Abstract
Severe acute respiratory syndrome (SARS) is an emerging infection caused by a novel coronavirus. It is characterised by a highly infectious syndrome of fever and respiratory symptoms, and is usually associated with bilateral lung infiltrates. The clinical syndrome of SARS often progresses to varying degrees of respiratory failure, with about 20% of patients requiring intensive care. Despite concern about potential aerosol generation, non‐invasive ventilation (NIV) has been reported to be efficacious in the treatment of SARS‐related ARF without posing infection risks to health care workers (HCW). Spontaneous pneumomediastinum and pneumothorax in SARS is common. The incidence of NIV‐associated barotrauma ranged from 6.6% to 15%. Patients who fail to tolerate NIV or fail NIV with progressive dyspnoea, tachypnoea and hypoxaemia should be intubated and mechanically ventilated. Mortality rates in intensive care units for SARS patients were high: 34–53% at 28 days, when some patients were still being ventilated. Strict adherence to infection control measures including isolation, use of appropriate personal protective equipment and negative pressure environment had been reported to eliminate cross‐infection to HCW.
Keywords: acute respiratory distress syndrome, infection control, mechanical ventilation, non‐invasive ventilation, severe acute respiratory syndrome
INTRODUCTION
The initial fever and respiratory symptoms of severe acute respiratory syndrome (SARS) often progress rapidly to acute respiratory failure (ARF) with varying levels of severity. Reported rates of intensive care (ICU) admission vary between 19 to 32%. 1 , 2 , 3 , 4 , 5 , 6 Early ARF usually peaks by 8 days after symptom onset. 1 , 2 But can be delayed with a protracted course. 2 In the ICU, mortality rates similar to or lower than those reported for the acute respiratory distress syndrome (ARDS) 7 have been reported. 1 , 5 The following recommendations on the ventilatory and ICU management of SARS are based on published data as well as our local experience.
INDICATIONS FOR ICU CARE
-
1
Patients who meet the criteria for acute respiratory distress syndrome (ARDS, defined by PaO2/FiO2≤ 200 mmHg) should be cared for in an ICU. Depending on availability, observation in a high dependency (HDU) setting or ICU is indicated in those who meet the criteria for acute lung injury ((ALI), defined by PaO2/FiO2 > 200–300 mmHg, or in those who presented with tachypnoea (respiratory rate > 30/min) and more than 50% progression of chest (bilateral or multilobular) shadows within 48 hours.) 8
-
2
Patients who develop signs of sepsis, septic shock or multiorgan failure. As SARS is characteristically accompanied by single organ (respiratory) failure 1 multiorgan failure is usually a result of superimposed hospital‐acquired bacterial infection.
MANAGEMENT OF RESPIRATORY FAILURE
Pharmacological agents
There is no specific treatment currently available for SARS. Empirical therapy includes broad‐spectrum antibiotics and various antiviral agents, with no proven efficacy. 3 , 4 , 6 , 9 , 10 Based on the hypothesis that the respiratory failure of SARS is secondary to an immunopathological phenomenon, 10 anti‐inflammatory agents like corticosteriods have been widely used in Canada, 4 China 5 , 12 , 13 , 14 and Hong Kong. 3 , 6 , 9 , 10 In particular, short‐term intravenous high dose (pulsed) methylprednisolone (500–1000 mg) has been advocated to treat progressive pulmonary infiltrates and hypoxaemia 9 , 11 , 13 with good response reported. 5 , 9 , 13 , 14 Half of the critically ill patients with SARS might benefit from initial intravenous corticosteroid. 15 Interferon and immunoglobin (including Pentaglobin) 11 , 14 , 16 have also been used.
Oxygen supplementation
Oxygen was required in 50–85% of SARS patients 9 , 12 , 14 and may be delivered via nasal cannulae at 1–6 L per minute (LPM) or via non‐rebreathing masks (NRM) at 8–15 LPM. Patients with continuing deterioration of respiratory status will require ICU care.
Ventilatory care
Non‐invasive ventilation
Non‐invasive ventilation (NIV) is a standard mode of ventilatory assist in early ARF and ARDS due to various causes. 17 , 18 While mortality benefit was not shown, NIV could reduce intubation rate 18 and thus the complications associated with intubation and mechanical ventilation. This may be of particular advantage in SARS, where anti‐inflammatory agents could predispose the patients to ventilator‐associated pneumonia (VAP).
Despite concern about potential aerosol generation, NIV has been reported to be effective in the treatment of SARS‐related ARF without posing infection risks to HCWs. 9 , 11 , 13 , 14 A study reported that NIV was indicated in ALI and early ARDS when desaturation (SaO2 < 93%) occurred despite oxygen supplementation (> 3–5 L/m), with persistent tachypnoea (≥ 30/min) and progressive deterioration on CXR. 11 Intubation could be avoided in up to two‐thirds of cases in a Hong Kong series (unpubl. data, 2003) and in two studies from Guangzhou. 5 , 19 The usual contraindications to NIV apply, including disturbed consciousness, uncooperative patient, high aspiration risk and haemodynamic instability.
Conventional mechanical ventilators (pressure support mode), BiPAP® or continuous positive airway pressure (CPAP) machines, preferably with leak compensation capability, may be used to deliver NIV. SARS‐related ARF responds readily to low positive pressures of CPAP 4–10 cm H2O 5 or inspiratory pressures (IPAP) of < 10 cm H2O and expiratory pressures (EPAP) of 4–6 cm water. Higher pressures should be avoided because of the common finding of spontaneous pneumomediastinum and pneumothorax in SARS. 10 The incidence of NIV‐associated barotrauma ranged from 6.6% 20 to 15% (unpubl. data, 2003).
To reduce aerosol generation, exhalation ports that generate round‐the‐tube airflow (e.g. Whisper‐Swivel II, (Respironics, Murrysville, Pennsylvania, USA)) are preferred to those producing jet outflow. A viral‐bacterial filter interposed between the mask and the exhalation port could further reduce environmental contamination. Strict adherence to infection control measures including isolation, 13 use of appropriate personal protective equipment (PPE) and negative pressure environment 9 had been reported to eliminate cross‐infection to healthcare workers. Further details on infection control are shown in Table 1.
Table 1.
| Staff education |
|---|
| High risk procedures, alternatives, and precautions |
| Limit opportunities for exposure: Limit aerosol generating procedures & limit number of HCWs present |
| Effective use of time during patient contact |
| How to ‘gown’ and ‘degown’ without contamination |
| Emphasis on importance of vigilance and adherence to all infection control precautions |
| Emphasis on importance of monitoring own health |
| Dissemination of information on SARS and other prevailing infections as they evolve |
| Personal protection equipment (PPE) |
| N95 respirator/surgical mask for airborne/droplet precautions |
| Contact precautions: Disposable gloves, gown, cap |
| Eye protection with non‐reusable goggles and face‐shield |
| Powered air purification respirators (PAPR) may be used when performing high‐risk procedures (1, 2) |
| Pens, paper, other personal items and medical records should not be allowed into or removed from the room |
| Immediate removal of grossly contaminated PPE and showering in nearby facility |
| Environment/Equipment |
| Conform to CDC recommendations for environmental control of tuberculosis: Minimum 6 air change per hour (ACH). Where feasible, increase to 3 12 ACH or recirculate air through HEPA filter |
| Preferred: Negative pressure isolation rooms with antechambers, with doors closed at all times |
| Equipment should not be shared among patients |
| Alcohol‐based hand and equipment disinfectants |
| Gloves, gowns, masks and disposal units should be readily available |
| Careful and frequent cleaning of surfaces with disposable cloths and alcohol‐based detergents |
| Use of video camera equipment or windows to monitor patients |
| Transport |
| Avoid patient transport where possible: Balance risks and benefits of investigations which necessitate patient transport |
| Special precautions for ICU |
| Viral/bacterial filter placed in expiratory port of bag‐valve mask |
| Two filters per ventilator: Between expiratory port and the ventilator, and another on the exhalation outlet of the ventilator |
| Closed‐system in‐line suctioning of endotracheal/tracheostomy tubes (Fig. 2a) |
| Heat and moisture exchanger (HME) preferred to heated humidifier: Careful handling of contaminated HME required (Fig. 2a) |
| Scavenger system for exhalation port of ventilator (e.g. Servo Evac 180, Fig. 2b): Optional if negative pressure with high air change (>12/h) is achieved |
| Preoxygenate patient and temporarily switch off machine when ventilator circuit disconnection required (e.g. change of ventilator tubings, HME, etc.) |
Figure 1.

Powered air purifying respirator (PAPR) (Air‐mate). (a) Frontal view. (b) Back view of battery with HEPA filter and duct supplying purified air to hood unit.
Figure 2.
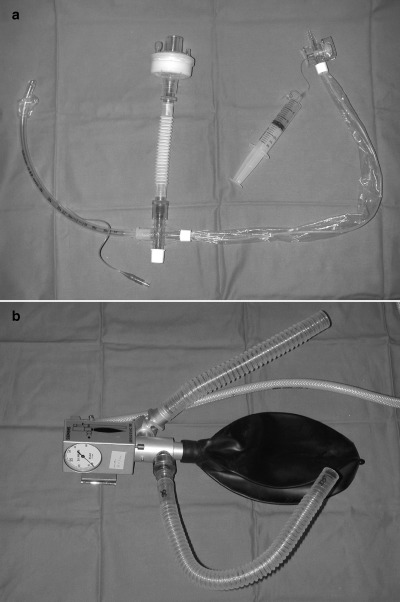
(a) Closed circuit (in‐line) suction system with heat and moisture exchanger (HME) shown as white flexitube connected to a viral/bacterial filter. (b) Servo‐Evac 180 for connection to exhalation port of ventilator. The Servo‐Evac 180 consists of a connection to the ventilator's expiratory port (curved flexitube) which empties expired air into a Evac Bag, which has a one‐way valve through which expired gas can be removed via an evacuation hose (white tube) connected to a suction source. The straight flexitube is open to the atmosphere to ensure the patient is not subjected to undue negative pressure or excessive resistance in case the suction sources is interrupted.
Mechanical ventilation (MV)
Patients who fail to tolerate NIV or fail NIV with progressive dyspnoea, tachypnoea and hypoxaemia should be intubated and mechanically ventilated. 5 , 11 Similar indications apply if NIV had not been used prior to intubation. Both pressure and volume control ventilation have been used to treat SARS‐related ARF. 2 Care should be taken to keep the tidal volume low at 5–6 mL/kg, and alveolar (plateau) pressures below 30 cm H2O. 2 PEEP levels should be titrated to as low as possible to maintain appropriate oxygenation. Since physical activity or even coughing may result in severe desaturation, sufficient sedation during early mechanical ventilation is useful to eliminate anxiety, thus improving pulmonary oxygenation. 21 A high rate of barotrauma (34%) had been reported, 1 again highlighting the need to avoid excessive volumes and pressures. Permissive hypercapnia resulting from cautious ventilator management necessitated short‐term neuromuscular blockade in 52–70% of ventilated cases. 1 , 2 Other ventilator modes used include airway pressure release ventilation and high frequency oscillatory ventilation but results have not been reported. 2
Apart from barotrauma, common complications of mechanical ventilation include ventilator‐associated pneumonia, acute renal failure, deep vein thrombosis and pulmonary embolism. 2 Low baseline PaO2/FiO2 ratios and high APACHE II scores were the only predictors for protracted ARDS and death in one study. 2 ICU mortality rates were high: 34–53% at 28 days, when some patients were still being ventilated. 1 , 2 To improve survival, research is required to identify early all SARS patients who may progress to severe ARDS and to develop effective treatment.
Meticulous infection control measures (Table 1) are mandatory in the care of ventilated SARS patients in ICU, where HCW are exposed to high risk procedures like bag‐mask ventilation, NIV, endotracheal intubation, actual or potential circuit disconnections, suctioning, tracheostomy and bronchoscopy with or without bronchoalveolar lavage. The duration of manual ventilation during resuscitation procedures should be reduced to a minimum. Endotracheal intubation should be performed by the most skilled person available 22 using rapid sequence induction: risk of aerosol generation is lowest when the patient is paralysed. All special precautions for ICU patients must be complied with. Contrary to recommendations to avoid NIV and nebulised therapy, 22 NIV has not been reported to be associated with increased infection risk for HCW, and doubts have been raised about the role of nebulised treatment on the spread of SARS within the hospital. 24 On the other hand, it is always prudent to limit opportunities for HCW exposure and to perform aerosol generating procedures in an airborne isolation environment. 25
In addition to ensuring the safety of HCWs, timely psychological support is critical to maintain staff morale. 2 The above strategies had been effective in preventing infection in ICU HCWs in hospitals in Singapore 2 and Hong Kong, and in the latter case over 80 HCWs exposed to NIV and mechanical ventilation of SARS patients were proved to be serologically negative for SARS‐CoV after the outbreak (unpubl. data, 2003).
REFERENCES
- 1. Fowler RA, Lapinsky SE, Hallet D et al. Critically ill patients with severe acute respiratory distress syndrome. JAMA 2003; 290: 367–73. [DOI] [PubMed] [Google Scholar]
- 2. Lew TWK, Kwek T, Tai D et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 2003; 290: 374–80. [DOI] [PubMed] [Google Scholar]
- 3. Lee N, Hui D, Wu A, Chan P et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003; 348: 1986–94. [DOI] [PubMed] [Google Scholar]
- 4. Booth CM, Matukas LM, Tomlinson GA et al. Clinical features and outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003; 289: 2801–9. [DOI] [PubMed] [Google Scholar]
- 5. Xiao ZL, Li YM, Chen RC, Li SY, Zhong SQ, Zhong NS. A retrospective study of 78 patients with severe acute respiratory syndrome. Chin. Med. J. 2003; 116: 805–10. [PubMed] [Google Scholar]
- 6. Tsui PT, Kwok ML, Yuen H, Lai ST. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. (Cited 8 August, 2003.] Available from URL: http://www.cdc.gov/ncidod/EID/vol9n09/03-0362.htm [DOI] [PMC free article] [PubMed]
- 7. Esteban A, Auzueto A, Frutos F et al. Characteristics and outcome in adult patients receiving mechanical ventilation: a 28‐day international study. JAMA 2002, 2003–13; 287: 345–55. [DOI] [PubMed] [Google Scholar]
- 8. Guangdong Department of Public Health. Guideline of the Management of ‘Atypical Pneumonia’ In Guangdong Hospitals. In: Office Document of Guangdong Department of Public Health (No. YWB 2003‐13). March 9 2003.
- 9. So LKY, Lau ACW, Yam LYC, Cheung MT, Poon E, Yung RYH. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet 2003; 361: 1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peiris JSM, Chu CM, Cheng VCC et al. Clinical progression and viral load in a community outbreak of coronavirus associated SARS pneumonia: a prospective study. Lancet 2003; 316: 1767–72l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo D & Qian SC. SARS treatment: experience from a team in Guangdong, China. Chin. Med. J. 2003; 116: 838–9. [PubMed] [Google Scholar]
- 12. Xue XY, Gao ZC, Xu Y et al. Clinical analysis of 45 patients with severe acute respiratory distress syndrome. Chin. Med. J. 2003; 116: 819–22. [PubMed] [Google Scholar]
- 13. Zhao Z, Zhang F, Xu M et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microb. 2003; 52: 715–20. [DOI] [PubMed] [Google Scholar]
- 14. Wu W, Wang JF, Liu PM et al. A hospital outbreak of severe acute respiratory syndrome in Guangzhou, China. Chin. Med. J. 2003; 116: 811–8. [PubMed] [Google Scholar]
- 15. Zheng JP & Zhong NS. Corticosteroid in the management of severe acute respiratory syndrome. Chin. J. Intern. Med. 2003; 42: 1–2. [Google Scholar]
- 16. Lu ZQ & Chen D. Effect of γ‐immunoglobin administration on the hospitalization time in patients with severe acute respiratory syndrome. Guangdong Med. J. 2003; 24: 151. [Google Scholar]
- 17. British Thoracic Society Standard of Care Committee. Non‐invasive ventilation in acute respiratory failure. Thorax 2002; 57: 192–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peter JV, Moran JL, Phillips‐Hughes J, Warn D. Noninvasive ventilation in acute respiratory failure—a metaanalysis update. Crit. Care Med. 2002; 30: 555–62. [DOI] [PubMed] [Google Scholar]
- 19. Chen SB, Liu XQ, Zheng ZG. Noninvasive continuous positive airway pressure ventilation in the management of critical patients with severe acute respiratory syndrome. Guangdong Med. J. 2003; 24 (SARS suppl.): 91–2. [Google Scholar]
- 20. Liu XQ, Chen SB, He GQ. Management of critical severe acute respiratory syndrome. Chin. J. Tuberc. Respir. Dis. 2003; 26: 329–33. [PubMed] [Google Scholar]
- 21. Li YM, Chen SB, Xu YD. Mechanical ventilation strategies in critical SARS patients. Chin. J. Emerg. Med. 2003; 12: 369–72. [Google Scholar]
- 22. Lapinsky SE & Hawryluck L. ICU management of severe acute respiratory syndrome. Intensive Care Med. 2003; 29: 870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centres for Disease Control and Prevention. Interim domestic infection control precautions for aerosol‐generating procedures on patients with severe acute respiratory syndrome (SARS). [Cited 20 May, 2003.] Abailable from URL: http://www.cdc.gov/ncidod/sars/aerosolinfectioncontrol.htm
- 24. Wong TW, Li CK, Tam W et al. A cluster or severe acute respiratory syndrome among medical students exposed to a single patient in Hong Kong. Emerg. Infec. Dis. 2003. [DOI] [PMC free article] [PubMed]
- 25. Centres for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Health Care Facilities. Centres for Disease Control and Prevention, Atlanta, 1994. [PubMed] [Google Scholar]


