Abstract
An 8‐year‐old, spayed female Domestic Short‐haired cat was referred for further evaluation of chronic lymphocytic–plasmacytic stomatitis and bilateral ocular disease. The cat had been treated with systemic glucocorticoids for several months. Initial ophthalmic examination revealed bilateral deep stromal corneal ulcers, exudative panuveitis and secondary glaucoma. Mature mild neutrophilia and monocytosis were detected on complete blood cell count. Abnormalities in the serum profile were hyperglycemia, mild azotemia, hyperglobulinemia and moderate polyclonal gammapathy. Urinalysis revealed glucosuria without ketonuria. Diabetes mellitus was diagnosed and treatment with long‐acting insulin was started. An enzyme‐linked immunosorbent assay was highly positive for leishmaniasis, and treatment with allopurinol was started. Although specific topical treatment was applied, melting ulcers progressed to corneal perforation and both eyes were enucleated. Ocular histology showed large numbers of intracellular organisms compatible with amastigotes of the genus Leishmania located in the uveal tract, cornea, sclera and retina. Results of inmunohistochemistry staining on ocular samples were positive for Leishmania. Bone marrow cytology demonstrated numerous macrophages with intracytoplasmatic Leishmania. Polymerase chain reaction results on bone marrow for Leishmania were positive. Three weeks later, hypoglycemic episodes permitted withdrawal of the insulin therapy. To the authors’ knowledge this is the first case of ocular and visceral leishmaniasis diagnosed in vivo and under systemic treatment in a cat.
Keywords: corneal ulcer, diabetes mellitus, feline, leishmaniasis, uveitis
An 8‐year‐old spayed female Domestic Short‐haired cat weighing 2.9 kg was referred to the Veterinary Teaching Hospital of the Autonomous University of Barcelona (HCV‐UAB) for further evaluation of chronic proliferative stomatitis and bilateral corneal ulcer. The referring veterinarian had diagnosed chronic lymphocytic–plasmacytic stomatitis by means of histopathologic evaluation of oral biopsies, 8 months prior to referral. At that time, results of serum biochemical profile and complete blood cell count (CBC) were within normal limits, and enzyme‐linked immunosorbent assay (ELISA) results for FeLV and FIV were negative. The stomatitis was treated with dental cleaning, tolfenamic acid (4 mg/kg, PO, every 24 h; Tolfedine, Vetoquinol s.a, Cedex, France), and enrofloxacin (5 mg/kg, subcutaneously, every 24 h; Baytril, Bayer, Barcelona, Spain) for 10 days. Owing to relapse of clinical signs, methylprednisolone acetate (20 mg/cat intramuscularly; Depomoderin, Pharmacia Upjohn, Barcelona, Spain) was added to the treatment, once weekly for 8 months.
One month prior to referral, the cat was re‐evaluated for ocular disease. Bilateral anterior uveitis, fever and weight loss were noted. An immunoglobulin (Ig)G antibody titer to Toxoplasma gondii was 1 : 50 (reference range < 1 : 40). Treatment was initiated with clindamycin (25 mg/kg, PO, every 12 h; Dalacín, Pharmacia, Barcelona, Spain) and topical chloramphenicol–dexamethasone (one drop, OU, every 6 h; Colircusí cloranfenicol and Maxidex; Alcon Cusí, Barcelona, Spain). One week later the ocular condition had not improved and deep stromal ulceration was detected OS. Topical corticosteroids were withdrawn and atropine topical ointment (one application, OS, every 12 h; Oftalmolosa cusí atropine, Alcon Cusí) was added to the treatment regimen. CBC and biochemistry were performed again and mild mature neutrophilia (13.5 × 103 cell/µL; reference range, 1.9–14.6 × 103 cell/µL), monocytosis (1.8 × 103 cell/µL; reference range, 0.055–0.78 × 103 cell/µL) and hypergammaglobulinemia (34 g/L; reference range, 12–32 g/L) were found. The cat was then referred to the HCV‐UAB.
At the time of referral, the cat was slightly depressed, thin and had poor hair coat quality. Other abnormalities detected on physical examination were increased rectal temperature (40.5 °C), mild dehydration, distended cranial abdomen, hepatomegaly, moderate diffuse gingivitis and marked bilateral proliferative faucitis. Initial ophthalmic examination revealed moderate blepharospasm, and severe mucoid discharge OU. Menace response and dazzle reflex were absent in both eyes. Pupillary light reflexes (PLRs) could not be evaluated OU. Schirmer tear test was normal OU. Diffuse transillumination and slit lamp biomicroscopic examination OU (Kowa SL‐14, Kowa Company, Tokyo, Japan) indicated moderate scleral injection, chemosis, deep stromal corneal ulceration, and diffuse endothelial corneal edema OU (Fig. 1). Both eyes presented with severe aqueous flare (+4). Intraocular pressure (IOP) measurements obtained by applanation tonometry (Tonopen XL, Mentor, Norwell, MA, USA) following instillation of topical anesthetic (Tetracaine hydrochloride 0.5%, Alcon‐Cusí) were 35 mmHg OD and 32 mmHg OS. The lens and fundus could not be evaluated OU. At this point, deep stromal corneal ulceration and exudative panuveitis with secondary glaucoma were diagnosed OU.
Figure 1.
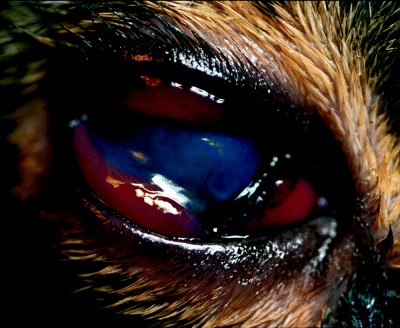
Right eye. Note the mucoid discharge, chemosis and diffuse endothelial corneal edema. The stromal corneal ulceration is behind the nictitating membrane.
Abnormalities found on CBC were mature mild neutrophilia (13.5 × 103 cell/µL; reference range, 1.9–14.6 × 103 cell/µL) and monocytosis (0.6 × 103 cell/µL; reference range, 0.055–0.78 × 103 cell/µL). Abnormalities on the serum profile were hyperglycemia (354 mg/dL; reference range, 73–134 mg/dL), mild azotemia (70 mg/dL; reference range, 42–64 mg/dL), hyperglobulinemia (5.32 mg/dL; reference range, 3.3–4.5 mg/dL) and polyclonal gammopathy (2.4 mg/dL; reference range, 1.3–2.2 mg/dL). Urinalysis abnormalities were glucosuria without ketonuria.
Based on the medical history, physical examination and laboratory test results, the most likely differential diagnoses included reactive hepatopathy due to diabetes mellitus (DM), colangiohepatitis complex, lymphoma, feline infectious peritonitis (FIP), FeLV, FIV, systemic mycosis, bartonellosis, leishmaniasis, herpesvirus infection and ehrlichiosis.
Additional diagnostic tests included abdominal and thoracic radiographs, abdominal ultrasonography, hepatic fine‐needle aspiration (FNA), glucose curve and ELISA serum tests for infectious diseases [Toxoplasma gondii, feline coronavirus (FeCoV), FeLV/FIV, Cryptococcus neoformans, Ehrlichia canis and Leishmania infantum]. Abdominal radiographs and ultrasonography demonstrated a diffusely enlarged liver with diffuse hyperechogenicity. Microscopic examination of hepatic FNA yielded normal hepatocytes and neutrophils. Persistence of hyperglycemia and glucosuria was detected. Type III DM (probably associated with long‐term glucocorticoid administration) and bilateral panuveitis with stromal corneal ulcer were diagnosed. Systemic glucocorticoids were withdrawn and the suspected type III DM was managed with long‐acting insulin (0.5 U/kg subcutaneously, every 12 h; Humulina ultralenta, Lilly) and clindamycin (25 mg/kg intravenously, every 12 h; Dalacín Pharmacia). Ophthalmic treatment consisted of topical oxytetracycline (one drop, OU, every 6 h (Colircusí aureomicina, Alcon Cusí), atropine ointment (one application, OU, every 12 h; Oftalmolosa cusí atropina, Alcon Cusí), flurbiprofen (one drop, OU, every 6 h; Ocuflur, Allergan) and dorzolamide (one drop, OU, every 8 h; Trusopt; Merck, Sharp and Dohme Chibret, Madrid, Spain).
One week after initial presentation, the cat was re‐evaluated. It had increased 0.4 kg in body weight, but was mentally depressed and the ocular condition had worsened. On ophthalmic examination, bilateral melting keratitis with deep stromal ulceration was present. Corneal cytology showed epithelial corneal cells and neutrophils, but no bacteria or parasites were observed. At this point, ELISA serum titers results were highly positive for leishmaniasis (300%; normal range < 40%), slightly positive for toxoplasmosis (1 : 50; reference range < 1 : 40) and FeCoV (1 : 340; reference range < 1 : 320), and negative for E. canis (1 : 20; reference range < 1 : 40) and FeLV/FIV. Latex agglutination test was negative for C. neoformans. A presumptive diagnosis of feline leishmaniasis and DM was made. The cat was managed with allopurinol (10 mg/kg, PO, every 12 h; Zyloric, Faes Laboratories, Vizcaya, Spain); long‐acting insulin (same dose), cephalexin (20 mg/kg, intravenously, every 8 h; Kefloridina; Lilly Laboratories, Madrid, Spain); tobramycin (one drop, OU, every 1 h; Tobrex; Alcon Cusí); flurbiprofen (one drop, OU, every 8 h; Ocuflur, Allergan, Madrid, Spain); feline serum (one drop, OU, every 1 h) and atropine ointment (one application, OU, every 12 h; Oftalmolosa cusi atropine, Alcon Cusí). Melting keratitis progressed to corneal perforation OU within a few hours. At this point, corneal graft and conjunctival flap was recommended, but due to the poor ocular prognosis, the owner decided to have a bilateral enucleation performed. Two days after admission, bilateral enucleation and bone marrow aspiration from the iliac crest were performed. The cat was discharged 2 days after surgery with allopurinol (same dose), long‐acting insulin (same dose), and enrofloxacin (5 mg/kg, PO, every 24 h, for 10 days; Baytril, Bayer).
Ocular histopathology of the enucleated eyes demonstrated a granulomatous inflammation located in fibrous and vascular layers with large number of intracellular organisms compatible with amastigotes of the genus Leishmania (2, 3). Inmunohistochemistry staining of ocular tissues confirmed the presence of Leishmania parasites (Fig. 4). Bone marrow cytology demonstrated numerous macrophages with intracytoplasmatic Leishmania. Polymerase chain reaction (PCR) test for Leishmania was positive. Therapy with allopurinol and long‐acting insulin was maintained.
Figure 2.
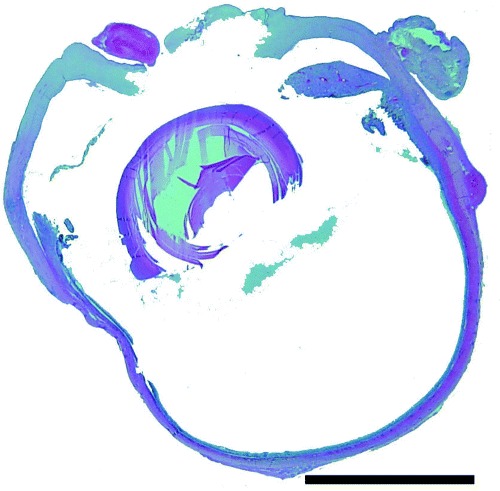
Histopathology of the right globe. Note the central corneal perforation, exudate in anterior chamber and vitreous. Hematoxlyn and eosin. Bar = 2000 µm.
Figure 3.
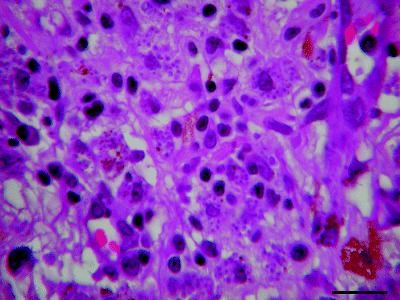
Macrophagic cells containing numerous amastigote forms of Leishmania species in the uveal tract. Hematoxlyn and eosin. Bar = 20 µm.
Figure 4.

Choroidal immunohistochemistry for detection of Leishmania amastigotes (brown staining) in the choroid. Bar = 800 µm.
One month later the cat had gained 0.6 kg in body weight, still had glucosuria and signs referable to oral discomfort were waning and waxing. Three weeks later, several hypoglycemic episodes made withdrawal of the insulin therapy necessary. Three months later, CBC and biochemical profile revealed mild polyclonal gammapathy (32 g/L reference range, 12–32 g/L) and a decrease in the leishmaniosis titer (140%). Nine months after the diagnosis, the cat was clinically healthy despite the fact that the owners had stopped allopurinol therapy 3 months earlier. CBC and biochemical profile revealed moderate polyclonal gammapathy (38 g/L, reference range, 12–32 g/L) and serum leishmaniasis titer result was higher than previously (174%). Therapy was restarted.
DISCUSSION
Leishmaniasis is a worldwide infectious disease of people and wild and domestic animals caused by diphasic protozoans of the genus Leishmania. It is a frequent cause of clinical disease in dogs and less common in cats and humans. 1 , 2 Natural leishmaniasis infection appears to be rare in the cat. Since 1911 about 50 cases of feline leishmaniasis have been reported. 3 , 4 , 5 , 6 , 7 The low prevalence of feline leishmaniasis in endemic areas could be due to under‐reporting or natural feline resistance to infection. 8 However, in endemic areas there is a high possibility of infection. Visceral forms are not usually described in feline leishmaniasis, 5 although some case reports of cutaneous manifestations have been published. 4 , 5 In some reports, feline leishmaniasis was related to natural acquired inmunodeficiency (FIV and FeLV infections), 1 although this association has not been confirmed. It is important to include leishmaniasis in the differential diagnosis of immunodeficient cats with systemic disease and polyclonal gammopathy. In our case, FIV and FeLV infection were ruled out, but as the patient was treated with glucocorticoids for 8 months, the immunological status may have been impaired. 9
Ocular manifestations are common in canine leishmaniasis, 10 but only two cases have been reported in cats. 6 , 11 In dogs, anterior uveitis is the most ocular common finding and may have an immunologic basis. 10 , 12 , 13 Melting ulcers, although not frequent, are a complicating component of corneal ulcer. Corneal epithelial cells, fibroblasts, polymorphonuclear leukocytes, some bacterias and possibly fungi produce an abnormal amount of proteases and collagenases that contributed to the progressive breakdown and rapid ‘melting’ of the corneal stroma. 14 In this case, histopathology confirmed the presence of a large number of Leishmania amastigotes and leukocytes in the corneal stroma. Collagenases could have been produced directly by Leishmania amastigotes or by polymorphonuclear corneal cells, although synthesis of collagenases by L. infantum has not been proven. To the authors’ knowledge this is the second description of panuveitis due to feline leishmaniasis, but it is the first with associated melting keratitis. In endemic areas, leishmaniasis should be included in differential diagnosis of feline uveitis and corneal melting ulcers.
Diagnosis of feline leishmaniasis is difficult because serology and serum protein electrophoresis patterns are generally less specific than in canine leishmaniasis. 15 In doubtful cases, immunohistochemical staining techniques and PCR analysis can be performed for Leishmania identification. In the case reported here, serum Leishmania titer was positive and bone marrow cytology and PCR techniques demonstrated Leishmania parasites, confirming visceral involvement. L. infantum has been described in some reports as the etiological agent of feline leishmaniasis. 4 , 7 Although Leishmania species could not be determined in this case, L. infantum was suspected, due to its endemicity in the Mediterranean basin 1 , 13 Some previous studies suggest the role of cats as potential peridomestic reservoirs for L. infantum in northeastern Spain. 16 In this report, ocular leishmaniasis was diagnosed by means of immunohistochemistry on ocular tissues examined after bilateral enucleation.
Type III or secondary DM was the most likely cause of hyperglycemia. This type of DM has been associated with hyperadrenocorticism, hyperthyroidism, excessive growth hormone secretion, chronic progesterone administration or exocrine pancreatic tumors. 17 In this case, DM could be the result of administering high doses of long‐acting glucocorticoids for several months. Insulin therapy and/or gradual tapering of glucocorticoid treatment allowed good control of blood glucose. Persistent hyperglycemia is an important factor in cats inducing glucose toxicity phenomena and inhibition of insulin synthesis or pancreatic secretion. 18 After starting insulin treatment and withdrawal of glucocorticoids, secretion of insulin is re‐established and hypoglycemic crises can occur. In this case, hypoglycemia episodes occurred 2 months after the initiation of therapy, and insulin treatment was stopped without any further observed relapses.
Owing to the small number of reported cases, feline leishmaniasis treatment is not defined. In this case, treatment with allopurinol, as reported for canine leishmaniasis, 1 was initiated and the clinical state of the patient improved as well as the gammopathy. The serum Leishmania titer also decreased with the treatment. Interestingly, the serum titer along with the polyclonal gammopathy increased again after the owner stopped the medication. Combining the treatments of the predisposing cause (when it is known) together with allopurinol seemed to be a good therapeutic option in this case.
To the authors’ knowledge, this is the first case of visceral feline leishmaniasis diagnosed in vivo with favorable response to therapy with allopurinol.
ACKNOWLEDGMENTS
The cat was treated at the Veterinary Teaching Hospital, Facultat the Veterinària, Universitat Autònoma de Barcelona. We thank our colleagues from Novavet Veterinary Clinic for referring the case and our colleagues from the Pathology Department for performing the histopathologic studies. We also thank Carolina Naranjo for her support with the histopathology pictures.
REFERENCES
- 1. Slappendel RJ, Ferrer L. Leishmaniasis In: Microbiology and Infectious Diseases of Dogs and Cats. (ed. Green C.) W.B. Saunders, Philadelphia, 1998;. 450–458. [Google Scholar]
- 2. Del Giudice P, Marty P. Cat‐associated zoonosis: don't forget rabies and leishmaniasis. Archives of Internal Medicine 2003; 62: 1945–1952. [DOI] [PubMed] [Google Scholar]
- 3. Barnes JC, Stanley O, Craig TM. Diffuse cutaneous leishmaniasis in a cat. Journal of the American Veterinary Medical Association 1993; 202: 416–418. [PubMed] [Google Scholar]
- 4. Ozon C, Marty P, Pratlong F et al. Disseminated feline leishmaniosis due to Leishmania infantum in southern France. Veterinary Parasitology 1998;. 75: 273–277. [DOI] [PubMed] [Google Scholar]
- 5. Hervas J, Chacon‐Manrique De Lara F, Sanchez‐Isarria MA et al. Two cases of feline visceral and cutaneous leishmaniosis in Spain. Journal of Feline Medicine and Surgery 1999; 1: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hervas J, Chacon M, De Lara F et al. Granulomatous (pseudotumoral) iridocyclitis associated with leishmaniasis in a cat. Veterinary Record 2001; 149: 624–625. [DOI] [PubMed] [Google Scholar]
- 7. Poli A, Abramo F, Barsotti P et al. Feline leishmaniosis due to Leishmania infantum in Italy. Veterinary Parasitology 2002; 106: 181–191. [DOI] [PubMed] [Google Scholar]
- 8. Kirkpatrick CE, Farrell JP, Goldschmidt MH. Leishmania chagasi and L. donovani: experimental infection in domestic cats. Journal of Experimental Parasitology 1984; 58: 125–131. [DOI] [PubMed] [Google Scholar]
- 9. MacDonald JM. Glucocorticoid therapy In: Textbook of Veterinary Internal Medicine, 5th edn. (eds Ettinger SJ, Feldman EC.) W.B. Saunders, Philadelphia, 2000;. 307–317. [Google Scholar]
- 10. Peña MT, Roura X, Davidson MG. Ocular and periocular manifestations of leishmaniasis in dogs: 105 cases (1993–98). Veterinary Ophthalmology 2000; 3: 35–41. [DOI] [PubMed] [Google Scholar]
- 11. Laruelle‐Magalon C, Toga I. Un cas de leishmaniose féline. Practice Médicale et Chirurgicale des Animaux de Compagnie 1996; 31: 255–261. [Google Scholar]
- 12. Roze M. Manifestations oculaires de la leishmaniose canine. Recueil de Medicine Veterinaire 1986; 162: 19. [Google Scholar]
- 13. Ferrer L. Leishmaniasis In: Current Veterinary Therapy, XI. (eds Kirk RW, Bonagura JD.) W.B. Saunders, Philadelphia, 1992;. 266–270. [Google Scholar]
- 14. Whitley D, Gilger B. Diseases of the canine cornea and sclera In: Veterinary Ophthalmology, 3rd edn. (ed. Gelatt KN.) Lippincott/Williams & Wilkins, Philadelphia, 1999;. 635–673. [Google Scholar]
- 15. Merchant SR, Taboada J. Systemic diseases with cutaneous manifestations. Veterinary Clinics of North America: Small Animal Practice 1995; 25: 945–959. [DOI] [PubMed] [Google Scholar]
- 16. Solano‐Gallego L, Quintana F, Iniesta L et al. A serosurvey of feline leishmaniosis in north‐east Spain. Proceedings of the American Congress in Veterinary Internal Medicine 2003, pp. 423–424.
- 17. Nelson RW. Diabetes mellitus In: Textbook of Veterinary Internal Medicine. (eds Ettinger SJ, Feldman EC.) W.B. Saunders, Philadelphia, 2000;. 1438–1460. [Google Scholar]
- 18. Behrend EN, Clark TP. Pathogenesis of diabetes mellitus In: Consultations in Feline Internal Medicine, 3. (ed. August JR.) W.B. Saunders, Philadelphia, 1997;. 125–131. [Google Scholar]


