Abstract
Background
Little information is available on the ciclosporin dose‐tapering regimen and clinical response in the treatment of feline hypersensitivity dermatitis.
Hypothesis/Objectives
To test a dose‐tapering regimen and assess efficacy and clinical safety for up to 18 weeks.
Animals
Eighty‐eight client‐owned cats with feline hypersensitivity dermatitis.
Methods
Cats that received either a placebo or ciclosporin at 2.5 mg/kg or 7 mg/kg daily for 6 weeks were given 7 mg/kg ciclosporin daily for 4 weeks. Depending on the clinical response, the dose was tapered from daily to every other day over the next 4 weeks and further to twice a week for an additional 4 weeks.
Results
After all cats received 7 mg/kg for 4 weeks, the dose could be tapered to every other day for the next 4 weeks in 70% of cats remaining in the study. During the next 4 weeks, 57, 15 and 22% of cats remaining in the study could be treated at twice a week, every other day or daily, respectively. After the first 4 weeks, the mean lesion score and owner‐assessed pruritus improved over baseline by 69 and 61%, respectively, and remained stable during the following 8 weeks. Approximately 65% of the cats in the study were reported to have an adverse event (AE), very often mild and resolving spontaneously. The most frequent AEs were gastrointestinal and included primarily vomiting and diarrhoea. Eighty per cent of AEs occurred when cats were on daily treatment.
Conclusions and clinical importance
Results suggest that the induction dose of 7 mg/kg ciclosporin can be tapered as soon as 4 weeks without deterioration of the clinical response. Establishment of the lowest effective dosing regimen of ciclosporin reduced the frequency of AEs.
Résumé
Contexte
Peu d'information est disponible sur la dose dégressive d'entretien de ciclosporine et sa réponse clinique dans le traitement des dermatites félines par hypersensibilité.
Hypothèses/Objectifs
Tester la dose dégressive et évaluer l'efficacité et l'innocuité clinique jusqu'à 18 semaines.
Sujets
Quatre‐vingt‐huit chats de propriétaires atteints de dermatite féline par hypersensibilité.
Méthodes
Les chats qui avaient reçu soit un placebo soit de la ciclosporine à la dose de 2.5 mg/kg ou 7 mg/kg chaque jour pendant 6 semaines, ont reçu 7 mg/kg de ciclosporine par jour pendant 4 semaines. En fonction de la réponse clinique, la posologie était ensuite diminuée sur 4 semaines de tous les jours à un jour sur deux et ensuite à deux fois par semaine sur les 4 semaines suivantes.
Résultats
Après que tous les chats aient reçu 7 mg/kg pendant 4 semaines, la dose a pu être baissée à un jour sur deux pendant les 4 semaines suivantes pour 70% des chats restants dans l'étude. Au cours des 4 semaines suivantes, 57, 15 et 22% des chats restants dans l'étude ont pu être traités respectivement deux fois par semaine, un jour sur deux ou chaque jour. Après les 4 premières semaines, le score lésionnel moyen et le prurit évalué par les propriétaires se sont améliorés de 69 et 61% respectivement, et sont restés stables pendant les 8 semaines suivantes. Approximativement 65% des chats de l'étude ont montrés un effet indésirable (AE), très souvent modéré et se résolvant spontanément. Les AE les plus fréquents étaient gastro‐intestinaux et comprenaient principalement vomissement et diarrhée. Quatre‐vingt pourcents des AEs se produisaient pour les chats recevant le traitement quotidiennement.
Conclusions et importance clinique
Les résultats suggèrent que la dose d'induction de 7 mg/kg de ciclosporine peut être diminuée dès 4 semaines sans détérioration de la réponse clinique. La mise en place de la dose minimale efficace de ciclosporine réduit la fréquence des AEs.
Resumen
Introducción
se tiene poca información acerca del régimen de reducción progresiva de dosis en el tratamiento con ciclosporina, así como acerca de la respuesta clínica en casos de dermatitis felina por hipersensibilidad.
Hipótesis/objetivos
probar un régimen de reducción de la dosis de ciclosporina y evaluar la eficacia y seguridad clínicas durante 18 semanas.
Animales
ochenta y ocho gatos de propietarios particulares con dermatitis por hipersensibilidad.
Métodos
los gatos que recibieron bien placebo o ciclosporina a dosis de 2.5 mg/kg o de 7 mg/kg diariamente durante 6 semanas, recibieron después 7 mg/kg diarios de ciclosporina durante 4 semanas. Dependiendo de la respuesta clínica, la dosis se redujo a aplicación diaria o aplicación en días alternos durante las cuatro semana siguientes y después a dos veces por semana durante otras cuatro semanas.
Resultados
después de recibir una dosis de 7 mg/kg al día durante cuatro semanas, la dosis pudo disminuirse a días alternos durante cuatro semanas en un 70% de los gatos que permanecían en el estudio. Durante las cuatro semanas siguientes, 57, 15 y 22% de los gatos que aún permanecían en el estudio pudieron tratarse dos veces en semana, en días alternos o a diario, respectivamente. Tras las primeras cuatro semanas, el valor medio de las lesiones y el valor de prurito asignado por el propietario mejoraron sobre el valor inicial en un 69 y un 61% de los casos, respectivamente, y permanecieron estables durante las semanas siguientes. Alrededor de un 65% de los gatos en el estudio presentaron efectos adversos (AE), con frecuencia leves y de resolución espontánea. Los AEs mas frecuentes fueron gastrointestinales e incluyeron primariamente vómitos y diarrea. Un 80% de los AEs se produjeron cuando los animales estaban en tratamiento diario.
Conclusiones e importancia clínica
los resultados sugieren que la dosis de inducción de 7 mg/kg de ciclosporina puede ser reducida a las cuatro semanas sin un deterioro de la respuesta clínica. El establecimiento de un régimen de dosificación efectiva a un nivel mas bajo redujo la frecuencia de AEs.
Zusammenfassung
Hintergrund
Es gibt nur wenig Information über das Reduzierungsschema für Ciclosporin und die klinische Verbesserung bei der Behandlung der felinen Dermatitis, die durch eine Hypersensibilität bedingt ist.
Hypothese /Ziele
Ein Reduzierungsschema und die Wirksamkeit sowie die klinische Sicherheit für bis zu 18 Wochen zu untersuchen.
Tiere
Achtundachtzig Katzen aus Privathaushalten mit feliner Dermatitis durch Hypersensibilität wurden in die Studie aufgenommen.
Methoden
Katzen, die entweder Plazebo oder Ciclosporin bei einer Dosis von 2,5 mg/kg oder 7 mg/kg 6 Wochen lang täglich erhalten hatten, bekamen 7mg/kg Ciclosporin täglich für 4 Wochen. Je nach klinischer Verbesserung wurde die Dosis über die nächsten vier Wochen von täglich auf jeden zweiten Tag und weiters auf zweimal pro Woche für weitere 4 Wochen reduziert.
Ergebnisse
Nachdem alle Katzen 7 mg/kg für 4 Wochen erhalten hatten, konnte diese Dosis in den nächsten vier Wochen bei 70% der Katzen, die in der Studie verblieben, auf jeden zweiten Tag reduziert werden. Während der nächsten 4 Wochen, blieben 57, 15 bzw 22% der Katzen in der Studie und wurden zweimal wöchentlich, jeden zweiten Tag bzw täglich behandelt. Nach den ersten 4 Wochen verbesserte sich die durchschnittliche Bewertung der Veränderungen und der durch die BesitzerInnen beurteilte Juckreiz um 69 bzw 61%, ausgehend vom Basiswert, und blieb dann während der nächsten 8 Wochen stabil. Bei ungefähr 65% der Katzen in dieser Studie wurde eine Nebenwirkung (AE) beschrieben, die oft milder Natur war und sich sponan wieder gab. Die häufigsten AEs waren gastrointestinaler Natur und bestanden aus Erbrechen und Durchfall. Achtzig Prozent der AEs traten auf, wenn die Katzen täglich behandelt wurden.
Schlussfolgerungen und klinische Bedeutung
Die Ergebnisse lassen darauf schließen, dass die Induktionsdosis von 7 mg/kg Ciclosporin schon nach 4 Wochen ohne eine Verschlechterung des klinischen Zustandes reduziert werden kann. Eine Festlegung der niedrigsten wirksamen Dosis für Ciclosporin reduzierte die Häufigkeit der AEs.
Abstract

Abstract

Introduction
Ciclosporin has been used for many years in the treatment of feline hypersensitivity dermatitis at various doses, generally ranging from 5 to 10 mg/kg.1, 2, 3, 4 The dose of 7 mg/kg given daily has recently been tested in a randomized, blinded parallel group, placebo‐controlled study and was shown to be efficacious and well tolerated.5 In cats with head and neck excoriations, self‐induced alopecia, eosinophilic plaque and/or miliary dermatitis, the administration of 7 mg/kg ciclosporin daily for 6 weeks resulted in a decrease in total lesion and owner‐assessed pruritus severity scores. In cats receiving the 7 mg/kg daily dose, the most common adverse events were mild gastrointestinal disorders. That dose‐determination study was aimed at establishing an efficacious and safe dose. Dosing duration was limited to 6 weeks and did not address the long‐term use of ciclosporin to treat feline hypersensitivity dermatitis.
In cats, limited information is available about long‐term efficacy and safety of ciclosporin. Studies suggest that feline hypersensitivity dermatitis can be well controlled with dose tapering;2, 6 however, the frequency and response to dose tapering has not been documented in cats. The present study was designed to assess the clinical response to a dose‐tapering regimen and the tolerability to ciclosporin administered for up to 18 weeks.
Materials and methods
Study design
The study was conducted in two phases. The first phase established 7 mg/kg as an effective dose of ciclosporin for the treatment of hypersensitivity dermatitis.5 The present study presents the outcome of the second phase, in which a dose‐tapering regimen was examined.
Briefly, the Phase 1 study was a blinded, randomized, parallel group, placebo‐controlled study, in which ciclosporin dosed orally at 2.5 or 7 mg/kg administered daily for 6 weeks was compared with a placebo control for the treatment of hypersensitivity dermatitis. The clinical response was assessed 3 and 6 weeks after the start of treatment.
The Phase 2 study explored the effect of a dose‐tapering regimen on the clinical response to ciclosporin. The open‐label study immediately followed the Phase 1 study and was not randomized nor blinded. Clinical observations were made by investigators at the inclusion visit V1 (day 0 of Phase 2; the same day as the final visit for Phase 1) and at weeks 4 (28 ± 3 days), 8 (56 ± 3 days) and 12 (84 ± 3 days).
Phase 1 and 2 studies were both multicentre, with sites located in the USA, France, the UK, Belgium and Switzerland. They were conducted in accordance with the procedures and principles of Good Clinical Practice. Cat owners signed an informed consent for their cat to be included and were free to withdraw their animals at any point.
Inclusion/exclusion criteria
Cats were included in Phase 1 after a diagnosis of hypersensitivity dermatitis was made by ruling out flea bite hypersensitivity, bacterial and fungal infections. Food allergies were also evaluated for case exclusion whenever possible. Cats were evaluated for feline leukaemia virus and feline immunodeficiency virus infections and excluded if found positive. Toxoplasma serology status was assessed prior to inclusion. In the USA, cats with Toxoplasma positive serology titres were excluded. In the EU sites, no exclusion was made based on Toxoplasma serology titres.
Cats completing Phase 1, except those which exhibited an adverse event resulting in study withdrawal or where there was poor owner compliance, were eligible to participate in Phase 2. To be included in the study, cats had to be a minimum of 6 months of age and ≥2 kg body weight. Exclusion criteria included the following: breeding, pregnant or lactating cats; presence of an active systemic infection; evidence of or history of any type of malignancy; uncontrolled ectoparasite infestation, requirement for vaccination during the study; and feeding uncooked, home‐prepared diets.
Assessment of the clinical response
The assessment to determine clinical effect for dose tapering was made by comparing the initial investigator examination (baseline of Phase 1; V1P) with the overall clinical improvement at each visit [visits 2 (V2) and 3 (V3)]. Clinical improvement was assessed as follows: 0, excellent (clinical signs observed during the first examination have completely disappeared); 1, good (clear amelioration of the clinical signs compared with initial examination); 2, acceptable (clinical improvement compared with initial examination but cat has responded only slightly to treatment); 3, poor (clinical status of the cat compared with initial examination has not changed); and 4, bad (clinical status of the cat compared with initial examination has deteriorated).
The same investigator evaluated the severity of the lesions and pruritus at every visit. The lesion severity was measured using SCORFAD (SCORing Feline Allergic Dermatitis), a scoring system previously described5 and partly validated.7 The SCORFAD is a numerical rating scoring system to assess the severity of excoriations, (Ex), self‐induced alopecia (SA), miliary dermatitis (MD) and eosinophilic granuloma (Eo) in 10 body areas, resulting in a Total Lesion Score (TLS) from 0 to 16. A visual analog scale was used to determine an Owner Pruritus Score (OPS) based on the owner's observations of their cat's behaviour during the previous 3 days. A line was marked at one end of the scale to indicate that the cat ‘was comfortable, with normal grooming behaviour’ or at the other end to show that the cat ‘was uncomfortable, and was grooming all the time’. In addition, a five‐point numerical rating scale from 0 to 4 was used to determine an Investigator Pruritus Score (IPS) as previously described.5 An overall assessment of the clinical response was made by the investigator and owner at each visit of the Phase 2 study. Assessments were scored from 0 to 4 like in phase 1 at each visit beginning on day 28 until study exit (day 84 of Phase 2 if the cat completed the study).
Dosage and administration
Ciclosporin was provided by Novartis Animal Health (Basel, Switzerland) as a 100 mg/mL micro‐emulsion liquid formulation contained in a glass bottle. The dosage of ciclosporin was delivered either via a minipump device attached to the bottle that was activated manually or by using a syringe, both of which were calibrated to deliver the required volume of treatment with an accuracy of 5%. The ciclosporin was administered to the cat either on the cat's food or directly into the cat's mouth. The owner was instructed to withdraw the cat's food for a sufficient period of time where possible to encourage the cat to eat the medicated food. If the cat refused the treatment for two consecutive dose attempts when offered with food, the owner was instructed to administer the treatment directly into the cat's mouth immediately after feeding.
From inclusion to study day 28 (±3 days), all cats were administered a once daily oral dose of 7 mg/kg ciclosporin, independent from the previous treatment received in Phase 1. From day 28, dose tapering was determined according to the investigator overall assessment of clinical response in comparison to the first day of inclusion in the Phase 1 study (V1P). All animals received ciclosporin at a target dose of 7 mg/kg, but the frequency of dosing could be altered to every other day (EoD) or twice weekly (TW) as presented in Table 1. The investigator could adjust the dose between visits back to the previous dose regimen at any time if deemed necessary. If an animal was returned to the previous dose regimen between visits, the animal was evaluated at the next scheduled visit and the dose regimen maintained or adjusted according to the clinical response as described above.
Table 1.
Decision procedure for the dose‐tapering regimen
| Inclusion (V1; day 0) | Day 28 (±3 days) | Day 56 (±3 days) |
|---|---|---|
|
7 mg/kg daily, all cats |
No response: score 3 or 4, drop out | Not applicable |
| Insufficient response: score 2, 7 mg/kg daily | No response: score 3 or 4, drop out | |
| Insufficient response: score 2, administer 7 mg/kg daily | ||
| Good or excellent response: score 0 or 1, administer 7 mg/kg every other day | ||
| Good or excellent response: score 0 or 1, 7 mg/kg every other day | No response: score 3 or 4, return to 7 mg/kg daily | |
| Insufficient response: score 2, administer 7 mg/kg every other day | ||
| Good or excellent response: score 0 or 1, administer 7 mg/kg twice a week |
The clinical improvement was scored as described in the Materials and methods (section ‘Assessment of the clinical response’).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Treatment acceptance was evaluated by the owner during dose administration.
Authorized and prohibited treatments
Throughout the duration of the Phase 2 study, cats received flea adulticide treatment at regular intervals according to the manufacturer's recommendations, except at one site which was located in a geographical area considered flea free. Medications or interventions likely to have a significant effect on the efficacy parameters, such as topical or systemic glucocorticosteroids (including ophthalmic preparations), megestrol acetate, antihistamines, clomipramine, amitriptyline and fluoxetine, topical calcineurin inhibitors and allergen‐specific immunotherapy, were prohibited during the study. Essential fatty acids and shampoos were authorized only if used before study initiation and maintained throughout the study. Drugs interfering with ciclosporin, such as ketoconazole, itraconazole, erythromycin and phenobarbital, were also prohibited if given continuously for ≥7 days. Any vaccinations were to be given prior to the study start in Phase 1. Other concomitant medications or treatments such as gastrointestinal parasiticides and medications having therapeutic effects unrelated to the treated dermatological disease were allowed as long as strictly documented. In cases of severe facial/neck pruritus, at the discretion of the investigator, an Elizabethan collar could be used at the beginning of the study and for up to a maximum of 10 days thereafter.
Housing and feeding
Cats remained with their owners in their usual home for the duration of the study. Owners were instructed not to change the home management or diet of their cats during the study and to allow for the cat(s) to be fed separately from other animals in the household to ensure that nonstudy animals did not ingest the test material when administered on food. Owners were encouraged to restrict their cats from hunting and scavenging, and to offer only a commercial or cooked diet for the duration of the study.
Assessment of safety
In addition to a full physical examination and body weight determination at each visit, blood samples were collected for haematology and biochemistry at inclusion and at study end, and in cases of premature withdrawal. Adverse events (AEs) were monitored by the owner and the investigator throughout the study. An AE was considered to be any observation that was unfavourable or unintended and occurred after the administration of ciclosporin, whether or not considered to be related to treatment. A serious AE was defined as an adverse reaction which resulted in death, was life threatening, resulted in significant disability or incapacity, or which resulted in permanent or prolonged signs. A significant AE included those that required active medical intervention and were considered by the investigator to be clinically significant.
All AEs, regardless of causality, were recorded during the study.
Statistical analysis
The dose tapering was measured on the cat population present for a full period separating two visits in Phase 2. For summary statistics and mean profile plots, missing values were imputed using the last observation carried forward method in order to obtain (approximately) comparable data for those animals which had missing values. Body weight was compared after and before the study using Student's paired t‐test within each subgroup. The influence of vomiting on body weight was assessed with a repeated‐measurements ANCOVA model, including subgroup, time, subgroup × time interaction, age, baseline weight and vomiting. Haematology and clinical chemistry parameters were compared between subgroups in an ANCOVA model including the subgroup and the baseline value. All statistical calculations were performed using the software SAS®, version 9.1.3, in particular using Proc Univariate and Proc Mixed (SAS Institute, Cary, NC, USA).
Results
Cat population and demographics
Of 100 cats participating in the Phase 1 study, 88 were eligible for inclusion into Phase 2. Sixty‐five cats completed the Phase 2 study; 23 withdrew during the study as shown in Figure 1. The cat population consisted of 38 male and 50 female cats, aged from 1 to 17 years at the time of enrolment, with a mean age (±SD) of 6.7 ± 3.8 years. Mean body weight (±SD) was 5.03 ± 1.48 kg, with a range of 2.3–10.4 kg. Thirteen breeds were represented, where approximately 80% of the study animals were domestic short hair or European short hair. Ninety‐five per cent of the cats were receiving commercial food; 60% were living indoors only, while 39% were living indoor and outdoor, and only one cat was living outdoors.
Figure 1.
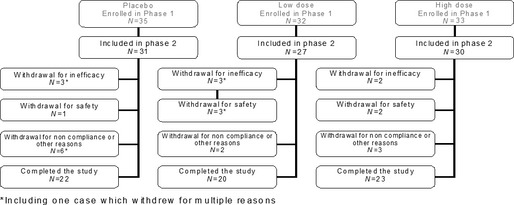
Flow chart of cases. *Including one case which withdrew for multiple reasons.
Treatment administration and dose‐tapering regimen
Ciclosporin was administered directly into the mouth in 47 cats and was taken mixed with food in 34 cases. In seven cases, mixed methods were used or the information was missing.
The dose could be tapered as shown in Table 2. After the cats received a daily dose of 7 mg/kg ciclosporin for 28 days in Phase 2, the dose was administered EoD in 50 of 71 (70%) cats remaining in the study, representing 57% of the total cat population included in the study. The interval between dose administration could be further extended to TW in 37 of 65 cats (57%) still in the study from day 56 to 84, representing 42% of the initial cat population. Only 25% of cats still in the study at V3 (18% of the initial population) remained on daily treatment at study end. In addition, the dose‐tapering regimen was similar regardless of observed lesion type at inclusion in the Phase 1 study. When considering cats still in the study between weeks 4 and 8 of Phase 2, the frequency of EoD treatment was 68, 70, 71 and 73% in cats with lesions of Eo, Ex, MD and SA, respectively. Between weeks 8 and 12, ciclosporin was administered TW in cats presenting with Eo (56%), Ex (54%), MD(55%) and SA (61%) lesions.
Table 2.
Frequency of ciclosporin administration after inclusion in Phase 2 study
| Administration frequency | V1–V2a | V2–V3a | V3–V4a |
|---|---|---|---|
| Unknown | — | 1 | — |
| Daily | 85 | 16 | 14 |
| Every other day | — | 50 | 10 |
| Twice weekly | — | — | 37 |
| Daily, then every other day | — | 1 | — |
| Every other day, then daily | — | 3 | 1 |
| Twice weekly, then daily | — | — | 1 |
| Twice weekly, then every other day | — | — | 2 |
| Withdrawn | 3 | 17 | 23 |
V1 denotes the inclusion visit of Phase 2 (day 0); V2, V3 and V4 are the visits on days 28, 56 and 84, respectively.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Response to therapy
Figure 2 shows that from inclusion in Phase 1 to week 4 in Phase 2, the mean (±SD) TLS decreased from 7.31 (±3.52) to 2.28 (±2.70), an improvement of 69% over Phase 1 baseline. During weeks 4–12 of Phase 2, the TLS score remained essentially stable. The mean (±SD) final TLS was 2.64 (±3.18), a 64% improvement (P < 0.0001) over Phase 1 baseline. The response was similar independent from the treatment received in Phase 1.
Figure 2.
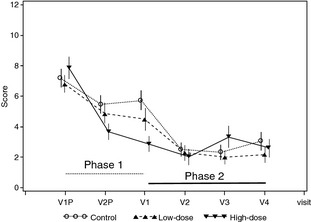
Total lesion score over time. V1P and V2P denote the inclusion visit in Phase 1 and day 21 visit, respectively. V1 denotes the last visit in Phase 1 (day 42 after inclusion) and inclusion visit of Phase 2 (day 0). V2, V3 and V4 visits are visits on days 28, 56 and 84, respectively, in Phase 2. Control, low dose, high dose refer to the phase 1 treatment groups.
Pruritus severity improved in a similar manner to TLS, as shown in Figure 3, illustrated by the owner assessment visual analog scale. The Phase 1 mean (±SD) baseline score of 66.3 (±23.2) decreased by week 4 of Phase 2 to 26.10 (±28.90) and remained stable until study end. At the final visit, the mean (±SD) OPS was 26.8 (±32.0). Overall, the mean OPS score improved (P < 0.0001) by 61 and 60% by weeks 4 and 12, respectively. In addition, 59 of 88 (67%) cats were considered as grooming all the time or often and being agitated or nervous at enrolment in Phase 1, while only 16 of 88 (19%) were still behaving in this way at Phase 2 study end. The proportion of cats grooming normally or grooming but being calm improved from eight of 88 (9%) to 57 of 88 (65%) from enrolment in Phase 1 to Phase 2 study end.
Figure 3.
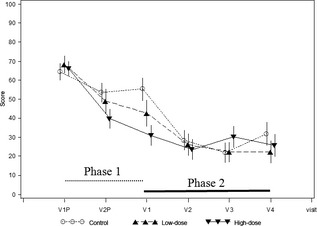
Pruritus intensity measured by the owner with the visual analog scale. Total lesion score over time. V1P and V2P denote the inclusion visit in Phase 1 and day 21 visit, respectively. V1 denotes the last visit in Phase 1 (day 42 after inclusion) and inclusion visit of Phase 2 (day 0). V2, V3 and V4 visits are visits on days 28, 56 and 84, respectively, in Phase 2. Control, low dose, high dose refer to the phase 1 treatment groups.
Overall, at study exit, there were no lesions or only mild lesions (TLS ≤ 1) in 50 of 88 (57%) cats. The frequency of complete resolution of lesions differed depending on the types of lesions initially observed at inclusion in Phase 1. There was a complete resolution of Eo, Ex, MD and SA lesions in 32 of 42 (76%), 44 of 68 (65%), 26 of 42 (62%) and 36 of 75 (48%) cats presenting with these respective lesions at inclusion in Phase 1.
At study end, the investigator assessed the overall efficacy as excellent (45%), good (28%), acceptable (9%), poor (13%) or bad (5%). A similar assessment was made by the owners (data not shown).
Concomitant therapies
Ciclosporin was used in conjunction with various medications, including macrocylic lactones and other antiparasitic agents, nutritional supplements, topical skin and otic cleansers, and antimicrobials. Of the 88 cats that were treated with ciclosporin, 18 were on concurrent systemic antimicrobials. Twenty‐eight of the cats treated with ciclosporin received a macrocylic lactone.
Tolerability
Adverse events were reported in 58 cats during the Phase 2 study. Table 3 presents the AEs in cats with ≥5% frequency using system organ classification. Digestive tract disorders were the most frequently reported AEs and included primarily vomiting (44% of cats with reported AEs) and diarrhoea (22% of cats with reported AEs). There were also reports of anorexia, lethargy, sneezing and weight loss. Eighty per cent of reported AEs occurred when cats were receiving a daily dose of ciclosporin.
Table 3.
Number of cats with reported adverse clinical signs of ≥5% frequency using system organ classificationa
| System organ class | Adverse clinical sign | Daily dosing | Other dosing |
|---|---|---|---|
| Behavioural disorders | Behavioural disorder (apathy, behavioural problem) | 2 | 1 |
| Hyperactivity | 1 | 0 | |
| Blood and lymphatic system disorders | Aplastic anaemia | 1 | 0 |
| Lymphadenopathy | 1 | 0 | |
| Lymphopenia/neutropenia | 3 | 0 | |
| Digestive tract disorders | Abdominal cavity disorder (mass) | 1 | 0 |
| Abdominal pain | 1 | 0 | |
| Ascites | 1 | 0 | |
| Diarrhoea/loose stool | 15 | 4 | |
| Digestive tract disorder (light stool colour) | 1 | 0 | |
| Gastritis | 1 | 0 | |
| Haemorrhagic diarrhoea | 1 | 0 | |
| Hypersalivation | 2 | 1 | |
| Pancreatic disorder (enlarged pancreas) | 1 | 0 | |
| Vomiting/spitting | 31 | 8 | |
| Eye disorders | Conjunctivitis/lacrimation increased/ocular discharge | 4 | 0 |
| Corneal ulcer | 1 | 0 | |
| Respiratory tract disorders | Bronchitis | 1 | 0 |
| Cough/rale | 3 | 0 | |
| Sneezing | 3 | 0 | |
| Disorders of skin and appendages | Alopecia | 1 | 0 |
| Dermatitis/desquamation/erythema/excoriation/pruritus | 6 | 2 | |
| Dermatomycosis | 1 | 0 | |
| Hair modification (hypertrichosis) | 0 | 1 | |
| Pyoderma/skin abscess | 2 | 0 | |
| Systemic disorders | Abnormal test resultb | 7 | 2 |
| Anorexia | 6 | 1 | |
| Hyperthermia | 2 | 0 | |
| Lethargy/malaise | 9 | 1 | |
| Polydipsia | 0 | 1 | |
| Trauma | 2 | 2 | |
| Weight gain | 0 | 1 | |
| Weight loss | 3 | 1 | |
| Total | 114 | 26 |
Includes all cases with ≥5% frequency. A cat may have had more than one adverse clinical sign.
Abnormal test results include one report each of increased thyroxine, blood urea nitrogen/creatinine ratio, alanine aminotransferase or globulin, positive titres for coronavirus or feline infectious peritonitis, and three reports of positive titres for Toxoplasma gondii.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Two cats had positive Toxoplasma gondii seroconversion during the study. In one case, a Toxoplasma titre was requested at study end because the cat was lethargic and had an elevation in creatinine phosphokinase; the cat remained clinically normal (IgG, 1:512; IgM negative). In the other case, Toxoplasma titres were measured during the course of the Phase 2 study because the cat was lethargic (IgG negative; IgM, 1:128). In both cases, no signs other than lethargy and/or elevated values of creatinine phosphokinase were noted. One other cat in the study was noted with anorexia and elevated liver enzymes (alanine aminotransferase and alkaline phosphatase). The values for alanine aminotransferase improved from baseline (prior to Phase 1, 1076 IU/L) until exit in Phase 2 (1002 IU/L). Alkaline phosphatase values remained elevated (prestudy baseline, 116 IU/L; study exit in Phase 2, 195 IU/L). The cat was withdrawn and recovered.
Cats showed a trend for slight and temporary weight loss when on initial daily dosing at 7 mg/kg, as shown in Figure 4 in Phases 1 and 2. In Phase 2, body weights for those animals that received any other dosing regimen than daily dosing showed no significant overall weight loss (P = 0.84). In addition, there were no correlations between body weight change and frequency of vomiting (P = 0.51).
Figure 4.
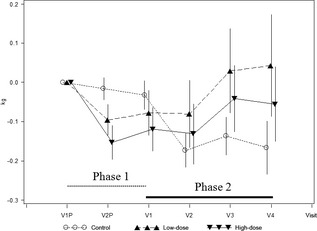
Body weight change. Total lesion score over time. V1P and V2P denote the inclusion visit in Phase 1 and day 21 visit, respectively. V1 denotes the last visit in Phase 1 (day 42 after inclusion) and inclusion visit of Phase 2 (day 0). V2, V3 and V4 visits are visits on days 28, 56 and 84, respectively, in Phase 2. Control, low dose, high dose refer to the phase 1 treatment groups.
Clinical chemistry and haematology values were evaluated prior to ciclosporin administration and at study end. There were no significant differences noted in haematology. Mean alanine aminotransferase, alkaline phosphatase and albumin were significantly lower in animals treated daily with ciclosporin in comparison to animals on all other dosing regimens. However, all mean parameter values remained within the normal reference ranges for cats (alanine aminotransferase, 34–100 IU/L; alkaline phosphatase, 14–102 IU/L; and albumin, 21–39 g/L).
A total of six cats were withdrawn per study protocol due to the development of an AE. Clinical signs included diarrhoea, depression, lethargy, anorexia and vomiting. One case was subsequently diagnosed with feline infectious peritonitis and was euthanized, one was diagnosed with T. gondii infection and another one was diagnosed with hyperthyroidism. The cat diagnosed with hyperthyroidism was withdrawn from the study by the owner. The owner elected not to treat, and the cat died from apparent uncontrolled hyperthyroidism after study exit. All animals, with the exception of the feline infectious peritonitis and hyperthyroidism cases, recovered and remained clinically normal.
Discussion
Following the daily administration of 7 mg/kg for a minimal duration of 4 weeks, the dose of ciclosporin could be tapered to EoD in up to 70% of cats remaining in the study. It was possible to taper the dose further to TW in 57% of cats completing the study. A similar frequency of dose tapering was previously reported in cats suffering from idiopathic pruritus, eosinophilic plaque, indolent ulcer and/or linear granuloma.2 Vercelli et al.2 showed that following 8 weeks during which cats received an inducing dose of ciclosporin, the dose could be administered TW in 73% of the cats and EoD in 36% of the cats.
Overall, it would appear that the frequency of dose tapering is overestimated if based on the cat population remaining in the study, but underestimated if based on the initial population included into the study. In a clinical study, the frequency of treatment interruption may be higher than in general practice conditions, because cats belonging to owners not complying with the protocol will be withdrawn. Furthermore, it should be noted that the frequency of dose tapering observed in a clinical study may not predict precisely what will be observed in clinical practice. In a clinical trial, strict and standardized rules are given to taper the dose, while in clinical practice, dose adjustment may be based on various considerations, including but not limited to clinical improvement. In addition, in this clinical trial, ciclosporin was generally used as monotherapy and the use of concomitant medications was restricted as much as possible. Cats treated in clinical practice may receive adjunct therapies that may contribute to clinical improvement. It is also likely that owner compliance to treatment is better in a clinical trial compared with clinical practice, because owner compliance is regularly monitored and drug accountability assessed. All these factors may contribute to the response to ciclosporin treatment and therefore affect the frequency of dose tapering. However, in a large proportion of cats in this study, the dose could be tapered to EoD and even TW without deterioration of the clinical response. In addition, a per protocol analysis was carried out to assess the influence of prohibited concurrent medications and noncompliance to the treatment administration or treatment regimen on the outcome. Cats wearing an Elizabethan collar for longer than the authorized 10 days or receiving systemic concomitant therapies, such as antibiotics for treatment of bacterial dermatitis (11 cats) or antihistamine (one cat), which might have contributed to the clinical improvement of some of the lesions, such as Eo,8, 9 were completely or partly excluded from this per protocol analysis. This analysis did not reveal any marked difference compared with the intent‐to‐treat analysis on dose tapering and clinical parameters, such as total lesion score and pruritus (data not shown), indicating a limited influence of compliance and concomitant therapies on the clinical outcome.
The frequency and the extent of dose tapering appeared to be much higher in cats than in dogs. In a systematic review on clinical studies in dogs,10 the frequency of EoD treatment following a 4 week induction period was found to be generally close to 50% and, as a rule, no more than 20% of dogs could be dosed twice a week for maintenance. Different algorithms were used in clinical studies to decide on the change of dose in dogs and cats. In dogs, the change of dosing frequency was guided by the relative improvement of the Canine Atopic Dermatitis Extent and Severity Index11 lesion score over baseline, while in cats the change of dosing regimen was based on the overall clinical assessment made by the investigator. The dose could be tapered when a good or excellent response was obtained, which corresponds approximately to a 50% improvement of the lesion score and pruritus.7 The same threshold was selected in dog studies for changing the dosing regimen.12, 13 It could have been expected that the frequency of dose reduction would be lower in cats than in dogs. In cats, the dose could not be tapered when moderate or severe pruritus was still exhibited, whilst in dogs a reduction of the lesion score was sufficient for dose tapering even with persistent pruritus. Pharmacokinetic features of ciclosporin in dogs and cats may explain the difference in dose‐tapering frequency. In cats, blood concentrations at the same dose of ciclosporin tend to be comparatively higher than in dogs.14, 15 However, the concentration of ciclosporin needed to inhibit the expression of immunomodulatory cytokines, such as interleukin‐2, interleukin‐4 and interferon‐γ, from peripheral blood mononuclear cell appears to be higher in cats than in dogs.16, 17 More information on the precise mode of action of ciclosporin in the skin and on the crucial target cells would be needed for the interpretation of this difference.
The dose‐tapering regimen was, initially, similar for all four types of lesions observed, but the complete resolution of lesions was less frequent in cats suffering from self‐induced alopecia than other types of lesions. Slow hair regrowth, generally seen in winter when the follicle activity is low,18 is unlikely to explain the incomplete resolution of self‐ induced alopecia. The study was conducted in different geographical areas, climatic conditions and over several seasons. It is possible that excessive grooming associated with behavioural disorders19, 20 may have contributed to the perpetuation of the alopecia. Severe prolonged pruritus can induce a state of anxiety and behavioural problems,21 forming a vicious cycle. The study may have included some cats with psychogenic alopecia, because a definitive diagnosis of psychogenic alopecia is difficult to make,19 and the anxious condition may not have resolved in spite of improvement in pruritus.
Interestingly, the duration of induction period at a starting dose of 7 mg/kg daily did not influence the clinical response at week 4. The total lesion score or pruritus severity was similar whether cats received ciclosporin for 4 weeks at 7 mg/kg (cats from the Phase 1 placebo group) or for 10 weeks (cats enrolled receiving the high dose in Phase 1). This indicates that extending the duration of treatment with the inducing dose of 7 mg/kg daily is not likely to result in a better clinical response. It is also noteworthy that the clinical response remained stable after dose tapering independent from the previous treatment received in Phase 1. The study showed that the change of dosing regimen resulted in a stable response, and that dose adjustment to compensate for a deterioration of the disease was rarely needed. A return to the previous dosing regimen between study visits was required in only 4% of the cats during Phase 2.
Treatment compliance was good, with only three cats being withdrawn during the study because the cat would not accept the medication either on food or dosed directly in the mouth. Up to 39% of cats took the formulation mixed with food for the majority of the study duration. The volume of the formulation mixed with food was very small (0.07 mL/kg), so any potential taste of the product could be masked if mixed with a palatable food.
In this study, 80% of reported AEs occurred during daily ciclosporin administration. The observed AEs were consistent with those already reported in cats receiving ciclosporin.6 Gastrointestinal AEs were the most frequently reported, with vomiting events seen in approximately 44% of the cases. Vomiting tended to resolve without discontinuation of the treatment. It is possible that some of the vomiting events were not related to ciclosporin, because vomiting was also observed in placebo‐treated cats enrolled in a previous study.5 Like vomiting, diarrhoea was generally mild and did not require medical intervention.
Weight loss was noted in cats treated daily with 2.5 and 7 mg/kg ciclosporin in the Phase 1 study and in those control cats (not previously exposed in Phase 1) when administered ciclosporin in the Phase 2 study. No correlation was found between weight loss and frequency of vomiting events. The weight loss was most probably a result of the combination of ciclosporin‐related gastrointestinal disturbance and effects on food intake, because these elements were reduced with decreased dose frequency. The majority of cats with weight loss and treated with ciclosporin tended to regain weight, resulting in no overall weight loss at study end.
Of the cats diagnosed with serious AEs, feline infectious peritonitis was most probably a previously undiagnosed condition. Toxoplasmosis in association with ciclosporin monotherapy has been anecdotally reported.22, 23, 24, 25 Cats that are seronegative for T. gondii may be at risk of developing clinical toxoplasmosis if they become infected while undergoing treatment with ciclosporin. Potential exposure of seronegative cats or cats suspected to be seronegative to T. gondii should therefore be minimized (e.g. keep indoors, avoid feeding raw meat or scavenging). The possible exacerbation of a pre‐existing serious systemic disease (e.g. feline infectious peritonitis) is consistent with the known pharmacology of this class of drug. In cases of clinical toxoplasmosis or other serious systemic illness, treatment with ciclosporin should be withdrawn and appropriate therapy initiated. Ciclosporin is an inhibitor of the MDR1 P‐glycoprotein transporter. Coadministration of ciclosporin with P‐glycoprotein substrates, such as macrocyclic lactones, could therefore potentially decrease the efflux of such drugs from blood–brain barrier cells, potentially resulting in signs of central nervous system toxicity. In this study, cats were required to have been treated with a flea adulticide to rule out flea allergy as a cause of their allergic disease. Twenty‐eight cats that were subsequently treated with ciclosporin received concomitant treatment with a macrocyclic lactone (27 were treated with selamectin and one cat received milbemycin and praziquantel). Approximately 70% of these 28 cats experienced an AE (primarily digestive tract disorders). One cat treated with selamectin was reported to have convulsions. The convulsions were attributed to possible chronic renal disease, and the cat was continued with ciclosporin off study with no further reports. While the number of animals on both ciclosporin and macrocyclic lactone therapy was limited and extrapolations should be made with caution, there did not appear to be an association between the concomitant use of these drugs and reports of neurological AEs.
In conclusion, these results suggest that the inducing dose of 7 mg/kg ciclosporin can be tapered as soon as 4 weeks without deterioration of clinical response. The dose regimen could be tapered to EoD or TW in greater than 53% of all cats entering the study. Although ciclosporin treatment appears generally to be well tolerated, these data also suggest that establishing the lowest effective dose regimen of ciclosporin treatment will improve the safety profile of the drug.
Acknowledgements
The authors would like to thank all the study investigators who contributed cases: Luc Beco, Andrea Cannon, Paul Coward, Chris Dale, Claude Favrot, Eric Florant, Jacques Fontaine, Peter Forsythe, Cecilia Friberg, Thierry Hazan, Julie Henfrey, John Hutt, Jean‐Francois Jamet, Janet Littlewood, Linda Messinger, Neil McEwan, Donald MacTaggart, Tim Nuttall, Pascal Prélaud, Tiffany Tapp, Randall Thomas and Patricia White. The authors would also like to thank the cat owners and the Atopica for Cat team at Novartis Animal Health.
Sources of Funding:
This study was funded by Novartis Animal Health, Basel, Switzerland.
Conflict of Interest:
J.S., E.R., S.K. and W.S. are full‐time employees of Novartis Animal Health. A.C., P.P., P.F. and J.F. have received related and unrelated consultancy fees, lecture fees or investigator fees from Novartis Animal Health.
References
- 1. Robson DC, Burton GG. Cyclosporin: applications in small animal dermatology. Vet Dermatol 2003; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Vercelli A, Raviri G, Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 2006; 17: 201–206. [DOI] [PubMed] [Google Scholar]
- 3. Noli C, Scarampella F. Prospective open pilot study on the use of ciclosporin for feline allergic skin disease. J Small Anim Pract 2006; 47: 434–438. [DOI] [PubMed] [Google Scholar]
- 4. Wisselink MA, Willemse T. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: a double blind, randomised prednisolone‐controlled study. Vet J 2009; 180: 55–59. [DOI] [PubMed] [Google Scholar]
- 5. King S, Favrot C, Messinger L et al A randomised double‐blinded placebo controlled study to evaluate an effective ciclosporin dose for treatment of feline hypersensitivity dermatitis. Vet Dermatol 2012; 23: 440–e84. [DOI] [PubMed] [Google Scholar]
- 6. Heinrich NA, McKeever PJ, Eisenschenk MC. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Vet Dermatol 2011; 22: 511–520. [DOI] [PubMed] [Google Scholar]
- 7. Steffan J, Olivry T, Forster SL et al Responsiveness and validity of the SCORFAD, an extent and severity scale for feline hypersensitivity dermatitis. Vet Dermatol 2012; 23: 410–e77. [DOI] [PubMed] [Google Scholar]
- 8. Bloom P.B. Canine and feline eosinophilic skin diseases. Vet Clin North Am Small Anim Pract 2006; 36: 141–160. [DOI] [PubMed] [Google Scholar]
- 9. Wildermuth BE, Griffin CE, Rosenkrantz WS. Response of feline eosinophilic plaques and lip ulcers to amoxicillin trihydrate–clavulanate potassium therapy: a randomized, double‐blind placebo‐controlled prospective study. Vet Dermatol 2012; 23: 110–e25. [DOI] [PubMed] [Google Scholar]
- 10. Steffan J, Favrot C, Mueller R. A systematic review and meta‐analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Vet Dermatol 2006; 17: 3–16. [DOI] [PubMed] [Google Scholar]
- 11. Olivry T, Mueller R, Nuttall T et al Determination of CADESI‐03 thresholds for increasing severity levels of canine atopic dermatitis. Vet Dermatol 2008; 19: 115–119. [DOI] [PubMed] [Google Scholar]
- 12. Steffan J, Alexander D, Brovedani F et al Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: a parallel, blinded, randomized controlled trial. Vet Dermatol 2003; 14: 11–22. [DOI] [PubMed] [Google Scholar]
- 13. Steffan J, Parks C, Seewald W. Clinical trial evaluating the efficacy and safety of cyclosporine in dogs with atopic dermatitis. J Am Vet Med Assoc 2005; 226: 1855–1863. [DOI] [PubMed] [Google Scholar]
- 14. Steffan J, Strehlau G, Maurer M et al Cyclosporin A pharmacokinetics and efficacy in the treatment of atopic dermatitis in dogs. J Vet Pharmacol Ther 2004; 27: 231–238. [DOI] [PubMed] [Google Scholar]
- 15. Mehl ML, Kyles AE, Craigmill AL et al Disposition of cyclosporine after intravenous and multi‐dose oral administration in cats. J Vet Pharmacol Ther 2003; 26: 349–354. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL‐2, IL‐4 and IFN‐γ, but not TNF‐α, in canine mononuclear cells. J Vet Med Sci 2007; 69: 887–892. [DOI] [PubMed] [Google Scholar]
- 17. Kuga K, Nishifuji K, Iwasaki T. Cyclosporine A inhibits transcription of cytokine genes and decreases the frequencies of IL‐2 producing cells in feline mononuclear cells. J Vet Med Sci 2008; 70: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 18. Ryder ML. Seasonal changes in the coat of the cat. Res Vet Sci 1976; 21: 280–283. [PubMed] [Google Scholar]
- 19. Mertens PA, Torres S, Jessen C. The effects of clomipramine hydrochloride in cats with psychogenic alopecia: a prospective study. J Am Anim Hosp Assoc 2006; 42: 336–343. [DOI] [PubMed] [Google Scholar]
- 20. Sawyer LS, Moon‐Fanelli AA, Dodman NH. Psychogenic alopecia in cats: 11 cases (1993–1996). J Am Vet Med Assoc 1999; 214: 71–74. [PubMed] [Google Scholar]
- 21. Oh SH, Bae BG, Park CO et al Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol 2010; 90: 582–588. [DOI] [PubMed] [Google Scholar]
- 22. Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J 2006; 84: 30–35. [DOI] [PubMed] [Google Scholar]
- 23. Last RD, Suzuki Y, Manning T et al A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet Dermatol 2004; 15: 194–198. [DOI] [PubMed] [Google Scholar]
- 24. Bernsteen L, Gregory C, Aronson LR et al Acute toxoplasmosis following renal transplantation in three cats and a dog. Aust Vet J 1999; 215: 1123–1126. [PubMed] [Google Scholar]
- 25. Beatty J, Barrs V. Acute toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J 2003; 81: 339. [DOI] [PubMed] [Google Scholar]


