Abstract
In order to develop a dose‐response model for SARS coronavirus (SARS‐CoV), the pooled data sets for infection of transgenic mice susceptible to SARS‐CoV and infection of mice with murine hepatitis virus strain 1, which may be a clinically relevant model of SARS, were fit to beta‐Poisson and exponential models with the maximum likelihood method. The exponential model (k= 4.1 × l02) could describe the dose‐response relationship of the pooled data sets. The beta‐Poisson model did not provide a statistically significant improvement in fit. With the exponential model, the infectivity of SARS‐CoV was calculated and compared with those of other coronaviruses. The does of SARS‐CoV corresponding to 10% and 50% responses (illness) were estimated at 43 and 280 PFU, respectively. Its estimated infectivity was comparable to that of HCoV‐229E, known as an agent of human common cold, and also similar to those of some animal coronaviruses belonging to the same genetic group. Moreover, the exponential model was applied to the analysis of the epidemiological data of SARS outbreak that occurred at an apartment complex in Hong Kong in 2003. The estimated dose of SARS‐CoV for apartment residents during the outbreak, which was back‐calculated from the reported number of cases, ranged from 16 to 160 PFU/person, depending on the floor. The exponential model developed here is the sole dose‐response model for SARS‐CoV at the present and would enable us to understand the possibility for reemergence of SARS.
Keywords: Dose‐response model, maximum likelihood method, SARS coronavirus, SARS outbreak in Hong Kong
1. INTRODUCTION
The causal pathogen of severe acute respiratory syndrome (SARS) is a newly isolated coronavirus (SARS‐CoV) that first appeared in late 2002 in Guangdong Province, People's Republic of China. In the spring of 2003, a large outbreak of this severe pneumonia occurred in Hong Kong and rapidly spread throughout the world. Ultimately, 8,096 cases of SARS were identified in 29 countries or areas and 774 patients reportedly died.( 1 ) The rapid transmission and high mortality rate made SARS a global threat for which no efficacious therapy was available and empirical strategies had to be used to treat the patients.
Since the SARS pandemic in 2003, developments of vaccines( 2 ) and antivirals( 3 ) have been rapidly proceeding for treatment of SARS patients and prevention of its reemergence. In parallel, mathematical models expressing the SARS propagation from person to person have been developed to discuss countermeasures against such a disease transmission from community to national scales.( 4 , 5 ) These models successfully explained the epidemic curve by estimating model parameters of infectious rate, removal rate, and so on. However, these models did not incorporate primary exposures or transmission via environmental reservoirs and the model parameters would not be consistent and highly influenced by numerous factors in environment and human behavior. Such models with parameters estimated on a case‐by‐case basis are of little use in any outbreaks occurring in other situations. On the other hand, dose‐response models to characterize the interaction between human and virus seems robust and applicable to assessing the risk of SARS via any possible routes of infection (e.g., aerosols containing virus particles, surface or hand contamination). Nevertheless, no dose‐response model for SARS‐CoV is available at the moment mainly due to unavailability of data sets challenging humans or animals with this virus.
In this article, we develop a dose‐response model for SARS‐CoV based on two data sets for infection of transgenic mice susceptible to SARS‐CoV and infection of mice with murine hepatitis virus strain 1 (MHV‐1) that may be a clinically relevant model of SARS.( 6 ) And also, as an example of model application, we analyze the epidemiological data of a SARS outbreak that occurred at an apartment complex in Hong Kong in 2003 with the developed model.
2. METHODS
2.1. Data Sets for Model Development
Coronaviruses cause acute and chronic respiratory, enteric, and central nervous system (CNS) diseases in many species of animals including humans. Previous to the emergence of SARS, there were two prototype human coronavirues OC43 and 229E, both etiologic agents of the common cold. SARS was the first example of serious illness in humans caused by coronaviruses.( 7 ) Since the outbreak in 2003, many researchers have worked to elucidate structures of viral genes and proteins, mechanisms of infection and replication, and its pathogenesis. Coronaviruses are divided into three groups (groups I to III) based on the genome sequences and SARS‐CoV may be a member of group II as well as murine hepatitis virus (MHV), bovine coronavirus, porcine hemagglutinating encephalomyelitis virus (HEV), equine coronavirus, and human coronavirues OC43 and NL63, which also cause respiratory infections. Among them, MHV that infects both mice and rats often has been studied as a suitable model of human coronavirus diseases.
Coronaviruses are generally restricted in their host range and viruses associated with disease in one species can be limited in their ability to replicate in other species.( 8 ) SARS‐CoV differs from this general pattern. This virus infects and replicates in mice, ferrets, hamsters, cats, and several species of nonhuman primates (cynomolgus and rhesus macaques, African green monkeys, and marmosets). Nevertheless, most attempts to reproduce completely human clinical disease and pathological findings in these animals failed. On the other hand, De Albuquerque et al. ( 9 ) demonstrated that intranasal infection of A/J mice with MHV‐1 produced pulmonary pathological features of SARS. From the fact that all MHV‐1‐infected A/J mice developed progressive interstitial pneumonitis, including dense macrophage infiltrates, giant cells, and hyaline membranes, resulting in death of all animals, they concluded that A/J mice infected with MHV‐1 would be a potentially useful small animal model of human SARS that defines its pathogenesis and suggests treatment strategies. We employed the data set (2 in Table I) challenging A/J mice with MHV‐1 as a surrogate data set for SARS‐CoV. The data set was obtained by monitoring the survival of four groups of the mice (n= 5 per group) for 21 days after intranasal inoculation with MHV‐1 at 5, 50, 500, and 5,000 PFU, respectively. In other approaches, transgenic (tg) mice expressing the human receptor for SARS‐CoV, which are very susceptible to SARS‐CoV, have been developed and used for pathogenesis studies.( 10 ) DeDiego et al. ( 11 ) reported a data set for infection of the tg mice with recombinant SARS‐CoV (1 in Table I). In their experiment, four groups of the tg mice (n= 2 to 6 per group) were intranasally inoculated with 240, 800, 2,400, and 12,000 PFU of rSARS‐CoV, respectively, and the survival was monitored 13 days. The details of data sets 1 and 2 are shown in the Appendix.
Table I.
Data Sets on Dose‐Response Relationship for Coronavirus Infection via Intranasal Route*
| No. | Virus | Genetic Group | Host | Reported Symptom | Number of Doses | Endpoint of Response | Reference | |
|---|---|---|---|---|---|---|---|---|
| Animal | Age | |||||||
| 1 | rSARS‐CoV** | 2 | tgMicet† | ? | R,CNS | 4 (1††) | Death | 11 |
| 2 | MHV‐1 | 2 | Mice | 6 to 8 weeks | R | 4(2) | Death | 9 |
| 3 | HCoV‐229E | 1 | Humans | 18 to 50 years | R | 4(4) | Illness (Cold) | 12 |
| 4 | MHV‐S | 2 | Mice | 3 days | CNS,H | 4(1) | Death | 13 |
| 5 | MHV‐S | 2 | Mice | 1 week | CNS,H | 4(1) | Death | 13 |
| 6 | MHV‐S | 2 | Mice | 2 weeks | CNS,H | 4(1) | Death | 13 |
| 7 | MHV‐2 | 2 | Mice | 3 weeks | H | 3(0) | Death | 14 |
| 8 | MHV‐2 | 2 | Mice | 4 weeks | H | 3(0) | Death | 14 |
| 9 | HEV‐67N | 2 | Mice | 1 week | CNS | 4(1) | Death | 15 |
| 10 | HEV‐67N | 2 | Mice | 4 weeks | CNS | 5(1) | Death | 15 |
| 11 | HEV‐67N | 2 | Mice | 8 weeks | CNS | 5(1) | Death | 15 |
| 12 | HEV‐67N | 2 | Rats | 1 week | CNS | 4(1) | Death | 16 |
| 13 | HEV‐67N | 2 | Rats | 4 weeks | CNS | 3(1) | Death | 16 |
| 14 | HEV‐67N | 2 | Rats | 8 weeks | CNS | 3(2) | Death | 16 |
| 15 | IBVA‐5968 | 3 | Chicks | 9 weeks | R,K | 6(4) | Death | 17 |
R = respiratory; CNS = central nervous system; H = hepatitis; K = kidney damage.
*Only data set 15 was obtained by intratracheal inoculation.
**Recombinant SARS‐CoV.
†Transgenic mice expressing the SARS‐CoV receptor.
††Number of dose points corresponding to other than 0 or 100% response.
The endpoint was death of mice for both data sets 1 and 2. Since neither infection nor illness of mice has been analyzed in these experiments, we assumed that all mice with the illness died. In general, mortality of animals with severe illness like SARS would be dependent on their physical strength, which indicates a potential of recovery from the illness, and availability of medical treatment. As described above, a high mortality was observed during the SARS outbreak, although the patients were treated with some empirical strategies. In the experiments using mice, higher mortality can be expected since the mice developing illness had never received any medical treatments and basically the physical strength of mice would be much lower than that of humans. This could support the above assumption and therefore we decided to use the data sets 1 and 2 to develop the dose‐response model with the endpoint of human illness, that is, SARS.
In order to compare the infectivity of SARS‐CoV with other coronaviruses, we also collected data sets (3 to 15 in Table I) challenging humans and animals with HCoV‐229E, MHV, HEV, and infectious bronchitis virus (IBV), which had been studied for many years before the emergence of SARS. Except for one data set (15 via tracheal inoculation), all these data sets were obtained by the intranasal inoculation, which is probably the primary route of infection with SARS‐CoV.
2.2. Fitting of Dose‐Response Models to Data Sets
Each data set in Table I was fitted to two types of dose‐response model, that is, exponential and beta‐Poisson models,( 18 ) with maximum likelihood method and goodness‐of‐fit to the models compared based on their likelihoods. The general forms of these models are as follows:
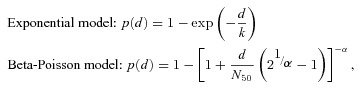 |
where p(d) is the risk of illness at the dose of d; and k, N 50, and α are parameters specific for the pathogen. Parameter k in the exponential model equals the reciprocal of the probability that a single pathogen will initiate the response. Parameters N 50 and α in the beta‐Poisson model are the median dose to get the response and the exponential fitting parameter, respectively.
The beta‐Poisson model was fitted to only four data sets 2, 3, 14, and 15 since it is not meaningful to fit this model to data sets including less than two dose points corresponding to other than 0 or 100% response. The model fitting was done via maximum likelihood estimation, using a quasi‐Newton method, implemented in the package of R version 2.6.2 for Windows.( 19 ) The sensitivity analysis of parameters of the best‐fitted model was performed with 10,000 bootstrap trials by a program written on R to estimate the doses corresponding to 10% and 50% responses (ID10 and ID50).
2.3. Model Application: SARS Outbreak at an Apartment Building in Hong Kong
During the pandemic of SARS in 2003, Hong Kong was the hardest hit reporting area with 1,755 cases and 299 deaths in a population of 6.7 million.( 1 ) The large community outbreak at an apartment complex named Amoy Gardens affected more than 300 among 20,000 residents in the early stage of the outbreak in Hong Kong. According to the report by Department of Health (DOH), Hong Kong SAR, the outbreak was begun with an index case who visited his family living on the 16th floor in Block E of Amoy Gardens.( 20 , 21 ) He stayed overnight and used the toilet there. DOH concluded that the sewage contamination associated with the index case (and other infected persons) excreting coronavirus that gained entry to households through the bathroom floor drain with dried U‐traps was the primary cause of the SARS outbreak. The building's ventilation system made a significant contribution to the entry of virus into households. According to McKinney et al.,( 22 ) many residents had installed high‐powered fans in their small bathrooms with capacities 6 to 10 times higher than the required capacity and the fans created large negative pressure and drew air from waste pipes. The WHO environmental team verified that sewer gas and aerosolized droplets, which the hydraulic action caused by flushing toilets generated, were being drawn into the bathrooms from the waste pipe system. Although DOH mentioned other possible causes of the SARS outbreak, such as person‐to‐person transmission, vectors acting as mechanical carriers for the virus, and environmental contamination, we assumed that all cases were infected via the airborne transmission, reported as the primary cause, in our model application.
DOH investigated epidemiological data of 321 confirmed SARS cases. Although the detailed data are not reported in the literature, the location of flats for 99 cases in Block E has been published in the modeling study by Li et al. ( 21 ) Since the number of residents at each flat during the outbreak has not been investigated, their group assumed that each flat housed four persons, which was the largest number of cases in any one flat, and calculated the risk of illness, equivalent to the attack rate, as the number of cases divided by the assumed number of residents.( 23 ) By using the dose‐response model developed here, we estimated the dose (d) of SARS‐CoV for cases in Block E from the attack rate (p(d)). The dose estimated on the above assumption might be lower than the true value because number of residents in some flats would be below four and there were possibly unoccupied flats. We also calculated the attack rate as the number of flats having at least one case divided by the total number of flats and estimated the dose, which would be higher than the real value, from the attack rate.
3. RESULTS
3.1. Dose‐Response Model for SARS‐CoV
Table II shows the result of fitting models to the data sets. The fits of the beta‐Poisson model to data sets 2, 3, 14, and 15 could not be rejected on statistical grounds, as indicated by pfit values greater than 0.05. Similarly, the exponential model provided statistically significant fits to all data sets except 14 and 15. As for data sets 2 and 3, both beta‐Poisson and exponential models were acceptable. We tested the null hypothesis that the beta‐Poisson model provided a statistically significant improvement in fit to these data sets rather than a more parsimonious (exponential) model by comparing the difference of variance between two models against the critical value (3.84) on the chi‐square distribution with 1 degree of freedom. As the result, since the hypothesis was rejected for both data sets, the exponential model was used for the analysis of viral infectivity in the latter part.
Table II.
Parameters and Likelihood of Dose‐Response Models Fitted to Data Sets on Coronavirus Infection
| No. | Virus | Host | Beta‐Poisson(BP) | Exponential (EXP) | Better Fit Model** | Unit of Dose | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Age | ln α | ln N 50 | Deviance | P fit | ln k | Deviance | P fit | ||||
| 1 | rSARS‐CoV | tgMice | ? | ‐* | ‐ | ‐ | ‐ | 5.82 | 0.97 | 0.81 | PFU | |
| 2 | MHV‐1 | Mice | 6 to 8 weeks | 1.23 | 5.71 | 0.54 | 0.76 | 6.15 | 0.61 | 0.89 | EXP | PFU |
| p† | SARS‐CoV | Mice | ? | 6.50 | 5.64 | 1.75 | 0.94 | 6.01 | 1.75 | 0.97 | EXP | PFU |
| 3 | HCoV‐229E | Humans | 18 to 50 years | −0.21 | 2.38 | 1.42 | 0.49 | 2.92 | 2.31 | 0.51 | EXP | TCD50 |
| 4 | MHV‐S | Mice | 3 days | ‐ | ‐ | ‐ | ‐ | 6.32 | 0.56 | 0.91 | PFU | |
| 5 | MHV‐S | Mice | 1 week | ‐ | ‐ | ‐ | ‐ | 8.62 | 0.56 | 0.91 | PFU | |
| 6 | MHV‐S | Mice | 2 weeks | ‐ | ‐ | ‐ | ‐ | 13.6 | 0.24 | 0.97 | PFU | |
| 7 | MHV‐2 | Mice | 3 weeks | (No death among 5 mice at doses from 7 × 102 to 7 × 104) | PFU | |||||||
| 8 | MHV‐2 | Mice | 4 weeks | (No death among 5 mice at doses from 6 × 103 to 6 × 105) | PFU | |||||||
| 9 | HEV‐67N | Mice | 1 week | ‐ | ‐ | ‐ | ‐ | 2.39 | 0†† | PFU | ||
| 10 | HEV‐67N | Mice | 4 weeks | ‐ | ‐ | ‐ | ‐ | 7.62 | 0.62 | 0.96 | PFU | |
| 11 | HEV‐67N | Mice | 8 weeks | ‐ | ‐ | ‐ | ‐ | 7.62 | 0.62 | 0.96 | PFU | |
| 12 | HEV‐67N | Rats | 1 week | ‐ | ‐ | ‐ | ‐ | 3.08 | 0†† | PFU | ||
| 13 | HEV‐67N | Rats | 4 weeks | ‐ | ‐ | ‐ | ‐ | 8.66 | 0.54 | 0.76 | PFU | |
| 14 | HEV‐67N | Rats | 8 weeks | −1.04 | 7.42 | 1.97 | 0.16 | 9.34 | 6.36 | 0.04 | BP | PFU |
| 15 | IBVA‐5968 | Chicks | 9 weeks | −5.11 | 109 | 2.93 | 0.57 | 11.4 | 26.6 | <0.01 | BP | CD50 |
*Beta‐Poisson model was not fitted to data sets including less than two dose points corresponding to other than 0 or 100% response.
**Beta‐Poisson model was employed if it provided a significant improvement of fit (p > 0.05) rather than exponential model.
†Data set p pooled data sets 1 and 2.
††Zero deviance means that all data were on the model.
The estimated parameter k in the exponential model for rSARS‐CoV (data set 1) was very close to that for MHV‐1 (data set 2). In order to decide whether these data sets could be pooled or not, the following statistic Δ was calculated and compared with the critical value (3.84) in the chi‐square distribution with 1 degree of freedom:( 18 )
where Y 1, Y 2, and Yp are deviances of fits of exponential model to data sets 1 and 2 and pooled data sets, respectively. As shown in Table II, we yielded ln k= 6.01 and Yp= 1.75 when the exponential model was fitted to the pooled data sets (data set p). The value of Δ (0.17) smaller than 3.84 indicated that data sets 1 and 2 could be pooled to p. Consequently, the exponential model (Fig. 1) with k= 4.1 × 102 was employed as the dose‐response model for SARS due to intranasal infection.
Figure 1.
Exponential dose‐response model fitted to the pooled data sets for SARS‐CoV.
3.2. Infectivity of SARS‐CoV
Fig. 2 illustrates the doses (ID10 and ID50) of SARS‐CoV and other coronaviruses corresponding to 10% and 50% responses calculated with the dose‐response models listed in Table II. ID10 and ID50 of SARS‐CoV were 43 (95% CI = 20 to 81 PFU) and 280 PFU (95% CI = 130 to 530 PFU), respectively. The doses (ID10= 2.0 TCD50; ID50= 13 TCD50) for HCoV‐229E seem relatively low; however, Schmidt et al. ( 24 ) reported that the 50% endpoint assay was about 10 to 30 times less sensitive than the plaque assay with HCoV‐229E. Accordingly, the estimated infectivity of SARS‐CoV would be comparable to that of HCoV that causes a mild cold in humans. Similar infectivity was also observed in data sets of three‐day‐old mice infected with MHV‐S (data set 4) and eight‐week‐old rats infected with HEV‐67N (data set 14). These animal coronaviruses belong to the same genetic group as SARS‐CoV. The infectivity of coronavirus might be related to the viral evolution, although a lot of unknown factors exist. The effect of host age was observed in data sets for MHV‐S (data sets 4 to 6) and HEV‐67N (data sets 9 to 14) and it was obvious that young mice and rats are more susceptible than old ones. In contrast, infection among children and adolescents was relatively uncommon( 25 ) and the mortality of senior people was comparatively high in the SARS pandemic.( 26 ) This age‐dependency of human susceptibility to SARS‐CoV is different not only from other coronaviruses but also from other human respiratory viruses. Avian influenza A (H5N1) virus is a highly virulent respiratory virus like SARS‐CoV. The epidemiologic investigations show that this virus primarily infects young people and Goicoechea( 27 ) made a comment that the receptor recognized by this virus, which is expressed in the lower respiratory tract, may be expressed in the upper airway in children, increasing the risk of infection. In case of respiratory syncytial virus (SRV), another well‐known human respiratory virus, the serious forms of the disease (principally bronchiolitis and interstitial pneumonia) are found most frequently in infants under six months of age and the disease is progressively milder with increasing age.( 28 )
Figure 2.
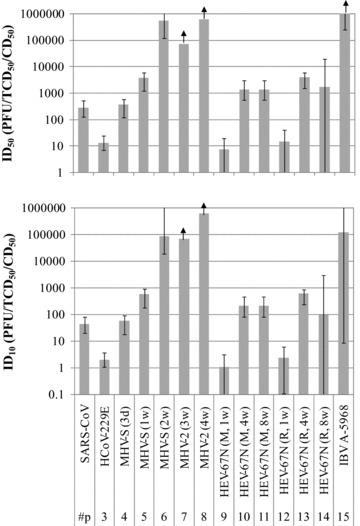
Doses (ID10 and ID50) of SARS‐CoV and other coronaviruses corresponding to 10 and 50% response. Error bars indicate 95% confident intervals.
3.3. Back‐Calculation of Viral Dose for Residents at Amoy Gardens
The doses of SARS‐CoV for residents in Block E, Amoy Gardens, estimated based on the assumed number of residents and the number of affected flats, are described in Table III. As Yu et al. ( 23 ) analyzed, the attack rate was dependent on the floor and the middle floors between levels 14 and 23 had the highest risk since the flat where the index case visited was located there. Therefore, the estimated dose of SARS‐CoV (63 to 160 PFU) for residents on the middle floor was also higher than the others. The estimated dose (42 to 117 PFU) for residents on the upper floor was slightly higher than that (16 to 49 PFU) on the lower floor. Li et al. ( 21 ) explained the reason as that viral particles were probably transmitted with upflow air movements between flats in this block. He and his colleagues( 23 ) have confirmed through air modeling that the exhaust fans propelled virus‐laden air into an outside air shaft, where it was carried upward by natural air currents and into other flats on the upper floor through open windows. Based on our estimate of dose, this transmission mode appears highly plausible, although we have been unable to locate data on viral load in either fecal matter or wastewater.
Table III.
Estimated Dose of SARS‐CoV for Residents in Amoy Gardens During the Outbreak in 2003
| (a) Estimation Based on the Assumed Number of Residents | ||||
|---|---|---|---|---|
| Floor (Level) | Number of Reported Cases | Number of Residents* | Attack Rate | Estimated Dose (PFU) |
| Lower (4–13) | 12 | 320 | 0.038 | 16 |
| Middle (14–23) | 46 | 320 | 0.144 | 63 |
| Upper (24–36) | 41 | 416 | 0.099 | 42 |
| Overall | 99 | 1056 | 0.094 | 40 |
| (b) Estimation Based on the Number of Affected Flats | ||||
|---|---|---|---|---|
| Floor (Level) | Number of Affected Flats** | Total Number of Flats | Attack Rate | Estimated Dose (PFU) |
| Lower (4–13) | 9 | 80 | 0.113 | 49 |
| Middle (14–23) | 26 | 80 | 0.325 | 160 |
| Upper (24–36) | 26 | 104 | 0.250 | 117 |
| Overall | 61 | 264 | 0.231 | 107 |
*Assumed that each flat has four residents.
**Number of flats where at least one case was reported.
4. DISCUSSION
4.1. Validation of Developed Model
Although we believe that there are no additional published data sets available for model development for SARS‐CoV other than those in Table I, partial information supporting our analysis has been provided by some researchers. Roberts et al. ( 29 ) developed mouse‐adapted SARS‐CoV through 15 serial passages in the respiratory tract of young BALB/c mice and they observed the lethality of mice dependent on the dose of the virus. Mice receiving a dose higher than 103.9 TCID50 of the virus died and the 50% lethal dose (LD50) was 104.6 TCID50. In contrast, ID50 of 2.8 × 102 PFU estimated with the developed model is low. Since ordinary SARS‐CoV cannot cause the death of mice, there is a possibility that the virulence of the virus was still low even after its adaptation to mice.
Nagata et al. ( 30 ) reported that cynomolgus monkeys inoculated intranasally with SARS‐CoV of 106 TCID50 did not show any clinical sign and symptoms, while slight histopathological changes and virus antigen‐positive cells were detected. Kuiken et al. ( 31 ) and McAuliffe et al. ( 32 ) also demonstrated the pulmonary replication of SARS‐CoV without any severe symptoms in monkeys receiving intranasal inoculation of 106 TCID50 viruses. The fact that as many as 106 viruses did not cause lethality in monkeys means that the susceptibility to this virus may be dependent on host species even within primates.
Of course, this model originated from data sets for mice not for humans. As Mizgerd and Skerrett( 33 ) pointed out, there are the differences between mice and humans relevant to pneumonia. However, since it is not realistic to obtain the data sets for human infection with SARS‐CoV by challenging to volunteers due to its high mortality, this model is still a valuable method to evaluate the risk of SARS. In prior work, it has been shown that data from animal experiments provide reasonable estimates for human susceptibility in inhaled pathogens.( 34 , 35 )
4.2 New Findings About SARS Epidemic Through Model Applications
It is widely considered that SARS is a respiratory illness. However, SARS patients may also exhibit gastrointestinal symptoms, splenic atrophy, and lymphadenopathy.( 7 ) Among them, diarrhea is a very frequent finding in SARS patients (30% to 40% of patients).( 7 ) Cheng et al. ( 36 ) reported that geometric mean titer for SARS‐CoV in feces of patients ranged from 5.1 × 101 copies/mL over 23 days after the onset of illness (dpi) to 8.9 × 104 copies/mL between 12 and 14 dpi. Number of gene copies has not been related yet to the plaque‐forming capability of this virus; however, there is no doubt that a huge amount of infectious viruses was excreted from patients for a certain period. According to the DOH report, at the beginning of the outbreak in Block E, a part of infectious viruses excreted from the index case must have reached other households through the waste pipe and ventilation systems. Considering this virus transportation, we can imagine that residents at the households inhaled small doses of SARS‐CoV (Table III) as estimated here by model application, although there are several unknown factors such as virus dispersion and inactivation during transportation.
4.3. Significance of Proposed Model for Preventing Reemerging SARS
The SARS pandemic was brought under control through a concerted global effort, and by July 5, 2003, no further human‐to‐human transmission took place. However, there are still several possibilities that might lead to the reemergence of SARS in humans.( 37 ) The potential source of SARS‐CoV might come from infected animals circulating in the geographical region, as highlighted by the four community‐acquired SARS cases between December 2003 and January 2004 in Guangdong, China. In these recent cases, fortunately, all patients only developed mild symptoms and secondary transmission did not occur since the animal virus had not fully adapted in humans yet. In addition to SARS‐CoV, two novel CoVs (HCoV‐NL63 and HCoV‐HKU1) that cause respiratory illness in humans were identified after the SARS outbreak. These facts indicate the possibility that a pandemic of SARS and other coronavirus infection may be brought by rapid viral evolution.
5. CONCLUSIONS
We proposed the exponential model with k= 4. 1 × 102 as a dose‐response model for SARS coronavirus (SARS‐CoV) based on the available data sets. With this model, the doses of SARS‐CoV corresponding to 10% and 50% responses (illness) were estimated at 43 PFU (95% CI = 20 to 81 PFU) and 280 PFU (95% CI = 130 to 530 PFU), respectively. The estimated infectivity of SARS‐CoV was comparable to those of HCoV‐229E, known as an agent of human common cold, and of some animal coronaviruses (MHV‐S and HEV‐67N) belonging to the same genetic group as SARS‐CoV.
The developed model was applied to the analysis of the epidemiological data of the SARS outbreak that occurred at an apartment complex in Hong Kong in 2003. From the reported number of cases, it was revealed that the apartment residents would be exposed to a dose of SARS‐CoV between 16 and 160 PFU per person, which depends on the floor, during the outbreak.
Although the susceptibility to SARS‐CoV seems to be host‐dependent, the developed model is the sole dose‐response model for SARS‐CoV at the present and would help us predict the reemergence of SARS in the future.
ACKNOWLEDGMENTS
We extend our special thanks to Ms. Stephy Y. M. Chan (the University of Hong Kong) for helping us collect the information on the SARS outbreak in Hong Kong. We acknowledge the support of the Japan Society for the Promotion of Science (JSPS) to Toru Watanabe, under JSPS Postdoctoral Fellowships for Research Abroad, and that of the Center for Advancing Microbial Risk Assessment (CAMRA) to Timothy A. Bartrand, Mark H. Weir, and Charles N. Haas. CAMRA is a U.S. EPA/Department of Homeland Security (DHS) Cooperative Center of Excellence funded under U.S. EPA STAR Grant R83236201. This work does not express official policy of either U.S. EPA or DHS.
Table 4.
APPENDIX: DATA SETS FOR MODEL DEVELOPMENT
| No. | Virus | Host Animal | Host Age | Dose | Tested | Positive | Negative |
|---|---|---|---|---|---|---|---|
| 1 | rSARS‐CoV | tgMice | ? | 240 | 3 | 1 | 2 |
| 800 | 3 | 3 | 0 | ||||
| 2,400 | 2 | 2 | 0 | ||||
| 12,000 | 6 | 6 | 0 | ||||
| 2 | MHV‐1 | Mice | 6 to 8 weeks | 5 | 5 | 0 | 5 |
| 50 | 5 | 1 | 4 | ||||
| 500 | 5 | 3 | 2 | ||||
| 5,000 | 5 | 5 | 0 | ||||
| 3 | HCoV‐229E | Humans | 18 to 50 years | 4 | 5 | 2 | 3 |
| 5 | 6 | 1 | 5 | ||||
| 16 | 9 | 6 | 3 | ||||
| 31 | 6 | 4 | 2 | ||||
| 4 | MHV‐S | Mice | 3 days | 20 | 4 | 0 | 4 |
| 200 | 4 | 1 | 3 | ||||
| 2,000 | 4 | 4 | 0 | ||||
| 20,000 | 4 | 4 | 0 | ||||
| 200,000 | 4 | 4 | 0 | ||||
| 5 | MHV‐S | Mice | 1 week | 200 | 4 | 0 | 4 |
| 2,000 | 4 | 1 | 3 | ||||
| 20,000 | 4 | 4 | 0 | ||||
| 20,0000 | 4 | 4 | 0 | ||||
| 6 | MHV‐S | Mice | 2 weeks | 2,000 | 4 | 0 | 4 |
| 20,000 | 4 | 0 | 4 | ||||
| 200,000 | 4 | 1 | 3 | ||||
| 7 | MHV‐2 | Mice | 3 weeks | 700 | 5 | 0 | 5 |
| 7,000 | 5 | 0 | 5 | ||||
| 70,000 | 5 | 0 | 5 | ||||
| 8 | MHV‐2 | Mice | 4 weeks | 6,000 | 5 | 0 | 5 |
| 60,000 | 4 | 0 | 4 | ||||
| 600,000 | 4 | 0 | 4 | ||||
| 9 | HEV‐67N | Mice | 1 week | 10 | 5 | 3 | 2 |
| 100 | 5 | 5 | 0 | ||||
| 1,000 | 5 | 5 | 0 | ||||
| 10,000 | 5 | 5 | 0 | ||||
| 10 | HEV‐67N | Mice | 4 weeks | 10 | 5 | 0 | 5 |
| 100 | 5 | 0 | 5 | ||||
| 1,000 | 5 | 2 | 3 | ||||
| 10,000 | 5 | 5 | 0 | ||||
| 100,000 | 5 | 5 | 0 | ||||
| 11 | HEV‐67N | Mice | 8 weeks | 10 | 5 | 0 | 5 |
| 100 | 5 | 0 | 5 | ||||
| 1,000 | 5 | 2 | 3 | ||||
| 10,000 | 5 | 5 | 0 | ||||
| 100,000 | 5 | 5 | 0 | ||||
| 12 | HEV‐67N | Rats | 1 week | 20 | 5 | 3 | 2 |
| 200 | 5 | 5 | 0 | ||||
| 2,000 | 5 | 5 | 0 | ||||
| 200,000 | 5 | 5 | 0 | ||||
| 13 | HEV‐67N | Rats | 4 weeks | 2,000 | 5 | 1 | 4 |
| 20,000 | 5 | 5 | 0 | ||||
| 200,000 | 5 | 5 | 0 | ||||
| 14 | HEV‐67N | Rats | 8 weeks | 2,000 | 5 | 3 | 2 |
| 20,000 | 5 | 3 | 2 | ||||
| 200,000 | 5 | 5 | 0 | ||||
| 15 | IBVA‐5968 | Chicks | 9 weeks | 0.32 | 11 | 0 | 11 |
| 3.2 | 11 | 1 | 10 | ||||
| 32 | 11 | 1 | 10 | ||||
| 320 | 11 | 0 | 11 | ||||
| 3,200 | 11 | 1 | 10 | ||||
| 32,000 | 11 | 1 | 10 |
REFERENCES
- 1. World Health Organization . Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at: http://www.who.int/csr/sars/country/table2004_04_21/en/index.html, Accessed on August 17, 2008.
- 2. Rockx B, Baric RS. Grand challenges in human coronavirus vaccine development In Thiel V. (ed). Coronaviruses, Molecular and Cellular Biology. Norfolk, UK : Caister Academic Press, 2007. [Google Scholar]
- 3. Keyaerts E, Vijgen L, Ranst MV. Current status of antiviral severe acute respiratory syndrome coronavirus research In Thiel V. (ed). Coronaviruses, Molecular and Cellular Biology. Norfolk, UK : Caister Academic Press, 2007. [Google Scholar]
- 4. Dye C, Gay N. Modeling the SARS epidemic. Science, 2003; 300:1884–1885. [DOI] [PubMed] [Google Scholar]
- 5. Ng TW, Turinici G, Danchin A. A double epidemic model for the SARS propagation. BMC Infectious Diseases, 2003; 3: Art. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Albuquerque N, Baig E, Xuezhong M, Shalev I, Phillips MJ, Habal M, Leibowitz J, McGilvray I, Butany J, Fish E, Levy G. Murine hepatitis virus strain 1 as a model for severe acute respiratory distress syndrome (SARS) In Perlman S, Holmes KV. (eds). The Nidoviruses: Toward Control of SARS and Other Nidovirus Diseases. New York : Springer, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss SR, Navas‐Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Reviews, 2005; 69(4):635–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subbarao K, Roberts A. Is there an ideal animal model for SARS? Trends in Microbiology, 2006; 14(7):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, Habal M, Liu M, Shalev I, Downey GP, Gorczynski R, Butany J, Leibowitz J, Weiss SR, McGilvray ID, Phillips MJ, Fish EN, Levy GA. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. Journal of Virology, 2006; 80:10382–10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCray PB Jr., Pewe L, Wohlford‐Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18‐hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of Virology, 2007; 81(2):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeDiego ML, Pewe L, Alvarez E, Rejas MT, Perlman S, Enjuanes L. Pathogenicity of severe acute respiratory coronavirus deletion mutants in hACE‐2 transgenic mice. Virology, 2008; 376:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bradburne AF, Bynoe ML, Tyrrell DAJ. Effects of a “new” human respiratory virus in volunteers. British Medical Journal, 1967; 3:767–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taguchi F, Aiuchi M, Fujiwara K. Age‐dependent response of mice to a mouse hepatitis virus, MHV‐S. Japanese Journal of Experimental Medicine, 1977; 47(2):109–115. [PubMed] [Google Scholar]
- 14. Hirano N, Takenaka S, Fujiwara K. Pathogenicity of mouse hepatitis virus for mice depending upon host age and route of infection. Japanese Journal of Experimental Medicine, 1975; 45(4):285–292. [PubMed] [Google Scholar]
- 15. Hirano N, Nomura R, Tawara T, Tohyama K. Neurotropisim of swine haemagglutinating encephalomyelitis virus (coronavirus) in mice depending upon host age and route of infection. Journal of Comparative Pathology, 2004; 130:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirano N, Haga S, Sada Y. Tohyama K. Susceptibility of rats of different ages to inoculation with swine haemagglutinating encephalomyelitis virus (a coronavirus) by various routes. Journal of Comparative Pathology, 2001; 125:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uenaka T, Kishimoto I, Uemura T, Ito T, Otsuki K. Cloacal inoculation with the Connecticut strain of avian infectious brouchitis virus: An attempt to produce nephropathogenic virus by in vivo passage using cloacal inoculation. Journal of Veterinary Medical Science, 1998; 60(4):495–502. [DOI] [PubMed] [Google Scholar]
- 18. Haas CN, Rose JB, Gerba CP. Quantitative Microbial Risk Assessment. New York : John Wiley and Sons, 1999. [Google Scholar]
- 19. R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna , Austria . Available at: http://www.R-project.org, Accessed on August 6, 2008. [Google Scholar]
- 20. Department of Health, Hong Kong . Outbreak of severe acute respiratory syndrome (SARS) at Amoy Gardens, Kowloon Bay, Hong Kong, main findings of the investigation. April 17, 2003. Available at: http://www.info.gov.hk/info/sars/pdf/amoy_e.pdf, Accessed on June 6, 2008.
- 21. Li Y, Duan S, Yu TS, Wong TW. Multi‐zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air, 2004; 15:96–111. [DOI] [PubMed] [Google Scholar]
- 22. McKinney LR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. Journal of Environmental Health, 2006; 68(9):26–30. [PubMed] [Google Scholar]
- 23. Yu ITS, Li Y, Wong TS, Tam W, Chan AT, Lee JHW, Leung DYC, Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New England Journal of Medicine, 2004; 350:1731–1739. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt OW, Cooney MK, Kenny GE. Plaque assay and improved yield of human coronaviruses in a human rhabdomyosarcoma cell line. Journal of Clinical Microbiology, 1979; 9(6):722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhong NS, Wong GWK. Epidemiology of severe acute respiratory syndrome (SARS): Adults and children. Paediatric Respiratory Reviews, 2004; 5:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu CM, Poon LLM, Cheng VCC, Chan KS, Hung IFN, Wong MML, Chan KH, Leung WS, Tang BSF, Chan VL, Ng WL, Sim TC, Ng PW, Law KI, Tse DMW, Peiris JSM, Yuen KY. Initial viral load and the outcomes of SARS. CMAJ, 2004; 171(11):1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goicoechea MG. Human H5N1 influenza. New England Journal of Medicine, 2007; 356(13):1375. [DOI] [PubMed] [Google Scholar]
- 28. Prince GA, Porter DD. The pathogenesis of respiratory syncytial virus infection in infant ferrets. American Journal of Pathology, 1976; 82:339–352. [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, Herman BD, Sheahan T, Heise M, Genrich GL, Zaki SR, Baric R, Subbarao K. A mouse‐adapted SARS‐coronavirus causes disease and mortality in BALB/c mice. PLoS Pathogens, 2007; 3(1):23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagata N, Iwata N, Hasegawa H, Sato Y, Morikawa S, Saijo M, Itamura S, Saito T, Ami Y, Odagiri T, Tashiro M, Sata T. Pathology and virus dispersion in cynomolgus monkeys experimentally infected with severe acute respiratory syndrome coronavirus via different inoculation routes. International Journal of Experimental Pathology, 2007; 88:403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, Van Amerongen G, Van Riel D, Laman JD, De Jong T, Van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, Van Der Werf S, Escriou N, Manuguerra JC, Stohr K, Peiris JS, Osterhaus AD. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet, 2003; 362:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McAuliffie J, Vogel L, Robert A, Fahle G, Fischer S, Shieh WJ, Butler E, Zaki S, St Claire M, Murphy B, Subbarao K. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology, 2004; 330(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 2008; 294:L387–L398. [DOI] [PubMed] [Google Scholar]
- 34. Bartrand TA, Weir MH, Haas CN. Dose‐response models for inhalation of Bacillus anthracis spores: Interspecies comparisons. Risk Analysis, 2008; 28(4):1115–1124. [DOI] [PubMed] [Google Scholar]
- 35. Armstrong TW, Haas CN. Quantitative microbial risk assessment model for Legionnaires’ disease: Animal model selection and dose‐response modeling. Risk Analysis, 2007; 27(6):1581–1596. [DOI] [PubMed] [Google Scholar]
- 36. Cheng PKC, Wong DA, Tong LKL, Ip SM, Lo ACT, Lau CSL, Yeung EYH, Lim WWL. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet, 2004; 363:1699–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poon LLM. SARS and other coronaviruses in humans and animals. In Perlman S, Holmes KV. (eds). The Nidoviruses: Toward Control of SARS and Other Nidovirus Diseases. New York : Springer, 2006. [Google Scholar]


