Abstract
Abstract
Objective
To describe ocular findings in kittens with congenital or early neonatal infection by Toxoplasma gondii and to determine if there are detectable differences in disease caused by three strains.
Animals studied
Six adult female cats and the offspring from seven litters.
Methods
Four kittens from uninfected specific pathogen‐free (SPF) queens and 21 kittens from SPF queens inoculated at various times late in gestation with Mozart, Maggie, or ME‐49 strain of T. gondii were used. Ocular examinations were performed on queens prior to and after delivery, and on kittens weekly to bi‐weekly for up to 27 weeks. Whole blood for serology was collected from all kittens at 5½ to 8 weeks of age and again at 12 weeks of age or later.
Results
No kittens from noninfected queens developed ocular lesions or antibody to T. gondii. Three of the 24 kittens from infected queens died or were euthanized early in the study. Chorioretinitis was detected in 15 of 21 living kittens from infected queens. Two developed concurrent anterior uveitis that resolved within 1 week. Posterior segment lesions varied ophthalmoscopically between strains. Of 21 kittens from T. gondii‐infected queens, six developed positive antibody titers to T. gondii during the study. All seropositive kittens were born to queens infected with Mozart strain of T. gondii.
Conclusion
Results of this study suggest that ocular toxoplasmosis can occur without other evidence of clinical illness in kittens infected in utero or in the neonatal period, and that T. gondii strains may have varying degrees of ocular pathogenicity in cats.
Keywords: chorioretinitis, neonatal toxoplasmosis, ocular toxoplasmosis, uveitis
Introduction
The protozoan parasite, Toxoplasma gondii, commonly infects both cats and humans. In humans, infection by T. gondii rarely causes noticeable clinical disease unless the person is immune suppressed or infected in utero. 1 T. gondii infection in cats is usually subclinical, although clinical disease has been well documented in both kittens and adults. 2 Cats with disseminated organism replication resulting in clinical toxoplasmosis usually have multiple organ involvement that commonly includes the eyes. In naturally infected cats with disseminated toxoplasmosis, the organism has been detected in association with inflammation in the retina, optic nerve, choroid, ciliary body, and iris. 3
The prevalence of ocular toxoplasmosis in cats that have no other evidence of clinical disease is difficult to estimate because the diagnosis is difficult to confirm. Serum antibodies against T. gondii have been detected in as many as 74.2% 4 of cats with uveitis as the primary clinical complaint. However, up to 53.4% of healthy cats are positive as well. 5 Thus, detection of serum antibodies cannot be used alone to document ocular toxoplasmosis. Using aqueous humor antibody testing has a better predictive value than serum antibody testing, but results do not always correlate to ocular toxoplasmosis in individual cats. 6 , 7
While the organism is commonly found in the eyes of cats with fatal, disseminated toxoplasmosis, 3 ocular histopathology rarely identifies the organism in the eyes of cats with uveitis only. 8 It has been suggested that uveitis in cats that are otherwise healthy might occur as a result of exposure to Toxoplasma antigens at nonocular locations. 9 However, T. gondii DNA has been detected by polymerase chain reaction (PCR) in the aqueous humor of naturally exposed and experimentally infected cats, confirming that the organism reaches the eye. 10 , 11 It is clear from serologic studies, aqueous humor antibody studies, and aqueous humor PCR studies that cats with uveitis commonly have been exposed to T. gondii, but it is unclear why some cats develop ocular disease and others do not.
Clinical manifestations of congenital toxoplasmosis in humans are often confined to the eye, but can be more generalized and cause devastating clinical illness. 12 Experimental infection of queens with T. gondii during gestation has resulted in disease in the neonate that ranges from clinically inapparent to grave. 13 , 14 Clinical ocular examinations were not performed in these studies so mild ocular disease in infected kittens that were otherwise healthy could have easily been missed. Additionally, as with humans, the severity of clinical disease induced in the neonate may depend upon when during gestation infection occurred. We hypothesized that, like people, ocular toxoplasmosis in cats may be related to infection in utero or the neonatal period.
The virulence of T. gondii isolates varies greatly in mice, and it has been proposed that exposure to oculotropic strains of T. gondii may explain the high incidence of ocular toxoplasmosis in an adult human population in Brazil. 15 Infection of adult cats with different T. gondii isolates results in varying manifestations of clinical disease (Lappin MR, unpublished data). Thus, the specific T. gondii isolate that infects an individual cat may in part determine whether disease will occur.
The goals of this study were to describe the ocular findings in kittens with congenital or early neonatal infection by T. gondii, and to determine if there are detectable differences in disease caused by three different strains.
MATERIALS AND METHODS
Adult cats
Six young adult female, specific‐pathogen free, domestic shorthair cats were purchased (Liberty Research, Inc., Waverly, NY, USA) and housed individually in infectious disease isolation facilities. The use of the cats was in accordance with ARVO resolution concerning the use of animals in research, and was approved by the Animal Care and Use Committee at Colorado State University. Queens were purchased while pregnant or were bred in house with a male from an internal breeding colony. Estimated dates of conception were either provided by the vendor or were estimated based on when the queens were housed with the male. All cats were tested and shown to be seronegative for T. gondii IgM and IgG, 5 FIV antibodies (Snap Combo, IDEXX Laboratories, Portland, ME, USA), coronavirus antibodies (Diagnostic Laboratory, Colorado State University, CO, USA), and FeLV p27 antigen (Snap Combo). All queens were examined by slit lamp biomicroscopy and binocular indirect ophthalmoscopy, and found to be free of ocular disease.
Experimentally induced T. gondii infection
The Mozart, Maggie, and ME‐49 strains of T. gondii were used in this study. The Mozart strain was initially isolated from the eye of a cat with uveitis (courtesy of Dr Michael Davidson, North Carolina State University), the Maggie strain was initially isolated from the peritoneal cavity of a cat with clinical toxoplasmosis, and the ME‐49 strain was initially isolated from a sheep. Strain CF‐1 mice were inoculated SQ with 104 T. gondii strain Mozart, Maggie, or ME‐49 sporulated oocysts. Six weeks after inoculation, the mice were anesthetized with isoflurane and euthanized by cervical dislocation. The brain was collected and homogenized in sterile 0.9% NaCl by repeatedly drawing the tissue through an 18‐gauge needle. Tissue cysts were quantitated, using a hemocytometer. Maggie (n = 300), Mozart (n = 300) or ME‐49 (n = 540) tissue cysts were orally administered to each cat by gavage after withholding food overnight.
Experimental design
Six queens were used to produce seven litters of kittens (Table 1). All queens and their kittens were housed in separate cages until the kittens were weaned, when they were gang housed. Control and experimental animals were housed in separate rooms.
Table 1.
Experimental T. gondii infection
| Queen (Litter) | T. gondii strain (Cyst numbers) | Inoculation (Days before queening) | Surviving kittens (Numbers and use) | Kitten ocular examinations (Week after birth) |
|---|---|---|---|---|
| 6 (g) | Maggie (300) | 21 | Seven kittens; all experimental | 4, 4½, 5, 5½, 6, 6½, 7, 7½, 8, 9, 9½, 10, 10½, 11, 12, 13, 14 |
| 3 (d) | ME‐49 (540) | 5 | Five kittens; all experimental Hand reared after 1 week | 4, 5, 5½, 6, 6½, 7, 7½, 8, 9, 9½, 10, 10½, 11, 12, 13, 14, 15½, 16½ |
| 1 (a) | Mozart (300) | 17 | Three kittens; all experimental | 5, 8, 11½, 12, 13, 13½, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25 |
| 5 (f) | Mozart (300) | 8 | Four kittens; one experimental, Two died, one euthanized | 2½, 3, 4, 4½, weekly weeks 5–27 |
| 4 (e) | Mozart (300) | 1 | Five kittens; all experimental | 3½, 4, 5, 5½, weekly weeks 6–27 |
| 2 (b) | Not infected | Not infected | Four kittens; two used as controls Two transferred to other studies | 10, 11, 12, 13, 14, 15, 16 |
| 3 (c) | Not infected | Not infected | Four kittens; two used as control Two transferred to other studies | 10, 11, 12, 13, 14, 15, 16 |
Fecal examinations
When available, fecal samples were collected daily for at least 3 weeks post infection, for microscopic examination for oocysts after sugar flotation. 2 Samples were stored at 4 °C until analysis could be performed.
Serologic testing
Whole blood was collected from queens and kittens on varying dates. For control queens, blood collections were prior to and 3 months after queening. For infected queens, blood collections were prior to and at least 10 weeks after infection with T. gondii. Whole blood was collected from all kittens at 12 weeks of age or later and at 5½ weeks (litter a), 6½ weeks (litter d), 7 weeks (litter f), and 8 weeks (litters e and g). After centrifugation, serum was collected and frozen at −70 °C until assayed for T. gondii IgM and IgG. 5 A titer of 1 : 64 was considered to be positive in both assays.
Physical and ocular examinations
Queens and kittens were observed and examined for changes in attitude, feeding, appearance, and behavior. Daily observations were carried out until the kittens were approximately 5 weeks old after which time the kittens were monitored at least three times per week. The queen of litter d died 7 days after parturition and from that time until weaning at 5 weeks of age, the kittens were hand fed with a commercially available kitten milk replacer (KMR®; PetAg Inc., Hampshire, IL, USA). All kittens were introduced to commercial cat food at 4 weeks of age. At approximately 12 weeks of age, kittens were gang housed with either other control or experimental cats from the study.
Ocular examinations utilizing slit lamp biomicroscopy and indirect ophthalmoscopy were performed after pupil dilation with 1% tropicamide. Queens were examined prior to and at least once after delivery. Kittens were examined on varying dates following the schedule detailed in Table 1. Ocular lesions were photographed using a fundus camera (RC‐2, Kowa Optimed, Inc., Washington D.C., USA) with either ASA 25 or ASA 64 color slide film. Photographs were computer scanned and lesion size estimated by computer measurement.
RESULTS
Fecal examinations
All infected queens shed oocysts between days 4 and 12 post infection documenting successful infection.
Serology
Sera from control queens and their kittens were negative for T. gondii IgM and IgG. Sera from experimental queens were negative for T. gondii IgM and IgG prior to inoculation with T. gondii, but were positive for IgG when tested 10 weeks after inoculation. Of the kittens from T. gondii‐infected queens, six of 21 kittens developed positive antibody titers for T. gondii during the study period. One kitten from litters a and f, and four kittens from litter e were positive for T. gondii IgG by 12 weeks of age. T. gondii IgM was not detected at any time. All of the kittens that seroconverted were born to queens infected with the Mozart strain of T. gondii.
Physical examinations
Queen 3 developed labored breathing 11 days post T. gondii inoculation (4 days post parturition) and died within 24 h. Necropsy results confirmed generalized toxoplasmosis. All other adult cats were apparently healthy throughout the study. All kittens remained healthy throughout the study with the exception of three kittens from litter f. Two kittens from litter f were eaten by the queen 3 and 5 days after birth. The kittens had not been observed to be clinically ill prior to their death. One kitten from litter f developed a mild head tilt 5 days after birth and was euthanized 10 days later due to progression of CNS signs. Necropsy and histopathologic examinations failed to identify the cause of neurologic signs in this kitten.
Ocular examinations
Ocular inflammation was not observed in adult cats or in control kittens. In all, 15 kittens developed lesions consistent with chorioretinitis. Chorioretinitis was detected in the six kittens that developed detectable T. gondii IgG. Lesion distribution, age at lesion appearance, and maximum titer are listed in Table 2. Concurrent anterior uveitis (trace flare, keratic precipitates) was detected in two kittens from litter e but resolved within 1 week. Of the 15 seronegative kittens born to experimentally infected queens, nine developed focal chorioretinitis that was detected between 6 and 13 weeks of age. None of these nine kittens were observed to have anterior uveitis.
Table 2.
Ocular examination results
| Litter‐kitten | Appearance of uveitis (weeks after birth) | Location | Highest Titer | |||
|---|---|---|---|---|---|---|
| OD | OS | OD | OS | IgM | IgG | |
| g‐1 | 8 | P | neg | neg | ||
| g‐2 | neg | neg | ||||
| g‐3 | neg | neg | ||||
| g‐4 | 9 | P | neg | neg | ||
| g‐5 | 9 | P | neg | neg | ||
| g‐6 | 9 | P | neg | neg | ||
| g‐7 | 9 | P | neg | neg | ||
| d‐1 | 6½ | 7½ | P | P | neg | neg |
| d‐2 | 13 | P | neg | neg | ||
| d‐3 | 9 | 10 | P | P | neg | neg |
| d‐4 | neg | neg | ||||
| d‐5 | 9½ | P | neg | neg | ||
| a‐1 | neg | neg | ||||
| a‐2 | neg | neg | ||||
| a‐3 | 12 | 12 | P | P | neg | 1 : 8192 |
| f‐1 | 11 | P | neg | 1 : 1024 | ||
| e‐1 | 11½ | P | neg | 1 : 1024 | ||
| e‐2 | 7 | 7 | P | A,P | neg | 1 : 4096 |
| e‐3 | neg | neg | ||||
| e‐4 | 9 | 9 | P | P | neg | 1 : 2048 |
| e‐5 | 10 | 12 | A | P | neg | 1 : 4096 |
P, posterior segment; A, anterior segment.
Many of the choroidal and retinal lesions in kittens from the Mozart strain‐infected queens (litters a, e and f) were first detected as variable sized (some > 2 disc diameter) areas of subtle tapetal color change that progressed to more pronounced gray discoloration. Some developed gray‐white subretinal elevations (Fig. 1). All chorioretinal lesions in kittens from the ME‐49 and Maggie strain‐infected queens had the appearance of small (up to ~0.2–0.3 disc diameters), gray dots in the tapetal fundus. All of these kittens had one or two small lesions (Fig. 2), with the exception of one kitten from litter d that had numerous lesions scattered throughout the tapetal fundus OU (Fig. 3). Lesions of the nontapetal fundus were not found in any kittens.
Figure 1.
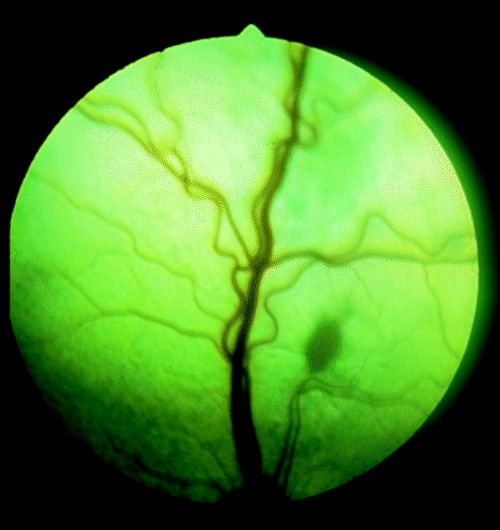
Oval shaped gray‐white subretinal exudate in a kitten born to a queen infected with the Mozart strain of T. gondii. A larger area of tapetal discoloration can be seen superior to the exudate.
Figure 2.
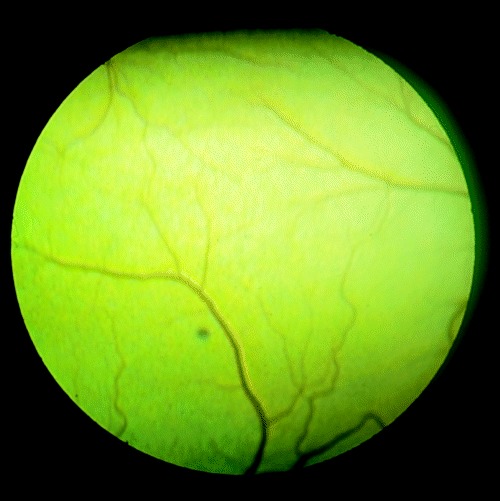
Focal pin‐point gray lesion of the tapetal fundus, typical of kittens born to queens infected with either the Maggie or ME‐49 strain of T. gondii.
Figure 3.
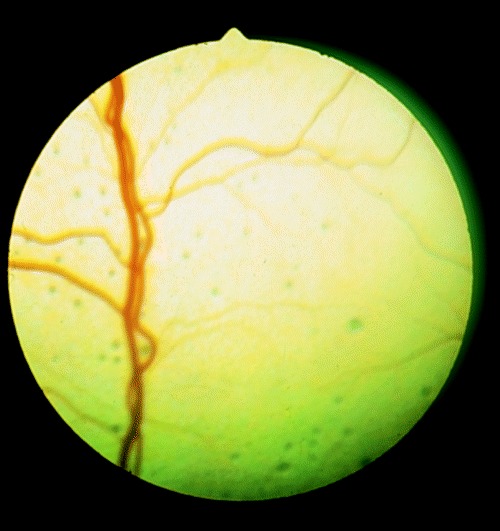
Multifocal pinpoint lesions of the tapetal fundus of a kitten born to a queen infected with ME‐49 strain of T. gondii.
The size and intensity of most lesions began to abate between 2 and 4 weeks after their initial appearance. Resolution of inflammatory lesions was complete in seven kittens 2–9 weeks after initial detection. Chorioretinal scars with tapetal discoloration and hyper‐reflectivity remained in larger areas of previous inflammation (Fig. 4). Smaller foci of inflammation often resolved without a detectable scar. Inflammation persisted or was resolving in eight kittens when the study ended.
Figure 4.
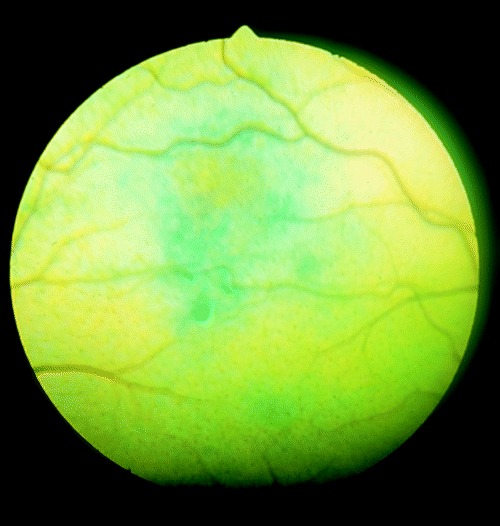
Large area of tapetal discoloration in a region of resolving inflammation.
DISCUSSION
An early study indicated transplacental transfer of T. gondii infection is uncommon in cats. 16 Since then, transplacental infection has been produced in several studies using a different strain of T. gondii. 13 , 14 , 17 It is likely that lactational transmission also occurs as infection rate is higher when kittens born to infected queens are allowed to nurse, and lactational transmission has been demonstrated in other species. 14 , 18
The presence or severity of clinical toxoplasmosis in the neonate is likely influenced by the organism strain, dose, and the stage of fetal development or age of the host at the time of infection. The infecting stage of the organism (tachyzoite, oocyst, cyst) may also be important. Ingestion of tachyzoites, the form most likely found in milk, was previously thought not to cause clinical disease, even in neonates. 2 , 14 Queens in this study were infected with cysts, late in gestation, in order to minimize clinical illness in the kittens. Transplacental infection has been shown to occur in queens infected as late as 13 days prior to parturition, but may not in queens infected later in pregnancy. 14 Three queens in the present study were infected 1, 5, and 8 days before parturition. It is likely that infection via suckling occurred in the kittens from these queens.
In this study, kittens from only one litter (f) died. These kittens were either eaten by the queen or were euthanized due to clinical illness, but systemic toxoplasmosis was not documented. In two previous studies, it was undetermined whether many of the transplacentally infected kittens developed clinical illness because euthanasia was performed prior to or immediately after birth. However, most of the kittens that were allowed to live were clinically ill and died or were euthanized within 8 days of age, although a few remained clinically normal until euthanasia at between 8 and 98 days of age. 13 , 14 Strain ME‐49 was used to infect the queens of those studies and may be related to the high kitten mortality rate. ME‐49 was used to infect only queen 3 in this study. Queen 3 was inoculated 5 days before and died 7 days after parturition. Because the queen was inoculated so late in gestation, infection in the kittens was more likely through ingestion of milk rather than transplacentally. The number of ingested organisms via suckling might have also been decreased because the kittens were hand reared after the queen’s death.
Ocular examinations of kittens with congenital or neonatal toxoplasmosis have not been previously reported; however, uveitis has been documented by necropsy in kittens transplacentally infected. 13 Clinical manifestations of congenital toxoplasmosis in humans are often confined to the eye. 12 This study shows that cats with neonatal infection can also have clinical manifestations confined to the eye. As all kittens were allowed to suckle from infected queens, it is impossible to determine if infection in these kittens occurred transplacentally or through ingestion of milk. Infection of some kittens by ingestion of oocysts from feces or from the perianal area of the infected queen or littermates is also possible but less likely as feces were removed daily before oocysts had time to sporulate and the number of organisms retained in the perianal area is likely to be small. Infection from direct contact with other excretions has not been documented. 19
Differences were found in kittens from queens infected with the Mozart strain of T. gondii compared to kittens from queens infected with ME‐49 or Maggie strains. In general, fundus lesions associated with the Mozart strain tended to be larger and appeared more exudative than those associated with either ME‐49 or Maggie strains. Additionally, kittens with ocular lesions from Mozart‐infected queens were the only kittens to develop detectable antibodies against T. gondii. The relationship of ocular disease to strain difference is not known and could be related to ocular tropism of the Mozart strain. However, because infection date, infection dose, and probably route of infection, varied between litters, the differences may be related to other factors. The kittens from the ME‐49‐infected queen were hand reared after 1 week of age, and so organism ingestion may have been less than kittens from the other litters. Parasite load could also influence development of ocular disease.
Why kittens from queens infected with ME‐49 and Maggie strains of T. gondii developed chorioretinitis consistent with ocular toxoplasmosis but failed to produce detectable levels of antibodies to T. gondii is unknown. It is possible that a low parasite load could result in antibody levels below the threshold of detection by the assays used and could also explain why lesions were small and few in all but one kitten. T. gondii has been isolated from congenitally infected rats that have remained seronegative, 20 and detection of antibodies in mice can be dependent on the infecting strain of T. gondii. 21 Similarly, route of infection and infecting strain may influence titer development of kittens. For humane reasons, none of the seronegative kittens with ocular disease were euthanized for bioassay or histopathology to confirm T. gondii infection. Thus, it is also possible that the ocular lesions were due to another cause and the kittens were not infected with T. gondii. This seems unlikely, however, as none of the control kittens developed similar lesions, the kittens were kept isolated in a controlled environment, and the queens were documented to be infected by T. gondii.
The results of this study suggest that ocular toxoplasmosis can occur without other evidence of clinical illness in kittens born to queens infected during pregnancy, and suggests that the time of infection, and possibly the route, is important to the development of ocular disease. Most cats become infected with Toxoplasma after weaning, by eating tissue cysts in prey animals. If neonatally infected cats are more likely to develop ocular toxoplasmosis, this may help to explain why so many cats in nature are infected but so few develop ocular toxoplasmosis. Results of this study also support the suggestion that some T. gondii strains are oculotropic. With the exception of one kitten, posterior segment lesions in kittens infected with ME‐49 and Maggie strains were less severe than those in kittens infected with the Mozart strain. Although the role strain played in development of ocular disease in this study is not clear because of the small number of litters studied, and the variations in protocol between litters, our results support a recent study of adult cats orally inoculated with ME‐49, Maggie, and Mozart cysts, where ocular lesions were detected in one of seven cats, one of five cats and three of five cats, respectively, suggesting strain may be important in development of ocular disease (Lappin MR, unpublished data).
Although transient anterior uveitis developed in two kittens in this study, chorioretinitis appeared to be the primary manifestation of ocular toxoplasmosis in the neonatal period. This differs from adult cats where T. gondii infection has been correlated more often with anterior segment inflammation. Further studies are needed to determine the importance of neonatal Toxoplasma infection in the development of chronic or recurrent anterior uveitis in the adult cat.
References
- 1. Roberts F & McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitology Today 1999; 15 (2): 51–57.DOI: 10.1016/s0169-4758(98)01377-5 [DOI] [PubMed] [Google Scholar]
- 2. Dubey JP & Beattie CP. Toxoplasmosis in cats In: Toxoplasmosis of Animals and Man. CRC Press Inc, Boca Raton, 1988; 117–124. [Google Scholar]
- 3. Dubey JP & Carpenter JL. Histologically confirmed clinical toxoplasmosis in cats: 100 cases. Journal of the American Veterinary Medical Association 1993; 203 (11): 1556–1566. [PubMed] [Google Scholar]
- 4. Lappin MR, Marks A, Greene CE et al. Serologic prevalence of selected infectious diseases in cats with uveitis. Journal of the American Veterinary Medical Association 1992; 201: 1005–1009. [PubMed] [Google Scholar]
- 5. Lappin MR, Greene CE, Prestwood AK et al. Prevalence of Toxoplasma gondii infection in cats in Georgia using enzyme‐linked immunosorbent assays for IgM, IgG, and antigens. Journal of Veterinary Parasitology 1989; 33 (3–4): 225–230. [DOI] [PubMed] [Google Scholar]
- 6. Chavkin MJ, Lappin MR, Powell CC et al. Toxoplasma gondii‐specific antibodies in the aqueous humor of cats with toxoplasmosis. American Journal of Veterinary Research 1994; 55 (9): 1244–1249. [PubMed] [Google Scholar]
- 7. Lappin MR, Roberts SM, Davidson MG et al. Enzyme‐linked immunosorbent assays for the detection of Toxoplasma gondii‐specific antibodies and antigens in the aqueous humor of cats. Journal of the American Veterinary Medical Association 1992; 201 (7): 1010–1016. [PubMed] [Google Scholar]
- 8. Peiffer RL & Wilcock BP. Histopathologic study of uveitis in cats: 139 cases. Journal of the American Veterinary Medical Association 1991; 198 (1): 135–138. [PubMed] [Google Scholar]
- 9. Davidson MG & English RV. Feline ocular toxoplasmosis. Veterinary Ophthalmology 1998; 1 (2–3): 71–80. [DOI] [PubMed] [Google Scholar]
- 10. Burney DP, Chavkin MJ, Dow SW et al. Polymerase chain reaction for the detection of Toxoplasma gondii within aqueous humor of experimentally inoculated cats. Veterinary Parasitology 1998; 79 (3): 181–186.DOI: 10.1016/s0304-4017(98)00172-1 [DOI] [PubMed] [Google Scholar]
- 11. Lappin MR, Burney DP, Dow SW et al. Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. American Journal of Veterinary Research 1996; 57 (11): 1589–1593. [PubMed] [Google Scholar]
- 12. Naessens A, Jenum PA, Pollak A et al. Diagnosis of congenital toxoplasmosis in the neonatal period: a multicenter evaluation. Journal of Pediatrics 1999; 135 (6): 714–719. [DOI] [PubMed] [Google Scholar]
- 13. Dubey JP, Mattix ME, Lipscomb TP. Lesions of neonatally induced toxoplasmosis in cats. Veterinary Pathology 1996; 33 (3): 290–295. [DOI] [PubMed] [Google Scholar]
- 14. Dubey JP, Lappin MR, Thuilliez P. Diagnosis of induced toxoplasmosis in neonatal cats. Journal of Veterinary Medical Association 1995; 207 (2): 179–185. [PubMed] [Google Scholar]
- 15. Glasner PD, Silveira C, Kruszon‐Moran D et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. American Journal of Ophthalmology 1992; 114 (2): 136–144. [DOI] [PubMed] [Google Scholar]
- 16. Dubey JP & Hoover EA. Attempted transmission of Toxoplasma gondii infection from pregnant cats to their kittens. Journal of the American Veterinary Medical Association 1977; 170 (5): 538–540. [PubMed] [Google Scholar]
- 17. Sato K, Iwamoto I, Yoshiki K. Experimental toxoplasmosis in pregnant cats. Japanese Journal of Veterinary Medical Science 1993; 55 (6): 1005–1009. [DOI] [PubMed] [Google Scholar]
- 18. Pettersen EK. Transmission of toxoplasmosis via milk from lactating mice. Acta Pathologica Microbiologica Immunologica Scandanavica 1984; 92 (B): 175–176. [DOI] [PubMed] [Google Scholar]
- 19. Dubey JP & Frenkel JK. Cyst‐induced toxoplasmosis in cats. Journal of Protozoology 1972; 19: 155–177. [DOI] [PubMed] [Google Scholar]
- 20. Dubey JP, Shen SK, Kwok OCH et al. Toxoplasmosis in rats (Rattus norvegicus): congenital transmission to first and second generation offspring and isolation of Toxoplasma gondii from seronegative rats. Parasitology 1997; 115 (1): 9–14. [DOI] [PubMed] [Google Scholar]
- 21. Lee YH, Kim KY, Kang MS et al. Detection of Toxoplasma antigens and antibodies in mice infected with different strains of Toxoplasma gondii . Korean Journal of Parasitology 1995; 33 (3): 201–210. [DOI] [PubMed] [Google Scholar]


