Abstract
Introduction
Alzheimer’s dementia (AD) is the most common form of dementia in the World. Pathologically, it is characterized by extracellular β-amyloid plaques and intraneuronal neurofibrillary tangles (NFTs). The latter is composed of irregular, pathological forms of the tau protein. Currently, FDA-approved symptomatic treatments are limited to the targeting of cholinergic deficits and glutamatergic dysfunctions. However, as understanding of β-amyloid plaques and NFTs expands, these dysfunctional proteins represent potential therapeutic interventions. The present review article evaluates active and passive immunotherapies in clinical development for AD to date and their potential to significantly improve the treatment of AD going forward.
Areas covered
All clinical trials that have targeted β-amyloid to date have produced somewhat disappointing results, leading to a shift in intervention focus to targeting tau protein. A key component in understanding the value of targeting tau in therapeutic paradigms has come from the conceptualization of prion-like pathological spread of tau isoforms from neuron to neuron, and referred to as ‘tauons’. Immunotherapies currently under investigation include approaches aiming at preventing pathological tau aggregation, stabilizing microtubules, and blocking of tauons.
Expert opinion
A multi-targeted approach that would use biologics targeting tau offers great promise to the development of effective AD therapeutic interventions.
Keywords: Tau, monoclonal antibodies, passive immunotherapy, Alzheimer’s disease, clinical trials
1. Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia, affecting 5.4 million individuals in the United States alone, and it is estimated that it will affect 13.8 million patients by the year 2050 [1]. AD is more common than other types of dementia, such as vascular dementia, Lewy body dementia, frontotemporal dementia (FTD), progressive supranuclear palsy (PSP), and Parkinson’s disease (PD). After the onset of AD dementia, the median survival time ranges from 3.3 to 11.7 years [2]. Despite the large research efforts supported by both public and private funds in the past three decades, no disease-modifying therapies are currently available in the clinic.
The two neuropathological hallmarks of AD are β-amyloid plaques and neurofibrillary tangles (NFT) [3,4]. Amyloid plaques are located outside of neurons, in the brain parenchyma. NFTs are primarily intra-neuronal filamentous inclusions or aggregates of aberrantly misfolded and hyperphosphorylated tau proteins [4]. From this basic knowledge, one can understand that the first therapies developed for AD aimed at lowering brain Aβ loads given amyloid are located outside of cells. However, after many timely and costly attempts, therapies aimed at β-amyloid neuropathology, including vaccination and passive immunotherapies (e.g. bapineuzumab, crenezumab and solanezumab [5,6]), have thus far yielded poor results in clinical trials [7]. Despite the significant reduction of β-amyloid levels, the progression of AD was unchanged [8]. Furthermore, 6% of patients that received the ANI 792 vaccine developed encephalitis [9–11], indicating that off-target effects, or generalized inflammation are one of the potential serious consequences of vaccine therapy. This is clinically relevant as AD therapy must be initiated as early as possible due to a clear indication that the pathophysiological processes of AD begin before the onset of cognitive decline. With this insight in mind, it is essential to confront the current obstacles to implementing therapeutic interventions. Geerts et al. describes a recent clinical trial complicated by interactions between Aβ and the glutamatergic systems [12], thus suggesting a novel research area which would greatly benefit from deeper exploration in order to unlock the full potential of therapeutic interventions that will be available in the future. There have been several initiatives that have trialed immunotherapy agents on preclinical asymptomatic patients, with results largely pending [13]. Two such initiatives, the Alzheimer Prevention Initiative (API), and the study for Treatment of Asymptomatic Alzheimer (A4), aim to identify therapies that are likely to prevent AD [14].
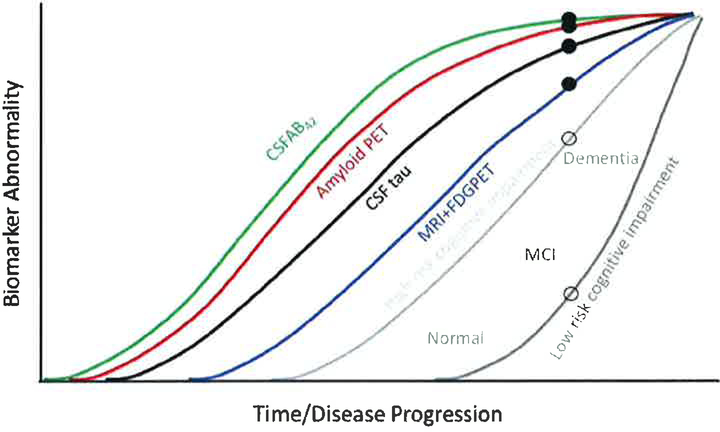
A model described by Jack et al. illustrates that biomarker changes precede clinically discernable changes in cognition in sporadic AD [15,16]. A similar theory was proposed by Bateman et al. in 2012 [17] to explain the progression of autosomal dominant AD, which also found a cascade which begins with increased Aβ42, followed by amyloidosis, tauopathy, brain atrophy, and decreased glucose metabolism, all of which manifest as clinical impairment and dementia. The model also suggests that there is a gradual worsening or accumulation of several biomarker abnormalities in a sigmoidal fashion at different time periods in the course of AD (Figure 1). Accordingly, early biomarker abnormalities precede symptom onset and clinical disease by several years. Of the two neuropathological hallmarks of AD, β-amyloid deposition may be detected earlier than major increases in tau. However, it is now accepted that the accumulation of tau abnormalities better correlates with dementia severity and duration than amyloid deposits [4].
Figure 1.
Modified from Jack et al. [91] that describes biomarker curves in reference to time/disease progression. This figure illustrates the patient dependent variability cognitive reserve in determining cognitive impairment. For example, a comparison between high risk and low-risk patient (open circle) may indicate similar biomarker levels (closed circle), however with a clear difference cognitive outcomes. Also stressed here is the progression of markers that can provide the basis of early screening, detection, and diagnosis. (Image recreated with Dr. Jack’s permission). Low and high-risk cognitive impairment is based on family history and environmental factors, such as traumatic brain injuries.
Because of the failure of amyloid-based therapeutic interventions to alter the course of clinical AD, in the past years, the focus of treatment has expanded to include other targets, such as tau and inflammation. A large body of work is been produced in animal models of AD about these novel targets, and others. However, in the present review, we center our discussion on the use of active and passive immunotherapies to remove misfolded and aggregated tau proteins that have been tested in clinical trials, with some of these biological agents showing great promise for future phase Il and Ill trials.
2. Tau proteins in physiology and pathology
Tau is a microtubule-associated protein mostly found in neurons of the central nervous system (CNS), though it is also expressed at low levels in astrocytes and oligodendrocytes [18]. It is highly soluble and found unfolded in the cytoplasm in normal cells, where it plays a major role in microtubule self-assembly, stabilization, and function [19,20]. Because of its microtubule regulation abilities, tau is involved in functions such as cell signaling, synaptic plasticity, and regulation of genomic stability [19,21–23]. Tau mRNA, encoded by the single MAPT gene located on chromosome 17q21, are alternatively spliced depending on the neuronal subtype. In humans, 6 major isoforms are generated by alternative splicing of exons 2, 3 and 10 [22], which generates tau proteins with 3 or 4 microtubule binding domains (denoted as 3R and 4R, respectively). Overall, in healthy adults, equal amounts of the 3R and 4R isoforms are present in the brain [24]. However, regional variations exist. For example, 3R is low in the cerebellum, while 4R is high in the globus pallidus. Interestingly, the increase in relative 4R expression, which includes exon 10, can lead to enhanced neurofibrillary tangles and tau aggregation [25–27] and is postulated to contribute to AD progression, as well as other tauopathies, such as PSP, corticobasal degeneration, and FTD [28].
Tau can undergo a number of post-translational modifications, such as phosphorylation, acetylation, nitration, glycation, glycosylation, ubiquitination, and truncation, which all can alter its function and cellular localization [23]. There are over 80 phosphorylation sites on tau proteins [29]. Several species of tau, such as soluble-misfolded, hyperphosphorylated, and mislocalized forms, are now being described as toxic. Hyperphosphorylation of tau decreases its bidding affinity for microtubules, which in turn leads to microtubule destabilization and disassembly [30]. These detached tau molecules have an affinity for one another, and they can self-aggregate to form oligomers of misfolded tau. The oligomers gain more units and evolve into tau deposits, which in turn fuse together and aggregate as neurofibrillary tangles [19,31]. These non-physiological forms of tau can lead to a cascade of interrelated pathological events, including impaired axonal transport, alteration of synaptic structure and function, mitochondrial dysfunction and transport alteration, activation of unfolded protein response, and ineffective protein degradation [22]. Collectively, the clinical syndromes caused by pathological aggregation of tau protein over an extended period of time are referred to as tauopathies. These include several neurodegenerative disorders, such as PSP [32], corticobasal degeneration [33], FTD [34], and chronic traumatic encephalopathy [35], among others.
Once released from presynaptic neurons, some studies have indicated that the misfolded tau aggregates are taken up by postsynaptic neurons where the misfolded tau acts as a ‘seed’, leading to the formation of additional tau aggregates [36–38]. In contrast, normal monomeric tau does not appear to have the seeding effect [37]. Thus, there is often a corollary to prion disease, ‘prionosis’, that describes the seeding and spreading of tau [39]. This new scheme regarding the spread of NFTs has been implicated in transneuronal propagation of tau pathology in cell-based and mouse models [39,40].
In AD, NFTs are principally found within neuronal perikarya, and are postulated to propagate and spread through a prion-like mechanism. In short, after initial formation in the cell body, NFTs are transported in the axons to presynaptic terminals where they are released into the synaptic cleft from where they can spread to interconnected cells, and possibly reach distant structures and regions [41–43]. In support of this model of tau propagation, mice expressing human wild-type tau and which received intracerebral injections of filamentous tau showed an increasing amount of abnormal tau forms that spread across brain regions and clustered in aggregates made of hyperphosphorylated tau [39]. The order in which NFTs appear in the brain suggests that misfolded tau may be trans-synaptically transferred from one neuron to the next [31,41,44]. Very interestingly, the flow of prion-like tau, referred to as tauons, follows a path that defines the six stages of tau pathology progression in AD described by Braak and Del Tredici [45,46], which develop across synaptically interconnected brain regions. Thus, it comes as no surprise that NFTs are found both in synaptosomes from human postmortem AD CNS, and in interstitial fluid [36,47].
Pathology of tau-related toxicity is due not only to loss of normal function but also the gain of new dysfunctions [48]. The propagation of NFTs highly correlates with cognitive disease progression [43,44]. NFTs first appear in the locus coeruleus of the pontine tegmentum, which projects to the perirhinal cortex [42]. From there, NFTs spread to limbic structures (hippocampus, amygdala, etc.) before extending to the interconnected neocortical areas [41,42].
Altogether, the scientific observations summarized above suggest that tau proteins come in many forms (both physiological and pathological) and shapes (i.e. conformations), both inside and outside of neurons. Consequently, one could think of developing specific tools to target the main tau forms participating in the progression of AD throughout the brain. Some of the most powerful and specific tools that could be used safely in patients are tau immunotherapies.
3. Rationale for tau active and passive immunotherapies
Immunotherapies aimed at preventing β-amyloid plaques formation have been successful at ameliorating symptoms in AD animal models; however, all trials conducted in humans resulted in the lack of significant cognitive improvements [49]. Thus, research focus is turning to tau as a more potent target for immunotherapy, though this approach needs to be investigated carefully given the important roles that tau serves in healthy tissues. Immunotherapy should principally target tau forms which have the propensity to form neurofibrillary tangles.
Targeting tau is further complicated by epitope selection. Some epitopes are more exposed and accessible to antibodies than others in tauons. First, all post-translational modifications listed in section 2 above are likely to alter the binding of antibodies to specific regions on tau proteins. Second, antibody efficacy would also be dependent on the structural properties of tau assembly, such as monomers vs. dimers vs. oligomers vs. fibrils vs. NFTs [45]. Third, and a very important consideration relates to the mechanism of action of antibodies. For example, some antibodies have strong potential to activate a systemic inflammatory process, which is not desirable. However, antibodies which bind to abnormal forms of tau and microglia for further degradation would be highly beneficial to lower tau pathology in the CNS. Although of great interest, targeting therapeutically relevant epitopes on tau proteins, and in a safe manner, is a complicated issue.
Several drug (not immunotherapies) disease-modifying therapies are under design to attempt to inhibit tau phosphorylation and aggregation, compensate for tau loss-of-function, or to inhibit the spread of pathologic tau [23,50–52]. The results from such drug approaches have generally been poor when attempting to inhibit tau phosphorylation [51], inhibit tau aggregates, or stabilize microtubules [51,53,54]. Therapies directed at the pathological seeding, the core of tauopathies, would be more tailored to prevent the progression of AD. These therapies include vaccinations and monoclonal antibody paradigms, and are under investigation as active and passive immunotherapies, respectively [22].
The rationale behind active immunotherapy, i.e. vaccinations, is to elicit an immune response that targets specific pathological conformers of phosphorylated tau in the biological context of each individual patient. Directing the vaccinations to these specific pathological targets has the advantage of preventing the mounting of B or T cell responses against the physiological forms of the intracellular tau proteins in the host, in contrast to passive immunization approaches. However, there is a concern of T-lymphocyte meningoencephalitis secondary to vaccination, which has been observed in other trials, and in particular for the β-amyloid immunotherapy AN-1792 [55]. Despite the risks, the convenience and potential for life long immunity of active vaccination is a very attractive feature, although it should be considered cautiously and off-target effects must be meticulously scrutinized at all stages of development of biologics. It is important to note that Rosenmann et al. demonstrated that mice inoculated with recombinant human tau protein developed tauopathy-like symptoms [56]. On the other hand, while passive immunotherapies maybe attractive for their potential to avoid complications of autoimmunity due to active immunization, one mouse study found an increase in mortality after receiving an anti-tau monoclonal antibody (mAB) [57]. While there is certainly excitement about anti-tau antibodies for their efficacy in both AD and PSP, caution must be taken as divergent tauopathies are thought to be responsible for AD and PSP [58].
Regardless of the source of antibodies, endogenous or exogenous, the mechanism of action of antibodies through immunotherapy on the brain parenchyma remains unclear. Most extracellular forms of tau might not be able to aggregate and might not be transmissible but this is still being explored [59,60].
In healthy brains, the blood-brain barrier (BBB) generally limits to an estimated 0.1–0.2% the crossing of circulating immunoglobulins [61]. Thus the ‘sink effect’ hypothesis has been proposed to explain the peripheral elimination of CNS targets. In short, in the sink model antibodies in the periphery sequester circulating antigens. This creates a void of antigens in the blood, which displaces antigens out of tissues, including CNS, to the blood in an attempt to restore homeostasis for this specific antigen [62]. For a review of the influence of various features of tau antibodies on the likelihood of therapeutic success, a recent discussion by Sigurdsson provides a more thorough summary [63]. However, it is important to note that the BBB is often compromised in AD [64], thus it is likely that antibodies can act directly on brain tissue in such condition.
Although the molecular mechanisms behind anti-tau antibodies efficacy are unknown to date, immunotherapies have proven successful in several AD animal models and were approved for human testing. Below, we discuss several active and passive immunotherapies currently under clinical investigation.
4. Active tau immunotherapies in clinical trials
4.1. AADvac1 (also known as Axon peptide 108 conjugated to KLH)
The first-in-human tau vaccine, AADvac1, is based on an antibody, DC8E8, which targets the important pathological tau-tau interaction (Table 1). The DC8E8 epitope (294KDNIKHVPGGGS305 ) was determined by X-ray crystallography, and the X-ray structure of the DC8E8Fab suggested that the four DC8E8 epitopes form protruding structures on the tau molecule. It was used to successfully generate a vaccine that is able to discriminate between pathological and physiological tau [65]. Antibodies generated against the DC8E8 epitope were primarily lgG1, indicating a predominant type 2 helper T-cel! immune response. Immunization of a tau rat model of AD with this vaccine resulted in a reduction in the levels of both early and late pathological forms of tau. Furthermore, a significant improvement in behavioral tests was reported. These results led to a phase I clinical trial, first-in-human, safety assessment of this vaccine in humans with mild/moderate AD.
Table 1.
The current state of tau immunotherapies evaluated in clinical trials. Aa: amino acids; Mod: moderate.
| Compound/clinicaltrials.gov ID | Characteristics & Mode of Action | Participant Characteristics | Status | Manufacturer/Study Sponsor |
|---|---|---|---|---|
| ACTIVE IMMUNOTHERAPIES | ||||
| AADvac-1 | Targets aa 294–305 Possible targets aa 268–283, 330–335, and 362–367 | Mild-Mod. AD | Phase I Trial completed | Axon Neuroscience |
| ACI-35 | Contains 16 copies of tau peptides phosphorylated on S396 (and maybe S404) | Mild-Mod. AD | Possibly in Phase lb Trial though unknown | AC Immune |
| PASSIVE IMMUNOTHERAPIES | ||||
| BIIB092 | IgG4 targeting extracellular, N-terminally fragmented forms of tau (eTau) | Mild AD | Phase II Trial underway | Bristol-Myers Squibb |
| ABBV-8E12 | IgG4 targeting aggregated extracellular form of pathological tau (aa 25–30). Does not require uptake into neurons. | Early stages of AD | Phase II Trial underway until October 2020 | C2N Diagnostics and AbbVie |
| RG7345 | Humanized mAB that targets tau phosphoepitope at S422 | Healthy male participants | Discontinued Phase I Trial | Hoffmann-La Roche |
| RO7105705 | IgG4 targeting the N-terminus of all six isoforms of human tau (binds both monomeric and oligomeric tau) | Healthy participants | Phase I Trial | AC Immune and Genentech |
| BIIB076 | IgG1 targeting monomeric and fibrillar forms of tau | Healthy participants | Phase I Trial | Biogen, Neurimmune |
| JNJ-63733657 | Binds the mid-region of tau to blocks seeding | Healthy Volunteers | Phase I trial | Janssen |
| LY3303560 | Binds aa 7–9 and 313–322. Neutralizes soluble tau aggregates | Mild-Mod. AD | Phase II trial | Eli Lilly and Co. |
| UCB0107 | Binds aa 235–246. Inhibits seeding | Healthy Men | Phase I trial | UCB S.A. |
Novak et al. reported that a phase l, 12 weeks, randomized, double-blind, placebo-controlled study of AADvac1 were started in June 2013 at four centers in Austria [66]. Patients 50 to 85 years of age with mild to moderate AD were randomly assigned in a 4:1 ratio to receive AADvac1 or placebo. The primary endpoint of the study was all-cause treatment-emergent adverse events with separate analysis of injection site reactions and other adverse events. Patients with a positive lgG titer against the tau peptide component of AADvac1 were considered responders. A total of 30 patients received AADvac1. Two patients withdrew because of serious adverse events (the first described as ‘a serious adverse event’ and the second developing raised troponin level). Of the 30 participants, 29 developed an lgG immune response. Overall, these results showed that AADvac1 had a favorable safety profile and excellent immunogenicity. This led to a phase Il study, named ADAMANT, in patients with Alzheimer’s disease. However, the results of this clinical trial are still pending (NCT02579252). AADvac1 is also in a phase I study for the treatment of non-fluent primary progressive aphasia (NCT03174886).
4.2. ACI-35
ACI-35 is a liposome-based vaccine that contains 16 copies of a synthetic tau fragment phosphorylated at the protein’s pathological phosphorylation residues S396 and S404 (Table 1). In preclinical trials conducted on Tau-P301L mice, a validated tauopathy model [67], long-term vaccination proved to be safe without marked inflammation. This is especially relevant because this approach utilizes multiple epitopes (16 copies), thus is likely to elicit aberrant CNS inflammation. On the behavioral and physiological level, an improvement in motor impairment was measured by a reduction in clasping score, an index of limb paralysis. Animals vaccinated with ACI-35 cocktail also demonstrated a slight, yet significant, improvement in longevity. Importantly, this vaccine did not generate any significant off-target inflammatory response, as assessed by quantification of inflammatory markers [68,69].
Currently, several review articles have discussed a Phase 1b trial that is underway for participants with mild to moderate AD to assess safety profile along with secondary outcomes including biomarkers, functional, and clinical change [69,70]. However, details are not available and at time of submission, and this trial is not listed in ClinicalTrials.gov or the World Health Organization’s clinical trial registry [69,70].
5. Passive tau immunotherapies in clinical trials
5.1. BIIB092 (also known as BMS-986168 and IPN007)
BIIB092 is a humanized lgG4 monoclonal anti-tau antibody (Table 1). This antibody is generated against N-terminally fragmented forms of tau (eTau) that were originally isolated from familial AD patient-derived pluripotent stem cells [71]. Safety of BIIB092 infusions was evaluated in a multi-center study with multiple ascending-dose phase I trial on patients with progressive supranuclear palsy (NCT02460094). Participants received doses of up to 2,100 mg infused once every four weeks for 12 weeks. A dose-dependent accumulation of the BIIB092 was found in serum and CSF. Additionally, a drastic reduction of CSF free Tau (90–96% after 39 days of treatment and 91–97% after 85 days of treatment) was detected. The study ran from September 2015 to summer 2017 at which point, it was determined to be safe and well-tolerated. In April 2017, Biogen began a 52 week, 61 sites efficacy study comparing BIIB092 to placebo in nearly 400 people with PSP (NCT03068468). In November 2017, Biogen also listed a phase II trial for AD that will run through 2020. This trial plans to enroll 528 participants with mild AD with a positive amyloid PET scan. The trial will compare monthly infusions of three different doses of BIIB092 to placebo (NCT03352557) [72].
5.2. ABBV-8E12
ABBV-8E12 is a humanized lgG4 monoclonal antibody against tau amino acids 25–30, and targeting aggregated, an extracellular form of pathological tau (Table 1). Critical is the fact that ABBV-8E12 does not require uptake into neurons. Kfoury et al., found that this antibody blocked seeding in a cell-based tau sensor assay [73]. Additional preliminary studies showed that in P301S tau-transgenic mice (mice that express mutant human microtubule-associated protein tau), ABBV-8E12 monoclonal antibodies reduced brain neurofibrillary pathology, seeding activity, and brain atrophy [74–77].
In July 2015, this antibody was given Orphan Drug designation for progressive supranuclear palsy. Between July 2015 and October 2016, a phase I study was conducted at 12 centers, comparing four doses of ABBV-8E12 to placebo in 30 patients with PSP [77]. ABBV-8E12 was found to have an acceptable safety profile, and in October 2016, a phase II clinical trial started assessing the efficacy and safety in patients with early AD. The trial is attempting to enroll 400 patients who meet diagnostic criteria for early stages of AD, and is set to run until October 2020 (NCT02880956), though recently an extension of these trials was listed to terminate in August 2027 (NCT03712787).
5.3. RG7345 (also known as RO6926496)
RG7345 is a humanized monoclonal antibody that targets the tau phosphoepitope at S422 (Table 1). This epitope is prominent in neuronal dendrites. Tau phosphorylated at this site is considered pathological. When phosphorylated at this location, tau relocalizes away from microtubules and toward the somato-dendritic compartment of the neuron [78]. Troquier et al. investigated the effects of active immunization, that targeted the pS422 tau epitope in the THY-Tau22 transgenic mouse model [79]. They reported decreased levels of brain insoluble tau and improvements in cognition (tested by the Y maze) following administration [70].
In January 2015, a Phase I single-ascending-dose study in 48 healthy males was started. The study compared the safety and pharmacokinetic measures of six different doses to placebo. In October 2015, the study was listed as having been discontinued, though no clear reason for this stoppage has been provided (NCT02281786) [70].
5.4. RO7105705 (also known as MTAU9937A and RG6100)
RO7105705 is an lgG4 anti-tau antibody currently being investigated for AD (Table 1). The initial aim for the development of this antibody grew out of a collaboration that focused on targeting extracellular, rather than intracellular, tau. It is advanced that this approach might reduce effector function, limit microglial activation and mitigate the inflammatory responses associated with AD [52].
In July 2016, a Phase I study of 71 volunteers, both healthy controls and patients suffering mild to moderate AD, were started to assess safety, tolerability, and pharmacokinetics. The study was expected to run until May 2017. However, no results are currently available (NCT02820896). In October 2017, Genentech started TAURIEL, a Phase II study with prodromal or probable AD participants ascertained by a positive amyloid PET or CSF Aβ42 finding, and mild clinical symptoms. This trial runs at 153 locations in North America, Australia, and various European countries; primary assessment is expected in 2020 (NCT03289143). In February 2019, another phase II started. The trial will randomize 260 subjects suffering Moderate Alzheimer’s Disease who are positive for the PET tau ligand [18F]GTP1, and is expected to run until September 2021 (NCT03828747).
5.5. BIIB076 (also known as NI-105 and 6C5 hulgG1/I)
BIIB076 is a human lgG1 recombinant monoclonal anti-tau antibody that was acquired by Biogen in 2010 (Table 1). BIIB076 binds with subnanomolar affinity to human and cynomolgus monkey recombinant tau. It is also known to bind monomeric and pre-formed fibrillar tau and human brain tau [80]. A safety profile study evaluated the toxicity potential and toxicokinetic profile of this mAB in cynomolgus monkeys. Both intravenous and subcutaneous formulations were assessed in amounts up to 16 times the highest predicted efficacious dose. Drug levels were measured in the serum while tau levels were measured in the cerebral spinal fluid. There were no adverse effects noted. CSF total and free tau levels were significantly reduced in the highest BIIB076 dose groups. Based on these results, BIIB076 was determined to be safe for inclusion in a Phase I clinical trial in healthy volunteers.
In February 2017, a six-center Phase I ascending dose trial was started in healthy volunteers. The primary outcome of the trial is to monitor adverse events, supplemented with secondary outcomes looking at pharmacokinetics. The trial is set to run until summer 2019 (NCT03056729).
5.6. LY3303560
LY3303560 is a humanized anti-tau antibody that binds and neutralizes soluble tau aggregates (Table 1). The antibody recognizes a conformational epitope whose primary epitope is in tau’s N-terminal region [81]. The target epitope is discontinuous and identifies an aberrant conformation of tau that correlates with the severity and progression of AD [82].
In April 2016, the first-in-human phase I trial was initiated at 12 sites. Participants were healthy volunteers and patients with mild cognitive impairment due to AD or mild to moderate AD diagnosed with a positive amyloid PET scan. Both intravenous and subcutaneous formulations were assessed in multiple escalating doses (NCT02754830). In January 2017, a second Phase I study began comparing just intravenous formulation and placebo. The study is measuring adverse events and pharmacokinetic parameters. The study will run until June 2019 (NCT03019536).
In April 2018, a Phase II study started with plans to compare two intravenous doses to placebo in patients with a gradual and progressive decline in memory. The trial is involving 60 sites in North America and Japan, and will run until October 2021 (NCT03518073).
5.7. JNJ-63733657
JNJ-63733657 is a monoclonal antibody that recognizes the mid-region of tau, as opposed to the N-terminus (Table 1). The hypothesis behind this approach is that this antibody may interfere with the propagation of pathogenic aggregated tau. In December 2017, a Phase I trial was initiated to evaluate the Mab’s safety and tolerability. Part 1 of the trial will assess intravenous infusions in healthy volunteers, while part 2 will include participants with mild AD. The trial is expected to run until October 2019 (NCT03375697).
5.8. UCB0107
UCB0107 is an mAB being tested in patients with PSP (Table 1). It binds to the mid-region of tau, similar to JNJ-63733657. This mAB targets the amino acids 235–246 of tau, which tie at the end of tau’s second proline-rich region. It was shown to reach nearly 100% inhibition of fibrillization at an antibody concentration of 300 nM [83].
In February 2018 a Phase I trial was started to assess safety and tolerability of intravenous infusion in healthy volunteers. The trial is expected to run for 20 weeks and ended in December 2018 (NCT03464227). The first reports are expected in late 2019.
6. Conclusion
In the present review, we discussed the most advanced active and passive immunotherapies for tau tested in clinical trials, which, theoretically, may slow or prevent the progression of brain pathology in AD patients. Some tau targets that have been or are currently under investigation include both peptideand conformation-based epitopes. Some epitopes are specific to pathological tau found exclusively in AD, while others could be used for additional tauopathies. With the understanding that tau undergoes a ‘seeding’ or ‘prionosis’ process, immunotherapies aiming at inhibiting tauons should be aggressively pursued as potential therapeutic interventions. As of 2019, the only human evidence has been safety testing in several phase I trials. Many of these studies looked at blood and/or CSF levels of tau with promising results. In addition to immunotherapies discussed here, there is a growing pipeline of novel tau immunotherapies being investigated in animal models. Most of these new biologics suggest that targeting tau aggregates can improve the brain pathology and clinical symptoms associated with AD, though these studies should be interpreted with caution as animal models do no recapitulate fully the complexity of human AD. Finally, although only little efficacy data are currently available on human subjects suffering AD for both active and passive immunotherapies, the idea is theoretically important and relevant.
7. Expert opinion
Transitioning from the single focus on amyloid to targeting tau is a critical new step towards a mature refining of the treatment of symptomatic dementia due to AD for the following reasons. First, one large impetus of moving from targeting amyloid to tau is that Aβ targets have largely not resulted in therapeutic successes. Second, amyloid has not correlated well with the clinical progression of AD. In contrast, tau and NFTs have been shown in both imaging- and autopsy-based studies to correlate more closely with cognitive decline than Aβ. This correlation seems to confirm the hypothesis that the spread of tau tangles from the hippocampus (HC) through the medial temporal cortex, and to the neocortex represents the anatomical pathological progression of AD in a prion-like manner, and that HC dysfunction manifests as amnestic cognitive changes. Thus, targeting tau appears more effective than Aβ from a therapeutic standpoint, because it better reflects disease progression and could alter the trajectory of decline more robustly than targeting amyloid. However, targeting both pathologies simultaneously holds even greater therapeutic potential than targeting only each protein separately and might depend on timing in such a way that amyloid would be targeted at the pre-symptomatic phase and tau targeted once cognitive symptoms have started. Thus, mABs targeting both proteins might need to be administered simultaneously at advanced stages of AD. While it is intuitive to posit a therapeutic intervention based on these findings, studies of this nature on human patients usually take 18–24 months of treatment to determine a possible change in disease trajectory and may be cost prohibitive.
The key finding summarized in this assay is that the biologics targeting tau are in full development and have entered phase II trials, which suggests that they are relatively safe, as they have met the mandatory safety protocols to pass phase I trials. The relative unknown, however, and as is also true of mABs targeting amyloid, is whether they exert their effect by crossing the BBB, or whether a peripheral sink effect takes place, which captures in the circulation and attracts tau outside of the CNS to reestablish homeostasis. This question remains unanswered, because mABs are massive in terms of molecular weight, and, due to their size, they lack the capacity for CNS penetration, though they may enter the brain in the context of AD given the BBB is often altered. Beyond that, however, no evidence shows that the mABs will cross into the neurons themselves to bind and clear abnormal or toxic forms of tau. Also not clear is wether the mABs that target tau would remove tau by capturing and moving the toxic forms out of the brain to the periphery, or simply neutralize tau in situ in the brain parenchyma without removal, or stimulate local phagocytosis by cells like microglia. Thus, it remains to be elucidated how mABs exert therapeutic effects at the cellular and tissular level despite understanding the molecular mechanisms involved in targeting the forms of extraneuronal tau.
Anti-tau therapeutic development holds potentially more promise than other targets as discussed above. The ultimate goal is to develop drugs that alter the course of AD-related symptomatic dementia. Biologics targeting tau hold more promise than most classes of AD drugs in development. Nonetheless, questions continue to linger as to whether it is more logical to target pre-dementia states (pre-clinical or MCI). Targeting symptomatic dementia appears to be the most logical choice, since tangle spread correlates well with clinical progression. However, it is not clear if the paradigm would be the same for PSP and other ‘pure’ tauopathy. Detecting tau presymptomatically would be cost prohibitive if using present methods of detection such as tau-PET.
Some questions must be answered before moving forward. First, and as mentioned above, is the mode of action a peripheral sink effect or the crossing of the BBB? Second, does targeting and clearing prion-like isoforms of tau to stop neuron-to-neuron seeding correlate with arresting the clinical progression? Third, can the stopping of tau pathology spreading be detected with tau imaging or CSF tau measurement as an in vivo biomarker of efficacy? Fourth, are clinical assessment instruments sensitive enough to detect these changes?
Targeting tau represents the maturation and evolution of the field from a therapeutic standpoint. Since this class of drugs is just now entering phase II clinical trials, therapeutic efficacy has not been established consistently, although safety protocols are very promising.
Deploying mABs that target tau offers many benefits. First, the mAB can be created to target a single epitope or multiple epitopes and, as a result, could target different forms of pathological tau. Second, mABs as biologics, are used routinely to treat many diseases, including migraine, malignancy, autoimmune diseases, and hyperlipidemia. Their inclusion within this group means that the feasibility and safety have been well established. Third, because of the pharmacology of mABs, they have predictable pharmacokinetics and half-lives and are typically dosed monthly, which is very relevant to physicians to decide the treatment regimens to administer to individual patients. Fourth, the pharmacodynamics of mABs can be tailored to bind and neutralize, stimulate phagocytosis by glia, or bind and clear tau.
More time is necessary to determine whether this class of drugs has therapeutic efficacy, because they will likely alter the molecular trajectory without obvious alteration of cognitive symptoms. Consequently, a typical trial will require 18–24 months to see separation of the curves between the investigational biologics and placebo. However, we strongly believe that AD therapeutics are expected to meet their fullest potential with multi-targeted approaches. Biologics targeting tau will be a crucial component of that armamentarium.
Article highlights.
Biomarker changes precede clinically discernable changes in cognition.
Therapy must be initiated as early as possible due to clear indication that the pathophysiological processes of Alzheimer’s dementia begin before the onset of cognitive decline.
Misfolded tau aggregates released from presynaptic neurons are taken up by postsynaptic neurons where tauons acts as ‘seeds’ to form additional tau aggregates via a process similar to prionosis.
The rationale behind active immunotherapy is to elicit an immune response that targets specific pathological conformers of tau-specific to the biology of a given patient.
Generalized inflammation is one of the potentially serious consequences of vaccine therapy.
This box summarizes key points contained in the article.
Acknowledgments
Funding
Supported by National Institute on Aging (5P20GM109025, 7K01 AG047279, and Keep Memory Alive Foundation).
Footnotes
Declaration of interest
No potential conflict of interest was reported by the authors.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Alzheimer’s A 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016. April;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 2.Todd S, Barr S, Roberts M, et al. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013. November;28 (11):1109–1124. [DOI] [PubMed] [Google Scholar]
- 3.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014. April;71 (4):505–508. [DOI] [PubMed] [Google Scholar]
- 4.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011. September;1(1):a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisardi V, Solfrizzi V, Imbimbo PB, et al. Towards disease-modifying treatment of Alzheimer’s disease: drugs targeting beta-amyloid. Curr Alzheimer Res. 2010. February;7(1):40–55. [DOI] [PubMed] [Google Scholar]
- 6.Imbimbo BP, Ottonello S, Frisardi V, et al. Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease. Expert Rev Clin Immunol. 2012. February;8(2):135–149. [DOI] [PubMed] [Google Scholar]
- 7.Panza F, Solfrizzi V, Imbimbo BP, et al. Amyloid-directed monoclonal antibodies for the treatment of Alzheimer’s disease: the point of no return? Expert Opin Biol Ther. 2014. October;14(10):1465–1476. [DOI] [PubMed] [Google Scholar]
- 8.Holmes C, Boche D, Wilkinson D, et at. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008. July 19;372(9634):216–223. [DOI] [PubMed] [Google Scholar]
- 9.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005. May 10;64(9):1553–1562. [DOI] [PubMed] [Google Scholar]
- 10.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005. May 10;64(9):1563–1572. [DOI] [PubMed] [Google Scholar]
- 11.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003. July 8;61(1):46–54. [DOI] [PubMed] [Google Scholar]
- 12.Geerts H, Spiros A, Roberts P. Impact of amyloid-beta changes on cognitive outcomes in Alzheimer’s disease: analysis of clinical trials using a quantitative systems pharmacology model. Alzheimers Res Ther. 2018. February 2;10(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tariot PN, Lopera F, Langbaum JB, et al. The Alzheimer’s prevention initiative autosomal-dominant Alzheimer’s disease trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement (N Y). 2018;4:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobel G Alzheimer’s prevention initiative. J Alzheimers Dis. 2010;21(3):1025–1035. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR Jr., Knopman DS, Jagust WJ , et at. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010. January;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neuroi. 2013. February;12(2):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012. August 30;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin RW, Iwaki T, Kitamoto T, et al. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab Invest. 1991. May;64(5):693–702. [PubMed] [Google Scholar]
- 19.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull. 2016. September;126(Pt 3):238–292. [DOI] [PubMed] [Google Scholar]
- 20.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012. July;2(7):a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forner S, Baglietto-Vargas D, Martini AC, et al. Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci. 2017;June;40(6):347–357. [DOI] [PubMed] [Google Scholar]
- 22.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017. May;133(5):665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtzman DM, Carrillo MC, Hendrix JA, et al. Tau: from research to clinical development. Alzheimers Dement. 2016. October;12(10):1033–1039.• review of the science.
- 24.Goedert M, Spillantini MG, Jakes R, et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989. October;63(4):519–526. [DOI] [PubMed] [Google Scholar]
- 25.Arai T, Ikeda K, Akiyama H, et al. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001. February;101(2):167–173. [DOI] [PubMed] [Google Scholar]
- 26.Schoch KM, DeVos SL, Miller RL, et al. Increased 4R-tau induces pathological changes in a human-tau mouse model. Neuron. 2016. June 1;90(5):941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love JE, Hayden EJ, Rohn IT. Aiternative splicing in Alzheimer’s disease. J Parkinsons Dis Alzheimers Dis. 2015. August;2(2). DOI: 10.13188/2376-922X.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005. January 3;1739(2–3):104–115.• mechanistic description of tauopathy in pathophysiology.
- 29.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010. January 28;362(4):329–344. [DOI] [PubMed] [Google Scholar]
- 30.Kopeikina KJ, Hyman BT, Spires-Jones TL. Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci. 2012. September;3(3):223–233.•• identificaiton of species of tau that are toxic for AD.
- 31.Shafiei SS, Guerrero-Munoz MJ, Castillo-Carranza DL. Tau oligomers: cytotoxicity, propagation, and mitochondrial damage. Front Aging Neurosci. 2017;9:83.• supporting paper for toxicity of tau.
- 32.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009. March;8(3):270–279. [DOI] [PubMed] [Google Scholar]
- 33.Kouri N, Whitwell JL, Josephs KA, et al. Corticobasal degeneration: a pathoiogicafly distinct 4R tauopathy. Nat Rev Neurol. 2011. May;7(5):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert M, Spillantini MG. Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer’s disease. Biochim Biophys Acta. 2000. July 26;1502(1):110–121. [DOI] [PubMed] [Google Scholar]
- 35.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy, Acta Neuropathol. 2016. January;131(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jl Ayers, Giasson BI, Borchelt DR. Prion-like spreading in tauopathies. Biol Psychiatry. 2018. February 15;83(4):337–346.••informs the field on the prion spreading of tau giving the rationale for mABs.
- 37.Goedert M The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimers Dement. 2016. October;12(10):1040–1050.• mechanistic description of the toxicity of tau.
- 38.Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain. 2017. February:140(2):266–278. [DOI] [PubMed] [Google Scholar]
- 39.Clavaguera F, Boimont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009. July;11(7):909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011. May;121(5):589–595. [DOI] [PubMed] [Google Scholar]
- 41.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011. October;70 (4):532–540.•• descbribes prion type seeding.
- 42.Braak H, Del Tredici K. Potential pathways of abnormal tau and alpha-synuclein dissemination in sporadic Alzheimer’s and Parkinson’s diseases. Cold Spring Harb Perspect Biol. 2016. November 1;8(11). DOI: 10.1101/cshperspect.a023630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H, Del Tredici K. Alzheimer’s disease: pathogenesis and prevention. Alzheimers Dement. 2012. May;8(3):227–233. [DOI] [PubMed] [Google Scholar]
- 44.Walker LC, Diamond Ml, Duff KE, et al. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013. March 1;70(3):304–310.• more about the mechanism of seeding.
- 45.Braak H, Rub U, Gai WP, et al. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003. May;110(5):517–536. [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Del Tredici K. Spreading of tau pathology in sporadic Alzheimer’s disease along cortico-cortical top-down connections. Cereb Cortex. 2018. September 1;28(9):3372–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina M, Hernandez F, Avila J. New features about tau function and dysfunction. Biomolecules. 2016. April 19;6(2). DOI: 10.3390/biom6020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Wu Z, Zhou B. Behind the curtain of tauopathy: a show of multiple players orchestrating tau toxicity. Cell Mol Life Sci. 2016. January;73(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemere CA. Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener. 2013. October 22;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panza F, Solfrizzi V, Seripa D, et al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. Biomed Res Int. 2016;2016:3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruthirakuhan M, Herrmann N, Suridjan I, et al. Beyond immunotherapy: new approaches for disease modifying treatments for early Alzheimer’s disease. Expert Opin Pharmacother. 2016. December;17(18):2417–2429. [DOI] [PubMed] [Google Scholar]
- 52.Lee SH, Le Pichon CE, Adolfsson O, et al. Antibody-mediated targeting of tau in vivo does not require effector function and microglial engagement. Cell Rep. 2016. August 9;16(6):1690–1700. [DOI] [PubMed] [Google Scholar]
- 53.Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology. 2014. January;76(Pt A):27–50. [DOI] [PubMed] [Google Scholar]
- 54.Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med. 2017. January 14;68:413–430. [DOI] [PubMed] [Google Scholar]
- 55.Monsonego A, Nemirovsky A, Harpaz I. CD4 T cells in immunity and immunotherapy of Alzheimer’s disease. Immunology. 2013. August;139(4):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenmann H, Grigoriadis N, Karussis D, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006. October;63(10):1459–1467. [DOI] [PubMed] [Google Scholar]
- 57.Mably AJ, Kanmert D, Mc Donald JM, et al. Tau immunization: a cautionary tale? Neurobiol Aging. 2015. March;36(3):1316–1332. [DOI] [PubMed] [Google Scholar]
- 58.Wagshal D, Sankaranarayanan S, Guss V, et al. Divergent CSF tau alterations in two common tauopathies: alzheimer’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015. March;86(3):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018. May 16;98(4):861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Z, Mengel D, Keshavan A, et al. Alzheimers dement. Alzheimer’s Dementia. 2019. March;15(3):487–496. Epub 2018 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013. July;10(3):459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menendez-Gonzalez M, Perez-Pinera P, Martinez-Rivera M, et al. Immunotherapy for Alzheimer’s disease: rational basis in ongoing clinical trials. Curr Pharm Des. 2011;17(5):508–520. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdsson EM. Tau immunotherapies for Alzheimer’s disease and related tauopathies: progress and potential pitfalls. J Alzheimers Dis. 2018;64(s1):S555–S565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med. 2017. November 6;214(11):3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kontsekova E, Zilka N, Kovacech B, et al. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res Ther. 2014;6(4):44.• important report about tau active immunotherapy.
- 66.Novak P, Schmidt R, Kontsekova E, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017. February;16(2):123–134.• report of initial results of vaccine therapy.
- 67.Terwel D, Lasrado R, Snauwaert J, et al. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem. 2005. February 4;280(5):3963–3973. [DOI] [PubMed] [Google Scholar]
- 68.Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau. P301L mice that model tauopathy. PLOS One. 2013;8(8):e72301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung SY, Fu WM. Drug candidates in clinical trials for Alzheimer’s disease. J Biomed Sci. 2017. July 19;24(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panza F, Solfrizzi V, Seripa D, et al. Tau-based therapeutics for Alzheimer’s disease: active and passive immunotherapy. Immunotherapy. 2016. September;8(9):1119–1134. [DOI] [PubMed] [Google Scholar]
- 71.Bright J, Hussain S, Dang V, et al. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015. February;36(2);693–709. [DOI] [PubMed] [Google Scholar]
- 72.Qureshi IA, Tirucherai G, Ahlijanian MK, et al. A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimers Dement (N Y). 2018;4:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kfoury N, Holmes BB, Jiang H, et al. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012. June 1;287(23):19440–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmes BB, Furman JL, Mahan TE, et al. Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci U S A. 2014. October 14;111(41):E4376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanamandra K, Jiang H, Mahan TE, et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol. 2015. March;2(3)278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013. October 16;80(2):402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West T, Hu Y, Verghese PB, et al. Preclinical and clinical development of ABBV-8E12, a humanized anti-tau antibody, for treatment of Alzheimer’s disease and other tauopathies. J Prev Alzheimers Dis. 2017;4(4):236–241. [DOI] [PubMed] [Google Scholar]
- 78.Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000. August;33(1):95–130. [DOI] [PubMed] [Google Scholar]
- 79.Troquier L, Caillierez R, Burnouf S, et al. Targeting phospho-Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012. May;9(4):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Czerkowicz J, Chen W, Wang Q, et al. Pan-tau antibody Biib076 exhibits promising safety and biomarker profile in cynomolgus monkey toxicity study. Alzheimer’s Dementia. 2017;13(7):P1271. [Google Scholar]
- 81.Alam R, Driver D, Wu S, et al. Preclinical characterization of an antibody [LY3303560] targeting aggregated tau. Alzheimer’s Dementia. 2017;13(7):P592–P593. [Google Scholar]
- 82.Vitale F, Giliberto L, Ruiz S, et al. Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice. Acta Neuropathol Commun. 2018. August 22;6(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novak P, Kontsekova E, Zilka N, et al. Ten years of tau-targeted immunotherapy: the path walked and the roads ahead. Front Neurosci. 2018;12:798. [DOI] [PMC free article] [PubMed] [Google Scholar]