Short abstract
Objective
To investigate the utility of the voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD).
Methods
Clinical data from patients who underwent screening for dementia using VSRAD and the Japanese version of COGNISTAT, the Neurobehavioral Cognitive Status Examination, were retrospectively investigated to specify the domains of cognitive function that correlate with the statistical mean value of positive Z-scores in the target volume-of-interest (VOI). A receiver operating characteristic (ROC) curve was constructed to assess the mean value of positive Z-scores in discriminating patients with AD.
Results
A total of 72 patients were included (18 male and 54 female; 15 patients with AD). The mean value of positive Z-scores in the target VOI was significantly correlated with standardized COGNISTAT scores for Orientation and Memory in all patients (r = –0.35 and –0.38, respectively). ROC curve analysis revealed that a cut-off of 1.57 for mean value of positive Z-scores in the target VOI provided 69.4% accuracy in discriminating patients with AD, with a sensitivity of 0.80 and specificity of 0.67.
Conclusions
The results evinced the value of VSRAD in diagnosing AD. The degree of atrophy represented by the target VOI may reflect impairments in Orientation and Memory, which are early stage symptoms observed in AD.
Keywords: Amygdala, COGNISTAT, entorhinal cortex, hippocampus, MRI
Introduction
The voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD) is a magnetic resonance imaging (MRI)-based volumetric software program used to evaluate the degree of atrophy of the middle temporal area of the brain, including the hippocampus, amygdala, and entorhinal cortex.1 The system has been widely used as a sensitive diagnostic tool to detect the early stages of Alzheimer’s disease (AD) in Japan. Voxel-based morphometry (VBM) utilizes the mean value of positive Z-scores in the target volume-of-interest (VOI) as an indicator for characterizing atrophy, by comparing a given subject’s grey matter concentration with that of the original healthy individual database template. When using the degree of atrophy in the target VOI, sufficient accuracy has been shown for discriminating patients with very mild AD from healthy controls.2
Although the VSRAD evaluates the degree of atrophy represented by the target VOI, it has also been suggested to be indicative of disease progression. For example, a longitudinal study showed that the mean value of positive Z-scores increased stepwise over time, in parallel with a decrease in Mini-Mental Examination (MMSE) score.2,3 To date, few studies have investigated the relationship between the mean value of positive Z-scores and neuropsychological test scores, including the revised version of Hasegawa’s Dementia Scale (HDS-R),4 the Alzheimer’s Disease Assessment Scale-Cognitive Part (ADAS-cog),5 the Wechsler Adult Intelligence Scale-III (WAIS-III),6 and the Wechsler Memory Scale-Revised (WMS-R).7 Li et al.8 observed a significant positive correlation between the mean value of positive Z-scores and the ADAS-cog score (P = 0.0129), and an inverse correlation with the WAIS-III Information subset score (P = 0.0294) in 18 patients with AD and 12 patients with mild cognitive impairment. There was also a tendency toward an inverse correlation between mean value of positive Z-scores and scores for the HDS-R, the WAIS-III Similarities subset, and the WMS-R Delayed Visual Reproduction subset (P = 0.0532, 0.0635, and 0.0609, respectively), while there was no other correlation with the intelligence quotient (IQ) or other subsets of the WAIS-III and WMS-R.8 A further study of 24 patients with dementia that included 20 patients with AD, found a significant inverse correlation between the mean value of positive Z-scores and the HDS-R score (P = 0.001), while there was no significant correlation with IQ or any subsets of the WAIS-III.9 Thus, the mean value of positive Z-scores is suggested to be related to the stage of AD; however, it is unclear what aspect of AD symptoms are reflected in this parameter, or whether the relationship is restricted to AD and not applicable to other types of dementia. In this context, elucidating the relationship between the mean value of positive Z-scores and neuropsychological function levels may help to resolve these questions and may improve the utility of VSRAD. In particular, there may be value in specifying the domains of cognitive function that correlate with atrophy of the middle temporal area.
The aim of the present study was to retrospectively investigate clinical data from patients who underwent screening for dementia using VSRAD and the Japanese version of the Neurobehavioral Cognitive Status Examination (COGNISTAT), a cognitive screening test which assesses five major cognitive ability areas: language, construction, memory, calculations, and reasoning,10 in order to specify the domains of cognitive function that correlate with the mean value of positive Z-scores in the target VOI, obtained using VSRAD. An additional aim was to evaluate the performance of VSRAD for differential AD diagnosis in clinical practice, i.e. in a heterogeneous population including other types of dementia, mild cognitive impairment, and psychiatric disorders. The present article is based on a study first reported by Oshikubo in 2018.11
Patients and methods
Study population
A systematic chart review was performed for all patients screened for dementia in the Department of Psychiatry, Teikyo University Hospital between February 2013 and December 2017. Both COGNISTAT and VSRAD were conducted during this period as a routine diagnostic procedure for all patients suspected of dementia, as per the results of the HDS-R and MMSE or due to atypical symptoms of depression, anxiety, or visual hallucinations in old age. Diagnosis of the patients was confirmed retrospectively by at least two of the authors according to the DSM-5 criteria,12 using clinical chart information, including symptoms, clinical course, and examination findings. Patients were required to have been screened for dementia using both COGNISTAT and VSRAD between February 2013 and December 2017 for study inclusion. The study protocol was approved by the Ethics Committee of Teikyo University (No. 17-043), and study information can be accessed via the following weblink (https://www.teikyo-u.ac.jp/affiliate/research/ethic_committee/).
COGNISTAT
The Japanese version of COGNISTAT was performed on the basis of the 2009 manual,13 following an improvement in patient status in cases of delirium or major depressive disorder comorbidity. Standardized scores of 10 subtests were obtained, comprising Orientation, Attention, Comprehension, Repetition, Naming, Constructional Ability, Memory, Calculation, Similarities, and Judgement. Scores were standardized as mean = 1 and SD = 1. Lower scores related to worse cognitive ability and scores >9 were considered to be within normal range.10 The consciousness levels of patients were determined as the state of arousal at assessment.
MRI procedure
The MRI assessments were performed using a Signa HDxt 3-T system (GE Healthcare, Chicago, IL, USA). The VSRAD utilises a database of normal data obtained using a 1.5-T MRI scanner, and the applicability of VSRAD to 3-T MRI data has been shown in a previously published study.14 For VSRAD, 3D sagittal sections of T1 weighted spin-echo images were obtained with the following parameters: FOV 25.6 cm × 25.6 cm, matrix 256 × 256, 1.0-mm slice thickness, and voxel size 1.0 mm × 1.0 mm × 1.0 mm. VSRAD advance or advance 2 software (Eisai Co., Tokyo, Japan) was used to determine the mean value of positive Z-scores in the target VOIs, as the degree of atrophy by comparing a given subject’s grey matter concentration voxel-by-voxel with that of the original healthy individual database template.
Statistical analyses
Data are presented as n prevalence or mean ± SD. One-way analysis of variance (ANOVA) was used for between-group comparisons, and Bonferroni correction was adopted for multiple testing of the mean value of positive Z-scores and 10 subtests of the COGNISTAT. The utility of VSRAD was evaluated using receiver operating characteristic (ROC) curve analysis: accuracy was determined as (true positives + true negatives)/all tests at the point closest to the upper left corner in the ROC curve, created with the mean values of positive Z-scores in the target VOI as the variable of interest and AD diagnosis as the classification variable. In order to investigate the relationship between the mean values of positive Z-scores in the target VOI and each subtest of the COGNISTAT, partial correlation analysis was conducted using age as the control variable. All statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA), and a P value < 0.05 was considered statistically significant.
Results
Charts from a total of 74 patients were reviewed, and of these, data from 72 patients were included for retrospective analyses. The study population comprised 18 male and 54 female patients (age, 73.0 ± 8.52 years; and COGNISTAT and VSRAD test periods, 47.7 ± 109.4 days). Two patients were excluded: one due to a comorbid status of delirium at the time of the COGNISTAT assessment, and the other because the VSRAD was not applicable due to a destructive lesion of the cerebrum.
Fifteen patients were diagnosed with AD (three with a history of delirium, and one with a history of major depressive disorder) and 12 were diagnosed with dementia other than AD, comprising: dementia with Lewy bodies (two patients), vascular dementia with a history of delirium (one patient), dementia due to traumatic brain injury (one patient), alcohol-induced dementia with a history of delirium (one patient), dementia due to multiple aetiologies (one patient), and six patients with unspecified dementia (one with a history of major depressive disorder and delirium, one with a history of major depressive disorder, and one with a history of delirium). Fourteen patients were diagnosed with mild cognitive impairment (one with a history of major depressive disorder and delirium, five with major depressive disorder, two with bipolar disorder, and one with an unspecified psychotic disorder) and were grouped separately from the patients with dementia. The additional 31 patients were diagnosed with other psychiatric disorders as follows: 11 patients were diagnosed with major depressive disorder (one with a history of delirium), six were diagnosed with bipolar disorder (two with a history of delirium), four were diagnosed with schizophrenia spectrum disorder, one had a history of delirium, one had adjustment disorder, one had dissociative disorder, one had panic disorder, one had somatic symptom disorder, one had autism spectrum disorder, three had an unspecified psychotic disorder (one with a history of delirium), and one had traumatic epilepsy. Table 1 summarizes patient demographics according to the four diagnosis groups, AD, dementia other than AD, mild cognitive impairment, and other psychiatric disorders.
Table 1.
Demographic characteristics, mean values of positive Z-scores in the target volume of interest, and standardized scores for each subtest of the COGNISTAT in patients categorized into four diagnosis groups.
| Characteristic | Diagnosis group |
Statistical significancea | ||||
|---|---|---|---|---|---|---|
| Alzheimer’s disease | Dementia other than Alzheimer’s disease | Mild cognitive impairment | Other psychiatric disorders | Total | ||
| N | 15 | 12 | 14 | 31 | 72 | |
| Sex, male: female | 1:14 | 2:10 | 5:9 | 10:21 | 18:54 | |
| Age, years | 76.7 ± 5.4 | 73.8 ± 9.4 | 73.8 ± 6.4 | 70.7 ± 9.7 | 73.0 ± 8.5 | NS |
| Mean value of positive Z-scoresb | 2.27 ± 0.94 | 1.33 ± 0.63** | 1.74 ± 0.78 | 1.45 ± 0.54*** | 1.66 ± 0.77 | P = 0.017 |
| COGNISTAT items | ||||||
| Orientation | 4.47 ± 4.21 | 7.00 ± 3.10 | 8.64 ± 3.03*** | 9.23 ± 1.59**** | 7.75 ± 3.35 | P = 0.00014 |
| Attention | 4.60 ± 4.52 | 5.50 ± 3.78 | 5.93 ± 4.20 | 7.35 ± 3.89 | 6.19 ± 4.13 | NS |
| Comprehension | 5.87 ± 4.22 | 4.92 ± 3.58 | 8.93 ± 2.79 | 9.10 ± 2.34** | 7.69 ± 3.52 | P = 0.0017 |
| Repetition | 7.73 ± 3.15 | 6.92 ± 2.15 | 8.50 ± 2.18 | 9.74 ± 1.59* | 8.61 ± 2.41 | P = 0.013 |
| Naming | 7.20 ± 2.62 | 8.58 ± 1.24 | 8.79 ± 1.48* | 9.48 ± 0.93**** | 8.72 ± 1.76 | P = 0.0032 |
| Constructional ability | 6.53 ± 2.42 | 7.00 ± 2.30 | 8.36 ± 1.99 | 8.29 ± 2.52 | 7.72 ± 2.44 | NS |
| Memory | 5.87 ± 1.25 | 6.58 ± 1.51 | 6.79 ± 1.12 | 7.65 ± 1.66*** | 6.93 ± 1.60 | P = 0.025 |
| Calculation | 8.00 ± 2.73 | 8.17 ± 2.48 | 9.43 ± 1.65 | 9.42 ± 1.18 | 8.92 ± 1.98 | NS |
| Similarities | 8.60 ± 1.40 | 8.25 ± 1.22 | 9.14 ± 1.23 | 9.57 ± 1.01 | 9.06 ± 1.26 | NS |
| Judgement | 9.60 ± 1.84 | 9.33 ± 1.83 | 9.93 ± 1.33 | 10.37 ± 1.25 | 9.94 ± 1.53 | NS |
Data presented as n prevalence or mean ± SD.
Corrected P value; bobtained using the voxel-based specific regional analysis system for Alzheimer’s disease.
*P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 (post hoc analysis compared with Alzheimer’s disease, corrected).
NS, no statistically significant between-group difference (P > 0.05).
The mean values of positive Z-scores in the target VOI and the standardized scores for each subtest of the COGNISTAT were grouped according to diagnosis (Table 1). Between-group comparisons showed that there were statistically significant differences in the mean value of positive Z-scores (P = 0.017), the standardized scores for Orientation (P = 0.00014), Comprehension (P = 0.0017), Repetition (P = 0.013), Naming (P = 0.0032), and Memory (P = 0.025; all ANOVA, corrected). Post hoc analysis on the basis of AD revealed statistically significant differences between AD and dementia other than AD and between AD and other psychiatric disorders regarding the mean value of positive Z-scores (P = 0.0056 and 0.0024, respectively); between AD and mild cognitive impairment and between AD and other psychiatric disorders in terms of Orientation (P = 0.0011 and 0.0000073, respectively), between AD and other psychiatric disorders regarding Comprehension and Repetition (P = 0.0091 and 0.029, respectively), between AD and mild cognitive impairment and between AD and other psychiatric disorders in terms of Naming (P = 0.0497 and 0.00010, respectively), and between AD and other psychiatric disorders regarding Memory (P = 0.0015) (Table 1).
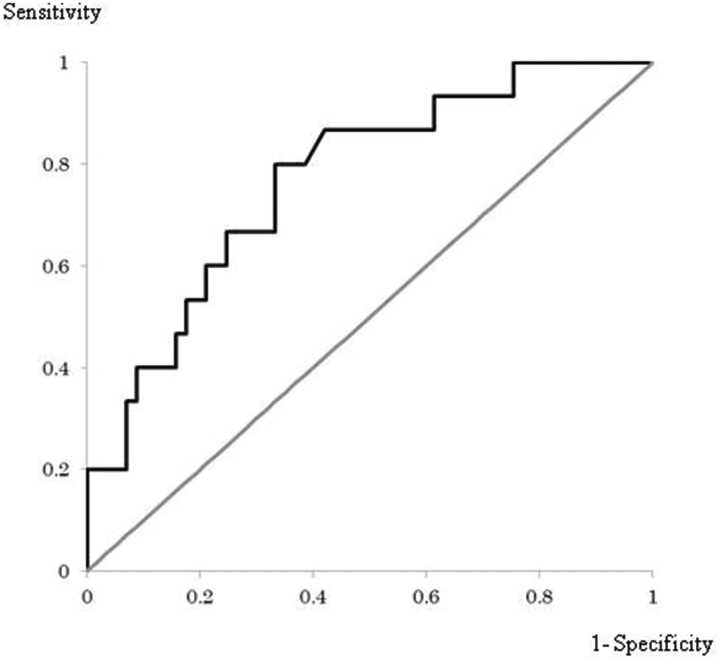
In the ROC analysis, the area under the curve was 0.770. A cut-off value of 1.57 for the mean value of positive Z-scores in the target VOI provided a sensitivity of 0.80, specificity of 0.67, and accuracy of 69.4% in discriminating patients with AD (Figure 1).
Figure 1.
An ROC curve using mean values of positive Z-scores in the target volume of interest (VOI; obtained using the voxel-based specific regional analysis system for Alzheimer’s disease) as the variable of interest and Alzheimer’s disease diagnosis as the classification variable. The area under the curve was 0.770, and a sensitivity of 0.80, specificity of 0.67, and accuracy of 69.4% were obtained with a cut-off value of 1.57 for the mean value of positive Z-scores in the target VOI.
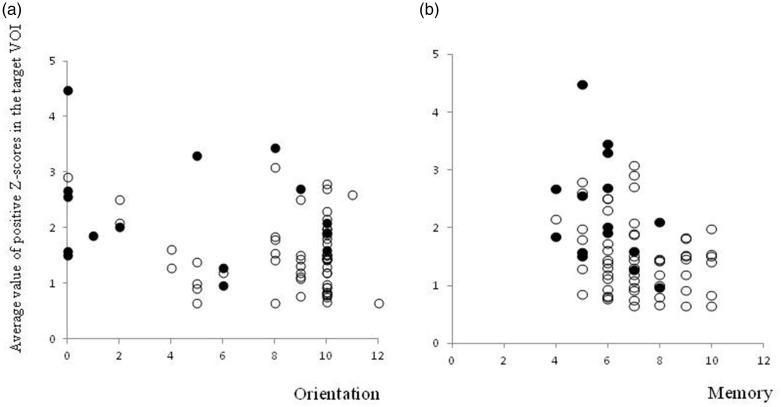
The correlation coefficients between the mean value of positive Z-scores and each subtest of the COGNISTAT in patients with AD and the total study population, obtained through partial correlation analysis using age as the control variable, are shown in Table 2. With a total of 72 patients, the mean value of positive Z-scores in the target VOI was statistically significantly correlated with the standardized scores for Orientation and Memory in the COGNISTAT (r = −0.35 and −0.38, uncorrected P = 0.0026 and 0.0013, respectively). Other subtests did not show any significant correlation in the total patient group, and there was no statistically significant correlation between the mean value of positive Z-scores and any subtest of the COGNISTAT in the 15 patients with AD. Scatter plots of the mean value of positive Z-scores, and Orientation and Memory in the COGNISTAT are shown in Figure 2.
Table 2.
Correlation coefficients between the mean value of positive Z-scores in the target volume of interest, obtained using the voxel-based specific regional analysis system for Alzheimer’s disease, and each subtest of the COGNISTAT.
| Patient group | n |
COGNISTAT subtest |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orientation | Attention | Comprehension | Repetition | Naming | Constructional Ability | Memory | Calculation | Similarities | Judgement | ||
| Alzheimer’s disease | 15 | -0.18 | 0.45 | -0.21 | 0.30 | -0.17 | -0.34 | -0.38 | -0.01 | 0.31 | 0.22 |
| Total | 72 | -0.35* | -0.01 | -0.14 | 0.09 | -0.18 | -0.20 | -0.38* | -0.16 | 0.07 | -0.01 |
*P < 0.005, uncorrected.
Figure 2.
Scatter plots of the mean value of positive Z-scores in the target volume of interest (y-axis) and the standardized scores for (a) Orientation and (b) Memory in the COGNISTAT (x-axis). Patients with Alzheimer’s disease and those without Alzheimer’s disease are shown as ● and ○, respectively. Statistically significant correlations were observed between the mean value of positive Z-scores and the standardized scores for both Orientation and Memory (r = –0.35 and –0.38, respectively, and uncorrected P = 0.0026 and 0.0013, respectively).
Discussion
In the present study, the performance of VSRAD for diagnosing AD in clinical practice was verified through investigating the clinical data of 72 patients screened for dementia using the COGNISTAT and VSRAD. There were significant differences in Orientation, Comprehension, Repetition, Naming, and Memory when comparing the standardized scores for each COGNISTAT subtest between four diagnosis groups: AD, dementia other than AD, mild cognitive impairment, and other psychiatric disorders. Post hoc analysis showed significant differences between AD and at least one of the other three groups in the standardized scores for these five COGNISTAT subtests.
In patients with other psychiatric disorders, the mean standardized scores for Orientation, Comprehension, Repetition, Naming, Calculation, Similarities, and Judgement were greater than nine, which is defined as the normal range in the COGNISTAT.10 In contrast, the means for Attention, Constructional Ability, and Memory ranged from seven to eight, indicating a mild to moderate impairment. These findings in patients with other psychiatric disorders may reflect why they were suspected of cognitive impairment in clinical practice. In patients with AD, the means for Calculation, Similarities, and Judgement ranged from eight to nine, indicating normal to mild impairment, which suggests that that most of these patients were in the mild stages of dementia. This may be the reason why significant differences between patients with AD and other diagnosis groups were observed in such domains as Orientation and Memory, which are the first observed symptoms of AD in the early stages.
It may be of note that a relatively high accuracy of 69.4% was obtained for discriminating patients with AD from other patients with dementia or cognitive impairment, in the present study that did not include healthy controls. The mean value of positive Z-scores in the target VOI may be a helpful indicator for discriminating AD from other dementia or psychiatric disorders, as post hoc analyses revealed statistically significant differences between AD and dementia (P = 0.0056) and between AD and other psychiatric disorders (P = 0.0024). To the authors’ knowledge, the present study is the first to date to verify the performance of VSRAD for differential diagnosis of AD in a heterogeneous population in clinical practice. A limitation in the present study was the lack of healthy control data, which precluded the comparison between the present and previous studies in terms of the accuracy of distinguishing patients with AD form healthy controls.2,14 Further investigations should be encouraged in larger multicentre studies, while caution may be needed for the possibility that VSRAD may misdiagnose such diseases as juvenile AD and frontotemporal dementia.15
Significant inverse correlations were observed between the mean value of positive Z-scores in the target VOI and the standardized COGNISTAT scores for Orientation and Memory in the whole study population (r = –0.35 and –0.38; uncorrected P = 0.0026 and 0.0013, respectively). The present correlation findings seemed to be valid on the basis of the following: First, previous studies have observed significant inverse correlations between the mean value of positive Z-scores and the HDS-R score,8,9 which includes items for orientation and memory; Secondly, in a study investigating the concurrent validity of the Japanese version of COGNISTAT, Orientation and Memory were the subtests that showed the strongest correlation with the MMSE as an external criterion (r = 0.87 and 0.76, respectively);16 and Thirdly, there was a relatively strong internal correlation between Orientation and Memory in the subjects used for standardizing the Japanese version of COGNISTAT (r = 0.42, P < 0.01).13 The present study results may be limited by the retrospective cross-sectional nature of the study and the lack of definition of a causal relationship, as well as the fact that VSRAD specifically evaluates the degree of atrophy of the middle temporal area. Nonetheless, the correlations observed in the present study may reflect functional relevance, and the relationship between memory deterioration and atrophy of the hippocampus or entorhinal cortex has been suggested in healthy subjects in several studies.17–19 In contrast, the COGNISTAT Orientation subtest comprises items concerning time, place, and identity (name and age),10 and it may be relatively difficult to provide a simple explanation for the present relationship concerning Orientation; however, the functional relevance may be supported by the speculation that the middle temporal area, including the hippocampus, provides long-term storage of environmental representations in spatial cognition.20
When analysing in the 15 patients with AD in the present study, no significant correlation was observed between the mean value of positive Z-scores and any subtest of the COGNISTAT. The limited sample size may be the reason for the discrepancy in Memory, as the correlation coefficients were basically the same between the analysis in all subjects and in patients with AD (r = –0.38 and –0.38, respectively). The explanation for the discrepancy in Orientation may be more complicated, as the correlation coefficient when analysing in all subjects was –0.35, while that in patients with AD was –0.18. Except for type II error, one of the reasons for the discrepancy may be that the Orientation subtest in the COGNISTAT comprises several items,10 which may reflect the function of different areas in the brain. For instance, impairment of the posterior parietal lobe, which is frequently observed in patients with AD and considered to be responsible for egocentric disorientation,21 was not analysed in VSRAD. A similar reason may be applicable to the result of a previously published study,9 in which no significant correlation with any subset of the WAIS-III was observed in 24 patients with dementia, including 20 patients with AD.
In conclusion, the results of the present study support the utility of VSRAD in clinical practice. Exploration of the domains of cognitive function that may be correlated to the degree of atrophy in the target VOI using VSRAD, revealed that orientation and memory, the first observed AD symptoms at early stages, may be potential candidates. Future studies involving patients with AD, in larger multicentre settings, may be helpful to further validate and improve the utility of VSRAD.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Mamoru Tochigi https://orcid.org/0000-0002-0143-2118
References
- 1.Hirata Y, Matsuda H, Nemoto K, et al. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci Lett 2005; 382: 269–274. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda H, Mizumura S, Nemoto K, et al. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol 2012; 33: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folstein M, Folstein S, McHugh P, et al. Mini-mental state examination user’s guide. Odessa: Psychological Assessment Resources, 2001. [Google Scholar]
- 4.Jeong JW, Kim KW, Lee DY, et al. A normative study of the Revised Hasegawa Dementia Scale: comparison of demographic influences between the Revised Hasegawa Dementia Scale and the Mini-Mental Status Examination. Dement Geriatr Cogn Disord 2007; 24: 288–293. [DOI] [PubMed] [Google Scholar]
- 5.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11: S13–S21. [PubMed] [Google Scholar]
- 6.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation, 1997. [Google Scholar]
- 7.Wechsler D. Wechsler Memory Scale–Revised manual. San Antonio: The Psychological Corporation, 1987. [Google Scholar]
- 8.Li X, Jiao J, Shimizu S, et al. Correlations between atrophy of the entorhinal cortex and cognitive function in patients with Alzheimer’s disease and mild cognitive impairment. Psychiatry Clin Neurosci 2012; 66: 587–593. [DOI] [PubMed] [Google Scholar]
- 9.Oshima K. Relationship between volume of the medial temporal lobe and both the cognitive and olfactory functions in patients with dementia. Journal of Kanazawa Medical University 2017; 42: 17–23 (In Japanese). [Google Scholar]
- 10.Kiernan RJ, Mueller J, Langston JW, et al. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann Intern Med 1987; 107: 481–485. [DOI] [PubMed] [Google Scholar]
- 11.Oshikubo G. Memory impairments associated with the atrophy of middle temporal area in the patients with Alzheimer’s disease. Psychiatry 2018; 33: 77–83 (In Japanese). [Google Scholar]
- 12.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, DSM-5. 5th ed Washington: American Psychiatric Association, 2013. [Google Scholar]
- 13.Matsuda O, Nakatani M. The manual for the Japanese version of Neurobehavioral Cognitive Status Examination (COGNISTAT). 2nd ed Tokyo: World Planning, 2009. (In Japanese). [Google Scholar]
- 14.Sone D, Imabayashi E, Maikusa N, et al. Voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD) on 3-tesla normal database: diagnostic accuracy in two independent cohorts with early Alzheimer’s disease. Aging Dis 2018; 9: 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernooij MW, van Buchem MA. Neuroimaging in dementia In: Hodler J, Kubik-Huch RA, von Schulthess GK. (eds) Diseases of the brain, head and neck, spine 2020–2023: diagnostic imaging. Cham: Springer, 2020. [PubMed] [Google Scholar]
- 16.Matsuda O, Kumazawa K, Sakuraba Y, et al. The Japanese version of Neurobehavioral Cognitive Status Examination (NCSE): 2nd announcement. Japanese Journal of Geriatric Psychiatry 2003; 14: 475–483 (In Japanese). [Google Scholar]
- 17.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A 1996; 93: 13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci 2004; 24: 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto M, Araki Y, Takashima Y, et al. Hippocampal atrophy and memory dysfunction associated with physical inactivity in community-dwelling elderly subjects: the Sefuri study. Brain Behav 2017; 7: e00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci 2008; 1124: 77–97. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain 1999; 122: 1613–1628. [DOI] [PubMed] [Google Scholar]