Abstract
Background and objective
Acute respiratory infection is the major cause of disease and death in children, particularly in developing countries. However, the spectrum of pathogenic viruses and atypical bacteria that exist in many of these countries remains incompletely characterized. The aim of this study was to examine the spectrum of pathogenic viruses and atypical bacteria associated with acute respiratory infection in children under the age of 16.
Methods
A total of 10 435 serum sera specimens were collected from hospitalized children presenting with acute respiratory infection symptoms. Indirect immunofluorescence assays were performed to detect immunoglobulin M antibodies against nine common pathogens: mycoplasma pneumonia, influenza virus B, respiratory syncytial virus, parainfluenza virus, adenovirus, influenza virus A, legionella pneumophila, coxiella burnetii and chamydophila pneumonia.
Results
Of the 10 435 specimens examined, 7046 tested positive for at least one pathogen. Among all of the tested pathogens, mycoplasma pneumonia had the highest detection rate (56.9%). Influenza virus A and influenza virus B epidemics occurred during both winter and summer. The detection rate of respiratory syncytial virus and adenovirus was higher in spring. Cases of mixed infection were more complex: 4136 specimens (39.6%) tested positive for ≥2 pathogens. There were statistically significant difference in detection rates of mycoplasma pneumonia, influenza virus B, respiratory syncytial virus, parainfluenza virus, adenovirus, influenza virus A, legionella pneumophila and chamydophila pneumonia among different age groups (P < 0.05).
Conclusions
The most common pathogens causing acute respiratory infection among children in Hubei of China were mycoplasma pneumonia, influenza virus B and respiratory syncytial virus. The detection rates for each pathogen displayed specific seasonal and age group variations.
Keywords: acute respiratory infection, child, detection rate, mixed infection, pathogen
Short abstract
Indirect immunofluorescence assays were performed to detect immunoglobulin M antibodies against nine pathogens to provide an important dataset for use in clinical diagnoses and the development of more effective therapeutic regimens in the future.
Abbreviations
- ADV
adenovirus
- COX
coxiella burnetii
- CPn
chamydophila pneumonia
- FluA
influenza virus A
- FluB
influenza virus B
- LOS
length of stay
- LPN
legionella pneumophila
- MP
mycoplasma pneumonia
- PIV
parainfluenza virus
- RSV
respiratory syncytial virus
Introduction
Acute respiratory infection (ARI) is one of the major diseases causing significant morbidity and mortality among children. Most cases of death caused by ARI are found in the developing world.1 Between 2000 and 2003, over two million children under the age of 5 died from ARI, with 75% of these deaths occurred in Africa or Southeast Asia.2 Worldwide for children under the age of 5, ARI (including pneumonia) accounts for 18% of deaths.3 Therefore, whether in developing or developed countries, ARI is a cause of tremendous human and economic burdens to families and society as a whole.4, 5
Currently, it is widely considered that the major pathogens responsible for ARI are bacteria,6, 7, 8 although atypical bacteria (e.g. Mycoplasma pneumoniae (MP)) and viruses also play crucial roles. In particular, during acute upper respiratory tract infections, the infection rates of MP and viruses (mainly influenza virus B (FluB), respiratory syncytial virus (RSV), parainfluenza virus (PIV) and adenovirus (ADV)) can be even higher than those of bacterial pathogens. In addition, new viruses are being discovered, such as human metapneumovirus and coronaviruses (NL63 and HKU1).9, 10 Many studies have also shown that MP and viruses display seasonal variations in infection and that mixed infections with multiple pathogens are common. Furthermore, many viruses show high infectivity, rapid transmission, short latency and acute manifestations; and they may also fail to induce lifelong immunity following diseases. The characteristics of these pathogens can lead to high morbidity and mortality, as well as disease pandemics.11, 12 Hence, the detection of viruses and atypical bacteria is crucially important.
The symptoms of respiratory viral infection and atypical bacterial infection in children are very similar and show no specificity. As a result, diagnoses of respiratory tract infections are made clinically instead of having microbiological confirmation, and the overuse of antibiotics for non‐bacterial respiratory tract infections is very common. By applying indirect immunofluorescence assays on specimens of hospitalized ARI children, we could detect immunoglobulin M antibodies against nine pathogens, and this provided important data for use in clinical diagnoses and development of more effective therapeutic regimens.
Methods
Ethics approval
The study was approved by the Medical Ethics Review Committee of Renmin Hospital, Wuhan University. All participants in this study were required to provide a written informed consent in accordance with Renmin Hospital of Wuhan University Ethics Committee; patients under supervision of a lawful caregiver if necessary.
Subjects
Between May 2010 and April 2012, 10 435 child patients, diagnosed as ARI by Emergency Department physician, were admitted to the Department of Pediatrics of Renmin Hospital of Wuhan University, one of the largest general hospitals in Hubei province, central China. The patient group, composed of 6642 men and 3793 women, ranged in age from 1 month to 16 years, with an average age of 37 months. Based on the growth and immune system development of the children, the patients were divided into four groups: under 1 year of age (<1; 3112 patients); 1–3 years of age (1–3; 3388 patients), 3–6 years of age (3–6; 2685 patients) and more than 6 years of age (>6; 1250 patients).
Samples and detection methods
Serum samples were collected from children who were diagnosed with ARI by Emergency Department physicians, based on clinical signs and symptoms. No radiographical studies were included. Venous blood (2–3 mL) was drawn from each patient and was immediately transferred to the Clinical Laboratory Center of People's Hospital of Wuhan University. Following coagulation, the blood samples were centrifuged at 1760 g to isolate the sera. If samples were not assayed immediately, they were stored at 4°C for no more than 24 h. Samples were then examined using immunofluorescence assays to identify immunoglobulin M antibodies against nine common pathogens of the upper respiratory tract: MP, FluB, RSV, PIV, ADV, FluA, LPN, COX and CPn. We used the immunoglobulin M antibody detection kit for upper respiratory tract pathogens (VIRCELL, Granada, Granada, Spain) according to the manufacturer's instructions. Microscopic examinations were performed with a EUROStar II immunofluorescence microscope (EUROIMMUN, Hanseatic City of Lubeck, Schleswig‐Holstein, Germany).
Statistical analysis
Differences between the pathogen detection rates for the various groups were examined using the χ2 test; the difference of length of stay (LOS) among different pathogens using t‐test (the results were expressed as means ± SD); P < 0.05 was considered to be statistically significant. Data analysis was performed using the SPSS17.0 software program (SPSS Inc, Chicago, Illinois, USA).
Results
Pathogen detection rates
Among the 10 435 specimens collected, at least one pathogen was identified in 7046 specimens, yielding an overall detection rate of 67.5%. MP showed a positive rate of 56.9% (Fig. 1), which was the highest among the nine examined pathogens. The next most common detected pathogen was FluB (35.4%). The detection rates of RSV, PIV, ADV, FluA, LPN, COX and CPn were 18.9%, 7.5%, 4.9%, 2.0%, 1.6%, 0.4% and 0.2%, respectively.
Figure 1.
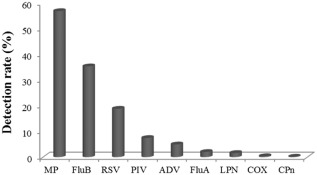
The immunoglobulin M (IgM) detection rates of nine respiratory tract pathogens. mycoplasma pneumonia (MP), influenza virus B (FluB) and respiratory syncytial virus (RSV) showed the highest detection rates. The others pathogens showed lower detection rates and are listed in order of decreasing prevalence: parainfluenza virus (PIV), adenovirus (ADV), influenza virus A (FluA), legionella pneumophila (LPN), coxiella burnetii (COX) and chamydophila pneumonia (CPn).
The seasonal epidemics for each pathogen
The detection rate of MP was consistently high throughout the year, although it was highest in the spring of 2010. Epidemics of FluB occurred in the summer of 2010, as well as in the winters and springs of 2010–2011 and 2011–2012. RSV showed the highest detection rates in the winter of 2010 and spring of 2011 (Fig. 2a). Seasonal differences in infection for PIV were not observed, and it showed a low detection rate throughout the year. ADV showed the highest detection rate in the spring. Seasonal differences in infection for LPN were not observed, and it showed a low detection rate throughout the year (Fig. 2b). Epidemics of FluA occurred in the summer of 2010, as well as the springs of 2011 and 2012, but its detection rates in the other seasons were otherwise very low. The detection rates of COX and CPn were very low throughout the year, but their detection rates in the summer of 2010 were slightly higher (Fig. 2c).
Figure 2.
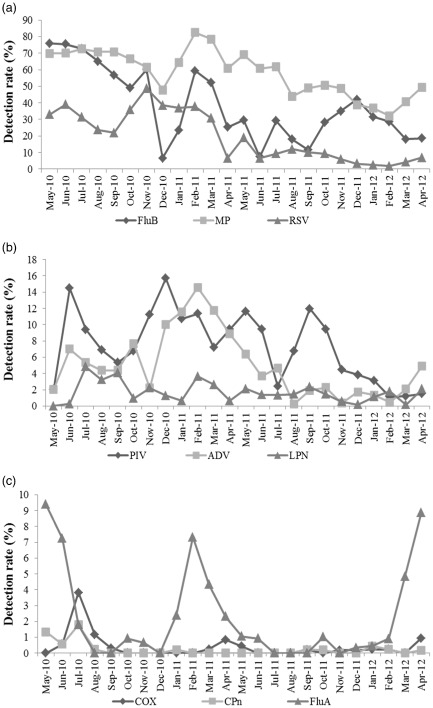
The monthly detection rates of the nine pathogens between May 2010 and April 2012. Panel (a) shows the seasonal changes of influenza virus B (FluB), mycoplasma pneumonia (MP) and respiratory syncytial virus (RSV);  , FluB;
, FluB;  , MP;
, MP;  , RSV. panel (b) shows the seasonal changes of parainfluenza virus (PIV), adenovirus (ADV) and legionella pneumophila (LPN);
, RSV. panel (b) shows the seasonal changes of parainfluenza virus (PIV), adenovirus (ADV) and legionella pneumophila (LPN);  , PIV;
, PIV;  , ADV;
, ADV;  , LPN. and panel (c) shows the seasonal changes of coxiella burnetii (COX), chamydophila pneumonia (CPn) and influenza virus A (FluA).
, LPN. and panel (c) shows the seasonal changes of coxiella burnetii (COX), chamydophila pneumonia (CPn) and influenza virus A (FluA).  , COX;
, COX;  , CPn;
, CPn;  , FluA. The pathogens are grouped by percentage found overall. The ‘Y’ axes have different labelling scale.
, FluA. The pathogens are grouped by percentage found overall. The ‘Y’ axes have different labelling scale.
Mixed infection modes of the pathogens
There were 4136 cases in which two or more pathogens were detected, representing 39.6% of the samples, and the modes of mixed infection were complex (Table 1). Among the specimens showing infection with two pathogens, the MP+FluB mixed infection was the most common, with 1573 cases (accounting for 38.0% of all mixed‐infection cases), followed by FluB+RSV with 320 cases (accounting for 7.7% of all mixed‐infection cases). Among the specimens showing mixed infection with three pathogens, the MP+FluB+RSV mixed infection was the most common, with 687 cases (accounting for 16.6% of all mixed‐infection cases), followed by MP+FluB+PIV with 133 cases (accounting for 3.2% of all mixed‐infection cases). Among the specimens showing mixed infection with four pathogens, the MP+FluB+RSV+PIV mixed infection was the most common, with 160 cases (accounting for 3.9% of all mixed‐infection cases). Other combinations of mixed infection were rarer and collectively accounted for 13.8% of all mixed‐infection cases.
Table 1.
Common mixed‐infection combinations of the nine respiratory tract pathogens (only the common mixed infection modes are listed)
| Mix infection modes | Cases (n) | Percentage (%) |
|---|---|---|
| MP+FluB | 1573 | 38.0 |
| MP+RSV | 320 | 7.7 |
| MP+PIV | 169 | 4.1 |
| FluB+RSV | 121 | 2.9 |
| MP+ADV | 116 | 2.8 |
| MP+FluB+RSV | 687 | 16.6 |
| MP+FluB+PIV | 133 | 3.2 |
| MP+FluB+ADV | 99 | 2.4 |
| MP+PIV+RSV | 90 | 2.2 |
| MP+FluB+RSV+PIV | 160 | 3.9 |
| MP+FluB+RSV+ADV | 66 | 1.6 |
| MP+FluB+RSV+FluA | 35 | 0.8 |
| Other mixed infection modes | 576 | 13.8 |
| Total | 4136 | 100.0 |
ADV, adenovirus; FluA, influenza virus A; FluB, influenza virus B; MP, mycoplasma pneumonia; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
The distribution of detection rates of the nine pathogens between the different age groups
The detection rates of the nine pathogens for each age group are shown in Figure 3a. The overall detection rates for those younger than 1, between 1 and 3, between 3 and 6 and over 6 years old were 39.2%, 75.3%, 85.0% and 79.4%, respectively; furthermore, the differences between the overall detection rates between the different age groups were statistically significant (P < 0.05), with the pathogen detection rate for the age group of <1 year old being the lowest. Among the nine pathogens, the differences in the detection rates of MP, FluB, RSV, PIV, ADV, FluA, LPN and CPn between different age groups were statistically significant (P < 0.05). The detection rates of MP, RSV, LPN, FluB, PIV and FluA in the age group <1 year old were significantly lower than those from the other age groups. The detection rate of ADV was significantly lower, and detection rate of CPn was significantly higher in the >6‐year age group compared with other age groups. The detection rates of COX did not show significant difference between age groups (P > 0.05). MP showed the highest detection rates over all age groups (47.4%, 49.8%, 51.1% and 48.1%), followed by FluB (30.7%, 31.8%, 34.4% and 33.6%) and RSV (17.2%, 17.5%, 19.8% and 17.1%). Figure 3b shows the constituent ratios of the detection rates of the nine pathogens for each age group. The ratios of all pathogens were lowest in the <1‐year‐old age group (except for that of CPn); the ratios of ADV, COX and MP were higher in the 1–3‐year‐old age group and the ratios of PIV, RSV, FluB and FluA were higher in the 3–6‐year‐old group.
Figure 3.
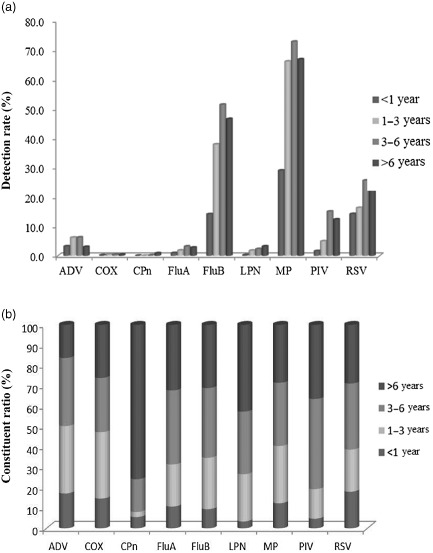
Panel (a) shows the detection rates of the nine respiratory tract pathogens for all age groups. The detection rates of all pathogens over all age groups showed significant differences (P < 0.05) except for those of coxiella burnetii (COX; P > 0.05).  , <1 year;
, <1 year;  , 1–3 years;
, 1–3 years;  , 3–6 years;
, 3–6 years;  , >6 years. Panel (b) shows the constituent ratios of the pathogen detection rates for each age group. The ratios of pathogen detection rates in the <1‐year‐old group were all smaller (except for that of chamydophila pneumonia (CPn)); the constituent ratios of the other pathogens for the different age groups were different.
, >6 years. Panel (b) shows the constituent ratios of the pathogen detection rates for each age group. The ratios of pathogen detection rates in the <1‐year‐old group were all smaller (except for that of chamydophila pneumonia (CPn)); the constituent ratios of the other pathogens for the different age groups were different.  , >6 years;
, >6 years;  , 3–6 years;
, 3–6 years;  , 1–3 years;
, 1–3 years;  , <1 year.
, <1 year.
Length of stay in the hospital among different pathogens
In this study, 2910 cases of children were diagnosed with a single infection (i.e. one pathogen only was detected). Among these cases, the LOS of 2872 cases of children infected with one of the five most prominent pathogens types (MP, FluB, RSV, PIV and ADV) and that of 3389 cases of children diagnosed with ARI but were negative for pathogens, were analyzed. The results are shown in Table 2. The LOS of children with no pathogens identified in their blood samples was shorter than children with a single pathogen, either MP, RSV or PIV, identified (P < 0.05) The longest LOS was MP‐positive patients, followed by PIV and RSV.
Table 2.
Length of stay (LOS) in the hospital for patients infected by one pathogen only among the five most prominent pathogens
| Pathogens | Cases (n) | LOS (mean ± SD) |
|---|---|---|
| Negative | 3389 | 5.92 ± 1.31 |
| MP | 2081 | 7.43 ± 1.74 |
| FluB | 492 | 6.13 ± 2.09 |
| RSV | 221 | 6.80 ± 2.18 |
| PIV | 46 | 7.06 ± 2.46 |
| ADV | 32 | 6.46 ± 2.52 |
ADV, adenovirus; FluB, influenza virus B; MP, mycoplasma pneumonia; PIV, parainfluenza virus; RSV, respiratory syncytial virus; SD, standard deviation.
Discussion
ARI is one of the major threats to the health of children; therefore, the detection of pathogens that causes ARI is now an area of research interest. In this study, we examined specimens from children with symptoms of respiratory tract infection. Our results showed that the overall antibody detection rate among the 10 435 patients was 67.5%, which was within the wide range of rates reported in other studies.13, 14, 15 These differences may be associated with various factors such as different pathogen epidemics,16, 17 geographical differences, population susceptibility and methodology.13, 18 It is also possible that because the subjects of this study were hospitalized children, the observed detection rates were higher than general screening. In addition, the overall detection rate was associated with the number of distinct types of pathogens being detected. Although we studied nine common respiratory tract pathogens in this study, other studies reported that metapneumovirus, human bocavirus, and human rhinovirus also account for a certain percentage of infections.12, 19 This difference suggests that our results may not reflect the full spectrum of ARI pathogens in the local area.
Among the examined pathogens, MP showed a high detection rate throughout the year and was highest in the spring of 2011. However, Bezerra et al.1 and Defilippi et al.20 reported that MP was most prevalent in summer. In addition, we found that epidemics of FluB occurred in the summer of 2010 as well as the winters and springs of 2010–2011 and 2011–2012, which was consistent with the study by Tang et al.,21 but inconsistent with the results of Mardy et al.,11 who found that epidemics of FluB occurred in the fall and winter. We found that the detection rates of RSV were higher in the winter of 2010 and the summer of 2011, similar to the findings reported by Fabbiani et al.22 and Noyola et al.23 Seasonal fluctuations of infection by PIV were not evident; on the other hand, Yeolekar et al.24 and Maitreyi et al.25 reported that infection by PIV was most common in the winter and spring. The detection rate of ADV was higher in spring, consistent with studies by Fabbiani et al.22 and Wang et al.26 Epidemics of FluA occurred in the summer of 2010 and springs of 2011 and 2012, but the positive rates were very low for all other seasons. This finding is consistent with the results of Chan et al.27 and demonstrates that this disease can have epidemic during the winter and spring as well as summer. Our study found that the epidemic seasons of certain pathogens were different from those found in other reports, which was most likely due to local climate and population differences. Climate is an important factor. An example of this is that RSV has a higher prevalence in summer in tropical countries (e.g. Vietnam)13 but a higher incidence in winter in temperate regions (e.g. Germany and South Korea).6, 17
Our study also revealed high incidences of mixed infections and that the modes of mixed infection were complex. Among mixed infections with two pathogens, the MP+FluB and FluB+RSV combinations were the most common. In a similar study, Peng et al.28 showed that FluA+FluB was the most common mode. The reason may be that MP, FluB and RSV were the most common pathogens of single infection; besides, there may be some similarities in their epidemic season. Mixed infection is likely the result of damage to airway mucosal cells caused by a prior pathogen (such as MP), allowing other pathogens to infect a patient. Mixed infections may also be associated with the relatively poor health and immune function of children, the long course of certain diseases, long hospitalizations or physical contact with other patients. It is also important to be aware that the detection rate of mixed infection is very dependent on methodology29 and how many types of pathogens are being studied.
We found that the detection rates between the different age groups were significantly different and that the <1‐year‐old group had the lowest rate. The detection rates for MP were the highest for each age group, which was significantly higher than that reported by a previous study,1, 20 but similar to that reported by Liu et al.30 In general, MP is more prevalent among older children1, 31 The detection rates of FluB observed in this study were much higher than those reported elsewhere;32, 33 in addition, the detection rates for the different age groups also showed significant differences, with the detection rate in the 3–6‐year‐old group being the highest. The detection rates of RSV in the different age groups were significantly different, with the highest detection rate being seen in the 3–6‐year‐old group; however, our RSV detection rates were higher than those of earlier studies.34, 35
The LOS was affected by many factors, such as the pathogen species, the timeliness of treatment, the hospital medical level, the children's family economic status and the individual differences with regard to medication; but most important was the pathogen species. Therefore, the LOS of children was analysed according to the pathogens responsible for the infection. A difference in the virulence of pathogens may lead to differences in LOS. Moreover, viral infections have varying self‐limiting cycles that may cause differences in LOS.
In summary, we used immunofluorescence assays to rapidly examine specimens from paediatric cases of ARI over a 2‐year period. We found that the most common pathogens causing ARI among the children of the Hubei area of China were MP, FluB and RSV; and the detection rates for each pathogen displayed specific seasonal variations. Mixed infections were more common than single infections. The detection rates of pathogens also displayed variations between different age groups. Our analysis enabled us to better understand the viral aetiology of local ARI in children. Despite the fact that certain ARI pathogens have yet to be identified, our approach may prove to be a powerful tool for aetiological diagnosis of ARI in children, and we will be able to provide children with timely diagnoses to inform appropriate treatment that may in turn prevent complications.
Acknowledgements
This work was supported by research grants from National Clinical Key Specialty Construction Projects, and National Mega Project on Major Infectious Diseases Prevention Grants 2012ZX10004‐207.
(Associate Editor: Marcos Restrepo).
References
- 1. Bezerra PG, Britto MC, Correia JB, Duarte Mdo C, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE, McNamara PS. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE 2011; 6: e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bryce J, Boschi‐Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005; 365: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 4. Fry AM, Chittaganpitch M, Baggett HC, Peret TCT, Dare RK, Sawatwong P, Thamthitiwat S, Areerat P, Sanasuttipun W, Fischer J et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE 2010; 5: e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010; 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weigl JA, Puppe W, Meyer CU, Berner R, Forster J, Schmitt HJ, Zepp F. Ten years' experience with year‐round active surveillance of up to 19 respiratory pathogens in children. Eur. J. Pediatr. 2007; 166: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet 2011; 377: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar S, Wang LH, Fan J, Kraft A, Bose ME, Tiwari S, Van Dyke M, Haigis R, Luo TQ, Ghosh M et al. Detection of 11 common viral and bacterial pathogens causing community‐acquired pneumonia or sepsis in asymptomatic patients by using a multiplex reverse transcription‐PCR assay with manual (enzyme hybridization) or automated (electronic microarray) detection. J. Clin. Microbiol. 2008; 46: 3063–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001; 7: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pyrc K, Berkhout B, van der Hoek L. The novel human coronaviruses NL63 and HKU1. J. Virol. 2007; 81: 3051–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mardy S, Ly S, Heng S, Vong S, Huch C, Nora C, Asgari N, Miller M, Bergeri I, Rehmet S et al. Influenza activity in Cambodia during 2006–2008. BMC Infect. Dis. 2009; 9: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect. Dis. 2006; 6: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anh HLD, van Doorn HR, My NN, Bryant JE, Thanh HTH, Quang HD, Tan LV, Tan TT, Wills B, Vinh CV et al. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS ONE 2011; 6: e18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr. Infect. Dis. J. 2006; 25: 680–686. [DOI] [PubMed] [Google Scholar]
- 15. Cabello C, Manjarrez ME, Olvera R, Villalba J, Valle L, Paramo I. Frequency of viruses associated with acute respiratory infections in children younger than five years of age at a locality of Mexico City. Mem. Inst. Oswaldo Cruz 2006; 101: 21–24. [DOI] [PubMed] [Google Scholar]
- 16. Ampofo K, Bender J, Sheng X, Korgenski K, Daly J, Pavia AT, Byington CL. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics 2008; 122: 229–237. [DOI] [PubMed] [Google Scholar]
- 17. Kim CK, Choi J, Callaway Z, Kim HB, Chung JY, Koh YY, Shin BM. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in Seoul, Korea, 2003–2008. J. Korean Med. Sci. 2010; 25: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadeghi CD, Aebi C, Gorgievski‐Hrisoho M, Muhlemann K, Barbani MT. Twelve years' detection of respiratory viruses by immunofluorescence in hospitalised children: impact of the introduction of a new respiratory picornavirus assay. BMC Infect. Dis. 2011; 11: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sung RY, Chan PK, Tsen T, Li AM, Lam WY, Yeung AC, Nelson EA. Identification of viral and atypical bacterial pathogens in children hospitalized with acute respiratory infections in Hong Kong by multiplex PCR assays. J. Med. Virol. 2009; 81: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Defilippi A, Silvestri M, Tacchella A, Giacchino R, Melioli G, Di Marco E, Cirillo C, Di Pietro P, Rossi GA. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir. Med. 2008; 102: 1762–1768. [DOI] [PubMed] [Google Scholar]
- 21. Tang LF, Wang TL, Tang HF, Chen ZM. Viral pathogens of acute lower respiratory tract infection in China. Indian Pediatr. 2008; 45: 971–975. [PubMed] [Google Scholar]
- 22. Fabbiani M, Terrosi C, Martorelli B, Valentini M, Bernini L, Cellesi C, Cusi MG. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J. Med. Virol. 2009; 81: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noyola DE, Zuviri‐Gonzalez A, Castro‐Garcia JA, Ochoa‐Zavala JR. Impact of respiratory syncytial virus on hospital admissions in children younger than 3 years of age. J. Infect. 2007; 54: 180–184. [DOI] [PubMed] [Google Scholar]
- 24. Yeolekar LR, Damle RG, Kamat AN, Khude MR, Simha V, Pandit AN. Respiratory viruses in acute respiratory tract infections in Western India. Indian J. Pediatr. 2008; 75: 341–345. [DOI] [PubMed] [Google Scholar]
- 25. Maitreyi RS, Broor S, Kabra SK, Ghosh M, Seth P, Dar L, Prasad AK. Rapid detection of respiratory viruses by centrifugation enhanced cultures from children with acute lower respiratory tract infections. J. Clin. Virol. 2000; 16: 41–47. [DOI] [PubMed] [Google Scholar]
- 26. Wang W, Cavailler P, Ren P, Zhang J, Dong W, Yan H, Mardy S, Cailhol J, Buchy P, Sheng J et al. Molecular monitoring of causative viruses in child acute respiratory infection in endemo‐epidemic situations in Shanghai. J. Clin. Virol. 2010; 49: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan PK, Mok HY, Lee TC, Chu IM, Lam WY, Sung JJ. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J. Med. Virol. 2009; 81: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 28. Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, Li Y, Wang F. Multipathogen infections in hospitalized children with acute respiratory infections. Virol. J. 2009; 6: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freymuth F, Vabret A, Cuvillon‐Nimal D, Simon S, Dina J, Legrand L, Gouarin S, Petitjean J, Eckart P, Brouard J. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J. Med. Virol. 2006; 78: 1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu CL, Wang GQ, Zhang B, Xu H, Hu LP, He XF, Wang JH, Zhang JH, Liu XY, Wei M et al. Mycoplasma pneumoniae pneumonia in hospitalized children diagnosed at acute stage by paired sera. Chin. Med. J. (Engl.) 2010; 123: 3444–3450. [PubMed] [Google Scholar]
- 31. Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008; 8: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shibli F, Chazan B, Nitzan O, Flatau E, Edelstein H, Blondheim O, Raz R, Colodner R. Etiology of community‐acquired pneumonia in hospitalized patients in northern Israel. Isr. Med. Assoc. J. 2010; 12: 477–482. [PubMed] [Google Scholar]
- 33. Razanajatovo NH, Richard V, Hoffmann J, Reynes JM, Razafitrimo GM, Randremanana RV, Heraud JM. Viral etiology of influenza‐like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS ONE 2011; 6: e17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed JA, Katz MA, Auko E, Njenga MK, Weinberg M, Kapella BK, Burke H, Nyoka R, Gichangi A, Waiboci LW et al. Epidemiology of respiratory viral infections in two long‐term refugee camps in Kenya, 2007–2010. BMC Infect. Dis. 2012; 12: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwofie TB, Anane YA, Nkrumah B, Annan A, Nguah SB, Owusu M. Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol. J. 2012; 9: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


