Abstract
Background
Despite scientific advancement in radiotherapy and chemotherapy, the 5-year survival rate of lung cancer patients is around 15%. The present study explored the anticancer potential of betulinic acid nanoparticles against lung cancer cells.
Material/Methods
The proliferative changes in lung cancer cells by betulinic acid nanoparticles were measured by MTT assay. Cell cycle analysis was performed by flow cytometry using propidium iodide stain. Transwell and wound healing assay were used for determination of HKULC2 cell metastatic potential.
Results
The betulinic acid nanoparticle treatment significantly (P<0.05) reduced proliferation of HKULC2, H1299, and H23 cells. The proliferation of HKULC2, H1299, and H23 cells was reduced to 33%, 28% and 24%, respectively on treatment with 10 μM of betulinic acid nanoparticles. The results from flow cytometry showed that betulinic acid nanoparticle exposure lead to cell cycle arrest in G1 phase in HKULC2 cells. Treatment with betulinic acid nanoparticles markedly decreased migration potential of HKULC2 cells. The invasive ability of HKULC2 cells was also suppressed markedly on exposure to betulinic acid nanoparticles. Western blotting of HKULC2 cells showed that betulinic acid nanoparticles promoted the expression of p21 and p53 and downregulated CD133, ALDH, BCL2, MCL1, and c-Myc expression. Betulinic acid nanoparticles reduced the expression of ABCG1 protein markedly.
Conclusions
The present study demonstrated that betulinic acid nanoparticles inhibit proliferation, metastatic ability, and arrest cell cycle in lung cancer cells through downregulation of ABCG1 oncogene expression. Therefore, betulinic acid nanoparticles may be used as therapeutic agent for the treatment of lung cancer.
MeSH Keywords: Apoptosis; Chemoembolization, Therapeutic; Neoplasm Recurrence, Local
Background
Lung cancer has a very high incidence rate and accounts for more than 225 000 novel cases every year in USA alone [1]. The most common type of lung cancer, which accounts for ~80% of cases, is non-small cell lung cancer [2]. The prognosis for the patients with non-small cell lung cancer is dismal with a 5-year survival rate around 15% [3]. Tumor recurrence has been reported in most cases of lung cancer despite the use of radical resection and adjuvant chemotherapy [4–6]. The poor response of lung cancer patients to the currently available chemotherapeutic agents demands development of effective and novel strategies to treat lung cancer. These tumors are comprised not only of cancer cells but also contain stromal cells such as inflammatory cells, fibroblasts, and vascular cells [7]. The behavior of tumors is determined by the tumor microenvironment in addition to genetic and epigenetic factors [8–10].
The family of ABC transporter genes consists of a member known as ATP-binding cassette transporter G1 (ABCG1) [11]. The major role of ABCG1 is to regulate the cholesterol homeostasis in various cells of the body [11]. The homeostasis of cholesterol plays a vital role in the smooth functioning and survival of the cells [12]. The only pathway used for elimination cholesterol from the cells by ABCG1 involves storing it in the form of high-density lipoprotein particles [13,14]. The intracellular transport of cholesterol is also facilitated by ABCG1 [15,16]. The expression of ABCG1 has been observed ubiquitously in various types of cells such as myeloid and endothelial cells as well as in lymphocytes [11]. It is reported that ABCG1 acts as the mediator for tumor immunity in various cells [11]. A deficiency of ABCG1 has been shown to reduce MB49-bladder and B16-melanoma cancer cell growth and proliferation in mice models [17]. The survival for bladder and melanoma cancer mice models has been shown to be prolonged by ABCG1 deficiency [17]. Development of treatments for cancers using therapeutic agents involves various pathways [18–20]. The present study investigated the anticancer potential of betulinic acid nanoparticles against lung cancer cells. The study showed that betulinic acid nanoparticles inhibit proliferation, metastatic ability, and arrest cell cycle in lung cancer cells through downregulation of ABCG1 oncogene expression.
Material and Methods
Cell culture
The HKULC2, H1299, and H23 cell lines were provided by the American Type Culture Collection (Manassas, VA, USA). The cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS). The medium was also mixed with antibiotics, penicillin (100 U/mL) and streptomycin (100 U/mL). The cell lines were cultured at 37°C under humidified atmosphere of 95% air and 5% CO2.
Cell viability assay
The proliferative rate of HKULC2, H1299, and H23 cells was assessed using an MTT assay. The HKULC2, H1299, and H23 cells were put into 96-well plates at 2×105 cells per well density and maintained for 24 hours. The cells were incubated in medium mixed with 1, 2, 3, 4, 5, 6, 7, and 10 μM of betulinic acid nanoparticles for 72 hours. At the completion of treatment, 20 μL of MTT (0.5 mg/mL) solution was put into each well of the plate and cells were incubated for 4 hours. The medium in the plates was decanted and 150 μL of dimethyl sulfoxide (DMSO) was added to each well of the plate. The cell proliferation was measured by recording absorbance of the plates in a microplate reader at 485 nm wavelength.
Cell cycle analysis
The HKULC2 cells were put into 6-well culture plate at 1×105 cells per well concentration and exposed for 72 hours to 5, 6, 7, and 10 μM of betulinic acid nanoparticles. Then cells were subjected to washing twice with phosphate-buffered saline (PBS) and subsequently centrifuged at room temperature for 10 minutes at 1200 g. The cell pellets were fixed in 70% ethyl alcohol at 4°C for 12 hours before incubation with DNase-free RNaseA and propidium iodide in accordance with the instructions from manufacturer. The cell cycle progression was detected using a flow cytometer (BD Accuri™ C6; BD Biosciences, Franklin Lakes, NJ, USA).
Wound healing assay
The changes in HKULC2 cell migration potential on treatment with 5, 6, 7, and 10 μM of betulinic acid nanoparticles was assessed by wound healing assay. Briefly, HKULC2 cells were put into 6-well plates at 2×105 cells/mL concentration and grown for 12 hours. The monolayer of the cells was starved for 24 hours before a 50 mL pipette tip was used draw a straight line wound through middle of the wells. The monolayers were washed twice with PBS and then exposed to 5, 6, 7, and 10 μM of betulinic acid nanoparticles for 72 hours. The mixture of 1.5% crystal violet dye and 3.5% ethyl alcohol was used for fixing and staining the cells over 25 minutes. The cell migration was observed randomly at 5 fields using an inverted light microscope (Nikon Corporation).
Transwell assay
The Matrigel was subjected to dilution with Dulbecco’s Modified Eagle Medium (DMEM) devoid of serum after thawing for overnight at 4°C. The upper chamber was coated with 50 μL of the mixture and then subjected to incubation for 1.5 hour at 37° C. HKULC2 cells at a concentration of 1 × 105 were distributed on the upper chamber in 200 μL of DMEM medium devoid of serum. The lower compartment contained 300 μL DMEM medium mixed with 10% FBS. The cells in upper compartment were incubated with 5, 6, 7, and 10 μM of betulinic acid nanoparticles for 72 hours at 37°C. The non-invaded cells on the upper compartment were removed by using cotton swab while as the cells in lower compartment were fixed for 15 minutes with 4% formaldehyde. The cells were stained by Giemsa method followed by microscopic observation for invasive potential.
Western blot analysis
The HKULC2 cells (1×106) after exposure to 5, 6, 7, and 10 μM of betulinic acid nanoparticles for 72 hours were trypsinized and collected. Then radio immunoprecipitation assay (RIPA) lysis buffer [Tris-base (50 mM), EDTA (1 mM), sodium chloride (150 mM), sodium dodecyl sulfate (0.1%), Triton X-100 (1%), sodium deoxycholate (1%)] was added to the cells. The lysate was collected after 30 minutes and centrifuged at 4°C for 15 minutes at 12 000 g. The bicinchoninic acid (BCA) assay kit was used for determination of protein concentration in the supernatants. The 5 μg protein samples were combined with 5×sodium dodecyl sulfate loading buffer and denatured for 15 minutes in boiling water bath. The proteins were subjected to resolution using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (80 V) and subsequently transferred to polyvinylidene difluoride membranes. The membrane blocking was achieved by incubation with 50 g/L skimmed milk for 1.5 hours at room temperature. Incubation with primary antibodies against: ABCG1, c-Myc, p21, B-cell lymphoma, myeloid cell leukemia 1, CD133 and aldehyde dehydrogenase (dilution, 1: 200; Santa Cruz Biotechnology, Inc.) was performed at 4°C for overnight. The membrane washing was carried out extensively with PBS and Tween-20 3 times followed by incubation at room temperature with goat anti-rabbit-labelled horseradish peroxidase-conjugated secondary antibodies for 1 hour. The enhanced chemiluminescence detection kit was used for developing the membranes for imaging and signals were analyzed by Image Lab v3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data presented are the mean±standard deviation of three experiments carried out independently. The intergroup comparisons were performed using ANOVA followed by Tukey’s or Dunnett’s test. Statistical analysis of the data was made by using SPSS 17.0 software (IBM Corp., Armonk, NY, USA). At P<0.05 the differences were taken statistically significant.
Results
Betulinic acid nanoparticles inhibited HKULC2, H1299, and H23 cell proliferation
The proliferation of HKULC2, H1299, and H23 cells was significantly (P<0.05) reduced on addition of betulinic acid nanoparticles to the culture plates (Figure 1). The cells were incubated with 1, 2, 3, 4, 5, 6, 7, and 10 μM of betulinic acid nanoparticles and changes in proliferation were measured by MTT assay. Treatment with betulinic acid nanoparticles at 1 μM decreased HKULC2, H1299, and H23 cell proliferation to 91%, 89%, and 86%, respectively at 72 hours. The proliferation of HKULC2, H1299, and H23 cells was decreased to 33%, 28%, and 24%, respectively on treatment with 10 μM of betulinic acid nanoparticles.
Figure 1.
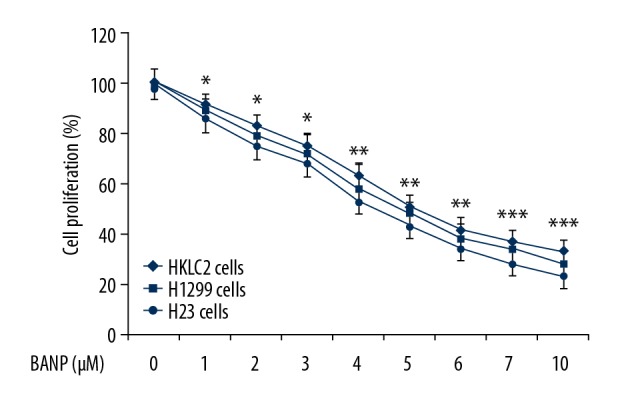
Betulinic acid nanoparticles reduce lung cancer cell proliferation. The HKULC2, H1299, and H23 cells were assessed for proliferation by MTT assay after treatment with betulinic acid nanoparticles. * P<0.05, ** P<0.02 and *** P<0.01 versus control.
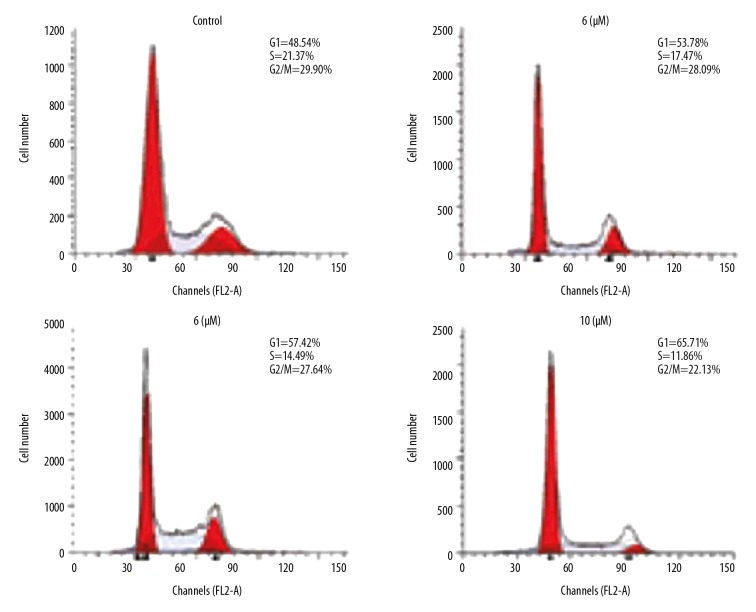
Betulinic acid nanoparticles arrested HKULC2 cell cycle
To further explore the effect of betulinic acid nanoparticles on HKULC2 cell proliferation, the changes in cell cycle distribution were studied (Figure 2). The results showed a significantly higher proportion of HKULC2 cells in G1 phase on treatment with betulinic acid nanoparticles for 72 hours. The HKULC2 cell population in S and G2/M phases showed a significant decrease on treatment in betulinic acid nanoparticles. The increase of cell population in G1 phase and decrease in S and G2/M phases was dependent on concentration of betulinic acid nanoparticles.
Figure 2.
Betulinic acid nanoparticles arrest HKULC2 cell cycle. The DNA content of the cells was observed after incubation with 5, 6, 7, and 10 μM of betulinic acid nanoparticles by flow cytometry.
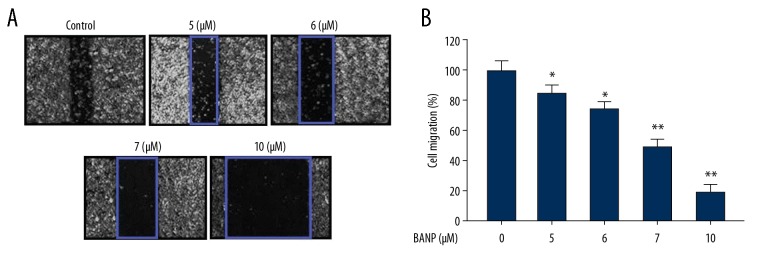
Betulinic acid nanoparticles inhibited HKULC2 cell migration
The results from wound healing assay showed that betulinic acid nanoparticles decreased migration potential of HKULC2 cells (Figure 3). The reduction of HKULC2 cell migration by betulinic acid nanoparticles was dependent on concentration. The decrease in HKULC2 cell migration was significant from 5 μM and the inhibitory effect was maximum on treatment with 10 μM of betulinic acid nanoparticles. The migration ability of HKULC2 cells was reduced by 4.5-fold on treatment with betulinic acid nanoparticles at 10 μM.
Figure 3.
(A, B) Betulinic acid nanoparticles reduced HKULC2 cell migration. The cell migration was detected following treatment with 5, 6, 7, and 10 μM of betulinic acid nanoparticles by wound healing assay. Magnification ×200. * P<0.05 and ** P<0.01 versus control.
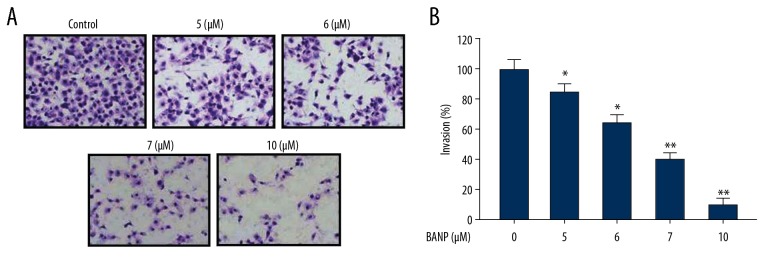
Betulinic acid nanoparticles inhibited HKULC2 cell invasion
Treatment of HKULC2 cells with betulinic acid nanoparticles reduced invasion ability in dose-based manner (Figure 4). Transwell assays showed a significant reduction in invasion ability of HKULC2 cells on treatment with 5, 6, 7, and 10 μM of betulinic acid nanoparticles. Treatment of HKULC2 cells with 10 μM of betulinic acid nanoparticles reduced invasion ability by 6.5-fold in comparison to untreated cells.
Figure 4.
(A, B) Betulinic acid nanoparticles inhibit HKULC2 cell invasion. The cell invasive ability was detected following treatment with 5, 6, 7, and 10 μM of betulinic acid nanoparticles by Transwell assay. Magnification 200×. * P<0.05 and ** P<0.01 versus control.
Betulinic acid nanoparticles altered protein expression in HKULC2 cells
The changes in protein expression by betulinic acid nanoparticles in HKULC2 cells were assessed at 5, 6, 7, and 10 μM (Figure 5). Western blot assay of HKULC2 cells showed that betulinic acid nanoparticles promoted the expression of p21 and p53 proteins and downregulated c-Myc expression. The levels of BCL2 and MCL1 in HKULC2 cells were suppressed on treatment with betulinic acid nanoparticles. Treatment of HKULC2 cells with betulinic acid nanoparticles downregulated the levels of CD133 and ALDH stem cell markers.
Figure 5.
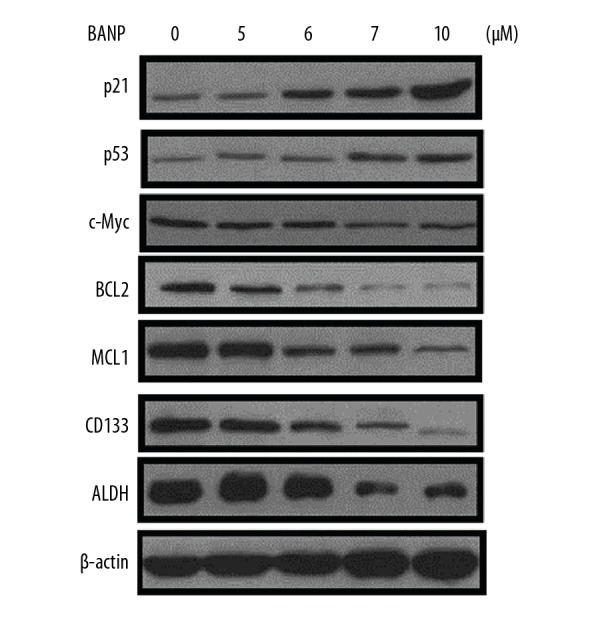
Effect of betulinic acid nanoparticles on protein expression in HKULC2 cells. The expression of various proteins in HKULC2 cells was assessed by western blot assay following treatment with betulinic acid nanoparticles.
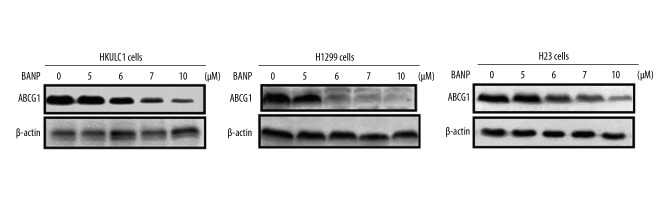
Betulinic acid nanoparticles downregulated ABCG1 expression in HKULC2, H1299, and H23 lung cancer cells
The ABCG1 protein expression in HKULC2, H1299, and H23 cancer cells was markedly higher (Figure 6). Betulinic acid nanoparticles reduced the expression of ABCG1 protein in all the 3 tested cancer cell lines significantly. The HKULC2, H1299, and H23 cells were treated with 5, 6, 7, and 10 μM of betulinic acid nanoparticles for 72 hours. Western blotting data showed reduction of ABCG1 expression in concentration dependent manner.
Figure 6.
Downregulation of ABCG1 expression in lung cancer by betulinic acid nanoparticles. Western blot analysis of HKULC2, H1299, and H23 cells treated with betulinic acid nanoparticles for determination of ABCG1 protein expression. The intensity β-actin expression was taken as loading control.
Discussion
Lung cancer is one among the common causes of deaths related to cancer and has very poor prognosis [2]. In the present study role of betulinic acid nanoparticles in inhibition of lung cancer cell proliferation and metastasis was investigated. The study also focused on the possible molecular mechanism involved in lung cancer proliferation inhibition by betulinic acid nanoparticles.
Tumor recurrence and metastasis have been found closely related to tumor stem cells’ resistance to drugs and immunosuppression [21,22]. The present study investigated the effect of betulinic acid nanoparticles on the metastatic potential of HKULC2 lung cancer cells. Transwell and wound healing assay showed that betulinic acid nanoparticles suppressed invasive and migration abilities of HKULC2 cancer cells effectively. The gap phase between G1 and S phases plays an important role in delaying growth of cells. and it is during this phase that progression to another stage of cell cycle is controlled by various intracellular and extracellular signals [23,24]. The G1 phase is considered to be a regulatory stage because during this phase cells may exit the cell cycle [25,26]. In the present study, betulinic acid nanoparticles increased the proportion of HKULC2 cells in the G1 phase. Treatment of HKULC2 cells with betulinic acid nanoparticles reduced population in the S and G2/M phases. These results proved that betulinic acid nanoparticles caused arrest of cell cycle in HKULC2 cells in G1 phase. The lung cancer stem cells containing surface marker CD133+ are bestowed with the ability to undergo self-renew and produce large population of non-tumorigenic cells [27]. The CD133+ lung carcinoma stem cells are highly tumorigenic and resistant to various anticancer drugs like cisplatin [28]. The stem cells present in breast cancer, brain tumor, multiple myeloma, and leukemia have been found to possess high activity of ALDH1 [29–36]. The higher expression of ALDH1 has also been found in lung cancer cells where it is increased by smoking and promotes transformation of lung cells into malignant tumor [37,38]. In pulmonary cancer cells, ALDH1 expression is considered to be the marker for tumor stem cells [39]. In the present study we studied the effect of betulinic acid nanoparticles on CD133+ and ALDH1 stem cell surface markers in HKULC2 cells. The results from western blotting showed that betulinic acid nanoparticle treatment of HKULC2 cells markedly reduced the expression of CD133+ and ALDH1.
Tumor growth is inhibited in absence of the cholesterol transporter ABCG1 because of modulation of carcinoma cell phenotype and survival of macrophages [17]. The excess cholesterol is eliminated from the cells in the form of high-density lipid particles by ABCG1 as reverse cholesterol transport pathway [13,14]. It has been reported that in mice, excess cholesterol and sterol esters are accumulated in lung ABCG1 macrophages [40–42]. The apoptotic rate of lung ABCG1 macrophages loaded with lipid has been reported to be very high [43]. It is believed that cholesterol accumulation in lung macrophages induced some harmful effects which can lead to lung cancer cell apoptosis [43]. The present study found markedly higher level of ABCG1 in HKULC2, H1299, and H23 lung cancer cells. However, when these lung cancer cells were treated with betulinic acid nanoparticles for 72 hours, the level of ABCG1 was significantly decreased. The expression of ABCG1 was suppressed by betulinic acid nanoparticles in concentration dependent manner. These results proved that inhibition of lung cancer cell proliferation and arrest of cell cycle by betulinic acid nanoparticles are related with the downregulation of ABCG1 expression.
Conclusions
In summary, the current study showed that betulinic acid nanoparticles exhibit cytotoxic effect on lung cancer cell, inhibit metastatic potential and arrest cell cycle through downregulation of ABCG1 oncogene expression. Therefore, betulinic acid nanoparticles can be used for treatment of lung cancer.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Tian C, Huang D, Yu Y, et al. ABCG1 as a potential oncogene in lung cancer. Expt Ther Med. 2017;13:3189–94. doi: 10.3892/etm.2017.4393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–86. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 5.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–17. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 6.Tsuboi M, Ohira T, Saji H, et al. The present status of postoperative adjuvant chemotherapy for completely resected non small cell lung cancer. Ann Thorac Cardiovasc Surg. 2007;13:73–77. [PubMed] [Google Scholar]
- 7.Tremblay G. Stromal aspects of breast carcinoma. Exp Mol Pathol. 1979;31:248–60. doi: 10.1016/0014-4800(79)90026-1. [DOI] [PubMed] [Google Scholar]
- 8.Fidler IJ. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–38. [PubMed] [Google Scholar]
- 9.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–37. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarr PT, Tarling EJ, Bojanic DD, et al. Emerging new paradigms for ABCG transporters. Biochim Biophys Acta. 2009;1791:584–93. doi: 10.1016/j.bbalip.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–38. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy MA, Barrera GC, Nakamura K, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Lan D, Chen W, et al. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774–79. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturek JM, Castle JD, Trace AP, et al. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells. J Clin Invest. 2010;120:2575–89. doi: 10.1172/JCI41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc Natl Acad Sci USA. 2011;108:19719–24. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sag D, Cekic C, Wu R, et al. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun. 2015;6:6354. doi: 10.1038/ncomms7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Li T, Zhang L, et al. A novel chalcone derivative S17 induces apoptosis through ROS dependent DR5 up regulation in gastric cancer cells. Sci Rep. 2017;7:9873. doi: 10.1038/s41598-017-10400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deligia F, Murineddu G, Gotti C, et al. Pyridinyl- and pyridazinyl-3,6-diazabicyclo[3.1.1]heptane-anilines: Novel selective ligands with subnanomolar affinity for 4-2 nACh receptors. Eur J Med Chem. 2018;152:401–16. doi: 10.1016/j.ejmech.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Deligia F, Deiana V, Gotti C, et al. Design of novel 3,6-diazabicyclo[3.1.1]heptane derivatives with potent and selective affinities for a4b2 neuronal nicotinic acetylcholine receptors. Eur J Med Chem. 2015;103:429–37. doi: 10.1016/j.ejmech.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Hou PC, Li YH, Lin SC, et al. Hypoxia induced downregulation of DUSP 2 phosphatase drives colon cancer stemness. Cancer Res. 2017;77:4305–16. doi: 10.1158/0008-5472.CAN-16-2990. [DOI] [PubMed] [Google Scholar]
- 22.Laudato S, Patil N, Abba ML, et al. P53 induced miR 30e 5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int J Cancer. 2017;141:1879–90. doi: 10.1002/ijc.30854. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–29. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 24.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 25.Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol. 1997;9:768–72. doi: 10.1016/s0955-0674(97)80076-2. [DOI] [PubMed] [Google Scholar]
- 26.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365–69. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 28.Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl AcadSci USA. 2009;106:16281–86. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–51. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 30.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–12. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hess DA, Craft TP, Wirthlin L, et al. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–20. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–69. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;6:752–60. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 34.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;15:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balicki D. Moving forward in human mammary stem cell biology and breast cancer prognostication using ALDH1. Cell Stem Cell. 2007;15:485–87. doi: 10.1016/j.stem.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Sreerama L, Sladek NE. Class 1 and class 3 aldehyde dehydrogenase levels in the human tumor cell lines currently used by the national cancer institute to screen for potentially useful antitumor agents. Adv Exp Med Biol. 1997;414:81–94. doi: 10.1007/978-1-4615-5871-2_11. [DOI] [PubMed] [Google Scholar]
- 38.Patel M, Lu L, Zander DS, et al. ALDH1A1 and ALDH3A1 expression in lung cancers: Correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–49. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–38. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Out R, Hoekstra M, Hildebrand RB, et al. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 41.Baldán A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–68. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 42.Baldán A, Tarr P, Vales CS, et al. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem. 2006;281:29401–410. doi: 10.1074/jbc.M606597200. [DOI] [PubMed] [Google Scholar]
- 43.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–82. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]