Abstract
Background:
Phenol exposure during pregnancy has been associated with preterm birth, but the potential effect of preconception exposure in either parent is unknown. There is a growing body of evidence to suggest that the preconception period is a critical window of vulnerability for adverse pregnancy outcomes.
Objective:
We examined whether maternal and paternal preconception urinary concentrations of select phenols were associated with the risk of preterm birth among couples attending fertility care.
Methods:
The analysis included 417 female and 229 male participants of the Environment and Reproductive Health (EARTH) Study who gave birth to 418 singleton infants between 2005 and 2018 and for whom we had phenol biomarkers quantified in at least one urine sample collected before conception. Mothers and fathers provided an average of 4 and 3 urine samples during the preconception period, respectively. We calculated the geometric mean of bisphenol A (BPA), bisphenol S (BPS), benzophenone-3, triclosan, and the molar sum of parabens (ΣParabens) concentrations for each participant’s preconception exposure. Risk ratios (RRs) of preterm birth (live birth before 37 completed weeks’ gestation) were estimated using modified Poisson regression models adjusted for covariates.
Results:
The mean (SD) gestational age among singletons was 39.3 (1.7) weeks with 8% born preterm. A log-unit increase in maternal preconception BPA (RR 1.94; 95% CI: 1.20, 3.14) and BPS (RR 2.42; 95% CI: 1.01, 5.77) concentration was associated with an increased risk of preterm birth. These associations remained after adjustment for maternal prenatal and paternal preconception exposure. Paternal preconception ΣParabens concentrations were associated with a possible increased risk of preterm birth (RR 1.36; 95% CI: 0.94, 1.96). No consistent pattern of association was observed for benzophenone-3 or triclosan biomarkers in either parent.
Discussion:
Maternal preconception urinary BPA and BPS concentrations, as well as paternal preconception urinary parabens concentrations were prospectively associated with a higher risk of preterm birth. Subfertile couples’ exposure to select phenols during preconception may be an unrecognized risk factor for adverse pregnancy outcomes.
Keywords: bisphenol, paraben, triclosan, benzophenone, preconception, preterm birth
Introduction
Preterm birth is a leading cause of perinatal and infant morbidity worldwide (Romero et al., 2014; Rubens et al., 2014) and is a relatively common perinatal outcome with around one in ten babies born with this condition in Europe and in the United States (U.S.) (Chawanpaiboon et al., 2019; EFCNI, 2010; WHO, 2012). Prenatal exposure to endocrine disrupting chemicals (EDCs) is considered a potential risk factor for preterm birth (Ferguson and Chin, 2017; Porpora et al., 2019). EDCs are exogenous chemicals that can interfere with any aspect of hormone action (Zoeller et al., 2012). Phenolic EDCs are of particular concern, as human exposure is ubiquitous and occurs through diet, use of personal care products, and many other sources (Frederiksen et al., 2014; Freire et al., 2019; Messerlian et al., 2017).
Bisphenol A (BPA) is a paradigmatic EDC that disrupts hormonal homeostasis at low doses in experimental studies (Vandenberg et al., 2012), and is classified as an ovarian and reproductive toxicant (ECHA, 2016; Peretz et al., 2014). Despite this, BPA is extensively used in the manufacturing of polycarbonate plastics, epoxy resins liners of canned food, and thermal paper, among numerous other applications (Mustieles et al., 2015; Vandenberg et al., 2007; von Goetz et al., 2017). Increasing public concern over BPA has led to its replacement in some consumer products often labeled as “BPA-free.” However, most replacements are structural analogs such as bisphenol S (BPS), which are also hormonally active (Rochester and Bolden, 2015) and are suspected of having similar adverse effects in experimental animals (Carvaillo et al., 2019). BPA to BPS replacement is a concern in many countries (Kataria et al., 2017; Molina-Molina et al., 2019; Ye et al., 2015), with the speed of substitution being relatively faster in the United States market compared to other countries (Wu et al., 2018). Other phenolic EDCs such as parabens and triclosan are frequently used in personal care products, food additives, and pharmaceuticals to avoid microbial growth (Ferguson et al., 2017; Giulivo et al., 2016), while benzophenone-3 is used as an ultraviolet filter in sunscreens, body lotions and make-up among other applications (Liao and Kannan, 2014). Despite concern about the potential endocrine action of these phenols (Ghazipura et al., 2017; Johnson et al., 2016; Nowak et al., 2018), epidemiologic evidence on birth outcomes is limited (Giulivo et al., 2016; Jamal et al., 2019; Messerlian et al., 2018a).
New and emerging data highlights the potential for EDCs to exert enduring epigenetic modifications in male and female gametes, as well as in other female reproductive organs (Luderer et al., 2019; Santangeli et al., 2017; Xin et al., 2015; Zama and Uzumcu, 2010). Further, epigenetic modifications likely contribute to preterm birth etiology (Mani et al., 2018; Menon et al., 2012) and have been recently identified as a key characteristic of female reproductive toxicants (Luderer et al., 2019). The preconception period is thus increasingly recognized as a highly sensitive window for environmental perturbation, via both maternal and paternal exposures (Braun et al., 2017; Toivonen et al., 2017). However, while some epidemiologic studies have previously explored the relationship between prenatal exposure to phenols and the risk of delivering preterm (Aung et al., 2019; Behnia et al., 2016; Cantonwine et al., 2010, 2015; Huo et al., 2018), the impact of preconception EDC exposures on preterm birth constitutes a knowledge gap (Toivonen et al., 2017). Therefore, our objective was to examine whether maternal and paternal urinary phenol biomarker concentrations measured before conception were associated with preterm birth in a prospective preconception cohort of couples attending a fertility clinic.
Methods
Study Cohort
The Environment and Reproductive Health (EARTH) Study is an ongoing prospective preconception cohort of couples from the Massachusetts General Hospital (MGH) Fertility Center. The EARTH Study aims to investigate how environmental and nutritional factors in both males and females from preconception throughout pregnancy influence fertility, gestation, and birth outcomes. The cohort has been previously described in detail (Messerlian et al., 2018b). Briefly, women 18–46 and men 18–55 years of age are invited to enroll independently or as a couple, and are followed from study entry throughout their fertility care, pregnancy, and labor and delivery. Participants complete general and lifestyle questionnaires, undergo anthropometric measurements, and provide a spot urine and blood sample at baseline and then subsequently during each fertility treatment cycle, as well as across trimesters among those females achieving conception.
The present study included 417 female and 229 male EARTH cohort participants who gave birth to a singleton infant between 2005 and 2018 and for whom we quantified phenol biomarkers in at least one urine sample collected before conception of the index pregnancy. One male participant enrolled without a female partner and had a singleton live birth, thus leaving 228 couples. Among the 418 singleton infants, preconception BPA measurements were available for 417 mother-child pairs and 229 father-child pairs, and paraben concentrations were available for 405 mother-child and 223 father-child pairs. Measurement of benzophenone-3 and triclosan began in 2010: preconception urinary concentrations were available for 278 mother-child pairs and 139 father-child pairs. Measurement of BPS did not begin until 2012: urinary concentrations were available for 163 mother–child pairs and 77 father–child pairs (See Fig. 1, Participant Flow Chart). Trained study staff explained the study details to all participants and answered any questions before obtaining their signed informed consent. The study was approved by the Institutional Review Boards of MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Figure 1.
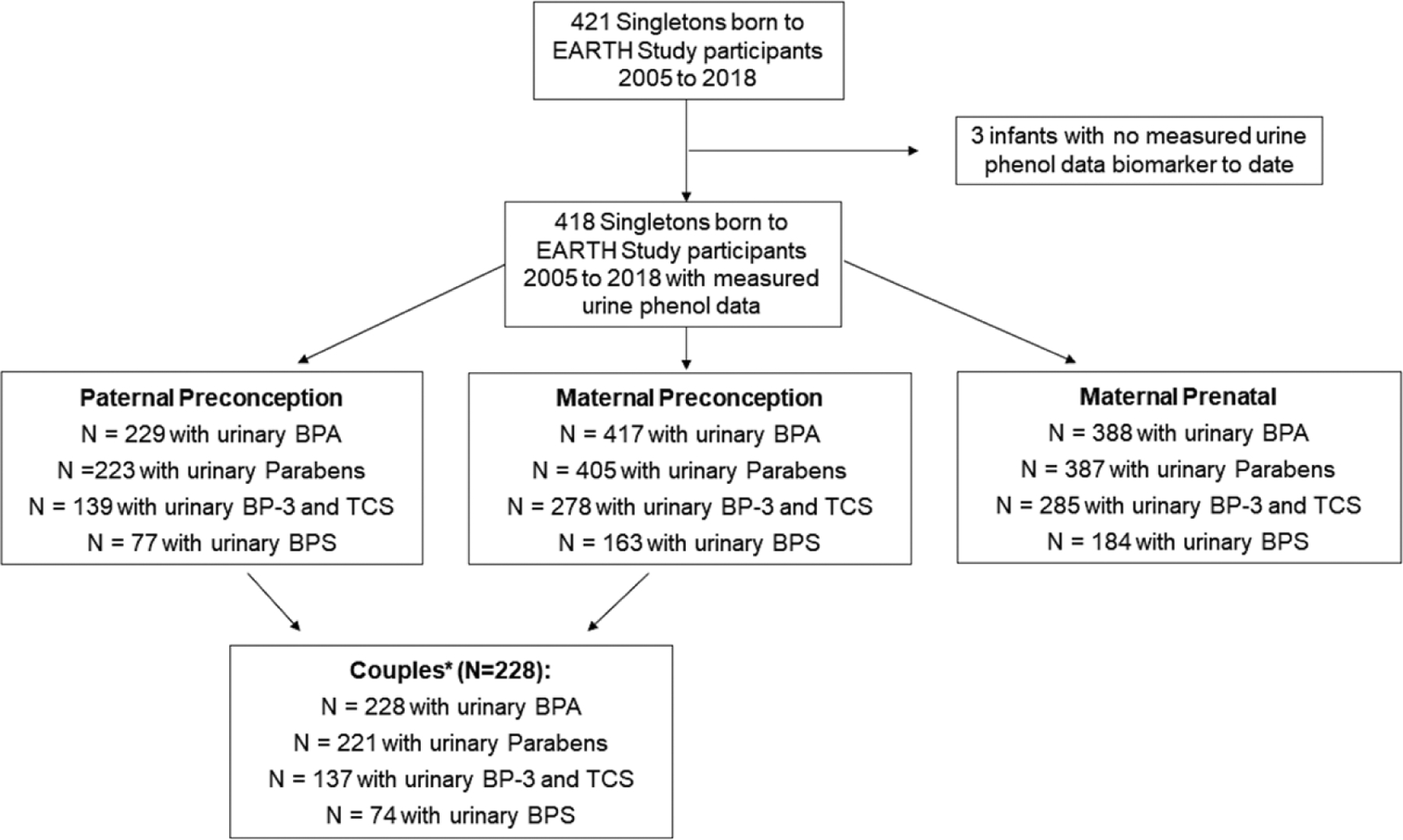
Participant flow-chart and phenol biomarker data available in the EARTH Study 2005–2018.
Abbreviations: bisphenol A (BPA); bisphenol S (BPS); benzophenone 3 (BP-3); triclosan (TCS).
* Note: One male participant joined without female partner, leaving 228 couples.
Exposure Assessment
Male and female participants provided one spot urine sample at study entry. Females provided up to two additional spot urine samples per fertility treatment cycle: the first sample collected during the follicular phase of the cycle (days 3 to 9) and the second obtained on the day of the fertility procedure [at time of oocyte retrieval, or embryo transfer for fresh or frozen in-vitro fertilization (IVF) treatment, or on the day of intrauterine insemination (IUI)]. During pregnancy, women also provided one spot urine sample per trimester at median 6, 21, and 35 weeks’ gestation. Males provided an additional spot urine sample per cycle on the day when their female partner underwent the fertility procedure. We used multiple urine samples per participant obtained from study entry up to and including the sample from the cycle of conception of the index pregnancy to estimate mean exposure in the preconception window.
Urine was collected in a polypropylene specimen cup and analyzed for specific gravity (SG) with a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), divided into aliquots, and frozen for long-term storage at −80 °C. All samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA) for quantification of urinary phenol concentrations using solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Zhou et al., 2014). The urinary concentrations of the following phenols were measured: BPA, BPS, methylparaben, ethylparaben, propylparaben, butylparaben, benzophenone-3 and triclosan. The limits of detection (LOD) ranged from 0.1 to 1.0 ng/ml. Concentrations below the LOD were assigned the LOD divided by the square root of two (Hornung, 1990). Due to low detection frequencies and small number of participants with ethylparaben quantified, we did not include or consider ethylparaben in further analyses. The weighted molar sum of parabens was calculated by dividing each urinary concentration by its molecular weight and then summing: ∑Parabens = [(Methylparaben * (1/152.15 g/mol)) + (Propylparaben * (1/180.20 g/mol)) + (Butylparaben * (1/194.23 g/mol))]. We then weighted this sum by the molecular weight of methylparaben (152.15 g/mol) to convert the molar concentration to units of ng/mL.
Outcome Assessment
Gestational age in days was abstracted from delivery records and validated using the American College of Obstetricians and Gynecologists (ACOG) guidelines to estimate gestational age for births following medically assisted reproduction (ACOG, 2014). The fertility treatment setting permitted us to estimate gestational age with high accuracy using in vitro fertilization (IVF) embryo transfer dates, substantially reducing misclassification of preterm birth due to inaccuracies in pregnancy dating (Savitz et al., 2002). For IVF pregnancies, gestational age was estimated as (outcome date – embryo transfer date +14 days + cycle day of transfer) (ACOG, 2014). For IUI and non-medically assisted/naturally conceived pregnancies, we used birth date minus cycle start date. Gestational age was corrected if medical delivery record estimates (gold standard) differed by over 6 days from the estimated gestational age using the described methods (corrected for three infants through additional chart verification). Preterm birth was defined as any live birth prior to 37 weeks of completed gestation (<259 days).
Covariates
At study entry, paternal and maternal age, education, race, and smoking status were obtained from self-reported questionnaires administered. A research study staff measured the height and weight of participants, and Body Mass Index (BMI) (kg/m2) was calculated. The physician administering fertility treatment diagnosed the underlying cause of infertility using the Society for Assisted Reproductive Technology (ART) definitions. Type of medically assisted reproduction used in the conception cycle of the index birth was abstracted from the electronic medical records by trained study staff and dichotomized: ART procedures (e.g., stimulated IVF cycles with fresh embryo transfers or cryo-thawed embryo transfer protocols) versus non-ART protocols (e.g., IUI with or without ovulation induction/stimulation; ovulation induction/stimulation with timed intercourse, or non-medically assisted/naturally conceived).
Statistical Analysis
To account for urinary dilution, we adjusted concentrations by multiplying each biomarker concentration by [(SGp-1)/(SGi-1)], where SGi is the SG of the participant’s urine sample and SGp is the mean SG for all male (mean=1.016) or all female (mean=1.015) participants included in the study samples (Pearson et al., 2009). The SG-adjusted biomarker concentrations were natural log-transformed to standardize the distribution and reduce the influence of extreme values. We estimated geometric mean paternal and maternal preconception phenol biomarker concentrations by averaging each participant’s concentration obtained from all urine samples collected from study entry and at each treatment cycle up to and including the cycle of the index conception of the singleton.
We calculated descriptive statistics for biomarker concentrations using SG-adjusted log concentrations, percentage of values below the LOD, as well as Spearman correlation coefficients for each natural log concentration between couples (paternal versus maternal preconception and across preconception and prenatal windows). We described the clinical and demographic characteristics of the study population with means (standard deviation (SD)) or proportions (n, %). We fit modified Poisson regression models to evaluate the association of continuous urinary biomarker concentrations and dichotomous preterm birth outcomes. Models were fit using a log link function and Poisson distribution to yield estimated risk ratios (RRs) and 95% confidence intervals (CIs) for preterm birth for every natural log-unit increase in biomarker concentration. We fit a separate model for each individual phenol biomarker.
We selected a priori covariates as potential confounders based on substantive knowledge using a directed acyclic graph (DAG, see Fig. 2) (Textor et al., 2016) and examined unadjusted and covariate-adjusted results. Models evaluating maternal preconception exposure were adjusted for: maternal age and BMI (continuous), maternal education (<college, college, graduate degree), smoking status (never smoked versus ever smoked, defined as a current or former smoker), and ART versus non-ART-based treatment. Both paternal and maternal covariates in reproductive epidemiologic studies are increasingly considered in confounding models (Bellavia et al., 2019). The study of paternal preconception exposure is still a novel field; we thus chose to be more conservative in models of father’s exposure. Paternal models were therefore adjusted for both the maternal covariates presented in our DAG in Figure 2, as well as for father’s age (continuous), BMI (continuous), and smoking (never vs. ever). We also adjusted for partner’s preconception exposure in subsequent models. To account for potential confounding by pregnancy-related exposure, we adjusted for mean prenatal log-concentrations across three trimesters for the biomarker of interest in additional multivariable models. Statistical analyses were conducted with SAS (version 9.4; SAS Institute Inc., Cary, USA).
Figure 2.
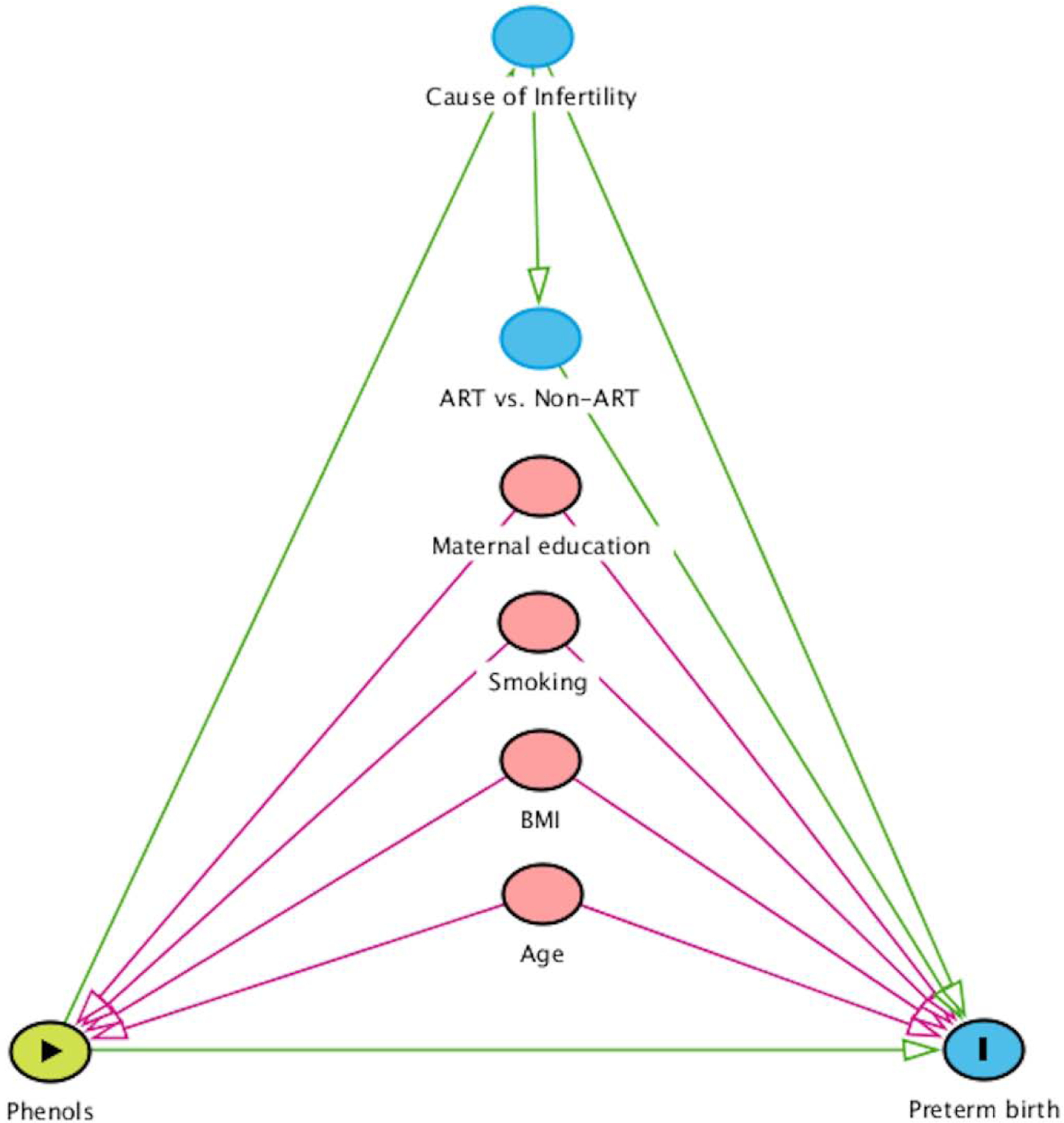
Hypothesized directed acyclic graph (DAG) between preconception exposure to phenols and preterm birth risk.
Abbreviations: body mass index (BMI); assisted reproductive technology (ART).
Sensitivity Analyses
To explore potential dose-response associations within positive findings, we created and fit models across biomarker quartiles. Given that the placenta shows sex-dependent functions susceptible to environmental insults (Rosenfeld, 2015), and previous sex-specific associations have been reported between prenatal phenol exposure and preterm birth (Aker et al., 2019; Cantonwine et al., 2015), we stratified analyses by infant sex and estimated sex-specific risk ratios and 95% CIs (Buckley et al., 2017). We calculated effect-measure modification p-values for the interaction term (sex × urine phenol biomarker concentration) and considered < 0.20 as potential evidence of effect modification by infant sex on the multiplicative scale. In the present study, we found a downward trend in preconception BPA concentrations among EARTH participants starting in 2012 up to 2018 (Mínguez-Alarcón et al., 2019). As such, we chose to further adjust for study period (≤ 2011 vs. ≥2012) in our maternal and paternal preconception BPA models. Furthermore, because the number of completed fertility cycles may influence both the number of urine samples collected for estimating exposure as well as adverse birth outcomes, we included a sensitivity analysis additionally adjusting for the number of fertility cycles in order to test the robustness of the main findings.
Results
Study cohort
Our cohort included 417 mothers and 229 fathers (228 couples) with an average age of 34.7 and 36.0 years and a mean BMI of 24.1 and 27.7 at recruitment, respectively (Table 1). Among the 418 singleton infants, mean gestational age was 39.3 (SD=1.7) weeks, with 8.1% (n=34) of infants born preterm (Table 2). Mean birth weight was 3360 (SD=550) grams, with 4.8% (n=20) of infants born low birth weight (<2500 grams) (Table 2).
Table 1.
Parental characteristics from 417 women and 229 men participating in the Environment and Reproductive Health (EARTH) Study (2005 – 2018).
| Parental Characteristic | Mothers | Fathers |
|---|---|---|
| N = 417 | N = 229 | |
| Age (years) | ||
| Mean ± SD | 34.7 ± 4.0 | 36.0 ± 4.6 |
| Age>35, n (%) | 172 (41) | 127 (55) |
| Race, n (%) | ||
| White | 353 (85) | 201 (88) |
| Black | 11 (3) | 4 (2) |
| Asian | 36 (8) | 15 (6) |
| Other | 17 (4) | 9 (4) |
| Body Mass Index (BMI, Kg/m2) | ||
| Mean ± SD | 24.1 ± 4.3 | 27.7 ± 6.1 |
| BMI >25, n (%) | 132 (32) | 156 (68) |
| Education, n (%) | ||
| < College | 54 (13) | 31 (17) |
| College Graduate | 135 (32) | 62 (34) |
| Graduate Degree | 228 (55) | 87 (49) |
| Smoking Status, n (%) | ||
| Never | 314 (75) | 159 (69) |
| Ever (former or current) | 103 (25) | 70 (31) |
| Infertility Diagnosis, n (%) | ||
| Male Factor | 100 (24) | 69 (30) |
| Female Factor | 132 (32) | 65 (28) |
| Unexplained | 185 (44) | 95 (42) |
| Primiparous, n (%) | 346 (83) | - |
Table 2.
Birth characteristics of 418 singletons from the Environment and Reproductive Health (EARTH) Study (2005 – 2018).
| Child Characteristics | Births 2005 – 2018 |
|---|---|
| N = 418 | |
| Male, n (%) | 216 (52) |
| Birth weight (grams) | |
| Mean ± SD | 3360 ± 550 |
| min-max | 1090 – 5040 |
| Low birth weight | |
| <2500 g, n (%) | 20 (4.8) |
| Gestational age at birth | |
| Mean weeks ± SD | 39.3 ± 1.7 |
| min-max | 29–42 |
| Mean days ± SD | 275 ± 12.0 |
| min-max | 205–294 |
| Preterm birth | |
| <37 weeks, n (%) | 34 (8.1) |
| Mode of Conception, n (%) | |
| ARTa | 236 (56) |
| Non-ARTb | 182 (44) |
Assisted Reproductive Technology (ART): fresh or frozen in-vitro fertilization protocols, including intracytoplasmic sperm injection.
Non-ART: intrauterine insemination with or without ovulation induction/stimulation; ovulation induction/stimulation with timed intercourse, or non-medically assisted/naturally conceived.
Urinary phenol concentrations
In total, 1693 maternal preconception and 547 paternal preconception urine samples were quantified for phenol biomarkers. Women and men provided on average 4.0 (range: 1–20) and 2.6 (range: 1–10) urine samples, respectively. Table S1 presents the distribution of specific gravity normalized mean urinary phenol biomarker concentrations. Within the measured phenols, the geometric mean of the SG-adjusted urinary concentrations for fathers and mothers were respectively: 1.4 and 1.1 ng/ml for BPA; 0.48 and 0.48 ng/ml for BPS; 30.7 and 142.9 ng/ml for ΣParabens; 63.4 and 161 ng/ml for benzophenone-3; and 14.6 and 14.0 for triclosan (Table S1). The frequency of detection was lowest for paternal preconception butylparaben (25.8%). The remaining paternal and maternal preconception phenol concentrations had moderate to high detection frequencies ranging from 63.2% to 99.7% (Table S1). The lowest Spearman correlation coefficients were observed for maternal and paternal preconception BPS concentrations (r=0.09) and maternal preconception and prenatal BPS concentrations (r=0.15). Other biomarkers presented moderate correlations for maternal and paternal preconception concentrations (r=0.23–0.59) and maternal preconception and prenatal concentrations (r=0.27–0.60) (Table S2).
Maternal preconception window
Covariate-adjusted models showed a positive association between maternal preconception urinary BPA concentrations and the risk of preterm birth (RR 1.94; 95% CI: 1.20, 3.14). This association was strengthened slightly in models additionally adjusting for maternal prenatal BPA concentrations (RR 2.20; 95% CI: 1.29, 3.75), and remained in models further adjusting for paternal BPA concentrations (RR 2.00; 95% CI: 1.00, 3.99) (Table 3). Sensitivity analyses revealed a linear trend across quartiles of maternal preconception BPA concentrations and preterm birth risk (P test for trend = 0.01) (Table S3). No significant difference was observed between male and female infants in the association of maternal preconception BPA concentration and preterm birth (P value for sex interaction term=0.32) (Table S5). Further adjustment for study period and number of fertility cycles did not materially change the association between maternal preconception BPA concentrations and preterm birth (RR 1.76; 95% CI: 1.02, 3.02) (Table S6).
Table 3.
Risk Ratios (RR) and 95% Confidence Intervals (95% Cis) for preterm birth (<37 weeks) per natural log-unit increase in maternal preconception urinary phenol concentrations among 417 mothers in the Environment and Reproductive Health (EARTH) Study, 2005–2018.
| Phenol Biomarker |
Preterm Birth Model 1 Unadjusteda |
Preterm Birth Model 2 Covariatesb |
Preterm Birth Model 3 Covariates + Prenatalc |
Preterm Birth Model 4 Covariates + Paternal Precond |
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Bisphenol A | ||||
| 1.95 (1.23, 3.09) | 1.94 (1.20, 3.14) | 2.20 (1.29, 3.75) | 2.00 (1.00, 3.99) | |
| p-values | 0.005 | 0.007 | 0.004 | 0.05 |
| Preterm Birth n/N | 34/417 | 34/417 | 31/384 | 18/228 |
| Bisphenol S | ||||
| 2.31 (1.04, 5.14) | 2.42 (1.01, 5.77) | 3.33 (1.13, 8.80) | DNC | |
| p-values | 0.04 | 0.05 | 0.03 | |
| Preterm Birth n/N | 9/163 | 9/163 | 7/145 | 2/74 |
| ∑Parabense | ||||
| 0.99 (0.76, 1.29) | 0.99 (0.76, 1.30) | 0.90 (0.63, 1.29) | 1.00 (0.65, 1.54) | |
| p-values | 0.93 | 0.95 | 0.57 | 0.99 |
| Preterm Birth n/N | 33/405 | 33/405 | 30/372 | 17/221 |
| Benzophenone-3 | ||||
| 0.91 (0.70, 1.19) | 0.91 (0.68, 1.22) | 0.93 (0.63, 1.39) | 1.55 (0.89, 2.70) | |
| p-values | 0.50 | 0.53 | 0.74 | 0.12 |
| Preterm Birth n/N | 21/278 | 21/278 | 18/254 | 8/137 |
| Triclosan | ||||
| 0.77 (0.58, 1.02) | 0.78 (0.59, 1.04) | 0.78 (0.53, 1.14) | 1.16 (0.68, 1.97) | |
| p-values | 0.07 | 0.10 | 0.19 | 0.59 |
| Preterm Birth n/N | 21/278 | 21/278 | 18/254 | 8/137 |
Note: n, number of preterm birth cases; N, sample size for maternal preconception biomarker concentrations; DNC, did not converge.
Models 1: Unadjusted.
Models 2: Adjusted for age (continuous), BMI (continuous), ART (yes/no), smoking (ever/never), education (categorical).
Models 3: Adjusted for age (continuous), BMI (continuous), ART (yes/no), smoking (ever/never), education (categorical) + prenatal biomarker exposure (continuous log-concentration).
Models 4: Adjusted for age (continuous), BMI (continuous), ART (yes/no), smoking (ever/never), education (categorical) + paternal preconception biomarker exposure (continuous log-concentration).
ΣParabens: the molar sum of parabens was estimated by dividing each concentration by its molecular weight and then summing: ∑Parabens = [(Methylparaben * (1/152.15)) + Propylparaben * (1/180.20)) + Butylparaben * (1/194.23))]. We then weighted this sum by the molecular weight of methylparaben (152.15) to convert the molar concentration to units of ng/ml.
We also observed that higher maternal preconception urinary BPS concentrations were associated with an elevated risk of preterm birth in the covariate-adjusted model (RR 2.42; 95% CI: 1.01, 5.77), with a similar pattern of strengthened associations after further adjustment for maternal prenatal BPS concentrations (RR 3.33; 95% CI: 1.13, 8.80) (Table 3). No differences by infant sex were observed with BPS (P value for sex interaction term=0.68) (Table S5). The positive association between maternal preconception BPS and preterm birth remained after adjusting for number of fertility cycles (RR 2.61; 95% CI: 1.03,6.57) (Table S6). Although mothers with higher preconception urinary triclosan concentrations had lower preterm birth risk (RR 0.78; 95% CI: 0.59, 1.04), confidence intervals became more imprecise after controlling for prenatal triclosan (RR 0.78; 95% CI: 0.53, 1.14) (Table 3). No consistent pattern of association compatible with the data was observed for maternal preconception ∑Parabens or benzophenone-3 concentrations (Table 3).
Paternal preconception window
No association with preterm birth was observed for paternal preconception urinary BPA concentrations (RR 0.82; 95% CI: 0.43, 1.55), BPS (RR 0.49; 95% CI: 0.08, 2.98), benzophenone-3 (RR 1.02; 95% CI: 0.53, 1.99) or triclosan (RR 0.97; 95% CI: 0.67, 1.41) (Table 4). Fathers with higher urinary concentrations of ΣParabens had singletons with a higher but imprecise risk of preterm birth in the main covariate-adjusted model (RR 1.36; 95% CI: 0.94, 1.96). This was unchanged with further adjustment for maternal preconception (RR 1.42; 95% CI: 0.97, 2.09) or pregnancy ΣParabens concentrations (RR 1.39; 95% CI: 0.95, 2.04) (Table 4). Further adjustment for number of fertility cycles did not materially change the association (RR 1.34; 95% CI: 0.93,1.93) (Table S7). An examination of the individual parabens revealed that higher paternal preconception concentrations of methylparaben (RR 1.35; 95% CI: 0.93, 1.98) and propylparaben (RR 1.27; 95% CI: 0.98, 1.65) were associated with a higher risk of preterm birth in covariate-adjusted models (Table S4). The associations between paternal preconception ΣParabens, methylparaben or propylparaben concentrations and preterm birth did not differ by infant sex (Table S5).
Table 4.
Risk Ratios (RR) and 95% Confidence Intervals (95% Cis) for preterm birth (<37 weeks) per natural log-unit increase in paternal preconception urinary phenol concentrations among 229 fathers in the Environment and Reproductive Health (EARTH) Study, 2005–2018.
| Phenol Biomarker |
Preterm Birth Model 1 Unadjusteda |
Preterm Birth Model 2 Covariatesb |
Preterm Birth Model 3 Covariates + Prenatalc |
Preterm Birth Model 4 Covariates + Maternal Precond |
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Bisphenol A | ||||
| 0.95 (0.52, 1.74) | 0.82 (0.43, 1.55) | 0.82 (0.41, 1.65) | 0.69 (0.35, 1.37) | |
| p-values | 0.86 | 0.54 | 0.58 | 0.29 |
| Preterm Birth n/N | 18/229 | 18/229 | 17/213 | 18/228 |
| Bisphenol S | ||||
| 0.49 (0.08, 2.98) | DNC | DNC | DNC | |
| p-values | 0.44 | |||
| Preterm Birth n/N | 2/77 | 2/77 | 1/68 | 2/74 |
| ∑Parabense | ||||
| 1.27 (0.90, 1.77) | 1.36 (0.94, 1.96) | 1.39 (0.95, 2.04) | 1.42 (0.97, 2.09) | |
| p-values | 0.17 | 0.10 | 0.09 | 0.07 |
| Preterm Birth n/N | 18/223 | 18/223 | 17/207 | 17/221 |
| Benzophenone-3 | ||||
| 1.01 (0.58, 1.73) | 1.02 (0.53, 1.99) | 0.92 (0.44, 1.94) | 0.81 (0.39, 1.69) | |
| p-values | 0.98 | 0.95 | 0.83 | 0.58 |
| Preterm Birth n/N | 8/139 | 8/139 | 7/128 | 8/137 |
| Triclosan | ||||
| 0.98 (0.67, 1.42) | 0.97 (0.67, 1.41) | 1.07 (0.59, 1.93) | 0.86 (0.51, 1.46) | |
| p-values | 0.90 | 0.88 | 0.82 | 0.58 |
| Preterm Birth n/N | 8/139 | 8/139 | 7/128 | 8/137 |
Note: n, number of preterm birth cases; N, sample size for paternal preconception biomarker concentrations; DNC, did not converge; Precon, maternal preconception.
Models 1: Unadjusted.
Models 2: Adjusted for maternal and paternal age (continuous), maternal and paternal BMI (continuous), ART (yes/no), maternal and paternal smoking (ever/never), education (categorical).
Models 3: Adjusted for maternal and paternal age (continuous), maternal and paternal BMI (continuous), ART (yes/no), maternal and paternal smoking (ever/never), education (categorical) + prenatal biomarker exposure (continuous log-concentration).
Models 4: Adjusted for maternal and paternal age (continuous), maternal and paternal BMI (continuous), ART (yes/no), maternal and paternal smoking (ever/never), education (categorical) + maternal preconception biomarker exposure (continuous log-concentration).
ΣParabens: the molar sum of parabens was estimated by dividing each concentration by its molecular weight and then summing: ∑Parabens = [(Methylparaben * (1/152.15)) + (Propylparaben * (1/180.20)) + (Butylparaben * (1/194.23))]. We then weighted this sum by the molecular weight of methylparaben (152.15) to convert the molar concentration to units of ng/ml.
Discussion
In this prospective analysis of couples seeking fertility care, maternal – but not paternal – preconception BPA and BPS concentrations were both associated with an elevated risk of singleton preterm birth. These associations remained relatively unchanged after accounting for maternal prenatal or paternal preconception urinary BPA or BPS concentrations, suggesting that the maternal preconception period may be a sensitive but underexplored critical window of exposure to bisphenols. Furthermore, paternal urinary paraben concentrations were also associated with an increased risk of early birth. Although we observed little difference by infant sex, small sample sizes within strata may have precluded our ability to detect differences. To our knowledge, this is the first work that has assessed couples’ preconception exposure to select phenols in relation to preterm birth.
Two previous maternal prenatal case-control studies have been conducted in the United States; one reporting that third trimester urinary BPA concentrations were positively associated with spontaneous preterm birth, but not all types of preterm birth (Cantonwine et al., 2015), and the other reporting higher maternal plasma BPA concentrations in mothers delivering preterm compared to women in term labor (Behnia et al., 2016). Additionally, a recent analysis in the same population studied by Cantonwine et al. (2015) observed an increased risk of spontaneous preterm delivery in relation to higher maternal urinary BPS concentrations measured during late pregnancy (Aung et al., 2019). In contrast, a study among Chinese pregnant women who provided a spot urine sample immediately after being admitted into hospital for delivery, reported inverse associations between urinary BPS concentrations and pregnancy duration (Wan et al., 2018). Nevertheless, the limitations of the study by Wang et al. (2018), including the possibility of reverse causation due to the timing of urine collection, should be considered. Although our maternal preconception BPA and BPS findings could be compatible with the abovementioned studies, differences between the preconception and prenatal windows preclude additional comparisons.
In the present study, maternal preconception exposure to bisphenols appeared to influence the risk of preterm birth. Although mechanistic data on preconception environmental exposures is markedly scarce, this latent effect is compatible with the known ability of BPA to alter the epigenetic programming of the ovary and other reproductive tissues including the uterus and placenta (Santangeli et al., 2017; Suvorov and Waxman, 2015; Ye et al., 2019). Indeed, BPA was recently highlighted as an example of a female reproductive toxicant that may act through epigenetic mechanisms (Luderer et al., 2019). In support of our novel maternal preconception BPS-preterm birth association, a recent in vivo study has shown that BPS can also exert epigenetic modifications in oocytes at very low doses, resulting in altered female reproductive outcomes such as decreased fertilization rates (Nevoral et al., 2018). Additionally, two murine-model studies support the existence of transgenerational female reproductive effects in response to low-dose BPS exposure during gestation (Shi et al., 2019b, 2019a). Our results are consistent with the increasing evidence that suggests similar or even stronger reprotoxic effects of BPS compared to BPA (Campen et al., 2018; Trasande, 2017; Žalmanová et al., 2017).
We previously observed that maternal preconception urinary BPA concentrations were inversely associated with offspring birth size in this same cohort (Mustieles et al., 2018). Our hypothesis of an early potential effect in the ovary, affecting oocyte quality and later resulting in reduced embryo viability and development and/or altered placental function leading to impaired fetal growth (Mustieles et al., 2018) is further supported by these preterm birth data. Importantly, such adverse pregnancy processes are also converging risk factors for preterm birth (Delnord and Zeitlin, 2019), which could have its roots in the early pre- and peri-conception period.
Although we observed that paternal paraben concentrations were associated with preterm birth, no previous epidemiologic reports with which to compare our results are available. However, in support of a potential association, parabens have been reported to increase oxidative stress in human spermatozoa (Samarasinghe et al., 2018), and butylparaben altered sperm DNA methylation in rats (Park et al., 2012). Some epidemiologic studies have reported associations between paraben exposure and altered semen quality (Zamkowska et al., 2018), while research from our own group in a smaller subsample of men did not (Meeker et al., 2011). Mechanistic studies evaluating the potential ability of parabens to exert heritable epigenetic modifications in spermatozoa could shed light on this field of study. Additional epidemiologic studies are needed to confirm or rule out the observed paternal associations with methyl- and propylparaben.
The absence of consistent preconception associations with benzophenone-3 and triclosan in our study may be compatible with previous mother-child cohorts assessing exposure during pregnancy, with most studies reporting inconsistencies. For example, null associations between prenatal urinary benzophenone-3 concentrations and preterm birth were observed among North-American women, while finding a protective association in response to higher urinary triclosan concentrations (Aung et al., 2019). Another study in Chinese pregnant women who provided a spot urine sample upon hospital admission for delivery found that benzophenone-3 concentrations were negatively associated with gestational age (Tang et al., 2013). In contrast, Aker et al. (2019) reported positive associations between maternal prenatal urinary benzophenone-3 concentrations and gestational age among Puerto Rican women, while triclosan concentrations were positively associated with gestational age in males, but negatively associated in females (Aker et al., 2019). Finally, Huo et al. (2018) did not observe associations of prenatal urinary triclosan concentrations and preterm birth risk among Chinese mothers (Huo et al., 2018). The considerable heterogeneity in these findings may be due to differences in exposure definition and measurement (e.g., gestational age at time of prenatal urine sample collection), variations in study populations, the evaluation vs. lack of evaluation of sex-dependent associations, or other differences in methods.
A strength of this study was the opportunity to assess both maternal and paternal exposure prior to conception, accounting for relevant potential confounders (Bellavia et al., 2019). Indeed, to our knowledge, this is the first work to examine phenol exposure in the preconception period in relation to the risk of preterm birth. Our findings may not be directly generalizable to fertile couples since subfertile mothers and fathers could be more susceptible to environmental insults. Notwithstanding, our results are consistent with previous studies reporting BPA-related adverse pregnancy outcomes in both subfertile and non-subfertile preconception cohorts (Mustieles et al., 2018; Smarr et al., 2015). Another strength is the ongoing follow-up of the EARTH Study, which allowed for a timely assessment of exposure to new chemical replacements such as BPS. BPS is suspected of causing similar reprotoxic effects as BPA, but has not undergone an exhaustive toxicological examination before its use in consumer goods (Trasande, 2017). Additionally, the assessment of diverse phenolic EDCs using multiple repeated urine samples allowed for improved exposure assessment thereby reducing exposure misclassification and its expected attenuation bias (Perrier et al., 2016). Notwithstanding, some degree of exposure misclassification cannot be ruled out considering the short biological half-lives and episodic nature of exposure to these non-persistent chemicals. While a limitation was the modest number of preterm birth cases, which precluded us from studying preterm birth subtypes (i.e., spontaneous preterm labor, preterm premature rupture of membranes, placental abruption, etc.), this is one of the few studies in its field that examined preterm birth risk under a prospective preconception cohort design. As we were not powered for estimating effect modification, small sample size within strata may have limited our ability to detect differences by infant sex. Future analyses with a higher number of male participants are warranted to confirm the reported associations with paternal preconception paraben exposure.
Conclusion
Maternal preconception urinary BPA and BPS concentrations as well as paternal preconception urinary paraben concentrations were prospectively associated with an increased risk of preterm birth. Our results suggest that couples’ exposure to select phenols during preconception, especially exposure to bisphenols, is an overlooked risk factor for adverse pregnancy outcomes. These findings may have significant public health implications given the importance of the outcome, the ubiquity of BPA exposure, its regrettable substitution with BPS and other structural analogs with similar reprotoxic potential, and that prevention strategies are needed to advance preconception care. While future studies should validate these associations, it may be prudent to inform couples planning conception about measures to reduce EDC exposure.
Supplementary Material
Highlights.
Prenatal exposure to some phenols was previously associated with preterm birth
Parental preconception exposure and preterm birth risk is understudied
Maternal preconception BPA and BPS exposure increased risk of singleton preterm birth
Fathers preconception paraben exposure may be associated with preterm birth
Preconception phenol exposure may be an unrecognized risk factor for preterm birth in subfertile couples
Acknowledgments:
The authors acknowledge all members of the EARTH Study team, including research staff Ramace Dadd and Myra Keller, and physicians and staff at Massachusetts General Hospital Fertility Center. We are also grateful to all study participants. The authors also gratefully acknowledge Xiaoliu Zhou, Tao Jia, and the late Xiaoyun Ye (CDC, Atlanta, GA) for measuring the urinary concentrations of phenol biomarkers.
Funding information: Work supported by grants ES009718 and ES000002 from the National Institute of Environmental Health Sciences (NIEHS). C.M. received funding from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ACOG, 2014. Committee opinion no 611: method for estimating due date. Obstet. Gynecol 124, 863–6. 10.1097/01.AOG.0000454932.15177.be [DOI] [PubMed] [Google Scholar]
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Cordero JF, Meeker JD, 2019. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ. Res 169, 41–51. 10.1016/j.envres.2018.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Ferguson KK, Cantonwine DE, McElrath TF, Meeker JD, 2019. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ. Res 169, 131–138. 10.1016/j.envres.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia F, Peltier M, Getahun D, Watson C, Saade G, Menon R, 2016. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J. Matern. Neonatal Med 29, 3583–3589. 10.3109/14767058.2016.1139570 [DOI] [PubMed] [Google Scholar]
- Bellavia A, Mitro SD, Hauser R, James-Todd T, 2019. Paternal bias: the impact of not accounting for paternal confounders in reproductive epidemiological studies. Am. J. Obstet. Gynecol 10.1016/j.ajog.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Messerlian C, Hauser R, 2017. Fathers Matter: Why It’s Time to Consider the Impact of Paternal Environmental Exposures on Children’s Health. Curr. Epidemiol. Reports 4, 46–55. 10.1007/s40471-017-0098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM, 2017. Statistical Approaches for Estimating Sex-Specific Effects in Endocrine Disruptors Research. Environ. Health Perspect 125, 067013 10.1289/EHP334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen KA, Kucharczyk KM, Bogin B, Ehrlich JM, Combelles CMH, 2018. Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S. Hum. Reprod 33, 895–904. 10.1093/humrep/dey050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine D, Meeker JD, Hu H, Sánchez BN, Lamadrid-Figueroa H, Mercado-García A, Fortenberry GZ, Calafat AM, Téllez-Rojo MM, 2010. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ. Heal 9, 62 10.1186/1476-069X-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD, 2015. Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. Environ. Health Perspect 123, 895–901. 10.1289/ehp.1408126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvaillo J-C, Barouki R, Coumoul X, Audouze K, 2019. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect 127, 047005 10.1289/EHP4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gülmezoglu AM, 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet. Glob. Heal 7, e37–e46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnord M, Zeitlin J, 2019. Epidemiology of late preterm and early term births – An international perspective. Semin. Fetal Neonatal Med 24, 3–10. 10.1016/j.siny.2018.09.001 [DOI] [PubMed] [Google Scholar]
- ECHA, 2016. ECHA. Member State Committee Support Document for Identification of 4,4’-Isopropylidenediphenol (Bisphenol A) as a Substance of Very High Concern Because of its Toxic For Reproduction (Article 57 C), EC 201-245-8, CAS 80-05-7. [Google Scholar]
- EFCNI, 2010. Too little, too late? Why Europe should do more for preterm infants. URL https://www.efcni.org/wpcontent/uploads/2018/03/EFCNI_report_light_copyright.pdf (accessed 6.18.19). [Google Scholar]
- Ferguson KK, Chin HB, 2017. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Curr. Epidemiol. reports 4, 56–71. 10.1007/s40471-017-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD, 2017. Personal care product use among adults in NHANES: associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J. Expo. Sci. Environ. Epidemiol 27, 326–332. 10.1038/jes.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Jensen TK, Jørgensen N, Kyhl HB, Husby S, Skakkebæk NE, Main KM, Juul A, Andersson A-M, 2014. Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. REPRODUCTION 147, 555–565. 10.1530/REP-13-0522 [DOI] [PubMed] [Google Scholar]
- Freire C, Molina-Molina J-M, Iribarne-Durán LM, Jiménez-Díaz I, Vela-Soria F, Mustieles V, Arrebola JP, Fernández MF, Artacho-Cordón F, Olea N, 2019. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ. Int 127, 592–600. 10.1016/j.envint.2019.04.013 [DOI] [PubMed] [Google Scholar]
- Ghazipura M, McGowan R, Arslan A, Hossain T, 2017. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod. Toxicol 73, 175–183. 10.1016/j.reprotox.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Giulivo M, Lopez de Alda M, Capri E, Barceló D, 2016. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res 151, 251–264. 10.1016/j.envres.2016.07.011 [DOI] [PubMed] [Google Scholar]
- Hornung RL, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Ocuupational Environ. Hyg 5, 46–51. [Google Scholar]
- Huo W, Xia W, Wu C, Zhu Y, Zhang B, Wan Y, Zhou A, Qian Z, Chen Z, Jiang Y, Liu H, Hu J, Xu B, Xu S, Li Y, 2018. Urinary level of triclosan in a population of Chinese pregnant women and its association with birth outcomes. Environ. Pollut 233, 872–879. 10.1016/j.envpol.2017.08.073 [DOI] [PubMed] [Google Scholar]
- Jamal A, Rastkari N, Dehghaniathar R, Aghaei M, Nodehi RN, Nasseri S, Kashani H, Yunesian M, 2019. Prenatal exposure to parabens and anthropometric birth outcomes: A systematic review. Environ. Res 173, 419–431. 10.1016/j.envres.2019.02.044 [DOI] [PubMed] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, Campbell M, Donald JM, Sen S, Bero L, Zeise L, Woodruff TJ, 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ. Int 92–93, 716–728. 10.1016/j.envint.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, Kannan K, Thurman JM, Morrison D, Brody R, Urbina E, Attina T, Trasande L, Trachtman H, 2017. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res 81, 857–864. 10.1038/pr.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2014. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: an assessment of human exposure. Environ. Sci. Technol 48, 4103–9. 10.1021/es405450n [DOI] [PubMed] [Google Scholar]
- Luderer U, Eskenazi B, Hauser R, Korach KS, McHale CM, Moran F, Rieswijk L, Solomon G, Udagawa O, Zhang L, Zlatnik M, Zeise L, Smith MT, 2019. Proposed Key Characteristics of Female Reproductive Toxicants as an Approach for Organizing and Evaluating Mechanistic Data in Hazard Assessment. Environ. Health Perspect 127, 75001 10.1289/EHP4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Ghosh J, Lan Y, Senapati S, Ord T, Sapienza C, Coutifaris C, Mainigi M, 2018. Epigenetic changes in preterm birth placenta suggest a role for ADAMTS genes in spontaneous preterm birth. Hum. Mol. Genet 28, 84–95. 10.1093/hmg/ddy325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R, 2011. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect 119, 252–7. 10.1289/ehp.1002238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Conneely KN, Smith AK, 2012. DNA methylation: an epigenetic risk factor in preterm birth. Reprod. Sci 19, 6–13. 10.1177/1933719111424446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Mustieles V, Minguez-Alarcon L, Ford JB, Calafat AM, Souter I, Williams PL, Hauser R, 2018a. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ. Int 114 10.1016/j.envint.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Mustieles V, Wylie BJ, Ford JB, Keller M, Ye X, Calafat AM, Williams PL, Hauser R, Environment Team Reproductive Health Study, 2017. Ultrasound gel as an unrecognized source of exposure to phthalates and phenols among pregnant women undergoing routine scan. Int. J. Hyg. Environ. Health 220, 1285–1294. 10.1016/j.ijheh.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T, Chiu Y-H, Nassan FL, Souter I, Petrozza J, Keller M, Toth TL, Calafat AM, Hauser R, 2018b. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum. Reprod. Open 1–11. 10.1093/hropen/hoy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Messerlian C, Bellavia A, Gaskins AJ, Chiu YH, Ford JB, Azevedo AR, Petrozza JC, Calafat AM, Hauser R, Williams PL, 2019. Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ. Int 126, 355–362. 10.1016/j.envint.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Molina JM, Jiménez-Díaz I, Fernández MF, Rodriguez-Carrillo A, Peinado FM, Mustieles V, Barouki R, Piccoli C, Olea N, Freire C, 2019. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ. Res 170, 406–415. 10.1016/j.envres.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Mustieles V, Pérez-Lobato R, Olea N, Fernández MF, 2015. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 10.1016/j.neuro.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Mustieles V, Williams PL, Fernandez MF, Mínguez-Alarcón L, Ford JB, Calafat AM, Hauser R, Messerlian C, 2018. Maternal and paternal preconception exposure to bisphenols and size at birth. Hum. Reprod 33, 1528–1537. 10.1093/humrep/dey234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevoral J, Kolinko Y, Moravec J, Žalmanová T, Hošková K, Prokešová Š, Klein P, Ghaibour K, Hošek P, Štiavnická M, Řimnáčová H, Tonar Z, Petr J, Králíčková M, 2018. Long-term exposure to very low doses of bisphenol S affects female reproduction. Reproduction 156, 47–57. 10.1530/REP-18-0092 [DOI] [PubMed] [Google Scholar]
- Nowak K, Ratajczak–Wrona W, Górska M, Jabłońska E, 2018. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol 474, 238–251. 10.1016/j.mce.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Park CJ, Nah WH, Lee JE, Oh YS, Gye MC, 2012. Butyl paraben-induced changes in DNA methylation in rat epididymal spermatozoa. Andrologia 44, 187–193. 10.1111/j.1439-0272.2011.01162.x [DOI] [PubMed] [Google Scholar]
- Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM, 2009. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J. Expo. Sci. Environ. Epidemiol 19, 336–42. 10.1038/jes.2008.48 [DOI] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, Flaws JA, 2014. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ. Health Perspect 122, 775–86. 10.1289/ehp.1307728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C, 2016. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology 27, 378–88. 10.1097/EDE.0000000000000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpora M, Piacenti I, Scaramuzzino S, Masciullo L, Rech F, Benedetti Panici P, 2019. Environmental Contaminants Exposure and Preterm Birth: A Systematic Review. Toxics 7, 11 10.3390/toxics7010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect 123, 643–50. 10.1289/ehp.1408989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ, 2014. Preterm Labor: One Syndrome, Many Causes. Science (80-. ) 345, 760–765. 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Sex-specific placental responses in fetal development. Endocrinology. 10.1210/en.2015-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C, 2014. Prevention of preterm birth: Harnessing science to address the global epidemic. Sci. Transl. Med 10.1126/scitranslmed.3009871 [DOI] [PubMed] [Google Scholar]
- Samarasinghe SVAC, Krishnan K, Naidu R, Megharaj M, Miller K, Fraser B, Aitken RJ, 2018. Parabens generate reactive oxygen species in human spermatozoa. Andrology 6, 532–541. 10.1111/andr.12499 [DOI] [PubMed] [Google Scholar]
- Santangeli S, Maradonna F, Olivotto I, Piccinetti CC, Gioacchini G, Carnevali O, 2017. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. Gen. Comp. Endocrinol 245, 122–126. 10.1016/j.ygcen.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Savitz DA, Terry JW, Dole N, Thorp JM, Siega-Riz AM, Herring AH, 2002. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am. J. Obstet. Gynecol 187, 1660–6. 10.1067/mob.2002.127601 [DOI] [PubMed] [Google Scholar]
- Shi M, Sekulovski N, MacLean JA, Whorton A, Hayashi K, 2019a. Prenatal Exposure to Bisphenol A Analogues on Female Reproductive Functions in Mice. Toxicol. Sci 168, 561–571. 10.1093/toxsci/kfz014 [DOI] [PubMed] [Google Scholar]
- Shi M, Whorton AE, Sekulovski N, MacLean JA, Hayashi K, 2019b. Prenatal exposure to bisphenol A, E and S induces transgenerational effects on female reproductive functions in mice. Toxicol. Sci 10.1093/toxsci/kfz124 [DOI] [PubMed] [Google Scholar]
- Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GMB, 2015. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ. Heal 14, 73 10.1186/s12940-015-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorov A, Waxman DJ, 2015. Early programing of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies. Reprod. Toxicol 57, 59–72. 10.1016/j.reprotox.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Chen M, Ding G, Chen X, Han X, Zhou K, Chen L, Xia Y, Tian Y, Wang X, 2013. Associations of prenatal exposure to phenols with birth outcomes. Environ. Pollut 178, 115–120. 10.1016/j.envpol.2013.03.023 [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT, 2016. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int. J. Epidemiol 45, 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Toivonen KI, Oinonen KA, Duchene KM, 2017. Preconception health behaviours: A scoping review. Prev. Med. (Baltim) 96, 1–15. 10.1016/j.ypmed.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Trasande L, 2017. Exploring regrettable substitution: replacements for bisphenol A. Lancet. Planet. Heal 1, e88–e89. 10.1016/S2542-5196(17)30046-3 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 33, 378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol 24, 139–77. 10.1016/j.reprotox.2007.07.010 [DOI] [PubMed] [Google Scholar]
- von Goetz N, Pirow R, Hart A, Bradley E, Poças F, Arcella D, Lillegard ITL, Simoneau C, van Engelen J, Husoy T, Theobald A, Leclercq C, 2017. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regul. Toxicol. Pharmacol 85, 70–78. 10.1016/j.yrtph.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Wan Y, Huo W, Xu S, Zheng T, Zhang B, Li Y, Zhou A, Zhang Y, Hu J, Zhu Y, Chen Z, Lu S, Wu C, Jiang M, Jiang Y, Liu H, Yang X, Xia W, 2018. Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ. Pollut 238, 717–724. 10.1016/j.envpol.2018.03.057 [DOI] [PubMed] [Google Scholar]
- WHO, 2012. The Global Action Report on Preterm Birth Born Too Soon. URL https://apps.who.int/iris/bitstream/handle/10665/44864/9789241503433_eng.pdf?sequence=1 (accessed 6.18.19). [Google Scholar]
- Wu L-H, Zhang X-M, Wang F, Gao C-J, Chen D, Palumbo JR, Guo Y, Zeng EY, 2018. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ 615, 87–98. 10.1016/j.scitotenv.2017.09.194 [DOI] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS, 2015. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol 43, 66–75. 10.1016/j.semcdb.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM, 2015. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol 49, 11834–9. 10.1021/acs.est.5b02135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Tang Y, Xiong Y, Feng L, Li X, 2019. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J. 33, 2732–2742. 10.1096/fj.201800934RRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žalmanová T, Hošková K, Nevoral J, Adámková K, Kott T, Šulc M, Kotíková Z, Prokešová Š, Jílek F, Králíčková M, Petr J, 2017. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci. Rep 7, 485 10.1038/s41598-017-00570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M, 2010. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front. Neuroendocrinol 31, 420–439. 10.1016/j.yfrne.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkowska D, Karwacka A, Jurewicz J, Radwan M, 2018. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health 31, 377–414. 10.13075/ijomeh.1896.01195 [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X, 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 944, 152–156. 10.1016/j.jchromb.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS, 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153, 4097–110. 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


