Abstract:
Asia is a highly heterogeneous region with vastly different cultures, social constitutions and populations affected by a wide spectrum of respiratory diseases caused by tropical pathogens. Asian patients with community‐acquired pneumonia differ from their Western counterparts in microbiological aetiology, in particular the prominence of Gram‐negative organisms, Mycobacterium tuberculosis, Burkholderia pseudomallei and Staphylococcus aureus. In addition, the differences in socioeconomic and health‐care infrastructures limit the usefulness of Western management guidelines for pneumonia in Asia. The importance of emerging infectious diseases such as severe acute respiratory syndrome and avian influenza infection remain as close concerns for practising respirologists in Asia. Specific infections such as melioidosis, dengue haemorrhagic fever, scrub typhus, leptospirosis, salmonellosis, penicilliosis marneffei, malaria, amoebiasis, paragonimiasis, strongyloidiasis, gnathostomiasis, trinchinellosis, schistosomiasis and echinococcosis occur commonly in Asia and manifest with a prominent respiratory component. Pulmonary eosinophilia, endemic in parts of Asia, could occur with a wide range of tropical infections. Tropical eosinophilia is believed to be a hyper‐sensitivity reaction to degenerating microfilariae trapped in the lungs.
This article attempts to address the key respiratory issues in these respiratory infections unique to Asia and highlight the important diagnostic and management issues faced by practising respirologists.
Keywords: avian influenza, eosinophilia, parasite, pneumonia, severe acute respiratory syndrome, tropical infection
INTRODUCTION
A vast spectrum of respiratory diseases caused by tropical pathogens unfamiliar to Western physicians, particularly parasitic and other exotic infections, occurs in Asia where the medical technological infrastructure is less established and often poorly accessible. Asia is a highly heterogeneous region with vastly different cultures and ethnic constitutions, health‐care systems, vaccination programs and socioeconomic developments. Rampant antibiotic resistance exhibited by respiratory pathogens such as Streptococcus pneumoniae, poverty, malnutrition, over‐population, environmental damage, poor public health infrastructure, failure to implement established health‐care interventions and host factors such as genetic susceptibility to diseases such as tuberculosis (TB) make it particularly challenging for clinicians in Asia. The increasing and worrying popularity of smoking remains a prime determinant to weakening host factors in many Asian countries particularly China where 63% of men smoke. 1 Background diseases such as COPD, diabetes mellitus and HIV infection (with India having the second largest population of infected patients in the world) further complicates the situation. 2
The warm and humid climate in many Asian countries provide suitable environment for many pathogens, vectors and intermediate hosts to flourish. Infectious diseases in Southeast Asia account for more than 17 million deaths annually. More than 2/3 of the 3.7 million childhood deaths are attributed to pneumonia, diarrhoeal illness and measles. 3 , 4 Many of these mortalities and morbidities are preventable if appropriate understanding, medical management and public health‐care intervention can be instituted. Understanding Asian pulmonary infectious diseases would help Western clinicians deal with imported cases.
In this article, we highlight some of the important aspects of pulmonary infections unique to Asia. We anticipate this article, along with forthcoming issues in the current series, will highlight and update practising pulmonologists in the evaluation and management of patients with pulmonary infectious diseases.
PNEUMONIA
Pneumonia is a leading cause of death from infectious diseases and is the sixth and third leading cause of death in the USA and Hong Kong respectively. 5 The mortality from pneumonia is estimated to be 8–14% 6 , 7 for hospitalized patients in the US and recently was estimated to be 7.3% in Asia. 8 Pneumonia is a vast subject and detailed discussion will occur in other articles in the series.
For Asian consideration, the microbiology for community‐acquired pneumonia should be addressed. Common pathogens causing community‐acquired pneumonia (CAP) in the West include S. pneumoniae, Mycoplasma pneumoniae, Chylamydia pneumoniae and Legionella pneumoniae, with similar pathogens in Japan and Korea. 9 Among 955 cases of Asian adult CAP patients, S. pneumoniae (29% of identifiable pathogens), Klebsiella pneumoniae (15.4%) and Haemophilus influenzae (15.1%) were the commonest bacterial pathogens, while M. pneumoniae (11%) and C. pneumoniae (13.4%) were the commonest ‘atypical’ pathogens. Legionella pneumophilia is probably only infrequently encountered and accounted for 1.1% of cases. 8 , 10 However, Gram‐negative bacilli, such as K. pneumoniae, accounted for 10% of identifiable pathogens in Malaysia and Singapore. 11 , 12 Mycobacterium tuberculosis accounts for 12.2–21% of CAP in Hong Kong and Singapore. 12 , 13 Burkholderia pseudomallei, the cause of melioidosis, accounts for 32% of the identifiable pathogens among hospitalized patients in Thailand. 14 M. pneumoniae (35.5%) and Staphylococcus aureus (41.9%) are particularly prominent in Bangladesh, Pakistan and India. 15 , 16 Although studies on the viral aetiology of CAP in Asia are scare, a recent Australian study showed that influenza, parainfluenza, adenovirus, respiratory syncytial virus and picornavirus account for 15% of CAP. 17 In addition, respiratory syncytial virus, influenza, adenovirus, parainfluenza virus and other viruses account for pneumonia in 41.2% of patients, thereby addressing the importance of viral aetiology in Asia. 18
Tuberculosis is a major cause of CAP in Asia and detailed discussion on this important topic is beyond the scope of this article. Nonetheless, it is important to register certain important facts in relation to CAP in relation to the tropics. M. tuberculosis is a highly infectious pathogen with airborne route for transmission. Eighty per cent of the global burden of TB is carried by 23 countries nearly all of which are tropical. 19 This is not only consequent from genetic susceptibility, socioeconomical deprivation, but also the effect of increasingly rampant HIV infection which imposes a 10‐fold risk for development and a higher mortality for TB among those affected. 20 , 21 Inadequate dust control, with its associated development of silicosis in Asia among miners and some builders, also predispose these populations to development of TB. 22 Helminthic infections, endemic in developing countries, induce a Th2 immune response which could predispose patients to development of TB. 23
The diagnosis of childhood TB is difficult at best as TB children are generally pauci‐bacillary and usually smear‐negative. Indeed, positive culture for TB is achieved only in 10–30% of treated children. 24 The endemic nature of TB, use of Bacille Calmette‐Guerin vaccination and HIV infection could affect the development of a positive tuberculin skin test. Undiagnosed TB, sputum smear negative or extra‐pulmonary, is responsible for 44% of HIV‐infected patients dying with wasting. 25 The World Health Organization case definition for smear‐negative pulmonary TB, based on demonstration of three negative sputum smears, radiographic features of active pulmonary TB and lack of response to broad‐spectrum antibiotics therapy, renders 14% of otherwise treatable conditions misdiagnosed as TB. 26
The use of anti‐TB drugs is already well standardized with preference given to using rifampicin and isoniazid as first line, with additional ethambutol and pyrazinamide added to the first 2 months of treatment, for a 6‐month regimen for most individuals. The strategy of Directly Observed Therapy Short‐course (DOTS) reduces drug resistance, which is desperately important for reducing the trend of increasing resistance to currently available first‐line drugs. 27 Rifampicin is relatively expensive for the poorest countries which only use short‐course therapy for sputum smear‐positive patients. Those who are sputum negative or have extra‐pulmonary diseases are treated with longer regimens that include streptomycin or thioacetazone. The latter can cause severe and sometimes fatal skin reactions in HIV‐infected individuals, and are less effective than rifampicin‐based regimens. 28 The development of multi‐drug‐resistant to TB, defined by resistant to both rifampicin and isoniazid, is a major worry in Asia. Prevention of TB remains a top priority for Asia in light of the difficulty in treatment both in drug administration and monitoring. The use of Bacille Calmette‐Guerin is only able to protect against TB meningitis and miliary TB, rather than adult cases. 29 The ideal situation would be to develop more effective TB vaccines.
The data show very considerable difference from the West in terms of the aetiology for CAP in Asia (Table 1). Antibacterial resistance in S. pneumoniae is increasing worldwide, affecting principally β‐lactams and macrolides, and resistance has been reported to be as high as 90% in Hong Kong. Among 955 cases of adult CAP patients from eight Asian countries, 17.5% and 35.1% of the S. pneumonia isolates showed penicillin intermediate (minimum inhibitory concentration (MIC) = 0.12–1 µg/mL) and penicillin resistance (MIC ≥ 2 µg/mL) respectively. 8 Among 665 adult patients from seven Chinese cities, 20.3% and 75% of S. pneumoniae isolates were resistant to penicillin and macrolides respectively. 30 Among 265 invasive isolates of S. pneumonia obtained between 1995 and 2001 in Hong Kong, 62.6% isolates were susceptible to penicillin, 20% were intermediately resistant, and 17.4% were resistant. The overall resistance to levofloxacin (MIC ≥ 8 µg/mL) was 3.8% but this increased to 15.2% among the penicillin‐resistant isolates. All levofloxacin‐resistant isolates were clonally related, had reduced susceptibility to penicillin, cefotaxime and clarithromycin, and were from patients over 50 years of age. 31
Table 1.
Prominent pathogens causing community‐acquired pneumonia in Asia
| Pathogen(s) | Country for particular prominence |
|---|---|
| Streptococcus pneumoniae | All Asia 8 , 16 |
| Mycoplasma pneumoniae | All Asia 8 , 15 |
| Haemophilus influenzae | All Asia 8 |
| Mycobacterium tuberculosis | South East and South Asia 12 , 13 |
| Klebsiella pneumoniae | South East Asia 8 , 11 , 12 |
| Staphylocccus aureus | South Asia 16 |
| Burkholderia pseudomallei | Thailand 14 |
| Chlamydia pneumoniae | Philippines 8 |
| Influenza, parainfluenza, respiratory syncytial virus, picornavirus | Australia 17 , 18 |
With the aforementioned data, it is hard to determine if Western guidelines on CAP are totally applicable to Asia (2, 3). Although resistant S. pneumoniae can still be treated with first‐line antibiotics such as β‐lactams at higher dosages, monotherapy with macrolides might not be so effective. 38 Findings from large observational studies suggest that current levels of β‐lactam resistance generally do not cause treatment failures when appropriate agents (i.e. amoxicillin, ceftriaxone, cefotaxime) and doses are used. However, discordant therapy with cefuroxime in patients with pneumococcal bacteraemia has been associated with an excessively high failure rate compared with other discordant therapies. Many experts believe that treatment failure is more likely for strains with penicillin resistance defined by MIC ≥ 4 µg/mL. In fact, these opinions are reflected by the new 2008 Clinical and Laboratory Standards Institute breakpoints (formerly NCCLS) for parenteral penicillin G of susceptible (≤ 2 µg/mL), intermediate (4 µg/mL) and resistant (≥ 8 µg/mL) for non‐meningeal infections such as CAP. 39
Table 2.
Available Western guidelines on management of community‐acquired pneumonia
| Society | Reference |
|---|---|
| British Thoracic Society (BTS) 2004 | BTS Pneumonia Guidelines Committee 32 |
| European Respiratory Society 2005 | Woodhead et al. 33 |
| Japanese Respiratory Society 2006 | Miyashita et al. 34 |
| CDC‐DRSPTWG (drug‐resistant Streptococcus pneumoniae Therapeutic Working Group) 2000 | Heffelfinger et al. 35 |
| Canadian Infectious Disease Society/Canadian Thoracic Society 2000 | Mandell et al. 36 |
| Infectious Diseases Society of America/American Thoracic Society 2007 | Mandell et al. 37 |
Table 3.
Comparison of recommendations of recently published guidelines for empirical antimicrobial therapy of community‐acquired pneumonia in adults (from North America, UK)
| Guideline | Outpatient † | General ward † | ICU/severe † |
|---|---|---|---|
| North American Guideline (ATS/IDSA; 2007) 37 | If no significant risks for drug‐resistant Streptococcus pneumoniae (DRSP) ‡ : macrolide § or doxycycline | β‐lactam (ceftriaxone, cefotaxime, ampicillin/sulbactam, ertapenem) + macrolide (can use doxycycline if macrolide not tolerated) | i.v. β‐lactam (ceftriaxone, cefotaxime, ampicillin/sulbactam) + i.v. azithromycin or i.v. fluoroquinolone ¶ ; |
| If risks for DRSP ‡ : anti‐pneumococcal fluoroquinolone ¶ | OR | If concern for Pseudomonas (e.g., presence of structural lung disease such as bronchiectasis; advanced COPD with steroid use): anti‐pseudomonal agent (piperacillin/tazobactam, imipenem, meropenem or cefepime) + anti‐pseudomonal fluoroquinolone (ciprofloxacin or high‐dose levofloxacin); If concern for MRSA (see text): add vancomycin or linezolid (defined as severe) Co‐amoxicla or second/third generation cephalosporin + (i.v. erythromycin or clarithromycin, ± rifampin); (i.v. levofloxacin + i.v. benzylpenicillin as alternative) | |
| OR | |||
| High‐dose amoxicillin (3 gm/day) or high‐dose amoxicillin/clavulanate (4 gm/day) + macrolide (if amoxicillin is used and there is a concern for H. influenzae, use macrolide active for β‐lactamase producing strains, e.g. azithromycin, clarithromycin) | Anti‐pneumococcal fluoroquinolone ¶ alone | ||
| British Thoracic Society (2004) 32 | Amoxicillin 500–1000 mg t.i.d. (Alternative—erythromycin or clarithromycin). | If admitted for non‐clinical reasons or previously untreated in the community: Amoxicillin (macrolide as alternative) | |
| If admitted for pneumonia and oral therapy appropriate: Amoxicillin + (erythromycin or clarithromycin); (levofloxacin or moxifloxacin as alternatives) | |||
| If parenteral appropriate: ampicillin or benzylpenicillin + (erythromycin or clarithromycin); (Alternative: i.v. levofloxacin, note i.v. moxifloxacin is not available in UK) |
Site of care; ICU, intensive care unit.
risks: antimicrobial therapy within the past 3 months, hospitalization within the past month, alcoholism, immune‐suppressive illness (including therapy with corticosteroids), multiple medical comorbidities, exposure to a child in a day care center.
§ Azithromycin, clarithromycin.
¶ Gemifloxacin, levofloxacin, moxifloxacin (gemifloxacin is only available in oral formulation).
Ketolides could be an alternative, but toxicity issues have recently restricted the use of telithromycin. Respiratory fluoroquinolones are attractive in being highly potent antibiotics with excellent blood and lung tissue penetration but their efficacy against M. tuberculosis could potentially mask such infections. The use of fluoroquinolones as first‐line anti‐CAP agents in Asia should be questioned. For treatment of multi‐drug‐resistant S. pneumoniae, ketolides and fluoroquinolones can be considered, and the potential use of cephalosporins, carbapenems, glycopeptides, lipopeptides, ketolides, lincosamides, oxazolidinones, glycylcyclines, quinolones, deformylase inhibitors are being evaluated.
SEVERE ACUTE RESPIRATORY SYNDROME
The horrors surrounding the onset of severe acute respiratory syndrome (SARS) in 2003, caused by a novel coronavirus (SARS‐CoV), are still vivid memories for most to date. SARS claimed the lives of 774 of 8098 affected cases scattered across 29 countries on all five continents. 40 There are three non‐distinct and highly individualized phases of SARS, namely, viral replication, inflammatory pneumonitis and then pulmonary fibrosis. Peak shedding of SARS‐CoV in nasopharyngeal aspirates and faeces peaks at 6– 11 and 9–14 days after disease onset respectively. Pathologically, SARS shows diffuse alveolar damage, secondary bacterial pneumonia and giant‐cell and macrophages infiltration into the alveoli and lung interstitium, pulmonary thromboemboli, and small airway and airspace fibrogranulation tissue proliferation. The latter resembles bronchiolitis obliterans with organizing pneumonia. 41 , 42
Diagnostic criteria proposed by the World Health Organization and Center of Disease Control and Prevention of USA are epidemiologically orientated, and rely on positive identification of SARS‐CoV. 43 , 44 , 45 These are, therefore, unhelpful at the bedside. The key diagnostic process relies on the demonstration of an epidemiological linkage, presence of pneumonia resistant to treatment and clinical features of SARS. It is thus extremely difficult, if not impossible, to diagnose the first case of SARS in any future outbreaks. The radiological patterns for consolidation are highly variable and include: bilateral patchy consolidation, nodular shadows, confluent consolidation, diffuse consolidation and even ARDS 46 (Fig. 1). Generally, radiographic opacities peak between 8 and 10 days after disease onset when bilateral disease usually occurs. 47 Pneumomediastinum and pneumothoraces, often spontaneous (but also occur with assisted ventilation), can complicate severe cases. Demonstration of a fourfold rise in anti‐SARS‐CoV titre confirms the diagnosis. Antibody response appears only around days 10–14 after onset of fever but may take up to 28 days. 44 Demonstration of a positive viral culture takes too long for prompt bedside decisions and is only useful as a confirmation. Nasopharyngeal aspirate, with a sensitivity of 80%, potentially infectious to staff during the collection, is best obtained in the first 5 days of the illness. 48
Figure 1.
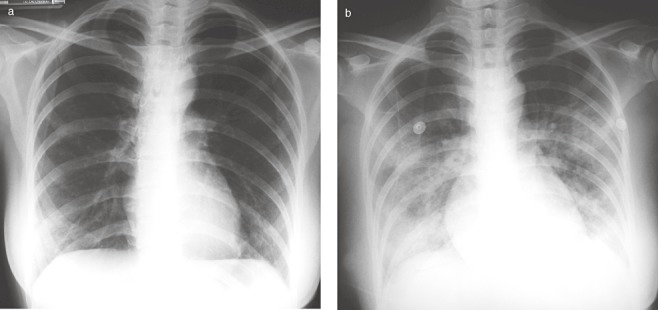
CXR of a 42‐year‐old woman who had contact with a SARS patient and then developed fever 3 days later (a) showing bilateral lower lobe mild ground glass consolidation, which rapidly progressed to bilateral ground glass consolidation after 24 h (b). SARS, severe acute respiratory syndrome.
The treatment for SARS is largely unsubstantiated with controlled trial data. Theoretically, an efficacious antiviral agent would abort the illness if given early. In 2003, SARS patients were treated with a broad spectrum antiviral agent, namely ribavirin, as well as corticosteroid, with apparent good initial responses clinically and radiologically for some patients, thereby leading to the use of this combination as a standard anti‐SARS regimen. 49 , 50 Ribavirin is now considered to be of no efficacy or even harmful. Patients treated with anti‐proteases like Kaletra (ritonavir 400 mg and lopinavir 100 mg for 14 days), combined with ribavirin, apparently had lower incidence of ARDS/death (2.4% vs 28.8%), steroid usage and nosocomial infections than historical controls. 51 Corticosteroids in combination with interferon alfacon‐1 appeared to improve the intensive care unit admission rate, mechanical ventilation need and mortality. 52
The judicial use of high‐dose methylprednisolone therapy for deteriorating SARS patients, with deteriorating radiographic consolidation, increasing oxygen requirement and respiratory distress, was associated with significant and sometime dramatic radiographic and clinical recovery. 53 Patients who received pulse steroid (methylprednisolone ≥ 500 mg/day) as initial steroid therapy had less oxygen requirement, better radiographic outcome and less likelihood of requiring rescue pulse steroid therapy than their counterparts. The use of high‐dose steroid was associated with sepsis, particularly ventilator associated pneumonia, systemic fungal infection and avascular necrosis of the hips and knees. 54 Administration of pentaglobin, an intravenous Ig enriched for IgM, on deteriorating SARS patients was associated with significant improvement in radiographic scores and oxygen requirement. 55
The control of SARS is predominantly through effective public health measures and infection control mechanisms. Although the development of vaccines for SARS is needed, there might not be adequate pharmaceutical interests or support unless further outbreaks occur. More research, including well‐planned and logistically ready clinical trials, need to be undertaken in SARS.
H5N1 AVIAN INFLUENZA
H5N1 influenza virus causes severe pneumonitis with a medium incubation period of 3 days. The virus is found in respiratory secretions 24 h before the illness and its density peaks at around 24–72 h. Predominant clinical features are fever, cough, dyspnoea, sore throat, myalgia and diarrhoea and vomiting, which may precede fatal encephalitis. Most patients develop multi‐organ failure with 80% mortality. 56 Blood tests show lymphopenia, thrombocytopenia, elevated creatinine and raised liver transaminases. Radiological features include ground glass consolidation progressing to ARDS (Fig. 2). Despite the widely reported prevalence among poultry and wild birds, there have only been 385 proven human cases with a mortality of 63.1% since 2003, due to ineffective human‐to‐human and animal‐to‐human transmission. 57
Figure 2.
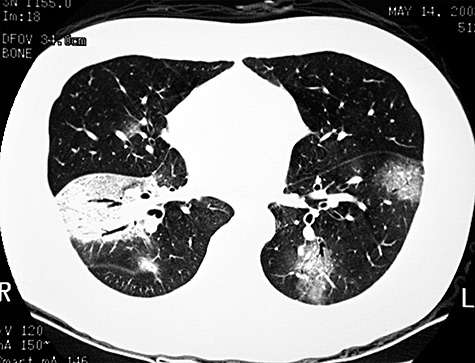
High‐resolution CT thoracic scan of a 32‐year‐old man who contracted H5N1 infection, who developed bilateral ground glass pneumonitis and ARDS showing bilateral ground glass consolidation in the lower lobes. The patient also developed multi‐organ failure requiring intensive care therapy.
Clinical diagnosis of human H5N1 infection is difficult and relies on the presence of an epidemiological link to endemic areas, contact with sick or dead poultry, or contact with a confirmed case of avian influenza. Suspected cases have aforementioned symptoms, fever (> 38°C) and contact within the previous 7 days with a confirmed or suspected human H5N1 patient, sick or dead bird, H5N1 contaminated environment, laboratory H5N1 specimen or history of consuming birds in an H5N1 endemic area. 58
Treatment of avian influenza infections in humans includes antiviral therapy and supportive care. Controlled clinical trials on the efficacy of antivirals (NA inhibitors), supportive therapy or adjuvant care have not been performed. NA inhibitors (oseltamivir and zanamivir) are used for treatment and prophylaxis with the human influenza A and B infection, and are efficacious for animal models with H5N1. 59 Oseltamivir has been used in H5N1 outbreaks and such therapy decreases the viral load in nasal secretions of patients for susceptible strains. 60 Resistance to oseltamivir can also occur in previously treated patients. 60
The use of oseltamivir and zanamivir in influenza A only provides a modest improvement in symptoms relief, and shortens the duration of fever by 1.1 days (out of 4.3 days) in otherwise healthy young patients if taken within 48 h of symptom onset. 61 The high fatality among treated H5N1 patients from Thailand and Vietnam strongly indicate a low, if any, clinical efficacy of oseltamivir in human H5N1 infection. 62 Oseltamivir has significant adverse reactions including nausea (7.0–10.7%), vomiting (2.1–8%), diarrhoea (3.2–5.5%), bronchitis (0.7–3.7%), headache (1.6–20.1%) and fatigue (0.8–7.9%), which could mimic influenza itself. 63 The use of steroid appears to be inevitable in desperately ill patients with ARDS. A recent meta‐analysis on post‐1995 cases shows significant survival benefit with long courses of low‐dose corticosteroids for 28‐day and hospital mortalities, thus leading to the authors to advocate the use of 200–300 mg hydrocortisone daily for 5–11 days. 64 , 65
Prevention and research on vaccine development of H5N1 human infections is a global health issue. Extensive research on pharmacotherapy, largely basic and pre‐clinical at present, is underway. Efforts to prevent H5N1 infections in poultry could also be a key factor in preventing animal‐to‐human transmission of this highly fatal disease. 66
MELIOIDOSIS
Melioidosis is an infectious disease caused by the bacterium B. pseudomallei, which is endemic in the soil and water of Southeast Asia, Northern Australia, Southern China, the Indian subcontinent of Asia. Patients acquire infection by inhaling contaminated dust, aspiration of contaminated water or direct inoculation of contaminated soil or water into skin wounds. Development of clinical illness may occur within 3–14 days but the infection can remain latent for decades. Clinical manifestations vary from totally asymptomatic to rapidly progressive septic shock with multi‐organ failure. Development of lobar or bronchopneumonia can be complicated by development of pleural effusion, empyema or lung abscesses (Fig. 3). Bacterial dissemination to liver and spleen with abscess formation therein, skin and soft tissue and central nervous system, bones and joints and the urinary tract can occur. Isolation of bipolar or irregular staining Gram‐negative rods in sputum or BAL fluid, and the use of selective culture media to identify B. pseudomallei are important diagnostically. Patients are treated with high‐dose ceftazidime, in combination with co‐trimoxazole, doxycycline or chloramphenicol for 2–4 weeks, followed by a combination of oral chloramphenicol, doxycycline or co‐trimoxazole. The Thai physicians, who have vast experience, administer oral antibiotics for 6–12 months depending on the clinical response and culture results. 67
Figure 3.
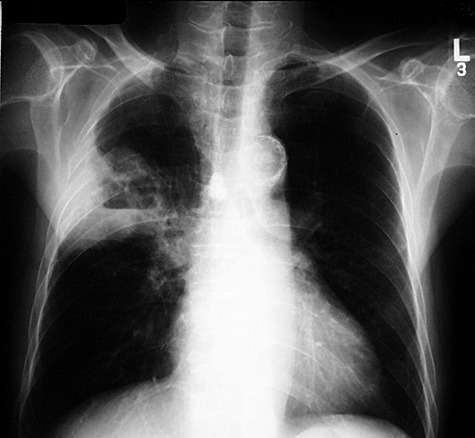
CXR of a 52‐year‐old man who had haemoptysis, fever and malaise showing right upper lobe lung abscess and mild surrounding consolidation. Serology confirmed the presence of a fourfold rise in IgG against Burkholderia pseudomallei, which was also detected on culture of BAL fluid obtained from the right upper lobe anterior segment.
DENGUE HAEMORRHAGIC FEVER
Dengue haemorrhagic fever is a mosquito‐borne viral inflection, is caused by four closely related virus serotypes of the genus Flavivirus and is endemic in Southern Asia, Philippines, India, Pakistan and Sri‐Lanka. This causes an acute increase in vascular permeability leading to leakage of plasma into extravascular compartment resulting in haemo‐concentration, hypotension or even shock. Clinically, infected individuals present with varying severity ranging from an asymptomatic state to fatal haemorrhagic disease. Febrile phase first develops with abrupt onset of high fever with constitutional symptoms (anorexia, vomiting, myalgia, headache and lethargy), abdominal pain and petechiae for 3–7 days. The toxic phase follows with an abrupt fall in temperature, and development of restlessness, tachycardia and then haemorrhagic manifestation (thrombocytopenic petechiae) or even shock. In severe cases, gastrointestinal and pulmonary haemorrhage can arise leading to ARDS. Abnormal CXR usually manifest as pleural effusion or pneumonia (Fig. 4). The final phase is the recovery phase characterized by dramatic improvement of general well‐being with improvement of the platelet count and gradual normalization over a few days. Diagnosis depends on demonstration of a positive IgM antibody to dengue virus antigen. Treatment entails meticulous infusion of intravenous fluids, but careful avoidance of blood transfusion, with avoidance of aspirin and NSAIDS. Prognosis is generally good for patients without shock and spontaneous recovery usually occurs within one week. 68
Figure 4.
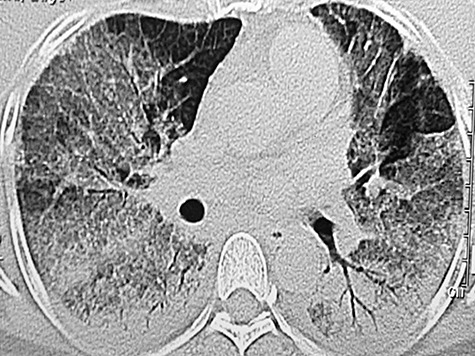
High‐resolution CT thoracic scan of a 62‐year‐old lady, a frequent visitor of Cambodia, who developed high fever, headache, respiratory failure, generalized petechiae and non‐productive cough showing bilateral ground glass consolidation. There was serological confirmation of dengue fever.
SCRUB TYPHUS
Scrub typhus is caused by accidental infection of humans, rather than the natural reservoirs, namely, wild rodents especially rats, with Orientia (formerly Rickettsia) tsutsugamushi carried by the vector larval trombiculid mites (chiggers), which usually inhabit grassy or low jungle areas. When bitten, the site develops a typical painless ulcer with black necrotic centre and a reddish rim (eschar), generally in the moist intertriginous surfaces (axilla, perineum and groin), trunks and proximal limbs. The patient then develops headache, malaise, anorexia and tender regional lymphadenopathy. High‐spiking fever with chills, severe headache and myalgia is followed a few days later with conjunctival injection and occasionally retinal haemorrahage, maculopapular rash and potential serious complications involving lung, heart, kidney and central nervous system. 69 Lung involvement is common and includes development of interstitial infiltration predominantly in the lower lung zones, and less frequently lobar or bronchopneumonia and rarely pleural effusion (Fig. 5). In severe cases, development of ARDS can occur with associated multi‐organ dysfunction and clotting defects. Diagnosis using more modern ELISA improves the accuracy in sero‐diagnosis, compared with the older Weil‐Felix test. Treatment with tetracycline, doxycycline or chloramphenical usually leads to disappearance of symptoms within 48 h. Azithromycin could be used for pregnant women while rifampicin is useful for cases with poor clinical response. Chemoprophylaxis with weekly doxycycline in high‐risk persons is effective. 70
Figure 5.
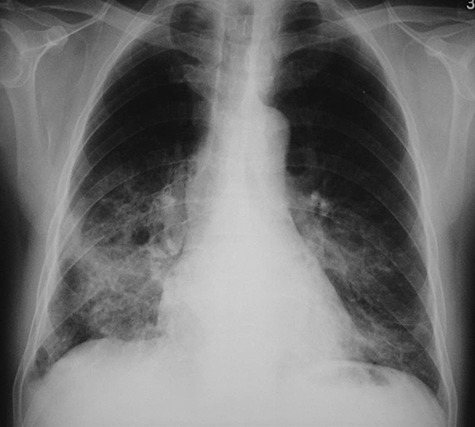
CXR of a 27‐year‐old man, a frequent hill walker in Hong Kong, who developed a right groin painless ulcer (eschar), fever, headache, myalgia and multi‐organ failure showing bilateral lower lobe ground glass consolidation. The presence of specific IgG against scrub typhus was detected by ELISA.
LEPTOSPIROSIS
Leptospirosis is a zoonotic disease caused by the genus Leptospira that infects human through exposure to infected rat urine, aggravated by heavy rainfall and presence of neutral or alkaline soil. The disease particular affects Thailand, Philippines, Australia and China, which have increasing incidence, while Taiwan and Korea show a decreasing incidence. Once the Leptospira spirochetes penetrate mucous membrane or skin, they disseminate to the liver, kidneys, heart and lungs. Patients present with a febrile illness with myalgia, headache, conjunctivitis, jaundice, meningitis, hepatitis, nephritis and haemorrhagic events. Involvement of the lung is common, but generally mild, with unproductive cough, chest pain and haemoptysis with associated thrombocytopaenia or disseminated intravascular haemorrhage. Haemorrhagic pneumonitis manifest radiologically as predominantly unilateral lower zone and peripheral non‐segmental pulmonary opacities (Fig. 6). Pleural effusion and lymphadenopathy are only rarely encountered. Fast resolution of shadows usually occurs without residual scarring. Demonstration of a fourfold rise in specific anti‐leptospiral antibodies is diagnostic using microscopic agglutination, ELISA and immunofluorescent antibody test. Treatment with oral doxycycline or penicillin G for 7 days is effective. 71
Figure 6.
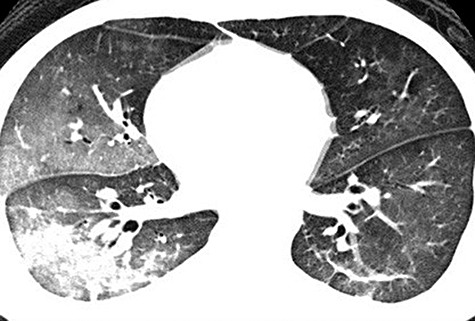
A high‐resolution CT thoracic scan of a 22‐year‐old man who underwent drowning in a heavily contaminated river who developed high fever, liver failure, anuria and haemoptysis, and a fourfold rise in anti‐leptospiral and IgG on ELISA showing bilateral ground glass consolidation.
SALMONELLOSIS
Salmonellosis is an endemic disease in many developing countries in Laos, Malaysia, Thailand, Nepal, India, Pakistan, China, Vietnam and Indonesia. The disease is caused by Salmonella species including S. typhi, S. typhimurium, S. cholerasuis, S. paratyphi and S. enteritidis. Salmonellosis is contracted by ingesting contaminated food or water with penetration of gastrointestinal lymphoid tissue followed by haematological dissemination to the liver, spleen, bone marrow and other parts of the body. There are four forms of pulmonary manifestations of salmonellosis. The commonest is acute bronchitis when patients present with mild productive cough. Pneumonia (usually caused by S. cholerasuis and S. typhi), empyema (S. thphimurium), and lung abscess formation (S. typhi) only constitute less than 1% of all cases. Pneumonia can be either lobar or bronchopneumonia, and predominantly affects the elderly and those with severe underlying diseases such as malignancy and diabetes mellitus, who have a high mortality. 72 The incidence of bacteraemia is very low in patients with normal immunity, but can be up to 100% in immune‐deficient hosts. Diagnosis entails the demonstration of a positive blood culture, and bone marrow culture is positive in more than 90% of cases. Widal test, which involves detection of antibody against the somatic (O) and flagella (H) antigens, is usually positive after 2–3 weeks. Chloramphenicol is the treatment of choice, although third generation cephalosporins, ciprofloxacin and ofloxacin are also effective. 73
PENICILLIOSIS MARNEFFEI
This is a condition caused by a Penicillium species particularly prominent in Southeast Asia, especially Thailand where it is commonly found in the soil. The organism is either inhaled and directly affects the lungs, or breaches the skin through contact with contaminated soil. Patients present with an acute febrile illness with anaemia, lymphadenopathy, hepatosplenomegaly and diffuse papular skin eruptions resembling molluscum contagiosum. Pulmonary manifestations include changes with reticulonodular or miliary infiltration, consolidation with or without cavities, pleural effusion, and hilar or mediastinal lymphadenopathy. In AIDS patients, pneumonia can be very severe and difficult to distinguish from other causes. Diagnosis is made by identification of sausage‐shaped Gram‐negative rods with central septation from sputum or BAL fluid. Blood culture is usually positive in disseminated infection. Patient should be treated with amphotericin although relapse is common among AIDS patients. 74
MALARIA
Malaria is a vector‐borne disease caused by Plasmodium (P. falciparum, P. vivax, P. ovale and P. malariae) which is endemic in the Indian subcontinent, Southeast Asia and the Middle East. Ninety per cent of deaths occurs in Africa south of the Sahara and mostly among children under 5 years of age. The bite of an infected female anopheline mosquito transmits sporozoites into the bloodstream which later enter the host liver cells. Exo‐erythrocytic schizogony leads to liberation of the merozoites that invade erythrocytes, followed by formation of erythrocytic schizogony and growth of trophozoite that infests Hb. The parasitized erythrocytes adhere to the endothelial surface and cause impairment of perfusion, nutrition and oxygen delivery in tissues especially the brain. Pulmonary manifestations occur in 3–10% of patients, and range from being asymptomatic to fatal pulmonary oedema caused by capillary leakage. The latter progresses to ARDS in 40–70% of cases. 75 Pleural effusion, usually of little clinical significance due to its small volume, and bronchitis occur. Malaria pneumonitis is less common than secondary bacterial pneumonia and occurs after severe cerebral symptoms of coma, convulsions, vomiting and aspiration. Pulmonary oedema could occur early due to heavy parasitaemia, but could occur later due to prolonged altered capillary permeability in severe malaria. The diagnosis of malaria is made by careful examination of well‐stained thick and thin peripheral blood films. Treatment is with oxygen therapy, modest fluid balance to avoid overloading, avoidance of steroid in cerebral malaria and the use of specific anti‐malarial drugs including quinine and possibly new combination of artesunate with mefloquine. 76
AMOEBIASIS
Amoebiasis, caused by the protozoan Entamoeba histolytica, is endemic in India and Southeast Asia. It is the third leading cause of death due to parasitic infections in the world. The organism is ingested via fecal‐contaminated water and food, and migrate to the large bowel where it lives for years. Most patients develop colitis and liver abscess. Pleuropulmonary amoebiasis occurs exclusively in patients with liver abscess, and characteristically presents as a right lower lobe lung abscess, manifesting radiologically as a triangular shadow with its base on the diaphragm. Symptoms include insidious onset of fever, night sweat, anorexia, right pleuritic chest pain, dry cough and haemoptysis. Development of hepatobronchial fistula leads to expectoration of characteristic chocolate‐coloured anchovy paste, which is the contents of the liver abscess. Confirmation of diagnosis is made by liver ultrasound and serological demonstration of the organism in BAL or pleural fluid. Patient are treated with metronidazole for 10–14 days and the lung abscess dealt with accordingly. 77
PARAGONIMIASIS
Paragonimiasis, caused by Paragonimus westermani, is a food‐borne trematode infection endemic in Japan, Korea, Taiwan, central China, Thailand, Laos, Vietnam, Cambodia, Malaysia, Indonesia, Philippines, India and Nepal. It is caused by ingestion of raw or poorly cooked freshwater crabs, which is the second intermediate host. Metacercaria then penetrate the small bowel wall through the abdominal cavity and appear in the pleural cavity 14 days after infection. Another 2 weeks later, the young worms enter the lungs where maturation occurs within several weeks to form parasitic cysts. The flukes lodge near bronchioles, where they lay eggs 6 weeks after initial infection, where the eggs are discharged in bronchial secretions. The latter is either expectorated or passed into the faeces after swallowing. 78 The flukes can migrate to the brain (the commonest extra‐pulmonary site) and to other organs. Affected individuals can be totally asymptomatic while others develop chronic cough, chest pain, repeated haemoptysis and symptoms due to extra‐pulmonary involvement. Three radiographic stages are identified (Fig. 7). First, during larvae migration, pleural effusion or pneumothorax with consolidation and band‐like opacities, consistent with haemorrhagic and exudative pneumonia in which the migrated larvae are detected. The consolidation resolves over several weeks during which CT scanning demonstrates the presence of the cysts within the consolidation. Second, nodular or cystic lesions (0.5–4 cm), characterizing the stage of worm maturation, predominantly in the periphery of the middle and lower lung zones, have a corona appearance (due to attachment of the worm to the wall of the cyst) are seen on CT scan. Focal bronchiectasis can also be present at this stage. During the recovery stage, following treatment induced death of the parasite, the lesions gradually disappear completely within 3–26 months. Definitive diagnosis is made by demonstrating the presence of golden brown oval 90 × 55 mm eggs with a flattened operculum in sputum, bronchoalveolar or pleural fluids, lung biopsy or stool. Sputum and blood eosinophilia and raised IgE are characteristic. Serological confirmation is more sensitive than the above using ELISA and IHA. 79 Treatment with praziquantel for 2 days can generate a cure rate of 90% and stops haemoptysis dramatically.
Figure 7.
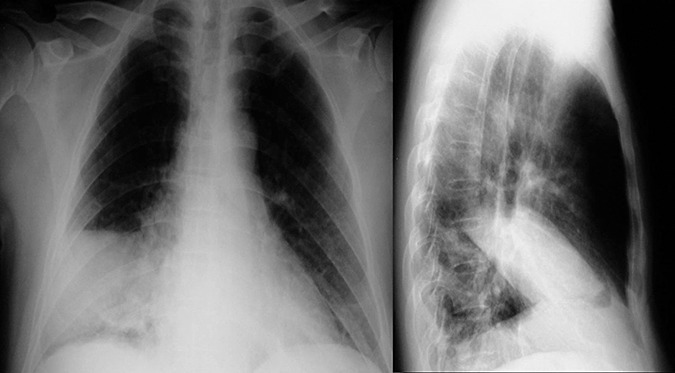
CXR (posterior‐anterior and lateral views) of a 42‐year‐old Chinese man, a frequent consumer of raw fresh water crabs, who developed daily haemoptysis, chronic cough, low‐grade fever, showing right middle lobe consolidation. BAL fluid from the affected lung segment showed the presence of brown oval eggs and serology showed the presence of specific anti‐IgG against Paragonimiasis westermani.
STRONGYLOIDIASIS
Strongyloidiasis is caused by penetration of human skin by the nematode which migrates to the bloodstream or lymph into the circulation and gradually to the small intestine. It is endemic in tropical and subtropical countries. 80 Most infected patients present with chronic diarrhoea, abdominal pain, skin rash and peripheral eosinophilia. Patients present with Loeffler's syndrome (transient coughing, wheezing, fever, symptoms of pneumonitis and eosinophilia) during larvae migration through the lungs. For patients who are receiving chronic steroid treatment or who have a chronic debilitating disease such as renal transplant, disseminated strongyloidiasis can occur, in addition to Loeffler's syndrome, with massive haemoptysis, ARDS and faecal Gram‐negative septicaemia. Bilateral lung shadows include the following features: bronchopneumonia, reticulonodular pattern, miliary nodules and ground‐glass shadows. Diagnosis is often made late thus contributing to a high mortality. Demonstration of the larvae in sputum, BAL fluid, duodenal aspirate and stool is useful, with accompanying eosinophilia. Patient should be treated with 5 days of thiabendazole or albendazole. 80
GNATHOSTOMIASIS
This disease is endemic in Thailand, Korea, Japan, Malaysia, Laos, Cambodia, Vietnam, Philippines, Bangladesh, Indonesia, India, Pakistan and China. Gnathostomiasis is caused by ingestion of raw or poorly cooked infected freshwater fish containing the third‐stage larvae of the small round worm Gnathostoma spinigerum. Upon ingestion, the larvae penetrate the gastric wall and migrate to the liver and other tissues, with associated eosinophilia. The worm tends to remain in the subcutaneous tissues but can migrate to any organs causing serious damage and even death. Within 48 h of ingestion, patients develop fever, malaise, anorexia, nausea, vomiting, diarrhoea, epigastric pain and urticaria. Migration through the liver is associated with right upper quadrant pain, while penetration through the diaphragm can produce pleuropulmonary symptoms. Intermittent migratory swelling due to subcutaneous migration could occur 3–4 weeks later. Pleuropulmonary involvement can comprise pleural effusion, pneumothorax, lobar consolidation and/or collapse. Definitive diagnosis, by demonstration of the parasite from clinical specimens, is impossible in most cases. Diagnosis is made on a history of intermittent subcutaneous migratory swelling, peripheral eosinophilia in an endemic area, and serological evidence. Patients are treated with surgical removal of the parasite whenever possible, particularly in the ocular and cutaneous forms. Albendazole is effective, if taken for 21 days, and steroid can be given to reduce the severity of pulmonary or subcutaneous symptoms. 81
TRICHINELLOSIS
The disease, endemic in Asia, usually results from ingestion of poorly cooked or raw meat infested by encysted larvae of Trichinella spiralis. The larvae migrate to the small intestine where mating occurs leading to release of newborn larvae that migrate throughout the body via the blood and lymphatic systems and finally penetrate striated muscles. Larval migration affects the heart, brain, lungs, kidney and skin. Within 24 h, patients present with an intestinal stage with abdominal cramp, vomiting and diarrhoea. This is followed by the larval migration stage 1–2 weeks later, with high fever, myalgia, periorbital oedema and elevation of muscle enzymes. Dyspnoea, chest pain and fatigue can develop due to involvement of the respiratory muscles especially the diaphragm. 82 CXR is usually normal but can show non‐specific patchy infiltration and possibly pulmonary oedema due to cardiac failure or frank larval invasion of the pulmonary vasculature. Convalescence occurs months to years later with complete recovery when the muscle larvae are eventually destroyed and calcified. Treatment with mebendazole can be used during the acute phase, and albendazole or thiabendazole to limit muscle invasion by larvae. Steroid can be used for severely symptomatic patients.
SCHISTOSOMIASIS
This is a disease endemic to China, Japan, Philippines, Taiwan, Thailand, Laos and the Indonesian island of Celebes, and is caused by penetration of the mercurial larvae of Schistosoma japonicum, S. mansoni and S. haematobium. After initial penetration, the adult form migrates to the bladder (S. haematobium) or mesenteric venules of the intestine (S. mansoni and S. japonicum) and lay eggs that circulate to the liver and shed in the stool. Granulomatous pulmonary endarteritis and dermatitis can occur at the site of penetration. Katayama syndrome, manifesting as fever, cough, general debility, hepatosplenomegaly and lymphadenopathy can also occur among non‐immune individuals. Radiographically, a non‐specific increase in lung markings and hilar lymphadenopathy can occur with associated eosinophilia. Chronic pulmonary schistosomiasis can result from long‐term parasitic egg deposition in the pulmonary vasculature, followed by granuloma formation and obstruction of blood flow, leading to the development of pulmonary hypertension and corpulmonale. 83 Diagnosis depends on demonstration of viable eggs in stool or intestinal wall, liver or bladder. BAL and transbronchial biopsy are usually fruitful in providing a diagnostic yield. Serological diagnosis using ELISA and western blot are currently used for such purpose. Confirmed cases are treated with a single dose of praziquantel.
ECHINOCOCCOSIS
This is caused by ingestion of contaminated food or water where the hexacanth embryos invade the intestine and migrate via the circulation to liver, lung, brain and elsewhere. Echinococcosis is endemic in the Middle East, India and Australia. Hydatid cysts develop and enlarge as space‐occupying lesions over months to years in affected organs. The lungs are the second most common site for hydatid, after the liver, and most patients present with a solitary lung cyst which can also be located in the fissure, pleural cavity and mediastinum. Symptoms usually result from the cysts being too sizeable or after their rupture leading to secondary bacterial infection and pneumothorax. Patients present with cough, dyspnoea, chest pain, haemoptysis, eosinophilia and expectoration of ‘grapeskins’. Intrapulmonary spread of the hydatid cysts, anaphylaxis, ARDS, empyema and lung abscess can also occur. 84 Physical examination can elicit a ‘hydatid thrill’ in the presence of a very large cyst. Demonstration on a CXR of the water lily sign (due to the presence of ruptured membranes floating on the fluid level of a partially empty cyst) and the crescent sign (air trapped between two layers of the ruptured cyst wall) are helpful but not diagnostic. CT thorax may identify the presence of a large and low‐density cyst containing numerous septae. Serology using ELISA is widely used to detect antibody. Percutaneous transthoracic needle aspiration under ultrasound or CT guidance with anti‐helminthic cover, for diagnosis of hepatic echinococcosis can be performed with minimal risks of anaphylaxis or secondary spread. Patients are treated with albendazole twice a day for 28 days with a 30% cure rate only. Localized cysts should be resected, whenever possible, if not responding to drug treatment.
PULMONARY EOSINOPHILIA IN THE TROPICS
Pulmonary eosinophilia is the concurrent appearance of peripheral eosinophilia with pulmonary infiltration, and is a complex syndrome. Peripheral eosinophilia, closely related to T‐cell activation, serves to inactivate mediators released from mast cells, modulate IgE‐mediated type I reactions and damage the larval stages of some helminthic parasites. 85 Eosinophilia is classified as primary or secondary depending on whether the aetiology is known or otherwise. Tropical pulmonary diseases associated with pulmonary eosinophilia includes TB, coccidioidomycosis, allergic bronchopulmonary aspergillosis, bronchocentric granulomatosis, chronic brucellosis, parasitic conditions and drugs. 86
Tropical eosinophilia is a syndrome characterized by respiratory symptoms and peripheral eosinophilia, which is endemic in India, Pakistan, Sri‐Lanka, Thailand, Malaysia, the Philippines and the islands of the South Pacific. It is believed to be a hyper‐sensitivity reaction to degenerating microfilariae trapped in the lungs. 87 Histologically, the presence of interstitial and alveolar histocyte infiltration progresses to eosinophilic pneumonia and chronic nodular interstitial disease with mixed cell infiltration, granulomatous reaction and fibrosis. Clinically, there is gradual onset of fatigue, low‐grade fever, night sweat, weight loss and anorexia. The presence of nocturnal cough, wheezing, dyspnoea, and tightness of the chest mimic asthma. Physical examination shows wheezing, crackles, lymphadenopathy and hepatosplenomegaly. There is leucocytosis, marked eosinophilia (absolute count 5000–60 000/mL) and very high serum IgE (1000 U/mL). Microfilariae are never found but there are detectable anti‐filarial antibodies in the blood. Radiographically, there could be generalized ground glass changes, and reticulonodular pattern affecting the middle and lower zones, hilar lymphadenopathy, diffuse interstitial nodular pattern, hyperinflation, and rarely consolidation, cavity formation and pleural effusion. 88 Lung function testing initially show obstructive pattern and later mixed obstructive‐restrictive pattern. Sputum examination shows eosinophilia. Spontaneous remission is known to occur, but treatment with diethylcarbamazine often leads to rapid recovery despite possible relapses, which could be amenable to a second course of treatment. 89
REFERENCES
- 1. Yang T, Fisher KJ, Li F, Danaher BG. Attitudes to smoking cessation and triggers to relapse among Chinese male smokers. BMC Public Health 2006; 6: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaidi AK, Awasthi S, DeSilva HJ. Burden of infectious diseases in South Asia. BMJ 2004; 328: 811–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unicef Child Mortality Statistics. [Accessed 17 February 2004.] Available from URL: http://www.childinfo.org/cmr/revis/db2.htm
- 4. Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003; 361: 2226–34. [DOI] [PubMed] [Google Scholar]
- 5. Census and Statistics Department, Department of Health . Hong Kong Hospital Authority Statistical Report 2005/06. [Accessed 16 July 2008.]http://www.ha.org.hk/visitor/ha_visitor_index.asp?Parent_ID=652&Content_ID=1170
- 6. Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS et al Prognosis and outcomes of patients with community‐acquired pneumonia. A meta‐analysis. JAMA 1996; 275: 134–41. [PubMed] [Google Scholar]
- 7. Marston BJ, Plouffe JF, File TM Jr, Hackman BA, Salstrom SJ et al Incidence of community‐acquired pneumonia requiring hospitalization. Results of a population‐based active surveillance Study in Ohio. The Community‐Based Pneumonia Incidence Study Group. Arch. Intern. Med. 1997; 157: 1709–18. [PubMed] [Google Scholar]
- 8. Song JH, Oh WS, Kang CI, Chung DR, Peck KR et al Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents 2008; 31: 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushima T, Miyashita N, File TM Jr. Etiology and management of community‐acquired pneumonia in Asia. Curr. Opin. Infect. Dis. 2002; 15: 157–62. [DOI] [PubMed] [Google Scholar]
- 10. Ishida T, Miyashita N, Nakahama C. Clinical differentiation of atypical pneumonia using Japanese guidelines. Respirology 2007; 12: 104–10. [DOI] [PubMed] [Google Scholar]
- 11. Liam CK, Lim KH, Wong CM. Community‐acquired pneumonia in patients requiring hospitalization. Respirology 2001; 6: 259–64. [DOI] [PubMed] [Google Scholar]
- 12. Hui KP, Chin NK, Chow K. Prospective study of the aetiology of adult community acquired bacterial pneumonia needing hospitalisation in Singapore. Singapore Med. J. 1993; 34: 329–34. [PubMed] [Google Scholar]
- 13. Chan CH, Cohen M, Pang J. A prospective study of community‐acquired pneumonia in Hong Kong. Chest 1992; 101: 442–46. [DOI] [PubMed] [Google Scholar]
- 14. Boonsawat W, Boonma P, Tangdajahiran T, Paupermpoonsiri S, Wongpratoom W et al Community‐acquired pneumonia in adults at Srinagarind Hospital. J. Med. Assoc. Thai. 1990; 73: 345–52. [PubMed] [Google Scholar]
- 15. Dey AB, Chaudhry R, Kumar P, Nisar N, Nagarkar KM. Mycoplasma pneumoniae and community‐acquired pneumonia. Natl. Med. J. India. 2000; 13: 66–70. [PubMed] [Google Scholar]
- 16. Asghar R, Banajeh S, Egas J, Hibberd P, Iqbal I et al Chloramphenicol versus ampicillin plus gentamicin for community acquired very severe pneumonia among children aged 2–59 months in low resource settings: multicentre randomised controlled trial (SPEAR study). BMJ 2008; 336: 80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charles PG, Whitby M, Fuller AJ, Stirling R, Wright AA et al The etiology of community‐acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin. Infect. Dis. 2008; 46: 1513–21. [DOI] [PubMed] [Google Scholar]
- 18. Yin CC, Huah LW, Lin JT, Goh A, Ling H et al Lower respiratory tract infection in hospitalized children. Respirology 2003; 8: 83–9. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Global Tuberculosis Control. WHO Report 2001. WHO, Geneva, Switzerland, WHO/CDS/TB/2001.287. [Google Scholar]
- 20. Wood R, Maartens G, Lombard CJ. Risk factors for developing tuberculosis in HIV‐1 infected adults from communities with a low or very high incidence of tuberculosis. J. Acquir. Immune. Defic. Syndr. 2000; 23: 75–80. [DOI] [PubMed] [Google Scholar]
- 21. Connolly C, Reid A, Davies G, Sturm W, McAdam KP et al Relapse and mortality among HIV infected and uninfected patients with tuberculosis successfully treated with twice weekly directly observed therapy in rural South Africa. AIDS 1999; 13: 1543–7. [DOI] [PubMed] [Google Scholar]
- 22. Hong Kong Chest Service, Tuberculosis Research Center, Madras, British Medical Research Council . A double‐blind placebo‐controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am. Rev. Respir. Dis. 1992; 145: 36–41. [DOI] [PubMed] [Google Scholar]
- 23. Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N et al Can eradication of worms change the face of AIDS and tuberculosis? Immunol. Today 1999; 20: 485–7. [DOI] [PubMed] [Google Scholar]
- 24. Starke JR. Tuberculosis in children In: Reichman LB, Hershfield ES. (eds) Tuberculosis: A Comprehensive International Approach. Marcel Dekker Inc, New York, 1993; 329–67. [Google Scholar]
- 25. Lucas SB, De Cock KM, Hounnou A, Peacock C, Diomande M et al Contribution of tuberculosis to slim disease in Africa. BMJ 1994; 308: 1531–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hargreaves NJ, Kadzakumanja O, Phiri S, Nyangulu DS, Salaniponi FM et al What causes smear‐negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? Int. J. Tuberc. Lung Dis. 2001; 5: 113–22. [PubMed] [Google Scholar]
- 27. De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int. J. Tuberc. Lung Dis. 1999; 3: 457–65. [PubMed] [Google Scholar]
- 28. Colebunders R, Bastian I. A review of the diagnosis and treatment of smear‐negative pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2000; 4: 97–107. [PubMed] [Google Scholar]
- 29. Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E et al Efficacy of BCG vaccine in the prevention of tuberculosis. Meta‐analysis of the published literature. JAMA 1994; 271: 698–702. [PubMed] [Google Scholar]
- 30. Liu YN, Chen MJ, Zhao TM, Wang H, Wang R et al A multicentre study on the pathogenic agents in 665 adult patients with community‐acquired pneumonia in cities of China. Zhonghua Jie He He Hu Xi Za Zhi 2006; 29: 3–8. [PubMed] [Google Scholar]
- 31. Ho PL, Que TL, Chiu SS, Yung RW, Ng TK et al Fluoroquinolone and other antimicrobial resistance in invasive pneumococci, Hong Kong, 1995–2001. Emerg. Infect. Dis. 2004; 10: 1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. BTS Pneumonia Guidelines Committee . BTS Guidelines for the Management of Community Acquired Pneumonia in Adults—2004 updated. Published on BTS website on 30.04.04. [Accessed 18 June 2005.] Available from URL: http://www.brit-thoracic.org.uk/Portals/0/Clinical%20Information/Pneumonia/Guidelines/MACAPrevisedApr04.pdf
- 33. Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M et al Guidelines for the management of adult lower respiratory tract infections. Eur. Respir. J. 2005; 26: 1138–80. [DOI] [PubMed] [Google Scholar]
- 34. Miyashita N, Matsushima T, Oka M, Japanese Respiratory Society . The JRS guidelines for the management of community‐acquired pneumonia in adults: an update and new recommendations. Intern. Med. 2006; 45: 419–28. [DOI] [PubMed] [Google Scholar]
- 35. Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR et al Management of community‐acquired pneumonia in the era of pneumococcal resistance: a report from the Drug‐Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 2000; 160: 1399–408. [DOI] [PubMed] [Google Scholar]
- 36. Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community‐acquired pneumonia: an evidence‐based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. The Canadian Community‐Acquired Pneumonia Working Group. Clin. Infect. Dis. 2000; 31: 383–421. [DOI] [PubMed] [Google Scholar]
- 37. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD et al Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin. Infect. Dis. 2007; 44 (Suppl. 2): S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Bambeke F, Reinert RR, Appelbaum PC, Tulkens PM, Peetermans WE. Multidrug‐resistant Streptococcus pneumoniae infections: current and future therapeutic options. Drugs 2007; 67: 2355–82. [DOI] [PubMed] [Google Scholar]
- 39. CLSI . MIC interpretive standards for Streptococcus pneumoniae. Table 2G In: Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth Informational Supplement. CLSI document M100‐S18. Clinical and Laboratory Standards Institute, Wayne, PA, 2008. [Google Scholar]
- 40. World Health Organization . Summary Probable SARS Cases with Onset of Illness from November 1 2002 to July 31 2003. [Accessed 26 September 2003.] Available from URL: http://www.who.int/csr/sars/country/table2003_09_23/en
- 41. Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST et al Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003; 361: 1773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tse GM, To KF, Chan PK, Lo AW, Ng KC et al Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J. Clin. Pathol. 2004; 57: 260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization . Case Definitions for Surveillance of Severe Acute Respiratory Syndrome (SARS). [Accessed 1 May 2003.] Available from URL: http://www.who.int/csr/sars/casedefinition/en/
- 44. Center for Disease Prevention and Control . Severe Acute Respiratory Syndrome (SARS). Diagnosis/Evaluation. [Accessed 6 February 2004.] Available from URL: http://www.cdc.gov/ncidod/sars/diagnosis.htm
- 45. Tsang KW, Mok TY, Wong PC, Ooi GC. Severe acute respiratory syndrome (SARS) in Hong Kong. Respirology 2003; 8: 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ooi CG, Khong PL, Lam B, Ho JC, Yiu WC et al Severe acute respiratory syndrome: relationship between radiologic and clinical parameters. Radiology 2003; 229: 492–9. [DOI] [PubMed] [Google Scholar]
- 47. Wong WM, Ho JC, Ooi GC, Mok T, Chan J et al Temporal patterns of hepatic dysfunction and disease severity in patients with SARS. JAMA 2003; 290: 2663–5. [DOI] [PubMed] [Google Scholar]
- 48. Poon LL, Wong BW, Chan KH, Leung CS, Yuen KY et al A one step quantitative RT‐PCR for detection of SARS coronavirus with an internal control for PCR inhibitors. J. Clin. Virol. 2004; 30: 214–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T et al A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003; 348: 1977–85. [DOI] [PubMed] [Google Scholar]
- 50. Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D et al Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003; 348: 1995–2005. [DOI] [PubMed] [Google Scholar]
- 51. Chu CM, Leung YY, Hui JY, Hung IF, Chan VL et al Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur. Respir. J. 2004; 23: 802–4. [DOI] [PubMed] [Google Scholar]
- 52. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B et al Interferon alfacon‐1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA 2003; 290: 3222–8. [DOI] [PubMed] [Google Scholar]
- 53. Tsang K, Zhong NS. SARS: pharmacotherapy. Respirology 2003; 8 (Suppl.): S25–30. [DOI] [PubMed] [Google Scholar]
- 54. Griffith JF, Antonio GE, Kumta SM, Hui DS, Wong JK et al Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology 2005; 235: 168–75 [DOI] [PubMed] [Google Scholar]
- 55. Ho JC, Wu AY, Lam B, Ooi GC, Khong PL et al Pentaglobin in steroid‐resistant severe acute respiratory syndrome. Int. J. Tuberc. Lung Dis. 2004; 8: 1173–9. [PubMed] [Google Scholar]
- 56. Apisarnthanarak A, Kitphati R, Thongphubeth K, Patoomanunt P, Anthanont P et al Atypical avian influenza (H5N1). Emerg. Infect. Dis. 2004; 10: 1321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WHO . Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. [Accessed 19 June 2008.] Available from URL: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_06_19/en/index.html
- 58. Sandrock C, Kelly T. Clinical review: updated of avian influenza A infections in humans. Crit. Care 2007; 11: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leneva IA, Goloubeva O, Fenton RJ, Tisdale M, Webster RG. Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals. Antimicrob. Agents Chemother. 2001; 45: 1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ et al Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005; 353: 2667–72. [DOI] [PubMed] [Google Scholar]
- 61. Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R et al Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 2000; 283: 1016–24. [DOI] [PubMed] [Google Scholar]
- 62. Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM et al Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 2004; 350: 1179–88. [DOI] [PubMed] [Google Scholar]
- 63. Tsang KW, Eng P, Liam CK, Shim YS, Lam WK. H5N1 influenza pandemic: contingency plans. Lancet 2005; 366: 533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D et al Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis. BMJ 2004; 329: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P et al Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomized study. Am. J. Respir. Crit. Care Med. 2005; 171: 242–8. [DOI] [PubMed] [Google Scholar]
- 66. Tsang KW, Shim YS, Wong TK, Liam CK, Eng P et al Possible case scenarios and logistic issues in H5N1 pandemic. Respirology 2006; 11: 520–2. [DOI] [PubMed] [Google Scholar]
- 67. Maneechotesuwan K. An exotic pulmonary infection in Thailand: melioidosis. Respirology 1999; 4: 419–22. [DOI] [PubMed] [Google Scholar]
- 68. Rigau‐Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ et al Dengue and dengue haemorrhagic fever. Lancet 1998; 352: 971–7. [DOI] [PubMed] [Google Scholar]
- 69. Berman SJ, Kundin WD. Scrub typhus in South Vietnam. Ann. Intern. Med. 1973; 79: 26–30. [DOI] [PubMed] [Google Scholar]
- 70. Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 2003; 16: 429–36. [DOI] [PubMed] [Google Scholar]
- 71. McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr. Opin. Infect. Dis. 2005; 18: 376–86. [DOI] [PubMed] [Google Scholar]
- 72. Saphra I, Winter JW. Clinical manifestations of salmonellosis in man. An evaluation of 7779 human infections identified at the New York Salmonella Center. N. Engl. J. Med. 1957; 256: 22–34. [DOI] [PubMed] [Google Scholar]
- 73. Hoag JB, Sessler CN. A comprehensive review of disseminated Salmonella arizona infection with an illustrative case presentation. South Med. J. 2005; 98: 1123–9. [DOI] [PubMed] [Google Scholar]
- 74. Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin. Infect. Dis. 1996; 23: 125–30. [DOI] [PubMed] [Google Scholar]
- 75. Gachot B, Wolff M, Nissack G, Veber B, Vachon F. Acute lung injury complicating imported Plasmodium falciparum malaria. Chest 1995; 108: 746–9. [DOI] [PubMed] [Google Scholar]
- 76. Vijayan VK. How to diagnose and manage common parasitic pneumonias. Curr. Opin. Pulm. Med. 2007; 13: 218–24. [DOI] [PubMed] [Google Scholar]
- 77. Shamsuzzaman SM, Hashiguchi Y. Thoracic amebiasis. Clin. Chest Med. 2002; 23: 479–92. [DOI] [PubMed] [Google Scholar]
- 78. Im JG, Chang KH, Reeder MM. Current diagnostic imaging of pulmonary and cerebral paragonimiasis, with pathological correlation. Semin. Roentgenol. 1997; 32: 301–24. [DOI] [PubMed] [Google Scholar]
- 79. Maleewong W. Recent advances in diagnosis of paragonimiasis. Southeast Asian J. Trop. Med. Public Health 1997; 28 (Suppl. 1): 134–8. [PubMed] [Google Scholar]
- 80. Chu E, Whitlock WL, Dietrich RA. Pulmonary hyperinfection syndrome with Strongyloides stercoralis. Chest 1990; 97: 1475–7. [DOI] [PubMed] [Google Scholar]
- 81. Sun T. Gnathostomiasis In: Sun T. (ed.) Parasitic Disorders: Pathology, Diagnosis, and Management, 2nd edn. Williams & Wilkins, Baltimore, MD, 1999; 225–31. [Google Scholar]
- 82. Winter MD, Ball ML, Altringham JD, Lee DL. The effect of Trichinella spiralis and Trichinella pseudospiralis on the mechanical properties of mammalian diaphragm muscle. Parasitology 1994; 109: 129–34. [DOI] [PubMed] [Google Scholar]
- 83. Barret‐Connor E. Parasitic pulmonary disease. Am. Rev. Respir. Dis. 1982; 126: 558–63. [DOI] [PubMed] [Google Scholar]
- 84. Safioleas M, Misiakos EP, Dosios T, Manti C, Lambrou P et al Surgical treatment for lung hydatid disease. World J. Surg. 1999; 23: 1181–5. [DOI] [PubMed] [Google Scholar]
- 85. Butterworth AE, David JR. Eosinophil function. N. Engl. J. Med. 1981; 304: 154–6. [DOI] [PubMed] [Google Scholar]
- 86. Muller GL. Clinical significance of the blood in tuberculosis. Commonwealth Fund, New York, 1943; 95–121. [Google Scholar]
- 87. Neva FA, Ottesen EA. Tropical (filarial) eosinophilia. N. Engl. J. Med. 1978; 298: 1129–31. [DOI] [PubMed] [Google Scholar]
- 88. Jain VK, Beniwal OP. Unusual presentation of tropicalpulmonary eosinophilia. Thorax 1984; 39: 634–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Joshi VV, Udwadia FE, Gadgil RK. Etiology of tropical eosinophilia: a study of lung biopsies and review of published reports. Am. J. Trop. Med. Hyg. 1969; 18: 231–40. [PubMed] [Google Scholar]


