: 151–157. [DOI] [PubMed] [Google Scholar]
- Jitrapakdee S, Unajak S, Sittidilokratna N, Hodgson RA, Cowley JA, Walker PJ et al. (2003) Identification and analysis of gp116 and gp64 structural glycoproteins of yellow head nidovirus of Penaeus monodon shrimp. Journal of General Virology 84: 863–873. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Lightner DV (1988) Rod‐shaped nuclear viruses of crustaceans: gut‐infecting species. Diseases of Aquatic Organisms 5: 123–141. [Google Scholar]
- Johnson K, Van Hulten MCW, Barnes AC (2008) “Vaccination” of shrimp against viral pathogens: phenomenology and underlying mechanisms. Vaccine 26: 4885–4892. [DOI] [PubMed] [Google Scholar]
- Kalagayan G, Godin D, Kanna R, Hagino G, Sweeney J, Wyban J et al. (1991) IHHN virus as an etiological factor in runt‐deformity‐syndrome of juvenile Penaeus vannamei cultured in Hawaii. Journal of the World Aquaculture Society 22: 235–243. [Google Scholar]
- Kanchanaphum P, Wongteerasupaya C, Sitidilokratana N, Boonsaeng V, Tassanakajon A, Withyachumnarnkul B et al. (1998) Experimental transmission of white spot syndrome virus (WSSV) from crabs to shrimp Penaeus monodon . Diseases of Aquatic Organisms 34: 1–7. [DOI] [PubMed] [Google Scholar]
- Kiatpathomchai W, Taweetungtragoon A, Jittivadhana K, Wongteerasupaya C, Boonsaeng V, Flegel TW (2005) Target for standard Thai PCR assay identical in 12 white spot syndrome virus (WSSV) types that differ in DNA multiple repeat length. Journal of Virological Methods 130: 79–82. [DOI] [PubMed] [Google Scholar]
- Krabsetsve K, Cullen BR, Owens L (2004) Rediscovery of the Australian strain of infectious hypodermal and haematopoietic necrosis virus. Diseases of Aquatic Organisms 61: 153–158. [DOI] [PubMed] [Google Scholar]
- Kumar AR, Ahamed VPI, Sarathi M, Basha AN, Hameed ASS (2008) Immunological responses of Penaeus monodon to DNA vaccine and its efficacy to protect shrimp against white spot syndrome virus (WSSV). Fish and Shellfish Immunology 24: 467–478. [DOI] [PubMed] [Google Scholar]
- Lester RJG, Doubrovsky A, Paynter JL, Sambhi SK, Atherton JG (1987) Light and electron microscope evidence of baculovirus infection in the prawn Penaeus plebejus . Diseases of Aquatic Organisms 3: 217–219. [Google Scholar]
- Leu JH, Tsai JM, Wang HC, Wang AH, Wang CH, Kou GH et al. (2005) The unique stacked rings in the nucleocapsid of the white spot syndrome virus virion are formed by the major structural protein VP664, the largest viral structural protein ever found. Journal of Virology 79: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lin O, Chen J, Wu JL, Lim TK, Loh SS et al. (2007) Shotgun identification of the structural proteome of shrimp white spot syndrome virus and iTRAQ differentiation of envelope and nucleocapsid subproteomes. Molecular and Cellular Proteomics 6: 1609–1620. [DOI] [PubMed] [Google Scholar]
- Lightner DV (1996a) A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp. World Aquaculture Society, Baton Rouge. [Google Scholar]
- Lightner DV (1996b) Epizootiology, distribution and the impact on international trade of two penaeid shrimp viruses in the Americas. Revue Scientifique et Technique, Office International des Epizooties 15: 579–601. [DOI] [PubMed] [Google Scholar]
- Lightner DV (2003) The penaeid shrimp viral pandemics due to IHHNV, WSSV, TSV and YHV: history in the Americas and current status In: Proceedings of the 32nd Joint UJNR Aquaculture Panel Symposium, Davis and Santa Barbara, California, USA, pp. 17–20. [Google Scholar]
- Lightner DV (2005) Biosecurity in shrimp farming: pathogen exclusion through use of SPF stock and routine surveillance. Journal of the World Aquaculture Society 36: 229–248. [Google Scholar]
- Lightner DV, Redman RM (1981) A baculovirus‐caused disease of the penaeid shrimp, Penaeus monodon . Journal of Invertebrate Pathology 38: 299–302. [Google Scholar]
- Lightner DV, Redman RM (1998a) Strategies for control of viral diseases of shrimp in the Americas. Fish Pathology 33: 165–180. [Google Scholar]
- Lightner DV, Redman RM (1998b) Shrimp diseases and current diagnostic methods. Aquaculture 164: 201–220. [Google Scholar]
- Lightner DV, Redman RM, Bell TA, Brock JA (1983a) Detection of IHHN virus in P. stylirostris and P. vannamei imported into Hawaii. Journal of the World Mariculture Society 14: 212–225. [Google Scholar]
- Lightner DV, Redman RM, Bell TA (1983b) Infectious hypodermal and hematopoietic necrosis, a newly recognized virus disease of penaeid shrimp. Journal of Invertebrate Pathology 42: 62–70. [DOI] [PubMed] [Google Scholar]
- Lightner DV, Redman RM, Bell TA (1983c) Observations on the geographic distribution, pathogenesis and morphology of the baculovirus from Penaeus monodon Fabricius. Aquaculture 32: 209–233. [Google Scholar]
- Lightner DV, Hedrick RP, Fryer JL, Chen SN, Liao IC, Kou GH (1987) A survey of cultured penaeid shrimp in Taiwan for viral and other important diseases. Fish Pathology 22: 127–140. [Google Scholar]
- Lightner DV, Redman RM, Hasson KW, Pantoja CR (1995) Taura syndrome in Penaeus vannamei (Crustacea: Decapoda): gross signs, histopathology and ultrastructure. Diseases of Aquatic Organisms 21: 53–59. [Google Scholar]
- Lightner DV, Redman RM, Poulos BT, Nunan LM, Mari JL, Hasson KW (1997) Risk of spread of penaeid shrimp viruses in the Americas by international movement of live and frozen shrimp. Revue Scientifique et Technique, Office International des Epizooties 16: 146–160. [DOI] [PubMed] [Google Scholar]
- Lightner DV, Hasson KW, White BL, Redman RM (1998) Experimental infection of western hemisphere penaeid shrimp with Asian white spot syndrome virus and Asian yellow head virus. Journal of Aquatic Animal Health 10: 271–278. [Google Scholar]
- Lightner DV, Pantoja CR, Poulos BT, Tang KFJ, Redman RM, Pasos‐de‐Andrade T et al. (2004) Infectious myonecrosis: new disease in Pacific white shrimp. Global Aquaculture Advocate 7 (5): 85. [Google Scholar]
- Lightner DV, Riggs A, Corbin JS, Ostrowski AC (2007) Taura syndrome virus in specific pathogen‐free Penaeus vannamei origination from Hawaii and in P. vannamei stocks farmed in France? Diseases of Aquatic Organisms 74: 77–79. [DOI] [PubMed] [Google Scholar]
- Limsuwan C (1991) Handbook for Cultivation of Black Tiger Prawns. Tansetakit, Bangkok (in Thai). [Google Scholar]
- Lin CK (1989) Prawn aquaculture in Taiwan. What went wrong? World Aquaculture 20: 19–20. [Google Scholar]
- Liu B, Yu Z, Song X, Guan Y, Jian X, He J (2006) The effect of acute salinity change on white spot syndrome (WSS) outbreaks in Feneropenaeus chinensis . Aquaculture 253: 163–170. [Google Scholar]
- Liu B, Yu Z, Song X, Guan Y (2007) Studies on the transmission of WSSV (white spot syndrome virus) in juvenile Marsupenaeus japonicus via marine microalgae. Journal of Invertebrate Pathology 95: 87–92. [DOI] [PubMed] [Google Scholar]
- Lo CF, Kou GH (1998) Virus‐associated white spot syndrome of shrimp in Taiwan: a review. Fish Pathology 33: 365–371. [Google Scholar]
- Lo CF, Ho CH, Chen CH, Liu KF, Chiu YL, Yeh PY et al. (1997) Detection and tissue tropism of white spot syndrome baculovirus (WSBV) in captured brooders of Penaeus monodon with a special emphasis on reproductive organs. Diseases of Aquatic Organisms 30: 53–72. [Google Scholar]
- Longyant S, Sithigorngul P, Chaivisuthangkura P, Rukpratanporn S, Sithigorngul W, Menasveta P (2005) Difference in susceptibility of palaemonid shrimp species to yellow head virus (YHV) infection. Diseases of Aquatic Organisms 64: 5–12. [DOI] [PubMed] [Google Scholar]
- Longyant S, Sattaman S, Chaivisuthangkura P, Rukpratanporn S, Sithigorngul W, Sithigorngul P (2006) Experimental infection of some penaeid shrimps and crabs by yellow head virus (YHV). Aquaculture 257: 83–91. [Google Scholar]
- Lotz JM (1997) Special topic review: viruses, biosecurity and specific pathogen‐free stocks in shrimp aquaculture. World Journal Microbiology and Biotechnology 13: 405–413. [Google Scholar]
- Lotz JM, Flowers AM, Breland V (2003) A model of Taura syndrome virus (TSV) epidemics in Litopenaeus vannamei . Journal of Invertebrate Pathology 83: 168–176. [DOI] [PubMed] [Google Scholar]
- Lu CC, Tang KFJ, Kou GH, Chen SN (1993) Development of a Penaeus monodon‐type baculovirus (MBV) DNA probe by polymerase chain reaction and sequence analysis. Journal of Fish Diseases 16: 551–559. [Google Scholar]
- Lu Y, Tapay LM, Brock JA, Loh PC (1994) Infection of the yellow head baculo‐like virus (YBV) in two species of penaeid shrimp, Penaeus stylirostris (Stimpson) and Penaeus vannamei (Boone). Journal of Fish Diseases 17: 649–656. [Google Scholar]
- Lu Y, Tapay LM, Loh PC, Brock JA, Gose R (1995) Development of a quantal assay in primary shrimp cell culture for yellow head baculovirus (YBV) of penaeid shrimp. Journal of Virological Methods 52: 231–236. [DOI] [PubMed] [Google Scholar]
- Lundin CG (1995) Global attempts to address shrimp disease. Report of the Land, Water and Natural Habitats Division, Environment Department. The World Bank, Washington. [Google Scholar]
- Manivannan S, Otta SK, Karunasagar I, Karunasagar I (2002) Multiple viral infection in Penaeus monodon shrimp postlarvae in an Indian hatchery. Diseases of Aquatic Organisms 48: 233–236. [DOI] [PubMed] [Google Scholar]
- Manivannan S, Kennedy B, Karunasagar I, Karunasagar I (2004) Prevalence of monodon baculovirus in wild Metapenaeus species along the southwest coast of India. Aquaculture 232: 63–67. [Google Scholar]
- Marcogliese DJ (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Revue Scientifique et Technique, Office International des Epizooties 27: 467–484. [PubMed] [Google Scholar]
- Mari J, Bonami JR, Lightner DV (1993) Partial cloning of the genome of infectious hypodermal and haematopoietic necrosis virus, an unusual parvovirus pathogenic for penaeid shrimps; diagnosis of the disease using a specific probe. Journal of General Virology 74: 2637–2643. [DOI] [PubMed] [Google Scholar]
- Mari J, Poulos BT, Lightner DV, Bonami JR (2002) Shrimp Taura syndrome virus: genomic characterization and similarity with members of the genus Cricket paralysis‐like viruses . Journal of General Virology 83: 915–926. [DOI] [PubMed] [Google Scholar]
- Marks H, Goldbach RW, Vlak JW, Van Hulten MC (2004) Genetic variation among isolates of white spot syndrome virus. Archives of Virology 149: 673–697. [DOI] [PubMed] [Google Scholar]
- Marks H, Van Duijse JJ, Zuidema D, Van Hulten MC, Vlak JM (2005) Fitness and virulence of an ancestral white spot syndrome virus isolate from shrimp. Virus Research 110: 9–20. [DOI] [PubMed] [Google Scholar]
- Mayer JD (2000) Geography, ecology and emerging infectious diseases. Social Science and Medicine 50: 937–952. [DOI] [PubMed] [Google Scholar]
- McColl KA, Slater J, Jeyasekaran G, Hyatt AD, Crane MS (2004) Detection of white spot syndrome virus and yellowhead virus in prawns imported into Australia. Australian Veterinary Journal 82: 69–74. [DOI] [PubMed] [Google Scholar]
- Miller P, Flaherty M, Szuster B (1999) Inland shrimp farming in Thailand. Aquaculture Asia 4: 27–32. [Google Scholar]
- Mohan CV, Sudha PM, Shankar KM, Hedge A (1997) Vertical transmission of white spot baculovirus in shrimps – a possibility? Current Science 73: 109–110. [Google Scholar]
- Mohan CV, Shankar KM, Kulkani S, Sudha PM (1998) Histopathology of cultured shrimp showing gross signs of yellow head syndrome and white spot syndrome during 1994 Indian epizootics. Diseases of Aquatic Organisms 34: 9–12. [DOI] [PubMed] [Google Scholar]
- Mohan CV, Phillips MJ, Bhat BV, Umesh NR, Padiyar PA (2008) Tools for preparedness and response: Farm level plans/husbandry measures. In: Changing trends in managing aquatic animal disease emergencies. Revue Scientifique et Technique, Office International des Epizooties 27: 161–173. [PubMed] [Google Scholar]
- Momoyama K, Hiroka M, Nakano H, Sameshima M (1998) Cryopreservation of penaeid rod‐shaped DNA virus (PRDV) and its survival in sea water at different temperatures. Fish Pathology 33: 95–96. [Google Scholar]
- Morales‐Covarrubias MS, Nunan LM, Lightner DV, Mota‐Urbina JC, Garza‐Aguirre MC, Chavez‐Snachez MC (1999) Prevalence of IHHNV in wild broodstock of Penaeus stylirostris from the upper Gulf of California, Mexico. Journal of Aquatic Animal Health 11: 296–301. [Google Scholar]
- Morse SS (1993) Examining the origins of emerging viruses In: Morse SS. (ed.) Emerging Viruses, pp. 10–28. Oxford University Press, New York. [Google Scholar]
- Morse SS (1995) Factors in the emergence of infectious diseases. Emerging Infectious Diseases 1: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte E, Yugcha E, Luzardo J, Castro F, Leclercq G, Rodriguez J et al. (2003) Prevention of IHHNV vertical transmission in the white shrimp Litopenaeus vannamei . Aquaculture 219: 57–70. [Google Scholar]
- Munday BL, Owens L (1998) Viral diseases of fish and shellfish in Australian mariculture. Fish Pathology 33: 193–200. [Google Scholar]
- Mushiake K, Shimizu K, Satoh J, Mori K, Arimoto M, Ohsumi S et al. (1999) Control of penaeid acute viremia (PAV) in Penaeus japonicus: selection of eggs based on the PCR detection of the causative virus (PRDV) from Receptaculum seminis of spawned broodstock. Fish Pathology 34: 203–207. [Google Scholar]
- Musthaq SS, Sudhakaran R, Ahmed VPI, Balasubramanian G, Hameed ASS (2006) Variability in the tandem repetitive DNA sequences of white spot syndrome virus (WSSV) genome and suitability of VP28 gene to detect different isolates of WSSV from India. Aquaculture 256: 34–41. [Google Scholar]
- Nadala ECB Jr, Lourdes M, Tapay SC, Loh PC (1997) Detection of yellowhead virus and Chinese baculovirus in penaeid shrimp by the western blot technique. Journal of Virological Methods 69: 39–44. [DOI] [PubMed] [Google Scholar]
- Nakano H, Koube H, Umezawa S, Momoyama K, Hiraoka M, Inouye K et al. (1994) Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in Japan in 1993: epidemiological survey and infection trials. Fish Pathology 29: 135–139. [Google Scholar]
- Namikoshi A, Wu JL, Yamashita T, Nishizawa T, Nishioka T, Arimoto M et al. (2004) Vaccination trials with Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture 229: 25–35. [Google Scholar]
- Nash G, Poernomo A, Nash MB (1988) Baculovirus infection in brackish water pond cultured Penaeus monodon Fabricius in Indonesia. Aquaculture 73: 1–6. [Google Scholar]
- Natividad JM, Lightner DV (1992a) Susceptibility of the different larval and postlarval stages of black tiger prawn, Penaeus monodon Fabricius, to monodon baculovirus (MBV) In: Shariff M, Subasinghe RP, Arthur JR. (eds) Diseases in Asian Aquaculture I, pp. 111–125. Fish Health Section, Asian Fisheries Society, Manila. [Google Scholar]
- Natividad JM, Lightner DV (1992b) Prevalence and geographic distribution of MBV and other diseases in cultured giant tiger prawns (Penaeus monodon) in the Philippines In: Fulks W, Main KL. (eds) Diseases of Cultured Penaeid Shrimp in Asia and the United States, pp. 139–160. Oceanic Institute, Honolulu. [Google Scholar]
- Natividad KT, Magbanua F, Migo VP, Alfafara CG, Albaladejo J, Nadala ECB Jr et al. (2002) Prevalence of yellow head virus in cultured black tiger shrimp (Penaeus monodon Fabricius) from selected shrimp farms in the Philippines In: Lavilla‐Pitogo CR, Cruz‐Lacierda ER. (eds) Diseases in Asian Aquaculture IV, pp. 45–55. Fish Health Section, Asian Fisheries Society, Manila. [Google Scholar]
- Natividad KT, Migo VP, Albaladejo J, Magbanua F, Noruma N, Matsumura M (2006) Simultaneous PCR detection of two shrimp viruses (WSSV and MBV) in postlarvae of Penaeus monodon in the Philippines. Aquaculture 257: 142–149. [Google Scholar]
- Nibert ML (2007) ‘2A‐like’ and ‘shifty heptamer’ motifs in penaeid shrimp infectious myonecrosis virus, a monosegmented double‐stranded RNA virus. Journal of General Virology 88: 1315–1318. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Sang‐oum W, Cheevadhanarak S, Flegel TW (2005) Taura syndrome virus (TSV) in Thailand and its relationship to TSV in China and the Americas. Diseases of Aquatic Organisms 63: 101–106. [DOI] [PubMed] [Google Scholar]
- Nunan LM, Poulos BT, Lightner DV (1998) The detection of white spot syndrome virus (WSSV) and yellow head virus (YHV) in imported commodity shrimp. Aquaculture 160: 19–30. [Google Scholar]
- Oanh DTH, Phuong NT, Preston N, Hodgson RAJ, Walker PJ (2005) Prevalence of white spot syndrome virus (WSSV) and monodon baculovirus (MBV) infection in Penaeus monodon postlarvae in Vietnam In: Walker PJ, Lester RG, Bondad‐Reantaso MG. (eds) Diseases in Asian Aquaculture V, pp. 395–403. Fish Health Section, Asian Fisheries Society, Manila. [Google Scholar]
- OIE (World Organization for Animal Health) (2006) Manual of Diagnostic Tests for Aquatic Animals. World Organization for Animal Health, Paris. [Google Scholar]
- OIE (World Organization for Animal Health) (2008) Aquatic Animal Health Code, 11th edn World Organization for Animal Health, Paris. [Google Scholar]
- Overstreet RM, Lightner DV, Hasson KW, McIlwain S, Lotz JM (1997) Susceptibility to Taura syndrome virus of some penaeid species native to the gulf of Mexico and the southeastern United States. Journal of Invertebrate Pathology 69: 165–176. [DOI] [PubMed] [Google Scholar]
- Owens L, Anderson IG, Kenway M, Trott L, Benzie JAH (1992) Infectious hypodermal and haematopoietic necrosis virus (IHHNV) in a hybrid penaeid prawn from tropical Australia. Diseases of Aquatic Organisms 14: 219–228. [Google Scholar]
- Padiyar A (2009) Epidemiology and Management of Penaeus monodon Farms in Andhra Pradesh, India. PhD Thesis. Deakin University, Australia. [Google Scholar]
- Paez‐Osuna F (2001) The environmental impact of shrimp aquaculture: causes, effects and mitigating alternatives. Environmental Management 28: 131–140. [DOI] [PubMed] [Google Scholar]
- Pantoja CR, Lightner DV, Holtshmit KH (1999) Prevalence and geographic distribution of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in wild blue shrimp Penaeus stylirostris from the Gulf of California, Mexico. Journal of Aquatic Animal Health 11: 23–34. [Google Scholar]
- Park JH, Lee YS, Lee S, Lee Y (1998) An infectious viral disease of penaeid shrimp newly found in Korea. Diseases of Aquatic Organisms 34: 71–75. [DOI] [PubMed] [Google Scholar]
- Pasharawipas T, Flegel TW, Sriurairatana S, Morrison DJ (1997) Latent yellow‐head infections in Penaeus monodon and implications regarding disease resistance or tolerance In: Flegel TW, Menasveta P, Paisarnat S. (eds) Shrimp Biotechnology in Thailand, pp. 45–53. National Center for Genetic Engineering and Biotechnology, Bangkok. [Google Scholar]
- Patil R, Palaksha KJ, Anil TM, Guruchannabasavanna , Patil P, Shankar KM et al. (2007) Evaluation of an immunodot test to manage white spot syndrome virus (WSSV) during cultivation of the giant tiger shrimp Penaeus monodon . Diseases of Aquatic Organiams 79: 157–161. [DOI] [PubMed] [Google Scholar]
- Paynter JL, Vickers JE, Lester RJG (1992) Experimental transmission of Penaeus monodon‐type baculovirus (MBV) In: Shariff M, Subasinghe RP, Arthur JR. (eds) Diseases in Asian Aquaculture I, pp. 97–110. Fish Health Section, Asian Fisheries Society, Manila. [Google Scholar]
- Peng SE, Lo CF, Liu KF, Kou GH (1998) The transition from pre‐patent to patent infection of white spot syndrome virus (WSSV) in Penaeus monodon triggered by periopod excision. Fish Pathology 33: 395–400. [Google Scholar]
- Phalitakul S, Wongtawatchai J, Meena Sarikaputi M, Viseshakul N (2006) The molecular detection of Taura syndrome virus emerging with White spot syndrome virus in penaeid shrimps of Thailand. Aquaculture 260: 77–85. [Google Scholar]
- Pongnak W (1999) Case study on the impact and conflict in using the nation’s freshwater land resources for farming penaeid shrimp. Greenline 4: 6–15. [Google Scholar]
- Poulos BT, Tang KFJ, Pantoja CR, Bonami JR, Lightner DV (2006) Purification and characterisation of infectious myonecrosis virus of penaeid shrimp. Journal of General Virology 87: 987–996. [DOI] [PubMed] [Google Scholar]
- Primavera JH, Quinitio ET (2000) Runt‐deformity syndrome in cultured giant tiger prawn Penaeus monodon . Journal of Crustacean Biology 20: 796–802. [Google Scholar]
- Rahman MM, Escobedo‐Bonilla CM, Corteel M, Dantas‐Lima JJ, Wille M, Alday‐Sanz V et al. (2006) Effect of high water temperature (33°C) on the clinical and virological outcome of experimental infections with white spot syndrome virus (WSSV) in specific pathogen‐free (SPF) Litopenaeus vannamei . Aquaculture 261: 842–849. [Google Scholar]
- Renault T (1996) Appearance and spread of diseases among bivalve molluscs in the northern hemisphere in relation to international trade. Revue Scientifique et Technique, Office International des Epizooties 15: 551–562. [DOI] [PubMed] [Google Scholar]
- Reyes A, Salazar M, Granja C (2007) Temperature modifies gene expression in subcuticular epithelial cells of white spot syndrome virus‐infected Litopenaeus vannamei . Developmental and Comparative Immunology 31: 23–29. [DOI] [PubMed] [Google Scholar]
- Robalino J, Browdy CL, Prior S, Metz A, Parnell P, Gross P et al. (2004) Induction of antiviral immunity by double‐stranded RNA in a marine invertebrate. Journal of Virology 78: 10442–10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robalino J, Bartlett T, Shepard E, Prior S, Jaramillo G, Scura E et al. (2005) Double‐stranded RNA induces sequence‐specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: Convergence of RNA interference and innate immunity in the invertebrate antiviral response? Journal of Virology 79: 13561–13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles‐Sikisaka R, Garcia DK, Klimpel KR, Dhar AK (2001) Nucleotide sequence of 3′‐end of the genome of Taura syndrome virus of shrimp suggests that it is related to insect picornaviruses. Archives of Virology 146: 941–952. [DOI] [PubMed] [Google Scholar]
- Robles‐Sikisaka R, Hasson KW, Garcia DK, Brovont KE, Cleveland KD, Klimpel KR et al. (2002) Genetic variation and immunohistochemical differences among geographic isolates of Taura syndrome virus of penaeid shrimp. Journal of General Virology 83: 3123–3130. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Bayot B, Amano Y, Panchana F, De Blas I, Alday V et al. (2003) White spot syndrome virus infection in cultured Penaeus vannamei (Boone) in Ecuador with emphasis on histopathology and ultrastructure. Journal of Fish Diseases 26: 439–450. [DOI] [PubMed] [Google Scholar]
- Roekring S, Nielsen L, Owens L, Pattanakitsakul S, Malasit P, Flegel TW (2002) Comparison of penaeid shrimp and insect parvoviruses suggests that viral transfers may occur between two distantly related arthropod groups. Virus Research 87: 79–87. [DOI] [PubMed] [Google Scholar]
- De La Rosa‐Velez J, Cedano‐Thomas Y, Cid‐Becerra J, Mendez‐Payan JC, Vega‐Perez C, Zambrano‐Garcia J et al. (2006) Presumptive detection of yellow head virus by reverse transcriptase‐polymerase chain reaction and dot‐blot hybridization in Litopenaeus vannamei and L. stylirostris culture on the northwest coast of Mexico. Journal of Fish Diseases 29: 717–726. [DOI] [PubMed] [Google Scholar]
- Rout N, Kumar S, Jaganmohan S, Murugan V (2007) DNA vaccines encoding viral envelope proteins confer protective immunity against WSSV in black tiger shrimp. Vaccine 25: 2778–2786. [DOI] [PubMed] [Google Scholar]
- Schultze H, Ulferts R, Schelle B, Bayer S, Granzow H, Hoffmann B et al. (2006) Characteration of white bream virus reveals a novel genetic cluster of nidoviruses. Journal of Virology 80: 11598–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapin S, Phongdara A (2006) Binding of shrimp cellular proteins to Taura syndrome viral capsid proteins VP1, VP2 and VP3. Virus Research 122: 69–77. [DOI] [PubMed] [Google Scholar]
- Senapin S, Phewsaiya K, Briggs M, Flegel TW (2007) Outbreaks of infectious myonecrosis virus (IMNV) in Indonesia confirmed by genome sequencing and use of an alternative RT‐PCR detection method. Aquaculture 266: 32–38. [Google Scholar]
- Shike H, Dhar AK, Burns JC, Shimizu C, Jousset FX, Klimpel KR et al. (2000) Infectious hypodermal and hematopoietic necrosis virus of shrimp is related to mosquito brevidensoviruses. Virology 277: 167–177. [DOI] [PubMed] [Google Scholar]
- Sithigorngul P, Rukpratanporn S, Sittidilokratna N, Pecharaburanin N, Longyant S, Chaivisuthangkura P et al. (2006) A convenient immunochromatographic test strip for rapid diagnosis of yellow head virus infection in shrimp. Journal of Virological Methods 140: 193–199. [DOI] [PubMed] [Google Scholar]
- Sittidilokratna N, Hodgson RAJ, Cowley JA, Jitrapakdee S, Boonsaeng V, Panyim S et al. (2002) Complete ORF1b‐gene sequence indicates yellow head virus is an invertebrate nidovirus. Diseases of Aquatic Organisms 50: 87–93. [DOI] [PubMed] [Google Scholar]
- Sittidilokratna N, Phetchampai N, Boonsaeng V, Walker PJ (2006) Structural and antigenic analysis of the yellow head virus nucleocapsid protein p20. Virus Research 116: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittidilokratna N, Dangtip S, Cowley JA, Walker PJ (2008) RNA transcription analysis and completion of the genome sequence of yellow head nidovirus. Virus Research 136: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittidilokratna N, Chotwiwatthanakun C, Wijegoonawardane PKM, Unajak S, Boonnad A, Wangnai W et al. (2009) A highly virulent isolate of yellow head nidovirus contains a deformed envelope glycoprotein gp116. Virology 384: 192–200. [DOI] [PubMed] [Google Scholar]
- Soowannayan C, Flegel TW, Sithigorngul P, Slater J, Hyatt A, Cramerri S et al. (2003) Detection and differentiation of yellow head complex viruses using monoclonal antibodies. Diseases of Aquatic Organisms 57: 193–200. [DOI] [PubMed] [Google Scholar]
- Soto MA, Lotz JM (2001) Epidemiological parameters of white spot syndrome virus infections in Litopenaeus vannamei and L. setiferus . Journal of Invertebrate Pathology 78: 9–15. [DOI] [PubMed] [Google Scholar]
- Soto MA, Shervette VR, Lotz JM (2001) Transmission of white spot syndrome virus (WSSV) to Litopenaeus vannamei from infected cephalothorax, abdomen, or whole shrimp cadaver. Diseases of Aquatic Organisms 45: 81–87. [DOI] [PubMed] [Google Scholar]
- Spann KM, Lester RJG (1996) Baculovirus of Metapenaeus bennettae from the Moreton Bay region of Australia. Diseases of Aquatic Organisms 27: 53–58. [Google Scholar]
- Spann KM, Cowley JA, Walker PJ, Lester RJG (1997) A yellow‐head‐like virus from Penaeus monodon cultured in Australia. Diseases of Aquatic Organisms 31: 169–179. [Google Scholar]
- Spann KM, Donaldson RA, Cowley JA, Walker PJ (2000) Differences in the susceptibility of some penaeid prawn species to gill‐associated virus (GAV) infection. Diseases of Aquatic Organisms 42: 221–225. [DOI] [PubMed] [Google Scholar]
- Spann KM, McCulloch RJ, Cowley JA, East IJ, Walker PJ (2003) Detection of gill‐associated virus (GAV) by in situ hybridization during acute and chronic infections of Penaeus monodon and P. esculentus shrimp. Diseases of Aquatic Organisms 56: 1–10. [DOI] [PubMed] [Google Scholar]
- Srisuvan T, Tang KF, Lightner DV (2005) Experimental infection of Penaeus monodon with Taura syndrome virus (TSV). Diseases of Aquatic Organisms 67: 1–8. [DOI] [PubMed] [Google Scholar]
- Srisuvan T, Pantoja CR, Redman RM, Lightner DV (2006a) Ultrastructure of the replication site in Taura syndrome virus (TSV)‐infected cells. Diseases of Aquatic Organisms 73: 89–101. [DOI] [PubMed] [Google Scholar]
- Srisuvan T, Noble BL, Schofield PJ, Lightner DV (2006b) Comparison of four Taura syndrome virus (TSV) isolates in oral challenge studies with Litopenaeus vannamei unselected or selected for resistance to TSV. Diseases of Aquatic Organisms 71: 1–10. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA, Holland JJ (1987) Rapid evolution of RNA viruses. Annual Review of Microbiology 41: 409–433. [DOI] [PubMed] [Google Scholar]
- Su J, Oanh DT, Lyons RE, Leeton L, Van Hulten MC, Tan SH et al. (2008) A key gene of the RNA interference pathway in the black tiger shrimp, Penaeus monodon: Identification and functional characterization of Dicer‐1. Fish and Shellfish Immunology 24: 223–233. [DOI] [PubMed] [Google Scholar]
- Subasinghe RP (2005) Epidemiological approach to aquatic animal health management: opportunities and challenges for developing countries to increase aquatic production through aquaculture. Preventive Veterinary Medicine 67: 117–124. [DOI] [PubMed] [Google Scholar]
- Subasinghe RP, Bondad‐Reantaso MG (2006) Biosecurity in aquaculture: International agreements and instruments, their compliance, prospects, and challenges for developing countries In: Scarfe AD, Lee CS, O’Bryen PJ. (eds) Aquaculture Biosecurity: Prevention, Control, and Eradication of Aquatic Animal Disease, pp. 9–16. Blackwell Publishing, Ames. [Google Scholar]
- Subasinghe RP, Bondad‐Reantaso MG (2008) The FAO/NACA Asia regional technical guidelines for the responsible movement of live aquatic animals: lessons learned from their development and implementation. Revue Scientifique et Technique, Office International des Epizooties 27: 54–63. [PubMed] [Google Scholar]
- Sunarto A, Widodo , Taukhid , Koesharyani I, Supriyadi H, Gardenia L et al. (2004) Current status of transboundary fish diseases in Indonesia: occurrence, surveillance, research and training In: Lavilla‐Pitogo CR, Nagasawa K. (eds) Transboundary Fish Diseases in Southeast Asia: Occurrence, Surveillance, Research and Training, pp. 91–121. SEAFDEC Aquaculture Department, Tigbauan, Iloilo. [Google Scholar]
- Supamattaya K, Hoffmann RW, Boonyaratpalin S, Kanchanaphum P (1998) Experimental transmission of white spot syndrome virus (WSSV) from black tiger shrimp Penaeus monodon to the sand crab Portunus pelagicus, mud crab Scylla serrata and krill Acetes sp. Diseases of Aquatic Organisms 32: 79–85. [Google Scholar]
- Szuster B (2006) Coastal shrimp farming in Thailand: searching for sustainability In: Hoanh CT, Tuong TP, Gowing JW, Hardy B. (eds) Environment and Livelihoods in Tropical Coastal Zones, pp. 86–98. CAB International, Oxon. [Google Scholar]
- Takahashi Y, Itami T, Kondo M, Maeda M, Fujii R, Tomonaga S et al. (1994) Electron microscopic evidence of bacilliform virus infection in kuruma shrimp (Penaeus japonicus). Fish Pathology 29: 121–125. [Google Scholar]
- Tang KFJ, Lightner DV (1999) A yellow head virus gene probe: nucleotide sequence and application to in situ hybridization. Diseases of Aquatic Organisms 35: 165–173. [DOI] [PubMed] [Google Scholar]
- Tang KFJ, Lightner DV (2002) Low sequence variation among isolates of infectious hypodermal and hematopoietic necrosis virus (IHHNV) originating from Hawaii and the Americas. Diseases of Aquatic Organisms 49: 93–97. [DOI] [PubMed] [Google Scholar]
- Tang KFJ, Lightner DV (2006) Infectious hypodermal and hematopoietic necrosis virus (IHHNV)‐related sequences in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Research 118: 185–191. [DOI] [PubMed] [Google Scholar]
- Tang KFJ, Durand SV, White BL, Redman RM, Pantoja CR, Lightner DV (2000) Postlarvae and juveniles of a selected line of Penaeus stylirostris are resistant to infectious hypodermal and hematopoietic necrosis virus infection. Aquaculture 190: 203–210. [Google Scholar]
- Tang KFJ, Poulos BT, Wang J, Redman RM, Shih HH, Lightner DV (2003a) Geographic variations among infectious hypodermal and hematopoietic necrosis virus (IHHNV) isolates and characteristics of their infection. Diseases of Aquatic Organisms 53: 91–99. [DOI] [PubMed] [Google Scholar]
- Tang KFJ, Durand SV, White BL, Redman RM, Mohney LL, Lightner DV (2003b) Induced resistance to white spot syndrome virus infection in Penaeus stylirostris through pre‐infection with infectious hypodermal and hematopoietic necrosis virus – a preliminary study. Aquaculture 216: 19–29. [Google Scholar]
- Tang KFJ, Pantoja CR, Poulos BT, Redman RM, Lightner DV (2005) In situ hybridization demonstrates that Litopenaeus vannamei, L. stylirostris and Penaeus monodon are susceptible to experimental infection with infectious myonecrosis virus (IMNV). Diseases of Aquatic Organisms 63: 261–265. [DOI] [PubMed] [Google Scholar]
- Tattersall P, Bergoin M, Bloom ME, Brown KE, Linden RM, Muzyczka N et al. (2005) Family Parvoviridae In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (eds) Virus Taxonomy, VIIIth Report of the International Committee on Taxonomy of Viruses, pp. 353–369. Elsevier, Academic Press, Amsterdam. [Google Scholar]
- Theilmann DA, Blissard GW, Bonning B, Jehle J, O’Reilly DR, Rohrmann GF et al. (2005) Family Baculoviridae In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (eds) Virus Taxonomy, VIIIth Report of the International Committee on Taxonomy of Viruses, pp. 177–185. Elsevier, Academic Press, Amsterdam. [Google Scholar]
- Tijsterman M, Plasterk RHA (2004) Dicers at RISC: The mechanism of RNAi. Cell 117: 1–4. [DOI] [PubMed] [Google Scholar]
- Tsai JM, Wang HC, Leu JH, Hsiao HH, Wang AH, Kou GH et al. (2004) Genomic and proteomic analysis of thirty‐nine structural proteins of shrimp white spot syndrome virus. Journal of Virology 78: 11360–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JM, Wang HC, Leu JH, Wang AH, Zhuang Y, Walker PJ et al. (2006) Identification of the nucleocapsid, tegument, and envelope proteins of the shrimp white spot syndrome virus virion. Journal of Virology 80: 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C, Huang HT, Chuang SH, Hsu JP, Kuo ST, Li NJ et al. (1999) Taura syndrome in Pacific white shrimp Penaeus vannamei cultured in Taiwan. Diseases of Aquatic Organisms 38: 159–161. [Google Scholar]
- Van VK (2004) Current status of transboundary fish diseases in Vietnam: occurrence, surveillance, research and training In: Lavilla‐Pitogo CR, Nagasawa K. (eds) Transboundary Fish Diseases in Southeast Asia: Occurrence, Surveillance, Research and Training, pp. 221–227. SEAFDEC Aquaculture Department, Tigbauan, Iloilo. [Google Scholar]
- Vanpatten K, Nunan LM, Lightner DV (2004) Seabirds as potential vectors of penaeids shrimp viruses and the development of a surrogate laboratory model utilizing domestic chickens. Aquaculture 241: 31–46. [Google Scholar]
- Vaseeharen B, Anand TP, Murugan T, Chen JC (2006) Shrimp vaccination trials with the VP292 protein of white spot syndrome virus. Letters in Applied Microbiology 43: 137–142. [DOI] [PubMed] [Google Scholar]
- De La Vega E, Degnan BM, Hall MR, Cowley JA, Wilson KJ (2004) Quantitative real‐time PCR demonstrates that handling stress can lead to rapid increases of gill‐associated virus (GAV) infection levels in Penaeus monodon . Diseases of Aquatic Organisms 59: 195–203. [DOI] [PubMed] [Google Scholar]
- Venegas CA, Nonaka L, Mushiake K, Nishizawa T, Muroga K (2000) Quasi‐immune response of Penaeus japonicus to penaeid rod‐shaped DNA virus (PRDV). Diseases of Aquatic Organisms 42: 83–89. [DOI] [PubMed] [Google Scholar]
- Vickers JE, Webb R, Young PR (2000) Monodon baculovirus from Australia: ultrastructural observations. Diseases of Aquatic Organisms 39: 169–176. [DOI] [PubMed] [Google Scholar]
- Vidal OM, Granja CB, Aranguren F, Brock JA, Salazar M (2001) A profound effect of hyperthermia on survival of Litopenaeus vannamei juveniles infected with white spot syndrome virus. Journal of the World Aquaculture Society 32: 364–372. [Google Scholar]
- Vijayan KK, Alavandi SV, Rajendran KV, Alagarswami K (1995) Prevalence and histopathology of monodon baculovirus (MBV) infection on Penaeus monodon and P. indicus in shrimp farms in the south‐east coast of India. Asian Fisheries Science 8: 267–272. [Google Scholar]
- Vlak JM, Bonami JR, Flegel TW, Kou GH, Lightner DV, Lo CF et al. (2005) Family Nimaviridae In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (eds) Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses, pp. 187–192. Elsevier, Academic Press, London. [Google Scholar]
- Waikhom G, John KR, George MR, Jeyaseelan MJP (2006) Differential host passaging alters pathogenicity and induces genomic variation in white spot syndrome virus. Aquaculture 261: 54–63. [Google Scholar]
- Walker PJ (2004) Disease emergence and food security: Global impact of pathogens on sustainable aquaculture production In: Brown AG. (ed.) Fish, Aquaculture and Food Security, pp. 44–50. The ATSE Crawford Fund, Melbourne. [Google Scholar]
- Walker PJ, Cowley JA, Spann KM, Hodgson RAJ, Hall MR, Withyachumnarnkul B (2001) Yellow head complex viruses: transmission cycles and topographical distribution in the Asia‐Pacific region In: Browdy CL, Jory DE. (eds) The New Wave: Proceedings of the Special Session on Sustainable Shrimp Farming, Aquaculture 2001, pp. 227–237. The World Aquaculture Society, Baton Rouge. [Google Scholar]
- Walker PJ, Bonami JR, Boonsaeng V, Chang PS, Cowley JA, Enjuanes L et al. (2005) Family Roniviridae In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (eds) Virus Taxonomy, VIIIth Report of the International Committee on Taxonomy of Viruses, pp. 975–979. Elsevier, Academic Press, Amsterdam. [Google Scholar]
- Wang YC, Chang PS (2000) Yellow head virus infection in the giant tiger prawn Penaeus monodon cultured in Taiwan. Fish Pathology 35: 1–10. [Google Scholar]
- Wang CS, Tang KFJ, Kou GH, Chen SN (1996) Yellow head disease‐like virus infection in the Kuruma shrimp Penaeus japonicus cultured in Taiwan. Fish Pathology 31: 177–182. [Google Scholar]
- Wang CH, Yang HN, Tang CH, Lu CH, Kou GH, Lo CF (2000a) Ultrastructure of white spot syndrome virus development in primary lymphoid organ cell cultures. Diseases of Aquatic Organisms 41: 91–104. [DOI] [PubMed] [Google Scholar]
- Wang YG, Lee KL, Najiah M, Shariff M, Hassan MD (2000b) A new bacterial white spot syndrome (BWSS) in cultured tiger shrimp Penaeus monodon and its comparison with white spot syndrome (WSS) caused by virus. Diseases of Aquatic Organisms 41: 9–18. [DOI] [PubMed] [Google Scholar]
- WB, NACA, WWF, FAO (2001) Thematic review on management strategies for major diseases in shrimp aquaculture In: Subasinghe R, Arthur R, Phillips MJ, Reantaso M. (eds) Proceedings of a workshop held in Cebu, Philippines on 28–30 November 1999, pp. 1–135. The World Bank, Network of Aquaculture Centres in Asia‐Pacific, World Wildlife Fund and Food and Agriculture Organization if the United Nations Consortium Program on Shrimp Farming and the Environment. Work in Progress for Public Discussion. Published by the Consortium. [Google Scholar]
- Webby RJ, Webster RG (2003) Are we ready for pandemic influenza? Science 302: 1519–1522. [DOI] [PubMed] [Google Scholar]
- Wei Q (2002) Social and economic impacts of aquatic animal health problems in aquaculture in China In: Arthur JR, Phillips MJ, Subasinghe RP, Reantaso MB, MacRae IH. (eds) Primary Aquatic Animal Health Care in Rural, Small‐scale, Aquaculture Development, pp. 55–61. FAO Fisheries Technical Paper No.406. Food and Agriculture Organization of the United Nations, Rome. [Google Scholar]
- Weppe M, Bonami JR, Lightner DV (1992) Demonstration de atlas cualidades de la cepa de P. stylirostris (AQUACORP SPR 43) resistente al virus IHHN. Memorias/Congreso Ecuatoriano Acuicultura, pp. 229–232 (in Spanish).
- Westenberg M, Heinhuis B, Zuidema D, Vlak JM (2005) siRNA injection induces sequence‐independent protection in Penaeus monodon against white spot syndrome virus. Virus Research 114: 133–139. [DOI] [PubMed] [Google Scholar]
- White BL, Schofield PJ, Poulos BT, Lightner DV (2002) A laboratory challenge method for estimating Taura syndrome virus resistance in selected lines of Pacific white shrimp, Litopenaeus vannamei . Journal of the World Aquaculture Society 33: 341–348. [Google Scholar]
- Wijegoonawardane PKM, Cowley JA, Phan TTN, Sittidilokratna N, Hodgson RAJ, Walker PJ (2008a) Genetic diversity in the yellow head nidovirus complex. Virology 380: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijegoonawardane PKM, Cowley JA, Walker PJ (2008b) Consensus RT‐nested PCR to detect yellow head virus genotypes in penaeid shrimp. Journal of Virological Methods 153: 168–175. [DOI] [PubMed] [Google Scholar]
- Wijegoonawardane PKM, Sittidilokratna N, Cowley JA, Gudkovs N, Walker PJ (2009) Genetic recombination in the yellow head complex of nidoviruses infecting Penaeus monodon shrimp. Virology (in press). [DOI] [PMC free article] [PubMed]
- Wilcox BA, Gubler DJ (2005) Disease ecology and the global emergence of zoonotic pathogens. Environmental Health and Preventive Medicine 10: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withyachumnarnkul B (1999) Results from black tiger shrimp Penaeus monodon culture ponds stocked with postlarvae PCR‐positive or‐negative for white‐spot syndrome virus (WSSV). Diseases of Aquatic Organisms 39: 21–27. [DOI] [PubMed] [Google Scholar]
- Withyachumnarnkul B, Boonsaeng V, Chomsoong R, Flegel TW, Muangsin S, Nash GL (2003) Seasonal variation in white spot syndrome virus‐positive samples in broodstock and post‐larvae of Penaeus monodon in Thailand. Diseases of Aquatic Organisms 53: 167–171. [DOI] [PubMed] [Google Scholar]
- Withyachumnarnkul B, Chayaburakul K, Lao‐Aroon S, Plodpai P, Sritunyalucksana K, Nash G (2006) Low impact of infectious hypodermal and hematopoietic necrosis virus (IHHNV) on growth and reproductive performance of Penaeus monodon . Diseases of Aquatic Organisms 69: 129–136. [DOI] [PubMed] [Google Scholar]
- Witteveldt J, Vlak JM, Van Hulten MC (2004a) Increased tolerance of Litopenaeus vannamei to white spot syndrome virus (WSSV) infection after oral application of the viral envelope protein VP28. Diseases of Aquatic Organisms 70: 167–170. [DOI] [PubMed] [Google Scholar]
- Witteveldt J, Cifuentes CC, Vlak JM, Van Hulten MC (2004b) Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. Journal of Virology 78: 2057–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveldt J, Vermeesch AM, Langenhof M, De Lang A, Vlak JM, Van Hulten MC (2005) Nucleocapsid protein VP15 is the basic DNA binding protein of white spot syndrome virus of shrimp. Archives of Virology 150: 1121–1133. [DOI] [PubMed] [Google Scholar]
- Wongteerasupaya C, Sriurairatana S, Vickers JE, Akrajamorn A, Boonsaeng V, Panyim S et al. (1995a) Yellow‐head virus of Penaeus monodon is an RNA virus. Diseases of Aquatic Organisms 22: 45–50. [Google Scholar]
- Wongteerasupaya C, Vickers JE, Sriurairatana S, Nash GL, Akarajamorn A, Boonsaeng V et al. (1995b) A non‐occluded, systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn Penaeus monodon . Diseases of Aquatic Organisms 21: 69–77. [Google Scholar]
- Wongteerasupaya C, Tongcheua W, Boonsaeng V, Panyim S, Tassanakajon A, Withyachumnarnkul B et al. (1997) Detection of yellow‐head virus of Penaeus monodon by RT‐PCR amplification. Diseases of Aquatic Organisms 31: 181–186. [Google Scholar]
- Wongteerasupaya C, Pungchai P, Withyachumnarnkul B, Boonsaeng V, Panyim V, Flegel TW et al. (2003) High variation in repetitive DNA fragment length for white spot syndrome virus (WSSV) isolates in Thailand. Diseases of Aquatic Organisms 54: 253–257. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhang X (2007) Characterization of a Rab GTPase up‐regulated in the shrimp Penaeus japonicus by virus infection. Fish and Shellfish Immunology 23: 438–445. [DOI] [PubMed] [Google Scholar]
- Wu JL, Nishioka T, Mori K, Nishizawa T, Muroga K (2002) A time‐course study on the resistance of Penaeus japonicus induced by artificial infection with white spot syndrome virus. Fish and Shellfish Immunology 13: 391–403. [DOI] [PubMed] [Google Scholar]
- Wu W, Zong R, Xu J, Zhang X (2008) Antiviral phagocytosis is regulated by a novel Rab‐dependent complex in shrimp Penaeus joponicus . Journal of Proteome Research 7: 424–431. [DOI] [PubMed] [Google Scholar]
- Xie X, Xu L, Yang F (2006) Proteomic analysis of the major envelope and nucleocapsid proteins of white spot syndrome virus. Journal of Virology 80: 10615–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wyrzykowski J, Alcivar‐Warren A (2003) Genetic analysis for TSV‐susceptible and TSV‐resistant Pacific white shrimp Litopenaeus vannamei using M1 microsatellite. Journal of the World Aquaculture Society 34: 332–343. [Google Scholar]
- Xu J, Han F, Zhang X (2007) Silencing shrimp white spot syndrome virus (WSSV) genes by siRNA. Antiviral Research 73: 126–131. [DOI] [PubMed] [Google Scholar]
- Yan DC, Dong SL, Huang J, Yu XM, Feng MY, Liu XY (2004) White spot syndrome virus (WSSV) detected by PCR in rotifers and rotifer resting eggs from shrimp ponds. Diseases of Aquatic Organisms 59: 69–73. [DOI] [PubMed] [Google Scholar]
- Yan DC, Dong SL, Huang J, Zhang JS (2007) White spot syndrome virus (WSSV) transmission from rotifer inoculum to crayfish. Journal of Invertebrate Pathology 94: 144–148. [DOI] [PubMed] [Google Scholar]
- Yang F, He J, Lin X, Li Q, Pan D, Zhang X et al. (2001) Complete genome sequence of the shrimp white spot bacilliform virus. Journal of Virology 75: 11811–11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Song XL, Huang J, Shi CY, Lu L (2007) Evidence of existence of infectious hypodermal and hematopoietic necrosis virus in penaeid shrimp cultured in China. Veterinary Microbiology 120: 63–70. [DOI] [PubMed] [Google Scholar]
- Yodmuang S, Tirasophon W, Roshrom Y, Chinnirunvong W, Panyim S (2006) YHV‐protease dsRNA inhibits YHV replication in Penaeus monodon and prevents mortality. Biochemical and Biophysical Research Communictions 341: 351–356. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu M (1996) Disease problems of salmonid fish in Japan caused by international trade. Revue Scientifique et Technique, Office International des Epizooties 15: 533–550. [DOI] [PubMed] [Google Scholar]
- Yu CI, Song YL (2000) Outbreaks of Taura syndrome in Pacific white shrimp Penaeus vannamei cultured in Taiwan. Fish Pathology 35: 21–24. [Google Scholar]
- Zhan WB, Wang YH, Fryer JL, Yu KK, Fukuda H, Meng QX (1998) White spot syndrome infection of cultured shrimp in China. Journal of Aquatic Animal Health 10: 405–410. [Google Scholar]
- Zhang S, Bonami JR (2007) A roni‐like virus associated with mortalities of the freshwater crab, Eriocheir sinensis Milne Edwards, cultured in China, exhibiting ‘sighs disease’ and black gill syndrome. Journal of Fish Diseases 30: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]


