Summary
Saffold virus (SAFV) is an emerging human cardiovirus that has been shown to be ubiquitous. Initial studies of SAFV focused on respiratory and gastrointestinal infection; however, it has also recently been associated with diverse clinical symptoms including the endocrine, cardiovascular, and neurological systems. Given the systemic nature of SAFV, and its high prevalence, understanding its pathogenicity and clinical impact is of utmost importance. This comprehensive review highlights and discusses recent developments in epidemiology, human pathogenicity, animal, and molecular studies related to SAFV. It also provides detailed insights into the neuropathogenicity of SAFV. We argue that human studies have been confounded by coinfections and therefore require support from robust molecular and animal research. Thereby, we aim to provide foresight into further research to better understand this emerging virus.
Keywords: animal model, CNS, neurotropic, pathogenicity, Saffold virus
Abbreviations Used
- CSF
Cerebrospinal fluid
- HFMD
hand‐foot‐mouth disease
- MS
multiple sclerosis
- PIV
parainfluenza virus
- SAFV
Saffold virus
- TMEV
Theiler's murine encephalomyelitis virus
- UTR
Untranslated region
1. INTRODUCTION
Members of the cardiovirus genus of Picornaviridae are single‐stranded RNA viruses, which were previously thought to mainly infect rodents.1, 2 In 2007, however, a novel human cardiovirus was identified through sequence‐independent genomic amplification from a historical stool sample of an 8‐month‐old child with fever of unknown origin.3 This virus was designated Saffold virus (SAFV) after the lead author of the research, Morris Saffold Jones. Phylogenetic analysis revealed that SAFV is closely related to the theilovirus species, which consists of Theiler's murine encephalomyelitis virus (TMEV), Theiler's rat virus, and Vilyuisk human encephalomyelitis virus.4 Since then, SAFV has been isolated from nasal and stool specimens of children with respiratory and gastrointestinal symptoms in many countries.3, 5, 6, 7, 8, 9, 10 To date, 11 genotypes of SAFV have been identified on the basis of phylogenetic analysis of the VP1 gene2 with SAFV‐2 and SAFV‐3 having high seroprevalence.6, 11
Initial work with SAFV was hindered by poor growth in laboratory cell lines.9, 12, 13 Subsequently, it was discovered that selected cell lines were indeed able to support the growth of SAFV (dependent on strain) thus the exponential increase in research data in recent years.11 These cell lines include Vero, HeLa, NIH/3 T3, CHO‐K1, Hep‐2, and Neuro2A.7, 13, 14, 15, 16, 17 Small studies demonstrating the role of SAFV in severe neurological deficits and death in children6, 11, 18 has garnered the attention of the research community. In this review, we highlight recent advances in SAFV research, with a focus on CNS infection.
2. MOLECULAR FEATURES OF SAFV
Saffold virus is a non‐enveloped single‐stranded RNA virus, with an icosahedral capsid of approximately 30 nm in diameter.7 The RNA genome is approximately 8050 nucleotides consisting of a single polyprotein coding region flanked by 2 UTRs at the 5’ and 3’ ends, with a variable length of poly (A) tail located at the terminus of the 3’ Untranslated region (UTR). The polyprotein region is divided into the leader (L) protein, the precursor P1, which encodes capsid proteins (VP1 to VP4), and precursors P2 and P3 regions, which encode 7 nonstructural proteins (2A‐2C and 3A‐3D)3 (Figure 1A). Similar to known picornaviruses, SAFV has a 5’ UTR of about 1040 nucleotides, containing internal ribosome entry sites that enable the initiation of translation through binding of canonical initiation factors and internal ribosome entry site–specific trans‐acting factors.19, 20 The L protein located at the N‐terminal portion of the polyprotein is thought to be highly important in the pathogenesis of the virus. The TMEV, a virus that is structurally and functionally similar to SAFV,21 has been extensively studied because of its unusual phenotype.2 TMEV is divided into 2 subgroups on the basis of their neurovirulence22; highly virulent strains (GDVII and FA) that cause acute fatal encephalomyelitis, and low virulent strains (DA and BeAn), which cause milder encephalomyelitis with chronic progression to a demyelinating syndrome similar to multiple sclerosis (MS).2, 23 The L protein of TMEV has been shown to play an essential role in the differences seen between the 2 TMEV subgroups.24 The typical characteristics of the L region of TMEV, and indeed other similar cardioviruses, contain a well‐conserved zinc‐finger motif, an acidic region and a serine/threonine‐rich domain.25 Interestingly, the serine/threonine‐rich domain of L protein is partially deleted in SAFV.7 On analysis, the homology of L between SAFV and TMEV DA strains is 78%.16 In certain strains of TMEV (such as the DA strain), an alternative translation initiation site downstream from authentic initiation site is able to synthesize a small out‐of‐frame 18‐kDa protein referred to as L*.26 The SAFV lacks the AUG initiation codon required to translate L* protein, although it is unclear if the ACG present in that region of SAFV is used to produce a truncated L* protein.2
Figure 1.
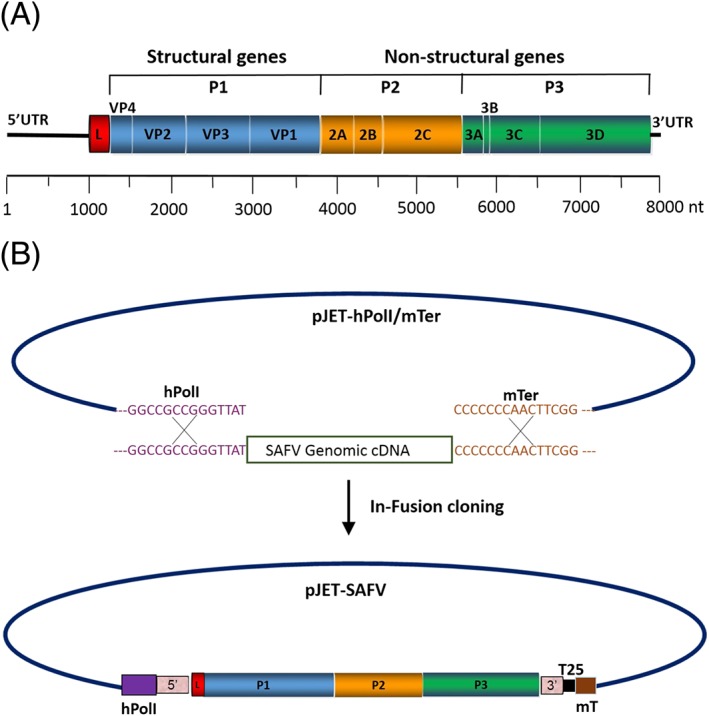
Diagram of Saffold virus (SAFV) genome. A, Diagram of SAFV genome showing summary of features. SAFV is a single‐stranded RNA and approximately 8050 bp in size. The single open reading frame (ORF) is flanked by UTRs at the 5’ and 3’ ends. The ORF is divided into the leader (L) protein, the P1 region encodes 4 structural proteins (VP1 to VP4), and P2 and P3 regions encode 7 nonstructural proteins (2A‐2C and 3A‐3D). B, Flow diagram describing the generation of an infectious SAFV by the human RNA polymerase 1 reverse genetics. The pJET‐SAFV plasmid was generated by insertion of SAFV cDNA amplicon into pJET‐hPolI/mTer using In‐Fusion cloning method. hPol1: human RNA polymerase 1; T25: poly (A) tail with 25 adenosines; mT: murine terminator
While the capsid proteins of SAFV have not been studied directly, we are able to deduce some of its features from related cardioviruses like TMEV. The capsid proteins VP1 to VP3 are exposed on the external surface of the virion and are responsible for the initiation of infection by host‐receptor bindings. The VP1 is the most exposed immunodominant protein and the most surface‐accessible capsid protein. The EF and CD loop structures are located in the VP2 and VP1 proteins, respectively, and are associated with host cell tropism and pathogenesis in cardioviruses.27, 28 The CD and EF loops are unique for each genotype of SAFV and are highly divergent among cardioviruses. Importantly, in addition to L protein, the CD loop of VP1 and the EF loop of VP2 have also been found to be important in virus persistence and host demyelination in TMEV.28, 29 We have previously suggested these are potential areas of study regarding SAFV.16
Reverse genetics has led to important advances in the understanding of the roles of both capsid and nonstructural proteins; the generation of infectious SAFV cDNA has allowed us to specifically modify the virus.16, 30 Previously, Himeda et al30 had generated infectious RNA in vitro from full‐length cDNA of SAFV using T7 RNA polymerase. Further, they constructed chimeric SAFV cDNA clones by replacing the VP1 and/or the VP2 gene of SAFV with those of TMEV of DA or GDVII strain. However, they were unable to rescue the recombinant viruses, even after 3 blind passages. Similarly, Shimizu et al31 generated chimeric SAFV and TMEV by replacing L protein of SAFV with that of TMEV DA strain and vice versa and studied their effects on interferons. Very recently, our laboratory generated an infectious cDNA clone of SAFV under the control of a human RNA polymerase 1 (hpol1) promoter (Figure 1B).16 In this method, the genomic viral cDNA is transcribed into an exact SAFV‐like RNA by hpol1 inside the cells. Compared to T7 polymerase‐driven reverse genetics systems, our approach has eliminated the need for troublesome in vitro RNA transcription from cDNA clones. It also eliminates the need to add extra bases during in vivo transcription at the 5’ and 3’ ends of viral transcripts.32 Overall, studies using chimeric SAFV containing TMEV L have shown that the L protein is at least partially responsible for differences in suppression of interferons, cell tropism, pathogenicity of the virus, and nucleocytoplasmic trafficking.16, 31, 33
3. SAFV EPIDEMIOLOGY AND HUMAN PATHOGENICITY
The SAFV has been detected in patients globally5, 6, 7, 9, 10, 11, 12, 17, 18, 21, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 (see Table 1 for summary). Although infection rates appear low (<10%) in symptomatic patients via polymerase chain reaction methods, neutralizing antibodies from respiratory tract samples have been found at a high percentage of asymptomatic populations (55‐100%).7, 17, 47, 51 This may point to either historical infection, or intrinsic immunity. The symptoms of SAFV infection in humans is diverse, with many studies demonstrating positive identification of SAFV in a variety of samples. Many studies have focused on respiratory and gastrointestinal symptoms in humans, which are presented below.
Table 1.
Summary of published papers of SAFV infections in humans
| Author/s | Country | Age Range (y) | Sample | Inclusion | n‐value | SAFV +ve | Other Pathogens | Remarks |
|---|---|---|---|---|---|---|---|---|
| Abed & Boivin5 | Canada | 0‐4 | NP aspirate | Otitis media, URTI (Exclusion: positive blood cultures or typical viral screen) | 3 | 3 | ||
| AFP: 57 | 5 | |||||||
| Blinkova et al6 | Pakistan and Afghanistan | 0‐15 | Stool | AFP (Exclusion: Polio positive) | Healthy household contacts of AFP patients: 9 Unrelated healthy patients: 41 | 1 | ||
| Branas et al45 | Spain | 0‐12 | NP aspirates | Respiratory tract infection | 608 samples from 552 patients | 8 | Adenovirus (2) and streptococcus pneumoniae (1) | Multiple samples from patients |
| 12‐95 | NP swabs | Immunosuppression, respiratory tract infection | 595 samples from 370 patients | 0 | ||||
| Respiratory secretions | Influenza‐like illness (Exclusion: FluA/B, RSV, RV, EV on culture, or RT‐PCR) | 460 | 1 | |||||
| Respiratory secretions | Single hospital (n = 278) and state‐wide with influenza‐like illness (n = 441) | 719 | 0 | Multiple samples for some patients | ||||
| Chiu et al21 | USA | CSF | Aseptic meningtis, encephalitis, or MS | 400 | 0 | |||
| Stool | Gastroenteritis or household contacts | 751 | 6 | Adenovirus (1), RV (1), norovirus (3), EV (1), sapovirus (1), and parechovirus (1) | All positive samples from children <2. Two children were asymptomatic multiple samples for most patients | |||
| 5 | Throat swab | Fever and sore throat | 1 | 1 | ||||
| Chua et al17 | Malaysia | 10‐12 | Serum | Post‐hepatitis B vaccination survery | 400 | >280 | >70% serum positive | |
| Dai et al38 | China | 0‐8 | Stool | 577 Diarrhea (>3 loose stools) and 60 healthy | 637 | 6 | RV (3) | |
| Drexler et al9 | Germany and Brazil | 0‐6 | Stool | Gastroenteritis | 844 | 6 | Enteric viruses (4) | |
| Itagaki et al12 | Japan | 2‐7 | NP swab | Exudative tonsillitis | 37 | 9 | NIL | |
| Itagaki et al39 | Japan | 0‐18 | NP specimens | Acute respiratory infection (Exclusion: viral coinfection) | 1525 | 54 | Diarrhea coexistent in n = 7 (20%) | |
| Ito et al52 | Japan | 2 | Stool, serum, and NP swab | Relapsing acute pancreatitis | 1 | 1 | Relapsing acute pancreatitis after HFMD | |
| Jones et al3 | USA | 8 mo | Stool | Fever of unknown origin | 1 | 1 | ||
| Khamrim et al41 | Thailand | 1‐5 | Stool | Gastroenteritis | 150 | 4 | RV (1) | |
| Khamrim et al43 | Japan | 0‐6 | Stool | Diarrhea | 454 | 7 | 4/7 coinfected with mix of rotavirus (1), bocavirus (3), and norovirus (2) | |
| Kobayashi et al51 | Japan | 0‐66 | Serum | Healthy | 114 | 95.6% positive for antibodies | ||
| Leguia et al10 | Peru | 2 | Oropharyngeal swab | Diarrhea and respiratory infection | 1 | 1 | ||
| Lin et al47 | Taiwan | 0‐15 | Throat swab | EV symptoms (URTI and/or D&V and/or rash) (Exclusion: EV positive [1228/1454]) | 227 | 22 | Antibody found in 43.7%‐77.8% of children 0‐ to 9‐y‐old | |
| Naeem et al44 | Afghanistan and Pakistan | 0‐15 | Stool | AFP (Exclusion: Polio and EV in stool) | 943 | 88 | ||
| Nielsen et al42 | Denmark | 6, 10, and 15 mo | Stool | Randomized samples | 1393 | 38 | Multiple samples from patients | |
| Nielsen et al50 | Denmark | 0‐77 | Formalin‐fixed paraffin embedded (FFPE) cardiac tissue | Myocarditis | 150 | 1 | Staphylococcus aureus, Haemophilus influenzae and non‐hemolytic streptococci in lung tissue, and enterovirus in respiratory secretion | Portmortem anaysls. SAFV +ve: patient 2‐y‐old, sudden death after fever. The SAFV also detectable in frozen blood and respiratory secretion but not CSF |
| Ren et al36 | China | 0‐16 | NP aspirate | Acute LRTI | 1032 | 4 | Mycoplasma pneumoniae (1), RSV (1), and EV (1) | SAFV +ve were <9yo |
| 0‐16 | Oropharyngeal swab | Acute URTI | 406 | 3 | ||||
| Ren et al35 | China | 0‐13 | Stool | Acute gastroenteritis | 373 | 12 | 11 coinfected with at least 1 known diarrhea‐causing virus, such as RV or norovirus | SAFV positive <3 years old |
| Tsukagoshi et al37 | Japan | 5 and 6 | NP specimens | Fever, Cranker sores, and URTI | 2 | 2 | ||
| Tsukagoshi et al40 | Japan | 0‐41 | NP swabs | Acute respiratory infection (Exclusion: coinfection with other respiratory viruses) | 423 | 9 | SAFV positive 1‐11 years old | |
| Wang et al48 | Australia | 0‐95 | NP aspirate (48.1%), NP swab (31.8%), nasal swab (5.4%), oropharyngeal swab (3.5%), and BAL (1.5%) | Acute respiratory infection | 1215 | 8 | Unknown pathogens | 75% SAFV +ve were from age < 2y n = 5 (62.5%) SAFV +ve were also +ve for another virus suggest autumn prevalance |
| Xu et al34 | China | <5 | Stool | Diarrhea | 631 | 3 | RV (2) and norovirus (1) | |
| Asymtomatic | 161 | 1 | NIL | |||||
| Yodmeeklin et al46 | Thailand | 0‐14 | Stool | Acute gastroenteritis | 608 | 9 | RV (2), adenovirus (2), EV (2), and cosavirus (1), Bocavirus (1) | SAFV positve age 1‐8, most <3‐y‐old |
| Zhang et al11 | China | 0‐14 | NP aspirate | Acute respiratory infection | 1647 | 17 | Parainfluenza (5), RSV‐B (4), adenovirus (2), bocavirus (2), coronavirus (2), FluA (1), FluB (1), and rhinovirus (1). | Significantly higher SAFV infection found in HFMD patients |
| Stool | Diarrhea | 2013 | 12 | Norovirus GII (6), RV (2), and adenovirus (2). | ||||
| Stool and some throat swabs, serum, and CSF | HFMD | 2392 | 86 | EV71 (23), coxsackie virus A16 (17), and other EV (18) | ||||
| Zoll et al7 | Netherlands | 0‐10 | Serum | Healthy | 210 | 90 children between 4 and 10 y of age—92% had neutralizing antibodies. 60 adults—98% had neutralizing antibodies | ||
| Finland | 30 | 77% of Finnish children had neutralizing antibodies | ||||||
| Africa (Mali and Cameroon) | 72 | 72 | 100% had neutralizing antibodies | |||||
| Indonesia (Java and Sumba) | 63 | 63 | 100% had neutralizing antibodies |
Abbreviations: AFP, acute flaccid paralysis; BAL, bronchoalveolar lavage; D&V, diarrhea and vomiting; EV, enterovirus; FluA/B, influenza A/B; HFMD, hand‐foot‐mouth disease; LRTI, lower respiratory tract infection; MS, multiple sclerosis; NP, nasopharyngeal; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rotavirus; SAFV, Saffold virus; URTI, upper respiratory tract infection.
As SAFV was first isolated from a stool sample, research had initially focused on gastrointestinal symptoms following a presumed fecal‐oral transmission route.3 Studies have looked at cohort of patients suffering from acute gastroenteritis and have identified SAFV as a potential source in 0.2% to 3% of symptomatic patients from their stool samples.9, 34, 36, 38, 41 Nielsen et al42 also demonstrated a similar SAFV positive rate (3%) in a surveillance study, which included asymptomatic patients. However, the data included most patients co‐enrolled into a randomized control trial investigating infection rates and the use of probiotics and included multiple samples from patients at different times. Other pathogens detected included adenovirus, bocavirus, cosavirus,46 enteroviruses,9, 47 norovirus,21, 34, 35 and rotavirus.21, 34, 36, 38, 41, 43 The significance of enterovirus coinfection will be discussed in the following paragraphs. The high coinfection rate with common pathogens causing gastroenteritis such as norovirus and rotavirus make correlations and conclusions very difficult. While most studies have noted a self‐limiting course of diarrhoeal illness, Ito et al52 identified SAFV as a potential cause of relapsing pancreatitis in a 2‐year‐old child after hand‐foot‐mouth disease (HFMD). However, this patient was also known to have Kawasaki disease, from which pancreatitis is an uncommon but recognized complication (presumed to be from infiltration of autoimmune and inflammatory cells).53
The SAFV has been isolated from nasopharyngeal aspirates of 0.2% to 24% of children suffering from nonspecific respiratory tract symptoms.5, 10, 11, 17, 21, 36, 39, 40, 45, 47 Itagaki et al39 have also isolated SAFV in patients with exudative tonsillitis. While some studies have isolated only SAFV in their patients,5, 12, 40 many studies have also isolated other pathogens in SAFV‐positive symptomatic patients. These include adenovirus,45 streptococcus pneumoniae, 45 mycoplasma pneumoniae, 36 respiratory syncytial virus,36 and enterovirus.36
The detection of coinfecting organisms is significant. This is especially prominent in the aforementioned studies and some others below investigating neurological symptoms. It may point to SAFV requiring the presence of coexisting infection to thrive, or coinfection may result in increased severity requiring presentation to medical institutions. Moreover, it clouds conclusions about primary organs of infection and the severity to which they are affected by SAFV. This is particularly the case with enteroviruses and mycoplasma pneumoniae, which have similar clinical manifestations to SAFV infection.54, 55 Enteroviruses may cause a plethora of symptoms, which include upper respiratory tract symptoms, myocarditis, aseptic meningitis, HFMD, polio‐like paralysis, make the significance of coinfection with SAFV very difficult to interpret. This may also act as a strong confounder in some studies discussed here.11, 36, 47, 52 Likewise with mycoplasma pneumoniae, which may demonstrate central nervous system, cardiac and gastrointestinal symptoms not dissimilar to enterovirus. With limited understanding of its systemic involvement, further studies are required to investigate the prevalence of SAFV in immunocompromised or critical care patients as well.
Due to the similarity of SAFV to TMEV, researchers began looking at SAFV in patients with neurological symptoms. The SAFV have been detected in stool samples of children suffering with non‐polio acute flaccid paralysis.6, 44 However, these studies did not investigate some other causes of acute flaccid paralysis, which include Guillian‐Barre syndrome and transverse myelitis. Nielsen et al18 found SAFV in the Cerebrospinal fluid (CSF) in 2 children out of 319 samples, younger than 4 years of age, but Chiu et al21 did not find SAFV in 400 CSF specimens of patients with aseptic meningitis, encephalitis and MS. Zhang11 identified a 3% prevalence of SAFV in the CSF of patients with HFMD. Neurological manifestations are recognized complications of HFMD, and indeed of other viral illnesses discussed above. Given that TMEV causes MS like disease in rodents,2, 23 researchers were interested to investigate SAFV in this light.
Several papers have pointed out the predilection of young children to SAFV infections. However, many cohort studies have also focused on testing children rather than adults. While Wang et al48 found SAFV in an adult population, other papers have not replicated their results.40, 45 The ability of SAFV infection to clinically affect adults is thus still controversial. More studies with adult samples are needed to shed more light on SAFV infection in an adult population.
Clinicians will note that the known symptoms of SAFV is not unlike other known and coinfected pathogens such as enterovirus,55 norovirus,56 rotavirus,57 and mycoplasma pneumoniae. 54 The mainstay of treatment of most viral infections tends to be supportive. Therefore, our current understanding of SAFV does not affect patient care. This may perhaps change when primer and polymerase chain reaction–based methods of identifying pathogens in patients become more widely used and readily available in a variety of health care settings.58, 59, 60
The current human studies discussed highlight the important systemic involvement of SAFV and give direction to further research in this area. However, a more cohesive understanding of human pathogenesis and symptoms is needed for translational therapeutic research to begin to tackle the health burden of SAFV. For a start, due to low detection rates of current infection, large sample sizes over many countries are needed to determine SAFV's true epidemiology. Moreover, meticulous data on SAFV‐only infections is needed to elicit its true symptoms and route of spread, followed by targeted coinfection studies to establish its role in overall disease process. All these must be supported by a strong molecular and animal base of research. Although this section forms a starting point for consolidation of current knowledge and understanding for researchers and clinicians, it also acknowledges the lack of depth in our understanding of this pathogen. The low infection rate (albeit high seroprevalence rate), coupled with a trend toward a self‐limiting course of disease and strong confounding factors, make honest cost‐benefit analyses essential to underpin further research into SAFV.
4. ANIMAL MODELS OF SAFV INFECTION
The use of animal models provide an effective way of studying virus infections at a systemic level. However, finding an appropriate model for SAFV infection has proven to be a challenge.2 To our knowledge, the first published research article on using an animal model of SAFV was by Hertzler et al15 They found that ic inoculation of SAFV‐2 to FVB/n (an inbred mouse strain commonly used for non‐clinical drug discovery) mice causes neuropathological changes consistent with acute encephalomyelitis. While the study above had started to uncover the pathogenesis of SAFV, hinting at invasive CNS infection, little progress was made thereafter. In 2016, however, 3 separate groups published work done on various mouse models, directly showing SAFV CNS infection.16, 61, 62 The first accepted paper was from our laboratory, which used SAFV‐3 on BALB/c mice and AG129 mice (mice with an intact immune system, but lacking alpha/beta interferon (IFN‐α/β) and IFN‐γ receptors63). We showed that BALB/c mice infected ip with SAFV‐3 showed neither clinical symptoms nor detectable viral titre in the CNS. Previous studies on TMEV using inbred 129Sv mice lacking IFN‐α/β receptors developed severe encephalomyelitis (acute TMEV infection), whereas mice lacking IFN‐γ receptors were highly susceptible to persistent infection in the white matter of the brain, causing demyelination.64, 65 We hence reasoned that the use of AG129 mice, lacking both IFN‐α/β and IFN‐γ receptors,63 would permit both acute and persistent infections if possible. We found that ip infection of SAFV and chimeric SAFV in 2‐week‐old AG129 mice initially caused ruffled fur, hunched posture, and subsequently progressed to hind‐limb paralysis and death.16 Interestingly although, 3‐ to 4‐week‐old mice did not die to paralysis nor death, and fully recovered showing no further symptoms.16 This was later supported by Sorgeloos et al62 when they demonstrated that infection of interferon receptor deficient (IFNAR‐KO) 129/sv mice permitted infection of the brain, spinal cord, heart, pancreas, and spleen. At the same time, Kotani et al61 showed that ic inoculation of SAFV causes non‐fatal infection of neonatal and 6‐week‐old ddY and BALB/c mice. They further showed demyelination in the spinal cord of infected neonatal ddY mice spine in one of their strains, but not in adult mice. It should be noted that while Kotani et al61 showed demyelination, they attributed it to a TMEV infection rather than an effect of SAFV. Both our laboratory16 and Sorgeloos et al62 have also shown that it is highly unlikely that SAFV causes demyelination, and similarly in humans, Galama et al66 suggested that an association between SAFV and MS is highly improbable.
While studies on animal models of SAFV have focused on CNS infection, SAFV has also been found in other organs/tissues such as the heart, spleen, muscles, and pancreas (Figure 2).61, 62 Sorgeloos et al62 found that SAFV exhibited a pronounced tropism for the pancreas and suggested further investigation of SAFV in pancreatic disease. This is further highlighted by Ito et al52 who suggested an association between acute pancreatitis and SAFV in humans. Furthermore, it is noteworthy that several viruses from the Picornaviridae family, such as coxsackie‐B virus, have been associated with type 1 diabetes mellitus (which involves the destruction of insulin producing cells in the pancreas).2, 67, 68, 69, 70, 71 However, a longitudinal study of children carrying HLA genotype (associated with high risk of type 1 diabetes mellitus) found no significant association between SAFV and diabetes.72 Overall, more work is needed to understand the pathogenesis of SAFV in the pancreas, and further work on recently established models could provide a means of doing so.
Figure 2.
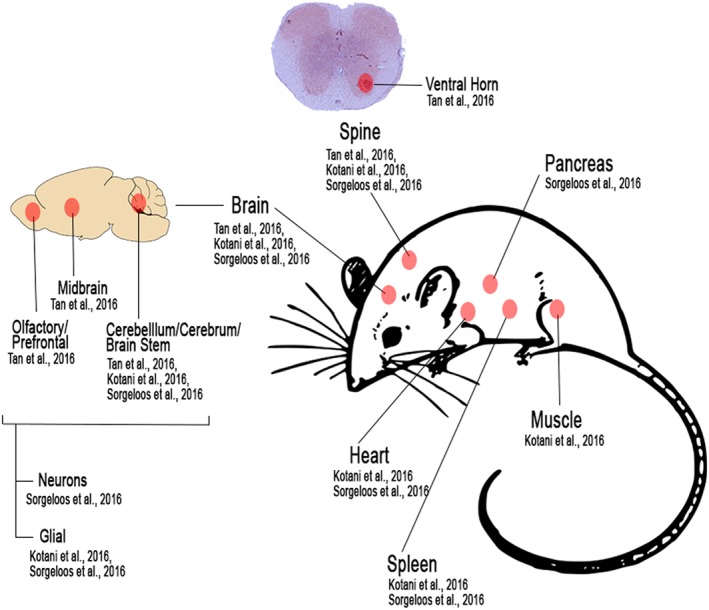
Summary of animal studies done of Saffold virus. Diagram shows locations in which SAFV have been reported to be detected in mice models. This includes the CNS (particularly in the ventral horn of the spine, and various regions in the brain), heart, spleen, pancreas, and muscle tissue
5. NEUROPATHOGENESIS
Initial research on SAFV focused heavily on respiratory and gastrointestinal tract infections,11 with neuropathogenesis of SAFV only being looked at relatively recently. While SAFV is thought to transmit via the fecal‐oral route,3, 6, 9, 35, 36 it is unclear as to how or why it infects the CNS, especially because no obvious selective pressure exists. It should be noted although, that many enteric viruses and enteroviruses, including the closely related TMEV, are neurotropic, and it has been suggested that gut cells share similar properties that could act as viral receptors.73 Regardless, researchers became increasingly interested in the neuropathogenesis of SAFV, with many recent studies focusing on infection of the CNS.16, 61, 62, 66 This interest started because of the similarities between SAFV and TMEV2, 23 and the possibility of invasive infection of the CNS by SAFV as a reason for MS. Subsequent research has suggested otherwise,16, 62, 66 even though infection of both neuronal and glial cells have been shown in animal models.61, 62
The L protein of TMEV has been shown to play an essential role in the establishment of persistent CNS infections in mice and therefore progression to demyelination.24 The native truncated L protein of SAFV may be a possible reason for the lack of demyelination in SAFV infection. We hence generated a chimeric SAFV with the L protein of TMEV DA strain (which causes demyelination).16 Initial results looked promising as the chimeric SAFV was able to infect macrophages. This is important as virus persistence in monocytes/macrophages is essential in TMEV induced demyelination.74, 75 However, the low infection rate of macrophages suggests that apart from L*, additional factors are required for virulence. Importantly, the structural capsid proteins of TMEV, which has been shown to be important for receptor binding,76 are completely different from that of SAFV and could explain the reason for low infectivity rates of macrophages. This low infectivity of macrophages is a potential reason for the lack of persistence despite the presence of TMEV DA L,16 as persistent TMEV DA infection is thought to be a result of infected macrophages crossing the blood‐brain barrier.77 However, it should be noted that the activation or differentiation state of macrophages have been suggested to play an important role in TMEV infection78 and hence the possibility of macrophage infection in vivo, while highly unlikely, cannot be completely ruled out.
The TMEV DA strain causes milder encephalomyelitis in the CNS, which then progresses to persistent infection and progressive demyelination reminiscent of MS.2 The rapidly fatal outcome of animals that permit infection of SAFV make it difficult to determine if the lack of demyelination reflects inability of the virus or insufficient time for development. The 3‐ to 4‐week‐old AG129 mice infected with SAFV only develop mild clinical symptoms between 7 and 10 dpi, but subsequently recover from the symptoms and show no further observable symptoms up to 35 dpi.16 They thus provide a model to study demyelination in SAFV viral persistence. Our laboratory,16 congruent with Kotani et al,61 failed to demonstrate persistent infection. This suggests that the pathological mechanism underlying the demyelination processes of SAFV and TMEV may be different.
It has been shown that infection and subsequent apoptosis of neurons are responsible for fatal outcomes in TMEV infection, while persistence in and subsequent apoptosis of glial cells such as oligodendrocytes causes progressive demyelination.79 This may suggest that clinical outcome depends on cell type infected. In vitro, SAFV has been shown to infect multiple cell types, including neurons, which result in apoptosis.14 Sorgeloos et al62 further showed SAFV's preference for astrocytes over neurons in mixed mouse primary neuron‐astrocytes cultures. In vivo, Kotani et al61 showed infection of glial cells, but not neurons, in both early adult (6‐week‐old) and neonatal brains of ddY and BALB/c mice. Sorgeloos et al62 however showed infection of both neuronal and glial cells. The possibility of acute infection of neurons could explain the ability of SAFV to cause sudden death in infected patients.18, 79 Two laboratory test results have demonstrated death and/or severe neurological symptoms in young interferon‐deficient mice infected with SAFV (within neurons).16, 62 However, the absence of neuronal infection in neonatal and 6‐week‐old ddY and BALB/c mice conferred survival from SAFV infection.61 One however needs to be cautious in interpreting the results in this fashion, as CNS is not the only location of SAFV infection, and coinfections with other viruses is not uncommon in human studies of SAFV (reviewed above). Overall, while results on neuropathogenesis seems varied between groups highlights CNS infection by SAFV differs depending on strain, age of infection, and breed of infected animal (and hence genetic makeup).
6. CONCLUSIONS AND FUTURE PERSPECTIVES
Despite recent research interest greatly increasing our knowledge about SAFV, we are still just beginning to scratch the surface. In this review, we discussed the epidemiology, pathogenesis, and molecular features of SAFV, providing detailed insights into CNS infections. We highlighted SAFV as a systemic virus, capable of producing devastating outcomes.
It may be easy to neglect SAFV given its low rate of debilitating infection in humans and low likelihood of progression to demyelinating disease.16, 61, 62, 66 However, due to its close relation to demyelinating viruses,2, 4, 21, 22, 23 the error‐prone RNA‐dependent RNA polymerase (RdRp) replication method,80 and high selective pressures toward high virulence,7 SAFV mutation to become a devastating virus is not altogether unthinkable. Research into SAFV and related viruses like TMEV help increase our understanding of the molecular mechanisms of their pathogenesis, thereby preparing ourselves for mutational changes in virulence, severity, and symptoms. The availability of an infectious cDNA clone of SAFV16, 30 could provide a powerful tool for this, allowing us to conduct reverse genetics studies and to understand the differences between SAFV and related viruses. Likewise, the identification of receptors for viral infection would further deepen our understanding of pathogenesis, as well as enable preparations of appropriate transgenic animal models for SAFV.2
There are no therapeutic options for SAFV currently. Knowledge from other RNA viruses such as EV71 and poliovirus has highlighted the ease of resistance development even with single mutations.81, 82 Therefore, studies into mechanisms of resistance for this RNA virus remain crucial yet unexplored.80 Such understanding may allow us to be better prepared for viral resistance and thus develop therapeutic options targeted at critical mechanisms in viral replication.
Reflecting on the recent Zika virus outbreaks, there are several lessons to be learnt. Strengthening research on lesser‐known viruses such as SAFV (and related cardioviruses) is key to preventing public health catastrophes.83 We hope SAFV will not mirror the course of Zika virus, but if it does, we need to be ready to halt progression before it reaches epidemic levels.
CONFLICT OF INTEREST
The authors have no competing interest.
ACKNOWLEDGEMENT
This work was supported by Temasek Life Science Laboratory.
Tan, S. Z. K. , Tan, M. Z. Y. , and Prabakaran, M. Saffold virus, an emerging human cardiovirus, Rev. Med. Virol. 2017;27:e1908. doi: 10.1002/rmv.1908.
REFERENCES
- 1. Pritchard AE, Strom T, Lipton HL. Nucleotide sequence identifies Vilyuisk virus as a divergent Theiler's virus. Virology. 1992;191:469–472. doi: 10.1016/0042-6822(92)90212-8 [DOI] [PubMed] [Google Scholar]
- 2. Himeda T, Ohara Y. Saffold virus, a novel human cardiovirus with unknown pathogenicity. J Virol. 2012;86:1292–1296. doi: 10.1128/JVI.06087-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45:2144–2150. doi: 10.1128/JCM.00174-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang Z, Kumar ASM, Jones MS, Knowles NJ, Lipton HL. Phylogenetic analysis of the species theilovirus: emerging murine and human pathogens. J Virol. 2008;82:11545–11554. doi: 10.1128/jvi.01160-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abed Y, Boivin G. New Saffold cardioviruses in 3 children, Canada. Emerg Infect Dis. 2008;14:834–836. doi: 10.3201/eid1405.071675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blinkova O, Kapoor A, Victoria J, et al. Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J Virol. 2009;83:4631–4641. doi: 10.1128/jvi.02085-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zoll J, Erkens Hulshof S, Lanke K, et al. Saffold virus, a human Theiler's‐like cardiovirus, is ubiquitous and causes infection early in life. PLoS Pathog. 2009;5:e1000416. doi: 10.1371/journal.ppat.1000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiu CY, Greninger AL, Chen EC, et al. Cultivation and serological characterization of a human Theiler's‐like cardiovirus associated with diarrheal disease. J Virol. 2010;84:4407–4414. doi: 10.1128/JVI.02536-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drexler JF, de Souza Luna LK, Stöcker A, et al. Circulation of 3 lineages of a novel saffold cardiovirus in humans. Emerg Infect Dis. 2008;14:1398–1405. doi: 10.3201/eid1409.080570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leguia M, Loyola S, Rios J, et al. Full genomic characterization of a Saffold virus isolated in Peru. Pathogens. 2015;4:816. doi: 10.3390/pathogens4040816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X‐A, Lu Q‐B, Wo Y, et al. Prevalence and genetic characteristics of Saffold cardiovirus in China from 2009 to 2012. Sci Rep. 2015;5:7704. doi: 10.1038/srep07704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itagaki T, Abiko C, Ikeda T, et al. Sequence and phylogenetic analyses of Saffold cardiovirus from children with exudative tonsillitis in Yamagata, Japan. Scand J Infect Dis. 2010;42:950–952. doi: 10.3109/00365548.2010.496791 [DOI] [PubMed] [Google Scholar]
- 13. Blomqvist S, Lappalainen M, Paananen A, Ylipaasto P, Roivainen M. Isolation of Saffold virus type 2 in green monkey kidney cells. J Med Virol. 2012;84:1497–1500. doi: 10.1002/jmv.23352 [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, Victorio CBL, Ng Q, Tan YJ, Chua KB. Saffold virus is able to productively infect primate and rodent cell lines and induces apoptosis in these cells. Emerg Microbes Infect. 2014;3:e15. doi: 10.1038/emi.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hertzler S, Liang Z, Treso B, Lipton HL. Adaptation of Saffold virus 2 for high‐titer growth in mammalian cells. J Virol. 2011;85:7411–7418. doi: 10.1128/JVI.00265-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan ZS, Chua BK, Xu Y, Prabakaran M. The pathogenesis of Saffold virus in AG129 mice and the effects of its truncated L protein in the central nervous system. Viruses. 2016;8. doi: 10.3390/v8020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua KB, Voon K, Yu M, Ali WN, Kasri AR, Wang LF. Saffold virus infection in children, Malaysia, 2009. Emerg Infect Dis. 2011;17:1562–1564. doi: 10.3201/eid1708.101380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen ACY, Böttiger B, Banner J, Hoffmann T, Nielsen LP. Serious invasive saffold virus infections in children, 2009. Emerg Infect Dis. 2012;18:7–12. doi: 10.3201/eid1801.110725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drexler JF, Baumgarte S, de Souza Luna LK, et al. Genomic features and evolutionary constraints in Saffold‐like cardioviruses. J Gen Virol. 2010;91:1418–1427. doi: 10.1099/vir.0.018887-0 [DOI] [PubMed] [Google Scholar]
- 20. Pestova TV, Kolupaeva VG, Lomakin IB, et al. Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci U S A. 2001;98:7029–7036. doi: 10.1073/pnas.111145798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu CY, Greninger AL, Kanada K, et al. Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc Natl Acad Sci U S A. 2008;105:14124–14129. doi: 10.1073/pnas.0805968105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jarousse N, Syan S, Martinat C, Brahic M. The neurovirulence of the DA and GDVII strains of Theiler's virus correlates with their ability to infect cultured neurons. J Virol. 1998;72:7213–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipton HL. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stavrou S, Ghadge G, Roos RP. Apoptotic and antiapoptotic activity of L protein of Theiler's murine encephalomyelitis virus. J Virol. 2011;85:7177–7185. doi: 10.1128/JVI.00009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paul S, Michiels T. Cardiovirus leader proteins are functionally interchangeable and have evolved to adapt to virus replication fitness. J Gen Virol. 2006;87:1237–1246. doi: 10.1099/vir.0.81642-0 [DOI] [PubMed] [Google Scholar]
- 26. Kong WP, Roos RP. Alternative translation initiation site in the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1991;65:3395–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCright IJ, Tsunoda I, Whitby FG, Fujinami RS. Theiler's viruses with mutations in loop I of VP1 lead to altered tropism and pathogenesis. J Virol. 1999;73:2814–2824. doi: 10.1080/13550280290049561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jnaoui K, Minet M, Michiels T. Mutations that affect the tropism of DA and GDVII strains of Theiler's virus in vitro influence sialic acid binding and pathogenicity. J Virol. 2002;76:8138–8147. doi: 10.1128/jvi.76.16.8138-8147.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adami C, Pritchard AE, Knauf T, Luo M, Lipton HL. A determinant for central nervous system persistence localized in the capsid of Theiler's murine encephalomyelitis virus by using recombinant viruses. J Virol. 1998;72:1662–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Himeda T, Hosomi T, Asif N, et al. The preparation of an infectious full‐length cDNA clone of Saffold virus. Virol J. 2011;8:110–110. doi: 10.1186/1743-422X-8-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimizu A, Himeda T, Okuwa T, Muraki Y, Ohara Y. Role(s) of leader protein of Saffold virus. Clin Exp Neuroimmunol. 2014;5:362–366. doi: 10.1111/cen3.12109 [DOI] [Google Scholar]
- 32. Meng T, Kiener TK, Kwang J. RNA polymerase I‐driven reverse genetics system for enterovirus 71 and its implications for vaccine production. Virol J. 2012;9:1–7. doi: 10.1186/1743-422x-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciomperlik JJ, Basta HA, Palmenberg AC. Three cardiovirus Leader proteins equivalently inhibit four different nucleocytoplasmic trafficking pathways. Virology. 2015;484:194–202. doi: 10.1016/j.virol.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu ZQ, Cheng WX, Qi HM, Cui SX, Jin Y, Duan ZJ. New Saffold cardiovirus in children, China. Emerg Infect Dis. 2009;15:993–994. doi: 10.3201/eid1506.090109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ren L, Gonzalez R, Xiao Y, et al. Saffold cardiovirus in children with acute gastroenteritis, Beijing, China. Emerg Infect Dis. 2009;15:1509–1511. doi: 10.3201/eid1509.081531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren L, Gonzalez R, Xie Z, et al. Saffold cardioviruses of 3 lineages in children with respiratory tract infections, Beijing, China. Emerg Infect Dis. 2010;16:1158–1161. doi: 10.3201/eid1607.091682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsukagoshi H, Masuda Y, Mizutani T, et al. Sequencing and phylogenetic analyses of Saffold cardiovirus (SAFV) genotype 3 isolates from children with upper respiratory infection in Gunma, Japan. Jpn J Infect Dis. 2010;63:378–380. [PubMed] [Google Scholar]
- 38. Dai XQ, Yuan CL, Yu Y, et al. Molecular detection of Saffold Virus in children in Shanghai, China. J Clin Virol. 2011;50:186–187. doi: 10.1016/j.jcv.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 39. Itagaki T, Abiko C, Aoki Y, et al. Saffold cardiovirus infection in children associated with respiratory disease and its similarity to coxsackievirus infection. Pediatr Infect Dis J. 2011;30:680–683. doi: 10.1097/INF.0b013e31821608a8 [DOI] [PubMed] [Google Scholar]
- 40. Tsukagoshi H, Mizuta K, Abiko C, et al. The impact of Saffold cardiovirus in patients with acute respiratory infections in Yamagata, Japan. Scand J Infect Dis. 2011;43:669–671. doi: 10.3109/00365548.2011.565796 [DOI] [PubMed] [Google Scholar]
- 41. Khamrin P, Chaimongkol N, Nantachit N, Okitsu S, Ushijima H, Maneekarn N. Saffold cardioviruses in children with diarrhea, Thailand. Emerg Infect Dis. 2011;17:1150–1152. doi: 10.3201/eid/1706.101983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielsen AC, Gyhrs ML, Holmes EC, Cui J. Co‐circulation and persistence of genetically distinct saffold viruses, Denmark. Emerg Infect Dis. 2012;18:1694–1696. doi: 10.3201/eid1810.120793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khamrin P, Thongprachum A, Kikuta H, et al. Three clusters of Saffold viruses circulating in children with diarrhea in Japan. Infect Genet Evol. 2013;13:339–343. doi: 10.1016/j.meegid.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 44. Naeem A, Hosomi T, Nishimura Y, et al. Genetic diversity of circulating Saffold viruses in Pakistan and Afghanistan. J Gen Virol. 2014;95:1945–1957. doi: 10.1099/vir.0.066498-0 [DOI] [PubMed] [Google Scholar]
- 45. Branas P, Garcia M, Prieto C, Folgueira L. Saffold virus respiratory infection in children and immunocompromised patients in Spain. J Infect. 2015;70:679–680. doi: 10.1016/j.jinf.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 46. Yodmeeklin A, Khamrin P, Chuchaona W, et al. Saffold viruses in pediatric patients with diarrhea in Thailand. J Med Virol. 2015;87:702–707. doi: 10.1002/jmv.24114 [DOI] [PubMed] [Google Scholar]
- 47. Lin TL, Lin TH, Chiu SC, et al. Molecular epidemiological analysis of Saffold cardiovirus genotype 3 from upper respiratory infection patients in Taiwan. J Clin Virol. 2015;70:7–13. doi: 10.1016/j.jcv.2015.06.100 [DOI] [PubMed] [Google Scholar]
- 48. Wang CY, Greer RM, Delwart E, Sloots TP, Mackay IM. A newly designed real‐time RT‐PCR for SAFV detects SAFV‐2 and SAFV‐3 in the respiratory tracts of ill children during 2011. J Clin Virol. 2012;55:173–176. doi: 10.1016/j.jcv.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aoki Y, Matoba Y, Tanaka S, et al. Isolation of Saffold virus type 2 from children with acute respiratory infections by using the RD‐18S‐niigata cell line. Jpn J Infect Dis. 2015;68:438–441. doi: 10.7883/yoken.JJID.2015.093 [DOI] [PubMed] [Google Scholar]
- 50. Nielsen TS, Nielsen AY, Banner J, Hansen J, Baandrup U, Nielsen LP. Saffold virus infection associated with human myocarditis. J Clin Virol. 2016;74:78–81. doi: 10.1016/j.jcv.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kobayashi M, Tsukagoshi H, Ishioka T, et al. Seroepidemiology of Saffold cardiovirus (SAFV) genotype 3 in Japan. J Infect. 2013;66:191–193. doi: 10.1016/j.jinf.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 52. Ito H, Miyagaki S, Sakaue S, et al. Saffold cardiovirus infection in a 2‐year‐old boy with acute pancreatitis. Jpn J Infect Dis. 2016. doi: 10.7883/yoken.JJID.2015.488 [DOI] [PubMed] [Google Scholar]
- 53. Asano T, Sasaki N, Yashiro K, et al. Acute pancreatitis with Kawasaki disease: analysis of cases with elevated serum amylase levels. Eur J Pediatr. 2005;164:180–181. doi: 10.1007/s00431-004-1589-4 [DOI] [PubMed] [Google Scholar]
- 54. Mycoplasmal pneumonia treatment and management . http://emedicine.medscape.com/article/1941994-overview.
- 55. Enteroviruses . http://emedicine.medscape.com/article/217146-overview.
- 56. Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/cmr.00075-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Esona MD, Gautam R. Rotavirus. Clin Lab Med. 2015;35:363–391. doi: 10.1016/j.cll.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 58. El Kholy AA, Mostafa NA, Ali AA, et al. The use of multiplex PCR for the diagnosis of viral severe acute respiratory infection in children: a high rate of co‐detection during the winter season. Eur J Clin Microbiol Infect Dis. 2016. doi: 10.1007/s10096-016-2698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Metzgar D, Frinder MW, Rothman RE, et al. The IRIDICA BAC BSI assay: rapid, sensitive and culture‐independent identification of bacteria and candida in blood. PLoS One. 2016;11(7):e0158186. doi: 10.1371/journal.pone.0158186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vincent JL, Brealey D, Libert N, et al. Rapid diagnosis of infection in the critically Ill, a multicenter study of molecular detection in bloodstream infections, pneumonia, and sterile site infections. Crit Care Med. 2015;43(11):2283–2291. doi: 10.1097/CCM.0000000000001249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kotani O, Naeem A, Suzuki T, et al. Neuropathogenicity of two Saffold virus type 3 isolates in mouse models. PLoS One. 2016;11:e0148184. doi: 10.1371/journal.pone.0148184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sorgeloos F, Lardinois C, Jacobs S, van Kuppeveld FJ, Kaspers B, Michiels T. Neurotropism of Saffold virus in a mouse model. J Gen Virol 2016;97:1350–1355. doi: 10.1099/jgv.0.000452 [DOI] [PubMed] [Google Scholar]
- 63. van den Broek MF, Müller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fiette L, Aubert C, Muller U, et al. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bihl F, Brahic M, Bureau JF. Two loci, TMEVP2 and TMEVp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system of mice. Genetics. 1999;152:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Galama JMD, Zoll JG, Lanke KH, et al. Saffold cardiovirus and multiple sclerosis: no evidence for an association. Ann Clin Transl Neurol. 2014;1:618–621. doi: 10.1002/acn3.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frisk G, Fohlman J, Kobbah M, et al. High frequency of coxsackie‐B‐virus‐specific IgM in children developing type I diabetes during a period of high diabetes morbidity. J Med Virol. 1985;17:219–227. doi: 10.1002/jmv.1890170303 [DOI] [PubMed] [Google Scholar]
- 68. Yin H, Berg AK, Tuvemo T, Frisk G. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes. 2002;51:1964–1971. doi: 10.2337/diabetes.51.6.1964 [DOI] [PubMed] [Google Scholar]
- 69. Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/nejm197905243002102 [DOI] [PubMed] [Google Scholar]
- 70. Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. BMJ. 1969;3:627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. King ML, Shaikh A, Bidwell D, Voller A, Banatvala JE. Coxsackie‐B‐virus‐specific IgM responses in children with insulin‐dependent (juvenile‐onset; type I) diabetes mellitus. Lancet. 1983;1:1397–1399. [DOI] [PubMed] [Google Scholar]
- 72. Tapia G, B¯Âs H, de Muinck EJ, et al. Saffold virus, a human cardiovirus, and risk of persistent islet autoantibodies in the longitudinal birth cohort study MIDIA. PLoS One. 2015;10:e0136849. doi: 10.1371/journal.pone.0136849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brahic M. Theiler's virus infection of the mouse, or: of the importance of studying animal models. Virology. 2002;301:1–5. doi: 10.1006/viro.2002.1607 [DOI] [PubMed] [Google Scholar]
- 74. Rossi CP, Delcroix M, Huitinga I, et al. Role of macrophages during Theiler's virus infection. J Virol. 1997;71:3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus‐induced demyelinating disease. J Virol. 1995;69:2525–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou L, Luo Y, Wu Y, Tsao J, Luo M. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J Virol. 2000;74:1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler's virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/cmr.17.1.174-207.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shaw‐Jackson C, Michiels T. Infection of macrophages by Theiler's murine encephalomyelitis virus is highly dependent on their activation or differentiation state. J Virol. 1997;71:8864–8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsunoda I, Kurtz CI, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382 [DOI] [PubMed] [Google Scholar]
- 80. Kok CC, McMinn PC. Picornavirus RNA‐dependent RNA polymerase. Int J Biochem Cell Biol. 2009;41:498–502. doi: 10.1016/j.biocel.2008.03.019 [DOI] [PubMed] [Google Scholar]
- 81. Meng T, Kwang J. Attenuation of human enterovirus 71 high replication fidelity variants in AG129 mice. J Virol. 2014. doi: 10.1128/jvi.00289-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pfeiffer JK, Kirkegaard K. A single mutation in poliovirus RNA‐dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc Natl Acad Sci U S A. 2003;100:7289–7294. doi: 10.1073/pnas.1232294100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ai J‐W, Zhang Y, Zhang W. Zika virus outbreak: ‘a perfect storm. Emerg Infect Dis. 2016;5:e21. doi: 10.1038/emi.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]


