Abstract
A 15‐month‐old spayed female ferret (Mustela putorius furo) presented for lethargy and weight loss of 2 weeks duration. Upon physical examination, a 2‐mm‐diameter focal area of opacity was noted in the left cornea. In addition, the ferret was quiet, in poor body condition, and dehydrated. A complete blood count and plasma biochemistry revealed a severe nonregenerative anemia, azotemia, hyperproteinemia, hypoalbuminemia, and mild hyperphosphatemia and hyperchloremia. Urinalysis revealed hyposthenuria. Whole body radiographs showed multifocal thoracic nodular disease, splenomegaly, and renomegaly. Abdominal ultrasonography confirmed bilaterally enlarged kidneys, hypoechoic liver and spleen, and a caudal abdominal hypoechoic mobile nodule. The ferret was humanely euthanized, and a postmortem examination with subsequent histopathology showed multifocal necrotizing pyogranulomas in the lung, spleen, kidneys, mesenteric lymph nodes, and serosa of the duodenum. Pyogranulomatous panophthalmitis was diagnosed in the left eye. The multisystemic granulomatous lesions were suggestive of ferret systemic coronavirus (FRSCV). The presence of coronavirus in the left eye was confirmed by positive immunohistochemistry. Reverse transcriptase polymerase chain reaction (RT‐PCR) on formalin fixed paraffin embedded tissue from the lung, spleen, and kidney was negative for FRSCV and positive for ferret enteric coronavirus (FRECV). Systemic coronavirus disease in ferrets closely resembles feline infectious peritonitis (FIP) in domestic cats, which can manifest with anterior uveitis, chorioretinitis, optic neuritis, and retinal detachment. To the authors’ knowledge, this is the first report of ocular lesions in a ferret with systemic coronavirus disease, suggesting that ferrets presented with similar ocular lesions should also be evaluated for evidence of coronavirus infection.
Keywords: coronavirus, ferret, Mustela putorius furo, panophthalmitis, pyogranulomatous, systemic
Introduction
Ferret systemic coronavirus (FRSCV) disease is an emerging condition in domestic ferrets, which was first reported in 2004. 1 Since then, additional cases have been reported in the United States, Europe, and Japan.2, 3, 4, 5 Clinical presentation and pathology in FRSCV disease closely resemble that in the noneffusive (dry) form of feline infectious peritonitis (FIP) in domestic cats, which is caused by a group 1 feline coronavirus (FCoV).1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Ferrets with systemic coronavirus disease typically display nonspecific clinical signs, including lethargy, vomiting, diarrhea, hyporexia or anorexia, weight loss, and loss of body condition.2, 8, 9, 10 Physical examination may identify intra‐abdominal masses, renomegaly, and splenomegaly.2, 8, 9, 10 Signs of central nervous system involvement include acute or progressive hind limb paresis, ataxia, seizures, opisthotonus, abnormal gait, and propioceptive deficits.2, 8, 9 Frequently noted hematologic changes include nonregenerative anemia, elevated globulins, decreased albumin, and thrombocytopenia.2, 8, 9, 10 Urinalysis abnormalities may include green urine, proteinuria, blood, and rare bilirubin crystals.8 Significant radiographic signs in ferrets with FRSCV include loss of lumbar musculature, decreased peritoneal detail, presence of mid‐abdominal soft tissue masses, and splenomegaly.11 The majority of ferret cases with systemic coronavirus disease were noted to have ultrasonographic evidence of peritonitis, abdominal lymphadenopathy, splenomegaly, abdominal soft tissue masses, nephromegaly, and changes in the renal cortex echogenicity.11
Gross lesions observed in FRSCV disease consist of multifocal to coalescing, white to tan, irregular nodules or plaques ranging from 0.5 to 2 cm diameter dispersed over serosal surfaces.2, 8, 9 The peritoneum, particularly the intestinal serosa and mesentery, is most commonly affected.2, 8, 9 Nodules can also be found on the surface or extending into the parenchyma of other organs; with the liver, kidneys, spleen, and lung most commonly affected.2, 8, 9 The mesenteric lymph nodes are also involved in most cases.2, 8, 9 Histopathologic examination of affected tissues is the gold standard to diagnose FRSCV.11 Histopathologic lesions are characterized by widespread pyogranulomatous perivasculitis and peritonitis, nearly identical to the noneffusive (dry) form of FIP.2, 8, 9, 10 Immunohistochemical (IHC) analysis using anti‐FCoV monoclonal antibody provides a definitive diagnosis by identifying positive staining for group 1 coronavirus antigen in foci of pyogranulomatous inflammation from ferrets with suspected coronavirus disease.2, 8, 9, 12 Reverse transcriptase polymerase chain reaction (RT‐PCR) assays have been developed and have the potential to differentiate genotypes of ferret coronavirus.6, 7
Ocular lesions, including anterior uveitis and chorioretinitis, are frequently reported in domestic cats with FIP.13, 14, 15, 16, 17, 18 To date, there have been no ocular lesions or clinical signs identified in ferrets with systemic coronavirus disease.2, 8, 9 To the authors’ knowledge, this is the first reported case of both clinical and histopathologic ocular lesions in a case of FRSCV disease.
Case Description
A 15‐month‐old spayed female ferret (Mustela putorius furo) presented to the Exotic and Zoological Medicine Service at Kansas State University's Veterinary Health Center for lethargy and weight loss of approximately 2 weeks duration. The ferret also had a history of a prolapsed rectum 1 month prior to presentation, which was treated and resolved with a temporary moist food diet prescribed by the animal's primary care veterinarian. The patient was housed with another apparently healthy ferret in a cage and allowed supervised exercise outside of the enclosure. There was a history of incomplete vaccination status, with only a single distemper and rabies vaccination administered as a juvenile. Diet consisted of ad libitum commercial ferret diet.
Upon physical examination under manual restraint, the ferret was quiet, dull, and responsive. The ferret was in thin body condition (2/5) and was estimated to be 5–7% dehydrated based on skin turgor. An ophthalmic examination was performed, including slit‐lamp biomicroscopy (Kowa SL‐15, Kowa Company Ltd., Tokyo, Japan), corneal application of fluorescein stain (BioGlo®; HUB Pharmaceuticals LLC, Rancho Cucamonga, CA, USA), and indirect ophthalmoscopy (Keeler Instruments Inc., Broomall, PA, USA). The ophthalmic examination of the left eye revealed mild ocular discharge, mild to moderate conjunctival hyperemia, and an approximately 2‐mm‐diameter yellow stromal opacity in the dorsolateral peripheral cornea that was confluent with the limbus (Fig. 1). There was superficial vascularization of the cornea in the area of the corneal opacity and multifocal areas of posterior synechia and dyscoria present. There was no aqueous flare present. The corneal lesion was consistent with a focal corneal abscess or neoplasia. The examination of the fundus in both eyes was unremarkable. Fluorescein staining was negative in both eyes. No significant lesions were noted in the right eye.
Figure 1.
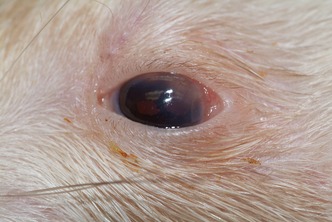
Photograph of the left eye of a 15‐month‐old female spayed ferret at the time of presentation for lethargy, weight loss, and pruritus. Ophthalmic examination revealed a focal area of yellow opacity approximately 2 mm in diameter in the dorsolateral peripheral cornea. The abnormal area of the cornea had superficial vascularization, and fluorescein staining was negative. There was also mild to moderate conjunctival hyperemia present, as well as multifocal areas of posterior synechia and dyscoria.
The ferret was sedated for diagnostic testing with midazolam (0.25 mg/kg IM; Caraco Pharmaceutical Laboratories Ltd., Detroit, MI, USA) and butorphanol (0.25 mg/kg IM; Torbugesic®; Fort Dodge Animal Health, Fort Dodge, IA, USA). A blood sample was collected from the cranial vena cava and submitted for a complete blood count (CBC) and plasma biochemistry. The CBC revealed a severe nonregenerative anemia (PCV 14%; reference interval19 40–70%). The platelet count was within normal limits (764 000/μL; reference interval19 171 700–1 280 600/μL); however, review of the blood smear supported a possible thrombocytosis based on the presence of clumping. The plasma biochemistry showed an azotemia with increased blood urea nitrogen (100 mg/dL; reference interval19 2.8–47 mg/dL) and creatinine (1.0 mg/dL; reference interval19 0.3–0.9 mg/dL) concentrations, as well as increased phosphorus (10.7 mg/dL; reference interval19 3.1–9.6 mg/dL) concentration, suggestive of renal insufficiency. Mildly elevated chloride (126 mmol/L; reference interval19 108–119.9 mmol/L), elevated total protein (9.4 g/dL; reference interval19 5.5–7.8 g/dL), decreased albumin (1.9 g/dL; reference interval19 2.8–4.4 g/dL), and elevated globulin (7.5 g/dL; reference interval19 2.67–3.4 g/dL) concentrations were also noted. A urine sample was collected by ultrasound‐guided cystocentesis, and urinalysis was performed, revealing hyposthenuria (1.014; reference interval19 1.042 ± 8) in the face of azotemia, confirming renal insufficiency. A urine dipstick (urinalysis reagent strip, Multistix® 10 SG; Siemens Healthcare Diagnostics, Tarrytown, NY, USA) showed an abnormal amount of blood (3+) and protein (3+) in the urine sample.20
Two view whole body radiographs revealed a triangular soft tissue opacity in the left mid‐thorax, a 7‐mm‐round soft tissue opacity in the right caudal thorax, and an ill‐defined soft tissue opacity in the right cranial thorax. Poor peritoneal detail was present. The kidneys were bilaterally enlarged, measuring 3× the length of the L2 vertebral body on the ventrodorsal view. The spleen also appeared enlarged on these images. Abdominal ultrasonography revealed a hypoechoic liver and an enlarged hypoechoic spleen with irregular margination. The kidneys were bilaterally enlarged with poor corticomedullary definition and fine hyperechoic striations throughout the cortices. Multiple abdominal lymph nodes were visible, measuring up to 0.6 cm in thickness. Lastly, a single, irregular hypoechoic mobile structure was visible in the caudal abdomen, measuring 0.7 × 1.3 cm.
Due to the guarded prognosis associated with combined renal compromise, severe anemia, high suspicion of neoplasia, and the patient's declining quality of life, the owner elected for humane euthanasia. The ferret was euthanized with pentobarbital (1 ml IV; Fatal Plus®; Vortech Pharmaceuticals, Ltd., Dearborn, MI, USA) and the remains submitted for necropsy.
Postmortem examination was performed and revealed focal, firm, nodular to slightly raised plaque‐like white to gray areas in the right and left cranial lung lobes and the right dorsal lung lobe, measuring up to 1 cm diameter. The kidneys were diffusely pale, and the cortical surfaces were irregular with multifocal to coalescing firm white nodules, which extended into the renal cortex on cut section and compressed the underlying medulla. The spleen was diffusely congested and contained multifocal to coalescing pinpoint to 0.4 cm diameter flat white foci, which extended into the parenchyma on cut section (Fig. 2). The mid‐abdominal region contained a 2 × 1.2 × 1 cm enlarged pale tan lymph node that was 1.6 cm caudal to the left adrenal gland. On cut section, the node was diffusely pale tan to white with coalescing nodular foci. The left cornea had a dorsolateral 2‐mm‐diameter focal area of opacity.
Figure 2.
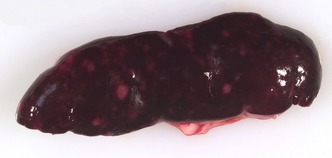
Photograph of the spleen from a 15‐month‐old female spayed ferret at gross necropsy. Note the diffuse congestion with multifocal to coalescing white pinpoint to 4 mm foci.
Histopathologic evaluation revealed granulomatous inflammation in the lungs, spleen, kidneys, mesenteric lymph nodes, duodenum, and pancreas (Fig. 3). Pyogranulomatous panophthalmitis was diagnosed in the left eye (Fig. 4). There was also pigment adhered to the anterior portion of the lens capsule, consistent with posterior synechia. Sheets of epithelioid macrophages admixed with degenerate neutrophils, which were surrounded by lymphocytes and plasma cells, expanded the ciliary body. The lesion extended into the iris and a focal area of the retina. The corneal stroma contained a similar inflammatory infiltrate, forming a pyogranulomatous nodule. There were no histopathologic lesions observed in the right eye. Gross and microscopic lesions in the affected eye were determined to be compatible with ferret systemic coronavirus disease. Subsequent coronavirus IHC using anti‐FCoV monoclonal antibody showed positive results within the affected eye (Fig. 5), lung (Fig. 3, inset), spleen, and kidney. After coronavirus IHC returned positive, formalin fixed paraffin embedded scrolls from lung, spleen, and kidney were submitted for ferret coronavirus RT‐PCR (Michigan State University, Diagnostic Center for Population and Animal Health, Lansing, MI, USA). These paraffin embedded fixed tissues were positive for ferret enteric coronavirus (FRECV).
Figure 3.
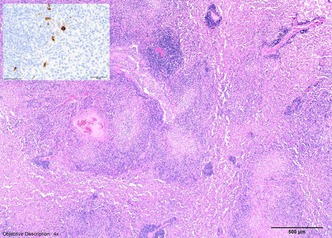
Lung, H&E. Severe pyogranulomatous infiltrates were noted within the lung parenchyma, which occasionally stained positively for coronavirus antigen using anti‐FCoV monoclonal antibody (inset). Inset: Feline Infectious Peritonitis virus immunohistochemistry.
Figure 4.
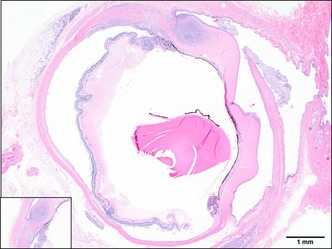
Left eye, H&E. Pyogranulomatous infiltrates within the cornea, sclera, iris, ciliary body, choroid, and retina. Inset: 4× of anterior uveitis and keratitis.
Figure 5.
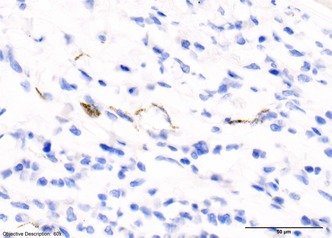
Left eye, Feline Infectious Peritonitis virus immunohistochemistry. The left eye stained positively for coronavirus antigen using anti‐FCoV monoclonal antibody.
Discussion
This is the first report of systemic coronavirus disease in a ferret with ocular lesions. Pyogranulomatous panophthalmitis was diagnosed in this ferret via histopathology. Uveitis is not frequently reported in ferrets.21, 22 When uveitis is reported, it is most commonly associated with ocular trauma or corneal ulceration.21, 22 One important exception is Aleutian parvoviral disease, which has been shown to cause uveitis in mink which were experimentally infected with the virus.21, 22 Other systemic diseases have been hypothesized to cause uveitis in ferrets, such as fungal disease, toxoplasmosis, and neoplasia.21, 22 Cryptococcus has been reported as a cause of bilateral exudative chorioretinitis in a ferret with acute onset blindness.23 This emphasizes the importance of diagnostic testing for systemic disease when anterior uveitis is noted without a primary ocular cause.21, 22 Keratitis consisting of a pyogranulomatous nodule and corneal vascularization was also noted in the present case. Nonulcerative keratitis in ferrets has been previously reported to be the result of immune mediated disease22 and multicentric lymphoma.21, 22
Given the absence of previously documented ocular lesions in cases of FRSCV and the similarities between FIP and FRSCV‐associated disease, a clinical comparison can be made to ocular abnormalities identified in cats with FIP. Domestic cats with the FIP frequently have ocular lesions.13, 14, 15, 16, 17, 18 Ocular manifestation of disease is more common in the noneffusive form of FIP.16 The kidneys were most often affected, followed by the brain and eyes.14 Furthermore, ocular signs (such as third‐eyelid protrusion, blepharospasm, or epiphora) may be the presenting complaint in some cats; and it is not uncommon for cats to present with ocular signs unaccompanied by signs of systemic disease.16 The most common ocular lesions described in cats with FIP include bilateral granulomatous anterior uveitis and chorioretinitis.13, 15, 16, 17, 18 It has been noted that the degree of inflammation is most severe in the uveal tracts, in which vasculitis and melanin accumulation were also present.15 Inflammation associated with ocular FIP can gradually progress to panophthalmitis or panuveitis with diffuse and severe corneal edema, marked anterior uveitis, marked vitritis, chorioretinitis, retinal detachments, or optic neuritis.16, 17 Anterior uveitis is usually more noticeable to the clinician16 and can manifest as aqueous flare, keratic precipitates, fibrinous exudate in the anterior chamber, hyphema, and color change of the iris.13, 15, 16, 17, 18 Posterior segment changes can include perivascular cuffing, granulomatous inflammation, chorioretinitis, optic neuritis, retinal hemorrhages, and retinal detachment.13, 16, 17 Although granulomatous inflammation is the characteristic of FIP, it is important to note that these lesions are not pathognomonic and similar changes can be seen in other systemic infectious diseases in cats.13
The ferret in this case presented with nonspecific clinical signs and a corneal opacity consistent with a focal abscess or neoplasia. A complete clinical and postmortem evaluation identified nonregenerative anemia, elevated total protein, decreased albumin, evidence of renal insufficiency, bilateral nephromegaly, the presence of thoracic and abdominal nodules, abdominal lymphadenopathy, and widespread pyogranulomatous inflammation. The array of abnormalities demonstrated in the present ferret case was strongly suggestive of FRSCV disease,2, 8, 9, 10 mainly due to the pyogranulomatous inflammation noted on histopathologic examination. Coronavirus was confirmed in the affected eye and systemic lesions by a positive IHC result, providing a definitive diagnosis of systemic coronavirus disease. Reverse transcriptase PCR was retrospectively submitted on formalin fixed paraffin embedded tissue, returning with a negative result for FRSCV and weak positive result for FRECV. Currently, it is unclear whether FRSCV is derived from FRECV by in vivo mutation, is a cocirculating distinct strain, or has originated from recombination between FRECV and another group 1 coronavirus.2, 6, 7, 11 The negative PCR result for FRSCV obtained in this case may simply be a false negative, resulting from the use of paraffin embedded fixed tissue samples. The addition of RT‐PCR on a more appropriate unfixed tissue sample may have been helpful in this case for further evaluation of the coronavirus genotype present; however, fresh tissue samples were unavailable.
This case illustrates that systemic coronavirus disease should be considered as a differential for anterior uveitis in ferrets, similar to FIP in domestic cats. A complete clinical evaluation should be performed in a ferret presenting with nonspecific clinical signs of systemic disease and evidence of anterior uveitis, chorioretinitis, or keratitis noted on physical examination.
References
- 1. Martinez J, Ramis AJ, Reinacher M et al Detection of feline infectious peritonitis virus‐like antigen in ferrets. The Veterinary Record 2006; 158: 523. [DOI] [PubMed] [Google Scholar]
- 2. Garner MM, Ramsell K, Morera N et al Clinicopathologic features of a systemic coronavirus‐associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius) . Veterinary Pathology 2008; 45: 236–246. [DOI] [PubMed] [Google Scholar]
- 3. Michimae Y, Mikami S, Okimoto K et al The first case of feline infectious peritonitis‐like pyogranuloma in a ferret infected by coronavirus in Japan. Journal of Toxicologic Pathology 2010; 23: 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Graham E, Lamm CL, Denk D et al Systemic coronavirus‐ associated disease resembling feline infectious peritonitis in ferrets in the UK. The Veterinary Record 2012; 171: 200. [DOI] [PubMed] [Google Scholar]
- 5. Shigemoto J, Muraoka Y, Wise AG et al Two cases of systemic coronavirus‐associated disease resembling feline infectious peritonitis in domestic ferrets in Japan. Journal of Exotic Pet Medicine 2014; 23: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wise AG, Kiupel M, Garner MM et al Comparative sequence analysis of the distal one‐third of the genomes of a systemic and an enteric ferret coronavirus. Virus Research 2010; 149: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Terada Y, Minami S, Noguchi K et al Genetic characterization of coronaviruses from domestic ferrets, Japan. Emerging Infectious Diseases 2014; 20: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray J, Kiupel M, Maes RK. Ferret coronavirus‐associated diseases. The Veterinary Clinics of North America. Exotic Animal Practice 2010; 13: 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiupel M, Perpiñán D. Viral diseases of ferrets In: Biology and Diseases of the Ferret, 3rd edn (eds Fox JG, Marini RP.) Wiley Blackwell, Danvers, 2014; 450–461. [Google Scholar]
- 10. Hoefer HL, Fox JG, Bell JA. Gastrointestinal diseases In: Ferrets, Rabbits, and Rodents Clinical Medicine and Surgery, 3rd edn (eds Quesenberry KE, Carpenter JW.) Elsevier Saunders, St. Louis, 2012; 27–45. [Google Scholar]
- 11. Dominguez E, Novellas R, Moya A et al Abdominal radiographic and ultrasonographic findings in ferrets (Mustela putorius furo) with systemic coronavirus infection. The Veterinary Record 2011; 169: 231–238. [DOI] [PubMed] [Google Scholar]
- 12. Martínez J, Reinacher M, Perpiñán D et al Identification of group 1 coronavirus antigen in multisystemic granulomatous lesions in ferrets (Mustela putorius furo) . Journal of Comparative Pathology 2008; 138: 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann K. Feline infectious peritonitis. The Veterinary Clinics of North America. Small Animal Practice 2005; 35: 39–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kipar A, Meli ML. Feline infectious peritonitis: still an enigma? Veterinary Pathology 2014; 52: 505–526. [DOI] [PubMed] [Google Scholar]
- 15. Montali RJ, Strandberg JD. Extraperitoneal lesions in feline infectious peritonitis. Veterinary Pathology 1972; 9: 109–121. [DOI] [PubMed] [Google Scholar]
- 16. Andrew SE. Feline infectious peritonitis In: Veterinary Clinics of North America: Small Animal Practice Infectious Disease and the Eye, Vol. 30 (ed. Stiles J.) WB Saunders, Philadelphia, 2000: 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Townsend WM. Canine and feline uveitis. The Veterinary Clinics of North America. Small Animal Practice 2008; 38: 323–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aroch I, Ofri R, Sutton GA. Ocular manifestations of systemic diseases In: Slatter's Fundamentals of Veterinary Ophthalmology, 5th edn (eds Maggs DJ, Miller PE, Ofri R.) Elsevier Saunders, St. Louis, 2013; 395–436. [Google Scholar]
- 19. Hein J, Spreyer F, Sauter‐Louis C et al Reference ranges for laboratory parameters in ferrets. The Veterinary Record 2012; 171: 218. [DOI] [PubMed] [Google Scholar]
- 20. Eshar D, Wyre NR, Brown DC. Urine specific gravity values in clinically healthy young pet ferrets (Mustela furo). Journal of Small Animal Practice 2012; 53: 115–119. [DOI] [PubMed] [Google Scholar]
- 21. Good KL. Ocular disorders of pet ferrets. The Veterinary Clinics of North America. Exotic Animal Practice 2002; 5: 325–339. [DOI] [PubMed] [Google Scholar]
- 22. Williams DL. The ferret eye Ophthalmology of Exotic Pets, 1st edn Wiley‐Blackwell, Oxford, 2012; 73–85. [Google Scholar]
- 23. Fox JG. Other systemic diseases In: Biology and Diseases of the Ferret, 3rd edn (eds Fox JG, Marini RP.) Wiley Blackwell, Danvers, 2014; 421–438. [Google Scholar]


