Abstract
Background and objective
High‐dose chemotherapy (HDCT) followed by autologous haematopoietic stem cell transplantation (HSCT) is widely used in paediatric cancer patients, but few data about noninfectious interstitial lung disease (ILD) following this treatment are available. Therefore, we aimed to evaluate the incidence, clinical features and risk factors of noninfectious ILD after HDCT in paediatric patients.
Methods
This was a retrospective cohort study of paediatric solid tumour patients who underwent HDCT and autologous HSCT between 1997 and 2012. ILD was diagnosed using clinical symptoms and radiography after excluding cardiac, renal and infectious causes. Risk factors were analysed using a Cox proportional hazard regression model.
Results
Three hundred and forty patients were enrolled, and the median age was 3 years (interquartile range 1–7). Eight patients (2.4%) were diagnosed with noninfectious ILD. The median duration of symptom onset was 30 months (range 7–74). Six (75%) of eight ILD patients died during the study period, even though steroids were administered for treatment. High‐dose cyclophosphamide use (hazard ratio = 11.37, 95% confidence interval = 1.38–93.32, P = 0.023) and sex (hazard ratio = 0.10, 95% confidence interval = 0.01–0.84, P = 0.034) were associated with late‐onset, noninfectious ILD upon multivariate analysis.
Conclusion
The incidence of noninfectious ILD after HDCT and autologous HSCT was not negligible, and the clinical features of ILD showed late onset and a poor prognosis. Female gender and high‐dose cyclophosphamide treatment may be risk factors for noninfectious ILD, but further studies with a larger number of ILD patients are suggested.
Keywords: chemotherapy, child, cyclophosphamide, interstitial lung disease, stem cell transplantation
Short abstract
We investigated noninfectious interstitial lung disease after autologous transplantation in 340 paediatric patients. The incidence was 2.4%. The symptom onset was late and the prognosis was poor. High‐dose cyclophosphamide and female gender were risk factors of interstitial lung disease.
Abbreviations
- CT
computed tomography
- DTPS
delayed pulmonary toxicity syndrome
- HDCT
high‐dose chemotherapy
- HSCT
haematopoietic stem cell transplantation
- ILD
interstitial lung disease
- MIBG
iodine‐131‐meta‐iodobenzylguanidine
- TBI
total body irradiation
Introduction
High‐dose chemotherapy (HDCT) followed by autologous haematopoietic stem cell transplantation (HSCT) is widely used as a treatment for advanced or refractory paediatric solid tumours.1, 2, 3, 4 Many chemotherapeutic agents, such as alkylating agents, anthracyclines, anti‐metabolites and vinca alkaloids, produce steep dose–response curves against different tumour cell types.5 In HDCT, the dose of chemotherapeutic agents is escalated at least 5–10 times.6 As this treatment may produce bone marrow failure, patients receive autologous HSCT to recover bone marrow function. However, concerns still exist regarding the toxic effects of HDCT on other organs.
Toxic effects to the lung after HSCT are common, and they contribute significantly to patient morbidity and mortality.7 As the prophylaxis and treatment of infectious complications continue to advance, noninfectious pulmonary complications have become more important.8 Toxicity to the lung after HDCT and autologous HSCT has been reported, and the implicated chemotherapeutic agents include melphalan, carmustine (BCNU) and busulfan.9, 10, 11 Noninfectious interstitial lung disease (ILD) is one of the most recognized lung injuries that can occur in adult patients with allogenic HSCT.12, 13 Even though many chemotherapeutic agents (bleomycin, busulfan, chlorambucil, cyclophosphamide, methotrexate, mitomycin and nitrosoureas) are known to cause ILD, few studies have investigated noninfectious ILD after autologous HSCT, especially in paediatric patients.14
Therefore, we intended to determine the incidence of noninfectious ILD, the clinical characteristics of patients and the risk factors associated with noninfectious ILD after HDCT and autologous HSCT in paediatric patients.
Methods
Study design
This was a retrospective cohort study conducted at a single tertiary institute. The medical records of consecutive patients (under 19 years of age) who had a solid tumour and were treated with HDCT chemotherapy and autologous HSCT from April 1997 to September 2012 were reviewed. Patients who had undergone additional allogenic HSCT after autologous HSCT were excluded (Fig. 1). All clinical research protocols were approved by the Institutional Review Board of Samsung Medical Centre (IRB No. 2013‐04‐024‐001).
Figure 1.
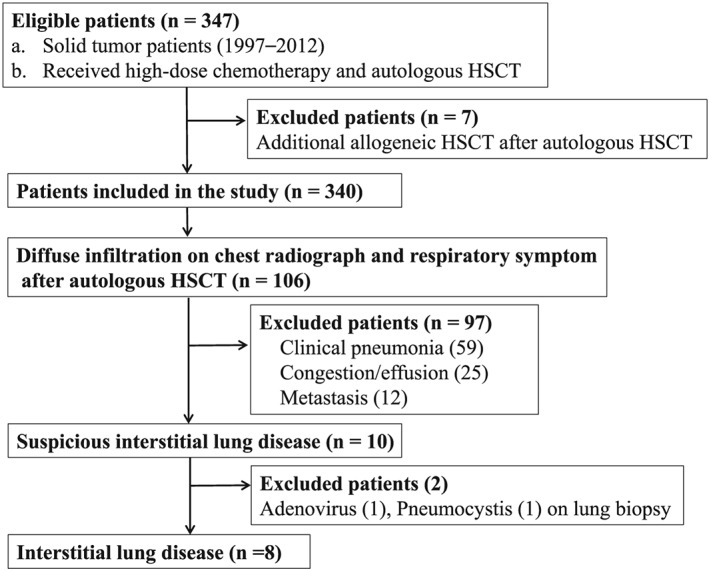
Flow diagram of patient selection, with inclusion and exclusion criteria
High‐dose chemotherapy regimen
Patients received HDCT according to their underlying pathology and risk group. The included conditioning regimens were carboplatin, etoposide and melphalan (CEM, first HDCT) or carboplatin, etoposide and cyclophosphamide (CECy, first HDCT) and carboplatin, thiotepa and melphalan (CTM, second HDCT) or thiotepa and melphalan (TM, second HDCT) for neuroblastoma patients. For brain tumour patients, carboplatin, thiotepa and etoposide (CTE, first HDCT) and cyclophosphamide and melphalan (CyM, second HDCT) were used. The CEM regimen consists of carboplatin (300 mg/m2 for 4 days), etoposide (200 mg/m2 for 4 days) and melphalan (140 mg/m2 for 1 day and 70 mg/m2 for 1 day). The CECy regimen includes carboplatin (650 mg/m2 for 3 days), etoposide (650 mg/m2 for 3 days) and cyclophosphamide (1800 mg/m2 for 3 days with 2‐mercaptoethane sulfonate sodium). Carboplatin (250 mg/m2 for 3 days), thiotepa (200 mg/m2 for 3 days) and melphalan (160 mg/m2 for 1 day) make up the CTM regimen. The TM regimen includes thiotepa (200–300 mg/m2 for 3 days) and melphalan (60 mg/m2) with or without iodine‐131‐meta‐iodobenzylguanidine (MIBG) treatment. Therapeutic MIBG (12 or 18 mCi/kg) was administered between the first and second HDCT sessions. The CTE regimen entails carboplatin (400–500 mg/m2 for 3 days), thiotepa (240–300 mg/m2 for 3 days) and etoposide (200–250 mg/m2 for 3 days). Finally, the CyM regimen consists of cyclophosphamide (1000–1500 mg/m2 for 3 or 4 days) and melphalan (50–60 mg/m2 for 1 or 2 days). The dosages of other HDCT regimens are similar to the dosage of chemotherapeutic agents stated earlier, except for some modifications. TBI was delivered in fractioned doses (333 cGy/fraction, 3 fractions). All stem cells were collected from peripheral blood during the recovery phase of chemotherapy or during the steady state with G‐CSF alone.
Definitions of noninfectious interstitial lung disease
A noninfectious ILD was defined when all the following were present: (i) clinical symptoms or signs of cough, dyspnea or desaturation; (ii) diffuse parenchymal infiltration on chest x‐ray or computed tomography (CT); (iii) lesions not caused by pneumonia, which can be diagnosed by clinical resolution of symptoms after antimicrobial or supportive treatment and/or detection of pathogenic microorganisms in respiratory secretions or lung biopsy tissue; (iv) lesions not caused by pulmonary congestion or effusion; and (v) no evidence of metastatic lung lesions.
Respiratory secretions were tested to identify infectious pathogens. Respiratory sampling included endotracheal aspirate, bronchoalveolar lavage (BAL) and nasopharyngeal aspirates, between 1997 and 2007 virus culture was performed to detect adenovirus, respiratory syncytial virus, influenza virus and parainfluenza virus, whilst multiplex polymerase chain reaction was used to detect adenovirus, respiratory syncytial virus, influenza virus, parainfluenza, coronavirus and rhinovirus after 2007. Gram stain and culture was also performed on tracheal or bronchial secretions. Fungi were cultured in separate media using tracheal suction or BAL fluid, and galactomannan enzyme‐linked immunosorbent assay for Aspergillus species was tested on serum or BAL fluid. For mycobacterium, acid‐fast bacillus stain, culture and DNA polymerase chain reaction were performed. Mycoplasma pneumonia was studied by antibody titer in blood and DNA detection in respiratory secretions. Pneumocystis jirovecii was evaluated by fluorescent staining of secretion smears.
All lung biopsies were conducted using video‐assisted thoracoscopic surgery at the acute or subacute stage of symptoms if the patients could tolerate the operation. The biopsy specimens were tested for adenovirus, pneumocystis jirovecci and cytomegalovirus by immunohistochemistry.
Statistical analysis
Twenty‐five parameters were analysed as potential risk factors for noninfectious ILD, including age at HDCT; gender; underlying tumour diagnosis; use of each chemotherapy agent (cisplatin, etoposide, cyclophosphamide, carboplatin, vincristine, ifosfamide, doxorubicin, methotrexate, actinomycin D and bleomycin); use of each HDCT agent (carboplatin, thiotepa, melphalan, cyclophosphamide, etoposide, busulfan and ifosfamide); MIBG treatment; interleukin‐2 usage; number of HDCT treatments; total body irradiation; and thoracic field radiation therapy.
The association between the development of noninfectious ILD and each potential risk factor was assessed using univariate and multivariate Cox proportional hazard regression models with stepwise selection. All tests were two‐tailed, and P‐values <0.05 were considered statistically significant. The SAS Program (Ver. 9.3, SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Characteristics of included patients
This study included a total of 340 patients. One hundred six patients had symptoms and radiology suggestive of ILD, 59 patients of these were diagnosed as clinical pneumonia (Fig. 1). In 41 patients (69.5%) out of those 59, pathogenic microorganisms were detected, which included respiratory syncytial virus (11), influenza virus (10), parainfluenza virus (8), Gram‐positive/Gram‐negative bacteria (4), adenovirus (2), Mycoplasma pneumoniae (2), coronavirus (1), rhinovirus (1), fungus (1) and pneumocystis (1). The other 18 patients (30.5%) were diagnosed with pneumonia according to the clinical course of the disease. The median age of participants was 3 years (interquartile range (IQR): 1–7). The most common primary diagnosis was neuroblastoma (46.5%), followed by medulloblastoma (18.2%). The number of HDCT and autologous HSCT treatments was 2 in 75.0% of patients and 1 in 25.0% of patients. The most common HDCT regimens were cyclophosphamide with melphalan (35.0%), followed by carboplatin and etoposide with cyclophosphamide (32.4%), and carboplatin and thiotepa with etoposide (29.4%). Some patients received total body irradiation (21.7%), thoracic area radiation therapy (38.2%), MIBG treatment (11.5%) and interleukin‐2 therapy (13.2%) (Table 1).
Table 1.
Characteristics of the included patients (n = 340)
| Total | Noninfectious ILD | P‐value | ||
|---|---|---|---|---|
| No, n (%) | Yes, n (%) | |||
| Gender, Female | 130 (38.2) | 123 (37.0) | 7 (87.5) | 0.006 |
| Median age at HSCT (IQR), years | 3 (1–7) | 3 (1–7) | 3 (2–3) | 0.113 |
| Median follow‐up to event after HSCT (IQR), months | 54 (15–96) | 54 (15–96) | 30 (9–61) | 0.168 |
| Median days to neutrophil engraftment (IQR) | 10 (9–11) | 10 (9–11) | 10 (8–12) | 0.530 |
| Median days to platelet engraftment (IQR) | 22 (17–30) | 22 (17–31) | 21 (19–28) | 0.968 |
| Diagnosis | 0.613 | |||
| Neuroblastoma | 158 (46.5) | 152 (45.8) | 6 (75.0) | |
| Medulloblastoma | 62 (18.2) | 60 (96.7) | 2 (2.5) | |
| Sarcoma | 24 (7.1) | 24 (100) | 0 (0.0) | |
| Retinoblastoma | 24 (7.0) | 24 (100) | 0 (0.0) | |
| Wilms' tumour | 12 (3.5) | 12 (100) | 0 (0.0) | |
| Germ cell tumour | 9 (2.6) | 9 (100) | 0 (0.0) | |
| Other brain tumour | 51 (15.0) | 51 (100) | 0 (0.0) | |
| Total body irradiation | 74 (21.7) | 70 (21.1) | 2 (25.0) | 0.678 |
| Thoracic RT | 130 (38.2) | 125 (37.7) | 4 (50.0) | 0.484 |
| MIBG treatment | 39 (11.5) | 33 (9.9) | 3 (37.5) | 0.042 |
| IL‐2 treatment | 45 (13.2) | 41 (12.3) | 3 (37.5) | 0.071 |
| Number of HDCT treatments | 0.209 | |||
| Once | 85 (25.0) | 85 (25.6) | 0 (0.0) | |
| Twice | 255 (75.0) | 247 (74.4) | 8 (100) | |
| HDCT regimen used in the first or second autologous HSCT session | 0.559 | |||
| CyM | 119 (35.0) | 117 (20.2) | 2 (12.5) | |
| CECy | 110 (32.4) | 104 (18.0) | 6 (37.5) | |
| CTE | 100 (29.4) | 100 (17.3) | 0 (0.0) | |
| TM | 96 (28.2) | 91 (15.7) | 5 (31.3) | |
| CEM | 63 (18.5) | 62 (10.7) | 1 (6.3) | |
| CE | 60 (17.6) | 60 (10.4) | 0 (0.0) | |
| BM | 24 (7.1) | 23 (4.0) | 1 (6.3) | |
| CTM | 17 (5.0) | 17 (2.9) | 0 (0.0) | |
| CT | 2 (0.6) | 1 (0.2) | 1 (6.3) | |
| TE | 2 (0.6) | 2 (0.3) | 0 (0.0) | |
| M | 1 (0.3) | 1 (0.2) | 0 (0.0) | |
| CEI | 1 (0.3) | 1 (0.2) | 0 (0.0) | |
BM, busufan and melphalan; CE, carboplatin and etoposide; CECy, carboplatin, etoposide and cyclophosphamide; CEI, carboplatin, etoposide and ifosfamide; CEM, carboplatin, etoposide and melphalan; CT, carboplatin and theiotepa; CTE, carboplatin, thiotepa and etoposide; CTM, carboplatin, thiotepa and melphalan; CyM, cyclophosphamide and melphalan; HDCT, high‐dose chemotherapy; HSCT, haematopoietic stem cell transplantation; IL, interleukin; IQR, interquartile range; M, melphalan; MIBG, iodine‐131‐meta‐iodobenzylguanidine; RT, radiation therapy; TE, thiotepa and etoposide; TM, thiotepa and melphalan., ,
Clinical features of noninfectious ILD patients
Eight (2.4%) out of 340 patients were diagnosed as having noninfectious ILD (Fig. 1). They had no history of serious respiratory infection before diagnosing ILD. The median age of these eight patients was 2 years (range 2–8). The median duration of symptom onset after HDCT and autologous HSCT was 30 months (range 7–74). All of these patients received systemic steroids as part of their treatment after ILD diagnosis, and five patients (subjects 1, 2, 3, 6 and 8) were administered methylprednisolone pulse therapy for 3 days. Six of the eight died during the study period. CT findings were subpleural thickening or consolidation, interstitial thickening and ground glass opacity. Lung biopsy showed interstitial fibrosis and/or thickening with inflammatory cell infiltration and pleural thickening. All eight patients received high‐dose cyclophosphamide as a conditioning regimen before autologous HSCT (Table 2).
Table 2.
Clinical features of patients with interstitial lung disease
| Subject | Age at HSCT (year) | Gender | Primary disease |
Conditioning regimen, first HDCT, second HDCT |
Biopsy findings |
Symptom, time after HSCT (month) |
CT findings | Outcome, time after HSCT (month) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | F | MBL |
1. CyM 2. BM |
Interstitial fibrous thickening with lymphocytic infiltration |
Dyspnea, chest discomfort 74 |
Subpleural thickening |
Death 84 |
| 2 | 8 | M | MBL |
1. CT 2. CyM |
Pleural thickening |
Dyspnea 5 |
Interstitial thickening ground glass opacity |
Death 15 |
| 3 | 1 | F | NBL |
1. CECy 2. TM |
Interstitial fibrosis and inflammatory cell infiltration |
Cough, dyspnea 16 |
Subpleural consolidation, ground glass opacity |
Death 39 |
| 4 | 3 | F | NBL |
1. CECy 2. TM |
Interstitial fibrosis and inflammatory cell infiltration |
Cough, dyspnea 28 |
Pleural and interstitial thickening ground glass opacity |
Death 46 |
| 5 | 2 | F | NBL |
1. CECy 2. TM |
Interstitial fibrosis and patch lymphocytic infiltration |
Cough, dyspnea 32 |
Fibrotic change with volume reduction, subpleural ground glass opacity |
Death 62 |
| 6 | 3 | F | NBL |
1. CECy 2. TM |
Interstitial thickening |
Cough, dyspnea 50 |
Perifissural, subpleural consolidation, uneven aeration |
Alive 63 |
| 7 | 2 | F | NBL |
1. CECy 2. CEM |
Interstitial fibrosis and lymphocytic infiltration |
Cough, dyspnea 7 |
Interstitial septal thickening, ground glass opacity |
Death 23 |
| 8 | 2 | F | NBL |
1. CECy 2. TM |
Fibrotic scar |
Dyspnea 74 |
Patchy supleural consolidation, interstitial thickening |
Alive 96 |
BM, Busulfan and melphalan; CECy, carboplatin, etoposide and cyclophosphamide; CEM, carboplatin, etoposide and melphalan; CT, computed tomography; CTE, carboplatin, thiotepa and etoposide; CyM, cyclophosphamide and melphalan; MBL, medulloblastoma; NBL, neuroblastoma; TM, thiotepa and melphalan.
Risk factors for noninfectious ILD
Based upon univariate analysis, risk factors for noninfectious ILD were female gender (P = 0.022), the use of cyclophosphamide for the HDCT regimen (P = 0.024) and MIBG treatment (P = 0.023) (Table 3). The usual dose of chemotherapy, which includes cyclophosphamide (P = 0.669), cisplatin (P = 0.5836), carboplatin (P = 0.3662), etoposide (P = 0.505), vincristine (P = 0.240), ifosfamide (P = 0.681), doxorubicin (P = 0.279), methotrexate (P = 0.822), actinomycin (P = 0.544) and bleomycin (P = 0.686), was not significantly associated with the development of noninfectious ILD.
Table 3.
Cox proportional‐hazard regression analysis for interstitial lung disease
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |||
| Male gender | 0.022 | 0.08 | 0.01 | 0.70 | 0.034 | 0.10 | 0.01 | 0.84 |
| Cyclophosphamide | 0.024 | 11.06 | 1.36 | 89.92 | 0.023 | 11.37 | 1.38 | 93.32 |
| MIBG treatment | 0.023 | 5.34 | 1.25 | 22.73 | ||||
CI, confidence interval; HR, hazard rate; MIBG, iodine‐131‐meta‐iodobenzylguanidine.
The multivariate analysis revealed that high‐dose cyclophosphamide (P = 0.023, hazard rate = 11.37, 95% CI: 1.38–93.32) and male gender (P = 0.034, hazard rate = 0.10, 95% CI: 0.01–0.84) were associated with noninfectious ILD (Table 3).
Discussion
In this study, the incidence of noninfectious ILD following HDCT and autologous HSCT in paediatric solid tumour patients was 2.4%. Cyclophosphamide as an HDCT regimen and female gender were significant predictors of the development of noninfectious ILD. Our patients showed a delayed onset of symptoms, and six of eight ILD patients (75.0%) died during the study period, even though all patients were administered steroid treatment for ILD.
The incidence of noninfectious ILD following autologous HSCT is not well known. In an adult study, the incidence of noninfectious ILD following allogenic bone marrow transplant was 0.1–5.1%.12, 13, 15 In a paediatric autologous HSCT study, two of 38 patients (5.3%) showed abnormal chest x‐ray, respiratory symptoms and restrictive pulmonary function tests, but infectious causes were not excluded.16 The incidence of noninfectious ILD after autologous HSCT may be different in different study groups, depending on patients' age, underlying disease or its treatment.
The clinical features of our patients do not fit well with the previously reported syndromes of noninfectious pulmonary complications after HSCT. Idiopathic pneumonia syndrome (IPS) is one common pulmonary complication after HSCT.17, 18 The American Thoracic Society defines IPS as follows: (i) widespread alveolar injury; (ii) the absence of infection; and (iii) the absence of cardiac, renal or iatrogenic aetiology,19 which is similar to our definition of noninfectious ILD except that it limits the injury site to the alveoli instead of the entire lung parenchyma. The mortality of IPS patients is 71–75%, which is similar to that of our patients.20, 21, 22 Because pulmonary complications follow a predictable timeline after HSCT,17 an onset time of a median of 20–42 days (range: 4–106 days) after HSCT is one major difference, in that all of our patients presented with symptoms after more than 100 days, and six of our patients (75%) developed symptoms after more than 12 months.19, 22, 23 Delayed pulmonary toxicity syndrome is another form of mild‐to‐moderate pulmonary injury that can occur after HDCT and autologous HSCT in breast cancer patients.24, 25, 26 Patients with delayed pulmonary toxicity syndrome develop symptoms with a mean onset of 10 weeks after HDCT containing cyclophosphamide, cisplatin and BCNU.24 Unlike our patients, they show good responses to steroid treatment and good prognosis.
Many drugs are known to cause ILD, and 3% of all ILD can be attributed to drug‐induced ILD.27 The incidence of drug‐induced ILD has not been well known, but the incidence density of 4.1 per million person‐years was reported in a study over a 12‐year period (1997–2008), and the time trend in incidence was not statistically significant.28 Cyclophosphamide is known to cause drug‐induced ILD, and the clinical and pathologic patterns of ILD are subacute ILD/non‐specific interstitial pneumonia, pulmonary fibrosis, acute respiratory distress syndrome and organizing pneumonia.14 Drug‐induced ILD, including that produced by cyclophosphamide, may not only develop during the administration of the medication but it can also present several years after treatment.29 Late‐onset pulmonary fibrosis was identified in two children treated with cyclophosphamide30 and has been reported up to 17 years later in children who received BCNU.31 Because the diagnosis of drug‐induced ILD is made by temporal eligibility and exclusion of other causes, it is difficult to diagnose patients who develop symptoms after finishing the medication. In this study, cyclophosphamide was associated with an 11.3‐times increased risk of ILD, and it raises a high suspicion for cyclophosphamide having a causative role in late‐onset drug‐induced ILD. Depletion of reduced glutathione and impaired antioxidant defences might be suggested as possible mechanisms.25
According to the univariate analysis conducted in this study, MIBG treatment and female gender were ILD risk factors. MIBG shares the same uptake and release mechanism as norepinephrine, and it is used for the detection of neuroendocrine tumours or targeted radiotherapy for advanced neuroblastoma.32, 33 123I‐MIBG is also taken up by the lung through energy‐requiring, sodium‐dependent transport mechanism, so MIBG scintigraphy is used to assess pulmonary endothelial cell injury.34 In 131I MIBG mega‐therapy groups, transient interstitial pneumonia and fatal pneumonia were reported, but the risk of pulmonary complications warrants further investigation. Even though gender was a risk factor in this study, the specific mechanism for this trend is not known. Gender‐based differences in bleomycin‐induced pulmonary fibrosis have been reported in an animal study.35 Male gender was also determined to be a risk factor for ILD in non‐small‐cell lung cancer patients who received gefitinib treatment.36 The gender effect of the development of ILD after autologous HSCT needs to be studied. Total body irradiation (TBI, >1200 cGy) among patients older than 40 years is also known to produce a high risk for IPS.22 However, TBI was not associated with ILD in this study, which may have been due to the young age of our patients or the lower dose of TBI (333 cGy/fraction, 3 fractions) that was used. Bleomycin and methotrexate, which are both drugs that are known to cause ILD,14 were used at conventional chemotherapy doses in this study group, but they had no significant association with noninfectious ILD.
Our large study number of autologous HSCT (n = 340) patients allowed us to analyse the risk factors associated with noninfectious ILD after autologous HSCT, which has previously only been anecdotally reported or investigated only in regard to one individual therapeutic agent or individual disease cases. Long follow‐up periods (median 4.5 years) allow for the detection of late‐onset noninfectious ILD.
There were some limitations in this study. We could not obtain pulmonary function tests as screening tests because most patients were too young to perform them. Additionally, approximately 10% of ILD patients may not present with abnormal chest x‐rays.37 Therefore, some ILD patients with minimal symptoms or with a normal chest x‐ray may not have been detected using this study definition, so the incidence could be higher than 2.4%. For this reason, caution should be used when interpreting any negative results for suspected risk factors in this population.
Conclusively, this study presents the incidence of late‐onset noninfectious ILD after autologous HSCT in paediatric solid tumour patients. Female gender and high‐dose cyclophosphamide treatment might be risk factors for noninfectious ILD, but further studies with a larger number of noninfectious ILD patients are suggested.
Lee, Y.‐K. , Huh, R. , Kim, J. , Ahn, K. , Sung, K. W. and Cho, J. (2016) Late‐onset noninfectious interstitial lung disease following autologous haematopoietic stem cell transplantation in paediatric patients. Respirology, 21: 1068‐1074. doi: 10.1111/resp.12787.
(Associate Editor: Yuben Moodley)
References
- 1. Chan KW, Petropoulos D, Choroszy M, Herzog C, Jaffe N, Ater J, Korbling M. High‐dose sequential chemotherapy and autologous stem cell reinfusion in advanced pediatric solid tumors. Bone Marrow Transplant. 1997; 20: 1039–43. [DOI] [PubMed] [Google Scholar]
- 2. Meyers PA, Krailo MD, Ladanyi M, Chan KW, Sailer SL, Dickman PS, Baker DL, Davis JH, Gerbing RB, Grovas A et al. High‐dose melphalan, etoposide, total‐body irradiation, and autologous stem‐cell reconstitution as consolidation therapy for high‐risk Ewing's sarcoma does not improve prognosis. J. Clin. Oncol. 2001; 19: 2812–20. [DOI] [PubMed] [Google Scholar]
- 3. Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG‐1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer 2005; 44: 348–57. [DOI] [PubMed] [Google Scholar]
- 4. Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M et al. Myeloablative megatherapy with autologous stem‐cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high‐risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005; 6: 649–58. [DOI] [PubMed] [Google Scholar]
- 5. Frei E 3rd, Canellos GP. Dose: a critical factor in cancer chemotherapy. Am. J. Med. 1980; 69: 585–94. [DOI] [PubMed] [Google Scholar]
- 6. Chen AR. High‐dose therapy with stem cell rescue for pediatric solid tumors: rationale and results. Pediatr. Transplant. 1999; 3(Suppl 1): 78–86. [DOI] [PubMed] [Google Scholar]
- 7. Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest 1996; 109: 1066–77. [DOI] [PubMed] [Google Scholar]
- 8. Soubani AO, Pandya CM. The spectrum of noninfectious pulmonary complications following hematopoietic stem cell transplantation. Hematol. Oncol. Stem Cell Ther. 2010; 3: 143–57. [DOI] [PubMed] [Google Scholar]
- 9. Akasheh MS, Freytes CO, Vesole DH. Melphalan‐associated pulmonary toxicity following high‐dose therapy with autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000; 26: 1107–9. [DOI] [PubMed] [Google Scholar]
- 10. Cao TM, Negrin RS, Stockerl‐Goldstein KE, Johnston LJ, Shizuru JA, Taylor TL, Rizk NW, Wong RM, Blume KG, Hu WW. Pulmonary toxicity syndrome in breast cancer patients undergoing BCNU‐containing high‐dose chemotherapy and autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2000; 6: 387–94. [DOI] [PubMed] [Google Scholar]
- 11. Kalaycio M, Pohlman B, Kuczkowski E, Rybicki L, Andresen S, Sobecks R, Bolwell B. High‐dose busulfan and the risk of pulmonary mortality after autologous stem cell transplant. Clin. Transplant. 2006; 20: 783–7. [DOI] [PubMed] [Google Scholar]
- 12. Schlemmer F, Chevret S, Lorillon G, De Bazelaire C, Peffault de Latour R, Meignin V, Michallet M, Hermet E, Wyplosz B, Houdouin V et al. Late‐onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir. Med. 2014; 108: 1525–33. [DOI] [PubMed] [Google Scholar]
- 13. Patriarca F, Skert C, Sperotto A, Damiani D, Cerno M, Geromin A, Zaja F, Stocchi R, Prosdocimo S, Fili C et al. Incidence, outcome, and risk factors of late‐onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplant. 2004; 33: 751–8. [DOI] [PubMed] [Google Scholar]
- 14. Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration 2004; 71: 301–26. [DOI] [PubMed] [Google Scholar]
- 15. Afessa B, Abdulai RM, Kremers WK, Hogan WJ, Litzow MR, Peters SG. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest 2012; 141: 442–50. [DOI] [PubMed] [Google Scholar]
- 16. Nenadov Beck M, Meresse V, Hartmann O, Gaultier C. Long‐term pulmonary sequelae after autologous bone marrow transplantation in children without total body irradiation. Bone Marrow Transplant. 1995; 16: 771–5. [PubMed] [Google Scholar]
- 17. Pena E, Souza CA, Escuissato DL, Gomes MM, Allan D, Tay J, Dennie CJ. Noninfectious pulmonary complications after hematopoietic stem cell transplantation: practical approach to imaging diagnosis. Radiographics 2014; 34: 663–83. [DOI] [PubMed] [Google Scholar]
- 18. Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am. Rev. Respir. Dis. 1993; 147: 1601–6. [DOI] [PubMed] [Google Scholar]
- 19. Panoskaltsis‐Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am. J. Respir. Crit. Care Med. 2011; 183: 1262–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am. Rev. Respir. Dis. 1993; 147: 1393–400. [DOI] [PubMed] [Google Scholar]
- 21. Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation 1997; 63: 1079–86. [DOI] [PubMed] [Google Scholar]
- 22. Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, Maloney DG, Deeg HJ, Martin PJ, Storb RF et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003; 102: 2777–85. [DOI] [PubMed] [Google Scholar]
- 23. Keates‐Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant. 2006; 38: 285–9. [DOI] [PubMed] [Google Scholar]
- 24. Wilczynski SW, Erasmus JJ, Petros WP, Vredenburgh JJ, Folz RJ. Delayed pulmonary toxicity syndrome following high‐dose chemotherapy and bone marrow transplantation for breast cancer. Am. J. Respir. Crit. Care Med. 1998; 157: 565–73. [DOI] [PubMed] [Google Scholar]
- 25. Todd NW, Peters WP, Ost AH, Roggli VL, Piantadosi CA. Pulmonary drug toxicity in patients with primary breast cancer treated with high‐dose combination chemotherapy and autologous bone marrow transplantation. Am. Rev. Respir. Dis. 1993; 147: 1264–70. [DOI] [PubMed] [Google Scholar]
- 26. Bhalla KS, Wilczynski SW, Abushamaa AM, Petros WP, McDonald CS, Loftis JS, Chao NJ, Vredenburgh JJ, Folz RJ. Pulmonary toxicity of induction chemotherapy prior to standard or high‐dose chemotherapy with autologous hematopoietic support. Am. J. Respir. Crit. Care Med. 2000; 161: 17–25. [DOI] [PubMed] [Google Scholar]
- 27. Thomeer MJ, Costabe U, Rizzato G, Poletti V, Demedts M. Comparison of registries of interstitial lung diseases in three European countries. Eur. Respir. J. Suppl. 2001; 32: 114s–8s. [PubMed] [Google Scholar]
- 28. Amar RK, Jick SS, Rosenberg D, Maher TM, Meier CR. Drug‐/radiation‐induced interstitial lung disease in the United Kingdom general population: incidence, all‐cause mortality and 29 characteristics at diagnosis. Respirology 2012; 17: 861–8. [DOI] [PubMed] [Google Scholar]
- 29. Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am. J. Respir. Crit. Care Med. 1996; 154: 1851–6. [DOI] [PubMed] [Google Scholar]
- 30. Alvarado CS, Boat TF, Newman AJ. Late‐onset pulmonary fibrosis and chest deformity in two children treated with cyclophosphamide. J. Pediatr. 1978; 92: 443–6. [DOI] [PubMed] [Google Scholar]
- 31. O'Driscoll BR, Hasleton PS, Taylor PM, Poulter LW, Gattameneni HR, Woodcock AA. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N. Engl. J. Med. 1990; 323: 378–82. [DOI] [PubMed] [Google Scholar]
- 32. Sisson JC, Wieland DM, Sherman P, Mangner TJ, Tobes MC, Jacques S Jr. Metaiodobenzylguanidine as an index of the adrenergic nervous system integrity and function. J. Nucl. Med. 1987; 28: 1620–4. [PubMed] [Google Scholar]
- 33. Miano M, Garaventa A, Pizzitola MR, Piccolo MS, Dallorso S, Villavecchia GP, Bertolazzi C, Cabria M, De Bernardi B. Megatherapy combining I(131) metaiodobenzylguanidine and high‐dose chemotherapy with haematopoietic progenitor cell rescue for neuroblastoma. Bone Marrow Transplant. 2001; 27: 571–4. [DOI] [PubMed] [Google Scholar]
- 34. Takabatake N, Arao T, Sata M, Abe S, Inoue S, Shibata Y, Takeishi Y, Kubota I. Involvement of pulmonary endothelial cell injury in the pathogenesis of pulmonary fibrosis: clinical assessment by 123I‐MIBG lung scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2005; 32: 221–8. [DOI] [PubMed] [Google Scholar]
- 35. Redente EF, Jacobsen KM, Solomon JJ, Lara AR, Faubel S, Keith RC, Henson PM, Downey GP, Riches DW. Age and sex dimorphisms contribute to the severity of bleomycin‐induced lung injury and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011; 301: L510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M. Predictive factors for interstitial lung disease, antitumor response, and survival in non‐small‐cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 2006; 24: 2549–56. [DOI] [PubMed] [Google Scholar]
- 37. Epler GR, McLoud TC, Gaensler EA, Mikus JP, Carrington CB. Normal chest roentgenograms in chronic diffuse infiltrative lung disease. N. Engl. J. Med. 1978; 298: 934–9. [DOI] [PubMed] [Google Scholar]


