Abstract
Background
Feline skin fragility syndrome (FSFS) is an acquired disorder characterized by altered collagen production resulting in an extremely thin and fragile skin. FSFS is associated with diseases characterized by excessive steroidal hormones that can inhibit collagen synthesis. It is also described concomitantly with severe inflammatory, infectious or neoplastic conditions where the pathogenesis remains largely unknown.
Objectives
To describe three cases of FSFS in cats that become cachectic secondary to different causes without glucocorticoid involvement. To describe the histopathological features of connective tissue for both fragile skin and the skin after healing.
Results
All cats developed cachexia in less than two months (body condition score ranging from 1–1.5). Concomitant diseases were diagnosed in Case 1 (aspiration pneumonia due to mega‐oesophagus) and Case 2 (feline immunodeficiency virus (FIV)). In Case 3, malnutrition was suspected as a primary cause. The main histological feature of fragile skin was an atrophic dermis with pale eosinophilic, thin and irregular collagen fibres with numerous red cores observed with Masson's stain. Elastic fibres were normal. Postrecovery histopathological findings at 11 (Case 1) and six months (Case 3) after diagnosis, indicated normalization of the collagen and of the whole skin as compared with controls.
Conclusions and clinical importance
To the best of the authors’ knowledge, this is the first report describing a reversible, nonsteroid‐induced FSFS, associated with rapidly developing cachexia in cats.
Background – Feline skin fragility syndrome (FSFS) is an acquired disorder characterized by altered collagen production resulting in an extremely thin and fragile skin. FSFS is associated with diseases characterized by excessive steroidal hormones that can inhibit collagen synthesis. It is also described concomitantly with severe inflammatory, infectious or neoplastic conditions where the pathogenesis remains largely unknown. Objectives – To describe three cases of FSFS in cats that became cachectic secondary to different causes without glucocorticoid involvement. To describe the histopathological features for connective tissue for both fragile skin and the skin after healing. Conclusions and clinical importance – To the best of the authors’ knowledge, this is the first report describing a reversible, nonsteroid‐induced FSFS, associated with rapidly developing cachexia in cats.

Résumé
Contexte
Le syndrome d'hyper fragilité cutané félin (FSFS) est une maladie acquise caractérisée par une production anormale de collagène résultant en une peau extrêmement fine et fragile. Le FSFS est associé à des maladies caractérisées par un excès d'hormones stéroïdiennes qui peut inhiber la synthèse de collagène. C'est également décrit concomitamment avec des atteintes néoplasiques ou infectieuses, avec une inflammation sévère dont la pathogénie reste largement méconnue.
Objectifs
Décrire trois cas de FSFS chez des chats devenus cachectiques secondairement à différentes causes sans lien avec des corticoïdes. Décrire les données histopathologiques des tissus conjonctifs à la fois pour la peau fragile et pour la peau après cicatrisation.
Résultats
Tous les chats ont développés une cachexie en moins de deux mois (score de condition corporelle allant de 1 à 1.5). Des maladies concomitantes étaient diagnostiquées dans le Cas 1 (pneumonie par aspiration due à un mégaœsophage) et le Cas 2 (FIV). Dans le Cas 3, la cause primaire suspectée était la malnutrition. La principale donnée histologique de la peau fragile était un derme atrophique avec des fibres de collagènes fines et irrégulières éosinophiliques, pales avec des centres rouges observés à la coloration de Masson. Les fibres élastiques étaient normales. Les données histopathologiques post‐cicatrisation à 11 (Cas 1) et six mois (Cas 3) après diagnostic, indiquaient une normalisation du collagène et de l'ensemble de la peau en comparaison avec les contrôles.
Conclusions et importance clinique
A la connaissance des auteurs, ceci est la première description d'un FSFS non induit par les corticoïdes, réversibles, associés à une rapide cachexie chez les chats.
Resumen
Introducción
El síndrome felino de fragilidad de la piel (FSFS) es un trastorno adquirido caracterizado por la alteración de la producción de colágeno, resultando en una piel extremadamente delgada y frágil. FSFS se asocia con enfermedades caracterizadas por exceso de hormonas esteroides que pueden inhibir la síntesis de colágeno. También se describe asociados con enfermedades inflamatorias, infecciosas o neoplásicas severas en las que la patogénesis permanece ampliamente desconocida.
Objetivos
Describir tres casos de FSFS en gatos que progresaron con caquexia secundario a causas diferentes sin presencia de glucocorticoides. Describir las características histopatológicas del tejido conectivo tanto para la piel frágil como para la piel después de la cicatrización.
Resultados
Todos los gatos desarrollaron caquexia en menos de dos meses (índice de condición corporal que oscilaba entre 1‐1,5). Enfermedades concomitantes fueron diagnosticadas en el caso 1 (neumonía por aspiración por megaesófago) y en el caso 2 (virus de la inmunodeficiencia felina (FIV)). En el caso 3, se sospechaba la malnutrición como una causa primaria. La característica histológica principal de la piel frágil era una dermis atrófica con fibras de colágeno eosinofílicas pálidas, delgadas e irregulares con numerosos centros rojos observados con la tinción de Masson. Las fibras elásticas eran normales. Los hallazgos histopatológicos tras su recuperación de la enfermedad a los 11 meses (caso 1) y los seis meses (caso 3) después del diagnóstico mostraron normalización de la apariencia del colágeno y de la piel completa en comparación con los controles.
Conclusiones e importancia clínica
Según conocimiento de los autores, este es el primer informe que describe una FSFS reversible no inducida por esteroides, asociada con caquexia de rápido desarrollo en gatos.
Zusammenfassung
Hintergrund
Das Hautfragilitätssyndrom der Katze (FSFS) ist eine angeborene Erkrankung, die durch eine veränderte Kollagenproduktion charakterisiert ist, die in einer extrem dünnen und fragilen Haut resultiert. FSFS wird im Zusammenhang mit Erkrankungen gesehen, bei denen eine hohe Steroidproduktion vorkommt, die die Kollagensynthese inhibieren kann. Es wird ebenfalls gleichzeitig mit hochgradig entzündlichen, infektiösen oder neoplastischen Zuständen, bei denen die Pathogenese weitgehend unbekannt ist, beschrieben.
Ziele
Die Beschreibung von drei Fällen eines FSFS bei Katzen, die aus verschiedenen Gründen sekundär kachektisch wurden ohne dass Glukokortikoide involviert waren. Eine Beschreibung der histopathologischen Merkmale von Bindegewebe sowohl in fragiler wie auch in abgeheilter Haut.
Ergebnisse
Alle Katzen entwickelten in weniger als zwei Wochen eine Kachexie (Body Condition Score reichte von 1‐1,5). Bei Fall 1 wurden Begleiterkrankungen diagnostiziert (Aspirationspneumonie aufgrund eines Megaösophagus) und bei Fall 2 (Felines Immundefizienzvirus (FIV). Bei Fall 3 wurde eine Fehlernährung als Primärursache vermutet. Die hauptsächlichen histologischen Merkmale der fragilen Haut waren eine atrophische Dermis mit schwach‐eosinophilen, dünnen und unregelmäßigen Kollagenfasern mit zahlreichen roten Stammfasern, die mittels Massonfärbung gesehen wurden. Die elastischen Fasern waren normal. Die histopathologischen Befunde nach der Erholungsphase wiesen 11 (Fall 1) und sechs Monate (Fall 3) nach der Diagnose auf eine Normalisierung des Kollagens und der ganzen Haut im Vergleich mit den Kontrollen hin.
Schlussfolgerungen und klinische Bedeutung
Nach bestem Wissen der Autoren handelt es sich hierbei um den ersten Bericht einer reversiblen, nicht durch Steroide induzierten FSFS, die im Zusammenhang mit einer sich rasch entwickelnden Kachexie bei Katzen auftrat.
要約
背景
猫皮膚脆弱症候群(FSFS)は、コラーゲンの産生異常を特徴とする後天性疾患であり、極度に菲薄した脆弱な皮膚をもたらす。FSFSは、コラーゲン合成を阻害するステロイドホルモンの過剰産生を引き起こす疾患と関連している。また、重症の炎症性疾患、感染性疾患または腫瘍との併発も報告されているが、その病因の大部分がいまだ不明である。
目的
グルココルチコイドが関与しない様々な原因に続発して悪液質に陥った猫のFSFSの3症例を紹介する。脆弱な皮膚と治癒後の皮膚の両方の結合組織の病理組織学的特徴を記述すること。
結果
すべてのネコが過去2ヶ月以内に悪液質に陥った(BCSは1〜1.5)。症例1(巨大食道による吸引肺炎)および症例2(猫免疫不全ウイルス(FIV))において、併発疾患が診断された。症例3では、栄養失調が主要な原因として考えられた。病理組織学的に脆弱な皮膚は、マッソン染色で観察される多数の赤色コアを有する淡色好酸球性の細く不規則なコラーゲン線維を伴う真皮の萎縮を認めた。弾性線維は正常であった。診断後11ヶ月(症例1)および6ヶ月(症例3)における回復後の病理組織学的所見は、コントロールと比較して、コラーゲンおよび皮膚全体の正常化を認めた。
結論および臨床的な重要性
著者の知る限りでは、これは急速に発症した猫の悪液質に関連した可逆的な非ステロイド誘発性FSFSを記述する最初の報告である。
摘要
背景
猫皮肤脆弱综合征(FSFS),是一种获得性皮肤病,其特征是改变胶原蛋白产物,导致皮肤菲薄和脆弱。FSFS发病特征,是过多的类固醇激素抑制胶原蛋白的合成。也有发现该病与严重的炎症、感染或肿瘤病并发,主要的发病原因尚不清楚。
目的
介绍三个猫的FSFS病例,在没有糖皮质激素摄入的情况下,由于不同病因继发了恶病质。描述脆弱皮肤和皮肤愈合后结缔组织的组织病理学特征。
结果
所有猫在两个月内发展为恶病质(体况评分1‐1.5分)。病例1(由于巨食道症导致的吸入性肺炎)和病例2(猫免疫缺陷病毒(FIV))被诊断出了并发病。营养不良被怀疑是病例3的原发病因。脆弱皮肤主要病理学特征是真皮萎缩,伴有嗜酸性、细弱和不规则的胶原蛋白,Masson's染色发现大量红芯。弹性纤维是正常的。在诊断的11个月(病例1)和六个月(病例3)后进行尸检,组织病理学发现提示胶原蛋白正常,整个皮肤也比较正常。
结论和临床意义
据作者所知,这是首篇非类固醇、可逆,由快速发展的恶病质导致的FSFS病例报告。
Resumo
Contexto
A síndrome da fragilidade cutânea felina (SFCF) é um distúrbio adquirido caracterizado pela produção alterada de colágeno, resultando em uma pele extremamente frágil e atrofiada. A SFCF está associada a doenças que cursam com excesso de hormônios esteroidais que podem inibir a síntese de colágeno. É também descrita concomitantemente com doenças inflamatórias, infecciosas e neoplásicas, e esta patogênese permanece desconhecida.
Objetivos
Descrever três casos de SFCF em gatos que se tornaram caquéticos secundariamente a diferentes causas, sem envolvimento de glicocorticoides. Descrever as características histopatológicas do tecido conectivo da pele frágil e da pele após resolução do quadro.
Resultados
Todos os gatos desenvolveram caquexia em menos de dois meses (escore de condição corpórea variando de 1‐1,5). No Caso 1, foram diagnosticadas concomitantemente pneumonia por aspiração secundária à megaesôfago. No Caso 2, foi diagnosticado o vírus da imunodeficiência felina (FIV). No Caso 3, a causa primária suspeita para SFCF foi a malnutrição. O principal achado histopatológico da fragilidade cutânea foi atrofia dérmica com eosinófilos pálidos, fibras colágenas finas e irregulares com numerosos grumos vermelhos corados com corante de Masson. As fibras elásticas estavam normais. Os achados histopatológicos pós recuperação, 11 meses (Caso 1) e seis meses (Caso 3) após o diagnóstico, demonstraram normalização do colágeno e de toda a pele, quando comparados aos controles.
Conclusões
De acordo com o conhecimento do autor, este é o primeiro relato descrevendo SFCF reversível, não induzida por esteroides e associada ao desenvolvimento rápido de caquexia em felinos.
Abbreviations
- BCS
body condition score
- FIV
feline immunodeficiency virus
- FSFS
feline skin fragility syndrome
Introduction
Feline skin fragility syndrome (FSFS) is an acquired disorder characterized by markedly thin skin that easily tears following minimal trauma.1 Hyperextensibility is not observed. Skin wounds are usually associated with absent or poor bleeding and are difficult to manage. The main histological changes include a thin epidermis and a severely atrophic dermis with disorganized collagen fibres that are reduced in number and size.2 Hair follicle atrophy can be variable whereas arrector pili muscles appear relatively enlarged. Masson's trichrome stain can demonstrate tinctorial abnormalities of collagen fibres whereas elastic fibres are normal.2, 3 Electron microscopy reveals collagen bundles that are convoluted and arranged haphazardly with fibrils of different diameters, and loosely packed.4
This syndrome is described in cats suffering from diseases with well‐known negative effects on collagen synthesis including hypercortisolism (spontaneous or iatrogenic) or excessive use of progestational drugs.5, 6, 7, 8, 9 It also has been reported in association with different diseases where the pathogenesis is not clear including hepatic lipidosis, feline infectious peritonitis (FIP), cholangiohepatitis, cholangiocarcinoma and multicentric lymphoma.4, 10, 11, 12, 13 To the best of the authors’ knowledge, histopathological descriptions postrecovery are not available due to the fact that cats usually don't recover or are euthanized shortly after diagnosis. There is one case of a reversible defect in a cat experimentally receiving phenytoin.14
This case report describes three cases of FSFS associated with cachexia secondary to different causes without the implication of steroidal hormones.
Case reports
Case1
A 9‐year‐old, 2.6 kg, castrated male domestic short hair (DSH) cat was presented with a markedly thin skin observed after clipping for abdominal ultrasound and the appearance of a skin tear at the base of the tail. The cat had been examined two weeks before, with a three month history of regurgitation associated with the loss of 1.5 kg. Complete blood analysis was unremarkable, serology for feline immunodeficiency virus (FIV), feline leukaemia virus (FeLV) and feline coronavirus were negative; hyperthyroidism was excluded (TT4 1.06, reference interval (r.i.) 1.21–3.29; fT4 8.1, r.i. 8.1–16.9). The abdominal ultrasound was normal.
The cat appeared slightly depressed with significant muscle wasting and a body condition score (BCS) of 1.5 of 5 and with estimated dehydration of 5–6%.15, 16 Serous nasal discharge, dyspnoea and respiratory stridor were also present. Dermatological examination revealed a dull sparse hair coat and easily visible blood vessels in the region of clipping. One single, nonbleeding, irregular skin tear of about 3 cm was observed on the sacral region (Figure 1). Radiographs revealed the presence of mega oesophagus and an alveolar pattern in the cranial lung lobes consistent with aspiration pneumonia. Oral antibiotic therapy was started once daily with cefadroxil (Cefacure Tabs, MSD Animal Health; Milan, Italy; 25 mg/kg) and enrofloxacin (Baytril, Bayer; Milan, Italy; 5 mg/kg). Specific management of feeding to reduce the risk of regurgitation was introduced. Histopathological findings were consistent with FSFS. The owner declined any further investigation to determine the cause of mega oesophagus. Three months later, the cat was 3.2 kg and the respiratory and cutaneous problems were resolved. A second biopsy of macroscopically normal skin was taken 11 months after the first visit. There has been no recurrence three years after the condition was diagnosed.
Figure 1.
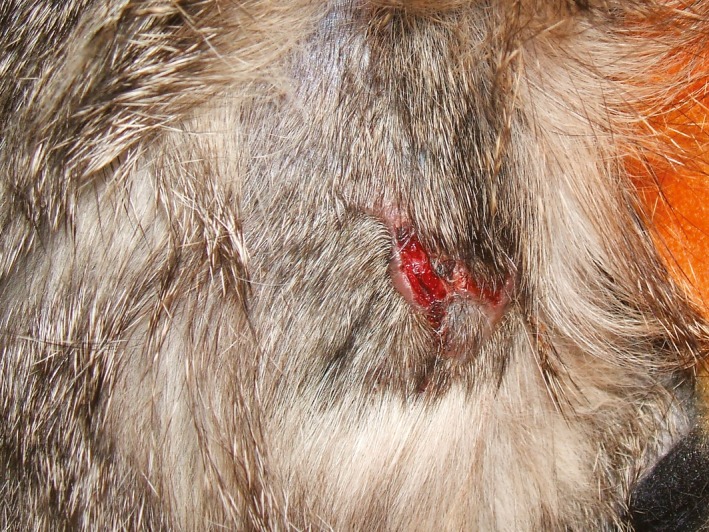
Acquired skin fragility syndrome in a cat. Case 1, skin tear observed on the sacral region. The lesion was 3 cm long and resembled an irregular “W”.
Case 2
A 13‐year‐old, 2.1 kg, male DSH feral cat was evaluated for a skin tear on the right shoulder that occurred during a restraint technique.
Ten weeks before, the cat had been hit by a car resulting in severe facial trauma with multiple fractures of the mandible and palate. The cat weighed 3 kg, was FIV positive, slightly anaemic (red blood cells (RBC) 4.70 (range 5–10) × 106/μL; haematocrit (Hct) 23 (30–45)%) with mild hyperechoic liver parenchyma and renal cortex based on ultrasound examination. After stabilization, three reconstructive surgeries were performed but normal mastication was not achieved; the cat's skin was normal during the four week recovery period. Six weeks after discharge, the cat was presented with severe cachexia (BCS of 1 of 5), hypothermia and hypoglycaemia. Abdominal ultrasonic findings were unchanged whereas the anaemia had progressed (RBC 4.64, Hct 21.3%) and the albumin decreased (2.1 (2.5–4) g/dL). Two linear, nonbleeding, skin tears of about 2–3 cm were observed on the right scapula and flank together with fine dark material consistent with flea faeces. Cutaneous histopathological evaluation confirmed a diagnosis of FSFS. The cat died spontaneously after several days of hospitalization. Postmortem examination was not performed.
Case 3
A 3‐month‐old, 0.9 kg, female DSH cat living in an outdoor feral cat colony was presented due to poor body condition and lethargy. The physical examination revealed a markedly decreased body condition with a BCS of 1.5 of 5 and an estimated dehydration of 6–7%. The cat was hospitalized to receive intravenous fluids and nutritional support. Serological tests for FIV and FeLV were negative. During normal restraint for removing the needle‐cannula, the skin tore on the forelimb and dorsal neck. Examination revealed generalized thinning of the skin, no evidence of scarring and hyperextensibility. The skin lacerations were sutured and a skin biopsy was collected. Histopathological evaluation and special stains for connective tissue were suggestive of FSFS. Meanwhile, the cat was adopted by a family and fed with a balanced diet. Cutaneous wounds healed and the kitten grew normally. At nine months of age, two skin biopsies were taken during ovariohysterectomy. At the time of writing, the cat was clinically healthy three years after the condition was diagnosed.
Histological examination and special stains
Skin biopsy specimens were collected using a 6–8 mm skin biopsy punch under local anaesthesia (cases 1 and 2) or general anaesthesia (Case 3). Samples were obtained from the interscapular area in cases 1 and 2 and from the left dorsolumbar region in Case 3. Postrecovery samples were taken from the interscapular region (cases 1 and 3) and dorsolumbar sacral region (Case 3). Control tissue was obtained from two cats with normal appearing skin and without history of skin diseases. Samples were processed and stained with haematoxylin and eosin (H&E), Masson's trichrome stain and Mallory's trichrome stain; specimens were examined by one of the authors (CB). The thickness of the epidermis (from the basement membrane (BM) to the stratum corneum) and of the dermis (from the BM to the panniculus, perpendicularly to the epidermis) was recorded in 10 different points across each biopsies. The mean value was calculated for each biopsy.
Fragile skin (cases 1–3)
When compared with the control skin, the dermis appeared reduced in thickness (mean: 1 mm versus 1.7 mm). The arrector pili muscles were prominent. The collagen fibres were diffusely pale eosinophilic, thin and irregular in shape. Masson and Mallory's trichrome stains demonstrated a reduced staining intensity of collagen fibres whereas elastic fibres were normal. Furthermore, Masson's stain revealed an altered tinctorial affinity of collagen fibres (red fibres) in affected cats compared to nonaffected cats (Figure 2).
Figure 2.

Histopathological findings of acquired skin fragility syndrome. (a) Histological section of the fragile skin of a cachectic cat (Case 1). The dermis is severely reduced in thickness and collagen fibres are diffusely pale, thin and irregular in shape. Haematoxylin and eosin (H&E) stain, ×100 magnification. (b) Histological section of the fragile skin of the same cachectic cat as in (a), after healing (Case 1). The dermal thickness and collagen fibre appearance have returned to normal morphology. H&E, ×40 magnification. (c) Histological section of the skin of a cat with steroid‐induced, acquired feline skin fragility syndrome. The dermis is severely reduced in thickness and the arrector pili muscles are prominent. H&E, ×40 magnification. (d) Altered tinctorial affinity of collagen fibres (red fibres) observed in the skin of an affected cat (Case 3). Masson's trichrome stain, ×200 magnification.
Macroscopically normal skin (cases 1 and 3)
The epidermis and dermis showed no significant differences compared to the control skin samples.
Discussion
Feline skin fragility syndrome (FSFS) is an acquired disorder most commonly reported in association with diseases where there are high levels of steroid hormones.5, 6, 7, 8, 9 It is presumed that reduced collagen synthesis due to endogenous or exogenous glucocorticoids contributes to the aetiopathogenesis of this syndrome. Less commonly, FSFS has been described in cats affected by severe inflammatory, infectious or neoplastic diseases without glucocorticoids involvement.10, 11, 12, 13 In these latter cases none of the cats recovered, contrary to this report. Hepatic lipidosis, a condition frequently observed in FSFS, was excluded in cases 1 and 2 by abdominal ultrasound and in Case 3 because the history was not consistent with this disease. In the cats in this series, skin fragility was diagnosed after the development of a cachectic condition. A common element observed in cases 1 and 2 was the relatively abrupt reduction in food intake and the consequent rapid and marked weight loss. Undoubtedly, the presence of infectious diseases likely contributed to the development of cachexia. In Case 2, FIV was considered to have a chronic course but it could have further hampered the recovery of the cat exacerbating its already limited ability to feed itself. In the kitten (Case 3), malnutrition was the only suspected cause. In this cat, the high‐energy requirements due to the young age may have prompted the development of cachexia, even in the absence of a concurrent illness.
These case reports seem to reinforce the hypothesis that lack of nutrient intake and severe catabolic state, as happens during cachexia, can negatively influence collagen production in some predisposed cats, leading to skin fragility. As demonstrated by histological examination and special stains, the skin of these cats was indistinguishable from the controls once the animals recovered from the cachectic status. Because corticosteroids also induce a catabolic state, the steroid‐induced FSFS and the cachexia‐related FSFS could share, at least partially, similar pathogenic pathways. Cachexia per se, or in association with other specific diseases, could play a crucial role in the pathogenesis of FSFS, mainly in those cases where exogenous steroids are not involved.
This report demonstrates that not all the cats with FSFS have a poor prognosis and, depending on the cause, a complete recovery is possible.
Acknowledgement
The authors are grateful to Veronica Greatti for providing the second skin biopsy in case 3.
Source of funding: This study was self‐funded.
Conflict of interest: No conflicts of interest have been declared.
References
- 1. Miller WH, Griffin CE, Campbell KL. Muller and Kirk's Small Animal Dermatology, 7th edition St. Louis, MO: Elsevier Inc., 2013; 695–723. [Google Scholar]
- 2. Gross TL, Ihrke PJ, Walder EJ et al. Skin Diseases of the Dog and Cat, Clinical and Histopathologic Diagnosis, 2nd edition Ames, IA, USA: Blackwell Science Ltd, 2005; 389–392. [Google Scholar]
- 3. Fernandez CJ, Scott DW, Erb HN et al. Staining abnormalities of dermal collagen in cats with cutaneous asthenia or acquired skin fragility as demonstrated with Masson's trichrome stain. Vet Dermatol 1998; 9: 49–54. [DOI] [PubMed] [Google Scholar]
- 4. Regnier A, Pieraggi MT. Abnormal skin fragility in a cat with cholangiocarcinoma. J Small Anim Pract 1989; 30: 419–423. [Google Scholar]
- 5. Rossmeisl JH Jr, Scott‐Moncrieff JC, Siems J et al. Hyperadrenocorticism and hyperprogesteronemia in a cat with an adrenocortical adenocarcinoma. J Am Anim Hosp Assoc 2000; 36: 512–517. [DOI] [PubMed] [Google Scholar]
- 6. Briscoe K, Barrs VR, Foster DF et al. Hyperaldesteronism and hyperprogesteronism in a cat. J Feline Med Surg 2009; 11: 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spada E, Proverbio D, Giudice C et al. Pituitary‐dependent hyperadrenocorticism and generalised toxoplasmosis in a cat with neurological signs. J Feline Med Surg 2010; 12: 654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross E, Moreland R, Wallack S. Feline pituitary‐dependent hyperadrenocorticism and insulin resistance due to a plurihormonal adenoma. Top Companion Anim Med 2012; 27: 8–20. [DOI] [PubMed] [Google Scholar]
- 9. Sharman M, FitzGerald L, Kiupel M. Concurrent somatotroph and plurihormonal pituitary adenomas in a cat. J Feline Med Surg 2013; 15: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zur G. Feline skin fragility syndrome in a cat with hepatic lipidosis In: Kwochka KW, Willemse T, von Tscharner C, eds. Advances in Veterinary Dermatology, Volume 3 Oxford, UK: Butterworth‐Heinemann, 1998: 495–496. [Google Scholar]
- 11. Trotman TK, Mauldin E, Hoffmann V et al. Skin fragility syndrome in a cat with feline infectious peritonitis and hepatic lipidosis. Vet Dermatol 2007; 18: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniel AGT, Silvia RRL, Archivaldo R Jr et al. Skin fragility syndrome in a cat with cholangiohepatitis and hepatic lipidosis. J Feline Med Surg 2010; 12: 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crosaz O, Vilaplana‐Grosso F, Alleaume C et al. Skin fragility syndrome in a cat with multicentric follicular lymphoma. J Feline Med Surg 2013; 15: 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barthold SW, Kaplan BJ, Schwartz A. Reversible dermal atrophy in a cat treated with phenytoin. Vet Pathol 1980; 17: 469–476. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin K, Bartges J, Buffington T et al. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 2010; 46: 285–295. [DOI] [PubMed] [Google Scholar]
- 16. Devey JJ. Crystalloid and colloid fluid therapy In: Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine. 7th edition St. Louis, MO: Elsevier, 2010; 488. [Google Scholar]


