ISBT Academy
Foundations of Good Practice
2A‐S01‐01
Donor Compensation and Remuneration – Is There Really a Difference?
Folléa G
European Blood Alliance, Montgermont, France
Background
Opposing voluntary non‐remunerated donations (VNRD) to compensated or paid donations have been debated since decades, particularly for donors supplying plasma for plasma derived products.
Aim
To review definitions for compensation, remuneration and non‐remuneration of blood and plasma donors, as well as ethical principles which should guide transactions regarding human blood and plasma, in order to analyse the ethical acceptability of these different modes of donations.
Methods
Review of reports from international organisations [e.g. Council of Europe (CoE)], reports in scientific literature and outcomes from meetings with main involved stakeholders, including Blood Establishments, Plasma Industry, Patients’ and Donors’ organisations.
Results
According to the Nuffield Council on Bioethics (NCB), recompense means payment to a person in recognition of losses they have incurred. This may take the form of either reimbursement of direct financial expenses incurred in donating (blood or plasma), or compensation for non‐financial losses (e.g. inconvenience, time). A reward is a material advantage gained by a person as a result of donating bodily material, which goes beyond ‘recompensing’ the person for the losses they incurred in donating. If reward is calculated as a wage or equivalent it becomes remuneration. To protect donors’ and patients’ safety, transactions of human bodily materials should comply with well acknowledged ethical principles: dignity (prohibition of making the human body and its parts as such a source of financial gain), non-maleficence (avoiding unnecessary or unreasonable harm), autonomy (avoiding any coercion/pressure) and justice (avoiding that the ‘burden of donation’ is being shifted to underprivileged populations). The NCB Intervention Ladder has been recognised as a useful tool for analysing the ethical acceptability of different forms of encouragement for donating bodily material. A comparison of each of the six ‘rungs’ of this Intervention Ladder with the CoE definition of VNRD (endorsed by WHO and ISBT) shows that rungs 1–4, classified as altruist‐focused, are fully compatible with this definition of VNRD, while rungs 5–6, classified as non‐altruist‐focused, do not comply with this definition. The assessment of current practices to encourage blood, blood component and plasma donations show that compensation could take the form of either altruistic or non‐altruistic encouragement. As an example, time off work far in excess of the time reasonably needed for donation and travel should be considered as remuneration. This led to suppress this practice in many countries. Similarly, monetary incentives given to students frequently donating plasma could be considered as financial motivation and, as such, ethically questionable. Awareness of ethical principles for blood and plasma donations and the NCB Intervention Ladder appear as indispensable means to help review and improve the current practices to encourage donations, in order to better guarantee both patients’ and donors’ safety.
Conclusions
Careful analysis should lead to identify non‐altruist forms of compensation of blood and plasma donors and to replace them by altruist forms of compensation. This would help to further develop VNRD as the best way to ensure both a safe and sustainable blood and plasma supply to meet the patients’ needs and a safe and sustainable donor population.
2A‐S01‐02
Towards Common Language and Understanding in Donor Health and Vigilance
Wiersum-Osselton JC
TRIP National Hemovigilance and Biovigilance Office and Sanquin Blood Supply, Leiden, The Netherlands
Background
The collection of blood from healthy donors is essential to ensure the provision of blood components for the treatment of patients. This is ethically acceptable provided the donors are informed of the objectives and risks to themselves and have consented. They should be screened and phlebotomised according to legal, professionally endorsed and evidence‐based procedures. National and supranational standards exist for donor selection, blood centre processes and quality management.
Recent years have seen a progressive increase of attention to monitoring and prevention of complications of blood donation. Comparison of data between centres, organisations and countries depends on common definitions for the events being monitored.
Method
In 2013 the International Society of Blood Transfusion's (ISBT) working party on haemovigilance launched a revision of the earlier set of definitions of complications of blood donation, with a brief to increase harmonization between the international set and the Northern American set of definitions. Comments and input were received from members of the working party as well as international experts. Prior to publication, organisations were asked to assess practicality of the proposed classification and recommended parameters.
Result
In December 2014 the revised definitions, incorporating recommendations for parameters to be recorded with reported complications, were published online with joint authorship and ownership of ISBT, the International Haemovigilance Network (IHN) and AABB (formerly: the American Association of Blood Banks). The definitions have formally been endorsed by the European Blood Alliance and Alliance of Blood Operators. They have been implemented in the Northern American donor vigilance reporting system (DonorHART[TRADEMARK]) which is available for capture of complications and associated parameters by blood establishments, allowing (currently univariable) adjustments of analyses and comparisons between organisations and the overall captured data.
Discussion
The availability of a classification with definitions for the monitoring of complications of blood donation is a first step towards making it feasible to share and compare data between organisations and countries. Basic data comparisons of aggregate data in the IHN database, ISTARE (International Surveillance database for Transfusion Adverse Reactions and Events), will in future be based on the revised classification.
Analyses of differences need to take account of differences in donor demographics and physiology: the recommended parameters will increase the feasibility of this. The harmonised definitions can contribute to improving collection centre practice, donor information and donor care.
Conclusion
International definitions for complications of blood donation are available as a tool for monitoring and improving blood donor care and safety.
2A‐S01‐03
Guidelines for Good Practice in Blood Banks
Schärer C
Swissmedic, Swiss Agency for Therapeutic Products, Bern, Switzerland
The implementation of Good Practices in blood establishments represents a globally accepted systematic approach ensuring that appropriate Quality Management Systems are in place for the collection, preparation, testing and distribution of blood and blood components. This approach provides a manufacturing control model that allows for a documented system of incorporating quality throughout the entire manufacturing process and describes the activities and controls needed to consistently produce products that comply with specifications and are safe for use.
Global initiatives have led to the development of internationally agreed harmonised guidelines on Good Manufacturing Practices (GMP) in blood establishments (e.g. WHO). In Europe, the first Good Practice Guidelines have been elaborated as an ad hoc co‐operation between the European Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM/CoE), and the Commission of the European Union. The document has become an integral part of the 18th Edition of the Council of Europe Guide to the Preparation, Use and Quality Assurance of Blood Components and identifies the quality system elements that must be met by blood establishments and hospital blood banks that are required to comply with EU Directive 2005/62/EC. It incorporates also quality system elements derived from the detailed principles of GMP (as referred to in Article 47 of EU Directive 2001/83/EC).
Since the time of elaboration of the Good Practice Guidelines in Europe there have been significant changes in the principles of GMP take into consideration new concepts and developments in technologies and manufacturing activities. Although a number the updates were not likely to be relevant to the Good Practice Guidelines all updates need to be reviewed to assess their relevance for blood establishments, in order to align, and to ensure maintenance of coherence with these updated GMP principles. A working group on the Guide to the Preparation, Use and QA of Blood Components (GTS) under the European Committee (partial agreement) on blood transfusion (CD‐P‐TS) is in charge to prepare the revision. Some aspects of this process will be discussed.
Power Tools in the Immunohaematology Toolbox
2A‐S02‐01
An Update on Human Blood Groups
Storry JR
Region Skåne, Office of Medical Services, Lund, Sweden
Discovery of blood groups is grounded in the immunohematology laboratory, where they most often have presented as an unsolved puzzle. Some have been simple to unravel, others requiring decades for their resolution. There are currently 35 different blood group systems of which 33 are erythroid in nature, the remaining two consisting of soluble antigens adsorbed from the plasma.
Something may start out as an antibody investigation of something unknown and lead to the discovery of a new blood group system. Much of progress is technique‐based. It wasn't until Coombs, Mourant and Race described the indirect antiglobulin test in 1945 that the field of blood groups really opened up and a world of polymorphism was discovered on the red blood cells of all human beings. Increased sensitivity in serological tests and techniques has revealed more blood group antigens and further diversity. This coupled with an increasing biochemical and genetic picture of erythrocyte membranes has led to the discovery of an array of functional proteins, glycoproteins and glycolipids and a broader understanding of RBC physiology.
In the past 3 years, five different blood group antigens have found homes in new blood group systems through different technological approaches. The first of these, FORS, was originally described in 1911, although not on human RBCs but on those of sheep and dogs. It was the investigation of an anomalous ABO subgroup, Apae, in two English families that led to the discovery of an unusual glycolipid on the red blood cells of the Apae family members. This was shown to be the Forssman glycolipid. FORS1 is similar to A antigen but is built by a different enzyme encoded by a different gene, and thus, is independent of ABO.
By using tools such as SNP arrays or exome sequencing and then comparing the results with such a database has enabled the elucidation of both Jra and the Vel blood group antigens, and permitted the identification of their carrier molecules. These techniques are particularly valuable when few samples of the rare phenotype exist but can be a little of a ‘needle‐in‐a‐haystack’ approach if too little if the test samples are unrelated to each other.
Sophistication in existing techniques can also lead to discovery. Mass spectrometric analysis of proteins has been around for a long time but the continuous improvement in sensitivity can lead to new discovery. This is exemplified by the biochemical approach that was used together with sensitive mass spectrometry to identify the proteins bearing Lan, Jra, and Vel. Thus, Jra and Lan were elevated to blood group systems in 2012 and Vel in 2014.
The fifth piece in this story is the discovery of a new antigen on a well‐known protein, CD59. A child with a rare CD59 deficiency was shown to have produced an antibody to the protein. The investigators have identified the molecular basis and therefore it attained blood group system status also in 2014. Blood group discovery is ongoing and it is likely that we will hear more in the near future.
2A‐S02‐02
Powerful Tools for Resolving Antibody Problems
Hamilton R
American Red Cross‐Southeastern Michigan Region, Detroit, MI, United States of America
The initial test protocols chosen by a laboratory for antibody detection and identification are designed for sensitivity in detection of clinically significant alloantibodies as well as ease of use and adaptability to the laboratory's workflow. For many laboratories both inside and outside the United States, the routine methods involve column agglutination (gel). Solid phase (SP) tests and tube hemagglutination tests may also be performed. The additional tests available for serological problem solving, therefore, depend on what type of testing is used in the routine, initial antibody identification studies.
Varying the test methodology is a valuable tool: solid phase, gel or tube testing may be chosen as a secondary method. Depending on the antibody problem, the increased OR decreased sensitivity of the secondary test may enhance the identification of alloantibodies or decrease the reactivity of autoantibodies, respectively. Similarly, the choice of enhancement media [polyethylene glycol (PEG)], low ionic strength solution (LIS) or a decision to omit such enhancement can change the reactivity of an antibody‐containing plasma to allow for antibody identification.
Chemicals that alter the antigens on a red cell membrane can assist antibody identification. Ficin and papain are commonly used to either enhance reactivity or destroy antigen sites. Other enzymes such as trypsin, chymotrypsin, pronase or neuraminidase can be used to assess the sensitivity of a target antigen. Sulfhydryl compounds, dithiothreitol (DTT) or 2‐aminoethylisothiouronium (AET), will also destroy certain blood group antigens. When combined with information from enzyme treatment, an investigation may be focused on antigens in certain blood group systems.
DAT‐positive autologous red cells, which cannot be used in antiglobulin testing, can be treated with EDTA‐glycine or chloroquine diphosphate to remove the bound IgG. The DAT‐negative autologous cells can then be used for phenotyping to predict alloantibodies that may be produced.
Separation of autologous red cells from a transfused sample by microhematocrit centrifugation or hypotonic saline wash will provide cells that can be used for red cell phenotyping. Testing antibody containing plasma or eluate with these autologous cells can confirm reactivity as being due to autoantibody or suggest alloantibody. Red cell genotyping is now commonly used to predict a red cell phenotype which can be used to suggest possible alloantibodies that may be produced or to aid in explaining unexpected serological findings.
Adsorption onto autologous red cells is used to remove warm or cold autoantibody and allow for detection of alloantibodies. Adsorption onto allogeneic red cells can remove autoantibody or separate multiple alloantibodies to facilitate identification.
In certain investigations, demonstrating that antibody reactivity can be neutralized by plasma, urine or specific blood group substances will suggest antibody specificity.
Specifics related to performance of these procedures, their interpretations and their limitations may be found in numerous reference books. Combined into a logical investigation, these additional tests will aid in resolution of most antibody containing samples.
2A‐S02‐03
How to Explain Discrepant Results Between Serology and Genotyping in Immunohaematology
Peyrard T
Institut National de la Transfusion Sanguine (INTS) & Laboratory of Excellence GR‐Ex, Paris, France
Blood types are routinely investigated by haemagglutination testing, considered the gold standard method. However, several limitations exist for serological techniques, which may be overcome by molecular testing. Genotyping is claimed only to predict a phenotype, as several discrepancies with phenotyping may occur. However, it should be reminded that false‐positive reactions also exist for phenotyping methods.
Out of human factors, that should definitely not be neglected (errors in sample manipulation, experimental protocol or data handling), many reasons explain discrepancies between serology and molecular testing results, with four possible combinations:
1‘false positive phenotype/true negative genotype’ Positive DAT (IgG) when IAT is required for serotyping.
Antigen‐negative patient recently transfused with antigen‐positive RBCs.
Polyclonal phenotyping antisera including an antibody to a low‐prevalence antigen.
Cross‐reacting typing reagents (e.g. some anti‐M clones with the He antigen).
RBC polyagglutination (e.g. activation of the cryptic Tn antigen, which may be reactive with some anti‐A clones).
Expression of epitopes by a variant homologous gene, reactive with some clones of reagents: D reactivity encoded by RHCE*ceCF (Crawford) and RHD*ce-D(5)-ce (DHAR) alleles, C reactivity encoded by RHD*DIVa.
Hybrid genes (C reactivity with some clones in presence of the RHD-ce(4-7)-D hybrid allele, included in the (C)ceS haplotype).
2‘false negative phenotype/true positive genotype’ Antigen‐positive patient recently transfused with antigen‐negative RBCs.
Partial antigen nonreactive with some clones (e.g. DVI).
Very weak antigen expression (DEL phenotype for D, Fyx for Fyb).
Poor quality of antisera (e.g. anti‐U marketed reagents are usually unable to screen U+var type).
RBC alteration, potentially responsible for antigen destruction.
3‘true negative phenotype/false positive genotype’ Silent alleles.
Mutation in the promoter sequence: Fy(a−b−) in people of African descent, In(Lu).
Mutation in the coding sequence: D− −, Rhnull (amorph type), Jknull.
Protein‐protein interaction at the RBC surface: inactivating mutations in the RHAG gene encode a Rhnull phenotype (regulator type); inactivating mutations in the GYPA (glycophorin A) gene are responsible for the lack of the high‐prevalence Wrb antigen, carried by the Band 3 (Diego) protein.
Mosaicism (two or more cell populations with different genotypes in one individual who has developed from a single fertilized egg).
Natural chimerism (exchange of cells between non‐identical twin fetuses).
Amplification of a contaminant allele, so‐called ‘mistaken allele’ (example of FUT2 and its pseudogene Sec1 with ahigh‐sequence identity).
4‘true positive phenotype/false negative genotype’ Allele drop out due to a polymorphism or mutation within one of the PCR primer‐binding sites.
Low quality or quantity of DNA: preferential amplification of the shorter allele in heterozygous individuals (short allele dominance).
In heterozygous individuals, preferential amplification of one allele when its denaturation is favored (low GC content).
No or weak amplification due to inhibitors in the DNA extract.
Despite limitations of molecular testing which may lead to false phenotype predictions and that we all need to be aware of, this is considered an essential tool to complex case solving in all immunohaematology reference laboratories.
Getting the Most Out of Blood Components
2B‐S03‐01
Optimising the Blood Component Production Process
Cid JC, Velásquez CAV, Lozano ML
Hospital Clínic, Barcelona, Spain
Background
Automation of blood component preparation (BCP) from whole blood (WB) collections can help to optimise the BCP process, and it is increasingly being widespread implemented.
Aim
This review summarizes the quality of blood components obtained with new automated devices.
Methods
We reviewed available literature on the quality of blood components obtained with new automated devices.
Results
Blood components obtained with the new devices met European standards. Of note, compared with platelet concentrates obtained with manual methods, automation of BCP improved the consistency of the final products.
Conclusion
The complete automation of BCP from WB collections is still in development and it represents a huge change in paradigm.
2B‐S03‐02
Introducing the Red Cell Storage Lesion
Devine D
Canadian Blood Services, Vancouver, BC, Canada
An essential feature of routine transfusion therapy is the ability to store blood components. However, the isolation of cells and their storage in non‐biological containers at non‐biological temperatures causes fundamental changes in cellular biochemistry above and beyond the normal ageing processes that cells undergo in the body. Over the time of the allowable red cell storage period, these changes are apparent in the routine examination of stored cells in vitro. With the storage of red blood cells in liquid form, changes occur over time which ultimately result in the inability of the cell to maintain its integrity at which point the cell ruptures. The processes occurring between the initial collection of blood and the lysis of the red cell result in a constellation of changes that we call the red cell storage lesion. The red cell storage lesion can be visualized morphologically with the change from normal discoid cell shape to a high proportion of echinocytic cells in a unit stored for several weeks. It can also be reflected in the measurement of a number of proteins and small molecules in the red cell.
Numerous factors influence the development of the red cell storage lesion including how red cell concentrates are prepared, the storage conditions and the use of additive solutions. The rate of red cell storage lesion development is clearly influenced by the composition of the additive solution used, and the role of the materials properties of the containers, for example with or without DEHP, is also a factor to consider. Inherent characteristics of the donor may also influence the rate of development of the storage lesion. Although the range of these characteristics is a source of active research, it is known that cells from donors with osmotic fragility syndromes, such as mild hereditary spherocytosis, or some forms of thalassemia store more poorly than typical donor cells. In addition, there is an interplay between donor characteristics and the manufacturing processes used to prepare red cell components.
Evidence of an effect of the red cell storage lesion in transfusion practice is variable. Some units with a mild storage lesion will readily recover their fresh profile a few hours after transfusion, while other units with severe storage lesion will not provide normal oxygenation or may be rapidly cleared in the spleen. Retrospective studies of the use of older red cells appear to support a negative effect on patient outcomes compared to fresh blood, but randomized controlled trials do not support the interpretation of these retrospective studies. Increasing concern over the possible role of cell‐derived membrane microparticles or exosomes in transfusion‐related immunomodulation has led to ongoing studies that seek to refine the question of the role of the storage lesion in the efficacy of transfusion.
2B‐S03‐03
Recent Advances in Platelet Processing and Storage
Lozano M
University Clinic Hospital, Barcelona, Spain
The first manuscripts regarding the preparation of platelet concentrates for transfusion were published in 1963. Since then we have walked a long way trying to have the safest and most efficacious platelet product to transfuse to our patients. In this presentation, the latest developments in platelet preparation and storage prepared from whole blood donations will be summarized. Probably one of the most important changes occurred in recent years in the preparation of platelet concentrates from whole blood donations has been the automation of the process, particularly in Western Europe. The first method introduced for preparing platelet concentrates for transfusion, the platelet rich method, has remained basically the same since the first description and is being performed using manual techniques. By contrast for the buffy coat (BC) method since its introduction in 1985 several devices have been developed that allow the automation of the process at different steps. The earlier ones allowed the separation of the centrifuged whole blood bag into red blood cells, plasma and the BC with the subsequent process of the individual BC. The discovery that the pooling of the BC increased the efficiency of platelet recovery, led to the development of a separator that combined centrifugation with the expression of the supernatant plasma rich plasma that appeared in 2003 processing one bag in each cycle. Later a new device was developed that allowed the processing of six pooled BC in each centrifugation run. Another significant change occurred in the preparation of platelet concentrates has been the introduction of platelet additive solutions (PAS) for resuspending platelets. It was introduced in the mid 1980s aiming at decreasing the amount of plasma in the platelet product so the plasma recovery for fractionation from the whole blood donation is maximized and at the same the quality of the final product is improved. It has been also shown that the transfusion of platelet concentrates in additive solution is associated with a decrease in the incidence of adverse reactions in the transfused patients. Currently several PASs are available. All PASs need some residual plasma left in the platelet concentrate for optimal storage. A plasma carryover of around 35% has been shown to be the optimal. In order to keep the structural and metabolic characteristics of platelet up to 7 days the addition of citrates, phosphate, acetate, magnesium and potassium is required. A great deal of research has been performed on the development of quantitatively and qualitatively better PC. However in spite of all these efforts, the aim of the perfect platelet concentrate for transfusion has not yet been met and the search still goes on.
Clinical Dilemmas with Red Cell Antibodies
2B‐S04‐01
Handling the Pre‐Transfusion Request for Blood in Complex Cases
Nance J
American Red Cross, Philadelphia, PA, United States of America
For the majority of acute or emergent transfusion requests, pretransfusion testing results are unremarkable and transfusion proceeds with no unanticipated delay. For a small percentage of cases, the pretransfusion testing yields results that require further testing. Most often, the patient's serum contains an antibody easily identified by routine methods. In some, the determination of blood type or the identification of the serum antibody is a great challenge. These cases often occur in patients chronically exposed to red cell antigens, through transfusion or pregnancy.
In pregnancy, if an antibody is detected, antigen negative blood must be transfused, either for the mother or for the baby (intrauterine transfusion). There are some countries that minimize the potential for alloimmunization by matching transfusion given to women with childbearing potential for at least K and often c. Thus, these antibodies are not formed pre‐pregnancy, usually allowing the birth of K+ or c+ infants and limiting hemolytic disease of the fetus or newborn (HDFN). There is likely value in antenatal RhIg and definite value in postnatal RhIg in the prevention of anti‐D. Both of these modalities may help prevent the initial alloimmunization of the mother to common antigens. When the mother forms antibodies to multiple common antigens or to an antigen of high prevalence, provision of compatible red cells is difficult. If detected early enough and if the mother is healthy (even if the hemoglobin is below ‘normal’ donor levels), pre‐delivery autologous donation is possible. If multiple donations are possible, one can be aliquotted into 2 or 3 portions for transfusion to the baby before or after birth, and the others for transfusion to the mother. For intrauterine transfusion, most often due to anti‐D HDFN, the blood should be as similar to the mother's red cell phenotype as possible to limit further alloimmunization.
The presence of alloantibodies can complicate the provision of red cells for chronic transfusion; e.g. Sickle Cell Disease or autoimmune hemolytic anemia (AIHA) patients. In many countries, these patients are extensively phenotyped (13 antigens) or genotyped (30+ antigens), thus knowing the antigens the patient lacks. Limited to extensive pheno‐ and geno‐matching is performed by some countries. Punnett Square analysis is helpful in determining the degree of match available with the typed donor pool.
There are rare AIHA cases in which the autoantibody has a relative specificity. Depending on the degree of hemolysis, it may be of value to match for the specificity during the acute crisis.
There are cases for which rare blood is exceeding difficult to obtain. This has been highlighted by presentations and publications of the ISBT Rare Donor Working Party. Antibodies to antigens of very high prevalence require local, regional or national rare donors programs to obtain red cell products. Obtaining Rhnull, Ko or D‐ U‐ red cells are most difficult. The use of regional, national and international collaborative centers with inventories of rare red cells is ultimately what enables these patients to receive the blood they need to survive.
2B‐S04‐02
Managing the Bleeding Emergency in a Patient with Red Cell Antibodies
Norgaard A, Gybel-Brask MGB , Christensen BKC , Zo El-Ghina RZ , Johansson PIJ , Dziegiel MDZ
Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark
Introduction
Treating patients with bleeding emergencies requires a well‐organized blood establishment and multidisciplinary cooperation. In this setting, a massive bleeding protocol can improve survival of life‐threatening bleedings substantially. To provide a similar level of care to patients with red antibodies is an organizational challenge.
Aims
Copenhagen Capital Region Blood Bank runs a continuous donor phenotype programme, a patient characterization and transfusion strategy, and an inventory strategy. The purpose is to secure a sufficient supply of antigen‐negative red cells for all clinical situations, from prophylaxis to emergency treatment.
Methods
New donors are screened for antigens corresponding to frequent and clinically significant antibodies to secure that a sufficient proportion of all stored blood is phenotyped. A supply of frozen red cells with rare phenotypes is kept corresponding to one blood volume. Finally, a donor‐recruiting and ‐alerting system and an inter‐blood‐establishment cooperation is in place to secure that antigen‐negative blood can be provided on a day‐to‐day basis. All patients at risk of transfusion get a type‐and‐screen allowing computer‐matched red cell transfusion for the next 4 days. If a patient is likely to form antibodies or these will pose a major risk, the patient is screened prophylactically, and blood is matched for clinically important antigens to prevent further immunization. Whenever a patient presents with antibodies, serological workout is done including the corresponding phenotype, and a preliminary transfusion strategy. The results are communicated to the clinical department including the time needed to provide compatible blood.
Results
The management of a patient with antibodies balances the bleeding emergency against the risk of haemolysis (often delayed) and the availability of compatible blood. In a bleeding patient, a purely prophylactic transfusion strategy is abandoned if antigen‐negative blood is not available. When surgery is scheduled in a patient with a clinically significant antibody, the surgeon is informed, and sufficient phenotype‐matched blood is reserved ahead. If antigen‐negative blood is limited or unavailable, the Blood Banks prepares a plan in cooperation with the surgeon, a bleeding expert and the Blood Bank doctor using a 360 degree patient‐centred approach. This may include patient blood management measures such as preoperative anaemia treatment, diagnosing coagulation defects, postponing surgery, auto‐transfusion, pharmacological measures, perioperative monitoring of platelet function and coagulation using whole‐blood functional assays. In the unexpected massive bleeding, acute transfusion of unmatched O blood may be necessitated. A prioritized serological workout is then done, and the inventory searched to promptly provide antigen‐negative blood. The clinical doctor is informed about incompatible transfusions and the patient is monitored to evaluate the impact of the antigen‐positive transfusion. In the rare case of severe haemolysis, exchange transfusion of compatible blood may be performed.
Summary
To provide a high level of care to bleeding patients with red cell antibodies, it is mandatory for the blood establishment to systematically organize donors, patient assessment, inventory and staff. Standard operating procedures for laboratory deliverables and communication pathways with bleeding experts and clinical departments should be effectively mobilized, both before elective surgery and during the unexpected bleeding.
2B‐S04‐03
Managing the Patient With Haemoglobinopathy and Multiple Red Cell Antibodies
Politis C 1, Hassapopoulou H 2, Halkia P 2, Kourakli A 3, Mougiou A 3, Zervou E 4, Kleronomos E 5, Sfyridaki K 5, Pappa C 6, Tsoumari I 6, Lafiatis I 6, Kavallierou L 1, Parara M 1, Richardson C 1
1Coordinating Haemovigilance Centre, Athens, Greece2AHEPA University Hospital Blood Centre and Thalassaemia Unit, Thessaloniki, Greece3RIO University Hospital Blood Centre and Thalassaemia Unit, Patras, Greece4University Hospital Blood Centre and Thalassaemia Unit, Ioannina, Greece5Venizeleio General Hospital Blood Centre and Thalassaemia Unit, Herakleion Crete, Greece6General Hospital Blood Bank and Thalassaemia Unit, Korinthos, Greece
Introduction
Haemoglobinopathies, particularly β‐thalassaemia and sickle cell disease (SCD), represent a major source of morbidity imposing considerable strain on health care resources. The recommended treatment – regular blood transfusion‐is associated with various risks including the formation of alloantibodies and autoantibodies against RBC antigens.
Aim
We examine the current status of alloimmunization against red cell antigens and autoimmunization in chronically transfused patients with thalassaemia and SCD in Greece.
Methods
983 patients (489 β‐thalassaemia major (TM),148 thalassaemia intermedia (TI),324 S‐ thalassaemia and 22 SCD; mean age 42 years) from six thalassaemia units were studied for alloimmunization and autoimmunization against RBC antigens. Alloantibody screening and identification was done using the 3‐cell panel followed by 11‐cell panel using the ID card micro typing system. Autoantibodies were identified by antiglobulin testing. Results before and after 2010 were compared and analysed in relation to number of transfusions, splenectomy and alternative antigen matching strategies depending on disease category and history of alloimmunization.
Results
Before 2010, 12.9% of all patients were positive for alloimmunization (14.3% in TI, 13% sickle thalassaemia, 9.8% TM and 18.2% in the small group of homozygous SCD) and 7.3% for alloimmunization and autoimmunization. Single alloantibodies were recorded in 6.4%, double alloantibodies in 1.4% and multiple alloantibodies in 3.9%. Alloantibodies of the Rhesus and Kell systems were commonest. Alloantibodies to RBC minor antigens were 32%. Incidence of multiple red cell antibodies with anti Cw, anti Jkβ, anti‐Kell specificities, and autoantibodies mainly of IGg type, were recorded in 8.16% of TI and 5.9% of SCD patients. Blood phenotypically matched for lacking RBC antigens and therapy with corticosteroids and immunosuppressants were administered to patients with multiple RBC antibodies. Late commencement of transfusion and splenectomy appear to be significant risk factors for both allo and autoimmunization. After 2010, only 14 cases with new alloimmunization were recorded (1.4%): 7 TM, 2 TI, 3 S‐Thalassaemia,2 SCD. Four patients had multiple alloantibodies. The incidence of alloimmunization in this period was 1:9405 units of transfused RBCs. A second parous patient with non‐transfused δβ‐thalassaemia presented with delayed haemolytic transfusion reaction (DHTR) and hyperhaemolysis that was attributed originally to Jkβ alloimmunization and worsened because of multiple antibodies anti‐M, anti‐Cw and anti‐Wra following the transfusion of 108 units of RBCs. No therapeutic intervention except splenectomy produced response. Autoimmunization in both periods. Thirteen of the 138 immunized patients (9.4%) were positive for autoimmunization typed IGg and C3d; ten were TM patients, two TI and one SCD. Forty‐two percent of all patients with allo‐ or autoimmunization had history of splenectomy.
Conclusions
Alloimmunization is commonest in TI followed by sickle‐thalassaemia and TM. One Thalassaemia Unit applies systematically the ABO, RhD, Kell strategy. The other five have introduced a systematic antigen matching policy (ABO, CcDEe, Kell) for all patients. In recent haemolysis or history alloimmunization all six Units are applying a better matching policy. The low risk of alloimmunization in the last 5 years shows the success of the nationally applied blood transfusion procedures. Multiple red cell alloantibodies represent a very small but difficult to manage risk for responders prone to combination with autoimmunization. DHTR with hyperhaemolysis is a rare but important clinical problem in SCD and thalassaemia.
Emerging Threats to Blood Safety
2C‐S05‐01
Can We Make the Pre‐Donation Screening Process More Effective?
Norda RAC , Frazzetto ML , Knutson F , Lubenow N
Uppsala University Hospital, Uppsala, Sweden
Blood establishments (BE) are ultimately responsible for the quality and safety of the blood or blood components collected and for the final acceptance or deferral of a donor. All donors must undergo a screening process, carried out just prior to the blood donation. Some BE's also do pre‐screening of donors, applying the process to prospective donors, collecting only blood samples and registering the donor at the first visit.
The screening process has several elements: (i) Identification of the donor according to legal requirements and linking the identity to all the relevant documentation, including labels for test tubes and blood bags for traceability. (ii) Appropriate information timely provided so that the donor can decide whether to donate or, if appropriate, to abstain. (iii) The donor health history evaluated, most commonly by a standardized questionnaire (DHQ) and complementary direct questions. (iv) The donor confirming his/her responses by signing the form. (v) A few physical parameters assessed. (vi) Blood samples, linked to the donation, collected to screen for hemoglobin and markers for infectious agents.
The DHQ serves to detect hidden problems with possible consequences for the recipient and exposures to infectious agents, as far as possible to establish whether the donor could be a healthy carrier of blood‐borne pathogens. Questions concern current and past life style, present and past country of residence, travels and work, health problems, chronic diseases, medical and surgical treatment. The complementary interview serves to clarify deviating answers and assesses that the donor has understood the information provided. The donor may interrupt the process at any time. However, coercion by group pressure or other factors have led to the practice of confidential self‐exclusion by some BE's.
The laboratory investigations of primary importance to the recipient involves screening assays for pathogens: antigens, antibodies and RNA/DNA. Tests for HBV, HCV and HIV are legally required at every donation. Continuous improvement has led to impressive shortening of the undetectable infectious period. There is continuous vigilance for emerging new infections or re‐emerging ‘old’ infections as threats to the blood supply. Safety measures include modified deferral rules and development of new screening tests. Certain test may be applied only to prospective or first‐time donors and in certain epidemiological situations.
In recent years there has also been modification of deferral criteria concerning sexual activities with high risk for acquiring transfusion‐transmittable infections (TTI). Permanent deferral has been replaced with a time‐based deferral in a number of countries. Prior to such changes, there has been modelling of the risks of TTI in the given epidemiological situation and estimates of donor compliance, as donor compliance was identified to be of critical importance. Attention was also directed to the content and distribution of information to donors. The DHQ has been adapted to IT‐environments and computerized.
Tools to study the effectiveness of process in itself can include quality control of the completed questionnaires. Hemovigilance including evaluation of recalled units, of verified screening‐positive donors and donor compliance studies are tools to study the outcome of the pre‐donation screening process.
2C‐S05‐02
Assessing the Risk of Transfusion‐Transmitted Emerging Infections
Domanovic DD
European Centre for Disease Prevention and Cotrol, Stockholm, Sweden
Enormous progress in the understanding and reducing of transfusion‐transmitted infections (TTI) has been achieved over recent decades. However, the risk from established blood‐borne infections is not completely eliminated, and newly emergent pathogens in the era of mass international travel and climate change are posing a continuous threat to blood safety. Assessing the risk is an important step in a response to, and managing the risks posed by, transfusion transmitted emerging infections (TTEI).
This is an overview of risk assessment methodologies, frameworks used in response to threats posed by TTEIs, and approaches and tools developed by the ECDC aiming to provide EU Member States and the EU Commission with the best advice available concerning infectious risks of donations and human use of substances of human origin (SoHO).
Basic methodology in developing a health risk assessment entails a preparation stage, the collection of event information, the literature search and systematic collection of information about the aetiological agent, an extraction of evidence, an appraisal of the evidence and the risk estimation. Scientific methods, transparency and sharing of information are essential at every stage. The complexity of the relationship between pathogens, donors, blood products, recipients and the environment defines transmissibility, imputability, severity, expectedness and case clustering of TTIs. A number of international efforts have been taken to identify, prioritize and develop fact sheets, toolkits and frameworks for assessing the threat of emerging infections and their impact on blood safety and availability.
ECDC supports member state blood authorities in dealing with outbreaks of infectious diseases not included in routine blood screening, through several projects and activities. The EUFRAT tool (European Up Front Risk Assessment Tool) was developed with the aim to allow member states to assess the risk of transmission of emerging infections by blood transfusion. ECDC's interactive maps of areas affected by West Nile virus (WNV) infection inform the blood competent authorities about areas with ongoing transmission of WNV to humans in order to support their implementation of blood safety measures. The risk of communicable disease transmission through blood transfusion is regularly discussed and assessed as part of the rapid risk assessment undertaken in the initial stages of an outbreak. ECDC has identified and prioritized eleven arthropod‐borne diseases that may pose a threat to the safety of SoHO. A priority list was the basis for developing a comprehensive, review‐based risk assessment and to define preventive interventions of their transmission through SoHO. The risk assessment of WNV infection, malaria and dengue included the use of newly created risk flow diagrams in the analysis of scientific evidence to develop an expert opinion. Flow diagram A deals with the possibility of disease transmission through a specific SoHO. Flow diagram B addresses the risk reducing measures that can be taken to mitigate the risk of transmission.
2C‐S05‐03
West Nile Fever, Malaria, Chikungunya and Dengue in Europe – The Mosquitoes Have Landed
Zaaijer HL
Sanquin Blood Supply Foundation, Amsterdam, The Netherlands
Mosquito‐borne infections are a threat to the safety of blood transfusion. Blood donors more often travel to regions were mosquito‐borne infections are endemic, and the areas where arboviruses are endemic are changing. Regarding the safety of blood transfusion some aspects of mosquito‐borne infections must be considered. It is clear that transfusion transmitted malaria and West Nile fever may harm the recipients seriously, and thus preventive measures are warranted. But despite massive outbreaks of dengue and chikungunya, reports of severe cases of dengue or chikungunya caused by infectious blood or blood products are rare. In addition, for highly endemic areas the question is whether it is justified to invest in the safety of blood, while at the same time patients have a high risk of acquiring the infection from mosquitoes in the hospital or at home. (This situation seems comparable to areas with high infection pressure of HEV gt3: screening of blood donors for HEV is not introduced because patients may acquire hepatitis E at any moment from other sources than blood). Contrary to dengue and West Nile fever, most cases of chikungunya are symptomatic. This offers a cheap alternative to expensive testing of blood donations for chikungunyavirus‐RNA in an endemic area during an outbreak: before releasing a unit of red blood cells, one may telephone the donor and ask whether he or she still is fine.
To cope with the changing areas of dengue, chikungunya and West Nile fever, non‐endemic countries may consider to simply defer each donor who travelled abroad for 4 weeks, thus covering threats posed by MERS‐CoV, Ebola, Congo‐Crimean hemorrhagic fever, etcetera too. In 1999 West Nile virus successfully invaded the Americas, spreading from New York towards Canada and Latin America within years. Since decades the area in Europe where West Nile fever occurs is stable, with a straight northern boundary running from the Algarve via the Camargue and the Po‐region to Hungary. Since 2005 chikungunya virus causes large epidemics along the borders of the Indian Ocean. Surprisingly, in La Reunion the vector was found to be Aedes albopictus, not Aedes aegypti as in former outbreaks. In 2007 an isolated outbreak of chikungunya occurred in the Emilia‐Romagna region in Italy, with more than 200 confirmed cases. In December 2013 chikungunya virus crossed the Atlantic and arrived in the Caribbean island of Saint Martin. A rapid spread to Central and South America followed, with 933.102 diagnosed cases in November 2014. Travelers to affected areas may import arboviruses to non‐endemic areas. Humans carrying West Nile virus are not infectious for mosquitoes, but humans transmit chikungunya and dengue virus to mosquitoes. In the autumn of 2014 secondary cases of chikungunya and dengue occurred in the South of France, in the vicinity of imported cases from abroad, illustrating that local mosquito populations in Europe may become infected and start transmission. Regarding malaria it must be realized that in the past many parts of Europe sustained transmission of malaria. Until 1959 a pocket of endemic malaria existed just north of Amsterdam.
Changing Patterns in Transfusion Practice
2C‐S06‐01
Managing Anaemia in an Outpatient Setting
Pendry K
NHS Blood and Transplant, Manchester, United Kingdom
Anaemia is a public health problem that affects populations from rich and poor countries. Anaemia is the result of a wide variety of causes, and globally, the commonest cause is iron deficiency. Using the WHO haemoglobin (Hb) thresholds, anaemia in adults is defined as: Hb <130 g/l in men, <120 g/l in non‐pregnant women and <110 g/l in pregnant women. It is estimated that globally the prevalence of anaemia is as follows: 42% of pregnant women, 30% of non‐pregnant women, 13% of men and 24% of the elderly population. Anaemia causes debilitating symptoms such as fatigue, breathlessness and cognitive impairment and can be associated with serious underlying health problems for example gastrointestinal malignancy or renal impairment. Anaemic patients have an increased risk of morbidity and mortality and are more likely to require hospitalisation. In addition, anaemic patients are more likely to receive blood transfusion even though this may be avoidable with timely investigation and treatment.
It is important to understand the reason for the anaemia so that appropriate treatment can be offered, both for the anaemia and for the underlying cause. In the UK, the National Comparative Audit of the use of blood in medical patients in 2011, showed that 29% of patients receiving a blood transfusion were likely to have a potentially reversible cause for their anaemia although less than half had had basic investigations such as haematinics undertaken prior to transfusion.
Many patients with anaemia can be managed in the outpatient setting. The development of a rapid access anaemia clinic allows for timely coordination of care which can result in the avoidance of emergency admissions, correction of anaemia prior to surgery, reduction in unnecessary transfusions and appropriate investigation and management. The development of investigation and treatment algorithms for anaemia allow for the preliminary management of such patients to be undertaken by specialist nurses who can then triage the patients to the appropriate specialist department such as haematology, gastroenterology or nephrology. These algorithms can also be used in the community, where primary care practitioners can initiate the appropriate investigations and treatment and refer to secondary care only when appropriate. A standardised approach to the care of pregnant women is also recommended, so that iron deficiency can be identified and effectively managed, thus reducing the risk of transfusion and also potential adverse effects for the infant. With the advent of electronic order communications for laboratory tests, there are opportunities for the development of decision support software that will guide the clinician through the algorithm according to the clinical scenario and the results of the investigations.
The timely investigation and management of patients with anaemia is an important facet of Patient Blood Management, resulting in improved patient outcomes, more efficient use of healthcare resources and a reduction in blood transfusion.
2C‐S06‐02
Autoimmune Haemolytic Anaemia (AIHA)
Allard S 1, Hill Q 2
1NHS Blood & Transplant, London, United Kingdom2St James's University Hospital, Leeds, United Kingdom
Autoimmune haemolytic anaemia (AIHA) is an acquired haemolytic disorder caused by autoantibodies to the patient's own red cell antigens. The overall incidence is ~1 per 100,000/year but incidence increases with age. The disorder may be classified as warm type (65%), cold type ie cold haemagglutinin disease (CHAD) (29%) and paroxysmal cold haemoglobinuria (PCH) (≤1%) or mixed type (5%). Cases may be primary or secondary to an associated disorder, most commonly a haemato‐oncological malignancy, systemic autoimmune disorder or infection. Following haematopoietic stem cell transplant, 3–4% of patients may develop AIHA that can be particularly challenging to manage.
Warm AIHA is caused predominantly by IgG antibodies active at 37°C. The direct antiglobulin test (DAT) is typically positive for IgG and may also be positive for C3. Symptoms can develop gradually with anaemia and jaundice or have a fulminant onset with severe life‐threatening haemolysis. CHAD is a chronic clonal disorder, often presenting in the elderly and is caused by IgM antibodies that bind red cells optimally in vitro at 4°C. The DAT is usually positive for C3 only. For all cases of suspected AIHA, laboratory testing should include a blood film, reticulocyte count, DAT and haemolytic markers such as bilirubin and LDH. If AIHA is confirmed additional testing is required to determine its type and exclude an associated disorder. For example in younger children with acute transient intravascular haemolysis, PCH can be diagnosed with the Donath Landsteiner test which demonstrates the biphasic nature of the IgG antibody.
There are very few high quality trials to inform evidence based therapy. Corticosteroids remain the first‐line approach for primary warm AIHA and are effective in 70–80%. Patients should also receive folic acid with consideration of concurrent therapy for gastric protection and prevention of steroid‐induced osteoporosis. In refractory/relapsed cases the best studied therapies are splenectomy and rituximab. Azathioprine, danazol, cyclosporin and mycophenolate mofetil can also be effective for some patients. Intravenous immunoglobulins may have a limited role as short‐term salvage therapy. Patients with primary CHAD should be advised to keep warm. For symptomatic patients requiring treatment, prednisolone and splenectomy should be avoided. Response to chlorambucil or cyclophosphamide may be limited with the best response described to rituximab; consider rituximab plus fludarabine if clonality as been demonstrated. Plasmapheresis may be useful in CHAD with severe anaemia, but the effect is transient.
Patients with severe anaemia may require blood transfusion pending onset of effective immunosuppression. The decision to transfuse must be based on the patient's clinical status and co‐morbidities as well as the haemoglobin. Approximately 30% of patients with AIHA have an underlying alloantibody, most commonly related to previous transfusion. In the face of a panreacting autoantibody, full serological testing including adsorption of the autoantibody to unmask and identify any alloantibody present is required but can take at least 4–6 h. Therefore to avoid delay to an urgent transfusion, ABO, extended Rh and Kell matched blood should be made available pending full serological investigations.
2C‐S06‐03
Alternative Strategies to Platelet Transfusion
Lozano M
University Clinic Hospital, Barcelona, Spain
Inspite of all the efforts made to look for alternatives, platelet transfusioncontinues being the mainstay of the treatment for patients suffering fromqualitative and quantitative platelet disorders. However in some instance it isadvisable to try to reduce the exposure to platelet transfusions in certainpatients because of severe side effects associated with the transfusions, difficulties in finding a suitable product for a given patient (e.g. with HLAand or HPA antibodies) and also efforts to reduce the risk of alloimmunizationin patients with congenital platelet disorders. Following we will reviewmeasures that can be considered when looking for alternatives strategies toplatelet transfusions. Preventive measures such as avoiding drugs interferingwith platelet function of avoidance of administering drugs intramuscularly, playa key in the managements of patients suffering from bleeding diathesis ingeneral, and in particular, in patients with thrombocytopenia and/orthrombocytopathies. Higher hematocrit is associated with a decrease in bleedingtime. Mechanism by which red blood cell transfusion could affect primaryhemostasis is uncertain but a hemorheologic effect of red blood cells increasingthe interaction of platelets with endothelium might explain, at least in part, the beneficial effect. In view of the available evidence it seems advisablekeep a hemoglobin levels of around 90–100 g/l in thrombocytopenic patientsto optimize the hemostatic effect of platelets. The use of hemostatic drugs hasproven to be effective in reducing the bleeding and transfusion needs inpatients suffering from plasmatic hemostatic defects or patients undergoingsurgery. Due to this fact they have been used in patients with plateletdefects. Tranexamic acid, desmopressin and activated recombinant factor VII arecurrently used. Although the evidence is sparse and contradictory tranexamicacid has become a part of the therapies used in the management of quantitative (particularly in the patients refractory to platelet transfusion) orqualitative platelet disorders. The dose generally recommended is 15–20 mg/kgevery 8 h orally or intravenously. Desmopressin at dose of 0.3–0.4 μk/kg, diluted in 50–100 ml sterile saline and infused slowly over 15–30 min been used for the treatment of platelet disorders (although not forGlanzmann's thrombasthenia due to poor response). Recombinant activated factorVII (rFVIIa) is approved in the European Union for treating Glanzmann'sthrombasthenia patients refractory to platelet transfusions due to antibodiesto GP IIb‐IIIa and/or HLA. The recommended dose is 90 μg/kg, repeated every 2 h until adequate hemostasis is achieved with at least three doses beforefailure declaration. In recent years thrombopoietic agents such as romiplostimand eltrombopag have been also tested in patients with congenital thrombocytopenias. Althoughplatelet transfusions continue to be the mainstay in the management of patientssuffering from qualitative and quantitative disorders, several strategies mightbe considered when trying to reduce or avoid platelet transfusions
Facets of Cell Therapy
2D‐S07‐01
New Perspectives on Peripheral Blood Stem Cell Collection. Facets of Cell Therapy. ISBT Academy Day
Chabannon C
Institut Paoli‐Calmettes, Marseille Cedex 9, France
Peripheral blood stem cell collection (PBSC) through apheresis is an essential technology to support clinical programs that deliver autologous or allogeneic hematopoietic stem cell transplantation (HSCT) to patients affected with severe malignant or non‐malignant diseases. For a long period of time, the field behaved as a ‘sleeping beauty’, witnessing little technological or medical changes. This is no longer the case with the recent introduction of a new generation of cell separators, of new drugs to mobilize stem cells, and the diversification of clinical applications, all of these unprecedented innovations occurring in a changing organizational, financial and regulatory environment.
The most widely used cell separator for decades is no longer commercialized nor supported by its manufacturer since the beginning of 2015. Alternative devices are now marketed by the same manufacturer and by competitors. Introduction of QM as part of authorization processes imposed by competent authorities or as part of peer‐driven initiatives such as FACT‐JACIE requests validation plans for these newly acquired equipment.
Commercialization of plerixafor, a first‐of‐its‐kind agent targeting the interaction between the chemokine CXCL‐12 and its major receptor CXCR‐4, offers new opportunities to improve CD34+ progenitor cell circulation in poor‐mobilizing patients affected with lymphoid malignancies who are candidates for high‐dose chemotherapy supported with autologous HSCT, and to streamline the mobilization and collection process.
Better biological characterization of collected and engineered cell products, as well as improved understanding of clinical consequences when technical variations occur, has renewed interest for an improved control of these processes.
Allogeneic HSCT is no longer seen as a ‘one‐shot’ process where hematopoietic chimerism is established from the single infusion of a stem cell product, the majority of which are obtained from PBSC collection in adult donors. Many patients will receive cell therapy products on several occasions: a stem cell graft after a conditioning regimen to support engraftment of donor‐derived hematopoietic cells, followed by one of several infusions of immune‐competent cells targeting either infectious agents or residual tumor cells in order to improve the outcome and quality of life of allo‐transplanted recipients. Most of these cell products are engineered from peripheral blood cells obtained through one or several donor apheresis sessions.
Finally, other types of medicinal products can nowadays be engineered from peripheral blood cells obtained by apheresis. These include anti‐cancer vaccines or genetically engineered T cells with anti‐tumor activity such as CAR (Chimeric antigen Receptor) T‐cells. This new generation of medicinal products will qualify as Advanced Therapy Medicinal Products (ATMPs), and will be manufactured and administered outside of the field of HSCT and in the context of an original collaboration between industry and healthcare stakeholders, among which apheresis facilities will be important players.
These major changes will be further discussed and illustrated.
2D‐S07‐02
Choosing the Right Blood Products for the Transplant Patient
Gabriel C
Austrian Red Cross, Blood Centre Linz, Linz, Austria
No abstract available.
2D‐S07‐03
Progress in In‐Vitro Red Cell Generation – Are We There Yet?
Douay L
Université Pierre et Marie Curie, Etablissement Français du Sang, Paris, France
We will report the various obstacles cleared during the past decade with the view of generating red blood cells in vitro from various sources of stem cells, for transfusion purposes. We will also consider the next developments to be performed for achieving this goal.
Starting from the natural source, the hematopoietic stem cells, the major advance resides in the establishment of the proof of principle for transfusion in human, by showing a normal life span of cultured Red blood cells compared to their native counterpart. The best available source of highly proliferative adult stem cells is cord blood, with the capacity of generating the equivalent of 50 units of packed RBC from one average unit. It is however a limited source in terms of hematopoietic stem cells and remains dependent on donations as observed from conventional blood supply.
Critical advances have allowed the in vitro production of functional RBC from pluripotent human stem cells, embryonic and induced pluripotent stem cells, in the past 5 years.
Because induced pluripotent stem cells (iPS) can proliferate indefinitely and be selected for a phenotype of interest, they appear the most favourable source of stem cells. Proof of concept of the generation of RBC from iPS has been made, but still needs to be optimized. We also discuss the key points that remain to be resolved to achieve an application for clinical transfusion.
Several crucial points remain to be resolved notably to ensure the safety of iPS of clinical grade, the optimization of the erythrocyte differentiation and cellular amplification.
Although there remain many biological and regulatory issues concerning the efficacy and safety of this new product, the major challenge today for future clinical applications is switching from the current limited 2‐dimensional production techniques to large‐scale 3‐dimensional bioreactors. In addition to requiring technological breakthroughs, the whole process also has to become cost‐efficient to match the current prices of high quality blood products.
Improving Patient Safety – The Role of the Transfusion Practitioner
2D‐S08‐01
My Role as a Transfusion Practitioner in a UK NHS Teaching Hospital
Moss RL
Imperial College Healthcare NHS Trust, London, United Kingdom
The recommendation for Transfusion Practitioners within UK hospitals was part of successive Department of Health documents for the Better Blood Transfusion initiative (BBT1 in 1998, BBT2 in 2002, BBT3 in 2007) and the role would work as part of Hospital Transfusion Team, made up with the Transfusion Laboratory Manager and Consultant in charge of blood transfusion. Over the intervening years most UK Trusts appointed to the Transfusion Practitioner role, employing experienced staff with a Nursing, Midwifery or Biomedical Science background.
A 2010 Transfusion Practitioner survey in England and North Wales stated that Transfusion Practitioners (TPs) have made a significant contribution in helping to improve transfusion practice at a local, regional and national level by promoting safe transfusion practice, the appropriate use of blood in medical and surgical patients, reducing wastage and increasing patient and public involvement ensuring that Better Blood Transfusion has become an integral part of NHS care.
Data from the Serious Hazards of Transfusion Scheme (SHOT) shows a growing safety culture in hospitals with respect to transfusion with the number of deaths directly attributable to transfusion reducing from 12 in 1996 to 1 in 2009. Red cell usage also fell by 15% from 2002 to 2007, thought largely due to a reduction in inappropriate use. Through the work of the Hospital Transfusion Team Trusts have been able to contribute to higher levels of compliance with respect to audit and inspection, such as the NHS Litigation Authority (NHSLA) Risk Management Standards and so contributing to significant financial savings for the NHS.
However, anecdotal evidence shows that the role and responsibility of the TP varies widely and has changed for most since it was introduced over 10 years ago, with significant variation in how TPs spend their time.
Within a London teaching hospital, the TP role encompasses all elements of the transfusion process and can be considered as the conduit for transfusion expertise for patients and clinical staff across the organisation pulling together available resources, whether that be information, financial or personnel and in‐putting into Trust programmes such as education and training for clinical staff, safe and appropriate use of blood for patients and risk and governance structures.
The role of the Transfusion Practitioner is varied and complex however can be summed up simply as an involvement in all aspects of the blood transfusion process relating to patients and staff, from the initial decision to take a blood sample to the final fate of any blood transfused.
References
1. Department of Health. Better Blood Transfusion (HSC1998/224; HSC 2002/009; HSC2007/001). http://www.transfusionguidelines.org.uk/uk-transfusion-committees/national-blood-transfusion-committee.
2. National Survey of Transfusion Practitioners in England and North Wales in April/May 2010. Chief Medical Officer's National Blood Transfusion Committee. http://www.transfusionguidelines.org.uk/uk-transfusion-committees/national-blood-transfusion-committee.
3. NHSLA (2013–2014) NHSLA Risk Management Standards for NHS Trusts providing Acute, Community or mental Health & learning Disability Services and Non‐NHS Providers of NHS Care. http://www.nhsla.com.
4. SHOT: Bolton‐Maggs (Ed), D Poles, A Watt and D Thomas on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2013 Annual SHOT Report (2014).
2D‐S08‐02
My Role as a Transfusion Practitioner in The Netherlands
Deelen R
The Netherlands
No abstract available.
2D‐S08‐03
The Role of the Transfusion Practitioner in Australia
Bielby LJ 1, Akers C 1, Francis S 2, Darby S 3, Campbell L 3, Hollis L 4, Quested B 5, Hogan C 6
1Blood Matters, West Melbourne, Vic., Australia2Clinical Excellence Commission, Sydney, NSW, Australia3Sir Charles Gairdner Hospital, Perth, WA, Australia4Sunshine Coast Hospital & Health Service, Nambour, Qld, Australia5BloodSafe, South Australian Department of Health & Australian Red Cross Blood Service, Adelaide, SA, Australia6Australian Red Cross Blood Service, Melbourne, Vic., Australia
Transfusion is a complex process involving many interlinking chains of events and a multidisciplinary group of health professionals, of which the transfusion practitioner (TP) is an integral part of the chain. The hospital based TP role commenced in 2002 in 2 states of Australia, and has expanded across the country. Currently there are 113 dedicated TP positions and many more staff involved as blood/transfusion champions. There are also 12 TP positions within the Australian Red Cross Blood Service (the Blood Service).
Over time both of these TP roles have evolved to meet the changes within the Australian blood sector. The primary focus of safety and appropriateness has now evolved to be more patient‐centred by incorporating patient blood management (PBM) initiatives. National PBM guidelines1, statements, strategies, criteria and healthcare standards2 specifically focused on all aspects of transfusion have influenced this evolution.
The roles and activities that are undertaken by the TP are diverse, and vary significantly between health services, and within each state and territory. In some states specific PBM roles have been established, while in others these aspects are incorporated into the TP role, or could be undertaken with collaboration by other specialist areas, such as pre‐anaesthetics/pre‐admission teams. The PBM activities could include being a resource consultant, a member of the multi‐disciplinary team for managing anaemia and/or providing support or conducting anaemia clinics. Within the Blood Service the role of the TP is considerably different to the hospital role, with the primary focus of facilitating specialist blood product support.
Education remains a fundamental component of the TP role and there are many varied ways that the education is conducted; including the use of online e‐learning (local/national), competency‐ based assessment, simulation scenarios and face to face presentations.
Governance activities are also a key aspect of the role, encompassing policy/clinical guideline development, activities supporting consent, auditing and reporting to meet national safety, appropriateness and haemovigilance requirements.
Currently in Australia the management of unnecessary blood wastage is a focus and many TPs are actively working with laboratories to understand the reasons for waste, and then implement strategies to assist in waste reduction.
Effective communication and change management skills are integral to the success of the role.
Education available in Australia to support the TP role and others working in the area including the Graduate Certificate in Transfusion Practice, BloodSafe eLearning Australia and an extensive range of learning experiences offered by the Blood Service.
In this tight economic environment there is constant pressure in all states regarding the funding of these positions.
Summary
Promotion of safe and appropriate transfusion remains the central focus of the TP role with a variety of other responsibilities such as governance, education, haemovigilance, promotion and implementation of PBM strategies. The TP is recognised as a key role within the transfusion team and the role continues to evolve with the changes in the Australian blood sector.
References
1. National Blood Authority 2012, PBM Guidelines – Modules 1, 2, 3 & 4.
2. ACSQHC 2011, National safety and quality health service standards.
Parallel Sessions
Immunological Mechanisms
3A‐S01‐01
Factors Influencing Aspects of Naitp
Gestur GV1, Kapur RK 1, Sonneveld MS 1, Natunen S 2, Partanen J 2, Sainio S 2, Wuhrer MW 3, van der Schoot CE 1
1Sanquin Research, Amsterdam, The Netherlands2Finnish Red Cross Blood Service, Helsinki, Finland3Leiden University Medical Center, Leiden, The Netherlands
Background
Immunoglobulin G (IgG) formed during pregnancy against human platelet antigens (HPA) of the fetus mediates fetal or neonatal alloimmune thrombocytopenia (FNAIT). Antibody titer or IgG subclass does not strictly correlate with disease severity, suggesting other unknown factors to play a role. IgG are glycoproteins, with an evolutionary conserved glycan present at Asn297 which is required for IgG binding to Fc‐receptors (FcγR). However, the exact composition of this glycan is quite heterogeneous. Particularly, the absence of core fucose increases the binding of IgG to FcγRIII on phagocytes and NK cells, possibly contributing to aggressiveness of the antibodies. This may also be influenced by extrinsic factors, such as oxidative stress and proinflammatory status of the patient.
Aims
To investigate if antibody glycosylation and other extrinsic factors may explain clinical features observed in FNAIT, and explain the lack of a strong relationship between IgG titer and platelet counts in the neonate.
Methods
Anti HPA1a specific antibodies were affinity purified and tryptic Fc‐derived glycopeptides analyzed by mass spectrometry (LC‐MS/MS). Phagocytosis of platelets opsonized with FNAIT sera and recombinant antibodies were carried out with granulocytes and sorted CD16+ (FcγRIIIa) and CD16- monocytes and interaction of opsonized platelets with antibodies and serum probed by cellular surface plasmon resonance (cSPR).
Results
We found the Fc glycosylation of anti‐platelet antibodies in FNAIT are associated with a slightly increased level of Fc galactosylation and sialic acid. A marked decreased of core‐fucosylation of anti‐HPA‐1a‐specific IgG1 from FNAIT patients, but not in total serum IgG1. Antibodies with low amount of fucose displayed higher binding affinity to FcγRIIIa and FcγRIIIb, enhanced phagocytosis of platelets, and correlated with the neonatal platelet counts in FNAIT. Curiously, we also identified C‐reactive protein (CRP) to enhance in vitro phagocytosis, and enhance in vivo platelet clearance in the presence of anti‐platelet IgG. CRP levels were elevated in some FNAIT patients possibly predisposing them to even deeper thrombocytopenia.
Conclusions
The combination of low level of core fucose in platelet specific IgG, elevated CRP, together with titer may together be a better predictor for thrombocytopenia alone. This can be used for clinical screening, and may serve as targets for future therapies.
3A‐S01‐02
Glycosylation Analysis of Monoclonal Anti‐D Antibodies Reveals Cell Line Dependent Glycosylation Patterns Differing from Polyclonal Anti‐D; Implications for Biological Efficacy
Kumpel BM1, Saldova R 2, Kapur R 3, Vidarsson G 4, Armour K 5, Olovnikova N 6, Abrahams J 2, Wuhrer M 7, Rudd PM 2
1NHS Blood and Transplant, Bristol, United Kingdom2National Institute for Bioprocessing Research and Training, Dublin, Ireland3Keenan Centre for Biomedical Research, St Michaels Hospital, Toronto, ON, Canada4Department for Experimental Immunohaematology, Sanquin Research, Amsterdam, The Netherlands5Department of Pathology, University of Cambridge, Cambridge, United Kingdom6Hematology Research Centre, Moscow, Russian Federation7Biomolecular Mass Spectrometry Unit, Leiden, The Netherlands
Background
Anti‐D (Rh) prophylaxis for the prevention of haemolytic disease of the fetus and newborn has been one of the most successful interventions of the past 50 years. However, the anti‐D immunoglobulin is derived from hyperimmunised donors and is insufficient and expensive for worldwide use. Monoclonal anti‐Ds have been tested in clinical trials but none have yet shown equivalent efficacy to anti‐D immunoglobulin at preventing D‐immunisation.
Aims
Determination of the composition of sugars in the carbohydrate chains attached to asparagine (ASN)297 of IgG Fc was undertaken to test the hypothesis that differences in these structures may account for the varying biological activity of monoclonal anti‐Ds.
Methods
The glycosylation of polyclonal anti‐D purified from a prophylaxis preparation (Rhophylac) and 23 monoclonal anti‐Ds produced from cell lines of four species was extensively characterised. The degree of fucosylation, bisecting N‐acetylglucosamine (GlcNAc), galactosylation and sialylation was compared with their ability to mediate haemolysis in FcgRIIIa‐mediated antibody dependent cell‐mediated cytotoxicity (ADCC) assays and with their efficacy in published human studies of D‐positive red cell clearance and prevention of D‐immunisation.
Results
Polyclonal anti‐D had approximately 81% fucosylation, 11% bisecting GlcNAc, 84% galactosylation (of which 5% was agalactosyl (G0) and 69% G2 IgG), and 33% sialylation (all sialic acids were in α2‐6 linkage to terminal galactose). Glycosylation of monoclonal anti‐D was characteristic of the species of producer cell line. Anti‐D from human B‐lymphoblastoid cell lines (BLCL) had similar glycosylation to polyclonal anti‐D but more bisecting GlcNAc and lower galactosylation and G2. Anti‐D from mouse/human heterohybridomas (HH), Chinese hamster ovary (CHO) cells and rat myeloma YB2/0 cell lines had low bisecting GlcNAc, low galactosylation and G2 (consequently high G0) and relatively low sialylation. All the sialic acid in CHO‐derived anti‐Ds was in α2‐3 linkage, whereas the other monoclonal anti‐Ds had α2‐6 or both linkages. HH monoclonal anti‐Ds had Galα1‐3Gal sugars. IgG1 anti‐Ds from YB2/0 cells had low fucosylation (<50%) in contrast to 69–100% in the other monoclonal anti‐Ds. The extent of fucosylation had a strong inverse relationship with ADCC activity (P = 0.0002), with a sharp reduction in activity of monoclonal anti‐Ds having more than approximately 85% fucose. Human studies have shown red cell clearance was faster than polyclonal anti‐D for two YB2/0‐derived anti‐Ds but very variable, slow and often incomplete by 1 week for HH‐ and CHO‐derived anti‐Ds. Monoclonal anti‐Ds from HH promoted D‐immunisation. Two BLCL monoclonal anti‐Ds were slightly less effective at red cell clearance and prevention of D‐immunisation than polyclonal anti‐D, despite similar fucosylation.
Conclusions
The level of fucose correlated inversely with ADCC. Monoclonal anti‐D with the low fucosylation phenotype from YB2/0 cell lines mediated accelerated red cell clearance, a necessary characteristic for effective prophylaxis. However, raised proportions of G0 in these monoclonal anti‐Ds, compared to polyclonal anti‐D, may be inflammatory. Because pregnant women have strong humoral immunity, additional selection for high galactosylation of monoclonal anti‐D may be beneficial to prevent possible inflammatory effects of rapid red cell sequestration leading to D‐immunisation in women.
3A‐S01‐03
Generation of De Novo Primary Anti‐Human Platelet Antibody (HPA)‐1a Responses Requires Placental Syncytiotrophoblast Microparticles
Eastlake J , Kumpel BM , Jackson D
NHS Blood and Transplant, Bristol, United Kingdom
Background
Anti‐human platelet antibody (HPA)‐1a responses are restricted to pregnant women, unlike other alloantibodies. Because syncytiotrophoblast microparticles (STMP) are shed into maternal blood throughout pregnancy, we hypothesised they might be essential for anti‐HPA‐1a formation. The HPA‐1 polymorphism is on beta3 integrin (CD61, GPIIIa) which is associated with alphaIIb (CD41, GPIIb) on platelets and alphaV (CD51) on syncytiotrophoblast.
Aims
The objective was to generate anti‐HPA‐1a de novo using a well established in vivo model to study long term human antibody responses in mice with severe combined immunodeficiency (SCID). The model was improved by the addition of interleukin (IL)‐4 and IL‐21 to stimulate Th2 responses and B cell differentiation to plasma cells, respectively.
Methods
STMP were prepared from fresh HPA‐1a‐positive placentas by abrading villous surfaces in saline followed by differential centrifugation to remove blood cells and to pellet STMP at 13,000 and 100,000 g. Expression of integrins and placental alkaline phosphatase (PLAP) on STMP was determined by flow cytometry. Peripheral blood mononuclear cells (PBMC) were prepared from HPA‐1b1b HLA DRB3*0101 genotyped male donors and platelets from HPA‐1 genotyped donors. SCID mice were injected intraperitoneally with PBMC (50 × 106), IL‐4 (100 ng/ml), IL‐21 (50 ng/ml) and antigens (40–80 μg STMP‐13 g, STMP‐100 g or HPA‐1a1a platelets). Further antigen injections were given 1 and 4 days later. Five blood samples were taken from 4 to 13 weeks. Concentrations of anti‐HPA‐1a and anti‐HPA‐1b were determined by a sensitive monoclonal antibody immobilisation of platelet antigen (MAIPA) assay, modified for small volumes of murine plasma.
Results
PLAP was expressed on 99% of STMP and CD61, CD51 and CD41 on 65–99% of STMP, indicating microaggregates of platelet microparticles with STMP. Two of 24 mice immunised with STMP produced high concentration anti‐HPA‐1a; one at week 6 after 40 μg STMP‐100 g (>100iu/ml) and one at week 10 after immunisation with 40 μg STMP‐30 g (25 iu/ml). There were no anti‐HPA‐1b responses and no responses from 6 control mice without STMP. Four of 12 mice immunised with HPA‐1a1a platelets produced low concentrations (mean 0.1iu/ml) of antibodies weakly reactive in MAIPA with both HPA‐1a1a and HPA‐1b1b platelets; two at weeks 9–11 and week 13 after 40 μg platelets and two at weeks 4–13 and week 11 after 80 μg platelets. Another mouse had only anti‐HPA‐1b at week 11 after 40 μg platelets.
Conclusions
This is the first report of human IgG anti‐HPA‐1a produced by experimentation. The low incidence of anti‐HPA‐1a was expected because there would be few antigen‐specific T and B cells per mouse (approximately 100 and 10, respectively) and functional immune responses require cellular interaction between dendritic cells (matured from monocytes of the injected PBMC), antigen‐specific T cells and B cells. The high concentrations of anti‐HPA‐1a produced in response to STMP indicates clonal, high affinity antibody; transient responses were probably due to lack of survival signals for plasma cells. It is likely that both STMP and contaminating platelet microparticles participated in anti‐HPA‐1a responses. In contrast, platelets alone induced low concentration anti‐GPIIIa responses that were pan reactive, suggestive of autoantibodies. Therefore our findings support the hypothesis that anti‐HPA‐1a, uniquely, requires primary immunisation by placental STMP.
3A‐S01‐04
Hyperimmunization With RhD Generates a Broad and Persistent Repertoire of IgM‐ and IgG‐ Antigen‐Specific B Cells
van der Schoot CE1, Della Valle L 1, IJspeert H 2, van der Burg M 2, Vidarsson G 1, Berkowska M 1
1Sanquin, Amsterdam, The Netherlands2Erasmus MC, Rotterdam, The Netherlands
Background
RhD‐negative women, pregnant with an RhD‐positive child, receive anti‐D immunoglobulins (Ig) from healthy hyperimmunized donors to prevent alloimmunization. Still, the characteristics of these anti‐D‐Igs and dynamics of the anti‐D response in time remain poorly characterized. The hyperimmunization of anti‐D donors can serve as a model system for alloimmunization.
Aim
To characterize the memory B cells involved in the allo‐immune response.
Methods
From six hyperimmune anti‐D donors RhD‐specific CD27-IgM+, CD27+IgM+, CD27+IgG+ and CD27-IgG+ B cells recognizing D+ red‐blood‐cell ghosts were sorted as single cells and expanded in culture (as described before Della Valle et al. J Immunology 2014). Then, clones producing anti‐D‐Igs were subjected to Ig gene analysis and the relationship of the RhD‐specific clones was studied. To investigate the evolution of anti‐D response over time, known anti‐D rearrangements were identified in samples from 3 donors from 3 remote time points (spanning upto 10 years) by massive parallel sequencing.
Results
RhD‐specific B cells were identified in all donors, with the highest frequency in recently immunized ones. They were equally distributed between IgM+ and IgG+ B cells. Rearranged Ig genes in RhD‐specific B cells carried somatic hypermutations and displayed exceptionally long IgH‐CDR3s. They showed a broad repertoire skewed towards usage of IGHV4-34, IGHV3-33 and IGHV3-30 genes. Frequently utilized IGHV4-34 genes showed limited selection for replacement mutations in IgH‐CDRs, implicating structural advantage of this gene in RhD recognition. At least 30% of identified anti‐D rearrangements represented clonally related cells. While the majority of clones spanned either IgM+ or IgG+ B‐cell subsets, others were shared by both IgM+ and IgG+ cells. The massive parallel sequencing data showed that in all 3 donors clonally related cells were identified at the different time points showing that these clones can have a very long life‐span of at least 10 years Remarkably, we identified in recent time points B cells harboring less mutations then their clonally related B cells isolated at earlier time points. Our data also suggest that the somatic hypermutation rate did not seem to increase over time.
Conclusion
The response to RhD involves a broad, but restricted Ig gene repertoire, which persists over time. These data support a model where both IgM+ and IgG+ B cells are important for the maintenance of a long lasting allo‐immune response. And in contrast to the classical idea on memory B cells, our data indicate that upon boosting the recruitment of new (less mutated) IgM+ memory B might play a more important role in the memory response than the IgG+ memory B cells reentering to the germinal center (GC).
3A‐S01‐05
Rhd Immunization Despite Adequate Immunoprophylaxis: Role of Fc Gamma Receptor Gene Polymorphisms
Stegmann TC , Veldhuisen B , Nagelkerke SQ , van Winkelhorst DW , de Haas M , Vidarsson G , van der Schoot CE
Sanquin Blood research, Amsterdam, The Netherlands
Background
One of the most effective immunological interventions in clinical medicine is the prevention of hemolytic disease of the newborn by prophylactic Rh immune globulin (Rh‐Ig) therapy. The administration of ante‐ and postnatal Rh‐Ig has reduced the risk of RhD immunization in the Netherlands from 17% to 0.31%. However, its mechanism of action is still unknown. One possibility is that it is related to a particular phenotype of the highly polymorphic IgG‐Fc receptor (FcγR) family, the receptors responsible for IgG‐mediated clearance of IgG‐opsonzied RBC, but potentially also affecting immune responses.
Aims
The aim of our study is to gain more insight into the working mechanism of anti‐D by identifying risk factors for developing anti‐D antibodies despite receiving Rh‐Ig prophylaxis, including the genetic makeup of the IgG‐Fc receptor (FcγR) locus.
Methods
Information about Rh‐Ig prophylaxis and additional clinical data for potential risk factors were collected through a structured questionnaire, and 160 Dutch women were identified immunized during pregnancy despite adequate prophylaxis. Of those, 95 were treated with IUT. DNA was collected from all, including 71 children severely affected by anti‐D antibodies. DNA was subjected to FcγR‐specific multiplex ligation‐dependent probe amplification (MLPA) assay, identifying both single nucleotide polymorphisms and copy number variations in the FCGR locus. The results were compared to a control group of 200 healthy donors.
Results
A history of red blood cell transfusion (P = 0.05) and caesarean section (P < 0.0001) were identified to be independent risk factors for RhD immunization. However, these risk factors did not explain the development of anti‐D antibodies for 47% of our cohort. We found no significant differences between our healthy controls and immunized pregnant group. However, when we compared our IUT‐treated group to the healthy controls we found an significant increase (P = 0.02) in prevalence of the FCGR2C ‐ORF, expressing a functional copy of the activating FcγRIIc, which is otherwise a pseudogene. In the same group, the prevalence of 2B.4 promotor haplotype, associated with a 1.5 fold increase of the inhibitory FcγRIIb, was (P = 0.007) increased as well as the FCGRIIIa‐158V (related to increased affinity) was overrepresented. To date the analysis of the FCGR‐polymorphisms of the children is not yet complete.
Conclusion
Caesarian section and red blood cell transfusion are risk factors that increase RhD immunization during pregnancies, accounting for about half failed Rh‐Ig prophylactic cases. Our data do not suggest that there is an increased risk for failure of prophylaxis in women with activating FcγR‐genotypes resulting in decreased opsonized RBC clearance. In the IUT group the higher affinity‐ FcγRIIIa‐allele was even overrepresented, possibly due to the more severe disease in these children. Also the significantly increased frequency of individuals expressing FcγRIIc, along with a polymorphism encoding for a higher expression of the inhibitory receptor FcγRIIb might be related to an increased immunization risk, since similar skewing has been observed in autoimmune diseases.
Adverse Reactions
3A‐S02‐01
Use of and Reactions to Fresh Frozen Plasma
Bolton-Maggs PHB
NHSBT, Manchester, United Kingdom
The UK National Haemovigilance Scheme, Serious Hazards of Transfusion (SHOT) was started in 1996 and now has data collated for 18 years of reporting. Initially only 22% of hospitals reported, but in recent years this has risen to almost complete (99.5%) reporting by National Health Service hospitals in the UK.
Fresh frozen plasma is associated with several adverse reactions including circulatory overload, allergic and anaphylactic reactions and transfusion‐related acute lung injury (TRALI). As a result of data accumulated from SHOT reports showing TRALI to be the most common cause of transfusion‐related death, a risk reduction project was started by the National Blood Service in England in 2003. Plasma‐rich components, particularly from female donors, had a higher risk. Male plasma is therefore preferred and by the end of 2007, 90% of FFP and plasma contributions to platelet pools were from male donors. Currently 100% of FFP is from male donors. Since this transition, reported TRALI cases and deaths have reduced from a peak of 36 in 2003 (7 deaths) to 10 in 2013 (1 death), none associated with FFP or platelets 2011‐2013 (while overall reporting to SHOT, all causes, has increased). Transfusion‐associated circulatory overload is more common but respiratory complications may be difficult to differentiate from each other.
Reporting of allergic, febrile and anaphylactic reactions (defined as occurring up to 24 h following transfusion) shows an increase over time in line with overall reporting to SHOT. Reports of anaphylaxis are stable at about 30–40 per year. From 2012 SHOT decided not to include mild reactions, this the annual total reduced (587 in 2011, 343 for 2014). Review of cases has proved instructive, demonstrating a need to ensure clinicians are aware that adrenaline is the treatment for anaphylaxis. There is widespread use of antihistamines and steroids for which there is little evidence in the literature. Review by component type demonstrates that febrile reactions are very uncommon with FFP but moderate and severe allergic and anaphylaxis are more likely with FFP than any other component. In recent years, in addition to standard FFP, two forms of pathogen‐inactivated (PI) FFP are available, solvent‐detergent sterilisation to produce pooled plasma, or methylene‐blue treatment of individual units. In the UK PI‐FFP is recommended particularly for those born since 1/1/96 (as a potential vCJD‐transmission‐reduction measure). Recent concern about reactions led to withdrawal of MB‐FFP in France, but careful analysis of SHOT data has shown no significant increase in reaction rates. SD‐FFP has some advantage with a demonstrated reduction in incidence of allergic reactions compared with standard FFP and is recommended for plasma exchange in thrombotic thrombocytopenic purpura.
While there is recognition that FFP should be used early in massive haemorrhage, other uses are frequently inappropriate and not evidenced‐based. A UK National Comparative Audit (2009) demonstrated inappropriate use, particularly for warfarin reversal and correction of abnormal coagulation tests in the absence of bleeding in adults and children. Recent reviews of coagulation in liver disease have led to a rethink about the management of abnormal coagulation in this setting.
3A‐S02‐02
75‐nm Nanofiltration of Plasma Removes Platelet Microparticles That Trigger Neutrophil Extracellular Traps (NETs) In Vitro
Burnouf T1, Sung PS 2, Hsiao SH 1, Chou ML 1, Hsieh SL 3
1Taipei Medical University, Taipei, Taiwan2National Yang Ming University, Taipei, Taiwan3Academia Sinica, Taipei, Taiwan
Background
Platelet derived microparticles (PMPs) are 0.05–1 mm phospholipid‐rich vesicles shed by platelets exposed to shear stress, activation, or undergoing apoptosis. They can be generated during blood components processing and storage. PMPs membranes have 50–100 times higher pro‐coagulant activity than platelets. Although likely contributing to plasma hemostatic effect, they may potentially lead to thrombotic and inflammatory adverse reactions in susceptible patients. One major complication of plasma transfusion is non‐immune transfusion related acute lung injury (TRALI), which is associated with the formation of neutrophil extracellular traps (NETs) resulting from neutrophil activation. Activated platelets have recently been shown to trigger NETs formation. We hypothesized here that (a) NETs could be promoted by PMPs and (b) that 75 nm‐nanofiltration of plasma, by potentially removing PMPs, could avoid NETs formation.
Aims
(i) Demonstrate the role of PMPs in NETs formation and (ii) show that removal of PMPs by plasma nanofiltration reduces NETS formation.
Methods
PMPs were prepared from apheresis human platelet concentrates that were centrifuged at 3000 xg for 15 min. Platelets were resuspended in Tyrode's buffer and activated by 0.1 U/ml thrombin for 60 min at 37°C under mild agitation to stimulate PMP release. Activation was stopped by 20 mM EDTA. PMPs were isolated by centrifugation at 3200 g for 10 min and at 20,000 g for 90 min at 22°C and resuspended in 1 ml Tyrode's buffer. PMP count and size was determined by tunable resistive pulse sensing (TRPS). Tyrode's buffer or plasma were spiked with 1.6 × 108 PMPs (final concentration) and were nanofiltered (0.001‐m2 Planova 75N filter; Asahi Kasei, Japan) under pre‐validated conditions and the plasma flow‐through was recovered. Human polymorphonuclear cells (PMNs) were prepared from healthy donors. Buffy coat was collected by Ficoll and centrifugation. Red blood cells were lysed and PMNs isolated by negative selection. The PMNs, cultured in RPMI‐1640 media, were incubated for 1 h at 37°C with various ratios of (i) PMP spiked buffer, (ii) PMP spiked plasma, (iii) PMP‐spiked buffer after nanofiltration, and (iv) PMP‐spiked plasma after nanofiltration. The PMNs were fixed by 4% paraformaldehyde in PBS. Neutrophil aggregation was checked by microscopy. NETs formation was evaluated by DNA, histone, and myeloperoxidase (MPO) staining using Hoechst 33342, anti‐human histone H1 and anti‐human MPO, respectively, and observed by confocal microscopy.
Results
The suspension obtained by thrombin activation of platelets contained approximately 1 × 1010 PMPs/ml with a size ranging from 50 to 200 nm. PMP‐spiked buffer and plasma incubated with PMNs at a PMP/PMN ratio of 1:10, 1:30, 1:100 induced strong PMNs aggregation and NETs formation. In contrast, PMN aggregation and NETs formation was absent or strongly reduced when using nanofiltered PMP‐spiked buffer or plasma. PMP‐buffer spiking experiment showed that a reduction factor of PMP >9 log by nanofiltration.
Summary/Conclusions
PMPs generated by thrombin activation of platelets, and spiked to both buffer or plasma, trigger strong aggregation of neutrophils and NETs formation in vitro. Neutrophil aggregation and NETs formation can be avoided or reduced dramatically by 75 nm‐nanofiltration of both PMP‐spiked buffer or plasma.
3A‐S02‐03
Delays in Transfusion – What Can We Learn After 5 Years Reporting to the Serious Hazards of Transfusion (SHOT) UK Haemovigilance Scheme
Ball J , Poles D , Bolton-Maggs PHB
Serious HAzards of Transfusion, Manchester, United Kingdom
Background
The UK National Patient Safety Agency (NPSA) received reports of 11 deaths and a further 83 incidents where patients suffered harm due to delay in transfusion (2006–2010). A national instruction (NPSA/2010/RRR017) was issued requiring all hospitals to review their local policies and procedures for the emergency provision of blood components, review any identified events and report these to the Serious Hazards of Transfusion (SHOT) haemovigilance scheme. SHOT extended this NPSA recommendation to include any delayed transfusions outside the emergency situation which resulted in patient harm or other adverse effects. A specific time‐frame for delay was purposely omitted as the impact on patient outcome is case‐dependant. Clinicians should decide case‐by‐case whether there was an unreasonable delay in treatment.
Aims
To review cumulative SHOT data 2010–2014 and identify the reasons for delayed transfusion.
Method
A 5‐year analysis of reports of delayed transfusion submitted to SHOT (2010–2014).
Results
There were 119 reports of delayed transfusion resulting in 10 (8%) deaths where delay was causal or contributory, and 6/10 related to problems with the massive haemorrhage protocol (MHP). These included: lack of familiarity with MHP, poor communication, delayed sample receipt, incorrect patient details from ambulance crew and confusion surrounding a ‘do not resuscitate’ order. In 4 further cases the delay was compounded by poor management particularly over weekend periods and shift changes.
Most transfusions (102/119) were indicated for acute situations; emergency (58/119) or urgent (44/119) with 26/119 relating to problems with MHP (including the 6 deaths). Failure to activate the MHP (8/26) and failure adhere to the MHP (5/26) were the commonest reasons for delay. In 10/26 cases the delays were due to other problems encountered during the incident, and in 3/26 the MHP call was delayed.
Most incidents were reported from wards (37/119), theatres (24/119), emergency departments (21/119) and critical care units (19/119). Causes were multi‐factorial but key root causes included failures in communication between the clinical and laboratory areas, missed information during the handover process, poor knowledge and assesment including failure to recognise haemorrhagic shock and delayed decision making. There were problems recorded with availability of components, poor communication, failure to activate or follow the MHP, sample labelling errors and wrong blood in tube events.
Conclusion
Reporting delayed transfusions has demonstrated some common themes related to management of MHP calls and timely provision of blood components. In addition to MHP issues, this wider review of delays highlights other areas of poor practice. The sharing of these lessons alerts others to examine the impact of changes in hospital practice and training, and can inform the development of future guidelines.
Transfusion is a complex process involving multi‐disciplinary working. Effective communication in these circumstances may be difficult but it is vital for the appropriate and efficient treatment of patients particularly in the acute setting. It is essential that all members of the team are kept fully informed of the progress of the case and carry out their role within the team according to the locally agreed protocol.
3A‐S02‐04
Are the ‘Rules’ for Times in Setup and Duration of Red Cell Transfusion Too Strict?
Foley K , Poles D , Bolton-Maggs PHB
Serious Hazards of Transfusion Office, Manchester, United Kingdom
Background
International consensus is that a transfusion must be set up within 30mins of the unit leaving controlled temperature storage and that a unit of red cells should be transfused over no more than 4 h. This is based on the potential risk of bacterial growth as the component warms, and the possible effect on the quality of red cells. The scientific basis for these rules is weak. A recent systematic review found that temperature exposure did not affect either of these parameters. Additional studies of red cells collected and stored in conditions similar to those used in the Blood Services found that repeated exposure to room temperature caused no significant damage to red cells, and repeated exposure to room temperature for up to 60mins did not significantly influence growth of bacteria in spiked units. There have been no reports to the UK haemovigilance scheme, Serious Hazards of Transfusion (SHOT), of transfusion transmitted bacterial infection since 2009, and only 40 reports since 1996 none of which were related to extended transfusion times.
Aims
To analyse clinical outcomes following extended transfusion time or delay in starting transfusion.
Methods
Aggregated data from reports to SHOT covering a 5 year period (2010–2014) were reviewed, including reports where a red cell unit was set up >30 min after removal from controlled temperature storage, or the total transfusion time was >5 h or both.
Results
There were 143 delays in set up with no adverse clinical outcomes reported. 79/143 (55%) were delayed by >60 min, and 39/143 (27%) by >2.5 h. 47/143 (33%) episodes were emergency or urgent transfusions, of which 12/47 were delayed >3 h.
In 239 incidents the transfusion duration of a single unit was >5 h, including 33/239 (16%) emergency/urgent transfusions. 152/239 (64%) took between 5 and 6 h to complete, but 35/239 (15%) exceeded 7 h. There were 19 delays in both parts, 4 of which were urgent transfusions. There were no adverse clinical outcomes (including no bacterial infections) associated with any of these episodes. 287/382 (75%) took place in wards or admissions units, but 49 (13%) in ITU/theatres/Emergency areas, and also 16 (4%) in the community.


Discussion
Delays in set‐up or prolonged infusion times are not associated with any clinical adverse events. However, it is worrying that a proportion of these related to urgent/emergency transfusions. Movement of patients between clinical areas and poor handover may contribute to this. Most delays occurred on inpatient or admissions wards, which may be a result of heavy workloads, patient movement or lack of staff training.
The current guidance of 30 min and 4 h may be unnecessarily stringent resulting in increased wastage. With more evidence it may be possible to further increase this limit. Blood services should consider further studies and consider making guidance more flexible.
Quality Issues and Blood Utilization
3A‐S03‐01
The Use of a Higher Trigger for Fibrinogen Substitution in Major Pediatric Surgery Does Not Lead to an Increase in Costs
Haas T1, Spielmann N 1, Restin T 2, Weiss M 1, Schmugge M 1, Cushing MM 3
1Zurich University Children's Hospital, Zurich, Switzerland2Zurich University Hospital, Zurich, Switzerland3Weill Cornell Medical College, New York, NY, United States of America
Background
Acquired hypofibrinogenemia is a common cause of bleeding during major pediatric surgery. In a recently performed randomized controlled trial at a University Children's Hospital in Switzerland, we demonstrated that transfusion requirements could be reduced significantly if higher trigger values for intraoperative fibrinogen substitution were applied (ClinicalTrials.gov identifier No. NCT01487837). A functional fibrinogen test was used (ROTEM, TEM International, Munich, Germany; FIBTEM test) to determine maximum clot firmness (MCF). The 17 children randomized to early substitution of fibrinogen (FIBTEM MCF < 13 mm) received significantly less RBCs during the 24 h after the start of craniosynostostis surgery (median, 28.2 ml/kg; IQR, 21.2–49.9 ml/kg) compared to the 13 children randomized to the conventional trigger (FIBTEM MCF < 8 mm) for fibrinogen substitution (median, 55.5 ml/kg; IQR, 27.5–61.8 ml/kg) (P = 0.03). In addition, platelets, FFP, and FXIII concentrate were administered based on a strict transfusion protocol.
Aims
The aim of this study was a post hoc cost analysis of the applied coagulation management with a special focus on comparing the two different trigger levels for fibrinogen substitution and the resulting overall costs per patient.
Methods
The total volume, as well as the number of bags or units, of all transfused allogeneic blood products and coagulation factors were recorded for each case. Total costs [median (IQR)] for all administered blood products and coagulation factors were collected according to the local prices per unit and total costs per patient were calculated. The total price for each unit or bag was charged, even if the entire volume was not administered (Table 1). Since the frequency of standard and point‐of‐care laboratory testing was similar between the two arms, the economic analysis did not include laboratory testing.
Results
Actual costs for coagulation management were collected for the 30 patients who underwent craniosynostosis surgery. The median cost of RBCs per patient was €187 (2 Units) in the early substitution as compared to €374 (1 Unit) in the conventional group (P = 0.13) (Table 2). In contrast, the median cost for fibrinogen concentrate was doubled (€563) in the early substitution group as compared to the conventional group (€282) (P = 0.07). Costs for administered FXIII concentrate were similar between both groups (P = 0.41). Costs for all administered blood products/coagulation factors per patient were not different between the early substitution group, €900 (€543–€1007) as compared to the conventional group €802 (€643–€1189) (P = 0.81).


Summary/Conclusions
The reported reduction in both blood loss and transfusions in children who underwent craniosynostosis surgery and were treated early with fibrinogen concentrate using a trigger of <13 mm FIBTEM MCF was not associated with an increase in costs for transfused blood products and coagulation factors as compared to a conventional fibrinogen trigger of <8 mm FIBTEM MCF.
3A‐S03‐02
Factors Influencing the Decision of Viral Testing: Applying Pharma‐Economics to the Current Testing Strategy Used by SA National Blood Service
Coleman CF , Vermeulen M
SA National Blood Service, Roodepoort, South Africa
Background
Viral testing has made great advances with the aim to maximise safety. Testing however comes at a great expense and the additional cost incurred compared to the net health gain requires constant assessment. The field of pharma‐economics aim to quantify this cost vs health gain and allows different health care interventions to be compared in order to aid decision making.
The decision of viral testing is however also influenced by the political environment and public opinion. Regulation from the World Health Organisation (WHO) also dictates mandatory testing.
Aims
The aim in this study is to assess the viability of the current viral testing strategy in SANBS for HIV, HBV and HCV using pharma‐economic modelling.
Methods
The cost formula as described by Farnham et al was applied as follows: (C – AT)/AQ, where C is the total cost of intervention, A is the number of infections averted, T is the treatment cost saved per infection averted and Q is the number of Quality Adjusted Life Years (QALYs) gained per infection prevented. A negative value implies that the cost of the testing is less than the cost of treatment.
QALYs for prevention of HIV, HBV and HCV as well as cost of treatment were not calculated but are conservative estimates based on literature review. QALYs used for HIV, HBV and HCV were 13.3, 10, and 10 respectively. Lifetime treatment cost for HIV was estimated as R1.5 million and R1 million for HBV and HCV.
Each assay was analysed both separately or as an incremental safety margin.
Results
Modelling returns negative values for NAT HIV and HBV testing but positive values for NAT HCV if the NAT testing cost is divided by three. Alternatively the total cost can only be assigned to HIV and HBV, which then still returns a negative value in the model.
Modelling returns negative values for Prism anti‐HIV, HBsAg and anti‐HCV testing when implemented alone (Table 1).

The cost of preventing an HIV, HBV and HCV infection per annum by serology testing only was calculated as R5 955, R10 082 and R558 185 respectively.
The cost of preventing an additional 271 infections (NAT yields HIV 78, HBV 190 HCV 3) by NAT per annum was calculated as R21 169 462.
Summary/Conclusions
The implementation of NAT is justified by the prevention of additional HIV and HBV infections. HCV detection is an added benefit but would not be cost effective if interpreted in isolation.
Anti‐HIV and HBsAg testing is still cost effective even in the presence of NAT. Modelling however suggest that anti‐HCV testing is not an viable option even if you assume that the 16 anti‐HCV only positives detected per annum are infectious which is unlikely as no HCV RNA was detected in any of these donors on plasma repeat testing or donor return samples since 2005. Anti‐HCV testing is however required by WHO guideline. SANBS should consider if this compliance is worth R28 million per annum.
3A‐S03‐03
Recent Trends in Blood Component Transfusions at a Population Level
Robillard P , Grégoire Y , Delage G , Germain M
Hema‐Quebec, Montreal, QC, Canada
Background
After many years of regular growth, many developed countries have recently experienced a decline in the demand of red blood cells (RBC). Data on other components like platelets (PLT) or plasma are lacking at a population level.
Objectives
The objectives of this study were to describe the recent trends in the epidemiology of blood component transfusions in the province of Quebec, Canada (population of 8 million) and to provide data on the age of blood components transfused.
Methods
In Quebec all blood banks are computerized with the same software and managed under the umbrella of the Quebec Health Ministry (QHM) allowing for central extraction of transfusion data. Héma‐Québec (HQ) is the sole blood supplier for the province. Data on all transfusions for years 2012–2014 were extracted by the QHM. They were matched with HQ database on products to calculate the age of products transfused. SAS software was used to conduct descriptive analyses.
Results
For the period 2012–2014 a total of 701,362 RBC units were transfused of which 52.6% were to male recipients. For PLT and plasma respective totals were 107,661 (61.0% to male) and 132,768 (56.4% to male). Age of RBC recipients was: <1 month: 0.9%; 1 month–17 years: 2.4%; 18–49 years: 13.6%; 50–59 years: 11.5%; 60–69 years: 21.3%; 70–79 years: 25.4%; 80+ years: 24.9%. Transfusion ratios per 1000 population are presented in table. There was a significant decrease over a 3‐year period of 9.0% for RBC, 8.7% for PLT and 15.5% for plasma transfusion ratios. This was true for all age groups except for the pediatric population for which ratios were stable. Mean age of RBCs was 22.8 ± 8.3 days (mean ± SD) and median was 22 days. Age of RBCs was significantly lower for child recipients (14.7 ± 9.4 days) than for adults (23.1 ± 8.1 days) (P < 0.001). Age of RBCs was also significantly lower for neonatal recipients (11.4 ± 7.3 days) than for other children (15.9 ± 9.7 days) (P < 0.001). Mean age of PLT was 4 days.

Conclusion
Despite already low RBC transfusion ratios compared to others (in 2013 they were 31.9 in UK and 37.8 in France compared to 28.6 in Quebec) demand for RBC as well as for other components is continuing to decrease. It appears that there is a significant change in transfusion practice. However, since 50% of RBC units were transfused to patients who are 70+ years and given the aging of our population this trend could reverse in the coming years.
3A‐S03‐04
Determination of the Top Indications for Red Blood Cell Use in Large European Hospitals to Identify Areas for Patient Blood Management (PBM) Interventions
van Kraaij MGJ1, Manzini PM 2, van Pampus EC 3, Pendry K 4, Wikman A 5, Meybohm P 6, Fischer D 6, Zacharowski K 6, Borg-Aquilina D 7, Laspina S 7, Georgsen J 8, Topholm-Bruun M 8, Geisen C 9, Mueller MGJ 9, Liumbruno G 10, Grant-Casey J 11, Singh Babra P 11, Seifried EC 9, Folléa G 12, Murphy MF 13
1Sanquin Blood Supply, Amsterdam, The Netherlands2Banca del Sangue e del Plasma CPVE, Torino, Italy3Laboratory of Medical Immunology, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands4Department of Transfusion, Central Manchester University Hospitals NHS Foundation, Manchester, United Kingdom5Department of Clinical Immunology and Transfusion Medicine, Karolinska University, Stockholm, Sweden6Department of Anesthesiology, IC Medicine and Pain Therapy, University of Frankfurt, Frankfurt, Germany7Hospital Blood Bank, Mater Dei University Hospital, Msida, Malta8Department of Clinical Immunology, Odense University Hospital, Odense, Denmark9German Red Cross Blood Service Baden‐Wuerttemberg‐Hessen, Frankfurt, Germany10Centro Trasfusionale, San Giovanni Calibita Rome University Hospital, Roma, Italy11NHS Blood & Transplant, Oxford Blood Centre, Oxford, United Kingdom12European Blood Alliance, Amsterdam, The Netherlands13Oxford University Hospitals and University of Oxford, Oxford, United Kingdom
Background
Patient blood management (PBM) is a multidisciplinary approach to optimize red blood cell (RBC) usage in patients. PBM strategies can be adopted for all patients, but up to now PBM has been studied mainly in surgical patients. Eight large hospitals from 7 different European countries are participating in a European Blood Alliance (EBA) initiative on PBM called PaBloE (Patient Blood Management in Europe). Before developing PBM strategies it is important to know the indications for RBC transfusion and whether there are differences between countries.
Aim
To determine the top indications for RBC use in 8 large European hospitals.
Methods
During the 2nd week of May 2014 all RBC transfusion episodes were recorded in the eight participating hospitals. The hospitals ranged in size from 953 to 3000 inpatient beds (median 1286). Data were collected for each transfusion episode on the number of units transfused, age and gender of the patient and the clinical category for transfusion. Data were submitted and analysed through Survey Monkey to the National Comparative Audit Program, NHS Blood & Transplant; hereby presenting the preliminary results.
Results
During the survey episode, 3367 RBC units were transfused to patients with a median age of 59 years (male/female ratio: 60% vs 40%) in the following clinical categories: medical: 61%, surgical: 36% and obstetric/gynaecological: 3%. The top three medical indications for RBC transfusion were: haematological anemias (30.1%) gastrointestinal (GI) bleeding (7.1%) and ICU patients (4.4%). Of haematological anaemias, RBC use was highest in AML/ALL/MDS patients and haemoglobinopathies (9.6% and 3.5% respectively). Among non‐haematological indications the percentage of RBC transfusions for iron deficiency and anaemia in chronic renal disease (1.4% and 2.2%, respectively) was remarkable, since other treatments are available. The top three surgical indications were: cardiothoracic surgery (9.6%), GI surgery (5.7%) and trauma (4.0%). Interestingly, only 3.5% of total units transfused for surgical indications were given to orthopaedic surgery patients, possibly demonstrating that PBM measures already may have been taken for this indication. For some medical and surgical indications the differences between hospitals were considerable, mainly due to bed size, diversity of population and specialist treatment.
Conclusions
By means of a simple survey, top indications for PBM measures could be identified among 8 large European hospitals. Almost two‐third of RBC transfusions have been given for medical indications. PBM should therefore be more focused on indications for medical RBC transfusions, especially haematological anaemias. Transfusion triggers, number of units transfused and alternatives for RBC transfusion should be targets for future studies on RBC transfusion for medical indications.
3A‐S03‐05
Quality Management and Inspection of Blood and Blood Components Following GMP Standards and the European Directives
Seidl C1, Huber H 2, Teskrat F 3, Aquilina A 4, Ceulemans J 5, Cardenas JM 6, Amil M 7, Seitz R 2, Pupella S 8, Grazzini G 8, Vuk T 9, Strengers P 10, de Wit J 11, van de Kerckhove P 12, Seifried E 1
1Institute of Transfusion Medicine and Immunohematology, Red Cross Blood Donor Se, Frankfurt am Main, Germany2Paul‐Ehrlich Institute, Langen, Germany3ANSM, St. Denis – Paris, France4Maltease Blood Transfusion Service, L'Mangia, Malta5Belgium Blood Transfusion Service Flanderen, Leuven, Belgium6Blood Transfusion Service Bilbao, Bilbao, Spain7Blood Transfusion Service, Porto, Portugal8Centro Nazionale Sangue, Rome, Italy9Croatian Blood Transfusion Service, Zakreb, Croatia10IPFA, Amsterdam, The Netherlands11Sanquin Foundation, Amsterdam, The Netherlands12EBA and Belgium Blood Transfusion Service Flanderen, Leuven, Belgium
Background
Quality criteria are essential to manufacture safe blood and blood components for the treatment of patients worldwide. Besides Good manufacturing standards (GMP) standards such as the European blood directives and the EDQM guidelines have been used be national authorities to ensure proper quality management. The European Commission (EC) has funded an initiative bringing together regulators [competent authorities (CA)] and manufacturers [blood establishments (BE)] to jointly develop criteria and standards aimed to assist: (i) BE in need to optimise their quality system and self‐inspection process and to prepare for regulatory inspections by CA. (ii) CA, which wish to use the manual and training guide as a reference for the implementation process of legislative requirements related to regulatory inspections.
Aim
This initiative is carried out by the EuBIS academy and has been supported by the CATIE training programme. In order to address these requirements on an international level the ISBT working party on quality management has performed a survey with support by EuBIS.
Methods
The survey has been performed recruiting ISBT partners covering several regions worldwide using a defined questionnaire covering quality management aspects and requirements of CA for licensing and control by inspections. The assessment of areas for improvement was based on the EuBIS guide, chapter 3 linked to GMP, EU directive and the EDQM guide using a grading of major, medium and minor for areas of improvement. Evaluation was performed using level of improvement, with level 1 (>30% in major and medium grade) and level 2 (<10% minor grade).
Results
The results are based on analysis 26 regional responses (50% Europe and 50% Africa, Northern and Southern America, South East Asia, Western Pacific and Eastern Mediterranian). 88% of BE indicated that their QM need to be changed or improved with 76% in need to adapt to national requirements. Improvement level comprised the following areas: Quality policy, personnel and organisation, documentation, self‐inspection, continuous improvements, non‐conformances and risk‐management. Improvement level 2: Premises (donor and collection area, processing and storage), laboratory testing, validation, storage and distribution.
Conclusion
These results underline the importance in defining common criteria for quality management and assisting the implementation and continuous improvement by training programmes as initiated by the EuBIS Academy and jointly supported by the ISBT working party.
Acknowledgement
The results are presented on behalf of the members of the EuBIS Academy and the ISBT Working Party on quality management. Initial funding has been provided by the Public Health Programme of the EC. The current study is supported by the European Blood Alliance (EBA) and the International Society of Blood Transfusion (ISBT).
3A‐S03‐06
Twenty Years of Joint Working to Monitor Blood Safety in the UK: 1995–2014
Reynolds CA1, Davison KL 1, Morrison R 2, Brailsford SR 2
1Public Health England, London, United Kingdom2NHS Blood and Transplant, London, United Kingdom
Background
NHS Blood and Transplant and Public Health England set up a jointly funded surveillance unit in October 1995 to enhance and share surveillance data associated with infected blood and tissue donors and recipients to support a safe supply within NHSBT and the wider UK blood services. The unit has since expanded surveillance to inform other areas such as organ infection.
Aims
To present UK trends in positive donation testing results in the context of annual estimates of risk and the number of confirmed transfusion transmitted infections (TTIs).
Methods
The unit obtains and stores data from UK testing centres and confirmatory laboratories, the NHSBT donor management database (PULSE) and the clinical team. Data were extracted for October 1995 to December 2014 and analysed by Poisson regression in STATA 13. Residual risk was calculated by modelling the infectious window period for HBV, HCV and HIV tests in use. The overall rate of infection among new donors was adjusted by the age and gender of the general UK population.
Results
Since 1995 the annual number of donors with positive test results has been low especially in repeat donors, previously screened. The HBV rate has been overall stable in new donors; the majority chronic infections linked to the donor's country of origin whilst decreasing in repeat donors (IRR 0.93 P = <0.001, 95% CI 0.91–0.95). Risk calculations have been consistently higher for HBV than HCV or HIV; just as most observed viral TTIs between 1995 and 2014 have been HBV, partly due to the long window period when infections can be missed by testing. HCV is mainly observed in white male donors and has decreased in donations from new (IRR 0.95, P = <0.001, 95% CI 0.94–0.95) and repeat donors (IRR 0.84, P = <0.001, 95% CI 0.82–0.86). The risk of infection is currently about 1 in 20 years, the last TTI in 1997 prior to NAT testing. HIV has been low and variable; the last reported TTI was in 2002 but predicted to be one every 2–3 years. HTLV was also low and variable, with infections mainly in females and linked to endemic countries through vertical transmission and/or heterosexual contact. Leucodepletion is likely to mitigate the risk of transmission via transfused blood. Syphilis prevalence appears to have increased in donations from new donors (IRR 1.05, P = <0.001, 95% CI 1.04–1.07) although testing detects past treated infections. Syphilis TTIs have not been recorded in the UK during the surveillance period; cold storage is thought to kill the bacterium. The rate of any marker among new blood donors in UK during 2013 was 109 per 100,000 i.e. approximately 1 in 900 donors. This increased to 139.7 per 100,000 when differences in the age and gender distribution between donors and the general population were adjusted for.
Summary
The findings of the NHSBT/PHE unit over 20 years have provided assurance regarding the high safety of the UK blood supply. These data have informed blood safety decisions including the change to a temporary deferral for sex between men, and enhanced public health care and knowledge.
Blood Pharming
3A‐S04‐01
Making Platelets from Stem Cells
Ghevaert C1, Moreau T 1, Colzani M 1, Howard D 1, Bouet G 1, Evans A 1, Arumugam M 1, Vasquez L 2, Tijssen M 1, Dalby A 1, Yang Y 2, Soranzo N 2, Pedersen R 1, Wright G 2, Cameron R 3, Best S 3
1University of Cambridge and NHS Blood and Transplant, Cambridge, United Kingdom2Wellcome Trust Sanger Institute, Cambridge, United Kingdom3University of Cambridge, Cambridge, United Kingdom
Background
Platelet transfusions to thrombocytopaenic patients are increasing by 7–10% per year. We are currently entirely reliant on donor‐derived platelets, which have limitations: short shelf‐life and precarious supply chain, risk of donor‐derived transmitted infections and issues of mismatch for patients with HLA class I antibodies.
Aims
we are aiming to develop protocols to produce platelets in vitro from a renewable source of stem cells (namely human pluripotent stem cells, hPSCs) using a methodology and reagents compatible with the production of a clinical grade commercially viable product.
Methods and results
we have developed a forward programming (FoP) approach to produce megakaryocytes (MKs) from hPSCs based on the overexpression of 3 key transcription factors (TFs) namely GATA1, FLI1 and TAL1. FoP of hPSCs with these 3 TFs leads to the formation of an MK precursor that can be maintained and expanded in culture for a period of 3 months using GMP‐compatible reagents (feeder‐free and chemically defined media) and minimal amount of cytokines. These precursor cells differentiate into fully mature MKs with a purity >90% and a cell yield >20 × 105 per starting hPSC. Functional and whole genome expression analysis show that the FoP MKs are comparable to MKs derived from primary cord blood stem cells. The FoP MKs are able to produce platelets with a functionality on a par with donor‐derived platelets. Platelet production in vitro by cultured MKs is still extremely inefficient compared to the number of platelets that are produced in vivo by bone marrow MKs (10 vs 1000 platelets per MK). To address this issue we are recreating the bone marrow vascular niche to reproduce the clues that positively regulate proplatelet formation by MKs and platelet release. We first developed a collagen‐based 3‐dimensional porous scaffold produced by a freeze‐dry process and chemical cross‐linking to stabilize the structure and remove active collagen moiety. Next in order to reproduce the cell‐to‐cell contact signaling received by the MKs in the bone marrow vascular niche, we generated a library of 50 recombinant proteins. These contain the ectodomain of proteins expressed on the surface a vascular endothelium and a series of tags to allow their immobilization on the 3D scaffold. Screening experiments showed that 2 of these proteins promoted proplatelet formation and platelet released by culture MKs leading to a ten‐fold increase in the yield of platelets in the 3D functionalized scaffold. Finally, in order to reproduce the shear rate encountered in the marrow sinusoid and therefore facilitating platelet release and harvest, we have developed a bespoke parallel‐flow two‐chambers bioreactor into which the scaffolds are integrated and from which platelets can be harvested in storage medium compatible with clinical applications.
Conclusions
we have developed a GMP‐compatible MK production system from human PSCs producing clinically relevant numbers of cells at high purity. We have used a bespoke tissue engineering approach to recreate the environment in order to harvest functional platelets from the cultured MKs to allow small‐scale proof of principle human studies.
3A‐S04‐02
In Vivo Survival Comparison of Human Cultured Reticulocytes and Adult Red Blood Cells in Mice
Taylor S , Blair A , Parsons SF , Anstee DJ
NHS Blood and Transplant, Bristol, United Kingdom
Background
Producing cultured red blood cells (cRBC) from haematopoietic stem cells could help to address the increasing demand for red blood cells for transfusion and the challenges of immune incompatibility. In addition, there is increasing evidence that transfusion of aged red cells may have adverse clinical effects in critically ill patients. cRBCs are likely to contain younger cells which could provide clinical advantages by surviving longer and possessing superior functional qualities. We have developed a liquid culture system for the production of cRBCs from adult or cord derived peripheral blood CD34+ cells. Using this method we can produce 5 ml of enucleated cRBCs, the largest yield reported to date from peripheral blood.
Aims
In this investigation we have carried out a detailed functional analysis, looking at the survival of cRBCs in transfused NSG mice compared to adult red cells from a fresh standard blood donation.
Methods
NOD/LtSz‐scid IL‐2Rγc null (NSG) mice were preconditioned by macrophage depletion prior to intravenous transfusion of red cells into the lateral tail vein. Separate groups of mice were injected with ex vivo generated cRBCs from either adult or cord peripheral blood or with adult red cells from a fresh blood donation. The animals were bled from the opposite tail vein at intervals starting 10 min post transfusion for up to 6 days. Human cells were identified by flow cytometry using an anti‐human CD235a (Glycophorin A) antibody.
Results
We have confirmed that macrophage depletion is a necessary requirement to allow uptake of human red cells in NSG mice. Following macrophage depletion, human cells could be detected in the transfused mice for up to 6 days post transfusion. Ex vivo generated cRBCs from adult or cord peripheral blood showed a marked improvment in survival over donor red cells: while 40% of adult donor red cells had been removed after 8 h in the mouse circulation, only 5% of cRBCs were lost during that time. Due to repopulating macrophages, prolonged exposure of the human cells in the mouse circulation resulted in the eventual removal of all non‐murine red cells, regardless of the source of cells.
Conclusions
Ex vivo cRBCs from human adult or cord peripheral blood show enhanced survival in the mouse circulation when compared to fresh adult donor red blood cells.
3A‐S04‐03
Comparison of CD34+ Cells Derived from Cord Umbilical Blood and Adult Peripheral Blood Cultured Ex Vivo in a 3 Stage Culture System
Green CA1, Karamatic Crew V 2, Cogan N 3, Taylor S 3, Anstee DJ 2
1NHS Blood and Transplant, Bristol, United Kingdom2Bristol Institute for Transfusion Sciences/IBGRL, Bristol, United Kingdom3Bristol Institute for Transfusion Sciences, Bristol, United Kingdom
Background
In future, the use of cultured red blood cells (cRBC) produced ex vivo is predicted to be invaluable in providing phenotype matched blood for patients requiring regular transfusion, particularly patients with haemoglobinopathies who are of black and minority (BME) heritage. Only 10% of U.K. blood donors are from the BME population, and the ethnic mix of patients requiring transfusion exceeds this input; 40% of cord donations are from BME populations and therefore the U.K. cord bank may be a valuable resource. So far, the only in‐man trial of cRBCs reported the use of CD34+ cells derived from adult peripheral blood (Douay et al Blood. 2011 Nov 10;118(19):5071–9). Our investigations of cultured cells have also used adult‐derived CD34+ cells. Understanding how cord‐derived erythroid cells function and develop during ex vivo culture is an important step toward their development as a novel cell therapy product.
Aim
To compare cell morphology, proliferation and enucleation rates of CD34+ cells derived from adult peripheral blood with CD34+ cells derived from frozen umbilical cord blood cultured ex vivo.
Methods
CD34+ cells were isolated, using positive selection (Miltenyi), from 10 frozen umbilical cord units and 10 cones (leucoreduction chambers obtained during aphaeresis) from adult platelet donors. Cells were cultured from each individual for 22 days in a 3‐stage culture medium at 37°C and 5% CO2 (Griffiths et al Blood. 2012 Jun 28;119(26):6296–306). Daily proliferation rates, enucleation rates and cytospin preparations stained with May‐Grünwald Giemsa were analysed.
Results
Cultures of adult and cord‐derived cRBCs had similar growth patterns, however, some notable differences were observed. While the proliferation rates of adult and cord cRBCs varied during the culture period, the overall expansion patterns between the two cohorts were not significantly different (ANOVA, P = 0.78). The proliferation rates at the end of cultures (day 22) were comparable, with an adult:cord ratio of 1.00:1.07. One of the marked differences was in the number of cells that reached the enucleated reticulocyte stage at the end of the culture period. In the adult‐derived cultures this was on average 76% compared to the 55% seen in cord‐derived cultures. An observed decrease in reticulocyte numbers after day 16 was typical of cord cultures, but was less prominent in the adult‐derived ones. Cord‐derived cultures contained greater numbers of non‐erythroid cells with macrophage/preadipocyte morphology, on average 3 fold higher, which formed higher numbers of erythroblastic islands in cord cultures compared to adult cultures. There was a notable relationship between the numbers of these cells and the reduced number of reticulocytes in the later stages of cord‐derived cultures when compared to the adult cultures (e.g. ANOVA, P = 0.03).
Summary
It is clear that cord and adult cRBCs have similar proliferation patterns when cultured with our method. Although cord‐derived cultures contained more erythroblastic islands, loss of enucleated cells at the late stages resulted in lower reticulocyte numbers compared to adult‐derived cultures. Eventually, cultured erythrocytes from a single donor source may be beneficial in reducing patient exposure to multiple donations and the likelihood of allo‐antibody production.
3A‐S04‐04
Enucleation in Cultured Erythroid Cells from Human Peripheral Blood and Immortalised Human Erythroid Progenitor Cell Lines
Griffiths RE1, Trakarnsanga K 2, Kurita R 3, Nakamura Y 3, Frayne J 1, Anstee DJ 1
1NHS Blood and Transplant, Bristol, United Kingdom2University of Bristol, Bristol, United Kingdom3RIKEN BioResource Center, Tsukuba, Japan
Background
Red cells obtained from laboratory culture of erythroid progenitors may provide an alternative source of blood for transfusion. The development of immortalised human erythroid cell lines from individuals with rare blood group phenotypes could provide an unlimited supply of red cells for patients when conventional blood donations are not available.
All of the immortalised human erythroid cell lines reported to date express embryonic or fetal globin. We have generated the first erythroid progenitor cell line BEL‐A1 (Bristol Erythroid cell Line from Adult progenitors, Trakarnsanga et al.2015 ISBT submitted abstract).
Aims
The aim of this study was to identify key proteins involved in the enucleation process in cultured erythroid cells from human peripheral blood and immortalised human erythroid progenitor cell lines.
Methods
In this study we use confocal microscopy to compare the distribution of key proteins involved in enucleation in cultured reticulocytes produced from adult peripheral blood progenitors with cultured reticulocytes derived from BEL‐A1 and the iPSC‐derived cell line HiDEP‐1 (Kurita et al. PLOS ONE 2013;8(3):e59890.)
We identify three different stages of the enucleation process in erythroid cells derived from adult progenitor cells. At enucleation actin and myosin IIb form a structure, known as the contractile actin ring (CAR) thought to be involved in the expulsion of the nucleus. We show that this actin and myosin structure persists in the reticulocyte after the nucleus is expelled forming an actin ‘core’ in the newly formed reticulocyte. The actin core is surrounded by a talin cloud and is adjacent to a similar structure rich in tubulin and containing the microtubule organising centre (MTOC) marker pericentrin. As the reticulocyte matures the actin core and the associated proteins disappear with only cell surface actin remaining. Cytoskeletal proteins are not colocalised with the components of the actin core. At enucleation cytoskeletal proteins including spectrin accumulate on one side of the reticulocyte adjacent to where the nucleus is extruded. After enucleation the skeletal proteins redistribute to the cell surface.
When HiDEP‐1‐derived erythroid cells were examined we observed that the reticulocytes were very fragile and did not exhibit the pattern of staining seen in cultured erythroid cells derived from adult progenitor cells. The actin core is missing from the HiDEP reticulocytes and they are negative for myosin IIb and pericentrin. Cytoskeletal proteins such as spectrin are seen in the cytoplasm of enucleating cells. These results suggest a defect in the mechanism of enucleation in HiDEP‐1.
In contrast in BEL‐A1 cells we can identify the same stages of enucleation as in adult derived reticulocytes.
Both of the cell lines examined give ~10% enucleation but only BEL‐A1 reticulocytes behave in the same way as adult derived reticulocytes.
Conclusions
These results indicate that immortalised cell lines derived from adult CD34+ cells provide an attractive approach to the in vitro production of red cells for transfusion.
3A‐S04‐05
Numerical Investigation of the Critical Diameter for a Stenosed Capillary to Prevent the Motion of Red Blood Cells
Flower RLP1, Nayanajith PGH 2, Saha SC 2, Sauret E 2, Gu Y-T 2
1Australian Red Cross Blood Service, Kelvin Grove, Qld, Australia2Queensland University of Technology, Brisbane, Qld, Australia
Background
The motion of blood is defined by the properties of red blood cells (RBCs). The complex three‐dimensional viscoelastic structure of the RBC enables passage through the smallest capillaries, which in some cases have an internal diameter less than size of the RBC, and exchange of oxygen and carbon dioxide through the wall of the capillary. As the internal diameter of the stenosed capillary is reduce blood flow rate is also reduced. According to the severity of the stenosis there is a risk of microvascular blockage and cessation of flow of blood through that capillary. Few of studies have been conducted to investigate the characteristics that define the deformability of RBCs and thus limitations of movement in stenosed capillaries.
Aims
To model the motion and deformation of the RBCs in a stenosed capillary using an advanced numerical simulation method and to determine the critical diameter at which RBC no longer traverse a stenosed capillary.
Methods
The three‐dimensional RBC membrane was modelled by a spring network. Initially the RBC membrane was assumed to be a 3.1 μm radius sphere with the membrane is discretised into 954 mass points. In the model the discretised mass points were interconnected by 2856 non‐linear springs. Then, the energy functions related with the in‐plane deformation, bending of the membrane, membrane area and volume constraint were considered. Finally, the forces acting on each particle were calculated based on the principle of virtual work to obtain the typical discoidal biconcave shape of the RBC membrane. In this study, we set the reference volume of the RBC (V0) to 60% of the initial volume of the sphere. RBC internal components (hemoglobin) and external fluid (plasma) were discretized into a finite number of particles and treated by the smoothed particle hydrodynamics method. Lagrangian form of SPH equation for the conservation of momentum was used to model the flow field. The capillary wall was constructed by a set of solid wall particles and Lenard‐Jones type repulsive forces are applied to the fluid particles to avoid the penetration through the capillary wall and the RBC membrane. The length and diameter of the capillary was set to 4 μm and 57.2 μm respectively. In the simulations, the behaviour for five identical RBCs was calculated in the context of various stenosed diameters in the capillary
Results
The modelling revealed that RBCs easily pass through the capillaries with minimal stenosis with ‘parachute‐like’ morphology when moving through the stenosed section. However, blood flows at lower speed as the stenosed section narrows, and RBCs adopted bullet like shapes in the narrowest section of the capillary. Further, in this model, blood flow stops when the stenosed diameter is 4.004 μm or below, for an inlet pressure of 1000 Pa.
Summary/Conclusions
A spring‐network model of the RBC and smoothed particle hydrodynamic concepts were applied in a model to predict a critical diameter preventing RBC passage through a stenosed capillary. In this model, for a given pressure gradient, the critical diameter to prevent passage of RBCs was 4.004 μm.
Donor Recruitment and Retention
3A‐S05‐01
Engaging Ethnic Minority Blood Donors
Charbonneau J
INRS, Montreal, QC, Canada
Background
Finding effective ways to recruit blood donors is crucial given rising demand for blood due to an aging population, strict donor deferral criteria, and the limited shelf life of blood products. Medical needs that require higher phenotype compatibility are on the rise. Because phenotype compatibility is higher within certain ethnic groups, this situation constitutes a sound justification for attempting to recruit new donors from such groups. Results from many studies show that ethnic minorities account for proportionally fewer donors. Blood collection services have made little effort to recruit donors from ethnic minorities, although the situation varies widely from one country to the next.
Aims
The aim of this lecture is to raise points of reflection based on initiatives developed in Quebec over the last 5 years.
Methods
In 2009, Héma‐Québec commissioned the INRS to carry out a research project on ethnic groups in Montreal and blood donation. Between March 2009 and May 2010, 83 interviews were conducted with donors and leaders of ethnic groups in Montreal. Héma‐Québec employees’ own perceptions were also explored. In parallel, the organization conducted 53 awareness‐raising activities in 2 years to rapidly recruit new donors from black communities in order to meet the medical needs of persons affected by sickle‐cell anemia.
Results
Following Héma‐Québec's initiatives, the number of donors climbed from 170 in 2009 to 1582 in August 2012. However, initiatives involving this population have required tremendous effort, and retaining these donors remains difficult: Only 24% of new donors made a second donation in the same year. Experiences with ethnic associations and donors raised many questions among the organization's staff. According to our research findings, 1) relationships to blood (cultural attitudes, motivations and practices) vary greatly from one ethnic group to another and, as a result, recruitment efforts need to be equally diverse; 2) in certain cases, all that appears necessary is to identify which sites are most conducive to the recruitment of ethnic donors, with no need to change the organization's usual strategies; and 3) regardless of the chosen strategy, the organization's management, marketing, blood drive planning, and customer service personnel all need to be better informed about these groups. In 2013, we developed a multi‐step training program for Héma‐Québec managerial staff based on these results, enabling the organization to provide its own training on ethnic diversity for all employees.
Conclusion
The research findings suggest the chief factor in successfully implementing such a process is the level of commitment on the part of the organization and each of its departments. Success is based on how well staff is trained, and staff confidence regarding interactions with ethnic minorities needs to be bolstered. Finally, it is essential that the objective of expanding blood collection services’ priorities to include the targeted recruitment of blood donors based on criteria such as being ‘foreign‐born’ (immigrant status) or belonging to an ‘ethnic minority’ take into account the way in which each society addresses questions of ethnic and cultural diversity.
3A‐S05‐02
Donor Mobilization and Blood Collection: Challenges and Opportunities – The Uganda Blood Transfusion Services
Byabazaire DK1, Craig RF 2, Saunders J 2
1UBTS, Kampala, Uganda2Pepal, London, United Kingdom
Background
The Uganda Blood Transfusion Service (UBTS) has almost doubled blood collection since 2004, from 106,996 units to 202,935 units in 2013. Over the same period, UBTS has received periodic technical assistance from WHO and Sanquine Consulting Services, and long‐term support from CDC via PEPFAR.
However, the level of blood collected remains under the 1% of the population recommended by WHO. To tackle this shortfall, UBTS embarked on a major project, funded by DFID via Uganda's Joint Population Program (JPP). UBTS is seeking new approaches to increase the efficiency and effectiveness of its activities in northern Uganda, with partnerships and integration a major area of opportunity.
Uganda currently fails to collect sufficient blood for its growing population. As a result, Uganda needs to devise new, innovative ways of mobilizing donors and achieving sufficient blood.
Aims
UBTS aims to increase the level of blood it collects in northern Uganda by approximately 25,000 units per calendar year. To achieve this, UBTS is looking at partnerships with both international and national organisations. The 2014‐launched JPP ‘Saving Lives of Mothers and Children’ focuses on northern Uganda, and is supported by the Uganda Red Cross Society and a British NGO, Pepal.
Methods
UBTS and its partners have trained new teams in Lira, Northern Region and Angal, West Nile Region, increasing blood collection in previously underserved areas of the country. A wide variety of training has been given to both new and existing teams – covering social marketing, community mobilization, data utilization, and leadership – some of which has been delivered in conjunction with NHSBT.
Results
During 2014, the project increased blood collection levels by 42% in the target areas. This meant that the five project teams collected 36,152 units in 2014, as compared to 25,419 units in 2013.
Summary/Conclusions
The project represents a first for UBTS in several areas:
Common Agenda‐ The funding for the ‘Saving Lives of Mothers and Children’ project has brought ‘blood’ on to the agenda of malaria, and child and maternal health partners. For UBTS, this opens the door to greater funding options.
Partnerships‐ A partnership with UK's blood services organisation, NHSBT, is providing complementary skills and resources which strengthen the implementation capacity of UBTS. Following leadership trainings led by NHSBT, blood collection in Northern Uganda has increased significantly.
Integration‐ Joint planning and activities in Northern Region with USAID‐funded NU‐HITES (Northern Uganda Health Integration to Enhance Services) have occurred under the project, integrating blood into other health programmes. The aim is to create integrated health messaging, sharing resources and skills to effect greater and more sustainable mobilization and results.
Next steps
Making blood a permanent part of malaria and mother and child funding in FY15/16 and beyond.
More, deeper and better partnerships nationally and internationally.
Entering Karamoja, in the underserved north‐east of the country, hand‐in‐hand with partners, integrating approaches, activities and resources.
3A‐S05‐03
Donor Recruitment: Meeting the Needs of Bame/Rare Blood Donors
Anand R , Clarke T , Watkins E
NHS Blood and Transplant, Birmingham, United Kingdom
Background
Changes in the ethnic make‐up of the UK population, combined with advancements in the treatment of patients with long term transfusion requirements, has increased the need for rare blood. Many patients with sickle cell disease and those with malignancies are living longer. Consequently, they require suitably matched blood for longer periods of time if they are to avoid future complications, such as alloimmunisation. The UK Black, Asian and Minority Ethnic (BAME) population stands at 14% but accounts for only ~4% of the blood donorbase. A shortage of the rare types of blood that are typically found in BAME individuals, increases the likelihood of red cell substitutions and puts pressure on stocks of group O Rh D negative red cells. In order to improve the recruitment of BAME/rare blood donors, NHS Blood and Transplant (NHSBT) must adapt its methods to reflect the changing BAME donor landscape and the needs of this diverse group.
Aims
To engage with BAME communities in order to better understand the barriers to blood donation and the common reasons for deferral, along with their motivations and specific needs in terms of the donation experience. To recruit and retain sufficient donors to meet the demand for rare blood, with units of the required type and quantity in the right location at the right time.
Methods
In 2014 NHSBT commissioned research within BAME communities in order to gain a better understanding of their relationship with the notion of blood donation. NHSBT also undertook a 4‐week Test and Learn (T&L) recruitment campaign, aimed at recruiting young Black Londoners who were identified via their musical and social media tastes and interests. It utilised powerful imagery and stark messaging, delivered via digital channels (Facebook/Spotify/weblinks) and radio adverts. T&L was complemented by an engagement/recruitment campaign with all black donors. These initiatives were forerunners to a new BAME recruitment strategy.
Results
T&L was incredibly successful, with 784 recruits from black communities; an increase of 260% and 300% on corresponding periods from the two previous years. 55% of recruits were under the age of 25% and 84% were under the age of 35. 65% of recruits resided in London and the Home Counties. The digital success of the campaign was demonstrated by 73% of recruits registering via the donor portal (website). Targeted messaging was well received and acted upon.
Conclusion
Research and direct interaction with BAME communities enabled NHSBT to successfully target specific groups with specific messaging; BAME donor registrations have increased as a result. T&L, which will be rolled out nationwide in 2015, has demonstrated NHSBT's intent to continue to refine and improve its techniques for BAME/rare donor recruitment and retention. BAME individuals were empowered to register as blood donors by a campaign that was appropriate to their needs. NHSBT should continue to market, plan and manage donation sessions with these needs in mind. The excellent results from T&L provide a solid foundation for an ongoing BAME strategy with donor and donation targets that will improve the supply of rare blood.
3A‐S05‐04
Sex and Racial/Ethnic Disparities in Initial Donation Success and Future Return Among First‐Time Donor Presenters
Steele WR , Flynn DP , Notari EP , Eder AF
American Red Cross, Rockville, MD, United States of America
Background
First‐time donors are critical to the ongoing sustainability of the blood supply. A significant proportion of donors in certain minority groups face higher rates of deferral, lower rates of successful donation and are less likely to return to donate. While it has been demonstrated that racial/ethnic and sex disparities exist in the donor population in regards to donation success and donor return, this study sought to examine these differences specifically in first‐time donors.
Aims
To describe the outcome of the first donation visit for all first‐time donors to the American Red Cross (ARC) in 2011, to examine differences in donation success at first‐time presentation by sex and race/ethnicity, and to look at donor return and successful donor return in the first 400 days following donation by these same demographic characteristics.
Method
Potential donors presenting for the first‐time to the ARC in 2011 were included. The outcome of the first presentation was classified as a successful or unsuccessful donation, hemoglobin deferral, temporary deferral up to 7 days (excluding hemoglobin), deferral 8–400 days, deferral >400 days or permanent/indefinite deferral. The first return presentation within 400 days of the initial visit was documented and classified as a productive (successful unit) or unproductive (deferral or unsuccessful unit). Initially successful donation (vs all other outcomes), donor return within 400 days (any outcome) and productive return (returning donors that gave a successful unit) were stratified by sex and racial/ethnic group. Frequencies, percentages and chi‐squares with Bonferroni correction were calculated.
Results
There were 1,210,685 first‐time presenting donors, constituting 17.3% of all presentations to the ARC in 2011. Females (53.5%), donors under the age of 30 (65.6%), and whites (54.6%) made up the majority of first‐time donors. At the initial presentation, 75.2% of first‐time donors were successful, 5.9% unsuccessful, 6.6% hemoglobin deferred, 6.6% deferred for a period up to 7 days, 4.1% deferred for 8–400 days and 1.7% were permanently/indefinitely deferred. When the initial presentation was classified as a successful donation vs all other outcomes, white males (85.9%) and Hispanic (83.9%) males had the highest percentage of initially successful donation while African‐American (46.3%) and Asian (55.5%) females had the lowest (Table). Overall 38.4% of the donors in the cohort returned to donate at least once. White females had the highest rate of overall return and African‐American males and females the lowest. For productive donation on return, the pattern approximated that of initial donation success with minor variations (Table). All groups were significantly different for all outcomes (P < 0.0001), except African‐American vs Asian males for first‐time success and Hispanic vs whites males for productive return.
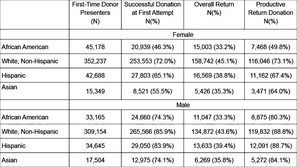
Summary
Significant disparities in initially successful donation, donor return and successful donor return exist by race/ethnicity and sex for first‐time donors. By understanding how race/ethnicity and sex are associated with donation success and donor return among first‐time donors, blood centers can work with regulators to adopt policies that increase the likelihood of giving a successful donation initially and develop interventions that will improve return rates in minority groups.
3A‐S05‐05
Socio‐Demographic Description of Danish Blood Donors
Burgdorf KB
Copenhagen University Hospital/Rigshospitalet, Copenhagen, Denmark
Background
Blood donation in the developed world is a well‐established part of the healthcare system, and recruitment and retention of voluntary non‐remunerated blood donors is essential to deliver safe and sufficient blood supply. Information about blood donors is important to create knowledge for the Danish Blood Bank to be considered when designing strategies for future maintenance of blood supply from the Danish Blood donor population. Detailed characterisation of the existing blood donor population may facilitate efficient recruitment strategies.
Aim
The aim of this study was to compare demographic variables between blood donors and the general population in Denmark.
Methods
Computerised registration of blood donors in Denmark has been nation‐wide since 2003 and data is amalgamated in the Scandinavian Donations and Transfusions (SCANDAT) database 2. We identified all donors (18–65 years old) who donated at least once in 2010 (N = 174,523). By comparison with the contemporary Danish non‐donor population (N = 3,236,753) we assessed associations with age, income, ethnicity, education, cohabitation, and degree of urbanization. We ascertained exposure information through linkage nation‐wide demographic registers at Statistics Denmark, using the individual civil registration number unique to Danish citizens as key.
Results
The age‐specific prevalence of blood donation broadly followed the same pattern in women and in men, peaking around ages 25–30 years and remaining fairly uniform across older age groups except for a lower prevalence in women older than 50 years (FIGURE). The prevalence of blood donation was lower for persons belonging to lower and highest income tiers, respectively, compared with the persons having middle to high incomes (Table). A similar pattern applied to degree of education, where the lowest blood donation prevalence was seen among individual without or with the longest educations. Cohabitation was associated with donation prevalence among men. Specifically, men living alone were least and men living with a female partner the most likely to have donated in 2010. In women blood donation prevalence was the highest among those cohabiting with another woman. Degree of urbanisation correlated positively with donation prevalence (Table). Finally, donation prevalence also varied by parental origin by being significantly higher for individuals with either or both parents born in Denmark.
Summary/Conclusions
Our data indicate that markers of socio‐economic deprivation such as low income, low level of education, male single living, and non‐Danish ethnicity are associated with low prevalence of blood donation. At the same time, socio‐economic affluence as evidenced by high income and long education also is associated with low blood donation prevalence.


Plenary Session: From Donor to Patient
3B‐PL1‐01
Blood Donation and Altruism
Ferguson E
School of Psychology University of Nottingham, Nottingham, United Kingdom
The simple answer to the simple question – ‘Why do people donate blood?’‐ is they are altruistic. This accepted wisdom drives recruitment and retention campaigns. The evidence for this assertion comes largely from three sources: (i) behaviourally blood donation fulfils all the criteria for altruism, (ii) when asked, in free response, why they donate blood, blood donors state most frequently that this is for pure altruistic reasons and (iii) many scholars writing on blood donation simply have made this assertion. However, there is a wide literature on altruism in biological, economic and psychological sciences that examines why altruism survives in the population and what motivates it. This has lead to a number of mechanisms being identified to explain altruism. In this talk I want to show that within transfusion medicine a too simplistic a view of altruism has been adopted and that by adopting a ‘mechanisms of altruism’ (MOA) approach we can gain a much fuller understanding of the motivations of blood donors. I set out a road map to achieving this and in so doing develop a more evidence based practice for intervention design around donor recruitment and retention. First, I argue that the MOA approach requires, not only relying on self‐report measures of motivations (theses will be biased by social desirability response set), but also on using behavioural methods from economics, psychology and biology. Crucially identifying points of correspondence between these methods will lead to identifying the underlying motivations of blood donors. Second, the MOA approach requires critically reviewing the current literature on blood donor motivation from the perspective of altruism mechanism. Third, intervention need to be developed that focuses on the key mechanisms of altruism that are pertinent to blood donation. I will show that this is not just simple pure altruism (caring about the welfare of others at personal expense) that actually drives blood donor behaviour. In reviewing the literature, and our own research incorporation behavioural economics, I will show that blood donors can be conceived of as warm‐glow giving (finding the act of donation emotionally rewarding) reluctant altruists [when faced with free‐riding they are motivated to cooperate because of (i) lack of trust that other will cooperate and (ii) the negative emotions that arise from both others free riding and lack of trust in others). This results in blood donors showing ‘saintly sinning’. That is, the extra ‘moral currency’ that blood donation brings allows them to be less generous in other contexts. I argue that this profile explains many phenomena observed with blood donation such as its lack of association with other forms of helping and why interventions based on pure altruism may be ineffective. I will suggest that interventions based on financial incentives, reluctant altruism and ‘voluntary reciprocal altruism’ are likely to be especially fruitful for recruitment and retention. I will show that many constructs from models in social psychology for volunteering or planned behavioural prediction in general can be subsumed under the MOA approach.
3B‐PL1‐02
Iron Regulation in Health and Disease
Swinkels DW
Radboud UMC, Nijmegen, United Kingdom
Iron is an essential but potentially harmful biometal. Mammalian cells require sufficient amounts of iron to satisfy metabolic needs or to accomplish specialized functions. Iron is delivered to tissues by circulating transferrin, a transporter that captures iron released into the plasma mainly from intestinal enterocytes or reticuloendothelial macrophages. The binding of iron‐laden transferrin to the cell‐surface transferrin receptor results in uptake of the metal cargo. Internalized iron is transported to mitochondria for the synthesis of haem or iron‐sulfur clusters and excess iron is stored and detoxified in cytosolic ferritin.
Iron metabolism is controlled at both the cellular and systemic level. At the cellular level iron transport, use and storage is regulated by the IRE (iron‐responsive element)/IRP (iron‐regulatory protein) system, a well known post‐transcriptional regulatory circuit that maintains iron homoeostasis in various cell types. At the systemic level, the hepatic iron peptide hormone hepcidin and its receptor and cellular iron exporter ferroportin control the major fluxes of iron into blood plasma: intestinal iron absorption and the delivery of recycled iron from macrophages. Because iron losses are comparatively very small, iron absorption and its regulation by hepcidin and ferroportin determine total body iron content. Hepcidin is in turn feedback‐regulated by plasma iron concentration and iron stores, and negatively regulated by the activity of erythrocyte precursors, the dominant consumers of iron. Hepcidin and ferroportin also play a role in host defense and inflammation, and hepcidin synthesis is induced by inflammatory signals.
Genetic inactivation of proteins of the hepcidin‐activating pathway causes iron overload. Hepcidin insufficiency and increased iron absorption are also characteristic of anemia due to ineffective erythropoiesis in which, despite high total body iron, hepcidin is suppressed by the high erythropoietic activity, worsening both iron overload and anemia in a vicious cycle. Hepcidin excess resulting from genetic inactivation of a hepcidin inhibitor TMPRSS6 leads to a form of iron deficiency refractory to oral iron (IRIDA). Increased hepcidin explains the iron sequestration and iron‐restricted erythropoiesis of anemia associated with chronic inflammatory diseases.
Transfusion medicine comprises management of iron balance of both blood donors and chronically transfused anemic patients. Iron stores are often depleted in frequent donors. This will impair hemoglobin synthesis and other iron‐containing proteins that are responsible for the clinical and pathological manifestations of iron deficiency. On the other hand, long‐term transfusions of anemic patients carries the risk of producing iron overload. In this condition, macrophages and hepatocytes can no longer retain all the surplus iron. Iron then enters plasma in amounts that exceed the transport capacity of circulating transferrin. As a consequence, non‐transferrin‐bound iron (NTBI) appears in the plasma as a heterogeneous assortment of mostly toxic iron complexes that have been reported as the major mediators of tissue damage in transfusional iron overload. Therapy with iron‐ chelating agents can clear plasma NTBI and remove excess iron from cells, and return body iron to safe levels.
The challenge in transfusion medicine is to understand the (patho)physiological role of iron to ensure optimal iron management of both blood donors and chronic transfusion recipients.
3B‐PL1‐03
Coagulopathy and Haemostasis in the Bleeding Trauma Patient: A Clinical View
Gaarder C
Department of Traumatology Oslo University Hospital, Norway
No abstract available.
Parallel Sessions
Transfusion and Prions
3C‐S06‐01
Update on Variant CJD and Transfusion Risk
Will G
National CJD Unit, Edinburgh, United Kingdom
Background
Data from the TMER epidemiological study provides compelling evidence that variant CJD has been transmitted from person to person by blood transfusion. There is, however, a disparity between the estimated prevalence of infection in the UK population derived from surveys of appendix specimens and the small number of observed cases of transfusion transmission.
Aims
To review current data to determine whether there may be missed cases of transfusion transmission and to consider the variables that may influence predictions of future numbers of cases, including the effect of host prion protein genotype and the efficiency of the intravenous route of transmission.
Methods
All cases of variant CJD are notified by the National CJD Research and Surveillance Unit (NCJDRSU) to the UK Blood Services (UKBS) in order to determine whether any of the cases have acted as blood donors. Details of individuals who received blood from such a donor are passed to the NCJDRSU to determine if there is a match with known cases. In the reverse study a search is made for donors to variant CJD cases to determine if there is a match with the NCJDRSU database. Death certificates are obtained from the Health and Social Care Information Centre in order to identify the cause of death in all matched recipients and donors.
Laboratory transmission studies have been carried out in collaboration with the Roslin Institute to determine the strain characteristics of the variant CJD agent after secondary transmission and in the spleen of a heterozygote individual with pre‐clinical infection.
Results
18 variant CJD cases had made donations issued for clinical use and 67 blood components were traced to recipients, 34 of whom survived <5 years after transfusion. The cause of death in these individuals did not suggest prion disease, but none had post‐mortem. Three recipients developed clinical variant CJD 7–9 years after transfusion and a further recipient who died of inter‐current illness was positive for abnormal prion protein in spleen. Review of 6 variant CJD cases with a history of blood transfusion not linked to a variant CJD donor provides weak evidence of additional transfusion transmitted cases.
Laboratory transmission studies suggest that there has been no major change in the strain characteristics of the variant CJD agent after secondary transmission. Infectivity has been identified in the spleen of a heterozygote individual with pre‐clinical infection, but preliminary evidence again shows no clear change in agent strain properties.
Summary/Conclusions
The mismatch between the observed number of transfusion‐transmitted cases of variant CJD and the estimates of the prevalence of infection in the UK population is unexplained. Current data do not suggest that significant numbers of such cases are missed by surveillance.
3C‐S06‐02
Assessing the Risk of Blood Borne Transmission of Classic Forms of Creutzfeldt‐Jakob Disease: A 20‐Year Lookback Study
Steele WR1, Flynn DP 1, Dodd RY 1, Schonberger LB 2
1American Red Cross, Rockville, MD, United States of America2Centers for Disease Control and Prevention, Atlanta, GA, United States of America
Background
Creutzfeldt‐Jakob disease (CJD) is a rapidly fatal neurodegenerative disease caused by abnormal prions. Each year there are approximately 300–400 cases of CJD in the United States (US). The vast majority of CJD cases are thought to occur sporadically with familial (10%) and iatrogenic (<1%) types making up only a minority of cases. The sporadic, familial and iatrogenic forms of CJD are commonly referred to as classic CJD and are distinct from variant CJD. While variant CJD has been documented as being transfusion transmitted, it is not known if classic CJD can be transmitted by blood transfusion. In 1995, the American Red Cross initiated with the US Centers for Disease Control and Prevention a lookback study of blood recipients to assess the risk of classic CJD transfusion transmission.
Aims
To assess the risk of blood borne transmission of classic CJD by identifying and enrolling blood donors with confirmed CJD and then identifying and enrolling these blood donors’ transfusion recipients to see if they subsequently die of CJD.
Methods
Blood donors who were subsequently diagnosed with CJD (CJD donors) are reported by blood centers, physicians and family members. Only donors with neurologist diagnosed CJD (most pathologically confirmed) and at least one reported recipient are enrolled. Consignees of all single donor components linked to the CJD donor are notified. Consignees are asked to provide information on the recipient including name, date of birth and social security number. Recipients are followed annually to determine their vital status by searching the National Death Index (NDI). The date and cause of death codes are recorded. The last NDI search covered deaths through the end of 2012.
Results
As of December 2014, there were 59 CJD blood donors and 717 recipients enrolled in the study. Of the 717 recipients of CJD donor blood (3112.5 person‐years of post‐transfusion observation), 556 are deceased (1502 person‐years), 139 are alive (1540 person‐years) and 22 (70.5 person‐years) are lost‐to‐follow up (LTF). Recipients who have survived for 5 or more years post‐transfusion are classified as long‐term survivors. These individuals are particularly important to the study because of the potentially long incubation of CJD. Currently there are 220 recipients who meet this criteria. Of these recipients, 116 are deceased, 98 are alive with 6 LTF. The total number of person‐years for the long‐term survivors is 2751.5 years. Of these long‐term surviving recipients, 86 (39 alive, 46 deceased and 1 LTF) received a component donated by the CJD donor 60 months or less prior to their donor's CJD diagnosis. None of the deceased recipients have CJD listed as their primary or contributing cause of death and to date we have not found evidence of transfusion transmission of classic CJD.
Conclusions
From this study, as well as other epidemiological studies, there is a lack of evidence that classic CJD is transfusion‐transmitted. Therefore the risk, if any, remains very small and theoretical. To verify the findings of the study, enrollment of CJD blood donors and their recipients is still active and ongoing.
3C‐S06‐03
Is There any Evidence That SCJD Could be Transmitted by Transfusion of Blood Components
Hewitt PE1, Mackenzie J 2, Llewelyn C 1, Will RG 2
1NHS Blood and Transplant, London, United Kingdom2National CJD Research and Surveillance Centre, Edinburgh, United Kingdom
Background
The Transfusion Medicine Epidemiology Review (TMER) is a collaborative project between the National CJD Research & Surveillance Unit (NCJDRSU) and the UK Blood Services (UKBS) whose remit is to investigate whether there is any evidence that Creutzfeldt‐Jakob disease (CJD) may have been transmitted via the blood supply. It was through this study that 3 cases of vCJD and one individual with sub‐clinical vCJD infection were identified as having received blood from donors who subsequently went on to develop vCJD. Here we report our findings in relation to sporadic CJD (sCJD).
Aims
To establish whether there is any evidence that sCJD could be transmitted through transfusion of blood components.
Methods
Information on blood donation and blood transfusion history is collected from cases of suspected CJD. The information is collected from the relatives of these cases by a research registrar from NCJDRSU when visiting the patient and the patient's relatives. Cases fulfilling the established diagnostic criteria for definite or probable sCJD and who have a reported history of blood donation or blood transfusion are retrospectively notified to UKBS by NCJDRSU in a blinded fashion on a 6‐monthly basis. A search is conducted by UKBS to trace the records of these donations/transfusions. Details of individuals who received blood components from a person who was later diagnosed with sCJD, or individuals who donated blood components to a person who subsequently developed sCJD, are passed back to NCJDRSU. Searches of the NCJDRSU database are carried out to check for matches between these individuals and CJD cases. These individuals are flagged with the Health and Social Care Information Centre (HSCIC) and, in the event of their death, NCJDRSU are notified by HSCIC and the cause of death is reviewed.
Results
Up to December 2014, 370 cases of sCJD who were reported to have donated blood components were sent to UKBS for tracing. Of these, 204 were reported to have donated after 1980 (from when UKBS records are generally available) and 29 were traced. Successful tracing depends on the accuracy of the information supplied to NCJDRSU by relatives of cases. 211 recipients of blood components were identified from these 29 donors. None have yet appeared on the NCJDRSU database. 144 have died, 43 are currently alive and the fate of 24 is unknown. Cause of death information is available on 139.
199 cases of sCJD, reported to have received a blood component transfusion, were sent to UKBS for tracing; 111 were reported to have been transfused after 1980. 23 sCJD cases with a history of transfusion were traced and 214 individuals were identified who had donated blood components to these cases (one case had received blood components from 107 donors). None have yet appeared on the NCJDRSU database. Of these 214 individuals, 4 have died, 205 are currently alive and the fate of 5 is unknown.
Summary/Conclusion
No donors or recipients identified in the study appear as CJD cases on the NCJDRSU database to date. This study is on‐going and further data are being accumulated.
3C‐S06‐04
Variant CJD and Blood Transfusion: Continuing Surveillance to Identify the Size of the Problem
Hewitt PE1, Mackenzie J 2, Llewelyn C 1, Will RG 2, Mackenzie J 2
1NHS Blood and Transplant, London, United Kingdom2National CJD Research and Surveillance Centre, Edinburgh, United Kingdom
Background
The UK TMER (Transfusion Medicine Epidemiology Review) study commenced in 1997 to address concerns that vCJD could be transmissible through blood transfusion. Three cases of variant CJD have been identified in recipients of blood donated by asymptomatic individuals who later developed the condition. The assumption that there is a population of asymptomatic ‘carriers’ of vCJD who may or may not develop symptoms, but could be a source of transmission of infection if acting as blood donors, is a major concern.
Aims
to identify whether there are further transmissions of vCJD through blood transfusion.
Methods
To date, of 177 cases of vCJD in the UK the last onset was in 2012 and death in 2013. Each case old enough to be a blood donor is checked with blood service records to establish whether blood donations were made. The fate of donations is traced, recipients identified, and passive surveillance establishes outcome in terms of survival period and cause of death. In the reverse process, history of blood transfusion in vCJD cases is obtained through history‐taking with next of kin, and transfusions are traced back to donors, who are similarly subject to passive surveillance.
Results
of 167/177 cases old enough to have been blood donors, 32 were reported to have ‘possibly’ donated blood, but only 24 had blood service records, and 18 had made donations which were issued for clinical use. Blood services identified components produced from these 18 donors; 67 of the components were traced to identified recipients. A small number of components could not be successfully traced. Half the recipients (34/67) survived <5 years after transfusion: cause of death is known and none suggest prion disease, but none had post‐mortem investigation for abnormal prion protein deposition. The three cases who developed clinical vCJD did so within 7–9 years of transfusion. A further 5 cases, who survived >5 years, were tested at post‐mortem for abnormal prion protein deposition and one was positive. Living recipients (14/67) have all survived >10 years following blood transfusion. Only 15 of the vCJD cases were reported to have been transfusion recipients, and post‐1980 transfusions have been confirmed in 10, including the 3 recipients already linked to 2 known infected donors. Apart from one case with onset within 1 year of transfusion, the other 6 cases could be transmissions; 6 donors linked to 2 recipients could not be traced, but continued surveillance of 105 donors linked to the other 4 cases has revealed no additional clinical diagnosis of vCJD.
Conclusion
Although no further cases of transfusion‐transmission have been identified through the TMER for several years, the risk of vCJD has influenced many changes in UK transfusion practice and continued surveillance is necessary to monitor the size of the problem.
3C‐S06‐05
Do Blood Components from Young Donors Pose a Transfusion Risk to Very Young Recipients?
Patel P1, Tettmar KI 1, Wickenden C 1, Azim N 1, Dicks S 1, Clarkson A 1, Zuczkowska A 1, Newham J 1, Lattimore S 2, Vasconcelos M 2, Brailsford S 2, Ijaz S 3, Kitchen A 1, Ushiro-Lumb I 1, Tedder RS 1
1NHS Blood and Transplant, London, United Kingdom2Joint PHE/NHSBT Epidemiology Unit, London, United Kingdom3Public Health England, London, United Kingdom
Background
NHS Blood and Transplant are exploring further strategies to minimise the risk of transfusion‐associated transmission of variant Creutzfeldt‐Jakob disease (vCJD). One such strategy is to utilise donations from donors born on or after 1 January 1996 onwards for neonatal, pre‐term infant and intrauterine transfusions. These donors become eligible to donate once they reach 17 years of age and subsequent donations are considered to carry a reduced risk to vCJD due to public health measures implemented before 1996. There is a concern, however, that these young donors may be at an increased risk of incident infection with certain viruses which may cause increased morbidity in the target recipient age group such as Cytomegalovirus (CMV), Epstein‐Barr virus (EBV) and Parvovirus B19 (B19).
Aims
This study addresses the need to verify the magnitude of risk posed by the young donors. Infections caused by CMV, EBV or B19 may have significant impact in the vulnerable groups of patients destined to receive blood components manufactured from young donors.
Methods
The prevalence of DNA viraemia was measured in 2117, 17‐year‐old blood donors (study group) and 2585 unselected donors of all ages (control group) by real‐time PCR. Samples positive for viral DNA were also tested for specific IgM. All samples were tested for IgG antibody to CMV, EBV and B19 by ELISA. 939 seronegative donors from the study group who returned to donate were re‐tested by serology and PCR for evidence of incident infection in the interim period.
Results
We found no difference in the prevalence of CMV, EBV and B19 DNA viraemia between study and control groups. Of the study group donors, 91% were seronegative for at least one of the three viruses. Seroprevalence of CMV and EBV was lower in the study group compared to the control group, and age specific incremental increase in CMV and EBV seroprevalence was observed in the control donors. Seroconversion rates in returning donors varied between the three viruses: 2.3% for CMV, 23.1% for EBV and 10.3% for B19. CMV seroconversion was more common in females, while B19 seroconversion was more common in males. Viral DNA was detected in 10/939 returning donors (CMV = 1, EBV = 3 and B19 = 6), all of whom had seroconverted.
Conclusion
This study shows that there is no difference in prevalence of detectable viral DNA between 17‐year‐old donors and the control group. There is however, a concern over subsequent donations from the young donors. Susceptibility to CMV and EBV is considerably greater in the 17‐year‐old donor than in older donors. Our results indicate that seroconversion for all three viruses were occurring in the young donor and there is an increased prevalence of viraemia in donors aged between 18 and 19 years. Haemovigilance must be maintained if components from young donors are to be targeted to the very young recipients.
Haemostasis and Platelets
3C‐S07‐01
The Emerging Role for Red Blood Cells in Hemostasis: Opportunity for Intervention
Cines B , Villa H , Muzykantov R
University of Pennsylvania, Philadelphia, PA, United States of America
Red blood cells (RBCs) exert prothrombotic activity, best exemplified by the risk of thrombosis in patients with hereditary and acquired hemolytic anemia, and by the salutary effect of phlebotomy in patients with polycythemia vera. RBCs increase blood viscosity, promote non‐linear flow, adhere to vasculature, promote platelet activation by releasing ADP and scavenging NO, release procoagulant microparticles and stroma, and actively participate in thrombin generation through expression of phosphatidylserine and subsequent surface assembly of prothrombinase complexes, among diverse other mechanisms that have been identified. These prothrombotic processes are enhanced at high hematocrit and by turbulent blood flow, alterations in vasculature induced by inflammation, and perturbations of membrane integrity. The latter includes intrinsic defects, such as sickle cell disease and paroxysmal nocturnal hemoglobinuria, as well as extrinsic phenomena, such as the intravascular lysis as a result of acute hemolytic transfusion reactions. Confirming the contribution of RBCs to coagulation, our recent studies of clotting in whole blood using T2 magnetic resonance demonstrated a concentration‐dependent effect of RBCs on promoting fibrin formation. At high hematocrits, a novel T2 signature emerged which was associated with impermeability of the clot to D2O and resistance to lysis by tPA. Electron microscopy revealed tessylated arrays of polyhedral RBCs within the clot interior surrounded by fibrin and platelets that were also readily identified in human venous > arterial thrombi.
We then reasoned that we could offset and indeed reverse these prothrombotic properties of RBCs using a ‘Trojan horse’ strategy of targeting antithrombotic drugs to RBC plasma membranes. We reasoned that RBC‐bound anti‐thrombotics would circulate longer than their unbound counterparts, and would enhance local delivery as they become incorporated within nascent clots, while sparing preformed impermeant mural hemostatic clots. To test this hypothesis, we have fused either an anticoagulant/anti‐inflammatory (e.g. soluble thrombomodulin, TM) or a pro‐fibrinolytic (e.g. a thrombin‐activatable single chain prourokinase) drug with a single chain variable fragment of a monoclonal antibody to RBC membrane antigens. The fusion proteins bind to RBCs safely and provide durable thromboprophylaxis after a single IV injection in murine models of arterial and venous thrombosis at doses orders of magnitude below soluble drug without causing excessive bleeding. The TM fusions can be reengineered to promote anti‐inflammatory or anti‐thrombotic activity as needed. RBC‐bound urokinase incorporated within clots generates channels that permit the perfusion of red cells prior to extensive lysis, thereby enhancing oxygen delivery to ischemic tissue. This strategy combines ease of design, high quantities of homogenous product, opportunity for humanization, and a practical delivery system that takes advantage of the natural biology of RBC participation in thrombosis.
3C‐S07‐02
The Effects of Three Novel Mutations in ITGB3 on Protein Expression In Vitro Expression System
Liu Y , Xu XG , Tao SD , Ying YL , He J , Zhu FM , Lv HJ
Blood Center of Zhejiang Province, Hangzhou, China
Background
Platelet membrane glycoprotein genes have highly polymorphisms. Some SNPs or mutations in these genes could lead to alternation of glycoprotein and associate with human platelet antigen systems (HPAs). We have identified three novel mutations located in the ITGB3 among the platelet donors in the Chinese individuals, including 1333G>A (V419M), 1813G>A (G579S) and 1476G>A (W466X), which result in single amino acid substitutions or terminator in the β3 integrin subunit. However, the influences of these mutation in ITGB3 on the β3 integrin protein expression are unclear.
Aims
The aim of this study was to explore the effects of mutations in ITGB3 on protein expression in vitro expression system.
Methods
The full‐length ITGB3 cDNA was cloned into the pcDNA 3.1/V5‐His TOPO® TA eukaryotic expression vector. The c.1333G>A, c.1813G>A and c.1476G>A constructs were generated by site‐directed mutagenesis and then were transformed into E. coli, respectively. The plasmid DNAs were extracted and sequenced to confirm the target mutations.The wild‐type and mutants recombination plasmids were transfected into Chinese hamster ovary (CHO) cells by nonliposomes method and the stable expression cells were harvested using G418 screening. The expressed proteins of CHO cells were detected by SDS‐PAGE and Western blotting, while GPIII a (CD61) antigen expression level in CHO cells was analyzed by flow cytometer.
Results
The eukaryotic expression vectors ofwild ITGB3 cDNA and three mutants were successful constructed and confirmed by cloning and sequencing. The stable expression CHO cells were also obtained after recombination plasmid transfection and screening with G418. The specific protein band about 90kD was confirmed in the wild‐type, V419M and G579S transfected cell lysates by SDS‐PAGE electrophoresis and western blotting with anti‐V5‐HTR antibody, but not found this specific band in the W466X transfected cells. Compared with the wild‐type transfected cells, the relative binding strength of anti‐human CD61‐FITC to the V419M transfected cells was 104%, while those of the W466X and the G579S transfected cells were reduced to 37% and 89% respectively.
Conclusions
Our data was indicated that the ITGB3 c.1476G>A mutation significantly decreased CD61 antigen expression, while the ITGB3 c.1333G>A and c.1813G>A mutation could not affect the CD61 antigen expression.
3C‐S07‐03
A Multi‐Centre Validation of Recombinant? 3 Integrins Coupled to Beads to Detect Human Platelet Antigen‐1 Antibodies in 498 Fetomaternal Alloimmune Thrombocytopenia Cases
Chong W1, Turro E 2, Metcalfe P 3, Yusuf R 3, Mérieux Y 4, Rigal D 4, Porcelijn L 5, Huiskes E 5, Lucas G 6, Bendukidze N 6, Green A 6, Fontão-Wendel R 7, Husebekk A 8, Dixey J 9, Guest A 9, Mushens R 9, Ouwehand W 2, Navarrete C 10
1NHS Blood and Transplant, London, United Kingdom2University of Cambridge and NHS Blood and Transplant (NHSBT) Cambridge, Cambridge, United Kingdom3National Institute for Biological Standards and Control (NIBSC), Potters Bar, United Kingdom4Etablissement Français du Sang Rhône‐Alpes (EFS), Lyon, France5Sanquin Diagnostic Services, Amsterdam, The Netherlands6NHS Blood and Transplant (NHSBT) Filton, Bristol, United Kingdom7Hospital Sirio Libanês Blood Bank, São Paulo, Brazil8University Hospital North Norway, Tromso, Norway9International Blood Group Reference Laboratory (IBGRL), Bristol, United Kingdom10NHS Blood and Transplant (NHSBT) Colindale and University College London (UCL), London, United Kingdom
Background
Fetomaternal alloimmune thrombocytopenia (FMAIT) is caused by maternal alloantibodies against paternally inherited human platelet antigens (HPA) on fetal platelets. Current tests for detecting HPA antibodies, such as the monoclonal antibody immobilisation of platelet antigens (MAIPA) assay, are relatively laborious and require access to panels of HPA‐typed platelets and validated glycoprotein‐capture monoclonal antibodies. Recombinant β3 integrin fragments, displaying the bi‐allelic HPA‐1 epitopes encoded by the single nucleotide polymorphism rs5918 (rHPA‐1a and rHPA‐1b) and coupled to fluorescently labelled beads, have been previously shown in a proof of principle study to detect HPA‐1a alloantibodies implicated in FMAIT.
Aims
To perform a clinical evaluation of the rHPA‐1 bead assay for detecting HPA‐1 antibodies in an international multi‐centre study using a large cohort of 498 independent FMAIT patient cases.
Methods
Fifty one blinded quality assurance (QA) samples, prepared and tested by MAIPA by the NIBSC laboratory, were used to standardise the assay and define the optimum parameters for analysis. Six laboratories tested the samples following a standardised protocol, with beads and assay reagents produced, prepared and distributed by the NHSBT H&I Colindale laboratory. Five laboratories retrieved samples from 498 FMAIT cases for testing, of which 282 were confirmed FMAIT cases with HPA‐1 and/or HPA‐5 antibodies detected by MAIPA at the time of investigation. The results of the bead assay were evaluated using a mathematical algorithm developed to classify samples into three categories; samples containing HPA‐1a antibodies, samples containing HPA‐1b antibodies and samples containing neither HPA‐1a nor HPA‐1b antibodies.
Results
Testing of the QA samples gave a mean concordance of 94% across the six laboratories compared to the reference MAIPA results. Subsequent testing of the 498 FMAIT samples showed a concordance of 97% compared with local historical MAIPA results, with no cross‐reactivity observed between the rHPA‐1 beads and FMAIT samples containing HPA‐5 antibodies. Of the 15 samples with discrepant results, eight were positive by MAIPA but negative by the beads, whilst seven were positive by the beads but negative by MAIPA. Overall, the bead assay achieved 98% sensitivity for HPA‐1a antibody detection in FMAIT and 98.7% specificity compared to the local MAIPA assay.
Summary/Conclusions
The rHPA‐1 bead assay is a rapid and simple‐to‐use assay for the sensitive detection of HPA‐1 antibodies, which utilises a common platform widely used for the detection of HLA antibodies. The use of this test would enable prompt detection of maternal HPA‐1a antibodies to support the diagnosis and treatment of suspected FMAIT cases.
3C‐S07‐04
A Chromatographically Purified, Virally Inactivated, Plasma‐Derived Anti‐Human Platelet Antigen‐1a Immunoglobulin G For Preventing Fetal and Neonatal Alloimmune Thrombocytopenia
Weng YJ1, Husebekk A 2, Burnouf T 1
1Taipei Medical University, Taipei, Taiwan2UiT The Arctic University of Norway, Tromsø, Norway
Background
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is caused by maternal antibody‐mediated destruction of fetal or neonatal platelets. Maternal immunization due to an incompatibility of platelet‐specific antigens between the fetus and the mother is the basis for FNAIT. In Caucasian population, fetal‐maternal incompatibility for human platelet antigen‐1a (HPA‐1a) is the most common cause of this disorder. Platelet specific antibodies induce fetal thrombocytopenia that may cause intracranial bleeding and death. To date, there is no way to prevent such alloimmunization, nor is there any safe and effective treatment for FNAIT until delivery. The strong pathological similarities that exist between FNAIT and hemolytic disease of the newborn, which can be prevented by the injection of anti‐rhesus immunoglobulin (IgG), supports the potential for a similar therapeutic approach against FNAIT based on antibody‐mediated immune suppression (AMIS).
Aim
Develop a state‐of‐the‐art scalable process for the purification and viral reduction of a human plasma‐derived anti‐HPA‐1a IgG that could possibly be used for preventing maternal alloimmunization.
Methods
HPA‐1a plasma was collected by apheresis from volunteer alloimmunized women who provided informed consent. Plasma was cryoprecipitated at 2–4°C, and the supernatant was treated by 5% caprylic acid pH 5.5 at 25°C. The supernatant was then directly incubated with solvent/detergent [S/D; 1% tri‐n‐butyl phosphate (TnBP)/1% Triton X‐100, >1 h, at 22 ± 1°C] to inactivate lipid‐enveloped viruses. The protein and SD mixture was then purified on a S‐HyperCel cation exchange column (Pall Life Sciences) to purify the IgG in binding/elution mode and remove the S/D agents and part of IgA and IgM. IgG polishing was achieved by HyperCel STAR AX anion‐exchanger (Pall Life Sciences) used in a flow‐through mode. The IgG‐rich flow‐through was subjected to 20 nm nanofiltration (Planova 20N, Asahi Kasei Medical) for viral removal, concentrated by tangential flow filtration and sterile‐filtered. Fractions were analyzed to determine protein content, protein profile by SDS‐PAGE and zone electrophoresis, IgG, IgA, and IgM by ELISA, and complement factor 3 (C3) and 4 (C4) by PEG enhanced immunoturbidimetry. Pak12 assay (Immucor, Inc.) was used for semi‐quantitative evaluation and follow‐up of anti‐HPA‐1a IgG along the purification process.
Results
The caprylic acid treatment precipitated most non‐Ig plasma proteins yielding approximately 90% pure immunoglobulin supernatant. S‐HyperCel and HyperCel STAR AX were found to ensure a high IgG recovery (>80%) and purity (>99.5%), as well as efficient IgA and IgM removal (undetectable). Complement factor C3 and C4 were <0.5 and <0.4 mg/dl, respectively. Pak12 assay showed that the developed purification process could successfully be used to extract the anti‐HPA‐1a IgG. The final IgG product could be nanofiltered on Planova 20N without significant loss of IgG, nor anti‐HPA‐1a antibodies.
Conclusions
We demonstrate that it is technically achievable to fractionate plasma of HPA‐1a‐alloimmunized women, using a manufacturing process combining cryoprecipitation, caprylic acid precipitation, cation‐exchange and anion‐exchange chromatography that allows to obtain essentially pure anti‐HPA‐1a hyperimmune IgG. The process includes three steps expected to contribute to robust viral reduction: caprylic acid precipitation, S/D treatment, and 20‐nm nanofiltration.
3C‐S07‐05
Molecular and Serologic Study of Simultaneous Occurence of Neonatal Alloimmune Thrombocytopenia and Neutropenia
Nogueira-Silva LCNS , Abbas SA , Barbosa Lopes L , Chiba A , Kuniyoshi A , Langhi-Junior DM , Moritz EM , Bordin JO
Universidade Federal de São Paulo, São Paulo, Brazil
Background
Neonatal alloimmune thrombocytopenia (NAIT) results from feto‐maternal platelet antigen (HPA) incompatibility leading to the production of maternal antibodies and destruction of fetal platelets during pregnancy. Similarly, neonatal alloimmune neutropenia (NAN) is caused by maternal alloimmunization to incompatible fetal neutrophil antigens (HNA) followed by transplacental transfer of the maternal anti‐neutrophil antibody causing fetal or neonatal neutropenia. Although there are many reports in the literature describing these rare disorders, the simultaneous occurrence of NAIT and NAN has not been systematically evaluated.
Aim
In this study we evaluated the incidence of simultaneous NAIT and NAN and investigated the prevalence and specificity of platelet, neutrophil and leucocyte antibodies when these rare syndromes happen concomitantly.
Methods
A previous cross sectional study of incidence of NAN included samples from 10,000 unselected neonates born in 4 obstetric units in Sao Paulo City (Brazil). From this cohort, we selected 19 cases of neonatal thrombocytopenia (platelet < 150 × 109/l) and neutropenia (neutrophil<2.0 × 109/l) occurring simultaneously. HPA (HPA‐1 to ‐11 and ‐15) and HNA (HNA‐1 and ‐3) genotyping was performed in the blood samples of mother and neonates by PCR‐SSP, PCR‐RFPL and xMAP technology (IDHPAXT, Progenika‐Grifols). Platelets antibodies were investigated in maternal serum by i) platelet enzyme‐linked immunosorbent assay (ELISA) (PaK12G, Immucor GTI Diagnostic, Inc), ii) monoclonal antibody immobilization of platelet antigens (MAIPA) assay, and iii) Luminex bead‐based platelet antibody detection method (PAKLx, Immucor GTI Diagnostic Inc). Neutrophil antibodies were investigated by granulocyte agglutination test (GAT), using a panel of donors previously genotyped for HNA‐1 and ‐3 systems, and Luminex technology (LABScreen®Multi, One Lambda, Inc). Anti‐HLA antibodies were investigated by ELISA (LAT Mixed, One Lambda Inc and PaK12G, Immucor GTI Diagnostic Inc), MAIPA and Luminex (LABScreen®Multi, One Lambda, Inc).
Results
Among the 19/10,000 (0.2%) simultaneous cases of neonatal thrombocytopenia and neutropenia, 15/19 (79%) showed feto‐maternal incompatibility for platelet and/or neutrophil antigens: HPA‐1 3/15 (20%), HPA‐2 3/15 (20%), HPA‐3 5/15 (33%), HPA‐5 4/15 (27%), HPA‐9 1/15 (7%), HNA‐1a 1/15 (7%), HNA‐1b 3/15 (20%), HNA‐1c 2/15 (13%), HNA‐3 2/15 (13%). Screening and identification of antibodies showed 3/15 (20%) samples with anti‐HPA (1 anti‐HPA‐5b, 1 anti‐HPA‐5a and 1 anti‐GPIIbIIIa), 10/15 (67%) samples with anti‐HLA class I, and 2/15 (13%) samples with anti‐HNA (1 anti‐HNA‐2 and 1 anti‐HNA‐2/‐3b). Concerning the 3 samples with anti‐HPA antibodies, 2/3 (67%) samples also presented an anti‐HNA‐2 antibody, one of which with multiple antibodies (anti‐HPA‐5b, anti‐HNA‐2, anti‐HNA‐3b and anti‐HLA class I), resulting in 2/10,000 (0.02%) cases in which alloimmunization was confirmed.
Conclusion
To our knowledge, this is the first study to investigate systematically the concomitant occurrence of NAIT and NAN. Overall, the present data indicate that the incidence of simultaneous occurrence of neonatal thrombocytopenia and neutropenia was 0.2% (19/10,000), and simultaneous NAIT and NAN was 0.02% (2/10,000). According to the literature, the systems most frequently involved in NAIT and NAN are HPA‐1 and HNA‐1 respectively. Interestingly, in our study, the involved antibodies were against HPA‐5, HNA‐2 and HNA‐3.
Near Patient Testing
3C‐S08‐01
Systematic Review of Thrombo‐Elastography
Hyde C
Public Health and Clinical Epidemiology, University of Exeter Medical School, United Kingdom
No abstract available.
3C‐S08‐02
Coagulopathy Management and the TEG: Clinical Experience
Fries D
Department for General and Surgical Intensive Care Medicine, Innsbruck, Austria
No abstract available.
3C‐S08‐03
Comparison of Thromboelastography and Conventional Coagulation Tests to Monitor Fresh Frozen Plasma Transfusions in Intensive Care Unit Patients
Praveen AJ , Kaur RK , Gombar SG , Palta AP
Government Medical College and Hospital, Chandigarh, India
Background
Transfusion of Fresh Frozen Plasma (FFP) is a commonly used therapy for coagulopathy in Intensive Care Unit (ICU) patients. Importantly, coagulograms are deranged in >30%, while only ~14% of patients experience bleeding. However, many of the FFP transfusions performed are inappropriate. The Thromboelastography (TEG) method assesses coagulation status and in order to monitor FFP transfusions in ICU patients, we compared the efficacy of TEG with the conventional coagulation tests i.e. Prothrombin time (PT), Prothrombin time index (PTI), activated partial thromboplastin time (APTT), and international normalized ratio (INR).
Aims
1. To determine whether TEG can be used to guide and monitor FFP transfusions in ICU patients.
2. To compare TEG with the conventional coagulation tests in ICU patients.
3. To investigate whether the TEG method is superior to the currently used coagulation tests.
Methods
A prospective observational study was conducted from January to December 2013. A total of 28 patients were observed during their ICU stay. Blood samples were collected before and 18–24 h post‐transfusion with FFP. Platelet counts and conventional coagulation tests i.e. PT, PTI, APTT and INR were manually measured and TEG were performed using TEG analyser (TEM‐A Framar Biomedica, Italy) simultaneously.
Results
27/28 patients showed abnormal results when measured by conventional coagulation tests. In contrast, only 13/28 showed abnormal TEG results before FFP transfusion. 3/28 patients experienced bleeding and displayed abnormal TEG and conventional coagulation (PT, APTT, PTI and INR) results. In addition, in the patients experiencing bleeding, a significant association of the TEG parameters – R time, α angle and Maximum Amplitude (MA), was observed (P < 0.05)(Table 1). Post FFP transfusion, 5 out of 28 patients were lost to follow‐up. 20/23 patients were observed to have deranged coagulogram, while only 13 patients had deranged TEG results. Only 1 patient experienced bleeding symptoms and showed abnormal TEG as well as conventional coagulation test results. Significant changes in K time were found in all patients following FFP transfusion (P < 0.05)(Table 2). MA values in TEG showed a weak correlation with platelet counts. With respect to bleeding, sensitivity of TEG was found to be similar to conventional coagulation test results. However, the specificity of TEG was found to be considerably higher (60%), when compared with the conventional coagulation tests (4%).
Table 1: Values of coagulation tests and TEG performed and their association with bleeding.

Table 2: Values of coagulation tests and TEG performed on patients blood samples pre and post FFP transfusion.

Conclusions
Using the TEG method can be considered as an effective tool to assess coagulation status of ICU‐patients due to several advantages including bed‐side analysis, faster turn‐over time, assessment of coagulation status of whole blood and the fibrinolytic system over conventional tests. Moreover, TEG is more specific and can therefore decrease the use of inappropriate FFP transfusions.
3C‐S08‐04
Do Platelet Counts Matter? Thromboelastometry, Coagulation, Platelet Parameters and Bleeding in Patients with Chronic Liver Disease and Thrombocytopenia
Boateng A , Riddell A , O'Bierne J , MacNaughton J , Sekhar M
NHS, London, United Kingdom
Background
There is considerable clinical variability in bleeding in patients with chronic liver disease (CLD) and thrombocytopaenia. The determinants of this variability include aetiology of thrombocytopenia, severity and co‐morbid factors. It is unclear whether bleeding propensity correlates with platelet number, size, maturity or functional activity. (Cines et al 2009). The impact of platelet function on the presence or absence of clinical bleeding in thrombocytopenic patients has not been adequately studied because of lack of appropriate methodology in thrombocytopaenic individuals. In patients with CLD, thrombocytopaenia impacts on clinical management decisions such as use of antiviral agents.
Aim
Determine correlation between numeric and functional platelet assessment of thrombocytopenic patients in patients with CLD using conventional and novel assays.
Method
Patients with CLD and thrombocytopenia had samples collected when they attended the outpatient clinic. Exclusion factors included plt count >100. No patients were transfused any blood products (FFP, PLT, Cryo, and PRC) for 3 months prior to testing. Tests performed included FBC, Immature platelet fraction [IPF], PT/INR, APTT, Clauss fibrinogen, factor [F] II, FV, F VII, F X, F VIII, FIX, FXI and FXII, VWF, ADAMTS13, and thrombin generation assays [TGA]. Rotational thromboelastometry (ROTEM) tests included NATEM[whole blood clotting], INTEM/APTT[intrinsic pathway], EXTEM/PT[extrinsic pathway], FIBTEM[platelet contribution blocked]; 4 variables including MCF [maximal clot firmness] were used in data analysis. Samples for ROTEM were tested within 2 h of collection.
Results
A total of 22 participants were studied. CLD due to alcohol, NASH, Hepatitis B and Hepatitis C were included. 1 patient had WHO gr 1 bleeding.
Platelet count for this group ranged between 35 and 91 × 109/l with 87% of IPF % within the normal range, and 86% within the normal fibrinogen range [1.5–4.0 g/dl]. There was no correlation between platelet count and bleeding, IPF and VWF levels.
In keeping with the coagulation profiles of this group of patients, PT and EXTEM MCF were abnormal in 60 and 73% respectively, APTT and INTEM MCF were abnormal in 46 and 66% respectively.
VWF levels and FVIII levels were elevated in all patients and the TGA parameter of endogenous thrombin generation (ETP) was normal in all patients in keeping with previous reports of patients with CLD.
NATEM MCF was abnormal in 75% due to thrombocytopaenia.
FIBTEM MCF was normal in 92% in keeping with majority of normal fibrinogen levels.
Abnormal ROTEM findings in the NATEM may be indicative of the contribution of thrombocytopaenia
Summary/Conclusion
Thrombocytopaenic CLD patients had significant coagulopathy, thrombocytopenia likely contributing to the abnormal ROTEM profiles. Clinical bleeding was rare. The elevated VWF and FVIII levels likely contribute to reduced bleeding in the face of thrombocytopaenia and coagulopathy. IPF was non‐contributory in this group of patients. In our cohort thrombocytopenia correlated with ROTEM measurements but not with clinical bleeding. Further functional studies are required to determine the clinical need for platelet transfusion in this group of patients rather than relying on the platelet count in isolation.
Quality, Training and IT
3C‐S09‐01
Bridging the Gap Between Theory and Practice in UK Transfusion Laboratories – Application of a Knowledge Based Competency Scheme (TACT)
Whitham C , White J , Milkins C , Rowley M
UK NEQAS (BTLP), Watford, United Kingdom
In the UK, Transfusion Laboratory Managers (TLMs) must comply with the Blood Safety and Quality Regulations, accreditation standards, and UK Transfusion Laboratory Collaborative recommendations to deliver comprehensive training and competency assessment for Biomedical Scientists working in transfusion laboratories. Changing pathology service delivery models add pressure and complexity to this task, as it becomes necessary to train and assess staff from other areas of Blood Sciences with little background in transfusion.
In November 2014, UK NEQAS (BTLP), in partnership with an IT company, launched a web‐based Training Assessment and Competence Tool (TACT). TACT is available 24/7 and complements existing practical competency schemes and external quality assessment. It provides TLMs with continual ‘real‐time’ monitoring of the application of essential knowledge of transfusion practice by generating multiple variations on a standard pre‐transfusion testing scenario and applying logic rules to allow automatic assessment based on national guidance. With 1050 members at the end of the initial launch phase, it is estimated that TACT is assessing approximately 18% of the 6000 UK transfusion staff potentially requiring transfusion knowledge based competency assessment, as extrapolated from questionnaire data at the inception of this project in 2012.
Current project developments include upgrading the TLMs’ interface to give more detailed information on staff progress and review of scenarios where errors are made, and further translation of UK national guidelines into more complex logic rules to expand TACT's automatic scoring elements to cover some identified knowledge gaps. For example, BTLP exercise 14R1 included a sample from a female patient, age 30, with a weak D type – contrary to UK guidelines, 93/356 (26%) UK laboratories recording anomalous D typing reactions and not undertaking further testing to characterise weak/partial D, reported this patient as D positive. 81/270 (30%) of those recording anything other than D positive, were prepared to issue a D positive red cell donation for this patient without characterising weak/partial D. TACT logic rules for anomalous D typing, soon to be released, will combine clinical context and patient demographics with recommendations from national guidelines. The learning outcomes from 14R1 and other similar scenarios will be automatically assessed by TACT, allowing TLMs to identify and address the training needs of individual staff.
Further developments to expand and refine TACT are being driven by the TACT Professional Steering Group. Content will be informed by errors identified as a result of EQA and haemovigilance. Feedback from members via surveys and TACT social media pages will be used to continually improve the system. Future project developments already identified include: introduction of emergency and out‐of hours scenarios (e.g. major haemorrhage), development of the educational aspect of TACT, introduction of different levels of complexity to assess all staff grades, and adapting TACT for use outside the UK. We aim to extract national benchmarking data on competency in specific areas to inform professional bodies of training needs, and guideline writing groups of areas of poor practice. National professional engagement should ensure TACT meets the needs of TLMs and Biomedical Scientists within the UK.
3C‐S09‐02
The Review of WHO Distance Learning Program of Blood Safety in China: 2002–2013
Zheng X
Blood Center of Zhejiang Province, Hangzhou, China
Background
During the past two decades, China has learned a lesson of the importance of blood safety and laid much emphasis on the issue. Besides the heavy investment in the physical facilities and equipment of blood transfusion services across the nation, systematic training and education for blood transfusion services staff is essential for the delivery of high‐quality transfusion service. In China, the situation is similar to many other countries: transfusion medicine education has not been systematically embedded in medicine education, as the result, it lacked a formal education program in transfusion medicine leading to a university degree or diploma. So on‐the‐job training is a practical way to solve the problem.
Aims
To improve the professional quality of national blood transfusion services personnel, on‐the‐job training and education was carried out.
Methods
In a country like China with vast geographic distances, it is a big challenge for training and education for blood service workers across the whole nation. The health authorities decided to use the distance learning method for training, and the Distance Learning Materials on Safe Blood and Blood Products developed by World Health Organization (WHO) was chosen as the training textbooks. Besides the WHO Distance Learning Materials, the training materials also include related laws, regulations, norms and standards of China as well as the supplementary materials ‘Blood component transfusion’. Blood transfusion service workers learn the materials by themselves in their spare time. Then they will take a conventional training for several days. As a means of assessment, all the learners must take national Blood Transfusion Services Personnel Certification Examination. Those who passed the examination were issued post training certificates, thus they got the corresponding qualification for doing their jobs.
Results
From 2002 to 2013, 90,426 blood transfusion service workers took the training. Among them, 85,032 took the examination and 66,760 passed, the passing rate was 78.51%. The number of trainees increased in recent years. In October 2013, 5472 trainees from 30 provinces and municipalities in China took the examination and the passing rate was 70.72%.
Summary
Government commitment and support is essential for carrying out a large scale training program across the nation. To assure the training quality, China has set up a professional qualification system accredited by health authorities which is unique in the world. All these measures lead to higher levels of blood transfusion service staff's knowledge and competence – and subsequently a positive impact on blood safety. Voluntary donations have increased from 5.5% in 1998 to 99% in 2008. The 12 years practice of this program was beneficial to blood safety promotion in this nation.
3C‐S09‐03
Deviation Management Using an Electronic Document Management System: Results of 2 Years of Experience
Gross MG1, Spyrantis AS 1, Capalbo GC 1, Sireis WS 1, Gubbe KG 2, Seifried ES 1, Schmidt MS 1
1GRC – Blood Donor Service Baden‐Wuerttemberg – Hessen, Frankfurt, Germany2GRC – Blood Donor Service Nord‐Ost, Plauen, Germany
Background
FDA`s Quality System Guide and EU‐GMP Guidelines Chapter 1 clearly highlight the importance of a reliable deviation management and corrective and/or preventive actions (CAPAs) in transfusion medicine. Therefore, the implementation of an efficient deviation management system is important. In 2005, a paper based version of the deviation management was implemented in our blood donor service and used in a day to day operation. The disadvantage of our paper based system was a reduced transparency regarding comparable reports, e.g. tracking of deviation reports and defining CAPAs for similar deviations within different locations of our blood donor service. In addition, CAPAs across several locations were exchanged within these locations and therefore often time consuming procedures.
Aims
The aim was an implementation of a deviation management system using an electronic document management system which is available in all 18 locations of our blood donor service. Furthermore, this deviation management system should be easily accessible for all employees and help to track deviations and define effective CAPAs within all locations.
Methods
The electronic document management software Saperion (Lexmark Perceptive Software) was used and defined customized workflows (adapted to organization`s needs) were implemented in 2012. The workflow starts with an electronic record of the deviation, which can be done by every employee. In the next step, the deviation report will be automatically transferred to the head of the responsible department for risk analysis using FMEA and to define CAPAs. Finally, the quality department review the complete file. Every report can be requested by a search tool and statistical analyses of trends can be easily performed.
Results
In total, 1529 deviation reports in all 18 locations were recorded and archived (2013: 705, 2014: 624). This number is comparable to the paper based version. The distribution of reports within different locations depends on the size of the locations: smaller locations report less deviations compared to bigger locations. The number of reports between the different departments is as followed: donation 33%, labour 27%, production 25%, sale 7%, IT 6%, and engineering 2%. About 24% of the reports were due to deviations in primary material, e.g. in blood bag systems.
Summary/Conclusions
The implementation of an electronically based deviation management system leads to effectively tracking and managing of deviations resulting in continuously improving of processes. Statistical analyses of trends are easy to perform. Most important, it results in clear responsibility of the process, an archived report of root cause analyses and effective CAPAs.
3C‐S09‐04
A Regional Blood Centre Experience in Blood Supply Management and KPI
Muon MCT , Pinheira A , Caeiro C , Pires I , Marques A
Portuguese Institute of Blood and Transplant, Coimbra, Portugal
Background
Coimbra Centre of Blood and Transplant – CCBT is one of the four Regional Blood Centre of the Portuguese Institute of Blood and Transplant – PIBT. It is located in the central part of Portugal, which is a south‐western European country with 10.6 million habitants.
An adequate blood supply chain became a new challenge to our organization in the recent years, due to the country economic constraints and the massive emigration of our blood donors.
CCBT performed 58,984 whole blood collections in 2014 to serve all its region hospitals and also contributes with supplies to Lisbon's area hospitals.
Aims
Coimbra Centre of Blood and Transplant aims to maintain the sustainability of an adequate blood supply chain to our client hospitals and institutions and to contribute for the national blood stock and monitor the KPI concerning outdated units.
Methods
The Portuguese Institute of Blood and Transplant began to implement the EDQM‐CoE 5 step BSM proceedings.
PIBT maintains online a web based tool where CCBT can assess in real time: the history of hospital blood use and the history of blood supplied by CCBT.


With these data, CCBT could establish forecast for overall annual supply, forecast and establish annual Blood Collection, Donor base, Supply Programme for the region according to human resources, logistics and budget. Daily web based monitoring red blood cells units use and inventories in Blood Establishments and Hospitals, Acting to maintain and replenish inventories at adequate levels. Periodical technical audit putted in place to monitor hospitals blood type demand and use. Several KPI were monitored in the meanwhile.
Results
BTCC in 2014 collected less 10% of whole blood, however it managed to fulfil the blood supply to all its region hospitals and also to other region as well and contributes and achieves the 6 days RBC stock target to all the country, maintaining at the present above 10 days stock level.
Due to technical audit to hospitals blood type RBC demand/use, over stocks and biased blood type RBC units demands are decreasing.
Outdated RBC in 2014 was 2.30% and outdated platelets was 0.43%.
Bone Marrow donors registrations and Blood donations are performed 100% in common collections sessions.
Collections sessions performance rose up from KPI = 1.021 to KPI = 1.317.
Conclusions
Our experience findings show some elements as good practice in Blood Supply Management (BSM):
1. KPI had great improvements since the organization began to implement the BSM proceeding.
2. Technical audit to hospitals RBC demand and use, including blood type, seemed to strengthen the practice of good use of blood.
3C‐S09‐05
Use of RFID Technology in Blood Component Transportation: An Italian Experience (DIMT Vicenza) in Unifying Working Centres
Sardella CS1, Md Pavan PL 1, Md Patrizia PD 2, Md Alghisi AA 3
1ULSS 4, Alto Vicentino, Santorso, Italy2ULSS 6 Vicenza, Vicenza, Italy3DIMT VICENZA ULSS 6, Vicenza, Italy
Background
Italian VENETO REGION is strongly recommending unification of blood processing centres, giving a minim limit of at least 40,000 blood bags processed yearly per centre in order to rationalize personnel availability, reduce expences and improve quality standards. The Provincial Department of Transfusion Medicine of Vicenza unified four blood‐processing centres in one, thereby processing about 45,000 blood bags per year. Radiofrequency identification (RFID) tags are gradually being introduced in blood management systems. It has recently being showed there is no risk for safety in blood components using ISO/IEC 18000‐3 mode 1 standard 13.56 MHz RFID tags. Up to date there is no global standard for the uses of RFID frequency, but if compared with ISBT128 barcode labels, RFID tags have numerous advantages, mainly data storage capacity and are resistant to environmental stress
Aims
The study aimed at verifying if we could reduce the use of staff and time in transporting blood units from collection centres to blood processing centre, and exportation to industry and/or other blood centres, maintaining security in traceability of blood components
Methods
All whole blood bags collected at our collection centres were tagged with RFID tags (43 × 43 mm) and tags placed under the standard ISBT 128 DIN label. In a 3‐bag collection, all bags were tagged at donation site. Tags were initialized after the DIN barcode was read. All tag data, together with barcode data were saved on a central server for donation check‐in. At blood collection centres, collected bags were placed on a reading gate (TRAY ID BC Multiple RFID reader/writer – 25 × 17 × 2 cm) in order to create a bleeding list. The bags were then placed in blood bag container, ready to be sent to the blood processing centre for centrifugation and separation. At arrival at the blood processing centre, the whole transportation bag containing maximum 15 whole blood bags, were placed in EmoPath (Massive RFD 50 × 50 × 50 cm tunnel reader.)
Results
Between 1 st July 2014 and 28 th February 2015, 28.707 whole blood bags were collected. 86.121 tags were utilized during this period. During the same period, more than 23.000 blood components were transferred with RFID technology to other blood centres. We had an average error rate of 2–4 tags not correctly identified daily, giving an error rate per month of 0.94% (incorrect writing of tags, or tags incorrectly applicated)
Summary/Conclusions
The low percentage of errors in identifying the blood components, permits us to work in security. We daily exchange an average of 270 whole blood bags and blood components daily, with limits of up to 360 bags daily. The rapid donation check in at the blood centre by using RFID's ability to read multiple units in closed containers, significantly reduced labour and we calculated in reducing check‐in/check‐out timing in about 1 1/2 h daily. This also enabled us to use only one person instead of the usual two for blood components check‐in/check‐out process, and thereby enabling us to utilize another person in other mansions
3C‐S09‐06
Slovenian National Telemedicine System for Remote Interpretation of Red Cell Laboratory Testing
Macek Kvanka M , Breskvar M , Bricl I , Simc M
Blood Transfusion Centre of Slovenia, Ljubljana, Slovenia
Background
The aim of the development and implementation of TM in the 2005–2008 period was to enable an expert from the central reference laboratory to give advice to the staff in any transfusion laboratory of regional hospitals where red cell laboratory tests were performed. After 2008, the reorganization of blood transfusion service started: the former transfusion departments of regional hospitals gradually became dislocated transfusion centres (TCs) of the Blood transfusion centre of Slovenia or the Maribor Centre for Transfusion Medicine (7 and 2 affiliated TCs respectively). These two blood establishments then took responsibility for the organization of the non‐stop work at the affiliated TCs. Physicians from the hospitals, who used to interpret routine pre‐transfusion testing when physician specialized in transfusion medicine (TMP) was not available, were no longer involved. Faced with the situation of the shortage of TMPs needed for the organization of continuous work (24/7) at dislocated TCs, telemedicine system that has already been implemented offers a solution. Consequently, its use was extended from giving advice in the case of complicated patients to the interpretation of pre‐transfusion and prenatal tests for all routine patients. The TM service is organized 24/7 and is used in 9 dislocated TCs when a TMP is not available. After receiving a request for blood components, the laboratory personnel perform the red cell tests using gel cards and create a TM session with captured images of the associated cards for each patient. The sessions are sent to a teleconsultant, a TMP working on the other location, who is responsible for several remote TCs (7 or 2) at the same time. After interpretation and validation, the test results are issued with the electronic signature.
Aims
Here experiences from the perspective of TM users and patients are presented.
Methods
In order to evaluate the significance of TM, statistical data obtained from the transfusion information system DATEC and TM system were analysed. The satisfaction of the TM users (teleconsultants and technicians) was evaluated by two surveys.
Results
Since the beginning of 2008, the number of TM sessions has increased from 295 to 21,222 in 2014. The proportion of patients from dislocated TCs whose red cell laboratory tests are interpreted by TM gradually increased to 50% on average in 2014. The TM system enables prompt responses: 54% of sessions are concluded within 30 min and 88% within 1 h. Eighty per cent of the TM users (8 teleconsultants and 32 technicians) have claimed that the TM service is indispensible and 20% that it is very important for everyday practice.
Conclusions
The use of TM has a strong impact on the improved and timely transfusion service for patients, improved relationship between BTS and hospitals, improved organization and rationalization of work in BTS and on substantial cost savings. TM allows pre‐transfusion tests all over the state to be interpreted by TMPs 24/7. Consequently, increased patient's safety is expected and the same quality of service for all the patients regardless of time and location is provided.
Component Storage
3C‐S10‐01
New Additive Solutions for Red Cells
de Korte D
Sanquin Blood Bank, Amsterdam, The Netherlands
Shortly after the introduction of component therapy in the 1960's, the first additives for red cell concentrates (RCC) were introduced. In first instance, adenine was added to the anticoagulant solution to improve metabolic state (maintenance of ATP) of erythrocytes during storage in plasma, but the next step was to remove most of the plasma, originally used as resuspension medium, and replace this by an additive solution, containing adenine and glucose (metabolism) and mannitol (protection against hemolysis). This development resulted in prolongation of RCC shelf‐life from 21 to 35 or 42 days. Over the years, there were some new solutions developed, but still SAGM (Europe) and AS‐1 (US) are more or less standard, nearly exclusive in combination with CPD as anticoagulant. Most of these new solutions were minor variations on SAGM or AS‐1, with minimal improvement of in vitro quality of erythrocytes. The replacement of mannitol by citrate in AS‐3 had a positive effect on membrane integrity during storage, with a significantly lower hemolysis at the end of storage. Also after cryopreservation and subsequent thawing, AS‐3 showed to have this protective effect, allowing a longer shelf‐life for thawed erythrocytes.
Development of new concepts, resulting in improved quality of RCC, was in first instance focused on achieving longer storage time. However, as a result from the concern about possible negative side effects caused by transfusion of older units, the research on new additive solutions is more focused on having a better quality during the currently accepted maximum storage time of 35–42 days. Around 1990, Meryman developed the concept of the so‐called chloride shift, which was succesfull used for development of new additive solutions. Through replacement of chloride by impermeable solutes, it is possible to manipulate the intra‐cellular pH of erythrocytes. With a high intra‐cellular pH the enzymes of the glycolysis remain active, resulting in high 2,3‐DPG and/or ATP values throughout storage. Because in this concept, the additive solution becomes alkaline, it is not longer possible to perform sterilization of the complete additive solution, because otherwise glucose will caramelize at pH > 5.5. During the past 10–15 years, several research groups, with the group of Greenwalt and Hess as pioneers, developed new additive solutions based on the Meryman concept. Recently, the first commercial product from these studies was launched, with an improved in vitro quality parameter profile and improved in vivo recovery data. Roughly, the quality at day 56 for the new solution was comparable to that at day 42 for the current standard solution.
An overview will be given on the stepwise development of new additive solutions, resulting in new approaches for erythrocyte storage. It will also be discussed how important high 2,3‐DPG values are in transfusion practice.
3C‐S10‐02
Storage of Washed Red Blood Cells in ESOL‐5 Increases Membrane Resistance to Osmotic Stress and Reduces Membrane Breakdown
Loh YS , Webb RG , Marks DC
Australian Red Cross Blood Service, Sydney, Australia
Background and Aims
Washing of red blood cells (RBCs) effectively reduces undesirable plasma proteins and soluble bioactive substances from the component supernatant. However washing and storage of RBCs in SAG‐M can lead to reduced 2,3‐diphosphoglycerate (2,3 DPG) concentration and increased haemolysis. ESOL‐5 is a new additive solution known to retain the pH and 2,3‐DPG concentration of RBCs. Therefore the aim of this study was to determine whether the in vitro quality of washed RBCs during storage could be improved by using ESOL‐5 in comparison to SAG‐M.
Methods
Two ABO/RhD matched leukoreduced RBCs were pooled and split to form matched pairs on day 4 post‐collection (n = 10). Red cells were stored in SAG‐M prior to washing. Both units were washed with 0.9% saline using a semi‐automated ACP‐215 cell washer. One unit was stored in SAG‐M (259.2 ± 11.1 ml), while the other unit in ESOL‐5 (260.5 ± 11.0 ml). All units were stored at 2–6°C and samples were taken after 3 h and subsequently on days 1, 2, 7, 14 and 21 post‐wash to measure in vitro quality and metabolic activity of RBCs. The volume, haemoglobin, RBC count and spun haematocrit were compared using two‐tailed paired t‐tests. All other in vitro quality parameters of RBCs in either ESOL‐5 or SAG‐M over storage were compared using two‐way repeated measures ANOVA with post hoc Bonferroni multiple comparisons at each time point, where P < 0.05 was considered significant.
Results
After washing the volume (288.9 ± 16.2, 282.7 ± 13.8 ml), haemoglobin (50.05 ± 3.32 g/unit, 49.78 ± 3.59 g/unit), and RBC count (5.67 ± 0.21 × 1012/l; 5.79 ± 0.14 × 1012/l) of RBCs stored in either ESOL‐5 or SAG‐M respectively were not significantly different. The IgA concentration of all washed RBCs was below the limit of detection (<0.05 g/l), and the total protein of all RBCs was <0.5 g/unit. The spun haematocrit (61.0 ± 2.5%, 54.8 ± 2.3%; P = 0.0020) of RBCs in ESOL‐5 was significantly higher. As expected, the pH (P = 0.0124) and concentration of glucose (P < 0.0001) of RBC in ESOL‐5 was significantly higher over storage. As a result, the glucose consumption rate (P = 0.0003) and lactate production rate (P = 0.0008) of RBCs in ESOL‐5 were significantly higher. In contrast, the osmotic fragility (P < 0.0001) and concentration of LDH (P = 0.0247) of RBCs in ESOL‐5 were significantly lower, indicating a greater resistance to osmotic stress and decreased membrane breakdown. At day 21, haemolysis (P < 0.001) and the number of annexin‐V/glycophorin‐A positive microparticles were significantly lower (P < 0.001) for RBC stored in ESOL‐5, while the concentration of ATP was significantly increased (P < 0.001). The concentrations of 2,3‐DPG and supernatant potassium of RBCs in either additive solution were not significantly different throughout storage.
Conclusion
Storage of washed RBCs in ESOL‐5 increased RBC membrane resistance to osmotic stress, leading to a reduction in LDH release and haemolysis. This may lead to an improved in vitro quality of washed RBCs and prolong the shelf‐life of RBCs after washing.
3C‐S10‐03
Degree of Hemolysis in Stored Red Cell Concentrates Prepared from Whole Blood Units with Lipemic Plasma
de Korte D , De Laleijne-Liefting L , Van der Meer PF , Lagerberg JW
Sanquin Blood Bank, Amsterdam, The Netherlands
Background
The effect of lipemic plasma on blood component quality was recently described by Bashir et al. (Vox Sang 2013;104:218). Addition of lipemic plasma to red cell concentrates (RCC) was found to result in an increased hemolysis during storage. These results were confirmed in our lab, but in this study we aimed to investigate to which extent this effect was also found in standard RCC prepared from whole blood units with lipemic plasma, but that contained only traces of plasma.
Methods
In routine blood bank practice, whole blood units are separated into plasma, buffy coat and leukodepleted RCC in SAGM. Plasmas are visually judged on being lipemic or not; if judged lipemic, the related RCC was selected for this study. During storage for 6 weeks, these RCC units (n = 20) were sampled at day 1, 35 and 42 for in vitro quality parameters including hemolysis, [K+], pH, glucose, lactate and ATP content. RCC from either lipemic (turbid, label hardly visible through container) or very lipemic (whitish, label not visible through container) plasma's were included.
Results
Results are shown in table. The concentration of triglycerides in the related plasmas was increased, showing that all plasmas were really lipemic. The increase of hemolysis during storage of RCC was significantly higher than found in our standard RCC, already starting with a higher value. For the other parameters pH, extracellular [K+] and intracellular ATP content no differences were found between control and study group. No correlation was found between degree of hemolysis and absolute triglyceride concentration in plasma, nor with visual score (lipemic or very lipemic).

Conclusions
Despite the low remaining amount of plasma (<15 ml) in buffy‐coat depleted leukoreduced RCC, there was a huge effect of the whole blood being lipemic on degree of hemolysis in these units during storage. Sofar, at our blood bank, only the lipemic plasma and the buffy coats from whole blood units with lipemic plasma are discarded. It should be discussed if not all products from such donations should be discarded and if the donor should not be excluded from donation in case of repeated lipemic donations.
3C‐S10‐04
Clinical Safety and Efficacy of Red Blood Cell Components Treated with the S‐303 Pathogen Inactivation System – A Randomized Controlled Double‐Blind Phase 3 Study in Patients Requiring Transfusion Support of Acute Anemia
Brixner V1, Kiessling AH 2, Madlener K 3, Leibacher J 4, Müller MM 4, Geisen C 4, Henschler R 5, North A 6, Huang N 6, Mufti N 6, Ernst C 6, Rico S 6, Corash L 6, Seifried E 4
1DRK Blutspendedienst Baden‐Württemberg – Hessen gGmbH, Frankfurt, Germany2Department of Thoracic and Cardiovascular Surgery, J. W. Goethe University, Frankfurt, Germany3Department of Haemostaseology and Transfusion Medicine, Bad Nauheim, Germany4German Red Cross Blood Donor Service Baden‐Wuerttemberg‐Hessen, Frankfurt, Germany5University Clinic Munich, Department for Transfusion Medicine, Munic, Germany6Cerus Corporation, Concord, United States of America
Background
The second generation S‐303 pathogen and leukocyte inactivation system for red blood cells (RBC) is intended to improve blood transfusion safety by reducing the risk of transfusion transmitted infections and transfusion‐associated graft vs host disease.
Aims
The clinical safety and efficacy in adult cardiovascular surgery patients requiring transfusion support for acute anemia was assessed in a randomized, double‐blind, controlled, multi‐center clinical trial.
Methods
Patients undergoing coronary artery bypass grafting, and/or valve replacement or repair were randomized to receive S‐303 treated or conventional RBC during a 7‐day treatment period. Clinical outcomes reflective of tissue oxygenation were assessed: renal insufficiency, hepatic insufficiency; and cardiopulmonary function as assessed by the 6 Min Walk Test. Adverse events (AE) were collected throughout the study. Immunogenicity was assessed by testing patient serum against S‐303 treated RBCs using a gel card agglutination test prior to transfusion, at the end of the study (Days 28–40) and at 90 ± 5 days.
Results
Eighty‐seven patients in two clinical centers were enrolled, and fifty‐one patients (Test 25, Control 26) who received study RBC were evaluable. A total of 73 S‐303 treated RBC and 75 control RBC components were transfused. Baseline characteristics and surgical variables were comparable between groups. Overall incidence of renal insufficiency was 15.7% (Test 5, Control 3; P = 0.41). None of the renal insufficiency events occurred in relationship to an acute drop in hemoglobin levels or administration of study RBC units, so a correlation of the effect of transfusion episodes to renal organ perfusion could not be established. Incidence of hepatic insufficiency was 2% (Test 1, Control 0, P = 0.37). Thirty‐seven patients (Test 17, Control 20) were able to perform the 6MWT at the time of first ambulation. There were no differences in the mean [SD] distance walked in meters (m) between days 0‐6 (Test 44.8 m [48.6], Control 53.1 m [41.8]; 95% CI −37.0, 26.6) or at day 13 or discharge (Test 95.5 m [69.7], Control 97.7 m [51.1]; 95% CI −30.8, 50.3). Most patients in both groups (84.3%) experienced an AE. There were no statistical differences in the overall incidence of AE rates (Test 22 vs Control 21, P = 0.412), or in possibly related AEs (Test 5 vs Control 3, P = 0.24). Overall, 22 (43.1%) patients experienced a serious adverse event (SAE), with similar distribution between groups (Test 13 vs Control 9, P = 0.20). Three SAEs were considered possibly related to the transfusion of study RBC (Test 1 vs Control 2). Five patients died during this study (Test 3 vs Control 2, P = 0.53). Deaths were not considered related to the administration of study RBC components. Observed AEs were within the expected spectrum of co‐morbidity and mortality for patients of similar age and with advanced cardiovascular diseases undergoing cardiovascular surgery requiring RBC transfusion. No patients exhibited an immune response to S‐303 treated RBCs.

Summary/Conclusions
Clinical safety and efficacy variables following the transfusion of S‐303 treated RBC were comparable to conventional RBC. S‐303 treated RBC appear to be safe to be transfused in support of acute anemia.
3C‐S10‐05
Microvesicle Production and the Reversibility of the Potassium Leak in Stored Red Cells from Healthy Donors
Bawazir WMS , Flatt JFF , Bruce LJB
National Health Service, Blood and Transplant, Bristol, United Kingdom
Introduction
The red blood cell (RBC) membrane acts as a barrier to monovalent cations however, a small diffusional permeability remains. This leak is continuously corrected by NaKATPase. Upon cold storage of RBCs, the NaKATPase becomes virtually inactive and hence the cation leak continues uncompensated. There are other changes that occur to RBCs during prolonged storage such as the decline of ATP and 2,3 DPG, and increase in oxidation and vesiculation. These changes are known as storage lesion.
Aims
This investigation aims to examine the reversibility of cation imbalance and the protein composition of vesicles released during storage.
Methods
Units of packed RBCs, suspended in CPD‐SAGM, from 8 healthy donors were stored at 4°C over 35 days. Each week two samples of RBCs were taken and one was rejuvenated with inosine and Li‐pyruvate and incubated for 2 h at 37°C. Hemox analysis was used to examine the haemoglobin affinity for oxygen and obtain the p50. Complete blood count was measured weekly. Flame photometry was used to measure intracellular sodium and supernatant potassium. Quantitative proteomics using tandem mass tag analysis was used to compare the composition of microvesicles at different stages of storage. SDS‐PAGE and immunoblotting were used to analyse proteins and phosphoproteins in the RBC membranes and the microvesicles (MVs) over the storage period.
Results
Rejuvenation of the stored RBCs was successful as ATP and 2,3 DPG levels were substantially restored. The haemoglobin affinity for oxygen was similarly restored to normal levels by rejuvenation due to the replenishment of 2,3 DPG. By day 35, the potassium concentration in the supernatant reached 35–60 mM. However, the cation imbalance was not significantly corrected by rejuvenation. Protein analysis of MVs showed that the total amount of protein released via MVs significantly increased after day 21 of storage, which coincides with increased band 3 tyrosine phosphorylation. These MVs were rich in GPI‐linked and lipid raft proteins. Protein composition of the MVs varied through the storage period. Confocal imaging showed that the lipid raft protein stomatin is lost via budding from echinocytic spicules.
Conclusion
Rejuvenation of stored RBCs successfully restored ATP and 2,3 DPG but minimally affected the cation imbalance. Analysis of MVs indicated that vesiculation is a lipid raft process. Variations in protein composition of MVs may be useful to identify markers of increased vesiculation.
Parallel Sessions
Cellular Microparticles in Transfusion
3D‐S11‐01
Blood Microvesicles: A Pathophysiologic Reality or a Laboratory Event?
Tissot JDT
Interregional Blood Transfusion SRC, Epalinges, Switzerland
Erythrocyte concentrates (ECs) are the major labile blood product being transfused worldwide, aiming at curing anemia of diverse origins. Most frequently ECs are stored at 4°C up to 42 days in additive solutions. Such storage induces cellular lesions, altering red blood cells (RBCs) metabolism, protein content and rheological properties. A hot debate exists regarding the impact of the storage lesions, thus the age of ECs on transfusion‐related clinical adverse outcomes. Several studies tend to show that poorer outcomes occur in patients receiving older blood products. However, no clear association was demonstrated up to date. While metabolism and early rheological changes are reversible through transfusion of the blood units, oxidized proteins cannot be repaired, and it is likely such irreversible damages would affect the quality of the blood product and the efficiency of the transfusion. In vivo, RBCs are constantly exposed to oxygen fluxes, and are thus well equipped to deal with oxidative challenges. Moreover, functional 20S proteasome complexes allow for recognition and proteolysis of fairly oxidized protein, and some proteins can be eliminated from RBCs by the release of microvesicles (EMVS). The present paper is aimed to review some biochemical aspects associated RBC storage and microvesiculation. The potential pathophysiologic effects of EMVS are also presented.
3D‐S11‐02
Microvesicles as Indicators and Mediators of Inflammatory Reactions in Response to Transfusion During Cardiac Surgery
Sullo NS , Wozniak MW , Farha AF , Dott WD , Nielsen PN , Murphy GJM
University of Leicester, Leicester, United Kingdom
Background
The REDWASH trial was a single blinded, randomised controlled trial that tested the hypotheses that removal of the red cell supernatant from stored allogenic red cells would attenuate inflammatory responses in recipients of large volume blood transfusions. A secondary hypotheses was that recipients of washed and unwashed red cells would have different levels of circulating microvesicles and that and these would underlie our observed differences in inflammatory responses and organ injury within the groups.
Aims
The aim was to establish a relationship between transfusion‐delivered storage lesion intermediates (microvesiscles) in cardiac surgery patients and inflammatory events that lead to MV generation. We also tested whether MV could serve as potential organ injury markers.
Methods
The study was performed on stored donor blood and blood and serum samples obtained from 40 cardiac surgery patients participating in the REDWASH trial that received on average 3 units of red cells with a mean storage age of 20 days. Complementary to that, samples from leukodepleted blood packs stored for 42 days were analysed, as well. Samples were analysed using nano‐particle tracking technology and flow cytometry. Data was analysed by ANCOVA and t‐tests and is reported as means ± SE.
Results
Two populations of MV were detected in stored blood packs: 50–150 and 150–300 nm in size. The smaller MV were present regardless of the blood pack age, and the larger MV increased in numbers during storage time. The larger MV were positive for red cells biomarker CD235a and phosphatidylserine (PS). Further analysis of REDWASH patients’ samples revealed that PS‐positive MV were also higher in numbers in patients transfused with standard (unwashed blood). That most likely led to activation of platelets, which resulted in higher levels of CD41‐positive microparticles. We also detected higher levels of pro‐coagulatory MV positive for tissue factor (CD142). In contrast MV that increased in samples from the washed group were of endothelial and monocytic origin. Size analysis showed that MV 50–200 nm large increased within the first 12 h after surgery in samples from washed group. Another population of MP, 100–200 nm in size, increased 48 h after surgery in samples from the standard group.

Conclusions
Transfusion of washed and unwashed RBC in cardiac surgery patients generates two distinctive populations of MV. That reflects different modes of inflammatory events where PS‐positive MV from stored blood packs activate leukocytes and platelets while changes in red cells concentrates during washing result in activation of endothelium and monocytes.
3D‐S11‐03
Inflammation and Organ Injury Following Transfusion of Red Cells – The Role of Microparticles and Oxidative Stress
Wozniak MJ1, Sullo N 1, Qureshi S 1, Patel N 2, Dott W 1, Nielsen P 1, Kumar T 1, Joel-David L 1, Mariani S 1, Wiltshire M 3, Cardigan R 3, Murphy GJ 1
1University of Leicester, Leicester, United Kingdom2Imperial Colledge London, London, United Kingdom3NHS Blood & Transplant, Cambridge, United Kingdom
Background
Allogeneic red blood cell (RBC) transfusion is associated with an increase in pulmonary and renal morbidity after cardiac surgery. Transfusion of RBC stored for longer periods increases the frequency of both lung and kidney injury. It has been hypothesised that these associations are attributable to the ‘storage lesion’ – accumulation of metabolites in the supernatant that includes pro‐inflammatory lyso‐lipids, high cell‐free haemoglobin and depletion of cellular ATP and 2,3‐DPG during storage.
Aims
The aims of this study were to identify components of the storage lesion that lead to inflammatory events resulting in kidney and lung damage using in vitro and porcine models, and in human subjects. We aimed to analyse mechanisms underlying organ injury in response to transfusion in cardiac surgery patients, and ways to improve patients’ outcomes.
Methods
Samples from 60 patients participating in REDWASH trial (a single blinded, randomised controlled trial of washing of allogeneic RBC prior to transfusion vs standard care – no washing, ISRCTN27076315) were analysed for inflammatory response markers. In a complementary study 28 pigs were randomised into 4 groups receiving crystalloid infusion, 14‐day old, washed 14‐day old, or rejuvenated‐washed 14‐day old RBC transfusion. Inflammatory markers, free haemoglobin and iron levels were measured in plasma and serum. Leukocyte invasion was determined by immunohistochemistry. The results from REDWASH trial and porcine model were verified and further analysed in an in vitro inflammatory models.
Results
Transfusion of stored RBC (~22 days old) into REDWASH patients was associated with increased leucocyte and platelet activation as compared to washed blood, and this effect was attenuated by washing. Our observations were reproduced in pigs. Further analysis revealed that oxidised lipids on the surface of microparticles transfused with unwashed blood were associated with activation of platelets and monocytes and depletion of either in an in vitro model reduced activation. Unexpectedly, transfusion of washed blood reduced lung injury but affected kidney function as indicated by serum creatinine levels. This was associated with increased free haemoglobin levels in samples from patients and pigs transfused with washed RBC, and with higher oxidative stress as indicated by increased HMOX‐1 levels in porcine tissues. Free haemoglobin also increased endothelial permeability in vitro and led to non‐standard endothelial activation manifested by expression of VLA5 and fibronectin retention. Lung and kidney injury following transfusion were alleviated in vivo in pigs and in vitro by rejuvenation of blood prior to transfusion.

Conclusion
Our results identify two types of inflammatory events that may be important in response to RBC transfusion. Oxidised lipids present in MP generated during storage activate leukocytes while free haemoglobin increases oxidative stress that can lead to endothelial activation. These may contribute towards kidney and lung injury in recipients. The latter can be attenuated by washing RBC, but is associated with increased kidney injury. However, in the porcine model both kidney and lung injury can be significantly reduced by rejuvenation and washing RBC prior transfusion. These data suggest that there may be interventions that may reduce adverse events following transfusion of RBC in cardiac surgery patients.
3D‐S11‐04
Microparticles from Stored Red Blood Cell Component Cause Acute Lung Injury in a Mouse Two Events Model
Xie RF1, Yang J 1, Hu P 2, Wang ZC 3, Yang YM 1, Gao L 1, Fan HH 1
1Shanghai Blood Center, Shanghai, China2East China Normal University, Shanghai, China3Hua Shan Hospital, Shanghai, China
Background
Transfusion relative acute lung injury (TRALI) may be induced by transfusion of stored red blood cells components (RBCs). Report has indicated that plasma from packed red blood cells induced acute lung injury in a rat two events model. Recently some researches have demonstrated there were some kinds of inflammatory factors existed in stored RBCs including anti leukocyte antibodies, LysoPCs, cytokines and microparticles derived from RBC, platelets and leucocytes. We hypothesize that RBC derived microparticles (RMP) may cause acute lung injury in a mouse two events model.
Aim
To analyze the RMP release from RBCs during 35 days of storage, and investigate the effects of RMP on formyl‐Met‐Leu‐Phe (fMLP) activated polymorphonuclear neutrophil (PMN) respiratory burst and LPS sensitized mouse acute lung injury.
Methods
Cell free supernatant was obtained by centrifugation of RBCs store in MAP (Mannitol‐Adenine‐Phosphate) solution at 3000 g for 20 min on day 1, 7, 14, 21, 28 and day35 of storage. RMP were isolated by centrifugation supernatant at 20,000 g for 1 h; RMP depletion was achieved by filtration supernatant through a 0.1 μm filter. RMP in supernatant were dual labeled with fluorescent mouse anti human CD235a monoclonal antibody and carboxyfluorescein succinimidyl ester (CFSE), then counted by flow cytometric analyze; Priming of the fMLP activated PMN oxidase activity with supernatant or isolated RMP was measured; Mice were injected intraperitoneally with 3 mg/kg lipopolysaccharide (LPS) followed by another injection of approximately 1.0–3.0 × 107 isolated RMP in PBS or equal volume of heat‐treated supernatant through tail vein in 2 h, then killed humanely after 2 h. Lungs were harvested for histology and pulmonary edema assessment. A bronchoalveolar lavage (BAL) was performed, and then protein content was measured.
Results
The amount of RMP increased significantly after 14 days of storage (from day1, day14 to day 35: 8.81 ± 4.96 × 102/μl, 3.85 ± 2.13 × 103/μl and 1.45 ± 0.43 × 104/μl respectively, P < 0.05). Either supernatant or isolated RMP priming PMN respiratory burst effectively. RMP depletion supernatant of 35 days stored RBCs showed significant reduction in their priming activity (P < 0.05). Mouse injected with LPS then infused with 3.0 × 107 RMPdemonstrated acute lung injury which included: (i) alveolar wall becomes thickened with increasing deposits of cells and protein in alveolar spaces in lung tissue stained with hematoxylin and eosin and examined with light microscopy; (ii) the rate of wet/dry lung weight was increased significantly (LPS vs LPS + RMP: 4.53 ± 0.09 vs 4.93 ± 0.18, P < 0.05); (iii) the protein concentration of bronchoalveolar lavage fluid (BVLF) were also increased significantly (LPS vs LPS + RMP: 145.13 ± 12.39 vs 247.65 ± 38.33 μg/ml, P < 0.05). Only the protein of BVLF but not was wet/dry lung weight reduced The effects of RMP depletion supernatant of 35 days stored RBCs on mice acute lung injury were reduced compared to original supernatant, but only protein concentration of BVLF reduced significantly.
Conclusion
Red blood cells derived microparticles accumulated during storage may prime polymorphonuclear neutrophil respiratory burst and cause acute lung injury in a mouse two events model. The effects of other inflammatory factors and relation with microparticles in stored RBCs still need to be assessed.
3D‐S11‐05
Packed Red Blood Cell Storage is Associated with Increased Microparticle and Procoagulant Phospholipid Levels
Flower RL 1, Aung HH 1, Tung JP 1, Dean MM1, Pecheniuk NM 2
1Australian Red Cross Blood Service, Brisbane, Australia2Faculty of Health, Queensland University of Technology, Brisbane, Australia
Background
Clinical studies have reported that PRBC storage duration is a potential risk factor for increased morbidity. During storage, one of the changes in PRBCs is the release of microparticles (MPs), which are <1 μm in size and express phosphatidylserine (PS), a procoagulant phospholipid (PPL). Therefore, MPs were hypothesised to contribute to hypercoagulable complications post‐transfusion.
Aims
To investigate the association between leucoreduced PRBC storage duration and progressive accumulation of MPs which execute procoagulant activity resulting in a hypercoagulable state post‐transfusion
Methods
Standard leucoreduced PRBC units (n = 9) were stored at 2–6°C for up to 42 days. At Day 1, and then at weekly intervals (Days 7, 14, 21, 28, 35 and 42), 15 ml from each PRBC unit was aseptically withdrawn and centrifuged twice at 3000 g for 10 min. The PRBC supernatants (PRBC‐SN) were removed and stored at −80°C for subsequent analyses. PRBC‐SNs from all units at all time‐points were analysed for (i) the presence of MPs originating from different cells by conventional flow cytometry (TRUcount and FACS Canto[TRADEMARK] II), (ii) the effect of PRBC storage duration on coagulation in a ‘spike in’ transfusion model by routine coagulation assays [prothrombin time (PT) and activated partial thromboplastin time (APTT)] and (iii) procoagulant activity by factor X‐activated clotting time (XACT) assay. In addition, all nine Day 42 PRBC‐SNs were filtered through a 0.22 μm membrane pore to assess (iv) the effect of filtration on the MP profile and procoagulant activity (XACT assay).
Results
(i) By Day 28 of storage, there was a significant increase in the total numbers of MPs present in the PRBC‐SNs (Day 1 vs Day 28; P < 0.01). Similarly, significant increases in the numbers of red blood cell‐derived MPs (P < 0.01) and PS‐expressing MPs (P < 0.05) were also observed by Day 28. Absolute counts of MPs derived from platelets and leucocytes, however, did not change over 42 days. (ii) Storage duration made no difference to coagulation, when assessed by routine clinical coagulation assays. (iii) Clotting times of PRBC‐SNs significantly decreased from Day 21 onwards (P < 0.05) whereas the concentration of PPL in the PRBC‐SNs, determined by comparison of these clotting times against a PPL standard reference, significantly increased. Levels of PS‐expressing MPs and red blood cell‐derived MPs positively correlated with amounts of PPL (r = 0.83, P = 0.02; r = 0.81, P = 0.03, respectively). (iv) The numbers of red blood cell‐derived MPs and PS‐expressing MPs in Day 42 PRBC‐SNs were significantly reduced by filtration (pre‐2189/μl vs post‐4/μl, P < 0.01; pre‐3047/μl vs post‐382/μl, P < 0.01, respectively); however, the PPL content, as measured by the XACT assay, was not.
Conclusion
Storage duration of leucoreduced PRBCs was associated with increases in red blood cell‐derived MPs, PS‐expressing MPs and PPL content; however, while filtration reduced the numbers of MPs it did not reduce PPL levels. The significance of this finding remains to clarify the contribution of the various sources of phospholipid or other factors in the stored PRBC‐SNs which may contribute to a hypercoagulable state post‐transfusion.
Paediatric Transfusion
3D‐S12‐01
The Risk of Necrotizing Enterocolitis (NEC) and Other Complications Associated with Red Blood Cells and Platelet Transfusions in Sick Neonates
Dame C
Department of Neonatology, Charité – Universitätsmedizin, Berlin, Germany
Introduction
Thresholds for transfusions of red blood cells (RBC) and platelets (PLT) in sick neonates are controversially discussed. Specific considerations are required for preterm vs term neonates and should attend to the underlying cause of anaemia or thrombocytopenia.
Objectives
To evaluate the risk of transfusion‐associated necrotizing enterocolitis (TANEC), retinopathy (ROP), and other disorders causing long‐term sequelae in preterm and term neonates.
Results
The current meta‐analysis on liberal vs restrictive haemoglobin thresholds for RBC transfusions in very low birth weight (VLBW) infants exhibiting anaemia of prematurity does not indicate significant differences in the combined outcomes of death or serious morbidity at 18–21 months corrected age. Any trial eligible for this meta‐analysis did not support an association between RBC transfusion strategies and NEC [RR 1.62 (0.083, 3.13), Cochrane 2011]. However, in almost all retrospective case‐control or cohort studies a recent transfusion (<48 h) is significantly associated with NEC. The risk for TANEC increases as younger and lighter the neonate is, and if the baby is ventilated or exhibiting patent ductus arteriosus (PDA). The risk of TANEC seems to be inversely related to pre‐transfusion haematocrit levels. The risk for severe ROP (³ 3°) is as higher as earlier RBC are transfused. It also increases with the total number and cumulative volume of RBC transfusions. There is no evidence that RBC transfusion affect the incidence of intraventricular haemorrhage (IVH). Single studies link the risk of bronchopulmonary dysplasia (BPD) to the volume and frequency of RBC transfusion, while others do not show this association. Uncertainty exists regarding the incidence of RBC transfusion‐related acute lung injury (TRALI) in neonates, and whether it putatively contributes to severe BPD.
Besides the general risks for transmission of infectious diseases and errors in administration as in RBC transfusions, PLT transfusions to sick neonates could be associated with other specific complications such as microembolisms, which are repetitively considered causing TANEC, IVH or TRALI. However, such associations have not been proven in randomised controlled clinical trials (RCTs) yet. Noteably, repeated PLT transfusions seem to be associated with hepatic dysfunction following NEC. Likely, the experimental observation is very important that transfusion of adult platelets results in a dysbalance of the neonatal haemostatic system with shortened epinephrine closure times, eventually explaining a specific risk for microembolisms. Normally, hyporeactivity of neonatal platelets is part of a delicately balanced haemostatic system, which is counteracted by high haematocrit levels, higher von Willebrand factor (vWF) concentrations, and predominance of longer vWF polymers.
Conclusions
Currently, no international consensus exists on transfusion guidelines for RBC or PLT to preterm and term neonates. However, transfusion‐associated complications, such as TANEC, ROP among others per se affecting long‐term outcomes of sick neonates, deserve more attention in clinical practice and justify careful evaluation of RBC and PLT transfusion thresholds by performing RCTs.
3D‐S12‐02
A Systematic Review and Meta‐Analysis of Risks of Red Cell Transfusion for Neonatal Morbidities or Mortality
Keir AK1, Pal S 2, Trivella M 3, Lieberman L 4, Callum J 5, Shehata N 6, Stanworth S 7
1University of Adelaide, North Adelaide, SA, Australia2Rosie Neonatal Unit, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom3Centre for Statistics in Medicine, University of Oxford, Oxford, United Kingdom4Transfusion Medicine, University Health Network, Toronto, ON, Canada5Transfusion Medicine and Tissue Banks, Sunnybrook Health Sciences Centre, Toronto, ON, Canada6Department of Medicine & Laboratory Medicine Pathobiology, University of Toronto, ON, Toronto, Canada7NHS Blood & Transplant/Oxford University Hospitals NHS Trust, Oxford, United Kingdom
Background
There is increasing evidence to indicate harmful consequences of red blood cell (RBC) transfusion in adults but adverse effects in the neonatal population have been less studied. Potential adverse associations postulated to be related to RBC transfusions and unique to neonates include necrotising enterocolitis (NEC), intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP) and chronic lung disease (CLD). There has been no systematic evaluation of the broad randomised and non‐randomised literature to collate evidence of harm related to transfusions in neonates. The aim of this systematic review was to describe adverse effects and associations attributed to RBC transfusions in neonates.
Methods
Relevant studies were identified from searches of MEDLINE (PubMed including the Cochrane Library) and EMBASE. Eligible studies were those examining the effects of small volume (10–20 ml/kg) RBC transfusions on neonates and had at least one pre‐defined outcome relevant to the review. Two independent authors conducted abstract, then full‐text article reviews, as well as data extraction and quality assessment. The primary outcome was mortality; other outcomes included CLD, ROP, NEC, IVH and adverse neurodevelopmental outcome. Separate meta‐analyses, where possible, were conducted for the different types of study design.
Results
Our search identified 6296 abstracts with a total of 190 full‐text publications reviewed. 61 were deemed eligible for inclusion, including 16 (26%) randomised studies and 45 (74%) non‐randomised studies. Thirty‐three (54%) studies used a retrospective design, the most common study type. The majority of studies were conducted in the United States (n = 29, 48%), followed by Europe (n = 13, 21%), South America (n = 6, 10%). Most studies were undertaken at a single site (n = 41, 67%). No differences in mortality were found between restrictive and liberal transfusion practices in the pooled randomised trials (RR: 1.24, 95% CI: 0.89–1.672, I2 = 0%). No differences were found between restrictive and liberal transfusion practices for CLD (RR: 0.95, 95% CI: 0.82–1.10, I2 = 2%), ROP (RR: 0.88, 95% CI: 0.60–1.27, I2 = 0%), NEC (RR: 1.45, 95% CI: 0.91–2.33, I2 = 0%) and IVH (RR: 0.59, 95% CI: 0.25–1.44, I2 = 0%) in pooled randomised trials. The results for pooling of non‐randomised studies with comparator groups indicated considerable heterogeneity for the outcomes. Risk of bias assessments highlighted common limitations, including lack of controlling for potential confounding factors in many of the non‐randomised studies.
Conclusions
There was no evidence of significant differences in a range of clinical outcomes between neonates exposed to higher and lower volumes of RBC transfusion. However, despite the number of identified studies, issues including methodological quality limit the strength of any conclusions. Specifically, there is a pressing need for standardised definitions of adverse events relevant to neonatal transfusion practice, as well as consistent reporting in future studies.
3D‐S12‐03
Evaluating the Use of Fresh Frozen Plasma and Cryoprecipitate in Paediatric Intensive Care
Poo SXW1, Andre LA 2, Morley SLM 3
1University of Cambridge, Cambridge, United Kingdom2Great Ormond Street Hospital, London, United Kingdom3NHS Blood and Transplant, Cambridge University Hospitals, Cambridge, United Kingdom
Background
Fresh frozen plasma (FFP) and cryoprecipitate are commonly used in critical care, but its clinical usefulness has been strongly debated and its administration in some cases may be considered inappropriate. Current BCHS guidelines suggest that pre‐ and post‐transfusion coagulation parameters should be measured in order to reduce the number of inappropriate transfusions. Furthermore, studies on FFP and cryoprecipitate administration in the paediatric intensive care setting are limited.
Aims
We examined in detail the use of FFP and cryoprecipitate in critically ill children; particularly auditing pre‐ and post‐transfusion blood measurements in accordance with the guidelines. We aimed to review the outcomes associated with FFP/cryoprecipitate transfusion matched by dose per weight of patients, and evaluate the risk‐benefit ratio of FFP/cryoprecipitate transfusion in the paediatric intensive care (PICU) setting.
Methods
The TraCC Study prospectively audited the presence and treatment of coagulopathy in UK paediatric intensive care units (PICU) in a multicentre cohort over a period of 6 months. This study investigates the subgroup of children that received either FFP or cryoprecipitate. We identified which pre‐ and post‐ transfusion coagulation samples had been collected for each child. Specifically, coagulation parameters (prothrombin time [PT], activated partial thromboplastin time [APTT] and fibrinogen) pre‐ and post‐transfusion were identified.
Results
The TraCC study included data from 2295 admissions in total. 301 (13.1%) children received FFP and 160 (7.0%) received cryoprecipitate at some point during their PICU stay. We identified 656 FFP/cryoprecipitate transfusion episodes; for which 312 (48%) and 344 (52%) had coagulation measurements both pre‐ and post‐transfusion. Of 187/312 (60%) who received FFP only; PT, APTT, and fibrinogen results were improved in 120/185 (65%), 133/186 (72%), and 88/151 (58%) transfusions; remained the same in 15/185 (8%), 10/186 (5%), and 10/151 (7%) transfusions; but deteriorated in 53/185 (27%), 43/186 (23%), and 53/151 (35%) transfusions respectively. The mean improvements of PT, APTT and fibrinogen in those transfused with FFP were 3.9s, 28.2s and 0.4 g/l respectively. 57 (18%) received cryoprecipitate only; of whom, PT, APTT, and fibrinogen results were improved in 37/52 (71%), 30/54 (56%), and 40/52 (77%) transfusions; remained the same in 1/52 (2%), 1/54 (2%), and 1/52 (2%) transfusion; but deteriorated in 14/52 (27%), 23/54 (43%), and 11/52 (21%) transfusions respectively.
Summary/Conclusions
We present a subset analysis of data from a large multi‐centre audit of coagulopathy in critically ill children in UK (the TraCC study). We have investigated the change in coagulation markers pre‐ and post‐ FFP and cryoprecipitate transfusion in this group. Where pre‐ and post‐transfusion coagulation parameters were available, preliminary results suggest some modest improvements in these markers when FFP or cryoprecipitate were given in the majority of patients. The response to transfusion was, however, variable, with a significant cohort showing no improvement. The clinical significance of these changes remains unclear. The effectiveness and safety of FFP/cryoprecipitate transfusion should be investigated further in this group.
3D‐S12‐04
Babies Misbehaving
Crighton G1, Scarborough R 1, Savoia H 2, McQuilten Z 1, Williams B 3, Henry A 4, Cole S 5, Wood E 1
1Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Vic., Australia2The Royal Children's Hospital, Melbourne, Vic., Australia3Royal Brisbane and Women's Hospital, Brisbane, Qld, Australia4St George Hospital, Sydney, NSW, Australia5The Royal Women's Hospital, Melbourne, Vic., Australia
Background/Aims
Fetomaternal or neonatal alloimmune thrombocytopenia (NAIT) is a rare but important condition, which may cause severe fetal and neonatal thrombocytopenia and can result in catastrophic bleeding with devastating long term sequelae. The Australian NAIT registry was established in 2009 to collect consistent national data on NAIT cases, with the aims of better defining the incidence, natural history and clinical outcome of pregnancies and children with NAIT in Australia, providing information on treatment strategies being employed, and promoting further research into this condition.
Methods
NAIT cases were notified by treating clinicians, as a result of NAIT investigations performed by the Australian Red Cross Blood Service, by blood products requested by clinicians e.g. intravenous immunoglobulin (IVIG) and HPA‐matched platelets, and/or by families directly approaching the registry.
Results
Data collection commenced in March 2009. As of 1 January 2015, 81 cases (79 pregnancies) had been notified. Nine cases were excluded due to insufficient clinical or laboratory data, one case was excluded because of duplicate reporting and in 10 cases laboratory investigations did not support a diagnosis of NAIT.
Of the remaining 61 NAIT cases (59 pregnancies), there were 59 live births (30 female, 28 male and 1 unknown) and 2 fetal deaths from intracranial haemorrhage (ICH). Mean birth weight of neonates was 2757 g (range 1030–4340 g) and mean gestation at delivery was 36.8 weeks (range 27–42 weeks).
Most commonly NAIT was identified because of a past history of a NAIT‐affected baby (26/61, 43%). Of those with clinical features, skin bleeding (petechiae and bruising) was most frequently reported (18/61, 30%). Antenatal and post‐natal ICH were identified in (6/61, 9.8%). Incidental thrombocytopenia without clinical symptoms was seen in 8/61 (13%).
Anti‐HPA‐1a was the most common antibody detected, in 46 cases (80%), followed by anti‐HPA‐5b in 3 cases (5%).
Median first platelet counts of all neonates was 40 × 109/l (range 2–290) compared with 124 × 109/l (range 3–290) for the 25 neonates whose mothers had received antenatal treatment. Despite antenatal treatment, two babies had platelet counts of <20 × 109/l at delivery and clinical symptoms.
Twenty‐six neonates received a total of 58 post‐natal platelet transfusions (mean 2.2 platelet transfusions per neonate); four neonates received five or more platelet transfusions. Median platelet increment with random donor platelets (e.g. non HPA‐matched) was 56 × 109/l compared with 111 × 109/l with HPA‐matched platelets.
Twenty‐one neonates received post‐natal IVIG, with a mean dose 2.95 g (range 2–6 g) and a mean number of 1.35 doses per neonate. One neonate received platelets, IVIG and IV methylprednisolone 1 mg/kg for 5 days.
Conclusions
Data from the Australian NAIT registry reflect the complexity of NAIT diagnosis and management. Diagnosis of NAIT should take into account maternal history, neonatal clinical presentation, platelet count, as well as parental investigations. There was a significant risk of severe thrombocytopenia and ICH. HPA‐matched platelet transfusions appeared more effective in raising neonatal platelet counts, however random donor platelet transfusions provided sufficient platelet increments for clinical benefit.
3D‐S12‐05
Therapeutic Plasma Exchange (TPE) for Guillain‐Barré Syndrome (GBS): Outcomes and Tolerability in a Paediatric and Adult Cohort
Dhieb A 1, Menif H1, Lahiani W 1, Rekik H 1, Dammak M 2, Mhiri C 2, Gargouri J 1
1CRTS Sfax, 99/UR/08‐33, Faculté de médecine de Sfax, Université de Sfax, Sfax, Tunisia2Neurology Service, Faculté de médecine de Sfax, Sfax University, Sfax, Tunisia
Background
Management of GBS is a challenge for both paediatric and adult patients. One established first‐line treatment is TPE, yet many neurologists are reluctant to utilise TPE for paediatric patients.
Aims
To compare outcomes and tolerability of TPE in a paediatric and adult cohort with GBS.
Methods
We retrospectively (1997–2014) analysed data of 184 patients: 38 (21%) were under 14 years of age (x = 7) and 145 (79%) were over 14 years (x = 45). At inclusion, there was no significant difference between the two groups in; duration of disease evolution (x = 9.7 days (paediatrics) vs 11.5 days (adults); P0.1), functional grading (x = 4 (paediatrics) vs 3.8 (adults); P0.1). Children had significantly more mechanical ventilation (MV) (26.3 vs 8.9%; P = 0.003). PCS+ and MCS3P (Haemonetics) were used until 2006 which were then replaced with Cobe Spectra (Terumo BCT). Blood was used as a priming fluid in low weight children (under 44 pounds). Peripheral venous access was the preferred route for treatment. 1.2 plasma volume was exchanged.
Results
Total procedures numbered 675: 144 paediatric and 531 adults. Average number of procedures and exchanged volumes were: 3.7 (1–6) vs 3.6 (1–7); (P 0.5) and 1.5 l (0.3–3.4 l) vs 3.4 l (0.4–5.6 l). There was no difference between paediatric and adult patients in regard to procedural adverse events (AE) [e.g. hypotension, hypoglycemia, hypocalcemia, shivering, dyspnea, shock (10.4% vs 7.9%. P0.3)]. Central catheter access was significantly higher in children (89% vs 51%; P < 0.01) as well as AE related to vascular access (9.7% vs 3.7%; P < 0.01). When the Cobe Spectra was initiated, paediatric AE significantly (P < 0.001) reduced (9%) compared to PCS+ and MCS3P (63.6%); therefore became comparable to adults (7.4%; P0.5). Two children (5.3%) and 3 adults (2.1%) died from septicaemia (4 cases) and respiratory failure (1 case).
Both paediatric and adults significantly improved their immediate functional grading, (grading gain = 0.9 (range 0–2); P < 0.001 and 0.7 (0–2); P < 0.001). Even though fewer paediatric patients had an unchanged functional grading (27 vs 43% in adults), there was no significant difference in the functional grading gains between the two cohorts (P0.3). Extubation following the third procedure was more frequent in paediatrics (5 from 10 MV paediatric patients) than in adults (3 from 13 MV adult patients).
Discussion
There are very few reports of paediatric GBS patients treated with TPE. In our paediatric GBS cohort, disadvantages of TPE were: venous access problems and blood exposure in low weight patients. Benefits were; good tolerability (when the Cobe Spectra was used), earlier efficacy compared to IVIG and seemingly earlier extubation than in adults. Our paediatric GBS patients were more likely deferred to TPE when they had severe pulmonary forms, this could explain the higher death rates compared to our adults, and to the literature (<5%). However, no death was due to the procedure.
Conclusion
In limited resource countries, TPE for GBS should be considered as a treatment since it is effective and less costly. Lastly, patients with GBS in the ICU treated with TPE recovered sooner than those treated with IVIG.
Platelet Transfusion in Clinical Practice
3D‐S13‐01
Do All Patients with Haematological Malignancies Need Prophylactic Platelets?
Stanworth SJ1, Wood E 2, Wandt H 3
1NHS Blood & Transplant/Oxford University Hospitals and the University of Oxford, Oxford, United Kingdom2Transfusion Research Unit and Monash Medical Centre, Monash University, Melbourne, Vic., Australia3Paracelsius Medzinische Privatuniversität Nürnberg, Nürnberg, Germany
Early all patients, whether receiving treatment with autologous hematopoietic stem cell transplantation (autoHSCT), intensive chemotherapy, or allogeneic HSCT (alloHSCT), will receive one or more transfusions of platelets, most as prophylaxis. Optimal use of prophylactic platelet transfusions for prevention of haemorrhage in all patients with treatment induced myelosuppression remains controversial. Two randomised controlled trials of prophylactic platelet transfusions have recently been completed in adults with thrombocytopenia due to haematological malignancies or their treatment (Stanworth et al; Wandt et al). Both found a no‐prophylaxis approach led to higher rates of World Health Organization (WHO) grade 2–4 bleeding overall. A key question is whether all subgroups of patients benefited equally from prophylactic platelet transfusions. Pre‐specified sub‐group analysis in the Trial of Prophylactic Platelets (TOPPS) study found that the reduction in proportion of patients experiencing WHO grade 2–4 bleeds (main trial outcome) seen in the prophylaxis arm was of greater magnitude in chemotherapy/allogeneic hematopoietic stem cell transplantation (chemo/allo HSCT) than autologous transplant (autoHSCT) patients (interaction P = 0.04).
Further evaluation of the effectiveness of prophylactic platelet transfusions between sub‐groups was explored by attempting to combine data between these two trials, and earlier historical trials. However, in an updated systematic review, meta‐analysis of results was limited by methodology, including lack of consistency in reporting bleeding outcomes and different time periods for which bleeding was reported. Specifically, meta‐analysis of data for autoHSCT patients could not be performed as there was evidence of considerable statistical heterogeneity between these two studies; I² = 90%. Given the challenges of analyzing bleeding data, a recurrent event analysis was considered, which allows the investigation of all available bleeding data, rather than just focusing on measures such as rates of bleeding in patients or time to first bleeding event. Multivariate analyses of TOPPS trial data investigated patient factors and clinical characteristics associated with bleeding. Of 16,982 daily bleeding records analysed for 592 patients, grade 2–4 bleeding was reported in 700 (4%). Skin bruising was the most common type of bleed, reported on 32% of days when bleeding was reported to have occurred. Baseline characteristics associated with the number of days of bleeding were investigated: patients in the no‐prophylaxis arm of the study, patients who received a red cell transfusion in the previous 3 days, female patients and chemo/allo HSCT patients had the highest hazard of a grade 2–4 bleed. Additional analyses also found increased hazard of bleeding associated with the number of days with a platelet count >10 × 109/l for chemo/allo HSCT patients and high patient temperatures.
In summary, there is evidence that the effectiveness of prophylactic platelet transfusions differs between sub‐groups of patients with haematological malignancies, with chemo/allo HSCT patients receiving prophylactic platelet transfusions showing a greater reduction in bleeding outcomes compared to patients following a no‐prophylaxis policy; there is no evidence that a prophylactic policy for platelet transfusion is a superior policy for patients receiving autoHSCT. Since many patients continue to bleed despite transfusion of prophylactic platelet transfusions, additional strategies are required to prevent or minimise bleeding in all patient groups.
3D‐S13‐02
Plateletes Made HLA‐Deficient by Acid Treatment Aggregate Normally and Escape Destruction by Complement and Phagocytes in the Presence of HLA‐Antibodies
Meinke SM1, Mörtberg AM 2, Karlström CK 1, Sandgren PS 2, Refsum ER 1, Douagi ID 1, Taune-Wikman ATW 2, Höglund PH 1
1Karolinska Institutet, Stockholm, Sweden2Karolinska University Hospital, Stockholm, Sweden
Background
Platelet transfusions are commonly used to prevent or stop spontaneous bleedings in thrombocytopenic patients. Platelet transfusion refractoriness, the repeated failure to achieve an adequate post‐transfusion platelet count increment, is a problem encountered in some cases, especially with patients receiving high numbers of transfusions. In the cases where refractoriness is mediated by the immune system antibodies against the HLA class I antigen are the most common cause. Such antibodies bind to HLA class I on the surface of the transfused platelets and mediate their rapid clearance from the circulation. Lysis by activation of the complement system and phagocytosis by Fc receptors‐expressing cells are supposed to be the main mechanisms of antibody‐mediated platelet destruction.
The refractoriness of immunized patients can be overcome by transfusion of HLA‐matched donor platelets, but the complexity of the HLA system makes it difficult to find enough suitable donors for all patients. In addition, donor platelets for acute situations are not available. A different approach to solve this problem is to create platelets that lack HLA molecules on their surface in order to protect them from antibody‐mediated destruction after transfusion. The trimolecular complex of the HLA class I molecule is sensitive to acidic environments. A short treatment at low pH (pH 2.9) thus removes the bound peptide and the associated β2 microglobulin, leaving only a denatured heavy chain on the cell surface.
Seven cases of refractory patients treated with HLA stripped platelets have been reported, of which four were successful. Contradictory results have been published regarding the efficacy of HLA removal and function of the treated platelets. So far, it has also not been shown whether acid treatment protects platelets from complement activation and phagocytosis in the presence of anti‐HLA antibodies.
Aims
In this project we aim to investigate how the acid‐treatment affects platelet function and whether acid‐treated platelets are protected from HLA antibody‐mediated destruction.
Methods
We have set up in vitro assays to assess complement‐mediated lysis and phagocytosis by monocytes. Flow‐cytometric analysis of activation markers and other surface molecules, as well as in vitro aggregometry were used to test platelet functionality.
Results
A short acid treatment removed between 70% and 90% of the native HLA class I complexes from the platelet surface. Acid‐treated platelets showed an increased expression of activation markers, but still responded to stimulation with further up‐regulation reaching similar expression levels as stimulated untreated platelets. Their ability to aggregate in response to different stimulating agents was comparable to untreated platelets. Treated platelets were completely protected from complement lysis in the presence of anti‐HLA antibodies. Anti‐HLA antibody‐mediated phagocytosis was clearly reduced.
Summary/Conclusions
Treatment of platelets with citric acid removes native HLA class I complexes and reduces the extent of antibody‐mediated destruction, but does not abolish their function in vitro. We believe that acid‐treated platelets remain an option for transfusion to HLA‐immunized patients as an alternative to HLA‐matched donors.
3D‐S13‐03
Next Generation Sequencing to Facilitate Donor Selection Using HLA Epitope‐Based Matching for Patients Immunologically Refractory to Random Platelet Transfusions
Davey SR , Brown C , Navarrete C
NHS Blood and Transplant, London, United Kingdom
Background
Patients with immunological platelet refractoriness due to the presence of HLA antibodies can present a significant challenge to a transfusion service. Numerous strategies for the selection of platelets to support these patients are available including the use of cross‐match negative, of antigen avoidance and of HLA matched products. HLA epitope‐based matching (HEM) is a more recent approach based on compatibility of antigenic determinants, so called ‘eplets’. Although studies reported to date have relied on epitope predictions based on low resolution HLA types obtained using methods such as Luminex xMAP® technology, accurate comparison of donor and patient intra and interlocus HLA is likely to require high resolution or allele level typing currently obtained using Sanger sequencing based typing (SBT). However, SBT is not suitable for the high throughput required to HLA type platelet donors due to high costs and logistical constraints.
Aim
Next Generation Sequencing (NGS) is a recent revolution in sequencing technology with the capacity to produce large amounts of data relatively quickly and cheaply. The H&I laboratory at NHSBT Colindale has recently developed and implemented NGS technology for HLA class I and II typing adult and cord blood donors for the British Bone Marrow Registry. We investigated whether this technology would also be appropriate for HLA typing of platelet donors.
Method
Whole gene amplification of HLA‐A, ‐B and ‐C was performed on purified DNA obtained from 180 apheresis platelet donors previously HLA typed at low resolution. The locus specific PCR products for each sample were then combined to form pooled amplicon which was subsequently normalised and followed by enzyme digestion. Resulting fragments were ligated to Illumina compatible barcoded adapters before combining into a single DNA library which was sequenced on the MiSeq (Illumina). FASTQ files generated were analysed using HLA specific software.
Results
Good quality data was obtained for all 180 samples sequenced by the MiSeq with a final Q30 score of 77%. The number of sequences representing each nucleotide ranged from 23 to 10,637 with an average depth of coverage of 2234 per base. HLA types at allelic level to the second, third or fourth field resolution were obtained on all samples, with a success rate of 98%, 95% and 99% for HLA‐A, ‐B and ‐C respectively. Individual test failures were due to poor PCR amplification rather than sequencing issues.
Summary/Conclusion
We have developed a high throughput DNA NGS sequencing approach to successfully type a minimum of 180 platelet donor samples for HLA class I loci. Definition of HLA types at second field resolution or beyond enables accurate HLA epitope definition. The use of NGS for platelet donor typing will facilitate selection of donors using HEM and may also benefit those patients with defined allele specific HLA antibodies. With the current difficulties and high cost of recruiting apheresis donors, the use of HEM and NGS typed donors may be important tools for the continuous provision of well matched products for immunologically refractory patients.
3D‐S13‐04
Platelet Components Associated with Adverse Reactions: Predictive Value of Mitochondrial Dna Relative to Biological Response Modifiers
Cognasse FC 1, Cognasse FC 1, Aloui CA 2, Nguyen KAN 2, Hamzeh-Cognasse HHC 2, Fagan JF 1, Sebban MS 3, Fromont EF 3, Pozzetto BP 2, Laradi SL 1, Garraud OG 4
1French National Blood System (EFS), Saint‐Etienne, France2Université de Lyon, GIMAP‐EA3064, Saint Etienne, France3Laboratoire Hubert Curien – UMR CNRS 5516, Saint‐Etienne, France4Institut National de Transfusion Sanguine (INTS), Paris, France
Background
It is admitted that Biological Response Modifiers (BRMs) that are secreted by the platelets themselves during storage have some responsibility on Adverse Events (AEs). Mitochondrial DNA (mtDNA) amounts in Platelet Component (PC) recently proved to be also significantly associated with AEs.
Aims
We explored as whether those two markers, i.e. pathogenic BRMs and mtDNA, can correlatively be considered predictors of transfusion pathology.
Methods
We investigated a series of AEs reported after PC transfusions; clinical observations, and relevant biological data were subjected to a mathematical modelling system aimed at predicting rare events.
Results
We observed that mtDNA was consistently released during the first days of PC storage; however, significant mtDNA release was earlier in ‘pathogenic’ as compared to as non‐pathogenic (control) stored PCs. PC supernatants with consistent levels of mtDNA were significantly associated with occurrences of AEs; elevated soluble CD40‐Ligand (sCD40L) is also proved to be significantly associated with AE occurrences. However, mtDNA associated significantly with none of the many (17) BRM tested, indicating that the mtDNA participation to PC transfusion‐linked inflammation is independent on that of BRMs otherwise known to be associated with AEs. Shown here is evidence that platelets generate distinct pathogenic secretion profiles of BRMs and mtDNA. We indeed aimed to predict the risk of hazardous outcomes by establishing statistical models based on the associations of BRMs and mtDNA within the incriminated platelet components and using decision trees. The calculated Area Under the Curve (AUC) relative to mtDNA was highly significant, despite less stringently predictive than to sCD40L, standing as a standard predictor of AE. The established model further predicts that distinct natures of AEs can be distinguished, that are both dependant on mtDNA levels and on PC storage length.
Summary/Conclusions
It is proposed that full consideration is given to mtDNA in PCs to test for their propensity to generate inflammation.
Progress in Developing Countries
3D‐S14‐01
NHS Blood and Transplant (NHSBT): Equipment Redistribution Programme
Long S , Morgan SJ , Odumeru D
NHS Blood and Transplant, Filton, United Kingdom
Background
NHSBT provides a comprehensive blood service to England and North Wales and aims to be the best organisation of its type in the world. As part of our Social Responsibility programme, we redistribute equipment to other blood services in the international community who find the funding of such equipment difficult.
Aims
The consolidation of NHSBT's facilities, and changes in technologies used, have led to a surplus of equipment and consumables in good condition. Previously, NHSBT would have scrapped such items, but now seeks to redistribute those with a reasonable functioning lifespan remaining.
Methods
The key issues for the programme are:
storage of high volume/bulky equipment.
funding and sustainability of donations.
formalising the process.
Storage: NHSBT has leased four warehouse units next to the Filton blood centre in Bristol. The current stock includes large numbers of Strub mobile donation beds, Compomat G4 and Optipress blood separators.
Funding and Sustainability: As a UK public sector organisation, NHSBT must justify the funding of storage and shipment costs. Although NHSBT does fund some shipments to blood services with whom it has a pre‐existing relationship, it has also sought to minimise costs by expanding its network of partners with a similar interest in equipment redistribution. These vital partner organisations include Blood for Life Foundation, Global Blood Fun and Mercy Trucks UK.
Formalising the process: In 2011, a Deed of Gift was introduced. This document is used in support of all free of charge ‘gift’ donations, and comprises a simple legal contract transferring ownership.
Results
NHSBT has explored the benefit of bringing some stock into its own warehouses, but these storage facilities tend not to be co‐located with a blood centre, and there are difficulties with storing the equipment in the fixed warehouse racks. Therefore, centralised off‐site storage is currently used. The partner network has resulted in a significant increase in donation activity. Partners, rather than NHSBT can become the main link to the recipient, and in those circumstances they manage the logistics and funding arrangements. Since January 2012, NHSBT, in partnership or directly, has gifted equipment to:
1. Cambodia, Lesotho, St Lucia, Uganda (beds).
2. Jamaica, Malta, Nigeria, Malawi (bloodmobiles).
3. Grenada, Lebanon, St Lucia (CompoGuards).
4. Ghana, Jamaica, Nepal, Nigeria, Uganda, Zimbabwe (various ítems of equipment and consumables).
5. Malawi (beds and one bloodmobile).
On average NHSBT now makes a major donation every quarter. In 2014, NHSBT, Scottish National Blood Transfusion Service and the Global Blood Fund worked jointly to collect and ship equipment to Nigeria and the Lebanon. These recipient blood services have secured vehicles, equipment and consumables that they might otherwise have found it difficult to obtain. The consequent benefits for donors and patients served in those countries have been significant.
Summary/Conclusions
NHSBT will aim to accelerate the opportunities to redistribute equipment with a reasonable functioning lifespan, in partnership with appropriate charitable organisations, and if possible other European blood services.
3D‐S14‐02
Revised Research Priorities For Blood Services In Sub‐Saharan Africa
Bates I1, Hassall O 1, Mvere DA 2, Owusu-Ofori S 3, van Hulst R 4, Ullum H 5
1LSTM, Liverpool, United Kingdom2NBSZ, Harare, Zimbabwe3NBSG, Kumasi, Ghana4University of Groningen, Groningen, Netherlands Antilles5Rigshospitlet, Copenhagen, Denmark
Background
At a workshop in Mombasa (Kenya 2008), transfusion service stakeholders from sub‐Saharan Africa (SSA) identified and prioritised the research needed to generate evidence specific to their needs. Since then, technologies, approaches, resources and health challenges have emerged, and important evidence has been garnered from subsequent research. This means that, building on the platform provided, research priorities for blood services in the region now need to be revised and re‐defined.
Aims
The original priorities will be addressed and updated. Strategies will be formulated for disseminating these new priorities, and for identifying and approaching potential sources of funding.
Methods
A two‐day workshop was held in South Africa (March 2015). Participants from Europe, USA and across SSA brought together diverse perspectives to revise the Mombasa agenda by considering each of the original challenges. These were:
1. biological safety.
2. blood donors.
3. hospital management of blood and blood transfusions.
4. adequate supplies and equitable distribution.
5. transfusion systems and sustainable financing.
Results
Overarching themes stressed the need to harmonise research policy in SSA, and to share information and IT systems within institutions and across countries and regions. Audits should be established, and reasons for non‐adherence or under‐reporting addressed. Reporting should include not only best practice, but also adverse events, it should be encouraged within a non‐punitive regimen that feeds into quality improvement processes.
Education is also an important factor across the board. Engaging content in schools curricula and an awareness‐raising media presence ensure a sustainable donor base; continuing professional development involves staff in research, updates them on practice guidelines and provides acumen in finance skills; regulators will understand their roles and the importance of blood as an ‘essential medicine’.
Validated models of stock management should be assessed for their relevance to African blood services, and a pilot implemented in more than one country. Blood services should exploit existing commercial expertise of stock management and distribution models from beyond the health care system.
Financing of blood services and the effectiveness of different blood service models is an under‐researched area. Blood services do not know the vein‐to‐vein cost of a unit of blood, and there is a need to measure and possibly standardise this. Non‐donor funds must be secured from alternative sources, including tax levies and commercial sponsorship from local firms or multi‐nationals. More evidence is needed about auxiliary therapies as alternatives (or in addition) to transfusion.
Participants recognised the need to disseminate the revised research priorities widely and in a variety of academic and non‐academic media.
Conclusions
The blood service is a people business. Innovative incentives for donor recruitment and care are needed on one hand, with a model of ‘whole‐staff’ team training that rebrands blood safety as a desirable career option on the other.
Equally, data‐driven models and tools remain crucial to inform practice and a solid evidence base to influence policy makers. Metrics such as the disability‐adjusted life year (DALY) index and the implications of not building an efficient transfusion service should be recognised as part of any full economic evaluation.
3D‐S14‐03
Review of Blood Services Research Capacity: A Case of National Blood Service Zimbabwe
Mapako T1,2, Mafirakureva N 1,2, Mutenherwa M 2, Bvuma G 1, Mvere DA 1, Marowa LM 1, Emmanuel JC 1, Bates I 3
1National Blood Service Zimbabwe, Harare, Zimbabwe2University of Groningen, The Netherlands3Liverpool School of Tropical Medicine (LSTM), Liverpool, United Kingdom
Background
There is growing need for blood services in developing countries to commission their own research and generate evidence applicable to their situations. In 2008, representatives of blood services in Africa held a workshop in Mombasa, Kenya, that highlighted the lack of indigenous researchers and the dire need for sub‐Saharan Africa blood services to define and implement their own research agenda. The workshop identified research priority areas and subsequently an EU‐funded T‐REC research capacity programme was launched in Zimbabwe and Ghana in 2011. National Blood Service Zimbabwe (NBSZ) has over the past 20 years been actively engaged in research activities. However there is currently no generic research capacity assessment tool, which would allow NBSZ and other blood services to assess their research capacities. In this study the overall research capacity of NBSZ was evaluated based on adapted tool and also on lessons learnt from the evaluation of the 4‐year T‐REC project.
Methods
Structured discussions with 85 NBSZ stakeholders including students, staff, management, board members and external actors collaborating or regulating national research and Service activities were held. The ‘benchmark’ against which NBSZ's research capacity was reviewed was adapted from one used previously to review African universities’ research systems. Information on each topic was obtained from at least two different sources and any discrepancies were resolved through discussions with NBSZ Executive Management. Recommendations emanating from these discussions were validated during a debrief meeting with senior NBSZ staff and at a national stakeholder workshop.
Results
The review of NBSZ's research capacity highlighted the structures and documents that are already in place to support research activities. NBSZ has a dedicated research function that is supported by a Research and Development Policy and budgetary support. Overall, T‐REC was very successful in generating research interest and expertise among professional staff in NBSZ and in enhancing the research ethos across the whole Service. Key areas for improvement were noted, in particular the need for better dissemination of NBSZ's research priorities, closer ties with academics and their institutions, and higher visibility of NBSZ in the national research arena and with the Ministry of Health and Child Care.
Discussion and conclusions
NBSZ has a vibrant research programme that is continually being strengthened and the T‐REC project has assisted this. If the identified recommendations are implemented, this will further buttress the research capacity of NBSZ. Our results indicate that it is feasible to apply a structured process to review the research capacity of blood services covering the following eight themes: research strategies and policies; institutional support services and infrastructure; supporting funding applications; project management and control; research for careers and promotions; development of skills and knowledge for research; external promotion of research and national research engagement. It is recommended that the blood services can adopt this adapted tool to assist themselves to periodically objectively review their research capacity. Similar research capacity programmes such as T‐REC are critically needed for blood services and should be actively sought, established and maintained.
3D‐S14‐04
Hemophilia Care in Africa: Challenges and Opportunities
Diop SD
Universite Cheikh Anta Diop, Dakar Fann, Senegal
Hemophilia is a rare bleeding disorders found in 1/10,000 newborn around the world. Significant progress has been made on hemophilia care in developed world during these two last decades including early diagnosis, availability of safer clotting factors concentrates, introduction of prophylaxis regimen as the gold standard of treatment and development of long acting factor concentrates which allow more efficiency and compliance. However, this disease remains still unknown in many sub‐Saharan African countries where only <10–20% of expected number of patients is identified. From the 173,000 hemophilia cases reported to the World Federation of Hemophilia in 2012, only 3750 were from African countries, i.e. 2.16%. Regarding treatment of the identified patients, mean per capita Factor VIII and IX use was <0.1 IU in African countries whereas it is established that one IU of FVIII clotting factor concentrate per capita should be the target minimum for countries wishing to achieve optimal survival for the hemophilia population. These data reflects the big challenges with management of hemophilia in Africa and the different reasons are: poor awareness, inadequate diagnostic facilities and scarce factor concentrates for therapy.
However, support from governments in some middle income countries is increasing and allow some progress. In South Africa, it was reported between 2004 and 2007, an increase in the number of identified patients, the clinic visits and per capita factor usage increased from 0.65 to 0.95. Low doses prophylaxis in 3–5 years old children have also been tested in Tunisia, Algeria and Morocco and shows >90% reduction in bleeding. In Senegal, comparison of the cohort data of 140 patients between the period 1995–2003 and 2004–2012 show significant progress: 67.9% increase in new patient's identification, 11.3 years reduction in mean age at diagnosis (from 15.5 to 4.2 years), lower mortality rate (from 15.3% to 6.8%) and age at death evolved from 6.5 to 23.3 years. In Egypt, minipools of cryoprecipitate with solvent‐detergent treatment have been processed in a closed bag system to improve its viral safety.
The priorities in establishing services for hemophilia in Africa include increasing availability and safety of blood products, developing coagulation laboratories capable of reliably performing coagulation tests, setting up patients registries and follow up in dedicated centers, training of care providers, organizing patients to set up associations and developing lay and medical cooperation, education of affected people and their families, providing low‐cost factor concentrates, improving social awareness.
Keywords
Hemophilia, clotting factor concentrates, Hemostasis, cryoprecipitates.
3D‐S14‐05
Blood Safety vs Limited Financial Resources: Innovative Solution
Shabaan DE
Egyptian National Blood Transfusion Center, Cairo, Egypt
Background
Transfusion of blood is an important issue of any health care system. It is necessary to ensure that blood administered is 100% safe. Transfusion transmitted infections (TTIs) are the most complex problem faced by BTS. Annually, unsafe blood transfusions are estimated to cause approximately 20 million new infections of HBV, HCV and HIV collectively*.
In Egypt, as many developing countries, challenges faced by a BTS are represented mainly in high prevalence of TTIs together with limited financial and technical resources to try and implement new screening technologies ensuring safe blood.
In 2008, Egyptian National Blood Transfusion Services (ENBTS) conducted an evaluation study to support policy decision to include NAT as part of routine donor blood screening. This resulted in routine NAT screening in National Blood Transfusion Center (NBTC) by the end of 2008. This was shortly followed by centralization of NAT screening for another 2 regional blood transfusion centers (RBTCs)in NBTC.
Aim
Challenging limited resources to implement routine NAT screening all over ENBTS, through two additional NAT labs, one to cover screening in Delta region and the other in Upper Egypt.
Methods
The government alone cannot fulfill the demanding needs of funds for NAT screening all over ENBTS. Hence it became imperative to find innovative sources of finance, one of which was to initiate cooperation with NGOs and civil institutions.
In 2013 cooperation between Egyptian MoH represented by ENBTS and Misr Al Khair foundation (one of the major civil institutes in Egypt) took place. Misr Al Khair had launched a strong preventive health program ‘Egypt Free from Virus C’. A crucial aspect in it was raising the standards and capabilities of blood banks for early detection of TTIs and supplying safe blood. As a result, a protocol was signed between the two parties where Misr Al Khiair would donate needed funds for extension of NAT screening within the ENBTS to include both Delta and Upper Egypt.
An implementation plan was set to create one NAT lab in Tanta RBTC. NAT screening centralization from 5 RBTCs in Delta region would take place in this lab.
Misr Al Khair donated the needed funds for preparing NAT lab in Tanta RBTC to fit technical demands of NAT screening. This was followed by procurement of equipment and assays assisted by experts from NBTS. With this accomplished, an action plan was set up by NBTC for sufficient training of assigned staff in Tanta RBTC. A competency test was conducted to the trained staff to ensure efficient training.
Results
Routine NAT screening in Tanta RBTC on Panther Grifols System using ULTRIO ELITE assay started in November 2014. Total samples screened till February 2015 are 11182. 5 NAT yield samples were detected in 4 months.
Conclusion
Introducing NAT screening was a rational decision especially in a country with high prevalence of hepatitis infection such as Egypt.
Cooperation between the government and civil organizations could help overcome major challenges.
Proper logistic planning within a blood service helps in adaptation of creative solutions regarding safe screening against TTIs.
3D‐S14‐06
Regulation of Blood Transfusion Services in Islamabad, Pakistan: Islamabad Blood Transfusion Authority Experience
Waheed UW , Zaheer HAZ
Ministry of National Health Services, Islamabad, Pakistan
Background
Empowered and effective regulatory system is an essential pre‐requisite for an efficient and reliable blood transfusion service. The regulation of blood sector implies enactment and enforcement of laws and rules by the government for safe blood transfusion practices. Islamabad, capital of Pakistan has an estimated population of two million. The blood transfusion authority for Islamabad was established in 2005, through the ICT Blood Safety Ordinance promulgated in 2002. Following the devolution of the subject of Health to the provinces in 2011, under the 18th constitutional amendment, the Islamabad Blood Transfusion Authority (IBTA) was revived by the Ministry of National Health Services in October 2013.
Aim
To standardize and regulate the blood transfusion services in Islamabadas peron the internationally recommended blood transfusion system model under the ICT Blood Safety Ordinance No. LXXIII of 2002.
Methods
Following its reveal in 2013, the IBTA developed a plan of action which was endorsed by its Advisory Board. The Plan laid out the framework of the scope of activities. This was followed by development of an evaluation tool for the inspection of the blood banks and a group of eminent experts were identified to act as IBTA inspectors. The inspection tool prepared was pre‐tested and revised accordingly. Announcements were published in the local press for receiving applications for registration and licensing. In some cases, reluctance was observed, for various reasons, among known partners to apply to IBTA. Many of them were fearful or reluctant to have inspections of their blood bank but effective advocacy, technical guidance, persuasion and reassurance ensured their cooperation. A series of capacity building activities were conducted to improve the standard of the technical and administrative human resource of the public and private sector blood banks in the Islamabad Capital Territory.
Results
The inspections of 23 blood banks were conducted in the year 2014 and licenses granted to 12 successful blood banks, 7 blood banks were placed on probation (to improve their deficiencies) while 4 have been closed down. At the end of the year, blood bank data was also collected from all the centers, compiled and analysed. Remarkably, the response from most of the blood banks was very encouraging and they invested considerable resources to fulfill their deficiencies and gaps and significantly upgraded their centres to meet the IBTA licensing requirements.
Conclusion
The Authority has shown significant achievements in a very short period of time. Continued strengthening of the Authority in the year 2015 will further improve the blood safety standards in the Federal Capital which will be emulated in the other provinces. Collaboration between IBTA and the provincial BTAs will go a long way in improving the standard of blood safety in Pakistan.
Donor Health
3D‐S15‐01
Effective Creation of Biomedical Research Infrastructure – Future Role of Blood Banks
Ullum HU1, Pedersen OBP 2, Sørensen ES 1, Petersen MSP 3, Nielsen KRN 4, Bruun MTB 5, Burgdorf KSB 1, Rostgaard KR 6, Thørner LWT 1, Hjalgrim HHJ 6, Erikstrup CE 3
1Rigshospitalet, København Ø, Denmark2Deaprtment of Clinical Immunology, Naestved Sygehus, Naestved, Denmark3Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark4Department of Clinical Immunology, Aalborg University Hospital, Aalborg, Denmark5Department of Clinical Immunology, Odense University Hospital, Odense, Denmark6Department for Epidemiological Research, Statens Serum Institut, Copenhagen, Denmark
Background
To improve medical therapy and prevention, results from high quality health research are needed. The investments in research infrastructure are often enormous for large scale human health studies. Blood donors are known to have a better health profile compared to the background population. However, in cause of time blood donors develop common diseases like any other population. Blood donors are generally willing to contribute to medical research and the infrastructure already available in blood banks closely resembles the infrastructure needed for large scale population studies. We suggest that provision of research infrastructure could be an additional key component of future blood bank services.
Aims
The Danish Blood Donor Study (DBDS) was established to study both health effects of blood donation, general health problems, and to establish a large prospective cohort for medical research.
Methods
Since March 2010 we have invited blood donors to participate in DBDS by giving informed consent and filling a four page questionnaire. We have access to plasma on index donation and on previous and subsequent donations. DNA is available and participant files are linked to national health registers with complete data on physical and mental health by relevant ICD‐10 codes in the Danish National Patient Registry and ATC‐codes in the Danish Prescription Registry.
Results
Approximately 98,000 participants are included in DBDS with 323,000 years of follow‐up. We have previously described a very high prevalence of iron depletion among our participants. Iron depletion is closely linked to subsequent drop in hemoglobin but not to self‐perceived health. To further study the potential health effects of iron depletion (restless legs syndrome and attention deficit hyperactivity disorder symptoms) as well as more general health issues such as (allergy/asthma, depression, hidradenitis) among donors a second questionnaire have been developed utilizing tablet computers. Because blood donors very often return to donate the DBDS platform provides a unique possibility for collection of additional bio‐specimens and information. There are currently 613,700 consecutive plasma samples and 20,000 extracted DNA samples available for further research. Among the participants, an estimated 1726 individuals have developed cancers (304 breast cancers, 112 colon cancers, 54 lung cancers, 156 prostate cancers, and 207 cases of malignant melanoma), 537 have had myocardial infarction, and 243 have been admitted to hospital with depression. There is linkage to 691,000 individual prescriptions of medicine with anti‐conception, antibiotics, and painkillers (NSAIDs) as the three largest groups of medication. By using infrastructure already available the costs per included participant is below 10 Euro. A large number of internal and collaborative projects are ongoing and The European Consortium for Blood Donor Health has been established together with other European blood donor research groups.
Summary/Conclusions
We have proven that blood bank infrastructure is ideal to perform large scale general health studies. Blood donors are content to expand their relationship from donating blood only to also donate knowledge in the quest for better therapies and preventions for future patients.
3D‐S15‐02
Development of Standard Definitions for Surveillance of Complications Related to Blood Donation
Goldman M1, Land K 2, Robillard P 3, Tomasulo P 2, Wiersum-Osselton J 4
1Canadian Blood Services, Ottawa, ON, Canada2Blood Systems, San Antonio, TX, United States of America3Hema‐Quebec, Montreal, QC, Canada4Sanquin Blood Supply, Amsterdam, The Netherlands
Background
Donor adverse reactions may lead to a poor donation experience, reduced likelihood of repeat donation, and rarely, donor injury or permanent disability. Clear definitions of donor reactions are important to each blood centre to establish baseline rates, evaluate risk factors and assess the impact of mitigating strategies or new collection procedures. Internationally, the use of comparable definitions would allow for comparison and benchmarking among blood systems, and data entry into haemovigilance systems such as AABB DonorHART.
Aims
We aimed to provide:
Simple definitions that are easy to apply in a standard way.
Minimal requirements for international comparison of reaction rates that meet the needs of a basic surveillance program.
Additional attributes that may be collected if possible that might be important for process improvement or lead to relevant research.
Methods
After discussion of basic aims, a revision subcommittee of the ISBT Working Group on Donor Vigilance was tasked with revising the 2008 ISBT standards for surveillance of complications related to blood donation. Classification schemes and definitions used in various jurisdictions were compared, and a draft version of the revised standard was circulated to members of the ISBT Working Group and the AABB Donor Haemovigilance Working Group. Comments were also obtained from other industry groups, such as the Alliance of Blood Operators (ABO) Medical Group, and the European Blood Alliance (EBA).
Results
A final version of the standard for surveillance of complications related to blood donation was developed and endorsed by the ISBT, AABB Haemovigilance committee, IHN, EBA, and ABO Medical Group (see table for list of categories, optional categories are shown in italics). Recommended information about each reaction and all donations in the blood system (numerator and denominator data) was also specified, in addition to basic information required about each jurisdiction's criteria (available on http://www.isbtweb.org, Haemovigilance Working Party section, definitions). Adjustments were made to the AABB haemovigilance definitions and to DonorHART to more easily align with the ISBT revised classification system.

Conclusions
A revised classification was developed and has already gained wide adoption.
3D‐S15‐03
Assessing the Impact of Donor Anxiety on the Yield of Apheresis Platelets. A Psychological Study
Elci EE 1, Ertas ZE 1, Eker IE 1, Cetin ATC 1, Gursel OG 1, Yilmaz SY 1, Cetinkaya RAC 2, Unlu AU 1, Savasci US 1, Kavacik MK 1, Yilmaz SY 1, Balikci AB 1, Acikel CA 1, Avci IYA1
1Gulhane Military Academy of Medicine, Ankara, Turkey2Military Hospital, Isparta, Turkey
Background
Most of the studies in the literature about the anxiety of plateletpheresis donors, are regarding to the effect and implication of this anxiety on further donations. The impact of this anxiety on the quality of the product is unknown.
Aims
We investigated the extent of donor trait anxiety levels and state anxiety levels during the donation and their effect on the yield of apheresis platelets. We hypothesized that anxiety of plateletpheresis donor creates greater product quality.
Methods
To examine this hypothesis, a prospective and descriptive cohort study was conducted with 63 voluntary, healthy, male plateletpheresis donors, without any psychiatric disturbance, who admitted to Blood Centre of Gulhane Military Academy of Medicine between 02 February 2015 and 02 March 2015. Trait Anxiety Inventory (TAI) and State Anxiety Invertory (SAI) were assessed among these donors before the donations. All plateletpheresises were performed by the same cell separator and the same technician.
Results
Median age of the donors’ was 25 (19–52) years and median frequency of donation was 2 (1–30). The mean scores of donors’ from SAI and TAI were 28 ± 7.2 (20–52) and 36 ± 7.7 (16–54) respectively. Mean platelet count of donors’ was 255 × 103 ± 50 (173–424)/μl, and mean platelet yield was 5.62 ± 1.1 × 1011 (4.3–7.5 × 1011) platelets. There was a significant positivelinear correlation between mean platelet yield and mean SAI score (Pearson r = 0.273; P = 0.03), as was between the mean TAI score and mean yield platelets (Pearson r = 0.404; P = 0.001). Both of the mean SAI score and mean TAI score were significantly positively correlated also with the mean platelet count (Pearson r = 0.392; P = 0.001 and Pearson r = 0.395; P = 0.001 respectively) and the mean leukocyte count (Pearson r = 0.375; P = 0.002 and Pearson r = 0.261; P = 0.039 respectively) of the donors’. The SAI scores of the donors’ who have high TAI scores was also high, in such a manner that there was a significant positive linear correlation between mean TAI score and mean SAI score (Pearson r = 0.594; P = 0.0001). Correlation with frequency of donation and SAI scores and also TAI scores of the donors’ was not found. The association of preapheresis variables associated with platelet yield including age, weight, height, body mass index, vital signs, donation frequency, socioeconomic status, sport, cigarette, SAI score, TAI score and baseline platelet count of the donor was analyzed by multivariate linear regression, using a backward elimination procedure. The baseline platelet count of the donor was an independent factor associated with platelet count in the multivariate model.
Conclusions
To our knowledge, this is the first study that assesses the impact of donor anxiety on the yield of apheresis platelets. Our results suggest that stress enhances platelet count, reactivity and immune‐modulatory capacity. Catecholamine induced platelet activation may explain the underlying mechanism. Further studies needed to evaluate also platelet activation and secretion among with the yield of platelets, associated with donor anxiety and the clinical consequences of these situations.
3D‐S15‐04
Emerging Trends in Serious Adverse Events of Donation in NHSBT 2010–2014
Anderson N , Barnes S , Hill L , Miflin G
NHS Blood and Transplant, Bristol, United Kingdom
Background
As part of the internal haemovigilance programme, NHSBT records Serious Adverse Events of Donation (SAED) in seven categories defined in the table below. These are broadly in line with the International Haemovigilance Network classification. For each recorded event, a full root cause analysis is undertaken to determine the likelihood that the event was related to the blood donation and also to identify any errors in the blood donation process that could have contributed to the adverse event occurring.
Aim
An analysis of reported SAEDS in NHSBT between January 2010 and December 2014 to identify any emerging trends in the event types, the likelihood of a relationship to blood donation and improvements that could be made to prevent them occurring.
Methods
An analysis of all SAED reports logged between January 2010 and December 2014 inclusive was undertaken.
Results
A total of 162 incidents were reported during the 5 year period.
Of the 162 reported events, 104 were in the top three imputibility groups, 70 definitely, 19 probably and 15 possibly, linked to the blood donation process. Neither of the deaths was related to blood donation. The trends have remained stable over the 5 year period but in 2013 there was an increased incidence of problems relating to needle insertion persisting for more than 1 year and fractures within 24 h of donation.

Caption 1: SAED reports logged between January 2010 and December 2014.
Conclusions
It is possible that donors are being encouraged to report long term problems with their arms by litigation experts promising a no‐win‐no‐fee arrangement. There is anecdotal evidence that the increase in 2013 maybe related to a change in the training programme for venepunctures in 2012. Measures have been implemented to review and improve the training given to staff performing venepunctures to reduce these problems. Many of the adverse events related to delayed vasovagal events occurring after the donor has left the session. The causes of delayed vasovagal events remain unclear and as such there is no evidenced based action plan for their reduction unlike vasovagal events that occur before, during of immediately after donation.
3D‐S15‐05
Two Decades of Risk Factors and Transfusion‐Transmissible Infections in Dutch Blood Donors
van de Laar TJW , Slot E , Janssen MP , Marijt-van der Kreek T , Zaaijer HL
Sanquin Blood Supply Foundation, Amsterdam, The Netherlands
Background
Risk behaviour based donor selection is widely used to mitigate the risk of transfusion transmissible infections (TTIs). Growing social opposition causes heavy debate on the need and effectivity of general donor deferral policies, especially in countries with low residual risks of infection.
Aims
To analyse trends in TTIs among Dutch blood donors, together with self‐reported risk behaviour of these infected donors during 20 years of routine donor screening.
Methods
Participants include all Dutch new and repeat donors who tested positive for HBV, HCV, HIV‐1/2, HTLV‐1/2 or syphilis during 1995–2014. Infected donors were interviewed by trained counsellors using a standardised questionnaire to identify the most likely route of transmission. Changes in the prevalence and incidence of TTIs were analysed using binomial regression models, differences in self‐reported risk behaviour between reported decades were determined using chi‐square tests.
Results
972 new donors and 381 repeat donors tested confirmed positive for one or more TTIs. Over the last 5 years, the prevalence of TTIs was 39 (HBV), 16 (HCV), 2.4 (HIV), 4.2 (HTLV) and 28 (syphilis) per 100.000 new donors. The prevalence of HBV and HCV decreased over the period 1995–2014 by 1.7% (P = 0.04) and 3.9% (P = 0.001) each year, syphilis showed a stepwise 50% increase after 2002 (P = 0.04). Repeat donors had stable but lower rates of TTI compared to new donors: during the last 5 years the ratio of infection in new vs repeat donors was 40:1 (HBV), 230:1 (HCV), 4:1 (HIV), 25:1 (HTLV), and 16:1 (syphilis). During the study period, the percentage of infected donors who were interviewed on risk‐behaviour increased from 70% to 93% (P < 0.001). In new donors, each TTI had its own typical risk profile: ‘blood‐blood’ for HCV, ‘sexual’ for HIV and syphilis and ‘endemic/country of birth’ for HBV and HTLV. Immigrants were responsible for 56% of TTIs among new donors. In contrast, 86% of infected repeat donors were indigenous Dutch, and sexual risk factors predominated for all TTIs. Male‐to‐male sexual contact was responsible for 35%, 20% and 15% of incident HIV, HBV and syphilis infections. Of infected repeat donors, 41% reported no blood‐related, sexual or endemic risk factors in the post‐test risk behaviour assessment, and 26% of infected repeat donors reported risk factors which, if disclosed during the pre‐donation interview, would have caused permanent exclusion from donation.
Summary/Conclusions
The prevalence and incidence of TTIs among Dutch blood donors remain low compared to donor populations in other western countries, and are 6–60 fold lower than their estimates in the adult general population of the Netherlands. Self‐reported risk factors are in line with the major routes of transmission in the general population, and did not significantly change over time. Full compliance to the donor health questionnaire would reduce the observed number of TTIs among Dutch donors with at least 26%.
Erythroid Regulation
4A‐S16‐01
Unraveling of the Role of Long Non‐Coding RNAs in Haematopoiesis
Alvarez-Dominguez JR , Lodish HF
Whitehead Institute of Biomedical Research, Cambridge, MA, United States of America
Hematopoiesis comprises a highly coordinated hierarchy of well‐defined cell state transitions. All hematopoietic effector cells ‐erythrocytes, myelocytes, and lymphocytes‐ derive from hematopoietic stem cells (HSCs) through a cascade of cell lineage specification, proliferation, and differentiation events. These events are directed by networks of interacting transcription factors, chromatin modifiers and regulatory RNAs. Recent evidence indicates that special types of RNAs, long non‐coding RNAs, are critical components of these networks.
lncRNAs resemble mRNAs in their biogenesis and regulation, yet do not function through encoded protein products. Instead, lncRNAs have the ability to recruit and regulate the activity of TFs and chromatin modifiers, organize chromosomal domains, and modulate mRNA splicing, translation, and degradation. Some lncRNAs are required for life, whereas others fulfill specialized roles in modulating proper formation of specialized cell lineages.
An analysis of the expression of lncRNAs across hematopoietic lineages reveals many with exquisite lineage specificity, suggesting that distinct collections of lncRNAs modulate formation or functioning of distinct blood cell types. Indeed, recent studies have described several lncRNAs that regulate blood cell formation through epigenetic control of gene expression, an emerging theme among functional lncRNAs.
The lncRNA H19, which contributes to growth control during embryogenesis, regulates adult HSC quiescence. Deletion of H19 leads to increased HSC activation and proliferation and impairs repopulating ability. This is mediated by de‐repression of Igf2 transcription and by increased Igf1r translation, resulting in increased signaling through the Igf1r.
Another well‐known lncRNA, Xist, regulates X chromosome dosage in females and its loss leads to blood cancer. Genetic deletion of Xist in HSCs leads to a myeloproliferative neoplasm and myelodysplastic syndrome (MPN/MDS), which is lethal at 100% penetrance in homozygote carriers. These effects emerge from altered dosage of X chromosome‐encoded oncogenes and tumor suppressors implicated in MPN and MDS, including the key myeloid transcription factor GATA1.
In lymphoid cells, the lncRNAs NeST and lincRNA‐Cox2 modulate expression of immune response genes downstream of inflammatory signaling via recruitment of chromatin modifiers, thus influencing the outcome of bacterial infection. Another lncRNA, Dleu2, is causally linked to B cell chronic lymphocytic leukemia (CLL). Loss of Dleu2 leads to monoclonal B cell lymphocytosis, which in some cases advances to diffuse large B cell lymphoma. Recent studies link Dleu2 to the in cis repression of gene neighbors that stimulate NF‐kB signaling, thus preventing CLL cell apoptosis.
In erythroid cells, we have described a dozen lncRNAs that are critical for proper formation of mature, enucleated red cells. One of them, lincRNA‐EPS, protects erythroid progenitors from apoptosis triggered by erythropoietin starvation. lincRNA‐EPS acts in the nucleus by repressing gene expression downstream of pro‐apoptotic signaling, most prominently the caspase activating protein Pycard.
Thus, lncRNAs are increasingly recognized as important players during blood cell development. In some cases, dysregulation of an lncRNA alone leads to malignant hematopoiesis, directly implicating lncRNAs in cancers such as leukemia. These observations highlight the clinical relevance of lncRNAs not only as useful markers for diagnosis and prognosis of cancers of the blood, but also as potential targets for novel therapies.
4A‐S16‐02
Mirna Regulation of Blood Group AB0 Genes
Kronstein-Wiedemann R 1, Milanov P 2, Gubbe K 1, Seifried E 2, Tonn T3
1German Red Cross Blood Donation Service North East, Dresden, Germany2German Red Cross Blood Donation Service Baden‐Württemberg‐Hessen, Frankfurt, Germany3Institute of Transfusion Medicine, Medical Faculty Carl Gustav Carus, TU Dresde, Dresden, Germany
The molecular genetic basis of the AB0 system has been known since 1990. More than 100 AB0 subgroup‐related variations were detected in the coding region of glycosyltransferases consisting of seven exons, resulting in weak blood group antigen expressions. Beside introns and exons, two regions were found to be critical for transcriptional activity of AB0 genes: a promotor sequence upstream of the translation start site and a CBF/NF‐Y‐binding enhancer element. However, the molecular mechanisms underlying some subgroups could not be explained by current methods. Here we asked, whether microRNA′s may play a role in the regulation of blood group A and B antigens. MicroRNAs are small noncoding RNAs that bind to the 3′ untranslated region (UTR) of target mRNAs resulting in translation repression or mRNA degradation. There is a lot of evidence for a role of miRNAs in the regulation of erythropoiesis, for example: positive regulation of terminal differentiation or negative regulation of early erythroid proliferation. Analysis of different databases (e.g. TargetScan) for matching miRNAs to the glycosyltransferases A and B, respectively, also indicates a potential role for microRNAs in the regulation of blood group antigens. MiRNA from homozygous and heterozygous carriers of different AB0 blood groups where isolated from the red blood cells of healthy blood donors and the presence of miRNA′s was analyzed using Nanostring technology. We found ~ 200 miRNA's to be present in mature red blood cells from healthy donors with partially different expression patterns depending on the blood group genotype. Most of miRNA's with potential binding sites for glycosyltransferases were not or only weakly expressed in mature red blood cells. Nevertheless miRNA‐331‐3p was significantly enhanced in red blood cells of Aweak variants. Sequencing of the 3′ untranslated region of 6 Aweak variants revealed the presence of more miRNA binding sites for certain miRNAs (miRNA‐1908) compared to normal controls. To evaluate whether glycosyltransferase A is a direct target of these miRNAs, DNA sequences of the 3′UTR of the respective blood group A glycosyltransferase and of weak blood group A variants were cloned into a luciferase expression system and co‐transfected with relevant miRNA's into K562 cells. Overexpression of miRNA‐331 and ‐1908 was able to reduce the expression of the respective transgene by 25–40% and the 3′UTR of the Aweak variant leads to a further reduction of luciferase expression of 10 ± 6%. Realtime PCR at different time points of erythroid differentiation shows a significant down regulation of relevant miRNA's in the first 5 days. Therefore primary hematopoietic CD34 stem cells were transduced with lentiviral miRNA expressing vectors and differentiated to red blood cells. MiRNA‐331 overexpression resulted in 10% blood group A negative red blood cells at day 8 of culture and a reduction in mean receptor expression by ~40%.This new concept of microRNAs as regulators of blood group glycosyltransferase expression may for the first time provide an explanation of the molecular basis for weak blood group receptor expressions in previously unexplainable Aweak phenotypes and may also play a role in regulation of other blood group variants (Rhesus, KELL).
4A‐S16‐03
The Expression Level Polymorphism of Ok Antigen/BSG Protein Highly Correlates with Recombinant PfRh5 Binding
Ye L , Zhao F , Han S , Yang Q , Zhu Z
Shanghai Blood Center, Shanghai, China
Background
Recently, basigin (BSG) which carries the OK antigens is found to be the receptor for Plasmodium falciparum reticulocyte‐binding protein homologue 5 (PfRh5). The PfRh5‐BSG interaction is essential for erythrocyte invasion by P. falciparum. The invasion of P. falciparum strains into BSG‐knockdown erythrocytes induced by shRNA was significantly reduced when erythrocytes showed a knockdown to 50–60% of BSG levels. The Ok(a−) erythrocytes also showed reduced invasion. However, because the Ok(a−) phenotype is extremely rare, it is still to be determined what kind of BSG polymorphisms are related to protection against P. falciparum malaria.
Aims
We aim to find out whether there is any individual difference in OK antigen/BSG protein expression levels on RBC and its potential impact on PfRh5 binding, as well as possible molecular mechanisms underlying differential expression.
Methods
Blood samples from random healthy donors were collected. Flow cytometry was used for quantitation of BSG and OK1 antigen. Sequencing of all BSG exons and UTRs was performed as reported. BSG mRNA level was analyzed by qRT‐PCR. The recombinant PfRh5 from the P. falciparum clone 3D7 was expressed with a C‐terminal 6‐histidine tag by inserted into the prokaryotic expression vector pEASY‐E2, then refolded, purified, and characterized. We developed an ELISA‐based erythrocyte binding assay (EBA) to measure the binding ability of recombinant PfRh5 to the surface of erythrocytes. Standard EBA was also performed for confirmation. All experimental data used for statistical analyses were obtained from three repeated experiments. MicroRNA sequencing and bioinformatics analyses were performed on one specific sample and its corresponding control.
Results
A total of 506 random blood samples were analyzed by flow cytometry. The OK antigen/BSG protein expression levels on erythrocytes vary among individuals. The ratio of the highest BSG expression level to the lowest level was 2.796, P = 0.003. 27 samples with diverse expression of OK/BSG were used for ELISA‐based EBA. There was significant correlation between recombinant PfRh5 binding and OK/BSG levels (R2 = 0.8245, Fig. 1). 43 samples were sequenced, including 12 samples with low expression and 6 samples with high expression. No SNP was found to correlate with the OK/BSG expression diversity. Furthermore, we analyzed BSG mRNA level in 11 samples, including 4 samples with low expression and 4 samples with high expression. No significant difference was found except one specific sample, whose BSG mRNA was remarkably up‐regulated (P < 0.001) but with low BSG protein expression. MicroRNA sequencing and bioinformatics analyses revealed that three microRNAs (miR‐15b‐5p, miR‐99b‐5p, and miR‐501‐3p) might be candidate regulators of BSG gene.

Fig. 1. ELISA‐based erythrocyte‐binding assay.
Conclusions
To the best of our knowledge, this is the first to describe the correlation between the OK/BSG protein expression diversity in random population samples and recombinant PfRh5 binding, suggesting differential expression of BSG protein might has potential relativity with erythrocyte invasion caused by P. falciparum. Although the genetic mechanisms of differential expression of OK/BSG have not been fully explained, our results obtained from a small amount of samples suggested post‐transcriptional regulation might be an important part. However, the biological functions of three candidate microRNAs are still needed to be confirmed.
4A‐S16‐04
Altered D Antigen Expression in a Patient with a Single‐Nucleotide Polymorphism in a GATA motif of the RHD Gene Promoter
Pambrun C 1, McAuley C 1, Sood C 2, Ochoa G2
1Dalhousie University, Halifax, NS, Canada2Progenika‐Grifols, Medford, MA, United States of America
Background
Over 400 RHD variant alleles have been reported to date. Most of them consist of single‐nucleotide polymorphisms in the coding sequence of the gene or at splice junctions. Polymorphisms in the promoter region, on the other hand, are very rare (1). Several transcription elements have been identified in the RHCE promoter, among them three binding motifs for the GATA‐1 transcription factor: two inverted motifs located at positions −454 to −449 and −2 to 3 from the transcription start site, and one motif located at positions −29 to −24 (2). These motifs are conserved in the RHD gene. A polymorphism in the GATA motif of the FY gene promoter has long been known to abrogate expression of Fya and Fyb antigens in erythroid cells (3). But similar findings have not been reported for Rh antigens.
Aims
To identify the molecular basis of discrepant RBC serological reactions with anti‐D reagents in a prenatal patient of Black and Caucasian mixed background.
Methods
RBC D antigen typing was performed on the ORTHO ProVue®, and by tube with both Immucor Series 5 and DBL Novaclone antisera. Genotyping was done on the Progenika/Grifols BLOODchip Reference microarray. DNA sequencing was performed by the Sanger dideoxy method with RHD‐specific primers spanning ~130 bp of 5'UTR, exon 1 and ~60 bp at the 5’ end of intron 1.
Results
Serological D typing reactions had a strength between 1+ and 4+, all showing a mixed‐field pattern (Table 1). Genotyping did not find any of the interrogated RHD variants, and detected common RHCE*Ce and RHCE*ce alleles. DNA sequencing detected a likely hemizygous A>C change at position −29 of the −29 to −24 GATA motif (AGATAA>CGATAA), which corresponds to position −115 from the start codon. No other changes were found in any of the exons or flanking intron regions of the gene. The allele defined by these changes, which we have named RHD*-155C, is likely present in hemizygosity and in cis with RHCE*Ce, as inferred from the genotype results as well as RHCE allele frequencies in Blacks and Caucasians.
Table 1.

Summary/Conclusions
Here we report the identification of a single‐nucleotide polymorphism within a GATA motif in the RHD promoter region in a prenatal patient with serologically weakened‐to‐normal mixed‐field agglutination reactions. This type of reaction may be explained by differences in transcriptional activity among RBCs. Family studies and in vitro studies are under way to gather evidence for a cause‐effect relationship between the RHD*-115C genotype and the altered D phenotype, as well as the incidence of alloimmunization among affected family members.
References
1. http://www.ncbi.nlm.nih.gov/nuccore/HE657774.
2. Cherif‐Zahar et al. (1994) Genomics, vol.19, p.68.
3. Tournamille et al. (1995) Nat. Genet., vol.10, p.224. Zimmerman et al. (1999) PNAS, vol.96, p.13973.
Pulmonary Adverse Effects
4A‐S17‐01
Good Bye TRALI, Good Morning TACO
Renaudier PR
Agence Régionale de Santé, Nancy, France
Pulmonary edema definition is based on pathophysiology <i.e. intra‐alveolar accumulation of fluid. The fluid is an exudate in hemodynamic edema and a transudate in lesional edema. The occurrence during or after a transfusion define respectively TRALI and TACO. The association with other etiological factors allows imputability. For 10 years, the attention was focused on TRALI. The choice of male or female donors nulliparous or absence of anti‐HLA/HNA helps prevent immune TRALI; but non immune TRALI persist. Pulmonary adverse effects observed in hemovigilance systems are now mostly TACO, some of which are serious and sometimes fatal. Pathophysiology of TACO has to be better understood at that point. Two points should be noted: (i)the distribution volume of a blood product is the intravascular space, unlike a crystalloid; (ii) most patients do not experience TACO after transfusion. Hemovigilance data establish further that TACO is mainly observed in the newborn, in the elderly and when the patient has a chronic anemia. To bring together these two groups of arguments, and by analogy with TRALI, we propose a two‐hits model: the second is the transfusion of a blood product remaining intravascular, the first is the inability to cardiac adaptation to the rapid increase in the circulating volume. In hematology, the 3 main causes of heart failure are (i) the use of anthracyclines, (ii) elderly, with age‐associated diseases such as hypertension and kidney disease, (iii) iron overload. Iron overload gradually leads to diastolic heart failure and arrhythmias, and systolic heart failure. According to this model, the transfusion itself may be the first hit.
4A‐S17‐02
Haemovigilance Data for Transfusion Associated Circulatory Overload (TACO) Provides a Framework for Informing Patient‐Tailored Rate and Volume of Transfusion
Grey SL
Bolton NHS Foundation Trust, Greater Manchester, United Kingdom
Background
TACO is a pulmonary complication of transfusion that causes cardiogenic pulmonary oedema due to iatrogenic fluid overload, and is a leading cause of transfusion related mortality and morbidity. UK Serious Hazards of Transfusion (SHOT) haemovigilance reporting data provides valuable insight into patients with comorbidities and characteristics that are associated with the development of TACO such as pre‐existing heart failure, positive fluid balance, hypoalbuminaemia, renal failure, age over 70 and low body weight. A safe rate of transfusion is considered to be 90–180 min per unit, but this is based upon experience rather than experimentally designed studies. This represents a lengthy treatment time for patients receiving regular elective transfusions.
Aims
To develop a medical exclusion criteria based upon data from SHOT haemovigilance reporting on TACO to inform which patients can safely receive 2 units of red cells for normovolaemic anaemia, over a shorter period, at a rate of 60 min per unit (not exceeding an infusion rate of 5 ml/kg/h).
Methods
The exclusion criteria were defined as: moderate to severe left ventricular dysfunction; severe aortic stenosis; hypoalbuminaemia (<35 g/l); low body weight (<50 kg); significant renal failure (eGFR < 30 ml/min); significant liver failure (bilirubin >μ30 mol/l); oedema; cough, dyspnoea and/or tachypnoea; tachycardia; un‐treated or uncontrolled hypertension.
Due to the variability of blood unit volumes, patients weighing <67 kg received volume selected units to ensure they were not subjected to an infusion rate exceeding 5 ml/kg/h.
Each patient received a standard rate (90 min per unit) transfusion followed by at least one 60 min per unit transfusion. Each patient had their vital sign observations measured within 24 h of transfusion to detect symptoms and signs of TACO. The assessment criteria were based upon International Society of Blood Transfusion (ISBT) and SHOT definitions of TACO, the National Early Warning Score trigger levels, and therefore included the following measurements, symptoms and signs: blood pressure; respiratory rate; heart rate; dyspnoea; cough; oedema.
Results
| Number of patients | 13 |
| Number of 60 min per unit transfusion episodes in total | 42 |
| Mean number of 60 min per unit transfusion/patient | 3.2 (range: 1–11). |
| Patients with symptoms and signs of TACO (based upon ISBT and SHOT definitions) | 0 |
Summary/Conclusions
The preliminary data suggest that 60 min per unit red cell transfusions (<5 ml/kg/h) appear to be safe when patients are medically selected against specific criteria that have been demonstrated by SHOT to be associated with TACO, and supports the use of body weight dosing of red cells. The study will continue, and also evaluate the patient and practitioner experience of shorter transfusions, and the impact on organisational economics.
4A‐S17‐03
An Analysis of Cases of Transfusion‐Associated Circulatory Overload (TACO) Using Different Definitions (UK Haemovigilance Scheme Data 2014)
Bolton-Maggs PHB 1, Lucero H2, Ball J 2, Poles D 2, Dr Cohen H 3
1NHSBT, Manchester, United Kingdom2SHOT, Manchester, United Kingdom32University College London Hospitals NHS Foundation Trust and University College, London, United Kingdom
Background
Increasing numbers of cases of TACO are reported to the UK haemovigilance scheme, Serious Hazards of Transfusion (SHOT) year on year. This is a serious complication with death or major morbidity (admission to intensive care and ventilation) in 48%. However it is likely that TACO may still be under‐reported. In the initial presentation other causes of transfusion‐associated respiratory distress must be considered including transfusion‐related acute lung injury and allergic reactions which can be difficult to distinguish from TACO.
Current diagnostic criteria for TACO are unsatisfactory. The International Society of Blood Transfusion (ISBT) definition (used in SHOT analysis until 2012) is under revision (draft revised ISBT – DRISBT) which includes cardiac imaging and BNP levels. For 2013 SHOT analysis applied modified ‘key features’ (KF), which identified significantly more ‘highly likely’ TACO cases than the ISBT definition alone by using clinical and other supportive evidence.
Aims
To evaluate the 4 different definitions for analysis of all cases of TACO reported to SHOT in 2014.
Method
We analysed 91 cases using 4 definitions, the original ISBT, DRISBT, SHOT ‘key features’ (KF) and a modified version of key features, with clinical prioritisation (CPKF). For this definition, cases were classified as ‘probable’ TACO if there were clinical features suggesting circulatory overload, e.g. dyspnoea without another cause, plus response to treatment for pulmonary oedema, even if imaging was not available. KF used clinical judgement based upon the specific features such as CXR evidence of pulmonary oedema, but also permitted more subjective assessments such as clinical examination findings and the patient's associated illness to determine overall probability. The clinical prioritisation of key features (CPKF) was based on the same principles but used a defined weighting to enhance reproducibility.
Results
The table shows that DRISBT resulted in classification of more cases as ‘highly likely’ (43%) compared to ISBT (19%) but more had the probability down‐graded due to absence of cardiac imaging, other supportive features (MAP, BNP, CVP) or onset after 12 h. Cardiac size was rarely reported (6/91 cases). Blood pressure was reported in 67/91 (74%) and BNP was only measured once. More cases were identified as ‘highly likely’ or ‘probable’ TACO using KF (86%) or CPKF (85%).
Table 1: Probability of TACO by 4 different definitions.

Conclusions
Both ISBT definitions exclude TACO in 25% SHOT‐reported cases, often due to insufficient data. We conclude that these definitions are unsatisfactory. TACO should be considered and reported when a patient develops circulatory overload requiring treatment even when initially triggered by other intravenous fluids. Increased recognition of volume intolerance should prompt clinicians to take preventative action.
4A‐S17‐04
Hemovigilance in the US: Independent Validation by the Private Sector
Whitaker BI1, Kleinman S 1, Fung M 2, Eder A 3, Katz L 4, Malasky J 1, Harris A 1, Dodd R 3
1AABB, Bethesda, MD, United States of America2University of Vermont, Burlington, VT, United States of America3American Red Cross, Rockville, MD, United States of America4America's Blood Centers, Washington, DC, United States of America
Background
National hemovigilance in the United States was established in 2010 as a voluntary reporting system for hospital transfusion services through the web‐based Centers for Disease Control and Prevention's (CDC) National Healthcare Safety Network (NHSN) Hemovigilance Module. The system was developed jointly by the US Department of Health and Human Services and its agencies and AABB, representing the private sector. The US system relies upon unmonitored adherence to detailed case‐definitions of adverse transfusion reactions, a written protocol, and self‐paced training for those reporting. Internationally, other hemovigilance systems have mechanisms in place to review and potentially reclassify reports of serious adverse reactions before they are accepted as part of the record.
To provide additional support and intervention analysis for hospitals, AABB established a patient safety organization (Center for Patient Safety, CPS) in which hospitals reporting to NHSN may participate and share their data. This invaluable tool allows the AABB CPS an avenue for retrospective review of the validity of reported data.
Aims
To evaluate data quality and consistency of the AABB CPS, data for respiratory reactions and transfusion transmitted bacterial infections (TTI) were reviewed using supplemental forms containing abstracted information.
Methods
The CPS accessed 2010–2012 hemovigilance data from participating hospitals to confirm that these data were representative and comparable to the larger CDC dataset (Harvey, 2014). Hospital demographics and baseline rates of reaction per unit transfused were calculated. Supplementary forms were developed by AABB subject matter experts to collect abstracted information from the transfused patient's record. A small expert group reviewed the initial hemovigilance reports for TRALI, TACO, TAD, TTI reactions from 2010 and 2011 with the supplementary reports and evaluated the reaction categorization.
Results
Forty‐seven hospitals reported transfusion reactions and monthly denominators of components transfused. From 2010 to 2012, 3482 adverse reaction reports met case definition and imputability inclusion criteria and were entered with corresponding monthly denominators that totaled 1,498,411 transfused components. The overall reaction rate was 2.32 per 1000 components transfused.
The expert group reviewed 14 reports of TTI and 42 pulmonary reactions (Dodd, 2015). Five of 14 TTI were found not to meet the reported case definition of definitive, as there was no evidence of an organism in the patient. The expert panel agreed with the reported imputability to the transfused component in one of the fourteen cases. Of six cases reported as definitive TRALI, the panel agreed with only one. For TACO, 27 cases were reported but the panel agreed there were insufficient data to support a report of TACO for 20 of the cases. Imputability was frequently unclear.
Summary/Conclusions
National hemovigilance in the US is still in its early days. Active participants in the CDC NHSN Hemovigilance Module and the AABB CPS are to be commended on the voluntary reporting of transfusion complications. In the absence of verification and validation steps, consistently accurate reporting based on self‐education and adherence to surveillance definitions has not yet been achieved. Enhanced and timely feedback from ongoing validation efforts is needed in order for national aggregate reporting to be meaningful.
4A‐S17‐05
Effect of Donor Age on Survival of Transfused Patients – Analysis of Over 1.5 Million Recipients from Scandinavia
Vasan K
Karolinska Institutet, Stockholm, Sweden
Background
The topic of possible effects of donor age on recipient health has generated recent interest following demonstration of beneficial effects on muscle regeneration and cognitive function when older mice are transfused with blood from younger mice. Subsequent animal studies have indicated that a key plasma protein named growth differentiation factor 11 (GDF‐11) found in blood of younger mice is responsible for reversal of age‐related cardiac‐hypertrophy and improvements in olfactory neurogenesis, cerebral vasculature and synaptic plasticity. Although there is no such evidence in humans, a randomized clinical trial have been initiated recently to study if Alzheimer's patients demonstrate improvements in cognition when transfused with plasma from healthy younger people.
Aims
We aimed to investigate if there is any effect of donor age (18–70 years) on survival following red blood cell (RBC) or plasma transfusions.
Methods
The SCANDAT2 database includes information on several million donors and recipients in Sweden (recruited from 1968) and Denmark (recruited from 1981). In SCANDAT2, we identified all recipients of RBC or plasma transfusions from known blood donors between 1995 and 2012, and calculated the donors’ ages at each of the original donations. For each recipient, we ascertained the age distribution of the donors contributing to products used within first 7 days of transfusion. We then stratified the recipients into groups who had exclusively received RBC or plasma from donors of a three age categories (<25; 25–50; 51–70 years). The recipients were followed until date of migration, death or end of 2012, and the association between donor age and recipient survival was assessed by Cox proportional regression models adjusted for age, sex, number of transfusions, calendar year, hospital and indication for transfusion. We carried out separate analyses for recipients of plasma and RBC cohorts, respectively, and for recipients with known dementia.
Results
The RBC cohort included 1,607,850 patients and the plasma cohort included 350,663 recipients. Transfused recipients were from both Sweden (n = 1,213,953) and Denmark (n = 744,560). Patients who exclusively received blood products from donors age 18–24 years did not have a better prognosis than recipients of products donors age 25–50, neither in RBC [hazard ratio (HR), 0.99; 95% CI 0.97–1.03], nor the plasma (HR, 1.02; 95% CI 0.97–1.08) recipient cohorts. Similar effects were observed when analyses were restricted to patients with known dementia: HRplasma 0.97 (95% CI, 0.73–1.27) and HRRBC 0.98 (95% CI, 0.88–1.08) (Table 1).
Caption 1: Effect of donor age on recipient survival among all individuals and among patients with dementia.
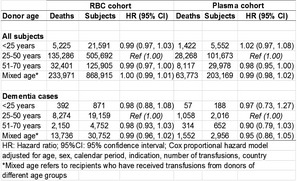
Conclusions
Based on the analysis of data from SCANDAT2, we provide large scale evidence of absence of donor age effect on recipient survival. Whether units from young blood donors may have other beneficial health effects remains unknown.
(Hyper)haemolysis
4A‐S18‐01
Management of Hyperhaemolysis After a Transfusion in Sickle Cell Patients
Bartolucci PB1, Anoosha AH 2, France FNP 3
1APHP‐UPEC‐INSERM, Créteil, France2APHP‐INSERM, Créteil, France3EFS‐UPEC‐INSERM, Créteil, France
Transfusion is a corner stone treatment of sickle cell disease complications. However one of the serious complications of transfusions is represented by the delayed hemolytic transfusion reaction (DHTR) with a significant mortality. It is due to a massive intravascular hemolysis of both transfused and autologous red blood cells. It occurs between 5 and 20 days after a transfusion and is accompanied by a vaso‐occlusive crisis and haemoglobinuria in almost all cases. The evolution can be life‐threatening because of the appearance of a severe acute chest syndrome or a multi organ failure. About half of the patients are transferred in Intensive Care Unit. The biological diagnosis of DHTR is made by the rapid decrease, or disappearance, of Hb A in sickle cell patients who were previously transfused. This event is associated with a significant increase of lactate dehydrogenase (LDH) and worsening of anemia due to hemolysis. Allo‐antibodies are not found in 30%. DHTR is under diagnosed because of the delay between the transfusion and the symptoms, which mimic vaso‐occlusive crisis. This can lead to further transfusions that worsen the evolution and are ineffective.
Because of the severity of this manifestation, many treatments have been tried to improve the outcome such as steroids, immunoglobulins, rituximab or eculizumab, without consensual guidelines.
Early diagnosis of DHTR should help to decide the appropriate treatment, and avoid reiteration of transfusions. Transfusion indications should be maximally restricted in patients with previous post‐transfusion hemolysis.
4A‐S18‐02
Recognition and Observation of Clearance of an Inappropriate RHD Positive Red Cell Transfusion to a RHD Variant Individual Through the Use of an ‘Enhanced’ Flowcytometry Method Post IV RhIg
Hazell MJ1, Grimsley S 1, Sekhar M 2, Fosbury E 2, Atugonza R 2, Lee E 1
1NHS Blood and Transplant, Bristol, United Kingdom2Royal Free London NHS Trust, London, United Kingdom
Background
The Rh System consists of two homologous genes, RHD and RHCE, that encode the RhD and RhCcEe polypeptides. Variants of RhD and RhCcEe are observed through gene rearrangements, deletions and point mutations in RHD and RHCE. The RhD variant (DAR) is characterised by the loss of nine of the 37 RhD epitopes, consequently, transfusion of RhD positive (RhD+) blood to DAR individuals can cause RhD immunisation. In DAR and RhD negative (RhD−) mothers, fetal maternal haemorrhage of RhD+ fetal blood can cause immunisation; this can be prevented by administering RhIg. Current UK guidelines stipulate a dose of 500 IU (100ug) per 4 ml bleed should be given within 72 h of birth to ensure clearance of RhD+ red blood cells (RBCs) from maternal blood.
Aim
A DAR phenotype patient was inappropriately transfused 300 ml of RhD+ blood and subsequently adminstered 45,000 IU of RhIg. Here we demonstrate an ‘enhanced’ flowcytometry (FC) method that identifies RhIg opsonised RhD+ RBCs and enables observation of their clearance.
Method
Quantification of RhD+ RBCs was conducted via FC with FITC‐BRAD3 (IBGRL Research Products). RhD+ RBCs opsonised with RhIg were determined via FC with a PE‐anti Human Ig (Jackson ImmunoResearch) and an ‘enhanced’ test combining both conjugates. Estimation of RhIg bound on RhD+ RBCs was determined through comparison to a standard curve of RhD+ RBCs incubated with known concentrations of RhIg. Plasma levels of RhIg were determined through Anti D quantitation.
Results
FC results estimated 173 ml of RhD+ RBCs 3 days post IV RhIg. The ‘enhanced’ FC method determined that 1.87% were coated with an estimated concentration of 0.3 μg/ml RhIg. By day 7, RhD+ RBCs present in circulation had reduced to 94 ml. Of these, the percentage coated with RhIg in circulation had peaked at 13.94%, but, RhIg coating had increased to 0.65 μg/ml. By day 12, no RhD+ RBCs remained in the circulation. FC data demonstrated that RhIg opsonisation of DAR RBCs occurred, but was minor relative to transfused RhD+ RBCs.

Summary/Conclusions
An anti‐RhD method distinguished between the patients DAR RBCs and RhD+ transfused RBCs. Using PE‐anti Human Ig, percentages of RhD+ RBCs opsonised with RhIg was observed, as was the presence of RhIg interaction with DAR RBCs. A combination of the two antibodies resulted in greater delineation of RBC populations, identifying the patient had received a unit of RhD‐ RBCs following DAR typing. The PE‐anti Human Ig and combination methods allowed for observation of the RhD+ RBCs clearance from circulation throughout RhIg treatment. At maximum binding (Day 7 post RhIg injection), 13.94% of RhD+ RBCs in circulation had bound RhIg, estimated at 0.65 μg/ml (0.33 IU/ml). Low percentages of RhIg opsonised RhD+ RBCs were hypothesised to be a result of rapid clearance from circulation and reduction in RhIg opsonisation due to RhIg interaction with DAR RBCs. By day 12 post transfusion, RhD+ RBCs had been cleared from circulation and at day 71 < 0.1 IU/ml RhIg was detected in the plasma.
4A‐S18‐03
Auto and Allo Red Blood Cell Antibodies in Sickle Cell Patients in Brazil
Amorim LA , Lopes MEDL , Vigorito MV , Monnerat BM , Porto PP , Souza ALS , Candol PC , Belmar EB , Albuquerque TA , Balducci DB , Sappi MS , Castilho SC
HEMORIO, Rio de Janeiro, Brazil
introduction and objectives
Sickle cell patients undergo, throughout life, a large number of transfusions, and so they have a high risk of developing antibodies against red blood cell (RBC) antigens. Previous studies show that 4–65% of transfused sickle cell patients develop alloantibodies. In our institution, the transfusion polices establish the use of K (K1) and Rh (RH1, RH2, RH3, RH4, Rh5) phenotyped RBC. If the patient develops an antibody against any other antigen, then the use of Kidd (JK1 and JK2) and Duffy (Fy1 and Fy2) matched cells is started, whenever possible. The aim of this study was to investigate the frequency and types of auto and alloantibodies in a population of sickle cell patients.
Methods
It was a retrospective 20 year survey of auto and alloimmunization in 3077 sickle cell patients followed in a single institution in Rio de Janeiro, Brazil. All the data related to blood transfusion, including antibody screening and identification results, were recovered from the electronic patient charts. Antibody screening was performed before each transfusion or once a year if there was no transfusion. Extended RBC phenotyping was performed for all the patients.
Results
1765 out of the 3077 patients were transfused (58.4%), according to the electronic records; the total number of transfused RBC units was 26,289 (mean per transfused patient: 14.9 unit, range 1–200). 471 out of 1765 transfused patients (26.7%) had an alloantibody. If we consider all the followed patients, then this rate was 15.3%; 67 patients (3.8% of all patients) had nonspecific cold agglutinins, probably not related to previous transfusion exposure; 57 patients (1.89% of all patients) had autoantibodies; from these, 36 (1.19%) had allo and autoantibodies and 21 (0.69%) had only autoantibodies. There were ten cases of auto anti‐e (0.33%) and 5 cases of auto anti‐C, probably secondary to partial antigen deletion. Amongst the alloantibodies, the most frequent one was anti‐E (89 cases), anti‐C (71 cases), anti‐S (40 cases) anti‐Kell (39 cases), anti‐e (38 cases) and anti‐D (27 cases). The rate of warm alloantibody production by transfused RBC was 1.79%.
Conclusions
The frequency of alloimmunization was not drastically reduced by antigen matched transfusion policy. The large number of anti‐e and auto anti‐C antibodies indicates that deletions and mutations in genes D and CE are not uncommon in sickle cell patients, suggesting that sickle cell patients and donors genotyping may be useful to prevent alloimmunization in this particular setting.
4A‐S18‐04
Post‐Transfusion Hyperhaemolysis Syndrome (PTHS) in a Pregnant Patient with Beta Thalassaemia Intermedia
Hebballi S
Royal Derby Hospitals NHS Foundation Trust, Sutton Coldfield, United Kingdom
Background
PTHS has been well recognised in patients with SCD. PTHS has also been described in thalasaemic patients, anaemia of chronic disorder, mantle cell lymphoma, dyserythropoietic anemia, myelofibrosis, CLL and HbH. PTHS seems more severe in children with thalassaemia and there were 3 case reports one received Cyclophosphamide and two required splenectomy/chemo and transplant.
Aims
We report a pregnant patient with thalassaemia intermedia who developed resistant PTHS (no response to steroids/IVG/Rituximab) followed by laparoscopic splenectomy.
Methods.
A 31‐year old patient (Kurdish) at 16 weeks gestation had Hb level 54 g/l and received 4 RBC units. A week later she was admitted with chest infection, Hb 51 g/l, reticulocytes 69 (range 20–100), with evidence of haemolysis: haptoglobulin <200, bilirubin 41 (range 0–17 μmol/l) LDH 1185 (range 220–450 IU/l) and high ferritin 864 (15–250 ng/l). Her base line ferritin was 400–500. She was given 3 RBC units and post‐transfuion Hb was 52 g/l with further rise in bilirubin and LDH. PTHS was diagnosed and prescribed standard therapy IV Methylprednisolone 1G/day × 3 days, IVIG 0.4 g/kg/day for 5 days, with no improvement. Hb further dropped to 43 and 39 g/l 2 weeks later. At 20 weeks gestation it was decided to terminate the pregnancy. With IVIG and steroids cover for 3 RBC units, post‐transfusion Hb level was 72 g/l but 4 days later Hb further dropped to 45 g/l. As there was no response to IVIG/steroids, Rituximab was added but a week later Hb level remained unchanged. It was noted that there was a gradual increase in spleen size from 21 cm booking to 23.5 cm. Therefore laproscopic splenectomy was organised. She received further immunosupprssive therapy. Pre‐op Hb level was 53 g/l and received 3 RBC units during the procedure with immediate post‐op Hb level 84 g/l. Through out admission DAT was negative with no red cell alloantibodies detected.
Result
Evidence of haemolysis still persisted a week post‐splenectomy with Hb 69 g/l, low retic count 17, bilirubin 480 and ferritin 1365. Histology of the spleen showed increased number of histiocytes, extramedullary haemopoiesis. Six months later Hb was stable at 95–105, bilirubin 30–40, high retic 250–300, LDH 300–400 and ferritin was down to 400–500. In PTHS, Hb level fell below pre‐transfusion level (destruction of both transfused and own RBC). Reticulocytopenia is a common findings and a rise in reticulocytes coincided with recovery. In view of the absence of serological evidence of RBC destruction it has been proposed that activate macrophages may play an important role with raised ferritin, a non‐specific marker for macrophage activation, correlating well with disease activity and clinical responses. All of the above were demonstrated in this case.
Conclusion
This is the first case report with β thalassaemia intermedia patient presenting with resistant PTHS. Recent publications have concluded that the presence of functional spleen in patients with SCD deserves further evaluation. Majortiy of the cases with PTHS in SCD respond to IVIG/steroids. In our case the patient did not respond to standard therapy and it might be due to excess histiocytes/macrophages activation in the enlarged spleen.
4A‐S18‐05
Resistant Post Transfusion Hyperhaemolysis (PTH) Syndrome in SCD Treated with Anti‐Macrophage Agent (Ciclosporin)
Trompeter S 1, Mactier C2, Stewart GW 2, Win N 1
1NHS Blood and Transplant, London, United Kingdom2University College Hospitals London NHS Foundation Trust, London, United Kingdom
Background
The majority of the cases of post transfusion hyperhaemolysis (PTH) respond to IVIg/steroid therapy, with occasional cases needing a further course of IVIg. However, there is a cohort of refractory PTH cases where Rituximab has been used. The context of rituximab as a profound imunosuppressant in the presence of a pre‐existing hyposplenism often with co‐existing infection raises concerns and indeed its use has been met with mixed success.
The pathogenesis of PTH seems complicated as both the transfused and patient's own RBC are destroyed after transfusion often without any detectable antibodies, and it has been proposed that the mechanism of destruction is through activated macrophages. We report a resistant PTH case which was treated successfully with ciclosporin.
Results
A 42 yearr old female with SCD and Lupus anticoagulant (blood group A Ro known to have antiS and antiJkb antibodies), and chronic thrombocytopenia went to Europe on holiday, and became non‐specifically unwell, and was transfused 4 units of blood despite her Hb being at her baseline of 80 g/l. Blood transfused was Orr with no further phenotype available, there was no record of an antibody screen. On return to the UK she was admitted with Hb 66 g/l and anti‐S, anti‐Jkb and new allo anti‐M and autoantibodies were detected, DAT was 1+ pos with IgG and C3d. Eluate study was non‐reactive. Three days after admission, her Hb dropped to 26 g/l with a low reticulocyte count. She was given 2 days of IV 500 mg Methylprednisolone and 2 days of IVIG 1 g/kg (60 g) and transfused with 3 RBC units. Hb rose to 77 g/l and then dropped again to 57 g/l the next day. She also noticed passing dark urine and had mild hepatosplenomegaly but nothing sufficient to account for the drop in Hb. She also received EPO 8000iu and antibiotics and was transferred to a teaching hospital. She was monitored closely and was treated with IVIG, IV methylprednisolone × 2 days followed by oral steroids. Sepsis and had bilateral pneumonia was treated with broad‐spectrum antibiotics. Eleven days after transfer Hb further dropped to 40 g/l and in addition to the above antibodies anti‐Fya was also detected. Samples were referred to International Blood Group Reference Laboratory (Bristol) which confirmed no mutation found on RHCE gene studies nor additional red cell alloantibodies detected. The patient was given another 2 days course of IVIG/Methylprednisolone and received 2 RBC units (antigen matched cross‐matched compatible units). The following week Hb dropped again, and the IvIg was repeated, a further unit was given and this time with ciclosporin and oral prednisolone was weaned off. Hb and platelet level slowly improved and remained stable and she was discharge 10 days later.
Summary
This patient had a limited and short‐lived response to standard treatment and the risk of exacerbating the existing sepsis was averted by the use of ciclosporin as a salvage treatment with a rapid and sustained response. Ciclosporin should be considered as an alternative treatment in PHT.
Transfusion Transmissible Infections
4A‐S19‐01
Current Prevalent, Incident and Residual Risk to HIV Infection in a Cohort of Volunteer Blood Donors in São Paulo City, Brazil
Araujo PR
Colsan‐Associação Beneficente de Coleta de Sangue‐São Paulo University‐UNIFESP, São paulo, Brazil
Background
Numerous studies have shown that the greatest threat to the safety of the blood supply is the donation of blood by seronegative donors during the infectious window period (WP) when the donors are undergoing seroconversion. Such people represent new, or incident, infections. When rates of seroconversion are combined with estimates of the probability that blood was donated during the donor's window period, the residual risks of transmitting infectious disease can be calculated. This risk of transmission depends upon four factors: method sensitivity, incidence, WP duration and procedural errors.
Aims
In this study, we evaluated current prevalence, incidence, and residual risk of transfusion‐transmissible HIV in a population of asymptomatic blood donors volunteers in the city ofSão Paulo, Brazil.
Methods
Donor demographics and donation information were captured as electronic data files with information including donation history, number and types of previous donations, age, gender, race, marital status, professional occupation, schooling and results of serologic screening and confirmatory test, allowing accurate retrieval of information for the calculation of prevalence and assessment for seroconversion in the repeat donors. In A total of 734,963 donations, including first‐time and repeat donors were included in the analysis, covering the period 2010 through February, 2015. The residual risk of infection was estimated using a published risk model that assumed that the residual risk is proportional to the probability of an undetected window period donation within the study period. The model separately calculated the residual risk component for first‐time and repeat donors.
Results
HIV seroprevalence among persons accepted for blood donation was 0.5% in 2010; 0.45% in 2011; 0.41% in 2012; 0.2% in 2013 to 2015. The HIV seroincidence was 1.0 per 200 person‐years at the 2010 to 2012. HIV seroincidence was 1.0 per 500 person‐years between 2013 and 2015. However, the number of HIV seroconversion cases increased at the same period. The median of HIV seroconversion cases in the period between 2010 and 2011 were three cases. Since2012 we observed an increase in HIV seroconversion cases. Ten cases in 2012; twelve cases in 2013; Nine cases in 2014 and seven cases in January and February, 2015. In HIV seroconversion cases we observed significant association with age over 25 years (odds ratio 1.6, confidence interval 1.1–2.4), male gender (odds ratio 1.7, confidence interval 1.2–2.6), unmarried (hazard ratio 2.5, confidence interval 1.4–5.0), low level of schooling and professional occupation (odds ratio 7.3, 95% confidence interval 4.4–1 2.1) and being born out of São Paulo state (hazard ratio 2.5, confidence interval 1.4–5.0). All HIV seroconversion cases presented a blood donation frequency of one donation per year.
Conclusion
A discrimination of risk factors for HIV transmission, which can be region/country specific, is necessary in order to develop strategies to mitigate residual HIV transfusion risk. Therefore, research to develop risk factor exclusion strategies must focus on predictors of HIV seroconversion/WP.
4A‐S19‐02
Improved Blood Safety by Dual Target Nat‐Screening for HIV‐1 – 5 Years of Experience at German Red Cross Frankfurt
Schmidt MS 1, Hourfar KH1, Nübling MN 2, Chudy MC 2, Kress JK 2, Gürtler LG 3, Eberle JE 3, Seifried ES 1
1German Red Cross Institute Frankfurt, Frankfurt, Germany2Paul‐Ehrlich‐Institute, Langen, Germany3Virologie University Munic, Munic, Germany
Background
The introduction of nucleic acid amplification technique (NAT) testing into blood donor screening was able to reduce the diagnostic window period to a minimum and to improve blood safety to a high level. Nevertheless RNA viruses are associated with a high frequency for mutations due to the missing proof‐ reading function of polymerases, as reverse transcriptase. Between 2007 and 2010 six blood donations with false negative NAT results were reported in Germany. Therefore NAT‐screening in two genome regions was introduced by our blood donation service in 2010 on a voluntary basis and became mandatory in Germany since beginning of 2015.
Material/Methods
Blood donor screening was done using in parallel the CE‐marked tests GRC HIV‐1 PCR kit (amplification within the 5′LTR region, LOD 8.9 IU/ml) and GRC HIV‐1 gag PCR kit (amplification within the gag region of HIV‐1, LOD 22.9 IU/ml). In total, 7 million blood donations were screened during the study period from 2010 to 2014 with the GRC dual target HIV‐1 NAT system. In case of discrepant results between both assays samples were sequenced in the primer binding regions (5′LTR and gag region) as well as in the hypervariable HIV‐1 V3 region. Additionally those specimens were analysed by four single target NAT assays and by five dual target NAT assays, respectively.
Results
Three out of seven million donations tested negative using the 5′LTR‐PCR but positive if amplification was performed in the gag‐region. HIV antibodies were detectable in all three donations. For two of these donations sufficient plasma was available for sequence analysis and testing in additional NAT‐assays. Nucleic acid sequence analysis identified a deletion of 24 base pairs within the 5′LTR probe binding region. Phylogenetic analysis revealed HIV‐1 group M subtype B virus and excluded genetically identical viruses in both donors. Three different LTR‐based mono‐target assays missed the two donations, with the exception of a low‐reactive result obtained by one of the assays. A pol‐based mono‐target assay achieved positive results with the two donations, as did five different dual‐target assays. In total the detection rates were 37.5% (3/8) for mono‐target assays and 100% (10/10) for dual‐target assays (P = 0.01) (Table 1).
Caption 1: Comparison of single and dual target HIV‐1 assays.
![]()
Discussion
Genetic modifications within the HIV‐1 genome are very frequent during the transcription process from RNA into DNA. Mutations can be classified into primer and probe mismatches and deletions. Although the primer and probe binding regions of screening assays are usually located in highly conserved genome regions (e.g. 5′LTR region, gag region or pol region) mutations cannot be excluded. The current data demonstrate that dual target NAT systems reduce the risk of false negative NAT screening results significantly. Therefore blood donor screening by dual target HIV NAT systems improves blood safety.
4A‐S19‐03
Nucleic Acid Amplification Tests in HCMV Infection by Blood Samples: A Meta Analysis
Bi H
Wuhan Blood Center, Wuhan, China
Background
to the patients with low immunity, blood screening of HCMV is necessary to prevent transfusion transmission of cytomegalovirus. However in China, the detection of HCMV have not yet been included into the routine blood screening programs, so there is no uniform standard. Although nucleic acid amplification test (NAT) has been regarded as the best method, but the diagnostic value of it for the HCMV infection by blood samples is not clear yet, because urine or milk are often used in the NAT for the HCMV infection.
Aims
to evaluate the diagnostic value of NAT for the HCMV infection by blood samples, with HCMV pp65 antigenemia assay as the reference standard, by meta analysis, benefiting the development of blood screening method of HCMV.
Methods
We identified all studies published between January 2000 and January 2015 from the following online databases: PubMed (MedLine), Web of Knowledge, Scopus, LILACS, EMBASE, CNKI, VIP and Wanfang. Search terms used were: ‘HCM’, and ‘Polymerase Chain Reaction’, and/or ‘Nucleic acid amplification testing’, and ‘pp65’. Only articles written in English or Chinese were included. Case reports and review articles were excluded. Studies with <10 subjects were also excluded. References of selected articles were reviewed to identify additional eligible studies. After collecting studies according to inclusion and exclusion criteria, data about of study background, design information and diagnostic parameters were extracted. Meta‐disc software was used to handle data of included studies and to examine heterogeneity. Sensitivity, specificity, and diagnostic odds ratio (DOR) were computed for each of the included studies. Pooled summary effect estimates were calculated, using a random effects model and SROC curve was drawn.
Results
We got potential relevant 1129 studies. According to eligibility criteria, 26 studies compared with HCMV pp65 antigenemia assay were included and outcomes were as follows: pooled sensitivity 0.88, 95% CI (0.86–0.89), pooled specificity 0.86 95% CI (0.86–0.87), and SROC AUC 0.94, but heterogeneity was limited. Some commercial FQ‐PCR kit was further analyzed in the stratified subgroups, Roche: pooled sensitivity 0.86, 95% CI (0.81–0.89), pooled specificity 0.86, 95% CI (0.85–0.88). QIAGEN: pooled sensitivity 0.92, 95% CI (0.88–0.95), pooled specificity 0.84, 95% CI (0.80–0.87). Nanogen: pooled sensitivity 0.92, 95% CI (0.90–0.94), pooled specificity 0.79, 95% CI (0.76–0.82). Da An: pooled sensitivity 0.94, 95% CI (0.89–0.97), pooled specificity 0.92, 95% CI (0.89–0.95).

Caption 1: Figures of meta analysis
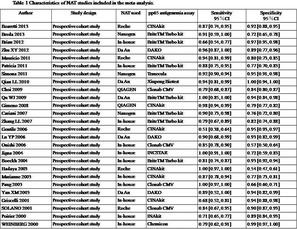
Conclusions
the diagnostic value of NAT for the HCMV infection through blood samples is high and NAT can be regarded as the best candidate to be developed for the blood screening method of HCMV.
4A‐S19‐04
Challenges of Using HIV as a Primary Risk Indicator: Need for Integrated Blood Donor Risk Management Model
Mapako T1,2, Parirewa JJ 1, Emmanuel JC 1, Mvere DA 1, Massundah E 1, Mavunganidze G 1, Marowa LM 1, Postma MJ 2, van Hulst M 2
1National Blood Service Zimbabwe, Harare, Zimbabwe2University of Groningen, Groningen, The Netherlands
Background
The use of risk modelling in blood safety is increasing getting momentum. NBSZ initiated blood donor risk profiling based on donation frequency (r‐coding) since 1994 and in 2006 a generic risk classification model was developed (include age and donation venue) which was mainly based on HIV risk considerations. The blood safety implications of this model, which include all the four routinely tested transfusion transmission infections (TTI) has not been studied. We aim to assess whether the use of HIV as a primary risk indicator is sufficient in Zimbabwe and the possible blood safety concerns for this.
Methods
Blood donor data on HIV, HBV, HCV and Syphilis from 2002 to 2011 was analysed. The NBSZ blood donor risk model developed in 2006, which has four levels [RC I (lower) – RC IV (higher)] of blood safety, was evaluated based on the TTIs seroprevalence results. The TTIs relative risk (RR) for usable (RC I & II) and unusable (RC III & IV) were determined and 95% CI determined. Sub‐group analysis by gender was also done to aid in explaining the results.
Results
A total of 627,072 donations were analysed. The overall TTIs seroprevalence by donor risk category are shown in Table 1. NBSZ donor risk classification seems to be performing well for all TTIs except for HBV, which has highest seroprevalence in RC II. The overall relative risk (RR) for TTIs in risk categories for unusable units over usable units is 3.5 (95% CI, 3.3–3.8), 1.3 (1.2–1.3), 1.3 (1.1–1.5) and 2.1 (1.9–2.4) for HIV, HBV, HCV and Syphilis respectively. The average RR in all risk categories for males being TTI positive compared to females is 0.70, 1.95, 1.6 and 1.2 for HIV, HBV, HCV and Syphilis respectively.
Caption 1: Blood donor TTIs seroprevalence by blood donor risk category in Zimbabwe (2002–2011).

Discussion and conclusions
The results for HIV, HCV and Syphilis indicate increasing safety concerns from low (RC I) to high (RC II) as we expect the model to perform. However, the high HBV seroprevalence in RC II is of concern to blood safety as these are usable blood units. Further analysis also noted that in risk category II, which is mainly composed of new donors in schools, the HBV risk is 68% higher when compared with other combined risk categories. Males are 95%, 60% and 20% more likely to be positive for HBV, HCV and Syphilis than females. However, they are 30% less likely to be HIV positive when compared to female donors.
In conclusion, our results points to blood safety concerns for HBV in Zimbabwe and hence there is need to review the current NBSZ blood donor risk classification model taking into consideration other TTIs and cost‐effectiveness analysis.
4A‐S19‐05
Molecular and Functional Characterization of Hepatitis B Virus Strains Infecting Blood Donors with High HBsAg and Undetectable HBV DNA Levels: Implications for Blood Safety and Screening Policy
Candotti DC , Leclerc CL , Servant-Delmas AS , Boizeau LB , Laperche SL
Institut National de la Transfusion Sanguine, Paris, France
Background
The implementation of nucleic acid testing (NAT) and the development of sensitive and specific serologic assays to detect the HBV surface antigen (HBsAg) and anti‐HBc antibodies significantly reduced the risk of HBV transfusion‐transmission. The apparent redundant testing for two direct viral markers prompted debates on maintaining HBsAg screening, particularly in low endemicity countries where blood donations are screened for anti‐HBc antibodies. However, frequencies of 2–20% of HBsAg‐confirmed positive/NAT negative donations have been reported depending on the HBV genotypes and the sensitivity limit of the molecular assays used. The nature of this discrepancy between HBsAg and DNA remains largely unknown and it is essential to evaluate any potential negative impact on blood safety before considering removing HBsAg testing.
Aims
The prevalence in blood donors and the molecular mechanisms responsible for a persistent undetectable or barely detectable level of viral replication in the presence of a sustained HBsAg production will be investigated. A high discrepancy between viral DNA and HBsAg levels suggests the presence of mutations that may negatively affect the molecular detection of viral variants or the HBV replication and/or the production of infectious viral particles.
Methods
In France, blood donations were tested for HBsAg (ABBOTT PRISMR HBsAg), anti‐HBc (ABBOTT PRISM™ AcHBc), and HBV DNA with ID‐NAT (Procleix‐Ultrio™). HBV DNA load was quantified (Cobas Taqman HBV; Roche; LOQ: 6 IU/ml) in samples reactive for at least one markers. HBsAg‐positive (≥100 IU/ml) donations tested HBV NAT non‐reactive/non repeatable reactive with undetectable or <6 IU/ml DNA load and HBV NAT reactive with DNA load <6 IU/ml were selected. HBV DNA was extracted from 5 to 12 ml of plasma directly and after ultracentrifugation. The whole HBV genome, the Pre‐S/S region and the BCP/PC region were amplified and sequenced.
Results
In 2010–2014, 47 out of 898 confirmed HBsAg‐positive donations tested ID‐NAT non‐reactive (5.2%). HBV DNA loads undetectable or below 6 IU/ml were observed in 23 (49%) samples with HBsAg levels ≥100 IU/ml (group 1). Viral loads ≤6 IU/ml were detected in 34 (3.8%) ID‐NAT reactive samples containing HBsAg levels ≥100 IU/ml (group 2). All donations tested anti‐HBc positive. The BCP/PC region was amplified and sequenced for 6/12 (50%) group 1 and 10/15 (67%) group 2 samples. The whole HBV genome of 1 group 1 strain (genotype D7) and 4 group 2 strains (3 genotypes A2 and 1 genotype E) was sequenced. Two sequences showed mutations in the pgRNA regulatory domain epsilon that may negatively affect HBV replication.
Conclusion
Presence of extremely low level of circulating DNA‐containing viral particles in ID‐NAT non‐reactive blood donations with concomitant high HBsAg levels was confirmed. Similar results obtained using independent molecular assays targeting different regions of the viral genome suggest an impaired viral replication rather than molecular detection issues. Mutations in a critical replication regulatory element support this hypothesis but further functional analysis is needed. The characterization of HBV variants associated with major HBsAg vs HBV DNA discrepancies appears essential to increase our knowledge about viral replication and HBsAg production control and to estimate the associated potential infectious risk.
4A‐S19‐06
Prevalence and Clinical Relevance of IL28B And IFN‐L4 Genetic Polimorphisms in the Context of Ethnic Ancestry Background of Populations in HCV/1b‐ Infected, PEG‐IFN‐A/Rbv Treated Patients in Uzbekistan
Sekler D
Bechtel National Incorporated, Tashkent, Uzbekistan
Background
Central and Eastern Asia, North Africa, and the Middle East are thought to have the highest prevalence of anti‐HCV antibodies (>3.5%). Implementation of antiviral therapy, combined treatment PEG‐IFN‐a plus Ribavirin is still the most effective treatment for patients with chronic hepatitis C in Central Asia. However, approximately half of treated patients fail to achieve a Sustained Virological Response. Genome‐wide association studies highlighted single nucleotide polymorphisms (SNPs) within the IFNL3/IL28B locus predict the treatment outcome for patients with HCV/1b. SNPs in newly discovered IFNL4 have population specific correlation with spontaneous clearance of HCV and improved the prediction rate of IFN‐therapy in African Americans, Caucasians and Japanese.
Uzbekistan is one of the most populous countries in Central Asia with very high HCV infection prevalence in the general population, at >6.4%, and >20% in ‘high‐risk’ groups.
Aim
Because the population of Uzbekistan consists of different genetic backgrounds, the aim of this study was to examine the prevalence and clinical relevance of IL28B and IFNL4 polymorphisms in the context of the ethnic ancestry populations background.
Methods
135 subjects with chronic HCV infection treated with PEG‐IFN‐a plus RBV at the Institute of Virology (MOH, Uzbekistan) were enrolled. Ethnic background was assessed from the questionnaires; Uzbek, Kyrgyz, Kazakh and Tajik ethnicities patients were included into the Central Asian ancestry (CA) group, Russian and Tatar ethnicities considered the Eastern Europe (EE) group.
IL28B genotyping: Genomic DNA was extracted from PBMC using a QIAamp DNA Kit (QIAGEN, Netherlands). All patients were genotyped for the SNPs rs8099917, rs12979860, rs8103142, rs11881222 and ss469415590 using a probe‐based assay as previously described. Two different probe based assays, Invader Plus and the TaqMan probe assay, were used, and their sensitivity and specificity were compared with those of direct sequencing. Data were analyzed using SPSS 17.0 (SPSS for Windows, Chicago IL).
Results
We found that rs12979860 and rs8099917 were informative markers of treatment response in Uzbekistan with different ethnicity. The rs8099917 genotype TT was the most common in the overall study population (67.8%), followed by rs12979860 genotype CC (49%). This is the first report showing the distribution and linkage between IFNL4 ss469415590 SNP and IL28B rs12979860 SNP in HCV patients in Uzbekistan. Interestingly, LD degree between rs12979860 and two SNPs within the IL28‐encoding gene identified herein was slightly different in two populations studied, i.e., a strong LD was observed among patients of EE ancestry. Moreover, a very low LD between rs8099917 and SNPs was found within the IL28B‐encoding region. Nevertheless, both genotypes of rs12979860 and rs8099917 were independent predictors of treatment outcome, suggesting different mechanisms of involvement of the IL28B genetic regions.
Caption 1: SNPs Showed Statistical Significance in Predicting Treatment.

Conclusions
The rs8099917 SNP was a better predictor of treatment outcome in subjects of CA ancestry than the rs12979860/ss469415590 SNPs. The reverse tended to be true for patients with EE ancestry. One explanation for this is the smaller size of this group; demanding a larger EE group investigations to confirm this trend. Predictive power of genetic markers ranges across different reports reinforcing importance of meta‐analyses within populations with different ethnic background.
Sex and Compliance
4A‐S20‐01
Blood Donation and Ethics – Personal Reflections on the ISBT Code of Ethics
Flanagan P
New Zealand Blood Service, Auckland, New Zealand
The Code of Ethics of the International Society of Blood Transfusion (ISBT) was first published in 1981. The Code has since been revised on two occasions, most recently in 2006 but overall the content remains the same. It has been endorsed by the World Health Organisation, International Federation of Red Cross and Red Crescent Societies and by the International Federation of Donor Organisations. The objective of the Code is ‘to define the ethical principles and rules to be observed in the field of Transfusion Medicine’ and identifies an intention that ‘these should form the basis of national legislation and regulation’. The Code is therefore a tool for advocacy and as such defines ‘what we believe should exist’ as opposed to simply reflecting the reality of the current environment.
The main driver to the establishment of the Code was increasing concern from professionals involved in the field of transfusion of the impact of the development of commercial plasma industry utilising plasma collected from individuals who received payment. The dominance of the commercial industry has increased significantly over the last 35 years and inevitably the legitimacy and relevance of the Code has been, and continues to be, challenged. The origin of the Code is closely linked to the adoption of World Health Assembly resolution 28.72 on ‘utilization and supply of human blood and blood products’ and to the principle of voluntary non‐remunerated donation (VNRD) and a social solidarity and cohesion perspective of the activities associated with blood donation.
Blood services exist in order to provide sufficient safe and effective blood products for patients. The role of the volunteer donor in this process is unique in modern healthcare. The health, safety and wellbeing of the donor is paramount and must not be compromised to meet patient needs. The Code as currently presented identifies two sets of requirements. The first relating to the rights and responsibilities of the donor and the second to the rights of the patient and the responsibilities of professionals involved in their care. The ethical principles underpinning the requirements are not however provided and there is no clarity as to which stakeholder the requirement applies. These issues will be addressed by a current review of the Code. This process should lead to the development of a framework which will support informed discussion on controversial issues and assist in building consensus moving forward.
Critics of the Code identify concerns relating to the difference between the identified requirements and current practice (the real world). Some of these concerns are valid and need to be considered as part of the current review to ensure that the Code continues to be relevant. Professionals do however have a responsibility to continue to promote what they believe is right but in doing so they should also demonstrate a real commitment to ensuring that practice progressively moves closer to this being achieved.
4A‐S20‐02
Donor Compliance in the Netherlands Regarding Male to Male Sex
Romeijn B 1, van Dongen A 1, Merz EM1, Kok GJ 2, de Kort WLAM 1
1Sanquin Bloedvoorziening, Amsterdam, The Netherlands2Maastricht University, Maastricht, The Netherlands
Background
Men who have had sex with men (MSM) are permanently deferred from blood donation in the Netherlands. The policy is based on the precautionary principle and was introduced in the 1980s to prevent blood‐borne viruses like HIV from entering the blood supply chain. Since then, blood testing has been introduced which has led to a significant reduction in (HIV‐)contaminated donations. Improvements in testing have also led to a reduction in the length of the window period between infection and detection of the infection. Therefore the question arises whether it is necessary to retain the ban on blood donation for MSM. To address this question and determine future donor deferral criteria, it was necessary to assess the donor compliance and reasons for non‐compliance with the current permanent deferral for MSM. Donor compliance is the degree to which donors truthfully disclose their behaviour to their blood supply organization. The level of compliance directly affects the efficacy of donor deferral criteria. To date there have been no studies to investigate donor compliance in the Netherlands regarding male‐to‐male sexual behaviour.
Aims
To assess the rate of donor compliance in the Netherlands regarding male to male sex, and to find out what reasons male non‐compliant donors have for not reporting their sexual experiences with men when donating blood.
Methods
We sent out an invitation for an anonymous online questionnaire to 54.734 (50.000 male and 5.000 female) active blood donors. The response rate was 37.7% (Men, n = 18.137; Women, n = 2.520). The content of the questionnaire was largely based on literature research and on results of a focus group, where MSM (n = 3) could express their views regarding the ban on blood donation and the potential lifting of this ban.
Results
We found that 1.4% of male donors were non‐compliant regarding disclosure of male to male sexual behaviour. Of these donors 67.2% reported that their sexual experience(s) with men happened just once, 45.1% a long time ago, or 37.2% occasionally. Also, feelings of discrimination (27.7%) and feelings of shame (38.3%) were found to play a role. Of the non‐compliant donors, 35.2% had an under‐inclusive definition of their sexual experience(s) with men. Under‐inclusive means that men who initially reported not to have had sex with a man, after reading a definition of male‐to‐male sex, did report to have had sex with a man.
Summary/Conclusions
Self reported donor compliance regarding male to male sexual behaviour is high in the Netherlands and corresponds with percentages found in other studies worldwide. It is likely that the majority of the current MSM non‐compliant donors would be eligible to donate under a revised policy without jeopardizing the safety of blood products. Reconsideration of the lifetime ban on blood donation for MSM is not discouraged by the current level of non‐compliance with the permanent deferral for MSM.
4A‐S20‐03
Getting Personal with UK Blood Donors – The Findings of a Large Scale Anonymous Behavioural Survey to Assess Understanding of, and Compliance with, Donor Selection Guidelines
Davison KL1, Reynolds CA 2, Andrews N 1, Brailsford SB 2
1Public Health England, London, United Kingdom2NHSBT/PHE Epidemiology Unit, London, United Kingdom
Background
Blood donor selection criteria are designed to select healthy individuals at low risk of infection; compliance with the donor selection guidelines (DSG) is important in ensuring blood safety. Compliance is thought to be affected by donor understanding of the guidelines, and their motivation for giving blood. On behalf of the four UK blood services, Public Health England undertook a large scale unlinked anonymous web‐based electronic survey to assess this. Here we describe the overall findings.
Aims
To determine the extent of compliance with the DSG concerning behaviours related to an increased risk of infectious diseases among blood donors and to gather information on reasons for non‐compliance.
Methods
Each month for 1 year, all eligible new donors and at least an equal number of repeat donors who had given whole blood were invited to participate via an email from the blood services, followed by two reminders. After the survey closed, response rates (with 95% confidence intervals, CI) were calculated, and the characteristics of the participating blood donors were described with their representativeness determined where possible. Responses to DSG questions relating to deferrals due to health and wellbeing, travel, overseas residence, history of infection, piercing, acupuncture and tattoos, sexual behaviour and drug use were reviewed and donors were categorised as compliant or not, and the rate (with 95% CI) of compliance estimated and compared among subgroups. Reasons for non‐compliance and understanding of the DHC were described.
Results
Of the 225,091 blood donors surveyed, 65,484 responded (29%), exceeding the 50,000 target. Responders were defined as providing demographic information at a minimum and 9 out of 10 responders completed the whole survey. Response rates were higher among repeat donors (31.3%, 95% CI 31.0–31.6%), females (30.7%, 95% CI 30.4–30.9%), older donors aged over 55 years (39.1%, 95% CI 38.7–39.6%), and those of white ethnicity (32.6%, 95% CI 32.4–32.8%). Compliance with the deferrals of interest among all responders exceeded 99.2% in each case, with evidence of a small but significant difference between new and repeat donors in some cases. The most frequently reported reason for non‐compliance was a lack of perceived risk by the donor. Responders who did not understand the eligibility criteria or did not agree with the rationale of DSG had lower rates of compliance. Test seeking was reported in 1.2% of responders, increasing to 1.6% in those who were non‐compliant.
Summary
UK blood donors responded well to the survey; response rates were good, completion was excellent and donors disclosed behaviours of a personal nature. Compliance with the DSG of interest was very good, however, seemed to be inversely associated with an understanding of the DSG, among other reasons. Responders were marginally more likely to be female, repeat donors of white ethnicity although among the very large number of donors, a broad range of subgroups were represented. Compliance among sub groups of responders will be further explored, along with a broader assessment of reason for non‐compliance, in order to inform blood donor policies.
4A‐S20‐04
‘Highlights on Donors’ Nightlives’ – Findings on Sexual Behaviours from the UK Blood Donor Survey
Davison KL1, Reynolds CA 2, Andrews N 1, Brailsford SB 2
1Public Health England, London, United Kingdom2NHSBT/PHE Epidemiology Unit, London, United Kingdom
Background
In accordance with the UK Donor Selection Guidelines (DSG), individuals with sexual behaviours associated with an increased risk of infection are deferred from blood donation to minimise the risk of an early, undetected infection being transmitted. Understanding the extent of these behaviours among blood donors and the degree of compliance with the current deferrals is important in informing donor selection policy. The UK blood donor survey asked donors about their recent sexual behaviour including partner gender, number of new partners, age at first sex, and whether the partner would be considered to have high‐risk behaviours by the DSG, e.g. person who has injected drugs (PWID) or (for females) a male who has had sex between men (SBM).
Aims
To determine the sexual profile of UK blood donors and assess compliance with DSG concerning sexual behaviours.
Methods
An anonymous and unlinked electronic survey of 225,000 new and repeat UK donors was undertaken over 12 months. Answers to the questions relating to sexual behaviour were reviewed. Donors were described by gender and age, and classified as compliant or not with DSG concerning sexual behaviours. The rates of compliance with 95% confidence interval (CI) were estimated.
Results
Of the 65,484 donors responding, 63,311 (96.7%) answered the sexual history questions. Of donors reporting sex ever, 71.7% (45,365/53,477) had had sex within 12‐months; 15.8% (7517/45,365) had at least one new partner. Sex within 12‐months was less common among male donors with same sex partners (34.7%, 87/251), but more common for young donors aged 17–24 (89%, 6718/7507), however the proportion with at least one new partner within 12‐months was higher among these subgroups with 82.2% (72/87) and 46.5% (3139/6718) respectively. History of STI was 5.5% (95% CI, 5.4–5.7%) among donors who had ever had sex, and 11% (95% CI, 7.5–15.7%) among males with same sex partners. Among all responders reporting ever having had sex, compliance with each of the sexual deferrals ranged between 99.8% (sex < 12 months partner had sex in an HIV endemic country, 95% CI 99.74–99.82%) and 99.95% (paid for sex, 95% CI 99.93–99.97%) with evidence of a small but significant difference between new and repeat donors for some deferrals. Among males who reported ever having sex, compliance with the 12‐month deferral of men who have sex with men (MSM) was 99.6% (95% CI 99.5–99.69%).
Summary
A high proportion of donors responded to the sexual behaviour questions, with many disclosing personal and sensitive information. Despite an overall high proportion of donors with recent sexual activity, this was lower among male donors reporting SBM, attributable to the MSM deferral. However, a high proportion of MSM and young donors reported more than one new partner within 12‐months and a history of STIs, suggesting riskier sexual behaviour among these groups. Compliance with sexual deferrals was very good, however further analyses among a range of sub‐groups will be undertaken to assess this further. These sexual profile data are unique for UK donors and will help to inform future sexual behaviour deferrals.
4A‐S20‐05
A New Evaluation of the Risk of Transfusion‐Transmitted HIV Prevented by a 12‐Month Deferral Before Donation for Men Who Have Sex with Men in France
Pillonel J1, Santos A 1, Lot F 1, Martinaud C 2, Djoudi R 3, Laperche S 4, Jauffret-Roustide M 5, Danic B 6
1Institut de Veille Sanitaire, Saint‐Maurice Cedex, France2Centre de Transfusion des Armées, Clamart, France3Etablissement Français du Sang, Saint‐Denis, France4Institut National de la Transfusion Sanguine, Paris, France5CERMES 3 – INSERM, Paris, France6Etablissement Français du Sang – Bretagne, Rennes, France
Background
In France, men who have sex with men (MSM) are permanently excluded from blood donation. This selection criterion has been the subject of debate, mainly because it can be felt to be discriminatory and outdated. In addition, this criterion is not fully respected since some MSM do not report before donating they have had sex with men.
Aims
We estimated the excess risk of HIV associated with the lack of compliance of MSM with the current criterion and assessed the rate of non‐compliance. We then estimated the impact of a new strategy in which men would be deferred for 12 months after the last sexual contact with a man.
Methods
The study‐period included 3 years, from 2011 to 2013. First, we estimated the fraction of the current HIV residual risk attributed to MSM, thanks to information on the mode of transmission obtained during the post‐donation medical interview. From these estimates and HIV epidemiological data in France, we assessed the non‐compliance rate with the current criterion. Lastly, using several assumptions of non‐compliance with a 12 month‐deferral found in the literature, we evaluated the impact of the new strategy on HIV residual risk.
Results
Twenty‐four HIV seroconversions were observed among blood donors who had made at least two donations during the study‐period, accounting for an incidence of 0.9 per 100,000 person‐years and a residual risk of one in 3,450,000 donations. Of them, 15 (64%) were MSM. If all MSM had abstained from donating blood during the study period, the risk would have been 1 in 9,000,000 donations, a 2.6 fold reduction in the current risk. Non‐compliance to permanent deferral of MSM, calculated in respect to the entire population of male blood donors, would be between 0.32% and 0.41% over the 2011–2013 period. Lastly, based on a non‐compliance varying from 0.08% to 0.33% with a 12 month‐deferral, the new strategy would result in an overall HIV residual risk from 1 in 6,000,000 (1.7 times lower than the current risk) to 1 in 3,300,000 donations (close to the current risk).
Conclusions
A change from lifetime exclusion of MSM to 12‐month deferral does not seem to increase the risk of transfusion‐transmitted HIV in France. As already reported, compliance is better for a 12‐month deferral than for lifetime exclusion; thus the number of HIV incident infections and HIV residual risk are expected to decrease. As compliance is a key parameter, we implemented in 2014 a sociological study among donors found HIV positive at the time of donation with the objective to explore knowledge, attitudes and motivations of these donors. This study based on in‐depth qualitative interviews and ethnographic observations will allow us to understand the social context of blood donation that should improve the pre‐donation medical interview and, therefore, the blood safety.
For the Blood Donor Epidemiological Surveillance Study Group of the French Institute for Public Health Surveillance.
Plenary Session: Novel Insights in Physiology & Transfusion
4B‐PL2‐01
NETS – the Second Function of Chromatin
Zychlinsky A
Max Planck Institute for Infection Biology, Berlin, Germany
Neutrophils are one of the first lines of defence of the immune system against microbes. These cells kill microorganisms effectively by phagocytosis and by the formation of extracellular structures, called Neutrophil Extracellular Traps (NETs). NETs are made of chromatin and specific neutrophil proteins and are released after a unique cell death program that requires the production of radical oxygen species (ROS) and the relocation of neutrophil elastase to the nucleus. NETs help limit and control infection and also can activate the acquired immune system. Thus, formation of NETs appears to be necessary for an efficient clearing of microbes but can also initiate and exacerbate autoimmune responses.
4B‐PL2‐02
Current Concepts in TRALI Pathogenesis
Looney MRL
University of California, San Francisco, San Francisco, CA, United States of America
Transfusion‐related acute lung injury (TRALI) is the leading cause of mortality from blood product transfusions. Clinical and experimental studies support a two‐event model of injury in which a variety of risk factors (‘first hit’) render the transfusion recipient susceptible to lung injury when transfused with a blood product containing antibody and non‐antibody mediators that provide the ‘second hit’ in the model. Our understanding of the mechanisms of antibody‐based TRALI have been advanced by the MHC Class I monoclonal antibody model of TRALI in mice. Challenge of LPS‐primed BALB/c mice with cognate MHC Class I antibody produces severe protein‐rich pulmonary edema and the lung injury response is critically dependent on the involvement of neutrophils and platelets. In the lung microcirculation, neutrophils and platelets spatially interact forming heterotypic aggregates that can be blocked with aspirin, which also reduces lung injury and mortality in a lipoxin‐dependent pathway. Activated platelets also trigger the formation of neutrophil extracellular traps (NETs), which can be found in the lungs and blood of experimental and clinical cases of TRALI. Several lines of evidence point to the pathogenicity of NETs in TRALI. Inhibiting the formation of NETs by targeting platelet activation (aspirin) ameliorates lung injury. Dismantling the NET structure using DNase1 or inhibiting extracellular histones, a key component of NETs, also robustly protects from lung injury. In summary, experimental modeling of TRALI has identified a novel pathophysiologic cascade involving neutrophils, platelets, and NETs and has in turn illuminated rational therapeutic approaches for the prevention or treatment of TRALI.
4B‐PL2‐03
TITRe2
Murphy GJ
University of Leicester, Leicester, United Kingdom
Strong associations between transfusion and adverse outcome in observational studies and a causal relationship between more liberal transfusion and death in RCTs of liberal vs restrictive transfusion thresholds in non‐cardiac surgery have led to recommendations that restrictive transfusion thresholds be applied as the standard of care. However, there is uncertainty as to whether this evidence is relevant to cardiac surgery patients and this is reflected by wide variation in clinical practice.
To address this uncertainty we sought to test the hypothesis that more liberal transfusion is harmful than more restrictive transfusion in anaemic cardiac surgery patients in a pragmatic RCT. The TITRe2 trial (http://www.controlled-trials.com/ISRCTN70923932), was a multi‐centre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and healthcare resource use following cardiac surgery. Two thousand patients in 16 UK cardiac centres were randomised to either a restrictive transfusion threshold of 7.5 g/dl or a liberal threshold of 9 g/dl with ischaemic and septic complications as a co‐primary endpoint. The trial design addressed important sources of bias that have been identified in previous studies. The results, along with those of other similar trials will be discussed.
Parallel Sessions
Red cell Enigmas
4C‐S21‐01
From GWAS to Function: Lessons from Blood Cells
Soranzo NS
WTSI, Cambridge, United Kingdom
Background
Haematopoiesis is the process whereby mature blood cell lineages are produced from the differentiation of self‐renewing haematopoietic stem cells. Human genetic variation is known to impact on blood cell traits that are regularly measured as part of a clinical assessment of full blood count. Understanding the heritability of these traits and their variation in different human populations can inform our knowledge of both disease mechanisms and discovery of new genes involved in haematopoiesis.
Aims
Here we review recent results of high‐powered meta‐analysis of genome‐wide association studies (GWAS) linking genomic loci to traits affecting blood formation in humans. A large number of these polymorphisms have been replicated in other human populations and validated to have functional effects in mice, zebrafish and fruitfly.
Methods
We discuss strategies and challenges (i) in finding functional and regulatory effects of genetic variants considering that the majority are found in non‐protein coding and outside of genic regions, (ii) in prioritizing most likely affected genes, (iii) in prioritizing genetic variants that are most likely to be the causal variant affecting phenotype, and (iv) approaches to functionally validate these effects via various experimental techniques. We evaluate new efforts in mapping the first blood epigenome, next generation sequencing‐based association studies and the implications of publicly available high dimensional epigenetic data supporting extensive genome‐wide regulatory evidence.
Results
Up to date, there are more than 211 genetic variants in 128 genomic loci that are associated to ten haematological traits. The variants are near to genes that have non‐random enrichments in genes implicated in haematopoietic biological processes and linked with Mendelian blood diseases. We further demonstrate the gain in using epigenetic data from blood tissues for annotating and prioritising genetic variants.
Summary
Overall, the findings highlight the value of hematopoiesis not only as a cell development model, but also as a highly tractable system for complex disease.
4C‐S21‐02
Mass Spectrometry Reveals the ENT1/SLC29A1 Transporter as a New Carrier of a Non‐ABH Blood Group Antigen
Ballif BA1, Hélias V 2, Saison C 2, Peyrard T 2, Cartron JP 2, Arnaud L 2
1University of Vermont, Burlington, VT, United States of America2Institut National de la Transfusion Sanguine (INTS), Paris, France
Background
The equilibrative nucleoside transporter 1 (ENT1/SLC29A1) is a major protein of the red cell membrane and, with the glucose transporter 1 (GLUT1/SLC2A1), constitutes ‘band 4.5’ in sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. While these two SLC transporters were identified as major carriers of the ABH antigens, they are not known to carry other blood group antigens unlike the AE1/SLC4A1 and UT1/SLC14A1 transporters that carry the Diego and Kidd antigens, respectively.
Aims and Methods
In an effort to elucidate the molecular basis of uncharacterized alloantibodies targeting antigens on red cells, we set up an approach that combined immunoprecipitation and mass spectrometry‐based protein identification. Previously we used this approach along with monoclonal antibodies to identify two ABC transporters, ABCB6 and ABCG2, as the carriers of the Lan and Jra antigens respectively (Helias et al. (2012) Nat. Genet., Saison et al. (2012) Nat. Genet.). Here we employed this same approach but with the serum of a group AB pregnant woman which reacted strongly with a high‐frequency blood group antigen that was apparently independent of all known systems and collections.
Results
By mass spectrometry analysis, her alloantibody was found to immunoprecipitate the ENT1/SLC29A1 transporter from red cell membranes. Sequencing of the SLC29A1 coding exons and flanking regions revealed that the woman was homozygous for a mutation predicted to have a deleterious effect on SLC29A1 splicing. The mutation was absent from all sequenced controls and the NCBI dbSNP database, and was not found by the NHLBI GO Exome Sequencing Project and the 1000 Genomes Project. Immunoblot analysis revealed that her red cells were totally deficient in the ENT1/SLC29A1 transporter.
Summary and conclusion
We identified the ENT1/SLC29A1 transporter as the target of an alloantibody produced by a pregnant woman who was homozygous for a null allele of SLC29A1. Our results place SLC29A1 as a third SLC transporter gene coding for a blood group system.
4C‐S21‐03
The Search in Resolving the Orphan Status of the Red Blood Cell Antigen August (Ata)
McBean R 1, Wilson B 2, Liew Y-W 2, Hyland C 1, Kupatawintu P 3, Emthip M 3, Flower R1
1Research and Development, Australian Red Cross Blood Service, Brisbane, Qld, Australia2Red Cell Reference Laboratory, Australian Red Cross Blood Service, Brisbane, Qld, Australia3Red Cell Reference Laboratory, National Blood Centre, Thai Red Cross Society, Bangkok, Thailand
Background
August (Ata) is a high‐frequency red blood cell (RBC) antigen found on the RBCs of over 99% of individuals. Previous studies suggest Ata is encoded by an autosomal dominant gene independent from ABO, Rh, Kidd, MNS, P1 and Xg blood group systems, though no further information on the basis of Ata is known. The At(a−) phenotype is extremely rare worldwide, having been identified in individuals of West African or West Indies ancestry only. Anti‐Ata has been described in At(a−) individuals though the clinical significance is largely uncharacterised due to rarity. Case studies implicating anti‐Ata in a mild case of haemolytic disease of the fetus and newborn (HDFN) and a severe haemolytic transfusion reaction have been reported. A case of mild HDFN in a twin pregnancy suspected to be caused by anti‐Ata was recently referred to our reference laboratory.
Aim
To undertake a family study utilising massively parallel sequencing (MPS) to resolve the orphan antigen status of Ata.
Methods
Blood samples were obtained from the mother, her husband and their newborn twins (n = 4). Serological investigations were performed to identify the antibody implicated in the HDFN. DNA samples were exome sequenced using the Agilent SureSelect DNA Human All Exon V5+ UTRs on an Illumina HiSeq 2500. Sequence alignment was performed using the Illumina CASAVA1.8.2 pipeline and mapped to the reference genome hg19.
Results
It was identified the mother was At(a−) and that anti‐Ata was implicated in her HDFN. MPS identified 126,575 variants, including single nucleotide variants (SNVs) and insertions and deletions (INDELs) within the four individuals. It was assumed the genetic basis of the mother's At(a−) phenotype would be previously unreported, thus variant filtering was performed to exclude variants with a NCBI dbSNP ID (n = 117,562). Next, variants which did not fit the hypothesised inheritance model were excluded and this was done on the basis of allele count (AC) scores. An AC defines the number of times within the dataset the variant has been observed; we excluded variants without a total AC score of 4 as it was assumed the mother would be homozygous for the variant causing her At(a−) phenotype (AC = 2), her husband would be wildtype homozygous (AC = 0) and their twins would be heterozygous (AC = 1 and AC = 1). Following this step, 871 variants remained and 58 of these were predicted to be protein‐coding.
Summary/Conclusions
MPS has allowed the identification of 58 variants as a putative genetic basis for the At(a−) phenotype. The genes associated with these variants are being examined and several will be studied further as they are hypothesised to be more likely candidates for encoding Ata. Indeed, a small selection of these variants are in genes already reported to encode proteins in the RBC membrane. In the near future we hope to confirm the resolution of the genetic basis of Ata and of the rare At(a−) phenotype in this family. Overall, this study highlights the utility of MPS in investigating novel genetic basis of RBC antigens.
4C‐S21‐04
Four Examples of Anti‐CD99 and Discovery of the Molecular Bases of the Rare CD99− Phenotype
Thornton NM1, Karamatic Crew V 2, Muñiz-Diaz E 3, García- Arroba J 3, Nogués N 3, Lee E 4, Jones C 5, Schistal E 6, Jungbauer C 6, Allhoff W 6, Bullock T 7, Marais I 7, Daniels G 7
1IBGRL, Bristol, United Kingdom2IBGRL/BITS, NHS Blood and Transplant, Bristol, United Kingdom3Blood and Tissue Bank of Catalonia, Barcelona, Spain4NHS Blood and Transplant, Colindale, United Kingdom5Peterborough City Hospital, Peterborough, United Kingdom6Austrian Red Cross Headquarters, Blood Service for Vienna, Lower Austria and Burgenland, Austria7IBGRL, NHS Blood and Transplant, Bristol, United Kingdom
Background
Xga (XG1) and CD99 (XG2) comprise the XG blood group system. CD99 is a high incidence antigen with a phenotypic relationship with Xga (XG1). Both antigens are encoded by closely linked homologous genes CD99 (X and Y chromosome) and XG (X chromosome). All Xg(a+) individuals are CD99 high expressors; all Xg(a−) females are CD99 low expressors and Xg(a−) males can be CD99 high or low expressors. Only two CD99‐ probands have been previously described, both were Japanese donors with anti‐CD99 in their plasma.
Aim
To present findings from the investigation of three unrelated patients found to have anti‐CD99 in their plasma, including serological and molecular characterisation of the CD99‐ phenotype and family studies.
Case studies and methods
Patient 1 (P1): 80 year old Spanish female, with a history of several pregnancies, samples received post‐knee surgery. Patient 2 (P2): 20 year old female from the UK, samples received during second pregnancy. Patient 3 (P3): 60 year old Austrian female with history of transfusion (received 30 RBC units during a surgery in 1990) and five successful pregnancies, awaiting a planned surgery. Serological investigation was performed by standard LISS tube and BioRad gel IAT techniques. RBCs were typed with Monoclonal anti‐CD99 (12E7). Genomic DNA and mRNA were isolated from whole blood of the patients and their families. 10 exons of CD99 were amplified by PCR from gDNA. CD99 cDNA was prepared from mRNA. All PCR products were directly sequenced.
Results
Anti‐CD99 was identified in the plasma of each patient and also the sister of P3, reacting by IAT with untreated and AET treated cells but not reacting with papain, trypsin, α‐chymotrypsin and pronase treated cells. The pattern of reactivity with cells of known sex and Xga type reflected the expected strength variation with predicted high and low CD99 expressors. At least one example of allogeneic CD99‐ cells was tested and found to be compatible with each example of anti‐CD99 identified.
Table 1. Results of Xga and CD99 typing of patients and family members.
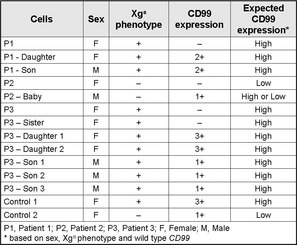
Sequencing of CD99 from gDNA, and subsequently cDNA, revealed all three patients to be homozygous for different deletions of a large portion of their CD99 gene: P1, exons 3–7 [c.(100 + 1_101‐1)_(361 + 1_362‐1)del]; P2, exons 4–8 [c.(148 + 1_149‐1)_(475 + 1_476‐1)del]; P3 and her sister, exons 2–8 [c.(67 + 1_68‐1)_(475 + 1_476‐1)del]. These deletions did not alter the translational reading frames, but would potentially cause loss of a number of amino acids (87 in P1, 109 in P2, 136 in P3) and encode severely truncated CD99 glycoproteins. In all three cases, each patient's children were heterozygous for their respective deletions.
Conclusion
We present evidence of four individuals with anti‐CD99 and the rare CD99‐ phenotype resulting from three different molecular backgrounds. Inheritance is shown through two generations of each patient's family. This is the first time the molecular bases for the CD99‐ phenotype have been described.
4C‐S21‐05
Molecular and Biochemical Characterization of the SMIM1 Protein Defining the New VEL Blood Group System
Kelley LPK1, Arnaud L 2, Hélias V 2, Cartron J-P 2, Ballif BA 1
1University of Vermont, Burlington, VT, United States of America2Institut National de la Transfusion Sanguine (INTS), Paris, France
Background
Recently SMIM1, formally a hypothetical gene, was found to underlie the VEL blood group system (Ballif et al. (2013) EMBO Mol. Med., Storry et al. (2013) Nat. Genet., Cvejic et al. (2013) Nat. Genet.). SMIM1 encodes a 78 amino acid protein that is predicted to contain a single transmembrane domain toward its C‐terminus. However, little is known about the function of SMIM1 and even its membrane orientation has been variably speculated (extracellular N‐ or C‐terminus). Our mass spectrometry analysis led us to hypothesize that the Vel antigen lies in the C‐terminus of SMIM1, and that posttranslational modifications of the N‐terminus of SMIM1 might regulate its trafficking to or insertion into the membrane.
Aims and Methods
To resolve the membrane orientation of SMIM1 and to test the role of posttranslational modifications on Vel antigen presentation we created an array of mutant SMIM1 expression constructs, expressed them in heterologous cell lines and analyzed the expressed proteins biochemically and by flow cytometry.
Results
Mass spectrometry identified a site of acetylation and several sites of phosphorylation on SMIM1 purified from red cells and harboring the Vel antigen. We confirmed that SMIM1 was significantly phosphorylated using immunoprecipitation, phosphatase treatment and electrophoretic mobility shift assays. However, flow cytometry analysis of cells expressing mutant SMIM1 expression constructs that abolish these sites of posttranslational modification did not show a significant role on cell‐surface expression of the Vel antigen. Finally, flow cytometry analysis of cells expressing various truncation mutants of SMIM1 definitively showed that the Vel antigen is presented by the C‐terminus of SMIM1.
Summary and conclusion
Molecular and biochemical analysis identifies SMIM1 to be posttranslationally‐modified in RBCs and heterologous cell lines at several residues, and that SMIM1 presents the Vel antigen as a type II membrane protein.
(Re)emerging Viral Infections
4C‐S22‐01
Hepatitis E and Transfusion‐ A Threat to Patients or a Threat to Pigs?
Tedder RS
NHS Blood and Transplant, London, United Kingdom
No abstract available.
4C‐S22‐02
Occurrence of Transfusion‐Transmitted Hepatitis E in France
Djoudi RD1, Gallian PG 2, Roques-Afonso AMRA 3, Bierling PB 4, Assal AA 5, Hauser LH 4, Izopet JI 6, Tiberghien PT 1
1Etablissement Français du Sang, La Plaine St Denis, France2Etablissement Français du Sang Alpes Alpes Méditerranée, Marseille, France3Virology, CHU Paul Brousse, Villejuif, France4Etablissement Français du Sang Ile de France, Créteil, France5Etablissement Français du Sang Aquitaine Limousin, Bordeaux, France6CNR VHE CHU Toulouse, Toulouse, France
Background
Hepatitis E virus (HEV) is subject to renewed interest in transfusion especially in immunocompromised patients. Seroprevalence within the French population has been recently evaluated to 25% and positive RNA blood donation frequency is estimated at 1/3000 (study on 12,000 plasma donations, Gallian et al, EID, 2014).
Aim
To describe the 16 cases of HEV transfusion‐transmitted infection (HEV‐TTI) reported in France between 2006 and 2013
Methods
Reporting of any adverse reaction which is or may be related to the transfusion of blood products is mandatory in France. Each notification is classified according to severity and imputability. For HEV‐TTI cases, imputability is considered certain if viral phylogenetic analysis conclude to identity between viruses present in the blood product and in the recipient as determined by homology on partial sequences of the open reading frame regions 1 and/or 2).
Results
The first documented case of HEV‐TTI was recorded in France in 2006. Since then till end of 2013, 16 cases of HEV‐TTI have been reported, most of these cases having occurred in 2012 and 2013 (Table). Blood products involved included red blood concentrates (n = 5), platelet concentrates [pooled whole blood‐derived (n = 3) and apheresis (n = 1)] and plasma (n = 7), including Solvent‐detergent (SD)‐plasma (n = 4, and plasma treated by amotosolen‐HCL+UV‐A illumination (Intercept)(n = 2), thus establishing resistance of HEV to such pathogen reduction technologies. Patients, aged 5–89 years old, were for half of them kidney (n = 5) or liver transplants (n = 3) recipients, having often undergone plasma exchange. Other underlying conditions included nephroblastoma, microangiopathy, acute auto‐immune hemolytic disease, myelodysplasia, chronic lymphocytic leukemia and acute leukemia. Transfusion‐transmitted hepatitis E resolved spontaneously in 7 patients while persisted as chronic hepatitis requiring ribavirine treatment in 9 other patients, all immunosuppressed, and among which 2 patients developed significant liver fibrosis. Viral strains involved were all of genotype 3. In most cases, viral phylogenetic analysis established transfusion imputability (considered certain in 15, and probable in the last case). Since HEV infection is not regularly sought, and in addition may be asymptomatic, exact frequency of transfusion‐associated hepatitis E is certainly underestimated. Lastly, a majority of these 16 cases were reported in one French region (Paris region) that transfuses 19% of blood products used nationally. Variable awareness by clinicians most probably contributes to such a finding.
Conclusion
Transfusion‐transmitted hepatitis E has emerged as a significant threat to transfusion safety. Immunosuppressed patients, in particular organ transplant recipients are at risk of transfusion‐transmitted chronic hepatitis E. Pathogen attenuation technologies such as SD or Intercept do not provide protection. HEV‐tested plasma for high risk patients is provided since January 2013 in France.
Caption 1: Reported transfusion‐associated hepatitis E in France.

4C‐S22‐03
Immunohaematological Prognostic Factors Associated with West Nile Virus Lineage 2 Disease Outcome in Greece, 2010–2013
Politis CP 1, Parara MP 1, Hassapopoulou EH 2, Iniotaki AI3, Papa AP 4, Richardson CR 1, Siorenta AS 3, Pervanidou DP 1, Baka AB 1, Kavallierou LK 1, A sariotou MA 1, Katsarou OK 5, Mougiou AM 6, Megalou AM 7, Magoula EM 8, Papadopoulou MP 9, Dadiotis LD 10, Alexandropoulou ZA 11, Hatzichristodoulou CH 1, Kremastinou TK 1
1Hellenic Centre for Disease Control and Prevention, Athens, Greece2Blood Centre Ahepa Hospital Thessaloniki, Thessaloniki, Greece3National Histocompatibility Centre Athens, Athens, Greece4Arboviruses National Refernce Laboratory Thessaloniki, Thessaloniki, Greece5Blood Centre Laiko Hospital Athens, Athens, Greece6Blood Centre Rio‐Patras, Patra, Greece7Blood Centre Evaggelismos Hospital Athens, Athens, Greece8Blood Centre Beroia Hospital, Beroia, Greece9Blood Centre Katerini Hospital, Katerini, Greece10Blood Centre Tzaneio Hospital Peiraeus, Peiraus, Greece11Blood Centre Asklipieion Hospital Athens, Athens, Greece
Background
Studies of the West Nile Virus (WNV) lineage 2 outbreak in Greece in 2010–2013 show a high case fatality rate (17%) and identified two risk factors associated with death related to West Nile neuroinvasive disease (WNND): age above 74 years and chronic renal failure. Other reports have indicated blood group A and D/Rh‐negativity as risk factors for symptomatic WNV infection.
Aims
We examined the association between disease outcome and ABO, D/Rh and Lewis blood group status as well as HLA class I and class II alleles in 132 WNV cases, including 78 with WNND. We evaluated the physical health of 70 patients 12–36 months after infection.
Methods
Blood samples were collected from the WNV patients in their homes in 2014. Eight blood establishments tested ABO, D/Rhesus and Lewis blood groups. The Lewis blood system controlling with the ABO and Se blood systems the secretor status of an individual was investigated for the first time in relation to the pathogenesis of WNV. Healthy blood donors from the WNV affected areas formed a control group (n = 51,339). HLA testing was performed by the National Histocompatibility Laboratory. An HLA control group was drawn from the healthy population of the same areas.
Results
Blood group A was significantly commoner in WNV cases than blood donors (51% vs 39%, P = 0.038). D/Rh negativity within blood group A was significantly higher in WNV cases (17.9%) than in blood donors (10.2%), P = 0.044). D/Rh negatives in blood group O were 7.1% in WNV and 11.4% in donors (P = 0.48). D/Rh negativity in group A was similar in the WNND and West Nile fever (WNF) groups (21.1% vs 16.2%, P = 0.94).
Secretor status, Lea(−) Leb (+), was detected in 58.2% of WNV cases and 67.5% of blood donors (P = 0.13. Its prevalence was 51.6% in WNF and 62.5% in WNND (P = 0.47).
HLA in 110 WNV cases showed that HLA‐C*08, DRB1*04:05, DRB1*10:01 and DQB1*02 alleles were all significantly less frequent in WNV cases than controls (P < 0.05 for each one). Statistically significant differences in HLA were not found betgween WNF and WNND.
Long term follow up of 70 WNV cases (66% WNND) showed full recovery from WNV symptoms in 16 patients including one with WNND acquired in 2012. Persistent morbidity with muscular weakness, anorexia and inability to deal with daily routine was observed in 69%, particularly in the WNND group (87.5%). Deterioration was observed in 6 patients (8.6%) with history of WNND.
Conclusions
This study, examining symptomatic WNV lineage 2 cases for the first time, confirms A/DRh‐ blood as a risk factor associated with WNV infection and level of morbidity. HLA‐C*08, DRB1*04:05, DRB1*10:01 and DQB1*02 alleles were all significantly less frequent in WNV cases than controls
Our study draws attention for the first time to the secretor status of blood group Lewis that might play a protective role against the WNV infection in humans. Further studies are required in order to confirm this finding and to examine the mechanisms for the pathogenesis of WNV infection.
4C‐S22‐04
Has the Introduction of CMV Untested Blood Components Affected the Rate of CMV PCR Positivity in CMV Negative Adults Receiving CMV Negative Allogeneic Haematopoietic Stem Cell Transplants?
Evans R1, Orr J 1, Wallis J 2, Waugh S 3, Jackson G 4, Collin M 4
1Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom2Department of Haematology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, United Kingdom3Department of Virology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom4Northern Centre for Bone Marrow Transplantation, Newcastle upon Tyne Hospitals, Newcastle upon Tyne, United Kingdom
Background
CMV is a herpes virus that can cause significant disease in the immunocompromised post allogeneic haematopoietic stem cell transplant (HSCT) recipient. In 2012 SaBTO (UK advisory committee on the safety of blood, tissues and organs) advised that leucodepletion of blood products was sufficient to prevent transfusion transmission of CMV from seropositive blood donors, and therefore, that unscreened blood products could be safely administered to allogeneic transplant recipients. This change was implemented in our centre in April 2012.
Aims
To assess any change in the incidence of CMV PCR positivity, following the use of CMV untested blood products for patients with low risk of CMV infection (CMV IgG negative donor and recipient).
Methods
A single centre retrospective audit of patient notes spanning the change in policy was performed (January 2010–April 2012 and April 2012–September 2014 respectively). Patients receiving allogeneic HSCT who were classed by the transplant team following serological testing as being CMV donor and recipient negative were included. CMV PCR results were obtained along with patient's transfusion history following transplant. Statistical analysis was performed using a chi‐square test.
Results
The demographics of the two groups are displayed in tables 1 and 2.
56 of 142 HSCT patients were CMV low risk prior to the use of CMV untested blood components. One patient became CMV PCR positive (prior to receiving any blood products post‐transplant) and subsequently tested positive on multiple occasions requiring treatment. This patient had an equivocal CMV IgG prior to transplant, and therefore, possible historical CMV infection.
43 of 152 HSCT patients were identified as CMV low risk following the change in policy, and were potentially exposed to unscreened blood products. Six became CMV PCR positive having been CMV IgG negative prior to transplant, a statistically significant difference (P = 0.030). Five of these patients only had a single low level CMV PCR positive result (all <2.4 × 104) which was negative on subsequent testing. The sixth patient had multiple positive CMV PCR results and required treatment.


Conclusion
We found a significant difference in incidence of CMV PCR positive results between our 2 groups following the introduction of use of CMV untested blood components (P = 0.030). This did not result in a significant increase in clinical CMV infections. The incidence of CMV positive PCR results in this population is greater than expected from community acquired CMV infection (around 1% per year). A larger scale study is required to determine if this is an isolated finding. Further work is also required to see if the transient PCR positivity observed in 5 of the post CMV untested blood component group is due to infection or passively acquired viral genome fragments and if this is clinically significant.
4C‐S22‐05
A Prospective Evaluation of Chronic b. Microti Infection In seroreactive Blood Donors
Bloch E1, Levin AE 2, Williamson PC 3, Cyrus S 3, Shaz B 4, Kessler D 4, Gorlin J 5, Bruhn R 1, Guiltinan A 1, Busch M 1
1Blood Systems Research Institute, San Francisco, CA, United States of America2Immunetics, Boston, MA, United States of America3Creative Testing Solutions, Tempe, AZ, United States of America4New York Blood Center, New York, NY, United States of America5Memorial Blood Center, St. Paul, MN, United States of America
Background
Babesia microti is the foremost infectious risk to the United States (US) blood supply for which an FDA licensed screening test is unavailable. Babesia species are globally ubiquitous and could pose a broader concern to international blood banking. However, data on the performance of potential screening assays and rates and duration of persistent Babesia infection in seroreactive blood donors are limited.
Aims
(i) Determine the proportions of B. microti seropositive donors that develop chronic vs transient/resolved infections. (ii) Establish a specimen repository for future study of mechanisms that underlie chronic Babesia infection.
Methods
During an FDA‐licensure trial, blood donors were prospectively screened (July 29‐November 15, 2013) using an ELISA (Immunetics) against B. microti in highly endemic (New York‐NY; n = 13,688), moderately endemic (Minnesota‐MN; n = 4583) and non‐endemic (New Mexico‐NM; n = 8451) regions. Blood donors with either repeat reactive (S/C ≥ 1.0) or gray zone (S/C ≥ 0.934–<1.0) results were enrolled into a prospective cohort study. Following initial screening and at each of 5 follow‐up visits (1–2 weeks, 3‐, 6‐, 9‐ and 12‐ months post index donation) index seroreactive donors were evaluated using B. microti ELISA, IFA and PCR in addition to peripheral blood smear and clinical questionnaires. Variables captured included donor demographics, travel outside state of residence, comorbid medical or surgical conditions (e.g. splenectomy), infections that may be associated, confused or co‐infect with B. microti (e.g. Lyme borreliosis, anaplasmosis, or malaria), time spent in grassy or wooded areas, tick bite/exposure and/or clinical symptoms of babesiosis.
Results
A total of 26,702 blood donors were screened in the parent trial. 134 (0.5%) donors screened seropositive (126 repeat reactive and 8 gray zone) of whom 87/134 (48 NY, 15 MN and 24 NM) consented to participate in the follow‐up study (median age 50 [18–89] years); 47% male; 77% non‐Hispanic Caucasian).
Importantly, 3/24 (12.5%) seroreactive donors in NM, a non‐endemic state, had travelled to Babesia endemic states in the year prior to donation. Fourteen (16.1%) subjects reported tick bites in the past year. 53/84 (63.1%) reported spending at least 1 h outdoors per week, with the majority (39/52 [73.58%] reporting >3 h/week. No (0%) donors reported splenectomy or history of organ transplantation.
Of those donors who were repeat reactive at index donation and completed a 12‐month follow‐up blood draw, 39/45 (86.67%) were still seroreactive at final visits, a median of 413 (372–475) days following index donation. A total of 9 PCR positive donors were identified during screening (8 from NY and 1 from MN) of which 4 participated in the follow‐up study. Three of the four PCR positive donors remained PCR positive at 6 months, 2 were still positive at 9 months and 1 remained PCR positive at final follow‐up (413 days).
Summary/Conclusions
The findings refine our understanding of Babesia infection and are consistent with prior reports of persistent seroreactivity in blood donors. Furthermore, although rare, protracted asymptomatic PCR positivity was also demonstrated. Therefore, repeat screening should be undertaken if donor reentry is to be considered. Finally, no clinical or behavioral risk factors were associated with persistent infection.
Transfusion Triggers
4C‐S23‐01
Intravenous Iron as Blood Saving Technique in Surgery, What is the Evidence?
Bisbe E
Hospital del Mar, Barcelona, Spain
Preoperative and postoperative anaemia are highly prevalent in major surgical procedure and condition the allogeneic transfusion (ABT). Preoperative anaemia and transfusion has been associated independently with worse postoperative outcome in patients undergoing major surgery. On the other hand, hematinic deficiency may blunt the recovery from postoperative anaemia. These both conditions could be corrected before elective procedures and/or in postoperative period, as the first pillar of Patient Blood Management (PBM).
In a NATA consensus statement on the management of peri‐operative anaemia, the panel suggested the administration of intravenous iron during the perioperative period for patients undergoing surgery who are expected to develop severe postoperative anaemia1 and have pure or functional iron deficiency. Many observational studies and few clinical assay show that intravenous iron is a safe and effective drug for treating preoperative anaemia with a median increase of 1–3 g/dl Hb in one month2,3. A randomized controlled trial shows that a dose of iv iron the day after surgery can be useful for improving postoperative anaemia, especially in patients with preoperative iron deficiency and/or severe anaemia4. Other studies show the utility of intravenous iron as a co‐adjuvant of few doses of erythropoietin for reducing ABT.5,6 The availability of new IV iron formulation that allows for fast administration of up to 1000 mg in a single dose, may reduce the number of treatment sessions, thus contributing to a wider use of IV iron as a blood saving strategy in surgical patients2.
However, it is noteworthy that many of the recommendations given for IV iron treatment are not supported by a high level of evidence. This indicates the need for further, large, randomized, controlled trials on the safety and efficacy of IV iron for treating anaemia in the perioperative setting
References
1. Beris P, Munoz M, Garcia‐Erce JA, et al. Perioperative anaemia management: consensus statement on the role ofintravenous iron. Br J Anaesth 2008;100:599–604.
2. Bisbe E, García‐Erce JA, Diez‐Lobo Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107(3):477–8.
3. Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology 2007; 107: 923–7.
4. Bisbe E, Molto L, Arroyo R, Muniesa J, Tejero M. A randomised, controlled trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia treatment after total knee arthroplasty. Br J Anaesth 2014.
5. Theusinger OM, Kind SL, Seifert B, et al. Patient blood management in orthopaedic surgery – a 4‐year follow‐up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus 2014;12:195–203.
6. Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta‐analysis of randomised clinical trials. BMJ. 2013;347:f4822.
4C‐S23‐02
Obstetric Bleeding and Transfusion
Jansen AJG1, Prick BW 2, Duvekot JJ 2
1Sanquin Blood Supply, Amsterdam, The Netherlands2Erasmus MC, Rotterdam, The Netherlands
Postpartum haemorrhage (PPH) is one of the top 5 causes of maternal mortality both in developed and developing countries. PPH is commonly defined as blood loss of ≥500 ml within 24 h after birth and severe PPH as blood loss of ≥1000 ml. The incidence is increasing over the last decade, with reports varying from 4% to 8% in the developed world up to 19% in developing countries. In the Netherlands we reported an incidence of severe PPH (defined as ≥1000 ml blood loss) of 4.5% in the period 2000–2008.
The causes of PPH can be classified into: (i) uterine atony, (ii) placental problems including retained placenta and abnormal placental implantation, (iii) genital tract trauma and (iv) systemic medical disorders (including inherited and acquired coagulation defects). As the majority of women who experience PPH complications have no identifiable clinical or historical risk factors, all women must be considered at risk and active third stage management is recommended in all women to prevent PPH. Risk factors associated with PPH can be divided into maternal and pregnancy characteristics, medical interventions and health care setting.
Maternal symptoms of PPH vary between none to death, but in general PPH leads to lower physical health related quality of life (HRQoL) scores, especially physical fatigue. Treatment of PPH involves medical, mechanical‐ and surgical methods or combinations of these methods. Despite several techniques to reduce blood loss during delivery, red blood cell (RBC) transfusion is often necessary in the treatment of women who suffered from PPH. Apart from the life‐saving restoration of the initial hemodynamic instability, RBC transfusion is also prescribed to treat the side‐effects of acute anaemia, including HRQoL, especially fatigue.
To determine the effect of RBC transfusion on HRQoL in patients with acute postpartum anaemia we performed a randomized non‐inferiority clinical trial. Women with acute anaemia (Hb 4.8–7.9 g/dl; 12–24 h postpartum and without severe anaemic symptoms) were allocated to RBC transfusion or a restrictive transfusion policy. With only small differences in physical fatigue scores and no differences in secondary outcomes we recommend implementation of a restrictive transfusion policy for this specific patient group. Additionally, this restrictive transfusion policy saves €438 per woman compared to a liberal transfusion policy. To determine clinical predictors for receiving RBC transfusion in women initially managed with a restrictive transfusion policy, we performed a secondary analysis. Independent predictors found were primiparity, multiple pregnancy, total blood loss during delivery and haemoglobin concentration postpartum. Adding HRQoL scores based on the Multidimensional Fatigue Inventory and EuroQoL‐5D questionnaires improved our model significantly. After external validation, the extended model may be an important tool for counselling and decision making in clinical practice.
Postpartum haemorrhage is an obstetrical emergency requiring an immediate response and a multidisciplinary approach. In this presentation we recommend:
1. an increased awareness of, and focus on prevention of postpartum haemorrhage;
2. implementation of a restrictive transfusion policy in women with acute PPH without severe anaemic complaints, and
3. implementation of HRQoL scores for counselling and decision making in clinical practice regarding transfusion policy after PPH.
4C‐S23‐03
Antepartum Fibrinogen Level as a Predictor of Bleeding Complications
Uhl L
Beth Israel Deaconess Medical CTR/Harvard Medical School, Boston, MA, United States of America
Background
Critical values for fibrinogen to identify patients at risk for hemorrhagic complications are based on average levels from non‐pregnant women and men. However, given that pregnant women experience a physiologic elevation in fibrinogen, the standard critical value of <100 mg/dl may not apply to this population, thereby indicating a need to further investigate fibrinogen levels and risk of hemorrhagic complications in the pregnant patient population.
Aims
To determine the correlation between fibrinogen concentration and bleeding complications among women presenting for delivery.
Methods
This was a nested case‐control study using a cohort of all women who delivered at our institution from October 1, 2000 to July 31, 2010 and in whom a fibrinogen level was obtained within 48 h prior to delivery. Cases consisted of women with one or more post‐partum bleeding complications (a ≥33% relative decrease in hematocrit, receipt of blood products, hemorrhage, and hysterectomy), and we included the first case delivery for a given woman. Controls were the next two consecutive deliveries without a bleeding complication and matched for number of fetuses. Case and control status were confirmed by individual chart review. We used logistic regression to calculate the odds ratio (OR) and 95% confidence intervals (CI) and calculated the area under the receiver operating characteristic curve (AUC).
Results
We identified 149 cases and 298 controls. The mean antepartum fibrinogen level was significantly lower in cases (459 ± 152 mg/dl) than controls (524 ± 119 ng/ml) for all bleeding complications combined (P < 0.001). This also was true for receipt of blood products (P < 0.01) and a ≥33% relative drop in hematocrit (P ≤ 0.04). For every 100‐unit increase in fibrinogen, the risk of a bleeding complication decreased by more than 30% (OR: 0.69; 95% CI: 0.59–0.81). However, the AUC was poor (0.62; 95% CI: 0.56–0.68). Below 200 mg/dl there were 11 cases and 0 controls, yielding perfect specificity but extremely low sensitivity (Table 1). We could not identify a cut‐off value that yielded acceptable values of sensitivity and specificity.

Conclusions
Antepartum fibrinogen level was significantly lower among women who developed bleeding complications, with the most pronounced difference for women who required blood products. However, these differences may not be large enough to provide clinically meaningful critical values. Nevertheless, consideration of a higher threshold for the critical value should be considered.
4C‐S23‐04
Prescription of Blood Components: Impact of a New Mandatory E‐Learning Program for Clinicians
Andersen Reeh MAR , Oevrehus AOE , Titlestad KET , Thue Lillevang STL
Odense University Hospital, Odense, Denmark
Background
In Denmark, prescription of red blood cells is remarkably high (48 per 1000 inhabitants) regardless of official guidelines by the National Health Board and continuing educational endeavours by transfusion medicine specialists to reduce usage to internationally recognized standards.
Aims
We wanted to investigate whether a new e‐learning program with intranet accessibility could influence knowledge of best prescription algorithms for blood components by clinicians within several specialties and all staff ranks. Secondary we desired to test participants’ knowledge about key aspects of evidence based transfusion medicine before and after completion of the program.
Methods
We developed a new e‐learning program using Adobe Captivate 7. It included five case stories and one status section. Besides, a questionnaire – pretest and posttest – comprising multiple choice questions was prepared. Each question assessed knowledge to take correct decisions concerning prescription of blood. The program was made obligatory for all clinicians in specialties using blood components in our hospital. Before going through the e‐learning program, each clinician received a personal link to the pretest and 3–6 weeks after completing the e‐learning program each participant received the posttest, evaluating exactly the same skills. Each correct answer in the multiple choice test gave one point, so a maximum of ten points was possible for each run. The results were analyzed by Wilcoxon matched‐pairs rank‐sum test.
Results
A total of 438 clinicians enrolled in the e‐learning program (participation: 48% of all doctors prescribing blood components). Both tests were completed by 266 clinicians from eight surgical and eleven medical specialties. The score before and after completing the e‐learning program for all doctors was [4.4/6.7 (P < 0.0001)]: In medical specialties (n = 101) [4.7/6.7 (P < 0.0001)], surgical specialties (n = 68) [4.3/5.8 (P < 0.0001)], anesthesiology (n = 72) [4.8/6.9 (P < 0.0001)]. Others (n = 25)(not calculated). The improvement per question for all was [−12 to +52% (average: 18.9%)]. We found a difference in the improvement in score between various categories of staff positions: (residents (n = 59)(2.9), senior residents [(n = 31) (1.8), staff doctors (n = 47) (1.8), consultants (n = 118) (1.6), professors (n = 8) (0.8)]. Before the e‐learning program, the residents had the lowest score of all groups (4.1), but after the e‐learning program, they obtained the highest score (7.0). The average total usage of red blood cells per month in our hospital tends to be lower after the e‐learning program was implemented (2031/1751).
Summary/Conclusions
Clinicians from all specialties and in all staff positions benefited from participating in this e‐learning program in terms of knowledge of evidence based prescription of blood. Residents improved their knowledge the most. It is crucial to ensure a strict organization to obtain satisfactory participation percentage. Further investigations will show if it is possible to improve the size of effect for each skill learned by general application of principles from learning theories adjusted for a medical setting.
4C‐S23‐05
Computerized Provider Order Entry with Clinical Decision Support Reduced Unnecessary Fresh Frozen Plasma Transfusion
Chu F-YC , Chang C-CC , Lee T-CL
Far Eastern Memorial Hospital, New Taipei City, Taiwan
Background
Transfusion of fresh frozen plasma (FFP) was indicated for supplement of coagulation factors in those with clinically significant coagulation deficiencies or undergoing major surgeries. Implementation of computerized provider order entry (CPOE) with clinical decision support (CDS) was expected to avoid unnecessary FFP utilization and thus promoted the evidence‐based transfusion practice.
Aims
To evaluate the effect of CPOE with CDS on FFP utilization.
Methods
The data of FFP transfusion from 2008 to 2014 were reviewed. The CPOE with CDS had been implemented since 2010, and associated education was given accordingly. An alert would be triggered if recent corresponding laboratory evaluation including prothrombin time (PT) and activated partial thromboplastin time (APTT) was not available or did not fulfill the criteria approved by local Transfusion Committee. The physician must reply the reason for FFP utilization on line. The indications for FFP transfusion as approved by local Transfusion Committee included patients with prolonged PT or APTT and having bleeding tendency or undergoing major surgeries, and those with massive blood transfusion or major bleeding after surgery, as well as congenital or acquired coagulation factor deficiencies. FFP utilization before and after implementation of the CPOE with CDS were compared. The trend of change of FFP utilization was evaluated by two‐proportion Z‐test.
Results
The inpatient service varied from 39,319 to 40,691 patient‐days the study period from 2008 to 2014. The total FFP unit transfusion was gradually decreased from 14,104 units in 2008 to 11,239 units in 2014. The average FFP utilization was decreased from 0.36 unit per hospitalized patient‐days in 2008 to 0.28 unit per hospitalized patient‐days in 2014. A significantly decreased trend of FFP utilization was observed after implementation of CPOE with CDS (Z = −7.19, P < 0.01).
Summary
COPE with CDS effectively decreased FFP utilization and facilitated the evidence‐based transfusion practice. The findings suggested that CPOE with CDS would be an essential part of patient blood management to comply with the evidence‐based transfusion guidelines.
Cell Therapies
4C‐S24‐01
Mesenchymal Stem Cell‐Derived Extracellular Vesicles, a Potential New Tool in Regenerative Medicine
Giebel BG , de Miroschedji KDM , Börger VB , Bremer MB , Görgens AG , Horn PAH
Institute for Transfusion Medicine, Essen, Germany
Background
MSCs have been used to treat a variety of different diseases such as myocardial infarction, stroke and graft‐vs‐host disease (GvHD). Despite contrary reports regarding the outcome of MSC treatments, increasing evidence suggests that MSCs exert their beneficial effects via small extracellular vesicles (EVs, 80–160 nm), such as exosomes and microvesicles, rather than by intercalating into affected tissues. Indeed, we observed beneficial therapeutic effects following MSC‐EV administration in a steroid‐refractory GvHD patient (Kordelas et al., 2014) and in animal models for stroke and preterm brain injury. At the functional level MSC‐EVs were shown to exert immunosuppressive functions in vivo and in vitro. Due to the contrary reports regarding the outcome of MSC treatments and the fact that MSCs are a very heterogeneous, ill‐defined class of fibroblast‐like cells, we consider that not all human MSCs release therapeutic effective EVs.
Aims and methods
To compare the immunomodulatory features of different MSC lineages, MSCs were raised from BM samples of 20 different stem cell donors. Their cell surface phenotype was analyzed by flow cytometry and their differentiation potential in conventional differentiation assays. EVs were harvested from MSC conditioned media using the PEG method followed by ultracentrifugation. Obtained MSC‐EV fractions were characterized by Western Blot and NTA. The MSC‐EVs’ immunomodulatory properties were studied in T cell proliferation and activation assays in which T cells were stimulated with the lectin phytohemagglutinin (PHA).
Results
Bona fide MSCs were obtained from all 20 donor samples. All of them expressed the cell surface antigens CD44, CD73, CD90, CD105, CD146 and CD166 and were negative for CD14, CD31, CD34 and CD45. They revealed osteogenic and adipogenic and as far as tested chondrogenic differentiation potentials. Huge differences regarding the averages cell size of the different MSC lineages were observed. All MSC‐EV fractions were positive for the tetraspanins CD9, CD63 and CD81 as well as for Tsg101 and HSP70 and revealed average size distributions ranging between 110 and 150 nm. However, only a proportion of the MSC‐EVs revealed immunosuppressive features in the T cell readouts.
Conclusions
MSC‐EV preparations of different MSC lineages differ in their in vitro immunomodulatory capabilities, suggesting that only a proportion of MSCs produce therapeutically active EVs. This might explain the controversy reports of MSC therapies in a variety of clinical settings. Even though all MSC‐EV fractions which we had used in in vivo studies were selected according to their capabilities to exert immunosuppressive functions in vitro, it will be important to also study the therapeutic impact of MSC‐EV fractions lacking in vitro immunosuppressive functions. Currently, we are improving the platform to produces MSC‐EVs for the clinical setting and search for surrogates to discriminate therapeutic effective and non‐effective MSC‐EV samples.
4C‐S24‐02
Human Platelet Lysates Exert a Neuroprotective Effect in a Dopaminergic Neuron Cell Model of Parkinson's Disease
Chou ML1, Gouel F 2, Devedjian JC 2, Devos D 2, Burnouf T 1
1Taipei Medical University, Taipei, Taiwan2Lille 2 University, Lille, France
Background
Neurodegenerative diseases have huge economic societal impact and induce immense emotional burden on patients and their caregivers. Reducing their progression with novel, effective, and affordable biotherapies, amenable for use in both developed and developing countries, is a great challenge for our societies worldwide. Platelets constitute abundant, natural, readily accessible sources of physiological mixtures of numerous growth factors (GFs) with proven effects on cell survival and proliferation. Platelet lysates (PL) are already used in several fields of human regenerative medicine as well as for cell therapy procedures. In particular, PLs contain a physiological balance of neurotrophic GFs thought to regulate the development, maintenance, function and plasticity of the vertebrate nervous system. We hypothesize now that PLs can potentially be used as disease modifying treatment strategy of neurodegenerative disorders.
Aims
To evaluate whether PLs exert an in vitro neuroprotective effect on immortalized dopaminergic human neuronal precursor cells used as a model for Parkinson's Disease (PD).
Methods
Various PLs were prepared from platelet concentrates collected by apheresis and frozen at −80°C. PL protein composition was characterized, and platelet growth factors (EGF, PDGF‐AB, FGF, HGF, VEGF, BDNF, TGFβ) measured by ELISA. Dopaminergic Lund Human Mesencephalic (LUHMES) cells were seeded on poly‐L‐ornithine/fibronectin coated flask and grown in Advanced DMEM/F12 medium (containing 2 mM l‐glutamine, 1 × N2, 40 ng/ml FGF‐2) supplemented in a 5% CO2/95% air atmosphere at 37°C. Cells were differentiated in a medium supplemented with 2 mM l‐glutamine, 1 × N2, 2.25 mM tetracycline, 1 mM cAMP and 2 ng/ml GDNF. At day 5 of differentiation, cells were exposed to 1–10% PL for 1 h prior to addition of 5 μM MPP+ neurotoxin. Various treatment combinations were also tested. Cell viability was assessed on day 7 of differentiation using a Resazurin assay and expressed as % of untreated control.
Results
PLs (N = 4) contained 9.6–10 ± 2.1 mg/ml of total proteins, and 0.88 ± 0.31, 30.34 ± 4.70, 0.25 ± 0.06, 0.17 ± 0.03, 0.12 ± 0.02, 23.59 ± 5.8, 26.84 ± 1.67 pg/μg protein of EGF, PDGF‐AB, FGF, HGF, VEGF, BDNF, TGFβ, respectively. Our studies (0.1–10%) showed that PLs did not exert a toxic effect on LUHMES cells and that as little as 2% PLs was sufficient to exert a strong neuroprotective effect of LHUMES exposed to MPP+ neurotoxin. Pre‐treatment of LHUMES cells with 2% PL for 1 or 3 h prior to MPP+ exposure showed similar neuroprotective effects. When cells were pre‐incubated with 2% PL for 1 or 3 h and then subjected to MPP+ treatment in the absence of PLs, a strong neurotoxic effect was observed indicating that continuous presence of PL is needed for neuroprotection. The neuroprotective effect of PL was found to last for at least for 24 h.
Conclusions
Our PLs preparations have a high content of neurotrophic factors. They strongly and efficiently protected dopaminergic cells from MPP+ neurotoxin in vitro. These novel data suggest possible novel therapeutic applications of PL in the treatment of PD, and possibly other neurodegenerative diseases.
4C‐S24‐03
Abstract Withdrawn.
4C‐S24‐04
If Multiple Myeloma Patients Previously Treated with Immunomodulatory Drugs Can Ensure Adequate Collection of CD34+ Cells Followed by Successful and Sustained Engraftment
Grubovic R1, Schiller G 2
1Institute for Transfusion Medicine of RM, Skopje, Macedonia2Hematological Malignancy, STC Unit, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States of America
Introduction
High‐dose chemotherapy followed by autologous transplantation of peripheral blood stem cells (PBSC) plays an important role in the management of multiple myeloma. In order for successful engraftment to occur, adequate number of high quality PBSC must be harvested prior to transplantation. Mobilization of PBSC in heavily pretreated patients is often associated with unsuccessful harvests and has been described in many patients with multiple myeloma (MM) who have been treated with immunomodulatory drugs. The aim of this study is to present our experience with mobilizing, harvesting and transplantation of PBSC in multiple myeloma patients previously treated with Thalidomide and/or its analog Lenalidomide.
Materials (or patients) and methods
This is a retrospective study performed at the Department of Medicine/Hematological Malignancy/Stem Cell Transplant Unit at the UCLA for a period of 5 years. Patients with multiple myeloma who had received pretransplant immunomodulatory drugs and were assigned autologous PBSC collection and transplantation were included in the study. Pts with renal failure were excluded from this analysis. Minimum dose required to ensure successful and sustained engraftment was 2 × 10(6)/kg CD34+ cells, whereas the optimal dose was 5 × 10(6)/kg CD34+ cells. PBSC harvesting was performed with COBE Spectra using conventional‐volume leucapheresis processing 2–2.5 total blood volume per apheresis. All MM patients underwent a single mobilization regimen that included Granulocyte Colony‐Stimulating Factor (G‐CSF) 10mcg/kg/day sc, Cyclophosphamide 2.5 g/m2 iv and Prednisone 2 mg/kg po.
Results
Autologous PBSC transplantation was performed in 103 multiple myeloma patients with high‐dose Melphalan. 212 autologous PSBC apheresis procedures were performed, with mean number of apheresis procedures 1.0 (range 1–12). Median age was 55.7 (range 32.6–75.1). 70 patients (68.9%) required just one collection to achieve the requisite number of cells, 15 patients required 2 collections (14.5%), and 14 patients (17.4%) had more than two collection procedures. There was only one patient with fewer than 2 × 10(6)/kg CD34+ cells (0.9%) collected and he underwent bone marrow harvesting. Median CD34+ cells collected were 6.7 × 10(6)/kg, with range from 1.4 to 33.5 × 10(6)/kg CD34+ cells. Median CD34+ cells infused were 5.8 × 10(6)/kg, with range from 1.4 to 28.1 × 10(6)/kg CD34+ cells. 80% of the patients didn't show any kind of symptoms during the transplantation time, and the 20% sustained mild reactions such as, cough (9.6%), vomiting (2.9%), nausea and hypertension (1.9%). Median time to engraftment was 10.7d (range 8.0–18.0 days). Most patients achieved neutrophil recovery (ANC > 500/mm3) in 10 days (33.1%); 11 days (23.6%), 9 days (19.8%) and 12 days (10.4%).
Discussion
Pretransplant therapy with immunomodulatory drugs did not show that had significant impact on the number of apheresis procedures or number of collected CD34+ cells in our study. We believe that we have identified successful method for mobilization of PBSC in multiple myeloma patients that can be easily applied to other MM patients undergoing therapy. Combination of G‐CSF, Cyclophosphamide and Prednisone happened to be an excellent mobilization strategy with mobilization failure <1% (0.9%). Collected and infused CD34+ cells number was more than the optimal 5 × 10(6)/kg CD34+ cells. All patients underwent transplantation without any serious adverse events and engraft soon after.
4C‐S24‐05
Identification of Key Regulators of Symmetric vs Asymmetric Cell Divisions During Human Hematopoietic Lineage Specification
Görgens AG 1, Radtke SR 2, Vitoriano SV 2, Murke FM 2, Möllmann MM 3, Dürig JD 3, Hanenberg HH 4, Horn PAH 2, Giebel BG2
1University Hospital Essen, Essen, Germany2University Hospital Essen, Institute for Transfusion Medicine, Essen, Germany3University Hospital Essen, Department of Hematology, Essen, Germany4Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN, United States of America
Hematopoietic stem cells (HSCs) contain lifelong potentials to self‐renew and to create progenitors of all mature blood cells. According to the current view, HSC homeostasis is controlled by both, HSC‐niches as well as asymmetric cell divisions. Our previous studies linked the process of asymmetric cell division of human hematopoietic stem and progenitor cells (HSPCs) to the expression kinetics of the stem cell surrogate antigen Prominin 1/CD133 (1). Furthermore, by characterizing human HSPCs subpopulations by means of their CD133 surface expression we gained evidence that CD133+ multipotent progenitors (MPPs) create CD133+ lymphomyeloid (LMPP) and CD133low erythromyeloid (EMP) daughter cells. The LMPP lineage was shown to contain lymphoid and neutrophil potentials, while the EMP lineage mainly creates eosinophils and basophils as well as erythrocytes and megakaryocytes (1) (Figure 1). Regarding lineage specification, we showed for the first time that under conventional culture conditions almost all MPPs divide asymmetrically to create a set of LMPP and EMP daughter cells, resulting in a loss of MPPs after the first cell division (3) (Figure 2). Thus, our data suggest that under conventional culture conditions asymmetric cell divisions are rather lineage instructive than self‐renewing (4). Now, aiming to identify key factors regulating the MPP division mode, we study whether conditions reported to promote HSC/MPP expansion interfere with the outcome and symmetry of the HSC/MPP cell division. In this context we co‐cultured human MPPs with murine and primary human stromal lines (human bona fide MSCs) and surprisingly observed that LMPPs are maintained and expanded but not MPPs. This contrary finding can be attributed to the former experimental definition of multipotent cells based on the classical model of hematopoiesis, according to which cells with dual lymphocyte and granulocyte (conventionally neutrophil) potentials can insufficiently be considered as multipotent. Currently, we test other culture conditions reported to expand human HSCs/MPPs in vitro. After confirming any of these conditions as HSC/MPP expansion condition, we will analyze its impact on the division mode of HSCs/MPPs using multi‐parametric flow cytometry, live‐cell imaging and functional differentiation assays at the single cell level.

Caption 1: Multipotent Hematopoietic Progenitors Divide Asymmetrically to Create Progenitors of the Lymphomyeloid and Erythromyeloid Lineages.

Caption 2: Revision of the Human Hematopoietic Tree: Granulocyte Subtypes Derive From Distinct Hematopoietic Lineages.
References
1. Beckman et al, Blood 2007.
2. Görgens et al, Cell Reports 2013.
3. Görgens et al, Stem Cell Reports 2014.
4. Görgens et al, Cell Cycle 2013.
Donor Iron: Status and Management
4C‐S25‐01
Optimising Donor Health and the Blood Supply: Update on the Interval Study
Roberts J , The INTERVAL Study Group
NHSBT – Oxford, Oxford, United Kingdom
Background
One approach to increase and stabilise the blood supply is to collect blood more frequently from existing donors. Currently, NHS Blood and Transplant recommend donation every 12 weeks (men) and 16 weeks (women) but European standards can be every 8 weeks.
Aims
The primary aim of INTERVAL is to determine whether donation intervals can be reduced whilst maintaining the health of the donor.
Design and methods
50,000 male and female donors were recruited at donation centres in England between June 2012 and May 2014. Donors were randomised to one of three gender‐specific donation frequencies for 2 years. The primary outcome is the number of donations per year and the main secondary outcome is quality of life (using Short Form Health Survey SF36v2). Further secondary outcomes are number of deferrals of donors due to low haemoglobin and other factors, iron status, cognitive function, physical activity and donor attitudes to giving blood and participating in research. Data have been collected via online questionnaires and research blood samples collected at the time of routine blood donation. Physical activity will be measured in a subset of 6000 donors using wrist‐worn accelerometers after their 2 year‐involvement. Extensive genotypic information will be available on donors.
All donors recruited into the study met NHSBT haemoglobin (Hb) screening criteria (males: >13.5, females: >12.5 g/dl). As part of the study protocol, full blood counts (FBCs) were performed using a Sysmex XN2000 analyser within 24 h of collection. Review of FBC data after one year showed many donors with abnormal indices and they were invited to donate a further blood sample for evaluation. Thresholds used were 3 SD from the mean of the study group (Hb, Plt) or BCSH guidelines (Hct). Donors with repeatedly abnormal results were if necessary referred to their GP.
A second phase of the study has enrolled participants to continue on their current intervals and randomised to different remindering schedules to determine the acceptability, feasibility of shorter donation intervals and the most effective remindering system.
Results
The study has achieved considerable successes including: integration of research protocols in routine donation practice; good questionnaire response (~80%) and sample retrieval (~97%) and good adherence by participants to allocated donation frequencies. Over half the donors have completed the study and the final end of study is June 2016.
The majority of donors with low Hb, high Hct or low Plt count had normal values upon re‐testing. However 66% of donors with initially high platelet counts (0.1% of enrolled donors) had repeatedly high counts and were referred to their GP for further investigation.
Conclusions
INTERVAL will generate scientific evidence on which to base future blood collection policies in England, and potentially elsewhere. It will yield, currently lacking, reliable data on the effect of donation frequency on blood supply and donors’ physical and mental well‐being. We plan to investigate tailoring donation intervals according susceptibility to iron deficiency.
A full list and contributions of the study group members can be found at http://www.intervalstudy.org.uk.
4C‐S25‐02
Handling Low Hemoglobin and Iron Deficiency in a Blood Donor Population
Magnussen K1, Ladelund S 2
1Copenhagen University Hospital, Rigshospitalet, Hvidovre, Denmark2Copenhagen University Hospital, Hvidovre, Hvidovre, Denmark
Background
Blood donors with low haemoglobin‐concentration (Hb) and iron deficiency are well known challenges in any blood bank setting. The handling is complex, and even though iron deficiency is a frequent cause of anaemia, there are differential diagnoses. In healthy blood donors ferritin is helpful in discerning between anaemia caused by iron‐deficiency and other causes, and while low Hb and low levels of ferritin, are the primary concern, some donors have too high levels, which must also be dealt with.
Aims
The primary aims were to standardize and optimize the handling of blood donors with Hb below the limit for donation (7.8 and 8.4 mmol/l for female and male donors respectively). Secondary aims were to deal with all issues related to high or low either Hb or ferritin in blood donors.
Methods
The problem was approached, by centralizing measurement of Hb, initiating ferritin measurements and establishing Centre for Donor Haemoglobin and Iron. An algorithm was created, taking mainly Hb and ferritin into account. The possible outcomes were: to send iron‐tablets and iron‐folder by mail, to give iron tablets with future donations, to refer the donor to general practitioner (GP) or, in most cases to do nothing. Pre‐existing staff was trained in donor‐communication and to handle the Hb and ferritin results. The donors where mainly contacted by mail, but when Hb was low or when there was insufficient effect of previously sent iron‐supplementation the donor was contacted by phone to improve compliance. Hb was measured on Sysmex‐XE2100D as part of a Complete Blood Count at every donation. Ferritin was measured one time in all donors, at every 10th donation and repeated when outside 60–300 μg/l at the previous donation. Ferritin measurement was also repeated if the previously measured Hb was low. The reason for not measuring ferritin at every donation was economy.
Results
From February 1st 2012 to February 1st 2015, 71,450 donors (53.5% women/46.5% men) donated 281,814 units of whole blood (48% women/52% men). The mean Hb increased from 8.59 to 8.64 mmol/l in the female donors (P < 0.001) while the increase in the male donors was smaller 9.55–9.57 mmol/l (P = 0.017). The Red‐Blood‐Cell Count increased from 4.57 to 4.66 and 5.02–5.12 × 1012/l in female and male donors respectively (P < 0.001). The decrease in % of female donors with low Hb from 4.0 to 3.5 was not significant. The % of male donors with low Hb decreased from 0.92 to 0.55 (P = 0.03). Of the donors that were referred to GP on suspicion or to rule out disease, not all called back to inform about the result. Of those that did inform 12 were diagnosed with leukaemia or cancer and 2 with Vitamin B12‐deficiency. Other finds were heterozygous thalassemia, hemochromatosis and polycythaemia vera.
Summary/Conclusions
While the aim was to keep the donors within our frame for Hb and ferritin, the main benefit of the program was to have a well‐functioning program for when the donors did fall outside anyway. The program with goal directed iron supplementation only to those that would benefit, has led to an increase in Hb and a reduction the number of donors with low Hb.
4C‐S25‐03
What Do Systematic Reviews of Iron Supplementation in Women Tell Us About the Functional Consequences of Donor Iron Deficiency?
Pasricha S1, Speedy J 2, Low M 3
1MRC Weatherall Institute of Molecular Medicine, Oxford, United Kingdom2Australian Red Cross Blood Servicw, Adelaide, SA, Australia3Walter and Eliza Hall Institute, Melbourne, Vic., Australia
Background
Donor iron deficiency is common, especially among premenopausal, female whole blood donors. While the epidemiology of donor iron deficiency is becoming increasingly understood, the functional consequences of this problem are less clearly understood. Randomised controlled trials of iron supplementation in women can provide insight into the functional consequences which are restored when iron is given.
Aim
To understand the functional consequences of donor iron deficiency through a systematic review of randomized controlled trials administering daily iron supplementation to blood donors.
Methods
We undertook a Cochrane systematic review of randomized controlled trials administering daily iron supplementation in premenopausal women. The search strategy covered key databases including Medline, Embase, WHO regional databases, and grey literature including theses databases. Two authors screened studies and extracted data. Data were synthesized using random effects meta‐analysis using RevMan.
Results
The search strategy identified 14540 reports, from which 58 studies were identified as eligible and 56 were included in the meta‐analysis. Iron supplementation increased haemoglobin (Mean difference (MD) 5.67 g/dl [4.47, 6.87]), ferritin (MD 11.44 ng/ml [9.48, 13.40]), and reduced the prevalence of anaemia (risk ratio (RR) 0.34 [0.20, 0.57]) and iron deficiency (RR 0.63 [0.51, 0.79]). Iron supplementation improved physical exercise performance in terms of both peak exercise – Absolute VO2 max MD 0.11 [0.02, 0.20]; relative VO2 max MD 2.36 [0.55, 4.17]; and submaximal exercise efficiency (Heart rate required −4.72 bpm [−8.64, −0.80]; % VO2 max needed to achieve exercise output −3.34 [−6.17, −0.51]). Limited data reported no evidence of benefit on self reported quality of life. Meta‐analysis suggested that iron supplementation improved fatigue among women (standardized mean difference for fatigue scores −0.37 [−0.65, −0.09]). Notably, subgroup analysis for effects of iron supplementation among the specific subgroup of non‐anaemic, iron deficient women, did not identify evidence of a benefit from iron supplementation on exercise performance, although improvements in haemoglobin (MD 5.36 g/dl [3.33, 7.40]), ferritin (MD 10.82 [7.64, 14.01]).
Conclusions
Iron supplementation improves haematologic and iron indices. Importantly, there is evidence that iron supplementation improves physical exercise performance and reduces fatigue. However, the impact of iron supplementation on functional outcomes among women with non‐anaemic iron deficiency is unclear.
4C‐S25‐04
Intravenous vs Oral Iron Supplementation for Blood Donors with Iron Deficiency
Macher S , Drexler C , Stojakovic T , Schröck M , Reinprecht C , Lindenau I , Sareban N , Schlenke P , Amrein K
Medizinische Universität, Graz, Austria
Background
Iron deficiency (ID) is one of the most common nutritional deficiencies worldwide. It is highly prevalent in premenopausal women and also frequently found in industrial countries. About 2−3% of the population participates in blood donation programs. Blood donations often contribute to ID, but for donor clearance, only a capillary hemoglobin (Hb) threshold is required. However, Hb does not reliably predict iron stores. ID is associated with restless legs syndrome, cognitive and physical symptoms and an increased risk for preterm birth. Currently, only in anemic donors, iron supplementation is routinely recommended. However, oral iron substitution is often associated with gastrointestinal side effects and poor compliance.
In our ongoing study, we compare the effect of a single intravenous high‐dose iron carboxymaltose preparation (1000 mg) for blood donors with iron deficiency (IV) with the effect of a corresponding dose (10 g) of oral iron over 10 weeks (PO).
Materials and methods
In our randomized, controlled clinical trial we include male and female blood donors who fulfil the criteria of a predonation hemoglobin value of ≤13.5 g/dl and a ferritin value of ≤30 ng/ml (target sample size 160 in total). Stratified by gender, participants are randomized with a web‐based randomization tool to IV or PO in a 1:1 ratio. 12 weeks after the first visit hemoglobin and ferritin values of both groups are determined.
Results
Out of 306 donors with Hb ≤13.5 g/dl at their blood donation visit 187 (166 female, 21 male) had a ferritin value of ≤30 ng/ml and of these, 112 had a ferritin ≤15 ng/ml.
To date, 32 participants (27 female, 5 male) have completed the trial. Hb and ferritin levels after treatment are shown in the Table. No serious adverse events occurred. Mean Hb and ferritin levels after the last donation but prior to any kind of iron supplementation were 11.5 ± 0.7 g/dl and 6.5 ± 3.9 ng/ml.
Table 1. Hb and ferritin values (mean ± SD) 12 weeks after iron supplementation.
In the IV group, 2/16 participants (12.5%) still had ferritin values <30 ng/ml after iron supplementation (both of whom donated blood after their first visit) compared to 8/16 (50%) participants of the PO group.

Conclusions
It is well known that iron deficiency is common in blood donors, especially in females. Both IV and PO iron supplementation improves iron stores but IV iron is more effective. The diagnosis and treatment of this common preanemic condition could be useful to maintain donors′health and is part of the duty of care for transfusion medicine services.
Red Cell Antigens
4D‐S26‐01
Recombinantly Produced Blood Group Proteins in Clinical Practice
Seltsam A
German Red Cross Blood Service NSTOB, Springe, Germany
The basic drawback of red blood cells (RBC)‐based assays is that a positive reaction between serum and test cells does not directly indicate the specificity of a red blood cell (RBC) antibody. These conventional assays use panels of human RBCs carrying a huge number of blood group antigens at the same time and the specificity of an antibody can only be concluded from negative reactions of the serum with antigen‐negative cells. This indirect way of antibody determination comes to its limits when antibody mixtures or rare RBC antibodies are present. Then advanced diagnostics using specialized serologic techniques and test cells with rare antigen profiles are required to identify these antibodies. Recently, a large number of CE‐marked recombinant blood group proteins (rBGPs) have become available in sufficient quantity and quality. The advantage of rBGPs over RBCs is that they can be used as single antigens in antibody identification assays so that a positive reaction of a serum with the recombinant protein directly indicates the presence and specificity of the target antibody. When used in soluble form in addition to RBC‐based diagnostics, rBGPs have been shown to increase sensitivity and specificity in cases with difficult‐to‐identify antibodies, resulting in a more efficient blood supply for immunized patients. rBGPs have also been successfully used in solid‐phase assays (ELISA, color‐coded microspheres and protein microarray chip‐based techniques), showing that they can be applied to a wide range of test systems. Although not all relevant antigen specificities have been established yet, the diagnostic potential of rBGPs could already be used in combined antibody detection assays including both RBC‐derived and recombinantely expressed antigens. Such complementary assays would already profit from the capacity of rBGPs for single‐step and direct antibody determination, greatly facilitating and accelerating the identification of common and rare RBC antibodies. Implementing rBGPs in pretransfusion antibody screening protocols introduces a paradigm shift in RBC antibody diagnostics and could help to reduce the risk of a haemolytic transfusion reaction, one of the leading causes of transfusion‐related death.
4D‐S26‐02
First Detailed Molecular Characterization of a Yus (Ge*01.‐02) Allele and Description of a Novel Gerbich (Ge*01.‐03) Allele Responsible for Rare Phenotypes in the Gerbich Blood Group System
Gourri EG1, Scharberg EAS 2, Peyrard TP 3, Frey BMF 1, Gassner CG 1
1Blood Transfusion Service Zurich, Swiss Red Cross, Zürich‐Schlieren, Switzerland2Red Cross Blood Service of Baden‐Wuerttemberg‐Hessen, Baden‐Baden, Germany3Institut National de la Transfusion Sanguine (INTS), Paris, France
Background
The Gerbich blood group system consists of 11 antigens located on glycophorin C (GPC) and D (GPD). GPD is a truncated version of GPC, and both are encoded by the same gene, GYPC. 1 ‘Yus’, ‘Gerbich’ and ‘Leach’ correspond to the rare Ge: −2, 3, 4, Ge: −2, −3, 4 and Ge: −2, −3, −4 types, respectively. Yus lacks the high‐prevalence Ge2 antigen (exon 2 deletion), and Gerbich lacks the high‐prevalence Ge2 and Ge3 antigens (exon 3 deletion).
Aim of the project
Serological determination of blood group Gerbich phenotypes is tedious and costly, and typing reagents are commercially unavailable. Therefore, we aimed to design a reliable, rapid and cost‐effective method to screen and/or confirm them at the genetic level.
Methods
Three unrelated patients with anti‐Gerbich had been reported to Blutspende Zurich and were serologically defined as two Gerbich and one Yus phenotype by the National Immunohematology Reference Laboratory in Paris. Positional PCRs, also accounting for the high intragenic homology, allowed for an approximate location of the deletion breakpoints present in the samples. PCR amplicons covering the suspected genomic region were sequenced. Diagnostic PCRs using Sequence Specific Priming (PCR‐SSP) were developed to detect the respective allelic breakpoints and the corresponding wild‐type sequences. They were used to genotype three additional samples from the Red Cross Blood Service of Baden‐Baden and one Gerbich sample from the SCARF exchange program. All Baden‐Baden samples had anti‐Gerbich, but lacked additional phenotypic information.
Results
All three Zurich samples were homozygous for one deletional GYPC allele, with two different molecular backgrounds. Both Gerbich phenotypes had the same ‘Gerbich allele’. The genomic extent of both newly observed alleles was in line with the expected phenotypes, e.g. a deletion of exon 2 (i1‐41 to i2 + 3511) in the Yus and a deletion of exon 3 (i2‐2640 to i3 + 859) for the Gerbich phenotype, respectively. All three Baden‐Baden samples were homozygous for either one, or the other of the previously observed alleles: two samples for the ‘Yus allele’ and one for the ‘Gerbich allele’. Sequencing confirmed presence of the same alleles as previously found in the Zurich samples. The SCARF sample was unambiguously recognized as Gerbich by our PCR‐SSP.

Conclusions
A total of 7 investigated samples, 6 of them with anti‐Gerbich allo‐antibodies, were unambiguously genotyped for their Yus and Gerbich phenotypes using a diagnostic PCR‐SSP approach including only four reactions. To our knowledge, this is the first report with a detailed molecular characterization of a ‘Yus allele’, originally reported together with the ‘Gerbich allele’ in 19892. One other deletional GYPC allele, e.g. encoding the Papua Neuguinean Gerbich phenotype have been reported previously3. An additional Baden‐Baden sample is currently under investigation and preliminary results are suggestive of another novel ‘Yus allele’. Therefore, the provisional ISBT terminology for the alleles encoding the Yus (GE*01.-02) and Gerbich (GE*01.-03) phenotypes will likely need splitting into two alleles each, and revision. Molecular characterization of alleles encoding the Leach phenotype would allow for a fully comprehensive genotyping of Yus, Gerbich and Leach. However, we were unable to obtain samples of the exceptional Leach type, until now.
4D‐S26‐03
Molecular Characterisation of 18 Individuals with the Lan Null or Weak Phenotypes Reveals Four New ABCB6 Null Alleles
Kittivorapart JK1, Karamatic Crew V 2, Thornton N 2, Daniels G 2
1Siriraj Hospital, Bangkok, Thailand2IBGRL/BITS, Bristol, United Kingdom
Background
Langereis (LAN) was first reported as a high incidence antigen in 1961, and was established as a blood group system after its coding gene ABCB6 was described in 2012. ABCB6 is located on chromosome 2q36 and comprises 19 exons. There is only one antigen, LAN, in the system and the Lan‐ phenotype is very rare. To date, there are at least 42 unique genotypes reported to encode Lan‐ or Lan‐weak phenotypes. Anti‐Lan can cause significant clinical transfusion reactions; it is therefore essential to identify this phenotype, and the introduction of high‐throughput molecular typing, the importance of describing causative mutations in the ABCB6 gene is highlighted.
Aims
This study aims to identify potential mutations in ABCB6 in archived Lan‐ and weak samples from the International Blood Group Reference Laboratory (IBGRL, Bristol, UK) in order to expand this rare blood group molecular database.
Study Design and Methods
Lan‐ cells, serum and DNA samples archived at the IBGRL, Bristol, over 40 year period were tested for the mutations in ABCB6. Genomic DNA was obtained from 14 Lan‐ and 4 Lan‐weak samples, and amplified by PCR for all 19 exons of ABCB6. Sanger sequencing was performed on 18 test and 4 control samples. PCR‐RFLP was developed to validate one newly identified mutation.
Results
A total of 16 ABCB6 mutations were identified in 18 test samples, of which four were novel mutations. Eleven samples were homozygous, and seven were compound heterozygous for inactivating and missense ABCB6 mutations (see Table). The observed novel mutations included two nonsense mutations c.247C>T in exon 1, encoding Gln83X, and c.1429C>T in exon 8, encoding p.Arg477X. A novel deletion c.1921_1923delGAC was found in exon 14, causing loss of Asp641. A novel intronic mutation was discovered in intron 12, c.1805+1G>A, in the splice site of exon 12, which would likely encode aberrant splicing of ABCB6. In this study we observed 10 previously reported ABCB6 mutations causing Lan‐ phenotypes: two deletions, one deletion/insertion, one duplication, four missense mutations, and two nonsense mutations. Two previously reported mutations resulted in weak Lan expression; c.826C>T (p.Arg276Trp) and c.1762G>A (p.Gly588Ser). The most frequently observed mutation was c.574C>T, which resulted in amino acid change p.Arg192Trp, found in six alleles from five samples; however, two of these samples were identical twins. Moreover, PCR‐RFLP was developed to analyse c.1429C>T, one of the novel mutations reported in this study, and provided further evidence that this mutation was genuine.
Caption 1: Table of mutations identified in homozygous samples.
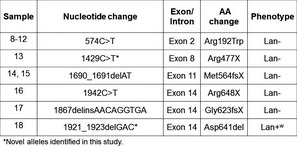
Caption 2: Table of molecular changes found in compound heterozygous samples.

Conclusion
Four novel mutations of the ABCB6 gene were disclosed in this study, extending the known number of different Lan‐ and Lan‐weak genotypes to at least 46. This implies that ABCB6 is one of highly variable genes augmented with the rarity of the Lan‐ or weak phenotype; it would present a challenge to establish high‐throughput molecular typing for many unique mutations of this gene.
4D‐S26‐04
Allele Drop‐Out as a Possible Cause for Discrepant V Genotyping
Moulds M1, Billingsley L 2, Noumsi T 1, Ochoa J 1
1Grifols, San Marcos, TX, United States of America2LifeShare Blood Centers, Shreveport, LA, United States of America
Background
The V antigen is common in persons of African descent and its expression is linked to VS. Because of the relatively high frequency of these antigens in the Black population, sickle cell disease (SCD) patients have an increased exposure rate when transfused with incompletely matched blood and V negative patients often make anti‐V. Most RHCE alleles coding for V have a guanine nucleotide (G) at RHCE positions c.733 and c.1006; thus, these two positions can be used to genotype for V. Genotyping to select V negative units for SCD patients may be more feasible since anti‐V sera are not readily available.
Aims
During validation of the IDCORE‐XT (Progenika) genotyping platform, we noted several discrepancies for the V antigen when compared to HEA (Immucor). The aim of the study was to perform a root cause analysis to determine which assay was producing incorrect results.
Methods
A comparison of 139 VS+V+ or VS+V− Black blood donors was made between the two genotyping platforms; HEA uses an elongation method while IDCORE‐XT uses probe‐hybridization for allele detection. When indicated, confirmation of red cell phenotype was done using several anti‐V sera. Genomic sequencing of RHCE exon 7 was performed to resolve discordant results. Later sequencing was done on an additional 45 samples genotyped as RHCE*ce733G,1006G by HEA in order to determine the prevalence of the SNP responsible for the discrepancy.
Results
Using HEA, 10 of the 139 samples (7.2%) genotyped as homozygous for RHCE*ce733G,1006T with a predicted phenotype of VS+V−. These same samples typed heterozygous (RHCE*ce733G,1006T; RHCE*ce733G,1006G) by IDCORE‐XT with a predicted phenotype VS+V+. Genomic sequencing revealed the presence of a polymorphism c.941T>C near the 5’ end of exon 7 in all 10 samples. An additional 45 samples (90 alleles) that were genotyped as homozygous RHCE*ce733G,1006G by HEA were also sequenced to estimate the prevalence of the c.941C SNP. Five of the alleles were RHCE*ceAR. Of the remaining 85 RHCE*ce733G alleles, 12 (14%) had the c.941C SNP. From these data we estimate that genotyping with HEA would result in incorrect V typing in 3.5% of Black blood donors.
Conclusions
These data suggest that the c.941T>C change adversely affects the PCR amplification step of the assay using primer elongation (HEA) resulting in allele drop‐out. The probe hybridization‐based assay (IDCORE‐XT) is not affected because the primers lay further outside. Blood from donors with the c.941C allele may be incorrectly typed as VS+V− by HEA and transfused to patients having undetectable anti‐V. Thus, the increased frequency of false negative V genotypes in VS+ samples could be of clinical significance in the alloimmunized SCD population.
At the time of this study both genotyping tests were labeled as RUO products in the USA.
4D‐S26‐05
Serological and Molecular Screening for Mar (RH51) Negative Donors and the First Case Report of Anti‐Mar‐Like Antibody in Germany
Scharberg A1, Weber S 1, Kömürcü N 1, Richter E 1, Rink G 2, Burkhart J 3, Bugert P 2
1Institute for Transfusion Medicine and Immunohematology, DRK‐BSD Ba‐Wu‐He, Baden‐Baden, Germany2Institute for Transfusion Medicine and Immunology, Mannheim, Germany3Institute for Transfusion Medicine, Blood Donor Service of the Bavarian Red Cross, Munich, Germany
Background
MAR is a high prevalence Rh antigen (RH51). The original anti‐MAR was found in 1994 in a Finnish woman whose red cells were Cx+ Cw+ D+ C+ c− E‐ e+. The antibody was reactive with all red cells except Rhnull, D– and Cx+/Cw+ and it was weakly reactive with Cw+/Cw+ and Cx+/Cx+ red blood cells. Antibodies made by people with Cw+/Cw+ and Cx+/Cx+ show MAR‐like specificity and do not react with Cw+/Cx+ red blood cells. Anti‐MAR is clinically significant. The first case of anti‐MAR‐like antibody in Germany was found in 2014. It was made by a recently transfused male patient who was Cw+/Cw+.
Aims
Because of a complete lack of MAR negative donors in the German Rare Donor Program we conducted a serological and molecular screening to find homozygous Cw positive and Cw/Cx blood donors. The red cells of these donors would be essential to provide compatible blood to patients with anti‐MAR or anti‐MAR‐like and for production of antibody identification panels with rare blood phenotypes.
Methods
Between March 2012 and November 2013 all blood donors of the daily routine in our center were tested for the Cw antigen on Beckman Coulter PK 7200 blood group analyzer using a monoclonal anti‐Cw (MS110). The reaction was enhanced by a 1% bromelain solution; the specific serum was diluted 1:100. All Cw positive donors with blood group O R1R1 were genotyped for the single nucleotide polymorphisms (SNPs) in the RHCE gene encoding Cw (122A>G) and Cx (106G>A) by using PCR with allele‐specific primers or TaqMan probes. The identified Cw+/Cw+ donors were tested for the MAR antigen with one anti‐MAR serum from SCARF in the indirect antiglobulin test using the gel technique. The red cells of one of the Cw+/Cw+ donors were frozen to be used for production of a special antibody identification panel containing rare blood phenotypes.
Results
152,102 of the 378,273 tested blood donors had blood group O (40.2%) and 1632 of the O group donors were R1R1and Cw positive (1.07%). The molecular testing of these donors revealed 8 Cw+/Cw+ individuals but none Cw+/Cx+. The Cw+/Cw+ individuals were tested negative with a weak/moderate reactive anti‐MAR serum from SCARF. A few weeks after the production of our first antibody identification panel containing Cw+/Cw+ red cells the panel helped to identify the first example of anti‐MAR‐like antibody in Germany.
Conclusions
Using serological and molecular methods 8 Cw+/Cw+ MAR‐like‐negative R1R1 blood donors could be identified among 152,102 donors with the blood group O. We found no single Cw+/Cx+ MAR negative donor in our study. The frequency of Cw+/Cw+ MAR‐like‐negative phenotype in our donor population is 1:19,000. The red cell units of these donors are currently used to create a stock of frozen red cell units. Cw+/Cw+ red cells were also included in an antibody identification panel and made it possible to identify the first example of anti‐MAR‐like antibody in Germany.
4D‐S26‐06
Sensitive Flow Cytometric Assay to Assess Activation of the Classical Pathway of Complement by Antibodies as Well as Interference of Complement Lytic Function by Complement Inhibitors
Weinstock C1, von Zabern I 1, Schmidt CQ 2, Höchsmann B 1, Schrezenmeier H 1, Anliker M 1
1Red Cross Blood Service Baden‐Württemberg – Hessen, Institute Ulm, Ulm, Germany2University of Ulm, Institute of Pharmacology of Natural Products and Clinical P, Ulm, Germany
Background/Aims
Auto‐ or alloantibodies directed against erythrocyte antigens vary in their potency to activate complement and to cause hemolytic disease. Antibody specificity and antibody subclass are only of limited predictive value as indicators for complement activating capacity. Unwanted complement activation may cause adverse effects, which may be ameliorated by the application of complement inhibitors. Therefore we designed a sensitive flow cytometric assay which allows to assess classical pathway activation by antibody as well as inhibition of complement mediated erythrocyte lysis.
Methods
Blood samples of patients with anti‐erythrocyteantibodies and of patients with confirmed paroxysmal nocturnal hemoglobinuria (PNH) were obtained from the University Hospital of Ulm and the Institute of Clinical Transfusion Medicine and Immunogenetics. Some alloantibodies were provided by contributors of the SCARF program. Alloantibodies and warm autoantibodies were incubated with RBC of PNH patients at 37°C, cold autoantibodies at 4°C for 20 min. After removal of the supernatant human serum was added as a source of complement and incubation was continued for 2 h at 37°C. When cold autoantibodies were tested, the highest temperature still showing residual agglutination was selected for an initial incubation for 1.5 h, which was followed by a final incubation for 30 min at 37°C. RBC were then washed, incubated with phycoerythrin (PE)‐labelled monoclonal anti‐CD59 (clone 0V9A2, eBioscience)and analyzed by flow cytometry (Höchsmann et al., Ann Hematol 2011;90:887). Eculizumab was obtained from Alexion; soluble recombinant complement receptor 1 (CR1) CCPs1‐3 was prepared as described (Tham et al., Blood 2011;118:1923); these substances were added to a final concentration of 2.3 μM and 23 μM, respectively, to inhibit lysis by human serum.
Results
In a homologous system, complement mediated hemolysis of RBC is ineffective sincecomplement inhibitors such as CD59 and CD55 (DAF) protect the cell from attack by activated complement. Patients with PNHharbor varying percentages of hematopoietic cells with a deficiency of all glycosylphosphatidylinositol (GPI)‐linked proteins including the complement inhibitors CD55 and CD59. Therefore, PNH RBC clones are especially sensitive to lysis by complement. This property was used to design an assay testing the complement activating capacity of cold reactive autoantibodies, isoagglutinins as well as different alloantibodies. Examples of complement activating antibodies were: anti‐A, ‐B, ‐H, ‐I, ‐P, ‐PP1Pk, ‐Vel, ‐Jr,‐Lan, ‐Jk3, ‐Fy3, ‐Co3, ‐Ku, ‐Le(a), some anti ‐Co(a), ‐Kp(b), ‐Jka, ‐Jkb as well as anti‐Fy(a), ‐Fy(b). Eculizumab and CR1 CCPs1‐3 (first three N‐terminal domains) interfered with complement lysis initiated by antibodies. Fig. 1 shows an example (A) of control without antibody (PNH RBC, 99% clone, were mixed with normal CD59 positive RBC and stained with PE‐labelled anti‐CD59); (B) complement‐mediated destruction of PNH RBC initiated by anti‐I which (C) was inhibited by the complement inhibitor CR1 CCPs1‐3.

Conclusion
RBC lacking complement inhibitors such as PNH cells are a sensitive and suitable tool for detecting the complement activating capacity of anti‐erythrocyte auto‐ and alloantibodies in a homologous (human) system. This test may, therefore, serve to estimate potential complement mediated harm; it can also be used to search for novel complement inhibitors and to compare their activity.
Pathogen Prevention
4D‐S27‐01
Preventing Bacterial Infection in Blood Components
Schmidt M
NHS Blood & Transplant/Oxford University Hospitals and the University of Oxford, Frankfurt, Germany
Background
The prevention of pathogen infections by blood components is a major goal in transfusion medicine. Nevertheless the residual risk of transfusion‐related bacterial infections is still 10–200 fold higher than the residual risk of virus infections (e.g HIV‐1, HCV or HBV). One reason is, that bacteria are competent microorganism and able to proliferate under different environmental conditions especially in different blood components. Secondly bacteria can harm patients by causing severe septic reactions and especially gram negative bacteria are able to induce endotoxic shock syndromes be their endotoxins (Lipopolysaccharides). Therefore the prevention of bacterial infections by blood components can be classified in general procedures and blood components specific procedures.
General procedures
Donor selection programs with a temporary deferral of blood donors after endoscopy, dental treatment, or fever are sufficient measures to prevent bacterial contaminations of blood components. Secondly an efficient donor arm disinfection with alcohol and iodine or chlorine with swabs is important to reduce the resident skin flora to a minimum. Within the beginning of this century all countries implemented the pre‐donation sampling with the diversion of the first 30–40 ml. All these general procedures are undoubtedly proven and implemented worldwide.
Blood components specific procedures
Many countries have already implemented detection of bacteria in platelets by using culture methods. Those systems convinced with a high analytical sensitivity. A residual risk for culture methods are sample errors by collecting the test volume within the first 24 h where the true bacterial concentration in the platelet concentrates is reduced. A second failure of culture methods could be the incubation temperature which is usually standardized to 35–36°C. Some bacteria have an optimized proliferation temperature at 30 or 20°C. Finally for some blood components with a reduced sample volume like stem cell concentrates, incubation in culture bottles with an input volume of 4–10 ml is not feasible. Some countries have implemented rapid bacterial detection systems like Bactiflow or NAT systems. These rapid detection systems can be used for platelets with a sample collection 48–72 h after blood donations. Therefore a small gap will be the first 48 h. Rapid bacterial detections systems can be used in principle for all blood components in modified versions. Next to bacterial detection systems different pathogen reduction methods were developed for platelets, plasma and packed red cells. The advantage of these methods is the reduction of all bacteria and potential new pathogens. A disadvantage might be the high price and limited efficiency for bacteria spores. After the inactivation bacterial endotoxins might still occur, this can potentially harm patients. Finally the reduction of the shelf life for platelets is in addition an option to prevent fatal bacterial transmissions. But a reduced shelf life will be a challenge for blood transfusion services and will increase the percentage of outdated components which represents a waste of limited resources.
Conclusion
General procedures are currently accepted and implemented worldwide, but they will not sufficient to prevent all bacterial transmissions. Culture methods and pathogen reduction methods focused on the prevention of bacterial contamination in platelets and might be not feasible for erythrocytes or stem cell components. Therefore the future is still waiting for a new general procedure that will be an addendum to current existing systems – such a system is eagerly awaited.
4D‐S27‐02
One Million and Counting: Bacterial Screening of Platelet Components by NHSBT, Is It An Effective risk Reduction Measure?
McDonald CP , Allen J , Roy A , Ball J , Robbins S , Moule R , Vasconcelos M , Brailsford S , Pitt T
NHSBT, London, United Kingdom
Background
The transmission of bacteria by transfusion remains a significant problem in transfusion medicine. NHSBT have implemented a number of bacterial risk reduction measures including improved donor arm disinfection and diversion. In the period (2006–2010), following these interventions there were 8 contamination incidents from platelet components which resulted in transmission to 11 patients with 3 deaths. In February 2011, NHSBT introduced bacterial screening of platelet components (PC) as a further risk reduction measure.
Aims
To increase the safety of the blood supply by screening PC for the presence of bacteria to prevent transfusion of contaminated units.
Method
Screening was performed on all NHSBT platelets using the BacT/ALERT microbial detection system. Sampling was undertaken between 36 and 48 h post‐donation. An 8 ml sample was incubated under both aerobic and anaerobic conditions on BacT/ALERT at 36°C for the extended 7 day platelet shelf life. Initial reactive (IR) bottles and related units were returned to National Bacteriology Laboratory (NBL) for confirmatory and reference work. IR bottles were Gram stained, subcultured and culture plates were incubated for 48 h. Bacterial species were identified by standard tests. Index packs, or if unavailable, associated units were repeat tested in duplicate on BacT/ALERT for confirmation. Positives were then processed as for IR bottles. A confirmed positive was defined as that in which the IR bottle and the index and/or associated packs were positive and the same organism was identified in both. In some cases bacteria were detected but not confirmed (indeterminate positive) or not detected and not confirmed (indeterminate negative), usually due to the unavailability of associated components.
Results
Between 20th February 2011 and 31st December 2014, 1,020,688 platelets were screened: 822,603 apheresis platelet components and 198,085 pooled platelet components, with an initial reactive rate respectively of 0.43% (n = 3551) and 0.32% (n = 634). The false positive rate was 0.22% (n = 2224) and 1219 initial reactive packs (0.12%) were described as indeterminate negative.
The overall confirmed positive rate was 0.03% (n = 320), apheresis 0.02% (n = 178) and pooled 0.07% (n = 142). In addition 0.04% (n = 422) of initial reactive packs were described as indeterminate positive.
Skin flora comprised 73% of the contaminants, oropharyngeal 19%, enteric 6% and environmental 2%. Potentially pathogenic organisms confirmed by screening included: Staphylococcus aureus (n = 9), Streptococcus pneumoniae (n = 8), Streptococcus dysgalactiae (n = 7), Listeria monocytogenes (n = 4), Escherichia coli (n = 4), Streptococcus agalactiae (n = 2), Klebsiella pneumoniae (n = 2), Serratia marcescens (n = 2), Campylobacter lari (n = 1) and Pseudomonas aeruginosa (n = 1).
Contaminated units containing enteric organisms were rapidly detected by BacT/ALERT, range 4–20 h, which prevented their transfusion.
There were no reported transmissions during this period, but three near misses from screen negative units were detected where visual inspection revealed the presence of clumps, thereby preventing their transfusion. Staphylococcus aureus was isolated in all three incidents.
Conclusions
The NHSBT bacterial screening protocol using the BacT/ALERT has been effective in increasing the safety of the blood supply. Over 1 million components have been screened and no transmissions have been reported during this period.
4D‐S27‐03
The 30 Min Rule: The Impact of Repeated 30°C Exposure on Bacterial Growth in Red Cells
Aplin K , Roy A , Allen J , Tidey K , McDonald CP , Pitt T
NHSBT, London, United Kingdom
Background
Storage of red cells at 2–6°C helps to maintain cell quality and prevent the proliferation of bacterial species. Handling of red cell units in UK hospitals is subject to stringent control measures. Current guidelines state that provided red cells removed from controlled temperature storage (CTS) in blood banks are returned within 30 min, they may be re‐issued. Units which fail to be returned to CTS within the stipulated time frame are discarded, resulting in potential wastage of red cells.
Aims
To determine whether single or multiple exposures to 30°C, for either 30 or 60 min, have a significant impact on bacterial growth in red cells. Temperature deviations were designed to validate the current 30 min rule, and investigate the effects of extending the rule to 60 min.
Methods
Leucodepleted red cell concentrates in SAGM (sets of four adult units and four paediatric units, in duplicate) were spiked with six transfusion relevant bacterial species; Staphylococcus epidermidis, Bacillus cereus, Pseudomonas putida, Enterobacter cloacae, Serratia liquefaciens and Yersinia enterocolitica, at a target concentration of 103 cfu/ml, on separate occasions. Spiked units, stored at 2–6°C for 35 days, were subjected to a schedule of 30°C temperature deviations. Units were sampled and enumerated immediately post spiking on day 1 and once only on days 7, 28 and 35. On each of days 15, 17 and 21, one adult and one paediatric unit were exposed to 30°C, once for either 30 or 60 min. The remaining units were exposed to 30°C three times on day 15 only, for either 30 or 60 min. Units were sampled and enumerated prior to and after each of the deviations on days 15, 17 and 21. Units exposed multiple times on a single day were returned to CTS for an equivalent time prior to the next 30°C exposure. The effect of 30°C exposures on bacterial count was compared with controls maintained at CTS (2–6°C).
Results
Most impact was noted upon bacterial counts for P. putida, divergence from the control was exhibited in all units subjected to prolonged 30°C exposures. Significant impacts were noted only for adult units subjected to multiple 60 min exposures on day 15. Following two exposures of 60 min, a > 1 logarithm difference from the control was observed. Little impact on growth of S. epidermidis and E. cloacae was observed, bacterial counts for both organisms steadily declined from 103 to 105 cfu/ml at the point of inoculation to ≤101 cfu/ml on day 35 of testing. Both S. liquefaciens and Y. enterocolitica reached concentrations exceeding 109 cfu/ml by day 7 of testing, however enumeration results were consistent with those of the controls until day 35. B. cereus showed no enhanced growth following repeated exposures to 30°C.
Conclusions
The results of the study validate the current 30 min rule with respect to the six bacterial strains tested, since growth in red cells was not significantly affected by either single or multiple exposures to 30°C. Results also indicate that an extension of the rule to 60 min might also be permissible.
4D‐S27‐04
Fatal Transfusion‐Transmitted Staphylococcus epidermidis: A Case Report
Ramirez-Arcos SM1, Kou Y 1, Pagotto F 2, Hannach B 1
1Canadian Blood Services, Ottawa, ON, Canada2Health Canada, Ottawa, ON, Canada
Background
Canadian Blood Services screens platelet concentrates (PCs) for bacterial contamination using BacT/ALERT aerobic culture bottles at approximately 24 h post‐collection. In September 2014, a splenectomised elderly male patient, suffering from leukemia, was transfused with two 5‐day‐old buffy coat platelet pools. Microbiology testing performed on a blood sample of the patient and remaining PCs revealed that the patient sample and one platelet pool were contaminated with Staphylococcus epidermidis. The transfused platelet pools had tested negative during initial platelet screening likely due to a bacterial concentration that was below the limit of detection (LOD) of the BacT/ALERT system.
Aim
To investigate a fatal transfusion case involving a bacterially‐contaminated buffy coat platelet pool.
Methods
The biochemical and antimicrobial phenotypes of the S. epidermidis strains isolated from the patient sample and the implicated platelet pool were determined using API Staph and Etest strips (bioMérieux), respectively. Automated ribotyping was applied to determine molecular relatedness of the strains. RBC and plasma units associated with the transfused pools were recalled and tested for sterility. A titration experiment was designed to determine the LOD of the BacT/ALERT system for this S. epidermidis strain: PC units were spiked at final concentrations from 0.00008–0.74colony forming units (CFU)/ml on day 0. PCs were incubated under platelet storage conditions for 5 days. On days 1, and 3–5 of incubation, samples were taken for BacT/ALERT culture and determination of bacterial concentration. The ability of this S. epidermidis strain to form surface‐attached aggregates (biofilms) was evaluated using a crystal violet assay. Furthermore, PCR amplification of biofilm‐associated icaA and icaD genes, slime production on Congo red agar, and bacterial attachment to platelet bags were determined.
Results
The phenotypic and molecular profiles of the S. epidermidis strains isolated from the patient and PCs were identical. None of the RBC or plasma units associated with the contaminated pool tested positive for bacterial contamination. The titration experiment indicated that, at the time of screening, the contaminated pool likely had a concentration of <0.74 CFU/ml (<227 CFU/unit) of S. epidermidis. This strain does not produce slime and does not carry the biofilm‐associated icaA and icaD genes. Interestingly, crystal violet assays showed that it developed a strong biofilm positive phenotype in PCs. Furthermore, attachment to the inner surface of platelet containers was demonstrated.
Summary/Conclusions
Despite several mitigation strategies, false negative cultures with the predominant PC contaminant S. epidermidis still occur. Missed detection of S. epidermidis is mainly linked to low initial concentrations with contribution of biofilm formation. Mathematical models have predicted that up to 70% of PCs contaminated with coagulase‐negative staphylococci at concentrations of 0.02 CFU/ml can be missed by BacT/ALERT screening. Importantly, patients transfused with blood products contaminated with S. epidermidis may not develop typical symptoms of an acute transfusion reaction. It is therefore essential to raise medical awareness of the pathogenicity of this seemly low virulence organism, which is exacerbated by its propensity to form biofilms during platelet storage.
4D‐S27‐05
Platelets and Infections, Storagetime and Storage Medium: Does It Matter?
Kreuger AL1, Middelburg RA 1, Wiersum-ossolton JC 2, Brimicombe RW 3, Kerkhoffs JLH 3, van der Bom JG 1
1Sanquin/LUMC, Leiden, The Netherlands2TRIP, National Hemovigilance and Biovigilance Office, Leiden, The Netherlands3Haga Hospital, Den Haag, The Netherlands
Background
Extended storage of platelet concentrates causes soluble immune modulators to accumulate. It also increases the ability to detect bacteria, by increasing the time to culture. Storage in platelet additive solution (PAS) reduces the biofilm capacity of bacteria, possibly leading to earlier detection of contamination. Plasma contains proteins which could influence bacterial growth.
Aim
The aim of this study is to determine the effect of storage time and storage medium of platelet products on bacterial infections in transfusion recipients.
Methods
We performed a case‐control study to compare the storage medium of platelet concentrates leading to transfusion‐transmitted bacterial infections, reported to TRIP (Transfusion Reactions In Patients, the Dutch national hemovigilance organization). All other reported reactions, associated with platelet transfusions, served as controls. For a case‐reference study data were collected in two hospitals, 1 using PAS, 1 using plasma‐stored platelets. All platelet transfusions between 2006 and 2013 were included. Cases were haematological patients who received a platelet transfusion and had a positive blood culture the next day. The observed storage time was calculated in cases as the number of products in 3 age categories (young ≤2 days, middle 3–4 days, old ≥5 days). As a control, the expected storage time was determined based on all transfusions given in that hospital, stratified by day of the week and Rhesus D type. For each age category and storage medium an observed‐expected ratio was calculated. This estimates the rate ratio for a positive blood culture after transfusion per platelet age category, using both other age categories as reference.
Results
Fourteen cases of transfusion‐transmitted bacterial infections had been reported in 2002–2013. In 8 (57.1%) a PAS‐stored unit was involved and in 6 (42.9%) a plasma unit. In all reported reactions, 20.5% of platelet products were stored in PAS. (RR for PAS 5.00, 95% confidence interval (CI) 1.73;14.46).376 positive blood cultures after transfusion of plasma‐stored platelets and 116 after transfusion of PAS‐stored platelets were included. The rate ratio for plasma‐stored platelets was 1.08 (CI 0.96;1.22) for young products, 0.90 (CI 0.82;0.99) for middle‐aged and 1.12 (CI 0.90;1.35) for old platelet units. For PAS‐stored platelets, the rate ratios were 0.97, (CI 0.81;1.12) for young, 0.92 (CI 0.73;1.12) for middle and 1.93 (CI 0.79;3.07) for old platelets.
Conclusion
We observed an association of storage medium and transfusion‐transmitted bacterial infections, with an increased incidence after exposure to PAS‐platelets. Storage time of plasma‐stored platelets appears not to affect the incidence of bacterial infections in haematology patients, but in PAS‐stored platelets the risk of infections may increase using older platelets.
4D‐S27‐06
Inactivation of Bacteria in Apheresis Platelets with Pathogen Reduction Performed 24 h After Inoculation
Wagner J 1, Benjamin RJ1, Hapip CA 1, Kaelber N 1, Annette A 1, Skripchenko A 1, Stassinopoulos A 2
1American Red Cross, Rockville, MD, United States of America2Cerus Corporation, Concord, CA, United States of America
Background
Pathogen reduction methods are designed to inactivate organisms present in blood components soon after collection. Blood Center operations are improved if inactivation of apheresis platelets can be delayed until the day after collection; however fast growing bacteria can increase in concentration during this time, potentially overwhelming the inactivation system.
Aims
This study investigates whether amotosalen and UVA treatment can sterilize units (defined as no growth on culture 5–7 days after collection) contaminated with blood collection‐relevant titers of either fast or slow growing organisms when inactivation is performed 24 h after inoculation.
Methods
Single (for K. pneumoniae and S. pyogenes) and double (for S. epidermidis and E. coli) units of platelets suspended in PAS‐III (35% plasma) were collected on the Amicus separator and subsequently inoculated with K. pneumoniae (Paul Ehrlich Institute, PEI‐B‐P‐08‐01), K. pneumoniae (American Type Culture Collection, ATCC 29015), S. pyogenes PEI‐B‐P‐20‐01, E. coli PEI‐B‐P‐19‐01 and S. epidermidis PEI‐B‐P‐06‐01. After inoculation, units were stored for 24 ± 0.3 h at 20–24°C with agitation. The contaminated single unit and one unit from each contaminated double unit (test) were treated with amotosalen and 3 J/cm2 UVA light, followed by 4 h incubation in a compound absorption device (CAD) and then transferred to a final storage container. For double units, the second unit served as an untreated control. All amotosalen treated and control unts were incubated at 20–24°C with agitation for 7 days. Samples from the inoculum solutions and samples of the apheresis units were taken on Day 1 before and after inactivation, Day 2 for aerobic and anaerobic BacT/ALERT testing, and Days 5 and 7 for plate counts and aerobic and anaerobic BacT/ALERT testing. Experiments with each bacteria were performed 3 times using blood from different donors except for S. epidermidis (n = 2).
Results
K. pneumoniae PEI and K. pneumoniae ATCC grew 105–106‐fold during the 24 h between inoculation and inactivation. E. coli grew 105–106‐fold during the 24 h between inoculation and inactivation in two of three experiments and in one experiment, had bacteria present but no observable growth. One‐hundred‐fold growth was observed for S. pyogenes but no growth was observed for S. epidermidis between inoculation and inactivation. All units treated with amotosalen and UVA light were culture negative on Days 2, 5, and 7 even though bacteria were present just prior to inactivation based on plate counts or units were culture positive in non‐inactivated control units. Results are summarized in Table 1.

Summary/Conclusions
A 24 h period between inoculation and pathogen reduction did not compromise the ability of amotasalen and UVA to inactivate apheresis platelet units to sterility, when contaminated with a variety of fast and slow growing organisms on the day of collection.
Haemoglobinopathies
4D‐S28‐01
Sickle Cell Disease in Subsaharan Africa
Mbanya NS
Faculty of Medicine and Biomedical Sciences, Yaounde, Cameroon
Background
Approximately 5% of the world's population carry trait genes for haemoglobin disorders, mainly, sickle cell disease (SCD) and thalassaemia. These SCDs refers to an inherited disorder in which red cells contain an abnormal haemoglobin (Hb) resulting from structural gene mutations of the beta globin chain, with Haemoglobin S (HbS) being the most prevalent genetic disorder worldwide, and affecting mainly those of African descent.
In SCD, the structural modification of erythrocytes results in blocked blood and oxygen flow to parts of the body, leading to episodes of pain and damages to vital organs, and also contributing to various clinical manifestations and complications.
Aim
This paper aims at reviewing SCD in SSA, including diagnosis and management, and the general impact on the affected individual and the society as well.
Methods
Data collected for this review has been obtained from published articles, books, internet websites and from personal experiences.
Results
Despite the high occurrence of SCD in SSA, there are few countries with national health policies and programmes regulating prevention and care. Even when these exists, they do not cover national territories. Hence SCDs remain neglected diseases, despite the huge socio‐economic impact on the affected (educational and psychological setbacks and discriminations), on the family (financial strains as there are virtually no functional health insurance schemes) and on the community as a whole.
Early (neonatal) screening for SCD, combined with care and parental education have been reported to drastically reduce infant morbidity and mortality. However, only few countries of SSA systematically carry out neonatal diagnosis. Thus, death before the age of 5 years is common. Standard screening tends to occur only when severe complications arise. There is lack of infrastructures, multidisciplinary teams and specialized centres for SCD care. Penicillin prophylaxis and pneumococcal immunizations are not routinely practised, despite established benefits.
The management of SCD patients has traditionally required nonspecific care such as rest, hydration and good nutrition, but also the use of several therapeutic drugs ranging from analgesic and anti‐inflammatory drugs, to blood and blood components, and the management of complications. The use of Hydroxyurea has shown very beneficial effects in reducing painful vaso‐occlusive crises, acute chest syndrome, preventing strokes and improving life expectancy. However, accessibility issues and other limitations make its use reduced in SSA.
Some studies indicate that 75% children receive some form of transfusion by the age of 6 years, usually not of phenotyped blood, with consequential frequent allo‐immunization. While chronic transfusion regimens have shown a lot of clinical benefits both in African and other studies, these are not always readily accessible in SSA where the procedure is greatly complicated by the limited availability of blood, the high prevalence of transfusion transmissible infections and scarcity of required resources for safe blood.
Haematopoieitc stem cell transplantation is curative but not available in SSA.
Conclusions
Prevention, care and management of SCD remain a major challenge in SSA. Coordinated multidisciplinary teams, including health and social workers, and non‐governmental organisations are indispensable for better impact on SCD outcome in SSA.
4D‐S28‐02
Transfusion Therapy in Sickle Cell Disease
Howard J
Guy's and St Thomas’ Hospital, London, United Kingdom
Blood transfusion therapy is used widely in the treatment of patients of sickle cell disease (SCD) and whilst it is often life saving it can also be associated with serious complications. The indications for blood transfusion in SCD include transfusion in the acute situation for the treatment of serious complications (e.g. for patients with acute chest syndrome, stroke or anaemia) and long term transfusion programmes for the prevention of chronic complications (e.g. secondary stroke prevention, recurrent pain crises or acute chest crises not responsive to hydroxycarbamide). Recent randomised controlled trials have described the role of transfusion therapy in primary stroke prevention in patients with raised trans‐cranial Doppler flow and with silent infarcts on MRI scan. Transfusion therapy is also used in specific circumstances such as prior to surgery or during pregnancy to prevent recurrent pain crises. The role of blood transfusion in other indications e.g. in the management of leg ulcers, priapism or pulmonary hypertension is not clear but there is observational evidence of benefit in some patients with these complications and indications for transfusion should be discussed on a case by case basis with a sickle expert.
Blood transfusion may be given as a simple or ‘top‐up’ transfusion, particularly in patients with a markedly low haemoglobin level, or as an exchange transfusion which will result in a more effective decrease in HbS level without causing an increase in blood viscosity. Exchange transfusions can be performed manually or can be automated (erythrocytopheresis) and the latter allows improved control of HbS levels without long term iron loading which is particularly useful for patients on long term transfusion regimens.
The complications of blood transfusion of particular relevance for patients with SCD are iron overload, which will require iron chelation therapy if severe and allo‐immunisation. Iron loading can be insidious, especially in patients who have been sporadically transfused over many years and should be monitored at least annually. Allo‐immunisation can be a serious complication and can result in delayed haemolytic transfusion reactions and haemolytic disease of the newborn. Patients with SCD should have an extended red cell phenotype performed before first transfusion and have extended red cell matching for full Rh and Kell typing. This will decrease but not eliminate the risk of allo‐immunisation. Hyperhaemolytic reactions may also occur post‐transfusion and may or may not be associated with the formation of a new allo‐antibody. These may cause rapid haemolysis with a rapid fall in haemoglobin and can re‐occur if patients are re‐transfused and hence have important ramifications for long term patient management. Immunosuppressive therapy can be of benefit in treatment and prevention of recurrence.
4D‐S28‐03
Exchange Transfusions for Sickle Cell Disease in Australia; To Exchange or Not to Exchange?
Crighton G1, Scarborough R 1, Greenway A 2, Mason K 3, Ho J 4, Wood E 1
1Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Vic., Australia2The Royal Children's Hospital, Melbourne, Vic., Australia3The Royal Melbourne Hospital, Melbourne, Vic., Australia4Institute of Haematology, Royal Prince Alfred Hospital, Sydney, NSW, Australia
Background
Erythrocytapheresis (ECP) or red blood cell (RBC) exchange transfusion is effective in managing both acute and chronic complications of sickle cell disease (SCD). Removing a patient's sickle RBCs and replacing them with normal donor RBCs can improve oxygen‐carrying capacity with reduced blood viscosity and iron neutrality. Australia has a growing SCD population but limited information on SCD patient numbers, management (including ECP) and clinical outcomes.
Aims
To determine numbers of centres managing SCD patients, uptake of ECP for SCD, and to characterise transfusion support provided for Australian SCD patients, including features of ECP programs.
Methods
An online survey distributed through the Haematology Society of Australia and New Zealand, the Australian and New Zealand Society of Blood Transfusion, the steering committee for Australia's new national haemoglobinopathy registry and also targeted to sites known to manage patients with SCD and/or perform ECP. Survey questions included SCD patient cohort characteristics, features of ECP programs and current transfusion support.
Results
Survey responses were received from 23 clinicians (22 Australian, 1 New Zealand) documenting current practice of 21 hospitals. Of these 21 institutions, 18 routinely provided care for SCD patients and of these, 13 had patients on a regular ECP program. Three clinicians provided ECP in the acute setting, one hospital was setting up a program and one clinician did not routinely provide or refer for ECP. 75% clinicians reported having an institutional protocol for ECP.
The majority of clinicians (78%) treated 1–20 patients, 3 clinicians treated 21‐ 50 patients and one institution treated 51–100 individuals. The highest numbers of SCD patients were seen in paediatric centres.
The most common acute indications for ECP were acute chest syndrome and acute ischaemic stoke (68% of respondents for each). The majority of clinicians (69% responses) providing regular ECP aimed for a target haemoglobin (Hb) S of <30%. However there was considerable variability in target Hb amongst clinicians (range 70–140 g/L).
With regard to transfusion, 65% clinicians reported matching for antigens to which the patient had developed alloantibodies and 41% aimed to provide phenotypically matched blood. The most common RBC modification requested was fresh RBCs (42% responses), followed by washed RBCs (33%) and sickle negative RBCs (25%).
Conclusions
Results from the survey support a rising incidence of SCD in Australia and NZ, with paediatric centres reporting the highest number of patients. Healthcare resource requirements are projected to increase, due to the significant improvement in life expectancy for SCD over the last 20 years. ECP is frequently being used in acute and chronic management of SCD. Documented variation in practice amongst clinicians includes indications for ECP, transfusion targets, RBC antigen matching practice and request for RBC modifications. The survey highlights the need for information on clinical outcomes and for national consensus on transfusion support in SCD. The new Australian Haemoglobinopathy registry has already gathered data on 200 patients and will provide further valuable information over time.
4D‐S28‐04
Occasional and Long Term Automated Red Blood Cells Exchange in Sickle Cell Disease Patients: Impact on Hospitalization Frequencies
Menif H1, Kharrat RK 1, Dhieb A 1, Louati N 1, Rekik TR 1, Rekik H 1, Ghorbel M 2, Elloumi M 2, Elloumi M 2, Gargouri J 1
1CRTS Sfax, 99/UR/08‐33, faculté de médecine de Sfax, université de Sfax, Sfax, Tunisia2Hematology service, faculté de Médecine de Sfax, Sfax University, Sfax, Tunisia
Aim
Effectiveness of automated red blood cells exchanges (ARE) in managing sickle cell patients (SCP) have been proven either during acute crisis or as prophylactic treatment of recurrent stroke or in pre‐operative management (ASFA 2013: categories I–III). In this study, we report our 10 years of ARE in SCP and evaluate the impact of chronic ARE on hospitalizations frequency.
Material and method
Twenty six SCP were enrolled. Seven patients were converted from chronic transfusion (5 had already occasional ARE) to chronic ARE: 4 with history of resistant vaso‐occlusive crisis (RR VOC), 2 patients for acute chest syndrome and 1 for acute stoke and multiorgan failure and 19 patients received occasional ARE: [preoperative: 12, resistant priapism: 4 and repeated resistant vaso‐occlusive crisis (RR VOC) in pregnancy: 3]. Seven patients had Hydrea [priapism: 1, perioperative: 1, RR VOC: 2, acute chest syndrome: 1 and acute stoke and multiorgan failure: 1). Erythrocytapheresis was performed on COBE Spectra (Terumo BCT) (fluid balance: 95–100%). Compatible cross‐matched, phenotyped and WBC‐depleted packed Red Blood Units (PRBU) were used to replace patients RBC. Peripheral venous access was the preferred route for treatment.
Results
We performed a total 200 ARE on 26 SCP [mean age at inclusion 26 (11–50) years and sex ratio 1.6). The 7 patients who experienced chronic ARE achieved a mean of 24 procedures (6–49), with a mean visit interval of 3.4 (2.2–7) months, [mean follow‐up period 5.4 (2.4–9.7) years]. Thirty ARE were performed occasionally on 19 patients (see table).

In 2 cases (priapism and painful VOC), symptoms resolved before the end of the procedure. In the chronic ARE group, mean hospitalization (frequency/duration) dropped from 2.4/year/patient before ARE inclusion (mean follow up: 7.3 year) to 1.6 after (mean follow up: 4.8 year) and from 19.7 to 8.6 day/year/patient. A non observant patient continued to have a mean of 3.8 hospitalization/year (mean 33 day/year). Adverse events were observed in 1 patient (3%) in the ‘occasional’ group and 9 patients (5%) in the chronic group (P0.001). A patient in the occasional group needed a CVC (two procedures prematurely stopped). Two patients in the chronic group had fistula from whom one showed repeated thrombosis.
Discussion
In our experience, ARE was safe, well tolerated, no serious adverse event were observed within the procedure. Despite less frequent procedures than reported by other centers and higher levels of abnormal hemoglobin, ARE were successful in bettering patient life quality, reducing hospitalization (frequency and duration) and thus reducing global disease cost in chronic transfused SCP. Vascular problem access could be a limitation factor and, as known, fistula at risk of repeated thrombosis.
Cell Therapy and Safety
4D‐S29‐01
Regulation and Acreditation in Cell Therapy
Chabannon C
Institut Paoli‐Calmettes, Marseille Cedex 9, France
Cell therapies are developed through engineering of living cells and tissues. These can be either primary material or established/immortalized cell lines; a common point is the origin of the primary material in human beings, that impose specific measures for cell or tissue procurement; the two major principles are (i) donor screening and validation, and (ii) traceability (the ability of the manufacturer/producer of cell or tissue therapies to continuously maintain a link between donor(s) and recipient(s)). Such requirements are largely covered in European directives that have been transcribed in national regulations throughout member states.
Processing or manufacturing of collected cells or tissues nowadays occurs in two different organizational and regulatory contexts. Products that are not ‘substantially manipulated’ (also referred to as ‘minimally‐manipulated’ products) undergo simple processing mostly designed to ensure appropriate storage and distribution; such processing does not affect the functional properties of cells and tissues, and is performed by trained professionals in cell therapy facilities or tissue banks mostly operated by public or private not‐for‐profit hospitals or blood banks. These products are prepared on the basis of an individual prescription, with a unique product for a designated recipient, as exemplified by the long‐standing practice of hematopoietic cell transplantation. Alternatively, cells or tissues that are extensively processed are considered as medicinal products, and – in Europe – fall in the recently created category of Advanced Therapy Medicinal Products (ATMPs). Such products are further classified in somatic cell therapies, gene therapies, products of tissue engineering, and combined ATMPs, depending on the nature and complexity of cell processing. Most examples of marketed ATMPs are still ‘one‐to‐one’ medicinal products; it is however expected that a growing proportion of these medicinal products will be produced as batches, paving the way for a truly pharmaceutical process. Although the European regulation defining ATMPs is now 8 years old, there remains a number of issues regarding these products, including but not limited to the coordination between industry and healthcare providers for cell procurement, ATMP administration and coverage of costs.
Quality management (QM) is deeply rooted in pharmaceutical manufacturing. It is also very much part of the practice of blood or tissue banking. It has proven more difficult to introduce QM in clinical practice; in this field, healthcare activities clearly lag behind the airline industry, to name one example. The field of hematopoietic cell transplantation (HCT) has provided a unique setting through its combination of clinical care, cell procurement and cell processing activities, and associated risks. HCT has allowed for the development of an integrated QM system (FACT in north‐America, JACIE in Europe) that has been implemented in a large proportion of HCT programs since the mid‐90s; the goal to promote excellence and improve harmonization of practices has translated in benefits in terms of hospital organization and delivery of care. Whether and how such organization must simultaneously adapt to the emergence of multiple QM initiatives in healthcare, driven either by competent authorities or by peers, and to continuous changes in regulations will be discussed.
4D‐S29‐02
Immunotherapy with Gene‐Modified T Cells: Promises and Challenges
Morris EC
United Kingdom
Immunotherapies for cancer, infection and immune disorders are now being tested widely in early phase clinical trials across Europe and the US.
Gene modification of T cells can alter specificity or function or both.
Altering the specificity of T cells can be achieved through the introduction of CARs or TCRs using retro or lentiviral vectors.
Chimeric antigen receptors (CARs) are modified single chain antibody fragments that recognise cell surface tumour antigen and are not limited by MHC restriction. However, CARs are unable to recognise tumour associated intracellular proteins, which are presented on the cell surface in the context of MHC. T cell receptors (TCRs) are required to target T cells to these antigens.
The first part of this lecture will discuss the rationale behind TCR and CAR modification of T cells, their use in clinical trials and some early results.
Modifying function, persistence, homing, cytotoxicity, cytokine secretion and differentiation status of T cells is being explored in research laboratories. This provides a platform for the generation of ‘designer’ immune cell‐based therapies. Preliminary findings will be discussed.
4D‐S29‐03
Alloreactive KIR‐2/3DL Negative NK Cell Subsets for Adaptive Immunotherapy
Seidl C , Klemm D , Stricker N , Hennecke J , Becker P , Seifried E
Institute of Transfusion Medicine and Immunohematology, Red Cross Blood Donor Se, Frankfurt am Main, Germany
Background
Natural killer (NK) cells mediate graft vs leukaemia (GvL) effects in hematopoietic stem cell transplantation (HSCT) and are used in clinical trials as adoptive immunotherapy after HSCT.
Aim
The GvL effect is supported by NK cells that solely express inhibitory killer cell immunoglobulin‐like receptors (KIR) missing their corresponding HLA ligand in the patient (KIR‐HLA mismatch). Here, we describe a technique for the isolation of patient specific alloreactive KIR+ donor‐NK subsets at GMP‐level by MHC Streptamers.
Methods
KIR‐ligand specific MHC Streptamers for KIR2DL1+, KIR2DL2/L3+, and KIR3DL1+ NK‐cells (HLA‐C*04:01‐CMV, HLA‐C*01:02‐TIMP, HLA‐B*57:01‐HIV) were used. Following negative isolation of NK cells from peripheral blood, functional activity was assessed by the CD107 degranulation assay against L721.221 transfectants.
Results
Flow cytometry showed a mean staining of 83% for KIR2DL1+ NK cells, 71% for KIR2DL2/L3+ NK cells, and 63% for KIR3DL1+ NK cells. Following magnetic assisted cell sorting we obtained a mean purity of 89% KIR2DL1+ and 73% KIR2DL3+ cells indicating a specific and reproductive ligation of MHC Streptamers to KIR on the NK cell surface. Importantly, sorted KIR+ NK cells showed lytic activity and higher degranulation activity against KIR‐HLA matched target cells compared to bulk NK cells.
Conclusion
This technology allows sorting of alloreactive KIR+ NK cell populations by negative isolation of all KIR‐expressing NK cells that will be inhibited by the patient′s HLA and only select the alloreactive NK cell subset for direct adoptive cell therapy or ex vivo NK cell expansion.
4D‐S29‐04
Efficient Induction and Expansion of Polyclonal Human CD8+ CD103+ Foxp3+ regulatory T cells by TGF‐? And Rapamycin Which Have a Therapeutic Function on Autoimmune Diease Model—CIA Mice
Sun JS1, Huo XH 2, Yang YY 1, Yang JY 1, Xie RX 1, Jiang XJ 1, Zhu YZ 1, Fan HF 1
1Shanghai Blood Center, Shanghai, China2Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China
Background
Regulatory T cell is a mature subpopulation of T cells with immune regulatory function which contains CD4+ Treg and CD8+ Treg. It has an important role in suppressing autoimmune disease, maintaining immune tolerance and makes it a potential candidate for cell‐therapy. However, because of very few number of nTreg cells, amplification of Treg cells in vitro for adoptive therapy is needed. Unfortunately, expanded CD4+ CD25+ Foxp3+ Tregs could transform into effect T cells easily in the condition of inflammatory. Several approaches for induction of Ag‐specific CD8+ Tregs have been reported, but there is currently no reliable protocol for the ex vivo induction and large‐scale expansion of human polyclonal CD8+ Foxp3+ Tregs.
Aims
In this study, we aim to establish the method of amplification of polyclonal CD8+ regulatory T cell in vitro and further study its purity, activity, expansion, inhibition, anergy, stability in inflammatory cytokines condition and function on autoimmune disease model— collagen‐induced arthritis (CIA) mice.
Methods
CD8+ Tregs were induced and expanded from CD8+ T lymphocytes (isolated from PBMCs) with anti‐CD3/28 beads, IL‐2, TGF‐β and rapamycin in vitro. In order to obtain sufficient Tregs, CD8+ Tregs were re‐stimulated for another three cycles in the condition above. The features and functionalities of expanded CD8+ Tregs were investigated and CIA mouse model was induced with type‐two collagen as an autoimmune disease model.
Results
CD8+ CD103+ Foxp3+ Tregs could be efficiently induced and expanded in vitro. The purity, activity can reach up to 90% and the expansion fold can reach 10000 times at least. Obtained CD8+ Tregs expressed high level of Foxp3, CD103, CD25, CD28, PD‐1, CD62L, CCR7, CTLA4, and CD39, secreted small amount of IL‐2, IFN‐γ, IL‐10 and TGF‐β, and did not secret IL‐17A. The expanded Tregs adopted vigorous suppression function on CD4+ CD25− T lymphocytes and anergy ability in vitro. The suppression of CD8+ CD103+ Foxp3+ Tregs in proliferation of effective T lymphocytes was mainly dependent on cell contact but not dependent on their cytotoxic activity. Moreover, CD8+ CD103+ Foxp3+ Tregs did not secret IL17A or secret small amount of IFN‐gamma in the presence of inflammatory cytokines such as IL‐1β and IL‐6 or IL‐21 and IL‐23 which were different from CD4+ CD25+ Foxp3+ Tregs. In CIA mice, CD8+ CD103+ Foxp3+ Tregs treatment could significantly alleviate the severity of arthritis, and reduce the cartilage destruction of the joints of CIA mice and the level of total anti‐type‐two collagen IgG antibody in serum as well, through the way to induce the mice self CD4+ Foxp3+ Treg generated in spleen and reduce the CD80/CD86 expression on mice mDCs to inhibit the proliferation of mice CD4+ T cells.
Conclusion
The results revealed that polyclonal human CD8+ Treg cells could be effectively expanded in vitro and its biology characters could be maintained after multi‐amplification. In addition, its therapeutic function on CIA mice was effective. This research can promote the further understanding of CD8+ Treg cells and cell therapy in autoimmune disease.
Donor Management
4D‐S30‐01
Managing Donors and Donation Sessions to Improve Donor Service and Session Productivity
Nyberg-Oksanen E 1, Sydenham V 2, Morgan S 2, Georgsen J 3, Jansen E 4, EBA Benchmarking Group (EBG)
1Finnish Red Cross Blood Service, Helsinki, Finland2NHSBT, Filton, United Kingdom3Odense University Hospital, Odense, Denmark4Blood Supply Chain Solutions, Amsterdam, The Netherlands
Background
The EBG has facilitated 3 collection productivity workshops (2008–2015). Productivity improvements are evident in some European blood services, but fewer than anticipated. To accelerate improvement, the EBG has organized ‘Flying Squad’ visits; with the host blood service, we study and identify improvement opportunities and disseminate key learnings. In a recent workshop, these learnings were presented to EBA subject experts, and more data was gathered.
Aims
European blood services sometimes lack data, experience, and state of the art technology to implement opportunities to improve productivity. With the decline in red cell demand across the developed world, alongside constrained health care budgets, the challenge is balancing increasing donor expectations with higher productivity targets.
Methods
Against each key element we have collected key performance indicators (KPIs) and benchmarked them across European blood services. We have also identified technology improvements that could help blood services improve performance.
Results and recommendations
Increase amount of successful donations: optimize selection process, adopt best practice for pre‐donation and educate donors to self‐defer. The KPI is the donor deferral rate. The technology enabler is the pre‐session electronic questionnaire and/or health check.
Implement a Pull system in your blood supply chain. Take into account the red cell stock levels per blood type for short term planning of attending donors. The KPI is the red cell stock level targets. The key is to tie the donor blood group to the technology enabler used e.g. appointment system or invitation software.
Reduce end-to-end donation time: reduce intermediate steps as much as possible, have multi‐skilled workforce to service any waiting donors and introduce self‐service possibilities for the donor. The KPI is minimum end‐to‐end donation time and waiting time. The technology enablers are an appointment system and a paperless donation journey.
Reduce staff non-donor time: optimize opening times, create continuous flow through scheduled donor attendance, and, in mobiles, reduce total set up and set down time. The KPIs are even distribution of donor arrivals, and optimal setup and set down time for mobile sessions. The technology enabler is an appointment system.
4D‐S30‐02
Comparison of Donor and General Population Demographics in Canada, the Netherlands, England and the United States: A Best Collaborative Group Study
O'Brien F1, Goldman M 1, Vassallo R 2, Steele WR 3, di Di Angelantonio E 4, van den Van den Hurk K 5, Custer B 2, Dubuc S 6, Germain M 6
1Canadian Blood Services, Ottawa, ON, Canada2Blood Systems, Scottsdale, AZ, United States of America3American Red Cross, Washington, DC, United States of America4NHS Blood and Transplant, London, United Kingdom5Sanquin, Amsterdam, The Netherlands6Hema‐Quebec, Montreal, QC, Canada
Background
The rapidly aging populations of most developed countries may increase demand for blood. The donor base may similarly be aging, with adverse consequences for future blood availability.
Aims
To compare age and gender of blood donors vs the general population in various developed countries.
Methods
The numbers of male and female allogeneic whole blood/apheresis donors and males and females in the general population in four countries in 2011 were obtained from local donor databases and institutions responsible for population statistics. Donor age limit criteria were provided by the authors. The numbers of donors were divided into age group categories for both males and females. The percentage of each demographic category was calculated as the percentage of total donors and done similarly for the general population. The difference in the percentage of the donor population vs the general population for each category was calculated for each country. A positive percentage indicates over‐representation of the age and gender category in the donor population compared to the general population, while a negative percentage indicates under‐representation. Percentages close to zero indicate that the percentage of donors is roughly equivalent to the general population.
Results
There was heterogeneity across blood donation services in males and females and in different age groups (see Table 1). Overall female donors tend to be over‐represented in younger age groups and under‐represented in older age groups in all countries. In North America, the youngest age group is over‐represented, while middle‐aged donors tend to be over‐represented in all countries except the USA. The oldest age group is universally under‐represented. The distribution of the general population showed similar higher proportions of older people in each country.

Summary/Conclusion
There are substantial differences in the representation of various population segments in the donor base amongst these four countries. Over‐representation of young donors in North America dissipates by young adulthood. Heavy reliance on middle‐aged donors is evident in most countries. These differences likely reflect variable donor recruitment and retention strategies, but perhaps also different interest in donation at different life stages. Lower representation of the oldest age group reflects the donor health criteria and different upper age criteria, but may also reflect less focus on retaining these donors. This study will ultimately include data from 11 participating countries at three different time points.
4D‐S30‐03
Donor Deferral in Poland in 2003–2004 and 2010–2013: What has Changed
Rosiek A , Plodzich A , Lachert E , Tomaszewska A , Letowska M
Institute of Hematology and Transfusion Medicine, Warsaw, Poland
Background
Appropriateblooddonor selection is essential to ensure the safety of blood donation and transfusion. Although inevitable, donor deferrals lead to losses in supplies of donated blood and may effect donor retention rates. Criteria for donor selection in Poland follow recommendations and guidelines as set forth by the EU directives particularly Directive 2004/33/EC.
Aims
To estimate the scope of donor deferrals and their causes in the period 2010–2013 as well as to compare the outcome with that of our earlier study regarding the period 2003–2004 data, i.e. before Poland's EU accession (first presented during the XV ISBT Regional Congress in Athens in 2005).
Methods
Retrospective analysis of 2010–2013 data reported by 21 Polish Regional Blood Transfusion Centers using standardized criteria for temporary and permanent blood donor deferrals. Comparison with results for the period 2003–2004 also reported by these Centers.
Results
In the period 2010–2013 the total of 876,944 donor deferrals was reported which translates to 164.59 deferrals/1000 donor presentations annually (from 146.19 in 2010 to 176.26 in 2013). A total of 46,946 permanent deferrals was reported in this period. This corresponds to the average of 8.84permanent deferrals/1000 presentations annually with a decreasing tendency (from 10.03 in 2010 to 8.14 in 2013). For comparison, the corresponding value for the period 2003–2004 was 14.93 at average. The most frequent reasons for permanent deferrals were severe chronic disorders (57% of all causes of permanent deferrals); less frequent were infectious diseases or infection risk (34%). The remaining causes of permanent deferral were within several percent range. In the same period 829,998 temporary deferrals were reported – 155.28 per 1000 presentations annually, with the tendency to increase in subsequent years (from 136.16 in 2010 to 168.12 in 2013). In the years 2003–2004 the corresponding value was 115.20 which reflects an increasing tendency in using temporary deferrals. The most frequent reasons for temporary deferrals (58%) were laboratory test abnormalities including low levels of Hb. Other reasons for temporary deferrals (transient health problems, infectious diseases, infection risk, abnormalities in medical examination) fell within the 9–12% range. The most prominent difference as compared to the period 2003–2004 was the lack of deferrals due to elevated ALAT values; this parameter has not been tested since 2010. It is also noteworthy to record a growing number of permanent deferrals due to chronic diseases which most likely reflects health problems of the aging Polish society. As 10 years ago also now, the number of permanent and temporary deferrals differs between blood transfusion centers.
Conclusions
Several important observations can be made based on the above data; the decrease in the number of permanent deferrals, increase of temporary deferrals, growing number of permanent deferrals due to chronic health problems, frequency of low Hb levels. Compared to data reported by other countries ours seem to suggest that Poland has a rather strict donor selection practice which may positively affect blood safety but with likely negative impact on blood supply.
4D‐S30‐04
Impact of the First Whole Blood Donation's Complications on Donor Retention and Complications at Further Donations
Gillet P1, Bertrand O 1, Govaerts B 2, Ceinos M 2, Rapaille A 1, de Bouyalsky I 1, Lambermont M 1
1Belgian Red Cross, Suarlée, Belgium2ISBA, Unité Catholique de Louvain, Louvain‐La‐Neuve, Belgium
Background
Previous studies have shown that the return rate for a second donation depends of the occurrence of donors’ complications including mostly vasovagal reactions (VVR) at the first donation.
Aims
his report evaluates the impact of the donor's retention in relation to the occurrence of VVR for the three first whole blood donations.
Methods
The study includes WB donations from January 2010 to December 2012. Among them, first time donors were followed during 15 months. Donor retention and occurrence of VVR were compared in donors with and without VVR at previous donations. Donor retentions were compared using a Chi‐square tests. And probabilities of VVR compared with exact Fisher tests and quantified through Odds ratios. Bonferroni correction was used to adjust all P‐values for multiple testing.
Results
Data for donors’ retention and VVR for the first three donations are shown in Table 1. After the first donation, the return rate (47.4% vs 29.0%) is statistically different (P < 0.001) in donors who encountered a VVR (3.4%). Return rates after a VVR at the first and/or second donations (29% and 35.7% respectively) are not statistically significant (P < 0.30). For the second donation, donors who experienced a VVR at the first donation have a higher risk (11.7%) to have a new VVR than those who did not have a VVR at the first donation (1.7%), (OR = 7.76, P < 0.001). Furthermore, donors who experienced a VVR at the first donation and not at the second have a higher risk to experience a VVR at third donation than those who did not experience a VVR at the two first donations (OR = 15.83, P < 0.001). Nevertheless, a first VVR occurring only at the second donation has no impact on the probability of VVR at third donation (P = 0.98). All these last tests conclusions concern of course only donors who show up at a next donation.
Table 1: History of first‐time donors who experienced or not a complication at first donation.

Conclusion
Our study confirms other reports concerning the importance of the occurrence of VVR at the first donation on the donors’ retention at the second donation. This study supports that it also concerns the third donations. Donors who have experienced a VVR at the first donation are at higher risk of VVR for the second and third donations. Thus, emphasis has to be paid on the implementation of prevention measures to reduce VVR among at risk donors (young donors of small weight, especially if they show signs of fear), at least during the two first donations: water loading, applied muscle tension and dietary salt supplement. Implementation of intention and commitment techniques must be encouraged such as systematic contact with donors who experience a VVR at first or second donation. Donors’ attrition must be followed since it can lead to potential blood components shortage.
4D‐S30‐05
Genetic Factors Associated with Ferritin Levels: A Study in The Australian Donor Population
Ji Y , Faddy H , Hyland C , Waller D , Flower R
Australian Red Cross Blood Service, Brisbane, Qld, Australia
Background
Blood donors are at risk of developing iron deficiency (ID) and/or iron deficiency anaemia (IDA) after regular donation, which has the potential to affect their health and subsequent donation career. Genetic variants, including various Single Nucleotide Polymorphisms (SNPs), have been shown to be associated with iron regulation and storage in various populations. Considering the importance of blood donor wellbeing, as well as the continual demand for certain blood components, it is of relevance to identify genetic factors involved in iron absorption and regulation, which may be associated with the maintenance of adequate iron levels in blood donors.
Aims
The aim of this study was to investigate whether selected SNPs in genes involved in iron metabolism are associated with ferritin levels in Australian blood donors.
Methods
Samples (n = 800) from randomly selected non‐first time blood donors in Queensland were collected. Plasma ferritin levels were quantified in each sample by ELISA before genomic DNA extraction. The genotype of the following four SNPs was determined: rs9296249 and rs3923809 in the BTB/POZ domain‐containing protein 9 (BTBD9) gene; and rs855791 and rs4820268 in the Transmembrane protease serine 6 (TMPRSS6) gene. For each SNP, tests for Hardy‐Weinberg equilibrium were performed. The association between each SNP alone and in combination, and ferritin levels were investigated using a general linear model.
Results
The allelic frequencies for all four SNPs did not differ from Hardy‐Weinberg equilibrium (P values above 0.05 for all). As expected, the variant allele was the least frequent for all SNPs (rs9296249: TT 55%, CT 38%, CC 7%; rs3923809: AA 44%, AG 45%, GG 11%, rs855791: GG 32%, AG 49%, AA 18%; rs4820268: AA 30%, AG 49%, GG 21%). The average plasma ferritin level in this donor cohort was 58.96 ng/ml, and levels ranged from 2.67 to 407.06 ng/ml. Each SNP in isolation, or in pairs, was not significantly (P > 0.05) associated with ferritin levels. In combination, rs9296249, rs855791 and rs4820268 (P < 0.05), as well as rs3923809, rs855791 and rs4820268 (P < 0.05) were significantly associated with ferritin levels, while rs9296249, rs3923809 and rs855791 (P > 0.05) and rs9296249, rs3923809 and rs4820268 (P > 0.05) were not. Ferritin levels were not associated (P > 0.05) with all four SNPs.
Summary/Conclusions
These preliminary analyses revealed that in combination these SNPs, specifically rs855791 and rs4820268 in TMPRSS6 together with either rs9296249 or rs3923809 in BTBD9, are associated with ferritin levels in blood donors. Additional analysis of other factors associated with altered ferritin levels, such as age, sex and details of the donors’ donation career, as well as exploring the specific alleles involved, is underway. This study provides support that genetic testing may be useful for the generation of predictive algorithms for the maintenance of iron stores in regular blood donors.
It is All About Ebola
5A‐S31‐01
Coping with Ebola Crisis in Transfusion Services in West Africa
Tapko JBT
AFSBT, Yaoundé, Cameroon
Background
Availability and accessibility to safe blood, remains challenging in Africa. The 2014 report on the Status of Blood Safety in the WHO African Region indicates that in 2010, 43 countries with a total population of 813,806,984 collected only 3, 486, 192 units of blood. Of 1462 facilities collecting, processing and distributing blood, 91.3% were hospital‐based and 17 countries still collected more than 50% of blood from family replacement donors. All units transfused were tested for HIV, but not for hepatitis B (HBV) and C.
In 16 West Africa Countries, home to 331,255,000 inhabitants, the donation rate ranged from 2.4% to 6.6%. Despite development of national blood policies and strategic plans, only few countries have well established national blood systems.
This is the context in which the Ebola Viral Disease (EVD) outbreak started in March 2014, with Guinea, Liberia and Sierra Leone being the most affected countries. With currently no specific treatment for EVD, convalescent whole blood (CWB) and convalescent plasma (CP) have been used as experimental treatment.
Aim
This study aimed at describing the status of blood transfusion services (BTSs) in West Africa in general and in Guinea, Liberia and Sierra Leone in particular, before and during the Ebola outbreak; and to analyze their capacity to respond to blood needs during the crisis.
Methods
Publications, reports and updates posted on various websites were reviewed for BTSs and blood transfusion practices. The information collected was analyzed to determine whether blood services were equipped for the Ebola outbreak.
Results
Overall, EVD cases were diagnosed in Guinea, Liberia, Mali, Nigeria, Senegal and Sierra Leone, but were rapidly controlled in Mali, Nigeria and Senegal. In Guinea, Liberia and Sierra Leone the number of cases increased dramatically to reach 24,282 (confirmed, probable and suspected cases), with 9976 reported deaths by 11 March 2015.
Of 16 West Africa countries, only 6 had well established BTSs. The three most affected countries lacked basic infrastructures and equipment for BTSs and depended mostly on external funding. Frequent stock‐outs of reagents and consumables, acute shortage of human resources and irregular power and water supplies were common. Less than 10% of blood was collected from VNRBD and blood was screened for TTIs using rapid tests Kits. The prevalence of TTIs in blood donors remained high ranging from 1.4% to 2.8% (HIV); and from 7.4% to 17.6% (HBV). All three countries lacked facilities for blood components production and transfused only whole blood.
With the EVD crisis BTSs were disrupted almost to the point of collapse, with substantial drop in blood donations, fear to donate and to receive blood, and the stigmatization of Ebola survivors.
Conclusions
These findings clearly expose the unpreparedness of Blood services in West Africa and particularly in the three Ebola‐affected countries to respond effectively and in timely manner to emergency situations, and to provide CWB and CP for clinical trials. The enormous international attention and resources attracted by the EVD provides a unique opportunity for re‐establishing well‐managed, nationally coordinated, and sustainable national blood systems.
5A‐S31‐02
Prospects for an Ebola Vaccine
Schuitemaker H
Academic Medical Center of the University of Amsterdam, Amsterdam, The Netherlands
Ebola Virus Disease is a severe and often fatal illness in humans with case fatality rates ranging from 25% to 90% in past outbreaks. The current West African outbreak of Ebola virus, a member of the filovirus family, has caused already more than 10.000 deaths and as such is by far the deadliest outbreak of the disease since its discovery in 1976. In response to the epidemic, the development of several already existing vaccine candidates has been accelerated and new candidates have been developed. The different candidates will be highlighted and the state of the art at the time of presentation will be given. The current vaccine development activities are mainly focused on the currently circulating strain. However, outbreaks due to other members of the filovirus family have frequently occurred in the past and are likely to happen again in the future. To be prepared for that, the development of a multivalent filovirus vaccine may be prioritized after the current outbreak is under control. Approaches for multivalent vaccines will be discussed as well.
Conflict of interest
Prof Schuitemaker is also the Head of Viral Vaccines Discovery and Translational Medicine at Janssen Pharmaceuticals.
5A‐S31‐03
Ebola Convalescent Plasma Therapy Trials – Does It Work?
van Griensven J
Institute of Tropical Medicine, Antwerp, Belgium
The evaluation of convalescent plasma (CP) for the treatment of Ebola Virus Disease (EVD) has been prioritized by the World Health Organization (WHO) in the current epidemic, predominantly affecting Guinea, Sierra Leone and Liberia. In each of the three countries, clinical trials have been initiated.
The Ebola_Tx trial, funded by the European Union, is designed to assess the feasibility, safety and efficacy of CP against EVD in Conakry, Guinea. Pathogen‐reduced CP is administered as two units (200–250 ml each) given consecutively on the same day, originating from two different donors. The primary outcome measure is survival 14 days after transfusion. The survival of patients treated with CP + supportive care will be compared to that of patients receiving supportive care alone, in an open‐label phase 2/3, open‐label, non‐randomised comparative study. All consecutive eligible and consenting patients of any age (including pregnant women) with confirmed EVD are enrolled; exclusion criteria are limited to contra‐indications for CP or patients arriving in a pre‐terminal condition. Available ABO compatible plasma is given, within 48 h after diagnosis, on a first‐come, first‐served basis, and patients with no compatible plasma available are enrolled as concurrent controls, complemented with historical controls. The first plasma collection started on February 9, 2015 and the first CP administration was done February 19, 2015. The main analysis will be done after 130 CP‐treated patients have reached day 14.
The Ebola_CP consortium emerged out of the Ebola_Tx initiative, with a number of additional partners and with main funding by the Wellcome Trust, to conduct a similar study in Sierra Leone using a similar protocol, CRF and database/management system. CP is administered as one single unit (450 ml) pathogen‐reduced CP from one single donor. The first control patient was recruited early March, 2015.
Finally, the EVD001 trial, funded by the Bill and Melinda Gates Foundation is a Phase I/II pilot study with viral load changes as primary outcome. Two units of 100 ml pathogen‐reduced CP from different donors are given on the first day, to be repeated after 48 hrs as indicated. In contrast with the above mentioned trials, children and pregnant women are excluded. The study was started in November, 2014 in Monrovia, Liberia.
In this presentation, an up to date overview of the different trials and available data on recruitment and findings will be given. If found to be effective, these studies will provide proof of concept of convalescent blood products to treat EVD.
Allo‐Immunisation
5A‐S32‐01
Mechanisms Underlying Red Cell Allo Immunization in Sickle Cell Disease
Pirenne F
Etablissement Francais du Sang, Créteil, France
Transfusion is a life sustaining therapy in sickle cell disease. Allo immunization against red blood cell (RBC) antigens can increase the risk of early death with hemolytic transfusion reaction. Between 20% and 50% of sickle cell disease (SCD) patients became allo immunized and this rate is higher than that for other transfused patients. One of the main causes of allo immunization is the high degree of antigen disparity between donors of Caucasian origin and SCD patients with an African background. However, some patients never get immunized despite frequent transfusion and exposition to a similar antigen disparity. It is therefore of high concern to understand the mechanisms underlying RBC allo immunization in SCD, in order to characterize high responders patients and provide them with specific prevention.
Findings with mouse models have shown that certain pro inflammatory stimuli enhance allo immunization but it was also reported that sickle mice, with or without pretreatment with a vira‐like inflammatory stimuli have a similar rate of allo immunization compared with wild type animal. The age of RBC units was also a parameter that increases the immunization rate. Other data are available in mice such the involvement of T Regulatory cells, CD4 T cells, and more recently the importance of the delay between the inflammatory stimuli and the transfusion for inducing allo immunization. Studies in SCD patients confirm some mice findings. It has been shown recently that red blood cell allo immunization is influenced by the patient inflammatory state at time of transfusion, but no correlation was found with the age of the RBC units. The immune system of poly transfused patients has been explored, based on their immunization status. A partial dysfunction of T regulatory cells activation was found in SCD patients, independently of their immunization status, but some authors identified T Regulatory cells as a major contributor to the allogeneic immune response in SCD. New finding implicate CD4+ T cells in allo immunization, showing activation differences between immunized and non‐immunized patients. Some other recent data suggests also that monocytes from non‐immunized patients in response to RBC break down products promote anti‐inflammatory state, less conductive to allo immunization. Many of these data, as well as genetic risk markers, could be exploited to identify responders or non‐responders as a path of resource conservation of valuable antigen negative RBC units, but they do not really provide the underlying mechanism of allo immunization. Prospective studies are necessary to study an ongoing process of allo immunization in order to determine the best targets for innovative prevention of allo immunization. Currently, Rituximab, based on its efficiency in many antibody‐related diseases, is the only treatment that has been used against RBC allo immunization in SCD.
5A‐S32‐02
Red Blood Cell Alloimmunization is Influenced by the Delay Between TLR‐Agonist Injection and RBC Transfusion
Elayeb RE , Tamagne MT , Bierling PB , Pirenne FP , Vingert BV
EFS IMRB, Creteil, France
Background
Murine models of red blood cell (RBC) transfusion show that inflammation associated with viruses or methylated DNA promotes RBC alloimmunization. These inflammations are induced by stimulation of TLR3 and TLR9. In the vaccination studies, the intensity of the antigen‐specific responses depends on the delay between antigen stimulation and adjuvant administration. A reduction of immune responses is observed when antigen administration occurs within the 6 h following the agonist injection. In mouse models of alloimmunization, the delay used between the injection of TLR agonists and transfusion is usually short.
Aim
The objective of this study is to demonstrate that the timing of TLR3‐agonist administration affects RBC alloimmunization.
Methods
Red blood cells expressing the HEL antigen (HEL RBCs) were obtained from transgenic HOD mouse. Poly(I:C), a TLR3 agonist, was administered to B10BR mice at various time points before transfusion of HEL RBCs. For each time point, splenic HEL‐presenting dendritic cells (DCs), HEL‐specific CD4+ T cells and anti‐HEL antibodies in the serum were measured.
Results
The phenotype of the activated immune cells is dependent on the delay between transfusion and TLR‐dependent inflammation. The production of anti‐HEL antibodies is highest when transfusion occurs 7 days after the agonist injection. The production of IL‐12 by HEL‐presenting CD8α+ DCs is highest in mice injected with Poly(I:C) 3 days before transfusion. Although the number of early‐induced HEL‐specific CD4+ T cells is similar between groups, these cells expresses high levels of CD134, CD40 and CD44 when mice are injected with Poly(I:C) 7 days before transfusion.
Conclusions
This study clearly shows that the delay between transfusion and TLR‐induced inflammation influences the immune response to transfused RBCs. Our findings provide new perspectives regarding the search for risk factors of RBC alloimmunization, particularly in sickle cell disease patients, who frequently experience inflammation and infection.
5A‐S32‐03
Incompatible Red Blood Cell Clearance Through Various IgG Subclasses Directed Against the Kell Glycoprotein in a Mouse Model of Alloimmune Anemias
Stegmann TC1, Waterman HR 2, Wang X 2, Vidarsson G 1, van der Schoot CE 1, Zimring JC 2
1Sanquin Blood research, Amsterdam, The Netherlands2BloodWorks Northwest, Seattle, WA, United States of America
Background
RBC alloimmunisation is characterized by rapid antibody mediated clearance of incompatible RBCs, this can lead to morbidity and mortality in the form of hemolytic transfusion reactions (HTR) and hemolytic disease of the newborn (HDN). Our recent studies in humans have found that IgG formed against Rhesus D in pregnancies or after transfusion can extremely low incorporation of core‐fucose in the IgG‐Fc‐glycan, enhancing binding to FcγRIIIa and FcγRIIIb and RBC clearance. After RhD, the Kell glycoprotein is one of the most immunogenic and clinically significant RBC antigen. In order to attempt to prevent or treat these pathologies, a better understanding of the mechanism by which antibody‐bound RBCs are cleared from the circulation is necessary.
Aim
To investigate the mechanisms behind anti‐Kell‐IgG‐mediated clearance of Kell‐RBC in mice and to see if it is affected by anti‐Kell core‐fucosylation.
Methods
We cloned the variable part of a human anti‐Kell antibody into vectors containing the constant domains of mouse IgG1, IgG2a, IgG2b and IgG3 to study incompatible RBC clearance for each IgG subtype specifically. Since we hypothesized that in analogy to human FcγRIII fucosylated antibodies have lower affinity for murine FcγRIV we produced anti‐Kell IgG1 and IgG2a as either normal high‐fucosylated IgG, or with low core‐fucose.
C57/BL6J WT mice, Complement C3 knock‐out, FcγR knock‐out and double knock‐out mice for both C3 and FcγR were passively immunized with the different subclasses of anti‐kell followed by a transfusion with a mixture of wild‐type C57BL/6J (labeled with DiO) and incompatible Kell + (labeled with DiI) RBCs. Clearance of transfused RBCs was assessed by enumerating labeled RBCs through flow cytometry and normalizing the circulating incompatible RBCs as a function of wild‐type RBCs within the same recipient.
Results
IgG2a is thought to be more potent antibody due to its increased binding affinity to complement and FcgRs. Surprisingly, we found that both IgG1 and IgG2a have almost similar effect on RBC clearance, whereas IgG2b and IgG3 also induce clearance albeit much less. The activating FcγRs were indispensible for clearance, but based on the subclass differences and the lack of enhanced clearance of low fucosylation the role of FcγRIV is less pronounced. The effect of complement was only seen at low concentration of IgG2a antibodies.
Conclusion
Anti‐Kell IgG1 and IgG2a have the ability to induce significant RBC clearance whereas IgG2b and IgG3 only induce weak RBC clearance. Activating FcγRs are necessary for RBC clearance whereas complement played only a minor role if any.
5A‐S32‐04
Blood Transfusion and Sickle Cell Disease in Senegal
Diop SD1, Seck MS 2, Senghor ABS 2, Faye BF 1, Gueye YBG 2, Dieng ND 2, Toure SAT 2, Diouf MD 2, Gadji MG 1, Sall AS 1, Toure Fall AOTF 1, Dièye TD 1
1Universite Cheikh Anta Diop, Dakar Fann, Senegal2CNTS, Dakar, Senegal
Background
Sickle cell disease is a major public health problem in Africa with 200,000–300,000 newborns each year in this continent. Blood transfusion (BT) remains a mainstay of treatment for sickle cell disease despite the use of hydroxyurea. Indications and practical modalities of BT differ from one country to another depending on the level of blood safety and transfusion technology.
Aims
The objective of this study was to evaluate the practice of BT in sickle cell disease in a sub Saharan African setting and to identify the different complications related to BT use in this cohort.
Methods
We analyze the data prospectively collected from the medical records monitoring BT in a cohort of 1245 sickle cell patients (87.4% SS, 10.5% SC, 1.1% Sβ0 thalassemia and 0.9% Sβ+ thalassemia) followed from 2005 to 2014 (4980 person‐years). Consecutively, we compare ferritin level, alloimmunization and prevalence of HIV, HBs Ag, HCV and syphilis in a group of 146 transfused SCD patients vs control group (no transfused SCD patients).
Results
In the total cohort, the number of transfused patients represented 28.5%. Indications for BT were acute anemia (58.1%), vaso‐occlusive crisis (12.8%), and pregnancy (7%). Chronic transfusion represented only 11% of BT modalities. Simple transfusion was practiced in 83.1% of patients and exchange transfusion was done in 16.9%. Prevalence of recorded transfusion incidents were 5.8%. Ferritinemia was normal in 93 patients (60.7%), between 300 and 1000 ng/ml in 60 patients (32%) and up to 1000 ng/ml in 10 patients (6.5%). Number of transfused blood units and number of blood transfusion episodes were the only significant risk factors for a ferritinemia level above 1000 ng/ml. Prevalence of Alloantibodies was 8.9% in transfused patients and there was no any significant difference in the prevalence of transfusion transmitted infectious (TTI's) agents in SCD patients according to their antecedent of transfusion.
Conclusions
Modalities of BT in SCD are different in our setting compared to developed country where chronic transfusion to prevent stroke and erythrapheresis are the gold standard. Allo immunization and iron overload have to be considered as major risk while risk of TTI's seems well controlled in our cohort
5A‐S32‐05
Migratory Properties of CD4+ T cells in Sickle Cell Disease Patients
Vingert BV1, Tamagne MT 1, Habibi AH 2, Pakdaman SP 1, Galactéros FG 1, Bierling PB 1, Pirenne FP 1
1Etablissement Français du Sang, Créteil, France2AP‐HP, Créteil, France
Background/Aims
The alloimmunization is partly under the control of the ability of CD4+ T cells to migrate to secondary lymphoid organs. The spleen is the major contributor of help for antibody responses. However, the spleen is often no‐functional in sickle cell disease (SCD) patients. In this study, the migratory functions of CD4+ T cells were assessed by the measurement of chemotaxis with CXCL12 and CCL19/CCL21 chemokines.
Methods
A chemotaxis assay was performed to determine the migration capacity of CD4+ T cells in two groups of SCD patients: 1/a group of SCD patients (n = 10) who never became immunized despite a high transfusion regimen and 2/a group of SCD patients (n = 10) who got immunized (at least against Jkb) after a low transfusion regimen. The control group consisted of race‐matched healthy blood donors (HD group, n = 10). In these 3 groups, the expressions of the chemokines CCR7, CXCR4, CXCR3, CXCR5, CCR6 were measured by flow cytometry on whole blood. A chemotaxis assay was performed to determine the migration capacity of CD4+ T cells also in response to CXCL12 (CXCR4 ligand), CCL19/CCL21 (CCR7 ligands) and CXCL13 (CXCR5 ligand).
Results
The CXCR4 expression was significantly decreased in the non‐alloimmunized patients compared to the alloimmunized patients or the HD group. This low expression of CXCR4 was correlated with a migration defect in non‐alloimmunized SCD patients in response to the CXCL12 chemokine. Moreover, CCR5 expression was higher on CD4+ T cells in low‐responder patients compared to the high‐responder group (P < 0.05). However the migration capacity measured in response to CXCL13 was the same in the 2 SCD groups of patients. Finally, the chemotaxis in response to CCL19 or CCL21 were preserved in the two groups of SCD patients.
Summary/Conclusions
In low‐responder SCD patients, we describe that a defect of CXCR4 expression could be correlated to a migration defect of the CD4+ T‐cell into lymphoid organs. If a facilitating migration with CXCL13 has not been detected for the CD4+ T lymphocytes in low‐responder patients, it could affect the follicular helper CD4+ T cells (TFH), indeed these cells also express CXCR5. The ability of CD4+ T cells to migrate into the spleen is necessary for the RBC alloimmunization. This study highlights the role of a functional asplenia in alloimmunization of SCD patients.
Massive Transfusion
5A‐S33‐01
Massive Hemorrhage Protocol: What's the Best Protocol?
Callum JL , Nascimento B , Alam AQ
Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Massive transfusion protocols only became common practice between the years of 2006 and 2010, primarily driven by retrospective reports from the military on the benefits of formula‐driven blood resuscitation. The terminology of ‘massive transfusion protocol’ was improved to ‘massive hemorrhage protocol (MHP)’ with the astute recognition that at the start of such a protocol it is unclear which patients will meet the classical definition of massive transfusion (10 units over 24 h). Despite the vast majority of the literature being reported from the trauma population, hospitals have generally adopted a single MHP for all patients. It remains unclear if the same protocol can be used for all types of hemorrhage, such as gastrointestinal, obstetrical, and post cardiac surgery hemorrhage.
MHPs assist with the prevention and management of the acute coagulopathy of trauma/shock (ACOTS). ACOTS is thought to primarily arise from shock leading to denuding of the endothelial glycocalyx, leading to exposure of thrombomodulin, binding of thrombomodulin to thrombin resulting in activation of protein C. Protein C activation results degradation of factor V and release of tissue plasminogen activator. The latter change results in rapid destruction of fibrin and fibrinogen, termed hyperfibrinolysis. Severe hyperfibrinolysis is seen in a minority of trauma patients but is associated with a very high mortality rate. Hypothermia and acidosis are thought to worsen the ACOTS. Lastly, underlying patient genetic differences, particularly the X‐link polymorphisms seen in interleukin‐1 receptor‐associated kinase (IRAK‐1), may predispose certain patients to higher rates of multiorgan failure if they survive to the intensive care unit. Blood group O patients also have higher rates of hemorrhage, compared to non‐O, likely due to lower levels of von Willebrand factor and factor VIII. In cardiac surgery, a lower preoperative fibrinogen level may be an important contributor to the risk of postoperative hemorrhage.
The goals of a MHP are to improve hemostasis, communication, and patient outcomes. The protocol must be specific for an individual hospital, depending on factors such as pre‐hospital transport times, distance from blood transfusion laboratory to trauma and operating rooms, patient populations served, and types of laboratory tests available. The key components of a MHP are the 6Ts: triggering of the protocol, laboratory testing, tranexamic acid, temperature maintenance, transfusion support, and termination of the protocol when hemostasis is achieved. The evidence and importance of each of these steps will be discussed in detail.
It is also critical that a formal quality assurance program supports the MHP. Poor compliance with the institutional MHP is associated with inferior survival in the trauma population. Each MHP activation should be followed by a formal debrief by the team and a discussion regarding key aspects of care to determine areas for improvement. Chart and live audits should be performed to determine compliance with key aspects to inform annual or biannual update of the MHP. Formal training and/or simulation should be a core part of the policy. In areas where the MHP is used infrequently, drills or simulations will be critical for high performance.
5A‐S33‐02
Epidemiology of Massive Transfusion: A Bi‐National Study from Sweden and Denmark
Halmin MAH1, Chiesa FC 1, Vasan SKV 1, Wikman AW 1, Norda RN 2, Rostaard KR 3, Vesterager Pedersen OP 4, Erikstrup CE 5, Nielsen KRN 6, Titlestad KT 7, Ullum HU 8, Hjalgrim HH 3, Edgren GE 1
1Karolinska Institutet, Stockholm, Sweden2Uppsala Universitet, Uppsala, Sweden3Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark4Vævs‐ og transfusionscenteret, Næstved Sygehus, Region Sjælland, Sjaelland, Denmark5Department of Clinical Immunology Aarhus University Hospital, Aarhus, Denmark6Department of Clinical Immunology, Aalborg University hospital, Aalborg, Denmark7Department of Clinical Immunology, Odense University Hospital, Odense, Denmark8Department of Clinical Immunology, Copenhagen University Hospital, Copenhagen, Denmark
Background
In recent years, massive transfusion protocols have received increasing attention. The potential risks associated with massive transfusion have been discussed and associations with both increased morbidity and mortality have been reported. However there is a paucity of comprehensive data on massively transfused patients and their long‐term outcomes. A better characterization of the epidemiology of massive transfusion is warranted to improve clinical decision making and to guide future studies.
Aims
The aim of this study was to estimate the incidence of massive transfusion and to describe characteristics and mortality of massively transfused patients.
Methods
We performed a retrospective cohort study based on the Scandinavian Donations and Transfusions (SCANDAT2) database, linking data on blood donation, blood components and transfused patients with inpatient‐ and population‐registers. We included all patients receiving 10 or more red blood cell (RBC) transfusions in up to 2 calendar days, in Sweden 1987–2010 and in Denmark 1996–2010. The patients were followed throughout 2012. Descriptive statistics were used to characterize the patients and the indications for massive transfusion. Post‐transfusion mortality was expressed as crude 30‐days mortality and long‐term mortality was estimated using the Kaplan‐Meier method and as standardized mortality ratios.
Results
53,836 patients were included. Of all blood components transfused during the study period, 7.7% constituted massive transfusion. The incidence of massive transfusion was higher in Denmark (2.5 per 10,000), than in Sweden (2.1 per 10,000). The most common indications for massive transfusion were major surgery, including cardiac/vascular‐ (34.8%), cancer‐ (10.8%) and other surgery (22.8%), followed by trauma (15.6%). Massive transfusion due to obstetrical bleeding constituted 2.6%. Median age at massive transfusion was 67 years and two thirds of the patients were male. The median number of blood components transfused per massive transfusion episode was 22. RBCs formed the majority of blood components transfused. However, the proportion of plasma increased slightly over time, being 33.3% in the most recent time‐period (2006–2010). The overall 5‐year mortality was high, 51.7%, but with large differences between indications, ranging from 84.7% among patients transfused for malignant disease who did not undergo surgery, to 1.4% among women transfused for obstetrical bleeding. Mortality increased gradually with age and among all patients massively transfused at age 80 years, only 26% were alive after 5 years. The relative mortality, early after transfusion, was high and decreased with time since transfusion. However it remained elevated throughout even 10 years after transfusion.

Summary/Conclusions
This large‐scale study based on nation‐wide data from Sweden and Denmark describes the incidence, indications and mortality associated with massive transfusion. We report a non‐negligible incidence and a high both absolute and relative mortality. The general pattern is similar for Sweden and Denmark and we believe that similar patterns would be found in other high resource countries. The study provides important information for clinicians and researchers designing future studies in this field.
5A‐S33‐03
Survey OF Factors Associated with Super‐Massive Transfusion and Mortality in Patients from the Australian and New Zealand Massive Transfusion Registry
Venardos KM , McQuilten Z , Aoki NJ , Andrianopoulos N , Wood E
Monash University, Melbourne, Vic., Australia
Background
Transfusion risks may be amplified in critically bleeding patients receiving massive transfusion (MT) due to the larger volumes of products administered. Evidence to guide MT remains limited and further data on optimal use of blood components is required.
Aims
To examinethe incidence of, transfusion support in, and long term outcomes for patients in the Australian and NZ Massive Transfusion Registry (ANZ‐MTR) receiving super‐massive (Super‐MT) and ultra‐massive (Ultra‐MT) transfusions.
Methods
The ANZ‐MTR generates observational data on transfusion management and outcomes in critically bleeding patients receiving MTs across many clinical settings. All adult patients receiving a MT (≥5 units RBC in 4 h) in any clinical context were identified at 16 Australian and NZ hospitals between April 2011‐Mar 2014. Patient data, including transfusion history, laboratory results and hospital admission data were extracted. Post‐hospital discharge mortality data were collected from the Australian National Death Index and NZ Ministry of Health to assess 30‐ and 90‐day and extended mortality status. MT cases were categorised into Super‐MT (≥20 units RBC in 48 h), and Ultra‐MT (≥40 units RBC in 48 h) using the RBC volume definitions suggested by the international BEST Collaborative. Coagulopathy was defined as INR > 1.5 and/or aPTT > 60 s. The association between patient characteristics and initial therapy with Super‐MT was modelled using multiple logistic regression. Predictors for survival were assessed using Cox proportional hazards regression.
Results
A total of 2659 MT patients were identified, of whom 221 (8.3%) received a Super‐MT and 19 (0.7%) an Ultra‐MT. Median (IQR) follow‐up time was 566 (92–899) days. Differences in patient profiles, management and outcomes were observed between MT groups (Table 1). Super‐MTs occurred across all clinical specialties but were predominantly trauma (21.7%) or cardiothoracic and vascular surgery (33.5%) cases. Eight obstetric (3.6%) and 13 GI (5.9%) haemorrhage Super‐MTs were identified. Ultra‐MTs were observed in trauma (42.1%) and surgical patients (57.9%) only.
Factors independently associated with a Super‐MT (Odds Ratio; 95% CI) were age (0.99; 0.97–0.99); Charlson Score (1.17; 1.06–1.27); more RBC transfused in the first 4‐h from MT onset (1.41; 1.35–1.48), FFP:RBC ratio at 4 h post‐MT onset (0.38; 0.21–0.68); and post‐MT onset platelet count (0.98;0.98–0.99), Hb (0.98; 0.97–0.99) and aPTT (1.01; 1.01‐1.01). Independent predictors of mortality (Hazard Ratio; 95% CI) in Super‐MT patients were 24 h RBC usage (1.03; 1.01–1.04); and post‐MT aPTT (1.01; 1.0–1.02).

Conclusions
Patients receiving Super and Ultra‐MTs are generally younger with fewer comorbidities but have significantly higher mortality rates than other MT patients in the ANZ‐MTR. Trauma and surgical cases account for most Super and Ultra‐MTs. Factors predictive of Super‐MTs were co‐morbidities, greater RBC and lower FFP:RBC ratio at 4 h post‐MT, and laboratory parameters post‐MT onset including longer aPTT, lower platelet count and lower Hb. Of the MT patients who had died by 90‐days, the majority died in hospital. The ANZ‐MTR provides a unique opportunity to explore differences in MT patient profile, clinical management and blood utilisation and short and long‐term outcomes.
5A‐S33‐04
Results of the Pragmatic Randomized Optimal Plasma and Platelet Ratios Trial (Proppr)
Hess R
University of Washington School of Medic, Seattle, WA, United States of America
Background
Until the last decade, most severely injured patients were resuscitated with crystalloid solutions to maintain intravascular volume and red cells to maintain oxygen transport with plasma, platelets, and cryoprecipitate to be given later based on laboratory transfusion triggers. Profound injuries seen in the Second Iraq War and occupation appeared to do better when more plasma and platelets were given early and best with the highest ratios. Multiple subsequent retrospective studies have confirmed the value of a high plasma and platelet to RBC unit ratio.
Aims
Assess the relative safety and efficacy of resuscitation with 1:1:1 unit ratios of plasma, platelets, and RBCs compared to 1:1:2 unit ratios in severe and profoundly injured trauma patients.
Methods
Twelve North American trauma centers screened severely injured trauma patients under protocols approved by local institutions and national governments and enrolled patients with an Assessment of Blood Consumption score ≥2 or attending surgeon gestalt of severe injury and receiving at least one blood component in the first 2 h of care. Patients were randomized to receive subsequent blood components in 1:1:1 or 1:1:2 unit ratios from blood component chests sealed until randomized and at the bedside within 10 min of request. Primary endpoints were 24 h and 30 day survival as required by the US Food and Drug Administration to conduct the trial under exception from informed consent regulations. Secondary endpoints included successful resuscitation, survival at the end of resuscitation, 23 safety endpoints, and trial management objectives.
Results
14,000 patients were screened and 680 were randomized. Mortality at 24 h and 30 days were not significantly different. More patients were successfully resuscitated in the 1:1:1 group and total survivorship was greater and hemorrhagic deaths were reduced in that group at the end of resuscitation. The absolute benefit in mortality was maintained after resuscitation but lost statistical significance as this mortality difference was subsequently diluted by CNS injury and multiple organ failure deaths which were equal in both arms. Transfusion and patient follow up goals were met and complications were equal across both groups.
Summary/Conclusions
The PROPPR trial is the first randomized trial to address the effect of ratios of resuscitation products given to severely injured trauma patients. The trial shows that a 1:1:1 ratio of blood components is as safe and at least as effective as a 1:1:2 ratio. Trials of resuscitation of uncontrolled hemorrhage are conducted in populations of very severely injured patients where risk on non‐hemorrhagic death cannot be rapidly determined and are subject to secondary dilution of therapeutic effect.
Transplantation
5A‐S34‐01
Extracorporeal Photopheresis for Graft‐vs Host Disease
Greinix H
Medical University of Vienna, Austria
No abstract available.
5A‐S34‐02
New Approaches for ABO‐Mismatched Solid Organ Transplantation
Ohdan H
Department of Gastroenterological and Transplant Surgery, Hiroshima University, Hiroshima, Japan
We have demonstrated a novel strategy for specific and persistent inhibition of antibody (Ab) production against blood group A or B carbohydrate determinants necessary for successful ABO‐incompatible transplantation. Similar to human blood group O or B individuals, mice have naturally occurring Abs against human blood group A‐carbohydrates in their sera. B cells with receptors for A‐carbohydrates in mice belonging to the CD5+CD11b+B‐1a subset, have phenotypic properties similar to those of human B cells. These cells could be temporarily eliminated by injecting synthetic A‐carbohydrates (GalNAcα1‐3, Fucα1‐2Gal) conjugated to bovin serum albumin (BSA) (A‐BSA) and anti‐BSA Abs. In mice that received the injection of A‐BSA/anti‐BSA Abs, the serum levels of anti‐A IgM were reduced, but immunization with human A erythrocytes resulted in increased serum levels of anti‐A Abs. When combined with cyclosporin A (CsA) treatment, which blocks B‐1a cell differentiation, and treatment with A‐BSA/anti‐BSA Abs, the serum levels of anti‐A Abs were persistently undetectable in the mice even after the immunization. B cells with receptors for A carbohydrates were markedly reduced in these mice. These results are consistent with the hypotheses that treatment with A‐BSA/anti‐BSA Abs temporarily depletes B cells responding to A‐determinants, and CsA treatment prevents the replenishment of these cells.
Additionally, we have demonstrated that CD1d‐restricted natural killer T (NKT) cells are required to produce anti‐A antibodies (Abs), probably through collaboration with B‐1a cells. After immunization of wild‐type (WT) mice with human blood group A red blood cells (A‐RBCs), interleukin (IL)‐5 exclusively and transiently increased, and the anti‐A Abs were elevated in sera; however, those were not observed at all in CD1d−/− mice, which lack NKT cells. Administration of anti‐mouse CD1d blocking mAb prior to the immunization abolished IL‐5 production by NKT cells and anti‐A Ab production in WT mice. Administration of anti‐IL‐5 neutralizing mAb also diminished anti‐A Ab production in WT mice, suggesting that IL‐5 secreted from NKT cells critically regulates anti‐A Ab production by B‐1a cells. In NOD/SCID/γcnull mice, into which peripheral blood mononuclear cells from type O human volunteers were engrafted, administration of anti‐human CD1d mAb prior to the A‐RBC immunization completely inhibited anti‐A Ab production. Thus, anti‐CD1d treatment might constitute a novel approach that could help in evading Ab‐mediated rejection in ABO‐incompatible transplants.
5A‐S34‐03
Use Of Non‐Irradiated Blood Components In Campath (Alemtuzumab) Treated Renal Transplant Patients
Hui YMT , Regan F , Willicombe M , Taube D
Imperial College Healthcare NHS Trust, London, United Kingdom
Background
Transfusion associated graft vs host disease (TA‐GvHD) is characterised by profound, progressive pancytopenia within 6 weeks following transfusion, with rash, diarrhoea, liver failure and death due to bone marrow failure. Prevention in susceptible individuals, through irradiation of components, is key as there is no effective treatment. Universal leucodepletion (since 1999 in the U.K.) significantly reduced, but did not eliminate, cases of TA‐GvHD, therefore irradiation is still recommended. In 2010, the British Committee for Standards in Haematology (BCSH) guideline on the use of irradiated components incorporated Campath (alemtuzumab) as an indication for irradiation following recommendation from the manufacturer (Genzyme). However, this was based on a single case of TA‐GvHD, developing 8 months following Campath, in a patient who had also received fludarabine. We considered this insufficient evidence to justify using irradiated components for Campath treated renal transplant patients at Imperial, given the large number already transplanted without irradiated components.
Aims
To review the use of non‐irradiated components in Campath conditioned renal transplant patients, to identify any resultant cases of TA‐GvHD.
To provide supporting evidence against the need for irradiated components in such patients in the U.K., where leucodepleted components are used.
Methods
We conducted a retrospective study of all our Campath conditioned renal transplant patients, who were also transfused, all with non‐irradiated components. We identified all those transfused up to 9 months following Campath, who survived to their routine 1 year follow‐up after transplantation: TA‐GvHD was excluded, as it would have developed within the follow‐up period. For patients who died within a year of transplantation or were not followed‐up for a full year, we reviewed their blood results and medical records to identify any laboratory and/or clinical features suggestive of TA‐GvHD. For patients transfused after 9 months following Campath, medical records were reviewed to establish if they survived at least 3 months following their last transfusion, so that TA‐GvHD could be excluded.
Results
870 Campath conditioned renal transplantations took place between November 2005 and July 2013, and 647 of these patients were also transfused. 616 of 647 were only transfused up to 9 months following Campath: 601 of these were alive at 1 year therefore TA‐GvHD was excluded. Of the remaining 15 patients, 12 died within a year of transplantation and 3 were not followed‐up for a full year. Closer review of blood results and medical records excluded TA‐GvHD in all 15 patients. 31 patients received transfusions 9 months or longer following Campath: all were alive at least 6 months following their last transfusion thus excluding TA‐GvHD.
Conclusions
Despite receiving non‐irradiated components, 647 Campath conditioned renal transplant patients were not put at additional harm with none developing TA‐GvHD, which provides some evidence against needing irradiated components in these patients. We recommend that others conduct similar reviews to see if our data can be replicated, and if there is sufficient evidence to change current guidance, at least in the U.K., where universal leucodepletion is practised. This is important as unnecessary use of irradiated components has both clinical delay and cost implications.
5A‐S34‐04
Extracorporeal Photopheresis (ECP) in Lung Transplant Rejection as an Effective Therapeutic Option in Refractory Patients: A Report of 360 Procedures in 17 Patients
de Miguel C1, Losa A 1, Aguilar M 1, Carreño MC 1, Sevilla J 2, Guillén M 2, Rojas M 1, Fernández C 1, Núñez MJ 1, Ussetti MP 1, Bueno JL 1, Cabrera R 1
1Hospital Universitario Puerta de Hierro Majadahonda, Majadahonda, Madrid, Spain2Hospital Niño Jesús, Madrid, Spain
Background
Extracorporeal photopheresis (ECP) is a rescue procedure for the treatment of the Bronchiolitis Obliterans Syndrome (BOS) following graft rejection in lung transplant.
Aims
The main objective of this study is to determine the ECP treatment efficacy in patients with BOS after lung transplantation.
Materials and Methods
We evaluated ECP procedures in lung transplantation patients with BOS refractory to immunosuppressive therapy (IT). Lymphopheresis sequence was 2 per week the first month, 2 every 2 weeks for 3 months and then 2 sessions per month. Photoinactivation with psoralen and UVA irradiation (Theraflex‐MacoPharma®) was performed in HIUNJM. The clinical response parameter was FEV1 improvement or stabilisation. Results were descriptive as median (range) or average (SD). We report apheresis procedures and products parameters as well as patients clinical outcome.
Results
Seventeen patients with BOS were treated with 360 ECP procedures; median 18 procedures per patient (6–35). Time between transplantation and first ECP was 63.6 months (18.4–125.9). The lymphopheresis procedures time was 203 (29) min. The patient's blood volume was 4.0 and processed blood volume 7.9 l. Lymphopheresis were performed mainly (84%) with Optia (Terumo BCT®). The product volume for photoinactivation was 82.8 (42.4) and the mononuclear cells count (average‐SD) 72.2 (41.0) × 109/l. Patients’ time under ECP treatment was 11.6 (1.4–36.6) months. Follow‐up (median‐range) was 10.8 months (2.6–36.6). Fourteen patients (82.4%) are still alive. Three (17.6%) of the patients were died. Fourteen patients (82.4%) achieved a response in (median–range) 1.2 months (0.2–25.4). From these 14 patients, 6 showed an improvement in their FEV1 (42.9%) and 8 achieved a stabilization (57.1%). None of the patients treated required a new lung transplant.
Conclusions
ECP is an effective rescue therapeutic for patients with BOS after lung transplant. ECP allows stabilizing the patient and delaying re‐transplantation.
5A‐S34‐05
Detection of Donor T and B Cells Specific C4d Fixing Alloantibodies Using Flow Cytometry: A Novel Diagnostic Approach
Jain D , Dorwal P , Tyagi N , Pande A , Mehra S , Raina V
Medanta the Medicity, Gurgaon, India
Background
Donor‐specific complement‐fixing alloantibody identification is known to play a major role in antibody‐mediated renal transplantation rejection and management. Specifically, the deposition of C4d in the peritubular capillaries in a kidney biopsy is a valuable marker of antibody‐mediated rejection (AMR). Various methods have been reported for the detection of antibodies in recipient sera, which can differentiate the all‐antibodies to be HLA or non‐HLA, complement‐fixing or non‐complement fixing, donor T and/or B cell specific. The C4d Flow‐PRA is one of the screening methods to identify the HLA specific complement fixing antibodies. However, the results are limited by the lack of donor specificity in this method as discudd.
Aim
Therefore we aimed to develop a non invasive assay that can pre‐transplant identify donor‐specific (T and/or B cell), C4d‐fixing alloantibodies.
Method
We hereby report a novel method christened Donor‐specific‐Flow‐C4d (DFC) of identifying donor‐specific (T and/or B cell), C4d‐fixing allo‐antibodies. Inter and intra‐assay reproducibility was confirmed by repeating the tests with the stored serum of the cases. With every run, commercial and pooled positive controls were used. The tests serums were run in duplicates. The Cut‐off was calculated based on a set of normal non‐sensitized control samples with the value calculated as three standard deviation of the mean. The results were compared with the NIH‐CDC method and C4d staining on renal biopsies.
Results
In present report we have discussed a series of cases representing a variety of cases in renal transplants wherein the newer method i.e. DFC was beneficial. The first two cases were of AMR, where initially there was a dilemma of AMR vs ACR as the result of histopathological finding of allograft biopsy and FCXM were not sufficient to confirm it as complement mediated rejections. The positivity on the DFC method confirmed the diagnosis of AMR due to donor B cell specific, complement fixing, allo‐antibodies for both of these cases. The third case was of a deferred pre‐transplant donor, wherein the recipient serum was confirmed to be positive for donor T and B cell specific; complement fixing, HLA alloantibodies without using an invasive procedure of allograft biopsy. This was helpful in pre‐ transplant prediction of a possibility of an AMR. The last case was a clear indication of correlation of the DFC and the histopathological findings. The patient was managed with the therapeutic protocols used for an ACR.
Summary and conclusion
With the added advantage of being non‐invasive and Donor T and B cell specificity, this newer method provides information pre‐transplant; whereas kidney biopsy based C4d evaluation can only be done post‐transplant. This method also emphasize the optimal use of Flow cytometry and useful for developing countries wherein the laboratories do not have the advance instrument like Luminex and Flow‐cytometers are only being used for hematological disorders. We postulate that this method incorporates the best features of all the available modalities (i.e. FCXM, C4d‐FlowPRA and NIH‐CDC).
Donor Session Management
5A‐S35‐01
Non‐Invasive Haemoglobin Testing at a National Level
Murphy WG , Selby T , Darragh E , Murray Y , McSweeney E
Irish Blood Transfusion Service, Dublin, Ireland
Several years ago we noticed that capillary haemoglobin levels (measured by Hemocue, in the lateral finger pulp) varied in relation to the corresponding venous haemoglobin level in a manner that depended on the sex of the donors and the season of the year1. This lead us to conclude that the Fåhraeus effect has a strong influence on the capillary haemoglobin level in healthy individuals, and that the location on the finger where the haemoglobin level is measured should affect the level measured. Using a non‐invasive device to measure haemoglobin levels we observed that the haemoglobin was approximately 1 g/dl higher close to the distal interphalangeal crease on the distal phalanx, compared to at the middle of the finger pulp.
Following a tendering process, we performed a detailed comparison of the non‐invasive Haemospect device, measuring at the base of the distal phalanx, with capillary blood haemoglobin measurements from the lateral finger pulp on Hemocue, and on venous blood samples. Mean Hemocue haemoglobin levels in healthy blood donors were 0.94 g/dl lower than the corresponding venous level for Hemocue levels within 1 g/dl of the cut‐off for men and women, while the corresponding Haemospect levels were 0.28 g/dl lower than the venous haemoglobin level. The false pass rate for Haemospect in routine donor screening was 2.4%, lower than the best published results for Hemocue capillary blood testing.
Countrywide rollout of the Haemospect device took place in July and August in 2014. Initially we used the mean of three readings; this was replaced after several weeks by the median of three where the initial reading was outside the acceptable range. This change simplified and accelerated the process and resolved a problem with failed readings that were probably due to build‐up of a thin layer of sweat between the finger and the device. Donor deferral rates for low haemoglobin have fallen from over 8% to under 3% in the winter. A seasonal effect is still apparent. Between the savings in disposables and the increase in donations procured the nett cost of the change was positive – i.e. the cost of the devices is offset by the savings accrued. Donor and staff satisfaction is high.
It has also become apparent that there is a normal range for finger pulp haemoglobin in the donor population that corresponds closely to, but is not the same as, the normal range for venous haemoglobin in the same population. However it not possible to predict where in the normal range for one of the values a donor's haemoglobin will be, based on the haemoglobin level for the other parameter. Thus a normal venous haemoglobin level (in a healthy donor) will predict for a normal capillary value, and vice versa, but will not predict where in the normal range the corresponding value will be. Whether measuring any value in healthy prospective donors gives a useful predictive value for any morbid quantity, over and above a general assessment of wellbeing, is not known.
Reference
1. Tong E et al. Vox Sang 2010;98:547–553.
5A‐S35‐02
Selecting an Alternative Skin Disinfection Method for Donors Allergic to Chlorhexidine at Canadian Blood Services
Ramirez-Arcos SM , Kou Y , Taha M , Goldman M
Canadian Blood Services, Ottawa, ON, Canada
Background
Adequate donor skin disinfection is important to ensure the safety of blood components. Currently, Canadian Blood Services uses the Chloraprep swabstick (2% chlorhexidine and 70% isopropyl alcohol) as the primary method for donor skin disinfection. For donors sensitive to chlorhexidine, a two‐step method (70% isopropyl alcohol scrub sponge is followed by an ampoule of 2% iodine tincture) is used. Both methods were validated in a study conducted in 2008; however, the production of the alternative two‐step method will be suspended in October of 2015. Since approximately 3% of donations at Canadian Blood Services use the two‐step method, there is a need to replace the alternative skin disinfection method to avoid a negative impact on blood product inventory. At the present time, the bacterial contamination rate of platelet concentrates at Canadian Blood Services is approximately 0.01% and any implemented new protocol must be as effective in skin disinfection.
Aim
To evaluate the efficiency of available, licensed alternatives for skin disinfection for donors sensitive to chlorhexidine.
Methods
This evaluation study was developed in two phases. A total of 127 and 134 study subjects were included in Phase 1 and Phase 2, respectively, aimed at detecting a 10% difference in efficiency (80% power, significance level 5%). Sixty‐five mm diameter contact plates containing agents for neutralization of remaining disinfectants were used. Contact‐plate cultures were done on the anticubital fossa on each arm of the subjects for 30 seconds before and after disinfection. In Phase 1, the swabstick Chloraprep and a 10% povidone‐iodine swabstick from vendor 1 were compared. During Phase 2, Chloraprep and a two‐step method [a 10% povidone‐iodine swabstick (vendor 2) followed by application of an isopropanol swabstick (vendor 3)] were compared. Within 3 h, plates were incubated at 37°C for 24 h, followed by colony counting. A comparison was made of the log10 reduction in colony counts on both arms of the same subject. A P‐value below 0.05 indicated a statistically significant difference.
Results
Phase 1 showed that the povidone‐iodine swabstick (vendor 1) was significantly less efficient than Chloraprep. The disinfection efficacy of the povidone‐iodine swabstick was variable and its application caused ergonomic concerns to the phlebotomists and skin irritations to a small number of subjects. By contrast, Phase 2 showed that the two‐step method was as efficient as Chloraprep with both methods achieving a 0–2 log10 reduction in colony counts in 93% of study subjects and a 3 log10 count reduction in 7% of study subjects. The two‐step method also had better acceptability by the phlebotomists and study subjects.
Summary/Conclusions
The two‐step method was as efficient as our primary skin disinfection method Chloraprep. Furthermore, it had better acceptability by both the phlebotomists and study participants. This method has therefore been selected to replace our current alternative method (70% isopropyl alcohol scrub sponge followed by an ampoule of 2% iodine tincture) for donors sensitive to chlorhexidine and will be implemented at Canadian Blood Services in the fall of 2015.
5A‐S35‐03
A Comparative Study Between the Non‐Invasive and Invasive Methods of Haemoglobin Estimation in Blood Donors
Rout DR , Marwaha N , Sachdev S
Postgraduate Institute of Medical Education and Research, Chandigarh, India
Background
Haemoglobin assessment is an essential pre‐requisite for blood donor selection. Pre‐donation screening of haemoglobin, done by finger prick, involves pain which could be a possible deterrent for donor retention. Using a new non‐invasive point of care testing device for haemoglobin assessment has the advantage of being a painless and comfortable technique for the donors.
Aim
To compare the point of care invasive and non‐invasive methods of haemoglobin estimation in blood donors, with the reference method of haemoglobin estimation.
Methods
With the institutional Ethics Committee's approval, the study was conducted on 1100 blood donors. The donors were selected as per the departmental standard operating procedures. Haemoglobin screening was done by the gravimetric Copper Sulphate method to select the blood donors for blood donation. Subsequently, upon consenting to participate in the study, the haemoglobin values were estimated by the portable haemoglobinometer (HemoCue 301+; HemoCue AB, Sweden), the noninvasive haemoglobin sensor (NBM‐200; OrSense, Ness Ziona, Israel) and the automated haematology analyser (ORION 60; Ocean technology, New Delhi, India). The automated haematology analyser was taken as the reference gold standard method and was calibrated everyday with the haematology reference control (Liquichek™ Hematology‐16 Control Low, Normal and High; Bio‐Rad Laboratories, Irvine, CA, USA). Statistical analyses were done; with P‐value < 0.05, considered significant.
Results
Samples from 18 donors were not accounted for analyses due to sampling errors. The mean haemoglobin values of 1082 blood donors were 14.87 ± 1.03 g/dl by the OrSense method, 15.03 ± 1.31 g/dl by the HemoCue method and 13.98 ± 1.27 g/dl by the haematology analyser method. The bias associated with the OrSense method was −0.89 g/dl (CI: −2.99, 1.21). The bias associated with the HemoCue method was −1.05 g/dl (CI: −2.87, 0.77). The Intraclass correlation coefficient between the haematology analyser and the OrSense method was 0.726 (CI: 0.691, 0.756) showing substantial agreement, while that for the HemoCue method was 0.851 (CI: 0.832, 0.867) showing almost perfect agreement. The Bland‐Altman plots showed the limits of agreement for the OrSense method as (1.216 g/dl, −2.999 g/dl) and that for the HemoCue method as (0.774 g/dl, −2.878 g/dl). The comparison of acceptance/deferral by the Copper Sulphate method and the projected acceptance/deferral by the HemoCue and the OrSense method is detailed in Table 1 along with their sensitivities, specificities, positive predictive values, negative predictive values and accuracies w.r.t. the reference gold standard method in Table 2.
Caption 1: Heamoglobin assessment by different methods.

Conclusion
The sensitivities and the specificities of the three test methods were almost similar for the eligibility assessment of blood donors for blood donation; though none of the test methods replicate the results of the venous haemoglobin values. The non‐invasive method though has the advantage of painless haemoglobin testing.
5A‐S35‐04
Introduction of the Electronically Donor Health Questionnaire (EDH) in Uppsala 2014
Norda RAC , Lindman G , Svenberg S , Schneider K , Lubenow N
Uppsala University Hospital, Uppsala, Sweden
Background
Due to the implementation of two parallel sets of deferral rules for sexual risk behaviour, one for blood components for transfusion and one for plasma for medicinal products, it was decided to introduce an electronical donor health questionnaire (EHD) based on the standard donor health questionnaire (DHQ) by the Swedish Society for Transfusion Medicine, and adapted by a working group of the Swedish Blood Alliance. In 2014 the EHD was introduced in Uppsala after updating ProSang and re‐building the premises. There was major change of the routines and the donor‐flow. The staff member was now responsible for whole chain from the interview, acceptance or deferral and the ensuing blood collection, whereas previously the administrative staff initially reviewed the DHQ. Implementation of the EHD also led to that information of all deferred donors was entered into ProSang with additional work for the staff.
Material and methods
The whole staff participated in a training program on the donor selection criteria, documentation of different temporary deferrals, interviewing and management of the EHD. Every EHD was retrospectively scrutinized for complete motivation for accepting deviating answers and for the decisions to accept or defer the donor. The correct documentation of decisions of temporary deferrals was also assessed. Besides direct individual feed‐back, regular feed‐back was provided at staff meetings and with information letters. After the implementation period a new quality control (QC) measure was introduced, checking accepted questionnaires with deviating answers for correct and complete management. The sample size (10%) was set to detect, at 95% significance level, if 99.9% were correctly handled according to national regulations and written specifications. Every deferral are were also checked as a learning process.
Results
EHD was introduced in February 2014. In October, an evaluation was performed. There were 9079 EHD's processed and 930 (10.2%) led to deferral. The most common reason for deferral was that the donor was invited for a control, not a donation. In 23 cases (0.2%) deviating answers were incorrectly accepted and blood collected. The blood components were recalled. In 0.4% the subsequent documentation was not fully correct or complete, requiring some extra administration. From November 2014 the new QC was introduced on a weekly basis. In all, there were 3573 EHDs filled out, 2348 had deviating answers and 237 were checked. There were 3 (1.3%) not correctly accepted. Comparing with period of implementation the performance had improved from 0.2% to 0.09%. With the new QC we also identified that 6 (3%) were not correctly managed. There were 237 deferred donors, and 115 (48, 5%) should not have filled out the EHD. Two donors (0.8%) were not correctly deferred. In 51 cases (14%) the documentation was not correctly handled and required some extra administration. The time required to perform the QC was on average 1 h for on the average 35 EHDs.
Conclusions
The EHD lead to an improved application of the standardised questioning of blood donors. A newly introduced QC by retrospectively assessing a set percentage of EHD will direct measures for further improvement.
5A‐S35‐05
Estimating the Impact of a Change in the Cancer Donor Deferral Criteria
Lattimore SL1, Barnes SB 2, Jones TJ 3, Miflin GM 2, Brailsford SB 2
1Public Health England, London, United Kingdom2National Health Service Blood and Transplant, London, United Kingdom3Cancer Intelligence Service, South West Public Health Observatory, Bristol, United Kingdom
Background
The current EU directive (and thus the UK Blood safety and Quality regulations, 2005) state that permanent deferral is required for all donors with a history of malignancy except those with a history of in situ cancer with complete recovery. There is however a lack of evidence to support the theoretical concern that cancer is transmitted via blood.
Aims
To estimate the number of potential blood donors that could be gained and retained if donor selection guidelines were altered whereby no deferral will required for pre‐malignant conditions, and no deferral following full recovery with no expectation of recurrence (i.e. cured) and the following conditions apply:
1. For cancers with negligible metastatic potential (e.g. basal cell carcinoma and carcinoma in situ of the cervix) the donor may be accepted immediately following successful removal and cure.
2. For all other cancers, at least 5 years would have elapsed since completion of active treatment.
3. However, permanent deferrals will remain for those with a history of haematological malignancies (e.g. leukaemia, lymphoma) and melanoma, as well as for malignancies known to be of viral origin (except for carcinoma in situe of the cervix).
Methods
Incidence counts, and counts of patients included/excluded from giving blood under previous guidelines and potential guidelines were taken from the National Cancer Data Repository (NCDR) 2010. All malignant cancers (ICD10 codes beginning with ‘C’), in‐situ cancers (ICD10 codes between D00 and D09), and neoplasms of uncertain behaviour (D37‐D48) were separated into appropriate categories according to the previous and potential blood donation guidelines. Tumours with ICD10 codes beginning with ‘D’ were included in the analysis as D00‐D09 are in‐situ cancers which are specifically mentioned in the current guidelines, and D37‐D48 are neoplasms of uncertain behaviour that may or may not be malignant, potentially affecting eligibility for blood donation. Haematological cancers were defined according to the ‘Haematological malignancies and cancer registration in England’ (Bagguley et al).
Results
In total, 1,612,905 patients with diagnosed tumours aged 17–74 (for patients in England alive on 31/12/2010) would be excluded from giving blood under the previous guidelines. Under the new guidelines 797,020 patients with diagnosed tumours aged 17–74 (for patients in England alive on 31/12/2010) would be excluded from giving blood.
Overall, 1,468,186 extra people aged 17–74 in England would be eligible to give blood on 31/12/2010 under the potential guidelines compared to the previous guidelines. During 2010, 125,442 extra people aged 17–74 in England would become eligible to give blood during 2010 under the potential guidelines that would not have been eligible under the previous guidelines
Conclusion
As the population in England shifts towards an older and more ethnically diverse population, the challenges of recruiting and retaining sufficient donors to secure an adequate, safe supply of blood in the future, will increase. It is therefore necessary to explore the potential impact that changes to recruitment and retention strategies will have to better plan programmes of work to ensure a safe supply of blood in the future.
Plenary Session: Oxygen, Red Cells & Transfusion
5B‐PL‐01
The Air They Breathe
Dzik W
Massachusetts General Hospital, Boston, MA, United States of America
Background
While all living creatures share the same atmosphere on Earth, they do not all breathe the same way. Adaptation to unique environments has given some animals respiratory capacities that far outstrip anything humanly possible.
Aims
To describe respiratory strategies used by different animals species which are alternatives to human physiology.
Methods
Published research on the respiratory physiology of deep diving mammals and high‐flying migratory birds will illustrate alternative approaches to the challenge of tissue oxygenation.
Results
The oxygen binding characteristics of hemoglobin are relatively constant across broad species of animals on Earth and yet the available oxygen is remarkably different in the varied environments in which animals live. Deep‐diving air‐breathing marine mammals can reach depths of 1 mile below the surface of the ocean and can hold their breath for up to 1 h under water. They achieve tissue oxygenation of muscles using a variety of willfully controlled changes in heart rate and blood flow collectively referred to as the ‘divers reflex’. They avoid nitrogen narcosis from enormous underwater pressures by emptying their lungs of air prior to dives. Myoglobin plays an important role in tissue oxygenation. Deep diving mammals are the finest anaerobic athletes on the planet. In contrast, birds use a method of breathing that is entirely different from mammals. Birds are able to oxygenate their blood during both inspiration and expiration. This remarkable process results in a far more efficient method of blood oxygenation than can be achieved in mammals (including humans), making birds the finest aerobic athletes on Earth.
Summary
Humans have become the dominant species on the planet and our behavior towards animals underscores the notion that we are evolutionarily superior to all other species. Viewed however from the perspective of oxygen delivery to tissues, athletic capacity, and adaptation to environmental stress, it is evident that other species have superior performance. A better understanding of the how other creatures use the same air that we breathe may lead to greater respect for species diversity and animal life.
5B‐PL‐02
ATP and Red Blood Cells: Signaling Mechanisms Involved in C‐Peptide‐Mediated Inhibition of Low O2‐Induced ATP Release from Human Erythrocytes
Sprague RS , Richards JP , Bowles EA , Stephenson AH , Ellsworth ML
Saint Louis University, St. Louis, MO, United States of America
Background
In vivo, insulin is produced from a prohormone, proinsulin, which is enzymatically cleaved releasing insulin and connecting peptide, or C‐peptide. Although C‐peptide was regarded as biologically inert it has now been shown to have function in a variety of cells including erythrocytes. When human erythrocytes pass through areas of skeletal muscle with increased O2 need, they release both O2 and the vasodilatorATP. This property of erythrocytes enables them to participate in the appropriate distribution of perfusion and oxygen supply. Low O2‐induced ATP release from erythrocytes ATP results from the activation of a discrete signaling pathway that requires increases in intracellular cAMP. We discovered that either insulin or C‐peptide alone inhibits, low O2‐induced ATP release from human erythrocytes. In addition, we determined that insulin this effect of insulin is the result of stimulation of phosphodiesterase 3 activity resulting in the hydrolysis of cAMP.
Aims
Here we investigated the mechanism(s) by which C‐peptide, in the absence of insulin, inhibits low O2‐induced ATP release from human erythrocytes.
Methods
Erythrocytes of healthy humans (n = 21) were obtained by venipuncture, isolated and incubated with C‐peptide alone (1 nM) or in the presence of inhibitors of either protein kinase C alpha (PKCα) or soluble guanylyl cyclase (sGC). Erythrocytes were equilibrated for 30 min in a thin‐film tonometer with 15% O2, 6% CO2, balance N2 (pO2 ~ 100 mmHg) prior to addition of inhibitors and/or C‐peptide (human, amino acid sequence 55–89) or its vehicle (saline). After 20 min, ATP levels were measured using a luciferin‐luciferase assay. Erythrocytes were then exposed to reduced O₂ (0% O2, 6% CO2, balance N2, pO2 ~ 20 mmHg) for 10 min and ATP levels were again measured. Statistical significance was determined using ANOVA followed by a Fisher's LSD test.
Results
In the absence of insulin, 1 nM C‐peptide fully inhibited ATP release in response to low O2. Pre‐incubation with either Gö6976, a PKCα inhibitor, or ODQ, a sGC inhibitor, prevented the C‐peptide‐mediated inhibition of low O2‐induced ATP release. When erythrocytes were incubated with both insulin and C‐peptide at 1 nM each, concentrations that could be present under physiological conditions, low O2‐induced ATP release was not inhibited.
Summary/Conclusions
In the absence of C‐peptide, insulin inhibits low O2‐induced ATP release from erythrocytes via stimulation of PDE3 activity. Here we show that C‐peptide in the absence of insulin also inhibits low O2‐induced ATP but via a different mechanism that requires the activity of PKC and sGC. Importantly, co‐incubation of human erythrocytes with physiological ratios and concentrations of C‐peptide and insulin produces no adverse effects on ATP release. While the inhibitory effects of C‐peptide are interesting, in vivo, C‐peptide never circulates in the absence of insulin. However, the use of C‐peptide in the treatment of diabetes is currently in clinical trials. Our results suggest that the administration of C‐peptide to insulin‐dependent individuals with diabetes could have beneficial effects on the microcirculation but the administration of excessive amounts of C‐peptide could be detrimental for optimal O2 delivery to skeletal muscle.
5B‐PL‐03
Age of Blood Evaluation (ABLE) Study: A Double Blind Multicentre Randomised Controlled Trial (ISRCTN44878718)
Lacroix R1, Hébert C 2, Fergusson A 3, Tinmouth T 4, Walsh S 5, Stanworth J 6
1Sainte‐Justine Hospital, Montreal, QC, Canada2Centre hospitalier universitaire de Montréal, Montréal, QC, Canada3Ottawa Hospital Research Institute, Ottawa, ON, Canada4Ottawa General Hospital, Ottawa, ON, Canada5Edinburgh Royal Infirmary, Edinburgh, United Kingdom6NHS Blood & Transplant/Oxford Radcliffe Hospitals, Oxford, United Kingdom
Background
Red blood cell (RBC) units can be stored up to 42 days (35 days in some country). Laboratory and observational studies have raised the possibility that prolonged RBC storage adversely affect clinical outcomes. The standard policy of blood providers is to deliver the oldest available RBC units (first in, first out policy) in order to limit blood wastage. No clinical data demonstrate that older stored RBC does not result in harms to vulnerable patients requiring blood transfusions.
Aims
To determine if the transfusion of fresh pre‐storage leukoreduced RBC (stored ≤7 days) will lead to a 5% or greater improvement in 90 day all‐cause mortality and clinically important decrease in morbidity in a vulnerable population of critically ill adults.
Methods
ABLE is a double blind, multicentre, parallel, pragmatic randomised controlled trial (RCT) that was conducted in 25 Canadian, 20 British, 10 French, 7 Dutch and 1 Belgian intensive care units (ICU). Critically ill adults who 1) have had a request for a first RBC unit transfusion during the first 7 days of their admission to the ICU, and 2) have an anticipated requirement for on‐going invasive and/or non‐invasive (CPAP or BIPAP) mechanical ventilation of 48 h were considered for inclusion. Participants were randomised to receive either 1) standard issue RBC or 2) RBC stored ≤7 days. All RBC were allogeneic and were prepared in accordance to international standards, including universal pre‐storage leukoreduction. The primary outcome was 90‐day all‐cause mortality. Secondary outcomes were: 1) ICU, hospital, and 30‐day mortality; 2) organ failure assessed using a multiple organ dysfunction score; 3) serious nosocomial infections; 4) adverse events. An intention‐to‐treat strategy was used for statistical analyses.
Results
From March 2009 to May 2014, 1211 patients were randomised to receive fresh RBC, 1219 patients, to receive standard issue RBC. All baseline data were similar in both groups. RBC units were stored 6.1 ± 4.9 days in the fresh group, 22.0 ± 8.4 days in the standard group (P < 0.001). The number of deaths 90 days post‐randomisation was 430 (35.3%) in the standard and 448 (37.0%) in the fresh group (absolute risk difference: 1.7%; 95% CI, −2.1% to 5.5%). Using survival analysis, the hazard ratio for median time to death was 1.1 higher in the fresh arm as compared to standard arm (95% CI, 0.9–1.2) (P = 0.33). There were no significant differences between groups for all secondary outcomes (major morbidities, length of respiratory, hemodynamic, and renal support, length of stay, and red cell transfusion reactions) or in any subgroups.
Conclusion
Providing fresh RBC units stored ≤7 days rather than blood delivered according to the standard policy did not improve the outcome of transfused critically ill adults. The standard delivery policy that is presently used is safe. There is no justification to require fresh blood for critically ill adults.
Posters
1. Management and Organisation
1.1 Organisational Issues
P‐001
Fulfilling the Unmet Need of Immuno‐Hematological Reference Laboratory Network in India – Regional Testing Centers (RTC)
Tiwari AKT1, Dara RC 1, Das SSD 2, Gupta G 3, Jacob LJ 4, Mathur AM 5, Menon RM 2
1Medanta‐The Medicity, Gurgaon, India2Apollo Hospitals, Kolkatta, India3SDMH, Jaipur, India4Hiranandani Hospital, Mumbai, India5Rotary TTK Blood Bank, Bangalore, India
Background
The Regional Testing Center (RTC) was created to fulfill the un‐met need of providing a specialized testing facility with right and timely consultation on red blood cell antibodies, grouping discrepancy, compatibility testing and sometimes even providing compatible red cells for patient with irregular antibodies. It began with a single hospital‐based blood center and later expanded to six centers in different geographical regions for ensuring shorter TAT and easy logistics. We would like to present the first seven‐month data of the immuno‐hematological problems observed at these six centers.
Aim
The aim was to analyze the data for the kind of immuno‐hematological problems and their solutions.
Materials and methods
Six centers were chosen across the country based on expertise and geographical location (1 east, 1 west, 1 north, 1 south and 2 central India). Personnel from these centers met to evolve consensus on the center‐codes, test‐menu, test‐codes, uniform request form, standard operating procedures (SOP), work‐sheet and reporting format. Ortho Clinical Diagnostics (OCD) partnered with these centers and supported with logistics and reagents. Three categories of TAT (8, 24 and 72 h) were defined according to the different patient condition and urgency; (i) life‐threatening emergency,(ii) NOT life‐threatening; transfusion needed and (iii) routine respectively. The RTC were manned round‐the‐clock to receive and process samples and this service was rendered at no cost. The samples were couriered by OCD, India and final reports were sent via e‐mail.
Results
A total of 371 samples were received at all 6 RTC centers for IH workup; 233 for antibody identification, 54 for ABO discrepancy/confirmation, 66 auto‐immune work‐up, 10 compatibility tests and 8 antibody titrations. Out of 233 antibody identification, 73.8% were single antibody, 16.7% had multiple antibodies, 2.1% could not be resolved and 7.4% did not have any irregular antibody. In 54 ABO discrepant cases, 42.5% were for confirmation, 31.5% were A sub‐groups, 11.1% were B sub‐groups, 11.1% were Bombay phenotype and 3.8% were weak D phenotype. In 66 auto‐immune work‐ups, 47% were warm type, 37.9% were cold type and 15.1% were mixed type auto‐antibodies. The compatibility tests were done in patients with multiple antibodies and Bombay phenotype and 17 compatible RBC units were issued to 10 patients in various hospitals. Mean TAT of all centers for three categories was 2.3, 13.0, 31.2 h respectively.
Caption 1: Test Codes.

Caption 2: Number of cases RTC‐ wise.

Conclusion
Initial results of RTC have been very encouraging in terms of laboratory and clinical outcomes within acceptable TAT. RTC program has to be further strengthened by disseminating the information on accessibility, procedures and outcomes to peripheral blood centers which can send discrepant samples; and capacity building within the RTC by imparting trainings, achieving standardization and accreditation.
P‐002
Stepwise Quality Improvement in a Small Scale Caribbean Blood Bank Long Term Success of Process Approach
Sille LF1, Duits AJ 1, Smid WM 2
1Red Cross Blood Bank Foundation Curacao, Willemstad, Netherlands Antilles2Sanquin Consulting Services, Amsterdam, The Netherlands
Background
Quality assurance and control are paramount in order to be able to guarantee the quality of blood products for patients. Large scale western blood banks introduced well‐defined quality systems starting in the eighties and nineties. For small scale blood banks the introduction of these systems is challenging, because of the high cost of introduction as related to the relative low volume of products.
We describe the process of quality improvement in the Red Cross Blood Bank Foundation Curacao as an example for small scale blood banks and describe related success factors.
Aims
What are the factors identified from a managerial perspective that are key for the process of quality improvement in a small scale setting.
Methods
Key players in the process evaluated the 8 years process and related the outcome of a yearly peer audit to the managerial approach.
Results
A number of factors where essential for the start of the process, first of all the need for improvement came from within the organization and consequently a director with a clear vision on quality was appointed by the board followed by a quality officer.
The process started with an in‐depth dialogue on quality and goals to be achieved between director and auditor that has remained the cornerstone for approach during the process. The input of the professionals involved was clearly defined with, director and quality officer having ownership at all times for the quality process and the auditor being available for the knowledge gap assessment and critical mirroring of the managerial approach. This cooperation was based on trust and mutual understanding
The situation analysis by SWOT analysis at the start was then compared with the desired future and GAP analysis and training need analysis. In dialogue the next appropriate step is identified and implemented by the director and quality officer. Yearly evaluation of the process follows during the next audit.
For a successful contribution to the process the auditor needs to adapt to the actual situation of the blood bank that differs in organizational terms but also in socio‐cultural terms to large scale blood banks.

The implemented process showed a clear progress with important hurdles to be taken being completion of documentation system and commitment of co‐workers in the change.
Summary/Conclusions
In conclusion for the implementation of a quality system in a small scale blood bank during 10 years the most important success factors were leadership and organizational clarity combined with a process approach with focus on the actual need over time to achieve sustainable improvement.
P‐003
Improving Communication with Clinical Teams When Patients Have Irregular Antibodies
Daniels H1, Stewart P 1, Clinton P 2, Paterson P 2
1Better Blood Transfusion/NHS Lanarkshire, Glasgow, United Kingdom2NHS Lanarkshire, Glasgow, United Kingdom
Background
Providing compatible blood for patients with irregular antibodies can be a complex and timely process; it often requires repeat blood samples and close communication with the clinical team around the patient's actual transfusion requirements, including date of planned surgery.
Most elective surgical patients are routed through the Surgical Pre Assessment (PA) clinic. This gives opportunity for samples to be tested in advance allowing irregular antibodies to be investigated and specificity confirmed. Hospital bed pressures now mean that most elective patients are admitted early on the day of surgery. Problems can arise however when the patient has antibodies and the laboratory has not been notified that blood is required on that day. Provision of compatible blood for patients with common antibody specificities can be met through screening available blood stocks but some will require blood to be ordered from the National Blood Services. This is usually necessary for patients with multiple antibodies or those of a non specific nature which may require issue of least incompatible blood. Delays of several hours or days can occur. In our hospitals this has led to cancellation of surgery on several occasions.
Aims
To provide better awareness to clinical teams around the complexity of providing compatible blood when a patient has antibodies.
To develop a flowchart to support transfer of information between PA, theatre schedulers and the laboratory.
Methods
The process was mapped to establish who had information relating to patients with antibodies and how they would be scheduled for surgery. Good systems were already in place to record antibody alerts in patient notes and patient management systems. Contact was made with surgical teams to explore how and when date of surgery was agreed and how this information could be relayed to the laboratory.
Results
Mapping of the process identified a single weak link in the overall process. We confirmed that patients with antibodies were having their status confirmed and this was being noted routinely on surgical pathways and patient notes. Alerts were also being added to the electronic patient management system (TRAKCARE). Each surgical team has a team member (either a theatre scheduler or the consultant Secretary) who can confirm the planned date of surgery and who notifies the patient. The lab however, were never being notified. This same person can see the antibody alerts on TRAKCARE. We now have agreement from these staff to call the laboratory with the date of surgery. The Biomedical Scientist can then ensure that screened blood is ordered and available as required.
Summary
A flow chart has been developed which clearly shows the process of events when a patient has antibodies. This shows clearly what should happen and when, including who adds alerts to TRAKCARE and who notifies the laboratory.
We have had no further cancellation of surgery and are now looking at the process of sample retention to avoid unnecessary repeats on the day of surgery.
We now have closer multidisciplinary working with clinical teams and the laboratory staff.
P‐004
Reduction of Peripheral Blood Stem Cell Collection Sessions with Extended‐Hour Operation of Apheresis Center
J Jacob S1, Choo YC 1, Tindle ST 1, Firpo-Betancourt AF 1, Guillet GG 1, Kent TK 1, Wu DWW 2
1Mount Sinai Hospital, New York, NY, United States of America2Montefiore Medical Center, Bronx, NY, United States of America
Background
At the Mount Sinai Apheresis Center, full‐time apheresis nurses used to work an 8.5‐h shift (8:00 am–4:30 pm) 5 days a week. To improve patient care quality by increasing operational efficiency with current nursing staffing, the nurse's shift was changed to 10.5 h per day for 4 days a week, starting from August 2013. Two daily nursing shifts are staggered for an extended 12.5 operation hours (7:30 am–8:00 pm) daily. Here we report the improved PBSC collection schedule with the extended operation hours. To our knowledge, such an exploration of technical aspects and systematic manner of nurse scheduling for PBSC collection service has not been reported.
Aim
To determine whether nursing schedule change effectively increases the number of total blood volume per collection day, thereby reducing PBSC collection days to achieve the collection target of CD34 positive cells for autologous PBSC patients.
Methods
After the nursing schedule change, transfusion medicine physicians (from Department of Pathology) worked in close collaboration with apheresis nurses to increase blood processing volume beyond 4 TBV, the previously maximum volume, when feasible, while ensuring patient comfort and safety by giving increased dose of calcium gluconate as prophylaxis infusion against hypocalcemia.
A retrospective study was performed to compare the pre‐change and post‐change data of the nursing schedule change for autologous PBSC collection patients mobilized by G‐CSF plus plerixafor.
Exclusion criteria were: (1) patients collected from July 30, 2013 to August 31, 2013, the transition month of nursing schedule change; (2) with peripheral blood CD34 positive cells (PB CD34) <10/ul or >120/ul (because extension of operation hours has little impact for collections of these patients). All other PBSC collection cases from Jan. 2, 2013 to Apr. 30, 2014 were included in the study. The end points in this study were: daily blood volume processed for collection, number of TBV per day, reduction of collection days, number and % of patients benefited from the reduction of collection days. SPSS (IBM) was used for statistics analysis in this study.
Results
Eighty patients undergoing 158 autologous PBSC collection sessions were analyzed in this study. Results showed that new nursing schedule (1) significantly increased number of TBV per collection day (P < 0.001); (2) thereby significantly reduced the number of collection days (P < 0.001); (3) significantly increased the number and the percentage of autologous PBSC patients (P < 0.001) who were benefited from reduction of collection days; (4) decreased the discomfort, inconvenience, cost, travel and time spent associated with the stem cell collection for the patients, and brought cost saving to the institution.
Conclusions
To our knowledge, this is the first report on positive impact of nurse scheduling change on PBSC collection service. The study is also unique in that all the autologous patients were mobilized with both G‐CSF plus plerixafor. The study further demonstrated that successful attainment of meaningful improvements in PBSC collection service while controlling costs and preserving quality of care. It requires close collaboration between health care from different disciplines and under different administrative controls.
Caption 1: Results before and after nursing schedule change.

P‐005
Successful Use of a Charity to Transport Blood Samples and Blood Components Between a District General Hospital and the National Health Service Blood and Transplant Birmingham
Jeyachandran SP1, Bower C 2, Smith D 2, Steen R 2, Chantry G 2, Woodward K 1, Ahmad H 1
1Burton Hospitals NHS Foundation Trust, Burton on Trent, United Kingdom2Shropshire & Staffordshire Blood Bikes, Shropshire, United Kingdom
Background
The transport of blood components are required to meet stringent standards as set out by the Blood Safety and Quality Regulations (BSQR) 2005 based on the 002/98/EC and 2004/33/EC European directive. In UK the blood components are provided by NHSBT which also provides the transport for these products. Such a standard transport of blood component has a significant cost implication to NHS. Moreover hospitals will have to rely on courier services to send urgent referral samples to NHSBT which adds additional cost to NHS. This is further compounded by the fact by some blood component such as platelets which has a short shelf life unlike red blood cells and fresh frozen plasma. Some charities are keen to help in the delivery of health care systems and we present this abstract in collaboration with Shropshire and Staffordshire Blood Bikes.
Aim
The main was to create a robust and validated system for transport of blood components and urgent samples to NHSBT using the volunteers from the charity who use the motor bikes for this purpose. The challenge was to ensure full adherence to the BSQR 2005.
Methods
After the expression of interest from the charity various meetings were held between the Hospital Transfusion Team and charity leadership to complete the feasibility. Advice was sought from the trust legal and governance team as well as professionals from NHSBT and blood bank managers from this region. A clinical risk assessment was completed and once the safety and feasibility was established we embarked on the validation of the transport boxes. Clinimed UBP‐110 &UBP‐130 boxes were selected for validation. The acceptance criteria for red blood cell is that the blood surface temperature was maintained between 2C and 10°C for up to 4 h and 20–24°C for platelets.
Once the validation was completed we embarked upon a training programme for all volunteers on the charity. A well structured programme was developed which includs annual training on Good Manufacturing Practice (GMP) and 2 yearly training and competency assessment on safe transport of blood components, spillage management, security breach and delivery and collection arrangements.
Results
We have successfully established a transport system for blood and blood components between our hospital and NHSBT Birmingham. There are 36 competent volunteer riders who have been trained and an on call rota has been established by the charity to provide an uninterrupted service. While we still use NHSBT blue light service for urgent deliveries the SSBB charity has taken over 75% of the adhoc deliveries. In the last 6 months the charity has so far completed around 90 deliveries to and from NHSBT Birmingham saving more than £5000.
Conclusion
This is a novel project with a charity that is not costing any money and providing a safe transport system. We are the first hospital in the west midlands region to do this novel project and this project has helped to develop processes and systems that can be copied by other NHST Trust and charities.
P‐006
Ownership – The Key to Sustainability of Safe Blood Development Project Outcomes
Smit Sibinga CTh1, Kajja I 2, Makhmudova M 3
11939, Zuidhorn, The Netherlands2Makerere University, Kampala, Uganda3RIH, Tashkent, Uzbekistan
Background
Many international development projects bring in consultants who earn a living without really being committed to the identified need of a country. They come and go, consuming a substantial part of project budget without paying proper attention to the need of sustainability of outcomes when the project has come to an end. To be successful, projects from the beginning on need to be owned by the beneficiary and therefore not presented as a piece meal but as a comprehensive programme of developing ownership through mutual understanding, collaboration and cooperation. The project should focus on feasibility based on the country's realities – cultural acceptance, economical status, existing infrastructure, education and levels of professional competence.
Aim
Active involvement creates commitment, that has to be stimulated and supported top down, from the abstract political level down to the practical operational levels and capacities.
Approach
A series of Safe Blood development projects (2001–2014) in 4 WHO regions was evaluated for success factors, such as commitment, leadership and ownership of the project. Applying an in‐country managerial and operational project structure through a committed Steering Committee (SC) composed of the key country stakeholders and operational Work Groups (WG) or Task Forces (TF) focused on the main objectives of a project (e.g. legal framework, quality management, voluntary blood donation, human capacity, clinical use) provides such opportunity.
Outcomes
Over the past 1.5 decade experiences were gained with project approaches and sustainability aspects in various countries in stages of developing health care and blood safety structures. There were situations where (i) beneficiaries wanted the work to be done by the external consultant (e.g. Macedonia, Kazakhstan); (ii) beneficiaries wanted to own the project (e.g. Montenegro, Mongolia, Namibia) and (iii) a gamma of situations in between (e.g. Sudan, Uganda, Uzbekistan, Kyrgyzstan, Zambia). In all situations where the piece meal approach was practiced, no ownership could be created and outcomes were just a paper report without concrete improvement.
In the latter two situations (the majority) ownership was developed, depending on the creativity, motivational and educational competencies of external consultants, local leadership development and support of health authorities. In those situations tangible implementation became visible and outcomes became the fundament of sustainability.
Conclusion
Creation of ownership of a Safe Blood developement project and its objectives is paramount for sustainability of outcomes and an important prerequisite for consistent improvement in a countrie's blood safety. A SC shall be composed of experienced managerial representatives of key stakeholders, to oversee and manage the project implementation. WGs or TFs shall be composed of operational stakeholder representatives from all over the country, interested in and clearly affiliated to the terms of reference. Both then shall be guided by experienced and truly competent international and/or local consultants.
P‐007
Patient Blood Management: Time for Iron
de las Nieves Lopez MA , Mata Cobos A , Gutierrez Ramon B , Dominguez Lomeña MJ , Palomo Hernandez AM , Bueno Hernandez C , Serna Juan S , Marin Fernandez A , Vazquez de la Villa A
INGESA, Melilla, Spain
Background
Iron deficiency and iron deficiency anemia (IDA) are leading causes of morbidity worldwide. Undernutrition plays a major role in IDA occurring in poor income countries, while physiological excessive needs and occult or overt abnormal bleeding are the main causes in countries where dietary iron intake is not an issue. Very often, IDA is an unrecognized problem, preventive recommendations are not applied, diagnosis is delayed, oral iron formulation prescription is far from being effective, and transfusion of packed red blood cells (PRBC) is finally used to treat severe IDA, applying hemoglobin (Hb) triggers as in the acute care setting.
Aims
PRBC transfusion pattern after a multilevel process implementation concerning prevention, early diagnosis and treatment of IDA during year 2014.
Methods
Setting. Public Healthcare Organization in north‐Africa spanish city with balanced population of berber and european ancestry of 80.000 ± 4.000 citizens for study years. Healthcare is delivered by four primary care centers and one second level of care hospital, with self‐ sufficiency of blood components and shared leadership in the core hematology laboratory, transfusion centre and hospital transfusion service.
Process description. Performance standards were introduced for early first step diagnosis of IDA in the laboratory, communication to general practitioners, uniform prescription patterns of oral iron formulations, including universal supplementation in pregnancy, and uniform delivery of intravenous first loading dose of iron for inpatients and outpatients in the emergency department. Patients with severe non active bleeding IDA (Hb < 8 g/dl) were closely followed in our day‐hospital for a total delivery of 1.200 mg of intravenous iron during ten days and until Hb was >8 g/dl. Iron sucrose (200 mg) was used for inpatients and in the emergency department, and iron carboxymaltose (500 mg) for appointed outpatient visits. Transfusion in IDA was reserved for fragile patients with cardiovascular risk factors or known cardiovascular disease and Hb < 8 g/dl or fixed threshold of Hb < 5.5 g/dl in any patient in parallel to iron reposition.
Data collection. Hospital activity was assessed for years 2011–2014, and included hospital discharges (DC), deliveries (DL), emergency department visits (EV) and PRBC units transfused.
Statistical analysis. Package SPSS v22 (IBM) was used for analysis of variance (ANOVA) of PRBC use throughout years and univariate analysis (ANCOVA) adjusting for DC, DL and EV as covariates. Yearly activity was analyzed in quarters to attain statistical power (P = 0.05 for the whole model).
Results
Global mean quarter activity (±SD) for DC, DL, EV and PRBC use were 1.756 ± 89, 624 ± 56, 15.230 ± 573, and 396 ± 46 respectively (411 ± 38 for years 2011–2013 and 350 ± 38 for 2014). According to yearly activity, 2014 showed the highest rate of DC (7.524), DL (2.770) and EV (62.422), whereas PRBC use showed the lowest (1.434), with a decline of 19% compared to 2013 (1.768). Analysis of variance showed a significant difference for PRBC use in 2014 (P = 0.014), which remained significant after adjusting for covariates (P = 0.008).
Conclusion
Improving IDA management is a target to avoid PRBC transfusion.
P‐008
Impact of Nursing Schedule Change on Therapeutic Apheresis Service
J Jacob S1, Firpo-Betancourt AF 1, Guillet GG 1, Kent TK 1, Choo YC 1, Tindle ST 1, Wu DWW 2
1Mount Sinai Hospital, New York, United States of America2Montefiore Medical Center, Bronx, United States of America
Background
Shortages of qualified apheresis nursing staff and the constraints of costs are demanding careful attention by management on the actual operation of services including scheduling arrangements. In order to reduce the high cost associated with therapeutic apheresis (TA) procedures performed by contracted outside apheresis service (COAS), our full‐time apheresis nurse's shift was changed in August 2013 from an 8.5‐h daily shift for 5 days a week to 10.5 h per day for 4 days a week. Two daily nursing shifts are staggered to provide an extended 12.5 operation hours (7:30 a.m. to 8:00 p.m.) daily. This change was an attempt to increase the number and percentage of TA procedures performed by full‐time nurse employees (FTN) without changing the total working hours per FTN, thereby increasing the operational efficiency. Here we report our experience as an accountable care organization (ACO) on an alternate scheduling of the hours of operations of our Apheresis Center.
Aim
To determine whether nursing schedule change effectively increases number of TA procedures performed by FTN, thereby reducing the number by COAS.
Methods
A retrospective study was conducted to compare the pre‐change and post‐change data of the nursing schedule change for TA procedures, including peripheral blood stem cell (PBSC) collections, during the period of January 1, 2013 to April 30, 2014. Exclusion criterion was: TA procedures performed from August 1, 2013 to August 31, 2013, the transition month of nursing schedule change. End points of this study were: Number and percentage of TA procedures performed per FTN per month, number and percentage of TA procedures by COAS per month, number and percentage of TA procedures by part‐time nurse employees (PTN) per month, working hours of each FTN per month, and number of collection days reduced for PBSC collection patients. SPSS (IBM) was used for statistics analysis in this study.
Results
A total of 1665 therapeutic procedures, including 435 PBSC collection sessions, was analyzed in this study. Our study results revealed that the new nursing schedule (i) did not significantly increase the number and percentage of TA procedures performed per month per FTN; (ii) did not significantly reduce the number and percentage of TA procedures performed per month by COAS (Multi‐fold reasons for the above outcome were analyzed.); (iii) did not significantly change the number of working hours of each FTN per month; (iv) did significantly reduce the number and percentage of TA procedures performed by PTN; and (5) did significantly reduce collection days for a subset of autologous PBSC collection patients, resulting in increased cost saving for these patients and the institution.
Caption 1: Results before and after nursing schedule change.

Conclusions
To our knowledge, this is the first report to explore the technical and systematic aspects of nurse scheduling for therapeutic apheresis service. The study revealed the positive impact of the schedule change for autologous PBSC collection patients, but did not demonstrate the desired positive impact on the entire TA service in the period studied. A study in a much longer period with controlled similar conditions might give a different outcome.
P‐009
Vast Distances, Low Population Densities – A Model Reform of the Blood Supply in Mongolia
Erdenebayar N 1, Smit Sibinga CTh2
1National Center for Transfusion Medicine, Ulaanbaatar, Mongolia21939, Zuidhorn, The Netherlands
Background
Landlocked Mongolia with 1.5 million km2 is the number 19 largest country, with only 3 million population. Almost 50% lives in the Ulaanbaatar capital area, the rest is spread over 21 aimags scattered around the country. Logistics of transportation are poorly developed, whether road, rail or air. Winters are harsh and Summers hot. Of the 26,000 blood collections/year (8.6/1000 population), almost 19,000 are collected in the capital, leaving the hospital‐based aimag blood centers with an average of 345 collections/year (range of 62–698), representing a pretty disbalanced picture.
Aim
Consolidation of the primary operational blood procurement processes to a more acceptable economy of scale in order to better control quality, efficacy, staff competency and cost‐effectiveness.
Approach
Besides the capital/northern area, 5 aimag clusters (regional centres) are planned with an average of 1000 collections/year for processing and testing. Aimag blood collections are transported to these regional supra‐aimag centres, which distribute cascade‐wise finished blood components to the aimag hospitals, of which some serve as a cascade stock facility, transporting components to even more remote aimag hospitals. Each hospital will be supplied with a rotating stock of red cells, plasma and cryoprecipitate for at least 1–2 weeks.
Outcomes
Aimag hospital blood banks will focus on collection, storage and patient blood management. Supra‐aimag regional blood centers will be supplied with blood collected in the remote aimags, process and re‐distribute finished products. The National Center for Transfusion Medicine (NCTM) in Ulaanbaatar will support the surrounding aimags and serve as a national center for transfusion medicine – management, education, development, purchase of consumables and equipment, maintenance and reference functions. This will contribute to an improvement in both the procurement and consumption of blood in the country – quality, efficacy, staff competency and cost‐effectiveness of the blood supply and patient blood management in the country. The hospital blood component stocks will contribute to a better and more efficacious blood management at the bed site, abandoning the current ad hoc inefficient and inappropriate clinical transfusion practice. As a positive spin‐off, ad hoc calls for donors (largely family/acquaintance) will be converted to regularly organized donor sessions where sufficient voluntary donors can come to donate their blood locally, synchronized with the hospital supply schedules of the regional supra‐aimag blood centres. Drivers supply and bring the collected blood back to the regional procurement centre.
Conclusion
Consolidating the 21 aimag hospital‐based blood centers into supra‐aimag centres (1 National and 4 regional) for processing and testing of collected blood, without creating too much distance to cover for supply of aimag hospitals, will improve on quality, efficacy and cost‐effectiveness as well as staff competency of this important supportive part of the health care system. The logistics of supply will not affect the satisfaction of the clinical demand through the development of an in‐hospital stock‐based structure. For the future a cyopreservation program might also allow platelets to be stored in these more remote areas.
P‐010
Establishment of National Blood Transfusion Services in Pakistan
Waheed UW , Zaheer HAZ
Ministry of National Health Services, Islamabad, Pakistan
Background
The Blood Transfusion Services in Pakistan are based on provincial structures guided by the Blood Safety Acts introduced between 1997 and 2004 in all provinces. Services are provided by public or private hospital blood banks or stand‐alone facilities. There is generally no separation between manufacturing units (Blood Centres) and ordering units (Blood Banks), which has an impact on the organization of services. The World Health Assembly resolutions 28.72 (1975) and 58.13 (2005) urge Member States to develop nationally coordinated blood transfusion services. Pakistan is a signatory to these resolutions and other similar resolutions on blood safety passed by the WHA during the last thirty five years. To fulfill these international obligations and the MDG 4, 5 and 6, the Government of Pakistan initiated blood safety systems reforms in 2010 including the establishment of a nationally coordinated de‐centralized and province oriented BTS.
Aims
To establish core elements of an independent rational structure of a national blood transfusion service that will ensure adequate, efficient and safe blood supply, in a cost effective manner through a network of new Regional Blood Centres linked to existing Hospital Blood Banks.
Methods
The agenda of re‐structuring the blood transfusion services in Pakistan is outlined in the National Blood Policy and Strategic Framework and is supported by the German Government.
Results
The Government of Pakistan through support from the Government of Germany initiated the process of restructuring the blood transfusion system by following a centralized model. The National Blood Transfusion Programme was established which created a high visibility environment to promote a modern national blood transfusion system as part of its health services. Required resources for national and provincial safe blood transfusion programmes were defined and approved in PC‐1s (project documents), while national and provincial commitment was enhanced by the German support through technical advisory services and the establishment of a network of fully equipped regional blood centres throughout the country. The SBTP has over the years through its various regular and diverse activities brought all the stakeholders on a single national platform. As a result of these networking opportunities, the SBTP is now the recognized national voice of Pakistan on all issues related to transfusion.
Recently, the SBTP under the supervision of Ministry of National Health Services adopted a systematic and participatory approach to revise the National Blood Policy & Strategic Framework (2014–20) to ensure a sustainable planning base for the future. The overarching priorities regarding blood transfusion services that are addressed in this framework include: equity; the development of leadership, planning and management capacity; quality of care and monitoring and evaluation.
Conclusion
Despite many challenges, the Programme continued to strengthen and scale up the implementation process by engagement of committed resources and the conduction of strategic system and regulatory reform efforts under the National Blood Policy and Strategic Framework. Apart from creating a conducive environment for sustainable blood programmes within their health systems, efforts are made to strengthen the capacity of system governance and safe blood transfusion practices through institutional and regulatory reforms.
P‐011
Risk‐Based Decision‐Making for Blood Safety
Ward SW 1, Kelly AKmr2
1Canadian Blood Services, Ottawa, Canada2Irish Blood Transfusion Service, Dublin, Ireland
Background
Since the 1980s, numerous measures aimed at maximizing blood safety have been implemented based on varying interpretations of the precautionary principle and an unsustainable pursuit of ‘zero risk’, without evident consideration of cost effectiveness and opportunity cost. The need for a collective, standardized decision‐making framework has become clear in the face of increasing complexity in blood safety decision‐making, driven by medical, scientific, ethical, economic, legal, and public policy factors. Risk is inherent from ‘vein‐to‐vein’, and there are now fewer resources available to mitigate these risks.
Aim
As such, the aim of the Risk‐Based Decision‐Making project has been to develop an integrated, internationally applicable framework, entrenched in donor safety and optimal patient outcomes, to guide major policy and operational change; this framework has been developed to improve consistency in decision‐making, facilitate proportional responses to risk, ensure that decisions are evidence‐based, increase trust in investment decisions, and allow for the redirection of resources to improve effectiveness.
Method
While there are many comprehensive, credible, and time‐tested risk frameworks available, the ABO risk framework differs from the others in that it has been designed specifically for blood safety risk assessment. The framework gauges quantitative and qualitative risk and overall risk tolerability in the context of patient and donor safety, and contains guidance regarding the use of health economic and outcomes assessments in the evaluation of risk and mitigation options. To achieve transparency and optimal input, the framework includes guidelines to enable stakeholder involvement on considerations such as possible gaps and problems, risks, opportunities, alternate solutions, unintended consequences, resources, linkages, and implementation implications. By enabling the integration of all of these dimensions into an overall risk profile in order to inform the decision‐making process, the framework offers clear advantages over other models.
Results
Under the aegis of the Alliance of Blood Operators, this project has been developed to include an overall risk framework with guidance on health economics and outcomes methodology for blood safety and stakeholder involvement guidelines to gather broad input and maximize support. Additional key elements of this framework are agreed‐upon risk management principles, guidance on conducting blood safety risk assessments, determining risk tolerability, and a way to apply these tools in the decision making process.
Conclusion
Ultimately, the Risk‐Based Decision‐Making Framework is a tool for moving away from the unattainable ‘zero‐risk’ paradigm towards a realistic system of monitored, managed risk that allows for more effective use of reduced resources.
P‐012
Organisational Change – Implementation of the Two‐Sample Rule for ABO/D Grouping Prior to the Issue of RBC
Buchan JP , Jeyachandran J
Burton Hospitals NHSFT, Burton‐upon‐Trent, United Kingdom
Background
ABO grouping is an important serological test performed on pre‐transfusion samples. Unless secure electronic patient identification systems are in place, a second sample should be requested for confirmation of the ABO group. Burton Hospitals Blood Bank does not utilise a patient identification system. The 2 Sample Rule was introduced following a near‐miss event of a Wrong Blood in Tube sample identified by a nurse providing a sample.
Aim
The aim of this study was to evaluate the effectiveness of the introduction of a two‐sample rule for transfusion of red blood cells (RBC) in a 450‐bedded acute hospital. The purpose of this was to reduce the risk of ABO incompatible transfusion.
Methods
A review of the Trusts practices was undertaken prior to implementing the 2 Sample Rule. A small‐scale local audit was undertaken for three months post implementation to evaluate effectiveness of implementation.
An organisational change management implementation approach was utilised. A systematic diagnosis of the current situation identified that current practice did not adhere to national pre‐transfusion compatibility testing recommendations, and could potentially result in a transfusion of an incorrect blood product (Never Event). Approximately 80% of patients had a historical ABO group identified. Ability to identify specific patient groups who did not have this allowed for targeted implementation steps.
Managing the change required involvement from the Hospital Transfusion Team, Hospital Transfusion Group and the Trusts Governance Groups to ensure support from the Directors and the utilisation of Governance structures to manage risk. A launch date for the 1st of August 2014 was agreed; chosen as it coincided with the Doctors change over period. A Patient Safety Week was held which included information stands, visits to clinical areas, distribution of information flyers and attending team meetings. Specific training was delivered directly to key areas where change in practice was required.
Results
During the three month period 320 patients required transfusion of RBC's. This equated to 750 units of blood. 29 patients received 92 units without a 2nd sample due to emergency transfusion (9% of patients, or 12.2% of units transfused). Of these 92 units issued, 56 were issued to group O patients. 36 units were issued to patients with other blood groups (15 patients or 4.8% of red blood cells issued). During the implementation phase, use of O‐Neg blood increased by 0.5%, and use of O‐Pos increased by 11.7%. On‐going monitoring shows this is reducing.
Conclusion
Implementation of the 2 Sample Rule required planning and assessment. Initially there was minimal resistance, but this was soon overcome when people understood the benefits and the numbers of patients affected. The ability to understand where to target change was hugely beneficial. Clear guidance is needed to define ‘2 samples’. The audit showed that there was an improvement in the understanding of the 2 Sample Rule, and that initial concerns regarding an increased usage in O‐Neg/O‐Pos blood was justified, but not to the extent expected.
P‐013
Blood Transfusion System in Albania – Achievements and Challenges
Durro V1, Saliasi S 2
1Ministry of Health, Tirana, Albania2Regional Health Authority, Tirana, Albania
Background
Organization of blood transfusion system in different country vary according to national characteristics and different national laws on blood safety in both EU and non‐EU countries,
Aim
The aim of this study is to present organization of blood transfusion system in Albania.
Method
The data are collected from Ministry of health and national blood transfusion centre archive.
Results
Looking the history of transfusion medicine in Albania, before 1990, 100% of collected blood provided from remunerated donations and has been see as an activity of professionals. The BTS in Albania had one National Blood Transfusion Centre (NBTC) and 26 hospitals blood banks which carry out all activity of blood bank. But now we have a different situation and organization in blood transfusion system. The supply with safety and qualitative blood collected by voluntary non remunerate regulate blood donors is declared as the priority of MoH. In this context during the 2005, MoH has undertaken very significant administrative, financial and technical interventions which consist of: (i) The adoption of legal framework in line with EU directive 2002/98/EC, and with WHO recommendations in the field of blood transfusion. There are actually developed and approved all guidelines and regulations predicted by the Law, regarding donor management, quality systems, hemovigilance, accreditation and inspection in conformity with EU Directives 2004/33/EC, 2005/61/EC, 2005/62/EC; (ii) Measures for establishing a voluntary unpaid donation system as: Implemented the National Strategy for Safe Blood Transfusion launched in 2006; approved National Voluntary Blood Program with activities; approved Agreement with the National Council of Televisions, that will make possible to transmit all spots on blood donation free of charge since 200; Establishment of a policy for reimbursing the partner involve in promotion of vnrbd from MoH since 2009; interrupted the admission of the paid first time blood donors since 2009, gradually phase out paid and family replacement donation and replace them with vnrbd from low‐risk population, maintain first time vnrbd and return them in regular vnrbd; (iii) administrative reorganization of BTS as: established the national blood transfusion committee, reorganization of BTS in NBTC, 5 regional blood banks (BB), 15 hospitals BB in regional hospitals and 11 BB in district hospitals; define the Competent authority for inspection of transfusion service and for authorization and accreditation, centralization of the processing of blood in 5 regional BB, Centralization of the testing for ITT by blood transfusion in NBTC in Tirana since 2010, The immunohaematology laboratory of NBTC is considered as reference for the whole country.
Conclusion
Change the structure of blood donors and increase the unit of blood collected as results of these innervations. The number of blood donation was increase from 14670 donations in 2006 to 29.232 in 2013. So in 2013, 90% of blood was collected from unpaid donation vs 52.6% in 2005. In 2013, 26% of blood was collected from vnrbd vs 11% in 2005, and 64% from family replacements blood donors (FRBD) vs 36.4% in 2005.
P‐014
Quantitative Methods in the Service of the Daily Work Management
Bujandric NB , Grujic JG , Dimitrijevic GD
The Blood Transfusion Institute of Vojvodina, Novi Sad, Serbia
Background
Blood establishments are responsible for many different activities such as the collection, testing, processing, storage and distribution of blood components, etc. In order to identify inefficiencies, there is a need to identify a time required to complete specific tasks, to determine the productivity level for specific work processes, to gain insight into the scope and content of laboratory work, and more.
Aims
To evaluate daily variations in the number of analyzes performed in the Clinical Transfusion Department of the Blood Transfusion Institute of Vojvodina.
Methods
A retrospective analysis of data which were obtained from the daily reports from January1st until December 31st 2014. The time period was divided into seven groups as Monday (1), Tuesday (2), Wednesday (3), Thursday (4), Friday (5), Saturday (6) andSunday (7). All of the results are expressed as mean ± SD. Comparisons between groups were performed using ANOVA and Tukey's simultaneous test, where appropriate. Data analysis includes number of: ABO grouping and Rh typing, Immediate Spin Crossmatch, AHG cross‐match, Direct Antiglobulin Test (DAT), Indirect Antiglobulin Test (IAT).
Results
The following frequency distribution of divided summary data was obtained: (1)17.1%; (2)17.4%; (3)17.6%; (4)15.8%; (5)12.5%; (6)8.1%; (7)11.5%. Number of AHG cross‐matches showed significant difference between groups 1‐4 and 5‐7 (one‐way ANOVA, F = 65.6, P < 0.01). The study was found that: (i) in 1–5 groups: 85.6% analyses were performed in the time period 7–19 h; 14.4% analyses were performed in the time period 19–7 h; (ii) in 6–7 groups: 78.9% analyses were performed in the time period 7–19 h and 21.1% in the time period 19–7 h. Immediate spin crossmatch was done due to the urgent request in 1.8% of pretransfusion testing, the most frequently on Wednesday (19%) and Friday (20%). Number of ABO/D and DAT in neonates showed significant difference between groups 1–6 and 7 (one‐way ANOVA, F = 15.7, P < 0.01).
Summary/Conclusions
Quantitative methods can help us in daily management of work tasks. Monitoring of working activities allows us to distinguish predictable and unpredictable working activities, the weekly variations, variation from day to day, variation which has a random character, etc.
P‐015
Analyze the Impact of the Strategic Foresight Capabilities on the Crisis Management With Regard to the Intermediary Role of the Human Resources Agility
Aghahosseini Eshkavandi M1, Rezaie Dolatabadi H 2, Nilipour Tabatabai SA 2
1Isfahan Blood Transfusion Organization, Isfahan, Iran2Isfahan University – Shakhes Pajouh Institute, Isfahan, Iran
Background
Nowadays, the term ‘strategic foresight’ is widely used to describe the activities and processes that help the decision‐makers for steering the firm future actions.
Aims
The study is performed to investigate the direct and indirect effects of Strategic Foresight capabilities on the crisis management, considering the mediating role of human resources agility in the Isfahan Province Blood Transfusion organization and 3 other accident‐prone provinces in 2013.
Methods
In this research the major components and variables of study has been identified at first and then accordingly the questionnaire was developed and given to the statistical sample The PLS structural equation model was used to test the research hypothesis.
Results
Based on obtained results the impact of the strategic foresight capabilities on the crisis management is 31%. the impact of the strategic foresight capabilities on the human resources agility is 89%. The impact of the human resources agility on the crisis management is 60%.
Summary/Conclusions
31% of the variation in the crisis management is associated to the strategic foresight capabilities. 89% of the variation in the human resources agility is related to strategic foresight capabilities and 60% of the variation in the crisis management is attributable to HR agility.
P‐016
Organizational Changes in the Polish Blood Donation System and Their Effect on Blood Transfusion Service
Antoniewicz-Papis J 1, Lorek M1, Lachert E 2
1National Blood Centre, Warsaw, Poland2Institute of Hematology and Transfusion Medicine, Warsaw, Poland
In the recent years Poland has undergone numerous economic, social and cultural changes as well as transformations all of which had crucial impact on the functioning of blood transfusion service (BTS). Apart from advancement in technological solutions, implementation of modern research methods related to blood donation, a sequence of organizational changes has also occurred which imposed modifications in blood donor management. The main aim of the paper was to evaluate the effect of organizational changes in blood collection system on the Polish blood transfusion service (BTS) including donor management. To this effect an analysis was performed which involved BTS statistical data from the 1998–2014 period. The data referred to type of blood collection sites, number of mobile blood collection sites (MCS) number of donations etc.
In 1998 blood was collected in 436 local blood collection sites (LCS), mainly located in hospitals. The number of mobile collection sites (MCS) was relatively small (1326) and they were organized mostly on the premises of large enterprises. It is noteworthy that as much as 60% of all blood donations was collected in LCS, 40% in the Regional Blood Transfusion Centers (RBTC) 6% of which during MCS. MCS were very effective; about 40 donations/1 MCS, vs 6 donations/working day in LCS. This was the first year of application of the main assumptions of the Polish Blood Transfusion Act related to Polish BTS centralization. By the end of 2014 only 137 LCS remained which covered about 45% of all blood collections. Approximately 31% were performed in RBTC and 21% during MCS. MCS – blood collections proved more effective than in LCS (24 vs 17 donations/working day for MCS and LCS respectively).
Data‐analysis demonstrated that every organizational change referring to transfusion service negatively affected donor activity, particularly in the initial phase. Huge effort was therefore required to convince donors to appreciate and accept the new solutions.
The growing number of MCS offered easier access to blood donation and enabled blood collection where no such opportunity was previously provided. In first years following implementation of the organizational changes the number of first time donors decreased by 3%; although the overall number of donations was maintained at a level of 1.2 million. The number of donors became stable in 2014. Despite the smaller number of LCS they are still important support for RBTCs and blood collection during MCS cannot substitute for LCS activity. Closing down of LCS triggers mobilization of donors in other LCS which results in substantial amount of collected blood.
Data‐analysis reveals that the blood donation system must be adjusted to the convenience of blood donors. MCS should be organized in locations with easy access for blood donors. Such movements call for coordinated educational and promotional actions undertaken by company management within the framework of corporate social responsibility.
1.2 Information Technology
P‐017
The Use of Cellular and Tablet Devices as a Teaching and Information Resource Tool
Squires E , Tormos M
Medical University of South Carolina, Charleston, United States of America
Background
Dissemination of rapidly expanding medical and scientific information to large numbers of incoming student physicians and allied health professionals represents a unique challenge in Academic Medical Institutions. Additionally, Transfusion Services provide these physicians with criteria (e.g. transfusion triggers) and transfusion ordering procedures that may differ from the hospitals in which they were originally trained; while for some, it may be their first exposure to blood product ordering. It was felt that the use of cellular (‘smart’) phone and tablet devices could supply timely and consistent Transfusion Service information in a convenient and easily adaptable method to new incoming physicians as well as more senior medical, nursing, and perfusion staff.
Aims
To develop a readily available tool to provide transfusion information to clinicians that can be controlled and updated as necessary by the transfusion service.
Methods
Initially, a printed reference card was provided to all incoming physicians at orientation, but it was rarely used and often lost. In addition, controlling use of current editions became difficult. To avoid these problems, the information was transferred to an ‘app’, a term loosely used to refer to a downloadable, non‐modifiable interactive document. Included is critical information on all available blood products, transfusion indications, transfusion risks, reaction symptoms, and reaction reporting procedures. A QR code (matrix or two‐dimensional bar code) was included to direct the smart phone or tablet browser to the appropriate website.
Results
New physicians are targeted with the inclusion of the web address and QR code in the New Physician Orientation materials. Work area signage including the QR code and intranet websites has also been posted in targeted clinical areas. The Graduate Medical Education Office is the host of the main control document. The same information is available through the Pathology Department website and the ‘Medical Toolbox’ section of the University Intranet, and will point to the GME website. The benefits that we expect to see include: convenient access to transfusion service information, the ability to revise guidelines and policies, the elimination of outdated information in the hands of physicians, reduced printing costs, and potentially increased use of easily available information on blood product use and transfusion reaction identification.
Conclusion
Rapid access to essential transfusion service information is critical to hospital physicians and nursing staff. Ensuring this information reaches hundreds of physicians‐in‐training increases the complexity of that task. With expanded use of smart phones and tablet devices in medical care, it seemed appropriate to utilize these devices to disseminate critical transfusion service information. In addition, the use of this technology has expanded the availability of this information to attending physicians, nursing staff, and perfusion staff, as well as incoming residents and fellows.
P‐018
Standardized 2D Label Design for Blood Products
Cabana EC1, Georgsen JG 2, Bolton WB 3, Brazier AB 4, Grabowski SG 5, Kirkpatrick BK 6, Lodge LL 7, Quan NQ 8, Skagestad RS 9, Wray BW 10, Campbell TC 11
1ICCBBA, San Bernardino, United States of America2Odense University Hospital, Odense, Denmark3Australian Red Cross Blood Service, Melbourne, Australia4NHS Blood and Transplant, Newcastle, United Kingdom5Gulf Coast Regional Blood Center, Houston, United States of America6Digi‐Trax, Lincolnshire, United States of America7Scottish National Blood Transfusion Service, Edinburgh, United Kingdom8Health Sciences Authority, Singapore, Singapore9Labcraft AS, Oslo, Norway10Computype, St. Paul, United States of America11One Blood, St. Petersburg, United States of America
Background
The ISBT 128 Standard was designed to ensure the highest levels of accuracy, safety, and efficiency for donors and patients. Blood establishments using ISBT 128 reported increasing challenges of including more information on blood component labels. Additionally, facilities seek to increase efficiency in processing hundreds of thousands of units each year without compromising safety. The current international ISBT 128 blood label was designed over 15 years ago and has four, sometimes five, standardized linear barcodes. It was proposed that a new label design, using a single 2D barcode (i.e. Data Matrix), be developed to satisfy these demands.
Aims
Moving towards a single 2D barcode on the label will:
• Increase efficiency by reducing the number of barcode scans.
• Allow for better organization of information.
• Decrease training and confusion on which barcode to scan.
Methods
An international committee was formed by ICCBBA with experts from various regions.
They began with published survey results (Transfusion Med. 2014 Apr; 24(2):89–98) from the UK which determined hospital views on the optimal placement of critical information on a label. Two of the authors participated on this committee. Then label information was categorized as either necessary or unnecessary. The Committee then surveyed blood facilities and hospitals in various regions to identify elements of the label that could be internationally standardized.
Different scanning mechanisms (e.g. hand held scanners and flatbed scanners) were taken into consideration when determining the best placement of the 2D barcode. Several draft labels were distributed for comment and a best‐fit design was created based on feedback.
Results
A new label design was created. Information that is important to the end‐user is in the upper half of the label. This includes the Donation Identification Number, Product Code, product name, ABO/RhD, expiry date, volume, and the Data Matrix symbol.
The lower right of the label includes test results and the processing facility (if different than the collection facility).
The lower left of the label contains the collection date, storage conditions, regulatory text, static information, and collection facility. Component specifications may be provided via a second 2D barcode (using QR symbology) that takes the user to a website that displays this information.
An ISBT 128 compound message will be used to encode the information into the Data Matrix symbol. Minimally it will include the Donation Identification Number, Product Code, ABO/RhD, and expiry date. Additional information may optionally be encoded.
Summary/Conclusions
The ISBT 128 standardized blood label will move towards the use of a single Data Matrix barcode and eventually eliminate linear barcodes on the label. This will improve efficiency at the blood center and hospital by requiring a single scan to capture all the information currently encoded within 4 or 5 linear barcodes. It will group key information for the transfusionist in the upper half of the label, while static information will appear in the lower half of the label. A transition period of ten years is anticipated. Facility software must be updated before the new label can be implemented. Design feedback can be submitted to ICCBBA.
P‐019
Blood Donor Extended Phenotyping For Duffy, Kidd, and Mns Blood Group Systems by the PK 7300 Analyzer With Ready‐to‐Use Reagents: Experience of the French National Blood Service
Adjerad LA1, Peyrard TP 2
1Etablissement Francais Du Sang, Lille, France2Institut National de la Transfusion Sanguine, Paris, France
Background
Blood donor extended phenotyping (Fya, Fyb, Jka, Jkb, S, s) is usually performed to prevent alloimmunization in chronically‐transfused patients and in order to quickly find compatible blood in patients with common alloantibodies. It is useful for rare blood donor screening, especially with regard to the Fy (a‐b‐) and S‐s‐ phenotypes in people of African ancestry. Furthermore, blood donor extended phenotyping is essential for the manufacturing of red blood cell antibody screening/identification panels. Blood donor extended phenotyping is usually carried out with low‐ to medium‐throughput methods, such as column‐agglutination or immunocapture techniques.
The determination of the Fya, Fyb, Jka, Jkb, S and s antigens with a high‐throughput device is now made possible with the PK 7300 blood group analyzer (Beckman‐Coulter, Fullerton, CA, USA), thanks to the use of a new set of ready‐to‐use reagents (Diagast, Loos, France).
Aims
To evaluate the performance of a new set of extended phenotyping ready‐to‐use reagents to be used on the high‐throughput PK7300 instrument.
Methods
A population of 2 352 donors previously typed for the Fya, Fyb, Jka, Jkb, S, s antigens (column agglutination or immunocapture technique) was investigated with the newly available set of ready‐to‐use reagents available on the PK7300 instrument. All samples showing discrepant results were referred to the French National Immunohematology Reference Laboratory (CNRGS – Paris) for further investigation (phenotyping and molecular testing when necessary).
Results
The throughput was estimated to be approximately 250 samples per hour in this study
All the results for Fya, Fyb, Jka, Jkb, S, s typing were concordant, except 18 cases of discrepancies for Fyb: negative result with the gel or immunocapture techniques and positive or ambiguous results with the new set of reagents for PK7300. Molecular testing has been performed for those samples and the results demonstrated a 100% concordance between the PK7300 reagents and molecular testing, since all of them showed a Fyx phenotype (weak expression of Fyb due to the c.265C>T mutation in the FY*02 allele).
Two donors showed a weak expression of Jkb, which was confirmed by phenotyping methods in the reference laboratory. Molecular testing is ongoing for those two cases in order to explore the genetic background of this depressed antigen expression.
Summary/Conclusions
Blood donor extended phenotyping on the PK7300 analyzer with the new set of ready‐to‐use reagents from the Diagast Company was found to be a reliable high‐throughput technique that showed a high capacity of detection of the Fyx phenotype. Despite a weak immunogenicity expected for the Fyx phenotype, the ability of a technique to screen for this variant in the blood donor testing background is a definite advantage, since such donors should logically be typed as Fy (b+). Altogether, our results suggest that this technique is fully relevant for a routine mass donor extended phenotyping program and could be an important source of new rare donors.
P‐020
Business Intelligence and Web Application for the Management of the Blood Supply Chain
Vio C , Vicarioto M , De Silvestro G , Marotti A
Azienda Ospedaliera Padua, Padova, Italy
Background
In the Department of Transfusion's Medicine of the Hospital of Padua there is steep decline in the donation rate and an increase in the consumption of blood components. Thus, optimal use of blood components requires even more attention. Managing transfusion to limit demand and maximise benefits for patients requires information, evidence and method. On the other hand blood gift by voluntary blood donors is decreasing. This requires new approaches on both sides of the blood supply chain.
Aims
Development of new informatics tools to analyse the database of the Transfusion Medicine Department, in the quickness identification of active donors with only one blood donation. There is the possibility to link the patient's necessity to the donor's accessibility.
Methods
A Business Intelligence platform, called QlikView, has been developed and applied on the database of the Transfusion Medicine Department. Different filters are used to monitor the uses of blood units for ward, diagnosis and single patients. The innovative analysis system works in‐memory, each items is clickable allowing to carry out queries and to analyse the database. Donor's associations have received an applicative web (EmoDonor‐InsielMercato) in order to monitor donation activities in real time. In addition, another web application (DonUp‐InsielMercato) links the requirement of blood units to donor's convocations. For safety reason, no information is saved in the programme, but if you need them the database can provide them.
Results
The total numbers of donors in 2013 was 25903 and in 2014 it was 25374, whereas donors making their first donation in 2013 were 3646 and in 2014 they were only 3186. In ours Transfusion Medicine Department in 2014 13712 patients were transfused 51469 units of red blood cells. In real time EmoDonor supplies active donors for 0 negative and active donors for the 0 positive blood group for the donor's voluntary associations. The same piece of information is available also to the donors with other blood groups. At the end, DonUp optimizes dynamics between donations and consumptions reducing the number of red blood cells unit expired in 2013 was 151 and in 2014 was 97.
Conclusions
The informatics systems are simple, quick, flexible and easy to use with common browsers by donors as well as doctors on blood bank in order to improve the management of the donors’ calls and the supply of important information for the decisional conclusion and programming. They are pivotal tools for clinical governance and patient blood management. In addition, each donor by mean of web technology can book his donation when it is necessary for the patient, but also when he/she is available.
Reference: http://www.QlikView.com.
P‐021
Introduction of Electronic Ordering and a Transfusion Summary Page Improves Workflow for Clinical and Laboratory Staff
Haberfield A , Miller K , Akers C , Webb A , Dauer R , Morgan S , Davis A
Alfred Health, Melbourne, Australia
Background
In 2012, to improve the practice of ordering blood products, we began introduction of electronic ordering (e‐ordering). An upgrade of the Laboratory Information System (LIS) in June 2014 resulted in the addition of the Transfusion Summary Page (TSP) as part of the Hospital Information System (HIS). It allowed clinical staff to check the availability of patients’ blood products in real time. Hospital‐wide use of e‐ordering occurred in July 2014.
Aims
To assess the uptake and ease of use of the TSP by clinical staff through survey completion
To assess the impact the introductions of these systems have had on the laboratory workflow by monitoring phone calls to the Blood Bank through phone log data.
Methods
Transfusion Summary page (TSP).
Built as part of the LIS upgrade in June, addition of this page to the HIS allows clinical staff to view transfusion status of their patients in real time. Information provided includes the patient's Group & Save status (date/time of expiry, specimen availability), phenotype/special requirements, recent haematology results. It also shows the crossmatch status and availability of other blood products. The page keeps a transfusion history of all blood products dispensed to the patient for traceability.
Following addition of the TSP to the HIS and a four month intensive education period to nursing staff hospital‐wide, a survey was disseminated amongst randomly chosen high frequency and infrequent end‐users to ascertain uptake and use of this new page.
Phone logs were reviewed to assess average number of phone calls per week pre‐ and post‐introduction of e‐ordering and the TSP.
Results
Results from the survey showed that four months post implementation, 63% of hospital staff knew of the TSP and how to access it. 95% of those surveyed found this page helpful and of these, 40% felt they no longer needed to phone the Blood Bank to enquire about their patient's blood products prior to collection. However, 70% of staff identified that ongoing education would be desirable and 30% felt they still did need to phone the Blood Bank for blood product enquiries.
Phone logs showed that following introduction of these IT initiatives, there has been an overall average of 30% reduction in phone calls to the Blood Bank when compared to phone logs prior to these initiatives.
Conclusion
Preliminary results from survey and review of phone logs suggest that the implementation of e‐ordering and the TSP has been effective in improving workflow amongst clinical and laboratory staff. Ongoing education is required and repeat survey to assess for any benefit from this education.
P‐022
Aiming for a Representative Sample: Evaluating Random Versus Non‐Probabilistic Strategies for Hospital Selection
van Hoeven LR1, Janssen MP 1, Koffijberg H 2, Roes KCB 1
1University Medical Center Utrecht, Utrecht, The Netherlands2MIRA Institute for Biomedical Technology and Technical Medicine, Enschede, The Netherlands
Background
A frequently encountered issue in research is that of selecting a representative sample from the study population. In literature the focus lies on random (probabilistic) sampling strategies, whereas in practice, random sampling of participants is not always feasible nor necessarily the optimal choice. Before putting a lot of effort in recruitment or data collection, it may be worthwhile to carefully consider potential sampling strategies. We illustrate a method for evaluating the effect of different sampling strategies on representativeness using the case of estimating blood use in Dutch hospitals.
Aims
The aims of the study are to: (i) investigate which sampling strategy is optimal for estimating national blood use; (ii) illustrate a method for evaluating various random and purposive (non‐probabilistic) sampling strategies.
Methods
In the case study we draw a sample of 12 hospitals (out of 89 in total), which should enable us to estimate blood use nationally. Five potential sampling strategies, both random and purposive, are simulated and evaluated: (i) only the largest hospitals; (ii) maximum variation (largest and smallest hospitals); (iii) random selection; (iv) regional variation (hospitals from each geographic region); (v) hospitals from two (out of seven) health care regions. The evaluation requires the a priori availability of a limited data set on population level in which a relation between a predictor and outcome exists that can be modelled. In this case, available data on hospital blood use and number of hospital beds are used. Simulations of each strategy result in different samples of hospitals, that are each used to fit a model that predicts blood use. The subsequent prediction errors are used to indicate the representativeness of the sampling strategy.
Results
The strategy leading to the lowest prediction error in the case study was maximum variation sampling, closely followed by random, regional variation and two regions sampling, with worst performance resulting from sampling the largest hospitals. Maximum variation sampling outperformed random sampling on both hospital and national level in respectively 85% and 76% of the simulations. Increasing sample size did not change the ranking of the strategies and led to only slightly better predictions, whereas a lower sample size increased preference for random strategies.
Conclusions
The optimal strategy for estimating blood use was maximum variation sampling. Using simulations it is possible to compare several probabilistic and nonprobabilistic sampling strategies on representativeness. The results enable researchers to make a more educated choice for an appropriate sampling strategy before data collection is started.
P‐023
Successful Creation of an Electronic Decision Support Tool to Educate Clinicians and Develop a Database to Monitor the Appropriate Use of Blood Components at Burton Hospitals Nhs Foundation Trust
Jeyachandran SP , Buchan J , Hopley A , Ahmad H
Burton Hospitals NHS Foundation Trust, Burton on Trent, United Kingdom
Background
In the UK 2.9 million blood components are issued every year and only 4% of the eligible population give blood. When used safely and appropriately, blood transfusion saves and improves patient's lives however it is not risk free as death and major morbidity can occur when things go wrong with transfusion. The national audits show that 15–20% of red blood cell transfusions and 20–30% of plasma/platelets transfusions are inappropriate.
One of the methods to restrict the inappropriate use of blood and blood component is through an electronic decision support tool to provide education at the point of requesting blood product with feedback message. The use of National Blood Transfusion Committee (NBTC) indicationcodes allow accurate audit
Aims
The principal aim was to create an electronic decision support system with feedback messages based on the selection of the indication codes for transfusion to assist with appropriate use of blood products.
Methods
Using our electronic patient record system (Meditech) a new custom defined screen wascreatedas a step in the blood ordering processto support decision making. The indication codes for the use of blood components recommended by National Blood Transfusion Committee were included as part of ordering process. The electronic decision support system allows us to integrate the codes in the ordering process. Feedback messages were programmed to provide information on the use of blood component.
To capture this data a functional database was created from the Meditech data repository application. This functional live database includes the reason for transfusion, patient's recent Hb level, indication codes, ordered by and other parameters required for monitoring the appropriate use of blood and blood components.
Results
We have successfully created a custom defined screen to help the clinicians make the right decision on the appropriateness of blood and blood component transfusion. A live functional database has been created to monitor the use of blood components. The data from this database will be used to provide feedback to other teams.
Conclusion
Decision making on the appropriate use of blood components is a critical process and should be based on the clinical findings, laboratory parameters and in line with national guidelines. We believe the use of newly created electronic decision support system as part of the blood ordering process can help clinicians to make the correct decision. The database will provide further insight in to the ordering behaviour of clinicians which we believe will help the Hospital Transfusion team to monitor the use of blood components.
Although this was created with Meditech electronic patient record system the same method and principle can be applied to other electronic patient record systems.
P‐024
IT Problem and Incident Management
Abbas MA
NBTC, Giza, Egypt
Background
Due to NBTS (National Blood Transfusion Services) IT (Information Technology) Automation project all over Egypt. Many IT Equipment will distribute in all RBTCs (Regional Blood Transfusion Centers) so we should have a method to handleit. Problem management is the process responsible for managing the lifecycle of all problems and IT is a category of services utilized by business. Thereare typically applications, infrastructure and Connections that are packaged and offered asservices by internal IT staff or external service providers.
Aims
Problem and incident Management can produce a set of data to generate some indicators which can use inmeasureof how a business is progressing. IT managers use these indicators to find out how they are performing and what they can do to make the IT operate better overall. Owner also use them when making decisions about a IT's prospects for future success. The primary objectives of Problem Management are to prevent problems and resulting incidents from happening, to eliminate recurring incidents and to minimize the impact of incidents that cannot be prevented, finallyis getting everything back to normal operation as soon as possible after an incident or problem.
Methods
Using one of NBTS IT forms for ‘Notifying Problems and Incident’ and register it to be categorized into three categories [SW (Software), HW (Hardware) and Connection (LAN, INTERENT)], during the interval of 14 workdays. Then make Pareto Analysis.
Results
• Collected Samples were 55 Notifications.
• Some of The result can be shown below as Table 1and 2 (Pareto Analysis).
• Table 1, Table 2.
• The average of incident |problemNotification = 3.92 per day.
• Average Time to fix≈ One day (The same day).
• (Hint: Except one incident not fixed yet).
• Most Complain Departments are [CB (Compensation), WH (WAREHOUSE) and HR (Human Recourses)].


Summary/Conclusion
On the long run by applying Problem and Incident Management techniques we can add many values likeHigh availability, Highproductivity of businessand reduction in cost for resolving repeatedincidents. Also, if we daily logging problems | incidentwe can categorize and prioritize events, when General Managers need to hire more IT staff we can hiring based on the gab or most cause of problems. when they decide to purchase new IT Equipment they can avoid most trouble devices. NBTC IT Staff recommend thatduring implementation of BMS (Blood Management system) or in Testing stage we should register all events (Problems, incident and errors) in software with expanding software word to be include all BMS Modules (Donation, Serology, Component … and so on) then calculate some useful KPIs (Key performance indicators) like percentage of incident related to, SW, HW, LAN, WAN Internet, Security, Average fixing time and downtime and … so on. In future this will build a knowledge base for supporting, reduces cost for maintained, deduct time to recover and generate indicators to help us in decision making.
1.3 Cost/Effectiveness
P‐025
Extracorporeal Membrane Oxygenation Transfusion: A Small Price to Pay for a Life‐Saving Treatment, or a Costly Uncontrolled Practice?
Sharpley FA1, White M 2, Dransfield M 2
1NHS, Oxford, United Kingdom2NHS Royal Brompton Hospital, London, United Kingdom
Background
Bleeding and coagulopathy are major problems in extracorporeal membrane oxygenation (ECMO). The foreign surface of the extracorporeal circuit promotes a hypercoagulable state demanding the need for systemic anti‐coagulation.
Despite careful monitoring, excessive anti‐coagulation can occur and multiple blood products are then required to maintain adequate flow through the ECMO circuit.
The excessive demand placed on the blood bank was one of the major concerns with extending the provision of ECMO from paediatric to adult patients (1) and yet this life‐saving modality has been instituted in over 10,426 adult patients since the early 1980s (2).
Aims
Whilst there is an awareness of the overall financial cost associated with the provision of ECMO (3), few have quantified the cost associated solely with transfusion.
This retrospective analysis aimed to quantify the blood products received by adult ECMO patients at a single tertiary cardiopulmonary intensive care unit in London over a two‐year period.
Methods
A database was created including all patients accepted for Veno‐Venous ECMO at the Royal Brompton Intensive Care Unit between 2012 and 2014. The number of days on ECMO was recorded and the patient status at time of discharge from the acute hospital. Each patient's blood product usage was retrospectively calculated including a breakdown of the number of units of packed red blood cells, platelets, fresh‐frozen‐plasma or cryoprecipitate. The cost of this was calculated using national 2014/15 blood prices.
Results
Sixty‐eight patients, 30 female and 38 males, underwent ECMO during this time. The average ECMO duration per patient was 10.8 days. Patients were transfused below defined parameters (Hb < 100 g/l; platelets < 100).
A cumulative total of 1999 blood products were used in this time, equating to: 16.6 packed red cells, 8.4 platelets, 3.1 fresh frozen plasma and 1.2 cryoprecipitate per patient. The overall associated cost was £ 259,426 or £3815 per patient.
Summary/Conclusions
This analysis has highlighted the significant resources and financial costs associated with blood product transfusion in ECMO patients. It must be remembered that these costs relate to transfusion alone and the overall cost of ECMO is considerably higher.
With 54.4% patients alive at time of discharge from the intensive care unit, ECMO remains a life‐saving treatment that we believe can still be justified for patients where all other hope is lost.
A reduction of the cost associated with transfusion is not impossible. Standardised transfusion criteria need to be developed for this cohort of patients. We hope that this analysis will encourage other centres to push for universal transfusion criteria.
References
1. Butch SH, Knafl P, Oberman HA (1996) Blood utilization in adult patients undergoing extracorporeal membrane oxygenated therapy. Transfusion. 36(1):61–3
2. Extracorporeal Life Support Organization http://www.elso.org/index.php?option=com_content{00AMP00}view=article{00AMP00}id=68{00AMP00}Itemid=435
3. Mishra V, Svennevig JL, Bugge JF (2010) Cost of extracorporeal membrane oxygenation: evidence from the Rikshospitalet University Hospital, Oslo, Norway. Eur J Cardiothorac Surg. 37:339–42
4. Ang AL, Teo D, Lim CH (2009) Blood transfusion requirements and independent predictors of increased transfusion requirements among adult patients on extracorporeal membrane oxygenation – a single centre experience. Vox Sang. 2009;96:34–43
P‐026
Simple Changes in Phenotyping Can Lead to Great Savings
Loo L , Chan C , Phang P , Ng N
Health Sciences Authority, Singapore, Singapore
Introduction
Commercial antisera are used to detect presence or absence of specific blood group antigens. They are mainly used in the Red Cell Reference Laboratory of Blood Services Group (BSG) to confirm the identification of alloantibodies in patients and the antigen‐negative status of donor blood for transfusion to patients with corresponding antibodies. The cost of rare commercial antisera used in phenotyping can be expensive and contributes significantly to the cost of transfusion in patients with antibodies.
Aim
Lean Six Sigma principles, a business management tool more commonly used in manufacturing industries, were applied in order to identify wastages, streamline and optimize the phenotyping process.
Methods
Phenotyping data between January and October 2014 was collated. Usage patterns and cost of commercial antisera used were systematically analyzed using DMAIC (Define, Measure, Analyze, Improve and Control).
Results
3069 cases of phenotyping were analyzed. 2655 (86.5%) of these were used to confirm the identification of alloantibodies in patients while the remaining 414 (13.5%) were used to determine antigen‐negative status of donor blood. Three most used antisera were commercial anti‐E (12.8%), anti‐c (9.3%) and anti‐P1 (8.4%). Further analysis using the Cause‐and‐Effect Diagram (Fishbone Analysis) revealed that while 57.3% of antisera was used for actual phenotyping of patients and donors, 42.7%, a much higher than expected percentage was utilized for quality control purposes. BSG's current standard operating procedures require both positive and negative QCs to be performed with each phenotyping case. However, this exceeds the international standards. Based on a recommended realignment of quality control frequency to once‐a‐day, total cost for commercial antisera could be reduced by 41.3% with potential savings of up to S$16,774. The three biggest reduction in costs were for anti‐D (66.1%), anti‐E (55.3%) and anti‐P1 (52.2%).
Conclusion
Lean Six Sigma methodology could be systematically applied to complicated processes such as phenotyping to reveal sub‐optimal practices and hidden costs. Recommendations can then be raised to effectively tackle these wastages and rightfully allocate precious resources without compromising international accreditation requirements.
P‐027
Abstract Withdrawn.
P‐028
The Application of ‘Lean’ to Improve NHS Blood and Transplant Platelet Concentrate Supply Chain
Edmondson D
NHSBT, Bristol, United Kingdom
Background
NHSBT created an operational improvement approach incorporating ‘lean’ (based on the Toyota Production System), focussing on the continuous elimination of waste such as inventory or overproduction, and the creation of value defined by the customer, using principles of pull and flow applied to processes. Operational improvement emphasises engagement and empowerment of frontline employees in problem solving and improvement, and is organised around a series of analytical, planning and improvement events.
Aims
NHSBT was facing steeply rising demand for platelet concentrates whilst needing to meet ongoing cost pressures. In order to meet this challenge the operational improvement approach was applied to deliver NHSBT strategy, which called for a customer focussed, integrated supply chain, addressing in particular product wastage including expiry of components.
Methods
The project consisted of a number of phases, following the ‘plan‐do‐check‐act cycle’, commencing in June 2012:
Current state mapping, data collection and information gathering, undertaken by a team leader and supported by a trained ‘lean’ facilitator.
‘Voice of the customer’ analysis in association with NHSBT Customer Services.
Value stream analysis, identifying waste and opportunities in the supply chain. This was sponsored by the Chief Executive and attended by a Blood Bank Manager and other senior stakeholders.
Future state mapping to determine ‘ideal’ and ‘to be’ processes.
Action plan and implementation consisting of a series of planning, design and improvement events over 18 months from November 2012 and involving operational staff and other stakeholders. This was targeted at each element of the supply chain, including donor recruitment, donation, manufacturing, distribution, stock management, storage and issue.
Benefits tracking.
Results
Sustained, statistically significant (t‐test P < 0.05) improvement in platelet component discard was achieved from February 2013, demonstrated through the application of statistical process control. Wastage fell from 9.99% to 8.16% (data to January 2015) and was comprised of a reduction in (i) platelets expiring before issue; (ii) failed apheresis platelet collections and (iii) buffy coat platelet wastage during manufacture. This created a saving against budget of £287K to end of March 2014 (baseline 12 months to November 2012), and recurring savings of over £440K per annum thereafter.
Additional benefits included:
Creation of information requirements and flow blueprint to support the adoption of a ‘sales and operations planning’ approach to the platelet supply chain.
Standard work in processes throughout the supply chain, including apheresis donor recruitment, collection, processing and stock management.
Creation of ‘pull’ based supply (rather than ‘push’) from point of issue through distribution, manufacturing and collection.
Summary
NHSBT successfully applied the principles of lean to address improvement opportunities identified through value stream analysis, and through a combination of targeted events and subsequent implementation of improved processes. This included directed collection by blood group, right sizing inventory and donor base, introducing improved pull and flow, together with reduction of defects (discarded components) in manufacturing and collection. This resulted in a sustained reduction in wastage of platelets, allowing NHSBT to meet demand at lower cost.
P‐029
Evaluation of Usability of the Ortho Vision™ Analyzer Using Industry Standards and Methods Established for Medical Devices
Casina TS , Warren K , Fanto-Holdaway PA
Ortho Clinical Diagnostics, Raritan, NJ, United States of America
Background
Instrument usability evaluation is a relatively new way to appraise a newly developed instrument's use by operators of the instrument in the laboratory setting. Studies to evaluate usability should be consistent with guidance that is provided by industry direction.
Aim
The ORTHO VISION™ analyzer is a new instrument designed to fully automate in vitro immunohematological testing of human blood using the column agglutination test. A multi‐site /multi person study was undertaken to evaluate the usability of the new instrument. An independent third party company was engaged to create a summative usability study, execute testing, evaluate results and provide the final output of the study. The overall objective of the study was to assure instrument design conformed to defined user needs and intended system use in actual use conditions. The usability tests evaluated the potential of use errors that could lead to patient injury, serious harm, therapy delays or suboptimal therapy.
Methods
Test methodology was developed in conjunction with guidance provided in: IEC 62366:2007 – Application of usability engineering to medical devices and the AAMI HE 75:2009 Human Factors Engineering Design of Medical Devices – Usability Testing. Usability evaluations were conducted at three laboratory settings. The study evaluated 5 areas of use of the system using standardized techniques to evaluate usability. The five tasks of evaluation included: System setup; Load samples, order tests and edit test results; Maintenance tasks; Respond to manual rack removed error and System handoff. Each task was evaluated for failure due to safety related concerns and failures due to test assistance required to complete the task. Fifteen individuals were engaged to perform the testing. All individuals were laboratory professionals that would fit the intended user profile. Each individual received 8 h of training in groups of 2 or 3 before the test plan was executed. Both objective test case execution observation and subjective post test case interviews were conducted.
Results
All tasks were completed by 14 of the test participants while one individual completed only 3 of the five tasks due to time constraints. There were no failures due to safety related use error. There were 12 failures that occurred due to a need for test assistance. The test pass rate was as follows: System setup (73%); Load samples, order tests and edit test results (73%); Maintenance tasks (80%); Respond to manual rack removed error (100%) and System handoff (93%). Interview feedback when asked to rate safety 1(least safe) – 7 (most safe), all tasks rated 6.7 or better while ease of use rated 6.1 or greater on a scale of 1 (difficult) – 7 (easy) for all tasks.
Conclusions
The multi‐site evaluation demonstrated that the ORTHO VISION™ Analyzer instrument showed a high degree of usability based on the results of testing by an independent testing entity. There were no safety related close calls observed and the Summative Validation was considered acceptable.
P‐030
Blood Utilization in Elective Surgery in a Tertiary Center in Lagos, Nigeria
Johnolabode SO1, Akinbami AA 2, Osikomaiya BI 2
1Babcock University, Lagos, Nigeria2Lagos State University College of Medicine, Lagos, Nigeria
Background
Blood transfusion remains a key component in the resuscitation of surgical patients. Shortages arising from a fall in supply, have contributed to the fact that blood remains very much a vital but limited asset in elective surgeries.
Aim
This study aimed to audit blood utilization in elective surgery in a tertiary institution.
Materials and Methods
A retrospective audit of blood usage from 1st January to 31st December 2013 was done using the patients’ wards, blood groups, type of surgery, the number of units requested, cross‐matched, transfused and returned units. The cross‐match transfusion ratio (CT) and the transfusion index (TI) were calculated. A statistical software package SPSS version 16 was be used for data analysis. The descriptive data was given as percentages.

Results
A total of one thousand two hundred and eleven surgical patients requested for blood for the year, and 2055 units of blood were cross‐matched. Only 17.32% of cross‐matched blood was used. The cross‐match to transfusion (CT) ratio was 2055/356 (5.77) while a transfusion index, the number of units used /number of patients cross‐matched was 356/1211 (0.29). Maximum Surgical Blood Order Schedule (MSBOS) for the year using Mead's criterion was 1.5 *Transfusion index = 1.5*0.29 = 0.43.

Conclusion
The values of 5.77, 0.29, and 0.44 for CT ratio, TI, and MSBOS respectively, derived in this study depicts inefficient blood usage. Blood ordering pattern needs a definite change and in surgeries with insignificant blood loss, only blood typing and screening should be done to prevent wastages.
P‐031
Crossmatching Test: Economic Impact in Scheduled Surgeries
Nunes C , Lichtner A , Costa C , Cardoso E , Plácido C , Barra A
Hospital Prof. Dr. Fernando da Fonseca, Amadora, Portugal
Background
In a time of shrinking budgets and the necessary push for increased efficiency that arises, we thought it would be useful to look at the request for Red Blood Cells (RBCs) that arrived to our service and to observe how they resulted into performed transfusions and the costs associated with them. According to the American Association of Blood Banks, a Crossmatch: Transfusion (C:T) ratio of 2 or less is appropriate for the surgical specialties requiring transfusions. When judging the economic costs associated with the incoming requests we looked only at the cost that is determined by law for each Crossmatching test. We did not look into other related costs. The Type and Screen (T&S) procedure takes up to 45 min. A subsequent crossmatch for the same patient takes only another 15 min. Our service receives the requests the day before the intervention and T&S all requests but in order to avoid waste and improve efficiency crossmatching is only performed after the decision of the transfusion medicine specialist.
Aims
Evaluating the efficiency of the transfusional program in the preparation of RBC Units for scheduled surgeries and its economic impact.
Methods
This work consists on a retrospective study of the requests for the scheduled surgeries that were made to the blood service of the Prof. Fernando Fonseca Hospital, during the period of August 2014, through January 2015. We studied the requests coming from Surgery, Orthopedics, Urology and Obstetrics & Gynecology.
Results
During this period there were 1685 RBC units requested, of which only 13% were transfused. We found that of the 892 interventions performed, 83% did not require transfusions. Owing to this and despite the best efforts of the Blood Service to improve efficiency by crossmatching only 43% of RBC units requested, the C:T Ratios were high (average: 3.28; max: 4.43), leading to a waste of 2.728€ in this period. Of all crossmatched units only a third was transfused.
Summary/conclusions
Changing the requesting pattern and behaviors of the several surgical specialties is paramount if we are to reduce expenses and improve the efficiency and safety of blood transfusions in our hospital.
P‐032
Effects of a 4‐Weeks, Individualized, Supervised Physical Therapy & Exercise Program on Hemophilic Patients
Elgamal SA
NBTS, Giza, Egypt
Background
Physical therapy is one of the foundations in the treatment of hemophilia. Haematological treatment alone is not sufficient to prevent and treat musculoskeletal bleeding. Coupling this treatment with sedentary lifestyle in such patients often leads to problems associated with inactivity, such as decreased strength, balance and coordination. In addition to the main problem of joints hemo‐arthrosis, these problems promote instability and changes to the joint loads, leading to appearance of new bleeds and increased joint damage. In many cases, surgical intervention, including full joint replacement is required. A physical therapy program can help prevent and manage bleeds and other musculoskeletal problems, contributing in reducing the need of surgical interventions.
Aims
Considering the limited access to factor replacement treatment in developing countries this study was designed to examine the efficacy of applying a professionally designed physical therapy and exercise program to patients with bleeding disorders. Further, comparing the results with the existing literature regarding physical activity & exercise in hemophilic patients to determine their possible benefits & recommendations. The scientific literature reviewed up to July 2010. Searches were performed in the WOS (Web of Science), Pubmed, Sportdiscus and Scopus databases using the following keywords (physical therapy, exercise, haemophilia & hemophilia).
Design
A single‐group, pre/post‐test clinical design was applied to Egyptian patients visiting the therapeutic unit in the Egyptian NBTS. All patients receive cryoprecipitate as treatment before every session. The physical therapy program included: Ultrasonic (pulsed‐mode, 1.5 w/cm2) on the target joints, electrical stimulation and individualized gradual active exercises. Target joints were (knee, ankle and elbow joints).
Methods
Thirty‐three patients (4 females, 29 males; 7–57 years of age) with mild to severe hemophilia A were enrolled in the study performed in physical therapy clinic in the Egyptian NBTS. Pre/post program measures included target‐joints’ muscles strength, joint range of motion, joint and extremity circumference. Each patient was prescribed a 4 weeks individualized program (three times per week). Twenty six participants (79%) completed the program.
Results
Pre/post‐program data were analyzed by paired t tests for all participants who completed the program. No exercise‐induced injuries, pain, edema, or bleeding episodes were reported. Significant improvements occurred in joint motion and strength, with no change in joint circumference.
According to the literature, the benefits of regular exercise and physical therapy for hemophilic patients are numerous and cover various physical and psychosocial aspects as well as other perspectives more directly related to the musculoskeletal symptoms. In respect of hemorrhagic symptoms and their after‐effects, exercise can decrease the frequency of bleeds, loss of bone mineral density and hemophilia‐related joint damage.
Conclusions
A professionally designed, supervised, individualized physical therapy and exercise program decreases risks of joints destructions, thus decreases the rate of joint‐replacement surgeries, and improve functional abilities & lifestyle of bleeding disorders patients in developing countries, where patients only receive on‐demand treatment (not prophylactic) due financial limitations.
1.4 Training and Education
P‐033
Blended Learning Model for Regulated Training in a Blood Center
Howard R , Orta-Gerber A
OneBlood, Inc, Fort Lauderdale, United States of America
Background
OneBlood, Inc. provides an innovative training and education model for regulated blood center staff. The Regulated and Business Application Training (RBAT) team replaced traditional on‐the‐job training with a blended learning model incorporating eLearning, simulation, and field training components.
Aims
The program's model provides standardized, compliant, and measurable training and education to approximately 2500 staff in a wide geographic area. RBAT possesses a dedicated training workforce, independent from manufacturing, to deliver a quality training experience. Trainers travel to staff reducing trainees’ travel costs and time away from home. This approach fosters a focused and stress free learning environment. Individual trainees progress through the program in a self‐paced manner via independent evaluations.
Methods
The training consists of basic, simulation, and field training components. Basic training provides self‐paced eLearning‐training modules delivered via a learning management system. Curricula include job‐related technical concepts, acknowledgement of standard operating procedures, and an introduction to the blood establishment computer system and business software basics. Electronic software captures the completion of basic training modules for the staff's training records. Following basic training, staff moves to simulation training, which provides realistic content focusing on applying knowledge gained in basic training. The staff practices technical skills, experiences real life work situations, and develops critical thinking skills in the training centers. OneBlood created six (6) realistic, simulation‐training centers in key locations across the company. The training centers house a blood center environment with a functional mobile bus for phlebotomy, a donor center with apheresis equipment, a blood manufacturing and distribution area, and a transfusion services laboratory. The training centers allow staff to learn in a safe and low stress environment with no operational distractions. Progression to field training is dependent upon individual performance. Field training provides on‐the‐job experience for building confidence, developing rapport with colleagues, practicing customer service, and perfecting technical skills.
Results
Preliminary data for 40 Donor Service Specialists (DSS), completing the new training model from June to November 2014, demonstrated that the average number of days in basic, simulation, and field training were 14, 15, and 18, respectively. Average time for new program completion was 7.3 weeks vs a 12‐week legacy program. We experienced a 42% reduction in training time, saving $2388.00 in costs per employee. Within the first six‐months of training model completion, ten (10) of the 40 DSS employees were issued 18 quality events. In comparison, 15 DSS employees, completing the last wave of the legacy program, were issued 25 quality events. The difference in the number of staff receiving quality events between the two programs was statistically significant (Chi‐square = 17.0505, P = 3.6E–05, P < 0.05). The new model reduced the number of quality events, saving $1462.00 in investigation time alone.
Conclusions
The blended learning model reduced time in training and decreased the number of quality events when compared to the legacy program.
P‐034
Improving Transfusion Education and Competency Assessment for UK Doctors
Graham JE1, Narayan S 2, Pendry K 3
1Health Education North West, Manchester, United Kingdom2Pennine Acute Hospitals NHS Trust, Manchester, United Kingdom3NHS Blood & Transplant/Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom
Background
Transfusion in the UK is safe, however, errors involving medical staff in the decision making or prescribing of blood products continues to occur. This is despite three yearly competency assessments being required by all staff clinically involved in the blood transfusion process.
Aims
This qualitative research aims to better understand how doctors are currently educated in transfusion medicine. To explore how the delivery of transfusion education could be improved and to examine the role of competency assessment in transfusion practice for this population.
Methods
Focus groups were held with healthcare personnel to explore views and experiences concerning education and competency assessment in transfusion medicine. Doctors from all disciplines and stages of training were included, in addition to personnel with practical experience of transfusion education. Participant contributions were audio‐recorded, transcribed and categorised into current education, ideal education and competency assessment. Analysis of recurrent themes created specific guidance on when, what, who and how to deliver transfusion education. Focus groups continued to be held until no new themes were emergent from data analysis.
Results
A total of eight focus groups were held involving 53 participants. Healthcare personnel included medical undergraduates through to consultant doctors, plus advanced nurse and transfusion practitioners. It was clear from the focus groups that formal transfusion education for doctors is most commonly delivered on‐line as part of mandatory hospital (Trust) induction and is viewed as a ‘tick‐box exercise’. In the clinical setting, education of junior doctors occurs through cascade training from senior clinical or nursing staff.
Recurrent themes from focus groups highlighted the following recommendations
• Deliver transfusion education to undergraduates, first year doctors (FY1) then every 3 years to all clinicians.
• Mandate transfusion education and deliver in ‘protected time’ away from clinical practice.
• Focus on the practicalities of transfusion and tailor to the stage of training and clinical role of the recipient.
• Deliver education face‐to‐face by a good educator who is knowledgeable in transfusion and understands the recipient's clinical role.
• The Hospital Transfusion Committee should be responsible for quality assuring Trust‐wide delivery of transfusion education. Ensure transfusion resources and guidelines are easily accessible.
• Establish a nationally recognised, multi‐disciplinary transfusion course, to addresses the principles and theories of transfusion medicine. Use small‐group, case‐based teaching/discussion and simulation training e.g. for acute transfusion reactions, massive haemorrhage management, to promote inter‐professional collaborative learning.
• Incorporate clinical competency assessment into the national course.
• Outside of this setting, attempting competency assessment was not seen to be beneficial, other than in practical skills such as prescribing for junior doctors.
Conclusion
Within the UK, the current method of e‐learning in transfusion medicine is ineffective. There is significant scope to improve the way transfusion education is delivered to doctors and other healthcare personnel. This could be achieved by adopting a nationally recognised, multidisciplinary transfusion course, which would employ face‐to‐face teaching tailored to differing learner styles. Combined with clinical competency assessment, this course would enhance delivery of Patient Blood Management and optimise safe transfusion practice.
P‐035
‘Qualified Apheresis Nurse’ Certification by The Japan Society of Transfusion Medicine and Cell Therapy to Promote Safety and Efficiency in Apheresis Through Encouraging Learning
Ikeda K1, Iseki T 2, Yamamoto K 3, Okuyama Y 4, Kanamori H 5, Matsuzaki K 6, Muroi K 7, Ohto H 8
1Okayama Red Cross Blood Center, Okayama, Japan2Chiba University Hospital, Chiba, Japan3Saitama Medical Center, Kawagoe, Japan4Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Tokyo, Japan5Kanagawa Cancer Center, Yokohama, Japan6Japanese Red Cross, Tokyo, Japan7Jichi Medical University Hospital, Yakushiji, Japan8Fukushima Medical University, Fukushima, Japan
Background
In 2013, peripheral blood stem cells were collected in 1500, 692 and 14 harvests from patients and from related and unrelated donors, respectively. Platelets and plasma were collected by the Japanese Red Cross Society (JRCS) in 873,092 platelet and 648,703 plasma harvests for transfusions and plasma fractionation products at 158 donation sites.
Aims
In order to promote safety and efficiency in apheresis through encouraging nurses practicing apheresis in Japan to learn and to be certified as Qualified Apheresis Nurse (QAN) by the Japan Society of Transfusion Medicine and Cell Therapy (JSTMCT).
Methods
A council for the JSTMCT‐certified QAN was formed consisting of 7 experts on apheresis, all of whom were JSTMCT members and some of whom also represented Japan Society for Hematopoietic Cell Transplantation, Japan Marrow Donor Program or JRCS. The council defined the eligibility and the procedures for application, pre‐examination lectures, examination, registration and renewal of the certification as follows: Apheresis was defined for the JSTMCT‐certified QAN as collection of blood components with aphereis devices excluding plasma exchange. To be eligible, nurses were required to (i) work at hospitals or blood donation sites operated by the JRCS; (ii) have more than 3 years of clinical experience at hospitals or blood donation sites; (iii) have experience of more than 1 year and 10 cases of apheresis; (iv) get recommended by director of nursing and by physician in charge of transfusion at hospitals or director of blood donation sites. The certification was supposed to be renewed every 5 years by acquiring defined credit points. A curriculum was formed, which included basics for transfusion and cellular therapy, colleting blood components at hospitals and blood donation sites, therapeutic apheresis, hematopoietic stem cell mobilization and transplantation, nursing for patients and donors in apheresis and Japanese guidelines for transfusion and in‐house cell processing. Applications were accepted in August and the applicants were notified of their eligibility in October. A 5‐h pre‐examination lecture and a 2‐h examination were held in November. The examination consisted of 80 mostly multiple‐choice questions. Applicants were required to answer at least 60% of the questions to get certified. The results of the examination were notified to the applicants in December with a questionnaire survey about the attitude of the applicants concerning the QAN‐certifying system.
Results
During the 5 fiscal years through 2014, a total of 210 nurses, of whom 82 and 128 belong to hospitals and blood donation sites, respectively, applied and a total of 203 nurses, of whom 76 and 127 belong to hospitals and blood donation sites, respectively, were accepted as eligible for the examination. Average scores at the examinations were 67.3%, 76.5%, 79.0%, 77.9% and 77.2%, respectively from 2010 to 2014. A total of 197 nurses, of whom 73 and 124 belong to hospitals and blood donation sites, respectively, passed the examination and were certified. Post‐examination questionnaire survey showed the applicants were strongly motivated and desired continued education after certification.
Summary/Conclusions
We introduced nurse‐certifying system to promote safe and efficient apheresis, for which post‐certification education was desired by applicants.
P‐036
Towards Evidence‐Based Transfusion Education: Evaluating Team Based Learning (Tbl) in the Postgraduate Setting
Graham JE1, Narayan S 2, Pendry K 3
1Health Education North West, Manchester, United Kingdom2Pennine Acute Hospitals NHS Trust, Manchester, United Kingdom3NHS Blood & Transplant/Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom
Background
There is global recognition of a need to improve transfusion education to further improve the safety of transfusion practice, with education a recognised effective method to improve appropriate blood use. Team‐Based Learning (TBL) is a small group, student centred, active teaching method that encourages cooperative learning. TBL has benefits both for the learner and faculty; helping students develop auxiliary skills to knowledge acquisition, such as interpersonal and team working skills and enabling the faculty to deliver small group teaching to a large number of students within one room facilitated by a single expert. The new dawn of Patient Blood Management (PBM) provides incentive to deliver evidence‐based practice; although this must be supported by the development of evidence‐based educational methods to deliver transfusion training.
Aims
This research aims to critically assess, according to Kirkpatrick's levels of learning evaluation, the effectiveness of TBL as a model to deliver transfusion medicine education to junior doctors; and to ascertain whether TBL is a practical method to deliver the transfusion curriculum in the postgraduate setting.
Method
Transfusion education, mapped to the 2012 Foundation Programme curriculum, was designed to be delivered over four sessions (one TBL orientation session and 3 transfusion sessions) adhering to the seven core elements of TBL. This included preparatory reading, knowledge assessment questions with immediate feedback scratch‐cards, real‐life scenarios addressing knowledge application and peer evaluation. The TBL transfusion programme was delivered by a single tutor to a cohort of junior doctors during routine Foundation Year 1 education sessions. Evaluation of TBL methodology consisted of quantitative and qualitative assessment of student reaction to TBL, objective and subjective evaluation of knowledge acquisition plus analysis of team continuity and TBL logistics.
Results
A total of 48 junior doctors received education delivered by TBL with an average attendance of 2.5/4 sessions (range 1–4). Six teams were originally created, reducing to 5 teams with an average team membership of 5.85 doctors per session (range 4–7), taken from a pool of 9.6 (8–11) team members. This resulted in a mean overall team continuity score (total team members attending/potential team members) of 65% over the four sessions (individual session range 40–88%). Student reaction to TBL was positive, scoring an overall mean of 83%. Objectively, knowledge acquisition, demonstrated by Readiness Assurance Testing (RAT), showed improved team knowledge over individual knowledge, with mean team RAT score exceeding maximum individual RAT score in 90% of cases. Subjectively, knowledge acquisition showed significant improvement, including increased confidence dealing with acute transfusion reactions, although student confidence levels with regards to prescribing declined. The amount of time spent preparing for sessions correlated with enjoyment, subjective knowledge gain and clinical confidence levels. Preparation time was reported as ‘adequate’ or ‘excellent’ in 86% of anonymous feedback.
Conclusion
TBL is an enjoyable and effective novel approach to deliver transfusion education to junior doctors, particularly in those who prepare adequately for sessions. Team continuity in this student population is poor and this must be factored in when delivering TBL sessions within the postgraduate medical setting.
P‐037
Transfusion Training for Clinical Nurses and Proposal an Updated Curricula for Pregraduate Nurse Education
Solaz N1, Bayik MB 1, Emekdas GE 2, Uluhan RU 1
1Turkish Blood Foundation, Istanbul, Turkey2Blood Banks and Transfusion Society of Turkey, Istanbul, Turkey
There have been numerous special training activities for blood bank staff so far by international and national organizations since decades but very limited interest has given on the special training activities on transfusion practice for clinical nurses who have a key position in ensuring the safety of blood transfusion in the hospital setting.
Blood Banks and Transfusion Society of Turkey (BBTST) and Turkish Blood Foundation (TBF) focused on ‘Bedside Transfusion Safety by Clinical Nurse’ and started a special post‐graduate training project at 2008. Aims of this are project are listed as below:
1. Evaluate actual curriculums of different Nurse Schools on transfusion practice
2. Evaluate actual information level of clinical nurses on transfusion practice before training
3. Evaluate information level of clinical nurses on transfusion practice after training
4. Evaluate impression of the participant nurses on the efficiency of the training program
5. Discuss the ideas of participant nurses on better clinical transfusion practice depending on their experiences
One day training program has below listed topics:
1. Legal and administrative aspects of transfusion
2. Blood group types
3. Blood components; definition, preparation, storage and transport
4. Pre‐transfusion competency tests
5. Transfusion indications of different blood components
6. Bedside transfusion practice
7. Transfusion complications
Lecturers were not only from BBTST and TBF but also selected from ‘Nurse Training Department’ of each hospital to get a higher identity by the participant nurses. Those selected trainers received special ‘training of the trainer’ program which was based on their lectures and given by BBTST and TBF trainers. Standard power point slides were prepared by BBTST and TBF. Pre and post tests were done to evaluate the accuracy of the training. A special survey also was done to learn the impression of the participants about the program and the lecturers.
More than 3500 nurses trained by this program. While percentage of successful nurses at pre training test was around 20% it was increased to around 98% at post training test which was the most objective evidence of the efficacy of this training program. Great favor and sympathy were given to this training program by the participant nurses and a major part of them stated that they were also recommending this training program to their colleagues.
Depending on this encouraging feedback from the field BBTST and TBF decided to continue and widen this training program and established new collaborations with other clinical organizations such as Intensive Care Society of Turkey, Society of Turkish Nurses, etc.
Final target of BBTST and TBF is preparing a proposal to Ministry of Health Education Department about: (i) Updating pre‐graduate nurse education curriculum on transfusion; (ii) Establishing a new position at hospitals as ‘Transfusion Surveillance Nurse’ like actually functioning ‘Infection Surveillance Nurse’.
P‐038
UK Skills for Health Collaboration to Develop a Blood Transfusion Training Passport
Dhesi AS1, Fulkes V 2, Hobson A 3, Cole S 4, Riley T 5, Nanuck J 6, Carr S 7, Coffey K 1
1NHSBT, London, United Kingdom2Guy's & St Thomas’ NHS Foundation Trust, London, United Kingdom3Royal Free London NHS Foundation Trust, London, United Kingdom4Princess Royal University Hospital, London, United Kingdom5Royal Brompton Hospital, London, United Kingdom6Queen Elizabeth Hospital, London, United Kingdom7St George's Hospital, London, United Kingdom
Background
Skills for Health (SfH) are a not‐for‐profit organisation and the Sector Skills Council for the whole UK health sector, licensed by Government. SfH major in workforce and organisational development. One of their aims is to raise standards in skills and training delivery.
For hospital Education Departments, that have elicited to follow SfH Training Frameworks, ensure their workforce are trained to the SfH UK Core Skills Training Framework, which include topics such as resuscitation, infection control, etc. SfH proposed the development of training passports to allow for previously achieved, recognised and standardised training to be transferable to other organisations.
Aim
To include a ‘Blood Component Transfusion’ subject guide into the SfH Clinical Skills Passport. As the passport is UK‐wide, national engagement was necessary. Inclusion of Blood Transfusion will support locally delivered blood transfusion training and ensure a minimum standard. This idea originally started following a discussion at The London Transfusion Practitioners (TP) group.
Methods
LearnBloodTransfusion e‐learning, existing regional passports and a regional training requirements survey were initially assessed and evaluated. Once a regional draft was developed SfH were contacted who worked with us to develop the final subject guide.
National subject matter experts were engaged for feedback.
Results
The Blood Component Transfusion (BCT) subject guide is currently available from the SfH website (http://www.skillsforhealth.org.uk) for reference/guidance and is not yet included in the process for Declaration of Alignment.
Due to costs, SfH were unable to launch the transferable training passport system. Instead the BCT subject guide will now be included in the next version of the SfH Core Skills Framework as an optional subject. It is hoped that the document will be live in March 2015.
SfH Core Skills Frameworks are available nationally for use but individual Trusts will need to elect if they use this as a resource with their Education Departments. Training records of staff registered as having completed training to the standard can be accessed via SfH.
Summary/Conclusion
It is hoped that the updated core skills framework will both raise the profile of transfusion training within Trust Education Departments and support TPs who have experienced reductions in allocated training time, to be able to deliver the minimum requirements that the learner need to know. It will not change the mode or style of delivery that TPs are currently using.
SfH have indicated that each of their subject frameworks are complemented with a dedicated e‐learning module. For blood transfusion a UK‐wide e‐learning package, LearnBloodTransfusion, already exists and is well recognised. SfH evaluated LBT and felt there was a gap in training availability for healthcare support workers requiring relevant and appropriate knowledge of transfusion in order to fulfil their roles. SfH will work with TPs to develop e‐learning for these staff groups to host on their own platform.
The impact of this collaborative working will not be realised until a number of hospitals adopt and roll out the core skills framework including the BCT subject guide.
P‐039
Creation of a Program of ‘Wet’ Workshops in Immunohematology (IH): The Indian Immunohematology Initiative (III) Experience
Perkins T1, Hamilton JR 2, Johnson ST 3, Combs MA 4
1Indian Immunohematology Initiative, Evanston, IL, United States of America2Red Cross Blood Services, Detroit, United States of America3Blood Center of Wisconsin, Milwaukee, United States of America4Duke University Medical Center, Raleigh, United States of America
Background/Aims
The III conducts programs based on the premise that hands‐on (‘wet’) training is most effective for teaching basic and intermediate methods of blood group antibody detection and identification. Lecture‐based instruction is most effective in association with hands‐on experiences in countries where there is limited clinical training provided in current educational programs. Another premise is that an IH laboratory must employ tube testing, even if non‐tube methods are available. Finally, it is known that case‐based training works effectively to establish both theoretical and practical understanding.
Methods
Learning objectives were developed to include basic skill in agglutination testing, use of the indirect anti‐human‐globulin test (IAT) and RBC panels to detect and identify antibodies, QC of IH reagents, and investigation of basic ABO discrepancies and autoantibodies. Potential workshop hosts were identified through networking at a local professional meeting (Indian Society for Blood Transfusion and Immunohematology, ISBTI). Workshop hosts are responsible for promotion of the program, participant selection and registration, and local arrangements. Equipment has been donated or purchased second‐hand, and reagent RBCs and sera have been donated by a US manufacturer (IMMUCOR™, Norcross, GA). Antibody‐containing donor plasma has been provided by multiple blood centers. Participants receive workbooks including worksheets, panel cell antigrams, and procedure summaries. Participants complete a course evaluation. Workshops have been funded by donations and ISBT grants.
Results
A standard 5‐day course of laboratory (wet) exercises has been established, including preparation of 3–5% RBC suspensions, grading agglutination reactions using diluted plasma, identification of single and multiple alloantibodies, daily reagent QC, and investigation of an ABO discrepancy and a warm autoantibody including elution and autoadsorption. This program is followed by a 1‐day case‐studies seminar open to local blood bank professionals who are invited to present cases with the faculty.
Initial workshops were associated with ISBTI meetings. However, this accommodated only 2 to 3‐day events, necessitated site‐to‐site transport of equipment, and depended on meeting‐organizer priorities. The model has evolved into 2 blood centers serving as fixed sites, each with a complete set of equipment and a commitment to hosting an annual workshop. Workshops have included between 12 and 25 participants with 2 faculty, but experience shows 15 participants works well based on typical classroom sizes, equipment, and faculty‐student ratio. Short lecture presentations precede analysis of each specimen, and supplement the workbooks.
The total number of workshop participants to date is 183 including 72 technical staff and 111 physicians at various levels of training. Evaluations have been extremely positive, an average of 4.9 on a scale of 1–5 with 5 being the highest.
Conclusion
Feedback indicates the need for much additional instruction including workshops in other areas of blood banking practice. Based on experiences with 14 workshops since 2006, it is clear the need for this type of instruction in the developing world is vast, and that this model is scalable.
P‐040
An Improvement in Transfusion Practices Through Continuous Education for Clinicians
Patidar G
Adlakha Medical Centre, Amritsar, India
Background
In view of development of new programs and practices in the field of transfusion medicine, blood centers need reliable methods for educating hospital staff to reduce the chances of error and hence frequency of transfusion reactions. To find out the root cause of the problem, first step is to analyse the blood requisition methods by the clinicians followed by educating them about the correct methods of sending requisitions for blood and blood components. A completeness of transfusion request form shows logical use of blood and blood components.
Aims
1. To analyse incomplete transfusion request forms from July 2014 to December 2014.
2. To study the impact on completeness of request form after change in the format of form and introduction of continuous medical educational (CME) for the clinicians.
Methods
A total of 6894 request forms received from the month of July 2014 to December 2014 at Blood Bank Adlakha Medical Centre, Amritsar were evaluated for completeness of the following fields:
1. Second identification (CR No. or Father/Husband Name)
2. Diagnosis
3. Pre transfusion hematological parameters
4. Quality and quantity of blood component required
5. History of previous transfusion and adverse transfusion reaction
6. Urgency of transfusion
7. Medical officer name and signature
8. Phlebotomist name and signature
We introduced a new transfusion request form which included additional fields for second identification (CR no. or father/husband name) which were missing in previous form. A series of CMEs on ‘Safe Transfusion Practices’ was organized for hospital staff from 1st August 2014 and improvement in clinicians’ behavior was analysed.
Results
1. A total of 60.83% requests were incomplete during the study period. Of these 91.42% were in the month of July, which decreased to 48.76% in the month of December 2014 with a total improvement of 42.66%.
2. Incomplete second identification (91.03%) and phlebotomist signature (75.27%) were the most common incomplete fields in the month of July 2014. In the month of December 2014, an improvement in incomplete second identification was observed (12.8%) however phlebotomist signature was still a major incomplete field (45.19%)
3. A statistically significant (P value = 0.004, paired ‘t’ test) improvement in completeness of forms was observed.
4. On the basis of information regarding history of previous transfusion and adverse transfusion reaction, a total of 108 antibody screening were performed using microcolumn gel technique and anti‐D was found in 4 cases, anti‐C in 2 cases, anti‐S and Anti‐M each in one case.
5. We were also able to increase use of blood components compared to whole blood

Summary
Results of medical audit and the expertise of blood center personnel can be used as a basis for developing a highly targeted educational program, which will have impact on clinicians’ behavior.
P‐041
Abstract Withdrawn.
P‐042
Training of Staff in Provision of Better Quality Service for Apprehensive ‘Difficult’ Donors. Improvement of the Blood Establishment Image
Antoniewicz-Papis J , Lorek M , Rutkowska M , Poplawska D
National Blood Centre, Warsaw, Poland
Maintaining a safe and adequate supply of blood and blood components depends on numerous factors one of which is an appropriate donor‐oriented attitude of the blood establishment (BE) staff. While blood donation campaigns impact on donor recruitment, donor retention is largely affected by provision of high quality donor service in a friendly BE environment.
The aim of the paper was assessment of the need for BE staff training and evaluation of the impact of such training on the BE image and increment of donor recruitment.
In the 2013–2014 period 8 training courses were organized, all addressed to 3 professional groups of BE staff:
• Donor registration staff,
• Physicians responsible for donor qualification and blood/blood component collection,
• Staff responsible for sample collection and for collection of blood and blood components
Lecture topics were based on donor complaints and queries as well as a survey of problems generated in direct contact between donors and BE staff.
A total of 360 BE personnel participated in the training program, including 90 registration staff, 90 physicians, and 180 personnel responsible for blood/blood component collection. Following the training, participants were given a survey on perceived training benefits, need for further training, and topic‐contribution to future sessions. The survey was also addressed to BE management staff.
The survey indicated success; the program met the expectations of about 99% of participants, 95% found the provided instructions helpful in every day work, 93% declared improvement in donor relations. Nevertheless, staff‐donor relations are still marked with emotional tension, pre‐dispositions, differences in interpretation. Detailed data is presented on the poster.
Major problems emerging from analysis of donor complaints are: long waiting pre‐donation time, post‐donation refreshments, accessibility of blood collection sites (more mobile units, fewer stationary donation sites), deferral for behavior‐related reasons.
Donors were frequently doubtful as to reasons for temporary and permanent deferral, questions in the donor‐questionnaire, loss of work‐day.
Major problems reported by the BE staff were mostly related to aggressive and highly emotional donor behavior, disregarding staff directions, excessive donor demands, donor self‐righteousness, differences in interpretation of some guidelines.
Knowledge from training sessions was regularly disseminated to other BE employees in form of briefings or hand‐outs. In‐training courses were also organized.
The participants could also suggest topics which they felt should be discussed. Some of the key topics were: coping with stress, how to communicate deferral, team work.
The management observed the following training‐outcome: more effective everyday work, fewer donor complaints, increment of donor recruitment, development of problem‐solving skills, improved staff self‐assurance, higher donor‐satisfaction, improved BE image and mounting donor‐trust.
Both participants and BE management recognize the need for implementation of regular training, at least once a year. Development of such training programs is ongoing.
P‐043
Abstract Withdrawn.
P‐044
Abstract Withdrawn.
P‐045
Abstract Withdrawn.
P‐046
How to Improve Pre‐Donation Interview Harmonization?
Py JY, Barnoux M , Sapey T , Leclerc C , Dehaut F
EFS Centre‐Atlantique, Orléans cedex 2, France
Background
Unlike many countries, pre‐donation interview is still done in France by a physician. In a very next future, nurses will begin to do it also. Some part of their training will consist of work in pairs with a physician. Considering the huge variation of practice among physicians, as shown by the dispersion of their individual exclusion rate, there is a need for new harmonization methods at once for them today and for nurses tomorrow.
Aims
We have chosen to work on a simulation tool of a serious game kind. As for every method of training in medicine field, the main problem is to identify the good practice without any doubt. Even if you have an official repository, there's always a degree of individual interpretation. So we've tried to build a consensus method to identify the good practice and understand deviances.
Methods
Some of our physicians choose to work on a part of the interview, e.g. management of the blood pressure measure. They create an electronic questionnaire of 10 relevant short cases which is proposed to each participant. They have to choose to accept or refuse donation, but they have the possibility to explain their choice and their doubts. The initial physicians group receives all the results, including these explanations, and proposes a synthesis about good practice and reasons of misunderstandings.
Results
We always found a clear majority choice. For the example of the management of blood pressure, the case the most indecisive was 70% for donation ‘accepted/mostly accepted’ vs 30% for donation ‘denied/mostly denied’. A very interesting point is that, even when there is a strong majority position (as 96% for one case), there is always a high level of response with a commentary (29% for the same case). It proves that choices are more complex than it seems, and a great benefit can be get out of such questioning in order to understand medical reasoning.
Summary/Conclusions
We believe that such a method is able to help us to harmonize medical reasoning, which is a prerequisite of the development of our simulation tool. We've planned to resubmit our questionnaires after a while so that we can measure our harmonization gain and its durability.
1.5 Risk Models, Standards and Regulation
P‐047
A Statistical Approach to Detect Test‐Seeking Behaviour Among Repeat Blood Donors
de Vos AS1, Lieshout-Krikke RW 2, Slot E 2, Janssen MP 3
1Sanquin Research, Amsterdam, The Netherlands2Department of Blood‐Borne Infections, Sanquin Research, Amsterdam, The Netherlands3University Medical Centre Utrecht, Utrecht, The Netherlands
Background
Following infection risk behaviour individuals may donate blood in order to determine their infection status. Such so called test‐seeking behaviour can compromise transfusion safety. Instances of test‐seeking are difficult to substantiate, as this depends on the willingness of the donor to admit to such behaviour during either the pre‐donation interview (leading to deferral without testing) or post‐test counselling. However, manifestation of test‐seeking behaviour in a population of repeat donors might be determined using statistical inference. This novel approach was applied to the Dutch repeat blood donor population.
Aims
To study how statistical inference can be used to detect test‐seeking behaviour among blood‐donors.
Methods
Donation intervals of all Dutch blood donors who acquired HBV (N = 18), HIV (N = 8), or HCV (N = 1) infections in 2008–2013 were compared to those of uninfected blood donors (N = 587,322). Test‐seeking behaviour following increased infection risk may influence the timing of donations. The intervals between donations which test positive for infection and the preceding negative donations would then differ from a random subset of all observed inter‐donation intervals, weighed by exposure time. Therefore we compared the cumulative distribution of the last intervals before positively tested donations and the cumulative distribution of all time‐weighed donation intervals, sorted by their length. We tested the difference between these two distributions using a Kolmogorov–Smirnov test.
Results
Two patterns of donation intervals among infected donors might indicate test‐seeking behaviour: (i) relatively short donation intervals due to high motivation to obtain test results, and (ii) relatively long donation intervals, as individuals several months or years from their last donation, who would otherwise not have returned to donate, will donate to obtain test results following an episode of risk behaviour. Despite a tendency for overrepresentation of short donation intervals among both plasma and whole blood donors who acquired infection, these differences were not statistically significant (P‐values of 0.31 and 0.45 respectively).
Summary/Conclusions
We have illustrated how statistical methods can help identify test‐seeking behaviour in a population of blood donors. In the Dutch setting there is insufficient proof of test‐seeking behaviour among repeat donors. This may be due to the low number of infected individuals during recent years.
P‐048
Is It Necessary to Shift to an All Apheresis Strategy for Platelets? Differences in the Residual Risks for HIV, HCV and Hbv in Different Types of Platelet Concentrates in Germany
Offergeld R , Ritter SE , Hamouda O , van der Heiden M
Robert Koch Institute, Berlin, Germany
Background
In Germany, approximately 40% of all platelet concentrates are pooled platelets made from four whole blood donations. A shift to an all apheresis supply for platelets is currently discussed to further reduce the small residual risk of transfusion transmitted infections by minimizing the number of donor exposures.
Aims
We compared residual risk estimates for pool and apheresis platelets using German donor vigilance data to define the possible impact of a shift to an all‐apheresis strategy for the tested infections.
Methods
We used an advanced incidence rate/window period model to estimate and compare residual risks due to HIV, HCV and HBV window period donations for pooled and apheresis platelets in Germany. Our model incorporates reported interdonation intervals before a positive donation and therefore reflects the look back procedures used in haemovigilance. We took data from the German National Blood Donor Surveillance System for the years 2006–2012 for the mathematic model. During this time 30,903,463 whole blood donations and 1,278,623 platelet apheresis donations were made. Among these, 832 whole blood and 19 apheresis repeat donors were found to be confirmed positive for HIV, HCV or HBV.
Results
The donor populations of whole blood and apheresis donors differed in age, gender, catchment area and interdonation interval. Platelet apheresis donors were more often male, young and donated in urban areas and donated more frequently than whole blood donors. The residual risk of an infectious pooled platelet concentrate due to a window period donation per million PTC is estimated to be 2.08 (95% CI: 1.98–2.18) for HIV, 1.39 (95% CI: 1.30–1.48) for HCV and 4.28 (95% CI 4.14–4.43) for HBV. The residual risk of an infectious apheresis platelet concentrate per million is estimated to be 1.70 (95% CI: 1.24–2.23) for HIV, 0.93 (95% CI: 0.65–1.05) for HCV and 6.92 (95% CI: 5.88–8.18) for HBV. The resulting ratio for the risk estimates between the two types of PCs (ATC res. risk/PTC res. risk) is not statistically significant for HIV (0.82, 95% CI: 0.60–1.08), showed a significantly lower residual risk for HCV in apheresis platelets (0.66, 95% CI: 0.45–0.77) and a significantly lower risk for HBV in pooled platelets. (1.62, 95% CI: 1.36–1.93).
Summary/Conclusions
Our findings do not support calls for a shift to an apheresis platelets‐only policy in Germany. The reported short interdonation intervals for some confirmed positive apheresis donations increased the estimated residual risk, especially for HBV with a relatively long diagnostic window period. Therefore, an increase in the minimal interdonation interval for apheresis platelets could be discussed to further increase transfusion safety.
P‐049
Donor Health: The Influence of (Previously) Diagnosed Medical Conditions on Donor Deferral
Prinsze FJ , de Kort WLAM , van den Hurk K
Sanquin Research, Amsterdam, The Netherlands
Background
Donor deferral is an unwanted aspect for donor and blood banks. Donor InSight (DIS) is a cohort study aimed at gaining insight into the characteristics, health and motivation of Dutch blood and plasma donors. In the Netherlands general practitioners (GP's) are the gatekeepers of the health system. A visit to a specialist occurs in general after a referral by the GP. In general 72% of the population have an average of 5.4 contacts with their GP per year, 43% of the population had a contact with a specialist in a year (2009) and about 47% has a chronic disease (Statistics Netherlands). A separate chapter of the DIS questionnaire is dedicated to these aspects of donor health.
Aim
The aim of this study was to investigate the health status of donors who had a (previously) known medical condition (as diagnosed by a physician) and whether they had a higher risk of deferral.
Methods
In 2007–2009 the questionnaire was sent to 50,000 donors. In total 31,388 (63%) responded and returned the questionnaire. Medical conditions were assessed by asking whether donors (previously) had one of 20 defined conditions as diagnosed by a physician. DIS data were linked to data from the blood bank information system (EProgesa, MAK) on deferrals in the next 5 years after completion of the questionnaire. Deferrals were grouped in five categories: (i) medical conditions; (ii) infectious disease, vaccination, travel and risky behaviour; (iii) Hb level and blood pressure; (iv) pregnancy related and (v) administrative and other reasons. The chi‐square test was used to evaluate the first results.
Results
The preliminary results showed that almost 30% of the donors contacted their GP in the three months prior to filling out the DIS questionnaire. Treatment by a (specialist) physician(s) in the past year was reported by 6172 (20%) of the donors. Donors with a previously diagnosed condition had more GP contacts (40%) and treatments by specialists (24%) than donors without a medical condition (23% and 16%) (P < 0.05). In the DIS population about 47% indicated to have at least one of the 20 specified conditions at a certain time; 21% of the donors used prescription drugs during the last 6 months.
The donors with a previously known medical condition had a slightly higher (P > 0.05) risk of deferral within the next year (30% vs 26%). However the first deferral after completion of the questionnaire was more often (P < 0.05) for medical reasons: 17% within the next year and 28% were deferred after 5 years (without 11% and 20%).
Summary/Conclusions
Donors with a previously known medical condition have a slightly increased risk of deferral compared to donors without such a medical condition. However it can be concluded that the donor population – even donors with a previously diagnosed condition – is relatively healthy and have little health care consumption as compared to the general population. Although donors with a diagnosed medical condition are deferred slightly more often on medical grounds it seems unnecessary to prevent them from donating.
P‐050
Inspection of Blood Banks in the State of Azad Jammu & Kashmir, Pakistan
Waheed UW1, Ahmed NA 2, Zaheer HAZ 1
1Ministry of National Health Services, Islamabad, Pakistan2AJK Blood Transfusion Authority, Department of Health, Government of AJK, Muzaffarabad, Pakistan
Background
The state of Azad Jammu and Kashmir having an area of 13,297 square kilometres and population of 4.25 million, lies in the north east of Pakistan and north west of India. The state is administratively divided into three divisions and ten districts. The healthcare coverage in the state remains inadequate. The blood transfusion services are an integral component of the health policy of AJK. The services are provided by public sector hospitals and an ever increasing number of NGO sector blood banks, many of them catering to the needs of the thalassaemia patients. One of the key issues in the blood transfusion services of AJK is weak regulation and governance of the system. The government of AJK passed an act in 2003 to regulate the blood system but only recently has the implementation of this act started in earnest.
Aim
To implement the AJK transfusion of safe blood act XVI of 2003.
Methods
The AJK BTA made an announcement in the newspapers for registration of blood banks. However, a very poor response was received for registration. In October 2014, the AJK authority approached the islamabad blood transfusion authority for assistance and support in the regulation of blood transfusion services in the state. The IBTA coordinated with AJK BTA and shared its developed and tested technical tools. IBTA then assisted in the planning and conduction of blood banks inspections in the districts of Mirpur, Bhimber, Kotli and Sudhanoti in the first phase of inspections. The blood banks were identified through an informal, unplanned mapping conducted with the assistance of local contacts. In addition, snowball sampling exercise was also applied for this purpose.
Results
On the whole, the standard of service delivery was found to be extremely poor and unsafe. Except for two blood banks, none of the blood banks were manned by qualified doctors or technicians. The private sector blood banks were run by unqualified and poorly technicians. The concept of haemovigilance was non‐existent. Out of the 31 blood banks visited, only two blood banks relatively fulfilled the minimum criteria and were granted licenses for one year. Two centres were directed to cease blood banking functions. The remaining 27 blood banks were placed on probation and informed about their deficiencies and remedial actions. They will be re‐inspected within the next three months.
Conclusion
An inspection report has been prepared and submitted to the department of health including recommendations which if adopted in earnest will significantly improve the blood safety standards and generate public confidence in the health care system in AJK. The remaining six districts in two divisions will be covered in the phase 2 and 3 within the next two months.
P‐051
Attributable Risk of Perioperative Bleeding as a Complications of Preoperative Plasma Transfusion on Postoperative Outcomes
Ngufor CN , Murphree DM , Upadhyaya SU , Madde NM , Kor DJK , Pathak JP
Mayo Clinic, Rochester, United States of America
Background
Despite great advances in improving perioperative outcomes in recent years, bleeding is still the most frequent serious complication during or early after major surgeries. While preoperative plasma transfusion (PPT) has been found in many studies to significantly impart perioperative bleeding (PB), and PB is significantly associated with prolonged intensive care unit length of stay (ICU‐LOS), morbidity and mortality, the effect of PB as a complication of PPT on these outcomes has not been firmly established.
Aims
The goal of this study is to establish the relationship between PB as a complication of PPT on important postoperative outcomes such as ICU‐LOS, need and duration of mechanical ventilation (MV), and mortality using advanced machine learning (ML) methods.
Methods
Different from previous approaches to the estimation of treatment effects in transfusion studies typically based on generalized linear models (GLM) such as logistic or linear regressions, this study applies ML methods to estimate the attributable risk (ψ) of PB as a complication of PPT on postoperative outcomes. Two robust and consistent estimators of the risk are considered: the Doubly Robust Inverse Probability of Censoring‐Weighted estimator (DR) estimator and the Targeted maximum likelihood estimator (TMLE). These estimators directly adjust for known confounding effects of patient baseline characteristics measured at the preoperative period. ML methods considered include: generalized boosted regression machines (GBM), random forest (RF), extreme logistic regression (ELR), elastic‐net regularized generalized linear models (GLMnet), and random k‐nearest neighbors (rKNN).
Screening for study participation was performed using Mayo Clinic's perioperative datamart, a validated transfusion repository that captures demographics, disease conditions, laboratory test results, medications, operative and postoperative measurements and outcomes for all patients admitted to acute care environments. To be considered for study participation, patients must have met the following criteria: age ≥ 18 years, noncardiac surgery, and International Normalization Ratio (INR) of at least 1.5 in the 30 days preceding surgery. Between 2008 and 2011, a total of 1,234 patients were identified among which 140 patients were administered PPT. The effect of PB on postoperative outcomes was independently studied for both PPT and non PPT patients. The discussions in this study are based on the PPT population.
Results
Using 5‐fold cross‐validation training procedure, estimates of DR and TMLE from ML methods show that the effect of ‘no’ PB as a complication of PPT significantly reduces ICU‐LOS, hospital LOS and duration of MV (P‐value < 1 x 10−16). Similarly, the need for MV is reduced by about 10–23% and mortality risk by about 2–10%. Results for generalized linear models tend to be inconsistent with respect to the TMLE estimator.

Summary/Conclusions
Using modern machine learning methods not frequently applied in this area of research, this study obtained robust, consistent, and meaningful measures to quantify the effect of perioperative bleeding as a complication of transfusion of blood products that may be useful for transfusion related decision support systems, ICU resource allocation, and help researchers answer questions like ‘what and how big is the effect of bleeding on patient outcomes’.
P‐052
Health is Contagious: Risk Management
Mottola M , Giaquinto A , Bruno C , Brighel F , Arcopinto C , Vigorita E , Mininni V , Zuccarelli B
A.O.R.N.„Ospedali dei Colli„, Napoli, Italy
Background
Risk management (RM) born as an answer to the ‘crisis of malpractice’ occurred in the US in the early 70s. Only around the 80s, many programs were started RM defined as ‘an approach to improving the quality of care dedicated to the identification of the circumstances leading to damaging events and control of these circumstances’. The analysis of these events allows us to identify the ‘weak points’ in the chain that are responsible for damage identified. The prevention strategy easier the damage is to make visible the errors through the multiplication of controls or cross‐checks. The technique of R.M. more effectively consists of the design of secure systems, researching and mapping regularly possibility or probability of error and damage and prevent through redesign of the operations and organization.
Aims
It was decided therefore to implement a specific project to detect the perception of clinical risk in our hospital.
Methods
Our SIT has programmed the formulation of a plan of RM according to a model based on the clear identification of the strengths and weaknesses of the organization, responsibilities, tasks, resources and skills, development objectives to be pursued and actions consistent to be implemented, and, in particular aimed at a systematic involvement of the staff with respect to the issues dealt with, in order to foster a real ethical change compared to safety issues, based on the constant promotion of the culture of the latter. The R.M. had already been implemented in our SIT in 2009–2011 by adopting a proactive approach while with the current RM it was decided to adopt a governance model evolved, effective and efficient, which harmonized the methodological choices already in use. The project was organized into the following phases: Identification and analysis of the risk profile; Activation of a monitoring system; setting and application of preventive measures; Monitoring and evaluation of the results achieved. Also, included: Revision the product information files in reference Haemovigilance; Redefinition of procedures and operating protocols and compilation of new forms; Organization path information.
Results
The results of our investigation are as follows:19 sheets compiled reporting clinical risk (1 in 2012, 5 in 2013,13 in 2014); 18 blood transfusion reactions reported; Incident Reporting voluntary and anonymous 2; Detected nonconformity by the Transfusion Centre: 118 in 2012,166 in 2013,126 in 2014; Reduction of the number of units of blood components removed for poor storage, breaking, deadline: 303 in 2012, 239 in 2013,133 in 2014; 50% return of the sheets of transfusion occurred in 2012, 75% in 2013, 90% in 2014.
Conclusions
The project has had the great merit of having involved all the professionals of the SIT and is intended as a work in progress, requiring systematic review for the purpose of risk mapping ever deeper, only way for the realization of systemic activity and integrated management of clinical risk.
P‐053
Perceived Risk of Blood Transfusions. A Cross‐European Comparison
Merz EM , de Prof. dr. Kort WLAM
Sanquin Blood Supply, Amsterdam, The Netherlands
Background
During the past decades, blood transfusions have become an ever safer clinical procedure in developed countries. Improved infectious disease testing has led to a minimalization of risks for transfusion recipients. Still, the general public perceives the process of blood transfusion as risky, although differently across Europe.
Aims
The current study examines variation in perceived transfusion safety across European countries and seeks to explain it with individual and country factors. In doing so, we contribute to the literature in two ways. First, we investigate variation in perceived transfusion safety across Europe. In particular, we consider current perception of transfusion safety compared to perceived safety ten years ago. Second, and most importantly, we test whether certain individual and country level factors can explain this variation.
Method
We apply multi‐level modelling to individual‐level data on perceived transfusion safety collected in 22 countries in the 2009 special Eurobarometer. We consider individual demographic characteristics and country level factors. Variables at the individual level included gender, age, education, current partner status, parent status and income. At the country level, we included the United Nation Development Programme (UNDP) Human Development Index (HDI), a composite statistic of a nation's health (i.e. a long and healthy life), education (i.e. access to knowledge) and income (i.e. a decent standard of living). Due to its composite nature, the HDI reflects both a country's economic as well as human development.
Findings
Results were largely in line with expectations derived from risk perception and power and status difference theories. Generally, women, elderly, the lower educated and those earning lower incomes perceived heightened risk. Most of the variation across Europe was explained by the Human Development Index. Risk perception regarding blood transfusions was lower in countries with higher Human Development Indices, i.e. countries with higher average education, life expectancy and GDP.
Summary/conclusions
Perceived risks of blood transfusion vary strongly across social categories within countries and across countries in Europe. Country context (i.e. HDI) appeared to be an important factor in explaining part of this variation. This study is a first attempt to shed light on the complex interplay between individual and context factors in shaping risk perception with regard to blood transfusion across various European countries. Given the importance of public risk perception to policy making and shaping public agendas, work detailing when universal and when country‐specific mechanisms determine risk perceptions with regard to blood transfusions is a key agenda for health scientists and policy makers.
1.6 Blood supply management and utilization
P‐054
Phenotyped Red Cells – Order Fulfilment and Inventory Management
Powley TL , Ismay S
Australian Red Cross Blood Service, Kelvin Grove, Australia
Background/Aim
The Australian Red Cross Blood Service (Blood Service) has reviewed its process for phenotyped red cell order fulfilment and inventory management with the view to moving to a standard national process to facilitate a commitment to maintaining improved phenotyped inventory, age of issue and order fulfilment.
The relative frequencies of phenotypes in our population, historic phenotyped red cell order data and applicable Transfusion Guidelines (ANZSBT [1] and NPAAC [2]) have been utilised to determine national inventory holdings. To support this inventory holding, a new national testing strategy for phenotyping has been implemented.
Methods
All donors are tested for extended Rh (C, c, E & e) and K with selected donors being further phenotyped for Fya, Fyb, Jka, Jkb, M, S and s. This extended phenotyping has been restricted to group O and A whole blood donors only with preference given to R1R1, R2R2 and rr, the most probable Rh genotypes to maximise the utility of the phenotyped inventory. Extended phenotyping requirements are driven by a ‘pull’ system with testing requirements determined based on actual inventory levels and the age of the inventory.
The Blood Service has developed a reporting tool to measure actual inventory holdings against planned inventory, selecting components for the phenotyped inventory based on a hierarchy of relative frequency and the age of the component.
Results
This method for managing phenotype testing ensures that we maintain an inventory that includes some of the more challenging phenotype combinations, while balancing the age of the component with the phenotype. The deficit in inventory holdings for individual phenotypes will be used in conjunction with the relative frequency to determine the number of donors that require phenotyping.
Requests for additional or other phenotypes, including rare phenotypes, are referred to Red Cell Reference Laboratory and Pathologist for further discussion with the customer on appropriate options for patient support and how the Blood Service can assist in fulfilling these orders. Realistic and acceptable lead time for order fulfilment is agreed, depending on the work required to fulfil the order.
A national search tool has been developed to enable a simple search of the national inventory based on the phenotype requirements and has the ability to search the frozen rare red cells inventory. The tool also provides functionality to search for suitable donors with forward appointments (<7 days or <14 days) or generate a file of suitable donors for our National Call Centre to contact for appointments.
Conclusions
The changes and new tools are designed to streamline the process for ordering phenotyped red cells and simplify inventory management and order fulfilment, ensuring consistent and improved customer service.
1. ANZSBT Guidelines for Pre‐transfusion Laboratory Practice, 5th Edition, March 2007.
2. National Pathology Accreditation Advisory Council Requirements for Transfusion Laboratory Practice (Second Edition 2013) Guidelines.
P‐055
Postoperative Red Cell Transfusion in Elective Unilateral Primary Total Hip Replacement: An Audit Cycle Over 8 Years
Rajkumar A , Rossi S , King J , Marlow R , Gilmour I , George M , Bankes M , Nunn D , Shah Z
Guy's and St. Thomas’ NHS Foundation Trust, London, United Kingdom
Background
There is a drive to minimise allogenic red cell transfusion following elective orthopaedic surgery due to cost, scarcity of resources and associated morbidity. NHS Blood and Transplant completed a National Comparative Audit of postoperative red cell transfusion in elective unilateral primary total hip replacement surgery in centres across the United Kingdom in 2007.
Aims
This audit cycle, spanning 8 years, aimed to monitor local transfusion practices and introduce interventions targeted at minimizing the burden and risk associated with allogenic red cell transfusion.
Methods
The methodology and practice standards outlined by the National Comparative Blood Transfusion Audit were followed. Local data collection was carried out at Guy's and St. Thomas’ NHS Foundation Trust in 2007, 2009, 2012, 2013 and 2014. In October of each year an analysis of 40 consecutive patients undergoing elective unilateral primary total hip replacement was performed. Exclusion criteria included other joint replacement, revision surgery and bilateral surgery.
Results
Interventions implemented across this audit cycle included: (i) A comprehensive education programme delivered to junior doctors surrounding trust transfusion policy; (ii) Automatisation of referral of anaemic patients from the pre‐operative assessment clinic to the rapid access anaemia clinic; (iii) Agreement amongst orthopaedic team to avoid surgery with an international normalised ratio (INR) outside normal range; (iv) Encouragement of operative team to make use of autologous transfusions where possible.
In line with the practice standard, 100% of patients had a pre‐operative haemoglobin (Hb) check in all years of data collection. The proportion of patients undergoing elective surgery with a Hb < 120 g/l decreased over the 8 year period (19% in 2007 to 5% in 2014). Concordantly, post‐operative red cell transfusion rates fell, with 2014 representing the first year achieving no post operative transfusions [13% (2007), 4% (2009), 15% (2012), 5% (2013), 0% (2014)].
Excessive transfusions were defined as those where the post‐transfusion Hb > 100 g/l. There was a reduction in the proportion of transfusions deemed excessive, indicating improved use of resources [100% (2007), 100% (2009), 17% (2012), 0% (2013)]. In line with these findings the mean volume transfused decreased from a peak of 4 units in 2009 to 2 units in 2013.
Data on the use of autologous transfusions was collected in 2013 and 2014. There was an increase in the proportion of patients undergoing intraoperative cell salvage and associated autologous re‐infusion [13% (2013) to 25% (2014)], mean volume re‐infused 206 mls (2014). However there was a decrease in the use of post‐operative Bellovac drains and associated autologous re‐infusion [50% (2013) to 10% (2014)], mean volume re‐infused 399 mls (2014).
Summary
This audit cycle demonstrates the utility of simple interventions to reduce avoidable post‐operative allogenic transfusions. Much of this benefit is derived in the advantage of many patients undergoing successful autologous transfusion.
P‐056
Tackling Red Blood Cell Waste in Victoria, Australia
Bielby LJ1, Akers C 1, Jackie J 1, Hogan C 2
1Blood Matters, West Melbourne, Australia2Australian Red Cross Blood Service, Melbourne, Australia
Background
In Australia the importance of minimising unnecessary waste of blood and blood products is supported by the National Blood Authority (NBA) wastage reduction strategy 2013–17(1), the National Stewardship statement(2), and in the Australian Commission of Safety and Quality in Healthcare National Standards(3).
Nationally 712,122 red blood cells (RBC) were issued (July 2013–June 2014), with 191,850 (27%) in Victoria. All RBC waste accounted for 37,264 units (5.2%) nationally and 11,746 (6.1%) in Victoria. National 2014–15 targets set by Governments are 3–5% depending on the number of RBC units received at each pathology site.
To support these strategies the Blood Matters program established and funded a RBC wastage reduction project, commencing July 2014.
Aim
To assist Victorian health services to further reduce RBC waste to, or below the specified national target of 3–5%.
Method
Work commenced with health/pathology services with RBC wastage rates above national targets. Issues associated with waste explored, and stategies implemented as outlined below (Table 1).
Some of these strategies were guided by the South Australian Blood Moves project and NBA Managing Blood and Blood Product Inventory Guidelines for Australian Health Providers, a 10 step guide (4).
Results
Twelve health/pathology services were visited (February 2015) with many others contacted by phone/email. With intervention, overall Victorian RBC waste has now reduced from 6.1–5.0% (July 2014–Jan 2015). While individual wastage rates show variable improvement (e.g. 24–9% and 10–0%), thus there is still considerable work to be done.
A Victorian blood fridge map is under development. Currently 233 sites documented where RBC can be issued; with 184 blood fridges located across the state. Compliance rates are currently being determined.
Caption 1: Issues and strategies.

Summary
Moving RBC stock is paramount to enable appropriate inventory management and reduce expiry related waste. Sharing blood fridge compliance data is essential to move stock between sites. Simplifying procedures, using MBOS and improving inventory management practices are challenges to be overcome to reach waste targets. Ongoing collaboration with health/pathology services is crucial to continued success of the project.
Acknowledgements
Health/pathology services for their collaboration and willing involvement. Australian Governments, Australian Red Cross Blood Service, South Australian Blood Moves project/ BloodSafe and the NBA for their support.
References
1. National Blood Authority (2013) Wastage reduction strategy 2013–17.
2. Australian Health Ministers’ Conference (2010) Statement on National Stewardship Expectations for the Supply of Blood and Blood Products.
3. Australian Commission of Safety and Quality in Healthcare (2012).
4. National Blood Authority (2014) Managing Blood and Blood Product Inventory.
P‐057
Tracking Medical Blood Transfusion in Scotland
Forrester KJ , Bailie K
SNBTS, Edinburgh, United Kingdom
Background
Information on the clinical use of blood components at a population level is crucial to blood services in improving their ability to plan for and meet the transfusion needs of patients. To date, this type of information has been limited to ‘snapshots in time’ using cross sectional studies and audit. In Scotland, the Account for Blood (AfB) data mart was developed to provide routine, up‐to‐date national information on the use of blood components. In addition to providing age and gender specific transfusion rates for geographically defined populations, linkage of transfusion data to NHSScotland (NHSS) hospital episode data provides information on the clinical context in which blood components are used. This linkage is straightforward for surgical populations as they are readily defined, but transfusion in non‐surgical settings is harder to characterise, due to the complexity of medical patients, their care pathways and the interventions that they receive.
Methods
In a pilot study to identify efficient and accurate ways to classify medical patients and the clinical reasons for non‐surgical transfusion, red cell transfusion events from AfB have been linked by patient identifier and date rules to Inpatient, Outpatient, Neonatal, Maternity and A&E episodes of care at NHSS hospitals. Where more than one episode of care can be linked to the transfusion event rules have been defined to prioritise the allocation of the most clinically relevant episode. The allocated episode of care provides the clinical context, consultant specialty and diagnostic data to describe the reason for transfusion.
Results
The linked data for 2011–2013 contained a total of 480,197 red cell units transfused to 88,640 patients (average age 66 years; 43% male). 99% red cell units could be linked to an episode of care. The majority (90%) were linked to an inpatient episode and by the exact date rule (transfusion date between admission and discharge inclusive). 63% red cell units transfused were linked to medical specialties of which the top three specialties accounted for 75%: Haematology (35%), General Medicine (29%) and Oncology (9%). Within Haematology the top ten red cell‐using diagnoses accounted for 80% of units transfused and included malignant and non‐malignant haematological conditions, with the conditions transfused the most being Myelodysplastic syndromes (19%) and Myeloid and Lymphoid leukaemia (25%). General medicine included predominantly respiratory, renal and gastrointestinal diagnoses, with the conditions transfused the most being Iron deficiency (8%) and other anaemia (10%). Red cell use increased from 2011 to 2012 for Haematology (13.5%), General Medicine (4.9%) and Gastroenterology (2.7%) but decreased from 2012 to 2013 (–1%, –8.4% and –4.6% respectively). In contrast there was a steady and significant increase from 2011 to 2013 in red cell use for Renal Medicine (20.5%) and Cardiology (15.1%).
Conclusion
As surgical blood use has reduced (currently <20% red cell units transfused), the future demand for blood will be driven by the needs of ‘medical’ patients. The ongoing routine linkage of transfusion data with hospital episode data provides a systematic, automated and temporal system for tracking trends in blood use at a population level.
P‐058
UK Blood Stocks Management Scheme: Audit of Fresh Frozen Plasma Issued to Hospitals in England and North Wales
MacRate E1, dr Taylor C 2, mr Hyam C 3
1NHSBT, Bristol, United Kingdom2The Dudley Group NHS Foundation Trust, Dudley, United Kingdom3Blood Stocks Management Scheme, Colindale, United Kingdom
Background
The UK Blood Stocks Management scheme (BSMS) has provided inventory benchmarking for UK blood services and hospitals for adult red cells and platelets since 2001. Hospitals benchmark their stock levels and component wastage against other hospitals with similar clinical or geographical settings. Frozen (haemostatic) components are now being added to the BSMS portfolio. BSMS has collected issue data for the hospitals supplied by National Health Service Blood and Transplant (NHSBT) hospitals which has already revealed some variances in practice.
Aim
NHSBT issues frozen components to hospitals in England and North Wales. Focusing on adult FFP, we have carried out a baseline audit of NHSBT issues to determine the range of inventory practice and to highlight future challenges for blood services in the provision of this component.
Method
The number of adult units of FFP issued by NHSBT was analysed by ABO group for a 12 month period (Jan 2014–Dec 2014). Hospital specialities were analysed from the BSMS registration database. BSMS Inventory Practice Surveys provided stock inventory information (Table 1).
Results
246 hospitals received adult FFP during the audit period.
The number of units issued ranged from 1 unit to 14,900 units. 49 hospitals received more than 100 units per month (high‐users).
6 of the ‘high‐user’ hospitals received more than 20% group AB FFP, 2 received ~90% group A FFP, demonstrating a wide variation between blood group mixes, even in the high‐users (Table 2)
Discussion
The 2009 National Comparative Audit for FFP usage determined the common reasons for FFP transfusion were liver disease, massive haemorrhage, ‘other’ surgery, and cardiac surgery. Warfarin reversal was also included though prothrombin complex is now widely used. Transfusing a patient whose blood group is unknown should be rare, only occurring in patients with massive haemorrhage.
This variance is not seen in red cells issues. With the exception of very low users, red cells are used in the same group‐mix as the population.
The bias towards certain ABO group FFP in high‐user hospitals is not being driven by time‐expiry. Deskilling within the laboratories could be one reason, leading to the thawing of a ‘universal’ FFP to prevent errors.
With treatment of massive trauma requiring rapid provision of FFP, some laboratories have introduced ‘pre‐thawed’ units. The shelf‐life of thawed product is 24 h, impacting on wastage. We are aware that some laboratories pre‐thaw only GroupAB and demand for Group A FFP may also increase, as the massive haemorrhage guidelines (currently in draft) recommend this group for patients whose blood group is unknown. Our data suggests some hospitals may have already implemented this as a universal product.


Summary
Hospitals should be encouraged to give blood group matched FFP when possible. With the introduction of frozen components on BSMS, hospitals will be able to benchmark their usage and wastage with others and there will be better understanding of differences.
Blood services will need to understand the reasons for increasing demand for Group A FFP to inform Demand Planning activities.
P‐059
ISBT Working Party on Blood Supply Management (BSM WP) International Survey on Blood Product Wastage
Yazer H 1, Abraham S 2, Follea G3, Beckman N 2
1Pitt, Pittsburgh, United States of America2Australian Red Cross, Melbourne, Australia3European Blood Alliance, Amsterdam, The Netherlands
Background
Ensuring a stable and reliable supply of blood products (BP) is important for the support of modern medical and surgical therapies. Although minimizing waste is an important factor in meeting the BP needs for patients, studies to identify the extent and causes of blood wastage in hospitals are lacking.
Aim
To describe the methodology by which an international BP wastage survey was designed, conducted, and validated with a view to building a self‐assessment tool.
Methods
A survey questionnaire was designed to assess and calculate BP wastage rates in hospitals (from hospital blood banks to patients prior to transfusion). Five mechanisms of waste were identified, and waste was calculated as a percent of issue (WAPI method). The survey team consisted of members of the BSM working party from the USA, Australia, Europe and Africa. One team member was skilled in using an online survey tool. Collaborators in transfusion medicine, either working in hospitals or working closely with hospitals in blood establishments, from around the world were asked to respond to the survey questionnaire. A pilot survey helped to ensure that the survey terminology was applicable and comprehensible in various parts of the developing and developed world. To disseminate the survey, ISBT headquarters provided the names and email addresses of some thought leaders in different parts of the world. The participation of these thought leaders was then solicited by personal communication from the survey team members, and they were encouraged to distribute the survey link to their regional colleagues, within hospitals and blood establishments collaborating with hospitals. A short description of the survey, and a link to the online data collection tool, was published in the ISBT newsletter to reach additional members. The link to the online survey was sent to all identified colleagues via personal emails.
Results
Over the course of 6 months, there were 85 completed survey responses received. There were 53/85 (62%) responses from Europe, 18/85 (21%) from North America, 5/85 (6%) from Oceania, 4/85 (5%) from Asia, 3/85 (4%) from the Middle East, 1/85 (1%) from Africa, and 1/85 (1%) from South America. Some respondents provided data from multiple institutions, thus there were 117 institutions represented in this survey: 57/117 (49%) respondents were from academic centres, 24/117 (21%) from graduate teaching centres, 17/117 (15%) from limited or non‐teaching facilities, 11/117 (9%) from paediatric hospitals, and 8/117 from other types of institutions (specialist maternity or cardiovascular surgery or cancer care hospitals, respondents from healthcare systems with multiple types of hospitals, and respondents who provided combined data from entire regions or countries).
Conclusions
The online questionnaire used for this survey has been validated and proved to be effective in objectively measuring BP wastage in a variety of hospitals around the world. The analysis of the outcomes from the survey will help in identifying the causes of wastage, finalizing a self‐assessment tool and help to reduce BP waste in hospitals.
P‐060
Patient Blood Management Organisation and Activities in Seven European University Hospitals
Pendry K1, Manzini PM 2, Wikman A 3, Meybohm P 4, Fisher D 4, Borg-Aquilina D 5, Laspina S 5, Topholm Bruun M 6, Georgsen J 6, van Pampus EC 7, van Kraaij M 8, Geisen C 9, Mueller MM 9, Liumbruno G 10, Grant-Casey J 1, Babra PS 1, Zacharowski K 4, Seifried E 9, Follea G 11, Murphy MF 1
1NHS Blood and Transplant, Manchester, United Kingdom2Citta della Salute e della Scienza di Torino, Torino, Italy3Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden4University Hospital, Frankfurt, Germany5Mater Dei University Hospital, Msida, Malta6Odense University Hospital, Odense, Denmark7Radboud University Medical Center, Nijmegen, The Netherlands8Sanquin Blood Bank, Amsterdam, The Netherlands9German Red Cross Blood Service Baden‐Wuerttemberg‐Hessen, Frankfurt, Germany10San Giovanni Calibita Rome University Hospital, Rome, Italy11European Blood Alliance, Amsterdam, The Netherlands
Background
Patient blood management (PBM) is a multi‐disciplinary evidence based approach to the care of patients who might need transfusion. In 2014, the European Blood Alliance (EBA) facilitated the collaboration of seven University Hospitals to form the PaBloE (Patient Blood Management in Europe) group.
Aims
As a baseline for further work, the group undertook a survey of PBM organisation and activities in the seven hospitals.
Methods
A questionnaire was circulated to the PBM lead for each of the seven hospitals using SurveyMonkey™. The results were collected and analysed in Excel.
Results
The seven hospitals range in size from 953 to 3000 inpatient beds (median 1286), all providing a full range of services. The median number (range) of units issued by component was as follows: red cells 21,363 (12,109–50,089), platelets 5.735 (1376–8432) and plasma 4566 (2231–13,587). Six out of seven hospitals have a Hospital Transfusion Committee (HTC), which oversees safe and appropriate use of blood. Only two have a specific person allocated to lead PBM and three have staff with time allocated to PBM activities. Five provide transfusion training for doctors. The cost of blood is allocated to clinical users in five hospitals and data on blood usage is presented to clinical teams in three hospitals although in only one is it possible to analyse information at patient level. Audit of blood use is undertaken in three hospitals. Electronic ordering of blood is possible in three and in progress in three. The date and time of transfusion are recorded electronically in three and two hospitals respectively. Provision of the reason for transfusion by the requester is mandatory in five sites.
Figure 1 shows the availability of PBM measures in the seven hospitals. There is significant variation in the type of PBM activity available between hospitals. For example, most hospitals have a major haemorrhage protocol (MHP) and agreed triggers for transfusion. All hospitals have cell salvage available for cardiac surgery, whereas cell salvage is provided by three sites for obstetrics and five sites for orthopaedics. Tranexamic acid is used variably by type of surgery; it is used by six sites for cardiac surgery but only by four sites for orthopaedics. A single unit transfusion policy has been adopted by two sites for red cells and by five sites for platelets. In three hospitals, the laboratory staff are empowered to challenge requests.
Caption 1: Number of sites undertaking PBM activities.
The main constraints to the implementation of PBM were identified as: inadequate human resources and IT support (in particular lack of a database for benchmarking blood use), lack of engagement from clinicians and management, and pre operative pathways that do not allow adequate time for anaemia management.

Conclusions
To date, there has been variable implementation of PBM at the seven large European hospitals that are participating in the PaBloE project. The information collected from the survey will serve as a baseline for the design of activities to increase the uptake of PBM activities both in these hospitals and through the EBA to other hospitals in Europe.
P‐061
Abstract Withdrawn.
P‐062
Where Did the Blood Go? Blood Use at the University Hospital of Cologne
Behrens L 1, Störmer M 1, Hellmich M 2, Gathof BS1
1Transfusion Medicine, University Hospital of Cologne, Cologne, Germany2Institute of Medical Statistics, Informatics, Epidemiology, University Cologne, Cologne, Germany
Background
Blood products such as red blood cell concentrates, RBS, are precious and restricted due to their short lifetime and the unavoidable sustained clinical demand. Indices such as the C/T ratio (crossmatched RBC/transfused RBC) help to evaluate the quality of the process of ordering and the actual use of RBC. In surgical patients C/T ration should not exceed 2.5.
Aims
To prevent over ordering of blood products, which unnecessarily restricts resources for other patients or may even increase outdating, a standard ordering catalogue had been established >20 years ago and revised regularly. This study was to evaluate the actual use in comparison to the orders. Comparison of present and past data might disclose trends in certain medical subjects with respect to future demands in blood products.
Methods
Data were collected based on standard request forms for November 2013 at this hospital including RBC, platelets and fresh frozen plasma as well as age, gender, Haemoglobin‐, Thrombocyte count and the medical indication for the transfusion. Missing records have been accomplished via electronic health records in ORBIS® and data‐sets have been processed with Excel® and SPSS®. To indicate changes in certain fields, data was compared to previous studies performed at the same institute for the year 1998, for urology for 1989‐1991 too.
Results
2398 order forms were evaluated leading to 6874 crossmatched RBCs and 3040 transfused RBCs. 1658 RBC were ordered for surgical, 1382 for non‐surgical indications.
Cardiac surgery was the predominant surgical use for RBC. Bypass operation was the leading indication with 266 doses, 8.75% of the total use [266/3040] and heart valve operation (239/3040, 7.86%). The C/T ratio for these were within the recommended range (bypass = 1.9, valve = 1.8). In abdominal surgery (not‐classified abdominal surgery C/T = 4.46 [125/28], esophagus operation C/T = 5.4), higher results were observed. For colorectal (C/T 3.4) and gastric (C/T 3.0) surgery results were higher (C/T 2.0 and 1.6 in 1998) than in the previous study at our hospital.
In urology C/T was for cystectomy 3.6, for prostatectomy 5.6 (in 1989–1991 9.2 and 3.9; in 1998 2.3 and 3.8 respectively). Besides the surgical demand of blood products, there is also a high need in non‐surgical fields particularly hemato‐oncology; these data will be reported elsewhere.
Conclusion
In general most of the results concerning the C/T ratio were satisfying. Results indicate room for improvement regarding the ordering process of RBCs, for example in urology and visceral surgery. The comparison with previous data in some surgical fields showed that results had been better in previous years. This indicates a reduction in preoperative ordering of RBCs is possible. Continuous evaluation and education can improve the logistics of blood use.
P‐063
Blood Transfusion Practices in Obstetric Care at a Tertiary Referral Hospital in Zimbabwe
Nyashadzaishe Mafirakureva N 1, Mberi YT 2, Khoza S 3, Mvere DA 1, Emmanuel JC 1, Postma MJ 4, van Hulst M 5
1National Blood Service Zimbabwe, Harare, Zimbabwe2School of Pharmacy, University of Zimbabwe, Harare, Zimbabwe3Department of Clinical Pharmacology, University of Zimbabwe, Harare, Zimbabwe4Unit of Pharmaco‐Epidemiology & Pharmaco‐Economics (PE2), Department of Pharmacy, Groningen, The Netherlands5Department of Clinical Pharmacy and Toxicology, Martini Hospital, Groningen, The Netherlands
Background
Blood transfusions are an essential element of obstetric care and may have a role in reducing maternal mortality, if used appropriately. Monitoring of transfusion practices provides information on current and future needs of blood. It may also lead to rational use of blood transfusions. Identifying common obstetric complications requiring transfusions is also critical for informing the development of appropriate operational maternal health programmes necessary for preventing their recurrence.
Aim
To describe the demographic characteristics of blood transfusion recipients, and diagnoses associated with the use of blood and blood components in obstetric care in Zimbabwe.
Study design and methods
Data on the characteristics of the blood transfusion recipients (age, sex, blood group), blood components received (type, quantity), discharge diagnoses and outcomes following transfusion (discharge status, length of stay in hospital), were retrospectively collected from Mbuya Nehanda Maternity Hospital, a tertiary referral hospital, during the period January 1, 2014 through December 31, 2014. Diagnoses were grouped into categories according to the block codes under Chapter 15 of the International Classification of Diseases, (ICD‐10).
Results
The total number of admissions in the maternity wards of the hospital was 12450. Of these, 416 (3.3%) received blood transfusion as part of obstetric care. The median age of the recipients was 30 years (range; 15 ‐ 55 years). Most of the recipients (n = 301; 84.3%) were in the age group 20–39 years. The majority of these recipients (n = 412; 99.0%) received a red blood cell transfusion. The median number of red blood cell units transfused per recipient was 2 units (range; 1–11 units). The majority of the transfusion recipients were diagnosed with complications of labour and delivery (n = 147; 35.3%), and pregnancy with abortive outcome (n = 141; 33.9%). The majority of the recipients required blood for the management of postpartum haemorrhage (130; 31.3%). The median length of stay in hospital was 4 days (range, 1–59) and in‐hospital mortality was 4.0%.
Caption 1: The distribution of transfusion recipients and blood components transfused over the ICD‐10 Chapter blocks.

Conclusions
The rate of blood transfusion is comparable to rates reported elsewhere in sub‐Saharan Africa. With most of the patients requiring blood for postpartum haemorrhage, further research is required to identify factors associated with excessive blood loss and the need for transfusions. The treatment of underlying anaemia, as a preventative measure, may also aid in reducing postpartum haemorrhage.
Keywords
blood usage, diagnosis, transfusion, International Classification of Diseases (ICD).
P‐064
Abstract Withdrawn.
P‐065
Taxonomy for Blood Product Supply Chain Selection
Cheng SY1, Rutherford C 1, Bailie K 2
1Heriot‐Watt University, Edinburgh, United Kingdom2Scottish National Blood Transfusion Service, Edinburgh, United Kingdom
Background
Management science theory has demonstrated how categorising products based on demand and product characteristics can help managers identify the best supply chain strategy for their products and customers. This paper presents the results of blood product segmentation carried out for the Scottish National Blood Transfusion Service (SNBTS) and proposes a taxonomy for supply chain selection in readiness for the centralisation of blood product processing in Scotland in 2017.
Aim
To develop a taxonomy for the selection of supply chain strategies appropriate for SNBTS products and customers.
Methods
A review of the literature revealed a range of taxonomies developed in different sectors to aid the selection of supply chain strategy. These findings informed the methodology adopted in this research. Transaction data at Blood Bank (customer) level for each of 111 blood products (including red cells, platelets, FFP & Cryoprecipitates) were exported from SNBTS databases into Microsoft Excel. Following data validation, single and multi‐criteria ABC analysis methods were applied using several criteria including: demand volume (transfused units); demand volatility; demand intermittency; product shelf‐life and delivery lead‐time to Blood Bank. Further statistical analyses were carried out to determine the nature of different product groupings before devising a classification system suitable for developing a differentiated supply chain strategy.
Results
Single criterion ABC analysis of aggregate transfused demand reveals a strong Pareto relationship with 19% of products (A category) accounting for 94% of total transfused demand. More detailed analysis using multi‐criteria methods reveals that 17% products exhibit statistically smooth demand (fast moving, low volatility). Only 2% of products are classified as Erratic (fast moving, high volatility) whereas 70% exhibit an Intermittent pattern (slow moving, low volatility). The remaining 11% of products have Lumpy demand patterns and are typically more difficult to manage as their high volatility and low demand makes forecasting less accurate requiring higher levels of safety stock. The risk of outage for these products is therefore relatively high and consequently products in this segment are prime candidates for risk pooling or postponement strategies. Combining these results with product shelf life, storage conditions and delivery lead‐time to customer location, a taxonomy for supply chain selection was designed enabling the allocation of each product to one of three supply chain types on a customer basis:
Caption 1: Matrix of product allocations for a large Blood Bank.

1. Make‐to‐stock (MTS): Lean processing and routine delivery direct to Blood Bank.
2. Deliver‐to‐Order (DTO): Lean processing and risk pooling; routine or special delivery as required to meet customer needs.
3. Make‐to‐Order (MTO): Agile responsive processing and delivery to meet individual customer needs.
The table below is an example matrix of product allocations for a large Blood Bank within a one hour lead‐time of the proposed central processing centre.
Conclusion
Supply chains must be carefully matched to product characteristics as well as customer requirement and circumstance (e.g. remoteness). This research provides a robust argument to support a proposed taxonomy for supply chain selection best suited to the needs of different customers (Blood Banks) and seeking operational efficiencies linked to product demand characteristics.
P‐066
Pre‐Operative Autologous Blood Donation is Still a Promising Tool. A 13 Years Single‐Centre Experience
Adamidou DA1, Markantonatou AM 1, Chouridi MC 1, Bartzoudis DB 2, Karafoulidou TK 1, Kokkinidou AK 1, Kyritsi KS 1, Kyrgianni SK 1, Sfiridou SS 1, Theodoridou ST 1
1Ippokrateio Hospital, Thessaloniki, Greece2Blood Bank, Katerini, Greece
Background
The limited blood resources together with the risks associated with allogeneic blood exposure has led in the past in the search of alternative transfusion practices. Pre‐operative autologous blood donation (PABD) has been widely adopted in certain surgical fields for the transfusion coverage.
Aims
To provide descriptive data based on our center's experience on the biological and demographic characteristics of patients that underwent PABD and to evaluate the technique's effectiveness.
Methods
We used the electronic blood bank record system (eΑΙΜΑ) in order to retrospectively collect data for a 13 (2001–2013) years period. Indication of PABD, number of RBC units required in advance, time between consecutive donations, laboratory parameters pre and post each donation were recorded. Based on the medical notes the perioperative total number of transfused RBC's (autologous + allogeneic) was measured. Any delay or cancellation of the scheduled surgery as well as reasons for autologous RBC's discard was taken into account. Any allogeneic break through transfusion or/and wastage of deposited autologous units was considered PABD's failure.
Results
Overall, 143 patients (112 females/31 males) with a median age of 45 (13–65) years underwent PABD for the coverage of 153 operations. These were mainly orthopedic (106) – 83 total hip or knee replacement and 23 disc‐fusions – plastic surgeries (32), urology (8) and others (7). 54 operations were done in public hospitals and 99 in private centers by the same surgical teams. The number of required RBC's was 2.4 (1–6) and patients managed to donate 2.1 (1–5) times in 7 (5–12) days intervals. For medical or practical reasons 50/143 patients did not manage to donate the entire number of prerequisite units. The starting hemoglobin level was 13.2 (12–16) gr/dl and was reduced to 11.5 (10–15) gr/dl just before the final donation.16 operations were delayed for 7 (2–49) days και 7were cancelled. Preemptive iron therapy was administered in 96 patients whereas 12 received erythropoietin. Only 3 minor adverse events were recorded during donation. In total 314 autologous units were donated whereas full data were found for the coverage of 102/153 operations for which 187autologous RBC units were deposited. Of those 106 were transfused corresponding to 43% wastage. Allogeneic blood was transfused in 10 patients (10%). 13% of the patients did not receive any transfusion. No other blood salvage techniques were implemented. Overall PABD was successful for the coverage of 60 surgeries (59%). Univariate analysis for the investigation of factors related to PABD effectiveness (patient's age and gender, starting Hb level, type of surgery, institution) did not reveal any significant association.
Conclusions
Based on our 13 years’ experience, PABD may be regarded as useful given that the failure rate‐ defined in a very strict way – (41%) is acceptable in comparison to literature. Further improvements in the technique together with exploration of a possible role of PABD in other surgical fields could offer an attractive tool in meeting increasing surgical blood needs.
P‐067
Review and Assessment of National Strategic Measures on Blood Donation in China Since 1978
Wang YW , L Li CQ , Liu ZL
Institute of Blood Transfusion, Chinese Academy of Medical Sciences, Chengdu, China
Background
Blood donation in China has gone through three different historical phases since 1978: paid, compulsory and voluntary non‐remunerated. During this quick and tough transition, Chinese government has undertaken strategic measures that have played an irreplaceable role and some of which have become milestone in the progress of blood donation.
Aims
To assess the effects of those strategic measures and provide references for blood management confronting with future challenges.
Methods
We summarized the strategic measures that have had marked impact on the legal framework, quality management and clinical blood use since 1978. Emphases were placed on several significant blood programs, the Voluntary Non‐Remunerated Blood Donor Program, Modification and centralization of blood service system, the nationwide nucleic acid testing (NAT) pilot program, Quality Management Program (QMP), Component Transfusion Program, and Capacity building Program. We collected and analyzed data associated with blood collection and blood safety following the implementation of these blood programs, trying to assess these strategic measures according to the revealed influence.
Results
It demonstrated that the concept of voluntary non‐remunerated blood donation has been widely accepted and supported in China since 1998, when the blood donation law was approved and gave rise to a new era of non‐remunerated blood donation. The rate of voluntary non‐remunerated blood donation has increased from 5.5% in 1998 to 100% in 2013 (Fig. 1). The blood test service mode changed from dispersed test in 1970s to centrally test in 2013. Nucleic acid testing (NAT) was nation wide advocated and implemented in the blood test in 2010, national coverage rate of NAT in blood banks system rose from 6.3% in 2010 to 32% in 2013, which further ensuring the safety of blood (fig 2). Percentage of component transfusion in clinical transfusion had a significant growth from 18% in 1989 to 90% in 2010. However, since the nation wide reform of performance appraisal system began in 2010, motivation of staffs working enthusiasm in blood service was reduced and the growing rate of blood collection volume decreased from about 12% before 2010 to below 1% after 2010.
Caption 1: The rate of voluntary non‐remunerated blood donation in clinical transfusion from 1998 to 2013.
Caption 2: Percentage of TTI in newly emerged HIV infections.

Summary/Conclusions
During the past decades, Chinese government has made countless efforts on strategic planning and adjustment for achieving blood safety on both national and regional level. Both achievements and negative effects are contributed by these strategic measures. In summery, significant progress has been made on both blood collection and safety since 1978. China is encountering many challenges with its blood donation system. The government still plays a responsible and irreplaceable role. Constant strategy assessment and further adjustment on blood safety is very much needed and will be effective to strengthen the country's blood service system.
P‐068
The Utilizattion Rate of Red Cell Concentrates in South Africa
Ngcobo MS , Tshilidzi M
South African National Blood Service, Durban, South Africa
Introduction
The medical services of any country could not exist without blood transfusions – life‐savers in many situations. The requirements of blood in a country depend on the population, health care structure, prevalence of conditions requiring regular transfusions, availability of surgical centers using modern sophisticated techniques, and awareness amongst clinicians regarding judicious use of blood.
Aim of the study
The aim of the study was to profile the usage rate of red cell concentrates (RCC) issued to South African hospitals (private and public) in 2013.
Methods
An analysis of the usage of RCC was done on all 335 public and 184 private hospitals that transfuse blood. The RCC usage was identified through records retrieved from Business Intelligence System of South African National Blood Transfusion Service and Western Cape Blood Transfusion Service.
Results
South Africa has a total population of approximately 51 770 561 people. Approximately 43 070 561 (83.2%) use public health care and 8,700,000 (16.8%) access private healthcare. There are 76,055 beds in public hospitals and 27,352 beds in the private sector. There are 1.77 beds and 3.14 beds per 1000 population in public and private healthcare sector respectively. Overall there are 2 beds per 1 000 population in South Africa.
A total of 849,250 RCC units were used in South Africa in 2013. Of these 552,370 (65.04%) were issued in public hospitals and 296,880 (34.96%) in private hospitals. The usage rate of RCC in South African public hospitals ranged from 0 to 25.18 units per bed per annum with a median of 3.76. The highest RCC usage per bed per annum was in tertiary hospitals with a median of 10.33 units per bed per annum. The district and regional hospitals had a median of 2.86 and 7.94 respectively. The red cell usage in the private sector hospitals was 9.49 units per bed per annum. There were 12.82 and 34.12 RCC units issued per 1000 population in the public sector and a private sector respectively. Overall there were 16.40 units of RCC per 1000 population issued in South Africa.
Summary and conclusions
The analysis clearly demonstrates that more red cell concentrates are transfused per patient in the private sector than in the public sector. The transfusion practice and hence RCC utilisation in the private sector mirrors that of the developed countries. The usage rate of 34.2 in the private sector approximates that of developed countries such as Spain, Denmark and Germany. The higher use in the private sector could among other factors be related to more sophisticated health care structure, prevalence of conditions requiring regular transfusions availability of surgical centers using modern sophisticated techniques in private sector as compared to the public healthcare sector.
The disparity in the red cell concentrates utilization rate between the public and the private sector could be a mirror of the inequalities between the public and the private sector.
P‐069
Economic Impact of the Reduction of the Ratio Red Cell Units Ordered Versus Issued in a Pediatric Hospital in Ecuador
Nieto MD
Pediatric Hospital Baca Ortiz, Quito, Equador
Background
The Blood Transfusion Service of the Pediatric Hospital Baca Ortiz (HPBO) started its activities in April 2011. On average, some 14,000 transfusions are undertaken per year, of which approximately 5500 are packed red blood cells (RBCs).
Aims
Evaluate the economic impact after implementation of strategies for the reduction of the rates of packed red cell units requested versus issued in HPBO from January 2012 to December 2014.
Methodology
A comparative analysis was done of transfusions of RBCs requested, issued and not returned by the wards, for the period 2012 until June 2013. All data were obtained from the management system for the blood bank in HPBO (Delphyn BB). Based on the analyzed results a process of sensitization and education was implemented with the prescribing physicians intended to reduce the rate of requested versus issued RBCs: briefings were held and analyzed and statistical data were shared with hospital units. The economic impact until December 2014 could be estimated and in January 2015 a comprehensive review of the strategies was undertaken for the two periods. In the assessment of the economic impact, only the costs of the reagents were taken into account, which were used during the pre‐transfusion testing to ensure compatibility of the RBC units. The main elements in the approach to induce changes in medical prescription of RBCs were training of prescribing doctors and systematic communication with them after confirmation of a planned surgery (within 24 h).
Results
In analyzing the two periods (January 2012–May 2013, June 2013–December 2014), the percentage of RBC units requested, following the implementation of the strategies described here above was reduced by 27%, by 3180 units. The percentage of RBC units not issued to the clinical wards was 48%, e.g. 2510 units. So, the total number of requested but unsent RBCs decreased by 65%. The economic impact was a significant decrease in reagent costs (33,885 USD or 85%).
Conclusions
Sensitization, education and implementation of simple actions resulted in a notable decrease in blood requests (RBCs) from the different clinical services, despite the fact that the total number of RBCs issued remained rather stable throughout the study period. It may be concluded that the real need for RBCs remained unchanged. According to the American Association of Blood Banks (AABB), it is considered that a C/T ratio (crossmatch/transfusion) of more than 2.0 usually indicates inappropriate medical prescribing of blood. The C/T ratio (a measure for efficiency in the context of blood transfusions, a quality indicator Q.I. for good clinical practice) decreased from 1.50(12,230/8339) to 1.18 (9050/7660). This study shows that the introduction of appropriate well designed interventions can reduce the number of unnecessary blood orders, without compromising the safety margin, making more efficient the management of blood stocks.
P‐070
Evaluation of the New Plasmapheresis System and Consideration of its Implementation
Svirnouskaya L1, Khulup Y 1, Novik V 1, Klimovich V 2, Novak V 1, Pasiukou V 1, Svirnovski I 1
1Research and Production Center for Transfusiology and Medical Biotechnology, Minsk, Belarus, Republic of Belarus2City Blood Center, Minsk, Belarus, Republic of Belarus
Background
In the majority of cases the incidence of TRALI can be reduced by the deferral of female donors or of female donors with a history of pregnancy. However, our studies of hospitals blood components supplying have revealed the shortage of male plasma for clinical use. In order to improve production of plasma is necessary to maximize male donors contribution in this component collecting. The same problem is significant for immune plasma collection especially when a peak of donor immune response takes a short time (about 10 weeks).
Aims
We aimed to evaluate the safety of proposed new plasma apheresis system with improved frequency of apheresis procedures.
Methods
Instead of the routine plasmapheresis practice at 2 weeks interval between plasma donations a new algorithm of apheresis procedures was developed. This new plasmapheresis system includes 3 cycles and 6 weeks intervals between cycles per year. Every cycle consists of 10 apheresis procedures at 1 week interval. The impact of moderate‐frequency plasmapheresis system on a donor status we evaluate on the base of changing such indicators as Hb, ferritin. whole protein, Ig G in donor blood at the beginning and at the end of every cycle. In parallel all data obtained in moderate‐frequency plasmapheresis donors (30 males and 30 females, group A) were compared with the same indicators in routine plasmapheresis donors (group B).
Results
During one year period in group B donors there was not any difference in indicators after every 10 plasma donations. In group A males and females demonstrated stable value of Hb and hematocrit as well as Ig G level. In contrast with male donors, in female donors group A it was found decreased ferritin level at the end of plasmapheresis cycles. Obtained indicators have been never less than normal values of ferritin (15–300 ng/ml). The same situation could be seen in female donors for whole protein level. It is necessary to underline that the values of serum ferritin and whole protein get back to previous value after 6 weeks interval between plasmapheresis cycles.
Conclusions
Our studies have demonstrated donor safety, i.e. 30 donations of 650 ml per year shown to be compatible with donor health, while the safety of new plasmapheresis system has been compared with routine practice. Implementation of moderate‐frequency plasmapheresis algorithm made possible to increase collecting of different kind of plasma for clinical use up to 30% (in comparison with routine plasmapheresis system). Male donors involving in the new plasmapheresis system gives the opportunity to minimize usage of female plasma and to decrease immune TRALI cases.
P‐071
What a Waste: Reasons for Blood Wastage Reported to the Singapore National Blood Center
Alcantara R , Leou K , Ang A
Health Sciences Authority Blood Services Group, Singapore, Singapore
Background
Blood product wastage is one of the key indicators to measure the quality of blood utilization and the efficiency of the transfusion service or blood bank. Since blood is a precious resource that must be carefully managed to ensure that each blood donor's contribution provides the greatest benefit to patients, blood wastage or discard should be avoided and kept to a minimum. With the continuous rise in the cost of blood and blood components due to the addition of new tests to improve blood safety, reduction of blood wastage is one of the greatest areas of potential cost savings for any blood bank.
Aim
The objective of the study is to determine the incidents or errors in Singapore hospitals that have resulted in blood component discard or wastage. The findings of this study will help us formulate corrective and preventive measures to reduce blood wastage.
Method
Information regarding blood wastage reported to Singapore's national blood service (Health Sciences Authority Blood Services Group) by seven hospitals from January 1, 2013 to December 31, 2013 were collected and analysed to determine the number of blood components wasted as well as the reason for discard.
Result
Discard rates for red cell, pooled platelets, single donation aphaeresis platelets, cryoprecipitate and FFP were 0.3%, 0.2.%, 0.5%,2.1% and 1.7% respectively during the period studied. A total of 757 units of blood components were discarded due to a variety of reasons as reported by the seven participating hospitals. 55.6% of the blood components wasted were FFP followed by red cell (31.2%). The most common reasons for blood component wastage were unit exceeded allowable shelf life (30.3%), blood bag leaks (18.1%) and request errors (12.4%). Other reasons for blood component wastage can be seen in table 1. The data also identified certain peculiarities wherein only ‘hospital G’ reported ‘haemolysis’ present in the blood bag (19 units) as a cause of discard while ‘hospital A’ reported significant higher rates (27 of 34 reported) of blood clots seen in the blood bags.
Caption 1: Reasons for Blood Component Discard.

Conclusion/Recommendation
Blood is a very important resource and hence should be used in a reasonable manner. The absolute amount of blood wastage is varied between hospitals as are the kinds of problems encountered. Despite the low discard rates reported, the absolute amount of discards within the year is substantial. Based on the data collected, more than half of the causes of blood wastage are preventable. Hospitals should routinely perform audits to monitor blood product wastage, investigate factors contributing to the wastage and establish systems to reduce wastage. Raising awareness of blood product wastage by educating physicians, nurses and other hospital staff involved in the blood transfusion process regarding best practices in blood component indications, product handling, transport and storage will result in less blood wastage. Hospital blood banks should also explore the possibility of implementing Lean Six Sigma process to reduce blood wastage.
P‐072
Continuous Improvement Approach (AC) Within Blood Products (PSL) Preparation Department (PRP) in Blood Bank of Pays De La Loire (PDL) France (EFS PDL).
Rivery ER , le Giraud YG , Bleis ILB , Decaris CD , Bezombes PB , Schneider TS
EFS Pays de la Loire, Nantes, France
Background
The French Blood Establishment (EFS) is the only civil operator of the transfusional in France. EFS PDL is one of 17 regional establishments. Every year, the Preparation Department of PDL transforms 178,300 products. The activity grows on average by 3% per year since 2007.
Aims
This department, crossroads of the transfusional supply chain (Supply Chain Management), is subjected to multiple constraints: quality, safety, deadline and cost. It was thus decided to work on a continuous improvement approach (AC) to reconcile these axes.
Methods
To do this, two apprentices in Supply Chain Management were recruited since November, 2012. It was asked to define the analysis of the progress (VSM), to establish a list of Mudas on all workstations, to federate in a long‐lasting way a Group 5S implying the team, to improve the ergonomic and the team work environment, to define quality indicators produced through the time of passage and to improve the efficiency.
Results
The implementation of this approach, that must reconcile work environment, quality, safety and efficiency, showed a synergy in the achievement of its objectives. For examples, 1125 useless movements were eliminated, the time of passage of Red Cells Concentrates were lowered by 27%, 649 h were gained and the efficiency progressed of 16%.
Summary/Conclusions
The actions possible for 2015/2016 for the Preparation Department will be to pass in the global analysis in connection with his suppliers (Donations Department/Analysis Department) and customers (Distribution Department) via a similar approach.
P‐073
Abstract Withdrawn.
P‐074
Effect of MSBOS on Transfusion Practices at Hip Arthroplasty in a University Hospital
Freixo AF , Monteiro CM , Leite AL , Delgado BD , Gomes HG , Araújo FA
São João University Hospital, Porto, Portugal
Background
Joint replacement surgery can result in substantial perioperative blood loss, placing patients at increased risk of blood transfusions. In 2012, the 1st version of Maximum Surgical Blood Ordering Schedule (MSBOS) was approved by the Hospital Transfusion Committee of the São João University Hospital. It was established that the amount of blood to be crossmatched for hip arthroplasty (HA) was 2 units of packed red blood cells (RBC). After regular audits to assess the C:T ratios, in 2013 the order was reduced to 1 unit of RBC and finally, in 2014, a Type and Screen order was implemented.
Aims
Describe the impact of implementation of MSBOS on transfusion practices in HA in our University Hospital.
Methods
We retrospectively evaluated all HA procedures between the 1st January 2012 and 31st October 2014 and accessed the quantity of blood units ordered and those actually transfused. We compared the crossmatched to transfused ratio in each version of MSBOS.
Results
During these 3 years period, 772 HA procedures were performed with a total of 414 (54%) blood requests to 384 patients according to our MSBOS policies. 52% of them were females and have a median age of 69 years old (min–max: 21–93). The mean value of pre‐ and post‐operative hemoglobin (Hg) level were 13.4 gr/dl (sd 1.7) and 10.2 gr/dl (sd 1.5), respectively, showing a mean loss of Hg of 3.2 gr/dL during surgery, without any statically significant difference between the 3 years. The C/T ratio over the last three years reduced from 6.8 to 1.3, as the MSBOS policy of the institution has been modified (Table 1).
Caption 1: Impact of MSBOS versions on transfusion practices.

Conclusion
The data collected revealed the importance of an adequate MSBOS that can influence the practices of the institution, helping to promote a restrictive policy. Other advantages of this approach is a reduction of costs in studying and cross‐matching units, problems of stock availability and wastage of units due to temperature deviations during transport.
P‐075
Abstract Withdrawn.
P‐076
Large Sustained Reduction in the Transfusion of Fresh Frozen Plasma After Establishment of a Culture Change
Sweeney JDS1, Tavares MF 2, DiQuattro PJ 2
1Miriam Hospital, Providence, United States of America2Roger Williams Hospital, Providence, United States of America
Background
Plasma is frequently transfused to correct abnormal coagulation tests in actively bleeding patients or prior to an invasive diagnostic or therapeutic intervention. The Prothrombin time or it's derivative, the INR, is the most common test used to guide the use of such plasma, yet considerable data exist that in clinical scenarios an INR prolongation does not correlate with clinical bleeding nor is it predictive of the risk of clinical bleeding in invasive procedures.
Aim
A program to interdict the use of plasma for such purposes was initiated in 2000–2001.
Methods
This program consisted of physician education, followed by a period of prospective engagement in which requests for plasma for patients with an INR of less than 3.0 were questioned and if the INR was greater than 3.0, a suggestion made to use intravenous vitamin K if the patient was known to be on a vitamin K antagonist. The effectiveness of the program was monitored by tabulating the number of units of plasma transfused divided by the number of discharges to control for patient volume and safety by tabulating in patient mortality and unexpected requests for red cell transfusion for bleeding patients. Any plasma used as an exchange fluid for apheresis procedures was excluded.
Results
Data are shown in the table. As can be seen, a 92–95% reduction in plasma is observed over a 15 year period. In patient mortality declined during this period and in no instance was a request received for peri‐procedure red cells or plasma after the cancellation of the plasma order. A cultural change in the use of plasma is evident in the last five years and physician engagement for questionable plasma orders is now rare.
Conclusion
It is possible to avoid a large volume of the plasma transfused with the intention of correcting a coagulopathy without any apparent patient harm indicating that the great majority of plasma transfused in this context is non‐efficacious and constitutes waste.

P‐077
Ready to Issue Pre‐Thawed Fresh Frozen Plasma: A Pilot of Successful Inventory Control Mechanism
Aggarwal G , Tiwari AK , Dara RC , Arora D , Acharya DP , Rawat GS , Sharma J , Raina V
Medanta The Medicity, Gurgaon, India
Background
Blood component therapy is now the standard of care in most of the modern hospitals even in a medium HDI country like India. Most commonly used blood components are red cells, platelets and fresh frozen plasma (FFP). FFP, which is stored at temperatures lower than – 30°C, has to be thawed at 37°C before it is issued for transfusion in a patient at the time of need. Thawing of FFP can be done using either a wet water or dry water bath. The turn around time (TAT) for processing and issue of FFP takes around 45–60 min which includes: (i) thawing; (ii) reverse ABO grouping and (iii) documentation. Out of these three processing steps thawing takes the longest. This TAT was a problem for patients requiring components urgently. This inspired the authors to experiment a new inventory control mechanism which involved pre‐thawed FFP and ABO group confirmation of plasma units ahead of time.
Aim
The study was conducted to compare the TAT, before and after implementation of new inventory control mechanism.
Materials and Method
Six month ambispective pilot study was conducted at a tertiary care hospital‐based blood bank in India. Retrospective data for last three months was compiled and analysed to reveal average number of ABO group specific plasma units issued per day for A, B, AB and O blood group. Rh blood group was not considered according to the hospital protocol. These averages were utilised in three month prospective ‘new inventory control mechanism’ pilot for defining optimum number of ABO group‐specific plasma to be kept as pre‐thawed inventory on daily basis. This inventory was stocked twice daily at 08:00 and 20:00 h. Stock‐out was defined as group specific inventory below 25% of optimum inventory at times other than defined and this was recorded at the time of actual patient need where additional units had to be thawed. The pre‐thawed plasma were stored at 2–6°C (in dedicated shelf in blood bank refrigerator) and units not issued within 24 h after thawing were shifted to ‘for fractionation’ inventory. TAT, stock‐outs and FFP units shifted for fractionation were prospectively monitored.
Results
During the study period, 3590 FFP units (40 per day) and 3464 FFP units (39 per day) were issued before and after the implementation of the new inventory management mechanism, respectively. There was a significant decrease in TAT [50 min (3590 units) vs 13.9 min (3464 units); P < 0.0001). 3–4 stock‐out were recorded per week. Total units shifted to fractionation were overall = 362;10.4% (A = 111/709;15.7%, B = 67/1488;4.5%, AB = 54/323;16.9%, O = 130/944;13.8%).
Discussion
Valuable time was saved in issuing of FFP with the implementation of the new inventory control mechanism. There was also increased satisfaction of physicians as evident from positive feedback. There was small increase in FFP units shifted out which seems to be insignificant.
P‐078
Rare Donors and Frozen Inventory Management
Powley TL , Ismay S
Australian Red Cross Blood Service, Kelvin Grove, Australia
Background
The cultural and ethnic diversity of the Australian population has been reshaped over many years by migration, with over one quarter of our resident population in 2013 being born overseas. The diversity of immigration has shifted from predominately European countries due to an increase in immigration from the Asia Pacific region. This change in population ethnicity presents some challenges for the Australian Red Cross Blood Service (the Blood Service) as we work towards a blood donor panel that reflects more closely the diversity of our population.
The Blood Service has a small but very supportive rare donor panel and maintains an inventory of frozen rare red cell donations. An increasing requirement for phenotypes that are considered very uncommon in our predominantly Caucasian population has prompted a review of our processes for managing our rare donors and our frozen inventory.
Methods
Our frozen rare inventory is held across three of our four processing centres and was historically managed independently. To improve management and equity of access, the inventory is now managed centrally and a national search tool has been developed to enable a simple search of the frozen rare red cells inventory which has the ability to include additional phenotype requirements. The tool also provides functionality to search for suitable donors with forward appointments (<7 days or <14 days) or generate a file of suitable donors for our National Call Centre to contact for appointments. The search tool also enables us to extract the required information about our rare donors to ensure we are able to update the International Rare Donor Register on a regular basis. It also supports systems to improve our ability to maintain contact with our rare donors and re‐engage those who have lapsed.
Results
The review of our rare donors provided us with the evidence that a ‘rare donor’ flag in our Blood Management System was not enough to avoid conversion of these donors from whole blood phlebotomy to apheresis plasma or platelets. These conversions resulted in a loss of opportunity to add to our frozen inventory and also impacted on the donor's availability when needed for a specific patient. To resolve this we have introduced a new code that restricts the phlebotomy choices to whole blood for these donors. This eliminates the risk of in‐donor centre conversions and it can be removed if the donor is ineligible or does not wish to donate whole blood.
Conclusions
With the improvements to our processes and the new tools to better manage our frozen inventory and our rare donors we are now looking at ways to identify and recruit a more diverse donor panel to increase the range and availability of our rare donors. Whilst support from our colleagues in the Asia Pacific has been utilised frequently and assisted us greatly, the logistics and risk involved in importing red cells is something we would hope to avoid whenever possible.
P‐079
Extended Phenotyping of Red Cells – Audit and Recommendations to Meet Demand
Anand R , Watkins E
NHS Blood and Transplant, Birmingham, United Kingdom
Background
The provision of blood for sickle cell patients and other patients with rare and/or multiple antibodies is a challenge for the blood service. NHS Blood and Transplant (NHSBT) aspires to have a sufficient supply of suitably tested units, i.e. those with an extended phenotype, to meet the urgent transfusion needs of these patients. The ‘Ro’ (cDe) Rh phenotype is the most common Rh type in patients with sickle cell disease. Approximately 50% of requests for Ro phenotyped products are supplied with substituted products, which are often phenotyped group O RhD negative red cells, putting pressure on stocks. A national process exists to manage the extended phenotyping of red cells, particularly Ro red cells but evidence suggests that it does not ensure sufficiency of supply.
Aims
To audit the current implementation of written standards for the extended phenotyping of blood donations.
Methods
NHSBT's testing process provided the written standards for audit. Retrospective data on extended phenotyping were reviewed for all whole blood donations received for testing during the month of October 2014. Extended phenotyping covers testing for any antigens other than ABO, D, C, E, c, e, or K and includes screening for the heterozygous sickle cell trait (HbS). The audit standard focuses particularly on the antigens S, s, Fya and Fyb.
Results
A total of 2816 Ro donations were received for testing; 1244 donations were received from donors with black/mixed black backgrounds. Only 64% of HbS negative, K negative Ro donations had an extended phenotype against an audit standard of 100%, and only 60% of HbS negative, K negative donations from black/mixed black donors had an extended phenotype. Only 25% of S negative donations from previously untested donors were tested for s. This impacts on the identification of donations that are negative for the U antigen, a type that is frequently in demand. 87% of Fya negative donations were tested for Fyb, however, the remaining 13% accounted for >900 donors. Almost 99% of the Ro donors who donated during October 2014 were screened for HbS, leaving 30 Ro donors who were not.
Conclusions
Current NHSBT standards for extended phenotyping are not being met and we recommend that the testing process be revised. The process should cover the extended phenotyping of donations from all new and existing Black, Asian and Minority Ethnic (BAME) donors, rather than just the current selection of donors from black/mixed black backgrounds. This would optimise the use of the donor pool. Extended phenotyping should be performed on all HbS negative, K negative, Ro units. The labelling of red cells based on historic phenotype data could improve the availability of suitably tested units. The tremendous efforts of NHSBT working with external partners and communities continue to yield an increase in the number of Ro and BAME donations being received. However, greater laboratory capacity is required if NHSBT is to optimise the use of the available donor pool and conserve its precious stocks of O RhD negative red cells.
P‐080
Rates of Appropriateness and Overtransfusion of Red Cells in Haematology Patients in Mater Dei Hospital, Malta
Borg-Aquilina DBA1, Attard DA 2, Dimech AD 2, Edwards NME 2, Galea GG 2, Gatt DG 2, Scerri RS 1, Laspina SL 2
1Malta National Blood Transfusion Service, Naxxar, Malta2Hospital Blood Bank, Mater Dei Hospital, Msida, Malta
Mater Dei Hospital (MDH) is the major public hospital in Malta (population ~425,000) receiving more than 90% of red cells (RC) collected by the only blood establishment in the country.
The aim of this audit was to obtain an objective view of RC usage in terms of transfusion appropriateness transfusion and overtransfusion. Rates of RC transfused to haematology patients were further analysed since haematology patients use significant amounts of RC compared to the rest of the patient population.
All units issued by the MDH blood bank from the 10th June till 10th August, 2013 were tracked, and information pertaining to the transfusion was researched in patient files. Data was collected in episodes, defined as the number of units issued before the next haemoglobin (Hb) check or within 24 h. Analysis took place using a spreadsheet program. Neonatal and paediatric transfusions were omitted from analysis as numbers were too small for significant results. An appropriate trigger for transfusion was calculated for each patient receiving a RC transfusion by considering Hb level, age, co‐morbidities, acute blood loss and symptoms of anaemia (British Committee for Standards in Haematology, 2013; EU Optimal Blood Use Project, 2008). A patient was judged to be overtransfused when the post‐Hb level (taken within 24 h of transfusion) was more than 2 g/dl above the appropriate transfusion trigger.
83.5% of data was collected (878 episodes analysed of a total of 1051 episodes). 125 episodes of issued RC were ultimately not transfused. Of episodes transfused, 44.22% were male patients whilst 55.78% were female patients. 68.79% of episodes were transfused to patients older than 60 years. Two units RC were transfused in 431 episodes (41.01% of episodes analysed). The overall picture for all specialties excluding Haematology showed that 20.14% of transfusions were not appropriate according to the pre‐defined criteria, and 29.86% of patients were overtransfused. 19.60% of patients had no post‐transfusion Hb check within 24 h of transfusion. 21.7% of episodes (154 episodes) analysed were transfused to patients with a haematological diagnosis. 18.18% of haematology patients were deemed to have been transfused RC inappropriately, and 13.64% were overtransfused. 43.51% of haematology patients did not have records of a Hb check 24 h after RC transfusion.
These results indicate a substantial quantity of RC transfusion is inappropriate, leading to a significant number of patients being overtransfused. In general, overtransfusion might also be attributed to the misconception that two units of RC must be transfused rather than one, without a Hb check between units to re‐assess appropriateness of transfusion. Whilst the rate of overtransfusion is lower for haematology patients than the general picture, it was noted that a considerable number of haematology patients had no post‐transfusion Hb check, thus overtransfusion rates are possibly higher. Future work includes education with emphasis on monitoring of post‐transfusion Hb after transfusion of one unit of RC, prior to taking a decision to transfuse a second unit in non‐bleeding haemodynamically stable patients. This audit will serve as a benchmark for future audits undertaken to evaluate effectiveness of education.
P‐081
Red Blood Cell Supply Management in the Regions of Ukraine
Chugriiev AN
Zhytomyr Regional Blood Centre, Zhytomyr, Ukraine
Total population of Ukraine during 1992–2012 decreased from 52 million to 45.6 million. From 2 to 3 million of able‐bodied population are working outside Ukraine. Proportion of people aged above 60 increased from 18.8% to 21.2%, while proportion of working age population decreased from 60.6% in 2007 to 59.8% in 2013. In Ukraine level of red blood cell (RBCs) usage for transfusion was 10 doses per 1000 population in 1992, 7.9 doses in 2012, in Europe it was on average 41 doses per 1000 and only in three countries – less than 20 doses. Out of total amount of procured RBCs proportion of RBCs used for transfusions in patient care institutions in Ukraine comprised 40.6% in 1992, 54.3% in 2012, proportion of RBCs discarded due to expiry date was 21.7%. Negative demographic trends in Ukraine, consistently low level of RBCs usage and significant number of RBCs unsuitable for transfusion due to expiration create conditions for predictable blood component shortage in the future. Questionnaire was received and sent back by 15 regional blood centers (blood transfusion stations). Questionnaire consists of 77 questions, grouped into six sections. Territory covered by participants accounts for 60.2% of population, 59.7% of physicians working there, 59.7% hospital beds, 64.3% RBCs transfused out of total amount of RBCs in Ukraine. Among specific factors that influence RBCs usage 80% of respondents identified ‘types of diseases’, 40% of respondents (6 regions) – ‘changes in the demographics’ and ‘access to health care’. Programme for collecting blood, donor data and supply is not compiled for next week in 12 of 15 regions. Compiling weekly programmes for collecting blood, donor data and supply does not take into account donors and orders by ABO and Rh‐systems. Among 15 regions seven create annual programme for collecting blood, donor data and orders at all levels: provincial, district, city, hospital. RBCs supply in blood service establishments (BSEs) is calculated using total amount of RBCs (11 regions) with existent restrictions of specific RBCs by ABO, Rh and pediatric doses (8 regions) without SOPs on prevention RBCs excess (6 regions). Seven regions monitor RBCs supply in hospitals. Computer supply management system exists in 5 regions without automatic update of hospital supply rate. Only 5 regions use SOPs for updating RBC supply balance. Seven regions carry out calculation and forecasting of RBCs supply for a certain period in future with procedures on lack and excess prevention. Six regions confirm combined usage of supply management, IT‐systems and SOPs in BSEs and hospitals. Ten regions evaluated RBCs sufficiency rate as 100% and more and 3 regions as 96–99%. Specified factors that influence supply and actual RBCs usage stipulate creation of blood supply management system in the following sequence:
• Τo evaluate RBCs usage in hospitals for the previous period.
• Τo calculate forecast of annual supply from BSEs and actual use in hospitals.
• Τo create annual programmes of collecting blood, donor data and RBCs supply from BSEs.
• Τo create a weekly balance of RBCs usage (demand) and supply from BSEs.
• Τo assess patients needs and level of their satisfaction.
P‐082
Is Electronic Remote Issue a Good Thing for the Laboratory?
Staves JS , Croxton TC , Hemmatpour SH , Guest CG , Burrows HB
Oxford University Hospitals NHS Trust, Oxford, United Kingdom
In the UK, the centralisation of hospital laboratory pathology services is 1 approach for ensuring these services are cost effective whilst providing improved patient care.
Blood transfusion has traditionally been seen to be a block on the centralisation of services as certain clinical specialities (maternity, trauma and surgical services) will require blood products urgently.
The introduction of IT driven alogrithms to allow the rapid release of red cells products from the laboratory (electronic issue) has also been taken directly to some clinical areas in the advent of electronic remote issue (ERI) from smart refrigerators. These systems allow clinical staff to release red cell products for transfusion to the patient as they are required without the need for laboratory involvement.
The impact of such systems has mainly been reported looking at the availability of red cell products to patients, the impact on the laboratory has not yet been reported.
The Oxford University Hospitals NHS Trust was an early implementer of ERI to supply red cell products to the operating theatres (OR) in the trust. In Oct 2013, ERI was rolled out to the recently merged Nuffield Orthopaedic Centre (NOC). This implementation included providing red cell products for ward based patients by ERI as well as to the OR. The NOC is a remote site approximately 3 miles from the main hospital and there is no laboratory service on site.
The impact on the laboratory has been monitored in terms of laboratory staff time to provide red cells for the NOC and the transportation of red cell units between sites.
The results show that the number of units issued each month to the NOC dropped by 77% with a corresponding reduction in the number of units returned to the lab. The reduction in the movement of units between sites saved considerable laboratory time from an average of 93 min of staff time per day to 20 min.
The Crossmatched/transfused ratio for the NOC dropped from 3.5 to 1 with only an occasional unit being issued but not transfused each month. This indicates that the number of units in stock but not available for issue to patient reduced considerably and this accounts for the reduction in the wastage of units associated with that fridge over the same time frame.
Caption 1: Table showing data.

In conclusion, the introduction of ERI for a remote site without a transfusion laboratory can offer considerable staff and transportation time savings with no clinical impact. The reduction in the time spent packing/unpacking and transporting units of red cells is considerable. The reduction in the C/T ratio also reduce wastage and aids stock control within the laboratory. The availability of red cells on a remote site in this manner allows patients to be transfused quickly and reduces the need for costly out of hours transportation.
P‐083
Wastage Evaluation of Blood and Blood Components in Iranian Transfusion Services in the Year 1392 (2013–2014)
Sharifi SS1, Shahabi MS 2, Zolfaghari Anaraki SZ 1
1Iranian Blood Transfusion Organization, Tehran, Iran2High Institute for Education and Research in Transfusion Medicine, Tehran, Iran
Background
Economy is one of the most important concerns in every country, so cost‐benefit analysis is a top priority in each process. Blood safety is the main issue in transfusion medicine and each cost‐benefit study should be considered from this standpoint. Finding and correction of the causes is a way of wastage reduction.
Although wastage is inevitable to some extent, but the majority is avoidable by proper planning and right management.
Aim
Finding the main causes of blood and blood components wastage in Iranian transfusion services
Methods
This study was a retrospective. Data was extracted from donation software without the possibility of any change by operator. Data contained the information from 31 provinces for a one‐year period (1392).
Results
A total of 2,520,076 volunteer donors registered during the year 1392, of which 2,001,791 were accepted and donated blood. The others were deferred for different reasons. Blood components were prepared from these donations as follows:
• Red blood cell concentrate 1,734,916 units
• Platelet concentrate 1,103,171 unites
• Fresh frozen plasma (FFP) 1,660,107 unites
• The wastage data are presented in Figures 1 and 2
Caption 1: Main causes of RBC concentrate wastage in 1392.

Summary/Conclusions
Our findings indicate that:
FFP has the highest rate of wastage among the other components (332,414 units). To reduce this amount, the following recommendations may be useful:
• Making contract with fractionation company to manufacture plasma derivatives.
• Need assessment to prevent resources losing.
The number of expired components available in blood centers is much greater than those in hospitals. This does not make sound and emphasizes the need for the implementation of comprehensive software in hospitals to record data throughout the process.
The FFP component is much more vulnerable to physical damage in both the blood centers and hospitals. This is quit logic and in accordance with global data.
Caption 2: Main causes of FFP wastage in 1392.

Platelet wastage due to expiration is greater than other products. This is best explained by the short shelf‐life of this component. It is recommended that proper measures to be taken with the aim of platelet releasing at the first day of donation. This will increase the availability of the component by one day.
Pricing the blood component and
P‐084
Abstract Withdrawn.
P‐085
Improving (and Measuring) Blood Traceability, Production Process Control and Efficiency With RFID‐Based Solution Rhesus© at Seville's Regional Transfusion Centre
Aznar Martín M1, Oyonarte S 1, Rodríguez MC 1, Miranda B 2, Gómez J 3, Benítez JP 3, Agea A 3
1CRTS Sevilla‐Huelva, Sevile, Spain2Biobanco de Andalucía, Granada, Spain3AT‐Biotech, Madrid, Spain
Background
Current Transfusion Management Systems are rather ineffective in controlling blood components and, very specially, their location, status and timestamps throughout the entire phlebotomy to transfusion process.
Consequently, traceability and management of blood units – especially when handling large volumes – are highly limited and adoption of best practices and compliance with regulatory requirements become a serious challenge.
Furthermore, the operational processes along the donation‐production‐transfusion cycle are generally very inefficient.
Aims
Main goal is to present a new technological solution which successfully addresses the aforementioned issues and is currently in full operation at Seville's Regional Transfusion Centre (RTC), that currently collects and processes around 77,000 blood donations per year.
Methods
Rhesus© is the solution of AT‐Biotech© consisting of a set of networked devices that control blood bags identified with a RadioFrequency Identification (RFID) label from the donation phase. Actually, diverse type of devices such as PDAs, printers, tunnels and portals are used in the different steps of the process to best suit the operation characteristics and to allow personnel using them seamlessly.
Rhesus© has been rolled out following a comprehensive analysis of particular operation procedures and physical layout. Then, the system functionality has been validated and the solution has entered into a robustness validation phase.
Results
To date, more than 75,000 blood components – red cells, platelets and plasma units – have been identified, stored and distributed to hospitals or fractionation industry using Rhesus©.
Haemovigilance, traceability and production process control has largely been improved, and compliance with GMP/GAMP has been facilitated. During this process, all units are controlled real time – what, when, who, where and how – in a total of 10 additional control points, resulting in an estimated total of 2,800,000 new data records per year.
Besides, the new solution has delivered other direct benefits:
• Increased operational efficiency.
• Better products quality, as a result of a better control of cold chain.
• Reduction of discarded and potential loss of blood bags.
• Improved health and safety conditions for workers – having time for handling and work under adverse conditions in storage chambers been reduced.
These benefits have been measured following industry standards, resulting in a total improvement of two digits %.
The solution also allows for indirect benefits, as a result of the observation of purposely built reports, big data analysis and process reengineering, resulting in expected very significant yearly savings.
For instance:
• Donation campaigns can be maximised by reducing donors waiting time – queue management – and analysing donations occurrences.
• Blood quality and delivery to/from the transfusion centre can be improved by analysing distributions behaviour.
• Expenditure on haemoderivates can be optimised by improving plasma quality – analysing donation‐to‐ultra‐freezer circuit steps.
Conclusions
RTC has deployed a new technological solution – Rhesus© – that soundly improves blood components traceability and production process control, helps GMP/GAMP compliance and increases operational efficiency and product quality, resulting in a total improvement of two digits %.
Rhesus also allows for further improvements on areas such as donation campaigns, blood distribution and plasma quality, resulting in expected very significant yearly savings.
P‐086
Return Blood to the Blood Bank: An Audit
Sood RS , Kumar T , Kumar V
Saket City Hospital, New Delhi, India
Background
Blood bank is designed to collect, store, process and rationally issue red blood cell and blood components that are screened and safe for transfusion to the needs of the patients. (1) Transfusion Service has an established policy mentioning in details the parameters for the acceptability of the returned units for re‐entry.
Aims
To audit blood /component bags returning to the blood bank, within and beyond the permissible limit of 30 min. (2)
Methodology
Compatibility report mentions the time of issue of blood /components. Once an issued component is received back to the blood bank, the time of receiving the same is clearly noted.
Data, related to return of blood/components, was retrospectively collected and analysed.
Results
Total 3733 units issued from July 2013 to July 2014 as:
• PRBC (Packed Red Blood Cells) – 1843: PRBC 1808; PA (Pediatric)‐PRBC 35
• Platelets – 743: RDPC – 622 SDPC – 121
• FFP (Fresh Frozen Plasma) – 1095: FFP 1092 PA‐FFP 3
• CRYO (Cryoprecipitate) – 38
• CPP (Cryo Poor Plasma) – 13
• WB (Whole Blood) – 1
Returning back of issued units from July 2013 to July 2014 was:
• PRBC – 18 (0.97%)
• FFP – 1(0.09%)
• RDPC – 3(0.48%)
All units were returned after the limit period of 30 min, hence discarded leading to wastage of not only human blood, but of the man‐power, the blood bags and the work hours as well.
Reasons for returning blood /components:
1. No need for transfusion‐6 PRBC; 1 FFP;3 RDPC
2. Patient having fever‐4PRBC
3. Suspicion of Transfusion Reaction‐1 PRBC
4. Operation Theatre (OT) patient transferred to ICU, blood requested in OT refused by ICU doctor‐2 PRBC
5. Patient Expired‐2PRBC
6. Attendant refused Transfusion‐1 PRBC
7. Patient went LAMA (Left against medical advice)‐2PRBC


Conclusions
1. Blood/Blood components request has to be based on rational criterias.
2. Many points are to be clarified before sending request by the doctor on duty rather than nurses.
3. Return blood should be within 30 min.
P‐087
Integrated Stock Management‐Two Years Experience
Cotton S1, Frith L 2, Addison E 3, Johnson J 4, Baker P 5, Staves J 6
1NHS Blood and Transplant, Sheffield, United Kingdom2NHS Blood and Transplant, Brentwood, UK, Brentwood, United Kingdom3Blackpool Teaching Hospitals NHS Foundation Trust, Blackpool, United Kingdom4The Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust, Bournemouth, United Kingdom5The Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, United Kingdom6Oxford University Hospitals NHS Trust, Oxford, United Kingdom
Background
In April 2012 hospitals in England were asked to express an interest in working alongside NHSBT to pilot an integrated stock management service. This was an opportunity for hospitals to influence the design of the service.
Aims
Build the capability to interface with hospital IT systems and track stock replenishment needs enabling NHSBT to automatically replenish stocks.
Understand areas of potential improvement in hospitals such as stock inventory levels, ad hoc delivery levels and wastage volumes.
Validate the operational effectiveness of the service over a period of time.
Methods
The service was based upon a collaborative approach, enabling NHSBT and eight pilot hospitals to manage and optimise the blood supply chain through the adoption of integrated processes.
Eight hospitals from four hospital trusts served by NHSBT were recruited as pilot sites. They represented a spread of hospitals with regard to size, clinical specialism and stock management practice.
Hospital data and information was collected and shared with NHSBT to enable a full understanding of the stock management process within the hospital. Stock levels, time expiry wastage and ad hoc delivery levels were modelled. Potential changes and timescales were discussed and agreed with hospitals. A suite of reports enabled monitoring of these factors over a period of time.
Results
An aggregated total of 6 years stock management service has been sustained at the pilot hospitals without significant incident.
Average (annualised) outcomes were:
• Ad hoc deliveries were reduced by approximately 30%.
• Red Blood Cell (RBC) time expired wastage was reduced by approximately 30% (excluding group AB units).
• Platelet wastage was reduced by approximately 25%.
• RBC stock levels held by hospitals were reduced by approximately 8%.
The feedback from the pilot hospitals has been overwhelmingly positive providing evidence of improvements in:
• Customer service – improved availability of platelets. The pilot gave hospitals the confidence to hold an increased stock of platelets. Clinicians report that this has enhanced the quality of patient care.
• Operational efficiency – hospital staff are able to focus their time on other tasks, spending less time on the ordering process.
• Risk reduction – routine stock orders are automatically generated for the hospital based on actual stock levels rather than the interpretation of a staff member.
Summary/Conclusions
The objectives of the new stock management service have been achieved.
In summary
NHSBT is investing in a new stock management system with hospitals to improve the whole blood supply chain and help hospitals to deliver efficiencies through automated stock replenishment.
The stock management system will see NHSBT and hospitals plan provision of blood stocks enabling hospital staff to focus on delivery of transfusion services to patients.
NHSBT are now developing stock management partnerships with a further 13 hospitals with a view to considering how this could be further rolled out in the future.
Through the stock management service NHSBT aim to improve the service to hospitals, and ensure the best use of precious blood donations.
1.7 Quality Management
P‐088
Abstract Withdrawn.
P‐089
Training for Blood Bank Accreditation Program (BBAP) by National Accreditation Board for Hospitals and Healthcare Providers (NABH): Study to Find Out Effectiveness of Program of Implementation (POI)
Choudhury NC 1, Kumar AK 2, Agarwal AA 3, Kalra KKK 2, Rana BKR 2
1Fortis Memorial Research Institute, Gurgaon, Delhi NCR, India2National Accreditation Board for Hospitals & Healthcare Provide, New Delhi, India3Transfusion Medicine Department, Fortis Escort Heart Institute, Okhla, New Delhi, India
Background
NABH is an accreditating body set up by Quality Council of India to provide accreditation and quality improvement services in healthcare domain. NABH is an Institutional member of ISQua. NABH has ten accreditation programs and BBAP started in 2007. India has a highly fragmented blood transfusion service with more than 2545 Blood Banks (BB). In seven years only 68 blood banks were accredited and 12 others on the process. To increase the number of accredited BB, NABH started three days training program called POI on NABH Standard to prepare BB for accreditation.
Aim
1. To find out effectiveness of POI among participants to initiate BBAP in respective blood banks.
2. To find out difference in knowledge level among participants before and after attending POI.
Methods
This program was organized in a class room interactive setting for three full days and two resource persons conducted the course. About 26 applicants were selected on the basis of their details in application. Before starting POI, one structured questionnaire (with identifier) was given to applicants and the same questionnaire was given at end of POI. Fifteen multiple choice questions were added in post course questionnaire to understand their leaning on quality management system. During the course, participants were trained on all clauses of NABH Standard; gap analysis; preparation of quality manual; organogram; quality policy (QP); calibration and validation of equipment; SOP writing; internal audit (IA) and management review; adverse incident reporting (AIR); nonconformity/ deviation; EQAS; QC/QA; document control; hospital transfusion committee (HTC); application process for BBAP; fees involved and future assistance channels. Both questionnaires were analyzed participant wise and results were evaluated.
Results
Participants observed great values in POI for BBAP. There was significant knowledge enhancement in post assessment. In preparing biomedical waste management policy and SOP, knowledge increased from 20% to 96%. Knowledge of preparation of QP and developing identification no. for equipment increased from 72% to 96%. Only 56% knew about IA and 32% knew how to implement. Knowledge in IA improved to 83%. About 28% had knowledge about AIR and it increased to 96%. Initially 32% participants knew structure of HTC but it was improved to 83% after the IP. Implementation of record retrieval was known to 28% and 96% become confident after IP. About 50% participants knew about all BB SOPs and it improved to 76% afterwards. About 9 participants came from accredited blood banks, therefore their knowledge was better. About 84% knew how to prepare organogram; 96% knew about equipment maintenance; 88% thought that documentation was in place; 68% already participated in EQAS program.
Summary/Conclusions
1. Knowledge about BBAP has greatly enhanced after the POI among participants. It is due to intense exposure to all clauses NABH Standard.
2. There is significant difference on level of knowledge among participants before and after the POI.
3. The feedback identified hand on approach, individual & group exercises, interactive sessions, presentations by participants and success story discussion made this program most successful and effective.
P‐090
Optimized Document Handling Assisted by Reporting Methods Within a Document Management System
Spyrantis AS , Capalbo GC , Gross MG , Sireis WS , Gubbe KG , Seifried ES , Schmidt MS
GRC‐Blood Donor Service Baden‐Wuerttemberg – Hessen/Frankfurt, Frankfurt, Germany
Background
The German Red Cross blood donor service in Baden‐Wuerttemberg – Hessen and its affiliates comprise of 18 independent institutes spread around six districts in Germany. More than 8000 released documents are currently valid within our organisation. Electronic document management systems (DMS) have evolved over the last years and have become an essential element of our quality system. Key features that have played a role include the ability to support workflow‐based functions as well as for their archiving capabilities, making them a favourable tool for document control or replacing paper‐based processes. Although the automation provided by such systems offers many benefits, a certain degree of administration, maintenance and further development is required to keep such systems at peak performance.
Aims
Harmonization of forms and SOPs remains a top priority, is however a very challenging task when dealing with 18 sites. An electronic DMS enables the identification of comparable documents, hence facilitating the harmonisation process. Moreover, the use of search tools can significantly assist in keeping the document database in an updated state, e.g. by identifying infrequently used documents and flagging them for revision or withdrawal.
Methods
We have developed a customized DMS on the Saperion platform. Data extraction providing an overview of released documents as well as the overall workflow status was generated by using an advanced Saperion search engine interface. Limitations to this method were overcome by additional advanced searches performed directly through the SQL server.
Results
Documents are electronically accessible on every workspace. Allocation of accessibility rights are based on three user groups: The largest group belongs to ‘readers’ (69%) whose access is restricted to viewing of released documents. The second largest group belongs to ‘Authors/Approvers’ (30%) which have read/write as well as document releasing rights. The remaining 1% corresponds to Quality Assurance staff which is also responsible for general administration and support. Searches on the database revealed a dramatic increase in forms opposed to any other document type (50% of all valid documents) over the past years. Valid documents were rated for the number of times they have been viewed. This method enabled us to identify important documents and make suggestions on the harmonisation of certain documents throughout our organisation. In addition, the identification of valid forms that have rarely been accessed helped optimise our document management system as authors of these documents either revised or replaced them. Although an automatic document revision cycle commences every 2 years, an analysis of revision frequencies not only reflects the effectiveness of the document harmonisation process, but also confirms that documents are frequently updated.
Summary/Conclusions
In this study we present the overall performance of a customized DMS, taking into account parameters such as overall user acceptance, usage as well as technical performance in an effort to further optimise document control.
P‐091
Application of Lean Six Sigma to Shorten the Non‐Value Added Waiting Time for Self Pick‐Up Blood Collection at the Hong Kong Red Cross Blood Transfusion Service (HKRCBTS)
Ng JTK , Liu GCL , Leung MTW , Chua EKM , Tsoi WC , Lin CK
Hong Kong Red Cross Blood Transfusion Service, Hong Kong, China
Background
Regular scheduled blood deliveries to hospital clients for blood stock replenishment are provided by HKRCBTS whereas ad hoc blood orders are collected by couriers arranged by hospital blood banks (‘self pick‐up’). There were observations that waiting time for ‘self pick‐up’ was long; ‘waiting time’, in this context, being defined as the time between physical arrival and departure of hospital couriers. The situation can be worsened if, at times, several couriers from different hospitals arrived in clusters and/or the ad hoc blood orders involve a large quantity of blood components.
Aims
To apply Lean tools in attempt to shorten the non‐value‐added waiting time of hospital couriers through streamlining of workflow at Storage and Issue Section (SIS) of HKRCBTS and enhancement of communication with client hospitals.
Methods
The DMAIC model was adopted to: (i) Define problems associated with long waiting time; (ii) Measure and collect current data as a baseline in Phase I; (iii) Analyse data using Ishikawa diagram (cause and effect) tool to identify the root causes of long waiting time; (iv) Formulate and implement Improvement plans; and (v) Establish Control plans.
Results
In Phase I, from June to August 2014, 1057 ad hoc blood requests were picked up by hospital couriers and the total waiting time involved was 272 h 8 min, which was equivalent to an average of 15 min 27 s waiting time per case or approximately 180 h waiting time per month. Root causes analysis indicated that factors such as limited availability of SIS staff, inappropriately pledged collection time, early arrival of hospital couriers, discrepancy in information stated on ‘self pick‐up’ documents and unexpectedly large quantity of blood requested could at times contribute to the causes of prolonged waiting time. To tackle these causes, an operation improvement protocol was proposed, which includes (i) assessment of the types and the amount of blood component required and the urgency of the ad hoc requests; (ii) providing more precise estimated collection time to requestors; (iii) assigning dedicated staff to handle such cases prior to courier arrival; and (iv) enhancing communication with client hospitals. In Phase II data collection, from October to November 2014, after implementation of the aforementioned measures, a total of 1197 ‘self pick‐up’ blood requests were encountered and the total waiting time was recorded as 172 h 30 min, which demonstrated a shortening in waiting time to 8 min 39 s per case or approximately 100 h per month.
Conslusions
Through application of Lean Six Sigma tools, elements contributing to long waiting time were analysed in details and process improvement opportunities were identified to aid shortening the long ‘self pick‐up’ waiting time. With streamlining of workflow at SIS after implementing the new operation improvement protocol, the average waiting time for self pick‐up collection process decreased by 44% and 80 h of non‐value added waiting time per month were saved. Control plan of integrating the improvement protocols into routine Standard Operating Procedures will be followed and clients’ satisfaction of our service performance will be monitored.
P‐092
From Quality to Quality Management in European Blood Establishments: Council of Europe's Contribution
Hecquet MLH
European Directorate for the Quality of Medicines & HealthCare/Council of Europe, Strasbourg, France
Background
During the last decade, the concept of quality in the field of blood transfusion has evolved greatly, while European Blood Establishments (BEs) have been required to comply with new regulatory requirements. Transfusion is a procedure that carries intrinsic risks and implementation of a Quality Management System (QMS) in BEs is the key to managing these risks and ensuring the efficacy, quality and safety of blood. Whereas Quality and Quality Assurance (QA) imply the development of procedures to ensure that requirements are fulfilled, a QMS additionally implies a quality policy and objectives, risk‐management and continuous improvement to direct all processes toward quality. Implementation of a QMS is required by EU Directive 2002/98/EC, and is prescribed in the Council of Europe (CoE) Guide to the Preparation, Use and Quality Assurance of Blood Components, EU GMP, PIC/S guidelines and ISO standards. Nevertheless, the concept is not always entirely understood and is often seen as a burden owing to a lack of appropriate on‐site support. This was confirmed in a survey performed by the European Directorate for the Quality of Medicines & Healthcare (EDQM) of the CoE in 2012. The data collected from 186 BEs (33 countries) and observations recorded during on‐site visits provide evidence of the difficulties encountered in understanding quality concepts and developing an integrated QMS, and show that existing systems tend to be blood product quality‐oriented.
Aims
To support BEs to develop an integrated QMS, since 2012, the EDQM has developed a programme which includes on‐site assessment and tailor‐made training and education schemes for BEs.
Methods
This programme delivers on‐site training or assessment schemes (Training Visits (TV), Mutual Joint Visits (MJV), Mutual Joint Audits (MJA)), and learning tools (Training Courses). The on‐site scheme type is selected based on a prior assessment of the existing QMS. The process‐ and risk‐based approach is taken, allowing BEs to rethink their system, consider the criticality of their processes, develop a risk‐based QMS and focus on continuous improvement. Training courses include learning modules on quality concepts, process mapping, managing quality documentation, staff training, validation, qualification, risk‐management, managing corrective and preventive actions, change control and internal auditing. The programme is open to EU and CoE Member States, and is run by qualified auditors/trainers from the field.
Results
Since 2012, the EDQM has run 9 B‐MJVs, 4 B‐TVs and 1 QM Training Course. BEs recognise this programme as essential, given the increasing relevance of QMS, the complexity of the activities undertaken and the difficulties in implementing all standards applicable in this field. Observations during the schemes ranked from critical to minor. Participating BEs were able to take actions, switch from a quality‐ to a QM‐oriented system and/or improve their QMS.
Conclusions
Blood quality can only be guaranteed through the development of a common European approach and operational tools in the field of QM, together with the implementation of an integrated QMS. The EDQM B‐QM programme is the first of its kind in Europe and provides non‐reprobative support to BEs.
P‐093
Lipemic Blood Donor Samples: To Test or Not
Makarovska-Bojadzieva TM , Neceva VN , Nikolova NJ , Velkova VE , Blagoevska MB , Samonikov-Tosevska JS , Mitevska LM , Todorovska OT
Institute of Transfusion Medicine, Skopje, Macedonia
Background
Lipemic donations are still the main single cause of wastage of blood in our transfusion practice which rang from 0.8% in 2007 to 2.3% in 2011 and being 1.4% in the last 3 years. The overall wastage of blood due to TTI reactive samples, hemolysis, inadequate quantity of blood and other unconformities in the same period was 3.6%. To date, there are no clear guidelines on the testing of lipemic donations mainly due to the lack of universal agreement on thresholds of unsuitability for testing or processing blood that exceedsdefinite cut‐offs for lipids.
Aim
To measure the triglycerides in the lipemic blood samples which are declared as unsuitable for testing according to our practice of visual inspection of serum turbidity.
Methods
The level of triglycerides was estimated in 402 donor samples which were classified as: no turbidity, slightly, moderately and markedly turbid samples. The obtained results were compared to the results of TTI markers (HBV, HCV, HIV and Syphilis) tested with EIA‐Enzygnost‐Siemens and CMIA‐Architect ABBOTT system.
Results
The calculated triglycerides levels showed almost constant twofold increase between each of the categories according to the visually estimated turbid appearance as shown on Table 1. None of the 402 samples showed TTI marker reactivity when tested.
Table 1. Turbidity of the sample and triglycerides level.

Conclusion
Although no anomalous test results were observed in the lipemic samples, we consider justified to continue the practice not to test and to discharge the lipemic donations according to the visual inspection method of turbidity as long as more evidence‐based data appear. For the present, we are focused on education of blood donors by introducing an informative material which is focused on the fat content of the pre‐donation meal to avoid postprandial lipemia.
P‐094
Abstract Withdrawn.
P‐095
Evolving Internal Quality Control Strategies for Transfusion Transmitted Infection (TTI) Testing in Blood Bank: Our Experience and Lessons Learnt
Sawant R , Mishra M , Gupta S , Deshpande A
Hinduja Hospital, Mumbai, India
Background
It is necessary to implement internal quality control (IQC) procedures as per good laboratory practices for continual and concurrent assessment of laboratory work and to ensure the correctness of the released results.
Aim
To study the feasibility of implementing robust IQC system capable of monitoring routine test performance as well as identifying errors due to other factors affecting the test results in our TTI laboratory.
Material and Methods
Levey Jennings plot for manufacturers controls is used for routine quality analysis of our TTI tests. We prepared ‘In‐house’ control sample by dilution of positive sample and aliquoting them at an OD just above the cut‐off OD of the kit control. Commercial, third party control was also run along with the kit and In‐house control. The laboratory mean and its %CV was calculated for each control and were compared with each other. A note of any change in reagent, equipment (including trouble shooting details) and staff performing the test was done. LJ chart was plotted and interpreted as per Westgard's rules. Data of HIV, HBV and HCV tests performed on Abbott Architect platform for 14 months period was analysed.
Results
Violation of Westgard's mandatory rules was observed on 22 occasions with the kit control, 21 times with in‐house control & 27 times with the commercial control. The lab mean for commercial control was found to be the most sensitive amongst the 3 controls in case of HBV & HCV testing. The cumulative mean of %CV for manufacturers positive controls was 6.04% in HBV, 5.83% in HCV and 7.73% in HIV testing, whereas the same for the commercial control was 5.66% in HBV, 3.47% in HCV and 5.52% in HIV testing. The % CV for manufacturers controls was within 10% on 100% occasions in case of HBV and HCV testing and 86% occasions in HIV testing, whereas the same with commercial control was 100% in HCV and 93% each in HBV and HIV testing. Overall, when the monthly CV was greater than twice the cumulative CV, a correlation with changes in reagent lot, equipment calibration or malfunction could be established.
Conclusion
Implementation of IQC in TTI testing is feasible. Atleast one additional control should be included along with the manufacturers control for early identification of changes in test performance.
P‐096
Transfusion Key Performance Indicators – One Years Experience
Johnson J
Royal Bournemouth & Christchurch Hospitals NHS Foundation Trust, Bournemouth, United Kingdom
Background
In 2014 a Transfusion Key Performance Indicator (KPI) Dash‐Board was designed to provide service users with a visual monthly, quarterly and annual Transfusion service quality and safety status report.
Aims
Establish quality data that users would find informative, interesting and useful.
Identify the way in which users would receive the report.
Ensure corrective actions could be identified where quality standards were not met.
Target re‐training where necessary.
Improve or maintain the quality of Transfusion processes.
Adapt the KPI to changing service needs.
Methods
Key Performance Indicators were identified from best professional practice guidelines, Clinical Pathology Accreditation Standards, Blood Safety and Quality Legislation, Annual Serious Hazards of Transfusion Reports, Safe Practice Notices and quality audits already being performed. Service Users were asked whether these were useful to them via the Hospital Transfusion Committee.
Each standard was audited and a record maintained on the Q‐Pulse Quality Management System Audit Module.
Results were collated into an Excel Spreadsheet which formed the basis of the Dash‐board KPI report.
The report was published quarterly via the Hospital Transfusion Committee and reported from there to the Trust Quality Assurance and Risk Committee. Any risks to service quality could be identified and if necessary further referred to Trust Board.
Results
Corrective and Preventative Actions were noted against each audit and targeted re‐training or process re‐evaluation/design ensued. The impact of each intervention could be seen in subsequent key performance indicator dashboard reports.
Quality was maintained or improved and results were evidence based.
The service provided by the laboratory was evidenced as of a good quality that met our user's needs.
Operational efficiency improved as users of the service worked with us to improve quality.
Patient safety and risk reduction were evidenced.
Summary/Conclusions
The objectives of the quality performance indicator dash‐board have been achieved.
In summary: We have developed twelve monthly KPI, seven quarterly KPI, and fourteen Annual KPI which are reported to our service users. Practice is improved where necessary by targeted re‐training and effectiveness monitored by subsequent KPI. Service Users have been sufficiently interested to contribute to and further develop the dash‐board. There is heightened awareness of Transfusion Safety and Quality within our organisation.
P‐097
Importance of External Quality Control in Serological Testing of Blood Donors for Transfusion Transmissible Infections
Andjelkovic Z
Blood Transfusion Institute Nis, Nis, Serbia
Introduction
Serological testing of blood donors for transfusion transmissible infections (TTI) in the Blood transfusion institute Niš involves testing blood donors for the presence of the hepatitis B surface antigen (HBsAg), antibodies against hepatitis C virus (anti‐HCV Ab), markers of human immunodeficiency virus infection (anti‐HIV1/2 Ab and HIV1 p24 Ag) and antibodies against Treponema pallidum (anti‐TP Ab).
Blood Transfusion Institute Nis participated in external quality control of blood donors testing for TTI markers for past 3 years. Control is organized by the European Directorate for the Quality of Medicines and Healthcare (EDQM) as blood proficiency testing scheme study on serological testing of blood donors for TTI.
Aim
Show the results of external quality control in serological testing of blood donors for the presence of TTI markers in Blood transfusion institute Nis for the period 2012–2014.
Materials and methods
During this period 4 panels of control serum samples were tested: 12 samples for the presence of HbsAg, 8 samples for the presence of anti‐HCV antibodies,8 samples for the presence of markers of HIV infection (HIV1/2 Ab, HIV1 p24 Ag), and 4 samples for the presence of anti‐TP antibody.
Volume of the control samples was 1.1 ml. In accordance with the protocol of the blood proficiency testing scheme study, initially reactive samples were not re‐tested in duplicate. Screening for the presence of HBsAg and anti‐HCV antibodies was performed by ELISA assays Hepanostika HbsAg ultra and Hepanostika anti HCV ultra (Biomerieux) on the device DaVinci Quattro. The presence of HIV infection markers (anti‐HIV1/2 Ab and HIV1 p24 Ag) and anti‐TP antibody was tested on the device Advia Centaur XP with CMIA assays – Advia Centaur CHIV, Advia Centaur Syph. (Siemens) and on the device Evolis with ELISA assays – Genscreen ULTRA HIV Ag/Ab and Syphilis EIA II Total Antibody (Biorad).
Results
A total of 32 control samples were tested and 19 samples were initially reactive for the presence of TTI markers: 6 samples for the presence of HbsAg, 4 samples for the presence of anti‐HCV antibodies,6 samples for the presence of anti‐HIV1/2 antibodies and HIV1 p24 antigen and 3 samples for the presence of anti‐TP antibody. In addition two samples had a score in the ‘gray zone’, i.e. signal/cut‐off (S/Co) 0.8–1, one sample for the presence of HBsAg and one for the presence of anti‐HCV antibodies. The remaining 11 samples had non‐reactive results in TTI testing.
Conclusion
External quality control is an objective assessment method of receiving, storing and serological testing of samples on TTI markers including the interpretation of the test results. Serological testing of blood donors for transfusion transmissible infections in Blood transfusion institute Nis is in accordance with the recommendations for the preparation, use and quality assurance of blood components of Council of Europe.
P‐098
Abstract Withdrawn.
P‐099
Quality Indicators in Transfusion Medicine
Condeço JC , Fonseca MGF , Ramoa AR , Escoval MAE
Instituto Português do Sangue e da Transplantação IP, Porto, Portugal
Background
Given the huge emphasis on quality in Transfusion Medicine and the concerns of professionals in this area for information on quality indicators, two organizations (the International Haemovigilance Network (IHN) and the International Society of Blood Transfusion through the Working Party on quality Management (ISBT WPQM) have launched initiatives to define quality indicators for Blood Establishments (BE) and Transfusion Medicine Services (TMS). These initiatives could lead to the development of credible and robust quality indicators, leading to harmonization of the quality of transfusion medicine care provided globally, and allow comparison between BE and TMS around the world, using a standard method of collection and processing of data. However, until now (2015) the fruits of these activities are scarce.
Aims
To have information on the vision that the Portuguese professional community of Transfusion Medicine would have on the quality indicators to be used, and how this vision will fit with the international evolution.
Methods
An electronic survey was conducted to collect information on the interest or not, to use a indicator. The indicators used, were those that was possible to collect from the national and international literature, covering all different steps of the transfusion chain and its metric was described.
Results
We received 35 responses from 63 emails sent to all BE and TMS of Portugal (response rate of 55.5%). If one considers the representation of BE in accordance with the red cell components they collected then the response rate translates into 62.1%. Of the 29 indicators presented to the BE 58.6% were consensual, (services indicated that the used or had interest in using). The only indicator in use by 100% (9 BE) was the ‘non‐compliance rate on results of quality control of blood components’. Of the 17 indicators presented to TMS only 29.4% are consensual. One of the proposed indicators for TMS, which stands out for having a different distribution of the others, is the ‘Erythrocyte concentrate rate made available by emergency procedure’ in which 7 (21%) of responses affirm use, but 19 (58%) express no use, but have an interest. There is greater consensus on the indicators presented in BE (58.6%) than in TMS (29.4%). This probably is the perception of the importance of legislation for this type of service and the easiest standardization activities.
Summary/conclusions
The setting and reaching consensus on quality indicators in transfusion medicine is essential, both to improve efficiency and patient safety (optimal use of blood and hemovigilance). Design of indicators and their implementation, should be based on models like SMART (properties of indicators themselves), SPICE (how the indicators should be used) and CREAM (defines what good performance indicators are).
A current challenge is implementing new forms of data standardization in order to obtain indicators which become transversal and also comparable. In conclusion, the establishment of cross‐reference indicators in transfusion medicine is critical; especially to allow benchmarking between BE and TMS, and a lot remains to be done in this area.
P‐100
Performance Indicators: A Tool for Continuous Quality Improvement (CQI)
Gajjar MDG , Bhatnagar M , Soni S , Shah C , Shah D , Patel H
B. J. Medical College & Civil Hospital, Ahmedabad, India
Background
A quality management system includes the organizational structure, responsibilities, policies, processes, procedures and resources, established by management, to achieve and maintain quality. The purpose of quality checks is to provide feedback to the operational staff about the state of a process that is in progress. Performance monitoring is an important tool which can be used for setting priorities for process improvement. We conducted a study to measure the impact of monitoring Performance Indicators and how it could be used as a tool for Continuous Quality Improvement (CQI).
Materials and Methods
The present study was a retrospective study where the performance indicator (PI) data of blood bank was analyzed for over four years. For certain parameters, benchmarks or thresholds are set that represent warning limits or action limits. The yearly data were collated from monthly data. ‘Shifts’ or ‘Trends’, if any, were identified and Corrective and Preventive Action (CAPA) taken accordingly. At the end, outcomes of the analysis were charted. Some Performance Indicators measured yearly were:
• Total number of blood donations with type of donations.
• Total number of blood grouping and antibody screening.
• Cross match: Transfusion Ratio.
• Total number of components discarded.
• Number of Adverse Transfusion Reactions.
Apart from this, the critical and other consumables used in the centre were monitored monthly for the usage pattern and available stock.
Results
After the yearly data evaluation, outcomes obtained were used to plan, correct and amend processes and systems in the blood center. It was observed that the workload of the center showed an upward trend. This helped us to plan for the purchase of consumables and management of manpower. The monitoring of usage and discard of blood helped in the efficient management of blood stocks. The C:T ratio was within acceptable limits which indicated maintenance of quality standards. Decrease in TTI reactive status showed that the donor screening was effective. There was no increase in the number of transfusion reactions, so good quality blood products were being prepared. The inventory monitoring enabled us to keep a check on the stock of critical items. The need for any new equipment could also be judged by the trends in workload.
Conclusion
Performance indicators are indispensible tools which various stakeholders in the Blood Transfusion centres should implement to improve on quality performance. As a consultant, performance monitoring allows to have the reins of blood bank in your hands and the assurance that quality work is being done at your centre.
P‐101
Efficient Incident Management in Blood Services With Kaizen Approach
Eldemir SE1, Ozet GO 2, Aksoy AA 1, Hafizoglu NH 1
1Turkish Red Crescent, Ankara, Turkey2Faculty of Medicine, Yildirim Beyazit University, Ankara, Turkey
Background
Relevant strategies are developed in order to improve processes in blood services and to implement these processes in a further effective and efficient way. Data on performance of main functional and independent processes are monitored via Blood Bank Information Management System (HemOnline) on an instant, daily and monthly basis. Turkish Red Crescent management determines critical control points of processes, numerical goals and indicators are managed with the HemOnline Blood Bank IMS. Errors, accidents, unwanted incidents or reactions, complaints are recorded via forms registered in the quality management documentation system; deviations are recorded in blood components in the HemOnline system. In improvement studies concerning deviation in indicators, Root Cause Analysis (RCA) is applied; then CAPA to prevent recurrence of the improperness/deviation determined are constituted. In the RCA, Fishbone Modeling is employed and causes are questioned under titles of personnel, product, process, management, equipment, environment, and raw material. Unwanted reactions are defined in Donor Reactions in the National Blood and Blood Product Guideline. Unexpected and unwanted conditions observed in donors during the blood donation are defined as unwanted reaction. Unwanted incidents, on the other hand, are defined as incidents causing death or life threat, permanent and significant disability or incapacity or hospitalization or increase in length of hospital stay for patients as a result of donation, process, storage of blood or blood components and transfusion of blood and blood components that are affected. Incidents that are determined and prevented in the last minute are defined as near‐misses. Near‐miss incidents do not affect health and/or safety of the blood donor or patient or properness of the product. Within this scope, errors, accidents, unwanted incidents or reactions, deviations in the collected blood components and complaints should be reported. The blood service departments designate such problems, examine and evaluate them; assess necessary corrective/preventive activities and their efficiency and prevent their recurrence.
Aims
Quality Management System's activity targets to evaluate KAIZEN approach.
Methods
All unwanted incidents recorded are presented in Table 1. Rational analysis of root causes of all unwanted incidents recorded is given in Table 2. The following methodology is followed while CAPA determined by the Improvement. Group in consequence of the RCA are recorded in the system.Main headings regarding Corrective Activities executed in the system are seen.
Results
When CAPA in September 2012–September 2013 are examined and root causes resulting in specific error/deviations are determined once again, new corrective/preventive activity rates are received as follows. Various root cause distribution in separate fields in the other category, except for the root causes displayed in Table 4, makes up 10% slice in total. Other title is omitted from the sample space. In this case, when CAPA that started in 2012 & started 2013 are examined; the improvement rate is 46.33%.

Caption 2: Unwanted incidents that occurred in the system between September 2012–September 2014 and were recorded, and rate of encountering.

Summary/conclusions
Standard practices constituted with simple and gradual improvements are progressed by the improvement groups, whereas the system's sustainability is assured. It is observed that error/deviation sources reception of the personnel participating in the studies, which focus on improving the process with the adapted KAIZEN methodology in the blood services from the beginning, has enhanced. Our affiliated blood service units individually select and apply the most efficient activity, which will eliminate the root causes resulting in error/deviation in these units, among suggestions they determine by including their precious time and opinions and this reflects on efficiency of the activity.
P‐102
Challenges in Change Control in Blood Establishments (BE) – How and When Correctly Change Location of Blood Separators in Use and Validate New Separators at the Same Time
Bolta ZB , Pajk JP
General Hospital Celje, Celje, Slovenia
Background
Since mandatory rules of quality assurance are in use in Slovenia, every centre has been allowed to process whole blood to blood components on new machines, after a successful validation and inspection carried out by the National Competent Authority. Simultaneously location change of separators in use has been done.
Aims
Our aim was to introduce a proper validation of blood processing on new machines and assure relocation of separators used in TC was done in a safe way.
Methods
After plans of change control of moving separators T‐ACE (Terumo) and validation of new machines MacoPress Smart (MPS) were made, the process started. In change control plan, the date, time, separators, personel and mainteinance service were exactly determined, as was the way of gentle handling with machines. Risk assesment for damaging machines during moving was determined as very low to zero.
Results
In two consecutive days relocation and instalation of existing and new separators was carried out. Visual inspection, functional testing and mechanical simulation of separation was done, assuring functionality of the sistem after relocation. Concurrently data transfer was confirmed. New machines were installed, functionally tested and already existing separation parameters were cloned to new machines while establishing data transfer and data processing in supporting software. We continued with validation in next 5 consecutive days with top/bottom (T/B), top/top (T/T) and platelets pooling sets, as are presented in Table 1.
All units were sampled and sent to quality control where, according to Council of Europe Recommendations; we checked the required parameters and obtained following results which are presented in Table 2.
In total 42 donations from exactly defined donors were tested for quality control where according to Council of Europe Recommendations and respecting the set protocol, the results of all tests met the expected percentage criteria for required parameters.
Regular weekly processing was also done on all separators simultaneously, while whole weekly lot of blood components prepared were quarantined till at least day 4 of validation, excepting pooled platelets and were later released based on sucesfull process control proved through validation.
Caption 1: Results of quality control parametres of blood components checked in validation and change control.

Caption 2: Number of days, type of separator, number of units, type of bags involved in validation and change control.
Conclusions
From our experience, we suggest proper discussion before planning the validation; plan must be written in a change control protocol before starting the process.
The plan must be written very clearly; real possibilities for fulfilling the plan must exist, enough material must be in stock. The personnel must be knowledgeable about the procedures and instruments. Difficulties at practical work must be reviewed and solved by a senior person and careful documentation must be kept. Change control and validation plans used in our center were safe enough to fulfill our expectations during simultaneous relocation and validation of the existing and new separators. The results of parameters met defined criterias from 83 to 100%.
P‐103
Quality Indicators and Occurrences in Laura Ayres Laboratory: An Overview
Vasconcelos E , Oliveira I , Maria V , Encarnação F , Rasteiro P , Saias M , Sousa E
IPST, Lisboa, Portugal
Background
Laura Ayres Laboratory (LAL) is characterized by processing and analysis of WB collected by 2 Hospital Collection Sites (HCS) of Algarve and distribution of the corresponding blood components.
Aims
To identify strengths and weaknesses of LAL through Quality Indicators (QI) and Occurrences.
Methods
The period of study was from 01/2013 to 03/2015. A strict non‐blame policy was established. QI had a monthly review and included Internal and External QC (%success = 93–95%), components QC (failure < 1.2%), ruptures/leaking during processing (<0.4%) and a > 82% capability of satisfying the Platelet Concentrates needs of Hospitals. Dysfunctions, referred to as occurrences, were gradually being identified. Reports were made through an Informatics‐Reporting‐System and forward to staff, IPST‐Quality‐Department and suppliers/clients when applicable. Classification of occurrences was made according to risk, reoccurrence, waste of resources and impact in the organization. A near‐miss was defined as a wrong product/service detected or corrected before being issued to Hospital; after delivery it was considered an error. It was expected that those involved in occurrences contributed for its understanding, correction and prevention.
Results
During this period, LAL received 29904 WB. QI were satisfactory, going beyond expectations. Occurrences are shown in table 1.
Discussion
QI demonstrated a good staff performance. Near‐miss accounted for the majority of occurrences (n = 0.83%), mostly related to collection. This process, under HCS responsibility, involves manual procedures and is prone to dysfunctions. Nevertheless, risk cannot be neglected; some failures led to WB discards, extra QC and influenced processing. Informatics‐system was quite vulnerable, near‐miss related to ‘incapability‐of‐recognizing‐blood‐group‐discrepancies’ carrying a high risk. Occurrences related to equipment/suppliers influenced the output of LAL and consequently were included in reports. Errors (n = 32), with the exception of ‘wrong‐expiration‐date‐of RCC’ which was a consequence of the Informatics‐system, had human origin. However, the cause of ‘RCC‐in‐wrong‐bag’ was not clarified, another hypothesis being a bag defect. Regarding bacterial screening, only one case was a true‐positive contamination. Furthermore, the cause might not have been related to LLA. In summary, QI and Occurrences allowed a quick overview of the organization. Staff is the strength of LAL. The Informatics‐system presented some weaknesses, but is currently under revision and consequently it is expected that in short term fragilities will be overcome.
Caption 1: Table 1: Occurrences in LAL.

P‐104
Blood Discarding – Quality Indicators Survey
Raka F , Makarovska-Bojadzieva T , Damevska-Todorovska O , Blagoevska M , Petkovic E , Grubovik R
National Institute of Transfusion Medicine‐Skopje, Skopje, Macedonia
Background
Quality indicators as a tool of the Quality Management System (QMS) serve to monitor and control the level of quality performance of the blood establishment enabling a better insight of the whole process within and outside the institution. The implementation of the system and continuous evaluation of the activities of the blood transfusion service can help achieve better outcome regarding safe blood.
Aims
To evaluate the rate of discard of blood components due to inappropriate collection, processing and storage since the introduction of the QMS in 2007.
Methods
The data on the number of discarded blood units were obtained from the Department of blood control of the National Institute for Transfusion Medicine (NITM)‐Skopje. This retrospective study involved the analysis of the four‐year period of time (from January 2009 through December 2012).
Results
During the period 2009–2012 at NITM‐Skopje were collected totally 105,761 blood units (median 26,440 per year). The total number of discard was 4010 (3.8%) with the average number of 1003 discarded units per year. According to the reason of discard, the highest percentage during the first year was due to reactive samples to TTI (transfusion transmissible infections samples), predominantly reactivity to HBsAg. The next two years of our study period showed switch in the major reason for discard. Lipemic blood units were presented in 1.4% of the collected units in 2010 showing a clear upward trend through the years, reaching the peak in 2011(2.29%). A downward trend of appearance was observed regarding faulty marking and other non‐conformances (0.45 in 2010 to 0.21% in 2012) as well as hemolysis (0.02 in 2009, 0.03 in 2010 to 0.003% in 2012) in the blood units. Underweight blood showed variables through the analyzed period of time (from 0.2% in 2009, 0.6% in 2010; 0.7% in 2011 and 0.6% in 2012), significantly increasing in the analyzed time interval. The discard rate of outdated RBCs showed no significant variables (5.07% in 2011; 4.15% in 2012).
Summary/conclusions
The percentage of discarded units is within internationally accepted limits. However, a good donor selection, regular training and evaluation of the staff, implementation of automation in order to improve processes and output, as well as monitoring of the whole system in order to introduce specific corrective and preventive measures when needed, will significantly reduce the discard rate of blood components and raise the level of quality.
P‐105
Non‐Conformities as Quality Indicators in ITM MK in the Period 2007–2014
Todorovska ODT , Velkova EV , Makarovska Bojadzieva MBT , Todorovski BLT , Veljanovski VN , Trajkovska TJ , Mitevska ML
Institute of Transfusion Medicine of RM, Skopje, Macedonia
Background
The Blood transfusion Service in the Republic of Macedonia is an essential and integral part of the health system in the country. Its goal is: self ‐sufficient supply of safe blood from voluntary non remunerated blood donors, for clinical use in many medical branches. Republic of Macedonia, with a population of 2,065,769 inhabitants, realized apx. 50,000 blood donations/year under WHO and CoE recommendations, according to the annual programmes made by the National Institute for Transfusion Medicine and the Macedonian Red Cross Organization, supported by the Ministry of Health. The reorganization of the BTS was done with harmonization of the legislation with the EU and the Law for Safety in Blood Supply was adopted by the Parliament of RM on September 5, 2007, corresponding to Eu Dir. 2002/98/EC and finished 2010 harmonizing with Eu. Dir. 2005/61/EC. ISO standardization is under implementation. The national SOP Manual in 2010 was harmonized with EU SOP Manual. Hospital Committees – 2013 introduced in all clinical hospitals in the country. Blood needs in the RM are apx. 55,000 donations per year. Also stem cells and PLT aferesis collections are in place.
Aim
To evaluate the non‐conformities as quality indicators in the transfusion medicine after the reorganization of the Institute of Transfusion Medicine in Macedonia and the introduction of QA/QC since 2007.
Table 1. Non‐conformities in reported to QA in ITM MK for 7 years period.

Material and methods
The department for QA/QC monthly and yearly reports and records for non‐conformities.
Results
The reports for non‐conformities ranged from 64 untill 2009, up to 52 in 2014, or 0.1% of 51,636 donations in MK. Most of them were coagula in RBC‐SAG concentrates, miss‐labeled component or blood samples, outdated blood component issued for clinical use, leakage from broken blood bag and not serious adverse reactions.
Conclusion
The analysis showes that most of the non‐conformities were due to the unproper mixing during blood collection with the corrective action undertaken – training of the staff in blood donation procedure, FFP leakage due to unproper handling during the production, storage and transportation. There were no reported serious adverse reactions. The introduction of QMS and regular training of the BT staff shows visible results and it is constantly needed and recommended, in clinics as well as in BTS.
P‐106
Full Audit Cycle of Use of Irradiated Cellular Blood Components in National Cancer Institute of Sri Lanka
Perera WWK1, Morawaka RL 2
1Kings College NHS Foundation Trust, London, United Kingdom2National cancer Institute of Sri Lanka, Maharagama, Sri Lanka
Background
National Cancer Institute (NCI), is a well‐established institution and only tertiary care center for cancer management in Sri Lanka. The Transfusion Medicine Department, NCI has yearly transfusion rate of over 56,000 units, and caters for special product requirements of needy patients such as leucoreduced, irradiated and washed blood products.
Transfusion Medicine department, NCI, has formally introduced irradiation facilities with NBTS, in January 2011. This was done in parallel with the guideline issue, and the introduction of alert card for patients who are in need of life‐long irradiated products, which was followed by a local circular. This was done accompanied with awareness programmme to stress the importance of minimizing the possibility of rare, but invariably fatal complication of Transfusion Associated Graph versus host disease.
Aims
Full audit cycle was performed to ascertain:
1. Compliance of requesting Irradiated cellular blood components for indicated patients.
2. Non‐Irradiated cellular blood components were transfused for whom Irradiated components are indicated.
Methods
Relevant blood request forms, transfusion laboratory documentation, and irradiation records were compiled and analyzed for one year in the primary audit. Compliance data for requests, as well as issues were presented according to guideline criteria, with recommendations and action plan.
Re‐audit was done after implementation of action plan, leaving adequate time period for changes to take place, with same audit criteria, and finding recorded and analyzed.
Results
In the primary audit it was shown that only 9.8% requests accompanied the irradiation requirement in the request. This was improved to 16.4% after awareness programme, which was recommended by primary audit.
The most likely indication of patients receiving non‐irradiated components was Hodgkin's Lymphoma, 56.2% being in the primary audit, was much lowered to 10% following awareness programme.
8.9% of patients who were indicated Irradiated cellular components, received non‐irradiated products in the primary audit, and was seen reduced to 1.16% in re‐audit.
Neither the primary nor re‐audit identified patients receiving Purine analogue treatment, an identified limitation.
Summary
The principal aim of the full audit cycle has been achieved by successfully implementing the changes by the clinical departments. However, it is recommended to have periodic audits to reassess the compliance, as well as, implement measures of increasing adherence to the guidelines to make this a productive exercise.
The results were presented at NCI, in 03rd July 2014, and the report was disseminated to, National Cancer Institute, and, National Blood Transfusion Service of Sri Lanka.
P‐107
Standardization of Plasma Collection for Fractionation
Navidrouyan M , Navidrouyan M , Talebian A , Banazadeh S
Iranian Blood Research and Fractionation Company, Tehran, Iran
Background
Iran was the first country in the Middle East that had fractionation facility. In spite of the valuable experiences in plasma industry, the fractionation plant was shut down due to the lack of virus inactivation technology. Iranian Blood transfusion Organization (IBTO) as the governmental organization is responsible for providing safe blood and blood derivatives to the Health care system. Since, the BTCs collect whole blood, the recovered plasma was the starting material of the fractionation facility. After closing the national fractionation company the policy makers decided to use the surplus plasma via Toll manufacturing in order to provide the plasma derived products for the country.
In this regard, Iranian Blood Research and Fractionation Company (IBRF) was determined to collect the surplus frozen recovered plasma of the BTCs and transport them to the Central Plasma Warehouse palletize the plasma boxes and store them until their transport to Fractionation Company.
Aim
Self‐sufficiency in plasma derived products is a goal in Iran's health‐care system. Our goal is to have a safe and sufficient plasma derivatives in our country.
In this regard, Iran has decided to perform toll manufacturing fractionation for surplus plasma.
Method
At first, IBTO selected 12 Blood Transfusion Centers (BTCs) from all around Iran and these BTCs were inspected by German Health Authorities. After approval of European authorities, sending plasma to the fractionators began. Now 19 out of 31 BTCs across Iran have already been active contributing to toll fractionation.
Frozen plasma which have been collected from approved BTCs are transported to IBRF, according to cold chain regulations. After arrival to IBRF plasma boxes are packaged on pallets and stored in validated freezer rooms.
A quality management system was established in IBRF according to GMP requirements as the following:
1. preparing related instructions and SOPs;
2. training of truck drivers and plasma warehouse and engineering staff according to approved procedures and GMP and retraining in determined intervals;
3. continuous monitoring of the approved processes;
4. internal inspection twice a year;
5. reviewing deviations and non‐conformances and providing corrective action for them;6organizing external inspections from regulatory authorities and fractionation Companies.
Results
In previous 10 years, about 800,000 l plasma were collected from BTCs, under the supervision of the Iran‐Food and Drug Organization and sent to fractionation companies. After implementing the quality management system, the performance of the mobile cold rooms (trucks) and plasma warehouse showed that they had optimum performance. The deviations were only minor which were overcome by remedial action.
Conclusion
Since the plasma is a precious material, Toll manufacturing is a suitable way to prevent it from wasting and saving it is a strategic action.
Iran's experience showed that it is possible to save plasma and provide the necessary plasma‐derived products from local plasma.
In‐addition, there are significant economic benefits in using local plasma. With performing toll manufacturing, Iran is now self‐sufficient in IVIG and Coagulation Factor IX and only Albumin and Coagulation Factor VIII is imported.
P‐108
Why Should Anti‐Leukocyte Filters be Examined Before Qualifying Them for the Routine Use, Although They Have Attests and Certificates of Conformity – Case Study
Budzynski L , Skrzypczak A
Regional Blood Center in Poznan, Poznan, Poland
Background
Disposable equipment, reagents, tests and other materials used for blood donations should be checked before qualifying them for the routine use. This rule applies to each batch, and even every single delivery. Particular attention shall be given to situations when dealing with new equipment, manufacturer or supplier.
Aims
The aim of the study is to discuss the results of research that was carried out before qualifying new filters for the removal of leukocytes in red blood cells concentrate for the routine use. These filters are manufactured in a country outside the European Union and had not been used earlier in our institution. They had the declaration of conformity for Class IIa medical devices, CE mark and an EC conformity certificate. Each batch had a certificate which reported positive results for chemical and biological tests. The manufacturer declared inter alia: the leukocyte content after the filtration <1 × 106 per unit, the average recovery of red blood cells greater than 95%, blood loss less than 30 ml after the filtration.
Materials and Methods
Fivety‐four units of red blood cells concentrate were leukoreduced by means of the filters of three different batches delivered at different times. The assessed parameters were the volume, RBC and WBC value. RBC and WBC before the filtration and RBC after filtration were marked with a hematology counter Pentra XL80 by Horiba using the impedance method. WBC value after the filtration was tested using the Beckman Coulter flow cytometer with a Leukosure Enumeration Kit set. The principle of this method is based on the removal of RNA with RNase and then staining the DNA with propidium iodide.
Results
With the first equipment delivery of 30 units of red blood cells concentrate. 80% of the components were obtained with the content of the leukocytes >1 × 106, with an average of 4.22 × 106. The average recovery of red blood cells was 89% and the average blood loss after filtration was 29 ml.
The second delivery was tested only in terms of the content of leukocytes after the filtration. Twelve units of red blood cells concentrate were examined. 58% of the components were obtained with the leukocyte content of >1 × 106, with the average of 1.64 × 106.
In the third delivery 12 filters were used as well. 42% of the components were obtained with the leukocyte content of >1 × 106, with the average of 1.54 × 106. The average recovery of red blood cells was 91% while all the ingredients fulfilled the declared blood loss.
Finally, no component was sent to the therapeutics, and the filters were not allowed to use. With each delivery the quality of the filters improves, which is indicated by the test results.

Summary/Conclusion
The CE mark, declaration of conformity, batch certificates and statements of compliance cannot replace the validation and the authorization studies. The described filters had all documents allowing them to be used in the EU but studies showed that the components produced with their help did not meet the quality standards for leukoreduced red blood cells.
P‐109
Evaluation of Mobile Blood Drive Survey Assessment
Ogan DO1, Ozet GO 2, Eldemir SE 1, dr Aksoy AA 1
1Turkish Red Crescent, Ankara, Turkey2Yildirim Beyazit University, Faculty of Medicine, Ankara, Turkey
Background
In line with ‘Safe Blood Donation Program’ determined in cooperation with the MoH, Turkish Red Crescent Blood Services has aimed to increase the number of voluntary blood donations and provide healthy and proper service to people in need, by raising awareness of our society on blood donation since 2005 until today. Accordingly, having started with 29 Blood Stations in 2005 (Figure 1), the process has been continuing its activities with 16 Regional Blood Centres and 62 Blood Donation Centres in 2014 (Figure 2).
Directorate General of Blood Services carries out TS EN ISO 9001:2008 QMS Standard in a bid to sustain quality of products and service. Quality management is a system based on customer satisfaction. Corporate mobile blood sessions are formed with a view to meeting the country's need for blood and gaining regular blood donors. Assuring corporate blood donor satisfaction constitutes the basis.
Data of the mobile blood drive assessment surveys are recorded by the Quality Management Head into the Process Performance Measure Software.
Aims
To measure satisfaction of corporate blood donors of BDC to reach volunteer blood donors.
Method
Between 01.01.2014 and 30.09.2014, 36,162 blood donation mobile blood sessions were formed. Number of the participants in our survey study is 3869 persons. The survey consisting of nine questions targets to receive opinion of blood services units within the body of Directorate General of Blood Services about their mobile blood sessions and their satisfaction status.
Findings
Of 1,399,390 units of blood donation collected by BDC on 01.01.2014–30.09.2014, 1,095,810 units of blood donation were collected through mobile blood sessions that was organized with corporate blood donors and Blood Donation Centers.
Our corporate‐blood donors, participation to voluntary, non‐remunerated and regular blood donation organisations of Blood Donation Centers (BDC) as a company, organized the mobile blood sessions with our BDC's. The scheduling, timing and also the place of the sessions are organized by corporate blood donors and our donor recruitment staff of BDC. Survey study concerning collaborative institutions and its findings are written in the table.
Summary/Discussion
According to the Turkish Red Crescent Quality Improvement Program, 95% satisfaction rate was our Key Performance Indicator and the aim. Due to the evaluation of mobile blood sessions survey assessments our corporate blood donors were satisfied about the blood donation campains and the rate is at the acceptable level. Due to quality improvement program, all parts of the survey has been analysed with the root cause analysis. Within this framework; 1208 training sessions were organized to the corporate blood donors and in this sessions 243,811 participants have been attended in the first 9 months of 2014. Moreover, blood service units performed assessments concerning personnel approaches and mobile blood session‐hour. According to evaluation, some of the staff cannot attend to the training sessions because of the timing or scheduling. Since volunteer and regular groups could be formed only with our corporate blood donors, blood donation organizations should be supported with trainings, satisfaction should be maintained at maximum level with encouraging programs, and our relations with institutions should be maintained within a strategic planning to enable our country to collect safe blood.
Caption 1: Survey study concerning collaborative institutions and its findings are written in the table.

Caption 2: Structuring of Turkish Red Crescent Blood Services in the Republic of Turkey in 2014.

P‐110
From Donor to Patient: Demonstration Study of The New Dutch Blood Transfusion Data Warehouse – Proton II
Kemper PF1, van Hoeven LR 2, Hooftman B 3, Janssen MP 2, Leyte A 4, de Bruijne MC 3, Koopman MMW 5
1Sanquin, Leiden, The Netherlands2Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, The Netherlands3EMGO+ Institute for Health and Care Research, VU Medical Center, Amsterdam, The Netherlands4OLVG hospital, Amsterdam, The Netherlands5Sanquin Bloodbank, Department of Transfusion Medicine; Sanquin Blood Supply, Amsterdam, The Netherlands
Background
Blood transfusion has health related, economical and safety implications. Still, the question whether, when, what and how much should be transfused in a specific patient population cannot be answered unequivocally. Blood is a biological product having a natural variability that can evoke reactions in the recipient. Clustering all blood transfusion related data creates the possibility to investigate the association of donor characteristics and patient outcomes (e.g., having HLA‐antibodies and the risk on TRALI). There is also the possibility to explore new determinants, for example hemoglobin (Hb) level of the donor, age, frequency of donation in relation to clinical effects in patients. Moreover, clustering data within a data‐warehouse facilitates benchmarking of both donor and patient blood management (e.g., transfusion triggers), and the evaluation of alterations in the supply chain (e.g., management outdating blood products).
Aims
We aim to:
1. Present the design and possibilities of the Dutch blood transfusion data‐warehouse;
2. Show an example of the first results regarding the relationship between the Hb level of the donor and the storage time of the red blood cell (RBC) product with the Hb change of the recipient.
Methods
The PROTON II Dutch blood transfusion data‐warehouse is built containing data on blood donors, blood products, and transfusion recipients. Donor and patient data were matched based on the unique identifier of the blood unit. PROTON II is still under construction; therefore on the recipient side complete data of one teaching hospital was available for the analysis. We selected patients (aged ≥ 18 years) who received one RBC transfusion during the study period (calendar year 2010) with at least one Hb measurement during a 24 h period before the transfusion and one measurement during the period of 1–48 h after the transfusion (n = 86). In case of multiple measurements, we selected the lowest level before and the first measurement after transfusion. Linear regression analysis was performed with Hb difference of the patient as dependent variable, predicted by the Hb level of the donor at the time of donation and the storage time of the RBC product in days as potential confounder.
Preliminary results
The first analysis showed that the Hb level of the donor was not significantly associated with the difference in Hb level of the transfusion recipient (β = 0.02; P = 0.82), nor was storage time of the RBC (β = −0.15; P = 0.15). Further analyses will make use of more hospital data if available and incorporate the time between Hb measurement and the transfusion, the age and gender of donor and recipient, as well as the match between blood groups of donor and recipient.
Summary/Conclusions
The PROTON II data‐warehouse allows studying the effect of various donor characteristics on patient outcomes, providing insight into potential improvements in effectiveness and safety in the blood transfusion chain. The preliminary results show no effect of the Hb level of the donor and storage time of the RBC product on Hb increase in transfusion recipients, most likely due to the limited number of observations.
2.1 Blood donor recruitment
P‐111
Improving First Time Donor Attendance Rates Through the Use of Tailored Donor Preparation Materials
Masser BM1, Hunder E 2, Foot J 2, Rozsa A 2, Hayman J 3, France C 4
1The University of Queensland, Brisbane, Qld, Australia2Australian Red Cross Blood Service, Melbourne, Vic., Australia3Monash University, Melbourne, Vic., Australia4Ohio University, Athens, GA, USA
Background
Donor recruitment is critical to ensuring a stable and sufficient donor panel for blood collection services (BCS). Many non‐donors are positive about blood donation and this motivates them to make an appointment to donate. However, as their appointment approaches, barriers to donating – such as anxiety – may become more salient and deter attendance.
Aims
Building on France and colleagues research demonstrating the positive effect of enhanced preparation on blood donor recruitment, the current study sought to determine whether communication materials designed to address donors’ concerns about donating may be an effective way to boost first donation appointment attendance rates.
Method
A field study comprising a 3 (brochure: none, email, hard copy) × 2 (National Call Centre [NCC] contact: none, call) between‐subjects design was conducted. Participants were 3646 non‐donors who made their first appointment to donate between July–October 2014. Participants in the brochure conditions received either a hard copy or an emailed link to an electronic form of a brochure modelled on France and colleagues donor preparation materials. In addition, participants in the NCC call condition also received a call scripted in line with France and colleagues donor preparation materials. The outcome measure was new donor attendance rate.
Results
Although first appointment attendance rates were high in the no brochure, no call control condition (81.10%), any contact from the BCS prior to the scheduled donation appointment boosted attendance. The relative risk of attending was highest in the NCC call and electronic brochure modelled on France and colleagues donor preparation materials condition at 1.11 (95% CI, 1.06–1.17, P = 0.0001). The attendance rate of donors in the NCC call and electronic brochure condition was improved by 11.2% on that observed in the control condition. Further the relative risk of attending in the NCC call and electronic brochure was higher than in the NCC call alone (1.04, 95% CI, 1.01–1.09, P = 0.0468) or the electronic brochure alone (1.05, 95% CI, 1.01–1.09, P = 0.0267) conditions.
Conclusion
The use of a tailored communication strategy that addresses new donors concerns and prepares them for donating bolsters new donors attendance rates.
P‐112
Perceptions of Maltese Blood Donors Regarding Reasons Encouraging or Deterring Blood Donation by Others
Borg-Aquilina DBA , Abela MA , Scerri RS , Aquilina AA
Malta National Blood Transfusion Service, Naxxar, Malta
Background
Malta, an island nation in the middle of the Mediterranean Sea, has a population of ~425,000. One Blood Establishment, the National Blood Transfusion Service, caters for all the whole blood (WB) collection in the country.
Aim
To determine the occupational backgrounds of donors and understand their perceptions regarding what encourages and deters other people to donate WB. This will reveal if there is a difference in perception for donation between the different occupational categories. Results will be used for marketing purposes.
Methods
A survey was organised amongst blood donors to evaluate the service provided to them. Donors consenting to take part in the survey were asked to complete the questionnaire during the 10 min post‐donation time they spent in the refreshment area. Donors deferred from donation were not asked to complete the survey. Results were analysed using a spreadsheet program.
Results
A total of 1107 donors consented to complete the survey over 5 weeks in July 2013. Donors who completed the Occupation question (1059 donors i.e. 95.66%), were categorised into Office/ Professional, Police/Soldiers, Manual Workers, Retired, Housewives, Students, Health Care Workers and Uncategorised groups. Altruistic reasons were sorted according to groups as described in the paper by Evans and Ferguson (2014).
In general, donors undertaking the survey were of the opinion that pure altruism was the main reason for WB donation, that is ‘the ultimate desire to help others at a personal cost, without reward’ (43.81%). The warm glow effect of donation followed as a reason for donation (16.90%) and was cited mostly by students. The least popular motives for others to donate blood were hedonistic reasons i.e. ‘…egoistic motive whereby helping is used to increase personal gain without concern for the recipient's welfare…’ mentioned by four donors (0.38%) (one Office/Professional and three Manual workers), and reluctant altruism (reported by 14 donors or 1.32%), most of whom were categorised under the Office/Professional group.
Regarding deterrents for WB donation according to donors, the commonest reason was fear (59.68%), in particular fear of the needle or venepuncture (30.69%) quoted mostly by the clergy, retired donors and students. This was followed by fear of the procedure (20.68%) cited mostly by Office/Professionals. One donor on the Office/Professional category mentioned that other donors might be afraid of being infected by blood donation. Other less common reasons for donors not to donate blood were lack of time (3.49%) and lack of information about blood donation (4.53%).
Summary/Conclusions
The information gathered in this survey will be used to tailor the marketing programme to better target donors according to the above results. Consolidation of pure altruistic motivation for WB donation for all donor groups is to continue. For deterrents of WB donation, an intensive education strategy is fundamental to manage fear perceptions, as well as to increase information available regarding WB donation to the general public.
P‐113
Abstract moved from Poster to Oral.
P‐114
Coagulation Disorders in Blood Donors: A Single‐Centre Analysis
Zighetti ML , Tagliabue L , Vozzo N , Pugliano M , Carpani G
Azienda Ospedaliera San Paolo, Milan, Italy
Background
Blood donor eligibility criteria are designed to protect both the blood donor and the recipient from harm and are based on informed medical evaluation, laboratory tests and nationwide regulatory rules.
The presence of severe coagulopathies in donorsisan exclusion criteriafor the donation. Two laboratory tests are commonly performed to detect possible coagulation disorders: prothrombin time (PT) which measures the optimal functioning of the extrinsic pathway as well ascommon pathway coagulation factors and activated partial thromboplastin time (aPTT), which measures the functioning of the intrinsic pathway coagulation factors and, also, the common pathway ones.
Aims
The scope of this study was to describe the results of a screening for coagulationdisorders performed in blood donors attending our Centre for their first donation.
Methods
We performed systematic screening for coagulation disorders in 4931 blood donors (median age 41 year, range 18–60) come to our Centrefrom January 2010 to December 2013.
Results
Among the 4931 blood donors nine subjects (0.18%) with prolonged aPTT and 4 (0.08%) with prolonged PT were identified. Causes for aPTT‐prolongation were mild vWF deficiency (0.02%), FXI deficiency (0.08%), FXII deficiency (0.02%), FXII + FXI deficiency (0.02%) and lupus anticoagulant (0.02%). Cause for PT‐prolongation was mild FVII deficiency (0.08%). In one subject the cause for the prolongation of the APTT remained unexplained. No bleeding diathesis was detected in the blood donors, none of the FXII deficient subjects or subject with lupus anticoagulant had a positive history of thromboembolism.
Conclusions
On the basis of these data the prevalence of mild coagulation disorders in our blood donors can be estimated to be 0.02–0.08%. Donors with a mild inherited coagulation disorders were permanently deferred from plasma donations.
P‐115
Why are You not Donating for VNRBD? A Description of Reasons
Villumsen JV
National Danish Blood Donor Association, Taastrup, Denmark
Background
The National Danish Blood Donor Association (NDBDA) asked non‐donors questions about their knowledge and preferences concerning blood donation. A key question was: Why are you not donating blood (VNRBD)?
Aims
To collect knowledge about how large a part of the grown‐up population who would be willing to sign up for VNRBD.
To get a general knowledge about why people do not want to sign‐up. Secondary purpose is to collect information on potential blood donors, so that we can develop the most effective recruitment of donors.
Methods
We collected 1199 answers through a digital questionnaire. The sample was chosen randomly, but it was ensured that it was representative for the Danish population. The first question to the panel was: Are you a blood donor? If they answered ‘yes'or ‘have been’ they were omitted. 1.199 persons were included. They answered 14 questions. The collection of data and sampling was done by yougov.dk.
The respondent was asked: What is the reason, that you are not donating blood (VNRBD)? They could choose between 14 predefined answers and ‘Another reason’ (this was always the last category). The 14 categories were presented in a random order. There was not limit on how many answers you could choose.
Results
The top three answers were:
123.6%, ‘I want to be a blood donor, but I haven't gotten around to do something about it’;219.7%, ‘I'm afraid of needles’;317.8% ‘I don't think that I can be a blood donor because of my life style, my sexual behavior or I travel a lot’;4Less than 11.3% choose any of the other categories.
Since a respondent can choose more answers we have looked into how many of the respondents that are potential donors. We defined a potential donor as one who had chosen at least one of the four positive answers, and the respondent should not have marked any of the six answers that would definitely disqualify them from donation.
362 (30.2%) would qualify as a potential donor. This group is a little younger than the entire population in this analysis, and males in this group are a majority (57.5%) compared to 48.7% of all the respondents.
602 (50.2%) answered at least one question, that indicates that they will never sign up.


We have only made a descriptive analysis. We have not intended to explain if any answers can be explained by socio‐economic or other factors.
Conclusions
30.2% of the interviewed persons are willing to sign up for VNRBD. They have marked at least one of the four answers, that were positive towards VNRBD, and they have NOT marked any of the six answers that would disqualify them as blood donors. For recruitment purpose it is good to know how many people are willing to sign up. If you are able to focus your recruitment on this share of the population your recruitment will be more effective.
P‐116
Filled Prescriptions as a Proxy for Morbidity in Register‐Based Studies of Blood Donor Health
Zimmer ASZ1, Pedersen OB 2, Ullum H 3, Erikstrup C 1
1Aarhus University Hospital, Aarhus N, Denmark2Næstved Hospital, Næstved, Denmark3Copenhagen University Hospital, Copenhagen, Denmark
Background
Traditionally, morbidity has been assessed in register‐based population studies as, e.g., admissions to hospital. This endpoint has also been used previously to assess morbidity among blood donors. However, the power in such studies may be limited due to few events. We used instead prescriptions to blood donors accessed through the National Prescription Register in Denmark (NPR) to assess morbidity. We compared the incidence of filled prescriptions between high and low frequency blood donors.
Aims
The aim of this study was to assess the power of filled prescriptions as a proxy for morbidity and to assess the health of blood donors in comparing high‐frequency and low‐frequency donors.
Methods
The study included 37,808 participants from The Danish Blood Donor Study (DBDS) initiated March 1St 2010. Participants filled out a questionnaire and blood samples were collected. Follow‐up data were obtained from the NPR.
The prescriptions were divided in to subgroups comprising heart‐medication, painkillers, antimicrobials, psychotropic drugs, and medications for asthma and allergy using the relevant ATC‐codes in the NPR.
Multivariable Cox proportional hazards analysis was used to examine the association between donation frequency and filled prescriptions from the subgroups defined above. Participants were followed from inclusion in the study and until event or censoring at December 31st 2012. Age was used as the underlying timescale and the analysis was adjusted for current smoking behavior. Donation frequency was defined as a dichotomous variable. In separate analyses we estimated whether donation frequency during either 1 year or 3 years prior to and including the enrollment donation was associated with filling a prescription. The cutoffs used were >3 donations and >8 donations, respectively, equaling approximately the 20% of donors donating most frequently. The analyses were performed for men and women separately.
Results
During 48,492 person‐years of observation, 22,198 participants received at least one prescription (Table 1). A high donation frequency in women was associated with a decreased risk of receiving a prescription of either painkillers (1 year: HR: 0.91, 3 years: HR: 0.91) or psychotropic drugs (1 year: HR: 0.85, 3 years: HR: 0.81). For antimicrobials we found a lower risk of receiving a prescription in men with high donation frequency (1 year: HR: 0.90, 3 years: HR: 0.91).

Conclusion
Half the active blood donors donating within the same year received a prescription for some kind of medication. The lower incidence of receiving a prescription among high frequency donors for various medications is consistent with the healthy donor effect.
P‐117
Knowledge, Attitude and Practice About Voluntary Blood Donation Among Young Student Population of Karachi
Nepal B1, Mukhtar Z 2
1Dr.Ishrat Ul Ebad Khan Institute of Blood Diseases, Karachi, Pakistan2Safe Blood Transfusion Service Project, Karachi, Pakistan
Background
Safe blood is a crucial and irreplaceable component in the medical management of many diseases. The Voluntary non‐remunerated blood donation is the ideal sources of quality blood, which forms less than 15% of the demand of the blood in Pakistan. Motivation among the youth, particularly students, is essential to make voluntary blood movement more successful.
Aims
To assess the knowledge, attitude and practice regarding the voluntary blood donation among the young student population of Karachi so that an effective approach can be made regarding motivation enrollment of voluntary non remunerated blood donors in future in Pakistan
Methods
A cross sectional prospective study was conducted among 700 students from different universities and colleges of Karachi. A well‐structured and pre‐tested questionnaire, in English, was used to access the knowledge, attitudes and practices about voluntary blood donation. A scoring mechanism was used to understand overall knowledge level. The participants were given a briefing about the objectives of the study and confidentiality about the personal data.
Obtained data was analyzed by using statistical package of social sciences (SPSS) version 17.0 in computer. Statistical significance level was set at P ≤ 0.05.
Results
The sample population consisted of 54% male and 46% female students in the age group of 18–28 years. Only 65% of the students have heard about voluntary blood donation and 28% of the students have given blood once in their lifetime and among them 19% are blood donors at the moment. 42% of the participants believed that there is a specific reason why they don't donate blood and 59% believed that there is a risk involved for the donors, when donating blood. 80% students wanted to promote voluntary blood donation. Fear and lack of awareness on blood donation are the reasons for not donating blood. Students gather information about voluntary blood donation from several sources mostly schools, colleges, family and friends.
Conclusions
This study showed that myths and misconceptions are leading the youngsters not to donate blood. Study also showed how increasing awareness and marketing through different ways can boost the culture of voluntary blood donation in society. Student population can be motivated to participate in different ways. There is a dire need to mobilize the electronic media for educating our youth about voluntary blood donation due to its access to masses.
P‐118
Evaluation of the Non‐Invasive Method of Hemoglobin Measurement in Polish Volunteers and Donors with Haemospect® Analyzer
Plodzich AP1, Lachert E 1, Antoniewicz-Papis J 1, Rosiek A 1, Chud-Wisniewska W 2, Letowska M 1
1Institute of Hematology and Transfusion Medicine, Warsaw, Poland2Regional Blood Transfusion Center, Warsaw, Poland
Background
Hb screening for donors and donor candidates is essential to prevent phlebotomy in anemic individuals and ensure normal hemoglobin (Hb) content in blood units. Hb measurements are routinely taken from vein and capillary blood. Recently however, noninvasive methods of Hb measurement have become available as alternative to currently used methods. The Haemospect® analyzer was designed for such purpose and is welcome both by donors and staff. It consists of a portable device and a clip‐sensor which is placed on the middle finger. Two‐size clips are available; S‐size grey and L‐size black. The analyzer is equipped with a pre‐measurement calibration module. The measurement is based on spectroscopy. A sensor head placed on the finger‐skin projects a white light into the underlying tissue via a waveguide. Some of the projected light is reflected and transmits information about the substances in the tissue. The data is then software processed and results are displayed in g/dl, g/l or mmol/l. The system was developed for Hb range of 9–18 g/dl (±10%).
Aim
To compare the Hb measurements obtained with analyzers: Haemospect, Beckaman Coulter and Hemocue.
Methods
Blood samples collected (9–22 September 2014) were screened for Hb concentrations with three different analyzer: Haemospect, Beckaman Coulter hematology analyzer and Hemocue. The first stage of the study involved comparison of Hb measurements from Haemospect and Beckaman Coulter hematology analyzer in samples collected from 44 IHTM volunteers. In the second stage, the Hb measurements from n = 73 donors (RBTC) obtained with Haemospect were compared with those obtained with Beckaman Coulter hematology analyzer and HemoCue. Since there are different Hb criteria for men and women donors (≥13.5 g/dl and ≥12.5 g/dl respectively) the test results for men and for women were analyzed separately.
Results
The first stage of the study involved 14 men and 30 women. No statistically significant differences were observed between the results obtained with Haemospect and Beckaman Coulter hematology analyzer in either group (n = 30 women; P > 0.05 and n = 14 men; P > 0.05). In 60% of cases the differences between measurements made with Haemospect and reference hematology analyzer did not exceed 1 g/dl; in five cases the difference exceeded 2 g/dl, but in medical interview two persons reported wrist injury and one woman was treated for anaemia. The second stage of the study involved 64 men and nine women (P > 0.05 and P > 0.05 respectively). No statistically significant differences were found between results obtained with Beckman Coulter hematology analyzer, Hemocue and Haemospect.
Conclusion
The study results demonstrate that Haemospect analyzer may be used by blood establishments for donor and donor candidate screening as an attractive alternative to vein and capillary tests. However, taking into account the 10% measurement error (declared by the manufacturer) we suggest that all Hb measurements that oscillate around the lower limit normal (≥13.5 g/dl and ≥12.5 g/dl for men and women respectively) are twice repeated. If all three measurements are at lower limit of normal the person should be deferred from donation of blood and blood components.
P‐119
The Role of Healthcare Providers in HIV or Other Infectious Diseases Test‐Seeking Behavior in Different Sub Populations of Brazilian Blood Donors
Goncalez TT1, Moreno ECM 2, Bruhn RB 1, Moreno ECM 2, S Sabino EC 3, Miranda CM 4, Carneiro-Proietti ABCP 5, Bolina-Santos EBS 6, Lopes MEL 7, Loureiro PL 4, Capuani LC 8, Takecian PLT 9, Custer BC 1, Goncalez TTG 10
1Blood Systems Research Institute, San Francisco, CA, USA2Fundação Hemominas/Hemocentro de Minas Gerais, Belo Horizonte, Minas Gerais, Bra, Belo Horizonte, Brazil3Departamento de Doenças Infecciosas, Faculdade de Medicina, Universidade de São, São Paulo, Brazil4Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil5Fundação Hemominas/Hemocentro de Minas Gerais, Belo Horizonte, Brazil6Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Belo Horizonte, Brazil7Fundação Hemorio/Hemocentro do Rio de Janeiro, Rio de Janeiro, Brazil8Fundação Pro‐Sangue/Hemocentro de São Paulo, São Paulo, São Paulo, Brazil9Institute of Mathematics and Statistics, University of São Paulo, Bra, São Paulo, Brazil10Blood Systems Research Institute, Epidemiology, San Francisco, CA, USA
Background
Blood banks may be attracting donors primarily interested in their own testing results, the so‐called ‘magnet effect’. Previous studies have shown that approximately 8–14% of Brazilian blood donors are test‐seekers.
Aim
This study investigated the frequency with which three different donor groups in Brazil: deferred for risk factors before donation, accepted and tested HIV (+), and accepted and tested negative on ID markers, reported that health care providers suggested infectious disease (ID) testing at blood centers.
Methods
Data from two REDS II studies, an HIV risk factor case‐control and a study of ID marker rates and risk factors among deferred donors, were analyzed. In both studies, participants at four blood centers completed a confidential audio computer‐assisted self‐interview (ACASI) which included two questions related to healthcare providers and test‐seeking.
Results
During the HIV case‐control study (April 2009 to March 2011), 341 HIV (+) and 791 ID marker negative donors were enrolled. Of those, 43 (12.6%) and 11 (1.4%) respectively, reported that healthcare providers suggested donation as a way to be tested for infections. From September 2010 to March 2011, of 4013 individuals deferred for higher risk behavior (HIV exposure, sexually transmitted infection (STI) exposure, high‐risk sexual partners, multiple heterosexual partners, and MSM activity), 468 (11.8%) reported being referred by a healthcare provider. Over all donor groups, physicians were frequently identified as the referring agent (69.1% of HIV (+), 9.1% of ID marker negative and 61.5% of deferred donors) (Table 1). Proportions of reported healthcare referrals varied from 0% to 17.9% depending on the donor group and blood center.

Summary/Conclusion
Efforts should be made to directly evaluate if health care providers are referring high risk individuals to blood banks, and if so, to educate them about the potential risk and impact this may pose on blood safety.
P‐120
Abstract Withdrawn.
P‐121
Have Economic Crisis Impacted on Blood Donors’ Motivations?
Alfieri S 1, Saturni V2, Marta E 1, Guiddi P 1
1Università Cattolica del Sacro Cuore, Milan, Italy2Associazione Volontari Italiani Sangue, Milan, Italy
Background
It is commonly acknowledged that the economic crisis has exasperated people's feelings of loneliness and reduced their future hopes. At the same time, unstable job conditions often don't allow people to commit to unpaid activities, such as voluntary work. Have the blood donors’ motivations changed with the upraise of the economic crisis compared to the pre‐crisis period? To answer this question we adopted Omoto & Snyder's (1995, 2000) functionalist approach. It states that volunteerism serves different functions for any one person, who may have different motivations from those held by other people. People are moved by a complex combination of motivational orientations that complete one another, considering in a conjoint manner the different motivations that could lead to the action. The authors identify five different motivations (see Table 1).
Aims
To investigate whether blood donors’ motivations changed in a peak period of the economic crisis (2014) compared to the pre‐crisis period (2008)
Methods
We compared 6‐year pre‐post (t1 ‘pre‐crisis’: 2008 – t2 ‘during the crisis’: 2014) data on a sample of blood donors in a single blood donation center situated in Northern Italy. T‐test was used for data analysis. 500 donors (Age range 18–60, M = 32.6, SD = 9.53; 54.5% male) were administered a survey at t1 and 660 (Age range 18–60, M = 37.8, SD = 10.16; 68% male) six years later at t2. In both surveys participants were administered a questionnaire with socio‐demographic items (age, gender, etc.) and a version of Omoto & Snyder's Motivations to Volunteer Scale adapted to blood donation.
Results
Statistical significant differences have been found on knowledge and ego‐protection motivations (respectively t(807) = 8.57, P < 0.001 and t(805) = 5.11, P < 0.001). Both motivations decreased over time, while the remaining one were stable from t1 to t2.

Conclusions
We can't draw casual inferences based on our cross‐sectional data, however results indicate that donors’ motivational priorities didn't vary across the time: values remained prominent as long as self‐enhancement and social motivations. On the other hand knowledge and self‐protection motivations decrease with the upraise of the crises. The latter is of particular interest: nowadays donors perceive themselves as luckier than others and such motivation is not a significant drive of their commitment.
P‐122
Screening a Cohort of Adolescents, Over Time, for Cardiovascular Disease Risk
Eason S , Sayers MH , Goudar S
Carter BloodCare, Bedford, TX, USA
Background
Cardiovascular disease is the major cause of mortality in developed nations. Elevated cholesterol has been associated with increased risk of future cardiovascular disease, which can begin in adolescence and progress asymptomatically for years. Information about the prevalence of risk in young individuals and how risk changes over time would be valuable.
Aims
Since our blood program performs routine total non‐fasting serum cholesterol on all donors at each donation, we decided to investigate adolescents (age 16–19) for their distribution of cholesterol levels and changes in each individual's level over a 5 year period.
Methods
Participants in the study were healthy volunteers who qualified as blood donors when they presented at school sponsored blood drives. Total non‐fasting serum cholesterol measurements (Beckman Coulter AU680) were evaluated at their first blood donation in 2010, when they were 16–19 years of age, using residual blood drawn for routine infectious disease screening. A subsequent measurement was taken on the same individuals when they presented for donation 4 years later in 2014. Cholesterol levels were defined as, Ideal <170 mg/dl (<4.25 mmol/l), Intermediate 170–199 mg/dl (4.25–5.00 mmol/l) and Poor ≥200 mg/dl (≥5.00 mmol/l) as suggested by the ‘Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents.’ Pediatrics 128 (2011): p. S213–S256.
Results
Review of donor records revealed 1484 donors, 842 females and 642 males, donating blood in years 2010 and 2014. When first tested in 2010, 391 (26.3%) of the participant group had a cholesterol ≥170 mg/dl (≥4.25 mmol/l). When reviewed by gender, 258 (30.60%) of the females and 133 (20.7%) of the males had cholesterol ≥170 mg/dl (≥4.25 mmol/l). On subsequent testing in 2014, 4 years after their donation in 2010, the number of individuals with a cholesterol ≥170 mg/dl (≥4.25 mmol/l) had risen to 656 (44.2%). When reviewed by gender, the percentage of females and males with cholesterol ≥170 mg/dl (≥4.25 mmol/l) had risen to 45.2% and 43.0% respectively.
Conclusions
Addition of total non‐fasting serum cholesterol to routine screening of blood donors provides an opportunity for insights into cardiovascular risk amongst adolescents. This study has demonstrated that a significant number of ostensibly healthy individuals in a population of blood donors can be identified for whom primary prevention could be targeted. If they are regular blood donors, they can be followed over time to monitor changes in their risk.
P‐123
Iron Stores in First‐Time and Regular Male Blood Donors in Taiwan
Wang H-h , Chen K-C , Lin C-L
Taichung Blood Center, Taichung, Taiwan
Background
In Taiwan, up to 70% of whole blood donors are repeat donors. Among frequent donors, iron store deficiency is a common side effect. For detecting iron deficiency, the most often used tests include hemoglobin (Hb), serum ferritin, serum iron, and total iron‐binding capacity (TIBC). In recent studies, it was reported that Hb testing lacked the sensitivity in iron deficiency screening, especially in the early stage of iron deficiency. Therefore, ferritin, iron, and TIBC in serum or plasma, which could directly reflect the body's iron stores, are increasingly available.
Aims
The objectives of this study were to investigate the iron status and the demographic characteristics of first‐time and regular male blood donors.
Methods
In Taiwan, the majority of blood donors are men; therefore, only men were included in our analysis. The whole blood donors selection criteria for men include age between 17 and 65 years, body weight ≧50 kg, and hemoglobin ≧13.0 g/dl, based on the rules approved by the Ministry of Health and Welfare, Taiwan. Furthermore, the minimum interdonation interval for 250 and 500 ml blood donations is 8 weeks and 12 weeks, respectively; men are restricted to donate 1500 ml each year. In this study, a regular voluntary blood donor was defined as the donor who donated 7500 ml during 5 years. Pre‐donation haemoglobin assessment was done by copper sulfate density method; serum ferritin, serum iron levels, and TIBC were estimated by indirect ELISA (enzyme‐linked immunoassy). Transferrin saturation (%) was calculated as serum iron level (μg/dl) divided by TIBC (μg/dl). Low ferritin and low transferrin saturation were defined as serum ferritin level less than 23.9 ng/ml and transferrin saturations less than 20%, respectively. Logistic regression analysis with and without (crude model) adjustment for age and body mass index was used to examine whether donor status was associated with low ferritin (or low transferrin saturation). All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
A total of 367 male blood donors, comprising 93 first‐time and 274 regular blood donors, were included in this study. Regular blood donors had higher prevalence of low ferritin (36.50% vs 1.08%, P < 0.001) and low transferrin saturation (27.13% vs 5.49%, P < 0.001) than first‐time donors. The average age of donors was 29.26 ± 9.68 years for the first‐time blood donors and that was 46.37 ± 9.45 years for the regular blood donors. Compared with the first‐time blood donors, regular blood donors had higher mean BMI (25.84 ± 2.62 vs 24.15 ± 3.37, P < 0.001). After the multivariate adjustments, regular blood donors had a 69.84 times greater risk of low ferritin (adjusted OR: 69.84; 95% CI: 8.07–604.22; P < 0.001). Furthermore, regular blood donors had a 6.49 times greater risk of low transferrin saturations (adjusted OR: 6.49; 95% CI: 2.19–19.27; P < 0.001).
Conclusions
These findings show that ferritin and transferrin saturation are significantly affected by regular blood donation, and regular blood donors appear to have reduced iron status compared with first‐time donors.
P‐124
Abstract Withdrawn.
P‐125A
Evaluation of Blood Donor Temporary Deferral Reasons
-Campa García-Bernardo MF 1, Freitas M 1, Lobo M 1, Rodrigues M 1, Muon M 2
1Portuguese Blood and Transplantation Institute (IPST), Coimbra, Portugal2Center of Blood and Transplantation of Coimbra, Coimbra, Portugal
Background
Safety of blood and blood products is a major problem all over the world. One of the most important steps in improving the safety of blood and blood products is donor selection. Hence the proper pre‐screening of the blood donors is essential to ensure quality of donors and to avoid risk of transfusion transmitted diseases to the recipient. By the process of screening donors are deferred for several reasons related to the donor as well as recipient safety. It is very essential to study and analyze the reasons for such deferral among prospective donors in order to categorize them into temporary and permanent deferrals.
Aims
Analyze the causes of donor temporary deferral in the Center of Blood and Transplantation of Coimbra. The objective of this study is to assess the current rate and reasons for donor deferral so that temporarily deferred donors with corrective reasons can be identified, properly informed and guided to improve their quality and thus later on continuous blood supply can be maintained.
Methods
Retrospective descriptive study of pre‐donation temporary deferral of prospective blood donors. All donors must undergo a screening process to assess their suitability. Based on the history and physical examination findings, all blood donors coming to the blood bank were classified in to fit for donation or as a deferred donor. Records of all pre‐donation deferral over a 1‐year period were analyzed to quantify the deferral rate and reasons. From January the 1st to December the 31st of 2014, 75.892 donors (38.524 female and 37.368 male) were studied in our Center.
Results
Total 75.892 pre‐donation screening interviews were conducted over the period of 1 year out of which 59.989 (79.05%) were found fit for donation. Total 14.300 (18.84%) donors were found unfit for temporary reasons and were deferred. The causes of temporary deferral are shown in the Table. The most common reason for overall deferral was low haemoglobin count (17.1%), closely followed by medication (14.4%) and general health illness (9.2%).
Table 1: Causes of temporary deferral (suspension).

Conclusions
One of the most important steps in improving the safety of blood and blood products is donor selection. Insight into the reasons of donor deferral is very important to avoid the permanent loss of the donor as blood donation programme is the life‐force of any blood bank. The deferral of donor due to any reason has a very negative impact and many temporarily deferred potential donors do not return to donate blood in the future. Hence analysis of rejection pattern will not only help in donor and recipient safety but also in maintaining a healthy donor pool in the long run. All the potential donors deferred due to temporary reasons should be informed at the time of deferral about the temporary cause and the time period of deferral. These donors should be appropriately counseled and managed to improve the efficiency of the donor programme.
P‐125
Non‐Invasive Hemoglobin Screening at Plasma Collection Centers
Lu LA
Jiangyou City Plasma Center, Jiangyou city, China
Background
Non‐invasive hemoglobin (Hb) testing methods have recently been introduced into blood donation centers, replacing established capillary and venous hemoglobin testing methods for pre‐donation donor screening. A significant number of works has already been published, describing the application of the non‐invasive (NI) approach to blood donors. However, NI techniques have limited exposure to the donor screening stage at plasma collection centers, reflected in the absence of published studies. The routine integration of non‐invasive Hb measurements may greatly improve the screening process by evading pain and bio‐hazard risks, in addition to simplifying operation.
Aims
The aims of this study are to evaluate the clinical performance and ease‐of‐use of the NBM‐200 system (OrSense Ltd., Israel) as a potential Hb screening method for plasma donors, in addition to discussion of its implications for the plasma collection process.
Methods
The NBM‐200 is based on Occlusion Spectroscopy technology in the red/near‐infrared range, and its measurement is performed using a multi‐wavelength finger sensor with pneumatic cuffs that produce an over‐systolic pressure at the finger base.
The NBM‐200 system was evaluated at the Sant Plasma Center (Mianyang city) and the Jiangyou City Plasma Center (Jiangyou City), both in the Sichuan province, China. For comparison, reference Hb tests were performed by an automated hematology analyzer (Sysmex XE2100, Japan), using extracted venous blood samples. Both methods were correlated and compared using the Bland‐Altman method.
Results
A total of 303 data pairs were obtained, 125 from male donors and 178 from female. NBM‐200 and reference Hb values were in the ranges of 9.9–16.2 g/dl and 7.4–17.3 g/dl respectively. The results showed an average difference (bias) of 0.20 g/dl and precision (standard deviation) of 0.95 g/dl. A Bland‐Altman 95% limits of agreement analysis yields lower and upper limits of −1.67 and 2.07 g/dl.
Conclusions
The non‐invasive NBM‐200 hemoglobin method shows correlating values to an established laboratory reference standard. Subjects showed satisfaction of the elimination of pain with the operators satisfied of the device's ease of use and the reduced infection risk. The significantly higher safety and comfort levels of the non‐Invasive approach may possibly improve donors return rate. This data suggests a promising potential of the NBM‐200 technique as a candidate for accurate, routine screening at plasma collection centers.
P‐126
Flying in the Face of Risk: Do UK Donors Comply with Travel Deferrals for Malaria?
Reynolds CA1, Davison KL 1, Morrison R 2, Brailsford SR 2
1Public Health England, London, United Kingdom2NHS Blood and Transplant, London, United Kingdom
Background
Malaria has been transmitted through transfusions in the UK in the past and several questions on the UK Donor Health Check (DHC) aim to identify persons with a travel risk for malaria. The UK transfusion guidelines policy on malaria is complex with deferral times and testing strategies based on a history of long stay or recent travel to a malarial area as well as donors reporting past malarial infection or fever. Donors are asked to report any travel in the last year and any long stays outside the UK, which may be their birth place. Donors who were born in endemic areas in Africa have been shown to remain PCR positive up to 7 years after arrival in the UK. We know anecdotally that donors have not reported travel, long stays or even past infection.
Aims
We sought to quantify compliance to the DHC travel questions where the donor had travelled to either Africa or India. Here we report the proportion with travel to Africa or India or a long stay and their compliance to the DHC.
Methods
Data on travel were collected from new and repeat blood donors between October 2013 and September 2014 as part of the online UK Blood Donor Survey. Donors were asked if they had been to Africa or India in the 12 months before their last donation. Compliance to the DHC was assessed by asking if they had made their trip(s) known at session and reasons if not reported. Demographic data were also collected. The data were downloaded to an MS Access database and Stata 13 used to estimate rates with 95% confidence intervals and to compare rates by demographic factors using logistic regression.
Results
Nearly two thirds (38,918) of responding donors had travelled outside of the UK in the last 12 months. A smaller proportion, 3.9% (2406/ 61,517) said they had travelled to either Africa (3.2% n = 1962) or India (0.8%, n = 493) in the year prior to their donation, 49 travelling to both areas. The vast majority with travel to Africa had reported it but 26; 0.04% (95% CI 0.03–0.06) were non‐compliant. These were mostly born in the UK (23), however, one non‐compliant donor was born in Africa, arriving in the UK in 2012 and thought their recent travel was not a risk to blood donation. ‘Not a risk’ was jointly the most common reason for not reporting travel (30%, 10) alongside forgetting to report it. Twenty percent of donors (12,473) reported a long stay, with 99.7% compliance although 177 appeared to be non‐compliant including three born in Africa and four in India.
Summary
Transfusion transmitted malaria, although rare in the UK, has occurred with fatal outcome. Although the majority of donors are compliant to the travel questions on the DHC some are not, including those with potential for transmission. It may be helpful to remind donors why they need to provide travel and long stay details, especially important given the drive to increase recruitment of Black and Minority Ethnic donors.
P‐127
Study of Hemoglobinopathies in Donors Related to ST Asl Caserta
Chiriano P , Di Girolamo MG , Sagliano SIC , Dell'Aversana MR , De Caprio G , Stabile A , Perillo M , Misso SIC
S.C.Servizio Trasfusionale Asl Caserta, Aversa, Italy
Background
According to the norm D.L.261/2007 and D.L. 219/2005, blood exams are compulsory on each blood donation, but the Guidelines of the SIMTI in 2007 allow, if necessary, their replacement with the determination of Hb or Hct before the donation to establis hits suitability and then the validation of the bag if the CBC performed later shows values that fall in to those considered normal by SIMTI. Some pathological or just physiologic conditions are only detectable by the CBC in the complete absence of symptoms or with signs and disorders that do not reach the level of consciousness of the subject. The carriers of thalassemia trait, for example, have Hb values that fall within the ranges of suitability for donation but values of meancorpuscular volume (MCV), much lower than the normal range.
Aim
Study of hemoglobinopathies in all donors related to ST Asl Caserta, in consideration of the prevalence ofthe 8–15% of thalassemia trait in Southern Italy.
Methods
All donors relating to ST Asl Caserta, in the year 2014, with hemoglobin values suitable for donation but MCV lower than 75 fl were deeper tested through serumiron, ferritin, transferrin and hemoglobin electrophoresis in HPLC.
Results
In the year 2014 were received 14,030 donors, 8623 male and 5407 female; of all these, 350 (2.5%) showed the MCV lowerthan 75 fl. The analysis of the results of the deepened tests showed that 121 (34. 5%) donors were suffering from irondeficiency anemia while 209 (59.7%) were suffering from beta thalassemia minor (see table); 20 donors (5.8%) presented values of serum iron, ferritin, transferrin and HbA2 that fell within the referencerange.

Summary/Conclusions
The statistical analysis of these data shows that the percentage of the donors affected by beta‐thalassemia minor perfectly matches the expecte done. All donors with iron deficiency anemia were routed to an iron therapy. Since the guidelines establish that the suitability of the donor must be evaluated according to the value of the haemoglobin, a more specialised analysis of the blood test, particularly as regards the MCV, may turn out important for the wealth of the donor.
P‐128
Donor Expectations and Counselling in Kumasi, Ghana
Sharma V1, Hassall O 1, Owusu-Ofori A 2, Bates I 1
1Liverpool School of Tropical Medicine, Liverpool, United Kingdom2Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Background
Donor counselling is a common recommendation in African and WHO blood policy documents, but there are limited details as to what it entails. Good counselling is an integral part of the donation experience and may help retain donors. Repeat donors are vital in maintaining consistent blood supply and studies show they are safest. Retaining donors is particularly important in Africa where blood supply is low and transfusion‐transmitted infection rates are high. Further research is required to develop appropriate counselling guidelines that meet local donor needs, improve the donation experience and help retain donors.
Aim
The aim of this study was to better understand donor expectations in Kumasi, Ghana and develop appropriate and context‐specific donor counselling guidelines.
Methods
This study relied on qualitative methods, rooted in the phenomenological approach. Participants were recruited at the donor clinic at the Komfo Anokye Teaching Hospital (KATH) and KATH mobile blood donation sessions. They included blood donors who had successfully donated blood that visit and donor clinic staff members. All participants were at least sixteen years of age. Only English or Twi speakers were included in this study.
Semi‐structured interviews were conducted with donors and staff members. Interviews were audio recorded, translated into English (if applicable) and transcribed. Data were grouped into broad themes and then further into more specific categories. Based on the results, two focus groups were conducted with donors. The aim of the focus groups was to generate discussion around donor expectations and determine donor priorities with regards to counselling.
Results
Five donor clinic staff and thirty donors were interviewed. One donor absconded part way through the interview. The focus groups consisted of 7–10 donors each.
Donor staff use a health and risk assessment questionnaire to learn more about donor health and behaviours prior to donation. Donors engaging in high risk behaviours (ex: multiple sexual partners or drug use) were counselled on how to minimise risks. According to staff, donors were provided with information concerning post‐donation care such as rest, dietary advice, fluid intake and donation frequency. Replacement donors and first time volunteers were encouraged to become regular volunteer donors.
Donor expectations of the donation process included pain, a more difficult process, shorter/longer process times, ensuring patient‐donor blood group matched, additional information. Most donors felt the health and risk assessment questions were important and relevant, but some felt they were too long, difficult to understand and uncomfortable to answer. Donors were most concerned about their health and well‐being and sought more post‐donation care and health advice. In addition, they would like to be better educated about the donation process and be given realistic expectations regarding process times.
Summary/Conclusions
Donor staff prioritised health, lifestyle behaviours and post‐donation care when counselling donors, but there were inconsistencies in the information donors were receiving. Donors, however, wanted to be counselled on their health, blood and post donation care. Blood service departments should consider this and develop a more detailed and appropriate framework for donor counselling.
P‐129
Abstract Withdrawn.
P‐130
Infectious Markers in Blood Donor Population in Tirana
Seferi I , Abazaj Zh , Metka A
National Blood Transfusion Centre, Tirana, Albania
Background
Blood donor population in Tirana is divided in two groups: voluntary non‐remunerated blood donors (VNRBD), and family replacement donors (FRD).
Aims
The aim of this study was to evaluate the prevalence and trends of transfusion transmitted infectious agents in our blood donors according to donor type age and sex of blood donors.
Methods
We included in the study all first‐time donors (VNRBD and FRD) that donated blood during 2013. The screening records were retrospectively evaluated with respect to screening outcome for HBsAg, anti‐HCV, HIV Ag/Ab and Syphilis. Testing was performed with Abbott‐Architect system. The total number of donors included in the study was 19315 (13922 FRD, 5393 VNRBD).
Results
The overall prevalence of infectious agents in our donor population was 4.94% for HBsAg, 0.7% for HCV, 0.05% for HIV, 0.20 for Syphilis. Prevalence of infectious agents in FRD donors was: HBsAg 5.7%, HCV 0.75%, HIV 0.07%, Syphilis 0.26% whereas in VNRBD was: HBsAg 2.9%, HCV 0.57%, HIV 0, Syphilis 0.05%. These data showed that the prevalence for all four infections is higher in FRD compared to VNRBD donors. The statistical comparison showed that the difference for HBsAg was highly significant (P < 0.001), for HCV was not significant, for HIV was significant (P = 0.049) and for Syphilis was highly significant (P = 0.004). We also observed the prevalence of these infectious markers in these donors divided according to age and sex. Results are shown in Tables 1 and 2.
Caption 1: Infectious agents according to donor sex.

Caption 2: Infectious agents according to donor age.

Summary/Conclusion
Our data showed an overall high prevalence of all infectious markers in our donor population. This high prevalence of infectious markers is dedicated mostly to the significant positivity rate amongst FRD donors compared to VNRBD. Based on these results non remunerated and repeat voluntary blood donors are needed in our country because they are basis for sufficiency and safety of blood.
P‐131
Low Haemoglobin Deferral in Blood Donors
Coutinho M , Ferreira A , Faria M , Seabra P , Coutinho J , Antunes M , Amil M
Centro Hospitalar do Porto, Porto, Portugal
Background
Low haemoglobin is a common cause for blood donors deferral. The correct screening of anemia is important to safeguard the health of donors as well as to maintain the quality of blood products.
Aim
To evaluate blood donors deferral due to low haemoglobin during 2014, in a blood and transfusion medicine department that collects about 11,000 whole blood units/year.
Methods
Donors with at least one episode of anaemia detected in 2014 were included. Anemia was defined as hemoglobin (Hb) value ≤13 g/dl in men and ≤12 g/dl in women according to WHO criteria. Hb was measured by capillary method (CompolabTS FreseniusKabi) and by complete blood count by automated methodology (Beckman Coulter LH 780). The following donor's data were collected recurring to blood center database. Anamnesis for anemia, comorbidities, number of blood donations before and eventually after anemia detection, complete blood count, iron kinetics and iron supplementation were analysed.
Results
460 (6.3%) out of 7266 donors were deferred due to anaemia in the considered period (356 women and 104 men, median age 46 years‐old, range 18–67). The median Hb was 11.6 g/dl (range 7.0–12) in women and 12.6 g/dl (range 12.5–13) in men. For 220 donors (48%) it was the first episode of anemia while in 240 (52%) previous episodes (ep) were documented, namely: 1 ep = 86, 2 ep = 72, 3 ep = 26 and >4 ep = 56 donors. In 48% donors we observed >11 previous blood donations, while 9% were first‐time donors. In 135 (29%) of the 460 donors deferred, the low Hb wasn′t confirmed and they performed a posterior donation, 216 (47%) donors didn′t return to our center after being deferred while in 109 (24%) iron kinetics was evaluated (84 women and 25 men). Thirty‐seven out of 84 women and 11 out of 25 men were treated with iron supplementation. Thirteen out of the donors treated with iron came back to donate. Anaemia relapse was observed in 16 (10%) out of 168 donors who performed a blood donation after anemia detection. The main comorbidities observed were: abnormally heavy bleeding at menstruation (n = 14), nutritional deficit (n = 2), uterine myoma (n = 2), haemorrhoids (n = 2), gastrointestinal bleeding (n = 1), gastric carcinoma (n = 1).
Summary/Conclusions
An adequate donor blood management is essential to keep donors linked to the Blood Center. When anaemia is detected, the donor should be treated and regular contact maintained to avoid unnecessary loss of potential healthy donors. Anaemia deferral results in the apparently loss of a large number of willing donors, so they should be encouraged to return to attempt donation. A high prevalence of anaemia deferral in donors with regular donations was observed. Although capillary methods are quick and easy, the procedure should be performed correctly, since 30% of the donors did not have anaemia confirmed and were able to donate thereafter.
P‐132
Voluntary and Directed Blood Donors – Problems and Attitude
Miah SS
Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Background
The blood has been recognized as a vital force since the beginning of human history. It is the essence of life and safe blood is a key component of effective health care. Moreover voluntary blood donors are the wealth of safe blood supply. In developing country like Bangladesh mass awareness and extension massages can lead towards the continued, safe and effective donation process. Advanced educational motivation and social drive in targeting the youths can lead to a successful and free donation campaigns. Focused more towards the directed donors that we recruit easily, can be motivated as a regular donor. Mass media can inspire more and more voluntary donor to achieve the national demand for safe blood.
Aims/Objectives
To find out the obstacles in voluntary blood donation.
Methods and Materials
This was an observational study carried out in the Department of Transfusion Medicine, Bangabandhu Sheikh Mujib Medical University, Dhaka.100 healthy adults between the ages of 18–60 years both male and female donors and others were interviewed using standardized questionnaire. Obtained data was analyzed using the statistical packages for social sciences (SPSS) for Windows version 16.
Study period
06 month (22 July 2013 – 22 January 2014).
Results
The study shows majority (95%) of the respondents including voluntary donors and directed donors were between 18 years to 40 years of age. Majority (93%) were male and females accounted for only 7%. The most common reason for having not donated blood among directed donor was emotional factor/ego (70.5%) followed by communication gap between blood bank authority and donor (42.6%), distance between blood centre and residence (42.6%), fear about needle prick (23%), occupational hazard (19.7%), fear about misuse of blood (11.5%). On the other hand in voluntary donor the most common reason are communication gap between blood bank authority and donor (23.1%) followed by distance between blood centre and residence (12.8%), fear about needle prick (10.3%), expectation of reward (7.7%), Emotional factor/ego (5.1%). So it indicated that negative attitude were more in directed donor than voluntary donor.
Conclusion
This study highlights the appropriate motivational campaigns based on the input provided by the participants. Blood donation service should be organized in a coordinated manner. The identified problem is actually reflecting our misbelieve, low socioeconomic drive, unprivileged turning and negative attitude towards organizing new community of donors. We need to be focused more in education and motivation in early schooling to sensitize the new donors. Moreover, Blood transfusion Dept. can devote more attention towards the prospective future donors to ensure safe and comfort donation during the process. That can also raise the chances of assembling repeated donation. It is our responsibility to give utmost effort to target the groups less willing to donate. Simultaneously capitalize the optimistic behavior of eager groups and renovate the directed donors into voluntary, non‐remunerated donors. When we recruit more and more voluntary donors that can enable and ensure the safe transfusion system.
P‐133
Abstract Withdrawn.
P‐134
A Study to Analyse Causes of Blood Shortage in Egypt
Mitri GWM
National Blood Transfusion Services, Cairo, Egypt
Background
Recently blood crises in Egypt have been worsened especially after 2011 revolution. According to the National Blood Transfusion Services (NBTS) in 2006; someone needs blood transfusion every 5 min and in 2012, this became every 4 min, the gap between the demand and the supply become a significant problem and people become less willing to donate blood, repeated pleas for people to spare few minutes to donate blood has been met with very little response.
Aims
To highlight the main causes which prevent people in Egypt to donate their blood and to find ways to solve them.
Methods
This study was done during year 2014 from March till December including (850) students form different universities in Egypt. They were (390) male and (460) female, ranging from 18 years to 24 years. They filled two questionnaires; (NBTS/DCD/001/05), and another questionnaire including questions concerning blood donation such as:
History of previous donation.
Willingness to repeat donation.
Causes of refusal if present.
Information regarding benefits of blood donation.
Information regarding how many patients in their country needs blood transfusion daily.
Results
Statistical study was done to determine the top five excuses of refusal blood donation, which revealed that:
31% of the participants were afraid from catching a disease through the blood donation process.
22% of them were afraid from the needle stick and afraid from being turned down.
18% have no trust in the government and they had doubts that their blood might be sold in private hospitals.
17% were discouraged from donating blood either because they have experienced the need for blood at certain point in their life and didn't find it or because their relatives did not have a favorable experience in receiving blood. So, they will only donate blood to family members or to someone they know personally.
12% thought that their blood is not suitable for donation either because of the illness they have had or because their blood type is not in demand.
Conclusions
Blood donations in Egypt is experiencing progressive deterioration in terms of blood donor numbers mainly due to bad reputation of the service and the untrusted government. So we recommended the following:
Awareness of blood donation importance and benefits would be more effective if started at younger age to accept the idea of regular blood donation. So, information sessions about blood donation process, benefits and adverse reactions could be part of the high school curriculum.
Increase the public orientation about the NBTS and illustrate how it serves the community through advertisement campaigns and social media.
Emphasize the highest safety and quality of the process of blood donation through creating awareness campaigns.
Professional donor care and management of the adverse donor reactions are strongly recommended.
P‐135
Non‐Invasive Methods of Measuring Haemoglobin to the Donor Selection – Pilot Study in Blood Transfusion Institute of Vojvodina
Jovanovic R , Bogdanovic S , Bujandric N , Antonic S
Blood Transfusion Institute of Vojvodina, Novi Sad, Serbia
Background
Assessing blood‐donor haemoglobin (Hb) is a worldwide screening requirement against inadequate donation. Hb values are mostly evaluated by measurement of capillary blood obtained from prick of the finger tip. Rapid noninvasive methods have recently become available and may be preferred by donors and staff. The Lmb Technologie GmbH NBM 200 is a non‐invasive finger blood analytes monitor. It measures and displays arterial blood hemoglobin and pulse rate values. Precision of NBM 200 according manufacturer manual is 1SD ± 10.0 g/l.
Aims
The present study was intended to evaluate these non‐invasive methods of measuring Hb to donor selection in Blood Transfusion Institute of Vojvodina in comparison to an invasive method and venous blood sample as references.
Methods
A study was carried in our Institution during the February 2015. The study included 200 blood donors (about 10% of the monthly number of blood donors in our Institution). Blood donors were screened for Hb levels in three different trials using three different methods: non‐invasive (Lmb Technologie GmbH NBM 200) in comparison to the established fingerstick method (copper sulphate test) and to levels obtained from venous samples on a cell counter (Nihon Kohden MEK 6420K) as reference.
Results
The study sample consisted of 142 males (71%) and 58 females (29%). Out of them, 123 (61.5%) were repeat donors; 54 (27%) first time donors; 23 (11.5%) second time donors. The results are summarized in Table 1.
Caption 1: Results of study.

Summary/Conclusions
In general the ideal Hb screening method should be portable, inexpensive, and noninvasive; should offer a low rate of donors’ exclusion due to falsely low Hb levels; and should provide accurate results to protect donors with low Hb levels. The results of this study demonstrate that the used noninvasive methods seem to be an alternative to invasive methods. Any effort should be considered to improve donor safety and good blood quality but as well to avoid unnecessary deferrals or an increase willingness to donate blood due to the fingerstick method.
P‐136
Not Just Blood Donors! A Research on the Multi‐Affiliations of Blood Donors
Alfieri S 1, Saturni V2, Marta E 1, Aresi G 1
1Università Cattolica del Sacro Cuore, Milan, Italy2Associazione Volontari Italiani Sangue, Milan, Italy
Background
In the world of activism there is a widespread phenomenon of multi‐affiliations, rather, those who actively participate in multiple associations. Some researches highlight that this can be negative for associations, since it reveals a lack of satisfaction and confidence of those who are engaged. Others, however, underline the aspects of enrichment and growth that this phenomenon can also bring to these same associations.
With the present work we propose to ask donors directly and look into how the multi‐affiliations can be linked to some spheres of their personal lives.
Aims
The present work proposes to investigate (1) how many donors participate also in other associations, (2) if there are differences ‘in terms of life satisfaction, membership, and reconciling family‐work‐volunteering’ between those who ‘only’ donate blood and those who instead participate in multiple associations.
Methods
Around 2559 donors of the Italian Association of Blood Donors (AVIS) participated in the research in north Italy (Range 18–65, M = 40.31, SD = 11.20; 66.4% males). Each participant was asked to complete a self‐report questionnaire consisting of socio‐demographic variables (age, gender, etc.), questions pertaining to other possible associative affiliations (i.e. groups or associations, etc.), the scales of Life Satisfaction (Pavot & Diener, 1993), of Membership (Marzana et al., 2014), and of Reconciling family‐work‐volunteering (Matthews et al., 2010).
Results
1. Four types of donors emerge: 46.7% are actively engaged (e.g. in volunteer associations, recreation clubs, etc.) also in other realities not involving donation; 35.8% belong ‘only’ to AVIS; 4.4% belong also to other donor associations (e.g. organs); and 13.1% belong concurrently to AVIS, other donor associations, but yet also to non‐donor associations.
2. Life satisfaction: the averages of this variable are high for all considered groups without statistically significant differences.
Membership: donors (a) who belong to AVIS in conjunction with other associations or groups and (b) who are engaged on all fronts, report averages statistically higher of membership compared to the other two groups, F(3,2496) = 68.43, P < 0.001.
Reconciling family-work-volunteering: statistically significant differences emerge only for the reconciliation between family‐volunteering and work‐volunteering, in a similar way: those who engage donation (only of blood or also of organs) manages to better reconcile both aspects, F(3,2427) = 10.83, P < 0.001, F(3,2403) = 3.02, P < 0.05.
Conclusions
Almost two‐thirds of surveyed donors are also active in other associations. Those who engage show that they are satisfied with their lives: in fact, regardless of the type of activity carried out, all of the surveyed donors report good levels of life satisfaction.
While donor associations allow better family‐volunteering and work‐volunteering reconciliation compared to other associations, they provide fewer opportunities for donors to feel part of a group, an aspect that they must reinforce in order to promote affiliation, and therefore the chance for donors to continue to donate.
P‐137
‘Give Blood for the First Time and Forever’. The New Promotion Campaign by AVIS, the Italian Volunteer Blood Donors Association
Saturni V1, Firenze C 1, Zuccon B 1, Cavazza F 1, Di Renzo R 2, Cortes N 2
1AVIS – Italian Volunteer Blood Donors Association, Milan, Italy2Heads Collective, Treviso, Italy
Background
AVIS (Italian Volunteer Blood Donors Association) is a not for profit and private association founded in 1927 with the aim of granting an adequate supply of blood and blood components. AVIS is constantly working in three main fields: the promotion of periodical, voluntary, anonymous, associated and non‐remunerated donation, the call for donors and the collection of blood and plasma.
Aims
In recent years AVIS has intensified efforts aimed at promoting blood donation, donor retention, healthy lifestyles and volunteering. The use of social networks became increasingly important and gave the opportunity to develop new codes of interaction and communication which could reach especially the younger members of the population.
Methods
‘Give blood for the first time and forever’ is the claim of the new AVIS campaign for the promotion of blood donation, which was createdin 2014 by the advertising agency Heads Collective. It features a tv and radio advert and six different image subjects conveyed through posters, postcards, banners, etc…
The main goal is to show that we usually get scared by new experiences such as blood donation, but we would like to live them again when they prove to be positive and unforgettable events.
With the aim of inviting people to tell their ‘first times’ through images, videos and text messages, in 2015 AVIS will launch a social contest connected to this campaign.
Results
For the very first time in the history of AVIS, the campaign achieved the ‘Pubblicità Progresso’ patronage, which is the most prestigious Italian acknowledgement for social and not for profit PR and advertising projects.
Conclusions
Promoting values such as solidarity and generosity and reaching people's hearts is not an easy task. It needs a strong, direct and emotional message wich everyone could understand. The new AVIS campaign proves that storytelling can be a perfect technique for this task: it's all about finding the right metaphors and the right images everybody can identify with.
P‐138
Summer Campaign – Overcoming the Seasonal Deficit – Activities of the Blood Transfusion Institute of Serbia in the Period 2005–2014
Rodic IR , Jovanovic Srzentic SJS
Blood Transfusion Institute of Serbia, Belgrade, Serbia
Introduction
Privatization of large systems, followed by the downsizing in the number of workers, revision of the terrain, and the reduction of territory jurisdiction of the Institute in the period 2002–2005, encumbered the implementation of organized voluntary blood donation in the field. In 2004, 60,921 units of blood were collected, with a visible tendency of decrease in the total number of blood donations and explicit deficits during the summer months.
Objective
To show the results of total blood donation, with an emphasis on the summer months, and developing a culture of donating blood while respecting the recommendations of World Health Organization.
Methodology
Analysis of field work and applied modern principles of promotion and information‐providing activities, with a special approach to actions in the local community.
Activities
The first Summer Campaign was organized in 2005 with the aim to promote voluntary donation and provide sufficient amounts of blood in the traditionally scarce summer period. It was implemented as a symbolic competition of cities and municipalities in Belgrade and the central region of Serbia for gold, silver and bronze medals ‘Champion of Solidarity’, which were officially awarded during the New Year's reception at the Belgrade City Hall. For the past 10 years, 2005–2014, the Summer Campaign has been launched on the World Blood Donor Day, and lasted until the end of August. Actions were organized in central city areas or in a transfusion van set in the main squares, with the support of local governments, celebrities as promoters, and volunteers of the Red Cross, who were in direct contact with the blood donors and citizens on site. In the decade of its existence, the Summer Campaign has become a significant event in the participating cities and municipalities. Modern principles of communication were used to support the activity: text messaging, web presentations, Facebook profiles, promotion at cultural and sport events, street stalls, and media support.
Results
The Summer Campaign spread from 44 cities and municipalities, in which 2307 people voluntarily donated blood in the first year, to 78 cities and municipalities, and 4647 blood donors, last year. In parallel, also the total number of blood donations at the Institute increased from 59,063 in 2005 to 64,864, or 8.9% more blood units, in 2014.
Conclusion
Realization of the above activities contributed to increasing the motivation of citizens to voluntarily donate blood, which significantly influenced the increase in the number of blood donations and preventing the deficit in the summer months. The 11th Summer Campaign has been planned in the annual calendar of voluntary blood donation in 2015, and the lessons learned will continue to be applied in the future.
P‐139
Information Services and Informaton Seeking Behavior of Blood Donors for the Reinforcement of Blood Donation in Greece
Chanos A1, Katsarou O 2, Kostagiolas P 3, Myriokefalitaki E 2, Anastasopoulou I 2, Gkotsi S 2, Niakas D 4
1Laiko General Hospital of Athens, Athens, Greece2Second Regional Blood Transfusion Center, Laikon General Hospital, Athens, Greece3Ionian University, Corfu, Greece4Hellenic Open University, Patra, Greece
Background
The term ‘information behavior’ comprises all human behaviors, regarding the search, evaluation, information management, information resources and obstacles encountered during the search process. This paper attempts to investigate the information behavior of blood donors in a public hospital.
Aims
Key expected results of this paper refer to (i) the importance of collecting and processing information as far as donors are concerned, (ii) the recording of the sources of information and respective information barriers, (iii) the extent to which blood donors’ behavior is affected through information searching process and (iv) the contribution of better information on transfusion center's proper functioning and quality of services.
Methods
A total of 352 donors participated in this survey, which was conducted in the Blood Centre of the University General Hospital of Athens ‘Laiko’. The study was conducted by distributing a targeted questionnaire which depicts the donor's demographic characteristics, the causes of blood donation, the ability to use computers and access to the internet, the information needs, the available information resources, the obstacles encountered in information searching process, the way that information and information behavior affect the blood donors’ relationship with the staff, the level of satisfaction with the donation service, and the blood donors behavior per se. The analysis conducted through SPSS (version 16) and includes descriptive statistics, statistical inference and factor analysis to reduce the variables in the questionnaire.
Results
From the statistical analysis of the questionnaires the following results were exported: (i) 75.4% of the blood donors are seeking information for the benefits of blood donation and 70.8% of the side effects of this procedure, (ii) 17.4% of donors are seeking information through other external sources, due to lack of available time from the medical staff, (iii) main sources of information are the internet (47.1%), friendliness (33.1%) and medical professionals (32.5%), (iv) the major obstacles in the seeking information procedure are the infrequent blood donation (21.8%), the validity (18.7%) and the volume (17.5%) of information on the internet, (v) 74.4% of donors recognize the substantial assistance of the information on the effective use of the service of blood donation, (vi) men seem to have less need for information giving less importance to information barriers, (vii) younger donors are seeking for new sources of information despite the fact that they are using more the existing sources, (viii) compared to the other non‐productive groups. the private employees seem to have greater need on information searching procedure, but less on seeking for new sources of information, (ix) the satisfaction of blood donation is influenced by the donors information on this process (45.4%).
Summary/Conclusions
The study results outlined the fact that the main source of information on blood donation is internet, with the highest percentage of donors having access to it. Furthermore, demographic factors are indicated as significant with respect to blood donors’ information behavior, whereas the quality of transfusion center services tends to be influenced by how well‐informed the blood donors are.
P‐140
Deferral of Donors Due to Piercing Events – Getting to the Point of the Matter
Morrison R1, Reynolds CA 2, Jegasothy E 3, Brailsford SR 2
1NHS Blood and Transplant, London, United Kingdom2NHSBT/PHE Epidemiology Unit, London, United Kingdom3Nuffield Institute, London, United Kingdom
Background
The UK Blood Services adhere to the Blood Safety and Quality Regulations which states that blood donors who have had recent piercing events including tattoos, body/skin piercings, needlesticks or acupuncture, unless performed by registered individuals or the NHS, are deferred for 4 months. If they return within 12 months they will be required to have an additional anti‐HBc test along with the regular mandatory tests. It is unknown how many donors are deferred as a result of the different types of piercing events. Here we explore the impact of piercing events on donor deferral in the south of England.
Aims
To collect data on the donors reporting a piercing event who donated in London and the south of England in order to:
Assess the prevalence of piercing events in the donor population.
Describe the characteristics of donors, including age and gender, who have piercing events.
Identify deferrals due to acupuncture carried out by a non‐registered practitioner.
Methods
Donor Health Check (DHC) questionnaires from donors attending between 03/06/2014 and 07/09/2014 who disclosed a recent piercing event were identified by the donor records staff at NHSBT Colindale and Filton. Information on donor demographics, piercing events and donation outcome were collected. The data were entered into a Microsoft Access database for analysis and secure storage. The number of donations tested at Filton (which covers the study area) by donor type was available from the Epidemiology Unit and were used to estimate prevalence of events among subgroups.
Results
During the study period, there were 311,474 donations made in the south of England. A total of 2604 donor health check forms reported a piercing; 1587 from Filton and 1017 from Colindale, representing less than 1% of all donations made during the study. Tattooing was the most frequently reported piercing event (43.3%, 1127/2604), followed by body/skin piercing (41.9%, 1093/2604) and acupuncture (13.2%, 344/2604). Where age known, the majority of body/skin piercings were in the 17–24 year olds (60.5%, 410/677), while most tattoos (35%, 268/770) and acupuncture (24%, 38/159) were in 25–34 year olds. The majority of piercing events were among females (78.1%, 2034/2604). Among all piercing events, 29.3% (762/2604) were deferred from donation; among those with recent acupuncture, 48.5% (167/344) were deferred because a non‐registered practitioner was used. The majority of donors deferred due to acupuncture were female (74.3%, 124/167) and repeat donors (69.7%, 106/167).
Summary/Conclusions
Piercing events were rare among donors in the south of England. When they did occur, most were due to tattooing; females and young donors were most commonly affected. Almost half of the donors reporting acupuncture were deferred due to recent treatment by a non‐registered practitioner, two thirds of whom were regular donors. Piercing deferrals affect a relatively small number of donors but may impact on donor retention in regular donors and disproportionately affect certain groups. Further data gathering and analysis about self‐deferrals prior to session, retention post deferral at session as well as the demographics of the pierced population are planned.
P‐141
Questions to be Asked: the Uniform German Blood Donor Questionnaire
Houareau CH1, Deitenbeck R 2, Sümnig A 3, Heiden M 4, Stötzer F 5, Northoff H 6, Offergeld R 7
1Robert Koch Institute, Berlin, Germany2German Red Cross Blood Service West, Hagen, Germany3Universitätsmedizin Greifswald, Department for Transfusion Medicine, Greifswald, Germany4Paul Ehrlich Institute, Department for Transfusion Medicine/Haematology, Langen, Germany5German Red Cross Service Baden‐Württenberg/Hessen, Mannheim, Germany6Zentrum fuer Klinische Transfusionsmedizin, Tuebingen, Germany7Department for Infectious Disease Epidemiology, Rober Koch Institute, Berlin, Germany
Background
To increase the quality of the different donor history questionnaires (DHQ), a uniform DHQ (UDHQ) was developed in Germany. It aimed at being plain, comprehensible, effective, and accepted by donors. This UDHQ was evaluated in a multi‐centre trial with 6500 first‐time donors. The analysis demonstrated the superiority of the UDHQ compared to the locally established DHQs. Nonetheless, the deferral rate increased by 4.6%. This was mainly due to acute illness and a newly introduced question about sexual contact with a partner within the past 4 months. To elucidate if too many young donors would be excluded if the UDHQ became mandatory, it was tested at a larger scale by incorporating first‐time and repeat blood‐donors.
Aims
The goal of this study was to make a stratified comparison of deferral rates of the UDHQ and the DHQ in first‐time and repeat donors within the German Red Cross Blood Service West (Hagen).

Methods
This study retrospectively analysed data on blood donations from 2010 (DHQ) and 2011 (UDHQ). These included age‐group, gender, donor status (first‐time, repeat), donation site (mobile, fixed), confidential unit exclusion, and donor eligibility. Differences on descriptive‐level were analysed with Chi2‐test according to Pearson. Direct backwards logistic regression based on the Wald statistic was performed.
Results
The analyses included 35,733 (DHQ) and 37,148 (UDHQ) blood donations. Both questionnaires showed the same confidential unit exclusion and almost the same permanent deferral rates in first‐time and repeat‐donors (Table 1). Overall temporary deferral rates differed significantly for both types of questionnaires (P < 0.001) and also stratified for first‐time and repeat‐donors (P < 0.001; Table 1). A logistic regression was performed to predict deferral. In this model first‐time donor status was the strongest predictor for deferral (OR = 2.7; P < 0.001). Also, the age‐groups 18–24 yrs. (OR = 1.9; P < 0.001) and 25–34 years (OR = 1.6; P < 0.001) were identified as predictors. Overall deferral due to new partners was 0.7% (260/37,148) and 2.6% (144/5644) in first‐time and repeat 0.4% (116/31504) donors respectively. The most frequent deferral criterion in all age‐groups was acute illness.
Caption 1: Deferral by donor status and donor history questionnaire version.
Summary/Conclusions
The DHQ and UDHQ did not change the rate of permanent deferral but lead to differences in temporary deferrals only. Even though 4.1% additional first‐time donors were deferred when the UDHQ was used, the logistic regression revealed stronger predictors for deferral than the questionnaire version. Although the basic claims data did not allow for conclusions on under‐lying reasons, being a first‐time donor or being in the younger age group of 18–34 years is a higher and independent risk for deferral than using the UDHQ. The impact of the additional 2.6% of deferrals of young first‐time donors due to a new sexual partner has to be further investigated.
P‐142
Cultural Context and Role of Communication in Promoting Adequate Blood Donation in Sub‐Saharan Africa: a Systematic Literature Review
Appiah BA1, Bates BA 2
1Centre for Science and Health Communication, Accra, Ghana2Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Background
Globally, there have been several reviews of aspects of blood donation, including deterrents and motivators of blood donation and efficacy of interventions to promote blood donations. However, many such reviews have not focused entirely on sub‐Saharan Africa or the influence of culture and communication on blood donation. Having such a focus could help in the design of culturally appropriate interventions to promote blood donation in sub‐Saharan Africa.
Objective
We identified influences of culture (for example, misconceptions) and communication on blood donation in Sub‐Saharan Africa.
Methods
Using systematic literature review, we performed database searches in, PsycINFO, CINAHL, EMBASE, Web of Science, Proquest Dissertations and Theses; Africa Journals Online (AJOL); Africa Index Medicus (AIM), and Global Health. We included 48 countries in sub‐Saharan Africa in the search terms. Scopus and Google Scholar were used to search references of selected papers that focused on sub‐Saharan Africa. The search took place from June 8, 2013 to 18 February 2014. We searched the databases without any limitation to the year of publication, and included studies in English or French. A coding form was developed using Qualtrics, an online survey creator. Both qualitative and quantitative studies on the subject were coded and analyzed together.
Results
A total of 3020 articles were identified, and 43 articles, including conference abstracts met inclusion criteria and were critically appraised. Aspects of culture that influence blood donation identified included misconceptions, religion, and role of relatives. The misconception that blood donation may cause decreased libido in men was common in seven studies. We identified various communication channels for increasing blood donation, including use of the mass media, mobile phones and face‐to‐face contacts.
Conclusion
Many culture‐ and communication‐related factors influence blood donation in Sub‐Saharan Africa. Culture‐related factors that could impact blood donation include myths and influences of families, religion and incentives. Communication factors include using the mass media as a channel for dissemination of knowledge about blood donation, a motivator for first‐time and repeat blood donation and as a non‐cash incentive; using mobile phones as an aid to blood donation; and face‐to‐face and other communication as a culturally‐appropriate strategy. When designing interventions, these factors and strategies should be considered.
P‐143
Regional and Socio‐Economic Drivers of Donor Activity in the Republic of Kazakhstan
Skorikova SV , Burkitbayev ZhK
Republican State Enterprise on Operative Management Right, Scientific and Produc, Astana, Kazakhstan
Background
The Republic of Kazakhstan is the ninth largest country in the world by area (3 million km2) and 60th by population (17 million). Vast distances and low population density call for non‐standard solutions in development of state‐of‐the‐art regional medical services, specialized inter‐regional centers and of appropriate blood service that meets both regional and nation‐wide needs.
Aims
To analyze the factors that drive regional trends of blood donation and develop guidelines for improving the donation rate in Kazakhstan.
Materials and Methods
The blood donation rates per 1000 of population, as well as socio‐economic (the proportion of the urban/rural population, the income level of people) and healthcare (surgical operations and the number of hospital beds per 10,000 of population, expenses per capita in the health system, post‐operation mortality, number of physicians in blood centers) indicators in 16 regions of Kazakhstan have been studied. Data were obtained from annual (2010–2014) reports of regional blood centers and official healthcare and statistical disclosures. Data were analyzed using descriptive statistics and correlation (r) tests with P < 0.05 considered as statistically significant.
Results
During the study period, 1,413,000 blood units were collected with average donation rate 17.0 per 1000 (target donation rate = 50). Different regions can be classified into regions with high, medium, low and very low donation rate (groups 1–4 respectively, 2014, Table).

Group 1 (highest donation rate), represents the state's capital. Here the leading national medical centers are located, the rate of surgeries per 10,000 population is double than in the other groups. Groups 2–3 did not differ from each other by the studied parameters. Group 4, characterized by predominantly (70%) rural population, had the lowest indicators of surgical operations and the number of hospital beds per 10,000 of population.
A statistically significant correlation was found between the donation rate per 1000 and expenses per capita in the health system (r = 0.73), number of physicians in blood centers (r = 0.54) and prevalence of rural population (r = −0.49). Correlation with other indicators was not statistically significant (P > 0.05).
According to regional health care authorities, the Blood Banks mainly well provide the needs of local clinics in blood components, including in regions with low/very low donation rates. In all regions in 2014 apheresis techniques were used (15.7% from total number of donations).
Summary/Conclusions
The main drivers of donor activity are the need of regional clinical institutions in blood components and the need in plasma for fractionation to ensure the Republic's demand for plasma products. The majority of regions retain large reserves for blood donation, a resource that may be utilized by significantly increasing the collection of apheresis plasma for fractionation in these regions. This strategy will allow addressing the nation‐wide needs for plasma products while uniformly distributing the loadamong citizens of all regions. The conditions for a significant increase in donation rate are adequate funding of the healthcare sector, qualified staffing of blood services and develop a special program for organizing blood donation among the rural population.
P‐144
Analysis of Reasons Behind Pre‐Donation Deferrals of Blood Donors: the National Blood Transfusion Centre (NBTC), Cairo Egypt
Samir Borhan Ragheb LB
NBTC, Cairo, Egypt
Background
It is well known that a large number of apparently healthy donors are not able to donate blood successfully due to varied reasons of deferral. These reasons are outlined in the donor questionnaire and it is the donation doctor's duty to conform to these selection criteria.
Donor deferral while a necessary preliminary step, acting as a first line of defence in insuring blood safety, has its drawbacks. Once these reasons leading to deferral have been established and analyzed, problems of safe donor shortage and consequent blood and blood component shortage will be addressed and ultimately reduced.
Aims
To analyze the rate and various reasons behind pre‐donation deferrals at the NBTC (National Blood Transfusion Centre), in order to reassess strategies in planning blood drives, and improve donor recruitment.
Methods
A retrospective analysis of donor records from the external donation department (donors gained from blood drives) at the NBTC, Cairo‐Egypt, for 12 months, from January 2014 to December 2014 was done. The rate of donor deferral and the percentage of deferrals associated with each category of deferral were found. The various deferral reasons were analyzed among voluntary, non‐remunerated donors, whether temporary or permanent deferrals.
Results
There were 40,765 donors in total throughout the 12 months, 30,311 Men (74.4%), and 9908 women (24.3%). Donor ages ranged from 18–65 year olds with the highest category being the 20–30 year olds at 15,271 (37.5%) and the lowest category being the 60–65 year olds at 32 donors (0.1%).
Out of this total 11,762 donors were deferred (28.9%) for various reasons, 11,522 were temporarily deferred, (28.3%) and 564 (1.4%) permanently deferral. Causes for deferral were divided into 10 main categories, ranging from; the age of the donor being less than 18 years old 1206 (3%), anaemia with a haemoglobin level below 12 g/dl in females and 13 g/dl in males 3409 (8.4%), history of jaundice 19 (0%), history of addictive drug intake 30 (0.01%), history of dental work less than 3 months from the donation date 2497 (6.1%), history of surgery 727 (1.8%), history of chronic diseases 570 (1.4%), illicit sexual relations 35 (0.1%), history of malaria (0%), other causes 3279 (8%).

Summary/Conclusions
A study of deferral reasons of blood donors will reflect the generic health of a population, and help to predict future blood supply. Temporary deferrals will have an expected temporary effect on blood supply, whilst permanent deferrals will categorically affect it. A clear understanding of reasons that hamper safe and sufficient blood supply need to be addressed and tackled through improving donor recruitment and retention strategies. Planning of future blood drive locations can also be improved through further research and identification of spatial patterns in blood donation, using geographic information systems.
Also while these results are specific to Cairo, they can be of use to other governorates and blood centres within Egypt. They also have external validity, so as to apply to other countries in the region, provided they have similar population structure, donor profiles and socio‐medical makeup.
P‐145
Blood Donors as Research Subjects: the Finnish Generisk Cohort
Mattila PMM1, Laatikainen LL 2, Kauko KK 1, Castren JC 1, Widen EW 2, Partanen JP 1, Ripatti SR 2
1Finnish Red Cross Blood Service, Helsinki, Finland2University of Helsinki, FIMM, Helsinki, Finland
Background
Blood donors committed to repetitively give blood and allow being contacted by Blood Service organisations are a potential source of healthy research subjects. On the other hand the recent biobanking law in Finland makes a broad consent possible enabling the usage of same set of samples in several studies.
To test feasibility of blood centers as research sample and data collection units we recently conducted a small‐scale pilot study with 300 participants. I this Cardio Compass‐study we collected classical and genetic risk markers for cardiovascular disease (CVD). Based on the positive experience on the Cardio Compass‐study, the FRC Blood Service provided blood donors an opportunity to participate in a larger GeneRISK cohort aiming to collect up to 15,000 participants to evaluate genetic and classical CVD risk scores and participants’ attitude to get novel type of information about their CVD risk. GeneRISK‐study is led by prof. Samuli Ripatti of University of Helsinki and the cohort consists of three subgroups: hospital patients of municipal CAREA hospital, private health care provider Mehiläinen Ltd, and the FRC Blood Service.
Aims
The aim of FRC Blood Service is to provide blood donors an opportunity to participate the GeneRISK study in the premises of FRC Blood Service. In the study various types of data are collected:
(i) a large questionnaire to collect health and lifestyle information; (ii) samples for lipid and sugar measurements; (iii) clinical measurements of weight, height, blood pressure and related parameters; and (iv) blood samples for genome analysis.
The objective of the GeneRISK‐study is to give participants information about their CVD risk based on both classical and genetic risks and to give them guidance how their can lower their individual risk by changing their lifestyle decisions. FRC Blood Service collects information how this type of study can be accomplished in rooms dedicated to routine blood donation and what is blood donors’ attitude to this kind of ‘novel way to help’.
Methods
The GeneRISK‐study in the FRC Blood Service includes the following main components:
1. A Questionnaire.
2. Pre‐booking an appointment via internet.
3. Physical measurements and blood sampling.
4. Lipid, blood sugar and genomic analyses.
5. Intervention in the form of guidance after the participant has received the personal risk scores.
6. Follow‐up.
Results
The study started March 2015 and the results of the pilot phase (March–April 2015 period) will be reported.
Summary/Conclusions
The present study offers blood donors new opportunities to help health care in addition to routine blood donation by participating in studies on common diseases. The FRC Blood Service learns how well its premises can be used for research purposes and whether blood donors appreciate this type of opportunity.
P‐146
Blood Donor Selection: Reasons for Exclusion from Donation
Di Lemma GG1, Danzi M 1, Di Girolamo MG 1, D’ Ambrosio R 1, Pecora P 2, Mabelli M 2, Tomeo R 1, Misso S 1
1S.C.Servizio Trasfusionale ASL Caserta, Aversa, Italy2Avis Casoria, Casoria, Italy
Background
The selection of donor blood and blood components still represents a fundamental moment of the transfusion process, the cornerstone on which to act to ensure the safety for both the giver and the receivers of the blood products, also to ensure their highest quality. For the protection of the donor, the Ministerial Decree of March 3, 2005, ‘Protocol for the evaluation of donor blood and blood components’ defines the requirements for acceptance of the candidate donor.
Aims
The purpose of our study was to examine the causes of exclusion from donation donor at the SIT Aversa.
Methods
Out of 1500 donors belonging to the SIT Aversa in 2014, 234 were not eligible (15.6%) of which 148 males (63.2%) and 86 females (36.7%).
They examined the medical records of donors and evaluated the reasons for the exclusion. Among the others:
1. the lack of certain physical requirements necessary for the donation (weight, Hb, blood pressure);
2. presence of infections;
3. recent surgery;
4. medication;
5. tattoos;
6. living in endemic areas.
Results
See tables.

Conclusions
Safety is the cornerstone of the transfusion system. The responsibility for the act of giving/receiving/return must be shared, otherwise it could become a one way act and end in itself, meaningless because it gives the opportunity to save lives, but paradoxically, it may put them at risk of infection. Analysis of the data showed that in both sexes, the lack of physical requirements has been the most frequent cause of exclusion from the donation. In particular, the age group involved is that 18–30 years (17.9% for males and 14.5% for females). In spite of many alarmist related to blood transfusions, the criteria for selection of donors and controls on donated blood, as provided by law, are rigid and strict and we must not forget that donating blood is not just a way to save more lives, but also because, the donor receives the result of the analysis and can easily keep an eye on his health.
P‐147
Distance to the Blood Bank is Important
Villumsen JV
National Danish Blood Donor Association, Taastrup, Denmark
Background
The National Danish Blood Donor Association (NDBDA) asked non‐donors questions about their knowledge and preferences concerning blood donation. A key question was how much time they would be willing to travel to do a donation.
Aims
To find out who long time a non‐donor would be willing to spend on travelling, to make it possible for us to evaluate the number and location of collections sites.
Methods
We collected 1199 answers through a digital questionnaire. The sample was chosen randomly, but it was ensured that it was representative for the Danish population. The first question to the panel was: ‘Are you a blood donor?’ If they answered ‘yes’ or ‘have been’ they were omitted. 1199 persons answered ‘no’ and continued the questionnaire. They answered 14 questions. The collection of data and sampling was done by yougov.dk.
We asked: ‘Pretend that you are a blood donor, how much time would you be willing to spend on transport to the blood bank (in minutes)?’.
Results
We found that 362 persons of the total of 1199 would be able and could be recruited for VNRBD. We defined these respondents as potential. They were asked, what would make them sign up for blood donation. Of 362 potential donors 157 (43.3%) answered ‘if the point of donation was on my work place, place of study or close by’. It seems like distance and especially a short distance is very important to convert non‐donors into new donors.
We also asked them to give the distance in minutes, and we found the following.
58.29% of the potentials answered 10–20 min.
28.73% of the potentials donors were willing to spend up to 30 min to go to the blood bank.
5.25% would only be willing to spend a maximum of 5 min.
Caption 1: Table Time of transportation.

Conclusions
It is important to make it easy for the donors to access the point of donation. We assess the time limit for additional minutes of transport among potential donors in Denmark are 20 min. Two reasons for keeping the distance to the point of donation short: (1) the voluntary donor shall change his daily procedure as little as possible (2) time is valuable, therefore they do not want to spend a lot of extra time on transportation. Additional travel may exclude potential donors from signing up.
P‐148
Analysis of Attitudes of Donors to Give Blood Voluntarily: an Assessment of a Questionnaire Survey and Tips for Future
Sönmezoglu M
Yeditepe University Hospital, Istanbul, Turkey
Background and Objectives
This study was conducted to assess the general view of public towards blood donation and the factors associated with it. Istanbul is a metropolis with a population around 14 million. There are two regional blood centers run by Turkish Red Crescent (TRC) and 20 hospital‐based blood banks supplying blood need of the city. Istanbul city blood need is around 400,000 units and 43% is supplied by TRC. Until 2010 before centralization development, our hospital is used to collect our own need from volunteer donors. To determine the problems encountered encouraging people eligible to donate, a questionnaire was prepared and applied at our center to 500 donor candidates.
Method
A descriptive cross‐sectional study was conducted in Istanbul among donor candidates applied to TC aged 18–65 years. A self‐reported questionnaire was completed for 450 participants. 20 questions about their past experience for donation, demographic information, why they donate, in which terms of conditions they are willing to donate, which factors are restraining were forwarded to the candidates at blood center and evaluated the results.
Results
Of the 450 participants, 409 of them (90.8%) were male, 94 (20.8%) reported that they had donated blood in the past and 360 (80%) stated that they were willing to donate blood in the future. 54 past donors (12%) reported that they had donated blood in the 12 months preceding the survey and 49 (10.8%) participants stated that they have been regular donors. Family member need is the leading cause for intention to donate, second was major public need like earthquake. Proposal for future is communication with the people about importance of blood donation of country and warning them constantly with mails or messages.
Conclusion
Altruism, social responsibility, peer influence, access to health communication, and knowledge about importance of blood donation are mentioned as some of the factors that motivate individuals to donate blood. But in daily life eligible people for donation expect to have sustained warning system for future donations.
P‐149
Abstract Withdrawn.
P‐150
Polycythemias: Role of the Transfusion Service
Giaquinto A , Mottola M , Bruno C , Arcopinto C , Brighel F , Vigorita E , Zuccarelli B
A.O.R.N., Ospedali dei Colli, Napoli, Italy
Background
Polycythemias are clinical conditions characterized by an increase in Hb, Hct and RBC and are divided into two groups.The first, includes the polycythemias by increased secretion of EPO that can be secondary to the reduction of pO2 or inappropriate secretion by a tumor. The second, includes the Polycythemia Vera (PV) that is part of a family of diseases known as ‘chronic myeloproliferative neoplasias’ characterized by a mutation in the JAK2 gene and the resulting clonal expansion dell'eritrone. The P.V. is a disease that, like many other neoplastic hematologic diseases infrequent, chronic and little aggressive, received until a few years ago little attention. However, if not treated properly, is associated with a poor quality of life.
Aims
The aim of this study was to evaluate the presence of polycythemias in our periodic blood donor population.
Methods
In the period 2013–2014, we selected 100 donors with polycythemias on a total of 15,000 donations (5000 donations annually). Donor selection to initiate further diagnostic investigations has been made considering the time of pre‐donation values: males RBC ≥ 5.5 million/μl, Htc ≥ 50%, Hb ≥ 17.5 g/dl; females RBC ≥ 5.0 million/μl, Htc ≥ 47.5%, Hb ≥ 16 g/dl. After a thorough medical history to rule out secondary erythrocytosis, general examinations to exclude concomitant diseases of the kidney, liver, including viral hepatitis, or the presence of a still undiagnosed diabetes and more instrumental tests (ECG, chest X‐ray, Ultrasound of the abdomen), we inserted as diagnostic criteria the evaluation of the V617F mutation of JAK2 gene, the dosage of EPO and serum homocysteine.
Results
Of the 100 selected donors according to the criteria listed above 90 were male and 10 female, with an age range overall between 45–60 years. Of these 90% were suffering from polycythemia secondary to smoking, 5% from sleep apnea syndrome, idiopathic erythrocytosis 4% and 1% from polycythemia vera with mutation of JAK2.
Conclusions
The Transfusion Medicine by treating donors which are by definition healthy person must be understood as the branch of medicine aims to promote and preserve health. An early diagnosis of Polycythemia Vera is important to prevent vascular complications, hemorrhagic and thrombotic, which may also represent the the first symptom.
P‐151
The Reasons for Refusal of Donor Candidates: Data of the Regional Blood Center of Gulhane Military Academy of Medicine
Savasci US , Yilmaz SY , Yakut UY , Guney MG , Cetinkaya RAC , Yilmaz SY , Avci IYA , Besirbellioglu BAB
Gulhane Military Academy of Medicine, Ankara, Turkey
Background
To protect the recipients from infectious diseases transmitted by transfusion and to maintain the good behavior of donors, the donors are screened prior to donation by donor questionnaire and physical examination.
Aim
In our study, we aimed to increase the harmonization of donors by examining the causes for rejection of the donors.
Methods
The records of the donor candidates applied to Blood Education Center and Blood Bank of Gulhane Military Academy of Medicine (BEC‐BB of GMAM) for donating between January 2012 and September 2012 were examined, retrospectively. The events after the donor sits on the seat for transfusion was defined as donor reaction and not included in the study as a reason for refusal of donor.
Results
9906 donor candidates applied to BEC‐BB of GMAM and the bloodletting team between January 2012 and September 2012. 20.2% of blood donor candidates (2010 people) were not accepted as blood donors in that time. Reasons for cancellation of blood donors were evaluated by dividing into groups based on blood donor assessment questionnaire, physical examination and laboratory test results (Table 1). 95.8% of those not accepted as donors (1923 people) were male and 4.2% of them (87 people) were female. When the reasons for cancellation are generally evaluated, it is determined that the most common cause was low hemoglobin concentration. On the other hand, the most common cause was dental treatment according to the donor questionnaire assessment. As the reasons for refusal of the women donor candidates was examined, it was seen that more than half (46 people, 52.87%) were unable to be blood donors because of low hemoglobin concentration.

Conclusion
For the supply of safe blood, donor selection must be done carefully in order both not to risk the donor from donating and the recipients from the complications of the transfusion. Compared with many other data in the period our study carried out, decreased hemoglobin concentration draws attention as the most common cause for rejection. Therefore, especially the units accepting transfusion in the field should take into account this issue and have to evaluate the donor with respect to decreased hemoglobin concentration by using hemoglobin measurement device. While dental treatment, another common cause of rejection, was expected to be among less common reasons for refusal with the Guide to National Blood and Blood Products 2011 version, the data of our study was not compatible with this expectation.
P‐152
Role of Haematological Parameters in Blood Donors’ Eligibility Assessment
Klausa E
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
The haemoglobin level is a basic criterion for evaluation of blood donors’ eligibility to give blood. It is measured in capillary and venous blood. In addition, such procedures as thrombapheresis and leukapheresis must be preceded by, respectively, the platelet number or white blood cell number measurements. Once a year, it is required to perform classification of morphological parameters in venous blood with white blood cell types in blood donors who regularly donate blood or blood components; such a requirement does not cover other (irregular, one‐time) blood donors. Haematological test together with biomarker (neopterin or suPAR) test results may constitute grounds for blood donors’ disqualification and provide information about bacterial or viral infections.
Aim
To analyze haematological parameters and white blood cell types in blood donors and patients.
Material
Haematological parameters were studied in 23 donors disqualified from donating blood due to the presence of HBV (four donors), HCV (13), HIV (three), HIV + HCV (one), Treponema pallidum + HIV (one), Treponema pallidum (one), and in five patients diagnosed with Staphylococcus aureus (two patients), Staphylococcus epidermidis (one), Streptococcus spp. (one), and Klebsiella oxytoca (one) infections.
Results
The reference values for monocyte levels were 4.0–8.0%. Among blood donors, increased monocyte levels, from 8.1 to 15.1%, were observed in 15 persons (i.e. 65.22%), including the range of: 8.1–9.0% in 7, 9.1–10.0% in 1, 10.1–11.0% in 4, 11.1–12.0% in 1, 13.1–14.0% in 1, 15.1–16.0% in 1. In the group of patients, all had increased monocyte values of 9.3 to 12.7%. Only in two cases (Staphylococcus aureus and Streptococcus spp. infections) high levels of leukocytes (10.8 tys./μl and 12.26 tys./μl) were detected. No other changes in haematological parameters and in the number of platelets were observed.
Summary
The retrospective analysis of morphological parameters and types of white blood cells in blood donors and patients infected with such pathogens as HBV, HCV, HIV, Treponema pallidum, Staphylococcus aureus, Klebsiella oxytoca, Staphylococcus epidermidis, Streptococcus spp., provided justification for the need to introduce, alongside measurement of the haemoglobin level, examination of complete morphology of peripheral blood and white blood cell types in the blood donor eligibility assessment. It is crucial that such tests be conducted once a year, not only in regular, but also all other donors. Special attention should be paid to levels of monocytes, involved in inflammation and in immune responses to pathogen infections.
P‐153
Social and Demographic Characteristics of Blood Donors in ITM RC Tetovo
Kocovska EK1, Timova TT 2
1ITM – RM RC Tetovo, Tetovo, Macedonia2ITM – Transfusion – Strumica, Strumica, Macedonia
Background
The Regional Center Tetovo comprises of the cities located in the north‐west of Macedonia – Tetovo, Gostivar, Kicevo, Struga, which are populated with predominantly Albanian population, thus showing the weakest results in blood donation in relation to the other regions. Therefore, the ethnic and social demographic analysis of the blood domors is very important.
Aim
The analysis of the blood donors by cities in the period between January and December 2014 was conducted in order to detect the behaviour of certain groups that are less involved in blood donations. The relations analyzed are field/facility, family/voluntary, men/women, Macedonians/Albanians, high school students/ university students, first‐time donors/regular donors.
Methods
the montly and yearly reports from the facilities that are delivered to the RC Tetovo, as well as registers from the field and the facility.
Results
In Tetovo, out of 2132 units of collected blood in 2014, a total of 935 (43.9%) were collected in the facility, while 1197 (56.1%) in the field. 2120 blood donors (99.4%) were volunteers, while 12 (0.5%) family donors. 1833 (85.9%) men, while 299 (14%) women. The first‐time domors were 681 (31.9%). There were 446 (20.9%) high school students, 278 (13%) university students. There were 873 Macedonians (40.9%), and 698 Albanians (44.7%), as well as 63 (2.9%) from other nationalities.
In Gostivar, out of 1560 units of collected blood in 2014, a total of 817 (52.3%) were collected in the facility, while 740 (47.4%) in the field. 1495 blood donors (95.8%) were volunteers, while 62 (3.9%) were family donors. 1270 (81.4%) were men, while 287 (18.3%) were women. The first‐time donors were 506 (32.4%). There were 378 (24.2%) high school students, 61 (3.9%) university students. There were 633 Macedonians (40%), and 698 Albanians (44.7%), as well as 226 (14.4%) from other nationalities.
In Struga, out of 1037 units of collected blood in 2014, a total of 1014 (97.7%) were collected in the facility, while 23 (2.2%) in the field. 1037 blood donors (100%) were volunteers. 909 (87.6%) were men, while 129 (12.4%) were women. The first‐time donors were 1132 (12.7%). There were no donors who were high school or university students. There were 420 Macedonians (40.5%), and 256 Albanians (24.6%), as well as 361 (34.8%) from other nationalities.
Summary/Conclusions
In all the facilities of the Regional Center Tetovo the dominant type of blood donor is a Macedonian male. The first‐time dononrs are in greaters numbers in the more developed centers with more employees. In the facilities with only one physician there is a high percentage of blood donation within the facilities. In the future, the accent should be put on motivating women donors, as well as university students from Albanaian ethnic background (since the relation of the population in the region is 80% Albanians and 20% Macedonians).
P‐154
Distribution of Donors in Each Age Group in Ampara Region Sri Lanka
Perera KM , Adikarama BMGM
General Hospital, Ampara, Malwana, Sri Lanka
Background
In Sri Lanka 100% blood donations are from voluntary non remunerated blood donors. Therefore it is crucial to have an idea about distribution of donors according to the age in the region to predict the future in order to maintain the 100% voluntary donor base uninterruptedly.
Objective
To evaluate the distribution of the donors in each age group in the donor pool in Ampara region.
Method
In Sri Lanka all hospital based blood banks are reporting monthly statistics of the donations to the National Transfusion Service. We started prospective study to collect data about demography of donors in January 2014. And study period is 12 months from January 2014.
Results
Total blood donations from both mobile blood collection and in house blood collection are 5341 during the study period of year 2014.
Out of the total collection 1352 (25.3%) are from age group 18–25, 1944 (36.3%) are from age group 26–35, 15,349 (28.7%) are from age group 36–45, 481 (9%) are from age group 46–55. 30 (0.5%) are from age group 56–60 respectively.
Caption 1: Distribution of donors in each age group 2014.

Conclusion
Out of the total donations most of the donations are from young population. Which is mostly from age groups of 26–36, 36–45, and 18–25.
Therefore can predict the 100% voluntary donor pool of the Ampara region will be static for at least another 10 year.
To maintain and to increase 100% voluntary donor pool in Ampara we have to conduct awareness programs for the whole population in Ampara region. And to encourage regular donors.
P‐155
Profile of Blood Donors Returning Themselves for their HIV, HBV, HCV Serostatus After a Blood Donation: a Cross‐Sectional Survey Among First Time Blood Donors of Regional Blood Centre of Ouagadougou, Burkina Faso
Nebie KY1, Sawadogo S 1, Kafando E 2, Ouattara S 1, Ouedraogo M 1, Ouedraogo O 1, Kienou K 1, Yooda P 1, Barro L 1, Dahourou H 1, Murphy E 3
1Centre National de Transfusion Sanguine, Ouagadougou, Burkina‐Faso2UFR/SDS, CHU Charles de Gaules, Ouagadougou, Burkina‐Faso3BSRI/University of South California, San Francisco, CA, United States of America
Background
Most countries in West Africa are experiencing a chronic shortage of blood products. In Burkina Faso as shown on several internal reports of the national blood transfusion centre, blood collection does not cover the theoretical needs of blood products of the population.
The low number of blood donors and the poorest of repeated donation are the main reason of these difficulties. Among many others explanations, it is reasonable to think that an inadequate management of donor serologic testing and results disclosure to them can be significant.
Indeed, many studies in this region on motivations to blood donation cite the need for some donors to know their HIV status. In another hand, ‘fear to discover a positive HIV status’ has also been identified as an obstacle to blood donation.
If in the European countries and North America, informing blood donors of the presence of infectious disease is mandatory and presented as a public health contribution of transfusion centres, in Burkina Faso, the donors have to freely choose to be or not to be inform on his HIV status. They then have to return to the blood centre, at least 2 weeks after blood donation and ask for their results.
In these circumstances, can the donors who choose to know their HIV status, be different from those who choose to not be inform?. The aim of this study was to answer this question.
Methods
We conducted a cross‐sectional study that includes all first time volunteer blood donors of the fixed site of Ouagadougou during the year 2011.
Data had been extracted from donors’ management software and registry of rendering results. These data includes socio demographics data, HIV, HBV and HCV testing results, as well as the return or none of the donor to be informed of its results.
Data had been process with EPI‐Info 7.
Results
An overall 1467 first time blood donors were included in the study. The rate of return for testing results was 33.26% among them. HIV, HBV and HCV serologic markers were found higher in blood donors who return for results than those who don't return (1.25% vs 0.96 for HIV, 16.88% vs 11.08% for HBsAg and 5.73% vs 3.88% for HCV) even if the differences were not significant regarding to statistical Chi square test.
Conclusion
The study shows a relatively low level of blood donors returning for their results on the basis of a voluntary approach. Unfortunately some of positive donors are detected on the occasion of the following blood donation often in an unprepared situation that generates frustration and misunderstanding.
P‐156
Abstract Withdrawn.
P‐157
Retaining School Leavers as Repeat Blood Donors in Malawi
Chandrasekar BC1, Latham TL 2, Dessoffy TRD 1, Njolomole SN 3
1University of Leeds, Leeds, United Kingdom2NHS Blood and Transplant, Bristol, United Kingdom3Malawi Blood Transfusion Service, Blantyre, Malawi
Background
Safe blood transfusion is a life‐saving intervention. However many African countries, including Malawi, suffer from an inadequate blood supply.
There are two main types of blood donors; voluntary non‐remunerated blood donors (VNRD) and replacement donors. Secondary school students aged between 16 and 19 are a major contributors to the pool of voluntary donors in Sub‐Saharan Africa as they are associated with a lower risk of Transfusion Transmitted Infections (TTIs) and can be easily recruited at a low cost. They are more inquisitive and can be given accurate in‐depth information on avoiding risky behaviour in order to remain as safe donors.
Malawi Blood Transfusion Service (MBTS) collects blood from only VNRD, as advocated by the World Health Organisation (WHO), but only contribute to 55% of the national blood supply. In Malawi, secondary school donors account for approximately 80% of the VNRD population. However, few continue to donate after secondary school and retaining school leavers will increase the number of voluntary donations, improving both blood safety and availability.
Aims
To explore why secondary school students stop donating blood after they leave school in Malawi.
Objectives
To briefly explore the views of donors and transfusion service staff on blood donation.
To identify the motivators and barriers to blood donation by secondary school students.
To identify the motivators and barriers to blood donation by school leavers.
Methods
A qualitative study was conducted at MBTS headquarter in Blantyre, Malawi. 24 participants including secondary school students, school leavers, and MBTS staff were identified through purposive and convenience sampling. Many variables including, number of years were considered, through maximum variation sampling to capture a wide range of ideas. Semi‐structures interviews and focus groups were utilised to explore participant views. Data was analysed thematically.
Results
All participants had positive opinions of blood donation and many recognised that there was an unmet need for blood in Malawi. Education, peer influence, and convenience are significant facilitators to blood donation by secondary students. Some school leavers are motivated to continue donating by altruism, reimbursement of transport costs, milestone awards, and a club for youth donors called Club 25. Poor communication, lack of trust in MBTS and distance are important barriers to blood donation faced by school leavers.
Conclusion
The decision to donate is complex and multifactorial. The loss of facilitating factors, such as peer influence and convenience, contributes to the high drop‐out rate. School leavers face further barriers to repeat donations which if addressed could increase voluntary donations. Education and peer support are found to a successful motivators for school donation, and are necessary in the long term to develop a relationship of trust between MBTS and its donors, thereby promoting a culture of post‐school donation. Many of the barriers to repeat donation could be addressed through rapid interventions that are within the influence of MBTS, such as maintaining regular contact with donors after they leave school and providing transport.
P‐158
Attitude to Blood Donation Among a Tertiary Hospital Workers in Nigeria
Adegoke M1, Jeminusi A 2, Ojo T 1, Sholeye O 1, Olatunji O 1
1Olabisi Oabanjo University Teaching Hospital, Sagamu, Nigeria2Department of Community Medicine and Primary Care, Olabisi Onabanjo University T, Sagamu, Nigeria
Background
Adequacy of donor blood to meet demand is no more a problem in the developed countries of the World, which have established organized means of collection and distribution. The story is however different in most of sub‐Saharan Africa where illiteracy, poverty and negative cultural beliefs remain obstacles to donation.
The inadequate donor blood supply in response to recent terrorist attacks in Nigerian Capital, Abuja has further highlighted the problem. Members of staff of hospitals who are close to blood transfusion centres and are expected to be better informed has been targeted in this study to assess willingness to donate blood as well as factors affecting their willingness or otherwise.
Aims
To assess willingness of health workers to blood donation as well as factors affecting their willingness or otherwise.
Method
Questionnaires were administered to consenting hospital staff from various departments of a tertiary health care centre with a blood transfusion unit, Respondents included staff of departments (Account and Finance, Administration, Catering, Health Information, Laundry, Medical, Nursing, Laboratory). Descriptive statistics (SPSS Version 17) were used to analyse data collected.
Result
Responses were received from two hundred and forty six (246) health workers, 76 male and 170 females, aged 20–60 years.
In Table 1, Columns A and B showed that the percentage of health workers who were willing to donate blood is uniformly higher than those who have been approached for donation, except for those in laundry and tailoring sections. Only a small proportion of Nurses (28%) who constitute the largest population of health workers has been approached for blood donation. Except for catering and Health Information staff, there is an identity between willingness to donate blood and the desire to help someone in need (columns B and C). The willingness therefore does not translate to actual donation. This is why the percentage that have donated closely approximates the percentage that have been invited to donate (Columns A and F), and not those who express willingness. Columns D and E showed that more people will rather donate for those they know than do it voluntarily. This is consistent with experiences in reports involving organ donation.
More people avoid blood donation out of fear than out of cultural or religious considerations (Table 2).
Table 1: Summary of percentage of health workers’ response to invitation and attitude to Blood donation.

Summary/Conclusions
Blood donation is still based largely on primordial Motivation of helping those we know, and Voluntary donation is relatively unpopular. All these are evidence of ineffectiveness of current local and national blood transfusion services. There is a need to improve awareness and advocacy with respect to blood donation among health workers and the general population.
Table 2: Factors discouraging blood donation.
P‐159
Awareness About the Conditions for Blood Donation of Blood Donors in the South Backa District of Vojvodina
Bogdanovic S , Bujandric N , Jovanovic R
Blood Transfusion Institute of Vojvodina, Novi Sad, Serbia
Background
The availability of sufficient quantities of safe blood is the main goal of transfusion services as well as medical institutions that use blood and blood components in the treatment of patients. The screening and self‐deferral process is unique instrument blood banks have of curtailing donations from individuals with very recently acquired infections that cannot be detected by laboratory assays. Screening questionnaire responses depend on donor knowledge of and attitude towards transfusion‐transmitted infections (TTI) risk and testing attributes such as socioeconomic status and education. Potential donors should have a certain level of awareness and knowledge concerning the blood donation process, TTIs and the impact of blood transfusion on a recipient's life prior to donation.
Aims
This paper is aimed to present awareness and sources of information blood donors about the conditions for blood donation in the South Backa district of Vojvodina.
Methods
A prospective multicenter cross‐sectional study was carried in our Institution during the period from October 2012 to April 2013. The study included 526 blood donors who completed a specially designed anonymous questionnaire. The questionnaire contained questions on demographic data and awareness and sources of information about the conditions for blood donation. Collected data were analyzed regarding to sex, age, number of blood donations, education, marital status, place of blood donation, place of residence, awareness and sources of information about the conditions for blood donation.
Results
The study sample consisted of 392 males (74.5%) and 134 females (25.5%). Out of them, 73.4% were 21 to 50 years old; 91.8% repeat donors; 70.3% high school educated; 48.9% married; 55.9% in institution blood donated; 86.5% permanent place of residence. 87.3% of blood donors answered that they were informed about the conditions for blood donation. The most frequent sources of information of blood donors were brochures (35.2%), lectures (23.8%), family member or friend (22.6%) and general practitioner (15.6%).
Summary/Conclusions
Blood donors in the South Backa district of Vojvodina are mostly well informed about conditions for blood donation. Information alone is not enough to make donors self‐defer or acknowledge their behavioral risks. Other steps such as informing them of nearby test centers are necessary that will hopefully decrease the incentive using the blood center for infectious disease testing. Therefore, there is a continuous need for promotion and improvement of active participation of blood donors in the selection process.
P‐160
Voluntary Blood Donation: Miles to Go, Celebrating World Blood Donor Day in June 2014
Sood RS , Asha S , Gupta VG , Jomol S , Rani S , Kumar T , Kumar D
Saket City Hospital, New Delhi, India
Background
WHO, the World Health Organisation is the directing and coordinating authority for health within United Nations system. The day 14th Jun is WHO's annual Blood Donor Day. This day forms part of WHO's campaign to raise awareness of the need for safe blood/components/products, to thank unpaid donors for their life‐saving contribution and to look forward to the continued efforts to achieve the strategic goal for all countries of obtainning 100% of blood supplies from voluntary unpaid donors by 2020. There have been significant increases in voluntary unpaid donations from low‐ and middle‐income countries, with an annual increase of 7.70 million donations from 2004 till 2011 (1). Blood from repeat voluntary blood donors is safer and regular donors have less chance of adverse reaction after donation (2,3,4).
Aim
To take this day as an opportunity to retain one time blood donors.
To recruit more new blood donors.
Methods
Extensive celebration of the World Blood Donor Day 2014 was organized by the Department of Transfusion Medicine & Immunhematology at Saket City Hospital, New Delhi, India.
Three days, i.e.12, 13, 14 June 2014, were celebrated by holding a free testing camp for past donors, all employees, patient‐attendants, anybody visiting the blood bank. Invitation and information was send to past donors via email.
It was a highly organised and coordinated event.
Visitors were educated on uses of blood/components, for various categories of patients, benefits of blood donation to them and also tested for their hemoglobin levels, blood group, hepatitis B and C status by ELISA testing.
Visitors were oriented to various areas of blood bank and to the work done in each area.
Last day evening was celebrated with a cake‐cutting ceremony, when a huge cake in form of red drop was cut and the cake and eateries were served to all present and invited. Past voluntary blood donors were especially invited for the ceremony.
Caption 1: CELEBRATING WBDD.

Caption 2: CELEBRATING WBDD.

Results
Most people coming to blood bank were willing to donate blood. They were instead enrolled as potential voluntary donors for future. Total of 312 donors enrolled as potential voluntary blood donors in these three days. They included 56 Apos, 105 Bpos, 27 ABpos, 89 Opos donors and 11 Aneg, 11 Bneg, four ABneg and nine Oneg blood donors. Each day one voluntary blood donor is motivated from among the enrolled donors for a voluntary blood donation.
Conclusion
The camp was not just for the checkup but to have an effective communication and acquaintance of the premises with the blood donors, communication and education being two important success factors towards our goal.
It is necessary to improve common man's understanding, and not just awareness, of the need for blood.
Important message to be delivered to all one time donors is the need for regular blood donation.
The need to invest resources and research in the area of promoting voluntary blood donation in order to ensure blood security is further established. This is important to reduce the global burden of disease due to lack of blood or unsafe blood.
P‐161
Abstract Withdrawn.
P‐162
When Will the Non‐Donors Prefer to Bleed (VNRBD)
Villumsen JV
National Danish Blood Donor Association, Taastrup, Denmark
Background
The National Danish Blood Donor Association (NDBDA) asked non‐donors questions about their knowledge and preferences concerning blood donation. One of the questions was: If you were a blood donor, when will it suits you to bleed.
Aims
We would like to find out when the non‐blood donors would prefer to go to the blood bank. If the blood collections sites have the right opening hours we remove one reason not to bleed.
Methods
We collected 1199 answers through a digital questionnaire. The sample was chosen randomly, but it was ensured that it was representative for the Danish population. The first question to the panel was: Are you a blood donor? If they answered ‘yes’ or ‘have been’ they were omitted. 1199 persons were included. They answered 14 questions. The collection of data and sampling was done by yougov.dk.
The respondent was asked: If you were a blood donor, when will it suits you to bleed (VNRBD)? They could choose between eight predefined answers, ‘Another reason’ and ‘I do not know’ (these two was always last). They could choose up to three answers: one answer: 711 respondents, two answers: 281 respondents and three answers 207 respondents.
Results
We found that the Danes asked to do VNRBD does not have one period of the day, when everybody would like to go. But we can see three periods when many would prefer to do the donation. Among the potential donors we find the following.
1. On their way to or from work or another purpose (30.1%) to this number you can also add a major part of the responders answered that they prefer the following hours 15–18 (28.7%) and 18–20 (26.5%). Which are when people are going home in Denmark.
2. Weekends (30.1%)3When I am at work or at school (22.4%).
We also found that less than 9% would prefer the blood bank in the time periods between 7 and 15 h. Only a minority of the non‐donors can come to the blood bank at any time of the day.
Please note in Denmark almost all bleedings are done at a prebooked time. In all blood banks the donor can book a donation at a web based booking system.
Conclusions
Main part of the respondents would prefer to do the bleeding when they are off work, but 22.4% would prefer to do the donation when they are at work or in school. The results does not give one answer on how to organize the blood collecting sites, the structure should be able to full fil the wishes of the donors, and they are not aligned.
P‐163
Donors’ Health as One of the Priorities of Blood Donor Management
Timova TT1, Stambolieva DS 1, Malinova TM 1, Baldzieva AB 2, Maninska LM 2, Kocovska EK 1, Vitlarova JV 1, Gjorgevska VG 1
1Institute of transfusion medicine, Strumica, Macedonia2General hospital, Strumica, Macedonia
Background
The primary obligation of blood centers is to protect the health of donors. Therefore it is necessary for all donors to undergo a screening process to assess their suitability. Only healthy persons with a good medical history can be accepted as donors of blood or blood components.
Aim
The purpose of this paper is to show that the basic steps in taking care of donors’ healthin the process of selecting donors who can donate blood without any damage to their health include a careful medical examination, an appropriate questionnaire and a correct evaluation of every potential donor.
Materials and Methods
The process of selecting donors who can donate blood without any damage to their health was carried out on 2255 potential donors who came to donate blood at the Transfusion Department – Strumica in 2014. The screening of donors was conducted through interviews, a questionnaire, direct questions, measuring Hb levels, measuring blood pressure and auscultation of heart.
Results
In 2104, 2255 individuals volunteered to donate blood in the Department of transfusion medicine in Strumica, 2104 (93.3%) of whom donated blood while 151 (6.7%) were deferred. The reasons for deferral are the following: low Hb in women (below 125 g/l) – 44 (29.1%), low Hb in men (below 135 g/l) – 31 (20.5%), infections treated with antibiotics – 4 (2.6%), hypertension – 15 (9.9%), hypotension – 11 (7.9%), cold‐4 (2.6%), tattoo in the last 12 months – 2 (1.3%), lower body weight (lower than 50 kg in women) – 3 (1.9%), a period less than 3 months since the last blood donation in men – 1 (0.7%), a period less than 4 months since the last blood donation in women – 2 (1.3%), surgeries in the last 12 months – 4 (2.6%), taking medicines – 2 (1.3%), diseases (diabetes mellitus – insulin dependent, heart diseases, allergies) – 10 (6.6%), vaccine – 4 (2.6%), older than 65 years – 2 (1.3%), younger than 18 years 5 (3.3%), risky sexual intercourse – 1 (0.7%), tooth extraction – 3 (1.9%), Hepatitis B – 1 (0.7%), other – 2 (1.3%). All of these potential donors were deferred for a certain period of time after which they could volunteer to donate blood again.
Conclusion
The design of the questionnaire is essential as it has to elicit relevant information about the health and life style of the donors. The assessment and the decision about the donor's health has to be made on the basis of appropriate questions and a careful medical examination. Appropriate screening contributes to the protection of donors’ health as one of the priorities of blood donor management. Deferred donors must be properly informed and given a clear explanation of the reasons for deferral.
P‐164
Abstract Withdrawn.
2.2 Blood collection incl. apheresis
P‐165
The ‘New Reinfusion Protocol’ Decreased the Post‐Procedure Residual Blood Loss During Plateletpheresis Procedures
Prashant A 1, Aseem Tiwari A 2, Vimarsh Raina V2, Kumar P 1
1Jaypee Hospital, Noida, India2Medanta‐The Medicity, Gurgaon, India
Background
In India, during last two decade the demand of single donor platelets (SDP) has increased considerably. The common contributors behind the growing demand for SDP are the increasing awareness of specific component therapy, repeat epidemics of dengue and the advancement in the surgical and medical practice. Results from published literature have demonstrated significant alteration in hematological parameters during plateletpheresis (PP). Routinely at the end of procedure the reinfusion of the residual blood in the circuit is done once only. The published data on hematological changes during plateletpheresis are with single reinfusion only. We have observed that spontaneous slow recovery of post procedure Hb% is a commonest cause for making donor unfit for repeat procedure. Therefore, we planned a study in which we could assess the hematological changes with the ‘new reinfusion protocol’ wherein the three simultaneous back to back reinfusions were done to return back the residual blood in the circuit.
Aims
The primary aim of the study was to assess the alteration in hematological parameters with previous established protocol of single reinfusion (conventional protocol) and with the new protocol of ‘three reinfusion’. Time taken for the completion of procedure (total procedure time) was also calculated with both the protocols.
Methods
Written consent was obtained from all the donors for the participation in study. All PP procedures were performed using a fully automatic platform, COM.TEC (Fresenius Kabi, Germany). All the procedures were done with double needle access kit only (C5L). We performed a total of 100 procedures with conventional protocol and 100 procedures with new protocols. Samples which were collected for the analysis of blood grouping (EDTA, 3 ml) and was utilized for the purpose of assessment of preprocedure hematological parameters. These samples were collected after the venepuncture of the antecubital vein when the donor was declared medically fit for the PP procedure. A sample for the post procedure changes in hematological parameters was collected after 1 h of the completion of procedure. The hematological parameters measured for the analysis were hemoglobin (Hb, g/dl), Hematocrit (Hct, %), platelet count (PC, ×109/l) and WBC count (×106/l). Statistical analyses were done using SPSS 16 software.


Results
Statistically significant (<0.01) drop in all hematological parameters were observed in ‘conventional reinfusion protocol’ group. With the new protocol, we did not observe a significant drop in hemoglobin and hematocrit. Drop in platelet and WBC counts remained significant in both the group. The mean procedure completion time was 60 min with new reinfusion protocol vs 54 min with the conventional reinfusion protocol.
Conclusion
As per the regulatory requirements none of the plateletpheresis donor could be repeated for plateletpheresis procedure until the platelet count is above 150 × 109/l and Hb is 12.5 g/dl. Inspite of having adequate post‐procedure platelet recovery, slow recovery of hemoglobin makes the donor unfit for plateletpheresis. The new protocol of reinfusion would increase the probability of reentry of a proportion of donors after 48 h of index procedure.
P‐166
Aggregates in Single Donor Platelet Concentrates Within 24 h from Collection
Castrillo Fernández ACF , Arcas Otero CAO , Rodríguez Calvo MIRC
Centro de Transfusión de Galicia, Santiago de Compostela, Spain
Platelet concentrates (PCs) may have visual, macroscopic aggregates (AG) after collection by apheresis or after production from whole blood.
We assessed the occurrence of residual AG in single donor platelet (SDP) in routine blood banking within 24 h after donation, during the two last years.
Material and Methods
All SDP were stored in platelet additive solution (PAS) SSP+ (MacoPharma); the final PAS/plasma ratio was 60/40. In routine, after collection PCs were rested for 1–2 h and visually inspected in the following 2–5 h (first inspection). If AG were seen, the products were checked again by a physician 18–24 h later (second inspection). PCs were stored under standard conditions at 22°C with continuous agitation. Inspection consisted of an assessment of swirl and the visible presence of AG before proceeding with Intercept pathogen reduction technology. If visible aggregates persisted and were evident in size or in number, the units were discarded. All PCs were visually inspected prior to supply.
From 2013 to 2014, 11,991 one dose SDP – 15% Amicus and 85% Trima Accel – were processed.
The presence of AG in PCs collected by Amicus and Trima Accel devices within the first hours after donation was recorded and this was likewise done for PCs with persisting macroscopically visible AG 24 h later.
We assessed variables like gender, hematocrit, platelet count, size and quantity of AG.
Results
The percentage of all units with AG beyond 24 h is 1.93%: Amicus 4.96% and Trima 1.39%; this difference is probably related to differences in apheresis device technologies. In Table 1 some parameters are shown.
Repeat donations of some donors were found to have resulted in platelet products that formed AG.
The presence of AG in female donors was 3.76%, and 1.68% in male donors. Hematocrit and platelet count were within normal limits.
A seasonal effect on the occurrence of AG was not observed.

Conclusion
We found that AG occurred in products collected on both apheresis devices but to a higher degree with Amicus. PCs containing AG were donated more often by females. Donor specific factors could contribute to AG formation. The occurrence of aggregates seems to be a multifactorial phenomenon. Temperature during the process, mixing with anticoagulant, ratio AS/plasma, resting period, agitation, gender, apheresis device used and individual variables from the donor should all be taken into account.
P‐167
A Prospective Analysis of the Productivity of MCS+ UPP‐999 (Haemonetics) and Amicus 3.21 (Fresenius Kabi) in 222 Apheresis Donors
Pajares Herraiz AL , Tordera JC , Torrades CT , Fernández Ramirez VF , Morillo LM , García García RG , Rodríguez Gambarte JD , Eguía Lopez BE , Flores Sanz MV
Centro Regional de Transfusion Toledo‐Guadalajara, Toledo, Spain
Background
Blood cell separators (CS) MCS+ and Amicus are automatic apheresis systems that separate and collect any combination of platelet products, plasma and concurrent red blood cells from donors, by means of discontinuous flow (MCS+) or continuous flow (Amicus 3.21). To obtain platelets and plasma we can use the UPP protocol and 999 collection set in MCS+, and in the Amicus 3.21 system we chose the protocol at the CS. Both systems reduce leukocyte load with continuous filtration and have automated preparation of platelet additive solution (PAS) stored platelets
Aims
Haemonetics changed the protocol to obtain platelet concentrates with additive solution (UPP‐999), so we were interested in comparing this system vs the other cell separator we are using, Amicus 3.21, to evaluate the productivity of two blood cell separators to obtain plasma and platelets in PAS, and to analyze the correlationbetween estimated (EP) andobtainedplatelets (OP) with both CS.
Methods
We prospectively analyzed 111 procedures (59 men/52 women) with Amicus3.21 and 111 (59 men/52 women) with UPP‐999, performed with apheresis donors to obtain platelets concentrates stored with PAS andadditionalplasma. Gender and age were controlled. Weight, height, blood volume, volume processed, platelets and hematocritPredonation (Coulter LH750), EP, OP, residual leukocytes, process time, platelet concentrate volume andplasmavolume were measured and adverse reactions with both CS were assessed. Data was recorded in a database (Excel 2007; Microsoft C., USA) and statistical analysis was done with IBM SPSS version 19 (Chicago, IL, USA) and MedCalc 12.2.1.0 (Ostende Belgium).
Results
There were no statistical differences in age (44.4 ± 9.1 vs 45.9 ± 7.8), blood volume (4645.3 ± 714.2 ml vs 4731.2 ± 870.7 ml) leucocytes/μl predonation (7789.4 ± 2037.8 vs 8116.2 ± 2027.1) and Hematocrit (%) (43.4 ± 3.5 vs 42.9 ± 3.7) between the two groups (Amicus vs UPP‐999). However Platelet count predonation (109/l) was distinct (258522.52 ± 39102.63 vs 289279.28 ± 42748.99) as more platelet load in donor is necessary to minimize the process time with the UPP procedure. There were significant differences in volume processed, process time, platelet concentrate volume, platelet yield, plasma volume and residual leukocytes (Table 1). The correlation index between EP and OP was 0.52 in the Amicus group and 0.36 in the UPP‐999 group. Adverse reactions, citrated realtionated, were more frequent in the UPP group (3.6%) than in the Amicus group (1.8%). All these mild adverse reactions were successfully resolved with oral calcium.

Conclusion
UPP‐999 and Amicus 3.21 collects platelets and plasma complying with the European Guidelines and Spanish CAT rules. More platelets yield and plasma volume in less time was obtained with Amicus 3.21 in comparison with the UPP‐999, with less adverse reactions and betterleukoreduction. However MCS+ allow us to perform apheresis in blood donation mobile units. In both groups we obtained more platelets than expected which is importantin designing anobjectivetoobtainplatelets concentrates.
P‐168
Post Donation Information: is it Useful, is it Timely?
Barnes SM1, Carroll J 2
1NHS Blood and Transplant, Leeds, United Kingdom2University of Limerick, Limerick, Ireland
Background
The European Directive states that we must provide prospective donors of blood and blood components with: ‘The reasons why it is important that donors inform the blood establishment of any subsequent event that may render any prior donation unsuitable for transfusion.’ This has been translated into UK statute law as part of the blood Safety and Quality Regulations 2005. NHS Blood and Transplant (NHSBT) give all donors an information card that asks them to contact the NHSBT if: ‘they become unwell (except for a cold or a cold sore) in the next two weeks’.
Aim
Do donors contact the blood service, if so how many?
When do they contact the service? Is it in time for the blood donation to be recalled?
Do they contact the service about the right things?
Is the reason for recall documented in the NHSBT computer system (Pulse) in a timely fashion?
There is no published work looking at this area of blood donation practice.
Methods
The first 164 donor contacts in 44 days starting 9/9/13 with Post donation Information were reviewed on the NHSBT Pulse computer system, the data obtained was analysed.
Results
The vast majority of the donors (98.2%) contacted NHSBT by phone through the National Call Centre (NCC) during normal working hours (77.4%). A smaller group (21.3%) contacted the service during extended hours, between 9–8 am or 5–7 pm weekdays or 9:30 am–5:30 pm, weekends when there are clinical staff available. Only two donors contacted the NCC outside these hours. These donor's donations are put on hold and in both cases a diagnosis was placed in Pulse by 10 am the next day.
Donors contacted NHSBT quickly, 79% within a week of donation and 97% within 2 weeks. They also reported symptoms swiftly, over 56% within the first 3 days, 90% within a week and 96% within 2 weeks. Only five donations (3%) had been transfused by the time the donor contacted NHSBT. There were no problems encountered with these donations.
A significant number of the contacts, 62.2%, were about common infections that could have been transmitted by the donation. The remaining 31 donors had a number of less common conditions most notable among these were the four Herpes Zoster infections. 10.4% of donors contacted NHSBT about colds and cold sores. The diagnosis was generally made and recorded during the first and only contact with the donor (93.9%).
Conclusions
PDI provides a very valuable safety net for the blood service and the recipient patients. 111 donations (67.7%) were discarded following 164 donor contacts to NHSBT. There is no way of determining how many donors developed an illness post donation. However the lack of any transfusion transmitted infections suggests that this system is working. Although concern is expressed by colleagues working with hospitals that a diagnosis is not available for donations recalled out of hours, there was a diagnosis available in all cases within 14 h. It is not clear how to reduce the 13.4% of late or unnecessary calls.
P‐169
Effects of Optia Red Cell Exchange Transfusion in Sickle Cell Disease on Blood Parameters Associated with Bleeding Risk
Cho G , Valliani S , Kiilu P
London North West Healthcare NHS Trust, London, United Kingdom
Background
Sickle Cell Disease (SCD) comprises a group of inherited blood disorders that result in complications related to haemolysis and vaso‐occlusion. Red cell exchange is a well established treatment in sickle cell disease for prevention and treatment of complications such as stroke. The procedure removes some of the patient's red cells as well as a lesser amount of plasma and platelets. The machine used was a Terumo BCT Optia (previously Caridian BCT, and COBE). A fall in platelet count after the procedure has been described but the effect on coagulation is not well documented.
Aims
To assess if automated red cell exchange tranfusion using an Optia can lead to a disturbance of blood parameters that could increase bleeding.
Methods
Retrospective analysis of automated red cell exchange transfusions in adult sickle cell disease patients performed on an Optia from January 2014 to February 2015. The patients were included if haemoglobin, haemoglobin S%, platelet count, prothrombin time (PT), and activated partial thromboplastin time (APTT) were available before and after the procedures. Only patients who had received more than one procedure were included.
The pre‐ and post‐transfusion results were used to calculate the percentage change in a parameter and because more than one set of results were available for each patient the average change over all procedures was calculated.
Results
Nine patients were included in this analysis. 6–8 units of red cells were used for each procedure.
The average % change in platelet count for all included procedures was −45.4%. The one patient who had preceding thrombocytopenia (subsequently diagnosed with ITP) had falls to a low of 37 × 109/l after one procedure (with a starting platelet count of 95). For the other patients average % change in platelets ranged from −40.5% to −58.6%.
The PT and APTT after automated red cell exchange were overall prolonged after exchange transfusion. For all procedures the % change in PT and APTT were 6.4% and 5.7%, respectively. Some went from within the normal range to outside. In addition on one occasion for one patient the PT change was 65.1% and for APTT it was 61.9%. The patient who is on warfarin had an average 10.8% prolongation of the PT, the highest out of all the patients.
Any adverse events were recorded during the procedures. The main problems were difficulties in securing vascular access and none involved bleeding.
Summary/Conclusions
Automated red cell exchange transfusion with the Terumo BCT Optia apheresis machine does lead to falls in the platelet count and prolongation of coagulation tests. Sometimes the pre‐transfusion results that are in the normal range change to abnormal (outside the normal range for the laboratory). However, no episodes of bleeding were reported during the procedure or afterwards. Our selection of patients included one on warfarin and one with ITP. No bleeding complications were detected in these patients but further work is needed.
P‐170
Vein Visualisation Technology and Young Blood Donors: Impact on Donation Success and Retention
Waller D , Mondy P , Brama T , Wall-Smith D , Irving DO , Fisher J , King A , Malkov K , Diaz P , Shuttleworth G , Bell B
Australian Red Cross Blood Service, Sydney, NSW, Australia
Background
The Australian blood donor population is ageing and there is a pressing requirement to recruit and retain younger donors. Vein visualisation technology (VVT) may help to improve the blood donation experience for young donors by lessening anxiety, reducing vasovagal events and minimising painful phlebotomies. VVT may also improve donor retention and contribute to phlebotomist confidence.
Aim
The aim of this research was to determine the impact of VVT on donation success and retention in younger Australian blood donors.
Methods
New and returning donors (aged 18–30 years) eligible to donate whole blood were prospectively recruited with 300 new and 600 returning donors planned. Donors were allocated in a block randomisation schedule of 2:1 active to control ratio at two donor centres. Two vein visualisation devices currently available in Australia were provided.
The study centres crossed over devices after the mid‐point (of new donor and returned inclusion). Participants completed the Blood Donor Reaction Inventory (BDRI) and the state component of the Spielberger State/Trait Anxiety Inventory (STAI). Participants also reported their intention to return, pain and fear of blood donation. Flow rates, collection volumes, phlebotomy complication rates and number of subsequent donations were taken from routinely collected data. Phlebotomists completed an in‐house developed questionnaire that measured acceptance of the technology including specific vessel choice and venesection success.
Results
No significant differences were found on donor measures between control and intervention groups on scores for anxiety, blood donation reactions nor intention to return (P > 0.05 on all measures). Phlebotomists reported that the technology was useful in 32.7% donations (190/580) overall and 46.7% (43/92) of donations when the phlebotomist stated that the vein was not visible to the naked eye. Staff who regarded the technology as useful had spent significantly less time working for the Blood Service (P < 0.05).
Summary/Conclusions
These results suggest that vein visualisation technology does not markedly improve the donation experience for younger donors. Indeed, self‐reported willingness to donate in the future was unaffected by the intervention. However, these devices may be useful as a training tool for junior phlebotomists. The detailed data regarding donor adverse events, donor retention, phlebotomy‐related injuries and collection volumes will also be presented.
P‐171
Exploratory Correlation Analysis of Procedural and Donor‐Related Parameters in the Occurrence of Aggregates in Platelet Products
Adler M1, Antoon M 2, Ayer I 1, Schaub T 1, Fontana S 1
1Interregionale Blutspende SRK AG, Bern, Switzerland2Terumo BCT Europe NV, Zaventem, Belgium
Background
Platelet (PLT) aggregate formation in whole blood‐ and apheresis‐derived PLT products has been known to occur. The underlying causes are thought to be multifactorial and subject to current research.
Aims
To investigate the presence of potential correlations between procedural and donor‐related parameters and the occurrence of aggregates in PLT products.
Methods
For each PLT apheresis procedure (Trima Accel™, Terumo Corp., Tokyo, Japan) performed between 2nd August and 30th December 2013, the PLT product was inspected for presence of aggregates 2 h following collection. If macroscopically visual aggregates were observed, the product was placed on the agitator under controlled conditions and re‐assessed 12 h later. The presence of aggregates was rated as ‘yes’ or ‘no’. For each of the evaluated products the apheresis machine datalog file was obtained to extract the corresponding procedural and donor parameters. STATISTICA™ V 12 (StatSoft Inc., Tulsa, OK, USA) was used to compare parameters in the aggregate‐free group and the group of products with aggregates respecting donor and procedure variables. Donor gender was evaluated with c2. Other parameters were t‐tested.
Results
Results are summarized in Table 1. Female gender was found to be significantly correlated with a higher proportion of aggregate‐containing products. Furthermore, total blood volume (TBV) as well as hematocrit (HCT) were shown being inversely correlated with a higher proportion of aggregates. While correlation of gender and TBV applied to both aggregates present after initial assessment and those present 12 h post‐collection, significant correlation of HCT was reached only between donor HCT and aggregate formation as observed 2 h after collection. The PLT product concentration was found to be correlated only in the group of aggregates present 12 h post‐collection. All other investigated parameters did not reveal significant correlation.

Conclusion
The correlation was found to be significant for donor gender, TBV and HCT. From a procedural point of view, only the storage PLT concentration was found to be significantly correlated with the occurrence of aggregates at 12 h post‐collection. As HCT and TBV are also a function of donor gender some interdependence may be present and will need to be investigated further.
P‐172
Evaluation of Quality of Platelet Concentrates and Plasma Collected Simultaneously from One Donor Using MCS+ Blood Separation System
Bojarska E , Szymczyk-Nuzka M , Stasiaczek G
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
The use of cell separators allows collecting from a single donor several types of blood components. MCS+ system enables collecting leucoreduced platelet concentrate suspended in additive solution (LR‐PC/AS) and programmed volume of plasma (FFP). Due to replacing plasma with additive solution for suspending platelets the volume of transfused plasma is reduced and it results with decreased risk of post transfusion adverse reactions occurrence. Simultaneously collected plasma can be used for fractionation purposes or as a quarantine plasma for clinical use.
Aims
The aim of the study was to evaluate quality control parameters of LR‐PC/AS and FFP collected from a single donor using automatic apheresis system. Additionally the total amount of platelets in LR‐PC/AS was compared with LR‐PC suspended in plasma.
Methods
Total amount of 63 LR‐PC and FFP collected using the MCS+ (Haemonetics) blood cell separator were examined. LR‐PC were automatically suspended in additive solution SSP+ (Macopharma). The volumes of the components were determined and then the amount of platelets was determined with Micros 60 ABX (Horiba), the amount of white blood cells with FacsCalibur (Becton Dickinson), pH with pH‐meter (inoLab). In case of FFP the residual platelets, leucocytes and erythrocytes content was determined with BD FacsCalibur while total protein concentration with Abbott‐Architect and factor VIII activity with ACL 9000.
Results
Results of the study are presented in Tables 1 and 2 as mean ± SD.
Conclusions
The study shows that both LR‐PC/AS and FFP can be collected simultaneously from a single donor in a period of time comparable with collecting one component at a time.
Examined blood components meet the current quality requirements of Polish and EU regulations.
The amount of platelets in LR‐PC/AS (3.6 × 10e11 per unit) is comparable or even slightly higher than observed in case of LR‐PC suspended in plasma (3.5 × 10e11 per unit).
Such system enables increasing the efficiency and effectiveness especially in periods of increased demand for blood components in case of a limited number of donors.
It provides flexibility in collecting blood components depending on demand.


Using AS‐SSP+ replaces significant amount of plasma so that it enhances the quality of PC and enables reducing the risk connected with transfusing redundant volumes of plasma and leads to achieving better standardization of blood components.
In case of clinical indications for PC and FFP transfusion for one patient, these blood components can be acquired from one donor leading to reduced risk of immunization.
Simultaneous collection of good quality LR‐PC/AD and FFP increases their availability without compromising donors’ safety.
P‐173
Clots in Red Blood Cell Products – Analysis of their Frequency and Causes
Vuk T , Radovcic MK , Ljubicic J , Lekic L , Benc H , Antolovic R , Ocic T
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
Even and undisturbed blood flow along with appropriate blood mixing with anticoagulant solution is a precondition for preventing clot formation in red blood cell (RBC) products. The literature data on the frequency of this nonconformity and causes of its occurrence are extremely scarce.
Aim
The aim of the study was to present results of long‐standing surveillance of the occurrence of clots in RBC products at the Croatian Institute of Transfusion Medicine in Zagreb. We believe that the results reported herewith can help other blood establishments to assess the frequency of this nonconformity and clarify the possible causes of its occurrence.
Methods
Records on nonconformities related to the occurrence of clots in RBC products were retrospectively analyzed for a 17‐year period (1998–2014). The presence of clots was determined by macroscopic examination of all units before storage. The frequency of RBC products with clots, causes, and trends observed over years were analyzed.
Results
This type of nonconformity was recorded at a mean frequency of 0.06% of RBC products. During the 1998–2010 period, when manual blood mixing was used, the frequency of RBC units with clots showed a declining trend, primarily owing to additional education of the blood collection staff having a higher rate of this nonconformity. An exception from this trend was found in 2006 and in part 2007, when the increased frequency of clots was influenced by defects of the cannulas inserted in the donation line (inappropriate cannula breaking with compromised blood flow). From 2010, automated mixers were gradually introduced in practice, with the process completed in 2011. In the period of their full implementation (2012–2014), the following findings were recorded: 117 donations with clots were donated by 97 men and 20 women, average age 40 years, which corresponded to the age and sex distribution of the general donor population. The mean length of donations with clots was longer (11.1 min) as compared with the mean length of donations recorded in the general donor population (6.35 min in a sample of 8007 donations in 2012). The automation of blood mixing did not additionally reduce the frequency of RBC products with clots. A negative correlation was observed with the frequency of donations interrupted for prolonged duration (r = −0.71), attributable to better surveillance of the length of donation. In addition, the proportion of filtered RBC concentrates was continuously increased over years, contributing to better detection of clots (slow filtration, clots visible on the filter). This was especially pronounced in the 2012–2014 period, when the proportion of filtered RBC concentrates increased from 56% to 93%. Therefore, positive correlation between the incidence of clot formation and filtered RBC concentrates was high in this period (r = 0.93).
Table 1. Frequency of clots in RBC products 1998–2014.

Summary/Conclusions
Study results showed the use of clot frequency as a blood collection quality indicator to be fully justified. Monitoring of clot formation enables quality evaluation of the materials (blood bags), as well as assessment of the factors related to the procedure of blood collection (blood flow, vein choice, etc.).
P‐174
Does IgG Level in Plasmapheresis Donors Correlate with the IgG Content in Plasma for Fractionation?
Moog R1, Rothe R 2, Blankenburg T 1, Tonn T 3
1German Red Cross Blood Donor Service North‐East, Cottbus, Germany2Blood Donor Service North‐East, Cottbus, Germany3Institute Dresden, Dresden, Germany
Background
Total protein is a quality parameter of plasma for fractionation.
Aim
To correlate baseline plasmapheresis donor total protein and IgG with total protein and IgG levels in plasma for fractionation.
Materials and Methods
Donors fulfilling current national and European eligibility criteria underwent whole blood donation and plasmapheresis. Plasma for fractionation was collected using the PCS and MCS+ (Haemonetics, Braintree, USA) as wells as the A 200 devices (Fenwal, Round Lake, USA) at eight plasmapheresis untis. Total protein and IgG were analysed (AU 640, Beckman Coulter, Germany) in peripheral baseline samples and in the plasma for fractionation over a period of 1 year. Fifteen samples were drawn from peripheral blood and plasma from fractionation at each of the eight plasma centers. The total number of plasmaphereses of the donor and the number of plasma donation during the study period were recorded. Whole blood donors without any plasma donation were used as a control.
Results
Total number of plasmapheresis ranged from 4 to 749 per donor. In the study period donors underwent 1–45 plasmapheresis. Total number of whole blood donations in the control group ranged from four to 103. Total protein and IgG levels averaged 7.38 ± 0.43 g/dl and 974.9 ± 167.4 mg/dl resp. in the control group showing higher levels than plasmapheresis donors. Mean baseline and product total protein and IgG levels from donors of eight plasma centers are showed in Figures 1 and 2.
Summary/Conclusion
Our data show that plasmapheresis donors show lower peripheral total protein and IgG levels than whole blood donors. There was a trend to lower total protein and IgG level in frequent plasmapheresis donors.
Caption 1: Total protein levels in donors and plasma products of eight different centers.

Caption 2: IgG levels of donors and plasma products of eight different centers.

P‐175
Influence of Apheresis Inlet Blood Flow on the Quality Product of Haematopoietic Progenitor Stem Cells Collection
Alonso Nogues EAN1, Morgades M 2, Carbonell L 1, Sancho JM 2, Vives S 2, Linio R 1, Ferra C 2, Ribera JM 2, Grifols JR 1
1Banc de Sang i Teixits, Badalona, Spain2Institut Català de la Salut‐Institut de Recerca contra la Leucèmia Josep Carrera, Badalona, Spain
Background
The collection of peripheral blood stem cell (PBSC) by aphaeresis depends on several factors. One of them is related to the total blood volume (TBV) processed so the inlet blood flow (IBF) and total processing time will condition the achievement of efficient successful procedures. In these, in addition to PBSC, the product can be indirectly contaminated by other cellular components presents in peripheral blood. Aphaeresis manuals used to recommend collecting with moderate IBF to avoid such effects.
Aims
The objective of this study is to evaluate how the IBF can influence the quality of PBSC collected.
Methods
We retrospectively evaluated 99 collection procedures performed on eligible patients for autologous transplantation in the period between 2011 and 2013. Paediatric patients were excluded. Patients (n = 99) diagnosed with non‐Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL) (n = 36), Multiple myeloma (MM) (n = 54), acute myeloid or lymphoblastic leukaemia (n = 7) and solid tumour (n = 2) were mobilized according to standard protocols with G‐CSF. Patients undergoing more than one collection procedure were excluded from the analysis. It was established a target CD34+ to be collected ≥2 × 10E6 CD34+/kg. Procedures were performed with COBE Spectra cell separator by a non‐tunnelled central venous catheter inserted the day before of the collection. CD34+ count pre and post‐aphaeresis was performed according ISHAGE protocol. PBSC were collected using a large volume leukaphaeresis protocol. IBF was established by each procedure to the maximum permitted in terms of ACD‐A proportion tolerance by the patient and the procedure 1:26–1:28. Median (range) and percentages were used for descriptive statistics. Continuous variables were compared by Kruskall–Wallis and median tests. Spearman rho coefficient was used to analyze the correlation between PBSC characteristics/product quality and speed.
Results
The quality indicators of collected product were fixed in cell viability ≥95%, CMN ≥70% and CN ≤200 × 10E6/ml. Our collection parameters achieved were (median; range): CD34+ pre‐aphaeresis (41.50/μl; 8–264/μl), blood volume processed (21.504 ml: 7.662–34.074 ml) and IBF/min (99.50 ml; 50–130 ml/min). PBSC characteristics and product quality: haematocrit (HCT) (4%; 2–7%), cell viability (99%; 83–100%), mononuclear cells (MNC) (84.5%; 35–98%) and nuclear cells (NC) (138 × 10E6/ml; 46–221 × 10E6/ml). 97% of PBSC collected reached a viability ≥95%, 88% MNC ≥ 70% and 96% NC≤200 × 10E6/ml. Any relevant adverse event was reported in the procedures analyzed. No statistically significant differences were found between the IBF and those parameters. There were also no statistical significant differences between IBF and purity/quality product in the overall diagnosis. In MM series there was a significant negative correlation between NC/IBF (ρ −0.294, P = 0.031) and between HCT/IBF (ρ −0.270, P = 0.048), therefore with higher IBF lower NC and HCT in the collected product. In NHL/HL, correlation between the NC/IBF (ρ 0.365, P = 0.029) and HCT/IBF (ρ 0.389, P = 0.019) was positive and significant, so at lower IBF greater the number of CN and HCT contaminating the collected product. No significant differences were found on IBF in both pathologies.
Conclusion
The use of high IBF is safe and effective, ensuring the quality product desired. It seems advisable to adjust it to the patient's underlying disease because it can lead differences in the final characteristics of the collected product.
P‐176
Abstract Withdrawn.
P‐177
Plateletapheresis in Nigeria: the University of Benin Teaching Hospital, Benin City Experience
Nwogoh B , Bazuaye GN , Ezire SE , Awodu AO
University of Benin Teaching Hospital, Benin City, Benin, Nigeria
Background
Apheresis activities in Nigeria is still at its beginning. The high cost of the machines and the overral cost of the procedure; paucity of trainned personnel to operate the machine and lack of technical support hinder the application of this science in transfusion and therapeutics in Nigeria.
Aim
To evaluate frequency of plateapheresis, donor characteristics, clinical indications and yield using Haemonetics MCS+ at the University of Benin Teaching Hospital, Benin City, Nigeria.
Method
Records of plateletapheresis over a 3 year period (January 2011 – December 2014) were evaluated. The Haemonetics MCS+ machine is used for plateletapheresis in our facility. Donors were mainly voluntary (students) and family members selected using the American Association of Blood Banks guideline for plateletapheresis donations. Group identical donors were preferred. Data including donor's age, sex, height, weight, body mass index, haematological parameters, blood group, primary diagnosis of recipient and details of apharesis procedures were analyzed using SPSS version 16. Variables between male and female donors were compared using student t test. P value was set at 0.05.
Result
A total of 139 plateletapheresis were done for 50 recipients in the 4 year period. This gives an average of 37.3 procedure//year and 2.6 plateletapharesis/patient. The mean age of donors was 27.9 ± 0.6 years with a M:F of 5.6:1 and a mean BMI of 24.3 ± 0.3. Repeat donation was recorded in 16 (11.5%) of donors. Blood group A donors were highest in 67 (48.2%), O 65 (46.8%), AB 4 (2.9%) and group B least in 3 (2.2%) donations. Rhesus D staus was positive in 137 (98.6%) of donors.
The highest number of recipients [14 (28%)] were patients with acute lymphoblastic leukaemia (ALL) while the highest number of procedures [45 (32.4%)] were done for patients who had haemopoietic stem cell transplantation. Details of the diagnosis of patients and the number of procedures they had are shown in Table 1.
The mean platelet count of donors were 239.1 ± 5.5 × 109 cells/l, the average yield was 3.6 ± 0.1 × 1011 cells, average volume processed was 3135.2 ± 61.0 ml in an average of 6.4 ± 0.2 cycles giving an average yield/processed volume of 1.2 ± 0.2 × 108 cells/ml. Details are shown in Table 2.
Female donors though had higher platelet counts but it was not statistically significant. The yield did not differ significantly between males and females.
Caption 1: Primary diagnosis, No of Patients and no of Platelet Concentrates received.

Caption 2: Haematological Parameters and Apharesis parameters of Platelet Donors.

Conclusion
Plateletapheresis is low in our setting owing to the high cost of the procedure. Acute leukaemia patients are the most beneficaries. Male donors were more but the yield do not differ significantly between males and females.
P‐178
Evaluation of Non‐Invasive Predonation Hemoglobin Screening in Blood Donors of Southeastern Serbia
Antic A1, Stanojkovic Z 1, Stojanovic D 2, Jelic M 3
1Blood Transfusion Institute Nis, Nis, Serbia2Department for transfusiology, Health Center, Leskovac, Serbia3Clinical Biochemical Laboratory, Military Hospital, Nis, Serbia
Background
Pre‐donation hemoglobin (Hb) screening is a mandatory test for selection of donors for blood donation. It is usually performed by invasive methods using capillary or venous blood. Today is also available non‐invasive hemoglobinometry, which eliminates the need for needle‐stick blood test. It is based on the principle of temporary blood‐flow occlusion made by a pneumatic finger cuff which generates a strong optical signal, enabling highly sensitive measurement of blood Hb. The method is comfort for potentially blood donors, painless, simple to perform and without the risk of infection for both the donor and medical stuff. The goal of this study was to analyze the feasibility of non‐invasive hemoglobinometry in our blood banks and to compare it with standard methods of Hb measurement using venous and capillary blood.
Material and Methods
This prospective study was conducted on 494 randomly selected potential blood donors at Blood Transfusion Institute Niš and Blood Transfusion Department in Lekovac. Potential blood donors were stratified by sex, weight, number of previous donations, pulse and blood pressure. Predonation Hb values were determined on non‐invasive NMB‐200 device (Lmb Technologie GmbH) (NI‐Hb) and compared with results obtained from HemoCue Hb201 (HemoCue, Sweden) for capillary Hb determination (HC‐Hb) and Beckman Coulter automated cell counter for venous EDTA samples (BC‐Hb). Data were analyzed and results were compared by using Means, Standard Deviations (SD), t‐test and correlation.
Results
The results obtained with NBM‐200 (mean 14.66 ± 1.12 g/dl, range: 11.1–17.4 g/dl) didn't show statistical significance in comparison with HC‐Hb (mean 14.76 ± 1.23 g/dl, range: 11.4–18.2 g/dl) and BC‐Hb (mean 14.53 ± 1.09 g/dl, range: 10.9–17.8 g/dl), P > 0.05 for both comparisons among groups. The correlation between NI‐Hb and HC‐Hb was 0.87, the bias was 0.32 g/dl, the correlation between NI‐Hb and BC‐Hb was 0.78 and the bias was 0.39 g/dl. In 476 cases (96.36%) there were no differences in decision about donation, but in 18 potential blood donors there was a difference in final donor status. Fourteen blood donors (2.83%) were deffered by HC‐Hb, but passed by NI‐HB and BC‐Hb, nine donors passed only BC‐Hb (1.82%) and five donors (1.01%) passed only NI‐Hb.
Conclusion
Non‐invasive hemoglobinometry has accuracy comparable to standard hemoglobin measurement methods and thus can be incorporated as routine predonation hemoglobin screening.
P‐179
Validation and Implementation of a Non‐Invasive Method for Haemoglobin Measurement (Haemospect) in a Plasmapheresis Centre in Hungary
Daniel ZD
Plazmaszolgálat Ltd., Budapest, Hungary
Background
Before plasma donationhaemoglobin (Hb) concentration of the donors have to be determined. It was obtained by a capillary method in our centre earlier but there was a growing demand to switch to a non‐invasive method especially from donor compliance point of view. The non‐invasive methods for Hb measurement has other positive features as well like reduced blood contamination risk, sparing soft goods for capillary method, less biohazard waste, work flow optimization for the staff. Seeking after the appropriate alternatives we chose Haemospect device and have performed a validation in our centre. After the validation we have implemented this non‐invasive method from October 2014.
Aims
To prove that the non‐invasive method can replace the capillary method for Hb measurement.
Methods
In course of the validationmore than 1500 data were collected at the plasmapheresis centre. The donors were screened for Hb values with three different methods. The capillary Hb was measured by the Hemocue HB 201+ after the finger prick. The venous Hb (vHb) was measured by the laboratory instrument Sysmex K4500. The donors were asked to undergo not only the usual capillary method but also the non‐invasive Hb measurement. An additional venous blood sample was drawn with the permission of the donors. The Hb cut‐off value for donation is 12.0 g/dl for women and 13.0 g/dl for men. The results of the capillary and the non‐invasive methods were compared to the results of the venous method. We used the venous method as a standard.
After validation and implementation we analysed further 1000 data of donors to whom additional venous blood sample analysis was required, such as new applicant donors and every 15th donation. Then the results of the non‐invasive methods were compared to the results of the venous method.
Results
The total number of tested donors was 1587 (1054 males and 533 females). The standard deviation of the non‐invasive method is the lowest. The median of capillary and non‐invasive Hb are comparable (Figure 1). The proportions of falsely allowed and falsely refused donors were similar to the capillary and the non‐invasive methods (Figure 1). The analysis of 1000 donors showed similar tendency as observed during the validation period (Figure 2).
Conclusion
The validation results showed comparable results between the capillary and the non‐invasive methods. It was possible to introduce this new method as a routine procedure. The statistical analysis showed that the non‐invasive method provides the donor safety. The additional data analysis of 1000 donors confirms that the data were similar compared to those obtained during the validation period. We have had positive experiences with the non‐invasive method so far.
Caption 1: Statistics during implementation.

Caption 2: Statistics during validation.

P‐180
Problems Faced During Apheresis Procedures from a Tertiary Care Hospital of North India
Dogra K , Coshic P , Chatterjee KD
All India Institute Medical Sciences, New Delhi, India
Background
Apheresis procedures for all departments (including department of clinical hematology) are carried in Department of Transfusion Medicine. In our set up, daily an average of 8–10 apheresis procedures are done. Various problems are encountered while performing these procedures in three different machines (viz. Haemonetics MCS+, Gambro Trima accel and Fresenius Com.Tec). In this study we have studied these adverse events under three different groups i.e donor related (local reactions or systemic reactions), equipment related and technical aberrations.
Materials and Methods
This study was conducted in Department of Transfusion Medicine for a period of 6 months i.e from 1st August, 2014 to 31st December, 2014. Total 441 apheresis procedures were performed during this period, out of which 425 were plateletpheresis, 13 were PBSC and three were TPE. 250 apheresis were on Haemonetics MCS+, 125 were on Trima accel and 66 were on Com.Tec.
Results
Total 441 apheresis procedures were performed. 33 (7.4%) adverse events were noted in relation to these procedures. Out of these 33 adverse events, 22 (66.7%) were donor related problems which included mainly 7 (21.2%) vascular injuries (hematoma formation), 9 (27.3%) citrate reactions and 6 (18.2%) vasovagal reactions. All donor reactions were mild. 7 (21.2%) were equipment related problems that included 2 (6.1%) air purge failures, 2 (6.1%) defective kits (leakage from separation chamber) and 3 (9%) high/low AC ratio. 4 (12.1%) were technical aberrations like 1 (3%) wrong programme selected (in case of Com.Tec), 2 (6.1%) ACD connected early or 1 (3%) donor line clamp not opened on time (in case of Trima accel). Out of these 33 cases, only eight procedures (24%) were incomplete and rests of 25 procedures were completed by intervening timely to rectify the problem.
Conclusion
Apheresis procedures performed on cell separators are safe and well tolerated. Most of the adverse events related either to the donors or the equipments or the techniques can be avoided if timely interventions are done to rectify these errors.
P‐181
Factors Influencing the Quality of Platelet Yield in Apheresis
Hiok JH , Mohamed Jamil NFM , Chong CJL , Rajudin RS , Arshad AZ , Mangahas MRA , Pennefather PJD
Health Sciences Authority, Singapore, Singapore
Background
40% of the annual platelet supply in Blood Services Group (BSG) is supported by apheresis platelet collection. One of the quality requirements is ensuring that the platelets collected fulfil its minimum platelet yield before released for transfusion. Platelet product that does not meet the minimum requirement of 3.0 × 1011 will be discarded.
Aims
Platelet units with failed yield accounted for 1.1% to 2.2% of the total platelet collection. The aim of this study is to identify possible causes that may have an influence on the platelet yield on both single and double units. This will help in improving the quality and collection efficacy of the platelets.
Methods
A retrospective study of the platelet data was carried out between June 2014 and Dec 2014 for all 3376 platelet donations. Using Lean Six Sigma as an application tool, three main areas of possible causes were identified. The team studied the relationship of low platelet yield with (i) donors’ platelet count (ii) donors’ haematocrit (iii) type of blood cell separator used. BSG currently uses three types of machines and they are MCS+, Amicus and Trima Accel. Instruments are programmed to a target yield of 3.6 × 1011 (all three machines) and 6.5 × 1011 (MCS+ and Trima Accel) for single and double respectively.
Results
Using the regression analysis tool, for single platelets, it was found that there was a weak relationship between donors’ platelet count and platelet yield (R2 value of 0.106). This was also reflected for double units (R2 value of 0.044). As for the correlation of the three types of cell separators used, donors’ platelet count and platelet yield, it showed that out of the three instruments, only MCS+ and Amicus presented a correlation with an R2 value of 0.278 and 0.272 respectively. For double collection, only MCS+ reflected a relationship with the two variables (R2 value of 0.226). In contrast, for both single and double collection, Trima Accel had no relationship with an R2 of an insignificant value.
The relationship between low yield and haematocrit was also found to be not substantial for both collections.
Summary/Conclusion
In conclusion, the correlation of the potential factors with low platelet yield explored in this study displayed relationship, albeit weak, with the use of MCS+ and Amicus, with the exception of Trima Accel, which showed no relationship at all.
Work processes might play a part in determining the above results. For donors’ data input, Trima Accel practises current platelet count (as recommended) when compared with the other two machines, which use donors’ platelet average count of last three donations. The team would follow‐up with a consolidation of retrospective data during the period when practice was uniformed for all instruments (every input based on donors’ average platelet count) so as to achieve a more conclusive outcome of this study.
P‐182
Abstract Withdrawn.
P‐183
Abstract Withdrawn.
P‐184
Experience on Platelet Collection with Amicus and Com.Tec in the Blood Bank in Rzeszów Poland
Sibilewska I1, Zawilinska E 1, Bzdek U 1, Borowska H 2
1Regional Center of Blood Donation and Treatment, Rzeszow, Poland2Uniwersytet Medyczny, Lublin, Poland
Background
From the beginning of 2014 the Blood Bank in Rzeszow employed two apheresis platform Amicus and Com.Tec (Fresenius) for platelet collection. We compared our experience with platelet collection with single (SN) and double needle (DN) procedures. The comparison is made with regard to processing time, platelet (PLT) yield, ACD used, collection rate (CR) and white blood cells content (WBC) of the product.
Aim
The aim of the study was to compare performance two platelet collection apheresis platforms Amicus and Com.Tec.
Materials and Method
We investigated data from 360 procedures from two apheresis platform both single and double needle procedure thus n = 90 each. Donors were randomly separated into four groups.
Results
In the pre apheresis settings 90 plateletpheresis procedures performer with each procedure and collection type revealed no significant differences in donor's sex, weight, height and thus total blood volume. The pre apheresis PLT count was also similar 270 × 1011/μl. Both devices were set to reach 3.3 × 1011 PLT and total volume of the end product do not exceed 300 ml. The blood volume processed to reach target PLT yield was higher in the Com.Tec than in Amicus for both SN and DN (SN 3097 vs 2526 ml; DN 3266 vs 2852 ml). The median separation time was also longer for Com.Tec (SN 78 vs 55; DN 47 vs 40 min). Collection rate of SN was significantly higher with the Amicus compared to the Com.Tec device (0, 08 ± 0.008 × 1011 vs 0.04 ± 0.008 × 1011PLT/min). The volume of ACD used during the SN procedure was almost double for Com.Tec (SN 470 vs 289 ml; DN 364 vs 337 ml). The required PLT count of ≥3.0 × 1011 was fulfilled for 80% and 90% platelet products obtained with Com.Tec using SN or DN mode respectively and for 97% products collected with Amicus. All products obtained with both instruments had WBC count lower than 1 × 106 as required by the guidelines. The mean number of platelets measured in the platelet product bag is slightly higher for Amicus than Com.Tec (SN 3, 85 × 1011 vs 3, 46 × 1011; DN 3, 74 × 1011 vs 3.54 × 1011).
Conclusion
Both instruments feasibly collect therapeutic dose of 3.0 × 1011 PLT/300 ml obliged by the polish guidelines. Additionally all the PLT products were leukoreduced. Nevertheless the Amicus collects desired number of PLT in a shorter time with less blood volume processed and also less ACD used. This suggests the Amicus being more convenient for the donor especially with regard to single needle procedure.
P‐185
Blood Savings Programs: Autotransfusion Study Program in the Center of Blood and Transplantation of Oporto (Portugal)
Almeida FQ , Lopes CP , Pereira MS , Pinto BC , Ferreira DS , Costa PP
CSTP‐IPST, IP, Porto, Portugal
Introduction
One of the objectives of the Blood Bank should be to strengthen strategies to optimize the blood supply and transfusion safety.
This study aims a review of requests of autotransfusions received in our center from January 2013 to December 2014.
Equipment and Methods
A prospective descriptive study of autotransfusion orders referred to our center was performed.To carry out this procedure a protocol was first established with hospitals to define the necessary documentation for the collection of autotransfusions and subsequent delivery to the Service of reference.
Further later was necessary to establish a communication system with all laboratories involved in this process providing information about the state of the collections in real time.
Were analyzed: age, gender, origin of the order, diagnosis, reason, pre‐donation hemoglobin, number of collected units, therapeutics and number of distributed units.
Results
During the study period 22 (6 Male/16 Female) autotransfusion blood orders were performed at our Center. The median age was 33 years old (range 12; 75). In 100% of the requests the reason was for scheduled surgery in public Hospitals 36%, Private Hospitals 64%. Median units is 1.55 units for patient (range = 1; 2). The distribution of orders for specialty was: 9.09% Orthopedic; 36.36% Neurosurgery; 4.55% Vascular Surgery; 50% Surgery.
22 autologous units were extracted and 100% of the ordered units (N = 22) were distributed. 19 patients were subsequently treated with oral iron from the beginning of the collections until surgery.
Three mild vagal reactions with quick recovery were recorded. Could not be analyzed the transfusion requirements.
Conclusions
Conducting blood‐saving techniques allows to respond the demands from elective surgery.
It is important that these programs are able to meet the real needs of each patient.
The development of specific protocols with hospitals of origin of the request, defining not only the necessary documentation for extraction but also timeslots between each extraction, preventing failures in registers and defining the necessary therapeutic protocols in each case (Iron, Epo, Vitamin B12, folic acid) that allows interventions with maximum security.
The implementation of new methods of collection, such as eritracitaféresis, can mean more flexibility to perform this procedure. We can collect 2 units in a procedure which means for the patient fewer trips to the blood bank and hospital.
It is important that these programs should be able to cover the real needs of each patient and develop a communication channel that allows evaluation.
P‐186
The Pre‐Donation Non‐Invasive Hemoglobin Screening: a Way to Reach Full Blood Donor Testing
Py JY , Barnoux M , Leclerc C , Dehaut F
EFS Centre‐Atlantique, Orléans cedex, France
Background
Pre‐donation hemoglobin screening (PDHS) is done in France using an invasive method (HemoCue). It's only mandatory for new donors, donors without donation during the two last years and donors with a low hemoglobin level on their last donation. All other donations are performed without PDHS. Consequently, among the donations found anemic with the successive venous control, 65% concern donors not tested.
Aims
Considering practical difficulties to extend the mandatory basis of PDHS with the invasive method, we are looking if a non‐invasive method could be the way to reach full blood donor testing.
Methods
EFS Centre‐Atlantique is a French regional blood center which collects annually nearly 200,000 whole blood donations (WBD). One location was chosen to test Haemospect (MBR Optical Systems GmbH & Co. KG). A preliminary feasibility study was done on four mobile teams, comparing the use of Haemospect either during the pre‐donation interview or on the chair just before donation. Results were compared with the successive venous control, and sometimes with the invasive PDHS.
Results
143 WBD were tested, 85 women and 58 men. With a threshold of 12 g/dl for women and 13 g/dl for men, the sensibility and specificity of the non‐invasive test compared with venous control were respectively of 0.50 and 0.97. These values are quite comparable with those of invasive methods. Among the 104 donors who were not concerned by the mandatory PDHS, one donor was found anemic, confirmed by venous control. After a quick appropriation time, it appears that both physicians and nurses are pertinently using the equipment, allowing us to use it at different steps of the donation process.
Summary/Conclusions
We concluded that Haemospect is a good way to move toward a full blood donor PDHS, which is lacking in France. Further studies are necessary to validate these first results.
P‐187
Apheresis Donors in the Center of Blood and Transplantation of Oporto (Portugal)
Almeida FQ , Lopes CP , Pereira CP , Pinto BC , Ferreira DS , Costa PP
CSTP‐IPST, IP, Porto, Portugal
Introduction
The cell separation process, known as apheresis, is carried out in our center since 2001. In our Center we perform apheresis, plasmapheresis and eritracitaférese programs. A donation by apheresis contains 6–8 times more platelets than a traditional donation, which means an obvious benefit to the patient.With this study we intend to do an inventory of donors who were considered able for donation by apheresis, those eliminated or suspended during the donor screening process and also the most used selected programs in the cell separation process.
Methods
We analyzed data related to donations by apheresis, performed in the Center of Blood and Transplantation of OPorto, during the years 2013–2014. Analysis was based in the following parameters: age, gender, number of donations, grounds for suspension/elimination and selected programs.
Results
During the study period were performed 3012 collections by apheresis, 1675 in the year 2013 to 1337 in 2014. Regarding the selected programs in 2013: one CUP (46.99%) and two CUP (14.27%), and 2014: one CUP (36.87%) and CUP + PFA (17.73%). Concerning the suspension rate we found that in 2013, 8.6% were suspended and 0.2% were eliminated. In 2014, there was a suspension rate of 7.2% and 0.3% to eliminated donors. As for the suspensions by age was found that 2.5% in the age group of 18–24 years; 53.33% in the age group of 25–44 years and 44.17% in the age group of 45–65 years. As to gender, 39.58% of donors were male and 60.42% female. 17.5% of donors had up to 10 donations, 50.42% between 11–25 donations and 32.08% over 25 donations. The suspension causes were analyzed in 2013: ongoing medication (18.75%); other reasons (12.50%), holiday on endemic area (10.42%) and infection (10.42%). In the year 2014: infection (19.80%), dental treatment in the last 7 days (17.71%), new sexual partner (13.54%) and non compatible hemoglobin levels (11.46%). 0.22% were eliminated of the eligible donors in 2013 and 0.28% in 2014. The causes of elimination in 2013 were ongoing medication 50%, cerebrovascular pathology 25% and cardiovascular pathology 25%. In 2014 were eliminated 25% of donors by upper age limit, 25% by autoimmune pathology, 25% by cardiovascular pathology and 25% by other reasons.
Conclusions
In our study it was verified that the suspension rate is more common in women, in donors with 10 to 25 previous donations and in the age group between 25 and 44 years. In 2013 the most common cause of suspension was ongoing medication and in 2014 it was infection. As for the eliminated donors the most frequent cause in 2013 was the ongoing medication (50% from the eliminated) and in 2014 varied from upper age limit, cardiovascular pathology and autoimmune pathology. Within the selected programs used in our center it prevails the collection of one CUP.
P‐188
Plasma Collection Technologies and Ensuring Plasma Safety in Russian Federation
Chechetkin AV
Russian Research Institute of Haematology and Transfusiology, St. Petersburg, Russia
Background
The demand for plasma continues to rise in healthcare organization, because it is widely used in the treatment of various diseases and pathological conditions. In addition, this blood component is necessary for the manufacture of plasma‐derived products. The realization of governmental program of blood service development led to improvement of technical equipment level in blood service establishments in Russia.
Aims
The aim was the investigation of collection methods structure and plasma safety ensuring technologies in blood service establishments in Russian Federation.
Methods
The research of parameters of plasma collection was carried out using the reports from blood service establishments in Russia during 2009–2013 followed by a statistical analysis and questionnaire survey.
Results
For the period 2009–2013, the percentage of plasma, collected by automatic plasmapheresis, has enlarged from 15.2% to 23.1%. The percentage of recovered plasma during this period ranged from 51.9% to 57.7%. The implementation level of automatic apheresis in different regions of the Russian Federation differs significantly. In Central federal district 28.1% plasma was collected by the automatic apheresis and 13.6% – in the far Eastern district in 2013. These regional characteristics are largely determined by technical equipment level of blood service establishments, the number of blood donors and the healthcare organizations need in fresh frozen plasma. The use of new technologies helped to intensify the process of plasma quarantine. The volume of quarantine plasma, collected by the blood service establishments for these years, has increased in 1.4 times, and more than 92% of plasma was subjected to quarantine in 2013. To improve the safety of plasma leukoreduction methods were used. For the period of 2009–2013 the percentage of leukocyte‐depleted plasma has increased from 10.0% to 22.2%. The detailed analysis detect that the leukoreduction technology for plasma was actively used in blood service establishments in Ural Federal district, where more than 83% of plasma was subjected to leukoreduction in 2013. In recent years the percentage of pathogen reduced plasma has increased in three times (7.6% in 2013). The pathogen reduction of plasma was actively used in blood service establishments in North Caucasus and Southern Federal districts. In these districts 13.7–17.4% of the collected plasma was subjected to pathogen reduction in 2013.
Conclusion
For the period 2009–2013 the advanced technology of donor's plasma collection and ensuring safety were introduced and developed in the activities of blood service establishments in Russia. The degree of use of such technologies is characterized by regional differences.
P‐189
Abstract Withdrawn.
2.3 Donor adverse events
P‐190
The Relative Importance of Risk Factors Across the Time Course of Donation: Case–Control Studies of Immediate and Delayed Vasovagal Reactions Events
Narbey DN1, Fillet AM 1, Fillet AM 1, Jbilou SJ 1, Tiberghien PT 2, Djoudi RD 1
1Etablissement français du sang, La Plaine Sain‐Denis, France2Etablissement français du sang, University of Franche‐Comté in the city of Besan, La Plaine Sain‐Denis, France
Background
Vasovagal reactions (VVR) are the most common adverse events during or after allogeneic blood donations. Different risk factors of VVR occurrence have been identified. In France, reporting of severe adverse effect occurring in donor within a delay of 7 days after the donation is mandatory.
Aims
To study the relative importance of the different factors for VVR across the time of donation, we performed retrospective case‐control studies with multivariate analysis of severe immediate and delayed VVR.
Methods
Cases of VVR were defined as immediate when occurring at the transfusion site and as delayed when occurring outside the transfusion site and within the 24 h following donation. Severity of adverse event was assessed as defined by the international haemovigilance network: grade 2 (moderate) to 4 (death). Adverse events with a high imputability, probable or likely and definite or certain, were considered. Two studies, one of immediate VVR and the other of delayed VVR, were performed with the same methodology. One control per case, matched by donation region and date, were drawn at random within donors without VVR. Various relevant variables (sex, age, body mass index (BMI), donation status, type of phlebotomy) were analyzed as well. By bivariate analysis, links between those variables and VVR were searched and interactions taken into account.
Results
Within the French haemovigilance data collected since 2011 to 2013, immediate VVR and delayed VVR occurred in 8410 and 833 donors respectively out of 8,834,214 donations (0.09% and 0.009% respectively).
In univariate analysis, first donation and young age (18–24 years) are strongly associated with immediate VVR occurrence, whereas sex (woman), type of phlebotomy (whole blood donation) and BMI (underweight) are slightly associated. By contrast, sex (woman) is strongly associated with delayed VVR occurrence, whereas donation status (first‐time), age (60–70 years), type of phlebotomy (whole blood donation) and BMI (underweight) are slightly associated.
In multivariate analysis, sex and BMI are interacting factors in both immediate and delayed VVR studies. Two risk factors, first‐time donation (OR 3.72, 95%IC [3.36–4.12]) and age group 18–24 years (OR 3.32, 95%IC [2.81–3.87]), are strongly associated with immediate VVR occurrence. Independently of BMI was (normal, overweight, obesity), woman is slightly associated with immediate VVR occurrence (OR 1.22 95%IC [1.11–1.34]; OR 1.56 95%IC [1.34–1.80]; OR 1.79 95%IC [1.35–2.37] respectively). Two risks factors, independently of BMI was (normal, overweight, obesity) woman (OR 7.31, 95%IC [4.96–10.77]; OR 7.89, 95%IC [4.84–12.87]; OR 3.72, 95%IC [1.42–9.38] and underweight man (OR 6.39, 95%IC [1.56–26.13], are strongly associated with delayed VVR occurrence. Lastly, apheresis is a risk factor either for immediate or delayed VVR occurrence.
Conclusion
Two different profiles of donors at risk of immediate and delayed VVR are described: a first donation by a young person and a donation by an old woman respectively. Our studies argue in favor of different underlying mechanisms for immediate and delayed VVR. In accordance, a recent prospective study (Evasion) performed by EFS demonstrated that muscle tensing and isotonic hydration could differentially reduce the incidence of immediate and delayed VVR. In addition, in order to prevent those VVR, we should implement appropriate surveillance of various donors.
P‐191
Abstract Withdrawn.
P‐192
Abstract Withdrawn.
P‐193
Abstract Withdrawn.
P‐194
Iron Deficient Blood Donors – Degree of Understanding and Actions Taken After Notification
Goldman M , Uzicanin S , O'Brien S , Scalia J , Scalia V
Canadian Blood Services, Ottawa, ON, Canada
Background
Frequent blood donation can lead to iron deficiency, as measured by serum ferritin. Internationally, many blood operators are initiating ferritin testing by various algorithms, but it remains unclear how to facilitate effective donor education.
Aims
To assess donor understanding and long term impact of notification of low ferritin.
Methods
In 2012, 600 whole blood donors were recruited at Ottawa collection sites and their ferritin was tested post‐donation. Donors with low ferritin (<25 μg/l) received a notification letter advising them to stop donating until after seeing their physician and their ferritin had returned to normal. They were not called to donate for 6 months. Two months post‐notification, a follow‐up online survey was conducted asking if donors had received the letter and taken actions to address their low ferritin. Donation frequency 2 years before and after ferritin testing was extracted from the database for all study donors and compared using the chi‐square test. Two years post‐notification, 21 donors completed qualitative interviews about their understanding and actions taken. The qualitative interviews were digitally recorded and transcribed, and analyzed using Grounded Theory.
Results
Of the 295 donors with low ferritin, 165 (56%) participated in the online survey. Of these, 162 (98%) said they received the notification letter and 99 (61%) discussed their low ferritin results with their physician. 62 (63%) donors reported being advised by their physician to take iron supplements and 50 (81%) of them did so. Only 29 (29%) donors said they were advised to stop donating or delay return. Two years post‐notification, 67% of low‐ferritin repeat female donors had returned to donate, of whom 78% made >1 donation, while 77% with normal ferritin had returned, of whom 92% made >1 donation (P = 0.02). Of male repeat low‐ferritin donors, 80% returned, of whom 72% made >3 donations, while 95% with normal ferritin returned, of whom 75% made >3 donations (P = 0.0035). In the qualitative interviews most donors were pleased to have been informed, but did not see low ferritin as serious; many delayed addressing low ferritin until their annual check‐up. Even after consulting a physician many donors were confused about being told their ferritin was low when their hemoglobin was acceptable to donate. Most that saw their physician were advised to take iron supplements, but discontinued due to adverse effects or inconvenience. Often supplement use was inappropriate (e.g. occasional use or when they felt the need). About half made dietary changes intending to boost their iron, but were misinformed (e.g. eating more green leafy vegetables). Many decided to lengthen the interval between donations, but rarely from physician guidance.
Summary/Conclusions
Donors notified of low ferritin frequently do not promptly consult a physician and when they do, the intervention and donor learning is inadequate. Some donors reduce donation frequency or stop, often without physician advice, but overall these data suggest that reliance on donor initiated physician consults does not fully address the needs of donors with low ferritin. Multiple interventions may be needed to enhance donor comprehension and actions over their donor career.
P‐195
Physical Donor Characteristics are Associated with Post Donation Symptoms: Donor Insight
van den Hurk K1, Peffer K 1, Habets K 1, Atsma F 2, Pasker-de ong PCM 1, van Noord PAH 1, Veldhuizen IJT 1, de Kort WLAM 1
1Sanquin Research, Amsterdam, The Netherlands2IQ Scientific Institute for Quality of Healthcare, Radboudumc, Nijmegen, The Netherlands
Background
Blood donation is generally safe, but can be accompanied by post donation symptoms such as fatigue or dizziness. Insight in physical characteristics associated with these symptoms, could help to estimate and deal with risk of symptoms.
Aims
(i) To identify how often post donation symptoms occur, and (ii) to investigate whether physical donor characteristics are associated with these symptoms.
Methods
Donor InSight (DIS) is a questionnaire survey linked to the Dutch donor database, in which 31,338 (63%) of the invited 50,000 donors (87% whole blood and 13% plasma donors) participated in 2007–2009. Donors were asked about both positive and negative ‘physical symptoms in the days after donation’. For the current analyses all DIS participants who made at least one whole blood or plasma donation before DIS, and of whom data on physical donor characteristics, haemoglobin (Hb), systolic blood pressure (SBP), body mass index (BMI) and estimated blood volume (BV), were available, were included. Mean Hb and SBP were calculated from the last 1–3 donations before completing the questionnaire. Height and weight from the questionnaire were used to calculate BMI and BV. All physical donor characteristics were recalculated into Z‐scores to enable comparisons of the relative strength of the associations. Logistic regression analyses were performed to examine whether differences in physical donor characteristics were associated with post donation symptoms. These analyses were stratified by sex and adjusted for age, number of donations in the past year, donation history (>4 vs fewer in total), donor type (whole blood or plasma donor), and additionally for the other three donor characteristics.
Results
Five percent of 13,676 men and 4% of 14,681 women reported positive (post donation) symptoms, of whom >60% reported feeling fit and around 20% experienced less headaches. Negative symptoms, like fatigue and dizziness, were more common: 8% of men and 18% of women reported these. Higher values for all studied physical donor characteristics were associated with higher odds on positive symptoms and with lower odds on negative symptoms, independent of age, number of donations, donation frequency and donor type (data not shown). Associations of Hb with symptoms largely disappeared after additional adjustment for the other physical donor characteristics; SBP, BMI and BV (see Table). In women only, higher Hb was independently associated with higher odds on positive symptoms. Associations of SBP and BMI with positive and negative symptoms remained significant and consistent, after adjusting for the other physical donor characteristics. BV was no longer associated with symptoms after donation, after additional adjustments. In general, associations of BMI with symptoms were strongest and most consistent.
Table 1. Odds ratio's for associations of physical donor characteristics per SD with symptoms after donation.

Conclusions
Dutch donors mostly report negative, but also positive post donation symptoms. Higher levels of donor characteristics are consistently associated with higher odds on positive, and lower odds on negative symptoms. BMI was most consistently, independently and strongly associated with symptoms, which points to the relevance of obtaining height and weight of donors as cheap parameters to allow risk estimation.
P‐196
Blood Donor Adverse Events UK 2013/14
Reynolds CA1, Davison KL 1, Andrews N 1, Brailsford SR 2
1Public Health England, London, United Kingdom2NHS Blood and Transplant, London, United Kingdom
Background
The UK blood services aim to make the donor experience as pleasant as possible to encourage donors to return. Reducing donor adverse events such as faints, re‐bleeds and bruises is important in achieving this. Data on donor adverse events is routinely collected in the UK relating to events at session or reported by donors following session. During 2013/14 225,000 donors throughout the UK were surveyed using an online anonymous unlinked questionnaire, this was an opportunity to ask donors about post‐donation events that they may not previously have reported.
Aims
To investigate whether donors were experiencing post‐donation adverse events that they were not reporting to the blood services and whether these events were being experienced by donors with particular characteristics.
Methods
Data on adverse events were collected from new and repeat blood donors for a year as part of the UK Blood Donor Survey. Donors were asked whether they had experienced fainting, bruising, bleeding or any other adverse event at their last donation. If so, they were asked if they had reported their adverse event to the blood service. The data were downloaded to an MS Access database and fields created to populate with faint, delayed faint, bruise, bleed and other and whether they had one or more than one type of event. Demographic data were also collected. Counts of the events and CHI tests to look for differences by demographic characteristics were performed in STATA 13. Rates were also compared to blood service data.
Results
Overall 32% of survey responders (19,816/62,703) said they had experienced an adverse event at their last donation with 4% experiencing multiple types of event eg bruising and bleeding. The vast majority of adverse events experienced were bruising (83%, n = 16,442) with delayed faint in 2% (n = 447). Adverse events were reported to the survey at approximately 10 times the rates recorded by the blood service. New donors, females and younger donors especially the 17–24 year old group were significantly more likely to experience an adverse event. About 16% of donors who had experienced an adverse event said the blood service was already aware or that they reported it. A quarter of those with a delayed faint (117/447) said they had reported it.
Summary
Adverse events were common, the majority related to bruising and mostly not reported. The low reporting rate may reflect the majority of events were bruising perhaps expected or considered minor by the donor. However, even faints were recorded at much higher rates by the survey than the blood service although donors may not have responded as reported if the event happened at session. Of concern are the numbers of donors who reported a delayed faint in the survey but had not notified the blood service. The survey does not have follow up data on whether donors experiencing an adverse event returned. The survey was useful in providing additional information on these unreported adverse events which will inform future donor strategies.
P‐197
Complications of Blood Donation: a Review of Arterial Punctures in Whole Blood Donors
Anand R , Watkins E , Miflin G
NHS Blood and Transplant, Birmingham, United Kingdom
Background
Accidental arterial puncture is a rare but potentially serious complication of blood donation. In a small number of cases it may lead to the development of haematoma or compartment syndrome. Very rarely, it may cause a pseudo‐aneurysm or an arteriovenous fistula. NHS Blood and Transplant (NHSBT) has a standard national procedure for managing a suspected/actual arterial puncture (AP) at a donor session. Once the bleeding has stopped and no other symptoms are observed, the nurse must provide the donor with post donation advice and a standard NHSBT leaflet, which gives advice on bruising, rebleeding, severe pain or other symptoms. All APs are recorded on the donor database using a specific code. If an AP is not recognised at the donation session, it may be reported by the donor via the national Blood Donor Helpline. Following an AP, the donor is contacted by a doctor or nurse for follow up.
Aim
To review the number of arterial punctures (AP) during a 2 year period and to establish how many of them were reported at the blood session and how many were reported after the donor had left the session, for example, by telephoning the donor helpline.
Methods
A review of information contained within the Medical Records pages of the donor database was performed for all AP incidents recorded between January 2013 and December 2014 inclusive. The data included both confirmed and suspected APs since the same medical code is applied to both.
Results
A total of 484 APs were entered onto the donor database during the 2 year period. The average number of AP incidents per month was 20 (range of 10 to 38). 441 (91%) APs were reported by staff while the donor was at the donation session. 35 (7%) APs were diagnosed after the donor had left the session. It is this group of donors that is most likely to develop arm complications. There were 8 (2%) cases in which the details were unclear. Two incidents led to a confirmed pseudo‐aneurysm.
Conclusions
Limited data is available in the literature on the incidence of APs during blood donation. A 2001 report from the American Red Cross highlighted one AP in every 34,000 donations. The equivalent figure for NHSBT is 1/8200 based on approximately 2 million donations per year. Anecdotal evidence suggests that NHSBT is much better at reporting incidents than some other blood services. Efforts should focus on reducing the number of APs. Such events are extremely unpleasant and may result in surgical intervention for the donor and can be costly to the blood service. Staff training and experience may influence the occurrence of these events but this information is not currently available. This review highlights the need for session staff to be alert to any signs of a suspected AP and to provide appropriate advice to the donor. Any suspected/actual arterial puncture must be accurately documented; there should not be any cases where it is unclear what happened and what advice has been provided to the donor.
P‐198
Prevention of Fainting Reactions After Whole Blood Donation: a Cluster Randomized Trial to Assess the Efficacy of Hydration with Isotonic Solution, Water and Applied Muscle Tension
Morand CM1, Roland CR 2, Coudurier CN 3, Thoret ST 2, Legrand DL 1, Tiberghien PT 4, Bosson JLB 2
1Etablissement Français du Sang Rhone Alpes, La Tronche, France2UGA‐Grenoble/CNRS CHU and Inserm CIC03/TIMC‐IMAG UMRI, Grenoble, France3Etablissement Français du Sang, St‐Denis, France4Etablissement Français du Sang, UMR 1098, Inserm Université de Franche‐Comté, Besançon, France
Background
Prevention of fainting reactions associated with whole blood donation is an important issue for donor safety and donor retention.
Aim
To assess the impact of three different pre‐donation hydration strategies: 500 ml of an isotonic drink vs 500 ml of water and an advice to drink (control arm) coupled or not with applied muscle tension, on faintness during the donation period and in the following 48 h.
Methods
A cluster randomized prospective trial between January 2014 and July 2014 enrolled whole blood donors (18–70 years old) from two French blood centers of the Rhône‐Alpes region. The study compared three arms for hydration criteria and two arms for muscle tension criteria with a cluster randomization (1 cluster = 1 donation unit). The randomization plan was stratified according to the type of donation unit: fixed/mobile. The documentation of fainting reaction and adverse reactions during and after blood donation was collected using standardized protocols. The main outcome was the cumulative incidence for each arm of presyncopal (feeling faint) and syncopal reactions requiring a Trendelenburg position on the donation site or any transient interruption of the daily activities (e.g. to sit down) outside the donation site. The secondary outcome was the cumulative incidence of these adverse events according to three periods of donation: (i) during donation; (ii) immediately after blood donation on the donation site; (iii) after donation within 48 h outside the donation site. We also assessed the impact of the different measures on the donor‐daily activities within the 48 h after the donation.
Results
4576 donors giving one donation were analyzed for the main criteria. Overall, faintness occurred in 5.5% of whole blood donation. Compared to the control arm (i.e. advice to drink), simple drinking 500 ml of plain water or an isotonic significantly reduced the rate of faintness [OR = 0.74 (95% CI, 0.55–0.99); P‐value = 0.041], independently of muscle tension. Analyzes according to time course of donation showed that: (i) muscle tension significantly reduced faintness during the donation [OR = 0.64 (95% CI, 0.42–0.98); P‐value = 0.041]; (ii) Isotonic drink significantly reduced delayed off‐site faintness [OR = 0.62 (95% CI, 0.40–0.98), P‐value = 0.040] and tiredness after donation [OR = 0.75 (95% CI, 0.59–0.94), P‐value = 0.014].
Conclusions
Drinking 500 ml of an isotonic solution or plain water and muscle tensing, at time of blood donation, is a useful measures to prevent fainting reactions overall. Furthermore, drinking an isotonic solution can provide a novel approach to reduce the risk of off‐site delayed fainting reactions as well as tiredness after whole blood donation.
P‐199
Blood Donors – Serious Side Effects (SSE) 2010–2014 EFS Châteauroux France
Sapey TS1, Riga AR 1, Py JYP 2
1Etablissement francais du sang, Chateauroux, France2Etablissement francais du sang, Orléans, France, Orléans, France
Background
In 2013 the national French incidence of SSE was 155.7 per 100,000 donations and 82% of SSE were grade 2 (French classification of SSE related to blood donors)
Aims
The purpose of our study was to describe the profile of blood donator candidate which had a SSE in our center.
Methods
The study contains all the SSE superior to grade 1 occurred on the site EFS Châteauroux (site and mobile blood collection) from January 2010 to October 31, 2014. We analyzed 37 parameters from the e‐fit files (e‐site French blood vigilance) and In‐log software.
Results
We identified 82 SSE for 72,553 blood donations (incidence: 113.02 SSE per 100,000 donations). 41 men and 41 women, middle age 39 years (18–66). Average height: 1.68 m (1.49–1.85); average weight: 68 kg (50–98); Body mass index (kg/m2): 24.13 (18.6–31.9). All donors were Caucasian and 30% unemployed.
We found 74 vasovagal syncope (VVS), five hematomas, two arterial injuries and an adverse reaction to citrate. In 90% the SSE was immediate and of grade 2 in 85% of cases. 37% of SSE were first donation in connection with whole blood in 87% of cases. Regarding the seniority of donors: the number of average donations (whole blood, plasma, platelets) was 16.5.
An SSE determined the stop of blood donation in 65% of cases with nearly 80% stoppage if it was a first donation.
73% of SSE as a VVS took place during blood collection or within 5 min following the end of the donation. 61% were men. 44% of cases were a first donation and 83% occurred in mobile blood collection. Average age: 36 years. The result was a permanent stop of all type of donations in 76% of cases.
27% of SSE as a VVS took place beyond 5 min after the end of the donation. 75% were women. 30% of cases were a first donation and 95% of SSE occurred in mobile blood collection. Average age 42 years. The result was a permanent stop of all type of donations in 40% of cases.
Conclusions
When the SSE as a VVS occurs during or within 5 min following the end of the donation, it leads to a permanent stop of any type of donation in 76% of cases.
P‐200
Abstract Withdrawn.
P‐201
Structured Reporting of Adverse Events in Blood Donors
Sadiq A , Maguire KA , Morris KG
Northern Ireland Blood Transfusion Service, Belfast, United Kingdom
Background
The international haemovigilance network has published definitions of donor adverse events of donation (DAEDs) and serious adverse events of donations (SAEDs). The Northern Ireland Blood Transfusion Service developed a protocol for recording of adverse events in donors according to these case definitions and categories.
Aims
The UK Blood Services intend to collect data which may be directly compared across the four blood services. Information distilled from this data will assist in developing corrective and preventive actions and their effectiveness in reducing adverse reaction rates may be monitored over time.
Methods
The donor health check declaration form includes a section for reporting and grading adverse events. The core blood management system (Pulse) captures this information for ease of analysis.
Results
In the first 6 months of reporting 40,000 donor attendances are represented. Over 500 adverse events were recorded. The categories are reviewed and adverse events are stratified according to demographics – age, gender and first time donor status. Risk reduction strategies are identified and following further analysis of the data will be deployed.
Summary/Conclusions
First time donors, younger age females are over represented in minor adverse events. Serious adverse events in blood donors are excessively rare. Avoidance of hypoglycemia, hypovolaemia and anticipatory anxiety strategies are outlined.
P‐202
Establishment of Donor Haemovigilance System in Blood Banks of Islamabad, Pakistan
Ansari AA1, Waheed UW 2, Zaheer HAZ 2
1Kulsum International Hospital, Islamabad, Pakistan2Ministry of National Health Services, Islamabad, Pakistan
Background
A blood regulatory Authority has the responsibility of data collection, management and analysis. This work allows the decision makers in BTS to identify where systems are weak and corrective actions required. Accordingly the Islamabad Blood Transfusion Authority streamlined data collection from all registered and licensed blood banks on all aspects of blood safety including donor haemovigilance. Donor haemovigilance systems allow monitoring of donor safety and assessment of the success of interventions designed to further improve donor safety. Before the start of regulatory work by IBTA in Islamabad, the adverse events during blood donation were not documented due to lack of proper documentation system and lack of awareness of the staff involved.
Aims
To improve the safety standards of blood donation by monitoring all adverse events in blood donors visiting public and private blood banks of Islamabad.
Methods
This was a retrospective study conducted from January to December 2014. A standardized data collection tool (pre‐tested) was designed by the IBTA for the collection of donor haemovigilance data from the 19 registered and licensed blood banks operating in Islamabad. The compliance to the submission of data was 100% and all the data gathered was analyzed through MS Excel 2010.
Results
The donor haemovigilance data shows that out of 61,043 blood donations in 2014, only 8.82% were voluntary blood donors and the rest were Family Replacement donors. A total of 235 donors experienced adverse events which included 1137 signs and symptoms which were all mild in nature. The adverse events included slow pulse 15.1% (n = 172), low BP 15.1% (n = 172), sweating 12.3% (n = 140), fainting 14.3% (n = 163), pallor skin 10.1% (n = 115), nausea 12.9% (n = 147), drowsiness 3.6% (n = 41), vomiting 4.5% (n = 52), cold extremities 3.1% (n = 36), haematoma 0.5% (n = 6), multiple pricks 2.3% (n = 27), shortness of breath 0.1% (n = 22), vasovagal 1.3% (n = 15), headache 1.0% (n = 12), bruising 0.7% (n = 8), weakness 0.1% (n = 2), falling 0.5% (n = 6).
Discussion
Donor haemovigilance data indicates that majority of the signs and symptoms were slow pulse, low BP and fainting. These events are often avoidable provided trained and committed staff is available. The information gained from the investigations and analyses of haemovigilance facilitate corrective and preventive actions to be taken to minimize the potential risks associated. Currently, it appears that the adverse events are under reported mainly due to the fear of reprisal and lack of recognition of adverse signs and symptoms.
Conclusion
It is crucial that all blood banks adopt a systematic approach to monitor the rates of donor adverse reactions. The current pilot study needs to be emulated in other provinces to bring about a general improvement in the functioning of the blood transfusion services in Pakistan. This practice is also likely to increase the trend of voluntary blood donation by reducing the potential adverse events in blood donors.
P‐203
Mitigation of Iron Deficiency – Effect of Routine Selective Ferritin Screening of Whole Blood Donors
Kamel T1, Bravo MDB 1, Custer S 2, Vassallo R 1
1Blood Systems, Scottsdale, AZ, United States of America2Blood Systems Research Institute, San Francisco, CA, United States of America
Introduction
Frequent donation may initiate or exacerbate iron deficiency in some donors. We implemented a ferritin screening program for donors with fingerstick hemoglobin (Hb) value just above the US blood donor cutoff of 12.5 g/dl.
Aim
To measure the impact of targeted deferrals on donor return and Hb recovery.
Methods
Allogeneic whole blood donors (WBDs) with near‐cutoff Hb values [females (F) 12.5–12.9 g/dl; males (M) 12.5–13.4 g/dl] had a serum ferritin determination. Donors were notified by letter, counseled, and deferred from red cell‐containing donations for 24 weeks when serum ferritin values indicated absent iron stores (AIS, <12 μg/l). WBDs with low ferritin [LF, 12–19 μg/l in F and 12–29 μg/l in M] were initially notified and deferred, but this practice ceased mid‐way through the initial study period. Donors with normal ferritin (NF) were not notified or deferred. We assessed return behavior and Hb recovery (Hb at subsequent presentation ≥ index Hb) by gender and donor experience: first time/lapsed (FT/LD, no donation in prior 24 months) and repeat donors (RD, ≥1 WB donation in prior 24 months).
Results
During a 13‐month period, ferritin testing for near‐cutoff Hb values on an index donation was triggered in 8531 M and 39,075 F donors. These donors were followed for an additional 12–25 month period. The overall donor return rate was 66% in M and 62% in F donors. Of donors who returned, Hb recovery was observed in 86% of M and 74% of F. In all gender and donor‐experience strata, deferred AIS/LF donors had longer time to return (not shown) and lower return rates than similar non‐deferred LF/NF donors (Table). In donors deferred with LF, Hb recovery of M and F FT/LD and RD was consistently better than non‐deferred LF donors. Hb recovery in deferred RD, even those with AIS, was higher than non‐deferred donors, including those with NF. This was not observed in FT/LD. Both M and F RD with AIS are more likely to recover Hb after a 24‐week deferral period than M or F FT/LD with AIS (M: 88% vs 68%; F: 80% vs 52%), and are less likely to be subsequently deferred (M: 5% vs 16%; F: 13% vs 33%), respectively.

Conclusions
Deferral decreased donor return, but resulted in improved Hb recovery in otherwise comparable donors. RD with AIS are more successful than FT/LD with AIS at increasing their Hb between donations, as reflected by their lower deferral rate.
P‐204
Prophylactic Oral Calcium Carbonate Suppplementation Reduced the Risk of Citrate Toxicty
Prashant A1, Aseem Tiwari A 2, Vimarsh Raina V 2, Kumar P 1
1Jaypee Hospital, Noida, India2Medanta‐The Medicity, Gurgaon, India
Background and Aims
Plateletpheresis (PP) generally considered to be a safe procedure. Citrate toxicity is the commonest side effect of plateletpheresis procedure. The unpleasant signs and symptoms of citrate toxicity make it difficult for a transfusion service to motivate the donor for repeat voluntary platelet donation. Therefore, this study was conducted with the primary objective to observe the effect of prophylactic oral calcium supplementation on the reduction of risk of citrate toxicity compared to the group receiving the placebo. Methods
Written consent was obtained from the donors for the participation in the study. A total of 200 donors participated in study. All PP procedures were performed using a fully automatic platform, COM.TEC (Fresenius Kabi, Germany). The parameters which were measured to see the effect of plateletpheresis were total calcium (T Ca) and ionized calcium level (Ica) before and after the procedure. Sample which was collected for transfusion transmitted disease screening (clotted serum, 3 ml) was utilized for the purpose of assessment of preprocedure biochemical parameters. The post procedure sample (clotted serum, 3 ml) was collected after 1 h of the completion of the procedure. The cohort of 200 donors was divided into two categories. In first category of the donors (N = 100) only sugar pellets were given and categorized as ‘placebo group’. In second category of the donors (N = 100) 1000 mg of prophylactic oral calcium carbonate was given before 1 h of start of the procedure and 1000 mg (total = 2000 mg) was given just before the start of procedure (‘prophylactic oral calcium supplementation group’). Schedule of placebo was same as calcium prophylaxis. Total calcium was measured on Vitros 5600 (Ortho Clinical Diagnostics, Johnson and Johnson, USA). Ionized calcium was measured on ABL800Basic (Denmark). Statistical analyses were done using SPSS 16 software.

Results
Prophylactic calcium supplementation reduced the relative risk of citrate toxicity by two fold. The donors who received the oral prophylactic calcium supplementation presented with perioral tingling only and did not require termination of procedure while in placebo group clinical presentation were generally mild to moderate and in one case required termination of procedure (Table I). Prophylactic calcium supplementation in a total dose of 2000 mg did not affect drop in ionized calcium level but the drop in total calcium was not significant compared to prophylactic calcium intake group (Table II).

Conclusion
Prophylactic calcium supplementation reduced the risk of citrate toxicity. It also reduces the severity of citrate toxicity. Re‐entry of plateletpheresis donors may improve if procedure remains pleasant.
P‐205
Negative Experiences and Pre‐Donation Blood Pressure at the Subsequent Donation in Blood Donors
Hoogerwerf MD1, Veldhuizen IJT 2, van den Hurk K 1, de Kort WLAM 1, Sluiter JK 3, Frings-Dresen MHW 3
1Sanquin Research, Amsterdam, The Netherlands2Radboud University Medical Center, Nijmegen, The Netherlands3Academic Medical Center, Amsterdam, The Netherlands
Background
Negative donation experiences, like being deferred or experiencing an adverse reaction, might upset blood donors. This may result in anticipatory stress responses, such as elevated blood pressure, at the subsequent visit.
Aims
The aim of the study was to explored associations between blood donors’ negative donation experiences and their blood pressure at the subsequent visit.
Methods
Blood pressure of donors with no history of negative experiences in three consecutive donations was compared to the blood pressure of donors with a negative experience during the second of the three donations. Blood pressure (systolic and diastolic) measured prior to the third donation was compared between the two groups, using linear regression analyses. Two types of negative experiences (adverse reactions and deferral) were analysed, stratifying for donation type and sex, and adjusting for age and pre‐donation blood pressure at baseline.
Results
In total 248,118 (50% female) donors were included in the analyses. 11% (26,380 donors, 61% female) had experienced a negative experience. Fainting and dizziness were associated with significant (P < 0.05) increases in systolic blood pressure: in men, of 3.2 mmHg (fainting) and 1.7 mmHg (dizziness); in women, of 2.2 mmHg (fainting) and 1.4 mmHg (dizziness). Deferral was associated with significant (P < 0.05) increases in both systolic (men 0.7 mmHg, women 0.3 mmHg) and diastolic (men 0.2 mmHg, women 0.3 mmHg) blood pressure.
Summary/Conclusions
Whole blood donations with negative experiences were associated with a statistically significant higher pre‐donation blood pressure at the subsequent visit. This indicates that negative experiences might cause an anticipatory stress reaction in a subsequent donation.
P‐206
Adverse Reactions in Blood Donors in the Center of Blood and Transplantation of Oporto (Portugal)
Almeida FQ , Lopes CP , Pereira CP , Pinto BC , Ferreira DS , Costa PP
CSTP‐IPST, IP, Porto, Portugal
Introduction
The Notification of incidents related to blood donation to the National System Hemovigilance Portuguese (HPV) is a legal requirement. Usually blood donation is considered a safe process with a relatively low rate of adverse reactions, but remains a major problem because it can negatively influence the recruitment and the fidelity of donors.
Equipment and Methods
During the years 2013–2014 the data related to all reports of adverse reactions in blood donors (RAD) were analyzed in theCenter of Blood and Transplantation of Oporto.
The following parameters were analyzed: age, gender, number of previous donations, time of the incident (immediate, delayed), quantification of the severity of the reaction and degree of resolution.
Results
During the study period the rate reported was 1090 (RAD) adverse reactions in 161,086 donations (27.71% Male/72.29% female).
Age: 26.88% RAD from 18–24 years; 52.11% from 25–44 years and 21.01% from 45–65 years.
Type of donors: 43.58% in first time donors; 35.32% in occasional donors and 21.10% in regular donors.
Gravity: 0.18% of total RAD were considered severe, in 2013 we note that 0.39% of the RAD were severe and 99.61% were not serious, while the total of 2014 RAD were not severe.
87.89% of the RAD were immediate, 6.42% were delayed and the remaining 5.69% were classified as other incidents related to the donation
Conclusions
In our study we verified that the RAD are more common in women, first time donors and casual donors. Most of the reported incidents are immediate reactions.
Implementing a system with well specified criteria of hemovigilance facilitates the recording and allows us more reliable information, making it possible to take measures to minimize these situations. Notification forms are necessary to facilitate the recording and analysis of data. We consider it important to the development of a communication channel that allows direct contact with the donor to ensure a record of the most reliable delayed reactions.
P‐207
Vasovagal Reactions in Blood Donors
Kaul Bazaz AB , Gupta AG
Rotary Blood Bank, Delhi, India
Background
Many modern techniques in surgery & medicine require the provision of substantial volumes of Blood & Blood components. In order to meet these requirements Rotary Blood Bank in New Delhi relies entirely on the willingness of volunteers to make regular blood donations.
Aim
To find out the prevalenceof donor reactions in outdoor Blood donation camps. For many potential blood donors, the incidence of fainting during or after the donation acts as a deterrent to their enrolment. So it is very important for transfusion services to do all it can to prevent these incidents.
Method
The incidence and pattern of vasovagal reactions were studied in a group of 1000 first time donors. When a donor experienced a vasovagal reaction during or after a blood donation, this fact was recorded on the donor form & records were analyzed in regards to various parameters such as age, sex, first time donor or repeat donor. The incidence of reaction was higher in females than males & these reactions were more common in college students and factory workers. Symptoms of pallor, sweating and dizziness without loss of consciousness, were referred as ‘mild reaction’ and more severe symptoms, such as vomiting and loss of consciousness, were recorded as ‘moderate reaction’. The occasional donor who experienced convulsions or an unusually prolonged vasovagal response was recorded as having had a ‘severe reaction’.
Result
A total of 1000 donor reactions (1.31%) were studies in 76,098 blood donors. Syncope and Giddiness was seen in 79% donors, Vomiting in 7.2%, and Hematoma seen in 13.8% donors. 84% were 1st time donors and 16% were repeat donors which indicates that first time donors have fear and apprehension. 48% reactions occurred during the donation process especially towards the end of the procedure and 52% took place post‐phlebotomy. 49% of the donors were in the 18–30 years of age group, 33% in the 31–40 years bracket and 18% in the >40 years age group. Weight was not seen to be strongly associated with blood donation related adverse events and only 115 who experienced adverse reaction were below 55 kg.
Conclusion
The result of this study has several important implications for those involved in the work of blood transfusion and in the medical management of voluntary blood donors. First it is important to recognize that any donor can experience vasovagal reaction. It is the responsibility of the transfusion service staff to be aware particularly of the increased risk of reaction with all persons donating blood for the first time. Second, when the possibility has been recognized, there are various precautions which can be taken and alertness to the earliest signs or symptoms of vasovagal reaction. Third, the reassurance of the donor who has experienced a reaction of the decreased likelihood of this occurring on a future occasion can be provided for the apprehensive donors. Donors should be encouraged for further donations and should be engaged in conversation during the course of donation.
P‐208
Do Adverse Reactions After Whole Blood Donation Have an Impact on Return Donation?
Danzi M1, Di Lemma G 1, D’ Ambrosio R 1, Di Girolamo MG 1, De Caprio G 1, Valiante A 1, Pecora P 2, Misso S 1
1S.C Servizio Trasfusionale ASL Caserta, Aversa, Italy2AVIS Casoria, Casoria, Italy
Background
Whole blood donations are generally considered to be safe procedures and voluntary donors normally tolerate blood donation. Occasionally adverse reactions of variable severity may occur in a few donors. A good hemovigilance of the donation process can be of operational value in identifying and understanding the predisposing factors for the reactions and also for making interventions to reduce themselves.
Aims
The objective of this study was to analyze the severity of adverse reactions in blood donors in order to define appropriate preventive and corrective measures to improve blood donation safety.
Methods
The study was conducted for 1 year from January 2014 to 31st of December 2014. The donor population analyzed consisted of 13,310 donors: italian donors are 12,278, males 7452 (60.7%) and female 4783 (38.9%) while foreign donors are 1032 (7.7%), males 720 (69.8%) and females 312 (30.2%). We have considered agitation, sweating, pallor, cold feeling, sense of weakness, nausea as mild adverse reactions and vomiting, and loss of consciousness as severe disorders.
Results
See table.

Conclusions
The adverse reactions that occured were mostly mild and resolved quickly. the analysis of the data obtained showed that the female donors are more involved for the following reasons: hypotension, iron deficiency and emotional factors. A small percentage of donors suffered a more severe reaction. Among this 145 (82.4%) donors (83 italian and 62 foreign donors) were first time donors and did not return to donate blood. Although the number of donors who developed reaction in relation to donating blood was low, it is nevertheless desirable to reduce risks to a minimum, working not only with the maximum environmental safety, but also with complete medical and nursing assistance.
P‐209
Quantification of the Healthy Donor Effect
Rigas AS1, Erikstrup C 2, Pedersen OB 3, Ullum H 1
1Rigshospitalet, København, Denmark2 Århus University Hospital, Århus, Denmark3Næstved Hospital, Næstved, Denmark
Background
Health Related Quality of Life (HRQL) represents people's subjective assessment of their mental and physical well‐being. HRQL is highly predictive of future health. A widely used scale for self‐reported Health Related Quality of Life (HRQL) is the short form 36 (SF‐36) which has been abridged into a 12 item short form (SF‐12) for epidemiological surveys. The SF‐12 measures an overall physical component summary score (PCS) and an overall mental component summary score (MCS). The correlation between SF‐12 and SF‐36 in Denmark are approximately 0.95. It is known that MCS increases with age and PCS decreases with age.
In Denmark as elsewhere all blood donors declare that they are healthy and fit at the time of donation and hence blood donors who are not eligible for donation cease to donate blood. The selection pressure known as the Healthy Donor Effect should therefore in theory yield higher HRQL scores for donors compared with non‐donors.
Aims
We aimed to explore the Healthy Donor Effect by describing the difference in self‐perceived HRQL between donors and non‐donors.
Methods
Data from the Danish Twin Study (DTS) collected in 2002 were compared with data from the Danish Blood Donor Study (DBDS) initiated in 2010. Data were available on SF‐12 scores, age, sex, and smoking status for both groups. Age was divided into five groups (1: 18–25 2: 25–35 3: 35–45 4: 45–55 5: >55). Multivariable linear regression analyses stratified by sex and age groups with MCS and PCS as outcome variables were performed.
Results
Data were available on 75,128 participants (34,843 participants from DTS and 40,285 participants from DBDS). DTS participants were older (mean difference: 4.6 years CI: 4.4–4.8 years P = <0.001), and DTS participants had a significant higher proportion of active tobacco users (DTS: 34.5% DBDS: 16.4% P < 0.001) when compared with DBDS participants.
In both groups we observed that MCS increased with age while PCS decreased with age. Donors had higher MCS than non‐donors but we observed that the difference between the two groups diminished with increasing age. Men under 35 years and women under 25 had lower PCS than non‐donors. However, with increasing age donors had significantly higher PCS than non‐donors.
Caption 1: Difference between donors and non‐donor.

Summary/Conclusions
The data shows, unsurprisingly, that donors in general have a better mental and physical health. When divided into age groups it appears that the selection pressure has a greater effect on the physical health than on the mental health. A major limitation of this study is the difference in year of data collection between the two studies allowing for calendar effects to contribute to the described differences between donors and non‐donors.
P‐210
Rates of Plasmapheresis Donor Complications and Procedural Problems in Relation to the Introduction of Express™ Software (Haemonetics)
Marijt-van der Kreek T , Wiersum-Osselton JC , Bokhorst AG
Sanquin Blood Supply, Amsterdam, The Netherlands
Background
In September 2013 the Dutch national blood establishment Sanquin Blood Supply switched to new software (Express™) for plasmapheresis procedures on the Haemonetics PCS®2 devices (H). This uses a higher flow rate during the draw and return phases and can reduce the duration of procedures. After the change staff at a number of collection centres reported an apparent increase of vasovagal reactions (VVR) during plasmapheresis procedures.
Aims
To compare the number and type of complications of plasma donation (VVR, hematomas or painful arm) and flow problems during plasmapheresis using PCS®2 before and after implementation of Express™ software.
In addition, to compare the duration of plasma collection procedures before and after the change in order to establish whether the desired reduction in collection time has been realised using the new software.
Methods
Data were extracted from the blood service information system eProgesa™ (MAK Systems) for all plasma donations in The Netherlands from 1‐1‐2013 to 31‐12‐2014. We analysed the incidence of recorded complications of plasma donation both before and after the change, according to donor gender. In order to examine for possible changes in staff alertness and reporting behaviour we compared the findings for procedures using the PCS®2 with those using the Autopheresis‐C® devices (Fenwal, F) in the same period.
Results
During the observation period a total of 571,252 plasmapheresis procedures were performed: 375,397 H and 195,857 F. Following the change a higher rate of VVR was recorded in female donors (from 0.7% to 1.0%); no increase was seen in male donors. The rate of VVR associated with F plasma donations in female donors also increased from 0.7% to 1.0%. For both types of devices an increased rate of flow problems was recorded (Table). Among procedures terminated before the standard volume had been collected, in some cases no explanatory complication code was recorded; this number was lower during the months after the software change (H: 43% → 38.7%; F: 41.5% → 25.7%). Following the change there was no change in rate of reported hematoma or painful arm.
After implementation of the new software the average collection duration (H) was 4 min shorter in female donors and 5 min shorter in male donors.

Summary/Conclusions
Following the introduction of the Express™ software an increase of recorded VVR was seen in female donors. In the same time frame a similar increase was seen with the F plasmapheresis procedures, as well as an overall increase of compliance in recording explanatory codes when procedures were terminated prematurely. It had been noticed that there was under‐recording of complication codes and this was drawn to the attention of collection centre staff late in 2013. Improved recording of donor complications could explain the observed increases. At the national level it cannot be concluded that the observed increase of vasovagal reactions was a consequence of the software change.
After the change the average time per H plasmapheresis procedure went down for both female and male donors.
P‐211
Regional Survey Study of Hazards of Blood Donation (HOBD) Donor Adverse Events Transfusion Services of the Arabic Speaking Countries of North Africa and the Middle East
Hammadi A1, Mediouni M 2, Boumazouzi R 3, Noubadji S 4, Hindaoui S 5, RanaAbdulrazak R 6, SNosseir sShennawy S 7, Gabra G 8, Boukhef MK 8
1National Blood Agency, Algiers, Algeria2Regionel blood center, Jendouba, Tunisia3Algiers blood center, Algiers, Algeria4Oran blood center, Oran, Algeria5Jadah blood center, Jadah, Saudi Arabia6Blood bank, Kuwait city, Kuwait7Egypte blood centers, Cairo, Egypt8Arabic Transfusion Medecine Forum, Birmingham, United Kingdom
Background
This is a prospective study to establish a national and regional systems for monitoring of the adverse events of blood donation in the Transfusion Services of the Arabic Speaking Countries of North Africa and the Middle East; The rules of ethics and duty of care to donors require blood services to put in place adequate systems to reduce as much as possible any harm that may be caused to donors through blood donation.
Aims
(1) Establish, across the region, blood collection procedures with maximum care and optimum safety procedures; (2) include the harmonisation and standardisation of the surveillance systems for monitoring of the quality of blood collection procedures and donor care using regionally agreed forms to report hazards of blood donation; (3) establish standards for qualifying the range of adverse events and recommending a regional approach to classify the clinical severity of these events; (4) to serve as basis of a region wide haemovigilance system for donor adverse events that can be easily adopted onto an electronic online surveillance scheme.
Materials and Methods
Form used: based on the standard severity classification of HOBD, devoloped and used by the Welsh Blood Service and the French grading classification; the study is not a comprehensive survey and the number of adverse incidents were chosen to facilitate the collection of data and provide optimmum information to serve as basis to meet the objectives of the study; the study was run over 6 months (from April to September 2012) aimed to prepare a preliminary report which was presented at ATMC10 in Kuwait as part of the agenda of the Standing Working Group on ‘Donors Donations and Blood Collection’; Monthly blood collection figures were required to allow calculation and analysis.
Results
1. Donors bled 88.071 (12,212 Female 13.79%; 75,927 Male 86.21%);
2. HOBD: 1566 (17.79%);
3. HOBD reported: a. Vasovagal reaction: 1073 (68.52%),b. Heamatoma: 166 (10.6%),c. Failed venipuncture; Incomplete donation: 216 (13.60%),d. Discomfort: 159 (10.15%);
4. Most of these adverts effects were observed during the donation and at session; vasovagal reaction is the most frequent category, donors that are more susceptible to present an advert event are men at first donation.
Summary
The advantage of this survey study lies in the exploitation and the use of the data collected
Establish National and Regional standard criteria for grading of the HOBD.
Establish National procedures for surveillance of HOBD in participating establishements that can be extended into the respective national services and a Regional Haemovigilance System.
Establish the incidence and prevalance of HOBD and develop the necessary interventions to reduce their incidence in order to improve the quality of donor care
Establishment of donor heamovigilance system is possible in the Arabic countries, this is a major improvement of the blood donor safety. It requires negotiation between the responsibles of the countries in question, and should be encouraged by the existence of formal regulations regarding blood donation and donor.
3.1 Blood Processing, Storage and Release
P‐212
Abstract Withdrawn.
P‐213
An In Vitro Assessment of the Haemostatic Potential of Stored Whole Blood from the Irish Donor Population
Maguire C , O'Brien P , Fitzgerald J
Blood Transfusion Laboratory, Dublin, Ireland
Background
Component Therapy(CT) has become standard in the treatment of massive haemorrhage. Following therecent reports of military success with whole blood (WB) resuscitation, there has been renewed interest in the use of WB in the treatment of massive haemorrhage.
Aims
To define a storage limit to ensure the optimal haemostatic potential of WB for the treatment of massive haemorrhage.
Methods
WB units were stored at 4°C ± 2°C for 30 days. Baseline testing of WB units was completed on day 2 post donation. WB units were tested at regular intervals between day 2 and day 30. Tests included full blood count, pH, lactate, Protrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, Thromboelastography and Coagulation factor assays (Factor V and VIII).
Citrated patient samples with abnormal coagulation results were selected by a third party for inclusion in the study. The preparation of the samples for the in vitro simulation of a transfusion of a WB trauma pack involved combining WB and abnormal citrate samples in a 2:3 ratio. Baseline and treated samples were assessed using Thromboelastography.
Results
Haemoglobin and haematocrit remained stable with a significant decrease in platelets (P = 0.0019). PT and aPTT increased over time, fibrinogen decreased slightly. pH decreased over time. Potassium and lactate levels increased throughout. TEG® variables in all units were normal through day 12. Abnormal values in some variables TEG® were noted on day 15. In vitro simulation of WB trauma pack showed improvements in all 4 TEG® parameters. However only increases in Maximum amplitude was significant (P = 0.0005).
Conclusion
This in vitro study demonstrated that the normal haemostatic potential of WB is preserved up to a minimum of 12 days under the standard refrigerated storage of WB for transfusion. Study also demonstrated that WB has a level of haemostatic correction potential up to day 16. Study provides significant in vitro evidence to support clinical investigation
P‐214
Preparation of Packed Red Blood Cells In Low‐Income Countries: Efficacy of Whole Blood Settling Method in Burkina Faso
Sawadogo SS1, Nébié NK 1, Kafando KE 1, Dahourou DH 2, Barro BL 2, Ouattara OS 2, Deneys DV 3
1University of Ouagadougou, Ouagadougou, Burkina‐Faso2Centre national de transfusion sanguine, Ouagadougou, Burkina‐Faso3Catholic University of Louvain, Brussels, Belgium
Background
Anemia is the main cause of blood transfusion in sub‐Saharan African countries because of high rates of malaria, malnutrition and other genetic dysfunctions of red blood cells. In these cases, transfusion of packed red blood cells (pRBCs) is the most appropriated. But in low‐income countries, production of blood components is difficult due to logistical constraints (lack of centrifuge, electricity, storage equipments…).
Regional Transfusion Center of Koudougou in Burkina Faso produces pRBCs by settling whole blood. Whole blood pockets are vertically held in place in the refrigerator during a given duration to allow red blood cells sedimentation by simple gravitation. The extracted plasma is destroyed and the resulting pRBCs is collected for therapeutic use.
Aim
Our study aims to evaluate the efficacy of the whole blood settling method.
Methods
We compared 860 units of pRBCs produced by whole blood settling (settled‐pRBC) to 215 units of pRBCs stored in 100 ml of SAGM‐solution prepared by centrifugation (centrifuged‐pRBC). Settled‐pRBCs comprised four sub‐groups of 215 units. The sub‐group I consisted of pRBCs prepared after a settling time of [24–48 h] hours without additive solution (settled‐pRBCs CPDA). The other three sub‐groups consisted of settled‐pRBCs stored in 100 ml of SAGM‐solution (settled‐pRBCs SAGM) produced after settling times of [24–48], ]48–72] and ]72–96] hours respectively. All the pRBCs were matched on the basis of the whole blood volume ± 10 ml. The volumes were calculated by dividing the net weight by density of each product (whole blood = 1.06, pRBC‐SAGM = 1.07 and pRBC‐CPDA = 1.08). The hematocrit and hemoglobin were determined with the BC3000 plus® automate (MINDRAY Coorporation Chenzen, China) on 2 ml of blood sampled by stripping method after agitation with AGITATOR TS‐2000®. The mean hematocrits were compared using anova and pairwise t‐tests.
Results
The centrifuged‐pRBCs had a mean hematocrit of 63.85% (SD: 4.25). The hematocrit was greater than 50% for all units in this group. Only 26.7% (57/215 units) of settled‐pRBCs SAGM had a hematocrit ≥ 50% compared to 87% for those with CPDA (P < 0.001). The mean hematocrits were respectively 57.92 (SD: 6.18), 45.03 (SD: 4.98), 47.15 (SD: 4.37), 47.28 (SD: 4.82) for settled‐pRBCs of sub‐group I, II, III and IV. Mean hematocrit of settled‐pRBCs of sub‐group III and IV were not statistically different (P = 0.46). In the other hand, it was different for sub‐group II compared to sub‐group III (P = 0.04) and IV (P = 0.004).
Conclusion
Indications of whole blood are currently very limited. Blood components therapy is the rule. Unfortunately, logistics to produce these components is most often out of reach in low‐income countries. Our study shows that, whole blood settling method could be an alternative and that, a settling time between 48 and 72 h is sufficient to obtain pRBCs with acceptable hematocrit. But it is crucial that further studies investigate the validity of this process.
P‐215
Evaluation of Processing and Storage Conditions Using a Dinch‐Based Blood Bag System
Rach J1, Gravemann U 1, Volgmann T 1, Handke W 1, Platenkamp K 2, Tijink M 2, Hakvoort K 2, Müller TH 1, Seltsam A 1
1Red Cross Blood Services, Springe, Germany2Fresenius Kabi, Emmer‐Compascuum, The Netherlands
Background
The plasticizer Hexamoll DINCH (1,2‐cyclohexane dicarboxylic acid diisononyl ester) is an alternative for DEHP (di‐2‐ethylhexyl phthalate) for medical devices as it may offer toxicological advantages over DEHP. However, DEHP is known to have a protective effect on red blood cell (RBC) membranes and the use of DINCH might result in higher haemolysis rates of RBC units during storage. Previous studies have shown that DINCH may result in higher haemolysis rates with SAGM, but that red cell quality might be preserved when using PAGGSM.
Aims
The aim of this study was to investigate the impact of a DINCH‐based blood bag system (phthalate‐free) on the quality of RBC units in PAGGS‐M during storage of 49 days. In particular, the influence of the time interval between collection of the whole blood units (WBUs) and preparation of the RBC units and the effect of weekly mixing of the RBC units during storage was tested.
Methods
In a five‐arm study, WBUs were processed either within 4 or after approx. 22 h after WB collection and storage on cooling plates. In both groups, half of the RBC units was mixed weekly, while the other half was left untouched. RBC units prepared after more than 22 h after WB collection and stored in a conventional DEHP/PVC blood bag system without mixing served as controls (fifth arm). The quality of the RBC units was assessed by a set of in vitro quality parameters at different time points.
Results
Haemolysis measured on day 49 was higher in RBC units stored in DINCH bags (mean 0.54% ± 0.17) than in RBC units stored in DEHP bags (mean 0.31% ± 0.12) when processed and stored under the same conditions (preparation after 22 h, no mixing). A shorter time interval between WB collection and RBC unit preparation and regular mixing both had a positive effect on haemolysis in the DEHP‐free test system. The mean haemolysis was lowest when WB was processed within 4 h and RBC units were mixed regularly (mean 0.22% ± 0.07; day 49). The highest rate of haemolysis was observed in DINCH RBC units prepared after more than 22 h and not mixed during storage (mean 0.54% ± 0.17; day 49). Haemolysis rate in control units was similar to those test systems that were either prepared after 4 h but not mixed or processed after 22 h and mixed regularly. For the other quality parameters only small and negligible differences were observed between groups.
Conclusions
The unfavourable effect on haemolysis of DINCH as alternative plasticizer to DEHP in blood bag systems might be compensated by regular mixing of the RBC units during storage. In addition, a shorter time interval between WB collection and RBC unit preparation appears to have an additional positive effect on the haemolysis rate of RBC units stored in DINCH bags.
P‐216
In Vitro Evaluation of the Effects of Timing of Irradiation on Stored Red Cell Concentrates
de Korte D1, Fitzpatrick A 2, Morrison A 3, Thibault L 4, Marks DC 5, Seldsam AW 6, Acker J 7
1Sanquin Blood Bank, Amsterdam, The Netherlands2Irish Blood Transfusion Service, Dublin, Ireland3Scottish National Blood Transfusion Service, Edinburg, United Kingdom4Héma Québec, Québec city, Canada5Australian Red Cross Blood Service, Sydney, Australia6German Red Cross Blood Service NSTOB, Springe, Germany7Canadian Blood Services, Edmonton, Canada
Background
There are very few comprehensive studies investigating the effect of irradiation on red cell concentrates (RCC) at varying points during their storage. Some studies showed effects on most in vitro parameters, but these are limited, showing that type of additive solution and amount of plasma might play a major role. In relation to a world‐wide concern about possible negative side effects caused by transfusion of older units, irradiation can be judged as artificial ageing.
Aims
This multi‐center, international study in a pool‐and‐split design was set up to investigate the effect of irradiation on erythrocyte in vitro quality after irradiation at several time points during storage.
Methods
Each center (in total 7 participating centers) used their standard RCC in this study (SAGM, AS‐3 or PAGGSM as additive solution) and produced 4 pools of 7 RCC (same gender per pool), which were each split back into 7 RCC. During storage for 6 weeks, one pool was irradiated each week. Units were sampled weekly for in vitro quality parameters including hemolysis, K+, pH, glucose, lactate and ATP.
Results
During storage an increase of hemolysis and K+ concentration was found, with K+ increasing significantly (P < 0.01) during the week immediately after irradiation. The earlier during storage that the units were irradiated, the higher the hemolysis and K+ concentration found at end of storage (table). ATP concentrations were affected by irradiation early during storage with end of storage concentrations falling below the currently accepted minimal value of 2.7 μmol/gHb. No specific effects of irradiation on pH or glucose consumption/lactate production were observed.

Conclusions
Irrespective of the timing of irradiation during storage of RCC, irradiation caused a rapid increase in extracellular K+, followed by a more gradual increase in hemolysis. Over the period of storage, the ATP concentration decreased faster in irradiated units. Results of this study will be used to formulate guidance on the maximal pre‐ and post‐irradiation storage time for RCC.
on behalf of Biomedical Excellence for Safer Transfusion (BEST) Collaborative
P‐217
Proteomic Analysis of RBC Membrane and Cell Function Changes in the Red Cell Storage Period
Ying YL , Chen S , Hong XZ , Xu XG , He J , Zhu FM , Lv HJ
Blood Center of Zhejiang Province, Hangzhou, China
Background
The red cell storage lesion (RCSL) comprises the biochemical and biomechanical changes during red blood cell (RBC) storage, which can reduce the cell function and influence the morbidity and mortality. Now some studies were explored the mechanisms of the RCSL and focused on the RBC membrane protein changes during storage.
Aims
The aim of this study was to elucidate the effects of membrane protein changes on the cell functions during the red blood storage.
Methods
The RBCs cell function parameters (pH, K+, Na+, free Hb, cell ATP, 2,3‐DPG) were determined by using standard biochemical techniques. Flow cytometry was used to monitor changes of CD47, CD55, CD59 and PS expression. Two‐dimensional gel electrophoresis and mass spectrometry were used to identify red blood cell membrane protein profile changes in the storage period.
Results
Potassium level was increased with the sodium level decreasing in the 6 weeks of RBC storage period. Cell ATP content was decreased to 64% from initial levels during storage period. 2,3‐DPG was declined significantly in the first week (92.6% decline, P < 0.001). CD47 expression was progressive decreased during storage period. All of them had statistically significant difference during storage period (P < 0.01), except CD55, CD59 and PS expression. The red blood cell membrane proteomics were analysis during the early, middle and late storage period. The protein points were increased in the first 2 weeks, and then decreased to stable level. Eleven protein points were chosen for analysis the kinds of the protein, in which eight different kinds of proteins were identified, including actin, hemoglobin subunit beta, tropomyosin alpha‐3 chain, glyceraldehyde‐3‐phosphate dehydrogenase, protein 4.1, and 55 kDa erythrocyte membrane protein. Proteins analysis was revealed that most of the modified proteins were located in the cytoskeleton. Some of the protein were shifted in isoelectric point, as a consequence of chemical oxidations or deamidations.
Conclusions
The changes of RBCs membrane protein may be generated as a result of protein attack by oxidation. The RBC function was closely related to the changes of membrane protein. Our data will help to reduce hazard of the red cell storage lesion.
P‐218
Evaluation of Platelet Concentrates After 5 Day Storage In Additive Solution (T‐Pas+, Terumo BCT)
Lachert E1, Plodzich A 1, Kubis J 1, Antoniewicz-Papis J 1, Rosiek A 1, Przybylska Z 2, Piotrowski D 2, Letowska M 1
1Institute of Hematology and Transfusion Medicine, Warsaw, Poland2Regional Blood Transfusion Center, Warsaw, Poland
Background
Factors such as preparation method, storage time and kind of additive solution affect the quality of platelet concentrates (PCs). Storage of PCs in additive solution has several advantages: reduction of the incidence of allergic and febrile reactions, facilitation of ABO‐incompatible PC transfusions, pathogen inactivation and greater volumes of plasma available for eg. fractionation. Furthermore, additive solution improves storage conditions and thus increases platelet shelf life with no loss to their viability and hemostatic functions. Development of new additive solutions is focused on creation of a storage environment that optimises platelet viability, energy metabolism, and the ability to undergo post transfusion haemostatic activation.
Aim
To investigate the effects of 5‐day storage of buffy‐coat‐derived platelets in a mixture of 30% plasma and 70% T‐PAS+.
Methods
Of 15 buffy‐coat derived pooled leukoreduced PCs were prepared in 70% T‐PAS+ (S‐PCs; study group) and compared with platelets stored in plasma (C‐PCs; control group). PCs from both groups were stored at 22°C with agitation. Samples for analysis were collected on days: 1, 3 and 5. We measured: platelet count, MPV, pH, HRS, CD 62 and CD 42b expression, level of glucose, pCO2, pO2, LDH concentration.
Results
In both groups the platelet count was on the same level up to storage Day 3 (C – 2.62–2.63 × 1011/unit; S – 2.55–2.61 × 1011/unit) and decreased on Day 5 by approximately 16% (C‐PCs) and 13% (S‐PCS) as compared to Day 1.
On storage Day 1 the pH values were within 7.50–7.60 for both groups. On Day 3 a statistically significant decrease of pH value in C‐PCs was observed. On Day 5 the pH value decreased by approximately 10% in C‐PCs but only by 6% in S‐PCs.
In both groups MPV increased with storage time. Statistically significant values were observed for Day 3 and 5.
In both groups the response to hypotonic stress reaction (HSR) decreased with storage time. Statistically significant values were observed on Day 5; 66.0 ± 20.7%, for C‐ PCs and 57.9 ± 19.0% for S‐ PCs.
Throughout the whole storage time no statistically significant differences in CD 42b antigen expression were reported for either of the PC groups.
On storage Day 5 there was a statistically significant increase in CD 42b antigen expression in C‐PCs.
On storage Day 5 we observed a statistically significant decrease of glucose concentration in C‐PCs (0.42 mg/dl).
On storage Day 5 we observed a statistically significant decrease of pCO2 in C‐PCs.
Throughout the whole storage period no statistically significant differences in pO2 values were reported.
We observed a statistically significant level of lactate dehydrogenase (LDH) release in C‐PCs stored in a mixture of 70% T‐PAS+ and 30% plasma.
Conclusion
In vitro results for platelet biochemical and functional parameters demonstrate that PCs can be stored in a mixture of 70% T‐PAS+ and 30% plasma for up to 5 days.
P‐219
Benefits of Implementing the Tomes Software Solution for T‐ACE II+
Pajares Herraiz AL1, Peréz Parrillas CP 1, Rodríguez Gambarte JD 1, Eguía Lopez BE 1, Flores Sanz MV 1, De Potter W 2
1Centro Regional de Transfusion Toledo‐Guadalajara, Toledo, Spain2Terumo BCT, Brussels, Belgium
Background
In 2011, 6 T‐ACE II+ Automatic Component Extractors from Terumo BCT were implemented at the regional Blood Center of Toledo. One‐hundred percent (100%) of the whole blood units (26.696 in 2014) were processed by the T‐ACE II+ extractors using both Conventional Blood Bags (to produce RBC and plasma) as Top & Bottom Blood Bags (to produce RBC, plasma and BC). This new generation of Whole Blood (WB) extractors is supported by a Software Solution called TOMEs which is used for data capture and programming of the devices, bi‐directional communication with the Blood Bank central Information System (BBIS), and allowing remote technical support. Terumo Operational Medical Equipment Software (TOMEs) was installed and connected in 2014. In the Toledo Regional Blood Center, TOMEs is configured to bi‐directionally communicate with the T‐ACE II+ devices and automatically export session data to the Blood Bank Information (e‐Delphyn®).
Aims
To evaluate the impact of TOMEs Software implementation on the discard rate of a semi‐automated production process (T‐ACEII+).
Method
By using the e‐Delphyn Blood Bank Information System 2 reports were created in order to compare the discarded products between 2 identical periods of 5 months. The first report shows the discarded products in a 5 month period prior to implementing TOMEs (data between 01/10/2013 and 28/02/2014). The second report shows the discarded products in a 5 month period after implementing TOMEs (data between 01/10/2014 and 28/02/2015)
Results
By implementing TOMEs in the Toledo Regional Blood Center an improvement of the manufacturing efficiency due to better traceability of the entire process leading to an increased adoption of standard operations procedures was observed. Furthermore, operator‐related improvements were possible. As a result we saw a significant drop in the number of discarded products during the same 5 month period in 2014 and 2015 (table 1). The manual process was reduced by automatically sending procedure information to the Blood Bank Information System which eliminated the manual data recording step and the manual weighing of Plasma (not quantified in this study). Reporting of real‐time procedural data was made easier by using custom‐made data views in TOMEs which was automatically created and/or exported on a daily, weekly or monthly basis, as needed. Thanks to TOMEs capabilities, Terumo BCT could remotely connect to the system (using the existing VPN infrastructure) for maintenance of TOMEs and troubleshooting of T‐ACE II+, which resulted in increased uptime of the separators.

Conclusion
Comparison of the discard rates pre‐ and post‐implementation of TOMEs showed a significant decrease in discard rates of products due to strict SOP compliance and improved operators performance. This fact not only contributed positively to the financial results of the blood bank, but most importantly led to optimization of blood donation utilization. Further studies could additionally focus on the outcomes of specialized software introduction on error reduction, time savings through reduced product manipulation, simplified reporting and enhanced troubleshooting through the remote connection capabilities.
P‐220
An In Vitro Study to Compare Interventions Designed to Attenuate or Reverse the Storage Lesion in Donor Red Cells
Wozniak MJ1, Sullo N 1, Qureshi S 1, Kumar T 1, Wiltshire M 2, Cardigan R 2, New H 3, Thomas S 2, Murphy GJ 1, Ang K 1
1University of Leicester, Leicester, United Kingdom2NHS Blood & Transplant, Cambridge, United Kingdom3Imperial College Healthcare NHS Trust, London, United Kingdom
Background
Associations between allogenic red blood cell (RBC) transfusion and adverse clinical outcomes in transfusion recipients have been attributed to the pro‐inflammatory effect of the ‘storage lesion’ that develops in stored RBC units. The storage lesion is characterised by (a) the accumulation of metabolites in the RBC supernatant such as potassium, lactate, free haemoglobin and inflammatory microparticles, and (b) changes in the stored erythrocytes such as depletion of adenosine triphosphate (ATP) and 2,3‐diphosphoglycerate (DPG).
Aims
To compare the attenuation of the RBC storage lesion using one of three approaches 1) storage in the hypotonic additive solution AS7, 2) washing RBC, or 3) rejuvenation and washing RBC. RBC in SAGM were used as controls.
Methods
Leukoreduced RBC were manufactured from 5 units of whole blood that had been pooled and split into identical units on each of 6 occasions and stored in SAGM or AS7 for 20 days. RBC were then washed using a Haemonetics ACP215 (SAGM or AS7), rejuvenated with Rejuvesol and washed (SAGM units only, Citra labs In, USA) or left untreated in either SAGM or AS7. Samples were analysed immediately and 1, 2, 5 and 14 days after washing.
Results
There was no difference between AS7 andSAGM stored red cells with respect to supernatant potassium, lactate and annexin‐V positive microparticle levels at day 20, however these were effectively removed by washing +/‐ rejuvenation. Red cell ATP levels were replenished by washing followed by re‐suspension in SAGM or AS7, and to a greater extent by rejuvenation. After washing or rejuvenation ATP levels slowly decreased and returned to the original levels over 5 days. Rejuvenation or storage in AS7 restored and preserved, respectively, deformability and resistance to osmotic stress compared to storage in SAGM or SAGM washed cells. Rejuvenation was the only treatment that restored 2,3‐DPG levels to those observed in fresh red cells and these levels remained stable for the first 2 days following rejuvenation. Free haemoglobin release was lower in units stored in AS‐7 and following washing was partially prevented by rejuvenation.
Conclusions
ATP levels were partially restored following washing and storage in SAGM, and to a greater extent AS‐7, but as expected maximal effect was seen with rejuvenation. Storage in AS‐7 alone reduced free haemaglobin levels and rejuvenation partially attenuated free haemaglobin release following washing in AS‐7 or SAGM. These data suggest that these interventions might attenuate the storage lesion of red cells compared with storage of red cells in SAGM.


P‐221
In Vitro Evaluation of Blood Components Prepared After Centrifugation Using Macopharma Macospin
Rahbek Asmussen MA1, Møller BM 1, Smed TS 1, Ravnsborg PR 1, Agerskov LA 1, Rasmussen LR 1, Collet CC 2, Leconte CL 2, Moisan KM 2
1Aarhus universitetshospital, Aarhus, Denmark2MACOPHARMA, Mouvaux, France
Background
In the blood processing workflow, centrifugation is a critical step that determines the quality of blood components. MacoPharma has recently designed a high‐capacity centrifuge.
The Macospin centrifugation device is intended for the separation of Blood components up to 5000 g from +4°C to +22°C.
Objective
The aim of the study, conducted by the Department of Clinical Immunology in Aarhus, Denmark and MacoPharma, was to evaluate the quality of blood components centrifuged with MacoSpin and to see if components manufactured meet the European and blood center requirements.
Material and methods
450 mL of whole blood was collected in MacoPharma Quadruple Top and Bottom bag system with LCRD2 filter for the preparation of plasma (PLS), buffy‐coat (BC) and Leucoreduced red cell concentrate (RCC).
Blood was stored overnight at ambient temperature. 124 Whole Blood units (test) were centrifuged with the MacoSpin device and 24 Whole Blood units (control) were centrifuged with Heraeus Cryofuge 6000i. Separation performed on MacoPressSmart Revo separator device.
Pool of 4 Buffy‐Coats was performed with PLX‐5 Fresenius‐Fenwal pooling set and 300 ml SSP+ for the preparation of Platelet Concentrate (PC). 31 Pooled Buffy‐Coat (test) were centrifuged with the MacoSpin device and 6 Pooled Buffy‐Coat (control) were centrifuged with Heraeus Cryofuge 6000i.
Samples were taken from Plasma, Buffy‐coat and Red Cell Concentrate prior and post filtration, and then Pooled BC, Waste and Platelet Concentrate. Measurement was performed by flow cytometer and routine haematology equipment.
Throughout the study of the MacoSpin, we have tested four different hard spin programs (1A to 1D) and three different soft spin programs (from 2A to 2C) based on Macopharma advice and agreed by the Department of Clinical Immunology in Aarhus.
*Including 24 Whole Blood centrifuged with up to 50 g imbalance (test) and 36 Whole Blood centrifuged with maximum 5 g (same as control).
**6 Pooled Buffy‐coat centrifuged with up to 50 g imbalance (test) and 10 Pooled Buffy‐coat centrifuged with maximum 5 g (same as control)
***Condition 1D and 2C were the worst conditions tested; they were removed from the study.
****Programs used in routine with max 5 g imbalance.
Results
Figure 2 Macospin and control results
We show results of the best programs used, 1A for first spin and 2B for second spin compared with routinely used centrifuge (control).
Figure 1. Macospin program Table

Figure 2. Macospin and control Results

Conclusion
The MacoSpin is very easy to use with short period of training, and the blood components meet the European and blood center requirements. Compared to routinely used centrifugation programs, it shows even less contamination of white blood cells, red blood cells and platelets in the plasma. Among the multiple programs tested with the device, 1A and 2B show higher quality results.
P‐222
Streamlining the Work Processes for Retrieval of Fresh Frozen Plasma (FFP)
Lim MZ , Chia KK , Su XT , Tan FH , Shu PH , Lam S
Health Sciences Authority, Singapore, Singapore
Background
The Blood Services Group (BSG) Singapore secures the nation's blood supply, ensuring that all patients in Singapore have access to adequate and safe blood. To supply the safest blood and blood components, post donation notification of problems that may affect blood components’ quality, efficacy or safety is an essential process.
Product problem notification (PPN) can be initiated through donor call back, hospital staffs, doctors or BSG staffs. When the BSG receives a PPN, a Product Problem Notification Form (PPNF) will be put up to retrieve all affected blood products in the inventory for quarantine or disposal. Plasma products stored at −35°C often require longer time to retrieve as the current retrieval process is deemed inefficient. As a result of exposure to the extreme cold temperature during retrieval, staffs may be subjected to safety and health concerns.
Aims
Our aim is to use the Lean Six Sigma methodology to review the inventory process for faster and easier retrieval of FFP.
Methods
Retrospective data collected from 1st January to 30th September 2014 was analyzed. By using Lean Six Sigma as a studying tool, 3 possible causes contributing to the current long processing time of FFP were identified and studied. Statistical analysis was performed with SigmaXL software using data of these 3 root causes, (i) blood group of FFP, (ii) types of PPNF received and (iii) days difference between PPNF received and date of collection (DOC).
Results
The statistical analysis showed that the longer the period between the date of PPNF receipt and DOC, the longer the time is taken for the FFP retrieval. The PPNF initiated by the BSG staffs for inventory look back due to reactive infectious disease testing results was also shown to have a longer retrieval time of FFP. There was no significant relationship between the blood group of FFP and the retrieval time (P > 0.05).
A new workflow is proposed to track each FFP in a labeled basket and to create freezer room location mapping of the labeled baskets. A pilot trial involving 136 baskets with 3264 products was conducted using the proposed method. The average processing time was reduced from 131 min −−− to 34.5 min (73.7% reduction) using the re‐defined process.
Conclusions
Overall, with the re‐defined process which track FFP in labeled basket with location mapping, FFP can be retrieved easily and therefore reduce the processing time by 73.7%. The storage of FFP is now better organized and the storage space can be fully utilized.
P‐223
Effects of ‘Super‐Cooling’ By Means of Magnetism on Cryopreservation of Blood Products In Vitro
Hetland GH
Oslo University Hospital, Oslo, Norway
Background
Prolonged preservation of blood products such as erythrocytes and leukocytes without the addition of cryoprotectans like glycerol or DMSO for storage at −80°C or in liquid N2, is of great interest if the time‐ and resource‐consuming washing step after thawing of the cells can be avoided. Moreover, in the blood bank routine, especially easily accessible erythrocyte concentrates that are safely stored beyond the current 30 days at 4°C, is of great value because that would reduce the extra blood production needed due to cassation.
Aims
This was to examine whether a supercooler such as the Magiquoal Supercooling refrigerator which maintains freezing temperatures by under‐cooling without actual freezing of liquids due to a magnetic field, could be used for cryopreservation of blood products.
Methods
SAGMAN‐erythrocytes (SAGMAN‐ery), platelets or leukocytes from buffy coats from healthy donors, and harvested hematopoietic stem cell (HSC) from cancer patients were stored for one or 3 weeks in the Magiquoal Supercooling refrigerator at storage temperature −7°C and in a normal refrigerator at storage temperature 4°C.
Results
Compared with storage in the common fridge, the supercooler was found to give lesser preservation of intracellular K+, but improved preservation of intracellular glucose, lactate, pH and Cl- in SAGMAN‐ery. However, whereas the supercooler seemed to preserve mature leukocytes, it gave lower viability of HSC, and platelets, that are normally maintained at 22°C in slow motion, lost their function in both of the above facilities.

Conclusions
The results indicate an acceptable effect of the Magiquoal Supercooling device on preservation of erythrocyte concentrates for 3 weeks and possibly also of leukocytes, but no advantage on preservation of platelets or HSC.
P‐224
Importance of Time and Removing Buffy Coat in Prestorage Leukocyte Filtration of the Red Blood Cell Concentrates
Ayhan FY1, Oymak Y 1, Karapinar T 1, Aydinok Y 2, Demirkan F 3, Ay Y 1, Vergin C 1
1Dr Behcet Uz Children's Hospital, Izmir, Turkey2Ege University Medical Faculty, Izmir, Turkey3Dokuz Eylul University Medical Faculty, Izmir, Turkey
Background
Human neutrophil elastase (HNE) is one of the bioactive substances released from granules of neutrophils. Increased HNE contamination in red cell concentrates (RCC) indicates neutrophil degranulation that may enhance systemic inflammatory process in the recipients. The HNE contamination in RCC is prevented by leukodepletion.
Aims
In this study, we performed the comparative evaluation of different component preparation methods regarding their impact on the efficacy of pre‐storage leukodepletion by quantification of HNE.
Methods
Leukocyte counting and HNE quantitation were performed on dual samples that were taken at 5th and 15th days of the storage from the RCC produced by three different blood centers operated by unaffiliated institutions.
Blood collection and component production were performed by using different methodology and equipment which include automated whole blood processing system (Atreus 3C, Terumo BCT, USA), top & top (T&T) blood bag system (Macopharma, France), top & bottom (T&B) blood bag system (Kansuk Laboratories, Turkey). The buffy‐coat removal process was performed in RCCs produced by T&B system but not in those produced by T&T system.
Pre‐storage leukodepletion was made by connecting a log4 leukocyte removal filter (Safetran, Safetran BioMedical, Taiwan) at the first 48 h in T&T blood bag system and automated whole blood processing system while the RCC processed with T&B blood bag systems was leukodepleted by connecting a log4 leukocyte removal filter (Bio r01 plus, Fresenius Kabi, Germany) within 24 h. During production and storage, the temperature of RCC was maintained at 4 ± 2°C.
Leukocyte counting was performed by using the Nageotte chamber. For the quantitative detection of HNE, plasma samples separated from RCC were tested with a commercial enzyme‐linked immunosorbent assay (Human PMN Elastase, Platinum ELISA, eBiosicence, Austria).
Results
Totally 54 and 64 samples were tested on 5th and 15th day of storage respectively. Leukocyte contamination was not detected in any samples tested after leukodepletion. The median (range) values of 5th day HNE concentration in T&B blood bag system, T&T blood bag system and automated whole blood processing separation system were 84.66 (10.07–443.87) ng/ml, 888.96 (77.04–1857.56) ng/ml and 1304.68 (354.23–1641.14) ng/ml, respectively (P = 0.0001). The median (range) values of 15th day HNE concentration in T&B blood bag system, T&T blood bag system and automated whole blood processing separation system were 95.07 (2.01–1584.85) ng/ml, 617.65 (219.04–1806.01) ng/ml and 689.33 (2.70–1584.32) ng/ml, respectively (P = 0.0001).
Conclusions
In this study, 5th and 15th day HNE concentrations were significantly lower in RCC of T&B blood bag system than that of other two systems. There weren't significant difference between HNE levels of RCC of T&T and automated whole blood processing separation systems on the 5th and 15th days of storage. The HNE contamination was almost 6 times higher in the T&T blood bag than the automated whole blood processing separation systems compared to the T&B blood bag system. Clinical significance of presented results are remained to be determined.
P‐225
Effects of Prestorage Leukoreduction on the Rate of Febrile Nonhemolytic Transfusion Reactions to Red Blood Cells
Kumar RK
Dayanand Medical College and Hospital, Ludhiana, India
Background
Febrile nonhemolytic transfusion reactions (FNHTRs) are relatively common complications associated with allogenic transfusion. White Blood Cells (WBCs or leukocytes) are considered to be an important cause of FNHTRs; the rate of WBC derived pro‐inflammatory cytokines increase with storage due to active synthesis of cytokines by these cells.The removal of the WBCs before storage will prevent the accumulation of cytokines during storage that leads to a reduction in the number of FNHTRs.
Aim
We have conducted a retrospective analysis comparing the rate of FNHTRs in prestorage leukoreduced and non leukoreduced RBCs transfusion.
Methods
A retrospective review of all the transfusion reactions (TRs) reported to the department over a period of two years from July 2012 to June 2014 was done. Patients were stratified by the date of reaction and by component received and then divided into two groups: (1) Patients who received allogeneic prestorage leukoreduced (PrSL) RBCs and (2) nonleukoreduced RBCs. All RBC units, both leukoreduced and non leukoreduced were prepared in our component laboratory. For the PrSL RBC units, leukoreduction was performed by using buffy coat method of component preparation by quadruple bags and integral bags containing Sepacell® Pure RC filter. (Manufactured in France by Fenwal ™ France and imported and marketed in India by Fenwal ™ India. Nonleukoreduced RBCs contain >109 WBCs. Leukoreduction by centrifugation and removal of buffy coat depleted RBCs give a log1 reduction (70%–80%) of leukocytes in the unit (<5 × 10⁸). Prestorage Leukoreduction by Fenwal disposal: Sepacell® Pure RC filter from ASAHI produce a 2 to 4 log reduction (99–99.9%) of the WBCs (<5 × 10 6). Patients were not stratified on the basis of diagnosis, by inpatient or out‐patient status at the time of the reaction. All data was categorized on a monthly basis. Reactions rates were calculated by dividing the number of reactions to each type of RBCs component by the total number of RBCs of that type transfused.
Results
37,232 RBCs units were transfused and out of which 14,149 (38%) were pre storage leukoreduced and 23,083 (62%) were non leukoreduced. A total of 142 (0.38%) transfusion reactions were reported during that time period, of which 62 (0.17%) were classified as FNHTRs. In the nonleukoreduced group 124 TRs were reported, of which 55 were classified as FNHTRs to RBCs and the overall rate of FNHTR to RBCs was 0.24%. In pre storage leukoreduced group, 18 TRs were reported, of which 7 were classified as FNHTRs to RBCs and the overall rate of FNHTR to RBCs was 0.05% (P ≤ 0.001). This represents a significant reduction in the rate of FNHTR after institution of prestorage leukoreduction
Conclusion
The rate of FNHTRs to allogenic RBC units after the implementation of pre‐storage leukoreduction has decreased significantly. Cytokines and chemokines accumulating during storage of cellular blood products are responsible for residual FNHTRs.
P‐226
Evaluation of CD41/CD61 and CD42b Platelet Receptors and Clotting Assay of Platelet Factor 3 During Long Term‐Storage of Platelet Concentrates
Nasiri S
High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
Background
Platelets play a key role in hemostasis, the process of stopping bleeding at the site of interrupted endothelium by adhesion and aggregation mechanisms. Platelet adhesion is mediated by GP Ib (CD42b) receptor which binds with von Willebrand factor (vWF) to collagen. Aggregation of platelets occurs as a result of functional GPIIb/IIIa (CD41/CD61) receptor which allows these receptors to bind with vWF or fibrinogen.
Aims
The purpose of this in vitro study was to evaluate the effects of long term storage of conventional platelet concentrates (PCs) on major platelet receptors CD42b and CD41/CD61 respectively by flow cytometry method and overall platelet procoagulant activity status with platelet factor 3 (PF3) assay.
Methods
Six random units of conventional platelet concentrate were prepared and after collection and resting period, they were placed in a standard platelet incubator with under continuous agitation at 22–24°C for eleven days. Samples of each PC unit were taken on 1, 5 and 11 days after their preparation. Two types of samples; one pooled sample for flow cytometry analysis and the individual samples for PF3 assay; were prepared and tested.
Results
Flow cytometric analysis for CD41, CD61 and CD42b were found 99.8%, 94.8% and 28% on the first day and reached to 68.3%, 75.1% and 3.4% respectively at the end of the storage period. PF3 assay were observed 95.0% and 41.7% at the beginning and end of storage period respectively.
Conclusions
It was concluded that platelet storage lesion (PSL) can occur during long‐term storage of platelets with a nearly constant slop and in spite of lower expression of CD42b, higher expression levels of CD41/CD61 and platelet procoagulant activity of PF3 were observed at the end of the storage period that confirm platelet procoagulant properties are moderately preserved for eleven days. Further investigations are required to improve quality of platelet concentrates to lower PSL such as change the preparation process, storage conditions or WBC removal.
P‐227
Structural and Functional Changes Associated With the Development of the Platelet Storage Lesion
Rijkers M
Sanquin Blood Supply, Amsterdam, The Netherlands
Background
Platelet products are stored at room temperature for a maximum of 7 days due to the risk of bacterial growth. During storage, platelets undergo modifications which alter the function and structure of platelets, called the platelet storage lesion (PSL). The von Willebrand Factor (VWF) receptor, especially glycoprotein Ibα (GPIbα), has been implicated in survival of platelets in a variety of murine models. Based on these findings it has been suggested that part of the PSL is due to loss of sialic acid on GPIbα. Whether sialic acid content of GPIbα also declines during storage of human platelets has not yet been addressed. Here, it was studied whether the loss of sialic acid on GPIbα contributes to the PSL of human platelets.
Aims
The aim of this study is to further characterize the PSL, focusing on the role of sialic acid on GPIbα in this process.
Methods
Platelets were stored in plasma under standard blood bank storage conditions. At different time points (t = 1, 2, 5, 7, 9, 13 and 16 days) ristocetin‐induced binding of VWF to platelets was determined. Additionally, the membrane expression of sialic acid, GlcNAc, GPIbα, GPV, GPIX, CD62P and annexin V was determined using flow cytometry.
Results
Up to day 9 no decline in surface‐staining of GPIbα and GPV was observed. Also the ristocetin‐induced binding of VWF to platelets declined at this time point. Unexpectedly, loss of sialic acid was not observed in platelets stored in plasma. Gradually increase of CD62P exposure indicates a continuous release of α‐granules during storage. A gradual increase in number of annexin V positive cells was also observed after prolonged storage.
Conclusion
Mouse studies showed a critical role of sialic acid on GPIbα for survival of platelets in vivo. Our in vitro experiments, where platelets were stored under standard blood bank conditions in plasma, revealed no loss of sialic acid. Also, GPIbα‐shedding was only observed upon prolonged storage for over 9 days. Overall, our results indicate that loss of sialic acid and/or GPIbα does not occur upon storage of platelets stored in plasma under blood bank conditions.
P‐228
A Novel Leukocyte Reduction Filter, Sepacell R‐S11, For Red Cell Concentrates Prepared By TB and TT Methods
Yokomizo T , Kai T , Yajima K , Fukuda T
Asahi Kase Medical, Oita, Japan
Background
Leukocyte reduction is widely used to reduce the risk of non‐hemolytic febrile transfusion reactions and alloimmunization by multiple blood transfusions. Around and after year 2000, universal leukocyte reduction was adopted in many developed countries. There are mainly two types of filter, one to filter whole blood and the other to filter red cell concentrates (RCCs) used during pre‐storage blood processing.
Aims
In this study, we conducted the tests with the leukocyte reduction filter, Sepacell™ R‐S11 for RCCs, in the residual leukocyte counts, hemoglobin contents per unit and filtration time, when filtering RCCs prepared by both top‐and‐bottom (TB) and top‐and‐top (TT) methods.
Methods
Six units of RCC were individually prepared from 500 ml of whole bloods with 70 ml of CPD solution stored at 22 ± 2°C for one day, by centrifugation with 3931 g‐force at maximum for 28.5 min in total and plasma / buffy coat (BC) removal by the TB method. Other six units of RCC were individually prepared from 500 ml of whole bloods with 70 ml of CPD solution stored at 22 ± 2°C for 8 h, by centrifugation with 2621 g‐force at maximum for 17.3 min in total and platelet rich plasma (PRP) removal by the TT method. Filter priming was performed with 110 ml of SAGM solution from filtrate bag in the opposite direction to RCC filtration, and then SAGM solution was combined with individual pre‐filtration RCC. After mixing, RCCs were filtered at 110 cm head height between pre‐filtration and filtrate bags.
Results
The test results are summarized in the table 1 and 2 below.

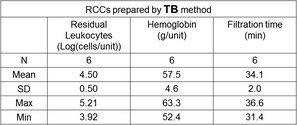
Conclusion
This study preliminarily shows the capability of Sepacell™ R‐S11 as the pre‐storage leukocyte reduction filter for RCCs prepared by TB and TT methods. Further evaluation should be performed in the larger scale including the post‐storage RCC quality parameters.
P‐229
Different Agitation Speed for Concentrated Platelets – Does It Matter?
Libek VL , Strugar AS , Milicev MM , Kulic AK , Djurdjevic JDJ , Gojkovic IG
CHC Zemun Belgrade, Belgrade, Serbia
Background
Platelets have an important role in hemostatic function of blood. Therefore, after collecting, they need to be properly stored in platelet incubator with agitator. The main role of agitator is to store platelet concentrates (PC) in continuous horizontal motion at a specified temperature (+22°C), ensuring no platelet clumping and maximum viability of platelets. Usually, stable frequency of agitator is 60 oscillation per minute (+2;−3), which maintain high level of quality and platelet number in every PC.
Aim
In order to determine effect of different agitator frequency on quality of PC, we measured platelet number in PC during 5 days of storage.
Methods
Single PCs were processed by separating components from whole blood units using LMB Novomatic separation device. Thirty PC were selected for measurement during 5 days. Thermo Scientific Forma Incubator was used for PC storage, as well as two LMB agitators on frequency of 60 and 40 oscillations/min. After counting the number of platelets on starting (0) day, every PC was divided in separate bags for measurement on different agitators, using sterile connection. Counting of platelets was continued on the fifth day of storage on both agitator frequencies. Data were processed using standard statistical methods.
Results
On day 0, average number of platelets in 30 PC was 1453.1 × 109/l (87.2 × 109/unit). On the first day of measurement, main platelet value in PC stored on 60 oscillations/minute was 1410.8 × 109/l (84.6 × 109/unit) and on 40 oscillations/min it was 1225.5 × 109/l (73.5 × 109/unit). At the end of five days storage, the average numbers of platelets in PC on agitator with 60 oscillations/minute was 1238.8 × 109/l (74.3 × 109/unit), in PC on agitator with 40 oscillations/minute the average platelet number was 1145.1 × 109/l (68.7 × 109/unit). There was high statistical significance in platelet number stored on different frequencies, in favor of 60 oscillations/minute speed on first day of counting (t = 138.3, P < 0.01), as well as on the fifth day (t = 9.14, P < 0.01).
Conclusion
Platelet number decreases on the fifth day of storage, both on faster and slower agitator. However, there is strong evidence that correct agitation frequency does matter for proper PC quality and the speed of 60 oscillations/minute should be used for adequate storage terms.
P‐230
Experimenting a New System for Whole Blood Processing
Gabriele AG , Poggi SP , Golini DG , Granata GG , De Felice MD , Propato MFP , Piccoli RP , Velati CV
Ospedale Maggiore, Bologna, Italy
Background
Recently a new system for whole blood processing (TACSI WB – Terumo) that automates centrifugation and separation of red cells, plasma and buffy coat in one solution has been introduced. Moreover, each device can produce 6 platelet concentrates from pooled buffy coats.
Aims
The aim of the study is verifying if this device maintains or improves the quality of blood components currently produced by the Immunohaematology and Transfusion Unit, optimising workflow, saving space and reducing manual steps. For platelet concentrates, the aim is verifying the actual opportunity of obtaining so many products.
Methods
At the Immunohaematology and Transfusion Unit, 2 TACSI devices (produced by TerumoBCT), including the accessories for all the steps of the process, have been installed.
As for a start, a collection of 380 blood units has been organised, with the aim of gradually increasing the number up to the current throughput. An appropriate number of quality controls have also been arranged.
365 kits were processed; of these, 5 have been discarded for technical reasons. 78 units have been checked, performing blood counts on whole blood, red cells and plasma; in 59/78 units cytofluorimetric assay for residual white cells has been performed. 20 platelet concentrates from 5 pooled buffy coat each have been produced; quality control analysis before and after concentration have been performed in all of them. Test results have been compared with the average values of the current blood production (Fresenius).
Results
The table A shows the values of the prestorage leukoreduced red cells (Fresenius/Terumo)

The table B shows the values of the platelet concentrates (Fresenius/Terumo)
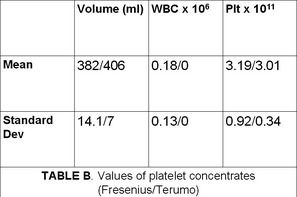
Conclusions
With the device TACSI WB, product standards have proven to be comparable, and in some cases superior, to those currently achieved. No substantial advantage over the present process has been observed as far as the time spent on the single procedure is concerned; nevertheless, increasing the number of procedures and optimising workflow, a shortening of the time spent in processing is obtained.
The biggest advantages that have been observed, however, are the reduction of the number of manual steps, the need for less devices in the unit, and the preparation of platelet concentrates that is dramatically streamlined.
P‐231
Evaluation of the TPL IMUFLEX CRC Set Including the New Sepacelltm R‐S01 Leukoreduction Filter for Red Blood Cell Units
Störmer M , Radojska S , Gathof BS
Transfusion Medicine, University Hospital of Cologne, Cologne, Germany
Background
Leukoreduction can reduce the number of non‐haemolytic febrile transfusion reactions, as well as the occurrence of alloimmunization in patients receiving multiple transfusions. Leukoreduction involves the removal of white blood cells (WBCs) by use of filter and may be performed either at the time of manufacture (pre‐storage) of red blood cell concentrates (RBCs), or concurrent with administration (post‐storage) of RBCs. In Germany the upper limit of residual WBCs in RBCs is ≤1 × 106/unit.
Aims
In this study we evaluated the suitability of the TPL IMUFLEX CRC blood collection set (TerumoBCT) including the new integrated Sepacell™ R‐S01 leukocyte removal filter for RBCs (Asahi Kasai Medical) for routine production and storage according to the German authorities requirements.
Methods
Whole blood (WB) donations of 500 ml were collected in CPD after donors gave informed consent. Separated RBCs in SAGM were filtered at room temperature through the Sepacell™ R‐S01 filter between 2 and 4 h after WB collection and stored under usual conditions for 49 days. Quality parameters including volume, red cells, residual leukocytes and platelets, haematocrit, haemoglobin, %haemolysis, ATP, free potassium, and visual inspection, were assessed so far until day 34, but data will continue to be collected until day 49. Finally sterility will be assessed at the end of storage.
Results
Overall 35 WB donations were performed on four different days. RBCs (n = 35, volume 299 ± 22 ml) that were produced 1‐h post‐collection showed normal values of haemoglobin (57.84 ± 5.52 g/unit) and haematocrit (59 ± 3%) at the day of collection. The percentage haemolysis also showed normal values at the day of collection (0.10 ± 0.05%, n = 35) and during storage so far (0.12 ± 0.03% on day 27, n = 22; 0.13 ± 0.03% on day 34, n = 11). Moreover the residual leukocyte count was below 1 × 106/unit (0.005 ± 0.014 × 106/unit, n = 35) in all produced units after leukocyte filtration (filtration time 41 ± 12 min).
Summary
The suitability of the new TPL IMUFLEX CRC blood collection set including the Sepacell™ R‐S01 leukocyte filter was evaluated for RBC production in our Institute. Up to now all quality parameters fulfilled authorities requirements. In conclusion, the whole system evaluated in the present study has proven to be a reliable and efficient WBC‐reduction system that consistently retains high‐quality results of RBCs.
P‐232
Hemolysis in In‐Date Red Cell Concentrates
Sweeney JDS 1, Gultawatvich G2, Tavares MF 2, DiQuattro PJ 2, Cheves TC 2
1Miriam Hospital, Providence, United States of America2Roger Williams Hospital, Providence, United States of America
Background
Hemoysis in liquid stored red cell concentrates is an important measure of quality, is known to increase with storage age and is a reflection of the red cell storage lesion. Regulatory agencies require that the percent hemolysis (PH) in systems licensed for red cell storage not exceed 1% (FDA) or 0.8% (EU) at the end of the storage period, typically 42 days. Furthermore there is on‐going concern that ‘older’ red cells exert a clinical deleterious effect, without clear definition of ‘fresh’ vs ‘older’ and hemolysis implicated by some investigators as the mediator of such an effect. Determination of the percent hemolysis is not routinely performed on in‐date products because it requires sampling which will break sterility. Percent hemolysis is largely determined by supernatant hemoglobin (SHb).
Aims
We sought to examin SHb and PH in a population of prestorage leukoreduced red cell concentrates at various times during their liquid shelf life in order to understand whether differences between products of different storage age would be associated with a material difference in SHb.
Methods
The red cell concentrates were removed from the refrigerator immediately prior to dispensing. After mixing, a sample was removed aseptically, one aliquot used for hemoglobin concentration and hematocrit measurement in a Horiba ABX‐MICROS 60 (Horiba Medical, Irving, CA) and another aliquot hard spun with removal of the supernatant. The hemoglobin concentration in the supernatant was measured using a three wavelength (562 nm, 578 nm and 598 nm) photometric method. The PH was measured from the total hemoglobin, the SHb and hematocrit. Data were tabulated as descriptive statistics and analyzed using non‐parametric tests. Correlation was Pearson's r.
Results
Of 218 red cells were sampled between day 7 and day 39 of liquid storage. Overall storage age showed a statistically significant correlation with PH (r = 0.26, P < 0.01) and SHb (r = 0.27 P < 0.01), as expected, but the strength of this association is weak. Data (median, range in parentheses) for the in date products stratified by arbitrary storage age categories is shown in the table. Although a statistically significance difference is present between categories (H = 21.3, P < 0.05), median SHb and PH differ only slightly.
Caption 1: Hemolyis in red cell concentrates stratified by storage age

Conclusion
There is considerable overlap in hemolysis even between ‘fresh’ (<10 days) and ‘older’ (>21 days) red cells. If hemolysis is a surrogate marker for any clinical deleterious effect of ‘older’ red cells, randomization using storage age in a univariate analysis might not be expected to show any clinical difference which may explain the absence of a storage age effect as reported in the recent ARIPI and RECESS studies.
P‐233
Analysis of RBC Transfusion Demands at a Tertiary University Hospital in Greece
Zervou EZ , Batsi CHB , Ziciadis ZK , Xanthi XE , Tzolou TA , Kavvadias SK , Tzoka TT
University Hospital, Ioannina, Greece
Background
Understanding of the clinical usage of RBC is important in transfusion practice improvement and planning for blood supply requirements.
Aim
Analysis of RBC transfusion demands by the clinic, blood group, storage time before transfusion and specific processing requirements (pre‐storage leucoreduction, irradiation, washing), at the University Hospital of Ioannina, Greece. We also analyzed the RBC transfusion demands for patients with autoantibodies or alloantibodies and the administration of Rh D + units in Rh D – patients and vice versa.
Material and methods
We used the electronic files in our Blood Bank (GI‐Blood IT system) during the year 2013.
Results
In this year 18,625 units were cross‐matched. 10,817 RBC units were transfused, 4391 (41%) of them leucoreduced. 258/10,817 (2%) units were irradiated and used for neonates and transplanted haematological patients. 722/ 1407 (7%) RBC units washed and the most of them used for thalassaemia and sickle cell anaemia patients. 6870/10,817 (63.5%) RBC units consumed in the Internal Medicine sector, 3110/10,817 (28.7%) in Surgical sector and 837/10,817 (7.8%) in ICUs for neonates, adults and cardiac surgery patients. The most of RBC units were used in: Thalassaemic (2397, 22%), Haematology (2003, 19%), Oncology (989, 9%), Internal Medicine clinic (957, 9%), Orthopedic (965, 9%), Cardiac Surgery (773, 7%), Surgery (706, 7%) and Urology (338, 3%).
The blood group distribution of RBC transfusions was: A + 3.345 (31%)/A‐517 (5%), Ο + 4.163 (38%) / O− 554 (5%), B + 1279 (12%) / B− 316 (3%), AB + 539 (5%) / AB‐104 (1%). The average storage time of transfused RBCs collected in our Blood Bank, by blood group was: A + 10 days, A−15, O + 8, O−17, B + 19, B−12, AB+21, AB‐17, while for RBCs imported from other centers it was A + 27 days, A− 32, O + 27, O – 35, B + 33, B‐26, AB+ 34, AB− 24.
50 patients received 691 RBC units with specific antigenic profile because of auto‐ and/or alloantibodies. 11 Rh D – patients received 24 Rh D + units. 106 Rh D + patients received 145 Rh D – units. 5/106 patients received 22/145 Rh D – units since they had D autoantibody or because of their phenotype, 123/145 units were imported mainly from other blood centers and used for Rh D + patients to avoid discard due to their expiration day. 46 patients (14A, 27AB, 5B) received 95 RBC units with different blood group (32 O, 43 B, 20 A). 64/95 units were sent from other centers on their last expiration day.
Conclusions
The greatest needs for RBC transfusion in our hospital are for patients with thalassemia and sickle cell anemia and also for hematological and oncologic patients. A better collaboration between domestic blood centers is necessary for the optimal use of blood components.
P‐234
Distribution of Abo and Rh Blood Groups in Turkish Patients
Uzun B , Güvel H , Güngör S , Sengel Ö
Izmir Katip Celebi University, Ataturk Training and Research Hospital, Izmir, Turkey
Background
ABO blood group system is defined by showing the presence of A and B antigens in erythrocytes and anti‐A and anti‐B antibodies in serum. The antibodies against A and B antigens in erythrocytes are produced in the first years of life as a response to environmental antigens (such as bacterial, viral or herbal antigens). It is important to type donor and recipient erythrocyte antigens, and determine and identify the antibodies produced against these antigens in order to provide transfusion safety.
Aims
In this study, it was aimed to investigate the blood group distribution of our patients who were treated in our hospital.
Methods
The blood group distribution of the patients in Izmir Katip Celebi University, Ataturk Training and Research Hospital were analyzed retrospectively using hospital database between January 2011 and December 2014. Blood groups were tested by manual and automatized gel centrifugation method. Biovue System (Ortho Clinical Diagnostics, France), DG gel System (Diagnostic Grifolls, Spain) and Across gel System (Across gel, DiaPro Medical Products, Turkey) kits were used in 2011, 2012 and 2013, and 2014, respectively.
Results
Blood group of 115.755 patients were studied in that time. 40% (45.799) of the patients were male while 60% (69.956) were female. The blood groups according to the years were shown in table.
Caption 1: The blood groups according to the years
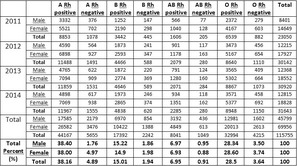
Conclusion
The blood group distribution of our patients were monitored parallel to data of our country. It was considered that the results would contribute to formation of a database for blood group distribution.
Keywords
ABO groups, Rh groups, blood groups.
P‐235
Quality Control of the Apheresis Platelet Concentrates and Collection Efficiency Using AmicusⓇ Cell Separation Platform
Robles de Vasconcelos FPRV , Freixo AMPF , Lopes MMFLA , Carvalho MC , Trigo RT , Gonçalves LBFRG , Regalado GR , Araújo FA
CH São João, Porto, Portugal
Background
The quality of a blood component is crucial in the guarantee of safe transfusion practices. The use of platelets (PLT) collected by apheresis has increased in the last years all over the world, due to its great benefits over random‐donor PLT, especially in patients with repeated platelet transfusion needs. The São João University Hospital is the biggest hospital in the north region of Portugal and its Blood Bank collected a total of 722 Apheresis Platelet Concentrates (APCs) in 2014.
Aims
The aim of this study was to evaluate the results of both the quality control (QC) tests and collection efficiency during the year of 2014.
Material and methods
Our apheresis platelet program includes a multidisciplinary working team formed by doctors and specialized nurses. The APCs are collected by 3 AMICUS® Cell Separator Platforms. Quality control testing parameters (volume, platelets and leukocyte count and pH at the end of shelf life) were done according to the European Council and the Portuguese laws. The collection efficiency (CE) was calculated as follow: CE = total PLT yield (1011) × 100/(pre‐apheresis PLT count + post‐apheresis PLT count)/2) × blood volume processed.
Results
The units tested meet the criteria defined by the Portuguese laws and by the Council of Europe recommendations (Table 1). 95% of the units evaluated showed an efficiency of >55% and 75% an efficiency of >70%.
Caption 1: Results of the blood units’ quality control and collection efficiency

Conclusions
A quality system and control is essential to provide safe blood components. Quality control of apheresis PLT collected in 2014 by AMICUS® Cell Separator Platform fully met the criteria defined by the Europe Council and the Portuguese laws. The capacity to achieve the best quality blood components with the least waste of time, blood volume processed and platelets loss by the donor is also of great importance for the blood bank. A mean collection efficiency of 74% in 2014 reflects not only the collection method itself but also the quality of our apheresis program.
P‐236
Evolution of Utmost Vital Emergency (UVE) Blood Transfusion from 2010 to 2014 in the French Establishment of the Blood Centre Atlantique
Sapey TS1, Lele IL 1, Jutant JT 2, Py JYP 3, Dehaut FD 4
1Etablissement francais du sang, Chateauroux, France2Etablissement francais du sang, Poitiers, France3Etablissement francais du sang, Orléans, France4Etablissement francais du sang, Tours, France
Background
Evolution of Utmost Vital Emergency (UVE) blood transfusion represents significant activity of delivery of labile blood Products (LBP).
Aim
The purpose of our study is to assess the part which represents UVE in comparison with all deliveries and its evolution from 2010 to 2014.
Methods
We analyzed from 2010 to 2014 all the deliveries of LBP in UVE to all establishments of blood transfusion (12) belonging in the French Establishment of the Blood Centre Atlantique in comparison with complete delivery.

Results
All results are in the table 1. On average over 5 years, the delivery in UVE represented 3.6% complete deliveries. The delivery in UVE of Red Blood Cells (RBC) and of platelets over 5 years was on average 2.8% for RBC and 3.9% for platelets in comparison with complete delivery but with a difference between every establishments of blood transfusion (RBC: 0.6–5.7%;platelets: 0–8.9%). There was a significant difference on evolution between 2010 and 2014 for RBC (P < 0.001) unlike platelets (P = NS). Delivery in UVE for plasma over 5 years was on average 11.2% with a significant difference between 2010 and 2014 (P < 0.001).
Conclusions
On average the delivery in UVE in the French Establishment of the Blood Centre Atlantique, all LBP was 3.6% over 5 years of our study. Plasma being most delivered LBP. There is a significant difference of the evolution of request between 2010 and 2014 concerning RBC and plasma reflecting the transfusion pack (ratio RBC / plasma) during massive blood transfusions.
P‐237
Abstract Withdrawn.
P‐238
Status of Additive Solution 7
Hess R
University of Washington School of Medic, Seattle, United States of America
Background
Red blood cell (RBC) additive solution 7 (AS‐7) is a second generation AS composed of bicarbonate, adenine, glucose, phosphate, and mannitol. Also known as experimental additive solution 81 (EAS‐81) it is now CE marked for 8‐week RBC storage after an overnight warm hold in Europe and approved in the US for 6‐week storage following processing with 8 h of collection. Additional testing has now been performed and submitted to the US Food and Drug Administration (FDA) seeking permission for processing after a 24‐h warm hold with 6‐week storage.
Aims
To review the background and current status of AS‐7.
Methods
Data from the developmental ex vivo and in vivo studies, initial licensure studies, and expanded indication studies will be reviewed to describe the mechanism of action and effectiveness of AS‐7 to function as an improved RBC storage medium. Evidence that bicarbonate buffering improves energy flux by increasing the buffer range and capacity to reduce eryptosis in stored RBC will be presented.
Results
RBCs stored in AS‐7 with bicarbonate/phosphate buffering show increased metabolic flux with greater glucose consumption and lactate production while having less decline in pH. This results in higher concentrations of intracellular adenosine 5′‐triphosphate (ATP) at all time‐points, higher 2,3‐diphosphoglycerate (DPG) in the second and third weeks of storage, and lower hemolysis and less microvesiculation at the end of storage than seen with first generation additive solutions. In vivo 24‐h 51Cr RBC recovery studies have now been conducted in more than 100 volunteers with mean recoveries of 86% after 24‐h room temperature hold and 6 weeks of storage at 1–6°C refrigerated storage.
Summary/Conclusions
AS‐7 is a robust new RBC additive solution that supports RBC metabolism and reduces RBC injury in the course of storage. This leads to more potent units, frequently with higher concentrations of ATP and DPG, and the presentation of fewer effete and hemolysed RBCs and microvesicles with each transfusion episode. The solution is also compatible with extended storage of RBC to at least 8‐weeks in times of national emergency.
3.2 Blood Components
P‐239
Von Willebrand Factor is Bound to Platelets in Apheresis Concentrates with Persistent Aggregates
Compernolle V , Devloo R , Feys HB , Vandekerckhove P
Belgian Red Cross‐Flanders, Ghent, Belgium
Background
Persistent aggregates (PA) in platelet concentrates are defined as particulate material that is visible to the naked eye following component preparation and that is persisting following a resting period, addition of additive solution and storage on an orbital shaker. In Flanders, 3.6% of platelet apheresis concentrates (Trima Accel®, TerumoBCT, CO) contain PA and are quarantined as a consequence. We recently compared PA to aggregate‐free (AF) concentrates (n = 180) and found a clear donor‐dependence. Furthermore, platelets in the PA containing concentrates were more sensitive to low doses of ristocetin in a light transmission aggregation experiment (day 6) indicating subtle differences between PA and AF platelets in von Willebrand factor (VWF) and/or the GPIb‐IX‐V platelet receptor (interaction).
Aims
Platelet adhesion and agglutination requires GPIb‐IX‐V to interact with VWF under shear forces which are present during apheresis. Moreover, VWF and GPIb‐IX‐V are highly polymorphic molecules allowing speculation for the observed donor‐dependency. We therefore hypothesized that these binding partners play a role in the establishment of PA under the conditions of apheresis.
Methods
We analyzed VWF‐GPIb‐IX‐V interactions under hydrodynamic flow on days 1 and 6 post donation using real time video microscopy and microfluidic flow chambers. VWF protein characterization was with flow cytometry and immunosorbent assays.
Results
VWF antigen levels were 6 μg/ml on average in both PA and AF concentrates (n = 13) and not significantly different. Despite normal protein levels, VWF activity can differ and therefore we performed immunosorbent assays measuring VWF binding to collagen and activated VWF using the conformation‐specific nanobody A11. Both parameters were similar among PA and AF concentrates. GPIb expression was determined in flow cytometry but was not significantly different. Next, we measured real‐time binding of fluorescently labeled platelets to VWF under hydrodynamic flow and found that there was a significant decrease in surface coverage in PA concentrates compared to AF controls on day 6. To determine whether this was a consequence of storage, we performed the same experiment on day 1 using newly collected samples and indeed found no such difference. Overall, these data indicate increased storage lesion for this particular parameter in platelet concentrates with PA compared to AF controls. However, this increase could still be a consequence of subtle differences in GPIb‐VWF since we found significantly more VWF bound to platelets on day 1 using fluorescently labeled anti‐VWF antibodies and flow cytometry. Moreover, the increased P‐selectin expression and cytokine release found on day 6 as published previously was also found on day 1 indicating that both degranulation and VWF binding are early effects of PA formation.
Conclusion
We found an increased lesion on platelets binding to immobilized VWF under flow in concentrates with PA. We hypothesize that primary binding of VWF to platelets increases cellular activation and granule secretion.
P‐240
Transfusion of CMV Seronegative T‐Deplete Allogeneic Stem Cell Transplant Recipients with CMV‐Unselected Blood Components Results in Zero CMV Transmissions in the Era of Universal Leucocyte‐Reduction: A UK Dual Centre Experience
Hall SM1, Danby R 2, Osman H 3, Peniket A 2, Rocha V 2, Craddock C 3, Murphy MF 1, Chaganti S 3
1NHS Blood and Transplant, Oxford, United Kingdom2Oxford University Hospitals, Oxford, United Kingdom3University Hospital Birmingham, Birmingham, United Kingdom
Background
Cytomegalovirus (CMV) infection remains a major cause of morbidity and mortality following allogeneic stem cell transplantation (SCT). CMV‐seronegative SCT recipients are particularly at risk of transfusion‐transmitted CMV (TT‐CMV), and until recently it has been recommended that they should receive blood components from CMV‐seronegative donors with significant resource implications. Increasing availability of precise and reliable CMV PCR assays has improved the ability to detect infection. Similarly, leucocyte‐reduction technology has advanced significantly over the last 20 years. Many UK centres now routinely provide CMV‐unselected (CMV‐U) products to CMV seronegative patient/ CMV seronegative donor (CMV neg/neg) allogeneic SCT recipients due to universal leucocyte‐reduction in the UK, along with routine CMV PCR monitoring.
Patients undergoing T‐cell depleted SCT are especially at risk of CMV infection. However, no recent studies examining TT‐CMV rates with transfusion of CMV‐U in CMV neg/neg recipient/donor pairs specify details of transplant conditioning or T‐cell depletion.
Aims
We set out to establish the rate of CMV transmission when CMV‐U, leucocyte‐reduced blood components are transfused to CMV neg/neg SCT recipients, the majority of whom have undergone T‐cell depletion.
Methods
We retrospectively analysed the incidence of TT‐CMV in CMV neg/neg allogeneic SCT recipients transfused with CMV‐U, leucocyte‐reduced blood components in two transplant centres in the UK. Patients were monitored for CMV infection by at least weekly, quantitative CMV PCR testing. Leucocyte‐reduction of blood components was in accordance with current UK standards i.e. pre‐storage leucocyte depletion resulting in >99% units containing <5 × 106 leucocytes, with 95% confidence.
Results
Among 76 CMV neg/neg transplants undertaken following the change to provision of CMV‐U components, 59 (76.6%) underwent in‐vivo T‐depletion, either with alemtuzumab (81.4%) or anti‐thymocyte globulin (18.6%). The most common indications for transplant were acute myeloid leukaemia (26.3%), non‐Hodgkin lymphoma (15.8%), myelodysplasia (10.5%) and Hodgkin lymphoma (10.5%). The majority of patients underwent reduced‐intensity conditioning (82.9%) and stem cell source was peripheral blood stem cells in 96.1%.
No episodes of CMV infection were detected in any of the 76 patients, with median follow‐up 22 months (range 3–46 months). Patients were transfused with 1442 CMV‐unselected, leucocyte‐reduced components equating to 1862 donor exposures during the study period. T‐deplete patients were transfused a total of 1237 components from 1585 donors.
Summary
We detected no episodes of CMV transmission in 76 CMV‐neg/neg allogeneic SCT patients closely monitored with qPCR testing. This is the first attempt at establishing the safety of leucocyte reduction as a strategy to prevent TT‐CMV in high‐risk allogeneic stem cell transplant patients undergoing T‐cell depletion with none of the currently published studies specifying infection rates in this patient group. Our data provide strong reassurance that routine use of CMV‐unselected blood components does not result in a significant risk of CMV transmission in SCT patients including those undergoing T‐deplete transplants.
P‐241
A Non Radioactive Method to Determine Recovery and Survival of Transfused Platelets
van der Meer PF1, Slichter SJ 2, Tomson B 1, Fitzpatrick L 2, Christoffel T 2, Bailey SL 2, Pellham E 2, Jones MK 2, Corson J 2, de Korte D 1, Claas FHJ 3, Mulder AM 3, Brand A 4
1Sanquin Blood Bank, Amsterdam, The Netherlands2Puget Sound Blood Center, Seattle, United States of America3Leiden University Medical Center, Leiden, The Netherlands4Sanquin Research, Leiden, The Netherlands
Background
For approval of new platelet products, radiolabeled autologous platelet recovery and survival measurements of both the ‘test’ platelets compared to the same subject's fresh platelets are required. However, a more relevant model might be to evaluate the ‘test’ platelets compared to an approved platelet product in the intended recipients; i.e., thrombocytopenic patients. Polymorphism of HLA antigens could be used to distinguish transfused ‘test’ donor platelets from any other platelets circulating in the recipient. We validated a panel of human monoclonal HLA‐class I antigen specific antibodies for expression on platelets aiming to discriminate between different (transfused) platelet populations. We compared the HLA tracking approach with the current standard 51Cr radiolabeling method.
Methods
Five patients were enrolled to receive a purposely HLA mismatched apheresis platelet transfusion to test 3 different HLA monoclonal antibodies. A 10 ml radiolabeled aliquot, as well as the remainder of the unlabeled apheresis platelets were transfused. A pre‐transfusion, as well as post‐transfusion samples were obtained at 1 h and twice daily on days 1, 2 and 3.
Post‐transfusion donor platelet recoveries and survivals were determined by (i) radioactive assays and (ii) platelet counts in combination with flow cytometric detection of an Alexa‐488‐labeled monoclonal antibody bound to the donor's incompatible antigen.
Results
Recoveries in all 5 patients were suitable for analysis, survival calculations in 4 patients only, since in one patient, 2 additional platelet transfusions were given on the same day. Platelet recoveries ranged between 30 and 55% by 51Cr radioactivity assays and between 31 and 51% by HLA tracking; the absolute differences among pairs ranged from ‘�6% to +1%’. Platelet survivals ranged between 2.9 and 3.9 days by 51Cr radioactivity assays and 2.6 and 4.4 by HLA tracking. Again, absolute differences between pairs were small, ranging between −0.7 days to +0.4 days.
Conclusion
Differential HLA tracking of transfused platelets proves a good alternative to radioactive measurements enabling recovery and survival of transfused platelets in the intended patient group.
We plan to extend the experiments by simultaneous transfusion of two different platelet products; both HLA mismatched. This will enable us to compare recovery and survival of test‐platelets to the standard product.
P‐242A
Microparticles Variability in Fresh Frozen Plasma: The Effect of Preparation Method, Storage Time and Donor‐Related Factors
Kriebardis AGK1, Antonelou MHA 2, Tzounakas VLT 2, Georgatzakou HTG 2, Papassider ISPII 2, Stamoulis KES 3
1Technological and Educational Institute of Athens, Athens, Greece2NKUA, Athens, Greece3National Blood Center, Acharnes, Greece
Background
Microparticles (MPs) are small membrane particles ubiquitously released by cells under physiological or pathological conditions. MPs exhibit critical roles in intercellular communication required in processes like immune regulation, coagulation and inflammation. Their possible implication in clinical settings has downgraded their vital role in normal development and homeostasis. MPs exhibiting procoagulant and thrombogenic activity may contribute to the hemostatic potential of fresh frozen plasma (FFP).
Aims
The aim of this study was the characterization of the MPs present in two standard FFP preparations used for transfusions, namely in FFP prepared from platelet rich plasma at RT and in FFP prepared directly from whole blood at 4°C.
Methods
Whole blood samples from eight male regular donors of blood group O and A were anticoagulated with either EDTA or citrate standard anticoagulant mixtures and processed for standard hematological and biochemical analysis. For storage analysis, FFP from the same donors was prepared from platelet‐rich plasma at RT (FFP‐1) or directly from whole blood at 4°C (FFP‐2). Each unit was divided in half, stored for various periods at −20°C and analyzed immediately after thawing or following storage for 24 h at 4°C by flow cytometry, prothrombinase activity assay, total antioxidant capacity and RBC membrane proteins. Differences between the FFP groups in each time point were identified using one way ANOVA. Pearson's and Spearman's (where needed) correlation tests were used to determine correlation coefficients (r).
Results
Although baseline hematological, biochemical and lifestyle characteristics did not differ significantly between donors, circulating MPs counts exhibited a considerable inter‐donor variation (13,733–21,319 MPs/μl of plasma) that was related to donor age, plasma lipoprotein profile and plasma/RBCs protein oxidation levels. FFP preparations were enriched in MPs compared to the plasma in vivo, particularly after long storage in the freezer. The FFP‐2 units contained more MPs (71,352 ± 18,588 MPs/μl), especially of PLTs‐origin compared to the FFP‐1 units (37,391 ± 8137 MPs/μl) at all storage times (P < 0.05). A comparable increase in the concentration of WBCs‐ and RBCs‐derived MPs was observed in both preparations with respect to the circulation levels. Preservation of the thawed units for 24 h at 4°C did not significantly affect MPs accumulation. MPs level in FFPs appeared further related to the in vivo levels of vesiculation and plasma antioxidant capacity as well as to the donors’ ABO blood group typing.
Conclusions
Preparation method, storage duration and various donor‐related factors, including the baseline oxidative status and the ABO blood group typing, significantly affect MPs accumulation in FFP. In vitro analysis of parameters that might influence FFP therapeutic potential seems precocious since the clinical effectiveness of FFP transfusion is still a topic of debate. However, there is a general need for clinical studies to determine how the MPs present in blood labile products might affect the transfusion recipients. Regarding FFP, it is important to determine not only how MPs are involved in the coagulation cascade but also the effect of each FFP hemostatic component on FFP quality and efficacy, as a parameter of preparation, storage, processing and donor‐associated topics.
P‐242
The UK National Frozen Blood Bank: A Five Year Review of Provision of Rare Phenotyped Units
MacLaren GRM , Gilbert JG , Callaghan TC , Win NW
NHS Blood and Transplant, Liverpool, United Kingdom
Background
Rare red blood cell (RBC) phenotype is classified as one which occurs in <1% of the population. Some phenotypes are extremely rare in the Caucasian population e.g. Bombay, and may be found only in certain ethnic groups e.g. U neg in the Black population. Sourcing these RBCs from a predominantly Caucasian donor population is difficult, as is locating RBCs suitable for patients with multiple antibodies, including antibodies with broad Rh specificity requiring Rhnull or ‐D‐ units.
The UK National Frozen Blood Bank (NFBB), which is part of NHS Blood and Transplant (NHSBT), receives, processes, stores and reconstitutes rare RBC units. A 24/7 service is available for emergency cases. Occasionally, NFBB may request import of units from abroad for a named patient. In this situation suitable donors/donations are identified in liaison with NHSBT International Blood Group Reference Laboratory (IBGRL) or by direct contact with other international blood banks. Following issue of rare units, efforts are made to replenish stocks including call up of rare red cell donors.
Aims
To review the performance of the NFBB in the provision of rare red cells over the 5 year period 2010–2014
Methods
Data were collected on the number and phenotype of units stocked, requested and issued, any requests not fulfilled, haemoglobin (Hb) content of thawed units, number and type of units imported from abroad and outcome information where available.
Results
Current stock is approximately 760 frozen units comprising 35 different phenotypes. Of these the following phenotypes are consistently below ideal stock level due to extreme rarity: Rhnull, ‐D‐, .D., hrs‐, hrb‐, Ko, KL, Jra‐, Joa‐, Inb‐, P1k, Gya‐, Ge‐2, Ge‐2,‐3.
During 2010–2014 the NFBB issued 414 components in response to 192 requests with 86.5% issued to UK hospitals and 13.5% issued to 11 non‐UK countries. The most commonly requested type was U neg (n = 101, 24%). Mean Hb content was 42 g/unit (range 17.6–52.7) with 88.5% donations compliant with the target of 36 g/unit. On 7 occasions units were imported from abroad (20 units, phenotypes ‐D‐, Bombay, Fy(a‐, b‐) plus extended phenotype, Rh null and R1wR1w).
Outcome information was received in for 204 (49%) units of which 108 (53%) were transfused. Where the reason for not transfusing was known (73 units), blood had mainly been requested to cover obstetric delivery (34/73 = 47%) or surgical procedure (29/73 = 37%) but was not required. A positive outcome (e.g. satisfactory Hb increment) was reported for 77/99 (78%) units. Reasons for poor increment included ongoing bleeding or haemolysis. One possible transfusion reaction was reported for one unit.
Data collected since May 2011 indicates 10% of potential requests could not be fulfilled; customers were referred to IBGRL in these cases for international search.
Conclusions
The NFBB, in collaboration with international colleagues and our dedicated donors, has proved a valuable resource for the provision of rare red cells.
In the next 5 years the NFBB aims to develop stronger links with other frozen blood banks internationally, to improve product availability, establish best practice and enable standardisation.
P‐243
Quality Parameters in Platelet Additive Solution (PAS) Added O Group Single Donor Platelets
Jain P , Tendulkar A , Gupta A , Kelkar R , Kulkarni A , Subramanian PG
Tata Memorial Hospital, Mumbai, India
Background
This study was conducted in a tertiary oncology setup wherein platelet transfusions are an integral part of the supportive care. Guidelines recommend ABO‐identical platelet transfusion preferably as against group switchovers. Incidence of acute hemolytic transfusion reactions (AHTR) after minor ABO incompatible platelet transfusion is increasing with the use of single donor platelets (SDP) containing ABO incompatible plasma. Approaches to avoid such events by examining the titre levels or performing plasma reduction are cumbersome. As a prelude to this study, we found that in our population, the incidence of high titre (anti A, anti B) ‘O’ group donors was high. Hence, we studied the feasibility of preparing ‘O’ SDP with Platelet additive solution (PAS)to obtain safer low titre units.
Aim
To study
Antibody titres (anti A, anti B) in ‘O’ SDP by adding PAS at source.
Quality parameters with reference to viability, morphology, sterility, metabolism and activation.
Materials and methods
SDP (n = 50) were prepared from O group donors on Amicus®(version 3.2, Fenwal Inc., USA) cell separator which provides 3 log leukodepletion. PAS III (SSP+™, MacoPharma, Moveaux, France)in a ratio 70:30 (PASIII: plasma) was added at source under sterile conditions (study arm). The units were studied on day of collection (day 0) and day 4, and compared with SDP containing 100% plasma (control arm). Anti‐A and anti‐B titres in study arm SDP were assessed by tube technique before and after addition of PAS to know the quantitative reduction in titres. The parameters studied were: Volume, yield, sterility, Mean platelet volume (MPV), Swirling, Kunicki score, pH, partial pressure of O2 and CO2, bicarbonate levels, Glucose levels, rate of glucose consumption (GCR), lactate, rate of lactate production (LPR), Oxygen consumption rate (OCR), Surface expression of CD62 (P‐selectin)
Results
Comparing the study and control groups, the difference in the volume and yield was negligible (P > 0.05). Sterility confirmed by bacterial culture was found to be maintained till day 5. Swirling was good and MPV was in normal range(8–11 fl). Kunicki morphology scores in the study arm were superior to control arm (P < 0.001). Metabolic parameters pO2, pCO2, OCR were similar in the two arms signifying good unit storage and stable oxygen consumption (P > 0.05). Bicarbonate levels, glucose levels and GCR, lactate levels and LPR were significantly low in study arm showing the superiority of PAS in storing platelets. pH was maintained above 6 in all study units. Platelet activation (CD62) by flow cytometry was similar in two groups (P > 0.05).In the study group, the median antibody titres (anti A, anti B) was 128 prior to PAS addition and reduced significantly to 16 post modification (P < 0.001).
Caption 1: Parameters studied for Study vs Control arm (Part 1)

Conclusion
O group SDPs can be prepared with PAS and the beneficial effects were significant with respect to antibody titres, morphology scores, LPR. Also, similar findings in both arms were seen for volume, yield, MPV, pO2, pCO2, OCR, GCR, bicarbonate and platelet activation. Patient is benefitted with PAS units, especially during platelet shortages which require ABO switchovers.
Caption 2: Parameters studied for Study vs Control arm (Part 2)

P‐244
Platelet Donors: Extremes of Platelet Function are Consistent Over Time
Cardigan R1, Garner SF 1, Furnell A 2, Kahan BC 3, Jones CI 4, Attwood A 2, Harrison P 5, Kelly AM 6, Goodall AH 7, Ouwehand WH 6
1NHS Blood and Transplant, Cambridge, United Kingdom2Department of Haematology, University of Cambridge, Cambridge, United Kingdom3Pragmatic Clinical Trials Unit, Queen Mary University of London, London, United Kingdom4Institute for Cardiovascular and Metabolic Research, University of Reading, Reading, United Kingdom5School of Immunity and Infection, University of Birmingham Medical School, Birmingham, United Kingdom6NHS Blood and Transplant and Department of Haematology, University of Cambridge, Cambridge, United Kingdom7Department of Cardiovascular Sciences, University of Leicester, Leicester, United Kingdom
Background
Platelet function is known to vary between individuals. This variation is believed to be at least partially genetically controlled and should therefore be stable over time in normal healthy individuals. Studies focusing on consistency of platelet function in blood donors undergoing regular platelet apheresis have been performed, but have only assessed consistency of function over periods up to three months.
Aim
We aimed to investigate consistency in platelet function in active platelet donors over periods ranging from months to approximately five years.
Methods
A cohort of 956 donors had their platelet function characterised by flow cytometry, measuring fibrinogen binding and P‐selectin expression after stimulation with either cross‐linked collagen related peptide or adenosine 5′‐diphosphate. Thirty seven donors who were initially found to be within the lower 10% of observed reactivity (low responders) and 52 within the upper 10% of observed overall reactivity for the entire cohort (high responders) were available for re‐testing between four months and five and a half years later. Their initial and repeat results were compared, as was the overall difference in results between the low and high responder groups.
Results
There was a good correlation between the initial and repeat platelet function results for all assays in 66 individuals (28 low responders and 38 high responders) retested between 4 and 20 months later (r2 ≥ 0.3738, P < 0.0001), after donating an average of 8 times between tests. Similarly, the 23 donors (9 low responders and 14 high responders) re‐tested approximately five years later after donating an average of 49 times also had a good correlation between initial and repeat testing results (r2 ≥ 0.2961, P ≤ 0.0069). Furthermore, the range of results observed in the groups of donors initially classified as either low or high responder remained significantly different from each other at the time of the second test (P ≤ 0.0005).
Conclusions
Platelet function responses remained consistent over time. This observation is compatible with the notion of there being a high level of heritability for this quantitative platelet trait. This implies that the potential impact of an individual's platelet function on the quality of apheresis platelets will remain a consistent feature for a given donor.
P‐245
Changes in Platelet Function in Thrombocytopenic Patients Following Transfusion Can be Demonstrated By Flow Cytometry
Cardigan R1, Kelly AM 2, Cavagnetto C 1, Wedderburn K 2, Garner SF 1
1NHS Blood and Transplant, Cambridge, United Kingdom2NHS Blood and Transplant and Department of Haematology, University of Cambridge, Cambridge, United Kingdom
Background
Although desirable, performing meaningful assays of haemostasis or platelet function in thrombocytopenic patients before and following transfusion remains a challenge. Routine assays such as the Platelet Function Analyser, or aggregometry, require platelet counts greater than those observed after transfusion. Flow cytometry has however been proposed as being suitable, as it enables single cell analysis, and provides data on platelet activation, irrespective of their concentration.
Aim
We aimed to assess the feasibility of using flow cytometry to both assess changes in platelet function in stable thrombocytopenic haematology patients after transfusion, and to potentially correlate these changes in the patient with known differences in platelet function of the donors.
Methods
Donors were from the Cambridge Platelet Function Cohort, whose platelet function had been previously characterised by flow cytometry, measuring fibrinogen binding and P‐selectin expression after stimulation with either cross‐linked collagen related peptide (CRP‐XL) or adenosine 5′‐diphosphate (ADP). Those selected were from either the lower 10% of observed reactivity (low responders) or the upper 10% (high responders).
Seventeen patients were studied both prior to, and one hour after transfusion. Of these, 10 received platelets from donors characterised as being low responders, and 7 from high responder donors. The patients’ platelet function was assessed using whole blood flow cytometry, with the resting, basal level of P‐selectin being measured along with the P‐selectin expression following stimulation with either CRP‐XL or ADP, as agonists. Results from all 17 patients were reviewed to study overall differences in activation before and after transfusion. Subsequently, the patients’ data were further analysed according to the responder status of the donors.
Results
Overall, irrespective of the source of their transfused platelets, patients demonstrated an increase in both basal platelet activation, and in responses to agonists after transfusion. The percentage of platelets expressing P‐selectin without incubation with agonist (i.e. basal activation) increased from a median of 20.3% (range 6.1–35.2%) to 32.7% (range 18.8–41.6%, P =< 0.0001). Similarly, following incubation with CRP‐XL, P‐selectin expression increased from 60.8% (range 32.9–87.7%) to 79.9% (range 48.3–91.9%, P = 0.0005). P‐Selectin expression in response to ADP stimulation also increased from 24.1% (range 11.4–45.3%) to 37.6% (range 21.3–51.3%, P = 0.0007). Analysis of the change in platelet function following transfusion according to whether the donor was a low or high responder however failed to show any significant differences between the groups (P ≥ 0.4791).
Conclusions
Our data showed that it is feasible to use flow cytometry to demonstrate changes in a patient's platelet function following transfusion. The degree of change in platelet function did not however correlate with the functional responder status of the platelet donor.
P‐246
The Stability of Pooled Cryoprecipitate Following Thawing
Backholer L1, Green L 2, Wiltshire M 1, Platton S 3, Stanworth SJ 4, Cardigan R 1
1NHS Blood and Transplant, Cambridge, United Kingdom2NHS Blood and Transplant/Barts Health NHS trust, London, United Kingdom3Barts Health NHS Trust, London, United Kingdom4NHS Blood and Transplant/Oxford University Hospitals NHS trust, Oxford, United Kingdom
Background
Cryoprecipitate as a concentrated source of fibrinogen may be transfused to treat acquired fibrinogen deficiency. There is interest in whether earlier administration of cryoprecipitate in the treatment of major haemorrhage may improve outcome. One option would be to have pre‐thawed cryoprecipitate available for these patients to avoid the delay in component provision due to the thawing process. The current post‐thaw shelf‐life of 4 h in the UK would preclude this. The purpose of this study was to investigate the stability of pooled cryoprecipitate, thawed and held at ambient temperature for an extended time to investigate the feasibility of extending the post‐thaw shelf‐life.
Methods
Pooled cryoprecipitate prepared according to standard NHSBT protocols (16 units, 8O & 8A) was thawed at 35°C ± 2°C for 20 min and then held at ambient temperature (18–24°C) for up to 72 h and sampled at 0, 4, 10, 24, 48 and 72 h following thawing. Samples were tested for FVIII (one‐stage clotting), fibrinogen activity (Clauss), FXIII (chromogenic), rotational thrombelastometry (ROTEM) and thrombin generation (TG).
Results
There was no significant decrease in levels of fibrinogen or FXIII over 72 h of storage, but there was a small (<10%) decrease in FVIII levels. Units at all time points met UK specification for FVIII and fibrinogen (75% of units >350 IU/unit for FVIII and >700 mg/unit for fibrinogen). There was no change in endogenous thrombin potential or peak thrombin, but a small increase in lag time and time to peak for thrombin generation was seen, with the latter correlating with changes in FVIII levels (R2 = 0.88). After 72 h there was no change in ROTEM parameters.
Conclusions
These stability data support the hypothesis that pre‐thawed cryoprecipitate retains adequate levels of pro‐coagulant factors and activity for up to 72 h. Other factors that need to be considered include potential for wastage and the risk of bacterial growth in units stored for prolonged periods at ambient temperature.
P‐247
Qualification of Three U.S. Blood Centers for Use of the Intercept Blood System for Platelets in 100% Plasma
Erickson AE
Cerus Corporation, Concord, United States of America
Background
The INTERCEPT™ Blood System for Platelets is used in Europe for the preparation of pathogen and leukocyte inactivated platelet components (PC) for transfusion. A recent CE mark label extension allows treatment of PC in 100% plasma using the INTERCEPT platelet processing set with dual storage containers (DS set). In 2014, the FDA approved the INTERCEPT Blood System for the preparation of pathogen reduced apheresis platelets suspended in InterSol. Three U.S. Blood Centers were qualified to process INTERCEPT platelets in 100% plasma for a Phase 1 in vitro study to support label extension in the U.S. for pathogen reduction of apheresis platelets in 100% plasma.
Aims
The purpose of this study was to qualify U.S. blood centers to produce INTERCEPT‐treated platelets in 100% plasma using the DS set. The Day 5 and 7 in vitro quality of INTERCEPT platelets was compared to untreated Control and to published ranges for conventional PCs in 100% plasma.
Methods
Apheresis PCs (3.5–8.0 × 1011 in 288–433 ml) were collected in ACD plasma on the Trima Accel® platform. Nine PCs (2 doubles, 7 singles) were stored per manufacturer's instructions (Control) while 32 PCs were treated with the INTERCEPT process before the end of the day after donation (Test). After treatment, Test PCs were stored under standard conditions as either singles (input dose <7.0, n = 21) or doubles (input dose ≥ 7.1, n = 11) depending on the PC input dose. On Days 5 and 7 post‐donation a sample was removed from each Test and Control replicate for evaluation using a full panel of in vitro function assays (Table 1).
Results
After treatment, 41/43 (95%) of Test components had ≥ 2.5 × 1011 platelets/unit, and 37/43 (86%) had ≥ 3.0 × 1011 platelets/unit. Estimated platelet dose recovery post‐treatment was 84% ± 12%. Thirty‐one of 32 (97%) Test PCs had Day 5 pH (22°C) ≥ 6.5, while 30/32 (94%) had Day 7 pH (22°C) ≥ 6.5. Once Trima collections were optimized for compatibility with IBS input specifications, all Test PCs maintained pH (22°C) ≥ 6.5 through Day 7. The mean and SD for in vitro function assays on Days 5 and 7 are listed in Table 1.
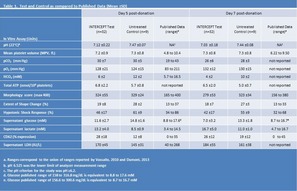
Conclusions
Over 7 days of storage, INTERCEPT platelets in 100% plasma displayed pH, ATP and morphology within published ranges known to correlate with in vivo recovery and survival and the ranges are consistent with hemostatic function for conventional platelets in 100% plasma.
P‐248
Proteomic Analysis of Platelets From Donors of Different Levels of Responsiveness
Schubert P1, Kelly AM 2, Lau T 1, Smethurst P 2, Jansen SBG 2, Cardigan RA 3, Devine DV 1
1Canadian Blood Services, Vancouver, Canada2Department of Haematology, University of Cambridge, Cambridge, United Kingdom3NHS Blood and Transplant, Cambridge, United Kingdom
Background
Donor‐to‐donor variation has been proposed as a factor in transfusion outcome. The levels of platelet responsiveness to agonists are known to vary between individual donors. Recently, the Platelet Responsiveness and Outcome from Platelet Transfusion (PROmPT) clinical trial assessed whether the outcome from transfusion differed between platelets from donors with a high or low combined measure of responsiveness (to ADP and collagen‐related peptide).
Aims
To compare proteomic profiles of platelets from donors, with high or low platelet responsiveness, participating in the PROmPT clinical trial.
Methods
In a semi‐blinded analysis approach, platelet samples were chosen from 9 and 7 donors of the low and high responder groups, respectively. Each platelet sample was pelleted by centrifugation, the supernatant was removed and stored separately and the pellet was washed and both were frozen. The supernatant was assessed for cytokines and chemokines released from the platelet using an antibody array analyzing 80 different cytokines/chemokines (RayBiotech). The platelet pellets were thawed in PBS‐buffered lysis solution containing 1% Triton and protease/phosphatase inhibitors. The protein kinase profile was analyzed using an antibody‐based array (R&D Systems). Kinase array results were verified by western blot analyses. Membranes were scanned on a Licor system and band intensities on western blots were determined using the Odyssey software.
Results
Three samples from each of the two responsiveness groups were analyzed and did not show between‐group differences on the profile. Furthermore, the levels of platelet factor 4 (PF4) – a platelet degranulation marker – were investigated. Although there had been sample‐to‐sample variations of about 8‐fold in PF4 levels, no trend between the two groups was visible. Similarly, kinase profiling carried out on one sample per group did not reveal any difference between the two responsiveness groups. This result was verified for all samples obtained using western blot analyses testing phosphorylation levels of p38MAPK, ERK and AMPK. Noteworthy, however, is that samples within each group show 11‐, 7‐ and 8‐fold variation, respectively, in their basal kinase activation level relative to the total kinase level. Lastly, we analyzed the expression level of selected proteins recently identified as markers for transfusion outcome, Rap1 and RhoGDI. Again, no difference in the expression profile of these proteins could be identified between the two responsiveness groups, but sample‐to‐sample variation of 5‐ and 7‐fold, respectively was observed.
Summary/Conclusions
The platelet proteomic analyses did not identify any differences between the samples of donors with low or high platelet responsiveness capacity. This observation is in agreement with the PROmPT study findings that corrected count increments in patients at one hour or 24 h following transfusion are similar whether platelets were from low or high responder donors. However, our proteomic profiling revealed some variation between the samples of individual donors that was unrelated to their broad responsiveness groupings in the study. Further analyses, such as regrouping according to transfusion outcome might provide a correlation to the protein profile.
P‐249
Red Cell Storage Lesion in the Light of Uric Acid Variation Among Regular Blood Donors
Tzounakas VLT1, Georgatzakou HTG 1, Kriebardis AGK 2, Papageorgiou EGP 2, Stamoulis KES 3, Foudoulaki-Paparizos LEFP 4, Antonelou MHA 1, Papassideri ISP 1
1NKUA, Athens, Greece2Technological and Educational Institute of Athens, Athens, Greece3National Blood Center, Acharnes, Greece4Regional Blood Transfusion Center, ‘Agios Panteleimon’ General Hospital of Nikea, Piraeus, Greece
Background
Red cell storage lesion is a donor‐related phenomenon that depends on the ability of stored units to cope with oxidative stimuli and defects. Inherent variation in the oxidative burden and antioxidant activity of the donated blood might determine to some extent the storability of RBCs and probably their post transfusion efficacy and effects.
Aims
This study aimed at the elucidation of donor‐specific and redox homeostasis‐associated hematological factors in vivo that might reveal the susceptibility of RBCs to storage lesion hallmarks during their preservation in standard systems.
Methods
Of 48 hematological characteristics in vivo including RBC and ferrum indexes, fragility, hemolysis, plasma total antioxidant capacity (TAC), biochemicals etc. were evaluated in 78 male donors (19–24 years old) who meet the criteria for blood donation. The probable effect of uric acid (UA) on RBCs storability was evaluated in 8 leukoreduced units of RBCs in CPD/SAGM produced from donors exhibiting high or low pre‐storage levels of serum uric acid. Several RBC storage quality parameters were examined throughout the 42 day storage period, including intracellular ROS and Ca2+ accumulation, TAC, RBC fragility and morphology, microparticles generation, hemolysis etc. Cluster and factor analysis were used to group donors or parameters respectively. Linear regression analysis was performed to further investigate association between UA and significant factors (P < 0.05).
Results
Cluster analysis grouped subjects in three groups according to their serum UA levels: high‐ (7.56 ± 0.59 mg/dl), upper border‐ (6.96 ± 0.51 mg/dl) and normal‐ (6.01 ± 0.63 mg/dl) UA donors. Iron and redox homeostasis indexes followed the UA‐based classification of both donors and profiles (cluster and factor analysis, respectively). Considering that UA accounts for up to 60% of the total antioxidant capacity of the plasma, these findings suggested that normal range‐UA‐variation in vivo may reveal intrinsic inter‐donor variation in the basal redox status. The probable association of UA with the RBCs storability was tested in RBC concentrates prepared from donors exhibiting low (4.73 ± 0.25 mg/dl) or high (7.52 ± 0.5 mg/dl) UA levels in vivo. Low UA‐units exhibited significantly elevated levels of intracellular Ca2+, non‐reversible spheroechinocytosis and band‐3 oligomerization along with lower TAC and flottilin‐2 throughout the storage period (P < 0.05). In addition, a trend for better oxidant/antioxidant equilibrium (clusterin, carbonylation, calpain levels etc.) was observed in high‐UA donors. Regression analysis indicated that UA was a statistically significant predictor for supernatant TAC, intracellular Ca2+ and spheroechinocytosis (P < 0.05).
Summary/Conclusions
Intrinsic variability in UA levels in vivo seems to be associated with RBCs storage lesion hallmarks like the degree of spheroechinocytosis. Although it is currently not known whether this association arises by direct antioxidant function of UA inside the RBCs unit or by its capability to reveal the redox homeostasis of the individual donor, normal range variation in UA might be used to evaluate the susceptibility of donated blood to storage lesion. The ability to pre‐evaluate storage lesion profile by using easily accessible factors is probably the first step towards the effective management of the donated blood (storage strategies, additive solutions, leukoreduction, appropriate storage period etc) by the blood bank services.
P‐250
Overhydrated Stomatocytosis Pedigrees Show Different Patterns of Stomatin Localisation Throughout Erythropoiesis
Flatt JF1, Stewart GW 2, Bruce LJ 1
1Bristol Institute for Transfusion Sciences, Bristol, United Kingdom2University College London, London, United Kingdom
Background
The Hereditary Stomatocytoses (HSt) are a group of disorders in which the cation permeability of the red cell membrane is pathologically increased. The molecular bases of virtually all of these disorders have now been identified; all involve mutations in large multi‐spanning membrane proteins. The most severe forms of HSt are overhydrated stomatocytosis (OHSt) and stomatin‐deficient cryohydrocytosis (sdCHC), which are caused by mutations in RhAG and GLUT1, respectively. Despite the distinct genetic backgrounds of these conditions, both feature a severe reduction or absence of stomatin in the mature red cell. The precise role of stomatin in the red cell and the mechanism underlying its deficiency in these disorders remain poorly understood. Previous genetic analysis of affected individuals has confirmed the presence of the wild‐type stomatin gene and successful translation of the protein during erythropoiesis. We have previously shown that the timing of stomatin loss differs between OHSt and sdCHC, since sdCHC erythroblasts successfully express stomatin at the plasma membrane whereas OHSt erythroblasts do not.
Aims
The aim of this study was to characterise the expression and fate of stomatin throughout erythropoiesis in two pedigrees of overhydrated stomatocytosis in comparison to stomatin‐deficient cryohydrocytosis.
Methods
CD34+ progenitor cells were isolated from the peripheral blood or cord blood of individuals with overhydrated stomatocytosis, stomatin‐deficient cryohydrocytosis and from a healthy control. Two OHSt pedigrees were studied, both carrying the same mutation in RhAG (Phe65Ser). Erythroid cells were cultured to reticulocyte stage. Cellular lysates were used for protein analysis by Western blot. Confocal microscopy and immunostaining were used to follow the localisation of stomatin at selected time points during erythropoiesis.
Results
It was found that the amount of stomatin and other membrane proteins in cultured OHSt cells were reduced during erythropoiesis, with evidence of proteolysis. Confocal microscopy revealed that the two OHSt pedigrees studied exhibited different patterns of stomatin localisation. In OHSt(A) cells, stomatin was largely confined to intracellular vesicles that colocalised with LAMP1, a lysosomal marker. In comparison, OHSt(B) cells also showed this pattern, but many also expressed stomatin at the cell surface. In contrast, sdCHC cells expressed normal amounts and localisation of stomatin throughout culture, even at late stages. In both OHSt and sdCHC reticulocytes, stomatin appears to be lost via internal trafficking pathways.
Summary/Conclusions
Our data suggest that stomatin undergoes proteolysis during erythropoiesis in both cases of OHSt, but not in sdCHC. However, we also found a difference in the localisation of stomatin during erythropoiesis between the 2 pedigrees of OHSt, which share the same causative mutation. Since the loss of stomatin is a secondary effect in these conditions, the variation observed within OHSt suggests that other factors contribute to the degradation and/or cell surface expression of this protein.
P‐251
Strides In Blood Component Production In Kenya
Githiomi RG , Oduor DR , Orgut IO , Cheptinga DC , Rukenya BR , Gitobu PG
Kenya National Blood Transfusion Service, Nairobi, Kenya
Background
Blood component is a part of whole blood that can be separated manually or via aphaeresis.[Red cells, platelets and plasma].
Blood product is a part of a blood component such as cryoprecipitate, albumin, immunogluloblin among others. Blood component production can be traced back to 1940 during the second world war. U.S. collected whole blood processed, tested, and stored plasma which was transported to Great Britain to save soldiers
The concept was picked by other developed countries and more blood components were introduced
Kenya National Blood Transfusion Service was founded in 2000 with three regional sites that were dealing with collection, testing and distributing of whole blood. Currently KNBTS has more regional sites as illustrated in fig 1 (Map of Kenya).
In 2006, blood component concept was born and RBTC Nakuru was identified as the pilot site.
The piloting was supported by JICA 2008. The first training on blood component was conducted at KNBTS Nairobi by AABB via PEPFAR support.
In October 2009, AABB trained more staff from all the six regional sites to enhance the blood component production.
Aims
To address blood demands
To ensure patients safety[specific components for specific needs]
To ensure quality/efficacy
Methods
q Closed system[Tube heat sealing]
Sedimentation[centrifugation]
Plasma expression
Weighing and documentation
Proper storage
Results
By the end of 2009, KNBTS could only produce 2% blood component out of the 167,000 annual blood collections.
2010 KNBTS conducted a blood component mentorship and the component production increased from 2% to 37% out of the total blood units collected
2012, a refresher training for the staff in blood component production unit was conducted and this saw the blood component raise from 45% to 52%
2013, blood component mentorship was conducted and this revealed that the blood component production was at 57%It was revealed that KNBTS was meeting 68% of Kenya blood needs
2014 September, Kenya raised its blood component production to 61% and was meeting almost 80% of transfusing facilities blood requests.
Currently Kenya is producing 70% blood component out of 186,000 annual blood collection and meeting 90% of red blood cells concentrates demands. It was also noted that the trend of component production is on the rise as illustrated in fig 2 (Results)


Summary/Conclusion
Currently KNBTS is at 70% in blood component productionSafety, quality/efficacy has been addressedSufficiency, KNBTS is currently meeting 80% of the transfusing facilities blood component needs.
P‐251A
Retrospective Analysis of OptiaⓇ Granulocyte Collection Efficiency Over a Two Year Period In King's College Hospital, London
Perera WWK , Hess D , Kuruppu D , Herath A , Haili C , McLornan D , Pagliuca A , Mufti G , Mijovic A
Kings College NHS Foundation Trust, London, United Kingdom
Background
King's College Hospital, London (KCH) facilitates the highest number of Polymorphonuclear leucocyte (PMN) collections in the UK, and is one of two centres licenced for such. In 2013, the granulocyte collection platform was changed from Spectra�’to Optia�’. This retrospective analysis compares PMN collection efficiency on the Optia�’ platform in both 2013 and 2014. No major procedural changes were introduced in this period
Aims
1. Compare donor‐ related and pre‐ procedural parameters of PMN donors in 2013 and 2014.
2. Compare the quality of the granulocyte product harvested during these periods.
3. Compare the granulocyte collection efficiency in 2013 and 2014.
Methodology and analysis
Total PMN collections for both 2013 and 2014 were compiled for analysis. Pre‐procedure blood counts were recorded from Electronic Patient records (EPR). Apheresis PMN collection details were recorded on the datasheets for each procedure, and PMN product parameters collated from PMN issue documentation. Standard PMN collection protocols were followed. All procedures utilised 6% Hydroxyethylstarch (HES), with a mean molecular weight (MW) of 130 KDa, as the sedimentation agent.
Median values were calculated for all parameters and data was analysed with non‐parametric Mann‐Whitney tests (MWT). P values <0.05 were considered significant. PMN collection efficiency was calculated using two equations: CE1 = Total PMN collected/ ((PMN Pre‐count +PMN post count)/2× total blood volume processed) x 100, and CE2 = Total PMN collected / (PMN pre‐count x Total blood volume processed) x 100.
Results
Available data from 91/97 procedures in 2013 were compared with 48/51 procedures in 2014. The median blood volumes processed were 5101 ml and 5292 ml respectively. No significant statistical difference were apparent in pre‐procedure haematocrit values. The pre‐ and post‐procedure total white blood cell (WBC) counts were 41.5 and 34.43 (2013) vs 40.4 and 30.2(2014) respectively.
In the collected product, total WBC was 104 × 103/ul in 2013, which increased to 120.5 in 2014. No difference was apparent as regards the procedure time, but total volume of the product was reduced from 426 ml in 2013–370 ml in 2014. The product haematocrit was lower in 2014 (0.13 vs 0.19). Collection efficiencies, both CE1 and CE2 were comparable in both years (22.99% vs 24.75% for CE1 and 21.29% vs.21.72% for CE2 in 2013 and 2014 respectively). PMN dose given per recipient Kg/ body weight was 3.2 × 108 in 2013, and 2.93 × 108 in 2014.
Conclusions
Optia�’ granulocyte collection figures in 2013 and 2014 were similar, showing reproducible results on this platform. No significant change in collection efficiency over this time period was noted and updates in Optia�’ software versions did not significantly affect PMN yield. Total PMN of about 3 × 108/Kg recipient weight was collected, with slightly reduced final product volume in 2014
Many studies suggest improved yield with high molecular weight preparations such as HES 6% (MW 480 KDa). However, prior to introducing changes in procedure, this demands safety and risk analysis, as these preparations have significantly longer plasma half‐life.
P‐252
Audit of Red Cell Concentrate Discards at Eastern Regional Blood Centre‐ Ampara Srilanka
Senanayake EWN , Adikarama BMGM , Sumanasekera RN
General Hospital Ampara, Panadura, Sri Lanka
Background
Currently with the increase in the aging population, continuous advancements in medicine and various surgical procedures the need for blood and blood products is increasing globally. Blood is a scarce resource that must be utilized with care with minimum wastage. Used appropriately it can improve the health care system. Because of the limitation of blood supply and the cost of preparation of blood it is our responsibility to ensure adequate and safe blood and products to all patients. Appropriate use of blood with minimum wastage is an important step to achieve this goal. The study of discard rates of Red Cell Concentrate is an important step in the management of blood supply and of unnecessary wastage in terms of manpower and cost. The Eastern Regional blood center in Srilanka located at Ampara is responsible for seven blood banks namely Ampara, Kalunai North, Kalmunai South, Dehiattakandiya, Mahaoya. Sammanthurai and Akkaraipattu. Most of the blood is from mobile donations, which are processed at the main cluster center. There is only limited data available in blood transfusion practices in developing countries such as Srilanka.
Aim
Retrospective audit from January 2014‐December 2014 to calculate the RCC discard rates due to Expiry at Regional Blood Center Ampara.
Method
Data was retrieved from monthly statistical reports. Discard rates of RCC by expiry was accessed in each of the blood banks monthly. This was compared with the RCC produced by the component laboratory and the stocks of RCC received during that month. Discard rates was calculated for the whole center as well as the main cluster center. Discard rate of RCC by Expiry = (Number of RCC discarded due to expiry)/(Inhouse production of RCC+Number of RCC recieved from other blood banks).
Results
There was a total of 732 RCC discarded due to Expiry at the Ampara region in 2014.The Discard Rate of RCC by Expiry in the whole cluster center in 2014 = 4.55% and the rate for main center = 4.93%. The values for the other blood banks in the cluster are shown below
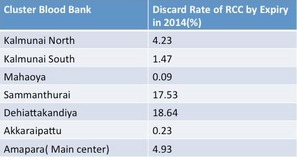
Conclusion
According to the available data it is clear that there is a significant wastage of RCC at the region. Immediate measures should be taken to look into the contributing factors of this high discard rate. There must be efficient stock management strategies introduction and reinforcement of haemovigilance programme. The appropriate officers must be educated to implement stock management strategies. This audit further confirms that regular audits of blood and blood products are crucial so that measures can be taken to maximize appropriate and judicious utilization of all components of blood.
P‐253
Effect of Fibrin Glue in Liver Regeneration after Laparoscopic Surgery
Stanojkovic Z1, Antic A 1, Dencic S 2, Stojanovic M 3, Stanojkovic M 4
1Blood Transfusion Institute Nis, Nis, Serbia2Health Center, Pirot, Serbia3Department of General Surgery, Clinical center, Nis, Serbia4Center of Clinical Biochemistry, Clinical Center, Nis, Serbia
Background
Fibrin glue (FG) is a natural chemical – adhesive system with an important role in blood coagulation and wound healing. It consists of two basic components – fibrinogen and thrombin, where activation of fibrinogen and its transformation into fibrin under the action of thrombin is the third phase of blood coagulation. It is known that the use of FG in laparoscopic cholecystectomy reduces the complication rate in terms of stopping diffuse bleeding in the liver parenchyma, preventing extravasation of bile and the reduction of abdominal adhesions. The main objective of this study was to determine whether the use of FG in laparoscopic surgery has an effect on the speed of healing and regeneration of liver tissue.
Material and methods
The study included a total of 40 experimental pigs in which was performed laparoscopic cholecystectomy and intraoperative standardized artificially damage of gallbladder boxes, which was repaired using FG in animals of experimental group (EG) or using standard means in animals of the control group (CG). FG was homemade (Blood Transfusion Institute Niš), prepared from two components, of which the first one was prepared from the cryoprecipitate with the addition of antifibrinolytic agents (aprotinin). The second component was a commercial bovine thrombin with calcium chloride. Animals were monitored for 30 days, 4 animals were sacrificed on the fifth, seventh, tenth, fourteenth and thirtieth day of follow‐up. During autopsy we have taken liver tissue and prepared for pathological research on which basis is calculated the histopathologic regeneration score (HRS: 0–3), which shows the level of liver regeneration.
Results
HRS was statistically significantly higher in EG on the fifth and seventh day (P < 0.05) and extremely higher on the tenth and fourteenth day (P < 0.0001). On the thirtieth postoperative day HSR in EG was 3.75 which is verified as a high level of regeneration and indicates the completion of liver regeneration after thirty days from the application of FG. In the course of liver regeneration, necrosis and hemorrhage fields were lower in EG compared to CG (P = 0.03, respectively). Verified cytoplasmic vacuolization was significantly higher in CG compared CG.
Conclusions
Application of fibrin glue in laparoscopic surgery affects the optimal and rapid flow of the process of healing and regeneration of the liver. Its application is recommended especially in the occurrence of diffuse intraoperative liver bleeding or in the bile duct injury, in patients who are on anticoagulant therapy, with liver cirrhosis and severe coagulation disorders.
P‐254
Audit on Discard of Platelets in the Eastern Regional Blood Center Ampara, Srilanka
Senanayake EWN , Sumanasekera RN , Adikarama BMGM
General Hospital Ampara, Panadura, Sri Lanka
Background
Ampara is the Eastern regional blood center in Srilanka catering to 7 blood banks. It is the responsibility of the cluster center to ensure adequate supply of blood and blood products to general Hospital Ampara and its surrounding blood banks. It is an isolated center so adequate bloodstocks need to be maintained always in cases of emergency, as it is difficult to acquire these products due to its distance from other cluster centers. Srilanka being a tropical country has a high prevalence of diseases such as dengue and Ampara being a rural area is endemic to venomous species of snakes. The patients admitted for above cases can progress quickly to DIC so platelet stocks need to be maintained for quick issue in these emergencies as platelets have only a shelf life of 5 days. The need for an audit of the platelet discard rates by expiry was identified in order to improve the clinical practice so a retrospective audit was carried out.
Aim
To assess the appropriate management and utilization of platelets, a 12‐month retrospective study for the year 2014 was carried out at Eastern regional blood center, Srilanka.
Method
Data was retrieved from monthly statistical forms. Discard of platelets only by expiry every month in each of the blood banks were analyzed with the amount of platelets received and the platelets produced in that respective month. Discard Rate (%) = Number of platelet discarded by expiry/(platelets received +platelets produced)
Results
Discard rate by Expiry of the Eastern region blood center in 2014 = 54.28%. Main Cluster center = 63.1% with a total number of platelet discard of 2238. The figures of other blood banks in the cluster are attached in the table below.

Conclusion
This retrospective platelet audit has enlightened us regarding our high platelet utilization and discard. Several measures have been considered to reduce high discard rates. Continuous education of clinicians, medical officers and nursing officers regarding platelet stock management and transfusion of non‐group specific platelet when there is a short supply of group specific platelets is considered. This further shows the importance of regular audits of blood and blood components so that necessary remedial measures can be taken to maximize appropriate and judicious utilization of all blood components.
P‐255
Quality Assessment of Filtered Platelet Concentrates Using Anti‐Leukocyte Filters from Different Manufacturers
Janowska – Stuchlak B , Skrzypczak A , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Backgroud
Due to the number of post‐ transfusion reactions caused by leukocytes (HLA alloimmunity, immunosuppression, transfer infection) in blood treatment it is currently preferable to perform blood transfusions using low leukocyte content components.
Platelet concentrates derived from apheresis or produced from leukocyte‐platelet buffy coats using Orbisac or Taxi automatic methods are finally of low leukocyte count. In our institution, most platelet concentrates are obtained manually from leukocyte‐platelet buffy coats. The components obtained in this way contain an average of 63.7 × 106 leukocytes in one container and to remove them anti‐leukocyte filters are used.
Aims
The aim of this study was to compare the quality of the filtered platelet concentrates and efficiency of filtration using anti‐leukocyte filters from different manufacturers.
Methods
The study involved 210 packages of pooled platelet concentrates. Each package was produced manually from 5 leukocyte‐platelet buffy coats and filtered using Fresenius Kabi, Terumo and Haemonetics anti‐leukocyte filters – 70 packages of each type of filter.
Samples were taken prior to the filtration and immediately after the filtration
The samples were evaluated for: The number of platelets before and after the filtration
Platelet loss caused by the filtration
Volume of platelet concentrate before and after the filtration
Loss of volume due to the filtration
The number of WBC before and after the filtration
The study was performed using a Horiba Pentra XL 80 hematology counter and a Beckman Coulter FC 500 flow cytometer. Measuring of the WBC and PLT count before the filtration is carried out on the basis of impedance changes. The limit of linearity for PLT is 0–2800 G/l and for WBC 0–120 G/I. The WBC count after the filtration was determined using a LeukoSure set intended for calculating the WBC count in low leucocyte blood components. This method uses propidium iodide, which binds only to DNA resulting in fluorescence emission of nucleated cells in the sample, in proportion to the contained DNA quantity.
Results
Table No. 1

Summary/Conclusions
1. All Platelet concentrates subjected to filtering were low leucocyte regardless of the type of the anti‐leukocyte filter
2. In all platelet concentrates subjected to filtering the platelet content met the quality requirements which was above 3.0 × 1011 for 1 package
3. The lowest volume loss (2%) was obtained using anti‐leukocyte Hemonetics filters
4. Platelet count loss was small and comparable using Hemonetics (7.5%) and Terumo (6.8%) filters whereas it was high (16.9%) using Fresenius Kabi filters.
P‐256
Red Blood Cell Components Processed from Hemochromatosis Patients Converted to Blood Donors Display Modest Alterations Compared to Standard Blood Components
Cognasse FC 1, Sut CS 2, Hamzeh-Cognasse HHC 2, Laradi SL 1, Bost VB 1, Aubrège CA 1, Acquart SA 1, Vignal MV 3, Boutahar NB 4, Arthaud CAA 1, Eyraud MAA 1, Pozzetto BP 2, Tiberghien PT 5, Garraud OG 6, Cognasse FC 1
1French National Blood System (EFS), Saint‐Etienne, France2Université de Lyon, GIMAP‐EA3064, Saint Etienne, France3Etablissement Français du Sang Rhône‐Alpes, Beynost, France4Département de Biochimie Hôpital Nord, CHU de Saint‐Etienne, Saint‐Etienne, France5Etablissement Français du Sang, Saint‐Denis, France6Institut National de Transfusion Sanguine (INTS), Paris, France
Background
Periodic bloodletting is the major treatment of Hereditary Hemochromatosis (HH) as red blood cells contain an abundant quantity of iron. Debates have been engaged concerning bloodletting in patients who could be converted into donors.
Aims
In this study, we investigate the quality of RBCCs (Red Blood Cells Concentrates) obtained from HH patients undergoing bloodletting and compare target parameters in HH RBCCs vs regular RBCSs, all along the allowed 42‐day storage.
Methods
RBCCs were obtained from HH patients and regular blood donors, by whole‐blood collection, and stored up to 42 days according to the French national regulation and blood bank conditions. The followed up parameters were; (i) Haematological and biochemical analysis; (ii) RBC membrane and soluble inflammatory markers; (iii) And the pro‐inflammatory potential of HH RBCC supernatant towards endothelial cells in an in vitro model.
Results
No major differences within the two groups were observed concerning biophysical, biochemical parameters and immunomodulatory soluble factors. We observed several significant ‘though minor’ differences concerning RBC membrane protein modulation during storage, i.e. an increased Annexin‐V expression as well as an increased hemolysis within the HH RBCC group. No difference within the two groups was observed regarding the bioactivity of immunomodulatory soluble factors during storage from RBC supernatant from HH patients and control donors concerning the effects on epithelial reporting cells in vitro.
Summary/Conclusions
RBCCs within regular RBCCs and HH RBCCs look equally suitable for transfusion purposes according to all criteria defined herein, with special focus on inflammation.
P‐257
Resting Levels of Platelet Activation in Platelet Donors Reflect Responses to In‐Vitro Activation
Cardigan R1, Kelly AM 2, Garner SF 1, Furnell A 3, Ouwehand WH 2
1NHS Blood and Transplant, Cambridge, United Kingdom2NHS Blood and Transplant and Department of Haematology, University of Cambridge, Cambridge, United Kingdom3Department of Haematology, University of Cambridge, Cambridge, United Kingdom
Background
We have previously demonstrated that there is a wide range of platelet responses to in-vitro activation between individuals, with some donors having platelets with low levels of responsiveness, while others are very responsive and are considered high responders. Futhermore, a donor's level of responsiveness is consistent over time. Reports have also demonstrated that the degree of platelet activation induced by apheresis procedures varies between donors, and there are indications that the appearance of aggregates in apheresis platelet concentrates may be a donor‐related phenomenon. It is therefore relevant to know if donors who are high responders also have higher basal, resting levels of platelet activation than low responders, since donors with higher resting levels of activation could be consequently prone to mechanical, apheresis‐induced activation and, or, also be associated with the presence of aggregates in platelet concentrates.
Aim
We aimed to investigate the potential association between donors’ platelet responses to agonists in-vitro and their resting, basal level of platelet activation.
Methods
A cohort of 450 donors had their platelet function characterised by flow cytometry, measuring P‐selectin expression and fibrinogen binding after in-vitro stimulation with either cross‐linked collagen related peptide or adenosine 5′‐diphosphate as agonists. In addition the degree of P‐selectin expression and bound fibrinogen in the absence of agonist was measured, as a refection of the level of resting, basal activation. Seventeen donors who were initially found to be within upper 10% of observed agonist responses (high responders) and 12 from the bottom 10% (low responders) were selected as being individuals most likely to have the greatest difference in basal activation, and were tested on a second occasion.
Results
Basal P‐selectin expression was significantly higher in high responder donors in both the original (P = 0.001) and repeat (P = 0.003) tests. Similarly, the level of bound fibrinogen in the high responder donors was significantly greater than in the low responder donors (P = 0.016) at the initial testing, but the difference was not maintained at the repeat testing (P = 0.107) predominantly due to a wide variation in the high responder group.
Conclusions
These observations support the hypothesis that in platelet donors there is a variation in the level of resting, basal platelet activation, which reflects the variation seen in responses to agonists. We are therefore examining if donors who have relatively high levels of basal platelet activation and high responses to agonists are also prone to apheresis induced platelet activation, and, or, are associated with donations containing aggregates.
P‐258
Pooled High Content Platelets from Reveos and Pathogen Inactivation with Intercept Blood System™
Loef HL , Lejskog KL , Vedin IV , Knutson FK
Akademiska Sjukhuset, Uppsala, Sweden
Background
The Reveos system (Terumo BCT) is a fully automated device able to process four whole blood units simultaneously into a plasma unit, a red cell unit and, one unit with white blood cells and an interim platelet unit (IPU). Multiple IPUs can be pooled to form a transfusable platelet product.
Aims
The aim of our study was to evaluate the possibility to use pooled IPUs to form ‘Large Platelet Units’ with a quality and volume acceptable for Intercept blood systems™ (IBS) treatment for ‘Large Platelet Units’
Methods
Whole blood units, 450 ml, collected in Reveos collection bags and held 17–21 h on a bench in room temperature were processed using the Reveos 3C procedure to generate IPUs (n = 110) for pooling. Before pooling each IPU rested one hour on bench and on an agitator two hours before pooling and filtration. For the pooling of IPUs we used four, six or seven to adjust PLT, Volume and Plasma content to meet Intercept Blood Systems™ specifications.
Result
Reveos 3C generate with different settings IPUs (n = 110) with a volume between 11.4 −37.6 ml (mean 22.1 ml)
Pooling of seven IPUs 15–20 ml with 280 ml PAS (SSP+, Macopharma) and 140 ml Plasma shown to be optimal, (n = 8) with an addition of PAS before the 2 h agitation.
Platelet: 5.46 ± 0.49 × 10e11/unit (IBS 2.5–7.0 × 10e11/unit), Volume 396 ± 8.3 ml (IBS 300–420 ml/unit), Plasma content 33.3% (IBS 32–47%)
Conclusion
Our study evaluate the possibility to use pooled IPUs to ‘Large Platelet Units’ with a quality meeting criterions for Intercept blood systems™ (IBS) treatment for ‘Large Platelet Units’ After pathogen inactivation it's possible to split the platelet concentrate into two units, with platelet concentration higher than 2.4 × 10e11/dose and reduce the cost of pathogen inactivation with 50%.
P‐259
Rate of Platelet Bacterial Contamination in Uganda
Hume A1, Ddungu H 2, Kajumbula H 3, Kyeyune-Byabazaire D 4, Jackson O 2, Ramirez-Arcos S 5, Tobian A 6
1CHU Ste‐Justine, Montreal QC, Canada2Uganda Cancer Institute, Kampala, Uganda3Makerere University, Kampala, Uganda4Uganda Blood Transfusion Service, Kampala, Uganda5Canadian Blood Services, Ottawa, Canada6The Johns Hopkins University, Baltimore, United States of America
Background
In high income countries (HIC), bacterial contamination (BC) of platelet units (PU) ranges from 0.01 to 0.07%; studies in Sub‐Saharan Africa (SSA) report BC rates as high as 9%.
Aims
We conducted a prospective, observational study to determine the rate of BC of PU in Kampala, Uganda, and the usefulness of performing Gram stains (GS) to prevent the transfusion of bacterially‐contaminated PU.
Methods
The study was conducted between March and September 2014 at the Uganda Cancer Institute and the Makerere University Microbiology Laboratory. 337 PU were evaluated. All were single, whole‐blood derived units collected and prepared by the Uganda National Blood Transfusion Service from healthy anonymous, volunteer donors. Informed consent for BC testing of PU prior to transfusion was obtained from recipients of study PU. Immediately prior to transfusion, 4 ml was aseptically removed from each PU for bacterial testing. For the first 42 units, chlorhexidine was used to clean the PU port; the ports of the remaining 295 PU were cleaned with 70% isopropanol. Three mL was inoculated into a BD Bactec™ Peds Plus™ Aerobic/F culture vial and incubated in a Bactec™ 9120 instrument. The other 1 ml was used for GS with the remainder (approximately 0.5 ml) refrigerated for follow‐up investigation of positive cultures. GS was deemed positive if >1 organism was observed by two people. Only PU with negative GS were transfused. Culture bottles were incubated for a maximum of 7 days or until positive. For initial positive cultures, a second culture bottle was inoculated with the remaining PU aliquot. PU cultures were considered true positives when initial and repeat cultures were positive for the same bacterium. PU cultures with initial positive but negative second cultures were categorized as false positives. Possible BC were those with positive GS and negative culture results.
Results
330/337 PU had negative GS and culture. 1/337 yielded a true positive culture identified as Streptococcus viridans; 3/337 cultures were false positives (Bacillus spp‐2, coagulase-negative Staphylococcus ‐1); these 4 PU had negative GS. 3/337 PU were possible BC as they had positive GS (Gram‐negative rods‐1, Gram‐positive rods‐1, Gram‐positive cocci‐1) with negative culture results (2/3 from PU for which chlorhexidine had been used). The median PU storage time at testing was 2 days although PU with positive GS or culture results had storage times of 3 (3 units) or 4 days (4 units).
Summary/Conclusions
Although only 0.3% (1/337) of cultures was confirmed, this may underestimate the true rate of BC. Two possible BC (PU cleaned with chlorhexidine) and 3 false positive cultures (second cultures done with only 0.5 ml of PU) have the potential to be confirmed BC. Hence, the positive BC rate of PU in Uganda could be as high as 1.8% (95%CI 0.66–3.83%). While this rate is lower than previously reported in SSA, it is higher than reports from HIC. As GS does not appear to be an effective method to capture BC of PU, alternate methods should be explored in SSA to prevent transfusing bacterially‐contaminated PU.
P‐260
Multi Component Collection with Apheresis Machines: In Vitro Quality of Platelet Concentrates In PasIII
Lagerberg JW , Karssing W , Van der Meer PF , De Korte D
Sanquin Blood Supply, Amsterdam, The Netherlands
Introduction
Both Trima Accel (Terumo BCT) and MCS+ (Haemonetics) apheresis machines are suitable to perform multi component collections (MCC). In one procedure, two (concentrated) platelet (PLT) products, a unit of plasma and a unit of red cells can be collected. After the MCC procedure, PASIII platelet additive solution is added to the PLT to produce a PLT concentrate (PC).
Aim
The aim of this study was to gain data on cellular composition and in vitro quality during storage in PASIII of the MCC‐TCs.
Methods
With Trima Accel and MCS+, 12 and 10 MCCs were performed, respectively. PCs were stored at 20–24°C for 8 days and analysed for haematological and metabolic parameters and activation. Differences between Trima‐ and MCS+‐PCs were analysed using Student's t‐test. P values less than 0.05 were considered significant.
Results
Results are summarized in the Table. All PCs complied with the requirements for volume. Both PLT concentration and total PLT count were lower for MCS+‐PCs compared to Trima‐PCs. One MCS+‐PCs did not comply with the requirement for total platelet count (2.2 × 1011/unit). At day 8 of storage, all PCs complied with the requirement for swirl and pH.
Caption 1: In vitro quality parameters of TCs collected with Trima and MCS+
PLT collected with MCS+ showed higher CD62P expression and binding of Annexin V.

Discussion
All PCs, collected with Trima Accel and stored in PASIII, complied with our Guidelines for Blood products. With the current settings, 1 MCS+‐PC did not comply with the requirement for minimum PLT count. MCS+‐derived PLT were significantly more activated as evidenced by higher CD62P expression and Annexin V binding. Further optimisation of MCS+‐MCC is needed to produce PCs which fully comply with the guidelines.
P‐261
Donor‐Variation Effect on Red Cell Storage Lesion
Tzounakas VLT1, Georgatzakou HTG 1, Kriebardis AGK 2, Voulgaridou AIV 3, Stamoulis KES 4, Foudoulaki-Paparizos LEFP 5, Antonelou MHA 1, Papassideri ISP 1
1NKUA, Athens, Greece2Technological and Educational Institute of Athens, Athens, Greece3Apostle Paul, Educational Institution, Thessaloniki, Greece4National Blood Center, Acharnes, Greece5Regional Blood Transfusion Center, ‘Agios Panteleimon’ General Hospital of Nikea, Piraeus, Greece
Background
A wide variability in the storage capacity and recovery of individual RBCs units from different donors has been observed. This inter‐donor variability is the most significant contributing factor for in‐bag hemolysis of stored packed RBC, even when storage solution, duration, unit volume and leukoreduction are taken into consideration. Certain aspects of RBC storage lesion, such as cell fragility, metabolites and microparticles (MPs) accumulation, oxidative stress‐sensitivity and antioxidant activity, have been proved to be strongly donor‐related.
Aims
This study aimed at the elucidation of the baseline variation in the hematological profile among eligible blood donors and at the discovery of probable links between in vivo characteristics and the quality of the subsequently produced stored RBC concentrates.
Methods
For the pre‐storage study, 137 male regular blood donors (18–25 years old) were recruited. Venous blood was collected into EDTA or citrate vacutainers. RBC storage capacity evaluation was performed in leukoreduced RBC units prepared from 10 young, non‐smoking male donors in CPD/SAGM during storage for 42 days at 4°C. All samples were examined for hematological, biochemical and corpuscular parameters such as RBC indexes, fragility, morphology, hemolysis, antioxidant status, membrane proteins, triglycerides, cholesterol, uric acid, HbF, electrolytes etc. Cytoscape and SPSS were used for network and statistical analysis respectively.
Results
The vast majority of the subjects displayed normal variation in most of the measurements (N = 48). However, as high as 10–40% of the donors exhibited aberrant values of cholesterol, uric acid, total antioxidant capacity (TAC), mean corpuscular fragility etc. Part of that variation was assigned to the presence of thalassemia traits among donors (N = 12). Network analysis revealed a complex system of interactions among parameters examined in vivo and a central role for MCHC, RDW, MCF, ALP, PCT and monocytes. Regarding storage analysis, variables like hemolysis, RBC fragility indexes, plasma oxidation status, nitric oxide (NO) and RBC membrane proteins (Hsp70, Band3 clustering, Prx2, clusterin etc.) exhibited high inter‐donor variability and fluctuation rate throughout the storage period. Notably, certain factors (NO, clusterin, MCF, AnnV+ RBCs and RBC indexes) fluctuated throughout the storage period proportionally to their pre‐storage or day 2 values (TAC and MPs concentration), as evidenced by the strong (P < 0.05 or P < 0.01) correlations between them (eg. fragility with hemolysis). Those correlations were integrated into undirected networks connecting pre‐storage or early storage data with those corresponding to the middle or late days of storage. Among other findings, intrinsic levels of HbF correlated with storage quality of RBC concentrates in terms of TAC, MCF and pro‐coagulant activity of MPs.
Summary/Conclusions
Eligible donors though meeting the established criteria for blood donation are widely heterogeneous in terms of hematological profile. Part of the diversity observed may lead to a different starting point for the development of RBCs storage lesion in individual units. Certain hematological parameters in vivo are correlated with the fluctuation of the same or different characteristics in stored cells. Presence of HbF is indicative of stored RBCs fragility and antioxidant capacity.
P‐262
Transfusion of CMV Unselected Blood Components May Lead to Inappropriate Donor Selection for Patients Subsequently Undergoing Allogeneic Stem Cell Transplant
Hall SM1, Danby R 2, Rocha V 2, Peniket A 2, Murphy MF 1
1NHS Blood and Transplant, Oxford, United Kingdom2Oxford University Hospitals, Oxford, United Kingdom
Background
Infection with cytomegalovirus (CMV) remains a major cause of morbidity and mortality after allogeneic stem cell transplantation (SCT); identification of a CMV‐seronegative donor for CMV‐seronegative recipients is vital in reducing risk of primary CMV infection. Historically, CMV‐seronegative recipients of grafts from seronegative stem cell donors have received products from CMV‐seronegative blood donors in order to prevent transfusion‐transmitted CMV infection. Leucocyte‐reduced (LR) blood products are considered to carry minimal risk of CMV transmission; many UK transplant centres now provide LR, CMV‐unselected blood products (CMV‐U) for CMV‐seronegative potential SCT recipients.
Previous CMV exposure in potential SCT recipients is determined by detection of serum CMV IgG but seronegative patients transfused with blood from CMV‐seropositive donors may passively acquire sufficient antibody to give positive results on routine testing with potential adverse consequences for subsequent donor selection.
Aims
To establish whether passive transfer of antibody during transfusion of CMV‐U components is a significant problem we set out to find the frequency of discordant (i.e. both positive and negative) CMV IgG results over time in patients being treated and assessed prior to allogeneic SCT.
Methods
We retrospectively reviewed all patients diagnosed with acute leukaemia or myelodysplasia who underwent SCT in our centre following the change to CMV‐U products.
Results
Thirty‐one adults diagnosed with acute leukaemia or myelodysplasia were identified. All patients had ≥1 CMV IgG checked, 29 patients (93.5%) had ≥2. Transfusions were given to 11 patients (35.5%) prior to baseline CMV IgG testing. Of those with ≥2 tests before SCT, 8 patients (27.6%) had discordant results.
All patients with discordant results were transfused between negative and subsequent positive results and were thought to have passively acquired CMV IgG via transfusion. Due to the retrospective nature of this study, we cannot prove unequivocally that discordant results occurred as a result of passive transfer of antibody; further testing to document the disappearance of CMV IgG would be required. Six of the 8 likely CMV naïve patients were recorded in the transplant database as seropositive.
Summary
False positive results in SCT recipients have multiple implications. Firstly CMV seronegative patients with a CMV‐seronegative vs seropositive donor have better outcomes. Secondly, SCT databases are frequently for registry data and retrospective analyses including the association of CMV status with clinical. We found 75% patients with discordant results were recorded with an incorrect serostatus.
The simple solution to this issue is to measure CMV IgG in potential SCT recipients prior to transfusion. However our data indicate that this is done in barely two‐thirds of cases.
In conclusion, transfusion history must be taken into account when interpreting CMV serology before SCT. False positive results are an unintended consequence of transfusing CMV‐U products to potential SCT recipients. This has the potential to cause morbidity due to the selection of a CMV‐positive rather than a CMV‐negative donor. The problem is preventable by increased recognition by transplant centres, specifically by measuring CMV IgG in potential transplant recipients prior to transfusion, undertaking repeat testing when discordant results are found and resolving discrepancies prior to transplant.
P‐263
Effect of Phosphate and Glucose in the Anticoagulant on the Quality of Red Cell and Platelet Concentrates
van der Meer PF , Korsten H , Lagerberg JH , de Korte D
Sanquin Blood Bank, Amsterdam, The Netherlands
Background
For decades, whole blood (WB) has been collected in citrate phosphate dextrose (CPD) anticoagulant. Citrate is the actual anticoagulant, but phosphate and glucose were added to improve the quality of the red cells when stored as whole blood. With the introduction of blood component therapy in the 1970s, the composition of the anticoagulant was never re‐evaluated, while studies show that platelets are stimulated by phosphate to produce lactic acid from glucose, thereby lowering pH during storage, resulting in poor platelet quality. Therefore, it was our aim to investigate the effect of phosphate and glucose in the anticoagulant on the in vitro quality of platelets, red cells and plasma.
Methods
The effect of phosphate in the anticoagulant was studied by collecting WB in acid citrate dextrose (ACD), and the effect of phosphate and glucose was studied by collecting in tri sodium citrate (TSC). Five hundred ml WB was collected either in our standard systems with 70 ml CPD, or in systems where CPD was replaced with 70 ml ACD or 50 ml TSC. WB was processed into a red cell concentrate, buffy coat (BC) and plasma. The BC was further processed into a ‘single’ platelet concentrate (PC) in plasma/SSP+ (35/65%). Storage experiments were performed with the red cells and PCs with regular sampling for in vitro measures. Plasma clotting parameters were determined.
Results
The results are summarized in the table. There were few differences in the platelet parameters whether collected in CPD or ACD, suggesting that phosphate has little effect on platelets. However, in the absence of glucose when collected in TSC, glucose was 1.0 ± 0.2 mmol/l on the day of PC preparation vs 7.7 ± 0.7 mmol/l when collected in CPD. Consequently, glucose was rapidly depleted, resulting in low ATP on day 8 (target value >4 μmol/1011 platelets) and high annexin A5 values (target, <30%) as sign of apoptosis.

TSC itself has a high pH, resulting in a pH>7.0 in the red cell concentrates, at which level diphosphoglycerate mutase remains active and 2,3‐DPG is synthesized. Hence, on day 21, 2,3‐DPG can still be demonstrated when collected in TSC. As for platelets, absence of phosphate had no effect on red cells. Under all conditions, ATP content was above the target value of 2.7 μmol/g Hb. aPTT, PT and fibrinogen concentration of plasma were not different among the groups.
Conclusion
In the era of blood component therapy, phosphate in the anticoagulant appears to serve no purpose. Removal of glucose from the anticoagulant leads to poor in vitro quality of platelets when stored in additive solution without extra glucose. Need for repeat with plasma stored platelets when stored in additive solution without extra glucose. Further studies are needed to confirm these findings, and storage studies with glucose added to the platelets need to be performed.
P‐264
In Vitro Quality of Platelet Concentrates from Overnight‐Held Buffy Coats in 2 Different Platelet Additive Solutions
van der Meer PF1, Lorinser J 1, Hoenderdaal R 1, Vlaar R 2, Gouwerok E 2, Lagerberg JH 1, de Korte D 1
1Sanquin Blood Bank, Amsterdam, The Netherlands2Sanquin Research, Amsterdam, The Netherlands
Background/Aim
In our blood center, the demand for red cells is decreasing while platelet demand is almost unchanged. Whole blood donations collected in the morning are currently not used for platelet preparation in platelet additive solution; only platelets in plasma are prepared. Because of the shifting demands, we anticipate that we have to make better use of our resources, and therefore we aimed to study the in vitro quality of platelet concentrates prepared from whole blood from morning collections that were processed the same day into buffy coats, and where the buffy coats were held overnight for platelet preparation the next day. Two platelet additive solutions (PASs) were evaluated: Intersol, and SSP+ which contains additional potassium and magnesium (that are known to largely prevent platelet activation). Both additive solutions contain acetate that can be oxidized by the platelets to provide energy.
Methods
Whole blood was collected in the morning hours and held for at least 4 h at room temperature. That afternoon, buffy coats were prepared, which were held for 12–14 h (overnight) at room temperature. Next morning, five buffy coats were pooled, and either Intersol (PAS3, Fresenius, Bad Homburg, Germany) or SSP+ (MacoPharma, Mouvaux, France) was added, and a platelet concentrate was prepared. Platelet concentrates were stored for 8 days with regular measurement of in vitro quality.
Results
The results are summarized in the Table. With Intersol, the platelets show considerable activation (CD62P expression) and apoptosis markers (annexin A5 binding), while with SSP+, these remain below our target values. Glucose metabolism was higher in Intersol, and by day 8, 4/25 units had depleted their glucose, while all units in SSP+ still had detectable glucose. ATP was higher when SSP+ was used.

Conclusion
Platelet concentrates in platelet additive solution, prepared from morning blood collections, conform to our requirements. Our results indicate that SSP+ is best able to maintain in vitro platelet quality with low activation and apoptosis measures, and glucose remains present until the end of the storage period.
P‐265
Washed Blood Components – An International Survey of Production and Use
Thomas S , Cardigan R
NHS Blood and Transplant, Watford, United Kingdom
Submitted by the authors on behalf of the BEST Collaborative
Background
Internationally, there is wide variation in practice in the secondary processing of blood components.
Aim
To find areas of commonality that might be used to promote more standardised practice.
Method
A detailed survey was compiled and circulated to members of the European Blood Alliance (EBA), Alliance of Blood Operators (ABO), and the Biomedical Excellence for Safer Transfusion (BEST) Collaborative.
Results
31 responses were received, along with two nil responses.
Washed components are issued to patients with severe allergic reactions to conventional components.
Washed components are issued to IgA deficient patients, but in 11/25 responses clinical evidence of previous allergic reaction is required
15 services collect IgA deficient components as well as providing washed components for IgA deficient patients
Washed red cells
30 services or hospitals reported supplying washed red cells; 39,000 of 14.5 million (0.3%) red cells issued
Starting material
Mostly whole blood derived red cells, with 7 different additive solutions, 2 anticoagulants (without additive)
3 use apheresis‐derived
Mean age at time of wash is 7.4 days (n = 9) but some regulations permit <42 day
8 use manual systems, 22 automated (some use both devices)
15 × Cobe 2991 – open system, post wash shelf life 24 h
8 × Haemonetics ACP215 – closed, post wash shelf life up to 14 day
Solutions
19 wash in saline, 10 in saline + glucose (ACP215), 3 wash with additive (some use more than one wash solution)
11 store in saline, 5 in saline + glucose, 8 in additive, 6 do not store
Post wash shelf life is most commonly 24 h (n = 23), but may be up to 14 days if a closed system and additive solution used for storage.
Washed platelets
21 services supply washed platelets; 4500 of 2.6 M (0.17%) of platelet issues
19 start with apheresis platelets, 9 use pooled, 7 use both
Aim for platelets less than 3 but up to 5 days old permitted
Mean age at time of wash is 2 days (n = 3)
14 use manual method, 9 automated (Cobe 2991), 2 use both
1 acidifies first, 1 pre‐dilutes in saline
13 wash in saline, 1 + ACD, 2 + dextrose; 5 different PAS used
4 h (n = 15) or 24 h (n = 4) shelf life post wash
All store in the same solution as used for the wash
Conclusions
Many services provide/use washed components to avoid transfusion reactions in certain patients, but there is variation in the indications for these components.
Three methods of red cell washing are in equal use (one manual and two automated), and with a variety of additive solutions and shelf lives assigned.
Most platelet washing is manual, although one device is also used; a variety of solutions are in use, but no centres had a shelf‐life of greater than 24 h.
There are areas where it would appear feasible to achieve greater harmonisation internationally, especially in respect of shelf‐life.
P‐266
Washed Red Cells in Australia and Abroad: Practice Patterns and Potential Areas for Improvement
Aung N , Oh DH , Hogan CJ
Australian Red Cross Blood Service, Melbourne, Australia
Background
Red cell washing is to remove plasma proteins for prevention of severe and recurrent allergic reactions. The patient groups include those with reactions to transfused plasma protein (IgA deficiency) and severe allergic reactions despite leucodepletion. In Australia, red cell washing is carried out manually by centrifugation.
Aims
To review the Australian Red Cross Blood Service (the Blood Service) practices regarding the indications and preparation of washed red cells across Australia, and in comparison to other Blood Services internationally.
Methods
Data on washed red cells from July 2013 to June 2014 were extracted via the Corporate Information Management and Reporting (CIMAR) system from the Blood Service National Blood Management System (NBMS).
Four international Blood Services were provided with survey questions through our International Bench Marking Department: American Red Cross (ARC), Canadian Blood Services (CBS), NHS Blood and Transplant (NHSBT) and BSI (a US based blood operator, also known as Blood Systems Inc.).
Results
The total red cell units supplied in Australia were 703,359 and 8302 units were washed red cells (1.18%) (Table1).
Nationally, 80.8% of washed red cells were provided to Victoria. 92.6% of these washed red cells were distributed to a single institution, which is the government funded state centre for thalassaemia.
International
In terms of indications, there was no major difference between Australia and other parts of the world.
Australia supplied the highest percentage of washed red cells (1.18) compared to other Blood Services (0.07–0.4) (Table 2).
Currently in Australia, the Blood Service uses a manual centrifuge method with 3 washes and total volume of 0.6 l saline. The majority of washed red cells were processed by an automated system in the UK. However, NHSBT also used a manual washing method. American and Canadian Blood Services only used an automated processor. Extra‐wash requests are rare in Australia and processed by 10 times washing with normal saline and total volume 2.0 l. Specifications of washed red cells vary among the Blood Services depending on the method used.
Caption 1: Number of washed red cells provided in each state and territory in Australia

Conclusions
Washed red cell provision not only costs more, but also is labour intensive. Use of automated cell processors should also be considered in Australia, as this is already implemented successfully in other international Blood Services.
Traditionally washed red cells have been used for prevention of febrile non‐haemolytic transfusion reactions. The majority of washed red cells here are used for thalassaemia patients. However, this practice arose predominantly in the pre‐leucodepletion era. Our findings suggest that there may be a relative overuse of washed red cells here. Australia should review the indications for washed red cells and also clarify these indications against historic practice.
Transitioning from washed red cell transfusion to standard leucodepleted products, in this patient group, may be potentially challenging and will require appropriate discussion and negotiation with clinicians and long‐standing washed red cell recipients.
Caption 2: International Comparison of Washed Red Cell Supplies

P‐267
Thrombolux Evaluation of Platelet Quality and Bacterial Contamination in Buffy Coat Platelet Concentrates
Vetlesen AV1, Brendemo MB 1, Stubhaug Taksdal TTS 1, Maurer EM 2, Hetland GH 1
1Oslo University Hospital, HF Ullevaal, Oslo, Norway2LightIntegra Technology Inc., Vancouver, Canada
Background
The outcome after platelet transfusion depends on the quality of the product and the patient's condition. Bacterial contamination of platelet concentrates (PCs) can cause serious transfusion reactions. The ThromboLUX (TLX) analyzer (LightIntegra Technology Inc.) uses dynamic light scattering to provide information on type, number and size of particles in PCs and the platelet response to temperature stress. The results are expressed as a ThromboLUX score on a scale from 0–40.
Aims
Determine if TLX analysis of pre‐donation samples can predict the quality of the corresponding buffy coat platelet concentrates (BC‐PCs) and to test the ability of TLX to detect bacteria in BC‐PCs.
Methods
Seven BC‐PCs, each pooled from 5 ABO‐compatible BCs, were analyzed along with platelet‐rich EDTA plasma (PRP) from pre‐donation samples from the 35 involved donors in TLX with 2rd generation software. Three different concentrations, 1 × 104, 1 × 105 and 1 × 106, of Staph. epidermidis or E. coli were added to BC‐PCs, and analysis on the TLX was performed without delay.
Results
There was a positive correlation between the TLX scores for the donor PRP samples and the corresponding seven BC‐PCs the day after production. When platelet quality was measured during storage at 22 ± 2°C on days 2, 3, 4, 5 and 6 after production, six of the products maintained their TLX score above the threshold value of 10, whereas one BC‐PC remained at TLX score <10 throughout the 6 day storage period. We were not able to detect the microbes in the BC‐PCs by TLX, and no qualitative effect on the TLX score was observed.
Summary
In conclusion, the TLX score may be used to omit donors with low platelet quality from the BC‐PC production line. Initial TLX score in BC‐PC is predictive for TLX score during platelet storage, but TLX may not be applicable for detection of pathogen contamination.
P‐268
Study of Functional Parameters in Stored Platelets and Riboflavin Inactivation Effect on Single Donor Plts
Gavalaki GM , Anastasopoulou AI , Polizoidou PM , Kanellopoulou KG , Marinaki MA , Gotsi GS , Chanos CA , Kotsi KP
Laikon General Hospital, Athens, Greece
Background
Platelet transfusions, although a common practice, present several risks and may produce adverse reactions to recipients. Retention of platelets is only 5 days due to the possibility of microbial contamination and the development of platelet storage lesions.
Aims
The purpose of the study was to evaluate the effect of the storage time to the in vitro quality and the possible change in functional characteristics of random donor platelets (RDPs) and the effect of riboflavin inactivation to single donor platelets (SDPs).
Methods
10 RDPs and 14 SDPs units were studied. 10 blood donor samples were used as controls. The RDPs qualification measurements were performed twice: on day 2 (~24 h after bood processing) and on day 5. The SDPs study was held the same day on rested derivatives (RT, no agitation), 2 h after the apheresis procedure and after the inactivation with riboflavin. Neither of the tests was performed in washed platelets. In addition to the mandatory legislation audit (product volume, PLTs and WBCs number, pathogens growth, PH, swirling) all samples were tested for: i)metabolic parameters (PCO2, PO2, glucose, LDH ii)platelet aggregation (activators: collagen 10 μg/ml and TRAP 100 μΜ) iii)quantification of platelet glycoproteins receptors (FACs analysis) iv)residual WBCs on SDPs (FACs analysis). SPSS20.0 programme was used for statistical analysis.
Results
Both RDPs and SDPs covered the requirements of international standards with complete lack of microbial contamination, satisfactory swirling and pH levels in all samples tested. A statistical significant PCO2 and glucose reduction was noticed both in RDPs and SDPs (P < 0.001), consistent to the literature data. Absence of RDPs aggregation with collagen was observed in both measurements. TRAP results (day 2) showed a slight decrease (p:0.043) compared to the control group but without significant statistical differences between the two measurements. SDPs aggregation study proved with no variations before and after the inactivation with riboflavin. RDPs glycoprotein receptors pattern seemed to have a significant statistical decrease of GPIb and GPIIb expression levels (p:0.009 and p:0.015 respectively), while a statistical significant increase was observed in GPIa (p:0.025) levels, during the storage time. Comparison (day 2) with the controls highlighted significant statistical increased expression of GPIIb (P < 0.001), GPIa (p:0.013), GPIIIa (p:0.43) and GMP (p:0.040). No differences were detected in the SDPs glycoprotein receptors tests, before and after the inactivation with riboflavin, except a decrease of GMP expression (p:0.021) after activation with TRAP. SDPs comparison with controls showed no differences.
Conclusions
In this preliminary study, mandatory requirements were covered in all samples. Lack of collagen aggregation is attributed to the production process and the measurement time. The observed changes on TRAP aggregation results and glycoprotein receptors patternare considered to be dueto the storage, but even after 5 days, platelet units retained their common properties, which allow them to be a safe concentrate for transfusion. The SDPS inactivation procedure does not seem to affect the in vitro platelet properties. Further studies, in both laboratory and clinical level, are required in order to evaluate the function and clinical effectiveness of stored and riboflavin‐treated platelets.
P‐269
Evaluation of Quality Parametres of Blood Components Obtained by Separation of Whole Blood With Luxomatic V2 Blood Extractor
Augustyniok Z , Galuszka W , Szulakowska A , Stasiaczek G , Szymczyk-Nuzka M
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Receiving blood components from whole blood is the primary activity of the preparation department. One of the most important devices in this process is automatic blood extractor for whole blood separation. LUXOmatic V2 is such a device intended for use in blood bank and transfusion centers and is able to separate centrifuged blood fully automated.
Aim
The aim of this study was to obtain blood components from whole blood (WB) such as plasma (PL), leucodepleted red cell concentrate (LRCC), buffy coat (BC) and pooled platelets concentrate (PC) with use of LUXOmatic V2 device and evaluate the quality in relation to Polish criteria. Also the quality of operation of the device and time of separation were taken into account.
Materials and methods
Obtained 29 units of whole blood in MacoPharma quadruple bags with in‐line filter, centrifuged with Rotosilenta device and processed using LUXOmatic V2 extractor. Time of separation for 18 processes was measured. Due to separation obtained 29 units of PL, LRCC and BC. Then 25 BC were used to produce 5 pooled PC using TACSI set and device.
Quality parameters of all those components were determined: amount of leukocytes on FACS Calibur flow cytometer and microscope Olympus CX31, hemoglobin, hematocrit and amount of platelets on hematology analyzer Micros 60ABX, pH on pH‐metr Inolab.
Results
Results of quality parameters studied in LRCC are in Table 1, PL in Table 2, BC in Table 3, pooled PC in Table 4.
Mean separation time was 2 min. 17 s (±21 s)


Summary
All quality results of tested blood components not only meet current criteria but also are very satisfying, especially amount of platelets in pooled PC and constant parameters in BC.
Time of separation is quite short.
Quality of operation of LUXOmatic V2 extractor is very high. Automatic valve breakers and side barcodes are very helpful and practical.
P‐270
Photochemical Treatment of Pooled Whole Blood Derived Plasma within 19 h from Collection
Isola H1, Dupuis A 1, Naegelen C 2, Marpaux N 2, Mourey G 2, Ravanat C 1, Laforet M 1, Gachet C 1, Morel P 2
1EFS Alsace, Strasbourg, France2EFS Bourgogne France‐Comté, Besançon, France
Background
The INTERCEPT Blood System (Cerus) is a pathogen inactivation (PI) method that utilizes amotosalen and UVA to inactivate contaminating viruses, bacteria, parasites and leukocytes in platelet concentrates and plasma. Apheresis plasma treated and frozen within 18 h (PFC‐IA) is used in France.
Aims
The objective of this study was to evaluate the quality of plasma derived from whole blood, pooled, treated with INTERCEPT, split and frozen within 18–19 h from collection.
Methods
Of 5 leukodepleted plasma units from whole blood of the same ABO group are pooled and divided into two sub‐units of 650 ml wich are each pathogen inactivated. Both sites prepared 20 pools (5 O; 15 non‐O). Each pool results after PI in 2 × 3 units of >200 ml subsequently frozen. The plasma quality was evaluated before treatment (T1) and after 2 weeks (T2), 6 months (T3) and 12 months (T4, 4 parameters) of storage below −25°C. 28 tests were carried out exploring cellular contamination, plasma proteins, coagulation factors, clotting time measurements, calibrated automated thrombin generation (CAT), fibrinolysis, coagulation and complement activation markers.
Results
see Table 1 for the evolution of selected indicators of plasma quality. Fibrinogen, Factor V, Factor VII, Factor VIII, Protein C, Protein S, alpha 2‐antiplasmin and ADAMTS13 show a moderate reduction following treatment with recoveries of 75% to 95% (68% for FVIII) and stability during storage. French requirements for FVIII to be >0.5 IU/ml and fibrinogen >2 g/l in at least 70% of the units are met with respectively 82% and 93% after 12 months (n = 40). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are increased by respectively 18% and 11% with the treatment. However, thrombin generation potential of plasma is adequately maintained with none of the parameters of the CAT markedly changed with treatment for the concentration of 20 pM tissue factor (TF) recommended for the test. The kinetics of the reaction is slightly affected at the suboptimal concentration of 1 pM of TF without influence on the total amount of thrombin generated (not shown). The measured concentrations of C3a and C5a show no activation of complement due to the treatment or frozen storage. C3a values actually decrease after treatment with INTERCEPT from 174 + 52 ng/ml (pre) to 72 + 40 ng/ml (post) and 87 + 37 ng/ml at 6 months. The evolution of C5a shows the same pattern evolving from 7.8 ± 1.9 ng/ml (pre) to 6.2 + 1.6 ng/ml (post) and 6.4 + 1.5 ng/ml at 6 months.
Table 1: quality indicators of INTERCEPT treated Whole Blood derived plasma (n = 40)

Conclusion
Pooled plasma derived from whole blood meets, after PI treatment and freezing within 18–19 h, the quality standards for PI therapeutic plasma. Plasma quality markers do not reveal significant alteration due to the photochemical treatment and frozen storage. The thrombin generation capacity of plasma is preserved demonstrating its normal hemostatic potential. Access to whole blood derived plasma provides an alternate or supplementary supply to apheresis plasma.
P‐271
Frozen Platelets as an Easy Available Alternative in Blood Components Supply
Bohonek MB , Landova LL
Central Military Hospital – Military University Hospital Prague, Prague, Czech Republic
Background
Massive bleeding and massive transfusion are associated with increased morbidity and mortality in severely injured patients and in some next indications (obstetric hemorrhage, ruptured esophageal varices and arterial aneurysmas, etc). Early and aggressive use of blood products in these patients may correct coagulopathy, control bleeding, and improve outcomes. Majority is preventable if red cells, plasma and platelets are available as soon as possible. Due to their very short shelf life, having a daily stockpile of fresh platelets is not possible for many hospital blood banks.
Aims
The alternative solution is a stock of frozen platelets which are successfully used in military medicine. Since September 2014 we used frozen platelets in routine practice and we preset up‐to date collected clinical and laboratory data.
Methods
Apheresis, lecodepleted, platelets, with >280 × 109 thrombocytes/unit, are (after a removal of supernatant) frozen in −80°C, with 5% DMSO, and stored in the same temperature for up to 2–4 years. For clinical use, there are thawed platelets, preferably group O, reconstituted in thawed plasma, group AB. The reconstitution is a simple process which consists of thawing platelets and plasma in water bath and adding plasma into platelets under mixing. The whole procedure takes 30 min at most. Shelf life of reconstituted frozen platelets is 6 h, during storage in 20–24°C and agitation. Before the clinical use, we performed the validation study with 15 produced units of frozen platelets, next we performed comparative study, where we compared patients transfused with frozen platelets (n = 14) and patients transfused with fresh platelets (n = 10).
Results
All 15 units of checked platelets from validation study meet specified criteria. Compared to fresh apheresis platelets, frozen platelets are (partially) activated, clot strength measured by TEG with citrated kaolin is reduced, and onset of clotting and clot amplification is faster. Clinical and laboratory data in a group of patients transfused with frozen platelets did not display any significant differences compared to the group transfused with fresh platelets. In period September 2014 – March 2015 we have transfused total 53 units of frozen platelets to 19 patients with no adverse effects and with the expected clinical effect.
Conclusion
Frozen platelets are a beneficial alternative not only for military blood banks, but also for civilian blood banks which do not have a permanent stock of fresh platelets available. Due to a relatively easy preparation, the cost of frozen platelets is not high and their storing in small portable deep freezers does not bring any significant additional expenses. Procedure of thawing and reconstitution of frozen platelets is very simple and fast, and it allows for having quality platelets products when dealing with massive bleedings and other urgent as well as for another special indication.
P‐272
Preparation of Thawed Washed Red Cells Suitable for Intra‐Uterine (IUT) and Neonatal Top‐Up Transfusion
MacLaren GRM , Gilbert JG , Healy DH , Chandrasekar AC , Callaghan TC
NHS Blood and Transplant, Liverpool, United Kingdom
Background
The National Frozen Blood Bank (NFBB) processes and stores phenotypically rare red cells for clinical use. Cells are frozen in glycerol, which is removed post‐thaw through automated methods to produce a thawed washed red cell (TWRC). Further processing of a TWRC had not previously been attempted. Between November 2013 and January 2014 there was a requirement to provide group O Rh17 (‐D‐/‐D‐) type red cells suitable for IUT and subsequent neonatal top‐up transfusion.
Aims
To produce a component that could meet specifications for IUT and top‐up transfusion on multiple occasions while minimising wastage.
Methods
At the point of initial request the NFBB had six compatible units in stock, two from the single matching UK donor and four previously sourced from Japan. A further two units from the UK donor were collected and frozen; they could not be used liquid as the donor was positive for antibodies to CMV so units were frozen to reduce the risk of viral transmission. As very small quantities were required for each neonatal transfusion one unit was split post glycerolisation and pre‐freezing by sterile connection to a 600 ml transfer pack, to create two low volume packs and thus minimise wastage. An additional unit was sourced from France.
Red cells were thawed in a 37°C waterbath and washed using the standard method (Haemonetics ACP215). For IUT the cells were then concentrated by centrifugation at 5000 g for 15 min. Supernatant was removed by a manual press and then approximately 15 ml was returned to the pack. The unit was sampled for measurement of haematocrit (Hct) and haemoglobin (Hb).


Results
Four units were provided for IUT. See Table 1.
Four units were provided for neonatal top‐up. See Table 2.
Conclusions
The NFBB was able to provide eight units for IUT and neonatal top‐up transfusion for a specific patient. Three units for IUT were provided from the single compatible UK donor. All met specification for both volume and haematocrit, but the haemogobin content fell short of the specification for IUT by up to 4 g/unit. All three units met all specifications for TWRC. The unit sourced from Japan failed to meet specifications for both IUT and TWRC, however it still proved a valuable component in the treatment of this patient.
Due to the rarity and specific clinical need for these components, it is unlikely that strict specifications will be developed and enforced. However, guidelines on the preparation of these components may be beneficial to encourage consistency and best practice.
P‐273
Assessment Of Full‐Automated Whole Blood Processing With Tacsi Wb At Lisbon Blood And Transplantation Center (Cstl)
Santos M1, Teles A 1, Rosa C 1, Imholz M 2, Teles A 1
1Instituto Português do Sangue e Transplantação, Lisbon, Portugal2Terumo BCT Europe NV, Zaventem, Belgium
Background
The CSTL successfully implemented TACSI PL for the processing of pools of buffy‐coat (BC) units into platelet concentrates (PC) 5 years ago and wanted to assess the feasibility of whole blood processing with TACSI WB. This device is able to process six whole blood units per run in 20 min into red cell concentrates in SAGM (RCC), plasma (PL) and buffy‐coats.
Aim
To evaluate the performance of TACSI WB for: i. product quality, according to the CE guidelines (17th edition), ii. results consistency; iii. compatibility to down‐stream process (pathogen reduction treatment ‐PRT).
Methods
Of 108 whole blood units collected into TACSI CRC kits with integrated RCC filter were processed with the TACSI WB device. After the first centrifugation step the resulting RCC were leukoreduced. RCC were assessed for volume, hemoglobin content (Hb), hematocrit (Htc) and residual white blood cells (WBC); BC units were tested for volume, platelet (PLT) counts, recovery percentage of PLTs and Htc; plasma was assessed for volume and residual cells. After some adjustments, initial investigation with 4‐BCs pools (n = 7) was followed by pools of 5‐BCs (n = 13). After pooling, the second centrifugation was carried out on the same TACSI WB device with the TACSI PL kit resulting in leukoreduced PCs. Final PCs were tested for volume, PLT and WBC counts and pH at day 5 of storage.
Results
All leukoreduced RCC were within the requirements. The Hb content was 56.5 ± 6.9 g/unit, Htc 62% ± 3 and residual WBCs 1 × 105 WBC/unit. Parameters used in the first separation resulted in a smaller BC volume, improving Hb content and optimizing plasma recovery. The obtained BC had an average volume of 50 ml, with PLT 1 × 1011 and Htc of 40% .Average plasma volume was 273 ml with minimal cellular contamination‐ WBC 0.9 × 106/l, RC 0.6 × 109/l and PLT 1.4 × 109/l, all far below EU thresholds. Final PCs derived from pooling of 4 BCs contained plasma in a percentage of 27% and were not compatible with the downstream PRT process. PC derived from pools of 5 BCs were within the guardbands of 35% plasma and were suitable for PRT with INTERCEPT, with an average volume of 373 ml, PLT 4.23 × 1011 and pH of 7.1 at day 5. Hence, all blood components processed with TACSI WB were in accordance with the European guidelines.
Conclusions
Our experience showed that the TACSI WB allowed an easy implementation and was less time‐consuming than the semi‐automated method. Blood components met European guidelines as well as local requirements. This system has the potential to improve the efficiency of blood processing and we inten d to proceed into a larger evaluation study.
P‐274
Introduction of Leukodepleted Red Blood Cells Concentrates In Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Wojciechowska – Chorebala A , Malyszczak E , Szymczyk – Nuzka M
Regional Centre of Blood Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Leukodepletion is the leukocytes removal from various blood components with specific filters or devices. Transfusion of such blood components can minimize the risk of post transfusion adverse effects resulting from the presence of leukocytes.
Since May 2013 we have been using, as the first Regional Centre of Transfusion Medicine and Blood Bank in Poland, only leukodepleted RBCs (LR‐RBCs).
It was the next step to improve haemovigilance system after we started to produce only leukodepleted, irradiated platelet concentrates.
Aims
The goal of this study was to evaluate this innovative solution by our Centre of Transfusion Medicine, by Transfusion Co‐ordinators and medical staff from hospitals.
Methods
We supply 33 hospitals in our region with all blood components.
Leukodepleted RBCs have been obtained in our centre using three methods:
A semimanual method with blood collection bags with in‐line filter,RBCs from TACSI WB device,Erythro‐apheresis with MCS+.
Since May 2013 only leukodepleted RBCs have been produced. We compared two periods of time in which transfusions were performed. One group, from 1st May 2012 to 30th April 2013, included non‐leukodepleted RBCs while the other, from 1st July 2013 to 31st June 2014, RBCs after leukodepletion. It was expressed per 10,000 therapeutic units of specific blood components, which were given to the clinical use. We counted the number of hospital complaints about RBCs connected with the presence of clots in RBCs bags. We summarized opinions and comments about using leukodepleted RBCs from hospitals after approximately one‐year experience.
Results
1. The number of adverse reactions after transfusions of leukodepleted RBCs significantly decreased‐ FNHTR (from 4.41 to 2.18); TAD (from 1.36 to 1.0) and TRALI (from 0.17 to 0) (Table 1).
2. From 1st January 2012 to 30th April 2013 there were 36 RBCs complaints from hospitals‐ concerning clots in the blood bags. In the corresponding period of time after introduction of leukodepletion no complaints about the above problem were reported.
3. Benefits connected with transfusion, which were reported by Transfusion Co‐ordinators: Previously waiting time was longer because filtration was performed immediately before transfusion,Leukodepleted RBCs for newborns and foetuses are available at once when required,Doctors confirmed that their patients had fewer adverse reactions, thus they didn't require any additional therapy.
4. Benefits for Blood Banks are connected with better blood components management.
Before introducing obligatory leukodepletion we had some issues when LR‐RBCs of rare phenotype were needed immediately; we also had a number of complaints because of clots found in blood bags.

Conclusions
1) Our study confirmed connection between introduction to clinical use only leukodepleted RBCs and decreased number of FNHTR, TAD and TRALI.
2) Introduction of safer and quicker available blood components was strictly associated with customer satisfaction and improved haemovigilance.
3) Leukodepletion of not all bags with RBCs was identified as contributor to RBCs wastage.
P‐275
Changes of Microparticles in RBC Concentrates After Irradiation
Lim CSL
Korea university, Seoul, South‐Korea
Background
Microparticles (MPs) have important pathological relevance for various diseases. Microparticles in blood components might contribute to transfusion‐related immunomodulation or other side effects. However, MP changes in transfusion units related to irradiation process have not been investigated.
Aims
The aim of this study was to evaluate how irradiation affects MP counts within transfusion units.
Methods
A total twentyRBC concentrates within 14 days after donation were exposed to gamma rays (Dose rate:25 cGy) by a cesium‐137 irradiator (IBL 437, CIS bio International, Germany). The numbers of MPs derived from RBC concentrates before and 24 h after irradiation were measured by flow cytometry. MPs were defined as particles with the size less than 1.0 μm, and their origin was characterized by staining of CD235a‐PE or CD41‐FITC. The numbers of microparticles within transfusion components in pre‐irradiation and post‐irradiation conditions were analyzed.
Results
The total number of MPs (±standard deviation) in the RBC concentrates (n = 20) was 21,858/μl (±22.691), ranging from 2.619/μl to 96.907/μl. Total MPs increased up to 22.635/μl (±31.604) after irradiation. Before irradiation, CD41‐positive MPs and CD235a‐positive MPs comprised 9.5% (1.031/μl) and 2.2% (263/μl) of total MPs, respectively. After irradiation of RBC concentrates, CD41‐positive MPs increased upto 12.1% (1.475/μl). But CD235a‐positive MPs decreased to 2.0% (214/μl) of total MPs. CD41‐positive MPs showed statistically significant numerical difference between pre‐irradiation and post‐irradiation (P = 0.014), while differences of total MPs (P = 0.785) and CD235a‐positive MPs (P = 0.369) was not significant between before and 24 h after irradiations.
Conclusions
RBC products have various microparticles related to unfavorable transfusion effects including platelet and cellular origins, and their characteristics are different before and after irradiation. Thus, the investigation of the potentially pathogenic effects of MPs in blood products associated with irradiation required.
P‐276
The Effect of the Platelet Concentration in the Donors Blood on the Final Concentration of Platelets in Platelet Concentrates
Przybylska E , Skrzypczak A , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Background
Platelets are disk‐shaped granular cells with no nucleus. Their main role is to maintain the hemostasis. The lifetime of platelets is about 10 days, and then they are removed from the blood system. In the case of thrombocytopenia or platelet dysfunction it is desirable to use platelet concentrates which can be prophylactic or therapeutic. Platelet concentrates are obtained using two methods: automatic (plateletpheresis) or manual (preparing Buffy Coats from whole blood). Platelet concentrates stored in incubators, with gentle mixing, and controlled temperature of 20–24°C have a period of the validity up to 5 days. Apart from the plates platelet concentrates contain a small amount of anticoagulant, plasma and leukocytes.
Aim
The aim of this study is to answer the question whether the initial concentration of platelets in donors blood may affect the final concentration of platelets in platelet concentrate.
Methods
The concentration of platelets in blood from 300 donors and also contents of PLT in 60 platelet concentrates produced from these donations were evaluated in RegionalBloodCenter in Pozna�’ in the period from January to August 2013.
The number of platelets was determined by impedance method using two hematology counters. The peripheral blood was testes using the analyzer Medonic Boule and platelet concentrates were tested using the analyzer Pentra XL 80 Horiba.
Results
1. The average concentration of platelets in 300 donors was 226 × 109/l.
2. The average content of platelets in 60 platelet concentrates pooled from Buffy Coats was 0.69 × 1011/unit. so 3.45 × 1011/in set.
3. The calculated Pearson's correlation coefficient is equal to 0.312.
Conclusion
1. Using a Guillford's scale you may say that the correlation is low and the factor is clear but weak.
2.The average number of platelets in the donor remains within the reference range of 150–450 × 109/l and is 226 × 109/l.
3. It can be said that the number of platelets in donors blood is stable and not subject to great fluctuations.
4. Random pooling of Buffy Coats without the prior knowledge of the initial number of platelets in donors allows for the production of platelet concentrates which fulfill the quality requirements.
P‐277
Multi Component Collection with Apheresis Machines: In Vitro Quality of Red Cell Concentrates in Sagm
Lagerberg JW , Karssing W , Van der Meer PF , De Korte D
Sanquin Blood Supply, Amsterdam, The Netherlands
Introduction
Both Trima Accel (Terumo BCT) and MCS+ (Haemonetics) apheresis machines are suitable to perform multi component collections (MCC). In one procedure, two platelet products, a unit of plasma and a unit of red cells can be collected. After the MCC procedure, SAGM is added to the red cells to produce a red cell concentrate (RCC). Leukocytes are removed by filtration.
Aim
The aim of this study was to gain data on cellular composition and in vitro quality during storage of the MCC‐RCCs.
Methods
With both Trima Accel and MCS+, 11 MCCs were performed. RCCs were stored at 2–6°C for 35 days and analysed for haematological and metabolic parameters. MCC‐RCCs were compared with RCCs prepared from overnight stored whole blood. Differences between groups were analysed using Student's t‐test. P values less than 0.05 were considered significant.
Results
Results are summarized in the Table.
The volume of RCCs collected with MCC was lower compared to standard RCCs. 4 RCCs (3× Trima, 1× MCS+) did not comply with the requirements for volume. MCC‐RCC haematocrit and total haemoglobin were significantly lower than standard RCCs, but complied with the guidelines. All MCC‐RCCs complied with the requirement for haemolysis, with lowest values for MCS+‐RCCs. ATP content on day 35 was comparable for all groups and complied with the requirements.
Caption 1: In vitro quality parameters of RCCs collected with Trima and MCS+

Summary/Conclusions
With the current settings, MCC‐RCC's volume andtotal haemoglobin were significantly lower compared to standard RCC and were not always conform to our Guidelines for Blood products. Haemolysis during storage in SAGM was lower for MCS+‐RCCs, but there is probably an association with the low haemoglobin and/or higher plasma content of these units. Further optimisation of MCC is needed to produce RCCs which fully comply with the guidelines.
P‐278
Evaluation of the Quality Of Blood Components Using Two Different Processing Methodologies
Dias FD , Valente EV , Machado EM , Spinola AS , Amil MA , Bini MB , Coutinho JC
Centro Hospitalar do Porto, Porto, Portugal
Background
Since 1998, prestorage leukoreduction of all cellular blood components has been made mandatory. Being recommended by Council of Europe Guide and mandatory by Portuguese law 267/2007 a minimum Hb of 40 g/unit and a LC <1 × 106 unit. Blood component must meet the minimum quality control (QC) requirements in regards of performance criteria: hemoglobin (Hb) content in the red blood cell (RBC) unit and residual leukocyte count (LC).
Aim
To compare Hb and LC in RBC units processed by different methodologies: Top and Top (TT) vs Top and Bottom (TB). To evaluate compliance of the QC criteria.
Methods
Between 2010 and 2014, Hb was measured by an automatic haematological counter (Coulter LH780) and LC by flow cytometry‐FacsCanto II, Leukocount Beckman Coulter kit. Two processing methods and two different RBC filters were compared. In 2010 and 2011, Pall Corporation filters at 2° to 6°C (Grifols SA, Spain) were used and after Bioflex RCC filter (Fresenius Kabi, Germany) used at room temperature. Statistical analysis was performed using non‐parametric Mann‐Whitney and Chi‐square test, P value <0.05 significant.
Results
Every year RBC were submitted to QC (2010 – 452 −3%; 2011 – 524–3.6%; 2012 – 494–3.9%; 2013 – 355–3.3%; 2014 – 349–3.3%). 98.8% of the tested RBC had >40 g/unit. The mean Hb value/year varied from 49.4 to 57.0 g/unit, being overall Hb content influenced by the processing method (53.8 ± 7.2 g/unit for TT vs 46.3 ± 6.1 g/unit for TB, P < 0.0001).
In 99% of the tested units the requirement of <1 × 106 LC/unit was reached. Mean LC count was 0.066 and 0.31 × 106 LC/unit, in 2014 and 2010 respectively, the mean LC was 0.262 ± 0.257 × 106/unit for Pall Corporation filters and 0.079 ± 0.124 × 106/unit for Bioflex RCC filter (P < 0.0001).
Twenty seven (1.2%) RBC units did not meet the minimum Hb content/unit (range, 38.1–39.9 g/unit). In 5 (0.66%) RBC units LC >1 × 106 were observed (range, 1.01–1.44 × 106 LC/unit). All LC above the minimum required were in 2010, at that time the leukoreduction carried out at 4 ± 2°C with the Pall filters. Concerning Hb content, 25.9% (n = 7) of the discarded units had low collected blood volume, 74.1% (n = 20) had no identifiable cause; however all were from female donors and 75% (n = 15) of them had a pre‐donation Hb level around 12.5 g/dl.
Conclusions
Processing methodology and RBC filters influence the final quality of blood components. TB method is qualitatively advantageous as it presents a lower quantity of plasma and a diminished LC. Despite a lower Hb content observed they meet the standard requirements.
A particular attention should be paid to female donors with low Hb to avoid unnecessary deferrals and nonetheless guarantee blood components quality. With this purpose a careful follow up of blood donors with anaemia has recently been implemented in our Blood Bank.
The 99.4% of leukoreduced RBC units within the QC criteria shows a high filter performance, change of RBC filter that allowed filtration at room temperature revealed to be a good decision for our current practice.
P‐279
In Vitro Effects of Fresh Blood vs Stored Red Blood Cell Concentrates in Patients with End Stage Renal Disease on Hemodialysis and Erythopoiesis Stimulating Agents Therapy
Georgatzakou HTG1, Tzounakas VLT 1, Kokkalis ACK 2, Kriebardis AGK 3, Antonelou MHA 1, Papassideri ISP 1
1NKUA, Athens, Greece2Chronic Hemodialysis Centre ‘Ionion’, Piraeus, Greece3Technological and Educational Institute of Athens, Athens, Greece
Background
Anemia is the most common complication in end stage renal disease (ESRD). Despite administration of erythropoiesis stimulating agents for its management, ESRD patients undergoing hemodialysis account for a substantial portion of the demand for blood transfusions. However, the physiology of ESRD recipients is quite different compared to the average recipient requiring transfusion after acute injuries or during surgical procedures.
Aims
In the current study we examined the interactions between ESRD blood and normal blood in vitro using crossing experiments of ESRD red blood cells (RBCs) or uremic plasma with fresh or stored in blood banks RBCs, plasma and supernatant.
Methods
ABO compatible RBCs from healthy subjects (N = 8) or pre‐storage leukoreduced RBC concentrates in CPD/SAGM (N = 5) were incubated with uremic plasma from ESRD patients (N = 20) for 24 h at 37°C and inversely, under the same conditions. Intracellular ROS levels, hemolysis, RBC osmotic and mechanical fragility as well as phosphatidylserine (PS) exposure were estimated post‐mixing and incubation compared to the unmixed, incubated total blood.
Results
(i) Incubation of fresh, healthy RBCs with uremic plasma collected before hemodialysis resulted in a significant decrease in intracellular ROS accumulation (by 23–30%, P < 0.05) and hemolysis levels (by 35%) but a significant aggravation in RBC mechanical fragility (53% higher). Uremic plasma pre‐treated with uricase failed to reproduce these beneficial effects. (ii) The protective effect of uremic plasma on ROS accumulation was subsequently confirmed by inverse experiments. (iii) On the contrary, when testing donated RBCs stored in CPD‐SAGM for various periods of time with uremic and control plasma there was a general deterioration in the intracellular ROS accumulation after mixing (on average 40% increase in ROS levels) with both plasmas tested. Uremic plasma, however, was beneficial for stored cells compared to control plasma regarding the post‐mixing susceptibility to stimulated ROS accumulation. Furthermore, normal plasma resulted in increased susceptibility of stored RBCs to hemolysis, but uremic plasma restrained significantly day 42 stored RBCs from hemolysis, compared to the non‐uremic one. Mechanical fragility of RBCs stored for short in CPD/SAGM was improved after mixing especially by normal plasma whereas the profile was inversed for day 21 RBCs. Minor effects on PS externalization and osmotic fragility were also noticed. (iv) Stored units supernatant significantly improved ROS generation in both ESRD and control RBCs however it was less effective on ESRD compared to control cells. Hemolysis was increased in ESRD cells by day 42 supernatants. Day 7 but not older supernatants provoked a significant decrease in the MFI and PS externalization of RBCs.
Conclusion
Uremic plasma exerts an antioxidant and antihemolytic effect on RBCs. Plasma components, including the natural ROS scavenger uric acid, mainly account for the antioxidant effect. The anti‐hemolytic effect of uremic plasma is also applied to the CPD/SAGM stored RBCs, however the antioxidant effect is restricted to decreasing the susceptibility of stored cells to oxidative stimuli. The RBCs unit supernatant is less effective in alleviating the oxidative stress of ESRD cells compared to the healthy cells and drives ESRD cells to hemolysis in a storage‐time dependent manner.
P‐280
The Clinical Application of Transfusion of Thawed Washed Red Blood Cells Received on the Acp‐215 Haemonetics – Experience from UFA Center
Ayupova R , Sultanbaev U , Zonova I , Magadeev K
Ufa Blood Transfusion Station, Ufa, Russian Federation
Background
The purpose of transfusion of red blood cells is to correct the anemia, in this connection the allogeneic leukocytes (the carrier of highly immunogenic antigens) which are not required for newborns patients, may lead to their alloimmunization, post‐transfusion non‐hemolytic temperature reactions, acute pulmonary insufficiency, immunosuppression, and become a source for leukocyte‐associated viral infections.
Aims
To evaluate the effectiveness of thawed washed red blood cells for the correction of anemia among children of the first month of life.
Methods
Washed thawed red blood cells were obtained by centrifugation of 2–3 days of storage banked blood, after the removal of plasma and buffy coat with addition of ‘Glycerol 57%’ and the average hematocrit of 70%. Frozen red cells were stored for 12–18 months and passed quarantine. The process of washing was carried out on the ACP215 Haemonetics with the addition of hypertonic saline 250 ml (12% NaCl), saline with glucose 2000 ml (0.9% NaCl, 0.2% dextrose) and preservative solution SAG‐M 350 ml. The final hematocrit of the dose in average volume of 300 ml was in average 55%. During the study 27 transfusion results of thawed washed red blood cells were analyzed which were used in treatment of newborns (Table 1).
Results
High clinical efficiency and positive trend in patient's condition during the treatment of patients with moderate anemia and reliable increase in hemoglobin, red blood cells, hematocrit.
Caption 1: The clinical effect of transfusion of thawed washed red blood cells

Conclusion
Sequestrated doses of red blood cells guarantee the prophylaxis against the infection of recipients with hepatitis B and C and HIV infection. The suspension of thawed washed red blood cells may be recommended to patients sensitized to plasma proteins, with the presence of anti‐HLA, specific antigranulocyte and anti‐platelet antibodies, to transfusion dependent patients with the purpose of prophylaxis of alloimmunization and particularly for the correction of anemia among the children of the first month of life.
P‐281
Survey on England's Practice on the Use of O RhD Negative Blood
Dhesi AS1, Bassey S 2, Hockley BH 1
1NHSBT, London, United Kingdom2Royal Cornwall Hospitals NHS Trust, Truro, United Kingdom
Background
Balance between supply and demand of ORhD negative red blood cells (OnegRBC) remains a challenge for almost every blood service. Blood Stocks Management Scheme data 2013–14 in England shows that although demand for red cells is reducing the demand for OnegRBC remains constant. Anecdotal evidence suggests that recommendations from previous audits are not being implemented. It was therefore suggested that a further multi‐regional or national audit of OnegRBC use should be undertaken.
Before this was done the National Blood Transfusion Committee requested the National Transfusion Laboratory Managers (NTLM) Group to carry out a survey to measure the uptake of recommendations.
Aims
To measure the level of uptake of recommendations from the 2010 National Comparative Audit of use of O RhD negative red cells (re‐audit) and decide if another audit on use is required.
Methods
An online survey was constructed using SNAP Surveys©. Data was analysed proportionally (n, %).
Results
50% (125 completed responses) of the total number of sites to which the survey was distributed were returned.
The findings from this survey suggest that recommendations from previous work are not being implemented. This assertion is supported by the following findings:
66% of organisations stockholding continues to be above the recommended maximum 10.5%
18% said they did not have a policy of transfusing OnegRBC to non ORhD negative patients to avoid time expiry
92% said they did not monitor transfusion of OnegRBC to non ORhD negative recipients to avoid time expiry as a KPI
42% did nothave a policy for transfusing ORhD positive blood to adult males of unknown blood group
34% did not investigate when >2 units OnegRBC used in emergencies
59% of hospitals could switch to group specific red cells by 15 min.
There are recommendations that are being followed around active management and practice to impact on levels of OnegRBC use. This assertion is supported by the following findings:
76% always followed up Massive Haemorrhage activations
66% always investigated when more than 2 units of O RhD negative unit were used in emergency
94% reviewed their OnegRBC stock levels, 30% at least annually
81% have a lab stock holding policy to transfuse OnegRBC to non‐ORhD negative patients
99% said they have a policy for active stock management of emergency units
Summary/Conclusion
This survey analysis supports the decision not to carry out another NCA audit of the use of O RhD negative red cells at this time. Previous recommendations still need some time to embed in practice.
The survey also highlighted that the current target of 10.5% ORhD negative stock holding does not relate to the appropriate use of this precious resource. As the total number of red cells issues reduces and OnegRBC remain constant the current target may longer be appropriate. A focus on more appropriate use will ensure that it is available to those patients for whom there is no alternative.
P‐282
Comparison of Red Cell Parameters Between a Full‐Automated and A Semi‐Automated Processing at the Regional Centre of Transfusion Medicine and Blood Bank of Wroclaw
Galuszka W , Augustyniok Z , Szulakowska A , Stasiaczek G , Szymczyk-Nuzka M
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Leukoreduced red blood cell concentrate (LR‐RCC) is a component obtained by removing most of the leukocytes and platelets from red blood cell concentrate (RCC) units aiming to reduce the risk of alloimunization to HLA antigens and viral infection (e.g CMV). The Regional Centre of Transfusion Medicine and Blood Bank of Wroclaw (BB Wroclaw) use two alternative methods to get LR‐RCC: the TACSI CRC kit and the quadruple bag system with in‐line filter (Leucoflex type, Macopharma).
Aim
To compare the quality of LR‐RCC and RCC filtration time produced with the TACSI CRC process and with quadruple bags with in line Leukoflex filter from June 2013 to December 2014.
Methods
Whole Blood Donations of 450 ml obtained in BB Wroclaw were processed with TACSI or with the Hettich centrifuge and automatic press T‐ACE II (Terumo BCT). Donations were kept at rest for a minimum of 2 h before processing. Leucocytes were subsequently removed using the in‐line filter. Quality parameters: hematocrit (Ht), hemoglobine (Hb) and white blood cells per unit (WBC/unit) were measured in the final LR‐RCC. A FACSCalibur[TRADEMARK] (Beckton‐Dickinson) flow cytometer was used to determine WBC/unit. A hematology analyzer Micros 60 ABX was used to measure Hb and Ht. T‐testing was used to compare volume, filtration time, Ht and Hb level and a Mann‐Whitney U test was applied to compare WBC/unit. Filtration time was measured for 16 consecutive RCC filtrationsin each process.
Results
The volume of RCC produced with the automated and manual process were respectively 255 ml ± 16 and 254 ml ± 14 and there was no statistically significant difference (P > 0.05). Ht was 58% ± 3% in automatically processed RCC and 56% +/4% in manually processed RCC; Hb level was 48 ± 5 and 47 ± 4 in both groups respectively. There was a statistically significant difference (P < 0.05) between Ht and Hb in the respective groups of RCC. The WBC/unit median for the TACSI process was 0.03 × 106 (min.0.0 × 106; max. 0.34 × 106) and for the Macopharma manual process this was 0.01 × 106 (min. 0.0 × 106 and max. 0.57 × 106). Log normal plotting showed that LR performance for TACS was higher (99.92% <1 × 106) than for Macopharma (99.68% <1 × 106). This difference was statistically significant (P < 0.05). The average filtration time was found to be significantly shorter (P < 0.05) using TACSI filters (678 ± 99 min) than Macopharma filters (1093 ± 265).
Conclusions
1. The residual white blood cell content in all LR‐RCC was <1 × 10e6/Unit. However it was significantly lower for TACSI processed RCC.
2. LR‐RCC processed with the TACSI kit have significantly higher values of Ht and Hb than those processed with the Macopharma kit.
3. The RCC filtration process is significantly shorter using TACSI filters than Macopharma filters.
4. All LR‐RCC met the requirements of the Polish and EU guidelines.
P‐283
Abstract Withdrawn.
3.3 Plasma Products
P‐284
The Development of a Process for the Collection, Manufacture, Storage and Issue of Plasma from Survivors of Ebola Virus
Pelle BJ , MacLennan S , Senior P , Mehenny S , Raymond S
NHS Blood and Transplant, Manchester, United Kingdom
Background
In the absence of other effective treatments for Ebola virus disease, there is interest in the use of convalescent plasma, which may contain neutralising antibodies, collected from survivors to treat new cases. There is animal data which suggest that this approach may be beneficial. The optimal time to collect plasma is not known, but is thought that antibody levels may be maximal 6–8 weeks following infection.
Aims
To establish a process for the consent, collection, manufacture, storage and availability for issue, of plasma from recovered individuals in a safe and controlled manner.
Methods
A project team was established to deliver the process in a short time frame. A high level process map was produced, which defined the key steps in the process and the actions associated with their completion. These steps ensured overall management, with the work being separated into four main areas: approval and preparation, pre‐screening and collection, manufacturing and testing, and stock monitoring and issue.
Authorisation to proceed was obtained from the Medicines and Healthcare Products Regulatory Agency (MHRA), whereupon the preparatory actions were initiated, including an assessment of associated risks and a full map of the process which identified all further actions.
A pre‐screening interview took place with the donor prior to the donation date and any additional testing requirements were identified.
The plasma was then collected by apheresis methodology in line with existing protocols and transported to a designated manufacturing site for production of the required components, including the use of pathogen reduction technology.
Upon completion of all mandatory and discretionary tests, the components were released from quarantine to undergo final labelling, then transported to a designated storage location available for issue upon agreement with the corresponding clinical experts.
Results
Mapping the process in full and identifying possible deviations from standard procedures at the earliest opportunity enabled the team to form a comprehensive action plan from the outset. The action plan, and the support of all stakeholders, ensured that all activities were performed in a safe and controlled manner
Conclusions
In collaboration with our colleagues in PHE and the RoyalFreeHospital in London, NHSBT has been able to set up a process for the collection, manufacture, storage and issue of convalescent plasma in order to support the treatment of individuals who are diagnosed with Ebola infection.
To date we have collected four units, two of which have been transfused.
NHSBT has also developed a protocol in conjunction with the EBA to manage European stock(s) of convalescent plasma to ensure that it is given to the most appropriate patients within Europe.
The process map provides a generic process that can be used in the future for the provision of convalescent plasma for the treatment of other pathogenic infections.
P‐285
Study of Coagulation Factor Activities in Apheresed Thawed Fresh Frozen Plasma Stored At 2–6°C For Fifteen Days
Chartois-Leaute AG , Toussaint-Hacquard M , Laviron B , Gross S , Schneider T
Etablissement Français du Sang, Nantes Cedex1, France
Background and aims
Current guidelines recommend prompt administration of fresh‐frozen plasma (FFP) in emergency medicine to prevent dilutional coagulopathy or disseminated intravascular coagulation after severe blood loss and to improve survival. Immediate availability of FFP is delayed by thawing procedures. Prethawing for possible use within the expiry time would result in large quantities of FFP being discarded as French regulation states that FFP should be used within 6 h after thawing while some countries permit the use of plasma stored for up to 5 days following thawing.
The aim of this study was to investigate the quality of FFP with regard to clotting factors and inhibitor activity when stored at 4 ± 2°C over a period of 15 days and to evaluate bacterial contamination at the end of the storage.
Materials and methods
30 apheresis plasma units (15 group A and 15 O) were sampled, deep‐frozen under standardized conditions and stored at a temperature below −30°C during 2–19 days before thawing.
At day 0, the units were thawed after which samples for laboratory tests were taken (D0). Then, the units were stored at 4 ± 2°C for 15 days, and sampled after 6 h and on days D1, D5 and D15. Daily aliquots of plasma were frozen at −70°C until analysis.
Measurements of clotting factor activity included fibrinogen, Factor II (FII), FV, FVII, FVIII, F IX, FX, FXI. Measurements of inhibitor activity included antithrombin III (ATIII), protein C (PC), and free protein S (FPS).
Thrombin generation assay by calibrated automated thrombography (CAT) was performed at D0, D5 and D15 to evaluate the global coagulation process in vitro.
Bacterial cultures (aerobic and anaerobic) were done on day 15 with the routine method including aerobic and aerobic cultures.
Results
From immediately after thawing to 15 days after, the activity of clotting factors and inhibitors showed the following patterns:
‐ Fibrinogen, FII, V, VII, IX, X, XI, ATII and PC remained stable over the entire study.
‐ FVIII changed significantly over time with an acute decline in activity between D0 and D1.
‐ PS decreased slowly but significantly from D1.
Thrombography displays a preservation of the hemostatic potency of FFP (activation and inhibition of thrombin generation) at D5 as at D15.
All cultures bottles were sterile at D15.
Conclusion
Plasma once thawed and stored at 4 ± 2°C retains most proteins at the required activities for a time‐period of up to 5 days.
Storage of thawed FFP up to 6 h, if not needed immediately, may be beneficial in emergency situation as a result of the immediate availability of plasma.
P‐286
Abstract Withdrawn.
P‐287
Identifying Major Donor and Process Parameters on FVIII and Fibrinogen Content of FFP24
Begue SB1, Donnadieu FD 2, Laforet ML 3, Delamaire MD 4, Salih FS 5, Errami AE 6, Belcour BB 7, Boivin SB 8, Léauté AEG 9, Civadier CC 10, Colombat M 11
1EFS, La Plaine‐Saint‐Denis, France2EFS Alpes Med., Marseille, France3EFS Alsace, Strasbourg, France4EFS Bretagne, Rennes, France5EFS Centre Atlantique, Tours, France6EFS Ile de France, Paris, France7EFs Lorraine Champagne, Nancy, France8EFS Nord de France, Lille, France9EFS Pays de la Loire, Nantes, France10CTSA, Clamart, France11EFs Aquitaine Limousin, Bordeaux, France
Background
Donor and process parameters are known to have a significant impact on the final FVIII:C and fibrinogen concentration in FFP24 (Fresh frozen plasmas frozen within 24 h post collection) prepared from whole blood collection.
Aim
This study aims to identify and quantify the effect of donor and process parameters on the FVIII:C and fibrinogen content on the FFP24. The outputs of this study are meant to optimize process control and QC sampling plans rationale.
Methods
In this multicentric study, 10 EFS Regional Blood Centers collected 401 FFP24 on BAT integrated plasma filter sets. FFP was frozen at <−45°C, stored at <−25°C for less than 14 days and then thawed in a + 37°C water bath for FVIII:C and fibrinogen testing. Experimental plan was such that blood group (‘O’ n = 211 and ‘Non‐O’ n = 190), ambient temperature holding‐time before freezing (‘<12 h’ n = 180 and ‘>12 h and <22 h’ n = 220) and disposable sets (Macopharma NPT6286LA n = 200 and Fresenius DGR7567B n = 201) categories were leveled in numbers. Donor's gender (156 females; 245 males) and age were recorded (age category: 18–29 years (n = 123); 30–39 years (n = 60); 40–49 years (n = 78); 50–70 years (n = 140)). FVIII:C was measured using a one‐stage assay (Stago or IL); Fibrinogen was measured using the Clauss method assay (Stago or IL). There were no significant differences between Stago and IL methods.
Results
FVIII:C: Anova analysis shows that gender and collection set have no significant effect on the final FVIII concentration (P = 0.76 and P = 0.196). Blood group, holding‐time before freezing and, for a weaker correlation, donors’ age has a significant influence on this parameter.
We observe a mean difference of −19% (−0.2 UI/ml) between ‘O’ blood group (0.87 UI/ml) and ‘Non‐O’ blood group (1.07 UI/ml) and a significant decrease of −11% (−0.12 UI/ml) between units frozen after an overnight ambient temperature storage at 19 h (0.92 UI/ml) and units frozen on the same day of collection at 8 h (1.04 UI/ml). Cf Table 1
Fibrinogen
Anova analysis shows that blood group (P = 0.75), holding‐time (P = 0.57) and collection sets (P = 0.77) have no significant effect on the final fibrinogen concentration while donor's gender and age prove to be major contributors. A mean difference of −8% (−0.23 g/l) is observed between female (2.92 g/l) and male donors (2.69 g/l) and a mean +10% (+0.30 g/l) increase is observed between young donors aged from 18 to 29 years and donors over 50 years. This age correlation to fibrinogen is not gender dependent. Cf Table2
Table 1: FVIII:C FFP content
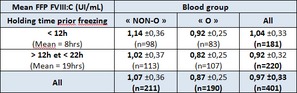
Table 2: Fibrinogen FFP content

Conclusions
FVIII:C activity of FFP24 is strongly depending on donor's blood group and ambient temperature holding‐time before freezing. A cumulative effect of those two factors may explain differences as high as 0.3 UI/ml between two sample groups.
Fibrinogen concentration is strongly dependent on donor's age and gender. A cumulative effect of those two factors may explain differences as high as 0.5 g/l between two sample groups.
P‐288
In Vitro Comparison of Octaplas‐LG, Standard Octaplas and Fresh Frozen Plasma in the Treatment of Coagulopathy Due to Liver Disease
Connaughton MA , Chin JL , O'b O'Brien P , Fitzgerald JF
St. Vincent's University Hospital, Dublin, Ireland
Background & Aims
Solvent detergent treated plasma (standard Octaplas‐SD) from non‐renumerated North American donors was introduced in Ireland circa 2002 to minimise transmission of variant Creutzfeldt Jacob Disease. Due to our institutional concerns over its safety and efficacy during liver transplantation, Fresh Frozen Plasma (FFP) is still used intraoperatively.1 Recently, Octaplas‐LG (ligand gel), a new prion filtered solvent detergent treated plasma with increased levels of protein S and plasmin inhibitor, has become available.2 We compared the efficacy of Octaplas‐SD, Octaplas‐LG and FFP in correcting coagulopathy in patients with liver cirrhosis.
Materials and methods
40 samples from 25 patients with chronic liver disease and cirrhosis (INR > 1.5) were studied. Quantitative analysis carried out on Octaplas‐SD, Octaplas‐LG and FFP, which includes full coagulation screen and factor concentrate measurements. Thromboelastography was used to perform qualitative analysis of in vitro correction of coagulant deficient samples with each plasma product.
Results
Octaplas‐LG has slightly higher levels of fibrinogen, Factor V, Factor VIII, Protein C, Protein S and Anti‐thrombin III, compared to Octaplas‐SD and FFP (P > 0.05). Qualitative analysis with thromboelastography showed no significant difference in correcting coagulopathy for all three plasma products in vitro (P > 0.05). The percentage improvement in R time (16.7%, 17.9% and 19.9%), K time (18.5%, 12.4%, 16.1%), angle (19.4%, 19%, 22.8%) and MA (5.7%, 2.8%, 3.7%), were not statistically significant for Octaplas‐SD, Octaplas‐LG and FFP respectively.
Caption 1: Determination of Coagulation Factor levels per blood group per plasma product.

Caption 2: Percentage change for the various thromboelastography parameters for each plasma product in vitro

Conclusion
We conclude that Octaplas‐LG and Octaplas‐SD are as effective as FFP in correcting coagulopathy for cirrhotic patients in vitro.
1.Magner JJ, Crowley KJ, Boylan JF. Fatal Fibrinolysis during orthotopic liver transplantation in patients receiving solvent/detergent‐treated plasma (Octaplas). J Cardiothorac Vasc Anesth 2007;21(3):410–413
2.Heger A, Svac T E, Neisser‐Svac A et al. Biochemical quality of the pharmaceutically licensed plasma Octaplas LG after implementation of a novel prion protein (PrPsc) removal technology and reduction of the solvent/detergent (S/D) process time. Vox Sanguinis 2009;97:219–225
P‐289
Abstract Withdrawn.
P‐290
Abstract Withdrawn.
P‐291
In Vitro Study of Hyper‐Oncotic Freeze Dried Plasma
Martinaud CM1, Civadier C 1, Ausset A 2, Mendes M 1, Sailliol S 1
1CTSA, Clamart, France2HIA Percy, Clamart, France
Background
Availability of blood products remains a challenge to manage massive hemorrhage. In hospital settings, red blood cells, platelets and fresh frozen plasma might be available in relevant delay thanks to an optimized organization. In prehospital settings as well as in austere settings or in war situations, overall survival of casualties suffering from hemorrhage mainly relies on red blood cells and crystalloid during the earliest management. French lyophilized plasma is a freeze‐dried plasma available in less than 6 min with a room temperature storage and a 2 years shelf life. It provides about 200 ml of plasma with the same security and hemostatic properties than its frozen counterpart. FLyP is routinely used in French armed forces, in France as well as in overseas operations. Through years, it becomes clear that one limitation of its use, as for every therapeutic plasma, is the total amount of volume transfused. Hyper‐oncotic products are supposed to limit the amount together with an osmolar effect. FLyP can be reconstituted with 100 ml of water and has been successful transfused in this volume to treat haemophilia in salvation situation.
Aims
We performed in vitro assays to test the characteristics of a hyper‐oncotic solution of FLyP as compared to iso‐osmolar FLyP.
Methods
Coagulation factors, thromboelastometry and thrombin generation were assessed on iso‐osmolar and hyper‐oncotic FLyP. Subsequently, an in vitro model of hemorrhage was used to test the hyper‐oncotic FLyP against iso‐osmolar.
Results
We showed that conventional tests revealed an almost 2 fold increased level of coagulation factors. Thromboelastometric assays were comparable. Surprisingly, hyper‐oncotic FLyP did not generate twice as much thrombin as iso‐osmolar FLyP. Finally, our dilution model did not argue for an improvement of the coagulation properties of the hyper‐oncotic solution.
Conclusions
Our results showed the feasibility of a hyper‐osmolar FLyP but raise questions about its hemostatic properties. Further analyses are required before allowing its use in patients.
P‐292
A Retrospective Analysis of the Production of Fresh Frozen Plasma Obtained from Whole Blood in Connection with the Introduction of Donor Consent Regarding Commercial Use of Plasma in the Regional Blood Center in Poznan
Gulinski L , Zawadzka A , Olbromski K
Regional Blood Center in Poznan, Poznan, Poland
Background
In Poland, the Blood Service is based on honorary donations. Regional Blood Centers are the only authorized entities for collection, processing, storage and distribution of blood and its components. There are 21 RegionalBloodCenters, one MilitaryBloodCenter and one BloodCenter of the Ministry of the Interior Affairs. The RegionalBloodCenter in Pozna�’ is one of the largest institutions in Poland. Substantive supervision over the Blood Centers is performed by the Institute of Hematology and Transfusiology in Warsaw (IHT). As part of its activities RegionalBloodCenter in Pozna�’ obtains fresh frozen plasma (FFP) using a manual method from whole blood, and also from apheresis. At the turn of the year 2012/2013 in order to emphasize the transparency of the Blood Collection System in Poland, the Institute of Hematology and Transfusiology introduced recommendations for the confirmation of donor consent for commercial (non‐clinical) use of plasma.
Aims
Analysis of the consequences of the implementation of donor consent for commercial use of plasma in the RegionalBloodCenter in Pozna�’.
Methods
The study involved data regarding the obtaining of FFP from whole blood in the RegionalBloodCenter in Pozna�’ from 2011 to 2014. The analysis included documentation related to the implementation of recommendations, and reports on the lack of consent for the commercial use of plasma.
Results
1. As part of the implementation of the IHT recommendations the formula of expressing the consent for commercial use of plasma was included in the Donor Health Questionnaire at the beginning of 2013. In 2013, as a result of lack of consent 851 units of plasma were destroyed.
2. At the beginning of 2014, information regarding blood components and the possibility of using plasma for the production of medicines was included in the questionnaire. In 2014, as a result of lack of consent 586 units of plasma were destroyed.
3. Obtained FFP from whole blood in years 2011–2014 is presented in Table 1.

Summary/Conclusions
1. In general, no significant differences in the number of collected plasma units can be observed due to the implementation of the donor consent for commercial use of plasma.
2. It seems that the educational activities undertaken by the RegionalBloodCenter employees proved to be successful and effective.
3. Having analyzed the positive effects of the direct education, it should be regarded as reasonable to inform donors in a transparent manner about the relation between honorary donations and particular elements of management in the Blood Collection System.
P‐293
Affinity Chromatography Separation of PLG from Human Plasma
Mousavi Hosseini K , Fallah Tafti M
Blood Transfusion Research Center, High Institute for Research and Education in, Tehran, Iran
Background
Many biological compounds can be derived from human plasma by fractionation. PLG is one of those compounds and when it activated to plasmin, it has the property of lysing fibrinogen, fibrin, and some other proteins. In another words, plasmin converts fibrin into soluble products and also hydrolyses some other proteins. Congenital plasminogen deficiency is an illness that results in inflamed growth on the mucous membranes, which are the moist tissues that line body opening such as the eyelids and the inside of the mouth. Propagation of the growths is usually triggered by infections or injury, but they may also occur spontaneously in the absence of known triggers. The prevalence of congenital plasminogen deficiency is about 1.6 per one million people.
Aims
Separation of PLG from human plasma by affinity chromatography method is the main purpose of this study.
Methods
In current work by adjustment of parameters such as ethanol concentration, pH, ionic strength, temperature, and protein concentration fractions I was obtained. We have used normal human plasma as starting material. By increasing of its concentration of ethanol from 0% to 8% in pH 7.2 at ‐3�-�C the centrifugation was carried out and the supernatant I was separated from precipitate I. Preparation of PLG with fraction I supernatant as raw material was followed by the affinity chromatography technique.
Results
In normal human plasma the concentration of PLG is about 200 mg/l. In our study we could obtain PLG with the concentration of 0.5%.,which in comparison with the concentration of PLG in normal plasma shows about 25 fold more concentration.
The SDS‐PAGE electrophoresis showed a strong band at 92 kDa related to PLG, which normally has a molecular weight of 90–92 kDa. The strongest band marked with 4 is related to non‐diluted PLG and weaker bands marked with 3 and 2 are related to ½ and ¼ diluted PLG respectively.
Summary/Conclusions
By affinity chromatography using affinity gel lysine Sepharose in separation of PLG from human plasma, the PLG concentration of 0.5% was obtained which in comparison with the concentration of PLG in normal plasma (200 mg/l) indicates 25‐fold more concentration. Our result shows affinity chromatography promising for separation of PLG from human plasma.
3.4 Pathogen Inactivation
P‐294
The Assessment of Platelet Function by Thrombelastography After Transfusion of Intercept Treated Platelets Reveals Satisfying Results
Leitner GC1, Rabitsch W 1, Kolovratova KV 2, H Horvath M 1
1Medical University, Vienna, Austria2Clinic for blood group serology and transfusion medicine, Vienna, Austria
Background
Blood safety is a ‘must’ in transfusion medicine. Over the last decades big efforts were made to increase blood safety and reduce the risk of transfusion transmitted infections (TTI). The means applied range from donor deferral to pathogen inactivation techniques (PI). The latest innovation in this field was the implementation of PI techniques for platelets. Nowadays bacterial contamination of platelet components (PC) is considered the most relevant risk to blood safety. Concerns arose about the function, the in vivo recovery and the possibly increased demand when using PI treated platelets. The European society of anaesthesiology (ESA) recommends among others the thrombelastography (TEG) for evaluating the need of platelet support. TEG is an in vitro test that measures viscoelastic changes of the entire clotting process. This prompted us to perform TEG before and 1 h after transfusion of PI platelets in a prospective, ongoing investigation.
Material and methods
At our institution the INTERCEPT Blood System is applied for the PI treatment of PCs. 21 Patients (pts) of our Bone Marrow Transplantation (BMT) unit diagnosed with either Acute Myeloid Lymphoma (12), Multiple Myeloma (3) or others (6) were included in this evaluation. We correlated the TEG results with the post‐transfusion platelet counts, corrected count increments (CCI), coagulation parameters, CRP and clinical conditions. The included patients (9 m/12f, median age 45 years, range 29–66) received a median of 7 PCs (range 1–37). At this time allogeneic BMT was performed in 15 pts and autologous BMT in 4 pts. CRP was elevated in 7 pts (>10 mg/dl, normal 0.5). In none of the patients the coagulation was impaired. One pt presented with 40% blasts in BM. Platelet support trigger is 10–20 G/l co‐triggered by clinical condition.
Results
In 47 transfusion episodes the TEG was measured before and 1 h after PC support. The median platelet dose transfused was 2.58 × 1011 per unit (range 1.96–4.1). The CCI was in median 10, range 0.7–28. The majority of pts showed marked improvement in all platelet related TEG parameters, irrespective of the CCI and the 1 h value. In 1 patient platelet transfusions failed to improve TEG, although CCI was 6.17. This patient was diagnosed with blast crisis in AML. None of the investigated patients experienced bleeding.
Conclusion
As verified by TEG measurements INTERCEPT PI inactivated platelets seem to have sufficient coagulation capacity. The measurement of the 1 h CCI values may not be indicative for platelet function in all cases. Overall clinical conditions have a great influence on the success of platelet support.
P‐295
Phosphatidylinositol‐3‐Kinase is Impaired Following Amotosalen and Ultraviolet a Light Treatment of Platelets Concentrates
Compernolle V , Van Aelst B , Devloo R , Vandekerckhove P , Feys HB
Belgian Red Cross‐Flanders, Ghent, Belgium
Background
Photochemical treatment of platelet concentrates significantly reduces chances of pathogen transmission. One technology uses the psoralen derivative amotosalen and ultraviolet A light illumination (AS‐PCT). It is currently used in several blood banks in Europe and was recently approved in the United States. We formerly demonstrated that AS‐PCT reduces thrombus formation on collagen under shear flow conditions, in vitro.
Aims
To elucidate the biomolecular mechanism causing this reduced thrombus formation, in vitro.
Methods
Biochemical analysis of signal transduction pathways in paired AS‐PCT treated or untreated platelets.
RESULTS AS‐PCT treatment significantly impaired activation of the platelet fibrinogen binding integrin αIIbβ3 in a dose‐dependent manner by PAR1 activating peptide, measured by PAC1 binding in flow cytometry. Because PAR1 is a G‐protein coupled receptor, rigorous analysis of downstream G‐protein signaling effectors was performed. Gq and G12/13 signaling were unaffected because intracellular calcium flux kinetics, pleckstrin phosphorylation and alpha‐granule release were all normal. Coupling to Gi was defective because integrin αIIbβ3 activation using adenosine diphosphate (ADP) and selective stimulation of solely P2Y12 was decreased in platelets treated with AS‐PCT. Because the beta‐gamma subunits of Gi are known to activate phosphatidylinositol‐3‐kinase (PI3K) and consequently phosphatidylinositol(3,4,5)‐trisphosphate formation, we measured Akt phosphorylation kinetics using western blotting of whole cell lysates. This was significantly less in AS‐PCT platelets in response to PAR1 activation confirming decreased activation of PI3K though Gi coupling. We next demonstrated that PI3K is affected as such since G‐protein coupled independent phosphorylation of Akt in response to receptor tyrosine kinases activation using thrombopoietin (c‐MPL receptor) and collagen related peptide (GPVI‐FcγRIIa) was substantially reduced. Because PI3K substrates (phosphatidylinositols) are imbedded in the platelet plasma membrane, we performed lipidomics on treated and untreated platelets. We found that AS‐PCT treatment causes covalent addition of amotosalen to unsaturated acyl side chains of multiple phospholipid species including phosphatidylinositols and speculate that this modification contributes to the observed phenotype.
Conclusion
We conclude that PI3K function is significantly impaired in AS‐PCT platelets explaining the diminished thrombus formation under shear flow. Further research will determine if this defect is caused by the altered platelet plasma membrane phospholipid composition.
P‐296
Observational Study Comparing Clinical Performances of Mirasol and Intercept‐Treated Platelet Concentrates in Routine
Garcia-Gala JM1, Muñoz-Turrilas C 2, Buesa-Garcia C 2, Martinez-Revuelta E 1, Martinez-Carballeira D 1, Garcia Menendez-Tevar F 1, Killian R 3, Cardoso M 3
1Hospital Universitorio Central de Asturias, Oviedo, Spain2Centro Comunitario Sangre Y Tejidos Asturias, Oviedo, Spain3TERUMO BCT, Brussels, Belgium
Background
During the period between February‐March 2014 and December 2014‐January 2015 153 transfusions of Mirasol‐treated platelets to 39 patients and 92 transfusions of Intercept‐treated platelets to 30 patients were followed ‐up at the Hospital Universitario Central de Asturias, Spain. Transfusions were performed per institution's standard practices that did not change during the entire observation period.
Aim
To compare clinical performances of Mirasol‐ and Intercept‐treated platelets used in the supportive therapy of thrombocytopenic patients in the Hospital Universitario Central de Asturias in two consecutive time periods.
Methods
Platelets were collected and processed at the Centro Comunitario de Sangre y Tejidos de Asturias, according to the site's SOPs and following European and Spanish guidelines for blood processing. Platelet concentrates (PC) were processed by pooling 5 whole blood derived BC with the TACSI PL device (Terumo BCT) or by apheresis collection with the Trima Accel (Terumo BCT) or Amicus (Fresenius). PC were treated either with the Mirasol PRT or with the Intercept system, according to manufacturer's instructions for use and were stored in PAS solution (SSP+, Macopharma) up to 7 days at 22°C on a platelet agitator. Platelets were transfused to thrombocythopenic patients at the Hospital Universitario Central de Asturias according to international and Spanish transfusion guidelines, as well as per institution's standard practices. Platelet transfusion responses were measured after 24 hs post‐transfusion as CI24 h and CCI24 h.Mean values for age, platelet doses and rates of successful transfusion as CCI24 h> 4500 were performed for all platelet transfusions in the two observational periods. Comparative analysis of patient responses to transfusion was done for the four major patient groups: allo‐, autologous transplantation, AML and NHL. All other patient groups were too small to allow for statistical analysis.
Results
Analysis of the data per observational periodsuggestedsimilar performances of both PRT‐treated PCs in this cohort of thrombocytopenic patients.Mean age of patients were 44 years (Intercept period) and 48 years (Mirasol period), P > 0.05. Mean platelet doses were similar at 3.5 × 10e11 for Intercept and Mirasol. Rates of successful transfusion as CCI24h>4500 were 45.6% and 46.6% for Intercept and Mirasol, respectively (P > 0.05). Platelet age at transfusion was significantly lower with Mirasol than with Intercept (3.9 vs. 5.8 days, respectively), P < 0.05. Moreover, clinically superior responses after Mirasol‐PC transfusion were observed in the groups of autologous‐ transplant, AML and non‐Hodgkin lymphoma.
Conclusion
Both Mirasol and Intercept technologies are simple and easy to adapt to the routine of the blood bank. Overall clinical performance was similar and no adverse reactions were reported for any of the two observational periods. Mirasol‐PCs do not require elimination step post‐treatment and hence makes transfusion of fresher platelets possible.
P‐297
Measurement of Nucleic Acid Modification Induced by Amotosalen and UVA Light Using a Real‐Time PCR Inhibition Assay Targeting Mitochondrial DNA as a QC Assay for Pathogen Reduction of Blood Components
Bakkour S1, Chafets DM 1, Wen L 1, Castro G 2, Dupuis K 2, Green JM 2, Stassinopoulos A 2, Busch MP 1, Lee TH 1
1Blood Systems Research Institute, San Francisco, United States of America2Cerus Corporation, Concord, United States of America
Background
The use of photochemically treated blood components to reduce the risk of transfusion‐transmitted infection has been increasing rapidly. Treatment with amotosalen and UVA light results in DNA and RNA adducts blocking replication, transcription and translation. Pathogens are thus inactivated, without affecting the therapeutic efficacy of platelets and plasma. It is important to document that the treatment dose delivered resulted in sufficient nucleic acid modification to prevent breakthrough infections. Current QA approaches measure the delivered UVA light dose with illuminator sensors, relying on process validation at each center. An HPLC‐based method can be used to measure the treatment dose by quantifying the percent residual amotosalen after illumination, before passage through the Compound Adsorption Device. However, a functional method directly measuring nucleic acid modification, such as long range PCR, is not currently available. Mitochondrial DNA (mtDNA) is an appropriate target since it is present in all blood products.
Aims
The goal of this study was to measure the extent of mtDNA modification induced by amotosalen/UVA treatment of platelets and plasma, using differential amplification of short‐ and long‐amplicon targets by real‐time PCR (rtPCR).
Methods
Apheresis platelets (N = 8) were either not treated or treated with amotosalen/UVA as mini‐units (30 ml) or full units (>300 ml). A blinded panel of 10 untreated and 10 treated samples, including several duplicate aliquots, was generated and stored frozen prior to testing. DNA was extracted and amplified using short‐ and long‐amplicon mtDNA rtPCR assays. Subsequent to completion of the blinded validation, samples from treated platelet units prepared from buffy coats for routine clinical use (N = 100) and untreated platelet units (N = 10) were tested in a blinded manner by rtPCR. To extend the applicability of the mtDNA PCR inhibition assay to apheresis and whole blood‐derived plasma (N = 4), we compared two sample processing methods with or without a concentration step via centrifugation. After optimization of sample processing, a blinded panel of 100 treated plasma units prepared for clinical use and 10 untreated plasma units was tested by short‐ and long‐amplicon mtDNA rtPCR.
Results
Significant inhibition of rtPCR was found in treated samples for both long and short amplicons, increasing with amplicon size (Table 1). Treated platelets showed a greater difference in the cycle threshold (Ct) between short and long amplicons, compared to untreated platelets. For analysis of plasma, which contains lower mtDNA amounts compared to platelets, a centrifugation step prior to DNA extraction allowed an enhanced detection of unmodified mtDNA compared to modified mtDNA, resulting in improved differentiation between untreated and treated plasma. Using the enhanced detection method, treated plasma showed a greater Ct difference between short and long mtDNA amplicons compared to non‐treated plasma in the blinded panel.
Table1: mtDNA real‐time PCR analysis before and after pathogen inactivation

Summary/Conclusions
A rtPCR method targeting short and long fragments in mtDNA allows the differentiation between blood products that have or have not been treated using pathogen reduction technology. This technique could be adopted as a tool to confirm the photochemical treatment process and monitor its effectiveness.
P‐298
Red Blood Cells Treated with the S‐303 System for Pathogen Inactivation Demonstrate In Vitro Characteristics Suitable for Transfusion – Phase III Clinical Trial in Cardiac Surgery Patients
Brixner V1, Leibacher J 2, Pfeiffer H-U 2, Müller MM 2, Geisen C 2, Henschler R 3, Janetzko K 2, Heldke S 4, Huang N 5, Ernst C 5, Rico S 5, Erickson A 5, Mufti N 5, Corash L 5, Seifried E 2
1DRK Blutspendedienst Baden‐Württemberg – Hessen gGmbH, Frankfurt, Germany2German Red Cross Blood Donor Service Baden‐Wuerttemberg‐Hessen, Frankfurt, Germany3University Clinic Munich, Department for Transfusion Medicine, Munic, Germany4BloodCenter of Wisconsin, Milwaukee, United States of America5Cerus Corporation, Concord, United States of America
Background
The risks of red blood cell (RBC) transfusion have been greatly reduced in the past decades thanks to improvements in donor screening, good manufacturing practices, and viral marker testing. Nevertheless, threats to the blood supply remain either from known pathogens which are not tested routinely and/or from emerging pathogens. The second generation S‐303 pathogen and leukocyte inactivation system was developed to reduce the risk of transfusion transmitted infections and transfusion associated graft vs. host disease.
Aims
Evaluation of the in vitro characteristics of RBC components produced for a randomized, controlled, double‐blind phase III clinical trial to assess the efficacy and safety of S‐303 treated RBC components in patients requiring transfusion support for acute anemia, during or shortly after a cardiac surgery.
Methods
Patients undergoing coronary artery bypass grafting, and/or valve replacement or repair, were randomized to receive S‐303 treated (Test) or conventional (Control) RBC during a 7‐day treatment period. Test and Control RBC components were either released for transfusion to patients or stored at 1–6°C for 35 days. Given that the patient's hemoglobin increase is proportional to the hemoglobin mass transfused, measuring the hemoglobin content of RBC components is considered a surrogate for the therapeutic efficacy of a RBC component. Therefore, the primary endpoint was the mean hemoglobin content per RBC component post‐production (PP). A priori definition of equivalence required the 95% CI limits for the mean treatment differences to be −5 g/unit to 5 g/unit. Secondary efficacy endpoints included end‐of‐storage (EOS; Day 35 – Day 38) measurements of hematocrit, hemolysis, normalized ATP, plasma‐free hemoglobin, and hemoglobin content.
Results
A total of 774 study RBC components were produced, and 754 RBC components (389 test, 365 control) were eligible for PP hemoglobin content analysis. Mean (±SD) PP hemoglobin content per Test RBC component (53.6 ± 5.6 g/unit) was slightly lower than Control RBC (56.3 ± 6.0 g/unit), however the primary endpoint was met as the mean treatment difference (Test‐Control) in hemoglobin content (95% CI −2.61 g/unit to −1.92 g/unit) was within the pre‐specified equivalence margins (95% CI ±5 g/unit). There were no differences in the proportion of components having EOS hemoglobin content (≥40 g/component; Test 98.7%, Control 99.2%; P = 0.69), or free hemoglobin (<6 g/l; Test 100%, Control 99.6%; P = 0.46), or hematocrit (50–70%; Test 99.3%, Control 99.2%; P = 1.00). There were differences in the proportion of components with EOS normalized ATP (>2 μmol/g; Test 21.8%, Control 0.90%; P < 0.001) and EOS hemolysis per the Quality of Medicines (EDQM) guideline for EOS hemolysis (<0.8%; Test 100%, Control 98.1%; P = 0.02).

Summary/Conclusions
S‐303 treated RBC demonstrated equivalence to untreated RBC regarding hemoglobin content and met EDQM guidelines hemoglobin content, hematocrit and hemolysis. The proportion of S‐303 components satisfying the EDQM guidelines for EOS hemolysis and the normalized ATP was higher for S‐303 treated RBCs than conventional RBCs. S‐303 treated RBCs show in vitro characteristics comparable to untreated RBC and are suitable for transfusion.
P‐299
Abstract Withdrawn.
P‐300
Chikungunya and Ross River Viruses are Effectively Inactivated by the Theraflex UV‐Platelets Technology
Marks DC1, Fryk J 1, Prow N 2, Hall R 2, Tolksdorf F 3, Sumian C 4, Gravemann U 5, Seltsam A 5, Faddy H 1
1Australian Red Cross Blood Service, Sydney, Australia2University of Queensland, Brisbane, Australia3MacoPharma International GmBH, Langen, Germany4MacoPharma, Tourcoing, France5Blood Centre of the German Red Cross, Springe, Germany
Background
Chikungunya virus (CHIKV) and Ross River virus (RRV) are mosquito‐borne viruses that are of concern for transfusion safety. Given the absence of an approved blood screening test for either virus in Australia, the Australian Red Cross Blood Service restricts fresh component manufacture from individuals diagnosed with a RRV or CHIKV infection, and for four weeks after they have recovered. In addition, similar restrictions are implemented for four weeks for donors returning from travel to countries at risk for CHIKV exposure. The use of pathogen inactivation (PI) technologies, such as the THERAFLEX UV‐Platelets system, could be an alternative approach to manage the potential risk of RRV or CHIKV transfusion transmission.
Aims
To examine the ability of the THERAFLEX UV‐Platelets system at different UVC doses to inactivate CHIKV and RRV in buffy coat‐derived platelet concentrates in additive solution.
Methods
CHIKV or RRV were isolated and spiked into buffy coat‐derived platelet concentrates in additive solution (SSP+) to give a final concentration of 6.71, or 5.30 log PFU/ml, respectively. For each virus, three buffy coat‐derived platelet concentrates were spiked. Spiked platelets were then treated with the THERAFLEX UV‐Platelets system at the following doses: 0.05, 0.1, 0.15 and 0.2 J/cm2 (standard dose). Pre‐ and post‐treatment samples were taken for each dose, and the level of viral infectivity was determined using a modified version of a conventional plaque assay. The reduction in viral infectivity was calculated for each dose to determine the effect of the inactivation process.
Results
Treatment resulted in the inactivation of both CHIKV and RRV. Viral inactivation of ≥6.34 log10 for CHIKV and ≥5.13 log10 for RRV, at the highest UVC dose (0.2 J/cm2) was observed. A dose‐dependency in viral inactivation was observed with increasing UVC doses for both viruses.
Summary/Conclusions
CHIKV or RRV transmission through transfusion is theoretically possible. Given this, studies are needed to gain an understanding of the threshold viral load in a blood component to cause disease through transfusion. This in turn will provide insight to the minimum level of inactivation to prevent infection through this route. Our study has shown that CHIKV and RRV, spiked into BC‐derived platelet concentrates in additive solution, were effectively inactivated by the THERAFLEX UV‐Platelets system. At the standard dose, the residual infectivity of virus was close to, or at the limit of quantification. Our results suggest that the THERAFLEX UV‐Platelets treatment could significantly contribute to the safety of platelet concentrates with respect to these arboviruses.
P‐301
Cytomegalovirus is Efficiently Inactivated in Human Plasma by the Theraflex MB‐Plasma System
Handke W1, Gravemann U 1, Lambrecht B 1, Sumian C 2, Reichenberg S 3, Seltsam A 1
1Red Cross Blood Services, Springe, Germany2Macopharma SA, Tourcoing, France3Macopharma Int., Langen, Germany
Background
Photodynamic treatment using methylene blue (MB) and visible light is in routine use for virus inactivation of human plasma. It has been shown to inactivate a broad range of different DNA and RNA viruses. Human cytomegalovirus (HCMV) is known to be transfusion transmissible and a recognized cause of morbidity and mortality in immunocompromised individuals.
Aims
Aim of the study was to investigate the efficacy of the THERAFLEX MB‐Plasma system to inactivate HCMV in human plasma.
Methods
Leukodepleted plasma was prepared from whole blood using standard blood banking technology. Donors were tested for anti‐CMV antibodies using the Enzygnost Anti‐CMV/ IgG+IgM test to exclude the presence of neutralizing antibodies in plasma. Only anti‐CMV negative plasma units were included in the study. Plasma units (n = 4, 285 ml) were spiked with virus suspension (30 ml) to reach a final volume of 315 ml. MB/light treatment was done according to the manufacturer's instructions. Samples were taken after spiking (load and hold sample), after addition of MB and after illumination with different light doses. The titer of HCMV (strain AD‐169, ATCC VR‐538) was determined by endpoint titration and large volume plating in microtitre plate assays on MRC‐5 cells (fibroblasts, ATCC CCL‐171).
Results
The results are summarized in the Table. HCMV was efficiently inactivated by more than 4.06 log steps. Already half of the standard light dose of 120 J/cm2 resulted in inactivation of HCMV to infectivity levels below the limit of detection.

Conclusions
It was shown that treatment of human plasma by THERAFLEX MB‐Plasma system efficiently inactivates HCMV and thereby significantly contributes to the viral safety of plasmas for transfusion.
P‐302
Parvovirus B19 Transmission by Transfusion of InterceptⓇ Blood System‐Treated Thrombocyte Concentrate
Niederhauser C , Stolz M , Fontana F , Andina N , Gowland P
Interregional Blood Transfusion SRC, Bern, Switzerland
Background
Parvovirus B19 (B19V) transfusion‐transmission has been reported, but it seems to be quite rare with red blood cell concentrates (RBC) and thrombocyte concentrates (TC). B19V contamination of plasma derivatives has led to widespread adoption of B19V nucleic acid screening of source and recovered plasma donations to interdict high‐titre viraemic units before pooling and fractionation. In Switzerland pathogen reduction of all produced TC has been mandatory since 2011.
Aims
Description of two possible transfusion‐transmitted B19V cases with TC which were treated with the Intercept® Blood System pathogen reduction system.
Methods
Nucleic acid test (NAT) screening for B19V is performed in pools of 480 donations on a twice weekly basis with a validated in‐house real time PCR assay. B19V positive pool samples containing >1E+6 IU/ml are resolved to the single donation. Those donations with viral loads >1E+4 IU/ml are removed from the plasma fractionation process. The corresponding RBC and TC are also removed from the transfusion process. TCs are routinely treated with the Intercept® Blood System pathogen reduction system. B19V serology (IgG and IgM) were performed with commercial assays (Biotrin, DiaSorin Ireland Ltd.). B19V DNA sequence analysis was performed on a partial coding NS1/VP1 unique fragment. B19 sequences were aligned using CLUSAL_W and phylogenetic trees were constructed using the nearest‐neighbour joining method.
Results
Two possible cases of B19V transmission with Intercept®‐treated pooled TC were detected. The delay in detecting by NAT the corresponding B19V‐contaminated blood unit is approximately five days. In both cases the TC was already transfused. The viral concentration in the original blood units were: case 1: 4.87E+10 IU/ml and case 2: 1.46E+08 IU/ml. Ten days after transfusion a B19V viral load (52 IU/ml) was detected in the TC recipient of case1, whereas no virus was detected in the TC recipient after four and nine days in case 2. The B19V serology with serum samples from recipient case 1 suggests a transient boost of the underlining B19 IgG immune status. B19V IgM remained negative in all samples analysed. The TC recipient from case 2 was B19 IgG and IgM negative in all samples analysed. B19V DNA sequence and phylogenetic analysis of the partial coding NS1/VP1 unique fragment (936 bp) revealed a 100% homology between the B19V isolated from the donor and recipient in case 1. No symptoms of an active B19V infection were observed in the recipients of the TC from both cases.
Summary/Conclusions
Pathogen reduction methods for blood components (including Intercept®) have been shown to be effective for a large number of viruses, however it has been reported that they are more efficient against enveloped viruses than against small, non‐enveloped viruses such as B19V (3.5–5.0 log degree of reduction). This report describes a B19V transmission via TC produced from a very high B19 viral load donation (>4.87E+10 IU/ml). No transmission was detected with a TC produced from a 1.46E+08 IU/ml B19V contaminated donation suggesting there is a B19 viral load limit at which pathogen inactivation is exceeded.
P‐303
Mirasol‐Treated Platelet Concentrates in Plasma Prepared from Overnight‐Held Buffy Coats
Bontekoe IJ , de Laleijne-Liefting LAE , van der Meer PF , Lagerberg JW , de Korte D
Sanquin Blood Supply, Amsterdam, The Netherlands
Background
Pathogen reduction technologies (PRT) are of growing interest as methods to improve the safety of blood components. Previous studies showed significant but acceptable loss of in vitro quality when platelet concentrates (PCs) from buffy coats (BCs) were treated with the Mirasol PRT. This treatment is based on the addition of riboflavin and illumination with UV light. As a standard, our blood bank uses ‘fresh’ BCs from overnight‐held whole blood. However, to allow optimal use of our whole blood collections, also overnight‐held BCs prepared from fresh whole blood are used for the preparation of PCs. Both logistic variations in processing, guarantee PC preparation within 24 h after whole blood collection.
Aims
To investigate the in vitro quality of Mirasol‐treated PC in plasma from overnight‐held BCs during 7 days of storage, compared to our current standard Mirasol‐treated PC.
Methods
Whole blood units were held 4–8 h before processing into red cell concentrates, plasma's and BCs. After overnight hold of the BCs for 7–13 h, 12 PCs (study group) were prepared from 1 unit of plasma and 5 BCs and subsequently Mirasol‐treated. On Day 2, 6 and 8, the PCs were sampled aseptically for in vitro measurements. As reference group, BCs from overnight‐held whole blood, processed into PCs and thereafter Mirasol‐treated, were analyzed; an unpaired t‐test was used.

Results
See table. Volume and associated platelet content of the study group was around 40 ml lower compared to the reference group, because the latter were not sampled for QC measurements at Day 1. At Day 8, the study group had lower pH values, resulting in 10/12 PCs fulfilling the requirement of pH37°C >6.3 (reference group 12/12). The activation marker CD62P and apoptosis marker annexin A5 showed the same increase during storage in both groups, but absolute levels on Day 2 and 8 were higher in the study group. A swirling effect was seen throughout the whole storage period and PCs were not bacterially contaminated.
Summary/Conclusions
Mirasol‐treated PCs prepared from overnight held BCs had lower pH and elevated activation and apoptotic parameters during 7 day storage, compared to Mirasol‐treated PCs from ‘fresh’ BCs (current standard product tested in the PREPAReS study). The clinical relevance, however, is unknown, and need to be demonstrated in a clinical trial.
P‐304
Assessment of the Clinical Performance of Mirasol‐Prt Treated Platelet Concentrates in Santiago De Compostela
Vilariño MD1, Villamayor M 1, Campos A 1, Castrillo A 2, Cardoso M 3
1Complejo Hospitalario Universitario de Santiago de Compostela, Santiago de Compostela, Spain2Centro de Transfusión de Galicia, Santiago de Compostela, Spain3TERUMO BCT, Brussels, Spain
Background
The Transfusion Center of Galicia and the University Clinic of Santiago de Compostela in Spain wanted to assess the performance in the clinical setting of platelet concentrates treated with the Mirasol pathogen reduction technology system.
Aim
This study aimed to evaluate the clinical performance of Mirasol‐treated platelet concentrates (M‐PC) used in the supportive therapy of thrombocytopenic patients
Methods
The protocol for a prospective observational study was submitted to the ethical committee of the University Clinic of Santiago de Compostela and granted approval before the start of the study. Patients receiving M‐PC had to sign an informed consent form before starting therapy. Initially, 41 patients and 106 transfusions were included in the study (see table 1). Four transfusions to one single patient were excluded after further analysis due to patient's refractoriness. Thrombocytopenic patients were maintained on platelet supportive therapy according to the Spanish and international transfusion guidelines, as well as per institution's standard practices. Post‐transfusion platelet counts were measured after 1 h and/or 24 h of transfusion. Platelets concentrates processed from whole blood collections with the OrbiSac technology (Terumo BCT) were treated with the Mirasol PRT system according to the manufacturer's (Terumo BCT) instructions for use. After treatment M‐PC were immediately released with no additional post‐treatment step. Platelets in PAS (SSP+, Macopharma) were stored up to 7 days at 22°C on an agitator. Furthermore, post‐transfusion surveillance of patients was maintained during the study period.
Results
Forty patients were transfused with 102 M‐PC at a rate of 2.6 M‐PC per patient. Average age of patients was 60 years old (range: 32–79). Average age of platelets was 4 days (range 2–7). Mean platelet dose 3.7 × 10e11 (range: 2.7–4.9). Table 1 illustrates patient groups. Transfusion responses (average values) for all patients included in the study and measured as CI1 h, CCI 1 h, CI 24 h and CCI24 h were: 22,500, 10,700, 11,000 and 5100, respectively. Sixty‐five percent of transfusions resulted in CCI1 h values ≥7500. Forty‐three percent of transfusions resulted in CCI24 h values ≥4500. Transfusion responses (CCI 24 h) per patient groups for AML, ALL, non‐Hodgkin lymphoma and other hematological diseases were: 3560, 3500, 6300 and 5100, respectively. No adverse reaction or severe adverse reaction was attributed to the transfusion of M‐PC by the hemosurveillance system in place during the observation period.
Table 1: Patient groups transfused with M_PC during the observation period

Conclusions
The use of M‐PC in the supportive treatment proved to be safe and effective for this cohort of thrombocytopenic patients. Mirasol‐PRT technology is very simple and easy to implement in the routine of the blood bank.
P‐305
Influence of Pathogen Inactivation of Platelet Concentrates on Transfusion Frequency, Number of Side Effects, and Application of Pooled Platelet Units
Nussbaumer W1, Astl M 1, Schennach H 1, Mayersbach P 1, Lin JS 2, Tappe D 2, Green J 2
1University Hospital Innsbruck, Innsbruck, Austria2Cerus, Concord, United States of America
Background
A photochemical treatment process utilizing amotosalen and UVA light (INTERCEPT[TRADEMARK] Blood System, Cerus, USA) has been developed for inactivation of viruses, bacteria, parasites, and leukocytes that can contaminate blood components intended for transfusion. INTERCEPT was approved by Austrian authorities in 2011 for a regional hospital based blood bank (University Hospital Innsbruck), and was introduced to routine use in April 2013.
Aims
The objective of this study was to characterize the platelet use and safety profile of INTERCEPT‐treated platelets components (PI‐PC) in comparison with non‐treated units (PC) administered across a broad patient population.
Methods
We report here a 21 month experience (1.4.2013 to 31.12.2014) with PI‐PC for all patients supported by our center, and compare it with an equal length period (1.1.2011 to 31.12.2012) when untreated PC were used before adoption of PI‐PC. Platelets of both groups were re‐suspended in SSP+ (Macopharma, Langen, BRD) at a 35%:65% ratio, and were used to support patients in all hospital departments. Platelet utilization and transfusion reactions reported in the observation periods were compared. In addition, we analyzed the use of pooled platelet units in three departments with high platelet consumption: hematology‐oncology (Hem‐Onc), heart surgery and graft surgery.
Results
Similar numbers of patients were transfused in the Pre‐adoption Control (C, 1797) and Post‐adoption Test (T, 1689) periods, with a comparable number of platelets units transfused (C 8611 and T 7662). PI‐PC successfully replaced PC for patients of all ages including 46 infants and 84 children transfused in the Post‐adoption period. The mean number of units transfused per patient was similar for the general population (C 4.8; median of 2, and T 4.5; median of 2), Hem‐Onc patients (C 10.8; median of 5 and T 9.9; median of 4), graft surgery patients (C 5.3; median of 2 and T 4.8; median of 3), and heart surgery patients (C 1.5; median of 1 and T 1.6; median of 1). Whereas the number of pooled platelets used increased for graft and heart surgery patients (C13.3% vs. T 40.9% and C 22.8% vs. T 54.0%, respectively), no notable difference was observed for Hem‐Onc patients. However, increased use of pooled platelets (C 706 units vs. T 1934 units) did not increase the total number of units transfused per patient. Rates of confirmed adverse reactions were similar between the T and C periods for both general population (C 1.3% T 1.4%) and Hem‐Onc patients (C 4.8% T 5.1%), with similar types of events observed, that included urticaria, pruritus, fever and chills.
Conclusion
Evaluation of use and safety of platelets before and after adoption of pathogen inactivation showed similar profiles for the support of all patients, including Hem‐Onc patients. Similar numbers of units were used per patient in all departments demonstrating comparability of PI‐PC and untreated platelets.
P‐306
Effects of Mirasol Pathogen Reduction Technology on Red Blood Cell Function Over the Course of Storage as Whole Blood or Packed Red Blood Cells
Yonemura S1, Larkin S 2, Doane S 1, Goodrich R 1, Kuypers F 2
1Terumo BCT, Lakewood, United States of America2Children's Hospital Oakland Research Institute, Oakland, United States of America
Background
Pathogen reduction technologies (PRT) represent a promising paradigm to address blood‐borne emerging infectious diseases (EID), but PRT must treat both components and whole blood (WB) for implementation in geographies where EIDs are of concern. The Mirasol PRT System uses riboflavin (Vitamin B2) and ultraviolet (UV) light to induce lesions in the nucleic acids of pathogens and nucleated cells, thereby inhibiting replication and function. This CE‐marked system is routinely used in Europe and the Middle East for platelets and plasma treatment and has been expanded to treat WB. Mirasol effectively reduces pathogen load and inactivates leukocytes, but its effect on packed red blood cells (pRBC) requires characterization.
Aims
This study assessed changes in RBC parameters during storage of Mirasol WB or pRBC in comparison to untreated WB or pRBC and gamma‐irradiated pRBCs.
Methods
Fifteen units of WB collected in CPD were evaluated, three each of Mirasol and Untreated stored as WB and three each of Mirasol, Gamma, and Untreated processed into leukoreduced pRBCs stored in AS‐3. Mirasol treatment involved illumination of WB in CPD mixed with 35 ml of 500 μM riboflavin solution at an 80 J/ml RBC UV energy dose. Hematology parameters were measured using an Advia 120 hematology analyzer and RBC deformability was assessed by ektacytometry. WB units were tested on days 2, 7, and 14 of storage while pRBCs were evaluated on days 1, 14, and 21.
Results
Differences among units were larger than differences over time or by treatment group; thus, changes over storage were assessed as percentages of starting values (see table). The hematology analyses for WB and pRBCs demonstrated little variation over the course of storage and among treatment groups for all RBC indices except reticulocyte count. A faster drop in reticulocyte count was observed for Mirasol in both WB and pRBCs. As expected WBC and platelet counts dropped rapidly over time in WB. No difference was found between Mirasol and Untreated in platelet count, but a more rapid reduction of WBC count for Mirasol was apparent. The ektacytometry results for pRBCs over all time points show trends for small shifts in the state of hydration (Omin and Ohyp values) for both Mirasol and Gamma relative to Untreated, but no difference was found in the maximum deformability index (DImax) among the three groups. When products were stored as WB no differences were observed between Mirasol and Untreated for any of these parameters.
Caption 1: Hematology and Ektacytometry Results

Summary/Conclusions
While sample sizes were small and donor‐to‐donor variability large, interesting trends were observed in this evaluation of Mirasol WB and pRBCs derived from Mirasol WB. Mirasol was effective in targeting nucleated cells as well as nucleic acid‐containing reticulocytes. The effect of removing reticulocytes on overall pRBC or WB quality parameters is expected to be small since reticulocytes comprise a small percentage of the RBC population, but is a question worthy of further investigation. Shifts in Omin and Ohyp suggest a slight dehydration of Mirasol and Gamma cells when stored as pRBC in AS‐3, but this effect is not observed in Mirasol WB.
P‐307
Production of Pathogen Inactivated Cryoprecipitate
Adamcova MA , Tesarova ET , Pacasova RP , Polokova NP
The University Hospital Brno, Brno, Czech Republic
Background
Cryoprecipitate is a diverse blood component containing specific clotting factors; factor I (fibrinogen), factor VIII, von Willbrand factor, factor V and factor XIII, as well as fibronectin and platelet microparticles. The complexity role of this blood product in the management of hemostasis has not been well studied, The most common current indication for the use of cryoprecipitace is hypofibrinogenemia in the setting of massive hemorrhage. At Transfusion and Tissue Department of The University Hospital Brno we prepared cryoprecipitate from male donors fresh frozen plasma after four months quarantine in the past. With starting pathogen inactivation method of platelet and plasma blood components for transfusion use – INTERCEPT Blood System™ in 2012 we also start working on the introduction of pathogen inactivated cryoprecipitate production as a source of cloting factors I and XIII ant its use for patients with massive trauma hemorrhage and/or for patient with acquired diluted surgical (obstetric) coagulopathy.
Methods
Plasma obtained by separation male donors (volume 650 ml) is treated by the INTERCEPT Blood System™ and it is shock frozen in one bag of 1000 ml capacity. A week later it is thawed overnight at 2–6°C, recentrifuged using a hard spin at temperature 4°C. After removing supernatant cryo poor plasma the concentrate is resuspended in 100 ml of own supernatant and again rapidly frozen. During validation following parameters were examined: content of fibrinogen in plasma after separation (input material), plasma after pathogen inactivation and in cryoprecipitate (final product), content of factor FXIII in plasma after separation (input material), in plasma after pathogen inactivation and in cryoprecipitate (final product), content of factor FVIII and von Willebrand factor in samples of pool of six cryoprecipitate units (final product).
Results
We tested content of fibrinogen and FXIII in 30 products from the beginning of the process to the final product (input material – plasma after separation, pathogen inactivated plasma, final product – cryoprecipitate). In tested samples there were blood groups A, 0 and AB. Results are in following table (*average content of 30 products per unit, **average content of 30 product per unit, ♠ average content of 3 pools,♠♠ average content of 3 pools)
Caption 1: Results

Conclusion
Validation of the cryoprecipitate is finished and we are able to offer to our clinicians colleaques product of the high quality with the reduction of risk related to transfusion. Till the middle of March 2015 we produced nearly 370 units of pathogen inactivated cryoprecipitate with average content of fibrinogen 0.80 g 212 of factor XIII respectively.
P‐308
Photochemical Inactivation of Middle East Respiratory Syndrome Coronavirus Using the Mirasol PRT System
Keil SD1, Marschner S 1, Gosney J 1, Edewaard K 1, Bowen R 2
1Terumo BCT, Lakewood, United States of America2Colorado State University, Ft. Collins, United States of America
Background
A novel coronavirus, first reported in June 2012, was isolated from a 60 year old patient suffering from respiratory disease in Jeddah, Saudi Arabia. This virus was later renamed Middle East respiratory syndrome coronavirus (MERS‐CoV). Through February 2015, the virus has been found in 23 countries, infected at least 1025 people and led to 399 fatalities (a 39% fatality rate). Although the virus has been most active in the Middle East, specifically Saudi Arabia, it has also been found in both Western Europe and the United States. It is believed most human cases are due to zoonotic transmission of the virus from camels to humans.
Although there is no evidence to date of transfusion transmission of this virus, it remains a possibility as the US Center for Disease Control confirmed the presence of viral RNA in a patient's serum sample in 2013. In this study we evaluated the efficacy of the Mirasol PRT System for Plasma on the reduction of MERS‐CoV.
Methods
Each of six Mirasol Treatment bags were filled with 200 ml of human plasma, followed by 35 ml of 500 μM riboflavin. The units also received a 15 ml aliquot of MERS‐CoV (EMC Strain). A 1–2 ml sample was removed from each unit both pre‐treatment and post‐treatment to measure the virus titer. Each of the resulting 12 samples (6 pre‐treatment and 6 post‐treatment) were assayed by plaque assay on Vero cells. In the case of pre‐treatment samples, the sample was diluted from 10−1 to 10−6, and 0.1 ml of each dilution was inoculated onto one well in a 6‐well plate of confluent Vero cells. For post‐treatment samples, 0.1 ml volumes of sample diluted to 10−1 were inoculated in triplicate using 6 well plates, for a total of 0.3 ml assayed per post‐treatment sample. The plates were rocked every 10–15 min for 45 min, after which assay plates were overlaid with 0.8% agarose in medium, incubated for 2 days (37°C, 5% CO2) and a second overlay containing 0.005% neutral red was added; plaques were counted the following day. Virus titers were calculated based on plaque count and dilution.
Results
None of the six samples collected from the Mirasol treated units contained detectable infectious virus and were below the limit of detection for the assay. The average pre‐treatment titer for the six replicates was 6.4 log PFU/ml and the limit of detection for the assay was ≤2.0 log PFU/ml. The observed reduction in this study was ≥4.4 log.
Conclusion
The Mirasol PRT System for Plasma effectively reduced the infectivity of MERS‐CoV to the limits of detection using an in vitro cell culture model. The ≥4.4 log reduction of MERS‐CoV observed in this study demonstrates that the Mirasol PRT System may provide protection against transfusion transmitted MERS‐CoV in plasma, platelets treated in plasma and platelets treated in platelet additive solution. All three systems are designed to have comparable reduction.
P‐309
Amotosalen and Ultraviolet a Light Inactivate Zika Virus in Plasma
Aubry MTP 1, Richard V 1, Green J 2, Broult J 3, Musso D1
1Institut Louis Malardé, Papeete, French Polynesia2Cerus Corporation, California, United States of America3Hôpital du Taaone, Pirae, French Polynesia
Background
Zika virus (ZIKV) is an arthropod‐borne virus (arbovirus) transmitted by the bite of infected mosquitoes. Symptoms of ZIKV infections are typically mild, but severe neurologic complications can occur. The potential for ZIKV transmission through blood transfusion was demonstrated during the largest ZIKV outbreak that occurred in French Polynesia from October 2013 to April 2014: 2.8% of the blood donors, asymptomatic at the time of blood donation, were found positive using ZIKV‐specific reverse transcription PCR (RT‐PCR). Prevention of transfusion‐transmitted ZIKV infections is challenging because most of the cases are asymptomatic, and are not detected during medical questionnaire, and nucleic acid testing for ZIKV is not routinely available. Pathogen inactivation of blood products is a proactive strategy that provides the potential to reduce transfusion‐transmitted diseases. Inactivation of arboviruses by amotosalen and ultraviolet A (UVA) illumination was previously demonstrated for chikungunya, West Nile (WNV) and dengue viruses (DENV). We report here the efficacy of this strategy for ZIKV inactivation in plasma.
Aims
According to the recommendations for evaluation of pathogen reduction efficacy, we performed a spiking experiment of plasma units with ZIKV in order to compare the viral titers and viral RNA loads before and after inactivation.
Methods
Plasma units were collected in California (USA) from DENV and WNV immunoglobulin G negative donors. ZIKV was propagated on African green monkey kidney cells (VERO) and concentrated. Four plasma units were spiked with ZIKV before transfer into an INTERCEPT disposable kit (INT3102B, Cerus Corporation). Three units were inactivated with amotosalen and 3 J/cm2 UVA light for ~6 min, the fourth one was not and was the positive control.
Detection of replicative ZIKV and viral titration was performed by inoculating pre‐ and post‐inactivated plasma unit samples on VERO cells. Five serial passages of plasma unit samples were performed in order to amplify any replicative virus. Infected cells were detected by an indirect immunofluorescence (IF) assay, and viral titers were expressed in 50% tissue culture infectious dose (TCID50/mL).
ZIKV RNA loads were measured after each passage on cell culture. RNA quantitation was performed by RT‐PCR and expressed in log10 copies/ml.
Results
Before inactivation, the mean viral titer was 6.57 log10 TCID50/ml. The culture of pre‐inactivated sample showed the presence of replicative viruses during the five serial passages. In contrast, no replicative virus was detected in post‐inactivated plasma units, even after five passages (Table).
Before inactivation, the mean viral RNA load was 10.25 log10 copies/ml. After inactivation, the mean viral RNA load was 9.51 log10 copies/ml, and decreased to 3.86 log10 copies/ml after the first passage. From the second and up to the fifth passage on VERO cells, the viral RNA remained undetectable indicating the absence of replicative virus.
Table: ZIKV titers and viral RNA loads before and after inactivation

Conclusions
Amotosalen combined with UVA light inactivated ZIKV in fresh frozen plasma (6.57 log10 by infectivity, and 10.25 log10 by RT‐PCR), and therefore may be used to prevent plasma transfusion‐transmitted ZIKV infections. This procedure is of particular interest in areas, as French Polynesia, in which several arboviruses are co‐circulating.
P‐310
Rfid Tags Robustness and Traceability in the Process of Methylene Blue Treated Plasma
de Valensart N , Lotens A , Najdovski T
Belgian Red Cross, Namur, Belgium
Background
Radio Frequency Identification (RFID) tags on bags increases possibilities of data recording during blood components processing and localisation in the storage facility. The illumination device and set for plasma Methylene Blue (MB) treatment can be equipped with RFID technology. Dedicated software is used for data transmission and management from sterile connection to illumination step of the plasma unit.
Aims
Evaluation of coagulation factors recovery and traceability with RFID technology during MB process. Test of tag robustness to freezing, storage and thawing in routine processing conditions.
Methods
Recovery experiment was performed on 12 whole blood (WB) plasmas treated with THERAFLEX MB‐Plasma set and illuminated with MacoTronic B2. Samples were drawn before and after MB treatment and dosage of Fibrinogen, FVIII and Total Protein were realised (mean ± SD). RFID traceability experiment was performed on 30 bags filled with water, connected to THERAFLEX MB‐Plasma set and tagged with RFID labels on the storage bag. Donation number and product code were registered in the RFID tag during the sterile connection to the treatment set. These data were transferred to MacoTrace software used to capture illumination data from MacoTronic B2. After MB treatment, bags bearing RFID tags were frozen in blast freezer (MBF42, Dometic®). Frozen units were stored at −25°C (n = 20) or at −80°C (n = 10) for 7 days. Frozen units were thawed either using a waterbath (n = 15) or a Plasmatherm DTM (Barkey®). Robustness of RFID tags was checked through all processing steps. Process data integrity and accuracy was challenged in 10 illumination cycles that were interrupted to simulate abnormal process conditions. Finally RFID use in routine conditions was assessed with 70 WB plasmas tagged with RFID labels, treated according to routine process, frozen and stored at −25°C.
Results
Recoveries after MB treatment were respectively at 83 ± 10% for Fibrinogen, 87 ± 7% for FVIII and 100 ± 2% for Total Protein. Data transfers to RFID tags occurred in hidden time during sterile connection and were accurate. Donation number, product code and lot number were correctly transferred to the MacoTrace software treatment queue. MacoTronic B2 synchronised with MacoTrace database, detected tags and, if products were present in the treatment queue, automatically started illumination. Software registered process data and final status of illuminated cycle on RFID tag. The 10 interrupted cycles were detected and accurately registered on RFID tags. After handling, freezing, cold storage and thawing RFID tags (n = 100) were readable. The routine treatment of 70 plasmas was fluent and no issues related to RFID tags have been detected.
Caption 1: Data contents in RFID tags at end of treatment

Conclusions
RFID technology used during the THERAFLEX MB‐Plasma process was functional, fast and easy to handle in routine use. In addition, RFID tags could be used for localisation in storage facility. RFID tags were readable at each processing step with an optimal traceability and data recording capability. RFID tags resisted to mechanical stress during processing, storage and in our thawing conditions. The only inconvenient is that RFID tags are not compatible with thawing devices using microwave technology.
P‐311
Double Dose Platelet Concentrate from Apheresis Treated with Amotosalen and UV‐A Light: First Evaluation
Castrillo Fernández ACF , Arcas Otero CAO , Abalo Martínez MAM , Adelantado Pérez MAP , Rodríguez Calvo MIRC
Centro de Transfusión de Galicia, Santiago de Compostela, Spain
Currently the Intercept platelet processing set with dual storage containers for treatment of platelet concentrates (PCs) in additive solution (AS) permits treat PCs, later they will be split en two therapeutic units, with one illumination cycle.
The objective of this study was to evaluate in vitro function for 7 days of storage, following Intercept treatment of double dose and also evaluate the processing efficiency.
Material and methods
Apheresis PCs were collected on the Amicus device and stored overnight with continuous agitation at 22°C. On the day after collection (day 1) the product was prepared for treatment with Intercept (Intercept blood system™,Cerus corp.), volume and platelet yield pre‐treatment was calculated. After treatment PC was split in half and stored in 2 bags, one was supplied later another was used by study. Nine PCs were studied, they were stored under standard conditions and samples were withdrawn on day 2, 5 and 7 of storage. The cell content and the mean platelet volume (MPV) were measured using a Sysmex XT‐2000i haematology analyser. The pH was measured at 22°C with a pH‐meter (Crison Micro pH 2001).The phenomenon of swirling was visually assessed, giving a numerical value of 0–2 (0 = no swirling, 1 = intermediate and 2 = patent swirling). Glucose, lactate and lactate dehydrogenase (LDH) determinations were carried out with an Olympus AU400 Chemistry Analyser.
Results
The average treatment dose and volume prior to split were 5.82 ± 0.24 × 1011 platelets and 416.11 ± 6.25 ml per unit respectively. The final average dose per unit was 2.62 ± 0.19 × 1011 platelets and the average volume was 197.7 ml. The pH of the PCs remained stable for 7 days with a positive swirling for all PCs. In three units the glucose levels at 7 day were low. In table 1, some parameters are shown.
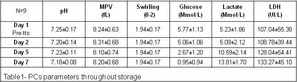
Conclusion
Intercept treated double dose PCs retained adequate in vitro platelet characteristics for 7 days storage but the platelet content is lower than our PCs standard. On the other hand this procedure has some logistic advantages and it saves cost.
P‐312
Intercept Blood System Inactivates Enterococcus Faecalis, Multiple Species Of Streptococcus and Serratia Liquefaciens in Platelet Components in Platelet Additive Solution and in Plasma
Hanson DFH
Cerus Corporation, Concord, United States of America
Background
The INTERCEPT™ Blood System for platelets has been shown to inactivate a wide spectrum of bacterial, viral and parasitic pathogens in platelet components (PC) suspended in 35% plasma/65% platelet additive solution (PAS) (Prowse C, 2013 Vox Sang 104(3):183) or in 100% plasma (Hanson D, et al., 2014 Transfusion 54(S):205A). Previous data included inactivation of Serratia marcescens and Streptococcus pyogenes. The SHOT study identified transfusion transmitted sepsis caused by additional species of Serratia and Streptococcus (SHOT annual report 2001/2002). The INTERCEPT mechanism of action is not species specific and evaluation of these additional species offers the opportunity to demonstrate this breadth of efficacy.
Aims
The aim of these studies was to demonstrate that the inactivation of Serratia liquefaciens, Streptococcus agalactiae, S. mitis,S. pneumoniae and Enterococcus faecalis by INTERCEPT is comparable to that previously demonstrated for S. marcescens and S. pyogenes.
Methods
Apheresis platelet components containing ~2.5–7.0 × 1011 platelets in ~330–400 ml of 100% plasma or 35% plasma/65% PAS were inoculated to a titer of ~106 organisms per mL with one of the five organisms under investigation, then treated with the INTRECEPT Blood System for platelets using large volume processing sets. Samples were taken before illumination to determine input titer and after illumination to detect and quantify any residual viable pathogens. Bacterial viability was determined by colony formation on rich agar (E. faecalis, S. liquefaciens and S. agalactiae) or on blood agar incubated in a CO2 atmosphere (S. mitis and S. pneumoniae). Historic data on inactivation of Streptococcus pyogenes and Serratia marcescens were obtained using PC of approximately 300 ml and small volume INTERCEPT platelet processing sets.
Results
Historical data for inactivation of S. marcescens and S. pyogenes in PC in PAS are shown in Table 1, along with the data obtained in the current studies. In the current study, inactivation of at least 6.5 log10 of one additional species of Serratia and four additional species of Streptococcus/Enterococcus was seen in PC suspended in 100% plasma or 35% plasma/65% PAS (Table 1).

Conclusions
INTERCEPT treatment inactivates high titers of multiple species of Serratia and Streptococcus in platelet components suspended in PAS and in plasma. These results support the applicability of inactivation data obtained using one species as a model for efficacy across a genus.
P‐313
In Vitro Evaluation of Pathogen Inactivated Apheresis RBC Using the S‐303 Treatment System
Nussbaumer W1, Volland H 1, Schennach H 1, Astl M 1, Andresen S 2, Mufti N 2, Erikson A 2
1University Hospital Innsbruck, Innsbruck, Austria2Cerus, Concord, United States of America
Background
The Second Generation S‐303 Treatment System for Red Blood Cells (RBCs) uses S’�303 to crosslink nucleic acids and prevent replication of contaminating pathogens and residual leukocytes. Glutathione (GSH) is included to quench non‐specific reactions. This pathogen inactivation system has shown robust pathogen inactivation for bacteria, viruses and parasites while retaining in vitro and in vivo RBC quality per AABB, FDA and EU Guidelines up to 35 days of storage.
Aims
The purpose of this study was to assess the quality of stored S‐303 treated RBCs compared to conventional (untreated) Control SAG‐M RBCs collected using the Alyx apheresis system.
Methods
Six replicates, each consisting of a double RBC apheresis units (total volume of 552 ± 15 ml), were collected using the Alyx system per manufacturer's instructions and institutional procedures. All red cell concentrates were suspended in SAG‐M and stored at 4 ± 2°C.
For each replicate Control units (268–288 ml) were prepared and held at 4 ± 2°C. Test units (265–289 ml) were treated with the S‐303 treatment process by combining RBC with GSH and a proprietary diluent solution followed by addition of S‐303 (final concentrations: 20 mM GSH and 0.2 mM S‐303, based on an RBC input volume of 280 ml). After an 18–24 h RT hold, units of S‐303 treated RBC were centrifuged and the treatment solution expressed and replaced with SAG‐M. S‐303 treated RBCs were stored at 4 ± 2°C for 6 weeks. Test and Control RBCs were sampled over 43 days of storage for analysis of in vitro parameters (Table 1).

Results
Following the S‐303 treatment process, the mean extracellular protein content was reduced 16‐fold in T compared to C and Hb recovery for T RBCs was 90%. Hemolysis, K+, and lactate increased over storage, whereas pH, ATP, Na+, and glucose decreased in T and C. On D43 the mean pH, K+, MCV and lactate were significantly lower in T compared to C whereas ATP, glucose, Hct, MCHC were significantly higher. None of these differences were physiologically relevant. Hemolysis was equivalent for T and C on D43.
Conclusions
S‐303 treated RBC units had Hb >40 g, Hct within 50–70% and hemolysis <0.8% after 43 days of storage. Apheresis collected RBC treated with the S‐303 treatment system met current EU guidelines for leukocyte‐depleted RBCs with respect to Hb, leukocytes, Hct, and hemolysis as has been observed for whole blood derived RBCs. All measured in vitro parameters of S‐303 treated RBCs indicate suitability for transfusion.
P‐314
Pathogen Inactivation with Amotosalen and UVA Light of Routinely Produced Double Dose Buffy‐Coat Concentrates Aimed for Transfusion: Preservation of Mitochondrial Function
Sandgren P
Karolinska University Hospital, Stockholm, Sweden
Background
The INTERCEPT Blood System for Platelets (PLT) utilizes amotosalen (S‐59) in combination with ultraviolet A (UVA) light to inactivate viruses, bacteria, protozoa and leucocytes that may contaminate PLT concentrates. Several in vitro studies have subsequently assessed the functional quality of INTERCEPT treated PLTs stored for up to 7 days after treatment. These results seem to be in line with in vivo results of INTERCEPT‐treated PLTs obtained from clinical trials. However, limited data are available on the mitochondrial function of routinely produced INTERCEPT‐treated double‐dose (DD) buffy‐coat (BC) PLT units which allows a single treatment procedure to produce two pathogen‐inactivated PLT units.
Aims
The main objective of this study was to evaluate potential effects on mitochondrial function after INTERCEPT treatment on routinely produced pools of 8 BCs aimed for transfusion. Also, processing efficiency was evaluated.
Materials and methods
Buffy‐coats(BCs)were separated from routinely collected 450 ml whole blood donations. Eight ABO matched BCs were selected, and manually pooled to undergo INTERCEPT‐treatment as a unit (n = 100 pools). Platelets were then aliquoted and suspended in 65% platelet additive solution (PAS) and 35% plasma. Potential changes in the mitochondrial membrane potential, a marker of pro‐apoptotic events and maintenance of oxidative phosphorylation capacity, were measured. In addition, cell count, blood gas analysis, aggregates and swirling in all DD INTERCEPT‐treated BC PLTs were studied preceding transfusion of 100 units.
Results
The mitochondrial membrane potential determined by JC‐1‐labelling was well maintained in all INTERCEPT‐treated units with >95% maintenance of mitochondrial function (96.36%±2.61%) after treatment (Table 1). Platelet count (232 ± 19 × 109/unit) was well above the European guideline requirements, and the pH (22°C) of all units was maintained at ~7.0 after INTERCEPT‐treatment on Day 2. This is in agreement with the Council of Europe recommended range of 6.4–7.4. All treated PLTs showed good swirling characteristics (score = 2) indicative of well‐maintained PLT morphology. No aggregates were observed in any of the units.
Conclusion
Our data demonstrate that photochemical pathogen inactivation of routinely produced DD‐BC PLT concentrates with the INTERCEPT Blood System had no influence on the mitochondrial function in all tested units.

P‐315
Reduction of Febrile and Allergic Reactions in Patients Receiving Platelets Treated With Riboflavin and UV Light
Jimenez-Marco T1, Truyols C 2, Pérez-Montaña A 2, Bautista-Gili AM 1, Girona-Llobera E 1
1Balearic Islands Blood Bank, Palma de Mallorca, Spain2Son Espases Balearic Islands University Hospital, Palma de Mallorca, Spain
Background
Pathogen Reduction Technology (PRT) system for blood components enhances blood safety by inactivating donor leukocytes, viruses, bacteria, parasites and as‐yet‐unidentified agents. Additionally, PRT for platelet (PLT) components has remarkable advantages such as replacing bacterial contamination screening tests, gamma irradiation, CMV tests and also reducing PLT transfusion reactions.
Aim
In order to investigate the occurrence of febrile and allergic reactions in patients receiving PLT treated with riboflavin and UV‐light in our region, a retrospective study of PLT transfusion reactions was performed at the Balearic Islands University Hospital during the years 2013–2014.
Methods
Since 2013, our regional Blood Bank provides PLT components prepared with riboflavin and UV‐light (Mirasol PRT system, TerumoBCT, Lakewood, CO, USA) for transfusing patients with thrombocytopenia. The incidence of febrile and allergic reactions in patients receiving transfusions of PLT treated with riboflavin and UV‐light at the Balearic Islands University Hospital during 2013–2014 was compared with the incidence of these reactions during a two‐year control period before PRT implementation (2007–2008).
The information regarding PLT transfusion reactions was provided by the Balearic Islands Hemovigilance Division and the UniversityHospital.
Results
During the control and the riboflavin and UV‐light PLT periods, 4893 and 5711 PLT components were transfused to patients with thrombocytopenia at the Balearic Islands University Hospital, respectively (P = 0.09).
The percentage of febrile reactions in patients receiving PLT transfusions was 2.5% and 1% for the control period and riboflavin and UV‐light PLT period, respectively (P = 0.023). The percentage of allergic reactions in patients receiving PLT transfusions was 1.02% and 0.75% for control period and PRT period, respectively (P = 0.005) (Table I).
Caption 1: Table I. Febrile and allergic reactions in patients receiving PLT transfusions in the Balearic Islands University Hospital

Conclusions
The occurrence of febrile and allergic reactions in patients receiving PLT transfusions at the Balearic Islands University Hospital decreased significantly after the implementation of PLT treated riboflavin and UV‐light. In addition, there have been no documented cases of platelet transfusion related sepsis or any other transfusion transmitted infections since the implementation of PRT.
P‐316
Mirasol PRT Treatment of Platelet Concentrates Derived from Four Buffy Coats Obtained with the Tacsi WB System: In Vitro Quality Evaluation
Labanca LL , Schiavo VS , Soster AS , Lucania GL , Mattiazzo LM , Palazzo MP , Giaccone MG , Bianchi MB , Eliantonio FE , Bordiga AMB
City of Science and Health of Turin, S. Anna Hospital, Turin, Italy
Background
Mirasol Pathogen Reduction Technology (PRT) uses riboflavin and UV light to reduce the pathogen load and to inactivate leukocytes in Platelet Concentrates (PCs) or Plasma.
Aims
To evaluate the in vitro cell quality of Mirasol treated PCs, derived from four Buffy Coat (BC) obtained with TACSI WB System, compared to identical untreated PCs during a seven‐day storage period.
Methods
Whole Blood (WB) units were collected in CRC kits and processed on TACSI WB System (Terumo BCT) to obtain Red Blood Cells, Plasma and BC. 20 BC‐derived PCs (BC‐PCs) were prepared on TACSI PL system by pooling four BCs and Intersol additive solution. 10 experimental replicates were performed. Each replicate was prepared from two blood group identical BC‐PCs through the use of a pool and split design, resulting in 10 control units and 10 paired test units. The test units were treated with Mirasol PRT (35 ml of 500 μM riboflavin solution and UV light) according to the manufacturers’ instructions (Terumo BCT). All units were stored with agitation at 22°C, away from direct light sources, for a total period of 7 days. Samples were taken on days 1, 5, and 7 for the following in vitro tests: platelet count and MPV (Sysmex XE‐2100), swirling, pH, Glucose and Lactate concentrations (ABL 800 Radiometer). All units were tested for sterility on day 7.
Results
The results of the in vitro measurements for untreated and Mirasol‐treated BC‐PCs on days 1, 5 and 7 are summarized in table 1. The plts concentrations were about 12 percent lower in Mirasol treated units, reflecting the dilution caused by the addition of the riboflavin, but remained fairly stable during the storage, with an accettable loss in all units. Treated units showed a higher metabolic rate with lower glucose concentration at the end of the storage. Glucose consumption and lactate production resulted increased compared to controls, but below the limits previously established for acceptable in vivo recovery and survival (glucose consumption: <0.05 mmol/1012 plts/h; lactate production: <0.110 mmol/1012 plts/h). The increased metabolism compared to control units corresponded to an increase in MPV at the end of the storage. pH values slightly decreased in all units but remained above the Council of Europe recommendations (pH ≥ 6.4). Although lower in treated units pH was above 6.85, the established limit for acceptable in vivo platelet recovery and survival. All units maintained a positive swirling up to day 7 and resulted negative to sterility controls.

Conclusions
This study confirms previously reported observations that Mirasol treatment of PCs leads to an increase in cellular metabolism. However metabolic rates were maintained at a suitable level through the 7 days of storage, falling within previously established ranges for acceptable in vivo survival and recovery. Both treated and untreated units complied with European quality requirements for product release until day 7 of storage. In conclusion, according to our findings, Mirasol treated PCs derived from four BC obtained with TACSI WB System, show accettable in vitro quality through 7 days of storage.
P‐317
Adequate Leukodepletion of Platelet Concentrates from Pools of 7 or 8 Buffy‐Coats for Photochemical Pathogen Inactivation Treatment of Double Transfusion Doses
Bergsten MB 1, Norda RN2, Hardarson BH 3, Landrö RL 3, Andersson AA 4, Abedi MA 5
1Landstinget Västmanland, Västerås, Sweden2Västmanland sjukhus Västerås / Laboratoriemedicin, Västerås, Sweden3University Hospital, Reykjavik, Iceland4Departement of Laboratory Medicine NU‐sjukvården, Trollhättan, Sweden5Departement of Laboratory Medicine Örebro University Hospital, Örebro, Sweden
Background
The European requirement for White Blood Cell (WBC) count in a platelet concentrate is below 1 × 106 per unit. It is of importance for the producing sites to have valid working processes which fulfill all requirements. The INTERCEPT™ Blood System (Cerus) utilizes amotosalen and UVA to inactivate contaminating viruses, bacteria, parasites and leukocytes in platelet concentrates and plasma. A treatment set allowing processing of a double dose of buffy‐coat (BC) or apheresis platelets and further storage in two bags before transfusion is available.
Aims
The production of double dose platelets from pools of 7 or 8 BC's is routinely implemented in our 4 Nordic sites. We looked back at QC data on a period ranging from 2010 to 2014 demonstrating the capability to produce double dose platelets treated with INTERCEPT meeting the leukodepletion guidelines using BC pooling sets (Fresenius‐Kabi) with Sepacell™ PLX‐5 filters (Asahi Kasei Medical).
Methods
Of 7 or 8 BC's separated on the day of donation or after overnight storage from 450 to 500 ml whole blood collections are sterile docked after overnight storage at 20–24°C with agitation to a pooling set with PLX‐5 filter K4R7039 using the ‘double train’ or the ‘octopus’ method. SSP+ 280 ml (Trollhättan) or 300 ml (other sites) solution (Macopharma) is added to the pool. After soft spin centrifugation, platelets are leukodepleted through a PLX‐5 filter on a Macopress Smart (Macopharma) in Trollhättan or Optipress (Fresenius Kabi) in the other sites. The double dose platelet concentrate is then treated for pathogen inactivation with INTERCEPT (amotosalen + UVA) using a Dual Storage DS set INT25. The capability of the filter to adequately leukodeplete below the required limit of 1 × 106 white blood cells (WBC) per unit in 90% of the units is evaluated.
Results
Of 160 platelet units were QC tested post INTERCEPT treatment (unless otherwise specified) in Õrebro, 162 in Reykjavik, 606 in Trollhättan and 184 in Västerås. Table 1 shows QC data in the product split in two doses. Platelet yields were above the limit of 2 × 1011/unit in 100% of the tested units in Õrebro, 99% in Reykjavik and 77% in Västerås (required 75%, not tested in Trolhättan). WBC count was below the limit of 1 × 106 in all of the tested units in the four sites with a maximum of 0.77 × 106. 17 batches of pooling sets including 21 batches of filters were tested.

Summary/conclusions
The pooling and leukodepletion of 7 or 8 BC with PAS can be done with conventional pooling sets including a leukodepletion filter to produce double dose platelet concentrates subsequently treated with INTERCEPT. WBC removal is consistently meeting the guidelines.
P‐318
Feasibility Evaluation of Implementing Amotosalen and UVA Treatment on Double Dose Leukoreduced Pooled Random Donor Platelets
Amorim LA , Ferreira TMF , Rodrigues JFRP , Lopes MEDL
HEMORIO, Rio de Janeiro, Brazil
Background
Optimized production methods for the production of platelet concentrates will lead to better utilization of whole blood collections, increased availability of platelet concentrates for transfusion, and significant reduction in use of disposables. Therefore, a new method was developed to prepare double dose, leukoreduced, pooled random donor platelets (RDPs). Additionally, viruses, bacteria and other micro‐organisms, which are not detected by routine laboratory tests, are a concern to the Brazilian medical community in relation to the safety of transfused blood products.
Aim
The objective of this study was to determine the feasibility of implementing the INTERCEPT™ Blood System for pathogen inactivation for the double dose RDPs produced using this optimized production method. The method was assessed, and the impact on the quality of the treated products was evaluated.
Methods
RDPs were separated from 450 ml ± 10% whole blood donations following local procedures. Within 36 h of collection, 8 ABO‐identical, whole blood‐derived RDPs were pooled into a standard 600 ml transfer container (n = 12 pools), and leukoreduced by filtration. The resulting mean platelet count for the pools was 1313 ± 125 × 109/l. The double dose RDP pools were then immediately treated with 150 μM amotosalen and 3 J/cm2 UVA, split in two platelet doses for transfusion, and stored at 20–24°C with flatbed agitation for 7 days. In vitro assays to assess platelet quality were performed on all pools on days 1 (pre‐treatment), 2, 5 and 7.
Results
This method for preparing double dose, leukoreduced RDP pools resulted in a 20% decrease in needed whole blood collections to produce the same number of platelet doses. Additionally, there was a 50% reduction in the use of filters and pooling systems, and a 30% reduction in the use of sterile connections. Due to reduction in pooling and centrifugation procedures labor time was reduced by 50%. Since the double dose INTERCEPT processing set was utilized, the time for producing 2 pathogen inactivated platelet doses was cut in half. For all INTERCEPT‐treated RDP pools, platelet quality was well preserved during 7 days of storage, and met local and EU guidelines for blood component specifications. The mean platelet dose was 2.6 ± 0.2 × 1011 per product, and further optimization is possible. The pH of the pools remained stable over 7 days of storage (mean 6.60 ± 0.17) with a platelet swirl rating of 2–3 for all pools. Additionally, all pools showed active metabolism with maintained pO2 levels (140 ± 30 mm Hg), adequate residual energy source (glucose, 190 ± 27 mg/dl), and sufficient buffering capacity.
Conclusions
INTERCEPT treatment of double dose, leukoreduced, pooled RDPs produced by the optimized manufacturing processes is feasible, and the quality of the INTERCEPT‐treated platelets is well maintained. Further optimization of the platelet dose is ongoing.
P‐319
Quality Parameters of Red Blood Cells Treated With Intercept Pathogen Inactivation System Using S‐303: A Phase III Clinical Trial in Cardiac Surgery Patients
Leibacher J 1, Brixner V 2, Pfeiffer H-U 2, Müller MM 2, Geisen C 2, Dombos S 2, Weber I 2, Wotapek T 2, Janetzko K 3, Henschler R 4, Erickson A 5, Mufti N 5, Seifried E 2
1German Red Cross Blood Donation Center, Frankfurt, Germany2German Red Cross Blood Donation Center Baden‐Württemberg Hessen gGmbH, Frankfurt, Germany3German Red Cross Blood Donation Center Baden Württemberg Hessen gGmbH, Mannheim, Germany4Uni‐Klinikum München (LMU), Germany, Munic, Germany5Cerus Corporation, Concord, CA, United States of America
Background
The INTERCEPT™ Blood System for Red Blood Cells (RBC) has been developed to prevent replication of contaminating pathogens and leukocytes in RBC components for transfusion. S‐303 is used in the system to crosslink nucleic acids and glutathione to quench nonspecific reactions of S‐303. The INTERCEPT Blood System for RBCs inactivates a variety of pathogens including gram‐negative/positive bacteria, enveloped/non‐enveloped viruses, and parasites; emerging pathogens and/or those not routinely tested may also be inactivated. A Phase III clinical trial was conducted to compare standard blood bank quality parameters of both S‐303 treated and conventional RBCs and additionally showing patient safety
Aims
A randomized, double‐blind, controlled, multi‐center Phase 3 clinical trial using the INTERCEPT Blood System for RBC was conducted to evaluate the quality of INTERCEPT RBCs and patient safety. The quality of INTERCEPT RBCs was evaluated post‐production (PP) for all components and after 35–38 days of storage (EOS) for not‐transfused components. Parameters assessed included those required by the European Directorate for Quality Medicines (EDQM) in addition to physical and metabolic attributes.
Methods
Leukocyte reduced RBC in SAG‐M, derived from buffy‐coat depleted whole blood were used as the input RBC component. Control RBCs were stored at 1–6°C. Test units were treated on D1 with the S‐303 process. Test RBCs were added to a diluent containing GSH followed by S‐303 addition (final concentrations of 20 mM GSH/0.2 mM S‐303, based on 280 mL RBC input). After 18–24 h hold at 20–25°C, RBCs were centrifuged and the treatment solution was expressed and replaced with SAG‐M. Test and Control RBCs were sampled post production (PP), stored at 1–6°C, and either transfused to cardiovascular surgery patients to evaluate transfusion efficacy and overall safety or sent to a quality control lab to assess quality parameters after 35–38 days of storage.
Results
Quality parameters not included as part of the endpoint analysis (PP hemoglobin and post ‐storage hematocrit, hemolysis and ATP) are listed in Table 1. The endpoint analyses are presented in a companion abstract (Brixner, et al). All parameters, except hematocrit, are significantly different between Test and Control RBCs. MCHC and glucose are increased in Test compared to Control, while Hb content, MCV, pH, potassium, lactate, and total protein are decreased. Total protein was 3.4 ± 1.4‐fold lower in Test compared to Control. The mean Test PP hematocrit met the EDQM specifications of 50–70% for leukocyte reduced RBCs in additive solution.

P‐320
Theraflex MB‐Plasma Treatment Does Not Interfere With the Antibody Integrity in Human Plasma
Handke W1, Gravemann U 1, Sumian C 2, Reichenberg S 3, Seltsam A 1
1Red Cross Blood Services, Springe, Germany2Macopharma SA, Tourcoing, France3Macopharma Int., Langen, Germany
Background
Convalescent plasma therapy is used to treat emerging infections which cannot yet be cured by drugs or prevented by vaccination. Using plasma from patients who survived such incurable infection it is of special interest to ensure that the donated plasma is pathogen‐free. THERAFLEX MB‐Plasma treatment is used to inactivate pathogens in human plasma. For plasma intended to be used as convalescent plasma for treatment of acute infections, it is essential that the functionality of antibodies is preserved after the MB treatment procedure. It is known that MB/light treatment can influence the integrity of labile plasma proteins (e.g. plasma factor VIII and fibrinogen) but does not grossly reduce the function and life span of proteins in fresh frozen plasma.
Aim
The aim of the study was to investigate whether antibody binding in human plasma is affected by the THERAFLEX MB‐Plasma treatment. For this purpose exemplarily the reactivity of anti‐HCMV antibodies was tested prior to and after MB/light treatment.
Methods
Plasma units were preselected for the presence of anti‐HCMV antibodies (n = 4). Pathogen inactivation was done using the THERAFLEX MB‐Plasma system and the MacoTronic B2 illumination device and samples were taken before and after illumination. In order to detect changes in antibody binding different dilutions of plasma samples were tested for reactivity of anti‐HCMV antibodies by ELISA.
Results
Reactivity of anti‐HCMV antibodies in human plasma was not reduced after MB/light treatment.
Conclusion
THERAFLEX MB‐Plasma treatment does not negatively affect the binding of anti‐HCMV antibodies in human plasma. Pathogen inactivation by MB/light treatment therefore could contribute to the safety of convalescent plasma without deteriorating its quality.
P‐321
Qualification of the S‐303 Treatment System at Colindale Blood Centre
Omonua A1, Hicks V 1, Symonds I 1, Davies J 1, Thomas S 1, Melucci Vigo G 2, Erickson A 3
1NHS Blood and Transplant, London, United Kingdom2Cerus BV, Amersfoort, Netherlands Antilles3Cerus Corporation, Concord, CA, United States of America
Background
The INTERCEPT System for Red Blood Cells (RBCs) is intended to inactivate pathogens and leucocytes in RBC components for transfusion by using S’�303 to crosslink nucleic acids, preventing replication of contaminating pathogens. A Phase 3 clinical investigation in patients with thalassemia major requiring chronic RBC transfusion support is currently in progress in Europe and will be expanded to include additional study sites. In the UK, NHSBT aims to provide INTERCEPT treated RBC for the investigation of the safety and efficacy of S‐303 treated RBCs using the INTERCEPT™ System for RBC, compared to conventional RBCs.
Aims
This study was designed to qualify the NHSBT blood centre in Colindale to process RBC components using the S‐303 treatment process for the planned Phase 3 clinical investigation.
Methods
On the day (D) of collection, D0, leucocyte‐depleted SAG‐M RBCs were prepared from CPD whole blood donations and stored at 4 ± 2°C. Test units were treated on D1 with the S‐303 process. Test RBCs (280 ± 20 ml) were added to a diluent containing glutathione (GSH) followed by S‐303 addition (final concentrations of 20 mM GSH/0.2 mM S‐303, based on 280 ml RBC input). After 18–24 h hold at 20–25°C, RBCs were centrifuged and the treatment solution was expressed and replaced with SAG‐M. RBCs were stored at 4 ± 2°C for 35 days and were sampled for testing on D1, D2, and D35.
Results
All units met the acceptance criteria (Table 1). The volume post treatment was 249–311 ml, with a loss of 2 ± 1 g of Hb due to the S’�303 process (n = 13). The post S‐303 treatment mean and SD for volume, Hb content, haematocrit (Hct), and hemolysis are listed in Table 1. All units had Hb values of ≥40 g ranging from 42 to 58 g. The final Hct was 53–59%, within the 50–70% criterion. After 35 days of storage haemolysis was 0.05–0.26% (Table 1).

Conclusions
The S‐303 Treatment System was successfully transferred to the Colindale Blood Centre. This study demonstrated that all S‐303 treated RBC units met the EU guidelines for leukocyte depleted RBCs in additive solution with respect to Hct, Hb content and haemolysis at end of storage.The results of this study will support an application to MHRA for clinical investigation authorization
P‐322
Validation of Double Dose Buffy‐Coat Platelet Processing Using the Intercept Dual Storage Processing Set Prepared From Seven Buffy‐Coats
Pajares Herraiz AL1, Rodríguez Gambarte JD 1, Flores Sanz MV 1, Eguía Lopez BE 1, Peréz Parrillas CP 1
1Centro Regional de Transfusion Toledo‐Guadalajara, Toledo, Spain
Background
INTERCEPT Blood System for platelets is intended for the ex vivo preparation and storage of whole blood‐derived buffy coat and apheresis platelet components and is used to inactivate a broad spectrum of pathogens as well as contaminating donor leukocytes in platelet products. The device uses amotosalen HCl (a photoactive compound) and long‐wavelength ultraviolet (UVA) illumination to photochemically treat platelets
Aims
To demonstrate that INTERCEPT platelets prepared from 7 ABO compatible whole blood derived buffy‐coats met the acceptance criteria for manufacturing (Spanish and EU guidelines) and for support of patients requiring platelet transfusions according to clinical practice guidelines and standard platelet infusion methods in Spain and Europe
Methods
Whole blood (WB) was collected into Top&Bottom bags, was overnight storage under controlled temperature and was separated into Red cells, Plasma and Buffy‐coats with T‐ACE II. The obtained Buffy‐coats fulfilled the double dose requirements for processing set as specified in TABLE 1, and were rested for a minimum of 2 h before pooling. Pre‐selection of buffy‐coats, based on platelets donor counts has been applied (minimun donor count 220 × 109/L). For each of the six replicates prepared, 7 ABO‐matched Buffy‐coat units were pooled with 280 ml of Intersol additive solution using the Octopus method. After a soft spin centrifugation platelets in intersol were extracted with a manual press and was leukoreduced through the in‐line filter and were INTERCEPT treated. At the end of treatment, the platelet unit was split and INTERCEPT products were stored for 7 days under continuous agitation. Platelet concentrates were monitored on day 1, 2, 5 and 7 for the following in vitro parameters: volume; swirling; platelet counts, MPV &, WBC (Coulter LH 750); pH, pCO2 and pO2 (Gem Premier 300); glucose consumption and lactate production (Olympus 5400).
Results
Platelet recovery after centrifugation was 81% in average, with Platelet content of 6.4 × 1011 ± 0.3. Platelet and volume loss due to INTERCEPT treatment were approximately 5.5% and 4.8% respectively (TABLE 1), within the expected range of values. During the 7 days storage, platelet concentrates splited were monitored and platelet count and volume in day 2 was 3 × 1011/unit and 186 ± 4 ml respectively. During storage the pH was stable and well maintained, within the values indicated by European and local requirements, above 6.4 through the end of shelf life. During storage we saw a little pH increase at day 5, but with no impact in platelets viability. Swirling with a minimum of 2 + is seen in all products during 7 days of storage. The platelet O2 consumption and the CO2 production are well maintained by the PL2410 platelet storage container during 7 days of storage. Platelet glucose consumption is in line with the production of lactate during the 7 days of storage. The average plasma ratio is 40%.

Conclusion
INTERCEPT treatment process for double dose platelets from seven Buffy‐coats is able to generate platelet products that meet the acceptance criteria for manufacturing (Spanish and EU guidelines) and for support of patients requiring platelet transfusions according to clinical practice guidelines.
P‐323
Effect of Increased Agitation Speed on the In Vitro Quality of UV‐C‐Treated Platelet Concentrates
van der Meer PF , Laleijne-Liefting LAE , Lagerberg JH , de Korte D
Sanquin Blood Bank, Amsterdam, The Netherlands
Background/Aim
Pathogen reduction of platelet concentrates can take place using the Theraflex UV‐Platelets procedure which is based on short‐length UV‐C light. However, penetration of the rays through plasma is not very high and therefore, the platelets need to be resuspended in a platelet additive solution (with ±35 ± 5% residual plasma) rather than 100% plasma. Further, the units need to be agitated vigorously during treatment to ensure that thin layers are formed where the UV‐C‐light can fully penetrate. The standard process uses an agitation speed of 109 rpm. Recently, studies showed that pathogen inactivation was improved by increasing the agitation speed to 180 rpm. We aimed to investigate the effect of this increased agitation speed on in vitro platelet quality.
Methods
Platelet concentrates from 5 pooled buffy coats in SSP+ (MacoPharma, Mouvaux, France) platelet additive solution were prepared. Three units were pooled and divided to prevent donor‐dependent differences. One unit served as untreated control (A), one unit was agitated at 109 rpm during UV‐C treatment (B), and one unit was agitated at 180 rpm during UV‐C treatment, which was the investigational procedure (C). Platelet concentrates were stored for 8 days with regular measurement of in vitro quality.
Results
The results are summarized in the Table. The platelets show a minor increase in activation (CD62P) and apoptotic parameters (annexin A5) by day 8. Lactate production is elevated because of the UV‐C‐treatment, resulting in a somewhat lower pH. There was no marked difference between agitation at 109 and 180 rpm. Under all conditions, in vitro data were well within acceptance limits.

Conclusion
Increase of the agitation speed has minor effect on the in vitro quality of UV‐C‐treated platelet concentrates. Both the treated platelet units at 109 rpm and 180 rpm have excellent in vitro quality, suggesting that a higher agitation speed should not impair the clinical performance of the pathogen‐reduced platelets.
3.5 Novel Blood Products
P‐324
Prion And Parvovirus Removal By Qyuspeed D (QSD) Filtration of Growth Media Supplemented With 10% Human Platelet Lysate Does Not Alter Ex Vivo Cell Expansion Capacity
Kao C1, Bailey A 2, Samminger B 2, Tanimoto J 3, Burnouf T 1
1Taipei Medical University, Taipei, Taiwan2Virusure, Vienna, Austria3Asahi‐Kasei Medical, Tokyo, Japan
Background
Human platelet lysates (HPL) can replace fetal bovine serum (FBS) for clinical‐grade ex vivo expansion of cells, including mesenchymal stem cells (MSC). HPL contains platelet‐derived growth factors that support cell growth and proliferation. HPL avoids the immunological and infectious risks associated with xenogenic FBS materials. In spite of the safety nets in place for donors screening and donation testing, infectious agents, including viruses and prions, can contaminate HPL. Therefore, HPL manufacturing processes should preferably undergo virus and prion reduction steps to ensure optimal pathogen safety. Recently, a new anion‐exchange hollow‐fiber chromatographic filtration membrane (QyuSpeed D, QSD) was shown to remove prions from growth media containing 10% FBS [1]. We hypothesized that such QSD device could be used for removing pathogens from HPL.
Aims
Determine whether growth media supplemented with HPL could be filtered through QSD filtration membrane under conditions (a) not affecting ex vivo cell expansion capacity and (b) removing blood‐borne pathogens.
Methods
HPL batches were prepared from platelet concentrates subjected to 3 freeze/thaw cycles, ‘serum‐converted’ by calcium chloride, and centrifuged. Growth media were supplemented with 10% HPL and ca. 50 ml filtered through 0.6 ml QSD hollow fiber. HPL‐supplemented growth media composition was determined by total protein assay, growth factors ELISA assay, SDS‐PAGE and chemical analysis. Capacity of HPL‐growth media, prior to or after QSD filtration, to support cell growth was compared using Chinese Hamster Ovary cells, gingival fibroblasts and periodontal ligament primary cells, and Wharton jelly cord blood (WJ) MSC. Cell expansion was evaluated by microscopy, cell counting, and MTT viability assays. Membrane markers were studied by flow cytometry. Doubling time and differentiation capacity of WJMSCs into adipogenic, osteogenic and chondrogenic lineages were determined. QSD removal capacity for prions and viruses was tested (duplicate runs each) with 97.4 ± 1.9 ml of growth medium containing 10% HPL after spiking with strain 263K hamster adapted scrapie (microsomal/cytosolic fraction) or Porcine Parvovirus (PPV). A Western blot assay was used for detecting PrPSc as a surrogate marker for infectivity. PPV infectivity was assessed by TCID50 infectivity assay on PK13 cells.
Results
QSD filtration did not induce detectable adverse effects on the chemical, protein and growth factor composition of culture media supplemented with 10% HPL, nor on the growth and viability of the four cells evaluated. Mean reduction factors over the first 20.5 ± 0.1 ml of QSD‐treated media were ≥ 5.42 ± 0.44 for PPV and ≥ 3.47 for 263K, and for the whole flow‐through volume 2.24 ± 0.53 for PPV and 3.22 for 263K.
Summary/Conclusions
HPL‐supplemented growth media can be filtered through QSD anion‐exchange hollow fiber membrane to remove prions and PPV under conditions that do not affect the capacity of HPL‐growth media to stimulate ex vivo expansion of various cells, including MSC.
Reference
[1] Chou ML, Bailey A, Avory T, et al.: Removal of transmissible spongiform encephalopathy prion from large volumes of cell culture media supplemented with fetal bovine serum by using hollow fiber anion‐exchange membrane chromatography. PLOS ONE in press.
P‐325
Stability and Inter‐Donor Variability of Serum Growth Factors Stored in Different Serum Eye Drop Packaging Systems
Marks DC , Webb RG , Tan JCG
Australian Red Cross Blood Service, Sydney, Australia
Background
Dry eye syndrome is a common disorder of the ocular surface characterised by decreased tear production, damage and inflammation resulting in severe discomfort and reduced vision. Serum eye drops (SED) have beneficial effects in patients suffering from dry eye syndrome. Although the stability of serum growth factors has been examined in a laboratory setting, consideration has not been given to the packaging system into which SED are dispensed. Furthermore, the minimum concentration of serum growth factors for SED to successfully relieve symptoms in patients is not known.
Aims
The aims of this study were to investigate the stability of serum growth factors in 20% serum stored in two different SED packaging systems at different temperatures, and to determine the range of these serum growth factor concentrations in healthy donors.
Methods
Whole blood was collected from healthy blood donors (n = 12) and left to clot at 2–6°C for 24 h. After centrifugation, serum was isolated and diluted to 20% serum with 0.9% sodium chloride, before dispensing into either tubing segments (Macopharma) or a vial (Meise) packaging system. In order to determine the stability of serum growth factors, tubing segments and vials were stored at a range of temperatures (‘�30°C for up to 6 months; 4°C for up to 48 h; 22°C for 9 h and 37°C for 6 h), with a set of tubing segments and vials kept for baseline testing without further storage. Epidermal growth factor (EGF), platelet‐derived growth factor (PDGF)‐AA, PDGF‐BB, transforming growth factor (TGF)‐β1, TGF‐β2 and fibronectin concentrations were measured using ELISAs, and the total protein concentration was also measured. Growth factor concentrations at each time point were compared to baseline using a one‐way ANOVA with post-hoc tests. Inter‐donor variation was also assessed by measuring serum growth factor concentrations from healthy male and female donors (n = 25).
Results
Fibronectin, TGF‐β1, TGF‐β2, PDGF‐AA and PDGF‐BB were stable in both tube segments and vials at all time‐points and storage temperatures when compared to baseline (P > 0.05 for all data points). While the EGF concentration of serum stored in vials was stable at −30°C for up to 6 months, 48 h at 4°C and 9 h at 22°C, it was only stable for 1 h at 37°C. Similar results were obtained for EGF concentrations in serum stored in tubing segments. There was considerable inter‐donor variation in the concentration of all serum growth factors, with up to 7.5‐fold variation in EGF concentration, 2.6‐fold variation in PDGF‐AA, 3.6‐fold variation in PDGF‐BB and 3.0‐fold variation in fibronectin concentration between donors. However, there was less inter‐donor variability in TGF‐β1 and TGF‐β2 concentrations (1.8‐ and 1.9‐fold respectively).
Conclusions
This investigation provides a better understanding of the stability of serum growth factors when stored in tubing segments and vials at different temperatures. Furthermore, it was determined that there is considerable individual variability in the concentration of specific growth factors. However, it is not yet known whether these differences influence the efficacy of SED.
P‐326
Lyophilized Platelet Rich Plasma, Possible Novel Blood Product In Regenerative Medicine Use
Mazzucco L
SS Antonio e Biagio Hospital, Alessandria, Italy
Background
Platelet Rich Plasma (PRP) is a blood component for not transfusional use, very promising in the field of regenerative medicine. It is recently employed for cells expansion in culture for clinical use, because it contains many Growth Factors (GF) and biomolecules families. Its preparation is not perfectly standardized but normally it has a 1 × 109 ml platelets concentration. An easy use of PRP is critical as regards its conservation and storage, since, the same way as other blood components, it must be stored at +22°C,+4°C, −40°C or at the time. The lyophilization process, normally used for the preparation of blood products but not for blood components, is an opportunity to overcome the problems of storage and variability of preparation, since it is possible to obtain in the future a standard sample with a defined characteristic. The lyophilized product must be controlled after the rehydration, to verify its main activity, that is to promote the cells proliferation (wound healing).
Aims
In this preliminary study we explore the inductions of cells proliferation in culture in the two PRP preparations (frozen and lyophilized), to compare the efficacy result and verify the same efficiency of both, checking the invariability of the product after lyophilization. In addition we dosed the GFs concentration.
Methods
PRP is prepared frompool of 10 consenting donors and divided into two parts: 50 ml frozen and e 50 ml lyophilized. In vitro experiments: cell culture of human fibroblast supplemented with and without Fetal Bovine Serum (FBS). As it was already demonstrated about the proliferative effect dose dependence, we used culture medium with different percentages of each product: 5%, 10%, 20%, 30% and 50% with cell response evaluated on 3rd day (g3) and on 6th day (g6) with MTS test. The GF (PDGF bb, TGFb, VEGF, IGF1) quantitatively assayed using Bio‐Plex test (Biorad) Results
The proliferative effect is compared to standard condition control (cells grown in absence of both PRP), the stimulus obtained of frozen or lyophilized PRP was on g3 1.6 times with FBS and 2.5 times without FBS; on g6 1.2 times with FBS and 3.4 times without FBS. The results with lyophilized PRP do not show ‘relevant’ differences compared to the frozen ones (difference ± 0.1 time). The higher growth is for fibroblasts on g6 and in absence of FBS. The dosage of PDGF bb, TGFb, VEGF, IGF1 reported extremely similar values in both preparations.
Conclusions
The results show that the PRP lyophilisation does not interfere with the induction activity to proliferation and it does not modify the main GF‐containing composition, as it is comparable to the standard one. Based on the data obtained in vitro we can suppose that the lyophilisation process does not influence effectiveness and activity of PRP. No microbial contamination due to method of preparation was detected. Therefore the lyophilization can be further evaluated to get real and significant advantages in terms of conservation and storage of the final product.
P‐327
The First Human Immortalised Cell Line Generated from Adult Erythroid Cells
Trakarnsanga K1, Wilson MC 1, Griffiths RE 2, Kurita R 3, Nakamura Y 3, Anstee DJ 2, Frayne J 1
1University of Bristol, Bristol, United Kingdom2Bristol Institute for Transfusion Sciences, NHSBT, Filton, Bristol, United Kingdom3Cell Engineering Division, RIKEN BioResource Center, Tsukuba, Ibaraki, Japan
An alternative supply to donor blood is desirable to meet an increasing worldwide demand for transfusion. Furthermore, supplies of certain rare blood groups are not readily or routinely available in the donor population. The production of red blood cells using in vitro systems is a promising approach to help meet these therapeutic needs. Such cells can be differentiated from adult, cord blood and pluripotent stem cells, but current methods do not yet produce the quantity of red cells required for therapeutic use. Furthermore, those generated from pluripotent stem cells have differentiation defects. The generation of immortalised adult erythroid cell lines could overcome these barriers by providing an unlimited supply of cultured red cells from individuals with desired blood group phenotypes. To date only a small number of immortalised human erythroid cell lines have been reported. These cell lines were generated from iPSC (HiDEP), CB (HuDEP and IE), or ESC derived erythroid cells. All express embryonic or fetal globin and have maturation defects.
We have created the first immortalised adult erythroid cell line, BEL‐A1 (Bristol Erythroid cell Line from Adult progenitors) from adult bone marrow CD34+ cells. The line was generated using the method of Kurita et al. In brief, CD34 + cells were transduced with Tet‐inducible HPV‐E6/E7 on day 1 in culture with doxycycline included from day 5. The cells have, to date been proliferating continuously for over 300 days, with a mean doubling time after day 100 of 43.2 h. The morphology of BEL‐A1 is that of proerythroblasts and early basophilic erythroblasts. There has been no change in morphology over the time period.
For further erythroid differentiation and maturation BEL‐A1 cells post day 90 were transferred to our established 3‐stage erythroid culture system, in which they differentiated efficiently along the erythroid pathway, becoming mature erythroblasts (orthochromatic) with 10–12% reticulocytes detected. Cells transferred to differentiation media every 30–45 days thereafter showed no difference in ability to differentiate. Flow cytometric analysis with antibodies to a panel of established red cell antigens showed the proliferating BEL‐A1 cells expressed erythroid specific markers GPA, GPC, Kell, CD147, CD71, CD44, Rh, RhAG and α4 and β1 integrins. Band 3 was detected in the basophilic population. Following transfer to differentiation medium the levels of GPA, GPC, CD147, Rh and RhAG were maintained. The level of Band 3 increased, whilst the level of Kell, CD44, CD71, α4 and β1 integrin decreased. These profiles are the same as for normal adult erythroid cells.
Before differentiation, centrifuged BEL‐A1 cells gave a pink cell pellet, but after differentiation a red pellet was observed indicative of increased haemoglobin synthesis. Western blot analysis of differentiated cells confirmed they produce predominantly β‐ (adult) globin, at levels similar to normal adult erythroid cells; fetal and embryonic globins were not detected.
BEL‐A1 is a valuable tool for the study of human erythropoiesis and can be easily manipulated by knock in and knock out gene studies. Further studies are underway to optimise our experimental procedures to obtain cell lines with more efficient enucleation rates.
P‐328
Lysates Produced from Irradiated and Expired Buffy Coat‐Derived Platelets Stored in Additive Solution Support Cell Proliferation
Loh YS , Kwok M , Ekman S , Marks DC
Australian Red Cross Blood Service, Sydney, Australia
Background and Aims
Platelets are storage pools of various growth factors, which when lysed are released into the supernatant, forming platelet lysate. Platelet lysate is a suitable substitute for foetal bovine serum (FBS) as a media supplement for the propagation of mesenchymal stem cells; however there is currently no standardised method for producing platelet lysate. Platelets manufactured by Australian standards must be irradiated and have a shelf‐life of 5 days. Therefore the aim of this study was to determine the effects of irradiation and storage of platelets on the quality and capacity of platelet lysate to support cell proliferation.
Methods
Two ABO/RhD matched buffy‐coat derived platelets were pooled and split to produce matching pairs (n = 16). From these, lysates were produced by repeated freeze‐thawing of platelets that were either untreated or irradiated on day‐2 post collection (n = 8), or irradiated and stored for either 2 or 6 days at 22°C with agitation (n = 8). Platelet derived endothelial cell growth factor (PD‐ECGF), transforming growth factor (TGF‐β1), platelet derived growth factor (PDGF)‐AB, PDGF‐BB, insulin growth factor (IGF)‐1, hepatocyte growth factor (HGF), endothelial growth factor (EGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet factor 4 (PF4), and soluble P‐selectin (sCD62P) were measured using enzyme‐linked immunosorbent assays (ELISAs). The ability of media supplemented with platelet lysate to support the proliferation of human dermal fibroblasts was assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium assay. All data indicates mean and SD value. The concentrations of growth factors were compared using two‐tailed paired t‐tests, cell proliferation with platelet lysate was compared to commercial medium using a one‐way ANOVA, and the influence of platelet storage and irradiation was analysed using a two‐way repeated measures ANOVA, with P < 0.05 considered significant in all cases.
Results
Lysis of platelets (1246 ± 138 × 106 platelets/μl) resulted in the release of many growth factors. There was a 33‐fold increase (range: 15–50‐fold) in the concentration of PD‐ECGF following lysis, whereas the concentration of bFGF, IGF, and HGF did not increase. All other growth factors were increased by 3‐ to 8‐fold following lysis. Irradiation of platelets did not result in significant changes in the concentrations of any of the growth factors. However, storage of platelets significantly increased the concentration of IGF‐1 (day‐2, 19,946 ± 1658 pg/ml; day‐6, 23,366 ± 1659 pg/ml; P = 0.0078) in platelet lysates, whereas the concentrations of PDGF‐BB (day‐2, 11,445 ± 1649 pg/ml; day‐6, 9794 ± 1026 pg/ml) and sCD62P (day‐2, 147 ± 15 pg/ml; day‐6, 120 ± 8 pg/ml) decreased significantly following storage (both P = 0.0078). Fibroblast proliferation increased in proportion with the amount of platelet lysate (0, 5, or 10%) added to the cell culture medium. Neither irradiation (P = 0.5205) nor storage (P = 0.9438) of platelets affected the ability of platelet lysate to support fibroblast growth. Fibroblast proliferation in medium containing 5% platelet lysate or in commercial medium (2% FBS, IGF‐1 and bFGF) was equivalent (P = 0.5414).
Conclusion
Platelet lysates produced from untreated or irradiated, and from either non‐expired or expired platelets contain sufficient growth factors to stimulate cell proliferation. The suitability of platelet lysates as a supplement for the propagation of MSCs should be further examined.
P‐329
Dynamic Light Scattering as a Tool for the Analysis of Platelet Quality Following Cryopreservation
Johnson LNJ , Raynel SMR , Tan ST , Marks DCM
Australian Red Cross Blood Service, Sydney, Australia
Background
Platelet cryopreservation alters many aspects of platelet quality as assessed by standard in vitro quality assays. However, it is unclear whether these in vitro assays are useful for predicting the efficacy of platelets when transfused. The ThromboLUX platelet function analyser uses dynamic light scattering (DLS) to assess the number and size of particles present in a platelet component (platelets and microparticles), as well as the response of platelets to temperature stress. This information is used to calculate a DLS score, which has been correlated to transfusion outcomes, with a DLS score less than 12 indicative of poor transfusion outcomes.
Aim
To determine the utility of the ThromboLUX system to assess the in vitro quality of cryopreserved platelets.
Methods
Apheresis platelets were cryopreserved with DMSO (5% final concentration) and stored at −80°C. Upon thawing, platelets were reconstituted in thawed plasma. Samples were taken from the same units before and after cryopreservation (n = 42). ThromboLUX testing was carried out according to the manufacturer's instructions and software settings (LightIntegra Technology Inc.; ThromboSight v1.18). Platelet number was determined with a haematology analyser (CellDyn Emerald) and platelet microparticles were assessed by flow cytometry (FACS Canto II).
Results
Cryopreserved platelets had a significantly lower DLS score than prior to freezing, with 12 cryopreserved units (28%) having a score below 12. ThromboLUX measurements demonstrated a significant increase in the proportion of particles classified as microparticles (50–550 nm) and a significant decrease in the proportion of platelets (550–2200 nm) following cryopreservation. However, the number of microparticles, as calculated by the ThromboLUX was significantly higher than that measured by flow cytometry (P < 0.0001), identifying the limitations of standard flow cytometry for the assessment of microparticles. Cryopreservation did not affect the mean size of particles classed as microparticles, but did result in significantly smaller platelets, compared to pre‐freeze. Exposure to temperature‐induced stress resulted in a greater change in particle distribution in the cryopreserved platelets, compared to pre‐freeze.

Conclusion
The ThromboLUX system can provide novel information that is complementary to standard invitro tests. Further, a DLS score greater than 12 has been correlated with acceptable transfusion outcomes using fresh components. However, the clear distinction between fresh and cryopreserved platelets, in terms of their temperatureresponses and number of microparticles, suggests that the ability of the DLS score for estimating clinical outcomes using cryopreserved platelets needs to be established.
P‐330
Adjuvant Clinical Effect of the Polydeoxyribonucleotide and Autologous Platelet Rich‐Plasma in the Repair of Breast Wound Dehiscence: A Case ‐Report
Di Domenico G1, Leonardi GM 1, Guerra Narducci V 2, Nocera C 1
1Immunohematology Service, ‘S.Giovanni Bosco’ Hospital, ASL NA1 Centro, Naples, Italy2Department of Emergency Surgery, ‘S.Giovanni Bosco’ Hospital, ASL NA1 Centro, Naples, Italy
Background
Breast augmentation is the most commonly performed aesthetic surgical procedure. Postoperative wound dehiscence are among the possible complications in breast augmentation. Polydeoxyribonucleotide (PDRN) is a deoxyribonucleotide linear polymer, which is a combination of purine and phosphodiester bonds forming the monometric unit of pyrimidine nucleotides; it is known to selectively act on the A2 purinergic receptor to help cell growth and neo‐angiogenesis. PDRN, is able to reach, in vivo, the inflammation sites, and interacts with platelets and fibronectin defining the formation of molecular complexes capable of activating the process of proliferation and tissue regeneration.
Aims
The aim of our study was to evaluate the clinical efficacy of the combined treatment by autologous platelet rich‐plasma (PRP) and PDRN in the repair of an inveterate breast wound dehiscence after aestetic plastic surgery of the breast.
Methods
We report a case of a 22‐year‐old woman who presented in May 2014 for an inveterate wound dehiscence post‐aestetic plastic surgery of the left breast, non‐responder to different types of treatments (hyaluronic acid medications and hyperbaric chamber −8 months of treatment). Dehiscence appeared in sub‐mammary position, size 4 × 10 cm in diameter, abundantly exudative and net margins. A positivity for Pseudomonas aeruginosa infection was detected after microbiological test of the wound. The patient was then subjected to debridement and antibiotics systemically and topically, to obtain the complete sterilization of the wound. Subsequently, the patient was subjected to a session of autologous platelet apheresis by cell separator MCS Plus, (Haemonethics); platelet bag was subsequently aliquoted in 8 aliquots of about 40 ml which were then frozen and stored at −30°C, while the supernatant plasma were obtained aliquots of autologous thrombin. At the same time, an intramuscular injection (1 ampule, 3 ml, 5.625 mg) of PDRN (Placentex® Integro 10%; Mastelli Srl, Sanremo, Italy) was administered to the patient at a dose of 1 fl/day for 30 days, stopped for 7 days and then followed by a second therapeutic cycle of 30 days. Dressings with autologous PRP were weekly performed by occlusive dressing with gauze of hyaluronic acid (HIALOFILL‐F, FIDIA, Italy). The total duration of the therapy has been of two months in ambulatory.
Results
The association of PDRN and autologous PRP induced since the first month of treatment a reduction of the exudate and of the diameter of the wound (55%), with rapid onset of granulation tissue. After two months of treatment it was observed a complete wound healing of the breast dehiscence, followed by a complete re‐epithelialization of the tissue.
Conclusions
In our study we report, for the first time in the literature, the efficacy of combined treatment with PDRN associated with autologous PRP in the wound healing of an inveterate surgical dehiscence of the breast. Our data lead to the hypothesis that the PDRN optimizing angiogenesis in regions injured skin, can enhance the therapeutic effects of the PRP and positively modify the wound healing process, improving the clinical outcomes and lower the need for additional therapies or hospital stay.
P‐331
Autophagic Vesicles on the Surface of Circulating Reticulocytes Explain the Elevated Levels of Phosphatidylserine Positive Red Cells in Sickle Cell Disease
Mankelow TJ1, Griffiths RE 1, Trompeter S 2, Flatt JF 1, Cogan NM 1, Massey EJ 1, Anstee DJ 1
1Bristol Institute for Transfusion Sciences, Bristol, United Kingdom2Joint Red Cell Unit, University College London NHS Foundation Trust, Haematology, London, United Kingdom
Background
Surface phosphatidylserine (PS) exposure is a well characterized signal for initiating phagocytosis of unwanted cells. In peripheral blood from patients with Sickle Cell Disease (SCD) the number of circulating red cells exposing PS on their surface is elevated. During maturation to an erythrocyte, a reticulocyte must eliminate any residual organelles and reduce its surface area and volume. We have previously described a form of exocytosis occurring in reticulocytes whereby large, intact, inside‐out PS‐exposed autophagic vesicles are extruded, during the maturation of reticulocytes to mature erythrocytes (1). We hypothesised that in vivo these vesicles are normally removed by passage through the spleen.
Aims
The aim of this study was to identify whether the elevated number of PS positive red cells in SCD is a result of incomplete reticulocyte maturation due to asplenia.
Methods
Confocal microscopy and flow sorting were used to analyse red cells from the peripheral blood from patients with Sickle Cell Disease (SCD) or splenectomised, but otherwise healthy patients.
Results
We show that in these patients the number of PS positve red cells is increased and PS is present on the cell surface of red cells in large (~1.4 μm) discrete areas which correspond to the presence of an inside‐out vesicle. We show that the autophagic vesicles found on cultured reticulocytes are identical to those observed on red cells from splenectomised individuals and SCD patients. Therefore, we identify the likely cause of PS‐exposure on red cells in patients with SCD is inside‐out autophagic vesicles.
Summary/Conclusions
Our data suggest the increased thrombotic risk associated with splenectomy and patients with haemoglobinopathies is a consequence of increased levels of circulating mature reticulocytes expressing inside out PS exposed autophagic vesicles because of asplenia. Since red cells produced in culture are mature reticulocytes and may express PS‐exposed autophagic vesicles at their surface these data point to the desirability of removing autophagic vesicles prior to transfusion of cultured red cells to asplenic patients.
Note. Mankelow and Griffiths are equally contributing authors.
1. Abstract at the 57th ASH Annual Meeting & Exposition, New Orleans. Blood (2013), Vol 122, No 21, 941a).
P‐332
Composition of Autologous and Allogeneic Serum Eye Drops
van der Meer PF1, Hoenderdaal R 1, Eggink CA 2, Swart J 3, de Korte D 1
1Sanquin Blood Bank, Amsterdam, The Netherlands2RadboudUMC, Nijmegen, The Netherlands3Oogheelkunde Rijswijk, Rijswijk, The Netherlands
Background/Aim
Autologous serum eye drops (SEDs) are used to treat extreme dry eye syndrome and to heal corneal ulcers. It is, however, not known which serum factor(s) is responsible for alleviation of these conditions.
To prepare SEDs in the hospital, multiple tubes are drawn from the patient, that are aseptically processed to serum and aliquoted in dropper bottles. For various reasons, patients are not always eligible to donate for autologous SEDs (age, poor venous access, low hemoglobin, underlying disease, et cetera), and therefore allogeneic SEDs from blood donors could serve as alternative. It was our aim to compare concentrations of various growth factors in autologous and allogeneic sera.
Methods
Ten patient sera were collected after informed consent, as well as 19 (heat inactivated) donor sera that were collected by us for other purposes. The following growth factors were analyzed: Vascular Endothelial Growth Factor (VEGF‐A), Hepatocyte Growth Factor (HGF), hyaluronic acid, Platelet‐Derived Growth Factor (PDGF‐BB), Epidermal Growth Factor (EGF), fibronectin and Vitamin A.
Results
The concentrations are shown in the table below. The biological variation is large, but the concentrations are in the same order of magnitude. HGF could not be demonstrated in the allogeneic sera due to the heat inactivation.

Conclusion
Autologous and allogeneic sera have equivalent concentrations of growth factors, and likely, allogeneic SEDs are a suitable alternative for autologous SEDs. A clinical comparison is currently planned. SEDs could then become available for patients that are currently unable to donate serum, but for whom SEDs are indicated.
4.1 Screening Strategies for TTI
P‐333
Hepatitis E Virus in Blood Donors in France: Incidence and Risk of Blood Contamination
Pillonel J1, Gallian P 2, Sommen C 1, Couturier E 1, Piquet Y 3, Djoudi R 4, Laperche S 5
1Institut de Veille Sanitaire, Saint‐Maurice Cedex, France2Etablissement Français du Sang – Alpes‐Méditerranée, Marseille, France3Etablissement Français du Sang – Aquitaine‐Limousin, Bordeaux, France4Etablissement Français du Sang, Saint‐Denis, France5Institut National de la Transfusion Sanguine, Paris, France
Background
As several cases of HEV transmission by blood transfusion had been observed in France, nucleic acid amplification technique (NAT) for HEV has been introduced in 2013 in pools of 96 plasma donations used for the preparation of solvent/detergent‐treated plasma (SD‐plasma).
Aims
To assess the risk of blood donation contamination by HEV and to estimate HEV incidence among blood donors in France
Methods
The risk of blood donation contamination was derived from the prevalence of HEV‐RNA in plasmas collected in 2013. Overall prevalence was extrapolated to all blood donations by adjusting data obtained on SD‐plasmas on the following risk factors: gender, age group and region of residence. As donors are immunocompetent, the presence of HEV‐RNA indicated a recent infection. Hence, HEV incidence (I) could be estimated as follows: I = Number of HEV‐RNA(+) donations / [ (Number of HEVAb(‐) + HEV‐RNA(+) donations) * (T/365) ] where T is the duration of HEV viremia (21–35 days). The number of HEVAb(‐) could be calculated thanks to the prevalence of HEV IgG estimated at 23% among blood donors in 2013 (Wantai immunoassay).
Results
Among 57,101 plasma donations tested for HEV‐RNA in 2013, 24 were positive (crude rate of 4.2 per 10,000 donations). After extrapolation, the total number of HEV‐RNA positive blood donations was estimated at 788, accounting for a rate of 2.65 per 10,000 donations (95% confidence interval (CI): 1.6–3.7) or 1 in 3800 donations (95% CI: 1 in 2700–1 in 6200). This rate was higher in men (5.0, 95% CI: 3.0–7.0) than in women (0.4, 95% CI: 0.0–1.2), increased with age, from 0.9 (95% CI: 0.0–2.2) for the 18–29 age group to 4.5 (95% CI: 1.9–7.1) for the 50–70 age group, and varied according to the region of residence. HEV incidence was estimated to be 0.45% (95% CI, 0.2–1.0) in 2013.
Conclusions
HEV incidence in French blood donors is high but is close to those observed in the United Kingdom (0.2%), the USA (0.7%) or Germany (0.35%). The risk of blood donation contamination by HEV has been estimated to be 1 in 3800 donations. This risk could have been underestimated due to the limited sensitivity of NAT method performed in pools of 96 plasma donations (2400 IU/ml per donation). On the opposite, a possible protective effect of anti‐HEV, which may be present concomitantly with the virus in blood products, could lead to an overestimation. Finally, the risk estimated in this study is related to the donation and not to the recipient. The clinical risk of an HEV infection in recipients has to be analysed according to characteristics of transfused patients: presence of anti‐HEV immunity, existence of chronic liver disease or immunodeficiency. Despite these limitations, our work is the first step in the decision making process regarding the implementation of HEV screening of blood donations in France.
For the Blood Donor Epidemiological Surveillance Study Group of the French Institute for Public Health Surveillance
P‐334
Failure of Donor Selection: What Can the Virus Tell Us?
van de Laar TJW1, Bezemer D 2, van Sighem A 2, van Swieten P 1, Zaaijer HL 1
1Sanquin Blood Supply Foundation, Amsterdam, The Netherlands2HIV Monitoring Foundation, Amsterdam, The Netherlands
Background
Despite risk‐behaviour based donor selection 296 Dutch blood donors tested positive for HIV, HBV or HCV in 2005–2014. As HIV, HBV and HCV are characterised by high genetic variability, and different genotypes have spread via distinct transmission networks in different parts of the world, individual viral genomes can be linked to geographic origin, route of transmission and time of infection of the donor.
Aims
To link viral typing results to the self‐reported risk behaviour of new and repeat blood donors infected with HIV, HBV or HCV, and to estimate the characteristics and magnitude of undisclosed risk factors.
Methods
Participants include all HIV RNA (n = 31) or HCV RNA (n = 43) positive Dutch donors in 2005–2014, and all HBV DNA (n = 80) positive Dutch donors in 2010–2014. Viral typing was based on amplification and sequencing of the HIV pol gene (1200 bp), HBV Core, Polymerase and Surface gene (±1950 bp), and HCV NS5B gene (707 bp). For each virus, phylogenetic trees were constructed containing donor sequences plus sequences from well‐characterised national and international HIV, HBV and HCV databases. Based on self‐reported risk behaviour, robust phylogenetic clusters (bootstrap values >70) were linked to specific routes of transmission and geographic location.
Results
Viral typing succeeded for 24/24 (100%), 66/76 (87%) and 41/41 (100%) of HIV, HBV and HCV positive donors respectively, for whom serum was available. HIV subtype B (67%) predominated, followed by CRF02_AG (13%) and subtype C (13%). For HBV, the majority of infections was caused by HBV genotypes A (47%) and D (41%). The HCV genotypic distribution was more diverse: 1a (37%), 1b (22%), 3a (17%), 2b (7%), 4d (5%), others (12%). Phylogenetic clustering revealed that 91% (10/11) of male donors with HIV subtype B were part of robust MSM clusters, 100% (4/4) of female donors with HIV subtype B are in heterosexual clusters, and 75% (6/8) of donors with HIV non‐B reported sexual contact with a partner from Africa. HBV genotype was strongly associated with country of birth: genotype A (the Netherlands), genotype D (Mediterranean/Middle East) and genotypes B and C (Asia). Within HBV genotype A, recent infections could be distinguished from HBV infections that were acquired in the distant past. At present, the vast majority of sexually acquired HBV infections in the Netherlands is caused by one specific HBV A2 strain affecting both MSM and heterosexuals. For HCV, the association between risk behaviour and HCV subtype is less evident, except for HCV subtypes acquired in non‐western countries.
Summary/Conclusions
Viral typing is a useful tool to estimate the presence and character of risk‐factors among donors with transfusion transmissible infections, enabling an estimation of risk factors for donors who avoid post‐test counselling or do not disclose risk behaviour.
P‐335
Abstract Withdrawn.
P‐336
Comparison of The Prevalence of Infectious Agents in English Blood and Deceased Tissue Donors
Kitchen AD
NHSBT, London, United Kingdom
Background
Although in the UK the same donor selection criteria are applied to all blood and tissue donors, in the case of deceased donors the accuracy of the donor risk information supplied is necessarily compromised. In addition not all deceased tissue donors would have been eligible to donate blood. Such differences could lead to differences in prevalence of infectious agents in the different donor groups and consequently donation residual risk.
Within the English Blood Service (NHS Blood and Transplant), the National Transfusion Microbiology Reference Laboratory (NTMRL) not only provides confirmation of screen reactive blood donations, it also performs the mandatory microbiological screening of deceased tissue donors, both serological and molecular targets.
Aims
A simple review of prevalence of the routinely screened for infectious agents in blood and deceased tissue donors is presented to determine if there are any potential differences in residual risk in the donor types. Such data may help inform selection and screening strategies.
Methods
Overall numbers of blood and deceased tissue donors screened together with numbers of confirmed infected donors, from beginning January 2009 to end December 2014, were retrieved from the annual blood donor epidemiology reports and from the laboratory database. Overall prevalence of infectious agents were compared between first time blood donors and deceased tissue donors, analysis by Chi‐squared test (Stats Direct, v2.8)
Results
During this period 11,972,192 blood donations, 1,026,418 from first time donors, and 19,349 deceased tissue donors were screened for the mandatory infectious agents. Confirmed infections are as detailed in the table below:
Chi‐squared analysis generated a c2 value of 320, representing a significant difference in prevalence of infectious agents in first time blood and deceased tissue donors.
Table 1

Summary/Conclusions
The number of confirmed infections in the deceased tissue donor group is significantly higher than in the blood donors. Although not all deceased tissue donors are also organ donors, a proportion are, and this finding should be considered to also reflect the situation in that particular group.
This finding is not unexpected given the inherent differences in the donor groups, even though the same selection criteria are applied. The continuous drive to increase organ and tissue donation requires the provision of effective screening strategies, specifically designed to ensure the safety of such donations, as such anti‐HBc is an additional test applied to all non‐blood donations.
These data are a reminder that there is reliance on effective laboratory screening, especially so in situations where the donor selection process is understandably compromised.
P‐337
Thirteen Years of Nat Screening in Blood Donors: New Insights on HIV, HCV and HBV Incident Cases, the French Experience
Laperche S1, Pillonel J 2, Roche-Longin C 3, Djoudi R 4
1Institut National de la Transfusion Sanguine, Paris, France2Institut de Veille Sanitaire, Saint Maurice, France3Centre de Transfusion des Armées, Clamart, France4Etablissement Français du Sang, Saint Denis, France
Background
Nucleic acid testing (NAT) has been introduced in 2001 in France (single testing since 2010), as in many other high‐income countries to improve blood safety by reducing the number of infectious seronegative donations entering in the blood supply. The study of epidemiological and molecular characteristics of NAT yield cases provides new insights of recent infections occurring in blood donor population and contributes to make decisions regarding donor selection.
Aims
To (i) investigate risk factors of NAT yield cases, (ii) characterize viruses circulating in these recently infected blood donors (BD) and (iii) calculate HIV and HCV incidence using the NAT method.
Methods
Surveillance of BD in France is based on questionnaires collating demographic and epidemiological data of the whole BD population and of each donor found positive for infectious markers. In addition, each positive donation is molecularly investigated at the National Reference center for HIV, HBV and HCV in transfusion. With the NAT method, incidence = [(RNA+/Ab‐ cases)/(donations*(T/365))], T =number of days between NAT and Ab detection (12 days for HIV‐1 and 59 days for HCV).
Results
From 2001 to 2013, 34.2 million donations were tested for HIV‐1 and HCV RNA and 10.7 million for HBV‐DNA (started in 2010). The number of NAT yield cases was 20, 14 and 9 (HBc negative) for HIV‐1, HCV and HBV respectively. Of the 20 HIV‐1, 16 were repeat BD (RBD) (mean delay interdonation 135 days excluding one BD with 1956 days), 18 were male (9 MSM) and 2 females (with partner from endemic area); the mean viral load was 5.5 (1.6–6.4) log cps/ml, 14 were gtB, 3 gtC, 3 gtCRF02. Among the 14 HCV, 10 were repeat BD (mean delay interdonation 379 days), 8 were male and 6 females, 36% had partner HCV positive; the mean viral load was 7 (1.39–7.7) log cps/ml, 8 were gt1a, 1 gt1b, 2 gt3a and 1gt4a.The 9 HBV cases included 5 RBD (mean delay interdonation 149 days), 5 males and 4 females (mean age 27 years), the 5 investigated declared a sexual risk; the mean viral load was low (from <6 to 456 UI/ml), 2 were gtA, 1gt D, 4 gtE, 2nt. 3 had anti‐HBs (37–414 mUI/ml).
During the 2001–2013 study‐period, incidence was estimated at 2.64 per 100,000 person‐years (95%CI: 0.85–7.26) in first‐time BD (FTBD) and 2.04 (95% CI, 1.21–3.39) in repeat donors for HIV and 0.47 (95% CI, 0.15–1.30) per 100,000 in FTBD and 0.23 (95% CI, 0.12–0.43) in repeat donors for HCV, showing a non‐significant difference between FTBD and repeat donors.
Conclusion
NAT effectiveness is very low in France. The proportion of RBD is higher in NAT yield cases than in seropositive BD: 80% vs 55% for HIV, 71% vs 8.8% for HCV and 55% vs 1.6% for HBV. Nevertheless, Incidence of HIV and HCV, reflecting the risk of recent infections that could escape screening laboratory tests, is not higher in first time donors.
for the BD Epidemiological Surveillance Study Group of the French Institute for Public Health Surveillance
P‐338
Which Infectious Blood Donors do We Miss in the Donor Selection Process? – Comparison of HIV or HCV Infected Blood Donors with Notified Cases from General Population
Preuβel K , Offergeld R
Robert Koch Institute, Berlin, Germany
Background
Potential risks for transfusion transmissible infections are usually identified by donor history questionnaires (DHQ) and donors with higher risks are deferred from donation. Still, a small proportion of donors is diagnosed with HIV or HCV infections.
Aims
We wanted to assess to which extend the currently used donor history questionnaires (DHQ) support the identification of infections by comparing unreported deferrable risks among infected blood donors and probable transmission routes of notified cases from the general population. Risk factors whose recording needs improvement should be identified.
Methods
We performed a retrospective case‐control study including adult notified HIV and HCV cases and confirmed positive blood donors in Germany, 2006–2013 from the National Donor Surveillance System. We used logistic regression analysis of possible transmission routes (adjusted for age group, sex and type of residence) and a multivariable logistic model to identify relevant risk factors which describe infected blood donors (compared to general infected population). We calculated population attributable fractions (PAF) that represent the proportion of donors that can be attributed to specific risk factors. Hence, we are able to estimate the possible effect of improved capture of infection risks for donor selection.
Results
HIV
Data about possible transmission routes of HIV infections were available for 78% of HIV diagnoses in the general population (17,377/22,295) and 37% of HIV infected blood donors (291/789). 97% of HIV infected blood donors reported sexual transmission risks. Among HIV infected blood donors 45% were MSM compared to 72% of persons with newly diagnosed HIV infections. In contrast 52% of blood donors reported heterosexual transmission compared to 23% of persons with newly diagnosed HIV infections. The identification of all heterosexual risk contacts might prevent acceptance of 47% HIV infected donors (PAF), according to our model with adjustment for age, sex and type of residence.
HCV
Possible transmission routes were reported for 54% of HCV diagnoses (25,105/46,836) and 33% of HCV infected blood donors (1128/3469). HCV infected blood donors were more likely to report heterosexual exposure, imprisonment, and piercing/tattoo in a multivariable analysis (adjusted for age, sex, type of residence) than notified HCV cases from the general population. Improved recording of piercing/tattoo could prevent acceptance of 16% HCV infected donors. Additionally, the identification of all relevant heterosexual exposures or imprisonment in the donor selection process could prevent 7% and 5% (PAF), respectively.
Conclusion
To reduce unreported deferrable risks, the donor selection process should be improved, especially with respect to the identification of sexual risk factors, invasive procedures like piercing/tattoo and imprisonment. This could be done by well‐designed DHQs, effective donor education with state of the art techniques and confidential environment in all steps of the donor selection process. Post donation interviews and epidemiological data from blood donors should be evaluated carefully.
P‐339
Incidence And Prevalence of Hepatitis E Virus Infections In Blood Donors From Central Germany
Kirchmaier F1, Schmidt M 1, Verheyen J 2, Seifried E 1, Hourfar K 1
1Institute of Transfusion Medicine & Immunohematology, Johann Wolfgang Goethe Univ, Frankfurt/Main, Germany2Institute of Virology, University of Duisburg‐Essen, University Hospital Essen, Essen, Germany
Background
Several transfusion transmitted hepatitis E infections have been reported, although the major infection pathway is via the fecal oral route. Studies on blood donors worldwide showed a quite high incidence of HEV‐RNA positive donations. The current study reports on the incidence and prevalence of HEV positive donations in central Germany between 2012 and 2014. Results are correlated with i.e. eating habits, place of residence etc.
Methods
A PCR assay for HEV‐RNA in combination of our automated NAT platform Zelos x100was used to screen blood donations. Additionally Microtiter‐plate based enzyme linked immunosorbent assays (ELISA) were used for Anti‐HEV testing. Testing for Anti‐HEV IgG and IgM was performed fully automated using a Siemens BEPIII‐Processor. Additionally results of a questionnaire will be presented that have been sent to RNA‐positive donors.
Results
From 2012 until today approximately 175,000 donations have been screened for HEV‐RNA. During the study period 90 HEV RNA positive donors have been identified. The majority of viremic donors showed a virus load of about 10,000 IU/ml or lower. However some donations approached a virus load of approximately 1 × 10e6 IU/ml. Additionally the incidence of HEV infections was relatively stable throughout the study period. The incidence of 1:1950 found in our blood donor population is accordance with reports from neighboring countries like the Netherlands.
Conclusions
Incidence of HEV‐infection is quite high in our blood donor population. Although several transfusion transmitted HEV‐transmissions have been reported, the introduction of HEV‐NAT for cellular blood components is still controversial. However, there is the growing need of the plasma industry to obtain plasma units that tested negative for HEV‐RNA. Therefore our laboratory has established NAT‐testing for HEV‐RNA for blood donations designated for production of solvent/detergent treated plasma.
P‐340
The Importance of Confirmatory Testing of Donations That are Reactive on Infectious Disease Screening
Zuczkowska AZ , Dr Kitchen AK
NHS Blood and Transplant, London, United Kingdom
Background
The National Transfusion Microbiology Reference Laboratory (NTMRL) is a highly specialised and expert laboratory within NHS Blood and Transplant (NHSBT). One of the core activities of NTMRL is the confirmatory testing of samples from donations which are repeat reactive on primary screening in NHSBT's routine testing laboratories. Confirmation is performed using a range of assays, both serological and molecular, in stringent and well‐defined algorithms that generate the data required to determine whether the reactivity seen is specific or non‐specific.
Aim
The aim is to demonstrate the value, supported by case studies, of the confirmatory testing of screen repeat reactivity to determine the true infection status of the donor, and thereby manage the donor appropriately. By applying stringent confirmatory protocols, donors whose reactivity is determined to be specific are referred for the appropriate intervention as soon as possible and those whose reactivity is determined to be non‐specific are identified and reinstated to active status as soon as possible. Subsequent donations from such donors may then be considered for issue on the basis of negative results in either the primary screening assay or, if still reactive in the primary screening assay, in an alternative screening assay.
Methods
Screening data have been obtained from the annual NHSBT Epidemiology reports. Confirmatory data and outcomes for 2012–2014 have been retrieved from the NTMRL database.
Results
The overall screening and confirmatory results are presented in the table below
The screening data demonstrate that 99.81% of donations screened by NHSBT for infectious diseases are screen negative. Out of 5,794,048 donations in 2012–2014, NTMRL received 11,087 (0.19%) samples for confirmatory investigations. Of these only 537 (4.84%) were confirmed to be infected and 10,238 (92.34%) were shown to have non‐specific reactivity and the donors were eligible for re‐instatement.

Conclusions
The majority of screen reactivity is non‐specific; this is to be expected in a low risk, pre‐selected population. The confirmed infected donors are deferred, usually permanently, and a significant number of donors may be reinstated (in the UK the reinstatement of confirmed non‐specifically reacting donors is permitted). Donors whose donations were previously screen reactive, but whose reactivity was confirmed as non‐specific, are bled normally. If the primary screening assay is still reactive, the donation is re‐screened on an alternative assay, selected on the basis of equal sensitivity, but with minimal cross reactivity with the primary assay. A negative result allows release of the components. This level of donor management is essential to minimise unnecessary distress to uninfected donors and ensure infected donors get medical advice. Further, the unnecessary loss of eligible donors from the donor panels can be avoided. This is all dependent upon having the appropriate confirmatory testing available to the blood service.
P‐341
Transmissible Disease Risk Factors Among Men Who Have Sex With Men – Comparison of Blood Donors vs. Community Men
O'Brien F , Osmond L , Goldman M
Canadian Blood Services, Ottawa, Canada
Background
Some men who have had sex with men (MSM) donate blood despite being ineligible. Most believe their blood is safe but it is unclear whether they have fewer transmissible disease risk factors than MSM in the general community.
Aims
To compare deferrable risk factors of male donors who have sex with men and MSM in the community.
Methods
There were two surveys. Between October, 2012 and March 2013 19,437 random male whole blood donors were invited to participate (9,669 (49.7%) participated) in an anonymous on‐line survey. The second survey sample was selected proportional to region and age of Canadians from an external company's community panel. Only those indicating MSM history participated (300 participants). In both surveys participants were asked about demographic questions, history of blood donation, MSM, sexually transmitted disease, intravenous and inhaled drug use, receiving money or drugs for sex, sexual contact with sex trade workers, intravenous drug users or someone who may have HIV. Time frames included ever or recent (6 or 12 months depending on the donor criteria). Logistic regression models compared risk factors among respondent to the two surveys with adjustment for age.
Results
Among the donors 0.8% said that they had a history of MSM, but only 0.66% had a male partner since 1977 (thus were ineligible to donate). Respondents of the community survey were older than donors (68.6% vs 19.8% >50 years old, P < 0.0001). Of the MSM donors, 14.9% said they had sex with a man in the past 6 months, 16.3% in the last year (but not the last 6 months) and 24.5% in the last 5 years (but not the last year) compared with 45.8%, 12.0% and 13.7% respectively of community MSM (P < 0.0001). Some remote risk factors were common in MSM donors and MSM from the community such as ever had sex with a sex trade worker (25.6% vs 23.1%, P = 0.6532). A few donors had recent risk factors such as sex with a sex worker in last 12 months (5.6%) and having a sexually transmitted disease in the last 6 months (1.7%) similar to community (P > 0.1). In the MSM community survey 48.5% said they had donated blood in the past.
Summary/Conclusions
MSM donors who failed to disclose their history during donor screening rarely had recent risk factors for transmissible disease similar to MSM from the community. Recent male‐to‐male sex was less common in MSM donors compared with MSM in the community survey. However, a history of blood donation at some point in the past was common among MSM in the community survey. Some may have been eligible at the time they donated. Routine general population surveys of the same community panel show about 39% of respondents have donated in the past and more frequently in people over 50. MSM in Canada may be at least equally as willing to donate as non‐MSM.
P‐342
How Do We Respond to New Knowledge Concerning HEV RNA Prevalence in German Blood Donor Base and the Risk of Transmission to Immuncompromised Recipients? HEV Nat‐Testing for Thrombapheresis and SDR Donations
Scheuch C , Driesel G , Baumann-Baretti B
Haema AG, Berlin, Germany
Background
Since 2012 the knowledge concerning HEV epidemiology has been rapidly enlarged. The IgG seroprevalence increases with age and represents 4.5 to 16.8% in the German population and about 6.8% in German blood donors. It is well established that HEV is endemic in Germany and other industrialized countries and most infections of genotype 3 are autochthonous. HEV infection has to be considered as a zoonosis because populations of boars and pigs are contaminated with the virus. Viral transmission occurs mainly through insufficiently cooked meat or direct animal contact. An alternative route of transmission is the transfusion of blood components. The prevalence of HEV‐RNA in German Blood donors was published between 0.015–0.14% (depending on NAT sensitivity).In 2000 mandatory testing of blood and plasma donations for ALAT activity as a surrogate marker for hepatitis was abrogated in Germany. HAV and HBV NAT testing was wide spread in blood donation services and HBsAg escape mutants, HEV and other hepatitis infections would be extremely rare. The Haema AG, an independent blood donor service in Germany, continued ALAT testing of all medicinal products. Products assaying ALAT value higher than 120 IU/l are discarded and donors are being temporarily disclosed.
Aims
To calculate the prevalence of HEV‐RNA within the blood donors of the Haema AG two groups of donors were examined:(A) 3600 donations with ALAT‐ activity <120 IU/l (B) 1145 donations with ALAT‐ activity >120 IU/l
Methods
For detection of HEV‐RNA in minipools of 48 donations we used 2 methods:The HEV Kit for the cobas 6800 instrument (Roche, LOD: 12.2 IU/ml) was used to test group (A). With the real‐Time RT‐PCR Kit (altona diagnostics) on the m2000rt instrument (LOD: 4.7 IU/ml) group (B) was tested. Viral loads were calculated in 24 cases.
Results
The prevalence of HEV‐RNA in the Haema donor population without conspicuous liver enzyme values (Group A) is about 0.17% (6/3600 NAT positive) and comparable to published data for German blood donors. In group B (pathologically elevated ALAT values ≥120 IU/l) the prevalence of HEV‐RNA was 10 times higher (1.66%, 19/1145 NAT positive) and a tendency of increasing viral load according to increasing ALAT was seen (table 1). The infected donors, 8 woman and 17 men were between 18 and 56 years old and did not present any clinical symptoms.

Conclusions
ALAT as a surrogate marker for hepatitis significantly reduces the release of HEV‐RNA positive blood products and Haema will maintain this testing for all blood products. The elevation of ALAT caused by HEV infection does not reliably correlate with HEV RNA Level in plasma. The HEV NAT screening seems to be the only precautionary measure to prevent transfusion‐transmitted HEV infection. Especially immunocompromised patients are endangered of developing chronic HEV infection with increased mortality.
In view of these facts, Haema has decided to initiate HEV‐NAT testing (analytical sensitivity of 100 IU/ml) for all thrombo‐ and erythrocytapheresis blood components to supply these patients with HEV free blood products.
P‐343
Nat Testing of Organ Donors: Results of Two Years Testing in Croatian Institute of Transfusion Medicine
Bingulac-Popovic J , Babic I , Maslovic M , Dogic V , Safic H , Miletic Lovric M , Jurakovic-Loncar N , Balija M , Jukic I
Croatian Institute for Transfusion Medicine, Zagreb, Croatia
Background
Croatia implemented nucleic acid testing on individual blood donation (ID‐NAT) as mandatory in March 2013. With implementation of NAT testing for blood donors, Croatia started to test blood samples in all organ, tissue and cell donors. Croatian Institute of Transfusion Medicine (CITM) in Zagreb is the only testing site for whole Croatia.
Aims
Evaluation of results and quality of two years NAT testing of organ, tissue and cell donors in Croatia.
Methods
Blood samples from organ, tissue and cell donors are sent to CITM from Croatian's hospitals and tissue banks. NAT is performed by using Procleix Ultrio Plus test on Tigris instruments (Grifols, Spain) which is a multiplex NAT for simultaneous detection of HIV‐1, HCV, and HBV. Samples from heart‐ and non‐heart beating organ donors have to be collected following a strict rules to avoid presence of inhibitors and false negative results. Maternal plasma taken after child delivery is used for indirect testing of umbilical cord bank samples. When organ donor NAT testing is positive, discriminatory testing for establishing HBV‐DNA, HCV‐RNA or HIV‐1 RNA is performed. Tissue and cell donor blood samples are retested if screening test is positive and in case of repeat reactive results discriminatory testing is performed. When requested, further molecular testing could be performed for establishing viral load with CAP/CTM tests, v 2.0 for HBV, HCV and HIV‐1 (Roche Diagnostics, USA), genotype with VERSANT HCV Genotype 2.0 Assay (Siemens, Germany), CMV‐DNA or EBV‐DNA viral load by using Artus RG PCR Kit (QIAQEN, Germany). Maximum time to get NAT screening results for urgent organ donors is eight hours, additional discriminatory testing requires another 4 h and further quantitative methods and genotyping require in total 20 h. Results of organ donor NAT testing are reported to national transplantation network which is a part of Eurotransplant network.
Results
We tested 961 blood samples in two years period: 301(31.3%) organ donors, 288 (30%) steam cells donors, 257 (26.7%) bone tissue samples, 110 (11.4%) corneal samples and 5 (0.5%) blood vessel samples. Results of NAT testing for 22/961 (2.3%) donors were positive. The results are summarized in Table 1. All samples with HBV and HCV NAT positive results also had serology positive results. Additional HCV genotyping in two organ donors with positive HCV‐RNA results determined presence of 3a genotype. Although NAT screening and anti‐HBc test gave positive results for all of three inconclusive cases, we did not have enough sample to discriminate HBV‐DNA and occult HBV infection.

Summary/Conclusions
Croatia is one of the countries with highest number of organ donors and transplantations per inhabitants in Europe. NAT testing of organ donors has great importance in organ transplantation to avoid the transmission of blood‐borne viruses to the recipient. By implementation of NAT testing of organ, tissue and steam cell donors in Croatia, the risk of HBV, HCV and HIV transmission is greatly reduced.
P‐344
Cytomegalovirus (CMV) and Transfusion Therapy: Prevalence of CMV Seropositivity in a Portuguese Blood Donor Population (2007–2014)
Plácido C1, Lichtner A 2, Barra A 2, Costa C 2, Plácido C 2, Nunes C 2, Ferreira M 2, Rebelo S 2, Santos C 2, Mota M 2
1Hospital Prof. Doutor Fernando Fonseca E.P.E, Amadora, Portugal2Hospital Prof. Doutor Fernando Fonseca E.P.E., Lisbon, Portugal
Background
Cytomegalovirus (CMV or human herpesvirus‐5) is a common infection and not clinically significant in immunocompetent individuals. It's the most important herpesvirus with reference to transfusion and, as all human herpesvirus, has the capability to lie dormant in tissues after an acute infection. The risk of CMV infectivity is still a major problem in immunocompromised patients requiring transfusion therapy, and should be minimized in CMV‐negative pregnant women, fetuses, premature infants and neonates, transplant recipients and other severely immunosuppressed patients.
The residual risk of transfusion‐transmitted CMV infection is between 2.3% and 3% for leucocyte‐reduced blood components, and the additional use of anti‐CMV screened blood components decreases this risk to less than 1%, which, in our point of view, justifies the non‐abandonment of CMV‐seronegative blood bank inventories, especially in high prevalent populations.
There is substantial variation in the donor rates of CMV seropositivity described in the literature (20–95%) and it seems to be inversely related to improved hygiene and living conditions. The same principle applies to the seroconversion rate per year.
Aims
To determine the prevalence of CMV seropositivity and CMV seroconversion rate per year in a Portuguese blood donor population over an eight‐year period of time (2007–2014), and compare it to those mentioned in other studies.
Methods
Blood samples from donors were analyzed during the period 2007–2014 (8 years), and tested for detection of anti‐CMV antibodies (‘total’ anti‐CMV ELISA‐based assays, capable of detecting both IgG and IgM class antibodies‐ Siemens Enzygnost® Anti‐CMV/IgG+IgM), as they belonged to a first‐time or to a previously tested CMV‐seronegative donor. The prevalence of CMV seropositivity and the seroconversion rate per year were retrospectively determined among this blood donors population.
Results
A total of 42.286 blood collections were analyzed. The prevalence of CMV infection was determined for each year: 2007 – 86.4%; 2008 – 87.2%; 2009 – 86.5%; 2010 – 88.9%; 2011 – 90.2%; 2012 – 89.7%; 2013 – 91.3%; 2014 – 89.4%. The CMV seroconversion rate per year was 1.43% (average age of seroconversion 36.4 years old; 81.8% men and 18.2% women).
Summary/Conclusions
All blood components transfused in Portugal are leucocyte‐reduced. We try to provide CMV seronegative blood components to all patients in high risk of CMV infection, which is particularly difficult because of the high prevalence of CMV seropositivity in our blood donors population (approximately 89%).
There were no reported cases of transfusion‐transmitted CMV infection during the time period analyzed, which confirms that our strategy is effective in preventing CMV infection, even in a high prevalent population as ours.
The existence of CMV‐seronegative blood bank inventories does not reflect practice countrywide, but is still important for some high‐risk groups of patients, and allows us to respond to the needs of our hospital and other institutions (whenever possible).
P‐345
Improvement of Blood Donation Safety Thanks to the New Amplified Chemiluminescence Biochip Technology
Chaumat P
BIO‐RAD, Marnes la Coquette, France
Background
Beyond the need for increased sensitivity to reduce the serological window, blood donation safety is also a question related to how can we secure the result delivered by the test and the instrument running the test?
We describe how we could increase blood donation safety thanks to Amplified Chemiluminescence BioChip Technology in a highly multiplexed highly sensitive serological test for the combined screening of HCV, HIV, HBV and HTLV viruses and Syphilis.
Aims
Using a new Biochip technology combining planar microarray and highly sensitive amplified chemiluminescence allowing simultaneous detection of multiple analytes, a new assay for serological screening of HIV Ag/Ab, HBs Ag, HCV Ag /Ab, HTLV I+II Ab, HBc Ab and Syphilis Ab was developed.
A major step was to implement a number of spots in the biochips to allow for unprecedented level of security and quality assurance to confirm correct completion of the test.
Methods
The highly multiplexed test is based on micro‐spotted biochip including enough positions for as many control spots as required to cover all of the critical test steps (patent application filed). In addition, a specific process allows for controlling spot integrity within the biochip at end of assay before final reading (patent application filed).
For the detection of the serological markers, deposited spots of the biochip include either well‐characterized purified antigens for antibody detection or antibodies for antigen detection.
Capture is detected via an enzyme‐based Amplified Chemiluminescence signal, captured with a highly sensitive cooled CCD sensor for long and simultaneous exposure of all of the biochips at the same time.
Results
A large evaluation including 9893 negative blood donations for specificity, performance and seroconversion panels for sensitivity has demonstrated the performance of the 6 markers and the benefit of control spots to guarantee safety of the blood donation screening result.
For instance HIV Ag, and HBc Ab detection limits have been found at 0.39 WHO UI/ml, 0.13 PEI UI/ml respectively.
Clinical sensitivity has been tested using at least 15 well‐documented HIV, HBV, and HCV seroconversion panels. The results show a significant window period reduction when compared to last generation Ag and Ab screening assays.
Preliminary specificity assessed on blood samples from European Blood Banks was found to be 99.99% for HBc and higher than 99.94% for the other markers.
Summary/Conclusion
Amplified Chemiluminescence BioChip Technology allows, in addition of improvement of sensitivity detection level, implementing much more quality controls in the test than ever met in traditional blood donation serological disease screening to guarantee the expected safety level in the result delivered.
P‐346
Performance Characteristics of a Transcription‐Mediated Amplification Assay for Parvovirus B19 And Hepatitis a Virus On a Fully Automated System
Ong E1, Walker S 1, Gao K 1, Janssen A 1, Cory R 1, Babizki M 1, Shin T 1, Hoang A 1, Lindquist A 1, Anderson G 1, Anderson O 1, Ibrahim S 1, Self D 1, Glass N 1, Pekny K 1, Gore S 1, Knight J 1, Koppelman MHGM 2, Linnen JM 1
1Hologic Inc., San Diego, United States of America2Sanquin Diagnostics, Amsterdam, The Netherlands
Background
The Procleix® Parvo/HAV assay is a nucleic acid test for the simultaneous qualitative detection of hepatitis A virus (HAV) RNA and quantitative detection of human parvovirus (B19) DNA. The assay is currently under development for the Procleix Panther system. A similar version of the assay is currently available for use on the Procleix Tigris system (in process test in US and CE‐IVD in EU).
Aims
Studies were performed to characterize the sensitivity, quantitation accuracy and precision, specificity, and reproducibility of the Procleix Parvo/HAV assay on the Panther system.
Methods
To assess analytical sensitivity, the HAV WHO International Standard (IS) (NIBSC: 00/560), HAV genotypes 1–3, and the 3rd WHO IS for Parvovirus B19 DNA (NIBSC: 12/208) were tested. Quantitation of 1st WHO International Reference Panel for Parvovirus B19 Genotypes 1–3 (NIBSC: 09/110) was also evaluated. To determine clinical specificity, 2010 individual donations from source and recovered plasma samples were screened for HAV and B19 (using a cutoff of 1000 IU/ml) using multiple kit lots. To assess clinical sensitivity, 212 B19 positive plasma specimens with viral load titers ranging from 5.93E+05 to 7.88E+12 IU/ml were tested. Assay reproducibility, assay precision, and the effect of other infectious agents and donor and donation factors (including interfering substances like albumin, hemoglobin, lipids, and bilirubin, and donors with autoimmune and other diseases) were determined.
Results
The Procleix Parvo/HAV assay showed a 95% limit of detection (LOD) and a limit of quantitation (LOQ) of 325 IU/ml for Parvovirus B19 WHO IS. The 95% LOD for HAV WHO IS was determined to be 1.06 IU/ml (95% fiducial limits: 0.90–1.30 IU/ml). The assay detected HAV genotypes 1–3 with >95% reactivity at 50 copies/ml and quantified B19 genotypes 1, 2, and 3 with equal accuracy. Clinical specificity was determined to be 100% (95% CI: 99.83–100.00%) for both viruses. Clinical sensitivity of the 212 B19 positive plasma specimens was 100% when tested in both 1:16 and in 1:512 pools. Assay comparisons showed good correlation to other Parvovirus assays, including a Parvo/HAV assay on the Tigris system and an in‐house Parvovirus PCR assay. Assay reproducibility over three days with multiple instruments, reagent lots, and operators showed the intra‐run factor contributing the largest source of variability. The presence of potentially cross‐contaminating infectious agents and other donor and donation factors had no impact on the sensitivity and specificity of the assay.
Summary/Conclusions
Preliminary results indicated that the Procleix Parvo/HAV assay was sensitive for qualitative detection of HAV (95% LOD: 1.06 IU/ml), showed good accuracy and precision for B19 genotypes 1, 2, and 3 quantitation, and showed very good specificity (100% for both HAV and B19). The results from testing B19‐positive pools showed that the assay may be effective in pool sizes ranging from 16 to 512. Furthermore, performance was not affected by other infectious agents and potentially interfering substances. In conclusion, based on the performance demonstrated in this study, the Procleix Parvo/HAV assay on the fully automated Panther system may be useful for screening of recovered and source plasma donations.
P‐347
Abstract Withdrawn.
P‐348
Screening Blood Donations by Nucleic Acid Amplification Testing: Are We Giving Benefit to the Donors?
Chaurasia R , Zaman S , Chatterjee K
All India Institute of Medical Sciences, Delhi, India
Background
Transfusion safety begins with healthy donors. A fundamental part of preventing transfusion transmitted infections (TTIs) is to notify and counsel reactive donors. Donor notification and counselling protect the health of the donor and prevent secondary transmission of infectious diseases.
Aim
We undertook this study to determine the response rate following notification of reactive status to the donors after initial screening by serology and NAT among blood donors attending our centre.
Methods
Of 113,014 donations were screened for TTIs, namely, HIV, HBV, HCV, and syphilis, by serology and NAT. All reactive donors were retested (wherever possible) and notified of their status by telephone or letter. All initial reactive screens were followed over six months.
Results
We evaluated 2838 (2.51%) cases with reactive screening test results (1.38% HBV, 0.54% HCV, 0.27% HIV, and 0.32% syphilis). Only 23.3% of donors (662) responded to notification. The response among voluntary donors was better as compared to the replacement donors (43.6% vs 21.2%). Only 373 (56.3%) responsive donors followed their first attendance at referral specialties. Over six months, only 176 of 662 (26.6%) reactive donors received treatment.
Conclusion
Our study shed light on the importance of proper donor counselling and notification of TTI status to all reactive donors who opt to receive this information. There is also an urgent need to formulate the nationally acceptable guidelines for notification and follow‐up of reactive donors so the onus of information and subsequent management shifts from donor to the transfusion facility/hospital.
P‐349
Prevalence and Risk Factors of Syphilis Infection and High‐Risk Behaviour Investigation Among Blood Donors
Zhang Z , Liu SL , Xi GX , Zhong L , Chen X , Wan LK , Li SP , He Y
Chengdu blood centre, Chengdu, China
Background
In China, the syphilis epidemic is growing rapidly within the general population and therefore poses a great threat to blood safety. Voluntary non‐remunerated blood donors are regarded as relatively safe; however, China still faces many challenges with blood safety. During pre‐donation screening the prospective donors are selected based on their Donor History Questionnaire answers. If a donor exposes oneself to high‐risk behaviours and conceals this, using the blood will be a great threat. The current study was set up because so far, few studies have evaluated these risks before.
Aims
The goal of this study was to investigate the serological epidemic trends and risk factors for syphilis among blood donors and to explore the credibility of blood donors’ answer about exposure to high‐risk behaviour in pre‐donation screening process.
Methods
The serological test results from blood donors were collected from the Chengdu Blood Centre during 2005–2013. A case‐control study was conducted on 368 only anti‐TP (TreponemaPallidumantibodies, marker for syphilis) positive blood donors and 736 negative blood donors. Conditional logistic regressions and Population Attributable Risks (PAR) were performed to examine risk factors.
Results
The serological epidemic for syphilis among blood donors in Chengdu showed an upward trend in the period 2005–2013(see table below). The study finds that risk behaviours are both in cases and controls. We can deduce that some blood donors including controls concealed their risk behaviours in pre‐donation process. Compared with anti‐TP negative blood donors, positive blood donors were more likely to have multiple sexual partners and commercial sex (50.8%vs.22.6%; 11.1%vs.4.6%). According to the multiple condition logistic regression model, within the donor populationthe results found in the following risk categories were respectively: multiple sexual partners (OR=4.733; 95%CI=2.188; 10.238; PAR=0.169), cosmetic surgery(OR=1.642; 95%CI = 1.236; 2.183, PAR=0.105), sexual contact with syphilis patients (OR=10.706; 95%CI=2.344; 48.898, PAR=0.013), unaware of sexual contacting with syphilis patients(OR=4.640; 95%CI=2.717; 7.927; PAR=0.048) increased the risk of syphilis infection. The total PAR was 33.5%.
Table 1. prevalence of syphilis among blood donors in Chengdu from 2005 to 2013

Conclusions
The prevalence of syphilis infection within the Chinese blood donor population is ascending each year. To ensure safe blood supply, there is an urgent need to strengthen Chinese pre‐donation health consultation and screening. More actions should be taken to defer donors who are exposed to high‐risk behaviours. Therefore, the blood donor management system needs to be improved in China.
P‐350
Abstract Withdrawn.
P‐351
The Implementation of Nucleic Acid Amplification Testing for Screening Blood Donors in Turkey, Preliminary Data
Saygan MB1, Beker CM 1, Demirel K 1, Aksoy A 1, Günçikan MN 1, Güllüoglu M 1, Sencan I 2, Özet G 1
1Turkish Red Crescent, Ankara, Turkey2Republic of Turkey Ministry of Health, Ankara, Turkey
Background
Turkish Red Crescent manages about 1.8–2.0 million blood donations annually. In the end of two years of preparation, Nucleic Acid Amplification Testing (NAT) systems have been installed at Turkish Red Crescent Laboratories in October, 2014.
Aims
In this study, we aimed to present data regarding the first three months.
Methods
The samples of 474,248 donations between November 1st, 2014 and February 1st, 2015 were tested in minipools of six (MP6) (Cobas s 201 platform and MPX v2.0 kit, Roche Diagnostics, Switzerland). Each sample at any reactive pool was subjected to single (individual) NAT. NAT was performed in parallel to serologic screening tests, simultaneously (HBsAg, anti-HCV, HIV 1-2 Ag+Ab and T.pallidum Total Ab, by enzyme immunoassay method on full automated microelisa systems). In order to determine the viral loads of those samples which were HBsAg nonreactive/HBV DNA reactive, quantitative PCR test (Cobas Ampliprep/Cobas TaqMan HBV Test, version 2.0, Roche Diagnostics, Switzerland) in addition to Anti‐HBc Total (Enzygnost Anti-HBc Total, Siemens, Germany) and Anti‐HBs (Liaison Anti-HBs II, DiaSorin, Italy) tests were performed on these samples. Those with a level of anti‐HBs above 10 mIU/ml have been classified as ‘reactive’.
Results
All serologic screening and NAT screening test results are summarized in Table 1. As for HCV and HIV, no serologic screening nonreactive and NAT reactive samples have been detected yet. Among 474,248 samples, 315 (0.07%) were detected as HBsAg nonreactive and HBV DNA reactive. Out of these 315 samples, on the quantitative PCR test, 130 (41.27%) have been found as with no HBV DNA present (TND; target not detected); the remaining 185 (58.73%) have been detected as ‘HBV DNA positive’. Out of these 315 samples, 146 (46.35%) have been demonstrated to have an HBV DNA level of <20 IU/ml; 29 (9.21%), a level of 20–99 IU/ml; 7 (2.22%), a level of 100–199 IU/ml, and 3 (0.95%), a level of 200–433 IU/ml. Among these 315 samples, 27 (8.57) have been found as anti‐HBc nonreactive and anti‐HBs reactive; 122 (38.73%) as Anti‐HBc reactive and Anti‐HBs nonreactive; 28 (8.89%) as both markers nonreactive; and 138 (43.81%) as both markers reactive (Table 2).
Table 1

Table 2

Conclusion
Among 472,191 HBsAg nonreactive donations, 315 samples were found as NAT reactive (HBV NAT yield, 1/1499) and 185 samples out of these 315 have been confirmed as ‘HBV DNA positive’ (HBV NAT yield, 1/2552). The remaining 130 samples have been demonstrated as ‘nonreactive’ with quantitative PCR. However, in order to determine whether these cases represent false positives in NAT screening test, follow up samples should be obtained from these donors and be tested again enabling us to find out if donors seroconvert. The preliminary data which we have demonstrate that the implementation of routine NAT testing will significantly reduce the residual risk regarding HBV infection by especially interdicting occult hepatitis B cases. To reach more precise and clear data from probable donors of infection window period, it is planned to further inquire into follow up samples in order to determine the exact stage of HBV infection of donors during the actual donation.
P‐352
Evaluation of the Specificity of CobasⓇ Mpx on the CobasⓇ 8800 System in US Blood Donations
Pate LL1, Bertuzis R 1, Huynh N 1, Hemyari P 1, Duncan J 1, Bhatti S 1, AuBuchon JP 2, Erickson Y 3, Waxman DA 4, Williamson PC 5, Yabes AY 6, Galel SA 1
1Roche Molecular Systems, Inc., Pleasanton, United States of America2Bloodworks Northwest, Seattle, United States of America3Mississippi Valley Regional Blood Center, Davenport, United States of America4Indiana Blood Center, Indianapolis, United States of America5Creative Testing Solutions, Tempe, United States of America6Annoel P. Yabes M.D., San Francisco, United States of America
Background
The cobas® MPX test is a qualitative multiplex donor screening test for detection of HIV‐1 (Groups M and O) RNA, HIV‐2 RNA, HCV RNA and HBV DNA, for use on the cobas® 6800/8800 systems. This real‐time PCR assay incorporates dual targets for HIV and dual probes for HCV. Fluorescent probes specific for HIV, HCV, HBV, and internal control enable separate detection and reporting of results for HIV, HCV, and HBV, eliminating the need for secondary viral target resolution. The cobas® 6800/8800 Systems incorporate fully automated sample preparation, amplification, and detection, ready‐to‐use reagents and controls, onboard refrigerated storage, and full traceability of reagents and consumables. The cobas p 680 instrument (p680) may be used to create minipools (MP) of donor samples.
Aims
To determine the clinical specificity of cobas® MPX in blood donor samples in the United States (US) in individual donation testing (IDT) and in MP of up to 6 donations (MP6).
Methods
Donations were tested by cobas® MPX on the cobas®8800 System, either by IDT (serum or plasma) or in plasma MP6 produced with the p680, across 4 test sites/3 reagent lots. Each donation was also tested in the same pool size (i.e., IDT or MP6) with the test of record, cobas® TaqScreen MPX Test, with COBAS® AmpliScreen Tests (CAS) for target virus discrimination (TaqScreen/CAS), using plasma samples. Reactive pools were resolved by testing each pool member. A donation was defined as reactive when reactive at the individual sample level. Discordant results were resolved with alternative nucleic acid testing (alt‐NAT) and donor follow‐up Donations were ‘status‐positive’ if positive for the same virus on both NAT screens, or on additional index or testing. Specificity was calculated as percentage of status‐negative donations nonreactive (NR) on cobas® MPX.
Results
Of 63,012 evaluable donations screened in MP6, 29 were positive on both NAT (26 HCV, 3 HBV). 2 HCV positive donors were seronegative; 1 seroconverted during f/u. Two donations were reactive only on TaqScreen MPX: 1 CAS(+)HCV, 1 CAS(−), both seronegative and negative on alt‐NAT. The CAS(+) donor was negative on f The CAS(−) donor declined status was considered unresolved.
62,982 donations screened in MP6 were status‐negative; all were NR on cobas® MPX, for a specificity of 100.0%. Of 10,534 pools containing only status‐negative donations, 10,524 were NR on cobas® MPX, for a pool specificity of 99.905% (Table 1).
Of 11,203 evaluable donations screened by IDT, there were 5 positive on both NAT screens (3 HCV, 2 HBV: all seropositive), three reactive only on TaqScreen MPX (all negative on CAS, serology, alt‐NAT and f/u), and six reactive only on cobas® MPX (5 HIV, 1 HBV: all negative on serology, alt‐NAT. The HIV donors were negative on of the HBV donor declined.
Of 11,198 status‐negative donations screened by IDT, 11,192 were NR on cobas® MPX for a specificity of 99.946% (Table 2).
Caption 1: Clinical Specificity of cobas® MPX in blood donations tested in MP6

Caption 2: Clinical Specificity of cobas® MPX in US Blood Donations – Individual Donation Testing

Summary/Conclusions
cobas® MPX demonstrated 100% clinical specificity in plasma pools of 6, 99.946% specificity in IDT, and 99.905% in overall pool specificity.
P‐353
Evaluation of the Specificity of CobasⓇ Mpx on the CobasⓇ 8800 System in US Source Plasma Donations
Pate LL1, Bertuzis R 1, Thakker M 1, Hemyari P 1, Duncan J 1, Yabes AY 2, Simon T 3, Alexander R 3, Galel SA 1
1Roche Molecular Systems, Inc., Pleasanton, United States of America2Annoel P. Yabes M.D., San Francisco, United States of America3CSL Plasma, Knoxville, United States of America
Background
The cobas®MPX test is a qualitative multiplex donor screening test for detection of HIV‐1 (Groups M and O) RNA, HIV‐2 RNA, HCV RNA and HBV DNA, for use on the cobas® 6800/8800 systems. This real‐time PCR assay incorporates fluorescent probes specific for HIV, HCV, HBV, and an internal control, with signal detection at 4 independent wavelengths. The assay separately detects and reports the results for HIV, HCV, and HBV, eliminating the need for secondary viral target resolution. Dual HIV target amplification and HCV dual probes improve detection of genetically diverse specimens. The cobas® 6800/8800 Systems incorporate fully automated sample preparation, amplification, and detection, ready‐to‐use reagents and controls, onboard refrigerated storage, and full traceability of reagents and consumables. An optional cobas p 680 pooling instrument creates minipools of up to 96 samples (MP96) for testing with the cobas® MPX.
Aims
To evaluate the clinical specificity of the cobas® MPX test on the cobas® 8800 System in source plasma (SP) donations in the United States.
Methods
Seronegative SP donations were tested in MP96 with both cobas® MPX and the nucleic acid test (NAT) of record, cobas® TaqScreen MPX Test with COBAS® AmpliScreen (CAS) Tests for virus target resolution (TaqScreen/CAS), at 1 site, using 3 reagent lots. Constituents of reactive pools underwent additional testing to resolve reactivity. A donation was defined as reactive when reactive at the individual sample level. Discordant results were resolved with alternative NAT testing of the index donation and by donor follow‐up (f.
Donations were ‘status‐positive’ if positive for the same virus on both NAT screens, or donor was positive on additional index or testing. Specificity was calculated as percentage of status‐negative donations nonreactive on cobas® MPX.
Results
Of 108,306 evaluable SP donations tested in MP96 with cobas® MPX, 12 donations were reactive on cobas® MPX at the individual sample level. Five donations (4 donors) were HCV RNA‐positive on both cobas® MPX and TaqScreen/CAS. Three of these donors enrolled in f/u and 2 seroconverted. Seven donations were reactive only with cobas® MPX: 3 HBV, 3 HIV, 1 HCV. 1 of the HBV reactive donations was confirmed by alternative NAT on the index donation. This donor made 3 subsequent donations not detected with the MP96 screen. HBV DNA was confirmed in multiple f/u samples, but the donor remained seronegative. 1 HCV and 2 HIV reactive donors were nonreactive on f 1 HIV and 2 HBV reactive donors declined to enroll in Altogether, 9 donations were status‐positive (5 from the HCV‐confirmed donors, 4 from the HBV‐confirmed donor). Of 1097 pools containing only status‐negative donations, 1090 were nonreactive on cobas® MPX, for a pool specificity of 99.362% (95% CI 98.689–99.691%). Of 108,297 status‐negative donations, 108,291 were nonreactive on cobas® MPX, for a specificity at the individual donation level of 99.994% (Table 1).
Caption 1: Clinical Specificity of cobas® MPX in US Source Plasma Donations Tested in MP96

Summary/Conclusions
cobas® MPX in MP96 demonstrated a clinical specificity of 99.994% in US source plasma donations. cobas® MPX detected one additional HBV infection in a seronegative donor.
P‐354
Implementation of the New CobasⓇ 6800/Cobas P 680 And CobasⓇ Mpx for Nat Screening of Blood Donations: A Safety and Efficiency Tool
Torres Sardina P , Ruiz Tovar M , García Carpio R , Richart A , Barea García LM
Centro de Transfusión de la Comunidad de Madrid, Madrid, Spain
Background
The need to shorten the HCV window period and the high frequency of occult hepatitis B (OBI) and HIV positive blood donations in Spain (1 case in 23,193 and in 100,000 respectively), makes nucleic acid testing (NAT) an indispensable tool to increase transfusion safety. Furthermore, during 2013–2015, our processing activity increased by 42%, reaching 1000 donations/day.
For these reasons, we implemented the new cobas® MPX test on the cobas® 6800 System, which offers increased sensitivity for HIV, HCV, and HBV, dual target amplification for HIV, and high capacity and speed of processing.
Aims
To evaluate the (1) time to implement the new system and assay, (2) time for processing samples, (3) assay performance, and (4) OBI yield in comparison to previous tests.
Material and methods
We recorded the time for installation and validation of the cobas® 6800, the cobas p 680 pooling instrument (p680), and cobas® MPX. Upon implementation, donations were tested by cobas® MPX in minipools of up to 6 samples (MP6) produced by the p680. Reactive pools were resolved by testing each pool member. A donation was defined as reactive when reactive at the individual sample level. We evaluated the time for processing donations, reactive rates of pools and donations, serology and viral load of reactive donations, and OBI yield in comparison to our previous tests, the cobas® TaqScreen MPX Test (MPX1) and cobas® TaqScreen MPX Test v2.0 (MPX2).
Results
The installation of the first cobas® 6800 and p680 was completed in 11 days. Installation of a second cobas® 6800 and p680 and creation of an LIS connection were accomplished over the next 4 weeks in parallel to validation of the new platform/assay and training. Validation testing included verification of detection of 2 archived HIV‐LTR mutation samples.
Upon routine implementation, pooling was performed during the night shift and testing in the morning (with MPX2, both were performed at night). When the number of samples/pools were ≤ 1,104/184, it took 3.5 h to obtain results and transfer them to the LIS, and 5.5 h if higher.
Between November 2014 and February 2015, a total of 81,702 donations were analyzed in 13,617 MP6. 94 pools were reactive (1/145; 0.69%). 70 pools resolved to an individual donation containing one or more viruses. There were 14 HIV+ and 11 HCV+ donations on cobas® MPX; all were seropositive. Of 45 HBV+ donations, 36 were hepatitis B surface antigen (HBsAg) positive. Of the 9 HBsAg negative, 8 were tested for anti‐HBc and all were reactive. OBI detection frequency with cobas® MPX, was 2.2 and 3 times higher than with the previous tests (Table). 24 reactive pools had no reactive individual members for a pool specificity of 13,523/13,547, or 99.82%.
Caption 1: OBI Detection According to the NAT Method Used for Donor Screening

Summary/Conclusions
The greater processing capacity of the cobas® 6800 has allowed us to increase NAT screening volume without increasing the number of lab technicians, and to move testing to the morning shift. cobas® MPX contributes to increased transfusion safety with higher sensitivity for OBI and ability to detect HIV mutations.
P‐355
Weaknesses of Blood Transfusion System in Burkina Faso: Evidences from Analysis of Two Parallel Systems
Sawadogo SS1, Kafando KE 1, Nébié NK 1, Dahourou DH 2, Ouattara OS 2, Kienou KK 2, Kientega KY 2, Traoré TF 2, Coulibaly CS 2, Deneys DV 3
1University of Ouagadougou, Ouagadougou, Burkina‐Faso2Centre national de transfusion sanguine, Ouagadougou, Burkina‐Faso3Catholic University of Louvain, Brussels, Belgium
Background
Since 1999, Burkina Faso has been implementing WHO's strategy for blood transfusion safety with the adoption of a national blood transfusion policy, the creation of blood transfusion services and the deployment of a quality system. But these actions are not yet rolled out to the whole territory. Many independent hospital blood banks (HBB) with relatively limited resources still coexist with a network of regional blood transfusion centers (RBTC) coordinated by a National Blood Transfusion Center (NBTC). So, the practices are not harmonized.
Aims
Our study aims to compare the proportions of positive tests to transfusion transmissible infections (TTIs) between blood donations collected by RBTC and HBB from 2009 to 2014.
Methods
The RBTC follow the WHO's recommendations. Blood is collected from voluntary non remunerated blood donors. Pre‐donation screening is systematic with a standardized process. Laboratory screening of HIV, hepatitis B and C and syphilis is also systematic with ELISA‐tests. But in HBB, blood is collected from family donors without pre‐donation screening. They use one step rapid‐tests to screen TTIs in blood units.
TTIs’ screening data on blood units collected in RBTC and HBB between 2009 and 2014 were recorded and analyzed. Chi square test was used to compare proportions of positive tests between the 2 samples.
Results
From 2009 to 2014, a total of 546,020 blood units were collected in Burkina Faso (63.37% by RBTC and 36.63% by HBB.
Compared to RBTC, in HBB, the mean proportion of positive tests to HIV (1.84 ± 0.14% vs 3.20 ± 0.48%) and syphilis (1.68 ± 0.44% vs 2.67 ± 0.41%) are respectively 1.73 and 1.87 times higher (P < 0.001). On the other hand, the mean proportion of positive tests to HBV (9.87 ± 0.81% vs 9.66 ± 0.34%) and HCV (5.74 ± 0.56% vs 3.19 ± 0.89%) were respectively 0.55 and 0.96 times lower (P < 0.001).
Conclusion
Our study confirms the importance of the voluntary unpaid blood donation and the pre‐donation selection of donors in blood transfusion safety. However, the high proportion of positive tests to TTIs in general compared with national prevalence's raise questions on the efficiency of the pre‐donation screening of blood donors. These questions are even more worrisome mainly for hepatitis when we compare the proportions of the positive tests in the RBTC and HBB. So, further studies must evaluate the efficacy of pre‐donation screening.
P‐356
Validation of the CobasⓇ Mpx Test on the CobasⓇ 8800 System in a Routine Blood Transfusion Screening Laboratory
Stolz M , Gowland P , Schmidseder C , Niederhauser C
Interregional Blood Transfusion SRC, Bern, Switzerland
Background
A highly sensitive and specific nucleic acid test (NAT) for blood‐borne viruses is essential for the safety of blood components. Extensive validation data for the new MPX test on the highly automated cobas® 8800 System in a routine blood transfusion laboratory setting is presented. The screening was performed on individual donations.
Aims
To establish the performance of thecobas® MPXtest on thecobas® 8800System in a routine laboratory setting.
Methods
The validation was performed with the Roche cobas® MPX test, detecting dual targets for HIV‐1 M (LTR and gag), HIV‐1 O, HIV‐2, HCV and HBV. The WHO international standards for HIV‐1, HIV‐2, HCV and HBV were used to define the detection limits, robustness, precision and repeatability. The specificity was performed with pooled plasma negative for HIV‐1, HIV‐2, HCV and HBV and cross‐contamination robustness was challenged with positive high titre samples. A virus stability study was also performed at various storage conditions. The cobas® MPX test reproducibility was tested with external quality control material from NRL, ISS and INSTAND and with genotype panels from NIBSC and PEI. The validation was performed according the European Pharmacopoeia guidelines.
Results
The limit of detection (LOD) was determined by probit analysis of results from 12‐member dilution rows (562 IU/ml down to 1.8 U/ml (HIV‐1), 100 IU/ml down to 0.3 IU/ml (HIV‐2, HCV) and 56.2 IU/ml down to 0.18 U/ml (HBV)) in triplicate. The following LOD were determined: HIV‐1: 12.9 IU/ml (10/152, NIBSC), HIV‐2: 5.0 IU/ml (08/150, NIBSC), HCV 8.3 IU/ml (96/798, NIBSC) and HBV: 1.1 IU/ml (10/264, NIBSC). Specificity, robustness against cross‐contamination, precision and repeatability assays all fulfilled the proposed acceptance criteria. Heparin inhibited the assay only at doses well above 100 I.E. Haemolytic blood samples taken from our routine testing are more prone for invalid results (up to 48%) than lipaemic samples (up to 22%). Virus spiked at 3‐times LOD in plasma was stable up to 10 days at room temperature. At higher temperatures HCV samples began to fail, first after 3 days at 45°C and later after 5 days at 30°C. HIV‐1 samples failed first at 5 days at 45°C and after 10 days at 30°C. HBV remained detectable throughout the study, even at 45°C for 10 days. Under these conditions untreated native viruses are more stable than heat‐inactivated WHO reference material.
Summary/conclusions
The expected specifications of our validation agreed with those presented by the manufacturer. The cobas® MPX test on the cobas® 8800 System performs well and fulfilled the requirements of a routine blood transfusion screening laboratory. Viruses in plasma are more stable than anticipated, making storage issues less of a concern for the reliability of the test result.
P‐357
Seroprevalence of Blood Borne Pathogens Among Croatian Solid Organ Donors 2006–2014
Miletic Lovric M1, Mihaljevic I 1, Balija M 1, Jukic I 1
1Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
The success of transplantation and graft survival are closely related to the absence of infection of recipient by infected graft, reactivated recipient's latent infections, nosocomial infections and community acquired infections. Since 2006, the Croatian Institute of Transfusion Medicine has been providing mandatory testing of solid organ donors (SOD) for blood borne pathogens (24/7) for the entire country. In 2013 molecular testing of SOD, ID‐NAT for HBV‐DNA, HCV‐RNA and HIV1‐RNA, was introduced.
Aims
To estimate the seroprevalence of blood borne pathogens among Croatian SOD, quality of samples measured against rate of dilution and turnaround time.
Methods
In the period from 5/11/2006 to 31/12/2014 a total of 944 solid organ donor's plasma samples were tested by means of Abbott Architect and bioMerieux Vidas tests, for HIV, HBV, HCV, CMV, EBV, T. pallidum (anti‐TP) and T. gondii (Toxo). Each positive result for HBsAg, anti‐HCV, isolated anti‐HBc and IgM (EBV VCA, CMV and Toxo) was retested on alternative analyzer/test (Architect i2000SR or Vidas). Anti‐TP positive result was retested and additionally tested for TPHA and RPR test.
Results
Seroprevalence of EBV, CMV and T. gondii among SOD was as high as expected, 97%, 92% and 70%, respectively. There was no HIV positive SOD, 0.6% were HBsAg positive, 10.6% anti‐HBc, 1.4% anti‐HCV and 1% anti‐TP. Isolated anti‐HBc positive result, as possible marker of occult hepatitis B virus infection (OBI), was detected in 3% of tested donors. 14% were only anti‐HBs positive, probably due to vaccination. 2.5% samples were diluted more than 50%. Average testing turnaround time was 2 h.
Conclusions
The results indicate the adequacy of the SOD selection, tests used and testing algorithms. Seroprevalence of HIV, HBV, HCV and T. pallidum is low and expected as it is high seroprevalence of ubiquitous pathogens suggesting the appropriate sensitivity of solid organ donor's selection methods in donor hospitals.
P‐358
Evaluation of the Procleix Ultrio Elite Assay (Grifols) for Nat HIV‐1/2, HCV and HBV Screening on Blood Donors and on Cell and Tissue Bank Specimens
Rodenbach M-P1, Goossens D 1, Cialone CH 1, Pisani GP 2, Lambermont M 1
1Belgian Red Cross, Suarlée, Belgium2Istituto Superiore di Sanità – CRIVIB, Rome, Italy
Background
HIV, HCV and HBV Nucleic‐Acid Testing is currently performed in our Blood Transfusion Establishment with the Procleix Ultrio Plus Assay on the Tigris instruments (Grifols) for blood donors screening in 8‐minipool and in individual testing on cell and tissue specimens. The new Procleix Ultrio Elite Assay is considered to have equivalent analytical performance than the Ultrio Plus Assay but only few studies have been published.
Aims
The aim of the study was to assess the performance of the Procleix Ultrio Elite Assay on 2 PANTHER instruments for analytical sensitivity, specificity, reproducibility, contamination and matrix comparison (plasma vs serum).
Methods
The analytical sensitivity has been evaluated by the determination of the 95% LOD (Limit of Detection) with dilutions of HIV‐1, HCV and HBV WHO standards by Probit analysis and with 2 qualification and 4 External Quality Assessment panels. Moreover, 31 positive known samples from donors (3 HIV‐1, 1 HIV‐2, 5 HCV and 22 HBV) have been tested.
The specificity has been studied on 60 pools of negative samples and on 60 samples of Cell and Tissue donors, including plasma and serum of living and cadaveric donors. Discriminatory tests in case of positive result have been carried out.
Reproducibility has been evaluated on both PANTHER instruments with 20 replicates of HIV‐1, HCV and HBV commercial controls with concentrations at above 3 times the LOD over 20 days.
The contamination has been studied by alternate testing of positive and negative samples in 4 runs.
Plasma and serum matrix have been compared with 20 negative donors and 7 positive donors for one marker and with a dilution test with an HBV donor.
Results
The 95% LOD (in IU/ml) were estimated at 14.5 (11.6–20.3) for HIV‐1, 3.2 (2.3–5.9) for HCV and 3.2 (2.0–9.3) for HBV. The qualification panels (tables 1 and 2) and the External Quality panels showed results in accordance with the expected results. All known positive samples tested were found positive.
The specificity was 99.17% as one cadaveric serum out of the 120 specimens was considered falsely positive.
Reproducibility studies with the 3 controls gave coefficients of variation from 3.4 to 5.9% and no contamination was observed.
For the matrix comparison test, one HBV sample was found positive in plasma but not in serum. The latter was related to a fluctuating HBV DNA donor with a presumed low viral charge. The dilution test proved however the serum to be at least as efficient as the plasma.
Caption 1: Qualification panel (AcroMetrix)

Caption 2: Qualification panel (EFS)

Conclusions
The results of our evaluation of the Procleix Ultrio Elite Assay were in accordance to the manufactured reported performance. The analytical sensitivity gives confidence to our screening strategy (8‐minipool) as it highly meets the international recommendations. Moreover, The PANTHER instrument offers significant technical advances such as 24 h valid calibration, continuous sample loading and drastically reduce maintenance.
P‐359
Analysis of Four Years Seroprevalence Data of Blood Donors Based on the Donor Type
Yilmaz SY 1, Cetinkaya RAC 2, Savasci US 1, Artuk CA 1, Yilmaz SY 1, Turker TT 1, Avci IYA 1, Kocak NK3, Besirbellioglu BAB 1
1Gulhane Military Academy of Medicine, Ankara, Turkey2Military Hospital, Isparta, Turkey3Gumussuyu Military Hospital, Istanbul, Turkey
Background
Microbiological screening tests are mandatory in order to ensure blood transfusion safety but not sufficient. Donor organizations and donor selection are an integral and important part of the safety chain of blood supplying system.
Aim
In this study, we aimed to analyze mandatory microbiological screening test results applied to blood donors between 2011 and 2015 at Regional Blood Center of Gulhane Military Academy of Medicine and demonstrate the possible relationship with the donor profile of these prevalence data.
Material and method
This study was conducted between March 2011 and February 2015 in Regional Blood Center of GMMA, Ankara, Turkey. Blood samples from 42,258 donors were tested for HBsAg, anti‐HCV, anti‐HIV‐1/2 and anti‐treponoma pallidum (TP) antibody within the scope of screening tests. All of these tests were performed by using a fully automated device (Architect, Abbott, IL, USA) with the microparticle enzyme immunoassay method (MEIA) method. All of the confirmatory tests were performed at laboratory of National Public Health Agency.
Results
Out of 42,258 donors, 22,434 (53.1%) were doing mandatory military service (soldier), 13,845 (32.8%) were replacement donors and 5979 (14.1%) were voluntary donors. Male gender was significantly predominant (97.9% men vs 2.1% women). Mean ages of soldiers, replacement donors and voluntary donors were 21.69 ± 1.98, 34.22 ± 8.96 and 31.06 ± 9.83, respectively. HBsAg was positive in 251 donors (0.6%), whereas positivity of anti‐HCV, anti‐HIV and anti‐TP were 8 (0.018%), 1 (0.002%) and 11 (0.026%), respectively. HBsAg positivity was found in %0.9 of soldiers (199/22,434), %0.3 (43/13,485) of replacement donors and %0.2 (9%5979) of voluntary donors. The distribution of microbiological screening test results according to the donor type were shown in the Table 1. There was a statistically significant difference in favor of the soldier donors in HBsAg positivity (P < 0.001), and in favor of the replacement donors in anti-TP positivity (P = 0.009). The source of the difference stems from the value of HBsAg positivity of soldiers. Statistically significant changes were found in volunteer donors when the donation period was evaluated year to year (P < 0.001) (Table 2).
When the risk factors affecting HBsAg positivity evaluated by reference with voluntary donors, ORs and 95% confidence intervals were calculated as 5.9, 3.0–11.6 and 2.1, 1.01–4.2 for soldier donors and replacement donors, respectively.
Table 1. The distribution of microbiological screening test results according to the donor type.

Table 2. Distribution of blood donor type in the time period of study.

Conclusion
Although innovation in microbiological screening tests with the advancing technology is so important for improving blood safety, donor selection is an issue that should not be ignored. According to our results, bloods that obtained from soldiers are six times more risky than obtained from voluntary donors. This data put forth importance of the donor selection. Our blood center has been spending significant effort to shift donor profile from soldier donors to voluntary donors. The determination of statistically significant change in favor of voluntary blood donors over the years reveals the impact of these efforts. Reliability of blood should ensure by fulfilling all the requirements of haemovigilance system and obtaining safe blood issue should not be left only the test results.
P‐360
Nat Testing of Blood Donors in Croatia: Results of Two Years Testing in Croatian Institute of Transfusion Medicine
Bingulac-Popovic J , Babic I , Maslovic M , Dogic V , Stojic Vidovic M , Miletic Lovric M , Jurakovic-Loncar N , Balija M , Jukic I
Croatian Institute for Transfusion Medicine, Zagreb, Croatia
Background
Croatia implemented nucleic acid testing on individual blood donation (ID‐NAT) as mandatory in March 2013. Croatian Institute of Transfusion Medicine (CITM) in Zagreb is the only testing site for whole Croatia. The test results are distributed through national transfusion IT system which interconnects all eight Blood Establishments (BE).
Aims
Evaluation of two years data on ID‐NAT organization, process and results and establishing plans for future improvements.
Methods
One selected courier service is delivering all blood donors samples by car or by plane for distantly located blood centers to CITM at day 0. ID‐NAT is performed at the same day by using Procleix Ultrio Plus test on 3 Tigris instruments (Grifols, Spain) which is a multiplex NAT for simultaneous detection of HIV‐1, HCV and HBV. The results are provided early morning of the day 1. All eight BE are connected to national transfusion IT system eDelphyn through which they share all information on blood donors, donations and results. Confirmatory testing with alternative NAT method is performed in incriminating donation and in follow‐up sample with CAP/CTM tests, v 2.0 for HBV, HCV and HIV‐1 (Roche Diagnostics); 95% LOD in plasma for HBV‐DNA, HCV‐RNA and HIV‐1 RNA: 9 IU/ml, 11 IU/ml, and 27.5 IU/ml.
Results
We tested 361.660 donations in two years period. Overall specificity of testing has been 99.96%. Rate of invalid runs was around 2.23%. The results of NAT testing were delayed 18 times in 520 work days, not more than for 10 h. Late shipment of blood samples caused by traffic or weather conditions (3/18, 17%) and hardware failure on Tigris instruments or other problems in testing (15/18, 83%) was the main source of delay of NAT testing and results. Blood products without NAT results, caused by delay of NAT testing, were not released. We found 23 HBV, 12 HCV and 5 HIV‐1 NAT and serology positive donations. There were 41 HBV NAT only positive donations later to be found anti‐HBc positive and were acknowledged as occult HBV infection (OBI). One HIV‐1 NAT only positive donation was given by donor in window period of infection. In all cases of NAT positive/serology positive and previously mentioned one window period HIV‐1 infection, follow‐up sample was also positive when tested with alternative NAT method. In 16 of 41 (39%) follow‐up samples of blood donors with OBI, HBV‐DNA was not determined with alternative NAT method which was explained by very low and fluctuating HBV‐DNA level.
Summary/Conclusions
Organization of NAT testing at one site has proved itself to be a good solution for improving blood transfusion safety without compromising optimal blood products supply and releasing them on time. Detection of 1 HIV‐1 window period infection and 41 OBI justifies implementation of ID‐NAT screening in Croatia. Improvements could be made with implementation of more sensitive test for confirmatory testing for HBV‐DNA with alternative NAT method in follow‐up samples.
P‐361
Evaluation of a New System – Lumipulse G Tp‐N for the Screening of Treponema Pallidum
Serafini RS , Iovino SI , Sbariggi LS , Mariotti AM , Pierdomenico LP
Sandro Pertini Hospital, Rome, Italy
Background
The Japanese Company Fujirebio, recently arrived in the European market, proposes this novel assay for the TP screening; it is a diagnostic assay with monotest cartridges that runs on a fully automated instrument of the latest generation – LUMIPULSE G1200, working with chemiluminescence technology.
Aims
The aim of our work was to evaluate the performance of this novel assay for the screening of Treponema pallidum (Lumipulse G TP‐N) and comparing it to the method in use in our transfusion center (Abbott Architect).
Method
Lumipulse G TP‐N is a two step immunoassay, using recombinant TP antigens (Tp15‐17 and TpN47), requiring a calibration every 30 days and with the Cut Off determined by the negative and the positive calibrators that are present in the kit.
A comparative study has been performed on 10,230 donors, tested in parallel on both LUMIPULSE G1200 and Abbott Architect systems. For samples showing discordant results, discrepancy testing was performed by using other diagnostic kits (SERODIA‐TP‐PA and INNO‐LIA SYPHILIS SCORE).
Results
Our data show an extremely good correlation between the two methods in terms of sensitivity but also show the superior specificity of the new system. In fact, taking into account all samples tested, Architect gave a total number of 57 false positive results (confirmed as real false positive) while the LUMIPULSE G1200 gave false positive results on just 5 samples.
Summary
Our experience with the LUMIPULSE G1200 system revealed its high reliability and good performances. Our results suggest that the Lumipulse G TP‐N assay can be proposed for routine syphilis screening tests. Moreover, its good specificity suggests its possible use to monitor and manage the donors that showed false positive results with other methods.
P‐362
Evaluation of Nucleic Acid Testing to Detect HBV Infections in Egyption Blood Donors
Farouk Badr R1, Farouk R 1, Ahmed Ali A 2
1Dar El Salam Regional Blood Transfusion Center, Cairo, Egypt2National Blood Transfusion Services, Cairo, Egypt
Background
Prevention of transfusion‐transmitted hepatitis B virus (HBV) has historically relied on serological screening of blood donors using progressively more sensitive HBV surface protein (HBsAg) assays; in some countries anti‐HBc assays have also been employed to detect chronic carriers with low‐level viremia who lack detectable HBsAg. Meanwhile HBV nucleic acid testing (NAT) was recently introduced to detect blood units that carry the risk of HBV infection while showing HBsAg and anti‐HBc‐negative results being donated during early acute infection stage, or in ‘occult’ HBV infections (OBIs), characterized by undetectable HBsAg, low viral load and presence of some serological markers (anti‐HBc and/or anti‐HBs).
Aims
To evaluate the performance of NAT in improving the blood safety through reducing the laboratory window phase of HBV infection detectability vs the routinely hepatitis B surface antigen (HBsAg) testing using Enzyme Linked Immunosorbent Assay (ELISA) techniques.
Methods
This descriptive study was conducted in a retrospective cross‐sectional manner by examining TTIs screening data of all blood donors who donated over a period of 2 years from January 1st 2013 to December 31st 2014 at Dar El‐Salam Regional Blood Transfusion Center, Cairo, Egypt.
All donors were accepted based on the Egyptian national blood donors’ selection criteria and expressed medical history. All collected blood samples were run in parallel for both ID (Individual‐Donation) nucleic acid screening assay (Procleix Ultrio Plus, Chiron/Gen‐Probe) and ELISA testing.
Samples found initially ELISA reactive, NAT non reactive were retested in duplicate by ELISA.
Samples found ELISA non reactive, NAT reactive were retested in triplicate by NAT and samples found reactive (even one of three) were further tested by discriminatory test for HIV, HCV and HBV.
Results
Out of 33,921 donors screened by ELISA, a total of 875 samples (2.57%) were found to be reactive; 198 (0.58%) were reactive for hepatitis B virus, 600 (1.77%) for hepatitis C virus, 35 (0.1%) for human immunodeficiency virus, 22 (0.06%) for syphilis antibodies and 20 (0.06%) for combined parameters. For the 33,046 ELISA non‐reactive blood samples subjected to NAT, 13 donor samples were found to be reactive and identified as NAT yields, all of them revealed HBV DNA in the discriminatory assays.
Summary/Conclusion
It is obvious from the study results that NAT managed to improve blood safety by detecting some cases of sero‐negative NAT positive HBV Blood donations; however, given many factors, mainly economic and technological, this cannot be generalized in all countries. To ensure blood safety, each country needs to develop its blood screening strategy for TTIs based on its own disease epidemiology characteristics, yield of infectious units detected by different serologic/NAT screening methods, and cost‐effectiveness of test methods. Nevertheless, NAT has improved the safety of blood products at the blood center in which this study was conducted, it is recommended to continue the NAT to ensure higher levels of blood safety. Further studies may be required to examine the health economics related to the routine use of NAT in blood centers mainly in terms of cost‐effectiveness and cost‐efficiency.
P‐363
Screening Blood Donors with Procleix Ultrio Elite and Procleix Ultrio Nat Assays: A Comparative Study
Chaurasia R , Zaman S , Chatterjee K
All India Institute of Medical Sciences, Delhi, India
Background
Continuous progress and improvements in nucleic acid amplification technologies (NAT) have resulted in an unprecedented technological turning point in the molecular diagnostic and characterisation of viral infections. Transcription‐based amplification is an isothermal nucleic acid amplification technique for the detection of viral genomes in plasma specimens from blood donors.
Aim
To compare the NAT yields of Procleix Ultrio assay (PUA) with newer Procleix Ultrio Elite assay (PUE).
Methods
Of 10,015 donor samples were tested with using 4th generation kits for HIV‐1/2, 3rd generation kits for HBsAg and HCV infections and both versions of NAT viz. PUA and PUE. We also subjected the NAT yields to viral load quantification and supplemental tests (anti‐HBc, anti‐HBs and anti‐HBe reactivities).
Results
A total of 21 NAT yield cases were detected, three cases were positive in both systems whereas 18 samples were reactive only by PUE. Of total 21 NAT yields, 18 HBV and 3 HCV yields were detected. Seventeen of HBV yields were occult infections and 1 window period (WP yield) infection. All 3 HCV yields were WP infections. PUE system's yield (1 in 477) was 7 times more efficient than the PUA (1 in 3338).
Conclusion
The current efforts and strategies have greatly helped to reduce transfusion‐transmitted infections. A considerable portion of this improvement is due to the introduction of NAT, rather than relying solely on measuring pathogen‐specific humoral immune responses in the donor. Although there is cross‐reactivity between the main virus types (HIV‐1 and HIV‐2), it is not sufficient to rely on an HIV‐1 specific assay to detect all cases of HIV‐2. Nevertheless, NAT in any platform is superior to serological immunoassays; the newer PUE system, is significantly improved over earlier versions in terms of sensitivity and automation.
P‐364
Prevalence of Transfusion‐Transmitted Infections in Turkish Red Crescent Blood Donors at Aegean Regional Blood Center, 2007–2014
Beker CM , Dündar IH , Gök G , Karahacioglu I , Aksoy A , Özet G
Turkish Red Crescent, Izmir, Turkey
Background
Laboratory testing of donated blood prior to transfusion is intended to ensure that recipients receive the safest possible blood products. It is mandatory to test donated blood for transfusion‐transmitted infections (TTIs) such as hepatitis B virus (HBV), hepatitis C virus, human immunodeficiency virus (HIV), and syphilis in Turkey.
Aims
This study was performed to review the screening and confirmatory test results and examine the seroprevalence rate of HBV, HCV, HIV, and syphilis in Turkish Red Crescent (TRC) blood donors at Aegean Regional Blood Center from 2007 to 2014.
Methods
Screening for HBV, HCV, HIV, and syphilis was done by using enzyme immunoassay kits (HBsAg 6.0, HIV Integral II, TP total antibody, Siemens, Germany; anti‐HCV Monolisa, Bio‐Rad, Germany). Initially reactive samples were tested in duplicate and repeatedly reactive samples were tested by confirmatory assays: LIA (Innogenetics, Belgium) for HCV and HIV; anti HBc total and neutralization test (Siemens, Germany) for HBV; FTA‐Abs (Euroimmun, Germany) for syphilis. The statistical data of the eight years between 2007 and 2014 was reviewed, retrospectively. Seroprevalence rate was defined as the percentage of seropositive donors.
Results
Among 1,734,879 donations, the number of test positives and the overall seroprevalence rate of HBV, HCV, HIV, and syphilis was 9859 (0.6%), 3775 (0.23%), 2002 (0.11%), and 2012 (0.12%) respectively. The number of blood donors found to have confirmed seropositivity and overall confirmation rate was as follows: 79% (7794/9859) for HBV, 7.7% (292/3775) for HCV, 2.6% (51/2002) for HIV, and 60.8% (1225/2012) for syphilis.


Conclusion
The seroprevalence of HBV, HCV, and HIV demonstrated a declining trend by year. The prevalence of HBV was lower than the other studies in Turkish blood donors (0.8–14.3%). The prevalences of HCV, HIV, and syphilis seem to be compatible with the data in Turkey (0.12–1.7%; 0.04–0.4%; 0–0.86% respectively). There was a slight fluctuation in the sreoprevalence rate of HIV by year, now it tends to decrease. Generally, decreasing of the seroprevalence for TTIs may be due to donor selection/qualification criteria and donor deferral registry system implemented by TRC. In recent years, we observed that the number of regular and voluntary blood donors essential for blood safety have been increasing day by day. So, we think the most important advance in blood safety is the conversion to a voluntary donor blood supply in Turkey.
P‐365
Introduction of Nucleic Acid Testing (Nat) for Blood Donations in National Blood Transfusion Service (NBTS), Sri Lanka
Gamini Perera IGJ
National Blood Transfusion Service, Moratuwa, Sri Lanka
Background
NBTS Sri Lanka comprises of a nationally coordinated network under the ministry of health with 90 Hospital Based Blood Banks (HBBs) 15 Cluster Blood Centers (CBCs), National Blood Center (NBC) in Colombo, which is the Headquarters and Coordinating center of NBTS.
In per with the mission to provide the safest blood to the nation, advanced serological tests for the screening of blood donations for Human Immunodeficiency Virus (HIV), Hepatitis B virus (HBV), and Hepatitis C virus (HCV) has been adopted by NBTS.
To add an additional layer of safety to reduce the viral detection window period of the blood supply and for the fulfillment of prerequisite in utilizing excess plasma of NBTS for fractionation; individual NAT testing was introduced as a pilot project at National Blood Center (NBC).
Aims
Ultimate goal of this exercise is to provide NAT tested blood for the whole country which is going to provide number of additional benefits and recognition of Safety Blood Products from NBTS.
Method
Funding for the testing facility is provided with the Netherland funded project for the development of the NBTS Sri Lanka. Two Tigris machines with other relevant equipments were set up in the temporary laboratory which is in the existing building of the NBC, until proper NAT laboratory will be coming up in the new wing of the NBC, which is under construction.
Five medical laboratory technologists’ two medical officers and a one consultant were trained for the relevant respective tasks.
local agent for the technology has deployed one service engineer and one application specialist at the Blood Bank additionally in order to do the maintenance and service to ensure the test is performed without any interrupt.
Blood samples are collected into 6 ml EDTA tubes from Voluntary Blood donors of NBC with other samples of donation.
Fully automated Tigris machine uses the Transcription Mediated Amplification (TMA) as the testing technology for the detection of HIV 1, Hepatitis B & C in all tested samples.
The whole exercise is tightly monitored and supervised by the various levels of the ministry of health and the NBTS management team.
Results
So far 73,839 samples are being tested in both machines with negative and positive results which are compatible with serological investigations.
Up to now there are two NAT yields, which are having two positives with NAT, while there are negative results from serological method (ELISA) for those particular samples.
There were 5717 invalid runs due to machine fallers, power fallers and user errors. Those are to be corrected in future.
Summary/Conclusions
Even though the Implementation of NAT in stepwise manner to cover the entire blood donation of the country for continuous improvement of blood safety is the final goal, there are many obstacles to overcome in this process. Continuous financial support, proper machine maintenance, efficient sample delivery, and timely issuing of results to distance blood collecting centers are some of those already noted.
P‐366
The Effect of Nucleic Acid Testing (NAT) in the Safety of Blood Transfusions in Nw Greece
Zervou EZ , Kourgia FK , Vini MV
University Hospital, Ioannina, Greece
Background
Testing blood donations for markers of infectious diseases plays an important role in establishing and maintaining the safety of blood transfusions. In accordance with national guidelines, the minimum requirements of blood donors testing include testing for syphilis, HBV, HCV and HIV. HTLV testing is also mandatory in some countries. The norm for viral testing has been serology, but increasingly NAT is been implemented where resources allow. NAT improves the safety of transfusions because shortens the serological silent window period and also detects the cases of occult HBV.
Aim
To determine the NAT yield (the infectious agent is not serologically detected but only by NAT) for HBV, HCV and HIV in the blood donations which conducted in the Blood Bank of University Hospital of Ioannina.
Methods
From 2006 until 2014, 65,219 donations (62,460 for whole blood and 2759 for apheresis platelets) were tested. For serological testing Abbott and Ortho immunoassays were used. For individual (ID) NAT testing the Procleix Ultrio (Novartis) until the end of 2013 and after this time the Cobas (Roche), were used. All initially reactive (IR) donations by NAT are retested in triplicate if the serological results are negative. IR donations are discarded. If retesting is negative, anti‐HBc is performed. If anti‐HBc is positive the donors are excluded from further donation. If anti‐HBc is negative the donors are eligible for further donation.
Results
In this period, 1 case of HIV in the window period and a total of 17 cases of occult hepatitis B and 11 cases of possible occult hepatitis B (NAT initially reactive, anti‐HBc positive but the repetition of NAT was negative, probably due to a low viral load) were identified.
Conclusion
The implementation of NAT detects infectious donations that could be missed by serological testing and so plays an important role in the safety of blood transfusions. In our center we found that the HBV NAT yield was 1/2329 (1/3836 clear occult HBV and 1/5929 possible cases) and the HIV NAT yield was 1/65,219 blood donations.
P‐367
Turkish Red Crescent Blood Donors’ Infectious Marker Seroprevalences For 2012–2014
Demirel U1, Saygan M 2, Günçikan MN 2, Birinci I 2, Aksoy A 2, Özet G 2
1Türkkizilayi, Istanbul, Turkey2Turkish red Crescent, Ankara, Turkey
Background
All donated blood should be tested for transfusion transmissible infectious diseases for blood safety.
Aims
We aimed to determine the seroprevalence rates of hepatitis B virus (HBV), hepatitis C virus (HCV), Human Immunodeficiency Virus (HIV) and Syphilis markers among blood donors of Turkish Red Crescent.
Materials and methods
We retrospectively analyzed the results of ELISA screening tests from all blood donors of Turkish Red Crescent who donated blood for the three year period between 2012 and 2014. For confirmation of initial repeat reactive ELISA results for HCV (Bio‐rad), HBV (Siemens), HIV (Siemens) and Syphilis (Siemens), we used Western Blot technique for HCV, and HIV (Innogenetics), Antibody Neutralization technique and Anti Hbc total for HBV (Siemens), and IFA (Euroimmun) for Syphilis Ab ELISA. Seroprevalence rates for males and females among new and repeat donors were calculated. The seroprevalence for new donors was compared with those of repeat donors; and a Mantel‐Haenszel Weighted Relative Risk (95% Confidence Interval) ‘MHRR (95%CI)’ was calculated for each of the pathogens.
Results
There were 1,232,690 new and 589,087 repeat male donors; 250,394 new and 73,770 repeat female donors. For HBV, the seroprevalence was 1.7222%, 0.1426%, 0.6470%, 0.0515% among new male, repeat male, new female, repeat female donors respectively. For HCV, the seroprevalence was 0.06%, 0.0170%, 0.0319%, 0.0054% among new male, repeat male, new female, repeat female donors respectively. For HIV, the seroprevalence was 0.0092, 0.0161%, 0.0012%, 0% (no cases) among new male, repeat male, new female, repeat female donors, respectively. For Syphilis, the seroprevalence was 0.2124%, 0.1431%, 0.1142%, 0.0691% among new male, repeat male, new female, repeat female donors respectively. The MHRR(95%) was 12.10(11.31–12.95), 3.64(2.97–4.47), 1.49(1.39–1.61), for HBV, HCV and Syphilis, respectively. For these, there was no confounding by sex. As for HIV, among repeat female donors, there was no seropositive persons, so we could not calculate a MHRR (95% CI); but instead, we calculated a relative risk just for new male donors vs repeat male donors, RR(95%CI) = 0.57(0.43–0.75).
Conclusions
We found that, for this period, the total risk for these pathogens were higher in the new donors except for HIV for which the risk was higher for repeat male donors. For female donors, we could not calculate a relative risk because one of the counts (repeat female donors) were zero. A larger future study might show a more precise seroprevalence and a relative risk for females. Maybe, the male donors which were at a larger risk of contracting HIV could be donating blood in order to get an HIV test. This might, in a way, be the reason for the relative risk of HIV being in the opposite direction of other pathogens. However, we currently have no data regarding any plausible explanations for this. The behavioral basis of this phenomenon would be interesting to reveal with a different study design in the future.
Kadri.demirel@kizilay.org.tr
P‐368
Evaluation Study of Roche Elecsys Assays on Cobas E 601 in Blood Donor Screening
Rahne Potokar U , Jovanovic P , Levicnik Stezinar S
Blood Transfusion Centre of Slovenia, Ljubljana, Slovenia
Background
Blood banks perform screening tests for HBsAg, anti‐HCV, HIV Ag/Ab, and syphilis for all donated blood units. Before the introduction of a new automated testing system, an evaluation of different testing systems is recommended. In the Blood Transfusion Centre of Slovenia an evaluation of screening assays on Roche cobas e 601 in comparison with the Abbott Architect system was performed.
Aims
The aim of this study was to evaluate the specificity of Roche Elecsys assays on a fully automated analyzer cobas e 601 on blood donor specimens in parallel with our routine system. A sensitivity testing was performed on seroconversion panels and previously characterized positive samples.
Methods
The specificity was evaluated on 1990 plasma specimens from unselected blood donors routinely screened on Architect i2000sr for HBsAg, anti‐HCV, HIVAg/Ab and syphilis (ARCHITECT HBsAg Qualitatve II, ARCHITECT Anti‐HCV, ARCHITECT HIV Ag/Ab Combo and Abbott ARCHITECT TP). The samples were retested on the same or the next day on cobas e 601 (Roche Elecsys HBsAg II, Roche Elecsys Anti‐HCV II, HIV Roche Elecsys HIV combi PT, Roche Elecsys Syphilis). The sensitivity was assessed by testing pre‐characterized samples expected to be positive (41 samples reactive for HBsAg, 23 samples reactive for anti‐HCV, and 38 samples reactive for anti‐Treponema pallidum). In addition, 3 commercial seroconversion panels were evaluated (SeraCare PHM934 for HbsAg, SeraCare PHV925 for anti‐HCV and SeraCare PRB966 for HIVAg/Ab).
Results
Based on the results from testing 1990 blood donations, the observed specificity of Roche Elecsys assays on cobas e 601 and Abbott Architect assays are comparable. The results are presented in Table 1.
The Roche Elecsys Anti‐HCV II assay was found more sensitive in a seroconversion panel in comparison with Abbott Architect Anti‐HCV. The presence of anti‐HCV was firstly detected 8 and 27 days after the first bleed respectively. Positive samples of the HIV seroconversion panel and positive samples of the HBV seroconversion panel were equally detected with both systems. Additionally, observed sensitivity on pre‐characterized positive samples was 100% on both systems for all tested markers.
Table 1. Comparison of the specificity of assays on Roche cobas e 601 and Abbott Architect
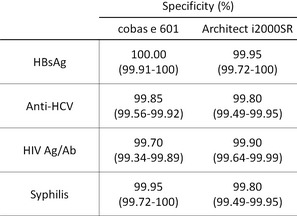
Conclusion
The observed performance of Roche Elecsys assays on cobas e 601 is comparable to Abbott Architect assays in blood donor screening. Both Roche cobas e 601 and Abbott Architect HBsAg, anti‐HCV, HIV Ab/Ag and syphilis assays can be considered very suitable for blood donor screening with very good specificity and sensitivity.
P‐369
Automated TPHA, an Appropriate Screening Tool for Syphilis in Blood Donors: Evaluation Report In Over 8000 Donors
Acharya AD , Tiwari AK , Arora DA , Dara RC , Aggarwal GA , Singh GS , Raina RV
Medanta The Medicity, Gurgaon, India
Introduction
World Health Organization estimated that approximately 12 million new cases of Syphilis are reported each year in the world with more than 90 percent from developing countries; rare in developed countries. Serological tests for syphilis (STS) contributed greatly to the detection of T. pallidum infection in blood donors and especially in those who were not identified during the medical selection. It is also a mandatory test for donor screening but various blood centres use different STS; most use non‐treponemal tests due to cost and ease of performance, despite reported high false positivity. Treponemal tests are more sensitive and specific than non treponemal tests that includes wide array of tests. There are hardly any published reports comparing various treponemal tests, which would have helped the blood centers decide upon the most appropriate treponemal test to be employed as the STS. We analyzed/ compared the effectiveness of two commonly available STS, Immuno‐chromatography Assay (ICA) and Treponema Pallidum Haemagglutination Assay (TPHA) in blood donors considering Fluorescent Treponemal Antibody Absorption assay (FTA‐Abs) as gold standard.
Aims
To compare and evaluate automated TPHA (a‐TPHA) and ICA as a STS screening test in blood donors
Materials and methods
The study was conducted in the Department of Transfusion Medicine in a large tertiary care hospital in India. Consecutive blood donors from June 2014 to February 2015 were evaluated simultaneously for anti‐treponemal antibodies by solid phase ICA (SD Bio Standard diagnostics, India), a‐TPHA (Immucor Diagnostics, USA) and FTA‐ Abs (Biocientifica SA, FTA ‐Abs, Argentina). Performances of both the assays were evaluated in terms of sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and Youden's index.
Results
8900 samples were evaluated during the study period. There were 8817 concordant negatives and 39 concordant positives verified by FTA‐Abs. 44 samples (31 were a‐TPHA positive and ICA negative; 13 ICA positive and a‐TPHA negative) were discordant. The sensitivity of a‐TPHA was found to be 92.54% which was substantially higher than that of ICA (56.72%) while the specificity of these two tests was similar. Youden's index which is considered to be global measure of a test performance used for the evaluation of overall discriminative power of diagnostic test was higher for a‐TPHA as compared to ICA.

Conclusion
The new a‐TPHA has a better sensitivity to the ICA thus seems to be a more appropriate treponemal test to be employed as the STS for screening in blood donors with additional advantages of automation and easier record keeping.
P‐370
Evaluation of the Specificity of the CobasⓇ WNV Test in US Blood Donations
Pate LL1, Bertuzis R 1, Huynh N 1, Hemyari P 1, Duncan J 1, Bhatti S 1, AuBuchon JP 2, Erickson Y 3, Waxman DA 4, Williamson PC 5, Yabes AY 6, Galel SA 1
1Roche Molecular Systems, Inc., Pleasanton, United States of America2Bloodworks Northwest, Seattle, United States of America3Mississippi Valley Regional Blood Center, Davenport, United States of America4Indiana Blood Center, Indianapolis, United States of America5Creative Testing Solutions, Tempe, United States of America6Annoel P. Yabes M.D., San Francisco, United States of America
Background
The cobas® WNV test is a qualitative blood donor screening test for the direct detection of West Nile Virus (WNV) RNA, for use on the cobas® 6800/8800 Systems. This real‐time PCR test incorporates the use of detection probes labeled with unique fluorescent reporter‐dyes measurable at defined wavelengths, enabling the simultaneous detection and discrimination of WNV RNA and the internal control (IC). cobas® WNV is highly sensitive for WNV lineages 1 and 2, and also detects other related flaviviruses.1 The cobas® 6800/8800 Systems incorporate fully automated sample preparation, amplification, and detection, ready‐to‐use reagents and controls, onboard refrigerated storage, and full traceability of reagents and consumables. Samples may be tested individually on the cobas® 6800/8800 Systems; a cobas p 680 pooling instrument (p680) may be used to produce minipools of donor samples for testing with cobas® WNV.
Aims
To determine the clinical specificity of the cobas® WNV test on the cobas® 8800 System in blood donor samples in the United States (US), both in individual donation testing (IDT) format and in minipools of up to 6 donations (MP6).
Methods
A total of 74,066 US blood donor samples were tested with cobas® WNV on the cobas® 8800 System; 10,823 donations were tested by IDT and 63,243 donations were tested in MP6 produced with the p680. Testing was performed across 4 test sites, using 3 cobas® WNV reagent lots. Each donation was also tested in the same format (i.e., by IDT or MP6) with the cobas® TaqScreen WNV Test on the cobas s 201 system (test of record). Discordant results were to be resolved according to a predetermined algorithm, using additional testing of the index unit and donor follow‐up (f/u) testing.
A donation was defined as status‐positive if reactive on both cobas® WNV and the cobas® TaqScreen WNV Test, or if the donor was positive for WNV RNA or anti‐WNV IgM on additional testing of the index unit or f/u testing. Specificity was calculated as the frequency of cobas® WNV nonreactive results among status‐negative donations (total donations minus status‐positive donations).
Results
10,823 donations were tested individually and all were nonreactive for WNV on both cobas® WNV and the cobas® TaqScreen WNV Test. Thus, the clinical specificity of cobas® WNV in donations tested individually was 100% (95% CI: 99.965% to 100%). 63,243 donations were tested in MP6 (in 10,573 pools) and all were non‐reactive for WNV with both tests, for a clinical specificity in donations tested in MP6 of 100% (95% CI: 99.994% to 100%). The overall clinical specificity of cobas® WNV in 74,066 donations was 100% (95% CI: 99.995% to 100%) (Table 1).
Caption 1: Clinical Specificity of cobas® WNV in US Blood Donations

Summary/Conclusions
Both the cobas® WNV and the cobas® TaqScreen WNV Test demonstrated 100% clinical specificity in US blood donations when tested as individual donations, in plasma pools of 6, and overall.
References
1. Roche. cobas® WNV Nucleic Acid Test for Use on the cobas® 6800/8800 Systems – CE/IVD – English 2014.
P‐371
Performance of the Liaison Xl Murex Hbsag Quant, HCV Ab, HIV Ab/Ag and Liaison Treponema Screen – Two Years Experience With Blood Donors Screening
Fialova Z1, Kollarova B 1, Biskova S 1, Janouskova M 2, Mecelova K 2, Novakova E 2, Bubenik B 3, Groborz P 3, Danhelova J 3, Farbiakova V 4, Szuscikova I 4, Janikova S 4, Urbaniecova K 4
1Hospital Sokolov, Sokolov, Czech Republic2Carlsbad Regional Hospital, Karlovy Vary, Czech Republic3Blood Center, Frydek – Mistek, Czech Republic4Hospital Trinec, Trinec, Czech Republic
Background
Serological tests for detection of HBsAg, HCV Ab, HIV Ab/Ag and Treponema Ab are mandatory for blood donors screening in the Czech Republic. Highly sensitive and specific tests and corresponding automation are needed for this purpose. The LIAISON XL (DiaSorin) is fully automated closed chemiluminescence analyzer offering wide test menu including LIAISON XL murex HBsAg Quant, LIAISON XL murex HCV Ab, LIAISON XL murex HIV Ab/Ag and LIAISON Treponema Screen assays appropriate for blood donors screening. In 2012 LIAISON XL system was introduced in blood bank segment in the Czech Republic and subsequently implemented in a routine blood donors testing.
Aims
A multicenter monitoring was carried out at four transfusion service sites in order to share experience in terms of validation and routine testing with respect to that the LIAISON XL systems replaced different ELISA and CLIA testing platforms.
Methods
In validation processes at each of four transfusion service places randomly selected blood donor samples and positive quality control samples were tested for HBsAg, HCV Ab, HIV Ab/Ag and Treponema Ab by LIAISON XL assays and by until used methods (ELISA or CLIA) in parallel and the results were compared and evaluated. Then in 2013–2014 results of routine blood donations testing on LIAISON XL platform were monitored. All repeatedly reactive samples of blood donors were forwarded to the National Reference Laboratory for Hepatitis and HIV in Prague for confirmation.
Results
During the validation at the site No. 1, 68 samples were tested by LIAISON XL methods and by ELISA HBsAg, HCV Ag/Ab, HIV Ag/Ab, Syphilis Ab (all BioRad) tests, with concordance 100% for HBsAg, HIV Ag/Ab, Treponema Ab, and 98.5% for HCV Ab (1 false positive sample by ELISA HCV Ag/Ab). At the site No. 2, 108 samples were measured by LIAISON XL assays and by ELISA tests HBsAg, HIV Ag/Ab (both BioMerieux), HCV (Ortho), Syphilis (DIESSE) with 100% concordance for all measured markers. At the site No. 3, 40 samples were measured on LIAISON XL and Architect (Abbott), the concordance between both systems was 100%. At the site No. 4, 90 samples were measured by LIAISON XL and by ELISA BioRad tests (mentioned at No. 1), the concordance was 100% for HBsAg, HCV Ab, Treponema Ab, and 98.9% for HIV Ab/Ag (1 false positive sample by LIAISON XL). During routine screening on LIAISON XL, 8.285, 16.014, 46.627 and 8.847 blood donor samples were tested at the sites No. 1, 2, 3 and 4, respectively. Percentage of repeatedly false positive samples measured for HBsAg: 0.10%; 0.12%; 0% and 0.02%, respectively, for HCV Ab: 0.22%; 0.24%; 0.08% and 0.18%, respectively, for HIV Ab/Ag: 0.25%; 0.17%; 0.08% and 0.17%, respectively, and for Treponema Ab: 0.05%; 0.01%; 0.03% and 0.07%, respectively.
Conclusions
LIAISON XL analyzer is at most flexible, user‐friendly and time and cost effective system with high‐quality test menu well suited for blood donors screening for infectious markers.
P‐372
Evaluation of Syphilis Screening for Blood Collected at Dar El‐Salam Regional Blood Transfusion Center, Cairo, Egypt
Farouk Badr R1, Farouk R 1, Ahmed Ali A 2
1Dar El‐ Salam Regional Blood Transfusion Center, Cairo, Egypt, Cairo, Egypt2National Blood Transfusion Services, Cairo, Egypt
Background
Provision of safe and effective blood and blood components intended for transfusion or manufacturing involves a diverse set of processes including: blood donors’ recruitment, blood collection, processing, and testing. Accurate screening of donated blood for Transfusion Transmissible Infections (TTIs) is one of the main processes to ensure blood safety. Therefore, blood transfusion services should establish and implement specific TTIs screening algorithms using efficient testing technology that takes into consideration the TTIs epidemiology status in the community/country, the needs, standards, infrastructure and resources of each country. A well designed algorithm can contribute significantly to improvement in blood safety, timely availability of blood, and efficient use of resources.
Aim
The purpose of this study is to evaluate the suitability of syphilis screening algorithm and technology at Dar El‐Salam Regional Blood Transfusion Center, Cairo, Egypt. Typically, this algorithm of screening for syphilis in blood donors depends on using Enzyme‐linked immune sorbent assay (ELISA) technique as an initial screening test (including duplicate testing for initially reactive samples) and Treponema pallidum hemagglutination test (TPHA) as a confirmatory test for repeatedly reactive samples.
Methods
This retrospective cross‐sectional study was carried out on the data retrieved from the blood screening records of Dar El‐Salam Regional Blood Transfusion Centre, Cairo, Egypt, for the period from the 1st of January 2013 through 31st of December 2014. All the donors during this period were selected based on the Egyptian blood donors’ selection criteria. Blood samples from collected units were initially screened for syphilis Ab sero‐reactivity using ELISA technique. All initially reactive samples were repeated in duplicate using the same ELISA. Only repeatedly reactive samples were sent for confirmatory testing using TPHA test for detection of specific antibodies to T. pallidum.
Results
A total of 33,921 donors were screened using ELISA during the study period, out of this number 235 (0.69%) were initially reactive to syphilis, and only 93 (0.27%) were repeatedly reactive after duplicate ELISA testing. Out of the 93 yield of ELISA repeatedly reactive samples, only 22 (0.06% of the total donors) were confirmed reactive using TPHA. This indicates that 71(almost 76%) of ELISA repeatedly reactive samples were proven to be non‐reactive using the TPHA test for confirmation.
Summary/Conclusions
The results clearly reveal that a considerable number of syphilis Ab reactive samples using EILSA were confirmed to be non‐reactive using TPHA as a confirmatory test for reactivity. This could be due to a variety of technical reasons. However, it highlights how critical is the selection process of initial screening assays. Accurate screening results depend on the consistent use of screening assays fulfilling the desired performance criteria, more precisely the balance between sensitivity and specificity, and designing a reliable testing algorithm fitting with different variables.
It is recommended to research in depth the exact reason behind any recorded false reactivity of screening test then to adapt the syphilis screening algorithm/technique accordingly, this would account for higher levels of cost‐effectiveness and ensure timely release of blood units by minimizing any delay associated with ELISA repeatedly reactive samples.
P‐373
Abstract Withdrawn.
P‐374
Detection of Treponema Pallidum DNA Using Real‐Time PCR in Blood Donors
Widuri S 1,2, Karuniawati A 3, Chunaeni S 1,2, Yasmon A 3, Aryati4
1Blood Transfusion Services2Red Cross, Surabaya – East Java, Indonesia3Department of Microbiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia4Department of Pathology Clinic, Soetomo Hospital, Surabaya, East Java, Indonesia
Background
Syphilis, a chronic and systemic disease, is caused by Treponema pallidum bacteria. During the course of the disease, syphilis can affect all organs of the body although there is a latency period without manifestations of lesions. Syphilis can be transmitted through sexual contact, blood transfusion and from mother to child (congenital syphilis). Anti‐Treponema pallidum IgM antibodies are produced after 2 weeks, followed by IgG antibodies 2–4 weeks post‐infection and in general patients are diagnosed using a serological test. This serological ELISA test is able to detect Treponema pallidum IgG and IgM antibodies specifically. Positive ELISA test results can lead to rejection of donor blood due to the presence of antibodies, even though this blood is not infectious anymore, since bacteria are absent. In case of primary syphilis, false negative results might occur because of this window period or due to the presence of antibodies from previous syphilis infections.
Aims
This study aims to determine the prevalence of antibodies against Treponema pallidum and therefore assess the transmission risk of syphilis in donated blood.
Methods
We used a Real-time PCR method for the specific detection of the PolA Treponema pallidum gene, which does not cross‐react with other bacteria. We analysed 350 blood samples from blood donors by both ELISA and real-time PCR methods to measure the presence of Treponema pallidum antibodies and DNA respectively.
Results
Detection of Treponema pallidum DNA by using Real-time PCR resulted in respectively 11.71% reactive and 88.29% non‐reactive blood samples. 5.71% of blood samples however, were found non‐reactive for antibodies against Treponema pallidum after serology testing while they showed positive for Treponema pallidum DNA.
Summary/conclusions
In the blood donor population tested (comprised 350 donors), we found that 11.71% of blood samples showed positive for Treponema pallidum DNA by the real-time PCR assay used. And following blood transfusion, 5.71% of recipients are still at risk for syphilis transmission due to insensitivity of the current serology assay used while the real-time PCR was able to detect Treponema pallidum DNA in these samples. These findings suggest that the real-time PCR assay is more sensitive than the current serology test and PCR techniques have therefore great value for the diagnosis of primary syphilis.
P‐375
Prevalence of Human Herpesvirus‐8 (HHV‐8) DNA Among South American Blood Donors: Lack of Association With the Genetic Ancestry of the Population
Torres OW 1, Hulaniuk ML 2, Bartoli SB 3, Fortuny LF 4, Burgos Pratx LBP 4, Frías FA 1, Nuñez NF 4, Corach CD 5, Caputo CM 5, Trinks TJ 6
1Hospital Materno Infantil Ramón Sardá, Buenos Aires, Argentina2Instituto de Ciencias Básicas y Medicina Experimental (ICBME), Hospital Italiano, Buenos Aires, Argentina3Servicio de Medicina Transfusional, Hospital Pablo Soria, San Salvador‐Jujuy, Argentina4Servicio de Medicina Transfusional, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina5Servicio de Huellas Digitales Genéticas.), Fac. de Farm. y Bioq.CONICET, Buenos Aires, Argentina6Inst de Ciencias Básicas y Med. Experimental. Hosp.Italiano‐ CONICET, Buenos Aires, Argentina
Background
HHV‐8 has been postulated as a new infectious agent for screening in blood donors. The viral spread among human populations varies widely with a striking geographical and ethnic distribution pattern described by its seroprevalence rates; for example, HHV‐8 is 10‐fold higher among Native Americans than among non‐native populations. Little is known, however, of the prevalence of HHV‐8 DNA in South American countries and its association with the highly admixed genetic ancestry of the population.
Aims
To determine ‐for the first time‐ the prevalence of HHV‐8 DNA among healthy blood donors from different South American populations and its association with the genetic ancestry.
Methods
After signing an informed consent statement upon enrolment, DNA was obtained from 667 unrelated blood donors, whose serum samples were routinely tested for transfusion transmission infections rendering negative results. Volunteers were grouped as Argentines from the metropolitan (n = 200) and North‐western (n = 106) areas, Bolivians (n = 187), Paraguayans (n = 98) and Peruvians (n = 76). The presence of HHV‐8 DNA was determined by a nested PCR protocol for partial amplification of ORF26.In 319 randomly‐selected samples (107 from the metropolitan region, 48 from North‐western Argentina, 57 from Bolivia, 55 from Paraguay and 52 from Peru), ethnicity was assessed in both maternal and paternal lineages by analysis of haplogroups in mitochondrial DNA and Y chromosome polymorphisms using real‐time PCR followed by High Resolution Melting. Chi‐square and Fisher's exact tests were used for statistical analysis. A P‐value of <0.05 was considered as statistically significant.
Results
HHV‐8 DNA was significantly more prevalent among blood donors from North‐western Argentina (24.5%) than among Argentines from the metropolitan area (3.5%), Bolivians (3.2%), Peruvians (6.6%) and Paraguayans (7.1%) (OR 8.45, 95% CI 3.47–20.56, P < 0.0001). There were no significant differences in the viral prevalence between men and women of the studied populations, although a possible high risk was observed among Bolivian men (P = 0.051). HHV‐8 infection was more prevalent among metropolitan Argentines under the age of 30 years (P < 0.05). Interestingly, North‐western Argentines older than 30 years old were more commonly infected than blood donors within the same age group from the other populations of this study (P < 0.03). Significant differences were observed in the ancestry components of the studied populations. When compared with blood donors from North‐western Argentina, Bolivia, Peru and Paraguay, the prevalence of Native American maternal and paternal haplogroups was the lowest for metropolitan Argentines(P < 0.01). However, no statistical significant differences were found when analysing the relationship between HHV‐8 DNA prevalence and genetic ancestry.
Conclusions
The results presented herein indicate that HHV‐8 infection is highly endemic in North‐western Argentina. The variation in the distribution of HHV‐8 in South America remains intriguing and may be associated with specific risk factors or behaviours, but not with the genetic ancestry of the population. In the present study, all HHV‐8 DNA positive samples were non‐reactive to all other infectious agents tested in blood banks and consequently these blood units were available for transfusion. Special caution should be taken with immunosuppressed recipient patients who are prone to acquire infections and develop the HHV‐8 associated diseases.
P‐376
Comparison Between the Prevalence of Hbsag in Voluntary Non‐Remunerated Blood Donors and Family Replacement Donors Screened in the Serology Department in the Egyptian National Blood Transfusion Center
Abbas N , Ekram D
NBTC Egypt, Cairo, Egypt
Background
Transfusion transmitted infections (TTIs) is a major challenge to the transfusion services all over the world. The problem of TTIs is directly proportionate to the prevalence of the infection in the blood donor community. Currently, prevention of (TTIs) depends upon proper pre‐donation selection of all voluntary non remunerated blood donors. The Pre donation questionnaire is considered the first line of defence against transfusion transmitted infections. mainly covering the history of diseases, dental procedures or tattooing, history of bilharziasis, family history of hepatitis and engagement in high‐risk behaviour e.g. homosexuality, intravenous drug abuse. Donors in the National Blood Transfusion Centre are regular voluntary non‐remunerated blood donors while in other governmental hospital blood donations depend mainly on family replacement donations which are screened in the national blood transfusion centre as a part of the centralization screening plan applied since 2008. This study aims to emphasize the importance of proper donor selection criteria to enhance blood safety through comparing between the prevalence of HBsAg in voluntary non‐remunerated blood donors and family replacement donors both screened in the Serology Department in the NBTC.
Methods
Study setting: The study took place in the Egyptian National Blood Transfusion Center (NBTC) which is the headquarters of the Egyptian NBTS. The study was conducted on all voluntary non remunerated donors and all the family replacement donors received from hospital blood banks screened in the NBTC
during the period from January 2014 to December 2014. Screening was done using EIA HBsAg.
Study design
A cross sectional retrospective study design used to examine statistical records (form F/NBTS/SD/037/01) of the NBTC over a period of one year for both NBTC donations and referring hospitals donations.
Results
A total of 55,154 donations comprised the study group. NBTC donation were 41,435 in total over one year. Referring hospitals donations were 13,719 in total over the same year. The total number of repeatedly reactive & confirmed positive donations for HBV in NBTC was 235 (0.5%), while in referring hospitals the total number of repeatedly reactive & confirmed positive donations for HBV was 167 (1.2%).
Conclusion
Many potentially HBV infectious donors were eliminated due to high selection criteria of the blood donation questionnaires together with the proper standardized testing procedures for TTIs. The prevalence of HBV among Voluntary non‐remunerated blood donors is less than its prevalence among family replacement donations emphasizing the importance of working on moving towards increasing the scale of voluntary non remunerated blood donors over family replacement donations as a crucial step for minimizing the risk of TTIs.
P‐377
Transfusion Transmissible Infections in Voluntary Non Remunerated Donors in Southern Nigeria
Ikusemoro AI1, Isoa EM 2, Borke ME 1, Nwagu MU 1, Olajide I 1
1DELSUTH Oghara, Benin city, Nigeria2Irrua Specialist Teaching Hospital, Irrua, Edo State, Irrua, Nigeria
Background
There is the need to ensure adequate screening of blood donors for transfusion transmissible infections (TTIs). This is essential to continue to maintain safe blood transfusion for the local population. The goal of any transfusion service is to provide adequate and safe blood and blood products that meet the needs of patients in the environment. Blood safety interventions in the developed nations have greatly reduced the overall risk of transfusion‐ transmitted infections (TTI). In Nigeria however, the blood transfusion service is at its teething stage and is not yet fully equipped to cater for the extensive screening tests obtained elsewhere despite the high burden of such infections.
Safe Blood for Nigeria Foundation (SBNF) is a Non Government Organization established 3 years ago situated in Benin city, Edo State. Edo state is one of the 36 states of Nigeria located in the South‐South geographical region of the country. SBNF is aimed at ensuring blood availability and safety through voluntary non remunerated blood donation.
Objective
The aim of this study was to estimate the prevalence of transfusion transmitted infections in voluntary non‐ remunerated blood donors at a blood mobile unit owned by Safe Blood for Nigeria Foundation situated in a city center in Benin city, Nigeria. This was from January 2014 to December 2014
Methods
All voluntary donors reporting to the blood mobile unit were screened for HBsAg, Hepatitis C Virus (HCV), HIV and Syphilis by using the rapid diagnostic testing (RDT), HIV was tested using Determine HIV 1/2 manufactured by abbott laboratories, appropriate enzyme‐linked immunosorbent assay. The study was designed for a duration of one year between January 2014 to December 2014.
Results
A total of 955 units of blood were collected from a study population with a mean age of 23 years and male donors (76.9%) more than females (23.1%). The seroprevalence of TTIs were HBV 3.1%, HCV 3.1%, HIV 2%, Syphilis 2.3%.
Conclusion
The prevalence of TTIs among voluntary non remunerated donors in our locality is low but still significant. We must ensure continuous testing and vigilance to provide safe blood for our population
Key words
Voluntary donors, Safe blood, Infections, Nigeria
P‐378
Abstract Withdrawn.
P‐379
Non‐Invasive pH‐Measurement – a Promising Approach for Monitoring Bacterial Growth in Platelet Concentrates
Sicker US1, Jamrad ZJ 1, Schurig US 1, Spindler-Raffel ESR 1, Karo JOK 1, Brachert JB 1, Beshir RB 1, Hägel WH 1, Becker BB 1, Weustenfeld SW 2, Volgmann TV 2, Müller TM 2, Spreitzer IS 1
1Paul‐Ehrlich‐Institut, Langen, Germany2Red Cross Blood Transfusion Service, NSTOB, Springe, Germany
Background
Determination of pH in Platelet Concentrates (PCs) is an established parameter for quality control in blood transfusion services. It is well known that the drop in pH value may be triggered by the increase of CO2 due to microbial growth. In previous studies (poster P‐693, Montag et al: Time Course of pH in Platelet Concentrates after Bacterial Contamination, 18th ISBT Congress 2008, Macao, China) the proof of this principle was already shown for PCs: in all so far tested microbial strains (Streptococcus pyogenes, Staphylococcus aureus, Klebsiella pneumoniae, Staphylococcus epidermidis, Bacillus thuringiensis [isolated spores], Escherichia coli and Candida albicans) an obvious drop in pH value occurred after a sufficient microbial count was reached in the preparation.
Aims
In this study the suitability of BCSI pH1000TM for monitoring PCs during the five days storage period and addressing bacterially contaminated PCs was evaluated and expanded with four more bacterial strains.
Methods
Pooled PCs (PPC) were spiked with low levels of four different platelet transfusion relevant bacteria species (Bacillus thuringiensis [isolated spores], Morganella morganii, Serratia marcescens, Enterobacter cloacae) in order to confirm the conclusion of previous pH studies (s. poster ISBT 2008). In defined intervals the bacterial count was determined by streaking samples on agar plates in order to picture the strain specific growth curve in PCs. Additionally, pH was measured at the same time points with the BCSI pH1000 (Blood Cell Storage Inc. (BCSI), Seattle, USA), and negative controls were processed in parallel.
Results
In the former study the suitability of the BCSI 1000 method could be shown with six bacteria strains and one yeast strain. The extension with four further strains (as mentioned above) showed similar results. All species showed a characteristic growth curve with subsequent drop, followed by a re‐increase in pH. It could be verified that microbial growth can be monitored by non‐invasive continuous measuring of pH value in PCs.
Summary/conclusions
Non‐invasive pH‐measurement is a suitable method for microbial screening of PCs and could be a useful tool for blood transfusion services to prevent the transfusion of contaminated PCs, if the pH measurement is conducted continuously and automatically to record the occurring pH drop in contaminated PCs.
P‐380
Prevalence of Transfusion Transmitted Infectious Diseases Markers in Macedonian Blood Donors‐A Comparrison Study
Nikolova JN , Makarovska-Bojadzieva TM , Velkova VE , Blagoevska BM , Samonikov-Tosevska JST , Damevska-Todorovska ODT
Institute of Transfusion Medicine, Skopje, Macedonia
Background
Transfusion transmissible infections (TTI) testing is one of the cornerstones of blood safety. Accurate estimation of the prevalence of TTI markers is essential for monitoring of the donor selection process as well as for the efficacy of the currently employed screening techniques.
Aim
To estimate the seroprevalence of mandatory tested TTI markers in blood donors in the period from 2013 to 2014 and to compare it with the estimated seroprevalence for the period from 2007 to 2012.
Methods
Blood samples were screened for HBsAg, anti‐HCV, anti‐HIV‐combo (assay for the p24 antigen and for HIV‐1/2 antibodies) and Syphilis (TP) using serological assays (EIA‐ Enzygnost ‐Siemens and CMIA‐ Architect system ABBOTT). Repeatedly reactive samples were retested with confirmatory assays: Vidas HBsAg Ultra and Immunoblot Deciscan HCV Plus.
Results
The total number of 63,764 and 145,800 blood samples were TTI markers tested in the period 2013–2014 and 2007–2012 respectively. The total number of: initially reactive (IR), repeatedly reactive (RR) and confirmed positive (CP) samples are shown on Table 1.
According to the results, the overall prevalence rate of HBV and HCV is increased from 0.23% to 0.37% and from 0.04% to 0.06% respectively. Also, we have estimated increased number of HIV and TP repeatedly reactive samples in the analyzed period.
Table 1. Prevalence of TTI markers in blood donors.
Conclusion
Despite of the decrement of the family donors from about 7% to 1% and the decrement of first time donors from about 20% to 10%, the prevalence of HBV and HCV has increased in blood donor population. According to our opinion, this is due to the changes and adaptation of our algorithm for TTI markers screening and confirmatory testing to the 17th Edition of Guide to the Preparation, Use and Quality Assurance of Blood Components (Council of Europe). We also think that these results provide more accurate information about seroprevalence rates of TTI markers among our blood donors.

P‐381
The Selection of Hepatitis B Core Antigen Antibodies Free Donor's Cohort
Tupoleva TA , Gulyaeva AA , Tikhomirov DS , Romanova TY , Filatov FP , Gaponova TV
National Research Center for Hematology, Moscow, Russian Federation
Background
Blood transfusion safety is essential for clinical transfusiology especially for multiple transfusions recipients. In Russian Federation hepatitis B virus (HBV) incidence remains high. The prevalence of the occult HBV reaches 2% among blood donors. Additional testing of donated blood for HBV core antigen antibodies (a‐HBc) improves viral safety by revealing of conceal infected donors. It is especially relevant for recipients of multiple transfusions from a large number of donors.
Aims
Assess the impact of a‐HBc positive donors’ dismissal on the total amount of donors.
Methods
Blood samples of 10,147 donors were screened for a‐HBc by Biorad Monolisa a‐HBc Plus and Abbott Anti‐HBc II. Among them 3920 (38.63%) were first‐time donors (FTD), 6227 (61.37%) – regular donors (RG). For statistical calculations of Pearson χ2 or Fisher's exact test was used EPI5 ver. 5.0 software.
Results
Among all blood samples 383 (3.77%) were a‐H�’c positive. In FTD a‐HBc rate was statistically significant higher than in RG (5.31% vs 2.81%, P = 0.000001, χ2 = 41.26). All a‐HBc positive donors were dismissed from blood donation. Monitoring of a‐HBc detection during 10 months presented in the Table 1. The monthly number of FTD and RG varied slightly. Implementation of the dismissal of a‐HBc positive donors reduced the overall incidence of a‐H�’c detection in RG from 8.8% to 0.2% (P = 0.00001), but not in FTD (overall incidence of a‐HBc varied from 3.6% to 7.8%, P > 0.05).
Table 1. The selection of donors by a‐HBc testing during ten months

Conclusions
A cohort of regular a‐HBc negative blood donors was selected. The dismissal of a‐HBc positive donors did not lead neither to the loss of amount of donors nor to the reduction of harvested production volume.
P‐382
Further Development of Hepatitis B and C Testing in Blood Donors in Tirana
Seferi I1, Abazaj Zh 1, Metka A 1, Xhetani M 2
1National Blood Transfusion Centre, Tirana, Albania2Faculty of Natural Sciences, Tirana, Albania
Background
Hepatitis investigation in Tirana NBTC consists in testing every blood donation for HBsAg and anti‐HCV with CMIA ABBOTT ARCHITECT. There is a high overall prevalence of hepatitis B in blood donors in Tirana 4.94%, whereas the prevalence of hepatitis C is 0.67%.
Aims
The aim of the study was to evaluate other infectious markers for hepatitis B and C, and to see if it is effective to implement them in routine blood testing for the detection of occult hepatitis B or early stage hepatitis C, since the implementation of NAT is very expensive, for resource limited countries.
Methods
Of 9000 donations during 2014 were tested additionally to routine testing for HBsAg, anti‐HCV, HIV Ag/Ab and Syphilis, also for anti‐HBc (IgG+IgM), anti‐HBs and HCVAg. All donations that resulted HBsAg negative, anti‐HBc (IgG+IgM) positive and anti‐HBs negative or positive with titer less than 100 mIU/ml, were additionally tested only with anti‐HBc IgM in order to more exactly evaluate the presence of an occult hepatits B. For hepatitis C the additional testing was HCV Ag.
Results
There were 19 donations which resulted HCV Ag positive but in all cases this was associated with anti‐HCV. There were only two grey zone results for HCV Ag with no accompanying antibodies for hepatitis C. One of this donors has been traced and recalled for testing which resulted negative for antibodies to hepatitis C after three and six months from the donation that was HCV Ag grey zone. We still have not traced the second one. There were 1700 donations (18.8%) that resulted HBsAg negative with anti‐HBc (IgG +IgM) positive and anti‐HBs either negative or titer less than 100 mIU/ml. All these 1700 donations were additionally tested for anti‐HBc IgM but none of them resulted positive.
Summary/Conclusions
Based on our data it is very difficult to evaluate occult hepatitis B by using anti‐HBc and anti‐HBs because it results in a very high number of donors lost (18.8%), therefore the only way for detecting occult hepatitis B in countries with high prevalence of hepatitis B remains implementation of NAT. Further investigation needs the implementation of HCV Ag in our country for the early detection of hepatitis C.
P‐383
Comparison of Different Techniques Used in a Transfusion Transmitted Infection Laboratory in the Blood Bank
Ranganathan S1, Iyer N 2, Sesikeran S 1
1Apollo Hospital, Hyderabad, India2Global Hospital, Hyderabad, India
Background
Serological assays used in the blood banks for the detection of potential Transfusion Transmitted Infections (TTIs) based on chemiluminescence technology are highly sensitive and may give biological false positive reactions .This may lead to discard of blood and unnecessary counselling of blood donors about their seropositive status with psychosocial implications. Nucleic Acid Amplification assay (NAT), recently introduced for blood screening has shown to improve blood safety by identifying additional NAT yield defined as seronegative but NAT reactive blood donations.This is a pilot study comparing different blood screening techniques used in the TTI laboratory in the blood bank.
Aims
1. To observe the NAT yield in a hospital based blood transfusion centre
2. A pilot study to compare positive results between serology, NAT and RT PCR (Cobas Taqman assay)
Methods
A total of 36,683 blood donations were screened for HBsAg,anti HCV,HIV 1&2 (antigen and antibodies)by a serological assay using the chemiluminescence principle (ECiQ, J&J/Architect, Abbott) over a period of three years between 2012–2014. Of the 36,683 donations screened, 36,214 seronegative donations were subjected to NAT in minipools of six using the Cobas Taqscreen MPX v1.0 (Roche Diagnostics)from January 2012‐August 2014,subsequently on the Cobas MPXv2.0 since September2014.The Cobas MPX v2.0 uses multi‐dye technology which is able to discriminate and identify the individual viral targets upon resolution of a reactive pool.
A pilot study was carried out to compare ten HBsAg sero‐ positive, five anti‐HCV and two anti‐HIV 1& 2 sero‐ reactive specimens with NAT. Donations with discrepant results between serology and NAT were further subjected to Western blot for HIV 1 and 2 (interpretation as per WHO Guidelines) and RTPCR for HCV RNA(as per CDC HCV 2013 Guidelines).
Results
Fifteen donations among the 36,214 seronegative blood donors were reactive by NAT giving a NAT yield of 1:2000 (0.04%). All these NAT positive cases were reactive for HBV on resolution .Ten samples which were HBsAg positive were also positive for HBV using NAT and as such there were no discrepancies between the serology by chemiluminiscence and NAT assays.
Out of five samples reactive by chemiluminiscence for anti‐HCV, two were positive by NAT and confirmed on the RT PCR assay, which shows that these two donors could have had ‘ current HCV infection’ and the other three which were negative on NAT and RT‐PCR may be classified as ‘ no current infection’ according to the CDC HCV guidelines .Out of two samples reactive for anti‐HIV ‐1 and 2 on chemiluminiscence, both samples were non‐reactive by Western blot and negative by NAT, showing that they were negative for HIV infection.
Conclusions
NAT technology may be helpful in identifying truly positive donors for HIV, HBV & HCV, besides improving blood safety. This is an initial attempt at our centre in comparing NAT with RTPCR and serology for its performance. This will hopefully pave the way for improved counseling of NAT reactive blood donors in the future.
P‐384
False Positive Results of Hbsag and Anti HCV in Donated Blood Units in the Transfusion Medicine Service in Gevgelia
Balamovski M , Ortakovska S
Institute of transfusion medicine, Skopje, Gevgelia, Macedonia
Introduction
In all Institutions for blood donation in the Republic of Macedonia, each blood unit donated by voluntary blood donors (VBD) is controlled obligatory on markers for virus transmitted diseases: HBsAg, anti HCV‐ab, anti HIV 1/2 and anti Treponema pallidum. From different reasons certain number of the positive results on the markers is not truly positive. Due to this, it is necessary to confirm which of them are truly positive, and which are false positive.
The aim of this paper is to realise and analyse the significance and risk of correct distinguishing between the false positive results and truly positive results on HBsAg and anti HCV‐ab markers for safety usage of blood and blood components received by the VBD's in our Institution.
Material and methods
In the period from 1990 to 2014, 14,652 blood units were controlled. The control of HBsAg was made at the beginning with Hemaglutination method, and later with Enzyme Immuno techniques from second and third generation. The control of anti HCV‐ab was made only with Enzyme Immuno Assay (EIA) from second ant third generation.
Results
Of 241 (1.64%) blood units were HBsAg positive and 114 (0.78%) were anti HCV‐ab positive from the controlled 14,652 blood units. All VBD's with positive results were controlled with new samples of blood for control, with double and independent control, with two different techniques in two health Institutions. During the re‐examination of the new samples of blood from the same donors whose results were previously positive, again, 209 (1.43%) were HBsAg positive and 85 (0.58%) were anti HCV‐ab positive. From the total amount of 14,652 tested blood units, false positive to HBsAg were 32 (0.22%) and to anti HCV‐ab were 26 (0.18%) blood units.
Conclusion
According to our knowledge that we got from the examinations, possible reasons for false positive results on the HBsAg and anti HCV‐ab markers in the blood donors are: cognate antibodies and antigens with those that are examined, disregarding of technical guidance for the conditions and procedures of working with the equipment and reagents for testing.
m_balamovski@yahoo.com
4.2 Hepatitis B (HBV)
P‐385
Detection and Identification of Occult Hepatitis B Virus Infection Among Blood Donors
Kumar RK , Kaur AK , Jain AJ
Dayanand Medical College and Hospital, Ludhiana, India
Background
Occult Hepatitis B Virus infection (OBI) is defined as the presence of circulating hepatitis B virus DNA as detected by HBV Nucleic Acid test (NAT), in the absence of detectable HBV surface antigen (HBsAg),with or without antibodies to hepatitis B core antigen (anti‐HBc) or hepatitis B surface antigen (anti HBs). HBV infection is a continuing threat to transfusion safety, especially in developing counties like India where detection of HBV is primarily based on screening for HBsAg as a marker of infection. Amongst the chronically transfused patients such as thalassemia the prevalence of HBV is 33% while in blood donors population is 1.2%.
Aims
A study was conducted to know the prevalence of OBI among blood donors and their serological and molecular characterization of NAT yield samples.
Methods
A total of 41,090 blood donor's samples from February 2013 to August 2014 were tested by ID‐NAT apart from routine serological screening for anti HIV 1–2, P24 antigen, anti HCV and Hepatitis B surface antigen (HBsAg) by Biomerieux (Vironostika® HIV Ag‐Ab, Hepanostika® HCV ultra and HBsAg ultra, France). All the samples were tested individually by Procleix® Utrio Plus® Assay (Novartis Emeryville, CA). Blood units which were HBsAg non reactive but ID‐NAT reactive (NAT yield) were further worked up with anti HBc, anti HBsAg, viral DNA load and viral genotyping. Serology and ID‐NAT were done on the samples from the pilot tubes initially (serum, plasma) followed by repeat testing from the blood bag. Individual donation (ID‐NAT) of the tube sample was tested as singlet; reactive results were further studied using the India user algorithm.
Results
Out of 41,090 samples, 29 were reactive for HBV DNA (NAT Yield). Among these 29 NAT Yields, 24 were individual NAT yield, 04 were HBV‐ HCV NAT Co‐yield and 01was HBV‐HIV NAT Co‐ yield. 24 HBV individual NAT yield samples were further tested for OBI. 14(58.3%) out of 24 NAT yield samples were reactive for anti‐HBc and 8 out of 14 having anti‐HBc had antibody against HBsAg. 2 out of 14 core antibody reactive samples has genotype D HBV infection and 3 samples had viral load tests less than 20 IU/ml. 10 out of 24 samples did not have any serological marker of HBV. 14 donors were OBI and 10 were in window period of HBV infection.
Conclusion
Blood productsfrom donors with OBI carry a high risk of HBV transmission by transfusion. Due to multiple antigen and antibody present in blood in response to HBV infection, at present there is no single test to detect the infection. In developing country like India with high seroprevalence of HBV infection, combination of at least two tests would be help us to improve the transfusion safety.
P‐386
Comparable Method for Confirm Hbsag Infection in Donated Blood
Chaiwong K
National Blood Centre, Thai Red Cross society, Bangkok, Thailand
Background
Serological testing of donated blood for hepatitis B virus infection began in 1986 at the National blood centre, Thai Red Cross society, using reverse passive haemagglutination (RPHA). The chemiluminescent microimmunoassay (CMIA) method was adopted in 2011. HBsAg screening required initial reactive (IR) samples to be re‐tested in duplicate for repeat reactivity. At least two reactive results from three tests were required to interpret samples as repeated reactive (RR), leading to sample rejection and donor deferral. Between 2012 and 2013, HBsAg IR and RR rates were observed to be 0.43% and 0.37% of total samples respectively. However, some of these deferred donors would subsequently test negative in follow‐up testing, complicating donor counseling. We evaluated HBsAg neutralization assay in parallel with routine duplicate testing to improve the specificity of HBsAg screening.
Aims
Evaluation of HBsAg neutralization within a HBsAg screening algorithm to optimize sample release and donor management.
Methods
Donor samples were screened for HBsAg with Abbott ARCHITECT HBsAg Qual II assay. The IR samples were re‐tested in duplicate to confirm RR, and the Abbott ARCHITECT HBsAg neutralization assay was tested in parallel. Neutralization results were categorized as positive (NP) if greater than 50% is reported; not confirmed (NC) if less than 50% reported and not applicable (NA) for samples without neutralization. The NC and NA samples were tested for HBV DNA (Roche Cobas MPX v2.0, Cobas s201 system), total anti‐HBc and anti‐HBs (both Abbott ARCHITECT). Statistics were calculated using MS Excel.
Results
Screening of 148,781 donor samples using HBsAg Qual II assay produced 630 (0.42%) IR results, of which 94% (594/630) were RR and 6% (36/630) were non‐reactive. In follow‐up HBV testing of deferred donors the sensitivity and specificity of RR testing was determined to be 100% and 23% respectively. When IR samples were tested for HBsAg neutralization, 77% (483/630) were neutralization positive. The remaining 33% (147/630) of IR samples were NA (81/147) or NC (66/147) and so were further tested for total anti‐HBc antibody and HBV DNA. Anti‐HBc was detected in 6.3% (40/630) IR samples, and HBV DNA was detected in 0.5% (3/630) IR samples. The sensitivity and specificity of HBsAg neutralization among IR samples was 92.35% and 100% respectively. Among repeat reactive samples, we observed a significant association of high HBsAg values (S/CO > 1000) with neutralization (P < 0.0001).
Conclusions
Compared to duplicate repeat testing, HBsAg neutralization assay improved the specificity of IR sample testing from 23% to 100%. Up to 12% more IR samples could be reported HBV negative and 71 donors avoided deferral, improving issuance of blood units and reducing donor deferral rates. With the significant relationship between high HBsAg S/CO and neutralization, the HBsAg testing algorithm was conservatively modified to apply HBsAg neutralization assay only to samples with S/CO<1000. Samples reporting NC or NA results would then be confirmed with NAT. Following this change in HBV screening algorithm, TRC was able to issue 2715 more blood components in 2014 and realized a calculated increase in income of 1,089,300 baht and savings of 86,730 baht in reagents cost.
P‐387
Enhancing Sensitivity of HBV DNA Confirmatory Testing to Discriminate Between True and False Repeat Reactive Nat Screening Results
Grabarczyk PG1, Kopacz AK 1, Liszewski GL 1, Kubicka-Russel DKR 1, Sulkowska ES 1, Kleinert AK 2, Roth KR 2, Lelie NL 3
1Institute of Haematology and Transfusion Medicine, Warsaw, Poland2GFE Blut mbH, Frankfurt, Germany3Lelie Research, Paris, France
Background
HBV DNA screening in Poland is performed in the regional blood banks by individual donation testing (IDT) or in minipools of 6 (MP6). Repeat reactive donations in IDT are subjected to a confirmation test procedure in the reference laboratory (IHTM, Warsaw). In 2013 we failed to confirm presence of HBV‐DNA by single TMA testing in 10/26 (38.5%) of anti‐HBc (and anti‐HBs) reactive donors who were likely occult carriers with extreme low viral load (VL).
Aim
Enhancement of the sensitivity of HBV‐DNA confirmatory testing in HBsAg‐negative, NAT screening reactive donations
Material and methods
NAT screening in 2013 and 2014 was performed in IDT using Ultrio Plus (UP) or Ultrio Elite (UE) (95% LOD 4.7 and 4.6 IU/ml) and in MP6 with MPX test v2 (95% LOD – 2.9 IU/ml). MP6 resolved and ID‐NAT repeat reactive donations were directed to IHTM for confirmation. Until July 2014 the procedure included single TMA and discriminatory testing using the Ultrio Plus (UP) or Elite (UE) assays and supplementary testing for anti‐HBc and anti‐HBs. Ten donations in which we have likely failed to confirm low VL HBV infection were further tested in replicate UE, dHBV (5x), GFE PCR assay (3x), and enriched GFE PCR(4x) using samples taken from the fresh frozen plasma (FFP) unit. In July 2014 a new confirmatory procedure was implemented based on duplicate testing with dHBV(UE) and if nonreactive followed by triplicate GFE PCR.
Results
Replicate TMA and real‐time PCR testing confirmed presence of very low VL in FFP units of 9/10 donors in whom HBV‐DNA was missed by single TMA testing (Table). High speed centrifugation of 5 ml plasma detected an additional two occult HBV infections (OBI) in 1 of 4 enriched assays. After implementing the combined replicate TMA and GFE PCR testing algorithm presence of HBV‐DNA was confirmed in 8/10 donations (5/5 identified by MP6 and 3/5 identified by IDT screening). In two anti‐HBc and anti‐HBs reactive (24 and 43 IU/l) donors presence of HBV‐DNA could not be confirmed, although OBI cannot be excluded since 2/4 UP replicate ID‐NAT screening tests were reactive in the regional blood center.
Caption 1: HBV‐DNA confirmatory test results (reactives/tested) – comparison of Real‐time PCR, enriched PCR (both GFE Blut) and replicate Ultrio Elite assays

Conclusion and discussion
Combined replicate TMA and PCR testing has improved sensitivity of HBV DNA confirmatory testing in HBsAg negative donations. High volume, high speed centrifugation only slightly increased the sensitivity of HBV‐DNA detection in the GFE PCR assay as was also observed by replicate testing of standard dilution series (data not shown). Multiple replicate testing based on both TMA and PCR in combination with supplemental serologic testing showed to be an effective confirmatory procedure to identify HBV infected donors with extreme low VL. One cannot exclude false repeat reactive ID‐NAT screening results if replicate NAT is nonreactive in FFP samples. However, if the donor is anti‐HBc (and anti‐HBs) positive in a low or intermediate HBV prevalence country like Poland a failure to confirm presence of HBV‐DNA may also be explained by lower sensitivity of HBV‐DNA detection in the FFP sample than in the primary screening tube.
P‐388
Pre‐Donation Screening for Hepatitis B Surface Antigen Reduced the Proportion of Blood Discards at Fort Portal Regional Blood Bank, Uganda
Mugume AM , Mukembo MM
Uganda Blood Transfusion Service, Kampala, Uganda
Background
Blood discards due to transfusion transmissible infections (TTIs) leads to significant waste of donated blood. Prior to July 2013, no blood donors were screened of TTIs before blood donation at the Uganda Blood Transfusion Service regional blood bank, Fort Portal, western Uganda. Evidence from data collected between October 2012 and March 2013 indicated that Hepatitis B virus was a significant contributor to the proportion of blood discarded (accounting for 40% of all blood discards) in addition to HIV (35%) and inadequate blood volume (12%), among other factors. We implemented a pilot intervention aimed at improving pre‐donation screening for hepatitis B surface antigen (HBsAg) to reduce the rate of blood discards due to Hepatitis B.
Aim
Reduce the proportion of blood discards at Fort Portal regional blood bank and minimize institutional costs.
Methods
The pilot project focused on reducing blood discards due to Hepatitis B virus at Fort Portal regional blood bank between July and October 2013. Eleven field staffs were trained on the use of a rapid strip for HBsAg tests. A total of 2419 blood donors were screened for HBsAg before donation. Data on blood donors screened before blood donation was entered into database and analyzed.
Results
The proportion of blood discards due to Hepatitis B in donated blood reduced from 40% in March to 23% at the end of October 2013. There was an un‐intended reduction on blood discards due to; HIV from 35% to 27% probably due to co‐infection, inadequate blood volume from 12% to 7.8% and expiry of blood from 4% to 0% due to strengthened blood collection and laboratory screening processes respectively.
Summary/Conclusions
Screening for Hepatitis B alone not only reduced the proportion of blood discards by 17% within 4 months but also minimized costs by USD1,468, made the final blood products safer and reduced blood discards due to HIV, Inadequate blood volume and expiry. These findings suggest a need for introducing free Hepatitis B vaccination to all regular blood donors to prevent costs due to future Hepatitis B blood discards.
P‐389
Significance of Anti HB Core Assay in Detecting Occult HBV Infection in Voluntary Blood Donors
Verma AV , Bazaz AB
Rotary Blood Bank, New Delhi, India
Background
Hepatitis B core antibody (Anti HBc) is the first antibody to appear following acute Hepatitis B infection and persist in high levels following resolution of infection and in chronically infected patients. A small number of Hepatitis B virus (HBV) carriers appear to circulate Hepatitis B surface antigen (HBsAg) at undetectable levels, and anti‐HBc may be the only serologic marker detectable in blood in these individuals. During the ‘window period’ when antigenemia with HBsAg has resolved and antibody to HBsAg (anti‐HBs) has not yet developed, HBc IgM antibody may be the only marker present.
Aim
To study the significance of HBc antibody screening in healthy voluntary donors to detect the Hepatitis B Occult infection. These donors were non‐reactive for HBsAg and HBV DNA.
Method
Highly sensitive enhanced chemiluminescence technology is being used for screening Anti HIV, Anti HCV, HBsAg and Anti HBc in all blood donor samples using Vitros 3600 Immunodiagnostics system. Further, to detect any sero‐negative ‘window period’ infected donor samples, all the samples were subjected to ID NAT.
Total of 14,220 voluntary blood donors were screened for all infectious disease markers. Samples, which showed ‘isolated HBc antibody ‘reactivity in VITROS were subjected to Biorad ELISA for HBc antibody which is based on indirect immunoassay, to rule out any false reactivity in VITROS. The donor samples which were ‘Non‐reactive’ in Biorad ELISA were excluded from the study. Samples, with confirmed ‘isolated anti HBc alone’ reactive in both were subjected to VITROS antiHBs assay to quantify the HBsAg antibody levels.
A total number of 25 randomly selected ‘Isolated anti HBc reactive’ samples with NAT Negative, HBsAg negative and Anti HBs Negative (<10 mIU/ml) donor samples were subjected to high sensitive HBV DNA PCR (HBV Viral Load) Quantitative assay with Cobas Taqman kit. This helped to identify if there is any low level of HBV DNA present in these samples of ‘isolated Anti HBc alone reactive’ donors, which is missed by NAT.
Results
Out of 14,220 Donors screened, 813 (5.7%) were found reactive for HBc antibody. All the 813 HBc antibody reactive samples were retested by ELISA, 493 samples were repeat reactive. These 493 samples were quantified for Anti HBs level using VITROS (Table 1)
Out of 85 samples, which showed ‘isolated HBc antibody’ reactivity with antiHBs <10 mIU/ml 25 samples were randomly selected and subjected to HBV DNA PCR (HBV Viral Load) Quantitative. Two samples (8%) showed the presence of HBV DNA which were missed by NAT. These samples with Occult HBV infection with very low level of HBV viral load showed the presence of ‘isolated HBc antibody’, which is the only serological marker to suggest HBV infection.

Conclusion
Anti HBcore antibody screening helps in detecting the Occult HBV infection in donors lacking detectable HBsAg and HBV DNA but having exposure to HBV infection. They are therefore potential source of HBV infection.
The VITROS Anti‐HBc Assay has the sensitivity of picking up the Occult HBV Donors which were missed by HBsAg assay and NAT test.
P‐390
Risk of Hepatitis B Infection Among Healthcare Workers: Policy Implementation by a Tertiary Healthcare Institution in India to Prevent It
Prashant Prashant A , Suryasnatadas S , Kumar D , Kumar P , Singh M
Jaypee Hospital, Noida, India
Background
Inspite of an availability of the safe, effective and affordable vaccine, hepatitis B infection is still the most common serious liver infection. It is caused by the hepatitis B virus (HBV) that can lead to cirrhosis. Most of the healthy adults who are exposed to the hepatitis B virus (HBV) recover on their own and develop protective antibodies. However, 10% of infected adults, 50% of infected children, and 90% of infected babies develop chronic hepatitis B infection. Health care workers who come into contact with human blood and body fluids are at an increased risk for exposure to the hepatitis B virus. The Centers for Disease Control and Prevention recommends that all health care workers who are exposed to blood or body fluids should be vaccinated against hepatitis B. The vaccine is safe and effective and can protect for a lifetime.
Aim
The aim of the present study was to find the level of protection among the healthcare workers at the time of joining by doing Anti‐HBsAb titer. The later was done amongst the nurses, doctors, housekeeping staff and general duty assistants. Those who were reported titer <10 μ/ml were started with hepatitis B vaccination and kept away for a month from a direct patient contact.
Methods
At the time of joining employees were informed about the study and its implementation. Three ml whole blood sample in red top vacutainer was collected. Consent was obtained from each employee for any addition tests, if required. At the time of sampling all the employees were asked to fill a format which had information about history of any previous exposure, needle stick injury and history of vaccination. Anti‐HBs antibody titer was done using antiHBs reagent on Vitros 3600 (orthoclinical diagnostics, JnJ, USA).Vaccine being provided is Engerix B (GSK Glaxo, Belgium). Testing of anti‐HBs antibody titer and vaccination in low titer individuals were offered free of cost.
Results
The data being presented here is for the duration of three and half months between the mid November 2014 to Feb 2015. Among the 694 subjects recruited during the study period we received duly filled registration format in 664 individuals only. We observed that only 65.8%(437/664) had history of previous vaccination only 60.8%(422/694) had titer above 10 miu/ml. Majority(87.1%, 237/272) were unvaccinated amongst those who had titer below 10 miu/ml.(Table 1). One fourth of (2/8) new joinees who had history of needle stick injury in past were found non‐immune to hep‐B infection (Table 2). Vaccination is being provided to all employees having titer below 10. The vaccine is being given in three doses over a 6 month period (0, 1, and 6 months).
Table 1: results of observation among the healthcare workers during the period of observation

Table 2: Immune status of healthcare workers who showed history of needle stick injury (N = 8)

Conclusions
Inclusion of anti‐HBS titer and hepatitis B vaccination to those who are unprotected are two important measures to reduce the exposure particularly in Indian scenario. Provision of anti‐HBs antibody testing and hepatitis B vaccination among the unprotected healthcare workers will increase the protection from the hepatitis B infection.
P‐391
Correlation Between Anti‐Cytomegalovirus IgM and IGG Antibodies in Iranian HIV Positive Patients
Tarabadi A1, Shaiegan M 1, Babaee G 2, Parsa N 3
1Institute for Research and Education in Blood Transfusiion Medicine, Tehran, Iran2Azad university karj, Tehran, Iran3National Institutes of Health, BethesdaI, Bethesda, United States of America
Background
Cytomegalovirus (CMV), a herpes virus, is the major cause of morbidity and mortality in the general population and an important pathogen in immune‐compromised hosts, including patients with AIDS, neonates, and transplant recipients. It is well documented that approximately 50% of the general population and 90% of HIV patients carry CMV. So, the aim of this study was to evaluate the status of IgM and IgG antibodies against CMV infection in Iranian HIV patients.
Material and methods
Serological testing of IgM and IgG anti‐CMV antibodies in 30 HIV positive patients were analyzed by ELISA technique. The patients ages ranged from 20 to 53 years old, compared to 30 healthy blood donors with HIV negative status as control ageing from 18 to 55 years old.
Results
IgM anti‐CMV antibody was presented in 7 (23.3%) patients, one (3.3%) borderline (doubtful), IgM antibody was not detected in controls (P = 0.0001). IgG anti‐CMV antibody was detected in all the patients and controls. There was a correlation between IgM anti‐CMV antibodies and HIV positivity (r = 0.874, P = 0.0001).
Conclusion
CMV co‐infection in HIV patients, has been proposed as a key factor in sustaining immune activation, which in turn could play an important role in determining immune senescence. As CMV disease may hurt many parts of the body, including GI tract, lungs and eyes, if it not treated; So, early detection of CMV antibodies appeared to be useful in an effective treatment of infected HIV positive patients.
Key word
HIV, CMV, IgG, IgM.
P‐392
Nucleic Acid Amplification Test (NAT) To Detect HBV Infection in Blood Donors
Allanki A
GLOBAL HOSPITALS, Hyderabad, India
Background
Nucleic acid amplification test(NAT) technology has the potential to detect viremia earlier than the current screening methods for blood donors, based on seroconversion i.e immunoassay. Immunoassays detect antibodies to viruses or viral antigens, however window period(WP) donations are missed. The detection of hepatitis B virus(HBV) in blood donors is achieved by screening for hepatitis B surface antigen(HBsAg), antibodies against hepatitis B core antigen(anti‐HBc), anti‐HBS and NAT for detecting HBV DNA along with human immunodeficiency virus(HIV) RNA and hepatitis C virus(HCV) RNA in a single multiplex assay which provides additional safety.
Methods
We performed NAT on Roche cobas s 201 system, using multiplex polymerase chain reaction(PCR) on 9316 blood donations, which were seronegative for HBsAg, anti‐HCV and anti‐HIV by enhanced chemiluminiscence method on vitros EciQ(Ortho‐Clinical Diagnostics).
Results
We identified 7 donors who were positive for HBV DNA(0.075%). None of these donors were vaccinated for hepatitis B. Of the 7 HBV positive donors, 4 donors were positive for anti‐HBc and negative for anti‐HBS, and the remaining 3 donors were negative for anti‐HBc. We did not find positivity for HIV or HCV RNA among the blood donors in our cohort.
Conclusion
Multiplex nucleic acid testing detected potentially infectious HBV during the window period before seroconversion, i.e occult HBV which added additional blood safety. HBV vaccination appeared to be protective.
4.3 Hepatitis C (HCV)
P‐393
Investigation of the Source of Low‐Level Reactivity Reported by a Qconnect Participant Testing for Anti‐HCV Antibodies
Dimech W , Vincini G , Karakaltsas M
NRL, Australia, Fitzroy, Australia
Background
In 2014 NRL, Australia introduced QConnect, a comprehensive quality control (QC) programme designed specifically for laboratories testing for infectious diseases. QConnect identifies QC samples most appropriate for each assay; provides access to EDCNet, an internet‐based QC monitoring software; generates annual uncertainty of measurement reports and provides participants access to case studies, reference materials and troubleshooting support. Of note, NRL developed QConnect limits specific to QC/assay combinations derived from data collected over a 10‐year period. In June 2014, a QConnect participant (Participant X) using the Abbott ARCHITECT Anti‐HCV CMIA (ARCHITECT HCV) started reporting unusually low results for the QC sample, QConnect Blue (Thermo Fisher Scientific, Fremont, CA. USA). An investigation of the possible sources of low‐level reactivity using EDCNet was instigated.
Aims
To investigate the source of low‐level reactivity of QConnect Blue QC results reported by Participant X.
Methods
QConnect participants periodically tested QConnect Blue on the ARCHITECT HCV assay and submitted results to EDCNet. Participants monitored the variation of their assays using EDCNet through various graphical and tabular reports and also compared their results with other participants using the same QC/assay combination. NRL established QConnect limits of 2.0–3.0 signal/cut‐off (S/Co) for the ARCHITECT HCV/QConnect Blue combination. This range was established using historical data derived from almost 108,000 test results obtained by multiple laboratories testing 13 lots of the QC sample over a 10 year period. Results reported by participants outside this range were flagged by EDCNet for investigation by NRL staff.
Results
During the calendar year 2014, a total of 59 participants reported 14,101 QConnect Blue results for the ARCHITECT HCV assay (mean 2.47 S/Co; coefficient of variation 8.22%). The mean number of results reported by individual participants was 239 (range: 1–1449). Only 296/14,101 (2.1%) QConnect Blue results were less than the lower QConnect limit of S/Co of 2.0. Of the 59 participants, 44 reported no results less than S/Co of 2.0. A further 12 participants reported <10 results below S/Co of 2.0. Only three participants reported >10 results below S/Co of 2.0, with Participant X reporting 165/296 (55.7%) of the results with S/Co <2.0. NRL, in collaboration with the participant and Abbott Diagnostics implemented an investigation. Using EDCNet graphing functions, different potential sources of low reactivity were eliminated. Other participants used the same reagent lots as Participant X without reporting low results. Low QConnect Blue results were reported by all operators and from all 12 Architect instruments associated with Participant X.
Conclusion
Participant X continues to report a high percentage of QConnect Blue results tested on the ARCHITECT HCV assay that are below the QConnect lower limit of the range. Investigations have excluded reagent lots, instruments and operators as being the source of the low QConnect reactivity. A root cause is most likely specific to processes used within Participant X's laboratory. QConnect allowed the detection of this situation and instigated an investigation by NRL, Abbott Diagnostics and the participant.
P‐394
Apparent Discrepancy Between the Virus Concentrations in the 2nd and 4th Who International HCV Standards
Gowland P , Stolz M , Niederhauser C
Interregional Blood Transfusion SRC, Bern, Switzerland
Background
WHO International Standards (WHO IS) are routinely used to calibrate in‐house control virus material and to validate commercially available and in‐house developed NAT assays according to the sensitivity requirements of local and international regulatory authorities. This is particularly true for assays developed for the blood‐borne viruses HCV, HBV and HIV. To achieve this goal, the given potency of the standards must be reliable.
Aims
To investigate the apparent discrepancy in the virus concentration of the 4th HCV WHO IS.
Methods
From July 2007 control samples produced from the 2nd HCV WHO IS (code 96/798) were tested as weekly quality control samples with the Procleix Ultrio assay (Grifols) on the Tigris System. These HCV quality control samples contained 15 IU/ml which is approximately 3–4 times the sensitivity limit determined during in‐house validation of the assay (3.7 IU/ml). Since January 2014 the 2nd HCV WHO IS was replaced by the 4th HCV WHO IS (code 06/102). This 4th HCV WHO IS was also used to validate the new cobas® MPX NAT screening assay (Roche Diagnostics) on the cobas® 8800 System in our institute. During this validation a 12 member dilution panel of the 4th HCV WHO IS from 100 IU/ml down to 0.1 U/ml were analysed in triplicate on 3 different days according to the guidelines of the European Pharmacopoeia.
Results
Between 2007 and 2013 the weekly quality control tests, on all 3 Tigris Systems installed in the laboratory, using the material produced with the 2nd HCV WHO IS had a negative rate of 2.5%. After the change from the 2nd to the 4th HCV WHO IS the negative rate rose to 29% of the analyses (a 12‐fold increase).
In 2014, our institute validated the sensitivity limits of thecobas® MPX on the cobas® 8800 System. The sensitivity limits for HIV and HBV claimed by the assay manufacturer were achieved but that for HCV was not reached. Instead of the claimed 7.0 IU/ml, a sensitivity limit of 15.7 IU/ml was obtained in the probit analysis using samples produced with the 4th HCV WHO IS. When the validation was repeated using the 2nd HCV WHO IS, the sensitivity limit attained (8.3 IU/ml) was similar to that claimed by the manufacturer. The difference in sensitivity limits calculated using the two materials suggests that the HCV concentration in the 4th WHO IS is 1.9 fold lower than that in the 2nd WHO IS.
Summary/Conclusions
A substantial discrepancy was detected between the viral concentration (IU/ml) of the 2nd and 4th HCV WHO IS (apparent factor 1.9). This discrepancy explains the problems encountered with the weekly HCV quality control analyses when the controls were produced with the 4th HCV WHO IS. The 4th HCV WHO IS may not be suitable as a standard for defining the sensitivity limit of new HCV NAT assays and may cause discrepancies in testing control material with very low viral loads. In our opinion the 4th WHO HCV IS needs to be recalibrated and a corrected value communicated.
P‐395
Abstract Withdrawn.
P‐396
Cathepsin B as a Reliable Biomarker For Laboratory Diagnostic of Hepatitis C Infection in Asymptomatic Brazilian Blood Donors
Melo Accardo CM1, Rodart IF 2, Mendes A 2, Pares MM 3, Nader HB 2, Tersariol ILS 2
1UNIFESP/EPM, São Paulo, Brazil2Department of Biochemistry, Federal University of São Paulo (UNIFESP), São Paulo, Brazil3Associação Beneficente de Coleta de Sangue – COLSAN, São Paulo, Brazil
Background
Cathepsin B is a lysosomal cysteine protease widely distributed in tissues that can degrade extracellular matrix (ECM) molecules such as collagen, fibronectin and proteoglycans and remodeling of ECM by controlled proteolysis. This enzyme can be release in serum in presence of apoptosis and inflammatory processes and in pathological events such as tumor progression, metastasis and viral infections.
Aim
In this study, was suggested the application of cathepsin B as a biomarker for diagnostic of hepatitis C infection in asymptomatic blood donors.
Methods
Two hundred and three serum samples of healthy blood donors (control group) and sixty‐seven serum samples of blood bag excluded after donation due serological viral positive results to HCV have the cathepsin B activity measured spectroflurometrically using the fluorogenic substrate Z‐FR‐MCA. Cathepsin B data are expressed in U/L as mean ± SE. The samples are also be tested for liver enzymes ALT and GGT and for LDH and hsCRP by biochemical procedures and are expresses as IU/L (mean ± SE). Determination of hyaluronic acid was perform using an ELISA‐like fluorimetric method. Real‐time PCR as used to determine the viral load of HCV (number of copies/reaction). The analyses of comparative data were perform using the following tests: ANOVA, scatter graphs and linear regression models on data analysis of correlations between: HA, ALT, GGT and hsCRP with HCV viral load and CB serum levels. Spearman test was use to verify correlation between the CB and liver biomarkers and receiver operating characteristics (ROC) analysis.
Results
Blood donors infected with HCV showed eight times more cathepsin B in serum than in control group. The concentration of cathepsin B in the serum of patients with HCV showed a strong positive correlation with viral load (rS = 0.584, P < 0.0001) as well as with the enzyme LDH (rS = 0.836, P < 0. 0001). Moderate correlation with hsCRP (rS = 0.428, P = 0.002) and with the marker of hepatic damage, GGT (rS = 0.384, P = 0.005) and with the non‐invasive markers for hepatic fibrosis, hyaluronic acid (rS = 0.430, P < 0.0001). Based on data obtained from ROC curve for serum levels of cathepsin B, it was possible to determine a cutoff value able to discriminate viral infection by HCV. The concentration values ‘�’�of cathepsin B ≥ 8.0 U/l showed values of sensitivity of 84% and specificity of 99% with positive predictive value of 97% and negative predictive value of 95%. Calculated accuracy value = 95.2%. Conclusions
The results suggests cathepsin B as a reliable biomarker for HCV infection showing good positive correlation with classical liver biomarkers and with inflammatory biomarkers.
P‐397
Abstract Withdrawn.
P‐398
Distribution of Hepatitis C Genotypes During 4 years of Testing in CITM (2011–2014)
Bingulac-Popovic J , Dogic V , Miletic Lovric M , Babic I , Jurakovic-Loncar N , Balija M , Jukic I
Croatian Institute for Transfusion Medicine, Zagreb, Croatia
Background
Hepatitis C virus (HCV) infection is the silent disease and a global health problem among people worldwide. It is estimated that approximately 1.7% of the Croatian population is infected with HCV infection, accounting for more than 75,000 people. Determining of HCV genotype is critical, because the selection and length of hepatitis C treatment depends about patient's genotype.
Aims of this study is to analyze the genotypes, anti‐HCV and HCV core Ag distribution among chronic hepatitis C (CHC) patients in Croatia.
Methods
The patients group have included−−−− 658 HCV infected patients who came in CITM for viral titre and genotype determination before and during 4 years of following therapy algorithm from 2011 to 2014. Simultaneously anti‐HCV and HCV antigen tests are performed in 242/658 of patients by Abbott Architect Anti‐HCV and HCV Ag tests. After extraction of viral RNA, reverse transcription and PCR amplification, HCV genotypes were determined by hybridization method (Versant HCV Genotype 2.0 assay, Siemens, Germany) on Auto‐Lipa 30 analyzer (Siemens, Germany).
Results
The most frequent HCV genotype among HCV infected patients is genotype 1, the subtypes 1b and 1a were found in 31.00%, and 25.07% of the patients, respectively. The next most prevalent genotype is 3a (35.26%), it follows genotype 4 found in 5.17% patients, genotype 2, found in 3.50% (1.82% genotype 2; 1.22% subtypes 2a/2c and 0.46% subtype 2b) patients. 241/242 patients tested for anti‐HCV were positive, 1/242 was negative (S/CO = 0.53 but HCV Ag positive 53 pg/ml). 12/242 patients tested for HCV Ag were negative and 230/242 had HCV Ag (M = 36 pg/ml, p25–p75 = 5–108 pg/ml).
Summary/Conclusions
We found a predominance of genotype 1 (56.07%), with an increase in the frequency of subtype 1b compared to genotype 1a, which is the most on therapy resistant HCV subtype. In studied CHC patients we did not found genotypes 5 and 6, which are not common for Croatian geographic region. Regional distribution HCV prevalence among CHC patients would be useful tool for planning of treatment strategy.
P‐399
Evaluation of A New Antibody Detection Test to Hepatitis C Infection
Sargento CSSS , Kyselova M , Ferraira C , Silva E , Achando P , Tomaz J
Centro Hospitalar e Universitário Coimbra, Coimbra, Portugal
Introduction
In Hepatitis C Infection, diagnostic serologic markers are essentially based on identifying anti‐HCV antibodies. The most commonly used assays are immunoenzimatic (ELISA) or its chemoluminiscent variants (CLIA; CMIA) while third generation test antibodies against core NS3, NS4, NS5 recombinant antigens. The range of diagnostic test still include quick and confirmatory tests with specific accuracy profiles. Multisure HCV (MP Diagnostics‐ USA), is a new multiparametric chromographic immunoassay detecting antibodies against Cp, NS3, NS4 and NS5. It shows a favorable profile due to fast response and low cost. However, its diagnostic accuracy in the clinical setting is still under debate.
Objectives
We aim to evaluate the diagnostic accuracy of Multisure HCV for HCV infection in a hospital‐based setting.
Material and methods: We included consecutive samples undergoing Multisure HCV testing. INNO‐LIA HCV score (Innogenetics‐Belgium) was used as the comparator in all samples. Both positive, doubtful and negative results in screening tests were included. 37 (30.83%) samples were from positive Anti‐HCV patients; 75 (62.5%)border‐line positive Anti‐HCV results blood donors; 6 repeated samples and 2 controls. All samples were tested for HCV‐RNA. Cohen's Kappa coefficient, sensibility and specificity of Multisure HCV were calculated.
Results
Of 120 samples were analysed. The tests showed some agreement between them (Cohen's Kappa coefficient: 0.734, P < 0.001).In this previous study, Multisure HCV showed a sensibility of 90.5% and specificity of 93.8%. In 2 (1.67%) cases Mutlisure HCV had invalid results.
Conclusions
Multisure HCV shows some agreement with INNO‐Lia. The absence of antigenic bands in the Multisure test and the different interpretation criteria of the techniques used condition most of the discrepant results. INNO‐Lia test demands the presence of 2 bands for positivity. The Multisure test requires only the presence of one band in Cp or NS3, with very thin bands, impairing interpretation. Larger studies should be performed to confirm our findings.
P‐400
Clinical Sensitivity Evaluation of the ElecsysⓇ Anti‐HCV II Assay (Roche) on Polish Nucleic Acid Testing (NAT) Yields
Grabarczyk PG , Kopacz AK , Liszewski GL , Kubicka-Russel DKR , Zwolinska PZ , Letowska ML
Institute of Haematology and Transfusion Medicine, Warsaw, Poland
Background
The Elecsys® Anti‐HCV Assay II (Roche Diagnostics) is a third‐generation assay utilized for blood donation screening and testing clinical samples using the electrochemiluminescence immunoassay method (ECLIA). In the assay, recombinant peptides equivalent to core antigen and NS3 and NS4 proteins are used to identify anti‐HCV antibodies. The assay is performed on a fully automated analyzer cobas e 601. The aim of the study was to evaluate the clinical sensitivity of the assay.
Material and methods
The specificity of the test was evaluated on 2508 donations from first time and repeat donors. Clinical sensitivity was assessed based on two seroconversion panels (PHV Care Sera 915 and 925), and one panel of seropositive samples (anti‐HCV positive, HCV RNA positive) infected with the most common genotypes in Poland (genotype 1–8 samples, genotype 3–1 and genotype 4–1 sample). From the single donations infected with subtypes 1a, 1b and 3a, two‐fold dilution panels from 1:50 to 1:600 were prepared. The panel testing was carried out in parallel with the Abbott Architect (reference test – RT). A separate group consisted of 91 NAT yield cases (RNA HCV positive, negative in serology screening with EIA (ELISA Enhanced SAVe HCV 3.0, Ortho) or chemiluminescence assay (Architect, Abbott or Vitros, Ortho). These samples were identified between 1999 and 2014. In the majority of donations, HCV RNA level (cobas Amplicor HCV Monitor v2.0, n = 78) and virus genotype (Versant HCV LiPA 2.0, n = 76) were determined.
Results
During screening, 8 repeat reactive results were obtained: 1 was HCV RNA positive, 2 were RNA HCV negative and Western blot (WB) indeterminate, and 5 were negative in both NAT and WB. The specificity was 99.8%. 3/4 bleedings in the panel PHV915 were reactive and one was borderline, while two bleedings were reactive in RT. 3/5 and 1/5 bleedings from the panel PHV925 were reactive in evaluated assay and RT respectively. All samples from the seropositive donations panel were reactive in Anti‐HCV II assay (100% sensitivity). In all three subtype dilution panels (subtypes 1b, 3a and 4), all results in Anti‐HCV II were reactive (dilutions from 1:50 to 1:1600), whereas the most diluted samples (1:400, 1:800 and 1:1600) were negative in RT. In NAT yield cases, the HCV RNA level ranged from 184 IU/ml to 47.8 million IU/ml, mean 2,752,114 IU/ml, median 459,000 IU/ml. The frequency of HCV subtypes was as follows: 1b – 42.1%; 1a – 1.3%; 3a – 47.4%; 4–9.2%. 11of 91 donations(12%) were assessed as repeat reactive. In 6 donations S/Co value was below 5, in 1 between 5 and 10, in 2 between 10 and 15 and in 2 above 35; 5/11 samples were primarily negative in screening with EIA, the remaining 6 in chemiluminescence assays. Of the 5 donations genotyped, four donations were infected with genotype 1b and one with 3a.
Conclusions
The Elecsys® Anti‐HCV Assay II is characterized by high clinical sensitivity, demonstrating infection detection in 12% out of 91 Polish donations previously identified as HCV window period phase donations.
P‐401
Increased Clinical Sensitivity of Monolisatm HCV Ag‐Ab Ultra V2 Comparing to Previous Assay Version Confirmed by Testing of Donation from Polish Donors in Early Phase of HCV Infection
Grabarczyk PG , Kopacz AK , Liszewski GL , Kubicka-Russel DKR , Nocen EN , Zwolinska PZ , Brojer EB , Letowska ML
Institute of Haematology and Transfusion Medicine, Warsaw, Poland
Background
MonolisaTM HCV Ag‐Ab ULTRA v2 Bio‐Rad is a qualitative enzyme linked immunosorbent assay (fourth generation, co called combo assay) simultaneously detecting antibodies to virus proteins (NS3 and NS4 non‐structural, and capsid) and its antigens (capsid). The assay is designed for blood donors screening and patients testing. In our previous study it has been shown that first version of this assay detected HCV infection markers in approx. 25% of Polish NAT yields (HCV RNA positive/anti‐HCV third generation assay negative).
The aim of the study was to verify whether the second version of the test, compared with the first assay version has a higher sensitivity for detection of HCV infection in Polish donors in the early phase of infection identified in the NAT screening program (window period donations (WP), HCV RNA positive/ anti‐HCV negative).
Material and methods
Testing using Monolisa HCV Ag‐Ab ULTRA V2 (Bio‐Rad, France) was performed on microplates in fully automated analyzer (EVOLIS). Specificity of the test was analyzed on 2016 donations from first‐time and repeat donors. Assay sensitivity was determined on 91 WP donations which were characterized for viral load (HCV RNA, Cobas Amplicor HCV Monitor v 2.0.) and HCV genotype (Versant HCV 2.0 LiPA). 59/91 donations has been tested with the previous assay version – Monolisa HCV Ag‐Ab ULTRA V1 (BioRad, France).
Results
Specificity of the Monolisa HCV Ag‐Ab ULTRA V2 was 99.80%: five repeatedly reactive donations were HCV RNA negative and in 3/5 Western blot (WB) was negative, in one positive and in one an indeterminate WB result was obtained. WP donation characteristics: HCV RNA level ranged from 184 IU/ml to 47.8 million IU/ml, mean 2,752,114 IU/ml, median 459,000 IU/ml; the distribution of HCV subtypes was as follows: 1b – 42.1%, 1a – 1.3%, 3a – 47.4%, 4 – 9.2%; Monolisa V2 ULTRA assay results are shown in Table 1. Monolisa HCV Ag‐Ab ULTRA reactive results were obtained in 32/91 (35.2%) donations and their frequency was higher in the new version compared to the previous assay version (15/59–25%) (Chi square P = 0.07, test McNamara P < 0.01). Reactive results of both assay versions concerned 13 samples; 2 and 7 samples were reactive exclusively in the first and second test version respectively. Improvement of clinical sensitivity of Monolisa HCV Ag‐Ab ULTRA was due to increased detection of genotype 3a from 7% in first version to 25% in latter assay version (Chi square P = 0.07, test McNamara P < 0.01).
Table 1. Reactivity of the plasma samples infected with HCV from donations collected in the early phase (WP) – comparison of the results for Monolisa

Conclusion
HCV Tests Monolisa Ag‐Ab (IV generation EIA, Bio‐Rad) detects HCV infection markers in more than 25% NAT yields primarily tested negative in III generation serology assays. The new assay version is characterized by improved detection sensitivity mainly due to increased genotype 3 detection.
P‐402
Mixed Hepatitis C Virus Genotypes/Subtypes Infection Identified by Deep Sequencing
Xu R
Guangzhou Blood Center, Guangzhou, China
Background
HCV mixed infection is commom in individuals and may lead to more severe disease, may confound assessment of response to antiviral therapy. Sanger sequencing only detect dominant genotype/subtype existed in HCV infected patients. Deep sequencing technologies are much faster and can perform large‐scale sequencing. Here we dientify infection of mixed hepatitis C virus (HCV) genotype/subtype using deep sequecning.
Aims
To identify infection of mixed hepatitis C virus (HCV) genotype/subtype by deep sequencing.
Methods
Plasma samples from two individuals infected with HCV were collected. Both persons were found disparate HCV genotypes between NS5B and E1 gene by RT‐PCR and then Sanger sequencing. We then amplified NS5B for three times and pooled together, which were subsequently applied to deep sequencing by the Ion Torrent system (Thermo Fischer). Sequencing results were analyzed and aligned by two methods in Genious software: ‘map to reference algorithm’ and ‘de novo assembly’. Mega5 were used to construct phylogenetic tree.
Results
Sanger sequencing revealed subtype 1b on NS5B and 2a on E1 for both samples. Deep sequencing on NS5B also revealed mixed infection with HCV 1b and 2a. Analyzed by ‘the map of reference algorithm’, subtype 1b and 2a accounted for 92.7% and 3.7% of the total reads (unused reads accounted for 3.5%) for sample 1, while 1b and 2a accounted for 84.3%, and 5.4% for sample 2 (10.3% unused reads), respectively. ‘De novo assembly’ analysis revealed the percentage for 1b and 2a were 96.3% and 3.7% for sample 1, and 94.1% and 5.9% for sample 2.
Conclusions
Deep sequencing is a powerful tool that can precisely identify mixed infection with multiple HCV genotype/subtype. De novo assembly performed better analysis on deep sequencing data.
P‐403
New HCV‐6 Variants Prevalent In Hainan Province China
Rong RX
Guangzhou Blood Center, Guangzhou, China
Background
Hepatitis C virus has a positive‐sense single‐stranded RNA genome of about 9.6 kb. So far, seven major genotypes (HCV‐1 to HCV‐7) have been described, containing 67 subtypes based on genetic diversity. HCV genotype 6 has the highest genetic diversity with the earliest origin of the seven HCV genotypes. Currently 26 subtypes of HCV‐6 have been assigned. Geographically, HCV‐6 isolates are typically found in Southeast Asia countries. In China, HCV‐6 is highly prevalent in Guangdong province, Guangxi province and Hainan province. Recently, we found a high rate of HCV infection and primary liver cancer in the population of Li minority of Hainan province, China. and the prevalent strains of HCV were mainly genotype 6 variants.
Aims
To investigate the molecular epidemiology of HCV‐6 in Hainan Li group which have unique life‐style and the closed ecological environment, and analyze the origin, evolutionary pattern, and epidemic trend of HCV‐6 variants.
Methods
Of 77 HCV RNA positive samples were collected from Li minority of Hainan province. Reverse‐transcription and polymerase chain reaction were performed to amplify the NSB5 and E1 region. The whole genomes of HCV‐6 strains were then amplified by DNA Walking on Bridges and Islands followed by sequencing. Each complete genome sequence was conducted molecular evolution analysis with Coalescent theory.

Results
Among 77 HCV RNA positive samples, HCV 1a, 1b,2a,and 6 were 9(11.7%),14(18.2%),12(15.6%) and 42(54.5%) respectively. All 42 HCV‐6 strains are new HCV‐6 variants with genetic diversity of 13–15%. The phylogenetic tree showed 3 clusters, the first cluster contained 28 strains which separated form 6a‐6xe. The second cluster contained 9 strains with 6w,6s,6t and 6 g. The third cluster included 5 strains close to 6xa.
Conclusions
The prevalent strains of HCV were mainly genotype 6 new variants in Li minority of Hainan province, China. In view of the unique life‐style and the closed ecological environment of ethnic Li population in Hainan, the population is a favorable epidemiological cohort in the study of origin, evolutionary pattern, and epidemic trend of HCV‐6.We will enlarge the sample size and do more study on it.
P‐404
Abstract Withdrawn.
P‐405
Interleukin 28b Polimorphisms Rs12979860 and Rs8099917 in Blood Donors and Patients with Chronic Hepatitis B and C
Sargento CSSS , Ferraira C , Silva E , Achando P , Kyselova M , Tomaz J
Centro Hospitalar e Universitário Coimbra, Coimbra, Portugal
Background
Several published studies, suggested that single nucleotide polymorphisms (SNPs) and viral markers in the IL28B gene locus are associated with increased risk of poor response to therapy. Knowledge of a patient IL28 genotype is likely to aid in clinical decision making with standard‐of‐care regimens. Il28B polymorphism is also strongly associated with spontaneous clearance of HCV.
rs12979860(rs60) genotype (CC, TT, CT) was the first polymorphism of the IL28B gene to be identified and was associated with a twofold improvement with treatment of HCV infection amongst European people.
The second polymorphism – rs8099917(rs17) genotype (GG, TT,GT) was found in individuals of Australian and northern and European ancestry.The GG genotype was strongly associated, in chronic HCV patients, with a null virological response (NVR), wheras patients with the GT or TT genotypes showed an increased probability of achieving an SVR.
Aims
We aimed to evaluate, by real time PCR (RT‐PCR), the percentage of these important genotypes as predictors of response to therapy in two diferent populations: healthy blood donors and chronic HCV/HBV patients.
Methods
Nucleic acid extraction: easyMAG (BioMerieux)
Laminar flow chamber classII – Telstar
Amplification reagents: TakaRa Bio inc
RT‐PCR system:Rotor ‐Gene Q‐Quiagen
Determining the polymorphisms rs 12979860 (rs60) and rs8099917 (rs17) in IL28B gene by RT‐PCR: Fast set IL28B‐Arrow Diagnostics and Sacace Biotechnologies.
A total of 769 samples were included:
273 voluntary healthy blood donors (18–63 years old); 81.1% male and 496 chronic HCV and HBV patients.
Results
273Blood donors genotyping: IL28B‐ rs60 CC: 50.9%; CT: 39.6%; TT: 9.5% and
496chronic HCV/HBV patients IL28B‐rs60: CC:45%; CT: 43%; TT:12% IL28B‐ rs17: GG: 5%; TG 36%; TT 59%.
The combination of variants analysed of the polymorphisms rs60 / rs17 was CC/TT (42%); CT/TG (29%); CT/TT (14%); TT/GG (5%); TT/TG (4%); CC/TG (3%); TT/TT (3%) and CT/GG (0%)
Conclusions
CCgenotype at rs60 is the most significant predictor of SVR. With genotype other than CC at rs60 likelihood of achieving SVR, depending on the combination of variants analysed of the polymorphisms rs60 / rs17.
In this study, we can conclude that the majority of these patients(42%) have the better combination polymorphisms (rs60/rs17) to predict a good response to therapy, as we could observe, when monitorized treatment.
Regarding our results on blood donors CC genotype in IL28B polymorphism rs60, is the most commun, who is also a good predictor.
P‐406
The Impact of Selecting Venues of Mobile Blood Drives Upon the Rate of Seropositive Incinerations
Fag El Nour M
Egyptian National Blood Transfusion service, Cairo, Egypt
Background
The main scope of our National Blood Transfusion services policy is the recruitment & retention of voluntary non‐remunerated blood donors from low risk population to provide adequate, safe and effective blood products and services.
Lately, our statistical analysis & Key performance Indicators for seropositive incinerations(especially HCV positive incinerations) showed an increase by 1.3%.
Egypt is known to be endemic for Hepatitis C virus.
National Blood Transfusion service (NBTS) testing strategy depends for TTIs screening upon using validated EIA kits for HBsAg &HCVag/ab, HIV1‐2 ag/ab &Syphilis ab. In addition to performing Nucleic acid testing using PROCLEIX® TIGRIS® for all our National Blood Transfusion Center blood collections.
Aim
To demonstrate the impact of selecting the venues of mobile blood drives in relation to the rate of seropositive incinerations in National Blood Transfusion center (NBTC).
Methods
A study was held to gather data about mobile blood donations in NBTC in the period from May 2013 till October 2014.
Concerning
Monthly Total collection of mobile blood donations ‐Monthly% of Seropositive Discarded units from total collections(EIA&NAT reactive)‐Monthly % of Positive HCV Discarded units from total collections (EIA&NATreactive)
A study was held upon the venues of different mobile blood drives with the collection of each blood venue in the same period from May 2013 till October 2014.
It was noticed that the Blood venues to ElFayoum Governorate compromises average 38% from the total collection of mobile blood collections (this blood venues are recently introduced to NBTC).
Analytical study was held upon the rate of total seropositive discarded units among ElFayoum mobile blood venues & also the rate of Positive HCV discarded units from this blood venue collection.
Results
By Referral to the 1st table it was noticed that:
In June, July, September & November 2013, January, February, March, May, June, August, September & October 2014
The rate of seropositive discarded units reached average 3.8% & the rate of Positive HCV discarded units reached average 2.7%
In May,August,October,December 2013, April & July 2014 are within our average rates of total& Pos HCV seropositive discarded units which were 2.5% & 1.4% respectively.
By referral to the 2nd table it was noticed that:
It demonstrated that Blood collection from El Fayoum Governorate venues were held at the same previously mentioned months that showed an increase in the % of total &HCV seropositive discarded units.
By calculating the average rate of total seropositive discarded units, it compromised 7% from El Fayoum collection.
By calculating the average rate of HCV seropositive discarded units,it compromised 5% from El Fayoum collection.
Conclusion
Mobile Blood Venues to El Fayoum Governorate affected our seropositive incineration rates especially the rate of HCV positive incineration.


During the selection of mobile blood venues,we must exclude venues to high risk populations to reduce the rate of seropositive incinerations among our blood collection.
MOH must investigate & afford medical care for the high risk population in El Fayoum Governorate.
4.4 HIV
P‐407A
Analysis of HIV‐1 Subtypes Among HIV‐Positive Blood Donors in Catalonia (Spain)
Bes MB1, Piron MP 1, Casamitjana NC 1, Rico AR 2, Esteban JIE 3, Gregori JG 4, Ortiz OP 1, Puig LP 1, Sauleda SS 1
1Banc de Sang i Teixits de Catalunya, Barcelona, Spain2CIBERehd Instituto de Salud Carlos III, Madrid, Spain3Hospital Universitari Vall d'Hebron, Liver Unit, Barcelona, Spain4Hospital Universitari Vall d'Hebron, Institu de Recerca, Barcelona, Spain
Background
HIV‐1 subtype B is predominant in Spain, although demographic changes due to immigration have resulted in an increased rate of non‐B subtypes in the HIV population. However, it is unclear whether these HIV variants remain confined to non Spanish population or have already spread among donors of Spanish origin.
Aims
In this study we investigated the prevalence and distribution of HIV‐1 subtypes in Catalan blood donors over the past 10 years and the association of HIV subtypes with transmission risk factors and demographic parameters.
Methods
In this study, we have included blood donors that were identified as HIV‐1 positive from January 2005 to December 2014. HIV‐1 subtyping was carried out by Nested‐PCR and sequencing of 1082 base pair from HIV‐1 pol region. Phylogenetic analyses were performed using neighbour‐joining method included in the MEGA 6 program and the reference sequences were obtained from the Los Alamos HIV Database.
Results
Over the past 10 years, 219 blood donors (88.6% male, mean age 34 ± 10) were found HIV‐1 positive out of 3,811,172 donations screened (1 in 17,500). HIV‐1 subtype was assessed in 156 individuals. According to country of origin, 128 donors were native Spaniards (82%), 19 from Latin America (12%) and the 9 donors remaining (6%) were from Eastern Europe, Africa and Arabia Saudi. As for risk factors for HIV acquisition, 64 individuals were men who have sex with men (MSM) (41%), 42 subjects declared heterosexual risk contacts (27%) and in 32% of donors the risk was unknown. HIV‐1 subtype B was the most prevalent variant (84.6%), while the rate of non‐B subtypes was 15.4%. Distribution of non‐B variants were as follows: 7 CRF02_AG (29.1%), 5 F (20.8%), 3 A1 (12.5%), 3 CRF_BF (12.5%), 2 CRF_BG (8.3%), 1 C (4.2%), 1 D (4.2%) and 1 G (4.2%). No correlation between the age of the donor and HIV‐1 subtype was observed. Surprisingly, although the percentage of non‐B subtypes was higher in immigrants than in native Spaniards, the differences were not statistically significant (25% vs 13.3%; respectively, P = 0.2). Additionally, we observed that the rate of non‐B subtypes among the Spanish donors was 8.6% in the period of 2005–2009 and increased to 17.1% in the period of 2010–2014, whereas in the non Spanish donors the prevalence of non‐B subtypes remains constant (25% in both periods). Finally, the frequency of HIV‐1 non‐B subtypes was higher in subjects infected by HIV‐1 following heterosexual contacts (30.9%) than in MSM (9.4%) (P = 0.010).
Conclusions
In our region, HIV‐1 donors are mostly infected by HIV‐1 subtype B, although identification of non‐B subtypes is increasing. HIV‐1 non‐B infections are mainly acquired through heterosexual risk practices.
P‐407
Epidemic and Diversity of HIV Genotype for Blood Donor Infection Individuals in China
Xu G , Da B , Ding W , Wu Y , He J , Zhu F , Lv H
Blood Center of Zhejiang Province, Hangzhou, China
Background
AIDS is becoming one of leading cause of death among infectious diseases. An estimated 780 thousand individuals in China were HIV infected in 2011, which is characterized with growing complexity in epidemic. It is showed that HIV epidemic from blood donations without medication was different from the general population. However, the data for diversity of HIV genotype of Chinese blood donor infection individuals is rare.
Aims
This study was to determine the prevalence of HIV infection and diversity of genotypes in blood donor infection individuals in Blood Centre of Zhejiang Province, China.
Methods
All blood donations were screened for anti‐HIV‐1/2 using two different manufacture's ELISA assays according to the manufacture's instructions from 2011 to 2013, and HIV‐1 RNA were also detected individually using the PROCLEIX® ULTRIO® assay in the Tigris instrument. The anti‐HIV‐1/2 positive samples by screening procedure are confirmed by Western blotting assays. HIV‐1 positive samples by PROCLEIX® ULTRIO® assay were used for extraction total RNA, then the env and gag regions of HIV‐1 gene were amplification by nested‐PCR, and the amplicons were purified with enzymes digestion and directly sequenced. HIV‐1 subtype was assigned according to the nucleotide sequences by BLAST and genotyping software.
Results
65 samples were anti‐HIV‐1/2 positive by Western blotting assay in the 367,851 blood donations, with frequency of 0.018%. Two individuals were confirmed HIV window period infection, which showed the HIV‐1 RNA positive and anti‐HIV‐1/2 negative in the first test, and showed anti‐HIV‐1/2 and HIV‐1 RNA positive in the further follow‐up test in one month. A total of 35 samples have analyzed the HIV‐1 genotype, which HIV recombinant form CRF01‐AE was the most prevalent subtype (35.3%), followed with CRF07‐BC(26.5%). The difference of average of viral load between CRF01‐AE and CRF07‐BC genotype showed no significance (P > 0.05).
Conclusions
In this study, the data for diversity of HIV genotype and viral load was obtained among blood donors in Blood Centre of Zhejiang Province, China and genotype CRF01‐AE was most prevalent of the HIV infections. Our findings on HIV epidemic and genotype profile in the blood donations can help to track the evolutionary trends of different virus lineages of circulating strains in Chinese population and expand the understanding of HIV testing, therapy, and reducing the risk of blood transfusion in China.
Funding: This research was supported by the Science Foundation of Zhejiang Health Bureau (No. 2013KYA053) and Science Technology Foundation of Zhejiang Province(No. 2013C03047‐2)
P‐408
First Report of Human Immunodeficiency Virus Breakthrough Transmission in Thailand After Mandatory 6‐Minipool Nucleic Acid Testing
Rujirojindakul P
Prince of Songkla University, Songkhla, Thailand
Background
In October 2013, a 29‐year‐old female repeat blood donor had anti‐human immunodeficiency virus (HIV) reactivity (CMIA HIV Ag/Ab Combo Architect system, Abbott). Her last donation in June 2013 (the 17th‐time donation) showed nonreactive results of anti‐HIV and 6‐minipool nucleic acid testing (6‐MP NAT) (cobas TaqScreen MPX test, s201 system, Roche). From her 17th donation, there were two recipients of blood products. The first patient who received fresh frozen plasma died due to severe trauma within seven days after transfusion. The second recipient transfused with packed red cell (PRC) showed anti‐HIV positive in November 2013. His previous anti‐HIV test before transfusion was negative.
Aims
To demonstrate the transfusion‐related HIV infection despite 6‐MP NAT
Methods
We investigated the homology of the HIV genome using post‐seroconverted plasma from the donor and PRC recipient. The HIV genes were sequenced and analysed for phylogenetic study together with nine HIV isolates from other patients living in Hat Yai, Songkhla. Archived plasma from the 17th donation was tested for HIV viral load.
Results
The phylogenetic analysis showed 99.8% similarity between the donor and patient. The HIV viral load in donor's plasma was below the detection limit (<20 copies/ml).
Summary/Conclusions
We reported the first case of HIV‐transfusion transmission through the 6‐MP NAT in Thailand. This result demonstrated that a residual risk might remain even individual‐donation NAT was used.
P‐409
Sensitivity of HIV Ag/Ab Combo Assay in Detecting Window Period Nat Yield Samples
Mah EM1, Bon AHB 1, Lam TPL 1, Radzi MMR 1, Halim MHMH 1, Lelie ML 2
1National Blood Centre, Kuala Lumpur, Malaysia2Lelie Research, Paris, France
Background
Total of 1,240,399 Malaysian blood donations screened for HIV‐RNA by ID‐NAT (Ultrio/Ultrio Plus, Grifols), for anti‐HIV and p24‐antigen by HIV‐Ag/Ab combo assay (Abbott PRISM) between 2008–2013. To recognize p24Ag positive window period (WP) donations we considered immunoblot (LIATEK) negative donations as HIV‐NAT yields.
Aim
Clinical sensitivity of HIV combo testing and HIV‐RNA screening was compared. We estimated two HIV Ag/Ab assays the p24‐antigen seroconversion point in copies/mL in HIV‐RNA ramp up phase. Residual risk of HIV transmission was calculated for ID‐NAT and the two combo assays and compared with the observed p24‐antigen negative WP‐NAT yield rate.
Methods
Seronegative, Ultrio (Plus) screening initially reactive (IR) donations were repeated in triplicate; then tested for discriminatory assays and viral load by real‐time PCR (Roche TaqMan), using frozen plasma unit when triplicate results repeatedly reactive. Concordant PRISM HIV Ag/Ab, Ultrio IR donations confirmed by LIATEK and dHIV assay. HIV‐NAT yield samples also tested for p24‐antigen reactivity with Abbott ARCHITECT HIV combo assay. p24‐antigen cutoff crossing point in HIV‐RNA ramp up phase was estimated by linear regression analysis on Log HIV Ag/Ab S/CO values plotted against Log HIV‐RNA concentration in copies/mL. Viral loads at p24‐antigen seroconversion points in combo assays and 50% lower limit of detection (LOD) of Ultrio assay were used for estimating residual HIV transmission risk according to WP risk day equivalent model. Minimum infectious dose (MID50) of 31.6 virions (63.2 copies of HIV‐RNA) was assumed for risk modeling.
Results
All 494 anti‐HIV positive confirmed donors had detectable HIV‐RNA, with 23 (1:53,930) confirmed HIV‐NAT yield. Replacement of anti‐HIV screening by HIV Ag/Ab testing on PRISM increased diagnostic sensitivity from 471/494 (95.3%) to 477/494 (96.5%); HIV‐RNA detection by ID‐NAT reached 494/494 (100%). PRISM combo assay detected 6/23 (26%) of HIV‐NAT yield samples, was less sensitive than ARCHITECT assay that detected 9/23 (39%). The p24‐antigen seroconversion point in HIV‐RNA ramp up phase was estimated by regression analysis at 42,600 and 166,000 copies/ml in ARCHITECT and PRISM assays. One anti‐HIV negative donor with HIV‐RNA level of 2.5 million copies/ml missed by both combo assays. Calculated residual risk for ID‐NAT, PRISM and ARCHITECT combo assays was 1/1,500,000, 1/94,000 and 1/108,000. Observed rate of PRISM, ARCHITECT HIV Ag/Ab combo assay non‐reactive HIV‐NAT yield samples was 1/73,000 and 1/89,000.
Conclusion
Estimated residual HIV transmission risk with combo assays was 15‐fold higher than with ID‐NAT. The observed p24‐Ag negative NAT yield rate slightly higher than calculated risk, which explained by one high viral load combo assay detection failure and by three donations with viral load below 40 copies/ml may not all been infectious. All 471 anti‐HIV positive donors were HIV‐RNA reactive, confirms robustness of dual target HIV detection system in Ultrio assay. Replacement of anti‐HIV screening assay by combo assay reduced the observed HIV transmission risk (WP‐NAT yield rate) from 1:54,000 to 1:73,000. Replacement by ID‐NAT alone strategy almost eliminates the HIV transmission risk. The regulators could consider accept ID‐NAT only screening strategy for dual target NAT assays, particularly for regular repeat donors whom anti‐HIV screening was only 78% (35/45) sensitive.
P‐410
Abstract moved from Poster to Oral.
P‐411
Establishing a Reentry Procedure for HIV Screening Reactive Donors in China
Li L1, Liu Z 1, Cheng W 2, Niu L 3
1Institute of Blood Transfusion, CAMS & PUMC, Chengdu, China2Anhui Blood Center, Hefei, China3Shanxi Changzhi Blood Center, Changzhi, China
Background
To ensure blood safety, blood donor screening strategy has employed highly sensitive reagents to screen blood. Initial S/CO ratios in the range of 0.50–0.99 for HIV‐1/2 were considered as ‘grey zone’ in China. However, these measures have resulted in false positive results, causing donor dissatisfaction and reducing the numbers of potential donors. Recently, formulating confirmation and reentry policy garnered the attention from decision‐making departments. There were few data on algorithm for the deferred reentry of volunteer blood donors due to false‐positive test results for anti‐HIV and/or HIV‐RNA. This present study would help to establish a blood donor reentry procedure for HIV screening reactive donors.
Aims
Establishing blood donor reentry procedure for HIV screening reactive donors in China.
Methods
The gold standard was based on the confirmatory results from follow‐up samples of blood donors who screened reactive for HIV, allowing to push forward an appropriate and suitable reentry strategy for screening reactive blood donors in China. All donor samples that screened reactive for anti‐HIV and/or HIV‐RNA in fourteen blood centers were sent to IBT HIV confirmed laboratory where anti‐HIV‐1/2 and HIV individual donor samples NAT (ID‐NAT) were tested again. If the results were reactive on the anti‐HIV‐1/2 test, then they would be tested with Recombinant ImmunoBlot Assay (RIBA). The result would be determined positive if RIBA was positive, otherwise, the donors would be tracked after eight weeks. If the results were reactive for ID‐NAT and but non‐reactive for anti‐HIV‐1/2 or if results were both non‐reactive for ID‐NAT and anti‐HIV‐1/2, donors would also be tracked after eight weeks. All follow‐up samples were tested by both ID‐NAT and anti‐HIV‐1/2 test. If the results were reactive for anti‐HIV‐1/2 but non‐reactive for ID‐NAT or if the results were non‐reactive for anti‐HIV‐1/2 but reactive for ID‐NAT test, another eight‐week follow‐up was conducted.
Caption 1: Blood Donor Reentry Procedure for HIV Screening Reactive Donor

Caption 2: Summary of Study Testing Results

Results
From Sept. 1st, 2013 to Aug. 31th, 2014, 277,096 samples were screened at 14 Chinese blood centers. Of these, 465 were anti‐HIV‐1/2 and /or HIV NAT reactive. Based on repeated testing after 8 weeks, 252 donors could be classified successfully: 45 (17.8%) true positives,207(82.1%) false positives. A total of 186 of 465 (40%) samples termed false positive were admitted for reentry and able to participate in further donations. 213 of 465 (45.8%) donors were abandoned for unsuccessful follow‐up, see Figure 1.
Conclusion
A blood donor reentry procedure of HIV screening reactive donors was formulated:
(i) If the ID‐NAT and the anti‐HIV‐1/2 test both were non‐reactive on the follow‐up sample, the donor would be allowed reentry. (ii) If the ID‐NAT was reactive on the follow‐up sample, the donor would be deferred permanently. (iii) If the ID‐NAT was non‐reactive, the anti‐HIV‐1/2 test was reactive and RIBA was non‐reactive after two follow‐ups, the donor would be allowed reentry. Based on, a blood donor reentry model of HIV screening reactive donors was formulated. Many donors (186) can re‐enter, 21 donors would be follow‐up after next 8 weeks and 45 donors would be permanently deferred from donation if our establish model for reentry is applied, see Figure 2.
P‐412
Abstract Withdrawn.
P‐413
Performance and Comparison of The New Liaison Xl Murex HIV Ab/Ag HT Assay with Liaison Xl Murex HIV Ab/Ag Assay for Blood Donation Screening
Janouskova M , Mecelova K , Novakova E
Carlsbad Regional Hospital, Karlovy Vary, Czech Republic
Background
Methods of high sensitivity and specificity and also of high throughput are demanded for routine blood donors testing for infectious markers. Recently a new high‐throughput assay LIAISON XL murex HIV Ab/Ag HT (DiaSorin) for qualitative determination of p24 HIV‐1 antigen and/or HIV‐1/2 antibodies was launched. On LIAISON XL platform two type of highly sensitive and specific HIV Ab/Ag combined qualitative assays are now available. While LIAISON XL murex HIV Ab/Ag assay using two reagent integrals is able to determine HIV antigen and antibody separately the new high‐throughput LIAISON XL murex HIV Ab/Ag HT method uses only one reagent integral and detects HIV antigen and/or antibody simultaneously without original resolution.
Aims
To compare the new LIAISON XL murex HIV Ab/Ag HT assay with currently used LIAISON XL murex HIV Ab/Ag assay in terms of routine blood donation screening for infectious markers.
Methods
150 randomly selected blood donor serum samples, 5 samples of External Quality Assessment and 2 samples of comercial positive control (Accurun) were tested by currently used LIAISON XL murex HIV Ab/Ag assay and by LIAISON XL murex HIV Ab/Ag HT assay. Measurements were performed on the same day and results and time of analyses were compared. To compare throughput of the HIV Ab/Ag tests in routin blood bank testing the samples were also simultaneously tested for additional mandatory infectious markers HBsAg, HCV Ab, Treponema Ab using LIAISON XL murex HBsAg Quant, LIAISON XL murex HCV Ab, LIAISON Treponema screen tests, respectively and time to the results was monitored.
Results
Concordance of the LIAISON XL murex HIV Ab/Ag HT and LIAISON XL murex HIV Ab/Ag results was 100% (150 blood donor samples negative, 2 (of 5) samples of External Quality Assessment positive and 3 samples negative, 2 Accurrun samples positive). Time to the HIV result of one sample is shorter by 23 min (42%) using LIAISON XL murex HIV Ab/Ag HT assay in comparison with the LIAISON XL murex HIV Ab/Ag assy. Time needed for screening of 50 blood donor samples for four mandatory infectious markers (HIV Ab/Ag, HBsAg, HCV Ab, Treponema Ab) on LIAISON XL analyzer is shorter by 40 min (21%) using LIAISON XL murex HIV Ab/Ag HT assay in comparison when LIAISON XL murex HIV Ab/Ag assay is used.
Conclusions
Implementation of the new LIAISON XL murex HIV Ab/Ag HT assay into the routine screening of blood donnors significantly reduces time of analysis and increases throughput of blood donor samples, especially when large series of samples common for blood transfusion service are tested.
P‐414
Evaluation of a New Assay: BioplexⓇ2200 HIV Ag‐Ab the 5th Generation Test for the HIV Screening
Fernández Menéndez JRFM , Portugal RP , Tomaz JT
Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
Background
The HIV infection remains a major public health problem, worldwide. The need to increase transfusion safety of our blood donations, reducing the window period, and the ‘obligation’ of the early detection of patients infected by HIV leads to the implementation of new, more sensitive and specific screening tests that allow simultaneous detection, differentiation and reporting of HIV p24 antigen 1, antibodies to HIV 1 (Groups M and O) and HIV 2 in the laboratory diagnosis of the different stages of HIV infection.
Aims
Evaluate the new immunoassay ‘5th generation’ BioPlex® 2200 HIV Ag‐Ab as an alternative to existing 4th generation tests on the market for the laboratory diagnosis of HIV infection.
Methods
40 known positive samples were studied for HIV with the screening test used in the routine of the viral serology laboratory of the Blood Service and Transfusion Medicine that belongs to the Centro Hospitalar e Universitário de Coimbra (CHUC). The study includes both patients and donors and also 20 negative control samples (10 patients and 10 donors). For routine screening of HIV were used two different methods: micro‐enzyme immunoassay ELISA HIV1 + 2 Ag/Ab from Bio‐Rad, in the automatic auto analyzer EVOLIS® Premium and chemiluminescence assay HIV1 + 2 Ag/Ab from Abbott Laboratories in the Architect i2000SR system.
Positive results were ‘confirmed’ using more specific tests: Western Blot from Bio‐Rad and the Innogenetics Inno‐Lia. For the determination of HIVAg was used the test Geenscreem™ HIV1 Ag Assay from Bio‐Rad.
These 60 samples were studied in Bioplex 2200 autoanalyzer in European Headquarters of Bio‐Rad, at Marnes La Coquette in Paris. Samples ‘outliers’ were studied at the Hospital Universitário de Getafe in Madrid.
Results
3 ‘False positives’(screening +/WB‐Inno‐Lia ‐) were negative on BioPlex
28 samples HIV1 + were identified as HIV1 on BioPlex
2 samples HIV2 + were identified as HIV2 on BioPlex
1 sample HIVAg+ was identified as HIVAg on BioPlex
1 sample screening HIV+ and indeterminate for WB‐HIV1 was identified as HIV1 on BioPlex
1 sample screening HIV+, WB indeterminate for HIV1 and HIVAg+ was identified as HIV1 on BioPlex
1 sample ELISA HIV+/Chemiluminescence‐, WB negative for HIV1/Inno‐Lia indeterminate for HIV1 was HIV1 + in BioPlex. The donor made a later blood collection with negative results (screening and confirmatory in CHUC) and also BioPlex negative
All the HIV negative samples were negative with BioPlex
1 sample was not pipette due to low sample volume
Conclusions
The BioPlex® 2200 HIV Ag‐Ab allows, in only one kit, to do a ‘complete’ laboratory study for the research of HIV in serum or plasma from donors and patients.’�
Detects and identifies simultaneously the p24 antigen of HIV‐1 and antibodies to HIV‐1 (Group M and O), HIV‐2, thereby differentiating infection by HIV‐1 or HIV‐2.
It is a very rapid test, the first result takes only 60 minutes and in each 36 seconds a new result goes out.
BioPlex® 2200 platform adapts to any algorithm or laboratory workflow. Fully automatic, needs no preparation of the sample and all the reagents are ready to use.
P‐415
A Cross‐Sectional Study Among Blood Donors on Sexual Risk Behaviors for HIV Infection
Grazzini G1, Raimondo M 2, Facco G 1, Regine V 2, Pupella S 1, Piccinini V 1, Suligoi B 2, The FRIHSS Study Group1,*
1Centro Nazionale Sangue, Rome, Italy2Istituto Superiore di Sanità, Rome, Italy
Background
In 2012, in Italy, blood donor surveillance data showed an HIV prevalence and incidence of respectively 14.3 and 5.3 per 100,000 donors. HIV risk factors reported by HIV‐positive donors during post‐donation interviews included mainly (70%) sexual contacts with occasional partners that were not reported in the pre‐donation face‐to‐face interview. Most missed deferrals for HIV were due to lack of perception of the risk of behaviours or its denial. This highlighted the importance of improving the pre‐donation selection tools and procedures. According to the Italian regulation, donors exposed to HIV risk behaviours must be deferred for four months upon the assessment of the physician in charge of blood donor selection; in case of ‘high’ risk donors must be permanently deferred.
Aims
The objective of this study was to evaluate the efficacy of a new standardized pre‐donation questionnaire as a tool to improve the identification of blood donors with sexual behaviours at risk or at high risk for HIV infection.
Methods
From June 2014 to March 2015, a cross‐sectional prospective study was carried out, consisting of the enrolment of first‐time and repeat blood donors from 6 different Blood Establishments (BEs). After filling in the routine pre‐donation questionnaire (RDQ) in use at the participating BEs, the enrolled donors were asked to fill in an additional standardized pre‐donation questionnaire (SDQ) including more detailed and explicitly posed questions about sexual risk behaviours, awareness of HIV risk and comprehension of educational material.
Results
As of February 2015, 6836 blood donors were enrolled: 83.7% repeat and 16.3% first‐time donors; 67.9% M and 32.1% F; average age 40,5 years (SD±12,1 years). Through the RDQ, 63/6836 (0.92%) were deferred for sexual risk behaviours in the last four months. Among 6360 enrolled eligible donors, 851 (13.4%) refused to fill in the SDQ. Considering the remaining 5509 donors who filled in the SDQ, 317 (5.7%) were deferred for sexual risk behaviours, including 12 donors who declared to be aware of the HIV positive serological status of their partners. Interestingly, among males who reported sexual contacts in the last four months, 4.1% declared having had sex with males. Among all donors eligible according to the RDQ (6360), 17.2% declared they had read the educational material provided by the BEs either superficially or not at all.
Conclusions
The reported preliminary data show that a more detailed pre‐donation questionnaire could facilitate the collection of additional significant information about HIV risk behaviours in comparison with the questionnaire currently used by the Italian BEs. Furthermore, more accurate procedures for the administration of pre‐donation educational material seem to be important in order to improve blood donors’ awareness about HIV sexual risk behaviours.
*Pagliarino M, Nucci A, Canil S, Velati C, Lazzarini M, Graziani G, Arnetoli F, Baldinotti V, Girelli G, Panzini E, Mallano S, Scelsi M, Pati I, Di Loreto M, Garozzo G, Maggiore R, Migliore S.
P‐416
An Evaluation of the Bio‐Rad Geenius™ System in the Virology Laboratory, Western Province Blood Transfusion Service
Pietersen NJ , Cable RT
WP Blood Transfusion Service, Cape Town, South Africa
Background
When testing for HIV, the most predominant strain tested worldwide, is HIV‐1. The less common HIV‐2 type is usually concentrated in West Africa. However, with refugees seeking asylum in SA, it brings on new concerns to update our routine and confirmatory testing with merging technology.
Our HIV confirmatory algorithm includes a HIV Combo assay and Rapid Diagnostic Test (RDT) of high specificity. The disadvantage is that it's read manually and once the results are recorded, the test cartridge is discarded – no permanent record of it is kept.
In keeping with our philosophy of striving to improve current processes, we evaluated the BIO‐RAD Geenius™ system which reads the test automatically and saves an electronic copy.
Aim
The aim was to investigate the prospect of the Geenius™ system to replace our current RDT and be incorporated into our HIV Confirmatory algorithm.
Method
A qualitative research approach was followed using purposive sampling from our donor base.
120 cartridges were donated by Bio‐Rad:
6 used for Controls60 used for known negative samples5 used for 5 known positive samples of varying strengths46 used for known cross reactive / false positive samples3 HIV‐2 indeterminate samples for re‐run. The procedure, as stated in the package insert, was followed. For the purpose of this study, all HIV‐2 Indeterminate results were excluded from our calculations, as these results are not negative or positive.
Results
All controls were valid.
The known negative samples showed a specificity of 100% (95% CI: 93.56–100%) with a Negative Likelihood Ratio = 1.00 and a Negative Predictive Value = 100% (95% CI: 93.56–100.00%).
The sensitivity of the tests only yielded 83.33% (95% CI: 36.10–97.24%) with a Positive Likelihood Ratio = 0.83 and a Positive Predictive Value = 100% (95% CI: 47.95–100.00%) due to a missed positive result, bearing in mind that it was calculated with five results.
The cross‐reaction samples also showed a specificity of 100% (95% CI: 58.93–100%), Negative Likelihood Ratio = 1.00 and Negative Predictive Value = 100% (95% CI: 58.93–100.00%).
Discussion
The Geenius™ has good features which will enhance the processes in Virology, but it has failed to detect one known positive result. The donor recently seroconverted with low levels of antibody, but the RDT found the sample low reactive.
Although the HIV‐2 Indeterminate (IND) results were ignored, it remains a concern in the blood transfusion environment. To reinstate a previously false‐reactive donor, all results – first‐line and second‐line – must be negative. An IND results poses a problem, resulting in another two‐month hold over for the donor.
Our RDT is also used to test Apheresis Platelets after hours. The result needs to be negative to issue the unit. An IND, results in the unit being discarded. Besides the hefty cost implications, finding willing donors over a weekend can become problematic.
P‐417
Hiv, Hepatitis B, Hepatitis C and HTLV Co‐Infection Among Blood Donors at Three Large Brazilian Blood Centers
Goncalez TTGON1, Moreno ECM 2, Bolina-Santos B-SE 3, Bruhn RB 4, Carneiro-Proietti ABFCP 2, Loureiro PL 5, Mendrone AM 6, Sabino ECS 7, Custer BC 4
1Blood Systems Research Institute, San Francisco, United States of America2Fundação Hemominas/Hemocentro de Minas Gerais, Belo Horizonte, Minas Gerais, Bra, Belo Horizonte, Brazil3Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, Belo Horizzonte, Brazil4Blood Systems Research Institute, Epidemiology, San Francisco, CA, United States, San Francisco, United States of America5Universidade de Pernambuco, Faculdade Ciências Médicas, Recife, Pernambuco, Braz, Recife, Brazil6Fundação Pró‐Sangue/Hemocentro de São Paulo, São Paulo, Brazil., São Paulo, Brazil7Departamento de Doenças Infecciosas, Faculdade de Medicina, Universidade de São, São Paulo, Brazil
Background
The frequency of co‐infections in large sample of blood donors in Brazil has not been reported.
Aim
The objective of this study was to describe rates and characteristics of donors infected by more than one virus: HIV, HBV, HCV and, HTLV.
Methods
Donor and donation data collected by the NHLBI‐ REDS‐II program in Brazil from January 2007 to March 2011 from three blood centers (Sao Paulo, Belo Horizonte and Recife) were included in the analysis. HIV (+) was defined as positive by 2 different EIAs and confirmed by WB. HCV (+) was defined as positive by 2 different EIAs. HBV (+) was defined as positive on 2 different EIA tests for HBsAg with or without antibody for hepatitis B core (anti‐HBc). HTLV (+) was defined as positive by 2 different EIAs. Chi‐square and multivariable logistic regression adjusted for gender, age, self‐reported skin color, level of education, previous donation history (repeat/first donation), donation type (replacement/community), and number of sexual partners in the last 12 months were used to compare donors with only one infection to those with two or more infections.
Results
During the study period 1,326,773 donations were evaluated. Of those, 1,308,262 (98.6%) were successfully screened, resulting in 3608 (0.27%) donations positive for HIV, HCV, HTLV or HBV. Of all screened donations, 711 were HIV (+) (0.05% prevalence), 1126 were HBsAg (+) (0.09% prevalence), 1131 were HCV (+) (0.09% prevalence) and 640 were HTLV (+) (0.05% prevalence). Of donors with HIV, 17 (2.4%) were co‐infected: 7 with HBV; 7 with HCV, 2 with HTLV and, 1 with HTLV and HCV. The overall proportions of viral co‐infection combinations varied from 0.8% to 3.0% (Table 1). The highest proportion of co‐infections was for HTLV with 3.5% of first time donors with HTLV also having other infections. No significant difference in demographics or type of donor (community or replacement) was observed between the HIV only and HIV co‐infected donors except for those HBV (+). For co‐infected donors; repeat donors were almost eight times more likely to be co‐infected with HIV and HBV (OR = 7.6, 95% CI = 1.7–34.4) than first time donors. HTLV and HVC co‐infection was significantly associated with age (>40 years old) when compared with HTLV alone and HCV alone.

Summary/Conclusion
This study found that 2.4% of HIV (+) donors in Brazil were also co‐infected with HCV, HBV or HTLV. While the numbers are small, we found a significant association between repeat donor status and HIV/HBV co‐infection and age with HTLV/HCV co‐infections. While the specific risk factors in these co‐infected donors are unknown, the co‐infection combinations suggest similar underlying routes of infection acquisition; including likely undisclosed risk behaviors at the time of the donation.
P‐418
A Paradigm Shift in the Prevalence of Transfusion Transmitted Infections Among Blood Donors in Bahir Dar Blood Bank, Northwest Ethiopia
Burssa DB1, Demewoz HD 1, Shiferaw MS 2
1Ethiopian Blood Bank Service, Addis Ababa, Ethiopia2Bahir Dar blood bank, Bahir Dar, Ethiopia
Background
Transfusion is one integral part of medical care given to patients but it has its own risk of transmitting blood born infections for recipients. This study compares and analyzes the trends of the prevalence of TTIs among blood donors in Bahir Dar at 2006 and 2014. Bahir Dar city is located 650 km from Addis Ababa in Northwest Ethiopia.
Aim
To see the prevalence of TTIs among blood donors in Bahir Dar blood bank at 2014/15.
Methods
A total of 3817 blood unit of blood was collected from July 2014 up to February 2015. All of the collected blood was screened for HIV, HBV, HCV & syphilis. Fourth generation Ag‐Ab ELISA test (Vironostica) was used for screening HIV, commercial ELISA kits were used for HBV& HCV and Syphilis was screened with RPR reagent.
Results
Of the 3817 donations, 89.5% were from VNRBDs and 10.5% were from family replacement. The overall prevalence of Transfusion transmitted infections among blood donors was 6.9%. The prevalence of HIV, HBV, HCV & Syphilis was 0.9%, 4.3%, 0.7% & 0.96% respectively. There is a marked reduction in the prevalence of TTIs among donors as compared to a study done in the same place at 2006, where the overall prevalence of TTIs among 324 donors (10.5 voluntary donors) were 43.2% of which HIV, HBV, HCV & syphilis accounted 11.7%, 25%,13.3% & 1.2% respectively. (Ethiop.J.Health Dev.2007;21(1):68–69).
Conclusions
This study showed that there is a significant reduction in blood borne infections among blood donors. This reduction is due to the increment of voluntary blood donors contribution and improved donor selection criteria. Even if there is a reduction in the prevalence still much has to be done to improve the safety of blood and blood products.
P‐419
Abstract Withdrawn.
4.5 Bacteria
P‐420
Does Pathogen Inactivation Offer an Alternative to Bacterial Screening for Platelet Components?
McDonald CP , Aplin K , Allen J , Ball J , Robbins S , Pitt T
NHSBT, London, United Kingdom
Background
NHSBT have implemented the bacterial risk reduction measures of improved donor arm disinfection, diversion and bacterial screening of platelet components to increase the safety of the blood supply.
Pathogen inactivation (PI) offers an alternative to bacterial screening. The NHSBT National Bacteriology Laboratory (NBL) have undertaken an evaluation of the Intercept PI Inactivation System (Cerus Corporation).
AimsTo determine the effectiveness of the system against key bacterial species isolated from actual platelet component transmissions, including species not covered in the literature. Also, to perform extensive testing for the presence of bacteria at the end of shelf life and estimated concentration breakpoints at which the system might fail.
Method
Eight bacterial species were spiked independently in pooled platelet components suspended in platelet additive solution. The bacteria used were: Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa, Streptococcus bovis, Streptococcus pneumoniae and Bacillus cereus (spore forming organism).
A pooled and split method was used for each organism tested in order to produce a homogeneous platelet component. Nine day 1 platelet bags were used for each bacterial species. Spiking concentrations were 100, 103 and 105 cfu/ml, 3 bags were used per concentration tested (2 for test and 1 as an untreated control).Bacteria were enumerated and enrichment culture performed immediately after spiking, prior to PI treatment (2 h after spiking), immediately after treatment, and at day 7 of shelf life. PI was performed in accordance with the manufacturer's instructions.
Conclusions
All bacteria species were inactivated at all concentrations with the exception of P. aeruginosa and B. cereus. P. aeruginosa broke through the inactivation process at 103 cfu/ml in both replicates tested to give a final day 7 pack concentration of 108 and 103 cfu/ml. Breakthrough also occurred in 1 replicate at 105 cfu/ml to give a final concentration of 103 cfu/ml at day 7. On repeat of this organism, breakthrough occurred at 105 cfu/ml concentration only in both replicates to give a final concentration of 108 cfu/ml.
B. cereus broke through at 103 and 105 cfu/ml concentrations in all replicates to give a final concentration at day 7 of 106–107 cfu/ml. The manufacturer makes no claim that their PI process inactivates spore forming bacteria i.e. B. cereus.
Discussion
PI offers an alternative to bacterial screening. A 100% inactivation occurred with all organisms tested at the 10 cfu/ml concentration, but at higher concentrations breakthrough occurred with P. aeruginosa and B. cereus. Our data indicates it would be prudent to minimise the time between platelet preparation and inactivation in order to reduce the risk of rapid growing bacteria attaining concentrations in excess of the breakpoint of the PI system. Further studies are required to determine bacterial growth kinetics, breakpoint concentrations and the most appropriate time to PI treatment to ensure patient safety.
P‐421
Platelet Transfusion Relevant Bacteria Reference Strains – Suitability Test of Different Candidate Strains
Spindler-Raffel E1, McDonald CP 2, Benjamin RJ 3, Aplin K 2, Gabriel C 4, Hourfar K 5, Jacobs MR 6, Keil SD 7, de Korte D 8, Mukhtar Z 9, Niekerk T 10, Ramirez-Arcos S 11, Rojo J 12, Satake M 13, Seltsam A 14, Stoermer M 15, Wagner SJ 16
1Paul‐Ehrlich‐Institut, Langen, Germany2NHS Blood and Transplant, London, United Kingdom3American Red Cross, Rockville, United States of America4Austrian Red Cross, Blood Centre Linz, Linz, Austria5German Red Cross, Frankfurt/Main, Frankfurt, Germany6Case Western Reserve University, Cleveland, United States of America7Terumo BCT Biotechnologies, Lakewood, United States of America8Sanquin Blood Supply Foundation, Amsterdam, The Netherlands9Institute of Biological, Biochemical and Pharmaceutical Sciences, Dow Medical Co, Karachi, Pakistan10South African National Blood Service, Weltevreden Park, South Africa11Canadian Blood Service, Ottawa, Canada12Centro Nacionale de la Transfusión Sanguínea, Ciudad de México, Mexico13Japanese Red Cross, Tokyo, Japan14German Red Cross, BSD Springe, Springe, Germany15Transfusion Medicine, University Hospital of Cologne, Cologne, Germany16Holland Laboratory, American Red Cross, Blood Component Dep. Rockville, Rockville, United States of America
Background
Bacterial contamination of platelets concentrates (PC) remains a persistent problem in transfusion. Platelet Transfusion Relevant Bacteria Reference Strains (PTRBRS) are a suitable tool for objective validation and assessment of various microbiological methods for blood safety and development of new techniques for screening and/or pathogen reduction. Furthermore these organisms will allow regulatory agencies as well as manufacturers and blood banks to decide on those approaches in an objective and standardized manner. Therefore the International Society of Blood Transfusion (ISBT) Working Party on Transfusion‐Transmitted Infectious Diseases, Subgroup on Bacteria, organized an international study on PTRBRS. The outcome of this study was the establishment of the first WHO Repository for PTRBRS.
Aims
To characterize new candidate strains for enlargement of the existing 4 strain repository (1st WHO Repository for PTRBRS) a second international collaborative study was performed. The growth of additional 11 candidate strains in PC under pragmatic conditions which simulated contamination during blood donation, as well as independence from donor's properties, was tested.
Methods
Eleven bacterial strains were evaluated in an international study under ‘real life’ and routine conditions which means inoculation directly into PC‐bags to determine their ability to proliferate in PC from multiple donors after low concentration spiking (<1 Colony Forming Unit per millilitre) simulating contamination during blood donation. Counts were performed at days 2, 4 and 7 to assess microbial growth kinetics. The candidate strains included 2 spore formers: Bacillus cereus PEI‐B‐P‐07‐S, Bacillus thuringiensis PEI‐B‐P‐57‐S. Gram‐negative species: Enterobacter cloacae PEI‐B‐P‐43, Morganella morganii PEI‐B‐P‐74, Proteus mirabilis PEI‐B‐P‐55, Pseudomonas fluorescens PEI‐B‐P‐77, Salmonella choleraesuis PEI‐B‐P‐78, Serratia marcescens PEI‐B‐P‐56. Gram‐positives: Staphylococcus aureus PEI‐B‐P‐63, Streptococcus dysgalactiae PEI‐B‐P‐71, Streptococcus bovis PEI‐B‐P‐61. The study was performed in fourteen centres (ten different countries) which tested every strain in triplicate.
Results
With the exception of the Morganella morganii strain, all strains showed moderate to excellent growth until day 7. The individual growth curves showed variation from slow to fast growth. Bacillus cereus, Bacillus thuringiensis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas fluorescens, Serratia marescens, Staphylococcus aureus and Streptococcus dysgalactiae showed a growth of significantly more than 2log10 CFU/ml up to 8log10 CFU/ml from day 2 on. For Enterobacter cloacae, Proteus mirabilis, Staphylococcus epidermidis, Streptococcus bovis and Streptococcus pyogenes this growth cut‐off was reached at day 4. Growth for Salmonella choleraesuis was lower than for other strains and showed a high variability among participants. In addition, the study provided information regarding the growth behavior and kinetics of different bacterial species in PC.
Conclusion
PTRBRS are a feasible tool for validation and assessment of various microbiological methods for improving blood safety. In the present collaborative study 11 candidate strains were characterized. Those that demonstrate growth independent of donor effects under pragmatic conditions will be nominated for inclusion in the WHO Repository.
P‐422
Insights into Bacterial Survival and Distribution During Buffy Coat Platelet Production
Ramirez-Arcos SM 1, Taha M 1, Schubert PS1, Maurer EM 2, Jenkins CJ 1, NetCAD1
1Canadian Blood Services, Ottawa, Canada2LightIntegra, Vancouver, Canada
Background
Canadian Blood Services produces single‐donor apheresis and pooled buffy coat (BC) platelet concentrates (PCs) which are screened for bacterial contamination with the BacT/ALERT system. Results of platelet screening showed a rate of positive cultures (true positives plus indeterminates) approximately two‐fold higher in apheresis PCs, which are leukoreduced during blood collection. Since BC pools are leukoreduced 24–28 h following blood collection, it is hypothesized that the overnight holding of collected whole blood (WB) at room temperature (RT) prior to BC PC production has a bactericidal effect.
Aims
Investigate the bactericidal effect of WB during overnight holding prior to BC pool production and evaluate changes in PC quality resulting from bacterial contamination.
Methods
WB units were inoculated with ~102–103 colony forming units (CFU)/mL of 8 bacteria (Staphylococcus epidermidis ST10002 and ST11003, Escherichia coli ATCC10798 and CBS11001, Serratia marcescens CBS07/2005 and CBS12/2010, and Klebsiella pneumoniae ATCC10031 and PEI‐B‐P‐08‐1) and subjected to the BC PC production process (N ≥ 2). Samples for bacterial enumeration were taken from WB before and after overnight holding at RT and from production fractions including RBC, plasma, BC and PCs. Bacterial growth was evaluated after 5 days of PC storage. On days 1 and 5 of storage, PC samples were taken for flow cytometry analyses of platelet activation and apoptosis. These were assessed by determining P‐selectin expression (CD62P) and phosphatidylserine exposure (AnnexinV) on the platelet surface, respectively. Furthermore, the ThromboLUX device (LightIntegra Technology Inc.) was used to test PC quality based on dynamic light scattering.
Results
The concentration of S. epidermidis remained unchanged after overnight holding of WB at RT. While both ATCC strainswere eliminated overnight, other Gram‐negative organisms proliferated in WB to significantly higher concentrations (P < 0.0001). There was no significant difference in the distribution of S. epidermidis‐bothand K. pneumoniae PEI‐B‐P‐08‐1 in the BC production fractions; however, S. marcescens‐bothand E. coli CBS11001 were preferentially found in the cellular fractions compared to plasma (P < 0.05). Despite varying initial concentrations on day 1, all non‐ATCC strains proliferated during platelet storage. CD62P expression levels in PCs carrying E. coli and K. pneumoniae were 50% and >80% on days 1 and 5 of storage, respectively. PCs with S. marcescens CBS07/2005 showed an artefactual decrease in CD62P expression by day 5 of storage which was linked to apoptosis. Only S. marcescens or E. coli growth in PCs resulted in ThromboLUX score values <10 by day 5 of storage indicating decreased product quality.
Conclusions/Summary
Some bacteria were eliminated during overnight WB holding likely due to plasma sensitivity properties. However, others remained viable or proliferated to high concentrations, indicating that WB holding during BC PC production does not have a broad‐spectrum bactericidal effect. Bacterial distribution in the BC production fractions was species‐dependent. Importantly, Gram‐negative pathogens proliferating in WB would be detected by routine BacT/ALERT testing. The effect on platelet quality depends on the bacterial strain and therefore is not a universal indicator of bacterial contamination in PCs. Overall, this study provides novel insights into the effects of BC PC manufacturing on bacterial contamination in PCs.
P‐423
Bacterial Screening of Platelets Does Not Guarantee Safety
Brailsford SR , Pitt T , McDonald CP , Roy A , Allen J , Reynolds CA , Tossell J
NHS Blood and Transplant, London, United Kingdom
Background
Bacterial screening of platelets was introduced by NHSBT in 2011 as an additional blood safety measure. Numbers of initial reactive and screen positive platelets are routinely monitored and reported. Any confirmed transfusion‐transmitted infections (TTIs) are reported to the Serious Hazards of Transfusion scheme (SHOT). Between February 2011 and August 2013 there were no proven false‐negative screens or bacterial TTIs.
Aims
To describe the outcome of three platelet donations which were falsely screen‐negative on NHSBT bacterial screening between September 2013 and December 2014
Methods
All platelet packs manufactured by NHSBT are screened for bacteria using the BacTAlert method. All packs are sampled after a minimum of 36 h post‐donation; 8 ml volume from each pack is cultured under aerobic and anaerobic conditions until the end of the 7 day shelf life. Platelets are issued as ‘negative‐to‐date’ after 6 h of incubation. Any hospital reporting a visually abnormal platelet pack is asked to return this for bacterial culture; associated packs are also recalled. If bacterial contamination is proven then the donor is followed up and, if appropriate, skin swabs taken to look for bacterial carriage.
Results
By December 2014 1,020,688 platelet packs had been screened and Staphylococcus aureus successfully detected on screening from 6 apheresis donations and four pooled platelets. However, there have been three hospital reports of visually abnormal apheresis platelets which were proven to be contaminated; all due to S. aureus. Separate reports were received in September 2013 (case 1), May 2014 (case 2) and December 2014 (case 3). In the first case a clump was noted in a day 5 platelet, S. aureus was also grown from the associated pack and the donor; all strains were indistinguishable on spa gene typing. In case 2 the pack was noticed to contain a clump on day four but no bacteria were isolated from the associated pack; strains from the index pack and donor were indistinguishable. In case 3, a day 5 platelet contained clumps, the two associated packs had already been transfused with no adverse events reported in either of the recipients. S. aureus was cultured from the index pack and donor; typing is ongoing. In all cases the screening results were falsely negative, but the S. aureus isolated from the contaminated packs appeared to have normal growth characteristics. Donors were eligible to donate; one donor disclosed a history of eczema, while the other two had no obvious skin problems but reported non‐healing lesions on an ankle and nostril respectively
Summary
NHSBT has identified four falsely screen‐negative packs from three donations since screening began in 2011. In all cases it was possible to show that the donor had S. aureus carriage and was the likely source of the platelet contamination. It is postulated that no bacteria were present in the screening sample, possibly due to low numbers in the pack or clumping, resulting in a negative screen. Although bacterial screening is an effective risk‐reduction method, it does have limitations particularly for the detection of some S. aureus strains.
P‐424
Surveillance Cultures on Day‐7 Apheresis Platelets Which Outdated on Day 5
Townsend J1, Bravo M 1, Vanderpool M 1, Tomasulo P 1, Custer B 2, Kamel H 1
1Blood Systems, Scottsdale, United States of America2Blood Systems Research Institute, San Francisco, United States of America
Background
To improve the sensitivity of in‐process platelet bacterial culture screening (primary test), we increased the percentage of the collection volume cultured. This study assessed the impact of a large volume proportional inoculum (LVPI).
Study design and methods
Apheresis Platelets (AP) are collected on Trima and Amicus separators. Primary testing was performed using BacT/ALERT 3D (BacT); a minimum of 3.8% of mother bag volume was inoculated 24–36 h post‐collection into 1–3 BacT aerobic bottles. Components with negative results were released 12 h post‐inoculation; bottles were monitored for 5 days. Platelets which expired on day 5 were maintained at manufacturing conditions and returned to the blood center. Over 12 months, 60% of the expired platelets were sampled (10 ml in 1 aerobic BacT bottle) on day 7 and incubated for 24 h (surveillance test). When a positive signal occurred, the bottle and the platelet unit were sent to a reference microbiology lab for evaluation and identification. Result classification followed modified AABB recommendations: true positive (TP), discordant negative (DN), false positive (FP) and indeterminate (IND).
Results
Of 9041 (1659 Amicus and 7382 Trima) day‐7 AP units cultured, there were 5 TP (3 Amicus and 2 Trima), 1 DN (Amicus) and 10 FP (1 Amicus and 9 Trima), table. Of the 5 TP, 3 were Amicus (0.00181, 95% CI 0.00037–0.00528) and 2 were Trima (0.00027, 95% CI 0.00003–0.00098). The two‐sided test of proportion comparing Trima to Amicus indicates a statistically‐significant difference in the proportion positive with P = 0.016. All TP alarmed within 8 h of incubation. Bacterial species isolated included coagulase‐negative Staphylococcus (n = 4) and Leclercia adecarboxylata (n = 1). There were 5 co‐components associated with the 5 TP units: 3 platelets and 1 RBC were transfused with no adverse event; a 5th platelet was discarded.
Caption 1: Summary of Surveillance Testing Results for Expired Platelets

Conclusions
The yield of surveillance culture of day‐7 Trima AP units screened with LVPI in this study is 271/million. Published studies with varying design and methods reported higher rates after post‐primary screening: 326/million (PGD test) and 480/million (plate culture) at point of transfusion [Jacobs et al. Transfusion 2011, 51:2573] and 662/million by surveillance test (Dumont et al. Transfusion 2010,50:589). Due to rarity of events and varied methodologies (primary tests’ sensitivities, day of secondary test, secondary tests’ sensitivities, etc.) direct comparison is fraught with difficulty.
P‐425
A New Generation Bacterial Identification System for Use in Blood and Transplantation Medicine
Allen J , Pitt T , McDonald CP
NHSBT, London, United Kingdom
Background
The National Bacteriology Laboratory (NBL) provides a centralised service for National Health Service Blood and Transplant (NHSBT), performing routine screening of a range of tissue and blood products, and acting as a reference laboratory for investigation of transfusion incidents and environmental monitoring of NHSBT sites. Accurate and timely bacterial identification is a pre‐requisite of the laboratory. However, currently used systems are based on phenotypic methods, which can be problematic when identifying certain transfusion and transplantation isolates and often take 48 h to achieve full, species‐level identification. Additionally, each identification has cumulative costs of around £10.
The development of Matrix‐Assisted Laser Desorption/Ionisation‐Time‐of‐Flight Mass Spectrometry (MALDI‐TOF‐MS) for bacterial identification has offered a more rapid and cost effective alternative, with specificity comparable to the gold‐standard, 16S rRNA sequencing. Identification is based on the molecular composition of the sample, determined by laser ionisation and separation of the molecular ions by their mass/charge ratio, which are plotted as mass spectra. As many spectral peaks correspond to species‐specific ribosomal proteins, this provides a unique fingerprint that can be matched against a database of known species.
Aims
The aim of this study was to assess the performance of the Vitek MS (Biomérieux) and MALDI Biotyper (Bruker) against a range of transfusion and transplantation isolates, and to compare both instruments for potential use within the laboratory.
Methods
Of 164 wild‐type bacterial isolates were selected from the NBL −80°C cryobank and passaged twice on Columbia blood agar, incubated at 35 ± 2°C for 24–72 h at each stage. A sample of each isolate was then applied directly to a MALDI target plate, in triplicate, and analysed using each system. Results were compared against the original identification reported by NBL, and any discrepant isolates were repeated, with identification confirmed by 16S rRNA sequencing.
Results
Of the 164 isolates, overall 91.4% and 93.3% were identified correctly to species level by the Vitek MS and MALDI Biotyper, respectively, including pathogens such as Staphylococcus aureus, Enterobacteriaceae and Clostridium species. While absence of less clinically‐significant species from the MALDI databases contributed some erroneous results, frequent inconsistencies were also noted for members of the Streptococcus mitis group and coagulase‐negative staphylococci. As genus‐level identification remained consistent (members were frequently reported as S. pneumoniae by the Biotyper and S. mitis/oralis by the Vitek MS), the variations seen may have been due to the homologous nature of ribosomal proteins between members of these groups, producing largely indistinguishable spectra. Unfortunately, each system failed to assign any result to 2 isolates, including infrequently isolated environmental species such as Bacillus gallactosidilyticus and Gordonia species. Expansion of the databases, either through planned periodic update by the manufacturer, or manual addition by the user, may rectify this problem if isolated in the future.
Summary
MALDI‐TOF‐MS provides a rapid, accurate and low cost identification and this study found the performance of each system to be comparable. Recent development of direct identification of bacteria from blood culture bottles may prove to be an additional advantage, and this will be evaluated in a future study by NBL.
P‐426
Bacterial Contamination in a Blood Bank Environment: Prevalence and Implications
Pape MP , Bakaloudi VB , Girtovitis FG , Ntinopoulou EN , Konstantinidou AK , Chatzikyrkou MC , Voulgaridou VV , Pantelidou DP , Chalkia PC , Floridis KF , Tzioura AT , Georgopoulou IG , Mantoudi TM , Moumtsaki EM , Protonotariou EP , Vasilaki OV , Skoura LS , Hasapopoulou-Matami EH
Ahepa Hospital, Thessaloniki, Greece
Introduction‐objectives
Surfaces frequently touched by healthcare workers are commonly bacterially contaminated. Routine cleaning and disinfection does not always remove pathogens from contaminated surfaces. Little is known about the prevalence or significance of environmental contamination in the Blood Bank setting. All kinds of pathogens, even normal flora, are important to infection control. Effective donor arm disinfection and diversion of the first 20–30 ml of blood to a satellite pouch, prevents blood bag contamination during collection. Processing, storing and distributing blood and blood components might expose to risk if the guidelines concerning cleaning and disinfecting are not adhered to.
Our aim was to evaluate the prevalence and type of bacterial contamination of high‐touch surfaces in the blood bank of a tertiary care University General Hospital.
Methods
The Blood Bank of the AHEPA Hospital collects and processes about 25,000 whole blood units per year, receives cellular components from other Blood Banks and distributes about 36,000 red cell concentrates, 17,300 FFP, 13,500 random platelet units and 850 plateletpheresis units. We chose the areas and items for sampling according to standard criteria for high‐touch surfaces. Surface swabbingprocedureswere applied using sterile cotton swabs. A total of 60 samples were taken from the donation area, plateletpheresis, processing, blood grouping, immunohematology and serologic testing laboratories. All swabs were inoculated in appropriate solid nutritious materials and incubated aerobically at 37°C for 24–48 h. Swab controls were also incorporated and enrichment broth techniques were also used in order to exclude false negative results. Growth was identified as per standard microbiological procedures.
Results
No growth appeared on the media used for swab controls. The positive culture rate was 60% (36/60 samples). Most of the isolated microorganisms were normal flora: 31/60 (51.66%). Pathogens were identified in 5/60 samples (Pseudomonas sp., Acinetobacter, Proteus), as shown in the table

Conclusion‐comments
Implementing microbiological surveillance programs and keeping records of the culture results, helps to be alert and avoid contamination. Due to the effectiveness of the sampling techniques used, our study showed high prevalence of environmental contamination. Normal flora prevailed. However, the detection of Acinetobacter, Pseudomonas, and Proteus in the setting of the Blood Bank is disturbing. They usually grow in soil or water. Transmission from sinks to hands and from hands to high‐touch surfaces might represent a potential hazard.
Sampling the hands of the staff and repeat sampling from the same sites, has to be applied in order to provide a means of monitoring trends over time.
P‐427
Molecular and Phenotypic Characterization of Microbial Contaminants Isolated of Umbilical Cord Blood Units for Transplant
Bello-López JB , Ibáñez-Cervantez GIC , Fernández-Sánchez VFS , Millán-Rocha MMR , Rojo-Medina JRM
National Center of Blood Transfusion, Mexico City, Mexico
Background
In order to ensure the safety of the Umbilical Cord Blood Units (UCBU) supply, suitable for transplantation, it is necessary to have strict quality controls. These controls include: CD34+ cell count, typing by Human Leukocyte Antigen (HLA), serological tests, clonogenic capacity and microbiological monitoring. The collection, manipulation, cryopreservation and transplantation of UCBU involve a large number of procedures that are carried out in different areas and can result in microbial contamination of the final UCBU.
Aims
To implement the use of 16S rRNA gene sequencing and ERIC‐PCR for the microbial strains typing obtained from criopreservated UCBU in the Cord Blood Bank (CBB) of the National Center of Blood Transfusion (NCBT) in a period of 10 years (2003–2013) to identify potential sources of microbial contamination and carry out measures for their prevention.
Methods
UCBU that showed initial microbial contamination were subject to strains isolation, identification and characterization by sequencing the 16S rRNA gene and Enterobacterial Repetitive Intergenic Consensus (ERIC‐PCR). Moreover, tests of antimicrobial resistance/sensitivity and phenotypic activities that may play an important role in microbial infection were detected.
Results
Microbial contamination (aerobic, anaerobic or both) were detected in 120 units (2.31%) in this 10 years. The most frequently isolated organisms were Enterococcus faecium (26.16%), followed by Staphylococcus epidermidis (15.88%), Escherichia coli (15.88%), Enterococcus faecalis (13.08%), Staphylococcus haemoliticus (6.54%), Klebsiella pneumoniae (6.54%), Enterococcus durans (6.54%), Lactobacillus helveticus (3.73%), Roseomonas spp. (2.81%) and Enterococcus hiriae (2.81%). The ERIC‐PCR essays revealed a wide genetic diversity in some strains although they belonged to the same genus and specie. The most common sources of contamination were: vaginal flora, digestive tract and skin flora. This results show that sulfonamides, folate antagonists, nitrofurans and first generation cephalosporins were the drugs with the best antimicrobial effect against all strains tested. The broad‐spectrum penicillins, third generation cephalosporins, aminoglycosides and fluoroquinolones showed lower inhibitory activity on the tested strains. All strains were proteolytic, 57.6% were amylase‐positive, 77.6% nuclease‐positive and 53.5% hemolysis‐positive.
Conclusions
This is the first study reporting Roseomonas spp. strains isolated from UCBU. Future molecular testing should be performed for the characterization of these strains of clinical interest. Although the incidence of contaminated UCBU found in this work is low, it is recommended continuing with quality protocols all the time (obtaining the CB in the operating room, process in CBB, and cryopreservation). Contamination sources identified in this work show the need for training in collecting cord blood, since all contaminants identified belong to the microbial flora of the donors.
Figure 1. Isolation strains bacterial percentage obtained from 107 contaminated Umbilical Cord blood (UCB) units.

Figure 2. ERIC‐PCR fingerprints of isolated strains from UCBU. A: E. durans; B: E. faecium; C: S. haemolyticus; D: S. epidermidis and E: Roseomonas sp

P‐428
Detection of Bacterial Contamination in Platelet Concentrates by Molecular Amplification of the Ribosomal 16S Gene
Levi JE1, Viana JD 1, Ferreira SCL 1, Matana SR 1, Rossi F 2, Patel P 3, Garson JA 4, Mendrone A Jr1
1Fundação Pró‐Sangue Hemocentro de São Paulo, São Paulo, Brazil2Microbiology Department, DLC, HC‐FMUSP, São Paulo, Brazil3Microbiology Services, NHS Blood and Transplant, London, United Kingdom4Division of Infection and Immunity, University College, London, United Kingdom
Background
Among cases of transfusion transmission of infectious agents, bacterial contamination ranks first in the number of events, morbidity and mortality. This occurs mainly by transfused platelets, which are stored at room temperature and under constant agitation. Automated culture is adopted by some blood banks for screening of bacterial contamination, but this is expensive and has a relatively long turnaround time. Recently some groups have evaluated the use of molecular amplification methods such as real‐time PCR based on the highly conserved 16S rRNA gene; this allows a single pair of ‘universal’ primers/probe to detect a very broad range of bacteria.
Aims
To establish a semi‐automated high‐throughput DNA amplification method for universal screening of bacteria in platelet concentrates.
Methods
Platelet concentrates were spiked with suspensions of Staphylococcus aureus, Staphylococcus hominis, Staphylococcus epidermidis, Enterobacter cloacae and Serratia marcescens to 1 and 10 colony‐forming units (CFU)/ml, to simulate contamination occurring during blood donation. These bacterial strains were recovered from platelet concentrates verified during routine screening. The platelet concentrates were stored at room temperature under agitation for 5 days and 500 μl aliquots were drawn every 24 h. The presence of bacteria was investigated by real‐time PCR and by the eBDS assay (Enhanced Bacterial Detection System, PALL) as a reference method. DNA was extracted by using a Large Volume kit in the MagNA Pure 96 (Roche) system. Real‐time PCR amplification was performed with a set of universal primers and probe targeting the 16S rRNA gene. Co‐amplification of human mitochondrial DNA served as an internal control. The amplification mixture was treated with ethidium monoazide (EMA) followed by photoactivation to eliminate contamination with spurious bacterial DNA in reagents (Garson J.A. et al, Transfusion 2014;54:870–878.).
Results
With the real‐time PCR it was possible to detect the presence of all bacterial species tested with an initial concentration of 10 CFU/ml 24 h after contamination of platelet concentrates, except for Staphylococcus hominis. The PCR assay also detected the presence of bacteria Serratia marcescens and Enterobacter cloacae with an initial concentration of 1 CFU/ml. The results of the molecular test could be obtained in 4 h. During the study period 5 units were found positive by routine testing with the eBDS system and were also positive by this molecular method.
Conclusions
The real‐time PCR assay may be a good alternative to conventional culture methods in the screening of bacterial contamination of platelet concentrates, enabling bacterial detection even with a low initial concentration of microorganisms, whilst offering good sensitivity and a fast turnaround time.
P‐429
The Pilot Studies for Lyme Disease (Lyme Borreliosis) in Blood Donors in RCKiK in Poznan
Laba A , Kalisz N , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Background
Lyme disease is caused by Borrelia burgdorferi spirochete. The transfer of the bacteria through the blood to the immunocompromised recipient can cause full‐blown Lyme disease with serious complications. So far there has been no such case.
Aim
The aim of studies was to verify if people qualified to donate blood who are regarded as healthy may have serological markers of Borrelia burgdorferi infection.
Material and methods
364 blood samples were tested from donors who knowingly subjected to the tests and filled a questionnaire regarding the exposure to a tick bite and exposure to a lyme disease in their workplace (i.e. agriculture or forestry), fact of being bitten and skin symptoms suggesting an infection. The tests were carried out by using Anty‐Borrelia ELISA‐ test for the presence of IgG and IgM antibodies in blood of the tested donors.
Results
In 21 samples (which make 5.76% of tested group) IgG and/or IgM antibodies were detected. In order to confirm the accuracy of the tests, donors were asked to give blood samples again in RCKiK in Pozna’ – 19 people turned up. In 6 samples with positive IgG results, positive IgM results also were detected. In 7 samples with positive IgG results, IgM in the range of the negative values were detected. In one sample with borderline IgG values, borderline IgM values were detected. In one sample with IgG borderline values, IgM in range of the negative values were detected. In 4 samples with negative IgG values, negative IgM values were detected. In six samples positive results of IgM antibodies were detected, although no donor reported the exposure to the risk of lyme disease in his/her workplace or the risk of a tick bite. No one noticed any skin symptoms suggesting an infection.
Conclusions
The presence of IgG and IgM antibodies for the Borrelia burgdorferi occurred in a small group of tested donors and it was confirmed in the next test using the same test.
No connection between the exposure to the risk of being bitten by a tick and the presence of antibodies was noted.
Current Polish regulations for blood donation system do not disqualify a donor because of the lyme disease. However, information from published sources does not exclude the possibility to pass on the Borrelia burgdorferi spirochete by the means of blood. That is why donors with positive results were temporarily disqualified and directed to the specialist for further diagnostics.
P‐430
Performance of a New Automated Syphilis Assay for the Detection of the Presence of Antibodies to Treponema pallidium
Schlauder GG1, Anderson M 1, Hershberger S 1, Asgedom H 2, Dixon L 2, Krysztof D 2, Bakker E 3, van Weert A 3, Bos H 3, Williams G 1
1Abbott Laboratories, Abbott Park, IL, United States of America2American Red Cross, Scientific Support Office, Gaithersburg, MD, United States of America3Sanquin, Amsterdam, The Netherlands
Background
Despite the low frequency of transfusion related cases of Syphilis, screening for the presence of antibodies to the bacterium Treponema pallidum is still commonly performed on blood donations in many countries. In an effort to improve the specificity of the detection of antibodies to T. pallidum, a new automated prototypechemiluminescence immunoassay was developed with the objective of improved performance for blood screening.
Aims
To evaluate the overall performance of a prototype chemiluminescence immunoassay for the detection of antibodies to the bacterium T. pallidum, on the automated ARCHITECT® platform.
Methods
The performance of a new automated chemiluminescence immunoassay for the detection of Syphilis antibodies was evaluated on the ARCHITECT iSR2000 instrument. Precision was tested using the Clinical and Laboratory Standards Institute (CLSI) document EP5‐A2 (20 days) on 3 reagent lots. Sensitivity was evaluated on 615 known Syphilis positive samples and as well as the one commercially available seroconversion panel. Specificity was evaluated on samples obtained from 17,252 blood donors from the United States of America (USA) and the Netherlands and 698 diagnostic specimens from the USA. Sensitivity and specificity testing were split across three reagent lots. Confirmation of reactive samples was performed using an algorithm with three confirmatory assays, INNO‐LIA™ Syphilis Score, Mikrogen Diagnostik's recomLine Treponema IgG, and recomLine Treponema IgM blots.
Results
The within laboratory CV was ≤4.12% for samples with values within the range of 1.00 to 3.75 S/CO (the upper limit of the PC) across all three lots evaluated. Overall clinical sensitivity was 100% on 615 known Syphilis positive samples tested. For the one available seroconversion panel, all positive bleeds were detected. The overall resolved specificity was 99.95% (17,194/17,203). Forty initial reactive samples were detected. All 40 samples were repeat reactive with 13 samples confirmed as positive and 18 identified as indeterminate by the confirmatory algorithm. The repeat reactive rate on blood donor samples, excluding confirmed positive samples, was 0.16% (27/17221). Resolved specificity for blood donor serum and plasma samples was 99.98% and 99.91%, respectively. Resolved specificity on 698 diagnostic specimens was 99.85% (683/684) with 12 samples confirmed as positive and two identified as indeterminate by the confirmatory algorithm.
Summary/Conclusions
These results indicate that the new automated prototype ARCHITECT Syphilis TP assay provides acceptable performance in specificity, sensitivity and precision. Sensitivity was comparable to the current on‐market ARCHITECT Syphilis TP antibody assay while specificity was improved over that published in the package insert of 99.78%.
P‐431
Bacterial Contamination of the Hands of Health Care Workers in a Blood Bank
Pape MP , Bakaloudi VB , Girtovitis FG , Ntinopoulou EN , Konstantinidou AK , Chatzikyrkou MC , Voulgaridou VV , Pantelidou DP , Chalkia PC , Floridis KF , Tzioura AT , Georgopoulou IG , Protonotariou EP , Vasilaki OV , Skoura LS , Hasapopoulou-Matami EH
Ahepa Hospital, Thessaloniki, Greece
Background
Various studies have investigated the rate of contamination of the hands of health care workers, not in the blood bank setting though. Shared working areas, close physical contact, and variable hygiene habits of the staff contribute to the transmission of infectious agents.
Aims
The aim of this study was to determine the prevalence of bacterial colonization of the hands of health care workers in the Blood Bank setting.
Methods
The study was conducted in the blood bank of the AHEPA University General Hospital which collects and processes about 22–25,000 whole blood units per year, receives cellular components from other Blood Banks and distributes annually about 36,000 red cell concentrates, 17,300 FFP, 13,500 random platelet units and 850 plateletpheresis units. A total of 35 staff members were randomly chosen and tested: seven doctors, 14 nurses, 11 laboratory technologists, three health visitors. The collection of the samples was performed without prior notification of the staff, in order to ensure that the participants would not take any extra hygiene measures beyond their routine practice. This series does not include repeat sampling. Cotton swabs, moistened with sterile normal saline solution (sterile solution of sodium chloride 0.9%), were used to collect samples according to a standardized procedure. A single swab was used for both hands of each person sampled. The sample was collected by swabbing the dorsum of each finger three times and the palm of each hand twice with a twirling motion of the swab. All swabs were inoculated in brain‐heart infusion broth and kept at 37°C overnight. Then 50 μl of the broth was plated in appropriate solid nutritious materials and incubated aerobically at 37°C for 24–48 h. Growth was identified as per standard microbiological procedures. Antibiotic susceptibility was not determined.
Results
All samples were culture‐positive. Among the isolated microorganismsBacillus sp (B. subtilis commonly found in the upper layers of the soil and considered a normal gut commensal in humans) predominated (21/35, 60%), followed by Coagulase Negative Staphylococci (CoNS 7/35, 20%). The Gram negative microorganisms Pseudomonas sp., Acinetobacter sp., E. coli and Pantoea Agglomerans were isolated from 6/35 samples (17.14%), In 4 cases more than one microorganism were isolated (in three of them Gram‐ bacteria were implicated, as shown in the table). No sample grew positive for staphylococcus aureus or fungi.

Conclusions
Besides common flora, this study has shown Gram negative pathogens in 17.14% (6/35) health care workers sampled without prior notification. This type of study is bound to make health care workers more conscious and increase adherence to the established hand hygiene guidelines. A program of repeat sampling is needed to monitor the degree of adherence.
P‐432
Platelet Transfusion Transmitted Relevant Bacteria – Growth Ability of Different Morganella morganii Strains in Platelets
Spindler-Raffel E1, McDonald CP 2, Benjamin RJ 3, Aplin K 2, Gabriel C 4, Jacobs MR 5, Ramirez-Arcos S 6, Satake M 7, Seltsam A 8, Wagner SJ 9
1Paul‐Ehrlich‐Institut, Langen, Germany2NHS Blood and Transplant, London, United Kingdom3American Red Cross, Rockville, United States of America4Austrian Red Cross, Blood Centre Linz, Linz, Austria5Case Western Reserve University, Cleveland, United States of America6Canadian Blood Service, Ottawa, Canada7Japanese Red Cross, Tokyo, Japan8German Red Cross, BSD Springe, Springe, Germany9Holland Laboratory, American Red Cross, Blood Component Department, Rockville, IL, United States of America
Introduction
Platelet concentrates (PC) are stored at 22 ± 2°C for optimal platelet viability and function. This temperature favours the growth of the majority of microorganisms up to clinical relevant levels. Platelet Transfusion Relevant Bacteria Reference Strains (PTRBRS) are a suitable tool for objective validation and assessment of microbiological methods for blood safety. Bacteria in PC can be inactivated by various self‐sterilisation effects of blood, or may survive, but not grow or grow in PC during storage to clinical significant levels. Different isolates of the same bacterial species may vary in their behaviour in PC.
Objective
To analyse the growth behaviour of three different Morganella morganii subsp. morganii strains in PC.
Materials and methods
Two different Morganella morganii subsp. morganii strains (PEI‐B‐P‐74, PEI‐A‐91) were evaluated in an international study (eight sites) inoculated directly in PC bags regarding their ability to proliferate in platelet concentrates after low spiking (<1 Colony Forming Unit per millilitre) simulating contamination occurring during blood donation. Microbial counts were performed at days 1, 2, 4 and 7. The third strain (PEI‐B‐P‐75) was tested under comparable conditions at one site. Genome sequencing was performed.
Results
Whereas one strain (PEI‐A‐91) showed logarithmic growth in all tested PC, the two other strains remained at low microbial counts or were not detected at all after 7 days storage. PEI‐A‐91 an isolate from a cord blood collection seems to be very robust against the antimicrobial properties of PC, which might include small numbers of leucocytes and phagocytes, various antibodies and nonspecific inhibitors. PEI‐B‐P‐74 showed poor growth in the observed PC. It seems that this strain‐type has an unsuccessful ‘survival strategy'in PC and the self‐sterilizing effects were successful. Only a spiking with more than 3000 CFU led to detectable growth. These data indicate that the potential for growth in PC varies at a strain level. Genome sequencing of the three strains showed a high diversity among the strains. Further investigations including genotypic and phenotypic tests will be necessary to clarify whether differences in bacterial genome can be linked to observed differences in growth behavior or ‘survival strategies'in PC.
Conclusion
PTRBRS are a feasible tool for objective validation and assessment of detection methods for contamination or pathogen reduction in blood components. The example of Morganella morganii subsp. morganii showed that growth ability may vary on strain level and underlined the importance of the validation of strains before selection and dedication as bacterial reference strains. Further investigations are necessary to delineate the mechanisms allowing survival of bacteria in PC.
P‐433
Skin Disinfection Prior to Injection: Standard Precautions
Perillo M1, Tomeo R 1, Gigliofiorito P 2, De Caprio G 1, Chiriano P 1, Sagliano SIC 1, Guida P 1, Misso S 1
1Medicina Trasfusionale ASL Caserta, Aversa, Italy2Plastic and reconstructive surgery unit, Università Campus Bio‐Medico, Roma, Italy
Background
Bacterial contamination is nowadays considered a major issue after blood transfusion. This is an unsolved problem and represents a common issue in this field, being tied with a higher morbidity and mortality. First source comes from the skin of the donor arm. In this case, prevention is the solution, and preventive disinfection is mandatory by lowering the bacterial concentration, which is located on the skin.
Aims
Aim of this work was to examine the effects of two common antiseptic solutions in use at our Department (SIMT ASL Caserta), Clorexidine 2% and Farmasept 0.175%, in order to establish the best way to prevent adverse reactions. The results will be valid as internal guidelines for our daily practice.
Methods
50 donors were enrolled in this study. Each participant had a skin microbial sample before and after disinfection. The disinfection was conducted by a senior nurse with a sterile gauze passed in a spiral fashion from the center of the target area to the periphery. We used 2 ml of liquid and left it in place for 1 min before having the bacterial culture. Samples were placed on three fields for 24 h at 37°C:
1. Columbia Agar with sheep blood at 5%.
2. Agar MacConkey.
3. AgarSabouraud.
Quantitative analysis was performed for each plate
Results
Before antiseptic detersion we detected a bacterial growth mainly Gram + based (1000 ufc/skin cm2). After disinfection no growth was fount in both series.
Summary/Conclusions
Both solutions showed a valid antimicrobial activity. No differenced were registered within the two groups. This study showed that preoperative disinfection is a valid tool to reduce minor and major complications after blood transfusion. Costs and timing are quite negligible if compared with the potential infective risks discussed previously. Within our unit nowadays donor skin’ antiseptic detersion is now a constant step during any daily procedure.
P‐434
Monocyte Activation Test – A Feasible Test for Measuring Pyrogens in Pathogen Inactivated Platelet Concentrates
Sicker US1, Spindler-Raffel ESR 1, Schurig US 1, Löschner BL 1, Janetzko KJ 2, Lindner ML 2, Hourfar KH 3, Spreitzer IS 1
1Paul‐Ehrlich‐Institut, Langen, Germany2DRK BSD Baden‐ Württemberg‐Hessen, Mannheim, Germany3DRK BSD Baden‐Württemberg‐Hessen, Frankfurt am Main, Germany
Background
Pathogen inactivated Platelet Concentrates (PC) treated with Amotosalen and irradiated with UV‐A light are approved in several blood transfusion services in Germany. These irradiated PCs can be stored up to 5 days without bacterial screening. Untreated platelets have to be tested for microbiological contamination between day 2 and 4 to be stored for 5 days. Transfusion associated accidents with PC are supposed to be prevented by Pathogen Inactivation (PI). Reduction of bacterial load up to 6 log10 levels shall be achieved in most cases of contamination. Therefore PI is supposed to be safe by inactivating most contaminating bacteria.
However bacteria affected by PI might release LPS and/or other immune‐activating structures into the PC transfusion unit, thus triggering pyrogenic reactions including shock (probably leading to fatal reactions for the recipient). Unknown and known irradiation products (cell fragments, remaining residual inactivating compound, and virus particle) can take effect due to synergistic effects on pyrogenicity, too.
Aims
Former results have demonstrated the feasibility of the Monocyte Activation Test (MAT; Ph. Eur. 2.6.30.) for detecting pyrogens in PPCs (poster DGTI 2013, Münster, Germany, September, 24–27.). This study is an approach for measuring bacterial pyrogens with the MAT in deliberately pathogen inactivated PPCs in order to investigate whether the inactivation process and the remaining debris could be harmful for the recipients.
Another approach was measuring the pyrogenicity of fixed amounts of bacteria in PPC‐matrix; therefore the four strains were inactivated by irradiation with UV‐C light before addition to the blood component and MAT measurement.
Methods
18 outdated PPCs (stored >5 days) are contaminated with four different strains (Staphylococcus epidermidis PEI‐B‐P‐06, Streptococcus pyogenes PEI‐B‐P‐20, Klebsiella pneumoniae PEI‐B‐P‐08 and Escherichia coli PEI‐B‐P‐19) established by PEI as the first WHO Repository for Platelet Transfusion‐Relevant Bacteria Reference Strains (PTRBRS). For each strain three PC‐bags were spiked with 1.5–2.5 CFU/bag. Pathogen Inactivation for each strain was performed near the limit of log‐reduction published by the manufacturer and was calculated with former growth curves of the applied strains. MAT was performed after 24 and 120 h. In the meantime, PPCs were stored under the routine storage conditions.
Separately, the four bacterial strains were inactivated by UV‐C light, diluted with sodium chloride and mixed with an aliquot of uncontaminated PPC (matrix). Samples were measured with the MAT.
Results
Bacterial pyrogens were detected with MAT in both experimental approaches with contaminated pathogen inactivated PCs and in samples composed with inactivated bacteria and PC.
Summary
To give evidence about the impact of pyrogenic PCs and possible complications it would be advantageous if PI‐PCs which caused febrile transfusion reactions in recipients would be tested with the MAT in the next future.
P‐435
Can Staphylococcus epidermidis from Blood Products be Dangerous for Patients Subjected to Arthroplasty? The Importance of Communication Between Blood Bank and Clinician
Batarilo I1, Saftic R 2, Gulan Harcet J 1, Strauss-Patko M 1, Vuk T 1, Slade M 1, Jukic I 1
1Croatian Institute of Transfusion Medicine, Zagreb, Croatia2Special hospital for Spine Surgery and Orthopedics, Zagreb, Croatia
Background
Bacterial contamination of blood products (BP) represents the highest infectious risk of transfusion treatments. The recipient's reaction depends of the pathogenicity and number of transfused bacteria, the recipient's state of health and underlying disease. Contaminated BP can cause serious infections in immunocompromised patients, and some bacteria like Staphylococcus epidermidis (SE) are very prone to the formation of biofilms on artificial materials. Platelet concentrates (PC) from whole blood unit are produced in the Croatian Institute for Transfusion Medicine (CITM) with the ‘buffy‐coat (BC)’ method as pools of four doses (4 BC and one associated plasma). Bacterial contamination usually occurs during blood collection as a result of inappropriate venipuncture site disinfection or present bacteremia in donors, but can also occur during the production and handling of the BP as well as during its transfusion. Microbiological control of BP is a part of statistical quality control.
Aim
Our goal was to show the events following initially positive bacteriological results of a PC pool, where SE was isolated. One red blood cell concentrate (RBC), associated with the initialy positive PC, was transfused to a patient during total hip arthroplasty.
Methods and results
From the initially positive PC sample, SE was isolated. BP produced from the same donations used for the initially positive PC pool were investigated. Three RBC were recalled from the hospitals where they were distributed and the corresponding plasmas were recalled from the CITM warehouse. Recalled products and the archive sample of the initially positive PC pool were inoculated and incubated in Bact / ALERT system. The archive sample reported a positive signal after 2 h of incubation. SE was isolated. Other products were negative by the end of a 7 days incubation period. The positive archive sample confirmed the PC pool contamination and ruled out the possibility of laboratory contamination. The last RBC was transfused to a patient (56), hospitalized in an orthopedic clinic, during hip arthroplasty, and plasma produced from the same blood unit was spent in the PC pool preparation. Since both BP of the last donation were utilized, it was not possible to discern whether the contamination of the PC pool occurred during the production and handling of BP or the last donation was contaminated, including the disputed RBC as well.The clinician was infomed about the possibility of RBC contamination. Vancomycin i.v. was administered in the patient's therapy. After the isolated bacteria was identified as SE, therapy was prolonged with Linezolid orally 10 days. Blood cultures were taken from the patient, and remained negative for the prescribed incubation period. The patient was afterwards monitored for 6 weeks with no clinical deterioration or signs of infection.
Conclusion
Transfusion transmitted bacteria can cause a wide range of infectious complications. In orthopedic surgery it is important to consider the tendency of SE toward artificial materials, with the possibility of biofilm formation which significantly complicates antibiotic therapy. Timely communication between CITM and clinician reduces such outcomes to a minimum.
P‐436
Survey for Bacterial Testing in Platelet Concentrates in Latin America
Ramirez-Arcos SM1, McDonald CM 2, Benjamin RB 3
1Canadian Blood Services, Ottawa, ON, Canada2NHS Blood and Transplant, London, United Kingdom3American Red Cross, Baltimore, United States of America
Background
Bacterial contamination of platelet concentrates (PCs) poses the highest post‐transfusion infectious risk in developed countries. Mitigation strategies that have contributed to reduce this risk include improved skin disinfection methods, first aliquot diversion and bacterial testing of PCs. Importantly, there is not extensive information about similar strategies implemented in developing countries. As part of the initiatives of the ISBT WP‐TTID, Latin American blood banks were surveyed and the results are presented herein.
Aims
To assess the status of platelet screening for bacterial contamination in Latin America.
Methods
A Survey Monkey with 10 comprehensive questions was sent to 43 blood banks in five countries: Argentina, Brazil, Colombia, Honduras and Mexico. The centers were asked about the type(s) of PCs produced, platelet shelf‐life and strategies used to improve platelet safety. Centers performing bacterial testing were questioned regarding the percentage of PCs tested, quarantine period after sampling, screening system(s), definitions to interpret testing results, haemovigilance data on septic transfusion reactions and implementation of pathogen reduction technologies. Respondents were further surveyed about annual PC production and distribution.
Results
One of the 43 centers does not perform bacterial testing in PCs. Seven out of the remaining 42 centers (16.7%) (2 from Argentina, 2 from Mexico and 3 from Brazil) answered all survey questions. Reported annual PC production/distribution varies within centers: 3000–13,800 (Mexico) and 3300–19,200 (Brazil). While 5 sites (71.4%) produce apheresis and platelet‐rich‐plasma PCs, one site (14.3%) only produces buffy coat (BC) PCs and the other (14.3%) produces apheresis and BC PCs. All seven centers store PCs for a maximum of 5 days. Regarding mitigation strategies, six centers (85.7%) have skin donor disinfection systems in place, five (71.4%) have implemented leukocyte reduction, five (71.4%) employ first aliquot diversion while two sites (28.6%) use swirling technique before platelet release. All centers screen PCs for bacterial contamination although screening practices vary between them. Three centers (42.9%) only test 1% PCs at the expiration date and the remaining four (57.1%) test 100% of PCs between 20–24 h after blood collection. Out of the latter four sites, three hold PCs after sampling for 24 h in quarantine before releasing them into inventory. Six of the seven (85.7%) sites use a culture method for PC screening while one site (14.3%) uses a rapid test. All sites have different systems to confirm and interpret results of platelet screening. Three of the seven sites (42.9%) have haemovigilance data on transfusion reactions although the data is unavailable to the public. None of the sites have implemented pathogen reduction technologies.
Conclusions
Although only a small percentage of centers completed the survey, our results highlight the inconsistency in the approaches used by different Latin American centers to reduce the risk of transfusing bacterially‐contaminated PCs. These results mimic the lack of consensus observed in North America and Europe as recently reported by Benjamin and McDonald (Transf Med Rev 2014;28:61). Our data provides further evidence of the need for standardization of platelet screening practices and results interpretation worldwide.
P‐437
The Importance of Visual Inspection of Platelet Components
Maguire KA , Morris KG
Northern Ireland Blood Transfusion Service, Belfast, United Kingdom
Background
The Northern Ireland Blood Transfusion Service has deployed automated culture bacterial detection systems for platelet components since 2004. This has enabled the extension of platelet component shelf life to 7 days and improved management of platelet inventory. At the point of issue, a day 6 platelet was held by blood bank issue staff because of its abnormal appearance. It should be noted the automated culture bacterial detection systems flagged negative.
Aims
The platelet component and implicated donor were investigated. Detailed investigation included histological investigation of abnormal material in the residual platelet component and DNA phage typing of isolates obtained from the residual platelet and donor skin.
Methods
The incident prompted a review of our bacterial detection system results and their clinical significance.
Results
The number of repeat reactive results and confirmed positive detections are tabulated. Repeat reactive results are <20 per 10,000 with <5 per 10,000 confirmed positive. The different methods and protocols for bacterial detection in UK Blood Services are reviewed. NIBTS delays sampling for a minimum 36 h interval post donation and employs 7 ml innocula of both aerobic and anaerobic culture bottles. The manufacturer's instructions (BacT/ALERT) specify terminal sub‐culture up to 12 days before confirming a negative result.
Summary/Conclusions
Bacterial detection systems have a false negative failure rate. The importance of visual inspection of the platelet component remains. The manufacturer's instructions in relation to confirming a negative result are stringent. It is time to revisit the debate around validated pathogen activation systems for platelet components.
P‐438
Propagation of Bacteria in Platelet Concentrates Using a Genetic Luciferase System
Bello-López JB , Ibáñez-Cervantez GIC , Fernández-Sánchez VFS , Millán-Rocha MMR , Rojo-Medina JRM
National Center of Blood Transfusion, Mexico City, Mexico
Background
Currently the use of molecular tools of Genetic Engineering in the study of microbial behavior in blood and blood components has replaced the employment of classical methods of Microbiology. Bacterial contamination tests of platelet concentrates (PC) have suggested that ‘intrinsic factors’ in these blood components inhibit the propagation of contaminants of clinical importance such as leukocytes, antibodies, complement, lysozyme, lipoproteins inhibit the growth of certain bacterial contaminants. This phenomenon has been described as auto-sterilization. Currently the bacterial bioluminescence offers great advantages directly related to the metabolic activity (ATP production) of bacteria.
Aim
Use a novelgenetic lux reporter system in the study of the propagation of bacterial contaminants in PC under standard storage conditions of blood banks by quantifying of ATP production by luminometry.
Methods
A miniTn5 transposon carrying the lux operon derived of Photorhabdus luminiscens (pUTminiTn5luxCDABEKm2) was used to construct four bacterial bioluminescents: Escherichia coli, Salmonella typhi, Proteus mirabilis and Pseudomonas aeruginosa. Luminescent strains were used for contamination tests with 20 CFU in PC bags and were stored under standard storage conditions in the blood bank (100 rpm at 22°C). The measurements of luminous activity and optical density were used to monitor bacterial proliferation during 7 days (168 h).
Results
In the exponential growth phase (log) of bacterial strains, a lineal correlation between Relative Light Units VS Biomass was observed (R 2 = 0.985, 0.976, 0.981) for E. coli::Tn5luxCDABEKm2, P. mirabilis::Tn5luxCDABEKm2 and P. auriginosa::Tn5luxCDABEKm2, respectively. The above indicates that metabolic activity (production of ATP) is directly related to biomass in this phase of microbial growth. While conducting experiments, the inability to propagate S. typhi::Tn5luxCDABEKm2 was detected. We can speculate that PC bags contain specific components that prevent the propagation of S. typhi but further studies must be done.
Figure 1. Bioluminescense in Relative Luminous Units (RLU) and biomass of (A) E. coli, (B) P. mirabilis, (C) S. tiphy and (D) P. aeruginosa.

Conclusion
The use of genetic lux reporter system for the quantification of luminous activity (by production ATP) is a rapid and sensitive alternative to study the propagation and auto‐sterilization of bacterial contaminants in PC bags.
4.6 Parasites
P‐439
Malaria and T. cruzi Screening in UK Deceased Organ Donors
Webster MH
NHSBT, London, United Kingdom
Background
The National Transfusion Microbiology Reference Laboratory (NTMRL) is a specialist laboratory within NHS Blood and Transplant (NHSBT) which, as part of its remit, undertakes the screening of some non‐blood donations: tissues, cord blood and stem cells, for product release. In conjunction with the Organ Donation and Transplantation (ODT) Directorate of NHSBT, NTMRL has recently undertaken the retrospective screening of organ donations, as soon as possible after transplantation has taken place, for Malaria and T.cruzi infection in donors with an identified risk according to the Standard Advisory Committee on the Safety of Blood, Tissue and Organs (SaBTO).
Aim
To verify the Malaria and/or T.cruzi infectivity status of deceased organ donors as soon as possible after the transplant.
Method
Blood samples from organ donors are referred to NTMRL by Specialist Nurses Organ Donation (SNODs) via local hospital laboratories with an accompanying form stating the screening tests requested and risk factors identified. Screening is primarily performed by EIA and follows documented algorithms that may lead to reference testing using an immunofluorescence antibody test (IFAT) and/or PCR.
Results
Between July 2014 and end February 2015, 43 ODT referrals were received for malaria and/or T.cruzi screening: 26 (60.4%) were for Malaria screening only, 5 of which were seropositive and had undetectable Plasmodium DNA. Three of the 5 seropositive donors had an identified history of malaria infection. Both Malaria and T.cruzi screening were requested for 14 (32%) samples, with none found to be positive. For 3 referred samples no testing was indicated as the identified risk did not apply under donor selection guidelines. No samples were referred for T.cruzi screening alone.
Summary
Five organ donors were identified as having serological evidence of malaria infection at some time with no detectable DNA parasitaemia. This information was relayed via the SNODs and NHSBT consultants to the recipient transplant centres. Timely results provided in the immediate post transplant period enables informed decision on the need for any clinical monitoring or intervention for the recipient. There have been some issues with the correct identification of donor risk factors regarding geographical location and dates of travel, and NTMRL is working with ODT to improve the current referral system.
P‐440
Antimalarial Use Among Transfusion Recipients in a Teaching Hospital in Kumasi, Ghana
Owusu-Ofori K1, Arthur P 2, Bates I 3
1Kwame Nkrumah University of Science and Technology, Kumasi, Ghana2KNUST Hospital, Kumasi, Ghana3Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Background
The appropriate use of antimalarials helps prevent the emergence and rapid spread of resistance. In malaria endemic regions, there is a lack of policies relating to the use of antimalarials in blood transfusion. To provide clear guidance to clinicians, evidence is needed on what the current practices are and why clinicians prescribe antimalarials.
Aim
The aim of study was to establish the rationale for anti‐malarial use in transfusion recipients in Komfo Anokye Teaching Hospital (KATH), Kumasi Ghana.
Methods
The study was conducted between February and May 2014 and involved reviewing patient charts and interviewing their doctors. Transfusion recipients from the four major departments of KATH were enrolled into the study after obtaining their informed consent. A detailed questionnaire was used to extract specific information from the participants’ folder concerning whether antimalarials were prescribed, when it was prescribed and whether there was a written indication. Subsequently, prescribers of the antimalarials were identified and interviewed to determine the rationale for prescribing antimalarials and whether they were guided by specific departmental policies.
Results
Three hundred transfusion recipient from the Departments of Obstetrics and Gynaecology (O&G) (32.3%), Paediatrics (27%), Internal Medicine (22.7%) and Surgery (18%).
The overall rate of antimalarial use in the study was 12.7% (38/300) but distribution was uneven among departments. Paediatrics had the highest rate of 39.5% (32/81) and surgery had the lowest (0%). Rate of use in Internal Medicine and O&G was 2.9% and 4.1% respectively. Of those who received antimalarials, 76.3% had a positive malaria test result in their folders.
There was concomitant antimicrobial administration in 23 of the 38 patients (60.5%) compared to 39.5% of those who did not receive antimalarials.
Of the 38 doctors who were interviewed for prescribing antimalarials, 18 (47.4%) rightly said there was no departmental policy on the use of antimalarials and 20 (52.6%) were not sure about the existence of a policy. Majority (81.6%) of prescribers gave the antimalarials for therapeutic use but 18.4% (7/38) prescribed the antimalarials for prophylaxis. All the prescribers interviewed stated that they will not routinely give antimalarials to every febrile transfusion recipient. However only 34.2% (13/38) of them said they will wait for laboratory confirmation before prescribing antimalarials. Those who thought laboratory confirmation was not needed said microscopy was not reliable and too dependent on the experience of the operator.
Conclusions
The study shows that antimalarials are most commonly prescribed among blood transfusion recipients in the Paediatrics Department. Prophylaxis accounts for 18.4% of antimalarial use in KATH. Although there are no departmental policies for antimalarial use in transfusion recipients, majority of the doctors appear to comply with the World Health Organisation's recommendation of laboratory confirmation of malaria before the use of antimalarials. The good practice observed however is at variance with the attitude of a third of prescribers who state that they will not wait for a laboratory confirmation of malaria before treatment with antimalarials. Further larger studies that focus on antimalarial use in paediatric transfusions are recommended to provide further understanding.
P‐441
Comparison of Policies to Address Imported Malaria in Five Non‐Endemic Countries
O'Brien F1, Delage G 2, Seed CR 3, Pillonel J 4, Fabra CC 5, Davison K 6, Kitchen A 7, Steele WR 8, Leiby DA 8
1Canadian Blood Services, Ottawa, ON, Canada2Hema‐Quebec, Montreal, QC, Canada3Australian Red Cross Blood Service, Perth, WA, Australia4Institut de Veille Sanitaire, Saint‐Maurice, France5Etablissement Francais du Sang, Poitiers, France6Public Health England Centre for Infectious Disease Surveillance & Control, London, United Kingdom7NHS Blood and Transplant, London, United Kingdom8American Red Cross, Washington, DC, United States of America
Background
In non‐endemic countries transfusion‐transmitted malaria risk is mostly from semi‐immune former residents of endemic areas with asymptomatic infections. Short‐term travellers present lower risk because they have less exposure and usually develop symptoms. Either selective testing policies or deferral policies are used to reduce risk to recipients and balance the impact on the sufficiency of the blood supply.
Aims
To compare donor selection policies in countries with selective testing (France, Australia, England) or deferral policies (Canada, USA).
Methods
Details of donor selection policies were extracted from blood donation standards, internal documents and from the investigators. Flow charts were constructed depicting donor questioning for selective testing or deferral.
Results
Short-term Travel: In the USA and Canada donors are deferred for 12 months after return from an endemic area. In France, after at least 4 months post‐travel donors are tested on their next donation. In Australia, donors who have travelled in the previous 3 years are tested, provided at least 4 months has passed since the travel. In England after at least 6 months and up to 12 months post‐travel donors are tested once.
Former Residents: Residency in an endemic area is defined in the USA as a continuous stay of 5 or more years emigrating <3 years ago, in Canada as a continuous stay of 6 months or more emigrating <3 years ago, in France born in, lived in the first 5 years as a child or spent 6 months or more at any time in life, in Australia and England resided for a cumulative period of 6 months or more at any time in life. In the USA and Canada donors are deferred for 3 years post‐emigration: in the USA the deferral is re‐started if there is intervening travel but treated as a US resident after living 3 years consecutively in a non‐endemic country. In France, England and Australia all former residents are tested at least once no matter how long since emigrating. In France after a 4 month waiting period each donation is tested for 3 years. In Australia and England after a 4 month waiting period since last travel (6 months in England) the first donation is tested.
History of Malaria: All donors with history of malaria are tested in France, England and Australia after an initial deferral period of 6 months in England and 4 months in France and Australia. They are permanently deferred in Canada, and deferred for 3 years after symptoms resolve in the USA.
Summary/Conclusions
All of these countries have different policies. Testing policies can address risk in all donors identified no matter how long since their last possible exposure. Deferral strategies presume risk has been resolved within a specified time period (3 years). Donors who travel are captured by these policies often with little to no risk. Selective testing offers shorter deferral periods and most donors successfully donate after testing. This is especially important in countries with diverse ethnic populations and a need for rare blood groups.
P‐442
Evaluation of Novalisa Malaria + Malaria AB Assay
Kitchen AD , Zuczkowska A
NHSBT, London, United Kingdom
Background
Malaria Ab screening of donations from ‘malaria risk’ donors is commonplace in a number of transfusion services globally. Screening is predominately performed by Services in malaria non‐endemic countries and is used to assess the malaria status of donors who have a history of travel or previous residency in an endemic area. Whilst this approach has been largely effective, there is reliance by many of these countries on the malarial Ab assay produced originally by Newmarket Laboratories (Kentford, England), currently Trinity Biotech (Bray, Ireland). Although the ‘Newmarket’ assay is still working well, failure of this assay in any way could have significant impact on the strategies of a number of blood services. The Microbiology Reference Laboratory of NHS Blood and Transplant (NHSBT) has for some time been reviewing and evaluating any additional malarial Ab assays that come to market. Evaluation is performed using a large, well‐provenanced panel of samples from infected individuals. Comparative evaluation of assays is available
Aims
To consider the suitability of the NovaLisa Malaria+ assay for donation screening.
Methods
A panel of 597 provenanced samples from malaria infected individuals at different stages of infection and different plasmodium species was built through collaboration between the Hospital for Tropical Diseases, London (HTD) and the National Transfusion Microbiology Laboratory (NTMRL), NHSBT's reference laboratory.
The panel samples were tested according to the manufacturer's instructions. Results were validated and recorded for comparative evaluation against data previously generated using other malarial antibody assays
Results
Analysis of the results was performed by plasmodium species. Results, samples detected in each group, are presented in the table.
Table 1.
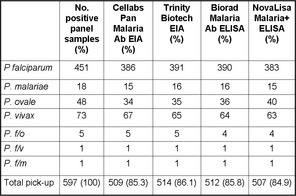
Summary/Conclusions
Donor/donation screening for malarial Ab reactivity is a complex area with limited assays available and limited data available to inform result interpretation. The original Newmarket assay remains the most suitable current commercially available assay, and consequently is the most widely used. Although the performance of the BioRad Malaria Ab assay is also good, this assay was unfortunately taken out of production some years ago.
The NovLisa Malaria+ assay was evaluated as a potential additional screening assay, but like other assays evaluated, its performance to date does not appear to be at a level that could be considered sufficient for donor/donation screening. In addition, its presentation, requiring a 1 + 100 initial sample dilution, does not make the assay an ideal choice as a screening assay. However, the assay may have value as a confirmatory assay in the investigation of malarial Ab screen reactivity. Additional evaluation work is planned.
P‐443
Transfusion‐Transmitted Malaria: Prevalence Among Blood Donors and a Survey Among Healthworkers in a District Hospital in Ghana
Owusu-Ofori K 1, Gadzo P 1, Bates I2
1Kwame Nkrumah University of Science and Technology, Kumasi, Ghana2Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Background
Transfusion‐transmitted malaria (TTM) has until recently received little attention worldwide and there is scanty published data. In endemic regions, malaria constitutes a screening challenge because healthy donors may carry plasmodium parasites without exhibiting signs and symptoms. Many sub‐Saharan African (SSA) countries including Ghana do not have policies that guide TTM practices or that prevents TTM. The absence of such policies can lead to non‐uniformity of practices by clinicians engaged in blood transfusion. Currently there are no studies in SSA that assess health workers knowledge and practices concerning TTM. There are only a few studies comparing malaria prevalence in donors and non donors.
Aims
The aim of the study was twofold:
1. To compare the prevalence of Plasmodium falciparum parasitaemia between blood donors and non donors.
2. To determine the knowledge and practices of healthcare workers in a district hospital to transfusion‐transmitted malaria.
Methods
This study was conducted in the Akatsi South district hospital in the Volta region of Ghana. One hundred blood donors and 100 healthy non donors were recruited to participate in the study to determine prevalence of P. falciparum. CareStart™rapid malaria antigen test kit was used to screen for P. falciparum malaria.
To determine the knowledge and practices ofhealth workers in the hospital, a structured questionnaire was randomly administered to 100 hospital personnel, including nurses, doctors, biomedical scientists and laboratory technicians.
Results
The predominant age group for both donors and non donors was 15–25 years. Males made up the majority in both donors and non‐donors, accounting for 55% and 57% respectively. The prevalence of P. falciparum was 10%, in both groups. All the infected individuals were within the 15–25 years age group.
Nurses made up the majority (63%) of respondents but only 2% were doctors. Forty‐five percent of respondents had not heard of TTM and 91% of the respondents had not attended any seminar or workshop on blood transfusion in the past 12 months.
Half of respondents said they were directly involved in transfusion in the hospital, by way of prescribing blood, monitoring transfusion or screening the donated blood. While 25% of respondents were not sure, 44% wrongly stated that blood was screened for Plasmodium before transfusion in their hospital.
Of the respondents (55) who knew about TTM, 54.5% including the 2 doctors and the only biomedical scientist indicated their willingness to transfuse P. falciparum positive blood in case of an emergency. On the other hand only 7.3% were willing to transfuse syphilis positive blood and none was willing to transfuse hepatitis B positive blood in case of an emergency.
Conclusion
Blood donors and non donors have a similar P. falciparum prevalence of 10%. Knowledge about transfusion transmitted malaria remains low among health workers in the Akatsi district hospital. There is the need for more training for healthcare workers in the area transfusion transmitted malaria to raise awareness and to improve practice. The different perceptions of risk to malaria, syphilis and hepatitis B require further investigations.
P‐444
Platelet Concentrates: High‐Risk Blood Components in the Transfusion‐ Transmission of Trypanosoma Cruzi in Non‐ Endemic Chagas Disease Areas
Cancino-Faure B 1, Fisa R 1, Riera C 1, Bula I 1, Guillén C 1, Berenguer D 1, Girona-Llobera E2, Jimenez-Marco T 2
1Universitat de Barcelona, Barcelona, Spain2Balearic Island Blood Bank, IUNICS, Majorca, Spain
Background
In non‐endemic areas such as Spain, the most important via of Trypanosoma cruzi infection is the transfusion, due to the number of Latin American immigrants from endemic areas which may be potential blood donors. Previously, it has been estimated that the risk of acquiring Chagas disease by receiving an infected unit is approximately 20%. Even though all blood components are potentially infectious, there is no empiric evidence in the literature that red blood cells or plasma are implicated in the transfusion transmission of T. cruzi. According to the reported cases of transfusion‐acquired T. cruzi infection, the risk of T. cruzi transfusion‐transmission appears to be higher with platelets.
Aims
The aim of this study was to investigate by quantitative real‐time polymerase chain reaction (qPCR) the parasitic load detected in leukoreduced plasma and PLT concentrates collected by apheresis from seropositive T. cruzi blood donors from endemic area and compare them with the parasite levels in peripheral whole blood (WB).
Methods
During 2011–2013, a prospective study was carried out in a group of blood donors originating from Chagas‐endemic areas, but now living in the island of Majorca, Spain. The serologic study was done by Enzyme‐Linked ImmunoSorbent Assay, Indirect Fluorescent Immunoassay and Western blot. Seropositive donor's samples were analyzed for the presence of parasite DNA in peripheral WB by qPCR, a test previously validated for the diagnosis of Chagas disease. Blood components were obtained by apheresis from seropositive donors with detectable parasitemia. The parasitic load in Leukoreduced plasma and PLT concentrates obtained by apheresis was also studied by qPCR.
Results
A total of 1201 donors from four endemic countries were studied, were 23 (1.9%) were seropositives for T. cruzi, having theBolivian donors the highest positive detection rate for T. cruzi at 16.03%. Fourteen out of 23 seropositive donors were qPCR positive with a mean ± SD Cq values of 35.0 ± 2.93, which represents <1 parasite equivalent/ml. A total of 8 apheresis donations were performed and 6 Leukoreduced plasma and 6 PLT concentrates were obtained from the seropositive donors with detectable parasite load. In all PLT concentrates (6/6) qPCR were positive with a mean ± SD Cq values of 30.7 ± 2.42, reflecting the highest parasitic load (5.33 ± 6.12 parasites equivalent/ml) comparing with plasma, where all qPCR were non‐detectable
Summary/Conclusions
These results illustrate the reported cases of transfusion‐acquired T. cruzi infection, where the platelets are the major blood component implicated in the transmission of the parasite. The higher parasitic load found in PLT concentrates from seropositive donors with detectable parasite load in peripheral WB compared to plasma and peripheral WB would explain the higher transfusion transmission rate of Chagas disease associated with PLT transfusions comparing with other blood components.
P‐445
Blood Donations and Malaria: Prevention Strategies
Tomeo R1, Perillo M 1, Damiano B 2, Pecora P 2, Mabelli M 2, Schiavone V 3, Chignoli V 3, Gigliofiorito P 4, Misso S 1
1SIT ASL CASERTA, Aversa, Italy2AVIS CASORIA, Afragola, Italy3P.O. PINETAGRANDE, Castelvolturno, Italy4CAMPUS BIOMEDICO, Roma, Italy
Background
Malaria is caused by the Plasmodium, an hemoparasite which is frequently transmitted after the bite of a mosquito better known as Anopheles. Even if uncommon, this disease may be passed with blood transfusion (0–2 cases over a million donations).
The growing flux of immigrants from areas where malaria is still endemic, are taking this phenomenon to our attention. Our structures need to be ready for these even rare conditions.
In Italy, it's forbidden for 3 years to donate when the donor has spent his first 5 years of life in countries where this disease is still endemic. If asymptomatic, they may be re‐admitted in the program.
This method may not be free of risk, but immigrants represent an high percent of donors within the donor’ population and cannot be preventively excluded.
Aims
This work was based on the research of Antibodies against the parasite within the immigrants that have been enrolled for blood donation.
Methods
350 potential donors were enrolled from April 2014 to February 2015 and screened for Malaria.
Control group was represented by 350 habitual donors. Quantitative analysis was determined by ELISA test by searching Antibodies against P. falciparum, P. vivax, P. ovale and P. malariae (Malaria EIA, Bio‐Rad)
Results
As shown table 1.
Table 1.

Summary/Conclusions
Our results show that 9.1% of the foreign donors was positive at the screening examination. 59.4% of these were between 18 and 30 y.o. and 65.6% of them were female. According to the epidemiologic data, 70% of them came from West Africa (Nigeria, Ghana, Benin, Senegal and Sierra Leone), 12.5% from Asia (India and Sri Lanka), 3.1% were from Central America. 9.4% of the foreigners were from East European countries where Malaria is not endemic.
If we consider that the anticorpal positivity lasts up to 10 years, we need to imagine that some patients are asymptomatic donors of the disease or present a partial immunity. This is a major issue and shows us that we need to introduce a lab screening tests to evaluate risk classes of donors in order to drastically reduce infective complications after blood transfusions.
4.7 Newly Emerging Pathogens and Other Transfusion Related Pathogens
P‐446
Risk Factors and Epidemiology of Human T‐Lymphotropic Virus Types 1 and 2 in Us Blood Donors
Vahidnia FV1, Stramer SL 2, Kessler DA 3, Krysztof DE 4, Dodd RY 4, Notari E 4, Glynn S 5, Custer B 1
1Blood Systems Research Institute, San Francisco, United States of America2American Red Cross, Rockville, United States of America3New York Blood Center, New York, United States of America4American Red Cross, Rockville, United States of America5National Heart, Lung and Blood Institute, Bethesda, United States of America
Background
Human T‐lymphotropic virus (HTLV) is a retrovirus transmitted through sexual contact, breastfeeding, injection drug use (IDU) and blood transfusion. HTLV‐infected individuals may develop adult T‐cell leukemia (ATL) and HTLV‐associated myelopathy/tropical spastic paraparesis (HAM/TSP). Blood donations in the US are routinely screened for markers of HTLV‐1 and ‐2.
Aims
To estimate the seroprevalence of HTLV infection in donors and to determine current relative distribution of behavioral risk factors and other exposures associated with prevalent HTLV type‐1 and type‐2 infections in the US.
Methods
This study was conducted as part of the Retrovirus Epidemiology Donor Study (REDS‐II). A consolidated donor/donation database from three major US blood collection organizations was developed. Consensus classification algorithms for HTLV were used to consistently classify donation testing results. In parallel, a case‐control study of risk factors was conducted. Donors with serology‐confirmed HTLV‐1 or ‐2 infection (cases) and donors who were confirmed false‐positive for retrovirus or hepatitis virus infections (controls) were interviewed about demographics and behaviors. Frequencies and adjusted odds ratios (AORs) from separate multivariable logistic regression analyses for HTLV‐1 and ‐2 cases compared to controls are reported.
Caption 1: Adjusted multivariable logistic regression odds ratios (AOR) for HTLV type‐1I and ‐2 infected donors, Donor Risk Factor Study.
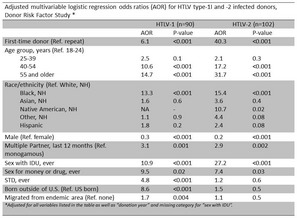
Results
Among 14,809,334 blood donations screened during 2011–2012, 516 HTLV‐confirmed seropositive cases were identified, with an overall prevalence of 3.5 infections per 100,000 donations (95% CI: 3.2–3.8). Risk factor interviews were completed for 198 cases and 1,587 controls. Six interviewed donors with untyped HTLV infection were excluded and interview data from 90 donors with HTLV‐1 and 102 with HTLV‐2 infection were analyzed. Mean age was 48.0 (SD: 12.4), 52.3 (SD: 11.0) and 41.7 (SD: 15.7) years for HTLV‐1, HTLV‐2 cases and controls, respectively. Being a first‐time donor, older, non‐white, non‐Hispanic female were significant demographic factors associated with HTLV‐1 and ‐2 infections. HTLV‐1 cases were more likely than controls to be Black (AOR: 13.3, 95% CI: 6.1–29.2), born outside of the US (AOR: 8.6, 95% CI: 4.0–18.4), have migrated (family or self) from an endemic area (AOR: 1.8, 95% CI: 1.2–2.7), report sex with an IDU (AOR: 10.9, 95% CI: 3.6–33.4), have multiple partners (AOR: 3.1, 95% CI: 1.5–6.1) or have a history of STD (AOR: 4.8, 95% CI: 2.4–9.7). HTLV‐2 cases were more likely to be Black (AOR: 15.4, 95% CI: 6.9–34.3), Native Americans (AOR: 10.7, 95% CI: 1.5–77.0), report sex with an IDU (AOR: 27.2, 95% CI: 9.7–75.8) or have multiple partners (AOR: 2.9, 95% CI: 1.5–5.7) (Table).
Summary/Conclusions
US blood donors with HTLV‐1 or ‐2 infection present with known risk factors, and the epidemiology of these infections in donors appears to be very stable over time when compared to earlier data. Black race and migration from endemic areas are mainly associated with HTLV‐1 infection, while HTLV‐2 is associated with Black and Native American donors. Sexual risk behavior and IDU continue to be risk factors for both viruses.
P‐447
Ten Years on – Followup of Cohorts with an Increased Risk of Variant CJD Through Donating or Receiving Blood
Hewitt PE1, Sinka K 2, Thorpe J 2, Checchi M 2, Mackenzie J 3
1NHS Blood and Transplant, London, United Kingdom2Public Health England, London, United Kingdom3National CJD Research and Surveillance Centre, Edinburgh, United Kingdom
Background and aims
Transmission of variant CJD (vCJD) through blood transfusion is implicated in the deaths of three people and the finding of abnormal prion deposition at post mortem in another. The prospect of a widespread, hidden threat of secondary spread of vCJD through transfusion led to the adoption of precautions to minimise this risk. Among these was identification of individuals assessed to be at increased risk of vCJD because of their transfusion or donation history. Alongside direct recipients of blood from donors who later developed vCJD (described elsewhere), two further groups of donors and recipients were identified using reverse risk assessment. These individuals are followed‐up to understand further the risks of transmission through blood. This paper assesses what we have learned after 10 years of follow‐up.
Methods
The two cohorts identified through reverse risk assessment were 1) donors to an individual who later developed CJD and 2) other recipients of blood from those donors. The risk assessment scenario attributed the source of vCJD in the index patient equally to a dietary source or blood transmission. The risk of blood transmission is shared equally among all the donors to that patient. There are between two and 103 donors to each index patient the probability‐based risk ranges from <1% to 25% per individual donor identified. A similar risk is attributed to other recipients from these donors.
These cohorts are monitored to detect whether any have developed CJD, using a combination of records flagging, post mortem notes review and where possible a post‐mortem examination of brain tissues to detect signs of asymptomatic disease.
Results
For 4 vCJD index patients, transfused in 1993 (2), 1994 and 2002, 112 donors and 34 other recipients of their blood were identified and informed of their increased risk for vCJD. Of these donors and recipients respectively, 6 (5%) and 16 (47%) have died. None were diagnosed with or died from CJD. The primary causes of death in both cohorts were cardiovascular, infection, and cancer. Only one individual had a neurological condition (non‐CJD dementia) listed among the secondary causes of death. In total, there have been 2,316 and 483 vCJD‐free years of follow‐up for the donors and other recipients respectively. For those still alive, the median time since the index patient was transfused to 01/01/2015 for the donors is 21 years (range 12–21 years) and since their own transfusion for the other recipients is 15.5 years (6–26).
Summary and conclusions
Reassuringly, many years have passed without detecting any clinical cases of vCJD in these donors and their other recipients. Each of these cohorts has survived disease‐free far longer than the estimated incubation time for dietary acquired CJD (donors) and transfusion acquired disease (other recipients) based on cases seen to date. However, since our understanding of the nature and prevalence of asymptomatic vCJD infection remains poor, and there is as yet no means to reliably detect such infection, absence of disease on its own may not be enough to completely rule out the risk in these individuals.
P‐448
A Highly Divergent Gemycircularvirus Genome Identified in a Hiv‐Positive Plasma Sample (French Blood Agency, National Plasma Bank): Characterization and Distribution
Biagini P
Viral Emergence and Co‐Evolution Unit, UMR 7268 ADES, Marseille, France
Background
Gemycircularviruses are newly discovered, small circular ssDNA viruses initially identified in insects, plant leaves and mammal faeces. The presence of these myco‐like viruses was very recently extended to cattle and humans according to the identification of 3 viral sequences in blood and tissue samples.
Interestingly, in an attempt to investigate the virome content of a HIV‐positive plasma sample, we identified several gemycircularvirus sequences.
Aims
In order to add clue about diversity and distribution of such viruses in human blood, we characterized the full‐length genome of this new gemycircularvirus; we further investigated 2 cohorts of HIV‐positive or HIV‐negative plasma samples for the presence of the viral sequence using a dedicated PCR detection system.
Methods
Four millilitres of a HIV‐positive plasma were treated with nucleases and further submitted to extraction. Viral nucleic acids were then used for the preparation of a NGS library and its subsequent analysis (MiSeq, Illumina). Gemycircularvirus sequences identified among reads were assembled; the resulting full‐length sequence (GemyC1c) was confirmed by using back‐to‐back PCR primers.
We subsequently investigated the presence of GemyC1c DNA in 128 HIV‐positive and 128 HIV‐negative plasma samples (national plasma bank, French blood agency) using a PCR detection assay targeting the coat (CP) gene.
Results
Analysis of the GemyC1c viral sequence revealed a highly divergent genome (2109 nt), as exemplified by the deduced CP protein exhibiting <45% amino acids identity with other gemycircularvirus CPs characterized so far. Interestingly, the CP aa identity was as low as 33% when compared to the sole viral sequence identified previously in humans.
Among the 256 plasma samples tested, no positive signal was identified by using the molecular protocol described.
Summary/Conclusions
We identified and characterized a highly divergent gemycircularvirus in a HIV‐positive blood donation. First prevalence results would suggest an uncommon frequency of GemyC1c‐related DNA in human plasma. Further investigations aiming to explore genetic diversity, distribution, and natural history of gemycircularviruses in human hosts are now needed. Molecular approaches, details of the study and recent developments will be exposed.
P‐449
Multiplex Elosa Detection and Identification of Viruses Using Oligonucleotide Microplates
Fournier-Wirth CFW1, Leon FL 1, Boutahar NB 2, Meyer AM 2, Brès J-CB 1, Cantaloube J-FC 1, Vasseur J-JV 2, Morvan FM 2
1EFS Pyrénées‐Méditerranée TransDiag, UMR 1058, Montpellier, France2IBMM, UMR 5247, Montpellier, France
Background
Transfusion safety in relation to agents transmitted by blood is a top priority in public health surveillance. The need of multiplex molecular methods is critical to face the continuing emergence of new viruses. The development of innovative tests allowing the simultaneous analysis of various pathogens at risk for transfusion remains a real challenge.
Aims
We have developed an innovative diagnostic approach based on the use of original capture nucleic acid probes (1, 2). This easy to handle, flexible and sensitive ELOSA assay (Enzyme linked oligosorbent assay) could be further dedicated to the genotyping or the multiplex screening of viruses.
Methods and results
After extraction from positive human plasma, viral genomes were amplified by RT‐PCR to generate long biotinylated amplicons. Then, the amplicons (diluted 1/10) were hybridized on original specific polythiolated probes grafted with high efficiency on maleimide‐activated microplates (96 wells). The probe/target hybridization was evaluated by ELOSA. The DELFIA streptavidine‐europium assay was achieved on a Victor Instrument. In a previous study (3), we showed that the presence of four‐thiol functions was enough to observe high signals.
Analytical ELOSA performances were first evaluated on the HCV, HBV and HIV WHO reference panels provided by the National Institute for Biological Standards and Control. Analytical sensitivities of 100, 10 and 50 IU/ml were obtained for HCV, HBV and HIV respectively. The strategy was extended to viral genotyping (3). Thus, specific probes were designed to perform a robust genotyping of HCV 1a/1b, 2a/2b/2c, 3a and 4a/4d strains. The ELOSA method was compared to the NS5b sequencing reference method. The results obtained using a blind panel of hundred samples of human plasma indicated a correlation of 100% between the two methods.
Finally, asymmetric amplifications were developed for the ELOSA detection of emerging viruses. Genomic RNA for West Nile (WNV NY 1999), Chikungunya (CHIK S27 Petersfield) and 4 strains of Dengue (DENV‐1 Hawaii, DENV‐2 New Guinea C, DENV‐3 H87 and DENV‐4 H241) viruses were obtained from VirCell. A generic flavivirus amplification and ELOSA detection of DENV 1/4, and WNV using generic flavivirus polythiolated probes were developed. ELOSA assays with specific probes for DEN, WNV and CHIK are in progress to test biological samples.
Conclusions
The polythiolated probes on maleimide‐activated plates developed in this approach increase sensitivity of ELOSA. This innovative strategy could be exploited in flexible, highly sensitive, easy to handle and cost‐effective platforms dedicated to the genotyping or the generic screening of viruses.1Patent « Multiple introduction of thiol functions in modified oligonucleotides » (PCT/EP2013/057122, 04/04/2013).2Patent « Modified oligonucleotides comprising thiol functions and their use for the nucleic acid detection » (PCT/EP2013/057150, 04/04/2013).3Lereau M., Fournier‐Wirth C., Mayen J., Farre C., Meyer A., Dugas V., Cantaloube J.F, Chaix C., Vasseur J.J., and Morvan F. Development of innovative and versatile polythiol probes for use on ELOSA or electrochemical biosensors: application in hepatitis C virus genotyping’. Anal. Chem. 2013, 85, 9204–12.
P‐450
Abstract Withdrawn.
P‐451
An Exploration of Risk Factors for Acquisition of Hepatitis E Virus Infection in Quebec Blood Donors
Delage G , Germain M , Dubuc S
Hema‐Quebec, Saint‐Laurent, QC, Canada
Background
The hepatitis E virus (HEV) has recently been recognized as an emerging threat to the blood supply. As part of the assessment concerning the risk that the hepatitis E virus represents to the Quebec blood supply, we tested 1952 blood donors for the presence of anti‐HEV using the Wantai assay.
Aim
This study reports our findings concerning the association of various risk factors for acquisition of HEV infection with anti‐HEV status.
Methods
The following data were collected by questionnaire on each donor at the time of blood sample collection: sex, age, population density in area of residence, level of education, current exposure to pigs, current consumption of well water (more than once per month), frequent (≥ once per week) consumption of cured meats, birth and travel outside of Canada and the U.S. Data were entered into an Excel file. Comparisons of frequencies were done using the Chi‐Square test. Multiple regressions were carried out for the following variables: age, sex and level of education. All statistical tests were carried out using the SAS application.
Caption 1: Comparison between positive and negative donors.
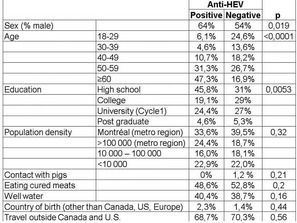
Results
131 of the 1 952 donors were found to be anti‐HEV‐positive. The table shows the comparison between positive and negative donors in the univariate analyses.
In the multi‐variate analysis only age remained as a significant predictive factor for anti‐HEV positivity. Seroprevalence of anti‐HEV increased from 1.76% in the 18–29 year‐old to 2.36% (30–39), 4.1% (40–49), 7.78% (50–59), 16.8% (≥60).
Conclusion
Apart from age, we did not find any risk factor predictive of anti‐HEV positivity in our blood donors. This is consistent with the results of another seroprevalence study carried out in North America (NHANES 2009‐10 Survey, Hepatology 2014; 60: 815–22). A more in‐depth evaluation of risk factors using a case‐control design will be carried out in an attempt to identify other lifestyle risk factors associated with evidence of previous HEV infection.
P‐452
Prevalence of Hepatitis E Virus (HEV) Infection in Blood Donors and Multi‐Transfused Patients in Greece
Zervou EZ1, Politis CP 2, Hassapopoulou EH 3, Vini MV 1, Parara MP 2, Kavallierou LK 2, Fountouli KF 4, Zaxarioudaki AZ 5, Hatzitaki MH 6, Martinis GM 7, Katopi DK 8, Megalou AM 9, Avrami DA 10, Halkia PH 11, Aggelou EA 12, Tsironi ET 13, Lafiatis IL 13, Richardson CR 14
1University Hospital of Ioannia, Ioannina, Greece2Hellenic centre for disease control and prevention, Athens, Greece3Blood Centre Ahepa Hospital, Thessaloniki, Greece4University Hospital of Ioannia Herakleion, Herakleion Crete, Greece5General Hospital Chania Crete, Chania, Greece6General Hospital of Larissa Koutlimpaneio, Larissa, Greece7University Hospital of Alexandoupoli, Alexandroupoli, Greece8General Hospital Alexandra, Athens, Greece9General Hospital Evangelismos, Athens, Greece10General Hospital of Red Cross, Athens, Greece11University Hospital of Ahepa, Thessaloniki, Greece12General Hospital of Rhodes, Rhodos, Greece13General Hospital of Lesvos, Lesvos, Greece14Panteion University Department of Statistics, Athens, Greece
Background
Recent data suggest that hepatitis E takes two epidemiologically distinct forms, one epidemic and the other locally acquired. The clinical spectrum of the latter ranges from asymptomatic infection to fulminant hepatitis; it can also cause chronic hepatitis and cirrhosis in immunocompromised patients.
Recent European epidemiological data indicate that the risk of HEV transmission by transfusions has been underestimated. Studies in Greece in 1995 showed higher prevalence of anti‐HEV IgG in haemodialysis patients (2.8%) than blood donors (1.02%). Given the high number of blood units transfused, prevalence was low in thalassaemia patients (1.4%).
Aims
To examine the current prevalence of HEV in Greek blood donors and thalassaemia patients nationwide.
Methods
Eleven Blood Services throughout Greece participated. Archived blood samples from 1200 blood donors (51.5% males) aged 19–65 years in the period 2013–2014 and 230 thalassaemic patients were examined for IgM and IgG antibodies using the MP Biomedicals HEV ELISA 4.0, an enzyme‐linked immunosorbent assay for the detection of total HEV antibodies in human plasma. It utilizes a proprietary recombinant antigen which is highly conserved between different HEV strains to detect the presence of specific antibodies including IgG, IgM and IgA. All positive samples for total antibodies were tested with the immunochromatographic MP Biomedicals ASSURE HEV IgM Rapid Test, intended for the rapid detection of HEV IgM antibodies in human plasma. Positive samples for HEV‐IgM and IgG antibodies and 200 randomly selected blood donors will be further tested for the presence of HEV‐RNA using Procleix HEV, Grifols.
Results
HEV IgG antibodies were detected in 36 (2.9%) blood donors (3.9% in males, 1.8% in females). Prevalence was higher in older donors (5.9% in the over‐50s vs 1.8% in younger donors). These differences were statistically significant (P = 0.040 for gender, P < 0.001 for age) but were heavily influenced by a relatively high prevalence of 13.3% among males in Herakleion, Crete, which requires further investigation. Most seropositive donors lived in urban areas. Seven (19.4%) had history of travel to endemic areas. Other risk factors for acquiring HEV, including meat preparation and cooking practices, or occupations related to livestock, were not confirmed. Associations with HBV and HCV markers were not detected in the seropositive blood donors.
Preliminary results on 69 thalassaemia patients showed only one (1.5%) seropositive. This subject is a female, 46 years of age, with history of 1380 blood transfusions, but no travel to endemic areas and no occupational risk of HEV. HBV and HCV markers are negative except for anti‐HBs attributed to vaccination against HBV.
Conclusions
The current prevalence of HEV in blood donors is higher than in 1995. Age and gender are significant factors affecting prevalence, while local acquisition of HEV should be further investigated in Heraklion, Crete. HEV prevalence remains unexpectedly low in thalassaemic patients.
P‐453
Evaluation of Sensitivity and Specificity of Roche Diagnostics Elecsys HTLV‐I/II in a Multicenter Study in Europe and Japan
Laperche S1, Sauleda S 2, Piron M 2, Schennach H 3, Mühlbacher A 3, Schottstedt V 4, Queirós L 5, Yanagihara K 6, Uno N 6, Imdahl R 7, Hey A 7, Watanabe T 8
1Institut National de la Transfusion Sanguine, Paris, France2Banc de Sang i Teixits, Barcelona, Spain3Central Institute for Blood Transfusion and Immunology, University Hospital, Innsbruck, Austria4DRK Blutspendedienst West, Hagen, Germany5Instituto Português do Sangue e da Transplantação, Oporto, Portugal6Department of Laboratory Medicine Nagasaki University Hospital, Nagasaki, Japan7Labor Schottdorf MVZ, Augsburg, Germany8Institute of Medical Science, The University of Tokyo, Tokyo, Japan
Background
The human T‐cell lymphotropic virus HTLV is a cell‐associated retrovirus and is related to leukemia/lymphoma and other myelopathies. Blood transfusion transmission of this virus is well described and many countries, in particular in high prevalence or endemic areas in Europe, Middle East, the Americas and Japan have introduced a HTLV antibody screening for blood donors.
Aims
To evaluate the clinical sensitivity and specificity of a new HTLV screening assay, Roche Diagnostics Elecsys HTLV‐I/II, using well characterized HTLV‐I and HTLV‐II positive samples and blood donors samples.
Methods
Elecsys HTLV‐I/II is a rapid, qualitative electrochemiluminescence immunoassay with a total duration of 18 min. It is based on recombinant antigens representing immunodominant regions of envelope and capsid proteins from HTLV‐I and HTLV‐II. Biotinylated antigens and antigens labelled with a ruthenium complex react with anti‐HTLV antibodies and form a sandwich complex binding to streptavidine‐coated magnetic particles. This assay is intended for use on cobas e immunoassay analyzers.
Results
Between May and September 2014, Elecsys HTLV‐I/II was evaluated at 7 centers in Europe, Japan and at Roche Diagnostics Germany with samples collected in different geographic origins. A total of 1149 HTLV positive samples (Japan (n = 420; provided by Joint Study on Predisposing Factors of ATL Development), South America (n = 134), Caribbean (n = 97), USA (n = 259), Europe/Middle East (n = 236), Africa (n = 3)) confirmed by HTLV I/II immunoblot, were included (926 HTLV‐I, 200 HTLV‐II, 23 HTLV subtype not identified) and tested with a sensitivity of 100%. Specificity,evaluated with (i) a random subset of 11575 blood donors (9551 serum, 2024 EDTA plasma) blood donors (repeat donors and first‐time donors) was found at 99.95% (95% confidence limit 99.89–100%) and with (ii) 2399 samples from a university hospital with a HTLV prevalence of approx. 7%, and daily routine specimens in a low prevalence area was found at 99.83% (95% confidence limit 99.56–99.99%).
Conclusions
Elecsys HTLV‐I/II is a very sensitive HTLV screening assay with an outstanding specificity and is suited to detect HTLV infections in a diagnostic purpose as well as in blood donors.
P‐454
Performance Characteristics of a TMA Assay for Dengue Virus Detection on a Fully Automated Platform and Evaluation of the Impact of Pooling Using Blood Donations from Puerto Rico
Bres VB1, Ocampo DO 1, Pekny KP 1, Gore SG 1, Hanhan MH 1, Self DS 1, Babizki MB 1, Janssen JA 1, Kougl JK 1, Millichamp MM 1, Ong EO 1, Stramer SLS 2, Linnen JML 1
1Hologic, Inc, San Diego, United States of America2American Red Cross, Gaithersburg, United States of America
Background
The Procleix® Dengue Virus assay is a qualitative in vitro nucleic acid test for the detection of dengue virus (DENV) RNA types 1–4 in human plasma and serum specimens. This transcription‐mediated amplification (TMA) assay was previously used on the Procleix Tigris® system to screen blood donations from Puerto Rico under an US FDA Investigational New Drug (IND) protocol. The same assay is now under development for use on the Procleix Panther® system.
Aims
The aims of this study were to determine the preliminary performance characteristics of the Procleix Dengue Virus assay on the Panther system compared to the Tigris system and evaluate the sensitivity of the assay in blood donations from Puerto Rico tested individually and in pools of 16.
Methods
To assess clinical specificity, fresh (1362) and frozen (3000) plasma specimens were tested on Panther system. For analytical sensitivity, dilutions of RNA transcripts of all four DENV types were evaluated. Results on the Panther system were analyzed by Probit (SAS Enterprise Guide 5.1) and compared to results from the Tigris system. Ninety‐five unique DENV clinical specimens obtained from whole blood donors from Puerto Rico that were collected by the American Red Cross from August 2012 to October 2013 were also evaluated. These samples, which were previously reactive in the Dengue Virus assay on the Tigris system, were tested individually and diluted 1:16 to mimic 16‐donation pools on the Panther system. The estimated copies/ml were obtained by testing the samples undiluted using a prototype real‐time TMA assay. Samples with estimated titers below ~15,000 copies/ml were tested diluted at 1:16 in 5 additional replicates to determine the minimum viral load required to consistently detect DENV in 1:16 pools.
Results
The Procleix Dengue Virus assay demonstrated 100% specificity with an invalid rate of 0.023% (1/4362) in routine donor specimens. The assay on the Panther system detected all 4 DENV types with a 95% Limit of Detection (LOD) ranging from 18.81 to 28.95 copies/ml using RNA transcripts for DENV 1‐4, which were comparable to the results on the Tigris system where the 95% LOD ranged from 20.50 to 29.43 copies/ml. DENV clinical specimens ranged from ~17,016,083 copies/ml to unquantifiable levels. All 95 specimens were reactive when tested undiluted. When tested diluted 1:16, only 56 of the 95 DENV clinical specimens were detected, showing 58.95% sensitivity. Sixteen of 18 specimens with estimated titers above ~1100 copies/ml were detected consistently when diluted 1:16. One of 55 samples tested below that titer was consistently reactive.
Summary/Conclusions
The Procleix Dengue Virus assay on the Panther system demonstrated high specificity and sensitivity and detected all 4 DENV types with similar sensitivity compared to the assay on the Tigris system. The study performed on 95 DENV‐positive donations suggested that pooling 16 samples limits detection of DENV RNA by more than 40% compared to individual donation testing due to the low viral titers found in many positive donations.
P‐455
Transfusion Transmitted Anaplasmosis from Pooled Platelets
Sweeney JDS1, Fine FB 2, Nixon CP 2, Young C 3, Knoll BM 2
1Miriam Hospital, Providence, United States of America2Rhode Island Hospital, Providence, United States of America3Rhode Island Blood Center, Providence, United States of America
Background
Ixodes Scapularis is a common tick in the Northeastern part of the US and a vector for the transmission of M.bancroti, B.bugdorfei and A.phagocytophilum. Tick borne diseases (TBD) have historically received little attention as transfusion transmitted infections. Recently, transfusion transmitted babesiosis (TTB) due to an intra‐erythrocytic protozoan has gained prominence as the most common microbe currently transmitted by blood transfusion in endemic areas of the US. Anaplasma phagocytophillum is an intraneutrophilic organism and a less common TBD. Approximately eight cases of transfusion transmitted anaplasmosis (TTA) have been has been reported in the US,seven from red cell transfusions and a single case of TTA presumed from apheresis platelets.
Aim
We describe a confirmed case of TTA from a platelet pool.
Methods
A 79 year old female Group AB, Rh(D) positive was admitted for an emergency coronary artery bypass surgery following a cardiac arrest. The cardiac surgery was performed uneventfully but she received a total of seven units of red cells and two platelet doses: one dose was a pooled platelets from five donors and the other a pool from four donors. All blood components were prestorage leukoreduced by filtration. She was discharged to rehabilitation 7 days after the date of surgery. She was readmitted, however, 5 days later with a 1 day history of chills and fever (104 F). Her hemoglobin was 10.5G/L, WCC 3.1 × 109/l, platelets 144 × 109/l with some Giant forms; Her AST (SGOT) was elevated at 101 IU/l (N: 10–42), slight increase in direct bilirubin at 0.5 mg/dl (N: 0–0.3). WBC differential showed 81% neutrophils and 6% band forms. Several neutrophils were observed to contain blue‐purple inclusion bodies consistent with the morulae of A. phagocytophilum. Her IgG A.phagocytopillum titer was borderline positive at 1:64 (N: <1:64). She was treated with doxycycline with a good clinical response and discharged to continue rehabilitation 18 days after admission. A tick bite could not be implicated as the cause as she either in hospital or in a rehabilitation unit during this time.
Results
There were a total of 16 donors (seven red cells and nine platelets), samples from all of whom were studied. Of the 16 donors, one whole blood donor who contributed platelets to the four ‐platelet pool tested positive for A. phagocytophilum by PCR and serology with an IgM titer of 3.5 and an IgG titer of 5.1 (N: <1.0 for each titer in this laboratory). This donor was a 23 year old female, AB Rh(D) positive who resides in an endemic area for I. scapularis. The red cell product from the donation was discarded. All other donors were found to be negative by PCR and serology.
Conclusion
This is the first confirmed case of platelet associated TTA from a leukoreduced whole blood derived platelet pool. The incubation period in this case of TTA was 10 days.
P‐456
Estimating the Risk of Dengue Transmission from Dutch Blood Donors Travelling to Suriname or to the Dutch Caribbean Islands
Oei W1, Lieshout R 2, Kretzschmar MEE 1, Coutinho RA 1, Zaaijer H 2, Jubithana B 3, Eersel M 3, Halabi Y 4, Gerstenbluth I 4, Maduro E 5, Tromp M 5, Janssen MP 1
1University Medical Centre Utrecht, Utrecht, The Netherlands2Sanquin blood Supply Foundation, Amsterdam, The Netherlands3Department of Public Health Suriname, Suriname, Suriname4Ministry of Health, the Environment and Nature Curacao, Curacao, Netherlands Antilles5Department of Public Health Aruba, Aruba, Aruba
Background
The risk of dengue transmission for travellers is well known, and methods to estimate the risk from donors travelling to such risk areas are available, for instance by using the European Up‐Front Risk Assessment Tool (EUFRAT) (http://eufrattool.ecdc.europa.eu).
Aims
This study aims to assess and validate the estimated risk from travelling donors obtained with the EUFRAT.
Methods
To estimate the risk of infection transmission, the incidence of infection in a risk area, travel characteristics and donation behaviour is required. Surveillance data on clinical dengue cases notified in Suriname and the Dutch Caribbean islands (Aruba, Curacao, St. Maarten, Bonaire, St. Eustatius, and Saba) in 2001–2011 was used to calculate local incidence rates. Information on travel behaviour of Dutch donors was collected from a questionnaire conducted in 2010. With the EUFRAT model the number of infected Dutch ‘travelling donors and the resulting number of infected blood recipients can be calculated. We compared model estimates with the number of dengue infections in Dutch travellers found by laboratory tests in the Netherlands.
Results
The average monthly incidence of dengue in Suriname and the Dutch Caribbean is calculated at (per 100,000 population) 29 and 234, respectively. The expected number of donors getting infected with dengue during travels to Suriname and the Dutch Caribbean accumulated from 2001 to 2011 was estimated at 5 (95% CI, 2–11) and 86 (45–179) respectively. The number of dengue infections accumulated by travelling donors in that period as inferred from the laboratory‐based study was 19 (9–61) and 28 (14–92). Given the independence of the data sources and simplicity of the model used to estimate the expected number transmissions, the estimates are remarkably close. The EUFRAT model estimated that there were in total 0.02 (0.001–0.06) and 0.40 (0.01–1.44) infected blood recipients resulting from the infected donors who travelled to Suriname or to the Dutch Caribbean in the period 2001–2011.
Summary/Conclusions
We have shown that the dengue risk among the Dutch travelling donors can be reliably estimated using basic transmission, travel and donation information. The risk from Dutch donors travelling to Suriname and the Dutch Caribbean was small in the considered time period. The model estimates were consistent with the number of dengue infections identified in the Netherlands among the general population travelling to these risk areas.
P‐457
An Epidemiology Study of Cytomegalovirus (CMV) Viraemia in Pre‐ and Post‐Seroconversion Donations in Hong Kong Blood Donors
Chan VCC , Tsoi WC
Hong Kong Red Cross Blood Transfusion Service, Hong Kong, China
Background
Transfusion transmitted cytomegalovirus infection (TT‐CMV) can be associated with significant morbidity and mortality in immunosuppressed seronegative recipients. In at‐risk patients, the incidence of TT‐CMV has been markedly reduced with the use of CMV seronegative or CMV‐safe leucodepleted cellular blood components. Notwithstanding such measures, breakthrough infections were reported in 1–3% of such patients. The residual risks may be due to infectious virions, which may be present during primary infections or reactivations of latent infections, missed detection by conventional serological testing for anti‐CMV in blood donors during window period. Moreover, leucodepletion might not efficiently remove cell‐free CMV from the plasma fraction.
Aims
Use nested polymerase chain reaction (PCR) method to assay for CMV DNA and determine the incidence of CMV viraemia in pre‐ and post‐seroconversion donation samples in Hong Kong blood donors.
Methods
To secure adequate supply of CMV seronegative cellular blood components for clinical use, about 100 donation samples, selected from first‐time donors and repeat donors with computer records of CMV seronegative results tested in the last donation, are screened for anti‐CMV IgG using Microparticle Enzyme Immunoassay on Abbott AXSYM (Abbott Park, IL) in daily routine operation. For the purpose of this study, the blood bank computer system was searched and 50 pairs of donations which showed seroconversion for anti‐CMV IgG in the subsequent donations were identified during the period from August 2011 to November 2012. DNA was extracted from archived samples (whole blood in EDTA) of these 50 pairs of donation and assayed for CMV DNA by nested PCR method (Roback JD, et al. Transfusion 2001;41:1249‐57). Analytic sensitivity of the assay was determined by testing on serial dilutions of a commercial positive CMV DNA control, HCMV (AD169 Strain) Quantitated Viral DNA (Advanced Biotechologies Inc, Columbia, MD).
Results
With regard to the demographic data of the 50 seroconversion donations, 30 (60%) were from males and 20 (40%) females; mean age and SD were 31.0 and 6.4 years old respectively; mean inter‐donation interval and SD were 200.0 and 85.3 days respectively. The limit of detection of the nested PCR assay for detecting CMV DNA was 100 copies/ml. CMV DNA was repeatedly detected in three (6%) of 50 post‐seroconverted samples originated from 2 male and 1 female donors. The inter‐donation intervals were 126, 129 and 272 days respectively. No CMV DNA was detectable in all pre‐seroconversion donations.
Conclusions
This study demonstrated that CMV DNA could be present in the post‐seroconversion donation samples in healthy repeat donors and could not be detected in the pre‐seroconversion samples. Although no window period donation was detected in this study, we cannot exclude the possibility of a future role for Nucleic Acid Testing (NAT) for CMV DNA in blood donor screening because there are hitherto no reliable data in the literature about the infectious dose of CMV which would lead to TT‐CMV infection in immunocompromised recipients.
P‐458
Abstract Withdrawn.
P‐459
Comparison of Two Commercial Hepatitis E Virus Elisa Kits in Australian Blood Donor Samples
Shrestha ACS1, Faddy HM 1, Flower RLP 1, Seed CR 1, Stramer SL 2
1Australian Red Cross Blood Service, Queensland, Australia2American Red Cross, Gaithersburg, United States of America
Background
Hepatitis E virus (HEV) is emerging globally as one of the causative agents of acute hepatitis. Transfusion‐transmitted HEV has been documented, demonstrating that this virus poses a risk to transfusion safety. Sero‐epidemiological studies are important for assisting with estimating the burden of disease. However, highly variable estimates of HEV seroprevalence have been reported using different antibody‐detection assays, as demonstrated for southwestern France where estimates have ranged from 16.6% to 52.5%. Non‐concordance of test results between assays is likely to be due to variation in sensitivity and specificity.
Aims
To compare the performance of commercially available HEV antibody detection assays using a panel of blood donor samples.
Methods
Surplus plasma samples from unique donors following an HEV seroprevalence study were selected. These included 194 samples that tested repeat reactive (RR) for HEV IgG (Wantai HEV IgG ELISA) and 200 age‐matched non‐reactive (NR) samples. Of the HEV IgG RR samples, 4 (2%) were RR by the Wantai HEV IgM ELISA. All samples were tested for: HEV IgG with the MP Diagnostics HEV ELISA; total (IgG, IgM and IgA) HEV antibody with the MP Diagnostics HEV ELISA 4.0; and, HEV IgM with the MP Diagnostics HEV IgM ELISA 3.0. The sample/cut‐off ratio for each sample was calculated for each assay, and results interpreted based on criteria from the manufacturers’ instructions. All samples were tested in singlet, with reactive samples retested in duplicate. A sample was considered RR when reactive in one or both re‐tests.
Results
Of the 194 Wantai HEV IgG RR samples, 92 (47%) tested RR with the MP Diagnostics HEV IgG ELISA, while 1 of the NR samples with the former assay tested RR with the latter. The agreement between these assays was poor (κ = 0.47). With the MP Diagnostics total HEV antibody assay, 126 of 194 (65%) Wantai HEV IgG‐positive samples tested RR and none of the NR samples tested RR. The agreement between these two assays was higher (κ = 0.65). None of the 4 Wantai HEV IgM RR samples tested RR for HEV IgM on the MP Diagnostics HEV IgM ELISA. Comparing the test results between the MP Diagnostics total HEV antibody ELISA and MP Diagnostics HEV IgG ELISA, 82 of 126 (88.17%) tested RR with the latter (κ = 0.65). However, 11 of the samples that tested negative with MP Diagnostics total HEV antibody ELISA were RR with MP Diagnostics HEV IgG ELISA.
Summary/Conclusions
In this seroprevalence study, a poor concordance of test results between the Wantai and MP Diagnostics ELISAs was observed. There was also poor concordance between the MP Diagnostics IgG and total antibody assays. Variability in results is likely due to differences in antigens, assay formats or other components (e.g. diluents) used in each assay. These observations are consistent with previous reports demonstrating significant variability between HEV ELISAs, highlighting that caution is required when interpreting the results of HEV serology.
P‐460
Hepatitis A Virus Antibody Levels in Seropositive Blood Donors
Faddy HF 1, Young MY 2, Cripps A 3, Nimmo G 4, Ji Y 1, Fryk J 1, Flower R1
1The Australian Red Cross Blood Service, Brisbane, Australia2School of Medicine, Griffith University, Meadowbrook, Australia3Griffith Health, Griffith University, Gold Coast, Australia4Pathology Queensland, Brisbane, Australia
Background
The incidence of hepatitis A virus (HAV) infection in Australia has declined since the 1990s from a peak in 1997 of 16.4 per 100,000 to an average of 0.9 per 100,000 in the last 5 years. The majority of cases are in travellers with occasional outbreaks due to contaminated food or water. For example, a recent outbreak was associated with the consumption of imported frozen berries. Funded vaccination is offered to Aboriginal and Torres Strait Islander children in high‐risk areas, but is not universally available. Immunisation is recommended for other high‐risk groups including travellers. Passive immunisation with human immune globulin (IG) is offered to HAV contacts that have not been vaccinated and do not meet national criteria for post‐exposure vaccination. Manufactured Australian IG is required to have ≥100 IU/ml HAV antibodies. Temporal patterns of HAV immunity, considering both infection and vaccination (which is associated with a lower antibody level), may impact the ability to meet such a standard.
Aims
The aim of this study was to quantify the level of HAV antibodies in a cohort of HAV antibody positive blood donor samples.
Methods
Blood donor samples collected in 2011 that were positive for HAV antibody were tested with a commercial HAV ELISA. HAV‐specific antibody levels were quantified from a standard curve that was run on each ELISA plate. The geometric mean titre (GMT) and geometric standard deviation (GSD) were calculated.
Results
The GMT of all samples was 1246.8 mIU/ml (GSD 11.8 mIU/ml) and was associated with age group (P < 0.001). GMT increased with increasing age, with samples from donors aged <20 years having a GMT of 326.5 mIU/ml (GSD 12.3 mIU/ml), while those from donors over 70 years had a GMT of 3120.4 mIU/ml (GSD 10.9 mIU/ml). Sex was not associated with GMT (P = 0.532).
Summary/Conclusions
As the GMT of HAV antibodies was higher in older donors, and as younger individuals in Australia are more likely to become immune due to vaccination rather than infection, the overall GMT in seropositive blood donations in Australia is likely to decrease in future decades. Waning HAV antibody levels in Australian IG may compromise its utility in passive immunisation. The periodic quantification of HAV antibody titres in blood donations in Australia would be of interest.
P‐461
Abstract Withdrawn.
P‐462
Prevalance of HTLV I/II Infection in Thai Blood Donors
Oota SO
National Blood Centre, Thai Red Cross Society, Bangkok, Thailand
Background
Human T‐lymphotropic virus (HTLV) is the causative agent of adult T‐cell leukemia/lymphoma and tropical spastic paraparesis. HTLV‐I and HTLV‐II can be transmitted via sexual contact, injecting drug use, breast feeding and transfusion of infected cellular blood products, thus HTLV co‐infections with TTI pathogens are likely among the high‐risk population. HTLV has previously been reported in Vietnam and Indonesia, but only Japan and Korea screen for HTLV‐I/II in blood donors in Asia. In Thailand, blood donations are routinely screened for HBV, HCV, HIV and Syphilis. Due to population migration around Asia and the awareness of providing a safe blood supply to patients, it is an important role of the blood transfusion centre to evaluate threats of emerging diseases that can be transmitted through blood transfusion.
Aim
To study the seroprevalence of HTLV I/II among blood donors in Thailand.
Methods
Residual samples from routine screening of blood donations from the National Blood Centre in Bangkok and 4 regional blood centres (RBC) ‐ Chiengmai RBC (North), Nakornratchasima RBC(Northeast), Lopburi RBC (central) and Phuket RBC (South) were used in these studies. A total of 10,082 seronegative samples, collected in 2013, and 977 samples confirmed to be positively reactive for HIV or HBV or Syphilis, collected in 2014, were tested for anti‐ HTLV I/II antibodies using the Abbott ARCHITECT chemiluminescent assay. Initially reactive (IR) samples were retested in duplicate. Repeatedly reactive (RR) samples were confirmed by Western Blot (WB) (MP Biomedicals, Singapore) and/or tested on the Abbott Prism HTLV I/II assay.
Results
Of the10,082seronegative samplestested, 24 samples were initially reactive but only seven samples were repeatedly reactive, giving a final specificity of 99.93% in Thai donor samples. All samples were negative on confirmation testing with WB and Prism HTLV I/II assay (table1). The majority of seropositive samples were from male donors (63.4%), and also from ages 21–30 (43.9%). Of the 977 seropositive samples (300 HIV positive, 460 HBV positive and 217 Syphilis positive), only one sample was IR and RR reactive for anti‐HTLV I/II antibodies.
Summary/Conclusions
Blood donor samples representative of every region of Thailand were screened for anti‐HTLV antibodies. The present data shows no evidence of established HTLV I/II infection among Thai blood donors. Biased sampling of high risk donors seropositive for STDs only detected one repeat reactive sample from an HBV positive donor. The known circulation of HTLV I/II in blood donors of neighboring Asian countries suggest periodic population surveillance will be useful to detect new emerging diseases and to protect the safety of the blood supply in Thailand.
P‐463
Chikungunya Virus RNA Reference Reagent for Use in Nucleic Acid Testing Characterized in a Collaborative Study
Rios M , Añez G , Jiang J , Heisey DAR , Kerby S
US‐FDA, Silver Spring, United States of America
Background
Chikungunya virus (CHIKV) is a RNA virus from the genus Alphavirus, family Togaviridae, transmitted by Aedes aegypti and Aedes albopictus mosquitoes. CHIKV human infections can be asymptomatic, but about 80% lead to a febrile illness characterized by high fever, polyarthralgia, headache, back pain, myalgia, nausea, vomiting, and rash known as chikungunya fever. There are no vaccines or specific treatments for CHIKV infection. Although there is no documentation to date of a transfusion‐transmitted CHIKV infection, transmission via this route is probable. Viremic blood donations have been identified during the current outbreak in the Caribbean and in previous outbreaks in Italy, Thailand and La Réunion. Laboratory diagnosis of CHIKV is made by serology, viral isolation and nucleic acid testing (NAT), the latter being considered the most sensitive viral detection method. There are various NAT assay protocols and laboratory‐developed tests for the detection of CHIKV in clinical samples but there are no Food and Drug Administration (FDA)‐approved CHIKV diagnostic or blood screening assays. The lack of a reference reagent for CHIKV RNA is a barrier for proper evaluation of available NAT assays as well as for the development of novel CHIKV assays.
Aims
We aimed to produce a well‐characterized CHIKV RNA reference reagent for use in NAT assays.
Methods
The CHIKV RNA reference reagent material consists of cell‐culture‐grown, heat‐inactivated CHIKV diluted in human plasma. Heat inactivation was confirmed by back titration and the material was further characterized by 8 well‐established laboratories according to a recommended plan as follows:. The participants were asked to test the reagent using their NAT assay(s) in qualitative assays with determination of RNA end‐point of log and half‐log dilutions, followed by calculation of estimated NAT‐detectable units/ml, after adjustment for the volume of reagent used for testing. They also performed quantitative testing when available.
Results
Results from the testing showed that the CHIKV Reference Reagent had an estimated overall mean of 7.56 log10 detectable units/ml, ranging from 6.2 log10 to 8.6 log10 units.
Summary
The Center for Biologics for Evaluation and Research/FDA CHIKV RNA Reference Reagent for NAT was established with a concentration of 7.56 log10 detectable units/ml.
P‐464
French Advisory Group for Safety of Substances of Human Origin: Definition, Principle and Summary of Measures Applied in 2013–2015
Aoustin L , Adda R , Boudjedir K , Ounnoughene N , Sandid I , Pouchol E , Labbe D , Ferry N
ANSM, Saind Denis Cedex, France
Background
The French advisory group for safety of Substances of Human Origin is dedicated to issuing recommendation about measures to be implemented in case of epidemiological alerts, in order to prevent transfusion‐transmitted or graft‐transmitted infections for pathogens that are not usually screened. The initial scope was initially focused on Arboviruses, but was, over the years, expanded to other blood‐borne pathogens responsible for Chagas disease, malaria, Coronavirus, Ebola.
Aims
The advisory group is steered by French National Agency for Medicine and Health Products Safety (ANSM) and comprises experts and representatives of implicated institutions: National Biomedicine Agency (ABM), National reference laboratories for each pathogen (CNR), Haemovigilance regional network (CRH), Military Blood services (CTSA), Ministry of Health (DGS), National Blood service (EFS) and French Institute for public health surveillance (InVS).
Epidemiological alerts are forwarded to ANSM by InVS. Depending on alert severity, ANSM triggers a meeting (or a teleconference in case of emergency) of the advisory group in order to discuss possible measures concerning donors or travelers returning from the alert area. Measures take into account available epidemiological data, measures already in place locally and impact on blood products or graft availability. Measures withdrawal, at the end of the epidemics, is also discussed.
Methods
Concerning Dengue, Chikungunya or West Nile virus (WNV) alerts, the advisory group is activated as soon as pre‐established specific criteria are met for metropolitan area, overseas area (Antilles and Reunion) and foreign countries, depending on previous viral circulation in these different areas. Possible measures applying to donor selection (temporary deferrals), donation screening (supplementary testing) or to products (quarantine, pathogen inactivation technics) are annually reviewed and updated but the possibility to apply them are discussed for each alert.
Results
In 2014, the advisory group managed 77 alerts and met 21 times to finally recommend 16 preventive measures and three measures withdrawal. Since 2011, a list of countries is established at the starting of WNV season (starting June 1st till November 30th) in order to temporarily defer donors even before the reporting of the first WNV cases. The list is established according to the cases of WNV reported in each country during the previous season.
Summary
A summary of the measures implemented during the 2014 season as well as the effectiveness of this strategy will be presented. In addition, the 2015 strategy will be developed.
Authors are acknowledging all advisory group members for their continuous involvement and their prompt and efficient cooperation.
P‐465
Confirmation of Anti‐HTLV Screen Reactivity
Kitchen AD , Dicks S
NHSBT, London, United Kingdom
Background
Screening of donations for HTLV infection is not universal, risk is restricted by population groups and geographically, but where HTLV is carried within the donor population, screening may be required. HTLV I & II antibody screening of donors/donations is mandatory in the UK. Screening was introduced in 2002,using pools of 24 samples for blood donations; non‐blood donations were screened individually. In 2013 the NHS Blood and Transplant switched to ID screening on the Abbott PRISM system. Although the antibody response to HTLV infection is usually that of high titre, high avidity antibody, NHSBT has identified occasional cases of failure to detect HTLV antibody due to the dilutional effect of pooling. Confirmation of HTLV infection in donors has been primarily serological, although in recent years detection of HTLV I & II pvDNA by nested PCR has been used increasingly, mainly to resolve inconclusive serology.
Aim
The increase in referrals following the move to ID screening together with the concomitant increase in samples with inconclusive serology triggered a review of the confirmatory approach. The need to have in‐house ability to sensitively detect HTLV I & II pvDNA was identified, and HTLV I & II pvDNA taqman PCRs were developed as a joint project with the Blood Borne Virus Unit, Public Health England (PHE), Colindale.
Methods
From 2002 until 2014 confirmation of screen reactives was performed using two alterative microplate EIAs, followed by, for those samples with reactivity in one or both of the EIAs, line assay and, for those with unclear serology at this point, western blot together with HTLV I+II pvDNA by nested PCR. From 2014 confirmation of screen repeat reactive samples has been performed using the alternative microplate EIAs, but then followed by both western blot and in‐house taqman pvDNA on any sample demonstrating reactivity in one or both of the primary EIAs.
Results
The screen reactive rates and confirmatory outcomes are presented in the table below.
Table 1.

Summary/Conclusions
Confirmation of all screen reactivity, for all infectious agents screened for, is critical in transfusion practice; potentially infected donors must be appropriately investigated, their status determined and subsequently informed so that they can benefit from appropriate management. In any selected, low risk donor population the majority of screen reactivity would be expected to be non‐specific, and confirmatory algorithms must reflect this. Resolving persisting inconclusive serology sometimes requires the use of molecular techniques.
P‐466
Verification of ‘Artus CMV QS‐RGQ Kit’ on the Qiasymphony SP and Rotor‐Gene Q Platform
Dogic V , Bingulac-Popovic J , Babic I , Boban M , Jurakovic-Loncar N , Sarlija D , Balija M , Jukic I
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
In intention to introduce full automation testing of CMV‐DNA, we performed a verification test ‘ARTUS CMV QS‐RGQ KIT'on the QIAsymphony SP and ROTOR‐GENE Q.
Aim
The aim of this study was to compare the performance characteristics of the two versions of viral DNA extraction: on QIAcube by the QIAamp MinElute Virus Spin Kit (Qiagen, Germany) and on QIAsymphony by the QIAsymphony DSP Virus/Pathogen Mini Kit (Qiagen, Germany).
Methods
Quantitative determination of CMV‐DNA was made by the QIAsymphony DSP Virus/Pathogen Mini Kit and artus CMV QS‐RGQ Kit (Qiagen, Germany). During the validation testing was done three series for 3 days including 11 samples containing Negative Control (NC) and four standards (QS1 = 1.00E+04; QS2 = 1.00E+03; QS3 = 1.00E+02 and QS4 = 1.00E+01) and three samples of known viral titer in duplicate. Also, linearity and accuracy testing (using panel of proficiency testing QCMD/2014 ‐ Glasgow, Scotland) was performed. Acceptance criteria of the testing procedure:
Precision: ± 0.5 log10 (95% CI) and 2 SD (standard deviation) Accuracy: tolerance ± 0.5 log10 Linearity deviation between dilutions: 1.0 ± 0.3 log10 R (correlation coefficient): > 0.950.
Results
Precision from ‘day to day’ was within the permitted range of ± 0.5 log10 (95% CI). The SD of repeated measurements of the control material for three concentration levels of CMV virus were 0.078, 0.103 and 0.122. The precision in the series was in the range of 0.0157 to 0.1348 log10, which is within the set criteria of ± 0.5 log10 (95% CI). Linearity of the new test was very good (R² = 0.9998). Comparison between two versions of viral DNA extraction showed excellent correlation R = 0.9993.
Conclusion
The results of the verification study QIAsymphony DSP Virus/Pathogen Mini Kit and artus CMV QS‐RGQ Kit were fully in accordance with the established criteria for quantitative molecular tests confirming that this test is good for routine use with an important advantage in the automation of CMV‐DNA testing.
P‐467
Malaria Infections in Transfusion Therapy from Immigrant Potential Donors: The Case of Campania Region in the South Italy
Rienzo M1, Romano R 2, Colicchio R 3, Pagliarulo C 4, Cicatiello AG 3, Pagliuca C 3, Scioscia E 4, Cirella M 2, Bottone A 2, Grimaldi V 5, Assentato E 6, Caputo M 6, Sem T 7, Vitale A 2, Pecora P 7
1Second University of Naples, Napoli, Italy2SIMT Division of Immunohematology and Transfusion Medicine, Avellino, Italy3Department of Molecular Medicine and Medical Biotechnology, Napoli, Italy4Department of Biological and Environmental Sciences, Benevento, Italy5Division of Immunohematology, Transfusion Medicine and Transplant Immunology, Napoli, Italy6Immunodiagnostic Transplantation and Regional Reference Center of NAT, Napoli, Italy7AVIS, Avellino, Italy
Aims
The project was designed to implement the monitoring in Campania Region (Italy) of the pathogens (Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae) responsible of infectious diseases and to develop an appropriate prevention and surveillance program. This study has led to record the distribution of these pathogens in the immigrant population and to assess the actual risks associated with transfusion therapy.
Methods
We have collected 4000 foreign donors coming from Asia, Africa, EST Europe, Centre and South America continents. They were resident in Campania Region (Italy). They were subjected to routine laboratory tests also to reveal the possible presence of these pathogens. To confirm the positivity of pathogen detection, we used blood samples in real‐time PCR assays that discriminate between microorganisms based on a signal from specific nucleic acid sequences.
Results
A total of 3758 foreign donors were collected. 2376 samples became from Asia (63.2%), 867 were from Africa (23.1%), 507 from East Europe (13.5%) and the remaining 8 donors were from Centre and South America (0.21%). Particularly, the Asiatic population is distributed as follow: the 28.5% becomes from Sri Lanka, the 11.2% from Bangladesh, the 8,1% from Pakistan, the 8% from Philippine and 3.8% from India. The African population is well represented by Morocco e Nigeria with the 5.1% and 3.81% of donors respectively. The East Europe was represented by donors from Ukraine (5.1%) and from Romaine (4.8%). Noteworthy, we found 685 samples to be positive for malaria antibodies. Particularly, 65.4% of them was from Asiatic countries, 34.2% from Africa and 0.44 from East Europe. Real‐time PCR assays are in progress to verify whether the infection was present also at the moment of blood collection.
Summary/Conclusions
These findings suggest that integrated human and entomological surveillance is crucial to monitor the spread of emerging vector‐borne diseases and to implement public health measures in order to avoid transmission and risks associated with transfusion therapy.
P‐468
Dengue Virus Types 1–4 RNA Who Candidate Standards for Use in Nucleic Acid Testing Characterized in an International Collaborative Study
Rios M , Añez G , Jiang J , Heisey DAR , Kerby S
US‐FDA, Silver Spring, United States of America
Background
The four types of Dengue virus (DENV‐1–4) are mosquito‐borne viruses from the genus Flavivirus, transmitted mainly by Aedes aegypti mosquitoes. DENV infection can be asymptomatic or lead to symptoms ranging from a mild disease known as dengue fever, which can progress to severe dengue/dengue hemorrhagic fever, a potentially life‐threatening condition. DENV has been detected in asymptomatic blood donors in both, endemic regions (Puerto Rico) and in non‐endemic regions experiencing outbreaks (the state of Florida in the United States). Cases of transfusion‐transmitted dengue have been reported. There are no vaccines or specific treatments for DENV infection. The most sensitive method for DENV detection is nucleic acid testing (NAT). While there is no Food and Drug Administration (FDA)‐approved DENV blood‐screening assay, a research NAT has been used under IND to screen blood donors in Puerto Rico. A standardized reference reagent for DENV RNA is needed to facilitate the development of novel DENV NAT assays suitable for blood screening and for the regulatory evaluation of performance of these new assays.
Aims
We aimed to characterize the candidate WHO standards for DENV types 1 to 4 RNA for use in NAT assays in an international collaborative study.
Methods
An international collaborative study was conducted to assess the suitability of candidate standard reagents for Dengue virus (DENV) types 1 to 4 RNA for use in nucleic acid amplification technology (NAT)‐based assays. Two candidate standards; one liquid frozen and one lyophilized were prepared for each DENV type and consisted of prototype laboratory grown viruses, infectivity‐inactivated and diluted in human plasma. Coded samples were sent to the participants who were asked to test candidates on four independent runs by qualitative testing and to determine the RNA end‐point by testing log and half‐log dilutions, followed by calculation of estimated NAT‐detectable units/ml after adjustment for the volume of reagent used, and by quantitative testing, when available. Data was collated and analyzed at the U.S. Food and Drug Administration.
Results
A total of 18 laboratories provided their results to date. Results from a preliminary, interim analysis of the DENV RNA WHO candidate standards data revealed that these materials had an estimated overall concentration of inactivated virus within the expected range.
Summary
Initial evaluation of results from the collaborator laboratories showed that the DENV WHO candidate standards are suitable for their intended purpose. A definitive analysis will be conducted when the remainders of the participating laboratories provide their results, and the candidate standards are proposed to the WHO Expert Committee for Biological Standardization as WHO International Standards.
P‐469
Abstract Withdrawn.
P‐470
Prevalence of Hepatitis E Virus in Mexican Blood Donors
Arroyo Perez JA , Padilla Hernandez MP , Rojo Medina JR
Centro Nacional de Transfusión Sanguínea, Mexico, Mexico
Background
Infections with hepatitis E virus (HEV) are the most frequent cause of non‐A, non‐B hepatitis worldwide. Since hepatitis E resembles not only hepatitis A, but in its early stages other hepatitis types as well, therefore differential diagnostic tests as part of the clinical and laboratory investigation are essential. HEV can cause sporadic as well as epidemic hepatitis. While HEV transmission usually occurs by eating and drinking contaminated foods and water, blood transfusion is another route of infection. During HEV infection, the first antibody to appear is immunoglobulin M (IgM) at week 4, followed by IgG at week 5. The usual length of IgM positivity is between 2 and 3 months. Viremia appears during acute HEV infection at week 2 and normally lasts for 2 to 3 weeks but can last for up to 112 days. In Mexico, HEV seroprevalence of blood donors has not been estimated.
Aim
To estimate the seroprevalence of IgG and IgM antibodies in blood donors from the metropolitan area of Mexico.
Methods
IgG and IgM antibodies against HEV were tested by commercial enzyme‐linked immunosorbent assay (ELISA) kits (Euroimmun, Luebeck, Germany), according to manufacturer's instructions. The assay was used to identify hepatitis E Virus infections in 630 blood donors of the Centro Nacional de la Transfusión Sanguínea in Mexico City. The mixed titer panel Zeptometrix K‐ZMC003 of hepatitis E was tested to evaluate the accuracy of the assay.
Results
From 630 samples analyzed, 5 masculine blood donors resulted IgG positive in the assay (0.79%), mean age 28 (± 4 years), and only one masculine of 32 years old resulted reactive to IgM (0.15%); this one, reported as a risk factor to work in a farm of pork meat production in the state of Michoacán, Mexico. The results of the mixed titer panel were the expected and validated the IgG and IgM assays.
Conclusions
HEV presents a potential risk of infection through blood transfusion in Mexico, with a medium prevalence of viremia among anti‐HEV IgM‐positive samples. Preventive strategies such as ad hoc questionnaires about eating habits (pork meat consumption and eat on the streets) in cities with high people concentration during the clinical evaluation, and serological screening of anti‐HEV IgM in donors with risk factors should be considered in order to reduce the possibility of transfusion‐transmitted HEV infections. Further studies must be done in other states of the country in order to define the mandatory HEV screening in blood donors.
P‐471
Abstract Withdrawn.
P‐472
Abstract Withdrawn.
P‐473
West Nile Virus (WNV) – Testing for the Presence of IGG Antibodies Carried by ELISA Method
Piersiala K , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Background
West Nile virus (WNV) is a RNA virus belonging to the genus Flavivirus in the family Flaviviridae. Tropical and migratory birds and blood‐sucking flies ‐ mostly mosquitoes and black flies are the reservoir of WNV. Mosquitoes play the main role in the transmission of infection to humans. In the tropical and subtropical areas WNV transmission occurs all over the year, whereas in the moderate climate it is possible in the summer, during heat waves. The majority of WNV infections are asymptomatic in humans. In some patients, there appear light symptoms (fever, headaches). It is possible to pass the infection vertically onto the fetus. Isolated cases of transmission of the virus during a blood transfusion have also been recorded. It was also demonstrated that there is a link between WNV infected, transplanted organs and the disease.
Aims
The aim of this study was to investigate the presence of WNV IgG class antibodies as markers of previous viral infection/disease in the population of Polish blood donors.
Methods
Tests were carried out for the presence of the West Nile virus IgG antibodies using the ELISA method (EUROIMMUN) in serum. The following interpretation of the results was adopted:
1. <16 RU/ml: negative.
2. ≥16 to <22 RU/ml: borderline.
3. ≥22 RU/ml: positive.
The incubation period for WNV is most often 2–15 days. The antibodies may occur in a short time after the infection. It is believed that the antibodies have the ability to inactivate the virus.
Results
The retrospective study involved 1633 healthy people from the area of Wielkopolska region, including 295 women and 1338 men in the period from October 2014 to February 2015. In the studied group two patients (0.12%) demonstrated the presence of IgG antibodies in whom the presence of the WNV IgM antibodies was excluded at the same time. The anti‐HIV, anti‐HCV, HBsAg tests, markers of syphilis infection, HCV RNA, HIV RNA and HBV DNA were also negative. The concentration in the case of the female donor was 34.355, and in case of the male donor 54.995, in both cases without any clinical symptoms. The blood was given to the patients after the surgery without any post‐transfusion reactions.

Summary/Conclusions
West Nile virus should be considered as an etiological factor for Polish patients who were diagnosed with fever of unknown origin with accompanying neurological disorders. The possibility to travel to areas in danger of WNV infection makes possible to transfer the infection also to Poland ‐ even in the winter. The existence of such a threat should be taken into consideration by the Polish epidemiologists.
P‐474
West Nile Virus (WNV) – Testing for the Presence of IGM Antibodies Carried by ELISA Method
Bukowska A , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Background
West Nile virus (WNV) is a RNA virus belonging to the genus Flavivirus in the family Flaviviridae. Tropical and migratory birds and blood‐sucking flies ‐ mostly mosquitoes and black flies are the reservoir of WNV. Mosquitoes play the main role in the transmission of infection to humans. The majority of WNV infections are asymptomatic in humans. In some patients there appear light symptoms (fever, headache). It is possible to pass the infection vertically onto the fetus. Isolated cases of transmission of the virus during a blood transfusion have also been recorded. It was also demonstrated that there is a link between WNV infected, transplanted organs and the disease.
Aims
The aim of this study was to investigate the presence of WNV IgM class antibodies as markers of early viral infection in the population of Polish blood donors.
Methods
Tests were carried out for the presence of the West Nile virus IgG antibodies using the ELISA method in serum, using commercially available kits from EUROIMMUN.
The following interpretation of the results was adopted:
1. Ratio <0.8 negative.
2. Ratio ≥ 0.8 <1.1 borderline.
3. Ratio ≥ 1.1 positive.
IgM antibodies appear in a short time after the infection, even after 8 days. They can remain effective for up to 2–3 months.
Results
The retrospective study involved 1633 healthy people from the area of Wielkopolska region, including 295 women and 1338 men, in the period from October 2014 to February 2015. In the studied group one person – a woman (0.06%) demonstrated the presence of IgM antibodies in whom the presence of HIV antibodies, anti‐HCV, HBsAg, and markers of syphilis infection was excluded at the same time. The HCV RNA, HIV RNA, HBV DNA tests were also negative. In the case of this female donor the result 1.504 (Plateletpheresis) was achieved without any clinical symptoms. The platelets were given to a male patient with acute myeloid leukemia without any post‐transfusion reactions. After 3 months the level of IgM antibodies in case of the female donor was determined again and gave the result of 0.943 (borderline).

Summary/Conclusion
West Nile virus should be considered as an etiological factor for Polish patients who were diagnosed with fever of unknown origin with accompanying neurological disorders. The virus multiplication in moderate climate is possible in the summer in hot weather (in Poland favorable conditions ‐ mainly in the southeast of the country). However, one case was detected in Wielkopolska region during the autumn/winter period. A borderline result of the IgM antibodies was achieved with lack of the IgG antibodies when determining the level of antibodies in the female donor again after 3 months.
5.1 Red Cell Immunology: Serology
P‐475
Red Cell Antibody Inhibition Using Unpurified Cell Culture Supernatant Containing Soluble Recombinant Blood Group Proteins
Doyle J1, Ridgwell K 2, Lambert M 1, Green F 2, Crumlish J 1, Keenan M 1, NiLoingsigh S 1
1Irish Blood Transfusion Service, Dublin, Ireland2Bristol Institute for Transfusion Sciences, Bristol, United Kingdom
Background
The provision of suitable red cell components for transfusion can be complicated by the presence of serologically complex mixtures of red cell antibodies in patients. Recombinant blood group proteins (rBGPs) have been shown to be useful in the elucidation of red cell antibodies. Assays using recombinant proteins have been reported in numerous forms, soluble recombinant proteins (SRP) can be used for antibody inhibition and immobilised recombinant proteins can be used in solid phase ELISAs, protein micro‐arrays, or on colour coded microspheres.
Aims
The study primarily evaluated the use of cell culture supernatant (CSN), as a source of unpurified recombinant protein in haemagglutination inhibition assays, thus facilitating the identification or exclusion of underlying allo‐antibodies. The use of CSN eliminates the protein purification step during recombinant protein production.
Methods
Antibodies to blood group antigens K, k, Kpb, Jsb, Fya, Fyb, and Lub were inhibited usingthe followingrBGPs; 3xFLAG‐K, 3xFLAG‐k, Fya‐3xFLAG, Fyb‐3xFLAG and Lub‐3xFLAG protein (K and k rBGPs also express Kpb and Jsb). rBGPs were analysed in two forms; 1) CSN containing SRP (CSN‐SRP) and 2) purified protein product for comparison. Recombinant proteins were provided by the Bristol Institute for Transfusion Sciences with funding from BIORAD. CSN‐SRPs (of unknown concentration) and purified rBGPs (of known concentration) were assessed for their ability to inhibit known clinically significant allo‐antibodies. All antibodies tested (n = 41) were titred to determine their strength and the strongest samples were selected for inhibition. Antibody isotype (IgG/IgM) was also determined by BIORAD monoclonal DAT. Antibody identification was performed by BIORAD IAT pre‐ and post‐inhibition. Both patient and reagent antibodies were tested.
Results
CSN‐SRP was extremely effective at inhibiting all patient antibodies tested (titre range from 1 to 512). Samples with an IgM component required a higher volume of CSN‐SRP to completely inhibit the antibody. A ratio of 1:10 (v/v) CSN‐SRP to plasma, incubated for 10 min at 37°C was the optimum method for antibody inhibition. All antibodies were inhibited with CSN‐SRP containing rBGP with the exception of one reagent monoclonal anti‐K (IgM). The inhibition of selected allo‐antibodies facilitated easier identification of other underlying allo‐antibodies, especially where multiple antibodies or antibodies to high frequency antigens were present (Figures 1 and 2).
Caption 1: Anti‐Kpb + underlying anti‐K pre‐inhibition.

Caption 2: Anti‐K detectable post inhibition of anti‐Kpb with K2 CSN‐SRP (cells 2 and 6 are K+). Ratio 1:10 (v/v, CSN‐SRP:antibody)

Conclusions
Successful antibody inhibition using purified protein has been previously reported. This study shows that CSN‐SRP is an acceptable alternative eliminating the need for protein purification. The relatively small volume of CSN required for inhibition prevents dilution of other antibodies present in the sample, allowing the effective detection or exclusion of other underlying antibodies.
P‐476
Abstract Withdrawn.
P‐477
Serological Detection of D Variants Using The DG Gel Cards
Traves C , Lozano E , Astasio C , Martorell D
Diagnóstic Grifols SA, Parets del Vallès, Spain
Background
The D antigen is highly immunogenic; a high proportion of D‐ people will produce anti‐D if exposed to the D antigen through pregnancy or transfusion. The frequency of D+ is about 85% in Caucasians, around 95% in Africans and >99.5% in the Asian population. About 1% to 2% of Europeans carry RHD alleles that encode weak or partial D antigens (D variants), and the incidence in individuals of African ethnicity is higher.
Monoclonal anti‐D reagents show a different pattern of reactivity with D variants as a consequence of the clone specificities and/or of the formulation. Weak and partial D samples identified by molecular analysis give us the possibility to properly characterize the monoclonal anti‐D reagents as well as monitoring their performance.
Aim
Different anti‐D formulations used in DG Gel cards from Diagnostic Grifols have been characterized using weak and partial D samples identified by molecular analyses.
Methods
Since 2010 a study over time with human blood samples expressing D variants collected in EDTA, CPD or preserved in SAG Manitol has been carry out. Samples were tested manually with different anti‐D DG Gel reagents by direct agglutination gel test: clone P3x61 and clone MS‐201 which do not detect the DVI variant, clones P3x290+P3x35+P3x61+P3x2122B10 and clones RUM‐1+ESD‐1M which detect the DVI variant, and a new anti‐D clones P3x61+ESD‐1M (not yet commercialized), which detects also the DVI variant.
D variants were supplied and identified at the Immunohematology Reference Laboratories, either by microarray genotyping using the BLOODchip® (Progenika Grifols, S.A) or by PCR‐SSP, using allele‐specific primers.
Results
Five hundred sixty seven D‐variant samples were analysed. All the reagents detect weak D type 1, 2 and 3 samples from 80% to 97% depending on the reagent, except with ESD1M+RUM‐1 detecting 74% of these samples. Weak D type 4.0/4.1, D weak type 4.2/DAR, DHK/DAU‐4, DIIIc, DVII and DFR are detected in most cases by all the reagents. DVI and DV/DBS variants only were detected with anti‐D reagents P3x290+P3x35+P3x61+P3x2122B10, ESD1M+RUM‐1 and P3x61+ESD‐1M. Weak D type 11, weak D type 15, weak D type 38 and Del, were not detected by any anti‐D reagents since these variants are not detected by conventional serological methods because the antigen site density is very low.
Conclusion
Anti‐D reagents from DG Gel 8 cards have been characterized using a high number of weak and partial D samples identified by molecular biology. Serological and molecular methods are both useful and complementary in D typing approach.
P‐478
Transfusion Support and Management for a Pregnant Thalassaemia Intermedia Patient with Anti‐Jra
Win N1, Adeyemo A 2
1NHS Blood and Transplant, London, United Kingdom2North Middlesex University Hospital, London, United Kingdom
Background
Jr is a high prevalance antigen in all population. Jr(a−) frequency is highest in Japanese population. Anti‐Jra may be stimulated by transfusion or pregnancy. Anti‐Jra may implicate fatal haemolytic disease of the fetus and newborn (HDFN) and may cause haemolytic transfusion reactions (HTR). It has been recommended to issue least incompatible units for most patients with anti‐Jra but Jr(a−) units should be selected for patients with strong anti‐Jra.
Aims
Jr(a−) individuals are extremely rare in the Caucasian population. We report a transfusion support, management and outcome of a pregnant thalassaemia intermedia patient with anti‐Jra.
Methods
A 27‐year‐old G1P0 with thalassaemia intermedia was seen at the booking clinic with Hb 65 g/l. Her stable Hb level ranged from 54 to 71 g/l. She had multiple blood transfusion history. Antibody investigation showed pan‐reacting antibodies and samples were referred to the National Health Service Blood and Transplant (NHSBT) who confirmed the presence of anti Jr(a) titre of 8. Antibody titre was monitored regularly. At 32 weeks she was admitted with severely growth restricted fetus with Hb level 60 g/l and anti‐Jra titre was 1:8. She was given steroids and commenced on intravenous immunoglobulin (IVIg) 0.4 g/kg/day × 5 days to cover blood transfusion prior to a planned caesarean section (CS). Four units of ‘most suitable’ cross matched blood were issued from NHSBT and she was transfused with 2 units with the 3rd to 5th IVIg infusions. The other 2 units of blood were retained in the event of a postpartum haemorrhage. CS was performed at 33 weeks with the delivery of a live female infant weighing 1.24 kg. Estimated blood loss at delivery was 550 ml.
Results
Transfusion was uneventful with no evidence of HDN in an infant. The patient was discharged 4 days later. Follow up showed no HTR.
Conclusion
There is no Jr(a−) donor in the UK. In general transfusion of Jr(a−) units to a patient with weak anti‐Jra may not cause adverse affect but further transfusion of incompatible units may cause a rise in antibody, resulting in an (AHTR). It has been recommended to issue least incompatible RBC units for patients with weak anti‐Jra if it was a one off transfusion. Those patients who require repeated or subsequent transfusion, there is a potential that the antibody titre may further rise and can cause AHTR. In our case the patient has low base line Hb level and poor bone marrow reserve, therefore concern was raised about the possibility of repeated transfusion during the course of pregnancy, and at delivery, with a potential for the development of HTR. IVIG/steroids have been successfully prescribed to prevent the anticipated transfusion reaction when given incompatible RBC units and Guidelines on the use of IVIG for haematologic conditions (Canadian expert panel 2007) have concluded that IVIG may be considered as an option among supportive therapies for urgent situations in this scenario (i.e. when compatible units are not available).
P‐479
Rare Haemolytic Disease of the Newborn Due to Anti‐Rh 46
Lee CY1, WIN N 1, Pang K 2, Brownell A 2
1NHS Blood and Transplant, London, United Kingdom2Queen's Hospital, Romford, United Kingdom
Background
The erythrocyte phenotype Rh:32,‐46 is rare. It is characterised by increased expression of D antigen, a markedly decreased expression of C and e antigen, the presence of low incidence antigen (Rh32) and the absence of high incidence antigen (Rh46). These individuals can make anti‐Rh46.
Aim
We described a plan for transfusion support when dealing with strong panreactive antibodies in an urgent clinical situation, prior to the identification of anti‐Rh46 and report the subsequent outcome of HDN due to anti‐Rh46.
Case study and method
A 25‐year‐old patient of African descent was admitted at 29 weeks gestation with antepartam haemorrhage (APH) with Hb level of 100 g/l. Antibody investigation revealed strong 4+ panreactive antibodies but specificity could not be determined. The patient's blood group phenotype was established as A R1R1, K‐, Jk(a+b−), S+s+, Lu(b+), Kp(b+), Fy(a−b−), Js(b+). Samples were forwarded to the International Blood Group Reference Laboratory (IBGRL, Bristol) for investigation.
Transfusion support was discussed, as antibody specificities were not known. Six extended antigen matched units were located and issued by the NHSBT. It was planned to give transfusions of incompatible units with IV steroids and IV Immunoglobulin cover. Fortunately, bleeding stopped and the patient gave birth to twins delivered by vaginal route. Post‐delivery Hb level was 83 g/l and did not require transfusion. Both twins were monitored closely. DAT was 4+ with Hb levels 160 and 140 g/l respectively, total bilirubin was 40 mmol/l.
Results
IBGRL found the patient to have the rare Rh:32, ‐46 phenotype and anti‐Rh46 present in her serum. After 24 days post delivery twin two Hb level was 68 g/l with bilirubin 137 mmol/l. The infant received a top up transfusion with rare ‐D‐ phenotype RBCs sourced from the UK National Frozen Blood Bank (NFBB). Both twins were discharged after 6 weeks of delivery.
Summary
The patient presented with APH and a panreactive antibody of unknown specificity. If antigen mismatched incompatible blood has to be transfused in an urgent situation when the benefit outweighs the risk, there is still a potential risk of haemolytic transfusion reaction, depending upon the nature of the unidentified antibodies. IVIG/steroids have been successfully prescribed to prevent the anticipated transfusion reaction when given incompatible RBC units and the Canadian expert panel for use of IVIG (2007) have concluded that IVIG may be considered as an option among supportive therapies for urgent situations in this scenario.
Anti‐Rh46 is considered to be of clinical significance and has been responsible for moderate to serious HDN. Fortunately, the patient did not require blood. Although there was no evidence of HDN at delivery, one twin developed late anaemia and needed top up transfusion with a ‐D‐ RBC unit provided from the NFBB. This case also highlighted the important role the UK NFBB plays in the provision of rare blood.
P‐480
Murine Monoclonal Antibodies Against VW (MNS9), MUR (MNS10) and MUT (MNS35) Antigens Associated with MNS Blood Group System
Uchikawa MU , Toyoda CT , Suzuki YS , Kaito SK , Yabe RY , Uchikawa MU , Nakajima KN
Kanto‐Koshinetsu Block Blood Center, Tokyo, Japan
Backgroud
Monoclonal antibodies (MoAbs) have been produced to a wide variety of structures on the red cell membranes and developed successfully to replace polyclonal sera as diagnostic blood grouping reagents. Miltenberger is a series of relatively rare phenotypes associated with the MNS blood group, related to each other through the overlapping specificities of a number of low frequency antigens. The Vw (MNS9) antigen was expected to be present on red cells with the GP.Vw (Mi.I). The Mur (MNS10) and MUT (MNS35) antigen were present on red cells with the GP.Mur (Mi.III), GP.Hop (Mi.IV), GP.Bun (Mi.VI), GP.Dane (Mi.IX) and GP.Kip, and with the GP.Hut (Mi.II), GP.Mur (Mi.III), GP.Hop (Mi.IV), GP.Bun (Mi.VI), GP.HF (Mi.X) and GP.Kip, respectively.
Aims
Hybridomas secreting murine MoAb to the Vw (MNS9), the Mur (MNS10) and the MUT (MNS35) antigens were isolated. These antibodies were produced in supernatant form and characterized for their use as a blood grouping reagent.
Methods
Immunization of BALB/c mice was performed by four intraperitoneal injections of 0.5 ml GP.Vw or GP.Bun red cells at 2‐week intervals. Splenocytes were fused with NS1 murine myeloma cells, and hybrid cells were selected by culture in RPMI/HAT medium according to conventional protocols. Culture supernatants were screened by saline or indirect antiglobulin methods using GP.Vw or GP.Bun red cells. The specificity of each monoclonal antibody was confirmed using a panel of GP.Vw, GP.Hut, GP.Mur, GP.Hil (Mi.V), GP.Bun, GP.HF and GP.Kip red cells. SDS‐polyacrylamide gel electrophoresis was performed on a 10% separating gel using a 3% stacking gel. Gel was electroblotted and immunostained with the murine MoAbs (anti‐Vw, ‐Mur or ‐MUT) followed by incubation with peroxidase labeled anti‐murine IgG.
Results
Anti‐Vw (CBC‐430, IgG1) reacted strongly by saline method with red cells of the GP.Vw, but did not with the GP.Hut, GP.Mur, GP.Hil, GP.Bun, GP.HF and GP.Kip. Immunoblotting of CBC‐430 produced characteristic bands with an apparent Mr. of 40,000 with GP.Vw. Anti‐Mur (CBC‐431, IgG1) only reacted by indirect antiglobulin method with the GP.Mur, GP.Bun and GP.Kip red cells, but did not with the GP.Vw, GP.Hut, GP.Hil, GP.HF red cells. Anti‐MUT (CBC‐412, IgG1) reacted strongly by saline mrthod with red cells of the GP.Hut, GP.Mur, GP.Bun, GP.HF and GP.Kip, but did not with the GP.Vw. Immunoblotting of CBC‐430 and CBC‐412 produced abnormal bands with apparent Mr. of 36,000 with GP.Mur and GP.Bun, and with GP.Hut, GP.Mur, GP.Bun and GP.HF, respectively.
Conclusion
The anti‐Vw (CBC‐430), anti‐Mur (CBC‐431) and anti‐MUT (CBC‐412) MoAbs had excellent characteristics in terms of specificity and potency, and are readily adapted for blood grouping reagents.
P‐481
Rapid Simultaneous Phenotyping of FYA, FYB, JKA, JKB, S(big) and S(small) in 5 min without Centrifugation Using Mdmulticard Lateral Flow Technique
Caesar A , Schwind P
Medion Grifols Diagnostics AG, Duedingen, Switzerland
Background
A lateral flow assay for simultaneous typing of ABO, RhD, Rhesus phenotype and K with stable end‐point and without a centrifugation step is in routine use since several years (MDmulticard).
Aims
The aim of this study was to evaluate the performance characteristics of the new parameters Fya, Fyb, Jka, Jkb, S and s in this device.
Methods
In 102 fresh blood samples, comprising 90 samples of blood donors, 10 authentic clinical samples of hospitalized patients and 2 samples of newborns, Fya, Fyb, Jka, Jkb, S and s blood groups were tested. The lateral flow based assay containing these parameters (MDmulticard, Medion Grifols Diagnostics, Duedingen, Switzerland) was compared with established CE certified techniques: Anti‐Fya,‐Fyb,‐Jka,‐Jkb for DG Gel, Anti‐S (Medion Grifols Diagnostics) and ID‐Anti‐s (Bio‐Rad, Cressier Switzerland).
The credit‐card sized lateral flow test device consists of a membrane, which is equipped in a cassette housing. Two equidistant detection areas with parallel lines of antibody reagents against Fya, Fyb, Jka, Jkb, S, and s are left and right of a central application zone. Both detection areas contain a process control spot (val) and an auto control spot (ctl). One cassette can be equipped for the simultaneous detection of up to 10 different blood group specificities.
For blood group typing, 100 μl of diluted whole blood or erythrocyte sediment are pipetted to the central application zone, followed by 300 μl of a rinsing solution. Results may be interpreted after 5 min. Positive results clearly impose as distinct red bands, whereas negative results lack the respective bands.
Results
All results of the blood samples tested were in full accordance with those of the CE certified comparative methods.
Summary/Conclusions
MDmulticard lateral flow technique was presented earlier with unique features, e. g. simultaneous multiparameter testing without the need of centrifiugation and results within 5 min. In this study we are able to reproduce these characteristics also in the detection of Fya, Fyb, Jka, Jkb, S and s. Moreover, for the first time, simultaneous determination of these blood groups, without the need of different phases and incubation times / temperatures in one single, homogeneous assay is demonstrated. Apart from this assay harmonization, testing times for certain parameters are significantly reduced compared to currently available technology, e.g. for the determination of Fy antigens from about 25 to 5 min.
P‐482
Reliable Detection of Duffy X (FYX) – A Weak Variant of Duffy B (FYB) by a New Reagent Using Lateral Flow Technique
Meyer S1, Caesar A 2, Trost N 1, Neuenschwander K 1, Frey BM 1, Gassner C 1, Schwind P 2
1Blood Transfusion Service Zurich, Swiss Red Cross, Zurich‐Schlieren, Switzerland2Medion Grifols Diagnostics AG, Duedingen, Switzerland
Background
With the advent of molecular techniques occasional blood group phenotyping errors became apparent. In particular, a number of weak phenotypes in several blood group systems were discovered, previously typed false‐negatively. For the Duffy (DARC) system and Fyx, this was reported as early as 1997 (1). Fyx is a weak Fyb phenotype with a pronounced quantitative reduction of the number of Fyb antigens on the erythrocyte surface.
Aims
Sensitivity of a novel Anti‐Fyb reagent should be evaluated, especially focusing on the ability to detect Fyx in a selected cohort with Fyx positive individuals.
Methods
Freshly drawn EDTA anticoagulated samples were from 42 random individuals with standard Fy serotypes, and 21 samples with standard Fy serotypes and additional FY* genotypes detected by MALDI‐TOF MS blood group genotyping (2). The 21 samples consisted of 9 heterozygous FY*A/ FY*02W.01/02, 2 homozygous FY*02W.01/02 and 10 heterozygous FY*B/FY*02W.01/02 genotypes, encoding the phenotypes Fy(a+bweak+), Fy(a−bweak+) and Fy(a−b+), respectively. Final panel‐composition had a strong statistical overrepresentation of samples positive for Fyx (33%), expected to range at about 2% in a normal Caucasian population.
The 9 previously known FY*A/ FY*02W.01/02 heterozygous individuals were of specific interest for evaluation purpose and included 3 samples with previously identified phenotypic Fyx positivity and 6 previously serologically ‘overseen’ Fyx cases. All samples were tested by one person without prior knowledge of the existent phenotypes.
Fyb serology was performed using a MDmulticard lateral flow blood grouping device (Medion Grifols Diagnostics, Duedingen, Switzerland). Comparable to routine testing for ABO, RhD, CcEe, Cw, and K, by the same method, 100 μl of diluted whole blood were transferred to the application zone of the MDmulticard cassette, followed by 300 μl of a rinsing solution. Results were interpreted after 5 min. Positive results were interpretable as distinct red bands.
Results
All samples with genotypic Fyx positivity were correctly recognized as Fyb positive by the MDmulticard technique. All 42 random phenotype‐only samples except one were concordant with the serological pre‐values (see table). The discrepant sample had a recorded Fy(a+b−) phenotype derived from routine serology, but showed a Fy(a+Fybweak+) phenotype resulting from the MDmulticard testing. Presumably and in line with unexpected, but plausible statistics, this sample supposedly had a Fy(a+bweak+) phenotype, undetected for Fyx by the previously used routine method. Retesting of the respective sample was prohibited by the mandatory ethical provisions, e.g. anonymization.
Table 1.

Summary/Conclusions
The new Anti‐Fyb reagent in conjunction with MDmulticard technology seemed to detect all Fyb and Fyx positive phenotypes, in all constellations, reliably. Technically, the combination of well selected clones with appropriate diagnostic techniques optimized the visualization of the hemagglutination end‐point and may therefore lead to novel methods with increased diagnostic sensitivity. As shown previously, genotyping may serve as a valuable tool to create more specific and better characterized testing panels (3).
(1) Murphy MT, et al, Trans Med 1997; 7: 135–141.
(2) Meyer S, et al, Transfusion 2014; 54: 3198–3207.
(3) Gassner C, et al, Transfus Med Hemother 2009; 36: 219–225.
P‐483
Abstract Withdrawn.
P‐484
Further Evidence for the Clinical Significance of Anti‐Jra
Needs ME , Win N
NHSBT‐Tooting Centre, London, United Kingdom
Background
Jra is a high‐prevalence antigen in all populations. Jr(a−) frequency is highest in the Japanese population. There are few case reports of delayed haemolytic transfusion reactions (DHTR) or haemolytic disease of the foetus/newborn (HDFN) implicating anti‐Jra. Recommendations are to issue ‘least incompatible’ units for patients with weak anti‐Jra, but Jr(a−) units for patients with strong ant‐Jra. Jr(a−) individuals are extremely rare in the White populations. Transfusion support of Jr(a−) patients is difficult to manage because of the rarity of Jr(a−) donors.
Aims
We discuss a patient with anti‐Jra who required multiple, repeated transfusions, resulting in a DHTR treated with IVIgG.
Case study
A 54‐year‐old patient was admitted after a road traffic accident. On admission, her Hb was 44 g/l. Her blood typed as A, D+ and a nonspecific weak antibody was detected. She received six units of RBCs and transfusion was uneventful. Four days later, the patient required pelvic surgery (Hb 87 g/l). The Hb dropped to 71 g/l the next day with a bilirubin level of 17mmol/l (range 0–14), and a positive DAT with anti‐IgG (3+) and anti‐C3d (3+). A weak pan reactive antibody was detected by IAT and 3 crossmatched ‘least incompatible’ RBCs units were transfused. Three days later, the Hb dropped from 130 to 114 g/l, with a further rise in bilirubin to 33mmol/l and one unit of ‘least incompatible’ RBCs was transfused. Samples were referred to NHSBT and 4 RBC units were requested.
This case was already known to NHSBT and the presence of anti‐Jra was confirmed. The pre‐op Hb level was 109 g/l, the bilirubin 42 mmol/l and LDH 867 IU/l (range 0–120). The patient received 4 ‘least incompatible’ units 4 days prior to this, but, despite that, there was no good increment in Hb level. As the patient presented with a low Hb, with evidence of haemolysis, a DHTR was suspected, and anti‐Jra was eluted from the patient's RBCs, which confirmed the diagnosis. As Jr(a−) units were not available, IVIgG 0.4 g/kg/day for 3 days was prescribed and ‘least incompatible’ units issued.
Result
The first dose of IVIgG was given on the day of surgery. It was planned only to give additional transfusion with definite indication. The post‐op Hb was 89 g/l. Three days after IVIgG therapy, the bilirubin level was 34 mmol/l and 7 days after, the bilirubin was normal with an Hb of 95 g/l. The patient was monitored closely, and, as the Hb level gradually rose, the issued units were not transfused.
Conclusion
We have described evidence of successful treatment of DHTR with IVIgG without additional transfusion in a patient with anti‐Jra, who had been repeatedly transfused with Jr(a+) RBCs. There is only one case report in the literature whereby a patient with anti‐Jra, after receiving repeated transfusion of incompatible units developed DHTR similar in our case. Severe DHTR, with a nadir Hb 38 g/l, has been successfully treated in a SCD patient with IVIgG/steroids without additional transfusion. Our outcome was similar.
P‐485
Production of Kidd Soluble Recombinant Membrane Proteins and Assessment of Antigenicity with Commercial Monoclonal/Polyclonal Antibody Reagents
Hazell MJ1, Clarke A 1, Green FA 1, Herman A 2, Ridgwell K 1
1NHS Blood and Transplant, Bristol, United Kingdom2University of Bristol, Bristol, United Kingdom
Background
The Kidd (Jk) blood group system consists of two multipass transmembrane proteins, Jka and Jkb, which are ~43 KDa products of polymorphic alleles in all populations tested. The Jka/Jkb polymorphism results from an Asp280Asn substitution in the Jk glycoprotein. Urea transport is the primary function of Jk, increasing transport across the red blood cell (RBC) membrane 1000 times faster than in RBCs lacking Jk (Jka−/Jkb−). Jk alloimmunisation is encountered during transfusion practice. Anti‐Jka/Jkb are potentially dangerous, they are difficult to work with, a common cause of delayed haemolytic transfusion reactions and have been reported in severe/fatal haemolytic disease of the fetus and newborn. Serological identification of Jk antibodies relies on negative exclusion of other clinically significant antibodies through indirect antiglobulin testing with enzyme treated/untreated panel cells.
Aim
To produce cell lines expressing recombinant transmembrane proteins with Jk blood group activity, and solubilised recombinant membrane protein (SRMP) extracts that allow Jk antibody identification in the clinical setting.
Method
cDNA encoding Jka protein was PCR amplified from a human bone marrow cDNA library using Jk specific primers. Recombinant DNA was inserted into the pcDNA3.1‐HIS6 eukaryotic expression vector producing the pcDNA3.1‐HIS6‐Jka construct. pcDNA3.1‐HIS6‐Jkb construct was created by site‐directed mutagenesis of the pcDNA3.1‐ HIS6‐Jka.
Human endothelial kidney (HEK) cells were lipofected with the pcDNA3.1‐HIS6‐ Jka/Jkb recombinant vectors and clones expressing Jk were selected using G418 treatment followed by fluorescence assisted cell sorting (FACS) with anti‐Jk3. Intra‐cellular localisation of Jk proteins was investigated using fluorescence microscopy (FM) by overlaying images of recombinant Jka/Jkb visualised with anti‐Jk3, with images of DAPI stained nuclear membranes and WGA488 labelled plasma membranes. Antigenicity was investigated using flow cytometry with anti‐Jka/Jkb commercial monoclonal and polyclonal antibodies.
Stably transformed HEK cells were used in haemaglutination inhibition assays (HIAs) with commercial polyclonal anti‐Jka/Jkb reagents and panel red cells.
Solubilisation of transformed HEK cells to produce extracts containing SRMPs was conducted in phosphate buffered saline (PBS) containing 1% non‐ionic detergent followed by centrifugation to exclude insoluble cell components.
Antigenicity of SRMPs in detergent extracts was investigated by enzyme linked immunosorbent assay (ELISA), capturing the SRMPs through the HIS6 tag and detecting Jk expression with commercial grade monoclonal/polyclonal anti Jka/Jkb, or vice versa.
Results
HEK cell lines transformed with Jka/Jkb proteins were positively selected through G418 treatment and FACS. Insertion of the Jka/Jkb protein into HEK plasma membranes was demonstrated through FM and specific antigenicity was observed using FC. Jka/Jkb transformed HEK cells in HIAs successfully inhibited agglutination of panel red cells with commercial grade polyclonal anti‐Jka/Jkb reagents. Transformed HEK cell lines were solubilised in PBS containing 1% non‐ionic detergent and antigenicity in extracts was detected by ELISA.
Summary
This work demonstrates that recombinant forms of complex blood group proteins, that rely on membrane interaction to retain conformation, can be expressed in plasma membranes of transformed eukaryotic cell lines and retain blood group antigenicity. In addition, antigenicity is retained in membrane fractions prepared from transformed cell lines solubilised in a PBS‐1% non‐ionic detergent solution.
P‐486
The Second Example of Alloanti‐D in a Weak D Type 33 Individual
Lambert MD1, Grimsley S 2, Ó’Donghaile D 1, Thornton N 2
1Irish Blood Transfusion Service, Dublin, Ireland2International Blood Group Reference Laboratory, Bristol, United Kingdom
Background
The RH blood group system contains the most clinically significant blood groups, after ABO. The RhD and RhCcEe proteins are encoded by two highly homologous genes, RHD and RHCE respectively. Currently there are 54 antigens in the RH blood group system, of which RhD is the most clinically significant, with alloanti‐D causing both haemolytic disease of the newborn and haemolytic transfusion reactions.
The RhD blood group phenotype can be further categorised as: Partial D, weak D, DEL and D‐. These phenotypes are the products of over 150 partial D, weak D, DEL and RhDnull alleles currently recognised (not including ‘sub‐alleles’) [www.isbtweb.org/working‐parties/red‐cell‐immunogenetics‐and‐blood‐group‐terminology/]. Alleles are assigned to partial or weak D groups largely dependent on the predicted ability or inability to develop alloanti‐D when exposed to RhD+ red cells.
Aims
Samples from a 68 year old female patient diagnosed with rectal carcinoma, requiring blood for anterior resection of the tumour, were referred for investigation due to the patient having apparent alloanti‐D, whilst having been historically typed as D+ and transfused D+ units.
Methods
Routine Rh typing was initially performed by AutoVue Innova, further RhD typing by tube agglutination using Bioscot anti‐D reagents (BS226 and BS232) and extended RhD typing was performed using the Quotient Advanced RhD Typing Kit. In total the patient's cells were tested with 16 different monoclonal anti‐D reagents. Antibody investigation was performed by standard BioRad IAT using untreated and papain‐treated panel cells. Antibody titration was performed by BioRad IAT (DCe/dce cell). Eluate was prepared using Immucor Elu‐Kit II and investigated by BioRad IAT. Cord cells were used to exclude the presence of anti‐LW. All exons of both RHD and RHCE were sequenced by Sanger sequencing at the IBGRL.
Results
The patient's cells typed as O RhD+ C+ c+ E‐ e+. All anti‐D reagents gave 4+ reactions with the patient's cells. Anti‐D was identified in the patient's plasma (IAT titre of 8). Autologous control and DAT were negative. An eluate prepared from the patient's cells contained no detectable anti‐D. The anti‐D present was considered to be alloanti‐D.
Sequencing confirmed the presence of normal RHCE*Ce/ce. However, RHD sequencing revealed that the patient was homozygous or hemizygous for a 520G>A mutation. This mutation results in a V174M transition and is characteristic of Weak D type 33 (RHD*01W.33) [Ann Hematol 2003;82:617‐620].
Conclusions
We report the second example of alloanti‐D in a weak D type 33 individual. The first was reported in 2011 [Transfusion Medicine 2011;21(Suppl.1):15]. Other reported cases of individuals with ‘weak D’ types producing alloanti‐D include weak D types 4.2, 11, 15 and 21. Such cases indicate the need to re‐evaluate the system for naming RhD variant phenotypes and RHD variant alleles to improve clarity of the nomenclature.
P‐487
Prevalence of Antibodies Against Low Frequency Antigens Specific of the Afro‐Caribbean Population in Transfused Sickle Cell Disease Patients
Le Floch ALF1, Gien D 2, Tournamille C 3, Muralitharan V 4, Adenet D 4, Rieux C 5, Chami B 4, Habibi A 6, Bierling P 7, Peyrard T 2, Pirenne F 7
1Etablissement Français du Sang, Créteil, France2INTS, GR‐Ex, Paris, France3EFS IdF, INSERM U955, GR‐Ex, Créteil, France4EFS IdF, Créteil, France5Comité de Sécurité Transfusionnelle et d'Hémovigilance, hôpital H. Mondor, Créteil, France6Sickle Cell Disease Reference Center, Créteil, France7EFS IdF, INSERM U955, GR‐Ex, UPEC, Créteil, France
Background
A low frequency antigen (LF) is characterized by a low incidence expression on common red blood cells within a reference population. The antibodies against LF antigens can be identified before a transfusion only if one of the red blood cells (RBC) used in the antibody screening test expresses the corresponding antigen. The LF antigens that are specific of the Afro‐Caribbean population are poorly expressed on the tested RBCs in countries with a European background, therefore the associated antibodies are not detected. Some LF antigens in the Afro‐Caribbean population can be considered as common, in patients but also in donors with the same ethnic background, leading to frequent exposition, and immunization risk of the transfused patients. This is the reason why cross match of RBC units with the patient plasma is standard care for sickle cell disease (SCD) patients in France.
Aim
In this study we aimed to evaluate the incidence of antibodies against LF antigens specific of individuals with an African background, in regularly transfused SCD patients in order (i) to determine which specificities are the most frequent and consequently, (ii) to adapt our guidelines to secure transfusion.
Methods
The plasma of 224 regularly transfused SCD patients has been tested against a panel of RBCs expressing LF antigens specific of the Afro‐Caribbean population: RH10, RH20, RH23, RH30, KEL6 and MNS6. The expression of RH20 and KEL6 in FY:‐1,‐2 donors and in these patients has been deduced by molecular analysis.
Results
9 antibodies against LF antigens specific of the Afro‐Caribbean population have been found in 8 patients (3.6%): 5 anti‐RH23, 2 anti‐RH30 and 2 anti‐MNS6. Molecular biology predicted 50% of RH20 antigen expression and 17% of KEL6 antigen expression in patients and donors.
Conclusions
This study demonstrates a low prevalence of antibodies against LF antigens specific of the Afro‐Caribbean population in transfused SCD patients. Surprisingly, we did not find any anti‐RH20 or anti‐KEL6, despite a potentially frequent mismatch for RH20 and KEL6, considering the frequency of expression of RH20 and KEL6, and also considering that patients received RBCs from donors with the same ethnic background with a frequency of about 70% at each transfusion. The question remains whether these two LF antibodies are poorly immunogenic or only evanescent, as well as their involvement in transfusion reactions. The benefit of specifically detecting these antibodies before transfusion with a specific panel of tested RBCs can be discussed as well as systematic cross matches.
P‐488
ABH Antigens Expession in Patients with Urogenital Tumors
Ensinck MA , Lebensohn N , Racca L , Garcia Borras S , Di Paolo O , Cotorruelo C , Biondi C
Facultad de Ciencias Bioquímicas. Universidad Nacional de Rosario, Rosario, Argentina
Backgraund
The A, B, and H antigens are complex carbohydrate structures found on glycoproteins and glycolipids present on the surface of erythrocytes, endothelial cells, and on most epithelial cells. These antigens are widely distributed in human tissues and undergo changes in expression during cellular differentiation and malignant development. Studies of associations between various cancers and the ABO blood groups have shown elevated relative risks for some categories of disease.
Aim
To investigate the expression of ABH antigens in tissue samples from patients with urogenital tumors.
Methods
72 patients with urogenital tumors were examined. Appropriate informed consent was obtained from all subjects and all procedures were performed according to the ethical standards established by the University of Rosario. All biopsies were fixed in buffered formaldehyde, paraffin embedded, and stained with hematoxilyn and eosin. Specific red cell adherence test was performed on paraffin sections to detect the intensity of isoantigens A, B and H on the epithelial cell surface by a three layer sandwich technique. Anti A, Anti B, antisera and Ulex europaeus lectin (Anti H) were used. 2–5% isologous indicator RBC's suspension were added to the sections and incubated for 30 min. The slides were inverted over a support in a petridish containing Tris buffered saline such that the undersurface of the slide just touched the solution, and kept for 5 min to settle unreacted RBCs down. The slides were observed under low power magnification and photographed immediately. Normal tissues containing blood group antigens, endothelium of blood vessels and RBCs acted as inbuilt positive controls, and adipose tissues acted as inbuilt negative controls. In the present study the isoantigenicity of the epithelium was graded according to degree of adherence of indicator RBCs as strongly positive adherence (++++) to negative adherence (−).
Results
The immunoadherence reaction to tissue sections using antibodies and red blood cells showed a loss of A, B or H antigens related to the stage of tumor. A loss of ABH reactivity within the most invasive sites of the tumors correlated significantly with the stage of tumor development and histological grade of malignancy. In the tissue sections studied, the endothelium of blood vessels was reactive with the erythrocytes (positive control), and adipose tissues did not react with the red blood cells (negative controls).
Conclusions
In the present work, therefore, we used the loss of the expression of ABH antigens as a marker of differentiation. As the expression of these antigens can be detected by monoclonal antibodies, they are a better objective marker of differentiation than the more commonly used subjective histologic assessment. The presence or absence of blood group antigens has been used to predict the clinical course of patients with superficial transitional cell carcinoma of the bladder. It is generally accepted that tumors are composed of heterogeneous cell populations with different biological behaviors. To obtain optimal prognostic information about the tumor, therefore, the entire tumor cell population should be studied. It was possible to show that loss of ABH antigens was associated with the spread of tumor.
P‐489
Management and Transfusion Support for a Pregnant Woman with the Parabombay (AH) Phenotype
Lee CY1, Phillips B 2, Sekhar M 3, Thornton N 4, Storry JR 5, Win N 1
1NHS Blood and Transplant, London, United Kingdom2The Whittington Hospital, London, United Kingdom3Royal Free Hospital, London, United Kingdom4International Blood Group Reference Laboratories, Bristol, United Kingdom5Office for Medical Services, Skane, Lund, Sweden
Background
Total absence of H antigen on red blood cells (RBCs) and in secretions together with a potent anti‐H defines the Bombay (Oh) phenotype, and is extremely rare. Also uncommon is the paraBombay (Ah or Bh) phenotype in which individuals have very low levels of ABH antigens (depending on the ABO genotype), usually as a result of mutations in the FUT1 gene in either secretor or non‐secretor individuals. They form a less potent anti‐H. There is little information on the clinical significance of anti‐H in paraBombay individuals.
Aims
Our aim was to develop a management plan and provide transfusion support for a patient with the paraBombay (Ah) phenotype.
Case study and methods
A G2P1 36‐year‐old Indonesian female was seen at the antenatal clinic. She was investigated following the birth of her first child due to a blood group discrepancy and was documented as paraBombay (Ah): with incomplete patient identification details. Standard serological techniques were used to confirm the phenotype. The patient's RBCs were evaluated also by flow cytometry using monoclonal anti‐A and anti‐H. Routine ABO and FUT2 genotyping was performed; FUT1 was sequenced. After evaluation of the patient's obstetric risk, delivery by caesarean section (CS) was planned. The patient was on prophylactic low molecular weight heparin, which was paused prior to CS due to the marginal increase in risk of bleeding during delivery. Iron deficiency anaemia was corrected. The plan was to issue two frozen‐thawed Oh units from the National Frozen Blood Bank, Liverpool UK. However, there was concern that the patient might be admitted unexpectedly before CS, hence 4 group A1 units (minimal expression of H antigen compared to A) were crossmatched and suitable units delivered to hospital as a backup 1 week before the planned CS. Since the anti‐H was both IgG and IgM, saline agglutination and IAT were used for crossmatch.
Results
The patient's RBCs were nonreactive with anti‐A, ‐B and ‐H lectin. The patient's plasma reacted 1+ with A1 cells, 4+ with B cells, and weak reactions were obtained with 3 of 10 panel cells by gel‐IAT. All panel RBCs were reactive by gel‐IAT following papain treatment, by manual polybrene and saline techniques. Anti‐H was confirmed. Weak reactivity with anti‐A,B was observed by an automated microplate technique. Flow cytometry also confirmed the presence of weak A antigen on the patient's RBCs, consistent with the paraBombay phenotype. The patient genotyped as ABO*A1.01/A2.01, and was heterozygous for the FUT2 polymorphism 385A/T, consistent with the secretor phenotype. Sequencing of FUT1 revealed homozygosity for FUT1*01W.02 (328G>A; Ala110Thr) which has been described previously in the Chinese population.
Conclusion
A management plan for transfusion support of this patient was discussed in the first trimester and her anaemia was corrected. CS was performed successfully using cell salvage without excess blood loss, and blood transfusion was not required. The infant was DAT‐negative without HDN. This case demonstrates the importance of, close liaison and planning among obstetric and haematology colleagues, the hospital transfusion laboratory and reference laboratories when dealing with antibodies to high prevalence antigen.
P‐490
Correlation Between HLA Class II (HLA‐DRB1*) with the Red Cell Alloimmunization in Patients with Sickle Cell Disease
Castilho SL , Vianna TVC , Frauches TF , Couto FC
HEMORIO, Rio de Janeiro, Brazil
Background
The transfusional therapy exposes patients to lots of risks such as alloimmunization to red cell antigens. From 6% to 36% of patients with the sickle cell disease in a chronic transfusion schedule will develop antibodies against red cell antigens, but the majority will not develop them even though they are exposed to many alloantigens. Several factors are known to influence the immunological response of the patients, however, we are investigating the presence of a individual inherent factor which is responsable for the failure of the immunologic system and non‐formation of the antibodies as a consequence. Some alleles of the MHC (Major Histocompatibility Complex) are being frequently found in alloimmunized pacients, indicating a possible correlatin between the MHC profile and the formation of alloantibodies against red cell antigens.
Aim
Relating the genotypic profile of the HLA class II (HLA‐DRB1*) with the red cell alloimmunization in patients with sickle cell disease.
Method
We have selected 157 patients with sickle cell disease who had received 2 red cell units at least. The presence of red cell antibodies and the number of units received were analyzed through the data of their immunohematologic studies gathered in a retrospectively form on the electronic platform called SACS. Patients that present natural antibodies and/or autoantibodies were considered non‐alloimmunized. The HLA‐DRB1* genotyping was realized according to the protocol INNO‐LiPA HLA‐DRB1* Plus from the extracted DNA from the samples destined to crossmatch tests. The allelic distribution of the HLA‐DRB1* genes was analyzed in all patients in both groups, allo and non‐alloimmunized. The analyze of the association related to the risk or protection to developing the red cell alloimmunization was realized comparing the presence and the absence of each allele in both groups. The statistic analyze of the associatrion were obteined through the Fisher's Exact Test, calculation of the Relative Risk (RR) and the Odds Ratio (OR). The significance level was established in 5%.
Results
The group we have studied presented ages between 0 and 45 years‐old, médium age of 16 years‐old and the médium numbers of transfused units was of 84. From 157 patients, 41 (26.1%) presented alloantibodies. The analyze of the association, showed that only the allele DRB1*15 presented a different significant statistic between the groups: RR = 1.73 (P = 0.015), OR = 2.36 (P = 0.0222), Fisher's Exact Test P = 0.03.
Conclusion
The data obtained on this work allows us to conclude that sickle cell patients with the allele DRB1*15 present 1,73 more chances of being alloimmunized than those that do not have it. Although the allele DRB1*01 presents a higher frequency in the non‐alloimmunized group, we could not correlate it to a higher protection against red cell alloimmunization. The red cell alloimmunization may deflagrate immunological changes as the appearing of auto‐antibodies and Bystander hemolisys and should therefore be avoided. Faced with important phenotypic differences between donors and patients, the knowledge of the HLA‐DRB1 * genotyping of patients with sickle cell disease may be useful in establishing a protocol transfusion which contemplate erythrocyte components with extended phenotyping to patients at risk.
P‐491
One Center's Experience with Drug‐Induced Immune Haemolysis
Mayer B , Bartolmäs T , Salama A
Charité ‐ Universitätsmedizin Berlin, Berlin, Germany
Background
Drug‐induced immune haemolytic aneamia (DIHA) is a rare but severe side effect of drug therapy.
Aims
Confusion exists about the clinical and serological presentations, causing DIHA to be underdiagnosed. In this study, we present data from 66 patients investigated at our laboratory during the last 18 years.
Methods
Serological studies were performed with standard techniques using gel cards. Drug‐dependent antibodies were investigated in the presence and absence of the drug and / or their ex vivo antigens (urine and / or plasma of patients treated with the drug).
Results
All patients presented with acute and severe haemolysis. Outcome was fatal in twelve patients (18%), in six of these patients ceftriaxone‐dependent antibodies were identified. The direct antiglobulin test (DAT) was positive in all but one patient: 58 patients (88%) had C3d‐positive DAT (± IgG ± IgM ± IgA), in six patients only IgG and in one patient only IgA was detectable. The eluate was negative in about half of the cases. The serum reacted positive in the IAT with untreated and / or papain‐treated RBCs due to drug‐induced autoantibodies and / or residual drug in more than 60% of all patients.
We identified drug dependent antibodies with specificities against Diclofenac (20), Piperacillin (12), Ceftriaxon (12), Oxaliplatin/Carboplatin (10), Rifampicin (3), Cefotaxim (2), radiographic contrast media (2), Cotrimoxazol (1), Clindamycin (1), Ibuprofen (1), Etoricoxib (1) and 5‐Fluorouracil (1).
Summary/Conclusions
DIHA is a rare but potentially life threatening condition. The key serological finding is a positive DAT, mainly with Anti‐C3d. In our study Diclofenac, Ceftriaxon, Piperacillin and Oxaliplatin were responsible for more than 80% of all cases of DIHA.
P‐492
Frequency of Alloimmunization in RHD Positive Pregnant Females and its Correlation with Neonatal Outcome
Jain A , Sankaralingam P , Marwaha N , Bagga R , Kumar P
Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Background
Hemolytic disease of fetus and newborn (HDFN) has been a major preventable cause of mortality and morbidity. Antibody screen during the antenatal period can detect the alloantibodies implicated in HDFN. HDFN may be caused by naturally occurring ABO red cell antibodies as well as alloantibodies directed against Rhesus (Rh) antigens and other minor blood group antigens.
Aims
To determine the frequency of alloimmunization in RhD positive pregnant females and its correlation with neonatal outcome.
Methods
In this prospective study, 1000 RhD positive pregnant females attending the antenatal clinic at our institute were included out of which 500 were of ‘high risk pregnancy’. Blood grouping (ABO and RhD) and extended Rh phenotyping (C, E, c, e) were done by tube technique. Antibody screening and identification was done using the commercial 3‐cell and 11‐cell panel respectively by gel technique (LISS‐Coombs’ AHG card, BioRad, Morat, Switzerland). Antibody titration was done using tube technique. In case of alloimmunized pregnant females, the husband and neonatal samples were taken for blood grouping, extended Rh phenotyping and typing for minor blood group antigens corresponding to the alloantibody specificity. The neonatal direct antiglobulin test (DAT) using the gel technique (LISS‐Coombs AHG gel Card, BioRad, Morat, Switzerland) was also done for those born to alloimmunized pregnant females. Type of treatment provided to the neonate in the form of phototherapy, double volume exchange transfusion (DVET) and/or intravenous immunoglobulin (IVIg) was also recorded. The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 15.0 for windows).
Results
Out of 1000 RhD positive pregnant females, 7 were alloimmunized (0.7%). All of them belonged to ‘high risk pregnancy’ category (P = 0.015) and were multigravida. Thus, the frequency of alloimmunization was 1.4% (7 out of 500 patients) in the ‘high risk pregnancy’ group. The antibody specificities were anti‐E (6 patients; 85.70%), anti‐c (5 patients; 71.43%), anti‐Cw (1 patient: 14.29%) and anti‐S (1 patient; 14.29%). In 5 out of the 7 alloimmunized pregnant females, anti‐E was found in combination with anti‐c. The anti‐c alloantibody titer ranged from 2 to 16. Out of the 7 alloimmunized pregnant females, 6 had a history of transfusion (P < 0.01). All the 7 neonates born to alloimmunized mothers were live births out of which 4 (57.14%) were term deliveries and 3 (42.86%) were preterm deliveries. Also, 4 out of these 7 (57.14%) neonates had a positive DAT (2+). The mean duration of phototherapy in the DAT positive neonates was significantly higher than in the DAT negative neonates (P < 0.01). None of the DAT negative newborns required double volume exchange transfusion (DVET), while 2 (50%) of the 4 DAT positive newborns required DVET. None of the 7 neonates born to the alloimmunized mothers required IVIg.
Summary/Conclusions
The frequency of alloimmunization was 0.7% in RhD positive pregnant females. High risk pregnancies and antenatal patients having a history of blood transfusion should be considered for regular antibody screening.
P‐493
Detection of IGG ABO Antibodies Using Hydrogel Medium
Nathalang ON1, Thattanon PT 1, Boonchu CB 1, Intharanut KI 2, Setthakarn MS 3, Duan SD 4, Wang HW 4, Ding SD 4, Li YL 4
1Faculty of Allied Health Sciences, Thammasat University, Pathumtani, Thailand2Graduate Program, Faculty of Allied Health Sciences, Thammasat University, Pathumtani, Thailand3National Blood Centre, Thai Red Cross Society, Bangkok, Thailand4SIBET, Souzhou, China
Background
ABO antibodies are clinically important in blood transfusion practice. Haemolytic transfusion reactions due to transfusions of group O platelets to a non‐group O patient have been reported in the literature. The prevalence of high titers of IgM and IgG anti‐A and anti‐B in Thais are high; therefore, screening for ABO antibody titers when providing ABO incompatible platelet transfusion is required.
Aims
The purpose of this study was to detect IgG anti‐A and anti‐B antibodies in group O Thai blood donors by indirect antiglobulin test (IAT) using conventional tube test (CTT) and hydrogel medium (HDM).
Methods
Altogether, 100 serum samples obtained from group O healthy blood donors of the National Blood Centre, Thai Red Cross Society, Bangkok, Thailand were included. IgG anti‐A and anti‐B titers were tested by IAT using CTT and HDM without washing step. The results of antibody titers and agglutination scores were compared.
Results
Among 100 blood donors, there were 51 males and 49 females (M:F = 1:1) and their ages ranged from 18 to 58 years. There were no association between IgG anti‐A and anti‐B agglutination scores and titers with age and gender. The IgG anti‐A, and anti‐B titers using CTT yielded higher agglutination scores than HDM (P < 0.001). However, a good correlation was obtained in the agglutination titers (anti‐A, r = 0.7583 and anti‐B, r = 0.7145). To assess the repeatability of IgG ABO antibody detection by IAT using HDM, the mean, standard deviation and coefficient of variation (CV) of 3 serum samples tested in quintuplicate. The CV of agglutination scores is within 5%.
Conclusions
From this study, the HDM can be used to perform IAT for determination of IgG ABO antibody titers in order to eliminate washing step. Furthermore, it provides not only reliable results but also reproducible testing.
P‐494
Establishing Alloimmunisation Rates and Safety of Electronic Issue in the Largest Automated Exchange Programme for Sickle Cell Disease in England
Trompeter S 1, McMillan A2
1NHS Blood and Transplant, London, United Kingdom2University College Hospitals London NHS Foundation Trust, London, United Kingdom
Background
Patients with SCD are amongst the most highly transfused patient populations over a lifetime. Typically a patient with SCD on an automated exchange will receive 10 units every 6 weeks, equalling 87 units per annum and a patient receiving top ups or manual exchanges will receive 3 units every 4 weeks, equalling 39 units per annum. Heavy donor exposure, particularly in those sporadically transfused, is recognised to cause formation of multiple alloantibodies, thus complicating the provision of compatible blood (Chou, et al 2012). For haemoglobin disorders and especially sickle cell disease, the ethnicity and Rh types of the donor population often differ from the patient population. Most hospitals will request a ‘wet’ crossmatch on all patients with SCD due to concern that the screening antibody panels may not adequately represent the antigens seen in black people and thuse may miss antibodies. The burden of a ‘wet’ crossmatch on the laboratory staff, the delay in issue when using the technique when large numbers of units are being requested is not to be underestimated. However, this institution permitted electronic issue in SCD patients with a negative antibody screen and no history of alloimmunisation.
Aims
To establish the alloimmunisation rates and safety of electronic issue in a hospital with the largest automated exchange programme for sickle cell disease (SCD) in England
Methods
A retrospective audit of transfusion exposure and alloimmunisation in patients receiving excange transfusions for sickle cell disease at UCLH. An in‐house database was interrogated to capture the patients and then the blood bank manager to establish transfusion details.
Results
There were 82 patients receiving regular exchange‐transfusion for their SCD. Of these 35 were alloimmunised prior to starting their regular transfusions at UCLH. Over the study period (7 years) 6 of those 46 non immunised developed antibodies (incidence 1.8% of patients p.a.). of the following phenotypes: anti Kpa, anti Lua, anti Lea, anti Jka and anti S. Of those who were already immunised 8 developed new alloantibodies (incidence 3% of patients p.a) of the following phenotypes: anti Kpa, anti M, anti C, anti Fy3, anti Lea, anti Lua and anti Goa. The anti C antibody was detected following a transcribing error of c for C when a patient's phenotype was passed on from a previous instituation. The overall incidence of new antibodies was 2.4% of patients p.a. or 0.0002 alloantibodies per unit transfused.
Summary
At a hospital where 82 patients receive automated red cell exchange on average 10 units 6 weekly mostly on weekdays only, the average number of units per day needing x‐matching is 35 units a day. One antibody would not have been picked up on a routine panel but this was in a patient who had previous antibodies and therefore was not eligibile for electronic issue. Electronic issue with appropriate safeguards is a safe mechanism of blood issue in SCD. Automated red cell exchange results in a similar rate to sickle cell patients in general despite this procedure being associated with much greater donor exposure than other techniques.
P‐495
Detection and Identification of Red Cell Alloantibodies in Multiply Transfused Thalassemia Major Patients
Jain RJ1, Jain P 1, Choudhury NC 2, Mahadik VK 1
1C.R.G. Hospital and R. D. Gardi Medical College, Ujjain, India2Fortis Hospital, Gurgaon, India
Background
Lifelong red blood transfusion remains the main treatment for β thalassemia major patients. Transfusion therapy could be complicated with the development of anti RBC antibodies (alloantibodies and/or autoantibodies). Some alloantibodies are hemolytic and may cause hemolytic transfusion reactions and limit the availability of further safe transfusion. Alloimmunisation to red cell antigens is one of the most important immunological transfusion reaction and causes delayed type of transfusion reaction.
Aims and objective
(i) To provide frequency and distribution pattern of various types of irregular red cell alloantibodies in patients with thalassemia major. (ii) To determine the mean red cell transfusion Requirement and mean transfusion duration.
Method
A prospective study was conducted from January 2014 to December 2014 at Transfusion Medicine and Blood Bank Dept. Seventy eight diagnosed thalassemia major patients were included in this study and samples collected and investigated for the development of alloantibody to red cell antigens by using Matrix Gel System (Tulip Diagnostics). Five to seven ml of blood was collected in plain tube and serum was separated. Separated serum was taken in two aliquots, labeled properly and stored in two different boxes at −30°C in deep freezer, till the antibody screening and identification performed. Tests for antibody screening and identification were performed on preserved sample to investigate prevalence of red cell alloimmunization by standardized laboratory techniques by same person. Antibody screening was carried out on serum employing commercial three‐cell panel (Matrix Gel System, Tulip Diagnostics) using standardized blood bank techniques. If patients were found to have an irregular red cell alloantibody then the antibody identification was performed using commercial 11 cell panel cells (Matrix Gel System, Tulip Diagnostics).
Results
A total of 78 patients were included in the study. Forty eight patients were males and thirty females. Mean age was 8.2 years. Irregular red cell alloantibodies were found in 6 patients (7.69%). Mean age of patients who developed red cell alloantibody was 12.48 years. Three patients developed single antibodies (50%) (2 patient anti‐K and 1 patient anti‐C), while other 3 developed multiple antibodies (50%) (anti‐D and anti‐E, anti‐D and anti‐C, anti‐E and anti‐K).
Conclusions
Red cell alloimmunisation should be kept in mind in the patients receiving multiple transfusions.In present study, alloimmunisation rate was 7.69%. Mean transfusion duration in these patients was 21.80 days, probably due to presence of alloantibody. We also suggest that red cell alloimmunization should not be overlooked in patients receiving regular blood transfusion. RBC alloantibody detection on regular interval and antibody negative blood transfusion is strongly recommended in transfusion dependent thalassemia patients.
P‐496
Alloimmunization in Surgery Patients After Massive and Multiple Blood Transfusions
Martinova FGM , Shurlieva EMSH
University Hospital for Emergency Medicine, Pirogov, Sofia, Bulgaria
Background
Determination of clinically significant antibodies before transfusion is of great importance in order to find compatible blood components and to prevent post‐transfusion reactions and complications. Alloimmunization to red blood cell (RBC) antigens occurs commonly in surgical patient received massive and multiple blood transfusions. Patients with alloimmunization demonstrate increased risk for new alloantibody formation with subsequent transfusion. Alloimmunization to human leukocyte antigens (HLA) can occur with RBC transfusion and may result in febrile non‐hemolytic transfusion reactions (FNHTR), platelet refractoriness, TRALI, TA‐GvHD.
Aim
The aim of the study is to determine alloimmunization against HLA and red blood cells antigens and to create pre‐transfusion diagnostic algorithm to find out compatible blood components in surgery patients received massive or multiple blood transfusions.
Methods
Anti‐erythrocyte antibodies were tested in 1498 surgery patient and anti‐HLA allo‐antibodies ‐ in 516 surgery patients (in the period of 1 year). Blood samples have been tested for antibody screening and identification by direct and indirect Coomb's tests, using ID card, panel test‐cells and reagent of Mycro Typing System (DiaMed). Microlymphocytotoxicity test (CLT) and Luminex‐based flowcytometry analysis were used for detection and identification of anti‐HLA allo‐antibodies, class I and class II.
Results
Anti‐erythrocyte antibodies were found in 271 patients(18,09%). 18 of them were Rh‐specific (anti – E; anti – D; anti – C; anti – Cw; anti – c; anti – e; anti – D+C), 12 – for antigens from other systems (Kell; Duffy; Kidd; Lewis; MNS) and other ‐ with unidentified specificity. In 30 out of 516 tested patients (5.81%) anti‐HLA class I antibodies were found, end in 27 patients ‐ anti‐HLA class II antibodies.
Conclusion
The number of transfusion exposure plays a role in the rate of alloimmunization. It is important to evaluate the immune response of patients with massive transfusions. Patients with clinically significant antibodies should receive antigen negative blood or fully matched red cell concentrates. In patients with anti‐HLA antibodies and in these incoming massive or multiple blood transfusions leucodepleted erythrocyte concentrates should be applied.
P‐497
Recombinant Soluble Blood Group Proteins: One Centre's Experience
Lambert MD1, Farrelly A 1, Seltsam A 2
1Irish Blood Transfusion Service, Dublin, Ireland2German Red Cross Blood Service NSTOB, Springe, Germany
Background
The identification of allo‐antibodies to high frequency antigens (HFAs) can be time consuming, even for national or international red cell reference laboratories. This can lead to delays in provision of suitable blood for these patients. Standard approaches for the identification of allo‐antibodies to HFAs involve testing the patients's plasma against cells negative for various HFAs.
With all the major blood group genes cloned, the production of recombinant blood group proteins (rBGPs) to any protein‐based blood group is possible. These rBGPs can be used in a soluble form to neutralise antibodies to HFAs [Transfusion 2014;54:1823–1830].
Aims
We investigated, both retrospectively and in‐parallel, different patient plasma with known or suspected antibodies to HFAs. We used the Imusyn soluble recombinant blood group proteins (srBGPs). The srBGPs include the following blood groups: C4A, C4B, YTA, LUB, DOA, DOB, KNA, JMH, SC, CR.
Methods
Patient plasma was neutralised with various srBGPs. 25 μl of test plasma was incubated at 37°C for 30 min with 2 μl (1 μg) of srBGP. The neutralised plasma was then tested by IAT using standard BioRad LISS‐Coombs Cards.
Results
A total of 20 plasmas were tested. Retrospective testing was performed on 12 plasmas: anti‐Lu8 (1), anti‐c+Jka+Yta (1), anti‐Yta (2), anti‐JMH (1), anti‐Lub (1), anti‐CR1‐related + possible anti‐Fya (1), anti‐CR1‐related (4) and anti‐Ch/Rg (1). In‐parallel testing was performed on 8 plasmas: Antibody to HFA (2), ‘HTLA’ antibody (5), anti‐CR1‐related (1).
Within the ‘Retrospective’ plasma group all antibodies were neutralised as expected except one anti‐CR1‐related. Anti‐Lu8 was successfully neutralised by srLUB protein. One of the anti‐Yta plasmas had an additional anti‐K identified, following use of srYTA. The plasma containing anti‐CR1‐related with possible anti‐Fya proved to contain just anti‐CR1‐related (during initial investigation all ‘CR1’ cells used were Fy(a−b−)).
Within the ‘In‐parallel’ plasma group the 2 antibodies to HFAs proved to be Oh with anti‐H (srCR and srSC were used but did not neutralise). A known anti‐CR1‐related from an African antenantal patient with an urgent blood request was neutralised by srKNA (compatibility testing was not performed with neutralised plasma). Of the 5 ‘HTLA’ antibodies investigated 3 proved to be anti‐CR1‐related and neutralised with srKNA. Two of these anti‐CR1‐related plasmas were from African antenatal patients requiring urgent investigation. A third ‘HTLA’ antibody was identified as anti‐Yta (srYTA successfully neutralised). The final HTLA antibody was tested with srJMH, which did not neutralise and was finally reported as ‘weak undefined’.
Conclusions
Soluble rBGPs proved very useful in investigating the specificity of antibodies to high frequency antigens. In particular srKNA proved very useful in neutralising anti‐CR1‐related in antenatal African patients requiring urgent investigation and provision of suitable blood, rapidly eliminating the possibility of anti‐Fy3. The Imusyn srBGPs have recently received CE‐marking and would be a useful addition to the repertoire of tests available to any laboratory investigating difficult serological samples, especially antibodies to HFAs.
P‐498
Introduction of an Additional Red Cell Antibody Screening Test at 36 weeks in Pregnant Women Receiving Routine Antenatal Anti‐D (RAADP) at 28–30 weeks of Pregnancy
Sulin K , Kuosmanen M , Tuomi K , Haimila K , Korhonen A , Natunen S , Reinman M , Sareneva I , Sainio S
Finnish Red Cross Blood Service, Helsinki, Finland
Background
The Finnish Red Cross Blood Service carries out antenatal blood group antibody screening, with RhD negative mothers screened three times at 8–12, 24–26 and 36 weeks. Although it is assumed that antibodies that become detectable only in the third trimester do not cause severe HDFN, information on these antibodies is considered important for remote delivery hospitals because of transfusions or postnatal care. In 2013, the National Institute for Health and Welfare announced recommendations to give RAADP (1250–1500 IU) at 28–30 weeks to women carrying an RhD positive fetus. After that, the majority of screening tests at 36 weeks were expected to be positive. As antibody identification is time consuming, an additional screening test at 36 weeks was introduced for RhD negative women receiving RAADP.
Aims
To assess the ability of the new screening test to detect late developing transfusion related antibodies without increasing the number of identifications required or missing high‐titer anti‐D immunizations requiring intervention.
Methods
All 36‐week screening samples were tested using two RhD positive screening cells (O1 and O2) (by LISS/Coombs gel cards; BioRad, Switzerland). If the reaction strength was a weak positive (≤1+), an additional RhD negative screening (O4, O5 and O6) was performed. Antibody identification was performed for all cases with reaction strength of ≥2+ in screening with O1 and O2 and for cases with a positive result in the additional RhD negative screening. The cut‐off point was set to ≤+1 in line with a pilot study where the reaction strength was weakly positive (≤1+) in most cases with anti‐D immunoglobulin given 6‐8 weeks earlier and in all cases with immune anti‐D where the titer was below the critical threshold of 16. Titers were performed using the tube method for indirect antiglobulin test.
Results
During the first year, the 36‐week screening test was positive in 2591 of 6069 (43%) RhD negative mothers. In 2227 (86%) cases, the reaction was weakly positive (≤1+). In the additional RhD negative screening test, three additional antibodies were identified (anti‐M (MNS), unidentified antibody, panagglutinin).
In 364 (14%) cases, the result was ≥2+. In the antibody identification, prophylactic anti‐D was identified in 340 mothers (although impossible to distinguish from low titer immune anti‐D). Immune anti‐D was detected in three mothers not receiving RAADP (titers 1–16), and in two mothers receiving RAADP (titers 4–32). In all cases with an anti‐D titer of ≥8, the reaction strength in screening with O1 and O2 was ≥3+. Furthermore, 19 additional antibodies were identified (anti‐Lea (LE) 2, anti‐Leb (LE) 1, panagglutinin 1, unidentified antibody 15).
Summary/Conclusions
Antibody identification was avoided in 86% of cases by using an additional automated RhD negative screening test. To reduce the need for identification further, a cut‐off level of ≥3+ for identification and titration can be considered. The 36‐week screening needs to be continued until RAADP has been fully adopted in Finland. The cohort size was too small to draw conclusions on the need for 36‐week screening in detection of transfusion relevant antibodies.
P‐499
Potential Interference of Daratumumab in Routine Blood Transfusion Policies and Serological Tests
Alonso Nogues EAN1, Muñiz-Díaz EM-D 2, Motlló CM 3, Boto NB 2, Oriol AO 3, Salgado MS 2, Torrent AT 3, Linio R 1, Josep Maria RJM 3, Grifols JR 1
1Banc de Sang i Teixits, Badalona, Spain2Immunohematology Laboratory, Banc de Sang i Teixits, Barcelona, Spain3Clinical Haematology Service, Catalan Institute of Oncology, Badalona, Spain
Background
Multiple myeloma (MM) remains an incurable disease despite important recent advances in treatment due to its innate resistance characterized by its heterogeneous molecular abnormalities. A novel therapeutic strategy that effectively targets specific molecules on MM cells is emergently appearing. Daratumumab (DARA) is a novel therapeutic IgG1 human CD38 monoclonal antibody for relapsed or relapsed and refractory disease in which other novel agents (bortezomib or immunomodulatory drugs) as well as autologous stem cell transplantation hasn't been successful. CD38, also known as cyclic ADP ribose hydrolase is a small multifunctional glycoprotein found on the surface of many immune cells: white blood cells (including CD4+, CD8+, B lymphocytes and natural killer cells) and red blood cells (RBCs). Because its presence on RBCs, DARA may interfere with routine blood bank serological tests.
Aim
To present our experience by studying a group of thirteen MM affected patients in whom DARA induced a positive antibody screen and a plasma panagglutination interfering in routine compatibility testing.
Methods
Our patients (eight men; five women) were enrolled in three different DARA trials (DARA as a monotherapy and in combination with other drugs) between April 2014, and February 2015. They were diagnosed of an IgA lambda (n = 4), IgG kappa (n = 7), IgG lambda (n = 1) and IgM kappa (n = 1) MM. Routine blood transfusion immunohemathological methods (tube and gel card) were used for pretransfusion testing when blood components were requested.
Results
A homogeneous panagglutinin (2+) was detected in all patients mainly reacting in the antiglobulin phase. No discrepant results on ABO/Rh(D) were found. Direct antiglobulin test and autocontrol were negative by both the tube method and the gel card technique (DG Gel®Coombs, Grifols). Elution (EluKit) was also negative. These results were identical regardless of the MM type, the amount of M‐protein or renal function. Adsorption techniques were unable to remove the panagglutinin, and the reactivity seemed to increase after the adsorption procedure. So, hidden alloantibodies by the panagglutinin couldn't be discarded. Cross‐matching was incompatible with all RBCs tested. Patient's genotyping (BLOODchip®Service, Grifols) was then performed in order to provide the most compatible RBCs. At the same time, we observed that panreactivity of all DARA‐treated patient samples was eliminated by using DTT‐treated RBCs (Chapuy CI. AABB 2014).
Conclusion
DARA potently interferes with routine pretransfusion immunohemathological tests by directly binding to RBC CD38 antigen. There were no differences between serum panreactivity in DARA‐treated patients despite being included in different trials and have different biological conditions. We can also confirm that DTT pre‐treatment of reagent RBCs is a good method to eliminated DARA‐interferences, allowing the safe provision of RBC units to DARA‐treated patients. Otherwise, it would be recommended to perform an accurate patients'RBC phenotyping or genotyping before enrolling the patient to the trial.
P‐500
Identification of an Exceptional Alloanti‐Eneh (Anti‐Mns40) in a Northern African Patient
Thonier V 1, Willemetz A 1, Babinet J 1, Biering V 1, Mazaux L 2, Kimmel C 3, Arnaud L 1, Peyrard T1
1Institut National de la Transfusion Sanguine, Paris Cedex 11, France2HOPITAL FOCH, Surennes, France3Etablissement Francais Du Sang, Versailles, France
Background
A 35 year‐old male patient from Maghreb (Northern Africa) was scheduled to undergo a cholecystectomy. No previous blood transfusion and no medical condition were reported. The first‐line immunohaematology laboratory suspected a weak antibody to a high‐frequency antigen, which led to postponement of the surgery. A blood sample was sent to our reference laboratory for further investigation.
Aims
To identify the specificity of the antibody.
Methods
Standard haemagglutination techniques (indirect antiglobulin test, autologous adsorptions) and genomic sequencing of the GYPA gene were performed.
Results
The patient's phenotype was A, D+C+E‐c‐e+, K‐, Fy(a−b+), Jk(a+b+), S‐s+. The antibody was nonreactive on either papain‐ or trypsin‐treated RBCs and was sensitive to DTT (IgM). Autologous controls and direct antiglobulin test were negative. Several RBCs with a rare blood type were tested: Ch‐, Kn(a−), Lu(b−), JMH‐, In(b−) and Ge:‐2. All of them were as reactive as the standard panel cells. In contrast, the red cells of two persons labeled as En(a−) were nonreactive with the patient's antibody. To confirm the specificity of this antibody, the Ena patient's phenotype was determined and was found to be En(a+) and also M+N‐, with a slightly weakened M reactivity. No difference in the intensity of serum was observed after autologous adsorptions. Serologic techniques were inconclusive: on one hand the adsorptions and autologous controls were in favor of an alloanti‐Ena, on the other hand the phenotype was in favor of an autoanti‐Ena. By sequencing the GYPA gene, we found in exon 3 the c.140C>T mutation (p.Thr47Met) at apparent homozygous state, known to encode the low‐frequency Vw antigen. The patient was homozygous for GYPA*M. The Vw+ phenotype was confirmed.The antithetical antigen of Vw is ENEH (MNS40), known to be a high‐frequency antigen. Unsurprisingly, no anti‐ENEH phenotyping reagent was available to confirm the ENEH‐ type. From these data, we concluded that the patient was ENEH‐ and that the identified antibody was an alloanti‐ENEH.
Summary/Conclusions
To the best of our knowledge, we report here the second case of anti‐ENEH alloimmunization. Finally the donor underwent his surgery with no transfusion needed. He was further urged to donate blood. The anti‐Ena specificity (anti‐MNS28) is a confusing umbrella term that incorporates different antibodies detecting various determinants on GPA. Anti‐Ena are extremely rare as alloantibodies, but are more likely to be autoantibodies (most of them are directed against a ‘trypsin resistant’ antigen). They are known to have different reactivity patterns with proteolytic enzymes. Like in our case when an anti‐EnaTS is suspected (nonreactive on trypsin‐ or papain‐treated red blood cells), the Vw phenotype could be determined to better characterize the anti‐Ena specificity. The Northern African origin of the patient was surprising to us, since most Vw+ subjects are known to be from Swiss ancestry. In addition, the Vw antigen was reported to be encoded by a GYPA*N allele while it is encoded by a GYPA*M allele in our case. Interestingly, the only compatible En(a−) donor with the patient was not ENEH‐. This case illustrates the need to better characterize the subjects labeled as En(a)−.
P‐501
Red Cell Autoimmunization and Transfusion Support: Three Year Analysis at a Tertiary Care Hospital
Makroo RN , Prakash B , Bhatia A , Chowdhry M
Indraprastha Apollo Hospital, New delhi, India
Background
The spectrum of autoantibodies ranges widely in terms of their thermal amplitudes and clinical significance. Pre transfusion testing and transfusion support in patients with autoantibodies has been a medical dilemma since ages.
Aims
This study aims at assessing the frequency of anti‐red cell autoantibodies and transfusion support in patients at a tertiary care hospital in North India.
Materials and methods
The records of all antibody screen positive patients who underwent antibody identification from Jan 2012 till December 2014 was retrieved and reviewed. All patients with anti red cell autoantibodies were evaluated for various parameters including age, sex, diagnosis, and serologic reactivity. Antibody detection and identification were performed by solid phase technology (Immucor Inc. Norcross GA) according to the manufacturer's specifications. Autologous or allogeneic adsorption was performed, as needed, to detect underlying alloantibody. DAT was performed using polyspecific as well as Monospecific reagents for IgG and C3d. Clinical records, transfusion requirements, and laboratory parameters suggesting hemolysis (hematocrit, reticulocyte count, bilirubin concentration, lactate dehydrogenase levels etc.) were reviewed. Response to transfusion was assessed wherever possible.
Results
A total of 722 antibody identifications were performed in the study period. Of these autoantibodies were detected in 286 patients. Among the autoimmunized, 213 had warm reactive autoantibodies, 71 had cold reactive antibodies, while 2 patients had biphasic antibodies. The age range was 12 months to 92 years with150 males and 136 females. In 47 patients one or more alloantibody was also identified after adsorption studies. Most common disease category was autoimmune disorder and haematological malignancies (18%). Nearly 21% of the patients with warm autoantibodies had clinical and lab features of hemolysis and DAT was positive in 61% of them. 55 patients with warm reactive antibodies received one or more PRC transfusions with units crossmatched either directly or with adsorbed plasma, while 19 were given group specific blood without crossmatching. No hemolytic transfusion reaction was noted and nearly 45 did show improvement in hematocrit post transfusion.
Conclusion
Autoantibodies are commonly encountered in medical practice. Clinical significance of these antibodies depends on the thermal amplitude and strength of DAT and reaction with panel cells. Many patients with serological evidence of warm autoantibodies do not show signs of haemolysis. The risk and benefits of transfusion in these patients should be judged based on clinical conditions and detailed pre transfusion workup results including adsorption studies.
P‐502
Frequency of Red Cell ABS in Thalassemic Patients at Dar Al‐Salam RBTC
Magdy Abdulla R
NBTS Egypt, Cairo, Egypt
Background
Thalassemia is a common hereditary hematologic disease in Egypt; it may lead to severe, progressive anemia which needs regular transfusions for life. One of the most important complications of regular blood transfusion is allo‐immunization which makes finding a suitable blood unit time consuming.
Aim
This study was performed to investigate the production of red cell alloantibodies and their frequency in thalassemia patients receiving regular blood transfusions at Dar AL‐Salam RBTC in Cairo Egypt, the results will be useful for setting guidelines for phenotype donor recall data base
Methods
The study conducted on 116 thalassemic patients presented to Dar AL‐Salam RBTC in Cairo Egypt. Patients subjected to: 1. screening for red blood cell Abs (panel of 3 blood group O‐cells) using appropriate IAT by CAT. Antibody identification for positive screening results (panel of 11 blood group O‐cells) using appropriate IAT by CAT. Red cell typing using monoclonal anti‐sera.
Results
Atotal of 116 patients screened for the presence of red cell allo‐antibodies 38 (32.7%) found to have allo‐antibodies. The alloantibodies identified as a percentage of the total antibodies, were anti E (33.3%), anti K (17.9%), anti D (15.3%), anti C (7.6%), anti c(7.6%), anti S (5.1%), anti JKa (5.1%),anti Fya (5.1%),anti Lea (2.5%)
Conclusions
Thalassemia patients have relatively high prevalence of RBC alloantibodies. The most frequent clinically significant anti bodies are anti E and K so when starting our donor data base for alloimmunised patients it is recommended to recall and retain negative E and K antigen donors thereby saving time and resources when selecting blood for such patients. To decrease the rate of alloantibody synthesis, phenotyping the patients before the 1st transfusion and evaluation of Abs status should be done before each transfusion.
P‐503
Abstract Withdrawn.
P‐504
Optimization of in House Reagents for Immunohematology Testing on Beckman Coulter PK7300 System
Hecimovic A , Stojic Vidovic M , Jurakovic Loncar N , Balija M
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
Since 1996 CITM is a manufacturer of ‘in house’ monoclonal reagents which are prepared from blood typing intermediates for further manufacturing use, by Merck Millipore. The assortment includes reagents for ABO, RhD, Rh phenotype and Kell antigen blood grouping and very soon it will includes reagents for M, S, Jka, Jkb antigen typing also.
It is essential that blood grouping reagents are prepared using reliable manufacturing procedures that are consistently capable of producing safe and efficacious products. The products must comply with requirements of the EU Directive (98/79/EC) on in vitro diagnostic (IVD) medical devices and other relevant international standards. These types of reagents, according to the classification of Directive 98/79/EC Annex II, fall under List A, or in the IVD of high risk to the health of the individual. Therefore, all processes are running within the quality system ISO 9001:2008 and ISO 13485:2003, which is in the process of setting.
Aim
Present the results of validation which is a part of the adjustment process of ‘in house’ reagents on PK7300BC system. The validation was carried out according to the instructions amending Decision 2002/364 /EC on common technical specifications for in vitro diagnostic medical devices (2009/886/EC) and the Guidelines for the blood transfusion services.
Methods
By dilution, blending and chemical additions ‘in house’ blood grouping regents are formulated for a variety of techniques including microaglutination technique for automatic system PK7300BC. Bromelain solution was prepared in 0.1% concentration.
3129 ABO and RH1 (D) tests (2% ‘weak D’) and 3199 Kell antigens and Rh phenotype (C,c,E,e) tests was carried out in parallel with the ‘in house’ reagents and CE mark commercial reagents (Sifin), according to the CITM algorithm for testing blood donors. Routine work was simulated during validation. Discrepant results were confirmed by gel microcolumn assay (Bio-Rad) and by tube technique (Ortho-Clinical Diagnostics).
Results
The accuracy of the interpreted results of ABO and RH1 (D) blood group system and Kell antigen was 100%. The accuracy of Rh phenotype was 99.95%; four discrepancies of ‘C’ antigen (false positive) are probably due to a technical problem, well/plate contamination. In the next donations, results of ‘C’ typing were correct. The weak types of RhD antigen, 1, 2, 3 and 14, are interpreted as RhD positive.
Conclusions
Results of validation are more than satisfactory, especially if we consider the reason for discrepant results of ‘C’ antigen. Interpretation of weak RhD types as RhD positive will reduce further testing of blood donors. The application of the ‘in house’ blood grouping reagents on Beckman Coulter PK 7300 automated system for testing DDK has proven to be very reliable. The facts such as the availability, sufficiency and saving money support this conclusion.
P‐505
Verification of IH‐1000 System in Croatian Institute of Transfusion Medicine
Kruhonja Galic Z , Jurakovic Loncar N , Jagnjic S , Bingulac Popovic J , Ðogic V , Hecimovic A , Jukic I
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
Automation in immunohematological laboratory reduces human analytical and postanalytical errors that can have significant consequences for patients health. Fully automated systems and correct interpretation of results minimize the possibility of transfusion hemolytic reactions.
Aims
The aim was to compare IH‐1000 (Bio‐Rad, Cressier FR, Switzerland) with Tango Optimo (Tango)(Bio‐Rad, Dreieich, Germany) which is routine method in Croatian Institute of Transfusion Medicine (CITM) for ABO/RhD typing and antibody screening and introducing IH‐1000 as another method in daily routine work.
Table 1. ABO and RhD typing.

Methods
For ABO/RhD typing we used 205 samples of patients, blood donors and pregnant women and 282 samples for antibody screening (Abs), among them 153 with previously positive results. ABO/RhD typing were done in Erytype S ABD+Rev. A1,B microtestplate (single strips) on Tango and in DiaClon ABO/D+Reverse Grouping ID‐Card on IH‐1000. Abs were performed in indirect antiglobulin test with Biotestcell P1, P2 in Solidscreen II Strip on Tango and with ID‐DiaCell I‐II by gel technology in LISS/Coombs ID Card BioRad on IH‐1000. All tests were performed and results validated after visual checking of the digital image, according to manufacturer instructions.
Results
In ABO typing 100% identical results were given, but in RhD typing there were some differences in weak D types (Table 1). In Abs 129 samples with previously negative results remained negative. Abs were positive on IH‐1000 for 7 samples with 1 anti‐c, 1 anti‐E, 2 anti‐Jka, 1 anti‐M, 2 anti‐N, which were on Tango negative. In 16 samples with positive (13 only visualy positive) Abs on Tango, IH‐1000 was negative: 3 pasive anti‐D, 6 anti‐M, 4 anti‐Lea, 2 anti‐Jka and 1 anti‐Jkb (Table 2). Other samples with positive Abs were concordant.
Conclusion
Automated system IH‐1000 is comparable with Tango for ABO/RhD typing. Differences in RhD typing depends on monoclonal anti‐D used. Most of the antibodies which IH‐1000 failed to detect are not clinically significant, unfortunatelly there were 3 samples (Tango negative, visualy positive) with suspected Kidd antibodies near the limit of detection. On the other hand Tango failed to detect 4 clinically significant antibodies. We can conclude that there is no superior method and automated system IH‐1000 is reliable for ABO/RhD typing and antibody screening in CITM.
Table 2. Antibody screening.

P‐506
Performance Characteristics of the Novaclone™ Anti‐D IGM + IGG Monoclonal Blend on the Galileo Neo® Automated Analyzer in Direct and Indirect Assay
Rübsamen D1, Muehl J 1, Marandiuc D 2, Matteocci A 3, Blincko S 1
1IMMUCOR Medizinische Diagnostik GmbH, Rödermark, Germany2Transfusion Center, University Medical Center of Johannes Gutenberg‐University, Mainz, Germany3Department of Transfusion Medicine, San Camillo Forlanini Hospital, Rome, Italy
Background
Mutations in the RHD gene, that codes the Rhesus D protein, lead to a wide range of weak and partial D phenotypes which have various reactivity to anti‐D antisera considering the test method and the clone. Patients with partial D may form allo‐antibodies against the complete D antigen, as well as Rhesus D negative patients if they were to encounter a partial D epitope. Therefore, the detection of the D antigen in patients and donors is of vital importance.
Aim
This poster describes the performance characteristics of the NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend on the Galileo NEO® automated analyzer in the hemagglutination and in the indirect antiglobulin test. NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend contains human monoclonal IgM anti‐D (D175‐2) and human monoclonal IgG anti‐D (D415 1E4) antibodies and can detect a wide range of weak and partial D antigens with both methods.
Methods
93 weak Ds, 24 partial Ds including 10 DVI specimens were analyzed with a hemagglutination assay and with an indirect antiglobulin test (Capture‐R® Select Solid Phase System) on Immucor's Galileo NEO® automated analyzer with respect to sensitivity of the NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend. Specimens were obtained from DRK Blutspendedienst in Frankfurt, Transfusion Center of the University Medical Center of the Johannes Gutenberg University Mainz, Germany and Forlanini Hospital in Rome. All weak and partial D donors were genotyped. All assays were run at Immucor's laboratory in Rödermark/Germany.
Results
The NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend detected 89% of the weak Ds, 64% of the partial Ds (excluding DVI) and, as expected, none of the DVI samples in the hemagglutination assay. All D antigens were detected with the NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend in Capture‐R® Select Solid Phase System.
Summary/Conclusion
The NOVACLONE[TRADEMARK] Anti‐D IgM + IgG Monoclonal Blend detected most D variant antigens in a hemagglutination assay and all of them in the in Capture‐R® Select Solid Phase System, i.e. there was a 100% concordance with the genotype of the samples. Therefore, it is highly suitable for the detection of partial D and weak D testing in patients and donors.
P‐507
Evaluation of Second Series Reagents for the New Daymate Automated Column Agglutination Technology for Patient Blood Grouping
Marandiuc DE , Seifert S , Conradi R , Runkel S , Hitzler WE
University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Background
The identification of ABO and Rhesus blood groups is essential in transfusion medicine.In conformity with the international guidelines, method adequate reagents and different clones are needed for correct blood grouping.
Aims
A second wave of reagents for the new automated column agglutination technology from DAYmedical SA was to be validated on the automate DAYmate M in a clinical setting by comparison to the institute's standard manual methods.
Methods
111 patient and 21 newborn samples were tested for AB0 forward and reverse blood group and Rhesus D antigen. Of those, 20 patient and 4 newborn samples were also tested for Rhesus (C, c, E, e) and Kell phenotype. DAYmate M used automated column agglutination on discs with 18 initially empty columns, which were filled with appropriate gel and reagents during the test. Manually, two methods were used: column agglutination onID‐System (Bio‐Rad Laboratories GmbH) and agglutination on Bioplate (Biotest AG) with reagents from BAG Health Care GmbH. Manual reverse grouping was performed on Bioplate with in house manufactured test erythrocytes. A confirmatory Rhesus D test with antiglobulin (AHG) was performed on DAYmate for all samples. Manually, only the Rhesus D negative samples were tested with AHG in tubes. Samples without a plausible result were retested and the difficult ones were clarified with additional manual testing (ID‐System, Bio‐Rad) or PCR.
Results
5 samples from polytransfused patients could not be typed for Rhesus antigens on DAYmate due to double population (DP) reactions. Manually, DP were found only in 4 of the 5 samples. 10 patient samples, 9 on DAYmate and 4 on Bioplate were not typed due to weak or negative reactions in the AB0 reverse group. The weak reactions were confirmed on ID‐cards for all the 10 samples. Due to weak reaction for Rhesus D antigen, 3 newborn samples were clarified with PCR as Rhesus D weak type 1, D weak type 3 and D partial VII, respectively. Two samples, one patient and one newborn were tested Rhesus D negative with all methods except the AHG confirmatory test on DAYmate, which was weak positive. Reruns of patient sample with DAYmate, manual methods and PCR did not confirm the AHG weak positive result. A rerun of the newborn sample on DAYmate with AHG confirmed the weak positive result, as well as a rerun on ID‐card with a very weak positive reaction. The results of the newborn sample could not be further clarified due to insufficient sample material.
Summary/Conclusions
All samples were correctly typed. DAYmate reagents have shown similar reactions to the manual method reagents. Difficult samples were further clarified with additional methods and were interpreted by a technician. The DAYmate automated system provided reliable blood grouping results.
P‐508
Automated ABO Antibody Titration on the Galileo Neo® Platform
Preuss E , Oldekamp S , Muehl J , Blincko S
IMMUCOR Medizinische Diagnostik GmbH, Rödermark, Germany
Background
ABO antibody titration is clinically relevant for many different applications such as organ and stem cell transplantations, ABO incompatible pregnancies and blood group O donor characterization in transfusions. Different methods are used to determine ABO antibody titers. To date, most commonly used techniques include manual tube tests and microcolumn gel card analysis. These techniques are known to be time consuming and associated with high manual effort.
Aim
In our study, we aimed to characterize the performance of fully automated ABO titration assays on the Immucor Galileo NEO® platform, which allow the titration of IgM and IgG antibodies from patient plasma. Titration assays have now been characterized for titration of A1, A2 and B IgG and IgM antibodies: The assays enable the titration of the IgM antibodies to 1:128 and IgG assays to 1:2048.
Methods
318 IgG and 105 IgM titrations were performed on the Galileo NEO® automated analyzer to investigate the reproducibility of the assays. 72 IgG and 54 IgM titrations were performed to investigate the effect of changing lots of reagents (Capture‐R® Select wells, Capture‐R® Ready Indicator Cells, Capture® LISS, Referencells® as appropriate). Instrument to instrument studies were performed on 2 Galileo NEO® instruments. 29 IgG titrations were performed and the results compared to the manual gel card method (Bio‐Rad) without pretreatment with DTT.
Results
Titration assays show good reproducibility when lots of reagents are kept constant and specimens are run on the same instrument (endpoint titer the same or maximally changed by one dilution). Moreover, reagent lot to lot studies and instrument to instrument studies demonstrated good reproducibility (endpoint titers maximally changed by 2 dilutions). The system throughput is 12 specimens in 30 min for the IgM assays and 5 specimens in 65 min for the IgG assays. Comparison of automated titration technology on the Immucor® Galileo NEO® platform to currently used gel card technology revealed a generally lower titer (typically 1 dilution).
Conclusions
The prototype automated Galileo NEO® ABO titration assays demonstrate good reproducibility across reagent lots and instruments. The typically one dilution difference vs the gel card method can be possibly explained by the presence of IgM affecting the gel card and that different antigen concentrations are employed to that in the automated assay. The ABO titration assays on the Galileo NEO® offer a complete picture of IgG and IgM titers and automate the currently labor intensive manual alternatives.
P‐509
Diagnostics of Antibodies Directed Against Red Blood Cell Antigens Before and After Haematopoietic Stem Cell Transplantation (HSCT) – Case Report
Smolarczyk A , Klausa E
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Haematopoietic stem cell (HSC) transplantation is performed in patients with malignant and non‐malignant haematological disorders, after chemotherapy. HSCs may be obtained from bone marrow or peripheral blood after appropriate mobilization. After transplantation, HSCs lodge in recipient's bone marrow niches which results in reconstitution of haematopoietic and immune systems consistent with donor's genotype. In allogeneic hematopoietic stem cell (alloHSC) transplantation, donors and recipients do not have to be related but as there is a need for a compliant human leukocyte antigen (HLA), donors are first sought among relatives.
Case description
A 33‐year‐old woman M.J. was diagnosed with a RAEB‐2 myelodysplastic syndrome in the transformation phase to acute myeloid leukemia with anemia and thrombocytopenia. The woman had given birth three times (2000, 2008, 2013; children's father blood group: B RhD+positive/Dccee) and never received blood components. She was qualified for transplantation of alloHSC from her brother's peripheral blood, HLA compliant, with low ABO incompatibility (donor's blood contained antibodies directed against antigens on recipient's erythrocytes) and high incompatibility in Rh and Kidd systems (recipient's blood contained antibodies directed against antigens on donor's erythrocytes).
Before transplantation, in the recipient and the donor, a blood group was determined and the presence of antibodies was examined by means of an indirect antiglobulin test (IAT), enzymatic test using a microcolumn gel method (card LISS/Coombs, card NaCl, Enzyme Test and Cold Agglutinins; Bio‐Rad), direct antiglobulin test (DAT) using a Bio‐Rad's card (IgG, IgA, IgM, C3c, C3d).
Recipient's test results:
1. Blood group: AB RhD+positive/DCW‐CCee, K‐, Jk (a−b+)
2. Antibodies anti‐c, anti‐E and anti‐Jka present in serum
3. DAT negative
Donor's test results:
1. Blood group: B RhD+positive/DCW‐Ccee, K‐, Jk (a+b−)
2. No antibodies in serum.
3. DAT negative.
In pre‐ and early post‐ transplant periods, the recipient M.J. required numerous transfusions of leukocyte‐depleted RBCs (25 units) and PCs (51 units from apheresis). After PC transfusions, post‐transfusion reaction symptoms such as headaches, lumbar area pains, restlessness, skin redness were often observed. Anti‐HLA antibodies were detected in recipient's serum. For further transfusions, blood components were obtained from male donors and PCs were only derived from apheresis, which reduced the incidence of post‐transfusion reactions. 13 days after transplantation, the recipient stopped receiving blood/blood components.
On the 35th day after transplantation the recipient underwent control tests.
Results
1. Trace amounts of antigen A on erythrocytes.
2. Presence of antigens Rhc and Jka.
3. DAT positive with anti‐IgG.
4. DAT negative with: anti‐IgA, anti‐IgM, complement components anti‐C3c and anti‐C3d.
5. Positive reactions with certain blood cell reagents in eluate by IAT.
6. Presence of anti‐c and anti‐E antibodies.
7. No ABO system's anti‐A.
8. No Kidd system's anti‐Jka.
Summary
Presence of anti‐c antibodies in recipient M.J.'s serum in the post‐transplant period may have resulted from their production by surviving lymphocytes. In order to evaluate the post‐transplant HSC anemia, which can manifest itself as warm‐type AIHA, presence of antigens and anti‐erythrocyte antibodies should be controlled.
P‐510
Evaluation of a New Fully Automated Immunohematological Testing Instrument for Performance
Casina TS , Golisano MK , Witkowski R
Ortho Clinical Diagnostics, Raritan, NJ, United States of America
Background
Evaluation of a new immunohematological testing system is necessary to show that the performance of the new instrument demonstrates equivalence from a method‐based perspective. Results are generated that are compared to the performance of a predicate method or instrument.
Aim
The ORTHO VISION[TRADEMARK] analyzer is a new instrument designed to fully automate in vitro immunohematological testing of human blood using the ORTHO BioVue® System column agglutination test (CAT). A multi‐site study was conducted to evaluate the performance of the new instrument compared to the ORTHO AutoVue® Innova System. Methods
Testing occurred in three laboratory settings and tests were completed the same day on both systems. Discordant samples were repeated using the site's manual ORTHO BioVue® System workstation. Testing was executed on a total number of 5351 valid samples acquired from the sites’ routine workload to meet required sample criteria. Data from direct agglutination testing and direct and indirect antiglobulin testing was assessed by comparison of interpreted tests and of column by column results to determine percent concordance between the two systems at the lower 95% confidence bound. The criteria for concordance was ≥99.4% for direct agglutinating testing and ≥98.0 for direct and indirect antiglobulin testing.
Results
High concordance between the two systems was observed for both direct agglutination testing and direct and indirect antiglobulin testing for each site and across all sites combined. Direct agglutination testing was performed on 3180 samples with 6024 interpreted results. 6019 results were concordant and 5 discordant. The system comparison demonstrated a concordance of 99.8% at a one‐sided lower bound 95% confidence interval for direct agglutination. Direct and indirect antiglobulin testing was performed on 2171 samples with 2345 interpreted results. 2314 results were concordant and 31 discordant. Following discordant result investigation and adjustment per the protocol, 2340 interpreted results were evaluated. 2313 of the 2340 results were concordant and 27 discordant. The concordance demonstrated was 98.4% at a one‐sided lower bound 95% confidence interval for direct and indirect antiglobulin testing.
Conclusions
The multi‐site evaluation demonstrated that ORTHO VISION[TRADEMARK] Analyzer system showed equivalent performance vs the predicate system in the intended use environment.
P‐511
The First Example of Anti‐Kanno Found Outside of Japan
Bullock T1, Folman C 2, van der Mark-Zoet J 2, Uchikawa M 3, Thornton N 4, Daniels G 4
1NHSBT, Bristol, United Kingdom2Diagnostic Services, Sanquin Blood Supply, Amsterdam, The Netherlands3Blood Group Section, Japanese Red Cross, Yamagata, Japan4IBGRL, NHSBT, Bristol, United Kingdom
Background
The high incidence antigen, KANNO and corresponding antibody, anti‐KANNO was first described in 2011 by the Japanese blood service. Anti‐KANNO was originally detected in 1991 in a Japanese female and a further 27 examples of the antibody were found in Japanese patients. The majority of the cases of anti‐KANNO have been found in females with a known history of pregnancy. Anti‐KANNO has the characteristics of a High Titre Low Avidity (HTLA) antibody. KANNO is sensitive to treatment with papain, trypsin, α‐chymotrypsin and pronase but resistant to treatment with 2‐Aminoethyl‐isothiouronium bromide (AET) and Dithiothreitol.
Case study and methods
Blood samples from a 36 year old pregnant female patient residing in The Netherlands but with a surname of Japanese origin, were investigated due to the detection of an unidentified antibody in her plasma at 24 weeks gestation. The pregnancy continued to term and a healthy baby girl was born with no clinical signs of HDN. Serological tests were performed by standard LISS tube IAT, PEG IAT and BioRad IAT techniques. For enzyme studies, cells were treated with the following enzymes, Papain, Trypsin, α‐Chymotypsin, Pronase and AET. The MAIEA assay was carried out with monoclonal anti‐CR1.
Results
The patient's plasma reacted weak to moderate strength in IAT tests but did not react in papain IAT tests with all cells tested. The auto control was negative. DAT was found to be negative. The patient's cells were found to be positive for the following papain sensitive antigens; Kna/McCa, Ch, Rg, Yta, JMH, Inb, Ena and Ge2. The patient's plasma gave a negative reaction with monoclonal anti‐CR1 in the MAIEA assay. Enzyme studies showed that the unknown antigen was sensitive to treatment with papain, trypsin and α‐chymotypsin, and resistant to treatment with AET. This distinctive enzyme pattern of reactivity matched the pattern of reactivity seen in previously reported cases of anti‐KANNO. Anti‐KANNO and KANNO‐ cells were sourced from Japan and the patient's cells were found to be KANNO‐ and her plasma was compatible with an example of KANNO‐ cells. Anti‐KANNO was detected in the newborn's plasma and her cells had a weakly positive DAT (IgG 1+), no antibodies were detectable in the eluate.
Conclusions
We provide evidence of the first example of anti‐KANNO in a KANNO‐ patient found outside of Japan. The distinctive pattern of reactivity seen in enzyme studies and the antibody characteristics matched the findings of previously reported cases of anti‐KANNO.
P‐512
Introduction of a ‘Blood Group Check’ Specimen
Watson H , Muir A , Avery S , Scott Y , Wallis J
Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background
The 2012 ‘Guidelines for pre‐transfusion compatibility procedures in blood transfusion laboratories’ state ‘Unless secure electronic patient identification systems are in place, a second sample should be requested for confirmation of the ABO group of a first time patient prior to transfusion, where this does not impede the delivery of urgent red cells or other components’.
Aim
A system was designed that would mandate that no patient would receive non group O cells on the basis of an unconfirmed blood group. Our system was modelled on the Toronto Sunnybrook design. Two samples have to be taken at different times and preferably by different people to avoid the documented practice of taking two samples at the same time and sending them separately.
Method
We sourced an EDTA specimen tube not used elsewhere and restricted stock holders to the Trust blood banks. We termed this the Blood Group Check sample (BGC). On receipt of a blood group request from a patient with no prior records the blood bank ascertains whether blood is needed within 12 h. If not the Laboratory indicates on the electronic patient record that a second group and save specimen is required before issue of non‐group O blood. A standard sample is requested at least 12 h after the first. If blood is required within 12 h the Laboratory immediately informs the clinical staff that a BGC sample is available for collection at Blood Bank and that only group O blood will be issued until this is received. A labelled sample bag containing the special sample tube is made ready for collection. This sample bag acts as a simplified request form. The clinical staff collects this prepared tube which is filled, signed and returned to the Blood Bank. Once the blood group is confirmed on this second sample group specific blood may be issued.
Results
A 3 month audit was performed during which 18,842 requests were processed. Major Haemorrhage requests were excluded. 73 BGC samples were prepared by the Laboratory for patients requiring urgent transfusion. 58 were returned by the clinical area. 8 standard group and save specimens tubes were sent to the lab despite the clinical area being told that these were not acceptable within 12 h of a first time sample. These were subsequently discarded. 1 sample was discarded due to mis‐labelling. Only 6 group O red cells were required to be issued prior to the receipt of a BGC sample to non‐group O patients.
Summary/Conclusion
The introduction of the BGC sample in the Newcastle Trust has been a qualified success and has been generally accepted by the clinical teams within the Trust. The understanding and acceptance of the policy requires careful implementation and training. No patient was transfused non‐group O blood on the basis of a single sample without a historical blood group. Increase in group O usage is small.
P‐513
Evaluation of System Mdmulticard® in Emergency Management
D'Ambrosio R1, Di Girolamo MG 1, Di Lemma GG 1, Danzi M 1, Schiavone V 2, Schiavone B 2, Chignoli V 2, Conte S 2, Misso S 1
1S.C. Servizio Transfusionale ASL Caserta, Aversa, Italy2P.O. Pineta Grande, Castel Volturno, Italy
Background
The delivery of blood units in emergency conditions is a highly critical step for each Blood Bank. In these cases, a delay in the transfusion of red blood cell (RBC)s units can be life‐threatening for the patients. The chosen procedure for blood group typing should be as rapid as possible, minimizing the likelihood of human error, the possible risk of alloimmunization and/or the wrong blood typing. Following the standards set by the Italian Society of immunohematology and Transfusion Medicine (SIMTI 2nd ed. 2010) and to ensure an appropriate interpretation level to the critical procedures that must be followed, we implemented a rapid method for the patients blood group typing which allows traceability and stability of results.
Aims
The aim of our study was to evaluate the MDmulticard Grifols system (Medion Grifols Diagnostics AG), for the AB0, Rh phenotype and Kell system under emergency.
Methods
MDmulticard AB0‐Rh D‐subgroup+ K take advantage of the lateral flow principle; the reagents consist in IgM monoclonal antibodies directed against AB0, Rh and Kell antigens immobilized on the device membrane, including internal positive and negative controls. 50 microliters of the patient blood sample are dispensed in the card and the RBC excess is washed using a specific diluent. The valid result is available within the following 5 min. After dispatching the emergency units to the ward, our internal protocol requires the automatic execution of AB0, Rh phenotype, Kell groups and indirect Coombs test with the CAT method (AutoVue by Ortho). The test execution is simple, fast and safe, and of easy interpretation. The MDmulticard guarantees total traceability and result stability for up to 72 h. They can also be photographed, preserved and archived.
Results
From January to December 2014 we received 1256 RBCs emergency requests, only 430 of which accompanied by a sample tube. The data analysis showed that the results obtained with MDmulticard were 100% confirmed by micro‐column, in spite of the different method.
Conclusions
The use of the MDmulticard as pre‐transfusion testing in emergency requests has allowed the distribution of compatible red cell phenotype; moreover the test execution is simple, fast and safe, and of easy interpretation. Furthermore a standard procedure in emergency is outlined, also by reducing the objective interpretation of the operator of the SIT. MDmulticard represent an efficient diagnostic tool, useful in emergency management as an additional method to the CAT determination that takes longer and a substitution of the tube/slide testing, which could generate misinterpretation despite its speed of execution.
P‐514
A Multicenter Comparative Study of Five Automates to Detect Irregular Antibodies (IA) and ABO‐RH.KEL1 Groups
Krause CK , Lauroua PL , Andre-Botte CAB , Auger SA , Besiers CB , Bouix OB , Chartier MC , Delamaire MD , Delugin LD , Giroux-Lathuile CGL , Mercadier AM , Mouchet CM , Volle PV , Gouvitsos JG , Rihet IR , Toulmonde ET , Djoudi RD
Etablissement Francais Du Sang, La Plaine Saint‐Denis cedex, France
Background
The sensitivity of methods to detect both irregular antibodies and double population of ABO‐RH.KEL1 groups is a critical point for safe transfusion.
Aims
Thus, we have conducted an assessment of the sensitivity of methods used in five automates available in laboratories of the Etablissement Français du Sang (EFS).
Methods
Blood groups and irregular antibodies have been detected by three methods i) a microplate ‘EMT’ method on QWALYS® (DIAGAST); ii) a gel microfiltration technique on ERYTRA® (Grifols), IH1000® (Bio‐Rad) and WADiana® (Grifols) and iii) a glass beads microfiltration technique on INNOVA® (OCD). Three evaluation campaigns have been performed. For each of them, the reagents preparation unit of EFS has prepared and sent to all centers involved in the study the same samples from donors and patients. They were:
1. 15 ABO‐RH.KEL1 groups containing or not containing double populations of red cells in various proportions (20%, 50%, 80%).
2. 15 irregular antibodies screening containing Anti‐RH, Anti‐KEL1, Anti‐FY, Anti‐JK, Anti‐MNS with various titers.
For each campaign, the samples have been analyzed by five automates, at least in duplicate by each automate and in a blind manner by the laboratories.
Results
None automate has detected all irregular antibodies. The reactivity of all non‐detected irregular antibodies was weak, at the limit of technical detection. These undetected antibodies are distributed as follows (by type of analyzer): Anti‐RH1 (ERYTRA®, WADiana®), Anti‐KEL1 (IH1000®, QWALYS®), Anti‐FY2 (IH1000®, WADiana®, INNOVA®, QWALYS®), Anti‐MNS3 (QWALYS®), Anti‐MNS4 (ERYTRA®, INNOVA®) Anti‐JK1 (IH1000®).
Results for ABO‐RH.KEL1 groups were compliant for samples without double populations of red cells. For those samples which did contain double populations, results vary depending on the type of automate and the proportions of the two populations of red cells. All samples containing double populations in the following proportions 50/50 and 20/80 (A/O, E+/E‐, C+/C‐, K+/K‐) have been detected by all automates. For the following proportions 20/80 (O/A and E‐/E+), 20/80 (D‐/D+) and 20/80 (D+/D‐) they have been detected respectively by IH1000®, ERYTRA®‐ IH1000® and all automates except QWALYS® and INNOVA®. On the contrary, samples with the following proportions 20/80 (C‐/C+, K‐/K+) have never been detected
Conclusion
Our study shows that the available automates demonstrate equivalent safety and prescriptive performances. The ‘ideal’ automate able to detect all antibodies and all double populations of red cells is not yet available. To detect double population of red cells within the ABO‐RH.KEL1 groups, the gel microfiltration technique appears to be more successful than the others techniques.
P‐515
Abstract Withdrawn.
P‐516
When Blood Transfusion Medicine Becomes Complicated Due to Interference by Monoclonal Antibody Therapy
Oostendorp M 1, Lammerts van Bueren JJ 2, Doshi P 3, Khan I 3, Ahmadi T 3, Parren PWHI 2, van Solinge WW 1, de Vooght KMK1
1University Medical Center Utrecht, Utrecht, The Netherlands2Genmab, Utrecht, The Netherlands3Janssen Pharmaceutical Companies LLC, Spring House, NJ, United States of America
Background
Monoclonal antibodies (mAbs) provide a rapidly growing class of therapeutics for the treatment of many diseases, but they can potentially interfere with laboratory tests.
Aims
To describe the interference of a therapeutic anti‐CD38 mAb in the indirect antiglobulin test, which may impact blood transfusion medicine, since negative cross matching during mAb treatment is impossible using the standard test.
Methods
The indirect antiglobulin test was performed in the LISS‐technique (Ortho) using 3 and 11 donor cell panels. The interference of anti‐CD38 was studied using fresh frozen plasma spiked with different mAb concentrations. Furthermore, we investigated whether two potentially neutralizing agents, an anti‐idiotype antibody and recombinant soluble CD38 extracellular domain, were able to inhibit the interference.
Results
The anti‐CD38 mAb caused agglutination in the indirect antiglobulin test in a dose‐dependent manner. Addition of an excess of anti‐idiotype antibodies or soluble CD38 protein to the patient's plasma abrogated the anti‐CD38 mAb interference and successfully restored irregular antibody screening and identification in patients.
Summary/Conclusions
Anti‐CD38 mAb therapy causes false positive results in the indirect antiglobulin test. The reliability of the test could be restored by adding a neutralizing agent against the anti‐CD38 mAb. Our study shows that the potential interference of targeted therapeutics with laboratory tests should be investigated during drug development. It is import that clinical laboratories are informed when patients receive mAb therapy and appropriate solutions to allow proper cross‐matching should be adapted.
P‐517
Frequency of Mia Antigen: A Pilot Study in Indian Blood Donors
Makroo RN , Bhatia A , Karna P
Indraprastha Apollo Hospital, New delhi, India
Background
The Miltenberger (Mi) classes represent a group of phenotypes for red cells that carry low frequency antigens associated with the MNSs blood group system. The incidence of the blood group antigen Mia among most populations is low (<0.01%), while in the Chinese and South East Asians it is up to 15%. The antigen frequencies in Indians is however not documented. The clinical significance of the Mia antigen lies in the development of anti Mia antibodies, which, although uncommonly, may result in transfusion reactions or Haemolytic Disease of Foetus and Newborn.
Aims
This pilot study aims at determining the Mia antigen positivity in the Indian blood donor population.
Methods
One thousand random blood donors were tested for Mia antigen using anti Mia antisera (Immucor Inc. USA) by tube technique.
Results
A total of 1000 donors were tested and only one was found Mia antigen positive. The Mia positive donor was a 42 year old Hindu male of North India ethnicity. The overall frequency of Mia antigen in this pilot study was 0.01%.
Conclusion
The frequency of Mia antigen in the Indian population is comparable to that reported in literature for other ethnic groups.
P‐518
Retrospective Analysis of Anti‐Kell Alloimmunization Frequency in Transfusion Patients of the Hospital ‘S.G.Bosco’, Italy
Di Domenico G , Leonardi GM , Borrelli E , Nocera C
Immunohematology Service, S.Giovanni Bosco, Hospital, ASL NA1 Centro, Naples, Italy
Background
The frequency of occurrence of anti‐Kell (anti‐K1) alloimmunization is actually not entirely estimated and at the same time the anti‐kell antibody (Ab) is one of the main antibodies most frequently found in the course of Ab identifications performed in clinical practice. Is remarkable to note that the anti‐Kell Ab can be found in patients with an infection even in the absence of direct exposure to the Kell antigen of the red blood cells.
Aims
The purpose of our work was to retrospectively evaluate the frequency of the anti‐Kell alloimmunization in transfused patients for both acute events (hemorrhagic Shoch, polytrauma) and chronic diseases (NDD anemia, myelodysplasia)
Methods
We analyzed the transfusion clinical records related to 14,560 patients (7800 M / 6760 F), median age 69 (range 1–98), transfused at the Immunohemathology Service of the ‘San Giovanni Bosco’ Hospital in Naples, Italy, between 2011 and 2014. The screening tests performed on the patients were: the ABO/Rh group typing, irregular antibodies identification by indirect antiglobulin test (IAT). The ABO typing was performed by a first determination though automatic equipment with polyclonal reagents cards on column of gel‐Sephadex (DIAMED); a second determination was performed by monoclonal antiserum (ALBACLONE) in the test‐tube. Reverse blood grouping has been tested on the plasma/serum using cell test Diacell A1, A2, B, O (DIAMED). For the IAT four different formulations were used: physiologic saline, low‐ionic‐strength saline (LISS), enzyme (Bromelin) and polyspecific anti‐human globulin serum (15’ at 4, 22 and 37°C), using a screening erythrocytes panel provided by Ortho‐Clinical Diagnostics. Screening tests for unexpected antibodies wee performed, according our fixed protocol, by two panel cells consisting of 11 (DIAMED) and 36 (IMMUCOR) different erythrocytes suspensions, using the same in vitro conditions of the IAT (physiologic saline, low‐ionic‐strength saline (LISS), enzyme (Bromelin) and polyspecific anti‐human globulin serum, 15 at 4, 22 and 37°C). The obtained results were statistically analyzed by the Fisher exact method.
Results
The frequency of irregular antibodies anti‐Kell was found in 20 of the 14,560 (0.13%) analyzed patients. Of these, seven patients (35%) were transfused for acute events (Hemorrhagic shock, polytrauma), four patients (20%) had a diagnosis of myelodisplastic syndrome and nine patients (45%) were affected by ndd chronic anemia. Among the latter patients, we identified one case of passive immunization anti‐Kell secondary to infection with Klebsiella Pneunomiae. In addition, 4 patients (20%) were already immunized to the Kell antigen from previous transfusions performed at other Immunohemathology Service.
Conclusions
The frequency of anti‐Kell alloimmunization observed in our work (0.13%) is substantially lower, demonstrating that the antibodies production against kell antigens of the RBC is an event, like other immunizations antibody, of multifactorial origin.
P‐519
Frequency of Clinically Important Blood Group Antigens in Albanian Blood Donors
Seferi I , Metka A , Abazaj ZH , Xhetani M
National Blood Transfusion Centre, Tirana, Albania
Background
There are very few studies in our country on frequencies of blood group antigens in general population and in blood donors. Determining the frequency of blood group antigens has biological and clinical importance for population studies and safe blood transfusion in multiply transfused patients.
Aims
Aim of this study was to evaluate the frequency of clinically important red cell antigen in donor population at NBTC Tirana.
Methods
We evaluated the frequency of ABO blood groups in 17,992 blood donors that donated blood during 2013 in NBTC Tirana. In 10,000 of them also RhD, C, c, E, e and Kell (K,k) was evaluated, whilst other clinically important blood group antigens such as Kidd (Jka,Jkb), Duffy (Fya, Fyb), Lutheran (Lua, Lub) MNSs, Lewis (Lea, Leb) and P1 were evaluated in 200 of them. Blood typing was performed by serologic method, using Diamed ID‐Systems, microplate technique for ABO and Rh blood groups, and gel‐card technique for the rest.
Results
The estimated frequency of ABO blood groups in our donor population is: O‐41.7%, A‐36.8%, B‐16.2%, AB‐5.3% which differs at a certain point from the ABO frequencies in Whites (O‐45%, A‐40%, B‐11%, AB‐4%). There is no significant difference between the estimated frequency of Rh antigens in our blood donors (D‐88.3%, C‐73.7%, c‐74.3%, E‐29.8%, e‐97.2%) and the known frequencies of these antigens in Whites (D‐85%, C‐79%, c‐80%, E‐30%, e‐98%).The frequency for Kell is K‐5.5%, k‐94.5. The estimated frequency for other clinically important blood group antigens in our donor group was as following: Jka‐58.5%, Jkb‐62.1%, Fya‐66.6%, Fyb‐82%, Lea‐13.5%, Leb‐69.37%, Lua‐6.3%, Lub‐86.5%, M‐76.5%, N‐78.3%, S‐56.7%, s‐78% and P1‐76.58%.
Summery/Conclusion
Estimating the frequencies of clinically important blood groups in donor population is very important because they can help for population studies and in the same time are helpful in predicting the likelihood of finding blood compatible with a serum that contains multiple antibodies as it is the case of thalassemia patients in our country.
P‐520
Automated Anti‐A/Anti‐B Titration in ABO Incompatible Renal Transplant Patients‐A New Breakthrough in Indian Blood Banking
Pathak PS1, Surjit S 1, Tamojit T 1, Satish S 1, Ruchi R 1, Anurag A 2, Vivek V 2
1Max Healthcare Ltd., New Delhi, India2Immucor India Pvt. Ltd., New Delhi, India
Background
Antibody titer is one of the few tests in blood banking that has proved to be difficult due to the variability of manual dilutions and the subjectivity determining the end‐point titer. The AABB Technical Manual states that titration is a semi‐quantitative method and is quite technique dependent, because many variables can affect testing results; the procedure is relatively imprecise. The use of automated methods offers the prospect of standardization and reproducibility of results.
Aim
To establish the routine Anti‐A & Anti‐B titration using a fully automated system and to minimize the risk of variability due to manual dilutions and subjective end‐point reading with documentation.
Method
In 6 months total 644 numbers of blood samples from renal transplant patients at a tertiary care hospital in Delhi, were tested as, 298 (46%) for anti‐A & 346 (54%) for anti‐B, specifically IgG antibodies. Testing was carried out by two different methods, 1) Fully automated immunohematology analyzer Galileo‐NEO (Immucor‐Norcross, GA) and, 2)Semi‐automated Bio‐Rad ID Gel Card. Samples were collected in EDTA and Plain vials. Results were generated via Solid Phase Red Cell AdherenceTechnology in Galileo‐NEO and by AHG method in Gel card. The instrument fully automate the assay process, including sample preparation, serial dilution, and interpretation, while in Gel Card, manual method of serial dilution and end point reading method was adopted.
Results
In this study of two different automated and semi‐automated methods; 483 (75%) results were founded as same titer report. 15% of titer reading were recorded 1 fold dilution increased and 7% were recorded 1 fold dilution decreased in Gel card rather than in Galileo‐NEO. 2% of samples also founded as 2 fold dilution increased while 1%samples founded 2 fold dilution decreased in Gel. These total 161(25%) samples which gave variable reaction were repeated in both method and found 78(12%) samples giving same result in both with reproducibility in automation while 83 (13%) giving 1 fold dilutions increased.
Summary/Conclusions
Antibody titrations are important in antenatal evaluations,transfusing ABO‐incompatible plasma products, and performing ABO‐incompatible organ transplants. As per AABB, The titer is determined from the highest dilution of serum that gives a reaction of 1+,macroscopic agglutination.Variations in technique can cause duplicate tests to give variable results. Serum containing antibody at a true titer of 32 may show variability on replicate tests. It is often seen in study that 1 fold increase & decreased is subject to making serial dilution and reading of reaction individually. The accepted titration methodology is the tube method, Titration using gel column technology may result in titers several dilutions higher than the tube method. Steiner et al reported antibody titers and scores in gel to be consistently higher than titers and scores in tubes.When a large number of ABO incompatible solid organ transplant programs in the country are seeking Transfusion Medicine for a true titer value,it is better to introduce an automated method of titration in spite of manual method to escape such type of variability in titer assay.
P‐521
Red Cell Antibodies in Egyptian Thalassemic Patients
Elsherbiny H
National Blood Transfusion Center, Cairo, Egypt
Background
Thalassemia is a common disease in Egypt that requires long life blood transfusion which may lead to allo‐immunization to red cell Ags that can significantly complicate transfusion therapy. Stem cell transplantation still remains the only cure currently available for patients with thalassemia.
Known blood group Ags are 302 in number till now, not all of them are highly immunogenic. Significant Abs can lead to red cell destruction and hemolytic transfusion reactions.
Aim
The aim of the study is to find out the frequency and factors influencing red cell allo‐immunization in thalassemic Egyptian patients.
Methods
The study conducted on 320 thalassemic patients; presented to Egyptian NBTC for blood transfusion. Age of the patients was ranging from 1 to 25 years. Patients subjected to: Screening for red cell Abs (panel of 3 blood group O‐ cells) using appropriate IAT by CAT. Antibody identification for positive screening results (panel of 11 blood group O‐ cells) using appropriate IAT by CAT. Red cell typing using monoclonal anti‐sera.
Results
A total of 320 patients screened for the presence of red cell allo‐antibodies. 84 (26.25%) found to have allo‐antibodies. Of these patients, 33 (39.30%) developed one allo‐antibody, 28 (33.30%) developed two allo‐antibodies and 23 (27.40%) patients developed more than two allo‐antibodies. The most frequent allo‐antibodies found was: anti‐K antibody (32/84) representing 38.1%, anti‐E (21/84) representing 25%, anti‐C (13/84) representing 15.5%, anti‐D (12/84) representing 14.3%, anti‐Fya (11/84) representing 13.1%, followed by anti‐Jka (10/84) representing 11.9% of alloantibodies found. The majority of patients were previously transfused for years with non‐ phenotyped blood, and the majority of red blood cell allo‐antibodies formation was against the Kell and Rh blood group systems.
Summary/Conclusions
Allo‐immunization to red cell antigens is a frequent finding among Egyptian thalassemic patients, which requires screening. It is important to have protocols for dealing with transfusion dependent patients. We recommend routinely performing RBCs phenotyping for all transfusion‐dependent thalassemic patients before starting RBCs transfusion. Evaluation of Abs status should be done before each transfusion, to give the patients Ags free blood transfusion for the relevant Abs.
P‐522
A Suspected Case Report of Blood Transfusion Induced Anti‐AnWj in Southern Medical Center of Taiwan
Chen HC
ChiMei medical center, Tainan, Taiwan
Background
The AnWj antigen is a high‐prevalence antigen, which belongs to 901 (901009) series of the ISBT classification. Its prevalence is higher than 99 percent of all population. AnWj antigen is resistant to papain, trypsin, and its agglutination titer becomes weaker after red cells were treated with 0.2 M DTT. It is not expressed on cord cells. Anti‐AnWj is a clinical significant antibody as it has been reported caused hemolytic transfusion reactions.
Case report
A 29‐year‐old female with a history of anemia had past history of right renal cancer. Since 2013, she has received regular chemotherapy treatment at our institution. She was typed as O group, antibody screening showed negative, and has transfused since January 2013. In July 2014, due to her clinical condition (Hb 7.0 g/dl), two PRBCs were required. The antibody screening showed positive (MP, IAT, BioVue AHG Card and Bio‐Rad gel card). The patient's serum reacted with all panel cells (all 2+), but DAT presented negative, autocontrol reveled negative as well. It indicated that this patient's serum contained an allo‐antibody reacted with high‐prevalence antigen. Testing with donor PRBCs all were 2+ crossmatch incompatible in both MP and gel card.
Aims
The present study focused on identifying rarely antibody and dealing with transfusion treatments.
Methods
Standard serological principles of AABB technical manual were applied to present study.
Results
The patient's RBC minor phenotype showed as follow:D+, C+E‐c‐e+, Le(a−b+), K‐k+, Fy(a+b−), Jk(a−b+), M‐N+S‐s+ Mia+, P1+, Dia −. Using enzyme‐treated cell (papain) reacted to serum, the agglutination titer displayed positive (2+). On the other hand, the agglutination titer declined (positive, ±) after responded to red cells treated with 0.2 M DTT. Besides, the patient's serum had positive reaction with i adult RBCs but negative with cord RBCs. Based on above results, the alloantibody verified to be presumed as Anti‐AnWj; nonetheless, it was less possible to offer AnWj Ag(−) for transfusion. According to previous studies, Lu (a−b−) RBCs could be a substitute choice because Lu (a−b−) RBCs express poorly the AnWj antigen. Since the shortage inventory of blood center, we could not obtain Lu (a−b−) RBCs. After noticing her ordering physician, we released O group stocked RBCs without performing crossmatch test. The patient did not have adverse transfusion reactions.
Conclusion
Anti‐AnWj is a rare antibody; if patients have this alloantibody, the chance of finding crossmatch compatibility RBCs t is extremely low. Transfusion with Lu (a−b−) RBCs could be another choice. Combined with patient's transfusion history and laboratory results, we strongly suspected this alloantibody is Anti‐AnWj. Referring patient's blood samples to the International Blood Group Reference Laboratory is a need while encountering the same condition case in the future.
P‐523
Importance of an Enzyme Treatment Method in Antibody Screening to Erythrocyte Antigen; Results from Japanese Study Group of Antigen Diversity in Asian Populations (ALLO‐ADP) Study Group
Watanabe H1, Takeshita A 1, Yamada C 1, Yurugi K 2, Tomoda Y 3, Uchikawa M 4, Kino S 5, Ohto H 6, Buseki Y 7, Fukuyoshi Y 8, Hashimoto M 9, Hiraoka A 10, Ishikawa S 11, Ito M 12, Kamimura M 13, Kasai D 14, Matsuura H 15, Kato A 16, Nakagiri I 17, Nishida S 18, Notoya T 19, Ochi N 20, Ogo H 21, Ohshima K 22, Ohtomo N 23, Okuyama Y 24, Otsu M 25, Sasada Y 26, Sone S 27, Takenouchi H 28, Yamamoto S 29, Yamamoto Y 30, Hanada D 3
1Hamamatsu University School of Medicine, Hamamatsu, Japan2Kyoto University, Kyoto, Japan3Asahikawa Medical College, Asahikawa, Japan4Japanese Red Cross Tokyo Metropolitan Blood Center, Koto‐ku, Japan5Japanese Red Cross Hokkaido Block Blood Center, Sapporo, Japan6Fukushima Medical University, Fukushima, Japan7Saitama Medical Center Jichi Medical University, Saitama, Japan8Kumamoto University Hospital, Kumamoto, Japan9Kobe University Hospital, Kobe, Japan10Hiroshima University Hospital, Hiroshima, Japan11Shinshu University Hospital, Matsumoto, Japan12Chiba University Hospital, Chiba, Japan13Niigata University Medical Dental Hospital, Niigata, Japan14Nagano Municipal Hospital, Nagano,, Japan15Fujita Health University Hospital, Toyoake, Japan16Yamamoto Kumiai General Hospital, Noshiro, Japan17Kawasaki Medical School Hospital, Kurashiki, Japan18Nara Medical University Hospital, Kashihara, Japan19Akita University Hospital, Akita, Japan20Nagoya City University Hospital, Nagoya, Japan21Okayama University Hospital, Okayama, Japan22Kanazawa Medical University Hospital, Kawakita, Japan23Tokyo Medical and Dental University, Bunkyo‐ku, Japan24Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Bunkyo‐ku, Japan25Yokohama City University Hospital, Yokohama, Japan26Kyoto Prefectural University of Medicine, Kyoto, Japan27The University of Tokyo Hospital, Bunkyo‐ku, Japan28University of Miyazaki Hospital, Miyazaki, Japan29Toho University Ohashi Medical Center, Meguro‐ku, Japan30Teikyo University Chiba Medical Center, Ichihara, Japan
Background
An indirect antiglobulin test (IAT) has been used for detection of irregular erythrocyte antibodies (Abs) in many laboratories. On the other hand, an enzyme treatment method (EM) is not routinely used in Ab detection test, but plays an adjunctive role in detection of some Abs for Rh and Kidd antigens (Ags) (AABB, 2014). It is superior for Ab determination in the early phase of Ab production in pregnant or transfused cases, while it often detects cold antibodies and benign autoantibodies. The application of EM in pregnant and newly transfused cases has not been well elucidated.
Aims
We tried to clarify the significance of EM in many samples from cases with or without previous history of blood transfusion.
Methods: Fifty nine Japanese institutions participated in this study. We used several methods for the detection of irregular Abs to following erythrocyte Ags. The frequencies of irregular Abs to D, C, c, E, e, f, Ce, P1, M, N, S, s, Mia, Lea, Leb, Jka, Jkb, Jk3, Fya, Fyb, K, k, Kpa, Kpb, Jsa, Jsb, Dia, Dib, Lua, Lub, Xga, Jra and H were studied. If a case was analyzed multiple times, it was counted only once. We analyzed the frequencies of Abs as well as the efficacy of EM. Several patients, who were initially negative for irregular erythrocyte Abs before receiving a blood transfusion, were continuously followed to find the conversion time for Abs detection by the methods of IAT and EM.
Results
The samples from 325,263 cases were analyzed by the beads‐column test which contained anti‐IgG‐specific antiglobulin for IAT. The samples from 213,353 and 111,910 cases were analyzed by IAT with or without EM, respectively. Anti‐E, anti‐C, anti‐Lea and anti‐Leb Abs were more frequently detected by IAT with EM compared to without EM (anti‐E; 0.503% vs 0.323%, P < 0.01, anti‐C; 0.037% vs 0.022%, P = 0.03, anti‐Lea; 0.474% vs 0.168%, P < 0.01, anti‐Leb; 0.055% vs 0.028%, P < 0.01, respectively). However, no difference was observed in anti‐D, anti‐e, anti‐c and anti‐Jka (anti‐D; 0.024% vs 0.026%, P = 0.79, anti‐e; 0.024% vs 0.024%, P = 0.97, anti‐c; 0.073% vs 0.076%, P = 0.74, anti‐Jka; 0.033% vs 0.023%, P = 0.13, respectively). Other clinically important Abs were not significantly different between them. Anti‐E was newly detected in 346 patients after receiving a blood transfusion. In 40 cases, anti‐E was detected earlier by EM than by IAT. In one case, anti‐E was detected earlier by IAT than by EM. In 147 cases, anti‐E was detected only by EM; while in 4 cases anti‐E was detected only by IAT. In 154 cases, anti‐E was concurrently detected by both methods. Similar results were obtained in the analysis of anti‐e, anti‐C and anti‐c Abs (Table 1).

Conclusions
Anti‐E and anti‐C were frequently detected by the Ab detection concomitant with IAT and EM, which was suitable for detection of early anti‐E, anti‐e, anti‐C and anti‐c Abs developing in casess who received a blood transfusion. We should appropriately use EM in the detection of small amount of these Abs in the cases who have recent history of blood transfusion and pregnancy.
P‐524
Recombinant Group Proteins Proved to be a Helpful Tool for Antibody Screening in Presence of an Anti‐KN(A)
Weinstock C , Kaiser S , Braun G , Schrezenmeier H , Anliker M
Red Cross Blood Service Baden‐Württemberg – Hessen, Institute Ulm, Ulm, Germany
Background
High‐titer, low‐avidity antibodies (formerly called HTLA) are directed against high‐prevalence blood group antigens. Thus, sera with HTLA antibodies react with all test cells available in a routine laboratory which hampers the identification of their specificity. HTLA antibodies have no clinical relevance, but they can mask underlying clinically relevant antibodies. In many cases unmasking of relevant antibodies is not possible, because the low avidity of HTLA antibodies prevents successful absorption to homologous cells. Recombinant proteins carrying blood group antigens are a promising tool for identification of the specificity of HTLA antibodies and for detection of underlying additional antibodies.
Methods/Results
A patient serum contained a broadly reacting antibody which was identified as anti‐Kn(a) (KN1, Knops system). The specificity of the antibody was indirectly confirmed by molecular typing of the patient as Kn*bb using a commercial typing kit for high prevalence blood groups (RBC‐Ready Gene Rare ID, inno‐train, Germany).
The Kn(a−) test cells available in the laboratory allowed only partially the exclusion of underlying clinically relevant antibodies. Therefore, we used recombinant CR1 protein carrying the Kn(a) antigen (Imusyn, Hannover, Germany) for inhibition of the anti‐Kn(a). 24 μl of solution containing the CR1 protein (0.5 mg/ml) were added to 300 μl of patient serum. For control 24 μl PBS were added to 300 μl patient serum. After centrifugation for 5 sec at 8000 x g the mixtures were incubated for 30 min at room temperature. Both mixtures were tested in the indirect antiglobulin test using gel cards (Grifols). Patient serum pre‐incubated with recombinant CR1 protein reacted negative with all test cells, whereas patient serum pre‐incubated with PBS still reacted positive.
Conclusion
Recombinant CR1 protein carrying the Kn(a) antigen thus was helpful in two ways: first, it confirmed the specificity of the anti‐Kn(a). Second, we could exclude underlying, clinically relevant antibodies in this patient serum.
P‐525
A Case: RHD Alloimmunization After Transfusion of One Unit of Apheresis Platelets
Li Y 1, Mamone L1, Grant M 1, Geier SS 1, James I 2, Luo X 1, Patel V 1, Liu Z 3, Nance S 4, Borge PD 5
1Temple University School of Medicine, Philadelphia PA, United States of America2Temple University Hospital, Philadelphia PA, United States of America3Institute of Blood Transfusion, Chengdu, China4American Red Cross, Philadelphia PA, United States of America5American Red Cross, Biomedical Services, Baltimore, MD, United States of America
Background
Controversy remains regarding the risk of RhD alloimmunization in Rh‐negative (neg) patients receiving Rh‐positive (pos) platelets with or without concomitant Rh immunoglobulin (RhIG) administration. Some studies have demonstrated no alloimmunization of recipients following Rh incompatible platelets transfusion, whereas others revealed Rh alloimmunization in this scenario with estimated rate to range from 1.44 to 3.8%.
Aims
We report a case of an AB‐neg 63‐year‐old woman who developed anti‐D following transfusion of one unit of O‐pos apheresis platelets (AP). This case study is to demonstrate the risks of RhD alloimmunization and to further explore potential risk factors associated with RhD alloimmunizationin in Rh‐neg patients receiving Rh‐pos AP. She had a history of cirrhosis, hepatocellular carcinoma, nonalcoholic steatohepatitis, diabetes mellitus, hypertension, thrombocytopenia, and multinodular thyroid disease and presented to our outpatient testing facility (8/25/14) for liver transplant candidate workup. She has no outside hospital transfusion history, no known red blood cell antibody history. Her pregnancy history was unknown.

Methods
Review of the literature and a clinical case. The antibody identifications were performed by gel method (ID‐Micro Typing System™ Gel Test™; Micro Typing Systems, Inc. Pompano Beach, FL, US, An Ortho‐Clinical DiagnosticsCompany). Human leukocyte antigen (HLA) class I antibodies were detected by single antigen‐bead based assay (LABScreen, One Lambda, Inc. Canoga Park CA, US).
Results
Her blood group was AB neg with a negative antibody screen on 8/25/2014. She was still negative for anti‐D antibodies on 8/29/14, when she was transfused with one O‐pos unit of AP. On 12/4/14 and 12/9/14, she showed Anti‐D antibodies with 3+ to 4+ by the gel method. On 12/9/14 she was transfused four units of AP (2u O pos, 1u B pos, 1uA neg) with platelet count increment increase of 10k/mm3 (from 22k/mm3 to 32k/mm3). On 1/26/15, the calculated percent reactive antibody (cPRA) was 88% based on HLA class I antibodies >1000 MFI, tested on single antigen solid phase beads. The antibodies were all against HLA‐B determinants with 1100 – 12,600 MFI. On 2/12/15, she showed anti‐D 3+ by gel method, and was transfused four units of AP (1u O neg, 1u O pos, 2u A pos) with platelet count increment of 4 k/mm3(from to 26k/mm3) (See Table 1). The patient developed chills and a fever of 102°F, 40 min after the last unit of AP was transfused. Transfusion reaction work‐up was non‐contributory.
Summary/Conclusions
This patient developed Rh alloimmunization after transfusion of just one unit of ABO compatible, Rh incompatible AP. Her immune statusand ABO compatibility may be contributory factors to her RhD alloimmunization. Further study in a larger patient population is warranted. We believe RhIG prophylaxis is justified for childbearing age women receiving Rh incompatible platelet products, especially ABO compatible Rh‐positive platelet products.
P‐526
Incidence of RH Negative Blood Type in Nakasero Blood Bank Blood Donors
Wambi WW
UBTS‐NAKASERO REGIONAL BLOOD BANK, Kampala, Uganda
Background
Rh blood group system is one of the thirty three current human blood group systems and is the most important system after ABO. The incidence varies according to the ethnic groups and it is known to be very low in the African populations (3%) according to a study by Steve Mack conducted in 2001 in children's Hospital in Oakland Research Institute.
In Uganda, the blood bank cannot predict the stock of Rh negative blood type and it becomes a challenge when there is need and yet the blood bank does not have the product in stock.
Aim of the study
To find out the incidence and the respective collection sites where there is a high incidence such that arrangements are always made to collect blood from those areas in order to be able to predict the Rh negative blood stock of Nakasero blood bank.
Methods
Data for 3 months (October, November & December 2014) was retrospectively reviewed & data analyzed using Statistical Package for Social Sciences. Rh factor, respective blood group and collection site was noted and consolidated. Results analyzed were from teams A, B, C, D, Jinja & Hoima (these are mobile teams), Donor room & ambassador house (fixed sites)
Results and discussion
The results reviewed show the incidence of 1.3%, 0.4%, 0.39%, and 0.1% for O Rh‐, A Rh‐, B Rh‐ & AB Rh‐ respectively. The overall incidence of Rh negative blood type irrespective of the specific blood groups is at 2.13% which is in agreement with a study conducted in 2009 by Ssebabi et al in northern Uganda which showed an incidence of 2% but less than the incidence of the same study which was conducted by the same researcher in Southern Uganda which showed the incidence of 6%. Hoima collection centre had the highest incidence (18% of the Rh negative blood collected from the eight collection sites; Ambassador house, donor room, team A,B,C,D, Jinja & Hoima collection centres). Apparently Uganda Blood Transfusion Service is collecting blood from such sites which have a relatively low incidence and there is a challenge as far as handling requests from hospitals for Rh negative blood are concerned.
Conclusions/Summary
There is a low incidence of Rh negative blood type in apparent Uganda Blood Transfusion Service collection sites. Uganda Blood Transfusion Service should consider the option of conducting a comprehensive study in other parts of the country such that the institution can be scheduling blood drives in those areas with high incidence in the event that there is need to have predictability of Rh negative blood stock in the blood bank such that all requests of Rh negative blood are attended to since Rh negative patients can only receive blood from Rh negative blood donors.
P‐527
The Importance of the Establishment of the Register of Typed Donors at the Blood Transfusion Institute of Serbia
Dukic Novakovic MDN , Jovicic MJ , Stamenic LS , Jovanovic Srzentic SJS , Kosovic SK
Blood Transfusion Institute of Serbia, Belgrade, Serbia
Background
Considering the need for adequate and timely transfusion treatment of the patients with presence of multiple red cell antibodies and/or antibodies to high frequency antigens, the Blood Transfusion Institute of Serbia, for the first time during 2012, started planning activities in aim to establish the national register of typed blood donors.
Aims
Presentation of the activities conducted in order to select and test suitable donors in the period 2012–2015.
Methods
According to previously defined criteria (multiple donors who donated blood at least three times in the past 3 years, up to 55 years old, mostly male, mostly from the city of Belgrade and its surroundings) the first stage of testing was systematic determination of the Rh system antigen, performed using tube method in duplicate. Donors with selected Rh phenotype were further typed to the presence of blood group antigens of the following systems Kell, Kidd, Duffy, MNS, Lewis and Lutheran using gel method. Respecting those criteria, in the period 2012–2015, the Rh phenotype was determined in more than 15,000 of O RhD +, A RhD +, O RhD ‐ and A RhD ‐ blood group donors. Test data are stored into a database that is designed in manner which enables possibility of searching donors according to certain criteria.
Results
Registry of typed donors currently contains data for 1041 blood donors. CCDee phenotype was confirmed in 12,88% donors, ccDEE in 10.41% and ccDee in 8.72% donors, the remaining 67.99% of donors have a phenotype ccddee. In the registry are represented donors with rare Rh phenotype: CCddee and ccddEE. In the Kell system the most common phenotype is kk with a frequency of 92.60%, Kk 7.20%, while phenotype KK confirmed in two donors (0.19%). Within the MNS blood group system the following prevalence of phenotypes was found: MM 35.11%, MN 48.49%, NN 16.39%, SS 11.47%, Ss 44.93% and ss 43.40%. Typing of donors to the antigens of Kidd and Duffy systems gave following data: Jk(a+b+) 49.29%, Jk(a+b−) 22.37%, Jk(a−b+) 28.34% and Fy(a+b+) 46.35%, Fy(a+b−)20.76%, Fy(a−b+) 32.80%. Rare phenotype Fy(a−b−) was detected in one donor (0.09%). The largest percentage of typed donors were males (85.85%). According to the age structure 13.28% of the donors are under the age of 25 years, 35.28% of donors have up to 35 years, and 36.76% of donors have up to 45 years of age.
Conclusion
Numerous requests for searching the existing database fully justified the undertaken activities, but also point to the need for further systematic mass typing of blood donors, and the introduction of more sensitive techniques to work. Thanks to the registry data, serching for adequate blood donors now is more efficiently, especially because of the fact that the blood donors are mostly from Belgrade and may already during the same day donate blood.
P‐528
Polish External Quality Assessment Scheme for Blood Transfusion Laboratories – 5 years Experience in Conducting the Program
Pelc-Klopotowska MPK , Michalik MM , Lopienska HL , Wojciechowska BW , Michalewska BM , Brojer EB
Institute of Heamtology and Transfusion Medicine, Warsaw, Poland
Background
Each medical laboratory in Poland is obliged to undergo external quality assurance four times a year. Polish External Quality Assessment Scheme for Blood Transfusion Laboratories (PEQAS for BTL) was established in 2010. The goal of the program was to improve the quality of pretransfusion testing and to educate laboratory medical staff.
Aim
To assess the frequency of errors in ABO and RhD typing, antibody screening and identification crossmatch during the program.
Materials and methods
A set of blood samples imitating samples of three patients and three donors for compatibility testing is sent to 367 participating laboratories. Results of the of 50 exercises performed over the last 5 years (2010–2014) were analysed
Results
ABO typing: The errors in ABO grouping in patients ranged from 0% up to 2,97% irrespective of analysed year; most of the errors were due to clerical mistakes. participants had more difficulties in ABO typing when the control material contained two populations of RBCs (O group and B group) and the mixed field reaction with anti‐B reagent occurred. In the first exercise with such samples the correct result ‘not identified ABO group’ was reported by 52.3% participants, the wrong result by 47.7%. In the second exercise in which two population of RBC were examined, the error rate was reduced to 2.8%.
RhD typing: Errors rates in D typing ranged from 0 to 0.52% when RBCs with normal D expression or D negative were tested. When patient's sample was D weak type 1 or type 2 the reported results of RhD depended on the applied technique. They were typed as RhD neg by 86% users of slide, 80% of users of tube, 75% of users of microcolumn manual technique, and by 48% of users of automated method. On the contrary, they were typed as RhD pos by 5%, 23%, 21% and 39% users respectively.
Antibody screening: Errors rate in antibody screening due to false negative results were initially (in 2010) 1,17% for anti‐D and 0.59 for anti‐K and dropped to 0% and 0.57% respectively in 2014. Weak anti‐S were missed in antibody screening by 7.53% labs, of which 95% were using the tube IAT technique. Surprisingly, less errors (5.36%) were found in crossmatch with heterozygous cells.
Conclusion
EQAS is an important tool for identification of issues which need to be corrected and trained.
P‐529
Unusual Auto‐Antibody in a Patient with Refractory Lymphoma
Shin J1, Tekle S 1, Lee E 2, Sekhar M 1, Win N 3
1Royal Free Hospital, London, United Kingdom2NHSBT Colindale RCI, London, United Kingdom3NHSBT Tooting, London, United Kingdom
Background
A 32 year old lady, with primary refractory diffuse large B cell lymphoma being treated on lenalidomide and dexamethasone, dropped her haemoglobin from 93 to 67 g/l. This was accompanied by a positive DAT (direct antiglobulin test), which was C3d 3+, indicating a strong complement reaction but a negative antibody screen. She was therefore transfused with 2 units of group specific red cells.
There was no evidence of bleeding and her haematinics were within normal limits. She had an inappropriately reduced reticulocyte count, in keeping with bone marrow suppression, but a raised LDH (lactate dehydrogenase) accompanying the low haemoglobin, suggestive of haemolysis. She had a conjugated hyperbilirubinaemia which was thought to be a side effect of lenalidomide. Her peripheral blood film showed marked red cell agglutination and occasional spherocytes.
Ten days after the transfusion (Day 15), her haemoglobin dropped from 97 to 48 g/l. DAT continued to be positive and antibody screening showed the presence of an anti‐I antibody reacting at 30°C. She was transfused 3 units of warmed red cells, with which her haemoglobin remained stable until discharge 4 days later. Of note, she was commenced on dexamethasone as part of her therapy for lymphoma, prior to any drop in haemoglobin, and she continued on it, during the episode of haemolysis.
Results
Her blood group previously B RhD positive, showed mixed field reaction at the time of anaemia. Serological results are tabulated in Figure 1 below. The IAT (indirect antiglobulin test) was negative by tube test; CAT (column agglutination technology) was also negative but pan‐reactive positive by enzyme technique. CAT demonstrated IgM saline agglutination at room temperature and 1+ agglutination at 30°C but negative at 37°C. This showed anti‐I antibody specificity. Blood group showed mixed field reactivity with C and E, with probable phenotype Ro. In summary, a high thermal amplitude cold antibody (auto anti‐I) was detected, reacting at 30°C.
Table 1: Chronological overview of haemoglobin and serological results.

Conclusion
Anti‐I autoantibody is usually considered harmless on account of its cold reacting state; it usually occurs in the setting of mycoplasma infection. Our patient demonstrates a strong haemolytic antibody, on a background of lymphoma, occurring in the face of ongoing steroid treatment. Despite progression of lymphoma, the antibody became undetectable without any additional treatment. Transfusion of warmed crossmatch‐compatible blood resulted in stable improvement.
P‐530
High Fidelity Antibody Immobilized Microbead Technology for Blood Typing
Kazumi KK1, Jung G 1, Gotoh M 2, Ishikawa Y 3, Uchikawa M 2
1Toray Industries, Inc., Kanagawa, Japan2Japanese Red Cross Kanto‐Koshinetsu Block Blood Center, Tokyo, Japan3Japanese Red Cross Central Blood Institute Blood Service Headquarters, Tokyo, Japan
Background
Donated blood samples undergo blood typing, biochemical/infectious disease tests, and nucleic acid amplification tests at Japanese Red Cross Society Blood Centers before being provided to clinical institutions as blood products for transfusion. ABO and RhD typing is technologically based on agglutination of red cells by IgM antibodies, and generally performed by automated systems. However, testing for rare blood groups is difficult in obtaining those antibodies, forcing alternative use of IgG antibodies and the accompanied laborious manual processing.
Aims
One of these rare blood groups is JR blood group. Jra antigen that belongs to JR blood group is high frequency in general population, and frequency of Jra antigen‐negative in Japanese population is 1 in 1600. Since hemolytic transfusion reaction might occur to patients with anti‐ Jra if they are transfused with Jra antigen‐positive blood, the Jra antigen‐negative blood transfusion is crucial for these patients. Currently the Jra typing is performed on only a part of blood products, and an increased number of testing by a simpler, more accurate method is long sought.
Methods
We developed a simple method for testing Jra antigen using highly sensitive antibody‐immobilized microbead technology. This system utilizes human monoclonal anti‐ Jra antibody (IgG) that were immobilized on the surface of micro‐beads while maintaining its high antibody activity. The test result can be observed with or without agglutination of red cells by mixing a reagent including the microbeads and red cell suspension. This microbead reagent is able to be adapted to the automated blood typing analyzer PK7300 (Beckman Coulter); by pre‐setting the reagent into a specialized bottle, and the Jra typing is determined in 30 min.
Results
We validated the test performance using more than three thousand blood samples and confirmed accuracy, simultaneous reproducibility, and day‐to‐day reproducibility of this test. Our bead technology could accurately determine Jra type (positive, weak positive, negative).
Caption 1: Automatic method by highly sensitive IgG‐immobilized micro‐bead.

Summary/Conclusions
We've developed a highly sensitive IgG antibody‐immobilized micro‐bead technology. By using the bead technology, we made the one step automate Jra blood typing possible. We are expecting this automate blood typing system is also applicable to other rare blood type determination tests and more.
P‐531
Evaluation of Second Series Reagents for the New Daymate Automated Column Agglutination Technology for Donor Blood Grouping
Marandiuc DE , Seifert S , Runkel S , Conradi R , Hitzler WE
University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Background
The identification of ABO and Rhesus blood groups is essential in transfusion medicine.In conformity with the international guidelines, method adequate reagents and different clones are needed for correct blood grouping.
Aims
A second series of reagents for the new automated column agglutination technology from DAYmedical SA was to be validated on the automate DAYmate M in a clinical setting by comparison to Neo® from Immucor®.
Methods
913 known donor samples were tested for AB0 forward and reverse blood group and Rhesus D antigen. 395 of them were also tested for Rhesus (C, c, E, e) and Kell phenotype. DAYmate M used automated column agglutination on discs with 18 initially empty columns, which were filled with appropriate gel and reagents during the test. Neo® used microplates with 96 wells for agglutination. On Neo® the Rhesus (C,c,E,e) and Kell antigens were tested with two different antisera. A confirmatory Rhesus D test was performed for all the samples. DAYmate used indirect antiglobulin testing and Neo® used Capture® technology (solid phase adherence).Samples without a plausible result were retested and the difficult ones were clarified with additional manual testing (ID‐System, Bio‐Rad), PCR or DNA sequencing.
Results
Of the 913 samples, 20 samples on DAYmate and 17 samples on Neo® were marked with ‘?’ for different reactions. Some were retested and others with a clear negative or positive reaction were visually read by a technician. 36 samples, 28 on DAYmate and 25 on Neo® were not typed due to weak or negative reactions in the AB0 reverse group. These weak reactions were confirmed manually on ID‐cards and the blood group was interpreted by a technician. For one sample, the confirmatory Rhesus D test on DAYmate was interpreted as positive, although the reaction was visually negative. The software was revised and this problem did not appear again. One sample was discrepantly typed for Rh e antigen. Neo® found the antigen with both anti‐e antisera and DAYmate tested the sample as Rh e antigen negative. The sample showed a weaker (3+) reaction with manual column agglutination with human antibodies. The presence of the antigen was confirmed with PCR. To clarify the results, the sample was sequenced. As expected, a new variant of Rhesus e antigen was found. This sample was tested positive with a second available anti‐e antisera for DAYmate. 22 samples were known as weak D or partial D. In the agglutination test, DAYmate typed one sample and Neo® two samples as Rhesus D negative. All 22 samples were typed positive with confirmatory test.
Summary/Conclusions
DAYmate automated blood grouping system showed similar reactions to Neo® automated system. The Rhesus e antigen of one sample was not recognized by DAYmate single clone (MS 62) antisera and consequently was identified as product of a new allele RHCE* M121L, I359T. In the standard laboratory routine, through testing with two reagents and different clones, this sample would have been identified as problematic with further need of clarification. The DAYmate automated system provided reliable blood grouping results.
P‐532
Transfuion Support for a Patient with Anti‐Inb
Lee CY
NHS Blood and Transplant, London, United Kingdom
Introduction
Indian blood group system (In) is a polymorphism in people from the Indian subcontinent and Arabs. Ina and Inb are of low and high incidence antigens of the Indian blood group and located on CD44 acts as a cellular adhesion molecule. Anti‐Inb had been reported to cause an immediate transfusion reaction report by Bhatia et al. However when reviewed the data, the evidence was not convincing. We report a case with a weak anti‐Inb which we provided with random cross matched compatible blood and the transfusion was uneventful.
Case history
A 31 year old Asian female patient was first seen in her local hospital in July 1999 (15 weeks gestation) in her 6th pregnancy with panreacting antibodies. The patient's blood group type was B+ R1r K‐. The reference laboratory confirmed the presence of a weak anti‐Inb (titre:2, reactive by tube LISS‐IAT). A baby girl was delivered in November 1999 and the DAT was negative and the infant typed as In(b+). On her subsequent seventh pregnancy in October 2008, a weak anti‐Inb (titre of 4, 2+ by tube LISS‐IAT) was detected. The pregnancy progressed uneventfully. The patient did not require transfusional support and she gave birth to a healthy baby boy. There was no evidence of haemolytic disease of the new born (HDN). She had a further baby in 2009 which was again uneventful.
She was admitted in June 2011, in her ninth pregnancy (25 weeks gestation). A very weak anti‐Inb was confirmed (titre of neat and weak by tube LISS‐IAT) by reference laboratory. On this admission she was diagnosed with severe pregnancy induced cardiomyopathy with an ejection fraction of 15–20% on an echocardiogram. She was managed medically on the coronary care unit. A follow up sample at 31 weeks gestation was tested at her local hospital. A panreacting antibody reacted 2+ to 3+ using Capture technique and anti‐IgG was detected. The following date, her condition acutely deteriorated and she had a cardiac arrest and an emergency caesarean was carried out. Two units of random ABO and D compatible K‐ red cells were transfused following the arrest to this patient. Post transfusion there was no evidence of haemolysis or significant change in the bilirubin or haemoglobin level. The baby's DAT was negative and there was no evidence of HDN.
Discussion
Anti‐Inb is a rare antibody that recognises an antigen of very high frequency and it is found predominantly in Asian Indians. Indian Inb blood group is extremely rare in European populations. It has been suggested in the literature to consider providing In(b−) units for patients with strong examples of the antibody. Therefore selection and provision of blood for transfusion support for patients with anti‐Inb imposes a special challenge in the United Kingdom. Although anti‐Inb has not been implicated in HDN, it has been claimed that anti‐Inb may cause haemolytic transfusion reactions (HTR) but precise information is limited.
P‐533
Comperative Study of Blood Antibody Screening and Crossmatching Using E.M.® Technology and Gel Method
Krania E , Katinari F , Kalkana C , Evliati L , Gerovangeli E , Megalou A
General Hospital of Athens, Evaggelismos, Athens, Greece
Introduction
The tests of Antibody Screening (AbS) and Crossmatching are performed to secure the compatibility between the patient and the donor. The solid phase method using the process of Erytrocytes Magnetized®
Technology (E.M.®Technology by DIAGAST) has been evaluated and compared to the DIAMED ID‐SYSTEM that has been used routinely in our laboratory.
Aim
The aim of this study was to evaluate the sensitivity and specificity of the E.M. ®Technology using the QWALYS®3 device compared to the DIAMED ID‐SYSTEM gel technique using the semi manual system on the AbS and the Crossmatch.
Methods
439 samples (plasma or serum) collected from patients of the ‘Evangelismos’ Hospital were tested for AbS with both E.M. ®Technology and the DIAMED ID‐SYSTEM gel technique.
175 samples collected from potential recipients were tested for Compatibility testing (crossmatching) with several blood units from the Evangelismos Hospital blood bank using both E.M. ®Technology and the DIAMED ID‐SYSTEM gel technique
Results
1/2 Ab S: From the 439 samples analyzed 414 were negative and 22 were positive in both methods. 3 samples were negative by E.M. ®Technology but positive by ID‐SYSTEM. Further investigation was carried out and a papain test was performed with the ID‐SYSTEM. The 2 samples were positive also on papain test, meaning that these Irregular Antibodies are of IgM nature, that is considered clinically not significant, and is not detected by EM.®Technology. The one sample that was negative with papain was also negative with the Dia Panel (x11) identification test. The result of the AbS in ID‐SYSTEM being weak positive (+/−), we can considered that it is a false positive result in ID‐SYSTEM.
2/2 Crossmatching: From the 175 samples analyzed 151 were negative and 16 positive with both techniques. 4 results were negative with E.M. ®Technology and positive with the ID‐SYSTEM (2 patients tested for two different blood units). The test was repeated on these samples with both techniques, 2 results were negative on the second test and two were found weak positive. The two weak positive were also tested for AbS and it was negative. On the absence of titration we can suppose that they were false positive results.
Also 4 results were negative with ID‐SYSTEM and positive with the E.M. ®Technology (3 patients one tested for two different blood units). The test was repeated on these samples with both techniques, 3 results were negative and one weak positive. The weak positive was also tested for AbS and it was negative. On the absence of titration we can suppose that they were false positive results.
Summary/Conclusions
E.M.®Technology provides a complete and reliable automation system without centrifugation steps for both AbS and Crossmatching tests. The performed comparative study reveals that E.M.®Technology is as sensitive and specific as the gel ID‐SYSTEM gel technique for thedetection of clinically relevant IgG antibodies. The QWALYS®3 device is easy to use and could be introduced in a regular lab working schedule.
P‐534
Pan‐Reactive Autoantibody and Alloantibody Detection at its Best
Waltham LM 1, Patnett K2, Tidman J 2
1Immucor, Solihull, United Kingdom2Queen Elizabeth Hospital Birmingham NHS Foundation Trust, Birmingham, United Kingdom
At Queen Elizabeth Hospital (QEH) NHS Trust, there is a large Haematology patient population of which a significant proportion exhibit pan‐reactive autoantibodies. Currently, these samples are referred to a reference centre for investigation, which can be costly in both time and departmental budget. To assist in investigating this serologically complex group of patients, three different specialty kits from Immucor were trialled over a 6 month period; the Immucor W.A.R.M. kit, used for the removal of warm‐reactive autoantibody from red blood cells (RBCs); the Immucor Gamma ELU‐KIT II, intended for use in the rapid acid elution of antibodies from intact RBCs; and the Immucor Gamma EGA Kit which is intended for use in dissociation of IgG from RBCs so that treated RBCs can be further antigen typed or used for serological testing.
Routine antibody screening for patient samples was performed using Immucor NEO analysers using a 3‐cell automated Capture‐R Ready antibody screen. All samples with a pan‐reactive autoantibody or positive direct antiglobulin test (DAT) were investigated further using a combination of the three different specialty kits, to determine whether any antibody specificity could be determined.
35 patient samples presented with a pan‐reactive antibody screen and/or a positive DAT and therefore required further investigation according to the laboratory algorithm: 8 of these samples had an underlying alloantibody specificity identified using the Immucor W.A.R.M. kit; 2 samples with autoanti‐e were identified with the Immucor Gamma ELU‐KIT II and it was possible to antigen phenotype 30 of the samples using the Immucor Gamma EGA Kit. Due to the patient population seen at QEH, the clinical need for investigation using the Gamma ELU‐KIT II was rarely indicated.
Predominantly positive results in all previous testing had not allowed this busy hospital Blood Transfusion laboratory to perform the traditional autoantibody testing on site. The comparison of the three kits demonstrated that the Immucor W.A.R.M. kit was easy to use and enabled the confirmation of the presence of the warm autoantibody and any additional alloantibodies to be determined. As a result of this work, the laboratory algorithm for antibody investigation will be amended. The Immucor W.A.R.M. kit and the Immucor Gamma EGA Kit are to be added to the testing repertoire of QEH to support further serological investigations of patients with autoantibodies. This will enable the laboratory to provide a more rapid turnaround time for the provision of cross‐matched blood and rationalise the requirement for send away testing.
P‐535
Management of Transfusion Needs in a Case of Cold Agglutinins and Hyperglobulinemia in a Patient of Peripheral T‐Cell Lymphoma (NHL)
Desai PD , Rajadhyaksha SBR , Navkudkar AN , Patil DP , Thakkar RNT , Jain PJ , Sharma NS
TATA Memorial Hospital, Mumbai, India
Background
Cold reacting autoantibodies may interfere in routine ABO blood group testing resulting in ABO discrepancy. Some lymphoma patients develop autoantibodies which if associated with hyperglobulinemia, create problems in serological testing for ABO grouping and compatibility tests which need to be resolved by specific serological techniques.
Case details
A 43 year old female, case of peripheral T‐cell lymphoma had a hemoglobin level 5.6 g/dl in December 2014 on presentation. Two units red cells were requested for transfusion. Cell and serum grouping showed discrepancy on automated column agglutination technique (CAT) system. Cell grouping showed ‘B Rh positive’ group and serum grouping showed ‘O’ group. Patient antibody screen was positive on three cell panel at room temperature, saline phase and IAT phase. Autocontrol was positive. The discrepancy persisted with conventional tube technique and could not be resolved by using prewarm technique on the same sample. Rouleaux formation was seen on microscopic examination of agglutinates. Clinical details and previous medical records of the patient were sought. Blood group was reported from other Blood Center in November 2014 and the patient was transfused two units of red cells. There was no recent history of GI infection or fever. Laboratory investigations revealed a normal LFT except a high serum protein (8.8 g/dl) and high serum globulin level (6 g/dl). Serum albumin level was normal. Based on the above workup, presence of cold antibody was suspected. Hence it was decided to repeat the prewarm technique on a fresh sample collected by using water bath to keep the collection tubes warm. Subsequently the blood group discrepancy got resolved by the prewarm technique and the patient's cell and serum grouping was ‘B Rh positive’. Repeat antibody screen was negative in saline, room temperature and IAT phase. However autocontrol was still positive. Crossmatch put up with two units of B Rh positive red cells showed rouleaux formation on tube technique. As rouleaux formation was suspected to be because of high serum globulin levels saline replacement technique was used. Compatible results were obtained with this technique.
Conclusion
This is a case of cold agglutinins with high serum globulin levels. Such cases may present with blood group discrepancy and problems during crossmatching. The problem can be resolved with use of prewarm technique ensuring that the samples are kept warm from the time of collection. Saline replacement can help in resolving problems during crossmatch which are due to high serum proteins.
P‐536
Validation of Immune‐Hematological Reagents Before Use Contributes to the Safety of Blood Supply
Abdelsalam DAS , Salah MS
NBTS, Cairo, Egypt
Background
Pre‐transfusion testing in the form of blood grouping, cross matching, screening and identification of red cell Abs are mandatory before blood transfusion. Available commercial reagents and anti‐sera are of different degrees of quality. It is required for each lab to have its own acceptance criteria for reagents.
Evaluation and validation of reagents before their acceptance for use should be done to ensure accurate results and to avoid hemolytic adverse transfusion events. Monitoring the performance of reagents during routine use should be done to ensure consistency and reliability following laboratory evaluation.
Aim
To highlight the importance of implementing a protocol for selection and validation of immune‐hematological reagents in the Egyptian national blood transfusion services, before their acceptance and distribution for use.
Methods
Selection and validation of immune hematological reagents done on the basis of laboratory and quality requirements and according to SOPs. All reagents were tested for: specificity, sensitivity, potency, appearance, ease of use, Suitability for methodology and stability.
Testing of reagents done by senior staff members of the red cell reference laboratory in the NBTC.
Caption 1: IMMUNE HEMATOIOGICAL REAGENTS.

Testing done using semi‐automated and manual techniques, using carefully selected samples and according to the manufactures’ instructions. Data collected in the relevant forms, analyzed and interpreted, then compared against existing specifications and acceptance criteria. Those fulfilling the international specifications and local acceptance criteria were accepted. Those not fulfilling the international specifications and criteria were rejected.
Results
Accepted and rejected reagents during the years 2011, 2012 and 2013, 2014 (till the end of December) are shown in table. No errors or adverse transfusion events were reported due to failure of the accepted reagents during this period of time.
Summary/Conclusions
Selection and Validation of immune hematological reagents is a process that needs to be planned carefully and the overall performance depends on several specifications and selection criteria. Proper selection of reagents before their use in lab testing ensures accurate results and contributes to the safety of blood supply.
P‐537
A2B or not A2B: Advanced Automation in Blood Group Serology: Validation Results of the Biorad IH‐1000
Quigley J1, McGarry P 1, Carrol L 1, Culliton M 1, Segurado R 1, Niland B 2, McCarron R 2, Fitzgerald J 1
1The National Maternity Hospital, Dublin 2, Ireland2Fannin Limited, Dublin, Ireland
Background
Automation for ABO/Rh D blood grouping was first introduced in the early 1960's. The introduction of the gel test developed by DiaMed (Cressier, Switzerland) in the late 1980's revolutionised blood group serology and is a technique widely used today on automated platforms. In recent years improved optical resolution has led to increased sensitivity and specificity for blood group determination.
Aims
Current BCSH and ISBT guidelines recommend the use of fully automated systems for blood grouping and antibody screening in order to reduce the risks of interpretation and transcription errors. According to SHOT data over the last decade, the majority of ABO blood grouping errors occurs in manual systems. But what about errors that occurs within automated systems? Here we investigate the concordance rate of two BioRad autoanalysers with a decade of technology between both platforms.
Methods
370 adults were tested for ABO/D group and antibody screen and 47 neonatal samples for ABO/D group. All samples were analysed on the DiaMed‐ID Classic ID‐GelStation (Cressier, Switzerland) and retested on the BioRad IH‐1000 (Cressier, Switzerland). Reaction strengths in each well were graded on a five point scale of 0–4.
Results
The rate of concordance of the two methods was very high, with absolute agreement on the 5‐point scale (0–4) for each ID‐well in both adults and neonatal samples ranging from 88% to 100%. Linear weighted Cohen's kappa statistics ranged from 0.955 to 0.997 in the adult sample ID‐wells, and equalled 1 for most of the neonatal ID‐wells.
Of the 370 adult samples tested, 0.2% (5/2220) of the ID‐wells yielded a >1+ reaction strength difference between both platforms (reverse group only). Further investigation revealed that the Classic ID‐GelStation over‐reported the reaction grade (4+) and the IH‐1000 under‐reported the reaction grade (2+), as visual inspection of the electronic screenshots were similar (3 ± 0.5 reaction grade).
In a single case a discrepancy in positive vs negative rating was observed. This occurred in a neonatal sample against the Anti‐A well, where the IH‐1000 graded the reaction strength against Anti‐A with a generous 2+ score (Group AB), while the Classic ID‐GelStation graded the reaction in this well as negative (Group B). This sample was 4+ against Anti‐B well on both analysers. The sample was referred to a reference laboratory and was confirmed as group AB with weak expression of the A antigen. With newborns it is common for the A antigen expression to be weakened and it is likely that the neonate is A2B or A3B. The clinical impact of the discrepancy (potential risk of transfusion of incompatible plasma) was felt to be low due to the weak expression of the A antigen. All other reactions in the neonatal cohort (n = 46, 98%) were identical between analysers.
Summary/Conclusions
Improved optical resolution in automated systems results in significant improvements in detection of weaker reactions in blood group serology. As an advanced in vitro diagnostic system, the BioRad IH‐1000 is more sensitive in its detection of weaker results and is therefore more accurate and superior than older platforms.
P‐538
A Comparison of Microcolumn and Solid‐Phase Automated Methods for Pretransfusion Testing
Raos MR , Lukic M , Plenkovic F , Tomac G , Golubic Cepulic B
University Clinical Hospital Center, Zagreb, Croatia
Background
On the market there are several automated systems which use different methods for pretransfusion testing. When making a choice about the methods and the system for pretransfusion testing, differences in the sensitivity and specificity must be taken into consideration together with the limitations and advantages of the system, such as: ease of use, reproducibility, cost effectiveness, etc. System which ensures safety of pretransfusion testing and meets the needs of the patients and the workflow of the laboratory should be chosen.
Aims
The aim of this study was to compare microcolumn automated method in the routine use (Techno TwinStation, Bio‐Rad) with the solid‐phase automated method (Neo, Immucor) for antibody screening and crossmatch.
Methods
Through 3 months, from October 2014 to December 2014, in total 715 routine patient samples for antibody screening and crossmatch and 1613 samples from the donor unit for crossmatch were analysed. All samples were tested in an automated way with Techno TwinStation (Techno) and Neo. Antibody screening was done with two‐cell screening set (ID‐DiaCell I‐II, Bio‐Rad) and three‐cell screening set (Capture‐R Ready Screen, Immucor). The specificities of antibodies were identified using Capture‐R Ready‐ID, ‐ID‐Extend I and ‐ID‐Extend II (Immucor). In case of doubtfull or discordant results antibody identification was done using Capture‐R Select with three‐cell and eleven‐cell set (Panoscreen and Panocell, Immucor) and also according to our standard procedures with eleven‐cell panel (ID‐DiaPanel and ID‐DiaPanel‐P, Bio Rad).
Results
Each method for pretransfusion testing missed to detect two antibodies. Differences found in specificities of antibodies were in two anti‐Jka antibodies (one confirmed and other suspected) detected only with solid‐phase antibody screening on Neo. Opposite, one anti‐M antibody was detected only with microcolumn antibody screening test on Techno. This antibody was further tested in tube method and was reactive on room temperature only, and not by antiglobulin test. With crossmatch test, another antibody, anti‐Lua, was detected with Techno, and not with Neo, and was also reactive in tube method on room temperature only.
Conclusions
Although both methods missed to detect the same number of antibodies, there are differencies in clinical significance of antibodies detected. While anti‐Jka antibodies detected with Neo are known to be clinically significant antibodies, anti‐M and anti‐Lua antibodies detected with Techno were not significant in this case. Use of both systems identified 4 additional antibodies (2 anti‐Jka, 1 anti‐M, 1 anti‐Lua), that would have not been detected if only one system was used. Each method has its limitations and in ‘our hands’ solid‐phase method showed better sensitivity for detecting anti‐Jka antibody. In conclusion, at least two methods are preferable in pretransfusion testing.
P‐539
Anti‐‘MIA’ Detection by Flow Cytometry: An Effective Method to Evaluate the Immune Characteristics of Miltenberg Antibodies
Lin TM , Wang CH , Yang YT , Chiang MTH
E‐DA hospital/I‐Show University, Kaohsiung City, Taiwan
Background
The Mi III phenotype is with a frequency of 7.3% among Chinese population in Taiwan. In blood bank, anti‐‘Mia’, used to describe antibodies that react to antibody screening cells with Mi III phenotype, is the most common RBC alloantibody in Taiwan with an incidence about 0.2%. The clinical significance of anti‐‘Mia’ has been debated; there have been reports of haemolytic disease of the newborn and delayed or even intravascular haemolytic transfusion reactions as a result of anti‐‘Mia’. The manual polybrene (MP) method detects anti‐‘Mia’ with screening cells with Mi.III phenotype is used in most blood banks in Taiwan. However, this method is rough to identify the immunological characteristics of anti‐‘Mia’.
Aim
In this study, we set up a flow cytometry method to quantify the IgG and IgM for anti‐‘Mia’, and compared it with sera treated with dithiothreitol (DTT) for IgG and IgM differentiation.
Material and methods
From June 2013 to May 2014, sera from 90 patients containing anti‐‘Mia’ detected by MP method were collected from E‐Da hospital. These antibodies were analyzed for immunoglobulin class and quantification by flow cytometry.
Results
Sixty‐four sera (71.1%) were mixture of anti‐‘Mia’ IgG and IgM, and 5 (5.6%) and 21 (23.3%) specimens had anti‐ ‘Mia’ IgG and IgM only, respectively. The agreements of immunoglobulin for anti‐‘Mia’ between flowcytometry and MP with DTT methods were 100%, 78.2% and 62.5% for mixed IgG‐IgM, IgM and IgG, respectively.
Conclusion
Based on our results, flowcytometry is suggested to be more appropriate than the MP method with higher sensitivity to differentiate IgG of anti‐‘Mia’ antibodies.
P‐540
Significance of Antibody Screening & Identification in Pretransfusion Testing a Retrospective Study
Premkumar R
Sakra World Hospital, Bangalore, India
Background
Genetic disparity of RBC Antigen between donor and recipient is responsible for RBC alloimmunisation.Though RBC transfusion is a lifesaving therapy in most of the patients, risk of alloimmunisation is always a concern for patients receiving multiple transfusion. Pregnancy also carries the risk of alloimmunisation. Very few studies on alloimmunisation are done on the general Indian Hospital patients.
Aim
The study was aimed at assuming the frequency and type of unexpected red cell antibodies in both patients going to receive transfusion and antenatal cases at a multispecialty tertiary care hospital in Bangalore.
Methods
It is a retrospective study. Antibody screening was carried out in 1912 patients including inpatients and antenatal mothers from January to December 2014.All positive cases were subjected to antibody identification. In patients receiving transfusion antigen negative red cells were cross matched and given. Antenatal cases were followed up every month with antibody titre till the time of delivery.
Results
It is a retrospective study in which evaluation of 1912 cases (870:50% males and 1042:54.4%females) done. All samples (patient and antenatal) were screened for the presence of unexpected antibodies. Antibody screening was positive in 19 patients (0.99%). In the serum samples of 37 patients only autoantibodies were identified, 4 cases revealed autoantibody also with underlying alloantibody. The total alloimmunisation rate was 0.99%, alloimmunisation in antenatal females was 0.15%.Among the antenatal females anti D was the most common (4 cases) Anti E and Anti K one each.
Interpretation and conclusion
Since clinically significant antibodies are frequently detected in our patient population, antibody screening and identification is mandatory to ensure safe transfusion practice. Since antibody against Rh, Kell and Le group antigens are more common and clinically significant, provision of Rh and Kell matched cells may be of protective value.
Clinically significant unexpected antibodies are capable of causing hemolytic transfusion reactions secondary to accelerated destruction of a significant proportion of transfused red blood cells. Therefore, screening for unexpected antibodies should be part of all pretransfusion testing, with antibody identification in the event of a positive result.
Antenatal detection of the non‐anti‐D causes of HDN requires Red cell antibody screening. If RCAS is positive, the following steps are to be taken. Antibody Identification should be done to identify the antibody. The spouse has to be screened for the presence of offending antigen and the pediatrician has to be alerted about delivery of a potentially sensitized infant. The blood bank should find a suitable antigen‐negative donor for transfusion to baby and mother.
P‐541
Cost Effectiveness of Applying Antibody Screening Test Routinely for the Egyptian Patients
Abdel Hakim M , Shabayek S , Ahmed A
National blood transfusion center, Giza, Egypt
Background
Pretransfusion Antibody screening test tests Patient's serum to ensure that he has no unexpected irregular antibodies to react with the donor cells causing transfusion reactions.
As well as, maternal serum to ensure that pregnant mother has no antibodies to react with fetal cells.
In National Blood Transfusion Center, Antibody screening was previously performed for all donors but for financial reasons it was done for certain patients only whose cross matching results shows reactivity with most of the units.
National Blood Transfusion Center started performing Antibody screening test for all the incoming patients since October 2014.
Aim
Study the cost effectiveness of applying Antibody screening testing for all the patients in National Blood Transfusion Center.
Method
Antibody screening is done by using home made screening cells from two or three individual donors containing these antigens: D, C, E, c, e, M, N, S, s, P1, Lea, Leb, K, k, Fya, Fyb, Jka, Jkb. Screening cells outdate every 4 weeks. Each new lot number of screening cells will vary related to antigen typing. In National Blood Transfusion Center antibody screening is done by cassette technique, dispensing the screening cells in the columns of the cassettes, incubate and centrifuge (according to the manufacture) then interpret the results, if all the readings are negative then Antibody screening is negative, if one or more reactive results appeared then Antibody Screening is positive and Antibody identification is recommended. Below is an example of a package insert.
Result
This study was done on the cases for 3 months (from 10/10/2014 till 17/1/2015).
Total number of patients: 4015.
Total number of negative screening: 3704 (92%).
Total number of positive screening: 191 (5%).
Total number of positive screening with negative cross matched units’ results: 84 (2%).
In Red Cell Research Laboratory the total number of positive screening after repetition and identification were done: 36 (1%).

Conclusion
This study shows that there are significant antibodies detected in Red Cell Research Laboratory in 36 patients out of 84 which represent about 42.8% and 1‐2% of the total patients. This indicates the benefits and the importance of early detection of significant antibodies especially in chronic patients who need regular blood transfusion. If blood units were transfused without antibody screening testing, new antibodies will be developed and sometimes there will be difficulty in finding suitable units for them.
P‐542
The Algorithm for Detection of RHD Antigen with Weak Expression Among First‐Time Blood Donors in the Regional Blood Center in Poznan in the years 2013–2014
Kolasinska K , Lewicka M , Olbromski K , Skalisz H
Regional Blood Center in Poznan, Poznan, Poland
Bacground
The red blood cells with weak RhD expression are present in 0.2–1% of the Caucasian population.
The automation of serologic tests in Regional Blood Center in Poznan allowed to design our own algorithm for detection of the blood donor's RhD antigen and to conduct the procedure for blood donors classified as RhD negative on the basis of tests performed using the immunohaematological analyzers with anti‐RhD IgM and anti‐RhD IgM+IgG reagents
Aims
The aim of this study was to compare the detection of weak RhD expression in Regional Blood Center in Pozna’ according to the standards of the Polish blood donation system in the years 2013‐2014
Methods
The study was conducted in 28893 healthy first‐time blood donors who gave blood or its components for clinical purposes in 2013–2014. The testing of RhD antigen was performed on immunoanalyzers (Techno TwinStation and PK 7300) using 2 different anti‐RhD monoclonal reagents (IgM and IgM+IgG). Negative or questionable results were confirmed by gel matrix technique. The results of weak RhD expression were confirmed by testing with various anti‐RhD reagents using the plate and test tube technique and with indirect antiglobulin test using the test tube method.
Results
Among 28893 first‐time blood donors the weak expression of RhD antigen was detected in 124 individuals (0.43%) with the following distribution of Rh phenotypes: Ccee67.92%, ccEe24.53%, ccee6,60%, CCee0.94%.
1. 47 cases (0.16%) of a weak D antigen expression were obtained using methods compliant with mandatory regulations for the Polish blood donation system (Ccee92.31%, ccee5.13% CCee2.56%).
2. 58 cases (0.20%) of a weak D antigen expression were obtained using one of the immuno‐analyzers with the negative reaction on the other one (Ccee73.47%, ccEe24.49%, ccee2.04%).
3. 19 cases (0.07%) of a very weak D antigen expression were detected by gel matrix technique (ccEe77.78%, ccee22.22%).
Conclusions
Testing of RhD antigen according to the obligatory IHIT regulations allowed for detection of 0.16% weak expression events. The introduction of automatic RhD testing in the Regional Blood Center in Poznan with opportunity to compare results achieved from two different immuno‐analyzers increased the detection of weak RhD expression by 0.20%. The introduction of the micro column method to the routine RhD testing led to the identification of another 0.07% blood donors with weak RhD antigen expression. In this group the negative or unclear reactions using manual and automatic methods would probably indicate the RhD negative classification. In order to detect the weakest variants of RhD antigen different testing methods should be used as they increase the efficiency of testing depending on Rh phenotype with weak RhD expression.
In order to increase the detection of weak RhD antigen expression the Regional Blood Center in Poznan introduced in 2015 the evaluation of RhD antigen on the basis of indirect antiglobulin test using micro column kit for all first‐time blood donors.
Using the PCR technique to search for the RHD gene in pools of serologically RhD negative donors would probably allow to identify another 0.2% cases with weak RhD antigen expression.
P‐543
The Lewis Phenotype Distribution Among Blood Donors from Northern Greece – Indirect Evidence of Their Secretor Status
Pape MP , Bakaloudi VB , Girtovitis FG , Ntinopoulou EN , Konstantinidou AK , Chatzikyrkou MC , Pantelidou DP , Chalkia PC , Floridis KF , Tzioura AT , Voulgaridou VV , Papageorgiou VP , Pavlopoulou AP , Pouloudi PP , Kltsa PK , Taliona GT , Lazaridou PL , Hasapopoulou-Matami EH
AHEPA Hospital, Thessaloniki, Greece
Background
People can be classified as secretors and non‐secretors according to their ability to secrete ABO (A, B, H) blood group antigens in saliva and other bodily fluids. The secretor status is under the genetic control of three systems: ABO, Lewis, Sese. The phenotype in the Lewis blood group indirectly defines the secretor status of an individual: Le(a−b+) = secretor, Le(a+b−) = non‐secretor. For the minority of the population with the Le(a−b−) phenotype, saliva testing is required to determine their secretor status. Innumerable articles correlate the ABO group, and/or the secretor status to a multitude of diseases and to the susceptibility or resistance to several pathogens. However, no solid conclusions can be drawn if the patient group is not compared to normal controls of the same ethnic background. To the best of our knowledge, there is only one report on the incidence of the secretor status in Greece (Kremastinou et al, 1996).
Aims
The aim of this retrospective study was to determine the frequency of the secretor status, besides the ABO and Rhesus group, in blood donors of our Blood Centre situated in Central Macedonia. Our data could then serve as the basis of comparative statistical analysis in studies attempting to correlate susceptibility or resistance to specific pathogens or particular diseases with the blood group and the secretor status in patients of the same region.
Methods
Both our manual (before 2012) and computerized registries (after 2012) were screened for donors typed in the Lewis system. We found 636 blood donors typed for both Lea and Leb antigens.
All donors were typed in the ABO system and RhD by two techniques ‐the slide technique and hemagglutination in microcolumns with glass beads, in a fully automated analyzer (Ortho AutoVue Innova, which is loaded with 6‐microcolumn cassettes preloaded with diluent and/or reagent and glass beads). Phenotyping in the Lewis blood group was performed using a commercial gel test technique (DiaMed): manual use of cards with microtubes containing monoclonal anti‐Le(a)/Le(b) within the gel matrix. Excel worksheets were used to classify the results.

Results
The distribution of the four Lewis blood group phenotypes is shown in the table.
Conclusions
The secretor and non secretor status was indirectly defined in 636 blood donors by phenotyping in the Lewis system. Secretors represented 67.45% and non secretors 19.02% of the study population, while 11.63% with the Le(a−b−) phenotype remain unclassified (they need saliva testing).
If we exclude the AB group and the B RhD‐ group due to the small number of individuals tested, the secretor status varied between 57.33 and 71.74% among the ABO groups. In the study by Kremastinou et al, some differences in the incidence of the phenotype Le(a−b+) were observed depending on the birth place (71–79% among 451 donors from Macedonia), but no correlation was made to the ABO blood group. The relatively lower incidence of secretors observed in group A RhD‐ individuals (57.33%) needs to be confirmed in a larger number of blood donors.
P‐544
Abstract Withdrawn.
P‐545
Transfusing ABO and RH (D) Matched Leukoreduced Red Cells to Beta Thalassemics: Prospective Analysis
Doda V , Arora S , Kotwal U , Dogra M
Dr Ram Manohar Lohia Hospital, New Delhi, India
Background
Beta thalassemia is one of commonest inherited hemoglobin disorder in Indian subcontinent with transfusion as the key intervention in decreasing morbidity and mortality in them. Transfusion therapy still remains a mainstay of treatment for majority of thalassemia patients despite successful cure with the use of hematopoietic stem cell transplantations. The risks of red blood cell (RBC) antigen alloimmunization in thalassemia patients are considerably greater than that in general population receiving transfusion. Factors majorly responsible for the alloimmunization rates include the differences in RBC antigen frequency between blood donor and the recipient population, the immune responsiveness of the patient and the immunomodulatory effects of the allogenic transfusions on the recipient's immune status
Aim
To analysed the frequency of allo‐ and auto‐antibody formation among thalassemics receiving ABO and Rh(D) leucoreduced transfusion from our blood bank.
Materials and methods
This study was a prospective analysis for 2 years (Jan 2013 to Jan 2015), involving serial screening for Allo/Auto‐Antibody in thalassemics receiving transfusion regularly from our centre. Screening was done every 6 months as well as if required in between (in case of incompatible crossmatch or reporting transfusion reaction). Rh and Kell phenotyping was done at the onset of the study at the time of enrolment of the patient. Screening of irregular antibody and identification was done using surgiscreen and resolve Panel A, ortho‐clinical diagnostics (USA) with polyspecific AHG cards (anti‐IgG and anti‐C3d).
Result
We prospectively analysed 62 patients enrolled in our study [37 male (59.6%) and 25 females (40.4%); Age Mean 10 years; Median 12 and Range 1–22 years]. Two patients (3.2%) were found to have alloantibody (both against Kell antigen). One was identified at the time of enrolment and other during the third screening. 9 patients (14.2%) were found to have autoantibodies as well. Among 30 patients phenotyped commonest Rh phenotype was DCce in 15 (50%) followed by DCe (R1R1) in 11 (36.6%) then DCEe in 2 and one each DCEce and ce (rr). Kell phenotype was negative in 57 (93%) patients, positive in one and mf in four patients
Conclusion
This was a pilot phase study before initiating Rh and Kell matched transfusion at our centre. The frequency of alloimmunization is 3.2% which is in concordance with an earlier study from our region by pahuja et al whereas on comparison with the sub continent region, where frequencies are reported from 18.8 to 3.8% due to heterogeneity between the donor and the patient population.
P‐546
The Demand for Packed Red Blood Cells in the First Period After Hematopoietic Stem Cell Transplantation
Iwankiewicz-Bahr EIB , Misiaszek AM
Regional Center of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Bone marrow transplantation is used to treat patients with various hematologic disorders. Hematopoietic stem cells (HSC), obtained in advance from the donor,are transplanted to a recipient. Allogeneic transplants are collected from related or unrelated donors.
ABO antigens do not serve as a transplantation barrier for marrow recipients. Potential donors who are fully HLA matched have an ABO incompatibility. Three types of ABO incompatibility exist: major, minor, and bidirectional. Major incompatibility is defined as the presence in the recipient's plasma alloagglutinins reactive with the donor's red cells. Minor incompatibility is defined as the presence alloagglutinins in the donors plasma reactive with the recipient's red cells. The bidirectional incompatibility is defined as the presence in both the donor and recipients plasma alloagglutinins reactive with recipient and donor cells.
From the commencement of transplantation begins the first period after and lasts about 100 days, which ends with the transplant engraftment and obtaining a stable condition.
In the period up to the adoption of the transplant recipients require transfusion of blood components e.g. packed red blood cells (PRBC)
Aims
What is the relationship of number of transfused units of packed red blood cells (PRBC) after transplantation depending on the blood group incompatibility in ABO donor‐recipient pairs and depending on the related or unrelated donor of HSC.
Methods
Observation consisted of 121 recipients (years 2012–2014), who were divided into groups depending on the scope of the compatibility system ABO and HSC antigens: Group 1: major incompatibility between donor and recipient (38 patients).Group 2: minor incompatibility between donor and recipient (38 patients).Group 3: bidirectional incompatibility (16 patients).Group 4: compatibility between donor and recipient (29 patients).
In addition, the patients were also divided according to the presence / or not relationship between donor and recipient into: Group A. recipients unrelated to donors (76 patients).Group B. recipients related to donors (45 patients).
After the transplant recipient received PRBC, before transfusion was performed compatibility testing.
Results
After transplantation patients were been transfused with a total of 760 units of PRBC.
Group 1. in major ABO‐incompatible patients received 300 units PRBC.Group 2. in minor ABO‐ incompatible 200 units.Group 3. bidirectional incompatible 116 units.Group 4.compatibility in ABO 144 units.
The recipients, who received HSC from related donor (group A) were given 135 units of PRBC, and recipients who received HSC from unrelated donor were given 625 units.
Conclusions
The recipients with ABO system incompatibility to the donor, require greater number of transfused units of PRBC comparing to compatible donors. The recipients, who received HSC from related donor require less PRBC units transfused comparing to recipients of unrelated donor. Looking for donors of HSC should tend to increase the number of related donors if it is possible to choose a donors compatible in ABO group system with recipients.
P‐547
Is Ortho Vision Analyser the Way Forward?
Scott Y , Muir A , Bevan L , Bolam M , Begum M , Wilson M , Main R
The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background
The Department participated in an Ortho Clinical Diagnostics (OCD) external evaluation (EET) which was designed as a method comparison to demonstrate that the ORTHO VISION[TRADEMARK] Analyser has similar performance to its predecessor; the ORTHO AutoVue® Innova. The method comparison and usability of the analyser was performed in a manner that was consistent with the general use of the ORTHO VISION[TRADEMARK] Analyser in a laboratory setting.
Aims
Method comparison was used to determine whether the concordances from the test results performed on the ORTHO VISION[TRADEMARK] Analyser were similar to those results from the ORTHO AutoVue® Innova.
Methods
The Freeman Laboratory provided unlinked unselected and selected samples from the daily workload to test in the trial. The selected samples were classified as desirable samples representing unusual or challenging conditions. Samples were first tested on the ORTHO AutoVue® Innova and then tested, within 24 h, on the ORTHO VISION[TRADEMARK] Analyser. Usability was assessed to ensure that any operators can operate the system in a safe and effective way.
Results
See Table 1.

Concordance ‐ Results from both ORTHO VISION[TRADEMARK] Analyser and ORTHO AutoVue® Innova were compared column by column. For results to be considered concordant either both results were to be negative or both were to be positive (the degree of positivity was irrelevant).
Discordance ‐ Any result that differed from one analyser to the other was classified as discordant and an investigation was initiated using documents and flow charts provided by OCD. Different levels of investigation existed for poor sample quality, instrument malfunction, mixed field errors, indeterminate results for panels, non‐panels and no interpretation of group.
Summary/Conclusion
We found 12 discordant results; 10 using the indirect antiglobulin test (IAT) and 2 using direct agglutination. All bar one were expected to be negative results. The ORTHO AutoVue® Innova gave 9 very weak (0.5) positive results in IAT which were negative in the ORTHO VISION[TRADEMARK] Analyser. In none of these samples was a specific antibody detected on further testing.
The ORTHO VISION[TRADEMARK] Analyser gave two weak (0.5) positive results one in IAT which on further testing no specific antibody was detected. The other gave a weak (0.5) positive reaction in direct agglutination immediate spin compatibility test which on investigation showed a non‐clinically significant anti‐Leb reacting with Leb positive donor cells. The remaining discordant result, a direct agglutination immediate spin compatibility test, was an expected positive (4+) reaction which the ORTHO VISION[TRADEMARK] Analyser resulted as a false negative giving a FIB flag (Fibrin top line) only.
Generally the ORTHO VISION[TRADEMARK] Analyser appears to be not affected as much by sample age etc. and the weak ‘false’ reactions seem to be eradicated with images much improved in both grey scale and colour. Overall the staff involved in the EET trial found the ORTHO VISION[TRADEMARK] Analyser easy to use with very intuitive software and reliable reproducible results.
P‐548
ABO‐Mismatched Platelet Transfusion: Evaluation of IGM and IGG ABO Antibody Titers and Hemolysins in Plasma and Platelet Products of Group O Blood Donors
Bastos EP , Bub CBB , Aravechia MG , Castilho L , Kutner JM
Blood Bank Department – Hospital Israelita Albert Einstein, São Paulo, Brazil
Background
Platelet concentrates from ABO‐identical donors are the components of choice for patients. However, since inventories are generally insufficient and because there is usually a higher frequency of group O donors, perfect matches are not always possible and therefore ABO‐mismatched platelet transfusion is an accepted practice when ABO‐identical platelets are unavailable. However, the transfusion of platelets containing plasma with high titers of ABO antibodies can cause clinically significant hemolysis. Most immune reactions related to platelet transfusion are caused by IgM ABO antibodies but IgG can also play an important role in these reactions. The way to improve the safety of group O platelets has focused on defining a safe level of IgM and IgG ABO antibodies.
Aim
In order to define a safe strategy to reduce the risk of passive hemolysis in ABO‐mismatched transfusion of platelets, we evaluated IgM and IgG ABO antibody titers in group O donors and compared the results with hemolysis grade. We also compared the IgM antibody titers in the donors’ plasma samples and in their platelet products.
Methods
A total of 82 group O donor plasmas (70 men and 12 women) and their respective platelet products were analysed. IgM ABO antibody titers in both plasma samples and platelet products were determined by direct agglutination in gel test using a dilution cutoff of 1:64. IgG ABO antibody titers were determined by treating the plasma sample with 0.01 dithiothreitol (DTT) using a dilution cutoff of 1:64 and tested by gel. Test for hemolysins and scoring was performed by standard procedure adding a fresh source of complement. Hemolysin was graded as complete hemolysis and partial hemolysis. IgM and IgG ABO antibody titers were compared with the result of hemolysins.
Results
At the dilution cutoff of 1 in 64, the prevalence of IgM high titers was 48% for anti‐A and 40% for anti‐B while the prevalence of IgG high titers was relatively higher, 65% for anti‐A and 45% for anti‐B. The overall prevalence of anti‐A and anti‐B hemolysins (complete hemolysis) in those high titers of ABO antibodies was 75% (39% IgM and 36% IgG). Anti‐A hemolysin was more prevalent than anti‐B hemolysin (53% and 22% respectively). When we compared IgM ABO antibody titers between the plasma sample and the platelet products we verified that plasma samples had higher titers of ABO antibodies (at least one dilution) than platelet products.
Conclusion
We found a high prevalence of anti‐A and anti‐B hemolysins with complete hemolysis in group O donor plasmas associated with IgM and IgG high titers. In our study, IgG ABO antibodies showed higher titers than IgM antibodies and platelet products showed lower IgM titers than plasma samples. Our data emphasize that strategies used to reduce the risk of platelet‐associated hemolytic transfusion reaction should include screening donor plasma for IgM and IgG high‐titer ABO antibodies.
P‐549
Evaluation of Mdmulticard® for RHD/Ab0 Serotyping
Di Girolamo MG , D'Ambrosio R , Di Lemma GG , Danzi M , Munno E , Tomeo R , Misso S
S.C. Servizio Transfusionale ASL Caserta, Aversa, Italy
Background
Following Italian recommendations blood group AB0 and RhD typing must be carried out on two samples taken at different times (D.M. March 3, 2005 art. 14, par. 2). In our Transfusion Center (TC), the first determination is performed with gel column technique and the second one on slide using mono and polyclonal antisera.
Aims
The aim of the work was to evaluate the MDmulticard ABO‐D Rh subgroups‐K system for patients (Medion Grifols Diagnostics AG) as an alternative to serological blood group ABO and RhD typing on slide.
Methods
Blood group AB0‐D‐Rh+ Kell phenotype including indirect Coombs test are performed on the first sample of transfusion recipients with automated gel column technique (ID‐System‐ DiaClon ABO/Rh for patients, DiaClon Rh‐subgroups+K) (Bio‐Rad, Ottobrunn, Germany). ABO and RhD typing on the second sample was performed in parallel with MDmulticard and on slide. MDmulticard test is a device based on lateral flow principle including IgM monoclonal antibodies directed against AB0, Rh and Kell antigens immobilized on a membrane, including internal positive (val) and negative (neg) controls. 50 microliters of the patient blood sample are dispensed in the card and the red blood cell excess is washed using a specific diluent. The valid result is available within the following 5 min. Moreover, it ensures total traceability given its stability for up to 72 h and it can also be photographed, preserved and archived.
Results
In the period July‐December 2014 4.203 blood group AB0‐D typing and 3,983 control bloodgroups (second determination) were performed. The results obtained with the MDmulticard test were quick, clearly interpretable and completely overlapped with those obtained with the slide and micro column test, without any discrepancy. However, three patients with genotype RhDweak type 1 (Kit RhD BeadChip[TRADEMARK], BioArray Solutions Ltd) that showed a +2 score in micro column gel‐test were clearly positive with MDMulticard compared with the slide test.
Conclusions
MDmulticard test can provide an useful complement to the methods already in use as it is a simple, fast, secure test. Indeed, this test has been implemented for the ABO‐D second blood grouping, substituting the slide test, due to its subjective interpretation, the increase in human errors and its low result stability, despite its speed of execution.
P‐550
Partial D Serotyping by Using a Commercial Kit in Patients Demonstrated Weak RHD Phenotype During Routine Blood Typing
Ayhan FY1, Uzun B 2, Karatas E 3, Askarogullari N 1, Caglayan D 4
1Dr Behcet Uz Children's Hospital, Izmir, Turkey2Katip Celebi University, Ataturk Teaching Hospital, Izmir, Turkey3Merkez Efendi Hospital, Manisa, Turkey4Torbali Hospital, Izmir, Turkey
Background
Having the potential for anti‐D alloimmunization, patients with weak RhD phenotype need to be taken additional care in blood grouping practice.
Aims
In this study, it is purposed to investigate the blood samples for the determination of RhD variants that were not identified in certainty during routine blood grouping process
Methods
An advanced RhD typing was performed in the red cell samples of the patients with weak RhD phenotypes in routine blood typing by using a commercial kit (ALBAclone ® Advanced Partial RhD, Alba Bioscience, UK) based on indirect agglutination.
Blood samples (n = 17) with weak RhD phenotype were collected from three unaffiliated hospital based transfusion centers. Advanced RhD typing was performed with tube method according to the producer recommendations at first. Then, typing was repeated in anti human globulin containing gel microcolons (Across Gel, Dia Pro, Turkey). Eleven different monoclonal antibodies derived from the in vitro culture of the IgG secreting human/mouse heterohybridomas were used for the differantiation of weak RhD phenotypes.
Results
While two patients were remained as unidentified, weak D Type 1&2 phenotype detected in 3 patients and partial RhD phenotypes were detected in 11 patients. Seven of the patients in whom partial Rh D phenotype determined were identified as DHK&DAU-4 phenotype and DFR phenotype was determined in remaining four patients. There was no discrepancy for indirect agglutination results between the tube and gel microcolon methods
Conclusions
RhD antigen is of great importance for transfusion medicine because of the high immunogenicity. The variants of RhD antigen need to be investigated because of the risk of alloimmunization in patients, particularly with pregnancy. Although the molecular typing is more efficient for solving the problem, advanced serotyping of RhD variants would be useful for the laboratories with limited sources.
P‐551
International Transfusion EQA Promotes Blood Group Reference Ability
Liu YL , Zhen LZH , Wu MHW , Xue MX , Ma LM , Liu YCHL
Jiangsu Province Blood Center, Nanjing, China
Background
Blood group reference in our laboratory started from 2001. In order to achieve rapid improvements in technical skills and to promote clinical application, our laboratory participated in the National Transfusion EQA Program (Shanghai, China) since 2003 and the International Transfusion EQA programs held by the Royal College of Pathologists of Australia (RCPA) during 2009–2013. The RCPA‐QAP had strict requirements on the involved laboratories and brought great improvements on detection techniques. Over 400 blood group reference laboratories participated in the program worldwide.
Aims
To analyze the feedback results from the RCPA‐QAP during 5 years and find the shortcoming of the test work and promote our technical approaches in blood group reference.
Methods
Our laboratory technicians were performed via serological methods in samples received from the RCPA. RCPA‐QAP tests included confirmation of patient information; identification of blood type antigens on red blood cells (3–25 antigens per sample), screening and identification of unexpected antibodies in patient serum, blood compatibility tests between patient serum and red blood cells from donors in order to optimize blood‐matching, baby cell blood type antigen tests, antibody absorption tests, HDN identification and blood compatibility, etc. Each examination and report was completed by an independent investigator. The feedback report was discussed by all members and consensus was reached.
Results
A total of 19 batches of samples were tested and reported and 10 (52.63%) batches reached full scores; qualified ratio of blood match tests was 94.74% (18/19). Thirty‐four sorts of irregular antibodies were identified in 19 batches involving 8 blood type systems. Total correct detection rate of antibodies were 85.3% (29/34); false negatives were found in 4 batches (five samples, 14.7%). Five (24.7%) samples were not exclusively identified (multiple choices); errors involving antibody identifications were detected in 9 (47.37%) batches.
Summary
The samples for RCPA‐QAP tests came from the clinic, which were real, complicated and representative. The feedback reports contained details of the design, analysis, methods and features of the antigens and antibodies in each sample, which were helpful to the examinees. During the participation of RCPA‐QAP, we purchased necessary equipment and reagents, learned new methods and promoted our comprehensive abilities in blood group reference. During 2009–2014, rare blood types from the donors including PP1Pk−, JKa−b−, Dib− and Fya−b−, rare antibodies from the recipients including anti‐Tja, anti‐Dib, anti‐Fya, anti‐Fyb were identified in our laboratory. These achievements guaranteed safe and effective blood transfusion in recipients with rare blood types. We look forward to working and communicating with more other laboratories in blood group reference programs.
P‐552
Immunohematological Challenges in Suspected Weak ABO Subgroup Blood Donor‐ A Case Report
Shashank Ojha SO1, Nagaraju P 1, Rajadhyaksha SB 2
1Tata Memorial Centre – ACTREC, Navi Mumbai, India2Tata Memorial Hospital, Mumbai, India
Background
Wrong ABO typing and ABO incompatibilities are the major cause of hemolytic transfusion reactions.There is a risk in doing only forward grouping and issuing blood on the basis of only immediate spin technique (IST) crossmatch. This may be important in case of weak ABO subgroups.We came across an interesting case in a blood donor to highlight this fact.
Aims
To highlight the importance of doing both forward and reverse grouping in blood donors to detect any discrepancy and prevent wrong blood typing. To discuss the approach for resolution of discrepancy in weak ABO subgroups. To highlight the necessity of doing AHG crossmatch to avoid missing ABO incompatibility in such cases
Methods
Forward and reverse blood grouping in donor and IST and AHG crossmatch was done by conventional tube technique as described in AABB Technical Manual(18th ed).For resolution of discrepancy adsorption‐ elution technique as well as Hemagglutination Inhibition technique for saliva secretor status was done as per methods given in AABB Technical Manual (18th ed). For further confirmation, retesting of forward and reverse blood grouping in donor and AHG crossmatch was done using Gel card system as per manufacturer's instructions. Samples were also tested in reference laboratory of National Institute of Immunohematology Mumbai for confirmation of findings.
Results
A discrepancy was observed in forward and reverse grouping by conventional tube technique and Gel card system. By forward grouping donor blood group was B Rh(D) positive and by reverse grouping it was AB. The discrepancy was resolved by adsorption‐ elution technique which showed the presence of weak A antigen. However Hemagglutination Inhibition technique for saliva showed donor as non secretor for A,B and H substances. From interpretation of above result probable donor blood group was AmB Rh(D) positive. Cross matching with serum of B Rh(D) positive patient gave compatible reaction with IST and 4+ reaction with AHG at 37°C. However Cross matching with serum of AB Rh(D) positive patient gave compatible reaction with both techniques.
Conclusions
The above case highlights that both the forward (red cell) grouping and reverse(serum) grouping are important to detect any ABO discrepancy and prevent wrong blood typing This kind of discrepancy is common in donors with weak ABO subgroups. The serologically determined weak ABO phenotypes require confirmation through genomic analysis. Secondly, blood of such donors should be cross matched with AHG technique to prevent ABO incompatible transfusion. AHG crossmatch acts as a second check to detect any such incompatibility missed in blood grouping and thus prevent ABO incompatible transfusion which may cause severe hemolytic transfusion reaction in the patient.
P‐553
Abstract Withdrawn.
P‐554
Distribution of Clinically Relevant Erythrocyte Antigens Among Blood Donors of Republika Srpska
Guzijan GG , Jukic JB , Mitrovic MS , Selak-Ðukelic SB
Institute for Transfusion Medicine of Republika Srpska, Banjaluka, Bosnia‐Herzegovina
Background
Identifying voluntary blood donors with rare phenotype characteristics is the basic precondition for creating a registry of blood donors with rare blood groups.
Aims
Determining the presence of phenotypes in the clinically most relevant blood group systems in regular blood donors at the Institute for Transfusion Medicine of Republika Srpska, the goal of which is to create a national registry of blood donors with rare blood groups.
Methods
Determination of antigens in the Rh system was performed by the automatic micro plate method, as well as by gel method. Determination of antigens in other blood group systems Kell, Kidd, Duffy, MNS, Lewis and Lutheran was performed by the gel method and test tube method. Altogether 384 blood donors were screened between 2012 and 2013.
Results
The analysis of Rh phenotypes showed most of the examinees were found to have the Rh phenotype CcDee,29.7% and 26.0% the ccddee, and the least of them had the Rh phenotype ccddEe 0.8%, Ccddee 1.6% and ccDee 2.3%. The antigen Cw was proved to be in 13 donors (3.4%), while the antigenP1 was detected in 291 donors (75.8%). In analyzing the Lewis antigen system, most of the blood donors were found to have the phenotype Le(a−b+), 74.0%.By analyzing the Lutheran antigen system, the phenotype Lu(a−b+) was detected in most of the donors typed for Lutherean antigens, in 93.0% of them. One individual was found to have the phenotype Lu (a+b−) (0.3%), and two donors the phenotype Lu(a−b−) (0.5%). The analysis of the Kell antigen system showed most of the donors were of the phenotype kk,93.2%, one individual was found to have the phenotype KK (0.3%) and 25 donors the phenotype Kk (6.5%). In analyzing the Kidd antigen system, most of the donors were found to have the phenotype Jk(a+b+),46.6% and 29.4% the Jk(a−b+), whereas24.0% had the phenotype Jk(a+b−).According to the study of MNantigen in the MNSsystem, most of the donors were typed as MN, 50.0% and 33.9% as MM, while the phenotype NN was detected in 16.1% donors. Analyzing the Sand santigens in the MNSsystem, the phenotypes Ss were found in most of the donors, 49.0% and ss in 43.2%, whereas the phenotype SS was detected in 7.8% donors. The analysis of the Duffyantigen system showed most of the donors were of the phenotype Fy(a+b+), 46.6% and34.6% of the Fy (a−b+),while the phenotype Fy (a+b−) was observed in 18.8% donors.
Conclusion
Data on the distribution of clinically relevant erythrocyte antigens among regular blood donors in the Republika Srpska is in line with the data set out in the literature for the white race.
P‐555
Immune Alloantibodies for Erythrocytes Antigens Detected Among RHD Positive Pregnant Women from 2011 To 2013 in RCBDT in Katowice
Szczudlo K , Skowronska J , Drybanska B
Regionalne Centrum Krwiodawstwa i Krwiolecznictwa w Katowicach, Katowice, Poland
Background
In Poland it is mandatory to conduct screenings for presence immune alloantibodies among pregnant women, this includes RhD positive and RhD negative. Screening conducted two times during the pregnancy allows early detection of haemolytic disease of fetus and newborn (HDFN) and implementation of appropriate clinical management.
Aims
Evaluation of the realization of the screening program for detecting immune alloantibodies among pregnant women in the area of supervision of the subordinate RCBDT in Katowice analysis and usage of the detected antibodies in HDFN diagnosis.
Methods
From 2011 to 2013 in the tested area 122,224 births occurred. Statement received by field serology laboratories supervised by RCBDT in Katowice showed that presents of immune alloantibodies was tested among 37 346 (30.5%) pregnant women, this include 17 645 (17%) RhD positive. Immune alloantibodies were detected in 196 (0.52%) pregnant women (this include 78 RhD positive). Identification of detected alloantibodies was conducted in Consultation Laboratory in RCBDT in Katowice with the use column micro method DiaMed (AHG, IgG and ENZ test) and tube method.
Results
Figure no 1.
Caption 1: Specifies and antibody titers tested among RhD positive women.

Conclusions
1. Demographic structure shows that RhD positive pregnant women pose approximately 83% polish population. The test for the presence of immune alloantibodies should be subjected to the group of 100,000 women and conducted analysis indicates that the test was performed among 17% pregnant women. Due to this fact a greater number of RhD positive women should be immunohematology tested during pregnancy.
2. The most commonly detected specifies immune alloantibodies among RhD positive pregnant women are: anti‐M with MNS group systems, anti‐E, anti‐c, anti‐Cw with Rh group systems and anti‐K with Kell group systems.
3. Alloantibodies anti‐M, anti‐E and anti‐Cw being common possess low titers and according to medical data the antibodies rarely cause HDFN.
4. Alloantibodies anti‐K and anti‐c, with maximal level was respectively 256 and 64 pose a serious threat of casing sever HDFN.
P‐556
Surviving Hemolytic Disease of the New Born from High Titer Anti‐E (1:254), A Case Report
Tsai S-YT1, Chang MT 1, Lin KHL 2, Huang HCH 2, Chen CPC 1, Lin ML 1
1Mackay Memorial Hospital, New Taipei City, Taiwan2Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
Background
The prevalence of hemolytic disease of the newborn (HDN) caused by maternal alloantibodies has been estimated to be 0.01%, so routine prenatal screening for alloantibodies was considered irrational in the Taiwanese population. Anti‐E and anti‐E+c were the most important alloantibodies resulting in HDN in Taiwan. (Wu, Chu, Chang, Shih, & Peng, 2003). Reviewing medical records of 1983‐1984 in MMH (Lin‐Chu et al., 1987) 6 cases of HDN due to maternal alloantibodies were found, in which 3 cases were anti‐E.
Aim
To report a baby survived with high titer maternal anti‐E.
Case report
A 38 years old mother, P3G3, has history of blood transfusion before pregnancy. Her first baby suffered from anemia and jaundice after normal spontaneous delivery at term and received blood transfusion. Her second baby born with hydrops fetalis expired within several hours following birth in 2006. She was found carrying anti‐E+ Mia. The AHG titer of anti‐E was 1: 2048 and anti‐ Mia 1:32. During her third pregnancy, her anti‐E antibody titer was consistently 1: 256 in eight tests in 2014. Since the mother Rh phenotyping was CcDe, Mia negative; the father was CcDEe, Mia negative, we hope that the fetus was E negative. Therefore, anti‐E titer and fetal Middle Cerebral Artery Peak Systolic Velocity (MCA PSV) were closely followed. MCA PSV is an important parameter in assessment of fetal anemia, showed only once slightly increased velocity out of six tests in this case. Anti‐E titer did not increase during pregnancy.
Her baby was born at 39 weeks gestation with Apgar score 9à 10, hemoglobin11.3 g/dl and total bilirubin 9.6 mg/dl. The baby Rh phenotype was E positive (CcDEe); DAT positive and anti‐E was eluted. Mia was negative on baby as expected. IVIG 1000 ng/kg and phototherapy were given the next day. Under the therapeutic regimen, the patient's condition improved gradually and discharged 10 days after birth.
Conclusion
We assume anti‐E+ Mia were induced by the pregnancy or blood transfusion. It is surprisingly that the baby survived with 1:256 anti‐E maternal antibody, it is also surprising that the titer did not increase during the pregnancy although baby is E positive. In Western country when mother anti‐E titer is higher than 1:64, intrauterine transfusion were performed in most of the cases after MCA PSV and amniocentesis evaluation. (Joy, Rossi, Krugh, & O'Shaughnessy, 2005)
Joy, S. D., Rossi, K. Q., Krugh, D., & O'Shaughnessy, R. W. (2005). Management of pregnancies complicated by anti‐E alloimmunization. Obstet Gynecol, 105(1), 24–28. doi: 10.1097/01.aog.0000149153.93417.66.
Lin‐Chu, M., Broadberry, R. E., Chyou, S. C., Hung, H. Y., Shen, E. Y., Hsu, C. H., & Ho, M. Y. (1987). Hemolytic disease of the newborn due to maternal irregular antibodies. Taiwan Yi Xue Hui Za Zhi, 86(6), 645–648.
Wu, K. H., Chu, S. L., Chang, J. G., Shih, M. C., & Peng, C. T. (2003). Haemolytic disease of the newborn due to maternal irregular antibodies in the Chinese population in Taiwan. Transfus Med, 13(5), 311–314.
P‐557
Performance Comparison of Fully Automated Systems Bio‐Rad IH‐500 vs Ortho Autovue® Innova
Schürmann M
ZLM GmbH, Essen, Germany
Background
Process optimization of automated immunohaematologic analyzers is becoming increasingly important. To achieve a faster availability of results, innovative techniques are required in order to speed‐up the overall process time around the standardized incubation time. The IH‐500's robotic transport arm is a genuinely innovative technique. The IH‐500's process flow has been evaluated and compared to Ortho AutoVue® Innova.
Aims
Prior to the launch of the fully automated analyzer IH‐500 from Bio‐Rad, a device comparison was performed with the Ortho AutoVue® Innova system to evaluate and optimize sample processing.
Methods
The evaluation focused on the comparison of the two systems and sample processing, as well as on the IH ‐500 device software ergonomics. The protocols demanded the testing of various blood group profiles and cross‐matches, which included both routine and emergency samples, and which were performed both in batch runs and continuous sample flow.
Results
Testing 50 routine samples (45 blood groups without rhesus formula, 5 blood groups with rhesus formula) in a batch run on IH‐500 convinced with a well‐structured process flow, which has shown to grant results being available 25% faster than the AutoVue®. Testing a continuous sample flow, on the other hand, showed that the result time of IH‐500® is by 28.85% longer as provided by the AutoVue®, increasing alongside the number of tested samples (in consideration of discrepant centrifugation times). The interruption of a batch run of 30 routine samples (30 blood groups including rhesus formula) at the time of pipetting the 4th sample in order to load an emergency sample, and loading another emergency sample 8 min after the first showed almost identical results: the average emergency result time on AutoVue® was 48.5 min, on IH‐500 46.5 min.
Conclusions
The comparison shows that the IH‐500's strengths lie particularly in the area of the batch run testing. Even when inserting emergency samples in a batch schedule the IH‐500 performs a better process time and faster availability of results. When processing a continuous sample flow, there are partially strong temporal differences to the disadvantage of the IH‐500. Furthermore, the IH‐500 convinces with excellent photo quality and subsequently unambiguous evaluation, as well as intuitive interaction with the device software.
P‐558
Clinical Case of Hemotransfusion Complicated by Hemolytic Reaction with Allotypic Antigens Kidd Incompatibility
Dubinkin V , Kalmykova O , Azimova M , Gaponova T
Federal State‐Funded Institution National Research Center for Hematology of the, Moscow, Russian Federation
Background
The test for individual compatibility (crossmatch) conducted in vitro in gel cards is a high‐sensitivity test, enabling to minimize the risks of post‐transfusion complications. At the same time, the biological features of allotypic antigens of some blood groups may not allow detecting incompatible pairs donor ‐ recipient and may lead to a hemolytic reaction during the hemotransfusion.
Aims
Retrospective analysis of the reason for incompatible hemotransfusion.
Methods
Patient H. 49 years old, with pancytopenia, splenomegaly, liver cirrhosis, hepatitis C; evolving reduction of blood values, dependence on the transfusion of hemocomponents. The medical history had numerous hemotransfusions with an episode of acute post‐transfusion complication, 4 pregnancies. There were 4 transfusions of erythrocytic suspension with individual selection of erythrocytes. Compatibility (crossmatch) was conducted, indirect antiglobulin test (IAT) LISS/Coombs AHG. All immunohematologic analyses were conducted in the gel cards of the company Dia‐Med in accordance with the manufacturer's instructions. At the first signs of homolytical reaction (biological test in vivo), hemotransfusion was immediately stopped.
Result
Patient H's erythrocytes phenotype ‐ 0(I)CCDeeKk. After hospitalization anti‐erythrocyte antibodies with anti‐Rhc specificity were detected. The individual selection of erythrocytes was conducted by allotypic antigens of the systems: A'0, rhesus, Kell, and crossmatch IAT LISS/Coombs AHG. The immunohematologic values of hemotransfusion are given in Table 1. Additional phenotyping of recipient H's erythrocytes using Kidd system revealed her homozygosity Jkb/Jkb. Retrospective phenotyping of the donors’ erythrocytes showed the presence of allotypic antigen Jka in the donors, in all cases of hemotransfusions. Homozygous erythrocytes of donor by Jka during the first hemotransfusion, obviously, were the reason for sensibilization of recipient H. and the appearance of additionally anti‐erythrocyte antibodies anti‐Jka, which caused intravascular hemolysis with subsequent hemotransfusions of heterozygous erythrocytes of the donors by Kidd. Heterozygous native erythrocytes of the donors Jka/Jkb during the crossmatch IAT did not agglutinate with the serum of recipient H. At the same time, the treatment of the donors’ erythrocytes with bromelain could clearly show incompatibility in these cases as well. The use of several 11 cellullar panels of standard erythrocytes made it possible to confirm the presence of anti‐erythrocyte antibodies with anti‐Rhc + anti‐Jka specificity.
Caption 1: Transfusion of erythrocytes to recipient H.

Conclusion
The methods of crossmatch test for the donor and the recipient in vitro in the gel cards do not guarantee the absence of hemolytic reaction during the hemotransfusion. Particularly, anti‐erythrocyte antibodies with anti‐Jka specificity may not be revealed on heterozygous erythrocytes of the donor by Kidd system in case of standard conditions of setting IAT in the gel cards. It is necessary to take into consideration the presence of minor mutually exclusive (antithetical) allotypic antigens of erythrocytes in the homozygous and heterozygous states. The biological test in vivo during hemotransfusions is an important element to ensure immunological safety and minimize the risks of the development of post‐transfusion complications, caused by the hemolytic reaction.
P‐559
Provision of Immunological Safety of Multiple Erythrocytes Transfusions for Hemotological Patients
Dubinkin V , Kalmykova O , Azimova M , Gaponova T
Federal State‐Funded Institution National Research Center for Hematology of the, Moscow, Russian Federation
Background
Immunological safety of hemotransfusions is based on test version in vitro for cross‐matching by different methods (detecting anti‐red‐cell antibodies) and biological test in vivo to reduce the risks of post‐transfusion complications. As well as selection of erythrocytes of the donor and the recipient by allotypic antigens (erythrocytes phenotyping) to reduce the risks of alloimmunization of the recepient.
Aims
Compassion of different methods of cross‐matching and erythrocytes phenotyping to ensure the immunological safety of multiple hemotransfusions for hematological patients.
Methods
The clinics of the Federal State‐Funded Institution National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation in 2014 performed 5829 hemotransfusions to 1321 patients (at the age from 20 to 76 years, M = 43), with diagnoses: hemophilia A, acute leukemia, aplastic anemia, thrombocytopenia, lymphoma, hemolytic anemia. In 93% of cases erythrocytes for transfusion were selected by 10 transfusion dangerous antigens: A, B, D, C, c, Cw, E, e, K1, K2. 3922 hemotransfusions were performed with crossmatch for 809 patients with 33% polyglucin. 1907 hemotransfusions were performed with crossmatch for 512 recipients in the gel cards Dia‐Med indirect antiglobulin test (IAT).
Result
Of 1321 hematological patients there were 13 cases with autohemolysins (1%) and 9 cases with anti‐erythrocyte alloantibodies (0.7%): 2‐ anti‐D, 2‐ anti‐K1, 1‐ anti‐D+C, 1‐ anti‐D+E, 1‐ anti‐Cw, 1‐ anti‐N, 1‐ anti‐M. During the crossmatch in the gel cards in the laboratory conditions in 3.1% of cases the erythrocytes of the donors were not compatible with the recipients and were not used for hemotransfusions. Meanwhile, there was not a single case of post‐transfusion reaction. At the same time, during the crossmatch with the help of polyglucin for 3922 hemotransfusions there were 9 post‐transfusion reactions (0.23% of cases). Selection of erythrocytes by 10 main transfusion‐dangerous antigens enabled to exclude sensibilization of the recipients by allotypic antigens of the donors’ erythrocytes.
Conclusion
The immunological safety of hemotransfusions to hemotological patients requiring multiple transfusions of erythrocytes can be provided only by means of a high‐sensitivity crossmatch. To minimize the risks of possible allosensitization of the patients one need a selection of erythrocytes by the main transfusion‐dangerous antigens of erythrocytes taking into consideration the homozygotes by antithetical pairs of allotypic antigens.
P‐560
Immunohaematological Diagnostics and Blood Treatment in a Rare Mixed Type Autoimmune Haemolytic Anaemia Caused by Autoantibodies Anti‐M of the MNS Blood Group System – A Case of 15‐year‐Old Boy
Bochenek-Jantczak D1, Szczudlo K 1, Domagala G 1, Drybanska B 1, Adamczyk P 2, Pobudejska-Pieniazek A 2, Szczepanski T 2
1Regionalne Centrum Krwiodawstwa i Krwiolecznictwa w Katowicach, Katowice, Poland2Samodzielny Publiczny Szpital Kliniczny nr 1, Zabrze, Poland
Intruduction
Autoimmune haemolytic anaemias(AIHA) which are caused by destroying red blood cells by autoantibodies pose a serious problem both diagnostic and clinical. The intensity of the course of disease differs from mild to serious life‐threatening haemolysis. The diagnostics which includes detection of antibodies and the evaluation of their immunohaematological features is crucial for identification and classification of AIHA. It is also extremely important in the process of predicting and monitoring the disease intensification and the implementation of effective treatment.
Aims
The presentation of autoimmune haemolytic anaemiacase with autoantibodies of IgG and IgM classes with rapid course with acute haemolysis.
Methods
Serological diagnostic of the case included: DAT execution with monospecific reagents (anti‐IgG, ‐IgM, ‐C3c, ‐C3d), evaluation activity of red blood cells of eluate, testing serum in IAT micromethod column and tube method (saline test).
Case report
15‐ year‐old boy in a bad condition was admitted to clinic in order to diagnose and treat the immune haemolytic anaemia. In a medical history of the patient just before the hospitalization the fever and symptoms of upper airways infection had occurred. The intensification of ailments, increasing weakness and yellowish discoloration of skin was the cause of previous hospitalization in a regional hospital where a rapid decrease of haemoglobin in 4.5 g/dl in a day was found. Then the child was taken to the clinic in a bad condition with haemoglobin of 4.8 g/dl and hyperbilirubinaemia of 142 μmol/l. Due to the suspicion of AIHA the immunohaematological diagnostics was commissioned as well as blood selection in Regional Centre of Blood Donation and Treatment in Katowice. During the tests it was asserted that:
1. There are autoantibodies anti‐M on the blood cells and in the serum and components of C3c and C3d complement.
2. There are cold type autoantibodies in the serum.
The titre of autoantibodies value predicted a high risk of haemolysis after blood transfusion. It was a treatment which included empirical antibiotic therapy, steroid therapy and symptomatic treatment. Irradiated,leukoreduced red blood cells which was compatible with ABO and Rh system without M‐antigen was selected and transfused for patient:
1. In the first 24 h – three units.
2. In the second 24 h – two units.
3. In the third 24 h – one unit.
The child's condition stabilised in the fourth 24 h and the standardization of morphology parameters was obtained after 10 days. At the present the condition of the patient is good without any ailments.
Report
Autoimmune haemolytic anaemia is a big challenge both diagnostics and clinical especially in case of children. Because of the fact that the immunohaematological test results not always confirm a patient's symptoms, in the AIHA diagnostics each case must be considered individually.
Conclusion
Good cooperation between laboratory diagnosticians and clinicians allows to conduct a careful analysis of the test results which effects in a significant increase of opportunities to make an immediate appropriate diagnosis and cure the patient.
5.2 Red Cell Immunology: Molecular
P‐561
Genotyping of Eighteen RBC Blood Group Alleles Using Multiplex Snapshot Reaction and Fragment Assay for Donors Screening
Xu XG , Hong XZ , Ma KR , Lan XF , Ying YL , He J , Zhu FM , Lv HJ
Blood Center of Zhejiang Province, China, Hangzhou, China
Background
Genotyping of RBC blood group alleles is a more effective method than traditionally serologic assay in some situations, such as discrepantly serologic results, lack of commercial antibodies, screening of rare blood group donors, and so on. Many molecular techniques (AS‐PCR, PCR‐RFLP, real‐time PCR, etc.) are applied in some blood group systems owing to the differences of single nucleotide polymorphisms (SNP) between antithetical alleles, but these techniques are almost low‐throughput approaches or only focusing on single blood group system and not suitable for large numbers of donors screening.
Aims
To overcome the limitation of conventional serology and establish a high‐throughput and low costs assay, we developed a multiple SNaPshot reaction and fragment assay to analyze eighteen SNP in eleven blood group systems.
Methods
Fourteen pairs of primers used in two multiplex PCR were designed to amplify the DNA fragments containing SNPs of blood group alleles. The multiplex PCR was optimized and the PCR products were confirmed and purified. Eighteen internal probes carrying different length poly‐A tail at 5′‐end to use in SNaPshot reaction, were also designed according to the SNP sequences. The SNaPshot reaction was performed with probes and the genotype was discriminated after fluorescence‐based fragment analysis. The genotypes of SNaPshot were compared to that of direct DNA sequencing method developed by our previous research.
Results
Two multiplex PCR systems were able to simultaneous amplify fourteen fragments encompassing eighteen SNP to detect the following alleles: FY*01/FY*02, FY*01N01, FY*01W.01, DO*01/DO*02.01, DO*01/DO*02.02, DO*01/DO*02.03, DO*04, DO*05, CO*01/CO02, KEL*01.01/KEL*02, JK*01/JK*02, LW*01/LW*02, SC*01/SC*02, DI*01/DI*02, LU*01/LU*02, MNS*01/MNS*02, MNS*03/MNS*04, YT*01/YT*02. The clear and typical profile was obtained by SNaPshot reaction followed by capillary electrophoresis on ABI 3730 DNA analyzer. Eighteen genotypes can be read from the GeneMapper electropherograms and the results were found to be concordant with direct DNA sequencing completely.
Summary
We developed a multiplex SNaPshot reaction and fragment assay for simultaneously genotyping eighteen RBC blood group alleles. It can provide a simple and high‐throughput tool for DNA genotyping in donors screening.
P‐562
New Silent RHD Allele and Weak D Alleles: Molecular Characterization and Associated Antigen Density
Silvy M1, Filosa L 2, Beley S 1, Granier T 1, Chiaroni J 1, Bailly P 1
1EFS Alpes‐Méditerranée, UMR 7268, Aix‐Marseille Université, Marseille, France2EFS Alpes‐Méditerranée, Marseille, France
Background
RhD is one of the most clinically important blood group antigens in the field of transfusion and prevention of fetomaternal incompatibilities. New variant RHD alleles are regularly identified and their characterization is essential to ensuring patient safety.
Aim
In an effort to expand the known RHD repertoire, we characterized six novel variant RHD alleles.
Material and methods
Samples reported to show D phenotype ambiguity by routinely used serologic analyses (n = 5) were addressed for characterizing the molecular basis of unusual D phenotypes. The sixth sample was investigated for confirming serological rare D‐C+E‐c‐e+ phenotype. First intension genotyping was performed by using BeadChip RHD kit (BioArray Solutions). RHD exons were sequenced and antigen density per red blood cell (RBC) was measured by flow cytometry using P3X249, LHM76/55, P3X241, and LHM169/80 antibodies (targeting epitopes 2.1 3.1, 5.4, and 6.3 respectively).
Results
Since RHD genotyping using BeadChip RHD kit failed to detect any polymorphism, RHD exons were sequenced to resolve typing inconsistency. Sequencing revealed six new RHD alleles bearing substitution(s) that occurred within the RHD coding sequence. Five samples had a single substitution, i.e. 73A>T (I25F), 143A>G (Y48C), 668T>C (F223S), 761C>G (S254X) or 1229T>C (F410S), and one had two polymorphisms: 105C>G and 1195G>A (D35E, A399T). All new alleles were apparent homozygous except RHD*RHD(I25F) which was found in trans to RHD*Dpsi. Ethnicity of patients/donor was unknown except for patient with RHD*RHD(F223S) who was from African ancestry.
The 761C>G transversion is predicted to encode a premature stop codon in RhD confirming the D‐ phenotype. Other amino‐acid (aa) changes encoded by the new alleles were located in the transmembrane‐ or intracellular‐segments of the RhD protein, a finding consistent with the characteristic location of weak D phenotypes. Antigen densities of RhD were ranging from 450 to 8804.
The aa changes located in first and second transmembrane‐segments (I25F, D35E and Y48C) may affect structure and/or stability at membrane level either directly the folding of the single RhD subunit or intersubunit interactions. A399T and F410S are located in the cytoplasmic tail of the protein in the Rh‐ankyrin complex known to display aa changes associated with the deficiency of RhD antigen expression. Moreover, the 1229T>C transition encoding F410S change is located within the Exon 10 consensus acceptor splice site and could affect mRNA splicing. Replacement of Phenylalanine by Serine at position 223 is predicted to occur in the fifth transmembrane‐segment. F223 is an aromatic hydrophobe aa involved in extracellular vestibule and its replacement by a small uncharged polar aa could explain the lower antigen density observed (450 antigen/RBC).
Conclusion
We described one novel silent RHD allele and five novel weak D alleles. The assignment of most of these new and rare alleles as weak D alleles was based on a combined consideration of the existence of a doubtful D phenotype, the location of predicted amino acid changes in the RhD protein and the number of D antigens per cell. Nonetheless, this should be regarded as speculative until a description of the immunogenicity against regular D is made available.
P‐563
Disruption of the Start Codon of the FUT1 Gene is the Molecular Background of the First Reported Case of European Non‐Secretor ‘Bombay’ Phenotype with a Weak H Antigen Expression
Letizia G, Vrignaud C , Peyrard T
Institut National de la Transfusion Sanguine (INTS), Paris, France
Background
In 1974, Rodier et al. reported the case of an Oh (so‐called ‘Bombay’) individual of Western Europe ancestry whose red blood cells expressed minute amount of H antigen, despite a non‐secretor status (Biomedicine. 1974;21(7):312-6). The molecular basis of the H antigen was unknown at that time, but the authors perspicaciously deduced that ‘Bombay’ phenotypes could be heterogeneous, with various levels of H expression.
Aims
To further investigate this index case by analyzing the molecular background responsible for H deficiency.
Methods
Frozen blood sample stored in our cryobank was thawed and phenotyping results were verified by standard serological methods and use of high‐titer polyclonal anti‐H from Indian ‘Bombay’ subjects to investigate H antigen expression. Automated genomic DNA extraction was performed. Coding regions of FUT1 (GenBank #: NM_000148.3) and FUT2 (NM_000511.5) were amplified by PCR and directly sequenced.
Results
Serological investigation confirmed a O, non‐secretor Le(a+b−) phenotype and a weak H expression. Apparent homozygosity was identified for a FUT1 mutation in the start codon: c.1A>C, predicted to switch the methionine initiation codon (AUG) to a leucine codon (CUG). The non‐secretor status was confirmed by homozygosity for a null FUT2 allele, commonly encountered in Caucasians (FUT2*01N.02 or se428).
Summary/Conclusions
The c.1A>C mutation was identified in FUT1 gene of a partially H‐deficient, non‐secretor European individual. This suggests that the fucosyl‐transferase activity of FUT1 enzyme is not entirely abolished. As a result, translation of FUT1 mRNA unexpectedly occurred despite an altered start codon. This mutation is unsurprisingly predicted to be deleterious by the Polyphen and SIFT applications. However, Peabody showed in 1989 that experimental modification of AUG start codon to CUG in the DHFR gene did not totally abolish the expression of the dihydrofolate reductase protein: the first amino‐acid actually remained a methionine, suggesting a ‘wobble’ initiation mechanism by mispairing between the Met‐tRNAiMet anticodon and CUG codon in a favorable Kozak context. In addition, evidences were recently published (Starck SR et al., Science. 2012;336(6089):1719-23) for a specific leucine‐dependent translation initiation pathway. These are potential explanations here for a partially functional FUT1 enzyme despite the c.1A>C mutation within the start codon. Translation initiation at the next downstream AUG codons seems unlikely since the first one is out of frame, and the second one (in‐frame) would lead to a lack of the transmembrane protein domain. Moreover, no other isoform of FUT1 protein has ever been described. The c.1A>C mutation appears to be rare as it is not referenced in the Blood Group Antigen Gene Mutation Database (BGMUT ‐ dbRBC). Whole‐exome sequencing studies confirm its rarity: it has been identified only once to date, at heterozygous state, among exome studies (in ClinSeq project, 355 participants from European descent). Finally, in a series of H‐deficient subjects that we have studied (unpublished data), the same mutation was found at heterozygous state in another European individual, associated to a frameshift mutation of FUT1 on the other allele; this subject expressed as well minute amount of H antigen, which is consistent with the results of the case reported here.
P‐564
MALDI‐TOF MS for MNS Typing – High Pheno/Genotype Concordance in 5743 Swiss
Meyer S1, Vollmert C 2, Trost N 1, Gottschalk J 1, Ries J 1, Marcovic A 1, Infanti L 3, Buser A 3, Amar El Dusouqui S 4, Castelli D 5, Weingand T 6, Sarraj A 7, Braisch MC 8, Thierbach J 8, Frey BM 1, Gassner C 1
1Blood Transfusion Service Zurich, Swiss Red Cross, Zurich‐Schlieren, Switzerland2Agena Bioscience, Hamburg, Germany3Blood Transfusion Service beider Basel, Swiss Red Cross, Basel, Switzerland4Blood Transfusion Service Genève, Swiss Red Cross, Genève, Switzerland5Blood Transfusion Service Svizzera Italiana, Swiss Red Cross, Lugano, Switzerland6Blood Transfusion Service Zentralschweiz, Swiss Red Cross, Luzern, Switzerland7Blood Transfusion Service Neuchâtel‐Jura, Swiss Red Cross, Neuchâtel, Switzerland8Blood Transfusion Service Ostschweiz, Swiss Red Cross, St. Gallen, Switzerland
Background
Transfusion of alloimmunized patients and its prevention may ideally be addressed by expanded blood group antigen matching protocols. For this purpose, beside ABO and RhD, a reasonable set of considerable antigens may include RhC/c(Cw)E/e, K/k, Jk(a, Fy(, M/N and S/s. Blood group genotyping has proven its capability in this context. However, in comparison to K/k, Jk(a and Fy(1, published performance data for MNSs genotyping are underrepresented.
Aim of the project
Estimate performance of MALDI‐TOF MS for MNSs genotyping: Resulting genotypes and existing serological values for MNSs should be compared and checked for concordance.
Methods
Genotyping relied on MALDI‐TOF MS based SNP‐detection at coding nucleotides 59(C/T) and 72(T/G) for M/N and G59 for He (actually on GYPB), 140(C/T/A) for Vw/Hut and 230(C/T) for Mt(a+) on GYPA, and 143(T/C) for S/s, intron 4+5(G/T) for U+W and intron 1+9103/+15014(C/T, C/G) zygosity typing for the ‘GYPB deletional U‐ phenotype’, on GYPB. The approach included 10 antigens of the MNSs blood group system, encoded by 8 SNPs on 10 GYPA/B alleles, multiplexed into one reaction. All genotyping results were compared to existing standard‐serological MNSs values of 5743 Swiss donor‐samples. Generic and allele‐specific PCR‐SSPs and Sanger‐DNA sequencing revealed genetic backgrounds in cases with confirmed pheno/genotype discordances.
Results
Concordant MN phenotypes comprised into 1711 MM (with 1Vw), 2807 MN (with 1He, 4Vw and 10Mt(a)), and 1208 NN (with 4Vw and 4Mt(a+)) all located on GYPA (pheno/genotype concordance rate 99.88%, 5726 of 5743). Four original MM, 10 MN and 3 NN phenotypes showed discrepancies in comparison to genotyping of which 4 MM (1 no follow up), 3 MN (1 no follow up), and 3 NN were due to serological mistypings. Sequencing of 7 MM genotypes with discrepant MN phenotypes revealed presence of GYPA/B hybrid genes, resulting in 1 Mur(GYP.504)‐like, and 6 Sch(GYP.401)‐like alleles, all known to encode N‐like phenotypes, while GYPA*02(N) negative. Genotyping for Ss on GYPB delivered full concordance of pheno/genotypes for 619 SS, 2416 Ss and 2702 ss samples (concordance‐rate 99.90%, 5.737/5.743). Discrepancies were due to serological mistypings (2Ss, 1ss) and ‘genotyping errors’. We identified a G145A(Gly49Arg) mutation in the GYPB*03 primer binding‐site and two donors with a presumably new GYPB*03 null‐allele with a G218A(Gly73Asp) substitution (1Ss, 2ss).
Summary
MNSs phenotyping errors had approximately the same frequency as ‘genotyping errors’, which could all be explained by rarely occurring GYPA/B genetic variants, or newly discovered alleles. Consequently, ‘genotyping errors’ may rather be interpreted as specific ‘indicators’, than profane ‘errors’. No repetitive genotyping was done and still, there was no evidence at all for any technical SNP‐typing error judging all the 57,430 SNP‐genotypes, obtained in this study. Only 57 samples, on top of the 5743 with complete data sets, had single, or multiple SNP‐genotype drop‐outs, resulting in the low drop‐out rate of only 0.98% (no result). MALDI‐TOF MS based MNSs genotyping proved to be extremely practical, robust and accurate, and ‐ for donors ‐ may well be considered as a valid stand‐alone method and/or valuable addition for serotyping.
(1) Meyer S, et al, Transfusion. 2014 Dec;54(12):3198–207.
P‐565
A Novel Do Mutation Giving Rise to a Gy(A−) Phenotype; a Serological and Molecular Study
Karamatic Crew V1, Marais I 1, Thornton N 1, Rubbins H 2, Skidmore I 2, Daniels G 1
1IBGRL/BITS, Bristol, United Kingdom2NHS Blood and Transplant, Birmingham, United Kingdom
Background
The first antigen of the Dombrock blood group system (ISBT system 014) was reported in 1965 and now the system comprises nine antigens, all located on the Dombrock glycoprotein (ART4) of 314 amino acids and five putative N‐glycosylation sites. The glycoprotein is attached to the red cell membrane through a glycosylphosphatidylinositol (GPI) linked anchor. Two of the system antigens, Doa and Dob are polymorphic, whilst the remaining seven antigens are all antigens of high frequency. The gene encoding ART4, DO (ART4), is located on chromosome 12 at 12p12.3 and organised into three exons distributed over 14 kb of genomic DNA. The Gy(a−) phenotype (Donull) is defined by the complete lack of all of the Dombrock system antigens and is reported to originate from at least seven different molecular backgrounds.
Aims and methods
A prenatal Caucasian female with no known history of transfusion or previous pregnancies presented with an unidentified antibody to a high frequency antigen in her plasma. Serological investigation of red cells and plasma from the patient was performed by standard LISS tube and column agglutination techniques. Her genomic DNA was isolated from whole blood and used as a template for PCR amplification and direct Sanger sequencing of all of the coding regions of the DO gene.
Results
Serological tests identified the presence of anti‐Gya in the patient's plasma, reacting moderate strength by IAT with untreated cells and marginally stronger with papain treated cells. The patient's plasma was compatible with three examples of Gy(a−) cells by IAT. Red cells from the patient were found to be Gy(a−) [and Hy‐, Jo(a−), DOYA−, DOLG−]. DO sequencing confirmed the patient to be DO*02 (DO*B) homozygous. Additionally, a novel homozygous deletion c.710delA in exon 2 was identified. This deletion would introduce a reading frame shift and a premature termination of protein synthesis at p.Leu246. This would most likely result in a truncated Dombrock glycoprotein and consequently no expression of Dombrock antigens.
Conclusion
We report serological and genetic evidence for a Gy(a−) phenotype resulting from a novel molecular background. A patient with anti‐Gya in her plasma revealed a novel Gy(a−) genotype resulting from a deletion c.710delA, encoding p.Gln237ArgfsX10. This novel DO allele adds to our expanding knowledge of the genetic complexity of the Dombrock blood group system.
P‐566
‘Irish’ Bombay Phenotype: Compound Heterozygosity for Novel FUT1 Alleles
Lambert MD1, Tilley L 2, Ní Loingsigh S 1, Power J 1, Fitzgerald J 1, Thornton N 2
1Irish Blood Transfusion Service, Dublin, Ireland2International Blood Group Reference Laboratory, Bristol, United Kingdom
Background
The human blood groups of the ABO, H and Lewis systems are determined by oligosaccharides. α‐(1,2)‐fucosyltransferases (coded for by FUT1 and FUT2) synthesise the H antigen, which is the precursor molecule of the A and B antigens. The product of FUT1 is responsible for H expression on red blood cells, while that of FUT2 is responsible for H expression in secretions. H‐deficient phenotypes arise due to inactivating mutations in FUT1 and FUT2 genes. The H‐ phenotype is called the Oh (Bombay) phenotype. Transfusion support for patients with the Oh phenotype is problematic due to the presence of anti‐H in their plasma, the ubiquity of H antigen in recipients and the rarity of Oh phenotype donors.
Aims
We investigated a 57 year old male patient with a history of bladder carcinoma, who required blood for a transurethral resection of the prostate. Samples were referred for investigations as he grouped O RhD+ with a suspected allo‐antibody to a high frequency antigen. There was no known history of transfusion. The patient's two brothers were also investigated.
Methods
Initial ABO typing was performed by AutoVue Innova. Further phenotyping was performed by direct agglutination tube methods. Antibody investigation was performed by standard BioRad and LISS tube IAT using untreated and papain‐treated cells. Adsorption‐elution tests were carried out using high‐titre immune anti‐A and anti‐B (1/1048 and 1/512 respectively) and anti‐H. Elution was performed with Immucor Elu‐Kit II. The coding regions of FUT1 (exon 4)and FUT2 (exon 2) were sequenced using Sanger sequencing.
Results
The patient's cells were negative with all examples of anti‐A, anti‐B and anti‐A,B tested and were negative with anti‐H from Oh indivdiuals and also Ulex europaeus lectin. Anti‐H was detected in the patient's plasma, reacting by IAT and direct agglutination methods, with all panel cells. Only Oh phenotype cells (n = 6) were compatible. Anti‐A, anti‐B and anti‐H were not present in eluates prepared from the patient's cells following adsorption with the respective antibodies.
FUT1 sequencing revealed compound heterozygosity for two novel mutations: c.310C>T (p.Q104X) and c.496G>T (p.G166C). FUT2 sequencing revealed homozygosity for mutations associated with the FUT2*01N.02 allele. The same mutations were found in samples from both brothers and they were confirmed to have the Oh phenotype and anti‐H in their plasma.
Conclusions
We identified a patient and his two brothers, with apparent compound heterozygosity for two novel FUT1 mutations: c.310C>T introducing a stop codon at residue 104, and c.496G>T changing glycine to cysteine at position 166. Both mutations are predicted to produce nonfunctional enzymes since no A, B or H antigens could be detected on their red cells. Due to the lack of Oh donors in Ireland, the patient's brothers have been assessed as donors.
P‐567
Complete Gene Sequencing of ABO Blood Group by Next‐Generation Sequencing
Altayar MA , Halawani AJ , Kiernan M , Madgett TE , Avent ND
School of Biomedical and Healthcare Sciences, Plymouth University, Plymouth, United Kingdom
The ABO blood group system is the most clinical significant in blood transfusion and transplantation medicine. Due to naturally occurring antibodies, mismatched transfusion of blood can cause rapid transfusion reactions. ABO is one of the most complex and polymorphic blood group genes, with an ever‐increasing number of variant alleles. These variant alleles not only affect the specificity of the enzymes but also the activity of the enzymes, which might result in a weak phenotype. Therefore the determination of ABO alleles is important for the safety of blood transfusion and transplantation medicine.
Although high‐throughput platforms have revolutionised the approach towards blood group genotyping (BGG), they are based on pre‐defined polymorphisms, which are not suitable for the discovery of new alleles. Next Generation Sequencing (NGS) circumvents this requirement and operates in discovery mode, which is critical for emerging alleles. NGS is capable of producing comprehensive, high‐throughput, rapid and accurate data resulting in extensive genotyping. ABO genotyping has frequently only focused on exons 6 and 7, neglecting the rest of the gene.
Following our successful NGS‐genotyping of blood group genes Duffy (DARC) and Kidd (SLC14A1), here we have used the Ion Torrent Personal Genome Machine™ (PGM™) sequencer to optimise and develop a reliable protocol for sequencing the entire ABO blood group gene including flanking regions.
In this pilot study, four long‐range polymerase chain reactions (LR‐PCR) were used to target the entire ABO gene plus over 5 kb upstream, regulatory regions (Promoter and CBF/NF‐Y),anddownstream in 16 randomly selected genomic DNA samples. DNA libraries were prepared by enzymatic fragmentation, ligation of barcoded adapters and size selection, before clonal amplification of templates was achieved using emulsion PCR and then the samples were loaded onto a 316 chip for sequencing.
Millions of reads with great coverage depth (100–1800x) were generated, which were then aligned to the reference gene sequence (NG_006669.1). These data were analysed and visualised with multiple software packages, such as CLC Genomic Workbench version 6.5. The serological phenotype data matched that of ABO genotyping. Bioinformatics analysis revealed a number of polymorphisms including single nucleotide polymorphisms (SNPs) and insertion/deletions (indels) distributed throughout exons, introns and the regulatory regions at around 4 kb upstream. Examples of amino acid changes due to the SNPs found in exons are those causing the differences between A and B alleles, previously described in exon 7 (Arg176Gly, Gly235Ser, Leu266Met and Gly268Ala), whilst other SNPs found in exons 3, 4 and 5 have been found to be of higher frequency in our samples than previously reported, including Arg63His (13/16 samples) and Ser74Pro (14/16 samples) and found in all ABO phenotypes. In addition, in two samples (of A and O phenotype), we showed the Trp181stop mutation, previously described only for the rare ABO*O.06 (O6) allele.
We suggest that NGS can provide a reliable approach to genotype ABO due to its powerful capabilities of comprehensive analysis and revealing novel alleles. NGS will supplant other genotyping platforms in the near future, becoming the potential methodology of choice for genotyping patients and donors for safe transfusion/transplantation practice.
P‐568
Implementing Next‐Generation Sequencing (NGS) for Extended Blood Group Genotyping: Molecular Typing of Sickle Cell Disease (SCD) Patients
Fichou YF1, Mariez MM 1, Dupont ID 2, Jamet DJ 2, Le Maréchal CLM 3, Férec CF 3
1Inserm UMR1078 ‐ EFS, Brest, France2EFS, Brest, France3Inserm UMR1078 ‐ EFS ‐ CHRU Brest ‐ UBO, Brest, France
Background
While we recently made the proof of principle that the molecular typing of 17 genes involved in 14 blood group systems may be carried out by next‐generation sequencing (NGS) (Fichou et al., Brit J Haematol 2014; 167:554–562), implementing this approach at the laboratory level for routine diagnosis remains a difficult task, most particularly because of the extensive technical procedures required at all steps of the protocol.
Aims
We then attempted 1/ to simplify the workflow and to limit the technical handling by taking advantage of the materials provided by the manufacturer; 2/ to reduce the cost of genotyping by substituting NGS by Sanger sequencing for the molecular typing of specific exons in homologous genes; and 3/ to test our approach in a cohort of patients in a clinical context.
Methods
DNA libraries from sickle cell disease (SCD) patients (N = 48) were generated with the Ion AmpliSeq[TRADEMARK] Library Kit 2.0 (Life Technologies) with custom primers previously designed. Libraries were quantified by the Ion Library Quantitation Kit (Life Technologies), mixed at equimolar ratios, prepared with the Ion Chef[TRADEMARK] Instrument (Life Technologies) and finally sequenced by either the Ion Torrent Personal Genome Machine® (PGM[TRADEMARK]) Sequencer (Life Technologies) or the Ion Proton[TRADEMARK] System (Life Technologies). Output data (i.e. BAM files) were exported to Alamut v2.4 (Interactive Biosoftware) for data analysis. Sanger sequencing was carried out in standard procedures.
Results
All DNAs could be successfully sequenced by NGS with an initial DNA amount as low as ~2 ng [range: 2–15 ng], as compared with the 10 ng recommended by the manufacturer. In our conditions, sequencing data generated with the Ion Proton[TRADEMARK] System are much more informative in terms of read depth (~100‐fold) than with the Ion Torrent PGM[TRADEMARK], resulting interestingly in a significant increase in the global coverage of the coding DNA sequences (94.4% vs 89.4%, respectively). Sanger sequencing was reintroduced to replace NGS in RHCE and GYPA exons 1 and 2, and also provided high‐quality data with limited technical handling. Phenotypes predicted from the NGS data of ACKR1 (Duffy), SLC14A1 (Kidd) and GYPB (MNS) were in full accordance with either those deduced from Sanger sequencing of these genes or serological data, when available. Furthermore phenotypes could be easily predicted from NGS data for all other blood groups. Rare variants, as well as novel variants, were also found in some samples.
Summary/Conclusions
Generating gene‐specific, PCR‐derived libraries for NGS, more specifically size‐selection on agarose gels was found to be the labor‐intensive step in our initial procedure. We have simplified the workflow by combining NGS and Sanger sequencing to optimize advantages provided by both technologies to generate high‐quality data in a cohort of SCD patients. Our novel strategy, which reduces both the cost and the technical handling, may now be part of a diagnosis process to investigate blood groups of the main interest in selected cohorts of blood donors or patients, and will further contribute to improve cross‐matching that is obviously highly valuable to the patients to be transfused.
P‐569
High‐Throughput Donor Typing with PCR‐SSO Multiplex‐Assays for Molecular Blood Group Diagnostics
Legler TJ , Holzhausen S
University Medical Center Goettingen, Göttingen, Germany
Background
Molecular genetic blood group typing has been integrated in Immunohematology routine of many Transfusion Centers worldwide. Genotyping is applied to investigate unclear phenotypes as well as to extend donor and patient typing in order to obtain maximum compatibility by providing perfectly matched blood units. BLOODchip ID CORE XT™ is a DNA‐based multiplex‐assay for blood donors that has not been tested in Germany.
Aim
The aim of this study was to evaluate and validate the performance of ID CORE XT™ in comparison with serological techniques of a German Transfusion Center.
Methods
We analyzed a batch of 96 DNA samples derived from Caucasian blood donors, previously phenotyped for RhCE, Kell, and also partly for Kidd, Duffy, MNS and Lutheran antigens with gel column agglutination technique and tube test, respectively. ID CORE XT™ (Progenika, a Grifols company, Spain) is a molecular genetic PCR‐SSO test based on Luminex xMAP®technologythat analyses 29 SNPs for the prediction of 37 antigens belonging to the blood group systems RhCE, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright and Lutheran. Genomic DNA extracted from peripheral blood was amplified by a single multiplex PCR. PCR products were hybridized, labeled with a fluorescent conjugate and detected with a Luminex® system. The data were analyzed with the IDCORE XT™ ANALYSIS SOFTWARE to obtain the genotypes and predicted phenotypes. Turnaround time to perform the batch analysis was determined.
Results
All 37 antigens revealed valid results. However, Diego, Dombrock, Colton and Cartwright were excluded from the evaluation because the donor samples have not been phenotyped for these antigens previously. In 3 (Kpa, N and Fya) of 4 discrepancies out of the 96 DNA samples genotyped for RhCE, Kell, Kidd, Duffy, MNS and Lutheran antigens the serological result was proved wrong after a second blood sample test. In 95 of 96 DNA samples, 1095 of 1096 typing results (99.91%) were concordant with the reference methodology. One discrepancy remained when typing for S and could not be resolved due to the unavailability of a second sample from this blood donor. The system was easy to perform, with turnaround time of 4.5 h with hands‐on time of approximately 40 min.
Conclusion
High‐throughput typing of donors using a PCR‐SSO multiplex‐assay such as ID CORE XT™ is a suitable tool to determine a wide spectrum of antigens in a single reaction. Maximized compatibility of blood units helps to prevent alloimmunization of patients, especially of those with life‐long transfusion dependent diseases such as thalassemia or sickle cell disease.
P‐570
A Novel Mutation (C.790C>T, P.ARG263STOP) in the RHAG Gene Leads to a Rhnull Phenotype in Japanese Individuals
Tanaka M
Japanese Red Cross Kinki Block Blood Center, Ibaraki, Osaka, Japan
Background
The Rhnull phenotype is a very rare blood group, in which all of the known Rh antigens (D, C/c, E/e) are lacking. Molecular analysis of Rhnull individuals has revealed that abnormalities occur either at the RH or RHAG gene. The Rhnull phenotype arises from two distinct genetic mechanisms, the amorph type and the regulator type. The amorph type is the homozygous genotype of a silent allele at the RH gene, whereas the regulator type has some mutations at the RHAG gene but none at the RH gene.
Aims
In this study, we aimed to perform the genetic analysis of a Japanese Rhnull family and identified a novel mutation in the RHAG gene.
Methods
Two Rhnull (AB) blood samples, from a 59‐year‐old Japanese male (proposita) and his 63‐year‐old sister (2 deliveries), were obtained in 1971. They had no history of Red Blood Cell (RBC) transfusion. For Rh phenotyping, standard serology techniques were used. Genomic DNA was isolated from whole blood samples using the QIAamp DNA Blood Kit (QIAGEN, Hilden, Germany). Genotyping for RBC antigens was performed using BloodChip Reference (GRIFOLS/Progenika, Barcelona, Spain). For the sequence analysis of the genes (RHD, RHCE, and RHAG), each of the exons from 1 to 10 was amplified by polymerase chain reaction using the set of intronic primers. All of the exons were sequenced using the 3130 genetic analyzer (Life Technologies /Applied Biosystems, CA, USA).
Results
The Rh phenotypes of 2 samples did not contain Rh and RhAG antigens on their RBCs and were identified as the Rhnull. In the results of the screening test for irregular RBC antibodies, anti‐Rh29 was detected from 2 Rhnull samples and anti‐C+e was detected from the sister (in 1971, these antibodies were not detected). From the genotyping, the proposita was predicted D+C+e+, and his sister was D+c+E+. Following the sequencing results of exons 1‐10 of the 2 samples, the RHD and RHCE genes were intact. To detect the presence of mutations in the RHAG gene, exons 1–10 of the RHAG gene were amplified and sequenced. The sequence results of the RHAG gene revealed a novel point mutation in exon 5, c.790C>T (accession number; AB938314), which led to a truncated protein (p.Arg263Stop) of RhAG, causing a loss of Rh antigen expression. Two Rhnull samples were identified as regulator Rhnull.
Conclusion
We found a novel mutation (c.790C>T, p.Arg263Stop) in exon 5 of RHAG from 2 Japanese subjects, which led to Rhnull phenotype.
P‐571
Abstract Withdrawn.
P‐572
Use of the Rhce Intron 2 Insert for RHCE*C Genotyping
Azcarate-Ania MN 1, Wynn P 2, Renovales-Garcia AM 1, Crews W 2, Sood C 3, Ochoa G3
1Centro Vasco de Transfusion y Tejidos Humanos, Galdakao, Spain2Carter BloodCare, Bedford, TX, United States of America3Progenika‐Grifols, Medford, United States of America
Background
Several commercial multiplex platforms use a targeted approach to blood group genotyping whereby individual assays interrogate in a gene the key polymorphic positions known to determine allelic variants. For polymorphisms that determine common and rare antigens these positions are predominantly located in the coding sequence of the gene, whereas for polymorphisms that cause altered expression of antigens (e.g. weak or null variants) they can also be located in the promoter region or at/near splice junctions. An exception to this trend is the assay that detects the RHCE*C allele (RHC henceforth), commonly based on the detection of a 109‐bp insert in intron 2 (1). Detection of this insert circumvents the problem posed by the identity of RHC and RHD sequences in exon 2, where the key polymorphic positions that allow discrimination between RHC and RHc alleles are located. This insert is specific to the RHC allele, which has made it a very valuable tool for the prediction of C antigen by targeted genotyping platforms.
Aims
To identify the molecular basis for the discrepancy between the C+ serology and the C‐ predicted phenotype of two apparently unrelated individuals: a Caucasian female patient seen in Texas and a Caucasian male donor from Spain.
Methods
C antigen typing on donor RBCs was performed by the microplate method with human MS24 monoclonal, and on patient RBCs by the tube method with Gamma‐clone monoclonal, both reagents from Immucor. Genotyping was done with genomic DNA extracted from EDTA whole blood on the Progenika‐Grifols BLOODchip Reference and ID CORE XT tests. Sequencing of RHCE exons 1, 2 and flanking intron regions was performed by the standard Sanger dideoxy method, and sequencing of RH intron 2 and exons 3, 7 by a semi‐quantitative modification of the same method.
Results
Serological C typing reactions had a strength of 4+ for the donor and 2+ for the patient. Genotyping on BLOODchip Reference and ID CORE XT found the RHC‐specific 109‐bp insert in intron 2 to be absent from both samples. BLOODchip Reference also found the donor to be homozygous or hemizygous RHD*weakDtype3. The results of DNA sequencing and their interpretation are shown in Table 1. The RH intron 2 region analyzed includes polymorphic positions upstream and downstream of the 109‐bp insert.
Table 1.

Summary/Conclusions
The serology and molecular results provide evidence for the replacement of a region of RHC intron 2 including the 109‐bp insert by RHD sequence. Identity of RHC and RHD sequences upstream of this region through the 5’ end of exon 2 prevents determination of the upstream boundary. The downstream boundary can be inferred to lay between IVS2+3273 and exon 3. We have named RHCE*Ce-D(IVS2)-Ce the allele resulting from this replacement. In trans for both, patient and donor is a common RHCE*ce allele. Interestingly, RHD*weakDtype3 is usually found in cis with RHC, which suggests a RHD*weakDtype3 - RHCE*Ce-D(IVS2)-Ce haplotype for the donor.
(1) Ann. Hum. Genet. (1993), vol.7, p.273.
P‐573
UK NHS Blood and Transplant Fetal RHD Screening – Giving Anti‐D Only to Those Who Need It!
Finning K , Tovey S , Desay K , Latham T , Daniels G
NHS Blood and Transplant, Bristol, United Kingdom
Background
Approximately 40,000 women per year in England and Wales receive antenatal anti‐D immunoglobulin when they cannot benefit from it, because they are carrying a D negative fetus. Prediction of fetal D status is possible by testing for the presence of RHD gene sequence in cell free fetal DNA in maternal peripheral blood.
Aims
A service implementation pilot was undertaken between April 2013 and March 2015 in three NHS trusts in the South West of England to explore the practicality of offering this test to all D negative pregnant women within the NHS.
Methods
Blood samples taken during routine midwifery visits before 26 weeks’ gestation (median gestation 16 weeks) were sent via local hospital laboratories to the International Blood Group Reference Laboratory at NHS Blood and Transplant in Filton using NHSBT transport. Automated DNA extraction and real‐time PCR technology was used to predict fetal D status as ‘D positive’, ‘D negative’, or ‘Inconclusive ‐ treat as D positive’. Reports were generated within the NHSBT Hematos system and sent by post and an electronic reporting system (sp‐ICE). If the fetus was predicted to be D negative, antenatal RhIg was not given unless the woman wanted to receive it. In a 6 month period, predicted fetal D blood group was compared to the cord blood group.
Results
In total, 2733 maternal blood samples were referred to IBGRL of which 2701 were tested. 32 samples were rejected owing to insufficient or unsuitable sample or incomplete information. The fetus was predicted to be D positive for 1462 samples (54%), D negative for 997 samples (37%) and an inconclusive result was issued for 242 samples (9%). Between 01/04/2013 and 30/09/13 the predicted fetal D blood group was compared to cord blood group at birth; no fetuses were incorrectly predicted to be D negative; one fetus was incorrectly predicted to be D positive. A 29% reduction in RhIg doses administered was seen in all three Trusts, equating to 35% of D negative women not receiving unnecessary RhIg in their pregnancy.
Summary/Conclusions
Fetal RHD genotyping can be applied within the NHS to allow use of RhIg in a more precise and indicated way, in line with practice in several other European countries. NHS Blood and Transplant will offer a fetal RHD screening service to hospitals in the South West from April 2015 and to all hospitals in England from April 2016.
P‐574
Genotyping to Reduce Anti‐D Immunoglobulin Usage in a Diverse Population Demographic: Fetal RHD Detection for Mothers Harbouring RHD Variants
Flower L1, Millard GM 1, McGowan EC 1, O'Brien H 1, Hyett JA 2, Gardener GJ 3, Hyland CA 1
1Australian Red Cross Blood Service, Brisbane, Australia2Department of High Risk Obstetrics, Royal Prince Alfred Hospital, Sydney, Australia3Mater Mothers’ Hospital, Mater Health Services, Brisbane, Australia
Background
A pilot clinical trial commenced in 2014 in Australia to assess the uptake and benefit for fetal RHD genotyping to target routine antenatal anti‐D prophylaxis in RhD negative pregnant women. Genotyping programs need to manage a unique geographic environment and a multi‐cultural maternity population expected to exhibit a diverse array of RHD genetic variants. These variants comprise two broad groups: One where the gene is largely intact and one where the gene is a RHD*D-CE-D hybrid.
Aims
We report on the initial performance of a screening system in two antenatal clinics and review the impact of maternal RHD variants on fetal RHD genotyping.
Methods
RhD negative pregnant women are recruited after 10 weeks gestation during routine antenatal visits and demographic variables recorded. Duplicate maternal blood samples (EDTA and STRECK) are collected and processed up to 7 days of collection. Automated cell‐free fetal (cff) DNA isolation and genotyping procedures are performed using QIAsymphony and Rotor Gene Q systems. Fetal RHD negative predictions are confirmed at a later gestation and women given the option to avoid anti‐D immunoglobulin.
An automated algorithm was used to detect maternal RHD genetic variants. These were further classified by SNP genotyping (Progenika Inc) or Sanger sequencing.
Results
The mean gestation of sampling for the first 407 subjects was 19 weeks; 17.8% had a BMI >30 kg/m2. The mean processing time was 65 h (1SD: 40 h). Total cfDNA concentrations ranged from 0.03 to 248 ng/μl for EDTA plasma samples and 0.02 to 0.117 ng/μl for STRECK tubes.
Fetal RHD exons were detected in 60.5% of cases; were not detected in 36.6% and were not assigned in 2.9% due to maternal variants.
There was a 50/50 breakdown of maternal variants into intact alleles (including one RHD*1227A) and hybrids. All the hybrids exhibited the maternal RHD exon 10 and lacked the maternal RHD exon 5 which enabled fetal RHD genotype predictions based on presence of exon 5 signals where STRECK sample collection tubes were used. However, discrepant results were obtained between the EDTA and STRECK tubes for two participants carrying the maternal variants hybrids RHD*D-CE(3-8)-D and RHD*DVI type III. The STRECK amplified fetal RHD exon 5 signals while the EDTA would have predicted a false negative result if only exon 5 had been interrogated.
Conclusion
The screening system is proving accurate for fetal RHD genotyping where there is no background maternal RHD gene. Although EDTA tubes allow reliable reporting in most cases, STRECK tubes may be of value in detecting fetal RHD signals when a maternal RHD*D-CE-D hybrid variant is present. In a nation‐wide screening program which will involve up to 45 000 mothers per annum the impact of these cases will accumulate. There is also projected value in defining the intact RHD variants as some carriers (such as those with the RHD*1227A) may not require anti‐D. We project that technologies such as massive parallel sequencing will have the potential to readily manage these cases and to provide large scale screening capability.
P‐575
A Novel Variant Sema7a Allele in a Compound Heterozygote, Resulting in Variant JMH Expression and Alloanti‐JMH Production
Tilley LA1, McNeill A 1, Moser MI 2, Romeiras MC 3, Thornton N 1, Daniels G 1
1NHS Blood and Transplant, Bristol, United Kingdom2Centro de Sangue e Transplantacao Lisboa/IPST.IP, Lisbon, Portugal3H CCabral/CHLC, Lisbon, Portugal
Background
The JMH blood group antigens are carried on the glycoprotein semaphorin 7A (Sema7A). The JMH‐ (JMH:‐1) phenotype is usually acquired and often transient. However; five rare, inherited variants of JMH also exist, lacking antigens JMH2‐6, resulting from homozygosity for missense mutations in SEMA7A. These variants have been identified in JMH+ individuals producing alloantibodies to high frequency antigens that do not react with JMH‐ cells. We recently reported a further variant allele, JMH*01.c.[709G>A;1545A>G], homozygosity for which was associated with loss of an apparent novel high frequency JMH antigen and production of alloanti‐JMH (Tilley et al. 2014. Transfus Med 24, suppl 2: 27).
Aims
Blood samples from a Caucasian female patient were investigated due to the presence of an unidentified antibody in her plasma.
Methods
Serological tests were performed by standard LISS tube IAT technique. Inhibition tests were carried out with soluble recombinant JMH protein (kindly provided by Imusyn, Germany). Genomic DNA was extracted and DNA sequencing performed for all exons (1 to 14) of SEMA7A.
Results
Anti‐JMH was identified in the patient's plasma, reacting by LISS IAT with untreated cells but not with papain treated cells. The autologous control and DAT were negative. Three examples of JMH‐ cells were compatible with the patient's plasma and no additional antibodies were detected. Complete inhibition of the antibody was achieved using soluble recombinant JMH protein, further confirming the JMH specificity. The patient's cells were found to be positive with four examples of anti‐JMH, indicating the possibility of a JMH variant. Sequencing of SEMA7A revealed heterozygosity for c.709G>A in exon 7, encoding p.Asp237Asn in the Sema7A protein, and c.1545A>G in exon 12 (silent) as identified previously in the homozygous state (Tilley et al. 2014). Two further novel heterozygous mutations were identified in exon 14, c.1864C>T and c.1865G>A, together encoding p.Arg622Tyr in the Sema7A protein. The patient's cells were found to be positive, although slightly weaker than JMH+ control cells, with the plasma of the JMH*01.c.[709A;1545G] homozygote patient.
Conclusions
Sequencing results in this case suggest the patient is a compound heterozygote for two variant SEMA7A alleles. The first of these, JMH*01.c.[709A;1545G], has been previously reported, whilst the second allele appears to be novel, carrying c.1864C>T and c.1865G>A, encoding p.Arg622Tyr in Sema7A. The positive reaction observed with the patient's cells and anti‐JMH from the previously described JMH*01.c.[709A;1545G] homozygote suggests that the JMH variant expressed in this case is different to the original proband. Therefore, it seems likely that the novel allele in this case, JMH*01.c.[1864CG>TA], also expresses a variant JMH antigen. Apparent compound heterozygosity for two variant SEMA7A alleles has resulted in variant JMH antigen expression on the cells of this patient and production of alloanti‐JMH. These findings further add to the complexity of the JMH blood group system.
P‐576
Renewable DNA Reference Panels for Blood Group Genotyping
Rios M , Volkova E , Liu M , Mitra B , Mercado TM , Davey RJ , Liu Z
US‐FDA, Silver Spring, United States of America
Background
Extended typing of red blood cells (RBC) is an effective strategy to reduce alloimmunization of patients who receive multiple transfusions, such as those with sickle cell anemia. Screening of large numbers of donors using traditional serological methods and matching for rare antigens is a resource intense activity often limited by the availability of reagents. Molecular blood group genotyping has been recognized as a reliable method that allows high‐throughput typing of blood groups for which serological reagents are not available. Well‐validated DNA reference panels are essential for the development, validation, manufacturing and quality control of the molecular methods. However, the availability of reference materials for blood group molecular genotyping is limited and comprehensive coverage of many blood group systems is currently difficult. Manufacturers of genotyping kits and genotyping laboratories use non‐renewable clinical materials for reference, some of which are poorly characterized, increasing the probability of mistypings.
Aim
To develop and make available renewable genomic DNA reference panels for manufacturers for development and validation of new assay kits. These reference panels will also be useful in genotyping laboratories for test calibration and monitoring of performance. The panels will help improve genotyping accuracy and reduce probabilities of adverse events in blood transfusions.
Methods
An agreement with a U.S. blood establishment allows access to fully characterized blood donor samples. The establishment has screened more than 30,000 donors using a multiplex molecular assay, allowing access to desired genetic variants. Donors for the reference panels were selected based on their historical RBC phenotype and DNA genotypes. Whole blood was collected after informed consent. Peripheral blood mononuclear cells were isolated and transformed with EBV in vitro for establishment of immortalized cell lines for use as renewable source of genomic DNA. Custom TaqMan assays and Sanger sequencing, covering multiple polymorphisms, were employed to confirm the genotypes associated with RBC antigens of interest. For the formulation of panel, cell lines were expanded, DNA isolated and lyophilized to ensure stability.
Results
We developed 39 unique TaqMan assays, 25 PCR‐SSP assays and a PCR‐RFLP, and performed genetic characterization of each sample for each genetic polymorphism. To date, we have tested 53 blood donors, covering polymorphisms associated with 41 target alleles in 18 blood group systems. The proposed panel will be formulated in steps. We have selected 20 cell lines that provide comprehensive coverage of our chosen alleles for DNA extraction and lyophilization to formulate the first prototype panel.. The prototype panels will be distributed to collaborating laboratories for characterization to establish their suitability as reference materials.
Conclusion
We have produced cell lines for use as renewable reference panels for RBC genotyping. The first prototype panel set has been developed using genomic DNA from 20 cell lines covering allele‐associated genetic polymorphisms in 18 blood groups. We expect to have a comprehensive panel ready for use in the foreseeable future.
P‐577
High‐Throughput Strategy for Molecular Identification of Vel‐Negative Blood Donors Using Leftover Nucleic Acids Extracted from Plasma Pools on Routine Viral Nat
Levi JE , Dezan M , Dinardo CL , Bosi SRA , Vega S , Salles NA , Mendrone A Jr
Fundação Pró‐Sangue Hemocentro de São Paulo, São Paulo, Brazil
Background
The Vel blood group system is defined by the presence of the Vel antigen in erythrocytes. The molecular mechanism responsible for the Vel‐negative phenotype is a deletion of 17 nucleotides in exon 3 of SMIM1, a small gene of 97 kb located in chromosome 1 and consisting of four exons. Vel‐negative phenotype is transmitted by autosomal recessive inheritance and is usually revealed when Vel‐negative blood recipients develop anti‐Vel antibodies, which are in general clinically relevant. Reagents (anti‐sera) used for the search of donors with this rare phenotype are mostly originated from human sources and difficult to obtain.
Aims
We sought to establish a high‐throughput low‐cost platform for molecular screening of blood donors for the associated 17 bp deletion.
Methods
Since 2013, pools consisting of plasma from 6 blood donors are screened at the NAT lab for the genome of HCV and HIV. DNA/RNA extraction is carried in a fully automated system (MDx QIAGEN®) in a microplate format. Nucleic acids are eluted into 50 uL and approximately 20uL are not used. After viral NAT results are released, this pure nucleic acid solution is discarded. We transferred this leftover material to another microplate containing a set of primers and probe identifying the 17 bp deletion characterizing the Vel‐negative genotype, and submitted to real‐time PCR. Whenever a pool showed reactivity, the samples were analyzed individually by the same procedure, and, when the specific deletion was confirmed, a zygosity assay was performed using previously established protocols. After validation, unselected consecutive samples from 4680 blood donors from Fundação Pró‐Sangue / Hemocentro de São Paulo were tested by this protocol.
Results
Seven hundred and eighty (780) minipools of 6 donations were tested and 20 showed initial reactivity. All 20 were dismembered and donations tested individually. 19 (0.40%) donors harbored the deletion in heterozygosis while one homozygous Vel‐/Vel‐ donor was identified 1 (0.02%). Serologic analysis was performed on the 20 samples. The 19 donors with a heterozygous deletion showed a weak expression of Vel antigen and absence of anti‐Vel antibody. The Vel‐negative donor with homozygous deletion had the anti‐Vel antibody identified in serum (Title 32, Score 43).
Conclusions
This Real‐Time PCR technology using DNA extracted from plasma pools proved to be a fast and accurate method to detect the deletion of 17 nucleotides (64_80del) of the SMIM1 gene. This methodology broadens our perspectives to screen for donors lacking other high‐frequency antigens and will assist in creating an inventory of frozen rare red blood cells units to be used in transfusion practice.
P‐578
A Patient of Caucasian Origin with an Apparent Fy(A−B−) Phenotype
Henny C , Lejon Crottet S , Niederhauser C , Hustinx H
Interregional Blood Transfusion SRC Ltd, Berne, Switzerland
Background
The Duffy (Fy) blood group protein, in its most abundant form, consists of 336 amino acids and is encoded by two exons of the DARC (FY) gene on chromosome 1q23.2. The major antigens of the Fy blood group system are Fy(a) and Fy(b), which are characterized by the change (c.) of a single nucleotide 125G>A. Several alleles, mostly comprising single nucleotide polymorphisms (SNPs) have been reported to weaken or silence the expression of the Fy protein. The most prominent ones are FY*02M.01 (c.265C>T, 298G>A), leading to a phenotype commonly known as Fy(x) and FY*02N.01 (c.‐67t>c), with a mutation in the promoter region leading to a Fy(a−b−) phenotype in homozygous cases. While the Fy(a−b−) phenotype is very common in people of African origin due to a certain protection against malaria parasites, this phenotype is very rare in people of Caucasian origin.
Aim
The sample of a patient of Caucasian origin was previously phenotyped as Fy(a−b−) in a hospital laboratory and sent to our reference laboratory for further characterization.
Methods
Phenotyping on ID/IAT‐cards (Bio‐Rad) was done using anti‐Fy(a) and anti‐Fy(b) polyclonal antibodies (in‐house). The adsorption‐elution analysis was performed using a polyclonal anti‐Fy(b) antibody. For FY genotyping the sequence specific primer (SSP)‐PCR kit RBC‐FluoGene vERYfy (Inno‐Train) as well as an in‐house SSP‐PCR method were applied. The sample was further characterized by exon sequencing using in‐house primers for amplification and sequencing.
Results
Repeated standard phenotyping of the sample confirmed the Fy(a) and Fy(b) negativity. Both SSP‐PCR genotyping methods were positive for nucleotide (nt.) 125A (FY*02), and nt.265T (FY*02M.01). The presence of nt.125G (FY*01) and nt.‐67c (FY*02N.01) could be excluded. Zygosity testing for allele FY*02M.01 revealed a heterozygous state. Sequencing of FY confirmed the FY*02/FY*02M.01 genotype and in addition the mutation c.151delT, found on the wild type allele. This deletion leads to a frameshift with an aberrant protein sequence starting from amino acid 51 and a premature stop codon at position 74. To the best of our knowledge the variant FY*02.151delT has not been reported previously. The adsorption‐elution test with polyclonal anti‐Fy(b) showed an almost negative result, which is in accordance with the Fy(x) phenotype.
Summary/Conclusions
Here we present a Caucasian patient with an apparent Fy(a−b−) phenotype, resulting from a combination of the new FY*02 null allele, FY*02.151delT, and the relatively frequent occurring FY*02M.01 allele (Berne 3.6%). As Fy(a−b−) red blood cells concentrates (RBC) are scarce in Switzerland and the patient is positive for the FY*02M.01 allele with no antibodies against clinical relevant blood groups present, Fy(a−b+) RBCs can be transfused.
P‐579
More Evidences of Problems for D Variant Determinations by Serology: Limitations of Commercial D Panels
Brito MA1, Fontão-Wendel R 1, Cardoso R 1, Simões AT 2, Pierrotti MR 1, Nucci C 1, Duarte KM 3, Cruz K 3, Wendel S 1
1Blood Bank Hospital Sirio Libanês, São Paulo, Brazil2Centro de Imunologia e Imunogenética, São Paulo, Brazil3Bio‐Rad Laboratories, Lagoa Santa ‐ MG, Brazil
Background
There are over 70 weak D alleles genotypes described so far. Several of these genotypes show high association with African ancestry1. Due to a high grade of African ancestry in Brazilian population, these genotypes are not uncommon in Brazil.
Aim
Panels of anti‐D for elucidation of D variants are widely used in Brazil; however, it demonstrates limitations for determination of weak D phenotypes.
Materials and methods
Twenty samples (20) typed as ‘D variant’ by gel‐spinning technique using commercial anti‐D panel ‘Extend Partial D Typing Kit’ (Bio‐Rad Laboratories, Inc) presented the following results: 15 samples had inconclusive results (positive with all antisera from the panel), three were typed as DFR phenotype, one as weak D type 38 and one as DAU‐4. These samples were investigated by molecular techniques using DNAs extracted from peripheral blood leukocytes (QIAamp®, Qiagen®, Germany), and analyzed for the presence of exons 3, 4, 5, 6, 7 and 9 of the RHD gene by PCR‐multiplex2.Weak D types 1, 2 and 3 were determined by PCR‐SSP that analyzes the ‘target’ mutation of each weak D type3.The DAU‐4 genotype was confirmed by two in‐house techniques: PCR‐SSP adapted from literature4, and PCR‐RFLP which primers were designed for exon 5 gene RHD using the TaqI enzyme for characterization of polymorphism 697T> A. The weak D type 38 genotype was determined by PCR-RFLP using the MnlI enzyme for characterization of mutation 833G> A (exon 6). To determine the hybrid gene RHD-CE-Ds, a multiplex‐PCR described in literature5was performed.
Results
From fifteen samples with inconclusive typing results, four (27%) were genotyped as weak D type 1 (809T > G); seven (47%) weak D type 2 genotype (1154G > C); one (6%) weak D type 3 (8C > G); two (14%) weak D type 38 (833G > A), and one (6%) DDN genotype (490 g > A), the latter subsequently confirmed by RHD gene sequencing. Three samples that were classified as DFR phenotype presented different results by molecular testing whose results were: one weak D type 38, one DVand one weak D type 2 / RHD -CE- Ds(two aberrant alleles). Only two samples (10%) tested by the panel demonstrated correlation with genotyping: weak D type 38 and DAU ‐4.
Conclusion
Although most of the samples presented an inconclusive result using the extended partial D typing kit, only 2/5 (40%) phenotyped samples demonstrated a good correlation with molecular testing. This demonstrates that caution should be used in the interpretation of this panel to confirm D variants, especially for weak D phenotypes in Brazil. It is important to know exactly these pheno/genotypes for better criteria definition for blood transfusions in such patients, including blood management stocks, especially for D‐ blood supply.
1. http://www.uni-ulm.de/~wflegel/RH/.
2. Maaskant‐van Wijk et al, Transfusion 1998; 38: 1015–1021.
3. Müller et al, Transfusion 2001; 41:45–52.
4. Wagner et al, Blood 2002; 100: 306–311.
5. Tax et al, Transfusion 2002; 42:634–644.
P‐580
Identification of the Mutation Affecting the KEL Gene Responsible for a K0 Phenotype
Mattaloni SM1, Arnoni C 2, Trucco Boggione C 1, Luján Brajovich ME 1, García Borrás S 3, Biondi C 3, Castilho L 4, Cotorruelo CM 1
1Laboratorio de Inmunohematología. Universidad Nacional de Rosario. CONICET, Rosario, Argentina2Colsan, San Pablo‐SP, Brazil3Laboratorio de Inmunohematología. Universidad Nacional de Rosario, Rosario, Argentina4Hemocentro‐Unicamp, Campinas‐SP, Brazil
Backgound
the Kell system is one of the most clinically relevant blood group. It comprises at least 34 antigens being KEL1/KEL2 (K/k), KEL3/KEL4 (Kpa/Kpb) and KEL6/KEL7 (Jsa/Jsb) antithetical and the most important epitopes. Most Kell antibodies are involved in the pathogenesis of hemolytic disease of the fetus and newborn and severe hemolytic transfusion reactions. Kell antigens are encoded by the KEL gene which comprises 19 exons. Single nucleotide polymorphisms (SNPs) are the most common cause of the different Kell phenotypes. Molecular defects may lead to the rare K0 (Knull) phenotype characterized by the absence of Kell antigens expression. Limited clinical data have been published regarding the significance of anti‐Ku, seen in K0 individuals.
Aims
the aims of this study were to characterize the molecular basis of a K0 phenotype encountered in a pregnant woman and report the clinical outcome the pregnancy.
Materials and methods
A peripheral blood sample of a pregnant patient was referred to our reference laboratory because of the presence of a panreactive antierythrocyte antibody. Standard serological analyses based on hemagglutination were performed using tube and gel techniques. Genomic DNA was obtained with a modified salting‐out method. Kel genotyping was performed by PCR‐RFLP strategies. All coding and intron‐exon splice regions were analysed by Sanger sequencing.
Results
Serological studies showed an IgG 37°C‐reactive alloantibody, titer 128. Extended red blood cell phenotype analysis failed to detect the expression of neither KEL1, KEL2, KEL3, KEL4, KEL6 nor KEL7 antigens suggesting a K0 phenotype with anti‐Ku. KEL genotyping showed KEL*02/02, KEL*04/04 and KEL*07/07 genotypes. Sequence analysis of the 19 exons and intron‐exon splice regions of KEL showed a guanine to an adenine substitution at the first nucleotide of intron 3 (IVS3+1 g>a) originating the allele KEL*02N.06.
Conclusions
The replacement of the conserved sequence gt at the donor splice site by at is responsible for an alternative assembly of mRNA with loss of part of exon 3 sequences. Alternatively, this mutation causes skipping of the entire exon 3. In both cases, a downstream premature stop codon is generated in exon 4 and no Kel protein synthesized. Molecular finding confirmed the suspected K0 phenotype. To our knowledge, this is first description of this unusual phenotype in an Argentinean patient. She gave birth to a preterm infant with hemolytic disease of the newborn, who was successfully treated with phototherapy. Identification of the mutation responsible for this exceptional phenotype allowed the development of a PCR‐SSP strategy to detect this allele in family members and provide appropriate genetic counseling regarding transfusion therapy and pregnancy.
P‐581
New Molecular Blood Group Typing with PCR‐SSO for MNS, Kell, Kidd and Duffy on MR.SPOT®
Conradi RC , Marandiuc DM , Jung AJ , Hitzler WEH
University Medical Center of the Johannes Gutenberg University, Mainz, Germany
Background
HLA typing with the PCR‐SSO method on MR.SPOT® device is well established in many HLA‐laboratories. Recently new kits for molecular typing of blood group polymorphisms (MNSs, Kell, Kidd, and Duffy) were developed for this device.
Aims
The experiences and the evaluation of the first version of the new PCR‐SSO test are presented.
Methods
New and historical serologic results from known blood donors were used to perform this study. PCR‐SSP was performed using BAGene KKD‐, MNS‐TYPE and the ERY SPOT® Common kit was used for PCR‐SSO typing both provided by BAG Health Care.
To compare the results of the new PCR‐SSO with the standard methods (serology and PCR‐SSP), 136 different DNA‐samples were analyzed with each method. From the 136 DNA‐samples 4 were isolated from frozen citrate whole blood, 20 from fresh CPDA whole blood and 112 from EDTA whole blood which was stored for 7–8 days. Mean of DNA‐concentration was 24.2 (10.1–61.7) ng/μl with a purity index (A260/A280) of 1.8 (1.65–1.94).
To evaluate the range of DNA concentration for the new PCR‐SSO, 8 additional samples were diluted to a final DNA concentration of 10, 15, 25 and 50 ng/μl.
To assess the reproducibility, 24 of the 136 DNA samples were prepared by 2 different technicians. All 24 samples had a DNA concentration of 15 ng/μl and were measured in the same PCR‐SSO run.
Results
In all 136 evaluated samples the results for Kell, Kidd and Duffy confirmed the pre typing. Due to limitations of serology, three discrepancies (weak‐ or null‐ alleles) were found between serology and DNA methods. In addition 3 samples with incorrect historical serologic results were found. A new serological typing confirmed the PCR results. 14/136 samples were misinterpreted for the MNSs system with the PCR‐SSO method.
Although different or no results were observed only for MNSs system (2/8 samples) in the concentration tests, a relation between the DNA concentration and false results was not seen.
For the 24 samples for reproducibility test no discrepancies were seen in the results obtained by the two operators for Kell, Kidd and Duffy. But again in the MNSs system 5 discrepancies in the results of the two operators were found.
Handling of the PCR‐SSO kits and the MR.SPOT device was without problems and the interpretation of results was clear and easy. Most of the minor problems with the software and result reports were quickly resolved by the manufacturer.
Conclusion
The new PCR‐SSO genotyping method on MR.SPOT® is easy to handle and generates correct and clear results for Kell, Kidd and Duffy alleles. The probes for the MNSs system which were used in this first test version have to be improved. Using the same method and reagents as for HLA typing makes the working and stock holding quite simple. Hopefully the manufacturer will soon solve the problems with the MNSs probes and develop kits for more alleles and blood group systems.
P‐582
Rhce Genetic Variation in Patients with Sickle Cell Disease and African Brazilian Donors
Vendrame TAPV1, Arnoni CPA 1, Muniz JGM 1, Person RDMP 1, Latini FMRL 1, Castilho LC 2
1Colsan, São Paulo, Brazil2Hemocentro Unicamp, Campinas, Brazil
Background
Alloimmunization remains a major problem in transfused patients with Sickle Cell Disease (SCD) and despite provision of Rh typed RBC units, Rh antibodies still occur due to the presence of RH variants, predominantly associated to altered RHCE alleles. Provision of RHCE allele matching for SCD patients should be considered to improve transfusion therapy and therefore molecular analysis of RHCE variants in patients and donors is essential. Based on this, we evaluated the RHCE genetic variation in a population of patients with SCD and African Brazilian donors in order to verify whether a RHCE genotype matching strategy is feasible.
Methods
RHCE genotyping was performed in 175 patients with SCD and 226 African Brazilian donors by PCR‐RFLP or exon sequence analysis .RHCE genotypes with altered alleles and predicted phenotypes were determined.
Results
The occurrence of RHCE altered alleles in patients was 28% and in donors was 20%. Table 1 shows the genotypes and predicted phenotypes found in both groups. Although the distribution of RHCE altered alleles in donors and patients was similar, we observed a higher prevalence of RHCE*ceAG, RHCE*ceVS.01 and RHCE*ceVS.02 alleles in patients.
Caption 1: Genotypes and phenotypes found in SCD patients and blood donors.

Conclusion
Our results showed that 12.5% of the patients with SCD presented RHCE variant alleles predicting partial c and e antigens and the hrS‐ and hrB‐ phenotypes. Although the selection of African Brazilian donors based on RHCE would be feasibly for at least one patient, this study also demonstrated that this RHCE genotyped donor pool is limited and needs to be increased to attend all the needs of the patients with RhCE variants. Knowledge of the RHCE genetic variation and expression of the variant antigens in patients and donors from the same population is necessary to determine the number of donors required to support patients with SCD with molecular RHCE matched units.
P‐583
Characterization of GP.MUR (MIA+, MUR+) Donors by Combination of Serological and Molecular Methods
Yang JC 1, Kamat A 2, Lin X 1, Rubio G 2, Billups N 2, Marston E 2, Yang JC1, Delaney M 3, Banerjee S 1, Blincko S 2, Frame T 2
1BioArray Solutions, An Immucor Company, Warren, New Jersey, United States of America2Immucor, Norcross, Georgia, United States of America3Bloodworks NW, Seattle, Washington, United States of America
Background
The GP.Mur (Mi.III) glycophorin, one of the eleven classes of the Miltenberger subsystem, is relatively common in Southeast Asia (including Southern China, Hong Kong, Taiwan, Malaysia, Thailand, Singapore, Vietnam, and the Philippines). The GP.Mur glycophorin is encoded by the hybrid gene, GYP*(B-A-B), which originated from a crossover between the Glycophorin A (GYP*A) and the Glycophorin B (GYP*B) genes. In transfusion, the GP.Mur glycophorin may elicit an immune response in individuals having the conventional glycophorin genes. Anti‐Mia antibody can cause both hemolytic transfusion reactions (HTRs) and hemolytic disease of the fetus and newborn (HDFN). Glycophorins in the Miltenberger subsystem can be phenotypically defined by using class‐specific antisera, although cross‐reactivity of the epitopes can be limiting for precise identification, and there is a short supply of the class‐specific antisera. Because molecular basis of these antigens have been determined, serological screening followed by molecular analysis appears to be a viable approach to identify people with the GP.Mur blood type.
Aim
The aim of this study was to develop a process to characterize blood donors with the GP.Mur phenotype.
Methods
The Mia+ blood donors (n = 22) were initially identified serologically by using anti‐Mia monoclonal antibody followed by confirmation using anti‐Mur monoclonal antibodies (a kind gift from Taiwan) and polyclonal GP.Mur antisera (Thailand Red Cross Society, Thailand) in the gel card, tube and automated antigen screening methods. The Mia+, Mur+ serologically identified donors were then analyzed using molecular methods including GYP Exon 3 hybrid gene sequence‐specific primer (SSP) PCR, nucleotide sequencing, and haplotype analysis with the GYP*A and GYP*B genes.
Results
We have identified 20 of 22 Mia+ donors having Mia+ and Mur+ phenotype by serological methods. All the 20 Mia+, Mur+ donors were confirmed to have the GYP*Mur allele. Three of the Mia+, Mur+ donors are heterozygous with the GYP*Bun (Mi.VI) (n = 2) or GYP*Hil (Mi.V) (n = 1) hybrid gene. Two other Mia+ donors with the Mia+ and Mur‐ phenotypes were confirmed to have the GYP*Vw (Mi.I) or GYP*Hut (Mi.II) allele.
Conclusion
These findings demonstrate concordance between serology typing and molecular analysis methods for screening GP.Mur donors. We have developed an efficient process to characterize the GP.Mur phenotype and the GYP hybrid genes by using combination of serological and molecular methods.
P‐584
Molecular Characterization of the JK Null Phenotype in the Maori and Polynesian Population in New Zealand
Wall LD1, Dunn P 2, Griner L 1
1New Zealand Blood Service, Auckland, New Zealand2Transplant Laboratory Leicester General Hospital, Leicester, United Kingdom
Background
The frequency of the Jk(a−b−) phenotype is very low in all populations except the Maori, Polynesians and the Finns. New Zealand Maori estimated as 0.27%and Polynesians have a high frequency of the JK nullphenotype estimated as 0.27%. These two diverse ethnic groups provide the majority of the global supply of JKnullblood for transfusion.
Aim
The aim of this study was to molecular characterize the JK null phenotype in the Maori and Polynesian population in New Zealand; from this information design a High Resolution Melting Analysis assay that will capture the alleles that cause the JK null phenotype. The risk of misinterpretation of results due to the presence of null alleles, only identified by molecular typing has been highlighted in the literature.
Methodology
Forty‐four samples, that were found to be Jk(a−b−) by 2 M urea lysis and haemagglutination were selected for this project. These samples were tested by High Resolution Melting on the LightCycler® 480. Samples that showed high resolution melt curve results other than homozygosity for Polynesian IVS5‐1g>a mutation (JK*02N.01) were further analysed.
Results
Thirty‐six of the forty‐four samples identified themselves as Maori or Polynesian. Forty of the 44 samples tested were homozygous for the Polynesian JK*02N.01 allele. A compound heterozygous 190C>T, and a homozygous 118G>A were identified. This sample also had 499 A>G (JK*02N.12) allele which has not been previously associated with 118G>A. A compound heterozygous 896G>A (JK*02N.07) together with JK*02N.01 allelewas identified and one sample had the 810G>A allele. One sample had 810G>A allele in exon 9 however it is heterozygous and further investigation will be required to elucidate the other silencing mutation.
Conclusion
Two novel alleles; namely a compound heterozygous 190C>T, and a homozygous 118G>A were identified. One novel nucleotide change in 499 A>G (JK*02N.12) allele which has not been previously associated with 118G>A was identified. The sample with unknown nucleotide changes is subject to further investigation. As previously reported the Maori and Polynesian population group has a high incidence of the intron 5, JK*02N.01 allele.
P‐585
Impact of Antigenic Exposures and the Role of Molecular Blood Grouping in Enhancing Transfusion Safety in Chronically Transfused Thalassemics
Agrawal S , Makroo RN , Bhatia A , Chowdhry M , Thakur UK , Rosamma NL
Indraprastha Apollo Hospital, New Delhi, India
Background
Red cell alloimmunization is a widely acknowledged complication of blood transfusion especially in the multiply transfused. Safe blood involves safety from transfusion transmissible infections as well as from other non‐infectious complications including alloimmunization. Current transfusion practice for thalassemics deals with compatibility of ABO and Rh(D) group alone but do not cater to the risk of development of alloimmunization. Serological phenotyping in multiply transfused patients is usually not reliable due to presence of multiple cell populations unless performed prior to the first transfusion. Under such circumstances, molecular blood group analysis offers an effective alternative for typing red cell antigens so as to provide better matched blood.
Aim of the study
To perform molecular blood group genotyping in chronically transfused thalassemia patients and assess the risk of antigenic exposure and incidence of alloimmunization with current transfusion protocols.
Table 1.

Methods
Forty seven thalassemics registered with us underwent molecular blood group genotyping using BioArray HEA BeadChip Technology (Immucor Inc. US) for Rh, Kell, Duffy, Kidd, MNS, Lutheran, Dombrock and other antigen systems in January 2014. Patient demographics and age at start of transfusion were recorded. One year transfusion records (from January to December 2013) were retrieved, which included the units transfused, Pre‐transfusion antibody screening status and antibody identification results, if any.
The Rh and Kell phenotype of the units transfused were also retrieved from the departmental records. These were compared with the Rh and Kell genotype of the patient to assess the antigenic exposure, if any, received by each patient during this 1 year and the frequency thereof.
Results
Molecular genotyping was done for 47 thalassemics. Six of these patients were already alloimmunized (3 with anti E and 3 with anti K) and were receiving the corresponding antigen negative units. We observed that random selection of ABO and Rh (D) matched units resulted in 56.6 ± 8.26% chance of Rh and Kell phenotype matching also. Of the 47 thalassemics studied, 44 had received one or more antigenic exposures at‐least once during the study period (Table 1). The 6 already alloimmunized patients were further exposed to antigens other than the ones they were immunized to.
During the study period, only one patient developed an alloantibody, anti E. The said patient's blood group was ARh(D) positive and genotype cceeK‐ and had received antigen exposures with antigens C (92%) and/or E (32%) at each transfusion.
Conclusion
Several factors apart from mere antigen exposure may influence the development of alloimmunization, as most of our patients received antigenic exposures during the study period and most likely in the past as well. Our data provides an impetus for future large scale comprehensive studies to understand the development of alloimmunization in such patients.
Molecular genotyping does have an edge over serology in improving transfusion safety by allowing better and more reliable antigen matching of blood. Since, the most common alloantibodies are directed against common Rh and Kell antigens, availability of molecular blood typing and better matching can go a long way in preventing further alloimmunization among the chronically transfused patients.
P‐586
Exon 3 Deletion of A1 Gene Causes Mixed‐Field Agglutination in the K562 Cell Study Model
Chen DP1, Sun CF 1, Wang WT 1, Tseng CP 2
1Chang Gung Memorial Hospital, Taoyuan, Taiwan2Chang Gung University, Taoyuan, Taiwan
Background
In the case of B3 with typical mixed‐field agglutination of RBCs in the presence of anti‐B or anti‐AB antibody, a number of genetic alternations have been reported. It is well known that the IVS3+5G→A mutation in the B gene destroys the consensus of the splice donor site leading to exon 3 skipping during mRNA splicing. The lack of exon 3 likely causes short stem region and produces B3 protein that is unstable and is concomitant with a decrease in B3 protein expression. Whether the phenomenon also appears in the A blood type is of question.
Aims
In this study, we evaluate whether exon 3 deletion of A gene also results in mixed‐field phenotype.
Methods
Site‐directed mutagenesis was used to generate the cDNA encoding A1 gene with exon 3 deletion. The cDNA was stably expressed in the K562 cells. The expression of A antigen was compared with the parental K562 cells that did not express A antigen and the stable K562 cell line expressing A1 cDNA by flow cytometry analyses.
Results
The exprssion of A antigen in the A1 stable cells and the parental K562 cells was set as 100% and 0%, respectively. The mean relative percentage of A antigen expression for the cells of A1 with exon 3 deletion was 63.9% of A1. Consistent with the observations of B3 which was exon 3 deletion of B gene, mixed field agglutination was observed for the cells expressing A1 with exon 3 deletion.
Conclusions
Exon 3 deletion results in mixed field phenotype in both A and B gene. However, the degree in antigen expression for exon 3 deletion in A gene was less severe when compared with the deletion occurred in B gene.
P‐587
Red Cell Molecular Genotyping – in vitro Diagnostic Regulation
Powley TL , Ismay S
Australian Red Cross Blood Service, Kelvin Grove, Australia
Background
Historically, regulation of in vitro medical devices (IVDs) in Australia was restricted mainly to infectious disease screening tests for HIV and HCV. In July 2010 this was expanded to include all medical devices associated with testing of human specimens. Under these new regulations, IVDs developed or manufactured in‐house by organisations are required to be registered with the Therapeutic Good Administration (TGA), or accredited by NATA, depending on the IVD classification. The Australian Red Cross Blood Service (Blood Service) has been working to standardise and reduce the number of in‐house IVDs, including the red cell molecular genotyping assays that have been in use for well over a decade.
Methods
An open tender was conducted to source an Australian Register of Therapeutic Goods (ARTG) listed molecular genotyping platform to replace the current in‐house assays. The range of tests that required replacement included RHD, RHC, RHc, RHE, RHe, K, k, JKA, JKB, FYA, FYB, nullFY, GATA(FY) and FYX. At the time the Blood Service went to market there were no ARTG listed platforms, and commercial companies entering the Australian market with new platforms were constrained by the transitional arrangements for these expanded regulatory requirements. The transitional arrangements were in place for existing IVDs, however from1 July 2015, IVDs that were not available in the Australian market prior to 1 July 2010 were no longer able to be sold without ARTG listing. This added an extra layer of complexity to the process, as finalisation of the tender and execution of the supply deed were subject to the successful tenderer being able to sell the platform in the Australian market whilst their submission for ARTG listing was being considered by the TGA.
Results
As a result of the tender process, the Immucor BioArray BeadChip[TRADEMARK] Red Cell Molecular Genotyping platform was introduced into the Red Cell Reference Laboratory in Australia. The platform uses BioArray BeadChip Kits (RHD BeadChip Kit and Precise type HEA BeadChip Kit) for genotyping patient and donor samples.
The implementation process was managed using our technology transfer framework, which incorporates the change management process and includes equipment qualification, method verification, documenting procedures, training and stakeholder communications. The service that is now offered is available for both patient and donor testing to resolve anomalous phenotypes, determination of phenotype variants and determination of phenotypes in multiply transfused patients or for samples that are not suitable for phenotyping. The move from our in‐house assays to a commercial alternative has provided the Blood Service with an expanded range of testing with results available in a shorter time frame providing a significant improvement in customer service.
P‐588
Risk of Alloimmunisation Associated with Aminoacid 223 Substitution in RH Protein: Analysis with the New Variant RHD*668
Tournamille C1, Martret J 1, de Brevern AG 2, Barrault A 1, Le Floch A 1, Bodivit G 1, Bierling P 1, Pirenne F 1
1EFS IdF ‐ INSERM U955 ‐ GREx, Creteil, France2INTS ‐ INSERM UMR‐S 1134 ‐ GREx, Paris, France
Backgroud
Within the RH blood group, the RhD antigen is the most immunogenic and is of high clinical significance because of the anti‐D implication in the hemolytic disease of the newborn and in the hemolytic transfusion reaction. Multiple variants of this antigen have been described with one or more amino acid substitions on the Rh protein. In this study, based on a new variant found in 4 individuals of African background with a very marked decrease of the RhD reactivity, we explored the consequences of the 223 amino acid variation on the Rh protein.
Aim
To determine the cause of the weak D reactivity, RBCs were tested with multiple reagents, the RH haplotype was investigated and the three‐dimensionnal structural model was analysed.
Methods
Samples referred to our laboratory were sequencing according to the RHD and RHCE genes. Samples were tested with different anti‐D monoclonal antibodies and by building a three‐dimensional structural model (3D) of the RhD protein. 3D structural models of the main variants in position 223 of the RhD protein were generated and compared to the reference one.
Results
RBCs from one proband phenotyped as Dw+, C‐,E‐, c+, e+ were clearly non‐reactive with Diagast (clones HM10, P3x21211F1 and P3x21223B10) and BIORAD (clones LHM174/102 and LHM57/17) monoclonal anti‐D reagents using manual testing. On the contrary, they were weakly positive with Diagast monclonal anti‐D reagents (clones P3x61, P3x35 and P3x290) and BIORAD monclonal anti‐D reagents (clones LHM70/45 and 59/19). The sequencing of RHD exons, in order to determine the cause of the depressed D expression, exhibited a single nucleotide change, 668T>C change in D exon 5, encoding Phe223Ser in the mature RhD polypeptide. No unknown nucleotide change was found in the RHCE gene except a 733C>G associated with the RH:20 antigen. The deduced haplotype is RHD*668/RHCE*ceVS.01.
The 3D structural model shows that the RhD wild type protein has hydrophobic interactions with 8 residues while the variant S223 has none. Similar analysis was done for the V233 variant (DAR or DFV mutants). V233 RhD protein shows hydrophobic interactions with 5 residues (4 in common with the RhD wild type protein).
Conclusions
In this study, we report a new polymorphism (668 T>C) located on a RHD allele in four RBC samples with weak D reactivity. This novel nucleotide change is predicted to substitute phenylalanine at amino acid position 223 to serine which is involved in hydrophobic interactions. Despite the absence of anti‐D in carriers of this new variant, our results support a potential risk of immunization when carriers are exposed to normal D antigen.
P‐589
Abrogation of FYA Expression by a Large Indel in the FY Coding‐Sequence
Kupatawintu P 1, Emthip M 1, El Hamss R 2, Sood C 2, Ochoa G2
1National Blood Centre, Thai Red Cross Society, Bangkok, Thailand2Progenika‐Grifols, Medford, United States of America
Background
Over ten FY null alleles have been reported to date, approximately evenly distributed over FY*B and FY*A backgrounds. The silencing mechanisms include single‐nucleotide substitutions in the promoter region or the coding sequence, and deletions in the coding sequence ranging between 1 and 14 nucleotides.
Aims
To identify the molecular basis for the discrepancy between the Fy(a−) serology and the Fy(a+) predicted phenotype of an Asian donor from Thailand.
Methods
Antigen typing was performed by the gel column method with the following reagents: Anti‐Fya Human Monoclonal IgG (DG‐FY‐02) from Bio‐Rad, Anti‐Fya Polyclonal IgG from CE‐Immundiagnostika, Anti‐Fya Polyclonal IgG from CSL. Genotyping was done with genomic DNA extracted from whole blood on the Progenika‐Grifols ID CORE XT test. Sequencing of the FY promoter region, exons 1 and 2, and flanking intron regions was performed by the standard Sanger dideoxy method.
Results
Serological Fya typing reactions were all negative, whereas Fyb typing was positive. No adsorption‐elution test was performed with anti‐ Fya. Genotyping on ID CORE XT found an apparently common FY*A/FY*B genotype and predicted a Fy(a+b+) phenotype. DNA sequencing identified a heterozygous indel in FY exon 2 consisting of a 201‐nucleotide deletion from position c.296 through c.496, and a 9‐nucleotide insertion (AGGCCACTG).
Summary/Conclusions
The DNA sequencing results provide an explanation for the Fya phenotype/genotype discrepancy. We have named the new allele defined by this indel FY*A(296del201,ins9). The FY*A background has been inferred from the serology data. The indel is predicted to encode a truncated in‐frame product that lacks 67 amino acid residues spanning the 2nd transmembrane domain through the 2nd intracellular domain of the protein, and includes an additional 3 residues. Interestingly, the deletion in FY*A(296del201,ins9) abuts the 14‐nucleotide deletion in the previously reported FY*A(282del14) null allele, which suggests the presence of a recombination hot spot around positions c.295‐296.
P‐590
Introduction and Qualification of an Automated Blood Group Genotyping System into a Routine Blood Donor Setting
Manfroi S 1, Artusi P 2, Biguzzi R 3, Lodi G 4, Nucci S 5, Cotti V 1, Innocenti C 1, Mattarozzi S 1, Zampino D 1, Velati C 1
1Unità Operativa Immunoematologia e Medicina Trasfusionale Area Metropolitana Bo, Bologna, Italy2Servizio Immunoematologia e Medicina Trasfusionale ‐ Policlinico Universitario, Modena, Italy3Dipartimento Patologia Clinica‐Azienda USL della Romagna, Cesena, Italy4Servizio Immunoematologia e Medicina Trasfusionale, Ospedale S.Anna, Cona Ferrara, Italy5Servizio Immunoematologia e Medicina Trasfusionale ‐ AUSL della Romagna, Rimini, Italy
Background
Our Transfusion Service as Blood Regional Centre of Emilia‐Romagna region (Italy) started a new project based on the identification of rare blood group donors using high throughput and LIS connected automated genotyping system (GS). In the process flow‐chart we identified critical steps, thus performance qualification (PQ) was designed and executed in order to verify and document the performance of GS and of the bidirectional interface with LIS.
Aims
The aim of this work, carried out in our Advanced Immunohematology Lab, is to describe the implementation and validation of a high throughput GS (BLOODchip ID CORE XT and BIDSXT software, Progenika‐Grifols, Spain) connected with our LIS (Eliot, Engineering, Italy).
Methods
A specially designed algorithm was implemented in our LIS to select recurring donors (criteria: age, ABO/Rh, number of donations) and to manage the electronic transfer of the samples to the genotyping platform. After automated DNA extraction (QIACube, Qiagen, Germany), samples are processed according to BLOODchip ID CORE XT protocol based on xMAP® technology and analyzed by Luminex® platform. Results are directly exported to LIS, which verifies the coherence of results with previous serological and/or molecular typing, if present, and detects the discrepancies. Discrepant results and weak typings are solved/verified by sequence‐specific primer PCR (PCR‐SSP) in order to validate the results. Subsequently, LIS selects extended genotyped donors for red blood cell (RBC) antigen serological typing (ABO, Rh, Kell, Kidd, Duffy, MNS) sending the work list to one of the validated fully automated systems of the Lab (NEO Immucor ®, IH1000 Bio‐Rad). The obtained serological results are transferred directly to the LIS, which finally checks the congruence of molecular and serological typings. Discrepant results are solved by the method (microplates, gel‐column agglutination) not applied in first instance. After validation, the results are integrated in donor immunohematological profile.
Results
FromDecember 2014 until February 2015 as a PQ we performed RBC genotyping on 149 DNA samples; we obtained a complete genotype in all except one of the 37 RBCs alleles analysed by the ID CORE XT kit, owing to out‐of‐range DNA quality. 116/149 samples had a previous molecular/serological typing and predicted phenotype was not concordant in 1/149 sample for Fybweak antigen (confirmed by PCR‐SSP). Genotyping of 33/149 samples without previous molecular/serological typing was followed by confirmatory PCR‐SSP, with a 100% concordance for all the shared antigens.
LIS donor selection was properly applied in all cases, the electronic transfer of sample data from LIS and vice versa was correct and molecular‐serological concordance was always respected.
Conclusion
The PQ results confirm sensitivity, repeatability, flexibility and robustness of this high‐throughput RBC GS. The bidirectional interface with LIS allows automated data transfer and traceability of operators. Furthermore, LIS manages and guarantees molecular/serological concordance of RBC antigen typing and it is in compliance to the minimum required standards for institutional accreditation of transfusion facilities.
P‐591
Evaluation of a Non‐Invasive Fetal Kell Blood Group Genotyping Approach by Next‐Generation Sequencing Using the Miseq Platform
Nogues N , Cobo R , Ramirez L , Farssac E , Ibañez M , Canals C , Vidal F , Muñiz-diaz E
Banc de Sang i Teixits, Barcelona, Spain
Background
Knowledge of the fetal Kell genotype in pregnancies of sensitized women with anti‐K is of great value to determine if the fetus is at risk of hemolytic anemia and allows a better management of such, otherwise, high‐risk pregnancies. Several non‐invasive approaches for fetal Kell genotyping have been developed but accurate detection is still problematic in some samples and, particularly, at gestational ages earlier than 20 weeks. The next‐generation sequencing (NGS) technology offers unprecedented possibilities of massive parallel analysis of gene‐targeted amplified sequences from maternal plasma cell‐free (cf) DNA. Initial proof of concept studies have shown promising results.
Aims
The aims of this study have been: (i) To design a NGS approach for the fetal Kell genotype analysis in plasma cfDNA and (ii) To evaluate its sensitivity and the reliability of the results in a validation study with a panel of clinical samples from sensitized pregnant women.
Materials and methods
A total of 17 plasma samples from 14 sensitized pregnant women with anti‐K have been prospectively collected during the past 3 years at a median gestational age of 22 weeks (range: 14–31). Cell‐free DNA has been extracted from 1 ml of these plasma samples as well as from artificial chimeric mixtures of plasma from individuals with known genotype (major KEL*02/KEL*02, minor KEL*01/KEL*02) using the QIAsymphony extractor. A 125 bp KEL gene fragment encompassing the KEL1/2 SNP has been amplified using gene‐specific primers with NGS suitable adaptors. The resulting amplification products have been pooled with other amplicons and sequenced in a MiSeq Desktop Sequencer (Illumina, San Francisco, CA). The NGS pipeline output paired sequence files (fastq format) have been used as input for the analysis with CLC Genomic Workbench software (CLCbio, Qiagen). The optimal analysis parameters (coverage, minor allele counts, percent of variant allele, etc.) have been adjusted in order to obtain the best performance for robust fetal KEL genotype determination.
Results
Initial tests were performed with plasma DNA from the artificial chimeric mixtures, having a 1% to 20% minor component represented. Parallel amplification of the AMEL gene XY homologous region and the SRY locus has been optimized as well to include a fetal sex marker. Optimal amplicon size, input plasma DNA and coverage have been assessed with a restricted panel of clinical samples representing the real scenario of application. Analysis of the remaining samples is currently ongoing.
Conclusions
This NGS method for non‐invasive fetal Kell genotyping using the MiSeq Platform has shown specific and sensitive detection of the fetal KEL*01 allele in clinical samples of sensitized pregnant women. This approach is also compatible with the simultaneous sequence analysis of multiple amplicons from diferent loci and/or individuals for other typing or diagnostic applications. In this sense, further developments may include other loci of blood group polymorphisms, potentially involved in hemolytic disease of the newborn.
P‐592
RhD Alleles and d Antigen Density Among Serologically d‐/c+e+ Albanian Blood Donors
Xhetani M1, Seferi I 2, Nurka T 2, Metka A 2
1Faculty of Natural Sciences, Tirana, Albania2National Blood Transfusion Center, Tirana, Albania
Background
The Rh blood group is associated to the expression of the highly homologous RHD and RHCE genes located on human chromosome 1, which respectively encode the RhD and the RhCE polypeptides. Owing to its high immunogenicity, D antigen can induce the production of alloantibodies resulting in a hemolytic transfusion reaction in alloimmunized patients or a hemolytic disease of the fetus and the newborn in alloimmunized, D‐negative pregnant women.
Aims
Appropriate assignment of the Rh status, RhD most importantly, has a critical importance in transfusion and obstetrical medicine. We aimed to observed molecular data on serologically RhD negative donors with discrepancies.
Methods
A total of 10639 blood donors were Rh phenotyped for D, C, c, E and e antigens. All samples were tested by using DiaClon monoclonal antibodies that detect the presence of the DVI variant (DiaMed GmbH, Cressier, Switzerland). The RhCcEe phenotype was determined by ID‐card human antibodies (DiaMed GmbH). Those who tested negative for RhD antigen were further subjected to weak D testing using anti‐D blend (clones TH‐28/MS‐26) (CE‐Immundiagnostika GmbH, Eschelbronn, Germany).
D weak samples as well as D‐negative samples with the presence of C or E antigen and underwent molecular characterization by analyzing the 10 RHD exons. Samples were first screened for weak D, type 1, 2 and 3 alleles by a Tm‐shift assay. The ten RHD exons in inconclusive samples were then PCR‐amplified, directly sequenced and data were analyzed with Sequencher® v5.1 sequence analysis.
Results
Among 10639 donors 89.00% were tested to be RhD‐positive and 10.85% RhD‐negative according to serology. D‐negative with the presence of C and E antigens and weak D‐positive were found 75 samples, while 77.3% (n = 58) of the samples were found to be weak D‐positive and 22.7% (n = 17) were mistyped by serology. With molecular testing we found that all the discrepant samples were D variant; approximately 62% of the variant D alleles were weak D, type 1 followed by weak D, type 3 (8.62%) and weak D type 2 (3.44%).
Conclusions
This extensive study reveals the serology discrepancy in our routine work in the laboratory. Albania is considered as a country with insufficient blood donation and the low frequency of D‐negative individuals in the overall population (i.e. 10.85%) make D‐negative units a critical problem in blood bank facilities. The molecular analysis that confirmed the prevalence of the three most common weak D variants in most variant D carriers may contribute to manage them as D‐positive and thus rationalize the use of D‐negative stock units. Those definitions allowed devising an improved D‐typing strategy, including two different methods; serology and molecular genotyping, especially for the RHD screening of regular volunteer blood donors.
Key words
Blood group, genotype, phenotype, Rh.
P‐593
The Importance of the Red Cell Genotype in Sickle Cell Disease
Trompeter S 1, McMillan A2, Lee E 1, Thornton N 3, Win N 1, Porter JB 2, Longair I 2
1NHS Blood and Transplant, London, United Kingdom2University College Hospitals London NHS Foundation Trust, London, United Kingdom3International Blood Group Reference Laboratory, NHSBT, Bristol, United Kingdom
Background
Sickle Cell Disease (SCD) is now the commonest inherited single gene disorder in England. Guidelines often promote a pre‐transfusion red‐cell phenotype preferably at diagnosis and this is often suggested as equal to a red‐cell genotype. It is becoming increasingly recognised that many phenotypes in this patient group may be incorrect particularly with reference to RH variants.
Aims
We report the case of a 39‐year‐old female with SCD. Her blood type was O R1r M+N‐S+s‐ Lu(a−b+) Kp(a−b+) kk, Fy(a−b−), Jk(a+b−) with auto‐Ce/e detected 16 years ago (serologically) She was admitted with on‐going haemolysis and PCR studies confirmed RhC variant with alloantibody anti‐Ce. Our case illustrates the importance of red cell genotyping in SCD.
Methods
The patient received a two unit top‐up transfusion due to symptomatic anaemia (Hb 60 g/l) secondary to a vaso occlusive crisis on the background of pulmonary hypertension, antibody screen was negative. The specificity of the initial unit transfused was C+c+E+e+D+K‐ and the second C‐c+D+E+e+K‐. Not further phenotyping had been done on the units. The patient was re‐admitted 16 days later febrile, anaemic and with symptoms of haemolysis, Hb was 60 g/l, HbA undetectable. She received IVIG 1 g/kg/day × 2 days and 500 mg methylprednisolone x2 and antibiotics. She was hypoxic and responded well to CPAP. DAT was weakly positive by IgG, elution negative. On antibody identification, variability was observed from the panel suggestive of the presence of multiple antibodies. The initial samples supplied to the reference laboratory was insufficient, but the results suggestive the present of anti‐s+Jkb. A selection of Rh and phenotype matched K‐ Fy(a−b−)Jk(b−)E‐s‐ units were supplied urgently, and XM was performed at hospital. The hospital biomedical scientist noted some units expressing C+ were strongly incompatible but C‐ units were compatible and the possibility of a C variant was raised. The patient received one compatible unit 7 days after readmission due to a further drop in Hb to 50 g/l and. Peak Hb73 g/l with HbA30%. She recovered well. On re‐testing 1 month later: Hb 70 g/l, HbA non‐detectable. Samples were referred to IBGRL.
Results
IBGRL identified the presence of anti‐Ce+E+s+Jkb+Fy3 and found the patient's as V+Vs+ and aberrant C expression. The predicated genotype of this patient as Dces/d(C)ces which explained the alloantibody anti‐Ce.
Conclusion
This patient illustrates the importance of the red cell genotype in sickle cell disease and also in rare phenotypes. Even if the patient had been given phenotyped blood she still would have developed alloantibodies. It is well recognised that the stimulating of the immune system in sickle cell disease can lead to a multiplicity of alloantibody formation which will make it extremely difficult to find compatible blood which can lead to life threatening delays in transfusion. NHSBT is now offering free red cell genotyping to all haemoglobinopathy patients including variants in a 18 month initiative and this data will be held on the central NHSBT database and be accessible from other hospitals.
P‐594
Abstract Withdrawn.
P‐595
Multi‐Ethnic Lewis Phenotype Prediction Using PCR‐SSP Genotyping on FUT2 and FUT3
Meyer S1, Neuenschwander K 1, Castilho L 2, Frey BM 1, Gassner C 1
1Blood Transfusion Service Zurich, Swiss Red Cross, Zurich‐Schlieren, Switzerland2Hemocentro Unicamp, Campinas, SP, Brazil
Background
Lewis antigens are ABH related carbohydrates and their expression is regulated by interaction of the two fucosyltransferases FUT2 (Secretor enzyme) and FUT3 (Lewis enzyme). In principle, active FUT3 transfers a fucose subterminal to Type 1 acceptor substrates resulting in Le(a+b−) phenotypes. Terminal addition of another fucose by active FUT2 transforms Lea to Leb, resulting in Le(a−b+). The third phenotype, Le(a−b−), is the result of an inactive FUT3, completely independent of FUT2 activity. Enzymatic inactivity of FUT2 and FUT3 is caused by a variety of inactivating single nucleotide polymorphisms (SNPs), whose distribution differs in various ethnic groups.
Aims
Since adsorbed onto red blood cells only, serological phenotyping of Lewis antigens is difficult under certain physiological conditions. Therefore, rapid and correct FUT2/FUT3 genotyping in different ethnic groups would be of great interest.
Methods
The Lewis phenotype was defined using standard serological procedures. For genotyping, an in‐house PCR‐SSP kit was developed to detect inactivating SNPs 428G>A and 385A>T of FUT2 and 59T>G, 202T>C, 484G>A and 1067T>A of FUT3, respectively. To avoid misinterpretation of 2 FUT3 null mutant signals from ‘cis‐alleles’ as compound heterozygous Le(a−b−) individuals, all FUT3 specific PCR‐SSPs were designed in bi‐specific manner, in order to allow for the distinction of ‘cis‐’ from ‘trans‐’ alleles. Individuals investigated were 150 blood donors of the Zurich area, 16 individuals of presumptive African ancestry, e.g. estimated by the presence of FY*02N.01 homozygosity, and 56 samples of Brazilian blood donors. All samples had existing serological prevalues of Lewis phenotypes, including a strong statistical overrepresentation of 100 and 8 Le(a−b−) phenotypes for individuals of Zurich and Africans, respectively.
Results
Considering above mentioned inactivating mutations and well‐known expression‐negative haplotypes of FUT3, e.g. le59,202, le202,484, le59,1067, for Caucasians, Africans, Asians and Amazonian populations(1-4), 12 specific PCR‐SSPs (four for FUT2 and eight for statistically relevant FUT3 haplotypes) were developed and delivered almost 100% (99.55%) concordance with serological prevalues for all samples. Only one sample showed a discrepancy between geno‐ and phenotyping, and subsequent sequencing delivered clear results and unambiguous phenotype prediction. However, serological retyping on a second sample could not be repeated until now.
Summary
The Lewis blood group system comprises the three common phenotypes Le(a+b−), Le(a−b+) and Le(a−b−). The kit consists of 12 PCR‐SSPs and provides a helpful and highly accurate diagnostic tool for Lewis genotyping with consecutive phenotype prediction. Since the Lewis blood group phenotype is difficult to assess in situations when affected by certain diseases and under atypical physiological conditions, genotyping the Secretor and Lewis genes FUT2 and FUT3 is therefore an attractive and accurate alternative.
(1) Soejima et al., 2009.
(2) Matzhold et al., 2009.
(3) Pang et al., 1998.
(4) Corvelo et al., 2013.
P‐596
Molecular Basis for D‐ Japanese: Identification of Novel Del and D‐ Alleles
Ogasawara K1, Suzuki Y 2, Sasaki K 1, Osabe T 2, Isa K 1, Tsuneyama H 2, Uchikawa M 2, Satake M 1, Tadokoro K 1
1Japanese Red Cross Central Blood Institute, Tokyo, Japan2Japanese Red Cross Kanto‐Koshinetsu Block Blood Center, Tokyo, Japan
Background and aims
The occurrence of D′ is approximately 0.5% in Japanese but DEL in apparently D′ individuals is relatively common compared with that in Caucasian populations. On the basis of molecular genetics, we examined D′ Japanese blood donors.
Methods
A standard serological technique was used for RhD typing and we selected 3526 D′ blood samples. Genomic DNA obtained from whole blood was used for RHD analysis by polymerase‐chain reaction (PCR) and sequencing. Multiplex‐PCR to detect all of the RHD exons and use of PCR‐sequence‐specific primer (SSP) to detect RHD deletion (RHD*01N.01) and c.1227G>A mutation (for RHD*01EL.01) were performed.
Results and discussion
Multiplex‐PCR and PCR‐SSP revealed that 3091 of 3526 D′ individuals (87.7%) were homozygous for RHD*01N.01, and 318 individuals (9.0%) had the RHD*01EL.01/RHD*01N.01 or RHD*01EL.01/RHD*01EL.01 genotype. The other 103 in the 3526 individuals (2.9%) had the known D-CE-D hybrid allele, RHD*01N.04, and the association of RHCE*Ce with RHD*01EL.01 as well as RHD*01N.04 was observed. The remaining 14 individuals had RHD*01N.01 hemizygous with one of the following alleles: RHD*01N.06 (3), RHD*01N.07 (1), RHD*04N.01 (1), RHD*DEL8 (1), RHD with c.761C>G (p.Ser254Ter) (2), RHD with c.1252T>A (p.Ter418Lysex26) (2), and apparently common RHD (4). Adsorption and elution tests with anti‐D revealed that the individuals with c.761C>G mutation were D′ while the individuals with c.1252T>A mutation were DEL. Both new DEL and RHD silencing alleles were deposited in the DNA Data Bank of Japan (DDBJ/EMBL/GenBank) under accession numbers LC004698 and LC004699. In the 3091 RHD*01N.01 homozygous individuals, more than 90% of them had the dce/dce, dcE/dcE, or dce/dcE phenotype, and the frequencies of the haplotypes dce and dcE were calculated to be 0.5207 and 0.4330, respectively. These frequencies seem to be very different from those in D′ English (dce and dcE were calculated to be 0.949 and 0.028, respectively) [1], RHD*01N.01 homozygous Chinese (0.949 and 0.006) [2], and RHD*01N.01 homozygous Koreans (0.893 and 0.099) [3].
Conclusions
The RHD genotype of more than 96% of D′ Japanese could be determined by conventional PCR‐SSP. In addition, we identified a novel DEL allele having c.1252T>A mutation and a novel RHD silencing allele having c.761C>G nonsense mutation.
References
1. Race RR, Mourant AE, Lawler SD, et al.: The Rh chromosome frequencies in England. Blood; 1948, 3: 689–695.
2. Shao CP, Maas JH, Su YO, et al.: Molecular background of Rh D‐positive, D‐negative, Del and weak D phenotypes in Chinese. Vox Sang; 2002, 83: 156–161
3. Kim JY, Kim SY, Kim CA, et al.: Molecular characterization of D′ Korean persons: development of a diagnostics strategy. Transfusion; 2005, 45: 345–352.
P‐597
The AQP1 del601G Mutation in Different European Romani (GYPSY) Populations
Flesch BK1, Morar B 2, Kalaydjieva L 2
1DRK Blutspendedienst West, Bad Kreuznach, Germany2Harry Perkins Institute of Medical Research, The University of Western Australia, Nedlands, Australia
Introduction
Iso‐immunization against the Colton blood group which is encoded by AQP1 is a rare event and a challenge for blood centers to provide compatible blood. In most published cases a frame shift or single amino acid exchanges were causative for the Colton negative phenotype only in single individuals. In contrast, an AQP1 601delG muation has been demonstrated as the only reason for the Colton deficiency in five unrelated Romani patients, three of them of Spanish decent, but not in other ethnic groups1, 2.
Aim
Screening for this mutation should be performed in different European Romani populations in order to find out whether the mutation is more frequent in this ethnic group.
Materials and methods
DNA was screened for the presence of the AQP1 del601G mutation by a TaqMan approach and positive samples were confirmed by DNA sequencing.
Results
Above 672 Romani/Gypsy individuals from Bulgaria, Romania, Slovakia, Lithuania, and Spain only one Spanish individual was carrier of the AQP1 601delG mutation [Table 1]. This person besides the mutated gene carried an AQP1 wild type gene and thus was predicted to carry a Colton positive phenotype.
Table 1: AQP1 601 delG mutation in different European Romani groups.

Conclusion
The data presentation summarised by migrational category highlights our hypothesis that the AQP1 601delG mutation originated late in the Romani diaspora, especially in Spanish Romani whose ancestors immigrated about 500 years ago to the Iberian Peninsula. Screening of additional Western European Romani would add information on the spread of the mutation in this region. The data collected from predominantly Eastern European individuals do not indicate an enhanced risk of Romani patients from these regions for iso‐immunization against the Colton blood group.
1. Saison C et al. Vox Sang 2012;103:137–44.
2. Flesch B et al. Bood Transfus 2014;12:73–77.
P‐598
Inflammatory Cytokines Genes Polymorphisms and Protection to Red Blood Cell (RBC) Alloimmunization in Polytransfused Patients with Sickle Cell Disease (SCD)
Sippert EAS , Siqueira LHS , Araújo Botelho MAB , Gaspardi ACG , Gilli SG , Saad SS , Addas Carvalho MAC , Castilho LC
Hemocentro‐Unicamp, Campinas, Brazil
Background
One of the most serious consequences of RBC transfusions in patients with SCD is alloimmunization. It has been observed that a group of polytransfused patients with SCD develops allo‐/autoantibodies against multiple RBC antigens, but not all patients develop alloantibodies following exposure to RBCs. Genetic factors controlling inflammatory responses are possible candidates, as the state of inflammation in recipients may activate the innate immune system and convert an inert or even a tolerogenic event into an immunogenic one. Interestingly, it has been described that IL1-511C and TNF-308G alleles may be related to a secretory phenotype of low capacity. Those alleles have also been associated with various immune and infectious diseases and evolutionary behavior in different diseases where the inflammatory component is relevant, such as the sickle cell anemia.
Aim
The aim of this study was to investigate the association of the inflammatory cytokines genes polymorphisms (TNF‐308G/A and IL1β ‐511C/T) with RBC alloimmunization risk among patients with SCD who received multiple transfusions.
Methods
The genotypic and allelic distributions of TNF‐308G/A and IL1β ‐511C/T genes polymorphisms were analyzed by Polymerase Chain‐Reaction (PCR) and Restriction Fragment Length Polymorphism (RFLP) for 171 patients with SCD (57 alloimmunized and 114 non‐alloimmunized to RBC antigens) and 105 healthy controls. The Hardy‐Weinberg equilibrium and the allelic and genotypic frequencies were obtained by Arlequin version 3.1 software. The frequencies of TNF‐308G/A and IL1β ‐511C/T polymorphisms were compared using the Fisher's exact test. The distribution of genotype frequencies of TNF‐308G/A and IL1β ‐511C/T polymorphisms in patients and controls were in Hardy‐Weinberg equilibrium (P > 0.05).
Results
Our results revealed a lower frequency of IL1β-511CC and TNF-308GG genotypes in alloimmunized patients compared to non‐alloimmunized (IL1β-511CC alloimmunized: 80.7%, non‐alloimmunized: 63.2%, P = 0.027, OR = 0.412, IC = 0.18‐0.86; TNF-308GG alloimmunized: 70.2%, non‐alloimmunized: 84.2% P = 0.055; OR = 0.44; IC = 0.20‐0.95). No significant difference was found in the distribution of allele frequencies among alloimmunized and non‐alloimmunized patients and healthy controls for TNF‐308G/A and IL1β ‐511C/T polymorphisms (P > 0.05).
Conclusion
Our results show that IL1β-511CC and TNF-308GG genotypes (possible low producers of IL1β and TNF alpha) can be associated with protection to RBC alloimmunization in polytransfused patients with SCD.
P‐599
Abstract Withdrawn.
P‐600
Red Cell Genotyping to Prevent and Manage Alloimmunization in Patients with Sickle Cell Disease
Londero D1, Melli C 2, Pusiol A 2, De Angelis V 3
1Azienda Ospedaliera‐Universitaria SMM, Udine, Italy2Azienda Ospedaliero‐Universitaria SMM, Udine, Italy3Azienda Ospedaliero‐Univeritaria SMM, Udine, Italy
Background
Patients with sickle cell disease (SCD) are at a specially high risk for developing alloantibodies to RBC antigens after transfusion because of racial disparity between patients and donors phenotypes. In addition, serological testing frequently will not detect partial Rh antigens widely described in black individuals and may result in typing discrepancies.
Aims
The aim of this study was to determine the predicted blood group phenotype by red cell genotyping allowing the selection of compatible blood products in two African siblings affected by SCD and on transfusion therapy.
Patients and methods
The patients were MQ, an 8‐year‐old girl and PQ, a 3‐year‐old boy, two siblings from Angola, multiply transfused. Rh phenotyping was performed by haemoagglutination using monoclonal reagents in gel test (Grifols) and on microplate (Immucor‐Gamma). Molecular RH typing was performed by using ready‐to‐use PCR‐SSP commercial kits (BAG‐Gene; Innotrain). RBC extended molecular typing was performed by using the BioArray BeadChip HEA kit (Immucor). DNA sequence analysis is in progress. Antibody screening and identification was performed by testing the patient'sserum against a panel of RBCs with microcolumn technology (Grifols) and with solid phase technology (Capture‐R, Immucor).
Results
RBCs of both siblings were serologically typed D+C+E‐c+e+. No weakness of C or e expression was noted. The direct antiglobulin test was negative in both cases. The indirect antiglobulin test (IAT) became positive for MQ, and antibody specificities were anti‐C and anti‐e. No antibody was detected in PQ serum. Molecular RH typing and DNA array analysis revealed both individuals to be ccee with variant alleles ceS /(C)ceS and with VS+/V+, hrB−, Fya−b− antigens.
Conclusions
DNA testing serves as an important complement to serologic testing, allowing superior matching of patient and donor and avoiding alloimmunization. In this case‐report, in fact, the association of serological and molecular results prevented anti‐C immunization in one patient and allowed to manage alloimmunization providing the second patient with genetically‐matched RBC units.
P‐601
Evaluation of Red Cell Genotyping in Thai Blood Donors
Kupatawintu P , Emthip M , Srisuddee A , Charoonruangrit U , Bejrachandra S
National Blood Centre, Thai Red Cross Society, Bangkok, Thailand
Background
Establishment of donor pool with red cell phenotypes to find antigen‐negative blood for patients has long been performed using serological typing. Recently, many high‐throughput genotyping commercial kits have been approached to blood banking system.
Aim
To evaluate the concordance of red cell genotyping in Thai blood donors using the Luminex xMAP platform with serologically typed in common blood group antigens.
Methods
One hundred and thirty eight group O Thai blood donors previously been serologically typed for C, E, c, e, Cw, M, N, S, s, U, P1, Lea, Leb, Mia, Jka, Jkb, Fya, Fyb, K, k, Kpa, Kpb, Dia, Lua and Lub were genotyped for 37 blood group antigens of the following blood group systems Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright and Lutheran using IDCORE XT[TRADEMARK], Grifols‐Progenika, Barcelona, Spain. The discrepancy results between serological phenotypes and red cell genotypes were repeated by serological typing and further investigated by using sequencing technique, performed by Progenika, Inc, Medford, Massachusetts, USA.
Results
Among 138 donors, no discordance result was found in C, E, c, e, Cw, M, S, s, U, Jka, Fyb, K, k, Kpa, Kpb, Dia, and Lua in this study. Discrepancy results were found in 10 donors of MN, Kidd, Duffy and Lutheran, to give 98.4%, 99.8%, 99.8%, 99.8% concordance, respectively. Seven donors with M+N+, Mia+ was GYPA*M/GYPA*M with GYP.MUR which gave false positive result by serological typing. One donor with Jk(a+b−) was JK*01W.01/JK*02N.07, which Jk*01/JK*02 result shown when using IDCORE XT[TRADEMARK]. One donor with Fy(a−b+) was FY*01/FY*02, which is suspected new FY*01 allele. One donor with Lu(a−b−) was LU*B/LU*B and KLF1*BGM12 with another mutation in exon 2 of KLF1 gene, which is suspected new KLF1 allele. The new alleles need further investigation.
Conclusions
Red cell genotyping using commercial kits in large‐scale genotyping of blood donors gave 98.4% concordance with serological phenotyping. However, the sequence‐specific polymerase chain reaction or sequencing is suggested in order to resolve discordant results.
P‐602
Additional Cases of the Fy*01w.01 Allele
López M1, Kupatawintu P 2, Morakot E 2, Puente F 3, Ochoa-Garay G 4, Tejedor D 1
1Progenika Biopharma, a Grifols Company, Derio, Spain2National Blood Centre, Thai Red Cross Society, Bangkok, Thailand3Banco de Sangre y Tejidos de Aragón, Zaragoza, Spain4Progenika‐Grifols, Medford, United States of America
Background and aim
The alleles encoding the antithetical Fya and Fyb antigens of the Duffy blood group, FY*01 and FY*02, are defined by a single nucleotide polymorphism, FY:c.125G>A. The weak expression of Fyb antigen (Fyx) is associated with nucleotide change FY:c.265C>T, which defines allele FY*02W.
A novel FY*01 allele with weakened expression of Fya antigen associated with c.265T was reported by Ardnt P et al. (1) in a fetus with alpha thalassemia.
More recently, Lopez GH, et al. (2) described a similar case in an Australian blood donor with a similar phenotype but with variants c.265T and c.298A. The names assigned by ISBT to these new alleles are: FY*01W.01 and FY*01W.02, respectively.
In this report we present additional cases of the FY*01W.01 allele, detected by genotyping in different ethnic populations.
Methods
Genotyping was performed on ID CORE XT (Progenika Biopharma, a Grifols Company). DNA sequencing of exon 2 of the FY gene was performed by the Sanger dideoxy method.
Results
ID CORE XT issued an ‘Unknown’ call for the Duffy genotype and predicted phenotype for four unrelated samples (see Table 1). DNA sequencing detected the presence of variants, c.125G (homozygous) and c.265T (heterozygous), and the absence of c.298Ain the four samples. The genotype of these samples is FY*01/01W.01 and the predicted phenotype Fy(a+b−).
These samples are carriers of the novel FY*01W.01 allele, but the common FY*01 allele in trans prevents detection of a weak Fya antigen.
Table 1. Description of the four ‘unknown’ unrelated samples.

Conclusion
The allele FY*01W.01 can be found in different ethnic groups. Further studies are needed to determine population frequencies.
The blood group genotyping assays should consider this allele in their algorithms for generating a predicted phenotype in Duffy blood group system.
Bibliography
1. Arndt PA, et al. Transfusion 2013; 53(Suppl):39A.
2. Lopez GH, et al. Vox Sanguinis 2014; 108: 52–57.
P‐603
Validation of a Commercial Kit Based on PCR‐SSP for Vel Typing
Villa MA , Paccapelo C , Truglio F , Sala V , Cosco M , Revelli N , Marconi M
Immunohematology Reference Laboratory, Milano, Italy
Background
Vel is a high incidence red blood cells (RBC) antigen first time described in 1952. Most people are Vel+, expressing the Vel antigen on the surface of their RBCs and only 1 in 5,000 individuals is Vel‐. Vel is generally expressed less strongly on cord red cells than on those of adults. Vel alloantibodies are never ‘naturally occurring’ but develop after immunization by transfusion or pregnancy. They can cause severe haemolytic transfusion reactions (HTR) and are involved in the hemolytic disease of the fetus and newborn (HDFN). A serological screen for this blood type currently exists, but it is not fully reliable because some people have a very weak expression of Vel antigen. In these cases the serological testing could give a false negative result and thus identifying the genotype would be a safer and more efficient way for donors and patients screening.
Aim
The molecular basis behind the Vel antigen remained obscure until 2013 and only after its recent identification, a reliable screening method has been developed. This new screening test is based on PCR‐SSP and identifies the Vel antigen in an effort to detect individuals who do not serologically express Vel, to make blood transfusions safer for Vel‐ individuals.
Methods
Genomic DNA was extracted from whole blood by QIAamp DNA Blood mini kit using QIAcube instrument (Qiagen, Valencia, CA, US) according to the manufacturer's instructions. We used RBC Ready Gene VEL Screen Kit (Inno‐Train Diagnostik GMBH, Kronberg/Taunus, Germany) to genotype 5 blood donors and 1 patient with anti‐Vel previously typed by serology as Vel ‐ with human serum. The kit primers are designed assuming that the SMIM1 gene codes for the Vel antigen. The Vel‐ phenotype is caused by a homozygous 17 base pair deletion in exon3 of the SMIM1 gene, which leads to a frame shift mutation and a premature stop codon. The Velweak phenotype is due to a heterozygous deletion in the same exon that results in one functional copy of the SMIM1 gene.
Results
We observed a discrepancy among serological and molecular results in all 5 donors that resulted heterozygous. Conversely a concordance was observed in the patient who resulted homozygous of the defective allele for gene SMIM1. Therefore, the Vel antigen was not produced in this patient.
Conclusion
Developing a genetic screen should make pregnancies and blood transfusions safer for Vel‐ individuals, in particular those at risk of severe post‐HTR and with allow a more effective HDNF prevention. This screening method is rapid and easy and could give a major contribution to the immunohematology field by identifying the genetic basis behind this rare blood group. For this reason, this method has recently been implemented in our Immunohematology Reference Laboratory for the identification of Vel‐ donors and for the management of alloimmunized Vel‐ patients.
P‐604
Molecular Blood Group Genotyping in Indian Thalassemics
Kulkarni S , Bhavika R , Ghosh K
National Institute of Immunohaematology, Mumbai, India
Background
Blood transfusion is the principal therapy for management of Thalassemic patients. Crossmatching with ABO and RhD matched donors is performed routinely for providing compatible blood to these patients. However RBCs are not routinely tested for all other minor blood group antigens. Therefore presence of alloantibodies against these antigens creates the potential for serologic incompatibility, makes the selection of appropriate units for future transfusion more difficult, delays the use of a potential life saving therapy and presents risk of hemolytic transfusion reaction.
In multi‐transfused thalassemic patients, hemagglutination fails to phenotype the patient's blood group antigens due to donor derived erythrocytes from previous transfusions or positive direct antiglobulin test. DNA technology has overcome the limitations of haemagglutination assays. Accurate determination of blood group antigen status will facilitate the procurement of antigen matched blood in patients who have not produced antibodies as well as in identification of appropriate antigen negative RBCs for transfusion in alloimmunised patients. Matching for the critical antigens of Rh, Kell, Kidd and Duffy blood group systems has been reported to minimize rate of alloimmunization.
Aims
To screen multitransfused thalassemic patients for clinically important blood group antigens(C, c, D, E, e, Fya, Fyb, Jka, Jkb, K, k) by serological and molecular methods.
Methods
Blood samples from 100 multitransfused beta‐thalassemia major patients were studied by hemagglutination and by PCR based technique for antigens or genes in Rh, Kell, Kidd and Duffy blood group system. Antibody detection and identification was performed using reagent panel red cells in all patients.
Results
Genotype and serological phenotype of 100 Thalassemic patients was compared for eleven antigens of four clinically significant blood group systems (Rh, Duffy, Kell and Kidd). 79% of multitransfused thalassemic patients gave discrepant results by serological (due to presence of donor red cells) and molecular methods. Out of the 79 patients giving discrepant results between phenotype and genotype; thirty three gave discrepancy with one, thirty with two, nine with three, six with four and one with five antigens. The antigens of Rh system (c, E) showed maximum and k and e showed no discrepancy between genotyping and serologic typing. A correlation between phenotype and genotype results showed concordance in Fya/Fyb in 70 of 100 patients, and Jka/Jkb in 62 of 100 patients. Five among nine alloimmunised patients were mistyped by haemagglutination.
Conclusion
Blood group genotyping has vital importance in transfusion management of chronically transfused patients especially if patients were not phenotyped before starting the initial transfusion. The determination of true blood group genotype will assist in the identification of suspected alloantibodies and in selection of antigen negative RBCs for transfusion.Red cell genotyping enabled determination of blood group when serology failed.
P‐605
Evaluation and Potential Use of Extended Compatibility Between Donors and Recipients for Transfusions in Thalassemia Patients
Belsito A1, Mancuso T 2, Costa D 1, Fiorito C 1, De Iorio G 1, Tartaglione I 1, Casamassimi A 1, Perrotta S 1, Napoli C 1
1Second University of Naples, Napoli, Italy2IMMUCOR GAMMA, Milano, Italy
Background
The implementation of Red Blood Cells (RBC) Genotyping in our laboratory allowed us typing β‐thalassemia patients for 38 erythrocyte antigens and some phenotypic variants. The comparative study included RhCE (C/c, E/e) and Kell (K/k, Jsa/Jsb, Kpa/Kpb), both typed through serological and molecular assays. To date, thalassemia patients have been usually phenotyped by serological assays and transfused with compatible RBCs for RhD, RhCE and Kell phenotype (Usual Match, UM). The RBC genotyping is a useful tool to find suitable donors for immunized patients with auto‐ or allo‐antibodies.
Aims
Our aim was to transfuse some of these patients following the compatibility for Duffy, Kidd and MNS systems (Extended Match, EM) or for Duffy and Kidd (Intermediate Match, IM), to evaluate the efficacy of the extended compatibility in alloimmunized and chronically transfused patients and to improve the outcome of RBC transfusions.
Methods
Among the 50 previously analyzed patients (with an average age of 25.5 years and a distribution of 1:2 male/female), we selected 12 with β‐Thalassemia major, of which 6 with and 6 without antibodies. All selected patients showed discrepancies or mismatches for multiple antigens between genotype and serological typing, received regular transfusions with a median interval of 15 days, and with previous transfusion reactions. For each patient we will also evaluate the characteristics of the units (Hb, volume), Hb before transfusion and transfusion interval (TI). We have selected 725 blood donors with a history of at least 3 donations (with an average age of 39.7 years and a distribution of 1:1 male/female) typed through serological and molecular methods. Molecular typing was performed by commercially available kits and platform HEA BeadChip[TRADEMARK] (Immucor, Warren, NJ, USA).
Results
For each analyzed patient we have found a number of <5 compatible donors (EM) (0.7%) and more than 15 (2.1%) donors (IM). These patients were previously typed only by serological methods and always transfused in the UM regimen. According to the obtained results, we have observed an increase of hemoglobin levels and reported a diminished frequency of transfusions by the mean TI increase, when patients were transfused with the IM regimen.
Conclusions
Data from this study highlight the discrepancies observed between molecular and serological methods and show that a patient/donor compatibility extended beyond the Rh and Kell phenotype may be associated with better survival of transfused RBC units, increased pre‐transfusion Hb levels and longer transfusion intervals, with a lower iron overload. In addition, in the long term, a minor incidence of both RBCs alloimmunization and delayed transfusion reactions are also expected. However, further efforts are needed to increase the number of extensively typed donors to reinforce the clinical data.
P‐606
A Rare P Null Erythrocyte Phenotype
Steffensen RNS , Vad JV , Aagaard Jensen BAJ
Aalborg University Hospital, Aalborg, Denmark
Background
In a serology blood type laboratory handling the rare P null phenotype, which is part of the GLOB blood type system (ISBT no. 028), is problematic. Here, we describe a male patient who was admitted to hospital for orthopaedic reasons and routine tests including ABO/D typing, erythrocyte antibody screening and compatibility tests were performed also. The patient never received blood transfusion.
Aims
To have a well‐established and approved strategy for choosing the most compatible blood component for patients in need of transfusion with the rare P null phenotype.
Methods
The antibody screening was performed with a 4‐cells red blood cell (RBC) panel with indirect antiglobulin test (IAT) using gelcards (BioRad). The antibody identification was carried out manually with an 11‐cells RBC panel using IAT and enzyme‐pretreated RBC (papain) in gelcards. Besides a 20–25°C panel was performed using tube test. Special analyses including direct antiglobulin test (DAT), full erythrocytes phenotype and adsorption analyses were also carried out. The patient's serum was exposed to an erythrocyte panel negative for the High‐Frequency antigens pp, P1k and P2k. Sequence analysis of exon 5 in the B3GALNT1‐gene encoding the functional P synthase was performed by Sanger sequencing. A TaqMan assay for the null phenotype allele was designed and used for screening of 405 Danish blood donors. LABType reverse Sequence Specific Oligonucleotide (rSSO) DNA Typing Products (One Lambda) using the Luminex xMAP technology were used for rapid and efficient genomic analysis of human leukocyte antigens (HLA)‐A,‐B,‐C,‐DRB1 and ‐DQB1.
Results
Antibody screening and antibody identification was found positive and autocontrols were negative in all tests performed. DAT was found negative. Compatibility was found with pp/Tja− (6 individuals), P1k (2 individuals) and P2k erythrocytes (2 individuals), indicating that the antibody is anti‐P. After adsorption with P1‐negative erythrocytes the serum was compatible with the panel. Erythrocytes from the patient were found compatible with anti‐P plasma from a P2k positive donor. The patient displayed a P1k phenotype. Sequence analysis revealed that the patient was homozygote for the null phenotype GLOB*01N.07 allele with an amino acid shift (Gly271Arg) based on an 811G>A nucleotide change, leading to absence of the P antigen. Samples from other relatives have been analyzed and both parents expressed the P antigen however they were found heterozygote for the null phenotype allele by genomic testing. The patient and relatives have no knowledge of existence of a common ancestor. HLA analyses confirmed that three generations (patient, parents and grandparents) demonstrated no HLA‐haplotypes in common. Furthermore 405 Danish blood donors have been tested for the same mutation and none was found to carry the null phenotype allele. The above results suggest that the transfusion strategy for this patient will consist of ABO, RhD compatible P1k, P2k (first priority) or pp (second priority) positive blood components.
Conclusions/Summary
This case report aims to increase awareness for the uncommon blood type P null. Considering anti‐P is known to cause a hemolytic transfusion reaction, the availability of blood for transfusion is therefore a challenge.
P‐607
Positive Association of HLA‐DRB1*15 with RH Alloimmunization in Polytransfused Patients with Sickle Cell Disease (SCD)
Sippert EAS1, Araújo Botelho MAB 1, Rodrigues CR 2, Visentainer JEV 2, Baleotti Junior WBJ 3, Gilli SG 1, Castilho LC 1
1Hemocentro‐Unicamp, Campinas, Brazil2Universidades Estadual de Maringá, Maringá‐PR, Brazil3Faculdade de Medicina de Marília, Marília‐SP, Brazil
Background
Blood transfusion is a vital therapy in treating and preventing the complications of SCD but despite transfusions of packed red blood cells (RBCs) significantly improve morbidity and mortality in this patient population, its use is complicated by the high incidence of RBC alloimmunization, which can result in delayed hemolytic transfusion reaction. However, not all patients develop antibodies after exposure to RBC transfusion. The hypothesis is that patient's alloimmunized to RBC antigens represent a genetically distinct group with an increased susceptibility of sensitization to blood group antigens. HLA system plays an important role in modulating the immune response and therefore the association of HLA class I and class II alleles has been investigated as a potential factor of susceptibility for RBC alloimmunization in some groups of polytransfused patients.
Aim
The purpose of this study was to investigate an association between HLA-DRB1 polymorphisms and RBC alloimmunization in polytransfused patients with SCD.
Methods
We studied 41 polytransfused patients with SCD aged 8–61 years (median, 40 years) who were alloimmunized against RBC antigens, of whom 35 (21 women and 14 men) presented antibodies against Rh (D, E, C, Cw, c, e) antigens. An additional group of 70 patients with SCD including 43 women and 27 men aged 4–63 years (median, 36 years) who received multiple blood transfusions and were not alloimmunized was also included in this study. The healthy control population studied was composed of 200 unrelated blood donors from the same geographical region of patients. HLA-DRB1 genotyping was performed using PCR‐SSO (One Lambda, Luminex, Plataform). HLA alleles and the phenotypic frequencies were obtained by direct counting. The association between HLA and the distinct groups was analyzed by Fisher's exact test.
Results
No significant difference in the frequencies of HLA-DRB1 was found in the comparison between all alloimmunized and non‐alloimmunized patients. When we analyzed the specificities of the antibodies produced we first observed that HLA-DRB1*15 was more frequent in SCD patients with anti‐E or anti‐D compared to non‐alloimmunized patients with SCD (anti‐E: 0.25 vs 0.08; P = 0.0088, OR = 4.79, IC5% = 1.45–15.62; anti‐D: 0.25 vs 0.1129, P = 0.035; OR = 4.77, IC95% = 1.11–18.57; respectively). However, an increased frequency of the HLA-DRB1*15 was also observed in alloimmunized patients who had antibodies against other Rh (C, c, e) antigens when compared to non‐alloimmunized patients (0.1714 vs 0.0642; P = 0.040, OR = 1.84; IC = 1.027–3.243).
Conclusion
Our results indicate that HLA-DRB1*15 can beassociated with susceptibility to RH alloimmunization in polytransfused patients with SCD and these allelic group may define a high‐responding phenotype to RhD and RhCE antigens.
P‐608
High‐Throughput Performance Testing of a Focused Blood Group Genotyping Panel in a Large Cohort oF DNA Samples
Nygren OH1, Young GJ 2, Lois A 1, Patel B 1, Keller MA 2
1Agena Bioscience, San Diego, United States of America2National Molecular Laboratory, American Red Cross, Philadelphia, United States of America
Background
Genotyping of blood group antigen genes represents an attractive alternative to serologic methods. MALDI‐TOF MS (matrix‐assisted laser desorption’ ionization time‐of‐flight mass spectrometry) has emerged as a powerful technique for blood group genotyping(Hemo ID[TRADEMARK] Panel, Agena Bioscience, Inc.). While the previously described Hemo ID Panel targets more than 100 antigens, a less comprehensive panel would be useful for high throughput screening of donors for the clinically relevant antigens. For this application, we developed and tested a new module (HemoID[TRADEMARK] DQS) that tests for antigens in RH, MNS, FY, LU, KEL, JK, DO, YT, SC, LW and DI. This assay set consists of two multiplex PCR reactions targeting 33 genomic variants and can be used to analyze 384 donor genomic DNA samples in 6 h with minimal hands‐on time. We report here the performance of that assay set using close to 1000 DNA samples.
Aims
Validate the HemoID DQS module in a clinical laboratory setting as well as compare its precision, robustness, throughput and turnaround time compared to already established technologies.
Methods
Verification of assay robustness, throughput and turnaround time was performed on 768 unique HapMap DNAs. Automation was enabled using a Hamilton liquid handler and a single 384 format MassARRAY[TRADEMARK] 4 For the challenge study, we obtained and tested a total of 88 pre‐genotyped samples (Immucor HEA BeadChip[TRADEMARK] or Serology). This study was performed in two labs with all samples being analyzed at a major blood center and 70 samples tested in‐house. Hemo ID software for automated genotype and predicted phenotype assignment was used to process data generated with the HemoID DQS Module. We investigated all unexpected discordant results between previously determined phenotypes and those predicted in this study using independent molecular methods such as allele‐specific PCR and targeted genomic sequence analysis.
Results
The verification study of 768 DNA samples, including testing, data interpretation, and report generation, was completed within 2 days by one operator. We observed >99% call rate and 100% concordance of genotypes and predicted phenotypes compared to the Hemo ID panel. If this testing were performed using the lower throughput 96 well format, it could be completed in <5 days. For the challenge study, 88 samples were analyzed by the major blood center while a subset of 70 samples were analyzed in‐house, both sites using the 96‐well format. For the challenge study, concordance across the 1626 variant calls available from the HEA BeadChip[TRADEMARK] was 99.4% at the major blood center and 99.3% for the 1477 variant calls in house. Discordant calls were most frequent in GYPB, where gene conversions and deletions are common.
Summary/Conclusions
Our performance testing data shows that the Hemo ID DQS Module is a robust assay set for molecular blood group genotyping. The assay has been tested with both reference DNA samples and blood donor samples previously tested with established methods and both datasets showed >99% concordance. The Hemo ID DQS Module enables high‐throughput predicted phenotyping of blood group antigens that is well suited for screening of blood donors.
P‐609
Usefulness of Red Cell Genotyping for Thai Transfusion‐Dependent Thalassemia Patients
Watanaboonyongcharoen P1, Pruksa C 1, Onpuns S 1, Rojnuckarin P 2
1King Chulalongkorn Memorial Hospital, Bangkok, Thailand2Chulalongkorn University, Bangkok, Thailand
Background
Thalassemia major is the most common chronic hemolytic anemia in Southeast Asia. Regular blood transfusion is the main treatment. Nonetheless, red blood cell (RBC) alloimmunization may cause delays in finding compatible blood and shortened in vivo transfused RBC survival. Autologous red cell phenotyping is an important part of antibody identification to find the matched units. In recently transfused patients, the procedure is inaccurate due to the presence of donor's RBC in recipients. RBC genotype may be used to accurately identify RBC antigens. However, the data of this method in patients with Southeast Asian ethnic background are lacking.
Aims
We compared serological phenotypes of red cell antigens and red cell genotyping in Thai thalassemia patients.
Methods
Transfusion‐dependent thalassemia patients were enrolled. Rh (C, c, E, e), Kell (K), Kidd (Jka, Jkb), Duffy (Fya, Fyb), MNS (M, N, S, Mia), and Diego (Dia) blood group systems were analyzed. Tube test and gel matrix test were used to evaluate red cell antigens serologically. Red cell genotyping was tested by the BLOODchip IDCoreXT kit and the Luminex genotyping platform.
Results
Of 68 transfusion‐dependent thalassemia patients, 46 (57.6%) were female. The median age was 16 years (range 2–66 years). Alloantibodies were detectable in 63% (43/68) and 56% of these patients (24/43) had multiple alloantibodies. Ninety‐six percent of the patients (65/68) had at least one inconclusive serological phenotype (a mixed field or weak reactivity) causing failure to obtain antigen‐matched blood. The inconclusive phenotypes were identified frequently in MNS (M, N, S) (68%), Kidd (51%), Rh (47%), Duffy (42%), MNS (Mia) (31%), and Diego (28%) blood group systems. Genotyping revealed that C+c‐ (66%) and Mia+ (15%) antigen types were more common compared with Western population. The genotype‐phenotype discordances were often found in Duffy (29%), MNS (M, N, S) (28%), and Diego (13%) blood group systems. In 3 patients who had conclusive serology, 1 of them had discordant results of the N antigen. In discrepant cases, DNA sequencing was performed. The sequencing results confirmed the genotypes derived from the kit. One case showed an inconclusive Duffy serology with an unknown Duffy genotype. The sequencing result revealed the unusual Duffy genotype of FY*A,FY*A(265T).
Summary/Conclusions
RBC serological phenotyping was not helpful in the majority of transfusion‐dependent thalassemia patients. Conversely, red cell genotyping is useful and allows us to obtain a better matched blood.
P‐610
Novel RHCE Allele (RHCE*M121L, I359T) That Encodes a Variant Rhesus Small E Antigen
Marandiuc DE1, Doescher A 2, Conradi R 1, Jung A 1, Hitzler WE 1
1University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany2DRK Blood Donation Center NSTOB, Institut Oldenburg, Oldenburg, Germany
Background
A second series of reagents for the new blood group automated system DAYmate from DAYmedical SA was validated in a clinical setting by comparison to Neo® from Immucor®. During the evaluation one blood donor sample tested discrepantly for Rhesus e antigen with the two systems.
Aims
The blood donor sample was to be further tested with additional manual methods, sequence specific primed polymerase chain reaction typing (PCR‐SSP) and sequenced based typing.
Methods
DAYmate used a new column agglutination technology on discs with initially empty columns with the antisera DAY (2) anti‐e. Neo® used microplate agglutination and two anti‐e antisera: ImmuClone® (1) and ImmuClone® (2). Manually, the sample was tested on Bio‐Rad ID‐Card ‘Rh‐Subgroups+Cw+K’ (human antibodies) and ID‐Card ‘DiaClon Rh subgroups+K.’ Also, the sample was tested on Bio‐Rad ID‐Cards (‘NaCl, Enzyme Test and Cold Agglutinins’ and ‘LISS/Coombs’) by adding different anti‐e antisera: BAG, DAY (1), DAY (2) and both ImmuClone® antisera. The cell lines of all antisera are presented in table 1. Standard Rhesus DNA typing was performed using a PCR‐SSP test from BAG Healthcare (BAGene RH‐TYPE). DNA and cDNA sequencing of the RHCE gene was performed with Taq‐polymerase cycle sequencing using fluorescent‐labeled dye terminator reactions. The sequencing data were analyzed and compared to known reference sequences with MacVector® Software. The density of Rhesus e antigen was analyzed with flow cytometry, using a technique described by WA Flegel et al. in 2002 in Transfusion.
Results
The donor erythrocytes reacted negative with DAY (2) anti‐e antisera and positive with both ImmuClone® antisera. A test rerun on DAYmate showed a very weak positive reaction. Manually, all the reagents reacted strong positive except the DAY (2) anti‐e antisera. The PCR‐SSP confirmed the presence of the Rhesus e allele. To clarify these results, the sample was DNA and cDNA sequenced. Two mutations were found. One A/T substitution on exon 3 at the nucleotide position 361, encoding a M121L amino acid substitution. The second mutation T/C was found on exon 8 at the position 1076 encoding the I359T amino acid substitution. The antigen density was performed with an antisera of the cell line MS 16+21+63 and found 22,273 antigens per erythrocyte, comparable to the control sample (CcD.Ee). The serological reactions of different antisera can be seen in table1.
Table 1. Serological reactions of the sample with different methods and anti‐e antisera.

Conclusion
We report the novel RHCE* M121L, I359T allele. The Rhesus e antigen encoded by this allele reacts negative with only the anti‐e antisera of the cell line MS 62. This case reaffirms the necessity of serological blood group antigen testing in the laboratory routine with at least two different clones.
P‐611
A Missing RHD‐CE‐D Hybrid in an RHD Positive Blood Donor: Implications for Future Blood Group Interpretative Algorithms
Flower L , Lopez GH , McGowan EC , Millard GM , Liew YW , Hyland CA
Australian Red Cross Blood Service, Brisbane, Australia
Background
A requirement of automated protocols for donor RHD blood group genotyping is that the interpretative algorithms include the array of partial D antigen types, exemplified typically by the DVI type. In these RHD-CE-D hybrids RHCE exons have been inserted into the RHD gene replacing the RHD sequence. From a panel of fully typed donors, 79 donors serologically typed as RhD positive were genotyped.
Aims
To investigate the extent of genetic variation in serologically unremarkable RhD positive donors and to characterize any novel RHD alleles detected.
Methods
RhD phenotyping was performed by accredited routine protocols. SNP genotyping was performed on BLOODchipv4 (Progenika Inc). RHD exon scanning was performed by both Progenika and in this laboratory. Sanger sequencing of the RHD gene was performed using published primers. Genotyping was repeated on samples collected on separate occasions. Extended RhD epitope mapping was performed using the HIRO D‐variant kit and by the D‐elute test using the D‐screen Diagast™ kit.
Results
For 76/79 donors, as expected, no variation from the reference RHD sequence was noted. Two donors represented weak D type 2 and 3 respectively, which is not remarkable. In one case, a long term donor always typed as RhD positive, the RHD result for SNP genotyping was ‘not valid’. Exon scanning did not detect RHD exons 5 and 6. Sequencing revealed that RHD exon 5 has been replaced by RHCE exon 5, with no conclusive determination of sequence breakpoints between 5 and 7. For all other RHD exons SNP mapping was as expected. RHD gene sequencing confirmed that for exons 1 to 4 and 7 to 10 the sequence was as expected for RHD. This variant is consistent with a RHD*D(1-4)-CE(5-6) D(7-10) however a deletion in the region of exon 6 has not been excluded. Epitope mapping showed that the red cells reacted with all monoclonal antibodies excepting HIRO‐6, P3X241 and HM10 which are directed against epitopes 5.1, 5.4 and 6.6 respectively.
Summary/Conclusions
A novel hybrid was detected in a donor consistently reported as an unremarkable RhD positive. For this RHD-CE-D hybrid the genetic and serological findings are consistent with partial D‐epitope expression. To our knowledge this RHD genetic profile has not been reported in the literature and is missing among the hybrids (over 17) that are registered on the ISBT data base. In this case management of the donor as RhD positive is appropriate. However, for this hybrid at least three epitopes are not detected. For an individual who is hemizygous (or homozygous) for this hybrid and becomes a recipient of transfused RhD‐positive red blood cells there is potential for formation of an anti‐D.
As various genotyping platforms, particularly massive parallel sequencing in which all blood group variants are detected, are more widely applied new genetic and serologic complexity in blood groups, particularly in the Rh system, is being revealed. For both SNP genotyping and massive parallel sequencing rapid updating of bioinformatic interpretative algorithms will be required to manage this emerging array of genetic diversity.
P‐612
Extensive Donor and Patients Typing for a Better Match in the Transfusion Medicine Department of Padua – Italy
Vio C , Vicarioto M , P Albergoni M , Sanavio D , Trovato E , De Silvestro G
Azienda Ospedaliera Padua, Padova, Italy
Background
Molecular Immunohematology for the extensive typing of donors and critical patients.
is essential in order to avoid immunizing potential recipients or inducing a haemolytic transfusion reaction due to serology testing discrepancies. An example is the Fyx, a weak form of Fyb, which may be mistyped as Fyb‐negative by serology thus leading to potential alloimmunization. In 2014 in our Department the percentage of the newly immunised patients for antigens non‐ABO and non‐Rh was equal to 0.11% over a total of 51,269 units of transfused red blood cells (RBCs) to 13232 patients, with high predominance of antibodies directed against Duffy and Kidd systems. In 2009 the percentage was equal to 0.10%.
The Transfusion Medicine Department of Padua (Italy) has adopted the routine molecular typing of selected RBCs donors and patients since September 2014.
Aims
Extensive molecular typing with an intuitive automatic system capable of generating and keeping a database for third level better match for a selected cohort of donors and patients; monitoring of group‐changes and chimerism in transplanted patients and evaluating the residual disease.
Methods
From September 2014 until February 2015 154 samples of both donors and patients of Caucasian and African origin were typed using ID CORE XT (Progenika Biopharma, a Grifols Company). This is a genetic test based on Luminex xMAP® technology for the simultaneous identification of 37 alleles of 10 blood group systems. The DNA was extracted from whole blood using EZ1 automated system (Qiagen).
Results
As reported in previous data (2013–2014) in 95 blood donors 8 FYX was detected. Four of them the genotype was FYA/FYB, while in the others it was FYB/FYB. Four in the FYX samples were typed with a serological method: in two samples the phenotype was Fy(a+b−); whereas in the other two samples the phenotype was Fy(a−b+). Furthermore FyGATA was detected in one donor with FYA/FYB genotype and Fy(a+b−) phenotype. In the 152 samples we found the following genotypes: 5 samples FYA/FYB (265T) FYX; 4 FYB/FYB (265T)FYX; 23 FYA; 56 FYB; 60 FYA/FYB; 4 FYB GATA the phenotype was (Fya−Fyb−). In particular, the FYB GATA samples come from three African patients with a forthcoming bone marrow transplant: based on literature they might be transfused with Fy(a−b+) units instead of the Fy(a−b−), which is difficult to find in a cohort of Caucasian donors since the frequency is very rare. FYX allele frequency is within the published frequencies.
Conclusions
The introduction of a molecular technology in our Department has led to the creation of a comprehensive database for the easy search of a given genotype and/or a predicted phenotype. This is useful for the better match of RBCs units for multi‐transfused or transplanted patients. Furthermore, in conjunction with serological methods, it helps to correctly type both donors and patients’ samples decreasing the alloimmunization rate and the usage of rare RBCs units.
References
1. Le Pennec PY et al. Vox Sang 1987; 52; 246–249.
2. G.M. Meny. The Duffy blood group system: a review. Immunohematology 2010;26:51–56.
P‐613
Molecular Characterization of Weaker Subgroups of B Group: First Report from India
Gogri V , Jadhav A , Kulkarni S , Vasantha K , Ghosh K , Gorakshakar C
National Institute of Immunohaematology, Mumbai, India
Background
ABO blood group system is the most important blood group system in transfusion medicine. Subgroups of A and B showing weaker expression of the respective antigens and are known as weaker variants of A and B respectively. These variants can be serologically distinguished when cell and serum grouping results do not match. They arise due to mutations in the ABO gene which is responsible for conferring differences in the specificity and activity of transferases that add A (or B) specific immunodominant sugars to the precursor molecule (H antigen). The weaker subgroups of B are rarer compared to A. It is important to characterize these weaker subgroups because, if a donor of weaker subgroup is mistyped and labeled as O group, transfusion of this blood to O group recipients may lead to decreased survival of the transfused cells due to ABO antibodies present in the recipient's serum.Molecular genotyping can help in characterization of these variants and is especially valuable for distinguishing acquired variant phenotypes from inherited ones. Molecular characterization is useful as it can reveal the true genotype when serological methods cannotidentify the variant especially if the individual is a non secretor. Thus, molecular genotyping will enable us to understand the different molecular mechanisms underlying the variant expression of these alleles.
Aim
To characterize the weaker subgroups of B detected serologically at molecular level by DNA analysis.
Materials and methods
EDTA and clotted blood samples were collected from two individuals referred to our laboratory for resolving discrepancies in blood grouping. Cell and serum grouping was performed by standard serological method using monoclonal antisera (Ortho diagnostics, USA). Serological techniques like adsorption‐elution test and saliva inhibition test were used to detect the weaker subgroups. For molecular testing, DNA was extracted by standard phenol‐chloroform method and DNA sequencing was carried out for exons 6 & 7 of the ABO gene.
Results
Both propositi were phenotyped as ABweak. At molecular level, these weaker variants were characterized as: A101/Bw07 and A101/ B301 respectively. In the first propositus father was also ABweak and showed the same variant Ballele ‘Bw07’.
Conclusion
This is the first study from India reporting molecular characterization of B weaker variants. Two B variant alleles Bw07 and B301 were identified. For correct identification and characterization of weaker variants of A and B, molecular techniques should be used in conjunction with serological methods.
P‐614
The Rare Kel:1,‐2 Phenotype May be Misconcluded in the Presence of the C.1153C>T Mutation in a Kel*02 Allele Background
Martin-Blanc S 1, Letizia G 1, Monfort M 2, Vrignaud C 1, Peyrard T 1
1Institut National de la Transfusion Sanguine (INTS), Paris, France2Laboratoire d'Immuno‐Hématologie Banque de sang, CHU Liège, Liège, Belgium
Background
Kell is a complex blood group system encoded by the KEL gene that currently includes 35 antigens officially recognized by the International Society of Blood Transfusion (ISBT). Some people may be subject to unusual K or k expression. Out of the exceptional Ko (Kell‐null) phenotype with a complete lack of Kell antigen expression, the Kmod (Kell‐mod) phenotype corresponds to weakly expressed Kell antigens, either encoded by a KEL*01 or KEL*02 allele. The highly sensitive adsorption‐elution test procedure is often required to detect Kmod individuals. The clear distinction between the Ko and Kmod phenotypes is sometimes not easy and molecular testing is a helpful tool to investigate such cases.
Aims
We report here a mutation in a KEL*02 allele responsible for a strongly decreased k expression.
Methods
We investigated four K+ unrelated patients referred to our reference laboratory for the following reasons: (i) very weak k reactivity; (ii) discrepant results with several anti‐k reagents; (iii) confirmation of a rare k‐ phenotype. Phenotype investigation was carried out by standard hemagglutination methods and adsorption‐elution testing was performed with a polyclonal anti‐k reagent and acid elution method. Genomic DNA was isolated from WBCs. Sequencing analysis was performed on each of the 19 exons of the KEL gene (NM_000420.2), including flanking intronic regions, as previously described (Martin‐Blanc S. et al. Transfusion 2013;53(11 Suppl 2):2859–66).
Results
Sequencing of the KEL gene showed that the four unrelated patients (with Northern African, African, Iberian and Italian ancestry) had a KEL*01/KEL*02 genotype (c.578C>T in exon 6). Sequencing analysis revealed the presence of the same mutation in exon 10, c.1153C>T, at heterozygous state. This missense variant is predicted to encode the p.Arg385Cys amino acid change. According to SIFT and PolyPhen software, this mutation is predicted to be ‘deleterious’ and ‘probably damaging’ with regard to the protein expression (score 0 and 0.954, respectively). Adsorption‐elution studies confirmed a very weak expression of the k antigen in the four patients.
Summary/Conclusions
The c.1153C>T nucleotide change (p.Arg385Cys) has been reported in the 1000 Genomes Project (rs143217520), with a frequency of 0.4% in people of African descent (no cases found in Europeans), and in the ESP6500 exome study, both in African‐Americans (0.09%) and European‐Americans (0.05%). However, the link between this mutation and its impact on the k blood group antigen expression was not established. The c.1153C>T mutation is here confirmed to be found in both k+w people of African and Western Europe ancestry. Our study also shows a likely higher prevalence of this mutation in the Mediterranean basin area. We propose that this novel KEL*02 allele should be included in the official list of alleles of the Kell blood group system by the ISBT Working Party on Red Cell Immunogenetics and Blood Group Terminology. Finally, we recommend that K+ people showing ambiguous k typing results should be fully investigated by KEL*01/02 genotyping and/or adsorption‐elution studies in order to confirm/infirm their rare k‐ phenotype, especially those of African or South European descent.
P‐615
A Novel RHD Allele Resulting in a Weak D Variant
Moser I1, Tomás C 2, Rodrigues MJ 1, Chabert T 1, Santos M 1
1Instituto Portugês do Sangue e da Transplantação, IP, Lisboa, Portugal2Hospital Egas Moniz, Serviço de Imunohemoterapia, Lisboa, Portugal
Background
The highly polymorphic D antigen of the Rh system has a large number of RHD variant alleles characterized through the serological study of D typing discrepancies and RH sequencing.
Aims
This report describes a new single nucleotide polymorphism (SNP) c.635G>A in the RHD gene.
Methods
A whole blood sample was studied due to an RhD discrepancy through: i)Rh phenotype confirmation, ii) RhD testing with anti‐D monoclonal panels, D‐Screen, Diagast, France and Advanced partial Rh D typing kit, Albaclone, UK, iii) sequencing of the exons 1‐10 of the RHD gene.
Results
The phenotype was Ror (probable genotype cD*e/cde) and had a nonspecific pattern with the extended anti‐D panels ‐ weak/negative with IgM sera and strong positive (4+) with IgG sera. Sequencing of RHD revealed homozygosity for c.635G>A in exon 5, encoding a Gly212Asp in the RhD protein.
Conclusions
The c.635G>A, associated with a ce haplotype, was assumed to be a novel RHD allele. Other SNP′s close or in the same nucleotide ‐ c.634G>T1, c.634G>C2 and c.635G>T3 give rise to three different D phenotypes due to amino acid 212 changes in the RhD protein: Weak D (WD) type 23 (p.Gly212Cys), Del (p.Gly212Arg) and D negative (p.Gly212Val). This probably happens because aminoacid 212 includes nucleotide 634 at the end of exon 4 and nucleotide 635 in the beginning of exon 5. As the c.635G>A mutation encoding p.Gly212ASP was predicted to be within the seventh transmembrane region of the RhD protein, it would be expected not to produce alloanti‐D and should be considered a WD variant. 1‐ Vox Sanguinis. 2001 Nov;81(4):254‐8; 2‐ Transfusion. 2009;49(3):465‐71; 3‐ BMC Genet. 2001;2:10.
P‐616
Case‐Report of a Newly Described RHD Allele
Quaglietta A , Esattore F , Di Saverio M , Carinci L , Accorsi P
Pescara Civil Hospital, Pescara, Italy
Background
The study of discrepancies between the serological determinations of the Rh(D) antigen performed by different reagents and methods can lead to the identification of new alleles and gives important indications for the transfusion specialist decision‐making.
Aims
This report describes the case of a 40 year old woman with a new RHD variant.
Method
The patient was of Brazilian origin; she was at the 16TH week of her first pregnancy and previously reported to be Rh (D) positive. Rh C and Rh E antigens were typed as negative on both microtiter plates and on card (CLB ‐ SANQUIN and BioVue Cards Ortho) but during serological determination of the Rh (D), a discrepancy between the systems in use was found (clone MS 201 from CLB ‐ SANQUIN on microtiter plates and clone MAD2e D7/B8 from BioVue Cards Ortho). The sample was then studied with the Extended Partial Rh D test (BIORAD) with PCR‐SSP (READY GENE CDE, READY D weak and ZygoFast, Inno‐Train, Austria) and DNA sequencing of RHD Exons 1 to 10. The latter was performed by Sanger dideoxy method by the BLOODchip Service (Grifols‐Progenika ‐ Medford, MA,USA).
Results
The sample was Rh (D) negative with CLB‐SANQUIN reagent while it was reactive (score 4+/MF) with BioVue Cards. Sample testing with the Extended Partial Rh (D) typing set gave inconclusive results. PCR‐SSP also gave contrasting results (possible D cat VI type 1, or weak D type 14) and zigosity testing of the sample gave a d/d result. The woman has given birth to a baby girl with unequivocally Rh (D) positive group and underwent post partum prophylaxis with anti‐D immunoglobulin. Subsequently, the woman came back to our observation for a new twin pregnancy and the sample was sent to Progenika for DNA sequencing. DNA sequencing of RHD Exons 1 to 10 showed: Exon 3 ‐ transmembrane domain (Nucleotide change C364A, Amino Acid Change p.122Thr) Exon 4 ‐ intracellular domain (Nucleotide change C602G, Amino Acid Change p.201 Arg) Exon 5 ‐ transmembrane domain (Nucleotide change C667G, Amino Acid Change p.223 val) Exon 5 ‐ transmembrane domain (Nucleotide change C744T, Amino Acid Change Silent) Exon 5 ‐ transmembrane domain (Nucleotide change C1025C, Amino Acid Change 342Thr). Seqscape software (ABI) was used to analyze sequence data by comparing the obtained sequence to a reference sequence from NCBI. The corresponding genotype 364A, 602G, 667G, 744T, 1025C predicted an unknown phenotype, never reported.
Conclusions
The introduction of molecular methods in an advanced Immunohematology lab could help in solving many of the discrepancies that could arise. The correct Rh(D) typing is of basic importance when assigning the result to pregnant patients or those candidates to transfusion in order to avoid unwanted alloimmunization. The use of highly advanced molecular technology could also help in identifying new weak or partial D, in this case with point mutations generated into the transmembrane or intracellular domains.
P‐617
A Novel JK*A Allele Associated with a Typing Discrepancy in a Brazilian Patient with Sickle Cell Disease (SCD)
Bub CBB1, Torres KBT 1, Aravechia MGA 1, Sood CS 2, Ocho GO 2, Castilho LC 1, Kutner JMK 1
1Hospital Israelita Albert Einstein, São Paulo, Brazil2Progenika, Grifols, Bizkaia, Spain
Background
The Kidd glycoprotein is encoded by the SLC14A1 gene with two major co‐dominant alleles, JK*A (JK*01) and JK*B (JK*02), which result from a single nucleotide polymorphism, 838A>G. The JK null phenotype had predominantly been associated with Polynesian and Finn persons, but with the availability of molecular genotyping, additional null and variant alleles have been identified in people of other ethnic backgrounds.
Aim
Here we report a novel JK*01 allele found in a Brazilian patient with SCD which abolishes Jka expression.
Methods
Antigen typing was done by gel column agglutination with a polyclonal anti‐Jka from Lorne laboratories Ltd, UK. DNA testing was done on ID‐CORE XT (Progenika‐Grifols, Spain). DNA sequencing was performed by the Sanger dideoxy method on genomic DNA extracted from whole blood.
Results
Serological typing showed the proband to have a Jk(a−b+) phenotype, but ID CORE XT predicted a Jk(a+b+) phenotype. Sequence analysis of JK exons 4‐11 revealed a heterozygous G>C mutation on a JK*01 background located 5 bp from the beginning of intron 10 (IVS10+5C, Genbank Accession Number KP202967). No other changes were identified. To the best of our knowledge, this polymorphism has not been previously reported.
Conclusion
While some microarray assays have incorporated JK null detection, these are usually limited to the more common Polynesian and Finnish mutations, IVS5‐1 g>a and c.871T>C respectively. As molecular assays continue to evolve and given the importance of Kidd antibodies, detection of additional mutations leading to JK nulls and variant alleles in different populations is essential. A family study to gather evidence for a cause‐effect relationship between the IVS10+5C polymorphism and the Jk(a−) phenotype is necessary. A population study to estimate the prevalence of this polymorphism in African Brazilians would be of interest as well.
P‐618
Integrated Serological and Molecular Methods Aimed at Determining RH Variants
Matteocci AM1, Mancuso TM 2, Castagna KC 1, Collaretti AC 1, Moscetti AM 1, Spaccino CS 1, Hailemariam TH 3, Banerjee SB 3, Pierelli LP 1
1S.Camillo/Forlanini Hospital, Rome, Italy2IMMUCOR Italia, Milan, Italy3IMMUCOR/BioArray Solutions, Warren NJ, United States of America
Background
A large number of rare RH antigens are originated by gene recombination between the RHD and RHCE alleles as well as other mutations at the RHCE and D loci. Although many of them are reported as detectable in serological studies, the contribution of molecular methods is becoming every day more important in the accurate determination of both the genetic background and the clinical implications of many phenotype variants.
Aims
Several molecular methods are available nowadays in the immuno‐hematology lab. This work presents examples of optimal integration between different methods, utilized with the aim of defining RH variants as much accurately as possible.
Methods
Antisera of different manufacturers (Bio‐Rad, AstraForMedic, AlbaClone, etc.) were used on Immucor NEO platforms to determine the status of RHD and RHCE in patients and donors routinely tested at S.Camillo/Forlanini Hospital, Rome. Samples with inconclusive D‐typing results were driven to a second level serological investigation ‐ ID Partial RhD typing kit (Bio‐Rad), or solid phase analysis with AlbaBioscience antisera ‐ in order to identify possible RhD variants. The same samples were tested in parallel with SSP kits (InnoTrain and BAGene) and RHD (or RHCE) BeadChip kit (Immucor/BioArray Solutions). A subset of 30 samples still showing undetermined typing was also tested with the research‐use‐only RHDxp BeadChip kit (Immucor) available at BioArray Solutions, Warren NJ.
Results
Of the samples that returned a discrepant result from routine serology, 422 were sent to second level serological investigation and molecular analysis. The most frequent RhD variants found in these samples were Weak D type1 (22.2%), Weak D type3 (9.7%), Weak D type2 (6.2%) and Weak D type4.0 or 4.3 (4.0%), while DVI, Weak D type4.1, 5, 11 and 15, DAU4 and DAU5, DCS‐1 or DFV, DIVa Type2, DNB, DFR, DIV Type4 were detected with frequencies lower than 2% each. Of note, 50 discrepant samples (11.8%) were not resolved by any of the methods above. By analyzing 30 of them with the RHDxp BeadChip panel, 4 were typed RHD*D-CE(2)-D, one was a RHD*DVII, one was a hybrid RHD*D-CE(10) and one was determined to be a RHD*DNU variant thanks to a confirmatory sequencing analysis of RHD exon 7.
A little group of samples from African subjects was found to have interesting RHD and/or RHCE variants. A pregnant woman was typed homozygous RHCE*CeRN, while a patient was heterozygous RHCE*ce/RHCE*ce733G. Both these variants were found in association with conventional RHD. A SCD patient had a combination of RHD*DIIIa-CE(4-7)-D and RHCE*ce/RHCE*ce48C,733G,1006T and a rare combination of RHD*04N.01/RHD*15 and RHCE*cE/RHCE*ce48C was found in another patient.
Conclusions
The integration of different typing methods, whether from serology and/or molecular biology, can be useful in providing crucial information in determining RH variants. Although commercially available kits demonstrate great performances in terms of accuracy, reliability and ease of use, there's no one product of choice that can detect all the known RHD and RHCE variants. Combining the available methods can help reduce the number of inconclusive data that require full RHCE and RHD gene sequencing.
P‐619
Evaluation of the Bioarray Hea Beadchip Method for Genotyping of Blood Group Antigens
Heroes A1, Van Sandt V 1, Naesens M 2, Parmentier K 3, Compernolle V 1, Emonds MP 1
1Rode Kruis Vlaanderen, Mechelen, Belgium2UZ Leuven, Leuven, Belgium3Karel de Grote‐Hogeschool, Antwerpen, Belgium
Background
Identification of human erythrocyte antigens (HEA) plays a crucial role in immunohematology and transfusion practice. In case of a recent transfusion or a positive direct antiglobulin test, serologic typing of HEA is compromised and genotyping is a valuable alternative. LIFECODES Sequence Specific Oligonucleotide (SSO) RBC(R) typing kit (Immucor) has been replaced by BioArray HEA BeadChip (Immucor) for genotyping of HEA, both bead‐based multiplex PCR methods detecting single nucleotide polymorphisms associated with multiple HEA's and phenotypic variants in a single test.
Aims
The purpose of this study was first to compare the genotyping results of BioArray HEA BeadChip (Immucor) with LIFECODES RBC typing (Immucor) results and with available phenotyping results using a proficiency panel, and second to evaluate the overall performance of HEA BeadChip technique.
Methods
The proficiency panel consisted of 17 reference DNA samples of which 12 samples were phenotyped. This panel was genotyped with BioArray HEA BeadChip (Immucor) according to the company's instructions for use as well as with LIFECODES RBC(R) typing kit (Immucor). Test material for overall performance evaluation of BioArray HEA BeadChip (Immucor) consisted of stored historic and newly extracted DNA with diverse concentration and purity from 762 whole blood samples of kidney donors and receptors. SSO RBC(R) typing evaluation was performed on 138 (RBC) and 21 (RBCR) routine samples. Test time, failure rate and software use were analysed for both techniques.
Results
All proficiency panel results of BioArray HEA BeadChip matched the reference results of the available serological typing results and of the RBC(R) typing for RhC/c/E/e, VS/V, K/k, Kpa M/N/S/s/U‐/Uvar, FyGATA/Fyx, Jka Js Lua Di Doa Co Joa, Hy, Lwa/b, Sc1/2 without any failures. Only 3.2% of the 762 samples tested in the performance panel, failed in the BioArray HEA BeadChip assay. 88% of these failed samples succeeded in a second run. SSO RBC and SSO RBCR tests were done in smaller batches than HEA BeadChip, and showed a first run failure rate of 5.8% and 28.6%, respectively. Low signal intensity or borderline results were observed mainly for antigen probes C/E/e, S/s, Fya/Fyb, Lua/Lub, Dia/Dib in the 762 HEA BeadChip samples and for probes e and Lub in tested routine SSO RBC(R) typing results. The overall test time to complete 96 samples was comparable in both genotyping techniques (± 6 h), however BioArray HEA BeadChip required more hands‐on time (2,5 h) compared to SSO RBC(R) typing (1 h), which can be partly overcome by pipetting automation. Finally we experienced MATCH IT! RBC software (RBC(R) typing) as being more user friendly than the BASIS software (BioArray HEA BeadChip).
Conclusions
BioArray HEA BeadChip (Immucor) showed full concordancy with all tested reference samples. Due to the well chosen antigen composition and low failure rate, it is an adequate and robust method for blood group antigen genotyping and therefore a good alternative to the SSO method. Its use in a high throughput setting such as blood donor testing, however, is questionable due to the necessity of automation and the inconvenience of the current interpretation software.
P‐620
A Novel Mutation in Fy*a Resulting in Aberrant Expression of Duffy Antigens
Tilley LA , McNeill A , Eggington J , Martin P , Thornton N , Daniels G
NHS Blood and Transplant, Bristol, United Kingdom
Background
The antithetical Fya and Fyb antigens are carried on the DARC glycoprotein encoded by FY on chromosome 1q21‐22. FY*A (FY*01) and FY*B (FY*02) differ by a single nucleotide, 125A>G, encoding Asp42Gly. The phenotype Fy(a−b−), commonly observed in people of African origin, results from homozygosity for a mutation within the erythroid‐specific GATA‐1 transcription factor binding site in the promoter region, resulting in no red cell expression of DARC. FY mutations may cause weak antigen expression; 265C>T (encoding Arg89Cys), carried most commonly on FY*B is associated with weakness of Fyb expression, known as Fyx, but has also been recently identified on FY*A. The FY*02M.01 allele carries 265C>T and an additional polymorphism 298G>A, encoding Ala100Thr.
Aims
A blood sample from an infant suffering from Thalassaemia Major was investigated because of aberrant Fy typing results.
Methods
Serological tests were performed by standard LISS tube IAT technique. Genomic DNA was extracted and standard PCR‐based allelic discrimination genotyping was carried out. DNA sequencing was performed for exons 1 (including promoter region) and 2 of FY. SfaNI restriction digestion to discriminate FY*A and FY*B alleles was performed at 37°C. Restriction fragments were separated by agarose gel electrophoresis, excised and purified for sequencing.
Results
Serological testing was limited due to paediatric sample size and unavailability of a repeat sample, however the patient's cells were shown to be negative with 2 examples of anti‐Fya and weakly positive with a third example. Cells were also negative with 2 examples of anti‐Fyb, including one known to detect Fyx. Genotyping indicated presence of both FY*A and FY*B alleles, and absence of the silencing GATA mutation. The discrepancy between apparent phenotype, Fy(a−/week b−), and that predicted by genotyping, Fy(a+b+), was further investigated by FY sequencing. Sequencing confirmed absence of the GATA mutation, and heterozygosity for 125A/G indicating presence of both FY*A and FY*B. Additionally, heterozygosity was observed for 265C>T and 298G>A in exon 2, resulting in Arg89Cys and Ala100Thr in DARC. These mutations are usually carried on FY*B, characteristic of FY*02M.01. One further heterozygous mutation was identified in exon 2, 680G>A, encoding Gly227Glu. FY exon 2 was amplified and digested with SfaNI, enabling FY*A and FY*B alleles to be discriminated and purified from agarose gel. Sequencing of FY*A revealed presence of 680G>A, while FY*B sequence showed 265C>T and 298G>A mutations.
Conclusions
This patient was found to carry FY*02M.01, in heterozygous combination with a novel variant FY*A allele carrying 680G>A, encoding Gly227Glu. Serological typing indicated an Fy(a−/week b−) phenotype, in disagreement with the Fy(a+b+) phenotype predicted by genotyping. The FY*A 680A allele appears to result in significant weakening of Fya expression, but it is notable that Fyb expression was also reduced compared to that expected from FY*02M.01, and was not detected by anti‐Fyb known to detect Fyx. It is possible that Fy antigens are further weakened due to some other unknown cause, or the variant FY*A allele is modifying expression of FY*02M.01 in some way. Unfortunately no sample remains for further serological testing, and attempts to obtain samples from family members were unsuccessful.
P‐621
A Novel Variant RHD (C.602G, C.667G, C.744T, C.957A, C.1025C, C.1063A) Allele in Brazilians
Arnoni CPA1, Vendrame TAPV 1, Person RDMP 1, Muniz JQM 1, Latini FMRL 1, Castilho LC 2
1Colsan, Sã, Brazil2Hemocentro Unicamp, Campinas, Brazil
Background
Rh is the most polymorphic blood group system with an increasing number of RhD variants. Although there is a lot of evidence regarding frequency and molecular basis of D variants, the majority of reports comprise Caucasian and African populations. Studying D variants in admixed populations, like Brazilian, is of interest, since there is an atypical ethnic background. In this study we report the molecular basis and serological profile of a novel RhD variant found in Brazilian blood donors.
Methods
A total of 360 blood donor samples with ambiguous serologic D typing results were genotyped by laboratory developed tests (LDTs) using a molecular strategy developed in our laboratory to identify D variants. Four of 360 samples showed an unknown set of SNPs. To characterize those samples, gDNA and RNA were extracted, cDNA was synthesized and all exons either from gDNA or cDNA were sequenced. Their serological profiles were evaluated with a large spectrum of monoclonal antibodies in 3 different techniques: tube, gel and solid phase.
Results
All 4 samples analyzed had a similar molecular profile. gDNA sequencing showed a 602C>G nucleotide change in exon 4, 667T>G and 744C>T changes in exon 5 and 957G>A, 1025T>C and 1063G>A nucleotide exchanges within exon 7. Analysis of cDNA sequencing showed the SNPs 602G, 667G, 744T and a deletion of exon 7. No changes were observed in exon 7 flanking regions of gDNA. Serologic patterns were consistent among the four samples with a similar monoclonal anti‐D profile and reduced reactivity in all techniques used.
Conclusion
We herein describe a novel variant RHD (c.602G, c.667G, c.744T, c.957A, c.1025C, c.1063A) allele with deletion of RHD exon 7 and atypical D antigen expression showing a prevalence of 1% (4/360) in Brazilian blood donors with altered expression of D antigen. Although the donors were not alloimmunized, further studies are necessary to assess the risk of RhD alloimmunization.
P‐622
Routine Complementary Molecular and Serologic Antigen Determination in a European, Mainly Hematologic Patient Population
Wagner FFW , Bittner R , Schmandt S
Red Cross Blood Service NSTOB, Springe, Germany
Background
Serologic antigen determination may fail, if the patient has been recently transfused or has a strong positive direct agglutinin test (DAT). This problem is well‐known for patients with inherited anemias like sickle cell disease and thalassemia. The impact on routine antigen determination in a routine hemato‐oncologic and preoperative setting as represented in our laboratory is less well documented.
Aims
In order to prepare for extended‐matched antigen strategies, we determine the antigen status for Fya, Fyb, Jka, Jkb, S and s for all patients with immunohematologic problems like irregular antibodies. To detect serologic failures, we devised a policy to complement serologic typing by a molecular check of a diagnostic SNP in the case of recent transfusion, positive DAT or positive results for all antigens. Here we report a compilation of the results in 599 patients.
Methods
Serologic antigen determination was done with commercial antisera. In the case of a positive direct agglutination test or recent transfusion, monoclonal direct agglutinating reagents were used for Jka, Jkb, S and s. A result was considered positive if a 2+ agglutination was seen or a 1+ agglutination was obtained for both antithetical antigens. Weakened reactivity or mixed field patterns were considered doubtful positive. The molecular check was performed using pooled capillary electrophoresis after DNA extraction with the QIAAmp Blood Mini kit.
Results: For 305 of 599 serologic antigen determinations, a molecular check was performed. Full concordance with normal antigen strength was seen for Jk in 243 cases (Fy 197, Ss 258), representing 80% (Fy 65% Ss 85%) of all cases. Discrepant results were seen for Jk in 6 cases (Fy 26, Ss 10). Serologically doubtful positive results were obtained for Jka in 31 cases (Jkb: 24; Fya: 12; Fyb:33; S:16; s: 4). The proportion of antigen negative samples among those with doubtful positive result were similar to the probabilities expected from the antigen distribution: For Jka, 22% of Jk(a?) samples were molecularly predicted to be antigen negative (Jkb: 20%; Fya: 66%, Fyb: 44%; S: 48%, s: 25%). Molecular results of 3 of 20 samples tested because of an ‘all antigens positive’ pattern differed from the serologic result, despite no reported previous transfusions and a negative DAT; however, in all cases the respective agglutination was not full strength.
Conclusion
In a routine setting in Europe, previous transfusions ‐ often not reported or even negated ‐ and a positive DAT often interfere with antigen determination. Weakened results and mixed field patterns are frequent and may both represent antigen positive and antigen negative status. A complementary molecular verification of the serologic result is strongly advocated.
P‐623
Fy*A(‐69C): A Novel Fy*a Expression Silencing Allele Occuring in a Caucasian Family
Pisacka M1, Marinov I 1, Kralova M 1, Kralova J 1, Koranova M 2, Bohonek M 2, Sood C 3, Ochoa-Garay G 3
1Institute of Haematology and Blood Transfusion, Prague 2, Czech Republic2Central Army Hospital, Prague 6, Czech Republic3Progenika Inc, Medford, United States of America
Background
Duffy antigens are carried by the glycoprotein DARC /ACKR1, a chemokine receptor encoded by the FY gene with two allelic forms, FY*A (FY*01) and FY*B (FY*02). Silencing FY expression has been described to arise from two mechanisms ‐ either a nucleotide change at position c.1‐67 of the FY promoter or rare cases of deletions or substitutions in exon 2 of the FY gene that result in Stop codons. The first type /known as ‘GATA‐mutation’/ silences expression of FY in erythrocytes only, while the second affects all tissues. Individuals with the latter type can produce anti‐Duffy antibodies whereas ‘GATA‐mutation’ individuals tolerate transfusions of erythrocytes with normal antigen expression. Duffy null phenotypes are associated with resistance to Plasmodium vivax infection. Thus, racial differences in frequency of this phenotype are the result of selective advantage of homozygous individuals in areas with endemic malaria, especially in Africa. This report presents a new FY*A gene promoter mutation in the GATA motif associated with silencing of Fya antigen expression in a family of Greek ancestry.
Aims
This investigation was prompted by a very rare apparent Fy(a−b−) phenotype in a blood donor of Caucasian origin, and by a discrepancy between the results of serology and genotyping.
Methods
Fya and Fyb typing was performed by column agglutination (Bio‐Rad) and by microtiter plate agglutination (Immucor). Fya expression was measured by flow cytometry (Becton Dickinson FACSCanto, with anti‐Fya polyclonal from Bio‐Rad, human anti‐Fya P3TIM monoclonal from BIOSCOT, and PE‐conjugated anti‐Human‐IgG‐Fc gamma from Affymetrix). Molecular testing was performed on genomic DNA extracted from whole blood (Qiagen). Genotyping was performed on BLOODchip‐Reference and ID CORE XT (Progenika‐Grifols). DNA sequencing of the FY gene promoter and coding sequence was performed by the Sanger dideoxy method. The linkage of the causative mutation (see below) to the FY*A allele was determined by Allele‐Specific PCR.
Results
A Fy(a−b−) phenotype was observed for the propositus and his siblings. Flow cytometry for Fya showed the mean fluorescence intensity to be the same as that of a Fy(a−b+) control with a common genotype. The genotype on BLOODchip Reference and ID CORE XT was found to be FY*A/FY*BW.01, and the predicted phenotype Fy(a+b+W). Sequencing of the FY promoter, exons and flanking intron regions detected polymorphisms c.1‐69T/C, c.125G/A, c.265C/T and c.298G/A. Allele‐Specfic PCR determined the c.1‐69C and c.125G polymorphisms to be on the same allele. The FY*A allele with c.1‐69C is listed in GenBank under accession number KP967558.
Conclusions
This study characterizes a novel GATA‐motif FY silencing allele, the first example linked with FY*A in Caucasians. The apparent Fy(a−b−) phenotype was caused by the presentation of the FY*A(-69C) allele together with FY*X (FY*02W.01); the anti‐Fyb reagents used did not react with this weak form of Fyb antigen. Our finding has practical implications in transfusion strategy, for the selection of donors for reagent red cells, and for genotyping algorithms. Larger population studies will be necessary to assess the frequency of this allele.
P‐624
Is RHD Genotyping for Weak D Clinically Significant? A Study on Israeli Patients and Donors
Yahalom V , Modan S , Cohen M , Shinar E , Asher O
Magen David Adom‐ National Blood Services, Ramat Gan, Israel
Background
Identification of RHD variants is required to avoid anti‐D alloimmunization, decision on RhIg prophylaxis and on appropriate products for transfusion. Serological RhD typing is limited and cannot distinguishD variants which trigger anti‐D formation from variants which do not.’Weak D (WD)’ phenotype can be resolved by genotyping, yet routine genotyping cannot detect all genetic changes. Genotyping methods can be improved and updated based on the prevalence of different variants within a specific population.
Aims
Describe RhD typing and characterization of D variants referred to the National Blood Group Reference Laboratory (NBGRL), Magen David Adom (MDA), Israel. This may promote development of a strategy for RhD typing and improve testing methodology.
Methods
Patients and blood donors with serological weak D (<+2) were studied. During 2010‐2013 RhD typing was based on serology testing using 3 anti‐D reagents from Immucor and Bio‐Rad (‘preliminary serology’) followed by ALBAclone (Quotient) and D‐Screen (Diagast) partial RhD typing kits (‘extended serology’). From 2011 unresolved cases were referred for additional molecular testing.Since 2014, RhD typing strategy has changed and all patients’ samples phenotyped as weak D by ‘preliminary serology’ were subjected to molecular testing if an informed consent was available. On donor samples, genotyping is done for women of childbearing age.RHD genotyping was analyzed using SSP‐PCR by Inno‐Train kits.
Results
During 2010‐2013 a total of 37 unresolved cases (27 blood donors and 10 patients) were subjected for molecular typing. D variants identified included: 2 WD type 1, 1 WD type 4.0/4.1, 2 WD type 4.2, 2 WD type 5,2 WD type 11, 1 WD type 14, 4 WD type 15, 1 DBS, 1 DAU, 3 DIII, 1 DVI, 6 DVII. Variable serological results were observed for patients with the same D variant genotype. Eleven serological weak D samples with a normal RHD genotype were sent for sequencing.In 2014, 65 serological weak D patients’ samples were referred for genotyping. In 23 patients (32%) genotyping was not feasible due to administrative restrictions. D variants identified were: 26 WD type 1, 2 WD type 3, 1 WD type 4.0/4.1, 1 WD type 4.2, 3 WD type 5, 1 DFR, 3 DAU, 1 DVI.Four serological weak D samples with a normal RHD genotype were sent for sequencing.
Conclusions
During 2014, genotyping by commercial kits resolved most weak D cases (90%). Most of these patients (67%) can be managed as RhD positive with no apparent risk for anti‐D production. This data is consistent with findings among 36 obstetric patients from the USA in whom 75% were not at risk for anti‐D formation (Haspel RL, Transfusion 2015, 55(3)). Of note is the absence of WD type 2. Although the cohort is small, it demonstrates the value of RHD genotyping in consulting and managing patients with serological weak D. More data on RHD genotyping is required on the diverse Israeli population.
P‐625
Association of RHD*Weak Partial 4.0 with Altered RHCE Alleles in Brazilians
Arnoni CPA1, Muniz JQM 1, Vendrame TAPV 1, Gazito DG 1, Person RDMP 1, Latini FMRL 1, Castilho LC 2
1Colsan, Sã, Brazil2Hemocentro Unicamp, Campinas, Brazil
Background
The RH blood group system has numerous variant alleles which may affect Rh antigens expression. RHD and RHCE altered alleles are inherited together in consequence of RH genes rearrangements and therefore various RHD variant alleles have been associated with altered RHCE alleles. Recently, it was reported that RHD*weak D type 4.0 is predominantly cis‐associated with an altered RHCE*ce (c.48C, c.105T, c.733G, c.744C, c.1025T) allele in the Tunisian and French populations. In an effort to characterize the haplotype of the RHD*weak D type 4.0 carriers in Brazilians and to determine the prevalence of this altered RHCE*ce allele in our population of interest we investigated the RHCE gene in 80 Brazilian blood donors genotyped as weak D type 4.0.
Methods
PCR‐RFLP assays and sequencing of RHCE exons 1‐10 and adjacent intronic sequences were performed in all 80 DNA selected samples.
Results
Seventeen of 80 RHD*weak D type 4.0 samples (21%) carried the variant RHCE*ce (c.48C, c.105T, c.733G, c.744C and c.1025T) in heterozygous, 52 (65%) were associated to the variant RHCE*ceVS.02, 2 (2.5%) were related to the RHCE*ceVS.04 and 4 (5%) carried the allele RHCE*ce.01. Five samples presented a normal RHCE gene.
Conclusion
RHCE sequence analysis revealed that, in the Brazilian population, Weak D type 4.0 is predominantly associated with the RHCE*ceVS.02 allele. Additionally, the variant RHCE*ce(c.48C, c.105T, c.733G, c.744C, c.1025T) allele recently characterized in Tunisian and French populations was detected in 17/80 (21%) of the Brazilian blood donors.
P‐626
Alterations in S (MNS3) Antigen Expression are Responsible for Discrepant Serologic Results
Brito MA1, Fontão-Wendel R 1, Cardoso R 1, Simões AT 2, Pierrotti MR 1, Achkar R 1, Biagini S 1, Wendel S 1
1Blood Bank Hospital Sirio Libanês, São Paulo, Brazil2Centro de Imunologia e Imunogenética, São Paulo, Brazil
Background
The S/s polymorphism is represented by a single amino acid substitution in GPB (glycoprotein B) at position 29 (Met‐29 for S antigen, Thr‐29 for s antigen) due to a mutation 143 T>C at exon 4 of GYPB. A point mutation at +5 (g>t) of intron 5 and two mutations in exon 5 (208G>T and 230C>T) from GPB were described as the molecular mechanisms underlying S‐s‐U+ variants.
Aim
We described a S variant antigen in a donor presenting a different serological and molecular pattern compared with the ones described so far1,2.
Materials and method: Blood donor, 44 yo, male, African descent, group O, 42 previous whole blood donations whose six donations were typed for S and s antigen, wherein five were S‐s+ (only by tube) and the last one was S+s+ (S+ was weak positive by tube and 2+ by gel test). Additionally his red cells were typed using a panel of anti ‐S antisera (7 polyclonal and 1 monoclonal) demonstrating 2+ reactivity (only by gel test) with four (all polyclonals) anti‐S tested. Adsorption/elution studies confirmed the presence of S antigen in his red cells. The donor was also typed for U (MNS5) and He (MNS6) antigens and was positive for both. Molecular studies confirmed the presence of alleles S and s (mutation 143 T>C, exon 4 of GYPB, by AS/PCR‐RFLP1) but it was also observed a heterozygous mutation in intron 5 (5 g>t) of GYPB by PCR‐RFLP1). Additional molecular tests demonstrate the mutation 230C>T exon 5 of GYPB by AS/PCR‐RFLP1 typical for GPB variants, and the Long‐distance AS‐PCR1 showed the presence of allele He(MNS*6) and the allele S in one haplotype, and presence of allele ‘N’(MNS*30) and allele s in the other donor haplotype.
Conclusions
This donor presented an unique serologic and molecular pattern compared with the most common mutations described for GPB variations1,2. The mutations 230T at exon 5 and 5t at intron 5, concomitantly with the presence of allele He(MNS*6), and the presence of antigen S in his red cells confirmed by studies of absorption/elution, probably demonstrated that this donor presented a ‘new’ variant S antigen due to one of his haplotypes. The other haplotype demonstrates a normal expression for GPB characterized by the antigens s+, U+, ‘N’+, He‐. So far, this donor must be considered S positive as we don′t know the antigenic potential of this genotype and should be consider S‐ if he becomes a patient.
1. Storry et al. TRANSFUSION 2003;43:1738–1747.
2. Omoto R. et al. Immunohematology 2008;4: 148–153.
P‐627
Molecular Strategy for High‐Throughput Screening of Brazilian Blood Donors Serologically Typed as RHD‐Negative
Levi JE , Chinoca J , Medeiros VR , Mendrone A Jr, Dinardo CL , Almeida-Neto C
Fundação Pró‐Sangue Hemocentro de São Paulo, São Paulo, Brazil
Background
RhD typing is a mandatory step for all blood donations and is performed using serological methods, which detect the presence of the RhD antigen in the erythroid cell surface. However, serological techniques may fail in identifying donors with low RhD expression, which may lead to recipients’ alloimmunization or hemolytic reactions.
Aims
Our objective was to evaluate a large group of blood donors phenotyped as RHD‐negative using serological methods, by high‐throughput amplification of the RHD gene, willing to identify possible carriers of RHD‐variants.
Methods
Serological determination of the RhD antigen was performed using the microplate method with 2 anti‐D sera (Blend IgM/IgG clones Th28/MS26 and MS26 clone‐1 IgG). Negative results were thereafter confirmed using IAT‐tube methodology. We manually prepared 100 pools of 10 sera from 1000 donors phenotypically characterized as RhD‐negative. DNA was extracted from these pools using Qiagen Blood Kit® and submitted to real‐time PCR in triplicates targeting RHD exons 5 and 7 (SAFE Protocol). The assay for the exon 5 is designed as it does not amplify the RHD pseudogene (RHDψ) nor some hybrid rearrangements RHD‐CE‐D.
Results
Reactivity was detected in 38 pools, 9 for both exons 5/7 and 29, only for exon 7. The 9 pools exhibiting dual reactivity were dismembered and individually tested using the same real‐time PCR assay. Twelve samples were identified as reactive for both exons (1.2%) and another 5 samples were positive only for exon 7. These 5 samples and the 29 exon 7‐only positive pools were tested individually for the presence of the RHDψ using conventional PCR. All but one sample confirmed the presence of the silent gene RHDψ (3.3%) similar to previous reports in the Brazilian population. RHD negative donors with both exon 5 and 7 are more intriguing and we are currently characterizing them by amplifying all RHD exons and sequencing.
Conclusions
We describe a method of identification of RHD variants amongst serologically‐determined RhD negative donors using sera pools reducing the process costs. However, the high prevalence of the RHDψ in our population makes the pooling strategy less cost‐effective. Inclusion of a primer/probe able to spot the RHDψ in addition to exon 5 and 7 would be of great advantage in this setting. The identification of donors carrying RHD variant alleles is relevant for transfusion practice, as it reduces alloimmunization and allows the identification of donors with rare RHD genotypes. Moreover, it may lead to the discovery of new alleles of RHD and RHCE genes, contributing to the continuous innovation in immunohematology.
P‐628
A Novel SSO Genotyping Method for Blood Group Alleles on MR.SPOT® System
Albrizio MA , Marell RM , Heiliger MH , Müller SM , Kirchgessner KK , Schaffert TS , Walter LW , Kissel KK
BAG Health Care GmbH, Lich, Germany
Background
Molecular methods are well established in the blood group diagnostic field. The need of a deep analysis of the blood group specificities is based on the knowledge that incompatible transfusions can cause severe hemolytic transfusion reactions. To guarantee a safe and fast blood group typing a new SSO genotyping method on MR.SPOT® system was established to detect clinical relevant blood group alleles.
Aims
Method evaluation of a new SSO genotyping method on MR.SPOT® system to detect clinical relevant blood group alleles.
Methods
Established serological methods were used as reference to determine the blood group phenotypes. In addition, the molecular typing of blood group antigens was performed using the SSP‐PCR method (BAGene kit, BAG Health Care). DNA isolation was performed on buffy coat and whole blood samples, using column based DNA extraction methods. To determine the influence of DNA extraction methods on test results bead‐isolated DNA samples from automated DNA extraction systems were tested. To assess the system sensitivity different DNA amounts (25, 50,150 and 200 ng) were tested. All samples used for the specificity test had a DNA concentration of 15 ng/μl. To evaluate the results reproducibility the SSO genotyping was performed by different users and with three different thermal cyclers.
Results
Kell, Kidd, Duffy and MNSs alleles were tested using serology, SSP and SSO technologies. The SSO molecular investigation performed using MR.SPOT® system revealed 100% concordance with SSP results, verifying the specificity of the test. Conversely, a few discrepancies were seen between serological and molecular SSO typing. In particular, six of the tested alleles (two MNSs, one Kell, one Duffy and two Kidd) were presumably incorrectly determined by serology and in one case (Kell) it was not possible to predict the phenotype of the sample. However further investigation is required to prove the serological findings. The different DNA amounts tested did not reduce test specificity but rather proved a good sensitivity. Test performance was not affected by different types of DNA extraction methods, different users and different thermal cyclers showing high stability and reproducibility. The automated and standardized workflow facilitates the test procedure allowing high throughput in a relative short time with low efforts. That means 96 samples can be processed in 4 h 30 from DNA extraction to results. User‐friendly software was developed to support the results analysis and assist in the establishment of a results report.
Conclusion
This work presents a new genotyping method for blood group typing of clinical relevant blood group alleles using SSO genotyping on MR.SPOT® system. All results for Kell, Kidd, Duffy and MNSs alleles showed 100% concordance to a reference method (SSP) and hence ensure a safe blood group typing. All tested parameters gave the expected results indicating that the test is robust and precise. The test was easy to handle thanks to the automatized and standardized workflow provided by the MR.SPOT® technology. Taking all findings into account the new SSO genotyping method on MR.SPOT® system provides a precise, fast and automated tool for high throughput typing of blood groups alleles.
P‐629
RHD Allelic Variability in North Argentine
Trucco Boggione C1, Sippert E 2, Luján Brajovich M 3, Gaspardi AC 2, Leri M 4, Mattaloni SM 3, Castilho L 2, Cotorruelo CM 3
1Laboratorio de Inmunohematología. Universidad Nacional de Rosario, Rosario, Argentina2Hemocentro‐Unicamp, Campinas‐SP, Brazil3Laboratorio de Inmunohematología. Universidad Nacional de Rosario CONICET, Rosario, Argentina4Banco Central de Sangre‐SIPROSA, Tucumán, Argentina
Background
The molecular background of the D negative and D variant phenotypes shows substantial ethnic variability. The population of Argentine is considered to be a mixture of white Caucasian Europeans, Amerindians and Africans. It has been observed that the indigenous and African component is higher in the north region of the country.
Aim
The aim of this study was to characterize the molecular background of D negative samples expressing C and/or E antigens (D‐, C/E+) and D variant (Dvar) phenotypes in individuals from the city of Tucumán, located in North Argentine.
Materials and methods
242 blood samples (D‐, C/E+: n = 187; Dvar: n = 55) were selected for this study. The Rh status was determined by hemagglutination using specific monoclonal antibodies. The D antigen was evaluated with three different IgM anti‐Ds and a blended anti‐D. When an immediate spin‐negative result was observed with the latter antiserum, the samples were tested by the indirect antiglobulin test. C, c, E and e antigens were also investigated. DNA samples from D‐, C/E+ individuals were initially screened for the presence of the 5′ untranslated region (UTR), intron 4 and the 3′ UTR of the RHD gene using PCR strategies. Samples carrying RHD specific fragments and DNA samples from the D variant donors were studied by RHD exon scanning, PCR‐SSP, PCR‐RFLP, microarray and sequencing.
Results
Among the 187 D negative samples expressing C and/or E antigens, 40 (21.4%) carried RHD specific fragments. Hybrid alleles were found in 27 (67.5%) of these samples: 21 RHD-CE-Ds, 1 RHD-CE(4-9)-D, 4 RHD-CE(3-9)-D, 1 RHD-CE(4-7)-D2. In the remaining 13 samples, 6 (15.0%) harboured DEL variants: 5 RHD(46T>C), 1 RHD(IVS3+1G>A) and 7 (17.5%) carried null alleles: 6 RHD(581insG), 1 RHDΨ in a sample expressing the C antigen (probably a RHDΨce/dCe genotype). Twelve different alleles were responsible for the 55 Dvar samples studied: 12 (21.8%) weak D type 1, 7 (12.7%) weak D type 2, 7 (12.7%) weak D type 3, 12 (21.8%) weak D type 4, 5 (9.1%) DVI type 4,1 (1.8%) DFR-2, 1(1.8%) weak D type 5 and 1 (1.8%) DMH. Interestingly, 3 novel mutations were found in 9 samples: 1 (1.8%) RHD(763G>A), 1 (1.8%) RHD(764G>A) and 7 (12.7%) RHD(359C>A).
Conclusions
The allelic variability found in North Argentine could be explained by the contribution of the Amerindian and African ethnicity to the genetic pool of the population. The finding of DEL alleles highlights the importance of genotyping D negative donors since these RBC units have the potential to cause anti‐D alloimmunization in truly D negative patients. The understanding RHD allele repertoire in the analyzed population will help to develop reliable strategies in Blood Banks and prenatal RHD genotyping.
P‐630
A Comparison of Serological and Molecular Methodologies to Determine D Variant Status in the RCI Reference Laboratory to Review the Clinical Implications of the Current Investigation Strategy
Davison CL1, Williams MW 1, Grimsley SG 2
1NHSBT, London, United Kingdom2IBGRL, Bristol, United Kingdom
Background
Accurate differentiation between D+ and D‐ blood groups is vital both in donation and pre‐transfusion testing. Variant forms of the D antigen can complicate this process, by producing anomalous haemagglutination reactions with standard D typing reagents. Reports of anti‐D formation in apparent D+ individuals highlight the limitations of current serological D screening methodology. Molecular methods for accurate determination of D variant type have been available for many years, but new commercial platforms offering robust, reliable and cost‐effective genotyping are available.
The availability of the inno-train RBC-FluoGene PCR-SSP end point fluorescence detection system to determine D weak and partial types offers the opportunity to introduce molecular technologies into routine practice, to improve the assessment of D status in variant cases, and thereby improve patient management
Aims
To compare the effectiveness of current D screening anti‐D typing reagents including the ALBAclone® Advanced Partial D typing kit haemagglutination tests against the inno-train RBC‐FluoGene D weak/variant kit in determining D variant types.
Methods
108 samples referred to National Health Service, Blood and Transplant, UK, Red Cell Immunohaematology reference laboratory in Tooting for anomalous D investigation were selected for analysis.
Initial Rh ‐D, ‐C, ‐c, ‐E, ‐e phenotypes were established by column agglutination technique using Bio‐Rad DiaClon cards (Bio‐Rad).
Further D typing was performed using two monoclonal anti‐D typing reagents D1:BIOSCOT® Anti‐D Cell Line RUM‐1 (DVI‐) (MerkMillipore) and D2: Seraclone® Anti‐D Cell Line (RH1) BS226 (Bio‐Rad).
Extended D variant typing was performed using the ALBAclone® Advanced Partial D typing kit by the indirect antiglobulin test.
Genomic DNA was extracted from whole blood samples via the Roche.
MagNA Pure DNA Extraction system (Roche Diagnostics).
Genotyping was performed using FluoGene SSP‐PCR RBC -D weak/variant kits, which identifies 95% of the most common D weak/variant alleles (inno‐train).
Results
The following table represents the ability of each method to assign D variant status to samples that can be clinically considered D+ (Weak D types 1 and 2) and samples that should be clinically considered D‐ (Weak D Partial types e.g DAR).
Summary
The primary screening methods (Bio‐Rad and 2D screen) were found to be rapid, but miss‐identified 4% (4/108) cases of partial D types as defined by ALBAclone® and FluoGene.

ALBAclone® offers significant improvements over the primary D screen in assigning D variants types, however application of known Weak D‐CcEe haplotype associations is vital in the final interpretation of results.
FluoGene was able to discriminate four additional DAR Weak D Partial types than ALBAclone®, this is a clinically significant finding as the transfusion advice would significantly differ in these cases.
Overall, FluoGene offers further improvement to accuracy in determining D variant types, with significant benefits to patient care.
However, ALBAclone® and FluoGene could not resolve 3% (3/108) of anomalous D cases respectively.
P‐631
Atypical Associations Between DI*a and DI*B Alleles and the Band 3 – Memphis Mutation in Amazonian Inhabitants
Albuquerque RL1, Sanguino C 1, Tezza C 1, Ali KA 1, Gomes A 1, Neto PM 2
1Amazonas Blood Bank, Manaus, Brazil2Universidade Federal do Estado do Amazonas, Manaus, Brazil
Background
The Diego blood group system has a great anthropological and transfusion medicine importance, being associated with immediate, delayed hemolytic reactions and hemolytic disease of new born. Its 22 antigens are located on the integral band 3 glycoprotein, wich is encoded by the SLC4A1 gene. We studied the frequency of DI*A, DI*B alleles, band 3 (166A) and band 3‐Memphis (A166G) in individuals from brazilian amazon.
Aim
To investigate atypical associations between DI*A, DI*B alleles, band 3 and band 3‐Memphis.
Methods
It was performed genetic sequencings with custom primers in 302 patients divided in 4 groups as folowed: Group A (anemic patients with erythrocyte membrane changes), group B (anemic patients without erythrocyte membrane changes), Group C (patients without anemia with erythrocyte membrane changes) and Group D (healthy individuals).
Results
It was found a frequency of 63 (20.9%) individuals having band 3‐Memphis, of which, 54 (17,9%) were heterozygous and 09 (3%) homozygous. The Diego system genotypic frequency was DI*A/DI*B (9,93%), DI*A/DI*A (0.90%), DI*B/DI*B (89,0%), of which the followed atypical associations were found: 14 (43,75%) DI*A alelle associated to homozigous 166A polymorphismand 16 (2,82%) DI*B allele associated to homozigous 166G polymorphism, interesting this one was not found only in the B group.
Conclusion
These findings are important for understanding the genetic associations between Diego blood group system and band 3 protein as well the comprehension of the atypical associations and its anthropological and haematological significance.
P‐632
High Resolution Genotyping of the RH Blood Group System by Next‐Generation Sequencing
Halawani AJ , Altayar MA , Kiernan M , Li X , Madgett TE , Avent ND
Plymouth University Peninsula Schools of Medicine and Dentistry, Plymouth, United Kingdom
The RH blood group system is the most complicated blood group system due to encoding by two highly homologous genes, RHD and RHCE. Typing the antigens of this system by conventional serology is not very appropriate to distinguish between D positive, weak D and partial D. It is only possible to assign weak D and partial D alleles accurately using blood group genotyping (BGG). Here, 10 samples of the RH system were genotyped, 5 D positive and 5 weak D samples using a long‐range PCR (LR‐PCR) approach coupled with next generation sequencing (NGS). For every sample, both genes, RHD and RHCE, were amplified by LR‐PCR, with three amplicons for RHD and four amplicons for RHCE. Then the PCR products were fragmented, ligated to barcoded adaptors and sequenced using NGS on an Ion Torrent PGM™ platform. We showed that LR‐PCR for RHD and RHCE completely correlated with their corresponding genomic sequence. For the D positive samples, there were no obvious SNPs on the RHD exons. The 5 weak D samples have been identified as following; two weak D Type 1 (exon 6 809T>G Val270Gly), two weak D Type 2 (exon 9 1154G>C Gly385Ala) and one weak partial D 4.1 with [exon 1 48G>C (Trp16Cys), exon 4 602C>G (Thr201Arg), exon 5 667T>G (Phe223Val), exon 6 819G>A (silent)]. The LR‐PCR method has confirmed that a novel heterozygous SNP, 208 C>T (Arg70Trp) in exon 2 is derived from the RHCE gene, although it had previously been identified by a Human Erythrocyte Antigen and Human Platelet Antigen panel as belonging to the RHD gene. More samples are currently being sequenced. Our approach, we believe, will facilitate the comprehensive genotyping of the antigens of the RH system, especially those with hybrid genes or insertions/deletions. Our method is able to demonstrate novel alleles by direct sequence analysis, a major drawback of current array‐based BGG platforms.
P‐633
ASP‐PCR Technique with no Direct DNA Extraction to Determine Weak RHD Types 1, 2 and 3
Rodrigues MJ
Lisbon Blood and Transplantation Centre/IPST.IP, Lisbon, Portugal
Background
A modified ‘in‐house’ Allele Specific Primer‐Polymerase Chain Reaction (ASP‐PCR) with no direct DNA extraction was developed, in 2013, for Hbs testing (Vox Sanguinis.105, S1; P‐500). As this method showed high sensitivity, specificity and reproducibility an ASP‐PCR for the most common RhD variants, the Weak RhD (WD) types 1, 2 and 3 was started, validated and implemented, in July 2014.
Aims
To perform a simpler and less expensive ‘in‐house’ ASP‐PCR technique with no direct DNA extraction to characterize WD types 1, 2 and 3.
Methods
118 blood samples were referred to the laboratory, between July 2014 and February 2015, due to RhD typing discrepancies. The samples were then characterized through the use of the Diagast D‐Screen, France and Albaclone Advanced Partial RhD Typing Kit, UK. Whenever the anti‐D panels results associated with the Rh phenotype indicated WD types 1, 2 or 3, the genetic characterization was performed with an ‘in‐house’ modified ASP‐ Multiplex PCR for Weak D types 1 and 3 and an ASP‐PCR for Weak D type 2, with mutagenic primers directed to the point mutations of the RHD gene c.809T>G, c.1154G>C and c.8C>G. An internal control was also designed from the exon 4 of the gene coding for the β‐Actin protein (NM_001017992). All the other samples ‐ not WD types 1,2 or 3 ‐ where studied with BAG Health Care, Germany and Inno‐Train Diagnostik GmbH, Germany Kits or by sequencing of exons 1–9 of the RHD gene.
Results
The modified ASP‐PCR had distinct amplification products of 118 bp for WD type 1, 205pb WD type 2, 150 bp WD type 3 and 398 bp for the Internal Control. It was possible to identify 78 (66%) WD types 1 (16–14%), 2 (38–32%) and 3 (24–20%)) and 30 (34%) other RhD variants, including WD, Del and partial D.
Discussion
A molecular test was developed to screen for the most common RhD variants in our studied population as an attempt to simplify it and to lower its costs. As this method is really easy cost effective, it can applied to identify other RhD variants and to genotypings.
It was also possible to reduce the amount of anti‐D prophylactic immunoglobulin used in pregnant women as well as to reduce the use of RhD negative units in patients with these WD phenotypes.
P‐634
Fetal RhD Genotyping in Plasma of RH Negative Pregnant Women by Real Time PCR
Ahmadi MH1, Amirizadeh N 1, Azarkeyvan A 1, Valikhani A 1, Sayyadipoor F 1, Navidrouyan M 2, Navidrouyan M 2
1High Institute for Research and Education in Transfusion Medicine, Tehran, Iran2Iranian Blood Research and fractionation, Tehran, Iran
Background
The Rh blood group system has an important role in Hemolytic disease of newborn. The prenatal determination of the fetal Rh genotype could lead to a substantial reduction of the use of anti‐D Ig and, in the other hand; it would prevent the unnecessary exposure of pregnant women carrying an RhD negative fetus to this that, despite strict control, is still associated with an elevated number of risks.
Material and methods
In this study we used 21 plasma sample from RhD negative pregnat women. DNA was excrated from plasma by Cinnapure DNA Kit. Real time PCR reaction was done with specific primers for RHD gene exons 5, 7 and 10 also beta‐globin and SRY genes. Phenotypes of red blood cells from mothers and babies were determinded by specific anti‐serums with agglutination method.
Results
Among the pregnant women, 11 were carrying male and 10 were carrying female fetuses. Out of 10 male fetuses, 9 were RhD‐positive and one was RhD‐negative. Cell free fetal DNA (CffDNA) presence in mother plasma was confirmed by amplification with SRY primers. Out of 10 female fetuses, 9 were RhD positive and one was RhD negative. All prenatal results except one were in concordance with postnatal RhD status and fetal sex without false positive or negative results. There was a false positive result in which the genotype of fetus was determined Rh positive but after birth the phenotype of baby was RhD negative. This may be due to a variant of RhD gene. Performing real‐time PCR on CffDNA showed accurate, efficient and reliable results, allowing rapid and high throughput non invasive determination of fetal sex and RhD status in clinical samples.
P‐635
The Utility of Duffy Blood Group Genotyping in Donors and Patients
Nathalang ON1, Intharanut KI 2, Siriphanthong KS 1, Nathalang SN 3, Kupatawintu PK 3
1Faculty of Allied Health Sciences, Thammasat University, Pathumtani, Thailand2Graduate Program, Faculty of Allied Health Sciences, Thammasat University, Pathumtani, Thailand3National Blood Centre, Thai Red Cross Society, Bangkok, Thailand
Background
DNA‐based techniques have been implemented to enhance immunohaematology typings. Duffy (FY) blood group genotyping is important in transfusion medicine as the Duffy alloantibodies can be associated with delayed haemolytic transfusion reactions and haemolytic disease of the fetus and newborn.
Aims
This study aimed to determine the FY allele frequencies in Thai blood donors by in‐house PCR with sequence‐specific primer (PCR‐SSP) and to predict the possibility of providing compatible blood for alloimmunised patients.
Methods
Five hundred blood samples from Thai blood donors of the National Blood Centre, Thai Red Cross Society were included. Only 200 samples were tested with anti‐Fya and anti‐Fyb using the gel technique. All 500 samples and 4 samples of the Guinea family with Fy(a−b−) phenotype were genotyped by PCR‐SSP. In addition, the possibility of providing antigen‐negative RBCs in alloimmunised patients was calculated according to the obtained FY allele frequencies.
Results
The comparison of FY phenotyping and genotyping results was in 100% concordance. Allele frequencies of FY*A and FY*B in 500 central Thais were 0.972(972/1000) and 0.028(28/1000), respectively. Even though Fy(a−b−) phenotype was not found in this study, FY*BES/FY*BES could be identified by PCR‐SSP in the Guinea family and confirmed by DNA sequencing. Hence, to provide two units of antigen‐negative RBCs for this rare phenotype, requires recruiting more than 500 Thai blood donors.
Conclusions
Our in‐house PCR‐SSP was shown as the best means for FY genotyping when compared with the serological technique. Furthermore, it is superior for typing mutitransfused patients, antigen matching patients, providing antigen‐negative RBC units to recipients and enhancing information of anthropologic studies among populations.
P‐636
New Insights into the Gender Differences in Hemolysis: Effect of Testosterone and Mapk‐Mediated Hemolysis in Mice
Kanias TK1, dos santos Santana LS 2, Osei-Hwedieh DOH 1, Brewer GWB 1, Benos PTB 2, Gladwin MTG 3
1Vascular Medicine Institute, Pittsburgh PA, United States of America2Department of Biomedical Informatics, University of Pittsburgh, Pittsbrgh PA, United States of America3Department of Medicine, University of Pittsburgh, Pittsburgh PA, United States of America
Red blood cell (RBC) hemolysis during storage may impair RBC recovery post‐transfusion, reduce RBC functionality, and release mediators that can adversely affect cardiovascular signaling. For example, RBC‐derived free hemoglobin and microparticles may promote endothelial dysfunction and vascular injury via interference with nitric oxide (NO) signaling leading to inflammation, oxidative stress, and hypertension. We hypothesize that donor genetic variation, including gender, largely determines RBC susceptibility to hemolysis in storage and after transfusion. We have previously demonstrated gender differences in predisposition to hemolysis in both human and 22 in‐bred mouse strains, where male RBCs consistently exhibited enhanced susceptibility to storage or stress‐induced hemolysis. The purpose of this study is to further clarify the association between sex hormones and hemolysis by gonadectomy studies in FVB/NJ mice, and by genome‐wide association analysis of hemolytic propensity in 22 inbred mouse strains.
Predisposition to hemolysis based upon gender or gonadectomy was evaluated in 3 independent experiments using orchiectomy or intact males, and ovariectomy or intact females (n = 10‐14 mice per group) from the FVB/NJ strain. RBC susceptibility to hemolysis was determined by osmotic and oxidative stress tests using the pink test method for osmotic fragility, and 2,2‐azobis (2‐methylpropionamidine) dihydrochloride (AAPH) or diamide to induce oxidative hemolysis. Genome‐wide association analysis was performed for genes grouped by gender and treatments for the identification of single‐nucleotide polymorphisms (SNPs) associated with hemolysis. Pathway enrichment analysis of the genes with significantly associated SNPs was done using Ingenuity Pathway Analysis (IPA) to identify signaling pathways associated with gender and hemolysis.
Susceptibility to osmotic and AAPH‐induced oxidative hemolysis was significantly higher in male compared with female FVB/NJ mice (67.4 ± 20.5% vs 44.9 ± 9.0% osmotic hemolysis, P = 0.0053; 81.7 ± 6.2% vs 54.4 ± 33.6% oxidative hemolysis, P = 0.0045; unpaired t‐test, males vs females, respectively). Ovariectomy had no significant effect on osmotic or AAPH‐induced oxidative hemolysis compared with intact females. Conversely, orchiectomy was correlated with decreased susceptibility to these hemolytic assays compared with intact males (51.6 ± 18.9% vs 67.4 ± 20.5% osmotic hemolysis; 61.1 ± 23.5% vs 81.7 ± 6.2% oxidative hemolysis; orchiectomy vs intact males, respectively). Subsequent experiments done on intact males, females, and orchiectomy males have strongly confirmed our observation that orchiectomy can significantly reduce predisposition to our stress‐induced hemolytic assays. Genome wide association analysis of 22 inbred mouse strains showed that gender differences in hemolytic propensity relate to gene networks involving mitogen and stress‐induced protein kinases, such as p38 MAPK, that are associated with membrane stability, and can be modulated by testosterone.
Our studies suggest that gender differences in hemolytic propensity can be driven by testosterone as orchiectomy, but not ovariectomy, was associated with enhanced resistance to osmotic or oxidative stress. These findings inform our central hypothesis that, in males, exposure to endogenous testosterone during erythropoiesis promotes mitogen and stress‐induced protein kinase activation leading to structural changes in erythrocyte cytoskeleton and membrane. We further propose that MAPK signaling may be coupled with pro‐hemolytic events involving the phosphorylation of vital cytoskeleton proteins, such as band 3, leading to alterations in membrane scaffolding, RBC volume, membrane fluidity, and eryptosis.
P‐637
A Novel Variant RhD Allele
Xhetani MX1, Seferi IS 2, Férec CF 3, Rumano MR 1, Fichou YF 4
1Faculty of Natural Sciences, Tirana, Albania2National Blood Transfusion Center, Tirana, Albania3INSERM U613, Etablissement Francais du Sang site de Brest, Brest, France4INSERM U1078, Etablissement Francais du Sang ‐ Bretagne, Brest, France
Background
Two highly homologous Rh system genes (RhD and RhCE) actually give rise to numerous gene conversions that, together with point mutations of the RhD gene itself, are at the origin of atypical RhD (RH1) proteins. So far dozen variant alleles have been reported in the RHD gene, most of them being associated with either weak or partial D phenotype (http://www.uni-ulm.de/~fwagner/RH/RB2/). The increase of use of several different monoclonal anti‐D and the introduction of genotyping methods, every year reveal several new variants.
Aims
In our daily routine for ABO and Rh typing of blood donors in National Blood Transfusion Center of Tirana, one case of weakly reacting D phenotype, in a donor was detected. We go further investigations with DNA testing for molecular characterization of RHD gene.
Methods
Rh typing for D, C, c, E and e antigens was carried by using DiaClon monoclonal antibodies that detect the presence of the DVI variant (DiaMed GmbH, Cressier, Switzerland). The RhCcEe phenotype was determined by ID‐card human antibodies (DiaMed GmbH). Sample was further subjected to weak D testing using anti‐D blend (clones TH‐28/MS‐26) (CE‐Immundiagnostika GmbH, Eschelbronn, Germany). After the final wash, the saline was decanted and one to two drops of anti‐human globulin serum (Biotec, purchased from Lab21 Healthcare Ltd, Dorset, UK) was added, and after incubation was considered to be weak D positive. Genomic DNA was extracted from a 2‐ml EDTA blood sample by the QIAmp DNA Blood Mini kit (Qiagen, purchased from Mediline d.o.o., Kamnik, Slovenia) according to the manufacturer's instructions. RHD gene variant screening was performed at the Laboratoire de Génétique Moléculaire des Groupes Sanguins (Etablissement Français du Sang ‐ Bretagne, Brest, France) with previously published methods. Briefly, sample was first screened for weak D, type 1, 2 and 3 alleles by a Tm‐shift assay. The ten RHD exons were then PCR‐amplified, directly sequenced and data were analyzed with Sequencher® v5.1 sequence analysis software (Genes Codes Corporation, Ann Harbor, MI, USA).
Results
The profile obtained in the Tm‐shift assay suggested that DNA were suspected to be homozygous for the deletion of the whole RHD gene. Sample was further studied by direct sequencing. One variant could be identified: the c.932A>G variant in exon 6 was found at the heterozygous state (Figure 1). This variant is assumed to result in the p.Y311C amino acid substitution, and affects a residue predicted to be located within the tenth transmembrane domain of the mature polypeptide.

Fig. 1 Direct sequencing of RHD exon 6 identified the novel c.932A>G variant allele at the heterozygous state (bottom panel). The arrow indicates the p.
Summary and conclusions
In our case, no cis‐linkage with any other variant could be detected by the genotyping approach, suggesting that the sporadic RHD c.932A>G substitution is a novel variant allele. We may be hypothesized that this founder variant, which is specific of the population of interest, is located within an unexplored region of the RHD gene and alters quantitatively its expression.
P‐638
Study of FUT2 Gene in Urinary Sediment Samples
Ensinck MA1, Racca L 1, Garcia Borras S 1, Yaber F 2, Provenzal O 2, Cotorruelo C 1, Biondi C 1
1Facultad de Ciencias Bioquímicas. Universidad Nacional de Rosario, Rosario, Argentina2Facultad de Ciencias Médicas. UNR, Rosario, Argentina
Backgraund
Histo‐blood group ABH antigens are major alloantigens in humans. These antigens are widely distributed in human tissues and are synthesised through a a(1,2) fucosylatranferase (FUT2) which incorporates molecules of fucose in common type oligosaccharide precursor. The FUT2 gene has a significant polymorphism with typical ethnic specificity. The nonsense mutation 428G→A (Trp143→stop) is characteristic for the dominating nonsecretor allele (se428) in Europeans and appears in about 20% of the Caucasian population. These individuals are no secretors who fail to express soluble A, B, H, and Leb histo‐boold group antigens in secretor iindividuals and secretor fluid because the absence of the Se (FUT2) gene.
Aim
To study from urinary sediment samples, the allelic varieties of the FUT2 gene by a PCR reaction.
Methods
Frozen saliva and urinary sediment samples from 136 unrelated of a population of Argentine were examined in this study. Appropriate informed consent was obtained from all subjects and all procedures were performed according to the ethical standards established by the University of Rosario. We determinated the secretor status in saliva with the hemagglutination inhibition technique using monoclonal antibodies anti‐A, anti‐B and lectin ulex europeaus. Agglutination of cells by antibody in tubes containing saliva samples indicates that the corresponding antigen is not present (non secretor status). Failure of known antibody to agglutinate indicator cells after incubation with saliva indicates that contains the corresponding antigen (secretor status).
For the molecular studies the sediment urinary samples were centrifugated and the genomic DNA was extracted from the pellet by an enzymatic digestion method. The DNA samples were analyzed by ASA‐PCR with specific primers for the G428A allele and for the wild type allele of the FUT2 gene. The PCR products (132 bp) were analyzed in 2% agarose gel containing ethidium bromide.
Results
The results obtained by serological and molecular methods presented 100% of concordance. Both techniques indicated that the 78% of the investigate individuals were secretors. The G428A polymorphism had present in 9.3%, smaller value to report in the bibliography for the Caucasian population.
Conclusions
This preliminary study demonstrates that allelic varieties of the FUT2 gen can be investigated using urinary sediment samples which were obtained with non‐invasive methods. This method designed in our laboratory is reproducible and robust. The allelic varieties of the other non‐secretor individuals different to the G428A might to correspond to other mutations.
P‐639
Eighteen Months Experience With Blood Group Sequencing Reveals and Confirms a Number of Known and New Mutations
Flesch BK , Schottstedt V , Spallek H , Baumann C , Stenzel A , Feick U , Steitz M , Janson A , Kuhn I , Just B
DRK Blutspendedienst West, Bad Kreuznach, Germany
Background
Conventional agglutination techniques are the gold standard of blood group typing. In cases of unclear typing results with weak blood group expression, mixed field reactions, blood group deficiencies and antibody formation in antigen positive individuals PCR‐SSP can add information on the molecular background. Beyond that DNA sequencing is a reliable tool to detect further mutations.
Aim
Eighteen months after introduction of DNA sequencing as a research approach an interim result of detected mutations should be drawn.
Material and methods
DNA sequencing was established for the RHD, RHCE, ABO, FUT1, FUT2, AQP1, ABCB6 and SMIM1 genes. Exons including short flanking intron sequences were amplified in a gene specific way by use of either published or in‐house primer sequences. Cycle sequencing was performed with the Big Dye Terminator v3.1 chemistry (ABI, Weiterstadt, Germany) followed by electrophoretic separation in an ABI Prism 310 DNA analyser. Determined DNA sequences were aligned to published reference sequences.
Results
In the respective time interval 48 patient samples submitted to the German Red Cross Blood Service West for immunohaematological analysis or samples from blood donors were subjected to DNA sequencing. In eight cases no reason for the unclear RhD or RhCE serologic typing was found, but in most samples known or new mutations were detected like different weak D (type 4.0, 11, 15, 20, 25, 31 and the new RHD 374T>A (Ile125Asn), RHD*DEL11, D el (IVS5‐38del4), DNB, DAU-2, DBS and ceMO. Additionally, known mutations of the Colton blood group (AQP1 601delG), Lan (ABCB6) and Vel (SMIM1) each resulting in a blood group negative phenotype were detected as well as mutations of the FUT1 gene resulting in the Bombay (hh) and the Para‐Bombay (H+ weak) phenotype.
Conclusions
DNA sequencing is a reliable tool to explain or confirm discrepancies between serological blood group testing and PCR‐SSP. It seems probable that looking at the molecular basis of blood groups into more detail will detect a higher number of mutations than assumed from the results of serological testing.
P‐640
The Prevalence of DEL‐Associated Allele RHD(M295I) in Dalmatia County of Croatia
Dajak DS1, Dogic V 2, Lukacevic J 3, Burilovic V 1, Körmöczi G 4, Mratinovic Mikulandra J 1
1University Hospital Center, Split, Croatia2CCroatian Institute of Transfusion Medicine, Zagreb, Croatia3Split University Hospital Center, Split, Croatia4Medical University of Vienna, Vienna, Austria
Background
Routine serology easily detect most of D variants, weak D or partial D, but it may not detect the least expressed D variants, collectively called DEL, which is considered to be serologically detectable only by adsorption‐elution techniques. Molecular typing overcomes the limitations of serologic methods and currently is the method of choice for detection of DEL phenotype in blood donors. The DEL phenotype arises from an array of genetic changes within RHD gene and is usually found in D‐ individuals with C or E phenotype. Previous studies found the frequency of DEL in Caucasians who were D‐ by routine serology and C/E+ is approximately 1 to 2 percent.
Aims
This work analyzes serological results, RHD genotype, and D antigen density from donors who typed D positive in IAT only; results of RHD genotypingof D‐ donors by routine serology with C and/or E antigens; and the prevalence of DEL‐associated alleles in blood donors in Split Dalmatia County.
Methods
Rh typing ofnew blood donors (Split‐Dalmatia County, between January, 2007 and April, 2013) was performed by direct and indirect glass beads column agglutination (Ortho BioVue System, Raritan NJ, USA). D variants detected by indirect agglutination only were investigated using extended serologic D antigen typing, molecular typing, and D antigen density determination by flow cytometry.
A total of 114 samples (between January, 2014 and December, 2014) obtained from D negative, C and/or E positive donors on standard serologic testing were tested for the presence of RHD gene in pools of 20 samples. The qPCR method (RT‐PCR System 7500, Applied Biosystems, USA) with TaqMan chemistry was used for exon 7 and 10 amplification (RHD-PCR screening). RHD genotyping of all qPCR‐positive samples was performed to define RHD alleles by the PCR‐SSP genotyping kits (Inno‐Train, Kronberg, Germany).
Results
Direct agglutination of D antigen was negative in 1630 donors of which 128 were C/E+, in six of them (0.37%) D antigen in IAT was weak positive in glass beads column technology. Extended serological testing of D variants in IAT gave negative or very weak positive results with other reagents and techniques.RH genotyping found that all D variants carry allele RHD(M295I), RH genotype CcDee. D antigen densities of D variants were very low, between 26 to 44 D antigens per red blood cell (RBC).
RHD-PCR screening of 114 serologically D‐, C/E positivedonors revealed four donors to be RHD positive, and all of them have allele RHD(M295I).
The prevalence of DEL‐associated alleleRHD(M295I) in D‐, C and/or E positive donors in Split Dalmatia County was 8,2% in total (Table 1). Obviously, DEL phenotype is more common in some parts of European population than initially thought. Also, in this study the only DEL genotype found was RHD(M295I).

Conclusion
More then half cases of DEL phenotype were detected by sensitive serology techniques for routine D typing, even RBCs with antigen density as low as 26 D antigens per RBC. However, correct assignment of all donors as D‐ or D+ is not possible using serotyping alone without genotyping.
P‐641
A Novel Variant in Regulatory Region of B Allele is Responsible for B Weak Phonetype
Wang JW , Ye XY , Gu PG , You GY , Fu QF
Shanghai Children's Medical Center,Shanghai Jiaotong University School of Med, Shanghai, China
Background
The ABO blood group system is highly important in clinical transfusion and transplantation medicine.Lot of rare subgroups of ABO blood group have been discoverd, which not only caused by the single nucleotide polymorphisms (SNP), but also relate to hybrid formation between the common alleles or mutation in the untranslated region (UTR) in ABO gene.
Aims
This study aims to investigate the molecular basis of ABO gene in a patient with serologic ABO blood group discrepancy.
Methods
The patient enrolled was from Shanghai Children's Medical Center. Serologic blood group identification, Coombs’ test and antibody screening were detected with DG Gel Confirm cards, Neutral cards, Coombs cards by WADiana/8XT Compact Analyzer (from Diagnostic Grifols, S.A). The enhancer, promoter, exon 1~7 and their adjacent intron region of ABO gene were amplified by using polymerase chain reaction (PCR) method, the PCR products were directly or by TA cloning sequenced to identify the gene mutation.
Results
The patient's red blood cells showed strong agglutination with monoclonal anti‐A (++++), mixed agglutination with monoclonal anti‐B and weak agglutination with anti‐H (++).The patient's serum showed no agglutination with A1 cell, B cell and O cell. The direct antiglobulin test (DAT), indirect antiglobulin test (IAT) and antibody screening were all negative.
The ABO gene sequencing result showed several variants in nocoding region: a 18 bp deletion in the promoter region from −35 to −18 bp, five 43 bp short tandem repeat in enhancer region (−3899 to −2618 bp), which was one more than the normal B allele. In addition, one heterozygous variation in exon 6 (297A>G) and seven heterozygous variations in exon7 (467C>T, 526C>G, 657C>T, 703G>A, 796C>A, 803G>C, 930G>A) of ABO gene were also identified in patient compared with the reference sequence of A101 allele.
Summary/Conclusions
Based on the Blood Group Antigen Gene Mutation Database, the patient was identified as A102/B101. The reason for B101 weakened expression was one more 43 bp tandem repeat in enhancer region and lack of 18 bp in the promoter region of B allele. The mutations in the enhancer and promoter of ABO gene may cause weak B phenotype.
P‐642
Distribution of RED Cell Genotypes Among Different Blood Groups and its Application in Transfusion in Developing Countries
Nicola MM , Ali A , Shabayek S , Habib S
Egyptian National Blood Transfusion Services, Cairo, Egypt
Background
Egypt is a developing country where many patients suffer from low socio‐economic level. Phenotyped Packed Red Blood Cells are recommended for multiply transfused patients. Many patients cannot afford the cost of phenotyping.
Red Cell Reference Laboratory in the National Blood Transfusion Center is one‐of‐a‐kind laboratory in Egypt that tests for red cell phenotypes. Therefore, National Blood Transfusion Center is the sole provider of phenotyped Packed Red Blood Cells if prescribed. Red Cell Reference Laboratory screens for matched units with the phenotyped patient.
National Blood Transfusion Center's Donation Department has a panel for regular phenotyped donors to match with the patients’ phenotypes to decrease the financial burden on patients.
Aims
To correlate between ABO types and genotypes (accordingly red cell phenotypes).
Methods
A statistical analysis was done to correlate ABO type with genotypes/phenotypes, using the database that was made in National Blood Transfusion Center's Donation Department, which includes a random sample of 735 regular donors of different ABO/Rh types and their corresponding genotypes.

Results
For a sample size of 735 regular donors (634 positive Rh, and 101 negative Rh), the following was found: 100% of the negative Rh donors have (rr) genotype; no comparative study could be made here.Positive Rh donors:R1r is the most prevalent genotype in the four blood groups as follows:41.2% of 233 A positive donors.37.58% of 149 B positive donors.39.13% of 207 O positive donors.42.22% of 45 AB positive donors. The distribution of other phenotypes is shown in Table 1.According to the data in table 2, the probability of patients who might develop antibodies to those specific antigens can be calculated: A pos:1. R0r: 1.72% of patients may develop anti‐e and 16.31% anti‐c.2. R1R1: 1.72% may develop anti‐e and 26.18% anti‐CB pos:1. R0r: 0.67% of patients may develop anti‐e and 25.5% anti‐c.2. R1R1: 0.67% may develop anti‐e and 28.19% anti‐C.O pos:1. R1R1: 0.48% of patients may develop anti‐e and 20.77% anti‐C.2. R0r: 0. 48% may develop anti‐e and 29.47% anti‐c.AB pos:1. R0r: 4.44% of patients may develop anti‐e and 15.56% anti‐c.2. R1R1: 4.44% may develop anti‐e and 31.11% anti‐C.

Conclusions
According to the study, the most prevalent genotypes in A, B and O Rh positive groups are R1r then R1R1 then R0r. But for AB Rh positive group the most prevalent genotypes are R1r then R2r then R1R1.
Based on the phenotypes found, a study will be held applying this proposal on unphenotyped patients, by administering units from an already‐phenotyped donor, to observe if this will help in decreasing the probability of the development of antibodies when compared to transfusion according to ABO group only.
P‐643
A New Era for Chronically Transfused Patients With ?‐Thalassemia: Molecular Typing Facilitates Transfusion and Reduces Red Blood Cell Alloimmunization
Castilho LC , Gaspardi ACG , Azevedo PRA , Sippert EAS , Pereira FBP , Pellegrino JPJ Jr
Hemocentro‐Unicamp, Campinas, Brazil
Background
Regular transfusions are essential for patients with thalassemia to maintain growth and development during childhood and to sustain good quality of life during adulthood; however, the development of red blood cell (RBC) alloantibodies and autoantibodies complicates transfusion therapy in such patients. Programs to prevent alloimmunization to RBC antigens have been designed and RH and K antigen matching is one of the most recommended approaches. Molecular testing has been successfully implemented in immunohematology laboratories and is proving to be a powerful tool, with potential advantages for finding better antigen matches for chronically transfused patients but replacement of serologic typing for RH and K antigens to molecular typing is not a practice yet.
Methods
In this study, we compared the Rh and K antigen phenotypes obtained by serologic methods with genotype predictions in 33 chronically transfused patients with β‐thalassemia receiving RH and K antigen matched products. We studied 33 patients (median age, 25 years) who were alloimmunized (n= 11) and non alloimmunized (n=22) to RBC antigens, of whom 5 presented antibodies against Rh antigens. Rh and K phenotyping was performed by hemagglutination in gel cards (Bio‐Rad Laboratories) and genotyping was performed by HEA BeadChip (Bioarray Solutions, Immucor).
Results
RH genotypes differed from the assumed Rh phenotypes in 13 patients with β‐thalassemia (Table 1). Six typing discrepancies were found among the alloimmunized patients and 7 discrepancies were identified among the non‐alloimmunized patients. In those cases, we confirmed that the transfused red blood cells were the source of the discrepancy between genotype and phenotype. The discovery of these discrepancies aided in the identification of alloantibodies and in the selection of the correct antigen‐matched products to those patients, increasing the blood availability and reducing the Rh alloimmunization. The patients who then were switched to the correct antigen‐matched RBCs had improved RBC survival with diminished frequency of transfusions.
Table 1: Rh typing discrepancies between phenotypes and genotype predictions.

Conclusion
As many adverse effects of blood transfusions have been solved or minimized, making it a therapeutic tool safer than ever, molecular typing brings a new era for the prevention of alloantibodies formation and haemolytic transfusion reactions. It provides the opportunity to implement this tool as the primary method for RH and K typing for patients with thalassemia, set to personalize medicine and to adapt blood products to the clinical needs of patients.
P‐644
Unclear Cause for a Weak C Antigen
Conradi RC1, Doescher AD 2, Marandiuc DM 1, Jung AJ 1, Hitzler WEH 1
1University Medical Center of the Johannes Gutenberg University, Mainz, Germany2DRK‐Blutspendedienst NSTOB, Oldenburg, Germany
Background
During the last years numerous new alleles of the rhesus system were found. Some years ago only the variants of the RHD gene were of major interest. In the past years the interest for RHCE variants increased. So it became a standard to clarify difficult serological Rhesus CE‐results with PCR methods.
Aims
In this paper a blood donor sample with weak serologic reactions for different anti‐C reagents is presented. The methods which were used to clarify the reason for the weak antigen and the results characteristic to this variant are described.
Methods
The different serologic tests are specified in table 1. Molecular typing was perfomed with the SSP‐PCR test from BAG (BAGene Rh‐Type). DNA and cDNA sequencing of the RHCE gene were performed with Taq‐polymerase cycle sequencing using fluorescent‐labeled dye terminator reactions. The sequencing data were analyzed with MAC Vector Software. Quantification of RHD and RHCE sequences by real‐time PCR was done as described by Doescher et al in 2001, Infus Ther Transfus Med, Vol 28, S1, Abs. V17.9P. The density of the antigen was analyzed with flow cytometry.
Results
The serological Rhesus blood group of the donor was D+, (C+), c+, E+, e‐. All reagents except the C‐antisera had clear reactions with the sample (positive or negative). The reactions with different anti‐C reagents and methods are described in table 1. Molecular typing with PCR‐SSP showed positive reactions with the primers for C‐, c‐ and E‐alleles. By sequencing the RHCE exons 1 and 2 and the bordering introns only the known polymorphisms for RHC and RHc were found but no mutation that could explain the weakened C‐antigen. Sequencing the promotorregion of the RHCE gene (5`UTR) showed a not yet described mutation at the position ‐978 (A+T). To examine the influence of this mutation on DNA transcription the cDNA of exons 1 and 2 of the RHCE gene was sequenced. Again, only the known polymorphisms for RHCc‐alleles were found and no explanation for the weakened C‐antigen. The hypothesis that a hybrid gene was responsible for the weak C‐antigen was dismissed due to a normal relative quantification of the RHD gen in relation to the RHCE gen. The Rhesus C‐antigen density was 3272 antigens per cell, which represented 20% of the Cc‐control sample.
Conclusion
A sample of a blood donor with the Rhesus sub type (C)cEE was presented. The weakened serological reactions with anti‐C were confirmed by measuring the antigen density. DNA‐typing and sequencing of the RHCE gene could not explain the weak serologic result. The new described mutation in the 5`UTR region at the position ‐978 (A+T)) did not influence the cDNA. A hybrid gene was excluded by quantification of RHD gene and RHCE gene. Interestingly for this case is that a loss of antigens was confirmed, but an explanation for the hereditary disposition was not yet found.

P‐645
Serological and Molecular Studies of a D–– Phenotype
Luján Brajovich M1, Trucco Boggione C 1, Mattaloni SM 1, García Borrás S 2, Rucci A 2, Berisvil C 3, Biondi C 2, Cotorruelo CM 1
1Laboratorio de Inmunohematología. Universidad Nacional de Rosario CONICET, Rosario, Argentina2Laboratorio de Inmunohematología. Universidad Nacional de Rosario, Rosario, Argentina3Banco de Sangre del Instituto de Hematología y Hemoterapia de la UNC, Córdoba, Argentina
Background
the Rh system is genetically controlled by the homologous RHD and RHCE genes that encode the RhD and RhCcEe polypeptides respectively. Deletions, point mutations and rearrangements between both genes are responsible for the great polymorphism of this system. In rare cases red blood cells totally fail to react with antisera that define one or more of the Rh antigens.
Aim
the aim of this work was to study a sample with no C, c, E or e antigen expression.
Materials and Methods
a peripheral blood sample of a patient was referred to our reference laboratory because of the presence of a panreactive antierythrocyte antibody. Extended red blood cell phenotype analysis was performed using tube and microplate techniques. The expression of the D, C, c, E and e antigens was also determinated by flow cytometry. An IgM anti‐D (clone MS‐201), IgM anti‐D (clone ESD1M), anti‐C (clone MS24), anti‐c (clone MS33), anti‐E (clone MS80 + MS258) and anti‐e (clones MS16 + MS21 + MS63) were used. R1R2 and antigen‐negative cells were tested as controls. PCR‐SSP strategies were used to study RHCE specific polymorphisms in exon 1 (nt 48), exon 2 (nt 201), intron 2 (ins 109pb), exon 3 (nt 383), exon 5 (nt 676), exon 9 (nt 1193) and the 3′ UTR region. PCR‐RFLP analysis with Bcl I, Taq I, Rsa I and Alu I endonucleases were performed to study exon 4 (nt 594), exon 5 (nt 697), exon 6 (nt 932) and exon 7 (nt 974) respectively. A Taq I site present in intron 1 and a Pst I site RHC/c‐associated polymorphism in intron 2 were also analysed.
Results
serological studies showed an IgG 37°C‐reactive alloantibody. Propositus’ red blood cells were positive for the anti‐Ds tested and failed to react with anti‐C, anti‐c, anti‐E and anti‐e antisera suggesting a D– phenotype with anti‐Rh17. The flow cytometric results demonstrated 43% and 47% overexpression of the D antigen with anti‐D clones MS‐201 and ESD1M respectively, compared to R1R2 cells (standard positive control). Accordingly with serological results, no antigen expression was detected with anti C, c, E and e monoclonal antibodies. Molecular analysis revealed the absence of RHCE sequences 3′ of the 4.2 Kb homology region encompassing exon 2. No RHCE specific polymorphisms were found in exons 3, 4, 5, 6 and 7 while RHCE specific nucleotides were detected in exon 9 and the 3′UTR. Therefore, the 3′ breaking point of this rearranged allele may occur within intron 7 and intron 8.
Conclusions
Molecular studies confirmed the serological findings and suggest that the D– phenotype resulted from a macroconversion event between the RHD and RHCE genes involving a segmental replacement of RHCE sequences with homologous RHD, generating a hybrid allele. The presence of RHD regions over most of its length in the recombinant structure found may account for the overexpression of the D antigen. To our knowledge, this is first description of this unusual phenotype in an Argentinean patient.
P‐646
A Simple Genotyping Procedure Without DNA Extraction to Identify Rare Blood Donors
Silvy M1, Bres JC 2, Grimaldi A 2, Movia C 2, Muriel V 2, Roubinet F 2, Chiaroni J 1, Bailly P 1
1EFS Alpes‐Méditerranée, UMR 7268, Aix‐Marseille Université, Marseille, France2Établissement Français du Sang, Blood Cell Grand Sud, Montpellier‐Marseille, France
Background
Transfusion‐induced allo‐immunization has severe clinical consequences including hemolytic transfusion reactions, impaired transfused RBCs longevity, and greater difficulty in finding compatible blood. Molecular analysis of genomic DNA now permits prediction of blood group phenotypes based on identification of single nucleotide polymorphisms. Implementation of molecular technologies in donor centers would be helpful in finding RBC units for special patient populations, but DNA extraction remains an obstacle to donor genotyping.
Aim
To proposea simple method compatible with high‐throughput that allows blood group genotyping using a multiplex commercial kit without the need for DNA extraction.
Materials and Methods
Results of genotyping without DNA extraction were compared to those of control DNA. Two procedures were assessed in preliminary testing (microwave irradiation of blood samples and pre‐heating/cooling blood in PCR mix). The pre‐PCR treatment of whole blood using heating/cooling procedure was optimized for accurate genotyping using the multiplex reverse sequence specific oligonucleotide (PCR‐RSSO) method using the LIFECODES RBC kit (Genprobe, Belgium). The accuracy of genotyping after pre‐PCR treatment using recombinant polymerase added prior to cycling was evaluated in a cohort of blood samples collected in EDTA tubes from random donors after inform consent.
Results
Optimization tests showed that blood group genotyping without DNA extraction was accurate using 5μl of whole blood with addition of KAPA2G Robust DNA hotstart polymerase prior to heating/cooling cycling. This procedure was used on a validation set i.e. prospective investigation of 209 donor blood samples investigated for 28 alleles within nine blood group systems.
Eight samples (3.8%) failed when genotyping was performed on blood while analysis gave correct results on DNA. Among the 5628 alleles investigated we considered as non reliable typing with a probe value less than 0.05 to the cut off. Thus, 45 samples (27 blood samples and 18 DNA) were tested twice and in all cases the second investigation showed both a high MFI (Mean fluorescence Intensity) and a correct deviation between probe values and cut off. Altogether, no discordance was noted between blood and DNA samples as long as deviation between probe value and cut off is ≥ 0.05. The prospective study designated 63 donors (30.1%) with rare blood, i.e., either negative for a high frequency antigen or with a rare combination of common antigens.
Conclusion
The procedure was optimized for simplicity of use in genotyping platform and would allow not only to supply antigen‐matched products to recipients but also to find rare phenotypes. This methodology could also be useful for establishing a donor repository for human platelet antigens (HPA) matched platelets since the same issues are involved for patients with neonatal alloimmune thrombocytopenia or posttransfusion purpura.
P‐647
RH Diversity in Mali: Characterization of a New Haplotype Rhd*Diva/Rhce*Ceti(D2)
Silvy M1, Ba A 2, Beley S 1, Chiaroni J 1, Bailly P 1
1EFS Alpes‐Méditerranée, UMR 7268, Aix‐Marseille Université, Marseille, France2EFS Alpes‐Méditerranée, Marseille, France
Background
Knowledge of RH variants in African populations is critical to improving transfusion safety in countries with populations of African ancestry and to providing valuable information and direction for future development of transfusion in Africa.
Aim
The purpose of this report is to describe RH diversity in individuals from Mali.
Study Design and Methods
Blood samples were collected from 147 individuals self‐identified as Dogon (n=101) and Fulani (n=46). All samples were phenotyped for D, C, E, c, and e antigens using the gel column agglutination method (Ortho Clinical Diagnostics, Illkirch, France). RHD and RHCE alleles were identified by Polymerase chain reaction (PCR) assay and sequencing on the 10 exons of the RHD and RHCE genes.
Results
Eight samples, <ie., 6 Dogon and 2 Fulani, were typed as D‐negative linked to homozygous RHD deletion in 4 Dogon and 1 Fulani or presence of RHD pseudogene (RHD*DPsi) and/or (C)ceS type 1 haplotype. No variant RHD allele was observed in 35 Dogon and 34 Fulani. In the remaining 78 samples, a variety of variant alleles were found. The most common RHD allele variant was RHD*DAU0. Five predicted partial‐D phenotypes were attributed to RHD*DAU3 or RHD*DIVa. Neither RHD*DAR nor RHD*DIIIa were found. A total of 11 RHCE*ce variant alleles and one RHCE*Ce variant allele were identified in this population study. In addition,2 samples carried a RHCE*cE allele with 48G>C transversion. The most frequent allele encoding partial e antigen was RHCE*ce(254G) that occurred in 20 Dogon and 11 Fulani and three samples were predicted to express a partial‐e because of RHCE*ce(254G) in trans to RHCE*cE. Regarding C antigen, 28 Fulani typed as C‐positive and 16/28 harbored at least one RHCE*Ce-D(4)-ce, two being homozygous and predicted to show a rare RH:32,‐46 phenotype. A new RHCE*ceTI with replacement of exon 2 by RHD designated herein as RHCE*ceTI(D2), was identified in Dogon. Inheritance study showed that RHCE*ceTI(D2) is in cis to RHD*DIVa. Samples bearing this haplotype typed C‐negative with anti‐C polyclonal antibody and monoclonal antibodies (MoAbs) MS24, P3X2551368+MS24, and MS273, but positive with anti‐RhCe MoAb‐BS58. The same pattern was observed in sample with RHD*DIVa/RHCE*ceTI.
Conclusion
Taken together, our results revealed an uneven distribution of some RH variant alleles in Mali Africa suggesting the need for further study in well‐documented cohorts. A wider study in donors of African descent will also be required to determine the frequency of the new haplotype RHD*DIVa/RHCE*ceTI(D2) associating an allele encoding partial D, variant ce antigens and aberrant reactivity with anti‐C, and to evaluate its potential impact on transfusion strategy.
P‐648
Improved Matched Red Cells in β Thalassemia Patients
Travali S , Barrotta G , Corallo G , Fidone C , Garozzo G , Giuca G , Licitra V , Spadola V , Bonomo P
ASP7, Ragusa, Italy
Background
199 patients with hemoglobinopathies are supported in our Institute, 75 of these have Thalassemia Major (TM), 23 Thalassemia Intermedia, 38 are thalasso‐drepanocytic patients and 48 drepanocytic patients. Treatment of TM is based on a transfusion regimen that guarantees a minimum pre‐transfusion hemoglobin (Hb) level of 9g/dL. On average, a dose of 9.8 mL (net of preservative solution) RBC/Kg body mass (range 7.6–12.5) is used, a transfusion interval on average of 24.2 days (range 18–28) is obtained, and units are matched for AB0, DCE and Kell groups. Several studies demonstrated that an extended match including additional antigen systems like Duffy, Kidd, MNSs, Dombrock and Lutheran would be expected to improve the transfusion yield in such patients.
Aims
Since the transfusion interval can significantly differ between patients, even when subjected to the same transfusion therapy, our first goal is to determine if extended blood matching improves the transfusion yield measured as daily decrease of Hb, with consequent lengthening of transfusion interval and maintenance of clinical pre‐transfusion parameters. An additional aim of this study is to determine the number of these patients having a “perfect match”in the donor pool, who could benefit from transfusion therapy with higher compatibility. Since each patient has to be treated with several units/year, this number can be limited by the need for multiple donors matched to each recipient
Methods
All patients with haemoglobinopathies were typed using a molecular method extended to 32 antigens (HEA BeadChiptm BioArray Immucor).
Four patients affected by TM not‐alloimmunizated, not‐splenectomized, not‐cardiopathic, have been selected, matched for body weight, age and clinical conditions but chosen in two different transfusion interval (see table 1).
Patients have been transfused for one year, in order to have a match extended to all antigens of Kell, Duffy, Kidd, MNSs, Dombrock, Lutheran, Colton. LW, Scianna and Diego systems (100% paziente A e D, 60.8% paziente C e 81.8% paziente B)
Results
Are shown in table 2
Table 1
Table 2
Conclusions
Data, obtained after one‐year treatment with match extended to 32 antigens, do not show significant improvements in patients A and B, who already had a better transfusion yield. A slight improvement have been obtained for patient C, with a reduced loss of Hb/die% (1.21 vs 1.17) with a reduction of packed red transfused (9360 ml vs 8960 ml), while a good improvement have been observed in patient D (1.38 vs 1.23) with a better reduction (9610 ml vs 8490 ml) resulting in a reduction of six units transfused and two entrances in less. In conclusion, our results indicate that an extended match improves transfusion yield, but to confirm we have to extend the study longer and with more patients.


P‐649
Frequency of Donor With Duffy Allele Fyx
Chiriano P , Sagliano SIC , D'Ambrosio R , Munno E , Dell'Aversana MR , Palumbo G , Perillo M , Valiante A , Misso SIC
S.C.Servizio Trasfusionale Asl Caserta, Aversa, Italy
Background
The Duffy blood group antigens are encoded by the Duffy gene Fy. This one has two major codominant alleles, FyA and FyB, which result from a SNP G125A, and the corresponding Fya and Fyb antigens differ by a single amino acid Gly42Asp. Individuals who are homozygous for a SNP C33T, present the phenotype Fy(a‐b‐) and do not express Duffy antigens on their RBCs. Additionally, a fourth allele, FyX, is found and defined as weak Fyb not detectable by all anti‐Fyb. The FY gene differentiates FyB and FyX (b+ weak) individuals on the basis of one substitution, C265T. The resulting Arg89Cys amino acid change reduces the binding of anti‐Fyb antibodies.
Aims
This research postulates that the FyX phenotype is conveyed by a fourth allele at the FY locus with a frequency of 2%; the aim is to evaluate the frequency of donor FyX relating to our ST ASL Caserta. The research relating to the FyX was carried out inside a wider project of perfect cross match between the phenotype of the donor and the one of the patient who had undergone transfusions through the identification of the extended erythrocyte phenotype with the use of molecular biology methods.
Methods
The research was carried out on 300 donors using a Bioarray Solution Head Bead Chip Kit (Immucor), which is a diagnostic test in vitro for the molecular determination of the allelic variants that indicate the phenotypes of the human erythrocyte antigens. The kit includes 24 polymorphisms associated to 38 erythrocyte antigens and to phenotype variants including the FyX characterized by C265T (Arg89Cys).
Results (see table)
Summary/Conclusion
The collected data perfectly mirror the distribution of the allelic variants of the gene Duffy over the population. Additionally even if the variant FyX results in having no clinical relevancy for the donor, the determination of FyX (b+weak), exclusively identifiable in molecular biology, turns out to be useful as regards the transfusion issues inside the context of perfect cross match.

5.3 Platelet Immunology
P‐650
Mitochondria‐Derived Reactive Oxygen Species Play an Important Role in Doxorubicin‐Induced Platelet Apoptosis
Wang WZ1, Yang Y 2, Xie X 2
1Fudan University, Shanghai, China2Shanghai Blood Center, Shanghai, China
Doxorubicin is an effective chemotherapeutic agent, however, its use is limited by some side effects, such as cardiotoxicity and thrombocytopenia. Doxorubicin‐induced cardiotoxicity has been intensively investigated, however, doxorubicin‐induced thrombocytopenia has not been clearly elucidated. Here we show that doxorubicin induced mitochondria‐mediated intrinsic apoptosis of platelets, including depolarization of mitochondrial inner transmembrane potential (ΔΨm), phosphatidylserine (PS) exposure, mitochondrial translocation of Bax, cytochrome C release, and caspase‐3 activation. Doxorubicin did not induce surface expression of P‐selectin and PAC‐1 binding. Whereas, doxorubicin obviously reduced ADP‐ and thrombin‐induced platelet aggregation, and impaired platelet adhesion on the von Willebrand factor (VWF) surface. In addition, we also show that doxorubicin induced intracellular reactive oxygen species (ROS) production and mitochondrial ROS generation in a dose‐dependent manner in platelets. The mitochondria‐targeted ROS scavenger Mito‐TEMPO blocked intracellular ROS and mitochondrial ROS generation. Furthermore, Mito‐TEMPO reduced doxorubicin‐induced platelet apoptosis. These data indicate that doxorubicin can induce platelet apoptosis, and mitochondrial ROS play a pivotal role in doxorubicin‐induced platelet apoptosis. Therefore, doxorubicin‐induced platelet apoptosis might contribute to doxorubicin‐triggered thrombocytopenia, and mitochondria‐targeted ROS scavenger would have potential clinical utility in platelet‐associated disorders involving mitochondrial oxidative damage.
P‐651
Diagnostic Potential of Multiple Assays of Anti‐Hpa‐1a for Prediction of Fetal Thrombocytopenia in HPA‐1a Alloimmunised Women
Kumpel BM1, Jackson DJ 1, Burg-Roderfeld M 2, Santoso SL 2
1NHS Blood and Transplant, Bristol, United Kingdom2Institute of Clinical Immunology and Transfusion Medicine, Justus‐Liebig Univ, Giessen, Germany
BACKGROUND: Assays of IgG anti‐HPA‐1a concentration are not always accurate predictors of the clinical severity of fetal and neonatal alloimmune thrombocytopenia (FNAIT). AIMS: Recently developed assays of anti‐HPA‐1a functional activity may enhance characterisation of pathogenicity. METHODS: Anti‐HPA‐1a in sera from seven women who had babies with FNAIT 2–9 years previously were analysed by monoclonal antibody immobilisation of platelet antigen (MAIPA) assay to determine concentration and surface plasmon resonance (SPR) assay to estimate avidity. The IgG subclass of anti‐HPA‐1a was determined by modified MAIPA. RESULTS: Platelet counts in all the fetuses or neonates before treatment with IVIg and/or intra‐uterine transfusion were less than 10 x 109/L. Three women had one intra‐uterine death (IUD) and one woman experienced multiple IUDs. Anti‐HPA‐1a varied over 100‐fold in concentration (1.2 to 177.0 IU/mL) between sera. IgG1 was the sole subclass in 6 sera and was greater than IgG3 in one. All sera reacted with immobilised antigen in a high avidity manner, with very little dissociation. CONCLUSIONS: The functional activity of anti‐HPA‐1a in sera of HPA‐1a alloimmunized women remained high for several years after childbirth. These antibodies bound strongly to antigen and were known from a previous study (Kapur R. et al., A prominent lack of IgG1‐fucosylation of platelet alloantibodies in pregnancy. Blood 2014;123:471–480) to have skewed Fc‐glycosylation (low levels of fucose) that would enhance interactions with IgG Fc receptor IIIa on macrophages. This small‐scale study suggests that testing sera of pregnant women with suspected FNAIT for these biological assays of anti‐HPA‐1a might improve diagnosis of fetal thrombocytopenia more accurately than anti‐HPA‐1a concentration alone.
P‐652
Performance Evaluation of Extended HPA Genotyping System (ID HPA XT) in Clinical DNA Samples
Szczypiorska-Mendoza M1, Artieda M 1, Azkarate Ania M 2, Puente F 3, Tejedor D 1, Martinez A 1, López M 1
1Progenika Biopharma, Derio, Spain2Centro Vasco de Transfusiones y Tejidos Humanos, Galdakano, Spain3Banco de Sangre y Tejidos de Aragón, Zaragoza, Spain
Background
Extended human platelet antigen typing information can help in the diagnosis and management of neonatal alloimmune thrombocytopenia, platelet refractoriness and post‐transfusion purpura.
Since serologic reagents for most of the HPA antigens are unavailable, especially for those with low‐frequency, genotyping of DNA is now the gold standard method for extended HPA typing.
ID HPA XT (Progenika Biopharma, a Grifols Company) is a genotyping test for the simultaneous identification of multiple common and rare alleles encoding human platelet antigens (HPA‐1 to HPA‐11 and HPA15). ID HPA XT analyzes 13 polymorphisms determining 18 antigens of the aforementioned human platelet systems.
Aims
The aim of this study was to evaluate the performance of ID HPA XT using selected clinical DNA samples that cover the presence and the absence of all antigens with high and low frequency, in comparison with the reference methods currently used in the blood bank centers.
Methods
Two hundred and eighty three (283) DNA clinical donor samples, obtained from two Spanish blood bank centres, were analysed with ID HPA XT, Luminex® xMAP technology based test. The sample selection criteria were to test at least 59 DNA samples for all antigens that show frequency higher than 10% (presence and absence) and, a minimum number of samples with less frequent antigens. The workflow of ID HPA XT consists of DNA amplification in a multiplex PCR using biotinylated dCTP, followed by hybridization of the PCR products onto oligonucleotide probes coupled to beads. These beads are labeled with streptavidin‐conjugated phycoerythrin and analyzed with Luminex®. The property software converts the signals onto genotypes and predicted phenotypes for the human platelet antigens tested. ID HPA XT results were compared to results obtained by well‐established molecular genotyping reference methods (BLOODchip® and bidirectional DNA sequencing). Specificity, sensibility, no calls and whole system failure rate were calculated.
Results
Two hundred and eighty three (283) DNA samples were run, covering 29 (80.5%) out of 36 phenotypes of 18 antigens. Results obtained by ID HPA XT are in agreement with the reference method (BLOODchip® and bidirectional DNA sequencing) in all cases. The ID HPA XT system failure rate showed 0% and the call rate was 100%. The sensibility and specificity was 100% for the 18 platelet antigen tested (presence and absence) (see Table 1).
Table 1. Specificity and sensitivity results of ID HPA XT in comparison to molecular genotyping methods (BLOODchip® and bidirectional DNA sequencing)

Summary and Conclusions
This performance evaluation study confirms the robustness of ID HPA XT test to correctly type common and rare allelic variants of genes encoding the human platelet antigens in clinical DNA samples. The high sensibility (100%) and specificity (100%) of the test make it valuable tool for extended HPA antigen genotyping which can be effectively applied in clinical routine.
P‐653
Abstract Withdrawn.
P‐654
HPA Beadchip Genotyping Study: Comparison Between DNA Extracted From Blood Samples and Buccal Swabs
Petermann R1, Chenet C 1, Quesne J 1, Bianchi F 1, Beranger T 1, Ferre N 1, Philippe S 1, Jallu V 1, Martageix C 1, Garraud O 2
1Institut National de la Transfusion Sanguine, Paris, France2Université de Lyon, Saint Etienne, France
Background
The HPA Beadchip genotyping kit (CE‐IVD BioArray Solutions, Immucor, Warren, NJ) has been introduced in the Platelet Immunology Department of the National Institute of Blood Transfusion in 2010. This technique allows to test for 11 human platelet antigen (HPA) groups simultaneously in a single platform; total time of assay is only 6 hours, including the DNA extraction procedure.
Aims
The aim of the study was to compare platelet genotypes using DNA extracted from blood samples and buccal swabs in patients suffering severe thrombocytopenia, presenting with refractoriness after platelet transfusion or being pre‐term neonates. Indeed, the current protocol for genotyping requires a range of DNA quantity between 80 to 640 ng at a concentration ranging from10 to 80 ng/μL.
Methods
This study was carried out in 2013 and 2014 and concerned a total of 112 samples. Two CEmarked in vitro diagnostics (IVD) DNA extraction techniques were implemented: i) the automated extraction on MagNA PURE Compact with the MagNA PURE Compact Nucleic Acid Isolation Kit I(Roche Diagnostics,Gmbh); ii) and the manual QIAamp DSP DNA Blood Mini kit (Qiagen,Gmbh).
The automated extraction was only used for blood samples while buccal swabs were extracted with either technique.
Results
On the 112 samples tested, 103 HPA genotyping results were 100% concordant even if the DNA concentration in buccal swabs is lower than the recommended protocol (≥ 0,5 ng/μL). 9 HPA genotyping buccal swabs results were non‐interpretable; different hypotheses have been proposed to explain these results, such as DNA degradation, low DNA concentration, punctual misreading of the chip…. These 9 samples are currently under study.
Conclusion
The HPA Beadchip genotyping kit allows a suitable genotyping of platelets antigens even at low DNA concentration. These first results indicate some interest to genotype on buccal swabs, an easy and non‐invasive collecting method which could be introduced in the future in routine screening.
P‐655
Evaluation of Platelet Cross‐Matching in the Management of Patients Refractory to Platelet Transfusions and Monitoring of Platelet‐Specific Antibodies
Ye X , Wang J , Deng J , Chen Y , Ding H , Xu X , Yuan S , Xia W
Guangzhou Blood Centre, Guangzhou, China
Background & Aims
This retrospective analysis was conducted to evaluate the efficacy of crossmatch‐compatible platelet transfusions in patients refractory to platelet transfusions and monitoring the platelet‐specific antibodies.
Methods
49refractory patients, defined as 24‐h corrected count increment (CCI)was <4500 following two consecutive platelet transfusions, were enrolled. A commercial solid‐phase adherence kit was used for crossmatching and Enzyme‐linked immunosorbent (ELISA) was used to detect platelet‐specific antibodies.
Results
A total of 194 crossmatched ABO compatible platelet units were given to refractory patients. A median of 4 unit crosssmatch platelets were transfused per patients (range, 2~11). The 24‐h CCI (mean±SD)was significantly higher for crossmatch‐compatible platelets(7800±7000) than for random selected platelets(3000±2100)(p<0.001). Mean percent reactivity in initial (36.64%) versus last (29.01%) crossmatch assay for each patient demonstrated no trend toward progressive alloimmunization (p>0.05). 88.46% incompatible crossmaches were due to anti‐HLA antibodies, alone (57.69% of cases) or together with anti‐HPA antibodies(30.77%).
Conclusion
HLA and/or HPA alloimmunization is an important factor to cause refractoriness to platelet transfusions. For most patients, medium‐term transfusion support with crossmatched platelets offers an eddfective and rapid first‐line approach to management of platelet transfusion refractoriness.


P‐656
Evaluation of the use of National Guidelines for Diagnosis and Management of Heparin‐Induced Thrombocytopenia (HIT) in the Croatian Institute of Transfusion Medicine in the Period From 2011 to 2014
Tomicic TM , Jagnjic JS , Kruhonja-Galic KGZ , Hecimovic HA , Jurakovic-Loncar JLN , Balija BM , Jukic JI
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Background
Heparin induced thrombocytopenia (HIT) is a serious complication of heparin administration. In the last decade, this clinical syndrome has come into the focus of interest, primarily because of the severe thromboembolic complications that may lead to lethal outcome. In addition, great improvements have been made in the treatment with direct thrombin inhibitors and in laboratory diagnosis of HIT. HIT II is immunologically (antibodies) mediated syndrome, characterized by decrease in platelet number for more than 50% in relation to value before heparin application, connected with increased inclination to thromboembolic incidents. The disease appears most frequently 5–10 days from beginning of mainly unfractionated heparin therapy.
The aim of this study was to evaluate the use of the national guidelines for dignosis and management of HIT in Croatian Institute of Transfusion Medicine (CITM) in the period from 2011 to 2014.
Material and Methods
Clinical and laboratory data for 266 consecutive patients with suspected HIT, referred to diagnostic laboratory for investigation between January 2011 to December 2014 were retrospectively analysed. According to the Croatian Society for Haematology and Transfusion Medicine guidelines on the diagnosis and management of HIT, “4T score”system has been used as a clinical judgement for HIT probability. Laboratory testing for HIT was done by antibody assays for anti‐heparin antibodies to platelet factor 4 (PF4) using particle gel method (HPF4 PaGia, Biorad, Switzerland) and enzyme‐immunologic method (HPF4 EIA‐IgG, Lifecodes, USA). Heparin induced platelet aggregation assay (HIPA) was used as functional confirmation assay. When anti‐heparin antibodies were demonstrated, heparin was discontinued immediately and continued with a heparin substitute (inhibitor FXa; fondaparinux or direct thrombin inhibitor; argatroban, lepirudin), depending on other clinical and laboratory findings.
Results
Pre‐test probability of HIT by “4T s”was done in 166 of 266 (62, 4%) patients. 45 of 166 (27, 1%) had low probability, 103 (62, 1%) moderate and 18 (10, 8%) high probability for HIT. Laboratory monitoring of platelet count before and during heparin therapy was done in 149 of 266 (56%) patients. Anti‐PF4 antibodies were found positive in 58 of 266 (21, 8%) patients by particle gel method (PaGia‐HPF4) and 42 (15, 9%) by enzyme‐immunologic method (EIA‐HPF4‐IgG). In 17 of 32 (53, 1%) EIA positive patients functional assay (HIPA) was positive. Thromboembolic complications were present in 18 patients with confirmed HIT II. 14 of 18 patients were treated with Factor Xa inhibitor; fondaparinux as the first line replacement therapy for heparin. 10 of 14 patients responded with complete recovery. 3 of 14 patients with cross reactive antibodies refractory to fondaparinux therapy were treated with direct thrombin inhibitor, one with lepirudin and other two with argatroban. One patient died, and two underwent amputation of the lower limb.
Conclusion
Implementation of national guidelines for diagnosing and treating of HIT upgrade the quality of laboratory diagnostic, reduce unnecessary testing, and improve interpretation of laboratory test results and their relevance and patient treatment. Fondaparinux as a replacement for heparin drug is effective in most patients with HIT II.
P‐657
Clinical Significance of Anti‐Hla Antibodies in Neonatal Alloimmune Thrombocytopenia
Bonstein L1, Atweh N 2, Tsuno NH 3, Sachs UJ 4
1Rambam Health Care Campus, Haifa, Israel2Platelet & Neutrophil Immunology Laboratory, Rambam Health care Campus, Haifa, Israel3Department of Transfusion Medicine, The University of Tokyo, Tokyo, Japan4Institute for Clinical Immunology and Transfusion Med. Justus Liebig University, Giessen, Germany
Background
Neonatal alloimmune thrombocytopenia (NAIT) is caused by maternal alloantibodies raised against paternally inherited alloantigens carried on fetal platelets. Platelets express both HLA class I and specific human platelet antigens (HPA). Although anti‐HLA class I antibodies are often detectable in pregnant women, NAIT is considered to be mainly associated with antibodies against HPA. Cases where NAIT has been caused by antibodies against HLA class I are relatively rare and the role of these antibodies in NAIT remains debatable.
We hereby describe a case of NAIT proved to be caused solely by anti‐HLA antibodies and discuss laboratory measures aimed at identification of pregnancies at risk of NAIT related to anti‐HLA class I antibodies.
Methods
This is a case of a young mother whodelivered her first son with a platelet count of 20x109 /L, minor petechiae and normal WBC count. Thrombocytopenia in the newborn resolved spontaneously two weeks after birth. Laboratory investigation included platelet immunofluorescence test (PIFT), monoclonal antibody‐specific immobilization of platelet antigens (MAIPA) assay, genotyping of both parents and the newborn for platelet antigens, including rare antigens and panel reactive antibodies (PRA) by Luminex for HLA antibody identification. A serum sample of this mother drawn during her second pregnancy and those of four other women with a similar obstetric history of neonatal thrombocytopenia were evaluated for the anti‐HLA antibody titer by MAIPA assay.
Results
The Rambam Platelet & Neutrophil Immunology Laboratory, as well as 32 other laboratories worldwide, that participated in the 2014 International Workshop organized by the ISBT Platelet Immunobiology Working Party failed to detect anti‐HPA antibodies in the mother's serum during her second pregnancy, despite using the most sensitive serological analysis and molecular methods. Only strong anti‐HLA antibodies with no single specificity were found in the analyzed samples by all the laboratories. Her second child was born by caesarean section with a platelet count of 50x109/L and maternal anti‐HLA antibodies were found in his serum and on his platelets. The anti‐HLA antibody titer of the mother, determined by MAIPA assay, was greater than 1:1024. Anti‐HLA antibody titer equal ³1:32 was found to correlate with low platelet counts in additional five cases tested, as opposed to the titer of £1:4 in cases with mild and not clinical significant neonatal thrombocytopenia.
Caption 1: Anti HLA titer and newborn's platelet counts

Conclusions
The presence of anti‐HLA class I antibodies should be considered as a potential cause of NAIT in cases with a very high titer of antibodies. The mechanism underlying the effect of these antibodies on fetal platelets needs to be further investigated.
P‐658
The efficacy of PRP gel in treating MRSA related surgical wound infections: an experimental study in rats
Cetinkaya RAC 1, Yilmaz SY 2, Unlu AU 2, Karabulut EK 3, Urkan MU 2, Kaya EK 2, Karabacak KK 2, Uyanik MU 1, Eker IE 2, Dogrul AD 2, Kilic AK 2, Avci IYA2, Yilmaz SY 2, Besirbellioglu BAB 2
1Military Hospital, Isparta, Turkey2Gulhane Military Academy of Medicine, Ankara, Turkey3Turkish Pharmaceutical and Medical Device Institution, Ankara, Turkey
Introduction
The wound healing properties of Platelet‐rich plasma (PRP) gel has been supported by studies. PRP gel has also become a promising agent for treating surgical site infections.
Aim
In this study, we investigated the antibacterial and wound healing properties of PRP against Methicillin-resistant Staphylococcus aureus subsp. aureus (MRSA N315) contaminated superficial soft tissue wound in a rat model.
Methods
We created 2 cm of midline subcutaneous wound in 48 Wistar Albino rats. Study groups comprised Sham (no treatment), PRP, MRSA, MRSA + PRP, MRSA + Vancomycin, MRSA + Vancomycin + PRP groups. We inoculated 0.1 mL (3×108 CFU/mL) of MRSA in contaminated groups. After 8 days, all rats were sacrificed, wounds were excised and subjected to histopathological examination and MRSA counts were determined.
Results
The MRSA counts in MRSA‐PRP, MRSA‐Vancomycin and MRSA‐Vancomycin‐PRP groups were 5.1 ′ 106 (SD ± 0.4), 4.3 ′ 106 (SD ± 0.7), 2.3 ′ 106 (SD ± 0.3), 1.1 ′ 106 (SD ± 0.4), respectively. The inflammation scores of MRSA + PRP, MRSA + Vancomycin and MRSA + Vancomycin + PRP groups were significantly lower than the MRSA group. PRP + Vancomycin + MRSA group inflammation score was significantly lower than the MRSA + PRP group.
Conclusions
All treatment groups were effective in contaminated wound healing and decreasing the MRSA counts. MRSA + PRP combination created identical inflammation scores to the PRP group. More in vivo studies are required to corroborate with our findings.
P‐659
New Assay for Fast and Accurate Detection of Anti‐HPA‐1A Allo‐Antibodies
Frassati C1, Di Cristofaro J 1, Basire A 1, Merieux Y 2, Picard C 1
1EFS Alpes Méditérranée, Marseille, France2EFS Rhone Alpes, Lyon, France
Background
Fetal/neonatal alloimmune thrombocytopenias (FNAIT) are the most frequent clinical condition involving anti‐HPA‐1a (Human Platelet Antigen) allo‐antibodies and cause the most morbidity. Therefore, rapid and accurate diagnosis anti‐HPA‐1a allo‐immunization is needed to determine appropriate treatment. The Capture‐P® Reading‐Screen® assay (C‐PRS®) is a new qualitative immunoassay to detect IgG anti‐HLA and anti‐HPA allo‐antibodies. The aim of this study is to evaluate the identification robustness of anti‐HPA‐1a allo‐antibodies by using the C‐PRS® assay, associated with HLA class I stripper reagents, on the fully automated bench top Galileo Echo® and compare this technology to the routinely used MAIPA method.
Study Design and Methods
59 sera, including 10 without allo‐antibodies anti‐HLA class I or anti‐HPA, 20 with anti‐HLA class I allo‐antibodies detected by Cytotoxicity Dependent‐Complement (CDC) or by Luminex (LifeCodesLSATM HLA class I, Gen‐Probe), 13 and 6 with anti‐HPA‐1a and anti‐HPA‐5b allo‐antibodies, among which 14 present anti‐HLA class I allo‐antibodies, and 10 with anti‐platelet glycoprotein auto‐antibodies were studied by C‐PRS® assay, with or without association with two different HLA class I stripper reagents, on the Galileo Echo®.
Results
All samples without allo‐antibodies were not reactive before and after stripper reagents incubation in C‐PRS® assay. Only anti‐HLA antibodies before and after stripper reagent detected by CDC and not by luminex remained positive. 11/13 samples of anti‐HPA‐1a allo‐antibodies were correctly identified after HLA assassin® reagent incubation. Furthermore, anti‐glycoprotein auto‐antibodies and anti‐HLA allo‐antibodies do not seem to interfere with the detection of anti‐HPA antibodies. Titration studies showed that the sensitivity of C‐PRS® for detecting anti‐HPA‐1a was one dilution superior to that of MAIPA for HPA1a/a homozygous platelet.
Conclusion
This study suggests that the C‐PRS® could be applicable for acute diagnosis of FNAIT using the HLA assassin® reagent, especially to detect anti‐HPA‐1a allo‐antibodies. This technique is fast and very easy, allows traceability according to laboratory quality standard and is flexible and therefore is suitable for emergency situations.
P‐660
Neonatal Alloimmune Thrombocytopenia: Diagnosis and Therapeutic Management in Algeria
Brouk HB1, Zermat BZ 1, Bertrand GB 2, Chergui NC 1, Bouhsane DB 1, Martageix CM 2, Djabri YD 1, Kaplan CK 2, Ouelaa HO 1
1Badji Mokhtar University, Annaba, Algeria2Institut Nationale de la Transfusion Sanguine, Paris, France
Background
Fetal/neonatal alloimmune thrombocytopenia (F/NAIT) is the most common cause of severe thrombocytopenia in the fetus and in an otherwise healthy newborn. The mother produces antibodies (IgG) against fetal antigens inherited from the father. These alloantibodies can cross the placenta, destroy fetal thrombocytes and may induce thrombocytopenia. In cases of severe thrombocytopenia, the major complication is intracranial hemorrhage (ICH) that occurs in 10–20% of cases, the consequences of which are harmful or fatal. In Algeria the diagnosis often remains unrecognized and requires laboratory demonstration of platelet antigen incompatibility, and detection of maternal platelet alloantibodies. The prospects for prevention against this alloimmunization are undefined because immunization mechanisms remain poorly identified.
The aim of this work is to diagnose neonatal alloimmune thrombocytopenia and draw attention of the practitioner on the frequency of this disease, morbidity and mortality associated with it.
Methods
We report our experience on the diagnosis and management of alloimmune thrombocytopenia. 249 cases of neonatal thrombocytopenia are included in this study. Diagnosis and management require, firstly, to detect maternal alloantibodies using the Platelet Antigen Monoclonal Antibody Immobilization Test (MAIPA) and, secondly, to detect the offending antigen by performing platelet genotyping (PCR‐SSP). Severe cases of NAIT required platelet transfusions.
Results and Discussion
The frequency of neonatal thrombocytopenia in Algeria is 0.82%, similar to the frequency previously reported in the literature. The incidence of NAIT is 1 case per 1650 births. Anti‐HPA antibodies were present in 15.07% of cases. Three cases of NAIT with maternal anti HPA‐1b, ‐3b and ‐5b alloantibodies were confirmed by platelet genotyping, with a favourable outcome. In addition; Platelet genotyping revealed two HPA‐3b, and one HPA‐5a incompatibilities without maternal alloantibodies detection. Despite platelet transfusions, two newborns with anti‐HPA‐3b and anti‐HPA–5a died following ICH.
Conclusion
Major progresses have been made to better diagnose and therapeutic management of F/NAIT. However, many questions are still unanswered about prevention and mechanisms of maternal immunization.
P‐661
Impact of Platelets Alloimmunization in Allograft
Astati S1, Croisille L 2, Maury S 3
1Military Hospital Mohamed V, Rabat, Morocco2Laboratoire d'immunologie leuco‐plaquettaire et d'histocompatibilité (E.F.S Ile, Creteil, France)3Service d'Hématologie Clinique et de thérapie cellulaire hôpital Henri Mondor, Créteil, France
Background
The prolonged thrombocytopenia after hematopoietic stem cell transplantation (HSCT) present factor of poor prognosis. There causes are varied and complex; whose platelet alloimmunization, which is responsible for refractoriness platelet transfusions.
Aims
Demonstrate the impact of platelets alloimmunization on platelet recovery.
Materiels and Methods
A retrospective analysis of 96 patients transplanted at the Department Hematology at Hospital Henri Mondor, Creteil, between January 2011 and December 2013 was performed. Only geno and pheno‐identical HSCT are studies. The objective is to demonstrate the impact of mismatches between donor and recipient for human platelet antigens (HPA) on recovery of platelet counts after transplantation.
Result
We tested each of the four HPA systems (HPA1, HPA3, HPA5 and HPA15), the platelet recovery in recipients ‘aa’ as they receive HSC donor ‘aa’ or ‘ab or bb’. We demonstrated no significant differences between groups with or no mismatch HPA, compared at 1, 3, 6, and 12 months after graft. While there was a trend (P = 0.07) in HPA3 system at 24 months to transplant. In HPA5 system, the differences were in the expected direction without statistically significant.
Conclusion
It would be important to demonstrate the impact of mismatch HPA on platelet recovery in a most important sample of patients to prevent the onset of refractoriness to platelet transfusions in the course of allogeneic haematopoietic stem cell.
5.4 Granulocyte Immunology
P‐662
Neonatal Alloimmune Neutropenia due to Maternal Alloimmunization Against Human Neutrophil Alloantigen‐1 and ‐3
Lopes LB1, Abbas SA 1, Moritz E 1, Chiba AK 1, Langhi-Junior DM 2, Baleotti WB 3, Suzuki RB 3, Fabron A Jr2, Ruiz MO 4, Chiattone C 2, Bordin JO 1
1Universidade Federal de São Paulo/UNIFESP, Marilia, Brazil2Faculdade de Ciências Médicas da Santa Casa de São Paulo, São Paulo, Brazil3Hemocentro da Faculdade de Medicina de Marília, São Paulo, Brazil4Laboratório de Imunologia de Marília, São Paulo, Brazil
Background
Neonatal alloimmune neutropenia (NAN) is a potentially lethal disorder that results from maternal alloimmunization to human neutrophil antigens (HNAs) present in the fetus. The alloantibodies more frequently involved in NAN are against the HNA‐1 and ‐2 systems, however, HNA‐3, HNA‐4 and HNA‐5 systems have also been associated with NAN.
Aims
In this study, we examined the frequency of neonatal neutropenia in Brazilians and investigated the presence of maternal anti‐HNA and anti‐HLA alloantibodies and the maternal‐fetal HNA‐1/3 incompatibility, when neonatal neutropenia was observed.
Methods
A cross sectional study included samples from 10,000 unselected neonates born in 4 obstetric units in Sao Paulo City (Brazil). Neonatal neutropenia was defined as neutrophil count<1.5 × 109/L in cord blood, resulting in the selection of 88 neutropenic newborns and their 83 mothers (3 pairs of twins and 1 triplet). Serologic studies for detecting maternal HNA alloantibodies were performed by granulocyte agglutination test (GAT), realized in duplicate using a specific panel of donors previously genotyped for HNA systems, and by LABScreen Multi‐HNA Kit (OneLambda®) that identify anti‐HNA‐1a,‐1b,‐1c,‐2 antibodies. Anti‐HLA‐I/II antibodies were investigated in maternal serum by LABScreen PRA Class I/II (One Lambda®), and LABScreen Multi‐HLA Kit (OneLambda®). In mothers with positive results in GAT, genotyping were performed by PCR‐SSP (HNA-1a,-1b,-1c alleles) and PCR‐RFLP (HNA-3a,-3b alleles) techniques. The PCR‐RFLP amplifies the rs2288904 sequence and the PCR product digested with enzyme Taqα1 specific to nucleotide guanine that codifies the HNA‐3a antigen (Lopes et al., Transfusion 2014;54(6):1619–21).
Results
Neonatal neutropenia was identified in 88/10,000 (0.9%) newborns. Serologic studies revealed 40/83 (48.2%) mothers with positive result in the GAT and 60/83 (72.3%) mothers with anti‐HLA I and/or II antibodies. Genotyping studies of the mothers with GAT(+) revealed 29/40 (72.5%) maternal‐fetal HNA incompatibilities, corresponding to 26/40 (65.0%) for HNA‐1 and 5/40 (12.5%) for HNA‐3. In 10/26 (38.5%) GAT(+) cases we found incompatibility for the HNA-1a allele; in 9/26 (34.6%) for HNA-1b; in 4/26 (15.4%) for HNA-1c; in 1/26 (3.8%) for both HNA-1a and -1c; and in 2/26 (7.7%) for both HNA-1b and -1c alleles. In all neutropenic cases related to HNA‐3 system mothers were typed as HNA‐3a/a and neonates as HNA‐3a/b. Using the LABScreen Multi‐HNA, HNA alloantibodies were identified in 7/40 (17.5%) mothers including 1 anti‐HNA‐1a, 2 anti‐HNA‐1b, 1 anti‐HNA‐1c and 5 anti‐HNA‐2. The specificity of HNA‐3b alloantibodies could be confirmed in 3/5 cases of HNA‐3 incompatibility using the panel of donors. Anti‐HLA I antibodies were detected in 18/29 (62.1%) GAT(+) maternal serum with HNA‐1/3 incompatibility. Two maternal‐fetal incompatibility cases occurred concomitantly for HNA‐1 and HNA‐3 systems; however, only in one case anti‐HNA‐3b alloantibodies could be identified using the panel of donors.
Conclusions
The observed frequency of neonatal neutropenia in Brazilians (0.9%) is similar to those described in North Americans and Europeans with a comparable positive serology rate of 48.2% associated with incompatibility maternal‐fetal for the HNA‐1/3 system (72.5%). As for the best of our knowledge this is the first study reporting the presence of anti‐HNA‐3b alloantibodies in newborns with NAN (17.2%).
P‐663
Activation of the NLRP3 Inflammasome Pathway
Li Q1, Zhang JM 1, Zhao FY 2, Zhu ZY 1
1Shanghai Blood Center, Shanghai, China2School of Life Science, East China Normal University, Shanghai, China
Background
The inflammasome was a multiprotein complex expressed on immune cells, such as macrophages and monocytes, and participated in host responses to various stimuli. The NLRP3 inflammasome would be triggered by a wide range of microbial pathogens and endogenous danger‐associated signals. The senescent erythrocytes, in a certain degree, could be identified as damaged cells. The clearance of aged erythrocytes was controlled by opsonized or unopsonized‐uptaken, which was involved in the IgG‐FcγR or CD47‐SIRPα signal pathway, respectively. Whether the NLRP3 inflammasome was activated when macrophage phagocytosed senescent erythrocytes was still unknown.
Aims
We planned to analyze whether the NLRP3 inflammasome would be triggered by erythrocytes opsonized or unopsonized‐uptaken.
Methods
4×106 THP‐1 cells were cultured in 6 cm dishes containing RPMI‐1640 medium (Gibco) supplemented with 10 IU/ml penicillin, 10 ug/ml streptomycin, 100 ng/ml Phorbol‐12‐myristate‐13‐acetate (PMA), and without fetal bovine serum. Then incubated in 5% CO2 at 37 �’� for 48h. Whole blood was collected from healthy donor at Shanghai Blood Center. The erythrocytes were washed three times by physiological saline solution. Then the RBCs were divided into three aliquots. One aliquot was incubated at 42 �’� for 2h to prepare senescent erythrocytes, one aliquot was sensitized with IgG anti‐D antibody. The rest one had no further treatment, and marked as young RBCs. The three aliquots of erythrocytes were co‐ incubated with PMA pre‐treated THP‐1 cells for three hours. The PMA induced THP‐1 was also stimulated with 30μM nigericin for 30 minutes to make NLRP3 inflammasome activity control. The supernatant was collected to detect the expression of IL‐1b, which was also analyzed on mRNA level. The NLRP3 inflammasome expression was detected by western blot with goat anti‐cryopyrin (sc‐34411, SANTA CRUZ).
Results
The ELISA results of IL‐1b was showed in Table 1. The relative mRNA level of THP‐1 with aged RBCs and THP‐1 with anti‐D sensitized erythrocytes was more than two folds against which of THP‐1 negative control. In addition, western blot showed NLRP3 inflammasome expression increased obviously in THP‐1 co‐cultured with senescent and anti‐D sensitized erythrocytes.
Table 1 The production of IL‐1 detected by ELISA

Summary/Conclusions
There was much information showed the mechanism of the clearance of senescent erythrocytes. In the study, we focused on macrophages which were phagocytosing aged RBCs. The NLRP3 inflammasome could control IL‐1b production. So, according to our results, we made a conclusion that the NLRP3 inflammasome pathway was activated when macrophages phagocytosed senescent erythrocytes through opsonized or unopsonized mechanism. As IL‐1b was one of the most important cytokines in infection and inflammatory disease, patients with certain diseases should be given fresh RBC products to increase the safety factor. The results were gained by experiments in vitro, in vivo tests would be further analyzed to confirm the findings.
P‐664
Newly Acquired Expertise: Implementation of Anti‐Neutrophil Antibody Assays
Bonstein L , Lauterbach R , Walter M , Chaho I
Rambam Health Care Campus, Haifa, Israel
Background
Anti‐neutrophil antibodies are involved in autoimmune neutropenia, neonatal allo‐immune neutropenia and transfusion‐related acute lung injury (TRALI). The identification of these unique antibodies is important for the clinical diagnosis of such conditions, sparing a lot of other extensive laboratory workup and sometimes invasive examinations. This approach also allows deferring antibody‐positive blood donors, hence preventing potential TRALI cases. Despite the importance of these assays, only 16 laboratories worldwide have been certified by the Granulocyte Immunology Working Party (GIWP) to conduct them.
Aim
To implementat the Rambam Platelet & Neutrophil Immunology Laboratory the assays required by the GIWP for the detection and identification of anti‐neutrophil antibodies, in order to provide a vital diagnostic tool at the national level.
Methods
Granulocyte antibodies were identified in various clinical conditions using the granulocyte agglutination test (GAT), granulocyte immunofluorescence test (GIFT), lympocyte immunofluorescence test (LIFT) and monoclonal antibody specific isolation of granulocyte antigens (MAIGA). Neutrophil antigen genotyping was established by the HNA‐Ready Gene assay (Inno train). Results of all the tests were validated by comparison with the results obtained by the Granulocyte Laboratory in Giessen, Germany which served as our mentoring and reference laboratory. The GIWP minimum targeted accuracy of 70% was required to be achieved. Repeatability was tested using three samples (positive, negative and borderline positive) which were evaluated three times, on three separate occasions.
Results
Molecular genotyping: All the ten evaluated samples were correctly genotyped for five different neutrophil antigens.Detection of anti‐neutrophil antibodies: Twenty four samples from patients suspected of having autoimmune neutropenia were tested simultaneously by both laboratories. Our results were consistent with those of the reference laboratory in all cases apart from one, which was found to be positive in the Giessen lab and negative in our lab. This 23/24 outcome gives 96% accuracy. High repeatability was achieved.Identification of anti‐neutrophil antibodies: Thirteen positive unknown serum samples were provided by the reference laboratory; in 12 of these samples antibody identification was consistent (92% accuracy). Twenty additional serum samples from donors who donated blood components involved in suspected TRALI cases were tested, yielding 95% accuracy.These successful validation results allowed the Rambam Laboratory to participate in the 2014 International Workshop organized by the ISBT Granulocyte Immunology Working Party, which is required for finalizing the certification process.
Summary
All results met the validation protocol requirements. This process enabled the implementation of anti‐neutrophil antibody detection and identification assays at the Rambam Platelet & Neutrophil Immunology Laboratory, which has become the 17th laboratory certified by GIWP to perform such evaluation.
P‐665
Differences in Procoagulant Activity Between Negative and Positive Duffy Antigen African Blood Donors
Ghilardi F 1, Zighetti ML 1, Sinigaglia E 1, Vismara G 1, Leo L 1, Camera M 2, Brambilla M 3, Lombardi M 1, Carpani G1
1Azienda Ospedaliera San Paolo, Milan, Italy2Dep. of Pharmacological and Biomolecular Sciences, Univ. degli Studi di Milano, Milan, Italy3Centro Cardiologico Monzino IRCCS, Milan, Italy
Background
The presence of the Duffy antigen receptor of chemokines (DARC) on the erythrocytes membrane defines the Duffy blood group system. DARC is a silent receptor and behaves as a reservoir of chemokines, its absence causing a higher level of circulating chemokines. A large part of Africans lack the DARC receptor on the membrane of their erythrocytes. Preclinical data suggest a link between the Duffy antigen blood group and coagulation.
Aims
This study aims to evaluate whether or not differences exist in monocyte‐associated Tissue Factor (TF) expression, plasma thrombin generation (TG), D‐Dimer and Von Willebrand Antigen (vWF:Ag) levels in Duffy‐null and Duffy‐positive African blood donors.
Methods
Twenty‐eight African blood donors (10 Duffy‐null and 18 Duffy‐positive) were enrolled in our Center (Table 1). TF expression on 50 ng/ml lipopolysaccharide (LPS)‐stimulated monocytes was assessed by whole blood flow cytometry at baseline and after 2, 4, 6 hours stimulation at 37°C. D‐Dimer and vWF:Ag (HemosIL[TRADEMARK], Instrumentation Laboratory, Italy) levels as well as TG (Thrombinoscope[TRADEMARK], Stago, Italy) were assessed in plasma samples at baseline.
Results
LPS‐stimulated monocytes showed a time‐dependent upregulation of TF expression which was higher, although not statistically significant,in Duffy‐null subjects at 4 and 6 hours. TG and vWF:Ag were significantly higher in Duffy‐null subjects (P<0.05), whereas D‐Dimer did not differ between the two groups.
Table 1. Demographic features and blood group of the study population

Conclusions
Duffy‐null subjects showed a trend towards a higher monocyte‐associated TF expression and a significantly higher thrombin generation capacity compared to the Duffy‐positive of the same ethnic group. These results, supporting a link between the absence of DARC and the increased procoagulant activity, might have clinical implications in the pathophysiology of inflammation and thrombosis in Duffy‐null subjects.
5.5 Fetal‐Maternal Immunology
P‐666
Glycosylation of Anti‐Platelet Antibodies in Different Phases of Pregnancy
Vidarsson GV1, Sonneveld MS 1, Koeleman CK 2, Natunen SN 3, Killie MK 4, Partanen JK 3, Kjeldsen-Kragh JK 4, Skogen BS 4, Wuhrer MW 2, van der Schoot CS 1
1Sanquin Research, Amsterdam, The Netherlands2Leiden University Medical Center, Leiden, The Netherlands3Finnish Red Cross Blood Service, Helsinki, Finland4University of Tromso,, Tromso, Norway
Background
Fetal or neonatal alloimmune thrombocytopenia (FNAIT) is a potentially life‐threatening disease where fetal platelets are destroyed by maternal anti‐platelet IgG alloantibodies. However, there is a discrepancy between antibody titer and clinical outcome preventing screening for these antibodies; of children of mothers with anti‐fetal‐platelet IgG, 31% develop thrombocytopenia but only 2.3% suffer from severe hemorrhagic disease. We recently found that N‐linked IgG Fc‐glycosylation can be particularly skewed towards low fucosylation in symptomatic individuals, increasing their affinity to FcγRIIIa and FcγRIIIb, and hence platelet destruction.
Aims
To investigate whether glycosylation patterns might be a useful parameter in screening for FNAIT
Methods
Anti‐human platelet antigen (HPA)‐1a specific IgG1 were affinity‐purified, trypsinized, and resulting glycopeptides analyzed with mass‐spectrometry in longitudinal samples, obtained throughout pregnancies and/or between pregnancies.
Results
We found a significant positive correlation between bisection, sialylation and fucosylation versus platelet count, but negative correlation with titer. Multiple regression analysis revealed glycosylation and titer to significantly predict platelet counts. Moreover, the level of fucosylation showed a significant drop after first pregnancy, but being constant throughout thereafter: between and years after pregnancy, with suggesting fucosylation to be a subject to immunological memory on the B cell level.
Conclusions
These results suggest that Fc‐glycosylation of anti‐platelet antibodies may explain the discrepancy between antibody titer and severity; potentially serving as additional marker to identify those urgently requiring medical intervention.
P‐667
Use of High‐Dose Intravenous Gammaglobulin for the Treatment of Severe Hemolytic Disease of the Newborn
Torres OW , Rey PR
Hospital Materno Infantil Ramón Sardá, Buenos Aires, Argentina
Introduction
Until recently, hemolytic disease of the newborn (HDN) accounted for 10% of all cases of perinatal mortality.At present because of intrauterine treatments (IUT, IVT), high‐dose intravenous gammaglobulin (hd IVIgG) administration or a both combined methods, perinatal survival outcomes are quite encouraging.The combined approach for this pathology leads to a survival above 85%, with lower prematurity ratesand an adequate long‐term neurological development. It is known that the risk of intrauterine death is higher if fetal hemolysis occurs during the first 20 weeks of gestation. The treatment with IVIgG before the 20th week of pregnancy in women with a history of HDN, and also in newborn severely affected has been proven to improve perinatal conditions.
Aims
To show the effectiveness of hd IVIgG treatment for severe HDN.
Materials and Methods
Cross‐sectional retrospective study of pregnant women and newborns with severe HDN treated in our institution between January 2006 and December 2014. Inclusion criteria: First antenatal visit before the 20th week of gestation, history of fetal or newborn death caused by HDN due to anti‐D in previous pregnancies and a male partner with homozygous phenotype for D factor.
Results
Out of 100 patients with severe HDN (2 twin pregnancies) treated during the prenatal period, 81 received IUT only, 8 received hd IVIgG exclusively (400 mg/kg weight) starting on the 14th week of gestation, and 11 women received a combined treatment (IUT + hd IVIgG).
A total of 191 IUT were carried out without complications and 112 series of hd IVIgG.
Perinatal data: GA: 34± 1.6 weeks. Deaths: 14 (14.0%) hydrops fetalis: 34 (34.0%). Live births: 88 (88.0%), weight: 2305 ± 323 g. Average hematocrit: 25%. According to the Sardá′s Protocol, all the neonates were trated with hd IVIgG (400 mg/kg weight, the first dose within 2 hours postpartum, and remaining doses at 24 and 48 hours respectively. Only 3 neonates needed exchange transfusion
The evolution during the first year of life for 68 (72.34%) newborns was normal.
In the case of fetuses whose mothers received hd IVIgG and required IUT, the procedure was carried out after the 26th week of gestation, reducing the fetal risks, more frequent with lower gestational ages. Anti‐D was always the responsible for hemolysis.
Conclusions
Our findings show that the early beginning of treatment with hd IVIgG reduces the severity of the anemia and the development of hydrops, thus improving fetal survival. This treatment can be provided separately or combined with IUT, according to the severity of the condition. The hd IVIgG is also useful for those newborns severely affected by maternal antibodies
P‐668
Comparison of Quantitation, Titre Scores and Flow Cytometry for Estimation of Maternal Anti‐D (RH1) and Anti‐C (RH4)
Green FA , Ridsdale C , Gilbert J , Ridgwell K , Bruce D
NHSBT, Bristol, United Kingdom
Background
Maternal anti‐D and anti‐c are the most common antibodies associated with moderate and severe haemolytic disease of the fetus and neonate (HDFN). Monitoring of antibody levels is important in predicting fetal risk and guiding medical intervention. UK practice is to quantitate these antibodies using the continuous flow analyser (CFA) which provides information on antibody levels in pregnancy (recorded as IU/mL) and the assignment of HDFN risk categories.
CFA (introduced in the UK over 45 years agos) is more reproducible and less subjective than manual antibody titration by tube, but is a complex test requiring specialised equipment, experienced operators and is limited to reference centres. Anti‐D and anti‐c levels could potentially be determined using flow cytometry (FC), or by titre score (TS) using column agglutination technology (CAT). CAT is in regular use for quantitation of other antibodies, and has been described in this context in a recent pilot study. The potential benefits of FC and CAT include ease of use (employing analysers in mainstream use for other laboratory purposes) and improved intra‐ and inter‐laboratory reproducibility.
Aim of the Study
To establish whether TS and FC can provide equivalent data to CFA that can meet the requirements of obstetric units in managing pregnancies at risk of HDFN.
Method
Antenatal plasma samples were tested by the following methods:
CFA: Plasma was tested against enzyme‐treated OR1R1 or Orr red cells. The strength of reaction was compared with NIBSC anti‐D and anti‐c standards.
CAT: Serial dilutions of sample were tested against CE‐marked pooled OR1r cells (NHSBT Reagents) on Bio‐Rad IAT cards. Reaction strength was determined using a semi‐automated Banjo ID card reader. Titre scores were assigned as described in the AABB technical manual (1996).
FC: Plasma was incubated with CE‐marked papain‐treated pooled OR1R1 or Orr red cells (NHSBT Reagents). Antibody levels were quantified against NIBSC standards using a Beckman Coulter Navios flow cytometer. Results were recorded in IU/mL.
Risk categories were assigned using BCSH guidelines (2007) for CFA and FC. Data from a pilot study was used to determine risk thresholds for TS.
Results
266 anti‐D and 174 anti‐c samples were tested by all methods. For anti‐D, consensus between the three methods in assigning risk category was 71.8%; between CFA and TS was 81.2% and between CFA and FC, 82.7%. Correlation between the methods was better for low and high risk categories (~80%) than for medium risk. For anti‐c, consensus between all methods was 78.7%; between CFA and TS was 82.2% and between CFA and FC, 86.2%. Correlation between methods was good for the low risk category (89%), but poor for the other categories.
Summary
This data suggests that TS or FC could be used as an alternative to CFA for anti‐D and anti‐c quantation. However, variation in the assignment of risk category was observed. A project extension in collaboration with fetal medicine units is required to link pregnancy outcomes with the assignment of intervention levels using these methods.
P‐669
Determination of Fetal Rhd Genotyping From Maternal Plasma in a Population With A High Frequency of the Rhd Pseudogene
Levi JE1, Chinoca K 1, Liao AW 2, Dezan M 1, Dinardo CL 1, Jens E 1, Brizot ML 2, Franscisco RVP 2, Zugaib M 2, Mendrone A Jr1
1Fundação Pró‐Sangue Hemocentro de São Paulo, São Paulo, Brazil2Dept. of Obstetrics, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
Background
Screening RhD negative pregnant women for the RHD gene by extracting DNA from plasma and submitting it to molecular amplification became a routine diagnostic procedure in several countries. The Obstetric clinics from the Hospital das Clínicas de São Paulo (Brazil) provides prenatal care to a large RhD‐ female population, monitoring for antibody titers and administering anti‐D immunoglobulin when appropriate.
Aims
To evaluate the accuracy of the SAFE protocol, with modifications, in this population, representing the mixture of ethnicities characteristic of the Brazilian people.
Methods
220 RhD‐ pregnant women were recruited for this study. Blood samples were collected at variable maternal ages (8‐28 weeks, mean 20 weeks), and plasma obtained in maximum 2 hours after drawing. 1 mL aliquots were extracted in a nucleic acid automated extraction platform (MagNA Pure Compact, Roche) employing a Large Volume kit. Extracted DNA was submitted to real‐time PCR amplification (Step One Plus, Applied Biosystems) using the SAFE group protocol, which targets exons 5 and 7 from the RHD gene.
Results
36 samples were excluded from the analysis due to preanalytical problems, abortion and missing follow‐up. Among the 184 samples analyzed, 128 were genotyped as RhD+ (70%) and 56 RhD‐ (30%) which was compared to phenotyping upon cord blood evaluation. Complete concordance (100%) was observed. Seven samples displayed exclusive amplification of exon 7 (3,8%). They were submitted to a real‐time PCR protocol specific for the RhD pseudogene (RhDΨ) and 5 (3%) were positive, further confirmed by analysis of maternal DNA from the buffy‐coat. We are currently collecting samples from the newborns to investigate their carrier status. The remaining 2 (1%) were negative for the presence of RhDΨ and are being submitted to exons 3‐9 PCR to be followed by direct DNA sequencing to investigate possible D variants.
Summary/Conclusions
The described method for fetal RhD determination from maternal plasma showed a high accuracy, is easy to perform, reproducible and fast. Parallel testing for the RhDΨ alelle may allow for direct recognition of carriers, precluding the need for further investigation of exon 5 failures. Due to the prevalence of this variant in our population, it may represent an improvement both for fetal medicine but also when searching for D variants among blood donors phenotyped as RhD‐. Implementation of this method will allow approximately 30% of the women to be discharged from the intensive surveillance over mothers bearing RhD+ fetuses, also sparing anti‐D immunoglobulin.
P‐670
Determination of the Fetal Kel Genotype From the Cell‐Free Fetal DNA in the Peripheral Blood of the Mother
Holuskova I , Durdova V , Bohmova J , Studnickova M , Lubusky M , Galuszkova D
Faculty Hospital, Olomouc, Czech Republic
Background
The KEL gene encodes the Kell antigens and is localizated on chromosome 7. It has two major codominant alleles K (KEL 1) and k (KEL2), which result from a single nukleotide polymorphism (698C→T), and the corresponding k and K antigens differ by a single amino acid change (T193M).
Objectives
Noninvasive determination of the fetal KEL genotype from cell‐free fetal DNA in plasma KEL of a homozygous pregnant woman.
Materials and Methods
Noninvasive determination of fetal KEL genotype from cell‐free fetal DNA in the plasma of pregnant women was carried out through minisequencing by capillary electrophoresis (so called SNaPshot). The assay is based on extending the sequence‐specific DNA primer by one base in KEL polymorphism (K/k, KEL1/KEL2). On the basis of an incorporated, fluorescently marked base, the additive of the complementary fetal allele K (KEL1) or k (KEL 2) can be identified and the KEL genotype can be determined by detecting the fluorescence of the respective base.
To establish the sensitivity threshold, the calibration of KEL was carried out with dilution ranges that were prepared using the plasmatic and cellular DNA of a “K”negative homozygous (k/k, KEL2/KEL2) and heterozygous (K/k, KEL1/KEL2) individual. In total, 141 random samples of cell‐free fetal DNA isolated from the plasma of women during their 1st trimester of gestation were tested.
Results
Using the dilution ranges, it was possible to detect less than 0.78% of K allele (KEL1), which corresponds to the concentration of DNA 0.04 ng/μl. In 113 random samples of “K”negative women (k/k, KEL2/KEL2), 7 fetuses with K allele were found corresponding so to about 4–5% of population frequency. In 8 cases, it was not possible to determine the fetal KEL genotype due to the KEL heterozygous genotype of the mother.
Conclusion
Minisequencing by capillary electrophoresis is appropriate for the detection of fetal K allele (KEL 1) from the cell‐free fetal DNA in the plasma of “K”negative pregnant women (k/k, KEL2/KEL2). Our results will be tested on the DNA samples of the newborns.
Financial supported by the grant IGA MZ ČR NT‐12225‐4/2011
P‐671
Prediction of the Fetal HPA‐1A Status From Maternal Plasma Dna Using Next‐Generation Sequencing
Orzinska A1, Guz K 1, Mikula M 2, Kluska A 2, Balabas A 2, Debska M 3, Uhrynowska M 1, Ostrowski J 2, Brojer E 1
1Institute of Hematology and Transfusion Medicine, Warsaw, Poland2Maria Sklodowska‐Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland3Medical Centre of Postgraduate Education, Department of Obstetrics and Gynaecolo, Warsaw, Poland
Background
In HPA‐1a negative pregnant women immunized against the HPA‐1a antigen, anti‐HPA‐1a antibodies can cause alloimmuno thrombocytopenia of a fetus or neonate (FNAIT). Thus, it is important to predict the fetus HPA‐1a status in an immunized mother to further manage the fetus at risk of the disease accordingly. The cell‐free fetal (cff) DNA circulating in the maternal plasma is widely used for determination of fetal blood groups but methods based on real‐time PCR detect a fetal allele with high accuracy only if the difference between a fetus and a mother concerns at least a few nucleotides. Obtaining of proper specificity in the amplification of a single nucleotide polymorphism (SNP), such as HPA*1A allele encoding platelet antigen 1a, requires modified protocols with using special primers or probes or a digestion of the maternal allele. Next generation sequencing (NGS) technology enables the high coverage sequencing of target SNP position and detection of low‐grade chimerism which takes place in plasma DNA of pregnant women.
Aim
To establish NGS technology usability in predicting the antenatal HPA*1A status.
Material and Methods
Blood from two HPA‐1a negative women at week 28 of pregnancy and two donors (for preparing an artificial mixture containing 5% plasma DNA of the HPA*1A/1B donor in plasma DNA of the HPA*1B/1B donor) was collected into EDTA vacutainer tubes. Plasma DNA was isolated using easyMag extractor (Biomerieux). The sequence of ITGB3 gene containing HPA*1A/1B SNP base position was amplified using a pair of primers (according to Ficko et al.) linked with barcoded sequencing adapters. PCR products were cleaned up with Agencourt AMPure XP beads and 100 pM DNA libraries were sequenced using Ion Torrent PGM on 316 chip (Life Technologies).
Results
The mean coverage for three samples was 49,893 total reads. The artificial mixture gave 3% HPA*1A positive reads (1732 of 57,537 reads). The samples from pregnant women yielded 1% (536 of 46,457 reads) and 5% (2223 of 43,385 reads) HPA*1A positive reads. Both NGS results were in agreement with the HPA*1A genotype of neonates obtained with real‐time PCR.
Conclusion
The use of NGS enables the prediction of fetal HPA*1A status with the accuracy required for diagnostic test.
P‐672
Non‐Invasive Prenatal Testing of HPA*1a in Prevfnait Programme for HPA‐1a Negative Pregnant Women in Poland
Orzinska A1, Guz K 1, Uhrynowska M 1, Debska M 2, Husebekk A 3, Brojer E 1
1Institute of Hematology and Transfusion Medicine, Warsaw, Poland2Medical Centre of Postgraduate Education, Department of Obstetrics and Gynaecolo, Warsaw, Poland3Institute of Medical Biology, University of Tromsø The Arctic University of Norw, Tromsø, Norway
Background
Human Platelet Antigen 1a (HPA‐1a) may cause maternal allo‐immunization of HPA‐1a negative pregnant woman and may lead to fetal/neonatal alloimmune thrombocytopenia (FNAIT). Determining feto‐maternal incompatibility using non‐invasive prenatal testing (NIPT) of HPA*1A status is essential for clinical decisions on antenatal interventions in cases of mothers with anti‐HPA‐1a antibodies.
Aim
Summary of 18 months period of prospective NIPT of HPA*1A done as part of the HPA‐1a screening programme “PREVFNAIT”which is currently performed by the Institute of Hematology and Transfusion Medicine (IHTM) in Poland (the Project Agreement No. Pol‐Nor / 203111/69/2013).
Methods
Plasma DNA was isolated on easyMag extractor (Biomerieux) from 87 HPA‐1a negative pregnant women (at week 28 of pregnancy), digested with MspI enzyme and examined by real‐time PCR on LCII 480 (Roche Diagnostics Ltd.) according to Scheffer et. al. publication. CCR5, SRY or bi‐allelic polymorphisms were tested to confirm the presence of total/ fetal DNA.
Results
In 51/87 cases, where neonatal HPA*1A genotype was available, NIPT gave correct fetal HPA*1 results (42 HPA*1A positive, 9 HPA*1A negative). In cases of HPA*1A negative results the presence of fetal DNA in non‐digested DNA was confirmed by detection of SRY (5 cases) or other paternal polymorphisms (5 cases). In one case the presence of HPA*1A variant in maternal genome made NIPT impossible.
Conclusions
The fetal and maternal HPA*1A genotypes were compatible in about 1/5 of pregnancies tested at IHTM. Real‐time PCR combined with digestion of maternal HPA*1B allele is a highly reliable method for predicting fetal HPA*1A status. In all seven cases of mothers with anti‐HPA‐1a antibodies, NIPT of HPA*1A determined fetuses as HPA*1A positive and it was possible to further manage the pregnancy clinically in reference hospitals.
P‐673
Prevention of Foetal/Neonatal Alloimmune Thrombocytopenia in Polish Neonates – Prevfnait Programme – Preliminary Results
Guz K1, Wrobel A 1, Uhrynowska M 1, Orzinska A 1, Debska M 2, Lopacz P 1, Berge G 3, Ahlen MT 4, Maslanka K 1, Husebekk A 3, Brojer E 1
1Institute of Hematology and Transfusion Medicine, Warsaw, Poland2Medical Centre of Postgraduate Education, Department of Obstetrics and Gynaecol, Warsaw, Poland3Institute of Medical Biology, University of Tromsø The Arctic University of Norw, Tromsø, Norway4Laboratory Medicine, University Hospital North Norway, Tromsø, Norway
Background
Foetal/Neonatal Alloimmune Thrombocytopenia (FNAIT) is severely under‐diagnosed in Poland. The awareness of the risk of this disease is still low despite HPA‐1a screening programs previously performed in selected regions of the country.
Aim
To present the preliminary results of the PREVFNAIT HPA‐1a screening programme, which is available throughout Poland for identifying pregnant women at risk of producing anti‐HPA‐1a antibodies in conjunction with current routine diagnostics offered to HPA‐1a negative women.
Material and methods
Blood samples from pregnant women at 8–20 week of pregnancy were collected at 260 collection sites located in various regions of Poland and delivered for HPA‐1a testing to IHTM in Warsaw.
1/ HPA‐1a screening: for samples collected within 7 days platelet rich plasma samples was tested by flow cytometry using anti‐human CD61‐FITC clone SZ21 (Beckman Coulter) on FACS Canto II cytometer with High Throughput Sampler (Becton Dickinson); for >7 day samples DNA isolated from whole blood was tested by HPA‐1 allelic discrimination on LC480II (Roche). All patients obtain the written report of the test results.
2/ Diagnostics algorithm of HPA‐1a negative women, their partners and neonates:
1At week 20: verification of HPA‐1 result and HLA DRB3*01:01 genotyping, HPA‐1 allelic discrimination of available partners,2At week 20, 28, 32, 38–40 and 6 weeks after delivery: detection of anti‐HPA‐1a antibodies using MAIPA,3After delivery: HPA‐1 allelic discrimination of the neonates,
All HPA‐1a negative women obtain medical consultation and mothers with anti‐HPA‐1a are monitored and treated, if necessary, in the reference hospital.
Results
Within 8 months of PREVFNAIT screening program 164/6600 HPA‐1a negative pregnant women were identified (2.5%). There was one woman phenotyped as HPA‐1a negative but genotyped as HPA‐1a/1b. 136 women were examined for DRB3*01:01 and 42 were positive. Available 135 partners were HPA‐1a/1a (97), HPA‐1a/1b (36) and HPA‐1b/1b (2). Anti‐HPA‐1a antibodies were detected in 9/136 woman (8 DRB3*01:01 positive and 1 negative). Their partners were HPA‐1a/1a. Treatment (IVIg or/and PLT transfusion) was applied in 7 women and 6 of them already delivered the baby ‐ 4 with no thrombocytopenia and 2 with mild thrombocytopenia (100 × 109/L and 124 × 109/L).
Conclusion
The introduction of large‐scale and automatic FACS or genotyping for HPA‐1a typing allows to centralize the screening and perform it at low cost. Diagnostic algorithm for HPA‐1a negative pregnant women allowed to detect anti‐HPA‐1a antibodies in 6.6% cases participating in PREVFNAIT and introduce the treatment if necessary.
P‐674
Preventive Matching for K‐Antigens in Female Blood Recipients Under 45 years of Age Reduces the Incidence of K‐Alloimmunisation in Pregnancy
Luken JS1, Schonewille H 1, Ligthart PC 1, van der Schoot CE 1, van der Bom JG 2, Koopman MMW 1, de Haas M 1
1Sanquin Blood Supply, Amsterdam, The Netherlands2Center for Clinical Transfusion Research, Leiden, The Netherlands
Background
Severe hemolytic disease of the fetus or newborn (HDFN) can develop as a result of maternal antibodies against Rh (mainly D, c and E) or K red blood cell antigens in pregnancy. A Dutch nationwide study revealed that 83% of pregnant women with anti‐K had a history of transfusion. To prevent transfusion induced K‐alloimmunisation, the Dutch transfusion guideline prescribes preventive matching for the K‐antigen in female transfusion recipients aged <45 years since 2004. Routine selection of K‐negative blood for these patients is feasible because the Dutch donor cohort is typed for Rh‐ and K‐antigens with antigen‐negativity displayed on the product label.
Aims
Evaluation of the effect of a preventive K‐matched transfusion strategy in female transfusion recipients aged <45 years on the prevalence of anti‐K affected pregnancies.
Methods
Sanquin Diagnostics serves as the national reference laboratory for laboratory monitoring of alloimmunisation in pregnancy, and analyzes on average 1849 (decreasing from 2407 in 2003 to 1582 in 2013) positive antibody screens at 12 weeks of gestation yearly. Data on antibody specificities from 2003 to 2013 in pregnant women were retrieved from the Sanquin laboratory database. To correct for changes in transfusion policy over time, anti‐K prevalence was compared to the prevalence of antibodies against Duffy (Fy), Kidd (Jk) and S antigens, for which no routine preventive measures are advised in the guidelines. Pregnancies at risk for development of HDFN were defined by K‐positivity of the father. Pregnancies with K‐negative fathers were indicated as not at risk for HDFN as well as pregnancies without record of the K‐typing of the father and without laboratory monitoring of anti‐K, because the referring hospital decided the pregnancy was not at risk.
Results
The number of pregnancies with antibodies against K, Fy, Jk and S antigens decreased from 257 (130.1/100,000 pregnancies) in 2003 to 113 (66.9/100,000 pregnancies) in 2013. During this period the birth rate decreased by 14.5% (National Registration Database) and a more restrictive transfusion policy was implemented. The prevalence of pregnancies complicated by anti‐K declined from 73.4/100,000 pregnancies in 2003 to 21.9/100,000 in 2013. Pregnancies with anti‐K, as percentage of all pregnancies with K, Fy, Jk and S antibodies, decreased from 56.4% to 32.7% indicating a higher reduction rate of anti‐K compared to other antibodies. The incidence of for the first time detected anti‐K during pregnancy decreased from 57.2/100,000 to 11.3/100,000. In this period the prevalence of pregnancies at risk for anti‐K‐mediated HDFN decreased from 12.7/100,000 to 3.6/100,000. In 2003, 77.9% of pregnancies with K‐immunisation had K‐negative fathers and in 2013 this decreased to 68%, indicating that less women became immunized by transfusion.
Conclusions
Compared to the reference year 2003, the number of alloimmunised pregnancies has decreased, with anti‐K at the highest rate. Although the alloimmunisation risk was also influenced by a more restrictive transfusion policy and a lower birth rate, a preventive K‐matched transfusion policy for female transfusion recipients aged <45 years has shown to be effective in reducing K‐alloimmunisation and the risk of severe HDFN.
P‐675
Investigation of Hemolytic Diseases of the Newborn in Southern Taiwan
Wang YH1, Chang WC 1, Tsai HW 1, Lin TM 2
1National Cheng Kung University Hospital, Tainan, Taiwan2E‐DA Hospital/I‐Shou University, Kaohsiung, Taiwan
Background
The destruction of the newborn RBCs occurs commonly due to maternal alloantibodies or maternal and fetal (or infant) ABO incompatibility.
Aim
In this retrospective study, we investigated the hemolytic diseases of the newborn (HDN) due to ABO blood group incompatibility (ABOi‐HDN) or other minor blood groups incompatibility in Southern Taiwan.
Methods and Results
From 2001 to 2014, a total of 336 pairs of maternal/baby blood samples from the blood bank of National Cheng Kung University Hospital were analyzed for ABO grouping, anti‐A/B titer, irregular antibody screening and direct Coombs’ test. Among them, 124 samples (36.9%) were found to be ABO incompatible, including 50 (40.3%) were group A and 74 (59.7%) were group B babies from mother of blood group O. In contrast, only 21 cases (6.25%) were demonstrated to be caused by RBC irregular antibodies. Of the 124 ABOi‐HDN blood samples, 67 (54%) had positive direct Coombs’ test, 30 infants (24.2%) were blood group A, while 37 infants (29.8%) were blood group B. Of the 21 cases caused by RBC irregular antibodies, 17 (80.9%) belonged to clinical significant warm type antibodies. Anti‐E and/or anti‐c were most frequently detected (9 cases, 42.9%) and only one was caused by Anti‐D antibody.
In conclusion, ABOi‐HDN had significantly higher incidence than minor blood group incompatibility (P < 0.001) in Southern Taiwan. In addition, the incidence of ABOi‐HDN in blood group B is higher compared with that of blood group A (P < 0.005). The anti‐E and anti‐c are shown to be the most common minor blood group causing incompatible HDN.
P‐676
Spontaneous Antepartal RHD Alloimmunization
Holuskova I , Studnickova M , Lubusky M , Galuszkova D , Durdova V
Faculty Hospital, Olomouc, Czech Republic
Aim of the Study
Determine the incidence of spontaneous antepartal RhD alloimmunization in RhD negative pregnant women with an RhD positive fetus.
Methods
A total of 906 RhD negative women with an RhD positive fetus and without the presence of anti‐D alloantibodies at the beginning of pregnancy were examined. RhD blood group of the pregnant women was determined in the I. trimester of pregnancy, RhD status of the fetus was determined after delivery. Screening for irregular antierythrocyte antibodies was performed in all women in the I. trimester of pregnancy, at 28–32 weeks gestation, immediately prior to delivery at 38–42 weeks gestation, and also at 6 months following delivery. Antibody screening was performed using the indirect antiglobulin (LISS/NAT) and enzyme (papain) test with their subsequent identification using a panel of reference erythrocytes by column agglutination method Dia‐Med. After delivery, the volume of fetomaternal hemorrhage was assesed in all RhD negative women and RhD alloimmunization prophylaxis was performed by administering the necessary IgG anti‐D dose; none of the women were administered IgG anti‐D antepartally.
Results
During screening for irregular antierythrocyte antibodies at 28–32 weeks gestation, anti‐D alloantibodies were diagnosed in 0.2% of the women (2/906); immediately prior to the delivery at 38–42 weeks gestation, anti‐D alloantibodies were diagnosed in 2.3% of the women (21/906) and repeatedly even at 6 months following delivery (21/157). In 82.7% of the women (749/906), examination at 6 months following delivery was not performed, therefore in these women spontaneous antepartal RhD alloimmunization cannot reliably be ruled out. If anti‐D alloantibodies were not present prior to the delivery, these women were all administered IgG anti‐D in a dose of at least 125 μg after delivery.
Conclusion
In RhD negative women with an RhD positive fetus, the incidence of spontaneous antepartal RhD alloimmunization was at least 2.3%. Most cases may theoretically be prevented by prophylactic administration of 250 μg of IgG anti‐D to all RhD negative women at 28 weeks gestation.
Supported by the grant from the Ministry of Health of the Czech Republic IGA NT 12225‐4/2011
P‐677
Antenatal Antibody Screening: Where do we Stand? Experience From India
Das S , Baliga P , Shastry S
Kasturba Medical College, Manipal, India
Background
For appropriate management of Hemolytic Disease of Fetus and Newborn (HDFN), it is important to detect irregular red cell antibody through antenatal antibody screening. In developed countries, screening is mandatory, while in a developing country like India, it is yet to come up due to various reasons.
Aim
To assess the frequency and specificity of maternal irregular red cell antibodies and its clinical relevance.
Methods
A prospective study was carried out from October 2013 to January 2015 at our tertiary care center from south India. All antenatal samples which the laboratory received for red cell antibody screening, were screened using a commercial 3‐cell screening panel. Antibody identification, along with further immunohematogical techniques as required, were performed for the mothers with positive screening. Demographic, transfusion and obstetric histories were recorded and neonatal outcomes were followed up.
Results
A total of 1410 antenatal mothers were screened for red cell antibodies. Among them 362 (25.67%) were Rh negative. We noted that, antenatal antibody screening is performed more frequently and routinely done for Rh negative pregnancies than for Rh positive pregnancies. A significantly higher proportion of alloimmunization was seen among D‐antigen negative pregnancies (8.8%) when compared to D‐antigen positive (1%). Most frequently anti‐D (61.5%) was identified among all the samples screened positive and in 6.63% Rh negative pregnancies. Other clinically significant antibodies included four cases of anti‐G, two cases each of anti‐c, anti‐D+C and anti‐Leb,and one case each of anti‐M, anti‐Lea and anti‐E. Two pregnancies were associated with auto‐immune antibodies and two cases remained inconclusive. Two Bombay blood group pregnancies were also managed. 11 neonates had features of HDFN, among which only two required exchange transfusion while rest were managed with phototherapy alone.
Conclusion
In the present study, though rate of alloimmunization in Rh negative pregnancies is more, we also noted significant rate of alloimmunization among Rh positive pregnancies (1:100). Thus, we advocate routine antenatal antibody screening for all antenatal mother irrespective of their Rh status.
P‐678
Successful Management of Haemolytic Disease of the Fetus and Newborn due to an Anti‐RH17 Antibody
Chandrasekar A1, Eggington J 1, Agarwal UA 2, Cox AM 3
1NHS Blood and Transplant, Liverpool, United Kingdom2Liverpool Women's NHS Foundation Trust, Liverpool, United Kingdom3Lancashire Women and Newborn Centre,, Burnley, United Kingdom
Background
Persons with rare D”‘phenotype may become alloimmunised to high frequency Rh system antigens through transfusions or pregnancy. Anti‐Rh17 antibody in pregnant women has been reported to cause sometimes moderate to typically severe and often fatal haemolytic disease of the fetus and newborn(HDFN). There are limited number of case reports in literature on prenatal diagnosis and successful management of HDFN of due to anti Rh17 antibodies.
Aim
We present our experience on successful management of HDFN due to anti Rh17 antibody following prenatal diagnosis. There have been four previous reports of successful outcome following intrautrine transfusions to treat HDFN due to anti Rh17.
Method
30 year old british asian lady with anti Rh 17 antibodies presented in her third pregnancy (Gravida 3, Para 2, live birth 1). Her first child is alive and well. Her antibody screen at the booking visit in her second pregnancy was negative. When she was admitted for induction at 39 weeks following intrauterine death in her second pregnancy, she was noted to have anti Rh17 antibodies with antibody titre of 1/256. Four months later she presented in the antenatal clinic in her third pregnancy and was closely monitored from 8 weeks gestation with antibody titres and ultrasound examination. Signs of HDFN were noted in 23 weeks gestation and Intrauterine transfusions with frozen thawed redcells were given at 23,24,28 and 32 weeks of gestation.
Results
During the course of the pregnancy, low titre anti‐e was demonstrated in maternal sample in addition to anti Rh17 antibodies which reached a titre of 1in 512. She also had anti‐CR1 related antibody which made the investgations and crossmatching more complicated and challenging. Baby boy was delivered 35 + weeks with planned caesarian section. Haemoglobin(Hb) at birth was 143 g/l and two days later 192 g/l two days later. The newborn was treated with phototherapy and did not receive exchange transfusion though thawed red cells were made available prior to delivery. Five weeks later the Hb dropped to 60 g/l and the baby was given top up transfusion with post transfusion Hb 90 g/l. However the Hb continued to decline due to bone marrow suppression caused by intrauterine transfusion regime. The baby was transfused two more units and commenced on erythropoietin after the second top up to stimulate the marrow. The Hb remained stable after third top up and did not require further interventions.
Summary
On literature review only ten cases could be identified with prenatal diagnosis of HDFN with successful outcome. One baby required phototherapy after birth, five required exchange transfusion, three were treated with IUT and exchange transfusion and one baby required IUT but did not require exchange or top up transfusions post delivery. Of the four IUT, two of them used washed maternal red cells, one received thawed maternal red cells and one of them was transfused red cells from unrelated blood donor. Good communication and planning between the foetal medicine centre, neonatal unit and the blood service is essential for successful management of HDFN due to rare antibodies.
P‐679
Blocked K Antigen on Neonatal Red Blood Cells‐ Which is the Critical Titre?
Novoselac JN , Raos MR , Tomac GT , Lukic ML , Golubic Cepulic BGC
University Hospital Centre Zagreb, Zagreb, Croatia
Background
Blocked erythrocyte antigens, first described in 1944, occured due to potent maternal anti‐D antibodies that blocked D antigens on foetal red blood cells(RBCs) causing them to be negative when typing with human immunoglobulin M anti‐D. The phenomenon is rare and where it does occur antibody doesn't have to be of high titre.
Antibodies to other blood group antigens can also cause false negative results when typing antibody coated foetal/neonatal RBCs.
There are only two case reports in literature that describe false negative K phenotyping due to blocking with maternal anti‐K, phenomenon registered at a titre level of 128 or greater.
Aim
We want to present a case report of a potent anti‐K antibody which blocked K antigens on neonatal RBCs and made them phenotyped as K negative.
Methods and Results
In September 2014 term male neonate was born from mothers fifth pregnancy. Soon after the birth he required RBC transfusion because of significant anemia. Neonate was typed as AB positive, DAT 3+.
Mother was typed as A positive, IAT positive, antibody specificity anti‐K. In 2008 she gave birth to a healthy female neonate, in 2009 she had two missed abortions in 12th and 16th weeks of pregnancy and in 2011 she also gave birth to a healthy girl. She never received any blood products.
From the beginning of the first pregnancy no control of IAT was made, so the antibody was first identified after this delivery.
Mother was typed as K negative, father as K positive (Kk) and neonate at first as K negative.
DAT performed on neonatal RBCs was 3+ , with anti‐IgG 2+ and ‐C3d 1+ , eluate contained anti‐K 2+. Neonatal RBCs from peripheral vein blood were typed with monoclonal anti‐ K (Diaclone MS‐56) as K negative. Neonatal sample was also tested with PCR‐SSP (Inno‐train's RBC‐Ready Gene kit), and was confirmed to be K1K2.
Titre of anti‐K in mothers sera in the tube technique was 32. Subclasses of anti‐K were tested using DAT IgG1/IgG3 card and positive results were obtained with total anti‐IgG(1:10) and ‐IgG1(1:100) which is a characteristic of the anti‐K antibody.
Soon after the first testing, neonate was transfused with K negative RBC, and repeated K typing wasn't reliable. Infants anemia persisted and required further RBC transfusion in two more occasions. He also had mild hyperbilirubinemia which was treated with phototherapy. Repeated typing was performed 3 months after the last transfusion, when DAT became negative, and showed that infant is K positive. At the age of five months he has normal complete blood count and doesn't require any therapy.
Conclusion
The findings presented in this case are consistent with blocking of K antigen sites by a potent anti‐K on the surface of neonate RBCs. Complete blocking of K antigen is not widely described, nor perhaps recognized. Manufacturers product inserts should note this possibility and laboratory staff should also have this situation in mind when performing such tests.
We proved blocking effect of anti‐ K at titre of 32, which is lower than previously reported.
P‐680
Prevelance of Irregular Antibodies in Pregnant Women in General Hospital Uzice and Clinical Outcome (Eight Years Experience)
Drndarevic D , Divac V , Smiljanic R , Djenadic D , Perisic Bozic M
General Hospital Uzice, Uzice, Serbia
Background
Clinically significant alloantibodies can cause mild to severe hemolytic disease of the fetus and newborn (HDFN) or in some cases prolonged anemia in neonatal period primarily by destroying erythroid progenitors. Erythrocyte antibody screening is part of routine prenatal testing. If the screening is positive, antibody identification is performed. In the last eight years we have introduced gel column agglutination technique as more sensitive in detection of irregular antibodies.
Aim
To determine prevalence of irregular red blood cell antibodies in pregnant women by retrospective analysis using data from Protocols of prenatal testing in period 01.03.2007.‐01.03.2015.
Methods
Blood samples were collected on EDTA. ABO/D typing and DAT were done by standard tube technique (BioRad and Sanqin reagents). Antibody screening was done by gel technique with DiaCell I, II, BioRad. Samples with positive antibody screening were further tested to determine specifity of antibodies by using 11 test cell ID DiaPanel, BioRad in gel technique.
Results
In investigated period 5109 pregnant women were tested. Screening for irregular antibodies was performed for all pregnant women when they first come for ABO/D typing. Control screenings were performed according to National standards. 224 antibody screenings were positive. After antibody identification:
45 (0,86%) had irregular antibodies, 71 had passive anti‐D in low titers because women come in too short period after receiving prophylactic dose of Rh immunoglobulin after invasive procedures like amniocentesis (titer was lower or negative on the next control), 62 had nonspecific antibodies usually connected with health state such as viremia, urinary infections, thyroid problems, allergic rhinitis, 46 were negative possibly due to sample reasons.
Prevalence of antibodies was: anti‐E 0,21% (11), anti‐D 0,12% (6), anti‐c 0,06% (3), anti‐C 0,04% (2), anti‐K 0,04% (2), anti‐Cw 0,04% (2), anti‐M 0,04% (2), anti‐N 0,02% (1), anti‐Lea 0,16% (8), anti‐Leb 0,06% (3). Four pregnant women had anti‐D+‐C ab (0,08%), and were send to tertiary institution for differentiation this antibodies and ‐G.
In one case of anti‐D IUT was performed in tertiary care institution, other 5 babies of the mothers with alloanti‐D had mild to severe HDFN, baby of mother with high titer of anti‐E had anemia after birth that needed transfusion, baby of one mother with anti‐K (titer 16) had anemia after fifth month of life but did not request transfusion, and twins of the mother with anti‐M IgG class (titer 16) had hyperbillirubinemia that was treated with phototherapy and mild anemia that did not request transfusion.
Summary
Management of the pregnancies with alloimunisation is a challenge. Still, there is no consensus of managing pregnancies with antibodies of variable clinical significance. Serological monitoring of the maternal antibodies pre and postnatal and good collaboration between clinicians and transfusiologist shows better results in prenatal diagnosis and in clinical outcome.
P‐681
Severe Case of Fetal Hemolytic Disease Caused By Anti‐Cw Requiring Serial Intrauterine Transfusions Complicated by Pancytopenia and Cholestasis
Macher S , Wagner TM , Rosskopf KR , Reiterer FR , Csapo BC , Klaritsch PK ,
Medizinische Universität Graz, Graz, Austria
Background
Only 2% of Anti‐Cw antibodies lead to fetal and neonatal hemolytic diseases (HDFN), mostly of mild or moderate severity. We report a severe case with serial intrauterine transfusions complicated by thrombocytopenia, neutropenia and a cholestatic liver disease in the neonatal period.
Case Report
A 37 years‐old woman, gravida 4, para 3, revealed anti‐Cw antibodies with a titer of 512 in her routine serologic screening examination at 10 weeks of gestation. In the following analyses at 18 and 26 weeks of gestation the titer remained unchanged. At 29 + 0 weeks the patient was referred to our tertiary care unit due to fetal ascites. A severe fetal hydrops including massive ascites, generalized skin edema and pleural effusions was diagnosed. Middle cerebral artery peak systolic velocity (MCA‐PSV) was suggestive for fetal anemia which was confirmed by cordocentesis. Fetal intravascular transfusion was performed immediately and was repeated at 29 + 3 and 30 + 3 weeks of gestation. At 31 + 1 and 31 + 6 weeks the MCA‐PSV was unsuspicous and fetal ascites resolved by 32 + 3 weeks of gestation. At 33 + 0 weeks spontaneous preterm premature rupture of membranes was followed by an uncomplicated vaginal delivery. The newborn girl, weighing 1935 g, had to be intubated and mechanically ventilated. She required 2 RBC transfusions, epoetin alfa and darbepoetin alfa therapy and one platelet transfusion. Serum ferritine levels were excessively elevated (maximum level 3455 mg/ml) and additionally leukopenia (minimum white blood cell count 1.2 G/L) and unconjugated and conjugated hyperbilirubinemia, discolored faeces and elevated liver enzymes compatible with a cholestatic liver disease were diagnosed and treated with ursodeoxycholic acid, vitamins and medium‐chain triglycerids riched formula feeding. The girl was discharged at the age of 8 weeks and 5 days with fully recovered blood count and a residual cholestatic liver disease.
Summary/Conclusion
Our case indicates that anti‐Cw antibodies have the ability to induce severe HDFN with subsequent complications in the neonatal period. Furthermore the case confirms the finding that progressive fetal anemia is not always paralleled by rising antibody titers. In cases of high serologic antibody titers close fetal surveillance using sonographic evaluation of the MCA‐PSV should be performed. The risk for severe hemolytic complications may increase with the number of pregnancies of sensitized women.
P‐682
Anti‐PP1PK Alloimmunization: Two Consecutive Full‐Term Pregnancies of Anti‐ PP1PK ‐Carrying Patient Without Need of Special Supportive Treatment And No Haemolytic Disease of the Fetus and the Newborn
Lazarova EL , Rousseau RC , Lambermont LM , Donner DC ,
Erasme Hospital, ULB, Brussels, Belgium
Anti‐PP1PK, formally called anti‐Tja, is found only in subjects of the very rare p phenotype (frequency of 5.8 in 1 million people) who lack the P, P1 and PK antigens. Anti‐PP1PK is associated with abortions early in the pregnancy and more rarely with haemolytic disease of the new born (HDN) of varying severity. Here we report a case of a PP1PK ‐carrying patient with three consecutive pregnancies, the last two followed in our institution, all of them with full term delivery and neither foetal distress nor neonatal problems.
A 32‐year‐old pregnant woman of Pakistani origin, gravida 2, para 1 was referred to our institution at 2 weeks’ gestation. Her serological study detected the presence of anti‐ PP1PK antibodies with initial titer of 32. Her past obstetric history revealed a first uncomplicated pregnancy in Pakistan when she delivered a healthy girl of 3000 g at 37 weeks of gestation with immediate neonatal adaptation and no health problems. During her second and third pregnancies, we checked the antibody titer every 2 weeks and it remained stable, with IgG titer of 8 after serum dithiotriotol treatment. An ultrasound examination was scheduled every two weeks with middle cerebral arterial (MCA) peak systolic velocity (PSV) measurement in order to detect an eventual fetal anemia which was not found. Spontaneous labor occurred at 38 weeks of gestation for the second and the 39 weeks of gestation for the third pregnancy and the patient delivered each time an infant without complications from anti‐ PP1PK antibodies. The last infant presented a weak transient direct antiglobulin test without hyperbilirubinemia. Microscopic examination of the placentas revealed foci of chronic villitis. Storage of maternal blood for autologous transfusion was done in prevention in case of obstetrical hemorrhage and need for blood transfusions.
Many hypotheses have been raised about the true role of anti‐PP1Pk antibodies in fetal‐maternal immunology, the importance of their nature, their titer and their preferential target; different pregnancy managements are proposed for p phenotype mothers. Here we present an exceptional case that did not need supportive treatment of any of her three pregnancies and delivered infants without HDN.
P‐683
The Place of Intrauterine Transfusion in the Management of Severe Fetal Anemia due to Maternal Red Cell Alloimmunization the Algerian Experience
Benmammar MA1, Lamara HS 2, Cherfi N 2,
1CTS Mohamed Benabadji Chu Mustapha Algiers, Algiers, Algeria2Mustapha Bacha teaching hospital of Algiers, Algiers, Algeria
Background
The hemolytic disease of the fetus/newborn has significantly decreased since the introduction of Rhesus (D) immunoprophylaxis in Algeria, the incidence of maternal red cell allo‐immunization is now about 1‐3% (700–2000 immunized women per year).
Antenatal treatment with intrauterine blood transfusions (IUTs) further reduced perinatal mortality and morbidity in severely anemic fetuses.
In Algeria this type of fetal therapy was introduced in the 2000s and the management of affected pregnancies has improved markedly year after year.
Aims
To evaluate intrauterine transfusions procedures and the outcome of severely anemic fetuses treated in utero.
Methods
Retrospective study of all intrauterine transfusions procedures performed between January 2008 and march 2013 at Mustapha Bacha teaching hospital center of Algiers.
Results
75 procedures were performed in 30 fetuses (10 intravascular transfusions, 43 intrauterine exchange transfusions, 14 IUET+IVT, 6 failures), the mean of gestational age at the first IUT was 27,5 weeks (21‐34 weeks), the mean of hemoglobin levels before IUT was 4,98 g/dl (0,8‐8,6 g/dl), the daily Hemoglobin drop was 0,4 g/dl, the mean of IUT per patient was 2,41(1‐5),the mean of gestational age at the delivery was 33,9 weeks, the overall survival rate in the study was 56,7% (17/30), fetal death 20%(6/30), neonatal death rate was 23,3% (7/30).
Conclusion
Mustapha Bacha teaching hospital center of Algiers is the first and the only one center in Algeria who performs intrauterine transfusions.
The relative high rate of fetal survival, confirm the success of this antenatal treatment.
P‐684
Knowledge of Saudi Pregnant Women Attending Antenatal Clinics in Jazan Province About Their Rhd Type and its Importance in Prevention of HDFN
Meshi AA1, Shangeety NA 2, Fagihi NO 3, Darraj AM 4, Hakami AH 5, Mangari MM 6, Shangeety HA 7, Al-Quby KA 1,
1King Fahd Central Hospital, Jazan, Saudi Arabia2PHC division, Nursing Adminstration, Jazan, Saudi Arabia3Al‐Ahad PHC, Jazan, Saudi Arabia4Al‐Ardah PHC, Jazan, Saudi Arabia5Gizan PHC, Jazan, Saudi Arabia6Damad PHC, Jazan, Saudi Arabia7Abu Arish PHC, Jazan, Saudi Arabia
Background
RhD‐associated haemolytic disease of foetus and newborn (HDFN) occurs when maternal anti‐D alloantibodies are actively transported by the placenta to the foetus and cause destruction of RhD‐positive foetal red cells. HDFN can be largely prevented by passive administration of anti‐D immunoglobulin to non‐sensitised women during pregnancy and immediately after delivery or a sensitisation event. About 1‐1.5% of women at risk, however, become immunised to the D antigen as a result of failure to administer protective doses of anti‐D IgG, either at all, or within 72 h of a sensitisation event. In Saudi Arabia, all pregnant women are routinely typed for ABO and RhD at their first visit to antenatal clinics in primary healthcare centres (PHCs). This is mainly to identify RhD‐negative women who are at risk of RhD‐alloimmunisation for counselling on the important of their RhD negativity in order to prevent HDFN.
Aim
To assess the level of knowledge of Saudi pregnant women in Jazan province about their RhD phenotype and to evaluate the effectiveness of current counselling programme provided to RhD‐negative pregnant women for HDFN prevention.
Methods
A cross sectional, questionnaire based study was conducted between November 2014 and January 2015 in antenatal clinics in the largest six primary healthcare centres in Jazan province, Saudi Arabia. A total of 576 pregnant Saudi women have participated in the study. Knowledge about RhD type of the pregnant women, their husbands, and their children, and adequacy of counselling services provided to them were analysed.
Result
A total of 576 pregnant women attending the six antenatal clinics have participated in this study. Their mean age was 32 years (range 18‐43 year) and the frequency of pregnancies ranged from 1‐10 (mean 3). All the 576 participating women revealed that they were not given any information about why they were tested for blood group or counselled on their results. The study revealed that 193 (33.5%) pregnant women did not know their RhD type. Among the remaining 383 who know their RhD type, 354 (92.4%) women were RhD‐positive while 29 (7.6%) women were RhD‐negative. Out of the 29 RhD‐negative women, only 5 (17.2) said they knew the RhD type of their children and husbands, and 12 (41.4%) answered that they are aware of the importance of their negative phenotype and its role in HDFN. none
Conclusion
Despite routine testing for RhD type at the first antenatal visit, this study shows that significant portion of pregnant women do not know their RhD type. It also reveals that there is lack of proper and effective counselling services in antenatal clinics in primary healthcare centres for RhD‐negative pregnant women. Therefore, a national counselling programme for RhD‐negative women on their RhD type in order to prevent RhD‐associated haemolytic disease of foetus and newborn would be urgently needed
P‐685
Determination of Fetomaternal Hemorrhage by Flow Cytometry and Red Blood Cell Alloimmunization in Pregnancy
Prochazkova RP , Zajic T , Bydzovska I , Kamenicka H ,
Regional Hospital Liberec, Liberec, Czech Republic
Background
Fetomaternal haemorrhage (FMH) is a state characterized by penetration of fetal red blood cells (RBCs) into maternal circulation before or during delivery. Significant anemia caused by FMH can result in death of the baby before or after birth, or significant illness in the newborn period. Excessive FMH leads to alloimmunization of mother resulting in an increased risk of hemolytic disease of the newborn.
Aims
Quantitative determination of FMH by flow cytometry and identification of unexpected RBC antibodies in pregnancy.
Methods
We present data from the prospective study from 12/2013 to 07/2014 (women after childbirth, abortion, amniocentesis and fetal death). FMH, Red cell antibody screening and ABO and D blood group was examined in a sample of 94 women. The fetal Cell Count™ kit is intended for the quantitative detection of human fetal RBCs in maternal blood by flow cytometric method (Beckman Coulter Flow Cytometer Cytomics FC 500), which offers a dual fluorescent detection of two intracellular antigens, Hemoglobin F (HbF) and Carbonic Anhydrase (CA). Both HbF and CA were detected in RBCs obtained from EDTA anti‐coagulated mother peripheral whole blood collected 2 h after surgery. The RBC antibody screening was repeated six weeks after the surgery or later to find new alloantibodies.
Results
Total number of samples was 94: 66 women after childbirth, 25 after abortion, 2 after amniocentesis and one fetal death. In 9 cases was founded clinically significant FMH (5x postpartum, 3x after abortion, 1x fetal death).
We present 2 cases of clinically unexpected massive FMH. The first case was a spontaneous vaginal delivery at 40th week of pregnancy with a 4.8% FMH. There was no alloimmunization of the mother during pregnancy or after child's birth. FMH decreased to 1.8% after 1 week and to 0.3% after 3 weeks.Excessive FMH did not affect the health of the baby. The newborn had a mild jaundice at the day of hospital discharge.
As second case was acute caesarean section at 40th week of pregnancy with a 6.9% FMH. The newborn was significantly anemic. Both mother and child had the same blood type. There was no alloimmunization of the mother. FMH was the reason of child's anemia, but the cause of FMH was hidden.
8 from 94 samples contained RBC antibodies within the first examination immediately after surgery (anti‐E, anti‐M, anti‐Cw, anti‐Lea and nonspecific antibodies). The RBC antibody screening of 62 women was repeated six weeks after the surgery or later. 11 of them contained new antibodies, once anti‐Lea and anti‐Leb, two time nonspecific antibodies and 8 times anti‐D (after IvIg prophylaxis).
Summary/Conclusions
FMH with possible clinical significance was founded in 9 cases ‐ excessive FMH only twice. We excluded immunohematologic cause in both cases of excessive FMH. We proved the presence of clinically significant and less significant antibodies caused by FMH after the actual/previous pregnancy or after RBC transfusion. IvIg prophylaxis reduced the overall risk of Rh immunization.
6.1 Neonatal and Pediatric Transfusion
P‐686
Assessing For Immunodeficiency – and the Need for Irradiated Blood Products – in Children With Suspected Digeorge Syndrome Undergoing Cardiac Surgery – a UK Survey
Mele S 1, Kelleher A 1, Turner PJ 2, New HV3,
1Royal Brompton Hospital, London, United Kingdom2Imperial College London/Imperial College Healthcare NHS Trust, London, United Kingdom3Imperial College NHS Trust/NHS Blood and Transplant, London, United Kingdom
Background
Transfusion‐associated graft versus host disease (TA‐GvHD) can occur if non‐irradiated blood is transfused to immunodeficient recipients, although few UK cases have been reported since the introduction of universal leucodepletion.
Patients with DiGeorge syndrome (DGS) typically have cardiac outflow tract (conotruncal) anomalies and a variable T‐cell immunodeficiency. Detection of a normal naïve T‐cell population by flow cytometry is an accurate means of excluding DGS‐associated immunodeficiency (Markert et al. JACI 2004;113:734‐41). Current UK guidelines (BCSH, 2011) recommend irradiated blood components for recipients undergoing cardiac surgery with clinical or laboratory findings suggestive of T‐cell immunodeficiency, to reduce the risk of TA‐GvHD. However, the use of irradiated blood products is controversial in this situation and not without risk (e.g. hyperkalaemia, delays in component provision). Exclusion of immunodeficiency in suspected DGS avoids requirement for irradiated blood but may not be done preoperatively.
Aims
To investigate the diagnosis of immunodeficiency and use of irradiated blood components by UK paediatric cardiac centres in patients with confirmed or suspected DGS.
Methods
A survey was sent to clinical and laboratory staff at the 11 UK paediatric cardiac surgical centres with follow‐up discussion where appropriate.
Results
Responses were received from 9/11 centres. 5 had an institutional protocol for the management of DGS. All 9 centres offered neonatal testing for patients with conotruncal heart defects (using FISH for 22q.11 deletion); 2 also performed broader genetic analysis by microarray. 2/11 undertook immune testing (T‐cell function and/or CD4/8 subset enumeration) but only in the event of a positive FISH/microarray. In the absence of a negative genetics test, 8/9 centres recommended irradiated blood products for children with conotruncal defects. 1 centre used irradiated products only if other phenotypic features of DGS were present (e.g. dysmorphism). If a morphologically‐normal thymus was identified intra‐operatively, 5/9 centres deemed this sufficient to allow subsequent non‐irradiated products (2/5 would also accept preoperative radiological evidence alone), while the remaining 4/9 centres would await the results of genetic testing.
Discussion and Conclusion
Participating paediatric cardiac centres all use neonatal FISH to diagnose the presence of DGS. However FISH may miss up to 10% of cases, results are often not available in time for cardiac surgery and it provides no information with regards to immunocompetence. Methods to assess immunodeficiency were variable: 5/9 centres relied on the assumption that presence of a thymus intra‐operatively excludes significant immunodeficiency. This is not infallible and is too late to prevent the use of irradiated products for that operation. Controversially 2/9 centres accepted radiological thymic detection, which is outside the international consensus.
No centre participating in the survey performed enumeration of naïve T‐cell populations to exclude significant immunodeficiency, a test which can be done rapidly in most NHS centres prior to surgery. Use of this test preoperatively in suspected DGS could assist in earlier decision‐making regarding the need for irradiated blood products, potentially reducing unnecessary use of perioperative irradiated components, and should be considered for future guidelines.
P‐687
Indications and use of Cryoprecipitate in the Pediatric Intensive Care Unit
Cushing MM1, DeSimone RA 1, Vasovic LV 1, Haas T 2,
1Weill Cornell Medical College, New York, United States of America2Zurich University Children's Hospital, Zurich, Switzerland
Background
Cryoprecipitate (cryo) is a source of high‐molecular weight plasma proteins including factor VIII, von Willebrand factor, factor XIII, fibrinogen and fibronectin. It is produced from the cold insoluble fraction of fresh frozen plasma obtained by thawing a unit of fresh frozen plasma (FFP) at 4°C. In current guidelines, cryo transfusion is suggested if fibrinogen levels are <100 mg/dL with clinically relevant bleeding. The recommended dose is 1 single unit of cryo per 10 kg (0.1 U/kg) of bodyweight, which corresponds to approximately 1.5 ml/kg for non‐pooled units and 2.2 ml/kg for pre‐pooled units. However, the indications for cryo are not well defined for any patient population, and no randomized controlled trials exist to evaluate the indications. In addition, there is limited information on patterns of cryo use in children.
Aims
The aim of this study was to describe the patterns and indications for cryo use in a large academic tertiary care center's Pediatric Intensive Care Unit (PICU).
Methods
Data was retrospectively collected from all patients transfused cryo in the PICU from September 2010 through February 2015, including clinical scenario, indications, dose, and fibrinogen levels pre‐ and post‐transfusion (tx), and other products transfused the same day as cryo.
Results
44 patients were transfused 49 doses of cryo in the PICU over 4.5 years. The mean age of recipients was 4.9 years (range: 7 days‐19 years) and 45% were female. The mean pre‐tx fibrinogen was 156 mg/dL (range: undetectable‐574 mg/dL). 25% (11/44) had no pre‐tx measurement of fibrinogen plasma level, while in 44% (14/44) no post‐tx fibrinogen measurement was performed. Only the Clauss assay was used for measurement of fibrinogen; no point‐of‐care testing (e.g. TEG/ROTEM) was performed. The mean dose of cryo was 6 ml/kg (range: 0.5‐38 ml/kg) and 0.36U/kg (range: 0.04‐1.4 U/kg). 41% were transfused prophylactically and 59% for bleeding. The most common indication was recent cardiac surgery 39% (17/44); then sepsis/DIC 30% (13/44); 14% (6/44) were transfused after non‐cardiac surgery; 9% (4/44) for hematological malignancy‐related DIC. 27% (12/44) died within a year of transfusion. Based on current guidelines, the indication chosen during physician order entry (POE) was correct in only 8/44 patients. On the day of cryo tx, red blood cells were transfused in 45% (20/44) of children, plasma tx was observed in 61% (27/44), and platelet tx occurred in 59% (26/44). Comparing fibrinogen levels pre‐ and post‐cryo tx, the overall mean increase was 64 mg/dL (P = 0.011). If children with additional plasma and/or platelet tx were excluded, the mean increase in fibrinogen was 132 mg/dL (P = 0.075).
Summary/Conclusions
The average cryo dose administered was 6 ml/kg or 0.36U/kg, which is above the dose suggested in current guidelines 1.5‐2.2 ml/kg or 0.1U/kg. The most common indication was recent cardiac surgery and then sepsis/DIC, but cryo was often transfused empirically without a known fibrinogen level. The correct indication for the transfusion was infrequently selected during POE. The implementation of age‐dependent point‐of‐care based algorithms using standard dosing for a targeted and timely approach to guide transfusion of cryo in the PICU would be helpful to resolve the lack of standardization with this blood component.
P‐689
Determination of the Mean Red Cell Transfusion Requirement Compared on the Basis of Iron Overload and Type of Chelation Therapy and Development of Alloantibodies in Multiply Transfused Thalassemia Major Patients
Jain PJ1, Jain RJ 1, Choudhury NC 2, Mahadik VK 1,
1C.R.G. Hospital and R. D. Gardi Medical College, Ujjain, India2Fortis Hospital, Gurgaon, India
Background
Transfusion‐dependent thalassemia patients, in the absence of chelation therapy, develop progressive accumulation of iron, which is responsible for tissue damage and, eventually, death. The factors, which influence the iron burden are type of chelation therapy and mean red cell transfusion requirement. Increasing red cell transfusion requirement, iron deposit and development of alloantibodies complicates transfusion therapy in thalassemia patients.
Aims
(1) To investigate the patients for the red cell transfusion requirement,compared on the basis of iron overload and type of chelation therapy. (2) To determine the rate of development of red cell alloantibodies in thalassemia major patients.
Method
A prospective study was conducted from February 2013 to December 2014. Ninety eight patients were included in this study and 3 consecutive samples collected after every 6 month and investigated for the red cell transfusion requirement,compared on the basis of iron overload and type of chelation therapy. Iron overload was measured by serum ferritin levels.
Results and Observations
In present study mean red cell transfusion requirement was 206.20 ml/kg/year (SD = 28.62). Majority of the children in this study i.e. 40 (41.67%) were undergoing hypertransfusion therapy and transfused red cells were in the range of 208‐248 ml/kg annually. It was observed that the requirement of red cells transfusion increases with age of the patients. Out of 96 patients, 86 (89.58%) thalassemic children were on chelation therapy. Maximum numbers of patients 37 (38.54%) were on oral chelation therapy after this 35 (36.45%) patients on combined chelation therapy (Desferroxamine & oral chelation). Only 14 (14.58%) were on parenteral (desferroxamine) chelation therapy. Out of 96 patients, 10 (10.41%) patients were not taking any chelation therapy. In present study the difference of mean red cell transfusion requirement among the all chelation therapy groups when compared with each other were found highly significant (P < 0.01).The mean red cell transfusion requirement were minimum in combination therapy group (combination of two iron chelators such as parenteral desferroxamine plus oral deferiprone) followed by parenteral desferroxamine chelation therapy group, oral chelation therapy group and maximum in patients those started chelation therapy but discontinued.
Irregular red cell alloantibodies were found in 8 patients (8.16%). Five patients developed single antibodies, while other three patients developed multiple antibodies(Matrix Gel, Tulip).
Conclusion
Red cell transfusion requirement & chelation therapy should be kept in mind in the patients receiving multiple transfusions. In present study the difference of mean red cell transfusion requirement among the all chelation therapy groups when compared with each other were found highly significant (P < 0.01). The mean red cell transfusion requirement was minimum in combination therapy group (combination of two iron chelators such as parenteral desferroxamine plus oral deferiprone) and maximum in patients who started chelation therapy but discontinued it and this difference was found highly significant (P < 0.01). Combination of two iron chelators (such as parenteral desferroxamine plus oral deferiprone) have been shown to produce additive and synergistic effects, may produce enhanced iron excretion, minimize side effects, decrease mean red cell transfusion requirement and improve compliance is strongly recommended in transfusion dependent thalassemia patients.
P‐690
Audit on Neonatal Donor Exposure
Allameddine A , Heaton M , Jenkins H , Morris H , Andrews S , Flynn S , Porada C , Sedman B ,
The Pennine Acute Trust, Oldham, United Kingdom
Background
Donor exposure in neonates is often inevitable, especially for those preterm requiring multiple blood transfusions. The current practice in issuing neonates with ABO Rh‐matched RBC has resulted in high wastage levels of RBC units. In order to reduce the wastage, the Hospital Transfusion Team (HTT) decided to issue neonates only with O Rh Negative units and to limit the number of packs issued in proportion to gestational age, as by BCSH recommendation. A complete set of six paediatric units is issued for neonates <28 weeks gestational age and only two units for those >28 weeks, allowing sharing a paedipack with up to 3 neonates. This change in practice has resulted in wastage reduction of about 50% of paediatric units. However, this raised the concern on whether this practice would increase the neonatal donor exposure.
Aims
An audit has been undertaken to assess the neonatal donor exposure following this change in practice.
Methods
We considered as inevitable (GREEN) an exposure to multiple donors for a neonate transfused within an interval of >20 days, avoidable (RED) for those within 10 days and AMBER when the interval is between 10 and 20 days.
Results
The audit considered the period between March‐September 2014. 21 neonates were included for 51 transfusions of 104 neonatal RBC units. 34 neonatal RBC units were wasted.
The audit showed that 10% of the patients were exposed to multiple donors with transfusions occurred within <10 days of interval (RED). A 20% were exposed to multiple donors but received transfusions occurring between 10 and 20 days (AMBER) and 70% with an interval > 20 days (GREEN), 50% of these had only one transfusion.
Conclusions
High risk neonates, including preterm infants are at high risk of donor exposure. Various strategies should be considered to minimize the exposure and a close collaboration between the HTT and the paediatric team should include sharing information about the likelihood of transfusion and age of red cells to guide allocation of paedipacks for top‐up transfusion.
P‐691
Profile and Haematological Indices of Patients Receiving Blood Transfusion in an Emergency Setting in Ghana: A Retrospective Review of Clinical Surveillance Data
Danfoku A-M1, Ansong D 2, Sylverken J 1, Akoto OA 3, Kwarteng OS 1, Nyanor I 1
1Komfo Anokye Teaching Hospital, Kumasi, Ghana2Kwame Nkrumah University of Science and Technolgy, Kumasi, Ghana3Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Introduction
Blood transfusion is a life‐saving treatment available in most paediatric wards in African Hospitals where infections such as malaria, malnutrition and sickle cell disease are prevalent This study was a review of all admission to a Paediatric Emergency Unit to assess the frequency of transfusions and its associated morbidity and mortality in typical tertiary hospital in Ghana.
Method
A systematic review of clinical data of patients admitted to the Paediatric Emergency Unit (PEU) of the Komfo Anokye Teaching Hospital in 2013 who received transfusion. Basic summary statistics for demography, and anthropometrics was done. The primary outcome measure was survival.
Results
2962 patients were admitted in 2013 to the PEU out of which 16.17% were haemo‐transfused. More males (56.78%) and children under 5 years (73.70%) were haemo‐transfused during the period of study. Infectious disease (23.26%) were the main presentation condition for transfusion. Majority of the patients haemo‐transfused (92.48%) were stabilised and discharged home or transferred to Speciality Wards but 7.31% died at the Emergency Unit.
Conclusions
The importance of blood transfusion in saving lives cannot be overemphasised considering the proportion of patients transfused irrespective of the underlying morbidities or comorbidities. Blood transfusion still remains a key therapeutic intervention in tertiary institution in sub‐Saharan Africa and efforts to monitor it use and outcome of use is essential in health institutions.
P‐692
Impact of Diet Modification on Serum Ferritin Level in Thalassemia Children Who are on Regular Blood Transfusion
Jabri GJ
Taibah university, Madina, Saudi Arabia
Background and Aim
Blood transfusion is the main treatment for people who have moderate or severe thalassemias. The main side effect of transfusion therapy is that the patient develops iron overload, which lead to serious damage to the internal organs. Many foods can play an important role in decreasing iron absorption from the intestine and that could improve the prognosis of these patients. The aim of our study is to detect the effect of diet adjustment in thalassemia children, to a more iron‐execreting and less iron‐absorption diet, on their level of serum ferritin.
Patients and Methods
In a randomized case control study, 36 thalassemia patients presented to the hematology/oncology center at the Maternity and children Hospital (MCH) in Almadinah Almounourah, KSA in the period from January 2014 to July 2014 were observed prospectively for their serum ferritin level. The cases were asked to follow a diet that limits iron absorption and increases iron excretion.
Results
Serum ferritin level is significantly decreased in cases after 6 months of diet iron restriction more than in controls (P value =0.03).
Conclusions
Restriction of foods that contain iron and increase intake of foods that execrate iron from body can significantly decrease serum ferritin level in thalassemia children. Recommendations. According to our results, control of diet and following food regimens that can minimize iron absorption and maximize iron execration are of great benefit for thalassemia children and can act as an adjuvant for drug chelation therapy.
6.2 Therapeutic Apheresis
P‐693
Efficacy and Safety of Therapeutic Plasma Exchange by Using Apheretic Devices in Paediatric Atypical Haemolytic Uremic Syndrome Patients (aHUS)
Hans RH , Sharma RRS , Marwaha NM , Suri DS , Kumar RK , Gupta AG , Singh SS
Post Graduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India
Background
Therapeutic Plasma Exchange (TPE) in atypical Haemolytic Uremic Syndrome (aHUS) is considered as first line treatment as per current ASFA guidelines. But there is very limited data available in literature regarding efficacy and safety of TPE procedures in paediatric aHUS patients.
Aim
To access the safety and efficacy of TPE by using apheretic devices in paediatric aHUS patients.
Materials and Methods
We did retrospective analysis of all TPE procedures performed in aHUS paediatric patients over a period of 13 years (2001‐2013). TPE procedures were done on two different apheretic devices (CS 3000 plus, Fenwal USA and Cobe spectra, Terumo BCT Lakewood, Colorado) daily or on alternate days depending on clinicial condition of the patient. Adverse events if any, were noted and analyzed. Pre and post procedural laboratory profiles were analyzed to assess the response to TPE therapy. Paired T test was applied for assessing statistical significance. One way Annova test was applied to assess the significance of age and time gap between onset of illness and initiation of TPE therapy on patient's outcome. Kruskall Wallis test and a Receiver‐operator characteristic (ROC) curve was plotted for calculating minimum number of TPE procedures required for a statistically significant response in these patients. Pearson's correlation test was applied to analyse correlation of time gap between onset of illness and initiation of TPE therapy with laboratory and biochemical parameters.
Results
A total of 169 TPE procedures (range 1‐22/patient with an average of 7.6 procedures/patient) were done for 30 paediatric patients with an average age of 6.6 yrs (1.2‐13 years). The Male:Female ratio was 3:1 with an average weight of 10 ± 2 Kgs. More than 3 TPE procedures were done in 24 patients. We observed an overall response rate of 87.5% with survival rate of 80% after 18 months of follow up. Sixteen patients were complete responder, 5 were partial responders and 3 were non responders. The time gap between onset of illness and start of TPE therapy was 1 to 4 days in complete responders, 5 to 7 days in partial responders and 8 to 9 days in non responders and was negatively correlated with patient outcome. Age of the patient did not reveal any significance with the outcome (P = 0.051). Adverse events were observed in 13 (7.69%) procedures.
Conclusion
TPE by using apheretic devices is safe and effective therapeutic modality in paediatric aHUS patients if instituted early in the course of disease with a minimum of 4 to 5 procedures.
P‐694
Audit of Plasma Exchange For Hyperviscosity at Nhsbt Tas Oxford: The Changing Role of Therapeutic Apheresis
Marrinan EA 1, Desborough M 2, Dlamini T 3, Methula B 3, Todd C 3, Clarke S 3, Benjamin S 3
1University of Oxford, London, United Kingdom2Oxford University Hospitals NHS Trust, Oxford, United Kingdom3NHSBT Therapeutic Apheresis Services (TAS) Oxford, Oxford, United Kingdom
Background
Therapeutic plasma exchange (TPE) has a role in the management of monoclonal gammopathy hyperviscosity. This audit was undertaken to gain a greater understanding of the current use of TPE within NHSBT TAS Oxford, a regional referral service for TPE.
Aims
To describe current patterns and outcomes of TPE for patients with monoclonal gammopathies, assessing:
a) Reasons for referral.b) Degree of hyperviscosity.c) Whether TPE is provided according to current guidelines.
Methods
Audit standards were taken from 2013 ASFA Guidelines.1 All patients with monoclonal gammopathy referred to NHSBT TAS Oxford for TPE between 09/06/13 ‐ 09/06/14 were identified by hand‐searching case notes.
Results
18 of 21 patients referred for TPE had Waldenstrom's macroglobulinaemia; two patients had two separate episodes of TPE (total 23 episodes). 12 episodes were for symptomatic hyperviscosity, 10 for prophylaxis prior to Rituximab treatment and one was an elective exchange prior to surgery.
The ASFA Guidelines advise prophylactic TPE prior to Rituximab treatment if the plasma IgM level is >50 g/l. Only 30% of patients referred for prophylactic TPE had an IgM >50 g/l, (mean IgM 43.2 g/l). The mean number of exchanges for each patient was 4 and mean interval between exchanges 14 days. When 9 prophylactic TPE patients were followed up, none developed a Rituximab ‘flare’ with treatment (results could not be found for one patient). A flare was defined as at least 25% increase in IgM between pre‐TPE samples before Rituximab and pre‐TPE samples after Rituximab. The time between first TPE and first Rituximab treatment varied from 1 to 92 days.
Conclusions
Referrals for TPE for asymptomatic patients were often made below the recommended threshold. This may represent a cautious approach from clinicians and reflect the lack of clear evidence about the nature and consequences of Rituximab ‘flares’, which have been well characterised.2 The factors predicting a flare have not been identified. This audit demonstrates that, with a cautious approach, 0% of patients who received prophylactic TPE went on to develop a Rituximab ‘flare’. The large variation in the time between first TPE and first Rituximab suggests that there is no clear consensus on how this prophylactic measure should be employed.
Whatever the contributing factors, the focus of referrals for TPE has shifted away from solely symptomatic treatment of hyperviscosity towards a proactive referral approach for the prevention of symptomatic hyperviscosity or treatment ‘flare’.
References
1. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice ‐ Evidence‐Based Approach from the Writing Committee of the American Society for Apheresis: The Sixth Special Issue. Journal of Clinical Apheresis 2013; 28: 145‐284.
2. Initial Immunoglobulin M ‘Flare’ after Rituximab Therapy in Patients Diagnosed with Waldenstrom Macroglobulinaemia. I Ghobrial, R Fonesca, et. al. Cancer. 2004; 101(11): 2593‐2598.
P‐695
Use of Recent Haematocrit And Haemoglobin S Levels Improves Apheresis Red Cell Exchange
Hood C , Potok D
NHS Blood & Transplant, Leeds, United Kingdom
Background
NHSBT's Therapeutic Apheresis Service treats patients with sickle cell disease using 6 to 8 weekly red cell exchange and transfusing 6 to 8 units of red cells per exchange.
In Leeds close collaboration with the Haemoglobinopathy Specialist Nurse and Consultant Haematologist at St James's Hospital Leeds has lead to closer monitoring of haematocrit (Hct) and Haemoglobin S (HbS) levels pre and post exchange. Regular joint reviews highlighted incremental rises in patient Hct post exchange, and as many patients are exchanged due to increased risk of stroke, maintaining an optimal haematocrit is a key element in minimising this risk.
Aim
The aim is, through a collaborative approach, to optimise the management of patients in the red cell exchange programme. The goal is to improve patient quality of life by reducing the number of hospital admissions and the risk of stroke and by monitoring their Hct and HbS closely, exposure to red cells could also be minimised.
Method
Case review found that regular exchanges were maintaining HbS well within the desired range but were sometimes resulting in increased Hct. Once this trend was identified, the parameters used to program the apheresis cell separator were changed from the 100% HbS level default calculated by the machine to the Pre exchange and target HbS levels which then determined the replacement red cell volume. Depletion exchanges which replace less volume were also used to maintain optimum haematocrit levels in appropriate cases. Post HbS levels were monitored and over time Hct was reduced to the desired level as result of the lower red cell replacement volumes now required.
The net results of these changes has been the ability to reduce the frequency of red cell exchange and the number of red cell units used per exchange in some patients.
Conclusion
Reducing red cell exchange frequency and/or the number of red cell units used per exchange can result in: Less exposure to allogeneic blood with potential for reduction in allo immunisation and cross matching issues Less frequent attendance for red cell exchange and associated central line insertion or peripheral vein access Reduction in red cell usage and potential usage of rarer phenotype units by using fewer units per exchange.
P‐696
Abstract Withdrawn.
P‐697
NHSBT National Therapeutic Apheresis Services Standardised Practice Results in Excellent Patient Outcomes in TTP
Wong H1, Benjamin S 2
1Oxford University Hospitals NHS Trust, Oxford, United Kingdom2NHBST TAS OX3 9BQ, Oxford, United Kingdom
Background
Thrombotic thrombocytopenic Purpura (TTP) is a rare life‐threatening medical emergency. Without prompt initiation of therapeutic plasma exchange (TPE), mortality approaches 90%. NHSBT provides a national TPE service for TTP in England. Treatment plans were updated in response to a new BCSH guideline in 2012[1].
Aims
Review of initial management of TTP by NHSBT TAS against BCSH Guideline, comparison of mortality to published registry outcomes and review of operational practice.
Methods
Data were collected retrospectively on all cases referred for TPE with presumed TTP 01/11/12‐31/10/13 to TAS units in Bristol, Leeds, Liverpool, Oxford and Sheffield.
Results
39 patients proceeded to TPE. 19 (48.7%) were subsequently confirmed to have autoimmune TTP, of those 6 (31.6%) were relapses. Secondary causes of MAHA included drugs, cancer, HIV and BMT. 56% presented with neurological symptoms and 41.6% went to ITU for first TPE. Review of treatments in all referrals demonstrated 100% compliance with use of S/D FFP for exchange. First TPE was initiated ‘out of hours’ in 33.3% cases. In only 1 patient was TPE initiated >24 h after referral (due to delayed transfer). 64.1% cases received 1.5PV as first TPE. Reasons for <1.5PV TPE included loss of venous access, patient deterioration, timing of first TPE and planned 1.0PV for suspected non autoimmune TTP. 16 (94%) patients with autoimmune TTP had CR (I unknown), supporting that prompt TPE reduces mortality. There were early deaths in 2 TTP patients who deteriorated on TPE, which commenced 12 and 16 h after referral. TTP mortality of 10.5% compares with 20% in Oklahoma Registry[2] and 8.5% in UK Regional TTP Registry[3].
Conclusions
This review shows timely and effective TPE is achievable. Until autoimmune TTP is excluded by ADAMTS13 assays, we recommend all first TPE should aim for 1.5PV as per BCSH guideline. Early deaths emphasise the need for prompt diagnosis and transfer by referring hospitals, so first TPE can be initiated within 8 h. This first national review of TPE at NHSBT for TTP shows excellent mortality figures for autoimmune TTP (where early appropriate treatment can result in compete recovery), comparable to published registry data. It is expected that the introduction of a 24/7 emergency on‐call TAS service from January 2015 will improve accessibility and delivery of prompt first TPE. This study highlights the benefits of a national NHSBT TAS service, where treatment regimes and education can be standardised to guidelines, resulting in excellent patient outcomes.
With thanks to TAS units for submitting data.
1. BCSH ‐ Guideline on the Diagnosis and Management of Thrombotic Thrombocytopenic Purpura and the other Thrombotic Microangiopathies. British Journal of Haematology 2012;158: 323‐335
2. Kremer H, Vesely S, Terrell D et al. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood 2010 Feb 25;115(8):1500‐11
3. Scully M, Yarranton H, Liesner R et al. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. British Journal of Haematology 2008;142(5):819‐26
P‐698
Off‐Line Extracorporeal Photochemotherapy (ECP) Utilizing Blood Bank Facilities and ISBT128
Sorensen BS1, Olesen GO 2, Iversen LI 3, Moller BKM 1
1Aarhus University Hospital, Aarhus N, Denmark2Aarhus University Hospital, Dept of Hematology, Aarhus C, Denmark3Aarhus University Hospital, Dept. of Dermatology, Aarhus, Denmark
Background
ECP is used for treatment of T‐cell mediated diseases. ECP comprises of 3 steps: (1) Collection of mononuclear cells by apheresis, (2) Addition of 8‐methoxysporalen (8‐MOP) followed by ultraviolet A (UVA) irradiation, (3) reinfusion of the treated cells. 8‐MOP is biologically inert unless exposed to UVA. When exposed, it covalently binds and crosslinks DNA leading to apoptosis. Apoptosis of mononuclear cells is considered the basis of the immunological effects. In‐line or off‐line techniques are used. Off‐line technique is considered more difficult to perform because the product is removed from the patient and demands for security, traceability and documentation are required. An advantage is the possibility to collect a product sample for quality control and research.
Aim
Change of routines from in‐line to off‐line technique provided basis for standardization and feasibility with the procedure using ISBT128 and blood bank facilities.
Methods
Collection is performed with Spectra Optia MNC software (TerumoBCT) on 2 consecutive days. Two chamber collections (21 + 4 ml) are performed providing a fixed product volume of 50 ml. Hct is measured on a sample taken from the sample tube of the Spectra Optia MNC kit. The product is diluted with 250 ml NaCl, end volume is 300 ml and Hct max 2%. The product bag is sterile connected to the irradiation bag and 3 ml 8‐MOP (Macopharma) is added to the product. Irradiation is performed in Macogenic G2 (Macopharma). Documentation and traceability are maintained by using a blood bank IT system and ISBT128. Identification control at reinfusion is identical to control procedures before blood transfusion.
Results
Seventeen patients received 261 procedures (May 2014‐Feb 2015), median age 64 years, 56% men.
Diagnoses
59% chronic GvHD, 24% cutaneous T‐cell lymphoma, 11% bronchiolitis obliterans, 11% lichen planus. Data are given as median (25% quartiles).
Patients
Total blood volume (TBV) 4724 ml (3440‐5465), WBC 9.4 × 109/L (4.8‐16.4), Hct 39% (32‐45), platelet 229 × 109/L (173‐358).
Procedure
Inlet volume 0.9 × TBV (0.5‐1.4 × TBV), inlet flow 62.5 ml/min (46.7‐76.8), time 85 min (50‐132).
Product
WBC 3.6 × 109 (1.8‐5.6), platelet 81 × 109 (52‐143), lymphocytes 44% (26‐58), monocytes 47% (36‐68), neutrophils 0% (0‐16), Hct (undiluted) 3% (2‐5).
No severe adverse events were observed. All patients experienced clinical improvement/steady state.
Discussion
We report a feasible ECP off‐line technique using blood bank facilities and ISBT128 without using clean room facilities. A product sample is used for quality control. We collect an adequate number of WBC, almost exclusively mononuclear cells. The number of WBC needed to achieve a clinical response is unknown, but the majority of cells should be mononuclear cells. Our procedure is standardized according to a fixed product volume, but time to perform the procedure is variable. Further, standardization of the duration of the procedure and quality assessment of the products is ongoing. We speculate that the collection of mononuclear cells is more efficient and uniform than for in‐line procedures. A study is planned to show if the procedure time and number of treatments may be reduced.
P‐699
Hematopoietic Stem Cell Collection on Spectra Optia: Is Collected CD34+ Cell Dose Predictable?
Nielsen CN , Topholm Bruun MTB , Stauffer Larsen TSL
Odense University hospital, Odense C, Denmark
Background
The ability to predict the final CD34+ cells dose collected should permit more precise management of hematopoietic stem cell (HSC) collection and in some cases either avoid a second unnecessary collection day or ending the procedure earlier. With Spectra Optia device, despite a continuous collection of the buffy coat, cells are transitorily accumulated in a chamber and intermittently flushed in the collected bag. As the Spectra Optia offers 2 sample bulbs on the collect bag, we studied if CD34+ cells collected at chamber 2 and 4 are linearly correlated to the final CD34 dose collected.
Aims and Methods
In a single center, 40 patients (13 Lymphoma and 27 Myeloma) were enrolled. A total of 62 autologous apheresis procedures were performed on the Spectra Optia separator (version 5, TerumoBCT). For each procedure, 3 samples of the collected product were taken: after 2 and 4 chambers were collected and at the completion of the run. CD34+ cells were counted by FACS. Different parameters related to patient, procedure and product were collected and retrospectively analyzed. Results are preliminary and presented as mean±SD.
Results
The overall performance of the Spectra Optia was satisfactory; CD34 CE2 (57.5 ± 17.7%) was shown really stable whatever patient conditions. CD34+ cells collecting rate vs number of collected chamber was not absolutely linear with a steeper slope later on in the procedure. A general slope could be estimated with a good correlation (y = 33.177, R² = 0.98). The CD34 precount and CD34 collecting rate vs number of chambers collected were higher in myeloma patients than lymphoma patients.
So it could potentially be valuable to have a different prediction analysis depending the pathology. We also observed a good correlation between CD34+ cells collected/L of blood processed and CD34 precount (R²=0.82) also allowing some prediction of blood to be processed to get the dose.
Summary/Conclusion
Those are preliminary results; more analysis are in progress but it looks doable to predict CD34 dose in the bag at the end of the procedure based on CD34 collected after few chambers flushed. As we observed a decline of the CD34% in product despite a higher absolute number of CD34 + collected and because CD34+ cells are accumulated in a chamber before being flushed to the collect bag, it will be interesting to analyse evolution of blood volume processed per chamber. This will also allow us to check whether CD34 collection rate/L of blood processed is linear.
P‐700
Cascade Plasmapheresis as a Preconditioning Regime in ABO Incompatible Living Donor Renal Transplant: A Case Report
Khetarpal A , Arora B
Artemis Hospitals, Gurgaon, India
Background
Major ABO incompatible (ABOi) living donor kidney transplant is being accepted to bridge the gap between the demand and supply of organs. The key factor to successful graft outcome is the prevention of hyperacute rejection, to establish accommodation as early as possible. Therapeutic Plasma Exchange (TPE) along with other desensitization protocols is commonly used to reduce the titer of blood group antibodies. Centers in the United States and Japan differ from those in Europe, where antigen‐specific immunoadsorption, rather than plasma exchange, is the preferred mode of removing antibodies.
Aims
The authors have evaluated the use of cascade plasmapheresis (CP) using a pore sized base semi‐selective filter column 2A20 (Evaflux, Kawasumi Laboratories, Japan) to reduce the preoperative titer of ABO antibodies to an acceptable titer of 16 or below as an alternative to conventional TPE.
Methods
After detailed investigations as per our centre's protocol, the patient, a 63 year old male, was inducted into the ABOi kidney transplant program. The Blood group of the patient and donor was O positive and AB positive respectively, revealing major ABO incompatibility. Recipient's baseline antibody titer was established using column agglutination technology (CAT; Ortho Clinical Diagnostics) as IgG Anti‐A: 256 and IgG anti‐B: 128. HLA cross‐match test was negative. Immunosuppression was commenced with Rituximab alongwith Tab Mycophenolate Mofetil. After an informed consent, a series of nine CP were performed over a period of 15 days exchanging 1‐1.5 volumes of plasma during each procedure. CP consisted of separating patient's plasma using a plastic disposable kit (PL1, Fresenius Kabi, Germany) on the apheresis equipment COM.TEC (Fresenius Kabi, Germany) and passing it through the pore size based semi‐selective filter column 2A20 (Evaflux, Kawasumi Laboratories, Japan). Each procedure was followed by administration of 5 gm IVIG. ABO antibody titer was performed after each procedure using CAT (Ortho Clinical Diagnostics). The sample for the titer was obtained within 12 h of carrying out the procedure.
Results
A gradual reduction in the titer of both IgG Anti‐A and IgG Anti‐B was seen with CP (Table 1). The transplant was undertaken on the 15th day and was uneventful. It was followed by another CP (10th procedure) on the same day of the transplant. Patient was commenced on triple immunosuppression with Tacrolimus, Mycophenolate Mofetil and Prednesolone. Follow up tires, initially done daily and then every other day (Table 2), were within normal limits indicating stable graft function. The patient was successfully discharged from the hospital on the 10th post operative day.
Conclusion
CP, which is more selective than conventional TPE in removing high molecular weight substances mainly immunoglobulins and some amount of albumin, is an effective desensitization modaility for ABOi kidney transplant. CP does not require replacement with fresh frozen plasma as opposed to conventional TPE obviating the risk of adverse effects to transfusion.


P‐701
Therapeutic Apheresis And Its Indications: Referred to Tehran Blood Transfusion Centre
Chegini A 1, Maghari A2, Ebrahimi A 3, Seraji B 1, Khorshidfar M 1
1High institue for research and education in transfusion medicine,Tehran,Iran, Tehran, Iran2Depart. of Biostatistics,Faculty of medical sciences,Tarbiat Modares University, Tehran, Iran3Department of hematology, faculty of medical sciences,Tarbiat Modares University, Tehran, Iran
Background
Apheresis is one of the treatment methods of various diseases of antibody and autoimmune mediated. Automated apheresis machines can remove pathogen elements from intravascular compartment.
Aims
The goal of this study is to survey disease prevalence which requires therapeutic apheresis studied during a one year period in Tehran Blood Transfusion Centre.
Methods
A retrospective study was carried during 2012‐2013 in Tehran Blood Transfusion Centre. All referred patients to TBTC were included in this study. Their illnesses were divided into 5 groups.
A‐neurologic B‐hematologic & oncologic C‐Nephrologic D‐Rheumathologic. Rare diseases were categorized in another group.
Results
During this one year period 397 patients were referred to apheresis department in TBTC. Of these, 178 patients were male (44.8%) and 219 patients were female (55.2%) and their ages varied from 2 years old up to 68 years old with a mean age of 42.2 ± 18.08. There were 16 children amongst the patients and 2282 procedures were carried out.
The most prevalent groups were as follow:
There were 250 neurologic patients (63%), 86 hematologic &oncologic patients (21.7%), 33 nephrologic patients (8.3%), 18 reumathologic patients (4.5%) and 10 others patients.
The most common disease in neurologic group was Guillan Barre syndrome (34%), myasthenia gravis place was in second place and the third in this group was Multiple Sclerosis (14%).
Heamathologic diseases included Thrombotic Thrombocytopenic Purpura (66.3%), HUS (9.3%), Myeloma Multiple (9.3%). Among the nephrologic diseases the most prevalent was kidney transplantation (45.4%) and in Reumathologic group the most prevalent was Systemic Lupus Erythmatos (61.1%). Among the patients 351 (88.4%) were alert and 46 (11.6%) were comatose. The bedridden patients in ICU were as follow: group A: 84 patients (33.6%), group B: 54 patients (63.8%), group c: 9 patients (27.3%), group D: 10 patients (55.6%).
Chance of recovery in conscious patients was 18 times greater than comatose. (P = 0.001)OR=18.58
64 (16.1%) patients supported with ventilator in ICU and 333 (83.9%) didn't need artificial ventilation. Mean age of treated patients was 41.2.
The younger patients survived where as the older ones did not.
(P = 0.001)
Summary
The most common diseases among the patients were: 1‐neurologic (Guillan Barre syndrome) 2 hematologic &oncologic (TTP)‐3‐nephrologic (kidney transplantation) 4‐Reumathologic (Systemic Lupus Erythmatos).
P‐702
Mononuclear Cells for Active Anticancer Immunotherapy – Cobe Spectra vs Optia Spectra
Gasova Z
Institute of Hematology and Blood Transfusion, Prague, Czech Republic
Background
Mononuclear cells (MNC) are increasingly used in the treatment of various types of diseases. Autologous MNC may be used for an active anticancer immunotherapy in the therapy of patients with prostate cancer or ovarian cancer. The collection regimen and the yield of cells may affect the effect of therapy.
Aims
We tried to find the optimum collection technique in patients with prostate cancer in order to collect enough target cells for their processing to anticancer vaccines. We evaluated the numbers of mononuclear cells, immature dendritic cells (iDC) in the product, and its contamination with platelets.
Methods
Parameters of 100 mononuclear cells concentrates collected from 100 patients with prostate cancer were evaluated. Collections were performed by means of leukocytapheresis technique, and the products were processed subsequently in laboratories Sotio in order to prepare anticancer vaccines – based on the dendritic cells function. MNC were collected by the use of Cobe Spectra and Optia Spectra, Terumo. Precollection parameters of blood counts in patients, volume of processed blood, number of leukocytes, MNC and platelets in the products were estimated (Sysmex XS 1000i, flow cytometry analyser Facs Calibur Becton‐Dickinson). The results were evaluated as medians and their ranges.
Results
Precollection laboratory results, and clinical condition of the patients enabled to perform leukocytapheresis in the planned extent. The volume of processed blood corresponded with processing of 1,4 to 1,6 total blood volumes (TBV) of the patients. The yield of iDC higher than 200 × 106 related to each 4 × 109 MNC was required by laboratories for the good quality vaccine.(a) Cobe Spectra, continuous regimen, 83 collections: we collected the number of 9 (4‐21)×109 MNC with 28 (10‐34) % of monocytes. The products contained 3,7 (1,3‐8,1) ×1011of platelets. Numbers of 319 (90‐1100)×106 iDC related to each 4 × 109 MNC were then prepared.(b) Optia Spectra: collections in cycles, 17 procedures: we collected 5,4 (2,9‐12)×109 of MNC. They contained 31 (21‐ 46) % of monocytes. Contamination of the product with platelets was lower than in Cobe Spectra and corresponded with 0,7 (0,4‐2,8) ×1011 of platelets. Numbers of 417 (209‐809)×106 iDC related to each 4 × 109 MNC were then prepared.
Conclusion
The collections by the use of Optia were efficient and enabled to prepare the sufficient dose of cells for therapy of the patients. Products prepared by Optia contained higher numbers of iDC and the lower numbers of platelets than Cobe. High number of platelets may negatively affect the efficiency of processing of MNC, quality of the anticancer product and then the effect of therapy. Any serious adverse reactions have not been observed, but the decrease of ionized serum calcium by 15 (13‐23) % was detected in the course of collections.
P‐703
Therapeutic Thrombocytapheresis for Symptomatic Thrombocytosis in Hemato‐Oncology Patients‐A Case Series
Hans RH , Prakash SP , Sharma RRS , Marwaha NM
Post Graduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India
Background
Thrombocytosis is defined as abnormal production and increase in platelet count exceeding 450‐500x109/ L. It may be essential due to myeloproliferative disorder or reactive secondary to cancer, asplenia or iron deficiency. Immediate control of rise in platelet count can be achieved with platelet removal by therapeutic thrombocytapheresis which is categorised as category II indication (ASFA 2013) for symptomatic patients.
Aims and Objectives
To access the efficacy of therapeutic thrombocytapheresis as life‐saving emergency procedure in symptomatic patients.
Material and Methods
A retrospective analysis of all therapeutic thrombocytapheresis procedures in symtomatic thrombocytosis patients was done over a period of 10 years (2004‐2014). Procedures were done on different apheretic devices (CS‐3000 Plus, Baxter, USA and Cobe spectra, Terumo BCT, Lakewood Co. USA). Patients’ pre and post procedural hematological profiles were analyzed by applying paired T test and pearson's correlation to assess the efficacy of thrombocytapheresis in them.
Results
We performed therapeutic cytapheresis on six patients (four‐ CML, one‐ essential thrombocythemia and one with ‐Thalassemia intermedia, post splenectomy). These patients presented in emergency medicine department of our tertiary care centre with acute symptoms of thrombocytosis such as severe abdominal pain, priapism, acrocynosis, parasthesias, burning pain in palm and sole of feet. The median age of our patients was 46 years. Male to female ratio was 2:1. The range of procedure per patient was 1‐11. The mean post procedure decline in platelet count was 40%(declining from a mean pre count of 2141 ± 960x109/ L to 1293 ± 647x109/ L post procedure) and was statistically significant (P < 0.001). There was no significant change in post total leukocyte count however mean post hemoglobin decreased significantly after procedure (P = 0.03). All patients improved symptomatically and discharged on antiplatelet therapy. Out of six, four patients are on regular follow up in hematology clinic.
Conclusion
Therapeutic thrombocytapheresis provides an immediate symptomatic relief and is an efficient useful emergency lifesaving procedure in cases of thrombocytosis.
P‐704
Effectiveness and Safety of Therapeutic Plasma Exchange in Paediatric Patients
Gajjar MDG1, Bhatnagar M 1, Patel R 1, Shah C 1, Shah D 1, Solanki V 2
1B J Medical College & Civil Hospital, Ahmedabad, India2B J Medical College & Civil Medical, Ahmedabad, India
Background
Therapeutic Plasma Exchange (TPE) is a well established modality of treatment in a variety of neurological, haematological, renal and autoimmune diseases. It is performed effectively and safely in adult patients, but the use of TPE is limited in paediatric patients due to lack of universally accepted indications and technical challenges like establishment of adequate vascular access, low blood volume, increased incidence of adverse events during procedure and poor co‐operation of patients during procedure.
Aim
To assess the effectiveness and safety of TPE in paediatric patients.
Materials and methods
The present report is from a tertiary care teaching hospital from western India. A total 122 TPE procedures were performed in 40 paediatric patients between the 3 to 15 years of age group with Guillain Barre Syndrome (GBS). TPE procedures were performed on Spectra Optia apheresis machine (Manufacturer TERUMO BCT) on daily or on alternate days depending on the clinical condition of the patient. The TPE kit was primed with group specific, screened and cross matched packed red blood cells for all procedures in order to avoid hypoxia and hypovolemia. Patient's total blood volume was calculated as per Nadler's formula and processed through central double lumen catheter.1‐1.5 plasma volume was exchanged with normal saline and fresh frozen plasma. Details of the procedural complications if any were noted and analyzed. Pre and post procedure renal functions along with haematological parameters were done at every procedure.
Results
A total of 122 TPE procedures (with an average of five procedures per patient) were performed on 40 paediatric patients over a period of one and half year from Jan‐2013 to Jul‐2014. In the 3‐5 years age group, average whole blood processed was 1137 ml, plasma volume removed was 515 ml, replacement fluid transfused was 571 ml, and ACD used was 93 ml. In 6‐8 years age group, average whole blood processed was 1423 ml, plasma volume removed was 625 ml, replacement fluid transfused was 667 ml, and ACD used was 119 ml. In 9‐11 years age group, average whole blood processed was 2009 ml, plasma volume removed was 961 ml, replacement fluid transfused was 927 ml and ACD used was 170 ml. Grades of improvement seen were from grade‐0 (complete paralysis) and grade‐I (only a trace or flicker of movement in the muscle) to grade‐III (movement possible against gravity but not against resistance) (Grading of muscle power is as per Medical Research Council Scale). Inadequate vascular access was most common complication observed in six procedures. Other complications were allergic reactions to fresh frozen plasma(FFP), hypovolemia, hypocalcaemia and vasovagal reactions.
Conclusion
TPE procedures in paediatric patients have been increasing and have been shown to be effective as first line or adjunctive therapy in selected diseases. It is a safe procedure when volume shifts, calcium supplementation and venous access are taken care of.
P‐705
Therapheutic Plasma Exchange: Single Center Experience
Navarro D , Ferreira AC , Gouveia S, Azevedo A , Mendes M , Araújo T , Nolasco F
Hospital Curry Cabral, Lisbon, Portugal
Background
Therapeutic plasma exchange (TPE) is used in an increasingly number of disorders. Our center includes a nephrology division, and is the biggest reference center for liver transplant in Portugal. We report our TPE experience during the last 5 year period (2009–2013).
Methods
Data collected from medical records. Statistical analysis was performed using STATA software. A P‐value <0.05 was considered statistically significant.
Results
In our center, 44 patients underwent on average 9.8 ± 13.5 TPE sessions. Reason for performing TPE was classified as either ‘Clinical Nephrology’ (23 patients) or ‘Transplant’‐related issue (21 patients).
Clinical nephrology disorders requiring TPE were: Vasculitis (n = 14) ‐ ANCA with or without anti‐GBM vasculitis (n = 8), Anti‐GBM disease (n = 2), Severe lupus (n = 2), Eosinophilic polyangiitis (n = 1), Cryofibrinogenemia (n = 1) ‐; Thrombotic microangiopathies (n = 7); Paraquat intoxication (n = 1); Hypertriglyceridemic pancreatitis (n = 1).
Transplant disorders were subdivided in AB0‐incompatible liver transplant (n = 9) and renal transplant (n = 12). Renal transplant disorders requiring TPE were: HUS relapse (n = 6), Humoral rejection (n = 5), Focal segmental glomerulosclerosis relapse (n = 1).
Table 1 shows the demographic, laboratory findings and outcomes of the population.
Excluding hepatic transplant recipients, 63% of 35 patients underwent renal biopsy. We found no correlation with better renal prognosis (3.9 ± 0.9 vs 2.9 ± 1.2, P = 0.6).
TPE sessions were inversely associated with death (1.3 ± 2.6 vs 10 ± 31.4 sessions, P = 0.04), and correlated with need of HD (9.9 ± 5.2 vs 5 ± 3.8 sessions, P = 0.03).
Patients needing TPE due to ‘Clinical Nephrology’ conditions had a significantly worse renal prognosis (ESRD rate 37% vs 5%, P = 0.03).
In the clinical nephrology group, patients with a higher number of TPE sessions trended towards better renal prognosis (11.5 vs 7.5 sessions, P = 0.1).
The 10 patients with pulmonary involvement had worse on entry creatinine (7.1 ±3.9 vs 5.4 ± 5.1 mg/dl, P = 0.3), but no correlation with renal prognosis or death.
In the transplant group, there was a trend towards worse renal function at end of follow‐up in the HUS relapse subgroup (1.5 ± 0.4 vs 2 ± 4, P = 0.1).
Caption 1 Demographics, laboratory findings and outcomes of the population
Conclusions
TPE is not a frequent treatment, but even in a small study its effects on survival and renal prognosis are noticeable. Further research is needed to establish TPE indications and prescription, and whether newer drugs can avoid its use.

P‐706
Treatment and Outcomes of Thrombotic Thrombocytopenic purpura at the Blood Transfusion Institute of Serbia
Milutinovic S , Bogdanovic G , Srejic V , Miljus D
Blood transfusion institute of Serbia, Belgrade, Serbia
Background
Thrombotic thrombocytopenic purpura (TTP) is a systemic disease that belongs to the group of trombotic microangiopathies. The disease is typically characterized by thrombocytopenia, microangiopathic hemolytic anemia, neurological symptoms, increased temperature and disturbance of renal function.
Aims
To presentat our experience with patients suffering from TTP who underwent therapeutic plasma exchange during the period from 2012 to 2014 at the Blood Transfusion Institute of Serbia.
Methods
All treated patients, their clinical features, laboratory parameters and treatment outcomes were monitored retrospectively. Volume changes ranged from 1.1–2.0, every day to normalization of LDH serum, haptoglobin and an increase in the number of Plt above 150 × 10 (9). We also considered if the disease was the first reported, the relapse, or the exacerbation, as well as the number of days needed for the recovery.
Results
Out of 25 patients, 15 (60%) were women. Median age of patients was 43 years (19‐66). Median age of male patients was significantly higher than in female patients (51 vs 30 years). All patients had thrombocytopenia and microangiopathic hemolytic anemia. In 84% (21/25) cases neurological symptoms were present, in 40% (10/25) kidney dysfunction, in 20% (5/25) elevated temperature. The first attack of disease was in 76% (19/25) of all cases, the first relapse in 3 patients, the third relapse in 2 patients, 1 patient had the sixth relapse, 3 patients had early ralapse, i.e. exacerbation after 7 days following the end of terapy. Average number of TPEs was 8 (3‐14), the average volume echanges 1.7 (1.1‐2.0). The average values of Hgb were 78 + /‐ 13 g/L, the average number of Plt 15 + /– 9 × 10 (9)/L, the average value of LDH 3624 + /− 1136 U/L, the average value of haptoglobin 10 + /− 10 mg/dL before the initiation of therapy. Therapeutic response was excellent, and in the further course of hospitalization patients showed clinical and biochemical improvment, except in 3 cases were additional 3 and/or 4 TIPs were necessary. Average number of days needed for the full recovery vere 10 (6‐ 17).
Conclusion
Therapeutic plasma exchange was proven to be an excellent therapeutic modality for TTP, severe and over 90% of lethal disease. Over the past three years during the application of this therapeutic procedure, we had no fatal outcomes, while only three patients had exacerbation and it was necessary to repeat the procedures.
P‐707
Abstract Withdrawn.
P‐708
Recurrent Pregnancy‐Related Thrombotic Thrombocytopenic Purpura Resistant to Intensive Therapeutic Plasma Exchange
Marandiuc DE , Conradi R , von Auer C , Maccagno G , Hitzler WE
University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Background
Thrombotic thrombocytopenic purpura (TTP) is a life threatening thrombotic microangiopathy, characterized by a deficiency of ADAMTS13, a cleaving protease that prevents accumulation of large von Willebrand factor multimers.
Aims
In this report, a recurrent pregnancy‐related TTP case resistant to intensive therapeutic plasma exchange (TPE) is presented. The purpose of TPE is to replace ADAMTS13 and to remove anti‐ADAMTS13 antibodies. TPE can increase the odds of surviving a TTP from 10% to 90%.
Methods
A 39 year old secundigravida white woman in the 18th week of pregnancy with a past medical history of TTP developed a second bout with corresponding clinical signs (chest and thighs petechiae) and laboratory parameters (platelets 7x109/L, hemoglobin 10.2 g/dL, LDH 1228 U/L, ASAT 69 U/L, schistocytes 35/1000 erythrocytes, ADAMTS13 < 1%, anti‐ADAMTS13 antibodies present, ultra large multimers of von Willebrand factor present). Concurrent, signs of preeclampsia were present (high blood pressure, proteinuria). First episode of TTP was 12 years prior during her first pregnancy, with multiple organ dysfunction syndrome and emergency c‐section resolved in full recovery of mother and child.
Results
Urgent TPE was begun, parallel to corticosteroid therapy and symptomatic treatment. After three‐day low platelet count (PC) under once daily TPE, the therapy was enhanced to twice per day, five consecutive days with a rapid impact on PC and hemolysis parameters. A PC around 140x109/L was only for three days stable under TPE once daily, so that the therapy was again enhanced. Despite 2xTPE daily for 18 days, rituximab weekly for 8 weeks, PC rapidly decreased and remained at a low level (around 15x109/L). The clinical state of the patient deteriorated, so that the pregnancy was terminated in the 21th week. TPE was continued once daily. 20 days postpartum, due to persistent renal failure, dialysis was started. After three months therapy, PC slowly increased, followed by a decrease of hemolysis parameters, so that TPE could gradually be tapered off. A total of 117 TPE were performed during 5,5 months. Dialysis continues 3 times per week to this date. Renal biopsy is planned for prognosis assessment.
Summary/Conclusions
During pregnancy, a differential diagnosis with other conditions that present anemia and thrombocytopenia is often difficult, considering that they may also occur concurrently. In TTP suspected cases, a life‐saving plasma exchange therapy must be immediately started even before the confirmatory results of ADAMTS13 activity are available. Rare cases of TTP are resistant to plasma exchange and repeated administration of rituximab. In this case, aggressive therapy, parallel to continuous TPE, ensured the patient a better outcome.
P‐709
The Use of Plasmapheresis for the Preparation of Patients for ABO‐Incompatible Renal Transplantation
Salimov EL1, Ragimov A 1, Kuznetsov O 1, Matveev A 1, Kaabak M 2, Dashkova N 1
1First Moscow State Medical University I.M.Sechenov, Moscow, Russian Federation2Russian Research Surgery Center n.a. Academician B.V. Petrovski of the RAMS, Moscow, Russian Federation
Transplantation from related donors is one of the solutions to the issue of shortage of donor organs. However, in many cases the only available donor has blood group antigens incompatible with the recipient's antigens. The use of plasma exchange to eliminate ABO‐antibodies in recipients makes it possible to transplant ABO‐incompatible kidneys. To evaluate the effectiveness of plasmapheresis in ABO‐incompatible transplantation, we studied the results of kidney transplants in 45 recipients with related ABO‐incompatible allotransplantation.
The most important issue of modern transplantology is the deficiency of donor organs. The possible solution is the development of ABO‐incompatible kidney transplant.
The study objective was to study the possibility of use of high‐volume therapeutic plasmapheresis to prepare the recipients for ABO‐incompatible kidneys transplant.
We studied 45 cases of preparation of patients for ABO‐incompatible transplant that were treated in the premises of kidney transplant department of Russian Research Surgery Center n.a. Academician B.V. Petrovski of the RAMS from 2008 till 2014. The main criterion for use of plasmapheresis was the level of ABO‐antibodies in recipient blood over 1:8.
Patients with body weight over 40 kg underwent plasmapheresis using the apparatuses made by Haemonetics (USA) PCS‐2 and MCS. There was performed the removal of 500‐4800 ml of plasma per one procedure (no less than 100% from total circulating plasma) r. In the patients with body weight less than 40 kg we used Haemophoenix apparatus with Rosa plasma filter using the method of filtration plasmapheresis.
Hematocrit fluctuated within the range 25 ‐ 35%. Replacement therapy was 70‐150% from removed plasma volume. Volume and character of replacement were determined by condition of hemodynamics, amount of diuresis, type and rate of secretion from drainage.
Out of 45 patients in 7 we failed to drop the titer of anti‐group antibodies due to which the transplants were cancelled. Also we recorded 8 cases of transplant loss and the death of two recipients. In three cases we needed to perform additional series of plasmapheresis after transplantation due to the increasing titer of anti‐group antibodies.
In general in 38 patients out of 45 (73%) with group incompatibility we managed to decrease the titer to subnormal values and to perform the transplantation of ABO‐incompatible kidney transplant.
Thus based on the data presented we can make the conclusion on effectiveness of extracorporeal hemocorrection, namely plasmapheresis, as part of the complex of preparation of patients to the ABO‐incompatible kidney transplant.
6.3 Evidence Based Transfusion Medicine Practice
P‐710
Emergency O Negative Red Cells for Women – Defining the Age of Cut off for ‘Child Bearing Potential’
Allard S1, Massey E 1, Seeney F 1, Jenks T 2
1NHS Blood & Transplant, London, United Kingdom2The Trauma Audit & Research Network, Salford, United Kingdom
Background
Immunisation against the RhD antigen can occur following pregnancy, transfusion or transplantation. Sensitised D negative women have the potential risk of haemolytic disease of the fetus and newborn (HDFN) in any subsequent pregnancy with a D positive baby. ORhD negative blood is therefore recommended for women of “childbearing potential”requiring urgent transfusion prior to compatibility testing. The age below which “child bearing potential”is defined does vary and ranges between 45 and 60 years in international guidelines.
Aims and methods
We attempted to assess the likelihood of pregnancy in women aged over 45, 50 and 55 years who had also received an urgent transfusion for trauma. We obtained data from the Office of National Statistics and the Trauma Audit and Research Network (TARN) in the UK.
Results
In a four year period in England and Wales (2009‐2012) there were 2,883,000 live births of which 7181 (0.25%), 517 (0.018%) and 54 (0.0019%) were to mothers aged over 45, 50 and 55 years respectively. The female population of England and Wales rose from 27,828,947 to 28,724,412 over the same period.
45,206 female trauma cases were reported to TARN between April 2009 and March 2013 of which 1808 received transfusion. Of the transfusion recipients 324, 213 and 103 were over 45, 50 and 55 years respectively.
We calculate the likelihood that a woman will receive an emergency transfusion for trauma and become pregnant once they are over the age of 45, 50 or 55 to be 0.0016, 0.00012 and 0.0000125 per million women respectively.
Summary and conclusion
Current UK guidelines stating that women <60 yrs should receive O Dneg blood are over‐cautious and we recommend reducing this to 50 yrs. Pregnancies where the fetus is affected by HDFN as a result of maternal transfusion over this age are likely to occur less frequently than once a decade in England & Wales based on current statistics.
P‐711
Anti‐D Immunisation in Pregnancy – An Ongoing Study From The Serious Hazards of Transfusion UK Haemovigilance Scheme (SHOT)
Keidan AJ 1, Davies T 2, Bolton-Maggs P 1, Poles D 1
1Serious Hazards of Transfusion (SHOT), Manchester, United Kingdom2NHSBT Patient Blood Management Team/SHOT office, Manchester, United Kingdom
Background
Despite antenatal and postpartum prophylaxis with anti‐D immunoglobulin (Ig), sensitisation to D antigen is still occurring. SHOT data show that anti‐D Ig is missed or administered late in many women (754 in the last 3 reporting years)
Aims
To improve understanding of this continuing problem, in 2012 SHOT began a prospective study of women with immune anti‐D detected for the first time in the current (index) pregnancy. More than 99% of UK hospitals submit reports to SHOT so the results will reflect UK practice.
Methods
Reporters are requested to provide data on booking weight, management of sensitising events during pregnancy and administration of routine anti‐D Ig prophylaxis (RAADP), both in the index pregnancy and the pregnancy immediately before the index pregnancy (if applicable).
By December 2014 (32 months) a total of 66 cases had been reported, 16 in women with no previous pregnancies (NPP) and 50 in women with previous pregnancies (PP).
Results
In 7 of 16 NPP cases sensitisation had occurred before RAADP administration but in only 3 of these were prior potentially sensitising events (PSE) documented. In one case the event immediately preceded delivery at 28 weeks. In the other two cases sensitising events occurred but the women did not receive prophylactic anti‐D Ig, because the event was not notified by the woman in one case, and due to incorrect medical management in the other.
In 9 of 16 NPP cases sensitisation occurred later in pregnancy (after 36 weeks) when RAADP had been given. Two of these 9 cases had grossly elevated BMI. In two cases PSE had occurred earlier in the pregnancy but had been correctly managed.
In 26 of 50 PP cases sensitisation must have occurred during the previous pregnancy as anti‐D was detected at booking in the index pregnancy, and in 8 of these no risks for immunisation were identified.
In 22 of 50 PP cases sensitisation occurred later in pregnancy so that the relative contribution of previous pregnancies is less clear, but analysis of the index pregnancy identified failures in management, particularly omission of RAADP and unreported potentially sensitising events.
Summary
Deficiencies in management of PSE indicate inadequate knowledge among healthcare professionals (medical, midwifery, laboratory) and also the women themselves who fail to seek advice. All healthcare professionals participating in the issue and administration of anti‐D Ig must maintain up to date knowledge of standards for management of D‐negative pregnancies and understand the rationale behind it. In addition, all departments involved in the issue and administration of anti‐D Ig should develop a flow chart or checklist reflecting national guidance to ensure that an appropriate dose of anti‐D Ig is issued and administered. D‐negative women must receive counselling at an early stage of pregnancy to ensure they seek medical advice after potentially sensitising events and are empowered to question their management.
These results also raise concerns about the efficacy of the currently recommended prophylactic anti‐D Ig regimens. Continuing data collection will provide important evidence on which to base future guidance.
P‐712
Patient Information and Consent For Transfusion – Results of A National Comparative Audit in the UK in 2014
Allard S1, Grant-Casey J 1, Court E 2, Browett M 3, Davidson A 1, Watson D 4, Robinson J 5, Lowe D 6
1NHS Blood & Transplant, London, United Kingdom2North Bristol NHS Trust, Bristol, United Kingdom3University Hospitals of Leicester NHS Trust, Leicester, United Kingdom4Scottish National Blood Transfusion Service, Glasgow, United Kingdom5Patient Representative, United Kingdom6Royal College of Physicians, London, United Kingdom
Background
The Advisory Committee on the Safety of Blood, Tissue and Organs (SaBTO), prompted by inconsistent practice across the UK, issued recommendations around the need for patient information and valid consent for blood transfusion.
Aims and methods
We undertook a national comparative audit in 2014 of patient information and consent in adults receiving elective red cell transfusion. Centres were asked to complete an organizational survey around availability of guidelines and training. Case notes were audited to assess if the indication for transfusion was documented, if consent had been obtained and if the patient had been given written information about blood transfusion. Patients were invited to complete a questionnaire and a staff survey was also undertaken.
Results
141 sites completed the organisational survey with 89% indicating that they had a policy on consent for transfusion, with the majority stating the need to provide information to patients. 164 sites provided patient data on 2784 cases for the case note documentation audit. The demographics were representative of the wider patient population requiring blood transfusion.
Of these, 81% had documentation of the clinical indication for transfusion in the notes. Evidence for documentation of patient consent for transfusion was found in 43%; this was largely verbal consent.
In nearly 80% of cases, consent was obtained by doctors and of these 72% were junior trainees. While 85% of staff stated that they had explained the reason for transfusion to the patient, only 63% stated that they had documented this; it was only evident in 37% of notes reviewed that the reason for transfusion had been explained to the patient.
The proportion of patients stating that they received information on risks was 38% and 8% for alternatives. These low levels are reflected in the case note audit with documentation that information was given on risks in 23% and on alternatives in 17%.The provision of written information to patients on transfusion was overall low as highlighted by the case note audit (19% documented as receiving these) as well as the patient feedback (28% recalled receiving these) and staff feedback (18% of staff provided these), demonstrating a major discordance with written policies within Trusts.
Despite the above, 75% of patients felt they had been given enough information on transfusion and had been able to ask questions. However 21% stated that they did not feel at all involved in the decision making process around receiving a blood transfusion.
Only 38% of medical and 24% of nursing respondents had used an eLearning module on patient consent and transfusion.
Summary and conclusions
There is an urgent need to improve practice around patient information and consent on transfusion with emphasis on documentation within the clinical records. Junior doctors in particular are involved in prescribing blood and this audit highlights an urgent need to strengthen their training in relation to consent and appropriate prescribing. The development and dissemination of patient leaflets needs review with a need to explore innovative methods to provide information to patients including use of information technology.
P‐713
Iatrogenic Blood Loss And its Correlation With Red Cell Transfusion in Acute Myeloid Leukaemia Patients
Tan D , Hwang J , Ang AL
Singapore General Hospital, Singapore, Singapore
Background
Reducing iatrogenic blood loss from phlebotomy is an important strategy in Patient Blood Management. It was shown to independently associate with developing hospital‐acquired anemia in acute myocardial infarct patients, and was significant in intensive care settings and neonates. No studies have been conducted on iatrogenic blood loss in acute leukaemia patients, who often have prolonged hospitalisations, frequent blood tests and phlebotomy from central venous lines (which requires a discard volume with each blood draw to avoid sample dilution from heparinised saline used to keep the lumens patent). This may increase their red blood cell transfusion (RBCT) requirements.
Aims
We evaluated the degree of iatrogenic blood loss among adult acute myeloid leukaemia (AML) patients, and assessed if this and other factors correlated with the number of RBCT. Factors associated with iatrogenic blood loss were also evaluated. We chose AML patients receiving consolidation chemotherapy as they are more stable with more predictable and comparable chemotherapy effects on blood counts and hospitalization.
Methods
We retrospectively analysed all our admissions for AML consolidation chemotherapy from September 2012 to December 2014. Patients were discharged only with resolution of severe neutropenia. RBCT was given to target a Hb of at least 7‐8 g/dL. Admissions with overt bleeding events or intensive care management were excluded. Data was obtained from computerised records and calculations were based on the assumptions below.
All patients had central venous lines for chemotherapy which were also used for phlebotomy. We counted phlebotomies for full blood count (FBC), electrolytes and blood cultures, but excluded tests like procalcitonin and liver function tests which were usually tested using the same sample as electrolytes, and PT/aPTT which was taken infrequently. Discard volume varied between 5‐10 ml, and we used the lower limit of 5 ml for calculation. Blood cultures did not necessitate a discard volume. The calculated volume of blood taken per test was the amount required to fill a BD Vacutainer® blood tube (3.0 ml and 5.0 ml for FBC and electrolytes respectively) and 10 ml into each blood culture bottle as per standard practice at our centre.
Results
18 patients (11 males and 7 females) with median age of 52 (23‐68) years had 47 suitable admissions. See Table 1 for their other baseline characteristics, phlebotomy losses and Hb parameters during admissions. During each admission, median total RBCT was 3 (0‐15) units and median average weekly RBCT was 1 (0‐2.9) unit. Independent factors associated with phlebotomy losses, total RBCT and average daily RBCT on multivariate analyses are shown in Table 2.
Caption 1 Baseline characteristics, Hb parameters and Phlebotomy volumes

Caption 2 Significant associatons on multivariate analysis

Summary/Conclusions
Our patients’ median phlebotomy volume was significant with equivalence to about half a whole blood donation. Phlebotomy volume also correlated with RBCT. Future interventions eg. standardised protocols to minimize discard volume and blood tests frequency (based on expected blood count nadirs during consolidation chemotherapy) and monitoring selected patients as outpatients instead of inpatients may help to minimize iatrogenic blood loss with potential benefits of reducing RBCT requirements in these patients, who are already at risk of requiring RBCT due to chemotherapy‐induced hypoproliferative anaemia.
P‐714
RBC Antigen Phenotype Matched Red Cell Transfusions for Thalassemia Patients to Prevent Alloimmunsation: How Much is Enough?
Elhence P
Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Background
Provision of RBC antigen phenotype matched blood beyond ABO and Rh D antigens is advocated in multiply transfused patients including thalassemics to prevent alloimmunisation to other RBC antigens. The extent of matching varies from extended to limited phenotype matching depending on whether all or some clinically relevant RBC antigens are matched. It is technically and logistically challenging task for transfusion services to provide such service and therefore there should be an attempt to differentiate between the relevant and the redundant.
Aims
To study the feasibility of providing extended phenotype matched blood from our inventory and to compare different protocols of phenotype matching for their efficacy in prevention of alloimmunisation in our thalassemia patients.
Methods
Extended RBC antigen phenotypes for 12 major clinically significant blood group antigens i.e. D, C, c, E, e, K, Jka/Fy and S/s were analysed for 57 minor phenotyped thalassemia patients. These were used to determine the prevalence of these phenotypes in a single day inventory (n = 1000) based on the RBC antigen frequency reported from the same center donor population. Five different phenotype matching protocols were compared to see which of the alloantibodies reported in 280 thalassemia patients at our center could have been prevented by the use of these protocols. Feasibility of providing blood for the best protocol from daily inventory was calculated.
Results
In our patient population frequency of none of theextended RBC antigen phenotype profile exceeded 3.5%. On the basis of the antigen frequency calculations these phenotypes were calculated to be between 3‐5% in our donor population. Hence for providing 2 units for a single patient from the inventory, 50‐100 units will have to be tested for all the 12 antigens. This demonstrates that is next to impossible to do this unless we have a fully phenotyped donor directory from which donors can be dedicated assigned to patients. Table 1 shows the allo antibodies in 280 registered thalassemia patients. 28 alloantibodies were found in 24 patients. 5 patients had auto antibodies which included one patient who subsequently formed an alloantibody. Table 2 shows the alloantibodies that could have been prevented by use of different protocols and blood availability thereof in the inventory. Using Protocol 2 it is prevent alloimmunisation in 83.3% of patients and matched blood availability varies from 23.5 ‐ 94% for three most common Rh phenotypes in thalassemia patients. Offering limited phenotyped matched blood only to patients once they have formed one alloantibody or autoantibody would further reduce the requirement to only 28 patients (10%) instead of all 280 patients.
Conclusion
This study shows that, it is neither possible nor necessary to provide extended phenotyped blood to prevent alloimmunisation to all thalassemia patients. Prophylactic Matching using protocol II and III would have prevented alloimmunisation in 83.3 and 91.6% of patients.


P‐715
Post‐Transfusion Hyperhaemolysis Syndrome (PTHS) in Sickle Cell Disease (SCD): An Autopsy Findings Supporting Macrophage Activation
Win N
NHS Blood and Transplant, London, United Kingdom
Background
PTHS is a potentially life threatening complication of transfusion and has been well described in patients with SCD. Although the first fatal case was reported in 1993, the editorial published in 2012 still described that the pathogenesis is yet unclear. Various hypotheses have been proposed, including bystander mechanism, suppression of erythropoiesis, macrophage hyperactivity, and excessive eryptosis. Ferritin levels correlate well with haemolysis and have been used as a biomarker of macrophage activation in PTHS.
Aims
A patient with SCD (HbSS) died of PTHS 2 days following transfusion. The autopsy demonstrated the increase in size and number of macrophages with haemophagocytosis of sickled and non‐sickled red cells, in the bone marrow, liver and spleen. These novel finding supports the role of activated macrophages in the pathogenesis of PTHS.
Methods
A 26‐year‐old West African male with SCD was admitted to hospital with painful crises. He was given IV fluid IV analgesia. The patient's blood group was A RhD positive. On admission the Hb level was 74 g/L and total bilirubin 207 mmol/L. No RBC antibodies detected in the patient's samples and crossmatched units were compatible. The patient was a university student and he received a blood transfusion to cover the period up to an examination. Twelve hours after receiving the first unit, the patient complaint of passing dark urine. His bilirubin level rose to 471 mmol/L and Hb level was 65 g/L. Acute haemolytic transfusion reaction was excluded with negative DAT and confirming that no red cell antibodies were detected in post‐transfusion samples. Hb level further dropped to 52 g/L and to 42 g/L within the next 10 hrs. Two cross‐matched compatible units were issued. Four hours after the first unit was transfused the patient had a cardiac arrest and failed resuscitation. The Hb level was recorded as 31 g/L. Consent was granted for an autopsy examination.
Results
The autopsy findings: The bone marrow in the femur showed expansion to the lower end. The liver and spleen were enlarged. The histology of marrow, liver and spleen showed marked haemophagocytosis of sickled and non‐sickled erythrocytes. This was emphasised by the increase in number and size of the tissue macrophages stained by CD68/PGM‐1 immunohistochemistry in the marrow, spleen and liver (Kupffer cells). The spleen also had background Gamna‐Gandy nodules of fibrosis, indicating chronic previous microinfarcts. All the internal organs had extramedullary haematopoiesis in their vessels.
Conclusion
As there is no evidence of red cell antibody mediated haemolysis in PTHS (as in this case) it has been proposed that the host factor of activated macrophages is the possible mechanism. We have documented at autopsy the widespread activated macrophages. This novel finding supports the macrophage activation theory. Hyperhaemolysis is a defined event in reporting to Serious Hazards of Blood Transfusion (SHOT) in UK and the first fatal case in UK (10‐year‐old child with SCD died of PTHS) was recorded in SHOT report 2010. This is the first fatal case reported in an adult SCD patient.
P‐716
Paroxysmal Nocturnal Haemoglobinuria In South‐East Norway 2000‐2010 – History, Diagnostics and Treatment
Nissen-Meyer LSH1, Tjønnfjord GET 2, Golebiowska EG 1, Kjeldsen-Kragh JKK 3, Akkök ÇAA 1
1Oslo University Hospital, Ullevål, Oslo, Norway2Oslo University Hospital, Rikshospitalet, Oslo, Norway3Labmedicin Skåne, Lund, Sweden
Background
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare disease characterised by chronic haemolysis, pancytopenia and atypical thrombosis. The condition is caused by defective glycophosphatidylinositol anchors in the cell membranes of red blood cells (RBC), platelets and white blood cells, leading to uninhibited complement activation, which again causes the clinical manifestations. In recent years, flow cytometry using specific antibodies to marker proteins has proved to be superior to the previously used biochemical tests (Hams test and sucrose test) for the diagnosis of PNH.
Aims
To observe changes in the PNH population depending on the new diagnostic tool. We collected the medical history, treatment details, laboratory findings and transfusion data of all patients diagnosed with PNH at the Department of Immunology and Transfusion Medicine, Ullevål University Hospital, in the decade 2000‐2010.
Methods
Blood samples from 28 patients with suspected PNH were analysed between 2000 and 2010. RBC, monocytes and granulocytes were examined with flow cytometry using antibodies to CD55 and CD59, and lack of these membrane proteins in at least two cell lineages was considered diagnostic for the disease. The results were evaluated together with the medical history and transfusion data of the patients.
Results
Flow cytometry identified 22 patients with PNH. Four of these had ‘classical’ PNH whereas 18 suffered a concurrent bone marrow disease. Five patients had atypical thrombosis. 10 patients were treated with anti‐thymocyte globulin and seven were treated conservatively. Of these 17, six responded well to therapy, six died and five were still transfusion‐dependent. Five patients with life‐threatening bone marrow failure were treated with allogeneic hematopoietic stem cell transplantation (HSCT); three of these died. Six patients received eculizumab; in three patients treated with this monoclonal antibody over time, transfusion‐dependence has been reduced or abolished.
Summary
Flow cytometry identified PNH in the majority of the patients we received samples from. Most patients had a PNH‐clone in the setting of another bone marrow disorder. Atypical thrombosis should be remembered as an indication for testing. Allogeneic HSCT, the only curative therapy, is no longer the first‐choice of treatment. Treatment with eculizumab is indicated in some patients with PNH.
P‐717
Anaemia and Iron Deficiency in Adults; How to Manage it for Nurses. A New Guide From the Royal College of Nursing
Whitmore EL1, Mason I 2
1NHS Blood and Transplant, London, United Kingdom2Royal Free London Foundation Trust, London, United Kingdom
Background
Iron deficiency anaemia (IDA) is the most common cause of anaemia in primary care and causes >57,000 emergency admissions to hospital/yr at a cost to the NHS of £55.48 m. Effective identification and management is often overlooked. Dealing with IDA prevents complications and blood transfusion use with a cost saving of £8.43 m per year.These guidelines have been developed by expert nurses from relevant specialties. They are aimed at all nurses, health care assistants, midwives and health visitors from all specialties and backgrounds.
Method
These guidelines have been developed and written as a joint multi disciplinary work piece by expert nurses from the relevant specialties and the Royal College of Nursing supported by Vifor. The document gives guidance on; Identifying IDA and escalating management understanding how IDA occurs, giving dietary advice and using oral iron supplements, the use of intravenous iron with practical tips for its delivery, it includes robust links to patient information and educational resources provided by NHSB Blood and Transplant and the supports the use and consideration of alternatives to bnlood and blood products where ever possible undfer the Patient Blood Management initiatives encouraging and supporting the patient's involvement in traement options and considerations.. Specialist guidance to nurses working in renal disease, gastroenterology, obstetrics and gynaecology and Patient Blood Management within all areas where transfusion would be considered.
Results
The main guidance contains; Definition of IDA, iron metabolism and pathophysiology, storage or iron, causes of functional and actual iron deficiency, measuring iron status, common symptoms of anaemia, history taking and examination, dietary iron, oral iron supplements, practical advice for administrating intravenous iron, and preoperative optimisation of patients and the use of or appropriate avoidence of blood transfusion.
The GI appendix discusses IDA in coeliac disease and inflammatory bowel disease, and gives case study examples to show the impact of effective nursing intervention on patient outcomes.
Conclusion
These newly published Royal College of Nursing Guidelines provide an invaluable resource about all areas of iron deficiency management for nurses from all backgrounds and specialties. The specialist appendices provide extra information for nurses working within renal disease, obstetrics and gyanecology and gastroenterology, and offers clear information and advice for the consideration and use of alternatives where transfusion of blood or blood products might be a consideration.
P‐718
Platelets From Patients Treated With Platelet Inhibitors are Highly Responsive
Bontekoe IJ1, Baharoglu MI 2, Koopman MMW 1, van der Meer PF 1, de Korte D 1
1Sanquin Blood Supply, Amsterdam, The Netherlands2Academic Medical Center, Amsterdam, The Netherlands
Background
The effectiveness of platelet transfusions, as treatment in bleeding patients receiving platelet aggregation inhibitors for secondary stroke prevention, is investigated in the Dutch ‘Platelet Transfusion in Cerebral Haemorrhage’ (PATCH) study. The question arose if the effect of a platelet transfusion could be measured with some laboratory test. For this purpose, stroke patients of the Neurology Department, who are also treated with platelet aggregation inhibitors, were asked to give their consent for a blood sample.
Aim
To investigate whether an effect could be measured with two selected assays, in blood samples of patients treated with platelet inhibitors while a platelet transfusion is mimicked.
Methods
From 7 patients who had a stroke and receiving platelet aggregation inhibitors (ascal, dipyridamol or clopidogrel), a blood sample was taken, 1 to 13 days after admission. Platelet CD62P‐expression was determined after activation with the agonists ADP, CRP or TRAP, each in 8 ascending concentrations. Also a coagulation test was performed by thromboelastography (TEG) with kaolin as activator (of the intrinsic pathway). Then a transfusion was mimicked in vitro by spiking the samples with 6% platelet concentrate. After incubation for 30 min at 37°C the same assays were repeated.
Results
The patients, 6 men and 1 woman, were aged 50‐88 year and had 251 ± 79x109/L platelets. The area‐under‐the‐curve of the agonist‐induced (AI) CD62P‐expression was comparable with reference values, see table. The TEG tracings showed a relative short (R‐ and K‐time) and strong coagulation reaction (high maximum amplitude), despite the use of aggregation inhibitors. Also, all samples showed a slight level of fibrinolysis (LY30).
The CD62P‐test was performed 5 times before and 4 times after spiking, where only small differences were observed after spiking. In TEG a significant decrease of R‐ and K‐times, increase of maximum amplitude and decrease of fibrinolysis was measured after spiking.

Summary/Conclusions
Both assays show that, despite the use of aggregation inhibitors, platelets were still highly responsive to agonists. It was expected that, caused by the aggregation inhibitors, a low response would be measured and that this response partly would be restored after spiking. The opposite seems to be the case. The CD62P‐test showed a normal responsiveness and a mimicked transfusion by spiking with platelets had no effect. In TEG an increased coagulation reaction was seen, which increased significantly after spiking. The TEG‐test seems the best of this two tests for determining the effect of a platelet transfusion.
Additional parameters can be determined in follow‐up research to describe and explain the fibrinolysis.
P‐719
Use of Manual and Electronic Infusion Devices to Red Blood Cells Transfusion: Seeking Evidences for Nursing Practice
Pedreira MP , Peterlini MAP , Kusahara DK , Pardo LP , Wilso Wilson AM , Avelar AA
Federal University of Sao Paulo, Sao Paulo, Brazil
Background
The level of damage of red blood cells (RBC) caused by infusion devices is an unsolved question and many of the equipment and supplies available for transfusion were not submitted to investigations that can support different clinical practices.
Aims
To compare plasma free hemoglobin, potassium and hemolysis ratio of RBC infused by macro drips infusion set and peristaltic infusion pumps.
Methods
Three of each analyzed devices (macro drips infusion sets, peristaltic infusion pump I and peristaltic infusion pump II) were studied and samples were collected before manipulation (A) of packed RBC, and after infusion (B) through the devices to evaluate plasma free hemoglobin and potassium, and hemolysis ratio (free hemoglobin × 100‐hematocrit/total hemoglobin). The laboratory was maintained with a temperature of 21(±1)oC and humidity of 55% to 58%. Six units of packed RBC (CPDA‐1) from different donors with storage time within recommended limits were maintained at room temperature one hour before the beginning of the experiments, reaching a mean temperature of 23.01(±1.19)oC during the infusions. The packed RBC were connected to gravitational infusion devices and placed 80 cm above the final tip of the infusion set; the packed RBC were installed in the infusion pumps according to manufacturer's recommendations (placed 60 cm above the final tip of the infusion set). The infusion rate in all devices was set at 100 ml/h. The data was analyzed by mean, standard deviation, t test and ANOVA variance analysis (P ≤ 0.05).
Results
The free hemoglobin level before RBC manipulation was similar (P = 0.932) in macro drips infusion sets (0.31 ± 0.09), peristaltic infusion pump I (0.25 ± 0.34) and peristaltic infusion pump II (0.25 ± 0.17). After infusion free hemoglobin level was higher in peristaltic infusion pump I (0.63 ± 0.98) than in macro drips infusion sets (0.26 ± 0.01) and peristaltic infusion pump II (0.27 ± 0.19) with no significant difference (P = 0.681). Potassium level was similar before (P = 0.939) and after (P = 0.376) RBC infusion by macro drips infusion sets (A‐39.03 ± 1.89; B‐39.14 ± 2.50), peristaltic infusion pump I (A‐39.39 ± 2.02; B‐39.87 ± 3.77) and peristaltic infusion pump II (A‐39.26 ± 2.25; B‐42.73 ± 2.75). The comparison of hemolysis ratio of RBC infused by macro drips infusion sets (0.15% ± 0.11%) and peristaltic infusion pump I (1.13% ± 1.82%) demonstrated differences (P = 0.007). No difference (P = 0.179) was observed between macro drips infusion sets (0.15% ± 0.11%) and the peristaltic infusion pump II (0.47% ± 0.35%). The peristaltic infusion pump I (1.13%±1.82%) lead to more hemolysis than the peristaltic infusion pump II (0.47%±0.35%), with a non‐significant difference (P = 0.071). Only the peristaltic infusion pump I presented hemolysis ratio higher than 0.8%.
Conclusions
The comparison of manual and electronic devices demonstrated differences in hemolysis ratio during infusion of RBC, with no significant variations on plasma free hemoglobin and potassium level. The peristaltic infusion pump I reach a mean hemolysis ratio of 1.13%. Despite the fact that all devices are commercially available for RBC infusion there are differences between the analyzed devices. The data collection of this study is continuing in order to obtain more results capable to support nursing practice and promote patient safety during RBC transfusion.
Acknowledgement
Grant for research FAPESP(2012/25284‐9) and CNPq(303006/2012‐9)‐researcher grant.
P‐720
Uptake and Impact of a Group‐Check Policy in The UK and Ireland
Milkins CE , White J , Rowley MR
UK NEQAS, Watford, United Kingdom
Background
UK NEQAS questionnaire data showed that 54% of UK laboratories routinely used electronic issue (EI) in 2012, with 60% of these requiring two separate samples for ABO grouping before using EI. Wrong blood in tube (WBIT) is the biggest cause of near‐miss reports to SHOT, and to reduce the risk of ABO incompatible transfusion due to WBIT, 2013 UK guidelines recommend that the ABO group is confirmed on a second sample regardless of how compatibility is established, where this does not impede an urgent transfusion. Concerns have been raised about the potential impact on the use of O negative red cells and workload, and of potential delays to urgent transfusions.
Aims
UK NEQAS wanted to assess the overall uptake of a second sample (group‐check) policy and to assess differences between methods of assessing compatibility, and between the different countries in the UK and Ireland.
Methods
Additional questions were added to the annual pre‐transfusion testing questionnaire in May 2014 relating to implementation of a group‐check policy, tests undertaken, transfusion policy when a 2nd sample is not available, and impact on workload. Data was linked to the use of EI and country.

Results
Questionnaires were analysed from 290/379 (77%) of eligible laboratories in the UK and Ireland. 44% had a group‐check policy, with a further 23% in the process of implementing one. 93/151 (62%) using EI have a group‐check policy compared with 34% not using EI. 83% undertake a full group and antibody screen on the 2nd sample, 4% an ABO/D group only, and 13% an ABO forward group only. Where transfusion is urgent, with no second sample available, 65% issue group O and 34% group‐specific blood, whilst one laboratory said they did not have a policy for this situation. 82% have to contact clinical areas <5 times in a 24 h period for a 2nd sample, 16% 5‐10 times, and 2% >10 times. 26% have exemptions to the policy, the majority (61%) within paediatrics. Uptake of this recommendation and use of EI varied between countries as shown in the table.
Conclusion
67% of laboratories had either implemented a group‐check policy or were in the process of doing so in May 2014, with considerable variation between countries. A higher proportion of laboratories using EI (than not), have a group‐check policy for all patients, possibly because the majority of EI users already had this policy for patients suitable for EI. It is difficult to assess the precise impact on workload without denominator data, however, the vast majority only request a 2nd sample <5 times in any 24 h period. All but one laboratory has a policy regarding emergency transfusion where no 2nd sample is available, with 65% choosing to issue group O rather than group specific. Future haemovigilance data may be an indicator of the number of cases of WBIT identified by the group‐check policy which may otherwise have resulted in ABO incompatible transfusion, and should also identify any related delays to transfusion. The questionnaire will be repeated in 2015.
P‐721
Patterns of Blood Use in Individual Patients
Forrester KJ1, Bailie K 1, Cheng SY 2
1SNBTS, Edinburgh, United Kingdom2Heriot‐Watt University, Edinburgh, United Kingdom
Background
Encouraging the appropriate use of blood components is a key objective for the Better Blood Transfusion programme. Illustrating patterns of transfusion activity can be useful in engaging clinicians in examining their practice and monitoring change. Using data held in the Scottish Account for Blood (AfB) data mart patients can be categorised by the number of units received; the 2013 annual total varies between 1 and 184 units per person transfused, with the median value being 2 units. For those patients in receipt of larger total volumes, this may constitute multiple small transfusions for transfusion dependent conditions or a single major haemorrhage episode. ‘One‐off’ transfusions could be appropriate in the context of a surgical procedure, but may not be so in a medical context.
Aim
To exploit patient level red cell transfusion data from AfB combined with NHS Scotland hospital episode data to examine individual ‘patient transfusion pathways’ and patterns of demand.
Methods
Syntetos and Boylan (2005) segmentation, traditionally applied in supply chain management, was used to characterise monthly red cell transfusion patterns for 2013. The average inter‐transfusion interval was plotted against the squared coefficient of variation in transfusion size. Transfusion patterns were categorised as ‘Smooth’ (short time between transfusions, small variation in number of units); ‘Intermittent’ (longer time between transfusions, small variation in number of units); ‘Lumpy’ (longer time between transfusions, larger variation in number of units); or ‘Erratic’ (shorter time between transfusions, larger variation in number of units). Pareto analysis was applied to further segment patients by ICD10 diagnosis codes.
Results
Of 32,932 patients included in the initial analysis the majority (95.5%) had an intermittent transfusion pattern; 3.5% exhibited a lumpy and 1% a smooth pattern. Within the smooth group the most common primary diagnosis was myelodysplasia (MDS) accounting for 27.4%. A smooth pattern would be expected in patients with transfusion dependent conditions such as MDS. However, more patients with MDS exhibited an intermittent pattern (67.4% of 319 cases). Within the intermittent group, one of the top five primary diagnoses was iron deficiency anaemia. Intermittent was the predominant transfusion pattern in these patients (97.3% of 910 cases), which may suggest inappropriate use of transfusion in the management of iron deficiency. Patients categorised as lumpy have a wide variety of primary diagnoses, each accounting for around 3% of cases. The most common diagnoses were fracture of femur, ‘other anaemias’ and gastrointestinal haemorrhage. Of the very few patients categorised as erratic all except one had haematological disease or cancer (conditions typically described by smooth or intermittent transfusion patterns); these may represent patients receiving atypical treatment, or other diagnosed or undiagnosed comorbidities.
Conclusion
This novel approach to characterising transfusion pathways can reveal expected and unexpected patterns of practice; further multi‐criteria segmentation is likely to illicit data with which to engage clinical teams in reviewing practice and ascertaining potential explanations for variation.
Reference
Syntetos, AA, Boylan, JE & Croston, JD (2005) On the categorization of demand patterns. J Oper Res Soc, 56, 495‐503.
P‐722
Patient Blood Management At Rigshospitalet Copenhagen University Hospital: Implementing Evidence‐Based Transfusion Practice to Reduce Unnecessary Exposure to Red Blood Cell Transfusion
Norgaard A , Seeberg JS , Honnens de Lichtenberg TH , Stensballe JS , Hansen MBH , Johansson PIJ
Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark
Introduction
Randomized clinical trials have shown that liberal red blood cell transfusion in the non‐bleeding may increase infection, circulatory overload and short term mortality in some patients. WHO recommends the implementation of evidence‐based Patient Blood Management, including restrictive transfusion practice and transfusion alternatives. In Denmark, liberal transfusion practice has been the rule, caused by a high haemoglobin concentration before transfusion and a traditional dose of 2 units. We established a Patient Blood Management programme in three phases: Transfusion optimization, perioperative blood management and preoperative anaemia treatment. We report the result of transfusion optimization including a 3‐year follow‐up.
Aims
To reduce unnecessary transfusion in our 1200‐bed tertiary hospital with a broad range of specialties including haematology, abdominal and vascular surgery, organ and stem cell transplantation, a cardiothoracic centre and 4 intensive care units.
Methods
Abefore‐and‐after study: In 2008 before intervention, a database was established and transfusion practice was analyzed. During 2009‐11 the whole hospital was introduced to transfusion optimization, all transfusing wards was offered intervention and 7 wards including surgery, anaesthesia, intensive care and medical departments were selected for a full‐scale intervention, based on their haemoglobin level before transfusion, the amount of blood transfused, and the level of evidence supporting the guidelines for their patient categories. The intervention comprised media communication, staff educational meetings, revision of local guidelines, and if necessary local multidisciplinary task forces. Transfusion data for participating wards were fed back to the staff, managers and quality organization quarterly from 2009. Data included all in‐hospital patients aged at least 16 years of age, excluding episodes of massive transfusion.
Results
Totally 101,215 patients were admitted during 2008‐2014. Nearly all wards participated, however a few did not complete the proposed intervention. At the hospital level mean pre‐transfusion Hb decreased from 9.0 to 8.3 g/dL (P < 0.001). Transfusion above the upper national guideline Hb‐trigger of 9.7 g/dL was reduced from 23% to 10% (P < 0.001). Transfusion compliance with the national guideline restrictive trigger increased from 6.8% to 19% (P < 0.001). The percentage of single‐unit transfusions increased from 72% to 78% (P < 0.001). Blood usage decreased with 41% in surgeries and 28% in admissions (P < 0.001). Percentages of transfused admissions decreased overall from 10% to 7.9%, more pronounced in intensive care units (from 48% to 29%) and surgical wards (from 10% to 5.7%), than in medical wards (from 10% to 8.2%). Transfusion rates in elective primary orthopaedic hip and knee surgery were halved and in CABG transfusion rate decreased from 42% to 27% (P < 0.001). The effect on transfusion rates in abdominal surgery varied.
Conclusion
The implementation of restrictive transfusion was associated with increased guideline compliance and a substantial reduction in the patients’ exposure to unnecessary blood transfusion. The effect was sustained during the 3 year follow‐up. This approach may be useful to other institutions, and may form the basis for implementing complete WHO‐defined Patient Blood Management.
P‐723
Abstract Withdrawn.
P‐724
Success of PBM in Japan Supported by Fees for Service
Inaba IS
Japanese Red Cross, Higashimatsuyama, Japan
Introduction
Frequent occurrence of serious or fatal side effects related to transfusion therapy prompted the Japanese Ministry of Health, Labour and Welfare and the Japanese Society of Blood Transfusion and Cell Therapy to work jointly to improve transfusion safety and appropriateness. The government drafted ‘Utilization standards for blood components’ in 1986, and several guidelines for blood utilization since 1989; six revisions have been performed to date. Our society cooperated with making these guidelines. Furthermore, the government formulated reimbursement strategies for blood management in 2006 to promote more appropriate transfusion therapy. Despite significant resource demands, new standards were widely adopted, anticipating modern concepts of PBM. Here we report the situation of Kanagawa Prefecture.
Subjects of the survey and methods
Target area: Kanagawa Prefecture (2014)
Investigation items:
1. Population 2014 of Kanagawa
2. The number of medical institutions providing blood transfusion and the number of beds at each hospital
3. The blood transfusion management charges in each case
Appropriate use of blood products, by type.
Results
(1) Population 2014 of Kanagawa: 9,100,346 (8.07% of all Japan)
(2) The number of medical institutions providing blood transfusion and the number of beds at each hospital
A total of 412 medical facilities in Kanagawa provided blood transfusion services in 2014. By size, 149 had fewer than 20 beds, 77 had 20‐99 beds, 84 had 100‐199 beds, 79 had 200‐499 beds, 21 had 500‐999 beds, and 2 had 1000 or more beds.
(3) The blood transfusion reimbursement in each case
Two tiers of reimbursement were established, each of which paid a basic fee for transfusion services that conformed to local (institutional) criteria, and paid more if the services also conformed to national criteria. Tier 1 reimbursement was provided in 38 cases, of which 37 met national guidelines. Tier 2 reimbursement was provided in 81 cases, of which 61 met national guidelines.
(4) Appropriate use of blood products, by type.
Caption 1: Transfusion‐related reimbursement to Kanagawa prefecture hospitals, by size (number of beds) 2014.

Merging Tier 1 and Tier 2 reimbursements in Kanagawa, national criteria for appropriate transfusion were met for 83% of red blood cells, 84% of fresh frozen plasma, and 90% of platelets. Hospitals receiving these reimbursements managed more than 80% of the allogeneic blood supply in Kanagawa prefecture.
Conclusions
A fee‐for‐service reimbursement system encouraged medical institutions to embrace national standards for appropriate blood transfusion. This system spread rapidly after 2012 due to financial incentives. More than 2000 institutions throughout Japan were participating in this system by the end of 2014. Considering that elsewhere in the world, many institutions struggle to have their own transfusion guidelines met by individual practitioners, our compliance rates with national standards are impressive. On the other hand, because compliance is locally assessed, future revisions should include external evaluation. This could be incorporated into an existing “I&A”program of inspection and accreditation.
P‐725
High Titre Anti‐A, Anti‐B in Ivig May Have Detrimental Effect on ABO Incompatible (ABOi) Renal Transplant
Lee CY1, Win N 1, Puliatti C 2
1NHS Blood and Transplant, London, United Kingdom2Barts Health NHS Trust, London, United Kingdom
Background
Anti‐A, B antibodies are major obstacles in a major ABO incompatible (ABOi) renal transplant (Donor Blood Type A or B and recipient Blood type O). ABOi may be associated with hyperacute rejection. It has been well‐established to provide ABOi renal transplant by removal and bring down the recipient anti‐A and or anti‐B antibody by plasma exchange before the transplant and following this Intravenous Immunoglobulin (IVIG) has also been prescribed as a form of Immunmodulation. The target is to bring down the anti‐A, anti‐B titre to 4 or below immediate pre‐transplant.
Aims
IVIG is a pooled blood product and contains anti‐A and anti‐B. Although attempt has been made to standardize haemagglutination testing for anti‐A, and anti‐B in IVIG recent studies have shown that anti‐A and anti‐B antibody titres varies with different IVIG preparations. IVIG use is increasing in transplant programme and high titre anti‐A or anti‐B may have adverse effect in this setting.
Methods
A 54‐year‐old patient was awaiting an ABO and HLA incompatible live donor renal transplant and was receiving plasma exchange with IVIG to reduce anti‐A titre and DSA (Donor Specific Antibody) levels. The patient was blood Group O and the donor was Blood group A. Both pre and post exchange samples were referred to the NHSBT reference laboratory for titre studies. Pre‐exchange anti‐A IgM titre 32 and IgG 16. Post ‐exchange both IgM and IgG titre 1:2. IgM was detected by saline agglutination method and serum was treated with 0.01 DTT (destroy IgM) followed by 37 C IAT technique using anti‐IgG reagent to detect IgG. NHSBT received another sample on the planned operation day: Unexpectedly both IgM and IgG titres were high 1:64. It brought to our attention that after plasma exchange the hospital had given IVIG therapy as part of the treatment for HLA desensitisation. Passive infusion of high titre anti‐A from IVIG product was suspected and NHSBT asked hospital clinicians to send samples to check titre from the index IVIG batch.
Results
NHSBT received the index IVIG batch and investigations confirmed the presence of high titre anti‐A and anti‐B; anti‐A (titre IgG and IgM 256) anti‐B (titre IgG and IgM 128). The Patient received further plasma exchanges and was able to bring down titre to 1:4 for both IgG and IgM.
Summary/Conclusions
IVIG contains anti‐A and anti‐B and has been well recognised that IVIG therapy may cause mild to severe haemolysis, especially if the recipient is blood group A and has been recommended to monitor and check Hb level 48 to 72 hrs after IVIG infusion. Haemolysis is associated with high dose IVIG therapy. The use of IVIG therapy is now common in a renal transplant setting and this case highlighted the importance to review the procedure regarding timing of infusion of IVIG and plasma exchange. It is also important to recheck anti‐A, anti‐B titre if IVIG has to be prescribed for any reasons immediately pre‐transplant for ABOi cases.
P‐726
Abstract Withdrawn.
P‐727
Four Years of Tailored Low Dose Prophylaxis in A Small Cohort of Kids With Severe Hemophilia A Using SD‐F Cryoprecipitate
El Ekiaby A1, Burnouf T 2, Radosevic M 3, Goubran H 4, El Ekiaby M 1
1Shabrawishi Hospital, Giza, Egypt2Taipei Medical University, Taipei, Taiwan3Human Protein Process Sciences, Lille, France4Saskatoon Cancer Center, College of Medicine, University of Saskatchewan, Saskaton, Canada
Background
Regular injection of clotting factor concentrates to prevent/reduce bleeding in patients with severe hemophilia is now considered the standard of care. Several protocols of prophylaxis are adopted with variable use of FVIII that ranges between 2500 iu/kg/year in the Dutch protocol to more than 5000 iu/kg/year in the Swedish protocol. The cost of these protocols cannot be supported in resource‐limited countries. In our center we have adopted the production of Solvent Detergent Treated and microbial filtered (SD‐F) cryoprecipitate. In previous studies, this product proved to contain FVIII with a pharmacokinetics profile similar to that of plasma derived and recombinant FVIII.
Aim of the study
Study the safety and the efficacy of tailored lower dose prophylaxis program using SD‐F cryoprecipitate in young kids with severe hemophilia A
Methods
8 kids with severe hemophilia A (FVIII clotting activity <1%) were sequentially enrolled in this program starting from January 2011 and up to February 2014. Age of enrollment was 2 – 4 years. All kids were negative for inhibitors to FVIII. IRB and patient family consent was obtained. X ray for elbow, knee and ankle joints was done to document baseline joint status. Kids were infused with 20 iu FVIII/kg once weekly. If one joint experienced more than one breakthrough bleed, the same dose was increased to twice weekly and if still there were more breakthrough bleeds the frequency of the same dose was increased to 3 times per week. Breakthrough bleeds were treated by infusion of 25 iu FVIII/kg once or more according to the severity of the bleed. The short‐term evaluation of this program was based on the annual bleeding rate (ABR) and Hemophilia Joint Health Score (HJHS)
Results
8 kids were followed on prophylaxis for a mean period of 33.55 months (12 – 38 months). The mean age at start was 31.11 months (24 – 38 months). The average FVIII consumption/kg/year was 1403 iu (892 – 2989 iu). Out of the 8 kids 6 are on SD‐F cryoprecipitate infusion once weekly, one twice weekly and one three times weekly. ABR was 2 (4 – 6), HJHS of the 8 kids was zero and none of them developed FVIII inhibitors. None of the kids developed adverse events from the infusion of SD cryoprecipitate and none developed transfusion transmitted HBV, HCV or HIV.
Conclusion
This study indicates the feasibility of lower dose prophylaxis in children with severe hemophilia A as the annual bleeding rate was similar to that seen in higher dose protocols. It also shows the efficacy and safety of SD‐F cryoprecipitate since the results are similar to those seen with plasma derived or recombinant FVIII. Of importance is the fact that none of the 8 patients developed FVIII inhibitors after 50 exposure days.
P‐728
Putting the Patient at the Heart of Pathology #Harvey's Gang
Whitmore EL 1, Robinson M2
1NHS Blood and Transplant, London, United Kingdom2Western Sussex NHS Trust, Worthing, United Kingdom
Background
Harvey, a seven year old with acute myeloid leukaemia wanted to know what happened to all his blood samples so the haematology laboratory staff at WorthingHospital (Western Sussex NHS Foundation Trust) arranged for him to visit. After his passing the Worthing Pathology Staff formed Harvey's Gang in his honour and supported by NHSBT, LabCold and Ortho now showcase pathology, key transfusion messages and explanations about the laboratory processes to other critically ill youngsters
Aim
To implement the principles of Patient Blood Management (PBM) by placing the patient in the heart of pathology and engaging laboratory staff with patients to enlighten critically ill youngsters and their families / carers about what happens to their samples and to myth bust Pathology.
Method
Planned are planned between the Chief BMS and the Paediatric team. Laboratory staff meet and present the young visitor with a white lab coat and a Trainee Scientist name badge.
Pathology reception, specimen reception and the laboratories are visited and a “Certificate of Attendance”, NHSBT childrens information comic/sticker books, pens, highlighter pens, rulers, “Ask me who am I”stickers, LabCold penguins, Ortho Rulers and of course sweets are presented. They all meet “Harvey”the new named Ortho Vision™ Blood Transfusion Blood Grouping Analyser.
Results
This approach has been adopted by other NHS Trusts in the South East Coast Region, and is part of the package that accompanies the new Ortho Vision™ Analyser as it is installed across the world, this has taken Harvey's gang to international levels and implementation by other pathology services. NHSBT has also adopted this as part of the Donor process by having named aphaeresis machines and donor and patient stories available to read. Each child has had photographs taken in the laboratoriess and the laboratory have received Thank you cards. The initative has featured in local press, radio and on television, the local Mayor and M.P have visited and the laboratory has received a congratulatory letter from CEO of IBMS. Most importantly in house Trust Press has featured the laboratory and raised the profile and morale of the laboratory staff and awareness of the vital role they play in day to day patient needs and hospital services.
Summary/Conclusions
This initiative has put patients, and their relatives/carers, into the laboratory which has highlighted PBM initiatives and Patient First campaigns locally, nationally and internationally.
Nine children and their families have visited the laboratory and taken the Harvey's Gang Tour, all expressed how this has resolved concerns and fears around the need and timing of blood tests and the provision of blood and blood products and has also raised the desire to encourage all who can to donate blood. It has raised the profile of the laboratory services and function within the Trust and raised the morale of staff that have seen first hand the impact and importance of the work they do by seeing the patient out of the test tube and in the laboratory.
P‐729
Development and Validation of a Checklist to Promote Safe Nursing Practices During Blood Transfusion
Avelar AA , Maurício EM , Peterlini MAP , Kusahara DM , Pedreira MP
Federal University of São Paulo, Sao Paulo, Brazil
Background
Adverse events due to transfusion therapy are associated with several factors, including errors during the administration process. According to the Serious Hazards of Transfusion (SHOT) from the United Kingdom the majority of the adverse events during blood components transfusion are related to errors in the identification of recipients.
Aims
To develop and validate a checklist to promote safe nursing actions during the administration of blood components.
Methods
Methodological study to develop and validate a checklist for safe blood components administration. A draft checklist was elaborated according to literature review data aiming to address critical points during the process of blood components administration, targeting to prevent errors and promote patient safety. The preliminary instrument was submitted to validation of construct and content with five specialists, through Delphi's technique. The validation ratio was set at 80% of concordance.
Results
After two rounds of the validation technique the checklist was considerate valid to be tested in clinical practice. The instrument comprises an identification segment (patient, blood component and healthcare professional) and four sections of actions to be performed before, during and after transfusion. Each one of the section includes four actions to be performed during planning and execution of blood components administration, resulting in 16 actions to be checked. The first checklist section, colored in yellow and named “before blood component request”includes the verification of patient information about risks and benefits, obtainment of informed consent, presence of favorable clinical conditions to transfusion and intravenous access adequate to transfusion, according to institutional protocols. The second section was entitled “when getting the blood component”and highlighted in orange, comprising blood bag inspection, confirmation of medical order, double check of patient and blood component identification and adequate equipment and supplies to administration. The third section “in the beginning of the infusion”was colored in green and comprises actions related to patient identification at bedside (two identifiers in bracelet/barcode, according to institutional protocol); assessment of patient vital signs, connection of the blood component in the intravenous catheter and infusion rate confirmation. The fourth section was tinted in blue and named as “during and after blood components infusion”has actions related to accompanying the patient during the first 15 min of the procedure, reassessment of patient vital signs and infusion quality one hour after the procedure, nursing interventions to patient clinical assessment and catheter withdraw or maintenance, and the documentation of the procedure.
Conclusion
The checklist reached consensus ratio after two rounds of the Delphi Technique application. Comprising an identification section with 10 items and 16 actions to be performed in the administration of blood components the instrument was considerate valid to be implemented in clinical practice. The clinical validation is one of the next steps in order to analyze the impact of the checklist in the promotion of patient safety during blood components administration.
Acknowledgement
CNPq grant for research n. 474906/2013‐2 and researcher grant n. 303006/2012‐9.
P‐730
The Development of a ‘Big’ Transfusion Dataset in a Large Teaching Hospital
Pendry K1, Royston R 2, Summerfield G 2, Whitehead C 2, Ardern J 2, Uttley J 2, Kettle R 2, Burgess A 2, Marsden M 2
1NHS Blood and Transplant, Manchester, United Kingdom2Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom
Background
The science of data management is developing to enable organisations to convert data held in electronic health records into information and knowledge that helps them achieve their objectives. There are several challenges to be overcome to enable this approach to be harnessed to support best practice in Transfusion Medicine and a pilot project has been undertaken at Central Manchester University Hospitals (CMFT) to address these.
Aims
To develop a transfusion dataset that links data on individual patients in the Patient Administration System (PAS) with the information held about the transfusion episode and to explore the utility of the dataset to support projects in Patient Blood Management (PBM) and Inventory Management
Methods
The Informatics and l Transfusion Teams collaborated to develop a transfusion dataset. The Trust is a large hospital with a full range of services and 1420 beds. In 2013, 21174 red cells and 5971 platelet units were issued. Trust computer systems, PAS (TorexR) and Blood Track (HaemoneticsR), were identified which contain information required to monitor blood usage and were linked together in the data warehouse to produce a range of reports. The data linkage was validated in test mode through preliminary analysis before application to the live data warehouse.
Results
A dataset has been developed which collects information about the transfusion episode (type of blood component, date and time of transfusion), patient demographics, specialty, and consultant, diagnostic information (healthcare resource group, primary diagnostic code (ICD‐10) and procedure code (OPCS4). In addition, data has been collected about transfusion alternatives from the anaesthetic electronic record, eg: use of tranexamic acid cell salvage. The data extracted from Blood Track also enables the monitoring of wastage of blood and the reasons for wastage. The dataset has proved to be a rich resource of easily accessible information. A variety of reports are available for the transfusion team and clinical users of blood through a ‘Central Intelligence’ portal on the Trust intranet site. Examples of data produced include: number of red cell and platelet units transfused per 1000 inpatient spells (48.2 and 17.1 units respectively in 2014/2015), transfusion rates for key operations, including cell salvage use and rate of use of tranexamic acid (see Figure 1). The data collected will be used to develop an accurate surgical blood order schedule amongst other projects.
Caption 1: Transfusion Rates and Tranexamic Acid Use in Key Surgical Procedures

Conclusions/Next steps
The transfusion database will be used to monitor the implementation of PBM and improved inventory management at CMFT. Key performance indicators will be developed. When the new electronic order comms system (SunquestR) is implemented, further information will be linked to the transfusion episode including the coded reason for and justification for transfusion, and the pre and post laboratory results. This will allow analysis of transfusion triggers as well as the development of decision support systems to drive best practice. The lessons learned from this project will be shared to support the development of a national transfusion database for England which will allow for benchmarking between hospitals and provide useful information for the blood supplier.
P‐731
Low Risk of Developing RHD Antibody: Five Year Experience of Using RHD Positive Platelets in RHD Negative Patients in a Tertiary Care Setting in India
Tiwari AKT , Dara RC , Arora DA , Aggarwal GA , Acharya DA , Singh GS , Raina VR
Medanta‐The Medicity, Gurgaon, India
Background
Platelet transfusion therapy has made significant contributions in several patient categories and many centres provide non‐ABO and Rh matched platelets due to prolific demand and limited availability. Though Rh antigens are not present on platelet surface but platelet concentrates may contain enough red cells leading to formation of anti‐RhD antibody in RhD negative recipient. Rh Immunoglobulins (RhIg) administration after RhD incompatible platelet transfusion is the suggested guideline to prevent RhD allo‐immunization but RhIg administration has problems of cost and availability especially in resource‐poor settings. It is in these very settings that Random Donor Platelet Concentrate (RDPC) is used more often than Single Donor Platelet Concentrate (SDPC). RDPC carry higher risk of allo‐immunization due to greater amount of RBCs in comparison to SDPC. At our institution platelets are transfused across the ABO and Rh blood group barrier without any RhIg immune‐prophylaxis.
Aim
Retrospective analyses of allo‐immunization after RhD incompatible platelet transfusion (RDPC and/or SDPC) in RhD negative patients during preceding 5 years.
Materials and methods
This study was conducted in Transfusion Medicine department of large tertiary care hospital in India from January 2010 to November 2014. Data was collected from Hospital Information System (HIS) for all RhD negative patients who received RhD positive platelets (RDPC and/or SDPC). Parameters included were age, gender, diagnosis, blood type, number of blood components transfused, blood group of component, transfusion date and date of antibody screen. RDPC were prepared from 450 ml of whole blood using SAGM triple blood bag system by Platelet Rich Plasma (PRP) method while SDPC were prepared on COMTEC (Fresenius Kabi) cell separator. Antibody screen was performed universally as an institution protocol for all the patients before initial transfusion and before all RBC transfusions using low ionic strength solution (LISS) based column agglutination technology and commercially available three cell reagent panel (R1wR1, R2R2 and rr phenotype, 0.8% Surgiscreen, Ortho Clinical Diagnostics). Positive antibody screening tests were followed by antibody identification using eleven‐cell identification panel (Ortho Clinical Diagnostics). Patients with positive antibody screen before first RhD incompatible platelet transfusion and who received RhD positive blood components (RBC/FFP) before the initial incompatible platelet transfusion were excluded. Naive patients who had neither pre‐existing antibody nor received Rh‐Incompatible platelets transfusion before admission to our hospital were included. In the patients included; only those who had repeat antibody screen at‐least 28 days after RhD incompatible platelet transfusion were considered.
Results
512 RhD negative patients received 2789 (2648 RDPCs and 141 SDPCs) platelet transfusions from RhD positive donors during the study period. Based on inclusion criteria only 332 (64.8%) RhD negative patients’ data was analyzed. Median follow up was 7 weeks (range=4‐22). Only three RhD‐negative patients developed anti‐RhD antibodies, two of them after receiving only RDPCs and one after receiving both RDPCs and SDPCs. None of patients receiving only SDPCs developed antibodies.
Conclusion
Issuing RhD positive platelets to RhD negative patients seems to be an acceptable practice since only 0.9% patients developed anti‐RhD antibody in this series. The risk is lower with SDPC as compared to RDPC.
P‐732A
A Randomized Controlled Trial for the Transfusion of Irradiated Red Blood Cell Units With a Potassium Adsorption Filter (PAF Study): Preliminary Results
Cid JC , Villegas VV , Velásquez CAV , Lozano ML ,
Hospital Clínic, Barcelona, Spain
Background
The transfusion of irradiated red blood cell (RBC) units with high amount of potassium (K+) causes concern about an increased risk of transfusion‐associated hyperkalemic cardiac arrest (TAHCA). To prevent K+ overload, a K+ adsorption filter (PAF; Kawasumi Laboratories, Inc., Tokyo, Japan) is available for use at bedside.
Aim
To evaluate the efficacy and safety of transfusing irradiated RBC units with PAF.
Methods
We designed a prospective, randomized, controlled, open, clinical trial (RCT) comparing the transfusion of irradiated RBC units with PAF or with standard blood infusion set (Transfusend 200‐micron filter; Sendal, Caceres, Spain). The efficacy and the safety of PAF was measured with the absolute change of haemoglobin (Hb) and the absolute change of K+ in patients’ blood samples collected before and after transfusing the irradiated RBC units. Patients were included if they were ≥18 years, received the transfusion of irradiated RBC units because of chronic anaemia, and gave informed consent. Patients were excluded if they were taking angiotensin‐converting enzyme inhibitor drugs. RBC units were irradiated on day +7 up to +14 days after WB collection, and received 25−50 Gy with a free‐standing irradiator with a caesium (137Cs) source. After irradiation, RBC units were stored at refrigerator until the day 14th after RBC irradiation, and were transfused to patients when required. The irradiated RBC units were transfused at a flow rate of 3−5 ml/min. One transfusion episode was considered as the transfusion of one or two irradiated RBC units with the same filter. We collected data regarding patient demographics, patient vital signs, laboratory data, and interval time until next RBC transfusion. We also collected data regarding irradiated RBC units (before and after filtration), and adverse events. We calculated to include 100 transfusion episodes (50 episodes transfused with the PAF, and 50 episodes transfused without the PAF) in order to have 80% power and a significance level of 10% of detecting a mean difference in the change of haemoglobin with or without the use of PAF of 10 g/L of haemoglobin. The comparison of the main outcomes of the two groups of the study was done with the unpaired t test, and the chi‐squared test, for quantitative and qualitative variables, respectively.
Results
Until now, 16 patients received 37 transfusion episodes with standard blood infusion set, and 13 patients received 32 transfusion episodes with PAF. The supernatant K+ in irradiated RBC units at the time of transfusion and after using PAF was 24.18 ± 1.99 mmol/L and 3.42 ± 2.91 mmol/L, respectively. The reduction of K+ levels with PAF was 94%±5%. In patients’ blood samples collected before and after transfusing the irradiated RBC units, the absolute change of Hb was 9.2 ± 8.9 g/L in the control group and 8.9 ± 6.0 g/L in the PAF group (P = 0.9). The absolute change of K+ was −0.06 ± 0.3 mmol/L in the control group and −0.08 ± 0.3 mmol/L in the PAF group (P = 0.8). Adverse events were not observed.
Conclusion
The transfusion of irradiated RBC units with the PAF was efficacious and safe. RCTs in at‐risk patients to suffer TAHCA are encouraged.
P‐732
Red Cell Usage in The North of England in 2014
Tinegate HN1, Babra P 1, Grant-Casey J 1, Hopkinson C 1, Hyare J 2, Murphy M 1, Pendry K 1, Rowley M 1, Seeney F 1, Watson D 1, Wallis J 3
1NHS Blood and Transplant, Newcastle upon Tyne, United Kingdom2University Hospitals, Leicester, Leicester, United Kingdom3Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background
Three published studies of red cell usage in the North of England (population approximately 3 million), were performed in 1999‐2000, 2004 and 2009. During this time, regional red cell use fell from 45.5 to 31 per 1000 population. This was mostly due to a progressive reduction in surgical use, particularly in elective surgery in the 50 to 80 age group. In 2014, an updated version of the survey was repeated in the whole of England and North Wales, where hospitals are supplied with blood by NHS Blood and Transplant (NHSBT). The intention was to provide baseline data prior to implementation of Patient Blood Management (PBM) and to indicate where PBM initiatives should be focused. Within this survey, data were analysed separately from 15 of the 16 Northern Region hospitals that contributed to the three previous surveys.
Aims
To obtain baseline data prior to implementation of PBM, to determine whether trends identified in previous surveys have continued, and to compare regional and national data
Methods
For each unit of red cells transfused, during two separate weeks (in February and May 2014), the gender and age of recipient, and clinical category (based on previous Northern red cell surveys) was recorded.
Results
Data were received on 3570 red cell units in the Northern region, and 46111 units nationally. This represents approximately 94% of red cells issued in the North during the study period, compared to 75% nationally. For the North, the median age of recipient was 70 years, and 55% of recipients were male. The majority of units (64.5%) were transfused for a medical indication, compared to 67% nationally. The percentage for the North was unchanged from 2009 figures, although absolute numbers of units transfused in all categories continue to fall. Surgical use accounted for 30% of red cells, and obstetrics/gynaecology for 5.5%. Red cell usage in the North was similar in most categories to national data, except in sickle cell disease (2 units, 0.1% for the North, 1350 units, 2.9% for England and North Wales as a whole)
Summary/conclusions
Regional and national data concur for all but one clinical category. Regional trends since 1999 explain why issue data from NHSBT show a national reduction in red cell usage of 23% in the period from 1999 to 2014. The one discrepancy between regional and national figures was in usage for sickle cell disease.
P‐733
Abstract Withdrawn.
P‐734
Transfusion Therapy Practices Among Brazilian Intensive Care Nurses: A Descriptive Survey
Pedreira MP , Isabela IS , Amato IA , Kusahara DK , Avelar AA , Mendes MTM , Peterlini MAP
Federal University of Sao Paulo, Sao Paulo, Brazil
Background
Transfusion therapy is a complex intervention requiring a multidisciplinary approach. The complexity and implicit risks have been largely studied demonstrating the relevance of evidenced‐based interventions and regular monitoring of performance standards. The assessment of critical care nursing transfusion practices can provide data capable of improving transfusion safety in critically ill patients.
Aims
To describe intensive care nurses (ICN) practices during transfusion therapy.
Methods
A survey was elaborated to explore aspects of ICN practices and experiences before, during and after the implementation of transfusion therapies in critical care patients. An instrument developed by the researchers was submitted to validation by experts through Delphi Technique. The survey has 68 affirmatives, and answers were measured by the Likert scale: always, sometimes, never and do not know. The instrument was inserted into an online database program and sent by email to 487 ICN, from neonatal, pediatric and adult intensive care units (ICU) across different regions of Brazil. Collected data was analyzed using mean, standard deviation, absolute and relative frequencies.
Results
The sample was composed by 72 (14.8%) nurses. The majority (95.8%) was female, average of 36.2(±8.4) years of age and 61.1% were clinical nurses, with 12.9(±8.4) years of experience in nursing, and 79.1% had formal learning on blood transfusion. The results demonstrated that indication for transfusion therapy is a physician's (88.8%) decision rather than multidisciplinary (8.3%) or protocol based (40.2%). In ICU the informed consent is obtained sometimes (23.6%) or never (44.4%) with the patient or family members. The double check (patient and blood product) is completed for 66.6%. Only 33.3% describe that always use an exclusive peripheral intravenous catheter to red blood cells (RBC) administration; central venous lines are employed sometimes (90.2%); peripherally inserted central catheters are never used according to 69.4%. The dilution of RBC with saline solution was described as a practice never (76.3%) or sometime done (13.8%). To RBC administration syringe infusion pumps and peristaltic infusion pumps are employed always or sometimes according to 18.4% and 18.0% of the ICN, respectively. Platelets and cryoprecipitate are infused always (69.4%) or sometimes (16.6%) immediately after thawing. Fresh frozen plasma is always (79.1%) infused as soon as possible, no more than 6 h after defrosting. Always (69.4%) and sometimes (29.1%) ICN check temperature, peripheral perfusion, respiratory and cardiac rate, before the procedure. In the ICU, sometimes for 51.3% of the ICN and never for 31.9%, there is enough number of nurses to allow staying with the patient at least within the first 15 min of transfusion. When transfusion reactions are identified the majority of nurses (93.0%) stops the infusion and calls the physician while verifying vital signs. According to ICN sometimes transfusion reaction occurs during RBC (73.6%), platelets (43.0%) and fresh frozen plasma (37.5%) infusion.
Conclusions
There was a wide variation of practices performed by Brazilian ICN during transfusion therapy due to organizational characteristics, knowledge based gaps and patient clinical needs.
Acknowledgement
Grant for research CNPq (474906/2013‐2) and CNPq researcher grant (303006/2012‐9)
P‐735
The Best Product for the Best Result: Packed Red Cells Without BC vs Red Cells From Apheresis
Dell'Aversana MR , Munno E , De Caprio G , Tomeo R , Chiriani P , Sagliano SIC , Misso S
Servizio Trasfusionale, Aversa, Italy
Background
The transfusion of red cell concentrates is shown to rapidly increase the supply of oxygen to the tissues, when the concentration of hemoglobin is low, in the presence of physiological compensation mechanisms inadequate. The tissue oxygenation depends of various factors: – Hb concentration; – Saturation of Hb, dependent on the voltage of oxygen and affinity of Hb for the oxygen ‐ volume of oxygen necessary to the tissues to perform their aerobic function. (Racc.SIMTI 2008) Considering these parameters becomes critical the choice of blood component transfused. At our SIT we produced packed red cells without BC and red cell from apheresis.
Aims
The aim of our study was to evaluate on a cluster of patients the effectiveness / efficiency transfusion of both products and any adverse events.
Methods
We selected 20 patients with myelodisplasia, whose diagnosis was made in 2010, and after failure of drug therapy with erythropoietin in 2012 have started to transfuse regularly (±every 15 days). This patients from January 2013 are transfused in randomly with packed red cells without BC and red cell by apheresis, with a storage time of 10 days and a compatibility in addition to the system ABO, Rh and Kell even for the system Duffy and Kidd. All patients were performed the following tests: – Pre transfusion Hb (Sysmex) – Post transfusion Hb (1 h and 24 h) (Sysmex) – Ferritin (every 15 days) – Blood group ABO Rh and Kell (Biorad) – Direct and Indirect Coombs tests (Biorad) – Phenotype erythrocyte extended in molecular biology (Bioarray BeadChip Immucor)
Results
See table. All patients at the end of the evaluation have direct and indirect test coombs negative.
Conclusions
The results show that no patient has been immunized because all were transfused with red blood cell with compatible phenotype. The perfect cross match improve the effectiveness transfusion as already seen in previous work reduces the risk of immunization. (Come migliorare la gestione terapeutica nelle MSD Blood transfusion abstract session 2015 in press). The frequency transfusion is slightly vary between administration of red cells from apheresis, and of red cells without BC, as well as the values “�”�of Hb post‐transfusion. Packed red blood cells from apheresis being a product with standard values of Hb, Hct and Wbc, as demonstrated by the quality controls carried out periodically (Hb: 63.2 ± 2 g / unit; Hct: 58.5 ± 1%), have a better efficacy transfusion compared to red blood cells from whole blood that have a greater variability in the quality parameters (Hb: 49.6 ± 3 g / unit Hct: 55.4 ± 3%). From the quality parameters obtained we would have expected better effectiveness and efficiency with RBCs from apheresis, although about 6 transfusions in less a year can be considered in order of the quality of life of the patient a good result
P‐736
Audit of the Use of Prothrombin Complex Concentrate for the Emergency Reversal of Warfarin
Smith NA1, Fenton M 2, Lumley M 1
1Heart of England NHS Foundation Trust, Birmingham, UK2College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK
Background
Patients on long‐term Vitamin K antagonist (VKA) therapy have major life‐threatening bleeding occurring in 2‐7% per year, with a 0.5 – 1% annual risk of fatal bleeding1.
Prothrombin Complex Concentrate (PCC) is a fractionated human plasma derived blood product containing coagulation factors II, VII, IX, X and proteins C and S. It rapidly corrects high INR's in over anti‐coagulated patients2. A 4‐factor PCC is recommended for VKA reversal in patients with a raised INR (>2.0) and major bleeding or require immediate surgery3. PCC is giving at 25‐50 units/kg slow iv (INR‐dependent) with Vitamin K4. PCC is very effective but associated with thrombotic complications.
Aim
To verify the continued appropriate, safe and accurate use of PCC in major bleeding.
Methods
A retrospective audit of PCC use was conducted over an 11 month period at Good Hope Hospital. PCC issues were gathered from the blood bank IT system and patient and infusion data acquired from electronic records. The audit standards from current trust guidelines on VKA reversal are based on the British Committee for Standards in Haematology guidelines.3, 4
Results
52 PCC recipients were identified, 61.5% were male, with a mean age of 76.6 years (80.8% ≥70; 50.0% ≥80). 65.4% of patients were anti‐coagulated for stroke prevention in atrial fibrillation and 55.8% had been treated for ≥ 3 years.
Only 5/52 (9.6%) of forms were returned. Pre PPC INRs were 2‐4 in 20/52, 4‐10 in 21 and >10 in 9 (unrecorded in 2). A mean dose of PCC was 1640 IU with only 36% of doses consider appropriate on re‐assessment with all other patients assessed retrospectively as underdosed, 47% receiving at least 500 iu less that indicated.
Post PCC INR was checked in 48/52 (92.3%) but only 5/52 (9.6%) were checked within 10 min. PCC reduced INR to 1.0 – 1.5 in 29/52, 1.6‐2.0 in 12/52 and > 2.0 in 7/52. 37/52 (84.6%) received Vitamin K.
14/52 (26.9%) died within 30 days of PCC administration. A thrombotic complication of PCC was recorded in 3/52 (5.8%).
There was a considerable variation between timings for recognition of need, request and administration of PCC with mean recognition to administration of 256 min (range 60‐978), with major part of delay inbetween recognition of bleeding and requesting PCC (Mean 220 min).
The mean previous percentage of INR tests in time of all PCC recipients was 51.8% which is lower than current targets.
Summary/Conclusions
Many patients did not achieve full INR reversal due to underdosing of PCC. There were major variations and delays in the PCC administration pathway mainly due to delay in recognition of major bleeding. Clinician re‐education about PCC may improve compliance.
The mortality rate was similar to previous audits, but higher than published data of 10.6%.1 Patients with major bleeding have lower mean INR tests in therapeutic range values.
References
1. Thromb Haemost 2011; 106: 429–438.
2. Thromb Haemost; 1997; 77: 477–480.
3. Br J Haematol; 2011; 154(3): 311–24.
4. Heart of England NHS Foundation Trust: Reversal of anti‐coagulation guideline
P‐737
Abstract Withdrawn.
P‐738
Certain Causes of Post‐Transfusion Hemolytic Complications
Bakhovadinov B1, Hakberdiev R 2, Kucher M 3, Hodjiev A 2, Tretyakova A 4
1St. Petersburg State Medical Institute, St. Petersburg, Russian Federation2Republican Scientific Blood Centre, Dushanbe, Tajikistan3St. Petersburg Medical Institute, St. Petersburg, Russian Federation4St. Peterburg Medical Institute, St. Petersburg, Russian Federation
Post‐transfusion hemolytic complications (PTHC) remain a significant problem.
Goal
To analyze the reasons for cases of PTHC that have been registered in the medical and therapeutic institutions of the Republic of Tajikistan.
Methods
A retrospective analysis has been conducted on materials from the Republican‐level and regional commissions in charge of investigating cases of PTHC; patient records from medical institutions in the Republic of Tajikistan have also been considered. During the period of 1989‐2014 a total of 86 cases of PTHC were recorded from a total of 840,000 doses of erythrocyte transfusions.
Discussion
The analysis demonstrated the following causes of PTHC: blood group incompatibility as per the ABO ‐ 32 system (in 37.2% of cases); as related to blood group antibody D on the Rh ‐ 34 system (39.53% of cases); as related to minor blood group antigens on the Rh and Kell (C,c,E,e,K) – 16 blood group systems (18.6%); and four (4) cases involving the transfusion of haemolysed erythrocyte‐containing mediums (4.6%). In 78 out of 86 cases (90.7%), the complications occurred as the result of errors committed by the doctors of the medical institutions, while in the remaining 8 cases (9.31%) errors were made by the medical staff of the blood service. Complications occurred as the result of doctors’ failure to adhere to the standards for conducting immunological investigations and the clinical use of blood components, including in connection with the doctors’ insufficient level of knowledge and the lack of equipment and technical conditions necessary for conducting pre‐transfusion testing in hospitals. In 34 cases patients’ D‐negative status was misclassified as D‐positive, while donors’ D‐positive status was misclassified as D‐negative. A total of 45 cases of PTHC developed among pregnant gynecological patients as a result of the incompatibility of their pregnancy and transfusions. In seven (7) cases, doctors did not even test patients’ blood groups, instead relying on the information in their passports. Tests were also not conducted on possible isoimmune antibodies to the erythrocyte antigens. Moreover, testing was not done in relation to patients’ compatibility with sensitive methods in accordance with the WHO standards for isoserological investigations.
Conclusions
Decreasing the PTHC was aided by training the relevant doctors and nurses in transfusion and implementing modern methods of phenotyping for donors’ and patients’ erythrocyte antigens as per the main antigens relevant to transfusions; this includes using the blood group system ABO by way of direct and indirect methods, as well as the Rh system (C,c, E, e) and Kell sytem (K) for patients in need of multiple transfusions and women of fertile age. A further step is to identify isoimmune antibodies and sensitive methods, and in the case of positive screening results, identifying the relevant antibodies. If a patient is identified as having clinically significant antibodies, then compatible erythrocytes must be chosen with reference to the particular antibodies. To guarantee the constant use of the antiglobulin‐mediated agglutination test when testing for compatibility between donor erythrocytes and patient serum with immunological antibodies.
P‐739
Plateletapheresis Transfusion in Dengue Patients: Evaluation of Optimum Utilization is Need of the Hour
Mathur A , Dontula S , Adulkar D , Jagannathan L
Rotary Bangalore TTK Blood Bank, Bangalore, India
Background
Dengue fever has emerged as a major public health problem in tropical and subtropical regions across the world including India. The practice of platelet transfusion has been adapted into the standard clinical practice in management of hospitalized dengue patients. Appropriate use of blood is required to ensure the availability of blood for patients in whom it is really indicated as well as to avoid unnecessary exposure of the patients to the risk of transfusion reactions and transmission of blood borne infection.
In India thousands of patients suffer from Dengue & many receive platelet transfusion but the clinical outcome is not widely evaluated. There is no defined transfusion protocol to follow by trnasfusionists. In the present study we compare two groups of thrombocytopenic patients including Dengue & analyzed the clinical out come.
Objective
To analyze the clinical outcome of platelet transfusion in Dengue patients & other thombocytopenic patients.
Material and methods
The study conducted at Reginal Blood Transfusion center of South India from July 2013 to December 2013. Total 110 thrombocytopenic cases were included. Among these patients, 65 patients were diagnosed as Dengue & remaining 45 had other clinical diagnosis like leukemia, aplastic anemia, cardiac surgery, neuro surgery & non hematological malignancies. All the pateints are tested for pre & post transfusion platelet counts. The plateletpheresis were done on Amicus cell separator & using single are procedure kits. The pre platelet count of the donor ranged from 186000 to 371000 per microL with the mean value 266000 per microL. The whole blood volume processed ranged from 1770 to 2315 ml with mean value 2164 ml.
Results
Out of 65 Dengue patients only 7 showed post transfusion platelet increment >15000/ microL & 58 patients had increment <15000/ microL.
Comparing to the other group, 34 out of 45 patients showed increment >15000/ microL & only 11 pateints had increment <15000/ microL.
The Chi square is 47.73 & P value is 0.001 which is highly significant.
Conclusion
The patients of dengue showed significantly less increment from one unit of SDP as compare to patients of other thromobocytopenic conditions. It is observed that in Dengue cases use of SDP has very limited therapeutic benefit. Therefore in the resourse constrain countries like India with high endemicity for Dengue there is a need to evaluate the role of platelet transfusion.
This study strongly recommend to have a transfusion protocol for dengue patients to save scare blood resurce & minimize infection exposure to patients
Post transfusion platelet increment has strong correlation with pre procedure donor platelet count & WB volume processed. It was observed that both these parameters have significant impact on patient platelet increment.
P‐740
The Sickle Cell Disease Environment Affects Old Stored Red Blood Cells: Consequences for Blood Transfusion
De Menorval MA1, Chadebech P 2, Bodivit G 2, Mekontso-Dessap A 3, Pakdaman S 2, Jouard A 2, Galacteros F 4, Bierling P 2, Habibi A 4, Pirenne F 2
1EFS ‐ IMRB U955, Creteil, France2EFS ‐ IMRB U955 ‐ UPEC ‐ GR‐Ex, Creteil, France3Service de réanimation médicale, Hopital Henri Mondor, AP‐HP, UPEC, Creteil, France4Centre de Référence des Syndromes Drépanocytaires Majeurs, AP‐HP, GR‐Ex, Creteil, France
Background
Transfusion of packed red blood cells (pRBC) is a life‐sustaining therapy in sickle cell disease (SCD). Besides improving oxygen delivery to tissues, the goal of transfusion is to reduce the percentage of circulating red blood cells (RBCs) with abnormal HbS. However, in some circumstances, pRBC transfusion can lead to severe post hemolytic transfusion reactions. In SCD, the age of blood products is not considered. However, adverse clinical consequences of transfusing old pRBCs have been highlighted when given to acute patients with the suggestion that storage lesions could be linked to these adverse effects. Storage lesions include metabolic damages such as 2‐3 DPG depletion, decrease in ATP concentration and pH, but also morphologic damages such as microparticle emission, loss of deformability, modification of the membrane.
Aims
In SCD, it is therefore of high interest to determine whether the length of RBC storage depending of the patient condition (steady or acute state) might have an impact on the life span and adverse effects of transfused pRBCs. In this work, we explored in vitro whether old stored pRBCs compared to fresh stored pRBCs could suffer from the SCD patient environment depending on his clinical condition. We also look to the storage parameters and effects on frozen RBC.
Methods
Plasmas were obtained from healthy donors and SCD patients in different clinical conditions: vaso‐occlusive crisis and/or acute chest syndrome, steady state patient with a pRBC transfusion protocol. Externalization of phosphatidylserine (PS), membrane markers expression linked to adhesion and complement inhibition and cell size were evaluated on stored RBCs obtained from packed units at day 3‐8, 14‐19, 25‐30 and 38‐42. The same parameters were evaluated after co‐incubation of stored pRBCs sampled at day 3‐8 and day 38‐42, and thawed pRBCs, with the different plasmas (donors and patients).
Results
During storage, there was no modification of the tested parameters, except a decrease of pH. After co‐incubation, we observed that the plasmas of SCD acute patients induced more damages (PS externalization, decrease in size) to RBCs than healthy donors and stable patient plasmas. Data also revealed that old RBCs were more sensitive to plasma co‐incubation than RBCs at the beginning of the storage and that toxicity was even higher when pRBCs were exposed to acute patient plasmas. Results obtained for thawed RBCs were similar to those obtained for young pRBCs (3‐8 days) whereas old RBCs were dramatically more sensitive to the co‐incubation.
Conclusions
These results bring evidence that the plasma of the SCD patients have a deleterious impact on pRBCs that could have in vivo consequences in term of survival and post‐transfusion hemolysis. This toxic effect is predominant when exposed pRBCs are older, and when the patient condition is acute. It is likely that in SCD, transfusion of young pRBCs will have a benefice. However, epidemiologic data regarding correlation between transfusion reaction and age of the units are needed to determine whether the age of RBC unit has to be taken into account, especially in severe acute SCD patients.
P‐741
The Risk of FNHTR and Other Transfusion Reactions in Recipients of Non‐Leukodepleted Platelet Concentrate Transfusion in Sardjito Hospital Yogyakarta Indonesia
Triyono TT1, Andayaningsih T 2
1Faculty of medicine,Gadjah Mada University, Yogyakarta, Indonesia2Sardjito Hospital/ Faculty of Medicine, Yogyakarta, Indonesia
Background
There is an increasing platelet transfusions for treatment and prophylaxis of bleeding in patients with hematologic disorders and malignancies. Because of limited resources, leukoreduced platelet concentrates is not yet implemented in most Indonesian hospitals. The high frequent of using platelet transfusions are followed by increased risk of transfusion reactions. Febrile non‐hemolytic transfusion reaction (FNHTR) is an acute transfusion reactions most often occur in the platelet concentrate (PC) transfusions. The incidence of FNHTR is associated with the presence of pyrogenic cytokines such as: interleukin‐1 (IL‐1), interleukin‐6 (IL‐6) and tumor necrosis factor‐a (TNF‐a) accumulation in PC products. Accumulation of these cytokines is associated with the presence of leukocytes in the product and the duration of storage before transfusion.
Aim
This study aimed to observe the relative risk of FNHTR and other transfusion reactions in transfusion of non leukodepleted compared to pre storage leukodepleted PC.
Methods
This was an observational study with prospective cohort design. Subjects were children aged 1 ‐ 18 years with indication of platelet transfusions in the children's ward Dr. Sardjito Hospital and met the inclusion and exclusion criteria. There were 54 patients who were devided into two groups. Twenty seven subjects recieved non‐leukodepleted PC and the other twenty seven were transfused by pre storage leukodepleted PC. Both groups were measured for axilar temperature every 30 min shortly before transfused until 2 h post transfusion. Positive for FNHTR was determined if subjects showed rising 10C or more of his/her body temperature without any other clinical signs or laboratory test results of another acute transfusion reaction such as Transfusion Related Acute Lung Injury (TRALI), allergic reaction, acute hemolytic, or acute bacterial infection. Statistical analysis was performed for the relative risk by determining the significant limit of P value < 0.05 with 95% confidence interval.
Results
We obtained the relative risk of FNHTR on non‐leukodepleted PC was 4.85 (P < 0.001) and 95% CI (2.23‐10.5). Urticaria were obtained 10.2% and 5.2% in non‐leukodepleted and leukodepleted PCs transfusion respectively. Erythema were observed 10.1% non‐leukodepleted and 9% leukodepleted PC transfusions.
Conclusion
There was a significant result by 4.85 for the relative risk of FNHTR on non‐leukodepleted PC. Other transfusion reaction i.e. urticaria and erythema were also observed.
P‐742
RH and Kell Antigens Blood Typing and Antigen‐Matching Protocol: Before and After
Spínola AI1, Sampaio M 2, Machado E 2, Ponte S 2, Oliveira F 2, Silva A 2, Couto M 2, Jorge C 2, Bini-Antunes M 2, Amil M 2
1Centro Hospitalar do Porto, E.P.E., Porto, Portugal2Hematology Department; Centro Hospitalar do Porto, E.P.E, Porto, Portugal
Background
Patients receiving multiple red blood cells(RBC) transfusions have increased risk of alloimunization. A Rh and Kell phenotyping protocol(RhKP) was implemented, in October 2010, for multitransfused patients attending the day‐care unit.
Aim
Compare prevalence of Rh and/or Kell(Rh/Kell) alloantibodies(alloAb) and transfusions requirements before and after alloimunization and RhKP. Assess RhKP compliance.
Methods
From 01/04/2010 to 31/09/2010(pre‐RhKP) and 01/04/2014 to 31/09/2014(post‐RhKP) transfusion requests for patients with ³5 previous transfusion episodes(TE)/year were evaluated. Prevalence of Rh/Kell alloAb and transfusion requirements before and after RhKP were determined. Excluded autoantibodies and alloAb against non‐Rh/Kell antigens. Statistical analysis was performed using non‐parametric Mann‐Whitney and Chi‐square test, P value <0.05 was significant.
Results
113 patients were evaluated (78 pre‐RhKP; 35 additional cases post‐RhKP), median(range) age 72 years (15‐96), with the following diagnosis: myelodysplastic syndrome 33, acute leukemia 15, chronic symptomatic anemia 12, chronic myeloproliferative disorders 10, chronic lymphoproliferative disorders 8, other anemia 8, hemorrhagic disorders 7, solid malignancy 6, multiple myeloma 5, hemoglobinopathies 3 and other diagnosis 6. Median number of TE/patient/year pre‐RhKP: 19.8(2‐117); and 17.3(5‐60) in the 35 newly transfused. Median of 2 units/TE.
Pre‐RhKP, Rh/Kell alloAb were detected in 20 out of 78 patients (25.6%); 12 had ³2 Rh/kell alloAb. Alloimmunization occurred after the 10th TE in 10 cases. Alloantibodies frequencies were: anti‐D 12, anti‐C 10, anti‐E 8, anti‐Kell 8, anti‐Cw 1 and anti‐c 1. Alloanti‐D was formed after platelet pool RhD+ in 4 patients, RBC RhD+ units in 3 and following RBC from donors with anti‐D in one. Five patients had no identifiable reason for alloanti‐D; 4 were women, median age of 75 years(42‐82).
Post‐RhKP, 6 patients alloimunized: 4 from the 35 new cases and 2 patients from the pre‐RhKP group. Of these 2 patients: one developed anti‐Kell (Kell+ RBC unit erroneously administrated) and other developed anti‐D probably due to a passive anti‐D from residual plasma in the RBC unit. Concerning the 4 new post‐RhKP cases: 2 formed anti‐Kell, 1 anti‐Kell+anti‐E and 1 anti‐E+anti‐c. RhKP was not compelled in 3 patients. One patient despite having transfused Rh/Kell antigen‐matching RBC, received 4 platelet pools in which Kell+ and E+ donors were included. Overall, alloimmunization prevalence was 23.9%(27/113).
Regarding the two periods analyzed, Rh/Kell alloAb prevalence was statistically different (P < 0.0001). However, transfusion requirements were not(P = 0.081). TE with one RBC unit increased (25.5% vs 29.4%, P < 0.0001), median interval between transfusions went from 19 to 14 days (P < 0.0001) and median pre‐transfusional hemoglobin levels from 7.9 to 7.6 g/dL (P < 0.0001). Four patients had additional adverse reactions: 2 volume overload; 1 febrile nonhemolytic reaction and 1 allergic reaction.
Conclusions
Providing compatible Rh/Kell units decreases the incidence of alloAb. RhKP was not always accomplished, mainly due to shortage of blood supplies. Women transfused with RhD incompatible units were over 41 years old and had no conditions of getting pregnant. Anti‐Kell and anti‐E found in one patient could be justified, as those antibodies are frequently non‐RBC stimulated antibodies. Shortening period of transfusion requirements was not related with alloimunization but with restrictive transfusion practices and disease progression.
P‐743
Managing a Case of Life‐Threatening Autoimmune Haemolytic Anaemia
Verma A1, Singh G2
1Pushpanjali Crosslay Hospital, Ghaziabad, India2Indraprastha Apollo Hospital, Ghaziabad, India
Background
An autoimmune response occurs when antibodies against an individual's own red blood cells (RBCs) are present. Most autoantibodies react with high‐incidence RBC antigens. Consequently, posttransfusion, they might agglutinate, sensitize or lyse RBCs. RBC survival might be shortened by these humoral antibodies and routine serologic detection is therefore important. Antibodies against Rh‐antigens, including anti‐e, are most commonly implicated in warm autoimmune haemolytic anaemia (AIHA).
Aims
The aim of this case study is to show whether haemoglobin is increased in a patient with AIHA, even when incompatible e‐positive RBC‐units are transfused to patients with anti‐e autoantibodies.
Case description
A 56 year old male was admitted with a 7‐day history of cough, associated with progressive breathing difficulties, fever, general weakness and a history of hypertension and smoking. The patient was found pallor, conscious and afebrile, without any focal neurological or sensory deficits. His pulse rate was measured 130/min, blood pressure 120/80 mmHg. Icterus, pedal edema and cyanosis were not visible. Chest and Cardiovascular examination was found normal and no organomegaly was observed. Also, no history was found of either drug intake or blood transfusion.
Results
Patient's Haemoglobin level was 2.8 g/dl, TLC 14300/lL and patient presented with neutrophilia. Platelet count was found 272,000/lL, PCV 9 and ESR 9 mm/h. Liver function tests showed a 3.4 total, direct 1.1 and indirect bilirubin concentration of 2.3 mg/dl respectively. Reticulocyte count was increased by 18%. Peripheral blood smear showed macrocytosis, serum LDH was found high (516.64 IU/l) while serum haptoglobin low (30 mg/dl). Cryoglobulins, cold agglutinins, ANA, ANCA, MPO, PR3 and anti‐phospholipid antibodies were not measured.
Serology studies showed no evidence of thrombocytopenic purpura and paroxysmal nocturnal haemoglobinuria. Concentration of Vitamin B12 was found >2000 IU/l, Folic acid 20 IU/l, Serum Iron 242 IU/l, TIBC 297 IU/l and Serum Ferritin 2000 IU/l. The patient was blood‐typed as O, Rh‐negative with strong (4+) pan‐agglutinin. DAT showed anti‐IgG and anti C3 positive and anti‐e was identified as autoantibody.
Therefore, final diagnosis was primary AIHA and negative compatible RBC units were not available. Along with the common treatments including antibiotics, Antacids, analgesics, injectable vitamin B12 and folic acid, 2 units of least incompatible anti‐e‐positive RBCs were transfused on day 1 and patient's haemoglobin raised from 2.8 to 3.6 g/dl. On day 3, Solumedrol 1 g was given by IV for 5 days. Additionally, 2 units of RBCs were given and haemoglobin further increased to 4.1 g/dl. Gradually, patient's condition improved, without breathing difficulties. Furthermore, two additional least incompatible e‐positive RBCs were given on day 4 and haemoglobin raised to 6.6 g/dl on day 5.
Conclusions
AIHA‐therapy is generally aimed at first treating the underlying disease if present. General measures to support cardiovascular function, are important for patients who are severly anaemic. Transfusions should not be avoided in situations of life threatening anaemia and least incompatible units can therefore be given. Thus, in the present primary AIHA case, by conservative treatment along with immunosuppressive and packed RBC transfusion, the patient was managed successfully.
P‐744
The Number and Types of Transfusion Reactions at the Institute for Transfusion Medicine Banjaluka From 2009 to 2015
Cejic CV , Jukic JB , Udovcic UD , Guzijan GG
Institute for transfusion medicine, Banja Luka, Bosnia‐Herzegovina
Introduction
Blood elements treatment (chemotherapy) is usually efficient treatment which is also safe for the patient. Though, chemotherapy can have unpleasant transfusion reactions and complications.
Goal
To show the total number of transfusion reactions from 2009 to 2015 at the Institute for transfusion medicine Banjaluka regarding the number of issued blood units in the same period.
Material and methods
We completed a retrospective analysis of data on registered transfusion reactions for the period given. The main source of data is the registration forms of transfunsion reactions filled in by the nurse. After the immunohematological analyses before and after posttransfusion sample, the transfusiologist makes decision on the type and the couse of transfusion reaction.
Results
During this period (2009 ‐ 2015) 124 transfusion reactions have been reported, i.e. 0.16% concerning the total ammount of issued blood units. From this number, 74 (59,7%) transfusion reactions were caused by applied erythrocyte preparation, and 50 (40.3%) developed after the use of frozen plasma. From the total number of transfusion reactions most of them were allergic ‐69 (55.6%), febrile non‐chemolysis transfusion reactions developed in 47 cases (37.9%), overcharged circulation in 3 cases (2.41%), 4 registered without symptoms (3,22%),and only 1 late chemolysis reaction (0.8%). During that period 78.299 units of blood were used, and from that nubmer, 0.15% led to unpleasent reactions and 65.240 units of fresh frozen plasma which caused allergic reactions in 0.19% cases.
Conclusion
Comparing our results with the results from the literature, in which the procentage of registered transfusion reactions is much bigger, we can conclude that we still have the problem of insufficient registering and unidentified rare forms of transfusion reactions by the doctors. Registration of unpleasent reactions during and after the blood transfusion as well as analyse of collected data should be obligatory and the impotrant link of modern transfusion treatment.
Key words
transfusion reactions, blood elements
P‐745
Evaluation of the Platelet Cross Matching in Oncology Patients
Mangwana SM , Arya D , Sharma SS
Sri Balaji Action Medical Institute, New Delhi, India
Background
Platelet transfusion is a critical and an essential part of managing cancer. Refractoriness to platelet transfusion is a complex process and poses a great challenge in the treatment of thrombocytopenic patients. Although advances have been made in the diagnosis and treatment of immune‐mediated platelet refractoriness, non‐immune causes, such as sepsis, remain problematic.
Aim
The prospective study was planned to correlate and evaluate the result of the platelet cross matching with the post transfusion count increment and to ascertain the effectiveness of routinely performing Platelet cross matching in Indian perspective as there is little evidence in India.
Methods
Study was performed from October 2014 to January 2015 on 30 oncology patientsreceiving multiple platelet transfusions. The pre transfusion platelet count of the patient was ascertained.ABO Compatiblewhole blood derived random donor platelets (RDP) were randomly transfused.Blood samples were collected within 1‐h of completing platelet transfusion for corrected count increment (CCI). Platelet cross‐match was performed using Solid‐phase red‐cell adherence (SPRCA) techniques. A Chi square test was used to correlate results of the cross match with the post transfusion counts.
Results
30 Oncology patients were given 142 units of ABO CompatibleRDPs within 72 h of collection. 18 patients (60%) were males while 12 patients(40%) females with a mean age of 46.87 ± 15.16 years. 18 of 30 patients were having solid organ malignancies, primarily epithelial with 12 patients having hematological malignancies. 135 of 142 units (95%) were negative platelet cross matched while 7 units (5%) were positive platelet cross match.Mean platelet dose given was 2.22 ± 1.88 x 1011/l. Mean CCI was 28,927 ± 23,007 which was more and statistically significant in females (36,865 ± 26,695) than in males (23,634 ± 19,176). 4 cases (13.33%) showed platelet refractoriness with CCI <5000, out of which 3 cases were of leukemia, 25% of hematological malignancies. All leukemia case were males.
Summary/Conclusion
Platelet transfusion therapy is life saving for oncology patients but platelet refractoriness always poses challenge due to allo‐ immunization to HLA and HPA antigens.Platelet cross match simplifies the selection of compatible platelet units. In this study, patients receiving cross‐matched products showed good increment.4 patients(13.33%) showed platelet refractoriness, more in hematological malignancies (25%) which could be due to HPA antigens.Platelet cross match using SPRCA is an effective and rapid first‐line approach for selecting compatible platelets as compared to HLA matched platelets in the treatment of thrombocytopenic cancer patients. Further study is required to conclude and correlate the platelet compatibility with refractoriness. Platelet cross‐match along with testing for anti‐platelet antibodies should be an important component in the management of oncology patients which is less time‐consuming and cost‐effective than the molecular testing.
P‐746
Preoperative Coagulation Tests in Patients Who had Undergone Liver Resection and Consuption of Blood Products During and 7 days After Surrgery
Skoko M , Culej JC , Banovic MB , Dodig JD , Sovic DS , Jularic AJ , Mihic Lasan IML , Stefic AS , Vrdoljak DVV , Vucemilo TV
University Hospital Center Sisters of Charity, Zagreb, Croatia
Background
The patient undergoing liver resection should be suitable for a general anaesthesia and a potentially haemorrhagic surgery. Metastases and resection lead to inpared function of the liver, reduced synthesis of cloting factors, but also reduced synthesis of inhibitors of hemostasis. The role of transfusion practicioner in University Hospital for Tumors is to take bleeding history and evaluate coagulation status. Preoperative coagulation testing include basic coagulation tests ‐ BCT (PT, APTT, fibrinogen and TT) or additional coagulation tests ‐ ACT (PT, APTT, fibrinogen, TT, ATIII, plasminogen, D‐dimers and protein C).
Aims
Can additional coagulation tests be helpful in assessment bleeding risk during and three days after surgery?
Methods
Twelve patients underwent preoperative coagulation testing which included BCT and ACT. All tests were done on Simens BCSXP analyzer with reagents from the same manufacturer.
Results
One of twelve patients had changes in BCT and ACT (prolonged APTT, reduced levels of ATIII, PC and plasminogen, increased value of D‐dimers) without the need for substitution therapy and without clinically evident perioperative bleeding. ACT showed increased value of D‐dimers in four patients, and reduced level of protein C in one patient who required 2 doses of fresh frozen plasma postoperatively because of the clinically significant disorders of hemostasis and bleeding. One patient received one dose of PRBC (packed red blood cells) with normal preoperative BCT and ACT for the correction of hemoglobin level.
Conclusion
Patient whose tests showed prolonged APTT during and postoperatively had no need for substitution therapy and no clinically evident bleeding. In our study additional testing was not useful in assessment of bleeding risk and consumption of blood products. Reduced level of protein C indicates thrombophilia. Since patients with liver metastases undergoing liver resection are complex there is a need for further study, on a larger number of patients, to determine how basic or additional coagulation tests can contribute to assessment of bleeding risk.
P‐747
Thinking Differently‐ Towards Better Clinical Decisions and Better Patient Blood Management Using the Simple ‘ABCDE’ Approach
Narayan S
Royal Oldham Hospital, as part of haematology rotation under Norht west deanery, Manchester, United Kingdom
Haemovigilance data and several studies highlight the lack of objective decision making that plagues many providers when it comes to the decision to transfuse. Rather than using arbitrary transfusion triggers or a single criterion to justify transfusion, multiple factors of the patient's status should be considered to reach a comprehensive evidence‐based transfusion decision. Patient blood management has already gained enormous momentum over the past several years and is now being universally adopted in hospitals across the country in the years to come. In addition this would help reduce the variation in clinical practice in seemingly similar populations. Shared decision making involving clinical teams and patients is an appropriate approach in making transfusion decisions in day to day clinical practice. We propose a novel, innovative and simplistic approach to promote better decision making and thus improving patient blood management.
ABC and its variations are initialism mnemonics for essential steps used by both medical professionals and lay persons (such as first aiders) when dealing with a patient. The protocol was originally developed as a memory aid for rescuers performing cardiopulmonary resuscitation, and is widely used in the care of the unconscious or unresponsive patient, although it is also used as a reminder of the priorities for assessment and treatment of patients in many acute medical and trauma situations, from first‐aid to hospital medical treatment. This stresses that each component is vital for life, and each is required, in that order, for the next to be effective. We propose a similar method to facilitate decision making with respect to transfusions which would promote evidence based decisions and safer patient care ‐ this would promote a truly improved patient blood management with improved outcomes and economic implications. It could also be used in transfusion education and would have the advantage that health care professionals are already familiar with the simple but effective algorithm and would be easily be able to adapt this to clinical decision making. This would improve staff and patient engagement and is truly an innovative way of promoting ‘Patient Blood Management’.
Caption 1: ABCDE approach to PBM.

This simple approach involves:
A: Evaluating the severity of anaemia in any given patient and also explore if an avoidable blood loss
B: Reviewing available blood results and considering best option in management
C: Looking for any correctable factors contributing to anaemia and also ensuring patient consent thus promoting patient involvement
D: Ensuring good clinical practice by reminding health care professionals:
1. Don't forget other measures (Vitamin K, tranexamic acid)
2. Don't forget to document
3. Don't hesitate to challenge
E: Looking for evidence supporting transfusion decisions and ensuring patient centred approach in management.
P‐748
Prognostic Effects of Perioperative Blood Transfusion in Esophageal Cancer Surgery and Related Factors
Kunori TK
Iwaki‐kyoritsu Hospital, Iwaki, Japan
Background
Blood transfusion is often used for esophageal cancer patients in perioperative period. The efficacy has been proved by significant improvement of blood components after surgery. However the prognostic effect is controversial.
Aims
To confirm the prognostic effects of blood transfusion, clinical course of patients after surgery was retrospectively analyzed.
Methods
(1) Operative methods and Patients: A hundred and ten patients (mean 65 years of age, male 88%; 1994‐2014) with esophageal cancer who underwent esophageal resection and lymph node dissection were analyzed. Pathological staging (TNM); stage 0,15 cases (13%), stage 1,29 (25%), stage 2, 27 case (24%), stage 3, 12 cases (11%), stage 4,31 cases (27%). All but 4 patients (4%) had pathologically squamous cell cancer. (2) Statistics: Comparison of groups was statistically tested by student‐t, rank‐test or chi‐square test. Odds ratio (OR; CI, 95% confidence interval) was used for specific events. Five‐year survival was estimated by Kaplan‐Meier method. Significance was determined if probability less than 5%. (3) Blood transfusion: No blood transfusion (A‐group), 42 cases (46%); frozen fresh plasma (FFP) only (B‐group), 11 cases (10%); packed red cells (RBC) only (C‐group), 27 cases (25%); FFP+RBC (D‐group), 23 (21%). 4) Operation status: Blood loss was estimated as 598 ± 368 m�’’ (mean± SD) in A‐group, 578 ± 260 (B), 877 ± 628 (C) and 1130 ± 680 m�’’ (D (significant A vs C, D). Duration was 512 ± 170 min (A‐group), 351 ± 38 (B), 524 ± 178 (C) and 481 ± 146 (significant B vs A, C,D).
Results
A. Effects of RBC transfusion. (1) Postoperative course: Although serum hemoglobin (Hb) decreased to 65‐74% of preoperative level, the recovery rate was higher in C‐D (RBC) group. (2) Complications after operation were most frequent (67%) in C‐group (56% in A, 9% in B and 45% in D). 3) OR was significant in C‐group for all complications (1.62; CI, 1.137‐2.311), high C‐reactive protein (1.893; 1.101‐3.254) and mortality (2.578; 1.022‐6.502). 4) Blood lymphocytes most decreased in C‐group; 55% of patients showed less than 800 per mm3, whereas the rate was 0‐10% in A, B and D group. 5) Five‐year survival: Survival rate of stage 0‐2 patients was lowest (54%) in C‐group (over 80% in the others). 6) Survival rate significantly decreased if more than 4 packs (8 units) of RBC were transfused.
B. Factors associated with the transfusion effects: (1) Plasma: Postoperative course was better in patients with FFP transfusion and without administration of purified albumin products (over 100 g). (2) Blood type: The adverse effect of RBC significantly increased in patients of blood type O; 5‐year survival was 30% (over 80% in type A and B), and OR for mortality was significantly high (2.62; 1.09‐6.36).
Conclusions
RBC transfusion may have adverse effects to the postoperative course after esophageal cancer surgery. The effects appeared to be interfered by the use of FFP (favorable), albumin products (unfavorable). Although the mechanism is unknown, some immunological response may be participated in, including lymphocytes and blood type antigens.
P‐749
Blood Component Utilization for Disseminated Intravascular Coagulation (DIC) Cases With Respect to Underlying Condition
Gupte SC , Jhaveri AG
Surat Raktadan Kendra and Research Centre, Surat, India
Background
Our Regional Blood Transfusion Centre supplies blood to about 500 hospitals in the district. Study presents the retrospective analysis of disseminated intravascular coagulation (DIC) cases receiving blood during the years 2001 to 2013.
Aims
To understand the transfusion requirement of blood/components for DIC due to various causes and evaluate if appropriate blood components are transfused.
Methods
The requisition forms and blood units issue details were entered in Microsoft office excel sheets. The data were analyzed using parameters like year wise requirement, age, sex utilization of blood with respect to cause of DIC etc.
Results
Total 1931 cases of DIC received 21,153 blood units including whole blood, red cell concentrate (RCC), fresh frozen plasma (FFP), random donor platelets (RDP), single donor platelets (SDP) and cryoprecipitate (Cryppt) during 13 years. Majority of the cases received FFP (58.8%) while whole blood was given in only 7% cases. Whole blood utilization was 25.5% in the year 2001 which was reduced to 1.2% in the year 2013. Majority of clinicians preferred RDP over SDP probably because of the high cost of SDP units. Cryppt was used in 9.4% cases. On average there were 148 cases every year requiring about 11units/case. The requirement per case was comparable in the male and female patients. Infants (< one year) needed minimum transfusions (six units/case) while 41 to 60 years’ age group needed maximum transfusions (17 units/case). Septicemia was the major cause of DIC associated in 31.5% cases. Second major cause was obstetric complications including intrauterine fetal death, post partum hemorrhage or HELLP syndrome, placental abnormality etc. In injury/Trauma including cases of head injury, crush injury, stabbing, bullet injury etc. highest number i.e. 24 units /case were utilized. Trauma cases associated with septicemia required 35 units /case. In septicemia associated with cirrhosis of liver or hepatitis 28.5% units /case were utilized.
Summary/Conclusion
Septicemia is the commonest cause for DIC followed by DIC due to obstetric complications. Maximum blood requirement is for DIC associated with Injury/Trauma. Whole blood use was significantly reduced during the period of study indicating increased awareness about the use of blood components.
P‐750
Evaluation of FFPS Utilisation In Inherited Factor XIII Deficiency Patients Reported to a Tertiary Care University Hospital in India: A Retrospective Cohort Analysis
Babu BB
Manipal University, Manipal, India
Background
Factor 13 also know as Fibrin stabilising factor. Its deficiency is exceedingly rare disorder in which a severe bleeding tendency takes place. The incidence is 1 in 5,000,000 people. It is mostly caused due to mutation in the A subunit gene which is autosomal recessive inherited pattern. While recombinant Factor XIII concentrates are the first line of therapy according to the World Federation of Haemophilia. FFP remain to be the second choice, though cost effective is reported to be causing adverse reactions.
Aim
To evaluate the usage pattern of FFPs in coagulation Factor ‐ XIII deficiency while also studying the clinico‐pathological, therapeutic and economic outcomes.
Method
A retrospective cohort analysis on patient admitted during 2004‐2014 was carried out with Factor 13 deficiency at KMC hospital, Manipal, Western Coastal Region of India.
Results
4 patients were diagnosed with Factor ‐13 deficiency. The mean age of onset of bleeding was 2.25 ± 1.25 year and were male patient. 2 patients were found to have strong family history of which 1 is an offspring from consanguineous marriage. Management was done using Fresh Frozen Plasma and Cryoprecipitate which are second line therapy for the disease according to World Haemophilia Federation. Patients received 1 unit of FFP as prophylactic treatment every month and 2‐4 units of FFP in any complaints of bleeding. After transfusion 2 of the patients developed allergic reactions after transfusion. FFP transfusion were mainly done on the basis of PT and APTT values.Cost of admission and treatment is ≈ 100$ per month. Complication was seen in which all 4 patients have hematoma, 2 patients have joint bleed, 3 patients had Umbilical Stump Bleeding and 2 had Intracranial Bleeding leading to General Tonic Clonic Seizure was treated with Phenytoin and Levetiracetam. Anaemia was also observed in patients with 2 having Grade 1 and Grade 2 anaemia and other 2 having grade 3 anaemia.
Conclusion: Though Recombinant Factor XIII concentrates are advocated against Factor XIII deficiency due to lack of availability physician rely on traditional transfusion of FFP and Cryoprecipitate.Quality of Life has also significantly decreased due to high cost of treatment and frequent admission in hospital.
P‐751
Chronic Transfusional Support in Patients With Myelodysplastic Syndromes (MDS) – A Single Centre Experience
Seabra P , Coutinho M , Faria M , Ferreira A , Gonçalves C , Coutinho J , Bini-Antunes M , Amil M
Centro Hospitalar do Porto, Porto, Portugal
Background
Red blood cell (RBC) transfusion is the primary management option for anaemia in elderly patients with MDS.
Aims
We performed a descriptive analysis of chronically transfused patients with MDS in a haematology department of a University Hospital during 2014.
Methods
We defined as chronically transfused every MDS patient with more than 5 RBC transfusion episodes per year. MDS were classified according to 2008 World Health Organization (WHO) classification. Patients were characterized with respect to: demographic data; number of transfused RBC units and transfusional frequency; pretransfusion haemoglobin values; alloimmunization; iron overload; and other treatments. Patients were transfused with RBC units matched for ABO, CcDdEe and Kell antigen, whenever RBC stocks would allow in accordance to the existing standard operating procedure.
Results
In 2014 81 patients were inchronic transfusional support. Twenty‐three had been diagnosed with MDS (15 men, 8 women, median age 79 years old, range 37‐90). Patients had been classified as follows: 9 (39.1%) refractory cytopenia with multilineage dysplasia; 4 (17.3%) refractory anaemia with excess blasts type 2; 3 (13%) unclassifiable MDS; 2 (8.7%) refractory cytopenia with unilineage dysplasia; 2 (8.7%) refractory anemia with ring sideroblasts; 2 (8.7%) refractory anemia with excess blasts type 1; 1 (4.3%) MDS with del (5q). About one third of the patients developed secondary acute leukemia. The median time of disease evolution was 2 years (range 0–11). Most of the patients (n = 19, 83%) started transfusional support in the first year after diagnosis. During 2014 the median number of RBC transfused per patient was 26 (range 5–55), being the median time between transfusions of 14 days (range 1–44) and the median pretransfusional haemoglobin 7.5 g/dL (range 4.2–9.6). Five patients (21.7%) formed alloantibodies after a median of 11 previously transfused RBC units. Eighteen patients had serum ferritin >1000 ng/ml and 50% out of them had documented iron overload. Therapies performed simultaneously with transfusion: quelation therapy: 2 patients; erythropoiesis‐stimulating agents (ESA): 11 patients; colony stimulating factor (G‐CSF): 2 patients; danazol: 1 patient; cyclosporine and prednisone: 1 patient; and lenalidomide: 1 patient. Patients doing ESA had a median time between transfusions of 12 days vs 15 days for the other patients. Eleven patients (47.8%) died during 2014, with an average of 1.8 years of transfusional support (range 0–6).
Summary/Conclusions
The requirement of frequent red cell transfusions is a marker of poor prognosis in patients with MDS. Despite the high number of units transfused per patient the prevalence of alloimmunization is not relevant and concordant with other previous publications, probably due to the patient's disease‐related imunossupressive status and the general compliance with the Rh and Kell matching protocol. Iron overload is frequent in MDS patients due to ineffective erythropoiesis and chronic transfusion, nevertheless the benefit of chelation is unproven. This probably explains why only two patients were under quelation therapy. In the group of patients treated with ESA a significant decrease in transfusional needs was not observed.
P‐752
Prophylactic Platelet Transfusions in Patients With Autologous Hematopoietic Stem Cell Transplantation
Protopopova EB1, Mochkin NE 1, Shestakov EA 1, Melnichenko VY 1, Zhiburt EB 2
1National Medical Pirogov Center, Moscow, Russian Federation2National Pirogov Medical Surgical Center, Moscow, Russian Federation
Background
Thrombocytopenia accompanies the autologous hematopoietic stem cells transplantation. Platelet transfusion is an important part of prevention and correction of bleeding during thrombocytopenia. Due to the features of blood component logistics, we practice prophylactic platelet transfusions at a concentration at or below 10,000 platelets/μL.
Aims: To define the needs for platelets in patients with autologous stem cell transplantation (ASCT). To evaluate the effectiveness of prophylactic transfusions during post‐transplant period and their impact on patient's length of stay (LOS) at hospital.
Methods: From January, 2013 to September, 2014 169 ASCT was performed in patients with autoimmune (n = 87) and blood malignancy (n = 82) diseases. Bleeding is not registered, cases of death ‐ 2. Blood malignancy group includes the following diseases: multiple myeloma (n = 18), non‐Hodgkin's lymphoma (n = 21) and Hodgkin's disease (n = 42). Autoimmune disease group is presented in most of multiple sclerosis (n = 84), cases of polyneuropathy (n = 2), ankylosing spondylitis (n = 1). During the study, 158 apheresis platelet units were transfused to 104 recipients, 38 of whom received multiple transfusions. Part of platelet recipients: blood malignancy ‐ 98,8% (n = 81), autoimmune diseases ‐26,4% (n = 23).
Results: LOS in patients with blood malignancies (26,9 ± 0,9 days) is less, than in patients with autoimmune diseases (32.4 ± 0.8 days) (t = 9.6; P < 0.01). Among the hematological recipients (n = 81) 46 patients have been transfused by single platelet unit, 24 patients received 2 units, 8 patients received 3 units, 2 patients received 4 units and 1 patient received 5 units. Transfusion of 3 or more units is associated with longer treatment (29.6 ± 1.4 days) in compare to transfusion of 0‐2 units (26.5 ± 0.6 days), (t = 2.1; P = 0.02). Platelet transfusions do not affect the LOS in patients with autoimmune diseases.
Conclusions: Prophylactic platelet transfusion at concentration below 10,000 platelets/μL prevent bleeding in patients with ASCT. Frequency of transfusions in patients with blood malignancies is 3,7 times higher than among patients with autoimmune diseases. The mean needs for platelets of patients with autoimmune diseases is 0,3 unit per patient; in patients with blood malignancies is 1,6 unit per patient, of which 53% of patients get single transfusion and 47% are repeat recipients. Patients with blood malignancies transfused with 3 or more platelet units had increased LOS. In patients with autoimmune diseases platelet transfusions do not affect the LOS.
P‐753
Outpatient Transfusions – Our Experience
Mihic Tomic MTB
CHC Dr Dragisa Misovic Belgrade Serbia, Belgrade, Serbia
Introduction
Anemias are categorized as very frequent pathological conditions occurring both individually and as a consequence of many other diseases.
Aim
Presentation and analysis of red blood cells consumption at the Outpatient Treatment Clinic of the CHC “Dr Dragiša Mišović‐Dedinje”during 2013‐2014, as a contribution to resolving professional, organizational and technical issues associated with the treatment of anemias in conditions found in the outpatient clinics.
Material and methods
Data regarding red blood cell consumption were collected using blood request forms forwarded to the Blood Transfusion Department, as well as blood/blood component issuing charts, patients` admission and release protocols and histories of the disease.
Results
During 2013–2014, total of 184 patients were transfused with red blood cells at the Outpatient Treatment Clinic of the CHC “Dr Dragiša Mišović‐Dedinje”. There were totally 394 half day hospitalizations, or 2.1 visits in the average. Among them, 71 (38,6%) were female and 113 (61,4%) were male patients, mean age around 70 years (the youngest patient was 45, and the oldest one was 94 years old). The lowest recorded haemoglobin value before transfusion was 3,7 g/dl, and the highest was 8,2 g/dl. At the admission to the Outpatient Treatment Clinic, all had obvious clinically expressed symptomes and parameters of (mostly) severe anemia confirmed by laboratory findings. In most patients, anemia developed as a consequence of a malignant disease and associated with radio and/or chemiotherapy treatment (I group, 129 or 70,1%), or as a consequence of haematological or hematooncological diseases (II group, 40 or 21,7%) and finally as a consequence of renal or cardiac diseases (III group, 15 or 8,2%). No unfavourable reactions were noted throughout the transfusion administrations. Since the patients from the first and the second group were polytransfused in most cases, 40 or 60 mg of I.V. urbason was administered prior to each transfusion for prevention purposes. Some patients from the third group were administered 1 to 2 ampoules of lasix in order to prevent circulation overload.
Conclusion
An ever increasing number of patients requires blood transfusion due to severe anemia (haemoglobin value below 4,0 g/dl). Capacity of the Outpatient Treatment Clinic of the CHC “Dr Dragiša Mišović‐Dedinje”, where the outpatient transfusion administration takes place, do not even closely satisfy the actual needs. That is why it is necessary to increase the number of the outpatient treatment clinics/hospitals where blood transfusion would be administered, and to consider the need to reestablish the practice of blood transfusion administration at patients` homes.
6.4 Haemorrhage and Massive Transfusion
P‐754
Abstract Withdrawn.
P‐755
Targeted Coagulopathy Management in Traumatic Massive Haemorrhage at a UK Major Trauma Centre
Jain N , Altemimi B
University Hospital Aintree, Liverpool, United Kingdom
Background
Trauma patients presenting with massive haemorrhage are at risk of acute coagulopathy of shock trauma (ACoST). Near‐patient coagulation testing (for example rotational thromboelastometry (ROTEM)) provides rapid, ‘real‐time’ results for goal‐directed clotting product transfusion1, which improves resuscitation, bleeding and coagulopathy management and therefore patient care. It also avoids complications associated with inappropriate transfusion and their financial implications.
Aims
To evaluate the use of ROTEM in traumatic massive haemorrhage over a 12 month period at University Hospital Aintree, a UK major trauma centre.
Methods
We conducted a retrospective review of ROTEM and haemorrhage management in trauma patients who activated the massive haemorrhage protocol (MHP) on admission to University Hospital Aintree, from 1st January 2014 to 31st December 2014.
Results
Eighty‐five trauma patients activated the MHP, of whom 64.7% had ROTEM checked following MHP activation. Of these 58.1% had clotting products transfused. The use and correct interpretation of ROTEM led to appropriate targeted management in 52.7% of patients, with no clotting product transfusion in 72.4% of these patients and transfusion of appropriate products in the remainder. Where clotting products were transfused, treatment response was not regularly evaluated with sequential ROTEM.
We identified 36.4% of the admission ROTEM results to be abnormal, with 35% of these patients having abnormalities in both EXTEM and FIBTEM. Overall, the most frequent abnormality occurred in EXTEM due to a combination of prolonged clotting time (CT) and reduced A10, followed by an abnormal FIBTEM.
Summary
Appropriate strategy for the management of ACoST is under debate. At our major trauma centre, we aim to manage traumatic massive haemorrhage using point‐of‐care testing to provide goal‐directed therapy with the correct clotting products for the correct coagulopathy.
Although we currently do not consistently provide goal‐directed clotting product transfusion, where it has been used and appropriately actioned, we have noted identification of critical coagulopathies and a consequential improvement in appropriate transfusion. This is evidenced by increased cryoprecipitate usage due to abnormal FIBTEM, representing poor elasticity of the fibrin‐based clot, which reflects an aspect of ACoST pathophysiology. Currently our MHP recommends a 1:1 transfusion of packed red cells to fresh frozen plasma until availability of ROTEM results, which may partly explain the apparent inappropriate transfusion. We hope that measuring ROTEM in traumatic massive haemorrhage patients on arrival to hospital and the use of sequential ROTEM measurements, depending on transfusion, haemorrhage control and clinical situation, will improve targeted coagulopathy management.
ROTEM provides rapid, ‘real‐time’ results that are more reflective and relevant to ongoing clinical situations than standard laboratory investigations. We aim to reduce laboratory coagulation investigations and increase ROTEM practice.
In order to improve targeted transfusion and clinical outcomes, training is necessary. We are instituting an educational package and a standardised algorithm to guide ROTEM interpretation using A5 to facilitate earlier targeted coagulopathy management and shall re‐audit our practice following these implementations.
References
1. Davenport R, Khan S. Management of major trauma haemorrhage: treatment priorities and controversies. Br J Haematol. 2011;155(5):537‐48.
P‐756
Management of Major Haemorrhage in Trauma Patients at the Royal London Hospital; Three Year Retrospective Data
Manohar SJ , Grist P , Maddison A , Allard S , Green L
Barts Health NHS Trust, London, United Kingdom
Background
The major haemorrhage protocol (MHP) for the management of trauma‐induced bleeding states that for every six units of red blood cells (RBC), four units of fresh frozen plasma (FFP) are transfused initially; if the bleeding continues cryoprecipitate and platelet transfusion are introduced later. Further, group and screen samples (G&S) and clotting tests should be taken as soon as possible. The results of PROPPR study are likely to change our MHP and we expect that the use of FFP and Platelet transfusion will increase.
Aims
The aims of this study were to assess the: a) blood wastage for trauma MHP; b) RBC:FFP ratio transfused; c) number of patients who had valid G&S; and d) proportion of patients who had high prothrombin time (PT, >16s), low fibrinogen (<2 g/dL) and low Platelet count (<100x109/L). Major haemorrhage was defined as transfusion of ≥6 RBC (or >4 units of FFP) within 24 hrs of trauma.
Methods
Data was collected retrospectively over a 3 year period (2012 to 2014) at the Royal London Hospital, using the transfusion laboratory database and patients’ electronic records system.
Results
A total of 510 patients (391 male, 119 female, median age 41 [range 3‐97]) were admitted with trauma‐associated major haemorrhage; of these 92/453 patients received massive transfusion (MT, ≥10 RBC). FFP, cryoprecipitate and platelets were transfused to 75%, 41% and 45% of patients respectively. The median RBC:FFP ratio was 1.00 (IQR 0.75 – 1.50). Overall wastage for RBC, FFP, Cryoprecipitate and Platelet transfusion were 238/3307 (6%), 385/2560 (12%), 143/674 (17%) and 9/433 (2%), respectively. In 21/510 (4%) patients there were no valid G&S. Overall 53/458 (12%), 245/345 (71%) and 61 (13%) of patients had low PT, low fibrinogen levels and low platelet count respectively. Of the 92 MT cases, 15 (16%) had a high PT, 52 (57%) had low fibrinogen, and 18 (20%) had low platelets.
Conclusions
(a) while the wastage of RBC and Platelet transfusion is low, more needs to be done to reduce plasma wastage; b) the RBC:FFP ratio is in accordance with the MHP; c) in 4% of patients G&S were missing; d) more patients (MT cases included) had low fibrinogen (~50%), than prolonged PT or low platelet count (<20%), indicating that the current MHP is inadequate in replacing fibrinogen.
P‐757
All Bleeding Stops Eventually: Analysis of Fatalities From Major Haemorrhage Recorded in Australia's Coronial Database
Gipson JS1, Wood EM 1, Cole-Sinclair MF 2, Woodford NW 3
1Transfusion Research Unit, Monash University, Melbourne, Australia2Department of Haematology, St Vincent's Hospital, Melbourne, Australia3Victorian Institute of Forensic Medicine, Melbourne, Australia
Background
Major haemorrhage (MH) is a leading cause of mortality across many clinical contexts. However, few data are available on numbers of deaths from MH and factors contributing to fatal outcomes, especially where onset of bleeding is out of hospital. The coroner provides a valuable public health role by investigating all sudden, unexpected deaths as well as those due to injury and medical misadventure, and recording these in a database available to public health researchers. Identifying common elements and clinical associations in fatal MH cases will inform clinical transfusion practice and public health efforts.
Aims
To describe and analyse MH fatalities in a large coronial database and explore possible common contributory factors.
Methods
Using keywords for MH(bleeding, exsanguination, haemorrhage, etc), we searched for closed (finalised) cases from the state of Victoria (population 5.5 million) recorded in Australia's National Coronial Information System from 2009‐2011. There were 13,298 closed cases: 1349 were identified using keywords; 648 cases were excluded as unlikely MH following review of cause of death. Autopsy and police reports, coronial findings and cause of death of the remaining 701 cases were reviewed to determine if MH caused or significantly contributed to death. Cases were assigned a clinical bleeding context and demographic data extracted. The presence and detail of coronial recommendations were examined for lessons to inform preventive work.
Results
382 cases of MH were included. These had a wide age range (13 months – 98 years); 69% were male. 36% fatalities were due to trauma, 27% gastrointestinal (GI) haemorrhage, 14% surgical/perioperative, 12% ruptured aneurysms/large vessels, 6% medical, 4% solid organ tumours eroding vessels and one case of peripartum haemorrhage. Onset was outside hospital in 78% cases; of these, 30% reached hospital alive. Of GI bleeds, the majority were due to oesophageal varices and 87% began out of hospital; 16% reached hospital. Of perioperative haemorrhages, 69% involved elective procedures; the remainder were urgent or emergency. 75% of all cases were managed in large metropolitan centres, 9% large regional, 5% small and medium metropolitan, 2% small and medium regional and 9% private. In cases where adverse outcomes were related to deficiencies in medical care, thematic analysis of coroners’ comments showed common associations to be poor clinical communication, failure to follow protocol, difficulties arising from rural practice, and need for greater training in recognition and management of MH. Coronial recommendations were made in 10 cases; three concerned management of bleeding and related to the need for greater education regarding commonly missed diagnoses.
Conclusion
Coronial investigations provide valuable data on the demographics, location, and clinical context of MH fatalities. This is the first analysis of MH using Australia's national coronial database. In addition to information on inpatient bleeding events, it provides valuable new data on bleeding contexts, management and outcomes of individuals where MH began before reaching hospital. The large number of out‐of‐hospital fatalities highlights the challenges of pre‐hospital management of MH and supports consideration of options for expansion of life‐saving support (for example, earlier availability of blood components, such as in ambulances).
P‐758
Management of Warfarin‐Associated Coagulopathy (Wac) in Patients With Acute Gastrointestinal Bleeding (GIB)
Andreeva JS1, Neudahina OA 2, Stradymova EA 2, Momotyuk KS 1, Appalup MV 1, Mayorova OA 1
1Moscow Blood Bank, Moscow, Russian Federation2City Clinical Hospital 15 Department of Health of Moscow, Moscow, Russian Federation
Objectives
There is no unified international standard therapy WAC complicated by massive or life‐threatening bleeding. The most common causes of major bleeding is Gastrointestinal bleeding (GIB).
The Background
Warfarin, an oral vitamin K antagonist, is used to prevent venous and arterial thromboembolism. The frequency of bleeding complications associated with WAC is 15‐20%, with fatal bleeds measuring as high as 1 to 3%. Laboratory control of anticoagulation during warfarin therapy is the international normalized ratio (INR). Target value is between 2.0‐3.5. The value of an INR less than 2.0 is associated with an increased risk of thromboembolic complications, while an INR of greater than 4.0 is associated with increased risk of bleeding. The risk of bleeding are patient age over 70 years, the first 90 days of treatment, the intensity of anticoagulation (INR greater than 4.5). There are several methods of reversing anticoagulation effect of warfarin: the omission of the dose of warfarin, administration of an oral or intravenous vitamin K1, use of fresh frozen plasma (FFP), prothrombin complex concentrate (PCC), recombinant activated Factor VII (rFVIIa). There are a significant number of concerns about the use of FFP in WAC: transfusion of large doses from 15 ml / kg, up to 50 ml / kg at an INR of greater than 6.0. INRs of patients treated with PCC were reversed 4 to 5 times more rapidly than those of patients treated with FFP. A complete correction of the INR within 15 min (mean INR of 1.3). Doses of PCC are different and depend on the initial level of INR. The higher the INR, the greater the dose of the PCC introduced (25, 30‐35, or 50 IU / kg). 2‐4 liter volume FFP gives the same effect as that of the PCC in a dose of 20‐50 IU / kg body weight. rFVIIa can quickly correct supratherapeutic INRs with doses ranging from 10 to 90 mg / kg. But, its high cost for an average patient and short half‐life of 2.5 to 3 h.
Aims
Comparison of management GBI in warfarin anticoagulated patients by FFP vs Four Factor PCC (FFPCC).
Methods
GIB events from 15 patients with WAC were analyzed. Median INR was 7.0 ± 1.8. Methods of hemostatic therapy were the transfusion of FFP and introduction FFPCC.
Results
The main method of hemostatic therapy GIB is the transfusion of FFP in large doses – more than 20 ml / kg. A significant reduction in the level of INRs were not observed, which required several repeat transfusions of FFP. Introduction FFPCC in a dose 600ME (Protrompleks 600) of patients with GIB with an INR of 7.0, to reduce the level to 2.0 in 4 h, while the volume of FFP transfusion was 600 ml.
Conclusion
Further studies are needed to provide for working out an algorithm with WAC complicated by massive or life‐threatening bleeding, which will focus on optimization hemostatic therapy to reduce the risk of volume overload.
P‐759
Reverting Vitamin K Antagonists With Prothrombin Complex Concentrate – A Three‐Year Retrospective Study
Plácido C1, Lichtner A 2, Barra A 2, Costa C 2, Nunes C 2, Cardoso E 1
1Hospital Prof. Doutor Fernando Fonseca E.P.E, Amadora, Portugal2Hospital Prof. Doutor Fernando Fonseca E.P.E ., Lisbon, Portugal
Background
Correcting coagulopathy has been a difficult challenge for hematologists throughout the world.
Practice guidelines recommend vitamin K for the reversal of anticoagulation in asymptomatic patients with elevated INR, in patients who require surgery and in patients with serious bleeding.
Prothrombin Complex Concentrate (PCC) is having progressive importance in bleeding management and reversal of International Normalized Ratio′s (INR) in patients taking oral vitamin K antagonists (VKA).
Aims
The main objective of our study is to determine the efficiency of PCC in the correction of INR in patients taking oral VKA and try to understand if the administration of vitamin K plays a role in the survival rates in these patients.
Methods
We carried out a retrospective, unicenter, descriptive study that included 54 patients taking oral VKA that required Octaplex® administration in order to try to control bleeding and/or correct INR. Of these patients 55% were female and 45% male. Average age was 76 years old. The data was collected from March 2011 to November 2014. The INR results were collected before and after the treatment with PCC.
Results
87% of the studied patients (47 patients) were taking warfarin and 13% (7 patients) acenocoumarol. The main cause of treatment was atrial fibrillation. PCC was administered in 78% due to bleeding and in 22% of the cases to prepare patients for surgery. The mean administered doses were approximately 800 IU. Pre‐treatment INR determination was 4,4 in the warfarin group and 5,6 in the acenocoumarol group. In 26% of the patients it was not possible to have an absolute INR value (INR>10), 12 patients in the warfarin group (26% of the group) and 1 in the acenocoumarol group (14% of the group). After treatment all patients had a measurable INR and 37% of them had an INR ≤1,5. The INR values dropped to 1,9 in the acenocoumarol group and to 2 in the warfarin group.
18 patients received concomitantly i.v. vitamin K administration. 17 of them 10 mg and one 20 mg.
We compared patients who only received PCC (33 patients) with those that received PCC and vitamin K (14 patients) and the impact on mortality. We excluded patients who did concomitant treatment with plasma (7 patients). We found a mortality of 36% in both groups, but we must point out their non‐uniformity.
None of the patients enrolled presented reported thrombotic complications.
Patients presented different bleeding sites: 23% intracranial (67% mortality), 40% gastrointestinal (38% mortality) and 37% other (33% mortality). The overall mortality was 37%.
Bleeding patients presented a mortality of 43% compared with no bleeding that presented mortality of 21%.
Summary/Conclusions
This study shows that Octaplex® treatment is efficient reversing the INR in patients taking oral VKA. In this population we didn't find any difference, regarding overall mortality, between patients who had taken vitamin K plus PCC and those who had only taken PCC. We found a higher mortality rate in these bleeding patients.
P‐760
Massive Haemorrhage Pathway and Key Performance Indicators – A Look at Six Months’ Data
Noble RN , Wei Lim SWL , Polyzois LP , Pendry KP
Central Manchester Foundation Trust, Manchester, United Kingdom
Background
Massive haemorrhage pathway activations occur by definition in the time‐critical acute setting. Key performance indicators (KPIs) give a locally or nationally agreed gold standard against which clinical and lab performance can be assessed. Using KPIs in analysis of massive haemorrhage activations can give a picture of clinical and lab performance on top of descriptive data.
Aims
We aimed to describe features including epidemiology, actions and outcomes of every massive haemorrhage pathway activation over a six month period to add to the evidence base and to allow analysis and comparison of clinical and lab performance, particularly in relation to key performance indicators.
Methods
A retrospective review of consecutive massive haemorrhage pathway activations across a large city centre tertiary hospital and adjacent tertiary paediatric hospital and maternity hospital over a six month period from 1/4/14 to 1/9/14 was conducted. Data was collected by case‐note retrospective review. Descriptive statistics and comparison with key performance indicators was utilised.
Results
97 cases of adult and 3 cases of paediatric massive haemorrhage activation occurred during the six month period. 27/100 activations came from a&e; 27/100 from wards; 16/100 from labour ward or associated theatre; 13/100 from critical care and 17/100 from surgical theatre. 63/100 cases did not use any emergency O negative blood, 7/100 used 1 unit, 21/100 used 2 units and 8/100 used more than two units of O negative blood. TEG was utilised in 25/100 cases. Tranexamic acid was utilised in 44/100 cases, including use in 7 out of 11 trauma cases. Baseline haemoglobin was checked within 4 h of activation in 87/100. Baseline coagulation screen and Clause fibrinogen was checked within 4 h of activation in 83/100 and 46/100 respectively. Median time from pathway activation to grouped red cells ready for dispatch from lab was 8 min. Lab was informed of stand down in 28/100 cases. Mortality at 24 h was 14/100 (14%) and at 30 days was 27/100 (27%).
Summary/Conclusions
Overall key performance indicator targets were not all met however this did not lead to any observable adverse outcome. 8/100 (8%) cases received more than 2 units of emergency O negative blood, in contrast to KPI target of less than 5%. Many cases did not have baseline bloods checked around time of event, especially fibrinogen. Tranexamic acid was underutilised with use in trauma cases below the key performance indicator target of 100%. Mortality rate was in keeping with previously studied periods.
6.5 Adverse Events, including TRALI
P‐761
Analysis of Acute Transfusion Reaction Rates to Fresh‐Frozen Plasma and Platelet Components in the UK
New HV 1, Tinegate H 2, Curnow E 2, Bolton-Maggs PHB 3, Cardigan R 2
1Imperial College Healthcare NHS Trust\NHS Blood and Transplant, London, United Kingdom2NHS Blood and Transplant, Newcastle Upon Tyne, United Kingdom3Medical Director, SHOT Manchester Blood Centre/Manchester University, Manchester, United Kingdom
Background
Methylene‐Blue treated imported fresh frozen plasma (MB‐FFP) is provided in the UK for recipients born on/after 1.1.96 and is used by some other countries. In 2012 France withdrew its use, partly due to finding increased allergic reactions to MB‐FFP. We have analysed 7 years of Acute Transfusion Reactions (ATRs) to plasma reported to the UK Serious Hazards of Transfusion (SHOT) haemovigilance scheme.
Aims
To compare rates of ATRs between standard FFP and MB‐FFP (2007–2013) or solvent detergent (SD) FFP (2009–2013). Since MB‐FFP is transfused to a different patient population in the UK compared with standard FFP, we also assessed differences in ATRs to platelets in these groups.
Methods
ATRs were categorised by severity and whether anaphylactic/severe allergic or hypotensive. For plasma, denominators for ATR rates were UK plasma issues data, using total numbers of paediatric split/adult sized units. For platelets, analysis of neonatal/infant (all recipients <1 year old) vs older child/adult platelet SHOT reaction rates was based on reports from 2008–2013, assuming that neonates/infants received ‘paediatric’ platelet split units and older recipients received full‐sized platelet units. For statistical analysis of FFP reaction rates, 95% CIs were calculated assuming Poisson distribution; comparisons between groups were made using Poisson regression models.
Results
Overall, reaction rates to MB‐FFP compared to those with standard FFP for 2007–2013 showed no difference for all ATRs, or non‐severe or severe allergic/anaphylactic reactions (see Table). However, MB‐FFP reaction rates for severe hypotensive reactions were significantly higher vs standard FFP (P = 0.0005), although the absolute numbers are very small. Of the 4 MB‐FFP hypotensive reactions, 2 were infant cardiac patients, one a neonate with bleeding on extracorporeal membrane oxygenation, the other also reacted to SD‐FFP. When MB‐FFP reaction rates were compared with SD‐FFP, overall ATR rates to MB‐FFP were higher, similar to standard FFP vs SD‐FFP. However, there was no significance for reaction rates subdivided by type of reaction, including severe hypotensive reactions.
In view of the severe hypotensive reactions to MB‐FFP in neonates/infants, platelet reactions were analysed for a similar pattern. For both FFP and platelets, neonates/infants had a higher rate of severe hypotensive reactions than older recipients. The rate of severe hypotensive reactions in neonates/infants was higher for FFP than platelets, but absolute numbers were small.
Caption 1: Comparison of UK FFP reaction rates.

Summary/Conclusions
Despite demonstration of a higher rate of severe hypotensive reactions to MB‐FFP vs FFP with statistical significance, the numbers are very small and the clinical significance is uncertain, possibly reflecting differences in recipient patient groups. Rates of all ATRs and of severe allergic/anaphylactic reactions are similar between MB‐FFP vs standard FFP. Therefore, the data do not indicate a change in current UK practice.
P‐762
Adverse Blood Transfusion Reactions: A Paediatric Prospective
Bakhru Dara V1, Jaiswal RM 1, Dara RC 2, Bakhru J 2, Acharya U 1, Kakkar M 1
1Mahatma Gandhi Medical College and University, Jaipur, India2Prakash Mother and Child Hospital, Jaipur, India
Background
Guidelines for transfusion in the neonatal period and childhood are available but its application varied widely. Similarly adverse events related to transfusion are well established, but because their occurrence in paediatric age group have not been well studied.
Aim
To collate the incidence and type of transfusion related complications in patients of paediatric age group.
Materials and methods
Over 10 years, between January 2005 and December 2014; the retrospective review of all transfusions given to paediatric in patients (≤ 18 years old) was performed. Data that includes age, gender, blood product transfused, type of transfusion reaction if any and its severity of the reaction was collected from transfusion record of all patients received transfusion.
Results
Total of 15,489 transfusions were performed during the study period that includes 2927 whole blood transfusion, 6831 red cell transfusions, 3717 platelet (PLT) transfusions and 2014 plasma transfusions in 6195 paediatric patients. Of these 1858 patients were neonates. During the study period 168 (1.08%) episodes of transfusion reactions were recorded. This corresponds to an incidence of 10.84 reactions per 1000 transfusions. Transfusion reactions were more common with platelets then other blood products (Table 1). Of these 168 transfusion reactions, 96(57.1%) were febrile non‐haemolytic transfusion reactions,54 (32.1%) allergic transfusion reactions, 14 (8.6%) hypotensive transfusion reactions, two (1.1%) were possible transfusion related acute lung injury (possible TRALI) and two (1.1%) was haemolytic transfusion reaction one was due to alloantibody and cause of another one remain unknown. The reactions were more common in male patients (6.8/1000 males vs. 4/1000 females P < 0.001). No deaths associated with transfusion reactions were observed.
Caption 1: No. of transfusions and transfusion reaction rate.

Conclusion
Administration of blood products to children is a common practice at all paediatric centres in the developing countries. Findings in our study indicate that incidence of transfusion reactions in the paediatric populations is more than the incidence reported in adult population with the similar life threatening complications.
P‐763
Incidence and Type of Adverse Transfusion Reactions Studied in Over 78,000 Patients Transfused Over a Period of Three Years: Report From a Developing Country
Singh GS , Tiwari AKT , Dara RC , Arora DA , Aggarwal GA , Acharya DA , Raina VR
Medanta‐The Medicity, Gurgaon, India
Background
Most of the developed countries have a well defined hemo‐vigilance system to report Adverse Transfusion Reactions (ATR) in patients and these are also well published. India has paucity of published data on ATR and therefore we would like to share three year retrospective ATR data from our centre.
aim: This study was undertaken to evaluate incidence and type of ATRs.
Materials and methods
Over three years’ data of ATR between January 2012 and January 2015 was systematically collected from Hospital Information System (HIS) in a hospital‐based blood transfusion center in a large tertiary care hospital. The center practices component therapy with lab‐side pre‐storage universal leucodepletion for Red Blood Cells in additive solution (RBC), and Platelet Concentrate (platelets). The apheresis platelets/Single Donor Platelets (SDP) were made on ComTec (Fresenius Kabi, Germany) which provided 3‐log leucodepletion. The data included age, gender, total number of components issued, components transfused, type of components implicated, ATR classified as per aetiology, causality (unknown/excluded/unlikely/possible/likely/certain) and recovery/outcome of the patient (unknown/permanently disabled/recovered with sequele /recovered/death).
Results
During the observed period, total of 1,67,161 blood components (73,408 RBC, 43,757 FFP, 41,348 platelets, 5817 apheresis platelets, 2831 Cryoprecipitate) were issued. Out of components issued 95.2% (n = 1, 59,154) of blood components were transfused to 78,318 patients. (Mean = 2.03 component/patient). ATR were reported in 196 different patients (141males, 55 females; mean age=49.7 years) giving a 0.2% incidence. Component implicated for ATRs were mainly RBCs 118; followed by FFP 41, PC 23 and SDP 14. Out of the total reported ATRs, 76.1% were allergic reactions, 23.4% were Febrile Non‐haemolytic Transfusion Reaction (FNHTR) and 0.5% (n = 1) Haemolytic Transfusion Reaction (HTR). This HTR was due to wrong blood in tube (WBIT) detected on root‐cause analysis. On causality assessment, 99.5% of ATRs were assessed as possible while 0.5% was certain. All patients including the one who had HTR had complete recovery. The low incidence (0.2%) in the present study matched the post‐universal‐leucodepletion incidence data (0.19%) in another published report.
Caption 1: Adverse Transfusion Reactions.

Conclusion
The incidence of adverse transfusion reactions was low possibly because of universal leucodepletion. Almost all reactions were allergic and NHFTR and patients recovered completely.
P‐764
Adverse Transfusion Outcomes: What Have we Learnt From Laboratory Models?
Flower RL , Dean MM , Bierman W , Meka D , Tung JP
Australian Red Cross Blood Service, Kelvin Grove, Australia
Background
Understanding mechanisms associated with adverse patient outcomes is necessary to inform improvements to product safety and improve transfusion outcomes. We have developed a number of laboratory models to characterise transfusion‐related immunomodulation in order to identify key factors associated with adverse transfusion outcomes. Here we present a summary of key learnings to date from our laboratory models.
Aims
To utilise a range of in vivo and in vitro models of transfusion to identify potential mechanisms associated with adverse patient outcomes post transfusion.
Methods
Ovine models were used to assess adverse outcomes associated with transfusion of supernatants (SN) derived from fresh, or, date‐of‐expiry, human packed red blood cell (PRBC) and platelet (PLT) components. An in vitro human whole blood culture model of transfusion was used to characterise changes in monocyte and neutrophil inflammatory responses (intracellular cytokine staining) following exposure to fresh and date‐of‐expiry PRBC‐SN and PLT‐ SN, antibodies targeting antigens relevant to transfusion‐related acute lung injury (TRALI; targeting HLA‐I, HLA‐II or HNA‐2a), and a range of biological response modifiers that have been reported to accumulate during routine storage of blood components (5‐,12‐ and 15‐HETE, IL‐8, sCD40L). The impact of dose was assessed using a 10% and 25% blood replacement strategy for PRBC‐SN and PLT‐SN and a 2 log dose response curve for 5‐, 12‐ and 15‐HETE, IL‐8 and sCD40L. All models also utilised a parallel approach of “transfusion”with and without co‐culture of 0.23 μg/ml LPS to model an inflammatory state in the recipient.
Results
In the ovine models, transfusion of date‐of‐expiry PRBC‐SN or PLT‐SN induced pathophysiological changes characteristic of TRALI, including haemodynamic and respiratory changes and pulmonary oedema. In both models, these outcomes were only observed when using date‐of‐expiry products in combination with underlying inflammation induced by administration of LPS. Using the PRBC‐SN and PLT‐SN from the ovine model in a human in vitro transfusion model we report that both PRBC‐SN and PLT‐SN mediated suppression of monocyte and neutrophil inflammatory responses in the absence of LPS, however both PRBC‐SN and PLT‐SN augmented the LPS‐induced monocyte and neutrophil inflammatory response. In these experiments, a dose response was also evident with further augmentation of the LPS‐induced inflammatory response with a 25% replacement volume. From our laboratory transfusion models using antibodies and BRMs, we also found that both monocyte and neutrophil responses were predominantly supressed in the absence of LPS, but the inflammatory response was augmented in the presence of LPS, particularly for 12‐HETE, where a clear dose response was also evident. Of note, the profile of immune modulation was dependant on the specific biological mediator used, indicating that differences in the composition of blood products is likely to mediate different outcomes.
Summary/Conclusions
Mechanisms associated with adverse patient outcomes post transfusion are multifactorial. Our laboratory models indicate that the patients’ underlying inflammation is a key parameter associated with development of adverse outcomes post‐transfusion. In addition, dose is an important factor mediating transfusion outcomes, albeit due to increased transfusion volume, or, due to accumulation of soluble mediators during routine storage.
P‐765
Transfusion Reactions – A 5 Year Overview
Scott Y , Avery S , Wallis J , Whitehead S
The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background
The incidence and severity of transfusion reactions is useful when seeking consent for transfusion from patients.
Aims
Retrospective analysis of all reported transfusion reactions over 5 years to estimate frequency and severity and probable causation in a large tertiary care hospital.
Methods
All transfusion reactions are reported on a standardised form from 1st January 2010 to 31st December 2014 were analysed according to the transfusion reaction type, clinical area, age of patient and component responsible.
Results
255 transfusion reactions were reported. 56.6% of patients were male. After investigation 119 were deemed not to have had a significant transfusion reaction. The remaining 136 reports were classified by type. 14% (19) were reported to national haemovigilence systems (SHOT/SABRE).


Clinical Area – Table 1
Paediatrics includes haematology/oncology and neonates. Paediatric cardio thoracic surgery comes under the surgical category
Reaction Type – Table 2
Age at time of reaction – Table 3
Reaction by product – Table 4
Reported reactions classed as not necessarily transfusion related included: Temperature rises of < 1°c from baseline with no other symptoms. Patients pyrexial pre‐transfusion or with other good reason for pyrexia. Rashes that on review were considered not transfusion related.
Conclusion
Confirmed transfusion reactions are relatively rare and mainly mild in nature. Reactions are relatively more common in children. This may in part be due to greater recognition by parents or other carers, or because reactions may genuinely be more common in children. Alternatively units that transfuse adults regularly, e.g. haematology day unit may deal with reactions without reporting giving a lower reporting rate.
The relatively high rate of reports that were not deemed to be significant reactions suggests that staff are on the whole vigilant and do report most possible reactions.
P‐766
Transfusion Transmitted Bacterial Infection, A 2‐Year Survey
Moncharmont P , Barday G , Meyer F
EFS Rhone Alpes Site de Lyon Gerland, Lyon, France
Background
Transfusion transmitted bacterial infection (TTBI) continues to be the cause of serious adverse reactions (AR) in recipients with platelet concentrates (PC) as the major source of TTBI.
Aims
The incidence of TTBI was evaluated in the recipients of blood components by studying all AR reports collected during a period of two years with this diagnosis.
Methods
The study involved patients from hospitals in the Rhone Alpes area, and who had been reported with TTBI. Each AR had been included in the national haemovigilance database. The severity of the AR, blood component(s) involved, clinical course and outcome, and imputability were evaluated.
Results
From January 1st 2012 to December 31st 2013, a total of 82,286 patients were transfused with 483,868 blood components. One thousand three hundred and fifty four adverse events were reported, of which 170 (12.6%) were AR with a diagnosis of TTBI. Of the 167 patients involved, 57 were females (34.1%) and 110 males (65.9%). In 134 reports, the patient had an associated disease, which could also explain the clinical signs observed and this included 102 infections (76.1%) before or at the time of the transfusion. One hundred and forty seven AR (86.5%) were not severe, 17 were severe (10.0%), 4 life threatening (2.3%) and 2 resulted in death (1.2%). The involved blood component was red blood cell concentrates in 146 cases (85.9%), PC in 23 (13.5%) and fresh frozen plasma in 1 (0.6%). Blood culture of the recipient was performed after the AR in 164 cases, and was positive in 79 cases (48,2%) and, negative in 85 (51.8%). Bacterial culture of the blood component was performed in 157 cases, and was positive in 7 cases (4.5%) and negative in 150 (95.5%). Among the 7 bacterial culture positive blood components, only one was truly contaminated. This was an apheresis PC contaminated with Escherichia coli, which was transfused to a female recipient who subsequently died. The imputablity of the blood component was excluded in 165 cases (97.0%), and certain in only 1 (0.6%). The incidence of TTBI was very low at 1 in 483,868 transfused blood components or 0.2 per 100,000 blood components.
Summary/conclusions
The incidence of TTBI remains very low over the two year period studied. Surprisingly, a great number of recipients were already infected before or at the time of the transfusion. The imputability of blood components in the AR was currently excluded.
P‐767
Analysis of Adverse Reactions Related to Transfusion Reported to Regional Centre of Transfusion Medicine And Blood Bank in Wroclaw, Poland From 2010 to 2014
Malyszczak E , Wojciechowska – Chorebala A , Szymczyk – Nuzka M
Regional Centre of Transfusion Medicine and Blood Bank, Wroclaw, Poland
Background
Adverse reactions related to transfusion are reported from hospitals in Poland to appropriate Centres of Transfusion Medicine. Continuous analysis of such situations helps improve haemovigilance, enables production of safer blood components and allows introducing preventive measures.
Aims
We assessed the incidence of adverse reactions related to RBCs, PC and FFP transfusions. We investigated benefits for patients connected with modification of blood component preparation technique.
Methods
We analysed adverse effects after transfusions, which were reported to our Blood Centre from 2010 to 2014. 472 cases were evaluated. The imputability of this effects were qualified as levels 1–3 (possible, probable, certain association with transfusion). We assessed the incidence of adverse effects after RBCs, PC and FFP. We evaluated posttransfusion allergic reactions after polled PC and apheresis PC. Between 2010 and 2014 new methods of blood components preparation were introduced in our Blood Centre. Buffy coat pooled platelet concentrates suspended in additive solution PAS III have been produced since 2012. We observed the incidence of post transfusion allergic reactions between 2010 and 2014 connected with buffy coat pooled PC suspended in PAS III or only in plasma. Two year periods of time were compared: 2010–2011 which included only pooled PC with plasma and the other – 2012–2013 suspended in PAS III.
Data concerning apheresis PC covers only one‐year, as the production was introduced in January 2014.
Results
1) The number of adverse reactions after transfusion between 2010–2014‐ (table 1).
2) The number of allergic reactions after transfusion of pooled PC were decreased in analyzed periods from 1.28 to 0.43.
The number of allergic reactions after transfusion of apheresis PC suspended in PAS III was 0.35 while apheresis PC suspended in plasma was 2.12.
The results were expressed per 1 000 therapeutic units of suitable PC, which were given to clinical use.

Conclusions
1) The most common adverse effects, which were reported to our Blood Centre, were febrile non‐haemolytic transfusion reactions after RBCs and allergic reactions after FFP and PC.
2) This study clearly indicates that the incidence of post transfusion allergic reactions significantly decreased following the implementation of pooled PC and apheresis PC in additive solution PAS III.
P‐768
Notify Library of Adverse Outcomes From Medical Products of Human Origin Expands to Include Blood
Whitaker BI1, Dodd R 2, Facco G 3, Fehily D 3, Marano G 3, Menitove J 1, Muniz E 4, Navarro A 5, Wiersum-Osselton J 6, Strong DM 7
1AABB, Bethesda, MD, United States of America2American Red Cross, Rockville, MD, United States of America3Istituto Superiore di Sanita, Roma, Italy4Banc de Sang i Teixits, Barcelona, Spain5BST‐OCATT, Barcelona, Spain6TRIP Hemovigilance and Biovigilance, The Hague, The Netherlands7Notify Project, Edmonds, WA, United States of America
Background
The Notify Library (www.Notifylibrary.org) is a joint global initiative, co‐sponsored by the World Health Organization (WHO) and the Italian National Transplant Centre (CNT) to support the sharing of published vigilance information for the purpose of education and transparency about adverse outcomes associated with Medical Products of Human Origin (MPHO), including blood, organs, tissues, and cells.
Aims
In order to develop a comprehensive reference tool including all MPHO, the addition of adverse events associated with blood collection and transfusion was considered critical to the mission of the library.
Methods
Using established haemovigilance resources and working with the existing Notify structure, the characterization of harm has been updated to include Harm to Donor, Harm to Recipient, Harm to Fetus/Offspring, and Risk of Harm. A taxonomy for adverse transfusion reactions and adverse blood donor reactions has been adopted to allow the incorporation of haemovigilance records into the database. Reports of adverse events that are added to the library are categorized by incident type (type of reaction or event), type of harm, MPHO substance, latency, alerting signals, frequency, imputability to MPHO and demonstration thereof, and reference. Only peer‐review published material, legal documents, and reports from well‐established haemovigilance/biovigilance systems are to be included in the library.
Results
There have been 1130 didactic cases entered into the Notify Library, 72 of which are related to blood or blood products. Over 2000 references have been reviewed by the editorial groups made up of experts from transplantation, transfusion, and assisted reproduction across WHO regions collaborating in ad‐hoc working groups to enter the case descriptions into the database.
The time and effort needed for the review and appropriate classification of exemplary cases and reports from the transfusion and blood donation literature quickly became burdensome with over 400 references initially identified. Clarification of the specific role and intended use for the searchable blood literature is underway.
A proposal for the establishment of a functional blood adverse occurrence review structure was developed at the Notify Project Technical Meeting in February 2015. This structure is designed not to burden the expert participants, while allowing for timely review and consultation to maintain the relevance of the searchable library. It will include recognition for individual participation and the involvement of early career clinicians, to encourage their understanding of and involvement with international haemovigilance efforts.
Summary/Conclusions
Lessons provided through review of the rich haemovigilance literature associated with blood collection and transfusion may be transferable to other MPHOs of more recent clinical use and serve as a didactic tool for trainees. In order to achieve the Notify Library aim of a comprehensive reference tool including all MPHOs, a comprehensive, mutually supported approach for incorporating the haemovigilance literature must be agreed upon for further progress.
P‐769
Abstract Withdrawn.
P‐770
Analysis of Adverse Reactions in Recipients After Transfusions of Platelet Concentrates vs Selection of a Preparation Method
Król D , Drybanska B , Dylag S
Regional Center of Blood Donation and Treatment, Katowice, Poland
Background
Transfusions of erythrocyte concentrates, platelet concentrates (PC), plasma, or other blood components may result in the occurrence of transfusion reactions, possible even in 10% of blood component recipients. Adverse reactions are observed during the transfusion or afterwards, and are associated with the transfused blood component specification, its composition, and the patient's clinical condition.
Most frequently, after blood component transfusions we observe mild or moderate reactions, including fever, shivers, urticaria or itch. In the case of transfusing blood components containing plasma, there may occur symptoms characteristic of allergic reactions, a response of the recipient's body to the protein present in the donor's plasma.
Blood components are applied in patients suffering from various diseases; therefore, they must be safe and available.
Aims
Analysis of the adverse reactions in recipients after transfusions of platelet concentrates reported in 2014 to the Regional Centre for Blood Donation and Treatment (RCKiK) in Katowice.
Methods
In 2014, 14,097 therapeutic packages of leukocyte‐poor platelet concentrates of different specifics, that is, pooled, obtained by the method apheresis (separators by Haemonetics, Terumo BTC), and thawed ones, were presented for treatment. All the pooled platelet concentrates were derived from buffy coats with the use of an Orbisac device (Terumo BCT). These, in turn, were obtained from conserved whole blood stored in Macopharma top‐and‐bottom containers. For suspension of the pooled blood platelets we applied plasma, as well as a mixture of plasma and platelets additive solution – PAS (SSP+, Macopharma), with the 2:3 plasma to SSP+ ratio (30–40% of plasma, 60–70% of SSP+). As a feedback, 14 cases of transfusion complications were reported by medical subjects, which accounts for 0.10% of the total number of transfused blood platelet concentrates.
Results
In the studied period 14 adverse reactions after blood platelet transfusions were reported to RCKiK: in three cases patients were found with lymphocytotoxic antibodies, after transfusions of pooled platelet concentrate in PAS, in nine cases patients displayed allergic reactions after transfusing apheresis‐derived platelet concentrates (8 cases) and pooled platelet concentrate in plasma (one case), in two cases the analysis of the reported transfusion reaction symptoms did not confirm a direct relation to the transfused blood component.
Allergic reactions occurred in 9 patients after transfusing blood platelet concentrates containing plasma. There was no report of an allergic reaction after a platelet concentrate in PAS, where the protein level was 24.78 g/l (7,6 g/PC), which constitutes 40% of the level of protein contained in plasma. The remaining parameters of the platelet concentrate with PAS were all up to the quality standards (V = 309 ml+23; BPC=4.31x1011+0.52; pH=7.24 +0.07).
Conclusions
The analysis of complications after a blood component transfusion is a source of information about the blood component and a selection of its preparation method. Introducing a new plasma‐reduced blood component seems to be effective in decreasing the risk of occurrence of allergic reactions.
P‐771
The Prevalence and Trend of ABO Mismatch Red Cell Transfusion in State Sector Hospitals of Sri Lanka From 2010 to 2014
De Silva SK , Sampath HMRC , Siriwardena CJ , Subasinghe NDNS , Dissanayake SAR
National Blood Transfusion Service, Colombo, Sri Lanka
Background
ABO mismatch red cell transfusions are an important entity within the serious hazards of transfusion. It is a significant cause of preventable acute hemolytic transfusion reactions which may lead to serious morbidity and mortality. The cause for such may occur at the blood bank setting or at the place of transfusion.
Aims
To determine the prevalence and trend of ABO mismatch red cell transfusions in state sector hospitals of Sri Lanka and to study the causes that leads to such, together with their outcome from 2010 to 2014.
Methods
A retrospective cross sectional study was conducted. Data was collected using a data extraction sheet. The number of total transfusions from 92 state sector hospitals was obtained from the blood transfusion statistics and the number of ABO mismatch blood transfusions was obtained from National Hemovigilence data. The prevalence of ABO mismatch blood transfusions was calculated per 100,000 red cell transfusions. Causes of the events were obtained by analyzing data from the National Hemovigilence System. Data entry and analysis were done accordingly.
Results
Over the 5 years 1,665,047 red cell transfusions occurred within state sector hospitals of Sri Lanka with 9074 cases of adverse reactions being reported out of which 101 cases were due to ABO mismatch blood transfusions with a prevalence of 6.07 per 100,000 transfusions. Analyzing the prevalence of ABO mismatch transfusions, it revealed a steep rise in trend from 2011 (4.76 per 100,000) to 2012 (8.69 per 100,000) and a drastic drop from 2013 (7.28 per 100,000) to 2014 (4.89 per 100,000). Causes of error were broadly categorized into blood bank errors (63.37%;n = 64) and errors at the site of transfusion (36.63%;n = 37). Patient testing was identified as the most common error occurring at the blood bank accounting for 48.44%(n = 31) of the blood bank errors. Patient identification was the most common place of error at the site of transfusion (54.05%;n = 20). Out of the total ABO mismatch red cell transfusions 73.27% (n = 70) showed mild to no symptoms and were managed in the ward setting while 26.73% (n = 27) cases had moderate to severe reactions. No deaths were reported due to ABO mismatch red cell transfusions within the study period.
Summery/Conclusion
ABO mismatch blood transfusions occurred at a prevalence of 6.07 per 100,000 transfusions within the state sector hospitals in Sri Lanka during the study period. A steep rise in prevalence was seen from 2011 to 2012 possibly due to improved hemovigilence reporting and then a drop from 2013 to 2014 which could have been due to corrective measures. Analyzing the causes, errors during patient testing was the main cause in the blood bank setting while errors occurring during blood administration were the leading cause at the site of transfusion. Majority of ABO mismatch transfusions were managed at the ward setting with minimal or no symptoms (73.27%;n = 74) while 26.73%(n = 27) showed moderate to severe reactions. There were no deaths reported within the study period.
P‐772
Trali: Associated With Donor Primary Autoimmune Neutropenia
Moore NM1, Roddy J 2, Motherway C 2, O'Leary H 2, Power J 3
1Irish Blood Transfusion Service MRTC, Cork, Ireland2University Hospital Limerick, Limerick, Ireland3Irish Blood Transfusion Service, Cork, Ireland
Background
Transfusion related acute lung injury (TRALI) is a severe transfusion trates during or within 6 h of transfusion. The most frequent cause of transfusion‐associated mortality reported to the FDA with a mortality rate of 0.4/million components ‐SHOT 2013.
Toy et al. identified recipient and blood product factors associated with increased risk of TRALI.
The aetiology of TRALI is usually associated with HLA and HNA antibodies, however where no antibody identified remains enigmatic. A number agents have been suggested as triggers into a primed recipient with a “one‐two”punch hypothesis postulated.1
Aim
Document a case of TRALI associated with donor Autoimmune Neutropenia proposed as a trigger in the patient, a primed recipient with a history of chronic alcohol abuse.
Method
HLA Typing: DNA‐PCR‐SSO. (IBTS ‐ NBC Dublin).
HLA leucocyte antibody: Fluorescent Bead Array.
Lymphocyte immunoflourescence test flow cytometry (LIFT).
HNA typing by PCR‐SBT and monoclonal antibodies.
Granulocyte Immunoflourescence Test (GIFT). (Histocompatibility and Immunogenetics Dept, NHSBT, Bristol, England.)
Results
54 year old female with upper GI unstable bleed (Hb 4.0 g/dl), requiring banding of oesophageal varices. Background of C2H3OH abuse, fatty liver. ALI occurred one hour into 5th FRCC (26 days old) with acute O2 desaturation, S. Tachy. Required Bipap ventilation unstable 2 weeks. CXR ‐ Interval deterioration diffuse consolidation of right lung, extensive patchy opacification of left.
BNP pre event, 444 pg/ml (>125 pg/ml) post 4286 pg/ml.
Patient: HLA Class I IgG antibody negative
HLA Class II IgG antibody positive
Granulocyte antibody (GIFT) negative
Lymphocytes ‐ elevated levels of membrane associated IgG
Donor
HLA Class I / Class II antibodies negative.
Granulocytes ‐ elevated membrane immunoglublins (IgM).
Crossmatch donor serum vs patient granulocyte negative.
Granulocyte specific IgG antibodies support diagnosis of Autoimmune Neutropenia.
Conclusions
Patient factors influence why some patients develop TRALI and others do not.
The female donor of the index FRCC transfusion reported as a probable dx of Auto immune Neutropenia. Although BNP levels ↑ pre and post transfusion acute clinical symptoms of TRALI occurred during transfusion of FRCC from this donor. Protracted ventilation would support diagnosis of TRALI.
Bux 2007 postulated a 2‐event entity for TRALI. The “first event”is an inflammatory condition in the host causing priming in the lung. The “second event”is transfusion of a blood product containing antibodies or factors that accumulate during storage, providing additional signals resulting in pulmonary oedema.
We propose chronic alcohol abuse acted as the “first event”with ¯ antioxidant glutathione in the lung, resulting in enhanced pulmonary inflammatory response.2
The “second event”was transfusion of granulocytic specific antibodies from the index donor with Autoimmune Neutropenia, notwithstanding controversy as to whether an auto antibody could trigger TRALI.
The strength of the auto antibody in the donor varies with time and could not be reproduced on further study, however was evidenced by neutropenia on several routine FBC over two years.
References
1. Shaz Beth. H. Blood 2012; 119(7):1620‐1621
2. Boé DM, et al. Clin Exp Res 2010 Oct; 34(10):1723‐1732.
P‐773
About Certain Immunological Aspects of Blood Safety
Bakhovadinov B1, Hakberdiev R 2, Kucher M 3, Hodjiev A 2, Tretyakova A 4
1St. Petersburg State Medical Institute, St. Petersburg, Russian Federation2Republican Scientific Blood Centre, Dushanbe, Tajikistan3St. Petersburg Medical Institute, St. Petersburg, Russian Federation4St. Peterburg Medical Institute, St. Petersburg, Russian Federation
Blood components were provided to every third patient in intensive therapy units. It has been established that the transfusion of fresh‐frozen plasma, erythrocyte aggregates, thrombocyte concentrates, and cryoprecipitates can lead to the development of TRALI during the first six hours following the start of transfusion.
Goal
To study the prevalence of TRALI in patients with surgical, obstetric‐gynecologic, oncologic, and polytrauma conditions subject to blood components transfusion.
Methods
The results of infusion‐transfusion therapies of 2000 patients were analyzed, of those 1 300 obstetric patients with bleedings, 300 with gastro‐intestinal bleedings, 200 with gynecological and oncological complications, and 200 with polytrauma. The seacrh for anti‐HLA and anti‐HNA antibodies in blood of 6 recepients and 12 blood and plasma donors was conducted.
Results
In total 24 969 blood components doses were transmitted to all recepients: 1 100 of dry antihemophylic plasma, 2 211 of dry and frozen cryoprecipitate, 14 519 of fresh frozen plasma, 500 of fresh citrated plasma, 5 239 of erythrocyte mass, 450 of washed erythrocytes, 150 of platelet concentrate, 550 of 10‐20% albumen solutions, 250 of protein. On average each recepient received 13,13 doses of blood components and solutions. Data allowed to identify 15 recepients (11 women and 4 men) with suspision on TRALI. The 11 of 15 recepinets had clearly identifyable clinical and roentgenologic signs of TRALI. The 4 remaining, due to paucity of data, were identified as «possibily» having TRALI. The friquency of TRALI and «possible TRALI» was 1 in 1 664,6 doses of blood components, which makes 0.060% per component's dose (0.044% and 0.016% accordingly). All female patients with complications had 3 to 7 pregnancies and births. The study of patients and donor's serums showed the presence of antibodies to HLA I antigens in one patient and two donors, to antigens of the II type in one patient and two donors. Anti‐HLA antibodies to antigens of both types were identified in four donors and two patients. Antibodies were not identified in 2 patients (men) and 4 donors (3 men, 1 woman).
Conclusions
The friquency of TRALI and «possible TRALI» in our research is in accordance with available literature. The presence of female blood and plasma donors with frequent pregnancies and births in anamnesis leads to higher risks of TRALI. In order to prevent TRALI it is necessary to launch a study on anti‐HLA and anti‐NHA in blood of female donors, and exclude those with antibodies from donorship. It is necessary to introduce restrictive practices of using hemotransfusion components, and especially of FFP, introduce alternative to homogeneous hemotransfusion tecknologies (blood and its components’ autodonation, collection, processing, refusion, surgical bleedings, usage of erythropoetins, hemocorrectors with the function of oxygen transmission (perftoranum, hemopure), cleaned factors of coagulation system, especially in obstetrical patients. Leukofiltration is a prophylactic of sensibilization of patients to HLA antigens, and accordingly the measure of the prophylactics of TRALI, connected with recepient's factors. Allogenic concentrates of platelets must not be prepared on the basis of female donors’ plasma, however on the basis of reconstituted solution ‐SSP+.
6.6 Haemovigilance and Transfusion Safety
P‐774
Donor Anti‐Hla Antibodies May Not Significantly Increase the Risk of Transfusion Reactions in Recipients of Apheresis Platelet Concentrates
Fontana S1, Infanti L 2, Thierbach J 3, Muriset M 1, NIederhauser C 1, Baumer H 1, Rotzetter K 1, Sigle J 4, Buser A 2
1Interregionale Blutspende SRK, Bern, Switzerland2Blutspendezentrum SRK Beider Basel, Basel, Switzerland3Blutspende SRK Ostschweiz, St. Gallen, Switzerland4Blutspende SRK Aargau‐Solothurn, Aarau, Switzerland
Background
Donor antibodies against HLA antigens may induce TRALI, FNHTR or other transfusion reactions in recipients of blood components containing plasma. The prevalence of HLA‐antibodies in apheresis platelet (PLT) donors is high and varies depending on the detection test used. The clinical impact on patients of HLA‐antibodies detected in PLT apheresis donors, of the HLA‐antibodies detection technique, and of the immunization history of donors, has not yet been assessed on a large transfusion data base.
Aims
To explore the clinical risk of transfusion reactions in recipients of apheresis PLT concentrates collected from donors with HLA‐antibodies detected by different laboratory tests in a broad donor and patient collective.
Methods
Retrospective cohort study on patients receiving PLT concentrates collected from donors previously tested for HLA‐antibodies. HLA‐antibodies were investigated by microbead single antigen essay (Luminex), ELISA (Lambda Antigen Tray), or a combination of GAT and GIFT. All consecutive patients of 2 large public hospitals transfused with PLT concentrates donated after HLA‐antibody testing of the donor were included in the study. Transfusions and transfusion reactions were evaluated if sufficiently documented in the patient records. Transfusion reactions were classified on the basis of IHN standard definitions. The proportions of transfusion reactions were compared by chi‐squared test in transfusions of PLT derived from donors with positive or negative HLA‐antibody test, from male or female donors, and from females with or without history of pregnancy.
Results
Out of 3'837 identified PLT concentrates, 2'976 transfusions were documented and 2'284 had charts evaluable for the occurrence of a transfusion reactions or not. The PLT concentrates were donated by 217 donors and transfused to 609 patients. The current results are summarized in Table 1. Donor, donation, and patient characteristics were roughly balanced between positive and negative HLA antibody results; further analyses aimed to identify confounding factors are ongoing.
Caption 1: Summary of the study results.

Conclusions
This first analyses exploring correlations between HLA antibodies and transfusion reactions in apheresis PLT concentrates doesn't indicate an increased risk due to donors with HLA antibodies, independently from the detection method, or to donors with immunization history (pregnancy). This conclusion may be limited to PLT in additive solution (>80% of the study products), is not applicable to HNA‐antibodies (no positive donors found in our study), and should be confirmed by the ongoing analysis of additional risk factors, which could bias the results. After the introduction of the mandatory pathogen reduction with amotosalen and UVA, Switzerland is increasing the proportion of buffy coat PLT, in which the antibodies are further diluted. Thus, further preventive measures reducing the risk of transfusion reactions after PLT transfusion in this setting may be not necessary.
P‐775
Haemovigilance Achievements to The Quality and Safety of Blood Transfusion
Escoval MA , Condeço J , Sousa G
Portuguese Blood and Transplantation Institute, Lisboa, Portugal
Background
The Portuguese Haemovigilance System (PHS) was implemented in 2008. Haemovigilance reporting covers all types and severity levels of reactions, errors and near miss events, within the transfusion chain, as well as hospitals and blood establishment's activities data. Since 2012 all reports are validated against the internationally adopted definitions. Annual reports are publicly available.
Aim
To analyse the impact of haemovigilance on the quality and safety of blood transfusion identifying outcomes, improvements and strategic challenges achieved.
Methods
A retrospective analysis of data reported to the PHS from 2008 to 2013, assessing the transfusion risk and the impact of recommendations/measures triggered, has been performed. The rate of reactions and errors was calculated per 10 000 blood components transfused.
Results
The reporting to the PHS, its consistency and validity, increased quantitatively and qualitatively since 2008. In 2013, participation by hospitals has been approximately 100%.
Adverse Transfusion Reactions (ATR) rate increased steadily until 2012 followed by a slight slowdown in 2013. (7.6 in 2008, 13.2 in 2012 and 11.8 in 2013). The transfusion risks identified were haemolytic reactions caused by ABO incompatibility and ATR with respiratory distress.
Transfusion related death rate is 0.03/10 000,seven cases, four haemolytic reactions caused by ABO incompatibility, a case of circulatory overload (TACO), an anaphylactic reaction and a case classified as other.
Haemolytic reactions rate, caused by errors, remain stable, however a slight decrease seems to have occurred in 2013 (0.3 in 2008‐2012, 0.1in 2013) following the decrease in the errors reporting rate (0.8 in 2008‐2012, 0.6 in 2013).All the errors were associated with patient misidentification.
Transfusion Respiratory complications seem to assume an increasing rate and severity 1.1 in 2008‐2012 to 1.4 in 2013, mainly due to the increase in TACO report (0.4 in 2008‐2012 to 0.7 in 2013). The risk of TRALI remains stable and low (only 2.7% of the components transfused in 2013 were plasma).
Recommendations were issued concerning ATR related to the quality and safety of the product and male only plasma utilization (2012), patient identification technologies/ procedures (2014), and technical audits have been implemented.
Donor Adverse Reactions rate registered also a steady increase until 2012, having been a slight slowdown in 2013. Severe reactions were 0.15 / 1000 donations stressing blood donation safety.
Conclusions
The most important haemovigilance achievements were transparency, awareness of the risks and prevention of preventable reactions.
The objective of transparency was largely achieved by high participation of hospitals and a validation policy of the reported events.
The PHS provided important data to quantify the transfusion risks. Even when it was not possible to reduce the risks, the results were essential to identify the areas of intervention.
The use of hemovigilance is essential to complete the quality cycle: identification of a problem, implementation of measures and monitoring outcomes. The objective of reducing preventable reactions by implementing recommendations has not yet been achieved, although errors show a downward trend.
The next challenge arises as a paradigm shift to ‘optimal blood use’ requiring the implementation of critical quality indicators
P‐776
Using a Regional Audit Cycle to Improve Platelet Transfusion Practice and Reduce Double Dose Transfusions
Sear F , Foukaneli T , O'Brien J
NHSBT, Cambridge, United Kingdom
Background
Platelet issues have been gradually rising since 2008. Between 2010‐2011 an increase in platelet issues of 16.6% was seen in the East of England in comparison with a national rise of 8.6%. In 2012 a regional audit was conducted to try and determine potential reasons for this increase. 18 of the 19 Trusts participated. Results demonstrated that patients came predominantly from haematology‐oncology and surgical departments. However the audit also revealed that of the 767 episodes of platelet transfusion audited, 101 were transfused with 2 or more units and 58 of these transfusions were deemed inappropriate.
Regional actions implemented following the audit included; piloting BSMS CUSUM charts to monitor platelet issues, regular sharing and discussion of platelet data and individual practice at regional meetings, promotion and use of education resources for clinical staff within hospitals, and changes to individual hospital practices. In 2014 a re‐audit was conducted to identify if practice had changed.
Aims
To identify whether there had been a change in the practice of double dose platelets transfusions.
Methods
15 of the 19 Trusts participated in the re‐audit. All Trusts retrospectively audited 40 cases of platelet transfusion. 540 episodes of transfusion and 260 individual patients were audited. Data was collected on; BCSH indication codes for transfusion, specialism, diagnosis, patient age, internal authorisation of platelets for issue, the number of transfusions where more than 2 units were requested and whether these were then issued and transfused.
Results
The 2 main cardiac hospitals within the region did not participate in the re‐audit. Taking this into account comparison of the results of participating hospitals in 2012 and 2014 demonstrated little change in the specialism's or diagnosis of the patients requiring platelets. The percentage of haematology patients receiving platelets dropped from 69% to 60% and the percentage to patients with massive haemorrhage dropped from 14% to 6%. 50% of transfusions were given to patients >65 years of age. The number of cases where 2 or more units of platelets were issued for transfusion as one dose was almost halved reducing from 13.8% to 7.6% and there was a demonstrated reduction in the percentage of these transfusions deemed inappropriate reducing from 37.8% to 32.3 In addition at the time of re‐audit, measured against the same period in 2013 the East of England had a decrease in platelet issues of 5.5% against a national rise in issues of 1.6%.
Summary
The regional audit cycle enabled us to identify the issues of double dose and inappropriate platelet transfusion and to implement appropriate actions to improve practice with engagement from the whole region.
Re‐audit demonstrated that practices have improved against a patient group which has remained the same. Inappropriate transfusion of more than one dose of platelets was a finding in both cycles although this had reduced in 2014. The total number of double dose transfusion requested had reduced by 45%. At the start of the audit cycle the region was demonstrating an upward trend and large rise in platelet issues, this now shows a decrease.
P‐777
Errors in Transfusion of Transplant Recipients: Time to Produce Guidelines
Watt A1, Bolton-Maggs PHB 2
1Serious Hazards of Transfusion, Manchester, United Kingdom2University of Manchester, Manchester, United Kingdom
Background
Patients receiving transplants, solid organ or haemopoietic stem cell transplants (HSCT) need careful attention in provision of blood component support, especially when donor and recipient are ABO or D nonidentical. Serious Hazards of Transfusion (SHOT), the UK confidential haemovigilance reporting scheme, records adverse transfusion incidents finds a surprising number of errors.
Aim
To review specific problems (errors) related to transfusion in transplantation.
Methods
A five year analysis of SHOT incident reports related to transfusion of patients undergoing transplantation was undertaken.
Results
A total of 214 cases was analysed from calendar years 2010‐2014, (2010 n = 42; 2011, n = 37; 2012, n = 37; 2013, n = 52; 2014 n = 46), including 150 HSCT and 62 solid organ transplants (2 mixed/unknown). The types and causes of errors are shown in the tables.
Table 1: Classification of errors

Table 2: Causes of error

The following problems were identified:
Lack of communication n = 128/214(59.8%), especially between the clinical team and the transfusion laboratory and between different clinical teams for patients receiving shared care between the transplant centre and their local hospital. Failure to inform the laboratory that a HSCT was taking place with ABO group change occurred in 53 cases.
Anti‐D immunoglobulin prophylaxis not given to D‐negative females of childbearing potential who receive D‐positive solid organ transplants, especially when organs are from living donors.
No national transfusion guidance for appropriate use of plasma‐rich components in the immediate post‐transplant period following an ABO‐incompatible (ABOi) solid organ transplant and a lack of understanding of the transfusion risks associated with passenger lymphocyte syndrome (PLS).
Conclusion
British Committee for Standards in Haematology (BCSH) transfusion guidelines cover some of the clinical and laboratory problems identified here, but errors are still seen which need attention.
There are no transplantation guidelines that fully inform transfusion professionals about group changes which would help avoid the errors reported here. SHOT recommends that transplantation and transfusion experts should collaborate to develop guidance for:
The procedures, particularly communication protocols, necessary for managing transplant patients, especially where the transplant is ABO/D mismatched.
The procedures necessary for managing female transplant patients who are of childbearing potential, where D‐positive transplants have been given to D‐negative recipients.
The specific transfusion requirements for recipients of ABOi solid organ transplants to include particular reference to risks associated with large volumes of plasma‐based components (fresh frozen plasma and platelets) until ABOi organs become accommodated and the risk of complications from PLS.
P‐778
Dangerous Clinical Practice Caught by Near Miss Analysis
Watt A1, Bolton-Maggs PHB 2
1Serious Hazards of Transfusion, Manchester, United Kingdom2University of Manchester, Manchester, United Kingdom
Background
A wrong blood in tube (WBIT) incident can lead to transfusion of an incorrect blood component, incompatible for ABO, which could have disastrous consequences for the patient. Wrong blood in tube is defined as:
blood taken from the wrong patient, but labelled with the intended patient's details.
blood taken from the intended patient, but labelled with another patient's details.
Most of these incidents are detected, so do not result in a wrong transfusion and are therefore classified as ‘near miss’. Serious Hazards of Transfusion (SHOT) the UK's confidential haemovigilance reporting scheme, encourages reporting of all near miss incidents and these have been fully analysed since 2010.
Aim
To investigate near miss WBIT incidents to determine whether the staff groups responsible, the causes of the errors and the means of detection of these incidents have changed over a five year.
Method
A retrospective analysis was performed of near miss WBIT incidents reported to SHOT in the five‐year period 01/01/2010 to 31/12/2014. The data were collated for:
Staff responsible for taking samples
Practices leading to WBIT
Circumstances leading to detection of WBIT
Results
Total near miss WBIT incidents have risen each year from n = 386 in 2010 to n = 686 in 2014, but the overall pattern remains unchanged. The majority of samples are taken by medical staff and the underlying poor practice remains failure or patient identification and labelling the sample away from the bedside.
The majority (78.3‐91.1%) are detected by the laboratory because a previous blood group on the patient gave different results.
During this five‐year period a further 14 cases were not detected and led to an incorrect transfusion of which 5 (37.5%) were ABO‐incompatible. In the first 3 years the ratio was approximately 1 wrong transfusion to every 100 near miss events, but in the last 2 years there have been more near misses and very few or no resultant wrong transfusions. This may relate to introduction of the check sample as recommended in new guidelines in 2012.
Caption 1: Practices leading to WBIT (percentages).

Caption 2: Percentage of each staff group responsible for taking samples.

Conclusion
Reports of near miss WBIT events continue to rise year on year and the causes have remained constant.
The vast majority of WBITs are detected before patient harm, mostly in the transfusion laboratory, whereas the staff responsible and the causes of WBIT sampling errors are entirely in the clinical area. The quality management systems and guidelines related to laboratory practice are calculated to detect WBITs, but that cannot be relied upon to detect every error.
Clinical practice should be improved to reduce the incidence of WBITs and these data from a five‐year period show the areas in which improvement could be made. In 2013 SHOT recommended that the transfusion process should be redesigned by process mapping and audit at local and national level to design out errors such as WBIT.
P‐779
Seventeen Years Analysis of Adverse Events Associated With Anti‐D Immunoglobulin
Davies T1, Bolton-Maggs PHB 2, Poles D 2
1NHSBT, Manchester, United Kingdom2SHOT, Manchester, United Kingdom
Introduction
Adverse events associated with anti‐D immunoglobulin (Ig) are included in the annual SHOT report as they provide a useful insight into process errors which may be applied to transfusion as a whole. Anti‐D Ig errors may be broadly categorised into: (1) failure to administer in a timely manner, placing women at risk of becoming sensitised to the D antigen, and (2) inappropriate administration, exposing women uneccessarily to a medicinal blood product manufactured from pooled human plasma
Methodology
Cases are categorised into the following types of event: Omission or late administration of anti‐D Ig, anti‐D ig administered to a D positive woman, administered to a woman with known immune anti‐D, administered erroneously to the mother of a D negative infant, administered to the wrong woman, administration of the wrong dose for the indication, and handling and storage errors such as administration of expired anti‐D Ig.
Additionally, the staff group of the person making the primary error is recorded: Nurse / Midwife, Laboratory Scientist or Medical Officer
Results
In the period between 1998 and 2014 2237 adverse incidents relating to anti‐D Ig were reviewed.
In 1462/2237 (65%) cases prophylaxis was delayed, omitted or underdosed, and in 775/2237 (35%) cases anti‐D was inappropriately administered or handled poorly.
In 1632/2237 (77%) cases, the primary error was made by midwives, nurses and doctors.
Commentary
Key systems failures in the process include lack of communication between healthcare teams, a lack of knowledge and training in healthcare professionals making treatment decisions, pressure of work and availability of experienced senior staff, and general poor practice including manual transcription of critical blood grouping information between paper records and a culture of completing the healthcare record before the intervention has actually been performed.
The clinical impact of such errors is unknown as there is no long term follow‐up of cases. SHOT is now conducting a survey of all cases of newly diagnosed anti‐D in pregnant women to gain a better understanding of the causes of immunisation.
Consistency of practice in hospitals is not helped by conflicting guidance for prophylaxis from NICE (National Institute for Health and Care Excellence) and BCSH (British Committee for Standards in Haematology), especially in the early stages of pregnancy, nor by poorly evidenced recommendations to change route of administration for particular products in larger women.
Conclusion
SHOT continue to promote partnership working between clinical area and laboratory to ensure the best possible treatment for pregnant women, and the use of a process flowchart to ensure consistency of practice. It is reassuring to discover from the 2013 National Comparative Audit of Transfusion that we get it right more than 90% of the time.
P‐780
Audit of Acute Transfusion Reaction Management and Knowledge
Bielby LJ1, Akers C 1, Glazebrook B 1, Beard P 1, Hennessy C 1, Hogan C 2
1Blood Matters, West Melbourne, Australia2Australian Red Cross Blood Service, Melbourne, Australia
Background
Transfusion is commonly used in a variety of clinical settings. Reactions are relatively uncommon, however they can be serious. An audit to assess transfusion reaction management and knowledge was undertaken by Blood Matters in 2013.
Aim
To measure if blood transfusion policies:
1. are in place?
2. include the management of transfusion reactions ?
3. are understood?are practiced?
Methodology
146 health services across four Australian jurisdictions were invited to participate in the three‐part audit of policy, management and staff knowledge.
Policy: Is the policy for the management and reporting of transfusion reactions consistent with Australian guidelines/standards(1,2)?Management: Up to 10 individual randomly selected retrospective transfusion reactions were investigated.Staff Knowledge: Recognition and management of transfusion reactions was surveyed using a combination of multi‐choice and open‐ended questions.
Results
Ninety‐eight health services submitted responses to the audit (67% return rate).
Policy: All health services had a policy that included management guidelines for transfusion reactions. Overall 95 (97%) included internal reporting mechanisms, e.g. documentation in the incident management system, while only 68 (69%) included information on the review process for reactions. Improvement is required in the initial approach to the management of mild reactions. Stopping the transfusion in mild reactions was only reported to be included in 91% (52/57) of policies. Improvement is also required in performing patient and product identity checks to ensure correct product to correct patient (33/40, 83%).
Management: 53 (55%) of the 97 health services responding to this section reported at least one acute reaction had occurred within audit timeframe. A total of 286 events were reported. 129 (45%) reactions occurred within the first hour of the transfusion, with 84 (29%) occurring in the first 30 min. A doctor was notified of the reaction in 279 (97%) of cases with 247 (86%) being reviewed by the medical officer. 222 (78%) of patients received some form of medication to treat symptoms, often in combination. The most commonly prescribed treatment was paracetamol.
Reporting reactions to local incident management system or transfusion committees occurred in 258 (91%) events. Only 63(22%) patients who experienced a reaction were reported as being provided with information about the reaction.
Staff knowledge: Responses received from 2092 staff, with 1806 (86%) from nurses/midwives. 1381 (66%) were able to accurately describe 4 symptoms that may be indicative of a transfusion reaction, the most common reported being fever (n = 1889, 90%). When asked about first line management of a reaction 2034 (97%) included stopping the transfusion. There was some variation depending on clinical role.
Staff were less clear about investigations required for a transfusion reaction, with apparently little difference in knowledge between the various clinical roles.
Conclusion
Policies and procedures need to include information on transfusion reactions. Staff education should be in line with these policies and procedures, to ensure all staff are aware of the requirements for monitoring for and managing any suspected transfusion reactions.
References
1) ANZSBT/RCNA 2011, Guidelines for the administration of blood products, 2nd edition.
2) ACSQHC 2011, National safety and quality health service standards.
P‐781
Breach In ‘Two Independent Sample’ Policy Led To Implementation of Additional Interventions For Reducing Errors: The Plan And Its Progress
Dara RC , Tiwari AS , Arora DC , Aggarwal G , Acharya DA , Rawat GR , Raina VR
Medanta– The Medicity, Gurgaon, India
Background
In any task, human error is almost always a constant component. Impact of an error in hospital blood bank is sometimes so profound that it can even result in patient's death. To detect incorrectly drawn sample i.e. wrong blood in tube [WBIT]; two independent samples for blood group was in place. This policy mandated doing blood groups on two different blood samples drawn at different times by different personnel. In last one year 12 WBIT were detected. Out of which “two independent sample”policy averted 11 WBIT cases preventing probable ABO‐incompatible transfusions. One WBIT error was missed despite the policy and led to wrong transfusion.
Aim
The objective was to do root‐cause analyses (RCA) of WBIT which was missed, identify the weaknesses in the system and to plan additional interventions to augment transfusion safety and monitor progress.
Materials and methods
The WBIT errors in the last one year were investigated to determine the breach in the system. Potential areas of errors (sample collection, requesting blood component, laboratory processing, blood issue, bedside check at the time of infusion) with the error prevention system in place (error reporting system, controlled patient registration and two independent samples) were analysed and probability of errors in respective areas were identified.
Results
Total of 12 WBIT were recorded for a period of twelve months (median, 1/month). No serious adverse events occurred in patients even with these WBIT events, with the transfusion of 70,323 blood components. On RCA of WBIT, breach in policy occurred because same personnel collected both the samples, simultaneously. Following interventions were incorporated to enhance transfusion safety. Cadre of trained transfusion nurses were formed to collect the recipient sample, request and transfuse the blood component under doctor's supervision. Manual blood request form and issue slip was replaced with computerised formats. Work flow changes were made at the issue counter to prevent near‐miss events. Near patient blood group test was introduced as an additional layer of safety. Adverse event reporting was strengthened by introducing transfusion episode entry in the Hospital Information System (HIS). Three months after interventions rate of WBIT was reduced to zero/month.
Conclusion
Separate cadre of transfusion nurses seems to be an effective intervention either alone or with other interventions has led to a reduction in WBIT. It is difficult to discern as to which intervention/combination is most effective. Data on the pre‐ and post‐implementation of interventions need to be collected for substantial period to demonstrate effectiveness.
Caption 1: WBIT errors.

P‐782
Should We Transfuse at Night?
Johnston AC , Patel R , Cho G , Chowdhury F
Northwick Park Hospital, Middlesex, United Kingdom
Background
The UK Serious Hazards of Transfusion (2005) report suggested overnight transfusions are inherently unsafe and should be avoided unless clinically essential. BCSH Guidelines (1999) recommended documentation of the reasons for such transfusions.
A National Comparative Audit (NCA) of overnight transfusions (2008) showed Northwick Park Hospital had poor results with the majority of transfusions happening outside daytime hours. It was also noted with all transfusions that there were high levels of wastage and poor traceability. There have since been initiatives implemented to improve these areas, with training of medical and nursing staff, use of Intranet screensavers, biomedical scientist education and avoidance of surgery at night.
Aims
To re‐audit overnight red cell transfusions and assess transfusion practice in areas which have reduced staffing overnight and ascertain if the reason for overnight transfusion is recorded.
To determine if transfusion practices have changed following initiatives implemented to improve traceability, reduce wastage and increase transfusion safety awareness compared to 2008.
Methods
The NCA proforma was used to collect data. Patient notes and pathology results were reviewed for transfusion episodes in non‐acute areas with units which left Blood Bank between 19:30 h‐07:30 h during October 2014.
Transfusion practice was assessed by:
1. Percentage of patients having observations within 15 min
2. Time to starting transfusion
3. The documented reasons for transfusion.
Wastage and traceability data are collected monthly by Blood Bank. This data was reviewed as part of the audit. Wastage includes units which were time expired, out of temperature control for too long, damaged packs or ‘medically ordered not used’ units.
Results
In total 104 red cell transfusion episodes were included.
The number of overnight transfusions in non‐acute areas fell from 65% of all transfusions in 2008 to 16% in 2014, but there was an increase from 0% to 12% in transfusions to facilitate next day discharge.
There was no significant change (57% in 2008 and 53% in 2014) in the documentation of reason for transfusion.
In 2014 69% of patients had their observations recorded at 15 min compared to 73% in 2008.
Transfusion traceability has increased from 94.6% of 10,817 units (2008) to 98.2% of 11,033 units (2014).
Blood component wastage figures show a 72% reduction since 2008, which amounts to a cost saving of £179,263 this year compared to 2008.
Conclusion
The initiatives to raise transfusion awareness have led to a massive decrease in the number of overnight transfusions which should reduce the frequency of incidents affecting patient safety. However, practice and safety measures during transfusions have not greatly improved since 2008, suggesting that the risk remains.
Measures used to generally promote good transfusion practice have had other significant benefits with reduced wastage (which is financially advantageous) and improved traceability.
We advocate continuing to raise the awareness of safe transfusion practice to improve patient safety during overnight transfusions because there are indicators that practice is still suboptimal, which may be due to reduced staffing levels at night in non‐acute areas.
P‐783
A Human Factors Review of Blood Sampling… As Done By Practitioners
Pickup L1, Atkinson S 1, Hollnagel E 2, Rawlinson S 3, Bowie P 4
1The University of Nottingham, Nottingham, United Kingdom2Center for Quality Improvement,, Southern Region, Denmark3East of Scotland Blood Transfusion Centre, Dundee, United Kingdom4NHS Education for Scotland, Glasgow, United Kingdom
Since 2000 the Serious Hazards of Transfusion (SHOT) Organisation indicates 50% of all near misses occur at the blood sampling phase (PHB Bolton‐Maggs et al 2014). In particular these data raise concerns for the stages of patient identification and the labelling of samples. The Health and Safety Executive (HSE 2009) highlights that ‘proper’ consideration of Human Factors is necessary for effective health and safety management of an organisation. This approach is adopted by other safety critical industries and actively encouraged by the recent NHS Concordat (NQB 2014), and deemed relevant to blood sampling (Murphy and Kay 2004), however, yet to be fully embraced within healthcare
A pilot study was undertaken by the University of Nottingham, for NHS Scotland and NES to understand why variability exists in patient identification and labelling of blood samples. The aim of this work was to evaluate ‘work as done’ rather than ‘perceived to be done’ and to identify factors contributing to the variability of work practices in the context of: clinical departments, working environments and organisational goals. The study adopted a qualitative approach and was completed in four Scottish hospitals; undertaking observations (n = 50) and interviews (n = 15) with a review of local incident data. The data was used to inform the Functional Resonance Analysis Method ‐ FRAM (Hollnagel 2012). This method models the interactions between the functions relevant to blood sampling and the potential variability in performance.
The output from this method is a visual and descriptive representation of the system, which assists in focusing safety management activities to dampen down unwanted variability.
The findings suggest that the technology to provide information, request a blood sample and provide labels prior to blood sampling were heavily influential to the reliability of obtaining a minimal data set and patient details. The accessibility, location and reliability of technology or its timely repair influenced how practitioners modified their work to minimise delay in obtaining a sample and manage workload associated with a high volume of patients.
The working contexts i.e. emergency departments, outpatients, influenced how blood sampling functions were organised; to balance clinical and safety goals in the context of organisational targets. Variability was discovered in the number of practitioners involved in a single blood sampling process and environmental factors that influenced practitioners work practices to mitigate the impact of common distractions and delays.
The ratio of staff to patients and skill mix within a working environment had implications for the time pressure and accuracy of some blood sampling functions. The writing surface of the label on a group and save sample was also identified as a contributing factor for the accuracy of sample labelling.
The FRAM model has enabled the visualisation of critical blood sampling functions to illustrate how constraints or a lack of resources can impact on the variability of the outcome. This study illustrates how variability in the practitioner's work practices seeks to balance a trade‐off between efficiency and thoroughness in order to achieve the required level of performance.
P‐784
Yield of Lookback And Reverse Lookback In Dutch Blood Transfusion, 2007‐2013
Huynh VT , Lieshout-Krikke RW , Spelmink S , Danovic F , Mäkelburg ABU , Tomson B , Koopman MMW , van Kraaij MG
Sanquin Blood Supply, Groningen, The Netherlands
Background
Transmission of infectious diseases by blood transfusion is minimized by donor selection and donor screening. When a repeat blood donor has been found to be infected, or when a patient shows signs of post‐transfusion‐infection, (reverse) lookback procedures are initiated to confirm or exclude transmission.
Aim
To determine the incidence of blood‐borne transmissions from Dutch blood donors to patients in 2007‐2013; and to evaluate the yield of the (reverse) lookback procedures. Bacterial screening of platelet concentrations is beyond the scope of the study.
Methods
The Sanquin Blood Supply tests each donation for presence of HBV, HCV, and HIV infection and for syphilis. Until 2011 all donations were tested for HTLV; since then only new donors are tested for HTLV. In 2008 HBV DNA screening was started to detect early and occult HBV infections [OBIs]. When a repeat donor was found to be infected, recipients up to 6 months previous to the last negative donation were traced and tested for the agent involved (look back). Regarding donors with OBI, all previous donations since 1992 were traced.
When a patient was found to be infected after transfusion, as recorded by the hospital, the donors involved were tested (reverse look back).
Results
During 2007‐2013, 5.95 million whole blood and apheresis donations were screened. A total of 74 repeat donors acquired HIV, HBV, HCV, or HTLV infection or syphilis; and in addition 14 donors with OBI were detected. This resulted in 54 look back procedures; HIV (n = 7), HBV (n = 14), HBV occult (n = 14), HCV (n = 1), HTLV (n = 2) and syphilis (n = 16). Reasons for not starting a lookback procedure were absence of donations in the window‐period or donations used for fractionation. In none of the traced recipients (excluding OBI, see below) HIV, HBV, HCV, HLTV infection or syphilis was detected. For occult HBV the look back procedure resulted in 273/ 442 (62%) traced recipients of which 80 (18%) could be tested. Six of 80 tested recipients were identified as HBV infected; 2 recipients were infected before transfusion; four recipients were associated with 2 OBI donors.
In 2007‐2013 28 reverse lookback procedures were performed: for HIV (n = 1), HBV (n = 11), HCV (n = 8), HTLV (n = 1), syphilis (n = 1), Yersinia enterocolitica (n = 1), malaria (n = 1), Salmonella typhi (n = 1), HEV (n = 2) and CMV (n = 1). Two reversed lookbacks were associated with one donor tested positive for OBI (included in the analysis above). One donor tested positive for Yersinia enterocolitica and one for subclinical malaria. One recipient developed salmonella‐sepsis after a pooled platelet transfusion; all 5 donors were found negative for Salmonella typhi. No donors were found positive for HIV, HBV, HCV or HTLV or syphilis.
Conclusion
The yield of lookback procedures initiated because of potentially infectious blood products in The Netherlands is low. Fourteen repeat donors were detected with OBI, who infected at least 4 patients. No transmission of HIV, HCV, syphilis or HTLV was found. Yersina enterocolitica and malaria were diagnosed in donors by reversed lookback.
P‐785
Incident Investigation ‐ Matching The Resource To The Impact
Davies T1, Bolton-Maggs PHB 2, Poles D 2, Jones J 3
1NHSBT, Manchester, United Kingdom2SHOT, Manchester, United Kingdom3Welsh Blood Service, Cardiff, United Kingdom
Background
The Serious Hazards of Transfusion scheme receives reports of reactions in patients and adverse events relating to the transfusion process.
In 2014, SHOT analysed 3019 reports of process errors and reactions in patients. We noted that 77.9% of all SHOT reports, including ‘near misses’, were error‐related.
Problem
Often error‐related reports are accompanied by detailed investigations, some of them disproportionate to the severity of the incident in the first place. These unnecessary investigations are expensive both in terms of time and expert resource diverted to them.
Many investigations make the mistake of raising actions which deal with only the direct causes of the incident, putting in a quick fix or simply adding extra levels of checking, while ignoring the root and underlying causes.
Solution
SHOT propose a ‘hierarchy of investigation’ based on risk assessment, where incidents are objectively scored according to impact and likelihood of reoccurrence.
Resources
In addition to new SHOT resources, the National Patient Safety Agency ‘Seven Steps to Patient Safety’ from 2004 includes key sections on reporting and learning from incidents, and which may be found on the NHS National Reporting and Learning Service website: http://www.nrls.npsa.nhs.uk/resources/collections/seven-steps-to-patient-safety/?entryid45=59787
Information gathered needs to be mapped or ordered in a useful way, either as a ‘Narrative Chronology’ or ‘Tabular Timeline’ and in many cases simply arranging events in chronological order may be enough to clarify perceptions and misunderstandings that inevitably arise around transfusion incidents.
Tools to further analyse data include ‘5 whys’, ‘Barrier Analysis’, or a ‘Fishbone’ diagram.
Identification of the contributory factors which have the highest impact on each problem enables an investigation to determine the root cause(s) that need to be addressed.
Another consideration is whether the Quality Management System (QMS) detected the error before it left the laboratory ‐ it may be that it only needs to be reported locally and local actions considered. The benefits of carrying out a concise investigation (perhaps a simple timeline or narrative is enough in many cases) must be balanced against the severity or service implications of the incident, and the likelihood that it will recur.
Discussion
The intention of the EU Blood Safety and Quality Directive is not that every single event or reaction needs to be reported, but only ‘serious’ ones as defined in the legislation. With each incident it is important to consider whether a patient or the safety and quality of a blood component was, or might have been put at risk. Consider also whether the incident resulted in hospitalisation or morbidity or whether hospitalisation or morbidity was prolonged.
Tracking and trending the types of incident and the staff involved can provide invaluable information in determining appropriate and meaningful corrective and preventative actions.
Good planning and targeted investigation ensures that it is systematic and complete, identifies the resources needed, identifies the personnel who will need to be involved, and how long it is likely to take.
Incident investigation based on risk assessment will make best use of time and resources in an already stretched Health Service.
P‐786
IGA Deficiency and Transfusion Reactions: A Case Report Illustrating Current Management Options
Musgrave M 1, Tinegate N 2, Cardigan R2
1The James Cook University Hospital, Middlesbrough, United Kingdom2NHS Blood and Transplant, Newcastle upon Tyne, United Kingdom
Introduction
In the UK and Western Europe, 1 in 500–800 individuals have IgA deficiency (IgAD). The commonest indications for testing, in the UK, are coeliac disease or the investigation of immunodeficiency. Historically, IgA deficiency has been thought to cause increased risk of severe allergic or anaphylactic transfusion reactions, however recent evidence from international haemovigilance registries has suggested this is rarely the case. The majority of IgAD individuals can receive transfusions of standard products without difficulty and only a minority react.
We maintain a register of IgAD individuals with a history of transfusion reactions. Currently, there are 12 living patients in the United Kingdom (population 64 million) on the register, of which only one has current transfusion requirements. We describe the transfusion management of this patient.
Case report
A 60 year old woman presented with acute myeloid leukaemia and received standard induction chemotherapy. She had previously been shown to be IgA deficient during testing for chronic fatigue. She had never been transfused. She became pancytopenic and required transfusion with leucocyte depleted red cells and platelets. In keeping with current NHS Blood and Transplant NHSBT guidance, she first received standard red cells, but experienced a moderate to severe allergic transfusion reaction. Subsequently she was transfused 6 washed red cell units and 5 platelet pools with plasma removed and resuspended with additive solution. These transfusions proceeded without problems.
Management of IgA deficient patients who have experienced reactions
NHSBT has over 300 IgAD donors. Despite this, IgAD red cells are rarely available at short notice and provision of IgAD platelets in a timely manner is impossible without creating a dedicated panel. This leads to delays in transfusion. The underlying management principle is that that transfusions should not be denied or delayed because of concerns about IgAD.
British Committee for Standards in Haematology (BCSH) guidelines for management of patients with a history of repeated transfusion reactions, regardless of IgA level, recommend use of washed red cells, which are readily available, (average IgA levels 0.006 g/l) and platelets suspended in additive solution (average IgA levels 0.08 g/l). Our patient, and two others on the NHSBT register of IgAD patients with a history of reactions, have received such components without problems, suggesting that the use of washed red cells and platelets in additive solution may be a viable alternative to those sourced from IgA deficient donors. For fresh‐frozen plasma there is no substitute for sourcing these from IgA deficient donors and therefore NHSBT is building stocks of IgAD plasma..
Conclusion
Evidence from the NHSBT registry of IgAD patients suggests that,on the rare occasions when IgAD patients do react to standard components, washed red cells and platelets suspended in additive solution may be an appropriate component, which can be provided with little delay. The maximum level of IgA in a donation to prevent reactions is not known.
P‐787
Key Factors to Better Understand the Haemovigilance Findings can be Obtained from Surveys
Lopez-Soques M1, Sanz C 2, Bosch MA 3, Gomez D 3, Alava F 4, Davins J 4, Muñiz-Diaz E 3
1Hospital del Mar, Barcelona, Spain2Hospital Clínic, Barcelona, Spain3Banc de Sang i Teixits, Barcelona, Spain4Departament de Salut, Barcelona, Spain
Background
Human factors are the main cause of adverse outcomes related to transfusion. However, there is little information about decisive circumstances surrounding blood administration. The Haematologists in charge of the 99 public or private Hospital Transfusion Laboratories in Catalunya (Spain) could provide valuable information. We surveyed hospital blood administration practices and laboratory policies among the haematologists.
Aims
The aim is to compare the results of the 2010 and 2013 surveys in search of positive changes in transfusion practice.
Method
A survey consisting of 28 questions was designed by the Haemovigilance Commission of our region in 2010. The regional Department of Health sent the questionnaire to all 99 Hospital Transfusion Services served by the regional Blood Establishment. Physicians could send their single choice answers via email. We classified institutions according to the number of packed red blood cell units (PRBC) transfused per year. (A‐Level hospitals: 1–2000 PRBC; B‐level hospitals: 2001–10,000 PRBC; andC‐ level hospitals: >10,000/year). The results of the survey were forwarded to all physicians, and some recommendations were made. In 2013, we surveyed practices again with a similar questionnaire.
Results
We obtained the initial data from 59 A‐level, 20 B‐level and 7 C‐level hospitals. Participation was 87% and 85% in 2010 and 2013, respectively. We observed improvement in 17 areas out of 28 (60%) surveyed: in bracelet usage (83% vs 87% in 2013) and in completing informed consent for transfusion(23 institutions >90% vs 34 institutions >90% in 2013); written blood prescription was available in 58% of institutions vs 63% in 2013 including 21% of electronic blood prescriptions already available; preventive measures for TACO were present in 62% of institutions in 2010, whereas in 2013, 94% had already implemented them. Traceability of blood units in patient records was fully established in 50 institutions compared with 69 in 2013. As regards Haemovigilance officer/s, 11% of the institutions had part time nurses in 2010, with only 1 fulltime nurse; in 2013, 30% of institutions had part time Haemovigilance officers, with 1 fulltime nurse. The implementation of pretransfusion checklists was 40% in 2013.
By contrast, Blood Transfusion Committee meetings were held more than 4 times/year in 52% of centres, compared to 46% in 2013. In 2010, 33% of Haematologists worked fulltime in the Hospital Transfusion Laboratory, compared with 13% in 2013. In most institutions (75.9% vs 73% in 2013) physicians reviewed the indications of components. Paediatric transfusion was available in 42.4% of centres; only in 16.6% were perfusion pumps available. In 2013, the Haematologists in charge dedicated from 1 to 10 h per week to transfusion‐related activities in 65% of hospitals.
Conclusion
We have been able to document some progress in blood administration practices and policies between both surveys.
The interpretation of Haemovigilance findings can be supported by complementary information found in surveys, in order to produce appropriate and realistic transfusion safety recommendations.
P‐788
Korean Hemovigilance System – Report of 7 years Operation
Hyun J1, Jo HJ 2, Choi YS 2, Lee DH 2, Han KS 3
1Hallym University College of Medicine, Hwaseong‐Si, Gyeonggi‐do, South‐Korea2Korea Centers for Disease Control and Prevention, Cheongwon‐Gun, Chungcheongbuk‐Do, South‐Korea3Seoul National University College of Medicine, Seoul, South‐Korea
Background
A government‐supported hemovigilance system is introduced in Korea from 2007. We have set up a national hemovigilance system supported by the Korean Ministry of Health and Welfare but independently operated by the Korean Society of Blood Transfusion. We have operated the system for 7 years including 2 years of the pilot study and annual reports have been published from 2009.
Aims
We intended to report the result of the 7 years operation of Korean hemovigilance system.
Methods
In the first year (Aug 2007–Jan 2008), we developed the reporting forms. During the next 2 years (Apr 2008–Apr 2010) of pilot study, we made a homepage of Korean hemovigilance system, recruited the participants to report and collected and analyzed the data. A national hemovigilance system operated by the Korean Society of Blood Transfusion supported by the Korean Ministry of Health and Welfare has been implemented since Aug 2010. We developed the on‐line reporting system and the data has been collected on the website. We have continued to encourage hospitals to participate in and report to the hemovigilance system. In 2014, we included the participation of hemovigilance system into the criteria of blood management fee which was newly created.
Results
The Korean hemovigilance system is a voluntary reporting system and all adverse events including transfusion reactions and incidents are reported. The numbers of participating hospitals and adverse events have increased every year and total 133 hospitals reported 1956 adverse events in 2014. The number of participating hospitals was dramatically expanded owing to the blood management fee in 2014 and the 133 participating hospitals account for about 74.6% of transfusions in Korea. After 7 years of the operation (including 2 years of the pilot study), total 5697 adverse events have been reported to the hemovigilance system. Overall 5116 adverse transfusion reactions were reported. Febrile non‐hemolytic transfusion reaction (3149, 61.6%) and allergic reaction (1510, 29.5%) were the most frequently reported adverse reactions and 42 cases (0.8%) of transfusion‐related acute lung injury were reported. There were 583 reports of incidents: near miss 494 (84.7%), incidents without adverse reactions 75 (12.9%), and incidents leading to adverse reaction 14 (2.4%). About the half of incidents (56.5%) occurred in related to blood sampling. The other 20.0% of the incidents occurred during transfusion in the ward and 11.2% occurred during performing pre‐transfusion tests in blood bank.
Summary/Conclusions
We have set up a national hemovigilance system and operated the system for 7 years and it seems to work very effectively. Data from the hemovigilance system is expected to support the development of blood safety strategies in Korea.
P‐789
Improving the Incidence of Missing and Illegible Wristbands Within the Ulster Hospital
Mackey P , Carson D , George H , Callaghan M
South Eastern Trust, Belfast, Northern Ireland
Background
The critical areas of sampling and administering blood components are dependent upon the check against a legible wristband validated with positive patient identification.
Approximately half of patients in the Ulster Hospital are seen daily by the phlebotomy team who audited the wristbands of over 5000 patients bled across an initial 26 day period. We identified an average of three missing or illegible wristbands per day in the group audited. This equates to 6 patients per day across the entire hospital and is equivalent to 2190 patients missing a wristband per calendar year. This group of patients would be at very high risk of blood sampling and administration errors in addition to other hospital misidentification mistakes.
Aims
To reduce the incidence of missing or illegible patient identification wristbands on the Hospital wards by 50% in the time period November 2013 to April 2014.
Methods
An exploratory audit of 82 staff was initially carried out to identify possible process measures involved in patients not having a legible wristband. 3 key areas of influence were identified: staff factors, wristband factors, and patient factors.
A number of improvement initiatives were initiated to correct the problem: Performance feedback was given to wards. A new Staff ‘ID ME’ education initiative was created and widely distributed. All wristbands in use were audited and examined for key criteria. Recommendations of the most suitable option was made to the wards. A patient empowerment document created and published in Trust welcome pack. Initially the project group exceeded its initial aim. However subsequent monitoring indicated a sudden unexpected increase in non‐compliance. Investigation revealed the Northern Ireland contract for patient wristbands was awarded to a new type of wristband that was not compatible with key essential criteria. The increasing use of this new wristband on the wards corresponded to the increase in incidents.
Liaison with the Patient Safety Team led to Trust guidance to only use recommended wristbands. Unfortunately this led to no appreciable effect on the type used.The team subsequently negotiated with Risk Management, Supplies and Senior Trust Personnel leading to agreement mid‐May 2014 to break from regional contract and to separately purchase and use wristbands that were key criteria compliant.
When this constraint was introduced and the recommended wristbands purchased, the number of patients with missing or illegible wristbands consistently fell below our target. This improvement was and continues to be sustained.

Conclusion
The incidence of inadequate wristbands appeared to be low and required a very large audit to quantify that was a consistent occurrence. This low level repetition leads to this issue becoming a cumulatively high risk problem over a period of time (an estimated 2190 patients at risk per year.). The project identified that the quality and type of the wristband was the single most important factor that influenced compliance with the wearing of a legible wristband. The project was successful in achieving a marked and sustained reduction in missing or illegible wristbands that may have impacted on patient transfusion safety.
P‐790
Validation of a Transfusion Safety System Based on RFID Technology
Millan A1, Mingo A 1, Gómez J 2, Benítez JP 2, Ortiz P 1, Massuet LL 1, Muñiz E 1, Puig LL 1
1BST, Barcelona, Spain2AT‐Biotech, Madrid, Spain
Background
Currently available Transfusion Safety Systems (TSS) are not fully effective in protecting against more frequent and/or severe transfusion incidents and, in general, do not have mechanisms for detection of near misses. Banc de Sang i Teixits (BST) in collaboration with a biotechnology organization has adapted the SST Rhesus© based on a combination of technologies ‐ especially, the identification by radio frequency (RFID) – and processes.
Aims
To present a new TSS that has been validated in the inpatient and ambulatory care units of the Institut Català d'Oncologia (ICO) of the Hospital Dr. Josep Trueta (Girona).
To analyze the incidences observed during its implementation and quantify the advantages comparing to other TSS in terms of quality and safety.
Methods
The SST allows: (i) To ensure the pretransfusion sample removal in the patient's bedside; (ii) To use the medical record number to identify patients unequivocally; (iii) To incorporate physical and procedural barriers.
From a detailed analysis of the most common errors that can occur during the transfusion process, and how SST can detect and avoid them, a series of indicators have been defined:
1. Number of Covered Errors (NCE): universe of errors covered by the TSS.
2. Transfusion Safety Index (TSI): measures the degree of security achieved by any TSS (relative impact = frequency × severity).
3. Transfusion Safety System Quality Index (TSSQI): measures the TSS quality based on aspects such as security, efficiency and control of the transfusion process.
4. Relative Improvement Factor (RIF): measures the TSS indicators’ relative improvement compared to other solutions.
Results
To date, 200 transfusions have been administered and the solution has detected 3 errors that could not be detected with other existing TSS:
1. A transfusion of an assigned component is avoided since the patient was incorrectly identified.
2. A wrong patient's pretransfusional sample extraction is avoided.
3. An extraction of a pretransfusional sample is blocked since the protocol is not followed.
4. 22 covered errors (NCE = 22 errors) have been defined and grouped in the following categories (Table 1: Categories of errors according the involved operations).
Our solution's NCE was 18 and, with regard to the RIF: (i) RIF‐NCE>3: TSS detects a number of failures more than 3 times higher than that of other known TSS, especially those errors occurring during the patient identification, sample removal, and in blood component administration; (ii) RIF‐TSI>1.5: TSS is at least 50% more secure than other known TSS.

Conclusions
BST has introduced a new technology solution which improves the transfusion safety in more than 50% compared to other TSS, tripling the number of errors detected before the transfusion.
So far, with 200 transfusions administered, TSS has detected 3 errors that with other SST had gone unnoticed.
This TSS allows the automatic registration and exploitation of the information generated during the transfusion process, in real time, as well as to detect errors with greater advance than other TSS. Both aspects have a positive impact on the quality, safety and efficiency of the transfusion process.
P‐791
Clinical Champions at the Bedside Improving Transfusion Safety for Patients
Catherwood AJ1, Goodwin J 1, Quested B 2
1Central Adelaide Local Health Network, Adelaide, Australia2Australian Red Cross Blood Service, Adelaide, Australia
Background
Central Adelaide Local Health Network (CAHLN) implemented a clinical champion portfolio nurse (PN) program to improve care and compliance with Standard Seven of the Australian Council on Healthcare Standards.
Aim
The project aimed to: improve the safety of patients in the clinical domain; and enhance support for accreditation requirements for NSQHCS Standard 7 from within the clinical domain.
Methods
A BloodSafe link nurse education program and support network was implemented for 90 nurses across CAHLN. Twelve months post implementation, evaluation of the PN program was conducted to assess its effectiveness in achieving its outcomes, and the effectiveness of education (initial and ongoing) provided to the PN's. The evaluation project was deemed a quality improvement initiative by the Human Research Ethics Committee for CALHN not requiring ethics approval.
The evaluation was conducted in 3 parts; data mining of the CALHN incident reporting system (SLS), engagement of stakeholders and then a mixed method (see table 1 & 2) of qualitative and quantitative data.
Results
Post implementation SLS data demonstrated:
28% increase in overall reports relating to blood and blood products 46% increase in reports originating from the clinical area.
Questionnaire responses:
88% of PN's reported they had changed their transfusion practice.84% of PN's reported they had changed the transfusion practice in their clinical area.89% of peers found the role beneficial for the clinical area.27% of senior peers stated the role had improved patient safety within their area.48% of PN's had supported accreditation requirements through education provision.76% of PN's had supported accreditation requirements with auditing activity.84% of PN's felt well supported through education.Overall those PN's who had attended the education day felt more supported.

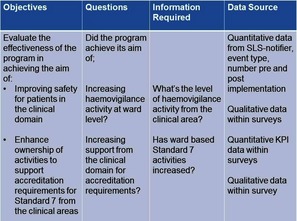
Summary/Conclusions
Results show the education program is effective. The PN program facilitated an increase awareness of the importance of haemovigilance reporting and activity, transfusion knowledge and subsequent change in bedside transfusion practice contributing to improved patient safety. It is now being developed for implementation across the state.
P‐792
Haemovigil: A Novel System to Prevent Bedside Phlebotomy and Transfusion Errors Due to Patient Mis‐Identification
Wankhede GR1, Pathak S 2
1Jeevan Jyoti Blood Bank and Components, Nagpur, India2Max Super Speciality Hospital, New Delhi, India
Background
Analysis of reports from various Haemovigilance programs indicate that the blood transfusion incidents such as Wrong‐Blood‐in‐Tube (WBIT) and transfusion of blood to wrong patient occur due to incorrect/lack of patient identification. These errors, along with issue of incorrect blood units, cause higher morbidity and mortality when compared to other transfusion reactions.
Aims
We studied the impact of using Haemovigil, a system designed to prevent errors in the transfusion process. Data was collected during 4 months and was compared to retrospective data to investigate whether the Haemovigil system is able to identify and therefore prevent (i) WBIT, (ii) issue of incorrect units and (iii) incorrect or absence of patient identification at bedside.
Methods
Retrospective data of 3 years (2011–2013) was analyzed to assess the number of sampling errors.
Haemovigil comprises of:
1. Wristbands ‐ consist of a unique 6‐digit number and 4 peel‐off labels with encrypted codes to be affixed on specimen tubes.
2. Software ‐ Which decrypts the code from the peel‐off labels on specimen tubes and generates the same 6‐digit number as on the wristband.
3. Digital Transporter ‐ An insulated reusable box with a digital lock to carry blood units, which can be locked by entering the unique 6‐digit number generated by the software and opened only by the same number also present on the Haemovigil Wristbands.
Results
Analysis of retrospective data of 3 years (Table 1).
We did not have data for incorrect patient identification or transfusion of blood to wrong patients. However, there were 2 incidents in that period where the patient was moved to a different bed while the nursing staff was not aware (near miss event).
A total of 800 patients were provided the Haemovigil Wristbands. The following results were obtained (Table 2).
Caption 1: Analysis of retrospective data of 3 years.

Since patient name and encrypted label affixed on specimen tube should match perfectly before release of the reserved units, one unit could not be released by the software as patient's name was different during unit release request. The Haemovigil system ensures that a final pre‐transfusion identity check is carried out; if not, the unit of blood cannot be accessed. One incidence was encountered in which the transporter box could not be opened as patient's coded wristband was taken off during transfer from the ward. The third incident occurred when untrained staff member was unable to open the transporter box. Importantly, when this this system was not put in place, untrained staff might have gone ahead with the transfusion without the correct patient identification details.
Caption 2: Analysis of use of Haemovigil.

Conclusions
The Haemovigil system makes it easier for phlebotomists to label patient specimens near the bedside. Even with thorough training, human errors cannot be completely eliminated. On the contrary, the Haemovigil system ensures that everyone in the transfusion chain is following the correct protocols.
We therefore recommend the use of Haemovigil, a cost effective, easy to implement intervention to prevent human errors occurring during specimen collection, blood unit allocation and patient identification prior to transfusion.
P‐793
Frequency of Adverse Transfusion Reactions Reported to Haemovigilance Programme of India
Marwaha N1, Singh S 2, Bisht A 2, Kaur R 3
1PGIMER, Chandigarh, India2NIB, National Coordinating centre, Haemovigilance Programme India, Noida, India3GMCH, Chandigarh, India
Background
Haemovigilance Programme of India (HvPI) was launched on 10th December, 2012 for surveillance of adverse reactions associated with blood and blood product transfusion, under the broad ambit of national Pharmacovigilance Programme. The National Institute of Biologicals, is the National Co‐ordinating Centre and a Core Group and a Haemovigilance Advisory Committee provide oversight to the programme. Reporting is web based through Haemovigil software and the adverse reaction report is uploaded through the Transfusion Reaction Reporting Form (TRRF). The TRRF is a one page form where patient details, blood component details, type and outcome of reaction are captured. A simplified form was introduced to encourage reporting to a new programme.
Aim
To analyse the frequency of various adverse transfusion reactions (ATRs) and assess the quality of data submitted.
Method
The transfusion reactions as reported in the TRRF are based on definitions of the Working Party on Haemovigilance of the International Society of Blood Transfusion (as adopted June, 2013) and were analysed with regard to following parameters as recorded in the TRRF:
1. Type of transfusion reaction.
2. Causality assessment.
3. Type of blood components transfused.
4. First time or repeat transfusion.
5. Clinical diagnosis of the patient.
6. Outcome after the transfusion reaction.
Results
Number of blood centres enrolled in HvPI till December 2014 is 191 out of which 54 centres have submitted transfusion reaction reports. A total of 1728 transfusion reactions were reported in 1679 patients, thus 49 patients had more than one transfusion reaction. Paediatric patients were 117 (6.9%), rest were adults. The male female distribution was 869 and 810 patients respectively. Mortality occurred in 10 patients, in 4 it was due to the transfusion reaction, in the remaining 6, death was attributable to underlying condition. Five patients recovered with sequelae, 1472 recovered completely and in 192 patients the outcome was not known. FNHTRs (42.9%) and mild allergic (29.1%) reactions constituted the most frequently reported ATRs. First time transfusion was reported in 237 FNHTRs and repeat in 175 reactions Anaphylactic/hypersensitivity reactions occurred in 162 patients (9.38%), in 108 patients reaction occurred after first transfusion episode.
Haemolytic transfusion reactions (HTRs) were reported in 69 out of 1679 patients (4.1%). Out of the 69 HTRs 11 (15.9%) were due to ABO mismatch 29(42.02%) due to non‐ABO alloantibodies and 29 (42.02%) due to non‐immune haemolysis. In immune HTRs due to other allo‐antibodies 23 out of 29 (79.3%) were patients with thalassemia (11) or other chronic haemolytic anaemias (12) on regular transfusion. TRALI was reported in 4 patients. TTBIs were reported in 16 out of 1679 patients (0.95%).Mortality resulted due to HTR (2 cases),TTBI (one neonate) and TRALI(one patient).
Conclusion
A successful haemovigilance programme has been established in India. Numbers of centres reporting to HvPI is steadily increasing. The TRRF has been expanded further to capture clinico‐laboratory details and causative factors.
P‐794
An Analysis of Laboratory Errors: What Goes Wrong and When Do Errors Occur?
Bolton-Maggs PHB1, Mistry H 1, Watt A 2, Davies T 2
1NHSBT, Manchester, United Kingdom2SHOT, Manchester, United Kingdom
Background
Laboratory errors in transfusion practice continue to put patients at risk. In 2009 the UK confidential haemovigilance reporting scheme (Serious Hazards of Transfusion, SHOT) highlighted that many of the wrong blood incidents in 2006–2009 occurred due to errors in the transfusion laboratory, with 144/274 (52.6%) occurring outside core hours (core hours are defined 8am–8 pm).
Aim
This review was undertaken to see if the pattern of laboratory‐related wrong blood incidents reported from 2010–2014 has changed over a period when many changes have taken place in UK laboratories intended to consolidate services and provide a leaner process with potential savings for the National Health Service.
Method
A retrospective analysis was performed of laboratory errors reported to SHOT 1/01/2010‐31/12/2014 which resulted in transfusion of an incorrect blood component.
This includes errors associated with:
Sample receipt‐ information missed or not heeded during ‘booking in’.Testing.Component selection.Component labelling, availability and handling and storage of blood components.Other.
Results
Over these 5 years of reporting 157/215 (73%) of wrong blood incidents occurred during core hours, 45/215 (21%) outside core hours, and time was not stated in 13/215 (6%). The laboratory steps at which the errors occurred are shown in the Table.
These errors contributed to 9 ABO‐incompatible red cell transfusions. Six errors occurred during core hours, (one patient experienced a haemolytic transfusion reaction after a component selection error). Three were outside core hours, with no resulting major morbidity.
Wrong blood events outside core hours have decreased from 144/274 (52.6%) reported in 2006–2009 to 45/215 (20.9%) in 2010–2014.

Conclusions
This 5‐year analysis confirms that most laboratory errors now occur during core hours in contrast to previous observations in 2006–2009. In previous years the errors were believed to occur out of hours because biomedical scientists who did not routinely work in transfusion were covering transfusion ‘out of hours.’ The United Kingdom Transfusion Laboratory Collaborative (UKTLC) was established in 2006 to improve and promote high standards with regard to staffing levels, technology, knowledge and skills both in and outside core working hours. Staff are now regularly competency‐assessed, however local investigation into errors must be carried out and a full root cause analysis performed to ascertain why they occurred.
The continuing level of laboratory error is disappointing. Pathology services in the UK are undergoing changes which impact on availability of expertise in transfusion laboratories. Laboratories have financial constraints with fewer resources allocated for training and education. It is clear that further measures are required to reduce the number of errors. The UKTLC has published new standards for transfusion laboratories in 2014. SHOT endorses these recommendations. All organisations providing blood transfusion services are urged to adopt these standards in the interests of patient safety.
P‐795
Biomedical Scientist Prompt Sheet for Wrong Blood in Tube Errors (WBIT)
Daniels H1, Stewart P 1, Clinton P 2, Paterson P 2
1Better Blood Transfusion/NHS Lanarkshire, Glasgow, United Kingdom2NHS Lanarkshire, Glasgow, United Kingdom
Background
Wrong Blood In Tube (WBIT) sampling errors have the potential to lead to transfusion of incompatible blood and are reportable to the Serious Hazards of Transfusion (SHOT) Haemovigilence Reporting Scheme. SHOT 2013 reports that WBIT incidents continue to rise, but are likely still under reported.
While many of these incidents are categorised as ‘near miss’ it is still vital that these serious errors are fully investigated to establish the root cause of the error and to ensure lessons can be learned from such errors.
Investigation however can be problematic if not undertaken immediately. These errors can be complex and difficult to establish what actually happened. They are usually detected when laboratory staff either receive a call from clinical staff to alert of a sampling/labelling error or the error is detected during testing. The BMS making initial contact with the clinical area has the opportunity to establish key information while the person who took the sample or detected the error can remember the incident. If this contact is made hours or days later that opportunity is often lost. Clinical staff bleed many patients and their recollection could possibly be compromised with time.
Aims
Development of a BMS prompt sheet. This flowchart prompts the BMS to direct initial questions appropriately, ensuring vital information is obtained as early as possible. The Transfusion Practitioner can then follow up as required.
Methods
The Transfusion Practitioner reviewed all WBIT errors over the previous 3 years to establish what information was required to support full investigation and what information may not always be available after the event. The key questions to help support root cause analysis are included and the BMS is encouraged to ask additional questions as they see appropriate.
Results
In the past laboratory staff often wrongly categorised WBIT errors as ‘wrong patient bled’ due to mismatch of historical blood groups. However, our investigations show that the majority of WBITs are actually ‘right patient bled but another patient's ID used’. This exercise has raised awareness of the complexity of WBIT incidents. It has also resulted in timely reporting to the Transfusion Practitioner who requires to start investigation within 5 days.
Summary
This has helped raise awareness of the importance of thorough and timely incident investigation. BMS staff in our three main laboratory sites are now using the prompt sheet and it offers them clear guidance on what information the Transfusion Practitioner requires to conduct a full investigation and reporting to SHOT. Accurate and detailed information is helping us track and trend all WBIT errors, enabling a clearer understanding of the human and system related factors that are contributing to the prevalence of these incidents.
P‐796
A Further Classification of Transfusion Near Misses into Type 1 and Type 2 Categories Could Improve Patient Safety
Lopez-Soques M , Garcia-Alvarez J , Basil C , Raya MCR , Tamayo N , Villaubi A , Gil G , Sanchez-Ron I , Carrasco P , Giraldo P , Fernandez-Reig M , Sala M
Hospital del Mar, Barcelona, Spain
Background
Some near misses are recovered in a planned manner, by appropriate Quality Management (QM) Systems; these could be called type 1 near misses (1). Other near misses are accidentally detected and could be classified as type 2 near misses. The description and recognition of type 2 near misses could lead to a reduction or mitigation of the role of luck or serendipity in patient safety.
Aims
to describe type 1 and type 2 near misses and their frequency in a 600‐bed institution and to investigate whether preventive actions were properly implemented in these ‘chance’ events.
Method
We selected in our Access QM database all episodes in which a patient could, but did not, receive an incorrect or delayed blood component. The database included: a short description of each near miss, the date, the origin and circumstances of the detection and the record of corrective actions. We classified the episodes into 2 categories: type 1, when the discovery happened in the course of a standard operating procedure and type 2, when the discovery was accidental. We recorded the number of components transfused from 2010 to feb.2015 and calculated the frequency of total and type 1 and 2 near misses. In addition, we reviewed the corresponding preventive actions.
Results
During this period, 58,335 blood components were administered and 32 transfusion near misses were collected (0.55 per 1000 blood components transfused); 20 near misses were classified as Type 1 (62.5%, 0.34 per 1000) and 12 (37.5%, 0.21 per 1000) were classified as Type 2). As table 1 shows, Type 1 near misses originated in several areas and Type 2 near misses were restricted to 3 origins: Hospital Transfusion Laboratory (50%), clinical areas (30%) and Blood Establishments (20%). Most type 2 events were detected in the morning shift (91.6%) and were detected in random safety rounds. In the review of preventive actions, type 2 near misses originated in the Hospital Transfusion Laboratory (ABO group transcription errors and issue of non‐fractionated Packed Red Blood Cells for fragile adults) had been addressed with new standard operating procedures and education. Type 2 near misses from clinical areas, including 2 Wrong Blood in Tube episodes, had been addressed properly, too, in order to prevent recurrence. Type 2 near misses from Blood Establishments (1 = lack of ‘tests results negative for HIV, HBV and HCV’ on the unit label, and 2 = incorrect outdating in 1 of four irradiated fractions) had been notified to the Blood Center and not addressed by new procedures.
Table 1.

Conclusion
Almost 40% of transfusion near miss events were classified as type 2, that is, found out by chance; preventive actions were in place to prevent recurrence.
To address potential type 2 near misses originated in Blood Establishments, we should add more labour‐related measures: safety rounds or checklist verifications.
By focusing on type 2 near misses we can reduce the role of luck and improve transfusion safety.
1. What is a near miss? S AM Nashef. Lancet, 2003; 361:180–1.
P‐797
Use of an Innovative Medical Device That Cancels the Risk of Error of ABO Typing of Donated Units Due to the Exchange of Test Tubes and That Effectively Increases Transfusion Safety
Agliastro R 1, De Francisci G 1, Bonaccorso R 1, Borghesan B 1, Masaniello F 1, Militello M 1, Orlando L 2, Vitrano A 2
1ARNAS Ospedali Civico Di Cristina Benfratelli, Palermo, Italy2Promedical Srl, Santa Flavia (PA), Italy
Background
Transfusion Safety depends on the measures and instruments adopted to ensure safety and efficiency at every stage of the transfusion chain from donor to recipient. An AB0‐incompatibility reaction can be determined by a ABO typing error of the blood unit due to the exchange of the samples or the labels at the time of donation causing a mismatch donor/blood unit. The exchange of the sample and/or the exchange of the label may not show up during processing, typing, distribution and infusion.
Aims
To reduce these exchange errors in our Transfusion Medicine Unit (OU), for several months we have been using blood‐collection units with an inline test tube (P) integrally attached to the first bag (WB‐Bag). The P is filled and separated from the WB‐Bag upon arrival at the OU. This makes it impossible to exchange samples between donors.
Methods
Since December 2014 we have used the new device (ND) for blood collection, a Triple Top & Bottom 450 ml bag set, code 8203TT (Promedical), in which the WB‐Bag is connected, by a tubing with a break‐away valve, to a PVC Medical Grade test tube (P) which has a diameter of 10 mm and height of 95 mm, for the determination of the ABO group unit. The P and WB‐Bag are labelled by the Nurse at the time of donation. Upon arrival at the OU the lab technician on duty for unit admittance verifies the correspondence of the WB‐Bag and P barcode labels, breaks the break‐away valve and fills the P squeezing it 2 times with the palm of the hand to expel the air, and welds the connecting tubing. The P are detached from the WB‐Bag and centrifuged at 4000 rpm. The P are then cut at a height of 75 mm with dedicated equipment (YODA‐automatic test tube cutter) by us previously qualified and validated. We use P on Autovue Innova (Ortho) to test each sample for ABO, Rh, Rh phenotype and unexpected antibodies (Tests).
Results
To date, we have used 8250 ND and performed Tests on each of the 8250 P. For the first 200 units tested, we carried out double Tests with P and EDTA test tubes: no discrepancy in results was detected. The use of ND did not create problems during blood collection and processing, and entered in our daily routine. The YODA has speeded up the process of preparation, cutting 24 test tubes at a time, and allowed us to standardize the height of the cut to 75 mm which does not interfere with the needle pick of the Autovue Innova. The P resists high speed centrifugation and freezing.
Conclusions
Our data shows that the ND with inline P for blood typing, has proven suitable for a Transfusion Medicine Unit like ours that processes more than 2200 blood units per month. The application of new technologies such as the ND minimizes the possibility of human error at the time of donation, that may cause a possible wrong ABO unit typing, and effectively increases Transfusion Safety.
P‐798
A Look on Transfusion Reactions and Antibodies Against Red Blood Cells in 21 years of Haemovigilance
Lorenzi M1, Chianese R 2, Giachino O 3, Ottone P 4, Pagliarino M 1, Bordiga A 1
1A.O.U. Città della Salute e della Scienza, Torino, Italy2SIMT ASL TO 4/CRCC Piemonte, Ivrea, Italy3SIMT ASL TO 2, Torino, Italy4SIMT A.O.U S.Luigi, Orbassano, Italy
Background
In most cases transfusion reactions are mild and Blood Transfusion Services receive delayed alerts. In such cases a decision must be made on the extent of diagnostic work up.
Aims
To evaluate if in patients who experienced a mild transfusion reaction additional immunohaematological tests are warranted to identify antibodies against RBC.
Methods
Databases of all five Transfusion Services of Turin area were recently merged. We searched the new database to calculate number and type of transfused units and number and type of transfusion reactions. We manually reviewed records of patients who had had a transfusion reaction and a positive antibodies screening after the reaction whereas the indirect antiglobulin test (IAT) had been negative before the transfusion.
We assessed time and results of immunohaematological tests, other units transfused at the same time and related clinical notes to ascertain if a timely work‐up of the reaction could have been useful in identifying antibodies.
Results
From 1994 until 2014 1,712,303 RBC units, 378,694 FFP units and 223,524 Plts units to 278,526 patients in 1,257,449 transfusion episodes were transfused. In these years we registered 5456 transfusion reactions in 4302 patients of which 19.4% attributable to PFC, 47.1% to RBC and 30.9% to Plts. The reactions were classified as FNH (36.8%), allergic (45.3%), dyspnoeic including TRALI (1,5%), hypotensive (0.4%), hemolytic including AB0 incompatibility (0.2%). Only in six cases a diagnosis of non ABO hemolytic transfusion reaction was reported. In 2897 (about 1 out of 1000) patients a IAT was performed, at any time, in the period after the transfusion reactions.
In 229 patients who experienced a transfusion reaction the antibody screen became reactive after the episode. In 209 we did not find any clue to a specific antierythrocite antibody. In only 20 cases a timely work‐up could have been useful. The incomplete work up was probably due to the mildness of the reaction, the delay in notifying the transfusion center about the reaction itself and the lack of urgent transfusion to be done to the patient.
Conclusions
Our database included a very large number of transfusions and transfusion reactions over 21 years. A complete immunohematologic work up after mild transfusion reaction seems not to be justified solely on the basis of increased number of alloantobodies discovered after transfusion reaction. However reactions related to RBC antibodies are probably underrated. Since transfusion reactions have a similar pattern of symptoms in the early phase is very important to take every opportunity in discovering every issue relating to RBC antibodies and AB0 incompatibility. We think that any transfusion reaction, even if mild, should be reported to the transfusion service in a timely manner and assessed properly to achieve better results in transfusion therapy and improve safety.
P‐799
Hospital Transfusion Committees – Impact on the Transfusion Safety
Escoval MAE , Condeço J , Ramoa A , Santos M , Sousa G
Portuguese Blood and Transplantation Institute, Lisboa, Portugal
Background
The existence of hospital transfusion committees seems to be a determining factor in the safety of the transfusion chain. It has also been suggested that the rates of reported transfusion reactions and near miss events are positively correlated with the safety of the transfusion chain, in a hospital, and reported errors as a proxy for unsafe transfusion. Reporting of non‐serious events could also be used as an indicator of transfusion safety when serious events are simultaneously declining, assuming that better reporting is associated with safety awareness and good surveillance of the patients.
Aim
To assess the impact of the existence of a Hospital Transfusion Committee (HTC) in the rate of adverse transfusion reactions (ATR), near miss events (NM) and transfusion errors (E) reported to the Portuguese Haemovigilance System (PHS).
Methods
The Portuguese hospital transfusion services have been invited to contribute to a survey available online from 5 to 27 May 2014, about the existence and activity of HTC. The respondents were classified in three categories: inexistence of HTC, active HTC and non‐active HTC. A retrospective analysis of red blood cells (RBC) transfused (84% of all blood components transfused in Portugal) recipients transfused, errors, NM events and ATR reported to the PHS in 2013, by the answering institutions, has been performed.
The rates of ATR, E and NM events were calculated per 10000 RBC transfused. The average of RBC transfused, the average rate of ATR, E and NM were calculated for each of the three categories. Four levels of annual RBC use (<2500, 2500–5000, 5000–7500 and >7500) were further defined and a similar analysis was performed.
Results
50.5% (47) of the total transfusion services (93) answered the survey. 44.7% (19) of the respondents have an active HTC, 14.9% have a non‐active HTC and 40.4% (19) don′t have HTC.
The average rate of ATR reporting was higher in the institutions where an active HTC exists (18.52) than where a non‐active HTC exists (13.17) or HTC doesn't exist (13.19). This rate was even higher in ATR/10,000 recipient transfused: active HTC (53.4), non‐active HTC (41.89) and nonexistence of HTC (34.39)
The average rate of errors reporting was lower in the institutions where an active HTC exists (2.75) than where doesn't exist (3.81).
The average rate for NM events reporting although was higher where an active HTC exists (14.40) than where doesn't exist (10.52), it was even higher in the institutions with a non‐active HTC (30.69).
When analysis by levels of RBC use was performed, the ATR rate, per 10000 RBC and 10000 recipients, was higher when a HTC exists, with the exception of 2500 to 5000 level. The errors rate was lower in the four levels of RBC use.
Conclusion
Although a bias can be present, related to the fact that the survey respondents may be the more committed in haemovigilance, data seem to support that hospitals with a HTC have a higher rate of transfusion reactions report and a lower rate of error report.
P‐800
Six Years of HIV, HBV and HCV Lookback at the Blood Bank of the Centro Hospitalar De São João
Vaz CV , Fernandes S , Moura J , Koch MC , Araújo F
Centro Hospitalar de São João, Porto, Portugal
Background
The Lookback is a strategy to identify recipients at risk of transfusion‐transmitted infection (TTI), by testing recipients of components from donors who were later diagnosed with the infection.
Despite the decrease of the residual risk provided by nucleic acid amplification technique screening assays, TTI from donors in the window period have been recently reported in German and Italy, resulting in the infection of two recipients.
Monitoring and characterizing transfusion‐transmitted risk of infections is essential to evaluate the safety of the blood supply, the impact of new testing strategies and their associated residual risk.
Aims
Review 6 years of HIV, HBV and HCV Lookback at Blood Bank of the Centro Hospitalar de São João (BBCHSJ)
Methods
All blood donations (139 968) were tested for markers of HBV, HCV and HIV infection using serological tests (Prism ChLIA, Abbott©) and minipool nucleic acid techniques (MP‐NAT). MP‐NAT was performed using Cobas Taqscreen MPX Test, Roche©, which detects simultaneously HIV‐1, HIV‐2, HBV and HCV in minipool of 6 samples.
When a repeat blood donor is diagnosed with an HIV‐1, HIV‐2, HBV or HCV, every recipient of the donor's components, in the 6 months prior to the last negative donation (if not applicable the process should go back up to a maximum of 5 years), is notified and tested for the correspondent virus.
This study identified and characterized every donor, component and recipient related to this process from 2009 to 2014, at BBCHSJ, the hospital blood bank with the most activity in Portugal.
Results
In the study period, from the 40 positive blood donors, 25 were first time blood donors and 15 repeat blood donors. 14 (93%) of the repeat blood donors were related to HIV‐1 and 1 (7%) to HCV (Table 1).
41 components from the repeat blood donors had been transfused to 42 recipients, 24 (57%) of whom were alive at Lookback time and all were notified. 20 (83%) blood recipients were tested and none was positive (Table 2).
Table 1. Blood donors involved in Lookback.

Table 2. Recipients and donations involved in Lookback.

Summary/Conclusions
No repeat blood donor was positive to HBV and no HIV or HCV positive recipients were identified on the Lookback interval studied.
The Lookback is an important process which enables identifying and referring infected patients for specialist care as well as evaluating the residual risk of TTI of the BBCHSJ.
P‐801
Effect of Intravenous Catheters on Red Blood Cells Hemolysis: An Integrative Review
Avelar AA , Mendes MTM , Jacinto AKJ , Kusahara DM , Peterlini MAP , Pedreira MP
Federal University of São Paulo, Sao Paulo, Brazil
Background
The occurrence of injury in erythrocytes during the transfusion process, with presence of free hemoglobin in plasma, increasing lactate dehydrogenase and extracellular potassium, and reducing haptoglobin, can arise due to mechanical trauma produced by type, smaller‐gauge and long length intravenous catheter. Such relation is not well established, especially to newborn and child that predominantly use smaller catheters.
Aims
To identify in the literature the influence of different intravenous catheters on the occurrence of hemolysis during infusion of red blood cells (RBC).
Methods
Integrative literature review. The inclusion criteria was set as: articles published in English, Spanish and Portuguese languages, indexed in Pubmed, The Cochrane Library, Scielo and LILACS databases, published until 2014, without inferior limit of date. Articles were excluded if unavailable in full version, after search in the databases and author's request. The keywords were catheter, vascular access devices, hemolysis, blood transfusion, erythrocyte and transfusion.
Results
We identified 42 (91.3%) articles indexed in Pubmed and 4 (8.7%) in The Cochrane Library. After the analysis, 7 (14.6%) were included, 4 (57.1%) were related to the hemolysis occurrence with peripheral catheters and 3 (42.8%) with peripherally inserted central catheters (PICC). Five (71.0%) studies highlighted blood transfusion in children. All selected studies, independent of the devices used, not described high levels of hemolysis. In most of the articles analyzed, the use of smaller‐gauge catheters (4; 57.1%) was associated with cell damage mainly in the presence of extreme conditions of pressure (2; 28.6%) and low infusion rate (1; 14.3%). The hemolysis occurrence can be associated with a longer exposure of cells to the pressure inside the catheter lumen, due to the slow flow and catheter length (1; 14.3%), mainly in PICC. Additionally, the time of RBC storage was intrinsically related to the elevation of hemolytic markers (3; 42.8%).
Conclusions
The occurrence of RBC hemolysis related to catheters characteristics was not conclusive, because other variables may be able to influence this effect. The cell damage may be influenced by smaller‐bore catheter, infusion in high pressure, the storage time, catheter length and low rate infusion. There is a lack of evidence that showed the effect of specific influence of catheters in the hemolysis occurrence which can be support nursing decision in choosing the most adequate intravenous catheters to perform safe RBC infusion.
Acknowledgements
Grant for research National Council for Scientific and Technological Development CNPq process No: 477055/2013‐3.
P‐802
Report on Systematic Transfusion Treatment Surveillance in Croatia 2013
Štimac R , Šarlija D , Vuk T , Mihaljevic I , Tomicic M , Jagnjic S , Ocic T , Balija M , Jukic I
Croatian Institute of Transfusion Medicine, Zagreb, Croatia
Aim
The aim is to present the 2013 Systematic Transfusion Treatment Surveillance (STTS) report that includes patient transfusion reactions, adverse events, reactions/complications in blood donors, epidemiological characteristics of blood borne diseases in the population of blood donors, and performance of look‐back procedures.
Methods
Transfusion reactions were categorized according to the Proposed Standard Definitions for Surveillance of Non Infectious Adverse Transfusion Reactions (ISBT, IHN, 2011) and their prevalence was expressed per number of units issued for transfusion treatment. Adverse events were classified according to the Medical Event Reporting System for Transfusion Medicine (MERS‐TM). Reactions/complications in blood donors were categorized according to the Standards for Collecting and Reporting Data on Reactions Related to Blood Donation (EHN, ISBT, 2008) and their prevalence expressed per number of blood donations. The prevalence and incidence of blood borne diseases were calculated per 100,000 blood units collected from new and repeat blood donors.
Results
A total of 322 reactions or 1.09/1000 blood products issued for transfusion treatment were reported. On report reassessment, 20 reactions were categorized as serious adverse reactions: 1 AHTR, 1 DHTR, 6 DSTR, 3 anaphylactic reactions, 2 ‘possible’ TRALI, 1 TAD, 4 TACO and 2 non‐immune hemolytic transfusion reactions.
On reassessment, 24 of 26 adverse events reported were categorized as serious adverse events, including 15 human errors (3 IBCT), 2 equipment failures and 7 events classified as ‘other’.
There were 2003 or 11/1000 reactions/complications recorded in whole blood donors and those undergoing apheresis, expressed per number of blood donations, including 1092 immediate vasovagal reactions (2 with accident), 608 hematomas, 215 delayed vasovagal reactions (4 with accident), 7 painful arm syndrome, 3 nerve injuries, 1 arterial puncture, 1 local allergic reaction and 2 ‘other’ reactions. During apheresis procedure, 74 reactions to citrate were recorded. Thirty‐one reactions or 0.2/1000 blood donations were categorized as serious reactions/complications.
This report also contains results of serologic and ID‐NAT confirmation testing, prevalence and incidence of blood borne diseases, and results of the look‐back procedures. The report is supplemented with commentaries and recommendations.
Conclusion
The STTS report has been continuously updated with new data and recommendations and is published in Transfuziološki vjesnik (Transfusion Medicine Newsletter), thus being widely available for continuous education of all those involved in transfusion treatment.
P‐803
Traceabilty Compliance ‐ A District General Hospital Experience
Ajeneye O1, Kilburn S 2, Mills G 2, Sirigireddy B 3
1Princess Royal University NHS Trust, Kent, United Kingdom2University of Portsmouth, Portsmouth, United Kingdom3Homerton University Hospital NHS Trust, London, United Kingdom
Introduction
The introduction of the European Union Directive in 2002 and Blood Safety and Quality Regulations (BSQR) in the U.K influenced mandatory compliance in the collection, storage, distribution, and traceability of blood products within the hospital.
Aim
This study explored reasons for the initial poor traceability compliance and further identified the risk factors that affected traceability compliance.
Methodology
A quantitative approach was adopted as it made it possible to measure the frequency of actions, and this data was used to answer the research questions. Data was collected using questionnaires, observations and audits. The data was analysed using the SPSS statistical package.
Results
During the period 2005–2008, wards within the Trust were grouped into three categories. The low usage group had an average traceability compliance of 68.5%, medium usage group had an average compliance of 73.8% and high usage group had an average compliance of 80.2%. The Kruskal–Wallis test also was used to compare the relationship between number of beds and average traceability compliance. The number of beds on the wards did not influence compliance rates (P = 0.70; median 0.49). The Mann–Whitney U test was used to compare the relationship between average compliance and the presence or absence of a trainer on the ward. A statistically significant difference was found; average traceability compliance was better with a trainer on the ward (P = 0.03; median 0.16). Sixty‐one per cent of respondents reported that 76–100% of their staff had been trained within the past year. It was evident that as the number of trained staff increased, compliance improved across all wards. Spearman's rank correlation coefficient showed a strong correlation between the proportion of trained staff and traceability compliance (r = 0.59; P = 0.002).
Conclusion
All wards within the Trust attained 100% traceability compliance by 2012. The findings from this study also reiterated the importance of training to frontline personnel involved in the collection and transfusion of blood products. The study provided an insight into the variety of changes adopted to meet the need of the end‐users of our transfusion services within the Trust. The study highlighted the importance of the training tools; improved procedure for the and return of labels and effective communication between the laboratory and end‐users.
P‐804
Patient Characteristics and Rate of Acute Transfusion Reactions to Platelet Transfusions in Uganda
Hume A1, Ddungu H 2, Kajumbula H 3, Kyeyune-Byabazaire D 4, Jackson O 2, Ramirez-Arcos S 5, Tobian A 6
1CHU Ste‐Justine, Montreal QC, Canada2Uganda Cancer Institute, Kampala, Uganda3Makerere University, Kampala, Uganda4Uganda Blood Transfusion Service, Kampala, Uganda5Canadian Blood Services, Ottawa, Canada6The Johns Hopkins University, Baltimore, United States of America
Background
There are no published reports from sub‐Saharan Africa, outside southern Africa, describing the characteristics of patients receiving platelet transfusions (PTs), nor the rate of acute transfusion reactions (ATRs) associated with PTs.
Aims
From March 2014 to September 2014, we performed a study to determine the rate of bacterial contamination of platelet units (PU) transfused to patients at Uganda Cancer Institute (UCI) in Kampala, Uganda. Secondary objectives were to determine characteristics of patients receiving PTs and the rate of ATRs associated with PTs. Here we report on the secondary objectives.
Methods
UCI patients were included if they consented to participate in the bacterial contamination study, and if their physician agreed that platelet transfusion could be delayed for the time required to remove an aliquot from the PU and perform a gram stain (GS). All PU were single donor, whole‐blood derived, non‐leukoreduced units collected and prepared by the Uganda Blood Transfusion Service from healthy, anonymous, volunteer donors. Recipient information including bleeding status and platelet count prior to PT were recorded. Patient monitoring included vital signs performed immediately prior to and at the end of the PT and at 2 h following PT. All records were reviewed by at least one of the physician to determine the presence of an adverse transfusion event (ATE). All ATE were reviewed by at least 2 transfusion medicine physicians, using CDC Hemovigilance definitions, to determine the presence of an ATR, severity and imputability.
Results
337 PU were included in the bacterial contamination study; of those 323 PU (representing approximately 38% of all PU administered at UCI during the study period) were transfused in 151 transfusion episodes to 50 patients. GS were negative for all transfused PU; 4 had initially positive blood cultures (1 confirmed, 3 not confirmed). PU not captured in this study were mainly excluded for logistical reasons (e.g. arrived at UCI outside study hours or during intermittent shortages of study materials). Patient characteristics are shown in Table 1. Platelet transfusion characteristics are shown in Table 2. ATRs occurred in 11 transfusion episodes, involving 13 PU for an overall rate of 4% (13/323) per PU or 7.2% (11/151) per transfusion episode. Of the 11 ATRs, 8 (5.3%) were febrile, non‐hemolytic ATRs, 3 with probable and 5 with possible imputability; 2 (1.3%) were allergic ATRs and 1 (0.7%) was transfusion‐associated dyspnea; all 3 were of definite imputability. All ATRs were non‐severe. No ATRs were observed during or after transfusion in the 4 PU with initially positive blood cultures.
Table 1. Patient characteristics of individuals that had a platelet transfusion.

Table 2. Platelet transfusion characteristics of 323 single donor units transfused to 50 patients

Summary/Conclusions
Although limited by the lack of inclusion of all PU administered at the UCI during the study period, this study does provide information about PT in a low‐resource setting such as Uganda and indicates that prophylactic PT are used, at least for some patients. The ATR rate for PT is similar to that reported in HIC prior to the introduction of leukoreduction; this finding is unlikely to be affected by the fact that not all PT administered during the study period were included in the study.
P‐805
Abstract Withdrawn.
P‐806
Authorisation of Blood Components: Ensuring Standardisation of Practice
Heyes JH 1, Riley T2, McSporran W 3, Patel R 4, Delieu L 5, Dunn E 6
1NHS Blood and Transplant, London, United Kingdom2Royal Brompton Hospital, London, United Kingdom3Royal Marsden Hospital, London, United Kingdom4Northwick Park Hospital, London, United Kingdom5Darent Valley Hospital, London, United Kingdom6Guys and St Thomas's NHS Trust, London, United Kingdom
Background
The authorisation of blood components has traditionally been regarded as the responsibility of the patient's clinician. However, since the change to section 130 of the Medicines Act which excluded blood components, there are now no legal barriers to other appropriately trained and competent nurses, midwifes and specialist practitioners making the decision to transfuse and authorising the administration. This extended practice was supported by a Governance Framework (2009) with input from the Royal College of Nursing and the Nursing and Midwifery Council. This is in response to the changing needs of clinical practice and is intended to improve patient care and expedite treatment for patients that need a transfusion.
To encourage and support hospitals implement this initiative the London and South East Regional Transfusion Committees have worked together to develop a toolkit that includes a policy template, competency and training records.
Aim
The aim is to achieve standardisation of practice and ensure that governance and patient safety is considered when non‐medical authorisation of blood components is implemented in NHS trusts.
The working group aims are to: Ensure that the decision to transfuse will be made by experienced, advanced nurses who have an in depth knowledge of the transfusion process and have completed competency assessment.Ensure that Advanced Nurses authorising transfusion have an in depth knowledge of their patients’ needs.Provide a high standard of care that will be effective, efficient and safe, prevent delays in the decision to transfuse and in the authorisation of transfusion.Ensure that Advanced Nurses undertaking the role are aware of their professional and legal responsibilities.Clarify the boundaries of the role undertaken by the specialist nurses and identify clear lines of accountability.
Methods
The London and South East working group was established to review current practice and ensure that non‐medical authorisation of blood components is beneficial and safe for patients having a blood transfusion. The group was made up of transfusion practitioners from both regions and was co‐ordinated by the patient blood management practitioner for London. All documentation produced was reviewed by the working group and comments from NHS Trust policy review committees were considered.
Results
The policy was written as a template to allow modification by the NHS Trust to reflect local practice. Included in the policy were notes for consideration that concentrate on ensuring robust governance procedures are in place to protect both patients and staff involved in the process. The establishment of a structured training programme is documented in the policy with consideration for how to assess competency and review practice. The use of this policy has been monitored using website analytics and feedback from the regions documented and incorporated into documentation during reviews.
Summary
To standardise the practice of non‐medical authorisation of blood components within London and the South East of England a working group was established to develop a regional toolkit. This toolkit has been designed to support the staff undergoing this extended practice and protect the patients.
P‐807
Haemovigilance in Szpital Wojewódzki (Regional Hospital) in Poznan Under the Surveillance of Regional Blood Center in Poznan
Sobisz-Blachowiak M , Olbromski K
Regional Blood Center in Poznan, Poznan, Poland
Background
Haemovigilance includes a number of activities to ensure the maximum safety of the recipient and optimal blood component. The most important task for all people involved in the process of treatment with blood components is to follow the established rules, regulations, procedures in order to minimize and preferably to eliminate any possible errors or mistakes. It is obvious that it is impossible to avoid all serious adverse events but it is essential to organize all activities to protect the patient from serious consequences of them and to avoid similar cases in the future. In the Szpital Wojewódzki in Pozna’ all activities relating with transfusion of blood components take place on the basis of the standard operating procedures (ISO) which are designed to allow full traceability of blood and blood components i.e. the possibility to reconstruct the course of each individual unit of blood or its component.
Aim
The aim of the study was to analyze the activities of the haemovigilance in Szpital Wojewódzki in Pozna’.
Methods
Analysis covered the use of blood components and data in the registers of serious post‐transfusion reactions, incidents and near misses in recipients in Szpital Wojewódzki in Pozna’ in years 2010–2014. Data was also obtained from annual reports submitted to the RegionalBloodCenter in Pozna’ in the context of haemovigilance.
Results
The number of transfusions was analysed and all associated adverse events that occurred in 5 years along with corrective actions. The study also includes the information from the RegionalBloodCenter in Pozna’ regarding the possibility of transmission of infection which required ‘Look back’ procedures. Data concerning the years is presented in the table below.

Conclusions
All adverse events that occurred in Szpital Wojewódzki in Pozna’ were reported to the RegionalBloodCenter in Pozna’. All cases reported by the RegionalBloodCenter in Pozna’ regarding the possibility of transmission of infection were investigated in accordance with the “Look back’ procedure.
In the years 2010–2014 none of the patients treated with blood components died or experienced such a deterioration of health which would result in prolonged hospitalization or long‐term illness in the Szpital Wojewódzki in Pozna’.
All undertaken activities of haemovigilance in Szpital Wojewódzki in Pozna’ significantly improve the safety of patients receiving blood components.
P‐808
Abstract Withdrawn.
P‐809
Aide Memoire to Support the Blood Transfusion Bedside Checklist
Dhesi AS1, Conran S 2, Fulkes V 3, Solanki S 4, Chituku M 5
1NHSBT, London, United Kingdom2Croydon Health Services NHS Trust, London, United Kingdom3Guy's & St Thomas’ NHS Foundation Trust, London, United Kingdom4HCA International, London, United Kingdom5West Middlesex University Hospital NHS Trust, London, United Kingdom
Background
Blood transfusions involve a complex sequence of activities to ensure the right patient receives the right blood, there must be strict checking procedures in place at each stage. Administration of an ABO incompatible transfusion is currently classified as a “Never Event”(NHS England 2013). Most incidents are due to failure in the bedside identity checks carried out between the patient and the blood component to be transfused.
The 2013 Serious Hazards of Transfusion (SHOT) report indicated that the majority of episodes resulting in an incorrect component transfusion resulted from multiple errors in the multidisciplinary transfusion process. For 70% of these cases the error could have been detected at the final pre‐administration check at the patient's bedside.
The London Regional Transfusion Committee (RTC) established a working group to action a recommendation from SHOT (2014); to develop a simple 5‐point aide memoire. This was agreed as an important tool during the final step to remind staff to check for the correct patient identifiers, the prescription for the correct component and confirmation of specific requirements.
The working group comprised of Transfusion Practitioners representing 4 NHS and Private sector hospitals.
Aim
To develop and evaluate the impact of implementing a 5‐point aide memoir card for use at the patient's bedside during pre‐transfusion checks.
Methods
Using the British Committee for Standards in Haematology Guidelines for the Administration of Blood Components (2009) and the Handbook of Transfusion Medicine (2013), the working group broke down each of the pre‐administration steps in to a comprehensive workflow. This was further analysed and simplified into the 5 key pre‐transfusion administration checks. The aide‐memoir is supported by an information leaflet for staff.
Mechanisms for qualitative and quantitative analysis of the impact and effect of implementation of the aide‐memoir to pilot wards/departments were identified: observational audit of transfusion practice pre and post implementation market research questionnaire to ascertain user feedback and/or recommendations for visual and content improvement.
Results
Members of the working group completed baseline audits and found that staff were omitting vital parts of the bedside check.
The prototype lanyard cards were ordered from printers to arrive in March 2015. These will be issued to the staff at the wards involved in the baseline audit. The pilot will run for 1 month, post‐implementation audit and market research will be completed in June 2015.
Summary/Conclusion
The aide memoir cards will be considered a successful intervention to support the final pre‐administration check list if the audits show a favourable increase from baseline.
Once the end users have had time to use the cards they will also be contacted as a focus group for market research to evaluate if the aide memoir cards were useful in their view/opinion, and if they would prefer any visual or content changes.
Once this work is completed a report will be made to the London RTC whether to issue these cards to all hospital within the London region.
P‐810
Overview of Transfusion Reactions Reported to a Cluster Center Blood Bank in Sri Lanka
De Alwis H , Muralitharan VM , Deveraja AD
National Blood Transfusion Service, Colombo, Sri Lanka
Background
Trincomalee Hospital Blood Bank is a cluster centre Blood Bank which provides blood and blood products to 4 Government Hospitals and 3 private Hospitals. Transfusion of blood and blood components can give rise to unexpected adverse events called as Transfusion reactions. These Immune and Non Immune adverse reactions can be present early or delayed. It can be range from mild to severe life threatening reactions. Monitoring and reporting of transfusion reactions are important part in Haemovigilance.
Aim
To analysis the prevalence and types of Transfusion Reactions reported to the cluster centre.
Method
Retrospective analysis of Transfusion reactions which are reported from government and private hospitals for a 1 year period From 2014 January to 2014 December. All these cases were investigated, monitored and reported to National Haemovigilance at National Blood Transfusion service.
Results
Total no of reported reactions from government and Private Hospitals were 155 and there were 4460 Red cell transfusions, 1974 FFP,438 Platelet and 502 Cryo precipitate Transfusions.
From them 2 Acute Febrile Haemolytic Transfusion Reactions, 134 (2%) Non Haemolytic Febrile Transfusion Reactions, 1 Transfusion Associated Cardiac overload and 17 (0.2%) Allergic Reactions were noted.1 case of suspected bacterial contamination was reported from Trincomalee Hospital but it was not proven. Among them large number of Febrile Non Haemolytic Transfusion Reactions were seen and most of them were due to Pack Red cell Transfusions. All Acute Febrile Haemolytic Transfusion Reactions were due to ABO incompatible Transfusions and they were detected as soon as the transfusion started.No deaths related to this. Also, there were no any transfusion reactions due to Cryoprecipitate.
Conclusion
Though such reactions were observed no fatalities were present. It is important to report and investigate all types of transfusion reactions to prevent further in future. Introduction of Hospital Transfusion Committee, continues education on bed side transfusion practice to Doctors and Nurses and Usage of leucodepleted blood products should be taken in order to prevent Transfusion reactions.
P‐811
Nurse‐Perceived Satisfaction of a Hospital‐Based Hemovigilance Team and Possibilities to Improve Their Efficiency
Costermans EAJ , Decadt ID , Van de Poel MVDP , Kesteloot KK , Devos TD
University hospital of Leuven, Leuven, Belgium
Background
At the university hospital of Leuven the hemovigilance team exists since 2002 and includes a physician, a clinical nurse specialist and a nurse. More than 40,000 blood transfusions are given at the hospital every year. No previous research was found concerning the efficiency and perceived satisfaction of a inhospital hemovigilance team.
Aims
We investigated the perceived satisfaction of the hemovigilance team at the nurse wards and searched for strengths, weaknesses and possibilities to improve their efficiency.
Methods
A semi‐quantitative questionnaire was distributed to the top 20 wards with the highest transfusion activity. Satisfaction was measured using a quantitative scale from 1 (very unsatisfied) to 5 (very satisfied). Quantitative results were analyzed with SPSS (P < 0.005). In order to define priorities, the non‐parametric correlation (Spearman's Rho) with the dependent variable was calculated for each aspect.
Results
We collected 57 questionnaires (90.5%). The presence of the hemovigilance team at the ward is perceived as rather rarely (4%) and mainly for information and education (10%), checking the blood refrigerators and freezers (8%) and support when blood scanning problems occur (4%).
The overall nurse‐perceived satisfaction of the hemovigilance team is rather high: 67% of the respondents is rather or very satisfied. Looking at the different aspects, especially the knowledge/expertise of the team (89%), the quality of their support (85%), reachability by phone (77%) and the quickness to offer support (71%) are positively evaluated.
Nurses are most dissatisfied of the feedback on blood transfusion practice (19%), support by implementing (16%) and communication (14%) concerning changes in blood transfusion procedures, the presence of the team at the ward (12%) and offered trainings (12%).
65% of the respondents believe that the hemovigilance team is an added value for the hospital. Only 17% feel a lack of support in their daily work.
An impact analysis regarding overall satisfaction shows the strengths of the team (figure 1): the knowledge/expertise, the quickness to offer support, the quality of the support, feedback on blood transfusion events and the reachability by phone or e‐mail (P < 0.01). Weaknesses are the presence of the team at the ward, feedback on blood transfusion practice and the collaboration between the team and the blood bank (P < 0.01). Respondents perceive overkill in accessibility (P < 0.05) and usefulness of procedures.
Finally, we analyzed the value of the hemovigilance team for the hospital. The knowledge/expertise of the team, the quickness to offer support and the quality of the support are seen as strong points (P < 0.01), whereas the collaboration with the blood bank is rather a weakness.
Figure 1: Impact analysis of the overall satisfaction of the hemovigilance team.

Summary/Conclusions
Most respondents are positive regarding the inhospital hemovigilance team. In order to improve the efficiency of the team, this study suggests better visibility and recognizability of the team, extended reachability, improved feedback on blood transfusion practice and focused trainings. Following this research, an action plan was drawn and implemented. To improve the visibility of the team, an internal website on hemovigilance has been created, an information desk has been set up and the team has been presented at several staff and head nurse meetings.
6.7 Alternatives to Blood Transfusion
P‐812
Erythropoietin to Reduce Allogeneic Transfusion in Patients Undergoing Total HIP or Knee Arthroplasty a Meta‐Analysis of Randomized Controlled Trials
So-Osman C1, Voorn VMA 2, van der Hout A 2, Vliet-Vlieland TPM 2, Nelissen RGHH 2, van den Akker-van Marle ME 2, Dahan A 2, Marang-van de Mheen PJ 2, van Bodegom-Vos L 2
1Sanquin Blood Supply, Leiden, The Netherlands2Leiden University Medical Centre, Leiden, The Netherlands
Background/Aims
A previous meta‐analysis showed that the use of erythropoietin to reduce allogeneic transfusion is effective in elective orthopedic surgery. However, this meta‐analysis did neither compare the effect of erythropoietin for individual indications such as total hip and knee arthroplasty, nor the safety and costs of the use of erythropoietin. Therefore we performed a meta‐analysis to assess the effectiveness, safety and costs of erythropoietin in total hip and knee arthroplasty separately.
Methods
A systematic literature search was performed to identify randomized controlled trials evaluating the effect of erythropoietin in total hip and knee arthroplasty. Study data were pooled using a random‐effects model. Methodological quality and strength of the evidence were assessed.
Results
Seven studies were included (2439 patients). Erythropoietin significantly reduced the exposure to allogeneic transfusion in both hip (RR 0.43; 95% CI 0.32–0.59) and knee (RR 0.38; 95% CI 0.27–0.53) arthroplasties with no subgroup differences (I2 = 0%). Mean red blood cell use significantly decreased in erythropoietin treated patients (mean difference −0.57; 95% CI −0.86 to −0.29) (not able to split for hip and knee). No significant differences in thromboembolic events or adverse events were found. One study evaluated the costs of erythropoietin. Erythropoietin increased healthcare costs with €785 per patient (€7300 per avoided transfusion).
Conclusion
Erythropoietin is effective and safe to use in patients undergoing total hip or knee arthroplasty. No differences in effect between hip or knee arthroplasty could be identified. However, the decision to use erythropoietin on a routine base must be balanced against its costs.
P‐813
2014 Survey of Intraoperative Cell Salvage: Equipment and Practice Across the United Kingdom
Gerrard RG1, Shreeve KS 2, Hockley BH 1
1NHS Blood and Transplant, Liverpool, United Kingdom2Welsh Blood Service, Pontyclun, United Kingdom
Background
In 2006, The UK Cell Salvage Action Group (UKCSAG) was established to help support the wider implementation of cell salvage as an alternative to donor blood and to facilitate a UK approach to its use. In the intervening years a Toolkit, including a range of education materials, has been developed.
To monitor uptake and implementation of intraoperative cell salvage (ICS) across the UK, a survey was conducted in 2007 and repeated in 2010. Improvements were seen in the areas of competency assessment, ICS policy implementation and uptake of ICS across specialities. However, it was also found that quality control of both operators and machines remained an issue. This survey was repeated in 2014 to evaluate progress with the implementation of ICS and to identify areas for improvement.
Aims
To evaluate progress with implementation of ICS.
To identify remaining obstacles to the implementation of an ICS service.
To measure the success of the Toolkit supplied by the UKCSAG.
To gain an overview of how training for ICS is being delivered and by whom.
To compare the specialties where ICS is being used in 2014 compared to in 2010.
To help focus future work priorities of the UKCSAG.
Methods
Questions were formulated by an iterative process and also based on previous surveys carried out by UKCSAG. The survey was conducted as an online exercise using SnapSurveys© software. Answers to each question were analysed proportionately (n, %).
Results
137 hospitals from all 4 countries in the United Kingdom responded to the survey. It identified new trends in ICS implementation. Some areas identified in the 2010 survey remain a challenge. Key findings in this 2014 survey are:
Level of ICS use (compared with 2010 survey) within each specialty is relatively unchanged other than obstetrics and gynaecology where use has increased.
There is a trend towards outsourcing of ICS services.
Shift to ICS becoming mainstream practice with ‘out of hours’ cover being provided by ‘on duty’ staff rather than ‘on call’ or dedicated operators.
Comprehensive training of ICS operators remains a challenge.
Between 50% and 60% of respondents said they did not quality control the machines or the operators.
13% of respondents are using “addressographs”to label salvaged blood rather than the recommended label.
Conclusions
A list of recommendations have been drawn up for key stakeholders. These include:
The requirement for, and provision of ICS should be reviewed/audited by every hospital transfusion committee and be part of the programme for Patient Blood Management/Better Blood Transfusion to be fully integrated into patient care.To minimise risks, organisations should ensure that there is a comprehensive training and assessment strategy in place, underpinned by evidence based policy and guidance.All ICS training received should be competency assessed.The use of addressograph labels introduces additional risk and is discouraged.Documentation on the ICS Toolkit should be systematically reviewed and updated by the UKCSAG at least once every 2 years.
P‐814
Patient Blood Management Awareness Amongst Clinicians from 6 European University Hospitals: A Multicentre Survey
Manzini PM1, Dall'Omo AM 1, D'Antico S 1, Lorenzi M 1, Valfrè A 1, Pendry K 2, Wikman A 3, Fischer D 4, Borg-Aquilina D 5, Laspina S 5, Van Pampus EC 6, Van Kraaij M 7, Grant-Casey J 8, Babra PS 8, Folléa G 9
1AOU Città della Salute e della Scienza di Torino, Torino, Italy2Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom3Karolinska University Hospital, Stockholm, Sweden4University Hospital Frankfurt, Frankfurt, Germany5Mater Dei University Hospital, Malta, Malta6Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands7Sanquin Blood bank, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands8NHS Blood & Transplant, Oxford Blood Centre, Oxford, United Kingdom9European Blood Alliance, Amsterdam, The Netherlands
Background
Patient Blood Management in Europe (PaBloE) is an European Blood Alliance (EBA) project gathering clinicians and transfusion experts from 7 European University Hospitals involved in patient blood management (PBM).
Aims
The aim of this survey was to evaluate the knowledge and application of PBM principles amongst clinicians working in 6 of the hospitals as a starting point to develop ways to implement best practice in the same hospitals.
Methods
A questionnaire on SurveyMonkey™ was sent to 2994 clinicians working in medical and surgery disciplines. The results were then analysed in Excel.
Results
To date, 560 responses (19%) have been obtained. Amongst respondents, surgeons are the most represented (43%, 23% general surgery and 20% working in solid organ transplant) followed by medical specialists (36%) and anaesthetists (21%). Respondents are equally divided in clinicians with a working experience <5 years, between 5 and 15 and more than 15 years after registration. 21% of respondents are not aware or declare not to know enough about correlation between pre‐operative anaemia (POA) and perioperative morbidity and mortality (9% and 36% among surgeons and medical specialists respectively). 15% of respondents think that treatment of POA is unlikely to favourably influence morbidity and mortality even before operations with expected blood loss >500 ml or declare not to have an opinion about this issue 0.91% of clinicians check haemoglobin level of their patients prior to scheduled surgery while 9% do not. 57% do not offer pre‐operative treatment of anaemia and only 33% of those who offer such treatment treat all patients. 38% of clinicians asked which is the best way to treat deficiency anaemia answered RBC transfusion prior to surgery. One third of interviewed clinicians use hypotension alone or tachycardia alone without identifiable causes as transfusion triggers.
Conclusions
A minority of clinicians (19%) working in large European University Hospital are aware of transfusion related issues as judged by the response rate to the survey. For 21% of respondents POA does not represent a perioperative risk, this perception being more frequent amongst medical specialists than surgeons. Moreover 15% of respondents believe that treatment of POA is unlikely to favourably influence morbidity and mortality even when the expected blood loss is higher than 500 ml. Nine percent of clinicians do not check haemoglobin level prior to surgery just focusing on blood loss risks instead of on patient global health. Often POA is not treated and 38% of clinicians think that the best way to treat it is RBC transfusion prior to surgery. This survey will help set up training programs to further develop awareness, implementation and assessment of PBM in European hospitals.
P‐815
Use of Tranexamic Acid in Total Hip and Total Knee Arthoplasty
Garcia-Gala JM 1, Perez de Ariba N 1, Martinez-Revuelta E 1, Solano-Tovar J 1, Argüello-Junquera M 1, Garcia Menendez-Tevar F 1
1Hospital Universitorio Central de Asturias, Oviedo, Spain
Currently there is evidence that the use of tranexamic acid decreases transfusion requirements in cardiac surgery, evidence is not as important in other types of surgery such as total hip arthoplasty (THA) or total knee arthoplasty (TKA).
Aim
In order to assess the efficacy of the use of tranexamic acid in reduced blood transfusion in THA and TKA we conducted this study
Methods
A case controls‐cases study was performed in patients undergoing surgery for THA and TKA. Tranexamic acid was administered as 1 g IV prior to surgery.. We excluded: prosthetic replacements, hip fractures, contraindications for the use of antifibrinolytic, and patients on autotransfusion program. We analyzed data on demographic, proportion of patients transfused, number of units transfused, volume of bleeding, re‐intervention for bleeding, stay hospital and thrombotic complications. Transfusión criteria used is defined in Hospital Guidelines for Transfusion.
Results
We included a total of 89 patients: 45 THA (26 cases / 19 controls) and 44 TKA (24 cases / 20 controls). There were no differences between cases and controls for each pathology with regard to age, sex, history of heart disease, lung disease, hemoglobin and kidney function. The pre‐surgery hemoglobin was 143 g/l in THA patients and 141 g/l in TKA.
In THA there was a smaller percentage of patients transfused between those receiving acid tranexamic (38.4%) compared to those without (42%) but the difference was no significant. We also did not find significant differences in the number of units transfused: in tranexamic acid patients received a mean of 0.64 ± 0.49 vs 0.89 ± 1.19 in the controls. There was significant difference in bleeding: 159.60 ± 69.6 cc in cases vs 392.11 ± 251.38 cc in patients who received tranexamic acid. No differences were observed in hospital saty.
In patients with TKA a lower percentage of transfused patients was observed among patients received tranexamic (20.8%) acid vs those who did not (45%), although without statistical significance (P = 0.08). the tranexamic acid patients received a mean of 0.29 ± 6.4 of packed red blood cells vs 1 ± 1.74 in controls, although no significant difference (P = 0.07). Ther was significant difference in bleeding in the resuscitation unit: 326.25 ± 249.6 cc in cases vs 472.5 ± 243.11 cc (P = 0.05) in patients not received tranexamic acid. No differences were observed day stay.
Conclusions
The use of tranexamic acid was associated with a lower bleeding THA and TKA.
In our study, minor bleeding in the group receiving tranexamic acid was not associated with lower percentage of transfused patients, due to the hemoglobin pre‐surgery pointed out the importance that patients come to surgery with a good hemoglobin.
7.1 Stem Cell and Tissue Banking, including Cord Blood
P‐816
Platelet‐Rich‐Plasma (PRP) Accelerates Diabetic Wound Healing Through Stimulating Angiogenesis by Recruiting Bone Marrow‐Derived Cells
Lu F , Dewei Z , Yu P
Affiliated Zhongshan Hospital of Dalian University, Dalian, China
Background
Many clinical trials have shown the effectiveness of platelets on chronic wound healing, but its mechanism of action is still poorly characterized. There is increasing evidence that angiogenesis is an important factor that affects diabetic wound healing and bone marrow‐derived stem cells (BMDCs) play a crucial role in angiogenesis.
Aims
The aim of this study is to investigate whether topical PRP accelerates diabetic wound healing through stimulating angiogenesis by recruiting BMDCs homing to the site of diabetic wound.
Methods
A single unit of human platelets was obtained by plateletpheresis. In vivo, platelets were topically applied on 1 cm2, full‐thickness wounds on db/db mice (n = 20/group) at postwound day 1 and 5 (plasma served as controls). Animals were sacrificed at postwound days 7 and 14. wounds were harvested for histology, immunohistochemistry, and immunofluorescence. Vasculogenic cytokine expression was evaluated via Western blot, reverse transcription‐polymerase chain reaction, and enzyme‐linked immunosorbent assay. Circulating CXCR4+ cells were analyzed by fluorescenceactivated cell sorting.
Results
PRP‐treated wounds were characterized by vasculature followed by abundant granulation tissue deposition at each time point. PRP treated wounds showed a 2.03‐fold increase in CD31 endothelial cell staining over controls. At day 7, reverse transcription‐polymerase chain reaction demonstrated a1.71‐fold and 1.69‐fold increase in SDF‐1 alpha and chemokine receptor type 4 (CXCR4) expression in treated wounds vs controls. CXCR4 was considered to be the only receptor for SDF‐1alpha. CXCR4 is often expressed by BM mononuclear cells (MNCs). Inhibition of CXCR4 decreases the homing ability of BMDCs to the site of injury, which indicates that the SDF‐1alpha / CXCR4 axis plays an important role in the homing of BMDCs to the site of injury. The numbers of circulating CXCR4+ cells increased in the PRP‐treated wounds vs controls, which demonstrate that the local recruitment of BMDCs was augmented by topical PRP.
Conclusions
Topical PRP is able to accelerate diabetic wound healing through stimulating angiogenesis by recruiting BMDCs. Thus, PRP therapy may be useful in the treatment of diabetic complications characterized by impaired neovascularization.
P‐817
The Effect of Hypoxia Preconditioning on the Expression of NT‐3, GFAP, Nestin, Oct‐4, Nanog Genes in Human Mesenchymal Stem Cells Derived From Umbilical Cord Blood
Kheirandish M1, Piri Gavgani S 2, Samiee S 2
1Iranain Blood Transfusion Organization, Tehran, Iran2IBTO, Tehran, Iran
Background
Human umbilical cord blood‐derived mesenchymal stem cell (hUB‐MSCs) has been regarded as an alternative and promising source for stem cell therapy. However, their implications are limited due to their reduced quality and function within a few days after transplantation.
Aims
In this study, we evaluated the effects of hypoxic‐preconditioning (HPC) on expression of Nt‐3, GFAP, Nestin, Oct‐4, Nanog genes and proliferative capacity of hUB‐MSCs in comparison with normoxic conditions to address a safe, non‐ genetic manipulated strategy for enhancing up‐coming MSC‐based therapies specially in the context of neurological disease.
Methods
Characterized hUB‐MSCs were cultured in Dulbecco's Modified Eagle's Medium – low glucose (DMEM‐LG) growth medium containing 10% FBS. Colony forming unit – fibroblast (CFU‐F) assay, viability and expression of surface marker were determined through each specific test. HPC protocol includes the following steps: 2 periods of hypoxia (15 min, 2.5% O2) and reoxygenation (30 min, 21% O2) prior to hypoxic challenge (72 h). Expression of pluripotency markers (Oct‐4, Nanog) and neural markers (Nt‐3, GFAP, and Nestin) was analysis by Real Time‐PCR.
Results
Our results indicated that cell population doubling rate was significantly increased in hypoxic‐preconditioned MSCs compared to normoxic group (P < 0.05). However MTT assays showed no significant differences between hypoxic‐preconditioned cells and normoxic group after 72 h of test time. Cell surface marker analysis through flow cytometry confirmed the presence of CD44, CD73, CD90 and CD105 markers and absence of CD45, CD14, HLA‐DR and CD271 in either hypoxic‐preconditioned or normoxic group. Our data showed that hUB‐MSCs expansion in hypoxic conditions leads to GFAP expression, expressed Nestin either hypoxic‐preconditioned or normoxic group. Besides, these two experimental groups were negative for Oct‐4, Nanog, Nt‐3 genes expression.
Conclusion
Conclusively, our results addressed hypoxic preconditioning as an effective strategy for enhancing cell viability and proliferation capacity of hUB‐MSCs and with impress the intracellular mechanisms leading to the expression some of neural genes.
P‐818
Abstract Withdrawn.
P‐819
CD14, CD16, HLA‐DR, HLA‐G Reliably Identifies Human Monocyte Origin and Their Subsets
Yin JP
Wuhan Blood Center, Wuhan, China
Background
Monocytes constitute 5–10% of peripheral leucocytes where they circulate for several days and can migrate into tissues to contribute to the macrophage pool. Monocytes and their progeny have established roles in defense against pathogens, homeostasis, and tissue repair. Monocytes were originally subclassified by their physical characteristics, but with the advent of monoclonal antibodies (mAbs) and their application in flow cytometry, monocytes were reclassified now.
Aims
To compare ‘positive inclusion’ and ‘negative exclusion’ gating techniques to identify monocyte subsets in the context of pathologically reduced HLA‐DR expression. And to verify whether the positively identified monocyte origin.
Methods
Monocytes and their subsets were concurrently identified through negative (exclusion of CD66b+ neutrophils, CD56+ NKcells, CD19+ B‐cells, and CD3+ T‐cells) and positive gating (inclusion of monocytes by expression of CD14, CD16, HLA‐G, and HLA‐DR) strategies on 30 occasions healthy controls (HC) and 21 patients with conditions associated with low monocyte HLA‐G and HLA‐DR expression.
Results
Bland‐Altman and Passing and Bablok regression statistics did not demonstrate any significant measurement bias between the two strategies of monocyte identification. Monocyte subset phenotype was then compared in 18 HC and 41 patients with acute liver failure, Compared with HC, in patients, the percentage of CD14++/CD16+HLA‐G+ monocytes was higher (7% vs 4%) whilst the percentage of CD14−/CD16+was lower (1.9% vs. 7%) (P ﹤ 0.001); HLA‐DR and CD86 MFIs on all monocyte subsets were lower, whilst CCR5, CD64, and CD11b MFIs were higher (P < 0.05). The relative expression by monocyte subsets of HLA‐G, HLA‐DR, CCR2, CCR5, CX3CR1, and CD11a was similar in patients and HCs. Repeat analysis of an identical antibody fluorochrome ‘backbone’ targeting HLA‐G, HLA‐DR, CD14, and CD16 was assessed in 189 samples across 5 different experiments. There was excellent agreement in the results obtained using the positive gating strategy (interclass correlation coefficients > 0.8).
Conclusions
Monocytes and their subsets can be reliably identified using an antibody‐fluorochrome ‘backbone’ of HLA‐G, HLA‐DR, CD14, and CD16. CD14+HLA‐DR-HLA‐G+CD16− monocytes were derived from the bone marrow stem cells, CD14+HLA‐DR-HLA‐G+CD16− monocytes were derived from Peripheral tissue stem cells. Both have stem/progenitor cell characteristics.
P‐820
Quality of Cryopreserved Cord Blood Units in the Public Cord Blood Bank of Korea
Lee HRL1, Shin SS 1, Roh EYR 1, Yoon JHY 1, Kim BJK 2, Song EYS 1
1Seoul National University Hospital, Seoul, South‐Korea2Boramae Hospital, Seoul, South‐Korea
Background
Cord blood (CB) units are cryopreserved for several years until they are used for suitable recipients. Therefore, the quality of cryopreserved CB units should ideally be maintained during cryopreservation. In Korea, 17 CB banks, including three government‐assigned public CB banks, have been operating with the permission of the Ministry of Health & Welfare, according to the law on the management and research of CB proclaimed in July 2011. Since May 2006, when the Seoul Metropolitan Government Public Cord Blood Bank (Allcord) was founded, approximately 30,000 CB units have been cryopreserved. To date, no quality assessment of cryopreserved CB units has been conducted in domestic CB banks in Korea.
Aims
We evaluated the quality of cryopreserved CB units according to the duration of cryopreservation, ranging from 1 year to 5 years, and investigated whether the quality of the cryopreserved CB units changed during cryopreservation.
Methods
We analyzed CB units that were rejected from the Seoul Metropolitan Government Public Cord Blood Bank inventory after conventional processing, because of unsuitability for allogeneic transplantation. Two hundred CB units that were cryopreserved from 1 year to 5 years were selected. After thawing the cryopreserved CB units, the total nucleated cell (TNC) count, CD34+ cell count, cell viability using 0.4% trypan blue staining and 7‐aminoactinomycin D (7‐AAD), apoptotic cells using caspase‐3 and annexin‐V, aldehyde dehydrogenase (ALDH) assay, and number of colony‐forming units (CFU) were analyzed. We conducted a comparative analysis to identify the presence of statistically significant differences in the recovery rates of the TNC and CD34+ cell counts and to compare the results of ALDH level, the cell viability test, the apoptosis test, and CFU analysis among groups according to the duration of cryopreservation.
Results
The recovery rates of the TNC count and CD34+ cell count did not differ significantly according to the duration of cryopreservation. Cell viability and the percentages of apoptotic cells among TNC and among CD34+ cells, did not reveal any increasing or decreasing trend according to the duration of cryopreservation. Also, the percentages of ALDH‐bright cells (ALDHbr cells) among TNC, ALDHbr cells among CD34+ cells, and CD34+ cells among ALDHbr cells, did not reveal any increasing or decreasing trend according to the duration of cryopreservation. Further, the numbers of CFU‐granulocyte/macrophage and CFU‐granulocyte/erythrocyte/macrophage/megakaryocyte, representing the clonogenic and proliferative potentials of hematopoietic stem cells, did not differ significantly according to the duration of cryopreservation.
Summary/Conclusions
These results suggest that there are no significant differences in the quality of CB units cryopreserved for up to five years. This is the first study investigating the quality of CB units cryopreserved at CB banks in Korea. Through the results of our study, we expect that the reliability of cryopreserved CB units as a source of hematopoietic stem cells for HSCT will be improved. Studies on the quality of CB units according to the duration of cryopreservation will be continued in our cord blood bank.
P‐821
Comparison of Three Methods for Processing Umbilical Cord Blood Units
Kondo ATK , Alvarez KCAA , Oliveira DCO , Colesanti MVC , Camillo ZMC , Lira SMCL , Nakazawa CYN , Yokoyama APHY , Sakashita AMS , Kutner JMK
Hospital Albert Einstein, Sao Paulo, Brazil
Background
Umbilical Blood cord is as an alternative source of hematopoietic progenitor cells in bone marrow transplantation. There are numerous advantages in cord blood use in transplantation, such as readily availability, no risk to the donor, and immunology immaturity, which allows the use of units with HLA mismatch, with results similar to fully compatible donors. However, some disadvantages have limited the use of these units in transplantation, such as slower hematologic recovery with higher graft failure rate. Countless studies have shown that greater compatibility HLA in association with the cellularity of the product are associated with improved graft rate and survival in transplantation. Moreover, literature reports serious complications during infusion of cord blood units that were not erythrocyte depleted before the cryopreservation and led to the blood banks to establish standard technique for cord bloodprocessing, with reduced plasma and red blood cells, concentrating the buffy coat. This aims to optimize storage units, since it reduces the volume to be cryopreserved, and reduces the risk of infusion reactions. This approach canhowever lead to a decrease in cellularity of the product and decreasing the product quality.
Aims
This study was performed to evaluate three methods of cord blood processing with its effectiveness in the concentration of buffy coat, with better cell recovery.
Methods
A retrospective analysis of units processed in the Albert Einstein umbilical cord blood bank was carried out from January to February 2015. Three methods were compared: group 1 – AXP method, group 2 – manual processing method, group 3 – SEPAX. The units were evaluated for cell recovery, viability and microbiological culture post processing.
Results
We analyzed 282 cord blood units in this study. In group 1 were processed 217 units, with an average recovery of 85.58%. In group 2, 15 units have been processed, and 6 were cryopreserved in two aliquots. The cell recovery in this group was 74.80%, however the units processed in this group had higher initial cellularity compared with the other groups (p 0.03), which may explain this lower recovery. Group 3 was composed of 50 units processed in SEPAX, with median cell recovery of 89.18%. In this group 16 units were processed on equipment SEPAX 1 and 34 in second version of equipment and there was no difference between the cell recovery equipment (90% vs 89%; p 0.8). There was no significant difference in cell recovery processing with AXP or SEPAX. The viability using flow cytometry (7AAD) was not different in the 3 groups, each group being 99.2%. No culture was positive, even in the manual processing method.
Conclusions
This study demonstrates the similarity of automated methods in umbilical cord blood processing, withou difference comparing Sepax 1 or Sepax 2. Only manual processing showed worse performance, however in this group the initial nucleated cells count was significant higher than other groups (0.002). There was no impact on the viability or products rate with positive culture comparing these three methods.
P‐822
Effect of MSC‐Derived Extracellular Vesicles on the Development and Function of Dendritic Cell Subtypes
Murke FM , Görgens AG , Bremer MB , Börger VB , Horn PAH , Giebel BG
Institute for Transfusion Medicine, University Hospital Essen, Essen, Germany
In more than 460 clinical trials mesenchymal stem/stromal cells (MSCs) have been administered to a variety of different patient cohorts. Apart of pro‐regenerative effects, MSCs exert immune modulatory functions and are able to suppress the function of a variety of different immune effector cells including dendritic cells (DCs). Against the initial dogma that MSCs act in a cellular manner, novel findings suggest that extracellular vesicles (EVs), such as exosomes (70–140 nm), mediate a huge proportion of the MSCs therapeutic effects. After showing that MSC‐EVs indeed suppressed symptoms in a graft‐versus‐host disease (GvHD) patient the MSC‐EVs’ target cells should be identified. Here, we aim to investigate the impact of MSC‐EVs on the development and maturation of human DCs, which are divided in CD1c+, CD141+, CD303+ and monocyte‐derived (MoDCs) subtypes.
We decided to establish an in vitro DC differentiation assay allowing for the in vitro generation of all DC subtypes first. To this end, human hematopoietic stem and progenitor cells (HSPCs) were raised under different conditions known to promote DC development. Arising immature DCs (iDCs) are identified and purified via multi‐color flow cytometry. The maturation of purified iDCs is stimulated by the addition of different TLR‐ligands (LPS, R848, polyI:C and CpG) and confirmed by flow cytometric analyses of the expression of co‐stimulatory (e.g. CD80, CD86 and CD40) and co‐inhibitory (e.g. PD‐L1) molecules and by functional assays, e.g. T cell activation assays. By adding purified MSC‐EVs to the differentiation cultures or the maturation conditions, respectively, the functional effects of MSC‐EVs on DC development will be studied. So far, the efficacy of different differentiation and maturation protocols has been compared. Furthermore, 9 color panels to discriminate the different DC subtypes have been set up.
Provided MSC‐EV will severally affect DC development (differentiation/maturation), the corresponding assay(s) should be qualified as potency assay(s) to compare the immune modulating capabilities of independent MSC‐EV fractions.
7.2 Collection, Processing, Storage and Release
P‐823
Safety and Efficacy of Apheresis Collection of Mobilized Hematopoietic Stem Cells from Peripheral Blood in Healty Donors – 14 years of Experience
Grubovic R1, Georgievski B 2, Useini S 1, Cevreska L 2, Velkova E 1, Genadieva-Stavric S 2, Veljanovski N 1, Pivkova A 2, Cadievski L 2, Grubovic M 1
1Institute for Transfusion Medicine of RM, Skopje, Macedonia2University Hematology Hospital, Skopje, Macedonia
Background
Allogeneic hematopoietic stem cell transplantation is an established therapy for many hematologic disorders. Since the discoveries of the potential of Peripheral Blood Stem Cells (PBSC) in the hematopoietic reconstitution mid 1980s and early 1990s PBSC gradually replaced bone marrow as the preferred source of stem cells. The introduction of hematopoietic cytokines that can mobilize large number of progenitors into circulation accelerated PBSC usage. The aim of our study is to present our 14 year experience with apheresis collecting of PBSC in donors. Material and Methods
This is a retrospective study performed in the Institute for Transfusion Medicine of Republic of Macedonia and University Hematology Hospital for period from 2001 till 2015. All donors were HLA typed and matched; they were fully informed on the donation procedure and signed an informed consent for donation. Minimum dose required to ensure successful and sustained engraftment was 2 × 106/kg CD34+ cells and 2 × 108/kg mono‐nucleated cells (MNC). PBSC harvesting was performed with continuous flow cell separator Baxter C53000 and COBE Spectra using conventional‐volume apheresis processing the 2–2.5 total blood volumes per apheresis. A femoral catheter was used for harvesting and Acid Citrate Dextrose formula A is used for anticoagulation. Recombinant human granulocyte colony‐stimulating factor (G‐CSF) is used to mobilize PBPC for collection. Harvesting of PBSC is usually performed after 4 to 5 days of G‐CSF subcutaneous administration at a dose of 10 μg/kg body weight.
Results
All the donors were siblings of the patients treated at the University Hematology Hospital. There were 117 apheresis procedures performed in 69 healthy sibling donors. There were 48 males and 21 females, aged 20–54. One to two apheresis procedures were required to collect adequate graft. The single procedure usually took 3–4 h and the volume of collected stem cells was 50–220 ml. The needed number of MNC and CD34+ cells was successfully collected by 1.7 apheresis. There were 8 ABO incompatible donors. Procedures for mobilization and collection of PBPC from healthy donors are generally well tolerated. The only adverse effects of the apheresis procedure were bone pain as reaction of G‐CSF and numbness of the extremities as reaction of ACD‐A (hypocalcemia), which occur rarely and were very mild. The collected PBSC were used in allogeneic stem cell transplantation in patients with: acute myeloid leukemia – 38 patients (55.1%), acute lymphoblastic leukemia – 13 patients (18.8%), chronic myeloid leukemia – 6 patients (8.7%), non‐Hodgkin lymphoma – 3 patient (4.3%), myeloproliferative disorders – 3 patients (4.3%), severe aplastic anemia – 3 patient (4.3%), chronic lymphoblastic leukemia – 1 patients (1.5%), Hodgkin disease – 1 patient (1.5%) and multiple myeloma – 1 patient (1.5%).
Conclusion
The apheresis collection of PBSC in healthy donors is an effective and safe procedure. We are developing a National Stem Cell Donors Registry as a part of Bone Marrow Donors Worldwide. In that way we hope we will help widen the world network of stem cell donors and enlarge the possibility for each patient to find the right match.
P‐824
Abstract Withdrawn.
P‐825
Apheresis Collection of Mobilized Peripheral Blood Stem Cells in Hematological Patients
Grubovic R1, Georgievski B 2, Useini S 1, Cevreska L 2, Velkova E 1, Genadieva-Stavric S 2, Veljanovski N 1, Pivkova A 2, Petkovikj E 1, Grubovic M 1
1Institute for Transfusion Medicine of RM, Skopje, Macedonia2University Hematology Hospital, Skopje, Macedonia
Background
Autologous peripheral blood stem cell (PBSC) transplantation is widely used to treat different hematologic malignancies. PBSC are the preferred source for 99% autologous stem cell transplantation (SCT). Optimal SCT outcome requires maximized stem cell collection efficiency. The aim of this study is to present our experience in apheresis collection of mobilized autologous PBSC in hematological patients.
Material and method
This is a retrospective study performed in the Institute for Transfusion Medicine of Republic of Macedonia and University Clinic for Hematology from January 2001 till January 2015. Patients with hematological diseases who underwent autologous PBSC collection and transplantation were included in the study. All subjects in the study were fully informed on the donation procedure and signed an informed consent for donation. Minimum dose required to ensure successful and sustained engraftment was 2 × 106/kg CD34+ cells and 2 × 108/kg mono‐nucleated cells (MNC). PBSC harvesting was performed with continuous flow cell separator Baxter C53000 and COBE Spectra using conventional‐volume apheresis processing 2–2.5 total blood volumes per apheresis. Mobilization regimens included granulocyte colony‐stimulating factor (G‐CSF) alone, or combination of G‐CSF and disease‐specific chemotherapy.
Results
There were 471 apheresis procedures performed in 223 hematologic patients, median 2.1 collections/patient (range 1–5) for the mentioned period. They were 138 males and 85 females, aged 18–65. Sufficient number of cells was collected by 2 apheresis procedures mostly (75%). The single procedure usually took 3–4 h and the volume of collected stem cells was 50–220 ml. They were patients with: multiple myeloma (39.6%), acute myeloid leukemia (29.4%), Hodgkin disease (15.3%), non‐Hodgkin lymphoma (12.2%), acute lymphoblastic leukemia (2.2%) and chronic lymphoblastic leukemia (1.3%). The only adverse effects of the apheresis procedure were bone pain as reaction of G‐CSF and numbness of the extremities as reaction of ACD‐A (hypocalcemia), which occur rarely and were very mild.
Conclusion
The needed number of MNC and CD34+ cells was successfully collected by ~2 apheresis. The tolerance of PBSC collection in our patients was good.
P‐826
Evaluation of Spectra Optia Apheresis System for Peripheral Blood Stem Cell Collection in Donors and Patients Over a Period of Four Years: A Single Center Study
Kuruppu KADDP , Herath A , Hess D , Perera K , Mijovic A
King's College Hospital, London, United Kingdom
Background
Peripheral Blood Stem Cells (PBSC) has been increasingly used for autologous and allogenic stem cell transplantation in United Kingdom. Kings College Hospital (KCH) is one of the largest transplant centres in UK, performing around 80 autologous and 80 allogenic transplantations a year. Apheresis unit at KCH collects PBSC from patients, sibling donors as well as from registry donors. Optia Apheresis platform has been used for PBSC collections since 2011.
Aim
Aim of this study is to establish the Collection efficiency of the Optia cell separator platform in relation to the donor and patient variables, different mobilization protocols, preharvest CD34+ cell counts and processed blood volume.
Method
In this single centre, 4‐year retrospective study, a total of 860 procedures were analyzed. Data were collected from the Apheresis unit, Stem cell Laboratory and from the Electronic Patient Records. The collection efficiency (CE) was calculated as (Product CD34+ cell count [×106])/(preharvest CD34+ count [μl] × Processed blood volume [l]) × 100 (Schlenke et al 2000).
Relevant data was initially entered into Microsoft Office Excel worksheet and subsequently transferred to SPSS (version 17.0 SSPS Inc.) data analysis software for further analysis. P‐values less than 0.05 were considered to be statistically significant.
Results
A total of 851 procedures were carried out in 361 patients and 251 stem cell donors; there were 402 males and 210 females. Nine procedures were excluded from analysis due to incomplete data.
The average number of procedures per person was 1.39, 1.2 in donors and 1.52 in patients. The overall CE of the Spectra Optia apheresis platform was 66.07%. The CE for donors and patients were 64.31 and 67.28%, respectively (P > 0.05). There was no statistically significant difference in CE with respect to gender, BMI or mobilization regimen. CE was not significantly different in the initial procedure compared to subsequent procedures, when the donors or patients had more than 1 collection. The CE was significantly different in donors and patients alike, in relation to their preharvest CD34+ cell count (P < 0.05). Interestingly CE decreased as the peripheral blood CD 34+ count (r 2 = −0.068; P < 0.05) and processed blood volume (r 2 = −0.078; P < 0.05) increased. Despite reduced CE, high blood CD 34+ counts enabled collection of sufficient cells in all donors (average 8.72 per kg recipient body weight) and patients (average 11.19 per kg body weight). The overall CE of Spectra Optia apheresis platform was found to be slightly higher than (66.07 vs 60.5%) the pilot study done in the same collection facility in 2012/2013.
Caption 1: Donor and Patient characteristics.

Conclusion
CE of the PBSC procedure on Spectra Optiacell separator is very satisfactory at 66.07%. Main factor affecting the CE was the preharvest CD 34+ count and the processed blood volume, which is inversely correlated with CE. We also showed that gender, BMI and mobilization regimen, do not affect collection efficiency.
P‐827
Flow Cytometry Using Annexin V and 7AAD for Assessment of Graft Quality in Allogeneic Hematopoietic Stem Cell Transplantation
Watz ESJ , Remberger M , Ishizaki E , Mattsson J , Uhlin M
Karolinska Institute, Stockholm, Sweden
Background
In about two thirds of patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) grafts are used that are donated by an unrelated HLA‐matched donor at distant sites. Factors as graft storage and transportation, previously shown to affect graft quality, are crucial in the HSCT process. To asses graft quality cell viability assays using nuclear stains such as 7‐aminoactinomycin (7AAD) are often used. However, 7AAD only detects necrotic or late apoptotic cells. Cells in early apoptosis are readily missed. This method may lead to overestimation of graft quality.
Aims
We aim to analyze graft quality using flow cytometry and staining with 7AAD and Annexin V, a marker for apoptotic cell death. We hypothesize that co‐staining with 7AAD and Annexin V may be a suitable marker for cell viability and graft quality in the clinical setting. The results will be correlated to clinical outcome.
Methods
Twenty nine patients who received allogeneic hematopoietic stem cell grafts (28 PBSC, 1 bone marrow) between June 2014 and February 2015 were included in the study. Graft quality was assessed by flow cytometry and co‐staining with Annexin V and 7AAD. Cells that were negative for both Annexin V and 7AAD were defined as live cells.
Results
Of these 29 patients 27 were still alive at time of analysis. The two patients that died, both had significantly less amount (%) of live cells in their grafts (P = 0.001) compared to patients that are alive. Patients that developed acute graft versus host disease grades II‐IV (GVHD, n = 10) also had significantly less amount (%) of live cells in the grafts (P = 0.02) in relation to those that have not developed acute GVHD. There was a correlation between time to platelet (PLT) engraftment (days to PLT>50 × 19 (9)/l) and amount (%) of live cells in the graft (r = −0.54, P = 0.004). No correlation to neutrophil engraftment was seen. There were no significant differences in the amount of live cells between patients with or without CMV infection or relapse.
Summary/Conclusion
Graft quality affect clinical outcome. Although only 29 patients were included in this preliminary study, we showed that patients who died or developed acute GVHD had less amounts (%) of live cells in their grafts. We also found a correlation between live cells in the graft and time to PLT engraftment. Co‐staining with Annexin V and 7AAD could be useful as a sensitive method for assessing graft quality in allogeneic HSCT.
P‐828
The Importance of FVIII and VWF Testing Before Autologous Plasma Collection
Philippova OI1, Trofimova SA 2, Lyschev AA 1, Koloskov AV 1
1North‐West State Medical University named I.I.Mechnicov, St. Petersburg, Russian Federation2Federal North‐West Medical Research Centre, St. Petersburg, Russian Federation
Background
The use of autologous plasma is one of blood saving methods. This technique is applied in pregnant women suffering the high risk of blood loss in a delivery process or a postpartum period. Autologous plasma banking is a laborious and cost effective procedure and it is important to evaluate the haemostatic potential of plasma before collection.
Aim
The objective of the study was to evaluate FVIIIc activity and VWF level in potential autologous plasma female donors before donation.
Materials and methods
The study included 100 healthy female plasma donors. The mean age was 29, 8 (range 18–42). The laboratory tests were focused on the detection of VWF level, the activity of factor VIIIc, activated partial thromboplastin time (ATTP) test and A‐O blood group typing.
Results
None of the individuals expressed any signs of bleeding. The APTT was in the range of normal values. In 8% of cases VWF level was less than 50%. 3 donors with the blood group O and 2 donors with blood group A expressed low activity of FVIIIc factor (less than 50%). In all other donors the FVIIIc and VWF levels were normal (101.7 ± 3.5% and 94.5 ± 3.0%) A direct strong correlation was found between the activity of FVIIc and VWF antigen levels. The low activity of FVIIIc corresponded to the low levels of VWF. Pearson's linear correlation coefficient was (R + 0, 72).
VWF level in blood group O was significantly lower in comparison with A (P < 0.01), B (P < 0.001) and AB (P < 0.05). FVIIIc activity in blood group O was also significantly lower compared to the other blood groups (P < 0.01).
Table. The level of VWF and FVIII activity in different ABO blood groups.
Conclusions.
It is important to test FVIII and VWF levels before autologous plasma collection in women to use it in obstetric bleedings. Further investigations are needed to assess the correlation of FVIII and VWF levels and haemostatic efficiency of plasma in hemorrhage treatment.
P‐829
Abstract Withdrawn.
P‐830
Mid Procedure Determination of Haematopoietic Stem Cell (HPSC) Dose in BM Harvest by Total Nucleated Cell Count (TNCC) and it's Comparison with Actual CD34 Counts: An Experience From Resource‐Poor Setting
Arora DA , Tiwari AK , Dara RCD , Aggarwal GA , Acharya DPA , Singh GS , Raina RV
Medanta The Medicity, Gurgaon, India
Background
There are various sources of haematopoietic stem cells (HPSC) commonly used clinically viz. Peripheral Blood Stem Cell (PBSC), Bone Marrow (BM) and Umbilical Cord (UC). While the number of CD34+ cells in UC is fixed, in PBSC CD34+ cells is decided on the basis of peripheral blood counts and can be augmented with a second harvest if needed. This is trickiest in case of BM, where the adequacy of target CD34+ cells depends on certain calculations done during the harvest procedure with the donor in an Operation Theatre under General Anaesthesia (GA). Since the recipient and donor are generally young children the second BM harvest is difficult. Therefore the calculation of haematopoietic cell dose mid‐procedure in Bone‐marrow harvest is crucial.
Aims
We studied the accuracy of a Total Nucleated Cell Count (TNCC) based formulacalculated during harvest in comparison to actual CD34+ cell counts done post‐harvest.
Methods
This study was carried out in large tertiary‐care hospital in National Capital Region (NCR), India. The data was retrieved from Hospital Information System (HIS) which included epidemiological data, disease condition, mobilization, harvest data, dose collected, infusion data, engraftment and follow up. All patients were screened and cleared for transplant after mandatory tests.
Total of five HLA‐ matched (6/6; HLA A, B and DRB1) family donors were taken for BM harvest. All the BM harvests were done fromiliac spine under GA. Each harvest was collected in single attempt and no repeat harvest was required. A dose of 5 × 106 CD34+ cells/kg patient body weight was targeted. Mid harvest sample was taken for TNCC. Another 2 ml was taken from the final product for determination of total CD34+ cells and compared with estimated dose collected based on TNCC. The maximum allowable volume was kept at 20 ml/kg donor body weight per harvest.
The estimated volume of BM to be harvested was calculated on basis of Mono‐nuclear Cells (MNC) count of the mid‐harvest sample as follows:
BM Volume (ml) = Desired MNC × weight of recipient (kg) × 1000 (for conversion to ml) Mid harvest TNCC × 10 (factor)
Similarly, the total cell dose was calculated from the mid‐harvest count as follows:
Cell dose (×108/kg) = Volume in bag (Litres) × mid‐harvest TNCC (× 109/litre)
Recipient's weight (kg)
For comparison, the CD34+ cells enumeration was done on harvest on FACSVerse (BD Biosciences, US) using ISHAGE protocol. The HPSC infusion was done and patient was monitored daily for engraftment and for chimerism at day 30. Patients were discharged after attaining clinical stability.
Results
Mean dose of HPSC estimated on basis of TNCC based formula was 5.02 × 108 MNC per kg body weight. Actual mean CD34+ cell count was 4.29 × 106 per kg body weight which was 85.69% of the estimated dose.
Caption 1: Donor‐Recipient demographics and comparison of HPSC dose calculated by TNCC method with actual CD34+ counts.

Conclusions
This data suggests that in resource‐poor settings where the facility of doing CD34+ counts is not readily available the volume of marrow to be collected can be decided on TNCC based formula.
P‐831
IDO‐Producing Splenic DC Cells Exhibited Tolerogenic Characteristics and Potential Inhibitory Activity Against Autoimmune Arthritis After Treated With Induced‐TREG Cells
Yang JY
Shanghai Blood Center, Shanghai, China
Background
As well known, Foxp3+ regulatory T cells play a crucial role in maintaining immune tolerance. It was reported that TGF‐β‐induced Tregs (iTregs) could effectively suppress established collagen‐induced arthritis (CIA). However, the mechanisms by which adoptive transferred iTregs suppress immune responses, particularly the interplay between iTregs and DCs in vivo, remained incompletely understood. In addition, indoleamine‐2,3‐dioxygenase (IDO), a catabolic enzyme, was indicated to play an important immunosuppressive role in arthritis. So, in this study, it was assessed the characteristics of splenic DC subsets of iTreg‐treated CIA mice. And whether these DCs could obtain suppressive activity and thereby maintaining oral tolerance and whether IDO was involved in this inhibitory effect were also determined.
Methods
First, iTregs were induced by TGF‐β in vitro and adoptive transferred into established CIA mice. After 10 days, splenic CD11c+DCs were isolated, termed ‘DCiTreg’. The phenotype, the expression of cytokines, the immunogenicity and the suppression on CD4+/CD8+T cell proliferation of DCiTreg were assessed. Second, DCiTreg were re‐infused into the new CIA mice, and clinical and histopathologic scores, cytokine and anti‐CII antibody secretion in serum were analyzed. Third, the IDO expression levels in different splenic DC subsets from iTreg‐treated CIA mice were determined by FACS, and IDO+DCs were further isolated. At last, the role of IDO in the inhibitory effect and in the induction of new Foxp3+iTreg were determined by 1‐MT blocking in vitro and in CIA mice.
Results
After iTregs adoptive transferred, isolated DCiTreg exhibited a series of tolerogenic characteristics. Compared with splenic DCs isolated from CIA mice (DCCIA), DCiTreg expressed obviously lower levels of MHC molecule (IA‐IE) and co‐stimulatory molecules (CD80/86 and CD40). And IL‐12p40 and IL‐6 production by DCiTreg were negligible, while high levels of IL‐10 and TGF‐β were expressed; especially enhanced level of IDO in DCiTreg was detected, and CD11b+DCs were found as a major contributor of IDO expression in iTreg‐treated CIA mice. In the proliferation assay, DCiTreg showed the poor ability to expand effector T cells and had the effective inhibitory potency. Meanwhile, after CD11b+IDO+DCiTreg re‐infused, a remarkable anti‐arthritic activity, improved clinical scores and histological end‐points were found. Also, serological levels of TNF‐α, IL‐6, IL‐17 and anti‐CII antibodies showed significantly low and TGF‐β production was high in the DCiTreg‐treated group. Conversely, DCCIA could not suppress CIA completely. And IDO+DCs could induce the generation and proliferation of functional Foxp3+Tregs in vitro and in CIA mice. However, DCiTreg lost the inhibitory ability on CIA when they pretreated 1‐MT.
Conclusion
These findings suggested that iTregs could inhibit CIA via tolerogenic splenic DCs formation. These tolerogenic splenic DCs could further effectively dampen the severity and progression of CIA in the IDO‐dependent manner, which was associated with modulation of inflammatory cytokine and anti‐CII antibody secretion and induction of new functional Foxp3+iTregs. Obviously, the long‐term protection against CIA was mediated through crosstalk between DCs and iTregs, suggesting that these tolerogenic DCs are key players in Treg‐induced ‘infectious tolerance’. The continuous cycling of this feedback loop might stabilize and even enhance inhibitory effect of iTreg treatment.
7.3 Clinical Applications
P‐832
Phenotypic Differences of CD4+ T Cells in Response to RBC Immunization in Transfused Sickle Cell Disease Patients
Vingert BV1, Tamagne MT 1, Habibi AH 2, Pakdaman SP 1, Ripa JP 1, Elayeb RE 1, Galactéros FG 1, Bierling PB 1, Ansart-Pirenne HA 1, Bartolucci PB 2, Pirenne FP 1
1Etablissement Français du Sang, Créteil, France2AP‐HP, Créteil, France
Background
Alloimmunization against red blood cells (RBC) is the main immunological risk associated with transfusion in patients with sickle cell disease (SCD). However, about 50 to 70% of SCD patients never get immunized despite frequent transfusion. In murine models, T CD4+ lymphocytes are a major contributor to the RBC alloimmunization.
Aims
Given the importance of CD4+ T cells in immune modulation, especially in diseases with antibody production, it is possible that CD4+ T cells in SCD patients may exhibit different phenotypes and function according to whether the patient is a responder or non‐responder to alloimmunization against RBCs.
Methods
The CD4+ T‐cell phenotypes and functions were explored and compared between a group of SCD patients (n = 11) who never became immunized despite a high transfusion regimen and a group of SCD patients (n = 10) who had become immunized (at least against Jkb) after a low transfusion regimen. The control group consisted of race‐matched healthy blood donors (HD group, n = 16). HLA‐DR, CD154, Tbet, OX40 and CD40 expression were studied to characterize the phenotype of CD4+ T cells from SCD patients. The expression of the three receptors of microbial structures, TLR2, TLR3 and TLR9 was determined. Jkb‐specific CD4+ T cells were studied in the alloimmunized group and in particular their cytokine production in response to Jkb peptides.
Results
Low TLR2/TLR3 expression and strong expression of CD40 on CD4+ T cells were associated with the non‐responder status, whereas spontaneous expression of IL‐10 by CD4+ T cells and weak Tbet expression were associated with the responder status. A Th17 profile was predominant in responders when stimulated by Jbk. Finally, the presence of memory Jkb‐specific Tfh cells producing IL‐10 and IL‐21 was shown for the first time in the blood of alloimmunized patients.
Summary/Conclusions
In summary, compared to HD group, we found CD4+ T cell‐related markers either associated or not with immune responses to RBC antigens. These findings implicate CD4+ T cells in alloimmunization in humans, and suggest that they may be exploited to differentiate responders from non‐responders. Precise identification of non‐responder patients could help to preserve rare matched units for patients with a higher risk of RBC allommunization. It would be especially beneficial when patients are already transfused, with no precise transfusion history.
P‐833
Intra‐Operative Autologous Donation Regulates Increase in Thoracic Fluid Content in Patients Undergoing Heart Valve Replacement on Cardiopulmonary Bypass
Narula J , Kiran U , Kapoor PM , Choudhury M , Chowdhury UK
All India Institute of Medical Sciences, New Delhi, India
Background
Intraoperative autologous donation (IAD) forms the mainstay of blood conservation strategy in cardiac surgery. Additional therapeutic benefit derived from it includes a decrease in the circulatory overload being handled by the compromised ventricles in volume loaded patients with pulmonary hypertension secondary to left heart disease (PH‐LHD). Institution of cardiopulmonary bypass (CPB) in such patients is associated with accumulation of extravascular lung water secondary to a multitude of factors. Electrical cardiometry (EC) provides an accurate, noninvasive, continuous measurements of thoracic fluid content (TFC) which is an indicator of total thoracic fluid volume (intravascular, intra‐alveolar and interstitial).
Aims
To evaluate the effect of perioperative volume shifts induced by IAD as measured by variations in TFC in patients undergoing heart valve replacement. Secondary goal of this study was to correlate IAD induced changes in TFC to the perioperative fluid balances.
Methods
Prospective randomized controlled trial conducted in 50 patients assigned to a control group (standard care, n = 25) or IAD group. 15% of the estimated blood volume of the patient extracted in the IAD group was simultaneously replaced with 1:1 colloid. TFC and other hemodynamic data were recorded across 7 perioperative time frames and perioperative fluid balances were calculated. All patients were managed according to a standard anesthetic technique and a well defined blood transfusion, fluid management and inotrope therapy protocol.
Results
IAD resulted in a significant reduction in TFC −10.1% (−15.0 to −6.1), right atrial pressure (RAP) −23% (−26.6 to −17.6), mean arterial pressure −12.6% (−22.2 to −3.8), peak −6.2% (−11.7 to −2.8) and mean −15.4% (−25.0 to −8.3) airway pressures and oxygenation index (OI) −10.34%(−16.4 to −4.8). Linear regression analysis showed good correlation between the amount of autologous blood removed and percentage change in TFC (%TFC), RAP, peak and mean airway pressures and OI. CPB induced increase TFC, airway pressures and oxygen index were significantly lesser in those undergoing IAD. IAD also ensured an early return of TFC to baseline values at 48 h after cardiac surgery.
All patients in control group received banked blood transfusion in the operation theatre vs 16 (64%) in the IAD group. The amount of allogenic blood transfused/kg of body weight was significantly higher in the control group [13.63 (8.3–18.5) ml] than the IAD group [6.9 (4.4–11.4) ml, P = 0.003]. The control group patients also had a higher blood component therapy requirement (P = 0.001) and greater incidence of massive blood transfusion (P = 0.043). Linear regression analysis revealed good correlation between %TFC and intra‐operative and cumulative fluid balance, CPB time, massive transfusion requirement and duration of mechanical ventilation.
Conclusions
Therapeutic benefits derived from IAD by decreasing TFC and decongesting volume loaded patients further warrant its routine use in patients with PH‐LHD. IAD associated reduced allogenic blood transfusion requirement optimizes fluid balance during cardiac surgery and causes an attenuated TFC increase. Alterations in TFC correlate well with perioperative changes in body fluid balance secondary to IAD and CPB. Addressing the deficiencies of the existing methods, this non‐invasive technology can be utilized during routine cardiac surgery to reliably predict fluid status of the body.
P‐834
Impact of ABO‐Blood Group Incompatibility on Transfusion Outcome and Survival of Patients Undergoing Single‐Unit Umbilical Cord Blood Transplantation: Experience of a Single Institution
Solves P , Carpio N , Gómez I , Carretero C , Hernani R , Martinez F , Sanz J , Sanz GF , Sanz MA
Hospital la Fe, Valencia, Spain
Background
Umbilical cord blood transplantation (UCBT) is a procedure that needs frequent blood products as part of their supportive therapy. In contrast to solid organ transplantation, UCBT is commonly performed across the ABO barrier. However, the association between ABO incompatibility and outcome of UCBT is controversial.
Aims
This study investigates the transfusion outcome and the effect of ABO and Rh (D) incompatibility in recipients of single‐unit UCBT.
Methods
We conducted a retrospective study for analyzing transfusion outcome of patients undergoing single unit UCBT for a 6‐year period in a single institution. Transfusion requirements for the first 3 months after UCBT were detailed. The study includes 142 patients (median age at time of transplant: 43.1 years, range 16.7–65.5), 87 were male and 55 were female. Patients had received UCBT from 2008 to 2014 for treatment of ALL (n = 40), AML (n = 58), NHL (n = 8) and other diseases (n = 36). Donor‐recipient ABO compatibility was as follows: ABO match in 61 patients (group A), major mismatch in 43 (group B), minor mismatch in 37 (group C) and bidirectional mismatch only in 1 patient that was included in major mismatch group for analysis. Regarding the Rh(D) compatibility, 100 patients were Rh(D) identical, 15 Rh(D) incompatible and 27 were Rh(D) minor incompatible. Most patients (n = 113) received myeloablative fludarabine based conditioning regimen (MA) and 29 received reduced intensity conditioning regimen (RIC). Platelet (PLT) prophylactic transfusions were administered at a trigger of less than 20,000/μl. Red blood cell (RBC) transfusions were performed for Hgb < 8 g/dl. Transfusion independence was defined as the day of the last transfusion, with no PLT transfusions in the following 7 days or no RBC transfusions in the following 30 days. All products were irradiated before transfusion with 25 Gy. Non‐parametric tests were used for comparisons among groups. Overall survival were estimated by Kaplan‐Meier and compared with log‐rank test.
Results
Patients received a median and range of 10 RBC (2‐78), 22 (1‐271) PLT and 0 FFP (0‐132) during first 90 days after UCBT. There was a significant correlation between RBC and PLT transfusions (rho: 0.876, P < 0.001). Patients with RIC had similar transfusion requirements than patients receiving MA regimen. PLT engraftment defined as PLT count ³ 20,000/ml occurred at a median of 44 days (10–184), and neutrophil engraftment defined as neutrophil count ³ 1000/ml occurred at a median of 21 days after UCBT (10–61). Days until engraftment or transfusion requirements were similar for groups A, B and C. RBC transfusion independence was reached by 69 patients, at a median of 31 days (0‐232), while PLT independence occurred in 82 patients at a median de 35.5 days (18‐414). There were also no differences among groups for transfusion independence. 90 days after UCBT, 101 patients (71%) were alive. Overall survival rates were similar for all groups: A = 78.6%; B = 75%; C = 70.2% at 90 days. There were no differences in transfusion outcome among patients according to the Rh compatibility.
Conclusions
ABO incompatibility did not significantly influence transfusion outcome or survival in patients undergoing single‐unit UCBT.
P‐835
Abstract Withdrawn.
P‐836
Abstract Withdrawn.
P‐837
Allelic Typing of KIR2DL1 and KIR3DL1 Genes to Define Low/High Affinity Receptor‐Ligand Combinations and Improve Donor Selection in Stem Cell Transplantation
Seidl C , Becker P , Stricker N , Hennecke J , Klemm D , Seifried E
Institute of Transfusion Medicine and Immunohematology, Red Cross Blood Donor Se, Frankfurt am Main, Germany
Background
NK cells are potent effectors of innate immunity against transformed cells enhancing the graft vs leukaemia effect. For patients in haplo‐identical and HLA‐matched SCT with malignant disorders, KIR genotyping has been introduced by several centers to define the NK cell alloreactivity. Recent studies on KIR – HLA ligand interactions reveal affinity differences based on structural differences leading to ‘low’ and ‘high’ affinity alleles of the same NK receptor gene.
Aim
Allelic typing strategies for these KIR could be used to select donors/grafts and or KIR positive NK subpopulations for immunotherapy.
Methods
Sequence analysis of KIR2DL1 and KIR3DL1 genes using amplified genomic DNA from different healthy donors was performed using primers as described by Hou et al. (2007) and Belle et al. (2008). These primers were used to build different sets of PCRs to generate overlapping amplicons containing KIR gene loci using long‐range PCR for sequencing (ABI Prism 3730xl Genetic Analyzer). KIR exon sequences were analyzed by GeneSearch software (Phenosystems) and compared to the IPD‐KIR database (www.ebi.ac.uk/ipd/kir/).
Results
Allelic level typing could be obtained with this strategy, in particular differentiating KIR3DL1 and KIR2DL1 low affinity alleles. In five cases, allelic subtyping revealed potential ‘new’ alleles.
Conclusion
KIR allelic subtyping to determine allelic functional polymorphism should lead to better prediction of donor‐NK cell alloreactivity to enhance the GvL effects in SCT and NK cell therapies.
P‐838
Is it Time to Revisit Conventional Plerixafor Protocol in Very Poor Stem Cell Mobilizers?
Khetarpal A , Arora B
Artemis Hospitals, Gurgaon, India
Background
Autologous hematopoietic peripheral blood stem cell (PBSC) transplantation offers a robust treatment option to patients with multiple myeloma or lymphoma. Conventional strategies to mobilize stem cells from the bone marrow into the peripheral blood (PB) which either include a combination chemotherapy plus granulocyte‐colony‐stimulating factor (G‐CSF) or G‐CSF alone fail in approximately 10–20% of patients. A new approach, offering a combination of G‐CSF and plerixafor (AMD 3100) is now available to achieve adequate stem cell mobilization in these patients. Though the conventional regimen suggests the initiation of PBSC harvest by apheresis be done 10–11 h after the administration of plerixafor, newer studies suggest the initiation of PBSC harvest 3–4 h after plerixafor administration.
Aims
We share our experience of using plerixafor in a patient of Anaplastic Large T cell Lymphoma (ALCL) first according to the conventional regimen followed by switching over to the newer regimen of initiating the harvest 3–4 h after administration.
Methods
After detailed investigations as per our centre's protocol, the patient was considered suitable to receive autologous peripheral blood stem cells for his condition. The stem cells were mobilized using a combination of chemotherapy, G‐CSF and plerixafor. Patient was administered GCSF (10mcg/kg) for 5 days with addition of plerixafor (0.24 mg/kg) on day +4. Harvest was done on day +5, 11 h after plerixafor administration. Plerixafor and G‐CSF were repeated on day +5 and a repeat harvest was done on day +6, starting 11 h after plerixafor administration.
The same patient was taken up for PBSC harvest after 1.5 months of initial harvest. The stem cells were mobilized using a combination of chemotherapy, G‐CSF and plerixafor as done previously. Pattern of G‐CSF administration remained the same (10mcg/kg) for 5 days. Plerixafor (0.24 mg/kg) was administered on day +5, at 5 AM, 4 h prior to the start of PBSC harvest, which was started at 9 AM on the same day.
For all harvests, 3 patient blood volumes were processed using COM.TEC (Fresenius, Germany) apheresis machine.
Results
Harvest failure, with poor mobilization of stem cells was observed using the conventional plerixafor regimen. The doses of CD 34+ cells obtained were 0.74 × 10*6/kg/body weight and 0.5 x10*6/kg/body weight on day +5 and +6 respectively. The combined dose was deemed inadequate for transplant and cryo‐preserved for later use.
A robust mobilization of the bone marrow was observed when plerixafor was administered 4 h prior to the apheresis procedure upon repeat harvest. The total dose of CD 34+ cells obtained was 6.3 × 10*6/kg/body weight and was considered adequate. The cells were cryo‐preserved and the patient was started on conditioning chemotherapy (BEAM regimen) in preparation to receive the harvest at a later date. The patient has since engrafted.
Summary/Conclusions
Administration of Plerixafor 10–11 h prior to the harvest may not result in adequate mobilization of stem cells. The time for plerixafor administration, 3–4 h prior to harvest, as considered in our case, needs to be further evaluated so as to provide adequate mobilization of the marrow especially in very poor mobilizers.
P‐839
Informed Consent Action Group the Development of a Regional Tool ~ the ICAG Pad
Whitmore EL 1, Goodwin S2
1NHS Blood and Transplant, London, United Kingdom2Surrey and Sussex Healthcare NHS Trust, Redhill, United Kingdom
Background
ICAG‐pad is an amalgamation of ideas brought together as a regional response to the 14 outcomes and recommendations made by SaBTo as a result of the public consultation on patient consent to transfusion conducted in March 2010, and to the recommendations made by the NHS England sanctioned Patient Blood Management national initiative 2014.
Aims
To change clinical practice in obtaining informed consent for transfusion in a none emergency/bleeding situation, increasing the safe, effective and appropriate use of transfusions and alternatives and including the patient in their treatment decisions.
Method
The front and inside cover of the A5 sized laminated pad is an aide memoir summarising the multiple and varied risks of transfusion which have been put into 4 risk categories with corresponding mitigating actions, and supporting the consideration of alternatives as an option. These are discussed with the patient and supported by NHSBT Patient Information Leaflets, national guidelines and local transfusion policy, giving appropriate information and reassurance.
Results
All NHS and independent sector hospitals in the SEC region have assessed its use and implementation in their own Trusts. Initial feedback has shown the need for a longer training and introduction period as time with new intake doctors, and with established clinicians is difficult to secure. Support and assistance is needed from nursing staff to promote and support medical colleagues to use the pads in daily practice. A short questionnaire has been prepared for sites to use at their own discretion to measure implementation and impact and collection and collation of that data will be gathered, reviewed and shared regionally, allowing a review of impact as an ongoing process. The decision to progress in this way rather than a single survey is in direct response to the feedback from regional users. Users external to SEC will also receive this tool and have agreed to share their findings. The recommendations and results of the 2014 National Comparative Audit on Consent and Patient Information are also now being used as a driver to engage previously reluctant members of Trusts to support improve and further measure consent practice in Trusts. A recent request and interest has been made in developing the ICAG Pad for specific area use in paediatrics and neonates. Work on this will begin in April 2015 with a scoping and impact exercise.
Summary/Conclusions
Although widely agreed that change to clinical practice and culture is slow it is also vital and this initiative has been welcomed and put into practice in over 50% of the sites it has been sent to within the region and has been requested by 7 sites outside of the SEC both NHS and Independent sites. Evidence from recent NCA audits as well as local concerns, Never Events and risk assessments and the 35 recommendations and actions from the PBM Action Plan are positive, evidence based drivers to continuing to implement change to clinical practice and culture around patient information and informed consent to improve patient safety and outcomes in transfusion.
P‐840
Extracellular Vesicles From Different Donor‐Derived Mesenchymal Stem Cells Differ in Their Immunomodulatory Properties
de Miroschedji KNM
University Hospital Essen, Essen, Germany
Background
Human mesenchymal stem cells (hMSCs) were administered in more than 400 NIH‐registered clinical trials to patients suffering from various diseases including myocardial infarction, stroke and graft‐versus‐host disease (GvHD). Initially, hMSCs were thought to replace lost cells in damaged tissues. Despite controversial reports regarding the efficacy of MSC‐treatments, MSCs seem to exert their beneficial effects rather by secretion of immunosuppressive factors than by cell replacement. In this context, extracellular vesicles (EVs, 70–140 nm), such as exosomes and microvesicles, were identified to execute the hMSCs’ therapeutic effects. Our recent successful treatment of a steroid‐refractory GvHD patient with MSC‐EVs further highlighted their therapeutic potential.
Aim
We assume that hMSCs of different bone marrow donors contain different immunosuppressive potential.
Methods
EVs from cell‐culture supernatants of hMSCs of 20 donors were harvested and their immunomodulatory properties were analyzed in a T cell proliferation/activation assay using flow‐cytometry.
Results and Conclusion
Regarding their immunosuppressive capabilities, huge differences were observed, suggesting that not all MSCs release therapeutically effective EVs. For the establishment of a MSC‐EV potency assay, we now compare the feasibility of different T cell activation assays and analyze their sensitivity by multicolour flow‐cytometry amongst others for the expression of known T cell activation markers. Our preliminary results will be presented.
P‐841
Flying High With Blood on Board
Watson H , Ranton H , Avery S , Scott Y , Hawes R , Wallis J
Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
Background
The Great North Air Ambulance Service (GNAAS) respond to approximately 1000 call outs per year bringing specialist trauma doctors and paramedics quickly to the scene of an accident. Of the patients that do not survive, major haemorrhage is a major contributing factor; studies have shown fluid resuscitation in patients in hypovolemic traumatic arrest to be poor. To improve the service and increase the chance of patient survival it was proposed to carry blood on board the helicopter allowing pre‐hospital transfusion.
Aims
Provide two units of group O RhD negative packed red cells to each of the two air ambulances GNAAS operate. The red cells must be packaged in such a way that maintains the core temperature of the blood so it does not exceed recommended guidelines and the cells must be replaced daily.
Methods
Minnesota Thermal Science have produced the Original Golden Hour® thermal shipping container designed to keep temperature dependent medical supplies safe for extended periods. First used by the military the Golden Hour box is capable of maintaining the required cold temperatures of packed red cells for up to 72 h, complying with The Blood Safety and Quality Regulations (2005). A box was purchased and tested to ensure these temperature regulations were met.
To supply the blood to the two bases the Northumbria Blood Bikes, a charitable organisation that supply an out of hours blood transport service that is free of charge, have agreed to take the prepared box to the base at Penrith, returning with the previous days box and then onto Durham Tees Valley airport to repeat the process. This will occur every day.
Results
The system went live in January 2015 and an audit undertaken to collect information regarding the patient. As well as patient demographics and clinical details, clinical management data is also collected. This includes details of pre hospital fluid and/or transfusion, any adverse reactions, if more blood would have been given if available and the clinical course of the patient. All logistical information is also gathered including the packing of the cool box and the paperwork required.
Conclusion
Currently the red cells have been used twice with no logistical problems and there has been no wastage of red cells. The audit is ongoing.
References
1. Lockey D, Crewdson K, Davies G: Traumatic cardiac arrest: who are the survivors?Ann Emerg Med 2006, 48(3):240‐4
2. Kent, Surrey and Sussex Air Ambulance Trust at http://www.kssairambulance.org.uk/accessed January 2015.
P‐842
Expansion and Orderly Differentiation of Megakaryocytic Progenitor Cells Generated From Cord Blood Hematopoietic Stem/Progenitor Cells and its Phase I/II Clinical Trials
Pei P , Xi J
Institute of Transfusion Medicine, AMMS, Beijing, China
Thrombocytopenia is a common and potentially fatal complication of high‐dose chemotherapy and hematopoietic stem cell transplantation. Infusion of platelets from unrelated donors is currently the only effective treatment to prevent fatal hemorrhage. Hematopoietic stem cells (HSCs) from bone marrow (BM), cord blood (CB), and peripheral blood (PB) can be used to generate functional hematopoietic progenitor cells, including megakaryocytic progenitors (MPs), megakaryocytes, and platelets. Umbilical cord blood is an abundant source of HSCs. Cord blood is also highly enriched in committed hematopoietic progenitor cells, including those of the megakaryocytic lineage. In vitro large scale production of hematopoietic progenitor cells from cord blood could represent an effective blood cell substitute. In the present study, our objective was to determine the safety and feasibility of ex vivo generated hematopoietic progenitor cells (HPCs) in patients with hematological malignancy. Based on promising results of our preclinical study, state food and drug administration (SFDA) of China approved our group to conduct a clinical trial of HPCs injection to patients with hematologic malignancy. We investigated the feasibility of large‐scale expansion, orderly differentiation and infusion of cord blood‐derived HPCs in the patients with advanced hematological malignancyes. No adverse effects were observed in patients who received ex vivo‐generated cells. Further, a moderate effect on platelet recovery was also observed. Administration of cord blood‐derived HPCs appeared safe and feasible for treatment of thrombocytopenia after chemotherapy.
8.1 HLA in Transfusion Medicine
P‐843
HLA‐DRB1*12 and HLA‐DRB1*15 are Associated with Antibody Response to RHD‐Antigen in Brazilians
Moritz E1, Souza CP 1, Cruz B 1, Lopes LB 1, Chiba AK 1, Martin FO 1, Costa SSM 2, Bordin JO 1
1Universidade Federal de São Paulo, São Paulo, Brazil2Hemocentro da Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil
Background
The D‐antigen of the Rh system is one of the most immunogenic blood group proteins. The anti‐D alloantibody is of clinical importance since it might cause either hemolysis of red blood cells (RBC) following blood transfusion or hemolytic disease of the newborn. The immunogenicity of D is now better understood due to (i) knowledge of the genetic basis of the protein involved, (ii) the molecular orientation into the RBC membrane, and (iii) the nature of the cellular immune responses. Since RhD differs by around 35 amino acids from the RhCcEe protein, immunogenic T‐cell epitopes are more likely to be generated than that for alleles characterized by a single amino acid difference. On the other hand, not all individuals form antibodies when exposed to a mismatched antigen. One of the possible factors determining whether an RBC antibody response occurs is the presence of suitable HLA class II, especially HLA‐DRB1 molecules, in the responder to present the allogenic RBC peptides.
Aims
To analyze whether the HLA-DRB1 polymorphisms are associated with alloimmunization to the RhD antigen alone or concomitantly with multiple RBC antibodies, in Brazilians subjects.
Methods
A cross‐sectional study was conducted including samples from 133 D‐negative and Partial D immunized individuals with anti‐D. In addition to anti‐D, in 35/133 (26%) samples one or more antibodies to RBC antigens were found. An additional 43 samples with multiple RBC antibodies of clinical importance (anti‐D not included) were also investigated. Immunized individuals were included in the study regardless the way of immunization (pregnancy, transfusion or transplantation). Alloantibodies were identified by gel column technology and confirmed by phenotyping and/or genotyping of the corresponding blood samples. HLA-DRB1 allele frequencies of these groups were compared to 348,030 controls without the presence of RBC antibodies. HLA-DRB1 genotyping was performed with sequence‐specific oligonucleotide primed PCR (PCR‐SSO) and use of the Luminex technology.
Results
HLA-DRB1*12 and HLA-DRB1*15 alleles were overrepresented in D‐negative and Partial D alloimmunized individuals (f = 0.0376 and f = 0.1579 respectively) compared to controls (f = 0.0166 and f = 0.0947; P = 0.0146 and P = 0.0006 respectively), conferring an odds ratio (OR) of 2.317 (95% confidence interval [CI], 1.231–4.361) to the HLA-DRB1*12 allele and 1.792 (95% CI, 1.289–2.492) to the HLA-DRB1*15 allele. To analyze the association of HLA-DRB1 alleles with the immune response against multiple RBC antigens, all samples with two or more RBC antibodies were investigated (n = 78), independently of the presence of anti‐D. In these cases HLA-DRB1*15 frequency was again increased when compared to controls (f = 0.1667 and f = 0.0947; P = 0.0034) conferring an OR of 1.912 (95% CI, 1.254–2.913).
Conclusions
This study suggests that individuals carrying the HLA-DRB1*12 and HLA-DRB1*15 alleles have an increased risk for RhD alloimmunization. However, the HLA-DRB1*15 allele does not appear to be associated specifically to the RhD response, as it is also overrepresented in individuals with multiple RBC antibodies. The HLA-DRB1 genotyping may contribute to a better understanding of the susceptibility factors involved in alloimmunization. These findings will aid in the selection of the best transfusion strategy.
P‐844
Association Between HLA‐DRB1 Alleles and RBC Alloimmunization in Brazilians
Pinheiro de Souza CS 1, Moritz EM1, Chiba AK 1, Costa SSM 2, Barbosa Lopes LB 1, Orlando Bordin JOB 1
1Universidade Federal de São Paulo, São Paulo, Brazil2Hemocentro da Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil
Background
Antibodies against red blood cell (RBC) antigens are often observed in polytransfused patients and pregnant women, although not all individuals form antibodies when exposed to a mismatched antigen. One of the possible factors determining whether an RBC antibody response occurs is the presence of suitable HLA class II molecules. Recent studies suggest that HLA-DRB1 alleles may influence the immune process by presenting selected peptides in their DR molecule, acting in the regulation of immune responses.
Aims
To analyze whether the HLA-DRB1 alleles are associated with alloimmunization against the clinically significant RBC antigen systems: Rh, Kell, Kidd, Duffy and MNS, in Brazilians.
Methods
A cross‐sectional study included samples from three groups: 110 alloimmunized patients, 101 not alloimmunized patients and 100 healthy individuals, collected at two hospitals in Sao Paulo city (Brazil). The alloimmunized group presented 186 antibodies: 18 anti‐D, 32 anti‐C, 39 anti‐E, 9 anti‐c, 4 anti‐e, 31 anti‐K, 10 anti‐Fya, 3 anti‐Fyb, 19 anti‐Jka, 6 anti‐Jkb, 13 anti‐S and 2 anti‐s. Alloantibodies were identified by gel column technology and confirmed by phenotyping of the corresponding blood samples. HLA-DRB1 genotyping was performed by sequence‐specific oligonucleotide primed PCR (PCR‐SSO) using Luminex technology. The frequency of HLA-DRB1 alleles and the relation to RBC alloimmunization were evaluated according to the P4 pocket polymorphisms (amino acids 13, 70, 71, 74 and 78).
Results
HLA-DRB1*15 allele was overrepresented in (i) D‐alloimmunized patients (f = 0.278) compared to not alloimmunized patients (f = 0.109; P = 0.0138) and healthy individuals (f = 0.135; P = 0.0439) and (ii) K‐alloimmunized patients (f = 0.226) compared to not alloimmunized patients (f = 0.109; P = 0.0322). The HLA-DRB1*01 allele was overrepresented in C, Jka and S‐alloimmunized patients (f = 0.172, f = 0.290 and f = 0.192 respectively) compared to not alloimmunized patients (f = 0.074, f = 0.074 and f = 0.115; P = 0.0295, P = 0.0005 and P = 0.0320 respectively). HLA-DRB1*04 was overrepresented in not alloimmunized patients (f = 0.139) when compared to C‐alloimmunized patients (f = 0.031; P = 0.0212), as well as HLA-DRB1*11 was overrepresented in healthy individuals (f = 0.140) compared to E‐alloimmunized patients (f = 0.051; P = 0.0377). Other associations have not shown significant results.
Conclusion
This study suggests that individuals carrying the HLA-DRB1*15 allele present an increased risk to D‐ and K‐alloimmunization, and HLA-DRB1*01 allele to C‐, JKa‐ and S‐alloimmunization. The associations between HLA-DRB1*15 and HLA-DRB1*01 alleles with alloimmunization to D, K and Jka antigens have been described in the European population, however this is the first study to report the association between HLA-DRB1*01 allele with C‐ and S‐alloimmunization. Moreover, the HLA-DRB1*04 and HLA-DRB1*11 alleles seem to offer a protective effect against the C and E‐alloimunization respectively.
P‐845
Screening of HLA Antibodies as a Strategy to Decrease TRALI
Pereira F , Malcata C
Instituto Portugues do Sangue e da Transplantação, Lisboa, Portugal
Background
The HLA system is very immunogenic and antibodies can appear after pregnancy or transfusion of blood components. It has been reported that approximately 10–20% of female donors with a history of pregnancy have HLA antibodies. The presence of these antibodies can cause transfusion reactions, the most serious of which is TRALI (Transfusion‐Related Acute Lung Injury). This reaction is known as the most frequent cause of morbidity and mortality after transfusion of blood and occurs in about 1 in 5000 transfusions.
Aims
The objective of this study was to identify the frequency of HLA antibodies in a population of female blood donors.
Methods
Study ofHLA antibodies was performed in a series of female blood donors at the Centro de Sangue e Transplantação de Lisboa‐Área do Sangue, using an ELISA technique (GTI QUICKSCREEN® and GTI B‐SCREEN® Gen‐Probe Inc, USA). The serum/plasma samples from 1326 female blood donors (649 apheresis donors and 677 blood donors), aged between 18 and 66 years, were analyzed.
Results
Two hundred and twenty eight donors (17.2%) had HLA antibodies, 88 Class I, 95 Class II and 45 were simultaneously positive for HLA Class I and II. The frequency of HLA antibodies was less than 1% in donors with no history of pregnancies, and increased significantly with the number of pregnancies (c2 for linear trend = 72.46, P < 0.0001), reaching 30.8% in donors with 3 pregnancies.
Conclusions
The frequency of HLA antibodies increases with the number of pregnancies. Nevertheless measures adopted as prophylaxis of TRALI, we suggest screening HLA antibodies in female donors with history of pregnancies.
P‐846
Determination of Anti‐HLA, Anti‐MICA and Anti‐Erythrocytes Antibodies in Multigravida Female Blood Donors
Fuentes Landa RFL
IMSS, Mexico city, Mexico
Background
The HLA Antigens are particulary efficient in obtaining the immune response as they can directly or indirectly be recognized by cells of the immune system. This efficiency is due to its high level of polymorphism, thus increasing the probability of incompatible transfusions or transplants of blood products, tissues or organs. The anti‐HLA antibodies are present in multigravida women, people with transfusional history and people with previous transplants. The MICA antibodies have shown great clinical relevance because they are involved with rejection of solid organ transplants. The erythrocytes antibodies have great clinical relevance in patients multitransfused. In Mexico 1 200 000 transfusions a year are performed, being its majority red cell concentrate transfusions, followed by the plasma transfusions. A risk attributable to the transfusion of fresh frozen plasma is Transfusion Related Accute Lung Injury (TRALI).
Aims
Determine the level of alloimmunization that multigravida female blood donors present in the Central Blood Bank of CMN SXXI, Mexico, and its relation with a possible post‐transfusion adeverse reaction resulting from the administration of their blood components in a therapeutic procedure.
Methods
Samples from 100 female blood donors with at least three previous pregnancies carried to term and ranging from 18 to 65 years old were studied. Samples from 20 female donors without previous pregnancies were used as controls. A solid phase technique with fluorochrome stained polystyrene beads, coated with specific HLA antigens was used for the indentification of anti‐HLA and anti‐MICA antibodies. The beads were incubated with donor serum and the reaction was carried out using phycoerythrin‐conjugated human IgG antibodies. Positive and negative reactions were identified using a Luminex® analyzer. Anti‐erythrocytes antibodies were detected incubating study sample serum with phenotype known erythrocytes, representative of the population of the patient or the receptor. The phenotype‐known erythrocytes used were from four different cells which covered most of the less‐frequent antigens in a popultion and at the same time lacked the most‐frequent antigens in that population.
Results
The antibody screening test showed that 66% of multigravida female donors were positive for anti‐HLA Class I and II antibodies. 60% of control subjects were positive for anti‐HLA Class I and II antibodies (P = 0.607 CI and P = 0.225 CII. Through specific %PRA test there were identified the presence of anti‐HLA antibodies in 70% of multigravida female donors and in 10% of control subjects (P = 0.000153 CI and P = 0.002 CII). In the search for anti‐MICA antibodies, 37% of multigravida female donors were positive and 49% were negative. The Results for controls showed that 15% were positive for anti‐MICA antibodies and 55% were negative (P = 0.106). In the determination of anti‐erythrocyte antibodies, in all cases the results were negative.
Summary
In this study it was found that the presence of anti‐HLA antibodies are present in the most multigravida female donors and the use of their blood components as therapeutic procedure could trigger a possible post‐transfusion adverse reaction, such as the TRALI.
P‐847
Immunological Refractoriness to Platelet Transfusions and Principles of Their Correction Sessions of Plasmapheresis
Rakhmani AR , Gaponova TG , Trotskaya VT , Dubinkin ID , Galuzyak VG , Mikhailova EM , Fidarova ZT , Parovichnikova EP
Federal State‐Funded Institution National Research Center for Hematology of the, Moscow, Russian Federation
Background
As a result of repeated platelet transfusions there often appears a refractory state, which, according to different authors, is observed in 30–83% of patients with acute leukemia and 33–100% – aplastic anemia. The most common cause of refractoriness is alloimmunization due to multiple transfusions of blood or blood components. PP is used to eliminate the alloimmune antibodies to overcome the immunological transfusion refractoriness to TC.
Aims
To assess the effectiveness of the sessions of plasmapheresis (PP) for immunological thrombocyte concentrate (TC) transfusion refractoriness.
Methods
From 2011 to 2015, in clinics of Hematological Scientific Center, 12 patients with refractoriness to transfusions of TC were observed. The median age of patients’ age was 37 years old (25–63). The ratio M/F was 2/10. Among them were 6 patients with aplastic anemia (AA). 4 – acute leukemia (AL). 2 patients with myelodysplastic syndrome (MDS). 10 patients had a history of multiple transfusions. 2 patients had no transfusions. All patients had refractoriness to transfusion in the form of hemorrhagic complications as a consequence of the absence of platelet growth. Additionally, 8 patients – febrile nonhemolytic reaction at 6 – allergic skin reactions at 1‐ bronchospasm. 3 patients had a history of thyroid disease (Hashimoto's thyroiditis, hyperthyroidism, hypothyroidism). 2 patients had aggravated obstetric and gynecological anamnesis (multiple pregnancies, abortions). 3 patients had a history of chronic viral hepatitis B. 5 patients with AA, 1 with MDS and 1 with AL had splenectomy performed. 5 patients with AA underwent antithymocyte globulin therapy (ATG). For 2 patients with AL courses of chemotherapy were conducted. All patients underwent sessions of PP = 4 M (2 to 15), with M = 3 intervals of the day (from 2 to 4 days). Removed plasma volume M = 1L (from 0.5–2) for 1 procedure.
Result
As a result of treatment in 66% of patients there was a decrease in transfusion refractoriness (no allergic reaction, relief of hemorrhagic syndrome). 33% of patients remained refractory to transfusion. Transfusions were carried out on individual selection 1 time a week. In the course of the PP sessions, a decrease of titer antibodies from M = 80% (31–100%) to M = 60% (23–100%) was observed. Degree of hemorrhagic syndrome according to the VOZ was M = 2 (1–4), after the PP‐1 M = (0–4). Prior to PP absolute increase of platelets after TC transfusion through 24 h was M = 14 × 109\L (2–32 × 109\L). After the PP‐M = 48 × 109\L. (2–98 × 109\L). The frequency of transfusions TC prior to PP was M = 4 time a week (every day – 2 times a week). After PP – M = 1 time a week (every day – 1 time a 2 weeks).
Conclusion
The PP sessions reduce the immunological transfusion refractoriness to TC. This leads to a reduction in titer HLA‐AT and AT frequency of response, reduction of the degree of a hemorrhagic syndrome, the absolute increase in growth after platelet transfusion with individual TC selection and also to reduce the frequency of transfusions TC.
P‐848
Abstract Withdrawn.
P‐849
Abstract Withdrawn.
8.2 Histocompatibility in Stem Cell Transplantation
P‐850
HLA Gene Frequency in Transplant Candidates Without Compatible Umbilical Cord Blood Units
Rojo-Medina JRM , Ibáñez-Cervantez GIC , Fernández-Sánchez VFS , Bello-López JMBL
National Center of Blood Transfusion, Mexico City, Mexico
Background
UmbilicalCord Blood Units (UCBU) transplantation is a therapeutic possibility for patients with a wide range of oncohematologic disorders. It is necessary to have registries of UCBU to ensure representation of ethnic diversity. The search for appropriately match donors is increasingly difficult for patients of mixed ethnicity. The issue of ethnic diversity in Cord Blood Banks (CCB) may be especially relevant in countries with patterns such as Mexico, with a population of 117 million people distributed in 31 states and a huge capital constituted by a combination of multiple origins, which highly difficult the UCBU matching.
Aims
To determine the gene frequency of Mexican candidates for transplantation without compatible UCBU.
Methods
A retrospective analysis from 2004 to 2014 of non‐compatible UCBU was performed in the archives of the CBB of the NCBT. HLA class I and II genotyping of 188candidates were performed by PCR‐SSP and/or PCR‐SSO. The population frequencies were obtained by including unrelated individuals without any particular bias to a phenotype. Results
One hundred‐eighty eight candidates for transplantation of UCBU did not have compatible units. The calculations of genetic distances between data for these candidates were analyzed with Phylip V. 3.6 (University of Washington, Seattle). The search of HLA‐gene origin found on the database of www.allelefrequencies.net was made. The four most frequent genes of HLA‐Class I and II were: for HLA‐A *02/02 (6.38%), *02/24 (4.78%), *24/24 (4.78%) and *24/68 (4.78%) for HLA‐B *35/40 (4.78%), *40/44 (4.25%), *15/45 (3.72%), and *14/40 (3.19%) and for HLA‐DRB1 *04/08 (4.25%), *01/07 (3.72%), *03/07 (3.72%) and *16/16 (3.19%).
Table 1. HLA I and II frequency for not compatible candidates for transplantation (The most frequent).
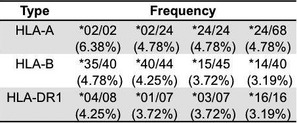
Conclusions
The ethnic origins of the analyzed data were represented in most cases by Caucasian‐Amerindian mixed races. Although, 1924 UCBU are currently available in the UCBB of the NCBT, there is still a group of patients for which the Center cannot find a compatible UCBU because of the mixed ethnic origin. For example, the population of northern Mexico is mostly Caucasian (30%), the center IS constituted by Amerindian/African (60%) and Oriental (10%) and finally in the south there are populations from pure Indian ethnicities (Figure 2). Most of the NCBT donors are natives from Mexico City with a population constituted by a combination of Amerindian and Caucasian origins; therefore some ethnic minorities like oriental, African and pure Indian are not represented. The NCBT is therefore establishing cooperation agreements with different states of the Mexican Republic to promote the donation of CBU in order to enrich the genetic diversity of cord blood units in this CBB, the longer public in this country to increase the cover of transplants mainly in the children population.
8.3 Histocompatibility in Organ Transplantation
P‐851
Abstract Withdrawn.
P‐852
Sensitisation Profile of Patients Awaiting Deceased Donor Kidney Transplantation, a Single Centre Retrospective Study
Kovarova PK , Maluskova AM , Fizkova DF , Pauliskova MP , Rudinska JR , Cermakova ZC
University Hospital Ostrava, Ostrava, Czech Republic
Background. Sensitisation to HLA antigens, formed as a result of transfusion, previous allografts or pregnancy, remains a significant problem in renal transplantation. The Luminex multiplexed technique permits a more comprehensive and sensitive assessment of anti‐HLA antibodies profiles in sensitised patients. However, the clinical importance of pre‐existing HLA antibodies at the time of transplantation, identified by Luminex single antigen beads (SAB), is not fully elucidated.
Aim. The aim of the present study was to evaluate pre‐transplant sensitisation defined by PRA‐CDC, Luminex screening and SAB assays, in patients awaiting renal transplantation at University Hospital Ostrava, in their relation to history of sensitising events and its clinical relevance.
Methods. The screening and identification of HLA antibodies was performed with Luminex‐based commercial kits (Labscreen Mixed and Single antigen beads Class I and II, One Lambda). The Panel reactive antibody (PRA) was assessed with the standard complement‐dependent cytotoxicity (CDC) assay (also with DTT treatment modification) using in‐house 60 cell panel.
Results. Between 2011 – 2014 was detected positivity, in CDC and/or Luminex assay in 116 patients awaiting renal transplantation in our centre. During this period showed positive results, in average, 21% and 35% patients on the waiting list in CDC and Luminex assays, respectively. These patients were divided into three groups according to positivity and negativity revealed in aformentioned tests. The first group comprised of 15 (12.5%) patients negative in Luminex LSM and positive CDC without transplant history. The positive reactivity in CDC was transient for all but one patient, with peak average PRA 13% and CDC‐DTT negative results. The second group included 23 (20%) patients with Luminex assay positive and no previous history of transplantation. All of the patients were I. class positive, CDC was positive in 16 (70%) of them with peak PRA 17%. HLA II. class antibodies were detected in 7 (30%) of these patients. The third group consisted of 79 (67.5%) patients awaiting retransplantation. There were 13 (16.5%) patients with only HLA I. class antibodies, peak PRA 55%, 3 of them proved to have non‐donor specific antibodies (DSA); 12 (15.2%) patients with only HLA‐DSA II. class antibodies and PRA 0%; 53 (67.1%) patients with HLA‐DSA I. and II. class antibodies with peak PRA 38%. The discrepant results between Luminex assay, CDC and historical alloimmunisation data lead us to adapt testing and result interpretation strategies as MFI cut off adjustment, sera dilution, sera EDTA treatment to abolish the prozone effect, and analysis of antibody epitopes. Based on data obtained we plan to enhance our antibody analysis with C1q Luminex assay and B cell cross‐match in order to further improve definition of safe and at‐risk HLA specificities especially in highly sensitised potential renal transplant recipients.
Conclusions. Particularly due to prozone effect and naturally occurring nonalloantibodies in Luminex SAB assay, the combination of different techniques for detecting clinically relevant HLA antibodies contributes to increasing their predictive performance.
P‐853
Abstract Withdrawn.
P‐854
Utility of HLA – Class II Donor Specific Antibody (DSA) as a Prognostic Marker in Renal Allograft Transplant Recipient: A Case Report
Sawant R , Jogale S , Deshpande A , Sirsat R
Hinduja Hospital, Mumbai, India
Background
Before kidney transplantation, recipients are routinely screened for preformed anti‐HLA antibodies (DSA) and prospective cross‐match (CDC). Low immunological risk is indicated when the CDC cross‐match result is negative and the patient does not have any significant DSA, both pre and post transplant. Clinical relevance of anti‐HLA DSA detected by Luminex assay in the development of rejection after transplant has been the focus of the transplant community.
Case report
A 50 years old male received live related renal allograft from his wife. There was a 1/6 (HLA ‐B*40) HLA antigen match between them. Pre transplant DSA and CDC cross‐match were negative. Patient was on induction immunosuppression with Pangraf (3.5 mg). Patient's pre transplant creatinine was 5.8 μmol/L and decreased to 1.2 μmol/L at discharge from the hospital after transplant. On day‐45 post transplant, patient's creatinine levels increased to 2.2 μmol/L. CDC cross‐match was carried out which showed negative results, anti‐HLA Class I DSA was negative (MFI = 270) and Class II DSA was borderline positive (MFI = 1041). Renal biopsy showed mild hydronephrosis changes and suggestive of acute humoral rejection. Patient was treated with plasmapheresis (5 sessions) and IVIG for acute humoral rejection and creatinine levels reverted to normal. On day 107 post transplant, DSA was repeated and was found to be negative for both Class I and Class II anti‐HLA antibodies. The graft is surviving till date, with a negative DSA, normal creatinine levels, while CDC cross‐match continues to be negative.
Conclusion
Anti‐HLA Class II DSA positivity in the absence of a positive CDC cross‐match can predict early rejection of renal allograft. There is a need to optimize DSA monitoring strategies post renal transplant.
P‐855
Abstract Withdrawn.


