Abstract
Abstract: A 5‐month‐old male neutered domestic shorthair cat was evaluated for spinal pain, ataxia, and anisocoria. Neuroanatomic localization indicated diffuse or multifocal central nervous system disease. On cerebrospinal fluid analysis, neutrophilic pleocytosis and intracellular protozoal merozoites were observed. The merozoites were oval, 2–4 μm in width and 4–6 μm in length, and had linear arrays of nuclear material concentrated at one pole. Serum was positive for Sarcocystis sp. antibodies and negative for Toxoplasma gondii antibodies. The organism was determined to be either Sarcocystis neurona or Sarcocystis dasypi based on sequence analysis of the internal transcribed spacer 1 ribosomal RNA genomic region. Clinical disease resolved following treatment with 3 different protocols for protozoal infection. This case is the first to demonstrate the antemortem diagnosis and survival of a domestic cat with Sarcocystis sp.‐associated encephalomyelitis. Clinicians and cytopathologists should include Sarcocystis sp. as a differential for feline inflammatory central nervous system disease characterized by neutrophilic pleocytosis.
Keywords: Cat, cerebrospinal fluid, molecular diagnosis, Sarcocystis dasypi, Sarcocystis neurona
A 5‐month‐old, 2.5‐kg, male neutered domestic shorthair cat was referred to the Purdue University Veterinary Teaching Hospital (PUVTH) for evaluation of spinal pain, ataxia, and anisocoria, with constriction of the right pupil and normal pupillary light reflexes. Over a 2‐week period, neurologic signs had progressed slowly from lethargy to decreased vocalization and dull mentation. The cat was housed indoors, current on vaccinations, had not traveled outside of Indiana, and had no known definitive exposure to livestock, horses, or toxins. The cat was 1 of 4 kittens born to a clinically healthy stray queen that was found in a rural area 5 days before giving birth. One kitten died within 4 weeks of birth with what was described as possible seizures; necropsy was not performed. Another kitten had clinical signs of circling to the left at 4 weeks of age, which resolved by 8 weeks of age; further diagnostic testing was not pursued. The remaining kitten had no apparent clinical signs of disease and was not evaluated further. Ten days before referral, results of an ELISA for feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) and an immunofluorescence assay (IFA) for feline coronavirus (Antech Diagnostics, Oak Brook, IL, USA) were negative. Treatment by the referring veterinarian consisted of meloxicam (dosage unknown), prednisone (2 mg/kg PO q 24 h, tapering dose), and generic clavamox (25 mg/kg PO q 12 h). Anisocoria subsided with initial treatment but ataxia and spinal pain persisted.
At presentation, the cat was 5% dehydrated and had a rectal temperature of 103.9°F. Results of physical examination and ophthalmologic examination were otherwise unremarkable. On neurologic examination, the cat was depressed, reluctant to walk, exhibited abnormal posture, and had generalized muscle fasiculations at rest, as if shivering. Postural reaction deficits were present in all 4 limbs while myotatic and flexor spinal reflexes were normal. Hyperesthesia was noted on palpation of the thoracolumbar spine; cervical spinal hyperesthesia was not apparent. Cranial nerves were unremarkable except for an abnormal gag reflex and equivocal right head tilt. Neuroanatomic localization was consistent with diffuse or multifocal central nervous system disease.
Primary differential diagnoses included cryptococcosis and toxoplasmosis; however, metabolic disorders, nutritional deficiencies, and toxin exposure were also considered. Initial treatment pending diagnostic workup consisted of buprenorphine (0.01 mg/kg IV q 8 h) and fluid therapy with a balanced isotonic electrolyte solution (Normosol‐R, Abbott Laboratories, Abbott Park, IL, USA) and 6 mEq KCl at a rate of 4.4 mL/kg/h. Treatment with prednisone was discontinued.
Results of hematologic and serum biochemical analyses, venous blood pH, creatine kinase activity, and resting plasma ammonia were within normal limits. Two days after presentation, cerebrospinal fluid (CSF) was collected from the cerebromedullary cistern. The CSF was grossly clear and colorless. The quantity of sample was insufficient for enumeration of the total nucleated cell count (TNCC) and protein quantification. A cytocentrifuged specimen was prepared using a few drops (<300 μL) of CSF (600 rpm for 5 minutes; Cytospin 3, Shandon, Thermo Scientific, Waltham, MA, USA) and stained with modified Wright's for cytologic examination. The resulting preparation was highly cellular without hemodilution. The differential cell count indicated marked neutrophilic pleocytosis (80% neutrophils, 11% lymphocytes, 9% large mononuclear cells). Neutrophils were nondegenerate and frequently hypersegmented. Intracellular protozoal merozoites were frequently observed within neutrophils (Figure 1A) and reactive large mononuclear cells (Figure 1B). Occasionally, extracellular basophilic, bow‐shaped organisms were observed free in the background (Figure 1C). The merozoites were ovoid, 2–4 μm in width and 4–6 μm in length. Nuclear material was concentrated in linear arrays at one pole of the organism. Rare extracellular aggregates of myelin were observed and attributed to iatrogenic contamination. The cytologic diagnosis was neutrophilic pleocytosis with protozoal organisms. Sarcocystis neurona and Toxoplasma gondii were the primary differential diagnoses. Clindamycin (10 mg/kg IM q 12 h) therapy was initiated, and palliative treatment with buprenorphine and maintenance fluid therapy (2.4 mL/kg/h) was continued. A day later, ponazuril (50 mg/kg PO q 24 h) was added to the existing treatment protocol and continued as a loading dose for 4 days.
Figure 1.
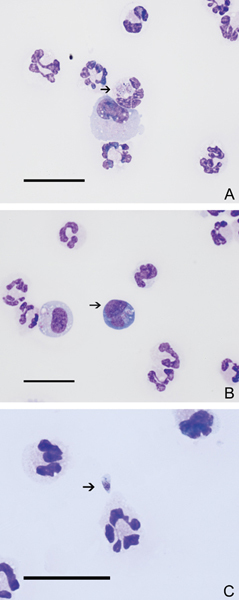
Cerebrospinal fluid from a cat with neurologic signs. Merozoites (arrows) in a neutrophil (A), in a large mononuclear cell (B), and extracellularly (C). Sarcocystis sp infection was diagnosed serologically. Modified Wright's, scale bars=25 μm.
Because an insufficient volume of CSF was available, frozen serum from the initial presentation was used for further diagnostic testing. A positive Sarcocystis sp. titer (1:6400) was obtained by direct agglutination (Department of Biomedical Sciences and Pathobiology, Virginia‐Maryland Regional College of Veterinary Medicine, Blacksburg, VA, USA). Titers (IFA) were negative for T. gondii IgG (<1:20; Louisiana Animal Diagnostic Laboratory, Baton Rouge, LA, USA) and feline coronavirus antibody (<1:400, negative at 1:1600; Antech Diagnostics). An ELISA for Cryptococcus neoformans antigen also was negative (Louisiana Animal Diagnostic Laboratory).
Molecular diagnosis of the Sarcocystis sp. was done by analysis of the small subunit ribosomal RNA (ssurRNA) gene and the rRNA internal transcribed spacer 1 (ITS‐1) using PCR and DNA sequencing (College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA). Genomic DNA was extracted (Flexi‐Gene DNA Kit protocol; Qiagen, Valencia, CA, USA) from the buffy coat layer of a microcentrifuged blood sample and quantified (NanoDrop ND‐1000 Spectrophotometer; NanoDrop Technologies Inc., Wilmington, DE, USA). The ssurRNA gene was amplified using previously published primers and PCR protocols. 1 , 2 The Sarcocystis rRNA ITS‐1 was amplified (platinum high fidelity Taq polymerase; Invitrogen, Carlsbad, CA, USA) using previously published primers JNB69 and JNB70 3 (Table 1) and the following cycling parameters: 2 minutes at 96°C, 60 rounds of 15 seconds at 94°C, 15 seconds at 60°C, 2 minutes at 68°C, a 10‐minute final extension at 68°C; and then held at 4°C. The products were evaluated, then cloned and analyzed using a previously described protocol. 1
Table 1.
List of primers used for molecular analysis targeting the small subunit ribosomal RNA gene (ssurDNA) and internal transcribed spacer 1 (ITS‐1) of the cat Sarcocystis sp clones.
| Primer | Sequence 5′–3′ | Target | Reference |
|---|---|---|---|
| A | AACCTGGTTGATCCTGCCAGT | ssurDNA | Soggin et al 2 |
| B | GATCCTTCAGCAGGTTCACCTAC | ssurDNA | Soggin et al 2 |
| AN | GCTTGTCTTAAAGATTAAGCCATGC | ssurDNA | Schoelkopf et al 1 |
| BN | CGACTTCTCCTTCCTTTAAG | ssurDNA | Schoelkopf et al 1 |
| JNB69 | CCTACCGATTGAGTGTTCCGGTGAAT | ITS‐1 | Tanhauser et al 3 |
| JNB70 | GCGTTCAGAAATCTGATGATTCCCTGA | ITS‐1 | Tanhauser et al 3 |
The cloned rDNA and ITS‐1 region were sequenced (Davis Sequencing, Davis, CA, USA) and evaluated using Sequencher 3.11 software (Gene Codes Corporation Inc., Ann Arbor, MI, USA). Similarity searches of the obtained sequences were performed (Basic Local Alignment Search Tool 4 ; http://blast.ncbi.nlm.nih.gov/blast.cgi) and multiple alignments for direct comparisons of the most similar sequences in the Genbank database (National Center for Biotechnology Information, Bethesda, MD, USA) were created using ClustalW 1.8 Algorithm (http://www.ebi.ac.uk/clustalw/). The most similar sequences and ITS‐1 sequences from Sarcocystis felis were also compared pairwise using LALIGN (http://www.ch.embnet.org/cgi-bin/lalign). 5
The consensus nucleotide sequence derived from 4 ssurRNA gene fragments was identical to 1 S. felis and 3 S. neurona sequences in the GenBank database, which cover the first approximately 450 nucleotides of the gene (ssurRNA gene, partial sequences; Accession Nos. AY656815, AY009112, AF252406, and U33149, respectively). When aligned with sequences from the cat, S. felis and S. neurona, the sole S. neurona full ssurRNA gene sequence in the database (GenBank Accession No. U07812) had a 2‐base deletion (GG) at positions 239 and 240, suggesting the parasite in this case was S. felis.
Two clones of the feline parasite rRNA ITS‐1 were obtained and sequenced. The clones differed by 3 single nucleotide polymorphisms (SNPs) at positions 356, 465, and 768. The feline ITS Clone 1 shared highest similarity with Sarcocystis dasypi (found in an armadillo; GenBank Accession Nos. AY082632 and AY082631) with a sole SNP at position 356 (T to A). The feline ITS Clone 1 and S. neurona UCD1 (identified in an otter; GenBank Accession No. AY082633) differed in 2 positions, 121 (C–T) and 356 (T–A). The feline ITS Clone 2 also shared the highest identity with S. dasypi (GenBank Accession Nos. AY082632 and AY082631), with 3 SNPs at positions 356 (T–G), 465 (T–C), and 766 (T–C). The feline ITS Clone 2 differed from S. neurona UCD1 (GenBank Accession No. AY082633) in the same 2 positions as Clone 1 and also at 2 additional positions, 465 and 766, that matched S. dasypi.
As shown in Table 2, the ITS‐1 clones from the feline parasite shared 99.7% identity. Similarly, 3 cloned sequences from S. dasypi also shared high identity (99.9–100%). The 3 S. neurona isolates that showed greatest similarity to the feline parasite ITS‐1 ranged in identity from 99.5 to 100%. Overall, the sequence variation within isolates (99.5–100%) was comparable to the variation seen between isolates (99.3–99.9%). Based on sequence similarity, the feline parasite was consistent with either S. dasypi or S. neurona; however, given the host species and geographic location, S. neurona was considered more likely.
Table 2.
Percent similarity between ribosomal RNA internal transcribed spacer 1 (ITS‐1) nucleotide sequences in the organism from the cat in this case and the 6 Sarcocystis sp. sequences (GenBank Accession No.) with the highest identity scores using the Basic Local Alignment Search Tool (NCBI).
| Cat, clone 1 | Cat, c lone 2 | S. dasypi (AY082631) | S. dasypi (AY082632) | S. dasypi (AY082633) | S. neurona (AY082644) | S. neurona (AY009113) | S. neurona (AF252407) | S. felis (AY190081) | S. felis (AY190082) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cat, clone 1 | 100 | 99.7 | 99.9 | 99.9 | 99.8 | 99.8 | 99.5 | 99.5 | 44.8 | 45.5 |
| Cat, clone 2 | 100 | 99.7 | 99.7 | 99.6 | 99.6 | 99.3 | 99.3 | 45.1 | 44.8 | |
| S. dasypi (AY082631) | 100 | 100 * | 99.9 | 99.9 | 99.6 | 99.6 | 45.6 | 46.4 | ||
| S. dasypi (AY082632) | 100 | 99.9 | 99.9 | 99.6 | 99.6 | 45.6 | 46.4 | |||
| S. dasypi (AY082633) | 100 | 99.8 | 99.5 | 99.5 | 44.8 | 45.3 | ||||
| S. neurona (AY082644) | 100 | 99.5 | 99.5 | 44.9 | 45.6 | |||||
| S. neurona (AY009113) | 100 | 100† | 44.8 | 45.2 | ||||||
| S. neurona (AF252407) | 100 | 44.8 | 45.2 | |||||||
| S. felis (AY190081) | 100 | 97.9 | ||||||||
| S. felis (AY190082) | 100 |
Between 2 cloned sequences from the same isolate.
†Between isolate sequences from 2 different hosts.
Similarity with S. felis ITS‐1 sequences are shown for comparison.
Seven days after presentation, the patient showed minor improvement and was discharged. Treatment with clindamycin (10 mg/kg PO q 12 h) and ponazuril (15 mg/kg PO q 24 h) was continued and the owner was instructed to continue monitoring at another referral institution located closer to the owner's home. The cat was admitted to a second referral institution where ponazuril was discontinued based on clinician preference. Treatment with clindamycin was continued and pyrimethamine (2 mg/kg PO q 24 h), folic acid (2 mg/kg PO q 24 h), and trimethoprim sulfonamide (unknown dosage) were added to the treatment protocol.
Twenty days after initial presentation, the cat was again presented to the PUVTH, for evaluation of emesis. Current oral medications included clindamycin, pyrimethamine, folic acid, and trimethoprim sulfonamide. Physical examination revealed 5% dehydration and no significant improvement in neurologic status compared with the initial presentation. Results of a CBC included marked leukopenia (WBC count 647/μL, reference interval 6000–18,000/μL) with lymphopenia (500/μL, reference interval 1500–7000/μL) and neutropenia (30/μL, reference interval 3000–12,000/μL). The leukopenia was attributed to the pyrimethamine and all medications except clindamycin were discontinued. Two days later, neutrophilia (8479/μL) and a toxic left shift (band neutrophils 420/μL, reference interval 0–300/μL) and monocytosis (1020, reference interval 50–850/μL) developed and alanine aminotransferase (ALT) activity was moderately elevated (1801 U/L, reference interval 20–108 U/L). Cytologic examination of a hepatic fine needle aspirate suggested hepatocellular hyperplasia based on the degree of anisocytosis and anisokaryosis and increased frequency of binucleation. The cat was treated with Unasyn (Pfizer, New York, NY, USA; 22 mg/kg IV BID) and famotidine (0.5 mg/kg SQ BID) for 5 days, and fluid therapy (Normosol‐R) with 12 mEq KCl. Clindamycin was continued for another 2 weeks after discharge, until reexamination. At that time, clinical signs had vastly improved, laboratory abnormalities had resolved, and protozoal treatment was discontinued.
A 6‐week posttreatment convalescent titer for Sarcocystis sp. was positive at a dilution of 1:200. The cat continued to improve neurologically with minimal ataxia 2 months after presentation. The only residual neurologic deficit at that time was delayed proprioception in the right thoracic limb. Spinal reflexes and results of cranial nerve evaluations were normal.
Discussion
The presence of Sarcocystis sp. merozoites in the CSF was a novel and unexpected finding as. To the authors' knowledge, Sarcocystis sp. merozoites have not been observed previously in CSF from any species. In a review of 62 cases, Singh et al 6 reported that CSF analysis in feline inflammatory central nervous system diseases provided a preliminary diagnosis in 63% of the cases and was most helpful in cases of feline infectious peritonitis, Cryptococcus infection, and lymphoma. 6 The authors' proposed that CSF analysis may be equally useful in protozoal diseases, however organisms were not observed in either of the 2 cases of T. gondii. Organisms were not observed, however, in the 2 cases of T. gondii infection in that study.
Felidae have been documented as definitive hosts for 15 different species of Sarcocystis. 7 , 8 The domestic cat has been identified as an intermediate host in the life cycle of S. felis and S. neurona, the latter being the causative agent of equine protozoal myelitis (EPM). 9 Owing to the economic loss caused by EPM, epidemiologic studies have examined the presence of serum S. neurona antibodies in cats, in an attempt to establish a causal link and establish a method of disease control. Antibodies to S. neurona were identified in 10 of 196 (5%) cats in Michigan for which sera were submitted for T. gondii testing. 10 Stanek et al 11 reported that 10% of cats presented to a mobile spay and neuter clinic had circulating antibodies to S. neurona. When feral cats on an equine farm known to have EPM were sampled, the prevalence was 40%. While these data support that a low to moderate percentage of domestic cats may have circulating antibodies to S. neurona, neurologic disease is rarely reported. Only 2 cases of clinical disease associated with Sarcocystis sp. in domestic cats have been reported to date. S. neurona encephalomyelitis was reported in a 4‐month‐old cat following routine castration. 12 In addition, Sarcocystis sp.‐associated meningoencephalitis was documented in a 3‐month‐old kitten after a history of trauma and in a Canada lynx. 7 , 13 In the latter cases, speciation was not performed; therefore, either S. neurona or S. felis could have been responsible for the infection.
Phylogenetically, the clinical infection in the present case was due either to S. dasypi or S. neurona, which had 99.9–100% and 99.5–100% ITS‐1 identity with the feline clones, respectively. The sole published sequence for S. dasypi is from a 9‐banded armadillo (Dasyctus) which has been identified as an intermediate host for S. dasypi 14 and S. neurona. 15 It is not possible to differentiate S. dasypi and S. neurona with the available sequences and it may be argued that the 2 are the same species. Further, the feeding of encysted armadillo muscle to opossums results in sporocysts identified as S. neurona. 15 Therefore, based on the high genetic similarity between these 2 species, the geographic restrictions of this case, and the known involvement of the domestic cat in the life cycle of S. neurona, clinical disease in this cat was attributed to S. neurona.
Because of the morphologic similarities of the apicomplexan protozoans, advanced diagnostic techniques are required for definitive diagnosis. Included in these techniques are serology, immunohistochemistry, electron microscopy, and molecular analysis; however, limitations are associated with each method. Limitations of serologic assays and immunohistochemical staining include antibody cross‐reactivity and false‐negative results. Anti‐Sarcocystis cruzi antibodies detect all known Sarcocystis sp. 16 and thus may be used as a general screening for infection; anti‐S. neurona antibodies are available but may yield false‐positive results. 10 IFA tends to overestimate seroprevalence compared with immunoblot assays 10 and therefore results should be confirmed with Western blot analysis. Serologic cross‐reactivity may also exist between the classes of apicomplexan protozoans. Although rare, false positives to IFA‐IgG for T. gondii have been reported in S. felis‐infected cats. 17 Electron microscopy may provide an accurate diagnosis provided enough protozoal structures are intact. Specifically, the absence of rhoptries in merozoites and division by endopolygeny can differentiate Sarcocystis sp. from T. gondii. 18 Electron microscopy was not performed in this case due to a limited quantity of sample.
While improved diagnosis may be obtained using molecular techniques, these also have limitations. With regards to Sarcocystis sp. the primers derived from the small subunit ribosomal RNA gene (ssurRNA [JD26/JD37]) amplify the S. felis gene as well those of other species, thus requiring sequencing of the product for analysis. However, the ssurRNA gene sequence is highly conserved among Sarcocystis sp. and even sequencing may not allow for reliable differentiation. Primer pair JNB25/JD396, which amplifies a genomic fragment of unknown function, 16 does not amplify S. felis DNA; however, it does amplify DNA from the closely related species S. neurona and Sarcocystis falcatula 8 , 19 which may then be differentiated.
Originally, this case was identified as S. felis based on analysis of the ssurRNA gene, showing a 2‐base difference from the full gene sequence for S. neurona (GenBank Accession No. U07812). However, when aligned with Sarcocystis partial gene sequences in the database and with published sequences not deposited in the database, 14 this difference was not definitive. Better speciation was then sought by comparing the ITS‐1 sequence using the primer pair JD26/JD37, as this region in S. felis shares only 45% and 46% identity with S. neurona and S. falcatula, respectively. 8 Two clones of the ITS‐1 region from the feline parasite shared 45% identity with S. felis and 99–100% and 95–100% identity with S. dasypi and S. neurona, respectively. Therefore, clinical disease was not due to S. felis and instead was the result of S. dasypi or S. neurona infection. This case demonstrates the importance of amplifying diverse, less conserved genes and supports amplification of the ITS‐1 sequence for differentiating Sarcocystis sp.
Various authors have suggested immunosuppression plays a role in the pathogenesis of Sarcocystis sp.‐associated disease but this has not been confirmed in domestic or wild felids. 7 , 8 , 20 , 21 The cat in this case was negative for FIV and FeLV, but it is possible the initial administration of prednisone exacerbated clinical disease. The route of infection in this case was unknown, but given the age of the cat and possibility of neurologic signs in its littermates, transplacental infection was considered.
Minimal information is known regarding clinical signs associated with S. neurona in species other than horses. 7 , 22 , 23 Clinical signs are often variable, depending on the area of the central nervous system that is parasitized. In horses, signs begin with asymmetric weakness and ataxia followed by rapid progression and mild mental dullness. 23 The most common cranial nerve deficits manifest as a head tilt, facial paralysis, and dysphagia. 23 Similarly, this cat presented with ataxia, an abnormal gag reflex, and head tilt. The previously reported 3‐month‐old kitten infected with Sarcocystis sp. had depression and left‐sided hemiparesis. 7
Despite the absence of a TNCC, a diagnosis of pleocytosis was made based on subjective assessment of the overall cellularity of the cytocentrifuged preparation and comparison to the expected cellularity of CSF with a normal TNCC; however, the magnitude of pleocytosis could not be accurately assessed. Cytopathologic findings in this case were unusual when compared with EPM in horses and other protozoal diseases. The marked neutrophilic pleocytosis was in contrast to the normal findings or mild mononuclear pleocytosis observed in CSF of infected horses. 24 While less common, mild neutrophilic pleocytosis has been reported in cats with central nervous system toxoplasmosis 6 and it is likely the neutrophilic predominance in this case was in direct response to the Sarcocystis sp. infection. Secondary bacterial encephalitis could not be entirely ruled out, as CSF culture was not performed. The magnitude of pleocytosis can be highly variable in protozoal diseases. The TNCC in CSF from T. gondii‐infected cats is generally mildly increased (<50/μL) 6 , 25 and values as high as 1450/μL have been reported in a Neospora caninum‐infected dog. 26 Similarily, marked variability is observed in total protein concentration, ranging from within the reference interval 25 to markedly elevated; a total protein concentration of 992 mg/dL was found in the dog with N. caninum infection. 26 Total protein was not determined in the present case due to insufficient sample quantity.
The development of neutropenia as a direct effect of S. neurona infection is unlikely. Because hematologic changes are not observed in infected horses, 27 drug‐related neutropenia was more likely in this cat. Idiosyncratic neutropenia has been documented with potentiated sulfonamides, 28 and pyrimethamine may also cause neutropenia by inhibiting folic acid. 25 Therefore, the development of monocytosis and a left shift may reflect an appropriate bone marrow response. Overwhelming inflammation in the central nervous system may have also contributed to the neutropenia.
The reason for the increased ALT activity in this cat was unclear. However, the lack of increase at initial presentation suggested possible drug‐related hepatotoxicosis. Of the administered drugs, trimethoprim sulfonamide was the most likely to have induced hepatocellular injury. Hepatopathy has been reported as an idiosyncratic reaction to potentiated sulfonamides in the dog. 28 A single case report of clindamycin‐induced acute cholestatic hepatitis has been reported in humans 29 ; however, clindamycin‐associated hepatotoxicity was considered unlikely in this case as ALT normalized during the course of treatment. The authors are unaware of hepatic injury reported with ponazuril or pyrimethamine. While less likely, direct effects of the Sarcocystis sp. cannot be ruled out. Hepatic sarcocystosis has been reported in the horse 30 and in a dog due to Sarcocystis canis. 18
To the authors' knowledge there is no published data regarding treatment of S. neurona in cats; ponazuril therapy was initiated given its effectiveness against S. neurona in horses (5–10 mg/kg for 28 days) 31 and its use in dogs and cats for the treatment of other protozoal infections. 16 , 32 , 33 , 34 The recommended anticoccidial dose in cats is 7.5–15 mg/kg 16 and doses >30 mg/kg and up to 50 mg/kg have been demonstrated to have better efficacy and equal safety margins in a group of research Beagles. 34 Based on these findings, a loading dose of 50 mg/kg was administered for 4 days and the lower dose was administered at discharge. In the absence of structured guidelines, ponazuril treatment was discontinued at the second institution and an accepted T. gondii treatment protocol was administered. 25 Before FDA approval of ponazuril, a similar protocol consisting of the folate inhibitor pyrimethamine, sulfonamides, and supplemental folic acid was used to treat S. neurona in horses. 23
A serum agglutination titer to Sarcocystis sp. of 1:6400 in conjunction with visualization of protozoal merozoites and the presence of clinical signs were strongly suggestive of active infection. However, it should be mentioned that cats experimentally infected with S. neurona developed titers up to 1:62,000 regardless of steroid administration, although they did not develop clinical disease. 35 While the titer was lower in the cat of this case, the difference may reflect exposure to a lower protozoal load than that used in experimental trials. The 32‐fold decrease in titer 6 weeks posttreatment supports resolution of active disease.
In summary, this case is noteworthy because it is the first case of Sarcocystis‐associated neurologic disease to be diagnosed by visualization of merozoites in the CSF. Diagnosis was confirmed by a positive serum titer and molecular analysis. This case also illustrates the clinical progression and resolution of disease through improvement in clinical signs and a 32‐fold reduction in convalescent titers. Owing to variation in the treatment protocol, conclusions regarding therapeutics and duration of treatment cannot be drawn and the optimal treatment protocol for S. neurona in cats remains to be determined. Nonetheless, clinicians and cytopathologists should include Sarcocystis sp. as a differential diagnosis for feline inflammatory central nervous system disease characterized by neutrophilic pleocytosis.
Acknowledgments
The authors would like to thank David Goodwin, Department of Biomedical Sciences and Pathobiology, Virginia‐Maryland Regional College of Veterinary Medicine, for performing the Sarcocystis titers and Dr. Catharine Scott‐Moncrieff for her assistance in manuscript preparation. Diagnosis was supported in part by Texas A&M AgriLife Research Project H8987.
References
- 1. Schoelkopf L, Hutchinson CE, Bendele KG, et al New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). J Wildl Dis. 2005;41:683–690. [DOI] [PubMed] [Google Scholar]
- 2. Soggin ML. Amplification of ribosomal RNA genes for molecular evolution studies In: Innis MA, ed. PCR Protocols: A Guide to Methods and Applications. New York, NY: Academic Press; 1990:307–314. [Google Scholar]
- 3. Tanhauser SM, Yowell CA, Cutler TJ, et al Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula . J Parasitol. 1999;85:221–228. [PubMed] [Google Scholar]
- 4. Altschul SF. Amino acid substitution matrices from an information theoretic perspective. J Mol Biol. 1991;219:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang X, Miller M. A time‐efficient, linear‐space local similarity algorithm. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- 6. Singh M, Foster DJ, Child G, et al Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis and outcome. J Feline Med Surg. 2005;7:77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubey JP, Higgins RJ, Barr BC, et al Sarcocystis‐associated meningoencephalomyelitis in a cat. J Vet Diagn Invest. 1994;6:118–120. [DOI] [PubMed] [Google Scholar]
- 8. Gillis KD, MacKay RJ, Yowell CA, et al Naturally occurring Sarcocystis infection in domestic cats (Felis catus). Int J Parasitol. 2003;33:877–883. [DOI] [PubMed] [Google Scholar]
- 9. Dubey JP, Saville WJ, Lindsay DS, et al Completion of the life cycle of Sarcocystis neurona . J Parasitol. 2000;86:1276–1280. [DOI] [PubMed] [Google Scholar]
- 10. Rossano MG, Murphy AJ, Vrable RA, et al Cross‐sectional study of serum antibodies against Sarcocystis neurona in cats tested for antibodies against Toxoplasma gondii . J Am Vet Med Assoc. 2002;221:511–514. [DOI] [PubMed] [Google Scholar]
- 11. Stanek JF, Stich RW, Dubey JP, et al Epidemiology of Sarcocystis neurona infections in domestic cats (Felis domesticus) and its association with equine protozoal myeloencephalitis (EPM) case farms and feral cats from a mobile spay and neuter clinic. Vet Parasitol. 2003;117:239–249. [DOI] [PubMed] [Google Scholar]
- 12. Dubey JP, Benson J, Larson MA. Clinical Sarcocystis neurona encephalomyelitis in a domestic cat following routine surgery. Vet Parasitol. 2003;112:261–267. [DOI] [PubMed] [Google Scholar]
- 13. Forest TW, Abou‐Madi N, Summers BA, et al Sarcocystis neurona‐like encephalitis in a Canada lynx (Felis lynx canadensis). J Zoo Wildl Med. 2000;31:383–387. [DOI] [PubMed] [Google Scholar]
- 14. DeLucia PM, Cheadle MA, Greiner EC. Prevalence of Sarcocystis sarcocysts in nine‐banded armadillos (Dasypus novemcinctus) from Florida. Vet Parasitol. 2002;103:203–205. [DOI] [PubMed] [Google Scholar]
- 15. Cheadle MA, Tanhauser SM, Dame JB, et al The nine‐banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona . Int J Parasitol. 2001;31:330–335. [DOI] [PubMed] [Google Scholar]
- 16. Dubey JP, Greene CE. Enteric coccidiosis In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed St. Louis, MO: Saunders Elsevier; 2006:775–784. [Google Scholar]
- 17. Elsheikha HM, Kennedy FA, Murphy AJ, et al Sarcocystosis of Sarcocystis felis in cats. J Egypt Soc Parasitol. 2006;36:1071–1085. [PubMed] [Google Scholar]
- 18. Allison R, Williams P, Lansdowne J, et al Fatal hepatic sarcocystosis in a puppy with eosinophilia and eosinophilic peritoneal effusion. Vet Clin Pathol. 2006;35:353–357. [DOI] [PubMed] [Google Scholar]
- 19. Elsheikha HM, Soltan DM, El‐Garhy MF. Inference of molecular phylogeny of Sarcocystis felis (Sarcocystidae) from cats based on nuclear‐encoded ribosomal gene sequences. J Egypt Soc Parasitol. 2006;36:441–453. [PubMed] [Google Scholar]
- 20. Anderson AJ, Greiner EC, Atkinson CT, et al Sarcocysts in the Florida bobcat (Felis rufus floridanus). J Wildl Dis. 1992;28:116–120. [DOI] [PubMed] [Google Scholar]
- 21. Greiner EC, Roelke ME, Atkinson CT, et al Sarcocystis sp. in muscles of free‐ranging Florida panthers and cougars (Felis concolor). J Wildl Dis. 1989;25:623–628. [DOI] [PubMed] [Google Scholar]
- 22. Dubey JP, Chapman JL, Rosenthal BM, et al Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet Parasitol. 2006;137:36–49. [DOI] [PubMed] [Google Scholar]
- 23. Dubey JP, Lindsay DS, Saville WJ, et al A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet Parasitol. 2001;95:89–131. [DOI] [PubMed] [Google Scholar]
- 24. MacWilliams P. Cerebrospinal fluid In: Schrefer JA, ed. Diagnositc Cytology and Hematology of the Horse. 2nd ed St. Louis, MO: Mosby; 2002:171–179. [Google Scholar]
- 25. Dubey JP, Lappin MR. Toxoplasmosis and neosporosis In: Greene CE, ed. Infectious Diseases in the Dog and Cat. 3rd ed St. Louis, MO: Saunders Elsevier; 2006:754–775. [Google Scholar]
- 26. Gaitero L, Anor S, Montoliu P, et al Detection of Neospora caninum tachyzoites in canine cerebrospinal fluid. J Vet Intern Med. 2006;20:410–414. [DOI] [PubMed] [Google Scholar]
- 27. MacKay RJ. Equine protozoal myeloencephalitis. Vet Clin North Am Equine Pract. 1997;13:79–96. [DOI] [PubMed] [Google Scholar]
- 28. Trepanier LA, Danhof R, Toll J, et al Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. J Vet Intern Med. 2003;17:647–652. [DOI] [PubMed] [Google Scholar]
- 29. Aygun C, Kocaman O, Gurbuz Y, et al Clindamycin‐induced acute cholestatic hepatitis. World J Gastroenterol. 2007;13:5408–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis CR, Barr BC, Pascoe JR, et al Hepatic sarcocystosis in a horse. J Parasitol. 1999;85:965–968. [PubMed] [Google Scholar]
- 31. Furr M, Kennedy T, MacKay R, et al Efficacy of ponazuril 15% oral paste as a treatment for equine protozoal myeloencephalitis. Vet Ther. 2001;2:215–222. [PubMed] [Google Scholar]
- 32. Swinger RL, Schmidt KA Jr., Dubielzig RR. Keratoconjunctivitis associated with Toxoplasma gondii in a dog. Vet Ophthalmol. 2009;12:56–60. [DOI] [PubMed] [Google Scholar]
- 33. Charles SD, Chopade HM, Ciszewski DK, et al Safety of 5% Ponazuril (toltrazuril sulfone) oral suspension and efficacy against naturally acquired Cystoisospora ohioensis‐like infection in Beagle puppies. Parasitol Res. 2007;101:S137–S144. [Google Scholar]
- 34. Reinemeyer CR, Lindsay DS, Mitchell SM, et al Development of experimental Cystoisospora canis infection models in Beagle puppies and efficacy evaluation of 5% Ponazuril (toltrazuril sulfone) oral suspension. Parasitol Res. 2007;101:S129–S136. [Google Scholar]
- 35. Dubey JP, Lindsay DS, Saville WJ. Serologic responses of cats against experimental Sarcocystis neurona infections. Vet Parasitol. 2002;107:265–269. [DOI] [PubMed] [Google Scholar]


